Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Epoetin alfa HEXAL 1,000 IU/0.5 ml solution for injection in a pre-filled syringe
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml of solution contains 2,000 IU of epoetin alfa
*
corresponding to 16.8 micrograms per ml
1 pre-filled syringe of 0.5 ml contains 1,000 international units (IU) corresponding to 8.4 micrograms
epoetin alfa
* Produced in CHO cell line by recombinant DNA technology
For a full list of excipients, see section 6.1.
Solution for injection in a pre-filled syringe (injection)
Clear colourless solution
4.1 Therapeutic indications
Treatment of symptomatic anaemia associated with chronic renal failure (CRF) in adult and paediatric
patients:
Treatment of anaemia associated with chronic renal failure in paediatric and adult patients on
haemodialysis and adult patients on peritoneal dialysis (See section 4.4).
Treatment of severe anaemia of renal origin accompanied by clinical symptoms in adult patients
with renal insufficiency not yet undergoing dialysis (See section 4.4).
Treatment of anaemia and reduction of transfusion requirements in adult patients receiving
chemotherapy for solid tumours, malignant lymphoma or multiple myeloma, and at risk of transfusion
as assessed by the patient's general status (e.g. cardiovascular status, pre-existing anaemia at the start
of chemotherapy).
Epoetin alfa HEXAL can be used to increase the yield of autologous blood from patients in a
predonation programme. Its use in this indication must be balanced against the reported risk of
thromboembolic events. Treatment should only be given to non-iron deficient patients with moderate
anaemia (haemoglobin (Hb) 10-13 g/dl (6.2-8.1 mmol/l)), if blood saving procedures are not available
or insufficient when the scheduled major elective surgery requires a large volume of blood (4 or more
units of blood for females or 5 or more units for males).
Epoetin alfa HEXAL can be used to reduce exposure to allogeneic blood transfusions in adult non-iron
deficient patients prior to major elective orthopaedic surgery, having a high perceived risk for
transfusion complications. Use should be restricted to patients with moderate anaemia (e.g. Hb
10-13 g/dl or 6.2-8.1 mmol/l) who do not have an autologous predonation programme available and
with an expected blood loss of 900 to 1800 ml.
4.2 Posology and method of administration
Treatment with Epoetin alfa HEXAL has to be initiated under the supervision of physicians
experienced in the management of patients with the above indications.
Treatment of symptomatic anaemia in adult and paediatric chronic renal failure patients:
In patients with chronic renal failure the medicinal product has to be administered intravenously (see
section 4.4).
Anaemia symptoms and sequelae may vary with age, gender, and overall burden of disease; a
physician´s evaluation of the individual patient´s clinical course and condition is necessary.
The haemoglobin concentration aimed for is between 10 and 12 g/dl (6.2-7.5 mmol/l) in adults, and
between 9.5 and 11 g/dl (5.9-6.8 mmol/l) in paediatric patients.
A sustained haemoglobin level of greater than 12 g/dl (7.5 mmol/l) should be avoided. If the
haemoglobin is rising by more than 2 g/dl (1.25 mmol/l) per month, or if the sustained haemoglobin
exceeds 12 g/dl (7.5 mmol/l) reduce the epoetin alfa dose by 25%. If the haemoglobin exceeds 13 g/dl
(8.1 mmol/l), discontinue therapy until it falls below 12 g/dl (7.5 mmol/l) and then reinstitute epoetin
alfa therapy at a dose 25% below the previous level.
Due to intra-patient variability, occasional individual haemoglobin values for a patient above and
below the desired haemoglobin level may be observed.
Patients should be monitored closely to ensure that the lowest approved dose of epoetin alfa is used to
provide adequate control of anaemia and of the symptoms of anaemia.
Iron status should be evaluated prior to and during treatment and iron supplementation administered if
necessary. In addition, other causes of anaemia, such as vitamin B
12
or folate deficiency, should be
excluded before instituting therapy with epoetin alfa. Non response to epoetin alfa therapy may have
the following causes: iron, folate, or vitamin B
12
deficiency; aluminium intoxication; intercurrent
infections; inflammatory or traumatic episodes; occult blood loss; haemolysis, and bone marrow
fibrosis of any origin.
Adult haemodialysis patients:
The treatment is divided into two stages:
Correction phase:
50 IU/kg 3 times per week by the intravenous route. When a dose adjustment is necessary, this should
be done in steps of at least four weeks. At each step, the increase or reduction in dose should be of
25 IU/kg 3 times per week.
Maintenance phase:
Dose adjustment in order to maintain haemoglobin values at the desired level: Hb between 10 and
12 g/dl (6.2-7.5 mmol/l).
The recommended total weekly dose is between 75 and 300 IU/kg which is administered in doses of
25-100 IU/kg three times per week given by the intravenous route.
The clinical data available suggest that those patients whose initial haemoglobin is very low (< 6 g/dl
or < 3.75 mmol/l) may require higher maintenance doses than those whose initial anaemia is less
severe (Hb > 8 g/dl or > 5 mmol/l).
Paediatric haemodialysis patients:
The treatment is divided into two stages:
Correction phase:
50 IU/kg 3 times per week by the intravenous route. When a dose adjustment is necessary, this should
be done in steps of 25 IU/kg 3 times per week at intervals of at least 4 weeks until the desired goal is
achieved.
Maintenance phase:
Dose adjustment in order to maintain haemoglobin values at the desired level: Hb between 9.5 and
11 g/dl (5.9-6.8 mmol/l).
Generally, children under 30 kg require higher maintenance doses than children over 30 kg and adults.
The following maintenance doses were observed in clinical trials after 6 months of treatment:
Dose (IU/kg given 3x/week)
The clinical data available suggest that those paediatrics patients whose initial haemoglobin is very
low (< 6.8 g/dl or < 4.25 mmol/l) may require higher maintenance doses than those whose initial
anaemia is less severe (Hb > 6.8 g/dl or > 4.25 mmol/l).
Adult peritoneal dialysis patients:
The treatment is divided into two stages:
Correction phase:
Starting dose of 50 IU/kg twice a week by the intravenous route.
Maintenance phase:
Dose adjustment in order to maintain haemoglobin values at the desired level: Hb between 10 and
12 g/dl (6.2-7.5 mmol/l). Maintenance dose between 25 and 50 IU/kg twice a week into 2 equal
injections.
Adult patients with renal insufficiency not yet undergoing dialysis:
The treatment is divided into two stages:
Correction phase:
Starting dose of 50 IU/kg 3 times per week by the intravenous route, followed if necessary by a dose
increase with 25 IU/kg increments (3 times per week) until the desired goal is achieved (this should be
done in steps of at least four weeks).
Maintenance phase:
Dose adjustment in order to maintain haemoglobin values at the desired level: Hb between 10 and
12 g/dl (6.2-7.5 mmol/l). Maintenance dose between 17 and 33 IU/kg 3 times per week by the
intravenous route.
The maximum dose should not exceed 200 IU/kg 3 times per week.
Treatment of patients with chemotherapy induced anaemia:
Epoetin alfa should be administered by the subcutaneous route to patients with anaemia (e.g.
haemoglobin concentration ≤ 10 g/dl (6.2 mmol/l). Anaemia symptoms and sequelae may vary with
age, gender, and overall burden of disease; a physician´s evaluation of the individual patient´s clinical
course and condition is necessary.
Due to intra-patient variability, occasional individual haemoglobin values for a patient above and
below the desired haemoglobin level may be observed. Haemoglobin variability should be addressed
through dose management with consideration for the haemoglobin target range of 10 g/dl (6.2 mmol/l)
to 12 g/dl (7.5 mmol/l). A sustained haemoglobin level of greater than 12 g/dl (7.5 mmol/l) should be
avoided; guidance for appropriate dose adjustment for when haemoglobin values exceeding 12 g/dl
(7.5 mmol/l) are observed are described below.
Patients should be monitored closely to ensure that the lowest approved dose of epoetin alfa is used to
provide adequate control of the symptoms of anaemia.
Epoetin alfa therapy should be continued until one month after the end of chemotherapy.









The initial dose is 150 IU/kg given subcutaneously 3 times per week. Alternatively, epoetin alfa can be
administered at an initial dose of 450 IU/kg subcutaneously once weekly.
-
If haemoglobin has increased by at least 1 g/dl (>0.62 mmol/l) or the reticulocyte count has
increased ≥ 40,000 cells/µl above baseline after 4 weeks of treatment, the dose should remain at
150 IU/kg 3 times a week or 450 IU/kg once weekly.
If the haemoglobin increase is < 1 g/dl (< 0.62 mmol/l) and the reticulocyte count has increased
< 40,000 cells/µl above baseline, increase the dose to 300 IU/kg 3 times per week. If after an
additional 4 weeks of therapy at 300 IU/kg 3 times per week, the haemoglobin has increased
≥ 1 g/dl (≥ 0.62 mmol/l) or the reticulocyte count has increased ≥ 40,000 cells/µl the dose
should remain at 300 IU/kg 3 times per week. However, if the haemoglobin has increased
< 1 g/dl (< 0.62 mmol/l) and the reticulocyte count has increased < 40,000 cells/µl above
baseline, response to epoetin alfa therapy is unlikely and treatment should be discontinued.
The recommended dosing regimen is described in the following diagram:
150 IU/kg 3x/week
or 450 IU/kg once weekly
Reticulocyte count increase ≥ 40,000/µl
Reticulocyte count increase
< 40,000/µl
Reticulocyte count increase ≥ 40,000/µl
Reticulocyte count increase
< 40,000/µl
Dosage adjustment to maintain haemoglobin concentration between 10 g/dl-12 g/dl (6.2-7.5 mmol/l):
If the haemoglobin is rising by more than 2 g/dl (1.25 mmol/l) per month, or if the haemoglobin
exceeds 12 g/dl (7.5 mmol/l), the dose should be reduced by approximately 25 to 50%. If the
haemoglobin exceeds 13 g/dl (8.1 mmol/l), discontinue therapy until it falls below 12 g/dl
(7.5 mmol/l) and than reinstitute epoetin alfa therapy at a dose 25% below the previous dose.
Adult surgery patients in an autologous predonation programme:
Epoetin alfa HEXAL should be given by the intravenous route.









At the time of donating blood, Epoetin alfa HEXAL should be administered after the completion of
the blood donation procedure.
Mildly anaemic patients (haematocrit of 33-39%) requiring predeposit of ≥ 4 units of blood should be
treated with Epoetin alfa HEXAL at a dose of 600 IU/kg body weight twice a week for 3 weeks prior
to surgery.
All patients being treated with Epoetin alfa HEXAL should receive adequate iron supplementation
(e.g. 200 mg oral elemental iron daily) throughout the course of treatment. Iron supplementation
should be started as soon as possible, even several weeks prior to initiating the autologous predeposit,
in order to achieve high iron stores prior to starting Epoetin alfa HEXAL therapy.
Treatment of adult patients scheduled for major elective orthopaedic surgery:
The subcutaneous route of administration should be used.
The recommended dose is 600 IU/kg epoetin alfa, given once a week for three weeks (days 21, 14 and
7) prior to surgery and on the day of surgery (day 0). In cases where there is a medical need to shorten
the lead time before surgery to less than three weeks, 300 IU/kg epoetin alfa should be given daily for
10 consecutive days prior to surgery, on the day of surgery and for four days immediately thereafter.
When performing haematologic assessments during the preoperative period, if the haemoglobin level
reaches 15 g/dl (9.38 mmol/l), or higher, administration of epoetin alfa should be stopped and further
doses should not be given.
Care should be taken to ensure that at the outset of the treatment patients are not iron deficient.
All patients being treated with epoetin alfa should receive adequate iron supplementation (e.g. oral
iron substitution of 200 mg Fe
2+
daily) throughout the course of epoetin alfa treatment. Iron
supplementation should be started prior to epoetin alfa therapy, to achieve adequate iron stores.
Epoetin alfa HEXAL is a sterile but unpreserved product and is for single use only. Administer the
amount required. This medicinal product must not be administered by intravenous infusion, or mixed
with other medicinal products.
1.
Intravenous injection
: over at least one to five minutes, depending on the total dose. In
haemodialysed patients, a bolus injection may be given during the dialysis session through a
suitable venous port in the dialysis line. Alternatively, the injection can be given at the end of
the dialysis session via the fistula needle tubing, followed by 10 ml of isotonic saline to rinse
the tubing and ensure satisfactory injection of the product into the circulation.
A slower injection is preferable in patients who react to the treatment with “flu-like” symptoms.
2.
Subcutaneous injection
: a maximum volume of 1 ml at one injection site should generally not be
exceeded. In case of larger volumes, more than one site should be chosen for the injection.
The injections are given in the thighs or the anterior abdominal wall.
Hypersensitivity to the active substance or to any of the excipients.
Patients who develop Pure Red Cell Aplasia (PRCA) following treatment with any
erythropoietin should not receive Epoetin alfa HEXAL or any other erythropoietin (see
section 4.4-Pure Red Cell Aplasia).
Uncontrolled hypertension.
Patients who for any reason cannot receive adequate antithrombotic prophylaxis.
The use of epoetin alfa in the indication “increasing the yield of autologous blood”is contraindicated
in patients with
-
myocardial infarction or stroke in the month preceding treatment,
increased risk of deep venous thrombosis such as history of venous thromboembolic disease.
The use of epoetin alfa in patients scheduled for major elective orthopaedic surgery and not
participating in an autologous blood predonation programme is contraindicated in patients with severe
coronary, peripheral arterial, carotid or cerebral vascular disease, including patients with recent
myocardial infarction or cerebral vascular accident.
4.4 Special warnings and precautions for use
In all patients receiving epoetin alfa, blood pressure should be closely monitored and controlled as
necessary. Epoetin alfa should be used with caution in the presence of untreated, inadequately treated
or poorly controllable hypertension. It may be necessary to add or increase antihypertensive treatment.
If blood pressure cannot be controlled, epoetin alfa treatment should be discontinued.
Epoetin alfa should be used with caution in the presence of epilepsy and chronic liver failure.
Chronic renal failure and cancer patients on epoetin alfa should have haemoglobin levels measured on
a regular basis until a stable level is achieved, and periodically thereafter.
In all patients, haemoglobin levels should be closely monitored due to a potential increased risk of
thromboembolic events and fatal outcomes when patients are treated at haemoglobin levels above the
target for the indication of use.
There may be a moderate dose-dependent rise in the platelet count within the normal range during
treatment with epoetin alfa. This regresses during the course of continued therapy. It is recommended
that the platelet count is regularly monitored during the first 8 weeks of therapy.
All other causes of anaemia (iron deficiency, haemolysis, blood loss, vitamin B
12
or folate
deficiencies) should be considered and treated prior to initiating therapy with epoetin alfa. In most
cases, the ferritin values in the serum fall simultaneously with the rise in packed cell volume. In order
to ensure optimum response to epoetin alfa, adequate iron stores should be assured:
-
iron supplementation, e.g. 200-300 mg Fe
2+
/day orally (100 -200 mg Fe
2+
/day for paediatric
patients) is recommended for chronic renal failure patients whose serum ferritin levels are
below 100 ng/ml
oral iron substitution of 200-300 mg Fe
2+
/day is recommended for all cancer patients whose
transferrin saturation is below 20%.
All of these additive factors of anaemia should also be carefully considered when deciding to increase
the dose of epoetin alfa in cancer patients.
Good blood management practices should always be used in the perisurgical setting.
In order to improve the traceability of erythropoiesis-stimulating agents (ESAs), the trade name of the
administered ESA should be clearly recorded (or stated) in the patient file.
Pure Red Cell Aplasia (PRCA)
Antibody-mediated PRCA has been very rarely reported after months to years of subcutaneous
erythropoietin treatment. In patients developing sudden lack of efficacy defined by a decrease in
haemoglobin (1 to 2 g/dl or 0.62 to 1.25 mmol/l per month) with increased need for transfusions, a
reticulocyte count should be obtained and typical causes of non-response (e.g. iron, folate or , vitamin
B
12
deficiency, aluminium intoxication, infection or inflammation, blood loss and haemolysis) should
be investigated.
unstable angina pectoris,
If the reticulocyte count corrected for anaemia (i.e., the reticulocyte “index”) is low (< 20,000/mm
3
or
< 20,000/microlitre or < 0.5%), platelet and white blood cell counts are normal, and if no other cause
of loss of effect has been found, anti-erythropoietin antibodies should be determined and bone marrow
examination should be considered for diagnosis of PRCA.
If anti-erythropoietin, antibody-mediated PRCA is suspected, therapy with Epoetin alfa HEXAL
should be discontinued immediately. No other erythropoietic therapy should be commenced because
of the risk of cross-reaction. Appropriate therapy such as blood transfusions may be given to patients
when indicated.
A paradoxical decrease in haemoglobin and development of severe anaemia associated with low
reticulocyte counts should prompt to discontinue treatment with epoetin and perform anti-
erythropoietin antibody testing. Cases have been reported in patients with hepatitis C treated with
interferon and ribavirin, when epoetins are used concomitantly. Epoetins are not approved in the
management of anaemia associated with hepatitis C.
Chronic renal failure patients
Immunogenicity data for subcutaneous use of Epoetin alfa HEXAL in patients at risk for
antibody-induced PRCA, i.e. patients with renal anaemia, are not sufficient. Therefore, in patients with
renal anaemia the medicinal product has to be administered intravenously.
In chronic renal failure patients the rate of increase in haemoglobin should be approximately 1 g/dl
(0.62 mmol/l) per month and should not exceed 2 g/dl (1.25 mmol/l) per month to minimise risks of an
increase in hypertension.
In patients with chronic renal failure, maintenance haemoglobin concentration should not exceed the
upper limit of the target haemoglobin concentration recommended in section 4.2. In clinical trials, an
increased risk of death and serious cardiovascular events was observed when erythropoiesis
stimulating agents (ESAs) were administered to target a haemoglobin of greater than 12 g/dl
(7.5 mmol/l).
Controlled clinical trials have not shown significant benefits attributable to the administration of
epoetins when haemoglobin concentration is increased beyond the level necessary to control
symptoms of anaemia and to avoid blood transfusion.
Shunt thromboses have occurred in haemodialysis patients, especially in those who have a tendency to
hypotension or whose arteriovenous fistulae exhibit complications (e.g. stenoses, aneurysms, etc.).
Early shunt revision and thrombosis prophylaxis by administration of acetylsalicylic acid, for example,
is recommended in these patients.
Hyperkalaemia has been observed in isolated cases. Correction for anaemia may lead to increased
appetite, and potassium and protein intake. Dialysis prescriptions may have to be adjusted periodically
to maintain urea, creatinine and potassium in the desired range. Serum electrolytes should be
monitored in chronic renal failure patients. If an elevated (or rising) serum potassium level is detected
then consideration should be given to ceasing epoetin alfa administration until hyperkalaemia has been
corrected.
An increase in heparin dose during haemodialysis is frequently required during the course of therapy
with epoetin alfa as a result of the increased packed cell volume. Occlusion of the dialysis system is
possible if heparinisation is not optimum.
Based on information available to date, correction of anaemia with epoetin alfa in adult patients with
renal insufficiency not yet undergoing dialysis does not accelerate the rate of progression of renal
insufficiency.
Adult cancer patients with symptomatic anaemia receiving chemotherapy
Erythropoietins are growth factors that primarily stimulate red blood cell production. Erythropoietin
receptors may be expressed on the surface of a variety of tumour cells. As with all growth factors,
there is a concern that epoetins could stimulate the growth of tumours. In several controlled studies,
epoetins have not been shown to improve overall survival or decrease the risk of tumour progression
in patients with anaemia associated with cancer.
In controlled clinical studies, use of epoetin alfa and other ESAs have shown:
decreased locoregional control in patients with advanced head and neck cancer receiving
radiation therapy when administered to target a haemoglobin of greater than 14 g/dl
(8.7 mmol/l),
shortened overall survival and increased deaths attributed to disease progression at 4 months in
patients with metastatic breast cancer receiving chemotherapy when administered to target a
haemoglobin of 12-14 g/dl (7.5-8.7 mmol/l),
increased risk of death when administered to target a haemoglobin of 12 g/dl (7.5 mmol/l) in
patients with active malignant disease receiving neither chemotherapy nor radiation therapy.
ESAs are not indicated for use in this patient population.
In view of the above, in some clinical situations blood transfusions should be the preferred treatment
for the management of anaemia in patients with cancer. The decision to administer recombinant
erythropoietins should be based on a benefit-risk assessment with the participation of the individual
patient, which should also take into account the specific clinical context. Factors that should be
considered in this assessment should include the type of tumour and its stage; the degree of anaemia;
life-expectancy; the environment in which the patient is being treated; and patient preference (see
section 5.1).
In cancer patients receiving chemotherapy, the 2 to 3 week delay between epoetin alfa administration
and the appearance of erythropoietin-induced red cells should be taken into account when assessing if
epoetin alfa therapy is appropriate (patient at risk of being transfused).
In order to minimise the risk for thrombotic events the haemoglobin level and rate of increase should
not exceed the haemoglobin limits detailed in section 4.2.
As an increased incidence of thrombotic vascular events (TVEs) has been observed in cancer patients
receiving erythropoiesis-stimulating agents (see section 4.8), this risk should be carefully weighed
against the benefit to be derived from treatment (with epoetin alfa) particularly in cancer patients with
an increased risk of thrombotic vascular events, such as obesity and patients with a prior history of
TVEs (e.g. deep vein thrombosis or pulmonary embolism). An investigational study (BEST study) in
women with metastatic breast cancer was designed to determine whether epoetin alfa treatment that
extended beyond the correction of anaemia could improve treatment outcomes. In that study the
incidence of fatal thromboembolic events was higher in patients receiving epoetin alfa than in those
receiving placebo (see section 5.1).
Adult surgery patients in an autologous predonation programme
All special warnings and precautions associated with autologous predonation programmes, especially
routine volume replacement, should be respected.
Patients scheduled for major elective orthopaedic surgery
In patients scheduled for major elective orthopaedic surgery the cause of anaemia should be
established and treated, if possible, before the start of epoetin alfa treatment. Thrombotic events can be
a risk in this population and this possibility should be carefully weighed against the benefit to be
derived from the treatment in this patient group.
Patients scheduled for major elective orthopaedic surgery should receive adequate antithrombotic
prophylaxis, as thrombotic and vascular events may occur in surgical patients, especially in those with
underlying cardiovascular disease. In addition, special precaution should be taken in patients with
predisposition for development of deep vein thrombosis (DVTs). Moreover, in patients with a baseline
haemoglobin of > 13 g/dl (> 8.1 mmol/l), the possibility that epoetin alfa treatment may be associated
with an increased risk of postoperative thrombotic/vascular events cannot be excluded. Therefore, it
should not be used in patients with baseline haemoglobin > 13 g/dl (> 8.1 mmol/l).
This medicinal product contains less than 1 mmol sodium (23 mg) per pre-filled syringe, i.e.
essentially “sodium-free”.
4.5 Interaction with other medicinal products and other forms of interaction
There is no evidence that treatment with epoetin alfa alters the metabolism of other medicinal
products.
However, since cyclosporin is bound by red blood cells there is potential for an interaction. If epoetin
alfa is given concomitantly with cyclosporin, blood levels of cyclosporin should be monitored and the
dose of cyclosporin adjusted as the haematocrit rises.
There is no evidence for an interaction between epoetin alfa and granulocyte colony-stimulating factor
(G-CSF) or granulocyte macrophage colony-stimulating factor (GM-CSF) with regard to
haematological differentiation or proliferation of tumour biopsy specimens
in vitro
.
4.6 Pregnancy and lactation
There are no adequate and well-controlled studies with epoetin alfa in pregnant women. Studies in
animals have shown reproductive toxicity (see section 5.3).
In chronic renal failure patients, Epoetin alfa HEXAL should be used in pregnancy only if the
potential benefit outweighs the potential risk to the foetus.
In pregnant or lactating surgical patients participating in an autologous blood predonation
programme, the use of epoetin alfa is not recommended.
4.7 Effects on ability to drive and use machines
Epoetin alfa HEXAL has no influence on the ability to drive and use machines.
In cancer patients and in chronic renal failure patients the most frequent adverse reaction during
treatment with epoetin alfa is a dose-dependent increase in blood pressure or aggravation of existing
hypertension. Monitoring of the blood pressure should be performed, particularly at the start of
therapy (see section 4.4). Other common adverse reactions observed in clinical trials of epoetin alfa
are deep vein thrombosis, pulmonary embolism, seizures, diarrhoea, nausea, headache, influenza like
illness, pyrexia, rash, and vomiting. Influenza like illness including headaches, arthralgia, myalgia,
and pyrexia may occur especially at the start of treatment. Frequencies may vary depending on the
indication (see table below).
Serious adverse drug reactions include venous and arterial thromboses and embolism (including some
with fatal outcomes), such as deep venous thrombosis, pulmonary emboli, arterial thrombosis
(including myocardial infarction and myocardial ischaemia), retinal thrombosis, and shunt thrombosis
(including dialysis equipment). Additionally, cerebrovascular accidents (including cerebral infarction
and cerebral haemorrhage) and transient ischaemic attacks have been reported in clinical trials of
epoetin alfa.
Aneurysms have been reported.
Hypersensitivity reactions, including cases of rash, urticaria, anaphylactic reaction, and angioneurotic
oedema have been reported.
Hypertensive crisis with encephalopathy and seizures, requiring the immediate attention of a physician
and intensive medical care, have occurred also during epoetin alfa treatment in patients with
previously normal or low blood pressure. Particular attention should be paid to sudden stabbing
migraine-like headaches as a possible warning signal.
Antibody-mediated pure red cell aplasia has been very rarely reported (in < 1/10,000 cases per patient
year) after months to years of treatment with epoetin alfa (see section 4.4).
The overall safety profile of epoetin alfa was evaluated in 142 subjects with chronic renal failure and
in 765 subjects with cancer who participated in placebo-controlled, double-blind clinical registration
trials. Adverse drug reactions reported by ≥ 0.2% of epoetin alfa-treated subjects from these trials,
additional clinical trials and from post-marketing experience are listed below by system organ class
and frequency.
Frequencies are defined as: Very common (≥ 1/10); common (≥ 1/100, < 1/10); uncommon (≥ 1/1,000,
< 1/100); rare (≥ 1/10,000, < 1/1,000); very rare (< 1/10,000), not known (cannot be estimated from
the available data).
Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness.
Blood and lymphatic system
disorders
Thrombocythaemia (cancer
patients)
Erythropoeitin antibody-
mediated pure red cell aplasia
1
Thrombocythaemia (chronic
renal failure patients)
Anaphylactic reaction
Hypersensitivity
Headache (cancer patients)
Seizures (chronic renal failure
patients)
Headache (chronic renal failure
patients)
Cerebral haemorrhage
2
Seizures (cancer patients)
Cerebrovascular accident
2
Hypertensive encephalopathy
Transient ischaemic attacks
Deep vein thrombosis
2
(cancer
patients)
Hypertension
Deep vein thrombosis
2
(chronic
renal failure patients)
Arterial thrombosis
Hypertensive crisis






Respiratory, thoracic, and
mediastinal disorders
Pulmonary embolism
2
(cancer
patients)
Pulmonary embolism
2
(chronic
renal failure patients)
Gastrointestinal disorders
Diarrhoea (cancer patients)
Vomiting
Diarrhoea (chronic renal failure
patients)
Skin and subcutaneous tissue
disorders
Angioneurotic oedema
Urticaria
Musculoskeletal, connective
tissue, and bone disorders
Arthralgia (chronic renal failure
patients)
Arthralgia (cancer patients)
Myalgia (cancer patients)
Myalgia (chronic renal failure
patients)
Congenital and
familial/genetic disorders
General disorders and
administration site conditions
Pyrexia (cancer patients)
Influenza like illness (chronic
renal failure patients)
Influenza like illness (cancer
patients)
Drug ineffective
Peripheral oedema
Pyrexia (chronic renal failure
patients)
Injection site reaction
Anti-erythropoietin antibody
positive
1
Injury, poisoning, and
procedural complications
Shunt thromboses including
dialysis equipment (chronic
renal failure patients)
1
The frequency cannot be estimated from clinical trials
2
Including cases with a fatal outcome.
Chronic renal failure patients
In chronic renal failure patients, haemoglobin levels greater than 12 g/dl (7.5 mmol/l) may be
associated with a higher risk of cardiovascular events, including death (see section 4.4).
Shunt thromboses have occurred in haemodialysis patients, especially in those who have a tendency to
hypotension or whose arteriovenous fistulae exhibit complications (e.g. stenoses, aneurysms, etc) (see
section 4.4).
An increased incidence of thromboembolic events has been reported in cancer patients receiving
ESAs, including epoetin alfa (see section 4.4).
In patients scheduled for major elective orthopaedic surgery, with a baseline haemoglobin of 10 to
13 g/dl (6.2-8.1 mmol/l), the incidence of thrombotic/vascular events (most of which were deep vein









thrombosis) in the overall patient population of the clinical trials appeared to be similar across the
different epoetin alfa dosing groups and placebo group, although the clinical experience is limited.
Moreover, in patients with a baseline haemoglobin of > 13 g/dl (8.1 mmol/l), the possibility that
epoetin alfa treatment may be associated with an increased risk of postoperative thrombotic/vascular
events cannot be excluded.
The therapeutic margin of epoetin alfa is very wide. Overdose of epoetin alfa may produce effects that
are extensions of the pharmacological effects of the hormone (critical increase of haemoglobin or
haematocrit levels). Phlebotomy may be performed if excessively high haemoglobin or haematocrit
levels occur. Additional supportive care should be provided as necessary.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: other antianaemic preparations, ATC code: B03XA01
Erythropoietin is a glycoprotein that stimulates, as a mitosis-stimulating factor and differentiating
hormone, the formation of erythrocytes from precursors of the stem cell compartment.
The apparent molecular weight of erythropoietin is 32,000 to 40,000 dalton. The protein fraction of the
molecule contributes about 58% and consists of 165 amino acids. The four carbohydrate chains are
attached via three N-glycosidic bonds and one O-glycosidic bond to the protein. Epoetin alfa obtained
by gene technology is glycosylated and is identical in its amino acid and carbohydrate composition to
endogenous human erythropoietin that has been isolated from the urine of anaemic patients.
Epoetin alfa HEXAL has the highest possible purity according to the present state of the art. In
particular, no residues of the cell line used for the production are detectable at the concentrations of
the active ingredient that are used in humans.
The biological efficacy of epoetin alfa has been demonstrated in various animal models
in vivo
(normal and anaemic rats, polycythaemic mice). After administration of epoetin alfa, the number of
erythrocytes, the Hb values and reticulocyte counts increase as well as the
59
Fe-incorporation rate.
An increased
3
H-thymidine incorporation in the erythroid nucleated spleen cells has been found
in
vitro
(mouse spleen cell culture) after incubation with epoetin alfa.
It could be shown with the aid of cell cultures of human bone marrow cells that epoetin alfa stimulates
erythropoiesis specifically and does not affect leucopoiesis. Cytotoxic actions of epoetin alfa on bone
marrow cells could not be detected.
721 cancer patients receiving non-platinum chemotherapy were included in three placebo-controlled
studies, 389 patients with haematological malignancies (221 multiple myeloma, 144 non-Hodgkin's
lymphoma, and 24 other haematological malignancies) and 332 with solid tumours (172 breast,
64 gynaecological, 23 lung, 22 prostate, 21 gastro-intestinal, and 30 other tumour types). In two large,
open-label studies, 2697 cancer patients receiving non-platinum chemotherapy were included,
1895 with solid tumours (683 breast, 260 lung, 174 gynaecological, 300 gastro-intestinal, and
478 other tumour types) and 802 with haematological malignancies.
In a prospective, randomised, double-blind, placebo-controlled trial conducted in 375 anaemic patients
with various non-myeloid malignancies receiving non-platinum chemotherapy, there was a significant
reduction of anaemia-related sequelae (e.g. fatigue, decreased energy, and activity reduction), as
measured by the following instruments and scales: Functional Assessment of Cancer
Therapy-Anaemia (FACT-An) general scale, FACT-An fatigue scale, and Cancer Linear Analogue
Scale (CLAS). Two other smaller, randomised, placebo-controlled trials failed to show a significant
improvement in quality of life parameters on the EORTC-QLQ-C30 scale or CLAS, respectively.
Erythropoietin is a growth factor that primarily stimulates red cell production. Erythropoietin receptors
may be expressed on the surface of a variety of tumour cells.
Survival and tumour progression have been examined in five large controlled studies involving a total
of 2833 patients, of which four were double-blind placebo-controlled studies and one was an open-
label study. The studies either recruited patients who were being treated with chemotherapy (two
studies) or used patient populations in which erythropoiesis stimulating agents are not indicated:
anaemia in patients with cancer not receiving chemotherapy, and head and neck cancer patients
receiving radiotherapy. The target haemoglobin concentration in two studies was > 13 g/dl
(8.1 mmol/l); in the remaining three studies it was 12 -14 g/dl (7.5-8.7 mmol/l). In the open-label
study there was no difference in overall survival between patients treated with recombinant human
erythropoietin and controls. In the four placebo-controlled studies the hazard ratios for overall survival
ranged between 1.25 and 2.47 in favour of controls. These studies have shown an consistent
unexplained statistically significant excess mortality in patients who have anaemia associated with
various common cancers who received recombinant human erythropoietin compared to controls.
Overall survival outcome in the trials could not be satisfactorily explained by differences in the
incidence of thrombosis and related complications between those given recombinant human
erythropoietin and those in the control group.
A systematic review- has also been performed involving more than 9,000 cancer patients participating
in 57 clinical trials. Meta-analysis of overall survival data produced a hazard ratio point estimate of
1.08 in favour of controls (95% CI: 0.99, 1.18; 42 trials and 8167 patients). An increased relative risk
of thromboembolic events (RR 1.67, 95% CI: 1.35, 2.06, 35 trials and 6769 patients) was observed in
patients treated with recombinant human erythropoietin. There is an increased risk for
thromboembolic events in patients with cancer treated with recombinant human erythropoietin and a
negative impact on overall survival cannot be excluded. The extent to which these outcomes might
apply to the administration of recombinant human erythropoietin to patients with cancer, treated with
chemotherapy to achieve haemoglobin concentrations less than 13 g/dl (8.1 mmol/l), is unclear
because few patients with these characteristics were included in the data reviewed.
A patient-level data analysis has also been performed on more than 13,900 cancer patients (chemo-,
radio-, chemoradio-, or no therapy) participating in 53 controlled clinical trials involving several
epoetins. Meta-analysis of overall survival data produced a hazard ratio point estimate of 1.06 in
favour of controls (95% CI: 1.00, 1.12; 53 trials and 13933 patients) and for the cancer patients
receiving chemotherapy, the overall survival hazard ratio was 1.04 (95% CI: 0.97, 1.11; 38 trials and
10,441 patients). Meta-analyses also indicate consistently a significantly increased relative risk of
thromboembolic events in cancer patients receiving recombinant human erythropoietin (see
section 4.4).
5.2 Pharmacokinetic properties
Measurement of epoetin alfa following multiple dose intravenous administration revealed a half-life of
approximately 4 hours in normal volunteers and a somewhat more prolonged half-life in renal failure
patients, approximately 5 hours. A half-life of approximately 6 hours has been reported in children.
Following subcutaneous injection, serum levels of epoetin alfa are much lower than the levels
achieved following intravenous injection, the levels increase slowly and reach a peak between 12 and
18 hours postdose. The peak is always well below the peak achieved using the intravenous route
(approximately 1/20th of the value).
There is no accumulation: the levels remain the same, whether they are determined 24 hours after the
first injection or 24 hours after the last injection.
The half-life is difficult to evaluate for the subcutaneous route and is estimated about 24 hours.
The bioavailability of subcutaneous injectable epoetin alfa is much lower than that of the intravenous
medicinal product: approximately 20%.
5.3 Preclinical safety data
In some preclinical toxicological studies in dogs and rats, but not in monkeys, epoetin alfa therapy was
associated with subclinical bone marrow fibrosis (bone marrow fibrosis is a known complication of
chronic renal failure in humans and may be related to secondary hyperparathyroidism or unknown
factors. The incidence of bone marrow fibrosis was not increased in a study of haemodialysis patients
who were treated with epoetin alfa for 3 years compared to a matched control group of dialysis
patients who had not been treated with epoetin alfa.).
In animal studies, epoetin alfa has been shown to decrease foetal body weight, delay ossification and
increase foetal mortality when given in weekly doses of approximately 20 times the recommended
human weekly dose. These changes are interpreted as being secondary to decreased maternal body
weight gain.
Epoetin alfa did not show any changes in bacterial and mammalian cell culture mutagenicity tests and
an
in vivo
micronucleus test in mice.
Long-term carcinogenicity studies have not been carried out. There are conflicting reports in the
literature regarding whether erythropoietins may play a major role as tumour proliferators. These
reports are based on
in vitro
findings from human tumour samples, but are of uncertain significance in
the clinical situation.
PHARMACEUTICAL PARTICULARS
Sodium dihydrogen phosphate dihydrate
Disodium phosphate dihydrate
Sodium chloride
Glycine
Polysorbate 80
Water for injections
Hydrochloric acid (for pH-adjustment)
Sodium hydroxide (for pH-adjustment)
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
6.4 Special precautions for storage
Store and transport refrigerated (2°C-8°C).
Do not freeze.
Keep the pre-filled syringe in the outer carton in order to protect from light.
For the purpose of ambulatory use, the patient may remove Epoetin alfa HEXAL from the refrigerator
and store it not above 25°C for one single period of up to 3 days.
6.5 Nature and contents of container
Pre-filled syringes (glass type I), with or without a needle safety guard, with plunger stopper
(Teflon-faced rubber) sealed in a blister.
The syringes contain 0.5 ml (1,000 IU) of solution.
Pack of 1 or 6 syringes.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
Epoetin alfa HEXAL must not be used
-
if the solution is cloudy or there are particles in it.
if the solution has been accidentally frozen.
The pre-filled syringes are ready to use (see section 4.2). The pre-filled syringe should not be shaken.
Syringes are embossed with graduation rings in order to enable partial use if required. Each graduation
ring corresponds to a volume of 0.1 ml. Only take one dose of Epoetin alfa HEXAL from each syringe
discarding unwanted solution before injection.
Using the pre-filled syringe with a needle safety guard
The needle safety guard covers the needle after injection to prevent needle stick injury. This does not
affect normal operation of the syringe. Depress the plunger slowly and evenly until the entire dose has
been given and the plunger cannot be depressed any further. While maintaining pressure on the
plunger, remove the syringe from the patient. The needle safety guard will cover the needle when
releasing the plunger.
Using the pre-filled syringe without a needle safety guard
Administer the dose as per standard protocol.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Hexal AG
Industriestrasse 25
D-83607 Holzkirchen
Germany
MARKETING AUTHORISATION NUMBER(S)
EU/1/07/411/001
EU/1/07/411/002
EU/1/07/411/027
EU/1/07/411/028
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency
NAME OF THE MEDICINAL PRODUCT
Epoetin alfa HEXAL 2,000 IU/1 ml solution for injection in a pre-filled syringe
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml of solution contains 2,000 IU of epoetin alfa
*
corresponding to 16.8 micrograms per ml
1 pre-filled syringe of 1 ml contains 2,000 international units (IU) corresponding to 16.8 micrograms
epoetin alfa
* Produced in CHO cell line by recombinant DNA technology
For a full list of excipients, see section 6.1.
Solution for injection in a pre-filled syringe (injection)
Clear colourless solution
4.1 Therapeutic indications
Treatment of symptomatic anaemia associated with chronic renal failure (CRF) in adult and paediatric
patients:
Treatment of anaemia associated with chronic renal failure in paediatric and adult patients on
haemodialysis and adult patients on peritoneal dialysis (See section 4.4).
Treatment of severe anaemia of renal origin accompanied by clinical symptoms in adult patients
with renal insufficiency not yet undergoing dialysis (See section 4.4).
Treatment of anaemia and reduction of transfusion requirements in adult patients receiving
chemotherapy for solid tumours, malignant lymphoma or multiple myeloma, and at risk of transfusion
as assessed by the patient's general status (e.g. cardiovascular status, pre-existing anaemia at the start
of chemotherapy).
Epoetin alfa HEXAL can be used to increase the yield of autologous blood from patients in a
predonation programme. Its use in this indication must be balanced against the reported risk of
thromboembolic events. Treatment should only be given to non-iron deficient patients with moderate
anaemia (haemoglobin (Hb) 10-13 g/dl (6.2-8.1 mmol/l)), if blood saving procedures are not available
or insufficient when the scheduled major elective surgery requires a large volume of blood (4 or more
units of blood for females or 5 or more units for males).
Epoetin alfa HEXAL can be used to reduce exposure to allogeneic blood transfusions in adult non-iron
deficient patients prior to major elective orthopaedic surgery, having a high perceived risk for
transfusion complications. Use should be restricted to patients with moderate anaemia (e.g. Hb
10-13 g/dl or 6.2-8.1 mmol/l) who do not have an autologous predonation programme available and
with an expected blood loss of 900 to 1800 ml.
4.2 Posology and method of administration
Treatment with Epoetin alfa HEXAL has to be initiated under the supervision of physicians
experienced in the management of patients with the above indications.
Treatment of symptomatic anaemia in adult and paediatric chronic renal failure patients:
In patients with chronic renal failure the medicinal product has to be administered intravenously (see
section 4.4).
Anaemia symptoms and sequelae may vary with age, gender, and overall burden of disease; a
physician´s evaluation of the individual patient´s clinical course and condition is necessary.
The haemoglobin concentration aimed for is between 10 and 12 g/dl (6.2-7.5 mmol/l) in adults, and
between 9.5 and 11 g/dl (5.9-6.8 mmol/l) in paediatric patients.
A sustained haemoglobin level of greater than 12 g/dl (7.5 mmol/l) should be avoided. If the
haemoglobin is rising by more than 2 g/dl (1.25 mmol/l) per month, or if the sustained haemoglobin
exceeds 12 g/dl (7.5 mmol/l) reduce the epoetin alfa dose by 25%. If the haemoglobin exceeds 13 g/dl
(8.1 mmol/l), discontinue therapy until it falls below 12 g/dl (7.5 mmol/l) and then reinstitute epoetin
alfa therapy at a dose 25% below the previous level.
Due to intra-patient variability, occasional individual haemoglobin values for a patient above and
below the desired haemoglobin level may be observed.
Patients should be monitored closely to ensure that the lowest approved dose of epoetin alfa is used to
provide adequate control of anaemia and of the symptoms of anaemia.
Iron status should be evaluated prior to and during treatment and iron supplementation administered if
necessary. In addition, other causes of anaemia, such as vitamin B
12
or folate deficiency, should be
excluded before instituting therapy with epoetin alfa. Non response to epoetin alfa therapy may have
the following causes: iron, folate, or vitamin B
12
deficiency; aluminium intoxication; intercurrent
infections; inflammatory or traumatic episodes; occult blood loss; haemolysis, and bone marrow
fibrosis of any origin.
Adult haemodialysis patients:
The treatment is divided into two stages:
Correction phase:
50 IU/kg 3 times per week by the intravenous route. When a dose adjustment is necessary, this should
be done in steps of at least four weeks. At each step, the increase or reduction in dose should be of
25 IU/kg 3 times per week.
Maintenance phase:
Dose adjustment in order to maintain haemoglobin values at the desired level: Hb between 10 and
12 g/dl (6.2-7.5 mmol/l).
The recommended total weekly dose is between 75 and 300 IU/kg which is administered in doses of
25-100 IU/kg three times per week given by the intravenous route.
The clinical data available suggest that those patients whose initial haemoglobin is very low (< 6 g/dl
or < 3.75 mmol/l) may require higher maintenance doses than those whose initial anaemia is less
severe (Hb > 8 g/dl or > 5 mmol/l).
Paediatric haemodialysis patients:
The treatment is divided into two stages:
Correction phase:
50 IU/kg 3 times per week by the intravenous route. When a dose adjustment is necessary, this should
be done in steps of 25 IU/kg 3 times per week at intervals of at least 4 weeks until the desired goal is
achieved.
Maintenance phase:
Dose adjustment in order to maintain haemoglobin values at the desired level: Hb between 9.5 and
11 g/dl (5.9-6.8 mmol/l).
Generally, children under 30 kg require higher maintenance doses than children over 30 kg and adults.
The following maintenance doses were observed in clinical trials after 6 months of treatment:
Dose (IU/kg given 3x/week)
The clinical data available suggest that those paediatrics patients whose initial haemoglobin is very
low (< 6.8 g/dl or < 4.25 mmol/l) may require higher maintenance doses than those whose initial
anaemia is less severe (Hb > 6.8 g/dl or > 4.25 mmol/l).
Adult peritoneal dialysis patients:
The treatment is divided into two stages:
Correction phase:
Starting dose of 50 IU/kg twice a week by the intravenous route.
Maintenance phase:
Dose adjustment in order to maintain haemoglobin values at the desired level: Hb between 10 and
12 g/dl (6.2-7.5 mmol/l). Maintenance dose between 25 and 50 IU/kg twice a week into 2 equal
injections.
Adult patients with renal insufficiency not yet undergoing dialysis:
The treatment is divided into two stages:
Correction phase:
Starting dose of 50 IU/kg 3 times per week by the intravenous route, followed if necessary by a dose
increase with 25 IU/kg increments (3 times per week) until the desired goal is achieved (this should be
done in steps of at least four weeks).
Maintenance phase:
Dose adjustment in order to maintain haemoglobin values at the desired level: Hb between 10 and
12 g/dl (6.2-7.5 mmol/l). Maintenance dose between 17 and 33 IU/kg 3 times per week by the
intravenous route.
The maximum dose should not exceed 200 IU/kg 3 times per week.
Treatment of patients with chemotherapy induced anaemia:
Epoetin alfa should be administered by the subcutaneous route to patients with anaemia (e.g.
haemoglobin concentration ≤ 10 g/dl (6.2 mmol/l). Anaemia symptoms and sequelae may vary with
age, gender, and overall burden of disease; a physician´s evaluation of the individual patient´s clinical
course and condition is necessary.
Due to intra-patient variability, occasional individual haemoglobin values for a patient above and
below the desired haemoglobin level may be observed. Haemoglobin variability should be addressed
through dose management with consideration for the haemoglobin target range of 10 g/dl (6.2 mmol/l)
to 12 g/dl (7.5 mmol/l). A sustained haemoglobin level of greater than 12 g/dl (7.5 mmol/l) should be
avoided; guidance for appropriate dose adjustment for when haemoglobin values exceeding 12 g/dl
(7.5 mmol/l) are observed are described below.
Patients should be monitored closely to ensure that the lowest approved dose of epoetin alfa is used to
provide adequate control of the symptoms of anaemia.
Epoetin alfa therapy should be continued until one month after the end of chemotherapy.









The initial dose is 150 IU/kg given subcutaneously 3 times per week. Alternatively, epoetin alfa can be
administered at an initial dose of 450 IU/kg subcutaneously once weekly.
-
If haemoglobin has increased by at least 1 g/dl (>0.62 mmol/l) or the reticulocyte count has
increased ≥ 40,000 cells/µl above baseline after 4 weeks of treatment, the dose should remain at
150 IU/kg 3 times a week or 450 IU/kg once weekly.
If the haemoglobin increase is < 1 g/dl (< 0.62 mmol/l) and the reticulocyte count has increased
< 40,000 cells/µl above baseline, increase the dose to 300 IU/kg 3 times per week. If after an
additional 4 weeks of therapy at 300 IU/kg 3 times per week, the haemoglobin has increased
≥ 1 g/dl (≥ 0.62 mmol/l) or the reticulocyte count has increased ≥ 40,000 cells/µl the dose
should remain at 300 IU/kg 3 times per week. However, if the haemoglobin has increased
< 1 g/dl (< 0.62 mmol/l) and the reticulocyte count has increased < 40,000 cells/µl above
baseline, response to epoetin alfa therapy is unlikely and treatment should be discontinued.
The recommended dosing regimen is described in the following diagram:
150 IU/kg 3x/week
or 450 IU/kg once weekly
Reticulocyte count increase ≥ 40,000/µl
Reticulocyte count increase
< 40,000/µl
Reticulocyte count increase ≥ 40,000/µl
Reticulocyte count increase
< 40,000/µl
Dosage adjustment to maintain haemoglobin concentration between 10 g/dl-12 g/dl (6.2-7.5 mmol/l):
If the haemoglobin is rising by more than 2 g/dl (1.25 mmol/l) per month, or if the haemoglobin
exceeds 12 g/dl (7.5 mmol/l), the dose should be reduced by approximately 25 to 50%. If the
haemoglobin exceeds 13 g/dl (8.1 mmol/l), discontinue therapy until it falls below 12 g/dl
(7.5 mmol/l) and than reinstitute epoetin alfa therapy at a dose 25% below the previous dose.
Adult surgery patients in an autologous predonation programme:
Epoetin alfa HEXAL should be given by the intravenous route.









At the time of donating blood, Epoetin alfa HEXAL should be administered after the completion of
the blood donation procedure.
Mildly anaemic patients (haematocrit of 33-39%) requiring predeposit of ≥ 4 units of blood should be
treated with Epoetin alfa HEXAL at a dose of 600 IU/kg body weight twice a week for 3 weeks prior
to surgery.
All patients being treated with Epoetin alfa HEXAL should receive adequate iron supplementation
(e.g. 200 mg oral elemental iron daily) throughout the course of treatment. Iron supplementation
should be started as soon as possible, even several weeks prior to initiating the autologous predeposit,
in order to achieve high iron stores prior to starting Epoetin alfa HEXAL therapy.
Treatment of adult patients scheduled for major elective orthopaedic surgery:
The subcutaneous route of administration should be used.
The recommended dose is 600 IU/kg epoetin alfa, given once a week for three weeks (days 21, 14 and
7) prior to surgery and on the day of surgery (day 0). In cases where there is a medical need to shorten
the lead time before surgery to less than three weeks, 300 IU/kg epoetin alfa should be given daily for
10 consecutive days prior to surgery, on the day of surgery and for four days immediately thereafter.
When performing haematologic assessments during the preoperative period, if the haemoglobin level
reaches 15 g/dl (9.38 mmol/l), or higher, administration of epoetin alfa should be stopped and further
doses should not be given.
Care should be taken to ensure that at the outset of the treatment patients are not iron deficient.
All patients being treated with epoetin alfa should receive adequate iron supplementation (e.g. oral
iron substitution of 200 mg Fe
2+
daily) throughout the course of epoetin alfa treatment. Iron
supplementation should be started prior to epoetin alfa therapy, to achieve adequate iron stores.
Epoetin alfa HEXAL is a sterile but unpreserved product and is for single use only. Administer the
amount required. This medicinal product must not be administered by intravenous infusion, or mixed
with other medicinal products.
1.
Intravenous injection
: over at least one to five minutes, depending on the total dose. In
haemodialysed patients, a bolus injection may be given during the dialysis session through a
suitable venous port in the dialysis line. Alternatively, the injection can be given at the end of
the dialysis session via the fistula needle tubing, followed by 10 ml of isotonic saline to rinse
the tubing and ensure satisfactory injection of the product into the circulation.
A slower injection is preferable in patients who react to the treatment with “flu-like” symptoms.
2.
Subcutaneous injection
: a maximum volume of 1 ml at one injection site should generally not be
exceeded. In case of larger volumes, more than one site should be chosen for the injection.
The injections are given in the thighs or the anterior abdominal wall.
Hypersensitivity to the active substance or to any of the excipients.
Patients who develop Pure Red Cell Aplasia (PRCA) following treatment with any
erythropoietin should not receive Epoetin alfa HEXAL or any other erythropoietin (see
section 4.4-Pure Red Cell Aplasia).
Uncontrolled hypertension.
Patients who for any reason cannot receive adequate antithrombotic prophylaxis.
The use of epoetin alfa in the indication “increasing the yield of autologous blood”is contraindicated
in patients with
-
myocardial infarction or stroke in the month preceding treatment,
increased risk of deep venous thrombosis such as history of venous thromboembolic disease.
The use of epoetin alfa in patients scheduled for major elective orthopaedic surgery and not
participating in an autologous blood predonation programme is contraindicated in patients with severe
coronary, peripheral arterial, carotid or cerebral vascular disease, including patients with recent
myocardial infarction or cerebral vascular accident.
4.4 Special warnings and precautions for use
In all patients receiving epoetin alfa, blood pressure should be closely monitored and controlled as
necessary. Epoetin alfa should be used with caution in the presence of untreated, inadequately treated
or poorly controllable hypertension. It may be necessary to add or increase antihypertensive treatment.
If blood pressure cannot be controlled, epoetin alfa treatment should be discontinued.
Epoetin alfa should be used with caution in the presence of epilepsy and chronic liver failure.
Chronic renal failure and cancer patients on epoetin alfa should have haemoglobin levels measured on
a regular basis until a stable level is achieved, and periodically thereafter.
In all patients, haemoglobin levels should be closely monitored due to a potential increased risk of
thromboembolic events and fatal outcomes when patients are treated at haemoglobin levels above the
target for the indication of use.
There may be a moderate dose-dependent rise in the platelet count within the normal range during
treatment with epoetin alfa. This regresses during the course of continued therapy. It is recommended
that the platelet count is regularly monitored during the first 8 weeks of therapy.
All other causes of anaemia (iron deficiency, haemolysis, blood loss, vitamin B
12
or folate
deficiencies) should be considered and treated prior to initiating therapy with epoetin alfa. In most
cases, the ferritin values in the serum fall simultaneously with the rise in packed cell volume. In order
to ensure optimum response to epoetin alfa, adequate iron stores should be assured:
-
iron supplementation, e.g. 200-300 mg Fe
2+
/day orally (100 -200 mg Fe
2+
/day for paediatric
patients) is recommended for chronic renal failure patients whose serum ferritin levels are
below 100 ng/ml
oral iron substitution of 200-300 mg Fe
2+
/day is recommended for all cancer patients whose
transferrin saturation is below 20%.
All of these additive factors of anaemia should also be carefully considered when deciding to increase
the dose of epoetin alfa in cancer patients.
Good blood management practices should always be used in the perisurgical setting.
In order to improve the traceability of erythropoiesis-stimulating agents (ESAs), the trade name of the
administered ESA should be clearly recorded (or stated) in the patient file.
Pure Red Cell Aplasia (PRCA)
Antibody-mediated PRCA has been very rarely reported after months to years of subcutaneous
erythropoietin treatment. In patients developing sudden lack of efficacy defined by a decrease in
haemoglobin (1 to 2 g/dl or 0.62 to 1.25 mmol/l per month) with increased need for transfusions, a
reticulocyte count should be obtained and typical causes of non-response (e.g. iron, folate or , vitamin
B
12
deficiency, aluminium intoxication, infection or inflammation, blood loss and haemolysis) should
be investigated.
unstable angina pectoris,
If the reticulocyte count corrected for anaemia (i.e., the reticulocyte “index”) is low (< 20,000/mm
3
or
< 20,000/microlitre or < 0.5%), platelet and white blood cell counts are normal, and if no other cause
of loss of effect has been found, anti-erythropoietin antibodies should be determined and bone marrow
examination should be considered for diagnosis of PRCA.
If anti-erythropoietin, antibody-mediated PRCA is suspected, therapy with Epoetin alfa HEXAL
should be discontinued immediately. No other erythropoietic therapy should be commenced because
of the risk of cross-reaction. Appropriate therapy such as blood transfusions may be given to patients
when indicated.
A paradoxical decrease in haemoglobin and development of severe anaemia associated with low
reticulocyte counts should prompt to discontinue treatment with epoetin and perform anti-
erythropoietin antibody testing. Cases have been reported in patients with hepatitis C treated with
interferon and ribavirin, when epoetins are used concomitantly. Epoetins are not approved in the
management of anaemia associated with hepatitis C.
Chronic renal failure patients
Immunogenicity data for subcutaneous use of Epoetin alfa HEXAL in patients at risk for
antibody-induced PRCA, i.e. patients with renal anaemia, are not sufficient. Therefore, in patients with
renal anaemia the medicinal product has to be administered intravenously.
In chronic renal failure patients the rate of increase in haemoglobin should be approximately 1 g/dl
(0.62 mmol/l) per month and should not exceed 2 g/dl (1.25 mmol/l) per month to minimise risks of an
increase in hypertension.
In patients with chronic renal failure, maintenance haemoglobin concentration should not exceed the
upper limit of the target haemoglobin concentration recommended in section 4.2. In clinical trials, an
increased risk of death and serious cardiovascular events was observed when erythropoiesis
stimulating agents (ESAs) were administered to target a haemoglobin of greater than 12 g/dl
(7.5 mmol/l).
Controlled clinical trials have not shown significant benefits attributable to the administration of
epoetins when haemoglobin concentration is increased beyond the level necessary to control
symptoms of anaemia and to avoid blood transfusion.
Shunt thromboses have occurred in haemodialysis patients, especially in those who have a tendency to
hypotension or whose arteriovenous fistulae exhibit complications (e.g. stenoses, aneurysms, etc.).
Early shunt revision and thrombosis prophylaxis by administration of acetylsalicylic acid, for example,
is recommended in these patients.
Hyperkalaemia has been observed in isolated cases. Correction for anaemia may lead to increased
appetite, and potassium and protein intake. Dialysis prescriptions may have to be adjusted periodically
to maintain urea, creatinine and potassium in the desired range. Serum electrolytes should be
monitored in chronic renal failure patients. If an elevated (or rising) serum potassium level is detected
then consideration should be given to ceasing epoetin alfa administration until hyperkalaemia has been
corrected.
An increase in heparin dose during haemodialysis is frequently required during the course of therapy
with epoetin alfa as a result of the increased packed cell volume. Occlusion of the dialysis system is
possible if heparinisation is not optimum.
Based on information available to date, correction of anaemia with epoetin alfa in adult patients with
renal insufficiency not yet undergoing dialysis does not accelerate the rate of progression of renal
insufficiency.
Adult cancer patients with symptomatic anaemia receiving chemotherapy
Erythropoietins are growth factors that primarily stimulate red blood cell production. Erythropoietin
receptors may be expressed on the surface of a variety of tumour cells. As with all growth factors,
there is a concern that epoetins could stimulate the growth of tumours. In several controlled studies,
epoetins have not been shown to improve overall survival or decrease the risk of tumour progression
in patients with anaemia associated with cancer.
In controlled clinical studies, use of epoetin alfa and other ESAs have shown:
decreased locoregional control in patients with advanced head and neck cancer receiving
radiation therapy when administered to target a haemoglobin of greater than 14 g/dl
(8.7 mmol/l),
shortened overall survival and increased deaths attributed to disease progression at 4 months in
patients with metastatic breast cancer receiving chemotherapy when administered to target a
haemoglobin of 12-14 g/dl (7.5-8.7 mmol/l),
increased risk of death when administered to target a haemoglobin of 12 g/dl (7.5 mmol/l) in
patients with active malignant disease receiving neither chemotherapy nor radiation therapy.
ESAs are not indicated for use in this patient population.
In view of the above, in some clinical situations blood transfusions should be the preferred treatment
for the management of anaemia in patients with cancer. The decision to administer recombinant
erythropoietins should be based on a benefit-risk assessment with the participation of the individual
patient, which should also take into account the specific clinical context. Factors that should be
considered in this assessment should include the type of tumour and its stage; the degree of anaemia;
life-expectancy; the environment in which the patient is being treated; and patient preference (see
section 5.1).
In cancer patients receiving chemotherapy, the 2 to 3 week delay between epoetin alfa administration
and the appearance of erythropoietin-induced red cells should be taken into account when assessing if
epoetin alfa therapy is appropriate (patient at risk of being transfused).
In order to minimise the risk for thrombotic events the haemoglobin level and rate of increase should
not exceed the haemoglobin limits detailed in section 4.2.
As an increased incidence of thrombotic vascular events (TVEs) has been observed in cancer patients
receiving erythropoiesis-stimulating agents (see section 4.8), this risk should be carefully weighed
against the benefit to be derived from treatment (with epoetin alfa) particularly in cancer patients with
an increased risk of thrombotic vascular events, such as obesity and patients with a prior history of
TVEs (e.g. deep vein thrombosis or pulmonary embolism). An investigational study (BEST study) in
women with metastatic breast cancer was designed to determine whether epoetin alfa treatment that
extended beyond the correction of anaemia could improve treatment outcomes. In that study the
incidence of fatal thromboembolic events was higher in patients receiving epoetin alfa than in those
receiving placebo (see section 5.1).
Adult surgery patients in an autologous predonation programme
All special warnings and precautions associated with autologous predonation programmes, especially
routine volume replacement, should be respected.
Patients scheduled for major elective orthopaedic surgery
In patients scheduled for major elective orthopaedic surgery the cause of anaemia should be
established and treated, if possible, before the start of epoetin alfa treatment. Thrombotic events can be
a risk in this population and this possibility should be carefully weighed against the benefit to be
derived from the treatment in this patient group.
Patients scheduled for major elective orthopaedic surgery should receive adequate antithrombotic
prophylaxis, as thrombotic and vascular events may occur in surgical patients, especially in those with
underlying cardiovascular disease. In addition, special precaution should be taken in patients with
predisposition for development of deep vein thrombosis (DVTs). Moreover, in patients with a baseline
haemoglobin of > 13 g/dl (> 8.1 mmol/l), the possibility that epoetin alfa treatment may be associated
with an increased risk of postoperative thrombotic/vascular events cannot be excluded. Therefore, it
should not be used in patients with baseline haemoglobin > 13 g/dl (> 8.1 mmol/l).
This medicinal product contains less than 1 mmol sodium (23 mg) per pre-filled syringe, i.e.
essentially “sodium-free”.
4.5 Interaction with other medicinal products and other forms of interaction
There is no evidence that treatment with epoetin alfa alters the metabolism of other medicinal
products.
However, since cyclosporin is bound by red blood cells there is potential for an interaction. If epoetin
alfa is given concomitantly with cyclosporin, blood levels of cyclosporin should be monitored and the
dose of cyclosporin adjusted as the haematocrit rises.
There is no evidence for an interaction between epoetin alfa and granulocyte colony-stimulating factor
(G-CSF) or granulocyte macrophage colony-stimulating factor (GM-CSF) with regard to
haematological differentiation or proliferation of tumour biopsy specimens
in vitro
.
4.6 Pregnancy and lactation
There are no adequate and well-controlled studies with epoetin alfa in pregnant women. Studies in
animals have shown reproductive toxicity (see section 5.3).
In chronic renal failure patients, Epoetin alfa HEXAL should be used in pregnancy only if the
potential benefit outweighs the potential risk to the foetus.
In pregnant or lactating surgical patients participating in an autologous blood predonation
programme, the use of epoetin alfa is not recommended.
4.7 Effects on ability to drive and use machines
Epoetin alfa HEXAL has no influence on the ability to drive and use machines.
In cancer patients and in chronic renal failure patients the most frequent adverse reaction during
treatment with epoetin alfa is a dose-dependent increase in blood pressure or aggravation of existing
hypertension. Monitoring of the blood pressure should be performed, particularly at the start of
therapy (see section 4.4). Other common adverse reactions observed in clinical trials of epoetin alfa
are deep vein thrombosis, pulmonary embolism, seizures, diarrhoea, nausea, headache, influenza like
illness, pyrexia, rash, and vomiting. Influenza like illness including headaches, arthralgia, myalgia,
and pyrexia may occur especially at the start of treatment. Frequencies may vary depending on the
indication (see table below).
Serious adverse drug reactions include venous and arterial thromboses and embolism (including some
with fatal outcomes), such as deep venous thrombosis, pulmonary emboli, arterial thrombosis
(including myocardial infarction and myocardial ischaemia), retinal thrombosis, and shunt thrombosis
(including dialysis equipment). Additionally, cerebrovascular accidents (including cerebral infarction
and cerebral haemorrhage) and transient ischaemic attacks have been reported in clinical trials of
epoetin alfa.
Aneurysms have been reported.
Hypersensitivity reactions, including cases of rash, urticaria, anaphylactic reaction, and angioneurotic
oedema have been reported.
Hypertensive crisis with encephalopathy and seizures, requiring the immediate attention of a physician
and intensive medical care, have occurred also during epoetin alfa treatment in patients with
previously normal or low blood pressure. Particular attention should be paid to sudden stabbing
migraine-like headaches as a possible warning signal.
Antibody-mediated pure red cell aplasia has been very rarely reported (in < 1/10,000 cases per patient
year) after months to years of treatment with epoetin alfa (see section 4.4).
The overall safety profile of epoetin alfa was evaluated in 142 subjects with chronic renal failure and
in 765 subjects with cancer who participated in placebo-controlled, double-blind clinical registration
trials. Adverse drug reactions reported by ≥ 0.2% of epoetin alfa-treated subjects from these trials,
additional clinical trials and from post-marketing experience are listed below by system organ class
and frequency.
Frequencies are defined as: Very common (≥ 1/10); common (≥ 1/100, < 1/10); uncommon (≥ 1/1,000,
< 1/100); rare (≥ 1/10,000, < 1/1,000); very rare (< 1/10,000), not known (cannot be estimated from
the available data).
Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness.
Blood and lymphatic system
disorders
Thrombocythaemia (cancer
patients)
Erythropoeitin antibody-
mediated pure red cell aplasia
1
Thrombocythaemia (chronic
renal failure patients)
Anaphylactic reaction
Hypersensitivity
Headache (cancer patients)
Seizures (chronic renal failure
patients)
Headache (chronic renal failure
patients)
Cerebral haemorrhage
2
Seizures (cancer patients)
Cerebrovascular accident
2
Hypertensive encephalopathy
Transient ischaemic attacks
Deep vein thrombosis
2
(cancer
patients)
Hypertension
Deep vein thrombosis
2
(chronic
renal failure patients)
Arterial thrombosis
Hypertensive crisis






Respiratory, thoracic, and
mediastinal disorders
Pulmonary embolism
2
(cancer
patients)
Pulmonary embolism
2
(chronic
renal failure patients)
Gastrointestinal disorders
Diarrhoea (cancer patients)
Vomiting
Diarrhoea (chronic renal failure
patients)
Skin and subcutaneous tissue
disorders
Angioneurotic oedema
Urticaria
Musculoskeletal, connective
tissue, and bone disorders
Arthralgia (chronic renal failure
patients)
Arthralgia (cancer patients)
Myalgia (cancer patients)
Myalgia (chronic renal failure
patients)
Congenital and
familial/genetic disorders
General disorders and
administration site conditions
Pyrexia (cancer patients)
Influenza like illness (chronic
renal failure patients)
Influenza like illness (cancer
patients)
Drug ineffective
Peripheral oedema
Pyrexia (chronic renal failure
patients)
Injection site reaction
Anti-erythropoietin antibody
positive
1
Injury, poisoning, and
procedural complications
Shunt thromboses including
dialysis equipment (chronic
renal failure patients)
1
The frequency cannot be estimated from clinical trials
2
Including cases with a fatal outcome.
Chronic renal failure patients
In chronic renal failure patients, haemoglobin levels greater than 12 g/dl (7.5 mmol/l) may be
associated with a higher risk of cardiovascular events, including death (see section 4.4).
Shunt thromboses have occurred in haemodialysis patients, especially in those who have a tendency to
hypotension or whose arteriovenous fistulae exhibit complications (e.g. stenoses, aneurysms, etc) (see
section 4.4).
An increased incidence of thromboembolic events has been reported in cancer patients receiving
ESAs, including epoetin alfa (see section 4.4).
In patients scheduled for major elective orthopaedic surgery, with a baseline haemoglobin of 10 to
13 g/dl (6.2-8.1 mmol/l), the incidence of thrombotic/vascular events (most of which were deep vein









thrombosis) in the overall patient population of the clinical trials appeared to be similar across the
different epoetin alfa dosing groups and placebo group, although the clinical experience is limited.
Moreover, in patients with a baseline haemoglobin of > 13 g/dl (8.1 mmol/l), the possibility that
epoetin alfa treatment may be associated with an increased risk of postoperative thrombotic/vascular
events cannot be excluded.
The therapeutic margin of epoetin alfa is very wide. Overdose of epoetin alfa may produce effects that
are extensions of the pharmacological effects of the hormone (critical increase of haemoglobin or
haematocrit levels). Phlebotomy may be performed if excessively high haemoglobin or haematocrit
levels occur. Additional supportive care should be provided as necessary.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: other antianaemic preparations, ATC code: B03XA01
Erythropoietin is a glycoprotein that stimulates, as a mitosis-stimulating factor and differentiating
hormone, the formation of erythrocytes from precursors of the stem cell compartment.
The apparent molecular weight of erythropoietin is 32,000 to 40,000 dalton. The protein fraction of the
molecule contributes about 58% and consists of 165 amino acids. The four carbohydrate chains are
attached via three N-glycosidic bonds and one O-glycosidic bond to the protein. Epoetin alfa obtained
by gene technology is glycosylated and is identical in its amino acid and carbohydrate composition to
endogenous human erythropoietin that has been isolated from the urine of anaemic patients.
Epoetin alfa HEXAL has the highest possible purity according to the present state of the art. In
particular, no residues of the cell line used for the production are detectable at the concentrations of
the active ingredient that are used in humans.
The biological efficacy of epoetin alfa has been demonstrated in various animal models
in vivo
(normal and anaemic rats, polycythaemic mice). After administration of epoetin alfa, the number of
erythrocytes, the Hb values and reticulocyte counts increase as well as the
59
Fe-incorporation rate.
An increased
3
H-thymidine incorporation in the erythroid nucleated spleen cells has been found
in
vitro
(mouse spleen cell culture) after incubation with epoetin alfa.
It could be shown with the aid of cell cultures of human bone marrow cells that epoetin alfa stimulates
erythropoiesis specifically and does not affect leucopoiesis. Cytotoxic actions of epoetin alfa on bone
marrow cells could not be detected.
721 cancer patients receiving non-platinum chemotherapy were included in three placebo-controlled
studies, 389 patients with haematological malignancies (221 multiple myeloma, 144 non-Hodgkin's
lymphoma, and 24 other haematological malignancies) and 332 with solid tumours (172 breast,
64 gynaecological, 23 lung, 22 prostate, 21 gastro-intestinal, and 30 other tumour types). In two large,
open-label studies, 2697 cancer patients receiving non-platinum chemotherapy were included,
1895 with solid tumours (683 breast, 260 lung, 174 gynaecological, 300 gastro-intestinal, and
478 other tumour types) and 802 with haematological malignancies.
In a prospective, randomised, double-blind, placebo-controlled trial conducted in 375 anaemic patients
with various non-myeloid malignancies receiving non-platinum chemotherapy, there was a significant
reduction of anaemia-related sequelae (e.g. fatigue, decreased energy, and activity reduction), as
measured by the following instruments and scales: Functional Assessment of Cancer
Therapy-Anaemia (FACT-An) general scale, FACT-An fatigue scale, and Cancer Linear Analogue
Scale (CLAS). Two other smaller, randomised, placebo-controlled trials failed to show a significant
improvement in quality of life parameters on the EORTC-QLQ-C30 scale or CLAS, respectively.
Erythropoietin is a growth factor that primarily stimulates red cell production. Erythropoietin receptors
may be expressed on the surface of a variety of tumour cells.
Survival and tumour progression have been examined in five large controlled studies involving a total
of 2833 patients, of which four were double-blind placebo-controlled studies and one was an open-
label study. The studies either recruited patients who were being treated with chemotherapy (two
studies) or used patient populations in which erythropoiesis stimulating agents are not indicated:
anaemia in patients with cancer not receiving chemotherapy, and head and neck cancer patients
receiving radiotherapy. The target haemoglobin concentration in two studies was > 13 g/dl
(8.1 mmol/l); in the remaining three studies it was 12 -14 g/dl (7.5-8.7 mmol/l). In the open-label
study there was no difference in overall survival between patients treated with recombinant human
erythropoietin and controls. In the four placebo-controlled studies the hazard ratios for overall survival
ranged between 1.25 and 2.47 in favour of controls. These studies have shown an consistent
unexplained statistically significant excess mortality in patients who have anaemia associated with
various common cancers who received recombinant human erythropoietin compared to controls.
Overall survival outcome in the trials could not be satisfactorily explained by differences in the
incidence of thrombosis and related complications between those given recombinant human
erythropoietin and those in the control group.
A systematic review- has also been performed involving more than 9,000 cancer patients participating
in 57 clinical trials. Meta-analysis of overall survival data produced a hazard ratio point estimate of
1.08 in favour of controls (95% CI: 0.99, 1.18; 42 trials and 8167 patients). An increased relative risk
of thromboembolic events (RR 1.67, 95% CI: 1.35, 2.06, 35 trials and 6769 patients) was observed in
patients treated with recombinant human erythropoietin. There is an increased risk for
thromboembolic events in patients with cancer treated with recombinant human erythropoietin and a
negative impact on overall survival cannot be excluded. The extent to which these outcomes might
apply to the administration of recombinant human erythropoietin to patients with cancer, treated with
chemotherapy to achieve haemoglobin concentrations less than 13 g/dl (8.1 mmol/l), is unclear
because few patients with these characteristics were included in the data reviewed.
A patient-level data analysis has also been performed on more than 13,900 cancer patients (chemo-,
radio-, chemoradio-, or no therapy) participating in 53 controlled clinical trials involving several
epoetins. Meta-analysis of overall survival data produced a hazard ratio point estimate of 1.06 in
favour of controls (95% CI: 1.00, 1.12; 53 trials and 13933 patients) and for the cancer patients
receiving chemotherapy, the overall survival hazard ratio was 1.04 (95% CI: 0.97, 1.11; 38 trials and
10,441 patients). Meta-analyses also indicate consistently a significantly increased relative risk of
thromboembolic events in cancer patients receiving recombinant human erythropoietin (see
section 4.4).
5.2 Pharmacokinetic properties
Measurement of epoetin alfa following multiple dose intravenous administration revealed a half-life of
approximately 4 hours in normal volunteers and a somewhat more prolonged half-life in renal failure
patients, approximately 5 hours. A half-life of approximately 6 hours has been reported in children.
Following subcutaneous injection, serum levels of epoetin alfa are much lower than the levels
achieved following intravenous injection, the levels increase slowly and reach a peak between 12 and
18 hours postdose. The peak is always well below the peak achieved using the intravenous route
(approximately 1/20th of the value).
There is no accumulation: the levels remain the same, whether they are determined 24 hours after the
first injection or 24 hours after the last injection.
The half-life is difficult to evaluate for the subcutaneous route and is estimated about 24 hours.
The bioavailability of subcutaneous injectable epoetin alfa is much lower than that of the intravenous
medicinal product: approximately 20%.
5.3 Preclinical safety data
In some preclinical toxicological studies in dogs and rats, but not in monkeys, epoetin alfa therapy was
associated with subclinical bone marrow fibrosis (bone marrow fibrosis is a known complication of
chronic renal failure in humans and may be related to secondary hyperparathyroidism or unknown
factors. The incidence of bone marrow fibrosis was not increased in a study of haemodialysis patients
who were treated with epoetin alfa for 3 years compared to a matched control group of dialysis
patients who had not been treated with epoetin alfa.).
In animal studies, epoetin alfa has been shown to decrease foetal body weight, delay ossification and
increase foetal mortality when given in weekly doses of approximately 20 times the recommended
human weekly dose. These changes are interpreted as being secondary to decreased maternal body
weight gain.
Epoetin alfa did not show any changes in bacterial and mammalian cell culture mutagenicity tests and
an
in vivo
micronucleus test in mice.
Long-term carcinogenicity studies have not been carried out. There are conflicting reports in the
literature regarding whether erythropoietins may play a major role as tumour proliferators. These
reports are based on
in vitro
findings from human tumour samples, but are of uncertain significance in
the clinical situation.
PHARMACEUTICAL PARTICULARS
Sodium dihydrogen phosphate dihydrate
Disodium phosphate dihydrate
Sodium chloride
Glycine
Polysorbate 80
Water for injections
Hydrochloric acid (for pH-adjustment)
Sodium hydroxide (for pH-adjustment)
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
6.4 Special precautions for storage
Store and transport refrigerated (2°C-8°C).
Do not freeze.
Keep the pre-filled syringe in the outer carton in order to protect from light.
For the purpose of ambulatory use, the patient may remove Epoetin alfa HEXAL from the refrigerator
and store it not above 25°C for one single period of up to 3 days.
6.5 Nature and contents of container
Pre-filled syringes (glass type I), with or without a needle safety guard, with plunger stopper
(Teflon-faced rubber) sealed in a blister.
The syringes contain 1 ml (2,000 IU) of solution.
Pack of 1 or 6 syringes.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
Epoetin alfa HEXAL must not be used
-
if the solution is cloudy or there are particles in it.
if the solution has been accidentally frozen.
The pre-filled syringes are ready to use (see section 4.2). The pre-filled syringe should not be shaken.
Syringes are embossed with graduation rings in order to enable partial use if required. Each graduation
ring corresponds to a volume of 0.1 ml. Only take one dose of Epoetin alfa HEXAL from each syringe
discarding unwanted solution before injection.
Using the pre-filled syringe with a needle safety guard
The needle safety guard covers the needle after injection to prevent needle stick injury. This does not
affect normal operation of the syringe. Depress the plunger slowly and evenly until the entire dose has
been given and the plunger cannot be depressed any further. While maintaining pressure on the
plunger, remove the syringe from the patient. The needle safety guard will cover the needle when
releasing the plunger.
Using the pre-filled syringe without a needle safety guard
Administer the dose as per standard protocol.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Hexal AG
Industriestrasse 25
D-83607 Holzkirchen
Germany
MARKETING AUTHORISATION NUMBER(S)
EU/1/07/411/003
EU/1/07/411/004
EU/1/07/411/029
EU/1/07/411/030
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency
NAME OF THE MEDICINAL PRODUCT
Epoetin alfa HEXAL 3,000 IU/0.3 ml solution for injection in a pre-filled syringe
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml of solution contains 10,000 IU of epoetin alfa
*
corresponding to 84.0 micrograms per ml
1 pre-filled syringe of 0.3 ml contains 3,000 international units (IU) corresponding to 25.2 micrograms
epoetin alfa
* Produced in CHO cell line by recombinant DNA technology
For a full list of excipients, see section 6.1.
Solution for injection in a pre-filled syringe (injection)
Clear colourless solution
4.1 Therapeutic indications
Treatment of symptomatic anaemia associated with chronic renal failure (CRF) in adult and paediatric
patients:
Treatment of anaemia associated with chronic renal failure in paediatric and adult patients on
haemodialysis and adult patients on peritoneal dialysis (See section 4.4).
Treatment of severe anaemia of renal origin accompanied by clinical symptoms in adult patients
with renal insufficiency not yet undergoing dialysis (See section 4.4).
Treatment of anaemia and reduction of transfusion requirements in adult patients receiving
chemotherapy for solid tumours, malignant lymphoma or multiple myeloma, and at risk of transfusion
as assessed by the patient's general status (e.g. cardiovascular status, pre-existing anaemia at the start
of chemotherapy).
Epoetin alfa HEXAL can be used to increase the yield of autologous blood from patients in a
predonation programme. Its use in this indication must be balanced against the reported risk of
thromboembolic events. Treatment should only be given to non-iron deficient patients with moderate
anaemia (haemoglobin (Hb) 10-13 g/dl (6.2-8.1 mmol/l)), if blood saving procedures are not available
or insufficient when the scheduled major elective surgery requires a large volume of blood (4 or more
units of blood for females or 5 or more units for males).
Epoetin alfa HEXAL can be used to reduce exposure to allogeneic blood transfusions in adult non-iron
deficient patients prior to major elective orthopaedic surgery, having a high perceived risk for
transfusion complications. Use should be restricted to patients with moderate anaemia (e.g. Hb
10-13 g/dl or 6.2-8.1 mmol/l) who do not have an autologous predonation programme available and
with an expected blood loss of 900 to 1800 ml.
4.2 Posology and method of administration
Treatment with Epoetin alfa HEXAL has to be initiated under the supervision of physicians
experienced in the management of patients with the above indications.
Treatment of symptomatic anaemia in adult and paediatric chronic renal failure patients:
In patients with chronic renal failure the medicinal product has to be administered intravenously (see
section 4.4).
Anaemia symptoms and sequelae may vary with age, gender, and overall burden of disease; a
physician´s evaluation of the individual patient´s clinical course and condition is necessary.
The haemoglobin concentration aimed for is between 10 and 12 g/dl (6.2-7.5 mmol/l) in adults, and
between 9.5 and 11 g/dl (5.9-6.8 mmol/l) in paediatric patients.
A sustained haemoglobin level of greater than 12 g/dl (7.5 mmol/l) should be avoided. If the
haemoglobin is rising by more than 2 g/dl (1.25 mmol/l) per month, or if the sustained haemoglobin
exceeds 12 g/dl (7.5 mmol/l) reduce the epoetin alfa dose by 25%. If the haemoglobin exceeds 13 g/dl
(8.1 mmol/l), discontinue therapy until it falls below 12 g/dl (7.5 mmol/l) and then reinstitute epoetin
alfa therapy at a dose 25% below the previous level.
Due to intra-patient variability, occasional individual haemoglobin values for a patient above and
below the desired haemoglobin level may be observed.
Patients should be monitored closely to ensure that the lowest approved dose of epoetin alfa is used to
provide adequate control of anaemia and of the symptoms of anaemia.
Iron status should be evaluated prior to and during treatment and iron supplementation administered if
necessary. In addition, other causes of anaemia, such as vitamin B
12
or folate deficiency, should be
excluded before instituting therapy with epoetin alfa. Non response to epoetin alfa therapy may have
the following causes: iron, folate, or vitamin B
12
deficiency; aluminium intoxication; intercurrent
infections; inflammatory or traumatic episodes; occult blood loss; haemolysis, and bone marrow
fibrosis of any origin.
Adult haemodialysis patients:
The treatment is divided into two stages:
Correction phase:
50 IU/kg 3 times per week by the intravenous route. When a dose adjustment is necessary, this should
be done in steps of at least four weeks. At each step, the increase or reduction in dose should be of
25 IU/kg 3 times per week.
Maintenance phase:
Dose adjustment in order to maintain haemoglobin values at the desired level: Hb between 10 and
12 g/dl (6.2-7.5 mmol/l).
The recommended total weekly dose is between 75 and 300 IU/kg which is administered in doses of
25-100 IU/kg three times per week given by the intravenous route.
The clinical data available suggest that those patients whose initial haemoglobin is very low (< 6 g/dl
or < 3.75 mmol/l) may require higher maintenance doses than those whose initial anaemia is less
severe (Hb > 8 g/dl or > 5 mmol/l).
Paediatric haemodialysis patients:
The treatment is divided into two stages:
Correction phase:
50 IU/kg 3 times per week by the intravenous route. When a dose adjustment is necessary, this should
be done in steps of 25 IU/kg 3 times per week at intervals of at least 4 weeks until the desired goal is
achieved.
Maintenance phase:
Dose adjustment in order to maintain haemoglobin values at the desired level: Hb between 9.5 and
11 g/dl (5.9-6.8 mmol/l).
Generally, children under 30 kg require higher maintenance doses than children over 30 kg and adults.
The following maintenance doses were observed in clinical trials after 6 months of treatment:
Dose (IU/kg given 3x/week)
The clinical data available suggest that those paediatrics patients whose initial haemoglobin is very
low (< 6.8 g/dl or < 4.25 mmol/l) may require higher maintenance doses than those whose initial
anaemia is less severe (Hb > 6.8 g/dl or > 4.25 mmol/l).
Adult peritoneal dialysis patients:
The treatment is divided into two stages:
Correction phase:
Starting dose of 50 IU/kg twice a week by the intravenous route.
Maintenance phase:
Dose adjustment in order to maintain haemoglobin values at the desired level: Hb between 10 and
12 g/dl (6.2-7.5 mmol/l). Maintenance dose between 25 and 50 IU/kg twice a week into 2 equal
injections.
Adult patients with renal insufficiency not yet undergoing dialysis:
The treatment is divided into two stages:
Correction phase:
Starting dose of 50 IU/kg 3 times per week by the intravenous route, followed if necessary by a dose
increase with 25 IU/kg increments (3 times per week) until the desired goal is achieved (this should be
done in steps of at least four weeks).
Maintenance phase:
Dose adjustment in order to maintain haemoglobin values at the desired level: Hb between 10 and
12 g/dl (6.2-7.5 mmol/l). Maintenance dose between 17 and 33 IU/kg 3 times per week by the
intravenous route.
The maximum dose should not exceed 200 IU/kg 3 times per week.
Treatment of patients with chemotherapy induced anaemia:
Epoetin alfa should be administered by the subcutaneous route to patients with anaemia (e.g.
haemoglobin concentration ≤ 10 g/dl (6.2 mmol/l). Anaemia symptoms and sequelae may vary with
age, gender, and overall burden of disease; a physician´s evaluation of the individual patient´s clinical
course and condition is necessary.
Due to intra-patient variability, occasional individual haemoglobin values for a patient above and
below the desired haemoglobin level may be observed. Haemoglobin variability should be addressed
through dose management with consideration for the haemoglobin target range of 10 g/dl (6.2 mmol/l)
to 12 g/dl (7.5 mmol/l). A sustained haemoglobin level of greater than 12 g/dl (7.5 mmol/l) should be
avoided; guidance for appropriate dose adjustment for when haemoglobin values exceeding 12 g/dl
(7.5 mmol/l) are observed are described below.
Patients should be monitored closely to ensure that the lowest approved dose of epoetin alfa is used to
provide adequate control of the symptoms of anaemia.
Epoetin alfa therapy should be continued until one month after the end of chemotherapy.









The initial dose is 150 IU/kg given subcutaneously 3 times per week. Alternatively, epoetin alfa can be
administered at an initial dose of 450 IU/kg subcutaneously once weekly.
-
If haemoglobin has increased by at least 1 g/dl (>0.62 mmol/l) or the reticulocyte count has
increased ≥ 40,000 cells/µl above baseline after 4 weeks of treatment, the dose should remain at
150 IU/kg 3 times a week or 450 IU/kg once weekly.
If the haemoglobin increase is < 1 g/dl (< 0.62 mmol/l) and the reticulocyte count has increased
< 40,000 cells/µl above baseline, increase the dose to 300 IU/kg 3 times per week. If after an
additional 4 weeks of therapy at 300 IU/kg 3 times per week, the haemoglobin has increased
≥ 1 g/dl (≥ 0.62 mmol/l) or the reticulocyte count has increased ≥ 40,000 cells/µl the dose
should remain at 300 IU/kg 3 times per week. However, if the haemoglobin has increased
< 1 g/dl (< 0.62 mmol/l) and the reticulocyte count has increased < 40,000 cells/µl above
baseline, response to epoetin alfa therapy is unlikely and treatment should be discontinued.
The recommended dosing regimen is described in the following diagram:
150 IU/kg 3x/week
or 450 IU/kg once weekly
Reticulocyte count increase ≥ 40,000/µl
Reticulocyte count increase
< 40,000/µl
Reticulocyte count increase ≥ 40,000/µl
Reticulocyte count increase
< 40,000/µl
Dosage adjustment to maintain haemoglobin concentration between 10 g/dl-12 g/dl (6.2-7.5 mmol/l):
If the haemoglobin is rising by more than 2 g/dl (1.25 mmol/l) per month, or if the haemoglobin
exceeds 12 g/dl (7.5 mmol/l), the dose should be reduced by approximately 25 to 50%. If the
haemoglobin exceeds 13 g/dl (8.1 mmol/l), discontinue therapy until it falls below 12 g/dl
(7.5 mmol/l) and than reinstitute epoetin alfa therapy at a dose 25% below the previous dose.
Adult surgery patients in an autologous predonation programme:
Epoetin alfa HEXAL should be given by the intravenous route.









At the time of donating blood, Epoetin alfa HEXAL should be administered after the completion of
the blood donation procedure.
Mildly anaemic patients (haematocrit of 33-39%) requiring predeposit of ≥ 4 units of blood should be
treated with Epoetin alfa HEXAL at a dose of 600 IU/kg body weight twice a week for 3 weeks prior
to surgery.
All patients being treated with Epoetin alfa HEXAL should receive adequate iron supplementation
(e.g. 200 mg oral elemental iron daily) throughout the course of treatment. Iron supplementation
should be started as soon as possible, even several weeks prior to initiating the autologous predeposit,
in order to achieve high iron stores prior to starting Epoetin alfa HEXAL therapy.
Treatment of adult patients scheduled for major elective orthopaedic surgery:
The subcutaneous route of administration should be used.
The recommended dose is 600 IU/kg epoetin alfa, given once a week for three weeks (days 21, 14 and
7) prior to surgery and on the day of surgery (day 0). In cases where there is a medical need to shorten
the lead time before surgery to less than three weeks, 300 IU/kg epoetin alfa should be given daily for
10 consecutive days prior to surgery, on the day of surgery and for four days immediately thereafter.
When performing haematologic assessments during the preoperative period, if the haemoglobin level
reaches 15 g/dl (9.38 mmol/l), or higher, administration of epoetin alfa should be stopped and further
doses should not be given.
Care should be taken to ensure that at the outset of the treatment patients are not iron deficient.
All patients being treated with epoetin alfa should receive adequate iron supplementation (e.g. oral
iron substitution of 200 mg Fe
2+
daily) throughout the course of epoetin alfa treatment. Iron
supplementation should be started prior to epoetin alfa therapy, to achieve adequate iron stores.
Epoetin alfa HEXAL is a sterile but unpreserved product and is for single use only. Administer the
amount required. This medicinal product must not be administered by intravenous infusion, or mixed
with other medicinal products.
1.
Intravenous injection
: over at least one to five minutes, depending on the total dose. In
haemodialysed patients, a bolus injection may be given during the dialysis session through a
suitable venous port in the dialysis line. Alternatively, the injection can be given at the end of
the dialysis session via the fistula needle tubing, followed by 10 ml of isotonic saline to rinse
the tubing and ensure satisfactory injection of the product into the circulation.
A slower injection is preferable in patients who react to the treatment with “flu-like” symptoms.
2.
Subcutaneous injection
: a maximum volume of 1 ml at one injection site should generally not be
exceeded. In case of larger volumes, more than one site should be chosen for the injection.
The injections are given in the thighs or the anterior abdominal wall.
Hypersensitivity to the active substance or to any of the excipients.
Patients who develop Pure Red Cell Aplasia (PRCA) following treatment with any
erythropoietin should not receive Epoetin alfa HEXAL or any other erythropoietin (see
section 4.4-Pure Red Cell Aplasia).
Uncontrolled hypertension.
Patients who for any reason cannot receive adequate antithrombotic prophylaxis.
The use of epoetin alfa in the indication “increasing the yield of autologous blood”is contraindicated
in patients with
-
myocardial infarction or stroke in the month preceding treatment,
increased risk of deep venous thrombosis such as history of venous thromboembolic disease.
The use of epoetin alfa in patients scheduled for major elective orthopaedic surgery and not
participating in an autologous blood predonation programme is contraindicated in patients with severe
coronary, peripheral arterial, carotid or cerebral vascular disease, including patients with recent
myocardial infarction or cerebral vascular accident.
4.4 Special warnings and precautions for use
In all patients receiving epoetin alfa, blood pressure should be closely monitored and controlled as
necessary. Epoetin alfa should be used with caution in the presence of untreated, inadequately treated
or poorly controllable hypertension. It may be necessary to add or increase antihypertensive treatment.
If blood pressure cannot be controlled, epoetin alfa treatment should be discontinued.
Epoetin alfa should be used with caution in the presence of epilepsy and chronic liver failure.
Chronic renal failure and cancer patients on epoetin alfa should have haemoglobin levels measured on
a regular basis until a stable level is achieved, and periodically thereafter.
In all patients, haemoglobin levels should be closely monitored due to a potential increased risk of
thromboembolic events and fatal outcomes when patients are treated at haemoglobin levels above the
target for the indication of use.
There may be a moderate dose-dependent rise in the platelet count within the normal range during
treatment with epoetin alfa. This regresses during the course of continued therapy. It is recommended
that the platelet count is regularly monitored during the first 8 weeks of therapy.
All other causes of anaemia (iron deficiency, haemolysis, blood loss, vitamin B
12
or folate
deficiencies) should be considered and treated prior to initiating therapy with epoetin alfa. In most
cases, the ferritin values in the serum fall simultaneously with the rise in packed cell volume. In order
to ensure optimum response to epoetin alfa, adequate iron stores should be assured:
-
iron supplementation, e.g. 200-300 mg Fe
2+
/day orally (100 -200 mg Fe
2+
/day for paediatric
patients) is recommended for chronic renal failure patients whose serum ferritin levels are
below 100 ng/ml
oral iron substitution of 200-300 mg Fe
2+
/day is recommended for all cancer patients whose
transferrin saturation is below 20%.
All of these additive factors of anaemia should also be carefully considered when deciding to increase
the dose of epoetin alfa in cancer patients.
Good blood management practices should always be used in the perisurgical setting.
In order to improve the traceability of erythropoiesis-stimulating agents (ESAs), the trade name of the
administered ESA should be clearly recorded (or stated) in the patient file.
Pure Red Cell Aplasia (PRCA)
Antibody-mediated PRCA has been very rarely reported after months to years of subcutaneous
erythropoietin treatment. In patients developing sudden lack of efficacy defined by a decrease in
haemoglobin (1 to 2 g/dl or 0.62 to 1.25 mmol/l per month) with increased need for transfusions, a
reticulocyte count should be obtained and typical causes of non-response (e.g. iron, folate or , vitamin
B
12
deficiency, aluminium intoxication, infection or inflammation, blood loss and haemolysis) should
be investigated.
unstable angina pectoris,
If the reticulocyte count corrected for anaemia (i.e., the reticulocyte “index”) is low (< 20,000/mm
3
or
< 20,000/microlitre or < 0.5%), platelet and white blood cell counts are normal, and if no other cause
of loss of effect has been found, anti-erythropoietin antibodies should be determined and bone marrow
examination should be considered for diagnosis of PRCA.
If anti-erythropoietin, antibody-mediated PRCA is suspected, therapy with Epoetin alfa HEXAL
should be discontinued immediately. No other erythropoietic therapy should be commenced because
of the risk of cross-reaction. Appropriate therapy such as blood transfusions may be given to patients
when indicated.
A paradoxical decrease in haemoglobin and development of severe anaemia associated with low
reticulocyte counts should prompt to discontinue treatment with epoetin and perform anti-
erythropoietin antibody testing. Cases have been reported in patients with hepatitis C treated with
interferon and ribavirin, when epoetins are used concomitantly. Epoetins are not approved in the
management of anaemia associated with hepatitis C.
Chronic renal failure patients
Immunogenicity data for subcutaneous use of Epoetin alfa HEXAL in patients at risk for
antibody-induced PRCA, i.e. patients with renal anaemia, are not sufficient. Therefore, in patients with
renal anaemia the medicinal product has to be administered intravenously.
In chronic renal failure patients the rate of increase in haemoglobin should be approximately 1 g/dl
(0.62 mmol/l) per month and should not exceed 2 g/dl (1.25 mmol/l) per month to minimise risks of an
increase in hypertension.
In patients with chronic renal failure, maintenance haemoglobin concentration should not exceed the
upper limit of the target haemoglobin concentration recommended in section 4.2. In clinical trials, an
increased risk of death and serious cardiovascular events was observed when erythropoiesis
stimulating agents (ESAs) were administered to target a haemoglobin of greater than 12 g/dl
(7.5 mmol/l).
Controlled clinical trials have not shown significant benefits attributable to the administration of
epoetins when haemoglobin concentration is increased beyond the level necessary to control
symptoms of anaemia and to avoid blood transfusion.
Shunt thromboses have occurred in haemodialysis patients, especially in those who have a tendency to
hypotension or whose arteriovenous fistulae exhibit complications (e.g. stenoses, aneurysms, etc.).
Early shunt revision and thrombosis prophylaxis by administration of acetylsalicylic acid, for example,
is recommended in these patients.
Hyperkalaemia has been observed in isolated cases. Correction for anaemia may lead to increased
appetite, and potassium and protein intake. Dialysis prescriptions may have to be adjusted periodically
to maintain urea, creatinine and potassium in the desired range. Serum electrolytes should be
monitored in chronic renal failure patients. If an elevated (or rising) serum potassium level is detected
then consideration should be given to ceasing epoetin alfa administration until hyperkalaemia has been
corrected.
An increase in heparin dose during haemodialysis is frequently required during the course of therapy
with epoetin alfa as a result of the increased packed cell volume. Occlusion of the dialysis system is
possible if heparinisation is not optimum.
Based on information available to date, correction of anaemia with epoetin alfa in adult patients with
renal insufficiency not yet undergoing dialysis does not accelerate the rate of progression of renal
insufficiency.
Adult cancer patients with symptomatic anaemia receiving chemotherapy
Erythropoietins are growth factors that primarily stimulate red blood cell production. Erythropoietin
receptors may be expressed on the surface of a variety of tumour cells. As with all growth factors,
there is a concern that epoetins could stimulate the growth of tumours. In several controlled studies,
epoetins have not been shown to improve overall survival or decrease the risk of tumour progression
in patients with anaemia associated with cancer.
In controlled clinical studies, use of epoetin alfa and other ESAs have shown:
decreased locoregional control in patients with advanced head and neck cancer receiving
radiation therapy when administered to target a haemoglobin of greater than 14 g/dl
(8.7 mmol/l),
shortened overall survival and increased deaths attributed to disease progression at 4 months in
patients with metastatic breast cancer receiving chemotherapy when administered to target a
haemoglobin of 12-14 g/dl (7.5-8.7 mmol/l),
increased risk of death when administered to target a haemoglobin of 12 g/dl (7.5 mmol/l) in
patients with active malignant disease receiving neither chemotherapy nor radiation therapy.
ESAs are not indicated for use in this patient population.
In view of the above, in some clinical situations blood transfusions should be the preferred treatment
for the management of anaemia in patients with cancer. The decision to administer recombinant
erythropoietins should be based on a benefit-risk assessment with the participation of the individual
patient, which should also take into account the specific clinical context. Factors that should be
considered in this assessment should include the type of tumour and its stage; the degree of anaemia;
life-expectancy; the environment in which the patient is being treated; and patient preference (see
section 5.1).
In cancer patients receiving chemotherapy, the 2 to 3 week delay between epoetin alfa administration
and the appearance of erythropoietin-induced red cells should be taken into account when assessing if
epoetin alfa therapy is appropriate (patient at risk of being transfused).
In order to minimise the risk for thrombotic events the haemoglobin level and rate of increase should
not exceed the haemoglobin limits detailed in section 4.2.
As an increased incidence of thrombotic vascular events (TVEs) has been observed in cancer patients
receiving erythropoiesis-stimulating agents (see section 4.8), this risk should be carefully weighed
against the benefit to be derived from treatment (with epoetin alfa) particularly in cancer patients with
an increased risk of thrombotic vascular events, such as obesity and patients with a prior history of
TVEs (e.g. deep vein thrombosis or pulmonary embolism). An investigational study (BEST study) in
women with metastatic breast cancer was designed to determine whether epoetin alfa treatment that
extended beyond the correction of anaemia could improve treatment outcomes. In that study the
incidence of fatal thromboembolic events was higher in patients receiving epoetin alfa than in those
receiving placebo (see section 5.1).
Adult surgery patients in an autologous predonation programme
All special warnings and precautions associated with autologous predonation programmes, especially
routine volume replacement, should be respected.
Patients scheduled for major elective orthopaedic surgery
In patients scheduled for major elective orthopaedic surgery the cause of anaemia should be
established and treated, if possible, before the start of epoetin alfa treatment. Thrombotic events can be
a risk in this population and this possibility should be carefully weighed against the benefit to be
derived from the treatment in this patient group.
Patients scheduled for major elective orthopaedic surgery should receive adequate antithrombotic
prophylaxis, as thrombotic and vascular events may occur in surgical patients, especially in those with
underlying cardiovascular disease. In addition, special precaution should be taken in patients with
predisposition for development of deep vein thrombosis (DVTs). Moreover, in patients with a baseline
haemoglobin of > 13 g/dl (> 8.1 mmol/l), the possibility that epoetin alfa treatment may be associated
with an increased risk of postoperative thrombotic/vascular events cannot be excluded. Therefore, it
should not be used in patients with baseline haemoglobin > 13 g/dl (> 8.1 mmol/l).
This medicinal product contains less than 1 mmol sodium (23 mg) per pre-filled syringe, i.e.
essentially “sodium-free”.
4.5 Interaction with other medicinal products and other forms of interaction
There is no evidence that treatment with epoetin alfa alters the metabolism of other medicinal
products.
However, since cyclosporin is bound by red blood cells there is potential for an interaction. If epoetin
alfa is given concomitantly with cyclosporin, blood levels of cyclosporin should be monitored and the
dose of cyclosporin adjusted as the haematocrit rises.
There is no evidence for an interaction between epoetin alfa and granulocyte colony-stimulating factor
(G-CSF) or granulocyte macrophage colony-stimulating factor (GM-CSF) with regard to
haematological differentiation or proliferation of tumour biopsy specimens
in vitro
.
4.6 Pregnancy and lactation
There are no adequate and well-controlled studies with epoetin alfa in pregnant women. Studies in
animals have shown reproductive toxicity (see section 5.3).
In chronic renal failure patients, Epoetin alfa HEXAL should be used in pregnancy only if the
potential benefit outweighs the potential risk to the foetus.
In pregnant or lactating surgical patients participating in an autologous blood predonation
programme, the use of epoetin alfa is not recommended.
4.7 Effects on ability to drive and use machines
Epoetin alfa HEXAL has no influence on the ability to drive and use machines.
In cancer patients and in chronic renal failure patients the most frequent adverse reaction during
treatment with epoetin alfa is a dose-dependent increase in blood pressure or aggravation of existing
hypertension. Monitoring of the blood pressure should be performed, particularly at the start of
therapy (see section 4.4). Other common adverse reactions observed in clinical trials of epoetin alfa
are deep vein thrombosis, pulmonary embolism, seizures, diarrhoea, nausea, headache, influenza like
illness, pyrexia, rash, and vomiting. Influenza like illness including headaches, arthralgia, myalgia,
and pyrexia may occur especially at the start of treatment. Frequencies may vary depending on the
indication (see table below).
Serious adverse drug reactions include venous and arterial thromboses and embolism (including some
with fatal outcomes), such as deep venous thrombosis, pulmonary emboli, arterial thrombosis
(including myocardial infarction and myocardial ischaemia), retinal thrombosis, and shunt thrombosis
(including dialysis equipment). Additionally, cerebrovascular accidents (including cerebral infarction
and cerebral haemorrhage) and transient ischaemic attacks have been reported in clinical trials of
epoetin alfa.
Aneurysms have been reported.
Hypersensitivity reactions, including cases of rash, urticaria, anaphylactic reaction, and angioneurotic
oedema have been reported.
Hypertensive crisis with encephalopathy and seizures, requiring the immediate attention of a physician
and intensive medical care, have occurred also during epoetin alfa treatment in patients with
previously normal or low blood pressure. Particular attention should be paid to sudden stabbing
migraine-like headaches as a possible warning signal.
Antibody-mediated pure red cell aplasia has been very rarely reported (in < 1/10,000 cases per patient
year) after months to years of treatment with epoetin alfa (see section 4.4).
The overall safety profile of epoetin alfa was evaluated in 142 subjects with chronic renal failure and
in 765 subjects with cancer who participated in placebo-controlled, double-blind clinical registration
trials. Adverse drug reactions reported by ≥ 0.2% of epoetin alfa-treated subjects from these trials,
additional clinical trials and from post-marketing experience are listed below by system organ class
and frequency.
Frequencies are defined as: Very common (≥ 1/10); common (≥ 1/100, < 1/10); uncommon (≥ 1/1,000,
< 1/100); rare (≥ 1/10,000, < 1/1,000); very rare (< 1/10,000), not known (cannot be estimated from
the available data).
Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness.
Blood and lymphatic system
disorders
Thrombocythaemia (cancer
patients)
Erythropoeitin antibody-
mediated pure red cell aplasia
1
Thrombocythaemia (chronic
renal failure patients)
Anaphylactic reaction
Hypersensitivity
Headache (cancer patients)
Seizures (chronic renal failure
patients)
Headache (chronic renal failure
patients)
Cerebral haemorrhage
2
Seizures (cancer patients)
Cerebrovascular accident
2
Hypertensive encephalopathy
Transient ischaemic attacks
Deep vein thrombosis
2
(cancer
patients)
Hypertension
Deep vein thrombosis
2
(chronic
renal failure patients)
Arterial thrombosis
Hypertensive crisis






Respiratory, thoracic, and
mediastinal disorders
Pulmonary embolism
2
(cancer
patients)
Pulmonary embolism
2
(chronic
renal failure patients)
Gastrointestinal disorders
Diarrhoea (cancer patients)
Vomiting
Diarrhoea (chronic renal failure
patients)
Skin and subcutaneous tissue
disorders
Angioneurotic oedema
Urticaria
Musculoskeletal, connective
tissue, and bone disorders
Arthralgia (chronic renal failure
patients)
Arthralgia (cancer patients)
Myalgia (cancer patients)
Myalgia (chronic renal failure
patients)
Congenital and
familial/genetic disorders
General disorders and
administration site conditions
Pyrexia (cancer patients)
Influenza like illness (chronic
renal failure patients)
Influenza like illness (cancer
patients)
Drug ineffective
Peripheral oedema
Pyrexia (chronic renal failure
patients)
Injection site reaction
Anti-erythropoietin antibody
positive
1
Injury, poisoning, and
procedural complications
Shunt thromboses including
dialysis equipment (chronic
renal failure patients)
1
The frequency cannot be estimated from clinical trials
2
Including cases with a fatal outcome.
Chronic renal failure patients
In chronic renal failure patients, haemoglobin levels greater than 12 g/dl (7.5 mmol/l) may be
associated with a higher risk of cardiovascular events, including death (see section 4.4).
Shunt thromboses have occurred in haemodialysis patients, especially in those who have a tendency to
hypotension or whose arteriovenous fistulae exhibit complications (e.g. stenoses, aneurysms, etc) (see
section 4.4).
An increased incidence of thromboembolic events has been reported in cancer patients receiving
ESAs, including epoetin alfa (see section 4.4).
In patients scheduled for major elective orthopaedic surgery, with a baseline haemoglobin of 10 to
13 g/dl (6.2-8.1 mmol/l), the incidence of thrombotic/vascular events (most of which were deep vein









thrombosis) in the overall patient population of the clinical trials appeared to be similar across the
different epoetin alfa dosing groups and placebo group, although the clinical experience is limited.
Moreover, in patients with a baseline haemoglobin of > 13 g/dl (8.1 mmol/l), the possibility that
epoetin alfa treatment may be associated with an increased risk of postoperative thrombotic/vascular
events cannot be excluded.
The therapeutic margin of epoetin alfa is very wide. Overdose of epoetin alfa may produce effects that
are extensions of the pharmacological effects of the hormone (critical increase of haemoglobin or
haematocrit levels). Phlebotomy may be performed if excessively high haemoglobin or haematocrit
levels occur. Additional supportive care should be provided as necessary.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: other antianaemic preparations, ATC code: B03XA01
Erythropoietin is a glycoprotein that stimulates, as a mitosis-stimulating factor and differentiating
hormone, the formation of erythrocytes from precursors of the stem cell compartment.
The apparent molecular weight of erythropoietin is 32,000 to 40,000 dalton. The protein fraction of the
molecule contributes about 58% and consists of 165 amino acids. The four carbohydrate chains are
attached via three N-glycosidic bonds and one O-glycosidic bond to the protein. Epoetin alfa obtained
by gene technology is glycosylated and is identical in its amino acid and carbohydrate composition to
endogenous human erythropoietin that has been isolated from the urine of anaemic patients.
Epoetin alfa HEXAL has the highest possible purity according to the present state of the art. In
particular, no residues of the cell line used for the production are detectable at the concentrations of
the active ingredient that are used in humans.
The biological efficacy of epoetin alfa has been demonstrated in various animal models
in vivo
(normal and anaemic rats, polycythaemic mice). After administration of epoetin alfa, the number of
erythrocytes, the Hb values and reticulocyte counts increase as well as the
59
Fe-incorporation rate.
An increased
3
H-thymidine incorporation in the erythroid nucleated spleen cells has been found
in
vitro
(mouse spleen cell culture) after incubation with epoetin alfa.
It could be shown with the aid of cell cultures of human bone marrow cells that epoetin alfa stimulates
erythropoiesis specifically and does not affect leucopoiesis. Cytotoxic actions of epoetin alfa on bone
marrow cells could not be detected.
721 cancer patients receiving non-platinum chemotherapy were included in three placebo-controlled
studies, 389 patients with haematological malignancies (221 multiple myeloma, 144 non-Hodgkin's
lymphoma, and 24 other haematological malignancies) and 332 with solid tumours (172 breast,
64 gynaecological, 23 lung, 22 prostate, 21 gastro-intestinal, and 30 other tumour types). In two large,
open-label studies, 2697 cancer patients receiving non-platinum chemotherapy were included,
1895 with solid tumours (683 breast, 260 lung, 174 gynaecological, 300 gastro-intestinal, and
478 other tumour types) and 802 with haematological malignancies.
In a prospective, randomised, double-blind, placebo-controlled trial conducted in 375 anaemic patients
with various non-myeloid malignancies receiving non-platinum chemotherapy, there was a significant
reduction of anaemia-related sequelae (e.g. fatigue, decreased energy, and activity reduction), as
measured by the following instruments and scales: Functional Assessment of Cancer
Therapy-Anaemia (FACT-An) general scale, FACT-An fatigue scale, and Cancer Linear Analogue
Scale (CLAS). Two other smaller, randomised, placebo-controlled trials failed to show a significant
improvement in quality of life parameters on the EORTC-QLQ-C30 scale or CLAS, respectively.
Erythropoietin is a growth factor that primarily stimulates red cell production. Erythropoietin receptors
may be expressed on the surface of a variety of tumour cells.
Survival and tumour progression have been examined in five large controlled studies involving a total
of 2833 patients, of which four were double-blind placebo-controlled studies and one was an open-
label study. The studies either recruited patients who were being treated with chemotherapy (two
studies) or used patient populations in which erythropoiesis stimulating agents are not indicated:
anaemia in patients with cancer not receiving chemotherapy, and head and neck cancer patients
receiving radiotherapy. The target haemoglobin concentration in two studies was > 13 g/dl
(8.1 mmol/l); in the remaining three studies it was 12 -14 g/dl (7.5-8.7 mmol/l). In the open-label
study there was no difference in overall survival between patients treated with recombinant human
erythropoietin and controls. In the four placebo-controlled studies the hazard ratios for overall survival
ranged between 1.25 and 2.47 in favour of controls. These studies have shown an consistent
unexplained statistically significant excess mortality in patients who have anaemia associated with
various common cancers who received recombinant human erythropoietin compared to controls.
Overall survival outcome in the trials could not be satisfactorily explained by differences in the
incidence of thrombosis and related complications between those given recombinant human
erythropoietin and those in the control group.
A systematic review- has also been performed involving more than 9,000 cancer patients participating
in 57 clinical trials. Meta-analysis of overall survival data produced a hazard ratio point estimate of
1.08 in favour of controls (95% CI: 0.99, 1.18; 42 trials and 8167 patients). An increased relative risk
of thromboembolic events (RR 1.67, 95% CI: 1.35, 2.06, 35 trials and 6769 patients) was observed in
patients treated with recombinant human erythropoietin. There is an increased risk for
thromboembolic events in patients with cancer treated with recombinant human erythropoietin and a
negative impact on overall survival cannot be excluded. The extent to which these outcomes might
apply to the administration of recombinant human erythropoietin to patients with cancer, treated with
chemotherapy to achieve haemoglobin concentrations less than 13 g/dl (8.1 mmol/l), is unclear
because few patients with these characteristics were included in the data reviewed.
A patient-level data analysis has also been performed on more than 13,900 cancer patients (chemo-,
radio-, chemoradio-, or no therapy) participating in 53 controlled clinical trials involving several
epoetins. Meta-analysis of overall survival data produced a hazard ratio point estimate of 1.06 in
favour of controls (95% CI: 1.00, 1.12; 53 trials and 13933 patients) and for the cancer patients
receiving chemotherapy, the overall survival hazard ratio was 1.04 (95% CI: 0.97, 1.11; 38 trials and
10,441 patients). Meta-analyses also indicate consistently a significantly increased relative risk of
thromboembolic events in cancer patients receiving recombinant human erythropoietin (see
section 4.4).
5.2 Pharmacokinetic properties
Measurement of epoetin alfa following multiple dose intravenous administration revealed a half-life of
approximately 4 hours in normal volunteers and a somewhat more prolonged half-life in renal failure
patients, approximately 5 hours. A half-life of approximately 6 hours has been reported in children.
Following subcutaneous injection, serum levels of epoetin alfa are much lower than the levels
achieved following intravenous injection, the levels increase slowly and reach a peak between 12 and
18 hours postdose. The peak is always well below the peak achieved using the intravenous route
(approximately 1/20th of the value).
There is no accumulation: the levels remain the same, whether they are determined 24 hours after the
first injection or 24 hours after the last injection.
The half-life is difficult to evaluate for the subcutaneous route and is estimated about 24 hours.
The bioavailability of subcutaneous injectable epoetin alfa is much lower than that of the intravenous
medicinal product: approximately 20%.
5.3 Preclinical safety data
In some preclinical toxicological studies in dogs and rats, but not in monkeys, epoetin alfa therapy was
associated with subclinical bone marrow fibrosis (bone marrow fibrosis is a known complication of
chronic renal failure in humans and may be related to secondary hyperparathyroidism or unknown
factors. The incidence of bone marrow fibrosis was not increased in a study of haemodialysis patients
who were treated with epoetin alfa for 3 years compared to a matched control group of dialysis
patients who had not been treated with epoetin alfa.).
In animal studies, epoetin alfa has been shown to decrease foetal body weight, delay ossification and
increase foetal mortality when given in weekly doses of approximately 20 times the recommended
human weekly dose. These changes are interpreted as being secondary to decreased maternal body
weight gain.
Epoetin alfa did not show any changes in bacterial and mammalian cell culture mutagenicity tests and
an
in vivo
micronucleus test in mice.
Long-term carcinogenicity studies have not been carried out. There are conflicting reports in the
literature regarding whether erythropoietins may play a major role as tumour proliferators. These
reports are based on
in vitro
findings from human tumour samples, but are of uncertain significance in
the clinical situation.
PHARMACEUTICAL PARTICULARS
Sodium dihydrogen phosphate dihydrate
Disodium phosphate dihydrate
Sodium chloride
Glycine
Polysorbate 80
Water for injections
Hydrochloric acid (for pH-adjustment)
Sodium hydroxide (for pH-adjustment)
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
6.4 Special precautions for storage
Store and transport refrigerated (2°C-8°C).
Do not freeze.
Keep the pre-filled syringe in the outer carton in order to protect from light.
For the purpose of ambulatory use, the patient may remove Epoetin alfa HEXAL from the refrigerator
and store it not above 25°C for one single period of up to 3 days.
6.5 Nature and contents of container
Pre-filled syringes (glass type I), with or without a needle safety guard, with plunger stopper
(Teflon-faced rubber) sealed in a blister.
The syringes contain 0.3 ml (3,000 IU) of solution.
Pack of 1 or 6 syringes.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
Epoetin alfa HEXAL must not be used
-
if the solution is cloudy or there are particles in it.
if the solution has been accidentally frozen.
The pre-filled syringes are ready to use (see section 4.2). The pre-filled syringe should not be shaken.
Syringes are embossed with graduation rings in order to enable partial use if required. Each graduation
ring corresponds to a volume of 0.1 ml. Only take one dose of Epoetin alfa HEXAL from each syringe
discarding unwanted solution before injection.
Using the pre-filled syringe with a needle safety guard
The needle safety guard covers the needle after injection to prevent needle stick injury. This does not
affect normal operation of the syringe. Depress the plunger slowly and evenly until the entire dose has
been given and the plunger cannot be depressed any further. While maintaining pressure on the
plunger, remove the syringe from the patient. The needle safety guard will cover the needle when
releasing the plunger.
Using the pre-filled syringe without a needle safety guard
Administer the dose as per standard protocol.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Hexal AG
Industriestrasse 25
D-83607 Holzkirchen
Germany
MARKETING AUTHORISATION NUMBER(S)
EU/1/07/411/005
EU/1/07/411/006
EU/1/07/411/031
EU/1/07/411/032
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency
What Epoetin alfa HEXAL contains
The active substance is epoetin alfa.
Epoetin alfa HEXAL 1,000 IU/0.5 ml solution for injection in a pre-filled syringe: one pre-filled
syringe of 0.5 ml solution for injection contains 1,000 international units (IU) corresponding to
8.4 micrograms epoetin alfa
Epoetin alfa HEXAL 2,000 IU/1 ml solution for injection in a pre-filled syringe: one pre-filled
syringe of 1 ml solution for injection contains 2,000 international units (IU) corresponding to
16.8 micrograms epoetin alfa
Epoetin alfa HEXAL 3,000 IU/0.3 ml solution for injection in a pre-filled syringe: one pre-filled
syringe of 0.3 ml solution for injection contains 3,000 international units (IU) corresponding to
25.2 micrograms epoetin alfa
Epoetin alfa HEXAL 4,000 IU/0.4 ml solution for injection in a pre-filled syringe: one pre-filled
syringe of 0.4 ml solution for injection contains 4,000 international units (IU) corresponding to
33.6 micrograms epoetin alfa
Epoetin alfa HEXAL 5,000 IU/0.5 ml solution for injection in a pre-filled syringe: one pre-filled
syringe of 0.5 ml solution for injection contains 5,000 international units (IU) corresponding to
42.0 micrograms epoetin alfa
Epoetin alfa HEXAL 6,000 IU/0.6 ml solution for injection in a pre-filled syringe: one pre-filled
syringe of 0.6 ml solution for injection contains 6,000 international units (IU) corresponding to
50.4 micrograms epoetin alfa
Epoetin alfa HEXAL 7,000 IU/0.7 ml solution for injection in a pre-filled syringe: one pre-filled
syringe of 0.7 ml solution for injection contains 7,000 international units (IU) corresponding to
58.8 micrograms epoetin alfa
Epoetin alfa HEXAL 8,000 IU/0.8 ml solution for injection in a pre-filled syringe: one pre-filled
syringe of 0.8 ml solution for injection contains 8,000 international units (IU) corresponding to
67.2 micrograms epoetin alfa
Epoetin alfa HEXAL 9,000 IU/0.9 ml solution for injection in a pre-filled syringe: one pre-filled
syringe of 0.9 ml solution for injection contains 9,000 international units (IU) corresponding to
75.6 micrograms epoetin alfa
Epoetin alfa HEXAL 10,000 IU/1 ml solution for injection in a pre-filled syringe: one pre-filled
syringe of 1 ml solution for injection contains 10,000 international units (IU) corresponding to
84.0 micrograms epoetin alfa
Epoetin alfa HEXAL 20,000 IU/0.5 ml solution for injection in a pre-filled syringe: one pre-
filled syringe of 0.5 ml solution for injection contains 20,000 international units (IU)
corresponding to 168.0 micrograms epoetin alfa
Epoetin alfa HEXAL 30,000 IU/0.75 ml solution for injection in a pre-filled syringe: one pre-
filled syringe of 0.75 ml solution for injection contains 30,000 international units (IU)
corresponding to 252.0 micrograms epoetin alfa
Epoetin alfa HEXAL 40,000 IU/1 ml solution for injection in a pre-filled syringe: one pre-filled
syringe of 1 ml solution for injection contains 40,000 international units (IU) corresponding to
336.0 micrograms epoetin alfa
The other ingredients are sodium dihydrogen phosphate dihydrate, disodium phosphate
dihydrate, sodium chloride, glycine, polysorbate 80, hydrochloric acid (for pH-adjustment),
sodium hydroxide (for pH-adjustment), and water for injections.
What Epoetin alfa HEXAL looks like and contents of the pack
Epoetin alfa HEXAL is presented as a clear, colourless solution for injection in a pre-filled syringe.
The syringes are sealed in a blister.
Epoetin alfa HEXAL 1,000 IU/0.5 ml solution for injection in a pre-filled syringe
The syringes contain 0.5 ml (1000 IU) of solution.
Epoetin alfa HEXAL 2,000 IU/1 ml solution for injection in a pre-filled syringe
The syringes contain 1 ml (2,000 IU) of solution.
Epoetin alfa HEXAL 3,000 IU/0.3 ml solution for injection in a pre-filled syringe
The syringes contain 0.3 ml (3,000 IU) of solution.
Epoetin alfa HEXAL 4,000 IU/0.4 ml solution for injection in a pre-filled syringe
The syringes contain 0.4 ml (4,000 IU) of solution.
Epoetin alfa HEXAL 5,000 IU/0.5 ml solution for injection in a pre-filled syringe
The syringes contain 0.5 ml (5,000 IU) of solution.
Epoetin alfa HEXAL 6,000 IU/0.6 ml solution for injection in a pre-filled syringe
The syringes contain 0.6 ml (6,000 IU) of solution.
Epoetin alfa HEXAL 7,000 IU/0.7 ml solution for injection in a pre-filled syringe
The syringes contain 0.7 ml (7,000 IU) of solution.
Epoetin alfa HEXAL 8,000 IU/0.8 ml solution for injection in a pre-filled syringe
The syringes contain 0.8 ml (8,000 IU) of solution.
Epoetin alfa HEXAL 9,000 IU/0.9 ml solution for injection in a pre-filled syringe
The syringes contain 0.9 ml (9,000 IU) of solution.
Epoetin alfa HEXAL 10,000 IU/1 ml solution for injection in a pre-filled syringe
The syringes contain 1 ml (10,000 IU) of solution.
Epoetin alfa HEXAL 20,000 IU/0.5 ml solution for injection in a pre-filled syringe
The syringes contain 0.5 ml (20,000 IU) of solution.
Epoetin alfa HEXAL 30,000 IU/0.75 ml solution for injection in a pre-filled syringe
The syringes contain 0.75 ml (30,000 IU) of solution.
Epoetin alfa HEXAL 40,000 IU/1 ml solution for injection in a pre-filled syringe
The syringes contain 1 ml (40,000 IU) of solution.
Pack size of 1 or 6 pre-filled syringe(s) with or without a needle safety guard.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Hexal AG
Industriestrasse 25
D-83607 Holzkirchen
Germany
Sandoz GmbH
Biochemiestr. 10
A-6250 Kundl
Austria
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
Hexal AG
Industriestrasse 25
D-83607 Holzkirchen
Germany
Tel: + 49 8024 908-0
Fax: + 49 8024 908-1290
e-mail: service@hexal.com
This leaflet was last approved in {MM/YYYY}.
Detailed information on this medicine is available on the European Medicines Agency web site:
------------------------------------------------------------------------------------------------------------------
Instructions on how to inject yourself (for patients receiving chemotherapy, adult patients
donating their own blood before surgery or adult patients scheduled for orthopaedic surgery
only)
This section contains information on how to give yourself an injection of Epoetin alfa HEXAL.
It is
important that you do not try to give yourself the injection unless you have received special
training from your doctor or nurse.
Epoetin alfa HEXAL is provided with or without a needle
safety guard and you will be shown how to use this by your doctor or nurse. If you are not sure about
giving the injection or you have any questions, please ask your doctor or nurse for help.
1.
Wash your hands.
2.
Remove one syringe from the pack and remove the protective cap from the injection needle.
Syringes are embossed with graduation rings in order to enable partial use if required. Each
graduation ring corresponds to a volume of 0.1 ml. If partial use of a syringe is required, remove
unwanted solution before injection.
3.
Clean the skin at the injection site using an alcohol wipe.
4.
Form a skin fold by pinching the skin between thumb and forefinger.
5.
Insert the needle into the skin fold with a quick, firm action. Inject the Epoetin alfa HEXAL
solution as you have been shown by your doctor. You should check with your doctor or
pharmacist if you are not sure.
Pre-filled syringe without needle safety guard
6.
Always keeping your skin pinched, depress the plunger slowly and evenly.
7.
After injecting the liquid, remove the needle and let go of your skin. Apply
pressure over the injection site with a dry, sterile pad.
8.
Discard any unused product or waste material. Only use each syringe for
one injection.
Pre-filled syringe with needle safety guard
6.
Always keeping your skin pinched, depress the plunger slowly and evenly
until the entire dose has been given and the plunger cannot be depressed
any further. Do not release the pressure on the plunger!
7.
After injecting the liquid, remove the needle while maintaining pressure on
the plunger and then let go of your skin. Apply pressure over the injection
site with a dry, sterile pad.
8.
Let go of the plunger. The needle safety guard will rapidly move to cover
the needle.
9.
Discard any unused product or waste material. Only use each syringe for one injection.
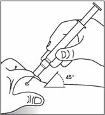

Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/epoetin_alfa_hexal.html
Copyright © 1995-2021 ITA all rights reserved.
|





































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































