Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
AVONEX 30 micrograms powder and solvent for solution for injection
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each BIO-SET vial contains 30 micrograms (6 million IU) of interferon beta-1a.
Following reconstitution with the solvent (water for injections) the vial contains 1.0 ml of solution.
The concentration is 30 micrograms per ml.
Using the World Health Organisation (WHO) International Standard for Interferon, 30 micrograms of
AVONEX contains 6 million IU of antiviral activity. The activity against other standards is not
known.
For a full list of excipients, see section 6.1.
Powder and solvent for solution for injection.
The vial contains a white to off-white cake.
4.1 Therapeutic indications
AVONEX is indicated for the treatment of
Patients diagnosed with relapsing multiple sclerosis (MS). In clinical trials, this was
characterised by two or more acute exacerbations (relapses) in the previous three years without
evidence of continuous progression between relapses; AVONEX slows the progression of
disability and decreases the frequency of relapses.
Patients with a single demyelinating event with an active inflammatory process, if it is severe
enough to warrant treatment with intravenous corticosteroids, if alternative diagnoses have been
excluded, and if they are determined to be at high risk of developing clinically definite multiple
sclerosis (see section 5.1).
AVONEX should be discontinued in patients who develop progressive MS.
4.2
Posology and method of administration
Treatment should be initiated under supervision of a physician experienced in the treatment of the
disease.
Adults:
The recommended dosage for the treatment of relapsing MS is 30 micrograms (1 ml solution),
administered by intramuscular (IM) injection once a week (see
section 6.6). No additional benefit has
been shown by administering a higher dose (60 micrograms) once a week.
Paediatric population:
The safety and efficacy of AVONEX in adolescents aged 12 to 16 years have
not yet been established. Currently available data are described in section 4.8 and 5.1 but no
recommendation on a posology can be made.
The safety and efficacy of AVONEX in children below 12 years of age have not yet been established.
No data are available
Elderly:
Clinical studies did not include a sufficient number of patients aged 65 and over to determine
whether they respond differently than younger patients. However, based on the mode of clearance of
the active substance there are no theoretical reasons for any requirement for dose adjustments in the
elderly.
The intramuscular injection site should be varied each week (see section 5.3).
Doctors may prescribe a 25 mm, 25 gauge needle to patients for whom such a needle is appropriate to
administer an intramuscular injection.
Prior to injection and for an additional 24 hours after each injection, an antipyretic analgesic is advised
to decrease flu-like symptoms associated with AVONEX administration. These symptoms are usually
present during the first few months of treatment.
At the present time, it is not known for how long patients should be treated. Patients should be
clinically evaluated after two years of treatment and longer-term treatment should be decided on an
individual basis by the treating physician. Treatment should be discontinued if the patient develops
chronic progressive MS.
Initiation of treatment in pregnancy (see section 4.6).
Patients with a history of hypersensitivity to natural or recombinant interferon -ß, human
albumin or to any excipients.
Patients with current severe depression and/or suicidal ideation (see sections 4.4 and 4.8).
4.4 Special warnings and precautions for use
AVONEX should be administered with caution to patients with previous or current depressive
disorders, in particular to those with antecedents of suicidal ideation (see section 4.3). Depression and
suicidal ideation are known to occur in increased frequency in the multiple sclerosis population and in
association with interferon use. Patients should be advised to immediately report any symptoms of
depression and/or suicidal ideation to their prescribing physician.
Patients exhibiting depression should be monitored closely during therapy and treated appropriately.
Cessation of therapy with AVONEX should be considered (see also sections 4.3 and 4.8).
AVONEX should be administered with caution to patients with a history of seizures, to those
receiving treatment with anti-epileptics, particularly if their epilepsy is not adequately controlled with
anti-epileptics (see sections 4.5 and 4.8).
Caution should be used and close monitoring considered when administering AVONEX to patients
with severe renal and hepatic failure and to patients with severe myelosuppression.
Hepatic injury including elevated serum hepatic enzyme levels, hepatitis, autoimmune hepatitis and
hepatic failure has been reported with interferon beta in post-marketing (see section 4.8). In some
cases, these reactions have occurred in the presence of other medicinal products that have been
associated with hepatic injury. The potential of additive effects from multiple medicinal products or
other hepatotoxic agents (e.g. alcohol) has not been determined. Patients should be monitored for signs
of hepatic injury and caution exercised when interferons are used concomitantly with other medicinal
products associated with hepatic injury.
Patients with cardiac disease, such as angina, congestive heart failure or arrhythmia, should be closely
monitored for worsening of their clinical condition during treatment with AVONEX. Flu-like
symptoms associated with AVONEX therapy may prove stressful to patients with underlying cardiac
conditions.
Laboratory abnormalities are associated with the use of interferons. Therefore, in addition to those
laboratory tests normally required for monitoring patients with MS, complete and differential white
blood cell counts, platelet counts, and blood chemistry, including liver function tests, are
recommended during AVONEX therapy. Patients with myelosuppression may require more intensive
monitoring of complete blood cell counts, with differential and platelet counts.
Patients may develop antibodies to AVONEX. The antibodies of some of those patients reduce the
activity of interferon beta-1a
in vitro
(neutralising antibodies). Neutralising antibodies are associated
with a reduction in the
in vivo
biological effects of AVONEX and may potentially be associated with a
reduction of clinical efficacy. It is estimated that the plateau for the incidence of neutralising antibody
formation is reached after 12 months of treatment. Data from patients treated up to two years with
AVONEX suggests that approximately 8% develop neutralising antibodies.
The use of various assays to detect serum antibodies to interferons limits the ability to compare
antigenicity among different products.
4.5 Interaction with other medicinal products and other forms of interaction
No formal interaction studies have been performed in humans.
The interaction of AVONEX with corticosteroids or adrenocorticotropic hormone (ACTH) has not
been studied systematically. The clinical studies indicate that MS patients can receive AVONEX and
corticosteroids or ACTH during relapses.
Interferons have been reported to reduce the activity of hepatic cytochrome P450-dependent enzymes
in humans and animals. The effect of high-dose AVONEX administration on P450-dependent
metabolism in monkeys was evaluated and no changes in liver metabolising capabilities were
observed. Caution should be exercised when AVONEX is administered in combination with medicinal
products that have a narrow therapeutic index and are largely dependent on the hepatic cytochrome
P450 system for clearance, e.g. antiepileptics and some classes of antidepressants.
4.6 Pregnancy and lactation
There is limited information on the use of AVONEX in pregnancy. Available data indicates that there
may be an increased risk of spontaneous abortion. Initiation of treatment is contraindicated during
pregnancy (see section 4.3).
Women of child-bearing potential
Women of child-bearing potential have to take appropriate contraceptive measures. If the patient
becomes pregnant or plans to become pregnant while taking AVONEX she should be informed of the
potential hazards and discontinuation of therapy should be considered (see section 5.3). In patients
with a high relapse rate before treatment started, the risk of a severe relapse following discontinuation
of AVONEX in the event of pregnancy should be weighed against a possible increased risk of
spontaneous abortion.
It is not known whether AVONEX is excreted in human milk. Because of the potential for serious
adverse reactions in nursing infants, a decision should be made either to discontinue breast-feeding or
AVONEX therapy.
4.7 Effects on ability to drive and use machines
No studies on the effects of AVONEX on the ability to drive and use machines have been performed.
Central nervous system-related adverse reactions may have a minor influence on the ability to drive
and use machines in susceptible patients (see section 4.8).
The highest incidence of adverse reactions associated with AVONEX therapy is related to flu-like
symptoms. The most commonly reported flu-like symptoms are myalgia, fever, chills, sweating,
asthenia, headache and nausea. Flu-like symptoms tend to be most prominent at the initiation of
therapy and decrease in frequency with continued treatment.
Transient neurological symptoms that may mimic MS exacerbations may occur following injections.
Transient episodes of hypertonia and/or severe muscular weakness that prevent voluntary movements
may occur at any time during treatment. These episodes are of limited duration, temporally related to
the injections and may recur after subsequent injections. In some cases these symptoms are associated
with flu-like symptoms.
The frequencies of adverse reactions are expressed in patient-years, according to the following
categories:
Very common (≥1/10 patient-years);
Common (≥1/100 to <1/10 patient-years);
Uncommon (≥1/1, 000 to <1/100 patient-years);
Rare (≥1/10, 000 to <1/1,000 patient-years);
Very rare (<1/10,000 patient-years);
Not known (cannot be estimated from the available data).
Patient-time is the sum of individual units of time that the patient in the study has been exposed to
AVONEX before experiencing the adverse reaction. For example, 100 person-years could be observed
in 100 patients who were on treatment for one year or in 200 patients who were on treatment for half a
year.
Adverse reactions identified from studies (clinical trials and observational studies, with a period of
follow-up ranging from two years to six years) and other adverse reactions identified through
spontaneous reporting from the market, with unknown frequency, are provided in the table below.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
lymphocyte count decreased, white blood cell
count decreased, neutrophil count decreased,
hematocrit decreased, blood potassium
increased, blood urea nitrogen increased
weight decreased, weight increased,
liver function tests abnormal
cardiomyopathy, congestive heart failure (see
section 4.4), palpitations, arrhythmia,
tachycardia
Blood and lymphatic system disorders
pancytopenia, thrombocytopenia
muscle spasticity, hypoesthesia
neurological symptoms, syncope
3
,
hypertonia, dizziness, paraesthesia,
seizures, migraine
Respiratory, thoracic and mediastinal
disorders
Gastrointestinal disorders
vomiting, diarrhoea, nausea
2
Skin and subcutaneous tissue disorders
rash, sweating increased, contusion
angioneurotic oedema, pruritus, rash vesicular,
urticaria, aggravation of psoriasis










Musculoskeletal and connective tissue
disorders
muscle cramp, neck pain, myalgia
2
, arthralgia,
pain in extremity, back pain,
muscle stiffness, musculoskeletal stiffness
systemic lupus erythematosus, muscle
weakness, arthritis
hypothyroidism, hyperthyroidism
Metabolism and nutrition disorders
Infections and infestations
General disorders and administration site
conditions
flu-like symptoms, pyrexia
2
, chills
2
, sweating
2
injection site pain, injection site erythema,
injection site bruising, asthenia
2
, pain,
fatigue
2
, malaise, night sweats
injection site reaction, injection site
inflammation, injection site cellulitis
1
,
injection site necrosis, injection site bleeding,
chest pain
anaphylactic reaction, anaphylactic shock,
hypersensitivity reactions (angioedema,
dyspnoea, urticaria, rash, pruritic rash)










hepatic failure (see section 4.4), hepatitis,
autoimmune hepatitis
Reproductive system and breast disorders
metrorrhagia, menorrhagia
depression (see section 4.4), insomnia
suicide, psychosis, anxiety, confusion,
emotional lability
1
Injection site reactions including pain, inflammation and very rare cases of abscess or cellulitis that
may require surgical intervention have been reported.
2
The frequency of occurrence is higher at the beginning of treatment.
3
A syncope episode may occur after AVONEX injection, it is normally a single episode that usually
appears at the beginning of the treatment and does not recur with subsequent injections.
Paediatric population:
Limited published data suggest that the safety profile in adolescents from 12 to
16 years of age receiving AVONEX 30 micrograms IM once per week is similar to that seen in adults.
No case of overdose has been reported. However, in case of overdose, patients should be hospitalised
for observation and appropriate supportive treatment given.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic Group: Interferons, ATC code: L03 AB07.
Interferons are a family of naturally occurring proteins that are produced by eukaryotic cells in
response to viral infection and other biological inducers. Interferons are cytokines that mediate
antiviral, antiproliferative and immunomodulatory activities. Three major forms of interferons have
been distinguished: alpha, beta and gamma. Interferons alpha and beta are classified as Type I
interferons and interferon gamma is a Type II interferon. These interferons have overlapping but
clearly distinguishable biological activities. They can also differ with respect to their cellular sites of
synthesis.
Interferon beta is produced by various cell types including fibroblasts and macrophages. Natural
interferon beta and AVONEX (interferon beta-1a) are glycosylated and have a single N-linked
complex carbohydrate moiety. Glycosylation of other proteins is known to affect their stability,
activity, biodistribution, and half-life in blood. However, the effects of interferon beta that are
dependent on glycosylation are not fully defined.






AVONEX exerts its biological effects by binding to specific receptors on the surface of human cells.
This binding initiates a complex cascade of intracellular events that leads to the expression of
numerous interferon-induced gene products and markers. These include MHC Class I, Mx protein,
2’ / 5’-oligoadenylate synthetase, β
2
-microglobulin, and neopterin. Some of these products have been
measured in the serum and cellular fractions of blood collected from patients treated with AVONEX.
After a single intramuscular dose of AVONEX, serum levels of these products remain elevated for at
least four days and up to one week.
Whether the mechanism of action of AVONEX in MS is mediated by the same pathway as the
biological effects described above is not known because the pathophysiology of MS is not well
established.
The effects of lyophilised AVONEX in the treatment of MS were demonstrated in a placebo-
controlled study of 301 patients (AVONEX n=158, placebo n=143) with relapsing MS characterised
by at least 2 exacerbations in the previous 3 years or at least one exacerbation per year prior to entry
when the duration of the disease was less than 3 years. Patients with an EDSS of 1.0 to 3.5 at entry
were included in the clinical trial. Due to the design of the study, patients were followed for variable
lengths of time. 150 AVONEX-treated patients completed one year on study and 85 completed two
years on study.
In the study, the cumulative percentage of patients who developed disability
progression (by Kaplan-Meier life table analysis) by the end of two years was 35% for placebo-treated
patients and 22% for AVONEX-treated patients. Disability progression was measured as an increase
in the Expanded Disability Status Scale (EDSS) of 1.0 point, sustained for at least six months. It was
also shown that there was a one-third reduction in annual relapse rate. This latter clinical effect was
observed after more than one year of treatment.
A double-blind randomised dose comparison study of 802 relapsing MS patients (AVONEX
30 micrograms n=402, AVONEX 60 micrograms n=400) has shown no statistically significant
differences or trends between the 30 micrograms and the 60 micrograms doses of AVONEX in clinical
and general MRI parameters.
The effects of AVONEX in the treatment of MS were also demonstrated in a randomised double-blind
study performed with 383 patients (AVONEX n=193, placebo n=190) with a single demyelinating
event associated with at least two compatible brain MRI lesions. A reduction of the risk of
experiencing a second event was noted in the AVONEX treatment group. An effect on MRI
parameters was also seen. The estimated risk of a second event was 50% in three years and 39% in
two years in the placebo group and 35% (three years) and 21% (two years) in the AVONEX group. In
a post-hoc analysis, those patients with a baseline MRI with at least one Gd-enhancing lesion and nine
T2 lesions had a two-year risk of suffering a second event of 56% in the placebo group and 21% in the
AVONEX treatment group. However, the impact of early treatment with AVONEX is unknown even
in this high-risk subgroup as the study was mainly designed to assess the time to the second event
rather than the long term evolution of the disease. Furthermore, for the time-being there is no well
established definition of a high risk patient although a more conservative approach is to accept at least
nine T2 hyperintense lesions on the initial scan and at least one new T2 or one new Gd-enhancing
lesion on a follow-up scan taken at least three months after the initial scan. In any case, treatment
should only be considered for patients classified at high risk.
Paediatric population
: Limited data of the efficacy/safety of AVONEX 15 micrograms IM once per
week (n=8) as compared to no treatment (n=8) with follow up for 4 years showed results in line to
those seen in adults, although the EDSS scores increased in the treated group over the 4 year follow-up
thus indicating disease progression. No direct comparison with the dose currently recommended in
adults is available.
5.2
Pharmacokinetic properties
The pharmacokinetic profile of AVONEX has been investigated indirectly with an assay that measures
interferon antiviral activity. This assay is limited in that it is sensitive for interferon but lacks
specificity for interferon beta. Alternative assay techniques are not sufficiently sensitive.
Following intramuscular administration of AVONEX, serum antiviral activity levels peak between 5
and 15 hours post-dose and decline with a half-life of approximately 10 hours. With appropriate
adjustment for the rate of absorption from the injection site, the calculated bioavailability is
approximately 40%. The calculated bioavailability is greater without such adjustments. Intramuscular
bioavailability is three-fold higher than subcutaneous bioavailability. Subcutaneous administration
cannot be substituted for intramuscular administration.
5.3 Preclinical safety data
Carcinogenesis:
No carcinogenicity data for interferon beta-1a are available in animals or humans.
Chronic Toxicity: In a 26-week, repeated-dose toxicity study in rhesus monkeys by intramuscular
route once per week, administered in combination with another immunomodulating agent, an anti
CD40 ligand monoclonal antibody, no immune response toward interferon beta-1a and no signs of
toxicity were demonstrated.
Local Tolerance: Intramuscular irritation has not been evaluated in animals following repeated
administration to the same injection site.
Mutagenesis: Limited but relevant mutagenesis tests have been carried out. The results have been
negative.
Impairment of Fertility: Fertility and developmental studies in rhesus monkeys have been carried out
with a related form of interferon beta-1a. At very high doses, anovulatory and abortifacient effects in
test animals were observed. Similar reproductive dose-related effects have also been observed with
other forms of alpha and beta interferons. No teratogenic effects or effects on foetal development have
been observed, but the available information on the effects of interferon beta-1a in the peri- and
postnatal periods is limited.
No information is available on the effects of interferon beta-1a on male fertility.
PHARMACEUTICAL PARTICULARS
Human serum albumin,
Dibasic sodium phosphate,
Monobasic sodium phosphate,
Sodium chloride.
AVONEX should be administered as soon as possible after reconstitution. However
,
the reconstituted
solution can be stored at 2 °C-8 °C for up to six hours, prior to injection.
6.4 Special precautions for storage
DO NOT FREEZE the powder or the reconstituted product.
For storage conditions of the reconstituted medicinal product see section 6.3.
Nature and contents of container
AVONEX is available as a package of four individual doses. Each dose is supplied in a 3 ml clear
glass vial with BIO-SET device and a 13 mm bromobutyl rubber stopper. It is provided with a 1 ml
pre-filled glass syringe of solvent for reconstitution (water for injections) and one needle.
6.6 Special precautions for disposal and other handling
Use the supplied pre-filled syringe of solvent to reconstitute AVONEX for injection. Do not use any
other solvent. Inject the content of the syringe into the vial of AVONEX by connecting the pre-filled
syringe to the BIO-SET device. Gently swirl the contents in the vial until all materials are dissolved;
DO NOT SHAKE. Inspect the reconstituted product: If it contains particulate matter or is other than
colourless to slightly yellow in colour, the vial must not be used. After reconstitution, draw all the
liquid (1 ml) from the vial back into the syringe for the administration of 30 micrograms AVONEX.
The needle for intramuscular injection is provided. The formulation does not contain a preservative.
Each vial of AVONEX contains a single dose only. Discard the unused portion of any vial.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
BIOGEN IDEC LIMITED
Innovation House
70 Norden Road
Maidenhead
Berkshire
SL6 4AY
United Kingdom
MARKETING AUTHORISATION NUMBER
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 13 March 1997
Date of latest renewal: 13 March 2007
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
Agency (EMEA) http://www.emea.europa.eu
NAME OF THE MEDICINAL PRODUCT
AVONEX 30 micrograms/0.5 ml solution for injection.
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each 0.5 ml pre-filled syringe contains 30 micrograms (6 million IU) of interferon beta-1a.
The concentration is 30 micrograms per 0.5 ml.
Using the World Health Organisation (WHO) International Standard for Interferon, 30 micrograms of
AVONEX contains 6 million IU of antiviral activity. The activity against other standards is not
known.
For a full list of excipients, see section 6.1.
Clear and colourless solution.
4.1
Therapeutic indications
AVONEX is indicated for the treatment of
Patients diagnosed with relapsing multiple sclerosis (MS). In clinical trials, this was
characterised by two or more acute exacerbations (relapses) in the previous three-years without
evidence of continuous progression between relapses; AVONEX slows the progression of
disability and decreases the frequency of relapses.
Patients with a single demyelinating event with an active inflammatory process, if it is severe
enough to warrant treatment with intravenous corticosteroids, if alternative diagnoses have been
excluded, and if they are determined to be at high risk of developing clinically definite multiple
sclerosis (see section 5.1).
AVONEX should be discontinued in patients who develop progressive MS.
4.2
Posology and method of administration
Treatment should be initiated under supervision of a physician experienced in the treatment of the
disease.
Adults:
The recommended dosage for the treatment of relapsing MS is 30 micrograms (0.5 ml
solution), administered by intramuscular (IM) injection once a week (see
section 6.6).
At the initiation of treatment, patients
may
either be started on a full dose of 30 micrograms (0.5 ml
solution)
or
on approximately half the dose once a week to help them to adjust to treatment and
thereafter increased to the full dose of 30 micrograms (0.5 ml solution).
In order to obtain adequate
efficacy, a dose of 30 micrograms (0.5 ml solution) once a week should be reached and maintained
after the initial titration period. A manual titration device to enable delivery of approximately half the
dose is available for patients initiating AVONEX treatment.
No additional benefit has been shown by administering a higher dose (60 micrograms) once a week.
Paediatric population:
The safety and efficacy of AVONEX in adolescents aged 12 to 16 years have
not yet been established. Currently available data are described in section 4.8 and 5.1 but no
recommendation on a posology can be made.
The safety and efficacy of AVONEX in children below 12 years of age have not yet been established.
No data are available
Elderly:
Clinical studies did not include a sufficient number of patients aged 65 and over to determine
whether they respond differently than younger patients. However, based on the mode of clearance of
the active substance there are no theoretical reasons for any requirement for dose adjustments in the
elderly.
The intramuscular injection site should be varied each week (see section 5.3).
Doctors may prescribe a 25 mm, 25 gauge needle to patients for whom such a needle is appropriate to
administer an intramuscular injection.
Prior to injection and for an additional 24 hours after each injection, an antipyretic analgesic is advised
to decrease flu-like symptoms associated with AVONEX administration. These symptoms are usually
present during the first few months of treatment.
At the present time, it is not known for how long patients should be treated. Patients should be
clinically evaluated after two years of treatment and longer-term treatment should be decided on an
individual basis by the treating physician. Treatment should be discontinued if the patient develops
chronic progressive MS.
Initiation of treatment in pregnancy (see section 4.6).
Patients with a history of hypersensitivity to natural or recombinant interferon-ß or to any
excipients.
Patients with current severe depression and/or suicidal ideation (see sections 4.4 and 4.8).
4.4 Special warnings and precautions for use
AVONEX should be administered with caution to patients with previous or current depressive
disorders, in particular to those with antecedents of suicidal ideation (see section 4.3). Depression and
suicidal ideation are known to occur in increased frequency in the multiple sclerosis population and in
association with interferon use. Patients should be advised to immediately report any symptoms of
depression and/or suicidal ideation to their prescribing physician.
Patients exhibiting depression should be monitored closely during therapy and treated appropriately.
Cessation of therapy with AVONEX should be considered (see also sections 4.3 and 4.8).
AVONEX should be administered with caution to patients with a history of seizures, to those
receiving treatment with anti-epileptics, particularly if their epilepsy is not adequately controlled with
anti-epileptics (see sections 4.5 and 4.8).
Caution should be used and close monitoring considered when administering AVONEX to patients
with severe renal and hepatic failure and to patients with severe myelosuppression.
Hepatic injury including elevated serum hepatic enzyme levels, hepatitis, autoimmune hepatitis and
hepatic failure has been reported with interferon beta in post-marketing (see section 4.8). In some
cases, these reactions have occurred in the presence of other medicinal products that have been
associated with hepatic injury. The potential of additive effects from multiple medicinal products or
other hepatotoxic agents (e.g. alcohol) has not been determined. Patients should be monitored for signs
of hepatic injury and caution exercised when interferons are used concomitantly with other medicinal
products associated with hepatic injury.
Patients with cardiac disease, such as angina, congestive heart failure or arrhythmia, should be closely
monitored for worsening of their clinical condition during treatment with AVONEX. Flu-like
symptoms associated with AVONEX therapy may prove stressful to patients with underlying cardiac
conditions.
Laboratory abnormalities are associated with the use of interferons. Therefore, in addition to those
laboratory tests normally required for monitoring patients with MS, complete and differential white
blood cell counts, platelet counts, and blood chemistry, including liver function tests, are
recommended during AVONEX therapy. Patients with myelosuppression may require more intensive
monitoring of complete blood cell counts, with differential and platelet counts.
Patients may develop antibodies to AVONEX. The antibodies of some of those patients reduce the
activity of interferon beta-1a
in vitro
(neutralising antibodies). Neutralising antibodies are associated
with a reduction in the
in vivo
biological effects of AVONEX and may potentially be associated with a
reduction of clinical efficacy. It is estimated that the plateau for the incidence of neutralising antibody
formation is reached after 12 months of treatment. Recent clinical studies with patients treated up to
three years with AVONEX suggest that approximately 5% to 8% develop neutralising antibodies.
The use of various assays to detect serum antibodies to interferons limits the ability to compare
antigenicity among different products.
4.5 Interaction with other medicinal products and other forms of interaction
No formal interaction studies have been performed in humans.
The interaction of AVONEX with corticosteroids or adrenocorticotropic hormone (ACTH) has not
been studied systematically. The clinical studies indicate that MS patients can receive AVONEX and
corticosteroids or ACTH during relapses.
Interferons have been reported to reduce the activity of hepatic cytochrome P450-dependent enzymes
in humans and animals. The effect of high-dose AVONEX administration on P450-dependent
metabolism in monkeys was evaluated and no changes in liver metabolising capabilities were
observed. Caution should be exercised when AVONEX is administered in combination with medicinal
products that have a narrow therapeutic index and are largely dependent on the hepatic cytochrome
P450 system for clearance, e.g. antiepileptics and some classes of antidepressants.
4.6 Pregnancy and lactation
There is limited information on the use of AVONEX in pregnancy. Available data indicates that there
may be an increased risk of spontaneous abortion. Initiation of treatment is contraindicated during
pregnancy (see section 4.3).
Women of child-bearing potential
Women of child-bearing potential have to take appropriate contraceptive measures. If the patient
becomes pregnant or plans to become pregnant while taking AVONEX she should be informed of the
potential hazards and discontinuation of therapy should be considered (see section 5.3). In patients
with a high relapse rate before treatment started, the risk of a severe relapse following discontinuation
of AVONEX in the event of pregnancy should be weighed against a possible increased risk of
spontaneous abortion.
It is not known whether AVONEX is excreted in human milk. Because of the potential for serious
adverse reactions in nursing infants, a decision should be made either to discontinue breast-feeding or
AVONEX therapy.
4.7
Effects on ability to drive and use machines
No studies of the effects of AVONEX on the ability to drive and use machines have been performed.
Central nervous system-related adverse reactions may have a minor influence on the ability to drive
and use machines in susceptible patients (see section 4.8).
The highest incidence of adverse reactions associated with AVONEX therapy is related to flu-like
symptoms. The most commonly reported flu-like symptoms are myalgia, fever, chills, sweating,
asthenia, headache and nausea. Flu-like symptoms tend to be most prominent at the initiation of
therapy and decrease in frequency with continued treatment.
Transient neurological symptoms that may mimic MS exacerbations may occur following injections.
Transient episodes of hypertonia and/or severe muscular weakness that prevent voluntary movements
may occur at any time during treatment. These episodes are of limited duration, temporally related to
the injections and may recur after subsequent injections. In some cases these symptoms are associated
with flu-like symptoms.
The frequencies of adverse reactions are expressed in patient-years, according to the following
categories:
Very common (≥1/10 patient-years);
Common (≥1/100 to <1/10 patient-years);
Uncommon (≥1/1, 000 to <1/100 patient-years);
Rare (≥1/10, 000 to <1/1,000 patient-years);
Very rare (<1/10,000 patient-years);
Not known (cannot be estimated from the available data).
Patient-time is the sum of individual units of time that the patient in the study has been exposed to
AVONEX before experiencing the adverse reaction. For example, 100 person-years could be observed
in 100 patients who were on treatment for one year or in 200 patients who were on treatment for half a
year.
Adverse reactions identified from studies (clinical trials and observational studies, with a period of
follow-up ranging from two years to six years) and other adverse reactions identified through
spontaneous reporting from the market, with unknown frequency, are provided in the table below.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
lymphocyte count decreased, white blood cell
count decreased, neutrophil count decreased,
hematocrit decreased, blood potassium
increased, blood urea nitrogen increased
weight decreased, weight increased,
liver function tests abnormal
cardiomyopathy, congestive heart failure (see
section 4.4), palpitations, arrhythmia,
tachycardia
Blood and lymphatic system disorders
pancytopenia, thrombocytopenia
muscle spasticity, hypoesthesia
neurological symptoms, syncope
3
,
hypertonia, dizziness, paraesthesia,
seizures, migraine
Respiratory, thoracic and mediastinal
disorders
Gastrointestinal disorders
vomiting, diarrhoea, nausea
2
Skin and subcutaneous tissue disorders
rash, sweating increased, contusion
angioneurotic oedema, pruritus, rash vesicular,
urticaria, aggravation of psoriasis










Musculoskeletal and connective tissue
disorders
muscle cramp, neck pain, myalgia
2
, arthralgia,
pain in extremity, back pain,
muscle stiffness, musculoskeletal stiffness
systemic lupus erythematosus, muscle
weakness, arthritis
hypothyroidism, hyperthyroidism
Metabolism and nutrition disorders
Infections and infestations
General disorders and administration site
conditions
flu-like symptoms, pyrexia
2
, chills
2
, sweating
2
injection site pain, injection site erythema,
injection site bruising, asthenia
2
, pain,
fatigue
2
, malaise, night sweats
injection site reaction, injection site
inflammation, injection site cellulitis
1
,
injection site necrosis, injection site bleeding,
chest pain
anaphylactic reaction, anaphylactic shock,
hypersensitivity reactions (angioedema,
dyspnoea, urticaria, rash, pruritic rash)










hepatic failure (see section 4.4), hepatitis,
autoimmune hepatitis
Reproductive system and breast disorders
metrorrhagia, menorrhagia
depression (see section 4.4), insomnia
suicide, psychosis, anxiety, confusion,
emotional lability
1
Injection site reactions including pain, inflammation and very rare cases of abscess or cellulitis that
may require surgical intervention have been reported.
2
The frequency of occurrence is higher at the beginning of treatment.
3
A syncope episode may occur after AVONEX injection, it is normally a single episode that usually
appears at the beginning of the treatment and does not recur with subsequent injections.
Paediatric population:
Limited published data suggest that the safety profile in adolescents from 12 to
16 years of age receiving AVONEX 30 micrograms IM once per week is similar to that seen in adults.
No case of overdose has been reported. However, in case of overdose, patients should be hospitalised
for observation and appropriate supportive treatment given.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic Group: Interferons, ATC code : L03 AB07.
Interferons are a family of naturally occurring proteins that are produced by eukaryotic cells in
response to viral infection and other biological inducers. Interferons are cytokines that mediate
antiviral, antiproliferative, and immunomodulatory activities. Three major forms of interferons have
been distinguished: alpha, beta, and gamma. Interferons alpha and beta are classified as Type I
interferons, and interferon gamma is a Type II interferon. These interferons have overlapping but
clearly distinguishable biological activities. They can also differ with respect to their cellular sites of
synthesis.
Interferon beta is produced by various cell types including fibroblasts and macrophages. Natural
interferon beta and AVONEX (interferon beta-1a) are glycosylated and have a single N-linked
complex carbohydrate moiety. Glycosylation of other proteins is known to affect their stability,
activity, biodistribution, and half-life in blood. However, the effects of interferon beta that are
dependent on glycosylation are not fully defined.






AVONEX exerts its biological effects by binding to specific receptors on the surface of human cells.
This binding initiates a complex cascade of intracellular events that leads to the expression of
numerous interferon-induced gene products and markers. These include MHC Class I, Mx protein,
2’ / 5’-oligoadenylate synthetase, β
2
-microglobulin, and neopterin. Some of these products have been
measured in the serum and cellular fractions of blood collected from patients treated with AVONEX.
After a single intramuscular dose of AVONEX, serum levels of these products remain elevated for at
least four days and up to one week.
Whether the mechanism of action of AVONEX in MS is mediated by the same pathway as the
biological effects described above is not known because the pathophysiology of MS is not well
established.
The effects of lyophilised AVONEX in the treatment of MS were demonstrated in a
placebo-controlled study of 301 patients (AVONEX n=158, placebo n=143) with relapsing MS
characterised by at least 2 exacerbations in the previous 3 years or at least one exacerbation per year
prior to entry when the duration of the disease was less than 3 years. Patients with an EDSS of 1.0 to
3.5 at entry were included in the clinical trial. Due to the design of the study, patients were followed
for variable lengths of time. 150 AVONEX-treated patients completed one year on study and 85
completed two years on study.
In the study, the cumulative percentage of patients who developed
disability progression (by Kaplan-Meier life table analysis) by the end of two years was 35% for
placebo-treated patients and 22% for AVONEX-treated patients. Disability progression was measured
as an increase in the Expanded Disability Status Scale (EDSS) of 1.0 point, sustained for at least six
months. It was also shown that there was a one-third reduction in annual relapse rate. This latter
clinical effect was observed after more than one year of treatment.
A double-blind randomised dose comparison study of 802 relapsing MS patients (AVONEX
30 micrograms n=402, AVONEX 60 micrograms n=400) has shown no statistically significant
differences or trends between the 30 micrograms and the 60 micrograms doses of AVONEX in clinical
and general MRI parameters.
The effects of AVONEX in the treatment of MS were also demonstrated in a randomised double-blind
study performed with 383 patients (AVONEX n=193, placebo n=190) with a single demyelinating
event associated with at least two compatible brain MRI lesions. A reduction of the risk of
experiencing a second event was noted in the AVONEX treatment group. An effect on MRI
parameters was also seen. The estimated risk of a second event was 50% in three years and 39% in
two years in the placebo group and 35% (three years) and 21% (two years) in the AVONEX group. In
a post-hoc analysis, those patients with a baseline MRI with at least one Gd-enhancing lesion and nine
T2 lesions had a two-year risk of suffering a second event of 56%
in the placebo group and 21% in the
AVONEX treatment group. However, the impact of early treatment with AVONEX is unknown even
in this high-risk subgroup as the study was mainly designed to assess the time to the second event
rather than the long-term evolution of the disease. Furthermore, for the time-being there is no well
established definition of a high risk patient although a more conservative approach is to accept at least
nine T2 hyperintense lesions on the initial scan and at least one new T2 or one new Gd-enhancing
lesion on a follow-up scan taken at least three months after the initial scan. In any case, treatment
should only be considered for patients classified at high risk.
Paediatric population
: Limited data of the efficacy/safety of AVONEX 15 micrograms IM once per
week (n=8) as compared to no treatment (n=8) with follow up for 4 years showed results in line to
those seen in adults, although the EDSS scores increased in the treated group over the 4 year follow-up
thus indicating disease progression. No direct comparison with the dose currently recommended in
adults is available.
5.2 Pharmacokinetic properties
The pharmacokinetic profile of AVONEX has been investigated indirectly with an assay that measures
interferon antiviral activity. This assay is limited in that it is sensitive for interferon but lacks
specificity for interferon beta. Alternative assay techniques are not sufficiently sensitive.
Following intramuscular administration of AVONEX, serum antiviral activity levels peak between 5
and 15 hours post-dose and decline with a half-life of approximately 10 hours. With appropriate
adjustment for the rate of absorption from the injection site, the calculated bioavailability is
approximately 40%. The calculated bioavailability is greater without such adjustments.
Intramuscular
bioavailability is three-fold higher than subcutaneous bioavailability. Subcutaneous administration
cannot be substituted for intramuscular administration.
5.3 Preclinical safety data
Carcinogenesis:
No carcinogenicity data for interferon beta-1a are available in animals or humans.
Chronic Toxicity: In a 26-week repeated dose toxicity study in rhesus monkeys by intramuscular route
once per week, administered in combination with another immunomodulating agent, an anti CD40
ligand monoclonal antibody, no immune response toward interferon beta-1a and no signs of toxicity
were demonstrated.
Local Tolerance: Intramuscular irritation has not been evaluated in animals following repeated
administration to the same injection site.
Mutagenesis: Limited but relevant mutagenesis tests have been carried out. The results have been
negative.
Impairment of Fertility: Fertility and developmental studies in rhesus monkeys have been carried out
with a related form of interferon beta-1a. At very high doses, anovulatory and abortifacient effects in
test animals were observed. Similar reproductive dose-related effects have also been observed with
other forms of alpha and beta interferons. No teratogenic effects or effects on foetal development have
been observed, but the available information on the effects of Interferon beta-1a in the peri- and
postnatal periods is limited.
No information is available on the effects of interferon beta-1a on male fertility.
PHARMACEUTICAL PARTICULARS
Sodium acetate trihydrate,
Acetic acid, glacial,
Arginine hydrochloride,
Polysorbate 20,
Water for injections.
6.4
Special precautions for storage
Store in a refrigerator (2
o
C -8
o
C).
AVONEX can be stored at room temperature (between 15
o
C and 30
o
C) for up to one week.
Store in the original package (sealed plastic tray) in order to protect from light (see section 6.5).
6.5
Nature and contents of container
1 ml pre-filled syringe made of glass (Type I) with a tamper evident cap and plunger stopper
(bromobutyl) containing 0.5 ml of solution.
Pack size: box of four or twelve pre-filled syringes of 0.5 ml. Each syringe is packed in a sealed
plastic tray, which also contains one injection needle for intramuscular use.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
AVONEX is provided as ready to use solution for injection in a pre-filled syringe.
Once removed from the refrigerator, AVONEX in a pre-filled syringe should be allowed to warm to
room temperature (15 °C -30 °C) for about 30 minutes.
Do not use external heat sources such as hot water to warm AVONEX 30 micrograms solution for
injection.
If the solution for injection contains particulate matter or if it is any colour other than clear colourless,
the pre-filled syringe must not be used. The injection needle for intramuscular injection is provided.
The formulation does not contain a preservative. Each pre-filled syringe of AVONEX contains a
single dose only. Discard the unused portion of any pre-filled syringe.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
BIOGEN IDEC LIMITED
Innovation House
70 Norden Road
Maidenhead
Berkshire
SL6 4AY
United Kingdom
MARKETING AUTHORISATION NUMBERS
EU/1/97/033/003
EU/1/97/033/004
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 13 March 1997
Date of latest renewal: 13 March 2007
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
Agency (EMEA) http://www.emea.europa.eu
A.
MANUFACTURER OF THE BIOLOGICAL ACTIVE SUBSTANCE AND
MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR
BATCH RELEASE
B.
CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURER OF THE BIOLOGICAL ACTIVE SUBSTANCE AND
MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturers of the biological active substance
Biogen Idec Inc., 14 Cambridge Center, Cambridge, Massachusetts 02142, USA.
Biogen Idec Inc., 5000 Davis Drive, POB 14627, Research Triangle Park, North Carolina, 27709,
USA.
Name and address of the manufacturers responsible for batch release
Biogen Idec B.V., Robijnlaan 8, 2132 WX Hoofddorp, The Netherlands.
Biogen Idec Denmark Manufacturing ApS, Biogen Idec Allé 1, DK-3400 Hillerød, Denmark.
The printed package leaflet of the medicinal product must state the name and address of the
manufacturer responsible for the release of the concerned batch.
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to restricted medical prescription (See Annex I: Summary of Product
Characteristics, section 4.2).
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
The holder of this marketing authorisation must inform the European Commission about the marketing
plans for the medicinal product authorised by this decision.
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
AVONEX 30 micrograms powder and solvent for solution for injection
STATEMENT OF ACTIVE SUBSTANCE(S)
Each vial contains 30 micrograms (6 million IU)
of interferon beta-1a.
Human serum albumin, sodium chloride, di- and monobasic sodium phosphate.
See the package leaflet for further information
.
PHARMACEUTICAL FORM AND CONTENTS
Powder and solvent for solution for injection.
Packs of four doses presented as: vial with BIO-SET device containing medicinal product, pre-filled
syringe of water for injections, one injection needle for intramuscular use.
METHOD AND ROUTE(S) OF ADMINISTRATION
For intramuscular use after reconstitution with solvent.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
























OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
BIOGEN IDEC LTD.
Innovation House
70 Norden Road
Maidenhead
Berkshire
SL6 4AY
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
AVONEX 30 micrograms/0.5 ml solution for injection
STATEMENT OF ACTIVE SUBSTANCE (S)
Each pre-filled syringe of 0.5 ml contains 30 micrograms (6 million IU)
of interferon beta-1a.
Sodium acetate trihydrate, acetic acid glacial, arginine hydrochloride, polysorbate 20, water for
injections.
See the package leaflet for further information.
PHARMACEUTICAL FORM AND CONTENTS
Box of four pre-filled syringes of 0.5 ml of solution.
Box of twelve pre-filled syringes of 0.5 ml of solution.
Each syringe is packed in a sealed plastic tray which also contains one injection needle for
intramuscular use.
METHOD AND ROUTE (S) OF ADMINISTRATION
Read the package leaflet before use.























6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING (S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
Avonex can be stored at room temperature (between 15
o
C-30
o
C) for up to one week.
Store in the original package (sealed plastic tray) in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
BIOGEN IDEC LTD.
Innovation House
70 Norden Road
Maidenhead
Berkshire
SL6 4AY
United Kingdom
12. MARKETING AUTHORISATION NUMBER (S)
EU/1/97/033/003 4 pack
EU/1/97/033/004 12 pack




















13. MANUFACTURER’S BATCH NUMBER
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE









PACKAGE LEAFLET: INFORMATION FOR THE USER
AVONEX 30 micrograms powder and solvent for solution for injection
(Interferon beta-1a)
BIO-SET Presentation
Read all of this leaflet carefully before you start using this medicine.
Even if you have used Avonex before, some of the information may have changed.
-
If you have any further questions, ask your doctor or your pharmacist
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this
leaflet,please tell your doctor or pharmacist.
Latest issue: 12/2008
This leaflet is changed from time to time.
Please check every time you get your prescription refilled to see if the leaflet has been updated.
In this leaflet
:
1. What AVONEX is and what it is used for
2. Before you use AVONEX
3. How to use AVONEX
4. Possible side effects
5.
How to store AVONEX
6.
Further information
7.
How to inject AVONEX
1.
WHAT AVONEX IS AND WHAT IT IS USED FOR
The active ingredient in Avonex is a protein called
interferon beta-1a.
Interferons are natural
substances made in your body to help protect you from infections and diseases. The protein in Avonex
is made up of exactly the same ingredients as interferon beta that is found in the human body.
Avonex
is used to treat Multiple Sclerosis (MS).
Treatment with Avonex can help to prevent you
from getting worse, although it will not cure MS.
Everyone has their own set of MS symptoms.
These can include:
-
feeling off-balance or light headed, walking problems, stiffness and muscle spasms, tiredness,
numbness in the face, arms or legs
-
acute or chronic pain, bladder and bowel problems, sexual problems and problems seeing things
-
difficulty in thinking and concentrating, depression.
MS also tends to flare up from time to time: this is called a relapse.
Keep this leaflet. You may need to read it again.




Avonex works best when you use it at the same time, once a week, on a regular basis.
Do not stop your Avonex treatment without speaking to your neurologist.
Avonex can help to reduce the number of relapses that you have and slow down the disabling
effects of MS.
Your doctor will advise you for how long you can use Avonex or when to stop.
Multiple sclerosis is linked to nerve (brain or spinal cord) damage. In MS, your body’s defence system
reacts against it’s own myelin – the ‘insulation’ that surrounds nerve fibres. When myelin is damaged,
the messages between the brain and other parts of the body are disrupted. This is what causes the
symptoms of MS. Avonex seems to work by stopping your body’s defence system from attacking the
myelin.
if you are allergic (hypersensitive)
to interferon beta, human serum albumin or any of the
other ingredients in Avonex
if you are pregnant,
do not start using Avonex
if you have severe depression
or think about committing suicide.
Talk to a doctor straight away if any of these apply to you.
Avonex and allergic reactions.
Because Avonex is based on a protein, there is a small chance of an
allergic reaction.
More about depression.
If you have severe depression or thoughts about suicide, you must not use
Avonex.
If you have depression, your doctor may still prescribe Avonex for you, but it's important to let your
doctor know if you have had depression or any similar problems affecting your moods.
Take special care with AVONEX
Talk to your doctor first:
If you have or have had in the past:
-
depression
or problems affecting your moods
thoughts about committing suicide.
Changes to your mood, thoughts about suicide, feeling unusually sad, anxious or worthless, should be
reported to your doctor immediately.
epilepsy
or other seizure disorders not controlled by medication
serious kidney or liver problems
a low number of white blood cells or platelets
, which can cause an increased risk of infection,
bleeding or anaemia







heart problems
, which can cause symptoms such as chest pain
(angina)
, particularly after any
activity; swollen ankles, shortness of breath
(congestive heart failure)
; or an irregular heartbeat
(arrhythmias)
.
Talk to your doctor
if you have any of these conditions
, or if they worsen whilst taking Avonex.
Tell your doctor you are using AVONEX:
-
if you are having a blood test. Avonex may interfere with the results.
Sometimes you will need to remind other medical staff that you are being treated with Avonex. For
example, if you are prescribed other medicines, or if you have a blood test, Avonex may affect the
other medicines or the test result.
If you are using other medicines, especially those used to treat epilepsy or depression. Avonex may
affect other medicines or be affected by them. This includes any other medicines including medicines
obtained without a prescription.
Pregnancy and breastfeeding
If you are pregnant, do not start using Avonex.
-
If you could get pregnant,
you need to use contraception while you use Avonex.
If you are planning a baby or if you become pregnant
while you are using Avonex, tell your
doctor. You and your doctor can discuss if you should carry on with treatment.
If you are already pregnant,
or think that you might be, talk to a doctor as soon as you can.
If you want to breastfeed
talk to your doctor first.
Driving and using machines
If you feel dizzy, do not drive.
Avonex makes some people feel dizzy. If this happens to you, or if
you get any other side effects that could affect your ability, do not drive or use machines.
Important information about some of the ingredients of AVONEX
This medicine is essentially ‘sodium-free’. It contains less than 23 mg (1 mmol) sodium in each
weekly dose.
The usual dose for adults and adolescents aged 12 years and over
One injection of Avonex, once a week.
Try to use Avonex at the same time on the same day each week.
Not for children
Avonex is
not to be used
in children below the age of 12 years.



You can inject Avonex yourself without the help of your doctor, if they have trained you to do this.
The instructions on how to inject yourself are at the end of this leaflet (see section 7
, How to inject
AVONEX
).
If you have trouble
handling the syringe, ask your doctor who may be able to help.
There are more details on how to inject Avonex
at the end of this leaflet.
Alternate needle:
Your pack of Avonex already includes a needle for injection. It may be possible for your doctor to
prescribe you a shorter and thinner needle, depending on your body type. Talk to your doctor to see if
this is appropriate for you.
If you have problems handling the syringe
, talk to your doctor about using a syringe grip. This is a
specially designed holder to help you with injecting Avonex.
Your doctor will tell you how long you need to keep using Avonex. It is important to continue using
Avonex regularly. Do not make changes unless your doctor tells you.
You should only have one injection of Avonex, once a week. If you have used more than one injection
of Avonex in a three-day period,
contact your doctor or pharmacist straight away for advice.
If you miss your usual weekly dose
, inject a dose as soon as you can. Then leave a week before using
Avonex again. Continue injecting on this new day every week. If you have a preferred day for using
Avonex, talk to your doctor about managing the dose, to get back to your preferred day.
Do not use two injections to make up for a missed injection.
Like all medicines, Avonex can cause side effects, although not everyone gets them.
Although the list of possible side effects can seem worrying, it’s possible that you may not have any of
them.
Serious side effects: get medical help







Serious allergic reactions
If you get any of these:
-
swelling of the face, lips or tongue
-
difficulty breathing
-
a rash.
Call a doctor immediately
. Do not use any more Avonex until you have spoken to a doctor.
Depression
If you get any symptoms of depression:
-
feeling unusually sad, anxious or worthless.
Call a doctor immediately.
Liver problems
If you get any of these symptoms:
-
yellowing of your skin or the whites of your eyes
(jaundice)
-
itching all over
-
feeling sick, being sick
(nausea and vomiting)
-
easy bruising of the skin.
Call a doctor immediately
as they may be signs of a possible liver problem.
Side effects seen in clinical trials
Side effects seen in clinical trials.
These are the side effects that people reported when Avonex was
being tested. The figures are based on how many people said they’d had them. It gives you an idea
how likely you are to get similar side effects.
Very common side effects
(at least 1 in 10 people are affected)
-
flu-like symptoms – headache, muscle aches, chills or a fever: see
Flu-like symptoms
, below
Common side effects
(less than 1 in 10 people are affected)
-
diarrhoea (
loose stools
)
feeling or being sick (
nausea or vomiting
)
numbness or tingling of skin
rash, bruising of the skin
increased sweating, night sweats
pain in your muscles, joints, arms, legs or neck
muscle cramps, stiffness in the joints and muscles
pain, bruising and redness at the injection site
changes to blood tests. Symptoms you might notice are tiredness, repeated infection,
unexplained bruising or bleeding.




Uncommon side effects
(less than 1 in 100 people affected)
-
changes to your monthly period
burning feeling at the site of injection.
Rare side effects
(less than 1 in 1,000 people affected)
-
If any of the effects trouble you, talk to your doctor.
These effects
have been seen in people using Avonex, but we do not know how likely they are to
happen.
-
an underactive or overactive thyroid
-
nervousness or anxiety, emotional instability, irrational thoughts or hallucinations (seeing or
hearing things that are not real), confusion or suicide
-
numbness, dizziness, seizures or fits and migraines
-
an awareness of your heartbeat
(palpitations)
, a rapid or irregular heartbeat, or heart problems
which would have the following symptoms: a reduced ability to exercise, inability to lie flat in
bed, shortness of breath or swollen ankles
-
liver problems as described above
-
nettle rash or blister-like rash, itching, worsening of psoriasis if you have it
-
swelling or bleeding at the site of injection, or chest pain after an injection
-
gaining or losing weight
-
changes to test results, including changes to liver function tests.
If any of the effects trouble you, talk to your doctor.
Effects of the injection
-
Feeling faint:
Your first injection of Avonex may be given by your doctor. It may make you
feel faint. You may even actually faint. This is unlikely to happen again.
Just after an injection, your muscles may feel tense or very weak
– as though you are having
a relapse. This is rare. It only happens when you inject and the effects soon pass. They may
happen any time after starting on Avonex.
If you notice any irritation or skin problems
after an injection, talk to your doctor.
Three simple ways to help reduce the impact of flu-like symptoms:
1.
Use your Avonex injection just before bedtime.
This may allow you to sleep through the
effects.
2.
Take paracetamol or ibuprofen
half an hour before
your Avonex injection and continue
taking it for up to a day. Speak to your doctor or pharmacist about a suitable dose.
3.
If you have a fever, drink plenty of water
to keep you hydrated.









Some people find that after injecting Avonex, they feel like they have flu
. Signs are:
-
These symptoms are not really flu.
You can’t pass it on to anyone else. They are more common when you first start using Avonex. As
you keep using your injections, the flu-like symptoms gradually decrease.
Keep out of the reach and sight of children.
Do not use after the expiry date stated on the label.
Once prepared in the syringe use as soon as possible. However, the prepared syringe can be stored in
the fridge (between 2 °C and 8 °C) for up to 6 hours before you inject. Do not freeze.
Bring it out half an hour before injecting.
Do NOT use AVONEX
if you notice:
The seal of the cap of the BIO-SET device is broken.
The sealed plastic tray is damaged or opened.
The liquid in the vial obtained after reconstitution is not colourless or slightly yellow in colour
or you can see particles floating in it.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active ingredient
is: Interferon-beta 1a 30 micrograms
The other ingredients
are: Human serum albumin, sodium chloride, dibasic sodium phosphate and
monobasic sodium phosphate.
What is in your Avonex pack
A box of Avonex Bioset has four doses of Avonex.
Each dose comes in a sealed plastic tray and has a white to off-white coloured powder in a glass
container (vial) and an injection syringe filled with water. These are mixed together to make up the
injection you take (solution for injection). A separate needle to give the injection is also included in
the tray.
Marketing Authorisation Holder is:
Biogen Idec Limited, Innovation House, 70 Norden Road, Maidenhead, Berkshire, SL6 4AY, United
Kingdom.
Biogen Idec BV, Robijnlaan 8, NL-2132 WX Hoofddorp, The Netherlands.
Biogen Idec Denmark Manufacturing ApS, Biogen Idec Allé 1, DK-3400 Hillerød, Denmark.
You can get a larger print version of this leaflet by calling the local representatives.
For any further information about this medicine, please contact the local representative of the
Marketing Authorisation Holder.
België/Belgique/Belgien
Biogen Idec Belgium N.V./S.A.
+32 2 2191218
Luxembourg/Luxemburg
Biogen Idec Belgium N.V./S.A.
+32 2 2191218
България
ТП ЕВОФАРМА
+359 2 962 12 00
Magyarország
Gedeon Richter Plc.
+36 1 432 6097
Česká republika
Biogen Idec Czech Republic
+420 222 191 640
Malta
Interpharma Co. Ltd.
+356 21354582
Danmark
Biogen Idec Denmark A/S
+45 77 41 57 57
Nederland
Biogen Idec International B.V.
+31 20 542 2000
Deutschland
Biogen Idec GmbH
+49 (0) 89 99 6170
Norge
Biogen Idec Norway AS
+47 23 00 52 50
Eesti
Richter Gedeon Eesti filiaal
+372 742 7056
Österreich
Biogen Idec Austria GmbH.
+43 1 484 46 13
Ελλάδα
Genesis Pharma SA
+30 210 8771500
Polska
Gedeon Richter Plc. S.A.
Przedstawicielstwo w Polsce.
+48 (22) 642-67-39
España
Biogen Idec Iberia SL
+34 91 310 7110
Portugal
Biogen Idec Portugal,
Sociedade Unipessoal, Lda.
+351 21 318 8450
France
Biogen Idec France
+33 (0)1 41 37 9595
România
MEDISON PHARMA SRL
+ 40 31 7104035
Ireland
Biogen Idec (Ireland) Ltd.
+353 (0)1 463 7799
Slovenija
Biogen Idec d.o.o.
+386 1 589 91 04
Ísland
Icepharma hf
+354 540 8000
Slovenská republika
Biogen Idec (Slovak Republic) s.r.o
+421 2 32410188
Italia
Biogen-Dompé s.r.l.
+39 02 583 831
Suomi/Finland
Biogen Idec Finland Oy
+358 207 401 200
Κύπρος
Genesis Pharma Cyprus Ltd
+3572 2 769946
Sverige
BiogenIdec Sweden AB
+46 8 594 113 60
Latvija
Gedeon Richter Plc.
+36 1 432 6097
United Kingdom
Biogen Idec Limited
+44 (0) 1628 50 1000
Lietuva
Gedeon Richter Plc.
+36 1 432 6097
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency (EMEA) web
site: http://www.emea.europa.eu
You should have had training in how to inject Avonex.
These notes are a reminder. If there’s anything you’re not sure about, check with your doctor or
pharmacist.
Avonex is injected into a muscle
, for example, the upper thigh muscle. Injection of Avonex
into the buttocks is not recommended.
Use a different injection site each week.
This means less risk of irritation to your skin and
muscle.
Do not use
any area of skin that is bruised, sore, or infected, or if there is an open wound.
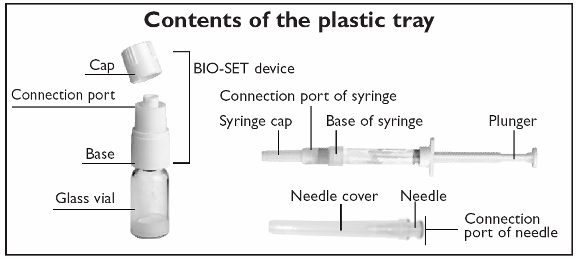
A Getting ready
1.
Take a tray out of the box.
Check the expiry date on the lid of the tray. Do not use it if it is out-of-date.
Peel back the paper lid completely. Check the blister tray contains:
•
one BIO-SET (vial + base + cap)
one injection needle (see picture “Contents of the plastic tray”).
2.
Wash your hands thoroughly
with soap and water and dry them.
3.
Prepare alcohol wipes and sticking plasters
(not supplied) if you need them.
4.
Find a clean, hard surface to lay out the items
needed for your injection. Lay the tray
down on it.
B. Preparing the injection
Remove cap off the vial
Twist, then pull cap off.
Do not touch the connection port.
Pull the cap off the syringe
Hold the base of the syringe. Pull the cap off.
Do not touch the connection port.
Do not push on the plunger.
Line up the syringe and vial
Place the Bio-Set on a flat surface.
Line up the two connection ports
so that they are in a straight line.
Hold the syringe by the base. Screw it firmly clockwise into the vial.
Push the syringe down until it clicks
Keep the Bio-Set on the flat surface and hold the syringe by the base.
Keep them in a straight line.
Tip:
If the syringe is at an angle to the Bio-Set, it will leak.
Push the syringe
until it clicks.
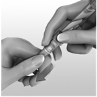
Mix the water and powder
Slowly inject all the water from the syringe into the vial.
Tip
: Don’t push the plunger quickly. This turns the solution to foam that
can’t be drawn into the syringe.
Push the plunger right down to get air out of the syringe.




Dissolve the powder fully
Pick up the vial and the syringe, keeping them attached and straight.
Gently swirl the vial until all the powder has dissolved.
Do not shake: this will make froth.
Fill the syringe
Turn the syringe and vial upside down, still in a straight line.
Tip:
If the syringe is at an angle to the Bio-Set, it will leak.
Slowly pull the plunger
until all the liquid is in the syringe.
Separate syringe and vial
Hold the filled syringe by the base. Turn it anti-clockwise to remove it
from the Bio-Set vial.
Do not touch the connection port on the syringe.
Check the liquid in the syringe
It should be clear and colourless.
If the solution is any colour except
colourless or slightly yellow, or if you can see particles floating in it,
do not inject.
Fit the needle
Unwrap the needle to expose the connection port. Keep the cover on.
Push and twist the needle clockwise onto the syringe.
Now pull off the plastic needle cover
.
Do not twist it.
Tip
: If you twist the needle cover to remove it, you may accidentally
remove the needle as well.
Remove any air
To remove air, point the syringe needle upwards. Gently tap to bring
air bubbles to the top.
Push the plunger carefully to remove the air
. Don’t let more than a
small drop of liquid escape.
Clean and stretch the injection site
If you need to, use an alcohol wipe to clean the skin at the injection
site you’ve chosen. Allow the skin to dry.
With one hand, stretch the skin around the injection site.
Relax your muscle.




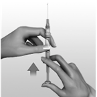

Make the injection
Insert the injection needle with a quick dart-like thrust
at right
angles to the skin, into the muscle.
The needle must go all the way in.
Press the plunger slowly until the syringe is empty.
Pull the needle out
Keep the skin stretched tightly or squeeze the skin around the injection
site, and pull out the needle.
If you use alcohol wipes, hold one on the injection site.
Put a plaster over the site of injection if you need to.
Dispose of the rubbish properly
After you have finished each injection, put the needle, syringe and vial
into a special container (such as a sharps bin), not in ordinary rubbish.
Waste paper and used wipes can be put in an ordinary rubbish bin.


PACKAGE LEAFLET: INFORMATION FOR THE USER
AVONEX 30 micrograms/0.5 ml solution for Injection
(Interferon beta-1a)
Pre-filled syringe presentation
Read all of this leaflet carefully before you start using this medicine.
Even if you have used Avonex before, some of the information may have changed.
-
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or your pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
Latest issue: 12/2008
This leaflet is changed from time to time.
Please check every time you get your prescription refilled to see if the leaflet has been updated.
In this leaflet
:
1.
What AVONEX is and what it is used for
2.
Before you use AVONEX
3.
How to use AVONEX
4.
Possible side effects
5.
How to store AVONEX
6.
Further information
7.
How to inject AVONEX
WHAT AVONEX IS AND WHAT IT IS USED FOR
The active ingredient in Avonex is a protein called
interferon beta-1a.
Interferons are natural
substances made in your body to help protect you from infections and diseases. The protein in Avonex
is made up of exactly the same ingredients as interferon beta that is found in the human body.
Avonex is used to treat Multiple Sclerosis (MS)
. Treatment with Avonex can help to prevent you
from getting worse, although it will not cure MS.
Everyone has their own set of MS symptoms.
These can include:
-
feeling off-balance or light headed, walking problems, stiffness and muscle spasms, tiredness,
numbness in the face, arms or legs
-
acute or chronic pain, bladder and bowel problems, sexual problems and problems seeing things
-
difficulty in thinking and concentrating, depression.
MS also tends to flare up from time to time: this is called a relapse.




Avonex works best when you use it at the same time, once a week, on a regular basis.
Do not stop your Avonex treatment without speaking to your neurologist.
Avonex can help to reduce the number of relapses that you have and slow down the disabling
effects of MS.
Your doctor will advise you for how long you can use Avonex or when to stop.
Multiple sclerosis is linked to nerve (brain or spinal cord) damage. In MS, your body’s defence system
reacts against it’s own myelin – the ‘insulation’ that surrounds nerve fibres. When myelin is damaged,
the messages between the brain and other parts of the body are disrupted. This is what causes the
symptoms of MS. Avonex seems to work by stopping your body’s defence system from attacking the
myelin.
if
you
are
allergic (hypersensitive)
to interferon beta, or any other of the other ingredients in
Avonex
if
you
are
pregnant
do not start using Avonex
if you have severe depression
or think about committing suicide.
Talk to a doctor straight away if any of these apply to you.
Avonex and allergic reactions.
Because Avonex is based on a protein, there is a small chance of an
allergic reaction.
More about depression.
If you have severe depression or thoughts about suicide, you must not use
Avonex.
If you have depression, your doctor may still prescribe Avonex for you, but it's important to let your
doctor know if you have had depression or any similar problems affecting your moods.
Take special care with AVONEX
Talk to your doctor first:
If you have or have had in the past:
-
depression
or problems affecting your moods
thoughts about committing suicide.
Changes to your mood, thoughts about suicide, feeling unusually sad, anxious or worthless, should be
reported to your doctor immediately.
epilepsy
or other seizure disorders not controlled by medication
serious kidney or liver problems
a low number of white blood cells or platelets,
which can cause an increased risk of infection,
bleeding or anaemia







-
heart problems,
which can cause symptoms such as chest pain
(angina)
, particularly after any
activity; swollen ankles, shortness of breath
(congestive heart failure)
; or an irregular heartbeat
(arrhythmias).
Talk to your doctor
if you have any of these conditions
, or if they worsen whilst taking Avonex.
Tell your doctor you are using AVONEX:
-
If you are having a blood test. Avonex may interfere with the results.
Sometimes you will need to remind other medical staff that you are being treated with Avonex. For
example, if you are prescribed other medicines, or if you have a blood test, Avonex may affect the
other medicines or the test result.
If you are using any other medicines, especially those used to treat epilepsy or depression. Avonex
may affect other medicines or be affected by them. This includes any other medicines including
medicines obtained without a prescription.
Pregnancy and breastfeeding
If you are pregnant do not start using Avonex.
-
If you could get pregnant,
you need to use contraception while you use Avonex.
-
If you are planning a baby or if you become pregnant
while you are using Avonex, tell your
doctor. You and your doctor can discuss if you should carry on with treatment.
-
If you are already pregnant,
or think that you might be, talk to a doctor as soon as you can.
-
If you want to breastfeed
talk to your doctor first.
Driving and using machines
If you feel dizzy, do not drive.
Avonex makes some people feel dizzy. If this happens to you, or if
you get any other side effects that could affect your ability, do not drive or use machines.
Important information about some of the ingredients of AVONEX
This medicine is essentially ‘sodium-free’. It contains less than 23 mg (1 mmol) sodium in each
weekly dose.
The usual dose for adults and adolescents aged 12 years and over
One injection of Avonex, once a week.
Try to use Avonex at the same time on the same day each week.
Not for children
Avonex is
not to be used
in children below the age of 12 years.
If you have decided to start treatment with Avonex, your doctor may recommend that you use about
half the dose of Avonex initially,
before increasing to the full dose. Your doctor
may
provide you with
an Avostartclip that attaches onto the syringe and reduces the dose of Avonex that will be injected.




Starting Avonex
If you are new to Avonex, your doctor may have advised that you start by taking a lower dose so that
you can adjust to the effects of Avonex
before increasing to the full dose. You
may b
e given an
Avostartclip. This Avostartclip will attach onto the syringe and allow about half a dose of Avonex to
be administered. For further details on use, please follow the instructions provided with the
Avostartclip.
You can inject Avonex yourself without the help of your doctor, if they have trained you to do this.
The instructions on how to inject yourself are at the end of this leaflet (see section 7
, How to inject
AVONEX
).
If you have trouble
handling the syringe, ask your doctor who may be able to help.
There are more details on how to inject Avonex
at the end of this leaflet.
Alternate needle:
Your pack of Avonex already includes a needle for injection. It may be possible for your doctor to
prescribe you a shorter and thinner needle, depending on your body type. Talk to your doctor to see if
this is appropriate for you.
If you have problems handling the syringe
, talk to your doctor about using a syringe grip. This is a
specially designed holder to help you with injecting Avonex.
Your doctor will tell you how long you need to keep using Avonex. It is important to continue using
Avonex regularly. Do not make changes unless your doctor tells you.
You should only have one injection of Avonex, once a week. If you have used more than one injection
of Avonex in a three-day period,
contact your doctor or pharmacist straight away for advice.
If you miss your usual weekly dose
, inject a dose as soon as you can. Then leave a week before using
Avonex again. Continue injecting on this new day every week. If you have a preferred day for using
Avonex, talk to your doctor about managing the dose, to get back to your preferred day.
Do not use two injections to make up for a missed injection.
Like all medicines, Avonex can cause side effects, although not everybody gets them.







Although the list of possible side effects can seem worrying, it’s possible that you may not have any of
them.
Serious side effects: get medical help
Serious allergic reactions
If you get any of these:
-
swelling of the face, lips or tongue
-
difficulty breathing
-
a rash.
Call a doctor immediately
. Do not use any more Avonex until you have spoken to a doctor.
Depression
If you get any symptoms of depression:
-
feeling unusually sad, anxious or worthless.
Call a doctor immediately.
Liver problems
If you get any of these symptoms:
-
yellowing of your skin or the whites of your eyes
(jaundice)
-
itching all over
-
feeling sick, being sick
(nausea and vomiting)
-
easy bruising of the skin.
Call a doctor immediately
as they may be signs of a possible liver problem.
Side effects seen in clinical trials
Side effects seen in clinical trials.
These are the side effects that people reported when Avonex was
being tested. The figures are based on how many people said they’d had them. It gives you an idea
how likely you are to get similar side effects.
Very common side effects
(at least 1 in 10 people are affected)
-
flu-like symptoms – headache, muscle aches, chills or a fever: see
Flu-like symptoms
, below
Common side effects
(less than 1 in 10 people are affected)
-
feeling or being sick
(nausea or vomiting)
numbness or tingling of skin
rash, bruising of the skin









increased sweating, night sweats
pain in your muscles, joints, arms, legs or neck
muscle cramps, stiffness in the joints and muscles
pain, bruising and redness at the injection site
changes to blood tests. Symptoms you might notice are tiredness, repeated infection,
unexplained bruising or bleeding.
Uncommon side effects
(less than 1 in 100 people affected)
-
changes to your monthly period
burning feeling at the site of injection.
Rare side effects
(less than 1 in 1,000 people affected)
-
If any of the effects trouble you, talk to your doctor.
These effects
have been seen in people using Avonex, but we do not know how likely they are to
happen.
-
an underactive or overactive thyroid
-
nervousness or anxiety, emotional instability, irrational thoughts or hallucinations (seeing or
hearing things that are not real), confusion or suicide
-
numbness, dizziness, seizures or fits and migraines
-
an awareness of your heartbeat (
palpitations
), a rapid or irregular heartbeat, or heart problems
which would have the following symptoms: a reduced ability to exercise, inability to lie flat in
bed, shortness of breath or swollen ankles
-
liver problems as described above
-
nettle rash or blister-like rash, itching, worsening of psoriasis if you have it
-
swelling or bleeding at the site of injection, or chest pain after an injection
-
gaining or losing weight
-
changes to test results, including changes to liver function tests.
If any of the effects trouble you, talk to your doctor.
Effects of the injection
-
Feeling faint:
Your first injection of Avonex may be given by your doctor. It may make you
feel faint. You may even actually faint. This is unlikely to happen again.
Just after an injection, your muscles may feel tense or very weak
– as though you are having
a relapse. This is rare. It only happens when you inject and the effects soon pass. They may
happen any time after starting on Avonex.
If you notice any irritation or skin problems
after an injection, talk to your doctor.




Three simple ways to help reduce the impact of flu-like symptoms:
1.
Use your Avonex injection just before bedtime
. This may allow you to sleep through the
effects.
2.
Take paracetamol or ibuprofen half an hour
before your Avonex injection and continue
taking
it for up to a day. Speak to your doctor or pharmacist about a suitable dose.
3.
If you have a fever, drink plenty of water
to keep you hydrated.
Some people find that after injecting Avonex, they feel like they have flu
. Signs are:
-
These symptoms are not really flu.
You can’t pass it on to anyone else. They are more common when you first start using Avonex. As
you keep using your injections, the flu-like symptoms gradually decrease.
Keep out of the reach and sight of children.
Do not use after the expiry date stated on the label.
Store in the original package (sealed plastic tray) in order to protect from light.
Store in the fridge (between 2 °C and 8 °C). Do not freeze.
Avonex can also be stored at room temperature (between 15 °C and 30 °C) for up to one week.
Do NOT use AVONEX
if you notice:
-
The pre-filled syringe is broken.
The sealed plastic tray is damaged or opened.
The solution is coloured or you can see particles floating in it.
The tamper evident cap has been broken.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active ingredient
is: Interferon-beta 1a 30 micrograms/0.5 ml
The other ingredients
are: Sodium acetate, trihydrate; acetic acid glacial, arginine hydrochloride,
polysorbate 20 and water for injections.
What is in your Avonex pack
Avonex Solution for Injection comes as a ready to use injection.
In a box of Avonex there are four or twelve ready to use (pre-filled) syringes, each with 0.5 ml of a
clear, colourless liquid inside. Not all pack sizes may be marketed. Each syringe is packed in a sealed
plastic tray. A separate needle to give the injection is also included in the tray.




Marketing Authorisation Holder is:
Biogen Idec Limited, Innovation House, 70 Norden Road, Maidenhead, Berkshire, SL6 4AY, United
Kingdom.
Biogen Idec BV, Robijnlaan 8, NL-2132 WX Hoofddorp, The Netherlands.
Biogen Idec Denmark Manufacturing ApS, Biogen Idec Allé 1, DK-3400 Hillerød, Denmark
You can get a larger print version of this leaflet by calling the local representatives.
For any further information about this medicine, please contact the local representative of the
Marketing Authorisation Holder.
België/Belgique/Belgien
Biogen Idec Belgium N.V./S.A.
+32 2 2191218
Luxembourg/Luxemburg
Biogen Idec Belgium N.V./S.A.
+32 2 2191218
България
ТП ЕВОФАРМА
+359 2 962 12 00
Magyarország
Gedeon Richter Plc.
+36 1 432 6097
Česká republika
Biogen Idec Czech Republic
+420 222 191 640
Malta
Interpharma Co. Ltd.
+356 21354582
Danmark
Biogen Idec Denmark A/S
+45 77 41 57 57
Nederland
Biogen Idec International B.V.
+31 20 542 2000
Deutschland
Biogen Idec GmbH
+49 (0) 89 99 6170
Norge
Biogen Idec Norway AS
+47 23 00 52 50
Eesti
Richter Gedeon Eesti filiaal
+372 742 7056
Österreich
Biogen Idec Austria GmbH.
+43 1 484 46 13
Ελλάδα
Genesis Pharma SA
+30 210 8771500
Polska
Gedeon Richter Plc. S.A.
Przedstawicielstwo w Polsce.
+48 (22) 642-67-39
España
Biogen Idec Iberia SL
+34 91 310 7110
Portugal
Biogen Idec Portugal,
Sociedade Unipessoal, Lda.
+351 21 318 8450
France
Biogen Idec France
+33 (0)1 41 37 9595
România
MEDISON PHARMA SRL
+ 40 31 7104035
Ireland
Biogen Idec (Ireland) Ltd.
+353 (0)1 463 7799
Slovenija
Biogen Idec d.o.o.
+386 1 589 91 04
Ísland
Icepharma hf
+354 540 8000
Slovenská republika
Biogen Idec (Slovak Republic) s.r.o
+421 2 32410188
Italia
Biogen-Dompé s.r.l.
+39 02 583 831
Suomi/Finland
Biogen Idec Finland Oy
+358 207 401 200
Κύπρος
Genesis Pharma Cyprus Ltd
+3572 2 769946
Sverige
BiogenIdec Sweden AB
+46 8 594 113 60
Latvija
Gedeon Richter Plc.
+36 1 432 6097
United Kingdom
Biogen Idec Limited
+44 (0) 1628 50 1000
Lietuva
Gedeon Richter Plc.
+36 1 432 6097
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency (EMEA) web
site: http://www.emea.europa.eu
You should have had training in how to inject Avonex
These notes are a reminder. If there’s anything you’re not sure about, check with your doctor or
pharmacist.
Avonex is injected into a muscle,
for example, the upper thigh muscle. Injection of Avonex
into the buttocks is not recommended.
Use a different injection site each week
. This means less risk of irritation to your skin and
muscle.
Do not use
any area of skin that is bruised, sore, or infected, or if there is an open wound.
1.
Remove one sealed plastic tray from the refrigerator
-
Check the expiry date on the lid of the tray. Do not use it if it is out-of-date.
Peel back the paper lid completely. Check the blister tray contains one pre-filled syringe
and one injection needle (see picture “Contents of the plastic tray”).
2.
Leave the syringe to warm up
-
Leave it at room temperature for half an hour. This makes the injection more
comfortable than injecting straight from the fridge.
Tip:
Do not use external heat sources such as hot water to warm the syringe.
3.
Wash your hands thoroughly
with soap and water and dry them.
4.
Prepare alcohol wipes and sticking plasters
(not supplied) if you need them.
Find a clean, hard surface to lay out the items
needed for your injection. Lay the tray down
on it.
B. Preparing the injection
Check the liquid in the syringe
It should be clear and colourless.
If the solution is cloudy, coloured
or contains any floating particles, do not use the pre-filled syringe.
Remove the syringe cap
The syringe has a white tamper-evident cap.
Make sure the cap is intact and has not been opened.
If it looks like it has been opened, do not use that syringe.
Hold the syringe so that the white cap is facing up.
Bend the cap at a right angle until it snaps off.
Do not touch the connection port.
Do not push on the plunger.



Fit the needle
Open needle to expose the connection port. Keep the cover on.
Press the needle onto the syringe.
Turn it clockwise until it locks into place.
Tip:
Make sure the injection needle is firmly attached to the syringe.
Otherwise it may leak.
If you have been prescribed a half dose of Avonex, you may need to
use the Avostartclip your doctor will provide.
Please follow the instructions provided with the Avostartclip.
Now pull off the plastic needle cover.
Do not twist it.
Tip:
If you twist the needle cover to remove it, you may accidentally
remove the needle as well.

Clean and stretch the injection site
If you need to, use an alcohol wipe to clean the skin at the injection
site you’ve chosen. Allow the skin to dry.
With one hand, stretch the skin around the injection site.
Relax your muscle.
Make the injection
Insert the injection needle with a quick dart-like thrust
at right
angles to the skin, into the muscle
.
The needle must go all the way in.
Press the plunger slowly until the syringe is empty.
If you are using the syringe that has the Avostartclip attached, you will
receive about half the dose of Avonex.
The syringe will not empty.
Pull the needle out
Keep the skin stretched tightly or squeeze the skin around the injection
site, and pull out the needle.
If you use alcohol wipes, hold one on the injection site.
Put a plaster over the site of injection if you need to.
Dispose of the rubbish properly
After you have finished each injection, put the needle and syringe into a
special container (such as a sharps bin), not in ordinary rubbish.
If you have used the Avostartclip, the syringe (and the Avostartclip) must
be thrown away afterwards. The unused portion of Avonex
must not
be
re-used.
Waste paper and used wipes can be put in an ordinary rubbish bin.



Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/avonex.html
Copyright © 1995-2021 ITA all rights reserved.
|





































































































































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































