Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
ALIMTA 100 mg powder for concentrate for solution for infusion
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each vial contains 100 mg of pemetrexed (as pemetrexed disodium).
After reconstitution (see section 6.6), each vial contains 25 mg/ml of pemetrexed.
Excipients
:
Each vial contains approximately 11 mg sodium.
For a full list of excipients see section 6.1.
Powder for concentrate for solution for infusion.
White to either light yellow or green-yellow lyophilised powder.
4.1 Therapeutic indications
Malignant pleural mesothelioma
ALIMTA in combination with cisplatin is indicated for the treatment of chemotherapy naïve patients
with unresectable malignant pleural mesothelioma.
Non-small cell lung cancer
ALIMTA in combination with cisplatin is indicated for the first line treatment of patients with locally
advanced or metastatic non-small cell lung cancer other than predominantly squamous cell histology
(see section 5.1).
ALIMTA is indicated as monotherapy for the maintenance treatment of locally advanced or metastatic
non-small cell lung cancer other than predominantly squamous cell histology in patients whose disease
has not progressed immediately following platinum-based chemotherapy. First line treatment should
be a platinum doublet with gemcitabine, paclitaxel or docetaxel (see section 5.1).
ALIMTA is indicated as monotherapy for the second line treatment of patients with locally advanced
or metastatic non-small cell lung cancer other than predominantly squamous cell histology (see section
5.1).
4.2 Posology and method of administration
ALIMTA must only be administered under the supervision of a physician qualified in the use of
anti-cancer chemotherapy.
ALIMTA in combination with cisplatin
The recommended dose of ALIMTA is 500 mg/m
2
of body surface area (BSA) administered as an
intravenous infusion over 10 minutes on the first day of each 21-day cycle. The recommended dose of
cisplatin is 75 mg/m
2
BSA infused over two hours approximately 30 minutes after completion of the
pemetrexed infusion on the first day of each 21-day cycle.
Patients must receive adequate anti-emetic
treatment and appropriate hydration prior to and/or after receiving cisplatin
(see also cisplatin
Summary of Product Characteristics for specific dosing advice).
ALIMTA as single agent
In patients treated for non-small cell lung cancer after prior chemotherapy, the recommended dose of
ALIMTA is 500 mg/m
2
BSA administered as an intravenous infusion over 10 minutes on the first day
of each 21-day cycle.
Premedication regimen
To reduce the incidence and severity of skin reactions, a corticosteroid should be given the day prior
to, on the day of, and the day after pemetrexed administration. The corticosteroid should be equivalent
to 4 mg of dexamethasone administered orally twice a day (see section 4.4).
To reduce toxicity, patients treated with pemetrexed must also receive vitamin supplementation (see
section 4.4). Patients must take oral folic acid or a multivitamin containing folic acid (350 to
1000 micrograms) on a daily basis. At least five doses of folic acid must be taken during the seven
days preceding the first dose of pemetrexed, and dosing must continue during the full course of
therapy and for 21 days after the last dose of pemetrexed. Patients must also receive an intramuscular
injection of vitamin B
12
(1000 micrograms) in the week preceding the first dose of pemetrexed and
once every three cycles thereafter. Subsequent vitamin B
12
injections may be given on the same day as
pemetrexed.
Monitoring
Patients receiving pemetrexed should be monitored before each dose with a complete blood count,
including a differential white cell count (WCC) and platelet count. Prior to each chemotherapy
administration blood chemistry tests should be collected to evaluate renal and hepatic function. Before
the start of any cycle of chemotherapy, patients are required to have the following: absolute neutrophil
count (ANC) should be ≥ 1500 cells/mm
3
and platelets should be ≥ 100,000 cells/mm
3
.
Creatinine clearance should be ≥ 45 ml/min.
The total bilirubin should be ≤ 1.5 times upper limit of normal. Alkaline phosphatase (AP), aspartate
transaminase (AST or SGOT) and alanine transaminase (ALT or SGPT) should be ≤ 3 times upper
limit of normal. Alkaline phosphatase, AST and ALT ≤ 5 times upper limit of normal is acceptable if
liver has tumour involvement.
Dose adjustments
Dose adjustments at the start of a subsequent cycle should be based on nadir haematologic counts or
maximum non-haematologic toxicity from the preceding cycle of therapy. Treatment may be delayed
to allow sufficient time for recovery. Upon recovery patients should be retreated using the guidelines
in Tables 1, 2 and 3, which are applicable for ALIMTA used as a single agent or in combination with
cisplatin.
Table 1 - Dose modification table for ALIMTA (as single agent or in combination) and
cisplatin – Haematologic toxicities
Nadir ANC < 500 /mm
3
and nadir platelets
≥ 50,000 /mm
3
75 % of previous dose (both ALIMTA and
cisplatin).
Nadir platelets <50,000 /mm
3
regardless of
nadir ANC
75 % of previous dose (both ALIMTA and
cisplatin).
Nadir platelets <50,000/mm
3
with bleeding
a
,
regardless of nadir ANC.
50% of previous dose (both ALIMTA and
cisplatin)
a
These criteria meet the National Cancer Institute Common Toxicity Criteria (CTC v2.0; NCI
1998) definition of ≥CTC Grade 2 bleeding







If patients develop non-haematologic toxicities ≥ Grade 3 (excluding neurotoxicity), ALIMTA should
be withheld until resolution to less than or equal to the patient’s pre-therapy value. Treatment should
be resumed according to the guidelines in Table 2.
Table 2 - Dose modification table for ALIMTA (as single agent or in combination) and
cisplatin– Non-haematologic toxicities
a, b
Dose for cisplatin (mg/m
2
)
Any Grade 3 or 4 toxicities except
mucositis
Any diarrhoea requiring
hospitalisation (irrespective of
grade) or grade 3 or 4 diarrhoea.
a
National Cancer Institute Common Toxicity Criteria (CTC v2.0; NCI 1998)
b
Excluding
neurotoxicity
In the event of neurotoxicity, the recommended dose adjustment for ALIMTA and cisplatin is
documented in Table 3. Patients should discontinue therapy if Grade 3 or 4 neurotoxicity is observed.
Table 3 - Dose modification table for ALIMTA (as single agent or in combination) and
cisplatin – Neurotoxicity
Dose for cisplatin (mg/m
2
)
a
National Cancer Institute Common Toxicity Criteria (CTC v2.0; NCI 1998)
Treatment with ALIMTA should be discontinued if a patient experiences any haematologic or
non-haematologic Grade 3 or 4 toxicity after 2 dose reductions or immediately if Grade 3 or 4
neurotoxicity is observed.
Elderly:
In clinical studies, there has been no indication that patients 65 years of age or older are at
increased risk of adverse events compared to patients younger than 65 years old. No dose reductions
other than those recommended for all patients are necessary.
Paediatric population
There is no relevant use of ALIMTA in the paediatric population in malignant pleural mesothelioma
and non-small cell lung cancer.
Patients with renal impairment:
(Standard Cockcroft and Gault formula or Glomerular Filtration Rate
measured Tc99m-DPTA serum clearance method): Pemetrexed is primarily eliminated unchanged by
renal excretion. In clinical studies, patients with creatinine clearance of ≥ 45 ml/min required no dose
adjustments other than those recommended for all patients. There are insufficient data on the use of
pemetrexed in patients with creatinine clearance below 45 ml/min; therefore the use of pemetrexed is
not recommended (see section 4.4).
Patients with hepatic impairment
: No relationships between AST (SGOT), ALT (SGPT), or total
bilirubin and pemetrexed pharmacokinetics were identified. However patients with hepatic impairment
such as bilirubin > 1.5 times the upper limit of normal and/or transaminase > 3.0 times the upper limit
of normal (hepatic metastases absent) or > 5.0 times the upper limit of normal (hepatic metastases
present) have not been specifically studied.
Method of administration:

















For Precautions to be taken before handling or administering ALIMTA, see section 6.6.
ALIMTA should be administered as an intravenous infusion over 10 minutes on the first day of each
21-day cycle. For instructions on reconstitution and dilution of ALIMTA before administration, see
section 6.6.
Hypersensitivity to the active substance or to any of the excipients.
Breast-feeding (see section 4.6)
.
Concomitant yellow fever vaccine (see section 4.5).
4.4 Special warnings and precautions for use
Pemetrexed can suppress bone marrow function as manifested by neutropenia, thrombocytopenia and
anaemia (or pancytopenia) (see section 4.8). Myelosuppression is usually the dose-limiting toxicity.
Patients should be monitored for myelosuppression during therapy and pemetrexed should not be
given to patients until absolute neutrophil count (ANC) returns to ≥ 1500 cells/mm
3
and platelet count
returns to ≥ 100,000 cells/mm
3
. Dose reductions for subsequent cycles are based on nadir ANC,
platelet count and maximum non-haematologic toxicity seen from the previous cycle (see section 4.2).
Less toxicity and reduction in Grade 3/4 haematologic and non-haematologic toxicities such as
neutropenia, febrile neutropenia and infection with Grade 3/4 neutropenia were reported when
pre-treatment with folic acid and vitamin B
12
was administered. Therefore, all patients treated with
pemetrexed must be instructed to take folic acid and vitamin B
12
as a prophylactic measure to reduce
treatment-related toxicity (see section 4.2).
Skin reactions have been reported in patients not pre-treated with a corticosteroid. Pre-treatment with
dexamethasone (or equivalent) can reduce the incidence and severity of skin reactions (see
section 4.2).
An insufficient number of patients has been studied with creatinine clearance of below 45 ml/min.
Therefore, the use of pemetrexed in patients with creatinine clearance of < 45 ml/min is not
recommended (see section 4.2).
Patients with mild to moderate renal insufficiency (creatinine clearance from 45 to 79 ml/min) should
avoid taking non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, and aspirin (> 1.3 g
daily) for 2 days before, on the day of, and 2 days following pemetrexed administration (see section
4.5).
In patients with mild to moderate renal insufficiency
eligible for pemetrexed therapy NSAIDs with
long elimination half-lives should be interrupted
for at least 5 days prior to, on the day of, and at least
2 days following pemetrexed administration (see section 4.5).
Serious renal events, including acute renal failure, have been reported with pemetrexed alone or in
association with other chemotherapeutic agents. Many of the patients in whom these occurred had
underlying risk factors for the development of renal events including dehydration or pre-existing
hypertension or diabetes.
The effect of third space fluid, such as pleural effusion or ascites, on pemetrexed is not fully defined.
A phase 2 study of pemetrexed in 31 solid tumour patients with stable third space fluid demonstrated
no difference in pemetrexed dose normalized plasma concentrations or clearance compared to patients
without third space fluid collections. Thus, drainage of third space fluid collection prior to pemetrexed
treatment should be considered, but may not be necessary.
Due to the gastrointestinal toxicity of pemetrexed given in combination with cisplatin, severe
dehydration has been observed. Therefore, patients should receive adequate antiemetic treatment and
appropriate hydration prior to and/or after receiving treatment.
Serious cardiovascular events, including myocardial infarction and cerebrovascular events have been
uncommonly reported during clinical studies with pemetrexed, usually when given in combination
with another cytotoxic agent. Most of the patients in whom these events have been observed had pre-
existing cardiovascular risk factors (see section 4.8).
Immunodepressed status is common in cancer patients. As a result, concomitant use of live attenuated
vaccines is not recommended (see section 4.3 and 4.5).
Pemetrexed can have genetically damaging effects. Sexually mature males are advised not to father a
child during the treatment and up to 6 months thereafter. Contraceptive measures or abstinence are
recommended. Owing to the possibility of pemetrexed treatment causing irreversible infertility, men
are advised to seek counselling on sperm storage before starting treatment.
Women of childbearing potential must use effective contraception during treatment with pemetrexed
(see section 4.6).
Cases of radiation pneumonitis have been reported in patients treated with radiation either prior,
during or subsequent to their pemetrexed therapy. Particular attention should be paid to these patients
and caution exercised with use of other radiosensitising agents.
Cases of radiation recall have been reported in patients who received radiotherapy weeks or years
previously.
4.5 Interaction with other medicinal products and other forms of interaction
Pemetrexed is mainly eliminated unchanged renally by tubular secretion and to a lesser extent by
glomerular filtration. Concomitant administration of nephrotoxic drugs (e.g. aminoglycoside, loop
diuretics, platinum compounds, cyclosporin) could potentially result in delayed clearance of
pemetrexed. This combination should be used with caution. If necessary, creatinine clearance should
be closely monitored.
Concomitant administration of substances that are also tubularly secreted (e.g. probenecid, penicillin)
could potentially result in delayed clearance of pemetrexed. Caution should be made when these drugs
are combined with pemetrexed. If necessary, creatinine clearance should be closely monitored.
In patients with normal renal function (creatinine clearance
>
80 ml/min), high doses of non-steroidal
anti-inflammatory drugs (NSAIDs, such as ibuprofen > 1600 mg/day) and aspirin at higher dose
(
>
1.3 g daily) may decrease pemetrexed elimination and, consequently, increase the occurrence of
pemetrexed adverse events. Therefore, caution should be made when administering higher doses of
NSAIDs or aspirin at higher dose, concurrently with pemetrexed to patients with normal function
(creatinine clearance
>
80 ml/min).
In patients with mild to moderate renal insufficiency (creatinine clearance from 45 to 79 ml/min), the
concomitant administration of pemetrexed with NSAIDs (e.g. ibuprofen) or aspirin at higher dose
should be avoided for 2 days before, on the day of, and 2 days following pemetrexed administration
(see section 4.4).
In the absence of data regarding potential interaction with NSAIDs having longer half-lives such as
piroxicam or rofecoxib, the concomitant administration with pemetrexed
in patients with mild to
moderate renal insufficiency
should be
interrupted
for at least 5 days prior to, on the day of, and at
least 2 days following pemetrexed administration (see section 4.4).
Pemetrexed undergoes limited hepatic metabolism. Results from
in vitro
studies with human liver
microsomes indicated that pemetrexed would not be predicted to cause clinically significant inhibition
of the metabolic clearance of drugs metabolised by CYP3A, CYP2D6, CYP2C9, and CYP1A2.
Interactions common to all cytotoxics:
Due to the increased thrombotic risk in patients with cancer, the use of anticoagulation treatment is
frequent. The high intra-individual variability of the coagulation status during diseases and the
possibility of interaction between oral anticoagulants and anticancer chemotherapy require increased
frequency of INR (International Normalised Ratio) monitoring, if it is decided to treat the patient with
oral anticoagulants.
Concomitant use contraindicated: Yellow fever vaccine: risk of fatal generalised vaccinale disease (see
section 4.3).
Concomitant use not recommended: Live attenuated vaccines (except yellow fever, for which
concomitant use is contraindicated): risk of systemic, possibly fatal, disease. The risk is increased in
subjects who are already immunosuppressed by their underlying disease. Use an inactivated vaccine
where it exists (poliomyelitis) (see section 4.4).
4.6 Fertility, pregnancy and lactation
Contraception in males and females
Women of childbearing potential must use effective contraception during treatment with pemetrexed.
Pemetrexed can have genetically damaging effects. Sexually mature males are advised not to father a
child during the treatment and up to 6 months thereafter. Contraceptive measures or abstinence are
recommended.
Pregnancy
There are no data from the use of pemetrexed in pregnant women but pemetrexed, like other
anti-metabolites, is suspected to cause serious birth defects when administered during pregnancy.
Animal studies have shown reproductive toxicity (see section 5.3). Pemetrexed should not be used
during pregnancy unless clearly necessary, after a careful consideration of the needs of the mother and
the risk for the foetus (see section 4.4).
Breastfeeding
It is not known whether pemetrexed is excreted in human milk and adverse reactions on the suckling
child cannot be excluded. Breast-feeding must be discontinued during pemetrexed therapy
(see
section 4.3)
.
Fertility
Owing to the possibility of pemetrexed treatment causing irreversible infertility, men are advised to
seek counselling on sperm storage before starting treatment.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. However, it
has been reported that pemetrexed may cause fatigue. Therefore patients should be cautioned against
driving or operating machines if this event occurs.
Summary of the safety profile
The most commonly reported undesirable effects related to pemetrexed, whether used as monotherapy
or in combination, are bone marrow suppression manifested as anaemia, neutropenia, leukopenia,
thrombocytopenia; and gastrointestinal toxicities, manifested as anorexia, nausea, vomiting, diarrhoea,
constipation, pharyngitis, mucositis, and stomatitis. Other undesirable effects include renal toxicities,
increased transaminases, alopecia, fatigue, dehydration, rash, infection/sepsis and neuropathy. Rarely
seen events include Stevens-Johnson syndrome and Toxic epidermal necrolysis.
Creatinine
clearance
decreased**
General
disorders and
administration
site conditions
*Refer to National Cancer Institute CTC version 2 for each grade of toxicity except the term
“creatinine clearance decreased”
** which is derived from the term “renal/genitourinary other”.
*** According to National Cancer Institute CTC (v2.0; NCI 1998), taste disturbance and alopecia
should only be reported as Grade 1 or 2.
For the purpose of this table a cut off of 5 % was used for inclusion of all events where the reporter
considered a possible relationship to pemetrexed and cisplatin.
Clinically relevant CTC toxicities that were reported in ≥ 1 % and
<
5 % of the patients that were
randomly assigned to receive cisplatin and pemetrexed include: renal failure, infection, pyrexia, febrile
neutropenia, increased AST, ALT, and GGT, urticaria and chest pain.
Clinically relevant CTC toxicities that were reported in < 1 % of the patients that were randomly
assigned to receive cisplatin and pemetrexed include arrhythmia and motor neuropathy.
The table below provides the frequency and severity of undesirable effects that have been reported in
> 5 % of 265 patients randomly assigned to receive single agent pemetrexed with folic acid and
vitamin B
12
supplementation and 276 patients randomly assigned to receive single agent docetaxel. All
patients were diagnosed with locally advanced or metastatic non-small cell lung cancer and received
prior chemotherapy.
System organ
class
Blood and
lymphatic
system disorders
Neutrophils/
Granulocytes
decreased
Gastrointestinal
disorders
Skin and sub-
cutaneous tissue




















































General
disorders and
administration
site conditions
*Refer to National Cancer Institute CTC version 2 for each grade of toxicity.
**According to National Cancer Institute CTC (v2.0; NCI 1998), alopecia should only be reported
as Grade 1 or 2.
For the purpose of this table a cut off of 5 % was used for inclusion of all events where the reporter
considered a possible relationship to pemetrexed.
Clinically relevant CTC toxicities that were reported in ≥ 1 % and
<
5 % of the patients that were
randomly assigned to pemetrexed include: infection without neutropenia, febrile neutropenia, allergic
reaction/hypersensitivity, increased creatinine, motor neuropathy, sensory neuropathy, erythema
multiforme, and abdominal pain.
Clinically relevant CTC toxicities that were reported in < 1 % of the patients that were randomly
assigned to pemetrexed include supraventricular arrhythmias.
Clinically relevant Grade 3 and Grade 4 laboratory toxicities were similar between integrated Phase 2
results from three single agent pemetrexed studies (n = 164) and the Phase 3 single agent pemetrexed
study described above, with the exception of neutropenia (12.8 % versus 5.3 %, respectively) and
alanine transaminase elevation (15.2 % versus 1.9 %, respectively). These differences were likely due
to differences in the patient population, since the Phase 2 studies included both chemonaive and
heavily pre-treated breast cancer patients with pre-existing liver metastases and/or abnormal baseline
liver function tests.
The table below provides the frequency and severity of undesirable effects considered possibly related
to study drug that have been reported in >5% of 839 patients with NSCLC who were randomized to
receive cisplatin and pemetrexed and 830 patients with NSCLC who were randomized to receive
cisplatin and gemcitabine. All patients received study therapy as initial treatment for locally advanced
or metastatic NSCLC and patients in both treatment groups were fully supplemented with folic acid
and vitamin B
12
.















Pemetrexed/
cisplatin
(N = 839)
Gemcitabine/
cisplatin
(N = 830)
Blood and
lymphatic
system
disorders
Neutrophils/
Granulocytes
decreased
Gastrointestinal
disorders
Diarrhoea without
colostomy
Skin and
subcutaneous
tissue disorders
Renal and
urinary
disorders
General
disorders and
administration
site conditions
*P-values <0.05 comparing pemetrexed/cisplatin to gemcitabine/cisplatin, using Fisher Exact test.
**Refer to National Cancer Institute CTC (v2.0; NCI 1998) for each Grade of Toxicity.
***According to National Cancer Institute CTC (v2.0; NCI 1998), taste disturbance and alopecia
should only be reported as Grade 1 or 2.
For the purpose of this table, a cut-off of 5% was used for inclusion of all events where the reporter
considered a possible relationship to pemetrexed and cisplatin.
Clinically relevant toxicity that was reported in ≥ 1% and ≤ 5% of the patients that were randomly
assigned to receive cisplatin and pemetrexed include: AST increase, ALT increase, infection, febrile
neutropenia, renal failure, pyrexia, dehydration, conjunctivitis, and creatinine clearance decrease.
Clinically relevant toxicity that was reported in < 1% of the patients that were randomly assigned to
receive cisplatin and pemetrexed include: GGT increase, chest pain, arrhythmia, and motor
neuropathy.





















































Clinically relevant toxicities with respect to gender were similar to the overall population in patients
receiving pemetrexed plus cisplatin.
The table below provides the frequency and severity of undesirable effects considered possibly related
to study drug that have been reported in > 5% of 441 patients randomly assigned to receive single
agent pemetrexed and 222 patients randomly assigned to receive placebo
in
the single-agent
maintenance pemetrexed study (Study JMEN). All patients were diagnosed with Stage IIIB or IV
NSCLC and had received prior platinum-based chemotherapy. Patients in both study arms were fully
supplemented with folic acid and vitamin B
12
.
Grade
3 - 4
toxicit
y
(%)
Grade
3 - 4
toxicit
y
(%)
Infections and
infestations
Blood and
lymphatic system
disorders
Gastrointestinal
disorders
Skin and
subcutaneous
tissue disorders
General disorders
and administration
site conditions
Abbreviations: ALT = alanine transaminase; AST = aspartate transaminase; CTCAE = Common Terminology
Criteria for Adverse Event; NCI = National Cancer Institute; SGOT = serum glutamic oxaloacectic
transaminase; SGPT = serum glutamic pyruvic transaminase.
*
Definition of frequency terms: Very common - ≥ 10%; Common - > 5% and < 10%. For the purpose of this
table, a cutoff of 5% was used for inclusion of all events where the reporter considered a possible relationship
to pemetrexed.
**
Refer to NCI CTCAE Criteria (Version 3.0; NCI 2003) for each grade of toxicity
Clinically relevant CTC toxicity of any grade that was reported in ≥ 1% and ≤ 5% of the patients that
were randomly assigned to pemetrexed include: decreased platelets, decreased creatinine clearance,
constipation, edema, alopecia, increased creatinine, pruritis/itching, fever (in the absence of
neutropenia), ocular surface disease (including conjunctivitis), increased lacrimation, and decreased
glomerular filtration rate.

































Clinically relevant CTC toxicity that was reported in < 1% of the patients that were randomly assigned
to pemetrexed include: febrile neutropenia, allergic reaction/hypersensitivity, motor neuropathy,
erythema multiforme, renal failure, and supraventricular arrhythmia.
The incidence of adverse reactions was evaluated for patients who received ≤ 6 cycles of pemetrexed,
and compared to patients who received > 6 cycles of pemetrexed. Increases in adverse reactions (all
grades) were observed with longer exposure; however, no statistically significant differences in
Grade 3/4 adverse reactions were seen.
Serious cardiovascular and cerebrovascular events, including myocardial infarction, angina pectoris,
cerebrovascular accident and transient ischaemic attack have been uncommonly reported during
clinical studies with pemetrexed, usually when given in combination with another cytotoxic agent.
Most of the patients in whom these events have been observed had pre-existing cardiovascular risk
factors.
Rare cases of hepatitis, potentially serious, have been reported during clinical studies with pemetrexed.
Pancytopenia has been uncommonly reported during clinical trials with pemetrexed.
In clinical trials, cases of colitis (including intestinal and rectal bleeding, sometimes fatal, intestinal
perforation, intestinal necrosis and typhlitis) have been reported uncommonly in patients treated with
pemetrexed.
In clinical trials, cases of interstitial pneumonitis with respiratory insufficiency, sometimes fatal, have
been reported uncommonly in patients treated with pemetrexed.
Uncommon cases of oedema have been reported in patients treated with pemetrexed.
Oesophagitis/ radiation oesophagitis has been uncommonly reported during clinical trials with
pemetrexed.
Sepsis, sometimes fatal, has been commonly reported during clinical trials with pemetrexed.
During post marketing surveillance, the following adverse reactions have been reported in patients
treated with pemetrexed:
Uncommon cases of acute renal failure have been reported with pemetrexed alone or in association
with other chemotherapeutic agents (see section 4.4).
Uncommon cases of radiation pneumonitis have been reported in patients treated with radiation either
prior, during or subsequent to their pemetrexed therapy (see section 4.4).
Rare cases of radiation recall have been reported in patients who have received radiotherapy
previously (see section 4.4).
Uncommon cases of peripheral ischaemia leading sometimes to extremity necrosis have been reported.
Rare cases of bullous conditions have been reported including Stevens-Johnson syndrome and Toxic
epidermal necrolysis which in some cases were fatal.
Rarely, haemolytic anaemia has been reported in patients treated with pemetrexed.
Reported symptoms of overdose include neutropenia, anaemia, thrombocytopenia, mucositis, sensory
polyneuropathy and rash. Anticipated complications of overdose include bone marrow suppression as
manifested by neutropenia, thrombocytopenia and anaemia. In addition, infection with or without
fever, diarrhoea, and/or mucositis may be seen. In the event of suspected overdose, patients should be
monitored with blood counts and should receive supportive therapy as necessary. The use of calcium
folinate / folinic acid in the management of pemetrexed overdose should be considered.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Folic acid analogues, ATC code: L01BA04
ALIMTA (pemetrexed) is a multi-targeted anti-cancer antifolate agent that exerts its action by
disrupting crucial folate-dependent metabolic processes essential for cell replication.
In vitro
studies have shown that pemetrexed behaves as a multitargeted antifolate by inhibiting
thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide
formyltransferase (GARFT), which are key folate-dependent enzymes for the
de novo
biosynthesis of
thymidine and purine nucleotides. Pemetrexed is transported into cells by both the reduced folate
carrier and membrane folate binding protein transport systems. Once in the cell, pemetrexed is rapidly
and efficiently converted to polyglutamate forms by the enzyme folylpolyglutamate synthetase. The
polyglutamate forms are retained in cells and are even more potent inhibitors of TS and GARFT.
Polyglutamation is a time- and concentration-dependent process that occurs in tumour cells and, to a
lesser extent, in normal tissues. Polyglutamated metabolites have an increased intracellular half-life
resulting in prolonged drug action in malignant cells.
The European Medicines Agency has waived the obligation to submit the results of studies with
ALIMTA in all subsets of the paediatric population in the granted indications
(see Section 4.2).
EMPHACIS, a multicentre, randomised, single-blind phase 3 study of ALIMTA plus cisplatin versus
cisplatin in chemonaive patients with malignant pleural mesothelioma, has shown that patients treated
with ALIMTA and cisplatin had a clinically meaningful 2.8-month median survival advantage over
patients receiving cisplatin alone.
During the study, low-dose folic acid and vitamin B
12
supplementation was introduced to patients’
therapy to reduce toxicity. The primary analysis of this study was performed on the population of all
patients randomly assigned to a treatment arm who received study drug (randomised and treated). A
subgroup analysis was performed on patients who received folic acid and vitamin B
12
supplementation
during the entire course of study therapy (fully supplemented). The results of these analyses of
efficacy are summarised in the table below:
Efficacy of ALIMTA plus cisplatin vs. cisplatin
in malignant pleural mesothelioma








Median overall survival (months)
Median time to tumour progression
(months)
Time to treatment failure (months)
(37.8 - 53.4) (13.8 - 26.6)
Abbreviation: CI = confidence interval
* p-value refers to comparison between arms.
** In the ALIMTA/cisplatin arm, randomized and treated (N = 225) and fully supplemented
(N = 167)
A statistically significant improvement of the clinically relevant symptoms (pain and dyspnoea)
associated with malignant pleural mesothelioma in the ALIMTA/cisplatin arm (212 patients) versus
the cisplatin arm alone (218 patients) was demonstrated using the Lung Cancer Symptom Scale.
Statistically significant differences in pulmonary function tests were also observed. The separation
between the treatment arms was achieved by improvement in lung function in the ALIMTA/cisplatin
arm and deterioration of lung function over time in the control arm.
There are limited data in patients with malignant pleural mesothelioma treated with ALIMTA alone.
ALIMTA at a dose of 500 mg/m
2
was studied as a single-agent in 64 chemonaive patients with
malignant pleural mesothelioma. The overall response rate was 14.1 %.
NSCLC, second-line treatment:
A multicentre, randomised, open label phase 3 study of ALIMTA versus docetaxel in patients with
locally advanced or metastatic NSCLC after prior chemotherapy has shown median survival times of
8.3 months for patients treated with ALIMTA (Intent To Treat population n = 283) and 7.9 months for
patients treated with docetaxel (ITT n = 288). Prior chemotherapy did not include ALIMTA.
An
analysis of the impact of NSCLC histology on the treatment effect on overall survival was in favour of
ALIMTA versus docetaxel for other than predominantly squamous histologies (n = 399, 9.3 versus
8.0 months, adjusted HR = 0.78; 95% CI = 0 .61-1.00, p = 0.047) and was in favour of docetaxel for
squamous cell carcinoma histology (n = 172, 6.2 versus 7.4 months, adjusted HR = 1.56;
95% CI = 1.08-2.26, p = 0.018). There were no clinically relevant differences observed for the safety
profile of ALIMTA within the histology subgroups.
Limited clinical data from a separate randomized, Phase 3, controlled trial, suggest that efficacy data
(overall survival, progression free survival) for pemetrexed are similar between patients previously pre
treated with docetaxel (n = 41) and patients who did not receive previous docetaxel treatment
(n = 540).
Efficacy of ALIMTA vs docetaxel in NSCLC - ITT population
Survival Time (months
)
Median (m)
95 % CI for median
(n = 283)
8.3
(7.0 - 9.4)
(n = 288)
7.9
(6.3 - 9.2)
HR
95 % CI for HR
Non-inferiority p-value (HR)





















Progression free survival (months)
Median
Time to treatment failure (TTTF – months)
Median
(n = 274)
8.8 (5.7 - 12.8)
46.4
Abbreviations: CI = confidence interval; HR = hazard ratio; ITT = intent to treat; n = total population
size.
(n = 264)
9.1 (5.9 - 13.2)
45.8
NSCLC, first-line treatment:
A multicentre, randomised, open-label, Phase 3 study of ALIMTA plus cisplatin versus gemcitabine
plus cisplatin in chemonaive patients with locally advanced or metastatic (Stage IIIb or IV) non-small
cell lung cancer (NSCLC) showed that ALIMTA plus cisplatin (Intent-To-Treat [ITT] population
n = 862) met its primary endpoint and showed similar clinical efficacy as gemcitabine plus cisplatin
(ITT n = 863) in overall survival (adjusted hazard ratio 0.94; 95% CI = 0.84-1.05). All patients
included in this study had an ECOG performance status 0 or 1.
The primary efficacy analysis was based on the ITT population. Sensitivity analyses of main efficacy
endpoints were also assessed on the Protocol Qualified (PQ) population. The efficacy analyses using
PQ population are consistent with the analyses for the ITT population and support the non-inferiority
of AC versus GC.
Progression free survival (PFS) and overall response rate were similar between treatment arms:
median PFS was 4.8 months for ALIMTA plus cisplatin versus 5.1 months for gemcitabine plus
cisplatin (adjusted hazard ratio 1.04; 95% CI = 0.94-1.15), and overall response rate was 30.6%
(95% CI = 27.3-33.9) for ALIMTA plus cisplatin versus 28.2% (95% CI = 25.0-31.4) for gemcitabine
plus cisplatin. PFS data were partially confirmed by an independent review (400/1725 patients were
randomly selected for review).
The analysis of the impact of NSCLC histology on overall survival demonstrated clinically relevant
differences in survival according to histology, see table below.
Efficacy of ALIMTA + cisplatin vs. gemcitabine + cisplatin in first-line non-small cell lung
cancer – ITT population and histology subgroups.
ITT population
and histology
subgroups
Median overall survival in months
(95% CI)
Adjusted
hazard tatio
(HR)
(95% CI)
ITT population
(N = 1725)
Abbreviations: CI = confidence interval; ITT = intent-to-treat; N = total population size.
a
Statistically significant for noninferiority, with the entire confidence interval for HR well below
the 1.17645 noninferiority margin (p <0.001).
Response
(n: qualified for response)
Response rate (%) (95 % CI)
Stable disease (%)
























Kaplan Meier plots of overall survival by histology
There were no clinically relevant differences observed for the safety profile of ALIMTA plus cisplatin
within the histology subgroups.
Patients treated with ALIMTA and cisplatin required fewer transfusions (16.4% versus 28.9%,
p<0.001), red blood cell transfusions (16.1% versus 27.3%, p<0.001) and platelet transfusions (1.8%
versus 4.5%, p=0.002). Patients also required lower administration of erythropoietin/darbopoietin
(10.4% versus 18.1%, p<0.001), G-CSF/GM-CSF (3.1% versus 6.1%, p=0.004), and iron preparations
(4.3% versus 7.0%, p=0.021)
.
NSCLC, maintenance treatment:
A multicentre, randomised, double-blind, placebo-controlled Phase 3 study (JMEN), compared the
efficacy and safety of maintenance treatment with ALIMTA plus best supportive care (BSC) (n = 441)
with that of placebo plus BSC (n = 222) in patients with locally advanced (Stage IIIB) or metastatic
(Stage IV) Non Small Cell Lung Cancer (NSCLC) who did not progress after 4 cycles of first line
doublet therapy containing Cisplatin or Carboplatin in combination with Gemcitabine, Paclitaxel, or
Docetaxel. First line doublet therapy containing ALIMTA was not included. All patients included in
this study had an ECOG performance status 0 or 1. Patients received maintenance treatment until
disease progression. Efficacy and safety were measured from the time of randomisation after
completion of first line (induction) therapy. Patients received a median of 5 cycles of maintenance
treatment with ALIMTA and 3.5 cycles of placebo. A total of 213 patients (48.3%) completed
≥ 6 cycles and a total of 103 patients (23.4%) completed ≥ 10 cycles of treatment with ALIMTA.
The study met its primary endpoint and showed a statistically significant improvement in PFS in the
ALIMTA arm over the placebo arm (n = 581, independently reviewed population; median of
4.0 months and 2.0 months, respectively) (hazard ratio = 0.60, 95% CI = 0.49-0.73, p < 0.00001). The
independent review of patient scans confirmed the findings of the investigator assessment of PFS. The
median OS for the overall population (n = 663) was 13.4 months for the ALIMTA arm and 10.6
months for the placebo arm, hazard ratio = 0.79 (95% CI = 0.65-0.95, p = 0.01192).
Consistent with other ALIMTA studies, a difference in efficacy according to NSCLC histology was
observed in JMEN. For patients with NSCLC other than predominantly squamous cell histology
(n = 430, independently reviewed population) median PFS was 4.4 months for the ALIMTA arm and
1.8 months for the placebo arm, hazard ratio = 0.47 (95% CI = 0.37-0.60, p = 0.00001). The median
OS for patients with NSCLC other than predominantly squamous cell histology (n = 481) was
15.5 months for the ALIMTA arm and 10.3 months for the placebo arm, hazard ratio = 0.70
(95% CI = 0.56-0.88, p = 0.002). Including the induction phase the median OS for patients with
NSCLC other than predominantly squamous cell histology was 18.6 months for the ALIMTA arm and
13.6 months for the placebo arm, hazard ratio = 0.71 (95% CI = 0.56-0.88, p = 0.002).






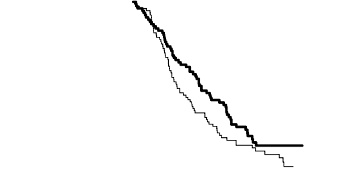








































The PFS and OS results in patients with squamous cell histology suggested no advantage for ALIMTA
over placebo.
There were no clinically relevant differences observed for the safety profile of ALIMTA within the
histology subgroups.
Kaplan meier plots of progression-free survival (PFS) and overall survival ALIMTA versus
placebo in patients with NSCLC other than predominantly squamous cell histology:
Progression-Free Survival
5.2 Pharmacokinetic properties
The pharmacokinetic properties of pemetrexed following single-agent administration have been
evaluated in 426 cancer patients with a variety of solid tumours at doses ranging from 0.2 to
838 mg/m
2
infused over a 10-minute period. Pemetrexed has a steady-state volume of distribution of
9 l/m
2
.
In vitro
studies indicate that pemetrexed is approximately 81 % bound to plasma proteins.
Binding was not notably affected by varying degrees of renal impairment. Pemetrexed undergoes
limited hepatic metabolism. Pemetrexed is primarily eliminated in the urine, with 70 % to 90 % of the
administered dose being recovered unchanged in urine within the first 24 hours following
administration.
In Vitro
studies indicate that pemetrexed is actively secreted by OAT3 (organic anion
transporter. Pemetrexed total systemic clearance is 91.8 ml/min and the elimination half-life from
plasma is 3.5 hours in patients with normal renal function (creatinine clearance of 90 ml/min).
Between patient variability in clearance is moderate at 19.3 %. Pemetrexed total systemic exposure
(AUC) and maximum plasma concentration increase proportionally with dose. The pharmacokinetics
of pemetrexed are consistent over multiple treatment cycles.
The pharmacokinetic properties of pemetrexed are not influenced by concurrently administered
cisplatin. Oral folic acid and intramuscular vitamin B
12
supplementation do not affect the
pharmacokinetics of pemetrexed.
5.3 Preclinical safety data
Administration of pemetrexed to pregnant mice resulted in decreased foetal viability, decreased foetal
weight, incomplete ossification of some skeletal structures and cleft palate.
Administration of pemetrexed to male mice resulted in reproductive toxicity characterised by reduced
fertility rates and testicular atrophy. In a study conducted in beagle dog by intravenous bolus injection
for 9 months, testicular findings (degeneration/necrosis of the seminiferous epithelium) have been

















































observed. This suggests that pemetrexed may impair male fertility. Female fertility was not
investigated.
Pemetrexed was not mutagenic in either the
in vitro
chromosome aberration test in Chinese hamster
ovary cells, or the Ames test. Pemetrexed has been shown to be clastogenic in the
in vivo
micronucleus test in the mouse.
Studies to assess the carcinogenic potential of pemetrexed have not been conducted.
PHARMACEUTICAL PARTICULARS
Mannitol
Hydrochloric acid
Sodium hydroxide
Pemetrexed is physically incompatible with diluents containing calcium, including lactated Ringer’s
injection and Ringer’s injection. In the absence of other compatibility studies this medicinal product
must not be mixed with other medicinal
products.
Reconstituted and infusion solutions
When prepared as directed, reconstituted and infusion solutions of ALIMTA contain no antimicrobial
preservatives. Chemical and physical in-use stability of reconstituted and infusion solutions of
pemetrexed were demonstrated for 24 hours at refrigerated temperature or 25°C. From a
microbiological point of view, the product should be used immediately. If not used immediately, in-
use storage times and conditions prior to use are the responsibility of the user and would normally not
be longer than 24 hours at 2°C to 8°C, unless reconstitution / dilution has taken place in controlled and
validated aseptic conditions.
6.4 Special precautions for storage
Unopened vial
This medicinal product does not require any special storage conditions.
For storage conditions of the reconstituted medicinal product, see section 6.3.
Nature and contents of container
Type I glass vial with rubber stopper containing 100 mg of pemetrexed.
Pack of 1 vial.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
1.
Use aseptic technique during the reconstitution and further dilution of pemetrexed for
intravenous infusion administration.
2.
Calculate the dose and the number of ALIMTA vials needed. Each vial contains an excess of
pemetrexed to facilitate delivery of label amount.
3.
Reconstitute 100-mg vials with 4.2 ml of sodium chloride 9 mg/ml (0.9 %) solution for
injection, without preservative, resulting in a solution containing 25 mg/ml pemetrexed. Gently
swirl each vial until the powder is completely dissolved. The resulting solution is clear and
ranges in colour from colourless to yellow or green-yellow without adversely affecting product
quality. The pH of the reconstituted solution is between 6.6 and 7.8.
Further dilution is
required
.
4.
The appropriate volume of reconstituted pemetrexed solution must be further diluted to 100 ml
with sodium chloride 9 mg/ml (0.9 %) solution for injection, without preservative, and
administered as an intravenous infusion over 10 minutes.
5.
Pemetrexed infusion solutions prepared as directed above are compatible with polyvinyl
chloride and polyolefin lined administration sets and infusion bags.
6.
Parenteral medicinal products must be inspected visually for particulate matter and
discolouration prior to administration. If particulate matter is observed, do not administer.
7.
Pemetrexed solutions are for single use only. Any unused product or waste material must be
disposed of in accordance with local requirements.
Preparation and administration precautions:
As with other potentially toxic anticancer agents,
care should be exercised in the handling and preparation of pemetrexed infusion solutions. The use of
gloves is recommended. If a pemetrexed solution contacts the skin, wash the skin immediately and
thoroughly with soap and water. If pemetrexed solutions contact the mucous membranes, flush
thoroughly with water. Pemetrexed is not a vesicant. There is not a specific antidote for extravasation
of pemetrexed. There have been few reported cases of pemetrexed extravasation, which were not
assessed as serious by the investigator. Extravasation should be managed by local standard practice as
with other non-vesicants.
MARKETING AUTHORISATION HOLDER
Eli Lilly Nederland B.V.
Grootslag 1-5
NL-3991 RA
Houten, The Netherlands
MARKETING AUTHORISATION NUMBER
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 20 September 2004
Date of latest renewal: 20 September 2009
10. DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines
Agency (EMEA)
http://www.ema.europa.eu.
NAME OF THE MEDICINAL PRODUCT
ALIMTA 500 mg powder for concentrate for solution for infusion
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each vial contains 500 mg of pemetrexed (as pemetrexed disodium).
After reconstitution (see section 6.6), each vial contains 25 mg/ml of pemetrexed.
Excipients:
Each vial contains approximately 54 mg sodium.
For a full list of excipients see section 6.1.
Powder for concentrate for solution for infusion.
White to either light yellow or green-yellow lyophilised powder.
4.1 Therapeutic indications
Malignant pleural mesothelioma
ALIMTA in combination with cisplatin is indicated for the treatment of chemotherapy naïve patients
with unresectable malignant pleural mesothelioma.
Non-small cell lung cancer
ALIMTA in combination with cisplatin is indicated for the first line treatment of patients with locally
advanced or metastatic non-small cell lung cancer other than predominantly squamous cell histology
(see section 5.1).
ALIMTA is indicated as monotherapy for the maintenance treatment of locally advanced or metastatic
non-small cell lung cancer other than predominantly squamous cell histology in patients whose disease
has not progressed immediately following platinum-based chemotherapy. First line treatment should
be a platinum doublet with gemcitabine, paclitaxel or docetaxel (see section 5.1).
ALIMTA is indicated as monotherapy for the second line treatment of patients with locally advanced
or metastatic non-small cell lung cancer other than predominantly squamous cell histology (see section
5.1).
4.2 Posology and method of administration
ALIMTA must only be administered under the supervision of a physician qualified in the use of
anti-cancer chemotherapy.
ALIMTA in combination with cisplatin
The recommended dose of ALIMTA is 500 mg/m
2
of body surface area (BSA) administered as an
intravenous infusion over 10 minutes on the first day of each 21-day cycle. The recommended dose of
cisplatin is 75 mg/m
2
BSA infused over two hours approximately 30 minutes after completion of the
pemetrexed infusion on the first day of each 21-day cycle.
Patients must receive adequate anti-emetic
treatment and appropriate hydration prior to and/or after receiving cisplatin
(see also cisplatin
Summary of Product Characteristics for specific dosing advice).
ALIMTA as single agent
In patients treated for non-small cell lung cancer after prior chemotherapy, the recommended dose of
ALIMTA is 500 mg/m
2
BSA administered as an intravenous infusion over 10 minutes on the first day
of each 21-day cycle.
Premedication regimen
To reduce the incidence and severity of skin reactions, a corticosteroid should be given the day prior
to, on the day of, and the day after pemetrexed administration. The corticosteroid should be equivalent
to 4 mg of dexamethasone administered orally twice a day (see section 4.4).
To reduce toxicity, patients treated with pemetrexed must also receive vitamin supplementation (see
section 4.4). Patients must take oral folic acid or a multivitamin containing folic acid (350 to
1000 micrograms) on a daily basis. At least five doses of folic acid must be taken during the seven
days preceding the first dose of pemetrexed, and dosing must continue during the full course of
therapy and for 21 days after the last dose of pemetrexed. Patients must also receive an intramuscular
injection of vitamin B
12
(1000 micrograms) in the week preceding the first dose of pemetrexed and
once every three cycles thereafter. Subsequent vitamin B
12
injections may be given on the same day as
pemetrexed.
Monitoring
Patients receiving pemetrexed should be monitored before each dose with a complete blood count,
including a differential white cell count (WCC) and platelet count. Prior to each chemotherapy
administration blood chemistry tests should be collected to evaluate renal and hepatic function. Before
the start of any cycle of chemotherapy, patients are required to have the following: absolute neutrophil
count (ANC) should be ≥ 1500 cells/mm
3
and platelets should be ≥ 100,000 cells/mm
3
.
Creatinine clearance should be ≥ 45 ml/min.
The total bilirubin should be ≤ 1.5 times upper limit of normal. Alkaline phosphatase (AP), aspartate
transaminase (AST or SGOT) and alanine transaminase (ALT or SGPT) should be ≤ 3 times upper
limit of normal. Alkaline phosphatase, AST and ALT ≤ 5 times upper limit of normal is acceptable if
liver has tumour involvement.
Dose adjustments
Dose adjustments at the start of a subsequent cycle should be based on nadir haematologic counts or
maximum non-haematologic toxicity from the preceding cycle of therapy. Treatment may be delayed
to allow sufficient time for recovery. Upon recovery patients should be retreated using the guidelines
in Tables 1, 2 and 3, which are applicable for ALIMTA used as a single agent or in combination with
cisplatin.
Table 1 - Dose modification table for ALIMTA (as single agent or in combination) and
cisplatin –Haematologic toxicities
Nadir ANC < 500 /mm
3
and nadir platelets
≥ 50,000 /mm
3
75 % of previous dose (both ALIMTA and
cisplatin).
Nadir platelets < 50,000 /mm
3
regardless of
nadir ANC
75 % of previous dose (both ALIMTA and
cisplatin ).
a
These criteria meet the National Cancer Institute Common Toxicity Criteria (CTC v2.0; NCI
1998) definition of ≥CTC Grade 2 bleeding
50 % of previous dose (both ALIMTA and
cisplatin).
Nadir platelets < 50,000 /mm
3
with bleeding
a
,
regardless of nadir ANC.







If patients develop non-haematologic toxicities ≥ Grade 3 (excluding neurotoxicity), ALIMTA should
be withheld until resolution to less than or equal to the patient’s pre-therapy value. Treatment should
be resumed according to the guidelines in Table 2.
Table 2 - Dose modification table for ALIMTA (as single agent or in combination) and
cisplatin– Non-haematologic toxicities
a, b
Dose for cisplatin (mg/m
2
)
Any Grade 3 or 4 toxicities except
mucositis
Any diarrhoea requiring
hospitalisation (irrespective of
grade) or grade 3 or 4 diarrhoea.
a
National Cancer Institute Common Toxicity Criteria (CTC v2.0; NCI 1998)
In the event of neurotoxicity, the recommended dose adjustment for ALIMTA and cisplatin is
documented in Table 3. Patients should discontinue therapy if Grade 3 or 4 neurotoxicity is observed.
Table 3 - Dose modification table for ALIMTA (as single agent or in combination) and
cisplatin – Neurotoxicity
Dose for cisplatin (mg/m
2
)
a
National Cancer Institute Common Toxicity Criteria (CTC v2.0; NCI 1998)
Treatment with ALIMTA should be discontinued if a patient experiences any haematologic or
non-haematologic Grade 3 or 4 toxicity after 2 dose reductions or immediately if Grade 3 or 4
neurotoxicity is observed.
Elderly:
In clinical studies, there has been no indication that patients 65 years of age or older are at
increased risk of adverse events compared to patients younger than 65 years old. No dose reductions
other than those recommended for all patients are necessary.
Paediatric population
There is no relevant use of ALIMTA in the paediatric population in malignant pleural mesothelioma
and non-small cell lung cancer.
Patients with renal impairment
(Standard Cockcroft and Gault formula or Glomerular Filtration Rate
measured Tc99m-DPTA serum clearance method): Pemetrexed is primarily eliminated unchanged by
renal excretion. In clinical studies, patients with creatinine clearance of ≥ 45 ml/min required no dose
adjustments other than those recommended for all patients. There are insufficient data on the use of
pemetrexed in patients with creatinine clearance below 45 ml/min; therefore the use of pemetrexed is
not recommended (see section 4.4).
Patients with hepatic impairment
: No relationships between AST (SGOT), ALT (SGPT), or total
bilirubin and pemetrexed pharmacokinetics were identified. However patients with hepatic impairment
such as bilirubin > 1.5 times the upper limit of normal and/or transaminase > 3.0 times the upper limit
of normal (hepatic metastases absent) or > 5.0 times the upper limit of normal (hepatic metastases
present) have not been specifically studied.
Method of administration:
b
Excluding neurotoxicity

















For Precautions to be taken before handling or administering ALIMTA, see section 6.6.
ALIMTA should be administered as an intravenous infusion over 10 minutes on the first day of each
21-day cycle. For instructions on reconstitution and dilution of ALIMTA before administration, see
section 6.6.
Hypersensitivity to the active substance or to any of the excipients.
Breast-feeding (see section 4.6)
.
Concomitant yellow fever vaccine (see section 4.5).
4.4 Special warnings and precautions for use
Pemetrexed can suppress bone marrow function as manifested by neutropenia, thrombocytopenia and
anaemia (or pancytopenia) (see section 4.8). Myelosuppression is usually the dose-limiting toxicity.
Patients should be monitored for myelosuppression during therapy and pemetrexed should not be
given to patients until absolute neutrophil count (ANC) returns to ≥ 1500 cells/mm
3
and platelet count
returns to ≥ 100,000 cells/mm
3
. Dose reductions for subsequent cycles are based on nadir ANC,
platelet count and maximum non-haematologic toxicity seen from the previous cycle (see section 4.2).
Less toxicity and reduction in Grade 3/4 haematologic and non-haematologic toxicities such as
neutropenia, febrile neutropenia and infection with Grade 3/4 neutropenia were reported when
pre-treatment with folic acid and vitamin B
12
was administered. Therefore, all patients treated with
pemetrexed must be instructed to take folic acid and vitamin B
12
as a prophylactic measure to reduce
treatment-related toxicity (see section 4.2).
Skin reactions have been reported in patients not pre-treated with a corticosteroid. Pre-treatment with
dexamethasone (or equivalent) can reduce the incidence and severity of skin reactions (see
section 4.2).
An insufficient number of patients has been studied with creatinine clearance of below 45 ml/min.
Therefore, the use of pemetrexed in patients with creatinine clearance of < 45 ml/min is not
recommended (see section 4.2).
Patients with mild to moderate renal insufficiency (creatinine clearance from 45 to 79 ml/min) should
avoid taking non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, and aspirin (> 1.3 g
daily) for 2 days before, on the day of, and 2 days following pemetrexed administration (see section
4.5).
In patients with mild to moderate renal insufficiency
eligible for pemetrexed therapy NSAIDs with
long elimination half-lives should be interrupted for at least 5 days prior to, on the day of, and at least
2 days following pemetrexed administration (see section 4.5).
Serious renal events, including acute renal failure, have been reported with pemetrexed alone or in
association with other chemotherapeutic agents. Many of the patients in whom these occurred had
underlying risk factors for the development of renal events including dehydration or pre-existing
hypertension or diabetes.
The effect of third space fluid, such as pleural effusion or ascites, on pemetrexed is not fully defined.
A phase 2 study of pemetrexed in 31 solid tumour patients with stable third space fluid demonstrated
no difference in pemetrexed dose normalized plasma concentrations or clearance compared to patients
without third space fluid collections. Thus, drainage of third space fluid collection prior to pemetrexed
treatment should be considered, but may not be necessary.
Due to the gastrointestinal toxicity of pemetrexed given in combination with cisplatin, severe
dehydration has been observed. Therefore, patients should receive adequate antiemetic treatment and
appropriate hydration prior to and/or after receiving treatment.
Serious cardiovascular events, including myocardial infarction and cerebrovascular events have been
uncommonly reported during clinical studies with pemetrexed, usually when given in combination
with another cytotoxic agent. Most of the patients in whom these events have been observed had pre-
existing cardiovascular risk factors (see section 4.8).
Immunodepressed status is common in cancer patients. As a result, concomitant use of live attenuated
vaccines is not recommended (see section 4.3 and 4.5).
Pemetrexed can have genetically damaging effects. Sexually mature males are advised not to father a
child during the treatment and up to 6 months thereafter. Contraceptive measures or abstinence are
recommended. Owing to the possibility of pemetrexed treatment causing irreversible infertility, men
are advised to seek counselling on sperm storage before starting treatment.
Women of childbearing potential must use effective contraception during treatment with pemetrexed
(see section 4.6).
Cases of radiation pneumonitis have been reported in patients treated with radiation either prior,
during or subsequent to their pemetrexed therapy. Particular attention should be paid to these patients
and caution exercised with use of other radiosensitising agents.
Cases of radiation recall have been reported in patients who received radiotherapy weeks or years
previously.
This medicinal product contains approximately 54 mg of sodium per vial. To be taken into
consideration by patients on a controlled sodium diet.
4.5 Interaction with other medicinal products and other forms of interaction
Pemetrexed is mainly eliminated unchanged renally by tubular secretion and to a lesser extent by
glomerular filtration. Concomitant administration of nephrotoxic drugs (e.g. aminoglycoside, loop
diuretics, platinum compounds, cyclosporin) could potentially result in delayed clearance of
pemetrexed. This combination should be used with caution. If necessary, creatinine clearance should
be closely monitored.
Concomitant administration of substances that are also tubularly secreted (e.g. probenecid, penicillin)
could potentially result in delayed clearance of pemetrexed. Caution should be made when these drugs
are combined with pemetrexed. If necessary, creatinine clearance should be closely monitored.
In patients with normal renal function (creatinine clearance
>
80 ml/min), high doses of non-steroidal
anti-inflammatory drugs (NSAIDs, such as ibuprofen > 1600 mg/day) and aspirin at higher dose
(
>
1.3 g daily) may decrease pemetrexed elimination and, consequently, increase the occurrence of
pemetrexed adverse events. Therefore, caution should be made when administering higher doses of
NSAIDs or aspirin at higher dose, concurrently with pemetrexed to patients with normal function
(creatinine clearance
>
80 ml/min).
In patients with mild to moderate renal insufficiency (creatinine clearance from 45 to 79 ml/min), the
concomitant administration of pemetrexed with NSAIDs (e.g. ibuprofen) or aspirin at higher dose
should be avoided for 2 days before, on the day of, and 2 days following pemetrexed administration
(see section 4.4).
In the absence of data regarding potential interaction with NSAIDs having longer half-lives such as
piroxicam or rofecoxib, the concomitant administration with pemetrexed in patients with mild to
moderate renal insufficiency should be interrupted for at least 5 days prior to, on the day of, and at
least 2 days following pemetrexed administration (see section 4.4).
Pemetrexed undergoes limited hepatic metabolism. Results from
in vitro
studies with human liver
microsomes indicated that pemetrexed would not be predicted to cause clinically significant inhibition
of the metabolic clearance of drugs metabolised by CYP3A, CYP2D6, CYP2C9, and CYP1A2.
Interactions common to all cytotoxics:
Due to the increased thrombotic risk in patients with cancer, the use of anticoagulation treatment is
frequent. The high intra-individual variability of the coagulation status during diseases and the
possibility of interaction between oral anticoagulants and anticancer chemotherapy require increased
frequency of INR (International Normalised Ratio) monitoring, if it is decided to treat the patient with
oral anticoagulants.
Concomitant use contraindicated: Yellow fever vaccine: risk of fatal generalised vaccinale disease (see
section 4.3).
Concomitant use not recommended: Live attenuated vaccines (except yellow fever, for which
concomitant use is contraindicated): risk of systemic, possibly fatal, disease. The risk is increased in
subjects who are already immunosuppressed by their underlying disease. Use an inactivated vaccine
where it exists (poliomyelitis) (see section 4.4).
4.6 Fertility, pregnancy and lactation
Contraception in males and females
Women of childbearing potential must use effective contraception during treatment with pemetrexed.
Pemetrexed can have genetically damaging effects. Sexually mature males are advised not to father a
child during the treatment and up to 6 months thereafter. Contraceptive measures or abstinence are
recommended.
Pregnancy
There are no data from the use of pemetrexed in pregnant women but pemetrexed, like other
anti-metabolites, is suspected to cause serious birth defects when administered during pregnancy.
Animal studies have shown reproductive toxicity (see section 5.3). Pemetrexed should not be used
during pregnancy unless clearly necessary, after a careful consideration of the needs of the mother and
the risk for the foetus (see section 4.4).
Breast-feeding
It is not known whether pemetrexed is excreted in human milk and adverse reactions on the suckling
child cannot be excluded. Breast-feeding must be discontinued during pemetrexed therapy
(see
section 4.3)
.
Fertility
Owing to the possibility of pemetrexed treatment causing irreversible infertility, men are advised to
seek counselling on sperm storage before starting treatment.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. However, it
has been reported that pemetrexed may cause fatigue. Therefore patients should be cautioned against
driving or operating machines if this event occurs.
Summary of the safety profile
The most commonly reported undesirable effects related to pemetrexed, whether used as monotherapy
or in combination, are bone marrow suppression manifested as anaemia, neutropenia, leukopenia,
thrombocytopenia; and gastrointestinal toxicities, manifested as anorexia, nausea, vomiting, diarrhoea,
constipation, pharyngitis, mucositis, and stomatitis. Other undesirable effects include renal toxicities,
increased transaminases, alopecia, fatigue, dehydration, rash, infection/sepsis and neuropathy. Rarely
seen events include Stevens-Johnson syndrome and Toxic epidermal necrolysis.
Tabulated list of adverse reactions
The table below provides the frequency and severity of undesirable effects that have been reported in
> 5 % of 168 patients with mesothelioma who were randomised to receive cisplatin and pemetrexed
and 163 patients with mesothelioma randomised to receive single agent cisplatin. In both treatment
arms, these chemonaive patients were fully supplemented with folic acid and vitamin B
12
.
Adverse reactions
Frequency estimate: Very common (≥1/10), Common (≥1/100 and <1/10), Uncommon (≥1/1000 and
<1/100), Rare (≥1/10,000 and <1/1000), Very rare (<1/10,000) and not known (cannot be estimated
from available data-spontaneous reports)
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Blood and
lymphatic
system
disorders
Neutrophils/
Granulocytes
decreased
Metabolism
and nutrition
disorders
Gastrointestinal
disorders
Skin and
subcutaneous
tissue disorders














































Renal and
urinary
disorders
Creatinine
clearance
decreased**
*Refer to National Cancer Institute CTC version 2 for each grade of toxicity except the term
“creatinine clearance decreased”
** which is derived from the term “renal/genitourinary other”.
*** According to National Cancer Institute CTC (v2.0; NCI 1998), taste disturbance and alopecia
should only be reported as Grade 1 or 2.
For the purpose of this table a cut off of 5 % was used for inclusion of all events where the reporter
considered a possible relationship to pemetrexed and cisplatin.
Clinically relevant CTC toxicities that were reported in ≥ 1 % and
<
5 % of the patients that were
randomly assigned to receive cisplatin and pemetrexed include: renal failure, infection, pyrexia, febrile
neutropenia, increased AST, ALT, and GGT, urticaria and chest pain.
Clinically relevant CTC toxicities that were reported in < 1 % of the patients that were randomly
assigned to receive cisplatin and pemetrexed include arrhythmia and motor neuropathy.
The table below provides the frequency and severity of undesirable effects that have been reported in
> 5 % of 265 patients randomly assigned to receive single agent pemetrexed with folic acid and
vitamin B
12
supplementation and 276 patients randomly assigned to receive single agent docetaxel. All
patients were diagnosed with locally advanced or metastatic non-small cell lung cancer and received
prior chemotherapy.
System organ
class
Blood and
lymphatic
system disorders
Neutrophils/
Granulocytes
decreased
Gastrointestinal
disorders



















































Skin and sub-
cutaneous tissue
disorders
General
disorders and
administration
site conditions
*Refer to National Cancer Institute CTC version 2 for each grade of toxicity.
**According to National Cancer Institute CTC (v2.0; NCI 1998), alopecia should only be
reported as Grade 1 or 2.
For the purpose of this table a cut off of 5 % was used for inclusion of all events where the reporter
considered a possible relationship to pemetrexed.
Clinically relevant CTC toxicities that were reported in ≥ 1 % and
<
5 % of the patients that were
randomly assigned to pemetrexed include: infection without neutropenia, febrile neutropenia, allergic
reaction/hypersensitivity, increased creatinine, motor neuropathy, sensory neuropathy, erythema
multiforme, and abdominal pain.
Clinically relevant CTC toxicities that were reported in < 1 % of the patients that were randomly
assigned to pemetrexed include supraventricular arrhythmias.
Clinically relevant Grade 3 and Grade 4 laboratory toxicities were similar between integrated Phase 2
results from three single agent pemetrexed studies (n = 164) and the Phase 3 single agent pemetrexed
study described above, with the exception of neutropenia (12.8 % versus 5.3 %, respectively) and
alanine transaminase elevation (15.2 % versus 1.9 %, respectively). These differences were likely due
to differences in the patient population, since the Phase 2 studies included both chemonaive and
heavily pre-treated breast cancer patients with pre-existing liver metastases and/or abnormal baseline
liver function tests.
The table below provides the frequency and severity of undesirable effects considered possibly related
to study drug that have been reported in >5% of 839 patients with NSCLC who were randomized to
receive cisplatin and pemetrexed and 830 patients with NSCLC who were randomized to receive
cisplatin and gemcitabine. All patients received study therapy as initial treatment for locally advanced
or metastatic NSCLC and patients in both treatment groups were fully supplemented with folic acid
and vitamin B
12
.


















Pemetrexed/
cisplatin
(N = 839)
Gemcitabine/
cisplatin
(N = 830)
Blood and
lymphatic
system
disorders
Neutrophils/
Granulocytes
decreased
Gastrointestinal
disorders
Diarrhea without
colostomy
Skin and
subcutaneous
tissue disorders
Renal and
urinary
disorders
General
disorders and
administration
site conditions
*P-values <0.05 comparing pemetrexed/cisplatin to gemcitabine/cisplatin, using Fisher Exact test.
**Refer to National Cancer Institute CTC (v2.0; NCI 1998) for each Grade of Toxicity.
***According to National Cancer Institute CTC (v2.0; NCI 1998), taste disturbance and alopecia
should only be reported as Grade 1 or 2.
For the purpose of this table, a cut-off of 5% was used for inclusion of all events where the reporter
considered a possible relationship to pemetrexed and cisplatin.
Clinically relevant toxicity that was reported in ≥ 1% and ≤ 5% of the patients that were randomly
assigned to receive cisplatin and pemetrexed include: AST increase, ALT increase, infection, febrile
neutropenia, renal failure, pyrexia, dehydration, conjunctivitis, and creatinine clearance decrease.
Clinically relevant toxicity that was reported in < 1% of the patients that were randomly assigned to
receive cisplatin and pemetrexed include: GGT increase, chest pain, arrhythmia, and motor
neuropathy.
Clinically relevant toxicities with respect to gender were similar to the overall population in patients
receiving pemetrexed plus cisplatin.





















































The table below provides the frequency and severity of undesirable effects considered possibly related
to study drug that have been reported in > 5% of 441 patients randomly assigned to receive single
agent pemetrexed and 222 patients randomly assigned to receive placebo in the single-agent
maintenance pemetrexed study (Study JMEN). All patients were diagnosed with Stage IIIB or IV
NSCLC and had received prior platinum-based chemotherapy. Patients in both study arms were fully
supplemented with folic acid and vitamin B
12
.
Infections and
infestations
Blood and
lymphatic
system disorders
Gastrointestinal
disorders
Skin and
subcutaneous
tissue disorders
General disorders
and administration
site conditions
Abbreviations: ALT = alanine transaminase; AST = aspartate transaminase; CTCAE = Common Terminology
Criteria for Adverse Event; NCI = National Cancer Institute; SGOT = serum glutamic oxaloacectic
transaminase; SGPT = serum glutamic pyruvic transaminase.
*
Definition of frequency terms: Very common - ≥ 10%; Common - > 5% and < 10%. For the purpose of this
table, a cutoff of 5% was used for inclusion of all events where the reporter considered a possible relationship
to pemetrexed.
**
Refer to NCI CTCAE Criteria (Version 3.0; NCI 2003) for each grade of toxicity
Clinically relevant CTC toxicity of any grade that was reported in ≥ 1% and ≤ 5% of the patients that
were randomly assigned to pemetrexed include: decreased platelets, decreased creatinine clearance,
constipation, edema, alopecia, increased creatinine, pruritis/itching, fever (in the absence of
neutropenia), ocular surface disease (including conjunctivitis), increased lacrimation, and decreased
glomerular filtration rate.
Clinically relevant CTC toxicity that was reported in < 1% of the patients that were randomly assigned
to pemetrexed include: febrile neutropenia, allergic reaction/hypersensitivity, motor neuropathy,
erythema multiforme, renal failure, and supraventricular arrhythmia.

































The incidence of adverse reactions was evaluated for patients who received ≤ 6 cycles of pemetrexed,
and compared to patients who received > 6 cycles of pemetrexed. Increases in adverse reactions (all
grades) were observed with longer exposure; however, no statistically significant differences in
Grade 3/4 adverse reactions were seen.
Serious cardiovascular and cerebrovascular events, including myocardial infarction, angina pectoris,
cerebrovascular accident and transient ischaemic attack have been uncommonly reported during
clinical studies with pemetrexed, usually when given in combination with another cytotoxic agent.
Most of the patients in whom these events have been observed had pre-existing cardiovascular risk
factors.
Rare cases of hepatitis, potentially serious, have been reported during clinical studies with pemetrexed.
Pancytopenia has been uncommonly reported during clinical trials with pemetrexed.
In clinical trials, cases of colitis (including intestinal and rectal bleeding, sometimes fatal, intestinal
perforation, intestinal necrosis and typhlitis) have been reported uncommonly in patients treated with
pemetrexed.
In clinical trials, cases of interstitial pneumonitis with respiratory insufficiency, sometimes fatal, have
been reported uncommonly in patients treated with pemetrexed.
Uncommon cases of oedema have been reported in patients treated with pemetrexed.
Oesophagitis/ radiation oesophagitis has been uncommonly reported during clinical trials with
pemetrexed.
Sepsis, sometimes fatal, has been commonly reported during clinical trials with pemetrexed.
During post marketing surveillance, the following adverse reactions have been reported in patients
treated with pemetrexed:
Uncommon Ccases of acute renal failure have been reported with pemetrexed alone or in association
with other chemotherapeutic agents (see section 4.4).
Uncommon Ccases of radiation pneumonitis have been reported in patients treated with radiation
either prior, during or subsequent to their pemetrexed therapy (see section 4.4).
Rare Ccases of radiation recall have been reported in patients who have received radiotherapy
previously (see section 4.4).
Uncommon Ccases of peripheral ischaemia leading sometimes to extremity necrosis have been
reported.
Rare cases of bullous conditions have been reported including Stevens-Johnson syndrome and Toxic
epidermal necrolysis which in some cases were fatal.
Rarely, haemolytic anaemia has been reported in patients treated with pemetrexed.
Reported symptoms of overdose include neutropenia, anaemia, thrombocytopenia, mucositis, sensory
polyneuropathy and rash. Anticipated complications of overdose include bone marrow suppression as
manifested by neutropenia, thrombocytopenia and anaemia. In addition, infection with or without
fever, diarrhoea, and/or mucositis may be seen. In the event of suspected overdose, patients should be



monitored with blood counts and should receive supportive therapy as necessary. The use of calcium
folinate / folinic acid in the management of pemetrexed overdose should be considered.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Folic acid analogues, ATC code: L01BA04
ALIMTA (pemetrexed) is a multi-targeted anti-cancer antifolate agent that exerts its action by
disrupting crucial folate-dependent metabolic processes essential for cell replication.
In vitro
studies have shown that pemetrexed behaves as a multitargeted antifolate by inhibiting
thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide
formyltransferase (GARFT), which are key folate-dependent enzymes for the
de novo
biosynthesis of
thymidine and purine nucleotides. Pemetrexed is transported into cells by both the reduced folate
carrier and membrane folate binding protein transport systems. Once in the cell, pemetrexed is rapidly
and efficiently converted to polyglutamate forms by the enzyme folylpolyglutamate synthetase. The
polyglutamate forms are retained in cells and are even more potent inhibitors of TS and GARFT.
Polyglutamation is a time- and concentration-dependent process that occurs in tumour cells and, to a
lesser extent, in normal tissues. Polyglutamated metabolites have an increased intracellular half-life
resulting in prolonged drug action in malignant cells.
The European Medicines Agency has waived the obligation to submit the results of studies with
ALIMTA in all subsets of the paediatric population in the granted indications
(see Section 4.2).
EMPHACIS, a multicentre, randomised, single-blind phase 3 study of ALIMTA plus cisplatin versus
cisplatin in chemonaive patients with malignant pleural mesothelioma, has shown that patients treated
with ALIMTA and cisplatin had a clinically meaningful 2.8-month median survival advantage over
patients receiving cisplatin alone.
During the study, low-dose folic acid and vitamin B
12
supplementation was introduced to patients’
therapy to reduce toxicity. The primary analysis of this study was performed on the population of all
patients randomly assigned to a treatment arm who received study drug (randomised and treated). A
subgroup analysis was performed on patients who received folic acid and vitamin B
12
supplementation
during the entire course of study therapy (fully supplemented). The results of these analyses of
efficacy are summarised in the table below:
Efficacy of ALIMTA plus cisplatin vs. cisplatin
in Malignant pleural mesothelioma
Median overall survival (months)
Median time to tumour progression
(months)










Time to treatment failure (months)
(34.8 - 48.1) (12.0 – 22.2) (37.8 - 53.4) (13.8 - 26.6)
Abbreviation: CI = confidence interval
* p-value refers to comparison between arms.
** In the ALIMTA/cisplatin arm, randomized and treated (N = 225) and fully supplemented
(N = 167)
A statistically significant improvement of the clinically relevant symptoms (pain and dyspnoea)
associated with malignant pleural mesothelioma in the ALIMTA/cisplatin arm (212 patients) versus
the cisplatin arm alone (218 patients) was demonstrated using the Lung Cancer Symptom Scale.
Statistically significant differences in pulmonary function tests were also observed. The separation
between the treatment arms was achieved by improvement in lung function in the ALIMTA/cisplatin
arm and deterioration of lung function over time in the control arm.
There are limited data in patients with malignant pleural mesothelioma treated with ALIMTA alone.
ALIMTA at a dose of 500 mg/m
2
was studied as a single-agent in 64 chemonaive patients with
malignant pleural mesothelioma. The overall response rate was 14.1 %.
NSCLC, second-line treatment:
A multicentre, randomised, open label phase 3 study of ALIMTA versus docetaxel in patients with
locally advanced or metastatic NSCLC after prior chemotherapy has shown median survival times of
8.3 months for patients treated with ALIMTA (Intent To Treat population n = 283) and 7.9 months for
patients treated with docetaxel (ITT n = 288). Prior chemotherapy did not include ALIMTA.
An
analysis of the impact of NSCLC histology on the treatment effect on overall survival was in favour of
ALIMTA versus docetaxel for other than predominantly squamous histologies (n = 399, 9.3 versus
8.0 months, adjusted HR = 0.78; 95% CI = 0 .61-1.00, p = 0.047) and was in favour of docetaxel for
squamous cell carcinoma histology (n = 172, 6.2 versus 7.4 months, adjusted HR = 1.56;
95% CI = 1.08-2.26, p = 0.018). There were no clinically relevant differences observed for the safety
profile of ALIMTA within the histology subgroups.
Limited clinical data from a separate randomized, Phase 3, controlled trial, suggest that efficacy data
(overall survival, progression free survival) for pemetrexed are similar between patients previously pre
treated with docetaxel (n = 41) and patients who did not receive previous docetaxel treatment
(n = 540).
Efficacy of ALIMTA vs docetaxel in NSCLC - ITT population
Survival Time (months
)
Median (m)
95 % CI for median
(n = 283)
8.3
(7.0 - 9.4)
(n = 288)
7.9
(6.3 - 9.2)
HR
95 % CI for HR
Non-inferiority p-value (HR)
Progression free survival (months)
Median
Time to treatment failure (TTTF – months)
Median



















(n = 274)
8.8 (5.7 - 12.8)
46.4
Abbreviations: CI = confidence interval; HR = hazard ratio; ITT = intent to treat; n = total
population size.
(n = 264)
9.1 (5.9 - 13.2)
45.8
NSCLC, first-line treatment:
A multicentre, randomised, open-label, Phase 3 study of ALIMTA plus cisplatin versus gemcitabine
plus cisplatin in chemonaive patients with locally advanced or metastatic (Stage IIIb or IV) non-small
cell lung cancer (NSCLC) showed that ALIMTA plus cisplatin (Intent-To-Treat [ITT] population
n = 862) met its primary endpoint and showed similar clinical efficacy as gemcitabine plus cisplatin
(ITT n = 863) in overall survival (adjusted hazard ratio 0.94; 95% CI = 0.84-1.05). All patients
included in this study had an ECOG performance status 0 or 1.
The primary efficacy analysis was based on the ITT population. Sensitivity analyses of main efficacy
endpoints were also assessed on the Protocol Qualified (PQ) population. The efficacy analyses using
PQ population are consistent with the analyses for the ITT population and support the non-inferiority
of AC versus GC.
Progression free survival (PFS) and overall response rate were similar between treatment arms:
median PFS was 4.8 months for ALIMTA plus cisplatin versus 5.1 months for gemcitabine plus
cisplatin (adjusted hazard ratio 1.04; 95% CI = 0.94-1.15), and overall response rate was 30.6%
(95% CI = 27.3-33.9) for ALIMTA plus cisplatin versus 28.2% (95% CI = 25.0-31.4) for gemcitabine
plus cisplatin. PFS data were partially confirmed by an independent review (400/1725 patients were
randomly selected for review).
The analysis of the impact of NSCLC histology on overall survival demonstrated clinically relevant
differences in survival according to histology, see table below.
Efficacy of ALIMTA + cisplatin vs. femcitabine + cisplatin in first-line non-small cell lung
cancer – ITT population and histology subgroups.
ITT population
and histology
subgroups
Median overall survival in months
(95% CI)
Adjusted
hazard
ratio (HR)
(95% CI)
ITT population
(N = 1725)
Abbreviations: CI = confidence interval; ITT = intent-to-treat; N = total population size.
a
Statistically significant for noninferiority, with the entire confidence interval for HR well below
the 1.17645 noninferiority margin (p <0.001).
Response
(n: qualified for response)
Response rate (%) (95 % CI)
Stable disease (%)






















Kaplan Meier plots of overall survival by histology
There were no clinically relevant differences observed for the safety profile of ALIMTA plus cisplatin
within the histology subgroups.
Patients treated with ALIMTA and cisplatin required fewer transfusions (16.4% versus 28.9%,
p<0.001), red blood cell transfusions (16.1% versus 27.3%, p<0.001) and platelet transfusions (1.8%
versus 4.5%, p=0.002). Patients also required lower administration of erythropoietin/darbopoietin
(10.4% versus 18.1%, p<0.001), G-CSF/GM-CSF (3.1% versus 6.1%, p=0.004), and iron preparations
(4.3% versus 7.0%, p=0.021)
.
NSCLC, maintenance treatment
:
A multicentre, randomised, double-blind, placebo-controlled Phase 3 study (JMEN), compared the
efficacy and safety of maintenance treatment with ALIMTA plus best supportive care (BSC) (n = 441)
with that of placebo plus BSC (n = 222) in patients with locally advanced (Stage IIIB) or metastatic
(Stage IV) Non Small Cell Lung Cancer (NSCLC) who did not progress after 4 cycles of first line
doublet therapy containing Cisplatin or Carboplatin in combination with Gemcitabine, Paclitaxel, or
Docetaxel. First line doublet therapy containing ALIMTA was not included. All patients included in
this study had an ECOG performance status 0 or 1. Patients received maintenance treatment until
disease progression. Efficacy and safety were measured from the time of randomisation after
completion of first line (induction) therapy. Patients received a median of 5 cycles of maintenance
treatment with ALIMTA and 3.5 cycles of placebo. A total of 213 patients (48.3%) completed
≥ 6 cycles and a total of 103 patients (23.4%) completed ≥ 10 cycles of treatment with ALIMTA.
The study met its primary endpoint and showed a statistically significant improvement in PFS in the
ALIMTA arm over the placebo arm (n = 581, independently reviewed population; median of
4.0 months and 2.0 months, respectively) (hazard ratio = 0.60, 95% CI = 0.49-0.73, p < 0.00001). The
independent review of patient scans confirmed the findings of the investigator assessment of PFS. The
median OS for the overall population (n = 663) was 13.4 months for the ALIMTA arm and 10.6
months for the placebo arm, hazard ratio = 0.79 (95% CI = 0.65-0.95, p = 0.01192).
Consistent with other ALIMTA studies, a difference in efficacy according to NSCLC histology was
observed in JMEN. For patients with NSCLC other than predominantly squamous cell histology
(n = 430, independently reviewed population) median PFS was 4.4 months for the ALIMTA arm and
1.8 months for the placebo arm, hazard ratio = 0.47 (95% CI = 0.37-0.60, p = 0.00001). The median
OS for patients with NSCLC other than predominantly squamous cell histology (n = 481) was
15.5 months for the ALIMTA arm and 10.3 months for the placebo arm, hazard ratio = 0.70
(95% CI = 0.56-0.88, p = 0.002). Including the induction phase the median OS for patients with
NSCLC other than predominantly squamous cell histology was 18.6 months for the ALIMTA arm and
13.6 months for the placebo arm, hazard ratio = 0.71 (95% CI = 0.56-0.88, p = 0.002).








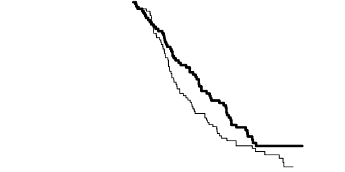








































The PFS and OS results in patients with squamous cell histology suggested no advantage for ALIMTA
over placebo.
There were no clinically relevant differences observed for the safety profile of ALIMTA within the
histology subgroups.
Kaplan Meier plots of progression-free survival (PFS) and overall survival ALIMTA versus
placebo in patients with NSCLC other than predominantly squamous cell histology:
Progression-Free Survival
5.2 Pharmacokinetic properties
The pharmacokinetic properties of pemetrexed following single-agent administration have been
evaluated in 426 cancer patients with a variety of solid tumours at doses ranging from 0.2 to
838 mg/m
2
infused over a 10-minute period. Pemetrexed has a steady-state volume of distribution of
9 l/m
2
.
In vitro
studies indicate that pemetrexed is approximately 81 % bound to plasma proteins.
Binding was not notably affected by varying degrees of renal impairment. Pemetrexed undergoes
limited hepatic metabolism. Pemetrexed is primarily eliminated in the urine, with 70 % to 90 % of the
administered dose being recovered unchanged in urine within the first 24 hours following
administration.
In Vitro
studies indicate that pemetrexed is actively secreted by OAT3 (organic anion
transporter. Pemetrexed total systemic clearance is 91.8 ml/min and the elimination half-life from
plasma is 3.5 hours in patients with normal renal function (creatinine clearance of 90 ml/min).
Between patient variability in clearance is moderate at 19.3 %. Pemetrexed total systemic exposure
(AUC) and maximum plasma concentration increase proportionally with dose. The pharmacokinetics
of pemetrexed are consistent over multiple treatment cycles.
The pharmacokinetic properties of pemetrexed are not influenced by concurrently administered
cisplatin. Oral folic acid and intramuscular vitamin B
12
supplementation do not affect the
pharmacokinetics of pemetrexed.
5.3 Preclinical safety data
Administration of pemetrexed to pregnant mice resulted in decreased foetal viability, decreased foetal
weight, incomplete ossification of some skeletal structures and cleft palate.
Administration of pemetrexed to male mice resulted in reproductive toxicity characterised by reduced
fertility rates and testicular atrophy. In a study conducted in beagle dog by intravenous bolus injection
for 9 months, testicular findings (degeneration/necrosis of the seminiferous epithelium) have been

















































observed. This suggests that pemetrexed may impair male fertility. Female fertility was not
investigated.
Pemetrexed was not mutagenic in either the
in vitro
chromosome aberration test in Chinese hamster
ovary cells, or the Ames test. Pemetrexed has been shown to be clastogenic in the
in vivo
micronucleus test in the mouse.
Studies to assess the carcinogenic potential of pemetrexed have not been conducted.
PHARMACEUTICAL PARTICULARS
Mannitol
Hydrochloric acid
Sodium hydroxide
Pemetrexed is physically incompatible with diluents containing calcium, including lactated Ringer’s
injection and Ringer’s injection. In the absence of other compatibility studies this medicinal product
must not be mixed with other medicinal
products.
Reconstituted and infusion solutions
When prepared as directed, reconstituted and infusion solutions of ALIMTA contain no antimicrobial
preservatives. Chemical and physical in-use stability of reconstituted and infusion solutions of
pemetrexed were demonstrated for 24 hours at refrigerated temperature or 25°C. From a
microbiological point of view, the product should be used immediately. If not used immediately, in-
use storage times and conditions prior to use are the responsibility of the user and would normally not
be longer than 24 hours at 2°C to 8°C, unless reconstitution / dilution has taken place in controlled and
validated aseptic conditions.
6.4 Special precautions for storage
Unopened vial
This medicinal product does not require any special storage conditions.
For storage conditions of the reconstituted medicinal product see section 6.3.
6.5 Nature and contents of container
Type I glass vial with rubber stopper containing 500 mg of pemetrexed.
Pack of 1 vial.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
1.
Use aseptic technique during the reconstitution and further dilution of pemetrexed for
intravenous infusion administration.
2.
Calculate the dose and the number of ALIMTA vials needed. Each vial contains an excess of
pemetrexed to facilitate delivery of label amount.
3.
Reconstitute 500-mg vials with 20 ml of sodium chloride 9 mg/ml (0.9 %) solution for injection,
without preservative, resulting in a solution containing 25 mg/ml pemetrexed. Gently swirl each
vial until the powder is completely dissolved. The resulting solution is clear and ranges in colour
from colourless to yellow or green-yellow without adversely affecting product quality. The pH
of the reconstituted solution is between 6.6 and 7.8.
Further dilution is required
.
4.
The appropriate volume of reconstituted pemetrexed solution must be further diluted to 100 ml
with sodium chloride 9 mg/ml (0.9 %) solution for injection, without preservative, and
administered as an intravenous infusion over 10 minutes.
5.
Pemetrexed infusion solutions prepared as directed above are compatible with polyvinyl
chloride and polyolefin lined administration sets and infusion bags.
6.
Parenteral medicinal products must be inspected visually for particulate matter and
discolouration prior to administration. If particulate matter is observed, do not administer.
7.
Pemetrexed solutions are for single use only. Any unused product or waste material must be
disposed of in accordance with local requirements.
Preparation and administration precautions:
As with other potentially toxic anticancer agents,
care should be exercised in the handling and preparation of pemetrexed infusion solutions. The use of
gloves is recommended. If a pemetrexed solution contacts the skin, wash the skin immediately and
thoroughly with soap and water. If pemetrexed solutions contact the mucous membranes, flush
thoroughly with water. Pemetrexed is not a vesicant. There is not a specific antidote for extravasation
of pemetrexed. There have been few reported cases of pemetrexed extravasation, which were not
assessed as serious by the investigator. Extravasation should be managed by local standard practice as
with other non-vesicants.
MARKETING AUTHORISATION HOLDER
Eli Lilly Nederland B.V.
Grootslag 1-5
NL-3991 RA, Houten
The Netherlands
MARKETING AUTHORISATION NUMBER
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 20 September 2004
Date of latest renewal: 20 September 2009
10. DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines
Agency (EMEA)
http://www.ema.europa.eu.
A.
MANUFACTURING AUTHORISATION HOLDER
RESPONSIBLE FOR BATCH RELEASE
B.
CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer responsible for batch release
Lilly France S.A.S.
2 rue du Colonel Lilly
67640 Fegersheim
France
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to restricted medical prescription (see Annex I: Summary of Product
Characteristics, 4.2)
CONDITIONS OR RESTRICTIONS WITH REGARDS TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
Pharmacovigilance system
The MAH must ensure that the system of pharmacovigilance, presented in Module 1.8.1. of the
Marketing Authorisation, is in place and functioning before and whilst the product is on the market.
Risk Management Plan
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version 3of the Risk Management Plan (RMP) presented in
Module 1.8.2. of the Marketing Authorisation and any subsequent updates of the RMP agreed by the
CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, the
updated RMP should be submitted at the same time as the next Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted:
•
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimization Activities
•
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
•
At the request of the European Medicine Agency EA.
The MAH will continue to submit yearly PSURs unless otherwise specified by the CHMP.
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
ALIMTA 100 mg powder for concentrate for solution for infusion
pemetrexed
STATEMENT OF ACTIVE SUBSTANCE(S)
Each vial contains 100 mg of pemetrexed (as pemetrexed disodium).
After reconstitution (see package leaflet), each vial contains 25 mg/ml of pemetrexed.
Mannitol, hydrochloric acid, sodium hydroxide (see package leaflet for further information).
PHARMACEUTICAL FORM AND CONTENTS
Powder for concentrate for solution for infusion.
1 vial
METHOD AND ROUTE(S) OF ADMINISTRATION
For single use only.
For intravenous use after reconstitution and dilution.
Read the package leaflet before use.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE
STORED OUT OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
EXP
Read the leaflet for the shelf life of the reconstituted product.
SPECIAL STORAGE CONDITIONS



























SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL
PRODUCTS OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL
Discard unused contents appropriately.
NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Eli Lilly Nederland B.V.
Grootslag 1-5
NL-3991 RA
Houten
The Netherlands
MARKETING AUTHORISATION NUMBER(S)
CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.

















MINIMUM PARTICULARS TO APPEAR ON SMALL IMMEDIATE PACKAGING
UNITS
The active substance is pemetrexed.
ALIMTA 100 mg: Each vial contains 100 milligrams of pemetrexed (as pemetrexed disodium).
ALIMTA 500 mg: Each vial contains 500 milligrams of pemetrexed (as pemetrexed disodium).
After reconstitution, the solution contains 25 mg/ml of pemetrexed. Further dilution by a healthcare
provider is required prior to administration.
The other ingredients are mannitol, hydrochloric acid and sodium hydroxide.
What ALIMTA looks like and contents of the pack
ALIMTA is a powder for concentrate for solution for infusion in a vial. It is a white to either light
yellow or green-yellow lyophilised powder.
Each pack of ALIMTA consists of one ALIMTA vial.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Eli Lilly Nederland B.V.
Grootslag 1-5
NL-3991 RA
Houten
The Netherlands
Manufacturer
Lilly France S.A.S.
rue du Colonel Lilly
F-67640 Fegersheim
France
For any information about this medicine, please contact the local representative of the
Marketing Authorisation Holder.
Belgique/België/Belgien
Eli Lilly Benelux S.A
Tél/Tel: + 32-(0)2 548 84 84
Luxembourg/Luxemburg
Eli Lilly Benelux S.A
Tél/Tel: + 32-(0)2 548 84 84
България
ТП "Ели Лили Недерланд" Б.В. - България
тел. + 359 2 491 41 40
Magyarország
Lilly Hungária Kft.
Tel: + 36 1 328 5100
Česká republika
ELI LILLY ČR, s.r.o.
Tel: + 420 234 664 111
Malta
Charles de Giorgio Ltd.
Tel: + 356 25600 500
Danmark
Eli Lilly Danmark A/S
Tlf: +45 45 26 60 00
Nederland
Eli Lilly Nederland B.V.
Tel: + 31-(0) 30 60 25 800
Deutschland
Lilly Deutschland GmbH
Tel. + 49-(0) 6172 273 2222
Norge
Eli Lilly Norge A.S.
Tlf: + 47 22 88 18 00
Eesti
Eli Lilly Holdings Limited Eesti filiaal
Tel:
+
372 6 817 280
Österreich
Eli Lilly Ges.m.b.H.
Tel: + 43-(0) 1 711 780
Ελλάδα
ΦΑΡΜΑΣΕΡΒ-ΛΙΛΛΥ Α.Ε.Β.Ε.
Τηλ: +30 210 629 4600
Polska
Eli Lilly Polska Sp. z o.o.
Tel.: +48 (0) 22 440 33 00
España
Lilly S.A.
Tel: + 34-91-663 50 00
Portugal
Lilly Portugal - Produtos Farmacêuticos, Lda
Tel: + 351-21-4126600
France
Lilly France S.A.S
Tél: +33-(0) 1 55 49 34 34
România
Eli Lilly România S.R.L.
Tel: + 40 21 4023000
Ireland
Eli Lilly and Company (Ireland) Limited
Tel: + 353-(0) 1 661 4377
Slovenija
Eli Lilly farmacevtska družba, d.o.o.
Tel: +386 (0)1 580 00 10
Ísland
Icepharma hf.
Sími + 354 540 8000
Slovenská republika
Eli Lilly Slovakia, s.r.o.
Tel: + 421 220 663 111
Italia
Eli Lilly Italia S.p.A.
Tel: + 39- 055 42571
Suomi/Finland
Oy Eli Lilly Finland Ab
Puh/Tel: + 358-(0) 9 85 45 250
Κύπρος
Phadisco Ltd
Τηλ: +357 22 715000
Sverige
Eli Lilly Sweden AB
Tel: + 46-(0) 8 7378800
Latvija
Eli Lilly Holdings Limited pārstāvniecība Latvijā
Tel:
+
371 6 7364000
United Kingdom
Eli Lilly and Company Limited
Tel: + 44-(0) 1256 315000
Lietuva
Eli Lilly Holdings Limited atstovybė
Tel. +370 (5) 2649600
This leaflet was last approved in
<---------------------------------------------------------------------------------------------------------------
The following information is intended for medical or healthcare professionals only:
Instructions for use, handling and disposal.
1.
Use aseptic techniques during the reconstitution and further dilution of pemetrexed for
intravenous infusion administration.
2.
Calculate the dose and the number of ALIMTA vials needed. Each vial contains an excess of
pemetrexed to facilitate delivery of the label amount.
3.
ALIMTA 100 mg:
Reconstitute each 100 mg vial with 4.2 ml of 9 mg/ml (0.9%) sodium chloride solution for
injection, without preservative, resulting in a solution containing 25 mg/ml pemetrexed.
ALIMTA 500 mg:
Reconstitute each 500 mg vial with 20 ml of 9 mg/ml (0.9%) sodium chloride solution for
injection, without preservative, resulting in a solution containing 25 mg/ml pemetrexed.
Gently swirl each vial until the powder is completely dissolved. The resulting solution is clear
and ranges in colour from colourless to yellow or green-yellow without adversely affecting
product quality. The pH of the reconstituted solution is between 6.6 and 7.8.
Further dilution is
required
.
4.
The appropriate volume of reconstituted pemetrexed solution must be further diluted to 100 ml
with 9 mg/ml (0.9 %) sodium chloride solution for injection, without preservative, and
administered as an intravenous infusion over 10 minutes.
5.
Pemetrexed infusion solutions prepared as directed above are compatible with polyvinyl
chloride and polyolefin lined administration sets and infusion bags. Pemetrexed is incompatible
with diluents containing calcium, including lactated Ringer’s Injection and Ringer’s Injection.
6.
Parenteral medicinal products should be inspected visually for particulate matter and
discolouration prior to administration. If particulate matter is observed, do not administer.
7.
Pemetrexed solutions are for single use only. Any unused product or waste material should be
disposed of in accordance with local requirements.
Preparation and administration precautions:
As with other potentially toxic anticancer agents,
care should be exercised in the handling and preparation of pemetrexed infusion solutions. The use of
gloves is recommended. If a pemetrexed solution contacts the skin, wash the skin immediately and
thoroughly with soap and water. If pemetrexed solutions contact the mucous membranes, flush
thoroughly with water. Pemetrexed is not a vesicant. There is not a specific antidote for extravasation
of pemetrexed. There have been a few reported cases of pemetrexed extravasation, which were not
assessed as serious by the investigator. Extravasation should be managed by local standard practice as
with other non-vesicants.
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/alimta.html
Copyright © 1995-2021 ITA all rights reserved.
|

































































































































































































































































































































































































































































































































































































































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































