Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Elonva 100 micrograms solution for injection.
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each pre-filled syringe contains 100 micrograms of corifollitropin alfa in 0.5 ml solution for injection.
Corifollitropin alfa is a glycoprotein produced in Chinese Hamster Ovary (CHO) cells by recombinant
DNA technology.
For a full list of excipients, see section 6.1.
Solution for injection (injection).
Clear and colourless aqueous solution.
Controlled Ovarian Stimulation (COS) in combination with a GnRH antagonist for the development of
multiple follicles in women participating in an Assisted Reproductive Technology (ART) program.
Posology and method of administration
Treatment with Elonva should be initiated under the supervision of a physician experienced in the
treatment of fertility problems.
In women with a body weight ≤ 60 kilograms a single dose of 100 micrograms should be
administered.
In women with a body weight > 60 kilograms a single dose of 150 micrograms should be
administered.
Stimulation day 1:
Elonva should be administered as a single subcutaneous injection, preferably in the abdominal wall,
during the early follicular phase of the menstrual cycle.
The recommended doses of Elonva have only been established in a treatment regimen with a GnRH
antagonist (see also section 4.1 and 4.4).
Stimulation day 5 or 6:
Treatment with a Gonadotropin Releasing Hormone (GnRH) antagonist should be started on
stimulation day 5 or day 6 depending on the ovarian response, i.e. the number and size of growing
follicles and/or the amount of circulating oestradiol. The GnRH antagonist is used to prevent
premature Luteinising Hormone (LH) surges.
Stimulation day 8:
Seven days after the injection with Elonva, treatment may be continued with daily injections of
(recombinant) Follicle Stimulating Hormone ((rec)FSH) until the criteria for triggering final oocyte
maturation (3 follicles ≥ 17 mm) have been reached. The daily dose of (rec)FSH may depend on the
ovarian response. In normal responders a daily dose of 150 IU (rec)FSH is advised. Administration of
(rec)FSH on the day of human Chorionic Gonadotropin (hCG) administration can be omitted,
depending on the ovarian response. In general, adequate follicular development is achieved on average
by the ninth day of treatment (range 6 to 18 days).
17 mm are observed, a single injection of 5,000 up to 10,000 IU hCG is
administered the same day or the day thereafter to induce final oocyte maturation. In case of an
excessive ovarian response, see the recommendations given in section 4.4 in order to minimise the risk
for developing ovarian hyperstimulation syndrome (OHSS).
Renal impairment: No clinical studies have been performed in patients with renal insufficiency. Since
the elimination of corifollitropin alfa might be impaired in patients with renal insufficiency, the use of
Elonva in these women is not recommended (see section 4.4 and 5.2).
Hepatic impairment: Although data in hepatically impaired patients are not available, hepatic
impairment is unlikely to affect the elimination of corifollitropin alfa (see section 5.2).
The use of Elonva in the paediatric population is not relevant within the approved indication.
Subcutaneous injection of Elonva may be carried out by the woman herself or her partner, provided
that proper instructions are given by the physician. Self administration of Elonva should only be
performed by women who are well-motivated, adequately trained and with access to expert advice.
Hypersensitivity to the active substance or to any of the excipients.
Tumours of the ovary, breast, uterus, pituitary or hypothalamus.
Abnormal (not menstrual) vaginal bleeding without a known/diagnosed cause.
Ovarian cysts or enlarged ovaries.
A history of Ovarian Hyperstimulation Syndrome (OHSS).
A previous COS cycle that resulted in more than 30 follicles
11 mm measured by ultrasound
A basal antral follicle count > 20.
Fibroid tumours of the uterus incompatible with pregnancy.
Malformations of the reproductive organs incompatible with pregnancy.
Special warnings and precautions for use
Before starting treatment, the couple’s infertility should be assessed as appropriate and putative
contraindications for pregnancy evaluated. In particular, women should be evaluated for
hypothyroidism, adrenocortical deficiency, hyperprolactinemia and pituitary or hypothalamic
tumours, and appropriate specific treatment given.
Elonva is intended for single subcutaneous injection only. Additional injections of Elonva
should not be given within the same treatment cycle.
In the first seven days after administration of Elonva, no (rec)FSH should be administered (see
also section 4.2).
As soon as three follicles
In patients with mild, moderate or severe renal insufficiency the excretion of corifollitropin alfa
might be impaired (see section 4.2 and 5.2). Therefore, the use of Elonva in these women is not
recommended.
There are limited data on the use of Elonva in combination with a GnRH agonist. Results of a
small uncontrolled study suggest a higher ovarian response than in combination with a GnRH
antagonist. Therefore, the use of Elonva is not recommended in combination with a GnRH
agonist (see also section 4.2).
Elonva has not been studied in patients with polycystic ovarian syndrome (PCOS). In these
women the use of Elonva is not recommended.
The ovarian response was shown to be higher after treatment with Elonva than after treatment
with daily recFSH. Therefore, women with known risk factors for a high ovarian response may
be especially prone to the development of OHSS during or following treatment with Elonva. For
women having their first cycle of ovarian stimulation, for whom risk factors are only partially
known, careful monitoring for potential ovarian hyperresponse is recommended.
Ovarian Hyperstimulation Syndrome (OHSS):
OHSS is a medical event distinct from uncomplicated ovarian enlargement. Clinical signs and
symptoms of mild and moderate OHSS are abdominal pain, nausea, diarrhoea, mild to moderate
enlargement of ovaries and ovarian cysts. Severe OHSS may be life-threatening. Clinical signs
and symptoms of severe OHSS are large ovarian cysts (prone to rupture), acute abdominal pain,
ascites, pleural effusion, hydrothorax, dyspnoe, oliguria, haematological abnormalities and
weight gain. In rare instances, venous or arterial thromboembolism may occur in association
with OHSS.
Signs and symptoms of OHSS are stimulated by administration of human Chorionic
Gonadotropin (hCG) and by pregnancy (endogenous hCG). Early OHSS usually occurs within
10 days after hCG administration and may be associated with an excessive ovarian response to
gonadotropin stimulation. Usually, early OHSS resolves spontaneously with the onset of
menses. Late OHSS occurs more than 10 days after hCG administration, as a consequence of
(multiple) pregnancy. Because of the risk of developing OHSS, patients should be monitored for
at least two weeks after hCG administration.
To minimise the risk of OHSS, ultrasonographic assesments of follicular development and/or
determination of serum estradiol levels should be performed prior to treatment and at regular
intervals during treatment. In ART there is an increased risk of OHSS with 18 or more follicles
of 11 mm or more in diameter. When there are 30 or more follicles in total it is advised to
withhold hCG administration.
Depending on the ovarian response, the following measurements can be used to prevent OHSS:
-
withhold further stimulation with a gonadotropin for a maximum of 3 days (coasting);
delay triggering final oocyte maturation with hCG administration until estradiol levels
stabilize or decrease;
administer a dose lower than 10,000 IU of hCG for triggering final oocyte maturation,
e.g. 5,000 IU hCG or 250 micrograms rec-hCG (which is equivalent to approximately
6,500 IU);
cryopreserve all embryos for future transfer;
withhold hCG and cancel the treatment cycle.
For luteal phase support, administration of hCG should be avoided.
Adherence to the recommended Elonva dose and treatment regimen and careful monitoring of
ovarian response is important to minimise the risk of OHSS.
Multiple pregnancies and births have been reported for all gonadotropin treatments. The woman
and her partner should be advised of the potential risks for the mother (pregnancy and delivery
complications) and the neonate (low birth weight) before starting treatment. In women
undergoing ART procedures the risk of multiple pregnancy is mainly related to the number of
embryos transferred.
Since infertile women undergoing ART, and particularly IVF, often have tubal abnormalities,
the incidence of ectopic pregnancies might be increased. It is important to have early ultrasound
confirmation that a pregnancy is intrauterine, and to exclude the possibility of extrauterine
pregnancy.
The incidence of congenital malformations after ART may be slightly higher than after
spontaneous conceptions. This is thought to be due to differences in parental characteristics (e.g.
maternal age, sperm characteristics) and the higher incidence of multiple pregnancies.
There have been reports of ovarian and other reproductive system neoplasms, both benign and
malignant, in women who have undergone multiple treatment regimens for infertility treatment.
It is not yet established whether or not treatment with gonadotropins increases the baseline risk
of these tumours in infertile women.
In women with generally recognized risk factors for thromboembolic events, such as a personal
or family history, severe obesity (Body Mass Index > 30 kg/m
2
) or thrombophilia, treatment
with gonadotropins may further increase this risk. In these women the benefits of gonadotropin
administration need to be weighed against the risks. It should be noted, however, that pregnancy
itself also carries an increased risk of thrombosis.
Interaction with other medicinal products and other forms of interaction
No interaction studies with Elonva and other medicines have been performed. Since corifollitropin alfa
is not a substrate of cytochrome P450 enzymes, no metabolic interactions with other medicinal
products are anticipated.
Pregnancy
No teratogenic risk has been reported, following controlled ovarian stimulation, in clinical use with
gonadotropins. When inadvertent exposure to Elonva during pregnancy occurs, clinical data are not
sufficient to exclude an adverse outcome of pregnancy. In animal studies reproductive toxicity has
been observed (see preclinical safety data in section 5.3). The use of Elonva during pregnancy is not
indicated.
Breast-feeding
The use of Elonva during breast-feeding is not indicated.
Effects on ability to drive and use machines
No studies on the ability to drive and use machines have been performed.
Elonva may cause dizziness. Women should be advised that if they feel dizzy, they should not drive or
use machines.
The most frequently reported adverse drug reactions during treatment with Elonva in clinical trials are
OHSS (5.2%, see also section 4.4), pelvic pain (4.1%) and discomfort (5.5%), headache (3.2%),
nausea (1.7%), fatigue (1.4%) and breast complaints (including tenderness) (1.2%).
The table below displays the main adverse drug reactions in women treated with Elonva in clinical
trials according to system organ class and frequency; common (≥ 1/100 to < 1/10), uncommon
(≥ 1/1,000 to < 1/100). Within each frequency grouping, adverse reactions are presented in order of
decreasing seriousness.
Gastrointestinal disorders
Abdominal pain, vomiting,
diarrhoea, constipation and
abdominal distension
Reproductive system and
breast disorders
OHSS, pelvic pain and
discomfort, breast complaints
General disorders and
administration site
conditions
In addition, ectopic pregnancy, miscarriage and multiple gestations have been reported. These are
considered to be related to the ART procedure or subsequent pregnancy.
More than one injection of Elonva within one treatment cycle or too high a dose of Elonva and/or
(rec)FSH are likely to increase the risk of OHSS. For measures to prevent and manage OHSS see
section 4.4.
PHARMACOLOGICAL PROPERTIES
Pharmacodynamic properties
Pharmacotherapeutic group: sex hormones and modulators of the genital system, gonadotropins
ATC code: G03GA09
Corifollitropin alfa is designed as a sustained follicle stimulant with the same pharmacodynamic
profile as (rec)FSH, but with a markedly prolonged duration of FSH activity. Due to its ability to
initiate and sustain multiple follicular growth for an entire week, a single subcutaneous injection of the
recommended dose of Elonva may replace the first seven injections of any daily (rec)FSH preparation
in a COS treatment cycle. The long duration of FSH activity was achieved by adding the carboxy-
terminal peptide of the β-subunit of human chorionic gonadotropin (hCG) to the β-chain of human
FSH. Corifollitropin alfa does not display any intrinsic LH/hCG activity.
Clinical trial information
In two randomized, double-blind, clinical trials, treatment with a single subcutaneous injection of
Elonva, 100 micrograms (trial A) or 150 micrograms (trial B), for the first seven days of COS resulted
in a significantly higher number of retrieved oocytes compared to treatment with a daily dose of 150
or 200 IU of recFSH, respectively. However, the difference was within the predefined equivalence
margins.





Primary efficacy variable
Trial B
Body weight > 60 kg
100 micrograms
Elonva
(N=268)
150 micrograms
Elonva
(N=756)
Mean number of oocytes
retrieved
In the 150 micrograms trial (trial B) pregnancy was also studied as primary efficacy parameter for
Elonva in direct comparison to recFSH, and similar success rates were established.
Primary efficacy variable
Trial B
Body weight > 60 kg
150 micrograms
Elonva
(N=756)
Ongoing pregnancy rate
(%)
Except for a slightly higher incidence of OHSS (not significant), the safety profile of a single injection
with Elonva was comparable to daily injections with recFSH (see also section 4.8).
The European Medicines Agency has deferred the obligation to submit the results of studies with
Elonva in one or more subsets of the paediatric population in hypogonadotrophic hypogonadism. See
4.2 for information on paediatric use.
Pharmacokinetic properties
Corifollitropin alfa has an elimination half-life of 69 hours (59-79 hours
0F
1
). After administration of the
recommended dose, serum concentrations of corifollitropin alfa are sufficient to sustain multiple
follicular growth for an entire week. This justifies replacement of the first seven injections of daily
(rec)FSH with a single subcutaneous injection of Elonva in COS for the development of multiple
follicles and pregnancy in an ART program (see section 4.2).
After a single subcutaneous injection of Elonva, maximum serum concentrations of corifollitropin alfa
are reached after 44 hours (34-57 hours
1
). The absolute bioavailability is 58% (48-70%
1
). The steady
state volume of distribution and clearance are 9.2 l (6.5 -13.1 l
1
) and 0.13 l/h (0.10 -0.18 l/h
1
),
respectively. The pharmacokinetic properties of corifollitropin alfa are independent of the
administered dose over a wide range (7.5-240 micrograms).
Body weight is a determinant of exposure to corifollitropin alfa. In clinical studies, serum
concentrations of corifollitropin alfa were similar after administration of 100micrograms
corifollitropin alfa to women with a body weight ≤60kilograms and of 150micrograms
corifollitropin alfa to women with a body weight > 60 kilograms.
Distribution, metabolism and elimination of corifollitropin alfa are very similar to other
gonadotropins, such as FSH, hCG and LH. After absorption into the blood, corifollitropin alfa is
distributed mainly to the ovaries and the kidneys. Elimination of corifollitropin alfa predominantly
occurs via the kidneys and may be impaired in patients with renal insufficiency (see section 4.2 and
1
Predicted range for 90% of subjects.















4.4). Hepatic metabolism contributes to a minor extent to the elimination of corifollitropin alfa.
Although data in hepatically impaired patients are not available, hepatic impairment is unlikely to
affect the pharmacokinetic profile of corifollitropin alfa.
Preclinical data revealed no special hazard for humans based on conventional studies of single and
repeated dose toxicity and safety pharmacology.
Reproduction toxicology studies in rats and rabbits indicated that corifollitropin alfa does not affect
fertility. Administration of corifollitropin alfa to rats and rabbits, prior to and directly after mating, and
during early pregnancy, resulted in embryotoxicity. In rabbits, when administered prior to mating,
teratogenicity has been observed. Both embryotoxicity and teratogenicity are considered a
consequence of the superovulatory state of the animal not able to support a number of embryos above
a physiological ceiling. The relevance of these findings for the clinical use of Elonva is limited.
PHARMACEUTICAL PARTICULARS
Sodium citrate
Sucrose
Polysorbate 20
Methionine
Sodium hydroxide (for pH adjustment)
Hydrochloric acid (for pH adjustment)
Water for injections
In the absence of compatibility studies, the medicinal product must not be mixed with other medicinal
products.
Special precautions for storage
Store in a refrigerator (2°C-8°C). For convenience, the patient is allowed to store the product at or
below 25°C for a period of not more than 1 month.
Do not freeze.
Keep the syringe in the outer carton in order to protect from light.
Nature and contents of container
Elonva is supplied in pre-filled luerlock syringes of 1 ml (type I hydrolytic glass), closed with a
bromobutyl elastomer plunger and a tip cap. The syringe is equiped with an automatic safety system to
prevent needle stick injuries after use and is packed together with a sterile injection needle. Each pre-
filled syringe contains 0.5 ml solution for injection.
Elonva is available in pack sizes of 1 pre-filled syringe.
Special precautions for disposal and other handling
Do not use Elonva if the solution is not clear.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
N.V. Organon
Kloosterstraat 6
5349 AB Oss
The Netherlands
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
Agency (EMEA)
0
http://www.emea.europa.eu/
NAME OF THE MEDICINAL PRODUCT
Elonva 150 micrograms solution for injection.
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each pre-filled syringe contains 150 micrograms of corifollitropin alfa in 0.5 ml solution for injection.
Corifollitropin alfa is a glycoprotein produced in Chinese Hamster Ovary (CHO) cells by recombinant
DNA technology.
For a full list of excipients, see section 6.1.
Solution for injection (injection).
Clear and colourless aqueous solution.
Controlled Ovarian Stimulation (COS) in combination with a GnRH antagonist for the development of
multiple follicles in women participating in an Assisted Reproductive Technology (ART) program.
Posology and method of administration
Treatment with Elonva should be initiated under the supervision of a physician experienced in the
treatment of fertility problems.
In women with a body weight ≤ 60 kilograms a single dose of 100 micrograms should be
administered.
In women with a body weight > 60 kilograms a single dose of 150 micrograms should be
administered.
Stimulation day 1:
Elonva should be administered as a single subcutaneous injection, preferably in the abdominal wall,
during the early follicular phase of the menstrual cycle.
The recommended doses of Elonva have only been established in a treatment regimen with a GnRH
antagonist (see also section 4.1 and 4.4).
Stimulation day 5 or 6:
Treatment with a Gonadotropin Releasing Hormone (GnRH) antagonist should be started on
stimulation day 5 or day 6 depending on the ovarian response, i.e. the number and size of growing
follicles and/or the amount of circulating oestradiol. The GnRH antagonist is used to prevent
premature Luteinising Hormone (LH) surges.
Stimulation day 8:
Seven days after the injection with Elonva, treatment may be continued with daily injections of
(recombinant) Follicle Stimulating Hormone ((rec)FSH) until the criteria for triggering final oocyte
maturation (3 follicles ≥ 17 mm) have been reached. The daily dose of (rec)FSH may depend on the
ovarian response. In normal responders a daily dose of 150 IU (rec)FSH is advised. Administration of
(rec)FSH on the day of human Chorionic Gonadotropin (hCG) administration can be omitted,
depending on the ovarian response. In general, adequate follicular development is achieved on average
by the ninth day of treatment (range 6 to 18 days).
17 mm are observed, a single injection of 5,000 up to 10,000 IU hCG is
administered the same day or the day thereafter to induce final oocyte maturation. In case of an
excessive ovarian response, see the recommendations given in section 4.4 in order to minimise the risk
for developing ovarian hyperstimulation syndrome (OHSS).
Renal impairment: No clinical studies have been performed in patients with renal insufficiency. Since
the elimination of corifollitropin alfa might be impaired in patients with renal insufficiency, the use of
Elonva in these women is not recommended (see section 4.4 and 5.2).
Hepatic impairment: Although data in hepatically impaired patients are not available, hepatic
impairment is unlikely to affect the elimination of corifollitropin alfa (see section 5.2).
The use of Elonva in the paediatric population is not relevant within the approved indication.
Subcutaneous injection of Elonva may be carried out by the woman herself or her partner, provided
that proper instructions are given by the physician. Self administration of Elonva should only be
performed by women who are well-motivated, adequately trained and with access to expert advice.
Hypersensitivity to the active substance or to any of the excipients.
Tumours of the ovary, breast, uterus, pituitary or hypothalamus.
Abnormal (not menstrual) vaginal bleeding without a known/diagnosed cause.
Ovarian cysts or enlarged ovaries.
A history of Ovarian Hyperstimulation Syndrome (OHSS).
A previous COS cycle that resulted in more than 30 follicles
11 mm measured by ultrasound
A basal antral follicle count > 20.
Fibroid tumours of the uterus incompatible with pregnancy.
Malformations of the reproductive organs incompatible with pregnancy.
Special warnings and precautions for use
Before starting treatment, the couple’s infertility should be assessed as appropriate and putative
contraindications for pregnancy evaluated. In particular, women should be evaluated for
hypothyroidism, adrenocortical deficiency, hyperprolactinemia and pituitary or hypothalamic
tumours, and appropriate specific treatment given.
Elonva is intended for single subcutaneous injection only. Additional injections of Elonva
should not be given within the same treatment cycle.
In the first seven days after administration of Elonva, no (rec)FSH should be administered (see
also section 4.2).
As soon as three follicles
In patients with mild, moderate or severe renal insufficiency the excretion of corifollitropin alfa
might be impaired (see section 4.2 and 5.2). Therefore, the use of Elonva in these women is not
recommended.
There are limited data on the use of Elonva in combination with a GnRH agonist.Results of a
small uncontrolled study suggest a higher ovarian response than in combination with a GnRH
antagonist. Therefore, the use of Elonva is not recommended in combination with a GnRH
agonist (see also section 4.2).
Elonva has not been studied in patients with polycystic ovarian syndrome (PCOS). In these
women the use of Elonva is not recommended.
The ovarian response was shown to be higher after treatment with Elonva than after treatment
with daily recFSH. Therefore, women with known risk factors for a high ovarian response may
be especially prone to the development of OHSS during or following treatment with Elonva. For
women having their first cycle of ovarian stimulation, for whom risk factors are only partially
known, careful monitoring for potential ovarian hyperresponse is recommended.
Ovarian Hyperstimulation Syndrome (OHSS):
OHSS is a medical event distinct from uncomplicated ovarian enlargement. Clinical signs and
symptoms of mild and moderate OHSS are abdominal pain, nausea, diarrhoea, mild to moderate
enlargement of ovaries and ovarian cysts. Severe OHSS may be life-threatening. Clinical signs
and symptoms of severe OHSS are large ovarian cysts (prone to rupture), acute abdominal pain,
ascites, pleural effusion, hydrothorax, dyspnoe, oliguria, haematological abnormalities and
weight gain. In rare instances, venous or arterial thromboembolism may occur in association
with OHSS.
Signs and symptoms of OHSS are stimulated by administration of human Chorionic
Gonadotropin (hCG) and by pregnancy (endogenous hCG). Early OHSS usually occurs within
10 days after hCG administration and may be associated with an excessive ovarian response to
gonadotropin stimulation. Usually, early OHSS resolves spontaneously with the onset of
menses. Late OHSS occurs more than 10 days after hCG administration, as a consequence of
(multiple) pregnancy. Because of the risk of developing OHSS, patients should be monitored for
at least two weeks after hCG administration.
To minimise the risk of OHSS, ultrasonographic assesments of follicular development and/or
determination of serum estradiol levels should be performed prior to treatment and at regular
intervals during treatment. In ART there is an increased risk of OHSS with 18 or more follicles
of 11 mm or more in diameter. When there are 30 or more follicles in total it is advised to
withhold hCG administration.
Depending on the ovarian response, the following measurements can be used to prevent OHSS:
-
withhold further stimulation with a gonadotropin for a maximum of 3 days (coasting);
delay triggering final oocyte maturation with hCG administration until estradiol levels
stabilize or decrease;
administer a dose lower than 10,000 IU of hCG for triggering final oocyte maturation,
e.g. 5,000 IU hCG or 250 micrograms rec-hCG (which is equivalent to approximately
6,500 IU);
cryopreserve all embryos for future transfer;
withhold hCG and cancel the treatment cycle.
For luteal phase support, administration of hCG should be avoided.
Adherence to the recommended Elonva dose and treatment regimen and careful monitoring of
ovarian response is important to minimise the risk of OHSS.
Multiple pregnancies and births have been reported for all gonadotropin treatments. The woman
and her partner should be advised of the potential risks for the mother (pregnancy and delivery
complications) and the neonate (low birth weight) before starting treatment. In women
undergoing ART procedures the risk of multiple pregnancy is mainly related to the number of
embryos transferred.
Since infertile women undergoing ART, and particularly IVF, often have tubal abnormalities,
the incidence of ectopic pregnancies might be increased. It is important to have early ultrasound
confirmation that a pregnancy is intrauterine, and to exclude the possibility of extrauterine
pregnancy.
The incidence of congenital malformations after ART may be slightly higher than after
spontaneous conceptions. This is thought to be due to differences in parental characteristics (e.g.
maternal age, sperm characteristics) and the higher incidence of multiple pregnancies.
There have been reports of ovarian and other reproductive system neoplasms, both benign and
malignant, in women who have undergone multiple treatment regimens for infertility treatment.
It is not yet established whether or not treatment with gonadotropins increases the baseline risk
of these tumours in infertile women.
In women with generally recognized risk factors for thromboembolic events, such as a personal
or family history, severe obesity (Body Mass Index > 30 kg/m
2
) or thrombophilia, treatment
with gonadotropins may further increase this risk. In these women the benefits of gonadotropin
administration need to be weighed against the risks. It should be noted, however, that pregnancy
itself also carries an increased risk of thrombosis.
Interaction with other medicinal products and other forms of interaction
No interaction studies with Elonva and other medicines have been performed. Since corifollitropin alfa
is not a substrate of cytochrome P450 enzymes, no metabolic interactions with other medicinal
products are anticipated.
Pregnancy
No teratogenic risk has been reported, following controlled ovarian stimulation, in clinical use with
gonadotropins. When inadvertent exposure to Elonva during pregnancy occurs, clinical data are not
sufficient to exclude an adverse outcome of pregnancy. In animal studies reproductive toxicity has
been observed (see preclinical safety data in section 5.3). The use of Elonva during pregnancy is not
indicated.
Breast-feeding
The use of Elonva during breast-feeding is not indicated.
Effects on ability to drive and use machines
No studies on the ability to drive and use machines have been performed.
Elonva may cause dizziness. Women should be advised that if they feel dizzy, they should not drive or
use machines.
The most frequently reported adverse drug reactions during treatment with Elonva in clinical trials are
OHSS (5.2%, see also section 4.4), pelvic pain (4.1%) and discomfort (5.5%), headache (3.2%),
nausea (1.7%), fatigue (1.4%) and breast complaints (including tenderness) (1.2%).
The table below displays the main adverse drug reactions in women treated with Elonva in clinical
trials according to system organ class and frequency; common (≥ 1/100 to < 1/10), uncommon
(≥ 1/1,000 to < 1/100). Within each frequency grouping, adverse reactions are presented in order of
decreasing seriousness.
Gastrointestinal disorders
Abdominal pain, vomiting,
diarrhoea, constipation and
abdominal distension
Reproductive system and
breast disorders
OHSS, pelvic pain and
discomfort, breast complaints
General disorders and
administration site
conditions
In addition, ectopic pregnancy, miscarriage and multiple gestations have been reported. These are
considered to be related to the ART procedure or subsequent pregnancy.
More than one injection of Elonva within one treatment cycle or too high a dose of Elonva and/or
(rec)FSH are likely to increase the risk of OHSS. For measures to prevent and manage OHSS see
section 4.4.
PHARMACOLOGICAL PROPERTIES
Pharmacodynamic properties
Pharmacotherapeutic group: sex hormones and modulators of the genital system, gonadotropins
ATC code: G03GA09
Corifollitropin alfa is designed as a sustained follicle stimulant with the same pharmacodynamic
profile as (rec)FSH, but with a markedly prolonged duration of FSH activity. Due to its ability to
initiate and sustain multiple follicular growth for an entire week, a single subcutaneous injection of the
recommended dose of Elonva may replace the first seven injections of any daily (rec)FSH preparation
in a COS treatment cycle. The long duration of FSH activity was achieved by adding the carboxy-
terminal peptide of the β-subunit of human chorionic gonadotropin (hCG) to the β-chain of human
FSH. Corifollitropin alfa does not display any intrinsic LH/hCG activity.
Clinical trial information
In two randomized, double-blind, clinical trials, treatment with a single subcutaneous injection of
Elonva, 100 micrograms (trial A) or 150 micrograms (trial B), for the first seven days of COS resulted
in a significantly higher number of retrieved oocytes compared to treatment with a daily dose of 150
or 200 IU of recFSH, respectively. However, the difference was within the predefined equivalence
margins.





Primary efficacy variable
Trial B
Body weight > 60 kg
100 micrograms
Elonva
(N=268)
150 micrograms
Elonva
(N=756)
Mean number of oocytes
retrieved
In the 150 micrograms trial (trial B) pregnancy was also studied as primary efficacy parameter for
Elonva in direct comparison to recFSH, and similar success rates were established.
Primary efficacy variable
Trial B
Body weight > 60 kg
150 micrograms
Elonva
(N=756)
Ongoing pregnancy rate
(%)
Except for a slightly higher incidence of OHSS (not significant), the safety profile of a single injection
with Elonva was comparable to daily injections with recFSH (see also section 4.8).
The European Medicines Agency has deferred the obligation to submit the results of studies with
Elonva in one or more subsets of the paediatric population in hypogonadotrophic hypogonadism. See
4.2 for information on paediatric use.
Pharmacokinetic properties
Corifollitropin alfa has an elimination half-life of 69 hours (59-79 hours
1F
2
). After administration of the
recommended dose, serum concentrations of corifollitropin alfa are sufficient to sustain multiple
follicular growth for an entire week. This justifies replacement of the first seven injections of daily
(rec)FSH with a single subcutaneous injection of Elonva in COS for the development of multiple
follicles and pregnancy in an ART program (see section 4.2).
After a single subcutaneous injection of Elonva, maximum serum concentrations of corifollitropin alfa
are reached after 44 hours (34-57 hours
1
). The absolute bioavailability is 58% (48-70%
1
). The steady
state volume of distribution and clearance are 9.2 l (6.5 -13.1 l
1
) and 0.13 l/h (0.10 -0.18 l/h
1
),
respectively. The pharmacokinetic properties of corifollitropin alfa are independent of the
administered dose over a wide range (7.5-240 micrograms).
Body weight is a determinant of exposure to corifollitropin alfa. In clinical studies, serum
concentrations of corifollitropin alfa were similar after administration of 100micrograms
corifollitropin alfa to women with a body weight ≤60kilograms and of 150micrograms
corifollitropin alfa to women with a body weight > 60 kilograms.
Distribution, metabolism and elimination of corifollitropin alfa are very similar to other
gonadotropins, such as FSH, hCG and LH. After absorption into the blood, corifollitropin alfa is
distributed mainly to the ovaries and the kidneys. Elimination of corifollitropin alfa predominantly
occurs via the kidneys and may be impaired in patients with renal insufficiency (see section 4.2 and
2
Predicted range for 90% of subjects.
















4.4). Hepatic metabolism contributes to a minor extent to the elimination of corifollitropin alfa.
Although data in hepatically impaired patients are not available, hepatic impairment is unlikely to
affect the pharmacokinetic profile of corifollitropin alfa.
Preclinical data revealed no special hazard for humans based on conventional studies of single and
repeated dose toxicity and safety pharmacology.
Reproduction toxicology studies in rats and rabbits indicated that corifollitropin alfa does not affect
fertility. Administration of corifollitropin alfa to rats and rabbits, prior to and directly after mating, and
during early pregnancy, resulted in embryotoxicity. In rabbits, when administered prior to mating,
teratogenicity has been observed. Both embryotoxicity and teratogenicity are considered a
consequence of the superovulatory state of the animal not able to support a number of embryos above
a physiological ceiling. The relevance of these findings for the clinical use of Elonva is limited.
PHARMACEUTICAL PARTICULARS
Sodium citrate
Sucrose
Polysorbate 20
Methionine
Sodium hydroxide (for pH adjustment)
Hydrochloric acid (for pH adjustment)
Water for injections
In the absence of compatibility studies, the medicinal product must not be mixed with other medicinal
products.
Special precautions for storage
Store in a refrigerator (2°C-8°C). For convenience, the patient is allowed to store the product at or
below 25°C for a period of not more than 1 month.
Do not freeze.
Keep the syringe in the outer carton in order to protect from light.
Nature and contents of container
Elonva is supplied in pre-filled luerlock syringes of 1 ml (type I hydrolytic glass), closed with a
bromobutyl elastomer plunger and a tip cap. The syringe is equiped with an automatic safety system to
prevent needle stick injuries after use and is packed together with a sterile injection needle. Each pre-
filled syringe contains 0.5 ml solution for injection.
Elonva is available in pack sizes of 1 pre-filled syringe.
Special precautions for disposal and other handling
Do not use Elonva if the solution is not clear.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
N.V. Organon
Kloosterstraat 6
5349 AB Oss
The Netherlands
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
Agency (EMEA)
1
http://www.emea.europa.eu/
MANUFACTURER OF THE BIOLOGICAL ACTIVE
SUBSTANCE AND MANUFACTURING AUTHORISATION
HOLDERS RESPONSIBLE FOR BATCH RELEASE
CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURER OF THE BIOLOGICAL ACTIVE SUBSTANCE AND
MANUFACTURING AUTHORISATION HOLDERS RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer of the biological active substance
N.V. Organon
Kloosterstraat 6
5349 AB Oss
The Netherlands
Name and address of the manufacturers responsible for batch release in the European Economic Area
N.V. Organon
Kloosterstraat 6 5349 AB Oss
P.O. Box 20 5340 BH Oss
The Netherlands
Organon (Ireland) Ltd.
Drynam Road, Swords, Co. Dublin
Ireland
The printed package leaflet of the medicinal product must state the name and address of the
manufacturer responsible for the release of the concerned batch.
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to restricted medical prescription (See Annex I: Summary of Product
Characteristics, Section 4.2).
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
The MAH must ensure that the system of pharmacovigilance, as described in version 7.0 presented in
Module 1.8.1. of the Marketing Authorisation Application, is in place and functioning before and
whilst the product is on the market.
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version 4.0 of the Risk Management Plan (RMP) presented
in Module 1.8.2. of the Marketing Authorisation Application and any subsequent updates of the RMP
agreed by the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, the
updated RMP should be submitted at the same time as the next Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted
•
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
At the request of the EMEA
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
OUTER CARTON, Elonva 100 micrograms solution for injection, 1 pre-filled syringe
NAME OF THE MEDICINAL PRODUCT
Elonva 100 micrograms solution for injection
corifollitropin alfa
STATEMENT OF ACTIVE SUBSTANCE
Each pre-filled syringe contains 100 micrograms of corifollitropin alfa in 0.5 ml solution for injection.
Other ingredients: sodium citrate, sucrose, polysorbate 20, methionine, sodium hydroxide (for pH
adjustment), hydrochloric acid (for pH adjustment), water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection
1 pre-filled syringe with an automatic safety (needle injury prevention) system and a sterile injection
needle. 0.5 ml
METHOD AND ROUTE(S) OF ADMINISTRATION
For single use only.
Read the package leaflet before use.
Subcutaneous use (SC)
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
Storage by the pharmacist
Store in a refrigerator. Do not freeze























Storage by the patient
There are two options:
1. Store in a refrigerator. Do not freeze.
2. Store at or below 25°C for a period of not more than 1 month.
Keep the syringe in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
N.V. Organon,
Kloosterstraat 6,
5349 AB Oss,
The Netherlands
MARKETING AUTHORISATION NUMBERS
GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
<Justification for not including Braille accepted>


















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
OUTER CARTON, Elonva 150 micrograms solution for injection, 1 pre-filled syringe
NAME OF THE MEDICINAL PRODUCT
Elonva 150 micrograms solution for injection
corifollitropin alfa
STATEMENT OF ACTIVE SUBSTANCE
Each pre-filled syringe contains 150 micrograms of corifollitropin alfa in 0.5 ml solution for injection.
Other ingredients: sodium citrate, sucrose, polysorbate 20, methionine, sodium hydroxide (for pH
adjustment), hydrochloric acid (for pH adjustment), water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection
1 pre-filled syringe with an automatic safety (needle injury prevention) system and a sterile injection
needle. 0.5 ml.
METHOD AND ROUTE(S) OF ADMINISTRATION
For single use only.
Read the package leaflet before use.
Subcutaneous use (SC)
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
Storage by the pharmacist
Store in a refrigerator. Do not freeze.























Storage by the patient
There are two options:
1. Store in a refrigerator. Do not freeze.
2. Store at or below 25°C for a period of not more than 1 month.
Keep the syringe in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
N.V. Organon,
Kloosterstraat 6,
5349 AB Oss,
The Netherlands
MARKETING AUTHORISATION NUMBERS
GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
<Justification for not including Braille accepted>


















PACKAGE LEAFLET: INFORMATION FOR THE USER
Elonva 100 micrograms solution for injection
corifollitropin alfa
Read all of this leaflet carefully before you start using this medicine.
-
Keep this leaflet. You may need to read it again.
If you have any further questions, please ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
In this leaflet
:
1.
What Elonva is and what it is used for
2.
Before you use Elonva
3.
How to use Elonva
4.
Possible side effects
5.
How to store Elonva
6.
Further information
1.
WHAT ELONVA IS AND WHAT IT IS USED FOR
Elonva is a medicine belonging to the group of gonadotropic hormones. Gonadotropic hormones play
an important role in human fertility and reproduction. One of these gonadotropic hormones is follicle-
stimulating hormone (FSH), which is needed in women for the growth and development of eggs in the
ovaries.
Elonva is used to help achieve pregnancy in women having infertility treatment, such as in vitro
fertilisation (IVF). IVF involves collecting the eggs from the ovary, fertilising them in the laboratory,
and transferring the embryos back into the womb a few days later. Elonva causes the growth of several
eggs at the same time by a controlled stimulation of the ovaries.
Do not use Elonva if you
-
are allergic (hypersensitive) to corifollitropin alfa or any of the other ingredients of Elonva (for
a list of all ingredients, see Section 6)
have cancer of the ovary, breast, womb, or brain (pituitary gland or hypothalamus)
have recently had unexpected vaginal bleeding, other than menstrual, without a diagnosed cause
have ovaries that do not work because of a condition called primary ovarian failure
have ovarian cysts or enlarged ovaries
have had ovarian hyperstimulation syndrome (OHSS), see below for further explanation
have previously had a treatment cycle of controlled stimulation of the ovaries that resulted in the
growth of more than 30 eggs with a size of 11 mm or larger
have a basal antral follicle count (the number of small follicles present in your ovaries at the
beginning of a menstrual cycle) higher than 20
have malformations of the sexual organs which make a normal
pregnancy impossible
have fibroids in the womb which make a normal pregnancy impossible
Take special care with Elonva
Ovarian hyperstimulation syndrome (OHSS)
Treatment with gonadotropic hormones like Elonva may cause
ovarian hyperstimulation syndrome
(OHSS)
. This is a condition where the eggs growing in the ovaries become larger than normal. This
may be noticed as severe abdominal swelling and pain in the stomach (abdomen), feeling sick or
diarrhoea. Therefore, close supervision by your doctor is very important. To check the effects of
treatment, ultrasound scans of the ovaries are usually made, and blood or urine samples may be
regularly taken (see also Section 4).
You may use Elonva only once during the same treatment cycle, as otherwise the chance of having
OHSS may increase.
Before starting to use this medicine, it is important to inform your doctor if you:
-
ever had ovarian hyperstimulation syndrome (OHSS)
have polycystic ovarian syndrome (PCOS)
Thrombosis
Treatment with gonadotropic hormones like Elonva may (just as pregnancy) increase the chance of
having a thrombosis. Thrombosis is the formation of a blood clot in a blood vessel, which occurs most
often in the legs or the lungs.
Please discuss this with your doctor, before starting treatment, especially if:
-
you know you already have an increased chance of having a thrombosis
you are severely overweight.
Multiple births or birth defects
There is an increased chance of having twins or even more than two babies if more than one embryo is
transferred back into the womb. Multiple pregnancies carry an increased health risk for both the
mother and her babies. Multiple pregnancies and specific characteristics of couples with fertility
problems (e.g. age) may also be associated with an increased chance of birth defects.
Pregnancy complications
If treatment with Elonva results in pregnancy, there is a higher chance of pregnancy outside the womb
(an ectopic pregnancy) in women with damaged fallopian tubes (the tubes which carry the egg from
the ovary to the womb). Therefore, your doctor should perform an early ultrasound examination to
exclude the possibility of pregnancy outside the womb.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
Pregnancy and breast-feeding
You should not use Elonva if you are already pregnant, or suspect that you might be pregnant, or if
you are breast-feeding.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Elonva may cause dizziness. If you feel dizzy, you should not drive or use machines.
Important information about some of the ingredients of Elonva
This medicinal product contains less than 1 mmol sodium (23 mg) per injection, i.e. essentially
‘sodium-free’.
Always use Elonva exactly as your doctor has told you. You should check with your doctor or
pharmacist if you are not sure.
you, or anyone in your immediate family, have ever had a thrombosis
Elonva is used in women having infertility treatment like in vitro fertilisation (IVF). During this
treatment Elonva is used in combination with a medicine to prevent too early ovulation (so called
GnRH-antagonist). Treatment with the GnRH-antagonist usually starts 4 to 5 days after the injection
of Elonva.
The use of Elonva in combination with a GnRH agonist (another medicine to prevent too early
ovulation) is not recommended. It may lead to a higher stimulation of your ovaries.
If your body weight is 60 kilograms or lower, a single dose of 100 micrograms of Elonva should
be injected on one of the first days of your period (menstruation), as instructed by your doctor.
If your body weight is more than 60 kilograms, a single dose of 150 micrograms of Elonva
should be injected on one of the first days of your period (menstruation), as instructed by your
doctor.
During the first seven days after the injection with Elonva, you should not use (recombinant) Follicle
Stimulating Hormone ((rec)FSH). Seven days after the injection of Elonva, your doctor may decide to
continue treatment with another gonadotropic hormone, like (rec)FSH. This may be continued for a
few days until enough eggs of adequate size are present. This can be checked by ultrasound
examination. Treatment with (rec)FSH is then stopped and the eggs are matured by giving hCG
(human Chorionic Gonadotropin). The eggs are collected from the ovary 34-36 hours later.
How Elonva is given
Treatment with Elonva should be supervised by a physician experienced in the treatment of fertility
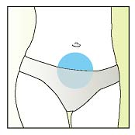
problems. Elonva must be injected under the skin (subcutaneously) into a skin fold, just below the
navel. The injection may be given by a healthcare professional (for example a nurse), your partner or
yourself, if carefully instructed by your doctor
.
Always use Elonva exactly as your doctor has told
you. You should check with your doctor or pharmacist if you are unsure. A step-by-step “instructions
for use” is given at the end of this leaflet .When the instructions are followed carefully, Elonva will be
given properly and with minimal discomfort.
Do not inject Elonva into a muscle.
If you use more Elonva or (rec)FSH than you should
If you use one pre-filled syringe of Elonva, it is not possible to inject more than you should.
Using too much Elonva or (rec)FSH may occur if Elonva is used more than once during a treatment
cycle, or if (rec)FSH is used during the first seven days after the injection with Elonva (see also “How
to use Elonva”). This may increase the risk of ovarian hyperstimulation syndrome (OHSS).
If you think you have used more Elonva or (rec)FSH than you should, contact your doctor
immediately.
If you forget to use Elonva
If you forgot to inject Elonva on the day you should have, contact your doctor immediately.
If you have any further questions on the use of this product, ask your doctor.
Like all medicines, Elonva can cause side effects, although not everybody gets them.
The chance of having a side effect is described by the following categories:
Common (affects 1 to 10 users in 100)
-
Ovarian hyperstimulation syndrome (OHSS)
Breast complaints (including tenderness)
Uncommon (affects 1 to 10 users in 1,000)
-
Pain in the stomach (abdomen)
Intestinal complaints (such as diarrhoea, constipation and abdominal distension)
A possible complication of treatment with gonadotropic hormones like Elonva is unwanted
overstimulation of the ovaries. The chance of having this complication can be reduced by carefully
monitoring the number of maturing eggs as well as your hormones during treatment. Your doctor will
take care of that. The first symptoms of ovarian overstimulation may be noticed as pain in the stomach
(abdomen), feeling sick or diarrhoea. Ovarian overstimulation may develop into a medical condition
called ovarian hyperstimulation syndrome (OHSS), which can be a serious medical problem. In more
severe cases this may lead to enlargement of the ovaries, collection of fluid in the abdomen and/or
chest (which may cause weight gain) or clots in the blood vessels.
Contact your doctor without delay if you have pain in the stomach (abdomen) or any of the other
symptoms of ovarian hyperstimulation, even if they occur some days after the injection has been
given.
Pregnancy outside the womb, miscarriage and multiple pregnancies have also been reported. These
side effects are not considered to be related to the use of Elonva, but to the Assisted Reproductive
Technology (ART) program or subsequent pregnancy.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, tell your
doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Elonva after the expiry date which is stated on the label and outer carton after “EXP”
(expiry date). The expiry date refers to the last day of the indicated month.
Storage by the pharmacist
Store in a refrigerator (2ºC-8°C). Do not freeze.
Storage by the patient
There are two options:
1.
Store in a refrigerator (2ºC-8°C). Do not freeze.
2.
Store at or below 25ºC for a period of not more than one month. Make a note of when you start
storing the product out of the refrigerator, and use it within one month of that date.
Keep the syringe in the outer carton in order to protect from light.
if it has been stored out of the refrigerator for more than one month.
if it has been stored out of the refrigerator at a temperature of more than 25ºC.
if you notice that the solution is not clear.
if you notice that the syringe or the needle is damaged.
An empty or unused syringe should not be disposed of via household waste. Ask your pharmacist or
doctor how to dispose of medicines no longer required. These measures will help to protect the
environment.
Pelvic pain and discomfort
The active substance is corifollitropin alfa. Each pre-filled syringe contains 100 micrograms in
0.5 millilitre (ml) solution for injection.
The other ingredients are: sodium citrate, sucrose, polysorbate 20, methionine and water for
injections. The pH may have been adjusted with sodium hydroxide and/or hydrochloric acid.
Elonva is available in two strengths: 100 micrograms and 150 micrograms solution for injection.
What Elonva looks like and contents of the pack
Elonva is a solution for injection (injection) in a pre-filled syringe with an automatic safety system,
which prevents needle stick injuries after use. The syringe is packed together with a sterile injection
needle.
One pre-filled syringe is available in a single pack.
Elonva is a clear and colourless solution for injection.
Marketing Authorisation Holder
N.V. Organon, Kloosterstraat 6, 5349 AB Oss, The Netherlands.
N.V. Organon, Kloosterstraat 6, 5349 AB Oss, The Netherlands.
Organon (Ireland) Ltd., Drynam Road, Swords, Co. Dublin, Ireland.
This leaflet was last approved in January 2010.
Detailed information on this medicine is available on the European Medicines Agency (EMEA) web
site:
2 H
http://www.emea.europa.eu/
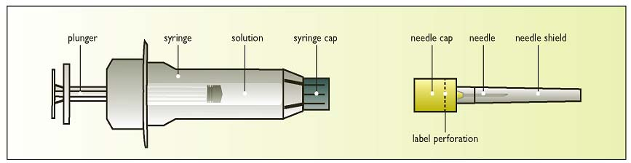
Components of the Elonva syringe with needle
Clean the skin area where the needle will enter with a disinfectant.
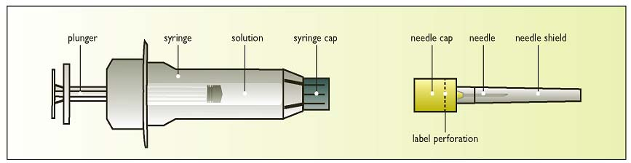
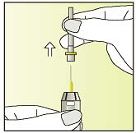
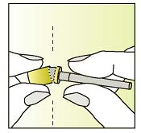
Break the label perforation and pull off the needle-cap
Leave the needle shield on the needle
Place the needle shield (containing the needle) on a clean dry
surface, while preparing the syringe.
Hold the syringe with the grey cap pointing upwards
Tap the syringe gently with your finger to help air bubbles rise to the
top.
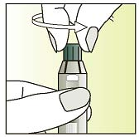
Keep the syringe pointing upwards
Unscrew the syringe cap counter-clockwise.
Keep the syringe pointing upwards
Screw the needle shield (containing the needle) clockwise onto the
syringe.
Keep the syringe pointing upwards
Remove the needle shield straight up and discard it
BE CAREFUL
with the needle




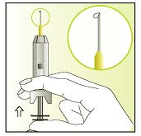
Now take the syringe between index and middle finger in the upward
position
Place your thumb on the plunger
Carefully push the plunger upwards until a tiny droplet appears at the
tip of the needle.
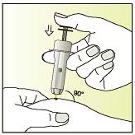
Pinch a fold of the skin between thumb and index finger
Insert the entire needle at an angle of 90 degrees into the fold of the
skin
CAREFULLY press the plunger until it can not go further and hold
the plunger down
COUNT TO FIVE to ensure that all of the solution is injected.
Release your thumb from the plunger
The needle will withdraw automatically into the syringe where it will
be locked permanently.

PACKAGE LEAFLET: INFORMATION FOR THE USER
Elonva 150 micrograms solution for injection
corifollitropin alfa
Read all of this leaflet carefully before you start using this medicine.
-
Keep this leaflet. You may need to read it again.
If you have any further questions, please ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
In this leaflet
:
1.
What Elonva is and what it is used for
2.
Before you use Elonva
3.
How to use Elonva
4.
Possible side effects
5.
How to store Elonva
6.
Further information
1.
WHAT ELONVA IS AND WHAT IT IS USED FOR
Elonva is a medicine belonging to the group of gonadotropic hormones. Gonadotropic hormones play
an important role in human fertility and reproduction. One of these gonadotropic hormones is follicle-
stimulating hormone (FSH), which is needed in women for the growth and development of eggs in the
ovaries.
Elonva is used to help achieve pregnancy in women having infertility treatment, such as in vitro
fertilisation (IVF). IVF involves collecting the eggs from the ovary, fertilising them in the laboratory,
and transferring the embryos back into the womb a few days later.
Elonva causes the growth of several eggs at the same time by a controlled stimulation of the ovaries.
Do not use Elonva if you
-
are allergic (hypersensitive) to corifollitropin alfa or any of the other ingredients of Elonva (for
a list of all ingredients, see Section 6)
have cancer of the ovary, breast, womb, or brain (pituitary gland or hypothalamus)
have recently had unexpected vaginal bleeding, other than menstrual, without a diagnosed cause
have ovaries that do not work because of a condition called primary ovarian failure
have ovarian cysts or enlarged ovaries
have had ovarian hyperstimulation syndrome (OHSS), see below for further explanation
have previously had a treatment cycle of controlled stimulation of the ovaries that resulted in the
growth of more than 30 eggs with a size of 11 mm or larger
have a basal antral follicle count (the number of small follicles present in your ovaries at the
beginning of a menstrual cycle) higher than 20
have malformations of the sexual organs which make a normal
pregnancy impossible
have fibroids in the womb which make a normal pregnancy impossible
Take special care with Elonva
Ovarian hyperstimulation syndrome (OHSS)
Treatment with gonadotropic hormones like Elonva may cause ovarian hyperstimulation syndrome
(OHSS). This is a condition where the eggs growing in the ovaries become larger than normal. This
may be noticed as severe abdominal swelling and pain in the stomach (abdomen), feeling sick or
diarrhoea. Therefore, close supervision by your doctor is very important. To check the effects of
treatment, ultrasound scans of the ovaries are usually made, and blood or urine samples may be
regularly taken (see also Section 4).
You may use Elonva only once during the same treatment cycle, as otherwise the chance of having
OHSS may increase.
Before starting to use this medicine, it is important to inform your doctor if you:
-
ever had ovarian hyperstimulation syndrome (OHSS)
have polycystic ovarian syndrome (PCOS)
Thrombosis
Treatment with gonadotropic hormones like Elonva may (just as pregnancy) increase the chance of
having a thrombosis. Thrombosis is the formation of a blood clot in a blood vessel, which occurs most
often in the legs or the lungs.
Please discuss this with your doctor, before starting treatment, especially if:
-
you know you already have an increased chance of having a thrombosis
you are severely overweight.
Multiple births or birth defects
There is an increased chance of having twins or even more than two babies if more than one embryo is
transferred back into the womb. Multiple pregnancies carry an increased health risk for both the
mother and her babies. Multiple pregnancies and specific characteristics of couples with fertility
problems (e.g. age) may also be associated with an increased chance of birth defects.
Pregnancy complications
If treatment with Elonva results in pregnancy, there is a higher chance of pregnancy outside the womb
(an ectopic pregnancy) in women with damaged fallopian tubes (the tubes which carry the egg from
the ovary to the womb). Therefore, your doctor should perform an early ultrasound examination to
exclude the possibility of pregnancy outside the womb.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
Pregnancy and breast-feeding
You should not use Elonva if you are already pregnant, or suspect that you might be pregnant, or if
you are breast-feeding.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Elonva may cause dizziness. If you feel dizzy, you should not drive or use machines.
Important information about some of the ingredients of Elonva
This medicinal product contains less than 1 mmol sodium (23 mg) per injection, i.e. essentially
‘sodium-free’.
Always use Elonva exactly as your doctor has told you. You should check with your doctor or
pharmacist if you are not sure.
you, or anyone in your immediate family, have ever had a thrombosis
Elonva is used in women having infertility treatment like in vitro fertilisation (IVF). During this
treatment Elonva is used in combination with a medicine to prevent too early ovulation (so called
GnRH-antagonist). Treatment with the GnRH-antagonist usually starts 4 to 5 days after the injection
of Elonva.
The use of Elonva in combination with a GnRH agonist (another medicine to prevent too early
ovulation) is not recommended. It may lead to a higher stimulation of your ovaries.
If your body weight is 60 kilograms or lower, a single dose of 100 micrograms of Elonva should
be injected on one of the first days of your period (menstruation), as instructed by your doctor.
If your body weight is more than 60 kilograms, a single dose of 150 micrograms of Elonva
should be injected on one of the first days of your period (menstruation), as instructed by your
doctor.
During the first seven days after the injection with Elonva, you should not use (recombinant) Follicle
Stimulating Hormone ((rec)FSH). Seven days after the injection of Elonva, your doctor may decide to
continue treatment with another gonadotropic hormone, like (rec)FSH. This may be continued for a
few days until enough eggs of adequate size are present. This can be checked by ultrasound
examination. Treatment with (rec)FSH is then stopped and the eggs are matured by giving hCG
(human Chorionic Gonadotropin). The eggs are collected from the ovary 34-36 hours later.
How Elonva is given
Treatment with Elonva should be supervised by a physician experienced in the treatment of fertility
problems. Elonva must be injected under the skin (subcutaneously) into a skin fold, just below the
navel. The injection may be given by a healthcare professional (for example a nurse), your partner or
yourself, if carefully instructed by your doctor
.
Always use Elonva exactly as your doctor has told
you. You should check with your doctor or pharmacist if you are unsure. A step-by-step “instructions
for use” is given at the end of this leaflet .When the instructions are followed carefully, Elonva will be
given properly and with minimal discomfort.
Do not inject Elonva into a muscle.
If you use more Elonva or (rec)FSH than you should
If you use one pre-filled syringe of Elonva, it is not possible to inject more than you should.
Using too much Elonva or (rec)FSH may occur if Elonva is used more than once during a treatment
cycle, or if (rec)FSH is used during the first seven days after the injection with Elonva (see also “How
to use Elonva”). This may increase the risk of ovarian hyperstimulation syndrome (OHSS).
If you think you have used more Elonva or (rec)FSH than you should, contact your doctor
immediately.
If you forget to use Elonva
If you forgot to inject Elonva on the day you should have, contact your doctor immediately.
If you have any further questions on the use of this product, ask your doctor.
Like all medicines, Elonva can cause side effects, although not everybody gets them.
The chance of having a side effect is described by the following categories:
Common (affects 1 to 10 users in 100)
-
Ovarian hyperstimulation syndrome (OHSS)
Pelvic pain and discomfort
Breast complaints (including tenderness)
Uncommon (affects 1 to 10 users in 1,000)
-
Pain in the stomach (abdomen)
Intestinal complaints (such as diarrhoea, constipation and abdominal distension)
A possible complication of treatment with gonadotropic hormones like Elonva is unwanted
overstimulation of the ovaries. The chance of having this complication can be reduced by carefully
monitoring the number of maturing eggs as well as your hormones during treatment. Your doctor will
take care of that. The first symptoms of ovarian overstimulation may be noticed as pain in the stomach
(abdomen), feeling sick or diarrhoea. Ovarian overstimulation may develop into a medical condition
called ovarian hyperstimulation syndrome (OHSS), which can be a serious medical problem. In more
severe cases this may lead to enlargement of the ovaries, collection of fluid in the abdomen and/or
chest (which may cause weight gain) or clots in the blood vessels.
Contact your doctor without delay if you have pain in the stomach (abdomen) or any of the other
symptoms of ovarian hyperstimulation, even if they occur some days after the injection has been
given.
Pregnancy outside the womb, miscarriage and multiple pregnancies have also been reported. These
side effects are not considered to be related to the use of Elonva, but to the Assisted Reproductive
Technology (ART) program or subsequent pregnancy.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, tell your
doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Elonva after the expiry date which is stated on the label and outer carton after “EXP”
(expiry date). The expiry date refers to the last day of the indicated month.
Storage by the pharmacist
Store in a refrigerator (2ºC-8°C). Do not freeze.
Storage by the patient
There are two options:
1.
Store in a refrigerator (2ºC-8°C). Do not freeze.
2.
Store at or below 25ºC for a period of not more than one month. Make a note of when you start
storing the product out of the refrigerator, and use it within one month of that date.
Keep the syringe in the outer carton in order to protect from light.
if it has been stored out of the refrigerator for more than one month.
if it has been stored out of the refrigerator at a temperature of more than 25ºC.
if you notice that the solution is not clear.
if you notice that the syringe or the needle is damaged.
An empty or unused syringe should not be disposed of via household waste. Ask your pharmacist or
doctor how to dispose of medicines no longer required. These measures will help to protect the
environment.
The active substance is corifollitropin alfa. Each pre-filled syringe contains 150 micrograms in
0.5 millilitre (ml) solution for injection.
The other ingredients are: sodium citrate, sucrose, polysorbate 20, methionine and water for
injections. The pH may have been adjusted with sodium hydroxide and/or hydrochloric acid.
Elonva is available in two strengths: 100 micrograms and 150 micrograms solution for injection.
What Elonva looks like and contents of the pack
Elonva is a solution for injection (injection) in a pre-filled syringe with an automatic safety system,
which prevents needle stick injuries after use. The syringe is packed together with a sterile injection
needle.
One pre-filled syringe is available in a single pack.
Elonva is a clear and colourless solution for injection.
Marketing Authorisation Holder
N.V. Organon, Kloosterstraat 6, 5349 AB Oss, The Netherlands.
N.V. Organon, Kloosterstraat 6, 5349 AB Oss, The Netherlands.
Organon (Ireland) Ltd., Drynam Road, Swords, Co. Dublin, Ireland.
This leaflet was last approved in January 2010.
Detailed information on this medicine is available on the European Medicines Agency (EMEA) web
site:
3 H
http://www.emea.europa.eu/
Components of the Elonva syringe with needle
Clean the skin area where the needle will enter with a disinfectant.

Break the label perforation and pull off the needle-cap
Leave the needle shield on the needle
Place the needle shield (containing the needle) on a clean dry
surface, while preparing the syringe.
Hold the syringe with the grey cap pointing upwards
Tap the syringe gently with your finger to help air bubbles rise to the
top.
Keep the syringe pointing upwards
Unscrew the syringe cap counter-clockwise.
Keep the syringe pointing upwards
Screw the needle shield (containing the needle) clockwise onto the
syringe.
Keep the syringe pointing upwards
Remove the needle shield straight up and discard it
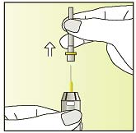
Be careful with the needle


Now take the syringe between index and middle finger in the upward
position
Place your thumb on the plunger
Carefully push the plunger upwards until a tiny droplet appears at the
tip of the needle.
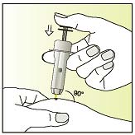
Pinch a fold of the skin between thumb and index finger
Insert the entire needle at an angle of 90 degrees into the fold of the
skin
CAREFULLY press the plunger until it can not go further and hold
the plunger down
COUNT TO FIVE to ensure that all of the solution is injected.
Release your thumb from the plunger
The needle will withdraw automatically into the syringe where it will
be locked permanently.


Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/elonva.html
Copyright © 1995-2021 ITA all rights reserved.
|

















































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































