Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Cayston 75 mg powder and solvent for nebuliser solution
QUALITATIVE AND QUANTITATIVE COMPOSITION
Cayston contains aztreonam lysine (formed
in situ
from 75 mg aztreonam) as a sterile lyophilised
powder in a vial and a 1 ml ampoule of sterile solvent (0.17% w/v sodium chloride). After
reconstitution of the powder in the solvent, the nebuliser solution contains 75 mg aztreonam (as
lysine).
For a full list of excipients, see section 6.1.
Powder and solvent for nebuliser solution.
White to off-white, lyophilised powder.
4.1 Therapeutic indications
Cayston is indicated for the suppressive therapy of chronic pulmonary infections due to
Pseudomonas
aeruginosa
in patients with cystic fibrosis (CF) aged 18 years and older.
The primary support for this indication is based on two single 28-day course placebo-controlled
studies. The data to support the sustainability of the observed short term benefit over subsequent
courses of treatment are limited (see section 5.1). Consideration should be given to official guidance
on the appropriate use of antibacterial agents.
4.2 Posology and method of administration
Patients should use a bronchodilator before each dose of Cayston. Short acting bronchodilators can be
taken between 15 minutes and 4 hours and long acting bronchodilators can be taken between
30 minutes and 12 hours prior to each dose of Cayston.
For patients receiving several respiratory therapies, the recommended order is:
other inhaled medicinal products
The recommended dose for adults is 75 mg three times per 24 hours for 28 days.
Doses should be taken at least 4 hours apart.
Multiple course, controlled efficacy data are not yet available (see section 5.1). Additional courses,
beyond the initial 28-day course, should be considered only at the discretion of the physician. If
additional courses are prescribed, a minimum of 28 days without Cayston is recommended.
Cayston is not recommended for use in children below the age of 18 years due to insufficient data on
safety and efficacy (see section 5.1).
Clinical studies with Cayston did not include sufficient numbers of patients aged 65 years and over to
determine whether they responded differently from younger patients. If Cayston is to be prescribed to
the elderly then the posology is the same as for adults.
Aztreonam is known to be excreted renally and therefore administration of Cayston in patients with
renal impairment (serum creatinine > 2 times upper limit of normal) should be undertaken with
caution. No dose adjustment is necessary in cases of renal impairment since the systemic
concentration of aztreonam following inhaled administration of Cayston is very low (approximately
1% of the concentration resulting from a dose of 500 mg aztreonam for injection).
There are no data on the use of Cayston in patients with severe hepatic impairment (ALT or AST
greater than 5 times the upper limit of normal). No dose adjustment is necessary in cases of hepatic
impairment.
Cayston is only for inhalation use.
Cayston should only be used with the Altera Nebuliser Handset and Altera Aerosol Head connected to
an Altera Control Unit or an eFlow rapid Control Unit. For instructions on reconstitution of the
medicinal product before administration, see section 6.6.
Hypersensitivity to the active substance or to any of the excipients.
4.4 Special warnings and precautions for use
If an allergic reaction to Cayston does occur, stop administration of the medicinal product and initiate
treatment as appropriate. The occurrence of rash may be indicative of an allergic reaction to Cayston.
Cross-reactivity may occur in patients with a history of allergy to beta-lactam antibiotics, such as
penicillins, cephalosporins, and/or carbapenems. Animal and human data demonstrate low risk of
cross-reactivity between aztreonam and beta-lactam antibiotics. Aztreonam, a monobactam, is only
weakly immunogenic. Caution is advised when administering Cayston to patients if they have a
history of beta-lactam allergy.
The following rare and severe adverse reactions, although these have not been observed to date with
Cayston, have been reported after parenteral use of other aztreonam containing products: toxic
epidermal necrolysis, anaphylaxis, purpura, erythema multiforme, exfoliative dermatitis, urticaria,
petechiae, pruritus, diaphoresis.
Bronchospasm is a complication associated with nebulised therapies. Patients were pre-treated with a
bronchodilator before dosing with study therapy. An acute reduction of ≥ 15% in forced expiratory
volume in 1 second (FEV
1
) following administration of study therapy was observed in 3% of patients
treated with Cayston and 4% of patients receiving placebo despite pre-treatment with a bronchodilator
before dosing with study therapy. Patients should use a bronchodilator before each dose of Cayston.
If a case of bronchospasm is suspected to be part of an allergic reaction appropriate measures should
be taken (see “allergic reactions” paragraph above).
In clinical studies, the efficacy and safety of Cayston were not tested in patients with FEV
1
%
predicted < 25% or > 75%. Patients with
Burkholderia cepacia
isolated from sputum within the
previous 2 years were excluded from the clinical studies.
Aztreonam for injection must not be used in the Altera or other nebulisers. Aztreonam for injection
has not been formulated for inhalation, and contains arginine, a substance known to cause pulmonary
inflammation.
The development of antibiotic-resistant
P. aeruginosa
and superinfection with other pathogens
represent potential risks associated with antibiotic therapy. Development of resistance during inhaled
aztreonam therapy could limit treatment options during acute exacerbations. In clinical studies of
Cayston, no increases of clinical significance were observed in the prevalence of antibiotic-resistant
P. aeruginosa
or other bacterial respiratory pathogens among patients treated three times daily with
Cayston. Among patients with multidrug-resistant
P. aeruginosa
, improvements in respiratory
symptoms and pulmonary function were observed following treatment with Cayston. An increased
prevalence of
Aspergillus
and
Candida
species were observed over time in patients treated with
several Cayston treatment courses. The clinical significance of this finding is unknown.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed. However, no evidence of any drug interactions with
Cayston were identified from clinical studies in which Cayston was taken concomitantly with
bronchodilators, dornase alfa, pancreatic enzymes, azithromycin, tobramycin, oral steroids (less than
10 mg daily/20 mg every other day) and inhaled steroids.
4.6 Pregnancy and lactation
There are no data from the use of aztreonam in pregnant women. Animal studies do not indicate direct
or indirect harmful effects with respect to reproductive toxicity (see section 5.3).
Systemic concentration of aztreonam following inhaled administration of Cayston is low compared to
a standard dose of aztreonam for injection (approximately 1% of the concentration resulting from a
dose of 500 mg aztreonam for injection).
Cayston should not be used during pregnancy unless the clinical condition of the woman requires
treatment with aztreonam.
Following administration of aztreonam for injection, aztreonam is excreted in human milk at very low
concentrations. Systemic concentration of aztreonam following inhaled administration of Cayston is
approximately 1% of the concentration resulting from a standard dose of aztreonam for injection.
Therefore, and because of low oral absorption, aztreonam exposure in breast-fed infants due to
mothers receiving Cayston is likely to be extremely low.
Cayston can be used during breast-feeding.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. However,
based on the safety profile and mechanism of action, Cayston is not expected to adversely affect the
ability to drive or use machines.
The safety of Cayston was evaluated in three Phase 3 studies in 344 predominantly adult patients
(77%) with chronic
P. aeruginosa
. In two Phase 3 placebo-controlled studies patients received
Cayston 75 mg 2 times (69 patients) or 3 times a day (146 patients) for 28 days. In one Phase 3 open-
label follow-on study 274 CF patients received up to nine 28-day treatment courses of Cayston 75 mg
2 times or 3 times a day.
The adverse reactions with suspected (at least possible) relationship to treatment in the placebo-
controlled studies are listed below by body system organ class and frequency.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Frequencies are defined as follows: very common (≥ 1/10) and common (≥ 1/100 to < 1/10).
Respiratory, thoracic and mediastinal disorders
Very common: wheezing, cough, pharyngolaryngeal pain, nasal congestion
Common: non-allergic bronchospasm, chest discomfort, rhinorrhoea
Skin and subcutaneous tissue disorders
Common: rash
General disorders and administration site conditions
Very common: pyrexia
The following rare and severe adverse reactions, although these have not been observed to date with
Cayston, have been reported after parenteral use of other aztreonam containing products: toxic
epidermal necrolysis, anaphylaxis, purpura, erythema multiforme, exfoliative dermatitis, urticaria,
petechiae, pruritus, diaphoresis.
Adverse reactions specifically associated with overdose of Cayston have not been identified. Since
the plasma concentration of aztreonam following administration of Cayston (75 mg) is approximately
0.6 µg/ml, compared to serum levels of 54 µg/ml following administration of aztreonam for injection
(500 mg), no safety issues associated with Cayston overdose are anticipated.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Antibacterials for systemic use, other beta-lactam antibacterials, ATC
code: J01DF01
Aztreonam exhibits activity
in vitro
against gram-negative aerobic pathogens, including
P. aeruginosa
. Aztreonam binds to penicillin-binding proteins of susceptible bacteria, which leads to
inhibition of bacterial cell wall synthesis, followed by filamentation and cell lysis.
Loss of susceptibility to aztreonam in CF patients with
P. aeruginosa
occurs either through selection
of strains with mutations located on the chromosome or rarely through acquisition of plasmid/integrin
mediated genes.
Known mechanisms of resistance to aztreonam mediated by mutation of chromosomal genes include:
hyperexpression of the Class C beta-lactamase AmpC and up-regulation of the efflux pump
MexAB-OprM. The known mechanism of resistance to aztreonam mediated by acquisition of genes
involves acquisition of extended spectrum beta-lactam enzymes (ESBLs) that hydrolyse the four-
member, nitrogen-containing ring of aztreonam.
ESBLs from Class A, B and D beta-lactamases generally have little or no activity against aztreonam.
Class A beta-lactamases reported to hydrolyse aztreonam include the VEB type (primarily Southeast
Asia), PER type (Turkey), and GES and IBC types (France, Greece, and S. Africa). There are rare
reports of organisms with metallo-beta-lactamases (MBLs), Class B, that are resistant to aztreonam,
VIM-5 (
K. pneumoniae
and
P. aeruginosa
- Turkey), VIM-6 (
P. putida
- Singapore) and VIM-7
(
P. aeruginosa
- United States), however, it is possible that these organisms were expressing multiple
resistance mechanisms and thus a MBL was not responsible for the observed resistance to aztreonam.
There are rare reports of Class D beta-lactamases from clinical isolates of
P. aeruginosa
, OXA-11
(Turkey) and OXA-45 (United States) that hydrolyse aztreonam.
A single sputum sample from a CF patient may contain multiple isolates of
P. aeruginosa
and each
isolate may have a different level of
in vitro
susceptibility to aztreonam. The
in vitro
antimicrobial
susceptibility test methods used for parenteral aztreonam therapy can be used to monitor the
susceptibility of
P. aeruginosa
isolated from CF patients.
In the Phase 3 placebo-controlled studies of Cayston, local aztreonam concentrations generally
exceeded aztreonam MIC values for
P. aeruginosa
, regardless of the level of
P. aeruginosa
susceptibility.
Treatment with a 28-day course of 75 mg 3 times a day Cayston therapy resulted in clinically
important improvements in respiratory symptoms, pulmonary function, and sputum
P. aeruginosa
CFU density, regardless of whether the highest aztreonam MIC for
P. aeruginosa
was above or below
the established susceptibility breakpoint for intravenous aztreonam administration (8 µg/ml). Based
on categorical analyses of the relationship between MIC and treatment response, a susceptibility
breakpoint for Cayston cannot be established. Over 6 courses of Cayston therapy,
P. aeruginosa
MIC
50
and MIC
90
did not change (± 2 dilution change), however there is a theoretical risk that patients
treated with Cayston may develop
P. aeruginosa
isolates resistant to aztreonam or other beta-lactam
antibiotics.
In studies of up to six 28-day courses of Cayston therapy, no increases of clinical significance have
been observed in the treatment-emergent isolation of other bacterial respiratory pathogens
(
Stenotrophomonas maltophilia, Alcaligenes xylosoxidans,
and
Staphylococcus aureus
).
Cayston was evaluated over a period of 28-days of treatment (one course) in two randomised, double-
blind, placebo-controlled, multicentre studies (CP-AI-005 and CP-AI-007). Patients participating in
these studies could subsequently receive multiple courses of Cayston in an open-label follow-on study
(CP-AI-006). Entry criteria included CF baseline FEV
1
% predicted between 25% and 75% and
chronic
P. aeruginosa
lung infection. Overall, 344 predominantly adult patients (77%) were treated in
these studies. Studies were conducted using the Altera Nebuliser System.
CP-AI-007 enrolled 164 adult (predominantly) and paediatric patients randomised in a 1:1 ratio
comparing inhaled Cayston 75 mg (80 patients) or placebo (84 patients) administered 3 times a day for
28-days (one course). Patients were required to have been off antipseudomonal antibiotics for at least
28 days before treatment with study drug.
Pulmonary function and respiratory symptoms significantly improved from baseline to Day 28 in
patients treated with one course of Cayston.
CP-AI-005 enrolled 246 adult (predominantly) and paediatric patients. All patients were treated with
Tobramycin Nebuliser Solution (TNS) 300 mg, 2 times a day in the four weeks immediately prior to
receiving Cayston or placebo either 2 or 3 times a day for 28 days. Patients continued on their
baseline medications, including macrolide antibiotics. Patients were randomised in a 2:2:1:1 ratio to
be treated with Cayston 75 mg 2 or 3 times a day or volume-matched placebo 2 or 3 times a day for
28 days immediately following the 28-day lead-in course of open-label TNS.
Cayston therapy resulted in significant improvements in pulmonary function and respiratory
symptoms at Day 28 in the 66 patients treated with one course Cayston 75 mg 3 times a day.
CP-AI-006 was an open-label follow-on study to CP-AI-005 and CP-AI-007 evaluating the safety of
repeated exposure to Cayston and the effect on disease-related endpoints over multiple 28-day courses.
Patients received Cayston at the same frequency (2 or 3 times a day) as they took Cayston or placebo
in the randomised studies. Patients continued on their baseline medications and whenever indicated
additional antibiotics were used in the majority of patients to treat exacerbations.
Each 28-day course
of Cayston was followed by a 28-day off drug period. Over six 28-day courses of therapy, measures
of pulmonary function (FEV
1
), CFQ-R respiratory symptoms scores, and log
10
P. aeruginosa
CFUs
showed a trend to improvement while the patients were on treatment compared with off treatment.
However, due to the uncontrolled nature of the study and concomitant medications no conclusion can
be drawn on the sustainability of the observed short term benefit over subsequent courses of treatment.
This medicinal product has been authorised under a so-called “conditional approval” scheme.
This means that further evidence on this medicinal product is awaited.
The European Medicines Agency will review new information on the product every year and this
SmPC will be updated as necessary.
5.2 Pharmacokinetic properties
Individual patients’ sputum aztreonam concentrations exhibited considerable variability. For the
combined Phase 3 placebo-controlled studies, ten minutes following a single dose of 75 mg Cayston
on Days 0, 14, and 28, the mean sputum concentrations in 195 patients with CF were 726 µg/g,
711 µg/g, and 715 µg/g, respectively, indicating no increased accumulation of aztreonam following
repeated dosing.
Individual patients’ plasma aztreonam concentrations exhibited considerable variability. One hour
following a single dose of 75 mg Cayston (at approximately peak plasma concentration), the mean
plasma level in patients with CF was 0.59 µg/ml. Mean peak plasma levels at Days 0, 14, and 28 of a
course with 75 mg Cayston 3 times a day were 0.55 µg/ml, 0.67 µg/ml, and 0.65 µg/ml, respectively,
indicating no systemic accumulation of aztreonam following 3 times a day dosing. In contrast, the
serum concentration of aztreonam following administration of aztreonam for injection (500 mg) is
approximately 54 µg/ml.
The elimination half-life of aztreonam from serum is approximately 2.1 hours following inhalation
administration, similar to what has been reported for aztreonam for injection. Systemically absorbed
aztreonam is eliminated by both active tubular secretion and glomerular filtration.
Pharmacokinetics in special populations
There was no clinically relevant effect of age or sex on the pharmacokinetics of Cayston.
Renal and hepatic impairment
Pharmacokinetic studies have not been performed in patients with renal or hepatic impairment.
Pharmacokinetic properties for aztreonam for injection
Peak levels of aztreonam are achieved at about one hour after i.m. administration. After identical
single i.m. or i.v. doses, the serum concentrations are comparable at 1 hour (1.5 hours from the start of
i.v. infusion), with similar slopes of serum concentrations thereafter. The serum half-life of aztreonam
averaged 1.7 hours in subjects with normal renal function, independent of the dose and route. In
healthy subjects 60-70% of a single i.m. or i.v. dose was recovered in the urine by 8 hours, and urinary
excretion was essentially complete by 12 hours.
5.3 Preclinical safety data
A 104-week rat inhalation toxicology study to assess the carcinogenic potential of ascending doses
(31, 56 and 120 mg/kg/day) of Cayston demonstrated no drug-related increase in malignant tumours.
The only evidence of Cayston-related carcinogenicity was a small increase in the incidence of benign
C-cell adenomas in females at 120 mg/kg/day. The clinical relevance of this effect is unknown. No
such added effect was observed at 56 mg/kg/day in which exposures exceeded 2.2 to 9 times the
human exposure, based on AUC or C
max
respectively.
Genotoxicity (Chromosomal aberration and mouse lymphoma mutation assay) studies with aztreonam
were negative indicating that the mechanism of benign C-cell adenomas in female rats was not
genotoxically mediated.
Fertility, teratology, perinatal and postnatal studies were conducted with aztreonam for injection in
rats at daily doses up to 750 mg/kg without adverse effects. The survival rate during the lactation
period was slightly reduced in the offspring of rats that received the highest dose.
PHARMACEUTICAL PARTICULARS
Solvent ampoule
Sodium chloride
Water for injections
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
After reconstitution, immediate use of Cayston is recommended. If not used immediately, the
reconstituted solution must be stored at 2°C - 8°C and used within 8 hours. In-use storage times and
conditions prior to use are the responsibility of the user.
6.4 Special precautions for storage
Powder vial and solvent ampoule: Store in a refrigerator (2°C - 8°C). May be stored outside a
refrigerator but below 25°C for up to 28 days.
For storage conditions of the reconstitued medicinal product see section 6.3.
6.5 Nature and contents of container
Powder vial: Type I amber glass vial with siliconised grey rubber stopper and aluminium tear off
overseal.
Solvent: 1 ml low density polyethylene ampoule.
Each 28-day pack of Cayston contains 84 vials of lyophilised Cayston and 88 solvent ampoules. The
four additional solvent ampoules are provided in case of spillage.
The following pack sizes are available:
•
Pack containing one 28 day pack of Cayston plus one Altera Nebuliser Handset
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
Cayston should only be reconstituted with the solvent provided. Following reconstitution, Cayston is
a clear, colourless to slightly coloured solution.
It is recommended that Cayston be administered immediately after reconstitution with solvent.
Cayston should not be reconstituted until a dose is ready to be administered. One glass vial containing
Cayston is opened by flipping up the metal tab, the metal ring is removed by carefully pulling the tab
(tweezers or small pliers may be used to remove the metal ring if necessary) and the grey rubber
stopper removed. The liquid is squeezed out of one solvent ampoule into the glass vial. The vial is
then gently swirled until contents have completely dissolved. The reconstituted Cayston is then
poured into the Altera Nebuliser Handset and the dose administered.
Cayston is administered by inhalation over a 2 to 3 minute period, using an Altera Nebuliser System
(consisting of a Cayston specific Altera Nebuliser Handset and Altera Control Unit). Cayston should
only be used with the Altera Nebuliser Handset and Altera Aerosol Head connected to an Altera
Control Unit or an eFlow rapid Control Unit. Cayston should not be used with any other type of
handset or aerosol head. Cayston should not be mixed with any other medicinal products in the Altera
Nebuliser Handset. Do not put other medicinal products in the Altera Nebuliser Handset.
Do not reconstitute or mix Cayston with any other solvent or medicinal product. Do not reconstitute
more than one dose at a time. Any unused product or waste material should be disposed of in
accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Gilead Sciences International Limited
Granta Park
Abington
Cambridge
CB21 6GT
United Kingdom
MARKETING AUTHORISATION NUMBER(S)
EU/1/09/543/001
EU/1/09/543/002
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
Agency http://www.ema.europa.eu.
A. MANUFACTURING AUTHORISATION HOLDER
RESPONSIBLE FOR BATCH RELEASE
B. CONDITIONS OF THE MARKETING AUTHORISATION
C. SPECIFIC OBLIGATIONS TO BE FULFILLED BY THE
MARKETING AUTHORISATION HOLDER
A. MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer responsible for batch release
Gilead Sciences Limited
IDA Business & Technology Park
Carrigtohill
County Cork
Ireland
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to medical prescription.
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
Pharmacovigilance system
The MAH must ensure that the system of pharmacovigilance, as described in version 1.0 presented in
Module 1.8.1. of the Marketing Authorisation Application, is in place and functioning before and
whilst the product is on the market.
Risk Management Plan
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version 2.0 of the Risk Management Plan (RMP) presented
in Module 1.8.2. of the Marketing Authorisation Application and any subsequent updates of the RMP
agreed by the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, the
updated RMP should be submitted at the same time as the next Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted
•
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
At the request of the European Medicines Agency
C. SPECIFIC OBLIGATIONS TO BE FULFILLED BY THE MARKETING
AUTHORISATION HOLDER
The Marketing Authorisation Holder shall complete the following programme of studies within the
specified time frame. The results of which shall be taken into account in the risk benefit balance
during the assessment of the application for a renewal.
1. The applicant commits to submit the results of study GS-US-205-0110 in September 2010.
2. Ongoing studies (ages 6 years and older):
Study GS-US-205-0110: Open-label, randomized Phase 3 study to evaluate the efficacy and safety of
AZLI
versus
Tobramycin Nebulizer Solutions (TNS) in an intermittent aerosolized regimen in patients
with CF. The final clinical study report will be available September 2010.
A review of all paediatric data from controlled studies will be provided by September 2010.
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
CAYSTON OUTER CARTON
(With a Blue Box - Not for co-packaging with the Altera Nebuliser Handset)
NAME OF THE MEDICINAL PRODUCT
Cayston 75 mg powder and solvent for nebuliser solution
aztreonam
STATEMENT OF ACTIVE SUBSTANCE(S)
Each powder vial contains 75 mg aztreonam.
After reconstitution, each ml of the nebuliser solution contains 75 mg aztreonam (as lysine).
Powder vial also contains L-Lysine
Solvent ampoule contains sodium chloride, water for injections
PHARMACEUTICAL FORM AND CONTENTS
Powder and solvent for nebuliser solution
84 single-use vials
88 single-use 1 ml ampoules of solvent
METHOD AND ROUTE(S) OF ADMINISTRATION
For inhalation use only. Reconstitute before use.
Read the package leaflet before use.
Powder should only be mixed with the solvent provided.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY

























SPECIAL STORAGE CONDITIONS
Store in a refrigerator (2°C - 8°C). May be stored outside a refrigerator but below 25°C for up to
28 days.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Do not dispose of medicines via wastewater or household waste. Ask your pharmacist about proper
disposal of medicines.
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Gilead Sciences International Ltd
Granta Park
Abington
Cambridge
CB21 6GT
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/09/543/001: 28 day pack of Cayston
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE





















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
OUTER CARTON
(Outer carton containing one 28 day pack of Cayston and one Altera Nebuliser Handset with a
Blue Box)
NAME OF THE MEDICINAL PRODUCT
Cayston 75 mg powder and solvent for nebuliser solution
aztreonam
STATEMENT OF ACTIVE SUBSTANCE(S)
Each powder vial contains 75 mg aztreonam.
After reconstitution, each ml of the nebuliser solution contains 75 mg aztreonam (as lysine).
Powder vial also contains L-Lysine
Solvent ampoule contains sodium chloride, water for injections
PHARMACEUTICAL FORM AND CONTENTS
Powder and solvent for nebuliser solution
84 single-use vials
88 single-use 1 ml ampoules of solvent
This pack contains one Altera Nebuliser Handset.
METHOD AND ROUTE(S) OF ADMINISTRATION
For inhalation use only. Reconstitute before use.
Read the package leaflet before use.
Powder should only be mixed with the solvent provided.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY























SPECIAL STORAGE CONDITIONS
Store in a refrigerator (2°C - 8°C). May be stored outside a refrigerator but below 25°C for up to
28 days.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Do not dispose of medicines via wastewater or household waste. Ask your pharmacist about proper
disposal of medicines.
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Gilead Sciences International Ltd
Granta Park
Abington
Cambridge
CB21 6GT
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/09/543/002: 28 day pack of Cayston plus one Altera Nebuliser Handset
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE






















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
CAYSTON OUTER CARTON
(No Blue Box - for use only for co-packaging with Altera Nebuliser Handset)
NAME OF THE MEDICINAL PRODUCT
Cayston 75 mg powder and solvent for nebuliser solution
aztreonam
STATEMENT OF ACTIVE SUBSTANCE(S)
Each powder vial contains 75 mg aztreonam.
After reconstitution, each ml of the nebuliser solution contains 75 mg aztreonam (as lysine).
Powder vial also contains L-Lysine
Solvent ampoule contains sodium chloride, water for injections
PHARMACEUTICAL FORM AND CONTENTS
Powder and solvent for nebuliser solution
84 single-use vials
88 single-use 1 ml ampoules of solvent
This pack is not to be sold separately.
METHOD AND ROUTE(S) OF ADMINISTRATION
For inhalation use only. Reconstitute before use.
Read the package leaflet before use.
Powder should only be mixed with the solvent provided.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY























SPECIAL STORAGE CONDITIONS
Store in a refrigerator (2°C - 8°C). May be stored outside a refrigerator but below 25°C for up to
28 days.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Do not dispose of medicines via wastewater or household waste. Ask your pharmacist about proper
disposal of medicines.
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Gilead Sciences International Ltd
Granta Park
Abington
Cambridge
CB21 6GT
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/09/543/002: 28 day pack of Cayston plus one Altera Nebuliser Handset
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE






















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
Cayston 75 mg powder and solvent for nebuliser solution
aztreonam
STATEMENT OF ACTIVE SUBSTANCE(S)
Each powder vial contains 75 mg aztreonam.
After reconstitution, each ml of the nebuliser solution contains 75 mg aztreonam (as lysine).
Powder vial also contains L-Lysine
Solvent ampoule contains sodium chloride, water for injections
PHARMACEUTICAL FORM AND CONTENTS
Powder and solvent for nebuliser solution
42 single-use vials
44 single-use 1 ml ampoules of solvent
METHOD AND ROUTE(S) OF ADMINISTRATION
For inhalation use only. Reconstitute before use.
Read the package leaflet before use.
Powder should only be mixed with the solvent provided.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY

























SPECIAL STORAGE CONDITIONS
Store in a refrigerator (2°C - 8°C). May be stored outside a refrigerator but below 25°C for up to
28 days.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Do not dispose of medicines via wastewater or household waste. Ask your pharmacist about proper
disposal of medicines.
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Gilead Sciences International Ltd
Granta Park
Abington
Cambridge
CB21 6GT
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/09/543/001: 28 day pack of Cayston
EU/1/09/543/002: 28 day pack of Cayston plus one Altera Nebuliser Handset
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE





















PACKAGE LEAFLET: INFORMATION FOR THE USER
Cayston 75 mg powder and solvent for nebuliser solution
Aztreonam
Read all of this leaflet carefully before you start taking this medicine.
-
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
What Cayston is and what it is used for
WHAT CAYSTON IS AND WHAT IT IS USED FOR
Cayston is an antibiotic used to suppress chronic lung infection caused by the bacteria
Pseudomonas
aeruginosa
in adult patients with cystic fibrosis. Cystic fibrosis, also known as mucoviscidosis, is a
life-threatening inherited disease that affects the mucus glands of internal organs, especially the lungs,
but also of the liver, pancreas, and the digestive system. Cystic fibrosis in the lungs leads to clogging
them with thick sticky mucus. This makes it hard to breathe.
if you are allergic
(hypersensitive) to aztreonam or any of the other ingredients of Cayston.
Take special care with Cayston
Your doctor needs to know:
- if you are
allergic to any other antibiotics
(such as penicillins, cephalosporins, and/or
carbapenems)
- if you do not tolerate or have chest tightness from taking other inhaled medicines
- if you have
kidney problems.
If any of these apply to you
tell your doctor
before using Cayston.
Cayston is not for use in children under the age of 18.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
Keep this leaflet. You may need to read it again.
Pregnancy and breast-feeding
It is important to tell your doctor if you are breast-feeding, if you are pregnant or planning to become
pregnant, or when you think you are pregnant. Your doctor will help you decide what is best for you
and your child.
There are no clinical data on the use of Cayston in pregnant women, therefore you should not take
Cayston during pregnancy unless specifically discussed with your doctor.
If you plan to breast-feed ask your doctor for advice before taking Cayston. You can breast-feed
during treatment with Cayston because the amount of Cayston likely to be passed to your child during
breast-feeding will be extremely small.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Cayston is not expected to affect your ability to drive or use machines.
Always take Cayston exactly as your doctor has told you. You should check with your doctor or
pharmacist if you are not sure.
Take Cayston 3 times a day for the 28-day treatment course.
Each of the three doses should
be taken by inhalation at least four hours apart, using an Altera Nebuliser Handset. You can use
either an Altera Control Unit or an eFlow rapid Control Unit with the Altera Handset.
Each dose consists of one vial of Cayston mixed with one ampoule of solvent. Cayston needs to
be mixed with a solvent before being inhaled through the Altera Nebuliser.
Put the prepared Cayston solution in the Altera Nebuliser Handset (see below). Each treatment takes
about 2 to 3 minutes to inhale.
Use a bronchodilator before each dose of Cayston. Short acting bronchodilators can be taken between
15 minutes and 4 hours and long acting bronchodilators can be taken between 30 minutes and 12 hours
prior to each dose of Cayston.
If you are using other therapies to treat cystic fibrosis, the recommended order is as follows:
1. bronchodilator
2. dornase alfa (a medicine that helps to dissolve the thick mucous produced in the lungs)
3. chest physiotherapy
4. any other inhaled medicines
and finally:
5.
Do not mix Cayston with any other medicines
in the Altera Nebuliser Handset.
-
Do not put the intravenous (injectable) form of aztreonam in the Altera Nebuliser Handset.
Intravenous aztreonam is not suitable for inhalation.
Do not put other medicines in the Altera Nebuliser Handset.
How to take Cayston using the Altera Nebuliser Handset
You will need the following:
One amber-coloured vial of Cayston
One plastic ampoule of solvent (0.17% w/v sodium chloride)
An Altera Nebuliser Handset containing an Altera Aerosol Head connected to an Altera Control
Unit (678G) or an eFlow rapid Control Unit (178G)
You must use the Cayston specific Altera Nebuliser Handset containing an Altera Aerosol Head.
Do not try to take Cayston using any other type of nebuliser handset (including the eFlow rapid
handset).
Check that your nebuliser works properly
before starting your treatment with Cayston. Read the
manufacturer’s instructions for use provided with your Altera Nebuliser System carefully.
Preparing your Cayston for inhalation
Do not prepare Cayston until you are ready to administer a dose.
Do not use Cayston if it has been stored outside a refrigerator for more than 28 days.
Do not use the solvent or prepared Cayston if it is cloudy or if there are particles in the solution.
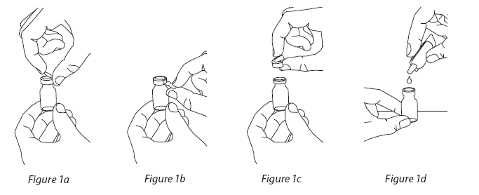
1.
Take one amber vial of Cayston and one ampoule of solvent
from the box. Solvent ampoules
must be separated by gently pulling them apart.
2.
Gently tap the amber vial
containing the Cayston so that the powder settles at the bottom. This
helps to ensure that you get the proper dose of medicine.
3.
Open the amber vial
by lifting up the metal flap on the top (Figure 1a) and pulling down
(Figure 1b) to carefully remove the entire metal ring and overcap from the vial (Figure 1c). Safely
dispose of the ring. Carefully remove the rubber stopper.
4.
Open the ampoule of solvent
by twisting off the tip. Squeeze out the contents completely into the
vial (Figure 1d). Next, gently swirl the vial until the powder has completely dissolved and the
liquid is clear.
It’s best to use Cayston immediately after you have made up the solution.
But, if you cannot use
the prepared dose straight away, replace the stopper in the vial and store in a refrigerator. Use the
prepared solution within 8 hours.
Preparing the Altera Nebuliser to take your Cayston
1.
Make sure the Altera
Nebuliser Handset
is on a flat, stable surface.
2.
Remove the medicine cap
by twisting anticlockwise.
Do not use Cayston if you notice that the package has been tampered with.



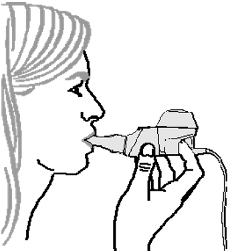
4.
Breathe normally
(inhale and exhale) through the mouthpiece. Avoid breathing through your
nose. Continue to inhale and exhale comfortably until the treatment is finished.
5.
When all of the medicine has been delivered,
you will hear a tone that means “treatment
complete” (2 beeps).
6.
When treatment is complete,
open the medicine cap to ensure that all medicine has been used. A
few drops of medicine may remain in the reservoir at the end of treatment. If there is more than a
few drops of liquid left, replace the medicine cap and restart treatment.
7.
Once treatment is complete,
disconnect the Control Unit and take apart the Altera Nebuliser
Handset for cleaning and disinfecting. For complete details on cleaning and disinfecting refer to
the manufacturer’s instructions for use provided with your Altera Nebuliser Handset.
What if I need to stop my treatment before I’ve finished?
8. If for any reason you must stop the treatment before you have finished, press and hold the On/Off
button for one full second. To re-start the treatment, press and hold the On/Off button for one full
second and then restart the treatment.
Replacing the Altera Nebuliser Handset
The Altera Nebuliser Handset is designed to last for three 28-day courses of Cayston when used as
directed. After this time replace your Altera Nebuliser Handset, including the aerosol head. If you
notice that the performance has changed before this time (for instance, if it takes longer to produce a
mist, more than 5 minutes), please refer to the Altera Nebuliser instructions for use.
If you take more Cayston than you should
If you have taken more Cayston than you should, talk to a doctor or pharmacist immediately.
If you forget to take Cayston
If you miss a dose, you can still take all 3 daily doses as long as they are at least 4 hours apart. If you
can’t leave a gap of 4 hours just skip the missed dose.
If you want to stop taking Cayston
Do not stop taking Cayston without first talking to your doctor.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Like all medicines, Cayston can cause side effects, although not everybody gets them.
If you get a rash, tell your doctor immediately
because this could mean that you have an allergic
reaction to Cayston.
The frequency of possible side effects listed below is defined using the following convention:
very common (affects more than 1 user in 10)
common (affects 1 to 10 users in 100)
uncommon (affects 1 to 10 users in 1,000)
rare (affects 1 to 10 users in 10,000)
very rare (affects less than 1 user in 10,000)
not known (frequency cannot be estimated from the available data).
Very common side effects
-
The following side effects have been observed after the use of aztreonam for injection, but not after
taking Cayston: swelling of the face, lips, tongue and/or throat with difficulty in swallowing or
breathing, sweating, skin irritation and flaking, itchy rash, flushing, small red spots and very rarely,
blistering of the skin. All these may be signs of an allergic reaction.
Tell your doctor if you have any of these effects.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Cayston after the expiry date which is stated on the vial label, solvent ampoule and the
carton. The expiry date refers to the last day of that month.
Powder vial and solvent ampoule:
Store in a refrigerator (2°C - 8°C). The unopened vials may also be stored outside the refrigerator but
below 25°C for up to 28 days.
Use Cayston immediately after preparation. If not used immediately, the prepared solution must be
stored at 2°C - 8°C and used within 8 hours. Do not prepare more than one dose at a time.
Do not use Cayston if you notice that the package has been tampered with.
Do not use Cayston if it has been stored outside a refrigerator for more than 28 days.
Do not use the solvent or prepared Cayston if it is cloudy or if there are particles in the solution.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
What Cayston and the solvent contain
The powder vial contains 75 mg aztreonam (which is the active substance) as lysine.
The solvent ampoule contains water for injections and sodium chloride.
What Cayston looks like and contents of the pack
Cayston is a sterile, white to off-white, lyophilised powder.
Cayston is contained in a 2 ml amber glass vial with a grey rubber stopper and aluminium tear-off
overseal.
The 1 ml solvent is contained in a plastic ampoule.
Each 28-day pack of Cayston contains 84 vials of lyophilised Cayston and 88 solvent ampoules. The
four additional solvent ampoules are provided in case of spillage.
The following pack sizes are available:
•
28 day pack of Cayston
•
Pack containing one 28 day pack of Cayston plus one Altera Nebuliser Handset
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
Gilead Sciences International Limited
Granta Park
Abington
Cambridge
CB21 6GT
United Kingdom
Gilead Sciences Limited
IDA Business & Technology Park
Carrigtohill
County Cork
Ireland
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
Gilead Sciences Belgium SPRL-BVBA
Tél/Tel: + 32 (0) 2 401 3550
Luxembourg/Luxemburg
Gilead Sciences Belgium SPRL-BVBA
Tél/Tel: + 32 (0) 2 401 3550
България
Gilead Sciences International Ltd
Teл.: + 44 (0) 20 7136 8820
Magyarország
Gilead Sciences International Ltd
Tel: + 44 (0) 20 7136 8820
Česká republika
Gilead Sciences International Ltd
Tel: + 44 (0) 20 7136 8820
Malta
Gilead Sciences International Ltd
Tel: + 44 (0) 20 7136 8820
Danmark
Gilead Sciences Sweden AB
Tlf: + 46 (0) 8 5057 1849
Nederland
Gilead Sciences Netherlands B.V.
Tel: + 31 (0) 20 718 3698
Deutschland
Gilead Sciences GmbH
Tel: + 49 (0) 89 899890-0
Norge
Gilead Sciences Sweden AB
Tlf: + 46 (0) 8 5057 1849
Eesti
Gilead Sciences International Ltd
Tel: + 44 (0) 20 7136 8820
Österreich
Gilead Sciences GesmbH
Tel: + 43 1 260 830
Ελλάδα
Gilead Sciences Ελλάς Μ.ΕΠΕ.
Τηλ: +30 210 8930 100
Polska
Gilead Sciences International Ltd
Tel: + 44 (0) 20 7136 8820
España
Gilead Sciences, S.L.
Tel: + 34 91 378 98 30
Portugal
Gilead Sciences, Lda.
Tel: + 351 21 7928790
France
Gilead Sciences
Tél: + 33 (0) 1 42 73 70 70
România
Gilead Sciences International Ltd
Tel: + 44 (0) 20 7136 8820
Ireland
Gilead Sciences Ltd
Tel: + 44 (0) 1223 897555
Slovenija
Gilead Sciences International Ltd
Tel: + 44 (0) 20 7136 8820
Ísland
Gilead Sciences Sweden AB
Sími: + 46 (0) 8 5057 1849
Slovenská republika
Gilead Sciences International Ltd
Tel: + 44 (0) 20 7136 8820
Italia
Gilead Sciences S.r.l.
Tel: + 39 02 439201
Suomi/Finland
Gilead Sciences Sweden AB
Puh/Tel: + 46 (0) 8 5057 1849
Κύπρος
Gilead Sciences Ελλάς Μ.ΕΠΕ.
Τηλ: + 30 210 8930 100
Sverige
Gilead Sciences Sweden AB
Tel: + 46 (0) 8 5057 1849
Latvija
Gilead Sciences International Ltd
Tel: + 44 (0) 20 7136 8820
United Kingdom
Gilead Sciences Ltd
Tel: + 44 (0) 1223 897555
Lietuva
Gilead Sciences International Ltd
Tel: + 44 (0) 20 7136 8820
This leaflet was last approved in {MM/YYYY}.
This medicine has been given “conditional approval”.
This means that there is more evidence to come about this medicine.
The European Medicines Agency will review new information on the medicine every year and this
leaflet will be updated as necessary.
Detailed information on this medicine is available on the European Medicines Agency web site:
http://www.ema.europa.eu.
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/cayston.html
Copyright © 1995-2021 ITA all rights reserved.
|























































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































