Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Cervarix suspension for injection
Human Papillomavirus vaccine [Types 16, 18] (Recombinant, adjuvanted, adsorbed)
QUALITATIVE AND QUANTITATIVE COMPOSITION
1 dose (0.5 ml) contains:
Human Papillomavirus
1
type 16 L1 protein
2,3,4
1
Human Papillomavirus = HPV
2
adjuvanted by AS04 containing:
3-
O
-desacyl-4’- monophosphoryl lipid A (MPL)
3
50
micrograms
3
adsorbed on aluminium hydroxide, hydrated (Al(OH)
3
) 0.5 milligrams Al
3+
in total
4
L1 protein in the form of non-infectious virus-like particles (VLPs) produced by recombinant DNA
technology using a Baculovirus expression system which uses Hi-5 Rix4446 cells derived from
Trichoplusia ni
.
For a full list of excipients, see section 6.1.
Suspension for injection.
Turbid white suspension. Upon storage, a fine white deposit with a clear colourless supernatant may
be observed.
Cervarix is a vaccine for the prevention of premalignant cervical lesions and cervical cancer causally
related to certain oncogenic Human Papillomavirus (HPV) types. See sections 4.4 and 5.1 for
important information on the data that support this indication.
The indication is based on the demonstration of efficacy in women aged 15-25 years following
vaccination with Cervarix and on the immunogenicity of the vaccine in girls and women aged 10-25
years.
The use of Cervarix should be in accordance with official recommendations.
Posology and method of administration
The recommended vaccination schedule is 0, 1, 6 months.
If flexibility in the vaccination schedule is necessary, the second dose can be administered
between 1 month and 2.5 months after the first dose and the third dose between 5 and 12 months
after the first dose.
Human Papillomavirus
1
type 18 L1 protein
2,3,4
The need for a booster dose has not been established (see section 5.1).
It is recommended that subjects who receive a first dose of Cervarix complete the 3-dose vaccination
course with Cervarix (see section 4.4).
Paediatric population
Cervarix is not recommended for use in girls below 10 years of age due to lack of data on safety and
immunogenicity in this age-group.
Cervarix is for intramuscular injection in the deltoid region (see also sections 4.4 and 4.5).
Hypersensitivity to the active substances or to any of the excipients.
Administration of Cervarix should be postponed in subjects suffering from an acute severe febrile
illness. However, the presence of a minor infection, such as a cold, is not a contraindication for
immunisation.
Special warnings and precautions for use
The decision to vaccinate an individual woman should take into account her risk for previous HPV
exposure and her potential benefit from vaccination.
As with all injectable vaccines, appropriate medical treatment and supervision should always be
readily available in case of a rare anaphylactic event following the administration of the vaccine.
Syncope (fainting) can occur following, or even before, any vaccination especially in adolescents as
a psychogenic response to the needle injection. This can be accompanied by several neurological
signs such as transient visual disturbance, paraesthesia and tonic-clonic limb movements during
recovery. It is important that procedures are in place to avoid injury from faints.
Cervarix should under no circumstances be administered intravascularly or intradermally.
No data are available on subcutaneous administration of Cervarix.
As with other vaccines administered intramuscularly, Cervarix should be given with caution to
individuals with thrombocytopenia or any coagulation disorder since bleeding may occur following an
intramuscular administration to these subjects.
As with any vaccine, a protective immune response may not be elicited in all vaccinees.
Cervarix will only protect against diseases that are caused by HPV types 16 and 18 and to some extent
against diseases caused by certain other oncogenic related HPV types (see section 5.1). Therefore,
appropriate precautions against sexually transmitted diseases should continue to be used.
Cervarix is for prophylactic use only and has no effect on active HPV infections or established clinical
disease. Cervarix has not been shown to have a therapeutic effect. The vaccine is therefore not
indicated for treatment of cervical cancer or cervical intraepithelial neoplasia (CIN). It is also not
intended to prevent progression of other established HPV-related lesions or existing HPV infections
with vaccine or non-vaccine types (see section 5.1 “Efficacy in women with evidence of HPV-16 or
HPV-18 infection at study entry.”).
Vaccination is not a substitute for routine cervical screening. Since no vaccine is 100% effective and
Cervarix will not provide protection against every HPV type, or against existing HPV infections,
routine cervical screening remains critically important and should follow local recommendations.
Duration of protection has not fully been established. Timing and need of booster dose(s) has not been
established.
There are no data on the use of Cervarix in subjects with impaired immune responsiveness such as
HIV infected patients or patients receiving immunosuppressive treatment. As with other vaccines, an
adequate immune response may not be elicited in these individuals.
There are no safety, immunogenicity or efficacy data to support interchangeability of Cervarix with
other HPV vaccines.
Interaction with other medicinal products and other forms of interaction
In all clinical trials individuals who had received immunoglobulin or blood products within 3 months
prior to the first vaccine dose were excluded.
Cervarix may be administered concomitantly with a combined booster vaccine containing diphtheria
(d), tetanus (T) and pertussis [acellular] (pa) with or without inactivated poliomyelitis (IPV), (dTpa,
dTpa-IPV vaccines), with no clinically relevant interference with antibody response to any of the
components of either vaccine. The sequential administration of combined dTpa-IPV followed by
Cervarix one month later tended to elicit lower anti-HPV-16 and anti-HPV-18 GMTs as compared to
Cervarix alone. The clinical relevance of this observation is not known.
Cervarix may be administered concomitantly with a combined hepatitis A (inactivated) and hepatitis B
(rDNA) vaccine (Twinrix) or with hepatitis B (rDNA) vaccine (Engerix B).
Administration of Cervarix at the same time as Twinrix has shown no clinically relevant interference
in the antibody response to the HPV and hepatitis A antigens. Anti-HBs geometric mean antibody
concentrations were significantly lower on co-administration, but the clinical relevance of this
observation is not known since the seroprotection rates remain unaffected. The proportion of subjects
reaching anti-HBs ≥ 10mIU/ml was 98.3% for concomitant vaccination and 100% for Twinrix given
alone. Similar results were observed when Cervarix was given concomitantly with Engerix B with
97.9% of subjects reaching anti-HBs ≥ 10mIU/ml compared to 100% for Engerix B given alone.
If Cervarix is to be given at the same time as another injectable vaccine, the vaccines should always be
administered at different injection sites.
Use with hormonal contraceptive
In clinical efficacy studies, approximately 60% of women who received Cervarix used hormonal
contraceptives. There is no evidence that the use of hormonal contraceptives has an impact on the
efficacy of Cervarix.
Use with systemic immunosuppressive medicinal products
As with other vaccines it may be expected that, in patients receiving immunosuppressive treatment, an
adequate response may not be elicited.
Fertility, pregnancy and lactation
Specific studies of the vaccine in pregnant women were not conducted. However, during the clinical
development program, a total of 3,993 pregnancies were reported including 2,009 in women who had
received Cervarix. Overall, the proportions of pregnant subjects who experienced specific outcomes
(e.g., normal infant, abnormal infants including congenital anomalies, premature birth, and
spontaneous abortion) were similar between treatment groups.
Animal studies do not indicate direct or indirect harmful effects with respect to fertility, pregnancy,
embryonal/foetal development, parturition or post-natal development (see section 5.3).
These data are insufficient to recommend use of Cervarix during pregnancy.
Vaccination should, therefore, be postponed until after completion of pregnancy.
The effect on breast-fed infants of the administration of Cervarix to their mothers has not been
evaluated in clinical studies.
Cervarix should only be used during breast-feeding when the possible advantages outweigh the
possible risks.
Effects on the ability to drive and use machines
No studies on the effects on the ability to drive or use machines have been performed.
In clinical studies that enrolled girls and women aged from 10 up to 72 years (of which 79.2% were
aged 10-25 years at the time of enrolment), Cervarix was administered to 16,142 subjects whilst
13,811 subjects received control. These subjects were followed for serious adverse events over the
entire study period. In a pre-defined subset of subjects (Cervarix = 8,130 versus control = 5,786),
adverse events were followed for 30 days after each injection.
The most common adverse reaction observed after vaccine administration was injection site pain
which occurred after 78% of all doses. The majority of these reactions were of mild to moderate
severity and were not long lasting.
Adverse reactions considered as being at least possibly related to vaccination have been categorised
by frequency.
Frequencies are reported as:
Very common (≥1/10)
Common (≥1/100 to <1/10)
Uncommon (≥1/1,000 to <1/100)
Infections and infestations
Uncommon: upper respiratory tract infection
Nervous system disorders
Very common: headache
Gastrointestinal disorders
Common: gastrointestinal symptoms including nausea, vomiting, diarrhoea and abdominal pain
Skin and subcutaneous tissue disorders
Common: itching/pruritus, rash, urticaria
Musculoskeletal and connective tissue disorders
Very common: myalgia
General disorders and administration site conditions
Very common: injection site reactions including pain, redness, swelling; fatigue
Common: fever (≥38°C)
Uncommon: other injection site reactions such as induration, local paraesthesia
A similar safety profile has been observed in subjects with prior or current HPV infection as compared
to subjects negative for oncogenic HPV DNA or seronegative for HPV-16 and HPV-18 antibodies.
Post marketing surveillance
Becaus e these events were reported spontaneous ly, it is not possible to reliably estimate their
frequency.
Blood and lymphatic system disorders
Allergic reactions (including anaphylactic and anaphylactoid reactions), angioedema
Syncope or vasovagal responses to injection, sometimes accompanied by tonic-clonic movements (see
section 4.4)
No case of overdose has been reported.
5. PHARMACOLOGICAL PROPERTIES
Pharmacodynamic properties
Pharmaco-therapeutic group: Papillomavirus vaccines, ATC code: J07BM02
Cervarix is an adjuvanted non-infectious recombinant vaccine prepared from the highly purified virus-
like particles (VLPs) of the major capsid L1 protein of oncogenic HPV types 16 and 18. Since the
VLPs contain no viral DNA, they cannot infect cells, reproduce or cause disease. Animal studies have
shown that the efficacy of L1 VLP vaccines is largely mediated by the development of a humoral
immune response.
HPV-16 and HPV-18 are estimated to be responsible for approximately 70% of cervical cancers.
Other oncogenic HPV types can also cause cervical cancer (approximately 30%). HPV 45, -31 and -33
are the 3 most common non-vaccine HPV types identified in squamous cervical carcinoma (12.1%)
and adenocarcinoma (8.5%).
The term “premalignant cervical lesions” in section 4.1 corresponds to high-grade Cervical
Intraepithelial Neoplasia (CIN2/3).
The efficacy of Cervarix was assessed in two controlled, double-blind, randomised Phase II and III
clinical trials that included a total of 19,778 women aged 15 to 25 years.
The phase II trial (study 001/007) enrolled only women who:
-
Were tested negative for oncogenic HPV DNA of types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56,
58, 59, 66 and 68
-
Were serone gative for HPV-16 and HPV-18 and
-
Had normal cytology
The primary efficacy endpoint was incident infection with HPV-16 and/or HPV-18. Twelve-month
persistent infection was evaluated as additional efficacy endpoint.
The phase III trial (study 008) enrolled women without pre-screening for the presence of HPV
infection, i.e. regardless of baseline cytology and HPV serological and DNA status.
The primary efficacy endpoint was CIN2+ (CIN2/3 or AIS) associated with HPV-16 and/or HPV-18
(HPV-16/18). Cervical Intraepithelial Neoplasia (CIN) grade 2 and 3 (CIN2/3) and cervical
adenocarcinoma in situ (AIS) were used in the clinical trials as surrogate markers for cervical cancer.
The secondary endpoints included 6- and 12-month persistent infection.
Persistent infection that lasts for at least 6 months has also been shown to be a relevant surrogate
marker for cervical cancer.
Prophylactic efficacy against HPV-16/18 infection in a population naïve to oncogenic HPV types
Women (N=1,113) were vaccinated in study 001 and evaluated for efficacy up to month 27. A subset
of women (N=776) vaccinated in study 001 was followed in study 007 up to 6.4 years (approximately
77 months) after the first dose (mean follow-up of 5.9 years). There were five cases of 12-month
persistent HPV-16/18 infection (4 HPV-16; 1 HPV-18) in the control group and one HPV-16 case in
the vaccine group in study 001. In study 007 the efficacy of Cervarix against 12-month persistent
HPV-16/18 infection was 100% (95% CI: 80.5; 100). There were sixteen cases of persistent HPV-16
infection, and five cases of persistent HPV-18 infection, all in the control group.
Prophylactic efficacy against HPV-16/18 in women naïve to HPV-16 and/or HPV-18
In study HPV-008, the primary analyses of efficacy were performed on the According to Protocol
cohort (ATP cohort: including women who received 3 vaccine doses and were DNA negative and
seronegative at month 0 and DNA negative at month 6 for the HPV type considered in the analysis)
This cohort included women with normal or low-grade cytology at baseline and excluded only women
with high-grade cytology (0.5% of the total population). Case counting for the ATP cohort started on
day 1 after the third dose of vaccine.
Overall, 74% of women enrolled were naïve to both HPV-16 and HPV-18 (i.e. DNA negative and
seronegative at study entry).
The mean follow-up for women included in study HPV-008 was approximately 39 months post dose 1
in TVC and 35 months post dose 3 in the ATP cohort.
Vaccine efficacy against the primary endpoint CIN2/3 or AIS is presented in Table 1. In a
suppl emental analysis, the efficacy of Cervarix was evaluated against HPV-16/18-related CIN3 or
AIS.
Table 1: Vaccine efficacy against high grade cervical lesions associated with HPV-16/18 (ATP
cohort)










N = number of subjects included in each group
n = number of cases
(1)
ATP: includes women who received 3 doses of vaccine, were DNA negative and seronegative
at month 0 and DNA negative at month 6 to the relevant HPV type (HPV-16 or HPV-18)
(2)
including 3 cases of CIN2/3 or AIS and 2 cases of CIN3 or AIS in which another oncogenic
HPV type was identified in the lesion, concomitantly with HPV-16 or HPV-18. These cases
are excluded in the HPV type assignment analysis (see under Table).
Further investigation of the cases with multiple HPV types considered the HPV types detected by
Polymerase Chain Reaction (PCR) in at least one of the two preceding cytology samples, in addition
to types detected in the lesion to distinguish the HPV type(s) most likely responsible to the lesion
(HPV type assignment). This post-hoc analysis excluded cases (in the vaccine group and in the control
group) which were not considered to be causally associated with HPV-16 or HPV-18 infections
acquired during the trial.
Based on the HPV type assignment post-hoc analysis, there were 1 CIN2/3 or AIS case in the vaccine
group versus 53 cases in the control group (Efficacy 98.1% (96.1% CI: 88.4; 100)) and 0 CIN3 or AIS
cases in the vaccine group versus 8 cases in the control group (Efficacy 100% (96.1% CI: 36.4; 100)).
In addition, statistically significant vaccine efficacy against CIN2/3 or AIS associated with HPV-16
and HPV-18 individually was demonstrated.
Vaccine efficacy against CIN1 associated with HPV 16/18 observed in the ATP cohort was 94.1%
(96.1% CI: 83.4;98.5). Vaccine efficacy against CIN1/2/3 or AIS associated with HPV 16/18
observed in the ATP cohort was 91.7% (96.1% CI: 82.4;96.7).
Vaccine efficacy against virological endpoints (6-month and 12-month persistent infection) associated
with HPV-16/18 observed in the ATP cohort is presented in Table 2.
Table 2: Vaccine efficacy against virological endpoints associated with HPV-16
/18 (ATP cohort)
6-month persistent infection
12-month persistent infection
20/7035
N = number of subjects included in each group
n = number of cases
(1)
ATP: includes women who received 3 doses of vaccine, were DNA negative and
seronegative at month 0 and DNA negative at month 6 to the relevant HPV type
(HPV-16 or HPV-18)
Efficacy against HPV-16/18 in women with evidence of HPV-16 or HPV-18 infection at study entry.
There was no evidence of protection from disease caused by the HPV types for which subjects were
HPV DNA positive at study entry. However, individuals already infected (HPV DNA positive) with
one of the vaccine-related HPV types prior to vaccination were protected from clinical disease caused
by the other vaccine HPV type.
Efficacy against HPV types 16 and 18 in women with and without prior infection or disease.
The Total Vaccinated Cohort (TVC) included all subjects who received at least one dose of the
vaccine, irrespective of their HPV DNA status, cytology and serostatus at baseline. This cohort
included women with or without current and/or prior HPV infection. Case counting for the TVC
started on day 1 after the first dose.
The efficacy estimates are lower in the TVC as this cohort includes women with pre-existing
infections/lesions, which are not expected to be impacted by Cervarix.
The TVC may approximate to the general population of women in the age range of 15-25 years.














Vaccine efficacy against high grade cervical lesions associated with HPV-16/18 observed in TVC is
presented in Table 3.
Table 3: Vaccine efficacy against high grade cervical lesions associated with HPV-16/18 (TVC
)
N = number of subjects included in each group
n = number of cases
(1)
TVC: includes all vaccinated subjects (who received at least one dose of vaccine) irrespective of
HPV DNA status, cytology and serostatus at baseline. This cohort includes women with pre-existing
infections/lesions
Vaccine efficacy against virological endpoints (6-month and 12-month persistent infection) associated
with HPV-16/18 observed in TVC is presented in Table 4.
Table 4:
Vaccine efficacy against virological endpoints associated with HPV-16/18 (TVC)
6-month
persistent infection
12-month
persistent infection
N = number of subjects included in each group
n = number of cases
(1)
TVC: includes all vaccinated subjects (who received at least one dose of vaccine) irrespective of HPV
DNA status, cytology and serostatus at baseline.
Overall impact of the vaccine on cervical HPV disease burden
In study HPV-008, the incidence of high grade cervical lesions was compared between the placebo
and vaccine group irrespective of the HPV DNA type in the lesion. In the TVC and TVC-naïve
cohorts, the vaccine’s efficacy was demonstrated against high-grade cervical lesions (Table 5).
The TVC-naïve is a subset of the TVC that includes women with normal cytology, and who were HPV
DNA negative for 14 oncogenic HPV types and seronegative for HPV-16 and HPV-18 at baseline.
Table 5: Vaccine efficacy against high-grade cervical lesions irrespective of the HPV DNA type
in the lesion
CIN2/3 or AIS
TVC-naïve
(1)
CIN3 or AIS
TVC-naïve
(1)


































N = number of subjects included in each group
(1)
TVC naïve: includes all vaccinated subjects (who received at least one dose of
vaccine) who had normal cytology, were HPV DNA negative for 14 oncogenic
HPV types and seronegative for HPV-16 and HPV-18 at baseline.
(2)
TVC: includes all vaccinated subjects (who received at least one dose of
vaccine) irrespective of HPV DNA status, cytology and serostatus at baseline.
Cervarix reduced definitive cervical therapy procedures (includes loop electrosurgical excision
procedure [LEEP], cold-knife Cone, and laser procedures) by 68.8% (96.1% CI: 50.0;81.2) in TVC
naïve and by 24.7% (96.1% CI: 7.4;38.9) in TVC.
Cross-protective efficacy
The cross-protective efficacy of Cervarix against histopathological and virological endpoints
(persistent infection) has been evaluated in study HPV-008 for 12 non-vaccine oncogenic HPV types.
The study was not powered to assess efficacy against disease caused by individual HPV types. The
analysis against the primary endpoint was confounded by multiple co-infections in the CIN2+ lesions.
Unlike histopathological endpoints, virological endpoints are less confounded by multiple infections.
Only HPV-31 showed consistent cross-protection for all endpoints (6m and 12m persistent infection,
CIN2/3 or AIS) and all study cohorts. Vaccine efficacy against 6 months persistent infection has also
been shown for HPV-33 and HPV-45 in all study cohorts.
Vaccine efficacy against 6-month persistent infection and CIN2/3 or AIS associated with individual
non-vaccine oncogenic HPV types is presented in Table 6 (ATP cohort).
Table 6: Vaccine efficacy for non-vaccine oncogenic HPV types
ATP
(1)
6-month persistent infection
HPV-16 related types (A9 species)
HPV-31
HPV-18 related types (A7 sp
ecies)
HPV-39
n= number of cases
(1)
ATP: includes women who received 3 doses of vaccine, were DNA negative and seronegative at month
































0 and DNA negative at month 6 to the relevant HPV type.
The limits of the confidence interval around the vaccine efficacy were calculated. When the value zero is
included, i.e. when the lower limit of the CI is <0, the efficacy is not considered statistically significant.
Immune response to Cervarix after the primary vaccination course
No minimal antibody level associated with protection against CIN of grade 2 or 3 or against persistent
infection associated with vaccine HPV types has been identified for HPV vaccines.
The antibody response to HPV-16 and HPV-18 was measured using a type-specific direct ELISA
(version 2, MedImmune methodology, modified by GSK) which was shown to correlate with the
pseudovirion-based neutralisation assay (PBNA).
The immunogenicity induced by three doses of Cervarix has been evaluated in 5,303
female subjects
from 10 to 55 years of age.
In clinical trials, more than 99% of initially seronegative subjects had seroconverted to both HPV
types 16 and 18 one month after the third dose. Vaccine-induced IgG Geometric Mean Titres (GMT)
were well above titres observed in women previously infected but who cleared HPV infection (natural
infection). Initially seropositive and seronegative subjects reached similar titres after vaccination.
Persistence of Immune Response to Cervarix
Study 001/007, which included women from 15 to 25 years of age at the time of vaccination,
evaluated the immune response against HPV-16 and HPV-18 up to 76 months after administration of
the first vaccine dose. In study 023 (a subset of study 001/007), the immune response continued to be
evaluated up to 101 months. 87 subjects in the vaccine group had immunogenicity data at the [M95-
M101] interval after the first vaccine dose with a median follow-up of 7.9 years. Of these subj ects,
100% (95% CI: 95.8;100) remained seropositive for HPV-16 and HPV-18 in the ELISA assay.
Vaccine-induced IgG GMTs for both HPV-16 and HPV-18 peaked at month 7 and then declined to
reach a plateau from month 18 up to the [M95-M101] interval with ELISA GMTs for both HPV-16
and HPV-18 at least still 10-fold higher than the ELISA GMTs observed in women who cleared a
natural HPV infection.
In study 008, immunogenicity up to month 36 was similar to the response observed in study 001. A
similar kinetic profile was observed with the neutralising antibodies.
In another clinical trial (study 014) performed in women aged 15 to 55 years, all subjects
seroconverted to both HPV types 16 and 18 after the third dose (at month 7). The GMTs were,
however, lower in women above 25 years. Nevertheless, all subjects remained seropositive for both
types throughout the follow-up phase (up to month 18) maintaining antibody levels at an order of
magnitude above those encountered after natural infection.
Evidence of Anamnestic (Immune Memory) Response
In study 024 (a subset of study 001/007), a challenge dose of Cervarix was administered to 65 subjects
at a mean interval of 6.8 years after the administration of the first vaccine dose. An anamnestic
immune response to HPV-16 and HPV-18 (by ELISA) was observed one week and one month after
the challenge dose, GMTs one month after the challenge dose exceeded those observed one month
after the primary 3-dose vaccination.
Bridging the efficacy of Cervarix from young adult women to adolescents



In two clinical trials performed in girls and adolescents aged 10 to 14 years, all subjects seroconverted
to both HPV types 16 and 18 after the third dose (at month 7) with GMTs at least 2-fold higher as
compared to women aged 15 to 25 years. On the basis of these immunogenicity data, the efficacy of
Cervarix is inferred from 10 to 14 years of age.
Pharmacokinetic properties
Evaluation of pharmacokinetic properties is not required for vaccines.
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, acute and repeated dose toxicity, local tolerance, fertility, embryo-foetal and postnatal
toxicity (up to the end of the lactation period).
Serological data suggest a transfer of anti-HPV-16 and anti-HPV-18 antibodies via the milk during the
lactation period in rats. However, it is unknown whether vaccine-induced antibodies are excreted in
human breast milk.
6. PHARMACEUTICAL PARTICULARS
Sodium chloride (NaCl)
Sodium dihydrogen phosphate dihydrate (NaH
2
PO
4
.2 H
2
O)
Water for injections
For adjuvants, see section 2.
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
Cervarix should be administered as soon as possible after being removed from the refrigerator.
However, stability data generated indicate that Cervarix presented in monodose containers remains
stable and can be administered in case it has been stored outside the refrigerator up to three days at
temperatures between 8°C and 25°C or up to one day at temperatures between 25°C and 37°C.
Special precautions for storage
Store in a refrigerator (2°C – 8°C).
Do not freeze.
Store in the original package in order to protect from light.
Nature and contents of container
0.5 ml of suspension in a vial (type I glass) for 1 dose with a stopper (rubber butyl) in pack sizes of 1,
10 and 100.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
A fine white deposit with a clear colourless supernatant may be observed upon storage of the vial. This
does not constitute a sign of deterioration.
The content of the vial should be inspected visually both before and after shaking for any foreign
particulate matter and/or abnormal physical appearance prior to administration.
In the event of either being observed, discard the vaccine.
The vaccine should be well shaken before use.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
GlaxoSmithKline Biologicals s.a.
Rue de l'Institut 89
B-1330 Rixensart, Belgium
MARKETING AUTHORISATION NUMBER(S)
EU/1/07/419/001
EU/1/07/419/002
EU/1/07/419/003
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 20 September 2007.
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
NAME OF THE MEDICINAL PRODUCT
Cervarix suspension for injection, multidose
Human Papillomavirus vaccine [Types 16, 18] (Recombinant, adjuvanted, adsorbed)
QUALITATIVE AND QUANTITATIVE COMPOSITION
1 dose (0.5 ml) contains:
Human Papillomavirus
1
type 16 L1 protein
2,3,4
1
Human Papillomavirus = HPV
2
adjuvanted by AS04 containing:
3-
O
-desacyl-4’- monophosphoryl lipid A (MPL)
3
50
micrograms
3
adsorbed on aluminium hydroxide, hydrated (Al(OH)
3
) 0.5 milligrams Al
3+
in total
4
L1 protein in the form of non-infectious virus-like particles (VLPs) produced by recombinant DNA
technology using a Baculovirus expression system which uses Hi-5 Rix4446 cells derived from
Trichoplusia ni
.
This is a multidose container. See section 6.5 for the number of doses per vial.
For a full list of excipients, see section 6.1.
Suspension for injection.
Turbid white suspension. Upon storage, a fine white deposit with a clear colourless supernatant may
be observed.
Cervarix is a vaccine for the prevention of premalignant cervical lesions and cervical cancer causally
related to certain oncogenic Human Papillomavirus (HPV) types. See sections 4.4 and 5.1 for
important information on the data that support this indication.
The indication is based on the demonstration of efficacy in women aged 15-25 years following
vaccination with Cervarix and on the immunogenicity of the vaccine in girls and women aged 10-25
years.
The use of Cervarix should be in accordance with official recommendations.
Posology and method of administration
The recommended vaccination schedule is 0, 1, 6 months.
Human Papillomavirus
1
type 18 L1 protein
2,3,4
If flexibility in the vaccination schedule is necessary, the second dose can be administered
between 1 month and 2.5 months after the first dose and the third dose between 5 and 12 months
after the first dose.
The need for a booster dose has not been established (see section 5.1).
It is recommended that subjects who receive a first dose of Cervarix complete the 3-dose vaccination
course with Cervarix (see section 4.4).
Paediatric population
Cervarix is not recommended for use in girls below 10 years of age due to lack of data on safety and
immunogenicity in this age-group.
Cervarix is for intramuscular injection in the deltoid region (see also sections 4.4 and 4.5).
Hypersensitivity to the active substances or to any of the excipients.
Administration of Cervarix should be postponed in subjects suffering from an acute severe febrile
illness. However, the presence of a minor infection, such as a cold, is not a contraindication for
immunisation.
Special warnings and precautions for use
The decision to vaccinate an individual woman should take into account her risk for previous HPV
exposure and her potential benefit from vaccination.
As with all injectable vaccines, appropriate medical treatment and supervision should always be
readily available in case of a rare anaphylactic event following the administration of the vaccine.
Syncope (fainting) can occur following, or even before, any vaccination especially in adolescents as
a psychogenic response to the needle injection. This can be accompanied by several neurological
signs such as transient visual disturbance, paraesthesia and tonic-clonic limb movements during
recovery. It is important that procedures are in place to avoid injury from faints.
Cervarix should under no circumstances be administered intravascularly or intradermally.
No data are available on subcutaneous administration of Cervarix.
As with other vaccines administered intramuscularly, Cervarix should be given with caution to
individuals with thrombocytopenia or any coagulation disorder since bleeding may occur following an
intramuscular administration to these subjects.
As with any vaccine, a protective immune response may not be elicited in all vaccinees.
Cervarix will only protect against diseases that are caused by HPV types 16 and 18 and to some extent
against diseases caused by certain other oncogenic related HPV types (see section 5.1). Therefore,
appropriate precautions against sexually transmitted diseases should continue to be used.
Cervarix is for prophylactic use only and has no effect on active HPV infections or established clinical
disease. Cervarix has not been show n to have a therapeutic effect. The vaccine is therefore not
indicated for treatment of cervical cancer or cervical intraepithelial neoplasia (CIN). It is also not
intended to prevent progression of other established HPV-related lesions or existing HPV infections
with vaccine or non-vaccine types (see section 5.1 “Efficacy in women with evidence of HPV-16 or
HPV-18 infection at study entry.”).
Vaccination is not a substitute for routine cervical screening. Since no vaccine is 100% effective and
Cervarix will not provide protection against every HPV type, or against existing HPV infections,
routine cervical screening remains critically important and should follow local recommendations.
Duration of protection has not fully been established. Timing and need of booster dose(s) has not been
established.
There are no data on the use of Cervarix in subjects with impaired immune responsiveness such as
HIV infected patients or patients receiving immunosuppressive treatment. As with other vaccines, an
adequate immune response may not be elicited in these individuals.
There are no safety, immunogenicity or efficacy data to support interchangeability of Cervarix with
other HPV vaccines.
Interaction with other medicinal products and other forms of interaction
In all clinical trials individuals who had received immunoglobulin or blood products within 3 months
prior to the first vaccine dose were excluded.
Cervarix may be administered concomitantly with a combined booster vaccine containing diphtheria
(d), tetanus (T) and pertussis [acellular] (pa) with or without inactivated poliomyelitis (IPV), (dTpa,
dTpa-IPV vaccines), with no clinically relevant interference with antibody response to any of the
components of either vaccine. The sequential administration of combined dTpa-IPV followed by
Cervarix one month later tended to elicit lower anti-HPV-16 and anti-HPV-18 GMTs as compared to
Cervarix alone. The clinical relevance of this observation is not known.
Cervarix may be administered concomitantly with a combined hepatitis A (inactivated) and hepatitis B
(rDNA) vaccine (Twinrix) or with hepatitis B (rDNA) vaccine (Engerix B).
Administration of Cervarix at the same time as Twinrix has shown no clinically relevant interference
in the antibody response to the HPV and hepatitis A antigens. Anti-HBs geometric mean antibody
concentrations were significantly lower on co-administration, but the clinical relevance of this
observation is not known since the seroprotection rates remain unaffected. The proportion of subjects
reaching anti-HBs ≥ 10mIU/ml was 98.3% for concomitant vaccination and 100% for Twinrix given
alone. Similar results were observed when Cervarix was given concomitantly with Engerix B with
97.9% of subjects reaching anti-HBs ≥ 10mIU/ml compared to 100% for Engerix B given alone.
If Cervarix is to be given at the same time as another injectable vaccine, the vaccines should always be
administered at different injection sites.
Use with hormonal contraceptive
In clinical efficacy studies, approximately 60% of women who received Cervarix used hormonal
contraceptives. There is no evidence that the use of hormonal contraceptives has an impact on the
efficacy of Cervarix.
Use with systemic immunosuppressive medicinal products
As with other vaccines it may be expected that, in patients receiving immunosuppressive treatment, an
adequate response may not be elicited.
Fertility, pregnancy and lactation
Specific studies of the vaccine in pregnant women were not conducted. However, during the clinical
development program, a total of 3,993 pregnancies were reported including 2,009 in women who had
received Cervarix. Overall, the proportions of pregnant subjects who experienced specific outcomes
(e.g., normal infant, abnormal infants including congenital anomalies, premature birth, and
spontaneous abortion) were similar between treatment groups.
Animal studies do not indicate direct or indirect harmful effects with respect to fertility, pregnancy,
embryonal/foetal development, parturition or post-natal development (see section 5.3).
These data are insufficient to recommend use of Cervarix during pregnancy.
Vaccination should, therefore, be postponed until after completion of pregnancy.
The effect on breast-fed infants of the administration of Cervarix to their mothers has not been
evaluated in clinical studies.
Cervarix should only be used during breast-feeding when the possible advantages outweigh the
possible risks.
Effects on the ability to drive and use machines
No studies on the effects on the ability to drive or use machines have been performed.
In clinical studies that enrolled girls and women aged from 10 up to 72 years (of which 79.2% were
aged 10-25 years at the time of enrolment), Cervarix was administered to 16,142 subjects whilst
13,811 subjects received control. These subjects were followed for serious adverse events over the
entire study period. In a pre-defined subset of subjects (Cervarix = 8,130 versus control = 5,786),
adverse events were followed for 30 days after each injection.
The most common adverse reaction observed after vaccine administration was injection site pain
which occurred after 78% of all doses. The majority of these reactions were of mild to moderate
severity and were not long lasting.
Adverse reactions considered as being at least possibly related to vaccination have been categorised
by frequency.
Frequencies are reported as:
Very common (≥1/10)
Common (≥1/100 to <1/10)
Uncommon (≥1/1,000 to <1/100)
Infections and infestations
Uncommon: upper respiratory tract infection
Nervous system disorders
Very common: headache
Gastrointestinal disorders
Common: gastrointestinal symptoms including nausea, vomiting, diarrhoea and abdominal pain
Skin and subcutaneous tissue disorders
Common: itching/pruritus, rash, urticaria
Musculoskeletal and connective tissue disorders
Very common: myalgia
General disorders and administration site conditions
Very common: injection site reactions including pain, redness, swelling; fatigue
Common: fever (≥38°C)
Uncommon: other injection site reactions such as induration, local paraesthesia
A similar safety profile has been observed in subjects with prior or current HPV infection as compared
to subjects negative for oncogenic HPV DNA or seronegative for HPV-16 and HPV-18 antibodies.
Post marketing surveillance
Becaus e these events were reported spontaneous ly, it is not possible to reliably estimate their
frequency.
Blood and lymphatic system disorders
Allergic reactions (including anaphylactic and anaphylactoid reactions), angioedema
Syncope or vasovagal responses to injection, sometimes accompanied by tonic-clonic movements (see
section 4.4)
No case of overdose has been reported.
5. PHARMACOLOGICAL PROPERTIES
Pharmacodynamic properties
Pharmaco-therapeutic group: Papillomavirus vaccines, ATC code: J07BM02
Cervarix is an adjuvanted non-infectious recombinant vaccine prepared from the highly purified virus-
like particles (VLPs) of the major capsid L1 protein of oncogenic HPV types 16 and 18. Since the
VLPs contain no viral DNA, they cannot infect cells, reproduce or cause disease. Animal studies have
shown that the efficacy of L1 VLP vaccines is largely mediated by the development of a humoral
immune response.
HPV-16 and HPV-18 are estimated to be responsible for approximately 70% of cervical cancers.
Other oncogenic HPV types can also cause cervical cancer (approximately 30%). HPV 45, -31 and -33
are the 3 most common non-vaccine HPV types identified in squamous cervical carcinoma (12.1%)
and adenocarcinoma (8.5%).
The term “premalignant cervical lesions” in section 4.1 corresponds to high-grade Cervical
Intraepithelial Neoplasia (CIN2/3).
The efficacy of Cervarix was assessed in two controlled, double-blind, randomised Phase II and III
clinical trials that included a total of 19,778 women aged 15 to 25 years.
The phase II trial (study 001/007) enrolled only women who:
-
Were tested negative for oncogenic HPV DNA of types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56,
58, 59, 66 and 68
-
Were serone gative for HPV-16 and HPV-18 and
-
Had normal cytology
The primary efficacy endpoint was incident infection with HPV-16 and/or HPV-18. Twelve-month
persistent infection was evaluated as additional efficacy endpoint.
The phase III trial (study 008) enrolled women without pre-screening for the presence of HPV
infection, i.e. regardless of baseline cytology and HPV serological and DNA status.
The primary efficacy endpoint was CIN2+ (CIN2/3 or AIS) associated with HPV-16 and/or HPV-18
(HPV-16/18). Cervical Intraepithelial Neoplasia (CIN) grade 2 and 3 (CIN2/3) and cervical
adenocarcinoma in situ (AIS) were used in the clinical trials as surrogate markers for cervical cancer.
The secondary endpoints included 6- and 12-month persistent infection.
Persistent infection that lasts for at least 6 months has also been shown to be a relevant surrogate
marker for cervical cancer.
Prophylactic efficacy against HPV-16/18 infection in a population naïve to oncogenic HPV types
Women (N=1,113) were vaccinated in study 001 and evaluated for efficacy up to month 27. A subset
of women (N=776) vaccinated in study 001 was followed in study 007 up to 6.4 years (approximately
77 months) after the first dose (mean follow-up of 5.9 years). There were five cases of 12-month
persistent HPV-16/18 infection (4 HPV-16; 1 HPV-18) in the control group and one HPV-16 case in
the vaccine group in study 001. In study 007 the efficacy of Cervarix against 12-month persistent
HPV-16/18 infection was 100% (95% CI: 80.5; 100). There were sixteen cases of persistent HPV-16
infection, and five cases of persistent HPV-18 infection, all in the control group.
Prophylactic efficacy against HPV-16/18 in women naïve to HPV-16 and/or HPV-18
In study HPV-008, the primary analyses of efficacy were performed on the According to Protocol
cohort (ATP cohort: including women who received 3 vaccine doses and were DNA negative and
seronegative at month 0 and DNA negative at month 6 for the HPV type considered in the analysis)
This cohort included women with normal or low-grade cytology at baseline and excluded only women
with high-grade cytology (0.5% of the total population). Case counting for the ATP cohort started on
day 1 after the third dose of vaccine.
Overall, 74% of women enrolled were naïve to both HPV-16 and HPV-18 (i.e. DNA negative and
seronegative at study entry).
The mean follow-up for women included in study HPV-008 was approximately 39 months post dose 1
in TVC and 35 months post dose 3 in the ATP cohort.
Vaccine efficacy against the primary endpoint CIN2/3 or AIS is presented in Table 1. In a
suppl emental analysis, the efficacy of Cervarix was evaluated against HPV-16/18-related CIN3 or
AIS.
Table 1: Vaccine efficacy against high grade cervical lesions associated with HPV-16/18 (ATP
cohort)










N = number of subjects included in each group
n = number of cases
(1)
ATP: includes women who received 3 doses of vaccine, were DNA negative and seronegative
at month 0 and DNA negative at month 6 to the relevant HPV type (HPV-16 or HPV-18)
including 3 cases of CIN2/3 or AIS and 2 cases of CIN3 or AIS in which another oncogenic
HPV type was identified in the lesion, concomitantly with HPV-16 or HPV-18. These cases
are excluded in the HPV type assignment analysis (see under Table).
Further investigation of the cases with multiple HPV types considered the HPV types detected by
Polymerase Chain Reaction (PCR) in at least one of the two preceding cytology samples, in addition
to types detected in the lesion to distinguish the HPV type(s) most likely responsible to the lesion
(HPV type assignment). This post-hoc analysis excluded cases (in the vaccine group and in the control
group) which were not considered to be causally associated with HPV-16 or HPV-18 infections
acquired during the trial.
Based on the HPV type assignment post-hoc analysis, there were 1 CIN2/3 or AIS case in the vaccine
group versus 53 cases in the control group (Efficacy 98.1% (96.1% CI: 88.4; 100)) and 0 CIN3 or AIS
cases in the vaccine group versus 8 cases in the control group (Efficacy 100% (96.1% CI: 36.4; 100)).
In addition, statistically significant vaccine efficacy against CIN2/3 or AIS associated with HPV-16
and HPV-18 individually was demonstrated.
Vaccine efficacy against CIN1 associated with HPV 16/18 observed in the ATP cohort was 94.1%
(96.1% CI: 83.4;98.5). Vaccine efficacy against CIN1/2/3 or AIS associated with HPV 16/18
observed in the ATP cohort was 91.7% (96.1% CI: 82.4;96.7).
Vaccine efficacy against virological endpoints (6-month and 12-month persistent infection) associated
with HPV-16/18 observed in the ATP cohort is presented in Table 2.
Table 2: Vaccine efficacy against virological endpoints associated with HPV-16
/18 (ATP cohort)
6-month persistent infection
12-month persistent infection
20/7035
N = number of subjects included in each group
n = number of cases
(1)
ATP: includes women who received 3 doses of vaccine, were DNA negative and
seronegative at month 0 and DNA negative at month 6 to the relevant HPV type
(HPV-16 or HPV-18)
Efficacy against HPV-16/18 in women with evidence of HPV-16 or HPV-18 infection at study entry.
There was no evidence of protection from disease caused by the HPV types for which subjects were
HPV DNA positive at study entry. However, individuals already infected (HPV DNA positive) with
one of the vaccine-related HPV types prior to vaccination were protected from clinical disease caused
by the other vaccine HPV type.
Efficacy against HPV types 16 and 18 in women with and without prior infection or disease.
The Total Vaccinated Cohort (TVC) included all subjects who received at least one dose of the
vaccine, irrespective of their HPV DNA status, cytology and serostatus at baseline. This cohort
included women with or without current and/or prior HPV infection. Case counting for the TVC
started on day 1 after the first dose.
The efficacy estimates are lower in the TVC as this cohort includes women with pre-existing
infections/lesions, which are not expected to be impacted by Cervarix.
The TVC may approximate to the general population of women in the age range of 15-25 years.














Vaccine efficacy against high grade cervical lesions associated with HPV-16/18 observed in TVC is
presented in Table 3.
Table 3: Vaccine efficacy against high grade cervical lesions associated with HPV-16/18 (TVC
)
N = number of subjects included in each group
n = number of cases
(1)
TVC: includes all vaccinated subjects (who received at least one dose of vaccine) irrespective of
HPV DNA status, cytology and serostatus at baseline. This cohort includes women with pre-existing
infections/lesions
Vaccine efficacy against virological endpoints (6-month and 12-month persistent infection) associated
with HPV-16/18 observed in TVC is presented in Table 4.
Table 4:
Vaccine efficacy against virological endpoints associated with HPV-16/18 (TVC)
6-month
persistent infection
12-month
persistent infection
N = number of subjects included in each group
n = number of cases
(1)
TVC: includes all vaccinated subjects (who received at least one dose of vaccine) irrespective of HPV
DNA status, cytology and serostatus at baseline.
Overall impact of the vaccine on cervical HPV disease burden
In study HPV-008, the incidence of high grade cervical lesions was compared between the placebo
and vaccine group irrespective of the HPV DNA type in the lesion. In the TVC and TVC-naïve
cohorts, the vaccine’s efficacy was demonstrated against high-grade cervical lesions (Table 5).
The TVC-naïve is a subset of the TVC that includes women with normal cytology, and who were HPV
DNA negative for 14 oncogenic HPV types and seronegative for HPV-16 and HPV-18 at baseline.
Table 5: Vaccine efficacy against high-grade cervical lesions irrespective of the HPV DNA type
in the lesion
CIN2/3 or AIS
TVC-naïve
(1)
CIN3 or AIS
TVC-naïve
(1)


































N = number of subjects included in each group
(1)
TVC naïve: includes all vaccinated subjects (who received at least one dose of
vaccine) who had normal cytology, were HPV DNA negative for 14 oncogenic
HPV types and seronegative for HPV-16 and HPV-18 at baseline.
(2)
TVC: includes all vaccinated subjects (who received at least one dose of
vaccine) irrespective of HPV DNA status, cytology and serostatus at baseline.
Cervarix reduced definitive cervical therapy procedures (includes loop electrosurgical excision
procedure [LEEP], cold-knife Cone, and laser procedures) by 68.8% (96.1% CI: 50.0;81.2) in TVC
naïve and by 24.7% (96.1% CI: 7.4;38.9) in TVC.
Cross-protective efficacy
The cross-protective efficacy of Cervarix against histopathological and virological endpoints
(persistent infection) has been evaluated in study HPV-008 for 12 non-vaccine oncogenic HPV types.
The study was not powered to assess efficacy against disease caused by individual HPV types. The
analysis against the primary endpoint was confounded by multiple co-infections in the CIN2+ lesions.
Unlike histopathological endpoints, virological endpoints are less confounded by multiple infections.
Only HPV-31 showed consistent cross-protection for all endpoints (6m and 12m persistent infection,
CIN2/3 or AIS) and all study cohorts. Vaccine efficacy against 6 months persistent infection has also
been shown for HPV-33 and HPV-45 in all study cohorts.
Vaccine efficacy against 6-month persistent infection and CIN2/3 or AIS associated with individual
non-vaccine oncogenic HPV types is presented in Table 6 (ATP cohort).
Table 6: Vaccine efficacy for non-vaccine oncogenic HPV types
ATP
(1)
6-month persistent infection
HPV-16 related types (A9 species)
HPV-31
HPV-18 related types (A7 sp
ecies)
HPV-39
n= number of cases
(1)
ATP: includes women who received 3 doses of vaccine, were DNA negative and seronegative at month
































0 and DNA negative at month 6 to the relevant HPV type.
The limits of the confidence interval around the vaccine efficacy were calculated. When the value zero is
included, i.e. when the lower limit of the CI is <0, the efficacy is not considered statistically significant.
Immune response to Cervarix after the primary vaccination course
No minimal antibody level associated with protection against CIN of grade 2 or 3 or against persistent
infection associated with vaccine HPV types has been identified for HPV vaccines.
The antibody response to HPV-16 and HPV-18 was measured using a type-specific direct ELISA
(version 2, MedImmune methodology, modified by GSK) which was shown to correlate with the
pseudovirion-based neutralisation assay (PBNA).
The immunogenicity induced by three doses of Cervarix has been evaluated in 5,303
female subjects
from 10 to 55 years of age.
In clinical trials, more than 99% of initially seronegative subjects had seroconverted to both HPV
types 16 and 18 one month after the third dose. Vaccine-induced IgG Geometric Mean Titres (GMT)
were well above titres observed in women previously infected but who cleared HPV infection (natural
infection). Initially seropositive and seronegative subjects reached similar titres after vaccination.
Persistence of Immune Response to Cervarix
Study 001/007, which included women from 15 to 25 years of age at the time of vaccination,
evaluated the immune response against HPV-16 and HPV-18 up to 76 months after administration of
the first vaccine dose. In study 023 (a subset of study 001/007), the immune response continued to be
evaluated up to 101 months. 87 subjects in the vaccine group had immunogenicity data at the [M95-
M101] interval after the first vaccine dose with a median follow-up of 7.9 years. Of these subj ects,
100% (95% CI: 95.8;100) remained seropositive for HPV-16 and HPV-18 in the ELISA assay.
Vaccine-induced IgG GMTs for both HPV-16 and HPV-18 peaked at month 7 and then declined to
reach a plateau from month 18 up to the [M95-M101] interval with ELISA GMTs for both HPV-16
and HPV-18 at least still 10-fold higher than the ELISA GMTs observed in women who cleared a
natural HPV infection.
In study 008, immunogenicity up to month 36 was similar to the response observed in study 001. A
similar kinetic profile was observed with the neutralising antibodies.
In another clinical trial (study 014) performed in women aged 15 to 55 years, all subjects
seroconverted to both HPV types 16 and 18 after the third dose (at month 7). The GMTs were,
however, lower in women above 25 years. Nevertheless, all subjects remained seropositive for both
types throughout the follow-up phase (up to month 18) maintaining antibody levels at an order of
magnitude above those encountered after natural infection.
Evidence of Anamnestic (Immune Memory) Response
In study 024 (a subset of study 001/007), a challenge dose of Cervarix was administered to 65 subjects
at a mean interval of 6.8 years after the administration of the first vaccine dose. An anamnestic
immune response to HPV-16 and HPV-18 (by ELISA) was observed one week and one month after
the challenge dose, GMTs one month after the challenge dose exceeded those observed one month
after the primary 3-dose vaccination.
Bridging the efficacy of Cervarix from young adult women to adolescents
In two clinical trials performed in girls and adolescents aged 10 to 14 years, all subjects seroconverted
to both HPV types 16 and 18 after the third dose (at month 7) with GMTs at least 2-fold higher as



compared to women aged 15 to 25 years. On the basis of these immunogenicity data, the efficacy of
Cervarix is inferred from 10 to 14 years of age.
Pharmacokinetic properties
Evaluation of pharmacokinetic properties is not required for vaccines.
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, acute and repeated dose toxicity, local tolerance, fertility, embryo-foetal and postnatal
toxicity (up to the end of the lactation period).
Serological data suggest a transfer of anti-HPV-16 and anti-HPV-18 antibodies via the milk during the
lactation period in rats. However, it is unknown whether vaccine-induced antibodies are excreted in
human breast milk.
6. PHARMACEUTICAL PARTICULARS
Sodium chloride (NaCl)
Sodium dihydrogen phosphate dihydrate (NaH
2
PO
4
.2 H
2
O)
Water for injections
For adjuvants, see section 2.
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
After first opening, immediate use is recommended. If not used immediately, the vaccine should be
stored in a refrigerator (2°C – 8°C). If not used within 6 hours it should be discarded.
Special precautions for storage
Store in a refrigerator (2°C – 8°C).
Do not freeze.
Store in the original package in order to protect from light.
For storage after first opening, see section 6.3.
Nature and contents of container
1 ml of suspension in a vial (type I glass) for 2 doses with a stopper (rubber butyl) in pack sizes of 1,
10 and 100.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
A fine white deposit with a clear colourless supernatant may be observed upon storage of the vial. This
does not constitute a sign of deterioration.
The content of the vial should be inspected visually both before and after shaking for any foreign
particulate matter and/or abnormal physical appearance prior to administration.
In the event of either being observed, discard the vaccine.
The vaccine should be well shaken before use.
When using a multidose vial, each 0.5 ml dose should be withdrawn using a sterile needle and syringe;
precautions should be taken to avoid contamination of the contents.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
GlaxoSmithKline Biologicals s.a.
Rue de l'Institut 89
B-1330 Rixensart, Belgium
MARKETING AUTHORISATION NUMBER(S)
EU/1/07/419/010
EU/1/07/419/011
EU/1/07/419/012
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 20 September 2007.
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
NAME OF THE MEDICINAL PRODUCT
Cervarix suspension for injection in pre-filled syringe
Human Papillomavirus vaccine [Types 16, 18] (Recombinant, adjuvanted, adsorbed)
QUALITATIVE AND QUANTITATIVE COMPOSITION
1 dose (0.5 ml) contains:
Human Papillomavirus
1
type 16 L1 protein
2,3,4
1
Human Papillomavirus = HPV
2
adjuvanted by AS04 containing:
3-
O
-desacyl-4’- monophosphoryl lipid A (MPL)
3
50
micrograms
3
adsorbed on aluminium hydroxide, hydrated (Al(OH)
3
) 0.5 milligrams Al
3+
in total
4
L1 protein in the form of non-infectious virus-like particles (VLPs) produced by recombinant DNA
technology using a Baculovirus expression system which uses Hi-5 Rix4446 cells derived from
Trichoplusia ni
.
For a full list of excipients, see section 6.1.
Suspension for injection in pre-filled syringe.
Turbid white suspension. Upon storage, a fine white deposit with a clear colourless supernatant may
be observed.
Cervarix is a vaccine for the prevention of premalignant cervical lesions and cervical cancer causally
related to certain oncogenic Human Papillomavirus (HPV) types. See sections 4.4 and 5.1 for
important information on the data that support this indication.
The indication is based on the demonstration of efficacy in women aged 15-25 years following
vaccination with Cervarix and on the immunogenicity of the vaccine in girls and women aged 10-25
years.
The use of Cervarix should be in accordance with official recommendations.
Posology and method of administration
The recommended vaccination schedule is 0, 1, 6 months.
Human Papillomavirus
1
type 18 L1 protein
2,3,4
If flexibility in the vaccination schedule is necessary, the second dose can be administered
between 1 month and 2.5 months after the first dose and the third dose between 5 and 12 months
after the first dose.
The need for a booster dose has not been established (see section 5.1).
It is recommended that subjects who receive a first dose of Cervarix complete the 3-dose vaccination
course with Cervarix (see section 4.4).
Paediatric population
Cervarix is not recommended for use in girls below 10 years of age due to lack of data on safety and
immunogenicity in this age-group.
Cervarix is for intramuscular injection in the deltoid region (see also sections 4.4 and 4.5).
Hypersensitivity to the active substances or to any of the excipients.
Administration of Cervarix should be postponed in subjects suffering from an acute severe febrile
illness. However, the presence of a minor infection, such as a cold, is not a contraindication for
immunisation.
Special warnings and precautions for use
The decision to vaccinate an individual woman should take into account her risk for previous HPV
exposure and her potential benefit from vaccination.
As with all injectable vaccines, appropriate medical treatment and supervision should always be
readily available in case of a rare anaphylactic event following the administration of the vaccine.
Syncope (fainting) can occur following, or even before, any vaccination especially in adolescents as
a psychogenic response to the needle injection. This can be accompanied by several neurological
signs such as transient visual disturbance, paraesthesia and tonic-clonic limb movements during
recovery. It is important that procedures are in place to avoid injury from faints.
Cervarix should under no circumstances be administered intravascularly or intradermally.
No data are available on subcutaneous administration of Cervarix.
As with other vaccines administered intramuscularly, Cervarix should be given with caution to
individuals with thrombocytopenia or any coagulation disorder since bleeding may occur following an
intramuscular administration to these subjects.
As with any vaccine, a protective immune response may not be elicited in all vaccinees.
Cervarix will only protect against diseases that are caused by HPV types 16 and 18 and to some extent
against diseases caused by certain other oncogenic related HPV types (see section 5.1). Therefore,
appropriate precautions against sexually transmitted diseases should continue to be used.
Cervarix is for prophylactic use only and has no effect on active HPV infections or established clinical
disease. Cervarix has not been show n to have a therapeutic effect. The vaccine is therefore not
indicated for treatment of cervical cancer or cervical intraepithelial neoplasia (CIN). It is also not
intended to prevent progression of other established HPV-related lesions or existing HPV infections
with vaccine or non-vaccine types (see section 5.1 “Efficacy in women with evidence of HPV-16 or
HPV-18 infection at study entry.”).
Vaccination is not a substitute for routine cervical screening. Since no vaccine is 100% effective and
Cervarix will not provide protection against every HPV type, or against existing HPV infections,
routine cervical screening remains critically important and should follow local recommendations.
Duration of protection has not fully been established. Timing and need of booster dose(s) has not been
established.
There are no data on the use of Cervarix in subjects with impaired immune responsiveness such as
HIV infected patients or patients receiving immunosuppressive treatment. As with other vaccines, an
adequate immune response may not be elicited in these individuals.
There are no safety, immunogenicity or efficacy data to support interchangeability of Cervarix with
other HPV vaccines.
Interaction with other medicinal products and other forms of interaction
In all clinical trials individuals who had received immunoglobulin or blood products within 3 months
prior to the first vaccine dose were excluded.
Cervarix may be administered concomitantly with a combined booster vaccine containing diphtheria
(d), tetanus (T) and pertussis [acellular] (pa) with or without inactivated poliomyelitis (IPV), (dTpa,
dTpa-IPV vaccines), with no clinically relevant interference with antibody response to any of the
components of either vaccine. The sequential administration of combined dTpa-IPV followed by
Cervarix one month later tended to elicit lower anti-HPV-16 and anti-HPV-18 GMTs as compared to
Cervarix alone. The clinical relevance of this observation is not known.
Cervarix may be administered concomitantly with a combined hepatitis A (inactivated) and hepatitis B
(rDNA) vaccine (Twinrix) or with hepatitis B (rDNA) vaccine (Engerix B).
Administration of Cervarix at the same time as Twinrix has shown no clinically relevant interference
in the antibody response to the HPV and hepatitis A antigens. Anti-HBs geometric mean antibody
concentrations were significantly lower on co-administration, but the clinical relevance of this
observation is not known since the seroprotection rates remain unaffected. The proportion of subjects
reaching anti-HBs ≥ 10mIU/ml was 98.3% for concomitant vaccination and 100% for Twinrix given
alone. Similar results were observed when Cervarix was given concomitantly with Engerix B with
97.9% of subjects reaching anti-HBs ≥ 10mIU/ml compared to 100% for Engerix B given alone.
If Cervarix is to be given at the same time as another injectable vaccine, the vaccines should always be
administered at different injection sites.
Use with hormonal contraceptive
In clinical efficacy studies, approximately 60% of women who received Cervarix used hormonal
contraceptives. There is no evidence that the use of hormonal contraceptives has an impact on the
efficacy of Cervarix.
Use with systemic immunosuppressive medicinal products
As with other vaccines it may be expected that, in patients receiving immunosuppressive treatment, an
adequate response may not be elicited.
Fertility, pregnancy and lactation
Specific studies of the vaccine in pregnant women were not conducted. However, during the clinical
development program, a total of 3,993 pregnancies were reported including 2,009 in women who had
received Cervarix. Overall, the proportions of pregnant subjects who experienced specific outcomes
(e.g., normal infant, abnormal infants including congenital anomalies, premature birth, and
spontaneous abortion) were similar between treatment groups.
Animal studies do not indicate direct or indirect harmful effects with respect to fertility, pregnancy,
embryonal/foetal development, parturition or post-natal development (see section 5.3).
These data are insufficient to recommend use of Cervarix during pregnancy.
Vaccination should, therefore, be postponed until after completion of pregnancy.
The effect on breast-fed infants of the administration of Cervarix to their mothers has not been
evaluated in clinical studies.
Cervarix should only be used during breast-feeding when the possible advantages outweigh the
possible risks.
Effects on the ability to drive and use machines
No studies on the effects on the ability to drive or use machines have been performed.
In clinical studies that enrolled girls and women aged from 10 up to 72 years (of which 79.2% were
aged 10-25 years at the time of enrolment), Cervarix was administered to 16,142 subjects whilst
13,811 subjects received control. These subjects were followed for serious adverse events over the
entire study period. In a pre-defined subset of subjects (Cervarix = 8,130 versus control = 5,786),
adverse events were followed for 30 days after each injection.
The most common adverse reaction observed after vaccine administration was injection site pain
which occurred after 78% of all doses. The majority of these reactions were of mild to moderate
severity and were not long lasting.
Adverse reactions considered as being at least possibly related to vaccination have been categorised
by frequency.
Frequencies are reported as:
Very common (≥1/10)
Common (≥1/100 to <1/10)
Uncommon (≥1/1,000 to <1/100)
Infections and infestations
Uncommon: upper respiratory tract infection
Nervous system disorders
Very common: headache
Gastrointestinal disorders
Common: gastrointestinal symptoms including nausea, vomiting, diarrhoea and abdominal pain
Skin and subcutaneous tissue disorders
Common: itching/pruritus, rash, urticaria
Musculoskeletal and connective tissue disorders
Very common: myalgia
General disorders and administration site conditions
Very common: injection site reactions including pain, redness, swelling; fatigue
Common: fever (≥38°C)
Uncommon: other injection site reactions such as induration, local paraesthesia
A similar safety profile has been observed in subjects with prior or current HPV infection as compared
to subjects negative for oncogenic HPV DNA or seronegative for HPV-16 and HPV-18 antibodies.
Post marketing surveillance
Becaus e these events were reported spontaneous ly, it is not possible to reliably estimate their
frequency.
Blood and lymphatic system disorders
Allergic reactions (including anaphylactic and anaphylactoid reactions), angioedema
Syncope or vasovagal responses to injection, sometimes accompanied by tonic-clonic movements (see
section 4.4)
No case of overdose has been reported.
5. PHARMACOLOGICAL PROPERTIES
Pharmacodynamic properties
Pharmaco-therapeutic group: Papillomavirus vaccines, ATC code: J07BM02
Cervarix is an adjuvanted non-infectious recombinant vaccine prepared from the highly purified virus-
like particles (VLPs) of the major capsid L1 protein of oncogenic HPV types 16 and 18. Since the
VLPs contain no viral DNA, they cannot infect cells, reproduce or cause disease. Animal studies have
shown that the efficacy of L1 VLP vaccines is largely mediated by the development of a humoral
immune response.
HPV-16 and HPV-18 are estimated to be responsible for approximately 70% of cervical cancers.
Other oncogenic HPV types can also cause cervical cancer (approximately 30%). HPV 45, -31 and -33
are the 3 most common non-vaccine HPV types identified in squamous cervical carcinoma (12.1%)
and adenocarcinoma (8.5%).
The term “premalignant cervical lesions” in section 4.1 corresponds to high-grade Cervical
Intraepithelial Neoplasia (CIN2/3).
The efficacy of Cervarix was assessed in two controlled, double-blind, randomised Phase II and III
clinical trials that included a total of 19,778 women aged 15 to 25 years.
The phase II trial (study 001/007) enrolled only women who:
-
Were tested negative for oncogenic HPV DNA of types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56,
58, 59, 66 and 68
-
Were serone gative for HPV-16 and HPV-18 and
-
Had normal cytology
The primary efficacy endpoint was incident infection with HPV-16 and/or HPV-18. Twelve-month
persistent infection was evaluated as additional efficacy endpoint.
The phase III trial (study 008) enrolled women without pre-screening for the presence of HPV
infection, i.e. regardless of baseline cytology and HPV serological and DNA status.
The primary efficacy endpoint was CIN2+ (CIN2/3 or AIS) associated with HPV-16 and/or HPV-18
(HPV-16/18). Cervical Intraepithelial Neoplasia (CIN) grade 2 and 3 (CIN2/3) and cervical
adenocarcinoma in situ (AIS) were used in the clinical trials as surrogate markers for cervical cancer.
The secondary endpoints included 6- and 12-month persistent infection.
Persistent infection that lasts for at least 6 months has also been shown to be a relevant surrogate
marker for cervical cancer.
Prophylactic efficacy against HPV-16/18 infection in a population naïve to oncogenic HPV types
Women (N=1,113) were vaccinated in study 001 and evaluated for efficacy up to month 27. A subset
of women (N=776) vaccinated in study 001 was followed in study 007 up to 6.4 years (approximately
77 months) after the first dose (mean follow-up of 5.9 years). There were five cases of 12-month
persistent HPV-16/18 infection (4 HPV-16; 1 HPV-18) in the control group and one HPV-16 case in
the vaccine group in study 001. In study 007 the efficacy of Cervarix against 12-month persistent
HPV-16/18 infection was 100% (95% CI: 80.5; 100). There were sixteen cases of persistent HPV-16
infection, and five cases of persistent HPV-18 infection, all in the control group.
Prophylactic efficacy against HPV-16/18 in women naïve to HPV-16 and/or HPV-18
In study HPV-008, the primary analyses of efficacy were performed on the According to Protocol
cohort (ATP cohort: including women who received 3 vaccine doses and were DNA negative and
seronegative at month 0 and DNA negative at month 6 for the HPV type considered in the analysis)
This cohort included women with normal or low-grade cytology at baseline and excluded only women
with high-grade cytology (0.5% of the total population). Case counting for the ATP cohort started on
day 1 after the third dose of vaccine.
Overall, 74% of women enrolled were naïve to both HPV-16 and HPV-18 (i.e. DNA negative and
seronegative at study entry).
The mean follow-up for women included in study HPV-008 was approximately 39 months post dose 1
in TVC and 35 months post dose 3 in the ATP cohort.
Vaccine efficacy against the primary endpoint CIN2/3 or AIS is presented in Table 1. In a
suppl emental analysis, the efficacy of Cervarix was evaluated against HPV-16/18-related CIN3 or
AIS.
Table 1: Vaccine efficacy against high grade cervical lesions associated with HPV-16/18 (ATP
cohort)










N = number of subjects included in each group
n = number of cases
(1)
ATP: includes women who received 3 doses of vaccine, were DNA negative and seronegative
at month 0 and DNA negative at month 6 to the relevant HPV type (HPV-16 or HPV-18)
(2)
including 3 cases of CIN2/3 or AIS and 2 cases of CIN3 or AIS in which another oncogenic
HPV type was identified in the lesion, concomitantly with HPV-16 or HPV-18. These cases
are excluded in the HPV type assignment analysis (see under Table).
Further investigation of the cases with multiple HPV types considered the HPV types detected by
Polymerase Chain Reaction (PCR) in at least one of the two preceding cytology samples, in addition
to types detected in the lesion to distinguish the HPV type(s) most likely responsible to the lesion
(HPV type assignment). This post-hoc analysis excluded cases (in the vaccine group and in the control
group) which were not considered to be causally associated with HPV-16 or HPV-18 infections
acquired during the trial.
Based on the HPV type assignment post-hoc analysis, there were 1 CIN2/3 or AIS case in the vaccine
group versus 53 cases in the control group (Efficacy 98.1% (96.1% CI: 88.4; 100)) and 0 CIN3 or AIS
cases in the vaccine group versus 8 cases in the control group (Efficacy 100% (96.1% CI: 36.4; 100)).
In addition, statistically significant vaccine efficacy against CIN2/3 or AIS associated with HPV-16
and HPV-18 individually was demonstrated.
Vaccine efficacy against CIN1 associated with HPV 16/18 observed in the ATP cohort was 94.1%
(96.1% CI: 83.4;98.5). Vaccine efficacy against CIN1/2/3 or AIS associated with HPV 16/18
observed in the ATP cohort was 91.7% (96.1% CI: 82.4;96.7).
Vaccine efficacy against virological endpoints (6-month and 12-month persistent infection) associated
with HPV-16/18 observed in the ATP cohort is presented in Table 2.
Table 2: Vaccine efficacy against virological endpoints associated with HPV-16
/18 (ATP cohort)
6-month persistent infection
12-month persistent infection
20/7035
N = number of subjects included in each group
n = number of cases
(1)
ATP: includes women who received 3 doses of vaccine, were DNA negative and
seronegative at month 0 and DNA negative at month 6 to the relevant HPV type
(HPV-16 or HPV-18)
Efficacy against HPV-16/18 in women with evidence of HPV-16 or HPV-18 infection at study entry.
There was no evidence of protection from disease caused by the HPV types for which subjects were
HPV DNA positive at study entry. However, individuals already infected (HPV DNA positive) with
one of the vaccine-related HPV types prior to vaccination were protected from clinical disease caused
by the other vaccine HPV type.
Efficacy against HPV types 16 and 18 in women with and without prior infection or disease.
The Total Vaccinated Cohort (TVC) included all subjects who received at least one dose of the
vaccine, irrespective of their HPV DNA status, cytology and serostatus at baseline. This cohort
included women with or without current and/or prior HPV infection. Case counting for the TVC
started on day 1 after the first dose.
The efficacy estimates are lower in the TVC as this cohort includes women with pre-existing
infections/lesions, which are not expected to be impacted by Cervarix.
The TVC may approximate to the general population of women in the age range of 15-25 years.














Vaccine efficacy against high grade cervical lesions associated with HPV-16/18 observed in TVC is
presented in Table 3.
Table 3: Vaccine efficacy against high grade cervical lesions associated with HPV-16/18 (TVC
)
N = number of subjects included in each group
n = number of cases
(1)
TVC: includes all vaccinated subjects (who received at least one dose of vaccine) irrespective of
HPV DNA status, cytology and serostatus at baseline. This cohort includes women with pre-existing
infections/lesions
Vaccine efficacy against virological endpoints (6-month and 12-month persistent infection) associated
with HPV-16/18 observed in TVC is presented in Table 4.
Table 4:
Vaccine efficacy against virological endpoints associated with HPV-16/18 (TVC)
6-month
persistent infection
12-month
persistent infection
N = number of subjects included in each group
n = number of cases
(1)
TVC: includes all vaccinated subjects (who received at least one dose of vaccine) irrespective of HPV
DNA status, cytology and serostatus at baseline.
Overall impact of the vaccine on cervical HPV disease burden
In study HPV-008, the incidence of high grade cervical lesions was compared between the placebo
and vaccine group irrespective of the HPV DNA type in the lesion. In the TVC and TVC-naïve
cohorts, the vaccine’s efficacy was demonstrated against high-grade cervical lesions (Table 5).
The TVC-naïve is a subset of the TVC that includes women with normal cytology, and who were HPV
DNA negative for 14 oncogenic HPV types and seronegative for HPV-16 and HPV-18 at baseline.
Table 5: Vaccine efficacy against high-grade cervical lesions irrespective of the HPV DNA type
in the lesion
CIN2/3 or AIS
TVC-naïve
(1)
CIN3 or AIS
TVC-naïve
(1)


































N = number of subjects included in each group
(1)
TVC naïve: includes all vaccinated subjects (who received at least one dose of
vaccine) who had normal cytology, were HPV DNA negative for 14 oncogenic
HPV types and seronegative for HPV-16 and HPV-18 at baseline.
(2)
TVC: includes all vaccinated subjects (who received at least one dose of
vaccine) irrespective of HPV DNA status, cytology and serostatus at baseline.
Cervarix reduced definitive cervical therapy procedures (includes loop electrosurgical excision
procedure [LEEP], cold-knife Cone, and laser procedures) by 68.8% (96.1% CI: 50.0;81.2) in TVC
naïve and by 24.7% (96.1% CI: 7.4;38.9) in TVC.
Cross-protective efficacy
The cross-protective efficacy of Cervarix against histopathological and virological endpoints
(persistent infection) has been evaluated in study HPV-008 for 12 non-vaccine oncogenic HPV types.
The study was not powered to assess efficacy against disease caused by individual HPV types. The
analysis against the primary endpoint was confounded by multiple co-infections in the CIN2+ lesions.
Unlike histopathological endpoints, virological endpoints are less confounded by multiple infections.
Only HPV-31 showed consistent cross-protection for all endpoints (6m and 12m persistent infection,
CIN2/3 or AIS) and all study cohorts. Vaccine efficacy against 6 months persistent infection has also
been shown for HPV-33 and HPV-45 in all study cohorts.
Vaccine efficacy against 6-month persistent infection and CIN2/3 or AIS associated with individual
non-vaccine oncogenic HPV types is presented in Table 6 (ATP cohort).
Table 6: Vaccine efficacy for non-vaccine oncogenic HPV types
ATP
(1)
6-month persistent infection
HPV-16 related types (A9 species)
HPV-31
HPV-18 related types (A7 sp
ecies)
HPV-39
n= number of cases
(1)
ATP: includes women who received 3 doses of vaccine, were DNA negative and seronegative at month
0 and DNA negative at month 6 to the relevant HPV type.

































The limits of the confidence interval around the vaccine efficacy were calculated. When the value zero is
included, i.e. when the lower limit of the CI is <0, the efficacy is not considered statistically significant.
Immune response to Cervarix after the primary vaccination course
No minimal antibody level associated with protection against CIN of grade 2 or 3 or against persistent
infection associated with vaccine HPV types has been identified for HPV vaccines.
The antibody response to HPV-16 and HPV-18 was measured using a type-specific direct ELISA
(version 2, MedImmune methodology, modified by GSK) which was shown to correlate with the
pseudovirion-based neutralisation assay (PBNA).
The immunogenicity induced by three doses of Cervarix has been evaluated in 5,303
female subjects
from 10 to 55 years of age.
In clinical trials, more than 99% of initially seronegative subjects had seroconverted to both HPV
types 16 and 18 one month after the third dose. Vaccine-induced IgG Geometric Mean Titres (GMT)
were well above titres observed in women previously infected but who cleared HPV infection (natural
infection). Initially seropositive and seronegative subjects reached similar titres after vaccination.
Persistence of Immune Response to Cervarix
Study 001/007, which included women from 15 to 25 years of age at the time of vaccination,
evaluated the immune response against HPV-16 and HPV-18 up to 76 months after administration of
the first vaccine dose. In study 023 (a subset of study 001/007), the immune response continued to be
evaluated up to 101 months. 87 subjects in the vaccine group had immunogenicity data at the [M95-
M101] interval after the first vaccine dose with a median follow-up of 7.9 years. Of these subj ects,
100% (95% CI: 95.8;100) remained seropositive for HPV-16 and HPV-18 in the ELISA assay.
Vaccine-induced IgG GMTs for both HPV-16 and HPV-18 peaked at month 7 and then declined to
reach a plateau from month 18 up to the [M95-M101] interval with ELISA GMTs for both HPV-16
and HPV-18 at least still 10-fold higher than the ELISA GMTs observed in women who cleared a
natural HPV infection.
In study 008, immunogenicity up to month 36 was similar to the response observed in study 001. A
similar kinetic profile was observed with the neutralising antibodies.
In another clinical trial (study 014) performed in women aged 15 to 55 years, all subjects
seroconverted to both HPV types 16 and 18 after the third dose (at month 7). The GMTs were,
however, lower in women above 25 years. Nevertheless, all subjects remained seropositive for both
types throughout the follow-up phase (up to month 18) maintaining antibody levels at an order of
magnitude above those encountered after natural infection.
Evidence of Anamnestic (Immune Memory) Response
In study 024 (a subset of study 001/007), a challenge dose of Cervarix was administered to 65 subjects
at a mean interval of 6.8 years after the administration of the first vaccine dose. An anamnestic
immune response to HPV-16 and HPV-18 (by ELISA) was observed one week and one month after
the challenge dose, GMTs one month after the challenge dose exceeded those observed one month
after the primary 3-dose vaccination.
Bridging the efficacy of Cervarix from young adult women to adolescents
In two clinical trials performed in girls and adolescents aged 10 to 14 years, all subjects seroconverted
to both HPV types 16 and 18 after the third dose (at month 7) with GMTs at least 2-fold higher as



compared to women aged 15 to 25 years. On the basis of these immunogenicity data, the efficacy of
Cervarix is inferred from 10 to 14 years of age.
Pharmacokinetic properties
Evaluation of pharmacokinetic properties is not required for vaccines.
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, acute and repeated dose toxicity, local tolerance, fertility, embryo-foetal and postnatal
toxicity (up to the end of the lactation period).
Serological data suggest a transfer of anti-HPV-16 and anti-HPV-18 antibodies via the milk during the
lactation period in rats. However, it is unknown whether vaccine-induced antibodies are excreted in
human breast milk.
6. PHARMACEUTICAL PARTICULARS
Sodium chloride (NaCl)
Sodium dihydrogen phosphate dihydrate (NaH
2
PO
4
.2 H
2
O)
Water for injections
For adjuvants, see section 2.
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
Cervarix should be administered as soon as possible after being removed from the refrigerator.
However, stability data generated indicate that Cervarix presented in monodose containers remains
stable and can be administered in case it has been stored outside the refrigerator up to three days at
temperatures between 8°C and 25°C or up to one day at temperatures between 25°C and 37°C.
Special precautions for storage
Store in a refrigerator (2°C – 8°C).
Do not freeze.
Store in the original package in order to protect from light.
Nature and contents of container
0.5 ml of suspension in a pre-filled syringe (type I glass) with a plunger stopper (rubber butyl) with or
without needles in pack sizes of 1 and 10.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
A fine white deposit with a clear colourless supernatant may be observed upon storage of the syringe.
This does not constitute a sign of deterioration.
The content of the syringe should be inspected visually both before and after shaking for any foreign
particulate matter and/or abnormal physical appearance prior to administration.
In the event of either being observed, discard the vaccine.
The vaccine should be well shaken before use.
Instructions for administration of the vaccine presented in pre-filled syringe
1.
Holding the syringe
barrel
(avoid holding the syringe plunger),
unscrew the syringe cap by twisting it anticlockwise.
2.
To attach the needle to the syringe,
twist the needle clockwise into the syringe
until you feel it lock.
3.
Remove the needle protector, which on
occasion can be a little stiff.
4.
Administer the vaccine.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
GlaxoSmithKline Biologicals s.a.
Rue de l'Institut 89
B-1330 Rixensart, Belgium
MARKETING AUTHORISATION NUMBER(S)
EU/1/07/419/004
EU/1/07/419/005
EU/1/07/419/006
EU/1/07/419/007
EU/1/07/419/008
EU/1/07/419/009
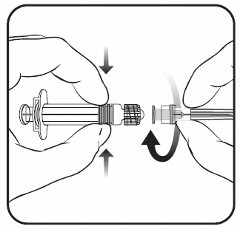





DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 20 September 2007.
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
A. MANUFACTURERS OF THE BIOLOGICAL ACTIVE
SUBSTANCE AND MANUFACTURING AUTHORISATION
HOLDER RESPONSIBLE FOR BATCH RELEASE
B. CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURERS OF THE BIOLOGICAL ACTIVE SUBSTANCE AND
MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturers of the biological active substance
GlaxoSmithKline Biologicals S.A.
89, rue de l'Institut
BE-1330 Rixensart
Belgium
GlaxoSmithKline Biologicals SA
Parc de la Noire Epine
rue Flemming
20-1300 Wavre
Belgium
GlaxoSmithKline Biologicals S.A.
Les Isnes
Rue Louis Genonceau, 13
BE-5023 Gembloux
Belgium
Name and address of the manufacturer responsible for batch release
GlaxoSmithKline Biologicals S.A.
89, rue de l'Institut
BE-1330 Rixensart
Belgium
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to medical prescription.
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
The MAH must ensure that the system of pharmacovigilance presented in Module 1.8.1. of the
Marketing Authorisation, is in place and functioning before and whilst the product is on the market.
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version 5 of the Risk Management Plan (RMP) presented in
Module 1.8.2. of the Marketing Authorisation Application and any subsequent updates of the RMP
agreed by the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human us e, the
updated RMP should be submitted at the same time as the next Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted
•
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
•
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
•
At the request of the EMEA
Official batch release: in accordance with Article 114 Directive 2001/83/EC as amended, the official
batch release will be undertaken by a state laboratory or a laboratory designated for that purpose.
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
MONODOSE VIAL, PACK OF 1, 10, 100
NAME OF THE MEDICINAL PRODUCT
Cervarix suspension for injection
Human Papillomavirus vaccine [Types 16, 18] (Recombinant, adjuvanted, adsorbed)
STATEMENT OF ACTIVE SUBSTANCE(S)
1 dose (0.5 ml) contains:
HPV type 16 L1 protein
1,2
HPV type 18 L1 protein
1,2
1
adjuvanted by AS04 containing:
3-
O
-desacyl-4’- monophosphoryl lipid A (MPL)
2
2
adsorbed on aluminium hydroxide, hydrated (Al(OH)
3
)
0.5 milligrams Al
3+
in total
Sodium chloride
Sodium dihydrogen phosphate dihydrate
Water for injections
PHARMACEUTICAL FORM AND CONTENTS
Suspension for injection
1 vial
1 dose (0.5 ml)
10 vials
10 x 1 dose (0.5 ml)
100 vials
100 x 1 dose (0.5 ml)
METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use
Intramuscular use
Shake before us e
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children























OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
Store in a refrigerator
Do not freeze
Store in the original package in order to protect from light
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Dispose of in accordance with local regulations
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
GlaxoSmithKline Biologicals s.a.
Rue de l’Institut 89
B-1330 Rixensart, Belgium
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/07/419/001 – pack of 1
EU/1/07/419/002 – pack of 10
EU/1/07/419/003 – pack of 100
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
16. INFORMATION IN BRAILLE
Justification for not including Braille accepted.



























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
MULTIDOSE VIAL, PACK OF 1, 10, 100
NAME OF THE MEDICINAL PRODUCT
Cervarix suspension for injection, multidose
Human Papillomavirus vaccine [Types 16, 18] (Recombinant, adjuvanted, adsorbed)
STATEMENT OF ACTIVE SUBSTANCE(S)
1 dose (0.5 ml) contains:
HPV type 16 L1 protein
1,2
HPV type 18 L1 protein
1,2
1
adjuvanted by AS04 containing:
3-
O
-desacyl-4’- monophosphoryl lipid A (MPL)
2
2
adsorbed on aluminium hydroxide, hydrated (Al(OH)
3
)
0.5 milligrams Al
3+
in total
Sodium chloride
Sodium dihydrogen phosphate dihydrate
Water for injections
PHARMACEUTICAL FORM AND CONTENTS
Suspension for injection
1 vial
2 doses (1 ml)
10 vials
10 x 2 doses (1 ml)
100 vials
100 x 2 doses (1 ml)
METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use
Intramuscular use
Shake before us e
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children























OTHER SPECIAL WARNING(S), IF NECESSARY
After first opening, use immediately or within 6 hours if stored in a refrigerator
SPECIAL STORAGE CONDITIONS
Store in a refrigerator
Do not freeze
Store in the original package in order to protect from light
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Dispose of in accordance with local regulations
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
GlaxoSmithKline Biologicals s.a.
Rue de l’Institut 89
B-1330 Rixensart, Belgium
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/07/419/010 – pack of 1
EU/1/07/419/011 – pack of 10
EU/1/07/419/012 – pack of 100
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
16. INFORMATION IN BRAILLE




























Justification for not including Braille accepted.

PARTICULARS TO APPEAR ON THE OUTER PACKAGING
PRE-FILLED SYRINGE WITH OR WITHOUT NEEDLE, PACK OF 1, 10
NAME OF THE MEDICINAL PRODUCT
Cervarix suspension for injection in pre-filled syringe
Human Papillomavirus vaccine [Types 16, 18] (Recombinant, adjuvanted, adsorbed)
STATEMENT OF ACTIVE SUBSTANCE(S)
1 dose (0.5 ml) contains:
HPV type 16 L1 protein
1,2
HPV type 18 L1 protein
1,2
1
adjuvanted by AS04 containing:
3-
O
-desacyl-4’- monophosphoryl lipid A (MPL)
2
2
adsorbed on aluminium hydroxide, hydrated (Al(OH)
3
)
0.5 milligrams Al
3+
in total
Sodium chloride
Sodium dihydrogen phosphate dihydrate
Water for injections
PHARMACEUTICAL FORM AND CONTENTS
Suspension for injection in pre-filled syringe
1 pre-filled syringe
1 dose (0.5 ml)
10 pre-filled syringes
10 x 1 dose (0.5 ml)
1 pre-filled syringe + 1 needle
1 dose (0.5 ml)
10 pre-filled syringes + 10 needles
10 x 1 dose (0.5 ml)
1 pre-filled syringe + 2 needles
1 dose (0.5 ml)
10 pre-filled syringes + 20 needles
10 x 1 dose (0.5 ml)
METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use





















Intramuscular use
Shake before us e
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
Store in a refrigerator
Do not freeze
Store in the original package in order to protect from light
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Dispose of in accordance with local regulations
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
GlaxoSmithKline Biologicals s.a.
Rue de l’Institut 89
B-1330 Rixensart, Belgium
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/07/419/008 – pack of 1 without needle
EU/1/07/419/009 – pack of 10 without needle
EU/1/07/419/004 – pack of 1 with 1 needle
EU/1/07/419/006 – pack of 10 with 10 needles
EU/1/07/419/005 – pack of 1 with 2 needles
EU/1/07/419/007 – pack of 10 with 20 needles
14. GENERAL CLASSIFICATION FOR SUPPLY























Medicinal product subject to medical prescription
16. INFORMATION IN BRAILLE
Justification for not including Braille accepted.






PACKAGE LEAFLET: INFORMATION FOR THE USER
Cervarix suspension for injection
Human Papillomavirus vaccine [Types 16, 18] (Recombinant, adjuvanted, adsorbed)
Read all of this leaflet carefully before you start receiving this vaccine.
-
Keep this leaflet. You may need to read it again.
This medicine has been prescribed for you. Do not pass it on to others.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
In this leaflet:
1. What Cervarix is and what it is used for
2. Before you receive Cervarix
3. How Cervarix is given
4. Possible side effects
5.
How to store Cervarix
6.
Further information
WHAT CERVARIX IS AND WHAT IT IS USED FOR
Cervarix is a vaccine intended to protect females against the diseases caused by infection with Human
Papillomaviruses (HPV).
These diseases include:
-
cervical cancer (cancer of the cervix i.e. lower part of the uterus or womb),
-
precancerous cervical lesions (changes in cells of the cervix that have a risk of turning into
cancer).
The Human Papillomavirus (HPV) types contained in the vaccine (HPV types 16 and 18) are
responsible for approximately 70% of cervical cancer cases. Other HPV types can also cause cervical
cancer. Cervarix does not protect against all HPV types.
When a female is vaccinated with Cervarix, the immune system (the body’s natural defence system)
will make antibodies against HPV types 16 and 18. In clinical trials Cervarix has been shown to
prevent HPV related diseases in women 15-25 years of age. Cervarix also stimulates production of
antibodies in females 10-14 years of age.
Cervarix is not infectious and so, it cannot cause HPV related diseases.
Cervarix is not used to treat HPV related diseases already present at the time of vaccination.
Cervarix should be used in accordance with official guidelines.
BEFORE YOU RECEIVE CERVARIX
Cervarix should not be given if
the person to be vaccinated:
•
is allergic (hypersensitive) to any of the active substances or any of the other ingredients of
Cervarix. The active substances and other ingredients of Cervarix are listed at the end of the
leaflet (see section 6). Signs of an allergic reaction may include itchy skin rash, shortness of
breath and swelling of the face or tongue.
If you have any further questions, ask your doctor or pharmacist.
•
has a severe infection with a high temperature. It might be necessary to postpone the vaccination
until recovery. A minor infection such as a cold should not be a problem, but talk to the doctor
first.
Take special care with Cervarix
You should tell the doctor if the person to be vaccinated:
•
has a bleeding problem or bruises easily.
•
has any disease which reduces her resistance to infection such as HIV infection
As with all vaccines, Cervarix may not fully protect all people who are vaccinated.
Cervarix does not protect people from diseases caused by infection with HPV types 16 or 18 if they
are already infected with Human Papillomavirus type 16 or 18 at the time of vaccination.
Although vaccination may protect you against cervical cancer, it is not a substitute for regular cervical
screening. You should continue to follow your doctor’s advice on cervical smear/Pap test (test to
screen for changes in cells of the cervix caused by an HPV infection) and preventative and protective
measur es.
As Cervarix will not protect against all types of Human Papillomavirus, appropriate precautions
against exposure to HPV and sexually transmitted diseases should continue to be used.
Cervarix will not protect against other diseases that are not caused by Human Papillomavirus.
The duration of protection after vaccination is currently unknown. In clinical trials, sustained
protection has been observed in females aged 15 to 25 years for up to 6.4 years after the first dose. The
need for booster dose(s) has not been investigated.
Using other medicines
Cervarix can be given with a combined booster vaccine containing diphtheria (d), tetanus (T) and
pertussis [acellular] (pa) with or without inactivated poliomyelitis (IPV), (dTpa, dTpa -IPV vaccines),
or with a combined hepatitis A and hepatitis B vaccine (Twinrix) or a hepatitis B vaccine (Engerix B),
at a separate injection site (another part of your body, e.g. the other arm) during the same visit.
Cervarix may not have an optimal effect if used with medicines that suppr ess the immune system.
In clinical trials, oral contraceptives (e.g. the pill) did not reduce the protection obtained by Cervarix.
Please tell the doctor if the person to be vaccinated is taking or has recently taken any other medicines,
including medicines obtained without a prescription or has recently received any other vaccine.
Pregnancy and breast-feeding
There are insufficient data concerning the use of Cervarix during pregnancy. If pregnancy occurs
during the course of vaccination your doctor should be consulted. It is recommended to postpone
vaccination until after completion of the pregnancy.
Ask your doctor for advice about breast-feeding before receiving Cervarix.
Driving and using machines
There is no information on the effect of Cervarix on your ability to drive or use machinery.
The doctor or nurse will give Cervarix as an injection into the muscle of the upper arm.
Cervarix is intended for females from 10 years of age onwards. A total of three injections will be
administered by your doctor or nurse according to the following schedule:
First injection: at chosen date
Second injection: 1 month after first injection
Third injection: 6 months after first injection
If necessary, the vaccination schedule can be more flexible. Please speak to your doctor for more
information.
When Cervarix is given for the first dose, it is recommended that Cervarix (and not another vaccine
against HPV) be given for the complete 3-dose vaccination course.
The vaccine should never be given into a vein.
If you forget a return visit for Cervarix:
It is important that you follow the instructions of your doctor or nurse regarding return visits. If you
forget to go back to your doctor at the scheduled time, ask your doctor for advice.
If you do not finish the complete vaccination course of three injections, you may not get the best
response and protection from the vaccination.
Like all medicines, Cervarix can cause side effects, although not everybody gets them.
Side effects that occurred during clinical trials with Cervarix were as follows:
♦
Very common (side effects which may occur in more than 1 per 10 doses of vaccine):
•
pain or discomfort at the injection site
•
redness or swelling at the injection site
•
headache
•
aching muscles, muscle tenderness or weakness (not caused by exercise)
•
tiredness
♦
Common (side effects which may occur in less than 1 per 10 but more than 1 per 100 doses of
vaccine):
•
gastrointestinal symptoms including nausea, vomiting, diarrhoea and abdominal pain
•
itching, red skin rash, hives (urticaria)
•
joint pain
•
fever (≥38°C)
♦
Uncommon (side effects which may occur in less than 1 per 100 but more than 1 per 1,000
doses of vaccine):
•
upper respiratory tract infection (infection of the nose, throat or trachea)
•
dizziness
•
other injection site reactions such as hard lump, tingling or numbness.
Side effects that have been reported during marketed use of Cervarix include:
•
allergic reactions. These can be recognised by:
itchy rash of the hands and feet,
swelling of the eyes and face,
difficulty in breathing or swallowing,
sudden drop in blood pressure and loss of consciousness.
These reactions will usually occur before leaving the doctor’s surgery. However, if your child gets
any of these symptoms you should contact a doctor urgently.
•
swollen glands in the neck, armpit or groin
•
fainting sometimes accompanied by shaking or stiffness.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Cervarix after the expiry date which is stated on the carton. The expiry date refers to the
last day of that month.
Store in a refrigerator (2°C – 8°C).
Do not freeze.
Store in the original package in order to protect from light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active subs tances are:
Human Papillomavirus
1
type 16 L1 protein
2,3,4
Human Papillomavirus
1
type 18 L1 protein
2,3,4
1
Human Papillomavirus = HPV
2
adjuvanted by AS04 containing:
3-
O
-desacyl-4’- monophosphoryl lipid A (MPL)
3
50
micrograms
3
adsorbed on aluminium hydroxide, hydrated (Al(OH)
3
) 0.5 milligrams Al
3+
in total
4
L1 protein in the form of non-infectious virus-like particles (VLPs) produced by recombinant
DNA technology using a Baculovirus expression system which uses Hi-5 Rix4446 cells derived
from the insect
Trichoplusia ni
.
The other ingredients are sodium chloride (NaCl), sodium dihydrogen phosphate dihydrate
(NaH
2
PO
4
.2 H
2
O) and water for injections.
What Cervarix looks like and contents of the pack
Suspension for injection.
Cervarix is a turbid white suspension.
Cervarix is available in vials for 1 dose (0.5 ml) in packs of 1, 10 and 100.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
GlaxoSmithKline Biologicals s.a.
Rue de l'Institut 89
B-1330 Rixensart, Belgium
For any information about this medicinal product, please contact the local representative of the
Marketing Authorisation Holder:
België/Belgique/Belgien
GlaxoSmithKline s.a./n.v.
Tél/Tel: + 32 2 656 21 11
Luxembourg/Luxemburg
GlaxoSmithKline s.a./n.v.
Tél/Tel: + 32 2 656 21 11
България
ГлаксоСмитКлайн ЕООД
ул. Димитър Манов бл.10
София 1408
Тел. + 359 2 953 10 34
Magyarország
GlaxoSmithKline Kft.
Tel.: + 36-1-2255300
Česká republika
GlaxoSmithKline s.r.o.
Tel: + 420 2 22 00 11 11
gsk.czmail@gsk.com
Malta
GlaxoSmithKline Malta
Tel: + 356 21 238131
Danmark
GlaxoSmithKline Pharma A/S
Tlf: + 45 36 35 91 00
dk-info@gsk.com
Nederland
GlaxoSmithKline BV
Tel: + 31 (0)30 69 38 100
nlinfo@gsk.com
Deutschland
GlaxoSmithKline GmbH & Co. KG
Tel: + 49 (0)89 360448701
produkt.info@gsk.com
Norge
GlaxoSmithKline AS
Tlf: + 47 22 70 20 00
firmapost@gsk.no
Eesti
GlaxoSmithKline Eesti OÜ
Tel: +372 667 6900
estonia@gsk.com
Österreich
GlaxoSmithKline Pharma GmbH.
Tel: + 43 1 970 75-0
at.info@gsk.com
Ελλάδα
GlaxoSmithKline A.E.B.E
Tηλ: + 30 210 68 82 100
Polska
GSK Commercial Sp. z o.o.
Tel.: + 48 (22) 576 9000
España
GlaxoSmithKline, S.A.
Tel: + 34 902 202 700
es-ci@gsk.com
Portugal
GlaxoSmithKline, Produtos Farmacêuticos, Lda.
Tel: + 351 21 412 95 00
FI.PT@gsk.com
France
Laboratoire GlaxoSmithKline
Tél: + 33 (0) 1 39 17 84 44
diam@gsk.com
România
GlaxoSmithKline (GSK) SRL
Tel: +40 (0)21 3028 208
Ireland
GlaxoSmithKline (Ireland) Ltd
Tel: + 353 (0)1 4955000
Slovenija
GlaxoSmithKline d.o.o.
Tel: + 386 (0) 1 280 25 00
medical.x.si@gsk.com
Ísland
GlaxoSmithKline ehf.
Sími: +354-530 3700
Slovenská republika
GlaxoSmithKline Slovakia s.r.o.
Tel: + 421 (0)2 48 26 11 11
recepcia.sk@gsk.com
Italia
GlaxoSmithKline S.p.A.
Tel:+ 39 04 59 21 81 11
Suomi/Finland
GlaxoSmithKline Oy
Puh/Tel: + 358 10 30 30 30
Finland.tuoteinfo@gsk.com
Κύπρος
GlaxoSmithKline (Cyprus) Ltd
Τηλ: + 357 22 39 70 00
Sverige
GlaxoSmithKline AB
Tel: + 46 (0)8 638 93 00
Latvija
GlaxoSmithKline Latvia SIA
Tel: + 371 67312687
lv-epasts@gsk.com
United Kingdom
GlaxoSmithKline UK
Tel: + 44 (0)808 100 9997
customercontactuk@gsk.com
Lietuva
GlaxoSmithKline Lietuva UAB
Tel: +370 5 264 90 00
info.lt@gsk.com
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency (EMA) web
-------------------------------------------------------------------------------------------------------------------------
The following information is intended for medical or healthcare professionals only:
Cervarix should be administered as soon as possible after being removed from the
refrigerator. However, stability data generated indicate that Cervarix presented in
monodose containers remains stable and can be administered in case it has been stored
outside the refrigerator up to three days at temperatures between 8°C and 25°C or up
to one day at temperatures between 25°C and 37°C.
A fine white deposit with a clear colourless supernatant may be observed upon storage of the vial. This
does not constitute a sign of deterioration.
The content of the vial should be inspected visually both before and after shaking for any foreign
particulate matter and/or abnormal physical appearance prior to administration.
In the event of either being observed, discard the vaccine.
The vaccine should be well shaken before use.
Any unused product or waste material should be disposed of in accordance with local requirements.
PACKAGE LEAFLET: INFORMATION FOR THE USER
Cervarix suspension for injection, multidose
Human Papillomavirus vaccine [Types 16, 18] (Recombinant, adjuvanted, adsorbed)
Read all of this leaflet carefully before you start receiving this vaccine.
-
Keep this leaflet. You may need to read it again.
This medicine has been prescribed for you. Do not pass it on to others.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
What Cervarix is and what it is used for
WHAT CERVARIX IS AND WHAT IT IS USED FOR
Cervarix is a vaccine intended to protect females against the diseases caused by infection with Human
Papillomaviruses (HPV).
These diseases include:
-
cervical cancer (cancer of the cervix i.e. lower part of the uterus or womb),
-
precancerous cervical lesions (changes in cells of the cervix that have a risk of turning into
cancer).
The Human Papillomavirus (HPV) types contained in the vaccine (HPV types 16 and 18) are
responsible for approximately 70% of cervical cancer cases. Other HPV types can also cause cervical
cancer. Cervarix does not protect against all HPV types.
When a female is vaccinated with Cervarix, the immune system (the body’s natural defence system)
will make antibodies against HPV types 16 and 18. In clinical trials Cervarix has been shown to
prevent HPV related diseases in women 15-25 years of age. Cervarix also stimulates production of
antibodies in females 10-14 years of age.
Cervarix is not infectious and so, it cannot cause HPV related diseases.
Cervarix is not used to treat HPV related diseases already present at the time of vaccination.
Cervarix should be used in accordance with official guidelines.
BEFORE YOU RECEIVE CERVARIX
Cervarix should not be given if
the person to be vaccinated:
•
is allergic (hypersensitive) to any of the active substances or any of the other ingredients of
Cervarix. The active substances and other ingredients of Cervarix are listed at the end of the
leaflet (see section 6). Signs of an allergic reaction may include itchy skin rash, shortness of
breath and swelling of the face or tongue.
If you have any further questions, ask your doctor or pharmacist.
Before you receive Cervarix
•
has a severe infection with a high temperature. It might be necessary to postpone the vaccination
until recovery. A minor infection such as a cold should not be a problem, but talk to the doctor
first.
Take special care with Cervarix
You should tell the doctor if the person to be vaccinated:
•
has a bleeding problem or bruises easily.
•
has any disease which reduces her resistance to infection such as HIV infection
As with all vaccines, Cervarix may not fully protect all people who are vaccinated.
Cervarix does not protect people from diseases caused by infection with HPV types 16 or 18 if they
are already infected with Human Papillomavirus type 16 or 18 at the time of vaccination.
Although vaccination may protect you against cervical cancer, it is not a substitute for regular cervical
screening. You should continue to follow your doctor’s advice on cervical smear/Pap test (test to
screen for changes in cells of the cervix caused by an HPV infection) and preventative and protective
measur es.
As Cervarix will not protect against all types of Human Papillomavirus, appropriate precautions
against exposure to HPV and sexually transmitted diseases should continue to be used.
Cervarix will not protect against other diseases that are not caused by Human Papillomavirus.
The duration of protection after vaccination is currently unknown. In clinical trials, sustained
protection has been observed in females aged 15 to 25 years for up to 6.4 years after the first dose. The
need for booster dose(s) has not been investigated.
Using other medicines
Cervarix can be given with a combined booster vaccine containing diphtheria (d), tetanus (T) and
pertussis [acellular] (pa) with or without inactivated poliomyelitis (IPV), (dTpa, dTpa -IPV vaccines),
or with a combined hepatitis A and hepatitis B vaccine (Twinrix) or a hepatitis B vaccine (Engerix B),
at a separate injection site (another part of your body, e.g. the other arm) during the same visit.
Cervarix may not have an optimal effect if used with medicines that suppr ess the immune system.
In clinical trials, oral contraceptives (e.g. the pill) did not reduce the protection obtained by Cervarix.
Please tell the doctor if the person to be vaccinated is taking or has recently taken any other medicines,
including medicines obtained without a prescription or has recently received any other vaccine.
Pregnancy and breast-feeding
There are insufficient data concerning the use of Cervarix during pregnancy. If pregnancy occurs
during the course of vaccination your doctor should be consulted. It is recommended to postpone
vaccination until after completion of the pregnancy.
Ask your doctor for advice about breast-feeding before receiving Cervarix.
Driving and using machines
There is no information on the effect of Cervarix on your ability to drive or use machinery.
The doctor or nurse will give Cervarix as an injection into the muscle of the upper arm.
Cervarix is intended for females from 10 years of age onwards. A total of three injections will be
administered by your doctor or nurse according to the following schedule:
First injection: at chosen date
Second injection: 1 month after first injection
Third injection: 6 months after first injection
If necessary, the vaccination schedule can be more flexible. Please speak to your doctor for more
information.
When Cervarix is given for the first dose, it is recommended that Cervarix (and not another vaccine
against HPV) be given for the complete 3-dose vaccination course.
The vaccine should never be given into a vein.
If you forget a return visit for Cervarix:
It is important that you follow the instructions of your doctor or nurse regarding return visits. If you
forget to go back to your doctor at the scheduled time, ask your doctor for advice.
If you do not finish the complete vaccination course of three injections, you may not get the best
response and protection from the vaccination.
Like all medicines, Cervarix can cause side effects, although not everybody gets them.
Side effects that occurred during clinical trials with Cervarix were as follows:
♦
Very common (side effects which may occur in more than 1 per 10 doses of vaccine):
•
pain or discomfort at the injection site
•
redness or swelling at the injection site
•
headache
•
aching muscles, muscle tenderness or weakness (not caused by exercise)
•
tiredness
♦
Common (side effects which may occur in less than 1 per 10 but more than 1 per 100 doses of
vaccine):
•
gastrointestinal symptoms including nausea, vomiting, diarrhoea and abdominal pain
•
itching, red skin rash, hives (urticaria)
•
joint pain
•
fever (≥38°C)
♦
Uncommon (side effects which may occur in less than 1 per 100 but more than 1 per 1,000
doses of vaccine):
•
upper respiratory tract infection (infection of the nose, throat or trachea)
•
dizziness
•
other injection site reactions such as hard lump, tingling or numbness.
Side effects that have been reported during marketed use of Cervarix include:
•
allergic reactions. These can be recognised by:
itchy rash of the hands and feet,
swelling of the eyes and face,
difficulty in breathing or swallowing,
sudden drop in blood pressure and loss of consciousness.
These reactions will usually occur before leaving the doctor’s surgery. However, if your child gets
any of these symptoms you should contact a doctor urgently.
•
swollen glands in the neck, armpit or groin
•
fainting sometimes accompanied by shaking or stiffness.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not us e Cervarix after the expiry date which is stated on the carton. The expiry date refers to the
last day of that month.
Store in a refrigerator (2°C – 8°C).
Do not freeze.
Store in the original package in order to protect from light.
After first opening, immediate us e is recommended. If not used immediately, the vaccine should be
stored in a refrigerator (2°C – 8°C). If not used within 6 hours it should be discarded.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active subs tances are:
Human Papillomavirus
1
type 16 L1 protein
2,3,4
Human Papillomavirus
1
type 18 L1 protein
2,3,4
1
Human Papillomavirus = HPV
2
adjuvanted by AS04 containing:
3-
O
-desacyl-4’- monophosphoryl lipid A (MPL)
3
50
micrograms
3
adsorbed on aluminium hydroxide, hydrated (Al(OH)
3
) 0.5 milligrams Al
3+
in total
4
L1 protein in the form of non-infectious virus-like particles (VLPs) produced by recombinant
DNA technology using a Baculovirus expression system which uses Hi-5 Rix4446 cells derived
from the insect
Trichoplusia ni
.
The other ingredients are sodium chloride (NaCl), sodium dihydrogen phosphate dihydrate
(NaH
2
PO
4
.2 H
2
O) and water for injections.
What Cervarix looks like and contents of the pack
Suspension for injection.
Cervarix is a turbid white suspension.
Cervarix is available in vials for 2 doses (1 ml) in packs of 1, 10 and 100.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
GlaxoSmithKline Biologicals s.a.
Rue de l'Institut 89
B-1330 Rixensart, Belgium
For any information about this medicinal product, please contact the local repr esentative of the
Marketing Authorisation Holder:
België/Belgique/Belgien
GlaxoSmithKline s.a./n.v.
Tél/Tel: + 32 2 656 21 11
Luxembourg/Luxemburg
GlaxoSmithKline s.a./n.v.
Tél/Tel: + 32 2 656 21 11
България
ГлаксоСмитКлайн ЕООД
ул. Димитър Манов бл.10
София 1408
Тел. + 359 2 953 10 34
Magyarország
GlaxoSmithKline Kft.
Tel.: + 36-1-2255300
Česká republika
GlaxoSmithKline s.r.o.
Tel: + 420 2 22 00 11 11
gsk.czmail@gsk.com
Malta
GlaxoSmithKline Malta
Tel: + 356 21 238131
Danmark
GlaxoSmithKline Pharma A/S
Tlf: + 45 36 35 91 00
dk-info@gsk.com
Nederland
GlaxoSmithKline BV
Tel: + 31 (0)30 69 38 100
nlinfo@gsk.com
Deutschland
GlaxoSmithKline GmbH & Co. KG
Tel: + 49 (0)89 360448701
produkt.info@gsk.com
Norge
GlaxoSmithKline AS
Tlf: + 47 22 70 20 00
firmapost@gsk.no
Eesti
GlaxoSmithKline Eesti OÜ
Tel: +372 667 6900
estonia@gsk.com
Österreich
GlaxoSmithKline Pharma GmbH.
Tel: + 43 1 970 75-0
at.info@gsk.com
Ελλάδα
GlaxoSmithKline A.E.B.E
Tηλ: + 30 210 68 82 100
Polska
GSK Commercial Sp. z o.o.
Tel.: + 48 (22) 576 9000
España
GlaxoSmithKline, S.A.
Tel: + 34 902 202 700
es-ci@gsk.com
Portugal
GlaxoSmithKline, Produtos Farmacêuticos, Lda.
Tel: + 351 21 412 95 00
FI.PT@gsk.com
France
Laboratoire GlaxoSmithKline
Tél: + 33 (0) 1 39 17 84 44
diam@gsk.com
România
GlaxoSmithKline (GSK) SRL
Tel: +40 (0)21 3028 208
GlaxoSmithKline (Ireland) Ltd
Tel: + 353 (0)1 4955000
GlaxoSmithKline d.o.o.
Tel: + 386 (0) 1 280 25 00
medical.x.si@gsk.com
Ísland
GlaxoSmithKline ehf.
Sími: +354-530 3700
Slovenská republika
GlaxoSmithKline Slovakia s.r.o.
Tel: + 421 (0)2 48 26 11 11
recepcia.sk@gsk.com
Italia
GlaxoSmithKline S.p.A.
Tel:+ 39 04 59 21 81 11
Suomi/Finland
GlaxoSmithKline Oy
Puh/Tel: + 358 10 30 30 30
Finland.tuoteinfo@gsk.com
Κύπρος
GlaxoSmithKline (Cyprus) Ltd
Τηλ: + 357 22 39 70 00
Sverige
GlaxoSmithKline AB
Tel: + 46 (0)8 638 93 00
Latvija
GlaxoSmithKline Latvia SIA
Tel: + 371 67312687
lv-epasts@gsk.com
United Kingdom
GlaxoSmithKline UK
Tel: + 44 (0)808 100 9997
customercontactuk@gsk.com
Lietuva
GlaxoSmithKline Lietuva UAB
Tel: +370 5 264 90 00
info.lt@gsk.com
This leaflet was last approved in
.
Detailed information on this medicine is available on the European Medicines Agency (EMA) web
-------------------------------------------------------------------------------------------------------------------------
The following information is intended for medical or healthcare professionals only:
A fine white deposit with a clear colourless supernatant may be observed upon storage of the vial. This
does not constitute a sign of deterioration.
The content of the vial should be inspected visually both before and after shaking for any foreign
particulate matter and/or abnormal physical appearance prior to administration.
In the event of either being observed, discard the vaccine.
The vaccine should be well shaken before use.
When using a multidose vial, each 0.5 ml dose should be withdrawn using a sterile needle and syringe;
precautions should be taken to avoid contamination of the contents.
Any unused product or waste material should be disposed of in accordance with local requirements.
PACKAGE LEAFLET: INFORMATION FOR THE USER
Cervarix suspension for injection in pre-filled syringe
Human Papillomavirus vaccine [Types 16, 18] (Recombinant, adjuvanted, adsorbed)
Read all of this leaflet carefully before you start receiving this vaccine.
-
Keep this leaflet. You may need to read it again.
This medicine has been prescribed for you. Do not pass it on to others.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
What Cervarix is and what it is used for
WHAT CERVARIX IS AND WHAT IT IS USED FOR
Cervarix is a vaccine intended to protect females against the diseases caused by infection with Human
Papillomaviruses (HPV).
These diseases include:
-
cervical cancer (cancer of the cervix i.e. lower part of the uterus or womb),
-
precancerous cervical lesions (changes in cells of the cervix that have a risk of turning into
cancer).
The Human Papillomavirus (HPV) types contained in the vaccine (HPV types 16 and 18) are
responsible for approximately 70% of cervical cancer cases. Other HPV types can also cause cervical
cancer. Cervarix does not protect against all HPV types.
When a female is vaccinated with Cervarix, the immune system (the body’s natural defence system)
will make antibodies against HPV types 16 and 18. In clinical trials Cervarix has been shown to
prevent HPV related diseases in women 15-25 years of age. Cervarix also stimulates production of
antibodies in females 10-14 years of age.
Cervarix is not infectious and so, it cannot cause HPV related diseases.
Cervarix is not used to treat HPV related diseases already present at the time of vaccination.
Cervarix should be used in accordance with official guidelines.
BEFORE YOU RECEIVE CERVARIX
Cervarix should not be given if
the person to be vaccinated:
•
is allergic (hypersensitive) to any of the active substances or any of the other ingredients of
Cervarix. The active substances and other ingredients of Cervarix are listed at the end of the
leaflet (see section 6). Signs of an allergic reaction may include itchy skin rash, shortness of
breath and swelling of the face or tongue.
If you have any further questions, ask your doctor or pharmacist.
Before you receive Cervarix
•
has a severe infection with a high temperature. It might be necessary to postpone the vaccination
until recovery. A minor infection such as a cold should not be a problem, but talk to the doctor
first.
Take special care with Cervarix
You should tell the doctor if the person to be vaccinated:
•
has a bleeding problem or bruises easily.
•
has any disease which reduces her resistance to infection such as HIV infection
As with all vaccines, Cervarix may not fully protect all people who are vaccinated.
Cervarix does not protect people from diseases caused by infection with HPV types 16 or 18 if they
are already infected with Human Papillomavirus type 16 or 18 at the time of vaccination.
Although vaccination may protect you against cervical cancer, it is not a substitute for regular cervical
screening. You should continue to follow your doctor’s advice on cervical smear/Pap test (test to
screen for changes in cells of the cervix caused by an HPV infection) and preventative and protective
measur es.
As Cervarix will not protect against all types of Human Papillomavirus, appropriate precautions
against exposure to HPV and sexually transmitted diseases should continue to be used.
Cervarix will not protect against other diseases that are not caused by Human Papillomavirus.
The duration of protection after vaccination is currently unknown. In clinical trials, sustained
protection has been observed in females aged 15 to 25 years for up to 6.4 years after the first dose. The
need for booster dose(s) has not been investigated.
Using other medicines
Cervarix can be given with a combined booster vaccine containing diphtheria (d), tetanus (T) and
pertussis [acellular] (pa) with or without inactivated poliomyelitis (IPV), (dTpa, dTpa -IPV vaccines),
or with a combined hepatitis A and hepatitis B vaccine (Twinrix) or a hepatitis B vaccine (Engerix B),
at a separate injection site (another part of your body, e.g. the other arm) during the same visit.
Cervarix may not have an optimal effect if used with medicines that suppr ess the immune system.
In clinical trials, oral contraceptives (e.g. the pill) did not reduce the protection obtained by Cervarix.
Please tell the doctor if the person to be vaccinated is taking or has recently taken any other medicines,
including medicines obtained without a prescription or has recently received any other vaccine.
Pregnancy and breast-feeding
There are insufficient data concerning the use of Cervarix during pregnancy. If pregnancy occurs
during the course of vaccination your doctor should be consulted. It is recommended to postpone
vaccination until after completion of the pregnancy.
Ask your doctor for advice about breast-feeding before receiving Cervarix.
Driving and using machines
There is no information on the effect of Cervarix on your ability to drive or use machinery.
The doctor or nurse will give Cervarix as an injection into the muscle of the upper arm.
Cervarix is intended for females from 10 years of age onwards. A total of three injections will be
administered by your doctor or nurse according to the following schedule:
First injection: at chosen date
Second injection: 1 month after first injection
Third injection: 6 months after first injection
If necessary, the vaccination schedule can be more flexible. Please speak to your doctor for more
information.
When Cervarix is given for the first dose, it is recommended that Cervarix (and not another vaccine
against HPV) be given for the complete 3-dose vaccination course.
The vaccine should never be given into a vein.
If you forget a return visit for Cervarix:
It is important that you follow the instructions of your doctor or nurse regarding return visits. If you
forget to go back to your doctor at the scheduled time, ask your doctor for advice.
If you do not finish the complete vaccination course of three injections, you may not get the best
response and protection from the vaccination.
Like all medicines, Cervarix can cause side effects, although not everybody gets them.
Side effects that occurred during clinical trials with Cervarix were as follows:
♦
Very common (side effects which may occur in more than 1 per 10 doses of vaccine):
•
pain or discomfort at the injection site
•
redness or swelling at the injection site
•
headache
•
aching muscles, muscle tenderness or weakness (not caused by exercise)
•
tiredness
♦
Common (side effects which may occur in less than 1 per 10 but more than 1 per 100 doses of
vaccine):
•
gastrointestinal symptoms including nausea, vomiting, diarrhoea and abdominal pain
•
itching, red skin rash, hives (urticaria)
•
joint pain
•
fever (≥38°C)
♦
Uncommon (side effects which may occur in less than 1 per 100 but more than 1 per 1,000
doses of vaccine):
•
upper respiratory tract infection (infection of the nose, throat or trachea)
•
dizziness
•
other injection site reactions such as hard lump, tingling or numbness.
Side effects that have been reported during marketed use of Cervarix include:
•
allergic reactions. These can be recognised by:
itchy rash of the hands and feet,
swelling of the eyes and face,
difficulty in breathing or swallowing,
sudden drop in blood pressure and loss of consciousness.
These reactions will usually occur before leaving the doctor’s surgery. However, if your child gets
any of these symptoms you should contact a doctor urgently.
•
swollen glands in the neck, armpit or groin
•
fainting sometimes accompanied by shaking or stiffness.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Cervarix after the expiry date which is stated on the carton. The expiry date refers to the
last day of that month.
Store in a refrigerator (2°C – 8°C).
Do not freeze.
Store in the original package in order to protect from light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active substances are:
Human Papillomavirus
1
type 16 L1 protein
2,3,4
Human Papillomavirus
1
type 18 L1 protein
2,3,4
1
Human Papillomavirus = HPV
2
adjuvanted by AS04 containing:
3-
O
-desacyl-4’- monophosphoryl lipid A (MPL)
3
50
micrograms
3
adsorbed on aluminium hydroxide, hydrated (Al(OH)
3
) 0.5 milligrams Al
3+
in total
4
L1 protein in the form of non-infectious virus-like particles (VLPs) produced by recombinant
DNA technology using a Baculovirus expression system which uses Hi-5 Rix4446 cells derived
from the insect
Trichoplusia ni
.
The other ingredients are sodium chloride (NaCl), sodium dihydrogen phosphate dihydrate
(NaH
2
PO
4
.2 H
2
O) and water for injections.
What Cervarix looks like and contents of the pack
Suspension for injection in pre-filled syringe.
Cervarix is a turbid white suspension.
Cervarix is available in pre-filled syringes with or without needles in packs of 1 and 10.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
GlaxoSmithKline Biologicals s.a.
Rue de l'Institut 89
B-1330 Rixensart, Belgium
For any information about this medicinal product, please contact the local representative of the
Marketing Authorisation Holder:
België/Belgique/Belgien
GlaxoSmithKline s.a./n.v.
Tél/Tel: + 32 2 656 21 11
Luxembourg/Luxemburg
GlaxoSmithKline s.a./n.v.
Tél/Tel: + 32 2 656 21 11
България
ГлаксоСмитКлайн ЕООД
ул. Димитър Манов бл.10
София 1408
Тел. + 359 2 953 10 34
Magyarország
GlaxoSmithKline Kft.
Tel.: + 36-1-2255300
Česká republika
GlaxoSmithKline s.r.o.
Tel: + 420 2 22 00 11 11
gsk.czmail@gsk.com
Malta
GlaxoSmithKline Malta
Tel: + 356 21 238131
Danmark
GlaxoSmithKline Pharma A/S
Tlf: + 45 36 35 91 00
dk-info@gsk.com
Nederland
GlaxoSmithKline BV
Tel: + 31 (0)30 69 38 100
nlinfo@gsk.com
Deutschland
GlaxoSmithKline GmbH & Co. KG
Tel: + 49 (0)89 360448701
produkt.info@gsk.com
Norge
GlaxoSmithKline AS
Tlf: + 47 22 70 20 00
firmapost@gsk.no
Eesti
GlaxoSmithKline Eesti OÜ
Tel: +372 667 6900
estonia@gsk.com
Österreich
GlaxoSmithKline Pharma GmbH.
Tel: + 43 1 970 75-0
at.info@gsk.com
Ελλάδα
GlaxoSmithKline A.E.B.E
Tηλ: + 30 210 68 82 100
Polska
GSK Commercial Sp. z o.o.
Tel.: + 48 (22) 576 9000
España
GlaxoSmithKline, S.A.
Tel: + 34 902 202 700
es-ci@gsk.com
Portugal
GlaxoSmithKline, Produtos Farmacêuticos, Lda.
Tel: + 351 21 412 95 00
FI.PT@gsk.com
France
Laboratoire GlaxoSmithKline
Tél: + 33 (0) 1 39 17 84 44
diam@gsk.com
România
GlaxoSmithKline (GSK) SRL
Tel: +40 (0)21 3028 208
Ireland
GlaxoSmithKline (Ireland) Ltd
Tel: + 353 (0)1 4955000
Slovenija
GlaxoSmithKline d.o.o.
Tel: + 386 (0) 1 280 25 00
medical.x.si@gsk.com
Ísland
GlaxoSmithKline ehf.
Sími: +354-530 3700
Slovenská republika
GlaxoSmithKline Slovakia s.r.o.
Tel: + 421 (0)2 48 26 11 11
recepcia.sk@gsk.com
Italia
GlaxoSmithKline S.p.A.
Tel:+ 39 04 59 21 81 11
Suomi/Finland
GlaxoSmithKline Oy
Puh/Tel: + 358 10 30 30 30
Finland.tuoteinfo@gsk.com
Κύπρος
GlaxoSmithKline (Cyprus) Ltd
Τηλ: + 357 22 39 70 00
Sverige
GlaxoSmithKline AB
Tel: + 46 (0)8 638 93 00
Latvija
GlaxoSmithKline Latvia SIA
Tel: + 371 67312687
lv-epasts@gsk.com
United Kingdom
GlaxoSmithKline UK
Tel: + 44 (0)808 100 9997
customercontactuk@gsk.com
Lietuva
GlaxoSmithKline Lietuva UAB
Tel: +370 5 264 90 00
info.lt@gsk.com
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency (EMA) web
-------------------------------------------------------------------------------------------------------------------------
The following information is intended for medical or healthcare professionals only:
Cervarix should be administered as soon as possible after being removed from the refrigerator.
However, stability data generated indicate that Cervarix presented in monodose containers remains
stable and can be administered in case it has been stored outside the refrigerator up to three days at
temperatures between 8°C and 25°C or up to one day at temperatures between 25°C and 37°C.
A fine white deposit with a clear colourless supernatant may be observed upon storage of the syringe.
This does not constitute a sign of deterioration.
The content of the syringe should be inspected visually both before and after shaking for any foreign
particulate matter and/or abnormal physical appearance prior to administration.
In the event of either being observed, discard the vaccine.
The vaccine should be well shaken before use.
Instructions for administration of the vaccine presented in pre-filled syringe
1.
Holding the syringe
barrel
(avoid holding the syringe plunger),
unscrew the syringe cap by twisting it anticlockwise.
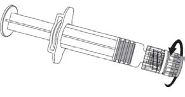



2. To attach the needle to the syringe,
twist the needle clockwise into the syringe
until you feel it lock.
3. Remove the needle protector, which on occasion
can be a little stiff.
4. Administer the vaccine.
Any unused product or waste material should be disposed of in accordance with local requirements.
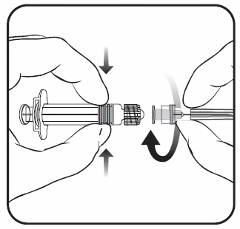


Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/cervarix.html
Copyright © 1995-2021 ITA all rights reserved.
|



















































































































































































































































































































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































