Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Actrapid 40 IU/ml solution for injection in a vial.
QUALITATIVE AND QUANTITATIVE COMPOSITION
Insulin human, rDNA (produced by recombinant DNA technology in
Saccharomyces cerevisiae
).
1 ml contains 40 IU of insulin human.
1 vial contains 10 ml equivalent to 400 IU.
One IU (International Unit) corresponds to 0.035 mg of anhydrous human insulin.
For a full list of excipients, see section 6.1.
Solution for injection in a vial.
Clear, colourless, aqueous solution.
4.1 Therapeutic indications
Treatment of diabetes mellitus.
4.2 Posology and method of administration
Actrapid is a fast-acting insulin and may be used in combination with long-acting insulin products.
Dosage
Dosage is individual and determined in accordance with the needs of the patient. The individual
insulin requirement is usually between 0.3 and 1.0 IU/kg/day. The daily insulin requirement may be
higher in patients with insulin resistance (e.g. during puberty or due to obesity) and lower in patients
with residual, endogenous insulin production.
In patients with diabetes mellitus optimised glycaemic control delays the onset of late diabetic
complications. Close blood glucose monitoring is therefore recommended.
An injection should be followed within 30 minutes by a meal or snack containing carbohydrates.
Dosage adjustment
Concomitant illness, especially infections and feverish conditions, usually increases the patient's
insulin requirement.
Renal or hepatic impairment may reduce insulin requirement.
Adjustment of dosage may also be necessary if patients change physical activity or their usual diet.
Dosage adjustment may be necessary when transferring patients from one insulin preparation to
another (see section 4.4 ).
For subcutaneous or intravenous use. Actrapid may also be administered intravenously, which should
only be carried out by health care professionals.
Actrapid is administered subcutaneously in the abdominal wall. The thigh, the gluteal region or the
deltoid region may also be used.
Subcutaneous injection into the abdominal wall ensures a faster absorption than from other injection
sites.
Injection into a lifted skin fold minimises the risk of unintended intramuscular injection.
The needle should be kept under the skin for at least 6 seconds to make sure the entire dose is injected.
Injection sites should be rotated within an anatomic region in order to avoid lipodystrophy.
The vials are for use with insulin syringes with a corresponding unit scale. When two types of insulin
are mixed, draw the amount of fast-acting insulin first, followed by the amount of long-acting insulin.
Actrapid is accompanied by a package leaflet with detailed instruction for use to be followed.
Hypersensitivity to the active substance or to any of the excipients (see section 6.1).
Hypoglycaemia.
4.4 Special warnings and precautions for use
Inadequate dosage or discontinuation of treatment, especially in type 1 diabetes, may lead to
hyperglycaemia
.
Usually, the first symptoms of hyperglycaemia set in gradually, over a period of hours or days. They
include thirst, increased frequency of urination, nausea, vomiting, drowsiness, flushed dry skin, dry
mouth, loss of appetite as well as acetone odour of breath.
In type 1 diabetes, untreated hyperglycaemic events eventually lead to diabetic ketoacidosis, which is
potentially lethal.
Hypoglycaemia
may occur if the insulin dose is too high in relation to the insulin requirement (see
sections 4.8 and 4.9).
Omission of a meal or unplanned, strenuous physical exercise may lead to hypoglycaemia.
Patients whose blood glucose control is greatly improved e.g. by intensified insulin therapy, may
experience a change in their usual warning symptoms of hypoglycaemia and should be advised
accordingly.
Usual warning symptoms may disappear in patients with longstanding diabetes.
Transferring a patient to another type or brand of insulin should be done under strict medical
supervision. Changes in strength, brand (manufacturer), type (fast-, dual-, long-acting insulin etc.),
origin (animal, human or analogue insulin) and/or method of manufacture (recombinant DNA versus
animal source insulin) may result in a need for a change in dosage. If an adjustment is needed when
switching the patients to Actrapid, it may occur with the first dose or during the first several weeks or
months.
As with any insulin therapy, injection site reactions may occur and include pain, itching, hives,
swelling and inflammation. Continuous rotation of the injection site within a given area may help to
reduce or prevent these reactions. Reactions usually resolve in a few days to a few weeks. On rare
occasions, injection site reactions may require discontinuation of Actrapid
A few patients who have experienced hypoglycaemic reactions after transfer from animal source
insulin have reported that early warning symptoms of hypoglycaemia were less pronounced or
different from those experienced with their previous insulin.
Before travelling between different time zones, the patient should be advised to consult the physician,
since this may mean that the patient has to take insulin and meals at different times.
Due to the risk of precipitation in pump catheters, Actrapid should not be used in insulin pumps for
continuous subcutaneous insulin infusion.
Actrapid contains metacresol, which may cause allergic reactions.
4.5 Interaction with other medicinal products and other forms of interaction
A number of medicinal products are known to interact with glucose metabolism. The physician must
therefore take possible interactions into account and should always ask his patients about any
medicinal products they take.
The following substances may reduce insulin requirement:
Oral hypoglycaemic agents (OHA), monoamine oxidase inhibitors (MAOI), non-selective beta-
blocking agents, angiotensin converting enzyme (ACE) inhibitors, salicylates, alcohol, anabolic
steroids and sulphonamides.
The following substances may increase insulin requirement:
Oral contraceptives, thiazides, glucocorticoids, thyroid hormones and beta-sympathomimetics, growth
hormone and danazol.
Beta-blocking agents may mask the symptoms of hypoglycaemia and delay recovery from
hypoglycaemia.
Octreotide/lanreotide may both decrease and increase insulin requirement.
Alcohol may intensify and prolong the hypoglycaemic effect of insulin.
4.6 Pregnancy and lactation
There are no restrictions on treatment of diabetes with insulin during pregnancy, as insulin does not
pass the placental barrier.
Both hypoglycaemia and hyperglycaemia, which can occur in inadequately controlled diabetes
therapy, increase the risk of malformations and death
in utero
. Intensified control in the treatment of
pregnant women with diabetes is therefore recommended throughout pregnancy and when
contemplating pregnancy.
Insulin requirements usually fall in the first trimester and subsequently increase during the second and
third trimesters.
After delivery, insulin requirements return rapidly to pre-pregnancy values.
Insulin treatment of the nursing mother presents no risk to the baby. However, the Actrapid dosage
may need to be adjusted.
4.7 Effects on ability to drive and use machines
The patient’s ability to concentrate and react may be impaired as a result of hypoglycaemia. This may
constitute a risk in situations where these abilities are of special importance (e.g. driving a car or
operating machinery).
Patients should be advised to take precautions to avoid hypoglycaemia whilst driving. This is
particularly important in those who have reduced or absent awareness of the warning signs of
hypoglycaemia or have frequent episodes of hypoglycaemia. The advisability of driving should be
considered in these circumstances.
As for other insulin products, in general, hypoglycaemia is the most frequently occurring undesirable
effect. It may occur if the insulin dose is too high in relation to the insulin requirement. In clinical
trials and during marketed use, the frequency varies with patient population and dose regimens.
Therefore, no specific frequency can be presented. Severe hypoglycaemia may lead to
unconsciousness and/or convulsions and may result in temporary or permanent impairment of brain
function or even death.
Frequencies of adverse drug reactions from clinical trials that are considered related to Actrapid, are
listed below. The frequencies are defined as: uncommon (≥1/1,000 to <1/100). Isolated spontaneous
cases are presented as very rare defined as <1/10,000, including isolated reports.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Nervous system disorders
Uncommon - Peripheral neuropathy
Fast improvement in blood glucose control may be associated with a condition termed “acute painful
neuropathy”, which is usually reversible.
Eye disorders
Uncommon - Refraction disorders
Refraction anomalies may occur upon initiation of insulin therapy. These symptoms are usually of
transitory nature.
Very rare - Diabetic retinopathy
Long-term improved glycaemic control decreases the risk of progression of diabetic retinopathy.
However, intensification of insulin therapy with abrupt improvement in glycaemic control may be
associated with temporary worsening of diabetic retinopathy.
Skin and subcutaneous tissue disorders
Uncommon - Lipodystrophy
Lipodystrophy may occur at the injection site as a consequence of failure to rotate injection sites
within an area.
General disorders and administration site conditions
Uncommon - Injection site reactions
Injection site reactions (redness, swelling, itching, pain and haematoma at the injection site) may occur
during treatment with insulin. Most reactions are transitory and disappear during continued treatment.
Uncommon - Oedema
Oedema may occur upon initiation of insulin therapy. These symptoms are usually of transitory nature.
Immune system disorders
Uncommon - Urticaria, rash
Very rare - Anaphylactic reactions
Symptoms of generalised hypersensitivity may include generalised skin rash, itching, sweating,
gastrointestinal upset, angioneurotic oedema, difficulties in breathing, palpitation, reduction in blood
pressure and fainting/loss of consciousness. Generalised hypersensitivity reactions are potentially life-
threatening.
Mild hypoglycaemic episodes can be treated by oral administration of glucose or sugary
products. It is therefore recommended that the diabetic patients carry some sugar lumps, sweets,
biscuits or sugary fruit juice.
A specific overdose of insulin cannot be defined. However, hypoglycaemia may develop over
sequential stages:
•
Severe hypoglycaemic episodes, where the patient has become unconscious, can be treated by
glucagon (0.5 to 1 mg) given intramuscularly or subcutaneously by a person who has received
appropriate instruction, or by glucose given intravenously by a medical professional. Glucose
must also be given intravenously, if the patient does not respond to glucagon within 10 to
15 minutes.
Upon regaining consciousness, administration of oral carbohydrate is recommended for the
patient in order to prevent relapse.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: insulins and analogues for injection, fast-acting, insulin (human). ATC
code: A10A B01.
The blood glucose lowering effect of insulin is due to the facilitated uptake of glucose following
binding of insulin to receptors on muscle and fat cells and to the simultaneous inhibition of glucose
output from the liver.
A clinical trial in a single intensive care unit treating hyperglycaemia (blood glucose above 10 mmol/l)
in 204 diabetic and 1344 non-diabetic patients undergoing major surgery showed that normoglycaemia
(blood glucose 4.4 – 6.1 mmol/l) induced by intravenous Actrapid reduced mortality by 42% (8%
versus 4.6%).
Actrapid is a fast-acting insulin.
Onset of action is within ½ hour, reaches a maximum effect within 1.5-3.5 hours and the entire
duration of action is approximately 7-8 hours.
5.2 Pharmacokinetic properties
Insulin in the blood stream has a half-life of a few minutes. Consequently, the time-action profile of an
insulin preparation is determined solely by its absorption characteristics.
This process is influenced by several factors (e.g. insulin dosage, injection route and site, thickness of
subcutaneous fat, type of diabetes). The pharmacokinetics of insulin products are therefore affected by
significant intra- and inter-individual variation.
Absorption
The maximum plasma concentration is reached within 1.5-2.5 hours after subcutaneous
administration.
Distribution
No profound binding to plasma proteins, except circulating insulin antibodies (if present) has been
observed.
Metabolism
Human insulin is reported to be degraded by insulin protease or insulin-degrading enzymes and
possibly protein disulfide isomerase. A number of cleavage (hydrolysis) sites on the human insulin
molecule have been proposed; none of the metabolites formed following the cleavage are active.
Elimination
The terminal half-life is determined by the rate of absorption from the subcutaneous tissue. The
terminal half-life (t
½
) is therefore a measure of the absorption rather than of the elimination
per se
of
insulin from plasma (insulin in the blood stream has a t
½
of a few minutes). Trials have indicated a t
½
of about 2-5 hours.
Children and adolescents
The pharmacokinetic profile of Actrapid has been studied in a small number (n=18) of diabetic
children (aged 6-12 years) and adolescents (aged 13-17 years). The data are limited but suggest that
the pharmacokinetic profile in children and adolescents may be similar to that in adults. However,
there were differences between age groups in C
max,
stressing the importance of individual dose
titration.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction.
PHARMACEUTICAL PARTICULARS
Zinc chloride
Glycerol
Metacresol
Sodium hydroxide (for pH adjustment)
Hydrochloric acid (for pH adjustment)
Water for injections
Insulin products should only be added to compounds with which it is known to be compatible.
Medicinal products added to the insulin solution may cause degradation of the insulin, e.g. if the
medicinal products contain thiols or sulphites.
30 months when stored between 2°C - 8°C.
4 weeks when used or stored at room temperature (below 25°C).
6.4 Special precautions for storage
Before use: store in a refrigerator (2°C - 8°C).
Do not store them in or too near the freezer section or cooling element.
Do not freeze.
During use: do not refrigerate. Do not store above 25°C.
Keep the vial in the outer carton in order to protect from light.
Protect from excessive heat and sunlight.
6.5 Nature and contents of container
10 ml glass vial (type 1) closed with a bromobutyl/polyisoprene rubber stopper and a protective
tamper-proof plastic cap.
Pack sizes: 1 and 5 vials x 10 ml and a multipack with 5 x (1 x 10 ml) vials.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
For intravenous use, infusion systems with Actrapid at concentrations 0.05 IU/ml - 1.0 IU/ml insulin
human in the following infusion fluids; 0.9% sodium chloride, 5% dextrose and 10% dextrose
inclusive 40 mmol/l potassium chloride, using polypropylene infusion bags, are stable at room
temperature for 24 hours. Although stable over time, a certain amount of insulin will initially be
absorbed to the material of the infusion bag. Monitoring of blood glucose is necessary during the
infusion.
Insulin preparations which have been frozen must not be used.
Insulin solutions should not be used if they do not appear water clear and colourless.
Actrapid should not be used in insulin pumps for continuous subcutaneous insulin infusion.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 07 October 2002
Date of latest renewal: 18 September 2007
10. DATE OF REVISION OF THE TEXT
NAME OF THE MEDICINAL PRODUCT
Actrapid 100 IU/ml solution for injection in a vial
QUALITATIVE AND QUANTITATIVE COMPOSITION
Insulin human, rDNA (produced by recombinant DNA technology in
Saccharomyces cerevisiae
).
1 ml contains 100 IU of insulin human.
1 vial contains 10 ml equivalent to 1000 IU.
One IU (International Unit) corresponds to 0.035 mg of anhydrous human insulin.
For a full list of excipients, see section 6.1.
Solution for injection in a vial.
Clear, colourless, aqueous solution.
4.1 Therapeutic indications
Treatment of diabetes mellitus.
4.2 Posology and method of administration
Actrapid is a fast-acting insulin and may be used in combination with long-acting insulin products.
Dosage
Dosage is individual and determined in accordance with the needs of the patient. The individual
insulin requirement is usually between 0.3 and 1.0 IU/kg/day. The daily insulin requirement may be
higher in patients with insulin resistance (e.g. during puberty or due to obesity) and lower in patients
with residual, endogenous insulin production.
In patients with diabetes mellitus optimised glycaemic control delays the onset of late diabetic
complications. Close blood glucose monitoring is therefore recommended.
An injection should be followed within 30 minutes by a meal or snack containing carbohydrates.
Dosage adjustment
Concomitant illness, especially infections and feverish conditions, usually increases the patient's
insulin requirement.
Renal or hepatic impairment may reduce insulin requirement.
Adjustment of dosage may also be necessary if patients change physical activity or their usual diet.
Dosage adjustment may be necessary when transferring patients from one insulin preparation to
another (see section 4.4).
For subcutaneous or intravenous use. Actrapid may also be administered intravenously, which should
only be carried out by health care professionals.
Actrapid is administered subcutaneously in the abdominal wall. The thigh, the gluteal region or the
deltoid region may also be used.
Subcutaneous injection into the abdominal wall ensures a faster absorption than from other injection
sites.
Injection into a lifted skin fold minimises the risk of unintended intramuscular injection.
The needle should be kept under the skin for at least 6 seconds to make sure the entire dose is injected.
Injection sites should be rotated within an anatomic region in order to avoid lipodystrophy.
The vials are for use with insulin syringes with a corresponding unit scale. When two types of insulin
are mixed, draw the amount of fast-acting insulin first, followed by the amount of long-acting insulin.
Actrapid is accompanied by a package leaflet with detailed instruction for use to be followed.
Hypersensitivity to the active substance or to any of the excipients (see section 6.1).
Hypoglycaemia.
4.4 Special warnings and precautions for use
Inadequate dosage or discontinuation of treatment, especially in type 1 diabetes, may lead to
hyperglycaemia
.
Usually, the first symptoms of hyperglycaemia set in gradually, over a period of hours or days. They
include thirst, increased frequency of urination, nausea, vomiting, drowsiness, flushed dry skin, dry
mouth, loss of appetite as well as acetone odour of breath.
In type 1 diabetes, untreated hyperglycaemic events eventually lead to diabetic ketoacidosis, which is
potentially lethal.
Hypoglycaemia
may occur if the insulin dose is too high in relation to the insulin requirement (see
sections 4.8 and 4.9).
Omission of a meal or unplanned, strenuous physical exercise may lead to hypoglycaemia.
Patients whose blood glucose control is greatly improved e.g. by intensified insulin therapy, may
experience a change in their usual warning symptoms of hypoglycaemia and should be advised
accordingly.
Usual warning symptoms may disappear in patients with longstanding diabetes.
Transferring a patient to another type or brand of insulin should be done under strict medical
supervision. Changes in strength, brand (manufacturer), type (fast-, dual-, long-acting insulin etc.),
origin (animal, human or analogue insulin) and/or method of manufacture (recombinant DNA versus
animal source insulin) may result in a need for a change in dosage. If an adjustment is needed when
switching the patients to Actrapid, it may occur with the first dose or during the first several weeks or
months.
As with any insulin therapy, injection site reactions may occur and include pain, itching, hives,
swelling and inflammation. Continuous rotation of the injection site within a given area may help to
reduce or prevent these reactions. Reactions usually resolve in a few days to a few weeks. On rare
occasions, injection site reactions may require discontinuation of Actrapid
A few patients who have experienced hypoglycaemic reactions after transfer from animal source
insulin have reported that early warning symptoms of hypoglycaemia were less pronounced or
different from those experienced with their previous insulin.
Before travelling between different time zones, the patient should be advised to consult the physician,
since this may mean that the patient has to take insulin and meals at different times.
Due to the risk of precipitation in pump catheters, Actrapid should not be used in insulin pumps for
continuous subcutaneous insulin infusion.
Actrapid contains metacresol, which may cause allergic reactions.
4.5 Interaction with other medicinal products and other forms of interaction
A number of medicinal products are known to interact with glucose metabolism. The physician must
therefore take possible interactions into account and should always ask his patients about any
medicinal products they take.
The following substances may reduce insulin requirement:
Oral hypoglycaemic agents (OHA), monoamine oxidase inhibitors (MAOI), non-selective beta-
blocking agents, angiotensin converting enzyme (ACE) inhibitors, salicylates, alcohol, anabolic
steroids and sulphonamides.
The following substances may increase insulin requirement:
Oral contraceptives, thiazides, glucocorticoids, thyroid hormones and beta-sympathomimetics, growth
hormone and danazol.
Beta-blocking agents may mask the symptoms of hypoglycaemia and delay recovery from
hypoglycaemia.
Octreotide/lanreotide may both decrease and increase insulin requirement.
Alcohol may intensify and prolong the hypoglycaemic effect of insulin.
4.6 Pregnancy and lactation
There are no restrictions on treatment of diabetes with insulin during pregnancy, as insulin does not
pass the placental barrier.
Both hypoglycaemia and hyperglycaemia, which can occur in inadequately controlled diabetes
therapy, increase the risk of malformations and death
in utero
. Intensified control in the treatment of
pregnant women with diabetes is therefore recommended throughout pregnancy and when
contemplating pregnancy.
Insulin requirements usually fall in the first trimester and subsequently increase during the second and
third trimesters.
After delivery, insulin requirements return rapidly to pre-pregnancy values.
Insulin treatment of the nursing mother presents no risk to the baby. However, the Actrapid dosage
may need to be adjusted.
4.7 Effects on ability to drive and use machines
The patient’s ability to concentrate and react may be impaired as a result of hypoglycaemia. This may
constitute a risk in situations where these abilities are of special importance (e.g. driving a car or
operating machinery).
Patients should be advised to take precautions to avoid hypoglycaemia whilst driving. This is
particularly important in those who have reduced or absent awareness of the warning signs of
hypoglycaemia or have frequent episodes of hypoglycaemia. The advisability of driving should be
considered in these circumstances.
As for other insulin products, in general, hypoglycaemia is the most frequently occurring undesirable
effect. It may occur if the insulin dose is too high in relation to the insulin requirement. In clinical
trials and during marketed use, the frequency varies with patient population and dose regimens.
Therefore, no specific frequency can be presented. Severe hypoglycaemia may lead to
unconsciousness and/or convulsions and may result in temporary or permanent impairment of brain
function or even death.
Frequencies of adverse drug reactions from clinical trials that are considered related to Actrapid, are
listed below. The frequencies are defined as: uncommon (≥1/1,000 to <1/100). Isolated spontaneous
cases are presented as very rare defined as <1/10,000, including isolated reports.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Nervous system disorders
Uncommon - Peripheral neuropathy
Fast improvement in blood glucose control may be associated with a condition termed “acute painful
neuropathy”, which is usually reversible.
Eye disorders
Uncommon - Refraction disorders
Refraction anomalies may occur upon initiation of insulin therapy. These symptoms are usually of
transitory nature.
Very rare - Diabetic retinopathy
Long-term improved glycaemic control decreases the risk of progression of diabetic retinopathy.
However, intensification of insulin therapy with abrupt improvement in glycaemic control may be
associated with temporary worsening of diabetic retinopathy.
Skin and subcutaneous tissue disorders
Uncommon - Lipodystrophy
Lipodystrophy may occur at the injection site as a consequence of failure to rotate injection sites
within an area.
General disorders and administration site conditions
Uncommon - Injection site reactions
Injection site reactions (redness, swelling, itching, pain and haematoma at the injection site) may occur
during treatment with insulin. Most reactions are transitory and disappear during continued treatment.
Uncommon - Oedema
Oedema may occur upon initiation of insulin therapy. These symptoms are usually of transitory nature.
Immune system disorders
Uncommon - Urticaria, rash
Very rare - Anaphylactic reactions
Symptoms of generalised hypersensitivity may include generalised skin rash, itching, sweating,
gastrointestinal upset, angioneurotic oedema, difficulties in breathing, palpitation, reduction in blood
pressure and fainting/loss of consciousness. Generalised hypersensitivity reactions are potentially life-
threatening.
Mild hypoglycaemic episodes can be treated by oral administration of glucose or sugary
products. It is therefore recommended that the diabetic patients carry some sugar lumps, sweets,
biscuits or sugary fruit juice.
A specific overdose of insulin cannot be defined. However, hypoglycaemia may develop over
sequential stages:
•
Severe hypoglycaemic episodes, where the patient has become unconscious, can be treated by
glucagon (0.5 to 1 mg) given intramuscularly or subcutaneously by a person who has received
appropriate instruction, or by glucose given intravenously by a medical professional. Glucose
must also be given intravenously, if the patient does not respond to glucagon within 10 to
15 minutes.
Upon regaining consciousness, administration of oral carbohydrate is recommended for the
patient in order to prevent relapse.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: insulins and analogues for injection, fast-acting, insulin (human). ATC
code: A10A B01.
The blood glucose lowering effect of insulin is due to the facilitated uptake of glucose following
binding of insulin to receptors on muscle and fat cells and to the simultaneous inhibition of glucose
output from the liver.
A clinical trial in a single intensive care unit treating hyperglycaemia (blood glucose above 10 mmol/l)
in 204 diabetic and 1344 non-diabetic patients undergoing major surgery showed that normoglycaemia
(blood glucose 4.4 – 6.1 mmol/l) induced by intravenous Actrapid reduced mortality by 42% (8%
versus 4.6%).
Actrapid is a fast-acting insulin.
Onset of action is within ½ hour, reaches a maximum effect within 1.5-3.5 hours and the entire
duration of action is approximately 7-8 hours.
5.2 Pharmacokinetic properties
Insulin in the blood stream has a half-life of a few minutes. Consequently, the time-action profile of an
insulin preparation is determined solely by its absorption characteristics.
This process is influenced by several factors (e.g. insulin dosage, injection route and site, thickness of
subcutaneous fat, type of diabetes). The pharmacokinetics of insulin products are therefore affected by
significant intra- and inter-individual variation.
Absorption
The maximum plasma concentration is reached within 1.5-2.5 hours after subcutaneous
administration.
Distribution
No profound binding to plasma proteins, except circulating insulin antibodies (if present) has been
observed.
Metabolism
Human insulin is reported to be degraded by insulin protease or insulin-degrading enzymes and
possibly protein disulfide isomerase. A number of cleavage (hydrolysis) sites on the human insulin
molecule have been proposed; none of the metabolites formed following the cleavage are active.
Elimination
The terminal half-life is determined by the rate of absorption from the subcutaneous tissue. The
terminal half-life (t
½
) is therefore a measure of the absorption rather than of the elimination
per se
of
insulin from plasma (insulin in the blood stream has a t
½
of a few minutes). Trials have indicated a t
½
of about 2-5 hours.
Children and adolscents
The pharmacokinetic profile of Actrapid has been studied in a small number (n=18) of diabetic
children (aged 6-12 years) and adolescents (aged 13-17 years). The data are limited but suggest that
the pharmacokinetic profile in children and adolescents may be similar to that in adults. However,
there were differences between age groups in C
max,
stressing the importance of individual dose
titration.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction.
PHARMACEUTICAL PARTICULARS
Zinc chloride
Glycerol
Metacresol
Sodium hydroxide (for pH adjustment)
Hydrochloric acid (for pH adjustment)
Water for injections
Insulin products should only be added to compounds with which it is known to be compatible.
Medicinal products added to the insulin solution may cause degradation of the insulin, e.g. if the
medicinal products contain thiols or sulphites.
30 months when stored between 2°C - 8°C.
6 weeks when used or stored at room temperature (below 25°C)
6.4 Special precautions for storage
Before use: store in a refrigerator (2°C - 8°C).
Do not store them in or too near the freezer section or cooling element.
Do not freeze.
During use: do not refrigerate. Do not store above 25°C.
Keep the vial in the outer carton in order to protect from light.
Protect from excessive heat and sunlight.
6.5 Nature and contents of container
10 ml glass vial (type 1) closed with a bromobutyl/polyisoprene rubber stopper and a protective
tamper-proof plastic cap.
Pack sizes: 1 and 5 vials x 10 ml and a multipack with 5 x (1 x 10 ml) vials.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
For intravenous use, infusion systems with Actrapid at concentrations 0.05 IU/ml - 1.0 IU/ml insulin
human in the following infusion fluids; 0.9% sodium chloride, 5% dextrose and 10% dextrose
inclusive 40 mmol/l potassium chloride, using polypropylene infusion bags, are stable at room
temperature for 24 hours. Although stable over time, a certain amount of insulin will initially be
absorbed to the material of the infusion bag. Monitoring of blood glucose is necessary during the
infusion.
Insulin preparations which have been frozen must not be used.
Insulin solutions should not be used if they do not appear water clear and colourless.
Actrapid should not be used in insulin pumps for continuous subcutaneous insulin infusion.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 07 October 2002
Date of latest renewal: 18 September 2007
10. DATE OF REVISION OF THE TEXT
NAME OF THE MEDICINAL PRODUCT
Actrapid Penfill 100 IU/ml solution for injection in a cartridge
QUALITATIVE AND QUANTITATIVE COMPOSITION
Insulin human, rDNA (produced by recombinant DNA technology in
Saccharomyces cerevisiae
).
1 ml contains 100 IU of insulin human.
1 cartridge contains 3 ml equivalent to 300 IU.
One IU (International Unit) corresponds to 0.035 mg of anhydrous human insulin.
For a full list of excipients, see section 6.1.
Solution for injection in a cartridge.
Clear, colourless, aqueous solution.
4.1 Therapeutic indications
Treatment of diabetes mellitus.
4.2 Posology and method of administration
Actrapid is a fast-acting insulin and may be used in combination with long-acting insulin products.
Dosage
Dosage is individual and determined in accordance with the needs of the patient. The individual
insulin requirement is usually between 0.3 and 1.0 IU/kg/day. The daily insulin requirement may be
higher in patients with insulin resistance (e.g. during puberty or due to obesity) and lower in patients
with residual, endogenous insulin production.
In patients with diabetes mellitus optimised glycaemic control delays the onset of late diabetic
complications. Close blood glucose monitoring is therefore recommended.
An injection should be followed within 30 minutes by a meal or snack containing carbohydrates.
Dosage adjustment
Concomitant illness, especially infections and feverish conditions, usually increases the patient's
insulin requirement.
Renal or hepatic impairment may reduce insulin requirement.
Adjustment of dosage may also be necessary if patients change physical activity or their usual diet.
Dosage adjustment may be necessary when transferring patients from one insulin preparation to
another (see section 4.4).
For subcutaneous use. The intravenous use of Actrapid from any pen or cartridge should be an
exception only in situations where vials are not available. In this case Actrapid should be drawn into
an insulin syringe, provided air is avoided, or infused with an infusion system. This procedure should
only be carried out by health care professionals.
Actrapid is administered subcutaneously in the abdominal wall. The thigh, the gluteal region or the
deltoid region may also be used.
Subcutaneous injection into the abdominal wall ensures a faster absorption than from other injection
sites.
Injection into a lifted skin fold minimises the risk of unintended intramuscular injection.
The needle should be kept under the skin for at least 6 seconds to make sure the entire dose is injected.
Injection sites should be rotated within an anatomic region in order to avoid lipodystrophy.
The cartridges are designed to be used with Novo Nordisk delivery systems (durable devices for
repeated use) and NovoFine or NovoTwist needles. Detailed instruction accompanying the delivery
system must be followed.
Actrapid is accompanied by a package leaflet with detailed instruction for use to be followed.
Hypersensitivity to the active substance or to any of the excipients (see section 6.1).
Hypoglycaemia.
4.4 Special warnings and precautions for use
Inadequate dosage or discontinuation of treatment, especially in type 1 diabetes, may lead to
hyperglycaemia
.
Usually, the first symptoms of hyperglycaemia set in gradually, over a period of hours or days. They
include thirst, increased frequency of urination, nausea, vomiting, drowsiness, flushed dry skin, dry
mouth, loss of appetite as well as acetone odour of breath.
In type 1 diabetes, untreated hyperglycaemic events eventually lead to diabetic ketoacidosis, which is
potentially lethal.
Hypoglycaemia
may occur if the insulin dose is too high in relation to the insulin requirement (see
sections 4.8 and 4.9).
Omission of a meal or unplanned, strenuous physical exercise may lead to hypoglycaemia.
Patients whose blood glucose control is greatly improved e.g. by intensified insulin therapy, may
experience a change in their usual warning symptoms of hypoglycaemia and should be advised
accordingly.
Usual warning symptoms may disappear in patients with longstanding diabetes.
Transferring a patient to another type or brand of insulin should be done under strict medical
supervision. Changes in strength, brand (manufacturer), type (fast-, dual-, long-acting insulin etc.),
origin (animal, human or analogue insulin) and/or method of manufacture (recombinant DNA versus
animal source insulin) may result in a need for a change in dosage. If an adjustment is needed when
switching the patients to Actrapid, it may occur with the first dose or during the first several weeks or
months.
As with any insulin therapy, injection site reactions may occur and include pain, itching, hives,
swelling and inflammation. Continuous rotation of the injection site within a given area may help to
reduce or prevent these reactions. Reactions usually resolve in a few days to a few weeks. On rare
occasions, injection site reactions may require discontinuation of Actrapid
A few patients who have experienced hypoglycaemic reactions after transfer from animal source
insulin have reported that early warning symptoms of hypoglycaemia were less pronounced or
different from those experienced with their previous insulin.
Before travelling between different time zones, the patient should be advised to consult the physician,
since this may mean that the patient has to take insulin and meals at different times.
Due to the risk of precipitation in pump catheters, Actrapid should not be used in insulin pumps for
continuous subcutaneous insulin infusion.
Actrapid contains metacresol, which may cause allergic reactions.
4.5 Interaction with other medicinal products and other forms of interaction
A number of medicinal products are known to interact with glucose metabolism. The physician must
therefore take possible interactions into account and should always ask his patients about any
medicinal products they take.
The following substances may reduce insulin requirement:
Oral hypoglycaemic agents (OHA), monoamine oxidase inhibitors (MAOI), non-selective beta-
blocking agents, angiotensin converting enzyme (ACE) inhibitors, salicylates, alcohol, anabolic
steroids and sulphonamides.
The following substances may increase insulin requirement:
Oral contraceptives, thiazides, glucocorticoids, thyroid hormones and beta-sympathomimetics, growth
hormone and danazol.
Beta-blocking agents may mask the symptoms of hypoglycaemia and delay recovery from
hypoglycaemia.
Octreotide/lanreotide may both decrease and increase insulin requirement.
Alcohol may intensify and prolong the hypoglycaemic effect of insulin.
4.6 Pregnancy and lactation
There are no restrictions on treatment of diabetes with insulin during pregnancy, as insulin does not
pass the placental barrier.
Both hypoglycaemia and hyperglycaemia, which can occur in inadequately controlled diabetes
therapy, increase the risk of malformations and death
in utero
. Intensified control in the treatment of
pregnant women with diabetes is therefore recommended throughout pregnancy and when
contemplating pregnancy.
Insulin requirements usually fall in the first trimester and subsequently increase during the second and
third trimesters.
After delivery, insulin requirements return rapidly to pre-pregnancy values.
Insulin treatment of the nursing mother presents no risk to the baby. However, the Actrapid dosage
may need to be adjusted.
4.7 Effects on ability to drive and use machines
The patient’s ability to concentrate and react may be impaired as a result of hypoglycaemia. This may
constitute a risk in situations where these abilities are of special importance (e.g. driving a car or
operating machinery).
Patients should be advised to take precautions to avoid hypoglycaemia whilst driving. This is
particularly important in those who have reduced or absent awareness of the warning signs of
hypoglycaemia or have frequent episodes of hypoglycaemia. The advisability of driving should be
considered in these circumstances.
As for other insulin products, in general, hypoglycaemia is the most frequently occurring undesirable
effect. It may occur if the insulin dose is too high in relation to the insulin requirement. In clinical
trials and during marketed use, the frequency varies with patient population and dose regimens.
Therefore, no specific frequency can be presented. Severe hypoglycaemia may lead to
unconsciousness and/or convulsions and may result in temporary or permanent impairment of brain
function or even death.
Frequencies of adverse drug reactions from clinical trials that are considered related to Actrapid, are
listed below. The frequencies are defined as: uncommon (≥1/1,000 to <1/100). Isolated spontaneous
cases are presented as very rare defined as <1/10,000, including isolated reports.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Nervous system disorders
Uncommon - Peripheral neuropathy
Fast improvement in blood glucose control may be associated with a condition termed “acute painful
neuropathy”, which is usually reversible.
Eye disorders
Uncommon - Refraction disorders
Refraction anomalies may occur upon initiation of insulin therapy. These symptoms are usually of
transitory nature.
Very rare - Diabetic retinopathy
Long-term improved glycaemic control decreases the risk of progression of diabetic retinopathy.
However, intensification of insulin therapy with abrupt improvement in glycaemic control may be
associated with temporary worsening of diabetic retinopathy.
Skin and subcutaneous tissue disorders
Uncommon - Lipodystrophy
Lipodystrophy may occur at the injection site as a consequence of failure to rotate injection sites
within an area.
General disorders and administration site conditions
Uncommon - Injection site reactions
Injection site reactions (redness, swelling, itching, pain and haematoma at the injection site) may occur
during treatment with insulin. Most reactions are transitory and disappear during continued treatment.
Uncommon - Oedema
Oedema may occur upon initiation of insulin therapy. These symptoms are usually of transitory nature.
Immune system disorders
Uncommon - Urticaria, rash
Very rare - Anaphylactic reactions
Symptoms of generalised hypersensitivity may include generalised skin rash, itching, sweating,
gastrointestinal upset, angioneurotic oedema, difficulties in breathing, palpitation, reduction in blood
pressure and fainting/loss of consciousness. Generalised hypersensitivity reactions are potentially life-
threatening.
A specific overdose of insulin cannot be defined. However, hypoglycaemia may develop over
sequential stages:
Mild hypoglycaemic episodes can be treated by oral administration of glucose or sugary
products. It is therefore recommended that the diabetic patients carry some sugar lumps, sweets,
biscuits or sugary fruit juice.
Severe hypoglycaemic episodes, where the patient has become unconscious, can be treated by
glucagon (0.5 to 1 mg) given intramuscularly or subcutaneously by a person who has received
appropriate instruction, or by glucose given intravenously by a medical professional. Glucose
must also be given intravenously, if the patient does not respond to glucagon within 10 to
15 minutes.
Upon regaining consciousness, administration of oral carbohydrate is recommended for the
patient in order to prevent relapse.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: insulins and analogues for injection, fast-acting, insulin (human). ATC
code: A10A B01.
The blood glucose lowering effect of insulin is due to the facilitated uptake of glucose following
binding of insulin to receptors on muscle and fat cells and to the simultaneous inhibition of glucose
output from the liver.
A clinical trial in a single intensive care unit treating hyperglycaemia (blood glucose above 10 mmol/l)
in 204 diabetic and 1344 non-diabetic patients undergoing major surgery showed that normoglycaemia
(blood glucose 4.4 – 6.1 mmol/l) induced by intravenous Actrapid reduced mortality by 42% (8%
versus 4.6%).
Actrapid is a fast-acting insulin.
Onset of action is within ½ hour, reaches a maximum effect within 1.5-3.5 hours and the entire
duration of action is approximately 7-8 hours.
5.2 Pharmacokinetic properties
Insulin in the blood stream has a half-life of a few minutes. Consequently, the time-action profile of an
insulin preparation is determined solely by its absorption characteristics.
This process is influenced by several factors (e.g. insulin dosage, injection route and site, thickness of
subcutaneous fat, type of diabetes). The pharmacokinetics of insulin products are therefore affected by
significant intra- and inter-individual variation.
Absorption
The maximum plasma concentration is reached within 1.5-2.5 hours after subcutaneous
administration.
Distribution
No profound binding to plasma proteins, except circulating insulin antibodies (if present) has been
observed.
Metabolism
Human insulin is reported to be degraded by insulin protease or insulin-degrading enzymes and
possibly protein disulfide isomerase. A number of cleavage (hydrolysis) sites on the human insulin
molecule have been proposed; none of the metabolites formed following the cleavage are active.
Elimination
The terminal half-life is determined by the rate of absorption from the subcutaneous tissue. The
terminal half-life (t
½
) is therefore a measure of the absorption rather than of the elimination
per se
of
insulin from plasma (insulin in the blood stream has a t
½
of a few minutes). Trials have indicated a t
½
of about 2-5 hours.
Children and adolescents
The pharmacokinetic profile of Actrapid has been studied in a small number (n=18) of diabetic
children (aged 6-12 years) and adolescents (aged 13-17 years). The data are limited but suggest that
the pharmacokinetic profile in children and adolescents may be similar to that in adults. However,
there were differences between age groups in C
max,
stressing the importance of individual dose
titration.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction.
PHARMACEUTICAL PARTICULARS
Zinc chloride
Glycerol
Metacresol
Sodium hydroxide (for pH adjustment)
Hydrochloric acid (for pH adjustment)
Water for injections
Insulin products should only be added to compounds with which it is known to be compatible.
Medicinal products added to the insulin solution may cause degradation of the insulin, e.g. if the
medicinal products contain thiols or sulphites.
30 months when stored between 2°C - 8°C.
6 weeks when used or carried as a spare (below 30°C).
6.4 Special precautions for storage
Before use: store in a refrigerator (2°C - 8°C).
Do not store them in or too near the freezer section or cooling element.
Do not freeze.
During use: do not refrigerate. Do not store above 30°C.
Keep the cartridge in the outer carton in order to protect from light.
Protect from excessive heat and sunlight.
6.5 Nature and contents of container
3 ml glass cartridge (type 1) with a bromobutyl rubber plunger and a bromobutyl/polyisoprene rubber
stopper.
Pack sizes: 1, 5 and 10 cartridges x 3 ml.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
For intravenous use, infusion systems with Actrapid at concentrations 0.05 IU/ml - 1.0 IU/ml insulin
human in the following infusion fluids; 0.9% sodium chloride, 5% dextrose and 10% dextrose
inclusive 40 mmol/l potassium chloride, using polypropylene infusion bags, are stable at room
temperature for 24 hours. Although stable over time, a certain amount of insulin will initially be
absorbed to the material of the infusion bag. Monitoring of blood glucose is necessary during the
infusion.
Cartridges should only be used in combination with products that are compatible with them and allow
the cartridge to function safely and effectively.
Actrapid Penfill is for single person use only. The container must not be refilled.
Insulin preparations which have been frozen must not be used.
Insulin solutions should not be used if they do not appear water clear and colourless.
Actrapid should not be used in insulin pumps for continuous subcutaneous insulin infusion.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 07 October 2002
Date of latest renewal: 18 September 2007
10. DATE OF REVISION OF THE TEXT
NAME OF THE MEDICINAL PRODUCT
Actrapid NovoLet 100 IU/ml solution for injection in a pre-filled pen
QUALITATIVE AND QUANTITATIVE COMPOSITION
Insulin human, rDNA (produced by recombinant DNA technology in
Saccharomyces cerevisiae
).
1 ml contains 100 IU of insulin human.
1 pre-filled pen contains 3 ml equivalent to 300 IU.
One IU (International Unit) corresponds to 0.035 mg of anhydrous human insulin.
For a full list of excipients, see section 6.1.
Solution for injection in a pre-filled pen.
Clear, colourless, aqueous solution.
4.1 Therapeutic indications
Treatment of diabetes mellitus.
4.2 Posology and method of administration
Actrapid is a fast-acting insulin and may be used in combination with long-acting insulin products.
Dosage
Dosage is individual and determined in accordance with the needs of the patient. The individual
insulin requirement is usually between 0.3 and 1.0 IU/kg/day. The daily insulin requirement may be
higher in patients with insulin resistance (e.g. during puberty or due to obesity) and lower in patients
with residual, endogenous insulin production.
In patients with diabetes mellitus optimised glycaemic control delays the onset of late diabetic
complications. Close blood glucose monitoring is therefore recommended.
An injection should be followed within 30 minutes by a meal or snack containing carbohydrates.
Dosage adjustment
Concomitant illness, especially infections and feverish conditions, usually increases the patient's
insulin requirement.
Renal or hepatic impairment may reduce insulin requirement.
Adjustment of dosage may also be necessary if patients change physical activity or their usual diet.
Dosage adjustment may be necessary when transferring patients from one insulin preparation to
another (see section 4.4).
For subcutaneous use. The intravenous use of Actrapid from any pen or cartridge should be an
exception only in situations where vials are not available. In this case Actrapid should be drawn into
an insulin syringe, provided air is avoided, or infused with an infusion system. This procedure should
only be carried out by health care professionals
Actrapid is administered subcutaneously in the abdominal wall. The thigh, the gluteal region or the
deltoid region may also be used.
Subcutaneous injection into the abdominal wall ensures a faster absorption than from other injection
sites.
Injection into a lifted skin fold minimises the risk of unintended intramuscular injection.
The needle should be kept under the skin for at least 6 seconds to make sure the entire dose is injected.
Injection sites should be rotated within an anatomic region in order to avoid lipodystrophy.
Actrapid NovoLet is designed to be used with NovoFine needles.
NovoLet delivers 2-78 units in increments of 2 units.
The pens should be primed before injection so that the dose selector returns to zero and a drop of
insulin appears at the needle top.
The dose is set by turning the selector, which returns to zero during the injection.
.
Actrapid is accompanied by a package leaflet with detailed instruction for use to be followed.
Hypersensitivity to the active substance or to any of the excipients (see section 6.1).
Hypoglycaemia.
4.4 Special warnings and precautions for use
Inadequate dosage or discontinuation of treatment, especially in type 1 diabetes, may lead to
hyperglycaemia
.
Usually, the first symptoms of hyperglycaemia set in gradually, over a period of hours or days. They
include thirst, increased frequency of urination, nausea, vomiting, drowsiness, flushed dry skin, dry
mouth, loss of appetite as well as acetone odour of breath.
In type 1 diabetes, untreated hyperglycaemic events eventually lead to diabetic ketoacidosis, which is
potentially lethal.
Hypoglycaemia
may occur if the insulin dose is too high in relation to the insulin requirement (see
sections 4.8 and 4.9).
Omission of a meal or unplanned, strenuous physical exercise may lead to hypoglycaemia.
Patients whose blood glucose control is greatly improved e.g. by intensified insulin therapy, may
experience a change in their usual warning symptoms of hypoglycaemia and should be advised
accordingly.
Usual warning symptoms may disappear in patients with longstanding diabetes.
Transferring a patient to another type or brand of insulin should be done under strict medical
supervision. Changes in strength, brand (manufacturer), type (fast-, dual-, long-acting insulin etc.),
origin (animal, human or analogue insulin) and/or method of manufacture (recombinant DNA versus
animal source insulin) may result in a need for a change in dosage. If an adjustment is needed when
switching the patients to Actrapid, it may occur with the first dose or during the first several weeks or
months.
As with any insulin therapy, injection site reactions may occur and include pain, itching, hives,
swelling and inflammation. Continuous rotation of the injection site within a given area may help to
reduce or prevent these reactions. Reactions usually resolve in a few days to a few weeks. On rare
occasions, injection site reactions may require discontinuation of Actrapid
A few patients who have experienced hypoglycaemic reactions after transfer from animal source
insulin have reported that early warning symptoms of hypoglycaemia were less pronounced or
different from those experienced with their previous insulin.
Before travelling between different time zones, the patient should be advised to consult the physician,
since this may mean that the patient has to take insulin and meals at different times.
Due to the risk of precipitation in pump catheters, Actrapid should not be used in insulin pumps for
continuous subcutaneous insulin infusion.
Actrapid contains metacresol, which may cause allergic reactions.
4.5 Interaction with other medicinal products and other forms of interaction
A number of medicinal products are known to interact with glucose metabolism. The physician must
therefore take possible interactions into account and should always ask his patients about any
medicinal products they take.
The following substances may reduce insulin requirement:
Oral hypoglycaemic agents (OHA), monoamine oxidase inhibitors (MAOI), non-selective beta-
blocking agents, angiotensin converting enzyme (ACE) inhibitors, salicylates, alcohol, anabolic
steroids and sulphonamides.
The following substances may increase insulin requirement:
Oral contraceptives, thiazides, glucocorticoids, thyroid hormones and beta-sympathomimetics, growth
hormone and danazol.
Beta-blocking agents may mask the symptoms of hypoglycaemia and delay recovery from
hypoglycaemia.
Octreotide/lanreotide may both decrease and increase insulin requirement.
Alcohol may intensify and prolong the hypoglycaemic effect of insulin.
4.6 Pregnancy and lactation
There are no restrictions on treatment of diabetes with insulin during pregnancy, as insulin does not
pass the placental barrier.
Both hypoglycaemia and hyperglycaemia, which can occur in inadequately controlled diabetes
therapy, increase the risk of malformations and death
in utero
. Intensified control in the treatment of
pregnant women with diabetes is therefore recommended throughout pregnancy and when
contemplating pregnancy.
Insulin requirements usually fall in the first trimester and subsequently increase during the second and
third trimesters.
After delivery, insulin requirements return rapidly to pre-pregnancy values.
Insulin treatment of the nursing mother presents no risk to the baby. However, the Actrapid dosage
may need to be adjusted.
4.7 Effects on ability to drive and use machines
The patient’s ability to concentrate and react may be impaired as a result of hypoglycaemia. This may
constitute a risk in situations where these abilities are of special importance (e.g. driving a car or
operating machinery).
Patients should be advised to take precautions to avoid hypoglycaemia whilst driving. This is
particularly important in those who have reduced or absent awareness of the warning signs of
hypoglycaemia or have frequent episodes of hypoglycaemia. The advisability of driving should be
considered in these circumstances.
As for other insulin products, in general, hypoglycaemia is the most frequently occurring undesirable
effect. It may occur if the insulin dose is too high in relation to the insulin requirement. In clinical
trials and during marketed use, the frequency varies with patient population and dose regimens.
Therefore, no specific frequency can be presented. Severe hypoglycaemia may lead to
unconsciousness and/or convulsions and may result in temporary or permanent impairment of brain
function or even death.
Frequencies of adverse drug reactions from clinical trials that are considered related to Actrapid, are
listed below. The frequencies are defined as: uncommon (
≥
1/1,000 to <1/100). Isolated spontaneous
cases are presented as very rare defined as <1/10,000, including isolated reports.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Nervous system disorders
Uncommon - Peripheral neuropathy
Fast improvement in blood glucose control may be associated with a condition termed “acute painful
neuropathy”, which is usually reversible.
Eye disorders
Uncommon - Refraction disorders
Refraction anomalies may occur upon initiation of insulin therapy. These symptoms are usually of
transitory nature.
Very rare - Diabetic retinopathy
Long-term improved glycaemic control decreases the risk of progression of diabetic retinopathy.
However, intensification of insulin therapy with abrupt improvement in glycaemic control may be
associated with temporary worsening of diabetic retinopathy.
Skin and subcutaneous tissue disorders
Uncommon - Lipodystrophy
Lipodystrophy may occur at the injection site as a consequence of failure to rotate injection sites
within an area.
General disorders and administration site conditions
Uncommon - Injection site reactions
Injection site reactions (redness, swelling, itching, pain and haematoma at the injection site) may occur
during treatment with insulin. Most reactions are transitory and disappear during continued treatment.
Uncommon - Oedema
Oedema may occur upon initiation of insulin therapy. These symptoms are usually of transitory nature.
Immune system disorders
Uncommon - Urticaria, rash
Very rare - Anaphylactic reactions
Symptoms of generalised hypersensitivity may include generalised skin rash, itching, sweating,
gastrointestinal upset, angioneurotic oedema, difficulties in breathing, palpitation, reduction in blood
pressure and fainting/loss of consciousness. Generalised hypersensitivity reactions are potentially life-
threatening.
A specific overdose of insulin cannot be defined. However, hypoglycaemia may develop over
sequential stages:
•
Mild hypoglycaemic episodes can be treated by oral administration of glucose or sugary
products. It is therefore recommended that the diabetic patients carry some sugar lumps, sweets,
biscuits or sugary fruit juice.
Severe hypoglycaemic episodes, where the patient has become unconscious, can be treated by
glucagon (0.5 to 1 mg) given intramuscularly or subcutaneously by a person who has received
appropriate instruction, or by glucose given intravenously by a medical professional. Glucose
must also be given intravenously, if the patient does not respond to glucagon within 10 to
15 minutes.
Upon regaining consciousness, administration of oral carbohydrate is recommended for the
patient in order to prevent relapse.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: insulins and analogues for injection, fast-acting, insulin (human). ATC
code: A10A B01.
The blood glucose lowering effect of insulin is due to the facilitated uptake of glucose following
binding of insulin to receptors on muscle and fat cells and to the simultaneous inhibition of glucose
output from the liver.
A clinical trial in a single intensive care unit treating hyperglycaemia (blood glucose above 10 mmol/l)
in 204 diabetic and 1344 non-diabetic patients undergoing major surgery showed that normoglycaemia
(blood glucose 4.4 – 6.1 mmol/l) induced by intravenous Actrapid reduced mortality by 42% (8%
versus 4.6%).
Actrapid is a fast-acting insulin.
Onset of action is within ½ hour, reaches a maximum effect within 1.5-3.5 hours and the entire
duration of action is approximately 7-8 hours.
5.2 Pharmacokinetic properties
Insulin in the blood stream has a half-life of a few minutes. Consequently, the time-action profile of an
insulin preparation is determined solely by its absorption characteristics.
This process is influenced by several factors (e.g. insulin dosage, injection route and site, thickness of
subcutaneous fat, type of diabetes). The pharmacokinetics of insulin products are therefore affected by
significant intra- and inter-individual variation.
Absorption
The maximum plasma concentration is reached within 1.5-2.5 hours after subcutaneous
administration.
Distribution
No profound binding to plasma proteins, except circulating insulin antibodies (if present) has been
observed.
Metabolism
Human insulin is reported to be degraded by insulin protease or insulin-degrading enzymes and
possibly protein disulfide isomerase. A number of cleavage (hydrolysis) sites on the human insulin
molecule have been proposed; none of the metabolites formed following the cleavage are active.
The terminal half-life is determined by the rate of absorption from the subcutaneous tissue. The
terminal half-life (t
½
) is therefore a measure of the absorption rather than of the elimination
per se
of
insulin from plasma (insulin in the blood stream has a t
½
of a few minutes). Trials have indicated a t
½
of about 2-5 hours.
Chrildren and adolescents
The pharmacokinetic profile of Actrapid has been studied in a small number (n=18) of diabetic
children (aged 6-12 years) and adolescents (aged 13-17 years). The data are limited but suggest that
the pharmacokinetic profile in children and adolescents may be similar to that in adults. However,
there were differences between age groups in C
max,
stressing the importance of individual dose
titration.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction.
PHARMACEUTICAL PARTICULARS
Zinc chloride
Glycerol
Metacresol
Sodium hydroxide (for pH adjustment)
Hydrochloric acid (for pH adjustment)
Water for injections
Insulin products should only be added to compounds with which it is known to be compatible.
Medicinal products added to the insulin solution may cause degradation of the insulin, e.g. if the
medicinal products contain thiols or sulphites.
30 months when stored between 2°C - 8°C.
6 weeks when used or carried as a spare (below 30°C).
6.4 Special precautions for storage
Before use: store in a refrigerator (2°C - 8°C).
Do not store them in or too near the freezer section or cooling element.
Do not freeze.
During use: do not refrigerate. Do not store above 30°C.
Keep the pen cap on in order to protect from light.
Protect from excessive heat and sunlight.
6.5 Nature and contents of container
Pre-filled pen (multidose disposable pen) comprising a pen injector with a cartridge (3 ml). The
cartridge is made of glass (type 1), containing a bromobutyl rubber plunger and a
bromobutyl/polyisoprene rubber stopper. The pen injector is made of plastic.
Pack sizes: 5 and 10 pre-filled pens x 3 ml.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
For intravenous use, infusion systems with Actrapid at concentrations 0.05 IU/ml - 1.0 IU/ml insulin
human in the following infusion fluids; 0.9% sodium chloride, 5% dextrose and 10% dextrose
inclusive 40 mmol/l potassium chloride, using polypropylene infusion bags, are stable at room
temperature for 24 hours. Although stable over time, a certain amount of insulin will initially be
absorbed to the material of the infusion bag. Monitoring of blood glucose is necessary during the
infusion.
Pens should only be used in combination with products that are compatible with them and allow the
pens to function safely and effectively.
Actrapid NovoLet is for single person use only. The container must not be refilled.
Insulin preparations which have been frozen must not be used.
Insulin solutions should not be used if they do not appear water clear and colourless.
Actrapid should not be used in insulin pumps for continuous subcutaneous insulin infusion.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 07 October 2002
Date of latest renewal: 18 September 2007
10. DATE OF REVISION OF THE TEXT
NAME OF THE MEDICINAL PRODUCT
Actrapid InnoLet 100 IU/ml solution for injection in a pre-filled pen
QUALITATIVE AND QUANTITATIVE COMPOSITION
Insulin human, rDNA (produced by recombinant DNA technology in
Saccharomyces cerevisiae
).
1 ml contains 100 IU of insulin human.
1 pre-filled pen contains 3 ml equivalent to 300 IU.
One IU (International Unit) corresponds to 0.035 mg of anhydrous human insulin.
For a full list of excipients, see section 6.1.
Solution for injection in a pre-filled pen.
Clear, colourless, aqueous solution.
4.1 Therapeutic indications
Treatment of diabetes mellitus.
4.2 Posology and method of administration
Actrapid is a fast-acting insulin and may be used in combination with long-acting insulin products.
Dosage
Dosage is individual and determined in accordance with the needs of the patient. The individual
insulin requirement is usually between 0.3 and 1.0 IU/kg/day. The daily insulin requirement may be
higher in patients with insulin resistance (e.g. during puberty or due to obesity) and lower in patients
with residual, endogenous insulin production.
In patients with diabetes mellitus optimised glycaemic control delays the onset of late diabetic
complications. Close blood glucose monitoring is therefore recommended.
An injection should be followed within 30 minutes by a meal or snack containing carbohydrates.
Dosage adjustment
Concomitant illness, especially infections and feverish conditions, usually increases the patient's
insulin requirement.
Renal or hepatic impairment may reduce insulin requirement.
Adjustment of dosage may also be necessary if patients change physical activity or their usual diet.
Dosage adjustment may be necessary when transferring patients from one insulin preparation to
another (see section 4.4 Special warnings and precautions for use).
For subcutaneous use. The intravenous use of Actrapid from any pen or cartridge should be an
exception only in situations where vials are not available. In this case Actrapid should be drawn into
an insulin syringe, provided air is avoided, or infused with an infusion system. This procedure should
only be carried out by health care professionals.
Actrapid is administered subcutaneously in the abdominal wall. The thigh, the gluteal region or the
deltoid region may also be used.
Subcutaneous injection into the abdominal wall ensures a faster absorption than from other injection
sites.
Injection into a lifted skin fold minimises the risk of unintended intramuscular injection.
The needle should be kept under the skin for at least 6 seconds to make sure the entire dose is injected.
Injection sites should be rotated within an anatomic region in order to avoid lipodystrophy.
Actrapid InnoLet is designed to be used with NovoFine short cap needles of 8 mm or shorter in length.
The needle box is marked with an
S.
InnoLet delivers 1-50 units in increments of 1 unit.
The pens should be primed before injection so that the dose selector returns to zero and a drop of
insulin appears at the needle top.
The dose is set by turning the selector, which returns to zero during the injection.
Actrapid is accompanied by a package leaflet with detailed instruction for use to be followed.
Hypersensitivity to the active substance or to any of the excipients (see section 6.1).
Hypoglycaemia.
4.4 Special warnings and precautions for use
Inadequate dosage or discontinuation of treatment, especially in type 1 diabetes, may lead to
hyperglycaemia
.
Usually, the first symptoms of hyperglycaemia set in gradually, over a period of hours or days. They
include thirst, increased frequency of urination, nausea, vomiting, drowsiness, flushed dry skin, dry
mouth, loss of appetite as well as acetone odour of breath.
In type 1 diabetes, untreated hyperglycaemic events eventually lead to diabetic ketoacidosis, which is
potentially lethal.
Hypoglycaemia
may occur if the insulin dose is too high in relation to the insulin requirement (see
sections 4.8 and 4.9).
Omission of a meal or unplanned, strenuous physical exercise may lead to hypoglycaemia.
Patients whose blood glucose control is greatly improved e.g. by intensified insulin therapy, may
experience a change in their usual warning symptoms of hypoglycaemia and should be advised
accordingly.
Usual warning symptoms may disappear in patients with longstanding diabetes.
Transferring a patient to another type or brand of insulin should be done under strict medical
supervision. Changes in strength, brand (manufacturer), type (fast-, dual-, long-acting insulin etc.),
origin (animal, human or analogue insulin) and/or method of manufacture (recombinant DNA versus
animal source insulin) may result in a need for a change in dosage. If an adjustment is needed when
switching the patients to Actrapid, it may occur with the first dose or during the first several weeks or
months.
As with any insulin therapy, injection site reactions may occur and include pain, itching, hives,
swelling and inflammation. Continuous rotation of the injection site within a given area may help to
reduce or prevent these reactions. Reactions usually resolve in a few days to a few weeks. On rare
occasions, injection site reactions may require discontinuation of Actrapid
A few patients who have experienced hypoglycaemic reactions after transfer from animal source
insulin have reported that early warning symptoms of hypoglycaemia were less pronounced or
different from those experienced with their previous insulin.
Before travelling between different time zones, the patient should be advised to consult the physician,
since this may mean that the patient has to take insulin and meals at different times.
Due to the risk of precipitation in pump catheters, Actrapid should not be used in insulin pumps for
continuous subcutaneous insulin infusion.
Actrapid contains metacresol, which may cause allergic reactions.
4.5 Interaction with other medicinal products and other forms of interaction
A number of medicinal products are known to interact with glucose metabolism. The physician must
therefore take possible interactions into account and should always ask his patients about any
medicinal products they take.
The following substances may reduce insulin requirement:
Oral hypoglycaemic agents (OHA), monoamine oxidase inhibitors (MAOI), non-selective beta-
blocking agents, angiotensin converting enzyme (ACE) inhibitors, salicylates, alcohol, anabolic
steroids and sulphonamides.
The following substances may increase insulin requirement:
Oral contraceptives, thiazides, glucocorticoids, thyroid hormones and beta-sympathomimetics, growth
hormone and danazol.
Beta-blocking agents may mask the symptoms of hypoglycaemia and delay recovery from
hypoglycaemia.
Octreotide/lanreotide may both decrease and increase insulin requirement.
Alcohol may intensify and prolong the hypoglycaemic effect of insulin.
4.6 Pregnancy and lactation
There are no restrictions on treatment of diabetes with insulin during pregnancy, as insulin does not
pass the placental barrier.
Both hypoglycaemia and hyperglycaemia, which can occur in inadequately controlled diabetes
therapy, increase the risk of malformations and death
in utero
. Intensified control in the treatment of
pregnant women with diabetes is therefore recommended throughout pregnancy and when
contemplating pregnancy.
Insulin requirements usually fall in the first trimester and subsequently increase during the second and
third trimesters.
After delivery, insulin requirements return rapidly to pre-pregnancy values.
Insulin treatment of the nursing mother presents no risk to the baby. However, the Actrapid dosage
may need to be adjusted.
4.7 Effects on ability to drive and use machines
The patient’s ability to concentrate and react may be impaired as a result of hypoglycaemia. This may
constitute a risk in situations where these abilities are of special importance (e.g. driving a car or
operating machinery).
Patients should be advised to take precautions to avoid hypoglycaemia whilst driving. This is
particularly important in those who have reduced or absent awareness of the warning signs of
hypoglycaemia or have frequent episodes of hypoglycaemia. The advisability of driving should be
considered in these circumstances.
As for other insulin products, in general, hypoglycaemia is the most frequently occurring undesirable
effect. It may occur if the insulin dose is too high in relation to the insulin requirement. In clinical
trials and during marketed use, the frequency varies with patient population and dose regimens.
Therefore, no specific frequency can be presented. Severe hypoglycaemia may lead to
unconsciousness and/or convulsions and may result in temporary or permanent impairment of brain
function or even death.
Frequencies of adverse drug reactions from clinical trials that are considered related to Actrapid, are
listed below. The frequencies are defined as: uncommon (
≥
1/1,000 to <1/100). Isolated spontaneous
cases are presented as very rare defined as <1/10,000, including isolated reports.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Nervous system disorders
Uncommon - Peripheral neuropathy
Fast improvement in blood glucose control may be associated with a condition termed “acute painful
neuropathy”, which is usually reversible.
Eye disorders
Uncommon - Refraction disorders
Refraction anomalies may occur upon initiation of insulin therapy. These symptoms are usually of
transitory nature.
Very rare - Diabetic retinopathy
Long-term improved glycaemic control decreases the risk of progression of diabetic retinopathy.
However, intensification of insulin therapy with abrupt improvement in glycaemic control may be
associated with temporary worsening of diabetic retinopathy.
Skin and subcutaneous tissue disorders
Uncommon - Lipodystrophy
Lipodystrophy may occur at the injection site as a consequence of failure to rotate injection sites
within an area.
General disorders and administration site conditions
Uncommon - Injection site reactions
Injection site reactions (redness, swelling, itching, pain and haematoma at the injection site) may occur
during treatment with insulin. Most reactions are transitory and disappear during continued treatment.
Uncommon - Oedema
Oedema may occur upon initiation of insulin therapy. These symptoms are usually of transitory nature.
Immune system disorders
Uncommon - Urticaria, rash
Very rare - Anaphylactic reactions
Symptoms of generalised hypersensitivity may include generalised skin rash, itching, sweating,
gastrointestinal upset, angioneurotic oedema, difficulties in breathing, palpitation, reduction in blood
pressure and fainting/loss of consciousness. Generalised hypersensitivity reactions are potentially life-
threatening.
A specific overdose of insulin cannot be defined. However, hypoglycaemia may develop over
sequential stages:
•
Mild hypoglycaemic episodes can be treated by oral administration of glucose or sugary
products. It is therefore recommended that the diabetic patients carry some sugar lumps, sweets,
biscuits or sugary fruit juice.
Severe hypoglycaemic episodes, where the patient has become unconscious, can be treated by
glucagon (0.5 to 1 mg) given intramuscularly or subcutaneously by a person who has received
appropriate instruction, or by glucose given intravenously by a medical professional. Glucose
must also be given intravenously, if the patient does not respond to glucagon within 10 to
15 minutes.
Upon regaining consciousness, administration of oral carbohydrate is recommended for the
patient in order to prevent relapse.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: insulins and analogues for injection, fast-acting, insulin (human). ATC
code: A10A B01.
The blood glucose lowering effect of insulin is due to the facilitated uptake of glucose following
binding of insulin to receptors on muscle and fat cells and to the simultaneous inhibition of glucose
output from the liver.
A clinical trial in a single intensive care unit treating hyperglycaemia (blood glucose above 10 mmol/l)
in 204 diabetic and 1344 non-diabetic patients undergoing major surgery showed that normoglycaemia
(blood glucose 4.4 – 6.1 mmol/l) induced by intravenous Actrapid reduced mortality by 42% (8%
versus 4.6%).
Actrapid is a fast-acting insulin.
Onset of action is within ½ hour, reaches a maximum effect within 1.5-3.5 hours and the entire
duration of action is approximately 7-8 hours.
5.2 Pharmacokinetic properties
Insulin in the blood stream has a half-life of a few minutes. Consequently, the time-action profile of an
insulin preparation is determined solely by its absorption characteristics.
This process is influenced by several factors (e.g. insulin dosage, injection route and site, thickness of
subcutaneous fat, type of diabetes). The pharmacokinetics of insulin products are therefore affected by
significant intra- and inter-individual variation.
Absorption
The maximum plasma concentration is reached within 1.5-2.5 hours after subcutaneous
administration.
Distribution
No profound binding to plasma proteins, except circulating insulin antibodies (if present) has been
observed.
Human insulin is reported to be degraded by insulin protease or insulin-degrading enzymes and
possibly protein disulfide isomerase. A number of cleavage (hydrolysis) sites on the human insulin
molecule have been proposed; none of the metabolites formed following the cleavage are active.
Elimination
The terminal half-life is determined by the rate of absorption from the subcutaneous tissue. The
terminal half-life (t
½
) is therefore a measure of the absorption rather than of the elimination
per se
of
insulin from plasma (insulin in the blood stream has a t
½
of a few minutes). Trials have indicated a t
½
of about 2-5 hours.
Chrildren and adolescents
The pharmacokinetic profile of Actrapid has been studied in a small number (n=18) of diabetic
children (aged 6-12 years) and adolescents (aged 13-17 years). The data are limited but suggest that
the pharmacokinetic profile in children and adolescents may be similar to that in adults. However,
there were differences between age groups in C
max,
stressing the importance of individual dose
titration.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction.
PHARMACEUTICAL PARTICULARS
Zinc chloride
Glycerol
Metacresol
Sodium hydroxide (for pH adjustment)
Hydrochloric acid (for pH adjustment)
Water for injections
Insulin products should only be added to compounds with which it is known to be compatible.
Medicinal products added to the insulin solution may cause degradation of the insulin, e.g. if the
medicinal products contain thiols or sulphites.
30 months when stored between 2°C - 8°C.
6 weeks when used or carried as a spare (below 30°C).
6.4 Special precautions for storage
Before use: store in a refrigerator (2°C - 8°C).
Do not store them in or too near the freezer section or cooling element.
Do not freeze.
During use: do not refrigerate. Do not store above 30°C.
Keep the pen cap on in order to protect from light.
Protect from excessive heat and sunlight.
6.5 Nature and contents of container
Pre-filled pen (multidose disposable pen) comprising a pen injector with a cartridge (3 ml). The
cartridge is made of glass (type 1), containing a bromobutyl rubber plunger and a
bromobutyl/polyisoprene rubber stopper. The pen injector is made of plastic.
Pack sizes: 1, 5 and 10 pre-filled pens x 3 ml.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
For intravenous use, infusion systems with Actrapid at concentrations 0.05 IU/ml - 1.0 IU/ml insulin
human in the following infusion fluids; 0.9% sodium chloride, 5% dextrose and 10% dextrose
inclusive 40 mmol/l potassium chloride, using polypropylene infusion bags, are stable at room
temperature for 24 hours. Although stable over time, a certain amount of insulin will initially be
absorbed to the material of the infusion bag. Monitoring of blood glucose is necessary during the
infusion.
Pens should only be used in combination with products that are compatible with them and allow the
pens to function safely and effectively.
Actrapid InnoLet is for single person use only. The container must not be refilled.
Insulin preparations which have been frozen must not be used.
Insulin solutions should not be used if they do not appear water clear and colourless.
Actrapid should not be used in insulin pumps for continuous subcutaneous insulin infusion.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 07 October 2002
Date of latest renewal: 18 September 2007
10. DATE OF REVISION OF THE TEXT
NAME OF THE MEDICINAL PRODUCT
Actrapid FlexPen 100 IU/ml solution for injection in a pre-filled pen
QUALITATIVE AND QUANTITATIVE COMPOSITION
Insulin human, rDNA (produced by recombinant DNA technology in
Saccharomyces cerevisiae
).
1 ml contains 100 IU of insulin human.
1 pre-filled pen contains 3 ml equivalent to 300 IU.
One IU (International Unit) corresponds to 0.035 mg of anhydrous human insulin.
For a full list of excipients, see section 6.1.
Solution for injection in a pre-filled pen.
Clear, colourless, aqueous solution.
4.1 Therapeutic indications
Treatment of diabetes mellitus.
4.2 Posology and method of administration
Actrapid is a fast-acting insulin and may be used in combination with long-acting insulin products.
Dosage
Dosage is individual and determined in accordance with the needs of the patient. The individual
insulin requirement is usually between 0.3 and 1.0 IU/kg/day. The daily insulin requirement may be
higher in patients with insulin resistance (e.g. during puberty or due to obesity) and lower in patients
with residual, endogenous insulin production.
In patients with diabetes mellitus optimised glycaemic control delays the onset of late diabetic
complications. Close blood glucose monitoring is therefore recommended.
An injection should be followed within 30 minutes by a meal or snack containing carbohydrates.
Dosage adjustment
Concomitant illness, especially infections and feverish conditions, usually increases the patient's
insulin requirement.
Renal or hepatic impairment may reduce insulin requirement.
Adjustment of dosage may also be necessary if patients change physical activity or their usual diet.
Dosage adjustment may be necessary when transferring patients from one insulin preparation to
another (see section 4.4 ).
For subcutaneous use. The intravenous use of Actrapid from any pen or cartridge should be an
exception only in situations where vials are not available. In this case Actrapid should be drawn into
an insulin syringe, provided air is avoided, or infused with an infusion system. This procedure should
only be carried out by health care professionals.
Actrapid is administered subcutaneously in the abdominal wall. The thigh, the gluteal region or the
deltoid region may also be used.
Subcutaneous injection into the abdominal wall ensures a faster absorption than from other injection
sites.
Injection into a lifted skin fold minimises the risk of unintended intramuscular injection.
The needle should be kept under the skin for at least 6 seconds to make sure the entire dose is injected.
Injection sites should be rotated within an anatomic region in order to avoid lipodystrophy.
Actrapid FlexPen is designed to be used with NovoFine short cap needles of 8 mm or shorter in
length. The needle box is marked with an
S.
FlexPen delivers 1-60 units in increments of 1 unit.
The pens should be primed before injection so that the dose selector returns to zero and a drop of
insulin appears at the needle top.
The dose is set by turning the selector, which returns to zero during the injection.
Actrapid is accompanied by a package leaflet with detailed instruction for use to be followed.
Hypersensitivity to the active substance or to any of the excipients (see section 6.1).
Hypoglycaemia.
4.4 Special warnings and precautions for use
Inadequate dosage or discontinuation of treatment, especially in type 1 diabetes, may lead to
hyperglycaemia
.
Usually, the first symptoms of hyperglycaemia set in gradually, over a period of hours or days. They
include thirst, increased frequency of urination, nausea, vomiting, drowsiness, flushed dry skin, dry
mouth, loss of appetite as well as acetone odour of breath.
In type 1 diabetes, untreated hyperglycaemic events eventually lead to diabetic ketoacidosis, which is
potentially lethal.
Hypoglycaemia
may occur if the insulin dose is too high in relation to the insulin requirement (see
sections 4.8 and 4.9).
Omission of a meal or unplanned, strenuous physical exercise may lead to hypoglycaemia.
Patients whose blood glucose control is greatly improved e.g. by intensified insulin therapy, may
experience a change in their usual warning symptoms of hypoglycaemia and should be advised
accordingly.
Usual warning symptoms may disappear in patients with longstanding diabetes.
Transferring a patient to another type or brand of insulin should be done under strict medical
supervision. Changes in strength, brand (manufacturer), type (fast-, dual-, long-acting insulin etc.),
origin (animal, human or analogue insulin) and/or method of manufacture (recombinant DNA versus
animal source insulin) may result in a need for a change in dosage. If an adjustment is needed when
switching the patients to Actrapid, it may occur with the first dose or during the first several weeks or
months.
As with any insulin therapy, injection site reactions may occur and include pain, itching, hives,
swelling and inflammation. Continuous rotation of the injection site within a given area may help to
reduce or prevent these reactions. Reactions usually resolve in a few days to a few weeks. On rare
occasions, injection site reactions may require discontinuation of Actrapid
A few patients who have experienced hypoglycaemic reactions after transfer from animal source
insulin have reported that early warning symptoms of hypoglycaemia were less pronounced or
different from those experienced with their previous insulin.
Before travelling between different time zones, the patient should be advised to consult the physician,
since this may mean that the patient has to take insulin and meals at different times.
Due to the risk of precipitation in pump catheters, Actrapid should not be used in insulin pumps for
continuous subcutaneous insulin infusion.
Actrapid contains metacresol, which may cause allergic reactions.
4.5 Interaction with other medicinal products and other forms of interaction
A number of medicinal products are known to interact with glucose metabolism. The physician must
therefore take possible interactions into account and should always ask his patients about any
medicinal products they take.
The following substances may reduce insulin requirement:
Oral hypoglycaemic agents (OHA), monoamine oxidase inhibitors (MAOI), non-selective beta-
blocking agents, angiotensin converting enzyme (ACE) inhibitors, salicylates, alcohol, anabolic
steroids and sulphonamides.
The following substances may increase insulin requirement:
Oral contraceptives, thiazides, glucocorticoids, thyroid hormones and beta-sympathomimetics, growth
hormone and danazol.
Beta-blocking agents may mask the symptoms of hypoglycaemia and delay recovery from
hypoglycaemia.
Octreotide/lanreotide may both decrease and increase insulin requirement.
Alcohol may intensify and prolong the hypoglycaemic effect of insulin.
4.6 Pregnancy and lactation
There are no restrictions on treatment of diabetes with insulin during pregnancy, as insulin does not
pass the placental barrier.
Both hypoglycaemia and hyperglycaemia, which can occur in inadequately controlled diabetes
therapy, increase the risk of malformations and death
in utero
. Intensified control in the treatment of
pregnant women with diabetes is therefore recommended throughout pregnancy and when
contemplating pregnancy.
Insulin requirements usually fall in the first trimester and subsequently increase during the second and
third trimesters.
After delivery, insulin requirements return rapidly to pre-pregnancy values.
Insulin treatment of the nursing mother presents no risk to the baby. However, the Actrapid dosage
may need to be adjusted.
4.7 Effects on ability to drive and use machines
The patient’s ability to concentrate and react may be impaired as a result of hypoglycaemia. This may
constitute a risk in situations where these abilities are of special importance (e.g. driving a car or
operating machinery).
Patients should be advised to take precautions to avoid hypoglycaemia whilst driving. This is
particularly important in those who have reduced or absent awareness of the warning signs of
hypoglycaemia or have frequent episodes of hypoglycaemia. The advisability of driving should be
considered in these circumstances.
As for other insulin products, in general, hypoglycaemia is the most frequently occurring undesirable
effect. It may occur if the insulin dose is too high in relation to the insulin requirement. In clinical
trials and during marketed use, the frequency varies with patient population and dose regimens.
Therefore, no specific frequency can be presented. Severe hypoglycaemia may lead to
unconsciousness and/or convulsions and may result in temporary or permanent impairment of brain
function or even death.
Frequencies of adverse drug reactions from clinical trials that are considered related to Actrapid, are
listed below. The frequencies are defined as: uncommon (
≥
1/1,000 to <1/100). Isolated spontaneous
cases are presented as very rare defined as <1/10,000, including isolated reports.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Nervous system disorders
Uncommon - Peripheral neuropathy
Fast improvement in blood glucose control may be associated with a condition termed “acute painful
neuropathy”, which is usually reversible.
Eye disorders
Uncommon - Refraction disorders
Refraction anomalies may occur upon initiation of insulin therapy. These symptoms are usually of
transitory nature.
Very rare - Diabetic retinopathy
Long-term improved glycaemic control decreases the risk of progression of diabetic retinopathy.
However, intensification of insulin therapy with abrupt improvement in glycaemic control may be
associated with temporary worsening of diabetic retinopathy.
Skin and subcutaneous tissue disorders
Uncommon - Lipodystrophy
Lipodystrophy may occur at the injection site as a consequence of failure to rotate injection sites
within an area.
General disorders and administration site conditions
Uncommon - Injection site reactions
Injection site reactions (redness, swelling, itching, pain and haematoma at the injection site) may occur
during treatment with insulin. Most reactions are transitory and disappear during continued treatment.
Uncommon - Oedema
Oedema may occur upon initiation of insulin therapy. These symptoms are usually of transitory nature.
Immune system disorders
Uncommon - Urticaria, rash
Very rare - Anaphylactic reactions
Symptoms of generalised hypersensitivity may include generalised skin rash, itching, sweating,
gastrointestinal upset, angioneurotic oedema, difficulties in breathing, palpitation, reduction in blood
pressure and fainting/loss of consciousness. Generalised hypersensitivity reactions are potentially life-
threatening.
A specific overdose of insulin cannot be defined. However, hypoglycaemia may develop over
sequential stages:
•
Mild hypoglycaemic episodes can be treated by oral administration of glucose or sugary
products. It is therefore recommended that the diabetic patients carry some sugar lumps, sweets,
biscuits or sugary fruit juice.
Severe hypoglycaemic episodes, where the patient has become unconscious, can be treated by
glucagon (0.5 to 1 mg) given intramuscularly or subcutaneously by a person who has received
appropriate instruction, or by glucose given intravenously by a medical professional. Glucose
must also be given intravenously, if the patient does not respond to glucagon within 10 to
15 minutes.
Upon regaining consciousness, administration of oral carbohydrate is recommended for the
patient in order to prevent relapse.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: insulins and analogues for injection, fast-acting, insulin (human). ATC
code: A10A B01.
The blood glucose lowering effect of insulin is due to the facilitated uptake of glucose following
binding of insulin to receptors on muscle and fat cells and to the simultaneous inhibition of glucose
output from the liver.
A clinical trial in a single intensive care unit treating hyperglycaemia (blood glucose above 10 mmol/l)
in 204 diabetic and 1344 non-diabetic patients undergoing major surgery showed that normoglycaemia
(blood glucose 4.4 – 6.1 mmol/l) induced by intravenous Actrapid reduced mortality by 42% (8%
versus 4.6%).
Actrapid is a fast-acting insulin.
Onset of action is within ½ hour, reaches a maximum effect within 1.5-3.5 hours and the entire
duration of action is approximately 7-8 hours.
5.2 Pharmacokinetic properties
Insulin in the blood stream has a half-life of a few minutes. Consequently, the time-action profile of an
insulin preparation is determined solely by its absorption characteristics.
This process is influenced by several factors (e.g. insulin dosage, injection route and site, thickness of
subcutaneous fat, type of diabetes). The pharmacokinetics of insulin products are therefore affected by
significant intra- and inter-individual variation.
Absorption
The maximum plasma concentration is reached within 1.5-2.5 hours after subcutaneous
administration.
Distribution
No profound binding to plasma proteins, except circulating insulin antibodies (if present) has been
observed.
Human insulin is reported to be degraded by insulin protease or insulin-degrading enzymes and
possibly protein disulfide isomerase. A number of cleavage (hydrolysis) sites on the human insulin
molecule have been proposed; none of the metabolites formed following the cleavage are active.
Elimination
The terminal half-life is determined by the rate of absorption from the subcutaneous tissue. The
terminal half-life (t
½
) is therefore a measure of the absorption rather than of the elimination
per se
of
insulin from plasma (insulin in the blood stream has a t
½
of a few minutes). Trials have indicated a t
½
of about 2-5 hours.
Chrildren and adolescents
The pharmacokinetic profile of Actrapid has been studied in a small number (n=18) of diabetic
children (aged 6-12 years) and adolescents (aged 13-17 years). The data are limited but suggest that
the pharmacokinetic profile in children and adolescents may be similar to that in adults. However,
there were differences between age groups in C
max,
stressing the importance of individual dose
titration.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction.
PHARMACEUTICAL PARTICULARS
Zinc chloride
Glycerol
Metacresol
Sodium hydroxide (for pH adjustment)
Hydrochloric acid (for pH adjustment)
Water for injections
Insulin products should only be added to compounds with which it is known to be compatible.
Medicinal products added to the insulin solution may cause degradation of the insulin, e.g. if the
medicinal products contain thiols or sulphites.
30 months when stored between 2°C - 8°C..
6 weeks when used or carried as a spare (below 30°C).
6.4 Special precautions for storage
Before use: store in a refrigerator (2°C - 8°C).
Do not store them in or too near the freezer section or cooling element.
Do not freeze.
During use: do not refrigerate. Do not store above 30°C.
Keep the pen cap on in order to protect from light.
Protect from excessive heat and sunlight.
6.5 Nature and contents of container
Pre-filled pen (multidose disposable pen) comprising a pen injector with a cartridge (3 ml). The
cartridge is made of glass (type 1), containing a bromobutyl rubber plunger and a
bromobutyl/polyisoprene rubber stopper. The pen injector is made of plastic.
Pack sizes: 1, 5 and 10 pre-filled pens x 3 ml.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
For intravenous use, infusion systems with Actrapid at concentrations 0.05 IU/ml - 1.0 IU/ml insulin
human in the following infusion fluids; 0.9% sodium chloride, 5% dextrose and 10% dextrose
inclusive 40 mmol/l potassium chloride, using polypropylene infusion bags, are stable at room
temperature for 24 hours. Although stable over time, a certain amount of insulin will initially be
absorbed to the material of the infusion bag. Monitoring of blood glucose is necessary during the
infusion.
Pens should only be used in combination with products that are compatible with them and allow the
pens to function safely and effectively.
Actrapid FlexPen is for single person use only. The container must not be refilled.
Insulin preparations which have been frozen must not be used.
Insulin solutions should not be used if they do not appear water clear and colourless.
Actrapid should not be used in insulin pumps for continuous subcutaneous insulin infusion.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: October 2002
Date of latest renewal: 18 September 2007
10. DATE OF REVISION OF THE TEXT
A. MANUFACTURER OF THE BIOLOGICAL ACTIVE
SUBSTANCE AND MANUFACTURING AUTHORISATION
HOLDERS RESPONSIBLE FOR BATCH RELEASE
B. CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURER OF THE BIOLOGICAL ACTIVE SUBSTANCE AND
MANUFACTURING AUTHORISATION HOLDERS RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer of the biological active substance
Name and address of the manufacturers responsible for batch release
Actrapid, Actrapid NovoLet, Actrapid InnoLet:
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
Actrapid Penfill and FlexPen:
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
Novo Nordisk Production SAS
45, Avenue d’Orléans
F-28002 Chartres
France
The printed package leaflet of the medicinal product must state the name and address of the
manufacturer responsible for the release of the concerned batch
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to medical prescription
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
Pharmacovigilance system
The MAH must ensure that the system of pharmacovigilance, as presented in Module 1.8.1 of the
Marketing Authorisation Application, is in place and functioning before and whilst the product is on
the market.
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version 1.0 (06 March 2009) of the Risk Management Plan
(RMP) presented in Module 1.8.2. of the Marketing Authorisation Application and any subsequent
updates of the RMP agreed by the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, the
updated RMP should be submitted at the same time as the next Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted
•
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
At the request of the EMEA
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING AND THE IMMEDIATE
PACKAGING
NAME OF THE MEDICINAL PRODUCT
Actrapid 40 IU/ml solution for injection in a vial
Insulin human (rDNA)
STATEMENT OF ACTIVE SUBSTANCE
1 ml solution contains 40 IU (1.4 mg) of insulin human (rDNA).
zinc chloride, glycerol, metacresol, sodium hydroxide, hydrochloric acid and water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection
1 x 10 ml
5 x 10 ml
METHOD AND ROUTE(S) OF ADMINISTRATION
Subcutaneous or intravenous use
Read the package leaflet before use
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children
OTHER SPECIAL WARNING(S), IF NECESSARY
EXP/
During use: use within 4 weeks
SPECIAL STORAGE CONDITIONS
Store in a refrigerator (2°C - 8°C)
Do not freeze
























Keep the vial in the outer carton in order to protect from light
During use: do not refrigerate or store above 25°C
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/02/230/001 1 x 10 ml
EU/1/02/230/002 5 x 10 ml
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
16. INFORMATION IN BRAILLE


















PARTICULARS TO APPEAR ON THE OUTER PACKAGING AND THE IMMEDIATE
PACKAGING
NAME OF THE MEDICINAL PRODUCT
Actrapid 100 IU/ml solution for injection in a vial
Insulin human (rDNA)
STATEMENT OF ACTIVE SUBSTANCE
1 ml solution contains 100 IU (3.5 mg) of insulin human (rDNA).
zinc chloride, glycerol, metacresol, sodium hydroxide, hydrochloric acid and water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection
1 x 10 ml
5 x 10 ml
METHOD AND ROUTE(S) OF ADMINISTRATION
Subcutaneous or intravenous use
Read the package leaflet before use
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children
OTHER SPECIAL WARNING(S), IF NECESSARY
EXP/
During use: use within 6 weeks
SPECIAL STORAGE CONDITIONS
Store in a refrigerator (2°C - 8°C)
Do not freeze
























Keep the vial in the outer carton in order to protect from light
During use: do not refrigerate or store above 25°C
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/02/230/003 1 x 10 ml
EU/1/02/230/004 5 x 10 ml
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
1 vial of 10 ml contains 1000 IU
16. INFORMATION IN BRAILLE


















PARTICULARS TO APPEAR ON THE OUTER PACKAGING AND THE IMMEDIATE
PACKAGING
NAME OF THE MEDICINAL PRODUCT
Actrapid 40 IU/ml solution for injection in a vial
Insulin human (rDNA)
STATEMENT OF ACTIVE SUBSTANCE
1 ml solution contains 40 IU (1.4 mg) of insulin human (rDNA).
zinc chloride, glycerol, metacresol, sodium hydroxide, hydrochloric acid and water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection
1 x 10 ml
This is part of a multipack and not for sale of individual vials
METHOD AND ROUTE(S) OF ADMINISTRATION
Subcutaneous or intravenous use
Read the package leaflet before use
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children
OTHER SPECIAL WARNING(S), IF NECESSARY
EXP/
During use: use within 4 weeks
SPECIAL STORAGE CONDITIONS
Store in a refrigerator (2°C - 8°C)
Do not freeze
Keep the vial in the outer carton in order to protect from light























During use: do not refrigerate or store above 25°C
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
16. INFORMATION IN BRAILLE

















PARTICULARS TO APPEAR ON THE PACKAGING
OUTER WRAPPER LABEL ON MULTIPACKS
NAME OF THE MEDICINAL PRODUCT
Actrapid 40 IU/ml solution for injection in a vial
Insulin human (rDNA)
STATEMENT OF ACTIVE SUBSTANCE
1 ml solution contains 40 IU (1.4 mg) of insulin human (rDNA).
zinc chloride, glycerol, metacresol, sodium hydroxide, hydrochloric acid and water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection
5 x (1 x 10 ml)
This is a multipack and not for sale of individual vials
METHOD AND ROUTE(S) OF ADMINISTRATION
Subcutaneous or intravenous use
Read the package leaflet before use
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children
OTHER SPECIAL WARNING(S), IF NECESSARY
EXP/
During use: use within 4 weeks
SPECIAL STORAGE CONDITIONS
Store in a refrigerator (2°C - 8°C)
Do not freeze
Keep the vial in the outer carton in order to protect from light
During use: do not refrigerate or store above 25°C























10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
16. INFORMATION IN BRAILLE

















PARTICULARS TO APPEAR ON THE OUTER PACKAGING AND THE IMMEDIATE
PACKAGING
NAME OF THE MEDICINAL PRODUCT
Actrapid 100 IU/ml solution for injection in a vial
Insulin human (rDNA)
STATEMENT OF ACTIVE SUBSTANCE
1 ml solution contains 100 IU (3.5 mg) of insulin human (rDNA).
zinc chloride, glycerol, metacresol, sodium hydroxide, hydrochloric acid and water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection
1 x 10 ml
This is part of a multipack and not for sale of individual vials
METHOD AND ROUTE(S) OF ADMINISTRATION
Subcutaneous or intravenous use
Read the package leaflet before use
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children
OTHER SPECIAL WARNING(S), IF NECESSARY
EXP/
During use: use within 6 weeks
SPECIAL STORAGE CONDITIONS
Store in a refrigerator (2°C - 8°C)
Do not freeze























Keep the vial in the outer carton in order to protect from light
During use: do not refrigerate or store above 25°C
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
1 vial of 10 ml contains 1000 IU
16. INFORMATION IN BRAILLE

















PARTICULARS TO APPEAR ON THE PACKAGING
OUTHER WRAPPER LABEL ON MULTIPACKS
NAME OF THE MEDICINAL PRODUCT
Actrapid 100 IU/ml solution for injection in a vial
Insulin human (rDNA)
STATEMENT OF ACTIVE SUBSTANCE
1 ml solution contains 100 IU (3.5 mg) of insulin human (rDNA).
zinc chloride, glycerol, metacresol, sodium hydroxide, hydrochloric acid and water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection
5 x (1 x 10 ml)
This is a multipack and not for sale of individual vials
METHOD AND ROUTE(S) OF ADMINISTRATION
Subcutaneous or intravenous use
Read the package leaflet before use
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children
OTHER SPECIAL WARNING(S), IF NECESSARY
EXP/
During use: use within 6 weeks
SPECIAL STORAGE CONDITIONS
Store in a refrigerator (2°C - 8°C)
Do not freeze
Keep the vial in the outer carton in order to protect from light
During use: do not refrigerate or store above 25°C























10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
1 vial of 10 ml contains 1000 IU
16. INFORMATION IN BRAILLE

















PARTICULARS TO APPEAR ON THE OUTER PACKAGING AND THE IMMEDIATE
PACKAGING
NAME OF THE MEDICINAL PRODUCT
Actrapid Penfill 100 IU/ml solution for injection in a cartridge
Insulin human (rDNA)
STATEMENT OF ACTIVE SUBSTANCE
1 ml solution contains 100 IU (3.5 mg) of insulin human (rDNA).
zinc chloride, glycerol, metacresol, sodium hydroxide, hydrochloric acid and water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection
1 x 3 ml
5 x 3 ml
10 x 3 ml
METHOD AND ROUTE(S) OF ADMINISTRATION
Subcutaneous use
Penfill cartridges for use with Novo Nordisk insulin devices
Read the package leaflet before use
Actrapid Penfill is for use by one person only
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children
OTHER SPECIAL WARNING(S), IF NECESSARY
EXP/
During use: use within 6 weeks
SPECIAL STORAGE CONDITIONS
























Store in a refrigerator (2°C - 8°C)
Do not freeze
Keep the cartridge in the outer carton in order to protect from light
During use: do not refrigerate or store above 30°C
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/02/230/005 1 x 3 ml
EU/1/02/230/006 5 x 3 ml
EU/1/02/230/007 10 x 3 ml
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
16. INFORMATION IN BRAILLE




















PARTICULARS TO APPEAR ON THE OUTER PACKAGING AND THE IMMEDIATE
PACKAGING
NAME OF THE MEDICINAL PRODUCT
Actrapid NovoLet 100 IU/ml solution for injection in a pre-filled pen
Insulin human (rDNA)
STATEMENT OF ACTIVE SUBSTANCE
1 ml solution contains 100 IU (3.5 mg) of insulin human (rDNA).
zinc chloride, glycerol, metacresol, sodium hydroxide, hydrochloric acid and water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection
5 x 3 ml
10 x 3 ml
METHOD AND ROUTE(S) OF ADMINISTRATION
Subcutaneous use
Actrapid NovoLet is designed to be used with NovoFine needles
Read the package leaflet before use
Actrapid NovoLet is for use by one person only
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children
OTHER SPECIAL WARNING(S), IF NECESSARY
EXP/
During use: use within 6 weeks
SPECIAL STORAGE CONDITIONS
Store in a refrigerator (2°C - 8°C)
























Do not freeze
Protect from light
During use: do not refrigerate or store above 30°C
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/02/230/008 5 x 3 ml
EU/1/02/230/009 10 x 3 ml
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
16. INFORMATION IN BRAILLE


















PARTICULARS TO APPEAR ON THE OUTER PACKAGING AND THE IMMEDIATE
PACKAGING
NAME OF THE MEDICINAL PRODUCT
Actrapid InnoLet 100 IU/ml solution for injection in a pre-filled pen
Insulin human (rDNA)
STATEMENT OF ACTIVE SUBSTANCE
1 ml solution contains 100 IU (3.5 mg) of insulin human (rDNA).
zinc chloride, glycerol, metacresol, sodium hydroxide, hydrochloric acid and water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection
1 x 3 ml
5 x 3 ml
10 x 3 ml
METHOD AND ROUTE(S) OF ADMINISTRATION
Subcutaneous use
Actrapid InnoLet is designed to be used with NovoFine S needles
Read the package leaflet before use
Actrapid InnoLet is for use by one person only
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children
OTHER SPECIAL WARNING(S), IF NECESSARY
EXP/
During use: use within 6 weeks
SPECIAL STORAGE CONDITIONS
























Store in a refrigerator (2°C - 8°C)
Do not freeze
Protect from light
During use: do not refrigerate or store above 30°C
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/02/230/010 1 x 3 ml
EU/1/02/230/011 5 x 3 ml
EU/1/02/230/012 10 x 3 ml
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
16. INFORMATION IN BRAILLE




















PARTICULARS TO APPEAR ON THE OUTER PACKAGING AND THE IMMEDIATE
PACKAGING
NAME OF THE MEDICINAL PRODUCT
Actrapid FlexPen 100 IU/ml solution for injection in a pre-filled pen
Insulin human (rDNA)
STATEMENT OF ACTIVE SUBSTANCE
1 ml solution contains 100 IU (3.5 mg) of insulin human (rDNA).
zinc chloride, glycerol, metacresol, sodium hydroxide, hydrochloric acid and water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection
1 x 3 ml
5 x 3 ml
10 x 3 ml
METHOD AND ROUTE(S) OF ADMINISTRATION
Subcutaneous use
Designed to be used with NovoFine or NovoTwist disposable needles up to a length of 8 mm.
Needles are not includedRead the package leaflet before use
Actrapid FlexPen is for use by one person only
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children
OTHER SPECIAL WARNING(S), IF NECESSARY
EXP/
During use: use within 6 weeks
SPECIAL STORAGE CONDITIONS
























Store in a refrigerator (2°C - 8°C)
Do not freeze
Protect from light
During use: do not refrigerate or store above 30°C
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/02/230/013 1 x 3 ml
EU/1/02/230/014 5 x 3 ml
EU/1/02/230/015 10 x 3 ml
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
16. INFORMATION IN BRAILLE




















PACKAGE LEAFLET: INFORMATION FOR THE USER
Actrapid 40 IU/ml solution for injection in a vial
Insulin human (rDNA)
Read all of this leaflet carefully before you start using your insulin.
–
If you have any further questions, ask your doctor, diabetes nurse or your pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor, diabetes nurse or your pharmacist.
WHAT ACTRAPID IS AND WHAT IT IS USED FOR
Actrapid is human insulin to treat diabetes.
Actrapid is a fast-acting insulin. This means that it will
start to lower your blood sugar about half an hour after you take it, and the effect will last for
approximately 8 hours. Actrapid is often given in combination with longer-acting insulin products.
► If you are allergic (hypersensitive)
to this insulin product, metacresol or any of the other
ingredients (see
7 Further information
). Look out for the signs of allergy in
5 Possible side
effects
► If you feel a hypo
coming on (a hypo is short for a hypoglycaemic reaction and is a symptom of
low blood sugar). See
4 What to do in an emergency
for more about hypos.
Take special care with Actrapid
►
If you have trouble
with your kidneys or liver, or with your adrenal, pituitary or thyroid glands
► If you are drinking alcohol:
watch for signs of a hypo and never drink alcohol on an empty
stomach
► If you are exercising
more than usual or if you want to change your usual diet
►
If you are ill:
carry on taking your insulin
► If you are going abroad:
travelling over time zones may affect your insulin needs and the
timing of your injections.
Many medicines affect the way glucose works in your body and they may influence your insulin dose.
Listed below are the most common medicines which may affect your insulin treatment. Talk to your
doctor or pharmacist if you take or have recently taken any other medicines, even those not prescribed.
Your need for insulin may change
if you also take: oral antidiabetic products; monoamine oxidase
inhibitors (MAOI); beta-blockers; ACE-inhibitors; acetylsalicylic acid; anabolic steroids;
sulphonamides; oral contraceptives; thiazides; glucocorticoids; thyroid hormone therapy; beta-
sympathomimetics; growth hormone; danazol; octreotide or lanreotide.
Pregnancy and breast-feeding
If you are pregnant, planning a pregnancy or breast-feeding:
please contact your doctor for
advice.
Driving and using machines
Keep this leaflet. You may need to read it again.
If you drive or use tools or machines:
watch out for signs of a hypo. Your ability to concentrate or to
react will be less during a hypo. Never drive or use machinery if you feel a hypo coming on. Discuss
with your doctor whether you can drive or use machines at all, if you have a lot of hypos or if you find
it hard to recognise hypos.
Talk about your insulin needs with your doctor and diabetes nurse. Follow their advice carefully. This
leaflet is a general guide.
If your doctor has switched you from one type or brand of insulin to another, your dose may have to
be adjusted by your doctor.
Eat a meal or snack containing carbohydrates within 30 minutes of the injection.
It is recommended that you measure your blood glucose regularly.
►
Check the label to make sure
it is the right type of insulin
►
Disinfect the rubber membrane
with a medicinal swab.
►
In insulin infusion pumps
► If the protective cap is loose or missing.
Each vial has a protective, tamper-proof plastic cap.
If it isn’t in perfect condition when you get the vial, return the vial to your supplier
► If it hasn’t been stored correctly
or been frozen (see
6 How to store Actrapid
)
►
If it does not appear water clear and colourless.
Actrapid is for injection under the skin (subcutaneously). Always vary the sites you inject, to avoid
lumps (see
5 Possible side effects
). The best places to give yourself an injection are: the front of your
waist (abdomen); your buttocks; the front of your thighs or upper arms. Your insulin will work more
quickly if you inject it around the waist.
Actrapid vials are for use with insulin syringes with the corresponding unit scale.
Actrapid may also be administered intravenously in special situations by medical professionals.
To inject Actrapid on its own
Inject the air into the vial: push the needle through the rubber stopper and press the plunger
Turn the vial and syringe upside down
Draw the right dose of insulin into the syringe
Pull the needle out of the vial
Make sure there is no air left in the syringe: point the needle upwards and push the air out
Check you have the right dose
To mix Actrapid with long-acting insulin
Roll the vial of long-acting insulin between your hands. Do this until the liquid is uniformly
white and cloudy
Draw as much air into the syringe as the dose of long-acting insulin you need. Inject the air into
the long-acting insulin vial, then pull out the needle
Draw as much air into the syringe as the dose of Actrapid you need. Inject the air into the
Actrapid vial. Then turn the vial and syringe upside down
Draw air into the syringe, in the same amount as the dose of insulin you need
4. Draw the right dose of Actrapid into the syringe.
Pull the needle out of the vial. Make sure there is no air left in the syringe: point the needle
upwards and push the air out. Check the dose
5. Now push the needle into the vial of long-acting insulin
6. Then turn the vial and syringe upside down
7. Draw the right dose of long-acting insulin into the syringe
8. Pull the needle out of the vial
9. Make sure there is no air left in the syringe, and check the dose
10. Inject the mixture straight away.
Always mix fast-acting and long-acting insulin in this order.
► Inject the insulin
under the skin. Use the injection technique advised by your doctor or diabetes
nurse
► Keep the needle under your skin
for at least 6 seconds to make sure that the full dose has been
delivered.
WHAT TO DO IN AN EMERGENCY
A hypo means your blood sugar level is too low.
The
warning signs of a hypo
may come on suddenly and can include: cold sweat; cool pale skin;
headache; rapid heart beat; feeling sick; feeling very hungry; temporary changes in vision; drowsiness;
unusual tiredness and weakness; nervousness or tremor; feeling anxious; feeling confused; difficulty in
concentrating.
If you get any of these signs,
eat glucose tablets or a high sugar snack (sweets, biscuits, fruit juice),
then rest.
Don’t take any insulin
if you feel a hypo coming on.
Carry glucose tablets, sweets, biscuits or fruit juice with you, just in case.
Tell your relatives, friends and close colleagues
that if you pass out (become unconscious), they
must: turn you on your side and seek medical advice straight away. They must not give you any food
or drink as it could choke you.
► If severe hypoglycaemia
is not treated, it can cause brain damage (temporary or permanent)
and even death
► If you have a hypo
that makes you pass out, or a lot of hypos, talk to your doctor. The amount
or timing of insulin, food or exercise may need to be adjusted.
You may recover more quickly from unconsciousness with an injection of the hormone glucagon by
someone who knows how to use it. If you are given glucagon you will need glucose or a sugary snack
as soon as you are conscious. If you do not respond to glucagon treatment, you will have to be treated
in a hospital. Seek medical advice after an injection of glucagon; you need to find the reason for your
hypo to avoid getting more.
You get a hypo if your blood sugar gets too low. This might happen:
•
If you take too much insulin
If you eat too little or miss a meal
If you exercise more than usual.
If your blood sugar gets too high
Your blood sugar may get too high (this is called hyperglycaemia).
The
warning signs
appear gradually. They include: increased urination; feeling thirsty; losing your
appetite; feeling sick (nausea or vomiting); feeling drowsy or tired; flushed, dry skin; dry mouth and a
fruity (acetone) smell of the breath.
If you get any of these signs,
test your blood sugar level and test your urine for ketones if you can.
Then seek medical advice straight away.
These may be signs of a very serious condition called diabetic ketoacidosis. If you don’t treat it, this
could lead to diabetic coma and death.
Repeatedly taking less insulin than you need
Less exercise than usual.
Like all medicines, Actrapid can cause side effects, although not everybody gets them. Actrapid may
cause hypoglycaemia (low blood sugar). See the advice in
4 What to do in an emergency.
Side effects reported uncommonly
(in less than 1 patient in 100)
Vision problems.
When you first start your treatment, it may disturb your vision, but the reaction
usually disappears.
Changes at the injection site (Lipodystrophy).
If you inject yourself too often at the same site, fatty
tissue under the skin at this site may shrink (lipoatrophy) or thicken (lipohypertrophy). Changing the
site with each injection may help to prevent such skin changes. If you notice your skin pitting or
thickening at the injection site, tell your doctor or diabetes nurse because these reactions can become
more severe, or they may change the absorption of your insulin if you inject in such a site.
Signs of allergy.
Reactions (redness, swelling, itching) at the injection site may occur (local allergic
reactions). These usually disappear after a few weeks of taking your insulin. If they do not disappear,
see your doctor.
Seek medical advice immediately:
• if signs of allergy spread to other parts of the body, or
• if you suddenly feel unwell and you start sweating; start being sick (vomiting); have difficulty
in breathing; have a rapid heart beat; feel dizzy; feel like fainting.
You may have a very rare serious allergic reaction
to Actrapid or one of its ingredients (called a
systemic allergic reaction). See also warning in
2 Before you use Actrapid.
Painful neuropathy
(nerve related pain). If your blood glucose levels improve very fast it may cause
a burning, tingling or electric pain. This is called acute painful neuropathy and it usually disappears. If
it does not disappear, see your doctor.
Swollen joints.
When you start taking insulin, water retention may cause swelling around your ankles
and other joints. This soon disappears.
Side effects reported very rarely
(in less than 1 patient in 10,000)
Diabetic retinopathy
(eye background changes). If you have diabetic retinopathy and your blood
glucose levels improve very fast, the retinopathy may get worse. Ask your doctor about this.
Having forgotten to take your insulin
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor, diabetes nurse or pharmacist.
Keep out of the reach and sight of children.
Do not use Actrapid after the expiry date which is stated on the label and the carton. The expiry date
refers to the last day of that month.
The vials that are
not being used
are to be stored in a refrigerator (2°C - 8°C).
Do not store them in or too near the freezer section or cooling element.
Do not freeze.
Keep the vials in the original package.
The vials that are being used
or about to be used are not to be kept in a refrigerator. You can carry
them with you and keep them at room temperature (not above 25ºC) for up to 4 weeks.
Always keep the vial in the outer carton when you’re not using it in order to protect it from light.
Actrapid must be protected from excessive heat and sunlight.
Actrapid should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active substance
is insulin human made by recombinant biotechnology. 1 ml contains
40 IU of insulin human. 1 vial contains 10 ml equivalent to 400 IU
The other ingredients are
zinc chloride, glycerol, metacresol, sodium hydroxide, hydrochloric
acid and water for injections.
What Actrapid looks like and contents of the pack
The solution for injection comes as a clear, colourless, aqueous solution.
It is supplied in packs of 1 or 5 vials of 10 ml or in a multipack of 5 x (1 x 10 ml) vials. Not all packs
may be marketed.
Marketing Authorisation Holder and Manufacturer
Novo Nordisk A/S
Novo Allé, DK-2880 Bagsværd, Denmark
This leaflet was last approved in
PACKAGE LEAFLET: INFORMATION FOR THE USER
Actrapid 100 IU/ml solution
for injection in a vial
Insulin human (rDNA)
Read all of this leaflet carefully before you start using your insulin.
–
If you have any further questions, ask your doctor, diabetes nurse or your pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor, diabetes nurse or your pharmacist.
WHAT ACTRAPID IS AND WHAT IT IS USED FOR
Actrapid is human insulin to treat diabetes.
Actrapid is a fast-acting insulin. This means that it will
start to lower your blood sugar about half an hour after you take it, and the effect will last for
approximately 8 hours. Actrapid is often given in combination with longer-acting insulin products.
► If you are allergic (hypersensitive)
to this insulin product, metacresol or any of the other
ingredients (see
7 Further information
). Look out for the signs of allergy in
5 Possible side
effects
► If you feel a hypo
coming on (a hypo is short for a hypoglycaemic reaction and is a symptom of
low blood sugar). See
4 What to do in an emergency
for more about hypos.
Take special care with Actrapid
► If you have trouble
with your kidneys or liver, or with your adrenal, pituitary or thyroid glands
► If you are drinking alcohol:
watch for signs of a hypo and never drink alcohol on an empty
stomach
► If you are exercising
more than usual or if you want to change your usual diet
► If you are ill:
carry on taking your insulin
► If you are going abroad:
travelling over time zones may affect your insulin needs and the
timing of your injections.
Many medicines affect the way glucose works in your body and they may influence your insulin dose.
Listed below are the most common medicines which may affect your insulin treatment. Talk to your
doctor or pharmacist if you take or have recently taken any other medicines, even those not prescribed.
Your need for insulin may change
if you also take: oral antidiabetic products; monoamine oxidase
inhibitors (MAOI); beta-blockers; ACE-inhibitors; acetylsalicylic acid; anabolic steroids;
sulphonamides; oral contraceptives; thiazides; glucocorticoids; thyroid hormone therapy; beta-
sympathomimetics; growth hormone; danazol; octreotide or lanreotide.
Pregnancy and breast-feeding
If you are pregnant, planning a pregnancy or breast-feeding:
please contact your doctor for
advice.
Driving and using machines
Keep this leaflet. You may need to read it again.
If you drive or use tools or machines:
watch out for signs of a hypo. Your ability to concentrate or to
react will be less during a hypo. Never drive or use machinery if you feel a hypo coming on. Discuss
with your doctor whether you can drive or use machines at all, if you have a lot of hypos or if you find
it hard to recognise hypos.
Talk about your insulin needs with your doctor and diabetes nurse. Follow their advice carefully. This
leaflet is a general guide.
If your doctor has switched you from one type or brand of insulin to another, your dose may have to
be adjusted by your doctor.
Eat a meal or snack containing carbohydrates within 30 minutes of the injection.
It is recommended that you measure your blood glucose regularly.
►
Check the label to make
sure
it is the right type of insulin
►
Disinfect the rubber membrane
with a medicinal swab.
►
In insulin infusion pumps
► If the protective cap is loose or missing.
Each vial has a protective, tamper-proof plastic cap.
If it isn’t in perfect condition when you get the vial, return the vial to your supplier
► If it hasn’t been stored correctly
or been frozen (see
6 How to store Actrapid
)
►
If it does not appear water clear and colourless.
Actrapid is for injection under the skin (subcutaneously). Always vary the sites you inject, to avoid
lumps (see
5 Possible side effects
). The best places to give yourself an injection are: the front of your
waist (abdomen); your buttocks; the front of your thighs or upper arms. Your insulin will work more
quickly if you inject it around the waist.
Actrapid vials are for use with insulin syringes with the corresponding unit scale.
Actrapid may also be administered intravenously in special situations by medical professionals.
To inject Actrapid on its own
Inject the air into the vial: push the needle through the rubber stopper and press the plunger
Turn the vial and syringe upside down
Draw the right dose of insulin into the syringe
Pull the needle out of the vial
Make sure there is no air left in the syringe: point the needle upwards and push the air out
Check you have the right dose
To mix Actrapid with long-acting insulin
Roll the vial of long-acting insulin between your hands. Do this until the liquid is uniformly
white and cloudy
Draw as much air into the syringe as the dose of long-acting insulin you need. Inject the air into
the long-acting insulin vial, then pull out the needle
Draw as much air into the syringe as the dose of Actrapid you need. Inject the air into the
Actrapid vial. Then turn the vial and syringe upside down
Draw air into the syringe, in the same amount as the dose of insulin you need
4. Draw the right dose of Actrapid into the syringe.
Pull the needle out of the vial. Make sure there is no air left in the syringe: point the needle
upwards and push the air out. Check the dose
5. Now push the needle into the vial of long-acting insulin
6. Then turn the vial and syringe upside down
7. Draw the right dose of long-acting insulin into the syringe
8. Pull the needle out of the vial
9. Make sure there is no air left in the syringe, and check the dose
10. Inject the mixture straight away.
Always mix fast-acting and long-acting insulin in this order.
► Inject the insulin
under the skin. Use the injection technique advised by your doctor or diabetes
nurse
► Keep the needle under your skin
for at least 6 seconds to make sure that the full dose has been
delivered.
WHAT TO DO IN AN EMERGENCY
A hypo means your blood sugar level is too low.
The
warning signs of a hypo
may come on suddenly and can include: cold sweat; cool pale skin;
headache; rapid heart beat; feeling sick; feeling very hungry; temporary changes in vision; drowsiness;
unusual tiredness and weakness; nervousness or tremor; feeling anxious; feeling confused; difficulty in
concentrating.
If you get any of these signs,
eat glucose tablets or a high sugar snack (sweets, biscuits, fruit juice),
then rest.
Don’t take any insulin
if you feel a hypo coming on.
Carry glucose tablets, sweets, biscuits or fruit juice with you, just in case.
Tell your relatives, friends and close colleagues
that if you pass out (become unconscious), they
must: turn you on your side and seek medical advice straight away. They must not give you any food
or drink as it could choke you.
► If severe hypoglycaemia
is not treated, it can cause brain damage (temporary or permanent)
and even death
► If you have a hypo
that makes you pass out, or a lot of hypos, talk to your doctor. The amount
or timing of insulin, food or exercise may need to be adjusted.
You may recover more quickly from unconsciousness with an injection of the hormone glucagon by
someone who knows how to use it. If you are given glucagon you will need glucose or a sugary snack
as soon as you are conscious. If you do not respond to glucagon treatment, you will have to be treated
in a hospital. Seek medical advice after an injection of glucagon; you need to find the reason for your
hypo to avoid getting more.
You get a hypo if your blood sugar gets too low. This might happen:
•
If you take too much insulin
If you eat too little or miss a meal
If you exercise more than usual.
If your blood sugar gets too high
Your blood sugar may get too high (this is called hyperglycaemia).
The
warning signs
appear gradually. They include: increased urination; feeling thirsty; losing your
appetite; feeling sick (nausea or vomiting); feeling drowsy or tired; flushed, dry skin; dry mouth and a
fruity (acetone) smell of the breath.
If you get any of these signs,
test your blood sugar level and test your urine for ketones if you can.
Then seek medical advice straight away.
These may be signs of a very serious condition called diabetic ketoacidosis. If you don’t treat it, this
could lead to diabetic coma and death.
Repeatedly taking less insulin than you need
Less exercise than usual.
Like all medicines, Actrapid can cause side effects, although not everybody gets them. Actrapid may
cause hypoglycaemia (low blood sugar). See the advice in
4 What to do in an emergency.
Side effects reported uncommonly
(in less than 1 patient in 100)
Vision problems.
When you first start your treatment, it may disturb your vision, but the reaction
usually disappears.
Changes at the injection site (Lipodystrophy).
If you inject yourself too often at the same site, fatty
tissue under the skin at this site may shrink (lipoatrophy) or thicken (lipohypertrophy). Changing the
site with each injection may help to prevent such skin changes. If you notice your skin pitting or
thickening at the injection site, tell your doctor or diabetes nurse because these reactions can become
more severe, or they may change the absorption of your insulin if you inject in such a site.
Signs of allergy.
Reactions (redness, swelling, itching) at the injection site may occur (local allergic
reactions). These usually disappear after a few weeks of taking your insulin. If they do not disappear,
see your doctor.
Seek medical advice immediately:
• if signs of allergy spread to other parts of the body, or
• if you suddenly feel unwell and you start sweating; start being sick (vomiting); have difficulty
in breathing; have a rapid heart beat; feel dizzy; feel like fainting.
You may have a very rare serious allergic reaction
to Actrapid or one of its ingredients (called a
systemic allergic reaction). See also warning in
2 Before you use Actrapid.
Painful neuropathy
(nerve related pain). If your blood glucose levels improve very fast it may cause
a burning, tingling or electric pain. This is called acute painful neuropathy and it usually disappears. If
it does not disappear, see your doctor.
Swollen joints.
When you start taking insulin, water retention may cause swelling around your ankles
and other joints. This soon disappears.
Side effects reported very rarely
(in less than 1 patient in 10,000)
Diabetic retinopathy
(eye background changes). If you have diabetic retinopathy and your blood
glucose levels improve very fast, the retinopathy may get worse. Ask your doctor about this.
Having forgotten to take your insulin
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor, diabetes nurse or pharmacist.
Keep out of the reach and sight of children.
Do not use Actrapid after the expiry date which is stated on the label and the carton. The expiry date
refers to the last day of that month.
The vials that are
not being used
are to be stored in a refrigerator (2°C - 8°C).
Do not store them in or too near the freezer section or cooling element.
Do not freeze.
Keep the vials in the original package.
The vials that are being used
or about to be used are not to be kept in a refrigerator. You can carry
them with you and keep them at room temperature (not above 25°C) for up to 6 weeks.
Always keep the vial in the outer carton when you’re not using it in order to protect it from light.
Actrapid must be protected from excessive heat and sunlight.
Actrapid should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active substance
is insulin human made by recombinant biotechnology. 1 ml contains
100 IU of insulin human. 1 vial contains 10 ml equivalent to 1000 IU
The other ingredients are
zinc chloride, glycerol, metacresol, sodium hydroxide, hydrochloric
acid and water for injections.
What Actrapid looks like and contents of the pack
The solution for injection comes as a clear, colourless, aqueous solution.
It is supplied in packs of 1 or 5 vials of 10 ml or in a multipack of 5 x (1 x 10 ml) vials. Not all packs
may be marketed.
Marketing Authorisation Holder and Manufacturer
Novo Nordisk A/S
Novo Allé, DK-2880 Bagsværd, Denmark
This leaflet was last approved in
PACKAGE LEAFLET: INFORMATION FOR THE USER
Actrapid Penfill 100 IU/ml solution for injection in a cartridge
Insulin human (rDNA)
Read all of this leaflet carefully before you start using your insulin.
–
Keep this leaflet. You may need to read it again.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor, diabetes nurse or your pharmacist.
WHAT ACTRAPID IS AND WHAT IT IS USED FOR
Actrapid is human insulin to treat diabetes.
Actrapid is a fast-acting insulin. This means that it will
start to lower your blood sugar about half an hour after you take it, and the effect will last for
approximately 8 hours. Actrapid is often given in combination with longer-acting insulin products.
► If you are allergic (hypersensitive)
to this insulin product, metacresol or any of the other
ingredients (see
7
Further information
). Look out for the signs of allergy in
5 Possible side
effects
► If you feel a hypo
coming on (a hypo is short for a hypoglycaemic reaction and is a symptom of
low blood sugar). See
4 What to do in an emergency
for more about hypos.
Take special care with Actrapid
► If you have trouble
with your kidneys or liver, or with your adrenal, pituitary or thyroid glands
► If you are drinking alcohol:
watch for signs of a hypo and never drink alcohol on an empty
stomach
► If you are exercising
more than usual or if you want to change your usual diet
► If you are ill:
carry on taking your insulin
► If you are going abroad:
travelling over time zones may affect your insulin needs and the
timing of your injections.
Many medicines affect the way glucose works in your body and they may influence your insulin dose.
Listed below are the most common medicines which may affect your insulin treatment. Talk to your
doctor or pharmacist if you take or have recently taken any other medicines, even those not prescribed.
Your need for insulin may change
if you also take: oral antidiabetic products; monoamine oxidase
inhibitors (MAOI); beta-blockers; ACE-inhibitors; acetylsalicylic acid; anabolic steroids;
sulphonamides; oral contraceptives; thiazides; glucocorticoids; thyroid hormone therapy; beta-
sympathomimetics; growth hormone; danazol; octreotide or lanreotide.
Pregnancy and breast-feeding
If you are pregnant, planning a pregnancy or breast-feeding:
please contact your doctor for
advice.
Driving and using machines
If you have any further questions, ask your doctor, diabetes nurse or your pharmacist.
If you drive or use tools or machines:
watch out for signs of a hypo. Your ability to concentrate or to
react will be less during a hypo. Never drive or use machinery if you feel a hypo coming on. Discuss
with your doctor whether you can drive or use machines at all, if you have a lot of hypos or if you find
it hard to recognise hypos.
Talk about your insulin needs with your doctor and diabetes nurse. Follow their advice carefully. This
leaflet is a general guide.
If your doctor has switched you from one type or brand of insulin to another, your dose may have to
be adjusted by your doctor.
Eat a meal or snack containing carbohydrates within 30 minutes of the injection.
It is recommended that you measure your blood glucose regularly.
►
Check the label to make sure
it is the right type of insulin
►
Always check
the cartridge, including the rubber plunger (stopper). Don’t use it if any damage
is seen or if there is a gap between the rubber plunger and the white label band. Take it back to
your supplier. See your delivery system manual for further instructions
►
Disinfect the rubber membrane
with a medicinal swab
►
Always use a new needle
for each injection to prevent contamination.
►
In insulin infusion pumps
►
If Penfill or the device containing Penfill is dropped, damaged or crushed
there is a risk of
leakage of insulin
►
If it hasn’t been stored correctly
or been frozen (see
6
How to store Actrapid
)
►
If it does not appear water clear and colourless.
Do not refill Actrapid Penfill.
Penfill cartridges are designed to be used with Novo Nordisk insulin delivery systems and NovoFine
or NovoTwist needles.
If you are treated with Actrapid Penfill and another insulin Penfill cartridge, you should use two
insulin delivery systems, one for each type of insulin.
Actrapid is for injection under the skin (subcutaneously). Always vary the sites you inject, to avoid
lumps (see
5 Possible side effects
). The best places to give yourself an injection are: the front of your
waist (abdomen); your buttocks; the front of your thighs or upper arms. Your insulin will work more
quickly if you inject it around the waist.
How to inject this insulin
► Inject the insulin
under the skin.
Use the injection technique advised by your doctor or diabetes
nurse and described in your delivery system manual
► Keep the needle under your skin
for at least 6 seconds to make sure that the full dose has been
delivered
► After each injection
be sure to remove and discard the needle and store Actrapid without the
needle attached. Otherwise, the liquid may leak out which can cause inaccurate dosing.
WHAT TO DO IN AN EMERGENCY
A hypo means your blood sugar level is too low.
The
warning signs of a hypo
may come on suddenly and can include: cold sweat; cool pale skin;
headache; rapid heart beat; feeling sick; feeling very hungry; temporary changes in vision; drowsiness;
unusual tiredness and weakness; nervousness or tremor; feeling anxious; feeling confused; difficulty in
concentrating.
If you get any of these signs,
eat glucose tablets or a high sugar snack (sweets, biscuits, fruit juice),
then rest.
Don’t take any insulin
if you feel a hypo coming on.
Carry glucose tablets, sweets, biscuits or fruit juice with you, just in case.
Tell your relatives, friends and close colleagues
that if you pass out (become unconscious), they
must: turn you on your side and seek medical advice straight away. They must not give you any food
or drink as it could choke you.
► If severe hypoglycaemia
is not treated, it can cause brain damage (temporary or permanent)
and even death
► If you have a hypo
that makes you pass out, or a lot of hypos, talk to your doctor. The amount
or timing of insulin, food or exercise may need to be adjusted.
You may recover more quickly from unconsciousness with an injection of the hormone glucagon by
someone who knows how to use it. If you are given glucagon you will need glucose or a sugary snack
as soon as you are conscious. If you do not respond to glucagon treatment, you will have to be treated
in a hospital. Seek medical advice after an injection of glucagon; you need to find the reason for your
hypo to avoid getting more.
You get a hypo if your blood sugar gets too low. This might happen:
•
If you take too much insulin
If you eat too little or miss a meal
If your blood sugar gets too high
Your blood sugar may get too high (this is called hyperglycaemia).
The
warning signs
appear gradually. They include: increased urination; feeling thirsty; losing your
appetite; feeling sick (nausea or vomiting); feeling drowsy or tired; flushed, dry skin; dry mouth and a
fruity (acetone) smell of the breath.
If you get any of these signs,
test your blood sugar level and test your urine for ketones if you can.
Then seek medical advice straight away.
These may be signs of a very serious condition called diabetic ketoacidosis. If you don’t treat it, this
could lead to diabetic coma and death.
Having forgotten to take your insulin
Repeatedly taking less insulin than you need
Less exercise than usual.
If you exercise more than usual.
Like all medicines, Actrapid can cause side effects although not everybody gets them. Actrapid may
cause hypoglycaemia (low blood sugar). See the advice in
4 What to do in an emergency.
Side effects reported uncommonly
(in less than 1 patient in 100)
Vision problems.
When you first start your treatment, it may disturb your vision, but the reaction
usually disappears.
Changes at the injection site (Lipodystrophy).
If you inject yourself too often at the same site, fatty
tissue under the skin at this site may shrink (lipoatrophy) or thicken (lipohypertrophy). Changing the
site with each injection may help to prevent such skin changes. If you notice your skin pitting or
thickening at the injection site, tell your doctor or diabetes nurse because these reactions can become
more severe, or they may change the absorption of your insulin if you inject in such a site.
Signs of allergy.
Reactions (redness, swelling, itching) at the injection site may occur (local allergic
reactions). These usually disappear after a few weeks of taking your insulin. If they do not disappear,
see your doctor.
Seek medical advice immediately:
• if signs of allergy spread to other parts of the body, or
• if you suddenly feel unwell and you start sweating; start being sick (vomiting); have difficulty
in breathing; have a rapid heart beat; feel dizzy; feel like fainting.
You may have a very rare serious allergic reaction
to Actrapid or one of its ingredients (called a
systemic allergic reaction). See also warning in
2 Before you use Actrapid.
Painful neuropathy
(nerve related pain). If your blood glucose levels improve very fast it may cause
a burning, tingling or electric pain. This is called acute painful neuropathy and it usually disappears. If
it does not disappear, see your doctor.
Swollen joints.
When you start taking insulin, water retention may cause swelling around your ankles
and other joints. This soon disappears.
Side effects reported very rarely
(in less than 1 patient in 10,000)
Diabetic retinopathy
(eye background changes). If you have diabetic retinopathy and your blood
glucose levels improve very fast, the retinopathy may get worse. Ask your doctor about this.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor, diabetes nurse or pharmacist.
Keep out of the reach and sight of children.
Do not use Actrapid after the expiry date which is stated on the label and the carton. The expiry date
refers to the last day of that month.
The Penfill that is not being used
is to be stored in a refrigerator (2°C - 8°C).
Do not store them in or too near the freezer section or cooling element.
Do not freeze.
Keet the Penfill in the original package.
The Penfill that is being used
or about to be used is not to be kept in a refrigerator. You can carry it
with you and keep it at room temperature (not above 30°C) for up to 6 weeks.
Always keep your cartridge in the outer carton when you’re not using it in order to protect it from
light.
Actrapid must be protected from excessive heat and sunlight.
Actrapid should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active substance
is insulin human made by recombinant biotechnology. 1 ml contains
100 IU of insulin human. 1 cartridge contains 3 ml equivalent to 300 IU
The other ingredients are
zinc chloride, glycerol, metacresol, sodium hydroxide, hydrochloric
acid and water for injections.
What Actrapid looks like and contents of the pack
The solution for injection comes as a clear, colourless, aqueous solution.
It is supplied in packs of 1, 5 or 10 cartridges of 3 ml. Not all packs may be marketed.
Marketing Authorisation Holder
Novo Nordisk A/S
Novo Allé, DK-2880 Bagsværd, Denmark
Manufacturer
The manufacturer can be identified by the batch number printed on the slip of the carton and on
the label:
If the second and third characters are W5, S6, P5, K7, or ZF Novo Nordisk A/S, Novo Allé,
DK-2880 Bagsværd, Denmark is the manufacturer
If the second and third characters are H7 or T6
Novo Nordisk Production SAS, 45 Avenue
d’Orléans F-28002 Chartres, France is the manufacturer.
This leaflet was last approved in
PACKAGE LEAFLET: INFORMATION FOR THE USER
Actrapid NovoLet 100 IU/ml solution for injection in a pre-filled pen
Insulin human (rDNA)
Read all of this leaflet carefully before you start using your insulin.
–
If you have any further questions, ask your doctor, diabetes nurse or your pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor, diabetes nurse or your pharmacist.
This side of the leaflet:
1.
What Actrapid is and what it is used for
What to do in an emergency
Overleaf:
How to use your NovoLet
WHAT ACTRAPID IS AND WHAT IT IS USED FOR
Actrapid is human insulin to treat diabetes.
Actrapid is a fast-acting insulin. This means that it will
start to lower your blood sugar about half an hour after you take it, and the effect will last for
approximately 8 hours. Actrapid is often given in combination with longer-acting insulin products.
► If you are allergic (hypersensitive)
to this insulin product, metacresol or any of the other
ingredients (see
7 Further information
). Look out for the signs of allergy in
5 Possible side
effects
► If you feel a hypo
coming on (a hypo is short for a hypoglycaemic reaction and is a symptom of
low blood sugar). See
4 What to do in an emergency
for more about hypos.
Take special care with Actrapid
► If you have trouble
with your kidneys or liver, or with your adrenal, pituitary or thyroid glands
► If you are drinking alcohol:
watch for signs of a hypo and never drink alcohol on an empty
stomach
► If you are exercising
more than usual or if you want to change your usual diet
► If you are ill:
carry on taking your insulin
► If you are going abroad:
travelling over time zones may affect your insulin needs and the
timing of your injections.
Many medicines affect the way glucose works in your body and they may influence your insulin dose.
Listed below are the most common medicines which may affect your insulin treatment. Talk to your
doctor or pharmacist if you take or have recently taken any other medicines, even those not prescribed.
Keep this leaflet. You may need to read it again.
Your need for insulin may change
if you also take: oral antidiabetic products; monoamine oxidase
inhibitors (MAOI); beta-blockers; ACE-inhibitors; acetylsalicylic acid; anabolic steroids;
sulphonamides; oral contraceptives; thiazides; glucocorticoids; thyroid hormone therapy; beta-
sympathomimetics; growth hormone; danazol; octreotide or lanreotide.
Pregnancy and breast-feeding
If you are pregnant, planning a pregnancy or breast-feeding:
please contact your doctor for
advice.
Driving and using machines
If you drive or use tools or machines:
watch out for signs of a hypo. Your ability to concentrate or to
react will be less during a hypo. Never drive or use machinery if you feel a hypo coming on. Discuss
with your doctor whether you can drive or use machines at all, if you have a lot of hypos or if you find
it hard to recognise hypos.
Talk about your insulin needs with your doctor and diabetes nurse. Follow their advice carefully. This
leaflet is a general guide.
If your doctor has switched you from one type or brand of insulin to another, your dose may have to
be adjusted by your doctor.
Eat a meal or snack containing carbohydrates within 30 minutes of the injection.
It is recommended that you measure your blood glucose regularly.
See overleaf for detailed instructions.
►
Check the label
to make sure
it is the right type of insulin
►
Always use a new needle
for each injection to prevent contamination.
►
In insulin infusion pumps
►
If NovoLet is dropped, damaged or crushed
there is a risk of leakage of insulin
►
If it hasn’t been stored correctly
or been frozen (see
6
How to store Actrapid
)
►
If it does not appear water clear and colourless.
Actrapid is for injection under the skin
(subcutaneously). Always vary the sites you inject, to avoid
lumps (see
5 Possible side effects
). The best places to give yourself an injection are: the front of your
waist (abdomen); your buttocks; the front of your thighs or upper arms. Your insulin will work more
quickly if you inject it around the waist.
WHAT TO DO IN AN EMERGENCY
A hypo means your blood sugar level is too low.
The
warning signs of a hypo
may come on suddenly and can include: cold sweat; cool pale skin;
headache; rapid heart beat; feeling sick; feeling very hungry; temporary changes in vision; drowsiness;
unusual tiredness and weakness; nervousness or tremor; feeling anxious; feeling confused; difficulty in
concentrating.
If you get any of these signs,
eat glucose tablets or a high sugar snack (sweets, biscuits, fruit juice),
then rest.
Don’t take any insulin
if you feel a hypo coming on.
Carry glucose tablets, sweets, biscuits or fruit juice with you, just in case.
Tell your relatives, friends and close colleagues
that if you pass out (become unconscious), they
must: turn you on your side and seek medical advice straight away. They must not give you any food
or drink as it could choke you.
►
If severe hypoglycaemia
is not treated, it can cause brain damage (temporary or permanent)
and even death
► If you have a hypo
that makes you pass out, or a lot of hypos, talk to your doctor. The amount
or timing of insulin, food or exercise may need to be adjusted.
You may recover more quickly from unconsciousness with an injection of the hormone glucagon by
someone who knows how to use it. If you are given glucagon you will need glucose or a sugary snack
as soon as you are conscious. If you do not respond to glucagon treatment, you will have to be treated
in a hospital. Seek medical advice after an injection of glucagon; you need to find the reason for your
hypo to avoid getting more.
You get a hypo if your blood sugar gets too low. This might happen:
•
If you take too much insulin
If you eat too little or miss a meal
If your blood sugar gets too high
Your blood sugar may get too high (this is called hyperglycaemia).
The
warning signs
appear gradually. They include: increased urination; feeling thirsty; losing your
appetite; feeling sick (nausea or vomiting); feeling drowsy or tired; flushed, dry skin; dry mouth and a
fruity (acetone) smell of the breath.
If you get any of these signs,
test your blood sugar level and test your urine for ketones if you can.
Then seek medical advice straight away.
These may be signs of a very serious condition called diabetic ketoacidosis. If you don’t treat it, this
could lead to diabetic coma and death.
Having forgotten to take your insulin
Repeatedly taking less insulin than you need
Less exercise than usual.
Like all medicines, Actrapid can cause side effects, although not everybody gets them. Actrapid may
cause hypoglycaemia (low blood sugar). See the advice in
4 What to do in an emergency.
Side effects reported uncommonly
(in less than 1 patient in 100)
If you exercise more than usual.
Vision problems.
When you first start your treatment, it may disturb your vision, but the reaction
usually disappears.
Changes at the injection site (Lipodystrophy).
If you inject yourself too often at the same site, fatty
tissue under the skin at this site may shrink (lipoatrophy) or thicken (lipohypertrophy). Changing the
site with each injection may help to prevent such skin changes. If you notice your skin pitting or
thickening at the injection site, tell your doctor or diabetes nurse because these reactions can become
more severe, or they may change the absorption of your insulin if you inject in such a site.
Signs of allergy.
Reactions (redness, swelling, itching) at the injection site may occur (local allergic
reactions). These usually disappear after a few weeks of taking your insulin. If they do not disappear,
see your doctor.
Seek medical advice immediately:
• if signs of allergy spread to other parts of the body, or
• if you suddenly feel unwell and you start sweating; start being sick (vomiting); have difficulty
in breathing; have a rapid heart beat; feel dizzy; feel like fainting.
You may have a very rare serious allergic reaction
to Actrapid or one of its ingredients (called a
systemic allergic reaction). See also warning in
2 Before you use Actrapid.
Painful neuropathy
(nerve related pain). If your blood glucose levels improve very fast it may cause
a burning, tingling or electric pain. This is called acute painful neuropathy and it usually disappears. If
it does not disappear, see your doctor.
Swollen joints.
When you start taking insulin, water retention may cause swelling around your ankles
and other joints. This soon disappears.
Side effects reported very rarely
(in less than 1 patient in 10,000)
Diabetic retinopathy
(eye background changes). If you have diabetic retinopathy and your blood
glucose levels improve very fast, the retinopathy may get worse. Ask your doctor about this.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor, diabetes nurse or pharmacist.
Keep out of the reach and sight of children.
Do not use Actrapid after the expiry date which is stated on the label and the carton. The expiry date
refers to the last day of that month.
The NovoLet that is not being used
is to be stored in a refrigerator (2°C - 8°C).
Do not store them in or too near the freezer section or cooling element.
Do not freeze.
The NovoLet that is
being used,
about to be used or carried as a spare is not to be kept in a
refrigerator. You can carry it with you and keep it at room temperature (not above 30ºC) for up to
6 weeks.
Always keep the pen cap on your NovoLet when you’re not using it in order to protect it from light.
Actrapid must be protected from excessive heat and sunlight.
Actrapid should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active substance
is insulin human made by recombinant biotechnology. 1 ml contains
100 IU of insulin human. 1 pre-filled pen contains 3 ml equivalent to 300 IU
The other ingredients are
zinc chloride, glycerol, metacresol, sodium hydroxide, hydrochloric
acid and water for injections.
What Actrapid looks like and contents of the pack
The solution for injection comes as a clear, colourless, aqueous solution.
It is supplied in packs of 5 or 10 pre-filled pens of 3 ml. Not all packs may be marketed.
Marketing Authorisation Holder and Manufacturer
Novo Nordisk A/S
Novo Allé, DK-2880 Bagsværd, Denmark
Now turn over for information on how to use your
NovoLet.
This leaflet was last approved in
Information on how to use Actrapid NovoLet
Please read the following instructions carefully before using your Actrapid NovoLet.
Actrapid NovoLet is a simple, compact pre-filled pen. You can dial doses from 2 to 78 units in
increments of 2 units. Actrapid NovoLet is designed to be used with NovoFine needles.
As a precautionary measure, always carry a spare insulin delivery device in case your NovoLet is lost
or damaged.
NovoLet
®
Push button scale
Push button
Check the label to make sure
that your Actrapid NovoLet contains the correct type of insulin. Take
off the pen cap.
•
Always use a new needle
for each injection to prevent contamination
Disinfect the rubber membrane
with a medicinal swab

Screw the needle straight and tightly
onto Actrapid NovoLet (picture
A
)
Pull off the big outer needle cap and the inner needle cap.
Do not discard the big outer
needle cap.
Small amounts of air may collect in the needle and cartridge during normal use.
To avoid injection of air and ensure proper dosing:
•
Hold Actrapid NovoLet with the needle pointing upwards
Tap the cartridge gently
with your finger a few times.
Any air bubbles will collect at the top of
the cartridge
Keeping the needle upwards, turn the cartridge for one click
in the direction of the arrow
(picture
B
)
Still with the needle upwards, press the push-button fully down
(picture
C
)
A drop of insulin must appear at the needle tip.
If not, change the needle and repeat the
procedure no more than 6 times.
If a drop of insulin still does not appear, the device is defective and must not be used.
Check that the push-button is fully down.
If it isn’t, turn the cap until the push-button is fully
depressed
Turn the cap in the direction of the arrow
(picture
E
)
to set the right dose. You’ll feel the cap
clicking, and the push-button will rise up
Don’t put your hand over the push-button
when you set the dose
.
If the push-button cannot
rise freely, some of your insulin will be pushed out of the needle
The scale on the cap shows 0, 2, 4, 6, 8, 10, 12, 14, 16 and 18 units.
For every click you feel
when you turn the cap, you set 2 units more. The push-button also rises as you turn the cap
The scale under the push-button
shows 20, 40 and 60 units.
Every time you fully turn the
cap, you set 20 units.
Remove the protective tab
from a NovoFine needle
Put the cap back on the pen,
with 0 next to the dosage indicator (picture
D
)
Hold your Actrapid
NovoLet horizontally.
Now you’re ready to set the dose you need

Dosage examples
To set 8 units:
Turn the cap until
8
is opposite the dosage indicator; four clicks
To select 26 units:
Turn the cap round 1 full turn, so
0
is opposite the dosage indicator again. You’ve now set 20 units.
Keep turning the cap until
6
is opposite the dosage indicator. On the push-button scale you’ll see a 20-
line.
Add the 6 from the dosage indicator to the 20 on the push-button scale. There, you’ve set
26
units
(picture
F
).
To check a dose you set
•
Note the highest figure you can see
on the push-button scale
Add the two together
to show the dose you set
If you have set a wrong dose,
simply turn the cap forwards or backwards until you set the right
number of units.
The maximum dose is 78 units
•
Don’t try to set a dose higher than 78 units.
Otherwise, insulin will leak out of the needle and
the dose will be incorrect
If you have, by mistake, tried to set a dose over 78 units, follow these steps:
Turn the cap back as far as you can. Turn it till the push-button is fully down and you can feel
resistance.
Then take the cap off and put it back on again, lining up the 0 next to the dosage indicator. Now
set the dose again.
Remember that 78 units is the maximum dose
After the dose is set, remove the cap to inject the insulin.
Go straight on to section
Injecting
the insulin
.
Deliver the dose by pressing the push-button fully down.
Be careful only to push the push-
button when injecting
Note the figure on the cap
next to the dosage indicator
Insert the needle into your skin.
Use the injection technique advised by your doctor


Keep the push-button fully depressed after the injection until the needle has been
withdrawn from the skin.
The needle must remain under the skin for at least 6 seconds. This
will ensure that the full dose has been delivered.
Always check that the push-button is completely down.
If not, turn the cap until the push-
button is fully depressed, then proceed as described in
Getting started
You may hear a clicking sound when you press the push-button.
Don’t use this to set or
check your dose; it may not be accurate
You can use the insulin level indicator to estimate how much is left,
but you can’t use it to
set or select your dose.
Replace the big outer needle cap and unscrew the needle. Dispose of it carefully.
Use a new needle for each injection.
Remove the needle after each injection and store NovoLet without a needle attached. Otherwise, the
liquid may leak out which can cause inaccurate dosing.
Health care professionals, relatives and other carers must follow general precautionary measures for
removal and disposal of needles to eliminate the risk of unintended needle penetration.
Close your Actrapid NovoLet fully with 0 next to the dosage indicator.
Dispose of your used Actrapid NovoLet carefully without the needle attached.
Your Actrapid NovoLet is designed to work accurately and safely. It must be handled with care.
Do not refill Actrapid NovoLet.
You can clean the exterior of your Actrapid NovoLet by wiping it with a medicinal swab. Do not soak
it, wash or lubricate it. This may damage the mechanism.
You can’t set a dose higher than the number of units left in the cartridge
PACKAGE LEAFLET: INFORMATION FOR THE USER
Actrapid InnoLet 100 IU/ml solution for injection in a pre-filled pen
Insulin human (rDNA)
Read all of this leaflet carefully before you start using your insulin.
–
If you have any further questions, ask your doctor, diabetes nurse or your pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor, diabetes nurse or your pharmacist.
This side of the leaflet:
1.
What Actrapid is and what it is used for
What to do in an emergency
Overleaf:
How to use your InnoLet
WHAT ACTRAPID IS AND WHAT IT IS USED FOR
Actrapid is human insulin to treat diabetes.
Actrapid is a fast-acting insulin. This means that it will
start to lower your blood sugar about half an hour after you take it, and the effect will last for
approximately 8 hours. Actrapid is often given in combination with longer-acting insulin products.
► If you are allergic (hypersensitive)
to this insulin product, metacresol or any of the other
ingredients (see
7
Further information
). Look out for the signs of allergy in
5 Possible side
effects
► If you feel a hypo
coming on (a hypo is short for a hypoglycaemic reaction and is a symptom of
low blood sugar). See
4 What to do in an emergency
for more about hypos.
Take special care with Actrapid
► If you have trouble
with your kidneys or liver, or with your adrenal, pituitary or thyroid glands
► If you are drinking alcohol:
watch for signs of a hypo and never drink alcohol on an empty
stomach
► If you are exercising
more than usual or if you want to change your usual diet
► If you are ill:
carry on taking your insulin
► If you are going abroad:
travelling over time zones may affect your insulin needs and the
timing of your injections.
Many medicines affect the way glucose works in your body and they may influence your insulin dose.
Listed below are the most common medicines which may affect your insulin treatment. Talk to your
doctor or pharmacist if you take or have recently taken any other medicines, even those not prescribed.
Keep this leaflet. You may need to read it again.
Your need for insulin may change
if you also take: oral antidiabetic products; monoamine oxidase
inhibitors (MAOI); beta-blockers; ACE-inhibitors; acetylsalicylic acid; anabolic steroids;
sulphonamides; oral contraceptives; thiazides; glucocorticoids; thyroid hormone therapy; beta-
sympathomimetics; growth hormone; danazol; octreotide or lanreotide.
Pregnancy and breast-feeding
If you are pregnant, planning a pregnancy or breast-feeding:
please contact your doctor for
advice.
Driving and using machines
If you drive or use tools or machines:
watch out for signs of a hypo. Your ability to concentrate or to
react will be less during a hypo. Never drive or use machinery if you feel a hypo coming on. Discuss
with your doctor whether you can drive or use machines at all, if you have a lot of hypos or if you find
it hard to recognise hypos.
Talk about your insulin needs with your doctor and diabetes nurse. Follow their advice carefully. This
leaflet is a general guide.
If your doctor has switched you from one type or brand of insulin to another, your dose may have to
be adjusted by your doctor.
Eat a meal or snack containing carbohydrates within 30 minutes of the injection.
It is recommended that you measure your blood glucose regularly.
See overleaf for detailed instructions.
►
Check the label to make sure
it is the right type of insulin
►
Always use a new needle
for each injection to prevent contamination.
►
In insulin infusion pumps
►
If InnoLet is dropped, damaged or crushed
there is a risk of leakage of insulin
►
If it hasn’t been stored correctly
or been frozen (see
6
How to store Actrapid
)
► If it does not appear water clear and colourless.
Actrapid is for injection under the skin
(subcutaneously). Always vary the sites you inject, to avoid
lumps (see
5 Possible side effects
). The best places to give yourself an injection are: the front of your
waist (abdomen); your buttocks; the front of your thighs or upper arms. Your insulin will work more
quickly if you inject it around the waist.
WHAT TO DO IN AN EMERGENCY
A hypo means your blood sugar level is too low.
The
warning signs of a hypo
may come on suddenly and can include: cold sweat; cool pale skin;
headache; rapid heart beat; feeling sick; feeling very hungry; temporary changes in vision; drowsiness;
unusual tiredness and weakness; nervousness or tremor; feeling anxious; feeling confused; difficulty in
concentrating.
If you get any of these signs,
eat glucose tablets or a high sugar snack (sweets, biscuits, fruit juice),
then rest.
Don’t take any insulin
if you feel a hypo coming on.
Carry glucose tablets, sweets, biscuits or fruit juice with you, just in case.
Tell your relatives, friends and close colleagues
that if you pass out (become unconscious), they
must: turn you on your side and seek medical advice straight away. They must not give you any food
or drink as it could choke you.
► If severe hypoglycaemia
is not treated, it can cause brain damage (temporary or permanent)
and even death
► If you have a hypo
that makes you pass out, or a lot of hypos, talk to your doctor. The amount
or timing of insulin, food or exercise may need to be adjusted.
You may recover more quickly from unconsciousness with an injection of the hormone glucagon by
someone who knows how to use it. If you are given glucagon you will need glucose or a sugary snack
as soon as you are conscious. If you do not respond to glucagon treatment, you will have to be treated
in a hospital. Seek medical advice after an injection of glucagon; you need to find the reason for your
hypo to avoid getting more.
You get a hypo if your blood sugar gets too low. This might happen:
•
If you take too much insulin
If you eat too little or miss a meal
If your blood sugar gets too high
Your blood sugar may get too high (this is called hyperglycaemia).
The
warning signs
appear gradually. They include: increased urination; feeling thirsty; losing your
appetite; feeling sick (nausea or vomiting); feeling drowsy or tired; flushed, dry skin; dry mouth and a
fruity (acetone) smell of the breath.
If you get any of these signs,
test your blood sugar level and test your urine for ketones if you can.
Then seek medical advice straight away.
These may be signs of a very serious condition called diabetic ketoacidosis. If you don’t treat it, this
could lead to diabetic coma and death.
Having forgotten to take your insulin
Repeatedly taking less insulin than you need
Less exercise than usual.
Like all medicines, Actrapid can cause side effects, although not everybody gets them. Actrapid may
cause hypoglycaemia (low blood sugar). See the advice in
4 What to do in an emergency.
Side effects reported uncommonly
(in less than 1 patient in 100)
If you exercise more than usual.
Vision problems.
When you first start your treatment, it may disturb your vision, but the reaction
usually disappears.
Changes at the injection site (Lipodystrophy).
If you inject yourself too often at the same site, fatty
tissue under the skin at this site may shrink (lipoatrophy) or thicken (lipohypertrophy). Changing the
site with each injection may help to prevent such skin changes. If you notice your skin pitting or
thickening at the injection site, tell your doctor or diabetes nurse because these reactions can become
more severe, or they may change the absorption of your insulin if you inject in such a site.
Signs of allergy.
Reactions (redness, swelling, itching) at the injection site may occur (local allergic
reactions). These usually disappear after a few weeks of taking your insulin. If they do not disappear,
see your doctor.
Seek medical advice immediately:
• if signs of allergy spread to other parts of the body, or
• if you suddenly feel unwell and you start sweating; start being sick (vomiting); have difficulty
in breathing; have a rapid heart beat; feel dizzy; feel like fainting.
You may have a very rare serious allergic reaction
to Actrapid or one of its ingredients (called a
systemic allergic reaction). See also warning in
2 Before you use Actrapid.
Painful neuropathy
(nerve related pain). If your blood glucose levels improve very fast it may cause
a burning, tingling or electric pain. This is called acute painful neuropathy and it usually disappears. If
it does not disappear, see your doctor.
Swollen joints.
When you start taking insulin, water retention may cause swelling around your ankles
and other joints. This soon disappears.
Side effects reported very rarely
(in less than 1 patient in 10,000)
Diabetic retinopathy
(eye background changes). If you have diabetic retinopathy and your blood
glucose levels improve very fast, the retinopathy may get worse. Ask your doctor about this.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor, diabetes nurse or pharmacist.
Keep out of the reach and sight of children.
Do not use Actrapid after the expiry date which is stated on the label and the carton. The expiry date
refers to the last day of that month.
The InnoLet that is not being used
is to be stored in a refrigerator (2°C - 8°C).
Do not store them in or too near the freezer section or cooling element.
Do not freeze.
The InnoLet that is being used,
about to be used or carried as a spare is not to be kept in a
refrigerator. You can carry it with you and keep it at room temperature (not above 30ºC) for up to
6 weeks.
Always keep the pen cap on your InnoLet when you’re not using it in order to protect it from light.
Actrapid must be protected from excessive heat and sunlight.
Actrapid should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active substance
is insulin human made by recombinant biotechnology. 1 ml contains
100 IU of insulin human. 1 pre-filled pen contains 3 ml equivalent to 300 IU
The other ingredients are
zinc chloride, glycerol, metacresol, sodium hydroxide, hydrochloric
acid and water for injections.
What Actrapid looks like and contents of the pack
The solution for injection comes as a clear, colourless, aqueous solution.
It is supplied in packs of 1, 5 or 10 pre-filled pens of 3 ml. Not all packs may be marketed.
Marketing Authorisation Holder and Manufacturer
Novo Nordisk A/S
Novo Allé, DK-2880 Bagsværd, Denmark
Now turn over for information on how to use your
InnoLet.
This leaflet was last approved in
Information on how to use Actrapid InnoLet
Please read the following instructions carefully before using your Actrapid InnoLet.
Actrapid InnoLet is a simple, compact pre-filled pen able to deliver 1 to 50 units in increments of
1 unit. Actrapid InnoLet is designed to be used with NovoFine
S
needles of 8 mm or shorter in length.
Look for an
S
on the needle box. The
S
stands for short cap.
As a precautionary measure, always carry a spare insulin delivery device in case your InnoLet is lost
or damaged.
Check the label to make sure
that your Actrapid InnoLet contains the correct type of insulin. Take
off the pen cap (as shown by the arrow).
Always use a new needle
for each injection to prevent contamination
Remove the protective tab
from a NovoFine
S
needle
Screw the needle straight and tightly
onto Actrapid InnoLet (picture
1A
)
Pull off the big outer needle cap and the inner needle cap.
You may want to store the big
outer needle cap in the compartment.
Disinfect the rubber membrane
with a medicinal swab

Small amounts of air may collect in the needle and cartridge during normal use.
To avoid injection of air and ensure proper dosing:
•
Hold Actrapid InnoLet with the needle pointing upwards and tap the cartridge gently
with
your finger a few times
to make any air bubbles collect at the top of the cartridge (picture
1B
)
A drop of insulin must appear at the needle tip.
If not, change the needle and repeat the
procedure no more than 6 times.
If a drop of insulin still does not appear, the device is defective and must not be used.
Always check that the push-button is fully depressed and the dose selector is set at zero
Dial the number of units required
by turning the dose selector clockwise (picture
2
). Do not
use the residual scale to measure your dose of insulin
You will hear a click for every single unit dialled.
The dose can be corrected by turning the
dial either way.
You cannot set a dose larger than the number of units left in the cartridge.
Dial 2 units
by turning the dose selector clockwise
Keeping the needle upwards, press the push-button
and the dose selector returns to zero
Insert the needle into your skin.
Use the injection technique advised by your doctor
Deliver the dose by pressing the push-button fully down
(picture
3
).
You will hear clicks as
the dose selector returns to zero
After the injection, the needle must remain under the skin for at least 6 seconds
to ensure
that the full dose has been delivered
Make sure not to block the dose selector while injecting,
as the dose selector must be allowed
to return to zero when you press the push-button
Remove the needle after each injection.
Replace the big outer needle cap and unscrew the needle (
picture
4). Dispose of it carefully.
Use a new needle for each injection.
Remove the needle after each injection and store InnoLet without a needle attached. Otherwise, the
liquid may leak out which can cause inaccurate dosing.
Health care professionals, relatives and other carers must follow general precautionary measures for
removal and disposal of needles to eliminate the risk of unintended needle penetration.
Dispose of your used Actrapid InnoLet carefully without the needle attached.

Your Actrapid InnoLet is designed to work accurately and safely. It must be handled with care.
Do not refill Actrapid InnoLet.
You can clean your Actrapid InnoLet by wiping it with a medicinal swab. Do not soak it, wash or
lubricate it. This may damage the mechanism.
PACKAGE LEAFLET: INFORMATION FOR THE USER
Actrapid FlexPen 100 IU/ml solution for injection in a pre-filled pen
Insulin human (rDNA)
Read all of this leaflet carefully before you start using your insulin.
–
If you have any further questions, ask your doctor, nurse or your pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor, nurse or your pharmacist.
This side of the leaflet:
1.
What Actrapid is and what it is used for
What to do in an emergency
Overleaf:
How to use your FlexPen
WHAT ACTRAPID IS AND WHAT IT IS USED FOR
Actrapid is human insulin to treat diabetes.
Actrapid is a fast-acting insulin. This means that it will
start to lower your blood sugar about half an hour after you take it, and the effect will last for
approximately 8 hours. Actrapid is often given in combination with longer-acting insulin products.
► If you are allergic (hypersensitive)
to this insulin product, metacresol or any of the other
ingredients (see
7
Further information
). Look out for the signs of allergy in
5 Possible side
effects
► If you feel a hypo
coming on (a hypo is short for a hypoglycaemic reaction and is a symptom of
low blood sugar). See
4 What to do in an emergency
for more about hypos.
Take special care with Actrapid
► If you have trouble
with your kidneys or liver, or with your adrenal, pituitary or thyroid glands
► If you are drinking alcohol:
watch for signs of a hypo and never drink alcohol on an empty
stomach
► If you are exercising
more than usual or if you want to change your usual diet
► If you are ill:
carry on taking your insulin
► If you are going abroad:
travelling over time zones may affect your insulin needs and the
timing of your injections.
Many medicines affect the way glucose works in your body and they may influence your insulin dose.
Listed below are the most common medicines which may affect your insulin treatment. Talk to your
doctor or pharmacist if you take or have recently taken any other medicines, even those not prescribed.
Keep this leaflet. You may need to read it again.
Your need for insulin may change
if you also take: oral antidiabetic products; monoamine oxidase
inhibitors (MAOI); beta-blockers; ACE-inhibitors; acetylsalicylic acid; anabolic steroids;
sulphonamides; oral contraceptives; thiazides; glucocorticoids; thyroid hormone therapy; beta-
sympathomimetics; growth hormone; danazol; octreotide or lanreotide.
Pregnancy and breast-feeding
If you are pregnant, planning a pregnancy or breast-feeding:
please contact your doctor for
advice.
Driving and using machines
If you drive or use tools or machines:
watch out for signs of a hypo. Your ability to concentrate or to
react will be less during a hypo. Never drive or use machinery if you feel a hypo coming on. Discuss
with your doctor whether you can drive or use machines at all, if you have a lot of hypos or if you find
it hard to recognise hypos.
Talk about your insulin needs with your doctor and nurse. Follow their advice carefully. This leaflet is
a general guide.
If your doctor has switched you from one type or brand of insulin to another, your dose may have to
be adjusted by your doctor.
Eat a meal or snack containing carbohydrates within 30 minutes of the injection.
It is recommended that you measure your blood glucose regularly.
See overleaf for detailed instructions.
►
Check the label
to make sure
it is the right type of insulin
►
Always use a new needle
for each injection to prevent contamination.
►
In insulin infusion pumps
►
If FlexPen is dropped, damaged or crushed
there is a risk of leakage of insulin
►
If it hasn’t been stored correctly
or been frozen (see
6
How to store Actrapid
)
► If it does not appear water clear and colourless.
Actrapid is for injection under the skin
(subcutaneously). Always vary the sites you inject, to avoid
lumps (see
5 Possible side effects
). The best places to give yourself an injection are: the front of your
waist (abdomen); your buttocks; the front of your thighs or upper arms. Your insulin will work more
quickly if you inject it around the waist.
WHAT TO DO IN AN EMERGENCY
A hypo means your blood sugar level is too low.
The
warning signs of a hypo
may come on suddenly and can include: cold sweat; cool pale skin;
headache; rapid heart beat; feeling sick; feeling very hungry; temporary changes in vision; drowsiness;
unusual tiredness and weakness; nervousness or tremor; feeling anxious; feeling confused; difficulty in
concentrating.
If you get any of these signs,
eat glucose tablets or a high sugar snack (sweets, biscuits, fruit juice),
then rest.
Don’t take any insulin
if you feel a hypo coming on.
Carry glucose tablets, sweets, biscuits or fruit juice with you, just in case.
Tell your relatives, friends and close colleagues
that if you pass out (become unconscious), they
must: turn you on your side and seek medical advice straight away. They must not give you any food
or drink as it could choke you.
► If severe hypoglycaemia
is not treated, it can cause brain damage (temporary or permanent)
and even death
► If you have a hypo
that makes you pass out, or a lot of hypos, talk to your doctor. The amount
or timing of insulin, food or exercise may need to be adjusted.
You may recover more quickly from unconsciousness with an injection of the hormone glucagon by
someone who knows how to use it. If you are given glucagon you will need glucose or a sugary snack
as soon as you are conscious. If you do not respond to glucagon treatment, you will have to be treated
in a hospital. Seek medical advice after an injection of glucagon; you need to find the reason for your
hypo to avoid getting more.
You get a hypo if your blood sugar gets too low. This might happen:
•
If you take too much insulin
If you eat too little or miss a meal
If your blood sugar gets too high
Your blood sugar may get too high (this is called hyperglycaemia).
The
warning signs
appear gradually. They include: increased urination; feeling thirsty; losing your
appetite; feeling sick (nausea or vomiting); feeling drowsy or tired; flushed, dry skin; dry mouth and a
fruity (acetone) smell of the breath.
If you get any of these signs,
test your blood sugar level and test your urine for ketones if you can.
Then seek medical advice straight away.
These may be signs of a very serious condition called diabetic ketoacidosis. If you don’t treat it, this
could lead to diabetic coma and death.
Having forgotten to take your insulin
Repeatedly taking less insulin than you need
Less exercise than usual.
Like all medicines, Actrapid can cause side effects, although not everybody gets them. Actrapid may
cause hypoglycaemia (low blood sugar). See the advice in
4 What to do in an emergency.
Side effects reported uncommonly
(in less than 1 patient in 100)
If you exercise more than usual.
Vision problems.
When you first start your treatment, it may disturb your vision, but the reaction
usually disappears.
Changes at the injection site (Lipodystrophy).
If you inject yourself too often at the same site, fatty
tissue under the skin at this site may shrink (lipoatrophy) or thicken (lipohypertrophy). Changing the
site with each injection may help to prevent such skin changes. If you notice your skin pitting or
thickening at the injection site, tell your doctor or nurse because these reactions can become more
severe, or they may change the absorption of your insulin if you inject in such a site.
Signs of allergy.
Reactions (redness, swelling, itching) at the injection site may occur (local allergic
reactions). These usually disappear after a few weeks of taking your insulin. If they do not disappear,
see your doctor.
Seek medical advice immediately:
• if signs of allergy spread to other parts of the body, or
• if you suddenly feel unwell and you start sweating; start being sick (vomiting); have difficulty
in breathing; have a rapid heart beat; feel dizzy; feel like fainting.
You may have a very rare serious allergic reaction
to Actrapid or one of its ingredients (called a
systemic allergic reaction). See also warning in
2 Before you use Actrapid.
Painful neuropathy
(nerve related pain). If your blood glucose levels improve very fast it may cause
a burning, tingling or electric pain. This is called acute painful neuropathy and it usually disappears. If
it does not disappear, see your doctor.
Swollen joints.
When you start taking insulin, water retention may cause swelling around your ankles
and other joints. This soon disappears.
Side effects reported very rarely
(in less than 1 patient in 10,000)
Diabetic retinopathy
(eye background changes). If you have diabetic retinopathy and your blood
glucose levels improve very fast, the retinopathy may get worse. Ask your doctor about this.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor, nurse or pharmacist.
Keep out of the reach and sight of children.
Do not use Actrapid after the expiry date which is stated on the label and the carton. The expiry date
refers to the last day of that month.
The FlexPen that is not being used
is to be stored in a refrigerator (2°C - 8°C).
Do not store them in or too near the freezer section or cooling element.
Do not freeze.
The FlexPen that is
being used,
about to be used or carried as a spare is not to be kept in a
refrigerator. You can carry it with you and keep it at room temperature (not above 30ºC) for up to
6 weeks.
Always keep the pen cap on your FlexPen when you’re not using it in order to protect it from light.
Actrapid must be protected from excessive heat and sunlight.
Actrapid should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active substance
is insulin human made by recombinant biotechnology. 1 ml contains
100 IU of insulin human. 1 pre-filled pen contains 3 ml equivalent to 300 IU
The other ingredients are
zinc chloride, glycerol, metacresol, sodium hydroxide, hydrochloric
acid and water for injections.
What Actrapid looks like and contents of the pack
The solution for injection comes as a clear, colourless, aqueous solution.
It is supplied in packs of 1, 5 or 10 pre-filled pens of 3 ml. Not all packs may be marketed.
Marketing Authorisation Holder
Novo Nordisk A/S
Novo Allé, DK-2880 Bagsværd, Denmark
Manufacturer
The manufacturer can be identified by the batch number printed on the slip of the carton and on the
label:
If the second and third characters are W5, S6, P5, K7, or ZF Novo Nordisk A/S, Novo Allé,
DK-2880 Bagsværd, Denmark is the manufacturer
If the second and third characters are H7 or T6 Novo Nordisk Production SAS, 45 Avenue
d’Orléans F-28002 Chartres, France is the manufacturer.
Now turn over for information on how to use your
FlexPen.
This leaflet was last approved in
Please read the following instructions carefully before using your Actrapid FlexPen.
Your FlexPen is a unique dial-a-dose insulin pen. You can select doses from 1 to 60 units in
increments of 1 unit. FlexPen is designed and tested to be used with NovoFine or NovoTwist
disposable needles up to a length of 8 mm. As a precautionary measure, always carry a spare insulin
delivery device in case your FlexPen is lost or damaged.
The colour of the pen in the illustrations differs from your FlexPen.
Maintenance
Your FlexPen is designed to work accurately and safely. It must be handled with care.
If it is dropped or crushed, there is a risk of damage and leakage of insulin.
You can clean the exterior of your FlexPen by wiping it with a medicinal swab. Do not soak it, wash
or lubricate it as it may damage the pen.
Do not refill your FlexPen.
Preparing your Actrapid FlexPen
Check the label to make sure that your FlexPen contains the correct type of insulin.
Disinfect the rubber membrane with a medicinal swab.
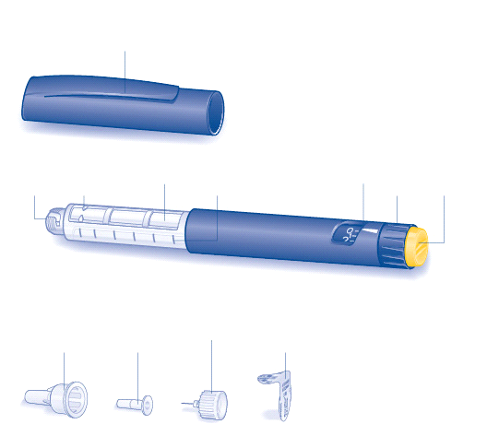
B
Remove the protective tab from a new disposable needle.
Screw the needle straight and tightly onto your FlexPen.
C
Pull off the big outer needle cap and keep it for later.
D
Pull off the inner needle cap and dispose of it.
Always use a new needle for each injection to prevent contamination.
Be careful not to bend or damage the needle before use.
To reduce the risk of unexpected needle sticks, never put the inner needle cap back on when you
have removed it from the needle.
Checking the insulin flow
Prior to each injection small amounts of air may collect in the cartridge during normal use. To
avoid injection of air and ensure proper dosing:
E
Turn the dose selector to select 2 units.




F
Hold your FlexPen with the needle pointing upwards and tap the cartridge gently with your finger a
few times to make any air bubbles collect at the top of the cartridge.
G
Keeping the needle upwards, press the push-button all the way in. The dose selector returns to 0.
A drop of insulin should appear at the needle tip. If not, change the needle and repeat the procedure no
more than six times.
If a drop of insulin still does not appear, the pen is defective, and you must use a new one.
Check that the dose selector is set at 0.
H
Turn the dose selector to select the number of units you need to inject.
The dose can be corrected either up or down by turning the dose selector in either direction until the
correct dose lines up with the pointer. When turning the dose selector be careful not to push the push-
button as insulin will come out.
You cannot select a dose larger than the number of units left in the cartridge.


Do not use the residual scale to measure your dose of insulin.
Insert the needle into your skin. Use the injection technique shown by your doctor or nurse.
I
Inject the dose by pressing the push-button all the way in until 0 lines up with the pointer. Be careful
only to push the push-button when injecting.
Turning the dose selector will not inject insulin.
J
Keep the push-button fully depressed after the injection until the needle has been withdrawn from the
skin.
The needle must remain under the skin for at least six seconds. This will ensure that the full dose has
been injected.
K
Lead the needle into the big outer needle cap without touching the big outer needle cap. When the
needle is covered, carefully push the big outer needle cap completely on and then unscrew the needle.
Dispose of it carefully and put the pen cap back on.



Always remove the needle after each injection otherwise the liquid may leak out which can
cause inaccurate dosing.
Caregivers should be most careful when handling used needles to avoid needle sticks.
Dispose of the used FlexPen carefully without the needle attached.
Do not share your FlexPen with anyone else.
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/actrapid.html
Copyright © 1995-2021 ITA all rights reserved.
|






























































































































































































































































































































































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































