Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
1.
NAME OF THE MEDICINAL PRODUCT
Effentora 100 micrograms buccal tablets
2.
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each buccal tablet contains 100 micrograms fentanyl (as citrate).
Excipient(s): Each tablet contains 8 mg of sodium.
For a full list of excipients, see section 6.1.
Flat-faced, white, round bevelled-edge tablet, embossed on one side with a “C” and on the other side
with “1”.
4.1
Therapeutic indications
Effentora is indicated for the treatment of breakthrough pain (BTP) in adults with cancer who are
already receiving maintenance opioid therapy for chronic cancer pain.
BTP is a transitory exacerbation of pain that occurs on a background of otherwise controlled persistent
pain
.
Patients receiving maintenance opioid therapy are those who are taking at least 60 mg of oral
morphine daily, at least 25 micrograms of transdermal fentanyl per hour, at least 30 mg of oxycodone
daily, at least 8 mg of oral hydromorphone daily or an equianalgesic dose of another opioid for a week
or longer.
4.2
Posology and method of administration
Treatment should be initiated by and remain under the guidance of a physician experienced in the
management of opioid therapy in cancer patients. Physicians should keep in mind the potential of
abuse of fentanyl. Patients should be instructed not to use two different formulations of fentanyl
concurrently for the treatment of breakthrough pain, and to dispose of any fentanyl product prescribed
for BTP when switching to Effentora. The number of tablet strengths available to the patients at any
time should be minimised to prevent confusion and potential overdose.
Effentora should be individually titrated to an “effective” dose that provides adequate analgesia and
minimises undesirable effects. In clinical studies, the effective dose of Effentora for BTP was not
predictable from the daily maintenance dose of opioid.
Patients should be carefully monitored until an effective dose is reached.
Titration in patients not switching from other fentanyl containing products
The initial dose of
Effentora should be 100 micrograms, titrating upwards as necessary through the
range of available tablets strengths (100, 200, 400, 600, 800 micrograms).
Titration in patients switching from other fentanyl containing products
Due to different absorption profiles, switching must not be done at a 1:1 ratio. If switching from
another oral fentanyl citrate product, independent dose titration with Effentora is required as
bioavailability between products differs significantly. However, in these patients, a starting dose
higher than 100 micrograms may be considered.
Method of titration
During titration, if adequate analgesia is not obtained within 30 minutes after the start of
administration of a single tablet, a second Effentora tablet of the same strength may be used.
If treatment of a BTP episode requires more than one tablet, an increase in dose to the next higher
available strength should be considered to treat the next BTP episode.
During titration, multiple tablets may be used: up to four 100 micrograms or up to four 200
micrograms tablets may be used to treat a single episode of BTP during dose titration according to the
following schedule:
•
If the initial 100 micrograms tablet is not efficacious, the patient can be instructed to treat the next
episode of BTP with two 100 micrograms tablets. It is recommended that one tablet should be
placed in each side of the mouth. If this dose is considered to be the effective dose, treatment of
successive episodes of BTP may be continued with a single 200 micrograms tablet of Effentora
.
•
If a single 200 micrograms tablet of Effentora (or two 100 micrograms tablets) is not considered to
be efficacious the patient can be instructed to use two 200 micrograms tablets (or four 100
micrograms tablets) to treat the next episode of BTP. It is recommended that two tablets should be
placed in each side of the mouth. If this dose is considered to be the effective dose, treatment of
successive episodes of BTP may be continued with a single 400 micrograms tablet of Effentora.
•
For titration to 600 micrograms and 800 micrograms, tablets of 200 micrograms should be used.
Doses above 800 micrograms were not evaluated in clinical studies.
No more than two tablets should be used to treat any individual BTP episode, except when titrating
using up to four tablets as described above.
Patients should wait at least 4 hours before treating another BTP episode with Effentora during
titration.
Once an effective dose has been established during titration, patients should continue to take this dose
as a single tablet of that given strength. Breakthrough pain episodes may vary in intensity and the
required Effentora dose might increase over time due to progression of the underlying cancer disease.
In these cases, a second tablet of the same strength may be used. If a second tablet of Effentora was
required for several consecutive times, the usual maintenance dose is to be readjusted (see below).
Patients should wait at least 4 hours before treating another BTP episode with Effentora during
maintenance therapy.
The maintenance dose of Effentora should be increased when a patient requires more than one tablet
per BTP episode for several consecutive BTP episodes. For dose-readjustment the same principles
apply as outlined for
dose titration
(see above).
Dose readjustment of the background opioid therapy may be required if patients consistently
present with more than four BTP episodes per 24 hours.
Discontinuation of therapy
Effentora should be immediately discontinued if no longer required.
Use in children and adolescents:
Effentora is not recommended for use in children and adolescents below 18 years due to a lack of data
on safety and efficacy.
Use in the elderly (older than 65 years):
In clinical studies patients older than 65 years tended to titrate to a lower effective dose than younger
patients. It is recommended that increased caution should be exercised in titrating the dose of
Effentora in elderly patients.
Hepatic or renal impairment:
Effentora should be administered with caution to patients with moderate or severe hepatic or renal
impairment (see section 4.4).
Patients with xerostomia:
Patients experiencing xerostomia are advised to drink water to moisten the buccal cavity prior to
administration of Effentora. If this recommendation does not result in an appropriate effervescence,
then a switch of therapy may be advised.
Method of administration:
Effentora tablet once exposed to moisture utilises an effervescent reaction to deliver the active
substance. Therefore patients should be instructed not to open the blister until ready to place the tablet
in the buccal cavity.
Opening the blister package
Patients should be instructed NOT to attempt to push the tablet through the blister because this could
damage the buccal tablet. The correct method of releasing the tablet from the blister is:
One of the blister units should be separated from the blister card by tearing it apart at the perforations.
The blister unit should then be flexed along the line printed on the backing foil where indicated. The
backing foil should be peeled back to expose the tablet.
Patients should be instructed not to attempt to crush or split the tablet.
The tablet should not be stored once removed from the blister package as the tablet integrity can not be
guaranteed and a risk of accidental exposure to a tablet can occur.
Tablet administration
Patients should remove the tablet from the blister unit and immediately place the entire Effentora
tablet in the buccal cavity (near a molar between the cheek and gum).
The Effentora tablet should not be sucked, chewed or swallowed, as this will result in lower plasma
concentrations than when taken as directed.
Effentora should be placed and retained within the buccal cavity for a period sufficient to allow
disintegration of the tablet which usually takes approximately 14-25 minutes.
Alternatively, the tablet could be placed sublingually (see section 5.2).
After 30 minutes, if remnants from the Effentora tablet remain, they may be swallowed with a glass of
water.
The length of time that the tablet takes to fully disintegrate following oromucosal administration does
not appear to affect early systemic exposure to fentanyl.
Patients should not consume any food and drink when a tablet is in the buccal cavity.
In case of buccal mucosa irritation, a change in tablet placement within the buccal cavity should be
recommended.
Hypersensitivity to the active substance or to any of the excipients.
Patients without maintenance opioid therapy (see section 4.1) as there is an increased risk of
respiratory depression.
Severe respiratory depression or severe obstructive lung conditions.
Treatment of acute pain other than breakthrough pain (e.g. postoperative pain, headache, migraine).
4.4
Special warnings and precautions for use
Patients and their carers must be instructed that Effentora contains an active substance in an amount
that can be fatal to a child, and therefore to keep all tablets out of the reach and sight
of children.
In order to minimise the risks of opioid-related undesirable effects and to identify the effective dose, it
is imperative that patients be monitored closely by health professionals during the titration process.
It is important that the long acting opioid treatment used to treat the patient’s persistent pain has been
stabilised before Effentora therapy begins and that the patient continues to be treated with the long
acting opioid treatment whilst taking Effentora.
As with all opioids, there is a risk of clinically significant respiratory depression associated with the
use of fentanyl. Particular caution should be used when titrating Effentora in patients with non-severe
chronic obstructive pulmonary disease or other medical conditions predisposing them to respiratory
depression, as even normally therapeutic doses of Effentora may further decrease respiratory drive to
the point of respiratory failure.
Effentora should only be administered with extreme caution in patients who may be particularly
susceptible to the intracranial effects of CO
2
retention, such as those with evidence of increased
intracranial pressure or impaired consciousness. Opioids may obscure the clinical course of a patient
with a head injury and should be used only if clinically warranted.
Intravenous fentanyl may produce bradycardia. In clinical trials with Effentora, no clear evidence for
bradycardia was observed. However, Effentora should be used with caution in patients with pre-
existing bradyarrhythmias.
In addition, Effentora should be administered with caution to patients with hepatic or renal
impairment. The influence of hepatic and renal impairment on the pharmacokinetics of the medicinal
product has not been evaluated, however, when administered intravenously the clearance of fentanyl
has been shown to be altered in hepatic and renal impairment due to alterations in metabolic clearance
and plasma proteins. After administration of Effentora, impaired hepatic and renal function may both
increase the bioavailability of swallowed fentanyl and decrease its systemic clearance, which could
lead to increased and prolonged opioid effects. Therefore, special care should be taken during the
titration process in patients with moderate or severe hepatic or renal impairment.
Careful consideration should be given to patients with hypovolaemia and hypotension.
Tolerance and physical and/or psychological dependence may develop upon repeated administration
of opioids such as fentanyl. However, iatrogenic addiction following therapeutic use of opioids is
rare.
This medicinal product contains 8 mg sodium per tablet. To be taken into consideration by patients on
a controlled sodium diet.
4.5
Interaction with other medicinal products and other forms of interaction
Fentanyl is metabolised mainly via the human cytochrome P450 3A4 isoenzyme system (CYP3A4),
therefore potential interactions may occur when Effentora is given concurrently with agents that affect
CYP3A4 activity. Coadministration with agents that induce 3A4 activity may reduce the efficacy of
Effentora. The concomitant use of Effentora with strong CYP3A4 inhibitors (e.g., ritonavir,
ketoconazole, itraconazole, troleandomycin, clarithromycin, and nelfinavir) or moderate CYP3A4
inhibitors (e.g., amprenavir, aprepitant, diltiazem, erythromycin, fluconazole, fosamprenavir,
grapefruit juice, and verapamil) may result in increased fentanyl plasma concentrations, potentially
causing serious adverse drug reactions including fatal respiratory depression. Patients receiving
Effentora concomitantly with moderate or strong CYP3A4 inhibitors should be carefully monitored
for an extended period of time. Dosage increase should be done with caution.
The concomitant use of other central nervous system depressants, including other opioids, sedatives or
hypnotics, general anaesthetics, phenothiazines, tranquillisers, skeletal muscle relaxants, sedating
antihistamines and alcohol may produce additive depressant effects.
Effentora is not recommended for use in patients who have received monoamine oxidase (MAO)
inhibitors within 14 days because severe and unpredictable potentiation by MAO inhibitors has been
reported with opioid analgesics.
The concomitant use of partial opioid agonists/antagonists (e.g. buprenorphine, nalbuphine,
pentazocine) is not recommended. They have high affinity to opioid receptors with relatively low
intrinsic activity and therefore partially antagonise the analgesic effect of fentanyl and may induce
withdrawal symptoms in opioid dependant patients.
4.6
Pregnancy and lactation
There are no adequate data from the use of fentanyl in pregnant women. Studies in animals have
shown reproductive toxicity (see section 5.3). The potential risk for humans is unknown. Effentora
should not be used in pregnancy unless clearly necessary.
Following long-term treatment, fentanyl may cause withdrawal in the new-born infant.
It is advised not to use fentanyl during labour and delivery (including caesarean section) because
fentanyl passes through the placenta and may cause respiratory depression in the foetus. If Effentora is
administered, an antidote for the child should be readily available.
Fentanyl passes into breast milk and may cause sedation and respiratory depression in the breast-fed
child. Fentanyl should not be used by breastfeeding women and breastfeeding should not be restarted
until at least 48 hours after the last administration of fentanyl.
4.7
Effects on ability to drive and use machines
No studies of the effects on the ability to drive and use machines have been performed. However,
opioid analgesics impair the mental and/or physical ability required for the performance of potentially
dangerous tasks (e.g., driving a car or operating machinery). Patients should be advised not to drive or
operate machinery if they experience somnolence, dizziness, or visual disturbance while taking
Effentora and not to drive or operate machinery until they know how they react.
Typical opioid undesirable effects are to be expected with Effentora. Frequently, these will cease or
decrease in intensity with continued use of the medicinal product, as the patient is titrated to the most
appropriate dose. However, the most serious adverse reactions are respiratory depression (potentially
leading to apnoea or respiratory arrest), circulatory depression, hypotension and shock and all patients
should be closely monitored for these.
The clinical studies of Effentora were designed to evaluate safety and efficacy in treating BTP and all
patients were also taking concomitant opioids, such as sustained-release morphine or transdermal
fentanyl, for their persistent pain. Therefore it is not possible to definitively separate the effects of
Effentora alone.
The following adverse reactions have been reported with Effentora during clinical studies and post
marketing experience. Adverse reactions are listed below as MedDRA preferred term by system organ
class and frequency (frequencies are defined as: very common ≥1/10, common ≥1/100 to <1/10,
uncommon ≥ 1/1,000 to <1/100, rare (≥1/10,000 to <1/1,000), not known (cannot be estimated from
the available data); within each frequency group, undesirable effects are presented in order of
decreasing seriousness:
Platelet count
decreased
Heart rate
increased
Haematocrit
decreased
Haemoglobin
decreased
Blood and
lymphatic
system
disorders
Dysgeusia
Somnolence
Lethargy
Tremor
Sedation
Hypoaesthesia
Migraine
Depressed
level of
consciousness
Disturbance in
attention
Balance
disorder
Dysarthria
Cognitive
disorder
Motor
dysfunction
Visual
disturbance
Ocular
hyperaemia
Blurred vision
Visual acuity
reduced
Abnormal
sensation in
eye
Photopsia
Ear and
labyrinth
disorders
Vertigo
Tinnitus
Ear discomfort














Respiratory,
thoracic and
mediastinal
disorders
Dyspnoea
Pharyngolaryn-
geal pain
Respiratory
depression
Sleep apnoea
syndrome
Gastro-
intestinal
disorders
Constipation
Stomatitis
Dry mouth
Diarrhoea
Abdominal pain
Gastro-
oesophageal
reflux disease
Stomach
discomfort
Dyspepsia
Toothache
Ileus
Mouth
ulceration
Oral
hypoaesthesia
Oral
discomfort
Oral mucosal
discolouration
Oral soft tissue
disorder
Glossodynia
Tongue
blistering
Gingival pain
Tongue
ulceration
Tongue
disorder
Oesophagitis
Chapped lips
Tooth disorder
Oral mucosal
blistering
Dry lip
Renal and
urinary
disorders
Skin and
subcutaneous
tissue
disorders
Pruritus
Hyperhidrosis
Rash
Cold sweat
Facial swelling
Generalised
pruritus
Alopecia
Musculoskel
etal and
connective
tissue
disorders
Muscle
twitching
Muscular
weakness
Metabolism
and nutrition
disorders
Infections and
infestations
Oral candidiasis Pharyngitis
Injury,
poisoning and
procedural
complications


















General
disorders and
administration
site conditions
Application
site reactions
including
bleeding,
pain,
ulcer,
irritation,
paraesthesia,
anaesthesia,
erythema,
oedema,
swelling and
vesicles
Peripheral
oedema
Fatigue
Asthenia
Drug
withdrawal
syndrome
Chills
Malaise
Sluggishness
Chest
discomfort
Feeling
abnormal
Feeling jittery
Thirst
Feeling cold
Feeling hot
Depression
Anxiety
Confusional
state
Insomnia
Euphoric
mood
Nervousness
Hallucination
Visual
hallucination
Mental status
changes
Drug
dependence
(addiction)
Disorientation
Tolerance, physical and/or psychological dependence may develop upon repeated administration of
opioids such as fentanyl.
Opioid withdrawal symptoms such as nausea, vomiting, diarrhoea, anxiety and shivering have been
observed in studies with Effentora.
Loss of consciousness and respiratory arrest have been observed in the context of overdose.
The symptoms of fentanyl overdose are expected to be similar in nature to those of intravenous
fentanyl and other opioids, and are an extension of its pharmacological actions, with the most serious
significant effects being altered mental status, loss of consciousness, hypotension, respiratory
depression, respiratory distress, and respiratory failure, which have resulted in death.
Immediate management of opioid overdose includes removal of the Effentora buccal tablet, if still in
the mouth, ensuring a patent airway, physical and verbal stimulation of the patient, assessment of the
level of consciousness, ventilatory and circulatory status, and assisted ventilation (ventilatory support)
if necessary.
For treatment of overdose (accidental ingestion) in the opioid-naive person, intravenous access should
be obtained and naloxone or other opioid antagonists should be employed as clinically indicated. The
duration of respiratory depression following overdose may be longer than the effects of the opioid
antagonist’s action (e.g., the half-life of naloxone ranges from 30 to 81 minutes) and repeated
administration may be necessary. Consult the Summary of Product Characteristics of the individual
opioid antagonist for details about such use.
For treatment of overdose in opioid-maintained patients, intravenous access should be obtained. The
judicious use of naloxone or another opioid antagonist may be warranted in some instances, but it is
associated with the risk of precipitating an acute withdrawal syndrome.











Although muscle rigidity interfering with respiration has not been seen following the use of Effentora,
this is possible with fentanyl and other opioids. If it occurs, it should be managed by the use of
assisted ventilation, by an opioid antagonist, and as a final alternative, by a neuromuscular blocking
agent.
5.
PHARMACOLOGICAL PROPERTIES
5.1
Pharmacodynamic properties
Pharmacotherapeutic group: analgesics; opioids; phenylpiperidine derivatives; ATC code N02AB03.
Fentanyl is an opioid analgesic, interacting predominantly with the opioid µ-receptor. Its primary
therapeutic actions are analgesia and sedation. Secondary pharmacological effects are respiratory
depression, bradycardia, hypothermia, constipation, miosis, physical dependence and euphoria.
The analgesic effects of fentanyl are related to its plasma level. In general, the effective concentration
and the concentration at which toxicity occurs increase with increasing tolerance to opioids. The rate
of development of tolerance varies widely among individuals. As a result, the dose of Effentora should
be individually titrated to achieve the desired effect (see section 4.2).
All opioid µ-receptor agonists, including fentanyl, produce dose dependent respiratory depression. The
risk of respiratory depression is less in patients receiving chronic opioid therapy as these patients will
develop tolerance to respiratory depressant effects.
The safety and efficacy of Effentora have been evaluated in patients taking the drug at the onset of the
breakthrough pain episode. Pre-emptive use of Effentora for predictable pain episodes was not
investigated in the clinical trials. Two double-blind, randomized, placebo-controlled crossover studies
have been conducted involving a total of 248 patients with BTP and cancer who experienced on
average 1 to 4 episodes of BTP per day while taking maintenance opioid therapy. During an initial
open-label phase, patients were titrated to an effective dose of Effentora. Patients who identified an
effective dose entered the double-blind phase of the study. The primary efficacy variable was the
patient’s assessment of pain intensity. Patients assessed pain intensity on a 11-point scale. For each
BTP episode, pain intensity was assessed prior to and at several time points after treatment.
Sixty-seven percent of the patients were able to be titrated to an effective dose.
In the pivotal clinical study (study 1), the primary endpoint was the average sum of differences in pain
intensity scores from dosing to 60 minutes, inclusive (SPID60), which was statistically significant
compared to placebo (p<0.0001).
Study 1: Mean (+/- SEM) Pain Intensity Difference at Each Time Point (Full Analysis Set)
SPID60 mean (+/- SD)
EFFENTORA=9.7(5.58) p<0.0001
Placebo=4.9(4.38)
Time from Administration of Study Drug (Minutes)
Treatment Group
±±±
EFFENTORA
Placebo
+ p<0.0001 E
FFENTORA
versus placebo, in favor of E
FFENTORA
, by an analysis of variance
PID=pain intensity difference; SEM=standard error of the mean
Study 2: Mean (+/- SEM) Pain Intensity Difference at Each Time Point (Full Analysis Set)
SPID30 mean (+/- SD)
EFFENTORA=3.2(2.60) p<0.0001
Placebo=2.0(2.21)
Time from Administration of Study Drug (Minutes)
Treatment Group
±±±
EFFENTORA
Placebo
* p<0.01 E
FFENTORA
versus placebo, in favor of E
FFENTORA
, by one-sample Wilcoxon signed rank test
+ p<0.0001 E
FFENTORA
versus placebo, in favor of E
FFENTORA
, by one-sample Wilcoxon signed rank test
PID=pain intensity difference; SEM=standard error of the mean
In the second pivotal study (study 2), the primary endpoint was SPID30, which was also statistically
significant compared to placebo (p<0.0001).





Statistically significant improvement in pain intensity difference was seen with Effentora versus
placebo as early as 10 minutes in Study 1 and as early as 15 minutes (earliest time point measured) in
Study 2. These differences continued to be significant at each subsequent time point in each
individual study.
5.2
Pharmacokinetic properties
General introduction
Fentanyl is highly lipophilic and can be absorbed very rapidly through the oral mucosa and more
slowly by the conventional gastrointestinal route. It is subject to first-pass hepatic and intestinal
metabolism and the metabolites do not contribute to fentanyl’s therapeutic effects.
Effentora employs a delivery technology which utilises an effervescent reaction which enhances the
rate and extent of fentanyl absorbed through the buccal mucosa. Transient pH changes accompanying
the effervescent reaction may optimise dissolution (at a lower pH) and membrane permeation (at a
higher pH).
Dwell time (defined as the length of time that the tablet takes to fully disintegrate following buccal
administration), does not affect early systemic exposure to fentanyl. A comparison study between one
400 mcg Effentora tablet administered either buccally (i.e., between the cheek and the gum) or
sublingually met the criteria of bioequivalence.
The effect of renal or hepatic impairment on the pharmacokinetics of Effentora has not been studied.
Absorption:
Following oromucosal administration of Effentora, fentanyl is readily absorbed with an absolute
bioavailability of 65%. The absorption profile of Effentora is largely the result of an initial rapid
absorption from the buccal mucosa, with peak plasma concentrations following venous sampling
generally attained within an hour after oromucosal administration. Approximately 50% of the total
dose administered is rapidly absorbed transmucosally and becomes systemically available. The
remaining half of the total dose is swallowed and slowly absorbed from the gastrointestinal tract.
About 30% of the amount swallowed (50% of the total dose) escapes hepatic and intestinal first-pass
elimination and becomes systemically available.
The main pharmacokinetic parameters are shown in the following table.
Pharmacokinetic Parameters* in Adult Subjects Receiving Effentora
Pharmacokinetic
parameter (mean)
Fraction
absorbed transmucosally










* Based on venous blood samples (plasma). Fentanyl citrate concentrations obtained in serum were
higher than in plasma: Serum AUC and Cmax were approximately 20% and 30% higher than plasma
AUC and Cmax, respectively. The reason of this difference is unknown.
** Data for T
max
presented as median (range).
In pharmacokinetic studies that compared the absolute and relative bioavailability of Effentora and
oral transmucosal fentanyl citrate (OTFC), the rate and extent of fentanyl absorption in Effentora
demonstrated exposure that was between 30% to 50% greater than that for oral transmucosal fentanyl
citrate. If switching from another oral fentanyl citrate product, independent dose titration with
Effentora is required as bioavailability between products differs significantly. However, in these
patients, a starting dose higher than 100 micrograms may be considered.
Mean Plasma Concentration Versus Time
Profiles Following Singles Doses of
EFFENTORA
and OTFC in Healthy Subjects
400 mcg
EFFENTORA
OTFC (normalized to 400 mcg)
Time after Dose Administration (hours)
OTFC data was dose adjusted (800 mcg to 400 mcg)
Differences in exposure with Effentora were observed in a clinical study with patients with grade 1
mucositis. C
max
and AUC
0-8
were 1% and 25% higher in patients with mucositis compared to those
without mucositis, respectively. The differences observed were not clinically significant.
Distribution
Fentanyl is highly lipophilic and is well distributed beyond the vascular system, with a large apparent
volume of distribution. After buccal administration of Effentora, fentanyl undergoes initial rapid
distribution that represents an equilibration of fentanyl between plasma and the highly perfused tissues
(brain, heart and lungs). Subsequently, fentanyl is redistributed between the deep tissue compartment
(muscle and fat) and the plasma.
The plasma protein binding of fentanyl is 80% to 85%. The main binding protein is alpha-1-acid
glycoprotein, but both albumin and lipoproteins contribute to some extent. The free fraction of
fentanyl increases with acidosis.


Biotransformation
The metabolic pathways following buccal administration of Effentora have not been characterised in
clinical studies. Fentanyl is metabolised in the liver and in the intestinal mucosa to norfentanyl by
CYP3A4 isoform. Norfentanyl is not pharmacologically active in animal studies. More than 90% of
the administered dose of fentanyl is eliminated by biotransformation to N-dealkylated and
hydroxylated inactive metabolites.
Elimination
Following the intravenous administration of fentanyl, less than 7% of the administered dose is
excreted unchanged in the urine, and only about 1% is excreted unchanged in the faeces. The
metabolites are mainly excreted in the urine, while faecal excretion is less important.
Following the administration of Effentora, the terminal elimination phase of fentanyl is the result of
the redistribution between plasma and a deep tissue compartment. This phase of elimination is slow,
resulting in a median terminal elimination half-life t
1/2
of approximately 22 hours following buccal
administration of the effervescent formulation and approximately 18 hours following intravenous
administration. The total plasma clearance of fentanyl following intravenous administration is
approximately 42 L/h.
Linearity/non-linearity
Dose proportionality from 100 micrograms to 1000 micrograms has been demonstrated.
5.3
Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, genotoxicity and carcinogenicity.
Embryo-foetal developmental toxicity studies conducted in rats and rabbits revealed no compound-
induced malformations or developmental variations when administered during the period of
organogenesis.
In a fertility and early embryonic development study in rats, a male-mediated effect was observed at
high doses (300 mcg/kg/day, s.c.) and is consistent with the sedative effects of fentanyl in animal
studies.
In studies on pre and postnatal development in rats the survival rate of offspring was significantly
reduced at doses causing severe maternal toxicity. Further findings at maternally toxic doses in F1
pups were delayed physical development, sensory functions, reflexes and behaviour. These effects
could either be indirect effects due to altered maternal care and/or decreased lactation rate or a direct
effect of fentanyl on the pups.
Carcinogenicity studies (26-week dermal alternative bioassay in Tg.AC transgenic mice; two-year
subcutaneous carcinogenicity study in rats) did not induce any findings indicative of oncogenic
potential.
6.
PHARMACEUTICAL PARTICULARS
Mannitol
Sodium starch glycolate type A
Sodium hydrogen carbonate
Sodium carbonate anhydrous
Citric acid anhydrous
Magnesium stearate
6.4
Special precautions for storage
Store in the original package in order to protect from moisture.
6.5
Nature and contents of container
Aluminium laminated blister of PVC/Al foil/Polyamide/PVC with paper/polyester lidding.
Blister packs are supplied in cartons of 4 or 28 tablets. Not all pack-sizes may be marketed.
6.6
Special precautions for disposal
Patients and carers must be advised to dispose of any unopened tablets remaining from a prescription
as soon as they are no longer needed.
Any used or unused but no longer required product or waste material should be disposed of in
accordance with local requirements.
7.
MARKETING AUTHORISATION HOLDER
Cephalon Europe
5 rue Charles Martigny
F-94700 Maisons-Alfort
France
8.
MARKETING AUTHORISATION NUMBER(S)
9.
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10.
DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency
(EMEA) http://www.emea.europa.eu.
1.
NAME OF THE MEDICINAL PRODUCT
Effentora 200 micrograms buccal tablets
2.
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each buccal tablet contains 200 micrograms fentanyl (as citrate).
Excipient(s): Each tablet contains 16 mg of sodium.
For a full list of excipients, see section 6.1.
Flat-faced, white, round bevelled-edge tablet, embossed on one side with a “C” and on the other side
with “2”.
4.1
Therapeutic indications
Effentora is indicated for the treatment of breakthrough pain (BTP) in adults with cancer who are
already receiving maintenance opioid therapy for chronic cancer pain.
BTP is a transitory exacerbation of pain that occurs on a background of otherwise controlled persistent
pain
.
Patients receiving maintenance opioid therapy are those who are taking at least 60 mg of oral
morphine daily, at least 25 micrograms of transdermal fentanyl per hour, at least 30 mg of oxycodone
daily, at least 8 mg of oral hydromorphone daily or an equianalgesic dose of another opioid for a week
or longer.
4.2
Posology and method of administration
Treatment should be initiated by and remain under the guidance of a physician experienced in the
management of opioid therapy in cancer patients. Physicians should keep in mind the potential of
abuse of fentanyl. Patients should be instructed not to use two different formulations of fentanyl
concurrently for the treatment of breakthrough pain, and to dispose of any fentanyl product prescribed
for BTP when switching to Effentora. The number of tablet strengths available to the patients at any
time should be minimised to prevent confusion and potential overdose.
Effentora should be individually titrated to an “effective” dose that provides adequate analgesia and
minimises undesirable effects. In clinical studies, the effective dose of Effentora for BTP was not
predictable from the daily maintenance dose of opioid.
Patients should be carefully monitored until an effective dose is reached.
Titration in patients not switching from other fentanyl containing products
The initial dose of
Effentora should be 100 micrograms, titrating upwards as necessary through the
range of available tablets strengths (100, 200, 400, 600, 800 micrograms).
Titration in patients switching from other fentanyl containing products
Due to different absorption profiles, switching must not be done at a 1:1 ratio. If switching from
another oral fentanyl citrate product, independent dose titration with Effentora is required as
bioavailability between products differs significantly. However, in these patients, a starting dose
higher than 100 micrograms may be considered.
Method of titration
During titration, if adequate analgesia is not obtained within 30 minutes after the start of
administration of a single tablet, a second Effentora tablet of the same strength may be used.
If treatment of a BTP episode requires more than one tablet, an increase in dose to the next higher
available strength should be considered to treat the next BTP episode.
During titration, multiple tablets may be used: up to four 100 micrograms or up to four 200
micrograms tablets may be used to treat a single episode of BTP during dose titration according to the
following schedule:
•
If the initial 100 micrograms tablet is not efficacious, the patient can be instructed to treat the next
episode of BTP with two 100 micrograms tablets. It is recommended that one tablet should be
placed in each side of the mouth. If this dose is considered to be the effective dose, treatment of
successive episodes of BTP may be continued with a single 200 micrograms tablet of Effentora
.
•
If a single 200 micrograms tablet of Effentora (or two 100 micrograms tablets) is not considered to
be efficacious the patient can be instructed to use two 200 micrograms tablets (or four 100
micrograms tablets) to treat the next episode of BTP. It is recommended that two tablets should be
placed in each side of the mouth. If this dose is considered to be the effective dose, treatment of
successive episodes of BTP may be continued with a single 400 micrograms tablet of Effentora.
•
For titration to 600 micrograms and 800 micrograms, tablets of 200 micrograms should be used.
Doses above 800 micrograms were not evaluated in clinical studies.
No more than two tablets should be used to treat any individual BTP episode, except when titrating
using up to four tablets as described above.
Patients should wait at least 4 hours before treating another BTP episode with Effentora during
titration.
Once an effective dose has been established during titration, patients should continue to take this dose
as a single tablet of that given strength. Breakthrough pain episodes may vary in intensity and the
required Effentora dose might increase over time due to progression of the underlying cancer disease.
In these cases, a second tablet of the same strength may be used. If a second tablet of Effentora was
required for several consecutive times, the usual maintenance dose is to be readjusted (see below).
Patients should wait at least 4 hours before treating another BTP episode with Effentora during
maintenance therapy.
The maintenance dose of Effentora should be increased when a patient requires more than one tablet
per BTP episode for several consecutive BTP episodes. For dose-readjustment the same principles
apply as outlined for
dose titration
(see above).
Dose readjustment of the background opioid therapy may be required if patients consistently
present with more than four BTP episodes per 24 hours.
Discontinuation of therapy
Effentora should be immediately discontinued if no longer required.
Use in children and adolescents:
Effentora is not recommended for use in children and adolescents below 18 years due to a lack of data
on safety and efficacy.
Use in the elderly (older than 65 years):
In clinical studies patients older than 65 years tended to titrate to a lower effective dose than younger
patients. It is recommended that increased caution should be exercised in titrating the dose of
Effentora in elderly patients.
Hepatic or renal impairment:
Effentora should be administered with caution to patients with moderate or severe hepatic or renal
impairment (see section 4.4).
Patients with xerostomia:
Patients experiencing xerostomia are advised to drink water to moisten the buccal cavity prior to
administration of Effentora. If this recommendation does not result in an appropriate effervescence,
then a switch of therapy may be advised.
Method of administration:
Effentora tablet once exposed to moisture utilises an effervescent reaction to deliver the active
substance. Therefore patients should be instructed not to open the blister until ready to place the tablet
in the buccal cavity.
Opening the blister package
Patients should be instructed NOT to attempt to push the tablet through the blister because this could
damage the buccal tablet. The correct method of releasing the tablet from the blister is:
One of the blister units should be separated from the blister card by tearing it apart at the perforations.
The blister unit should then be flexed along the line printed on the backing foil where indicated. The
backing foil should be peeled back to expose the tablet.
Patients should be instructed not to attempt to crush or split the tablet.
The tablet should not be stored once removed from the blister package as the tablet integrity can not be
guaranteed and a risk of accidental exposure to a tablet can occur.
Tablet administration
Patients should remove the tablet from the blister unit and immediately place the entire Effentora
tablet in the buccal cavity (near a molar between the cheek and gum).
The Effentora tablet should not be sucked, chewed or swallowed, as this will result in lower plasma
concentrations than when taken as directed.
Effentora should be placed and retained within the buccal cavity for a period sufficient to allow
disintegration of the tablet which usually takes approximately 14-25 minutes.
Alternatively, the tablet could be placed sublingually (see section 5.2).
After 30 minutes, if remnants from the Effentora tablet remain, they may be swallowed with a glass of
water.
The length of time that the tablet takes to fully disintegrate following oromucosal administration does
not appear to affect early systemic exposure to fentanyl.
Patients should not consume any food and drink when a tablet is in the buccal cavity.
In case of buccal mucosa irritation, a change in tablet placement within the buccal cavity should be
recommended.
Hypersensitivity to the active substance or to any of the excipients.
Patients without maintenance opioid therapy (see section 4.1) as there is an increased risk of
respiratory depression.
Severe respiratory depression or severe obstructive lung conditions.
Treatment of acute pain other than breakthrough pain (e.g. postoperative pain, headache, migraine).
4.4
Special warnings and precautions for use
Patients and their carers must be instructed that Effentora contains an active substance in an amount
that can be fatal to a child, and therefore to keep all tablets out of the reach and sight
of children.
In order to minimise the risks of opioid-related undesirable effects and to identify the effective dose, it
is imperative that patients be monitored closely by health professionals during the titration process.
It is important that the long acting opioid treatment used to treat the patient’s persistent pain has been
stabilised before Effentora therapy begins and that the patient continues to be treated with the long
acting opioid treatment whilst taking Effentora.
As with all opioids, there is a risk of clinically significant respiratory depression associated with the
use of fentanyl. Particular caution should be used when titrating Effentora in patients with non-severe
chronic obstructive pulmonary disease or other medical conditions predisposing them to respiratory
depression, as even normally therapeutic doses of Effentora may further decrease respiratory drive to
the point of respiratory failure.
Effentora should only be administered with extreme caution in patients who may be particularly
susceptible to the intracranial effects of CO
2
retention, such as those with evidence of increased
intracranial pressure or impaired consciousness. Opioids may obscure the clinical course of a patient
with a head injury and should be used only if clinically warranted.
Intravenous fentanyl may produce bradycardia. In clinical trials with Effentora, no clear evidence for
bradycardia was observed. However, Effentora should be used with caution in patients with pre-
existing bradyarrhythmias.
In addition, Effentora should be administered with caution to patients with hepatic or renal
impairment. The influence of hepatic and renal impairment on the pharmacokinetics of the medicinal
product has not been evaluated, however, when administered intravenously the clearance of fentanyl
has been shown to be altered in hepatic and renal impairment due to alterations in metabolic clearance
and plasma proteins. After administration of Effentora, impaired hepatic and renal function may both
increase the bioavailability of swallowed fentanyl and decrease its systemic clearance, which could
lead to increased and prolonged opioid effects. Therefore, special care should be taken during the
titration process in patients with moderate or severe hepatic or renal impairment.
Careful consideration should be given to patients with hypovolaemia and hypotension.
Tolerance and physical and/or psychological dependence may develop upon repeated administration
of opioids such as fentanyl. However, iatrogenic addiction following therapeutic use of opioids is
rare.
This medicinal product contains 16 mg sodium per tablet. To be taken into consideration by patients
on a controlled sodium diet.
4.5
Interaction with other medicinal products and other forms of interaction
Fentanyl is metabolised mainly via the human cytochrome P450 3A4 isoenzyme system (CYP3A4),
therefore potential interactions may occur when Effentora is given concurrently with agents that affect
CYP3A4 activity. Coadministration with agents that induce 3A4 activity may reduce the efficacy of
Effentora. The concomitant use of Effentora with strong CYP3A4 inhibitors (e.g., ritonavir,
ketoconazole, itraconazole, troleandomycin, clarithromycin, and nelfinavir) or moderate CYP3A4
inhibitors (e.g., amprenavir, aprepitant, diltiazem, erythromycin, fluconazole, fosamprenavir,
grapefruit juice, and verapamil) may result in increased fentanyl plasma concentrations, potentially
causing serious adverse drug reactions including fatal respiratory depression. Patients receiving
Effentora concomitantly with moderate or strong CYP3A4 inhibitors should be carefully monitored
for an extended period of time. Dosage increase should be done with caution.
The concomitant use of other central nervous system depressants, including other opioids, sedatives or
hypnotics, general anaesthetics, phenothiazines, tranquillisers, skeletal muscle relaxants, sedating
antihistamines and alcohol may produce additive depressant effects.
Effentora is not recommended for use in patients who have received monoamine oxidase (MAO)
inhibitors within 14 days because severe and unpredictable potentiation by MAO inhibitors has been
reported with opioid analgesics.
The concomitant use of partial opioid agonists/antagonists (e.g. buprenorphine, nalbuphine,
pentazocine) is not recommended. They have high affinity to opioid receptors with relatively low
intrinsic activity and therefore partially antagonise the analgesic effect of fentanyl and may induce
withdrawal symptoms in opioid dependant patients.
4.6
Pregnancy and lactation
There are no adequate data from the use of fentanyl in pregnant women. Studies in animals have
shown reproductive toxicity (see section 5.3). The potential risk for humans is unknown. Effentora
should not be used in pregnancy unless clearly necessary.
Following long-term treatment, fentanyl may cause withdrawal in the new-born infant.
It is advised not to use fentanyl during labour and delivery (including caesarean section) because
fentanyl passes through the placenta and may cause respiratory depression in the foetus. If Effentora is
administered, an antidote for the child should be readily available.
Fentanyl passes into breast milk and may cause sedation and respiratory depression in the breast-fed
child. Fentanyl should not be used by breastfeeding women and breastfeeding should not be restarted
until at least 48 hours after the last administration of fentanyl.
4.7
Effects on ability to drive and use machines
No studies of the effects on the ability to drive and use machines have been performed. However,
opioid analgesics impair the mental and/or physical ability required for the performance of potentially
dangerous tasks (e.g., driving a car or operating machinery). Patients should be advised not to drive or
operate machinery if they experience somnolence, dizziness, or visual disturbance while taking
Effentora and not to drive or operate machinery until they know how they react.
Typical opioid undesirable effects are to be expected with Effentora. Frequently, these will cease or
decrease in intensity with continued use of the medicinal product, as the patient is titrated to the most
appropriate dose. However, the most serious adverse reactions are respiratory depression (potentially
leading to apnoea or respiratory arrest), circulatory depression, hypotension and shock and all patients
should be closely monitored for these.
The clinical studies of Effentora were designed to evaluate safety and efficacy in treating BTP and all
patients were also taking concomitant opioids, such as sustained-release morphine or transdermal
fentanyl, for their persistent pain. Therefore it is not possible to definitively separate the effects of
Effentora alone.
The following adverse reactions have been reported with Effentora during clinical studies and post
marketing experience. Adverse reactions are listed below as MedDRA preferred term by system organ
class and frequency (frequencies are defined as: very common ≥1/10, common ≥1/100 to <1/10,
uncommon ≥ 1/1,000 to <1/100, rare (≥1/10,000 to <1/1,000), not known (cannot be estimated from
the available data); within each frequency group, undesirable effects are presented in order of
decreasing seriousness:
Platelet count
decreased
Heart rate
increased
Haematocrit
decreased
Haemoglobin
decreased
Blood and
lymphatic
system
disorders
Dysgeusia
Somnolence
Lethargy
Tremor
Sedation
Hypoaesthesia
Migraine
Depressed
level of
consciousness
Disturbance in
attention
Balance
disorder
Dysarthria
Cognitive
disorder
Motor
dysfunction
Visual
disturbance
Ocular
hyperaemia
Blurred vision
Visual acuity
reduced
Abnormal
sensation in
eye
Photopsia
Ear and
labyrinth
disorders
Vertigo
Tinnitus
Ear discomfort














Respiratory,
thoracic and
mediastinal
disorders
Dyspnoea
Pharyngolaryn-
geal pain
Respiratory
depression
Sleep apnoea
syndrome
Gastro-
intestinal
disorders
Constipation
Stomatitis
Dry mouth
Diarrhoea
Abdominal pain
Gastro-
oesophageal
reflux disease
Stomach
discomfort
Dyspepsia
Toothache
Ileus
Mouth
ulceration
Oral
hypoaesthesia
Oral
discomfort
Oral mucosal
discolouration
Oral soft tissue
disorder
Glossodynia
Tongue
blistering
Gingival pain
Tongue
ulceration
Tongue
disorder
Oesophagitis
Chapped lips
Tooth disorder
Oral mucosal
blistering
Dry lip
Renal and
urinary
disorders
Skin and
subcutaneous
tissue
disorders
Pruritus
Hyperhidrosis
Rash
Cold sweat
Facial swelling
Generalised
pruritus
Alopecia
Musculoskel
etal and
connective
tissue
disorders
Muscle
twitching
Muscular
weakness
Metabolism
and nutrition
disorders
Infections and
infestations
Oral candidiasis Pharyngitis
Injury,
poisoning and
procedural
complications


















General
disorders and
administration
site conditions
Application
site reactions
including
bleeding,
pain,
ulcer,
irritation,
paraesthesia,
anaesthesia,
erythema,
oedema,
swelling and
vesicles
Peripheral
oedema
Fatigue
Asthenia
Drug
withdrawal
syndrome
Chills
Malaise
Sluggishness
Chest
discomfort
Feeling
abnormal
Feeling jittery
Thirst
Feeling cold
Feeling hot
Depression
Anxiety
Confusional
state
Insomnia
Euphoric
mood
Nervousness
Hallucination
Visual
hallucination
Mental status
changes
Drug
dependence
(addiction)
Disorientation
Tolerance, physical and/or psychological dependence may develop upon repeated administration of
opioids such as fentanyl.
Opioid withdrawal symptoms such as nausea, vomiting, diarrhoea, anxiety and shivering have been
observed in studies with Effentora.
Loss of consciousness and respiratory arrest have been observed in the context of overdose.
The symptoms of fentanyl overdose are expected to be similar in nature to those of intravenous
fentanyl and other opioids, and are an extension of its pharmacological actions, with the most serious
significant effects being altered mental status, loss of consciousness, hypotension, respiratory
depression, respiratory distress, and respiratory failure, which have resulted in death.
Immediate management of opioid overdose includes removal of the Effentora buccal tablet, if still in
the mouth, ensuring a patent airway, physical and verbal stimulation of the patient, assessment of the
level of consciousness, ventilatory and circulatory status, and assisted ventilation (ventilatory support)
if necessary.
For treatment of overdose (accidental ingestion) in the opioid-naive person, intravenous access should
be obtained and naloxone or other opioid antagonists should be employed as clinically indicated. The
duration of respiratory depression following overdose may be longer than the effects of the opioid
antagonist’s action (e.g., the half-life of naloxone ranges from 30 to 81 minutes) and repeated
administration may be necessary. Consult the Summary of Product Characteristics of the individual
opioid antagonist for details about such use.
For treatment of overdose in opioid-maintained patients, intravenous access should be obtained. The
judicious use of naloxone or another opioid antagonist may be warranted in some instances, but it is
associated with the risk of precipitating an acute withdrawal syndrome.











Although muscle rigidity interfering with respiration has not been seen following the use of Effentora,
this is possible with fentanyl and other opioids. If it occurs, it should be managed by the use of
assisted ventilation, by an opioid antagonist, and as a final alternative, by a neuromuscular blocking
agent.
5.
PHARMACOLOGICAL PROPERTIES
5.1
Pharmacodynamic properties
Pharmacotherapeutic group: analgesics; opioids; phenylpiperidine derivatives; ATC code N02AB03.
Fentanyl is an opioid analgesic, interacting predominantly with the opioid µ-receptor. Its primary
therapeutic actions are analgesia and sedation. Secondary pharmacological effects are respiratory
depression, bradycardia, hypothermia, constipation, miosis, physical dependence and euphoria.
The analgesic effects of fentanyl are related to its plasma level. In general, the effective concentration
and the concentration at which toxicity occurs increase with increasing tolerance to opioids. The rate
of development of tolerance varies widely among individuals. As a result, the dose of Effentora should
be individually titrated to achieve the desired effect (see section 4.2).
All opioid µ-receptor agonists, including fentanyl, produce dose dependent respiratory depression. The
risk of respiratory depression is less in patients receiving chronic opioid therapy as these patients will
develop tolerance to respiratory depressant effects.
The safety and efficacy of Effentora have been evaluated in patients taking the drug at the onset of the
breakthrough pain episode. Pre-emptive use of Effentora for predictable pain episodes was not
investigated in the clinical trials. Two double-blind, randomized, placebo-controlled crossover studies
have been conducted involving a total of 248 patients with BTP and cancer who experienced on
average 1 to 4 episodes of BTP per day while taking maintenance opioid therapy. During an initial
open-label phase, patients were titrated to an effective dose of Effentora. Patients who identified an
effective dose entered the double-blind phase of the study. The primary efficacy variable was the
patient’s assessment of pain intensity. Patients assessed pain intensity on a 11-point scale. For each
BTP episode, pain intensity was assessed prior to and at several time points after treatment.
Sixty-seven percent of the patients were able to be titrated to an effective dose.
In the pivotal clinical study (study 1), the primary endpoint was the average sum of differences in pain
intensity scores from dosing to 60 minutes, inclusive (SPID60), which was statistically significant
compared to placebo (p<0.0001).
Study 1: Mean (+/- SEM) Pain Intensity Difference at Each Time Point (Full Analysis Set)
SPID60 mean (+/- SD)
EFFENTORA=9.7(5.58) p<0.0001
Placebo=4.9(4.38)
Time from Administration of Study Drug (Minutes)
Treatment Group
±±±
EFFENTORA
Placebo
+ p<0.0001 E
FFENTORA
versus placebo, in favor of E
FFENTORA
, by an analysis of variance
PID=pain intensity difference; SEM=standard error of the mean
Study 2: Mean (+/- SEM) Pain Intensity Difference at Each Time Point (Full Analysis Set)
SPID30 mean (+/- SD)
EFFENTORA=3.2(2.60) p<0.0001
Placebo=2.0(2.21)
Time from Administration of Study Drug (Minutes)
Treatment Group
±±±
EFFENTORA
Placebo
* p<0.01 E
FFENTORA
versus placebo, in favor of E
FFENTORA
, by one-sample Wilcoxon signed rank test
+ p<0.0001 E
FFENTORA
versus placebo, in favor of E
FFENTORA
, by one-sample Wilcoxon signed rank test
PID=pain intensity difference; SEM=standard error of the mean
In the second pivotal study (study 2), the primary endpoint was SPID30, which was also statistically
significant compared to placebo (p<0.0001).
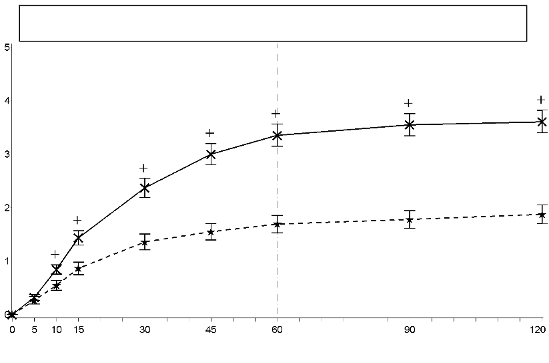

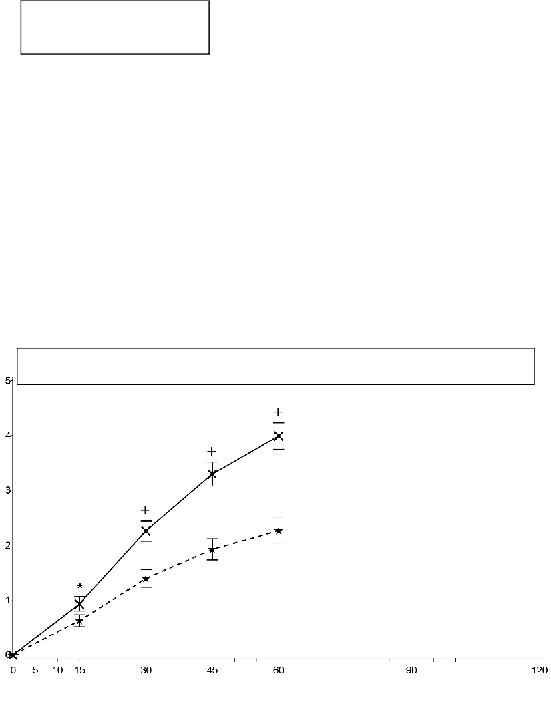


Statistically significant improvement in pain intensity difference was seen with Effentora versus
placebo as early as 10 minutes in Study 1 and as early as 15 minutes (earliest time point measured) in
Study 2. These differences continued to be significant at each subsequent time point in each
individual study.
5.2
Pharmacokinetic properties
General introduction
Fentanyl is highly lipophilic and can be absorbed very rapidly through the oral mucosa and more
slowly by the conventional gastrointestinal route. It is subject to first-pass hepatic and intestinal
metabolism and the metabolites do not contribute to fentanyl’s therapeutic effects.
Effentora employs a delivery technology which utilises an effervescent reaction which enhances the
rate and extent of fentanyl absorbed through the buccal mucosa. Transient pH changes accompanying
the effervescent reaction may optimise dissolution (at a lower pH) and membrane permeation (at a
higher pH).
Dwell time (defined as the length of time that the tablet takes to fully disintegrate following buccal
administration), does not affect early systemic exposure to fentanyl. A comparison study between one
400 mcg Effentora tablet administered either buccally (i.e., between the cheek and the gum) or
sublingually met the criteria of bioequivalence.
The effect of renal or hepatic impairment on the pharmacokinetics of Effentora has not been studied.
Absorption:
Following oromucosal administration of Effentora, fentanyl is readily absorbed with an absolute
bioavailability of 65%. The absorption profile of Effentora is largely the result of an initial rapid
absorption from the buccal mucosa, with peak plasma concentrations following venous sampling
generally attained within an hour after oromucosal administration. Approximately 50% of the total
dose administered is rapidly absorbed transmucosally and becomes systemically available. The
remaining half of the total dose is swallowed and slowly absorbed from the gastrointestinal tract.
About 30% of the amount swallowed (50% of the total dose) escapes hepatic and intestinal first-pass
elimination and becomes systemically available.
The main pharmacokinetic parameters are shown in the following table.
Pharmacokinetic Parameters* in Adult Subjects Receiving Effentora
Pharmacokinetic
parameter (mean)
Fraction
absorbed transmucosally










* Based on venous blood samples (plasma). Fentanyl citrate concentrations obtained in serum were
higher than in plasma: Serum AUC and Cmax were approximately 20% and 30% higher than plasma
AUC and Cmax, respectively. The reason of this difference is unknown.
** Data for T
max
presented as median (range).
In pharmacokinetic studies that compared the absolute and relative bioavailability of Effentora and
oral transmucosal fentanyl citrate (OTFC), the rate and extent of fentanyl absorption in Effentora
demonstrated exposure that was between 30% to 50% greater than that for oral transmucosal fentanyl
citrate. If switching from another oral fentanyl citrate product, independent dose titration with
Effentora is required as bioavailability between products differs significantly. However, in these
patients, a starting dose higher than 100 micrograms may be considered.
Mean Plasma Concentration Versus Time
Profiles Following Singles Doses of
EFFENTORA
and OTFC in Healthy Subjects
400 mcg
EFFENTORA
OTFC (normalized to 400 mcg)
Time after Dose Administration (hours)
OTFC data was dose adjusted (800 mcg to 400 mcg)
Differences in exposure with Effentora were observed in a clinical study with patients with grade 1
mucositis. C
max
and AUC
0-8
were 1% and 25% higher in patients with mucositis compared to those
without mucositis, respectively. The differences observed were not clinically significant.
Distribution
Fentanyl is highly lipophilic and is well distributed beyond the vascular system, with a large apparent
volume of distribution. After buccal administration of Effentora, fentanyl undergoes initial rapid
distribution that represents an equilibration of fentanyl between plasma and the highly perfused tissues
(brain, heart and lungs). Subsequently, fentanyl is redistributed between the deep tissue compartment
(muscle and fat) and the plasma.
The plasma protein binding of fentanyl is 80% to 85%. The main binding protein is alpha-1-acid
glycoprotein, but both albumin and lipoproteins contribute to some extent. The free fraction of
fentanyl increases with acidosis.


Biotransformation
The metabolic pathways following buccal administration of Effentora have not been characterised in
clinical studies. Fentanyl is metabolised in the liver and in the intestinal mucosa to norfentanyl by
CYP3A4 isoform. Norfentanyl is not pharmacologically active in animal studies. More than 90% of
the administered dose of fentanyl is eliminated by biotransformation to N-dealkylated and
hydroxylated inactive metabolites.
Elimination
Following the intravenous administration of fentanyl, less than 7% of the administered dose is
excreted unchanged in the urine, and only about 1% is excreted unchanged in the faeces. The
metabolites are mainly excreted in the urine, while faecal excretion is less important.
Following the administration of Effentora, the terminal elimination phase of fentanyl is the result of
the redistribution between plasma and a deep tissue compartment. This phase of elimination is slow,
resulting in a median terminal elimination half-life t
1/2
of approximately 22 hours following buccal
administration of the effervescent formulation and approximately 18 hours following intravenous
administration. The total plasma clearance of fentanyl following intravenous administration is
approximately 42 L/h.
Linearity/non-linearity
Dose proportionality from 100 micrograms to 1000 micrograms has been demonstrated.
5.3
Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, genotoxicity and carcinogenicity.
Embryo-foetal developmental toxicity studies conducted in rats and rabbits revealed no compound-
induced malformations or developmental variations when administered during the period of
organogenesis.
In a fertility and early embryonic development study in rats, a male-mediated effect was observed at
high doses (300 mcg/kg/day, s.c.) and is consistent with the sedative effects of fentanyl in animal
studies.
In studies on pre and postnatal development in rats the survival rate of offspring was significantly
reduced at doses causing severe maternal toxicity. Further findings at maternally toxic doses in F1
pups were delayed physical development, sensory functions, reflexes and behaviour. These effects
could either be indirect effects due to altered maternal care and/or decreased lactation rate or a direct
effect of fentanyl on the pups.
Carcinogenicity studies (26-week dermal alternative bioassay in Tg.AC transgenic mice; two-year
subcutaneous carcinogenicity study in rats) did not induce any findings indicative of oncogenic
potential.
6.
PHARMACEUTICAL PARTICULARS
Mannitol
Sodium starch glycolate type A
Sodium hydrogen carbonate
Sodium carbonate anhydrous
Citric acid anhydrous
Magnesium stearate
6.4
Special precautions for storage
Store in the original package in order to protect from moisture.
6.5
Nature and contents of container
Aluminium laminated blister of PVC/Al foil/Polyamide/PVC with paper/polyester lidding.
Blister packs are supplied in cartons of 4 or 28 tablets. Not all pack-sizes may be marketed.
6.6
Special precautions for disposal
Patients and carers must be advised to dispose of any unopened tablets remaining from a prescription
as soon as they are no longer needed.
Any used or unused but no longer required product or waste material should be disposed of in
accordance with local requirements.
7.
MARKETING AUTHORISATION HOLDER
Cephalon Europe
5 rue Charles Martigny
F-94700 Maisons-Alfort
France
8.
MARKETING AUTHORISATION NUMBER(S)
9.
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10.
DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency
(EMEA) http://www.emea.europa.eu.
1.
NAME OF THE MEDICINAL PRODUCT
Effentora 400 micrograms buccal tablets
2.
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each buccal tablet contains 400 micrograms fentanyl (as citrate).
Excipient(s): Each tablet contains 16 mg of sodium.
For a full list of excipients, see section 6.1.
Flat-faced, white, round bevelled-edge tablet, embossed on one side with a “C” and on the other side
with “4”.
4.1
Therapeutic indications
Effentora is indicated for the treatment of breakthrough pain (BTP) in adults with cancer who are
already receiving maintenance opioid therapy for chronic cancer pain.
BTP is a transitory exacerbation of pain that occurs on a background of otherwise controlled persistent
pain
.
Patients receiving maintenance opioid therapy are those who are taking at least 60 mg of oral
morphine daily, at least 25 micrograms of transdermal fentanyl per hour, at least 30 mg of oxycodone
daily, at least 8 mg of oral hydromorphone daily or an equianalgesic dose of another opioid for a week
or longer.
4.2
Posology and method of administration
Treatment should be initiated by and remain under the guidance of a physician experienced in the
management of opioid therapy in cancer patients. Physicians should keep in mind the potential of
abuse of fentanyl. Patients should be instructed not to use two different formulations of fentanyl
concurrently for the treatment of breakthrough pain, and to dispose of any fentanyl product prescribed
for BTP when switching to Effentora. The number of tablet strengths available to the patients at any
time should be minimised to prevent confusion and potential overdose.
Effentora should be individually titrated to an “effective” dose that provides adequate analgesia and
minimises undesirable effects. In clinical studies, the effective dose of Effentora for BTP was not
predictable from the daily maintenance dose of opioid.
Patients should be carefully monitored until an effective dose is reached.
Titration in patients not switching from other fentanyl containing products
The initial dose of
Effentora should be 100 micrograms, titrating upwards as necessary through the
range of available tablets strengths (100, 200, 400, 600, 800 micrograms).
Titration in patients switching from other fentanyl containing products
Due to different absorption profiles, switching must not be done at a 1:1 ratio. If switching from
another oral fentanyl citrate product, independent dose titration with Effentora is required as
bioavailability between products differs significantly. However, in these patients, a starting dose
higher than 100 micrograms may be considered.
Method of titration
During titration, if adequate analgesia is not obtained within 30 minutes after the start of
administration of a single tablet, a second Effentora tablet of the same strength may be used.
If treatment of a BTP episode requires more than one tablet, an increase in dose to the next higher
available strength should be considered to treat the next BTP episode.
During titration, multiple tablets may be used: up to four 100 micrograms or up to four 200
micrograms tablets may be used to treat a single episode of BTP during dose titration according to the
following schedule:
•
If the initial 100 micrograms tablet is not efficacious, the patient can be instructed to treat the next
episode of BTP with two 100 micrograms tablets. It is recommended that one tablet should be
placed in each side of the mouth. If this dose is considered to be the effective dose, treatment of
successive episodes of BTP may be continued with a single 200 micrograms tablet of Effentora
.
•
If a single 200 micrograms tablet of Effentora (or two 100 micrograms tablets) is not considered to
be efficacious the patient can be instructed to use two 200 micrograms tablets (or four 100
micrograms tablets) to treat the next episode of BTP. It is recommended that two tablets should be
placed in each side of the mouth. If this dose is considered to be the effective dose, treatment of
successive episodes of BTP may be continued with a single 400 micrograms tablet of Effentora.
•
For titration to 600 micrograms and 800 micrograms, tablets of 200 micrograms should be used.
Doses above 800 micrograms were not evaluated in clinical studies.
No more than two tablets should be used to treat any individual BTP episode, except when titrating
using up to four tablets as described above.
Patients should wait at least 4 hours before treating another BTP episode with Effentora during
titration.
Once an effective dose has been established during titration, patients should continue to take this dose
as a single tablet of that given strength. Breakthrough pain episodes may vary in intensity and the
required Effentora dose might increase over time due to progression of the underlying cancer disease.
In these cases, a second tablet of the same strength may be used. If a second tablet of Effentora was
required for several consecutive times, the usual maintenance dose is to be readjusted (see below).
Patients should wait at least 4 hours before treating another BTP episode with Effentora during
maintenance therapy.
The maintenance dose of Effentora should be increased when a patient requires more than one tablet
per BTP episode for several consecutive BTP episodes. For dose-readjustment the same principles
apply as outlined for
dose titration
(see above).
Dose readjustment of the background opioid therapy may be required if patients consistently
present with more than four BTP episodes per 24 hours.
Discontinuation of therapy
Effentora should be immediately discontinued if no longer required.
Use in children and adolescents:
Effentora is not recommended for use in children and adolescents below 18 years due to a lack of data
on safety and efficacy.
Use in the elderly (older than 65 years):
In clinical studies patients older than 65 years tended to titrate to a lower effective dose than younger
patients. It is recommended that increased caution should be exercised in titrating the dose of
Effentora in elderly patients.
Hepatic or renal impairment:
Effentora should be administered with caution to patients with moderate or severe hepatic or renal
impairment (see section 4.4).
Patients with xerostomia:
Patients experiencing xerostomia are advised to drink water to moisten the buccal cavity prior to
administration of Effentora. If this recommendation does not result in an appropriate effervescence,
then a switch of therapy may be advised.
Method of administration:
Effentora tablet once exposed to moisture utilises an effervescent reaction to deliver the active
substance. Therefore patients should be instructed not to open the blister until ready to place the tablet
in the buccal cavity.
Opening the blister package
Patients should be instructed NOT to attempt to push the tablet through the blister because this could
damage the buccal tablet. The correct method of releasing the tablet from the blister is:
One of the blister units should be separated from the blister card by tearing it apart at the perforations.
The blister unit should then be flexed along the line printed on the backing foil where indicated. The
backing foil should be peeled back to expose the tablet.
Patients should be instructed not to attempt to crush or split the tablet.
The tablet should not be stored once removed from the blister package as the tablet integrity can not be
guaranteed and a risk of accidental exposure to a tablet can occur.
Tablet administration
Patients should remove the tablet from the blister unit and immediately place the entire Effentora
tablet in the buccal cavity (near a molar between the cheek and gum).
The Effentora tablet should not be sucked, chewed or swallowed, as this will result in lower plasma
concentrations than when taken as directed.
Effentora should be placed and retained within the buccal cavity for a period sufficient to allow
disintegration of the tablet which usually takes approximately 14-25 minutes.
Alternatively, the tablet could be placed sublingually (see section 5.2).
After 30 minutes, if remnants from the Effentora tablet remain, they may be swallowed with a glass of
water.
The length of time that the tablet takes to fully disintegrate following oromucosal administration does
not appear to affect early systemic exposure to fentanyl.
Patients should not consume any food and drink when a tablet is in the buccal cavity.
In case of buccal mucosa irritation, a change in tablet placement within the buccal cavity should be
recommended.
Hypersensitivity to the active substance or to any of the excipients.
Patients without maintenance opioid therapy (see section 4.1) as there is an increased risk of
respiratory depression.
Severe respiratory depression or severe obstructive lung conditions.
Treatment of acute pain other than breakthrough pain (e.g. postoperative pain, headache, migraine).
4.4
Special warnings and precautions for use
Patients and their carers must be instructed that Effentora contains an active substance in an amount
that can be fatal to a child, and therefore to keep all tablets out of the reach and sight
of children.
In order to minimise the risks of opioid-related undesirable effects and to identify the effective dose, it
is imperative that patients be monitored closely by health professionals during the titration process.
It is important that the long acting opioid treatment used to treat the patient’s persistent pain has been
stabilised before Effentora therapy begins and that the patient continues to be treated with the long
acting opioid treatment whilst taking Effentora.
As with all opioids, there is a risk of clinically significant respiratory depression associated with the
use of fentanyl. Particular caution should be used when titrating Effentora in patients with non-severe
chronic obstructive pulmonary disease or other medical conditions predisposing them to respiratory
depression, as even normally therapeutic doses of Effentora may further decrease respiratory drive to
the point of respiratory failure.
Effentora should only be administered with extreme caution in patients who may be particularly
susceptible to the intracranial effects of CO
2
retention, such as those with evidence of increased
intracranial pressure or impaired consciousness. Opioids may obscure the clinical course of a patient
with a head injury and should be used only if clinically warranted.
Intravenous fentanyl may produce bradycardia. In clinical trials with Effentora, no clear evidence for
bradycardia was observed. However, Effentora should be used with caution in patients with pre-
existing bradyarrhythmias.
In addition, Effentora should be administered with caution to patients with hepatic or renal
impairment. The influence of hepatic and renal impairment on the pharmacokinetics of the medicinal
product has not been evaluated, however, when administered intravenously the clearance of fentanyl
has been shown to be altered in hepatic and renal impairment due to alterations in metabolic clearance
and plasma proteins. After administration of Effentora, impaired hepatic and renal function may both
increase the bioavailability of swallowed fentanyl and decrease its systemic clearance, which could
lead to increased and prolonged opioid effects. Therefore, special care should be taken during the
titration process in patients with moderate or severe hepatic or renal impairment.
Careful consideration should be given to patients with hypovolaemia and hypotension.
Tolerance and physical and/or psychological dependence may develop upon repeated administration
of opioids such as fentanyl. However, iatrogenic addiction following therapeutic use of opioids is
rare.
This medicinal product contains 16 mg sodium per tablet. To be taken into consideration by patients
on a controlled sodium diet.
4.5
Interaction with other medicinal products and other forms of interaction
Fentanyl is metabolised mainly via the human cytochrome P450 3A4 isoenzyme system (CYP3A4),
therefore potential interactions may occur when Effentora is given concurrently with agents that affect
CYP3A4 activity. Coadministration with agents that induce 3A4 activity may reduce the efficacy of
Effentora. The concomitant use of Effentora with strong CYP3A4 inhibitors (e.g., ritonavir,
ketoconazole, itraconazole, troleandomycin, clarithromycin, and nelfinavir) or moderate CYP3A4
inhibitors (e.g., amprenavir, aprepitant, diltiazem, erythromycin, fluconazole, fosamprenavir,
grapefruit juice, and verapamil) may result in increased fentanyl plasma concentrations, potentially
causing serious adverse drug reactions including fatal respiratory depression. Patients receiving
Effentora concomitantly with moderate or strong CYP3A4 inhibitors should be carefully monitored
for an extended period of time. Dosage increase should be done with caution.
The concomitant use of other central nervous system depressants, including other opioids, sedatives or
hypnotics, general anaesthetics, phenothiazines, tranquillisers, skeletal muscle relaxants, sedating
antihistamines and alcohol may produce additive depressant effects.
Effentora is not recommended for use in patients who have received monoamine oxidase (MAO)
inhibitors within 14 days because severe and unpredictable potentiation by MAO inhibitors has been
reported with opioid analgesics.
The concomitant use of partial opioid agonists/antagonists (e.g. buprenorphine, nalbuphine,
pentazocine) is not recommended. They have high affinity to opioid receptors with relatively low
intrinsic activity and therefore partially antagonise the analgesic effect of fentanyl and may induce
withdrawal symptoms in opioid dependant patients.
4.6
Pregnancy and lactation
There are no adequate data from the use of fentanyl in pregnant women. Studies in animals have
shown reproductive toxicity (see section 5.3). The potential risk for humans is unknown. Effentora
should not be used in pregnancy unless clearly necessary.
Following long-term treatment, fentanyl may cause withdrawal in the new-born infant.
It is advised not to use fentanyl during labour and delivery (including caesarean section) because
fentanyl passes through the placenta and may cause respiratory depression in the foetus. If Effentora is
administered, an antidote for the child should be readily available.
Fentanyl passes into breast milk and may cause sedation and respiratory depression in the breast-fed
child. Fentanyl should not be used by breastfeeding women and breastfeeding should not be restarted
until at least 48 hours after the last administration of fentanyl.
4.7
Effects on ability to drive and use machines
No studies of the effects on the ability to drive and use machines have been performed. However,
opioid analgesics impair the mental and/or physical ability required for the performance of potentially
dangerous tasks (e.g., driving a car or operating machinery). Patients should be advised not to drive or
operate machinery if they experience somnolence, dizziness, or visual disturbance while taking
Effentora and not to drive or operate machinery until they know how they react.
Typical opioid undesirable effects are to be expected with Effentora. Frequently, these will cease or
decrease in intensity with continued use of the medicinal product, as the patient is titrated to the most
appropriate dose. However, the most serious adverse reactions are respiratory depression (potentially
leading to apnoea or respiratory arrest), circulatory depression, hypotension and shock and all patients
should be closely monitored for these.
The clinical studies of Effentora were designed to evaluate safety and efficacy in treating BTP and all
patients were also taking concomitant opioids, such as sustained-release morphine or transdermal
fentanyl, for their persistent pain. Therefore it is not possible to definitively separate the effects of
Effentora alone.
The following adverse reactions have been reported with Effentora during clinical studies and post
marketing experience. Adverse reactions are listed below as MedDRA preferred term by system organ
class and frequency (frequencies are defined as: very common ≥1/10, common ≥1/100 to <1/10,
uncommon ≥ 1/1,000 to <1/100, rare (≥1/10,000 to <1/1,000), not known (cannot be estimated from
the available data); within each frequency group, undesirable effects are presented in order of
decreasing seriousness:
Platelet count
decreased
Heart rate
increased
Haematocrit
decreased
Haemoglobin
decreased
Blood and
lymphatic
system
disorders
Dysgeusia
Somnolence
Lethargy
Tremor
Sedation
Hypoaesthesia
Migraine
Depressed
level of
consciousness
Disturbance in
attention
Balance
disorder
Dysarthria
Cognitive
disorder
Motor
dysfunction
Visual
disturbance
Ocular
hyperaemia
Blurred vision
Visual acuity
reduced
Abnormal
sensation in
eye
Photopsia
Ear and
labyrinth
disorders
Vertigo
Tinnitus
Ear discomfort














Respiratory,
thoracic and
mediastinal
disorders
Dyspnoea
Pharyngolaryn-
geal pain
Respiratory
depression
Sleep apnoea
syndrome
Gastro-
intestinal
disorders
Constipation
Stomatitis
Dry mouth
Diarrhoea
Abdominal pain
Gastro-
oesophageal
reflux disease
Stomach
discomfort
Dyspepsia
Toothache
Ileus
Mouth
ulceration
Oral
hypoaesthesia
Oral
discomfort
Oral mucosal
discolouration
Oral soft tissue
disorder
Glossodynia
Tongue
blistering
Gingival pain
Tongue
ulceration
Tongue
disorder
Oesophagitis
Chapped lips
Tooth disorder
Oral mucosal
blistering
Dry lip
Renal and
urinary
disorders
Skin and
subcutaneous
tissue
disorders
Pruritus
Hyperhidrosis
Rash
Cold sweat
Facial swelling
Generalised
pruritus
Alopecia
Musculoskel
etal and
connective
tissue
disorders
Muscle
twitching
Muscular
weakness
Metabolism
and nutrition
disorders
Infections and
infestations
Oral candidiasis Pharyngitis
Injury,
poisoning and
procedural
complications


















General
disorders and
administration
site conditions
Application
site reactions
including
bleeding,
pain,
ulcer,
irritation,
paraesthesia,
anaesthesia,
erythema,
oedema,
swelling and
vesicles
Peripheral
oedema
Fatigue
Asthenia
Drug
withdrawal
syndrome
Chills
Malaise
Sluggishness
Chest
discomfort
Feeling
abnormal
Feeling jittery
Thirst
Feeling cold
Feeling hot
Depression
Anxiety
Confusional
state
Insomnia
Euphoric
mood
Nervousness
Hallucination
Visual
hallucination
Mental status
changes
Drug
dependence
(addiction)
Disorientation
Tolerance, physical and/or psychological dependence may develop upon repeated administration of
opioids such as fentanyl.
Opioid withdrawal symptoms such as nausea, vomiting, diarrhoea, anxiety and shivering have been
observed in studies with Effentora.
Loss of consciousness and respiratory arrest have been observed in the context of overdose.
The symptoms of fentanyl overdose are expected to be similar in nature to those of intravenous
fentanyl and other opioids, and are an extension of its pharmacological actions, with the most serious
significant effects being altered mental status, loss of consciousness, hypotension, respiratory
depression, respiratory distress, and respiratory failure, which have resulted in death.
Immediate management of opioid overdose includes removal of the Effentora buccal tablet, if still in
the mouth, ensuring a patent airway, physical and verbal stimulation of the patient, assessment of the
level of consciousness, ventilatory and circulatory status, and assisted ventilation (ventilatory support)
if necessary.
For treatment of overdose (accidental ingestion) in the opioid-naive person, intravenous access should
be obtained and naloxone or other opioid antagonists should be employed as clinically indicated. The
duration of respiratory depression following overdose may be longer than the effects of the opioid
antagonist’s action (e.g., the half-life of naloxone ranges from 30 to 81 minutes) and repeated
administration may be necessary. Consult the Summary of Product Characteristics of the individual
opioid antagonist for details about such use.
For treatment of overdose in opioid-maintained patients, intravenous access should be obtained. The
judicious use of naloxone or another opioid antagonist may be warranted in some instances, but it is
associated with the risk of precipitating an acute withdrawal syndrome.











Although muscle rigidity interfering with respiration has not been seen following the use of Effentora,
this is possible with fentanyl and other opioids. If it occurs, it should be managed by the use of
assisted ventilation, by an opioid antagonist, and as a final alternative, by a neuromuscular blocking
agent.
5.
PHARMACOLOGICAL PROPERTIES
5.1
Pharmacodynamic properties
Pharmacotherapeutic group: analgesics; opioids; phenylpiperidine derivatives; ATC code N02AB03.
Fentanyl is an opioid analgesic, interacting predominantly with the opioid µ-receptor. Its primary
therapeutic actions are analgesia and sedation. Secondary pharmacological effects are respiratory
depression, bradycardia, hypothermia, constipation, miosis, physical dependence and euphoria.
The analgesic effects of fentanyl are related to its plasma level. In general, the effective concentration
and the concentration at which toxicity occurs increase with increasing tolerance to opioids. The rate
of development of tolerance varies widely among individuals. As a result, the dose of Effentora should
be individually titrated to achieve the desired effect (see section 4.2).
All opioid µ-receptor agonists, including fentanyl, produce dose dependent respiratory depression. The
risk of respiratory depression is less in patients receiving chronic opioid therapy as these patients will
develop tolerance to respiratory depressant effects.
The safety and efficacy of Effentora have been evaluated in patients taking the drug at the onset of the
breakthrough pain episode. Pre-emptive use of Effentora for predictable pain episodes was not
investigated in the clinical trials. Two double-blind, randomized, placebo-controlled crossover studies
have been conducted involving a total of 248 patients with BTP and cancer who experienced on
average 1 to 4 episodes of BTP per day while taking maintenance opioid therapy. During an initial
open-label phase, patients were titrated to an effective dose of Effentora. Patients who identified an
effective dose entered the double-blind phase of the study. The primary efficacy variable was the
patient’s assessment of pain intensity. Patients assessed pain intensity on a 11-point scale. For each
BTP episode, pain intensity was assessed prior to and at several time points after treatment.
Sixty-seven percent of the patients were able to be titrated to an effective dose.
In the pivotal clinical study (study 1), the primary endpoint was the average sum of differences in pain
intensity scores from dosing to 60 minutes, inclusive (SPID60), which was statistically significant
compared to placebo (p<0.0001).
Study 1: Mean (+/- SEM) Pain Intensity Difference at Each Time Point (Full Analysis Set)
SPID60 mean (+/- SD)
EFFENTORA=9.7(5.58) p<0.0001
Placebo=4.9(4.38)
Time from Administration of Study Drug (Minutes)
Treatment Group
±±±
EFFENTORA
Placebo
+ p<0.0001 E
FFENTORA
versus placebo, in favor of E
FFENTORA
, by an analysis of variance
PID=pain intensity difference; SEM=standard error of the mean
Study 2: Mean (+/- SEM) Pain Intensity Difference at Each Time Point (Full Analysis Set)
SPID30 mean (+/- SD)
EFFENTORA=3.2(2.60) p<0.0001
Placebo=2.0(2.21)
Time from Administration of Study Drug (Minutes)
Treatment Group
±±±
EFFENTORA
Placebo
* p<0.01 E
FFENTORA
versus placebo, in favor of E
FFENTORA
, by one-sample Wilcoxon signed rank test
+ p<0.0001 E
FFENTORA
versus placebo, in favor of E
FFENTORA
, by one-sample Wilcoxon signed rank test
PID=pain intensity difference; SEM=standard error of the mean
In the second pivotal study (study 2), the primary endpoint was SPID30, which was also statistically
significant compared to placebo (p<0.0001).
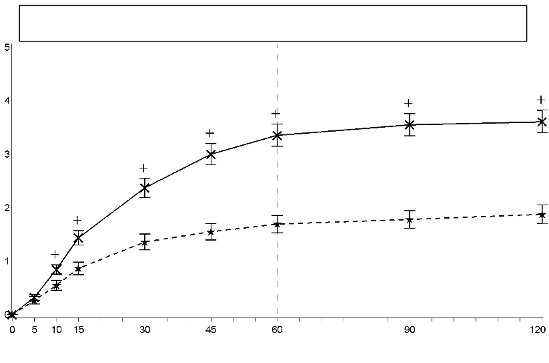

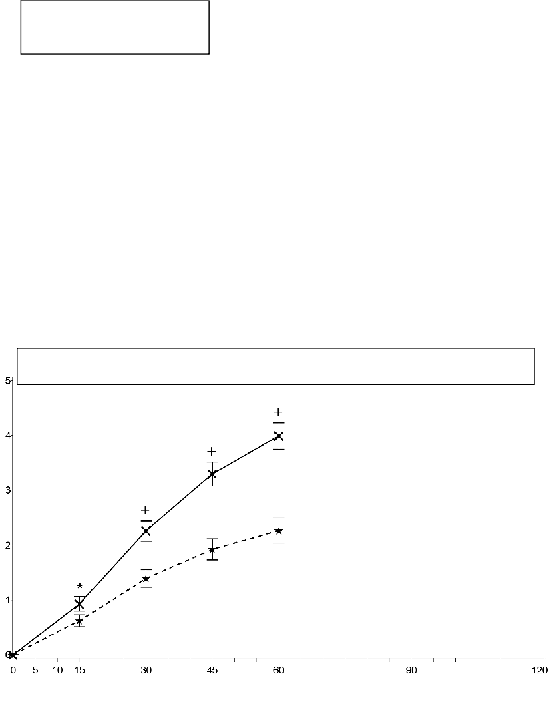


Statistically significant improvement in pain intensity difference was seen with Effentora versus
placebo as early as 10 minutes in Study 1 and as early as 15 minutes (earliest time point measured) in
Study 2. These differences continued to be significant at each subsequent time point in each
individual study.
5.2
Pharmacokinetic properties
General introduction
Fentanyl is highly lipophilic and can be absorbed very rapidly through the oral mucosa and more
slowly by the conventional gastrointestinal route. It is subject to first-pass hepatic and intestinal
metabolism and the metabolites do not contribute to fentanyl’s therapeutic effects.
Effentora employs a delivery technology which utilises an effervescent reaction which enhances the
rate and extent of fentanyl absorbed through the buccal mucosa. Transient pH changes accompanying
the effervescent reaction may optimise dissolution (at a lower pH) and membrane permeation (at a
higher pH).
Dwell time (defined as the length of time that the tablet takes to fully disintegrate following buccal
administration), does not affect early systemic exposure to fentanyl. A comparison study between one
400 mcg Effentora tablet administered either buccally (i.e., between the cheek and the gum) or
sublingually met the criteria of bioequivalence.
The effect of renal or hepatic impairment on the pharmacokinetics of Effentora has not been studied.
Absorption:
Following oromucosal administration of Effentora, fentanyl is readily absorbed with an absolute
bioavailability of 65%. The absorption profile of Effentora is largely the result of an initial rapid
absorption from the buccal mucosa, with peak plasma concentrations following venous sampling
generally attained within an hour after oromucosal administration. Approximately 50% of the total
dose administered is rapidly absorbed transmucosally and becomes systemically available. The
remaining half of the total dose is swallowed and slowly absorbed from the gastrointestinal tract.
About 30% of the amount swallowed (50% of the total dose) escapes hepatic and intestinal first-pass
elimination and becomes systemically available.
The main pharmacokinetic parameters are shown in the following table.
Pharmacokinetic Parameters* in Adult Subjects Receiving Effentora
Pharmacokinetic
parameter (mean)
Fraction
absorbed transmucosally










* Based on venous blood samples (plasma). Fentanyl citrate concentrations obtained in serum were
higher than in plasma: Serum AUC and Cmax were approximately 20% and 30% higher than plasma
AUC and Cmax, respectively. The reason of this difference is unknown.
** Data for T
max
presented as median (range).
In pharmacokinetic studies that compared the absolute and relative bioavailability of Effentora and
oral transmucosal fentanyl citrate (OTFC), the rate and extent of fentanyl absorption in Effentora
demonstrated exposure that was between 30% to 50% greater than that for oral transmucosal fentanyl
citrate. If switching from another oral fentanyl citrate product, independent dose titration with
Effentora is required as bioavailability between products differs significantly. However, in these
patients, a starting dose higher than 100 micrograms may be considered.
Mean Plasma Concentration Versus Time
Profiles Following Singles Doses of
EFFENTORA
and OTFC in Healthy Subjects
400 mcg
EFFENTORA
OTFC (normalized to 400 mcg)
Time after Dose Administration (hours)
OTFC data was dose adjusted (800 mcg to 400 mcg)
Differences in exposure with Effentora were observed in a clinical study with patients with grade 1
mucositis. C
max
and AUC
0-8
were 1% and 25% higher in patients with mucositis compared to those
without mucositis, respectively. The differences observed were not clinically significant.
Distribution
Fentanyl is highly lipophilic and is well distributed beyond the vascular system, with a large apparent
volume of distribution. After buccal administration of Effentora, fentanyl undergoes initial rapid
distribution that represents an equilibration of fentanyl between plasma and the highly perfused tissues
(brain, heart and lungs). Subsequently, fentanyl is redistributed between the deep tissue compartment
(muscle and fat) and the plasma.
The plasma protein binding of fentanyl is 80% to 85%. The main binding protein is alpha-1-acid
glycoprotein, but both albumin and lipoproteins contribute to some extent. The free fraction of
fentanyl increases with acidosis.


Biotransformation
The metabolic pathways following buccal administration of Effentora have not been characterised in
clinical studies. Fentanyl is metabolised in the liver and in the intestinal mucosa to norfentanyl by
CYP3A4 isoform. Norfentanyl is not pharmacologically active in animal studies. More than 90% of
the administered dose of fentanyl is eliminated by biotransformation to N-dealkylated and
hydroxylated inactive metabolites.
Elimination
Following the intravenous administration of fentanyl, less than 7% of the administered dose is
excreted unchanged in the urine, and only about 1% is excreted unchanged in the faeces. The
metabolites are mainly excreted in the urine, while faecal excretion is less important.
Following the administration of Effentora, the terminal elimination phase of fentanyl is the result of
the redistribution between plasma and a deep tissue compartment. This phase of elimination is slow,
resulting in a median terminal elimination half-life t
1/2
of approximately 22 hours following buccal
administration of the effervescent formulation and approximately 18 hours following intravenous
administration. The total plasma clearance of fentanyl following intravenous administration is
approximately 42 L/h.
Linearity/non-linearity
Dose proportionality from 100 micrograms to 1000 micrograms has been demonstrated.
5.3
Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, genotoxicity and carcinogenicity.
Embryo-foetal developmental toxicity studies conducted in rats and rabbits revealed no compound-
induced malformations or developmental variations when administered during the period of
organogenesis.
In a fertility and early embryonic development study in rats, a male-mediated effect was observed at
high doses (300 mcg/kg/day, s.c.) and is consistent with the sedative effects of fentanyl in animal
studies.
In studies on pre and postnatal development in rats the survival rate of offspring was significantly
reduced at doses causing severe maternal toxicity. Further findings at maternally toxic doses in F1
pups were delayed physical development, sensory functions, reflexes and behaviour. These effects
could either be indirect effects due to altered maternal care and/or decreased lactation rate or a direct
effect of fentanyl on the pups.
Carcinogenicity studies (26-week dermal alternative bioassay in Tg.AC transgenic mice; two-year
subcutaneous carcinogenicity study in rats) did not induce any findings indicative of oncogenic
potential.
6.
PHARMACEUTICAL PARTICULARS
Mannitol
Sodium starch glycolate type A
Sodium hydrogen carbonate
Sodium carbonate anhydrous
Citric acid anhydrous
Magnesium stearate
6.4
Special precautions for storage
Store in the original package in order to protect from moisture.
6.5
Nature and contents of container
Aluminium laminated blister of PVC/Al foil/Polyamide/PVC with paper/polyester lidding.
Blister packs are supplied in cartons of 4 or 28 tablets. Not all pack-sizes may be marketed.
6.6
Special precautions for disposal
Patients and carers must be advised to dispose of any unopened tablets remaining from a prescription
as soon as they are no longer needed.
Any used or unused but no longer required product or waste material should be disposed of in
accordance with local requirements.
7.
MARKETING AUTHORISATION HOLDER
Cephalon Europe
5 rue Charles Martigny
F-94700 Maisons-Alfort
France
8.
MARKETING AUTHORISATION NUMBER(S)
9.
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10.
DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency
(EMEA) http://www.emea.europa.eu.
What Effentora contains
The active substance is fentanyl. Each tablet contains either:
•
100 micrograms fentanyl (as citrate)
•
200 micrograms fentanyl (as citrate)
•
400 micrograms fentanyl (as citrate)
•
600 micrograms fentanyl (as citrate)
•
800 micrograms fentanyl (as citrate)
The other ingredients are mannitol, sodium starch glycolate type A, sodium hydrogen carbonate,
sodium carbonate anhydrous, citric acid anhydrous, magnesium stearate.
What Effentora looks like and contents of the pack
The buccal tablets are flat-faced, round bevelled-edge tablet, embossed one side with a “C” and on the
other side with “1” for Effentora 100 micrograms, with “2” for Effentora 200 micrograms, with “4”
for Effentora 400 micrograms, with “6” for Effentora 600 micrograms, with “8” for Effentora
800 micrograms.
Each blister contains 4 buccal tablets, supplied in cartons of 4 or 28 buccal tablets.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Cephalon Europe
5 rue Charles Martigny
94700 Maisons-Alfort
France
Manufacturer
Cephalon France
20, rue Charles Martigny
94700 Maisons-Alfort
France
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder or call the following number:
België/Belgique/Belgien
Cephalon B.V.
Tél/Tel: + 31 497 55 10 50
Luxembourg/Luxemburg
Cephalon B.V.
Tél/Tel: + 31 497 55 10 50
България
Cephalon Europe
Teл.: + 33 1 49 81 80 19
Magyarország
Cephalon Europe
Tel.: + 33 1 49 81 81 60
Česká republika
Cephalon Europe
Tel: + 33 1 49 81 80 44
Malta
Cephalon (UK) Limited
Tel: + 44 800 783 4869
Danmark
Cephalon Europe
Tlf: + 33 1 49 81 80 40
Nederland
Cephalon B.V.
Tel: + 31 497 55 10 50
Deutschland
Cephalon GmbH
Tel: + 49 89 89 55 70 0
Norge
Cephalon Europe
Tlf: + 33 1 49 81 81 64
Eesti
Cephalon Europe
Tel: + 33 1 49 81 80 42
Österreich
Cephalon GmbH
Tel: + 49 89 89 55 70 0
Ελλάδα
Cephalon Europe
Τηλ: + 33 1 49 81 80 50
Polska
Cephalon Sp.z.o.o.
Tel.: + 48 22 50 40 890
España
Cephalon Pharma, S.L.
Tel: + 34 93 567 78 80
Portugal
Companhia Portuguesa Higiene Pharma -
Produtos Farmacêuticos, S.A.
Tel: + 351 21 444 96 00
France
Cephalon France
Tél: + 33 1 49 81 81 81
România
Cephalon Europe
Tel: + 33 1 49 81 81 73
Ireland
Cephalon Pharma (Ireland) Limited
Tel: + 44 800 783 4869
Slovenija
Cephalon Europe
Tel: + 33 1 49 81 81 88
Ísland
Cephalon Europe
Sími: +33 1 49 81 80 96
Slovenská republika
Cephalon Europe
Tel: + 33 1 49 81 11 03
Italia
Cephalon S.r.l.
Tel: + 39 06 591935231
Suomi/Finland
Cephalon Europe
Puh/Tel: + 33 1 49 81 80 25
Κύπρος
Cephalon Europe
Τηλ: + 33 1 49 81 81 04
Sverige
Cephalon Europe
Tel: + 33 1 49 81 82 24
Latvija
Cephalon Europe
Tel: + 33 1 49 81 81 23
United Kingdom
Cephalon (UK) Limited
Tel: + 44 800 783 4869
Lietuva
Cephalon Europe
Tel: + 33 1 49 81 81 45
This leaflet was last approved in {MM/YYYY}.
Detailed information on this medicine is available on the European Medicines Agency (EMEA) web
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/effentora.html
Copyright © 1995-2021 ITA all rights reserved.
|




















































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































