Summary for the public
What is Equilis Prequenza?Equilis Prequenza is a vaccine for use in horses. It contains parts (subunits) of three equine influenza (flu) strains (‘A/equine-1/Prague/1/56’, ‘A/equine 2/Newmarket/1/93’ and ‘A/equine-2/Newmarket/2/93’). The vaccine is available as a suspension for injection.
What is Equilis Prequenza used for?Equilis Prequenza is used to vaccinate horses from six months of age against equine influenza. Equine influenza is a highly contagious disease that is very common in horses but that rarely causes death. The vaccine reduces the signs of equine influenza and the excretion (shedding) of the virus after infection.
The vaccine is given as an injection into a muscle.
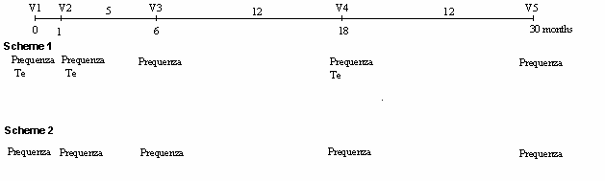
Horses should receive a primary vaccination, consisting of two injections given four weeks apart. This should be followed by a third vaccination five months later, and afterwards by yearly revaccinations.
How does Equilis Prequenza work?Equilis Prequenza contains fragments (subunits) of the influenza virus against which the vaccine is indicated for. These fragments provoke a protective response of the immune system (the body’s natural defences) but cannot cause the disease.
Vaccines work by ‘teaching’ the immune system how to defend itself against diseases. When the product is given to horses, it helps the animals’ immune system to react more quickly when the animal is naturally exposed to the virus. This helps to protect against equine influenza. The vaccine also contains an ‘adjuvant’ to stimulate a better response.
How has Equilis Prequenza been studied?The safety of Equilis Prequenza was studied in several studies under laboratory and field conditions in a large number of horses, from 2 months of age. It was concluded that the product is well tolerated by horses of different ages. Equilis Prequenza was also studied in pregnant mares. No negative influence on gestation, foaling and offspring of mares was observed after vaccination at different times during pregnancy.
The effectiveness of Equilis Prequenza has been studied in several trials under laboratory and field conditions. Most of the studies used Equilis Prequenza Te, a vaccine that protects against the three equine influenza strains (the same as Equilis Prequenza), as well as tetanus. The main measure of effectiveness was the production of protective levels of antibodies against the influenza components. The studies also compared the clinical signs and virus excretion of a group of vaccinated animals with those of a control group, i.e. which did not receive the vaccine.
What benefit has Equilis Prequenza shown during the studies?The studies showed that Equilis Prequenza is an effective vaccine against equine influenza to reduce clinical signs and virus excretion after infection, in horses from 6 months of age. Horses developed protection two weeks after primary vaccination. The duration of protection was five months after primary vaccination and 12 months after the first revaccination.
What is the risk associated with Equilis Prequenza?Swelling (max. diameter 5 cm) may occur, either as hard or soft swelling. The swelling is expected to decrease within two days. Pain at the injection site can occur occasionally. In some cases fever may occur for one day, and up to three days in exceptional circumstances.
What are the precautions for the person who gives the medicine or comes into contact with the animal?In case of accidental self-injection, seek medical advice immediately and show the package leaflet or the label to the doctor.
What is the time to allow before the animal can be slaughtered and the meat used for human consumption (withdrawal period)?The withdrawal period of the product is zero days.
What is the time to allow before milk can be taken from the animal for human consumption?Zero days.
Why has Equilis Prequenza been approved?The Committee for Medicinal Products for Veterinary Use (CVMP) concluded that the benefits of Equilis Prequenza exceeded the risks for the immunisation of horses from six months of age against equine influenza to reduce clinical signs and virus excretion after infection. The Committee recommended that Equilis Prequenza should be given a marketing authorisation.
The benefit-risk balance may be found in module 6 of this EPAR.
Other information about Equilis PrequenzaThe European Commission granted a marketing authorisation valid throughout the European Union, for Equilis Prequenza to Intervet International BV on 8 July 2005. Information on the prescription status of this product may be found on the label/outer package.
Authorisation details
| Name: Equilis Prequenza |
| EMEA Product number: EMEA/V/C/000094 |
| Active substance: Purified haemagglutinin subunits from equine influenza viruses |
| INN or common name: Adjuvanted vaccine against equine influenza |
| Species: Horses |
| ATCvet Code: QI05AA01 |
| Marketing Authorisation Holder: Intervet International BV |
| Revision: 6 |
| Date of issue of Market Authorisation valid throughout the European Union: 08/07/2005 |
Contact address:
Intervet International B.V.
Wim de Körverstraat 35
5831 AN Boxmeer
The Netherlands
|























































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































