Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
BYETTA 5 micrograms solution for injection, prefilled pen
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each dose contains 5 micrograms(μg) synthetic exenatide in 20 microlitres(μl), (0.25 mg exenatide
per ml).
Excipients:
Each dose contains 44 µg metacresol.
This medicinal product contains less than 1mmol sodium per dose, i.e. essentially “sodium-free”.
For a full list of excipients, see section 6.1.
Solution for injection, pre-filled pen.
Clear, colourless solution.
4.1 Therapeutic indications
BYETTA is indicated for treatment of type 2 diabetes mellitus in combination with:
- metformin
- sulphonylureas
- thiazolidinediones
- metformin and a sulphonylurea
- metformin and a thiazolidinedione
in patients who have not achieved adequate glycaemic control on maximally tolerated doses of these
oral therapies.
4.2 Posology and method of administration
BYETTA therapy should be initiated at 5 μg exenatide per dose administered twice daily (BID) for at
least one month in order to improve tolerability. The dose of exenatide can then be increased to 10 μg
BID to further improve glycaemic control. Doses higher than 10µg BID are not recommended.
BYETTA is available as either a 5 µg or a 10 µg exenatide per dose pre-filled pen.
BYETTA can be administered at any time within the 60-minute period before the morning and
evening meal (or two main meals of the day, approximately 6 hours or more apart). BYETTA
should
not
be administered after a meal. If an injection is missed, the treatment should be continued with the
next scheduled dose.
Each dose should be administered as a subcutaneous injection in the thigh, abdomen, or upper arm.
BYETTA is recommended for use in patients with type 2 diabetes mellitus who are already receiving
metformin, a sulphonylurea or a thiazolidinedione. When BYETTA is added to existing metformin
and/or thiazolidinedione therapy, the current dose of metformin and/or thiazolidinedione can be
continued as no increased risk of hypoglycaemia is anticipated, compared to metformin or
thiazolidinedione alone. When BYETTA is added to sulphonylurea therapy, a reduction in the dose of
sulphonylurea should be considered to reduce the risk of hypoglycaemia (see section 4.4.).
The dose of BYETTA does not need to be adjusted on a day-by-day basis depending on self-
monitored glycaemia. However, blood glucose self-monitoring may become necessary to adjust the
dose of sulphonylureas.
Specific patient groups
Elderly
BYETTA should be used with caution and dose escalation from 5 µg to 10 µg should proceed
conservatively in patients >70 years. The clinical experience in patients >75 years is very limited.
Patients with renal impairment
No dosage adjustment of BYETTA is necessary in patients with mild renal impairment (creatinine
clearance 50 – 80 ml/min).
In patients with moderate renal impairment (creatinine clearance:30-50 ml/min), dose escalation from
5 µg to 10 µg should proceed conservatively (see section 5.2).
BYETTA is not recommended for use in patients with end-stage renal disease or severe renal
impairment (creatinine clearance <30 ml/min) (see section 4.4).
Patients with hepatic impairment
No dosage adjustment of BYETTA is necessary in patients with hepatic impairment (see section 5.2).
Children and adolescents
The safety and effectiveness of exenatide have not been established in patients under 18 years of age.
(see section 5.2).
Hypersensitivity to the active substance or to any of the excipients.
4.4 Special warnings and precautions for use
BYETTA should not be used in patients with type 1 diabetes mellitus or for the treatment of diabetic
ketoacidosis.
BYETTA should not be used in type 2 diabetes patients who require insulin therapy due to betacell
failure.
Intravenous or intramuscular injection of BYETTA is not recommended.
In patients with end-stage renal disease receiving dialysis, single doses of BYETTA 5 μg increased
frequency and severity of undesirable gastrointestinal effects. BYETTA is not recommended for use in
patients with end-stage renal disease or severe renal impairment (creatinine clearance <30 ml/min).
The clinical experience in patients with moderate renal impairment is very limited.
There have been rare, spontaneously reported events of altered renal function, including increased
serum creatinine, renal impairment, worsened chronic renal failure and acute renal failure, sometimes
requiring hemodialysis. Some of these events occurred in patients experiencing events that may affect
hydration, including nausea, vomiting, and/or diarrhoea and/or receiving pharmacological agents
known to affect renal function/hydration status. Concomitant agents included angiotensin converting
enzymes inhibitors, angiotensin-II antagonists, nonsteroidal anti-inflammatory medicinal products and
diuretics. Reversibility of altered renal function has been observed with supportive treatment and
discontinuation of potentially causative agents, including BYETTA.
BYETTA has not been studied in patients with severe gastrointestinal disease, including gastroparesis.
Its use is commonly associated with gastrointestinal adverse reactions, including nausea, vomiting,
and diarrhoea. Therefore, the use of BYETTA is not recommended in patients with severe
gastrointestinal disease.
There have been rare, spontaneously reported events of acute pancreatitis. Patients should be informed
of the characteristic symptom of acute pancreatitis: persistent, severe abdominal pain. Resolution of
pancreatitis has been observed with supportive treatment but very rare cases of necrotizing or
hemorrhagic pancreatitis and/or death have been reported. If pancreatitis is suspected, BYETTA and
other potentially suspect medicinal products should be discontinued. Treatment with BYETTA should
not be resumed after pancreatitis has been diagnosed.
The concurrent use of BYETTA with insulin, D-phenylalanine derivatives (meglitinides), or
alpha-glucosidase inhibitors has not
been studied and cannot be recommended.
The experience in patients with BMI ≤25 is limited.
This medicinal product contains metacresol, which may cause allergic reactions.
Weight loss
Weight loss greater than 1.5 kg per week has been observed in approximately 5% of clinical trial
patients treated with exenatide. Weight loss of this rate may have harmful consequences.
Hypoglycaemia
When BYETTA was used in combination with a sulphonylurea, the incidence of hypoglycaemia was
increased over that of placebo in combination with a sulphonylurea. In the clinical studies patients on
a sulphonylurea combination, with mild renal impairment had an increased incidence of
hypoglycaemia compared to patients with normal renal function. To reduce the risk of hypoglycaemia
associated with the use of a sulphonylurea, reduction in the dose of sulphonylurea should be
considered.
Interactions
The effect of BYETTA to slow gastric emptying may reduce the extent and rate of absorption of orally
administered medicinal products. BYETTA should be used with caution in patients receiving oral
medicinal products that require rapid gastrointestinal absorption and medicinal products with a narrow
therapeutic ratio. Specific recommendations regarding intake of such medicinal products in relation to
BYETTA is given in section 4.5.
4.5 Interaction with other medicinal products and other forms of interaction
The effect of BYETTA to slow gastric emptying may reduce the extent and rate of absorption of orally
administered medicinal products. Patients receiving medicinal products of either a narrow therapeutic
ratio or medicinal products that require careful clinical monitoring should be followed closely. These
medicinal products should be taken in a standardised way in relation to BYETTA injection. If such
medicinal products are to be administered with food, patients should be advised to, if possible, take
them with a meal when BYETTA is not administered.
For oral medicinal products that are particularly dependent on threshold concentrations for efficacy,
such as antibiotics, patients should be advised to take those medicinal products at least 1 hour before
BYETTA injection.
BYETTA is not expected to have any clinically relevant effects on the pharmacokinetics of metformin
or sulphonylureas. Hence no restriction in timing of intake of these medicinal products in relation to
BYETTA injection are needed.
Gastroresistant formulations containing substances sensitive for degradation in the stomach, such as
proton pump inhibitors, should be taken at least 1 hour before or more than 4 hours after BYETTA
injection.
Paracetamol
Paracetamol was used as a model medicinal product to evaluate the effect of exenatide on gastric
emptying. When 1000mg paracetamol was given with 10 µg BYETTA (0 h) and 1h, 2h and 4h after
BYETTA injection, paracetamol AUCs were decreased by 21 %, 23 %, 24 % and 14 % respectively;
C
max
was decreased by 37 %, 56 %, 54 % and 41 %, respectively; t
max
was increased from 0.6h in the
control period to 0.9h, 4.2h, 3.3h, and 1.6h, respectively. Paracetamol AUC, C
max
and t
max
were not
significantly changed when paracetamol was given 1 hour before BYETTA injection. No adjustment
to paracetamol dosing is required based on these study results.
HMG CoA reductase inhibitors
Lovastatin AUC and C
max
were decreased approximately 40 % and 28 %, respectively, and T
max
was
delayed about 4 h when BYETTA (10 μg BID) was administered concomitantly with a single dose of
lovastatin (40 mg) compared with lovastatin administered alone. In the 30-week placebo-controlled
clinical trials, concomitant use of BYETTA and HMG CoA reductase inhibitors was not associated
with consistent changes in lipid profiles (see section 5.1). Although no predetermined dose adjustment
is required, one should be aware of possible changes in LDL-C or total cholesterol. Lipid profiles
should be monitored regularly.
Digoxin, lisinopril and warfarin
A delay in t
max
of about 2h was observed when digoxin, lisinopril or warfarin was administered 30 min
after exenatide. No clinically relevant effects on C
max
or AUC were observed. However, since market
introduction, increased INR has been reported during concomitant use of warfarin and BYETTA. INR
should be closely monitored during initiation and dose increase of BYETTA therapy in patients on
warfarin and/or cumarol derivatives (see section 4.8).
Ethinyl estradiol and levonorgestrel
Administration of a combination oral contraceptive (30 µg ethinyl estradiol plus 150 µg
levonorgestrel) one hour before BYETTA (10 µg BID) did not alter the AUC, C
max
or C
min
of either
ethinyl estradiol or levonorgestrel. Administration of the oral contraceptive 30 minutes after BYETTA
did not affect AUC but resulted in a reduction of the C
max
of ethinyl estradiol by 45%, and C
max
of
levonorgestrel by 27-41%, and a delay in t
max
by 2-4 h due to delayed gastric emptying. The reduction
in C
max
is of limited clinical relevance and no adjustment of dosing of oral contraceptives is required.
4.6
Pregnancy and lactation
There are no adequate data from the use of BYETTA in pregnant women. Studies in animals have
shown reproductive toxicity (see section 5.3). The potential risk for humans is unknown. BYETTA
should not be used during pregnancy and the use of insulin is recommended. If a patient wishes to
become pregnant, or pregnancy occurs, treatment with BYETTA should be discontinued.
It is unknown whether exenatide is excreted in human milk. BYETTA should not be used if breast
feeding.
4.7
Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. When
BYETTA is used in combination with a sulphonylurea, patients should be advised to take precautions
to avoid hypoglycaemia while driving and using machines.
Table 1 lists adverse reactions reported from Phase 3 studies. The table presents adverse reactions that
occurred with an incidence ≥5 % and more frequently among BYETTA-treated patients than insulin-
or placebo-treated patients. The table also includes adverse reactions that occurred with an incidence
≥1 % and with a statistically significantly higher and/or ≥2X incidence among BYETTA-treated
patients than insulin- or placebo-treated patients.
The reactions are listed below as MedDRA preferred term by system organ class and absolute
frequency. Patient frequencies are defined as: very common (≥1/10), common (≥1/100, <1/10) and
uncommon (≥1/1,000 to <1/100).
Table 1: Adverse reactions reported in long term phase 3 controlled studies
1
Body system/adverse reaction
terms
Metabolism and nutrition
disorders
Hypoglycaemia (with
metformin and a
sulphonylurea)
2
Hypoglycaemia (with a
sulphonylurea)
Gastrointestinal disorders
Gastroesophageal reflux
disease
Skin and subcutaneous tissue
disorders
General disorders and
administrative site conditions
N= 1788 BYETTA-treated intent-to-treat (ITT) patients.
1
Data from Phase 3 comparator-controlled studies versus placebo, insulin glargine or 30 % soluble
insulin aspart/ 70 % insulin aspart protamine crystals (biphasic insulin aspart) in which patients also
received metformin, thiazolidinediones or sulphonylurea in addition to BYETTA or comparator.
2
In insulin-comparator controlled studies in which metformin and a sulphonylurea were concomitant
medicinal products, the incidence for these adverse reactions was similar for insulin- and BYETTA-
treated patients.






























3
Does not conform to criteria previously cited; acute pancreatitis events were uncommon in all
treatment groups.
Hypoglycaemia
In studies in patients treated with BYETTA and a sulphonylurea (with or without metformin), the
incidence of hypoglycaemia was increased compared to placebo (23.5 % and 25.2 % versus 12.6 %
and 3.3 %) and appeared to be dependent on the doses of both BYETTA and the sulphonylurea.
There were no clinically relevant differences in incidence or severity of hypoglycaemia with exenatide
compared to placebo, in combination with a thiazolidinedione, with or without metformin.
Hypoglycaemia was reported in 11% and 7% of patients treated with exenatide and placebo
respectively.
Most episodes of hypoglycaemia were mild to moderate in intensity, and resolved with oral
administration of carbohydrate.
Nausea
The most frequently reported adverse reaction was nausea. In patients treated with 5 µg or 10 µg
BYETTA, generally 40-50 % reported at least one episode of nausea. Most episodes of nausea were
mild to moderate and occurred in a dose-dependent fashion. With continued therapy, the frequency
and severity decreased in most patients who initially experienced nausea.
The incidence of withdrawal due to adverse events was 8 % for BYETTA-treated patients, 3 % for
placebo-treated and 1 % for insulin-treated patients in the long-term controlled trials (16 weeks or
longer). The most common adverse events leading to withdrawal for BYETTA-treated patients were
nausea (4 % of patients) and vomiting (1 %). For placebo-treated or insulin-treated patients, <1 %
withdrew due to nausea or vomiting.
BYETTA-treated patients in the open-label extension studies at 82 weeks experienced similar types of
adverse events observed in the controlled trials.
Injection site reactions
Injection site reactions have been reported in approximately 5.1 % of subjects receiving BYETTA in
long-term (16 weeks or longer) controlled trials. These reactions have usually been mild and usually
did not result in discontinuation of BYETTA.
Immunogenicity
Consistent with the potentially immunogenic properties of protein and peptide pharmaceuticals,
patients may develop anti-exenatide antibodies following treatment with BYETTA. In most patients
who develop antibodies, antibody titres diminish over time and remain low through 82 weeks.
Overall the percentage of antibody positive patients was consistent across clinical trials. Patients who
develop antibodies to exenatide tend to have more injection site reactions (for example: redness of
skin and itching), but otherwise similar rates and types of adverse events as those with no anti-
exenatide antibodies. In the three placebo-controlled trials (n=963) 38 % of patients had low titre anti-
exenatide antibodies at 30 weeks. For this group, the level of glycaemic control (HbA
1c
) was generally
comparable to that observed in those without antibody titres. An additional 6 % of patients had higher
titre antibodies at 30 weeks. About half of this 6 % (3 % of the total patients given BYETTA in the
controlled studies), had no apparent glycaemic response to BYETTA. In two insulin-comparator
controlled trials (n=475) comparable efficacy and adverse events were observed in BYETTA-treated
patients regardless of antibody titre.
Examination of antibody-positive specimens from one long-term uncontrolled study revealed no
significant cross-reactivity with similar endogenous peptides (glucagon or GLP-1).
Spontaneous reports
Since market introduction of BYETTA, the following additional adverse reactions have been reported:
Immune system disorders: anaphylactic reaction, very rarely.
Metabolism and nutritional disorders: dehydration, generally associated with nausea, vomiting and/or
diarrhoea.
Nervous system disorders: dysgeusia, somnolence.
Gastrointestinal disorders: eructation, constipation, flatulence.
Renal and urinary disorders: altered renal function, including acute renal failure, worsened chronic
renal failure, renal impairment, increased serum creatinine (see section 4.4).
Skin and subcutaneous tissue disorders: alopecia (rarely), macular rash, papular rash, pruruitis,
urticaria, angioneurotic oedema.
Investigations: international normalised ratio increased with concomitant warfarin, some reports
associated with bleeding (see section 4.5).
Signs and symptoms of overdose may include severe nausea, severe vomiting and rapidly declining
blood glucose concentrations. In the event of overdose, appropriate supportive treatment (possibly
given parenterally) should be initiated according to the patient’s clinical signs and symptoms.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Other blood glucose lowering drugs, excl. insulins, ATC code:
A10BX04.
Mechanism of action
Exenatide is a glucagon-like peptide-1 (GLP-1) receptor agonist that exhibits several
antihyperglycaemic actions of glucagon-like peptide-1 (GLP-1). The amino acid sequence of
exenatide partially overlaps that of human GLP-1. Exenatide has been shown to bind to and activate
the known human GLP-1 receptor
in vitro
, its mechanism of action mediated by cyclic AMP and/or
other intracellular signaling pathways.
Exenatide increases, on a glucose-dependent basis, the secretion of insulin from pancreatic beta cells.
As blood glucose concentrations decrease, insulin secretion subsides. When exenatide was used in
combination with metformin alone, no increase in the incidence of hypoglycaemia was observed over
that of placebo in combination with metformin which may be due to this glucose-dependent
insulinotropic mechanism. (see section 4.4).
Exenatide suppresses glucagon secretion which is known to be inappropriately elevated in type 2
diabetes. Lower glucagon concentrations lead to decreased hepatic glucose output. However,
exenatide does not impair the normal glucagon response and other hormone responses to
hypoglycaemia.
Exenatide slows gastric emptying thereby reducing the rate at which meal-derived glucose appears in
the circulation.
Pharmacodynamic effects
BYETTA improves glycaemic control through the immediate and sustained effects of lowering both
postprandial and fasting glucose concentrations in patients with type 2 diabetes.
Clinical efficacy
The clinical studies comprised 3945 subjects (2997 treated with exenatide), 56% men and 44%
women, 319 subjects (230 treated with exenatide) were ≥70 years of age and 34 subjects (27 treated
with exenatide) were ≥75 years of age.
BYETTA reduced HbA
1c
and body weight in patients treated for 30 weeks in three placebo-controlled
studies, whether the BYETTA was added to metformin, a sulphonylurea or a combination of both.
These reductions in HbA
1c
were generally observed at 12 weeks after initiation of treatment. See
Table 2. The reduction in HbA
1c
was sustained and the weight loss continued for at least 82 weeks in
the subset of 10 µg BID patients completing both the placebo-controlled studies and the uncontrolled
study extensions (n=137).
Table 2: Combined results of the 30 week placebo controlled studies (intent to treat patients)
HbA
1c
(%) change from
base line
Proportion of patients
(%) achieving HbA
1c
≤7%
Proportion of patients
(%) achieving HbA
1c
≤7% (patients
completing studies)
Change of weight from
baseline(kg)
Two placebo-controlled studies were conducted: one of 16 and one of 26 weeks duration, with 121
and 111 BYETTA and 112 and 54 placebo treated patients respectively, added to existing
thiazolidinedione treatment, with or without metformin. Of the BYETTA patients, 12% were treated
with a thiazolidinedione and BYETTA and 82% were treated with a thiazolidinedione, metformin and
BYETTA. BYETTA (5 µg BID for 4 weeks, followed by 10 µg BID) resulted in statistically
significant reductions from baseline HbA
1c
compared to placebo (-0.7% versus +0.1%) as well as
significant reductions in body weight (-1.5 versus 0 kg) in the 16 week study. The 26 week study
showed similar results with statistically significant reductions from baseline HbA
1c
compared to
placebo (-0.8% versus -0.1%). There was no significant difference in body weight between treatment
groups in change from baseline to endpoint (-1.4 versus -0.8 kg).
When BYETTA was used in combination with a thiazolidinedione, the incidence of hypoglycaemia
was similar to that of placebo in combination with a thiazolidinedione. The experience in patients > 65
years and in patients with impaired renal function is limited. The incidence and type of other adverse
events observed were similar to those seen in the 30-week controlled clinical trials with a
sulphonylurea, metformin or both.
In insulin-comparator studies BYETTA (5 µg BID for 4 weeks, followed by 10 µg BID) in
combination with metformin and sulphonylurea significantly (statistically and clinically) improved
glycaemic control, as measured by decrease in HbA
1c
. This treatment effect was comparable to that of
insulin glargine in a 26-week study (mean insulin dose 24.9 IU/day ,range 4-95 IU/day, at the end of
study) and biphasic insulin aspart in a 52-week study (mean insulin dose 24.4 IU/day, range 3-78
IU/day, at the end of study). BYETTA lowered HbA
1c
from 8.21 (n=228) and 8.6% (n=222) by 1.13
and 1.01% while insulin glargine lowered from 8.24 (n=227) by 1.10% and biphasic insulin aspart
from 8.67 (n=224) by 0.86%. Weight loss of 2.3 kg (2.6 %) was achieved with BYETTA in the 26
week study and a loss of 2.5 kg (2.7 %) in a 52-week study whereas treatment with insulin was
associated with weight gain. Treatment differences (BYETTA minus comparator) were -4.1 kg in the













26-week study and –5.4 kg in the 52-week study. Seven-point self monitored blood glucose profiles
(before and after meals and at 3 am) demonstrated significantly reduced glucose values compared to
insulin in the postprandial periods after BYETTA injection. Premeal blood glucose concentrations
were generally lower in patients taking insulin compared to BYETTA. Mean daily blood glucose
values were similar between BYETTA and insulin. In these studies the incidence of hypoglycaemia
was similar for BYETTA and insulin treatment.
BYETTA has shown no adverse effects on lipid parameters. A trend for a decrease in triglycerides has
been observed with weight loss.
Clinical studies with BYETTA have indicated improved beta-cell function, using measures such as the
homeostasis model assessment for beta-cell function (HOMA-B) and the proinsulin to insulin ratio.
A pharmacodynamic study demonstrated in patients with type 2 diabetes (n=13) a restoration of first
phase insulin secretion and improved second phase insulin secretion in response to an intravenous
bolus of glucose.
A reduction in body weight was seen in patients treated with BYETTA irrespective of the occurrence
of nausea although the reduction was larger in the group with nausea (mean reduction 2.4kg versus
1.7kg) in the long term controlled studies of up to 52 weeks.
Administration of exenatide has been shown to reduce food intake, due to decreased appetite and
increased satiety.
The European Medicines Agency has deferred the obligation to submit the results of studies with
BYETTA in one or more subsets of the paediatric population in type 2 diabetes mellitus (see section
4.2 for information on paediatric use).
5.2 Pharmacokinetic properties
Absorption
Following subcutaneous administration to patients with type 2 diabetes, exenatide reaches median
peak plasma concentrations in 2 h. Mean peak exenatide concentration (C
max
) was 211 pg/ml and
overall mean area under the curve (AUC
0-inf
) was 1036 pg •h/ml following subcutaneous
administration of a 10 μg dose of exenatide. Exenatide exposure increased proportionally over the
therapeutic dose range of 5 μg to 10 μg. Similar exposure is achieved with subcutaneous
administration of exenatide in the abdomen, thigh, or arm.
Distribution
The mean apparent volume of distribution of exenatide following subcutaneous administration of a
single dose of exenatide is 28 l.
Metabolism and Elimination
Nonclinical studies have shown that exenatide is predominantly eliminated by glomerular filtration
with subsequent proteolytic degradation. In clinical studies the mean apparent clearance of exenatide
is 9 l/h and the mean terminal half-life is 2.4 h. These pharmacokinetic characteristics of exenatide are
independent of the dose.
Special populations
Patients with renal impairment
In patients with mild (creatinine clearance 50 to 80 ml/min) or moderate renal impairment (creatinine
clearance 30 to 50 ml/min), exenatide clearance was mildly reduced compared to clearance in
individuals with normal renal function (13 % reduction in mild and 36 % reduction in moderate renal
impairment). Clearance was significantly reduced by 84% in patients with end-stage renal disease
receiving dialysis (see section 4.2).
Patients with hepatic insufficiency
No pharmacokinetic study has been performed in patients with hepatic insufficiency. Exenatide is
cleared primarily by the kidney, therefore hepatic dysfunction is not expected to affect blood
concentrations of exenatide.
Gender and race
Gender and race have no clinically relevant influence on exenatide pharmacokinetics.
Elderly
Long-term controlled data in elderly are limited, but suggest no marked changes in exenatide exposure
with increased age up to about 75 years old. In a pharmacokinetic study in patients with type 2
diabetes, administration of exenatide (10µg) resulted in a mean increase of exenatide AUC by 36% in
15 elderly subjects aged 75 to 85 years compared to 15 subjects aged 45 to 65 years likely related to
reduced renal function in the older age group (see section 4.2).
Children and adolescents
In a single-dose pharmacokinetic study in 13 patients with type 2 diabetes and between the ages of 12
and 16 years, administration of exenatide (5μg) resulted in slightly lower mean AUC (16% lower) and
Cmax (25% lower) compared to those observed in adults.
5.3 Preclinical safety data
Non-clinical data reveal no special hazards for humans based on conventional studies of safety
pharmacology, repeat-dose toxicity, or genotoxicity.
In female rats given exenatide for 2 years, an increased incidence of benign thyroid C−cell adenomas
was observed at the highest dose, 250 µg/kg/day, a dose that produced an exenatide plasma exposure
130-fold the human clinical exposure. This incidence was not statistically significant when adjusted
for survival. There was no tumorigenic response in male rats or either sex of mice.
Animal studies did not indicate direct harmful effects with respect to fertility or pregnancy. High doses
of exenatide during mid-gestation caused skeletal effects and reduced foetal growth in mice and
reduced foetal growth in rabbits. Neonatal growth was reduced in mice exposed to high doses during
late gestation and lactation.
PHARMACEUTICAL PARTICULARS
metacresol
mannitol
glacial acetic acid
sodium acetate trihydrate
water for injections
This medicinal product must not be mixed with other medicinal products.
3 years.
Shelf life for pen in use: 30 days.
6.4
Special precautions for storage
Store in a refrigerator (2 ºC - 8 ºC).
Do not freeze.
In use
Store below 25 ºC.
The pen should not be stored with the needle attached.
Replace cap on pen in order to protect from light.
6.5 Nature and contents of container
Type I glass cartridge with a (bromobutyl) rubber plunger, rubber disc, and aluminium seal. Each
cartridge is assembled into a disposable pen-injector (pen).
Each pre-filled pen contains 60 doses of sterile preserved solution (approximately 1.2ml)
Pack size of 1 and 3 pens. Not all pack sizes may be marketed.
Injection needles are not included. The following are examples of disposable needles that can be used
with the BYETTA pen: 29, 30 or 31 gauge (diameter 0.25 - 0.33mm) and 12.7, 8 or 5mm length.
6.6 Special precautions for disposal and other handling.
The patient should be instructed to discard the needle after each injection.
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
Instructions for use
BYETTA is for use by one person only.
The instructions for using the pen, included with the leaflet, must be followed carefully.
The pen is stored without needle.
BYETTA should not be used if particles appear or if the solution is cloudy and/or coloured.
BYETTA that has been frozen must not be used.
MARKETING AUTHORISATION HOLDER
Eli Lilly Nederland B.V., Grootslag 1-5, NL-3991 RA Houten, The Netherlands.
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
NAME OF THE MEDICINAL PRODUCT
BYETTA 10 micrograms solution for injection, prefilled pen
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each dose contains 10 micrograms(μg) synthetic exenatide in 40 microlitres(μl), (0.25 mg exenatide
per ml).
Excipients:
Each dose contains 88 µg metacresol.
This medicinal product contains less than 1mmol sodium per dose, i.e. essentially “sodium-free”.
For a full list of excipients, see section 6.1.
Solution for injection, pre-filled pen.
Clear, colourless solution.
4.1 Therapeutic indications
BYETTA is indicated for treatment of type 2 diabetes mellitus in combination with:
-metformin
-sulphonylureas
-thiazolidinediones
-metformin and a sulphonylurea
-metformin and a thiazolidinedione
in patients who have not achieved adequate glycaemic control on maximally tolerated doses of these
oral therapies.
4.2 Posology and method of administration
BYETTA therapy should be initiated at 5 μg exenatide per dose administered twice daily (BID) for at
least one month in order to improve tolerability. The dose of exenatide can then be increased to 10 μg
BID to further improve glycaemic control. Doses higher than 10µg BID are not recommended.
BYETTA is available as either a 5 µg or a 10 µg exenatide per dose pre-filled pen.
BYETTA can be administered at any time within the 60-minute period before the morning and
evening meal (or two main meals of the day, approximately 6 hours or more apart). BYETTA
should
not
be administered after a meal. If an injection is missed, the treatment should be continued with the
next scheduled dose.
Each dose should be administered as a subcutaneous injection in the thigh, abdomen, or upper arm.
BYETTA is recommended for use in patients with type 2 diabetes mellitus who are already receiving
metformin, a sulphonylurea or a thiazolidinedione. When BYETTA is added to existing metformin
and/or thiazolidinedione therapy, the current dose of metformin and/or thiazolidinedione can be
continued as no increased risk of hypoglycaemia is anticipated, compared to metformin or
thiazolidinedione alone. When BYETTA is added to sulphonylurea therapy, a reduction in the dose of
sulphonylurea should be considered to reduce the risk of hypoglycaemia (see section 4.4.).
The dose of BYETTA does not need to be adjusted on a day-by-day basis depending on self-
monitored glycaemia. However, blood glucose self-monitoring may become necessary to adjust the
dose of sulphonylureas.
Specific patient groups
Elderly
BYETTA should be used with caution and dose escalation from 5 µg to 10 µg should proceed
conservatively in patients >70 years. The clinical experience in patients >75 years is very limited.
Patients with renal impairment
No dosage adjustment of BYETTA is necessary in patients with mild renal impairment (creatinine
clearance 50 – 80 ml/min).
In patients with moderate renal impairment (creatinine clearance:30-50 ml/min), dose escalation from
5 µg to 10 µg should proceed conservatively (see section 5.2).
BYETTA is not recommended for use in patients with end-stage renal disease or severe renal
impairment (creatinine clearance <30 ml/min) (see section 4.4).
Patients with hepatic impairment
No dosage adjustment of BYETTA is necessary in patients with hepatic impairment (see section 5.2).
Children and adolescents
The safety and effectiveness of exenatide have not been established in patients under 18 years of age.
(see section 5.2).
Hypersensitivity to the active substance or to any of the excipients.
4.4 Special warnings and precautions for use
BYETTA should not be used in patients with type 1 diabetes mellitus or for the treatment of diabetic
ketoacidosis.
BYETTA should not be used in type 2 diabetes patients who require insulin therapy due to betacell
failure.
Intravenous or intramuscular injection of BYETTA is not recommended.
In patients with end-stage renal disease receiving dialysis, single doses of BYETTA 5 μg increased
frequency and severity of undesirable gastrointestinal effects. BYETTA is not recommended for use in
patients with end-stage renal disease or severe renal impairment (creatinine clearance <30 ml/min).
The clinical experience in patients with moderate renal impairment is very limited.
There have been rare, spontaneously reported events of altered renal function, including increased
serum creatinine, renal impairment, worsened chronic renal failure and acute renal failure, sometimes
requiring hemodialysis. Some of these events occurred in patients experiencing events that may affect
hydration, including nausea, vomiting, and/or diarrhoea and/or receiving pharmacological agents
known to affect renal function/hydration status. Concomitant agents included angiotensin converting
enzymes inhibitors, angiotensin-II antagonists, nonsteroidal anti-inflammatory medicinal products and
diuretics. Reversibility of altered renal function has been observed with supportive treatment and
discontinuation of potentially causative agents, including BYETTA.
BYETTA has not been studied in patients with severe gastrointestinal disease, including gastroparesis.
Its use is commonly associated with gastrointestinal adverse reactions, including nausea, vomiting,
and diarrhoea. Therefore, the use of BYETTA is not recommended in patients with severe
gastrointestinal disease.
There have been rare, spontaneously reported events of acute pancreatitis. Patients should be informed
of the characteristic symptom of acute pancreatitis: persistent, severe abdominal pain. Resolution of
pancreatitis has been observed with supportive treatment but very rare cases of necrotizing or
hemorrhagic pancreatitis and/or death have been reported. If pancreatitis is suspected, BYETTA and
other potentially suspect medicinal products should be discontinued. Treatment with BYETTA should
not be resumed after pancreatitis has been diagnosed.
The concurrent use of BYETTA with insulin, D-phenylalanine derivatives (meglitinides), or
alpha-glucosidase inhibitors has not
been studied and cannot be recommended.
The experience in patients with BMI ≤25 is limited.
This medicinal product contains metacresol, which may cause allergic reactions.
Weight loss
Weight loss greater than 1.5 kg per week has been observed in approximately 5% of clinical trial
patients treated with exenatide. Weight loss of this rate may have harmful consequences.
Hypoglycaemia
When BYETTA was used in combination with a sulphonylurea, the incidence of hypoglycaemia was
increased over that of placebo in combination with a sulphonylurea. In the clinical studies patients on
a sulphonylurea combination, with mild renal impairment had an increased incidence of
hypoglycaemia compared to patients with normal renal function. To reduce the risk of hypoglycaemia
associated with the use of a sulphonylurea, reduction in the dose of sulphonylurea should be
considered.
Interactions
The effect of BYETTA to slow gastric emptying may reduce the extent and rate of absorption of orally
administered medicinal products. BYETTA should be used with caution in patients receiving oral
medicinal products that require rapid gastrointestinal absorption and medicinal products with a narrow
therapeutic ratio. Specific recommendations regarding intake of such medicinal products in relation to
BYETTA is given in section 4.5.
4.5 Interaction with other medicinal products and other forms of interaction
The effect of BYETTA to slow gastric emptying may reduce the extent and rate of absorption of orally
administered medicinal products. Patients receiving medicinal products of either a narrow therapeutic
ratio or medicinal products that require careful clinical monitoring should be followed closely. These
medicinal products should be taken in a standardised way in relation to BYETTA injection. If such
medicinal products are to be administered with food, patients should be advised to, if possible, take
them with a meal when BYETTA is not administered.
For oral medicinal products that are particularly dependent on threshold concentrations for efficacy,
such as antibiotics, patients should be advised to take those medicinal products at least 1 hour before
BYETTA injection.
BYETTA is not expected to have any clinically relevant effects on the pharmacokinetics of metformin
or sulphonylureas. Hence no restriction in timing of intake of these medicinal products in relation to
BYETTA injection are needed.
Gastroresistant formulations containing substances sensitive for degradation in the stomach, such as
proton pump inhibitors, should be taken at least 1 hour before or more than 4 hours after BYETTA
injection.
Paracetamol
Paracetamol was used as a model medicinal product to evaluate the effect of exenatide on gastric
emptying. When 1000mg paracetamol was given with 10 µg BYETTA (0 h) and 1h, 2h and 4h after
BYETTA injection, paracetamol AUCs were decreased by 21 %, 23 %, 24 % and 14 % respectively;
C
max
was decreased by 37 %, 56 %, 54 % and 41 %, respectively; t
max
was increased from 0.6h in the
control period to 0.9h, 4.2h, 3.3h, and 1.6h, respectively. Paracetamol AUC, C
max
and t
max
were not
significantly changed when paracetamol was given 1 hour before BYETTA injection. No adjustment
to paracetamol dosing is required based on these study results.
HMG CoA reductase inhibitors
Lovastatin AUC and C
max
were decreased approximately 40 % and 28 %, respectively, and T
max
was
delayed about 4 h when BYETTA (10 μg BID) was administered concomitantly with a single dose of
lovastatin (40 mg) compared with lovastatin administered alone. In the 30-week placebo-controlled
clinical trials, concomitant use of BYETTA and HMG CoA reductase inhibitors was not associated
with consistent changes in lipid profiles (see section 5.1). Although no predetermined dose adjustment
is required, one should be aware of possible changes in LDL-C or total cholesterol. Lipid profiles
should be monitored regularly.
Digoxin, lisinopril and warfarin
A delay in t
max
of about 2h was observed when digoxin, lisinopril or warfarin was administered 30 min
after exenatide. No clinically relevant effects on C
max
or AUC were observed. However, since market
introduction, increased INR has been reported during concomitant use of warfarin and BYETTA. INR
should be closely monitored during initiation and dose increase of BYETTA therapy in patients on
warfarin and/or cumarol derivatives (see section 4.8).
Ethinyl estradiol and levonorgestrel
Administration of a combination oral contraceptive (30 µg ethinyl estradiol plus 150 µg
levonorgestrel) one hour before BYETTA (10 µg BID) did not alter the AUC, C
max
or C
min
of either
ethinyl estradiol or levonorgestrel. Administration of the oral contraceptive 30 minutes after BYETTA
did not affect AUC but resulted in a reduction of the C
max
of ethinyl estradiol by 45%, and C
max
of
levonorgestrel by 27-41%, and a delay in t
max
by 2-4 h due to delayed gastric emptying. The reduction
in C
max
is of limited clinical relevance and no adjustment of dosing of oral contraceptives is required.
4.6 Pregnancy and lactation
There are no adequate data from the use of BYETTA in pregnant women. Studies in animals have
shown reproductive toxicity (see section 5.3). The potential risk for humans is unknown. BYETTA
should not be used during pregnancy and the use of insulin is recommended. If a patient wishes to
become pregnant, or pregnancy occurs, treatment with BYETTA should be discontinued.
It is unknown whether exenatide is excreted in human milk. BYETTA should not be used if breast
feeding.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. When
BYETTA is used in combination with a sulphonylurea, patients should be advised to take precautions
to avoid hypoglycaemia while driving and using machines.
Table 1 lists adverse reactions reported from Phase 3 studies. The table presents adverse reactions that
occurred with an incidence ≥5 % and more frequently among BYETTA-treated patients than insulin-
or placebo-treated patients. The table also includes adverse reactions that occurred with an incidence
≥1 % and with a statistically significantly higher and/or ≥2X incidence among BYETTA-treated
patients than insulin- or placebo-treated patients.
The reactions are listed below as MedDRA preferred term by system organ class and absolute
frequency. Patient frequencies are defined as: very common (≥1/10), common (≥1/100, <1/10) and
uncommon (≥1/1,000 to <1/100).
Table 1: Adverse reactions reported in long term phase 3 controlled studies
1
Body system/adverse reaction
terms
Metabolism and nutrition
disorders
Hypoglycaemia (with
metformin and a sulphonylurea)
2
Hypoglycaemia (with a
sulphonylurea)
Gastrointestinal disorders
Gastroesophageal reflux disease
Skin and subcutaneous tissue
disorders
General disorders and
administrative site conditions
N= 1788 BYETTA-treated intent-to-treat (ITT) patients.
1
Data from Phase 3 comparator-controlled studies versus placebo, insulin glargine or 30 % soluble
insulin aspart/ 70 % insulin aspart protamine crystals (biphasic insulin aspart) in which patients also
received metformin, thiazolidinediones or sulphonylurea in addition to BYETTA or comparator.
2
In insulin-comparator controlled studies in which metformin and a sulphonylurea were concomitant
medicinal products, the incidence for these adverse reactions was similar for insulin- and BYETTA-
treated patients.
3
Does not conform to criteria previously cited; acute pancreatitis events were uncommon in all
treatment groups.






























Hypoglycaemia
In studies in patients treated with BYETTA and a sulphonylurea (with or without metformin), the
incidence of hypoglycaemia was increased compared to placebo (23.5 % and 25.2 % versus 12.6 %
and 3.3 %) and appeared to be dependent on the doses of both BYETTA and the sulphonylurea.
There were no clinically relevant differences in incidence or severity of hypoglycaemia with exenatide
compared to placebo, in combination with a thiazolidinedione, with or without metformin.
Hypoglycaemia was reported in 11% and 7% of patients treated with exenatide and placebo
respectively.
Most episodes of hypoglycaemia were mild to moderate in intensity, and resolved with oral
administration of carbohydrate.
Nausea
The most frequently reported adverse reaction was nausea. In patients treated with 5 µg or 10 µg
BYETTA, generally 40-50 % reported at least one episode of nausea. Most episodes of nausea were
mild to moderate and occurred in a dose-dependent fashion. With continued therapy, the frequency
and severity decreased in most patients who initially experienced nausea.
The incidence of withdrawal due to adverse events was 8 % for BYETTA-treated patients, 3 % for
placebo-treated and 1 % for insulin-treated patients in the long-term controlled trials (16 weeks or
longer). The most common adverse events leading to withdrawal for BYETTA-treated patients were
nausea (4 % of patients) and vomiting (1 %). For placebo-treated or insulin-treated patients, <1 %
withdrew due to nausea or vomiting.
BYETTA-treated patients in the open-label extension studies at 82 weeks experienced similar types of
adverse events observed in the controlled trials.
Injection site reactions
Injection site reactions have been reported in approximately 5.1 % of subjects receiving BYETTA in
long-term (16 weeks or longer) controlled trials. These reactions have usually been mild and usually
did not result in discontinuation of BYETTA.
Immunogenicity
Consistent with the potentially immunogenic properties of protein and peptide pharmaceuticals,
patients may develop anti-exenatide antibodies following treatment with BYETTA. In most patients
who develop antibodies, antibody titres diminish over time and remain low through 82 weeks.
Overall the percentage of antibody positive patients was consistent across clinical trials. Patients who
develop antibodies to exenatide tend to have more injection site reactions (for example: redness of
skin and itching), but otherwise similar rates and types of adverse events as those with no anti-
exenatide antibodies. In the three placebo-controlled trials (n=963) 38 % of patients had low titre anti-
exenatide antibodies at 30 weeks. For this group, the level of glycaemic control (HbA
1c
) was generally
comparable to that observed in those without antibody titres. An additional 6 % of patients had higher
titre antibodies at 30 weeks. About half of this 6 % (3 % of the total patients given BYETTA in the
controlled studies), had no apparent glycaemic response to BYETTA. In two insulin-comparator
controlled trials (n=475) comparable efficacy and adverse events were observed in BYETTA-treated
patients regardless of antibody titre.
Examination of antibody-positive specimens from one long-term uncontrolled study revealed no
significant cross-reactivity with similar endogenous peptides (glucagon or GLP-1).
Spontaneous reports
Since market introduction of BYETTA, the following additional adverse reactions have been reported:
Immune system disorders: anaphylactic reaction, very rarely.
Metabolism and nutritional disorders: dehydration, generally associated with nausea, vomiting and/or
diarrhoea.
Nervous system disorders: dysgeusia, somnolence.
Gastrointestinal disorders: eructation, constipation, flatulence.
Renal and urinary disorders: altered renal function, including acute renal failure, worsened chronic
renal failure, renal impairment, increased serum creatinine (see section 4.4).
Skin and subcutaneous tissue disorders: alopecia (rarely), macular rash, papular rash, pruruitis,
urticaria, angioneurotic oedema.
Investigations: international normalised ratio increased with concomitant warfarin, some reports
associated with bleeding (see section 4.5).
Signs and symptoms of overdose may include severe nausea, severe vomiting and rapidly declining
blood glucose concentrations. In the event of overdose, appropriate supportive treatment (possibly
given parenterally) should be initiated according to the patient’s clinical signs and symptoms.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Other blood glucose lowering drugs, excl. insulins, ATC code:
A10BX04.
Mechanism of action
Exenatide is a glucagon-like peptide-1 (GLP-1) receptor agonist that exhibits several
antihyperglycaemic actions of glucagon-like peptide-1 (GLP-1). The amino acid sequence of
exenatide partially overlaps that of human GLP-1. Exenatide has been shown to bind to and activate
the known human GLP-1 receptor
in vitro
, its mechanism of action mediated by cyclic AMP and/or
other intracellular signaling pathways.
Exenatide increases, on a glucose-dependent basis, the secretion of insulin from pancreatic beta cells.
As blood glucose concentrations decrease, insulin secretion subsides. When exenatide was used in
combination with metformin alone, no increase in the incidence of hypoglycaemia was observed over
that of placebo in combination with metformin which may be due to this glucose-dependent
insulinotropic mechanism. (see section 4.4).
Exenatide suppresses glucagon secretion which is known to be inappropriately elevated in type 2
diabetes. Lower glucagon concentrations lead to decreased hepatic glucose output. However,
exenatide does not impair the normal glucagon response and other hormone responses to
hypoglycaemia.
Exenatide slows gastric emptying thereby reducing the rate at which meal-derived glucose appears in
the circulation.
Pharmacodynamic effects
BYETTA improves glycaemic control through the immediate and sustained effects of lowering both
postprandial and fasting glucose concentrations in patients with type 2 diabetes.
Clinical efficacy
The clinical studies comprised 3945 subjects (2997 treated with exenatide), 56% men and 44%
women, 319 subjects (230 treated with exenatide) were ≥70 years of age and 34 subjects (27 treated
with exenatide) were ≥75 years of age.
BYETTA reduced HbA
1c
and body weight in patients treated for 30 weeks in three placebo-controlled
studies, whether the BYETTA was added to metformin, a sulphonylurea or a combination of both.
These reductions in HbA
1c
were generally observed at 12 weeks after initiation of treatment. See
Table 2. The reduction in HbA
1c
was sustained and the weight loss continued for at least 82 weeks in
the subset of 10 µg BID patients completing both the placebo-controlled studies and the uncontrolled
study extensions (n=137).
Table 2: Combined results of the 30 week placebo controlled studies (intent to treat patients)
HbA
1c
(%) change from
base line
Proportion of patients
(%) achieving HbA
1c
≤7%
Proportion of patients
(%) achieving HbA
1c
≤7% (patients
completing studies)
Change of weight from
baseline(kg)
Two placebo-controlled studies were conducted: one of 16 and one of 26 weeks duration, with 121
and 111 BYETTA and 112 and 54 placebo treated patients respectively, added to existing
thiazolidinedione treatment, with or without metformin. Of the BYETTA patients, 12% were treated
with a thiazolidinedione and BYETTA and 82% were treated with a thiazolidinedione, metformin and
BYETTA. BYETTA (5 µg BID for 4 weeks, followed by 10 µg BID) resulted in statistically
significant reductions from baseline HbA
1c
compared to placebo (-0.7% versus +0.1%) as well as
significant reductions in body weight (-1.5 versus 0 kg) in the 16 week study. The 26 week study
showed similar results with statistically significant reductions from baseline HbA
1c
compared to
placebo (-0.8% versus -0.1%). There was no significant difference in body weight between treatment
groups in change from baseline to endpoint (-1.4 versus -0.8 kg).
When BYETTA was used in combination with a thiazolidinedione, the incidence of hypoglycaemia
was similar to that of placebo in combination with a thiazolidinedione. The experience in patients > 65
years and in patients with impaired renal function is limited. The incidence and type of other adverse
events observed were similar to those seen in the 30-week controlled clinical trials with a
sulphonylurea, metformin or both.
In insulin-comparator studies BYETTA (5 µg BID for 4 weeks, followed by 10 µg BID) in
combination with metformin and sulphonylurea significantly (statistically and clinically) improved
glycaemic control, as measured by decrease in HbA
1c
. This treatment effect was comparable to that of
insulin glargine in a 26-week study (mean insulin dose 24.9 IU/day ,range 4-95 IU/day, at the end of
study) and biphasic insulin aspart in a 52-week study (mean insulin dose 24.4 IU/day, range 3-78
IU/day, at the end of study). BYETTA lowered HbA
1c
from 8.21 (n=228) and 8.6% (n=222) by 1.13
and 1.01% while insulin glargine lowered from 8.24 (n=227) by 1.10% and biphasic insulin aspart
from 8.67 (n=224) by 0.86%. Weight loss of 2.3 kg (2.6 %) was achieved with BYETTA in the 26
week study and a loss of 2.5 kg (2.7 %) in a 52-week study whereas treatment with insulin was
associated with weight gain. Treatment differences (BYETTA minus comparator) were -4.1 kg in the
26-week study and –5.4 kg in the 52-week study. Seven-point self monitored blood glucose profiles













(before and after meals and at 3 am) demonstrated significantly reduced glucose values compared to
insulin in the postprandial periods after BYETTA injection. Premeal blood glucose concentrations
were generally lower in patients taking insulin compared to BYETTA. Mean daily blood glucose
values were similar between BYETTA and insulin. In these studies the incidence of hypoglycaemia
was similar for BYETTA and insulin treatment.
BYETTA has shown no adverse effects on lipid parameters. A trend for a decrease in triglycerides has
been observed with weight loss.
Clinical studies with BYETTA have indicated improved beta-cell function, using measures such as the
homeostasis model assessment for beta-cell function (HOMA-B) and the proinsulin to insulin ratio.
A pharmacodynamic study demonstrated in patients with type 2 diabetes (n=13) a restoration of first
phase insulin secretion and improved second phase insulin secretion in response to an intravenous
bolus of glucose.
A reduction in body weight was seen in patients treated with BYETTA irrespective of the occurrence
of nausea although the reduction was larger in the group with nausea (mean reduction 2.4kg versus
1.7kg) in the long term controlled studies of up to 52 weeks.
Administration of exenatide has been shown to reduce food intake, due to decreased appetite and
increased satiety.
The European Medicines Agency has deferred the obligation to submit the results of studies with
BYETTA in one or more subsets of the paediatric population in type 2 diabetes mellitus (see section
4.2 for information on paediatric use).
5.2 Pharmacokinetic properties
Absorption
Following subcutaneous administration to patients with type 2 diabetes, exenatide reaches median
peak plasma concentrations in 2 h. Mean peak exenatide concentration (C
max
) was 211 pg/ml and
overall mean area under the curve (AUC
0-inf
) was 1036 pg •h/ml following subcutaneous
administration of a 10 μg dose of exenatide. Exenatide exposure increased proportionally over the
therapeutic dose range of 5 μg to 10 μg. Similar exposure is achieved with subcutaneous
administration of exenatide in the abdomen, thigh, or arm.
Distribution
The mean apparent volume of distribution of exenatide following subcutaneous administration of a
single dose of exenatide is 28 l.
Metabolism and Elimination
Nonclinical studies have shown that exenatide is predominantly eliminated by glomerular filtration
with subsequent proteolytic degradation. In clinical studies the mean apparent clearance of exenatide
is 9 l/h and the mean terminal half-life is 2.4 h. These pharmacokinetic characteristics of exenatide are
independent of the dose.
Special populations
Patients with renal impairment
In patients with mild (creatinine clearance 50 to 80 ml/min) or moderate renal impairment (creatinine
clearance 30 to 50 ml/min), exenatide clearance was mildly reduced compared to clearance in
individuals with normal renal function (13 % reduction in mild and 36 % reduction in moderate renal
impairment). Clearance was significantly reduced by 84% in patients with end-stage renal disease
receiving dialysis (see section 4.2).
Patients with hepatic insufficiency
No pharmacokinetic study has been performed in patients with hepatic insufficiency. Exenatide is
cleared primarily by the kidney, therefore hepatic dysfunction is not expected to affect blood
concentrations of exenatide.
Gender and race
Gender and race have no clinically relevant influence on exenatide pharmacokinetics.
Elderly
Long-term controlled data in elderly are limited, but suggest no marked changes in exenatide exposure
with increased age up to about 75 years old. In a pharmacokinetic study in patients with type 2
diabetes, administration of exenatide (10µg) resulted in a mean increase of exenatide AUC by 36% in
15 elderly subjects aged 75 to 85 years compared to 15 subjects aged 45 to 65 years likely related to
reduced renal function in the older age group (see section 4.2).
Children and adolescents
In a single-dose pharmacokinetic study in 13 patients with type 2 diabetes and between the ages of 12
and 16 years, administration of exenatide (5μg) resulted in slightly lower mean AUC (16% lower) and
Cmax (25% lower) compared to those observed in adults.
5.3 Preclinical safety data
Non-clinical data reveal no special hazards for humans based on conventional studies of safety
pharmacology, repeat-dose toxicity, or genotoxicity.
In female rats given exenatide for 2 years, an increased incidence of benign thyroid C−cell adenomas
was observed at the highest dose, 250 µg/kg/day, a dose that produced an exenatide plasma exposure
130-fold the human clinical exposure. This incidence was not statistically significant when adjusted
for survival. There was no tumorigenic response in male rats or either sex of mice.
Animal studies did not indicate direct harmful effects with respect to fertility or pregnancy. High doses
of exenatide during mid-gestation caused skeletal effects and reduced foetal growth in mice and
reduced foetal growth in rabbits. Neonatal growth was reduced in mice exposed to high doses during
late gestation and lactation.
PHARMACEUTICAL PARTICULARS
metacresol
mannitol
glacial acetic acid
sodium acetate trihydrate
water for injections
This medicinal product must not be mixed with other medicinal products.
3 years.
Shelf life for pen in use: 30 days.
6.4 Special precautions for storage
Store in a refrigerator (2 ºC - 8 ºC).
Do not freeze.
In use
Store below 25 ºC.
The pen should not be stored with the needle attached.
Replace cap on pen in order to protect from light.
6.5 Nature and contents of container
Type I glass cartridge with a (bromobutyl) rubber plunger, rubber disc, and aluminium seal. Each
cartridge is assembled into a disposable pen-injector (pen).
Each pre-filled pen contains 60 doses of sterile preserved solution (approximately 2.4ml)
Pack size of 1 and 3 pens. Not all pack sizes may be marketed.
Injection needles are not included. The following are examples of disposable needles that can be used
with the BYETTA pen: 29, 30 or 31 gauge (diameter 0.25 - 0.33mm) and 12.7, 8 or 5mm length.
6.6 Special precautions for disposal and other handling.
The patient should be instructed to discard the needle after each injection.
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
Instructions for use
BYETTA is for use by one person only.
The instructions for using the pen, included with the leaflet, must be followed carefully.
The pen is stored without needle.
BYETTA should not be used if particles appear or if the solution is cloudy and/or coloured.
BYETTA that has been frozen must not be used.
MARKETING AUTHORISATION HOLDER
Eli Lilly Nederland B.V., Grootslag 1-5, NL-3991 RA Houten, The Netherlands.
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
A. MANUFACTURING AUTHORISATION HOLDERS
RESPONSIBLE FOR BATCH RELEASE
B. CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURING AUTHORISATION HOLDERS RESPONSIBLE FOR
BATCH RELEASE
Name and address of the manufacturer responsible for batch release
Lilly Pharma Fertigung und Distribution GmbH & Co. KG
Teichweg 3
35396 Giessen
Germany
B. CONDITIONS OF THE MARKETING AUTHORISATION
•
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE
IMPOSED ON THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to medical prescription.
•
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
Pharmacovigilance system
The MAH must ensure that the system of pharmacovigilance, as described in version 2.4
presented in Module 1.8.1. of the Marketing Authorisation Application, is in place and
functioning before and whilst the product is on the market.
Risk Management Plan
The MAH commits to performing the studies and additional pharmacovigilance activities
detailed in the Pharmacovigilance Plan, as agreed in version Revision 10 of the Risk
Management Plan (RMP) presented in Module 1.8.2 of the Marketing Authorisation
Application and any subsequent updates of the RMP agreed by the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, any
updated RMP should be submitted at the same time as the following Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted:
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
At the request of the European Medicines Agency
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
{CARTON OF ONE}
{CARTON OF THREE}
NAME OF THE MEDICINAL PRODUCT
Byetta 5 micrograms solution for injection, prefilled pen
Exenatide
STATEMENT OF ACTIVE SUBSTANCE(S)
Each dose contains 5 micrograms exenatide.
Mannitol, glacial acetic acid, sodium acetate trihydrate, water for injections.
Contains metacresol. See leaflet for further information.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection, pre-filled pen
1 pen (60 doses)
3 pens (3 X 60 doses)
METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet and pen user manual before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
If the seal is broken before first use, contact your pharmacist.
EXP
{
MM/YYYY
}
Discard pen 30 days after first use.





















SPECIAL STORAGE CONDITIONS
Store in a refrigerator.
Do not freeze.
Once in use: Store below 25 ºC for 30 days.
Do not store with needle attached.
Recap pen to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Eli Lilly Nederland B.V.
Grootslag 1-5, 3991 RA Houten
The Netherlands
MARKETING AUTHORISATION NUMBER(S)
EU/1/06/362/001
EU/1/06/362/002
GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.




















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
{CARTON OF ONE}
{CARTON OF THREE}
NAME OF THE MEDICINAL PRODUCT
Byetta 10 micrograms solution for injection, prefilled pen
Exenatide
STATEMENT OF ACTIVE SUBSTANCE(S)
Each dose contains 10 micrograms exenatide.
Mannitol, glacial acetic acid, sodium acetate trihydrate, water for injections.
Contains metacresol. See leaflet for further information.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection, pre-filled pen
1 pen (60 doses)
3 pens (3 X 60 doses)
METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet and pen user manual before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
If the seal is broken before first use, contact your pharmacist.
EXP
{
MM/YYYY
}
Discard pen 30 days after first use.























SPECIAL STORAGE CONDITIONS
Store in a refrigerator.
Do not freeze.
Once in use: Store below 25 ºC for 30 days.Do not store with needle attached.
Recap pen to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Eli Lilly Nederland B.V.
Grootslag 1-5, 3991 RA Houten
The Netherlands
MARKETING AUTHORISATION NUMBER(S)
EU/1/06/362/003
EU/1/06/362/004
GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.



















PACKAGE LEAFLET: INFORMATION FOR THE USER
BYETTA 5 micrograms solution for injection, pre-filled pen
BYETTA 10 micrograms solution for injection, pre-filled pen
(exenatide)
Read all of this leaflet carefully before you start using this medicine.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or your pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
1. What BYETTA is and what it is used for
2. Before you use BYETTA
3. How to use BYETTA
4. Possible side effects
5. How to store BYETTA
6. Further information
1. WHAT BYETTA IS AND WHAT IT IS USED FOR
BYETTA is an injectable medicine used to improve blood sugar control in adults with type 2 (non
insulin dependent) diabetes mellitus.
BYETTA is used with other diabetic medicines called metformin, sulphonylureas or
thiazolidinediones. Your doctor is now prescribing BYETTA as an additional medicine to help control
your blood sugar. Continue to follow your food and exercise plan.
You have diabetes because your body does not make enough insulin to control the level of sugar in
your blood or if your body is not able to use the insulin properly. BYETTA helps your body to
increase the production of insulin when your blood sugar is high.
If you are allergic (hypersensitive) to exenatide or any of the other ingredients of BYETTA,
listed at the end of this leaflet.
Take special care with BYETTA:
When using it in combination with a sulphonylurea, as low blood sugar (hypoglycaemia) can
occur. Ask your doctor or pharmacist if you are not sure if any of your other medicines contain a
sulphonylurea.
BYETTA should be injected under the skin and not into a vein or into the muscle.
If you have severe problems with your stomach emptying (including gastroparesis) or food
digestion the use of BYETTA is not recommended. BYETTA slows stomach emptying so food
passes more slowly through your stomach.
The use of BYETTA with insulins is not recommended.
There is little experience with BYETTA in patients with kidney problems. The use of BYETTA
is not recommended if you have severe kidney disease or you are on dialysis.
There is no experience with BYETTA in children and adolescents less than 18 years and,
therefore, use of BYETTA is not recommended in this age group.
BYETTA slows stomach emptying and can affect medicines that need to pass through the stomach
quickly.
Ask your doctor if the time at which you take any tablets (for example, antibiotics) should be changed.
For tablets that you need to take with food, it may be best if they are taken at a meal at a time when
BYETTA is not being administered.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
Using BYETTA with food and drink:
Use BYETTA at any time within the 60 minutes (1 hour)
before
your meal. (See 3 “How to use
BYETTA”).
Do not
use BYETTA
after
your meal.
Pregnancy and breast-feeding:
It is not known if BYETTA may harm your unborn child. Tell your doctor if you are, you think you
might be, or are planning to become pregnant as BYETTA should not be used during pregnancy.
It is not known if BYETTA passes into your milk. BYETTA should not be used if breast-feeding.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines:
If you use BYETTA in combination with a sulphonylurea, low blood sugar (hypoglycaemia) can
occur. Hypoglycaemia may reduce your ability to concentrate. Please keep this possible problem in
mind in all situations where you might put yourself and others at risk (e.g. driving a car or operating
machinery).
Important information about some of the ingredients of BYETTA:
This medicine contains less than 1mmol sodium per dose, i.e. essentially “sodium-free”.
This medicine contains metacresol which may cause allergic reactions.
Always use BYETTA exactly as your doctor or diabetes nurse has told you. You should check with
your doctor, diabetes nurse or pharmacist if you are unsure.
Two presentations of BYETTA are available: BYETTA 5 micrograms(µg) and BYETTA
10 micrograms(µg). Your doctor may tell you to use BYETTA 5 µg twice a day to start with. After
using BYETTA 5 µg twice a day for 30 days the doctor may increase your dose to BYETTA 10 µg
twice a day. One injection of your pre-filled pen will give you your dose. Do not change your dose
unless your doctor has told you to.
BYETTA should be injected at any time within the 60 minutes (1 hour)
before
your morning and
evening meals, or before your two main meals of the day, which should be about 6 hours or more
apart.
Do not
use BYETTA
after
your meal.
BYETTA is injected under the skin (subcutaneous injection) of your upper leg (thigh), stomach area
(abdomen), or upper arm.
You will
not
need to test your sugar levels on a day-by-day basis to set the dose of BYETTA.
However, if you are also using a sulphonylurea your doctor may tell you to check your blood sugar
levels to adjust the dose of sulphonylurea.
See the accompanying Pen User Manual for instructions for using the BYETTA Pen.
Your doctor or nurse must teach you how to inject BYETTA before you use it for the first time.
Injection needles are not included. The following are examples of disposable needles that can be used
with your BYETTA pen:
•
29 (thin), 30 or 31 (thinner) gauge (diameter 0.25 - 0.33mm) and
•
12.7, 8 or 5mm length.
Ask your doctor or nurse, which needle gauge and length is best for you.
Use a new injection needle for each injection and dispose of it after each use. This medicine is for you;
never share a BYETTA pen with others.
If you use more BYETTA than you should:
If you use too much BYETTA you may need medical treatment right away. Too much BYETTA can
cause nausea, vomiting, dizziness, or symptoms of low blood sugar.
If you forget to use BYETTA:
If you miss a dose of BYETTA, skip that dose and take your next dose at the next prescribed time.
Do
not
take an extra dose or increase the amount of your next dose to make up for the one you missed.
If you stop using BYETTA:
If you feel you should stop using BYETTA consult your doctor. If you stop using BYETTA this can
affect your blood sugar levels.
If you have any further questions on the use of this medicine, ask your doctor, diabetes nurse or
pharmacist.
Like all medicines, BYETTA can have side effects although not everybody gets them.
Very common, more than 1 in 10 patients experienced: nausea, (nausea is most common when first
starting BYETTA, but decreases over time in most patients), vomiting or diarrhoea.
When BYETTA is used with a medicine that contains a sulphonylurea, episodes of low blood sugar
(hypoglycaemia, generally mild to moderate) can occur very commonly. The dose of your
sulphonylurea medicine may need to be reduced while you use BYETTA. The signs and symptoms of
low blood sugar may include headache, drowsiness, weakness, dizziness, confusion, irritability,
hunger, fast heartbeat, sweating, and feeling jittery. Your doctor should tell you how to treat low blood
sugar.
Common, less than 1 in 10 but more than 1 in 100 patients experienced: dizziness, headache, reduced
appetite, weight decreased, feeling jittery, pain in the stomach area, bloating, indigestion, increased
sweating, loss of energy and strength or heartburn, injection site reactions (redness).
In addition some other side effects have been reported: angiodema, hypersensitivity (rashes, itching
and rapid swelling of the tissues of the neck, face, mouth or throat), decrease in kidney function,
dehydration, sometimes with a decrease in kidney function, unusual taste in the mouth, drowsiness,
constipation, burping, flatulence, hair loss (rarely). Changes in INR (measurement of blood thinning)
have been reported when used together with warfarin.
Cases of inflammation of the pancreas (pancreatitis) have been reported in patients receiving
BYETTA. Pancreatitis can be a serious, potentially life-threatening medical condition.
Tell your doctor if you have had pancreatitis, gallstones, alcoholism or very high triglycerides.
These medical conditions can increase your chance of getting pancreatitis, or getting it again,
whether or not you are taking BYETTA.
Call your doctor if you experience
severe and persistent
stomach pain, with or without
vomiting, because you could have pancreatitis.
Some severe allergic reactions (anaphylaxis) have been reported very rarely.
You should see your doctor immediately if you experience symptoms such as
•
Swollen face, tongue or throat
Hives and difficulties to breathe
BYETTA may reduce your appetite, the amount of food you eat, and your weight.
Take care if you lose weight too quickly as this may not be good for you (for example, it can cause
dehydration, dizziness upon standing, and gallstones).
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use BYETTA after the expiry date, which is stated on the label and the carton.
Store in a refrigerator (2 °C – 8 ºC). Once in use, your BYETTA pen should be kept below 25 ºC.
Replace the cap on the pen in order to protect from light. Do not freeze. Throw away any BYETTA
pen that has been frozen.
Use a BYETTA pen for only 30 days. Dispose of a used BYETTA pen after 30 days, even if some
medicine remains in the pen.
Do not use BYETTA if you notice particles in the solution, or if it is cloudy or coloured.
Do not store the BYETTA pen with the needle attached. If the needle is left on, medicine may leak
from the BYETTA pen or air bubbles may form in the cartridge.
Medicines should not be disposed of via waste water or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active substance is exenatide.
Two pre-filled pens are available one to deliver doses of 5 micrograms (µg) and one
10 micrograms (µg).
Each dose of BYETTA 5 micrograms solution for injection contains 5 micrograms exenatide in
20 microlitre.
Each dose of BYETTA 10 micrograms solution for injection contains 10 micrograms exenatide
in 40 microlitre.
The other ingredients are metacresol (44 micrograms/dose in BYETTA 5 micrograms solution
for injection and 88 micrograms/dose in BYETTA 10 micrograms solution for injection),
mannitol, glacial acetic acid, sodium acetate trihydrate and water for injections.
What BYETTA looks like and contents of the pack
BYETTA is a clear and colourless liquid (solution for injection) filled in a glass cartridge within a pen.
When the pen is empty, you cannot use it again. Each pen has 60 doses to provide 30 days of twice–a–
day injections.
It is available in pack sizes of 1 and 3 pens. Not all pack sizes may be marketed.
Marketing Authorisation Holder:
Eli Lilly Nederland B.V., Grootslag 1-5, NL-3991 RA Houten, The Netherlands.
Manufacturer:
Lilly Pharma Fertigung und Distribution GmbH & Co. KG, Teichweg 3, D- 35396 Giessen, Germany.
Each millilitre (ml) of the solution for injection contains 0.25 milligrams (mg) of exenatide.
For any information about this medicinal product, please contact the local representative of the
Marketing Authorisation Holder:
Belgique/België/Belgien
Eli Lilly Benelux S.A/N.V.
Tél/Tel: +32-(0) 2 548 84 84
Luxembourg/Luxemburg
Eli Lilly Benelux S.A/N.V.
Tél/Tel: +32-(0) 2 548 84 84
България
ТП "Ели Лили Недерланд" Б.В. - България
тел. + 359 2 491 41 40
Magyarország
Lilly Hungária Kft.
Tel: + 36 1 328 5100
Česká republika
Eli Lilly ČR, s.r.o.
Tel: + 420 234 664 111
Malta
Charles de Giorgio Ltd.
Tel: + 356 25600 500
Danmark
Eli Lilly Danmark A/S
Tlf: +45 45 26 60 00
Nederland
Eli Lilly Nederland B.V.
Tel: + 31-(0) 30 60 25 800
Deutschland
Lilly Deutschland GmbH
Tel. + 49-(0) 6172 273 2222
Norge
Eli Lilly Norge A.S
Tlf: + 47 22 88 18 00
Eesti
Eli Lilly Holdings Limited Eesti filiaal
Tel:
+
3726441100
Österreich
Eli Lilly Ges.m.b.H
Tel: +43-(0) 1 711 780
Ελλάδα
ΦΑΡΜΑΣΕΡΒ-ΛΙΛΛΥ Α.Ε.Β.Ε
Τηλ: +30 210 629 4600
Polska
Eli Lilly Polska Sp. z o.o.
Tel.: +48 (0) 22 440 33 00
España
Lilly S.A.
Tel: + 34 91 663 50 00
Portugal
Lilly Portugal - Produtos Farmacêuticos, Lda
Tel: +351 21 4126600
France
Lilly France S.A.
Tél.: +33-(0)1 55 49 34 34
România
Eli Lilly România S.R.L.
Tel: + 40 21 4023000
Ireland
Eli Lilly and Co. (Ireland) Limited,
Tel: +353-(0) 1 661 4377
Slovenija
Eli Lilly farmacevtska družba, d.o.o.
Tel: +386 (0)1 580 00 10
Ísland
Icepharma hf.
Sími: + 354 540 8000
Slovenská republika
Eli Lilly Slovakia, s.r.o.
Tel: + 421 220 663 111
Italia
Eli Lilly Italia S.p.A.
Tel: + 39-055 42571
Suomi/Finland
Oy Eli Lilly Finland Ab
Puh/Tel: + 358-(0) 9 85 45 250
Κύπρος
Phadisco Ltd
Τηλ: +357 22 715000
Sverige
Eli Lilly Sweden AB
Tel: +46 (0) 8 737 88 00
Latvija
Eli Lilly Holdings Limited pārstāvniecība Latvijā
Tel:
+
371 67364000
United Kingdom
Eli Lilly and Company Limited
Tel: +44-(0) 1256 315000
Lietuva
Eli Lilly Holdings Limited atstovybė
Tel. +370 (5) 2649600
This leaflet was last approved in XXX.
Detailed information on this medicine is available on the website of the European Medicines Agency
Section 1.
WHAT YOU NEED TO KNOW ABOUT YOUR BYETTA PEN
Read this section completely before you begin. Then, move on to Section 2 – Getting Started.
BYETTA 5 micrograms solution for injection, pre-filled pen
(exenatide)
Read
these instructions carefully BEFORE using your BYETTA
pen.
Also read the BYETTA Package
leaflet that comes with the BYETTA pen carton.
You need to use the pen correctly in order to get the most benefit from BYETTA. Failure to follow
these instructions completely may result in a wrong dose, a broken pen or an infection.
These instructions do not take the place of talking with your healthcare professional about
your medical condition or your treatment. If you are having problems using your BYETTA
pen, contact your healthcare professional.
IMPORTANT INFORMATION ABOUT YOUR BYETTA PEN
BYETTA is injected twice a day, the pen contains enough medicine for 30 days. You do not
have to measure any doses, the pen measures each dose for you.
DO NOT TRANSFER THE MEDICINE IN THE BYETTA PEN TO A SYRINGE.
If any part of your pen appears broken or damaged, do not use the pen.
This pen is not recommended for use by people who are blind or who cannot see well enough.
Help will be needed by a person trained to use the pen.
Healthcare professionals or other caregivers should follow local or institutional policies
regarding needle handling.
Follow the instructions for hygienic injection technique recommended by your healthcare
professional.
Follow Section 2 only to set up a new pen before first use.
Section 3 of this manual should be used for every injection.
What kinds of needles can be used with my BYETTA pen?
•
Injection needles are not included. The following are examples of disposable needles that can be
used with your BYETTA pen:
- 29 (thin), 30 or 31 (thinner) gauge (diameter 0.25-0.33mm) and
- 12.7, 8 or 5mm length.
Ask your healthcare professional which needle gauge and length is best for you.
Do I use a new needle for each injection?
•
Yes. Do not reuse needles.
Remove the needle immediately after each injection. This will help prevent leakage of
BYETTA, keep out air bubbles, reduce needle clogs, and decrease the risk of infection.
Never push the injection button on the pen unless a needle is attached.
How do I throw away my needles?
•
Throw away used needles in a puncture-resistant container or as recommended by your
healthcare professional.

•
Do not throw away the pen with a needle attached.
Do not share your pen or needles.
How do I store my BYETTA pen?
•
Store in a refrigerator (2° to 8°C).
•
Do not freeze. Throw away any BYETTA pen that has been frozen.
•
Once in use, your BYETTA pen should be kept below 25°C.
•
Replace the cap on the pen in order to protect from light.
•
Do not store the BYETTA pen with the needle attached. If the needle is left on, medicine may
leak from the BYETTA pen or air bubbles may form in the cartridge.
Keep your pen and needles out of the reach and sight of children.
How long can I use a BYETTA pen?
•
Use a BYETTA pen for only 30 days after setting up a new pen for first use.
Dispose of a used BYETTA pen after 30 days, even if some medicine remains in the pen.
Mark the date when you first used your pen and the date 30 days later in the spaces below:
Date of First Use
Date to Throw Away Pen
Do not use BYETTA after the expiry date, which is stated on the label and the carton.
How do I clean my BYETTA pen?
•
If needed, wipe the outside of the pen with a clean, damp cloth.
White particles may appear on the outside tip of the cartridge during normal use. You may
remove them with an alcohol wipe or alcohol swab.
Please see the accompanying BYETTA Package Leaflet. For additional information, contact
your healthcare professional.
Section 2. GETTING STARTED
Read and follow the directions in this section only after you’ve read Section 1-What You Need
To Know About Your BYETTA Pen.
Set up your new pen just before you use it for the first time. Follow the
New Pen Setup
only
once
.
For routine use,
do not repeat
the New Pen Setup. If you do, you will run out of BYETTA before 30
days of use.
NEEDLE PARTS
(Needles Not Included)
ready to pull dose knob out
ready to turn to dose position
dose knob pushed in and ready to
reset
NEW PEN SETUP – DO THIS ONE TIME ONLY
Check pen label to make sure it is your 5 µg pen.
Pull off the blue pen cap.
Check BYETTA in the cartridge. The liquid should be clear, colourless, and free of particles. If it is
not, do not use.
Note
: A small air bubble in the cartridge is normal
Remove paper tab from outer needle shield.
Push
outer needle shield containing the needle
straight
onto the pen, then
screw
needle on until
secure.













Pull off outer needle shield.
Do not
throw away. The outer needle shield will be used when you
are removing the needle from the pen after the injection.
Pull off inner needle shield and throw away. A small drop of liquid may appear. This is normal.
is in the dose window. If not, turn dose knob clockwise
until it stops
and the
Pull dose knob out until it
stops
and the is in the dose window.
Turn dose knob clockwise until it stops
at
. Make sure that the 5 with the line under it is in
the centre of the dose window.
Note
: If you cannot turn the dose knob clockwise to the
, see
Commonly Asked Questions
,
number 8, in Section 4 of this user manual.
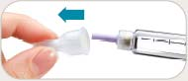









Point the needle of the pen up and away from you.
Use thumb to firmly push the injection button in until it stops
, then continue holding the
injection button in while
slowly counting to 5.
If you do not see a stream or several drops come from the needle tip, repeat Steps C & D.
Pen preparation is complete when the is in the centre of the dose window AND you have
seen a stream or several drops come from the needle tip.
Note
: If you do not see liquid after 4 times, see
Commonly Asked Questions
, number 3, in Section 4
of this user manual.
STEP E Complete New Pen Setup
Turn dose knob clockwise until it stops
and the
New Pen Setup is now done. Do not repeat Section 2 for routine use, if you do, you will run out
of BYETTA before 30 days of use.
You are now ready for your first dose of BYETTA.
Go to Section 3, Step 3, for instructions on how to inject your first routine dose.
Note:
If you cannot turn the dose knob, see
Commonly Asked Questions
, number 8, Section 4 of this
user manual.
Now that you have done the New Pen Setup, follow Section 3 for
all
of your injections.

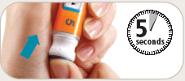




Check pen label to make sure it is your 5 µg pen.
Pull off the blue pen cap.
Check BYETTA in the cartridge.
The liquid should be clear, colourless, and free of particles. If it is not, do not use.
Note
: A small air bubble will not harm you or affect your dose.
Remove paper tab from outer needle shield.
Push
outer needle shield containing the needle
straight
onto the pen, then
screw
needle on until
secure.
Pull off outer needle shield.
Do not
throw away. The outer needle shield will be used when you
are removing the needle from the pen after the injection.
Pull off inner needle shield and throw away. A small drop of liquid may appear. This is normal.




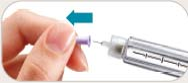

is in the dose window. If not, turn dose knob clockwise
until it stops
and the
Pull dose knob out until it stops
and the is in the dose window.
Turn dose knob clockwise until it stops
at
. Make sure that the 5 with the line under it is in
the centre of the dose window.
Note
: If you cannot turn the dose knob clockwise to the
, see
Commonly Asked Questions
,
number 8, in Section 4 of this user manual.
Insert needle into skin using
hygienic
injection technique recommended by your healthcare
professional.
Use thumb to firmly push injection button in until it stops
, then continue holding the
injection button in while
slowly counting to 5
in order to get a full dose.








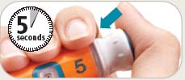

Injection is complete when the is in the centre of the dose window.
The pen is now ready to reset.
Note
: If you see several drops of BYETTA leaking from the needle after the injection, the injection
button was not pushed in all the way. See
Commonly Asked Questions
, number 4, in Section 4 of
this user manual.
Turn dose knob clockwise until it stops
and the
Note
: If you cannot turn the dose knob, or if your pen leaks, your full dose has not been delivered. See
Commonly Asked Questions
, numbers 4 and 8, in Section 4 of this user manual.
STEP 6 Remove and Dispose of the Needle
Remove the needle after each injection.
Carefully put the outer needle shield back over the needle.
Replace blue pen cap on pen before storage.
Throw away needles in a puncture-resistant container or as recommended by your healthcare
professional.
STEP 7 Store Pen for Next Dose
Store your BYETTA pen properly. (See
Storing Your BYETTA Pen
in Section 1 of this user
manual for more information.)
When it is time for your next routine dose, go to
Section 3, Step 1
, and repeat Steps 1 - 7.






Section 4. COMMONLY ASKED QUESTIONS
1. Do I need to do the New Pen Setup before every dose?
•
No. The New Pen Setup is done only
once
, just before each new pen is used for the first time.
The purpose of the setup is to make sure that your BYETTA pen is ready to use for the next 30
days.
If you repeat the New Pen Setup before each routine dose, you will not have enough
BYETTA for 30 days
. The small amount of BYETTA used in the New Pen Setup will not
affect the 30-day supply of BYETTA.
2. Why are there air bubbles in the cartridge?
•
A small air bubble is normal. It will not harm you or affect your dose.
If the pen is stored with a needle attached, air bubbles may form in the cartridge.
Do not
store
the pen with the needle attached.
3. What should I do if BYETTA does not come out of the needle tip after four tries during New
Pen Setup?
•
Remove the needle by carefully putting the outer needle shield back over the needle. Unscrew
and dispose of properly.
Attach a new needle and repeat
New Pen Setup, Steps B – E
, in Section 2 of this user manual.
Once you see several drops or a stream of liquid coming out of the tip of the needle, the setup is
complete.
4. Why do I see BYETTA leaking from my needle after I have finished my injection?
It is normal for a single drop to remain on the tip of your needle after your injection is complete. If you
see more than one drop:
•
You may not have received your full dose.
Do not
inject another dose.
Consult with your
healthcare professional about how to handle a partial dose.
To prevent this, for your next dose,
firmly push and hold
the injection button in and
slowly
count to 5
(see
Section 3, Step 4: Inject the Dose
).
5. What do the arrows mean?
The arrows mean you are ready for the next step. These arrows
show the direction to pull or
turn the dose knob in the next step. This symbol means the dose knob is pushed in and the pen is
ready to reset.
6. How can I tell when the injection is complete?
The injection is complete when:
•
You have firmly pushed the injection button in all the way
until it stops
You have slowly counted to 5
while you are still holding the injection button in and the needle
is still in your skin
is in the centre of the dose window.
7. Where should I inject BYETTA?
BYETTA should be injected into your abdomen, thigh, or upper arm using the injection technique
recommended by your healthcare professional.
Front Back




8. What if I cannot pull, turn, or push the dose knob?
Check the symbol in the dose window. Follow the steps next to the matching symbol.
Pull the dose knob out until appears.
is in the dose window and the dose knob will not turn:
The cartridge in your BYETTA pen may not have enough liquid to deliver a full dose. A small
amount of BYETTA will always remain in the cartridge. If the cartridge contains a small
amount or looks empty, obtain a new BYETTA pen.
are in the dose window and the dose knob cannot be pushed in:
The dose knob was not turned all the way. Continue turning the dose knob clockwise until
is in the centre of the dose window.
and part of are in the dose window and the dose knob cannot be pushed in:
The needle may be clogged, bent, or incorrectly attached.
Attach a new needle. Make sure needle is on straight and screwed on all the way.
Firmly push the injection button in all the way. BYETTA should come from needle tip.
If is in the dose window and the dose knob will not turn:
•
The injection button was not pushed in all the way and a complete dose was not delivered.
Consult with your healthcare professional about how to handle a partial dose
.
Follow these steps to reset your pen for your next injection:
- Firmly push the injection button in all the way
until it stops
. Keep holding the injection button
in and
slowly count to 5
. Then turn the dose knob clockwise until
window.
- If you cannot turn the dose knob, the needle may be clogged. Replace the needle and repeat
the step above.
For your next dose, be sure to
firmly push and hold
the injection button in and
slowly count to
5
before removing needle from skin.
Please see the accompanying Package Leaflet. For additional information contact your
healthcare professional.









Section 1.
WHAT YOU NEED TO KNOW ABOUT YOUR BYETTA PEN
Read this section completely before you begin. Then, move on to Section 2 – Getting Started.
BYETTA 10 micrograms solution for injection, pre-filled pen
(exenatide)
Read
these instructions carefully BEFORE using your BYETTA
pen.
Also read the BYETTA Package
leaflet that comes with the BYETTA pen carton.
You need to use the pen correctly in order to get the most benefit from BYETTA. Failure to follow
these instructions completely may result in a wrong dose, a broken pen or an infection.
These instructions do not take the place of talking with your healthcare professional about
your medical condition or your treatment. If you are having problems using your BYETTA
pen, contact your healthcare professional.
IMPORTANT INFORMATION ABOUT YOUR BYETTA PEN
BYETTA is injected twice a day, the pen contains enough medicine for 30 days. You do not
have to measure any doses, the pen measures each dose for you.
DO NOT TRANSFER THE MEDICINE IN THE BYETTA PEN TO A SYRINGE.
If any part of your pen appears broken or damaged, do not use the pen.
This pen is not recommended for use by people who are blind or who cannot see well enough.
Help will be needed by a person trained to use the pen.
Healthcare professionals or other caregivers should follow local or institutional policies
regarding needle handling.
Follow the instructions for hygienic injection technique recommended by your healthcare
professional.
Follow Section 2 only to set up a new pen before first use.
Section 3 of this manual should be used for every injection.
What kinds of needles can be used with my BYETTA pen?
•
Injection needles are not included. The following are examples of disposable needles that can be
used with your BYETTA pen:
- 29 (thin), 30 or 31 (thinner) gauge (diameter 0.25-0.33mm) and
- 12.7, 8 or 5mm length.
Ask your healthcare professional which needle gauge and length is best for you.
Do I use a new needle for each injection?
•
Yes. Do not reuse needles.
Remove the needle immediately after each injection. This will help prevent leakage of
BYETTA, keep out air bubbles, reduce needle clogs, and decrease the risk of infection.
Never push the injection button on the pen unless a needle is attached.

How do I throw away my needles?
•
Throw away used needles in a puncture-resistant container or as recommended by your
healthcare professional.
•
Do not throw away the pen with a needle attached.
Do not share your pen or needles.
How do I store my BYETTA pen?
•
Store in a refrigerator (2° to 8°C).
•
Do not freeze. Throw away any BYETTA pen that has been frozen.
•
Once in use, your BYETTA pen should be kept below 25°C.
•
Replace the cap on the pen in order to protect from light.
•
Do not store the BYETTA pen with the needle attached. If the needle is left on, medicine may
leak from the BYETTA pen or air bubbles may form in the cartridge.
Keep your pen and needles out of the reach and sight of children.
How long can I use a BYETTA pen?
•
Use a BYETTA pen for only 30 days after setting up a new pen for first use.
Dispose of a used BYETTA pen after 30 days, even if some medicine remains in the pen.
Mark the date when you first used your pen and the date 30 days later in the spaces below:
Date of First Use
Date to Throw Away Pen
Do not use BYETTA after the expiry date, which is stated on the label and the carton.
How do I clean my BYETTA pen?
•
If needed, wipe the outside of the pen with a clean, damp cloth.
White particles may appear on the outside tip of the cartridge during normal use. You may
remove them with an alcohol wipe or alcohol swab.
Please see the accompanying BYETTA Package Leaflet. For additional information, contact
your healthcare professional.
Section 2. GETTING STARTED
Read and follow the directions in this section only after you’ve read Section 1-What You Need
To Know About Your BYETTA Pen.
Set up your new pen just before you use it for the first time. Follow the
New Pen Setup
only
once
.
For routine use,
do not repeat
the New Pen Setup. If you do, you will run out of BYETTA before 30
days of use.
NEEDLE PARTS
(Needles Not Included)
ready to pull dose knob out
ready to turn to dose position
dose knob pushed in and ready to
reset
NEW PEN SETUP – DO THIS ONE TIME ONLY
Check pen label to make sure it is your 10 µg pen.
Pull off the blue pen cap.
Check BYETTA in the cartridge. The liquid should be clear, colourless, and free of particles. If it is
not, do not use.
Note
: A small air bubble in the cartridge is normal
Remove paper tab from outer needle shield.
Push
outer needle shield containing the needle
straight
onto the pen, then
screw
needle on until
secure.













Pull off outer needle shield.
Do not
throw away. The outer needle shield will be used when you
are removing the needle from the pen after the injection.
Pull off inner needle shield and throw away. A small drop of liquid may appear. This is normal.
is in the dose window. If not, turn dose knob clockwise
until it stops
and the
Pull dose knob out until it
stops
and the is in the dose window.
Turn dose knob clockwise until it stops
at . Make sure that the 10 with the line under it is
in the centre of the dose window.
Note
: If you cannot turn the dose knob clockwise to the , see
Commonly Asked Questions
,
number 8, in Section 4 of this user manual.









Point the needle of the pen up and away from you.
Use thumb to firmly push the injection button in until it stops
, then continue holding the
injection button in while
slowly counting to 5.
If you do not see a stream or several drops come from the needle tip, repeat Steps C & D.
Pen preparation is complete when the is in the centre of the dose window AND you have
seen a stream or several drops come from the needle tip.
Note
: If you do not see liquid after 4 times, see
Commonly Asked Questions
, number 3, in Section 4
of this user manual.
STEP E Complete New Pen Setup
Turn dose knob clockwise until it stops
and the
New Pen Setup is now done. Do not repeat Section 2 for routine use, if you do, you will run out
of BYETTA before 30 days of use.
You are now ready for your first dose of BYETTA.
Go to Section 3, Step 3, for instructions on how to inject your first routine dose.
Note:
If you cannot turn the dose knob, see
Commonly Asked Questions
, number 8, Section 4 of this
user manual.
Now that you have done the New Pen Setup, follow Section 3 for
all
of your injections.





Check pen label to make sure it is your 10 µg pen.
Pull off the blue pen cap.
Check BYETTA in the cartridge.
The liquid should be clear, colourless, and free of particles. If it is not, do not use.
Note
: A small air bubble will not harm you or affect your dose.
Remove paper tab from outer needle shield.
Push
outer needle shield containing the needle
straight
onto the pen, then
screw
needle on until
secure.
Pull off outer needle shield.
Do not
throw away. The outer needle shield will be used when you
are removing the needle from the pen after the injection.
Pull off inner needle shield and throw away. A small drop of liquid may appear. This is normal.






is in the dose window. If not, turn dose knob clockwise
until it stops
and the
Pull dose knob out until it stops
and the is in the dose window.
Turn dose knob clockwise until it stops
at . Make sure that the 10 with the line under it is
in the centre of the dose window.
Note
: If you cannot turn the dose knob clockwise to the , see
Commonly Asked Questions
,
number 8, in Section 4 of this user manual.
Insert needle into skin using
hygienic
injection technique recommended by your healthcare
professional.
Use thumb to firmly push injection button in until it stops
, then continue holding the
injection button in while
slowly counting to 5
in order to get a full dose.










Injection is complete when the is in the centre of the dose window.
The pen is now ready to reset.
Note
: If you see several drops of BYETTA leaking from the needle after the injection, the injection
button was not pushed in all the way. See
Commonly Asked Questions
, number 4, in Section 4 of
this user manual.
Turn dose knob clockwise until it stops
and the
Note
: If you cannot turn the dose knob, or if your pen leaks, your full dose has not been delivered. See
Commonly Asked Questions
, numbers 4 and 8, in Section 4 of this user manual.
STEP 6 Remove and Dispose of the Needle
Remove the needle after each injection.
Carefully put the outer needle shield back over the needle.
Replace blue pen cap on pen before storage.
Throw away needles in a puncture-resistant container or as recommended by your healthcare
professional.
STEP 7 Store Pen for Next Dose
Store your BYETTA pen properly. (See
Storing Your BYETTA Pen
in Section 1 of this user
manual for more information.)
When it is time for your next routine dose, go to
Section 3, Step 1
, and repeat Steps 1 - 7.





Section 4. COMMONLY ASKED QUESTIONS
1. Do I need to do the New Pen Setup before every dose?
•
No. The New Pen Setup is done only
once
, just before each new pen is used for the first time.
The purpose of the setup is to make sure that your BYETTA pen is ready to use for the next 30
days.
If you repeat the New Pen Setup before each routine dose, you will not have enough
BYETTA for 30 days
. The small amount of BYETTA used in the New Pen Setup will not
affect the 30-day supply of BYETTA.
2. Why are there air bubbles in the cartridge?
•
A small air bubble is normal. It will not harm you or affect your dose.
If the pen is stored with a needle attached, air bubbles may form in the cartridge.
Do not
store
the pen with the needle attached.
3. What should I do if BYETTA does not come out of the needle tip after four tries during New
Pen Setup?
•
Remove the needle by carefully putting the outer needle shield back over the needle. Unscrew
and dispose of properly.
Attach a new needle and repeat
New Pen Setup, Steps B – E
, in Section 2 of this user manual.
Once you see several drops or a stream of liquid coming out of the tip of the needle, the setup is
complete.
4. Why do I see BYETTA leaking from my needle after I have finished my injection?
It is normal for a single drop to remain on the tip of your needle after your injection is complete. If you
see more than one drop:
•
You may not have received your full dose.
Do not
inject another dose.
Consult with your
healthcare professional about how to handle a partial dose.
To prevent this, for your next dose,
firmly push and hold
the injection button in and
slowly
count to 5
(see
Section 3, Step 4: Inject the Dose
).
5. What do the arrows mean?
The arrows mean you are ready for the next step. These arrows
show the direction to pull or
turn the dose knob in the next step. This symbol means the dose knob is pushed in and the pen is
ready to reset.
6. How can I tell when the injection is complete?
The injection is complete when:
•
You have firmly pushed the injection button in all the way
until it stops
You have slowly counted to 5
while you are still holding the injection button in and the needle
is still in your skin
is in the centre of the dose window.
7. Where should I inject BYETTA?
BYETTA should be injected into your abdomen, thigh, or upper arm using the injection technique
recommended by your healthcare professional.
Front Back




8. What if I cannot pull, turn, or push the dose knob?
Check the symbol in the dose window. Follow the steps next to the matching symbol.
Pull the dose knob out until appears.
is in the dose window and the dose knob will not turn:
The cartridge in your BYETTA pen may not have enough liquid to deliver a full dose. A small
amount of BYETTA will always remain in the cartridge. If the cartridge contains a small
amount or looks empty, obtain a new BYETTA pen.
If and part of the are in the dose window and the dose knob cannot be pushed in:
•
The dose knob was not turned all the way. Continue turning the dose knob clockwise until
is in the centre of the dose window.
If part of and part of are in the dose window and the dose knob cannot be pushed in:
•
The needle may be clogged, bent, or incorrectly attached.
Attach a new needle. Make sure needle is on straight and screwed on all the way.
Firmly push the injection button in all the way. BYETTA should come from needle tip.
If is in the dose window and the dose knob will not turn:
•
The injection button was not pushed in all the way and a complete dose was not delivered.
Consult with your healthcare professional about how to handle a partial dose
.
Follow these steps to reset your pen for your next injection:
- Firmly push the injection button in all the way
until it stops
. Keep holding the injection button
window.
- If you cannot turn the dose knob, the needle may be clogged. Replace the needle and repeat
the step above.
For your next dose, be sure to
firmly push and hold
the injection button in and
slowly count to
5
before removing needle from skin.
Please see the accompanying Package Leaflet. For additional information contact your
healthcare professional.
in and
slowly count to 5
. Then turn the dose knob clockwise until
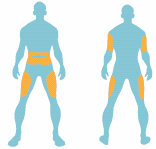








Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/byetta.html
Copyright © 1995-2021 ITA all rights reserved.
|













































































































































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































