Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
EMADINE 0.5 mg/ml, Eye Drops, solution
QUALITATIVE AND QUANTITATIVE COMPOSITION
1 ml of solution contains emedastine 0.5 mg (as difumarate)
Excipients: Benzalkonium chloride 0.1 mg/ml
For a full list of excipients, see section 6.1
Eye drops, (solution).
Clear, colourless solution.
4.1 Therapeutic indications
Symptomatic treatment of seasonal allergic conjunctivitis.
4.2 Posology and method of administration
EMADINE has not been studied in clinical trials beyond six weeks.
Posology
The dose is one drop of EMADINE to be applied to the affected eye(s) twice daily.
When used with other ophthalmic medicines, an interval of ten minutes should be allowed between
applications of each medicinal product.
Elderly population
EMADINE has not been studied in elderly patients older than 65 years, and therefore its use is not
recommended in this population.
Paediatric population
EMADINE may be used in paediatric patients (3 years of age and older) at the same posology as in
adults.
Hepatic and Renal impairment Use
EMADINE has not been studied in these patients and therefore, its use is not recommended in this
population.
To prevent contamination of the dropper tip and solution, care should be taken not to touch the
eyelids, surrounding areas or other surfaces with the dropper tip of the bottle.
Hypersensitivity to emedastine or to any of the excipients.
4.4 Special warnings and special precautions for use
Ocular corneal infiltrates:
Ocular corneal infiltrates were reported in conjunction with the use of EMADINE. In case of corneal
infiltrates, the product should be discontinued and appropriate management should be implemented.
Excipients
Benzalkonium chloride, which is commonly used as a preservative in ophthalmic products, has been
reported to cause punctate keratopathy and/or toxic ulcerative keratopathy. Since EMADINE contains
benzalkonium chloride, close monitoring is required with frequent or prolonged use.
In addition benzalkonium chloride may cause eye irritation and is known to discolour soft contact
lenses. Contact with soft contact lenses is to be avoided. Patients must be instructed to remove contact
lenses prior to the application of EMADINE and wait 15 minutes after instillation of the dose before
reinsertion.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed .
Pregnancy
There are no adequate data from the use of emedastine in pregnant women. Studies in animals have
shown reproductive toxicity (see section 5.3). The potential risk for humans is unknown. Nevertheless,
considering the absence of effects of emedastine on adrenergic, dopaminergic and serotonin receptors,
EMADINE can be used during pregnancy if the dosage recommendation in section 4.2 is respected.
Lactation
Emedastine has been identified in the milk of rats following oral administration. It is not known
whether topical administration to humans could result in sufficient systemic absorption to produce
detectable quantities in breast milk. Caution should be exercised if EMADINE is administered during
breast-feeding.
4.7 Effects on ability to drive and use machines
As with any ocular medication, if transient blurred vision occurs at instillation, the patient should wait
until the vision clears before driving or using machinery.
In 13 clinical studies involving 696 patients, Emadine was administered one to four times daily in both
eyes for up to 42 days. In clinical trials, approximately 7% of patients experienced an adverse drug
reaction associated with the use of Emadine; however, less than 1% of these patients discontinued
therapy due to these adverse drug reactions. No serious ophthalmic or systemic adverse drug reactions
were reported in the clinical trials. The most frequent adverse drug reaction was eye pain, reported at
an overall incidence of 2.0%.
The following adverse reactions listed below were observed in clinical studies or with post marketing
experience. They are ranked according to system organ class and classified according to the following
convention: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1000 to <1/100), rare
(≥1/10,000 to <1/1000), very rare (<1/10,000), or not known (cannot be estimated from the available
data). Within each frequency grouping, adverse reactions are presented in decreasing order of
seriousness.
Cardiac disorders
Not known: tachycardia
Nervous system disorders
Common: headache
Uncommon: sinus headache, dysgeusia
Eye disorders
Common: eye pain, eye irritation, blurred vision, eye pruritus, dry eye, corneal staining, conjunctival
hyperaemia
Uncommon: corneal infiltrates, foreign body sensation in eyes, lacrimation increased, asthenopia,
ocular hyperaemia
Skin and subcutaneous tissue disorders
Uncommon: rash
Psychiatric disorders
Uncommon: abnormal dreams
No data are available in humans regarding overdose by accidental or deliberate ingestion. In case of
accidental ingestion of the content of a bottle of EMADINE, the potential of emedastine to increase
the QT interval should be borne in mind and appropriate monitoring and management should be
implemented.
PHARMACOLOGICAL PROPERTIES
Pharmacodynamic properties
Pharmacotherapeutic group:
decongestants and antiallergics; other antiallergics,
Emedastine is a potent selective and topically effective histamine H
1
antagonist (K
i
= 1.3 nM).
In vitro
examinations of emedastine’s affinity for histamine receptors (H
1
, H
2
, and H
3
) demonstrate
10,000-fold selectivity for the H
1
receptor, K
i
’s = 1.3 nM, 49,064 nM and 12, 430 nM, respectively.
In
vivo
topical ocular administration of emedastine produces a concentration-dependent inhibition of
histamine-stimulated conjunctival vascular permeability. Studies with emedastine have not shown
effects on adrenergic, dopaminergic, and serotonin receptors.
Pharmacokinetic properties
Absorption
Emedastine is absorbed systemically, as are other topically administered drug substances. In a study
involving ten normal volunteers dosed bilaterally twice daily for 15 days with EMADINE 0.5 mg/ml
eye drops solution
,
plasma concentrations of the parent compound were generally below the
quantitation limit of the assay (0.3 ng/ml). Samples in which emedastine was quantifiable ranged from
0.30 to 0.49 ng/ml.
The human oral bioavailability of emedastine is approximately 50% and maximum plasma
concentrations were achieved within one-two hours after dosing.
Metabolism
Emedastine is principally metabolised by the liver
.
The elimination half-life of topical emedastine is
ten hours. Approximately 44% of an oral dose is recovered in the urine over 24 hours, with only
3.6% of the dose excreted as parent drug substance. Two primary metabolites, 5-and
6-hydroxyemedastine, are excreted in the urine as both free and conjugated forms. The 5′-oxo
analogues of 5-and 6-hydroxyemedastine and the N-oxide are also formed as minor metabolites.
Emedastine difumarate demonstrated low acute toxicity in a number of species by various routes of
administration. No clinically significant local or systemic effects were observed in long-term topical
ocular studies in rabbits.
Corneal limbal mononuclear cell infiltrates were noted in 1/4 male monkeys treated with 0.5 mg/ml
and in 4/4 males and 1/4 females treated with1.0 mg/ml. Scleral mononuclear cell infiltrates were
present in 1/4 males and 1/4 females treated with 0.5 mg/ml and in 2/4 males and 1/4 females treated
with1.0 mg/ml. Mean peak plasma levels were approximately 1 ng/ml and 2 ng/ml for the 0.5 and
1.0 mg/ml treatments respectively.
Emedastine was found to increase the QT interval in dogs; the NOEL corresponds to levels 23-fold
higher than those found in patients (7 ng/ml as compared with 0.3 ng/ml, i.e., the limit of detection for
emedastine).
Emedastine difumarate was not found to be carcinogenic in studies in mice and rats. Emedastine
difumarate was not genotoxic in a standard battery of
in vitro
and
in vivo
genotoxicity assays.
In a teratology study in rats, foetotoxic but not teratogenic effects were observed at the highest dose
evaluated (140 mg/kg/day); no effects were observed at a lower level (40 mg/kg/day) which
corresponds to an exposure well in excess of that produced by the therapeutic recommended dose. No
reproductive toxicity was observed in a study in rabbits.
PHARMACEUTICAL PARTICULARS
Benzalkonium chloride 0.1 mg/ml
Trometamol,
Sodium chloride,
Hypromellose,
Hydrochloric acid/sodium hydroxide (to adjust pH)
Purified water.
EMADINE should not be used for longer than 4 weeks after first opening.
Special precautions for storage
Nature and content of container
EMADINE is supplied in 5 ml and 10 ml opaque plastic DROP-TAINER bottles.
Not all pack sizes may be marketed.
Instructions for use and handling, and disposal
MARKETING AUTHORISATION HOLDER
Alcon Laboratories (UK) Ltd.
Pentagon Park
Boundary Way
Hemel Hempstead
Herts HP2 7UD
United Kingdom
MARKETING AUTHORISATION NUMBERS
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 27
th
January 1999.
Date of last renewal: 29
th
January 2004
DATE OF REVISION OF THE TEXT
NAME OF THE MEDICINAL PRODUCT
EMADINE 0.5 mg/ml Eye Drops, solution, single-dose container.
QUALITATIVE AND QUANTITATIVE COMPOSITION
1 ml of solution contains emedastine 0.5 mg (as difumarate).
For a full list of excipients, see section 6.1.
Eye drops, solution.
Clear, colourless solution.
4.1 Therapeutic indications
Symptomatic treatment of seasonal allergic conjunctivitis.
4.2 Posology and method of administration
EMADINE has not been studied in clinical trials beyond six weeks.
Posology
The dose is one drop of EMADINE to be applied to the affected eye(s) twice daily.
When used with other ophthalmic medicines, an interval of ten minutes should be allowed between
applications of each medicinal product.
For single use only; one container is sufficient to treat both eyes. Any unused solution should be
discarded immediately after use.
Elderly Population
EMADINE has not been studied in elderly patients older than 65 years, and therefore its use is not
recommended in this population.
Paediatric Population
EMADINE may be used in paediatric patients (3 years of age and older) at the same posology as in
adults.
Hepatic and Renal impairment Use
EMADINE has not been studied in these patients and therefore, its use is not recommended in this
population.
Hypersensitivity to emedastine or to any of the excipients.
4.4 Special warnings and special precautions for use
Ocular corneal infiltrates:
Ocular corneal infiltrates were reported in conjunction with the use of EMADINE. In case of corneal
infiltrates, the product should be discontinued and appropriate management should be implemented.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed.
4.6 Pregnancy and lactation
Pregnancy
There are no adequate data from the use of emedastine in pregnant women. Studies in animals have
shown reproductive toxicity (see section 5.3). The potential risk for humans is unknown. Nevertheless,
considering the absence of effects of emedastine on adrenergic, dopaminergic and serotonin receptors,
EMADINE can be used during pregnancy if the dosage recommendation in section 4.2 is respected.
Lactation
Emedastine has been identified in the milk of rats following oral administration. It is not known
whether topical administration to humans could result in sufficient systemic absorption to produce
detectable quantities in breast milk. Caution should be exercised if EMADINE is administered during
breast-feeding.
4.7 Effects on ability to drive and use machines
As with any ocular medication, if transient blurred vision occurs at instillation, the patient should wait
until the vision clears before driving or using machinery.
In 13 clinical studies involving 696 patients, Emadine was administered one to four times daily in both
eyes for up to 42 days. In clinical trials, approximately 7% of patients experienced an adverse drug
reaction associated with the use of Emadine; however, less than 1% of these patients discontinued
therapy due to these adverse drug reactions. No serious ophthalmic or systemic adverse drug reactions
were reported in the clinical trials. The most frequent adverse drug reaction was eye pain, reported at
an overall incidence of 2.0%.
The following adverse reactions listed below were observed in clinical studies or with post marketing
experience. They are ranked according to system organ class and classified according to the following
convention: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1000 to <1/100), rare
(≥1/10,000 to <1/1000), very rare (<1/10,000), or not known (cannot be estimated from the available
data). Within each frequency grouping, adverse reactions are presented in decreasing order of
seriousness.
Cardiac disorders
Not known: tachycardia
Nervous system disorders
Common: headache
Uncommon: sinus headache, dysgeusia
Eye disorders
Common: eye pain, eye irritation, blurred vision, eye pruritus, dry eye, corneal staining, conjunctival
hyperaemia
Uncommon: corneal infiltrates, foreign body sensation in eyes, lacrimation increased, asthenopia,
ocular hyperaemia
Skin and subcutaneous tissue disorders
Uncommon: rash
Psychiatric disorders
Uncommon: abnormal dreams
No data are available in humans regarding overdose by accidental or deliberate ingestion. In case of
the deliberate ingestion of the contents of many unit doses of EMADINE, the potential of emedastine
to increase the QT interval should be borne in mind and appropriate monitoring and management
should be implemented.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group:
decongestants and antiallergics; other antiallergics.
Emedastine is a potent selective and topically effective histamine H
1
antagonist (K
i
= 1.3 nM).
In vitro
examinations of emedastine’s affinity for histamine receptors (H
1
, H
2
, and H
3
) demonstrate
10,000-fold selectivity for the H
1
receptor, K
i
s = 1.3 nM, 49,064 nM and 12, 430 nM, respectively.
In
vivo
topical ocular administration of emedastine produces a concentration-dependent inhibition of
histamine-stimulated conjunctival vascular permeability. Studies with emedastine have not shown
effects on adrenergic, dopaminergic, and serotonin receptors.
5.2 Pharmacokinetic properties
Absorption
Emedastine is absorbed systemically, as are other topically administered drug substances. In a study
involving ten normal volunteers dosed bilaterally twice daily for 15 days with EMADINE 0.5 mg/ml
eye drops, solution
,
plasma concentrations of the parent compound were generally below the
quantitation limit of the assay (0.3 ng/ml). Samples in which emedastine was quantifiable ranged
from 0.30 to 0.49 ng/ml.
The human oral bioavailability of emedastine is approximately 50% and maximum plasma
concentrations were achieved within one-two hours after dosing.
Metabolism
Emedastine is principally metabolised by the liver. The elimination half-life of topical emedastine is
ten hours. Approximately 44% of an oral dose is recovered in the urine over 24 hours, with only 3.6%
of the dose excreted as parent drug substance. Two primary metabolites, 5-and 6-hydroxyemedastine,
are excreted in the urine as both free and conjugated forms. The 5′-oxo analogues of 5-and
6-hydroxyemedastine and the N-oxide are also formed as minor metabolites.
5.3 Preclinical Safety Data
Emedastine difumarate demonstrated low acute toxicity in a number of species by various routes of
administration. No clinically significant local or systemic effects were observed in long-term topical
ocular studies in rabbits.
Corneal limbal mononuclear cell infiltrates were noted in 1/4 male monkeys treated with 0.5 mg/ml
and in 4/4 males and 1/4 females treated with 1.0 mg/ml. Scleral mononuclear cell infiltrates were
present in 1/4 males and 1/4 females treated with 0.5 mg/ml and in 2/4 males and 1/4 females treated
with 1.0 mg/ml. Mean peak plasma levels were approximately 1 ng/ml and 2 ng/ml for the 0.5 and
1.0 mg/ml treatments respectively.
Emedastine was found to increase the QT interval in dogs; the NOEL corresponds to levels 23 fold
higher than those found in patients (7 ng/ml as compared with 0.3 ng/ml, i.e., the limit of detection for
emedastine).
Emedastine difumarate was not found to be carcinogenic in studies in mice and rats. Emedastine
difumarate was not genotoxic in a standard battery of
in vitro
and
in vivo
genotoxicity assays.
In a teratology study in rats, foetotoxic but not teratogenic effects were observed at the highest dose
evaluated (140 mg/kg/day); no effects were observed at a lower level (40 mg/kg/day) which
corresponds to an exposure well in excess of that produced by the therapeutic recommended dose. No
reproductive toxicity was observed in a study in rabbits.
PHARMACEUTICAL PARTICULARS
Trometamol
Sodium chloride
Hypromellose
Hydrochloric acid/sodium hydroxide (to adjust pH)
Purified water
After first opening a foil pouch: 7 days.
6.4 Special precautions for storage
6.5 Nature and content of container
EMADINE is supplied in low density polyethylene single-dose containers which contain 0.35 ml.
Five single-dose containers are then presented in a foil pouch.
The following pack sizes are available: 30 x 0.35 ml single-dose containers and 60 x 0.35 ml
single-dose containers. Not all pack sizes may be marketed.
6.6 Instructions for use and handling, and disposal
For single use only; one container is sufficient to treat both eyes. Any unused solution should be discarded
immediately after use.
MARKETING AUTHORISATION HOLDER
Alcon Laboratories (UK) Ltd.
Pentagon Park
Boundary Way
Hemel Hempstead
Herts., HP2 7UD
United Kingdom
MARKETING AUTHORISATION NUMBERS
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: (30
th
May 2002 SD MAA) 27
th
January 1999
Date of last renewal: 29
th
January 2004
DATE OF REVISION OF THE TEXT
A.
MANUFACTURING AUTHORISATION HOLDERS
RESPONSIBLE FOR BATCH RELEASE
B.
CONDITIONS OF THE MARKETING AUTHORISATION
MANUFACTURING AUTHORISATION HOLDERS RESPONSIBLE FOR BATCH
Names and addresses of the manufacturers responsible for batch release
EMADINE 0.5 mg/ml Eye Drops, solution
S.A. Alcon-Couvreur N.V.,
Rijksweg 14,
B-2870 Puurs,
Belgium.
Alcon Cusí, S.A.,
Camil Fabra 58,
08320 El Masnou,
Barcelona,
Spain.
EMADINE 0.5 mg/ml Eye Drops, solution, single-dose container.
Laboratoires Alcon,
23 Rue Georges Ferrenbach,
F-68240 Kaysersberg
France
The printed package leaflet of the medicinal product must state the name and address of the
manufacturer responsible for the release of the concerned batch.
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to medical prescription
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
BOX OF 1 BOTTLE, 5 ml & 10 ml.
NAME OF THE MEDICINAL PRODUCT
EMADINE 0.5 mg/ml Eye Drops, solution.
Emedastine
STATEMENT OF ACTIVE SUBSTANCE
Emedastine 0.5 mg/ml as difumarate
Contains: benzalkonium chloride 0.1 mg/ml, trometamol, sodium chloride, hypromellose,
hydrochloric acid/sodium hydroxide (to adjust pH), purified water.
PHARMACEUTICAL FORM AND CONTENTS
Eye drops, solution;
1 x 5 ml
1 x 10 ml
METHOD AND ROUTE OF ADMINISTRATION
Ocular use. Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNINGS, IF NECESSARY
EXP:
Discard four weeks after first opening.
Opened:
SPECIAL STORAGE CONDITIONS






























10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Alcon Laboratories (UK) Ltd
Pentagon Park
Boundary Way
Hemel Hempstead
Herts., HP2 7UD
United Kingdom
12. MARKETING AUTHORISATION NUMBERS
EU/1/98/095/001 1 x 5 ml
EU/1/98/095/002 1 x 10 ml
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.





















MINIMUM PARTICULARS TO APPEAR ON SMALL IMMEDIATE PACKAGING UNITS
BOTTLE LABEL 5 ml & 10 ml
NAME OF THE MEDICINAL PRODUCT AND ROUTE OF ADMINISTRATION
EMADINE 0.5 mg/ml Eye Drops.
Emedastine.
Ocular use.
Read the package leaflet before use.
EXP:
Discard four weeks after first opening.
Opened:
CONTENTS BY WEIGHT, BY VOLUME OR BY UNIT





















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
Carton for 30 x 0.35 ml containers & carton of 60 x 0.35 ml containers
NAME OF MEDICINAL PRODUCT
EMADINE 0.5 mg/ml Eye Drops, solution, single-dose container.
Emedastine
STATEMENT OF ACTIVE SUBSTANCE
Emedastine 0.5 mg/ml as difumarate.
Contains: trometamol, sodium chloride, hypromellose, hydrochloric acid, sodium hydroxide and
purified water.
PHARMACEUTICAL FORM AND CONTENTS
Eye drops, solution;
0.35 ml x 30.
0.35 ml x 60
METHOD AND ROUTE OF ADMINISTRATION
Ocular use. Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNINGS, IF NECESSARY
For single use only; one container is sufficient to treat both eyes. Preservative free..
Exp:
Discard any unused contents of a single use container immediately after use.
Discard any unused containers one week after first opening pouch.























SPECIAL STORAGE CONDITIONS
10 SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Alcon Laboratories (UK) Ltd.
Pentagon Park
Boundary Way
Hemel Hempstead
Herts., HP2 7UD
United Kingdom.
MARKETING AUTHORISATION NUMBERS
EU/1/98/095/003 0.35 ml x 30
EU/1/98/095/004 0.35 ml x 60
14 GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.




















PARTICULARS TO APPEAR ON THE OUTER PACKAGING OR, WHERE THERE IS NO
OUTER PACKAGING, ON THE IMMEDIATE PACKAGING
NAME OF THE MEDICINAL PRODUCT
EMADINE 0.5 mg/ml Eye Drops, solution, single-dose container.
Emedastine
STATEMENT OF ACTIVE SUBSTANCE
Emedastine 0.5 mg/ml as difumarate.
Contains: trometamol, sodium chloride, hypromellose, hydrochloric acid, sodium hydroxide and
purified water.
PHARMACEUTICAL FORM AND CONTENTS
Eye drops, solution; 0.35 ml x 5.
METHOD AND ROUTE OF ADMINISTRATION
Ocular use. Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNINGS, IF NECESSARY
For single use only; one container is sufficient to treat both eyes. Preservative free.
EXP:
Discard any unused contents of a single use container immediately after use.
Discard any unused containers one week after first opening pouch.




















SPECIAL STORAGE CONDITIONS
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Alcon Laboratories (UK) Ltd.
Pentagon Park
Boundary Way
Hemel Hempstead
Herts., HP2 7UD
United Kingdom.
12. MARKETING AUTHORISATION NUMBERS
EU/1/98/095/003 0.35 ml x 30
EU/1/98/095/004 0.35 ml x 60
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
Tear open pouch at level of cut.


















PACKAGE LEAFLET
EMADINE 0.5 mg/ml Eye Drops, solution.
Emedastine
Read all of this leaflet carefully
before you start using this medicine.
Keep this leaflet.
You may need to read it again. If you have any further questions, ask your doctor or
your pharmacist.
This medicine has been prescribed for you
.
Do not pass it on to others. It may harm them even if their
symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
What EMADINE is and what it is used for
1. WHAT EMADINE IS AND WHAT IT IS USED FOR
EMADINE is a medicine
for the treatment of seasonal allergic conjunctivitis of the eye (allergic
conditions of the eye). It works by reducing the intensity of the allergic reaction.
Allergic conjunctivitis.
Some materials (allergens) like pollens, house dust or animal fur may cause
allergic reactions resulting in itching, redness as well as swelling of the surface of your eye.
if you are allergic
to emedastine or any of the other ingredients.
Ask your doctor for advice.
Take special care with EMADINE ...
Do not use EMADINE in children under the age of 3 years.
If you wear contact lenses
please see section ‘Important information about some of the
eingredients of Emadine below’.
EMADINE is not recommended
for use in patients over 65 years of age, as it has not been
studied in clinical trials in this age group.
EMADINE is
not recommended
for use in patients with kidney or liver problems.
Please tell your doctor or pharmacist if you are taking (or have recently taken) any other
medicines
including medicines obtained without a prescription.
If you are using other eye drops at the same time as EMADINE, follow the advice at the end of
section 3 “How to use EMADINE”.
Pregnancy and breast feeding
If you are pregnant, or might get pregnant
, if you are breast-feeding. Ask your doctor for
advice.
Driving and using machines
You may find that your vision is blurred for a time just after you use EMADINE. Do not drive or use
machines until your vision is clear.
Important information about some of the ingredients of EMADINE
A preservative in EMADINE (benzalkonium chloride) may cause eye irritation and is known to
discolour soft contact lenses. Therefore do not wear contact lenses whilst using EMADINE. Wait
15 minutes after using EMADINE before putting your lenses back in.
Always use EMADINE exactly as your doctor has told you. You should check with your doctor or
pharmacist if you are not sure.
Adults and children over 3 years:
One drop in the eye, twice a day
.
Use this much unless your doctor tells you something differently. Use it for as long as your doctor tells
you to. Only use drops in both eyes if your doctor tells you to.
Only use the drops
in your eyes.
Turn the page for more advice
3. HOW TO USE EMADINE (
continued)
How much to use
< see side 1
Get the EMADINE bottle and a mirror.
Take the bottle and twist off the cap.



Hold the bottle, pointing down, between your thumb and middle finger.
Tilt your head back. Pull down your eyelid with a clean finger, until there is a ‘pocket’ between
the eyelid and your eye. The drop will go in here (picture 1).
Bring the bottle tip close to the eye. Use the mirror if it helps.
Don’t touch your eye or eyelid, surrounding areas or other surfaces with the dropper.
It
could infect the drops left in the bottle.
Gently press on the base
of the bottle to release one drop of EMADINE at a time.
Don’t squeeze the bottle,
it is designed so that just a gentle press on the bottom is needed
(picture 2).
If you use drops in both eyes, repeat the steps for your other eye.
Put the bottle cap back on firmly immediately after use.
If you accidentally swallow EMADINE or inject it contact a doctor immediately.
It may affect
your heart rhythm.
If a drop misses your eye
, try again.
If you get too much in your eyes,
rinse it all out preferably with sterile saline or, if not available, with
warm water. Don’t put in any more drops until it’s time for your next regular dose.
If you forget to use EMADINE,
use one drop as soon as you remember, and then go back to your
normal routine.
Do not use a double dose
to make up for the one missed.
If you are using other eye drops,
leave at least 10 minutes between using EMADINE and the other
drops.
Like all medicines EMADINE
can cause side effects although not everyone gets them. These can be
unpleasant, but most of them disappear quickly.
You can carry on taking the drops, unless the effects are serious, If you are worried, talk to your
doctor or pharmacist.
Common side effects
(affects 1 to 10 users in 100)
Effects in the eye:
eye pain, eye irritation, blurred vision, corneal staining, dry eye, itchy eye, eye
redness
General side effects:
headache
Uncommon side effects
(affects 1 to 10 users in 1,000)
Effects in the eye:
corneal disorder, abnormal eye sensation, increased tear production, tired eyes
General side effects:
difficulty sleeping, sinus headache, bad taste, rash
Not Known
(frequency cannot be estimated from the available data)
General side effects
: increased heart rate
If any of these side effects gets serious or if you notice any side effects not listed, please tell your
doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use EMADINE after the expiry date which is stated on the bottle and box after “EXP”. The
expiry date refers to the last day of the month.
You must throw away the bottle four weeks after you first opened it,
to prevent infections. Write
down the date you opened each bottle in the space below and in the space on the bottle label and box.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help protect the environment.
The active substance is
emadastine 0.5 mg/ml as difumarate.
The other ingredients are:
benzalkonium chloride, trometamol; sodium chloride; hypromellose;
purified water. Tiny amounts of hydrochloric acid or sodium hydroxide are sometimes added to keep
acidity levels (pH levels) normal
What EMADINE looks like and the contents of the pack
EMADINE is a liquid (a solution) supplied in a pack containing a 5 ml or 10 ml plastic
(DROP-TAINER) bottle with a screw cap. Not all pack sizes may be marketed.
The marketing authorisation
holder
Alcon Laboratories (UK) Ltd.,
S.A. Alcon-Couvreur N.V.,
Herts., HP2 7UD,
United Kingdom.





For any information about this medicine, please contactthe local representative of the Marketing
Authorisation Holder.
België/Belgique/Belgien
Luxembourg/Luxemburg
SA Alcon-Couvreur NV
+ 32 (0)3 890 27 11 (België/Belgique/Belgien)
Lietuva
Alcon Pharmaceuticals Ltd. atstovybė
+ 370 5 2 314 756
България
Алкон България ЕООД
+
359 2 950 15 65
Magyarorszag
Alcon Hungária Gyógyszerkereskedelmi Kft.
+ 36-1-463-9080
Česká republika
Alcon Pharmaceuticals (Czech Republic) s.r.o
.
+ 420 225 377 333
Nederland
Alcon Nederland BV
+ 31 (0) 183 654321
Danmark
Alcon Danmark A/S
+ 45 3636 3434
Norge
Alcon Norge AS
+ 47 23 25 25 50
Deutschland
Alcon Pharma GmbH
+ 49 (0)761 1304-0
Österreich
Alcon Ophthalmika GmbH
+ 43 (0)1 596 69 70
Ελλάδα
Κύπρος
Άλκον Λαμποράτορις Ελλάς ΑΕΒΕ
+ 30 210 68 78 300 (Ελλάδα)
Polska
Alcon Polska Sp. z o.o.
+ 48 22 820 3450
Eesti
Alcon Eesti
+ 372 6 313 214
Portugal
Alcon Portugal – Produtos e Equipamentos
Oftalmológicos, Lda.
+ 351 214 400 300
España
Alcon Cusí, S.A.
+ 34 93 497 7000
România
S.C. Alcon Romania S.R.L.
: + 40 21 203 93 24
France
Laboratoires Alcon
+ 33 (0)1 47 10 47 10
Slovenija
Alcon d.o.o.
+ 386 1 422 5280
Ireland
Malta
United Kingdom
Alcon Laboratories (UK) Ltd.
+ 44 (0) 1442 34 1234 (United Kingdom)
Slovenská republika
Alcon Pharmaceuticals Ltd – oz
+ 421 2 5441 0378
Ísland
Alcon Danmark A/S
+ 45 3636 3434
Suomi/Finland
Alcon Finland Oy
+358 207 871 600
Italia
Alcon Italia S.p.A.
+ 39 02 81803.1
Sverige
Alcon Sverige AB
+ 46 (0)8 634 40 00
E-post: receptionen@alconlabs.com
Latvija
Alcon Pharmaceuticals Ltd
+ 371 7 321 121
This leaflet was last approved on xxxxxx
Detailed information on this medicine is available on the European Medicines Agency website:
www.ema.europa.eu
PACKAGE LEAFLET
EMADINE 0.5 mg/ml Eye Drops, solution, single-dose container.
Emedastine
Read all of this leaflet carefully
before you start using this medicine.
Keep this leaflet.
You may need to read it again. If you have any further questions, ask your doctor or
your pharmacist.
This medicine has been prescribed for you
.
Do not pass it on to others. It may harm them even if their
symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
What EMADINE is and what it is used for
1. WHAT EMADINE IS AND WHAT IT IS USED FOR
EMADINE is a medicine
for the treatment of seasonal allergic conjunctivitis of the eye (allergic
conditions of the eye). It works by reducing the intensity of the allergic reaction.
Allergic conjunctivitis
. Some materials (allergens) like pollens, house dust or animal fur may cause
allergic reactions resulting in itching, redness as well as swelling of the surface of your eye.
•
if you are allergic
to emedastine or any of the other ingredients.
Ask your doctor for advice.
Take special care with EMADINE...
Do not use EMADINE in children under the age of 3 years.
EMADINE is not recommended
for use in patients over 65 years of age, as it has not been
studied in clinical trials in this age group.
EMADINE
is not recommended
for use in patients with kidney or liver problems.
Please tell your doctor or pharmacist if you are taking (or have recently taken) any other
medicines
including medicines obtained without a prescription.
If you are using other eye drops at the same time as EMADINE, follow the advice at the end of
section 3 (How to use EMADINE).
Pregnancy and breast feeding
If you are pregnant, or might get pregnant, if you are breast-feeding
. Ask your doctor for advice
Driving and using machines
You may find that your vision is blurred for a time just after you use EMADINE. Do not drive or use
machines until your vision is clear.
Always use EMADINE exactly as your doctor has told you. You should check with your doctor or
pharmacist if you are not sure.
How much to use
Adults and children over 3 years: One drop in the eye, twice a day
.
Use this much unless your doctor tells you something differently. Use it for as long as your doctor
tells you.
Only use drops in both eyes
if your doctor tells you to.
Only use the drops
in your eyes.
Turn the page for more advice
3. HOW TO USE EMADINE
(
continued)
How much to use
< see side 1
Do not use a container you’ve already opened. Do not use any sealed containers from a foil
wrapper that was opened more than a week ago.
Tear off the foil wrapper and take out the strip of 5 containers.
Do not use if the solution is cloudy or has bits in it.
Hold the strip with the long flat end uppermost and separate off one container by pulling it
towards you while holding the others firm. You will need to snap it apart where it joins the
others (picture 1).
Keep the single container out. Put the others back in the foil wrapper.
Make sure you have a mirror handy and wash your hands.
Hold the long flat end of the container between your thumb and forefinger and open it by
twisting off the other end (picture 2).
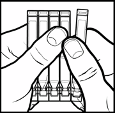


Tilt your head back. Pull down your eyelid with a clean finger, until there is a ‘pocket’ between
the eyelid and your eye. The drop will go in here.
Hold the container between your thumb and fingers with the open end pointing down.
Bring the container tip close to the eye. Use the mirror if it helps.
Don’t touch your eye or eyelid, surrounding areas or other surfaces with the container tip.
It could infect the drops.
Gently squeeze the container to release one drop into the pocket between the eyelid and eye
(picture 3).
If your doctor has told you to use drops in both eyes, repeat the steps for your other
eye-using the same container.
Throw away the container and any left over solution at once.
Throw away any unused containers one week after opening the foil wrapper-even if the
containers are still sealed.
If you accidentally swallow EMADINE or inject it contact a doctor immediately.
It may affect
your heart rhythm.
If a drop misses your eye
, try again.
If you get too much in your eyes,
rinse it all out preferably with sterile saline or, if not available, with
warm water. Don’t put in any more drops until it’s time for your next regular dose.
If you forget to use EMADINE
use one drop as soon as you remember, and then go back to your
normal routine.
Do not use a double dose
to make up for the one missed.
If you are using other eye drops,
leave at least 10 minutes between using EMADINE and the other
drops.
Like all medicines EMADINE
can cause side effects although not everyone gets them. These can be
unpleasant, but most of them disappear quickly.
You can carry on taking the drops, unless the effects are serious. If you are worried, talk to your
doctor or pharmacist.
Common side effects
(affects 1 to 10 users in 100)
Effects in the eye:
eye pain, eye irritation, blurred vision, corneal staining, dry eye, itchy eye, eye
redness
General side effects:
headache
Uncommon side effects
(affects 1 to 10 users in 1,000)
Effects in the eye:
corneal disorder, abnormal eye sensation, increased tear production, tired eyes
General side effects:
difficulty sleeping, sinus headache, bad taste, rash
Not Known
(frequency cannot be estimated from the available data)
General side effects
: increased heart rate
If any of these side effects gets serious or if you notice any side effects not listed, please tell your
doctor or pharmacist.
Keep out of the reach and sight of children
Do not use EMADINE after the expiry date which is stated on the bottle and box after “EXP”. The
expiry date refers to the last day of the month.
You must throw away the container as soon as you have used it.
Once the foil wrapper has been
opened any unused containers must be thrown away one week after you first opened it.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help protect the enviroment
The active substance
is emadastine 0.5 mg/ml as difumarate.
The other ingredients are:
trometamol; sodium chloride; hypromellose; purified water. Tiny
amounts of hydrochloric acid or sodium hydroxide are sometimes added to keep acidity levels (pH
levels) normal.
What EMADINE looks like and the contents of the pack
EMADINE is a liquid (a solution) supplied in single-dose plastic containers which contain 0.35 ml.
Five single-dose containers are provided in a pouch. EMADINE is supplied in packs containing 30 or
60 units. Not all pack sizes may be marketed.
The marketing authorisation holder
Alcon Laboratories (UK) Ltd.,
23 Rue Georges Ferrenbach
Herts., HP2 7UD,
United Kingdom.
For any information about this medicine , please contact the local representative of the Marketing
Authorisation Holder..
België/Belgique/Belgien
Luxembourg/Luxemburg
SA Alcon-Couvreur NV
+ 32 (0)3 890 27 11 (België/Belgique/Belgien)
Lietuva
Alcon Pharmaceuticals Ltd. atstovybė
+ 370 5 2 314 756
България
Алкон България ЕООД
+
359 2 950 15 65
Magyarorszag
Alcon Hungária Gyógyszerkereskedelmi Kft.
+ 36-1-463-9080
Česká republika
Alcon Pharmaceuticals (Czech Republic) s.r.o
.
+ + 420 225 377 333
Nederland
Alcon Nederland BV
+ 31 (0) 183 654321
Danmark
Alcon Danmark A/S
+ 45 3636 3434
Norge
Alcon Norge AS
+ 47 23 25 25 50
Deutschland
Alcon Pharma GmbH
+ 49 (0)761 1304-0
Österreich
Alcon Ophthalmika GmbH
+ 43 (0)1 596 69 70
Ελλάδα
Κύπρος
Άλκον Λαμποράτορις Ελλάς ΑΕΒΕ
+ 30 210 68 78 300 (Ελλάδα)
Polska
Alcon Polska Sp. z o.o.
+ 48 22 820 3450
Eesti
Alcon Eesti
+ 372 6 313 214
Portugal
Alcon Portugal – Produtos e Equipamentos
Oftalmológicos, Lda.
+ 351 214 400 300
España
Alcon Cusí, S.A.
+ 34 93 497 7000
România
S.C. Alcon Romania S.R.L.
: + 40 21 203 93 24
France
Laboratoires Alcon
+ 33 (0)1 47 10 47 10
Slovenija
Alcon d.o.o.
+ 386 1 422 5280
Ireland
Malta
United Kingdom
Alcon Laboratories (UK) Ltd.
+ 44 (0) 1442 34 1234 (United Kingdom)
Slovenská republika
Alcon Pharmaceuticals Ltd – oz
+ 421 2 5441 0378
Ísland
Alcon Danmark A/S
+ 45 3636 3434
Suomi/Finland
Alcon Finland Oy
+358 207 871 600
Italia
Alcon Italia S.p.A.
+ 39 02 81803.1
Sverige
Alcon Sverige AB
+ 46 (0)8 634 40 00
E-post: receptionen@alconlabs.com
Latvija
Alcon Pharmaceuticals Ltd
+ 371 7 321 121
This leaflet was last approved on
Detailed information on this medicine is available on the European Medicines Agency website:
www.ema.europa.eu
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/emadine.html
Copyright © 1995-2021 ITA all rights reserved.
|



















































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































