Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Axura 10 mg film-coated tablets
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each film-coated tablet contains 10 mg of memantine hydrochloride equivalent to 8.31 mg memantine.
For a full list of excipients, see section 6.1.
Film-coated tablet.
Pale yellow to yellow, oval shaped film-coated tablet with breaking line and engravings "1-0" on one
side and "M M" on the other side
The tablet can be divided into equal halves.
4.1 Therapeutic indications
Treatment of patients with moderate to severe Alzheimer’s disease.
4.2 Posology and method of administration
Treatment should be initiated and supervised by a physician experienced in the diagnosis and
treatment of Alzheimer’s dementia. Therapy should only be started if a caregiver is available who will
regularly monitor the intake of the medicinal product by the patient. Diagnosis should be made
according to current guidelines.
Axura should be administered once a day and should be taken at the same time every day. The
film-coated tablets can be taken with or without food.
Adults:
Dose titration
The maximum daily dose is 20 mg per day. In order to reduce the risk of undesirable effects the
maintenance dose is achieved by upward titration of 5 mg per week over the first 3 weeks as follows:
Week 1 (day 1-7):
The patient should take half a 10 mg film-coated tablet (5 mg) per day for 7 days.
Week 2 (day 8-14):
The patient should take one 10 mg film-coated tablet (10 mg) per day for 7 days.
Week 3 (day 15-21):
The patient should take one and a half 10 mg film-coated tablet (15 mg) per day for 7 days.
From Week 4 on:
The patient should take two 10 mg film-coated tablets (20 mg) per day.
Maintenance dose
The recommended maintenance dose is 20 mg per day.
Elderly:
On the basis of the clinical studies, the recommended dose for patients over the age of 65 years
is 20 mg per day (two 10 mg tablets once a day) as described above.
Children and adolescents :
Axura is not recommended for use in children below 18 years due to a lack
of data on safety and efficacy.
Renal impairment:
In patients with mildly impaired renal function (creatinine clearance 50 -
80 ml/min) no dose adjustment is required. In patients with moderate renal impairment (creatinine
clearance 30 - 49 ml/min) daily dose should be 10 mg per day. If tolerated well after at least 7 days of
treatment, the dose could be increased up to 20 mg/day according to standard titration scheme. In
patients with severe renal impairment (creatinine clearance 5 – 29 ml/min) daily dose should be 10 mg
per day.
Hepatic impairment:
In patients with mild or moderate hepatic impaired function (Child-Pugh A and
Child-Pugh B) no dose adjustment is needed. No data on the use of memantine in patients with severe
hepatic impairment are available. Administration of Axura is not recommended in patients with severe
hepatic impairment.
Hypersensitivity to the active substance or to any of the excipients.
4.4 Special warnings and precautions for use
Caution is recommended in patients with epilepsy, former history of convulsions or patients with
predisposing factors for epilepsy.
Concomitant use of N-methyl-D-aspartate(NMDA)-antagonists such as amantadine, ketamine or
dextromethorphan should be avoided. These compounds act at the same receptor system as
memantine, and therefore adverse reactions (mainly central nervous system (CNS)-related) may be
more frequent or more pronounced (see also section 4.5).
Some factors that may raise urine pH (see section 5.2 “Elimination”) may necessitate careful
monitoring of the patient. These factors include drastic changes in diet, e.g. from a carnivore to a
vegetarian diet, or a massive ingestion of alkalising gastric buffers. Also, urine pH may be elevated by
states of renal tubulary acidosis (RTA) or severe infections of the urinary tract with
Proteus bacteria
.
In most clinical trials, patients with recent myocardial infarction, uncompensated congestive heart
failure (NYHA III-IV), or uncontrolled hypertension were excluded. As a consequence, only limited
data are available and patients with these conditions should be closely supervised.
4.5 Interaction with other medicinal products and other forms of interaction
Due to the pharmacological effects and the mechanism of action of memantine the following
interactions may occur:
The mode of action suggests that the effects of L-dopa, dopaminergic agonists, and
anticholinergics may be enhanced by concomitant treatment with NMDA-antagonists such as
memantine. The effects of barbiturates and neuroleptics may be reduced. Concomitant
administration of memantine with the antispasmodic agents, dantrolene or baclofen, can modify
their effects and a dose adjustment may be necessary.
Concomitant use of memantine and amantadine should be avoided, owing to the risk of
pharmacotoxic psychosis. Both compounds are chemically related NMDA-antagonists. The
same may be true for ketamine and dextromethorphan (see also section 4.4). There is one
published case report on a possible risk also for the combination of memantine and phenytoin.
Other active substances such as cimetidine, ranitidine, procainamide, quinidine, quinine and
nicotine that use the same renal cationic transport system as amantadine may also possibly
interact with memantine leading to a potential risk of increased plasma levels.
There may be a possibility of reduced serum level of hydrochlorothiazide (HCT) when
memantine is co-administered with HCT or any combination with HCT.
In post-marketing experience isolated cases with international normalized ratio (INR) increases
have been reported in patients concomitantly treated with warfarin. Although no causal
relationship has been established, close monitoring of prothrombin time or INR is advisable for
patients concomitantly treated with oral anticoagulants.
In single-dose pharmacokinetic (PK) studies in young healthy subjects no relevant active substance-
active substance interaction of memantine with glyburide/metformin or donepezil was observed.
In a clinical study in young healthy subjects no relevant effect of memantine on the pharmacokinetics
of galantamine was observed.
Memantine did not inhibit CYP 1A2, 2A6, 2C9, 2D6, 2E1, 3A, flavin containing monooxygenase,
epoxide hydrolase or sulphation
in vitro
.
4.6 Pregnancy and lactation
For memantine, no clinical data on exposed pregnancies are available. Animal studies indicate a
potential for reducing intrauterine growth at exposure levels, which are identical or slightly higher
than at human exposure (see section 5.3). The potential risk for humans is unknown. Memantine
should not be used during pregnancy unless clearly necessary.
It is not known whether memantine is excreted in human breast milk but, taking into consideration the
lipophilicity of the substance, this probably occurs. Women taking memantine should not breast-feed.
4.7 Effects on ability to drive and use machines
Moderate to severe Alzheimer’s disease usually causes impairment of driving performance and
compromises the ability to use machinery. Furthermore, Axura has minor to moderate influence on the
ability to drive and use machines such that outpatients should be warned to take special care .
In clinical trials in mild to severe dementia, involving 1,784 patients treated with Axura and 1,595
patients treated with placebo, the overall incidence rate of adverse reactions with Axura did not differ
from those with placebo; the adverse reactions were usually mild to moderate in severity. The most
frequently occurring adverse reactions with a higher incidence in the Axura group than in the placebo
group were dizziness (6.3% vs 5.6%, respectively), headache (5.2% vs 3.9%), constipation (4.6% vs
2.6%), somnolence (3.4% vs 2.2%) and hypertension (4.1% vs 2.8%).
The following Adverse Reactions listed in the Table below have been accumulated in clinical studies
with Axura and since its introduction in the market. Within each frequency grouping, undesirable
effects are presented in order of decreasing seriousness.
Adverse reactions are ranked according to system organ class, using the following convention: very
common (≥ 1/10), common (≥1/100 to < 1/10), uncommon (≥ 1/1,000 to < 1/100), rare (≥1/10,000 to
< 1/1,000), very rare (< 1/10,000), not known (cannot be estimated from the available data).
Infections and infestations
Venous
thrombosis/thromboembolism
Respiratory, thoracic and mediastinal
disorders
Gastrointestinal disorders
General disorders and administration
site conditions
1
Hallucinations have mainly been observed in patients with severe Alzheimer´s disease.
2
Isolated cases reported in post-marketing experience
Alzheimer’s disease has been associated with depression, suicidal ideation and suicide. In post-
marketing experience these events have been reported in patients treated with Axura.
Only limited experience with overdose is available from clinical studies and post-marketing
experience.
Symptoms
:
Relative large overdoses (200 mg and 105 mg/day for 3 days, respectively) have been
associated with either only symptoms of tiredness, weakness and/or diarrhoea or no symptoms. In the
overdose cases below 140 mg or unknown dose the patients revealed symptoms from central nervous
system (confusion, drowsiness, somnolence, vertigo, agitation, aggression, hallucination, and gait
disturbance) and/or gastrointestinal origin (vomiting and diarrhoea).











In the most extreme case of overdose, the patient survived the oral intake of a total of 2000 mg
memantine with effects on the central nervous system (coma for 10 days, and later diplopia and
agitation). The patient received symptomatic treatment and plasmapheresis. The patient recovered
without permanent sequelae.
In another case of a large overdose, the patient also survived and recovered. The patient had received
400 mg memantine orally. The patient experienced central nervous system symptoms such as
restlessness, psychosis, visual hallucinations, proconvulsiveness, somnolence, stupor, and
unconsciousness.
Treatment
:
In the event of overdose, treatment should be symptomatic. No specific antidote for
intoxication or overdose is available. Standard clinical procedures to remove active substance material,
e.g. gastric lavage, carbo medicinalis (interruption of potential entero-hepatic recirculation),
acidification of urine, forced diuresis should be used as appropriate.
In case of signs and symptoms of general central nervous system (CNS) overstimulation, careful
symptomatic clinical treatment should be considered.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Other Anti-dementia drugs, ATC code: N06DX01.
There is increasing evidence that malfunctioning of glutamatergic neurotransmission, in particular at
NMDA-receptors, contributes to both expression of symptoms and disease progression in
neurodegenerative dementia.
Memantine is a voltage-dependent, moderate-affinity uncompetitive NMDA-receptor antagonist. It
modulates the effects of pathologically elevated tonic levels of glutamate that may lead to neuronal
dysfunction.
Clinical studies:
A pivotal monotherapy study in a population of patients suffering from moderate to severe
Alzheimer’s disease (mini mental state examination (MMSE) total scores at baseline of 3 - 14)
included a total of 252 outpatients. The study showed beneficial effects of memantine treatment in
comparison to placebo at 6 months (observed cases analysis for the clinician´s interview based
impression of change (CIBIC-plus): p=0.025; Alzheimer´s disease cooperative study – activities of
daily living (ADCS-ADLsev): p=0.003; severe impairment battery (SIB): p=0.002).
A pivotal monotherapy study of memantine in the treatment of mild to moderate Alzheimer’s disease
(MMSE total scores at baseline of 10 to 22) included 403 patients. Memantine-treated patients showed
a statistically significantly better effect than placebo-treated patients on the primary endpoints:
Alzheimer´s disease assessment scale (ADAS-cog) (p=0.003) and CIBIC-plus (p=0.004) at week 24
last observation carried forward (LOCF). In another monotherapy study in mild to moderate
Alzheimer’s disease a total of 470 patients (MMSE total scores at baseline of 11-23) were randomised.
In the prospectively defined primary analysis statistical significance was not reached at the primary
efficacy endpoint at week 24.
A meta-analysis of patients with moderate to severe Alzheimer’s disease (MMSE total scores < 20)
from the six phase III, placebo-controlled, 6-month studies (including monotherapy studies
and studies
with patients on a stable dose of acetylcholinesterase inhibitors) showed that there was a statistically
significant effect in favour of memantine treatment for the cognitive, global, and functional domains.
When patients were identified with concurrent worsening in all three domains, results showed a
statistically significant effect of memantine in preventing worsening, as twice as many placebo-treated
patients as memantine-treated patients showed worsening in all three domains (21% vs. 11%,
p0.0001).
5.2 Pharmacokinetic properties
Absorption:
Memantine has an absolute bioavailability of approximately 100%. t
max
is between 3 and
8 hours. There is no indication that food influences the absorption of memantine.
Distribution:
Daily doses of 20 mg lead to steady-state plasma concentrations of memantine ranging
from 70 to 150 ng/ml (0.5 - 1 µmol) with large interindividual variations. When daily doses of 5 to
30 mg were administered, a mean cerebrospinal fluid (CSF)/serum ratio of 0.52 was calculated. The
volume of distribution is around 10 l/kg. About 45% of memantine is bound to plasma-proteins.
Biotransformation:
In man, about 80% of the circulating memantine-related material is present as the
parent compound. Main human metabolites are N-3,5-dimethyl-gludantan, the isomeric mixture of 4-
and 6-hydroxy-memantine, and 1-nitroso-3,5-dimethyl-adamantane. None of these metabolites exhibit
NMDA-antagonistic activity. No cytochrome P 450 catalysed metabolism has been detected
in vitro.
In a study using orally administered
14
C-memantine, a mean of 84% of the dose was recovered within
20 days, more than 99% being excreted renally.
Elimination:
Memantine is eliminated in a monoexponential manner with a terminal t
½
of 60 to
100 hours. In volunteers with normal kidney function, total clearance (Cl
tot
) amounts to
170 ml/min/1.73 m² and part of total renal clearance is achieved by tubular secretion.
Renal handling also involves tubular reabsorption, probably mediated by cation transport proteins. The
renal elimination rate of memantine under alkaline urine conditions may be reduced by a factor of 7 to
9 (see section 4.4). Alkalisation of urine may result from drastic changes in diet, e.g. from a carnivore
to a vegetarian diet, or from the massive ingestion of alkalising gastric buffers.
Linearity:
Studies in volunteers have demonstrated linear pharmacokinetics in the dose range of 10 to
40 mg.
Pharmacokinetic/pharmacodynamic relationship:
At a dose of memantine of 20 mg per day the CSF
levels match the k
i
-value (k
i
= inhibition constant) of memantine, which is 0.5 µmol in human frontal
cortex.
5.3 Preclinical safety data
In short term studies in rats memantine like other NMDA-antagonists have induced neuronal
vacuolisation and necrosis (Olney lesions) only after doses leading to very high peak serum
concentrations. Ataxia and other preclinical signs have preceded the vacuolisation and necrosis. As the
effects have neither been observed in long term studies in rodents nor in non-rodents, the clinical
relevance of these findings is unknown.
Ocular changes were inconsistently observed in repeat dose toxicity studies in rodents and dogs, but
not in monkeys. Specific ophthalmoscopic examinations in clinical studies with memantine did not
disclose any ocular changes.
Phospholipidosis in pulmonary macrophages due to accumulation of memantine in lysosomes was
observed in rodents. This effect is known from other active substances with cationic amphiphilic
properties. There is a possible relationship between this accumulation and the vacuolisation observed
in lungs. This effect was only observed at high doses in rodents. The clinical relevance of these
findings is unknown.
No genotoxicity has been observed following testing of memantine in standard assays. There was no
evidence of any carcinogenicity in life long studies in mice and rats. Memantine was not teratogenic in
rats and rabbits, even at maternally toxic doses, and no adverse effects of memantine were noted on
fertility. In rats, foetal growth reduction was noted at exposure levels, which are identical or slightly
higher than at human exposure.
PHARMACEUTICAL PARTICULARS
Tablet core:
Microcrystalline cellulose
Croscarmellose sodium
Colloidal anhydrous silica
Magnesium stearate
Hypromellose
Macrogol 400
Titanium dioxide (E 171)
Iron oxide yellow (E 172)
6.4 Special precautions for storage
This medicinal product does not require any special storage conditions.
6.5 Nature and contents of container
Blister packs containing either 7,
10, 14 or 20 tablets per blister strip (Alu/PP). Pack sizes of 14, 28,
30, 42, 50, 56, 98, 100, 112, 980 (10 x 98) or 1000 (20 x 50) tablets are presented.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
MARKETING AUTHORISATION HOLDER
Merz Pharmaceuticals GmbH
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany
MARKETING AUTHORISATION NUMBER(S)
EU/1/02/218/002
EU/1/02/218/003
EU/1/02/218/007
EU/1/02/218/008
EU/1/02/218/009
EU/1/02/218/010
EU/1/02/218/012
EU/1/02/218/013
EU/1/02/218/014
EU/1/02/218/015
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 17/05/2002
Date of latest renewal: 17/05/2007
10.
DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency
(EMA) http://www.ema.europa.eu
NAME OF THE MEDICINAL PRODUCT
Axura 10 mg/g oral drops, solution.
QUALITATIVE AND QUANTITATIVE COMPOSITION
1 g of solution contains 10 mg of memantine hydrochloride equivalent to 8.31 mg memantine.
Each activation of the pump (one downward stroke) delivers 0.5 ml (0.5g) of solution containing 5 mg of
memantine hydrochloride equivalent to 4.16 mg of memantine
Excipients: Each one gram of solution contains 100 mg sorbitol (E420) and 0.5 mg potassium, see
section 4.4.
For a full list of excipients, see section 6.1.
Oral drops, solution.
The solution is clear and colourless to light yellowish.
4.1 Therapeutic indications
Treatment of patients with moderate to severe Alzheimer’s disease.
4.2 Posology and method of administration
Treatment should be initiated and supervised by a physician experienced in the diagnosis and
treatment of Alzheimer’s dementia. Therapy should only be started if a caregiver is available who will
regularly monitor the intake of the medicinal product by the patient. Diagnosis should be made
according to current guidelines.
Axura should be taken once daily at the same time each day. The solution can be taken with or without
food. The solution must not be poured or pumped into the mouth directly from the bottle or the pump,
but should be dosed onto a spoon or into a glass of water using the pump. For detailed instructions on
the preparation and handling of the product see section 6.6.
Adults:
Dose titration
The maximum daily dose is 20 mg once daily. In order to reduce the risk of undesirable effects the
maintenance dose is achieved by upward titration of 5 mg per week over the first 3 weeks as follows:
Week 1 (day 1-7):
The patient should take 0.5 ml solution (5 mg) equivalent to one downward stroke per day for 7 days.
Week 2 (day 8-14):
The patient should take 1 ml solution (10 mg) equivalent to two downward strokes per day for 7 days.
Week 3 (day 15-21):
The patient should take 1.5 ml solution (15 mg) equivalent to three downward strokes per day for
7 days.
From Week 4 on:
The patient should take 2 ml solution (20 mg) equivalent to four downward strokes once a day.
Maintenance dose
The recommended maintenance dose is 20 mg (2 ml solution, equivalent to four downward strokes) per
day.
Elderly:
On the basis of the clinical studies, the recommended dose for patients over the age of 65 years
is 20 mg per day (2 ml solution, equivalent to four downward strokes) as described above.
Children and adolescents:
Axura is not recommended for use in children below 18 years due to a lack of
data on safety and efficacy.
Renal impairment:
In patients with mildly impaired renal function (creatinine clearance 50 -
80 ml/min) no dose adjustment is required. In patients with moderate renal impairment (creatinine
clearance 30 - 49 ml/min) daily dose should be 10 mg (1 ml solution, equivalent to two downward
strokes).. If tolerated well after at least 7 days of treatment, the dose could be increased up to 20
mg/day according to standard titration scheme. In patients with severe renal impairment (creatinine
clearance 5 – 29 ml/min) daily dose should be 10 mg (1 ml solution, equivalent to two downward
strokes) per day.
Hepatic impairment:
In patients with mild or moderate hepatic impaired function (Child-Pugh A and
Child-Pugh B) no dose adjustment is needed. No data on the use of memantine in patients with severe
hepatic impairment are available. Administration of Axura in patients with severe hepatic impairment
is not recommended.
Hypersensitivity to the active substance or to any of the excipients.
4.4 Special warnings and precautions for use
Caution is recommended in patients with epilepsy, former history of convulsions or patients with
predisposing factors for epilepsy.
Concomitant use of other N-methyl-D-aspartate (NMDA)-antagonists such as amantadine, ketamine
or dextromethorphan should be avoided. These compounds act at the same receptor system as
memantine, and therefore adverse reactions (mainly central nervous system (CNS)-related may be
more frequent or more pronounced (see also section 4.5).
Some factors that may raise urine pH (see section 5.2 “Elimination”) may necessitate careful
monitoring of the patient. These factors include drastic changes in diet, e.g. from a carnivore to a
vegetarian diet, or a massive ingestion of alkalising gastric buffers. Also, urine pH may be elevated by
states of renal tubulary acidosis (RTA) or severe infections of the urinary tract with
Proteus bacteria
.
In most clinical trials, patients with recent myocardial infarction, uncompensated congestive heart
failure (NYHA III-IV), or uncontrolled hypertension were excluded. As a consequence, only limited
data are available and patients with these conditions should be closely supervised.
Excipients:
The oral solution contains sorbitol. Patients with rare hereditary problems of fructose
intolerance should not take this medicine.
4.5 Interaction with other medicinal products and other forms of interaction
Due to the pharmacological effects and the mechanism of action of memantine the following
interactions may occur:
The mode of action suggests that the effects of L-dopa, dopaminergic agonists, and
anticholinergics may be enhanced by concomitant treatment with NMDA-antagonists such as
memantine. The effects of barbiturates and neuroleptics may be reduced. Concomitant
administration of memantine with the antispasmodic agents, dantrolene or baclofen, can modify
their effects and a dose adjustment may be necessary.
Concomitant use of memantine and amantadine should be avoided, owing to the risk of
pharmacotoxic psychosis. Both compounds are chemically related NMDA-antagonists. The
same may be true for ketamine and dextromethorphan (see also section 4.4). There is one
published case report on a possible risk also for the combination of memantine and phenytoin.
Other active substances such as cimetidine, ranitidine, procainamide, quinidine, quinine and
nicotine that use the same renal cationic transport system as amantadine may also possibly
interact with memantine leading to a potential risk of increased plasma levels.
There may be a possibility of reduced serum level of hydrochlorothiazide (HCT) when
memantine is co-administered with HCT or any combination with HCT.
In post-marketing experience isolated cases with international normalized ratio (INR) increases
have been reported in patients concomitantly treated with warfarin. Although no causal
relationship has been established, close monitoring of prothrombin time or INR is advisable for
patients concomitantly treated with oral anticoagulants.
In single-dose pharmacokinetic (PK) studies in young healthy subjects no relevant active
substance-active substance interaction of memantine with glyburide/metformin or donepezil was
observed.
In a clinical study in young healthy subjects no relevant effect of memantine on the pharmacokinetics
of galantamine was observed.
Memantine did not inhibit CYP 1A2, 2A6, 2C9, 2D6, 2E1, 3A, flavin containing monooxygenase,
epoxide hydrolase or sulphation
in vitro
.
4.6 Pregnancy and lactation
For memantine, no clinical data on exposed pregnancies are available. Animal studies indicate a
potential for reducing intrauterine growth at exposure levels, which are identical or slightly higher
than at human exposure (see section 5.3). The potential risk for humans is unknown. Memantine
should not be used during pregnancy unless clearly necessary.
It is not known whether memantine is excreted in human breast milk but, taking into consideration the
lipophilicity of the substance, this probably occurs. Women taking memantine should not breast-feed.
4.7 Effects on ability to drive and use machines
Moderate to severe Alzheimer’s disease usually causes impairment of driving performance and
compromises the ability to use machinery. Furthermore, Axura has minor or moderate influence on the
ability to drive and use machines such that outpatients should be warned to take special care.
In clinical trials in mild to severe dementia, involving 1,784 patients treated with Axura and 1,595
patients treated with placebo, the overall incidence rate of adverse reactions with Axura did not differ
from those with placebo; the adverse reactions were usually mild to moderate in severity. The most
frequently occurring adverse reactions with a higher incidence in the Axura group than in the placebo
group were dizziness (6.3% vs 5.6%, respectively), headache (5.2% vs 3.9%), constipation (4.6% vs
2.6%), somnolence (3.4% vs 2.2%) and hypertension (4.1% vs 2.8%).
The following Adverse Reactions listed in the Table below have been accumulated in clinical studies
with Axura and since its introduction in the market. Within each frequency grouping, undesirable
effects are presented in order of decreasing seriousness.
Adverse reactions are ranked according to system organ class, using the following convention: very
common (≥ 1/10), common (≥1/100 to < 1/10), uncommon (≥ 1/1,000 to < 1/100), rare (≥1/10,000 to
< 1/1,000), very rare (< 1/10,000), not known (cannot be estimated from the available data).
Infections and infestations
Venous
thrombosis/thromboembolism
Respiratory, thoracic and mediastinal
disorders
Gastrointestinal disorders
General disorders and administration
site conditions
1
Hallucinations have mainly been observed in patients with severe Alzheimer´s disease.
2
Isolated cases reported in post-marketing experience
Alzheimer’s disease has been associated with depression, suicidal ideation and suicide. In post-
marketing experience these events have been reported in patients treated with Axura.











Only limited experience with overdose is available from clinical studies and post-marketing
experience.
Symptoms:
Relative large overdoses (200 mg and 105 mg/day for 3 days, respectively) have been
associated with either only symptoms of tiredness, weakness and/or diarrhoea or no symptoms. In the
overdose cases below 140 mg or unknown dose the patients revealed symptoms from central nervous
system (confusion, drowsiness, somnolence, vertigo, agitation, aggression, hallucination and gait
disturbance) and/or gastrointestinal origin (vomiting and diarrhoea).
In the most extreme case of overdose, the patient survived the oral intake of a total of 2000 mg
memantine with effects on the central nervous system (coma for 10 days, and later diplopia and
agitation). The patient received symptomatic treatment and plasmapheresis. The patient recovered
without permanent sequelae.
In another case of a large overdose, the patient also survived and recovered. The patient had received
400 mg memantine orally. The patient experienced central nervous system symptoms such as
restlessness, psychosis, visual hallucinations, proconvulsiveness, somnolence, stupor, and
unconsciousness.
Treatment:
In the event of overdose, treatment should be symptomatic. No specific antidote for
intoxication or overdose is available. Standard clinical procedures to remove active substance material,
e.g. gastric lavage, carbo medicinalis (interruption of potential entero-hepatic recirculation),
acidification of urine, forced diuresis should be used as appropriate.
In case of signs and symptoms of general central nervous system (CNS) overstimulation, careful
symptomatic clinical treatment should be considered.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Other Anti-dementia drugs, ATC code: N06DX01.
There is increasing evidence that malfunctioning of glutamatergic neurotransmission, in particular at
NMDA-receptors, contributes to both expression of symptoms and disease progression in
neurodegenerative dementia.
Memantine is a voltage-dependent, moderate-affinity uncompetitive NMDA-receptor antagonist. It
modulates the effects of pathologically elevated tonic levels of glutamate that may lead to neuronal
dysfunction.
Clinical studies:
A pivotal monotherapy study in a population of patients suffering from moderate to severe
Alzheimer’s disease (mini mental state examination (MMSE) total scores at baseline of 3 - 14)
included a total of 252 outpatients. The study showed beneficial effects of memantine treatment in
comparison to placebo at 6 months (observed cases analysis for the clinician´s interview based
impression of change (CIBIC-plus): p=0.025; Alzheimer´s disease cooperative study – activities of
daily living (ADCS-ADLsev): p=0.003; severe impairment battery (SIB): p=0.002).
A pivotal monotherapy study of memantine in the treatment of mild to moderate Alzheimer’s disease
(MMSE total scores at baseline of 10 to 22) included 403 patients. Memantine-treated patients showed
a statistically significantly better effect than placebo-treated patients on the primary endpoints:
Alzheimer´s disease assessment scale (ADAS-cog) (p=0.003) and CIBIC-plus (p=0.004) at week 24
last observation carried forward (LOCF). In another monotherapy study in mild to moderate
Alzheimer’s disease a total of 470 patients (MMSE total scores at baseline of 11-23) were randomised.
In the prospectively defined primary analysis statistical significance was not reached at the primary
efficacy endpoint at week 24.
A meta-analysis of patients with moderate to severe Alzheimer’s disease (MMSE total scores < 20)
from the six phase III, placebo-controlled, 6-month studies (including monotherapy studies and studies
with patients on a stable dose of acetylcholinesterase inhibitors) showed that there was a statistically
significant effect in favour of memantine treatment for the cognitive, global, and functional domains.
When patients were identified with concurrent worsening in all three domains, results showed a
statistically significant effect of memantine in preventing worsening, as twice as many placebo-treated
patients as memantine-treated patients showed worsening in all three domains (21% vs. 11%,
p0.0001).
5.2 Pharmacokinetic properties
Absorption:
Memantine has an absolute bioavailability of approximately 100%. t
max
is between 3 and
8 hours. There is no indication that food influences the absorption of memantine.
Distribution:
Daily doses of 20 mg lead to steady-state plasma concentrations of memantine ranging
from 70 to 150 ng/ml (0.5 - 1 µmol) with large interindividual variations. When daily doses of 5 to
30 mg were administered, a mean cerebrospinal fluid (CSF)/serum ratio of 0.52 was calculated. The
volume of distribution is around 10 l/kg. About 45% of memantine is bound to plasma-proteins.
Biotransformation:
In man, about 80% of the circulating memantine-related material is present as the
parent compound. Main human metabolites are N-3,5-dimethyl-gludantan, the isomeric mixture of 4-
and 6-hydroxy-memantine, and 1-nitroso-3,5-dimethyl-adamantane. None of these metabolites exhibit
NMDA-antagonistic activity. No cytochrome P 450 catalysed metabolism has been detected
in vitro.
In a study using orally administered
14
C-memantine, a mean of 84% of the dose was recovered within
20 days, more than 99% being excreted renally.
Elimination:
Memantine is eliminated in a monoexponential manner with a terminal t
½
of 60 to
100 hours. In volunteers with normal kidney function, total clearance (Cl
tot
) amounts to
170 ml/min/1.73 m² and part of total renal clearance is achieved by tubular secretion.
Renal handling also involves tubular reabsorption, probably mediated by cation transport proteins. The
renal elimination rate of memantine under alkaline urine conditions may be reduced by a factor of 7 to
9 (see section 4.4). Alkalisation of urine may result from drastic changes in diet, e.g. from a carnivore
to a vegetarian diet, or from the massive ingestion of alkalising gastric buffers.
Linearity:
Studies in volunteers have demonstrated linear pharmacokinetics in the dose range of 10 to
40 mg.
Pharmacokinetic/pharmacodynamic relationship:
At a dose of memantine of 20 mg per day the CSF
levels match the k
i
-value (k
i
= inhibition constant) of memantine, which is 0.5 µmol in human frontal
cortex.
5.3 Preclinical safety data
In short term studies in rats memantine like other NMDA-antagonists have induced neuronal
vacuolisation and necrosis (Olney lesions) only after doses leading to very high peak serum
concentrations. Ataxia and other preclinical signs have preceded the vacuolisation and necrosis. As the
effects have neither been observed in long term studies in rodents nor in non-rodents, the clinical
relevance of these findings is unknown.
Ocular changes were inconsistently observed in repeat dose toxicity studies in rodents and dogs, but
not in monkeys. Specific ophthalmoscopic examinations in clinical studies with memantine did not
disclose any ocular changes.
Phospholipidosis in pulmonary macrophages due to accumulation of memantine in lysosomes was
observed in rodents. This effect is known from other active substances with cationic amphiphilic
properties. There is a possible relationship between this accumulation and the vacuolisation observed
in lungs. This effect was only observed at high doses in rodents. The clinical relevance of these
findings is unknown.
No genotoxicity has been observed following testing of memantine in standard assays. There was no
evidence of any carcinogenicity in life long studies in mice and rats. Memantine was not teratogenic in
rats and rabbits, even at maternally toxic doses, and no adverse effects of memantine were noted on
fertility. In rats, foetal growth reduction was noted at exposure levels, which are identical or slightly
higher than at human exposure.
PHARMACEUTICAL PARTICULARS
Potassium sorbate
Sorbitol E420
Purified water
4 years.
Once opened, the contents of the bottle should be used within 3 months.
6.4 Special precautions for storage
The bottle with the mounted pump may only be kept and transported in a vertical position.
.
6.5 Nature and contents of container
Brown glass bottles (Hydrolytic Class III) containing either 50, 100 g or 10 x 50 g solution.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
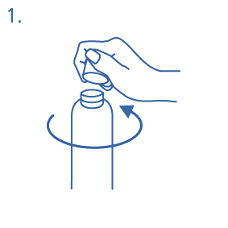
Prior to first use the dosing pump has to be screwed on the bottle. For removing the screw cap from
the bottle the cap must be turned anticlockwise and unscrewed completely (fig.1).
Mounting the dosing pump on the bottle:
The dosing pump has to be removed from the plastic bag (fig. 2) and placed on top of the bottle,
sliding the plastic dip tube carefully into the bottle. Then the dosing pump needs to be hold onto the
neck of the bottle and screwed clockwise until it is firmly attached (fig 3). For the intended use the
dosing pump is only screwed on once when starting the use, and should never be unscrewed.
Use of the dosing pump for dispensing:
The dosing pump head has two positions and is easy to turn – anticlockwise (unlocked position) and
clockwise (locked position). The dosing pump head should not be pushed down while in the locked
position. The solution may only be dispensed in the unlocked position. To do this, the dosing pump
head has to be turned in the direction of the arrow about one eighth of a turn, until a resistance is felt
(fig. 4)


The dosing pump is then ready for use.
Preparing the dosing pump:
When used for the first time, the dosing pump does not dispense the correct amount of oral solution.
Therefore, the pump must be prepared (primed) by pushing the dosing pump head down completely
five times in succession (fig. 5).
The solution thus dispensed is discarded. The next time the dosing pump head is pushed downwards
completely, it dispenses the correct dose (1 dosing unit/stroke is equivalent to 0.5 ml oral solution, and
contains about 5 mg of the active substance memantine hydrochloride; fig. 6).
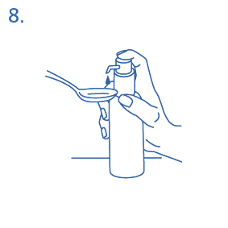
Correct use of the dosing pump:
The bottle should be placed on a flat, horizontal surface, for example a table top, and only use it in a
vertical position.. A glass with a little water or a spoon should be hold below the nozzle and the dosing
pump head has to be pushed down in a firm but calm and steady manner (not too slowly) right down to
the stop (fig. 7, fig. 8).



The dosing pump head can then be released and is ready for the next downward stroke.
The dosing pump may only be used with the memantine hydrochloride solution in the bottle provided,
not for other substances or containers. If the pump does not function as described during intended use
and according to instruction, the patient should consult the treating physician or a pharmacist. The
dosing pump should be locked after use.
MARKETING AUTHORISATION HOLDER
Merz Pharmaceuticals GmbH
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany
MARKETING AUTHORISATION NUMBER(S)
EU/1/02/218/005
EU/1/02/218/006
EU/1/02/218/011
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 17/05/2002
Date of latest renewal: 17/05/2007
10.
DATE OF REVISION OF THE TEXT
MM/YYYY
Detailed information on this product is available on the website of the European Medicines Agency
(EMA) http://www.ema.europa.eu

NAME OF THE MEDICINAL PRODUCT
Axura 5 mg film-coated tablets.
Axura 10 mg film-coated tablets
Axura 15 mg film-coated tablets.
Axura 20 mg film-coated tablets.
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each film-coated tablet contains 5 mg of memantine hydrochloride equivalent to 4.15 mg memantine.
Each film-coated tablet contains 10 mg of memantine hydrochloride equivalent to 8.31 mg memantine.
Each film-coated tablet contains 15 mg of memantine hydrochloride equivalent to 12.46 mg memantine.
Each film-coated tablet contains 20 mg of memantine hydrochloride equivalent to 16.62 mg memantine.
For a full list of excipients, see section 6.1.
Film-coated tablet.
The 5 mg film-coated tablets are white to off-white, oval-oblong film-coated tablets with imprint ‘5’ on
one side and imprint ‘MEM’ on the other side.
The 10 mg film-coated tablets are pale yellow to yellow, oval shaped film-coated tablet with breaking
line and engravings "1-0" on one side and "M M" on the other side The tablet can be divided into
equal halves.
The 15 mg film-coated tablets are orange to grey-orange, oval-oblong film-coated tablets with imprint
‘15’ on one side and imprint ‘MEM’ on the other side.
The 20 mg film-coated tablets are pale red to grey-red, oval-oblong film-coated tablets with imprint ‘20’
on one side and imprint ‘MEM’ on the other side.
4.1 Therapeutic indications
Treatment of patients with moderate to severe Alzheimer’s disease.
4.2 Posology and method of administration
Treatment should be initiated and supervised by a physician experienced in the diagnosis and
treatment of Alzheimer’s dementia. Therapy should only be started if a caregiver is available who will
regularly monitor the intake of the medicinal product by the patient. Diagnosis should be made
according to current guidelines.
Axura should be administered once a day and should be taken at the same time every day. The
film-coated tablets can be taken with or without food.
Adults:
Dose titration
The recommended starting dose is 5 mg per day which is stepwise increased over the first 4 weeks of
treatment reaching the recommended maintenance dose as follows:
Week 1 (day 1-7):
The patient should take one 5 mg film-coated tablet per day (white to off-white, oval-oblong) for 7 days.
Week 2 (day 8-14):
The patient should take one 10 mg film-coated tablet per day (pale yellow to yellow, oval shaped
)
for
7 days.
Week 3 (day 15-21):
The patient should take one 15 mg film-coated tablet per day (greyorange, oval-oblong) for 7 days.
Week 4 (day 22-28):
The patient should take one 20 mg film-coated tablet per day (grey-red, oval-oblong) for 7 days.
Maintenance dose
The recommended maintenance dose is 20 mg per day.
Elderly:
On the basis of the clinical studies, the recommended dose for patients over the age of 65
years is 20 mg per day (20 mg once a day) as described above.
Children and adolescents:
Axura is not recommended for use in children below 18 years due to a lack
of data on safety and efficacy.
Renal impairment:
In patients with mildly impaired renal function (creatinine clearance 50 – 80
ml/min) no dose adjustment is required. In patients with moderate renal impairment (creatinine
clearance 30 - 49 ml/min) daily dose should be 10 mg per day. If tolerated well after at least 7 days of
treatment, the dose could be increased up to 20 mg/day according to standard titration scheme. In
patients with severe renal impairment (creatinine clearance 5 – 29 ml/min) daily dose should be 10 mg
per day
.
Hepatic impairment:
In patients with mild or moderate hepatic impaired function (Child-Pugh A and
Child-Pugh B) no dose adjustment is needed. No data on the use of memantine in patients with severe
hepatic impairment are available. Administration of Axura in patients with severe hepatic impairment
is not recommended.
Hypersensitivity to the active substance or to any of the excipients.
4.4 Special warnings and precautions for use
Caution is recommended in patients with epilepsy, former history of convulsions or patients with
predisposing factors for epilepsy.
Concomitant use of N-methyl-D-aspartate(NMDA)-antagonists such as amantadine, ketamine or
dextromethorphan should be avoided. These compounds act at the same receptor system as
memantine, and therefore adverse reactions (mainly central nervous system (CNS)-related) may be
more frequent or more pronounced (see also section 4.5).
Some factors that may raise urine pH (see section 5.2 “Elimination”) may necessitate careful
monitoring of the patient. These factors include drastic changes in diet, e.g. from a carnivore to a
vegetarian diet, or a massive ingestion of alkalising gastric buffers. Also, urine pH may be elevated by
states of renal tubulary acidosis (RTA) or severe infections of the urinary tract with
Proteus bacteria
.
In most clinical trials, patients with recent myocardial infarction, uncompensated congestive heart
failure (NYHA III-IV), or uncontrolled hypertension were excluded. As a consequence, only limited
data are available and patients with these conditions should be closely supervised.
4.5 Interaction with other medicinal products and other forms of interaction
Due to the pharmacological effects and the mechanism of action of memantine the following
interactions may occur:
The mode of action suggests that the effects of L-dopa, dopaminergic agonists, and
anticholinergics may be enhanced by concomitant treatment with NMDA-antagonists such as
memantine. The effects of barbiturates and neuroleptics may be reduced. Concomitant
administration of memantine with the antispasmodic agents, dantrolene or baclofen, can modify
their effects and a dose adjustment may be necessary.
Concomitant use of memantine and amantadine should be avoided, owing to the risk of
pharmacotoxic psychosis. Both compounds are chemically related NMDA-antagonists. The
same may be true for ketamine and dextromethorphan (see also section 4.4). There is one
published case report on a possible risk also for the combination of memantine and phenytoin.
Other active substances such as cimetidine, ranitidine, procainamide, quinidine, quinine and
nicotine that use the same renal cationic transport system as amantadine may also possibly
interact with memantine leading to a potential risk of increased plasma levels.
There may be a possibility of reduced serum level of hydrochlorothiazide (HCT) when
memantine is co-administered with HCT or any combination with HCT.
In post-marketing experience isolated cases with international normalized ratio (INR) increases
have been reported in patients concomitantly treated with warfarin. Although no causal
relationship has been established, close monitoring of prothrombin time or INR is advisable for
patients concomitantly treated with oral anticoagulants.
In single-dose pharmacokinetic (PK) studies in young healthy subjects no relevant active substance-
active substance interaction of memantine with glyburide/metformin or donepezil was observed.
In a clinical study in young healthy subjects no relevant effect of memantine on the pharmacokinetics
of galantamine was observed.
Memantine did not inhibit CYP 1A2, 2A6, 2C9, 2D6, 2E1, 3A, flavin containing monooxygenase,
epoxide hydrolase or sulphation
in vitro
.
4.6 Pregnancy and lactation
For memantine, no clinical data on exposed pregnancies are available. Animal studies indicate a
potential for reducing intrauterine growth at exposure levels, which are identical or slightly higher
than at human exposure (see section 5.3). The potential risk for humans is unknown. Memantine
should not be used during pregnancy unless clearly necessary.
It is not known whether memantine is excreted in human breast milk but, taking into consideration the
lipophilicity of the substance, this probably occurs. Women taking memantine should not breast-feed.
4.7 Effects on ability to drive and use machines
Moderate to severe Alzheimer’s disease usually causes impairment of driving performance and
compromises the ability to use machinery. Furthermore, Axura has minor or moderate influence on the
ability to drive and use machines such that outpatients should be warned to take special care.
In clinical trials in mild to severe dementia, involving 1,784 patients treated with Axura and 1,595
patients treated with placebo, the overall incidence rate of adverse reactions with Axura did not differ
from those with placebo; the adverse reactions were usually mild to moderate in severity. The most
frequently occurring adverse reactions with a higher incidence in the Axura group than in the placebo
group were dizziness (6.3% vs 5.6%, respectively), headache (5.2% vs 3.9%), constipation (4.6% vs
2.6%), somnolence (3.4% vs 2.2%) and hypertension (4.1% vs 2.8%).
The following Adverse Reactions listed in the Table below have been accumulated in clinical studies
with Axura and since its introduction in the market. Within each frequency grouping, undesirable
effects are presented in order of decreasing seriousness
.
Adverse reactions are ranked according to system organ class, using the following convention: very
common (≥ 1/10), common (≥1/100 to < 1/10), uncommon (≥ 1/1,000 to < 1/100), rare (≥1/10,000 to
< 1/1,000), very rare (< 1/10,000), not known (cannot be estimated from the available data).
Infections and infestations
Venous
thrombosis/thromboembolism
Respiratory, thoracic and mediastinal
disorders
Gastrointestinal disorders
General disorders and administration
site conditions
1
Hallucinations have mainly been observed in patients with severe Alzheimer´s disease.
2
Isolated cases reported in post-marketing experience
Alzheimer’s disease has been associated with depression, suicidal ideation and suicide. In post-
marketing experience these events have been reported in patients treated with Axura.











Only limited experience with overdose is available from clinical studies and post-marketing
experience.
Symptoms:
Relative large overdoses (200 mg and 105 mg/day for 3 days, respectively) have been
associated with either only symptoms of tiredness, weakness and/or diarrhoea or no symptoms. In the
overdose cases below 140 mg or unknown dose the patients revealed symptoms from central nervous
system (confusion, drowsiness, somnolence, vertigo, agitation, aggression, hallucination, and gait
disturbance) and/or gastrointestinal origin (vomiting and diarrhoea).
In the most extreme case of overdose, the patient survived the oral intake of a total of 2000 mg
memantine with effects on the central nervous system (coma for 10 days, and later diplopia and
agitation). The patient received symptomatic treatment and plasmapheresis. The patient recovered
without permanent sequelae.
In another case of a large overdose, the patient also survived and recovered. The patient had received
400 mg memantine orally. The patient experienced central nervous system symptoms such as
restlessness, psychosis, visual hallucinations, proconvulsiveness, somnolence, stupor, and
unconsciousness.
Treatment:
In the event of overdose, treatment should be symptomatic. No specific antidote for
intoxication or overdose is available. Standard clinical procedures to remove active substance material,
e.g. gastric lavage, carbo medicinalis (interruption of potential entero-hepatic recirculation),
acidification of urine, forced diuresis should be used as appropriate.
In case of signs and symptoms of general Central Nervous System (CNS) overstimulation, careful
symptomatic clinical treatment should be considered.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Other Anti-dementia drugs, ATC code: N06DX01.
There is increasing evidence that malfunctioning of glutamatergic neurotransmission, in particular at
NMDA-receptors, contributes to both expression of symptoms and disease progression in
neurodegenerative dementia.
Memantine is a voltage-dependent, moderate-affinity uncompetitive NMDA-receptor antagonist. It
modulates the effects of pathologically elevated tonic levels of glutamate that may lead to neuronal
dysfunction.
Clinical studies:
A pivotal monotherapy study in a population of patients suffering from moderate to severe
Alzheimer’s disease (mini mental state examination (MMSE) total scores at baseline of 3 - 14)
included a total of 252 outpatients. The study showed beneficial effects of memantine treatment in
comparison to placebo at 6 months (observed cases analysis for the clinician’s interview based
impression of change (CIBIC-plus): p=0.025; Alzheimer´s disease cooperative study- activities of
daily living (ADCS-ADLsev): p=0.003; severe impairment battery (SIB): p=0.002).
A pivotal monotherapy study of memantine in the treatment of mild to moderate Alzheimer’s disease
(MMSE total scores at baseline of 10 to 22) included 403 patients. Memantine-treated patients showed
a statistically significantly better effect than placebo-treated patients on the primary endpoints:
Alzheimer’s disease assessment scale (ADAS-cog) (p=0.003) and CIBIC-plus (p=0.004) at week 24
last observation carried forward (LOCF). In another monotherapy study in mild to moderate
Alzheimer’s disease a total of 470 patients (MMSE total scores at baseline of 11-23) were randomised.
In the prospectively defined primary analysis statistical significance was not reached at the primary
efficacy endpoint at week 24.
A meta-analysis of patients with moderate to severe Alzheimer’s disease (MMSE total scores < 20)
from the six phase III, placebo-controlled, 6-month studies (including monotherapy studies
and studies
with patients on a stable dose of acetylcholinesterase inhibitors) showed that there was a statistically
significant effect in favour of memantine treatment for the cognitive, global, and functional domains.
When patients were identified with concurrent worsening in all three domains, results showed a
statistically significant effect of memantine in preventing worsening, as twice as many placebo-treated
patients as memantine-treated patients showed worsening in all three domains (21% vs. 11%,
p0.0001).
5.2 Pharmacokinetic properties
Absorption:
Memantine has an absolute bioavailability of approximately 100%. t
max
is between 3 and
8 hours. There is no indication that food influences the absorption of memantine.
Distribution:
Daily doses of 20 mg lead to steady-state plasma concentrations of memantine ranging
from 70 to 150 ng/ml (0.5 - 1 µmol) with large interindividual variations. When daily doses of 5 to
30 mg were administered, a mean cerebrospinal fluid (CSF)/serum ratio of 0.52 was calculated. The
volume of distribution is around 10 l/kg. About 45% of memantine is bound to plasma-proteins.
Biotransformation:
In man, about 80% of the circulating memantine-related material is present as the
parent compound. Main human metabolites are N-3,5-dimethyl-gludantan, the isomeric mixture of 4-
and 6-hydroxy-memantine, and 1-nitroso-3,5-dimethyl-adamantane. None of these metabolites exhibit
NMDA-antagonistic activity. No cytochrome P 450 catalysed metabolism has been detected
in vitro.
In a study using orally administered
14
C-memantine, a mean of 84% of the dose was recovered within
20 days, more than 99% being excreted renally.
Elimination:
Memantine is eliminated in a monoexponential manner with a terminal t
½
of 60 to
100 hours. In volunteers with normal kidney function, total clearance (Cl
tot
) amounts to
170 ml/min/1.73 m² and part of total renal clearance is achieved by tubular secretion.
Renal handling also involves tubular reabsorption, probably mediated by cation transport proteins. The
renal elimination rate of memantine under alkaline urine conditions may be reduced by a factor of 7 to
9 (see section 4.4). Alkalisation of urine may result from drastic changes in diet, e.g. from a carnivore
to a vegetarian diet, or from the massive ingestion of alkalising gastric buffers.
Linearity:
Studies in volunteers have demonstrated linear pharmacokinetics in the dose range of 10 to
40 mg.
Pharmacokinetic/pharmacodynamic relationship:
At a dose of memantine of 20 mg per day the CSF
levels match the k
i
-value (k
i
= inhibition constant) of memantine, which is 0.5 µmol in human frontal
cortex.
5.3 Preclinical safety data
In short term studies in rats memantine like other NMDA-antagonists have induced neuronal
vacuolisation and necrosis (Olney lesions) only after doses leading to very high peak serum
concentrations. Ataxia and other preclinical signs have preceded the vacuolisation and necrosis. As the
effects have neither been observed in long term studies in rodents nor in non-rodents, the clinical
relevance of these findings is unknown.
Ocular changes were inconsistently observed in repeat dose toxicity studies in rodents and dogs, but
not in monkeys. Specific ophthalmoscopic examinations in clinical studies with memantine did not
disclose any ocular changes.
Phospholipidosis in pulmonary macrophages due to accumulation of memantine in lysosomes was
observed in rodents. This effect is known from other active substances with cationic amphiphilic
properties. There is a possible relationship between this accumulation and the vacuolisation observed
in lungs. This effect was only observed at high doses in rodents. The clinical relevance of these
findings is unknown.
No genotoxicity has been observed following testing of memantine in standard assays. There was no
evidence of any carcinogenicity in life long studies in mice and rats. Memantine was not teratogenic in
rats and rabbits, even at maternally toxic doses, and no adverse effects of memantine were noted on
fertility. In rats, foetal growth reduction was noted at exposure levels, which are identical or slightly
higher than at human exposure.
PHARMACEUTICAL PARTICULARS
Tablet cores for 5/10/15/20 mg film-coated tablets:
Microcrystalline cellulose
Croscarmellose sodium
Colloidal anhydrous silica
Magnesium stearate
Tablet coat for 5/10/15/20 mg film-coated tablets:
Hypromellose
Macrogol 400
Titanium dioxide (E 171)
Additional for 10 mg film-coated tablets:
Iron oxide yellow (E 172)
Additional for 15 mg and 20 mg film-coated tablets:
Iron oxide yellow and red (E 172)
6.4 Special precautions for storage
This medicinal product does not require any special storage conditions.
6.5 Nature and contents of container
Each pack contains 28 film-coated tablets in 4 PVDC/PE/PVC/Al-blister or PP/Al-blisters with 7 film-
coated tablets of 5 mg, 7 film-coated tablets of 10 mg, 7 film-coated tablets of 15 mg and 7 film-coated
tablets of 20 mg.
6.6 Special precautions for disposal
MARKETING AUTHORISATION HOLDER
Merz Pharmaceuticals GmbH
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany
8. MARKETING AUTHORISATION NUMBER(S)
EU/1/02/218/016
EU/1/02/218/023
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 08/05/2008
Date of latest renewal:
10. DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency
(EMA) http://www.ema.europa.eu
NAME OF THE MEDICINAL PRODUCT
Axura 20 mg film-coated tablets.
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each film-coated tablet contains 20 mg of memantine hydrochloride equivalent to 16.62 mg memantine.
For a full list of excipients, see section 6.1.
Film-coated tablet.
Pale red to grey-red, oval-oblong film-coated tablets with imprint “20” on one side and imprint “MEM”
on the other side.
4.1 Therapeutic indications
Treatment of patients with moderate to severe Alzheimer’s disease.
4.2 Posology and method of administration
Treatment should be initiated and supervised by a physician experienced in the diagnosis and
treatment of Alzheimer’s dementia. Therapy should only be started if a caregiver is available who will
regularly monitor the intake of the medicinal product by the patient. Diagnosis should be made
according to current guidelines.
Axura should be administered once a day and should be taken at the same time every day. The film-
coated tablets can be taken with or without food.
Adults:
Dose titration
The maximum daily dose is 20 mg per day. In order to reduce the risk of undesirable effects the
maintenance dose is achieved by upward titration of 5 mg per week over the first 3 weeks as follows.
For up-titration other tablet strengths are available.
Week 1 (day 1-7):
The patient should take one 5 mg film-coated tablet per day for 7 days.
Week 2 (day 8-14):
The patient should take one 10 mg film-coated tablet per day for 7 days.
Week 3 (day 15-21):
The patient should take one 15 mg film-coated tablet per day for 7 days.
From Week 4 on:
The patient should take one 20 mg film-coated tablet per day.
Maintenance dose
The recommended maintenance dose is 20 mg per day.
Elderly:
On the basis of the clinical studies, the recommended dose for patients over the age of 65
years is 20 mg per day as described above.
Children and adolescents:
Axura is not recommended for use in children below 18 years due to a lack
of data on safety and efficacy.
Renal impairment:
In patients with mildly impaired renal function (creatinine clearance 50 – 80
ml/min) no dose adjustment is required. In patients with moderate renal impairment (creatinine
clearance 30 - 49 ml/min) daily dose should be 10 mg per day. If tolerated well after at least 7 days of
treatment, the dose could be increased up to 20 mg/day according to standard titration scheme. In
patients with severe renal impairment (creatinine clearance 5 – 29 ml/min) daily dose should be 10 mg
per day
.
Hepatic impairment:
In patients with mild or moderate hepatic impaired function (Child-Pugh A and
Child-Pugh B) no dose adjustment is needed. No data on the use of memantine in patients with severe
hepatic impairment are available. Administration of Axura in patients with severe hepatic impairment
is not recommended.
Hypersensitivity to the active substance or to any of the excipients.
4.4 Special warnings and precautions for use
Caution is recommended in patients with epilepsy, former history of convulsions or patients with
predisposing factors for epilepsy.
Concomitant use of N-methyl-D-aspartate(NMDA)-antagonists such as amantadine, ketamine or
dextromethorphan should be avoided. These compounds act at the same receptor system as
memantine, and therefore adverse reactions (mainly central nervous system (CNS)-related) may be
more frequent or more pronounced (see also section 4.5).
Some factors that may raise urine pH (see section 5.2 “Elimination”) may necessitate careful
monitoring of the patient. These factors include drastic changes in diet, e.g. from a carnivore to a
vegetarian diet, or a massive ingestion of alkalising gastric buffers. Also, urine pH may be elevated by
states of renal tubulary acidosis (RTA) or severe infections of the urinary tract with
Proteus bacteria
.
In most clinical trials, patients with recent myocardial infarction, uncompensated congestive heart
failure (NYHA III-IV), or uncontrolled hypertension were excluded. As a consequence, only limited
data are available and patients with these conditions should be closely supervised.
4.5 Interaction with other medicinal products and other forms of interaction
Due to the pharmacological effects and the mechanism of action of memantine the following
interactions may occur:
The mode of action suggests that the effects of L-dopa, dopaminergic agonists, and
anticholinergics may be enhanced by concomitant treatment with NMDA-antagonists such as
memantine. The effects of barbiturates and neuroleptics may be reduced. Concomitant
administration of memantine with the antispasmodic agents, dantrolene or baclofen, can modify
their effects and a dose adjustment may be necessary.
Concomitant use of memantine and amantadine should be avoided, owing to the risk of
pharmacotoxic psychosis. Both compounds are chemically related NMDA-antagonists. The
same may be true for ketamine and dextromethorphan (see also section 4.4). There is one
published case report on a possible risk also for the combination of memantine and phenytoin.
Other active substances such as cimetidine, ranitidine, procainamide, quinidine, quinine and
nicotine that use the same renal cationic transport system as amantadine may also possibly
interact with memantine leading to a potential risk of increased plasma levels.
There may be a possibility of reduced serum level of hydrochlorothiazide (HCT) when
memantine is co-administered with HCT or any combination with HCT.
In post-marketing experience isolated cases with international normalized ratio (INR) increases
have been reported in patients concomitantly treated with warfarin. Although no causal
relationship has been established, close monitoring of prothrombin time or INR is advisable for
patients concomitantly treated with oral anticoagulants.
In single-dose pharmacokinetic (PK) studies in young healthy subjects no relevant active substance-
active substance interaction of memantine with glyburide/metformin or donepezil was observed.
In a clinical study in young healthy subjects no relevant effect of memantine on the pharmacokinetics
of galantamine was observed.
Memantine did not inhibit CYP 1A2, 2A6, 2C9, 2D6, 2E1, 3A, flavin containing monooxygenase,
epoxide hydrolase or sulphation
in vitro
.
4.6 Pregnancy and lactation
For memantine, no clinical data on exposed pregnancies are available. Animal studies indicate a
potential for reducing intrauterine growth at exposure levels, which are identical or slightly higher
than at human exposure (see section 5.3). The potential risk for humans is unknown. Memantine
should not be used during pregnancy unless clearly necessary.
It is not known whether memantine is excreted in human breast milk but, taking into consideration the
lipophilicity of the substance, this probably occurs. Women taking memantine should not breast-feed.
4.7 Effects on ability to drive and use machines
Moderate to severe Alzheimer’s disease usually causes impairment of driving performance and
compromises the ability to use machinery. Furthermore, Axura has minor or moderate influence on the
ability to drive and use machines such that outpatients should be warned to take special care.
In clinical trials in mild to severe dementia, involving 1,784 patients treated with Axura and 1,595
patients treated with placebo, the overall incidence rate of adverse reactions with Axura did not differ
from those with placebo; the adverse reactions were usually mild to moderate in severity. The most
frequently occurring adverse reactions with a higher incidence in the Axura group than in the placebo
group were dizziness (6.3% vs 5.6%, respectively), headache (5.2% vs 3.9%), constipation (4.6% vs
2.6%), somnolence (3.4% vs 2.2%) and hypertension (4.1% vs 2.8%).
The following Adverse Reactions listed in the Table below have been accumulated in clinical studies
with Axura and since its introduction in the market. Within each frequency grouping, undesirable
effects are presented in order of decreasing seriousness.
Adverse reactions are ranked according to system organ class, using the following convention: very
common (≥ 1/10), common (≥1/100 to < 1/10), uncommon (≥ 1/1,000 to < 1/100), rare (≥1/10,000 to
< 1/1,000), very rare (< 1/10,000), not known (cannot be estimated from the available data).
Infections and infestations
Venous
thrombosis/thromboembolism
Respiratory, thoracic and mediastinal
disorders
Gastrointestinal disorders
General disorders and administration
site conditions
1
Hallucinations have mainly been observed in patients with severe Alzheimer´s disease.
2
Isolated cases reported in post-marketing experience
Alzheimer’s disease has been associated with depression, suicidal ideation and suicide. In post-
marketing experience these events have been reported in patients treated with Axura.
Only limited experience with overdose is available from clinical studies and post-marketing
experience.
Symptoms:
Relative large overdoses (200 mg and 105 mg/day for 3 days, respectively) have been
associated with either only symptoms of tiredness, weakness and/or diarrhoea or no symptoms. In the
overdose cases below 140 mg or unknown dose the patients revealed symptoms from central nervous
system (confusion, drowsiness, somnolence, vertigo, agitation, aggression, hallucination, and gait
disturbance) and/or gastrointestinal origin (vomiting and diarrhoea).
In the most extreme case of overdose, the patient survived the oral intake of a total of 2000 mg
memantine with effects on the central nervous system (coma for 10 days, and later diplopia and
agitation). The patient received symptomatic treatment and plasmapheresis. The patient recovered
without permanent sequelae.











In another case of a large overdose, the patient also survived and recovered. The patient had received
400 mg memantine orally. The patient experienced central nervous system symptoms such as
restlessness, psychosis, visual hallucinations, proconvulsiveness, somnolence, stupor, and
unconsciousness.
Treatment:
In the event of overdose, treatment should be symptomatic. No specific antidote for
intoxication or overdose is available. Standard clinical procedures to remove active substance material,
e.g. gastric lavage, carbo medicinalis (interruption of potential entero-hepatic recirculation),
acidification of urine, forced diuresis should be used as appropriate.
In case of signs and symptoms of general central nervous system (CNS) overstimulation, careful
symptomatic clinical treatment should be considered.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Other Anti-dementia drugs, ATC code: N06DX01.
There is increasing evidence that malfunctioning of glutamatergic neurotransmission, in particular at
NMDA-receptors, contributes to both expression of symptoms and disease progression in
neurodegenerative dementia.
Memantine is a voltage-dependent, moderate-affinity uncompetitive NMDA-receptor antagonist. It
modulates the effects of pathologically elevated tonic levels of glutamate that may lead to neuronal
dysfunction.
Clinical studies:
A pivotal monotherapy study in a population of patients suffering from moderate to severe
Alzheimer’s disease (mini mental state examination (MMSE) total scores at baseline of 3 - 14)
included a total of 252 outpatients. The study showed beneficial effects of memantine treatment in
comparison to placebo at 6 months (observed cases analysis for the clinician´s interview based
impression of change (CIBIC-plus): p=0.025; Alzheimer´s disease cooperative study – activities of
daily living (ADCS-ADLsev): p=0.003; severe impairment battery (SIB): p=0.002).
A pivotal monotherapy study of memantine in the treatment of mild to moderate Alzheimer’s disease
(MMSE total scores at baseline of 10 to 22) included 403 patients. Memantine-treated patients showed
a statistically significantly better effect than placebo-treated patients on the primary endpoints:
Alzheimer´s disease assessment scale (ADAS-cog) (p=0.003) -and CIBIC-plus (p=0.004) at week 24
last observation carried forward (LOCF). In another monotherapy study in mild to moderate
Alzheimer’s disease a total of 470 patients (MMSE total scores at baseline of 11-23) were randomised.
In the prospectively defined primary analysis statistical significance was not reached at the primary
efficacy endpoint at week 24.
A meta-analysis of patients with moderate to severe Alzheimer’s disease (MMSE total scores < 20)
from the six phase III, placebo-controlled, 6-month studies (including monotherapy studies
and studies
with patients on a stable dose of acetylcholinesterase inhibitors) showed that there was a statistically
significant effect in favour of memantine treatment for the cognitive, global, and functional domains.
When patients were identified with concurrent worsening in all three domains, results showed a
statistically significant effect of memantine in preventing worsening, as twice as many placebo-treated
patients as memantine-treated patients showed worsening in all three domains (21% vs. 11%,
p0.0001).
5.2 Pharmacokinetic properties
Absorption:
Memantine has an absolute bioavailability of approximately 100%. t
max
is between 3 and
8 hours. There is no indication that food influences the absorption of memantine.
Distribution:
Daily doses of 20 mg lead to steady-state plasma concentrations of memantine ranging
from 70 to 150 ng/ml (0.5 - 1 µmol) with large interindividual variations. When daily doses of 5 to
30 mg were administered, a mean cerebrospinal fluid (CSF)/serum ratio of 0.52 was calculated. The
volume of distribution is around 10 l/kg. About 45% of memantine is bound to plasma-proteins.
Biotransformation:
In man, about 80% of the circulating memantine-related material is present as the
parent compound. Main human metabolites are N-3,5-dimethyl-gludantan, the isomeric mixture of 4-
and 6-hydroxy-memantine, and 1-nitroso-3,5-dimethyl-adamantane. None of these metabolites exhibit
NMDA-antagonistic activity. No cytochrome P 450 catalysed metabolism has been detected
in vitro.
In a study using orally administered
14
C-memantine, a mean of 84% of the dose was recovered within
20 days, more than 99% being excreted renally.
Elimination:
Memantine is eliminated in a monoexponential manner with a terminal t
½
of 60 to
100 hours. In volunteers with normal kidney function, total clearance (Cl
tot
) amounts to
170 ml/min/1.73 m² and part of total renal clearance is achieved by tubular secretion.
Renal handling also involves tubular reabsorption, probably mediated by cation transport proteins. The
renal elimination rate of memantine under alkaline urine conditions may be reduced by a factor of 7 to
9 (see section 4.4). Alkalisation of urine may result from drastic changes in diet, e.g. from a carnivore
to a vegetarian diet, or from the massive ingestion of alkalising gastric buffers.
Linearity:
Studies in volunteers have demonstrated linear pharmacokinetics in the dose range of 10 to
40 mg.
Pharmacokinetic/pharmacodynamic relationship:
At a dose of memantine of 20 mg per day the (CSF)
levels match the k
i
-value (k
i
= inhibition constant) of memantine, which is 0.5 µmol in human frontal
cortex.
5.3 Preclinical safety data
In short term studies in rats memantine like other NMDA-antagonists have induced neuronal
vacuolisation and necrosis (Olney lesions) only after doses leading to very high peak serum
concentrations. Ataxia and other preclinical signs have preceded the vacuolisation and necrosis. As the
effects have neither been observed in long term studies in rodents nor in non-rodents, the clinical
relevance of these findings is unknown.
Ocular changes were inconsistently observed in repeat dose toxicity studies in rodents and dogs, but
not in monkeys. Specific ophthalmoscopic examinations in clinical studies with memantine did not
disclose any ocular changes.
Phospholipidosis in pulmonary macrophages due to accumulation of memantine in lysosomes was
observed in rodents. This effect is known from other active substances with cationic amphiphilic
properties. There is a possible relationship between this accumulation and the vacuolisation observed
in lungs. This effect was only observed at high doses in rodents. The clinical relevance of these
findings is unknown.
No genotoxicity has been observed following testing of memantine in standard assays. There was no
evidence of any carcinogenicity in life long studies in mice and rats. Memantine was not teratogenic in
rats and rabbits, even at maternally toxic doses, and no adverse effects of memantine were noted on
fertility. In rats, foetal growth reduction was noted at exposure levels, which are identical or slightly
higher than at human exposure.
PHARMACEUTICAL PARTICULARS
Tablet core:
Microcrystalline cellulose
Croscarmellose sodium
Colloidal anhydrous silica
Magnesium stearate
Tablet coat:
Hypromellose
Macrogol 400
Titanium dioxide (E 171)
Iron oxide yellow and red (E 172)
6.4 Special precautions for storage
This medicinal product does not require any special storage conditions.
6.5 Nature and contents of container
Blister packs containing 14 film-coated tablets per
PVDC/PE/PVC/Al-blister or PP/Al-
blister strip.
Pack sizes of 14, 28, 42, 56, 98 or 840 (20 x 42) film-coated tablets are presented.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
MARKETING AUTHORISATION HOLDER
Merz Pharmaceuticals GmbH
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany
MARKETING AUTHORISATION NUMBER(S)
EU/1/02/218/017
EU/1/02/218/018
EU/1/02/218/019
EU/1/02/218/020
EU/1/02/218/021
EU/1/02/218/022
EU/1/02/218/024
EU/1/02/218/025
EU/1/02/218/026
EU/1/02/218/027
EU/1/02/218/028
EU/1/02/218/029
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 08/05/2008
Date of latest renewal:
10. DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency
(EMA) http://www.ema.europa.eu
A. MANUFACTURING AUTHORISATION HOLDER(S)
RESPONSIBLE FOR BATCH RELEASE
B. CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURNG AUTHORISATION HOLDER(S) RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer(s) responsible for batch release
Merz Pharma GmbH & Co. KGaA
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to restricted medical prescription (See Annex I: Summary of Product
Characteristics, section 4.2).
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
Pharmacovigilance system
The MAH must ensure that the system of pharmacovigilance, as described in version 2.0 (7 May
2007) presented in Module 1.8.1. of the Marketing Authorisation, is in place and functioning before
and whilst the product is on the market.
Risk Management Plan
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version 4 (7 February 2008) of the Risk Management Plan
(RMP) presented in Module 1.8.2. of the Marketing Authorisation and any subsequent updates of the
RMP agreed by the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, the
updates RMP should be submitted at the same time as the next Periodic Safety Update Report (PSUR).
In addition, an updated RMP should be submitted
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
At the request of the EMA
Periodic Safety Update Reports (PSURs)
The MAH will continue to submit yearly PSURs, unless otherwise specified by the CHMP.
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
CARTON FOR BLISTER PACK
NAME OF THE MEDICINAL PRODUCT
Axura 10 mg film-coated tablets
Memantine hydrochloride
STATEMENT OF ACTIVE SUBSTANCE(S)
Each film-coated tablet contains 10 mg memantine hydrochloride equivalent to 8.31 mg memantine.
PHARMACEUTICAL FORM AND CONTENTS
Film-coated tablets.
14 film-coated tablets.
28 film-coated tablets.
30 film-coated tablets.
42 film-coated tablets.
50 film-coated tablets.
56 film-coated tablets.
98 film-coated tablets.
100 film-coated tablets.
112 film-coated tablets.
METHOD AND ROUTE(S) OF ADMINISTRATION
Oral use. Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS


























10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Merz Pharmaceuticals GmbH
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/02/218/012 14 film-coated tablets.
EU/1/02/218/007 28 film-coated tablets.
EU/1/02/218/001 30 film-coated tablets.
EU/1/02/218/013 42 film-coated tablets.
EU/1/02/218/002 50 film-coated tablets.
EU/1/02/218/008 56 film-coated tablets.
EU/1/02/218/014 98 film-coated tablets.
EU/1/02/218/003 100 film-coated tablets.
EU/1/02/218/009 112 film-coated tablets.
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE
Axura 10 mg tablets




















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
CARTON FOR 50 AND 98 TABLETS AS INTERMEDIATE PACK / COMPONENT OF A
MULTIPACK (WITHOUT BLUE BOX)
NAME OF THE MEDICINAL PRODUCT
Axura 10 mg film-coated tablets
Memantine hydrochloride
STATEMENT OF ACTIVE SUBSTANCE(S)
Each film-coated tablet contains 10 mg memantine hydrochloride equivalent to 8.31 mg memantine.
PHARMACEUTICAL FORM AND CONTENTS
Film-coated tablets. 50 film-coated tablets.
Component of a multipack comprising 20 packs, each containing 50 film-coated tablets. Not for separate
sale
Film-coated tablets. 98 film-coated tablets.
Component of a multipack comprising 10 packs, each containing 98 film-coated tablets.Not for separate
sale.
METHOD AND ROUTE(S) OF ADMINISTRATION
Oral use. Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF




























11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Merz Pharmaceuticals GmbH
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/02/218/010 20 x 50 film-coated tablets
EU/1/02/218/015 10 x 98 film-coated tablets
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE




















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
OUTER WRAPPER LABEL ON MULTIPACKS (20 x 50 TABLETS AND 10 x 98 TABLETS)
WRAPPED IN FOIL (INCLUDING BLUE BOX)
NAME OF THE MEDICINAL PRODUCT
Axura 10 mg film-coated tablets
Memantine hydrochloride
STATEMENT OF ACTIVE SUBSTANCE(S)
Each film-coated tablet contains 10 mg memantine hydrochloride equivalent to 8.31 mg memantine.
PHARMACEUTICAL FORM AND CONTENTS
Multipack comprising
20 packs, each containing 50 film-coated tablets.
10 packs, each containing 98 film-coated tablets
METHOD AND ROUTE(S) OF ADMINISTRATION
Oral use. Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE




























11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Merz Pharmaceuticals GmbH
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/02/218/010 20 x 50 film-coated tablets
EU/1/02/218/015 10 x 98 film-coated tablets
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE


















MINIMUM PARTICULARS TO APPEAR ON BLISTERS OR STRIPS
NAME OF THE MEDICINAL PRODUCT
Axura 10 mg film-coated tablets
Memantine hydrochloride
NAME OF THE MARKETING AUTHORISATION HOLDER
Merz Pharmaceuticals GmbH
EXP {MM/YYYY}
See embossed stamp.
Lot {number}
See embossed stamp.



















PARTICULARS TO APPEAR ON THE OUTER PACKAGING AND THE IMMEDIATE
PACKAGING
CARTON AND LABEL FOR BOTTLE
NAME OF THE MEDICINAL PRODUCT
Axura 10 mg/g oral drops, solution
Memantine hydrochloride
STATEMENT OF ACTIVE SUBSTANCE(S)
One activation of the pump (one downward stroke) delivers 0.5 ml (0.5g) of solution containing 5 mg of
memantine hydrochloride equivalent to 4.16 mg of memantine
The solution also contains potassium sorbate and sorbitol E420.
See package leaflet for further information.
PHARMACEUTICAL FORM AND CONTENTS
Oral drops, solution.
50 g.
100 g.
METHOD AND ROUTE(S) OF ADMINISTRATION
Oral use. Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS






























Do not store above 30ºC.
When opened, use within 3 months.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Merz Pharmaceuticals GmbH
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/02/218/005 50 g.
EU/1/02/218/006 100 g.
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE




















PARTICULARS TO APPEAR ON THE OUTER PACKAGING AND THE IMMEDIATE
PACKAGING
CARTON AND LABEL FOR 50 g BOTTLE AS INTERMEDIATE PACK / COMPONENT OF
A MULTIPACK (WITHOUT BLUE BOX)
NAME OF THE MEDICINAL PRODUCT
Axura 10 mg/g oral drops, solution
memantine hydrochloride
STATEMENT OF ACTIVE SUBSTANCE(S)
Each activation of the pump (one downward stroke) delivers 0.5 ml (0.5g) of solution containing 5 mg of
memantine hydrochloride equivalent to 4.16 mg of memantine
The solution also contains potassium sorbate and sorbitol (E420).
See package leaflet for further information.
PHARMACEUTICAL FORM AND CONTENTS
Oral drops, solution. 50 g.
Component of a multipack comprising 10 packs, each containing 1 bottle with 50 g oral solution. Not for
separate sale.
METHOD AND ROUTE(S) OF ADMINISTRATION
Oral use. Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS





























Do not store above 30ºC.
When opened, use within 3 months.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Merz Pharmaceuticals GmbH
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE

















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
OUTER WRAPPER LABEL ON MULTIPACKS (10x 50 g) WRAPPED IN FOIL
(INCLUDING BLUE BOX)
NAME OF THE MEDICINAL PRODUCT
Axura 10 mg/g oral drops,solution
memantine hydrochloride
STATEMENT OF ACTIVE SUBSTANCE(S)
Each activation of the pump (one downward stroke) delivers 0.5 ml (0.5g) of solution containing 5 mg of
memantine hydrochloride equivalent to 4.16 mg memantine
The solution also contains potassium sorbate and sorbitol E420.
See package leaflet for further information.
PHARMACEUTICAL FORM AND CONTENTS
Oral Drops, solution
Multipack comprising 10 packs, each containing 1 bottle with 50 g oral drops, solution.
METHOD AND ROUTE(S) OF ADMINISTRATION
Oral use. Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
Do not store above 30ºC.
When opened, use within 3 months.





























10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Merz Pharmaceuticals GmbH
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE

















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
CARTON FOR 28 TABLETS - TREATMENT INITIATION PACK – 4 WEEK TREATMENT
SCHEDULE
NAME OF THE MEDICINAL PRODUCT
Axura 5 mg film-coated tablets.
Axura 10 mg film-coated tablets.
Axura 15 mg film-coated tablets.
Axura 20 mg film-coated tablets.
Memantine hydrochloride
STATEMENT OF ACTIVE SUBSTANCE(S)
Each film-coated tablet contains 5 mg of memantine hydrochloride equivalent to 4.15 mg memantine.
Each film-coated tablet contains 10 mg of memantine hydrochloride equivalent to 8.31 mg memantine.
Each film-coated tablet contains 15 mg of memantine hydrochloride equivalent to 12.46 mg memantine.
Each film-coated tablet contains 20 mg of memantine hydrochloride equivalent to 16.62 mg memantine.
PHARMACEUTICAL FORM AND CONTENTS
Each pack with 28 film- coated tablets for a 4 week treatment schedule contains:
7 film-coated tablets.of Axura 5 mg
7 film-coated tablets of Axura 10 mg
7 film-coated tablets of Axura 15 mg
7 film-coated tablets of Axura 20 mg
METHOD AND ROUTE(S) OF ADMINISTRATION
Oral use. Read the package leaflet before use.
For continuation of your treatment please consult your doctor.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY



























SPECIAL STORAGE CONDITIONS
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Merz Pharmaceuticals GmbH
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/02/218/016 28 film-coated tablets
EU/1/02/218/023 28 film-coated tablets
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE
Axura 5 mg, 10 mg, 15 mg, 20 mg tablets






















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
Axura 20 mg film-coated tablets
Memantine hydrochloride
STATEMENT OF ACTIVE SUBSTANCE(S)
Each film-coated tablet contains 20 mg memantine hydrochloride equivalent to 16.62 mg memantine.
PHARMACEUTICAL FORM AND CONTENTS
Film-coated tablets.
14 film-coated tablets.
28 film-coated tablets.
42 film-coated tablets.
56 film-coated tablets.
98 film-coated tablets.
840 (20x42) film-coated tablets.
METHOD AND ROUTE(S) OF ADMINISTRATION
Oral use. Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS


























10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Merz Pharmaceuticals GmbH
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/02/218/017 14 film-coated tablets
EU/1/02/218/018 28 film-coated tablets
EU/1/02/218/019 42 film-coated tablets
EU/1/02/218/020 56 film-coated tablets
EU/1/02/218/021 98 film-coated tablets
EU/1/02/218/022 840 (20 x 42) film-coated tablets
EU/1/02/218/024 14 film-coated tablets
EU/1/02/218/025 28 film-coated tablets
EU/1/02/218/026 42 film-coated tablets
EU/1/02/218/027 56 film-coated tablets
EU/1/02/218/028 98 film-coated tablets
EU/1/02/218/029 840 (20 x 42) film-coated tablets
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE




















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
CARTON FOR 42 TABLETS AS INTERMEDIATE PACK / COMPONENT OF A
MULTIPACK (WITHOUT BLUE BOX)
NAME OF THE MEDICINAL PRODUCT
Axura 20 mg film-coated tablets
Memantine hydrochloride
STATEMENT OF ACTIVE SUBSTANCE(S)
Each film-coated tablet contains 20 mg memantine hydrochloride equivalent to 16.62 mg memantine.
PHARMACEUTICAL FORM AND CONTENTS
Film-coated tablets. 42 film-coated tablets.
Component of a multipack comprising 20 packs, each containing 42 film-coated tablets. Not for
separate sale.
METHOD AND ROUTE(S) OF ADMINISTRATION
Oral use. Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE



























11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Merz Pharmaceuticals GmbH
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/02/218/022 840 (20 x 42) film-coated tablets
EU/1/02/218/029 840 (20 x 42) film-coated tablets
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE
















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
OUTER WRAPPER LABEL ON MULTIPACKS (20x 42 TABLETS) WRAPPED IN FOIL
(INCLUDING BLUE BOX)
NAME OF THE MEDICINAL PRODUCT
Axura 20 mg film-coated tablets
Memantine hydrochloride
STATEMENT OF ACTIVE SUBSTANCE(S)
Each film-coated tablet contains 20 mg memantine hydrochloride equivalent to 16.62 mg memantine.
PHARMACEUTICAL FORM AND CONTENTS
Multipack comprising 20 packs, each containing 42 film-coated tablets.
METHOD AND ROUTE(S) OF ADMINISTRATION
Oral use. Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE

























11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Merz Pharmaceuticals GmbH
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/02/218/022 840 (20 x 42) film-coated tablets
EU/1/02/218/029 840 (20 x 42) film-coated tablets
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE
















MINIMUM PARTICULARS TO APPEAR ON BLISTERS OR STRIPS
NAME OF THE MEDICINAL PRODUCT
Axura 20 mg film-coated tablets
Memantine hydrochloride
NAME OF THE MARKETING AUTHORISATION HOLDER
Merz Pharmaceuticals GmbH
EXP {MM/YYYY}
See embossed stamp.
4. BATCH NUMBER
Lot {number}
See embossed stamp.

















PACKAGE LEAFLET: INFORMATION FOR THE USER
Axura 10 mg film-coated tablets
Memantine hydrochloride
Read all of this leaflet carefully before you start taking this medicine.
-
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
1.
What Axura is and what it is used for
2.
Before you take Axura
3.
How to take Axura
4.
Possible side effects
5.
How to store Axura
6.
Further information
WHAT AXURA IS AND WHAT IT IS USED FOR
How does Axura work
Axura belongs to a group of medicines known as anti-dementia medicines.
Memory loss in Alzheimer’s disease is due to a disturbance of message signals in the brain. The brain
contains so-called N-methyl-D-aspartate (NMDA)-receptors that are involved in transmitting nerve
signals important in learning and memory. Axura belongs to a group of medicines called NMDA-
receptor antagonists. Axura acts on these NMDA-receptors improving the transmission of nerve
signals and the memory.
What is Axura used for
Axura is used for the treatment of patients with moderate to severe Alzheimer’s disease.
if you are allergic (hypersensitive) to memantine hydrochloride or any of the other ingredients
of Axura tablets (see section 6).
Take special care with Axura
-
if you have a history of epileptic seizures
if you have recently experienced a myocardial infarction (heart attack), or if you are suffering
from congestive heart failure or from an uncontrolled hypertension (high blood pressure).
In these situations the treatment should be carefully supervised, and the clinical benefit of Axura
reassessed by your doctor on a regular basis.
If you suffer from renal impairment (kidney problems), your doctor should closely monitor your
kidney function and if necessary adapt the memantine doses accordingly.
The use of medicinal products called amantadine (for the treatment of Parkinson´s disease), ketamine
(a substance generally used as an anaesthetic), dextromethorphan (generally used to treat cough) and
other NMDA-antagonists at the same time should be avoided.
Axura is not recommended for children and adolescents under the age of 18 years.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
In particular, Axura may change the effects of the following medicines and their dose may need to be
adjusted by your doctor:
amantadine, ketamine, dextromethorphan
dantrolene, baclofen
cimetidine, ranitidine, procainamide, quinidine, quinine, nicotine
hydrochlorothiazide (or any combination with hydrochlorothiazide)
anticholinergics (substances generally used to treat movement disorders or intestinal cramps)
anticonvulsants (substances used to prevent and relieve seizures)
barbiturates (substances generally used to induce sleep)
dopaminergic agonists (substances such as L-dopa, bromocriptine)
neuroleptics (substances used in the treatment of mental disorders)
oral anticoagulants
If you go into hospital, let your doctor know that you are taking Axura.
Taking Axura with food and drink
You should inform your doctor if you have recently changed or intend to change your diet
substantially (e.g. from normal diet to strict vegetarian diet) or if you are suffering from states of renal
tubulary acidosis (RTA, an excess of acid-forming substances in the blood due to renal dysfunction
(poor kidney function)) or severe infections of the urinary tract (structure that carries urine), as your
doctor may need to adjust the dose of your medicine.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking any medicine.
Tell your doctor if you are pregnant or planning to become pregnant. The use of memantine in
pregnant women is not recommended.
Women taking Axura should not breast-feed.
Driving and using machines
Your doctor will tell you whether your illness allows you to drive and to use machines safely.
Also, Axura may change your reactivity, making driving or operating machinery inappropriate.
Always take Axura exactly as your doctor has told you. You should check with your doctor or
pharmacist if you are not sure.
Dosage
The recommended dose of Axura for adults and elderly patients is 20 mg once a day. In order to
reduce the risk of side effects this dose is achieved gradually by the following daily treatment scheme:
one and a half 10 mg tablet
two 10 mg tablets once a
day
The usual starting dose is half a tablet once a day (1x 5 mg) for the first week. This is increased to one
tablet once a day (1x 10 mg) in the second week and to 1 and a half tablet once a day in the third
week. From the fourth week on, the usual dose is 2 tablets once a day (1x 20 mg).
Dosage in patients with impaired kidney function
If you have impaired kidney function, your doctor will decide upon a dose that suits your condition. In
this case, monitoring of your kidney function should be performed by your doctor at specified
intervals.
Administration
Axura should be administered orally once a day. To benefit from your medicine you should take it
regularly every day at the same time of the day. The tablets should be swallowed with some water.
The tablets can be taken with or without food.
Duration of treatment
Continue to take Axura as long as it is of benefit to you. Your doctor should assess your treatment on a
regular basis.
If you take more Axura than you should
-
In general, taking too much Axura should not result in any harm to you. You may experience
increased symptoms as described in section 4. „Possible side effects“.
If you take a large overdose of Axura, contact your doctor or get medical advice, as you may
need medical attention.
If you forget to take Axura
-
If you find you have forgotten to take your dose of Axura, wait and take your next dose at the
usual time.
Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Like all medicines, Axura can cause side effects, although not everybody gets them.
In general, the observed side effects are mild to moderate.
Common (affects 1 to 10 users in 100):
Headache, sleepiness, constipation, dizziness, shortness of breath, high blood pressure and drug
hypersensitivity
Uncommon (affects 1 to 10 users in 1,000):







Tiredness, fungal infections, confusion, hallucinations, vomiting, abnormal gait, heart failure
and venous blood clotting (thrombosis/thromboembolism).
Very Rare (affects less than 1 user in 10,000):
Seizures
Not known (frequency cannot be estimated from the available data):
Inflammation of the pancreas and psychotic reactions
Alzheimer's disease has been associated with depression, suicidal ideation and suicide. These events
have been reported in patients treated with Axura.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Axura after the expiry date which is stated on the carton and the blister after EXP. The
expiry date refers to the last day of that month.
This medicinal product does not require any special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active substance is memantine hydrochloride. Each film-coated tablet contains 10 mg of
memantine hydrochloride equivalent to 8.31 mg memantine.
The other ingredients are microcrystalline cellulose, croscarmellose sodium, colloidal anhydrous
silica, and magnesium stearate, all in the tablet core; and hypromellose, macrogol 400, titanium
dioxide (E171) and iron oxide yellow (E172) all in the tablet coating.
What Axura looks like and contents of the pack
Axura film-coated tablets are presented as pale yellow to yellow, oval shaped film-coated tablet with
breaking line and engravings "1-0" on one side and "M M" on the other side
Axura film-coated tablets are available in blister packs of 14 tablets, 28 tablets, 30 tablets, 42 tablets,
50 tablets, 56 tablets, 98 tablets, 100 tablets, 112 tablets, 980 (10 x 98) tablets or 1000 (20 x
50) tablets.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Merz Pharmaceuticals GmbH
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany.
Merz Pharma GmbH + Co. KGaA
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany.
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder.
België/Belgique/Belgien
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Duitsland/Allemagne/Deutschland
Tél/Tel: +49 (0)69 1503 – 1
Luxembourg/Luxemburg
Ets. Hanff Frères
B.P. No. 1706
L-1017 Luxembourg
Tél: +352 45 07 07
България
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Германия
Tel.: +49 (0)69 1503 – 1
Magyarország
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Németország
Tel: +49 (0)69 1503 - 1
Česká republika
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Německo
Tel: +49 (0)69 1503 – 1
Malta
Clinipharm Co. Ltd
Farrugia Buildings
Triq tat-Torba
Attard BZN 12
Tel: +356 21 43 74 15
Danmark
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Tyskland
Tlf: +49 (0)69 1503 – 1
Nederland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Duitsland
Tel: +49 (0)69 1503 – 1
Deutschland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Tel: +49 (0)69 1503 - 1
Norge
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Tyskland
Tlf: +49 (0)69 1503 – 1
Eesti
H. Abbe Pharma GmbH
Pirita tee 20
EE-10127 Tallinn
Tel.: +372 6 460980
Österreich
Merz Pharma Austria GmbH
Guglgasse 17
A-1110 Wien
Tel: +43 (1) 869 16 04
Ελλάδα
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Γερμανία
Τηλ: +49 (0)69 1503 - 1
Polska
Natur Produkt Zdrovit Sp. z o.o.
ul. Nocznickiego 31
PL-01-918 Warszawa
Tel: +48 22 56 98 200
España
Grünenthal Pharma, S.A.
C/Dr. Zamenhof, 36
E-28027 Madrid
Tel: +34 (91) 301 93 00
Portugal
Grünenthal, S.A.
Rua Alfredo da Silva, 16
P-2610-016 Amadora
Tel: +351 / 214 72 63 00
France
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Allemagne
Tél: +49 (0)69 1503 – 1
România
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Germania
Tel.: +49 (0)69 1503 – 1
Ireland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Germany
Tel: +49 (0)69 1503 – 1
Slovenija
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Nemčija
Tel: +49 (0)69 1503 - 1
Ísland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Þýskaland
Tel: +49 (0)69 1503 – 1
Slovenská republika
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Nemecko
Tel: +49 (0)69 1503 - 1
Italia
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Germania
Tel: +49 (0)69 1503 - 1
Suomi/Finland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Saksa
Puh/Tel: +49 (0)69 1503 – 1
Κύπρος
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Γερμανία
Tel: +49 (0)69 1503 - 1
Sverige
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Tyskland
Tel: +49 (0)69 1503 – 1
Latvija
H. Abbe Pharma GmbH
Bauskas 58a
LV-1004 Riga
Tel.: +371 7 103203
United Kingdom
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Germany
Tel: +49 (0)69 1503 – 1
Lietuva
H. Abbe Pharma GmbH
M. Marcinkeviciaus g. 19-1
LT-2021 Vilnius
Tel.: +370 52 711710
This leaflet was last approved in MM/YYYY.
Detailed information on this product is available on the website of the European Medicines Agency
(EMA) http://www.ema.europa.eu
PACKAGE LEAFLET: INFORMATION FOR THE USER
Axura 10 mg/g oral drops, solution
Memantine hydrochloride
Read all of this leaflet carefully before you start taking this medicine.
-
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
1.
What Axura is and what it is used for
2.
Before you take Axura
3.
How to take Axura
4.
Possible side effects
5.
How to store Axura
6.
Further information
WHAT AXURA IS AND WHAT IT IS USED FOR
How does Axura work
Axura belongs to a group of medicines known as anti-dementia medicines
.
Memory loss in Alzheimer’s disease is due to a disturbance of message signals in the brain. The brain
contains so-called N-methyl-D-aspartate (NMDA)-receptors that are involved in transmitting nerve
signals important in learning and memory. Axura belongs to a group of medicines called NMDA-
receptor antagonists. Axura acts on these NMDA-receptors improving the transmission of nerve
signals and the memory.
What is Axura used for
Axura is used for the treatment of patients with moderate to severe Alzheimer’s disease.
if you are allergic (hypersensitive) to memantine hydrochloride or any of the other ingredients
of Axura oral drops, solution (see section 6).
Take special care with Axura
-
if you have a history of epileptic seizures
if you have recently experienced a myocardial infarction (heart attack), or if you are suffering
from congestive heart failure or from an uncontrolled hypertension (high blood pressure).
In these situations the treatment should be carefully supervised, and the clinical benefit of
Axura reassessed by your doctor on a regular basis.
If you suffer from renal impairment (kidney problems), your doctor should closely monitor your
kidney function and if necessary adapt the memantine doses accordingly.
Keep this leaflet. You may need to read it again.
-
The use of medicinal products called amantadine (for the treatment of Parkinson´s disease),
ketamine (a substance generally used as an anaesthetic), dextromethorphan (generally used to
treat cough) and other NMDA-antagonists at the same time should be avoided.
Axura is not recommended for children and adolescents under the age of 18 years.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
In particular, Axura may change the effects of the following medicines and their dose may need to be
adjusted by your doctor:
amantadine, ketamine, dextromethorphan
dantrolene, baclofen
cimetidine, ranitidine, procainamide, quinidine, quinine, nicotine
hydrochlorothiazide (or any combination with hydrochlorothiazide)
anticholinergics (substances generally used to treat movement disorders or intestinal cramps)
anticonvulsants (substances used to prevent and relieve seizures)
barbiturates (substances generally used to induce sleep)
dopaminergic agonists (substances such as L-dopa, bromocriptine)
neuroleptics (substances used in the treatment of mental disorders)
oral anticoagulants
If you go into hospital, let your doctor know that you are taking Axura.
Taking Axura with food and drink
You should inform your doctor if you have recently changed or intend to change your diet
substantially (e.g. from normal diet to strict vegetarian diet) or if you are suffering from states of renal
tubulary acidosis (RTA, an excess of acid-forming substances in the blood due to renal dysfunction
(poor kidney function)) or severe infections of the urinary tract (structure that carries urine), as your
doctor may need to adjust the dose of your medicine.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking any medicine.
Tell your doctor if you are pregnant or planning to become pregnant. The use of memantine in
pregnant women is not recommended.
Women taking Axura should not breast-feed.
Driving and using machines
Your doctor will tell you whether your illness allows you to drive and to use machines safely.
Also, Axura may change your reactivity, making driving or operating machinery inappropriate.
Important information about some of the ingredients of Axura
This medicinal product contains sorbitol. If you have been told by your doctor that you have an
intolerance to some sugars, contact your doctor before taking this medicinal product. Your doctor will
advise you.
Furthermore, this medicine contains potassium, less than 1 mmol (39 mg) per dose, i.e. essentially
potassium-free.
Always take Axura exactly as your doctor has told you. You should check with your doctor or
pharmacist if you are not sure.
Dosage
The recommended dose of Axura for adults and elderly patients is four strokes, equivalent to 20 mg
once a day.
In order to reduce the risk of side effects this dose is achieved gradually by the following daily
treatment scheme:
The usual starting dose is one downward stroke (1 x 5 mg) once daily for the first week. This dose is
increased in the second week to two downward strokes once daily (1 x 10 mg), and in the third week
to three downward strokes (1 x 15 mg) once daily. From the fourth week the recommended dose is
four downward strokes once daily (1 x 20mg)
Dosage in patients with impaired kidney function
If you have impaired kidney function, your doctor will decide upon a dose that suits your condition. In
this case, monitoring of your kidney function should be performed by your doctor at specified
intervals.
Administration
Axura should be administered orally once a day. To benefit from your medicine you should take it
regularly every day at the same time of the day. The solution should be taken with a little water. The
solution can be taken with or without food.
For detailed instructions on the preparation and handling of the product see end of this leaflet.
Duration of treatment
Continue to take Axura as long as it is of benefit to you. Your doctor should assess your treatment on a
regular basis.
If you take more Axura than you should
-
In general, taking too much Axura should not result in any harm to you. You may experience
increased symptoms as described in section 4. „Possible side effects“.
If you take a large overdose of Axura, contact your doctor or get medical advice, as you may
need medical attention.
If you forget to take Axura
-
If you find you have forgotten to take your dose of Axura, wait and take your next dose at the
usual time.
Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of this product, ask your doctor or pharmacist.







Like all medicines, Axura can cause side effects, although not everybody gets them.
In general, the observed side effects are mild to moderate.
Common (affects 1 to 10 users in 100):
Headache, sleepiness, constipation, dizziness, shortness of breath, high blood pressure and
drug hypersensitivity
Uncommon (affects 1 to 10 users in 1,000):
Tiredness, fungal infections, confusion, hallucinations, vomiting, abnormal gait, heart failure
and venous blood clotting (thrombosis/thromboembolism)
Very Rare (affects less than 1 user in 10,000):
Seizures
Not known (frequency cannot be estimated from the available data):
Inflammation of the pancreas and psychotic reactions
Alzheimer's disease has been associated with depression, suicidal ideation and suicide. These events
have been reported in patients treated with Axura.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Axura after the expiry date which is stated on the carton and the bottle label after the EXP.
The expiry date refers to the last day of that month.
Once opened, the contents of the bottle should be used within 3 months.
The bottle with the mounted pump must be kept and transported in an upright position only.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active substance is memantine hydrochloride.
Each one gram of oral drops, solution contains 10 mg of memantine hydrochloride equivalent to
8.31 mg memantine.
The other ingredients are: potassium sorbate, sorbitol (E420) and purified water.
What Axura looks like and contents of the pack
Axura oral drops, solution is presented as a clear, colourless to light yellowish solution.
Axura oral drops, solution is available in bottles of 50 g, 100 g or 10 x 50 g.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Merz Pharmaceuticals GmbH
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany.
Merz Pharma GmbH + Co. KGaA
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany.
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder.
België/Belgique/Belgien
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Duitsland/Allemagne/Deutschland
Tél/Tel: +49 (0)69 1503 – 1
Luxembourg/Luxemburg
Ets. Hanff Frères
B.P. No. 1706
L-1017 Luxembourg
Tél: +352 45 07 07
България
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Германия
Tel.: +49 (0)69 1503 – 1
Magyarország
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Németország
Tel: +49 (0)69 1503 - 1
Česká republika
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Německo
Tel: +49 (0)69 1503 – 1
Malta
Clinipharm Co. Ltd
Farrugia Buildings
Triq tat-Torba
Attard BZN 12
Tel: +356 21 43 74 15
Danmark
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Tyskland
Tlf: +49 (0)69 1503 – 1
Nederland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Duitsland
Tel: +49 (0)69 1503 – 1
Deutschland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Tel: +49 (0)69 1503 - 1
Norge
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Tyskland
Tlf: +49 (0)69 1503 – 1
Eesti
H. Abbe Pharma GmbH
Pirita tee 20
EE-10127 Tallinn
Tel.: +372 6 460980
Österreich
Merz Pharma Austria GmbH
Guglgasse 17
A- 1110 Wien
Tel: +43 (1) 869 16 04
Ελλάδα
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Γερμανία
Τηλ: +49 (0)69 1503 - 1
Polska
Natur Produkt Zdrovit Sp. z o.o.
ul. Nocznickiego 31
PL-01-918 Warszawa
Tel: +48 22 56 98 200
España
Grünenthal Pharma, S.A.
C/Dr. Zamenhof, 36
E-28027 Madrid
Tel: +34 (91) 301 93 00
Portugal
Grünenthal, S.A.
Rua Alfredo da Silva, 16
P-2610-016 Amadora
Tel: +351 / 214 72 63 00
France
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Allemagne
Tél: +49 (0)69 1503 – 1
România
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Germania
Tel.: +49 (0)69 1503 – 1
Ireland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Germany
Tel: +49 (0)69 1503 – 1
Slovenija
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Nemčija
Tel: +49 (0)69 1503 - 1
Ísland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Þýskaland
Tel: +49 (0)69 1503 – 1
Slovenská republika
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Nemecko
Tel: +49 (0)69 1503 - 1
Italia
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Germania
Tel: +49 (0)69 1503 - 1
Suomi/Finland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Saksa
Puh/Tel: +49 (0)69 1503 – 1
Κύπρος
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Γερμανία
Tel: +49 (0)69 1503 - 1
Sverige
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Tyskland
Tel: +49 (0)69 1503 – 1
Latvija
H. Abbe Pharma GmbH
Bauskas 58a
United Kingdom
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
LV-1004 Riga
Tel.: +371 7 103203
D-60318 Frankfurt
Germany
Tel: +49 (0)69 1503 – 1
Lietuva
H. Abbe Pharma GmbH
M. Marcinkeviciaus g. 19-1
LT-2021 Vilnius
Tel.: +370 52 711710
This leaflet was last approved in MM/YYYY.
Detailed information on this product is available on the website of the European Medicines Agency
(EMA) http://www.ema.europa.eu
Instruction for proper use of the pump
The solution must not be poured or pumped directly into the mouth from the bottle or pump. Measure
the dose onto a spoon or into a glass of water, using the pump.
.
Take the screw cap off the bottle:
The cap must be turned anticlockwise, unscrewed completely and removed (fig. 1).
Mounting the dosing pump on the bottle:
Take the dosing pump out of the plastic bag (fig. 2) and place it on top of the bottle. Slide the plastic
dip tube carefully into the bottle. Hold the dosing pump onto the neck of the bottle and screw it
clockwise until it fits firmly (fig. 3). The dosing pump is only screwed on once when starting the use,
and should never be unscrewed
.
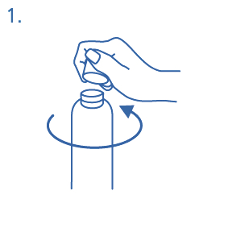
How the dosing pump works:
The dosing pump head has two positions and is easy to turn:
-
anticlockwise to unlock and
-
clockwise to lock.
The dosing pump head should not be pushed down while in the locked position. The solution may only
be dispensed in the unlocked position. To unlock, turn the pump head in the direction of the arrow
until it cannot be turned any further (about one eighth of a turn, fig. 4). The dosing pump is then ready
for use.
Preparing the dosing pump:
When used for the first time, the dosing pump does not dispense the correct amount of oral solution.
Therefore, the pump must be prepared (primed) by pushing the dosing pump head down completely
five times in succession (fig. 5).
The solution thus dispensed is discarded. The next time the dosing pump head is pushed downwards
completely, it dispenses the correct dose (fig. 6).


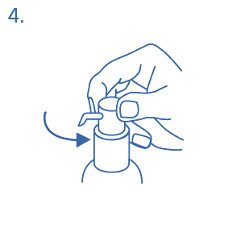

Correct use of the dosing pump:
Place the bottle on a flat, horizontal surface, for example a table top, and only use it in a upright
position. Hold a glass with a little water or a spoon below the nozzle. Push down the dosing pump
head in a firm but calm and steady manner - not too slowly (fig. 7, fig. 8).
The dosing pump head can then be released and is ready for the next downward stroke.
The dosing pump must only be used with the Axura solution in the bottle provided, not for other
substances or containers. If the pump does not function properly, consult your doctor or a
pharmacist.Lock the dosing pump after using Axura.
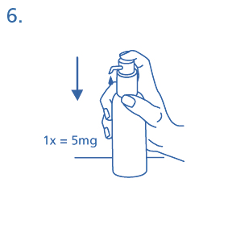


PACKAGE LEAFLET: INFORMATION FOR THE USER
Axura 5 mg film-coated tablets
Axura 10 mg film-coated tablets
Axura 15 mg film-coated tablets
Axura 20 mg film-coated tablets
Memantine hydrochloride
Read all of this leaflet carefully before you start taking this medicine.
-
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
1.
What Axura is and what it is used for
2.
Before you take Axura
3.
How to take Axura
4.
Possible side effects
5.
How to store Axura
6.
Further information
WHAT AXURA IS AND WHAT IT IS USED FOR
How does Axura work
Axura belongs to a group of medicines known as anti-dementia medicines.
Memory loss in Alzheimer’s disease is due to a disturbance of message signals in the brain. The brain
contains so-called N-methyl-D-aspartate (NMDA)-receptors that are involved in transmitting nerve
signals important in learning and memory. Axura belongs to a group of medicines called NMDA-
receptor antagonists. Axura acts on these NMDA-receptors improving the transmission of nerve
signals and the memory.
Axura is used for the treatment of patients with moderate to severe Alzheimer’s disease.
if you are allergic (hypersensitive) to memantine hydrochloride or any of the other ingredients
of Axura tablets (see section 6).
Take special care with Axura
-
if you have a history of epileptic seizures
if you have recently experienced a myocardial infarction (heart attack), or if you are suffering
from congestive heart failure or from an uncontrolled hypertension (high blood pressure).
In these situations the treatment should be carefully supervised, and the clinical benefit of Axura
reassessed by your doctor on a regular basis.
If you suffer from renal impairment (kidney problems), your doctor should closely monitor your
kidney function and if necessary adapt the memantine doses accordingly.
Keep this leaflet. You may need to read it again.
The use of medicinal products called amantadine (for the treatment of Parkinson´s disease), ketamine
(a substance generally used as an anaesthetic), dextromethorphan (generally used to treat cough) and
other NMDA-antagonists at the same time should be avoided.
Axura is not recommended for children and adolescents under the age of 18 years.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
In particular, Axura may change the effects of the following medicines and their dose may need to be
adjusted by your doctor:
amantadine, ketamine, dextromethorphan
dantrolene, baclofen
cimetidine, ranitidine, procainamide, quinidine, quinine, nicotine
hydrochlorothiazide (or any combination with hydrochlorothiazide)
anticholinergics (substances generally used to treat movement disorders or intestinal cramps)
anticonvulsants (substances used to prevent and relieve seizures)
barbiturates (substances generally used to induce sleep)
dopaminergic agonists (substances such as L-dopa, bromocriptine)
neuroleptics (substances used in the treatment of mental disorders)
oral anticoagulants
If you go into hospital, let your doctor know that you are taking Axura.
Taking Axura with food and drink
You should inform your doctor if you have recently changed or intend to change your diet
substantially (e.g. from normal diet to strict vegetarian diet) or if you are suffering from states of renal
tubulary acidosis (RTA, an excess of acid-forming substances in the blood due to renal dysfunction
(poor kidney function)) or severe infections of the urinary tract (structure that carries urine) as your
doctor may need to adjust the dose of your medicine.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking any medicine.
Tell your doctor if you are pregnant or planning to become pregnant. The use of memantine in
pregnant women is not recommended.
Women taking Axura should not breast-feed.
Driving and using machines
Your doctor will tell you whether your illness allows you to drive and to use machines safely.
Also, Axura may change your reactivity, making driving or operating machinery inappropriate.
The Axura treatment initiation pack is only to be used for the beginning of the treatment with Axura.
Always take Axura exactly as your doctor has told you. You should check with your doctor or
pharmacist if you are not sure.
Dosage
The recommended treatment dose of 20
mg per day is achieved by a gradual increase of the Axura
dose during the first 3 weeks of treatment. The treatment scheme is also indicated on the treatment
initiation pack. Take one tablet once a day.
Week 1 (day 1-7):
Take one 5 mg tablet once a day (white to off-white, oval-oblong) for 7 days.
Week 2 (day 8-14):
Take one 10 mg tablet once a day (pale yellow to yellow, oval shaped) for 7 days.
Week 3 (day 15-21):
Take one 15 mg tablet once a day (grey-orange, oval-oblong) for 7 days.
Week 4 (day 22-28):
Take one 20 mg tablet per day (grey-red, oval-oblong) for 7 days.
Maintenance dose
The recommended daily dose is 20 mg once a day.
For continuation of the treatment please consult your doctor.
Dosage in patients with impaired kidney function
If you have impaired kidney function, your doctor will decide upon a dose that suits your condition. In
this case, monitoring of your kidney function should be performed by your doctor at specified
intervals.
Administration
Axura should be administered orally once a day. To benefit from your medicine you should take it
regularly every day at the same time of the day. The tablets should be swallowed with some water.
The tablets can be taken with or without food.
Duration of treatment
Continue to take Axura as long as it is of benefit to you. Your doctor should assess your treatment on a
regular basis.
If you take more Axura than you should
-
In general, taking too much Axura should not result in any harm to you. You may experience
increased symptoms as described in section 4. „Possible side effects“.
If you take a large overdose of Axura, contact your doctor or get medical advice, as you may
need medical attention.
If you forget to take Axura
-
If you find you have forgotten to take your dose of Axura, wait and take your next dose at the
usual time.
Do not take a double dose to make up for a forgotten dose.







If you have any further questions on the use of this product, ask your doctor or pharmacist.
Like all medicines, Axura can cause side effects, although not everybody gets them.
In general, the observed side effects are mild to moderate.
Common (affects 1 to 10 users in 100):
Headache, sleepiness, constipation, dizziness, shortness of breath, high blood pressure and
drug hypersensitivity
Uncommon (affects 1 to 10 users in 1,000):
Tiredness, fungal infections, confusion, hallucinations, vomiting, abnormal gait, heart failure
and venous blood clotting (thrombosis/thromboembolism)
Very Rare (affects less than 1 user in 10,000):
Seizures
Not known (frequency cannot be estimated from the available data):
Inflammation of the pancreas and psychotic reactions
Alzheimer's disease has been associated with depression, suicidal ideation and suicide. These events
have been reported in patients treated with Axura.
If any o f the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Axura after the expiry date which is stated on the carton and the blister after EXP. The
expiry date refers to the last day of that month.
This medicinal product does not require any special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active substance is memantine hydrochloride. Each tablet contains 5/10/15/20 mg of memantine
hydrochloride equivalent to 4.15/8.31/12.46/16.62 mg memantine.
The other ingredients for Axura 5/10/15 and 20 mg film-coated tablets are microcrystalline cellulose,
croscarmellose sodium, colloidal anhydrous silica, magnesium stearate, all in the tablet core; and
hypromellose, macrogol 400, titanium dioxide (E 171) and additional for Axura 10 mg film-coated
tablets is iron oxide yellow (E172) and for Axura 15 mg and Axura 20 mg film-coated tablets are iron
oxide yellow and red (E 172), all in the tablet coating.
What Axura looks like and contents of the pack
Axura 5 mg film-coated tablets are presented as white to off-white, oval-oblong with imprint ‘5’ on one
side and imprint ‘MEM’ on the other side.
Axura 10 mg film-coated tablets are presented as pale yellow to yellow, oval shaped film-coated tablet
with breaking line and engravings "1-0" on one side and "M M" on the other side The tablet can be
divided into equal halves.
Axura 15 mg film-coated tablets are presented as orange to grey-orange, oval-oblong with imprint ‘15’
on one side and imprint ‘MEM’ on the other side.
Axura 20 mg film-coated tablets are presented as are pale red to grey-red, oval-oblong with imprint ‘20’
on one side and imprint ‘MEM’ on the other side.
One treatment initiation pack contains 28 tablets in 4 blisters with 7 tablets of Axura 5 mg, 7 tablets of
Axura 10 mg, 7 tablets of Axura 15 mg and 7 tablets of Axura 20 mg.
Marketing Authorisation Holder
Merz Pharmaceuticals GmbH
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany.
Merz Pharma GmbH + Co. KGaA
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany.
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder.
België/Belgique/Belgien
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Duitsland/Allemagne/Deutschland
Tél/Tel: +49 (0)69 1503 – 1
Luxembourg/Luxemburg
Ets. Hanff Frères
B.P. No. 1706
L-1017 Luxembourg
Tél: +352 45 07 07
България
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Германия
Tel.: +49 (0)69 1503 – 1
Magyarország
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Németország
Tel: +49 (0)69 1503 - 1
Česká republika
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Německo
Tel: +49 (0)69 1503 – 1
Malta
Clinipharm Co. Ltd
Farrugia Buildings
Triq tat-Torba
Attard BZN 12
Tel: +356 21 43 74 15
Danmark
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Tyskland
Nederland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Duitsland
Deutschland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Tel: +49 (0)69 1503 - 1
Norge
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Tyskland
Tlf: +49 (0)69 1503 – 1
Eesti
H. Abbe Pharma GmbH
Pirita tee 20
EE-10127 Tallinn
Tel.: +372 6 460980
Österreich
Merz Pharma Austria GmbH
Guglgasse 17
A- 11110 Wien
Tel: +43 (1) 869 16 04
Ελλάδα
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Γερμανία
Τηλ: +49 (0)69 1503 - 1
Polska
Natur Produkt Zdrovit Sp. z o.o.
ul. Nocznickiego 31
PL-01-918 Warszawa
Tel: +48 22 56 98 200
España
Grünenthal Pharma, S.A.
C/Dr. Zamenhof, 36
E-28027 Madrid
Tel: +34 (91) 301 93 00
Portugal
Grünenthal, S.A.
Rua Alfredo da Silva, 16
P-2610-016 Amadora
Tel: +351 / 214 72 63 00
France
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Allemagne
Tél: +49 (0)69 1503 – 1
România
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Germania
Tel.: +49 (0)69 1503 – 1
Ireland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Germany
Tel: +49 (0)69 1503 – 1
Slovenija
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Nemčija
Tel: +49 (0)69 1503 - 1
Ísland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Þýskaland
Tel: +49 (0)69 1503 – 1
Slovenská republika
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Nemecko
Tel: +49 (0)69 1503 - 1
Italia
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Germania
Tel: +49 (0)69 1503 – 1
Suomi/Finland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Saksa
Puh/Tel: +49 (0)69 1503 – 1
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Γερμανία
Tel: +49 (0)69 1503 - 1
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Tyskland
Tel: +49 (0)69 1503 – 1
Latvija
H. Abbe Pharma GmbH
Bauskas 58a
LV-1004 Riga
Tel.: +371 7 103203
United Kingdom
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Germany
Tel: +49 (0)69 1503 – 1
Lietuva
H. Abbe Pharma GmbH
M. Marcinkeviciaus g. 19-1
LT-2021 Vilnius
Tel.: +370 52 711710
This leaflet was last approved in MM/YYYY.
Detailed information on this product is available on the website of the European Medicines Agency
(EMA) http://www.ema.europa.eu
PACKAGE LEAFLET: INFORMATION FOR THE USER
Axura 20 mg film-coated tablets
Memantine hydrochloride
Read all of this leaflet carefully before you start taking this medicine.
-
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
1.
What Axura is and what it is used for
2.
Before you take Axura
3.
How to take Axura
4.
Possible side effects
5.
How to store Axura
6.
Further information
WHAT AXURA IS AND WHAT IT IS USED FOR
How does Axura work
Axura belongs to a group of medicines known as anti-dementia medicines.
Memory loss in Alzheimer’s disease is due to a disturbance of message signals in the brain. The brain
contains so-called N-methyl-D-aspartate (NMDA)-receptors that are involved in transmitting nerve
signals important in learning and memory. Axura belongs to a group of medicines called NMDA-
receptor antagonists. Axura acts on these NMDA-receptors improving the transmission of nerve
signals and the memory.
Axura is used for the treatment of patients with moderate to severe Alzheimer’s disease.
if you are allergic (hypersensitive) to memantine hydrochloride or any of the other ingredients
of Axura tablets (see section 6).
Take special care with Axura
-
if you have a history of epileptic seizures
if you have recently experienced a myocardial infarction (heart attack), or if you are suffering
from congestive heart failure or from an uncontrolled hypertension (high blood pressure).
In these situations the treatment should be carefully supervised, and the clinical benefit of Axura
reassessed by your doctor on a regular basis.
If you suffer from renal impairment (kidney problems), your doctor should closely monitor your
kidney function and if necessary adapt the memantine doses accordingly.
Keep this leaflet. You may need to read it again.
The use of medicinal products called amantadine (for the treatment of Parkinson´s disease), ketamine
(a substance generally used as an anaesthetic), dextromethorphan (generally used to treat cough) and
other NMDA-antagonists at the same time should be avoided.
Axura is not recommended for children and adolescents under the age of 18 years.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
In particular, Axura may change the effects of the following medicines and their dose may need to be
adjusted by your doctor:
amantadine, ketamine, dextromethorphan
dantrolene, baclofen
cimetidine, ranitidine, procainamide, quinidine, quinine, nicotine
hydrochlorothiazide (or any combination with hydrochlorothiazide)
anticholinergics (substances generally used to treat movement disorders or intestinal cramps)
anticonvulsants (substances used to prevent and relieve seizures)
barbiturates (substances generally used to induce sleep)
dopaminergic agonists (substances such as L-dopa, bromocriptine)
neuroleptics (substances used in the treatment of mental disorders)
oral anticoagulants
If you go into hospital, let your doctor know that you are taking Axura.
Taking Axura with food and drink
You should inform your doctor if you have recently changed or intend to change your diet
substantially (e.g. from normal diet to strict vegetarian diet) or if you are suffering from states of renal
tubulary acidosis (RTA, an excess of acid-forming substances in the blood due to renal dysfunction
(poor kidney function)) or severe infections of the urinary tract (structure that carries urine),as your
doctor may need to adjust the dose of your medicine.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking any medicine.
Tell your doctor if you are pregnant or planning to become pregnant. The use of memantine in
pregnant women is not recommended.
Women taking Axura should not breast-feed.
Driving and using machines
Your doctor will tell you whether your illness allows you to drive and to use machines safely.
Also, Axura may change your reactivity, making driving or operating machinery inappropriate.
Always take Axura exactly as your doctor has told you. You should check with your doctor or
pharmacist if you are not sure.
Dosage
The recommended dose of Axura for adults and elderly patients is 20 mg once a day.
In order to reduce the risk of side effects this dose is achieved gradually by the following daily
treatment scheme. For up-titration other tablet strengths are available.
At the beginning of treatment you will start by using Axura 5 mg film-coated tablets once a day. This
dose will be increased weekly by 5 mg until the recommended (maintenance) dose is reached. The
recommended maintenance dose is 20 mg once a day, which is reached at the beginning of the
4th week.
Dosage in patients with impaired kidney function
If you have impaired kidney function, your doctor will decide upon a dose that suits your condition. In
this case, monitoring of your kidney function should be performed by your doctor at specified
intervals.
Administration
Axura should be administered orally once a day. To benefit from your medicine you should take it
regularly every day at the same time of the day. The tablets should be swallowed with some water.
The tablets can be taken with or without food.
Duration of treatment
Continue to take Axura as long as it is of benefit to you. Your doctor should assess your treatment on a
regular basis.
If you take more Axura than you should
-
In general, taking too much Axura should not result in any harm to you. You may experience
increased symptoms as described in section 4. „Possible side effects“.
If you take a large overdose of Axura, contact your doctor or get medical advice, as you may
need medical attention.
If you forget to take Axura
-
If you find you have forgotten to take your dose of Axura, wait and take your next dose at the
usual time.
Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Like all medicines, Axura can cause side effects, although not everybody gets them.
In general, the observed side effects are mild to moderate.
Common (affects 1 to 10 users in 100):
Headache, sleepiness, constipation, dizziness, shortness of breath, high blood pressure and
drug hypersensitivity
Uncommon (affects 1 to 10 users in 1,000):
Tiredness, fungal infections, confusion, hallucinations, vomiting, abnormal gait, heart failure
and venous blood clotting (thrombosis/thromboembolism)
Very Rare (affects less than 1 user in 10,000):
Seizures
Not known (frequency cannot be estimated from the available data):
Inflammation of the pancreasand psychotic reactions
Alzheimer's disease has been associated with depression, suicidal ideation and suicide. These events
have been reported in patients treated with Axura.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Axura after the expiry date which is stated on the carton and the blister after EXP. The
expiry date refers to the last day of that month.
This medicinal product does not require any special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active substance is memantine hydrochloride. Each film-coated tablet contains 20 mg of
memantine hydrochloride equivalent to 16.62 mg memantine.
The other ingredients are microcrystalline cellulose, croscarmellose sodium, colloidal anhydrous
silica, magnesium stearate, all in the tablet core; and hypromellose, macrogol 400, titanium dioxide (E
171), iron oxide yellow and red (E 172), all in the tablet coating.
What Axura looks like and contents of the pack
Axura film-coated tablets are presented as pale red to grey-red, oval-oblong film-coated tablets with
imprint ‘20’ on one side and imprint ‘MEM’ on the other side.
Axura film-coated tablets are available in blister packs of 14 tablets, 28 tablets, 42 tablets, 56 tablets,
98 tablets or 840 (20 x 42) tablets
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Merz Pharmaceuticals GmbH
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany.
Merz Pharma GmbH + Co. KGaA
Eckenheimer Landstr. 100
D-60318 Frankfurt/Main
Germany.
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder.
België/Belgique/Belgien
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Duitsland/Allemagne/Deutschland
Tél/Tel: +49 (0)69 1503 – 1
Luxembourg/Luxemburg
Ets. Hanff Frères
B.P. No. 1706
L-1017 Luxembourg
Tél: +352 45 07 07
България
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Германия
Tel.: +49 (0)69 1503 – 1
Magyarország
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Németország
Tel: +49 (0)69 1503 - 1
Česká republika
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Německo
Tel: +49 (0)69 1503 – 1
Malta
Clinipharm Co. Ltd
Farrugia Buildings
Triq tat-Torba
Attard BZN 12
Tel: +356 21 43 74 15
Danmark
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Tyskland
Tlf: +49 (0)69 1503 – 1
Nederland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Duitsland
Tel: +49 (0)69 1503 – 1
Deutschland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Tel: +49 (0)69 1503 - 1
Norge
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Tyskland
Tlf: +49 (0)69 1503 – 1
Eesti
H. Abbe Pharma GmbH
Pirita tee 20
EE-10127 Tallinn
Tel.: +372 6 460980
Österreich
Merz Pharma Austria GmbH
Guglgasse 17
A- 1110 Wien
Tel: +43 (1) 869 16 04
Ελλάδα
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Γερμανία
Τηλ: +49 (0)69 1503 - 1
Polska
Natur Produkt Zdrovit Sp. z o.o.
ul. Nocznickiego 31
PL-01-918 Warszawa
Tel: +48 22 56 98 200
España
Grünenthal Pharma, S.A.
C/Dr. Zamenhof, 36
E-28027 Madrid
Tel: +34 (91) 301 93 00
Portugal
Grünenthal, S.A.
Rua Alfredo da Silva, 16
P-2610-016 Amadora
Tel: +351 / 214 72 63 00
France
Merz Pharmaceuticals GmbH
România
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Allemagne
Tél: +49 (0)69 1503 – 1
Eckenheimer Landstraße 100
D-60318 Frankfurt
Germania
Tel.: +49 (0)69 1503 – 1
Ireland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Germany
Tel: +49 (0)69 1503 – 1
Slovenija
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Nemčija
Tel: +49 (0)69 1503 - 1
Ísland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Þýskaland
Tel: +49 (0)69 1503 – 1
Slovenská republika
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Italia
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Germania
Tel: +49 (0)69 1503 - 1
Suomi/Finland
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Saksa
Puh/Tel: +49 (0)69 1503 – 1
Κύπρος
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Γερμανία
Tel: +49 (0)69 1503 - 1
Sverige
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Tyskland
Tel: +49 (0)69 1503 – 1
Latvija
H. Abbe Pharma GmbH
Bauskas 58a
LV-1004 Riga
Tel.: +371 7 103203
United Kingdom
Merz Pharmaceuticals GmbH
Eckenheimer Landstraße 100
D-60318 Frankfurt
Germany
Tel: +49 (0)69 1503 – 1
Lietuva
H. Abbe Pharma GmbH
M. Marcinkeviciaus g. 19-1
LT-2021 Vilnius
Tel.: +370 52 711710
This leaflet was last approved in MM/YYYY.
Detailed information on this product is available on the website of the European Medicines Agency
(EMA) http://www.ema.europa.eu
Nemecko
Tel: +49 (0)69 1503 - 1
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/axura.html
Copyright © 1995-2021 ITA all rights reserved.
|


































































































































































































































































































































































































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































