ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS



















































































































































































































































































































































 | |||||
 |
| ||||
 | |||||
|
|||||
 | |||||

|
Summary for the public
What is Enbrel? Enbrel is a medicine that contains the active substance etanercept. It is available as vials containing a powder that is made up into a solution for injection, and as prefilled syringes and pens containing a solution for injection. Each vial or syringe contains either 25 or 50 mg of etanercept. The pens contain 50 mg of etanercept. What is Enbrel used for? Enbrel is an anti-inflammatory medicine. It is used for the treatment of the following diseases:
For more information, see the Summary of Product Characteristics (also part of the EPAR). The medicine can only be obtained with a prescription. How is Enbrel used? Enbrel treatment should be started and supervised by a specialised doctor who has experience in the diagnosis and treatment of the diseases that Enbrel is used to treat. Enbrel is given by injection under the skin. For adults, the usual recommended dose is 25 mg twice a week or 50 mg once a week. Treatment with 50 mg twice a week can also be used during the first 12 weeks of treatment for psoriasis. For patients below 18 years of age, the dose depends on body weight. The patient or carer can give the injection if they have been trained appropriately. For more information, see the Package Leaflet. Patients who take Enbrel must be given the special alert card that summarises important safety information about the medicine. How does Enbrel work? The active substance in Enbrel, etanercept, is a protein that has been designed to block the activity of a chemical messenger in the body called tumour necrosis factor (TNF). This messenger is found at high levels in patients with the diseases that Enbrel is used to treat. By blocking TNF, etanercept reduces the inflammation and other symptoms of the diseases. Etanercept is produced by a method known as ‘recombinant DNA technology’: it is made by a cell that has received a gene (DNA), which makes it able to produce etanercept. How has Enbrel been studied? Enbrel has been studied in five main studies in rheumatoid arthritis, involving about 2,200 patients and lasting from three months to two years. Three studies compared Enbrel with placebo (a dummy treatment) in patients who had taken anti-arthritis medicines in the past. One of these studies examined Enbrel’s effects as an add-on to methotrexate in 89 patients. In the fourth study, Enbrel was compared with methotrexate in 632 patients who had not taken methotrexate before. The fifth study compared the effectiveness of Enbrel, methotrexate and a combination of both in 686 patients. Enbrel was also compared with placebo in 51 children with polyarticular juvenile idiopathic arthritis, 205 adults with psoriatic arthritis, 357 adults with ankylosing spondylitis, and 1,263 adults and 211 children with plaque psoriasis. In all of the studies, the main measure of effectiveness was the change in symptoms. What benefit has Enbrel shown during the studies? Overall, in the studies of rheumatoid arthritis, about two-thirds of the patients receiving Enbrel had a reduction in symptoms of 20% or more after three months. This compared with around a quarter of the patients receiving placebo. In the study of patients who had not taken methotrexate before, those receiving 25 mg Enbrel twice a week had less joint damage than those taking methotrexate alone after 12 and 24 months. In the fifth study, Enbrel on its own or in combination with methotrexate was more effective than methotrexate alone. For all other diseases studied, Enbrel had produced a greater improvement in symptoms than placebo after three to four months. What is the risk associated with Enbrel? The most common side effects with Enbrel (seen in more than 1 patient in 10) are injection site reactions (including bleeding, bruising, redness, itching, pain and swelling) and infections (including colds, and lung, bladder and skin infections). Patients developing a serious infection should stop Enbrel treatment. For the full list of all side effects reported with Enbrel, see the Package Leaflet. Enbrel should not be used in people who may be hypersensitive (allergic) to etanercept or any of the other ingredients. Enbrel must not be used in patients who have or are at risk of sepsis (when bacteria and toxins circulate in the blood and start to damage the organs), or in patients with infections. Before using Enbrel, doctors must check that the patient is free of infections including tuberculosis. Why has Enbrel been approved? The Committee for Medicinal Products for Human Use (CHMP) decided that Enbrel’s benefits are greater than its risks and recommended that it be given marketing authorisation. Other information about Enbrel The European Commission granted a marketing authorisation valid throughout the European Union for Enbrel to Wyeth Europa Ltd on 3 February 2000. The marketing authorisation is valid for an unlimited period. Authorisation details
|
| |||||||||||||||||
|
Product Characteristics
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
Enbrel 25 mg powder and solvent for solution for injection.
Each vial contains 25 mg of etanercept.
Etanercept is a human tumour necrosis factor receptor p75 Fc fusion protein produced by recombinant
DNA technology in a Chinese hamster ovary (CHO) mammalian expression system. Etanercept is a dimer
of a chimeric protein genetically engineered by fusing the extracellular ligand binding domain of human
tumour necrosis factor receptor-2 (TNFR2/p75) to the Fc domain of human IgG1. This Fc component
contains the hinge, CH
2
and CH
3
regions, but not the CH
1
region of IgG1. Etanercept contains 934 amino
acids and has an apparent molecular weight of approximately 150 kilodaltons. The potency is determined
by measuring the ability of etanercept to neutralise the TNFα-mediated growth inhibition of A375 cells.
The specific activity of etanercept is 1.7 x 10
6
units/mg.
For a full list of excipients, see section 6.1.
Powder and solvent for solution for injection (powder for injection).
The powder is white. The solvent is a clear, colourless liquid.
Rheumatoid arthritis
Enbrel in combination with methotrexate is indicated for the treatment of moderate to severe active
rheumatoid arthritis in adults when the response to disease-modifying antirheumatic drugs, including
methotrexate (unless contraindicated), has been inadequate.
Enbrel can be given as monotherapy in case of intolerance to methotrexate or when continued treatment
with methotrexate is inappropriate.
Enbrel is also indicated in the treatment of severe, active and progressive rheumatoid arthritis in adults
not previously treated with methotrexate.
Enbrel, alone or in combination with methotrexate, has been shown to reduce the rate of progression of
joint damage as measured by X-ray and to improve physical function.
Polyarticular juvenile idiopathic arthritis
Treatment of active polyarticular juvenile idiopathic arthritis in children and adolescents from the age of
4 years who have had an inadequate response to, or who have proved intolerant of, methotrexate. Enbrel
has not been studied in children aged less than 4 years.
2
Psoriatic arthritis
Treatment of active and progressive psoriatic arthritis in adults when the response to previous disease-
modifying antirheumatic drug therapy has been inadequate. Enbrel has been shown to improve physical
function in patients with psoriatic arthritis, and to reduce the rate of progression of peripheral
joint
damage as measured by X-ray in patients with polyarticular symmetrical subtypes of the disease.
Ankylosing spondylitis
Treatment of adults with severe active ankylosing spondylitis who have had an inadequate response to
conventional therapy.
Plaque psoriasis
Treatment of adults with moderate to severe plaque psoriasis who failed to respond to, or who have a
contraindication to, or are intolerant to other systemic therapy, including ciclosporin, methotrexate or
psoralen and ultraviolet-A light
(PUVA) (see section 5.1).
Paediatric plaque psoriasis
Treatment of chronic severe plaque psoriasis in children and adolescents from the age of 8 years who are
inadequately controlled by, or are intolerant to, other systemic therapies or phototherapies.
Enbrel treatment should be initiated and supervised by specialist physicians experienced in the diagnosis
and treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing
spondylitis, plaque psoriasis or paediatric plaque psoriasis. Patients treated with Enbrel should be given
the Patient Alert Card.
Enbrel is available in strengths of 25 and 50 mg.
Posology
Rheumatoid arthritis
25 mg Enbrel administered twice weekly is the recommended dose. Alternatively, 50 mg administered
once weekly has been shown to be safe and effective (see section 5.1).
Psoriatic arthritis and ankylosing spondylitis
The recommended dose is 25 mg Enbrel administered twice weekly, or 50 mg administered once weekly.
Plaque psoriasis
The recommended dose of Enbrel is 25 mg administered twice weekly or 50 mg administered once
weekly. Alternatively, 50 mg given twice weekly may be used for up to 12 weeks followed, if necessary,
by a dose of 25 mg twice weekly or 50 mg once weekly. Treatment with Enbrel should continue until
remission is achieved, for up to 24 weeks. Continuous therapy beyond 24 weeks may be appropriate for
some adult patients (see section 5.1). Treatment should be discontinued in patients who show no response
after 12 weeks. If re-treatment with Enbrel is indicated, the same guidance on treatment duration should
be followed. The dose should be 25 mg twice weekly or 50 mg once weekly.
Special populations
Elderly (
≥
65 years)
No dose adjustment is required. Posology and administration are the same as for adults 18-64 years of
age.
3
Paediatric population
Juvenile idiopathic arthritis (age 4 years and above)
The recommended dose is 0.4 mg/kg (up to a maximum of 25 mg per dose) after reconstitution of 25 mg
Enbrel in 1 ml of solvent, given twice weekly as a subcutaneous injection with an interval of 3-4 days
between doses.
Paediatric plaque psoriasis (age 8 years and above)
The recommended dose is 0.8 mg/kg (up to a maximum of 50 mg per dose) once weekly for up to 24
weeks. Treatment should be discontinued in patients who show no response after 12 weeks.
If re-treatment with Enbrel is indicated, the above guidance on treatment duration should be followed.
The dose should be 0.8 mg/kg (up to a maximum of 50 mg per dose) once weekly.
Renal and hepatic impairment
No dose adjustment is required.
Method of administration
Enbrel is administered by subcutaneous injection.
Comprehensive instructions for the preparation and administration of the reconstituted Enbrel vial are
given in the package leaflet, section 7, "Instructions for preparation and giving an injection of Enbrel."
Hypersensitivity to the active substance or to any of the excipients.
Sepsis or risk of sepsis.
Treatment with Enbrel should not be initiated in patients with active infections, including chronic or
localised infections.
Infections
Patients should be evaluated for infections before, during, and after treatment with Enbrel, taking into
consideration that the mean elimination half-life of etanercept is approximately 70 hours (range 7 to
300 hours).
Serious infections, sepsis, tuberculosis, and opportunistic infections, including invasive fungal infections,
have been reported with the use of Enbrel (see section 4.8). These infections were due to bacteria,
mycobacteria, fungi and viruses. In some cases, particular fungal and other opportunistic infections have
not been recognised, resulting in delay of appropriate treatment and sometimes death. In evaluating
patients for infections, the patient’s risk for relevant opportunistic infections (e.g., exposure to endemic
mycoses) should be considered.
Patients who develop a new infection while undergoing treatment with Enbrel should be monitored
closely. Administration of Enbrel should be discontinued if a patient develops a serious infection. The
safety and efficacy of Enbrel in patients with chronic infections have not been evaluated. Physicians
4
should exercise caution when considering the use of Enbrel in patients with a history of recurring or
chronic infections or with underlying conditions that may predispose patients to infections, such as
advanced or poorly controlled diabetes.
Tuberculosis
Cases of active tuberculosis, including miliary tuberculosis and tuberculosis with extra-pulmonary
location, have been reported in patients treated with Enbrel.
Before starting treatment with Enbrel, all patients must be evaluated for both active and inactive (‘latent’)
tuberculosis. This evaluation should include a detailed medical history with personal history of
tuberculosis or possible previous contact with tuberculosis and previous and/or current
immunosuppressive therapy. Appropriate screening tests, i.e., tuberculin skin test and chest X-ray, should
be performed in all patients (local recommendations may apply). It is recommended that the conduct of
these tests should be recorded in the patient’s alert card. Prescribers are reminded of the risk of false
negative tuberculin skin test results, especially in patients who are severely ill or immunocompromised.
If active tuberculosis is diagnosed, Enbrel therapy must not be initiated. If inactive (‘latent’) tuberculosis
is diagnosed, treatment for latent tuberculosis must be started with anti-tuberculosis therapy before the
initiation of Enbrel, and in accordance with local recommendations. In this situation, the benefit/risk
balance of Enbrel therapy should be very carefully considered.
All patients should be informed to seek medical advice if signs/symptoms suggestive of tuberculosis (e.g.,
persistent cough, wasting/weight loss, low-grade fever) appear during or after Enbrel treatment.
Hepatitis B virus reactivation
Reactivation of hepatitis B virus (HBV) in patients who are chronic carriers of this virus who are
receiving TNF-antagonists, including Enbrel, has been reported. Patients at risk for HBV infection should
be evaluated for prior evidence of HBV infection before initiating Enbrel therapy. Caution should be
exercised when administering Enbrel to patients identified as carriers of HBV. If Enbrel is used in
carriers of HBV, the patients should be monitored for signs and symptoms of active HBV infection, and,
if necessary, appropriate treatment should be initiated.
Worsening of hepatitis C
There have been reports of worsening of hepatitis C in patients receiving Enbrel. Enbrel should be used
with caution in patients with a history of hepatitis C.
Concurrent treatment with anakinra
Concurrent administration of Enbrel and anakinra has been associated with an increased risk of serious
infections and neutropenia compared to Enbrel alone. This combination has not demonstrated increased
clinical benefit. Thus, the combined use of Enbrel and anakinra is not recommended (see sections 4.5 and
4.8).
Concurrent treatment with abatacept
In clinical studies, concurrent administration of abatacept and Enbrel resulted in increased incidences of
serious adverse events. This combination has not demonstrated increased clinical benefit; such use is not
recommended (see section 4.5).
Allergic reactions
The needle cover of the pre-filled syringe contains latex (dry natural rubber) that may cause
hypersensitivity reactions when handled by, or when Enbrel is administered to, persons with known or
possible latex sensitivity.
5
Allergic reactions associated with Enbrel administration have been reported commonly. Allergic reactions
have included angioedema and urticaria; serious reactions have occurred. If any serious allergic or
anaphylactic reaction occurs, Enbrel therapy should be discontinued immediately and appropriate therapy
initiated.
Immunosuppression
The possibility exists for TNF-antagonists, including Enbrel, to affect host defences against infections
and malignancies since TNF mediates inflammation and modulates cellular immune responses. In a study
of 49 adult patients with rheumatoid arthritis treated with Enbrel, there was no evidence of depression of
delayed-type hypersensitivity, depression of immunoglobulin levels, or change in enumeration of effector
cell populations.
Two juvenile idiopathic arthritis patients developed varicella infection and signs and symptoms of aseptic
meningitis, which resolved without sequelae. Patients with a significant exposure to varicella virus should
temporarily discontinue Enbrel therapy and be considered for prophylactic treatment with Varicella
Zoster Immune Globulin.
The safety and efficacy of Enbrel in patients with immunosuppression have not been evaluated.
Malignancies and lymphoproliferative disorders
Solid and haematopoietic malignancies (excluding skin cancers)
Reports of various malignancies (including breast and lung carcinoma and lymphoma) have been received
in the postmarketing period (see section 4.8).
In the controlled portions of clinical trials of TNF-antagonists, more cases of lymphoma have been
observed among patients receiving a TNF-antagonist compared with control patients. However, the
occurrence was rare, and the follow-up period of placebo patients was shorter than for patients receiving
TNF-antagonist therapy. In the postmarketing setting, cases of leukaemia have been reported in patients
treated with TNF-antagonists. There is an increased background risk for lymphoma and leukaemia in
rheumatoid arthritis patients with long-standing, highly active, inflammatory disease, which complicates
risk estimation.
Based on current knowledge, a possible risk for the development of lymphomas, leukaemia or other
haematopoietic or solid malignancies in patients treated with a TNF-antagonist cannot be excluded.
Caution should be exercised when considering TNF-antagonist therapy for patients with a history of
malignancy or when considering continuing treatment in patients who develop a malignancy.
Malignancies, some fatal, have been reported among children, adolescents and young adults (up to 22
years of age) treated with TNF-antagonists (initiation of therapy ≤ 18 years of age), including Enbrel, in
the postmarketing setting. Approximately half the cases were lymphomas. The other cases represented a
variety of different malignancies and included rare malignancies typically associated with
immunosuppression. A risk for the development of malignancies in children and adolescents treated with
TNF-antagonists cannot be excluded.
Skin cancers
Melanoma and non-melanoma skin cancer (NMSC) have been reported in patients treated with
TNF-antagonists, including Enbrel. Postmarketing cases of Merkel cell carcinoma have been reported
very infrequently in patients treated with Enbrel. Periodic skin examination is recommended for all
patients, particularly those with risk factors for skin cancer.
6
Combining the results of controlled clinical trials, more cases of NMSC were observed in patients
receiving Enbrel compared with control patients, particularly in patients with psoriasis.
Vaccinations
Live vaccines should not be given concurrently with Enbrel. No data are available on the secondary
transmission of infection by live vaccines in patients receiving Enbrel. It is recommended that paediatric
patients, if possible, be brought up to date with all immunisations in agreement with current immunisation
guidelines prior to initiating Enbrel therapy. In a double-blind, placebo-controlled, randomised clinical
study in adult patients with psoriatic arthritis, 184 patients also received a multivalent pneumococcal
polysaccharide vaccine at week 4. In this study, most psoriatic arthritis patients receiving Enbrel were
able to mount effective B-cell immune response to pneumococcal polysaccharide vaccine, but titres in
aggregate were moderately lower, and few patients had two-fold rises in titres compared to patients not
receiving Enbrel. The clinical significance of this is unknown.
Autoantibody formation
Treatment with Enbrel may result in the formation of autoimmune antibodies (see section 4.8).
Haematologic reactions
Rare cases of pancytopenia and very rare cases of aplastic anaemia, some with fatal outcome, have been
reported in patients treated with Enbrel. Caution should be exercised in patients being treated with Enbrel
who have a previous history of blood dyscrasias. All patients and parents/caregivers should be advised
that if the patient develops signs and symptoms suggestive of blood dyscrasias or infections (e.g.,
persistent fever, sore throat, bruising, bleeding, paleness) whilst on Enbrel, they should seek immediate
medical advice. Such patients should be investigated urgently, including full blood count; if blood
dyscrasias are confirmed, Enbrel should be discontinued.
Neurological disorders
There have been rare reports of CNS demyelinating disorders in patients treated with Enbrel (see section
4.8). Additionally, there have been very rare reports of peripheral demyelinating polyneuropathies
(including Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy, demyelinating
polyneuropathy, and multifocal motor neuropathy). Although no clinical trials have been performed
evaluating Enbrel therapy in patients with multiple sclerosis, clinical trials of other TNF antagonists in
patients with multiple sclerosis have shown increases in disease activity. A careful risk/benefit evaluation,
including a neurologic assessment, is recommended when prescribing Enbrel to patients with pre-existing
or recent onset of demyelinating disease, or to those who are considered to have an increased risk of
developing demyelinating disease.
Combination therapy
In a controlled clinical trial of two years duration in rheumatoid arthritis patients, the combination of
Enbrel and methotrexate did not result in unexpected safety findings, and the safety profile of Enbrel
when given in combination with methotrexate was similar to the profiles reported in studies of Enbrel and
methotrexate alone. Long-term studies to assess the safety of the combination are ongoing. The long-term
safety of Enbrel in combination with other disease-modifying antirheumatic drugs (DMARD) has not
been established.
The use of Enbrel in combination with other systemic therapies or phototherapy for the treatment of
psoriasis has not been studied.
7
Renal and hepatic impairment
Based on pharmacokinetic data (see section 5.2), no dose adjustment is needed in patients with renal or
hepatic impairment; clinical experience in such patients is limited.
Congestive heart failure
Physicians should use caution when using Enbrel in patients who have congestive heart failure (CHF).
There have been postmarketing reports of worsening of CHF, with and without identifiable precipitating
factors, in patients taking Enbrel. Two large clinical trials evaluating the use of Enbrel in the treatment of
CHF were terminated early due to lack of efficacy. Although not conclusive, data from one of these trials
suggest a possible tendency toward worsening CHF in those patients assigned to Enbrel treatment.
Alcoholic hepatitis
In a phase II randomised placebo-controlled study of 48 hospitalised patients treated with Enbrel or
placebo for moderate to severe alcoholic hepatitis, Enbrel was not efficacious, and the mortality rate in
patients treated with Enbrel was significantly higher after 6 months. Consequently, Enbrel should not be
used in patients for the treatment of alcoholic hepatitis. Physicians should use caution when using Enbrel
in patients who also have moderate to severe alcoholic hepatitis.
Wegener's granulomatosis
A placebo-controlled trial, in which 89 adult patients were treated with Enbrel in addition to standard
therapy (including cyclophosphamide or methotrexate, and glucocorticoids) for a median duration of
25 months, has not shown Enbrel to be an effective treatment for Wegener’s granulomatosis. The
incidence of non-cutaneous malignancies of various types was significantly higher in patients treated with
Enbrel than in the control group. Enbrel is not recommended for the treatment of Wegener’s
granulomatosis.
Hypoglycaemia in patients treated for diabetes
There have been reports of hypoglycaemia following initiation of Enbrel in patients receiving medication
for diabetes, necessitating a reduction in anti-diabetic medication in some of these patients.
Inflammatory bowel disease (IBD) in patients with juvenile idiopathic arthritis (JIA)
There have been reports of IBD in JIA patients being treated with Enbrel (see section 4.8).
Special populations
Elderly patients (≥ 65 years)
In the Phase 3 studies in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis, no overall
differences in adverse events, serious adverse events, and serious infections in patients age 65 or older
who received Enbrel were observed compared with younger patients. However, caution should be
exercised when treating the elderly and particular attention paid with respect to occurrence of infections.
Concurrent treatment with anakinra
Adult patients treated with Enbrel and anakinra were observed to have a higher rate of serious infection
when compared with patients treated with either Enbrel or anakinra alone (historical data).
In addition, in a double-blind, placebo-controlled trial in adult patients receiving background
methotrexate, patients treated with Enbrel and anakinra were observed to have a higher rate of serious
infections (7%) and neutropenia than patients treated with Enbrel (see sections 4.4 and 4.8). The
8
combination Enbrel and anakinra has not demonstrated increased clinical benefit, and is therefore not
recommended.
Concurrent treatment with abatacept
In clinical studies, concurrent administration of abatacept and Enbrel resulted in increased incidences of
serious adverse events. This combination has not demonstrated increased clinical benefit; such use is not
recommended (see section 4.4).
Concurrent treatment with sulfasalazine
In a clinical study of adult patients who were receiving established doses of sulfasalazine, to which
Enbrel was added, patients in the combination group experienced a statistically significant decrease in
mean white blood cell counts in comparison to groups treated with Enbrel or sulfasalazine alone. The
clinical significance of this interaction is unknown. Physicians should use caution when considering
combination therapy with sulfasalazine.
Non-interactions
In clinical trials, no interactions have been observed when Enbrel was administered with glucocorticoids,
salicylates (except sulfasalazine), nonsteroidal anti-inflammatory drugs (NSAIDs), analgesics, or
methotrexate. See section 4.4 for vaccination advice.
No clinically significant pharmacokinetic drug-drug interactions were observed in studies with digoxin or
warfarin.
Pregnancy
There are no studies of Enbrel in pregnant women. Developmental toxicity studies performed in rats and
rabbits have revealed no evidence of harm to the foetus or neonatal rat due to etanercept. Preclinical data
about peri- and postnatal toxicity of etanercept and of effects of etanercept on fertility and general
reproductive performance are not available. Thus, the use of Enbrel in pregnant women is not
recommended, and women of child-bearing potential should be advised not to get pregnant during Enbrel
therapy.
Lactation
It is not known whether etanercept is excreted in human milk. Following subcutaneous administration to
lactacting rats, etanercept was excreted in the milk and detected in the serum of pups. Because
immunoglobulins, in common with many medicinal products, can be excreted in human milk, a decision
should be made whether to discontinue breast-feeding or to discontinue Enbrel while breast-feeding.
No studies on the effects on the ability to drive and use machines have been performed.
Summary of the safety profile
The most commonly reported adverse reactions are injection site reactions (such as pain, swelling,
itching, reddening and bleeding at the puncture site), infections (such as upper respiratory infections,
bronchitis, bladder infections and skin infections), allergic reactions, development of autoantibodies,
itching, and fever.
9
Serious adverse reactions have also been reported for Enbrel. TNF-antagonists, such as Enbrel, affect the
immune system and their use may affect the body’s defenses against infection and cancer. Serious
infections affect fewer than 1 in 100 patients treated with Enbrel. Reports have included fatal and
life-threatening infections and sepsis. Various malignancies have also been reported with use of Enbrel,
including cancers of the breast, lung, skin and lymph glands (lymphoma).
Serious haematological, neurological and autoimmune reactions have also been reported. These include
rare reports of pancytopenia and very rare reports of aplastic anaemia. Central and peripheral
demyelinating events have been seen rarely and very rarely, respectively, with Enbrel use. There have
been rare reports of lupus, lupus-related conditions, and vasculitis.
Tabulated list of adverse reactions
The following list of adverse reactions is based on experience from clinical trials in adults and on
postmarketing experience.
Within the organ system classes, adverse reactions are listed under headings of frequency (number of
patients expected to experience the reaction), using the following categories: very common (≥1/10);
common (≥1/100 to <1/10); uncommon (≥1/1000 to <1/100); rare (≥1/10,000 to <1/1000); very rare
(<1/10,000); not known (cannot be estimated from the available data).
Infections and infestations:
Very common: Infections (including upper respiratory tract infections, bronchitis, cystitis, skin
infections)*
Uncommon: Serious infections (including pneumonia, cellulitis, septic arthritis, sepsis)*
Rare:
Tuberculosis, opportunistic infections
(including invasive fungal, protozoal, bacterial and
atypical mycobacterial infections)*
Neoplasms benign, malignant and unspecified (including cysts and polyps):
Uncommon: Non-melanoma skin cancers* (see section 4.4)
Rare:
Lymphoma, melanoma (see section 4.4)
Not known:
Leukaemia, Merkel cell carcinoma (see section 4.4)
Blood and lymphatic system disorders:
Uncommon: Thrombocytopenia
Rare:
Anaemia, leukopenia, neutropenia, pancytopenia*
Very rare:
Aplastic anaemia*
Immune system disorders:
Common:
Allergic reactions (see Skin and subcutaneous tissue disorders), autoantibody formation*
Rare:
Serious allergic/anaphylactic reactions (including angioedema, bronchospasm)
Not known:
Macrophage activation syndrome*, anti-neutrophilic cytoplasmic antibody positive vasculitis
Nervous system disorders:
Rare:
Seizures
CNS demyelinating events suggestive of multiple sclerosis or localised demyelinating
conditions, such as optic neuritis and transverse myelitis (see section 4.4)
10
Very rare:
Peripheral demyelinating events, including Guillain-Barré syndrome, chronic
inflammatory demyelinating polyneuropathy, demyelinating polyneuropathy, and
multifocal motor neuropathy (see section 4.4)
Eye disorders:
Uncommon: Uveitis
Cardiac disorders:
Rare:
Worsening of congestive heart failure (see section 4.4)
Respiratory, thoracic and mediastinal disorders:
Uncommon: Interstitial lung disease (including pneumonitis and pulmonary fibrosis)*
Hepatobiliary disorders:
Rare: Elevated liver enzymes
Skin and subcutaneous tissue disorders:
Common: Pruritus
Uncommon: Angioedema, urticaria, rash, psoriasiform rash, psoriasis (including new onset or
worsening and pustular, primarily palms and soles)
Rare:
Cutaneous vasculitis (including leukocytoclastic vasculitis), Stevens-Johnson syndrome,
erythema multiforme
Very rare:
Toxic epidermal necrolysis
Musculoskeletal and connective tissue disorders:
Rare:
Subacute cutaneous lupus erythematosus, discoid lupus erythematosus, lupus-like
syndrome
General disorders and administration site conditions:
Very common: Injection site reactions (including bleeding, bruising, erythema, itching, pain, swelling)*
Common:
Fever
*see , below.
Additional information
Malignancies and lymphoproliferative disorders
One hundred and twenty-nine (129) new malignancies of various types were observed in 4,114
rheumatoid arthritis patients treated in clinical trials with Enbrel for up to approximately 6 years,
including 231 patients treated with Enbrel in combination with methotrexate in the 2-year
active-controlled study. The observed rates and incidences in these clinical trials were similar to those
expected for the population studied. A total of 2 malignancies were reported in clinical studies of
approximately 2 years duration involving 240 Enbrel-treated psoriatic arthritis patients. In clinical studies
11
conducted for more than 2 years with 351 ankylosing spondylitis patients, 6 malignancies were reported
in Enbrel-treated patients. In a group of 2,711 plaque psoriasis patients treated with Enbrel in double-
blind and open-label studies of up to 2.5 years, 30 malignancies and 43 nonmelanoma skin cancers were
reported.
In a group of 7,416 patients treated with Enbrel in rheumatoid arthritis, psoriatic arthritis, ankylosing
spondylitis and psoriasis clinical trials, 18 lymphomas were reported.
Reports of various malignancies (including breast and lung carcinoma and lymphoma) have also been
received in the postmarketing period (see section 4.4).
Injection site reactions
Compared to placebo, patients with rheumatic diseases treated with Enbrel had a significantly higher
incidence of injection site reactions (36% vs. 9%). Injection site reactions usually occurred in the first
month. Mean duration was approximately 3 to 5 days. No treatment was given for the majority of
injection site reactions in the Enbrel treatment groups, and the majority of patients who were given
treatment received topical preparations, such as corticosteroids, or oral antihistamines. Additionally, some
patients developed recall injection site reactions characterised by a skin reaction at the most recent site of
injection, along with the simultaneous appearance of injection site reactions at previous injection sites.
These reactions were generally transient and did not recur with treatment.
In controlled trials in patients with plaque psoriasis, approximately 13.6% of patients treated with Enbrel
developed injection site reactions compared with 3.4% of placebo-treated patients during the first 12
weeks of treatment.
Serious infections
In placebo-controlled trials, no increase in the incidence of serious infections (fatal, life-threatening, or
requiring hospitalisation or intravenous antibiotics) was observed. Serious infections occurred in 6.3% of
rheumatoid arthritis patients treated with Enbrel for up to 48 months. These included abscess (at various
sites), bacteraemia, bronchitis, bursitis, cellulitis, cholecystitis, diarrhoea, diverticulitis, endocarditis
(suspected), gastroenteritis, hepatitis B, herpes zoster, leg ulcer, mouth infection, osteomyelitis, otitis,
peritonitis, pneumonia, pyelonephritis, sepsis, septic arthritis, sinusitis, skin infection, skin ulcer, urinary
tract infection, vasculitis, and wound infection. In the 2-year active-controlled study where patients were
treated with either Enbrel alone, methotrexate alone or Enbrel in combination with methotrexate, the rates
of serious infections were similar among the treatment groups. However, it cannot be excluded that the
combination of Enbrel with methotrexate could be associated with an increase in the rate of infections.
There were no differences in rates of infection among patients treated with Enbrel and those treated with
placebo for plaque psoriasis in placebo-controlled trials of up to 24 weeks duration. Serious infections
experienced by Enbrel-treated patients included cellulitis, gastroenteritis, pneumonia, cholecystitis,
osteomyelitis, gastritis, appendicitis,
Streptococcal
fasciitis, myositis, septic shock, diverticulitis and
abscess. In the double-blind and open-label psoriatic arthritis trials, 1 patient reported a serious infection
(pneumonia).
Serious and fatal infections have been reported during use of Enbrel; reported pathogens include bacteria,
mycobacteria (including tuberculosis), viruses and fungi. Some have occurred within a few weeks after
initiating treatment with Enbrel in patients who have underlying conditions (e.g., diabetes, congestive
heart failure, history of active or chronic infections) in addition to their rheumatoid arthritis (see
section 4.4). Enbrel treatment may increase mortality in patients with established sepsis.
12
Opportunistic infections have been reported in association with Enbrel, including invasive fungal,
protozoal, bacterial (including
Listeria
and
Legionella
), and atypical mycobacterial infections. In a pooled
data set of clinical trials, the overall incidence of opportunistic infections was 0.09% for the 15,402
subjects who received Enbrel. The exposure-adjusted rate was 0.06 events per 100 patient-years. In
postmarketing experience, approximately half of all of the case reports of opportunistic infections
worldwide were invasive fungal infections. The most commonly reported invasive fungal infections were
Pneumocystis
and
Aspergillus
. Invasive fungal infections accounted for more than half of the fatalities
amongst patients who developed opportunistic infections. The majority of the reports with a fatal
outcome were in patients with
Pneumocystis
pneumonia, unspecified systemic fungal infections, and
aspergillosis (see section 4.4).
Autoantibodies
Adult patients had serum samples tested for autoantibodies at multiple timepoints. Of the rheumatoid
arthritis patients evaluated for antinuclear antibodies (ANA), the percentage of patients who developed
new positive ANA (≥1:40) was higher in patients treated with Enbrel (11%) than in placebo-treated
patients (5%). The percentage of patients who developed new positive anti-double-stranded DNA
antibodies was also higher by radioimmunoassay (15% of patients treated with Enbrel compared to 4% of
placebo-treated patients) and by
Crithidia luciliae
assay (3% of patients treated with Enbrel compared to
none of placebo-treated patients). The proportion of patients treated with Enbrel who developed
anticardiolipin antibodies was similarly increased compared to placebo-treated patients. The impact of
long-term treatment with Enbrel on the development of autoimmune diseases is unknown.
There have been rare reports of patients, including rheumatoid factor positive patients, who have
developed other autoantibodies in conjunction with a lupus-like syndrome or rashes that are compatible
with subacute cutaneous lupus or discoid lupus by clinical presentation and biopsy.
Pancytopenia and aplastic anaemia
There have been postmarketing reports of pancytopenia and aplastic anaemia, some of which had fatal
outcomes (see section 4.4).
Interstitial lung disease
There have been postmarketing reports of interstitial lung disease (including pneumonitis and pulmonary
fibrosis), some of which had fatal outcomes.
Concurrent treatment with anakinra
In studies when adult patients received concurrent treatment with Enbrel plus anakinra, a higher rate of
serious infections compared to Enbrel alone was observed and 2% of patients (3/139) developed
neutropenia (absolute neutrophil count < 1000/mm
3
). While neutropenic, one patient developed cellulitis
that resolved after hospitalisation (see sections 4.4 and 4.5).
In general, the adverse events in paediatric patients with juvenile idiopathic arthritis were similar in
frequency and type to those seen in adult patients. Differences from adults and other special
considerations are discussed in the following paragraphs.
The types of infections seen in clinical trials in juvenile idiopathic arthritis patients aged 2 to 18 years
were generally mild to moderate and consistent with those commonly seen in outpatient paediatric
populations. Severe adverse events reported included varicella with signs and symptoms of aseptic
meningitis, which resolved without sequelae (see also section 4.4), appendicitis, gastroenteritis,
depression/personality disorder, cutaneous ulcer, oesophagitis/gastritis, group A streptococcal septic
shock, type I diabetes mellitus, and soft tissue and post-operative wound infection.
13
In one study in children with juvenile idiopathic arthritis aged 4 to 17 years, 43 of 69 (62%) children
experienced an infection while receiving Enbrel during 3 months of the study (part 1, open-label), and the
frequency and severity of infections was similar in 58 patients completing 12 months of open-label
extension therapy. The types and proportion of adverse events in juvenile idiopathic arthritis patients
were similar to those seen in trials of Enbrel in adult patients with rheumatoid arthritis, and the majority
were mild. Several adverse events were reported more commonly in 69 juvenile idiopathic arthritis
patients receiving 3 months of Enbrel compared to the 349 adult rheumatoid arthritis patients. These
included headache (19% of patients, 1.7 events per patient year), nausea (9%, 1.0 event per patient year),
abdominal pain (19%, 0.74 events per patient year), and vomiting (13%, 0.74 events per patient year).
There were 4 reports of macrophage activation syndrome in juvenile idiopathic arthritis clinical trials.
There have been reports of inflammatory bowel disease in JIA patients being treated with Enbrel from
post-marketing sources, including a very small number of cases indicating a positive rechallenge (see
section 4.4).
In a 48-week study in 211 children aged 4 to 17 years with paediatric plaque psoriasis, the adverse events
reported were similar to those seen in previous studies in adults with plaque psoriasis.
No dose-limiting toxicities were observed during clinical trials of rheumatoid arthritis patients. The
highest dose level evaluated has been an intravenous loading dose of 32 mg/m
2
followed by subcutaneous
doses of 16 mg/m
2
administered twice weekly. One rheumatoid arthritis patient mistakenly
self-administered 62 mg Enbrel subcutaneously twice weekly for 3 weeks without experiencing
undesirable effects. There is no known antidote to Enbrel.
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Immunosuppressants, Tumour Necrosis Factor alpha (TNF-α) inhibitors,
ATC code: L04AB01
Tumour necrosis factor (TNF) is a dominant cytokine in the inflammatory process of rheumatoid arthritis.
Elevated levels of TNF are also found in the synovium and psoriatic plaques of patients with psoriatic
arthritis and in serum and synovial tissue of patients with ankylosing spondylitis. In plaque psoriasis,
infiltration by inflammatory cells, including T-cells, leads to increased TNF levels in psoriatic lesions
compared with levels in uninvolved skin. Etanercept is a competitive inhibitor of TNF binding to its cell
surface receptors, and thereby inhibits the biological activity of TNF. TNF and lymphotoxin are
pro-inflammatory cytokines that bind to two distinct cell surface receptors: the 55-kilodalton (p55) and
75-kilodalton (p75) tumour necrosis factor receptors (TNFRs). Both TNFRs exist naturally in
membrane-bound and soluble forms. Soluble TNFRs are thought to regulate TNF biological activity.
TNF and lymphotoxin exist predominantly as homotrimers, with their biological activity dependent on
cross-linking of cell surface TNFRs. Dimeric soluble receptors, such as etanercept, possess a higher
affinity for TNF than monomeric receptors and are considerably more potent competitive inhibitors of
14
TNF binding to its cellular receptors. In addition, use of an immunoglobulin Fc region as a fusion element
in the construction of a dimeric receptor imparts a longer serum half-life.
Mechanism of action
Much of the joint pathology in rheumatoid arthritis and ankylosing spondylitis and skin pathology in
plaque psoriasis is mediated by pro-inflammatory molecules that are linked in a network controlled by
TNF. The mechanism of action of etanercept is thought to be its competitive inhibition of TNF binding to
cell surface TNFR, preventing TNF-mediated cellular responses by rendering TNF biologically inactive.
Etanercept may also modulate biologic responses controlled by additional downstream molecules (e.g.,
cytokines, adhesion molecules, or proteinases) that are induced or regulated by TNF.
Clinical trials
This section presents data from four randomised controlled trials in adults with rheumatoid arthritis, one
study in polyarticular juvenile idiopathic arthritis, one study in adults with psoriatic arthritis, one study in
adults with ankylosing spondylitis, one study in paediatric patients with plaque psoriasis, and four studies
in adults with plaque psoriasis.
Adult patients with rheumatoid arthritis
The efficacy of Enbrel was assessed in a randomised, double-blind, placebo-controlled study. The study
evaluated 234 adult patients with active rheumatoid arthritis who had failed therapy with at least one but
no more than four disease-modifying antirheumatic drugs (DMARDs). Doses of 10 mg or 25 mg Enbrel
or placebo were administered subcutaneously twice a week for 6 consecutive months. The results of this
controlled trial were expressed in percentage improvement in rheumatoid arthritis using American
College of Rheumatology (ACR) response criteria.
ACR 20 and 50 responses were higher in patients treated with Enbrel at 3 and 6 months than in patients
treated with placebo (ACR 20: Enbrel 62% and 59%, placebo 23% and 11% at 3 and 6 months,
respectively: ACR 50: Enbrel 41% and 40%, placebo 8% and 5% at months 3 and 6, respectively; p<0.01
Enbrel vs. placebo at all timepoints for both ACR 20 and ACR 50 responses).
Approximately 15% of subjects who received Enbrel achieved an ACR 70 response at month 3 and
month 6 compared to fewer than 5% of subjects in the placebo arm. Among patients receiving Enbrel, the
clinical responses generally appeared within 1 to 2 weeks after initiation of therapy and nearly always
occurred by 3 months. A dose response was seen; results with 10 mg were intermediate between placebo
and 25 mg. Enbrel was significantly better than placebo in all components of the ACR criteria, as well as
other measures of rheumatoid arthritis disease activity not included in the ACR response criteria, such as
morning stiffness. A Health Assessment Questionnaire (HAQ), which included disability, vitality, mental
health, general health status, and arthritis-associated health status subdomains, was administered every
3 months during the trial. All subdomains of the HAQ were improved in patients treated with Enbrel
compared to controls at 3 and 6 months.
After discontinuation of Enbrel, symptoms of arthritis generally returned within a month. Re-introduction
of treatment with Enbrel after discontinuation of up to 24 months resulted in the same magnitudes of
responses as patients who received Enbrel without interruption of therapy based on results of open-label
studies. Continued durable responses have been seen for up to 48 months in open-label extension
treatment trials when patients received Enbrel without interruption; longer-term experience is not
available.
The efficacy of Enbrel was compared to methotrexate in a randomised, active-controlled study with
blinded radiographic evaluations as a primary endpoint in 632 adult patients with active rheumatoid
arthritis (<3 years duration) who had never received treatment with methotrexate. Doses of 10 mg or
15
25 mg Enbrel were administered subcutaneously (SC) twice a week for up to 24 months. Methotrexate
doses were escalated from 7.5 mg/week to a maximum of 20 mg/week over the first 8 weeks of the trial
and continued for up to 24 months. Clinical improvement, including onset of action within 2 weeks with
Enbrel 25 mg, was similar to that seen in the previous trials and was maintained for up to 24 months. At
baseline, patients had a moderate degree of disability, with mean HAQ scores of 1.4 to 1.5. Treatment
with Enbrel 25 mg resulted in substantial improvement at 12 months, with about 44% of patients
achieving a normal HAQ score (less than 0.5). This benefit was maintained in Year 2 of this study.
In this study, structural joint damage was assessed radiographically and expressed as change in Total
Sharp Score (TSS) and its components, the erosion score and Joint Space Narrowing (JSN) score.
Radiographs of hands/wrists and feet were read at baseline and 6, 12, and 24 months. The 10 mg Enbrel
dose had consistently less effect on structural damage than the 25 mg dose. Enbrel 25 mg was
significantly superior to methotrexate for erosion scores at both 12 and 24 months. The differences in
TSS and JSN were not statistically significant between methotrexate and Enbrel 25 mg. The results are
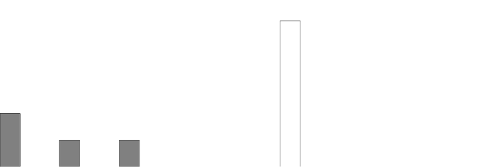
shown in the figure below.
Radiographic Progression: Comparison of Enbrel vs. Methotrexate in Patients with RA of
<3 Years Duration
2.5
12 Months
2.5
2.2
24 Months
2.0
2.0
1.5
1.3
1.5
1.2
1.3
1.0
0.8
0.9
1.0
0.9
0.6*
0.6
0.5
0.4* 0.4 0.4
0.5
0.0
0.0
TSS Erosions JSN
TSS
Erosions JSN
MTX
Enbrel 25 mg
*p < 0.05
In another active-controlled, double-blind, randomised study, clinical efficacy, safety, and radiographic
progression in RA patients treated with Enbrel alone (25 mg twice weekly), methotrexate alone (7.5 to
20 mg weekly, median dose 20 mg), and the combination of Enbrel and methotrexate initiated
concurrently were compared in 682 adult patients with active rheumatoid arthritis of 6 months to 20 years
duration (median 5 years) who had a less than satisfactory response to at least 1 disease-modifying
antirheumatic drug (DMARD) other than methotrexate.
Patients in the Enbrel in combination with methotrexate therapy group had significantly higher ACR 20,
ACR 50, ACR 70 responses and improvement for DAS and HAQ scores at both 24 and 52 weeks than
patients in either of the single therapy groups (results shown in table below). Significant advantages for
Enbrel in combination with methotrexate compared with Enbrel monotherapy and methotrexate
monotherapy were also observed after 24 months.
16


























































Clinical Efficacy Results at 12 Months: Comparison of Enbrel vs. Methotrexate vs.
Enbrel in Combination with Methotrexate in Patients with RA of 6 Months To 20 Years
Duration
Endpoint
Methotrexate
(n = 228)
Enbrel
(n = 223)
Enbrel +
Methotrexate
(n = 231)
ACR Responses
a
ACR 20
58.8%
65.5%
74.5%
†,
φ
ACR 50
36.4%
43.0%
63.2%
†,
φ
ACR 70
16.7%
22.0%
39.8%
†,
φ
DAS
5.5 5.7 5.5
Week 52 score
b
3.0 3.0
2.3
†,
φ
Remission
c
14% 18% 37%
†,
φ
HAQ
Baseline 1.7 1.7 1.8
Week 52 1.1 1.0
0.8
†,
φ
a: Patients who did not complete 12 months in the study were considered to be non-responders.
b: Values for Disease Activity Score (DAS) are means.
c: Remission is defined as DAS <1.6.
Pairwise comparison p-values: †
= p < 0.05 for comparisons of Enbrel + methotrexate vs.
methotrexate and φ
= p < 0.05 for comparisons of Enbrel + methotrexate vs. Enbrel.
Radiographic progression at 12 months was significantly less in the Enbrel group than in the methotrexate
group, while the combination was significantly better than either monotherapy at slowing radiographic
progression (see figure below).
17
Baseline score
b



Radiographic Progression: Comparison of Enbrel vs. Methotrexate vs. Enbrel in Combination with
Methotrexate in Patients with RA of 6 Months To 20 Years Duration (12 Month Results)
3.0
2.80
Methotrexate
Enbrel
Enbrel + Methotrexate
2.5
2.0
1.68
1.5
1.12
1.0
0.52*
0.5
0.21*
0.32
0.0
-0.5
-0.30
†
-0.23
†
,φ
-0.54
†
,φ
-1.0
TSS
Erosions
JSN
Pairwise comparison p-values: * = p < 0.05 for comparisons of Enbrel vs.
methotrexate, † = p < 0.05 for comparisons of Enbrel + methotrexate vs. methotrexate
and φ = p < 0.05 for comparisons of Enbrel + methotrexate vs. Enbrel.
Significant advantages for Enbrel in combination with methotrexate compared with Enbrel monotherapy
and methotrexate monotherapy were also observed after 24 months. Similarly, the significant advantages
for Enbrel monotherapy compared with methotrexate monotherapy were also observed after 24 months.
In an analysis in which all patients who dropped out of the study for any reason were considered to have
progressed, the percentage of patients without progression (TSS change ≤ 0.5) at 24 months was higher in
the Enbrel in combination with methotrexate group compared with the Enbrel alone and methotrexate
alone groups (62%, 50%, and 36%, respectively; p<0.05). The difference between Enbrel alone and
methotrexate alone was also significant (p<0.05). Among patients who completed a full 24 months of
therapy in the study, the non-progression rates were 78%, 70%, and 61%, respectively.
The safety and efficacy of 50 mg Enbrel (two 25 mg SC injections) administered once weekly were
evaluated in a double-blind, placebo-controlled study of 420 patients with active RA. In this study, 53
patients received placebo, 214 patients received 50 mg Enbrel once weekly and 153 patients received
25 mg Enbrel twice weekly. The safety and efficacy profiles of the two Enbrel treatment regimens were
comparable at week 8 in their effect on signs and symptoms of RA; data at week 16 did not show
comparability (non-inferiority) between the two regimens.
Paediatric patients with polyarticular juvenile idiopathic arthritis
The safety and efficacy of Enbrel were assessed in a two-part study in 69 children with polyarticular
juvenile idiopathic arthritis who had a variety of juvenile idiopathic arthritis onset types. Patients aged 4
to 17 years with moderately to severely active polyarticular juvenile idiopathic arthritis refractory to, or
intolerant of, methotrexate were enrolled; patients remained on a stable dose of a single nonsteroidal
anti-inflammatory drug and/or prednisone (< 0.2 mg/kg/day or 10 mg maximum). In part 1, all patients
received 0.4 mg/kg (maximum 25 mg per dose) Enbrel subcutaneously twice weekly. In part 2, patients
18




































































with a clinical response at day 90 were randomised to remain on Enbrel or receive placebo for four
months and assessed for disease flare. Responses were measured using the JRA Definition of
Improvement (DOI), defined as ≥ 30% improvement in at least three of six and ≥ 30% worsening in no
more than one of six JRA core set criteria, including active joint count, limitation of motion, physician
and patient/parent global assessments, functional assessment, and erythrocyte sedimentation rate (ESR).
Disease flare was defined as a ≥ 30% worsening in three of six JRA core set criteria and ≥ 30%
improvement in not more than one of the six JRA core set criteria and a minimum of two active joints.
In part 1 of the study, 51 of 69 (74%) patients demonstrated a clinical response and entered part 2. In part
2, 6 of 25 (24%) patients remaining on Enbrel experienced a disease flare compared to 20 of 26 (77%)
patients receiving placebo (p=0.007). From the start of part 2, the median time to flare was ≥ 116 days for
patients who received Enbrel and 28 days for patients who received placebo. Of patients who
demonstrated a clinical response at 90 days and entered part 2 of the study, some of the patients
remaining on Enbrel continued to improve from month 3 through month 7, while those who received
placebo did not improve.
Studies have not been done in patients with polyarticular juvenile idiopathic arthritis to assess the effects
of continued Enbrel therapy in patients who do not respond within 3 months of initiating Enbrel therapy
or to assess the combination of Enbrel with methotrexate.
Adult patients with psoriatic arthritis
The efficacy of Enbrel was assessed in a randomised, double-blind, placebo-controlled study in 205
patients with psoriatic arthritis. Patients were between 18 and 70 years of age and had active psoriatic
arthritis (≥ 3 swollen joints and ≥ 3 tender joints) in at least one of the following forms: (1) distal
interphalangeal (DIP) involvement; (2) polyarticular arthritis (absence of rheumatoid nodules and
presence of psoriasis); (3) arthritis mutilans; (4) asymmetric psoriatic arthritis; or (5) spondylitis-like
ankylosis. Patients also had plaque psoriasis with a qualifying target lesion ≥ 2 cm in diameter. Patients
had previously been treated with NSAIDs (86%), DMARDs (80%), and corticosteroids (24%). Patients
currently on methotrexate therapy (stable for ≥ 2 months) could continue at a stable dose of
≤ 25 mg/week methotrexate. Doses of 25 mg of Enbrel (based on dose-finding studies in patients with
rheumatoid arthritis) or placebo were administered SC twice a week for 6 months. At the end of the
double-blind study, patients could enter a long-term open-label extension study for a total duration of up
to 2 years.
Clinical responses were expressed as percentages of patients achieving the ACR 20, 50, and 70 response
and percentages with improvement in Psoriatic Arthritis Response Criteria (PsARC). Results are
summarised in the table below.
19
Responses of Patients with Psoriatic Arthritis in a Placebo-
Controlled Trial
Percent of Patients
Placebo
Enbrel
a
Psoriatic Arthritis Response
n = 104
n = 101
ACR 20
Month 3
15
59
b
Month 6
13
50
b
ACR 50
Month 3
4
38
b
Month 6
4
37
b
ACR 70
Month 3
0
11
b
Month 6
1
9
c
PsARC
Month 3
31
72
b
Month 6
23
70
b
a: 25 mg Enbrel SC twice weekly
b: p < 0.001, Enbrel vs. placebo
c: p < 0.01, Enbrel vs. placebo
Among patients with psoriatic arthritis who received Enbrel, the clinical responses were apparent at the
time of the first visit (4 weeks) and were maintained through 6 months of therapy. Enbrel was
significantly better than placebo in all measures of disease activity (p < 0.001), and responses were
similar with and without concomitant methotrexate therapy. Quality of life in psoriatic arthritis patients
was assessed at every timepoint using the disability index of the HAQ. The disability index score was
significantly improved at all timepoints in psoriatic arthritis patients treated with Enbrel, relative to
placebo (p < 0.001).
Radiographic changes were assessed in the psoriatic arthritis study. Radiographs of hands and wrists were
obtained at baseline and months 6, 12, and 24. The modified TSS at 12 months is presented in the table
below. In an analysis in which all patients who dropped out of the study for any reason were considered
to have progressed, the percentage of patients without progression (TSS change ≤ 0.5) at 12 months was
higher in the Enbrel group compared with the placebo group (73% vs. 47%, respectively, p ≤ 0.001). The
effect of Enbrel on radiographic progression was maintained in patients who continued on treatment
during the second year. The slowing of peripheral joint damage was observed in patients with
polyarticular symmetrical joint involvement.
Mean (SE) Annualized Change from Baseline in Total Sharp Score
Placebo
Etanercept
Time
(n = 104)
(n = 101)
Month 12
1.00 (0.29)
-0.03 (0.09)
a
SE = standard error.
a. p = 0.0001.
20





Enbrel treatment resulted in improvement in physical function during the double-blind period, and this
benefit was maintained during the longer-term exposure of up to 2 years.
There is insufficient evidence of the efficacy of Enbrel in patients with ankylosing spondylitis-like and
arthritis mutilans
psoriatic arthropathies due to the small number of patients studied.
No study has been performed in patients with psoriatic arthritis using the 50 mg once-weekly dosing
regimen. Evidence of efficacy for the once-weekly dosing regimen in this patient population has been
based on data from the study in patients with ankylosing spondylitis.
Adult patients with ankylosing spondylitis
The efficacy of Enbrel in ankylosing spondylitis was assessed in 3 randomised, double-blind studies
comparing twice-weekly administration of 25 mg Enbrel with placebo. A total of 401 patients were
enrolled, from which 203 were treated with Enbrel. The largest of these trials (n= 277) enrolled patients
who were between 18 and 70 years of age and had active ankylosing spondylitis defined as visual analog
scale (VAS) scores of ≥ 30 for average of duration and intensity of morning stiffness plus VAS scores of
≥ 30 for at least 2 of the following 3 parameters: patient global assessment; average of VAS values for
nocturnal back pain and total back pain; average of 10 questions on the Bath Ankylosing Spondylitis
Functional Index (BASFI). Patients receiving DMARDs, NSAIDS, or corticosteroids could continue them
on stable doses. Patients with complete ankylosis of the spine were not included in the study. Doses of
25 mg of Enbrel (based on dose-finding studies in patients with rheumatoid arthritis) or placebo were
administered subcutaneously twice a week for 6 months in 138 patients.
The primary measure of efficacy (ASAS 20) was a ≥20% improvement in at least 3 of the 4 Assessment
in Ankylosing Spondylitis (ASAS) domains (patient global assessments, back pain, BASFI, and
inflammation) and absence of deterioration in the remaining domain. ASAS 50 and 70 responses used the
same criteria with a 50% improvement or a 70% improvement, respectively.
Compared to placebo, treatment with Enbrel resulted in significant improvements in the ASAS 20, ASAS
50 and ASAS 70 as early as 2 weeks after the initiation of therapy.
21
Responses of Patients with Ankylosing Spondylitis in a
Placebo-controlled Trial
Percent of Patients
Ankylosing Spondylitis
Response
Placebo
N = 139
Enbrel
N = 138
ASAS 20
2 weeks
22
46
a
3 months
27
60
a
6 months
23
58
a
ASAS 50
2 weeks
7
24
a
3 months
13
45
a
6 months
10
42
a
ASAS 70
2 weeks
2
12
b
3 months
7
29
b
6 months
5
28
b
a: p<0.001, Enbrel vs. placebo
b: p = 0.002, Enbrel vs. placebo
Among patients with ankylosing spondylitis who received Enbrel, the clinical responses were apparent at
the time of the first visit (2 weeks) and were maintained through 6 months of therapy. Responses were
similar in patients who were or were not receiving concomitant therapies at baseline.
Similar results were obtained in the 2 smaller ankylosing spondylitis trials.
In a fourth study, the safety and efficacy of 50 mg Enbrel (two 25 mg SC injections) administered once
weekly vs. 25 mg Enbrel administered twice weekly were evaluated in a double-blind, placebo-controlled
study of 356 patients with active ankylosing spondylitis. The safety and efficacy profiles of the 50 mg
once-weekly and 25 mg twice-weekly regimens were similar.
Adult patients with plaque psoriasis
Enbrel is recommended for use in patients as defined in section 4.1. Patients who “failed to respond to” in
the target population is defined by insufficient response (PASI<50 or PGA less than good), or worsening
of the disease while on treatment, and who were adequately dosed for a sufficiently long duration to
assess response with at least each of the three major systemic therapies as available.
The efficacy of Enbrel versus other systemic therapies in patients with moderate to severe psoriasis
(responsive to other systemic therapies) has not been evaluated in studies directly comparing Enbrel with
other systemic therapies. Instead, the safety and efficacy of Enbrel were assessed in four randomised,
double-blind, placebo-controlled studies. The primary efficacy endpoint in all four studies was the
proportion of patients in each treatment group who achieved the PASI 75 (i.e., at least a 75%
improvement in the Psoriasis Area and Severity Index score from baseline) at 12 weeks.
Study 1 was a Phase 2 study in patients with active, but clinically stable, plaque psoriasis involving ≥
10% of the body surface area who were ≥ 18 years old. One hundred and twelve (112) patients were
randomised to receive a dose of 25 mg of Enbrel (n=57) or placebo (n=55) twice a week for 24 weeks.
22





















Study 2 evaluated 652 patients with chronic plaque psoriasis using the same inclusion criteria as study 1
with the addition of a minimum psoriasis area and severity index (PASI) of 10 at screening. Enbrel was
administered at doses of 25 mg once a week, 25 mg twice a week or 50 mg twice a week for 6 consecutive
months. During the first 12 weeks of the double-blind treatment period, patients received placebo or one
of the above three Enbrel doses. After 12 weeks of treatment, patients in the placebo group began
treatment with blinded Enbrel (25 mg twice a week); patients in the active treatment groups continued to
week 24 on the dose to which they were originally randomised.
Study 3 evaluated 583 patients and had the same inclusion criteria as study 2. Patients in this study
received a dose of 25 mg or 50 mg Enbrel, or placebo twice a week for 12 weeks and then all patients
received open-label 25 mg Enbrel twice weekly for an additional 24 weeks.
Study 4 evaluated 142 patients and had similar inclusion criteria to studies 2 and 3. Patients in this study
received a dose of 50 mg Enbrel or placebo once weekly for 12 weeks and then all patients received open-
label 50 mg Enbrel once weekly for an additional 12 weeks.
In study 1, the Enbrel-treated group had a significantly higher proportion of patients with a PASI 75
response at week 12 (30%) compared to the placebo-treated group (2%) (p<0.0001). At 24 weeks, 56% of
patients in the Enbrel-treated group had achieved the PASI 75 compared to 5% of placebo-treated
patients. Key results of studies 2, 3 and 4 are shown below.
Responses of Patients with Psoriasis in Studies 2, 3 and 4
Study 2
Study 3
Study 4
----------Enbrel---------
--------Enbrel-------
-------Enbrel------
Placebo
25 mg
BIW
50 mg
BIW
Placebo
25 mg
BIW
50 mg
BIW
Placebo
50 mg
QW
50 mg
QW
n = 166
wk 12
n =
162
wk
12
n =
162
wk
24
a
n =
164
wk
12
n =
164
wk
24
a
n = 193
wk 12
n = 196
wk 12
n = 196
wk 12
n = 46
wk 12
n = 96
wk 12
n = 90
wk 24
a
Response
(%)
PASI 50
14
58* 70 74* 77
9
64*
77*
9
69*
83
PASI 75
4
34* 44 49* 59
3
34*
49*
2
38*
71
DSGA
b
,
clear or
almost
clear
5
34* 39 49* 55
4
39*
57*
4
39*
64
*p ≤ 0.0001 compared with placebo
a. No statistical comparisons to placebo were made at week 24 in studies 2 and 4 because the original
placebo group began receiving Enbrel 25 mg BIW or 50 mg once weekly from week 13 to week 24.
b. Dermatologist Static Global Assessment. Clear or almost clear defined as 0 or 1 on a 0 to 5 scale.
Among patients with plaque psoriasis who received Enbrel, significant responses relative to placebo were
apparent at the time of the first visit (2 weeks) and were maintained through 24 weeks of therapy.
Study 2 also had a drug withdrawal period during which patients who achieved a PASI improvement of at
least 50% at week 24 had treatment stopped. Patients were observed off treatment for the occurrence of
rebound (PASI ≥150% of baseline) and for the time to relapse (defined as a loss of at least half of the
improvement achieved between baseline and week 24). During the withdrawal period, symptoms of
psoriasis gradually returned, with a median time to disease relapse of 3 months. No rebound flare of
23













disease and no psoriasis-related serious adverse events were observed. There was some evidence to
support a benefit of re-treatment with Enbrel in patients initially responding to treatment.
In study 3, the majority of patients (77%) who were initially randomised to 50 mg twice weekly and had
their Enbrel dose decreased at week 12 to 25 mg twice weekly maintained their PASI 75 response through
week 36. For patients who received 25 mg twice weekly throughout the study, the PASI 75 response
continued to improve between weeks 12 and 36.
In study 4, the Enbrel-treated group had a higher proportion of patients with PASI 75 at week 12 (38%)
compared to the placebo-treated group (2%) (p<0.0001). For patients who received 50 mg once weekly
throughout the study, the efficacy responses continued to improve with 71% achieving PASI 75 at week
24.
In long-term (up to 34 months) open-label studies where Enbrel was given without interruption, clinical
responses were sustained and safety was comparable to shorter-term studies.
An analysis of clinical trial data did not reveal any baseline disease characteristics that would assist
clinicians in selecting the most appropriate dosing option (intermittent or continuous). Consequently, the
choice of intermittent or continuous therapy should be based upon physician judgment and individual
patient needs.
Paediatric patients with plaque psoriasis
The efficacy of Enbrel was assessed in a randomised, double-blind, placebo-controlled study in 211
paediatric patients aged 4 to 17 years with moderate to severe plaque psoriasis (as defined by an sPGA
score ≥ 3, involving ≥ 10% of the BSA, and PASI ≥ 12). Eligible patients had a history of receiving
phototherapy or systemic therapy, or were inadequately controlled on topical therapy.
Patients received Enbrel 0.8 mg/kg (up to 50 mg) or placebo once weekly for 12 weeks. At week 12, more
patients randomised to Enbrel had positive efficacy responses (e.g., PASI 75) than those randomised to
placebo.
Paediatric Plaque Psoriasis Outcomes at 12 Weeks
Enbrel
0.8 mg/kg Once
Weekly
(N = 106)
Placebo
(N = 105)
PASI 75, n (%)
60 (57%)
a
12 (11%)
PASI 50, n (%)
79 (75%)
a
24 (23%)
sPGA “clear” or “minimal”, n (%)
56 (53%)
a
14 (13%)
Abbreviation: sPGA-static Physician Global Assessment
a. p < 0.0001 compared with placebo
After the 12-week double-blind treatment period, all patients received Enbrel 0.8 mg/kg (up to 50 mg)
once weekly for additional 24 weeks. Responses observed during the open-label period were similar to
those observed in the double-blind period.
During a randomised withdrawal period, significantly more patients re-randomised to placebo
experienced disease relapse (loss of PASI 75 response) compared with patients re-randomised to Enbrel.
With continued therapy, responses were maintained up to 48 weeks.
24


Antibodies to Enbrel
Antibodies to etanercept have been detected in the sera of some subjects treated with etanercept. These
antibodies have all been non-neutralising and are generally transient. There appears to be no correlation
between antibody development and clinical response or adverse events.
In subjects treated with approved doses of etanercept in clinical trials for up to 12 months, cumulative
rates of anti-etanercept antibodies were approximately 6% of subjects with rheumatoid arthritis, 7.5% of
subjects with psoriatic arthritis, 2% of subjects with ankylosing spondylitis, 7% of subjects with
psoriasis, 9.7% of subjects with paediatric psoriasis, and 3% of subjects with juvenile idiopathic
arthritis.
The proportion of subjects who developed antibodies to etanercept in longer-term trials (of up to
3.5 years) increases over time, as expected. However, due to their transient nature, the incidence of
antibodies detected at each assessment point was typically less than 7% in rheumatoid arthritis subjects
and psoriasis subjects.
In a long-term psoriasis study in which patients received 50 mg twice weekly for 96 weeks, the incidence
of antibodies observed at each assessment point was up to approximately 9%.
5.2 Pharmacokinetic properties
Etanercept serum values were determined by an Enzyme-Linked Immunosorbent Assay (ELISA) method,
which may detect ELISA-reactive degradation products, as well as the parent compound.
Etanercept is slowly absorbed from the site of subcutaneous injection, reaching maximum concentration
approximately 48 hours after a single dose. The absolute bioavailability is 76%. With twice-weekly doses,
it is anticipated that steady-state concentrations are approximately twice as high as those observed after
single doses. After a single subcutaneous dose of 25 mg Enbrel, the average maximum serum
concentration observed in healthy volunteers was 1.65 ± 0.66 μg/ml, and the area under the curve was
235 ± 96.6 μg•hr/ml. Dose proportionality has not been formally evaluated, but there is no apparent
saturation of clearance across the dosing range.
A biexponential curve is required to describe the concentration time curve of etanercept. The central
volume of distribution of etanercept is 7.6 l, while the volume of distribution at steady-state is 10.4 l.
Etanercept is cleared slowly from the body. The half-life is long, approximately 70 hours. Clearance is
approximately 0.066 l/hr in patients with rheumatoid arthritis, somewhat lower than the value of 0.11 l/hr
observed in healthy volunteers. Additionally, the pharmacokinetics of Enbrel in rheumatoid arthritis
patients, ankylosing spondylitis and plaque psoriasis patients are similar.
Mean serum concentration profiles at steady state in treated RA patients were C
max
of 2.4 mg/l vs.
2.6 mg/l, C
min
of 1.2 mg/l vs. 1.4 mg/l, and partial AUC of 297 mgh/l vs. 316 mgh/l for 50 mg Enbrel once
weekly (n=21) vs. 25 mg Enbrel twice weekly (n=16), respectively. In an open-label, single-dose,
two-treatment, crossover study in healthy volunteers, etanercept administered as a single 50 mg/ml
injection was found to be bioequivalent to two simultaneous injections of 25 mg/ml.
In a population pharmacokinetics analysis in ankylosing spondylitis patients, the etanercept steady state
AUCs were 466 μg•hr/ml and 474 μg•hr/ml for 50 mg Enbrel once weekly (N= 154) and 25 mg twice
weekly (N = 148), respectively.
25
Although there is elimination of radioactivity in urine after administration of radiolabelled etanercept to
patients and volunteers, increased etanercept concentrations were not observed in patients with acute
renal or hepatic failure. The presence of renal and hepatic impairment should not require a change in
dosage. There is no apparent pharmacokinetic difference between males and females.
Methotrexate has no effect on the pharmacokinetics of etanercept. The effect of Enbrel on the human
pharmacokinetics of methotrexate has not been investigated.
Special populations
Elderly patients
The impact of advanced age was studied in the population pharmacokinetic analysis of etanercept serum
concentrations. Clearance and volume estimates in patients aged 65 to 87 years were similar to estimates
in patients less than 65 years of age.
Paediatric patients with polyarticular juvenile idiopathic arthritis
In a polyarticular juvenile idiopathic arthritis trial with Enbrel, 69 patients (aged 4 to 17 years) were
administered 0.4 mg Enbrel/kg twice weekly for three months. Serum concentration profiles were similar
to those seen in adult rheumatoid arthritis patients. The youngest children (4 years of age) had reduced
clearance (increased clearance when normalised by weight) compared with older children (12 years of
age) and adults. Simulation of dosing suggests that while older children (10-17 years of age) will have
serum levels close to those seen in adults, younger children will have appreciably lower levels.
Paediatric patients with plaque psoriasis
Patients with paediatric plaque psoriasis (aged 4 to 17 years) were administered 0.8 mg/kg (up to a
maximum dose of 50 mg per week) of etanercept once weekly for up to 48 weeks. The mean serum
steady-state trough concentrations ranged from 1.6 to 2.1 mcg/ml at weeks 12, 24, and 48. These mean
concentrations in patients with paediatric plaque psoriasis were similar to the concentrations observed in
patients with juvenile idiopathic arthritis (treated with 0.4 mg/kg etanercept twice weekly, up to
maximum dose of 50 mg per week). These mean concentrations were similar to those seen in adult
patients with plaque psoriasis treated with 25 mg etanercept twice-weekly.
5.3 Preclinical safety data
In the toxicological studies with Enbrel, no dose-limiting or target organ toxicity was evident. Enbrel was
considered to be non-genotoxic from a battery of
in vitro
and
in vivo
studies. Carcinogenicity studies, and
standard assessments of fertility and postnatal toxicity, were not performed with Enbrel due to the
development of neutralising antibodies in rodents.
Enbrel did not induce lethality or notable signs of toxicity in mice or rats following a single subcutaneous
dose of 2000 mg/kg or a single intravenous dose of 1000 mg/kg. Enbrel did not elicit dose-limiting or
target organ toxicity in cynomolgus monkeys following twice weekly subcutaneous administration for 4
or 26 consecutive weeks at a dose (15 mg/kg) that resulted in AUC-based serum drug concentrations that
were over 27-fold higher than that obtained in humans at the recommended dose of 25 mg.
26
6.1 List of excipients
Powder:
Mannitol (E421)
Sucrose
Trometamol
Solvent:
Water for injections
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
6.3 Shelf life
3 years.
Chemical and physical in-use stability has been demonstrated for 48 hours at 2°C - 8°C after
reconstitution. From a microbiological point of view, the reconstituted medicinal product should be used
immediately. If not used immediately, storage times and conditions prior to use are the responsibility of
the user and would normally not be longer that 6 hours at 2°C - 8°C, unless reconstitution has taken place
in controlled and validated aseptic conditions.
6.4 Special precautions for storage
Store in a refrigerator (2°C - 8°C). Do not freeze
For storage conditions of the reconstituted medicinal product, see section 6.3.
6.5 Nature and contents of container
Clear glass vial (4 ml, type I glass) with rubber stoppers, aluminium seals, and flip-off plastic caps.
Enbrel is supplied with pre-filled syringes containing water for injection. The syringes are type I
glass-fitted with stainless-steel needles. Cartons contain 4 vials of Enbrel with 4 pre-filled solvent
syringes and 8 alcohol swabs.
6.6 Special precautions for disposal and other handling
Instructions for use and handling
Enbrel is reconstituted with 1 ml water for injections before use, and administered by subcutaneous
injection. Enbrel contains no antibacterial preservative, and therefore solutions prepared with water for
injections should be administered as soon as possible and within 6 hours following reconstitution. The
solution should be clear and colourless to pale yellow, with no lumps, flakes or particles. Some white
foam may remain in the vial – this is normal. Enbrel should not be used if all the powder in the vial is not
dissolved within 10 minutes. If this is the case, start again with another vial.
27
Comprehensive instructions for the preparation and administration of the reconstituted Enbrel vial are
given in the package leaflet, section 7, ”INSTRUCTIONS FOR PREPARATION AND GIVING AN
INJECTION OF ENBREL”.
Any unused product or waste material should be disposed of in accordance with local requirements.
Wyeth Europa Ltd.
Huntercombe Lane South
Taplow, Maidenhead
Berkshire, SL6 0PH
United Kingdom
EU/1/99/126/001
Date of first authorisation: 3 February 2000
Date of last renewal: 03 February 2010
Detailed information on this product is available on the website of the European Medicines Agency
http://www.ema.europa.eu
28
Enbrel 25 mg powder for solution for injection.
Each vial contains 25 mg of etanercept.
Etanercept is a human tumour necrosis factor receptor p75 Fc fusion protein produced by recombinant
DNA technology in a Chinese hamster ovary (CHO) mammalian expression system. Etanercept is a dimer
of a chimeric protein genetically engineered by fusing the extracellular ligand binding domain of human
tumour necrosis factor receptor-2 (TNFR2/p75) to the Fc domain of human IgG1. This Fc component
contains the hinge, CH
2
and CH
3
regions, but not the CH
1
region of IgG1. Etanercept contains 934 amino
acids and has an apparent molecular weight of approximately 150 kilodaltons. The potency is determined
by measuring the ability of etanercept to neutralise the TNFα-mediated growth inhibition of A375 cells.
The specific activity of etanercept is 1.7 x 10
6
units/mg.
For a full list of excipients, see section 6.1.
Powder for solution for injection (powder for injection).
The powder is white.
Rheumatoid arthritis
Enbrel in combination with methotrexate is indicated for the treatment of moderate to severe active
rheumatoid arthritis in adults when the response to disease-modifying antirheumatic drugs, including
methotrexate (unless contraindicated), has been inadequate.
Enbrel can be given as monotherapy in case of intolerance to methotrexate or when continued treatment
with methotrexate is inappropriate.
Enbrel is also indicated in the treatment of severe, active and progressive rheumatoid arthritis in adults
not previously treated with methotrexate.
Enbrel, alone or in combination with methotrexate, has been shown to reduce the rate of progression of
joint damage as measured by X-ray and to improve physical function.
Polyarticular juvenile idiopathic arthritis
Treatment of active polyarticular juvenile idiopathic arthritis in children and adolescents from the age of
4 years who have had an inadequate response to, or who have proved intolerant of, methotrexate. Enbrel
has not been studied in children aged less than 4 years.
29
Psoriatic arthritis
Treatment of active and progressive psoriatic arthritis in adults when the response to previous disease-
modifying antirheumatic drug therapy has been inadequate. Enbrel has been shown to improve physical
function in patients with psoriatic arthritis, and to reduce the rate of progression of peripheral
joint
damage as measured by X-ray in patients with polyarticular symmetrical subtypes of the disease.
Ankylosing spondylitis
Treatment of adults with severe active ankylosing spondylitis who have had an inadequate response to
conventional therapy.
Plaque psoriasis
Treatment of adults with moderate to severe plaque psoriasis who failed to respond to, or who have a
contraindication to, or are intolerant to other systemic therapy, including ciclosporin, methotrexate or
psoralen and ultraviolet-A light
(PUVA) (see section 5.1).
Paediatric plaque psoriasis
Treatment of chronic severe plaque psoriasis in children and adolescents from the age of 8 years who are
inadequately controlled by, or are intolerant to, other systemic therapies or phototherapies.
Enbrel treatment should be initiated and supervised by specialist physicians experienced in the diagnosis
and treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing
spondylitis, plaque psoriasis or paediatric plaque psoriasis. Patients treated with Enbrel should be given
the Patient Alert Card.
Enbrel is available in strengths of 25 and 50 mg.
Posology
Rheumatoid arthritis
25 mg Enbrel administered twice weekly is the recommended dose. Alternatively, 50 mg administered
once weekly has been shown to be safe and effective (see section 5.1).
Psoriatic arthritis and ankylosing spondylitis
The recommended dose is 25 mg Enbrel administered twice weekly, or 50 mg administered once weekly.
Plaque psoriasis
The recommended dose of Enbrel is 25 mg administered twice weekly or 50 mg administered once
weekly. Alternatively, 50 mg given twice weekly may be used for up to 12 weeks followed, if necessary,
by a dose of 25 mg twice weekly or 50 mg once weekly. Treatment with Enbrel should continue until
remission is achieved, for up to 24 weeks. Continuous therapy beyond 24 weeks may be appropriate for
some adult patients (see section 5.1). Treatment should be discontinued in patients who show no response
after 12 weeks. If re-treatment with Enbrel is indicated, the same guidance on treatment duration should
be followed. The dose should be 25 mg twice weekly or 50 mg once weekly.
Special populations
Elderly (
≥
65 years)
No dose adjustment is required. Posology and administration are the same as for adults 18-64 years of
age.
30
Paediatric population
Juvenile idiopathic arthritis (age 4 years and above)
The recommended dose is 0.4 mg/kg (up to a maximum of 25 mg per dose) after reconstitution of 25 mg
Enbrel in 1 ml of solvent, given twice weekly as a subcutaneous injection with an interval of 3-4 days
between doses.
Paediatric plaque psoriasis (age 8 years and above)
The recommended dose is 0.8 mg/kg (up to a maximum of 50 mg per dose) once weekly for up to 24
weeks. Treatment should be discontinued in patients who show no response after 12 weeks.
If re-treatment with Enbrel is indicated, the above guidance on treatment duration should be followed.
The dose should be 0.8 mg/kg (up to a maximum of 50 mg per dose) once weekly.
Renal and hepatic impairment
No dose adjustment is required.
Method of administration
Enbrel is administered by subcutaneous injection.
Comprehensive instructions for the preparation and administration of the reconstituted Enbrel vial are
given in the package leaflet, section 7, "Instructions for preparation and giving an injection of Enbrel."
Hypersensitivity to the active substance or to any of the excipients.
Sepsis or risk of sepsis.
Treatment with Enbrel should not be initiated in patients with active infections, including chronic or
localised infections.
Infections
Patients should be evaluated for infections before, during, and after treatment with Enbrel, taking into
consideration that the mean elimination half-life of etanercept is approximately 70 hours (range 7 to
300 hours).
Serious infections, sepsis, tuberculosis, and opportunistic infections, including invasive fungal infections,
have been reported with the use of Enbrel (see section 4.8). These infections were due to bacteria,
mycobacteria, fungi and viruses. In some cases, particular fungal and other opportunistic infections have
not been recognised, resulting in delay of appropriate treatment and sometimes death. In evaluating
patients for infections, the patient’s risk for relevant opportunistic infections (e.g., exposure to endemic
mycoses) should be considered.
Patients who develop a new infection while undergoing treatment with Enbrel should be monitored
closely. Administration of Enbrel should be discontinued if a patient develops a serious infection. The
safety and efficacy of Enbrel in patients with chronic infections have not been evaluated. Physicians
31
should exercise caution when considering the use of Enbrel in patients with a history of recurring or
chronic infections or with underlying conditions that may predispose patients to infections, such as
advanced or poorly controlled diabetes.
Tuberculosis
Cases of active tuberculosis, including miliary tuberculosis and tuberculosis with extra-pulmonary
location, have been reported in patients treated with Enbrel.
Before starting treatment with Enbrel, all patients must be evaluated for both active and inactive (‘latent’)
tuberculosis. This evaluation should include a detailed medical history with personal history of
tuberculosis or possible previous contact with tuberculosis and previous and/or current
immunosuppressive therapy. Appropriate screening tests, i.e., tuberculin skin test and chest X-ray, should
be performed in all patients (local recommendations may apply). It is recommended that the conduct of
these tests should be recorded in the patient’s alert card. Prescribers are reminded of the risk of false
negative tuberculin skin test results, especially in patients who are severely ill or immunocompromised.
If active tuberculosis is diagnosed, Enbrel therapy must not be initiated. If inactive (‘latent’) tuberculosis
is diagnosed, treatment for latent tuberculosis must be started with anti-tuberculosis therapy before the
initiation of Enbrel, and in accordance with local recommendations. In this situation, the benefit/risk
balance of Enbrel therapy should be very carefully considered.
All patients should be informed to seek medical advice if signs/symptoms suggestive of tuberculosis (e.g.,
persistent cough, wasting/weight loss, low-grade fever) appear during or after Enbrel treatment.
Hepatitis B virus reactivation
Reactivation of hepatitis B virus (HBV) in patients who are chronic carriers of this virus who are
receiving TNF-antagonists, including Enbrel, has been reported. Patients at risk for HBV infection should
be evaluated for prior evidence of HBV infection before initiating Enbrel therapy. Caution should be
exercised when administering Enbrel to patients identified as carriers of HBV. If Enbrel is used in
carriers of HBV, the patients should be monitored for signs and symptoms of active HBV infection, and,
if necessary, appropriate treatment should be initiated.
Worsening of hepatitis C
There have been reports of worsening of hepatitis C in patients receiving Enbrel. Enbrel should be used
with caution in patients with a history of hepatitis C.
Concurrent treatment with anakinra
Concurrent administration of Enbrel and anakinra has been associated with an increased risk of serious
infections and neutropenia compared to Enbrel alone. This combination has not demonstrated increased
clinical benefit. Thus, the combined use of Enbrel and anakinra is not recommended (see sections 4.5 and
4.8).
Concurrent treatment with abatacept
In clinical studies, concurrent administration of abatacept and Enbrel resulted in increased incidences of
serious adverse events. This combination has not demonstrated increased clinical benefit; such use is not
recommended (see section 4.5).
Allergic reactions
Allergic reactions associated with Enbrel administration have been reported commonly. Allergic reactions
have included angioedema and urticaria; serious reactions have occurred. If any serious allergic or
32
anaphylactic reaction occurs, Enbrel therapy should be discontinued immediately and appropriate therapy
initiated.
Immunosuppression
The possibility exists for TNF-antagonists, including Enbrel, to affect host defences against infections
and malignancies since TNF mediates inflammation and modulates cellular immune responses. In a study
of 49 adult patients with rheumatoid arthritis treated with Enbrel, there was no evidence of depression of
delayed-type hypersensitivity, depression of immunoglobulin levels, or change in enumeration of effector
cell populations.
Two juvenile idiopathic arthritis patients developed varicella infection and signs and symptoms of aseptic
meningitis, which resolved without sequelae. Patients with a significant exposure to varicella virus should
temporarily discontinue Enbrel therapy and be considered for prophylactic treatment with Varicella
Zoster Immune Globulin.
The safety and efficacy of Enbrel in patients with immunosuppression have not been evaluated.
Malignancies and lymphoproliferative disorders
Solid and haematopoietic malignancies (excluding skin cancers)
Reports of various malignancies (including breast and lung carcinoma and lymphoma) have been received
in the postmarketing period (see section 4.8).
In the controlled portions of clinical trials of TNF-antagonists, more cases of lymphoma have been
observed among patients receiving a TNF-antagonist compared with control patients. However, the
occurrence was rare, and the follow-up period of placebo patients was shorter than for patients receiving
TNF-antagonist therapy. In the postmarketing setting, cases of leukaemia have been reported in patients
treated with TNF-antagonists. There is an increased background risk for lymphoma and leukaemia in
rheumatoid arthritis patients with long-standing, highly active, inflammatory disease, which complicates
risk estimation.
Based on current knowledge, a possible risk for the development of lymphomas, leukaemia or other
haematopoietic or solid malignancies in patients treated with a TNF-antagonist cannot be excluded.
Caution should be exercised when considering TNF-antagonist therapy for patients with a history of
malignancy or when considering continuing treatment in patients who develop a malignancy.
Malignancies, some fatal, have been reported among children, adolescents and young adults (up to 22
years of age) treated with TNF-antagonists (initiation of therapy ≤ 18 years of age), including Enbrel, in
the postmarketing setting. Approximately half the cases were lymphomas. The other cases represented a
variety of different malignancies and included rare malignancies typically associated with
immunosuppression. A risk for the development of malignancies in children and adolescents treated with
TNF-antagonists cannot be excluded.
Skin cancers
Melanoma and non-melanoma skin cancer (NMSC) have been reported in patients treated with
TNF-antagonists, including Enbrel. Postmarketing cases of Merkel cell carcinoma have been reported
very infrequently in patients treated with Enbrel. Periodic skin examination is recommended for all
patients, particularly those with risk factors for skin cancer.
Combining the results of controlled clinical trials, more cases of NMSC were observed in patients
receiving Enbrel compared with control patients, particularly in patients with psoriasis.
33
Vaccinations
Live vaccines should not be given concurrently with Enbrel. No data are available on the secondary
transmission of infection by live vaccines in patients receiving Enbrel. It is recommended that paediatric
patients, if possible, be brought up to date with all immunisations in agreement with current immunisation
guidelines prior to initiating Enbrel therapy. In a double-blind, placebo-controlled, randomised clinical
study in adult patients with psoriatic arthritis, 184 patients also received a multivalent pneumococcal
polysaccharide vaccine at week 4. In this study, most psoriatic arthritis patients receiving Enbrel were
able to mount effective B-cell immune response to pneumococcal polysaccharide vaccine, but titres in
aggregate were moderately lower, and few patients had two-fold rises in titres compared to patients not
receiving Enbrel. The clinical significance of this is unknown.
Autoantibody formation
Treatment with Enbrel may result in the formation of autoimmune antibodies (see section 4.8).
Haematologic reactions
Rare cases of pancytopenia and very rare cases of aplastic anaemia, some with fatal outcome, have been
reported in patients treated with Enbrel. Caution should be exercised in patients being treated with Enbrel
who have a previous history of blood dyscrasias. All patients and parents/caregivers should be advised
that if the patient develops signs and symptoms suggestive of blood dyscrasias or infections (e.g.,
persistent fever, sore throat, bruising, bleeding, paleness) whilst on Enbrel, they should seek immediate
medical advice. Such patients should be investigated urgently, including full blood count; if blood
dyscrasias are confirmed, Enbrel should be discontinued.
Neurological disorders
There have been rare reports of CNS demyelinating disorders in patients treated with Enbrel (see section
4.8). Additionally, there have been very rare reports of peripheral demyelinating polyneuropathies
(including Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy, demyelinating
polyneuropathy, and multifocal motor neuropathy). Although no clinical trials have been performed
evaluating Enbrel therapy in patients with multiple sclerosis, clinical trials of other TNF antagonists in
patients with multiple sclerosis have shown increases in disease activity. A careful risk/benefit evaluation,
including a neurologic assessment, is recommended when prescribing Enbrel to patients with pre-existing
or recent onset of demyelinating disease, or to those who are considered to have an increased risk of
developing demyelinating disease.
Combination therapy
In a controlled clinical trial of two years duration in rheumatoid arthritis patients, the combination of
Enbrel and methotrexate did not result in unexpected safety findings, and the safety profile of Enbrel
when given in combination with methotrexate was similar to the profiles reported in studies of Enbrel and
methotrexate alone. Long-term studies to assess the safety of the combination are ongoing. The long-term
safety of Enbrel in combination with other disease-modifying antirheumatic drugs (DMARD) has not
been established.
The use of Enbrel in combination with other systemic therapies or phototherapy for the treatment of
psoriasis has not been studied.
Renal and hepatic impairment
Based on pharmacokinetic data (see section 5.2), no dose adjustment is needed in patients with renal or
hepatic impairment; clinical experience in such patients is limited.
34
Congestive heart failure
Physicians should use caution when using Enbrel in patients who have congestive heart failure (CHF).
There have been postmarketing reports of worsening of CHF, with and without identifiable precipitating
factors, in patients taking Enbrel. Two large clinical trials evaluating the use of Enbrel in the treatment of
CHF were terminated early due to lack of efficacy. Although not conclusive, data from one of these trials
suggest a possible tendency toward worsening CHF in those patients assigned to Enbrel treatment.
Alcoholic hepatitis
In a phase II randomised placebo-controlled study of 48 hospitalised patients treated with Enbrel or
placebo for moderate to severe alcoholic hepatitis, Enbrel was not efficacious, and the mortality rate in
patients treated with Enbrel was significantly higher after 6 months. Consequently, Enbrel should not be
used in patients for the treatment of alcoholic hepatitis. Physicians should use caution when using Enbrel
in patients who also have moderate to severe alcoholic hepatitis.
Wegener's granulomatosis
A placebo-controlled trial, in which 89 adult patients were treated with Enbrel in addition to standard
therapy (including cyclophosphamide or methotrexate, and glucocorticoids) for a median duration of
25 months, has not shown Enbrel to be an effective treatment for Wegener’s granulomatosis. The
incidence of non-cutaneous malignancies of various types was significantly higher in patients treated with
Enbrel than in the control group. Enbrel is not recommended for the treatment of Wegener’s
granulomatosis.
Hypoglycaemia in patients treated for diabetes
There have been reports of hypoglycaemia following initiation of Enbrel in patients receiving medication
for diabetes, necessitating a reduction in anti-diabetic medication in some of these patients.
Inflammatory bowel disease (IBD) in patients with juvenile idiopathic arthritis (JIA)
There have been reports of IBD in JIA patients being treated with Enbrel (see section 4.8).
Special populations
Elderly patients (≥ 65 years)
In the Phase 3 studies in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis, no overall
differences in adverse events, serious adverse events, and serious infections in patients age 65 or older
who received Enbrel were observed compared with younger patients. However, caution should be
exercised when treating the elderly and particular attention paid with respect to occurrence of infections.
Concurrent treatment with anakinra
Adult patients treated with Enbrel and anakinra were observed to have a higher rate of serious infection
when compared with patients treated with either Enbrel or anakinra alone (historical data).
In addition, in a double-blind, placebo-controlled trial in adult patients receiving background
methotrexate, patients treated with Enbrel and anakinra were observed to have a higher rate of serious
infections (7%) and neutropenia than patients treated with Enbrel (see sections 4.4 and 4.8). The
combination Enbrel and anakinra has not demonstrated increased clinical benefit, and is therefore not
recommended.
35
Concurrent treatment with abatacept
In clinical studies, concurrent administration of abatacept and Enbrel resulted in increased incidences of
serious adverse events. This combination has not demonstrated increased clinical benefit; such use is not
recommended (see section 4.4).
Concurrent treatment with sulfasalazine
In a clinical study of adult patients who were receiving established doses of sulfasalazine, to which
Enbrel was added, patients in the combination group experienced a statistically significant decrease in
mean white blood cell counts in comparison to groups treated with Enbrel or sulfasalazine alone. The
clinical significance of this interaction is unknown. Physicians should use caution when considering
combination therapy with sulfasalazine.
Non-interactions
In clinical trials, no interactions have been observed when Enbrel was administered with glucocorticoids,
salicylates (except sulfasalazine), nonsteroidal anti-inflammatory drugs (NSAIDs), analgesics, or
methotrexate. See section 4.4 for vaccination advice.
No clinically significant pharmacokinetic drug-drug interactions were observed in studies with digoxin or
warfarin.
Pregnancy
There are no studies of Enbrel in pregnant women. Developmental toxicity studies performed in rats and
rabbits have revealed no evidence of harm to the foetus or neonatal rat due to etanercept. Preclinical data
about peri- and postnatal toxicity of etanercept and of effects of etanercept on fertility and general
reproductive performance are not available. Thus, the use of Enbrel in pregnant women is not
recommended, and women of child-bearing potential should be advised not to get pregnant during Enbrel
therapy.
Lactation
It is not known whether etanercept is excreted in human milk. Following subcutaneous administration to
lactacting rats, etanercept was excreted in the milk and detected in the serum of pups. Because
immunoglobulins, in common with many medicinal products, can be excreted in human milk, a decision
should be made whether to discontinue breast-feeding or to discontinue Enbrel while breast-feeding.
No studies on the effects on the ability to drive and use machines have been performed.
Summary of the safety profile
The most commonly reported adverse reactions are injection site reactions (such as pain, swelling,
itching, reddening and bleeding at the puncture site), infections (such as upper respiratory infections,
bronchitis, bladder infections and skin infections), allergic reactions, development of autoantibodies,
itching, and fever.
Serious adverse reactions have also been reported for Enbrel. TNF-antagonists, such as Enbrel, affect the
immune system and their use may affect the body’s defenses against infection and cancer. Serious
infections affect fewer than 1 in 100 patients treated with Enbrel. Reports have included fatal and
36
life-threatening infections and sepsis. Various malignancies have also been reported with use of Enbrel,
including cancers of the breast, lung, skin and lymph glands (lymphoma).
Serious haematological, neurological and autoimmune reactions have also been reported. These include
rare reports of pancytopenia and very rare reports of aplastic anaemia. Central and peripheral
demyelinating events have been seen rarely and very rarely, respectively, with Enbrel use. There have
been rare reports of lupus, lupus-related conditions, and vasculitis.
Tabulated list of adverse reactions
The following list of adverse reactions is based on experience from clinical trials in adults and on
postmarketing experience.
Within the organ system classes, adverse reactions are listed under headings of frequency (number of
patients expected to experience the reaction), using the following categories: very common (≥1/10);
common (≥1/100 to <1/10); uncommon (≥1/1000 to <1/100); rare (≥1/10,000 to <1/1000); very rare
(<1/10,000); not known (cannot be estimated from the available data).
Infections and infestations:
Very common: Infections (including upper respiratory tract infections, bronchitis, cystitis, skin
infections)*
Uncommon: Serious infections (including pneumonia, cellulitis, septic arthritis, sepsis)*
Rare:
Tuberculosis, opportunistic infections
(including invasive fungal, protozoal, bacterial and
atypical mycobacterial infections)*
Neoplasms benign, malignant and unspecified (including cysts and polyps):
Uncommon: Non-melanoma skin cancers* (see section 4.4)
Rare:
Lymphoma, melanoma (see section 4.4)
Not known:
Leukaemia, Merkel cell carcinoma (see section 4.4)
Blood and lymphatic system disorders:
Uncommon: Thrombocytopenia
Rare:
Anaemia, leukopenia, neutropenia, pancytopenia*
Very rare:
Aplastic anaemia*
Immune system disorders:
Common:
Allergic reactions (see Skin and subcutaneous tissue disorders), autoantibody formation*
Rare:
Serious allergic/anaphylactic reactions (including angioedema, bronchospasm)
Not known:
Macrophage activation syndrome*, anti-neutrophilic cytoplasmic antibody positive vasculitis
Nervous system disorders:
Rare:
Seizures
CNS demyelinating events suggestive of multiple sclerosis or localised demyelinating
conditions, such as optic neuritis and transverse myelitis (see section 4.4)
37
Very rare:
Peripheral demyelinating events, including Guillain-Barré syndrome, chronic
inflammatory demyelinating polyneuropathy, demyelinating polyneuropathy, and
multifocal motor neuropathy (see section 4.4)
Eye disorders:
Uncommon: Uveitis
Cardiac disorders:
Rare:
Worsening of congestive heart failure (see section 4.4)
Respiratory, thoracic and mediastinal disorders:
Uncommon: Interstitial lung disease (including pneumonitis and pulmonary fibrosis)*
Hepatobiliary disorders:
Rare: Elevated liver enzymes
Skin and subcutaneous tissue disorders:
Common: Pruritus
Uncommon: Angioedema, urticaria, rash, psoriasiform rash, psoriasis (including new onset or
worsening and pustular, primarily palms and soles)
Rare:
Cutaneous vasculitis (including leukocytoclastic vasculitis), Stevens-Johnson syndrome,
erythema multiforme
Very rare:
Toxic epidermal necrolysis
Musculoskeletal and connective tissue disorders:
Rare:
Subacute cutaneous lupus erythematosus, discoid lupus erythematosus, lupus-like
syndrome
General disorders and administration site conditions:
Very common: Injection site reactions (including bleeding, bruising, erythema, itching, pain, swelling)*
Common:
Fever
*see , below.
Additional information
Malignancies and lymphoproliferative disorders
One hundred and twenty-nine (129) new malignancies of various types were observed in 4,114
rheumatoid arthritis patients treated in clinical trials with Enbrel for up to approximately 6 years,
including 231 patients treated with Enbrel in combination with methotrexate in the 2-year
active-controlled study. The observed rates and incidences in these clinical trials were similar to those
expected for the population studied. A total of 2 malignancies were reported in clinical studies of
approximately 2 years duration involving 240 Enbrel-treated psoriatic arthritis patients. In clinical studies
conducted for more than 2 years with 351 ankylosing spondylitis patients, 6 malignancies were reported
in Enbrel-treated patients. In a group of 2,711 plaque psoriasis patients treated with Enbrel in double-
38
blind and open-label studies of up to 2.5 years, 30 malignancies and 43 nonmelanoma skin cancers were
reported.
In a group of 7,416 patients treated with Enbrel in rheumatoid arthritis, psoriatic arthritis, ankylosing
spondylitis and psoriasis clinical trials, 18 lymphomas were reported.
Reports of various malignancies (including breast and lung carcinoma and lymphoma) have also been
received in the postmarketing period (see section 4.4).
Injection site reactions
Compared to placebo, patients with rheumatic diseases treated with Enbrel had a significantly higher
incidence of injection site reactions (36% vs. 9%). Injection site reactions usually occurred in the first
month. Mean duration was approximately 3 to 5 days. No treatment was given for the majority of
injection site reactions in the Enbrel treatment groups, and the majority of patients who were given
treatment received topical preparations, such as corticosteroids, or oral antihistamines. Additionally, some
patients developed recall injection site reactions characterised by a skin reaction at the most recent site of
injection, along with the simultaneous appearance of injection site reactions at previous injection sites.
These reactions were generally transient and did not recur with treatment.
In controlled trials in patients with plaque psoriasis, approximately 13.6% of patients treated with Enbrel
developed injection site reactions compared with 3.4% of placebo-treated patients during the first 12
weeks of treatment.
Serious infections
In placebo-controlled trials, no increase in the incidence of serious infections (fatal, life-threatening, or
requiring hospitalisation or intravenous antibiotics) was observed. Serious infections occurred in 6.3% of
rheumatoid arthritis patients treated with Enbrel for up to 48 months. These included abscess (at various
sites), bacteraemia, bronchitis, bursitis, cellulitis, cholecystitis, diarrhoea, diverticulitis, endocarditis
(suspected), gastroenteritis, hepatitis B, herpes zoster, leg ulcer, mouth infection, osteomyelitis, otitis,
peritonitis, pneumonia, pyelonephritis, sepsis, septic arthritis, sinusitis, skin infection, skin ulcer, urinary
tract infection, vasculitis, and wound infection. In the 2-year active-controlled study where patients were
treated with either Enbrel alone, methotrexate alone or Enbrel in combination with methotrexate, the rates
of serious infections were similar among the treatment groups. However, it cannot be excluded that the
combination of Enbrel with methotrexate could be associated with an increase in the rate of infections.
There were no differences in rates of infection among patients treated with Enbrel and those treated with
placebo for plaque psoriasis in placebo-controlled trials of up to 24 weeks duration. Serious infections
experienced by Enbrel-treated patients included cellulitis, gastroenteritis, pneumonia, cholecystitis,
osteomyelitis, gastritis, appendicitis,
Streptococcal
fasciitis, myositis, septic shock, diverticulitis and
abscess. In the double-blind and open-label psoriatic arthritis trials, 1 patient reported a serious infection
(pneumonia).
Serious and fatal infections have been reported during use of Enbrel; reported pathogens include bacteria,
mycobacteria (including tuberculosis), viruses and fungi. Some have occurred within a few weeks after
initiating treatment with Enbrel in patients who have underlying conditions (e.g., diabetes, congestive
heart failure, history of active or chronic infections) in addition to their rheumatoid arthritis (see
section 4.4). Enbrel treatment may increase mortality in patients with established sepsis.
Opportunistic infections have been reported in association with Enbrel, including invasive fungal,
protozoal, bacterial (including
Listeria
and
Legionella
), and atypical mycobacterial infections. In a pooled
data set of clinical trials, the overall incidence of opportunistic infections was 0.09% for the 15,402
39
subjects who received Enbrel. The exposure-adjusted rate was 0.06 events per 100 patient-years. In
postmarketing experience, approximately half of all of the case reports of opportunistic infections
worldwide were invasive fungal infections. The most commonly reported invasive fungal infections were
Pneumocystis
and
Aspergillus
. Invasive fungal infections accounted for more than half of the fatalities
amongst patients who developed opportunistic infections. The majority of the reports with a fatal
outcome were in patients with
Pneumocystis
pneumonia, unspecified systemic fungal infections, and
aspergillosis (see section 4.4).
Autoantibodies
Adult patients had serum samples tested for autoantibodies at multiple timepoints. Of the rheumatoid
arthritis patients evaluated for antinuclear antibodies (ANA), the percentage of patients who developed
new positive ANA (≥1:40) was higher in patients treated with Enbrel (11%) than in placebo-treated
patients (5%). The percentage of patients who developed new positive anti-double-stranded DNA
antibodies was also higher by radioimmunoassay (15% of patients treated with Enbrel compared to 4% of
placebo-treated patients) and by
Crithidia luciliae
assay (3% of patients treated with Enbrel compared to
none of placebo-treated patients). The proportion of patients treated with Enbrel who developed
anticardiolipin antibodies was similarly increased compared to placebo-treated patients. The impact of
long-term treatment with Enbrel on the development of autoimmune diseases is unknown.
There have been rare reports of patients, including rheumatoid factor positive patients, who have
developed other autoantibodies in conjunction with a lupus-like syndrome or rashes that are compatible
with subacute cutaneous lupus or discoid lupus by clinical presentation and biopsy.
Pancytopenia and aplastic anaemia
There have been postmarketing reports of pancytopenia and aplastic anaemia, some of which had fatal
outcomes (see section 4.4).
Interstitial lung disease
There have been postmarketing reports of interstitial lung disease (including pneumonitis and pulmonary
fibrosis), some of which had fatal outcomes.
Concurrent treatment with anakinra
In studies when adult patients received concurrent treatment with Enbrel plus anakinra, a higher rate of
serious infections compared to Enbrel alone was observed and 2% of patients (3/139) developed
neutropenia (absolute neutrophil count < 1000/mm
3
). While neutropenic, one patient developed cellulitis
that resolved after hospitalisation (see sections 4.4 and 4.5).
In general, the adverse events in paediatric patients with juvenile idiopathic arthritis were similar in
frequency and type to those seen in adult patients. Differences from adults and other special
considerations are discussed in the following paragraphs.
The types of infections seen in clinical trials in juvenile idiopathic arthritis patients aged 2 to 18 years
were generally mild to moderate and consistent with those commonly seen in outpatient paediatric
populations. Severe adverse events reported included varicella with signs and symptoms of aseptic
meningitis, which resolved without sequelae (see also section 4.4), appendicitis, gastroenteritis,
depression/personality disorder, cutaneous ulcer, oesophagitis/gastritis, group A streptococcal septic
shock, type I diabetes mellitus, and soft tissue and post-operative wound infection.
In one study in children with juvenile idiopathic arthritis aged 4 to 17 years, 43 of 69 (62%) children
experienced an infection while receiving Enbrel during 3 months of the study (part 1, open-label), and the
40
frequency and severity of infections was similar in 58 patients completing 12 months of open-label
extension therapy. The types and proportion of adverse events in juvenile idiopathic arthritis patients
were similar to those seen in trials of Enbrel in adult patients with rheumatoid arthritis, and the majority
were mild. Several adverse events were reported more commonly in 69 juvenile idiopathic arthritis
patients receiving 3 months of Enbrel compared to the 349 adult rheumatoid arthritis patients. These
included headache (19% of patients, 1.7 events per patient year), nausea (9%, 1.0 event per patient year),
abdominal pain (19%, 0.74 events per patient year), and vomiting (13%, 0.74 events per patient year).
There were 4 reports of macrophage activation syndrome in juvenile idiopathic arthritis clinical trials.
There have been reports of inflammatory bowel disease in JIA patients being treated with Enbrel from
post-marketing sources, including a very small number of cases indicating a positive rechallenge (see
section 4.4).
In a 48-week study in 211 children aged 4 to 17 years with paediatric plaque psoriasis, the adverse events
reported were similar to those seen in previous studies in adults with plaque psoriasis.
No dose-limiting toxicities were observed during clinical trials of rheumatoid arthritis patients. The
highest dose level evaluated has been an intravenous loading dose of 32 mg/m
2
followed by subcutaneous
doses of 16 mg/m
2
administered twice weekly. One rheumatoid arthritis patient mistakenly
self-administered 62 mg Enbrel subcutaneously twice weekly for 3 weeks without experiencing
undesirable effects. There is no known antidote to Enbrel.
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Immunosuppressants, Tumour Necrosis Factor alpha (TNF-α) inhibitors,
ATC code: L04AB01
Tumour necrosis factor (TNF) is a dominant cytokine in the inflammatory process of rheumatoid arthritis.
Elevated levels of TNF are also found in the synovium and psoriatic plaques of patients with psoriatic
arthritis and in serum and synovial tissue of patients with ankylosing spondylitis. In plaque psoriasis,
infiltration by inflammatory cells, including T-cells, leads to increased TNF levels in psoriatic lesions
compared with levels in uninvolved skin. Etanercept is a competitive inhibitor of TNF binding to its cell
surface receptors, and thereby inhibits the biological activity of TNF. TNF and lymphotoxin are
pro-inflammatory cytokines that bind to two distinct cell surface receptors: the 55-kilodalton (p55) and
75-kilodalton (p75) tumour necrosis factor receptors (TNFRs). Both TNFRs exist naturally in
membrane-bound and soluble forms. Soluble TNFRs are thought to regulate TNF biological activity.
TNF and lymphotoxin exist predominantly as homotrimers, with their biological activity dependent on
cross-linking of cell surface TNFRs. Dimeric soluble receptors, such as etanercept, possess a higher
affinity for TNF than monomeric receptors and are considerably more potent competitive inhibitors of
TNF binding to its cellular receptors. In addition, use of an immunoglobulin Fc region as a fusion element
in the construction of a dimeric receptor imparts a longer serum half-life.
41
Mechanism of action
Much of the joint pathology in rheumatoid arthritis and ankylosing spondylitis and skin pathology in
plaque psoriasis is mediated by pro-inflammatory molecules that are linked in a network controlled by
TNF. The mechanism of action of etanercept is thought to be its competitive inhibition of TNF binding to
cell surface TNFR, preventing TNF-mediated cellular responses by rendering TNF biologically inactive.
Etanercept may also modulate biologic responses controlled by additional downstream molecules (e.g.,
cytokines, adhesion molecules, or proteinases) that are induced or regulated by TNF.
Clinical trials
This section presents data from four randomised controlled trials in adults with rheumatoid arthritis, one
study in polyarticular juvenile idiopathic arthritis, one study in adults with psoriatic arthritis, one study in
adults with ankylosing spondylitis, one study in paediatric patients with plaque psoriasis, and four studies
in adults with plaque psoriasis.
Adult patients with rheumatoid arthritis
The efficacy of Enbrel was assessed in a randomised, double-blind, placebo-controlled study. The study
evaluated 234 adult patients with active rheumatoid arthritis who had failed therapy with at least one but
no more than four disease-modifying antirheumatic drugs (DMARDs). Doses of 10 mg or 25 mg Enbrel
or placebo were administered subcutaneously twice a week for 6 consecutive months. The results of this
controlled trial were expressed in percentage improvement in rheumatoid arthritis using American
College of Rheumatology (ACR) response criteria.
ACR 20 and 50 responses were higher in patients treated with Enbrel at 3 and 6 months than in patients
treated with placebo (ACR 20: Enbrel 62% and 59%, placebo 23% and 11% at 3 and 6 months,
respectively: ACR 50: Enbrel 41% and 40%, placebo 8% and 5% at months 3 and 6, respectively; p<0.01
Enbrel vs. placebo at all timepoints for both ACR 20 and ACR 50 responses).
Approximately 15% of subjects who received Enbrel achieved an ACR 70 response at month 3 and
month 6 compared to fewer than 5% of subjects in the placebo arm. Among patients receiving Enbrel, the
clinical responses generally appeared within 1 to 2 weeks after initiation of therapy and nearly always
occurred by 3 months. A dose response was seen; results with 10 mg were intermediate between placebo
and 25 mg. Enbrel was significantly better than placebo in all components of the ACR criteria, as well as
other measures of rheumatoid arthritis disease activity not included in the ACR response criteria, such as
morning stiffness. A Health Assessment Questionnaire (HAQ), which included disability, vitality, mental
health, general health status, and arthritis-associated health status subdomains, was administered every
3 months during the trial. All subdomains of the HAQ were improved in patients treated with Enbrel
compared to controls at 3 and 6 months.
After discontinuation of Enbrel, symptoms of arthritis generally returned within a month. Re-introduction
of treatment with Enbrel after discontinuation of up to 24 months resulted in the same magnitudes of
responses as patients who received Enbrel without interruption of therapy based on results of open-label
studies. Continued durable responses have been seen for up to 48 months in open-label extension
treatment trials when patients received Enbrel without interruption; longer-term experience is not
available.
The efficacy of Enbrel was compared to methotrexate in a randomised, active-controlled study with
blinded radiographic evaluations as a primary endpoint in 632 adult patients with active rheumatoid
arthritis (<3 years duration) who had never received treatment with methotrexate. Doses of 10 mg or
25 mg Enbrel were administered subcutaneously (SC) twice a week for up to 24 months. Methotrexate
doses were escalated from 7.5 mg/week to a maximum of 20 mg/week over the first 8 weeks of the trial
and continued for up to 24 months. Clinical improvement, including onset of action within 2 weeks with
42
Enbrel 25 mg, was similar to that seen in the previous trials and was maintained for up to 24 months. At
baseline, patients had a moderate degree of disability, with mean HAQ scores of 1.4 to 1.5. Treatment
with Enbrel 25 mg resulted in substantial improvement at 12 months, with about 44% of patients
achieving a normal HAQ score (less than 0.5). This benefit was maintained in Year 2 of this study.
In this study, structural joint damage was assessed radiographically and expressed as change in Total
Sharp Score (TSS) and its components, the erosion score and Joint Space Narrowing (JSN) score.
Radiographs of hands/wrists and feet were read at baseline and 6, 12, and 24 months. The 10 mg Enbrel
dose had consistently less effect on structural damage than the 25 mg dose. Enbrel 25 mg was
significantly superior to methotrexate for erosion scores at both 12 and 24 months. The differences in
TSS and JSN were not statistically significant between methotrexate and Enbrel 25 mg. The results are
shown in the figure below.
Radiographic Progression: Comparison of Enbrel vs. Methotrexate in Patients with RA of
<3 Years Duration
2.5
12 Months
2.5
2.2
24 Months
2.0
2.0
1.5
1.3
1.5
1.2
1.3
1.0
0.8
0.9
1.0
0.9
0.6*
0.6
0.5
0.4* 0.4 0.4
0.5
0.0
0.0
TSS Erosions JSN
TSS
Erosions JSN
MTX
Enbrel 25 mg
*p < 0.05
In another active-controlled, double-blind, randomised study, clinical efficacy, safety, and radiographic
progression in RA patients treated with Enbrel alone (25 mg twice weekly), methotrexate alone (7.5 to
20 mg weekly, median dose 20 mg), and the combination of Enbrel and methotrexate initiated
concurrently were compared in 682 adult patients with active rheumatoid arthritis of 6 months to 20 years
duration (median 5 years) who had a less than satisfactory response to at least 1 disease-modifying
antirheumatic drug (DMARD) other than methotrexate.
Patients in the Enbrel in combination with methotrexate therapy group had significantly higher ACR 20,
ACR 50, ACR 70 responses and improvement for DAS and HAQ scores at both 24 and 52 weeks than
patients in either of the single therapy groups (results shown in table below). Significant advantages for
Enbrel in combination with methotrexate compared with Enbrel monotherapy and methotrexate
monotherapy were also observed after 24 months.
43


























































Clinical Efficacy Results at 12 Months: Comparison of Enbrel vs. Methotrexate vs.
Enbrel in Combination with Methotrexate in Patients with RA of 6 Months To 20 Years
Duration
Endpoint
Methotrexate
(n = 228)
Enbrel
(n = 223)
Enbrel +
Methotrexate
(n = 231)
ACR Responses
a
ACR 20
58.8%
65.5%
74.5%
†,
φ
ACR 50
36.4%
43.0%
63.2%
†,
φ
ACR 70
16.7%
22.0%
39.8%
†,
φ
DAS
5.5 5.7 5.5
Week 52 score
b
3.0 3.0
2.3
†,
φ
Remission
c
14% 18% 37%
†,
φ
HAQ
Baseline 1.7 1.7 1.8
Week 52 1.1 1.0
0.8
†,
φ
a: Patients who did not complete 12 months in the study were considered to be non-responders.
b: Values for Disease Activity Score (DAS) are means.
c: Remission is defined as DAS <1.6.
Pairwise comparison p-values: †
= p < 0.05 for comparisons of Enbrel + methotrexate vs.
methotrexate and φ
= p < 0.05 for comparisons of Enbrel + methotrexate vs. Enbrel.
Radiographic progression at 12 months was significantly less in the Enbrel group than in the methotrexate
group, while the combination was significantly better than either monotherapy at slowing radiographic
progression (see figure below).
44
Baseline score
b



Radiographic Progression: Comparison of Enbrel vs. Methotrexate vs. Enbrel in Combination with
Methotrexate in Patients with RA of 6 Months To 20 Years Duration (12 Month Results)
3.0
2.80
Methotrexate
Enbrel
Enbrel + Methotrexate
2.5
2.0
1.68
1.5
1.12
1.0
0.52*
0.5
0.21*
0.32
0.0
-0.5
-0.30
†
-0.23
†
,φ
-0.54
†
,φ
-1.0
TSS
Erosions
JSN
Pairwise comparison p-values: * = p < 0.05 for comparisons of Enbrel vs.
methotrexate, † = p < 0.05 for comparisons of Enbrel + methotrexate vs. methotrexate
and φ = p < 0.05 for comparisons of Enbrel + methotrexate vs. Enbrel.
Significant advantages for Enbrel in combination with methotrexate compared with Enbrel monotherapy
and methotrexate monotherapy were also observed after 24 months. Similarly, the significant advantages
for Enbrel monotherapy compared with methotrexate monotherapy were also observed after 24 months.
In an analysis in which all patients who dropped out of the study for any reason were considered to have
progressed, the percentage of patients without progression (TSS change ≤ 0.5) at 24 months was higher in
the Enbrel in combination with methotrexate group compared with the Enbrel alone and methotrexate
alone groups (62%, 50%, and 36%, respectively; p<0.05). The difference between Enbrel alone and
methotrexate alone was also significant (p<0.05). Among patients who completed a full 24 months of
therapy in the study, the non-progression rates were 78%, 70%, and 61%, respectively.
The safety and efficacy of 50 mg Enbrel (two 25 mg SC injections) administered once weekly were
evaluated in a double-blind, placebo-controlled study of 420 patients with active RA. In this study, 53
patients received placebo, 214 patients received 50 mg Enbrel once weekly and 153 patients received
25 mg Enbrel twice weekly. The safety and efficacy profiles of the two Enbrel treatment regimens were
comparable at week 8 in their effect on signs and symptoms of RA; data at week 16 did not show
comparability (non-inferiority) between the two regimens.
Paediatric patients with polyarticular juvenile idiopathic arthritis
The safety and efficacy of Enbrel were assessed in a two-part study in 69 children with polyarticular
juvenile idiopathic arthritis who had a variety of juvenile idiopathic arthritis onset types. Patients aged 4
to 17 years with moderately to severely active polyarticular juvenile idiopathic arthritis refractory to, or
intolerant of, methotrexate were enrolled; patients remained on a stable dose of a single nonsteroidal
anti-inflammatory drug and/or prednisone (< 0.2 mg/kg/day or 10 mg maximum). In part 1, all patients
received 0.4 mg/kg (maximum 25 mg per dose) Enbrel subcutaneously twice weekly. In part 2, patients
45




































































with a clinical response at day 90 were randomised to remain on Enbrel or receive placebo for four
months and assessed for disease flare. Responses were measured using the JRA Definition of
Improvement (DOI), defined as ≥ 30% improvement in at least three of six and ≥ 30% worsening in no
more than one of six JRA core set criteria, including active joint count, limitation of motion, physician
and patient/parent global assessments, functional assessment, and erythrocyte sedimentation rate (ESR).
Disease flare was defined as a ≥ 30% worsening in three of six JRA core set criteria and ≥ 30%
improvement in not more than one of the six JRA core set criteria and a minimum of two active joints.
In part 1 of the study, 51 of 69 (74%) patients demonstrated a clinical response and entered part 2. In part
2, 6 of 25 (24%) patients remaining on Enbrel experienced a disease flare compared to 20 of 26 (77%)
patients receiving placebo (p=0.007). From the start of part 2, the median time to flare was ≥ 116 days for
patients who received Enbrel and 28 days for patients who received placebo. Of patients who
demonstrated a clinical response at 90 days and entered part 2 of the study, some of the patients
remaining on Enbrel continued to improve from month 3 through month 7, while those who received
placebo did not improve.
Studies have not been done in patients with polyarticular juvenile idiopathic arthritis to assess the effects
of continued Enbrel therapy in patients who do not respond within 3 months of initiating Enbrel therapy
or to assess the combination of Enbrel with methotrexate.
Adult patients with psoriatic arthritis
The efficacy of Enbrel was assessed in a randomised, double-blind, placebo-controlled study in 205
patients with psoriatic arthritis. Patients were between 18 and 70 years of age and had active psoriatic
arthritis (≥ 3 swollen joints and ≥ 3 tender joints) in at least one of the following forms: (1) distal
interphalangeal (DIP) involvement; (2) polyarticular arthritis (absence of rheumatoid nodules and
presence of psoriasis); (3) arthritis mutilans; (4) asymmetric psoriatic arthritis; or (5) spondylitis-like
ankylosis. Patients also had plaque psoriasis with a qualifying target lesion ≥ 2 cm in diameter. Patients
had previously been treated with NSAIDs (86%), DMARDs (80%), and corticosteroids (24%). Patients
currently on methotrexate therapy (stable for ≥ 2 months) could continue at a stable dose of
≤ 25 mg/week methotrexate. Doses of 25 mg of Enbrel (based on dose-finding studies in patients with
rheumatoid arthritis) or placebo were administered SC twice a week for 6 months. At the end of the
double-blind study, patients could enter a long-term open-label extension study for a total duration of up
to 2 years.
Clinical responses were expressed as percentages of patients achieving the ACR 20, 50, and 70 response
and percentages with improvement in Psoriatic Arthritis Response Criteria (PsARC). Results are
summarised in the table below.
46
Responses of Patients with Psoriatic Arthritis in a Placebo-
Controlled Trial
Percent of Patients
Placebo
Enbrel
a
Psoriatic Arthritis Response
n = 104
n = 101
ACR 20
Month 3
15
59
b
Month 6
13
50
b
ACR 50
Month 3
4
38
b
Month 6
4
37
b
ACR 70
Month 3
0
11
b
Month 6
1
9
c
PsARC
Month 3
31
72
b
Month 6
23
70
b
a: 25 mg Enbrel SC twice weekly
b: p < 0.001, Enbrel vs. placebo
c: p < 0.01, Enbrel vs. placebo
Among patients with psoriatic arthritis who received Enbrel, the clinical responses were apparent at the
time of the first visit (4 weeks) and were maintained through 6 months of therapy. Enbrel was
significantly better than placebo in all measures of disease activity (p < 0.001), and responses were
similar with and without concomitant methotrexate therapy. Quality of life in psoriatic arthritis patients
was assessed at every timepoint using the disability index of the HAQ. The disability index score was
significantly improved at all timepoints in psoriatic arthritis patients treated with Enbrel, relative to
placebo (p < 0.001).
Radiographic changes were assessed in the psoriatic arthritis study. Radiographs of hands and wrists were
obtained at baseline and months 6, 12, and 24. The modified TSS at 12 months is presented in the table
below. In an analysis in which all patients who dropped out of the study for any reason were considered
to have progressed, the percentage of patients without progression (TSS change ≤ 0.5) at 12 months was
higher in the Enbrel group compared with the placebo group (73% vs. 47%, respectively, p ≤ 0.001). The
effect of Enbrel on radiographic progression was maintained in patients who continued on treatment
during the second year. The slowing of peripheral joint damage was observed in patients with
polyarticular symmetrical joint involvement.
Mean (SE) Annualized Change from Baseline in Total Sharp Score
Placebo
Etanercept
Time
(n = 104)
(n = 101)
Month 12
1.00 (0.29)
-0.03 (0.09)
a
SE = standard error.
a. p = 0.0001.
47





Enbrel treatment resulted in improvement in physical function during the double-blind period, and this
benefit was maintained during the longer-term exposure of up to 2 years.
There is insufficient evidence of the efficacy of Enbrel in patients with ankylosing spondylitis-like and
arthritis mutilans
psoriatic arthropathies due to the small number of patients studied.
No study has been performed in patients with psoriatic arthritis using the 50 mg once-weekly dosing
regimen. Evidence of efficacy for the once-weekly dosing regimen in this patient population has been
based on data from the study in patients with ankylosing spondylitis.
Adult patients with ankylosing spondylitis
The efficacy of Enbrel in ankylosing spondylitis was assessed in 3 randomised, double-blind studies
comparing twice-weekly administration of 25 mg Enbrel with placebo. A total of 401 patients were
enrolled, from which 203 were treated with Enbrel. The largest of these trials (n= 277) enrolled patients
who were between 18 and 70 years of age and had active ankylosing spondylitis defined as visual analog
scale (VAS) scores of ≥ 30 for average of duration and intensity of morning stiffness plus VAS scores of
≥ 30 for at least 2 of the following 3 parameters: patient global assessment; average of VAS values for
nocturnal back pain and total back pain; average of 10 questions on the Bath Ankylosing Spondylitis
Functional Index (BASFI). Patients receiving DMARDs, NSAIDS, or corticosteroids could continue them
on stable doses. Patients with complete ankylosis of the spine were not included in the study. Doses of
25 mg of Enbrel (based on dose-finding studies in patients with rheumatoid arthritis) or placebo were
administered subcutaneously twice a week for 6 months in 138 patients.
The primary measure of efficacy (ASAS 20) was a ≥20% improvement in at least 3 of the 4 Assessment
in Ankylosing Spondylitis (ASAS) domains (patient global assessments, back pain, BASFI, and
inflammation) and absence of deterioration in the remaining domain. ASAS 50 and 70 responses used the
same criteria with a 50% improvement or a 70% improvement, respectively.
Compared to placebo, treatment with Enbrel resulted in significant improvements in the ASAS 20, ASAS
50 and ASAS 70 as early as 2 weeks after the initiation of therapy.
48
Responses of Patients with Ankylosing Spondylitis in a
Placebo-controlled Trial
Percent of Patients
Ankylosing Spondylitis
Response
Placebo
N = 139
Enbrel
N = 138
ASAS 20
2 weeks
22
46
a
3 months
27
60
a
6 months
23
58
a
ASAS 50
2 weeks
7
24
a
3 months
13
45
a
6 months
10
42
a
ASAS 70
2 weeks
2
12
b
3 months
7
29
b
6 months
5
28
b
a: p<0.001, Enbrel vs. placebo
b: p = 0.002, Enbrel vs. placebo
Among patients with ankylosing spondylitis who received Enbrel, the clinical responses were apparent at
the time of the first visit (2 weeks) and were maintained through 6 months of therapy. Responses were
similar in patients who were or were not receiving concomitant therapies at baseline.
Similar results were obtained in the 2 smaller ankylosing spondylitis trials.
In a fourth study, the safety and efficacy of 50 mg Enbrel (two 25 mg SC injections) administered once
weekly vs. 25 mg Enbrel administered twice weekly were evaluated in a double-blind, placebo-controlled
study of 356 patients with active ankylosing spondylitis. The safety and efficacy profiles of the 50 mg
once-weekly and 25 mg twice-weekly regimens were similar.
Adult patients with plaque psoriasis
Enbrel is recommended for use in patients as defined in section 4.1. Patients who “failed to respond to” in
the target population is defined by insufficient response (PASI<50 or PGA less than good), or worsening
of the disease while on treatment, and who were adequately dosed for a sufficiently long duration to
assess response with at least each of the three major systemic therapies as available.
The efficacy of Enbrel versus other systemic therapies in patients with moderate to severe psoriasis
(responsive to other systemic therapies) has not been evaluated in studies directly comparing Enbrel with
other systemic therapies. Instead, the safety and efficacy of Enbrel were assessed in four randomised,
double-blind, placebo-controlled studies. The primary efficacy endpoint in all four studies was the
proportion of patients in each treatment group who achieved the PASI 75 (i.e., at least a 75%
improvement in the Psoriasis Area and Severity Index score from baseline) at 12 weeks.
Study 1 was a Phase 2 study in patients with active, but clinically stable, plaque psoriasis involving ≥
10% of the body surface area who were ≥ 18 years old. One hundred and twelve (112) patients were
randomised to receive a dose of 25 mg of Enbrel (n=57) or placebo (n=55) twice a week for 24 weeks.
49





















Study 2 evaluated 652 patients with chronic plaque psoriasis using the same inclusion criteria as study 1
with the addition of a minimum psoriasis area and severity index (PASI) of 10 at screening. Enbrel was
administered at doses of 25 mg once a week, 25 mg twice a week or 50 mg twice a week for 6 consecutive
months. During the first 12 weeks of the double-blind treatment period, patients received placebo or one
of the above three Enbrel doses. After 12 weeks of treatment, patients in the placebo group began
treatment with blinded Enbrel (25 mg twice a week); patients in the active treatment groups continued to
week 24 on the dose to which they were originally randomised.
Study 3 evaluated 583 patients and had the same inclusion criteria as study 2. Patients in this study
received a dose of 25 mg or 50 mg Enbrel, or placebo twice a week for 12 weeks and then all patients
received open-label 25 mg Enbrel twice weekly for an additional 24 weeks.
Study 4 evaluated 142 patients and had similar inclusion criteria to studies 2 and 3. Patients in this study
received a dose of 50 mg Enbrel or placebo once weekly for 12 weeks and then all patients received open-
label 50 mg Enbrel once weekly for an additional 12 weeks.
In study 1, the Enbrel-treated group had a significantly higher proportion of patients with a PASI 75
response at week 12 (30%) compared to the placebo-treated group (2%) (p<0.0001). At 24 weeks, 56% of
patients in the Enbrel-treated group had achieved the PASI 75 compared to 5% of placebo-treated
patients. Key results of studies 2, 3 and 4 are shown below.
Responses of Patients with Psoriasis in Studies 2, 3 and 4
Study 2
Study 3
Study 4
----------Enbrel---------
--------Enbrel-------
-------Enbrel------
Placebo
25 mg
BIW
50 mg
BIW
Placebo
25 mg
BIW
50 mg
BIW
Placebo
50 mg
QW
50 mg
QW
n = 166
wk 12
n =
162
wk
12
n =
162
wk
24
a
n =
164
wk
12
n =
164
wk
24
a
n = 193
wk 12
n = 196
wk 12
n = 196
wk 12
n = 46
wk 12
n = 96
wk 12
n = 90
wk 24
a
Response
(%)
PASI 50
14
58* 70 74* 77
9
64*
77*
9
69*
83
PASI 75
4
34* 44 49* 59
3
34*
49*
2
38*
71
DSGA
b
,
clear or
almost
clear
5
34* 39 49* 55
4
39*
57*
4
39*
64
*p ≤ 0.0001 compared with placebo
a. No statistical comparisons to placebo were made at week 24 in studies 2 and 4 because the original
placebo group began receiving Enbrel 25 mg BIW or 50 mg once weekly from week 13 to week 24.
b. Dermatologist Static Global Assessment. Clear or almost clear defined as 0 or 1 on a 0 to 5 scale.
Among patients with plaque psoriasis who received Enbrel, significant responses relative to placebo were
apparent at the time of the first visit (2 weeks) and were maintained through 24 weeks of therapy.
Study 2 also had a drug withdrawal period during which patients who achieved a PASI improvement of at
least 50% at week 24 had treatment stopped. Patients were observed off treatment for the occurrence of
rebound (PASI ≥150% of baseline) and for the time to relapse (defined as a loss of at least half of the
improvement achieved between baseline and week 24). During the withdrawal period, symptoms of
psoriasis gradually returned, with a median time to disease relapse of 3 months. No rebound flare of
50













disease and no psoriasis-related serious adverse events were observed. There was some evidence to
support a benefit of re-treatment with Enbrel in patients initially responding to treatment.
In study 3, the majority of patients (77%) who were initially randomised to 50 mg twice weekly and had
their Enbrel dose decreased at week 12 to 25 mg twice weekly maintained their PASI 75 response through
week 36. For patients who received 25 mg twice weekly throughout the study, the PASI 75 response
continued to improve between weeks 12 and 36.
In study 4, the Enbrel-treated group had a higher proportion of patients with PASI 75 at week 12 (38%)
compared to the placebo-treated group (2%) (p<0.0001). For patients who received 50 mg once weekly
throughout the study, the efficacy responses continued to improve with 71% achieving PASI 75 at week
24.
In long-term (up to 34 months) open-label studies where Enbrel was given without interruption, clinical
responses were sustained and safety was comparable to shorter-term studies.
An analysis of clinical trial data did not reveal any baseline disease characteristics that would assist
clinicians in selecting the most appropriate dosing option (intermittent or continuous). Consequently, the
choice of intermittent or continuous therapy should be based upon physician judgment and individual
patient needs.
Paediatric patients with plaque psoriasis
The efficacy of Enbrel was assessed in a randomised, double-blind, placebo-controlled study in 211
paediatric patients aged 4 to 17 years with moderate to severe plaque psoriasis (as defined by an sPGA
score ≥ 3, involving ≥ 10% of the BSA, and PASI ≥ 12). Eligible patients had a history of receiving
phototherapy or systemic therapy, or were inadequately controlled on topical therapy.
Patients received Enbrel 0.8 mg/kg (up to 50 mg) or placebo once weekly for 12 weeks. At week 12, more
patients randomised to Enbrel had positive efficacy responses (e.g., PASI 75) than those randomised to
placebo.
Paediatric Plaque Psoriasis Outcomes at 12 Weeks
Enbrel
0.8 mg/kg Once
Weekly
(N = 106)
Placebo
(N = 105)
PASI 75, n (%)
60 (57%)
a
12 (11%)
PASI 50, n (%)
79 (75%)
a
24 (23%)
sPGA “clear” or “minimal”, n (%)
56 (53%)
a
14 (13%)
Abbreviation: sPGA-static Physician Global Assessment
a. p < 0.0001 compared with placebo
After the 12-week double-blind treatment period, all patients received Enbrel 0.8 mg/kg (up to 50 mg)
once weekly for additional 24 weeks. Responses observed during the open-label period were similar to
those observed in the double-blind period.
During a randomised withdrawal period, significantly more patients re-randomised to placebo
experienced disease relapse (loss of PASI 75 response) compared with patients re-randomised to Enbrel.
With continued therapy, responses were maintained up to 48 weeks.
51


Antibodies to Enbrel
Antibodies to etanercept have been detected in the sera of some subjects treated with etanercept. These
antibodies have all been non-neutralising and are generally transient. There appears to be no correlation
between antibody development and clinical response or adverse events.
In subjects treated with approved doses of etanercept in clinical trials for up to 12 months, cumulative
rates of anti-etanercept antibodies were approximately 6% of subjects with rheumatoid arthritis, 7.5% of
subjects with psoriatic arthritis, 2% of subjects with ankylosing spondylitis, 7% of subjects with
psoriasis, 9.7% of subjects with paediatric psoriasis, and 3% of subjects with juvenile idiopathic
arthritis.
The proportion of subjects who developed antibodies to etanercept in longer-term trials (of up to
3.5 years) increases over time, as expected. However, due to their transient nature, the incidence of
antibodies detected at each assessment point was typically less than 7% in rheumatoid arthritis subjects
and psoriasis subjects.
In a long-term psoriasis study in which patients received 50 mg twice weekly for 96 weeks, the incidence
of antibodies observed at each assessment point was up to approximately 9%.
5.2 Pharmacokinetic properties
Etanercept serum values were determined by an Enzyme-Linked Immunosorbent Assay (ELISA) method,
which may detect ELISA-reactive degradation products, as well as the parent compound.
Etanercept is slowly absorbed from the site of subcutaneous injection, reaching maximum concentration
approximately 48 hours after a single dose. The absolute bioavailability is 76%. With twice-weekly doses,
it is anticipated that steady-state concentrations are approximately twice as high as those observed after
single doses. After a single subcutaneous dose of 25 mg Enbrel, the average maximum serum
concentration observed in healthy volunteers was 1.65 ± 0.66 μg/ml, and the area under the curve was
235 ± 96.6 μg•hr/ml. Dose proportionality has not been formally evaluated, but there is no apparent
saturation of clearance across the dosing range.
A biexponential curve is required to describe the concentration time curve of etanercept. The central
volume of distribution of etanercept is 7.6 l, while the volume of distribution at steady-state is 10.4 l.
Etanercept is cleared slowly from the body. The half-life is long, approximately 70 hours. Clearance is
approximately 0.066 l/hr in patients with rheumatoid arthritis, somewhat lower than the value of 0.11 l/hr
observed in healthy volunteers. Additionally, the pharmacokinetics of Enbrel in rheumatoid arthritis
patients, ankylosing spondylitis and plaque psoriasis patients are similar.
Mean serum concentration profiles at steady state in treated RA patients were C
max
of 2.4 mg/l vs.
2.6 mg/l, C
min
of 1.2 mg/l vs. 1.4 mg/l, and partial AUC of 297 mgh/l vs. 316 mgh/l for 50 mg Enbrel once
weekly (n=21) vs. 25 mg Enbrel twice weekly (n=16), respectively. In an open-label, single-dose,
two-treatment, crossover study in healthy volunteers, etanercept administered as a single 50 mg/ml
injection was found to be bioequivalent to two simultaneous injections of 25 mg/ml.
In a population pharmacokinetics analysis in ankylosing spondylitis patients, the etanercept steady state
AUCs were 466 μg•hr/ml and 474 μg•hr/ml for 50 mg Enbrel once weekly (N= 154) and 25 mg twice
weekly (N = 148), respectively.
52
Although there is elimination of radioactivity in urine after administration of radiolabelled etanercept to
patients and volunteers, increased etanercept concentrations were not observed in patients with acute
renal or hepatic failure. The presence of renal and hepatic impairment should not require a change in
dosage. There is no apparent pharmacokinetic difference between males and females.
Methotrexate has no effect on the pharmacokinetics of etanercept. The effect of Enbrel on the human
pharmacokinetics of methotrexate has not been investigated.
Special populations
Elderly patients
The impact of advanced age was studied in the population pharmacokinetic analysis of etanercept serum
concentrations. Clearance and volume estimates in patients aged 65 to 87 years were similar to estimates
in patients less than 65 years of age.
Paediatric patients with polyarticular juvenile idiopathic arthritis
In a polyarticular juvenile idiopathic arthritis trial with Enbrel, 69 patients (aged 4 to 17 years) were
administered 0.4 mg Enbrel/kg twice weekly for three months. Serum concentration profiles were similar
to those seen in adult rheumatoid arthritis patients. The youngest children (4 years of age) had reduced
clearance (increased clearance when normalised by weight) compared with older children (12 years of
age) and adults. Simulation of dosing suggests that while older children (10-17 years of age) will have
serum levels close to those seen in adults, younger children will have appreciably lower levels.
Paediatric patients with plaque psoriasis
Patients with paediatric plaque psoriasis (aged 4 to 17 years) were administered 0.8 mg/kg (up to a
maximum dose of 50 mg per week) of etanercept once weekly for up to 48 weeks. The mean serum
steady-state trough concentrations ranged from 1.6 to 2.1 mcg/ml at weeks 12, 24, and 48. These mean
concentrations in patients with paediatric plaque psoriasis were similar to the concentrations observed in
patients with juvenile idiopathic arthritis (treated with 0.4 mg/kg etanercept twice weekly, up to
maximum dose of 50 mg per week). These mean concentrations were similar to those seen in adult
patients with plaque psoriasis treated with 25 mg etanercept twice-weekly.
5.3 Preclinical safety data
In the toxicological studies with Enbrel, no dose-limiting or target organ toxicity was evident. Enbrel was
considered to be non-genotoxic from a battery of
in vitro
and
in vivo
studies. Carcinogenicity studies, and
standard assessments of fertility and postnatal toxicity, were not performed with Enbrel due to the
development of neutralising antibodies in rodents.
Enbrel did not induce lethality or notable signs of toxicity in mice or rats following a single subcutaneous
dose of 2000 mg/kg or a single intravenous dose of 1000 mg/kg. Enbrel did not elicit dose-limiting or
target organ toxicity in cynomolgus monkeys following twice weekly subcutaneous administration for 4
or 26 consecutive weeks at a dose (15 mg/kg) that resulted in AUC-based serum drug concentrations that
were over 27-fold higher than that obtained in humans at the recommended dose of 25 mg.
53
6.1 List of excipients
Powder:
Mannitol (E421)
Sucrose
Trometamol
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
6.3 Shelf life
3 years.
Chemical and physical in-use stability has been demonstrated for 48 hours at 2°C - 8°C after
reconstitution. From a microbiological point of view, the reconstituted medicinal product should be used
immediately. If not used immediately, storage times and conditions prior to use are the responsibility of
the user and would normally not be longer that 6 hours at 2°C - 8°C, unless reconstitution has taken place
in controlled and validated aseptic conditions.
6.4 Special precautions for storage
Store in a refrigerator (2°C – 8°C). Do not freeze.
For storage conditions of the reconstituted medicinal product, see section 6.3.
6.5 Nature and contents of container
Clear glass vial (4 ml, type I glass) with rubber stoppers, aluminium seals, and flip-off plastic caps.
Cartons contain 4 vials of Enbrel with 8 alcohol swabs.
6.6 Special precautions for disposal and other handling
Instructions for use and handling
Enbrel is reconstituted with 1 ml water for injections before use and administered by subcutaneous
injection. Enbrel contains no antibacterial preservative, and therefore, solutions prepared with water for
injections should be administered as soon as possible and within 6 hours following reconstitution. The
solution should be clear and colourless to pale yellow, with no lumps, flakes or particles. Some white
foam may remain in the vial – this is normal. Enbrel should not be used if all the powder in the vial is not
dissolved within 10 minutes. If this is the case, start again with another vial.
Comprehensive instructions for the preparation and administration of the reconstituted Enbrel vial are
given in the package leaflet, section 7, ”INSTRUCTIONS FOR PREPARATION AND GIVING AN
INJECTION OF ENBREL”.
Any unused product or waste material should be disposed of in accordance with local requirements.
54
Wyeth Europa Ltd.
Huntercombe Lane South
Taplow, Maidenhead
Berkshire, SL6 0PH
United Kingdom
EU/1/99/126/002
Date of first authorisation: 3 February 2000
Date of last renewal: 03 February 2010
Detailed information on this product is available on the website of the European Medicines Agency
http://www.ema.europa.eu
55
Marketing Authorisation Holder:
Wyeth Europa Ltd.
Huntercombe Lane South
Taplow, Maidenhead
Berkshire, SL6 0PH
United Kingdom
Manufacturer:
Wyeth Pharmaceuticals
New Lane
Havant
Hampshire, PO9 2NG
United Kingdom
409
For any information about this medicinal product, please contact the local representative of the Marketing
Authorisation Holder.
België/Belgique/Belgien
Luxembourg/Luxemburg
Kύπρος
Wyeth Hellas (Cyprus Branch) AEBE
Tηλ: +357 22 817690
Pfizer S.A. / N.V.
Tél/Tel: +32 (0)2 554 62 11
Česká Republika
Pfizer s.r.o.
Tel: +420-283-004-111
Malta
Vivian Corporation Ltd.
Tel: +35621 344610
Danmark
Nederland
Wyeth Pharmaceuticals B.V.
Tel: +31 23 567 2567
Pfizer ApS
Tlf: +45 44 201 100
Deutschland
Pfizer Pharma GmbH
Tel: +49 (0)30 550055-51000
Norge
Pfizer AS
Tlf: +47 67 526 100
България/Eesti/Latvija/Lietuva/Slovenija
Wyeth-Lederle Pharma GmbH
Teл .
/Tel/T
ā
lr: +43 1 89 1140
Österreich
Pfizer Corporation Austria Ges.m.b.H.
Tel: +43 (0)1 521 15-0
Ελλάδα
Pfizer Hellas A.E.
Τηλ: +30 210 6785 800
Polska
Pfizer Polska Sp. z o.o.
Tel.: +48 22 335 61 00
España
Pfizer, S.A.
Télf: +34 91 490 99 00
Portugal
Laboratórios Pfizer, Lda.
Tel: (+351) 21 423 55 00
France
România
Pfizer Romania S.R.L
Tel: +40 (0) 21 207 28 00
Pfizer
Tél +33 (0)1 58 07 34 40
Ireland
Wyeth Pharmaceuticals
Tel: +353 1 449 3500
Slovenská Republika
Pfizer Luxembourg SARL, organiza
č
ná zložka
Tel: + 421 2 3355 5500
Ísland
Icepharma hf.
Tel: +354 540 8000
Suomi/Finland
Pfizer Oy
Puh/Tel: +358 (0)9 430 040
410
Italia
Wyeth Lederle S.p.A.
Tel: +39 06 927151
Sverige
Pfizer AB
Tel: +46 (0)8 550 520 00
Magyarország
Pfizer Kft.
Tel: +36 1 488 3700
United Kingdom
Wyeth Pharmaceuticals
Tel: +44 845 367 0098
This leaflet was last approved in
Detailed information on this product is available on the website of the European Medicines Agency
http://www.ema.europa.eu
7. USING THE MYCLIC PRE-FILLED PEN TO INJECT ENBREL
This section is divided into the following subsections:
Introduction
Step 1: Preparing for an Enbrel injection
Step 2: Choosing an injection site
Step 3: Injecting the Enbrel solution
Step 4: Disposing of the used MYCLIC pen
Introduction
The instructions below explain how to use the MYCLIC pen to inject Enbrel. Please read the instructions
carefully, and follow them step by step. Your doctor or nurse will tell you how to inject Enbrel. Do not
attempt to administer an injection until you are sure that you understand how to use the MYCLIC pen
properly. If you have questions about how to inject, please ask your doctor or nurse for help.
Diagram 1
The
MYCLIC
pre-filled pen
White needle cap
Green activation button
Clear inspection window
Step 1: Preparing for an Enbrel injection
1.
Select a clean, well-lit, flat surface.
2.
Gather the items that you will need for your injection, and place them on the chosen surface:
411

a.
One MYCLIC pre-filled pen and
one alcohol swab
(take these from the carton of pens
you keep in your refrigerator). Do not shake the pen.
b.
One cotton ball or gauze
3.
Inspect the solution in the pen by looking through the clear inspection window. Only inject the
solution in the pen if it is clear, colourless or pale yellow, and free from easily visible particles. If it is
not, use a different syringe, then contact your pharmacist for assistance.
4.
Leave the white needle cap in place and wait approximately 15-30 minutes to allow the Enbrel
solution in the pen to reach room temperature. Waiting until the solution reaches room temperature
may make the injection more comfortable for you. Do not warm in any other way.
Always leave the
pen out of sight and reach of children.
Whilst waiting for the solution in the pen to reach room temperature, read Step 2 (below), and choose an
injection site.
Step 2: Choosing an injection site (see diagram 2)
1.
The recommended injection site is the middle of the front of the thighs. If you prefer, you may
alternatively use the stomach area, but make sure you choose a site at least 5 cm away from the belly
button (navel). If someone else is giving you the injection, the outer area of the upper arms may also
be used.
Diagram 2
2.
Each injection should be given at least 3 cm from where you last injected. Do not inject into tender,
bruised or hard skin. Avoid scars or stretch marks. (It may be helpful to keep notes on the location of
the previous injections.)
3.
If you have psoriasis, you should try not to inject directly into any raised, thick, red, or scaly skin.
412
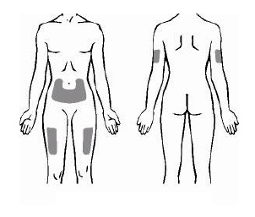
Step 3: Injecting the Enbrel solution
1.
After waiting approximately 15-30 minutes for the solution in the pen to warm to room temperature,
wash your hands with soap and water.
2.
Clean the injection site with the alcohol swab using a circular motion, and allow it to dry. Do not
touch this area again before injecting.
3.
Pick up the pen, and remove the white needle cap by pulling it straight off (see Diagram 3). To avoid
damaging the needle inside the pen, do not bend the white needle cap while you are removing it, and do
not re-attach it once it has been removed.
After removal of the needle cap, you will see a purple needle
safety shield extending slightly from the end of the pen. The needle will remain protected inside the pen
until the pen is activated.
Diagram 3
White needle cap
Purple needle safety shield
413

4.
Lightly pinching the skin between the thumb and index finger of your free hand may make the injection
easier and more comfortable.
5.
Without pressing the green activation button on top of the pen
, hold the pen at a right angle (90º) to
the injection site, and press the open end of the pen firmly against the skin, so that the needle safety
shield is pushed completely inside of the pen. A slight depression in the skin will be seen (see Diagram
4). Note that the green activation button will remain locked, and the pen will not activate, unless the
needle shield is completely pushed inside the pen.
Diagram 4
Needle safety shield
disappears inside the pen
6.
Whilst pushing the pen firmly against the skin to ensure that the needle safety shield is fully
depressed inside the pen, press
and immediately release
the green button on top of the pen with your
thumb to start the injection (see Diagram 5). On pressing the button, you will hear a click.
Continue
to hold the pen firmly against your skin until
you hear a second click,
or until 10 seconds after
the first click (whichever happens first).
Note – Remember to remove your thumb from the button once you hear the first click, or else there
will be no second click when the injection is complete. You do not need to keep your thumb on the
button in order to inject Enbrel.
414

Diagram 5
7.
On hearing the second ‘click’ (or, if you do not hear a second ‘click’, after 10 seconds have passed),
your injection will be complete (see diagram 6). You may now lift the pen from your skin (see
diagram 7). As you lift the pen, the purple needle safety shield will automatically extend to cover the
needle.
Diagram 6
Diagram 7
Purple needle safety shield
extends to cover needle
Inspection window will
have turned purple
8.
The pen’s inspection window should now be completely purple, confirming that the dose has been
injected correctly. If the window is not completely purple, contact your nurse or pharmacist for
assistance, since the pen may not have injected the Enbrel solution completely. Do not try to use the
pen again, and do not try to use another pen without agreement from your nurse or pharmacist.
9.
If you notice a spot of blood at the injection site, you should press the cotton ball or gauze over the
injection site for 10 seconds. Do not rub the injection site.
415


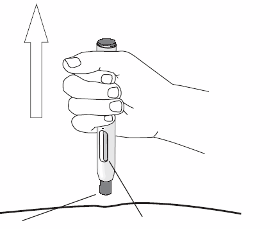

Step 4: Disposing of the used MYCLIC pen
•
The pen should be used once only - it should never be re-used. Dispose of the used pen as instructed
by your doctor, nurse or pharmacist.
If you have any questions, please talk to a doctor, nurse or pharmacist who is familiar with Enbrel.
416
Source: European Medicines Agency  - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line. https://theodora.com/drugs/eu/enbrel.html Copyright © 1995-2021 ITA all rights reserved. |