Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Extavia 250 microgram/ml powder and solvent for solution for injection.
QUALITATIVE AND QUANTITATIVE COMPOSITION
Extavia contains 300 microgram (9.6 million IU) of recombinant interferon beta-1b per vial.
Recombinant interferon beta-1b* 250 microgram (8.0 million IU) per ml when reconstituted.
* produced by genetic engineering from strain of
Escherichia coli
.
For a full list of excipients, see section 6.1.
Powder and solvent for solution for injection.
White to off-white powder.
4.1 Therapeutic indications
Extavia is indicated for the treatment of
Patients with a single demyelinating event with an active inflammatory process, if it is severe
enough to warrant treatment with intravenous corticosteroids, if alternative diagnoses have been
excluded, and if they are determined to be at high risk of developing clinically definite multiple
sclerosis (see section 5.1).
Patients with relapsing-remitting multiple sclerosis and two or more relapses within the last two
years.
Patients with secondary progressive multiple sclerosis with active disease, evidenced by
relapses.
4.2 Posology and method of administration
The treatment with Extavia should be initiated under the supervision of a physician experienced in the
treatment of the disease.
Adults
The recommended dose of Extavia is 250 microgram (8.0 million IU), contained in 1 ml of the
reconstituted solution (see section 6.6), to be injected subcutaneously every other day.
Children and adolescents
No formal clinical trials or pharmacokinetic studies have been conducted in children or adolescents.
However, limited published data suggest that the safety profile in adolescents from 12 to 16 years of
age receiving Extavia 8.0 million IU subcutaneously every other day is similar to that seen in adults.
There is no information on the use of Extavia in children under 12 years of age and therefore Extavia
should not be used in this population.
Generally, dose titration is recommended at the start of treatment.
Patients should be started at 62.5 microgram (0.25 ml) subcutaneously every other day, and increased
slowly to a dose of 250 microgram (1.0 ml) every other day (see Table A). The titration period may be
adjusted, if any significant adverse reaction occurs. In order to obtain adequate efficacy, a dose of
250 microgram (1.0 ml) every other day should be reached.
Table A: Schedule for dose titration*
Treatment day Dose Volume
1, 3, 5 62.5 microgram 0.25 ml
7, 9, 11 125 microgram 0.5 ml
13, 15, 17 187.5 microgram 0.75 ml
≥ 19 250 microgram 1.0 ml
* The titration period may be adjusted if any significant adverse reaction occurs.
The optimal dose has not been fully clarified.
At the present time, it is not known for how long the patient should be treated. There are follow-up
data under controlled clinical conditions for patients with relapsing-remitting multiple sclerosis for up
to 5 years and for patients with secondary progressive multiple sclerosis for up to 3 years. For
relapsing-remitting multiple sclerosis, efficacy has been demonstrated for therapy for the first two
years. The available data for the additional three years are consistent with sustained treatment efficacy
of Extavia over the whole time period.
In patients with a single clinical event suggestive of multiple sclerosis, efficacy has been demonstrated
over a period of three years.
Treatment is not recommended in patients with relapsing-remitting multiple sclerosis who have
experienced less than 2 relapses in the previous 2 years or in patients with secondary-progressive
multiple sclerosis who have had no active disease in the previous 2 years.
If the patient fails to respond, for example a steady progression in Expanded Disability Status Scale
(EDSS) for 6 months occurs or treatment with at least 3 courses of adrenocorticotropic hormone
(ACTH) or corticosteroids during a one-year period is required despite Extavia therapy, treatment with
Extavia should be stopped.
Initiation of treatment in pregnancy (see section 4.6).
Patients with a history of hypersensitivity to natural or recombinant interferon beta, human
albumin or to any of the excipients.
Patients with current severe depression and/or suicidal ideation (see sections 4.4 and 4.8).
Patients with decompensated liver disease (see sections 4.4, 4.5 and 4.8).
4.4 Special warnings and precautions for use
Immune system disorders
The administration of cytokines to patients with a pre-existing monoclonal gammopathy has been
associated with the development of systemic capillary leak syndrome with shock-like symptoms and
fatal outcome.
Gastrointestinal disorders
In rare cases, pancreatitis was observed with Extavia use, often associated with hypertriglyceridaemia.


Nervous system disorders
Extavia should be administered with caution to patients with previous or current depressive disorders,
in particular to those with antecedents of suicidal ideation (see section 4.3). Depression and suicidal
ideation are known to occur in increased frequency in the multiple sclerosis population and in
association with interferon use. Patients treated with Extavia should be advised to immediately report
any symptoms of depression and/or suicidal ideation to their prescribing physician. Patients exhibiting
depression should be monitored closely during therapy with Extavia and treated appropriately.
Cessation of therapy with Extavia should be considered (see also sections 4.3 and 4.8).
Extavia should be administered with caution to patients with a history of seizures, to those receiving
treatment with anti-epileptics, particularly if their epilepsy is not adequately controlled with anti-
epileptics (see sections 4.5 and 4.8).
This product contains human albumin and hence carries a potential risk for transmission of viral
diseases. A risk for transmission of Creutzfeld-Jacob disease (CJD) cannot be excluded.
Laboratory tests
Thyroid function tests are recommended regularly in patients with a history of thyroid dysfunction or
as clinically indicated.
In addition to those laboratory tests normally required for monitoring patients with multiple sclerosis,
complete blood and differential white blood cell counts, platelet counts, and blood chemistries,
including liver function tests (e.g. aspartate aminotransferase serum glutamic-oxaloacetic transaminase
(SGOT), alanine aminotransferase serum glutamate pyruvate transaminase (SGPT) and gamma
glutamyltransferase), are recommended prior to initiation and at regular intervals following
introduction of Extavia therapy, and then periodically thereafter in the absence of clinical symptoms.
Patients with anaemia, thrombocytopenia or leukopenia (alone or in any combination) may require
more intensive monitoring of complete blood cell counts, with differential and platelet counts. Patients
who develop neutropenia should be monitored closely for the development of fever or infection. There
have been reports of thrombocytopenia, with profound decreases in platelet count.
Hepatobiliary disorders
Asymptomatic elevations of serum transaminases, in most cases mild and transient, occurred very
commonly in patients treated with Extavia during clinical trials. As for other beta interferons, severe
hepatic injury, including cases of hepatic failure, has been reported rarely in patients taking Extavia.
The most serious events often occurred in patients exposed to other medicinal products or substances
known to be associated with hepatotoxicity or in the presence of co-morbid medical conditions (e.g.
metastasising malignant disease, severe infection and sepsis, alcohol abuse).
Patients should be monitored for signs of hepatic injury. The occurrence of elevations in serum
transaminases should lead to close monitoring and investigation. Withdrawal of Extavia should be
considered if the levels significantly increase or if they are associated with clinical symptoms such as
jaundices. In the absence of clinical evidence for liver damage, and after normalisation of liver
enzymes, a reintroduction of therapy could be considered with appropriate follow-up of hepatic
functions.
Renal and urinary disorders
Caution should be used and close monitoring considered when administering interferon beta to
patients with severe renal failure.
Cardiac disorders
Extavia should also be used with caution in patients who suffer from pre-existing cardiac disorders.
Patients with pre-existing significant cardiac disease, such as congestive heart failure, coronary artery
disease or arrhythmia, should be monitored for worsening of their cardiac condition, particularly
during initiation of treatment with Extavia.
While Extavia does not have any known direct-acting cardiac toxicity, symptoms of the flu-like
syndrome associated with beta interferons may prove stressful to patients with pre-existing significant
cardiac disease. During the post-marketing period very rare reports have been received of worsening
of cardiac status in patients with pre-existing significant cardiac disease temporarily associated with
the initiation of Extavia therapy.
Rare cases of cardiomyopathy have been reported. If this occurs and a relationship to Extavia is
suspected, treatment should be discontinued.
General disorders and administration site conditions
Serious hypersensitivity reactions (rare but severe acute reactions such as bronchospasm, anaphylaxis
and urticaria) may occur. If reactions are severe, Extavia should be discontinued and appropriate
medical intervention instituted.
Injection site necrosis has been reported in patients using Extavia (see section 4.8). It can be extensive
and may involve muscle fascia as well as fat and therefore can result in scar formation. Occasionally
debridement and, less often, skin grafting are required and healing may take up to 6 months.
If the patient experiences any break in the skin, which may be associated with swelling or drainage of
fluid from the injection site, the patient should be advised to consult with his/her physician before
continuing injections with Extavia.
If the patient has multiple lesions Extavia should be discontinued until healing has occurred. Patients
with single lesions may continue on Extavia provided the necrosis is not too extensive, as some
patients have experienced healing of injection site necrosis whilst on Extavia.
To minimise the risk of injection site necrosis patients should be advised to:
−
use an aseptic injection technique,
rotate the injection sites with each dose.
The incidence of injection site reactions may be reduced by the use of an auto-injector. In the pivotal
study of patients with a single clinical event suggestive of multiple sclerosis an auto-injector was used
in the majority of patients. Injection site reactions as well as injection site necroses were observed less
frequently in this study than in the other pivotal studies.
The procedure for self-administration by the patient should be reviewed periodically, especially if
injection site reactions have occurred.
Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. Serum samples in controlled
clinical trials were collected every 3 months for monitoring of development of antibodies to Extavia.
In the different controlled clinical trials, between 23% and 41% of the patients developed serum
interferon beta-1b neutralising activity confirmed by at least two consecutive positive titres; of these
patients, between 43% and 55% converted to a stable antibody negative status (based on two
consecutive negative titres) during the subsequent observational period of the respective study.
The development of neutralising activity is associated with a reduction in clinical efficacy only with
regard to relapse activity. Some analyses suggest that this effect might be larger in patients with higher
titre levels of neutralising activity.
In the study of patients with a single clinical event suggestive of multiple sclerosis, neutralising
activity measured every 6 months was observed at least once in 32% (88) of the patients treated
immediately with Extavia; of these, 47% (41) returned to negative status over a 3-year period. Within
this period, the development of neutralising activity was not associated with a reduction in clinical
efficacy (with regard to time to clinically definite multiple sclerosis (CDMS), and time to confirmed
EDSS progression).
New adverse events have not been associated with the development of neutralising activity.
It has been demonstrated
in vitro
that Extavia cross-reacts with natural interferon beta. However, this
has not been investigated
in vivo
and its clinical significance is uncertain.
There are sparse and inconclusive data on patients who have developed neutralising activity and have
completed Extavia therapy.
The decision to continue or discontinue treatment should be based on clinical disease activity rather
than on neutralising activity status.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed.
The effect of alternate-day administration of 250 microgram (8.0 million IU) of Extavia on drug
metabolism in multiple sclerosis patients is unknown. Corticosteroid or ACTH treatment of relapses
for periods of up to 28 days has been well tolerated in patients receiving Extavia.
Due to the lack of clinical experience in multiple sclerosis patients, the use of Extavia together with
immunomodulators other than corticosteroids or ACTH is not recommended.
Interferons have been reported to reduce the activity of hepatic cytochrome P450-dependent enzymes
in humans and animals. Caution should be exercised when Extavia is administered in combination
with medicinal products that have a narrow therapeutic index and are largely dependent on the hepatic
cytochrome P450 system for clearance, e.g. anti-epileptics. Additional caution should be exercised
with any co-medication which has an effect on the haematopoetic system.
No interaction studies with anti-epileptics have been carried out.
4.6 Pregnancy and lactation
Pregnancy
There is limited information on the use of Extavia in pregnancy. Available data indicates that there
may be an increased risk of spontaneous abortion. Initiation of treatment is contraindicated during
pregnancy (see section 4.3).
Women of child-bearing potential
Women of child-bearing potential should take appropriate contraceptive measures. If the patient
becomes pregnant or plans to become pregnant while taking Extavia, she should be informed of the
potential hazards and discontinuation of therapy should be considered (see section 5.3). In patients
with a high relapse rate before treatment started, the risk of a severe relapse following discontinuation
of Extavia in the event of pregnancy should be weighed against a possible increased risk of
spontaneous abortion.
Lactation
It is not known whether interferon beta-1b is excreted in human milk. Because of the potential for
serious adverse reactions in nursing infants a decision should be made whether to discontinue breast-
feeding or Extavia therapy.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed.
Central nervous system-related adverse events associated with the use of Extavia might influence the
ability to drive and use machines in susceptible patients.
a) At the beginning of treatment adverse reactions are common but in general they subside with
further treatment. The most frequently observed adverse reactions are a flu-like symptom
complex (fever, chills, arthralgia, malaise, sweating, headache, or myalgia), which is mainly due
to the pharmacological effects of the medicinal product, and injection site reactions. Injection site
reactions occurred frequently after administration of Extavia. Redness, swelling, discoloration,
inflammation, pain, hypersensitivity, necrosis and non-specific reactions were significantly
associated with 250 microgram (8 million IU) Extavia treatment.
Generally, dose titration is recommended at the start of treatment in order to increase tolerability
to Extavia (see section 4.2). Flu-like symptoms may also be reduced by administration of non-
steroidal anti-inflammatory medicinal products. The incidence of injection site reactions may be
reduced by the use of an auto-injector.
b) The following adverse event listing is based on reports from clinical trials
(Table 1, adverse
events and laboratory abnormalities)
and from the post-marketing surveillance
(Table 2,
reporting rates based on spontaneous adverse drug reaction reports classified as very common
≥1/10, common ≥1/100 to <1/10, uncommon ≥ 1/1,000 to < 1/100, rare ≥1/10,000 to <1/1,000,
very rare < 1/10,000)
of Extavia use. Experience with Extavia in patients with multiple sclerosis
(MS) is limited, consequently those adverse events which occur very rarely may not yet have
been observed.
Adverse events and laboratory abnormalities with incidence rates ≥10% and the
respective percentages under placebo; significantly associated side effects <10%
Single Event
suggestive of
Multiple
Sclerosis
(BENEFIT)
Secondary
Progressive
Multiple
Sclerosis
(European
Study)
Secondary
Progressive
Multiple
Sclerosis
(North
American
Study)
Relapsing-
Remitting
Multiple
Sclerosis
Adverse Event
and
Laboratory Abnormalities
Extavia
250 microgram
(Placebo)
n=292 (n=176)
Extavia
250 microgram
(Placebo)
n=360 (n=358)
Extavia
250 microgram
(Placebo)
n=317 (n=308)
Extavia
250 microgram
(Placebo)
n=124 (n=123)
Infections and infestations
Infection
Blood and lymphatic system disorders
Lymphocyte count
decreased (<1500/mm³)
×
Λ
°
Absolute neutrophil count
decreased (<1500/mm³)
×
Λ
* °
White blood cell count
decreased (<3000/mm³)
×
Λ
* °
Metabolism and nutrition disorders
Blood glucose decreased
(<55 mg/dl)
×



















Single Event
suggestive of
Multiple
Sclerosis
(BENEFIT)
Secondary
Progressive
Multiple
Sclerosis
(European
Study)
Secondary
Progressive
Multiple
Sclerosis
(North
American
Study)
Relapsing-
Remitting
Multiple
Sclerosis
Adverse Event
and
Laboratory Abnormalities
Extavia
250 microgram
(Placebo)
n=292 (n=176)
Extavia
250 microgram
(Placebo)
n=360 (n=358)
Extavia
250 microgram
(Placebo)
n=317 (n=308)
Extavia
250 microgram
(Placebo)
n=124 (n=123)
Psychiatric disorders
Depression
Nervous system disorders
Headache
Λ
Eye disorders
Conjunctivitis
Ear and labyrinth disorders
Ear pain
Cardiac disorders
Palpitation *
Vascular disorders
Vasodilatation
Respiratory, thoracic and mediastinal disorders
Upper respiratory
infection
Gastrointestinal disorders
Diarrhoea
Hepatobiliary disorders
Alanine aminotransferase
increased (SGPT >5 times
baseline)
× Λ
* °
Aspartate
aminotransferase
increased (SGOT
>5 times baseline)
× Λ
* °










































Single Event
suggestive of
Multiple
Sclerosis
(BENEFIT)
Secondary
Progressive
Multiple
Sclerosis
(European
Study)
Secondary
Progressive
Multiple
Sclerosis
(North
American
Study)
Relapsing-
Remitting
Multiple
Sclerosis
Adverse Event
and
Laboratory Abnormalities
Extavia
250 microgram
(Placebo)
n=292 (n=176)
Extavia
250 microgram
(Placebo)
n=360 (n=358)
Extavia
250 microgram
(Placebo)
n=317 (n=308)
Extavia
250 microgram
(Placebo)
n=124 (n=123)
Skin and subcutaneous tissue disorders
Skin disorder
Musculoskeletal and connective tissue disorders
Hypertonia°
Renal and urinary disorders
Urinary retention
Urinary protein positive
(>1+)
×
Reproductive system and breast disorders
Dysmenorrhoea





























Single Event
suggestive of
Multiple
Sclerosis
(BENEFIT)
Secondary
Progressive
Multiple
Sclerosis
(European
Study)
Secondary
Progressive
Multiple
Sclerosis
(North
American
Study)
Relapsing-
Remitting
Multiple
Sclerosis
Adverse Event
and
Laboratory Abnormalities
Extavia
250 microgram
(Placebo)
n=292 (n=176)
Extavia
250 microgram
(Placebo)
n=360 (n=358)
Extavia
250 microgram
(Placebo)
n=317 (n=308)
Extavia
250 microgram
(Placebo)
n=124 (n=123)
General disorders and administration site conditions
Injection site reaction
(various kinds)
Λ
* °
§
Injection site necrosis * °
Significantly associated with Extavia treatment for patients with first event suggestive of MS, p
<0.05
Significantly associated with Extavia treatment for RRMS, p <0.05
Significantly associated with Extavia treatment for SPMS, p <0.05
Injection site reaction (various kinds) comprises all adverse events occurring at the injection site,
i.e. the following terms: injection site haemorrhage, injection site hypersensitivity, injection site
inflammation, injection site mass, injection site necrosis, injection site pain, injection site
reaction, injection site oedema, and injection site atrophy
“Flu-like symptom complex” denotes flu syndrome and/or a combination of at least two adverse
events from fever, chills, myalgia, malaise, sweating.
The most appropriate MedDRA term is used to describe a certain reaction and its synonyms and
related conditions.
Reporting rates (very common ≥1/10, common ≥1/100 to <1/10, uncommon ≥1/1,000
to <1/100, rare ≥1/10,000 to <1/1,000, very rare <1/10,000) based on spontaneous
adverse drug reaction reports
Uncommon
≥
1/1,000 to <1/100
Rare
≥
1/10,000 to
<1/1,000
Blood and
lymphatic system
disorders
Anemia,
Thrombocytopenia,
Leukopenia































Uncommon
≥
1/1,000 to <1/100
Rare
≥
1/10,000 to
<1/1,000
Hyperthyroidism,
Hypothyroidism,
Thyroid disorder
Metabolism and
nutrition disorders
Blood triglycerides
increased,
Anorexia
Depression (see
also section 4.4)
Confusion,
Anxiety,
Emotional lability,
Suicide attempt
(see also section
4.4)
Cardiomyopathy,
Tachycardia,
Palpitation
Respiratory,
thoracic and
mediastinal
disorders
Gastrointestinal
disorders
Alanine amino-
transferase
increased,
Aspartate amino-
transferase
increased
Blood bilirubin
increased,
Gamma-glutamyl-
transferase
increased,
Hepatitis
Skin and
subcutaneous
tissue disorders
Urticaria,
Rash,
Pruritus,
Alopecia
Musculoskeletal,
connective tissue
and bone
disorders
Reproductive
system and breast
disorders
General disorders
and
administration site
conditions
Flu-like
symptoms*,
Chills*,
Fever *,
Injection site
reaction*,
Injection site
inflammation*,
Injection site pain
Chest pain,
Malaise,
Sweating
* frequencies based on clinical trials






















The most appropriate MedDRA term is used to describe a certain reaction and its synonyms and
related conditions.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Interferon beta-1b has been given without serious adverse events compromising vital functions to
adult cancer patients at individual doses as high as 5,500 microgram (176 million IU) intravenously
three times a week.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Interferons, ATC Code: L03AB08
Interferons belong to the family of cytokines, which are naturally occurring proteins. Interferons have
molecular weights ranging from 15,000 to 21,000 Daltons. Three major classes of interferons have
been identified: alpha, beta, and gamma. Interferon alpha, interferon beta, and interferon gamma have
overlapping yet distinct biological activities. The activities of interferon beta-1b are species-restricted
and therefore, the most pertinent pharmacological information on interferon beta-1b is derived from
studies of human cells in culture or in human
in vivo
studies.
Interferon beta-1b has been shown to possess both antiviral and immunoregulatory activities. The
mechanisms by which interferon beta-1b exerts its actions in multiple sclerosis are not clearly
understood. However, it is known that the biological response-modifying properties of interferon
beta-1b are mediated through its interactions with specific cell receptors found on the surface of
human cells. The binding of interferon beta-1b to these receptors induces the expression of a number
of gene products that are believed to be the mediators of the biological actions of interferon beta-1b. A
number of these products have been measured in the serum and cellular fractions of blood collected
from patients treated with interferon beta-1b. Interferon beta-1b both decreases the binding affinity
and enhances the internalisation and degradation of the interferon-gamma receptor. Interferon beta-1b
also enhances the suppressor activity of peripheral blood mononuclear cells.
No separate investigations were performed regarding the influence of Extavia on the cardiovascular
system, respiratory system and the function of endocrine organs.
Clinical trials
Relapsing-remitting multiple sclerosis (RR-MS)
One controlled clinical trial with Extavia in patients with relapsing-remitting multiple sclerosis and
able to walk unaided (baseline EDSS 0 to 5.5) was performed. Patients receiving Extavia showed a
reduction in frequency (30%) and severity of clinical relapses, as well as the number of
hospitalisations due to disease. Furthermore, there was a prolongation of the relapse-free interval.
There is no evidence of an effect of Extavia on the duration of relapses or on symptoms in between
relapses, and no significant effect was seen on the progression of the disease in relapsing-remitting
multiple sclerosis.
Secondary progressive multiple sclerosis (SP-MS)
Two controlled clinical trials with Extavia involving a total of 1,657 patients with secondary
progressive multiple sclerosis (baseline EDSS 3 to 6.5, i.e. patients were able to walk) were
performed. Patients with mild disease and those unable to walk were not studied. The two studies
showed inconsistent results for the primary endpoint time to confirmed progression, representing delay
of disability progression:
One of the two studies demonstrated a statistically significant delay in the time to disability
progression (Hazard Ratio = 0.69, 95% confidence interval (0.55, 0.86), p=0.0010, corresponding to a
31% risk reduction due to Extavia) and in the time to becoming wheelchair-bound (Hazard Ratio =
0.61, 95% confidence interval (0.44, 0.85), p=0.0036, corresponding to a 39% risk reduction due to
Extavia) in patients who received Extavia. This effect continued over the observation period of up to
33 months. The treatment effect occurred in patients at all levels of disability investigated and
independent of relapse activity.
In the second trial of Extavia in secondary progressive multiple sclerosis, no delay in the time to
disability progression was observed. There is evidence that the patients included in this study had
overall less active disease than in the other study in secondary progressive multiple sclerosis.
In retrospective meta-analyses including the data of both studies, an overall treatment effect was found
which was statistically significant (p=0.0076; 8 million IU Extavia versus all placebo patients).
Retrospective analyses in subgroups showed that a treatment effect on disability progression is most
likely in patients with active disease before treatment commences (Hazard Ratio 0.72, 95% confidence
interval (0.59, 0.88), p=0.0011, corresponding to a 28% risk reduction due to Extavia in patients with
relapses or pronounced EDSS progression, 8 million IU Extavia versus all placebo patients). From
these retrospective subgroup analyses there was evidence to suggest that relapses as well as
pronounced EDSS progression (EDSS >1 point or >0.5 point for EDSS >=6 in the previous two years)
can help to identify patients with active disease.
In both trials secondary progressive multiple sclerosis patients receiving Extavia showed a reduction
in frequency (30%) of clinical relapses. There is no evidence of Extavia having an effect on the
duration of relapses.
Single clinical event suggestive of multiple sclerosis
One controlled clinical trial with Extavia was performed in patients with a single clinical event and
Magnetic Resonance Imaging (MRI) features suggestive of multiple sclerosis (at least two clinically
silent lesions on the T2-weighted MRI). Patients with monofocal or multifocal onset of the disease
were included (i.e. patients with clinical evidence for a single or at least two lesions, respectively, of
the central nervous system). Any disease other than multiple sclerosis that could better explain signs
and symptoms of the patient had to be excluded. This study consisted of two phases, a placebo-
controlled phase followed by a pre-planned follow-up phase. The placebo-controlled phase lasted for
2 years or until the patient developed clinically definite multiple scleroiss (CDMS), whichever came
first. After the placebo-controlled phase, patients entered a pre-planned follow-up phase with Extavia
to evaluate the effects of immediate versus delayed start of Extavia treatment, comparing patients
initially randomised to Extavia (“immediate treatment group”) or to placebo (“delayed treatment
group”). Patients and investigators remained blinded to the initial treatment allocation.
In the placebo-controlled phase, Extavia delayed the progression from the first clinical event to
clinically definite multiple sclerosis (CDMS) in a statistically significant and clinically meaningful
manner, corresponding to a risk reduction of 47% (Hazard Ratio = 0.53, 95% confidence interval
(0.39, 0.73), p<0.0001). Within the study period of two years, CDMS occurred in 45% of the placebo
group compared to 28% of the Extavia group (Kaplan-Meier estimates). Extavia prolonged the time to
CDMS by 363 days, from 255 days in the placebo group to 618 days in the Extavia group (based on
the 25th percentiles). This treatment effect was still evident after the additional year of follow-up at
which stage the risk reduction was 41% (Hazard Ratio = 0.59, 95% confidence interval (0.42, 0.83),
p=0.0011). Within the study period of three years, CDMS occurred in 51% of the delayed treatment
group compared to 37% of the immediate treatment group (Kaplan-Meier estimates). The persistence
of the treatment effect was observed although the majority of patients from the placebo-group was
treated with Extavia in the third year of the study.
The robustness of the treatment effect was also shown by the delay of progression to multiple sclerosis
according to the McDonald criteria. In two years, the risk in the placebo group was 85% and 69% in
the Extavia group (Hazard Ratio = 0.57, 95% confidence interval (0.46, 0.71), p<0.00001).
After 3 years, a pre-planned interim analysis showed EDSS progression (confirmed increase in EDSS
of greater than or equal to 1.0 compared to baseline) occurred in 24% of the patients in the delayed
treatment group compared to 16% in the immediate treatment group [Hazard Ratio = 0.6, 95%
confidence interval (0.39, 0.92), p=0.022]. There is no evidence for benefit in terms of confirmed
disability progression in the majority of patients receiving “immediate” treatment. Follow-up of
patients is continuing in order to provide additional data. No benefit, attributable to Extavia, in quality
of life (as measured by FAMS – Functional Assessment of MS: Treatment Outcomes Index) was seen.
Subgroup analyses according to baseline factors demonstrated evidence of efficacy in all subgroups
evaluated. Significant effects were also obtained in patients with less disseminated and less active
disease at the time of the first event, the risk in two years for progression to CDMS in patients with
monofocal onset was 47% for placebo and 24% for Extavia, without gadolinium (Gd-) enhancement
41% and 20%, with less than 9 T2 lesions 39% and 18%. Further subgroup analyses indicated a high
risk for progression to CDMS within 2 years in monofocal patients with at least 9 T2-lesions (55%
risk for placebo, 26% for Extavia) or Gd-enhancement (63% versus 33%). In multifocal patients, the
risk for CDMS was independent from MRI findings at baseline, indicating a high risk for CDMS
because of the dissemination of the disease based on clinical findings. However, the long-term impact
of early treatment with Extavia is unknown even in these high risk subgroups as this study was mainly
designed to assess the time to CDMS rather than the long term evolution of the disease. Furthermore,
for the time being there is no well established definition of a high risk patient, although a more
conservative approach is to accept at least nine T2 hyperintense lesions on the initial scan and at least
one new T2 or one new Gd-enhancing lesion on a follow-up scan taken at least 1 month after the
initial scan. In any case, treatment should only be considered for patients classified as high risk.
Therapy with Extavia was well accepted in the study of patients with a single clinical event as
indicated by a high rate of trial completion (92.8% in the Extavia group). To increase tolerability of
Extavia in the study of patients with a first clinical event, a dose titration was applied and non-
steroidal anti-inflammatory medicinal products were administered at start of therapy. Moreover, an
autoinjector was used by the majority of patients throughout the study.
RR-MS, SP-MS and single clinical event suggestive of MS
Extavia was effective in all multiple sclerosis studies to reduce disease activity (acute inflammation in
the central nervous system and permanent tissue alterations) as measured by magnetic resonance
imaging (MRI). The relation of multiple sclerosis disease activity as measured by MRI and clinical
outcome is currently not fully understood.
5.2 Pharmacokinetic properties
Extavia serum levels were followed in patients and volunteers by means of a not completely specific
bioassay. Maximum serum levels of about 40 IU/ml were found 1-8 hours after subcutaneous injection
of 500 microgram (16.0 million IU) interferon beta-1b. From various studies mean clearance rates and
half-lives of disposition phases from serum were estimated to be at most 30 ml·min
-1
·kg
-1
and 5 hours,
respectively.
Extavia injections given every other day do not lead to serum level increases, and the
pharmacokinetics does not seem to change during therapy.
The absolute bioavailability of subcutaneously administered interferon beta-1b was approximately
50%.
5.3 Preclinical safety data
No acute toxicity studies have been carried out. As rodents do not react to human interferon beta,
repeated dose studies were carried out with rhesus monkeys. Transitory hyperthermia was observed, as
well as a significant rise in lymphocytes and a significant decrease in thrombocytes and segmented
neutrophils.
No long-term studies have been conducted. Reproduction studies with rhesus monkeys revealed
maternal toxicity and an increased rate of abortion, resulting in prenatal mortality. No malformations
have been observed in the surviving animals.
No investigations on fertility have been conducted. No influence on the monkey oestrous cycle has
been observed. Experience with other interferons suggest a potential for impairment of male and
female fertility.
In one single genotoxicity study (Ames test), no mutagenic effect has been observed. Carcinogenicity
studies have not been performed. An
in vitro
cell transformation test gave no indication of tumorigenic
potential.
PHARMACEUTICAL PARTICULARS
Powder
Human albumin
Mannitol (E421)
Solvent
Sodium chloride
Water for injections
This medicinal product must not be mixed with other medicinal products except for the supplied
solvent mentioned in section 6.6.
After reconstitution immediate use is recommended. However, in-use stability has been demonstrated
for 3 hours at 2-8 °C.
6.4 Special precautions for storage
Do not store above 25°C.
Do not freeze.
For storage conditions of the reconstituted product, see section 6.3.
6.5 Nature and contents of container
Powder
3 ml vial (clear type I glass) with a butyl rubber stopper (type I) and aluminium overseal.
Solvent
1.2 ml pre-filled syringe (type I glass) with 1.2 ml solvent.
Unit pack containing 5 vials with powder and 5 pre-filled syringes with solvent
Unit pack containing 14 vials with powder and 14 pre-filled syringes with solvent
3-month multipack containing 42 (3x14) vials with powder and 42 (3x14) pre-filled syringes
with solvent
3-month multipack containing 45 (3x15) vials with powder and 45 (3x15) pre-filled syringes
with solvent
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
Reconstitution
To reconstitute lyophilised interferon beta-1b for injection, the pre-filled syringe with solvent should
be used with a needle or a vial adapter to inject the 1.2 ml of the solvent (sodium chloride 5.4 mg/ml
(0.54%) solution for injection) into the Extavia vial. The powder should dissolve completely without
shaking. After reconstitution, 1.0 ml of the solution should be drawn from the vial into the syringe for
the administration of 250 micrograms Extavia.
Inspection prior to use
The reconstituted product should be inspected visually before use. The reconstituted product is
colourless to light yellow and slightly opalescent to opalescent.
The product should be discarded before use if it contains particulate matter or is discoloured.
Disposal
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
MARKETING AUTHORISATION NUMBERS
EU/1/08/454/001
EU/1/08/454/002
EU/1/08/454/005
EU/1/08/454/006
EU/1/08/454/007
Unit pack containing 15 vials with powder and 15 pre-filled syringes with solvent
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
MANUFACTURERS OF THE BIOLOGICAL ACTIVE SUBSTANCE AND
MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR
BATCH RELEASE
CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURERS OF THE BIOLOGICAL ACTIVE SUBSTANCES AND
MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturers of the biological active substance
Boehringer Ingelheim RCV GmbH & Co KG
Dr.-Boehringer-Gasse 5-11
A-1121 Vienna
Austria
Bayer Healthcare Pharmaceuticals Inc.
5650 Hollis Street
Emeryville, CA 94608
USA
Name and address of the manufacturer responsible for batch release
Novartis Pharmaceuticals UK Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON THE
MARKETING AUTHORISATION HOLDER
Medicinal product subject to restricted medical prescription (See Annex I: Summary of Product
Characteristics, section 4.2).
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
PSURs: The PSUR cycle of Extavia is aligned with the cross-referred product, Betaferon, until
otherwise specified.
Pharmacovigilance system: The MAH must ensure that the system of pharmacovigilance, as described
in version 8.0 presented in Module 1.8.1. of the Marketing Authorisation Application, is in place and
functioning before and whilst the product is on the market.
Risk Management Plan: The Marketing Authorisation Holder commits to performing the studies and
additional pharmacovigilance activities detailed in the Pharmacovigilance Plan, as agreed in
version 1.0 of the Risk Management Plan (RMP) presented in Module 1.8.2. of the Marketing
Authorisation and any subsequent updates of the RMP agreed by the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, any
updated RMP should be submitted at the same time as the following Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted
•
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
At the request of the EMEA
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
Extavia 250 microgram/ml powder and solvent for solution for injection
Interferon beta-1b
STATEMENT OF ACTIVE SUBSTANCE(S)
1 ml contains 250 microgram (8 million IU) interferon beta-1b when reconstituted.
1 vial contains 300 microgram (9.6 million IU) interferon beta-1b.
Excipients:
Powder: Human albumin, mannitol.
Solvent: Sodium chloride, water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Powder and solvent for solution for injection.
5 vials with powder and 5 pre-filled syringes with 1.2 ml solvent.
14 vials with powder and 14 pre-filled syringes with 1.2 ml solvent.
15 vials with powder and 15 pre-filled syringes with 1.2 ml solvent.
METHOD AND ROUTE OF ADMINISTRATION
For subcutaneous use after reconstitution with 1.2 ml of solvent.
Single use.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY






















After reconstitution immediate use is recommended. In-use stability demonstrated for 3 hours at
2-8°C.
SPECIAL STORAGE CONDITIONS
Do not store above 25°C.
Do not freeze.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER
5 vials with powder and 5 pre-filled syringes with solvent
15 vials with powder and 15 pre-filled syringes with solvent
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE
14 vials with powder and 14 pre-filled syringes with solvent


























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
OUTER CARTON OF MULTIPACK (INCLUDING BLUE BOX)
NAME OF THE MEDICINAL PRODUCT
Extavia 250 microgram/ml powder and solvent for solution for injection
Interferon beta-1b
STATEMENT OF ACTIVE SUBSTANCE(S)
1 ml contains 250 microgram (8 million IU) interferon beta-1b when reconstituted.
1 vial contains 300 microgram (9.6 million IU) interferon beta-1b.
Excipients:
Powder: Human albumin, mannitol.
Solvent: Sodium chloride, water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Powder and solvent for solution for injection
42 vials with powder and 42 pre-filled syringes with 1.2 ml solvent.
3-month multipack comprising 3 cartons, each containing 14 vials with powder and 14 pre-filled
syringes with 1.2 ml solvent.
45 vials with powder and 45 pre-filled syringes with 1.2 ml solvent.
3-month multipack comprising 3 cartons, each containing 15 vials with powder and 15 pre-filled
syringes with 1.2 ml solvent.
METHOD AND ROUTE OF ADMINISTRATION
For subcutaneous use after reconstitution with 1.2 ml of solvent.
Single use.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY






















After reconstitution immediate use is recommended. In-use stability demonstrated for 3 hours at
2-8°C.
SPECIAL STORAGE CONDITIONS
Do not store above 25°C.
Do not freeze.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER
3-month multipack comprising 45 vials with powder and 45 pre-filled
syringes with solvent
3-month multipack comprising 42 vials with powder and 42 pre-filled
syringes with solvent
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE


























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
INTERMEDIATE CARTON OF MULTIPACK (WITHOUT BLUE BOX)
NAME OF THE MEDICINAL PRODUCT
Extavia 250 microgram/ml powder and solvent for solution for injection
Interferon beta-1b
STATEMENT OF ACTIVE SUBSTANCE(S)
1 ml contains 250 microgram (8 million IU) interferon beta-1b when reconstituted.
1 vial contains 300 microgram (9.6 million IU) interferon beta-1b.
Excipients:
Powder: Human albumin, mannitol.
Solvent: Sodium chloride, water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Powder and solvent for solution for injection
14 vials with powder and 14 pre-filled syringes with 1.2 ml solvent.
Component of a 3-month multipack comprising 3 cartons, each containing 14 vials with powder and
14 pre-filled syringes with 1.2 ml solvent.
15 vials with powder and 15 pre-filled syringes with 1.2 ml solvent.
Component of a 3-month multipack comprising 3 cartons, each containing 15 vials with powder and
15 pre-filled syringes with 1.2 ml solvent.
METHOD AND ROUTE OF ADMINISTRATION
For subcutaneous use after reconstitution with 1.2 ml of solvent.
Single use.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY






















After reconstitution immediate use is recommended. In-use stability demonstrated for 3 hours at
2-8°C.
SPECIAL STORAGE CONDITIONS
Do not store above 25°C.
Do not freeze.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER
3-month multipack comprising 45 vials with powder and 45 pre-filled
syringes with solvent
3-month multipack comprising 42 vials with powder and 42 pre-filled
syringes with solvent
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE


























MINIMUM PARTICULARS TO APPEAR ON BLISTERS OR STRIPS
BLISTER OF PRE-FILLED SYRINGE
NAME OF THE MEDICINAL PRODUCT
Solvent for reconstitution of Extavia
1.2 ml sodium chloride solution 5.4 mg/ml
NAME OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Read the package leaflet before use.













MINIMUM PARTICULARS TO APPEAR ON SMALL IMMEDIATE PACKAGING UNITS
LABEL OF PRE-FILLED SYRINGE
NAME OF THE MEDICINAL PRODUCT AND ROUTE OF ADMINISTRATION
Solvent for reconstitution of Extavia
1.2 ml sodium chloride solution 5.4 mg/ml
CONTENTS BY WEIGHT, BY VOLUME OR BY UNIT
0.25 / 0.5 / 0.75 / 1.0 / 1.2















PACKAGE LEAFLET: INFORMATION FOR THE USER
Extavia 250 microgram/ml powder and solvent for solution for injection
Interferon beta-1b
Read all of this leaflet carefully before you start using this medicine.
-
Keep this leaflet. You may need to read it again.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
What Extavia is and what it is used for
Annex – self-injection procedure
WHAT EXTAVIA IS AND WHAT IT IS USED FOR
What Extavia is
Extavia is a type of medicine known as interferon used to treat multiple sclerosis. Interferons are
proteins produced by the body that help it fight against attacks on the immune system such as viral
infections.
How Extavia works
Multiple sclerosis (MS)
is a long-term condition that affects the central nervous system (CNS),
particularly the functioning of the brain and spinal cord. In MS inflammation destroys the protective
sheath (called
myelin
) around the nerves of the CNS and stops the nerves from working properly. This
is called
demyelination
.
The exact cause of MS is unknown. An abnormal response by the body’s immune system is thought to
play an important part in the process which damages the CNS.
The damage to the CNS
can occur within an MS attack
(relapse)
. It can cause disability temporarily,
such as difficulty walking. Symptoms may disappear completely or partly.
Interferon beta-1b has been shown to change the response of the immune system and to help to reduce
disease activity.
How Extavia helps fight your disease
Single clinical event indicating a high risk of developing multiple sclerosis:
Extavia has been
shown to delay progression to definite multiple sclerosis.
Relapsing-remitting multiple sclerosis:
People with relapsing-remitting MS have occasional attacks
or relapses during which symptoms become noticeably worse. Extavia has been shown to cut down the
number of attacks and make them less severe. It reduces the number of hospital stays due to the
disease and prolongs the time without relapses.
If you have any further questions, ask your doctor or pharmacist.
Secondary progressive multiple sclerosis:
In some cases people with relapsing-remitting MS find
that their symptoms increase and they progress to another form of MS called secondary progressive
MS. With this, people find themselves becoming increasingly impaired, whether or not they have
relapses. Extavia can reduce the number and severity of the attacks, and slow the progression of
disability.
What Extavia is used for
Extavia is for use in patients
► who have experienced for the first time symptoms which indicate a high risk of developing
multiple sclerosis.
Your doctor will rule out any other reasons which could explain these
symptoms before you are treated.
► who suffer from relapsing-remitting multiple sclerosis, with
at least two relapses
within
the last two years
.
► who suffer from secondary progressive multiple sclerosis with active disease shown by
relapses.
if you are pregnant
. You should not start treatment with Extavia (see “Pregnancy”).
if you are allergic
(hypersensitive)
to natural or recombinant interferon beta, human albumin or
any of the other ingredients of Extavia.
if you currently suffer from severe depression and/or suicidal thoughts
(see “Take special
care” and section 4, “Possible side effects”).
if you have a severe liver disease
(see “Take special care”, “Using other medicines” and
section 4, “Possible side effects”).
►
Tell your doctor,
if any of the above applies to you.
Take special care with Extavia
Your doctor also needs to know the following before you are given Extavia:
If you have
monoclonal gammopathy.
This is
a disorder of the immune system where an
abnormal protein is found in the blood.
Problems with your small blood vessels
(capillaries)
may develop when using medicines like Extavia
(systemic capillary leak syndrome)
. This can
lead to shock
(collapse)
and even be fatal.
If you have had depression or are depressed or previously had thoughts of suicide.
Your
doctor will closely monitor you during treatment. If your depression and/or suicidal thoughts are
severe, you will not be prescribed Extavia (see also “Do not use Extavia”).
If you have ever had seizures or if you are taking medicines to treat epilepsy
(anti-
epileptics)
(see also “Using other medicines” and section 4, “Possible side effects”), you doctor
will monitor your treatment carefully.
If you have
severe kidney problems
, your doctor may monitor your kidney function during
treatment.
►
Tell your doctor
if any of these applies to you.
Your doctor also needs to know the following whilst you are using Extavia:
If you experience symptoms such as itching all over your body, swelling of your face
and/or your tongue or sudden shortness of breath.
These may be symptoms of a serious
allergic reaction
(hypersensitivity)
, which may become life threatening.
If you feel noticeably more sad or hopeless than before the treatment with Extavia, or if
you develop thoughts of suicide.
If you become depressed while you are on Extavia, you may
need special treatment and your doctor will closely monitor you and may also consider stopping
your treatment. If you suffer from severe depression and/or suicidal thoughts, you will not be
treated with Extavia (see also “Do not use Extavia”).
If you notice any unusual bruising, excessive bleeding after injury or if you seem to be
catching a lot of infections
. These may be symptoms of a fall in your blood cell count or in the
number of platelets in your blood (cells, which help the blood to clot). You may need extra
monitoring by your doctor.
If you have loss of appetite, fatigue, feeling sick
(nausea)
, repeated vomiting, especially if
you notice widespread itching, yellowing of the skin or of the whites of the eyes, or easy
bruising.
These symptoms may suggest problems with your liver. Changes to liver function
values occurred in patients treated with Extavia during clinical studies. As for other beta
interferons, severe liver damage, including cases of liver failure, have been reported rarely in
patients taking Extavia. The most serious were reported in patients taking other medicines or
who were suffering from diseases that can affect the liver (e.g. alcohol abuse, severe infection).
If you experience symptoms such as irregular heart beat, swelling such as of the ankles or
legs, or shortness of breath.
This may suggest a disease of the heart muscle
(cardiomyopathy)
which has been reported rarely in patients using Extavia.
If you notice pain in your belly which is radiating to your back , and/or you feel sick or
have a fever.
This may suggest an inflammation of the pancreas
(pancreatitis)
, which has been
reported with Extavia use. This is often associated with an increase of certain blood fats
(triglycerides)
.
►
Stop using Extavia and tell your doctor immediately
if any of these happens to you.
Other things to consider when using Extavia:
You will need blood tests
to determine your blood cell count, blood chemistry and your liver
enzymes. These will be performed
before you start using Extavia, regularly after treatment
with Extavia has been initiated and periodically whilst you are on it,
even if you have no
particular symptoms
.
These blood tests will be in addition to the tests which are normally done
to monitor your MS.
If you have a heart disease, the flu-like symptoms which often occur at the start of
treatment may prove stressful to you.
Extavia must be used with caution, and your doctor will
monitor you for worsening of your heart condition, particularly at the start of treatment. Extavia
itself does not affect the heart directly.
The functioning of your thyroid gland will be checked
regularly or whenever thought
necessary by your doctor for other reasons.
Extavia contains human albumin and therefore carries
a potential risk for transmission of
viral diseases
. A risk of transmission of Creutzfeld-Jacob disease (CJD) cannot be ruled out.
During treatment with Extavia
your body may produce substances called
neutralising
antibodies,
which may react with Extavia
(neutralising activity)
. It is not yet clear whether these
neutralising antibodies reduce the effectiveness of the treatment. Neutralising antibodies are not
produced in all patients. Currently it is not possible to predict which patients belong to this
group.
Injection site reactions:
During Extavia treatment you are likely to experience injection site reactions.
Symptoms include
redness, swelling, change in the skin colour, inflammation, pain, and hypersensitivity. Dead skin and
tissue around the injection site
(necrosis)
are reported less frequently. Injection site reactions usually
become less frequent over time.
Injection site skin and tissue breakdown can result in scars forming. If this is severe a doctor may have
to remove foreign matter and dead tissue
(debridement)
and, less often, skin grafting is required and
healing may take up to 6 months.
To reduce the risk of getting injection site reaction you must:
−
use a sterile
(aseptic)
injection technique,
rotate the injection sites with each injection (see Annex Self-Injection procedure).
Injection site reactions may occur less frequently if you use an auto-injector device. Your doctor can
tell you more about this.
If you experience any break in the skin, which may be associated with swelling or fluid leaking
out from the injection site:
►
Stop injections with Extavia
and talk to your doctor.
►
If you have only one sore injection site
(lesion)
and the tissue damage
(necrosis)
is not too
extensive
you may continue using Extavia.
►
If you have more than one sore injection sites
(multiple lesions)
you must stop using Extavia
until your skin has healed.
Your doctor will regularly check the way you inject yourself
, particularly if you have experienced
injection site reactions.
Children and adolescents
There have been no formal clinical trials undertaken in children or adolescents.
However, there is some data available in children and adolescents from 12 to 16 years. This data
suggests that the safety profile from this age is the same as in adults for use of Extavia 8 million IU
under the skin
(subcutaneously)
every other day. Extavia should not be used in children under 12 years
of age as there is no information on this use.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
No formal interaction studies have been carried out to find out whether Extavia affects other
medicines or is affected by them.
Using Extavia with other medicines that modify the immune system response is not recommended,
except anti-inflammatory medicines called
corticosteroid
s or the
adrenocorticotropic hormone
(ACTH)
.
Extavia should be used with caution with:
-
medicines which need a certain liver enzyme system
(known as
cytochrome P450 system
) for
their removal from the body, for example medicines used to treat epilepsy (such as phenytoin).
medicines which affect the production of blood cells.
Using Extavia with food and drink
Extavia is injected under the skin so any food or drink you consume is not thought to have any effect
on Extavia.
Pregnancy
Women at risk of becoming pregnant should use contraception during treatment with Extavia.
► If you are pregnant or you think you may be,
tell your doctor. Extavia therapy should not be
started if you are pregnant (see also “Do not use Extavia”).
► If you wish to become pregnant
, discuss this with your doctor first.
► If you become pregnant while using Extavia
, stop your treatment and contact your doctor
immediately. Your doctor will decide together with you whether your Extavia treatment will be
continued or not.
Ask your doctor or pharmacist for advice before taking any medicine.
Breast-feeding
It is not known whether interferon beta-1b passes into human breast milk. However, it is theoretically
possible that a breast-fed baby could experience serious side effects to Extavia.
►
Discuss it with your doctor first
to decide whether to stop breast-feeding or to stop using
Extavia.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving or using machines
Extavia may cause side effects in the central-nervous system (see section 4 Possible side effects). If
you are especially sensitive, this might influence your ability to drive or use machines.
Treatment with Extavia should be started under the supervision of a doctor who is experienced in the
treatment of multiple sclerosis.
Always use Extavia exactly as your doctor has instructed you. You should check with your doctor or
pharmacist if you are unsure.
Every other day
(once every two days), 1.0 ml of the prepared Extavia solution (see Annex) injected
under the skin
(subcutaneously)
. This equals 250 microgram (8 million IU) interferon beta-1b.
In general, treatment should be started at a low dose of 0.25 ml
(62.5 micrograms). Your doses
will then be increased gradually to the full dose of 1.0 ml (250 micrograms).
The dose should be increased at every fourth injection in four steps (0.25 ml, 0.5 ml, 0.75 ml, 1.0 ml).
Your doctor may decide together with you to change the time intervals for dose increase depending on
side effects you may experience at the start of treatment.
Preparing the injection
Before injection, the Extavia solution has to be prepared
from a vial of Extavia powder and 1.2 ml
of liquid from the pre-filled solvent syringe. This will either be done by your doctor or his/her assistant
or by yourself after you have been carefully trained.
Detailed instructions for self-injection
of Extavia under the skin
are provided in the Annex at the
back of this leaflet. These instructions also tell you how to prepare the Extavia solution for injection.
The injection site must be changed regularly
. See “Take special care with Extavia” and follow the
instructions under “Rotating injection sites” in the Annex at the back of this leaflet.
At present it is not known how long treatment with Extavia should last.
The length of treatment will
be decided by your doctor together with you.
If you use more Extavia than you should
Giving many times the dose of Extavia recommended for the treatment of multiple sclerosis has not
led to life-threatening situations.
►
Talk to your doctor
if you inject too much Extavia or injected too often.
If you forget to use Extavia
If you have forgotten to give yourself an injection at the right time do it as soon as you remember and
then follow on with the next one 48 hours later.
Do not inject a double dose to make up for a forgotten individual dose.
If you stop using Extavia
Talk to your doctor if you stop or wish to stop treatment. Stopping Extavia is not known to cause acute
withdrawal symptoms.
► If you have any further questions on the use of this product, ask your doctor or pharmacist.
Like all medicines, Extavia can cause side effects, although not everybody gets them.
At the beginning of treatment side effects are common but in general they become less with further
treatment.
The most common side effects are:
►
Flu-like symptoms
such as fever, chills, painful joints, malaise, sweating, headache, or
muscular pain. These symptoms may be reduced by taking paracetamol or non-steroidal anti-
inflammatory medicines such as ibuprofen.
►
Injection site reactions.
Symptoms can be redness, swelling, discolouration, inflammation,
pain, hypersensitivity, necrosis. See “Take special care” in section 2 for more information and
what to do if you experience an injection site reaction. These may be reduced by the use of an
auto-injector device. Please talk to your doctor for further information.
To reduce side effects at the start of treatment, your doctor should start you on a low dose of Extavia
and increase it gradually (see section 3, “How to use Extavia”).
Extavia may also cause serious side effects. If any of the side effects get serious, or if you notice any
side effects not listed in this leaflet, please tell your doctor or pharmacist.
►
Tell your doctor immediately and stop using Extavia:
-
if you experience
symptoms such as
itching all over your body, swelling of your face and/or
your tongue or sudden shortness of breath.
if you feel
noticeably more sad or hopeless than before the treatment with Extavia, or if
you develop thoughts of suicide.
if you notice
any unusual bruising, excessive bleeding after injury or if you seem to be
catching a lot of infections.
if you have
loss of appetite, fatigue, feeling sick
(nausea)
, repeated vomiting, especially if
you notice widespread itching, yellowing of the skin, or of the whites of the eyes or easy
bruising.
if you experience symptoms like
irregular heart beat, swelling such as of the ankles or legs,
or shortness of breath.
if you notice
pain in your belly which is radiating to your back , and/or you feel sick or
have a fever.
The following side effects listing is based on reports from clinical trials with Extavia (List 1)
and
from side effects reported on the marketed product (List 2).
Side effects which have occurred in clinical trials with Extavia very commonly (1 or
more in every 10 patients) and at a higher percentage than those observed with placebo.
The list also includes side effects which occur in less than 1 in 10 patients but were
significantly associated with the treatment:
reduced number of white
blood cells
, swollen
lymph glands
decrease of
sugar in the blood
headache, dizziness,
sleeplessness, migraine,
numbness or tingling feeling
(paresthesia)
conjunctivitis, abnormal vision
irregular, rapid beating or pulsation of the
heart
(palpitation)
redness and/or facial flushing due to widening of
blood vessels,
increased
blood pressure
runny nose, cough, hoarseness due to infection of the upper
respiratory tract,
sinusitis, cough
increased,
shortness of breath
diarrhoea, constipation, nausea, vomiting,
abdominal
pain
rises in the blood levels of
liver
enzymes (will show up in blood tests)
muscle
stiffness
(hypertonia)
, painful muscles
(myalgia)
, muscular debility
(myasthenia)
,
back
pain, pain in
extremities
such as fingers and toes
holding urine
(urine retention)
, protein in the
urine
(will show up in urine tests), urinary
frequency, urinary incontinence, urinary urgency
painful periods
(menstruation)
,
menstrual disorder,
heavy uterine bleeding especially between
menstrual periods,
impotence
injection site
reaction (including redness, swelling, discoloration, inflammation, pain, allergic
reaction
(hypersensitivity)
, see “Take special care with Extavia”), skin breakdown and tissue
destruction
(necrosis)
at injection site (see “Take special care with Extavia”), flu-like
symptoms, fever, pain, chest pain,
accumulation of fluid
in arm, leg or face,
lack/loss of
strength,
chills, sweating, malaise
Side effects reports on the marketed product (from spontaneous reporting):
► Very common
side effects (means 1 or more in every 10 patients are likely to get
these):
injection site reaction*,
injection site inflammation*,
injection site pain*.
(* frequencies based on clinical trials)
► Common side effects (means between 1 and 10 in every 100 patients are likely to get
these):
skin breakdown and tissue destruction
(necrosis)
at injection site*
(* frequencies based on clinical trials)
► Uncommon
side effects (means
between 1 and 10 in every 1,000 patients are likely to
get these):
the number of white cells and red cells in the blood may fall, the number of platelets
(which help the blood to clot) may fall,
increase in blood pressure,
change in the results of liver tests (increase in the blood levels of enzymes produced by
the liver),
swollen and usually itching patches of skin or mucous membranes
(urticaria)
,
► Rare side effects (means
between 1 and 10 in every 10,000 patients are likely to get
these):
serious allergic
(hypersensitivity)
reactions,
disturbance in the functioning of the thyroid gland (too much or too little hormone is
produced),
a certain type of blood fats
(triglycerides)
may increase, see “Take special care with Extavia”
(this will show up in blood tests),
severe loss of appetite leading to weight loss
(anorexia)
,
disease of the heart muscle (
cardiomyopathy)
,
irregular, rapid beating or pulsation of the heart
(palpitation)
,
sudden shortness of breath
(bronchospasm)
,
shortness of breath
(dyspnoea)
,
pancreatitis, see “Take special care with Extavia”,
blood levels of a specific liver enzyme (gamma GT) and a reddish yellow pigment (bilirubin),
which is produced by your liver, may rise (this will show up in blood tests),
Keep out of the reach and sight of children.
Do not use after the expiry date which is stated on the pack.
Do not store above 25°C. Do not freeze.
After preparing the solution you should use it immediately. However, if you are not able to do so, it
will remain usable for a period of 3 hours, if kept at 2-8°C (in a refrigerator).
Do not use Extavia if you notice it contains particles or is discoloured.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active substance is
interferon beta-1b. Each vial contains 300 microgram (9.6 million IU)
interferon beta-1b per vial. After reconstitution, each millilitre contains 250 microgram
(8 million IU) interferon beta-1b.
The other ingredients are
−
in the powder:
mannitol and human albumin.
in the solvent:
sodium chloride, water for injections.
What Extavia looks like and contents of the pack
Extavia is a powder and solvent for solution for injection.
The powder is white to off-white.
The Extavia powder is provided in a 3-millilitre vial.
The solvent for Extavia is provided in a 1.2 ml pre-filled syringe and contains 1.2 ml sodium chloride
5.4 mg/ml (0.54% (w/v)) solution for injection.
Extavia is available in pack sizes of:
−
5 vials of interferon beta-1b and 5 pre-filled syringes containing solvent.
14 vials of interferon beta-1b and 14 pre-filled syringes containing solvent.
15 vials of interferon beta-1b and 15 pre-filled syringes containing solvent.
3-month multipack containing 42 (3x14) vials of interferon beta-1b and 42 (3x14) pre-filled
syringes containing solvent.
3-month multipack containing 45 (3x15) vials of interferon beta-1b and 45 (3x15) pre-filled
syringes containing solvent.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
Manufacturer
Novartis Pharmaceuticals UK, Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
Novartis Pharma N.V.
Tél/Tel: +32 2 246 16 11
Luxembourg/Luxemburg
Novartis Pharma GmbH
Tél/Tel: +49 911 273 0
България
Novartis Pharma Services Inc.
Тел.: +359 2 489 98 28
Magyarország
Novartis Hungária Kft. Pharma
Tel.: +36 1 457 65 00
Česká republika
Novartis s.r.o.
Tel: +420 225 775 111
Malta
Novartis Pharma Services Inc.
Tel: +356 2298 3217
Danmark
Novartis Healthcare A/S
Tlf: +45 39 16 84 00
Nederland
Novartis Pharma B.V.
Tel: +31 26 37 82 111
Deutschland
Novartis Pharma GmbH
Tel: +49 911 273 0
Norge
Novartis Norge AS
Tlf: +47 23 05 20 00
Eesti
Novartis Pharma Services Inc.
Tel: +372 66 30 810
Österreich
Novartis Pharma GmbH
Tel: +43 1 86 6570
Ελλάδα
Novartis (Hellas) A.E.B.E.
Τηλ: +30 210 281 17 12
Polska
Novartis Poland Sp. z o.o.
Tel.: +48 22 550 8888
España
Novartis Farmacéutica, S.A.
Tel: +34 93 306 42 00
Portugal
Novartis Farma - Produtos Farmacêuticos, S.A.
Tel: +351 21 000 8600
France
Novartis Pharma S.A.S.
Tél: +33 1 55 47 66 00
România
Novartis Pharma Services Inc.
Tel: +40 21 31299 01
Ireland
Novartis Ireland Limited
Tel: +353 1 260 12 55
Slovenija
Novartis Pharma Services Inc.
Tel: +386 1 300 75 50
Ísland
Vistor hf.
Sími: +354 535 7000
Slovenská republika
Novartis Slovakia s.r.o.
Tel: +421 2 5542 5439
Italia
Novartis Farma S.p.A.
Tel: +39 02 96 54 1
Suomi/Finland
Novartis Finland Oy
Puh/Tel: +358 (0)10 6133 200
Κύπρος
Novartis Pharma Services Inc.
Τηλ: +357 22 690 690
Sverige
Novartis Sverige AB
Tel: +46 8 732 32 00
Latvija
Novartis Pharma Services Inc.
Tel: +371 67 887 070
United Kingdom
Novartis Pharmaceuticals UK Ltd.
Tel: +44 1276 698370
Lietuva
Novartis Pharma Services Inc.
Tel: +370 5 269 16 50
This leaflet was last approved in
Annex: SELF-INJECTION PROCEDURE
The following instructions are intended to explain how to prepare Extavia for administration and how
to inject Extavia yourself. Please read the instructions carefully and follow them step by step. Your
doctor or his/her assistant will help you to learn the process of self-administration. Do not attempt to
inject yourself until you are sure that you understand how to prepare the injection solution and give the
injection to yourself.
PART I: STEP BY STEP INSTRUCTIONS
The instructions include the following main steps:
A) General advice
B) Getting ready to inject
C) Reconstituting the solution, step by step
D) Drawing up the injection
E) Making the injection manually (to make an injection with an auto-injector, refer to the
instructions for use provided with the auto-injector)
F) Quick review of the process
Getting off to a good start!
You will find that within a few weeks your treatment will become a natural part of your routine. As
you get started, you may find the following tips helpful:
Set up a permanent storage area in a convenient location out of the reach of children so that
your Extavia and other supplies are always easy to find.
For details on storage conditions see section 5 of the leaflet, “How to store Extavia”.
Try to give yourself the injection at the same time each day. This makes it easier to remember
and easier to plan a block of time when you will not be interrupted.
Please refer to section 3 of the leaflet, “How to use Extavia”, for further details on how to use
Extavia.
Prepare each dose only when you are ready for an injection. After mixing Extavia, you should
administer the injection immediately (if Extavia is not used immediately, see section 5 of the
leaflet, “How to store Extavia”).
Important tips to keep in mind
Be consistent - use Extavia as described in section 3 of the leaflet, “How to use Extavia”.
Always double-check your dosage.
Keep your syringes and syringe disposal unit out of the reach of children; lock the supplies if
possible.
Never re-use syringes or needles.
Always use a sterile
(aseptic)
technique as described in here.
Always place the used syringes in the proper disposal unit.
B) Getting ready to inject
Choosing an injection site
Before preparing your injection, decide where you are going to inject. You should inject Extavia into
the fatty layer between the skin and muscle (that is, subcutaneously, about 8 to 12 mm under the skin).
The best places for injections are where the skin is loose and soft, and away from joints, nerves, bones,
for example the abdomen, arm, thigh or buttocks.
Important:
Do not use any area where you can feel lumps, bumps, firm knots, pain or an area that is
discoloured, indented, scabbed, or where the skin is broken. Talk to your doctor or healthcare
professional about these or any other unusual conditions you may find.
You should rotate the injection site at every injection. If some areas are too difficult for you to reach,
you may need a family member or friend to help you with these injections. Follow the sequence
described in the schedule at the end of the Annex (see II) Rotating injection sites) and you will come
back to your first injection site area after 8 injections (16 days). This will give each injection site a
chance to fully recover before receiving another injection.
Please refer to the rotation schedule at the end of this Annex to learn how to choose an injection site.
An example of a medication record is also included. This should give you an idea of how you can keep
track of your injection sites and dates.
You will need the medicine:
•
1 Extavia vial (with powder for solution for injection)
1 pre-filled syringe of solvent for Extavia (sodium chloride solution (0.54% (w/v))
To reconstitute and inject your medicine you will need the following components:
•
a needle suitable for reconstitution
•
30-gauge needle for injection
•
alcohol wipes
You will also need a disposal unit for used syringes and needles.
Alternatively, you can use an application kit for the administration of Extavia (supplied separately
from your medicine), which contains vial adapters for reconstituting your medicine, 30-gauge needles
for injecting your medicine and alcohol wipes, as well as instructions on how to use these components.
The 30-gauge needles provided with the application kit for the administration of Extavia can be used
either for manual injection
OR
with an ExtaviJect 30G auto-injector.
For skin disinfection use an appropriate disinfectant.
C) Reconstituting the solution, step by step
1 - Wash your hands thoroughly with soap and water before beginning this process.
2 - Open the Extavia vial and put it on the table. It is best to use your thumb rather than your nail as it
could break.
3 - Clean the top of the vial with an alcohol wipe, moving the wipe in one direction only. Leave the
wipe on top of the vial.
4 – Take out the pre-filled solvent syringe from its package. Remove the tip cap from the pre-filled
syringe by twisting it off. Be careful not to touch the exposed end of the syringe. Do not push the
plunger.
5 - Take the needle suitable for reconstitution out of its wrapping and place it firmly onto the tip
(nozzle) of the syringe. Remove the needle guard from the needle. Do not touch the needle.
6 - Holding the Extavia vial on a stable surface, slowly insert the needle of the syringe (containing
1.2 ml of liquid) all the way through the stopper of the vial.
7 - Push the plunger down slowly, directing the needle toward the side of the vial to allow the liquid
to run down the inside of the vial.
Injecting solvent directly onto the powder will cause excess foaming
.
Transfer all the solvent to the vial. Release the plunger.
8 - After the solvent in the syringe has been completely injected into the Extavia vial, hold the vial
between your thumb, forefinger and middle finger with the needle and syringe resting against your
hand and swirl the vial gently to completely dissolve the powder of Extavia.
Do not shake the vial.
9 - Examine the solution carefully. It should be clear and contain no particles. If the solution is
discoloured or contains particles, discard it and start again with a syringe and vial out of your
package.
If foam is present –- which can happen if the vial is shaken or swirled too vigorously – let the vial sit
undisturbed until the foam settles.
D) Drawing up the solution for injection
10 - In order to redraw the solution back into the syringe, turn the syringe-vial-assembly, needle
pointing upwards. Redraw the needle a little bit back within the vial so that the needle tip rests at the
lowest point of the vial.
Keep the needle tip in the liquid,
and slowly pull the plunger back all the way to withdraw the
solution into the syringe.
11 - Any excessive air rises to the top of the solution. Remove the air bubbles by gently tapping the
syringe and pushing the plunger to the 1 ml mark, or to the volume prescribed by your doctor.
If too much solution goes into the vial along with the air bubbles, pull the plunger back a little to
withdraw the solution back from the vial into the syringe. Do this until all the air is gone and there is
1 ml of reconstituted solution in the syringe.
12 - Disconnect the syringe and the needle. Leave the needle in the vial.
13 - Take the
30-gauge
needle out of its wrapping and place it firmly onto the tip (nozzle) of the
syringe.
14 - Dispose of the vial and the remaining unused portion of the solution into the disposal unit.
15 - You are now ready to inject.
If, for some reason, you are not able to inject Extavia immediately, you can keep the reconstituted
solution in the syringe in a refrigerator for up to 3 hours before using. Make sure the needle cap is
fixed properly while the syringe is stored in the fridge. Do not freeze the solution, and do not wait
longer than 3 hours to inject it. If more than 3 hours pass, discard the medicine and prepare a new
injection. When you use the solution, warm it up in your hands before injecting to avoid pain.
Making the injection manually (to make an injection with an auto-injector, refer to the
instructions for use provided with the auto-injector)
1 - Choose an area for the injection (see advice at the start and the
diagrams at the end of this Annex), and make a note of it in your
medication record.
2 - Use an alcohol swab to clean the skin at the injection site. Let the
skin air-dry. Throw the swab away.
For skin disinfection use an appropriate disinfectant.
3 - Remove the cap from the needle by pulling and not twisting it.
4 - Gently pinch the skin together around the disinfected injection site
(to raise it up a little).
5 - Holding the syringe like a pencil or a dart, push the needle straight
into the skin at a 90˚ angle with a quick, firm motion.
6 - Inject the medicine using a slow, steady push on the plunger. (Push
the plunger all the way in until the syringe is empty.)
7 - Discard the syringe in the disposal unit.
F) Quick review of the process
1.
Take out the contents needed (1 vial, 1 pre-filled syringe, 2 alcohol wipes, needle suitable for
reconstitution, 30-gauge needle for injection).
2.
Take off the cap from the vial and clean the rubber stopper with an alcohol wipe.
3.
Unwrap the needle suitable for reconstitution and fix it to the syringe from which you removed
the tip cap before.
4.
Transfer the solvent from the syringe to the vial by pushing the plunger in all the way.
5.
Turn the syringe-vial-assembly over, then withdraw plunger to redraw the solution.
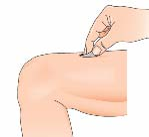
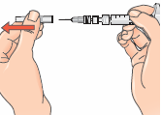
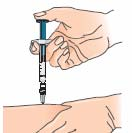
6.
Disconnect syringe and needle, leave the needle in the vial and discard both.
7.
Unwrap the 30-gauge needle and connect it to the syringe.
8.
Remove needle cap just before injection - you are now ready to inject.
NOTE: The injection should be administered immediately after mixing (if the injection is delayed,
refrigerate the solution and inject it within 3 hours). Do not freeze.
PART II: ROTATING INJECTION SITES
You need to choose a new site for each injection to allow the area time to recover and help prevent
infection. Advice on which areas to choose is given in the first part of this Annex. It is a good idea to
know where you plan to inject before you prepare your syringe. The schedule shown in the diagram
below will help you to vary the sites appropriately. For example, give the first injection into the right
side of the abdomen, choose the left side for the second injection, then move to the right thigh for the
third, and so on through the diagram until all suitable areas of the body have been used. Keep a record
of where and when you last gave yourself an injection. One way to do that is to note the injection site
on the enclosed medication record card.
By following this schedule, you will come back to your first area (e.g. the right side of the abdomen)
after 8 injections (16 days). This is called a Rotation Cycle. On our example schedule each area is split
again into 6 injection sites (which adds up to 48 injection sites altogether), left, right, upper, middle
and lower part of each area. If you come back to an area after one Rotation Cycle choose the most
distant injection site within this area. If an area becomes sore, talk to your doctor or nurse about
choosing other injection sites.
Rotation Schedule
To help you rotate the injection sites appropriately we recommend that you keep a record of the date
and location of your injection. You can use the following rotation schedule.
Work through each rotation cycle in turn. Each cycle will be 8 injections (16 days), given in area 1
through to area 8 in turn. By following this sequence, you will give each area a chance to recover
before receiving another injection.
Lower right section of each area
Middle left section of each area
Upper right section of each area
Lower left section of each area
Middle right section of each area
Upper left section of each area
PART III: EXTAVIA
Medication record
Instructions for keeping track of your injection sites and dates
Start with your first injection (or your last injection if you are not a new Extavia user).
Select an injection site. If you have already been using Extavia start with the area that has not
been used during the last rotation cycle, i.e. the past 16 days).
After your injection, fill in the used injection site and date in the table in your injection record
(See the example: Keeping track of your injection sites and dates).
EXAMPLE OF A MEDICATION RECORD:
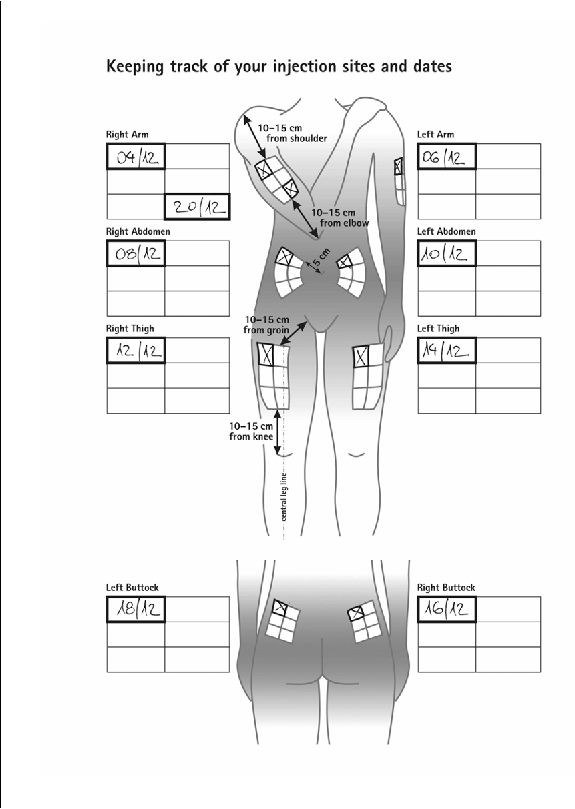



Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/extavia.html
Copyright © 1995-2021 ITA all rights reserved.
|

















































































































































































































































































































































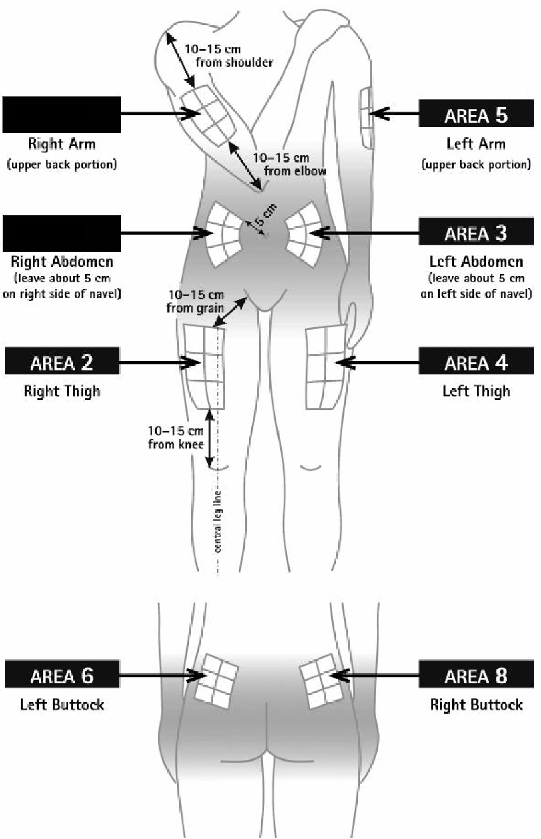














 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































