Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Faslodex 250 mg solution for injection
QUALITATIVE AND QUANTITATIVE COMPOSITION
One pre-filled syringe contains 250 mg fulvestrant in 5 ml solution.
For a full list of excipients, see section 6.1.
Clear, colourless to yellow, viscous solution.
4.1 Therapeutic indications
Faslodex is indicated for the treatment of postmenopausal women with estrogen receptor positive,
locally advanced or metastatic breast cancer for disease relapse on or after adjuvant anti-estrogen
therapy, or disease progression on therapy with an anti-estrogen.
4.2 Posology and method of administration
Posology
Adult females (including the elderly)
The recommended dose is 500 mg at intervals of one month, with an additional 500 mg dose given
two weeks after the initial dose.
Special population
Renal impairment
No dose adjustments are recommended for patients with mild to moderate renal impairment
(creatinine clearance ≥30 ml/min). Safety and efficacy have not been evaluated in patients with severe
renal impairment (creatinine clearance <30 ml/min), and, therefore, caution is recommended in these
patients (see section 4.4).
Hepatic impairment
No dose adjustments are recommended for patients with mild to moderate hepatic impairment.
However, as fulvestrant exposure may be increased, Faslodex should be used with caution in these
patients. There are no data in patients with severe hepatic impairment (see sections 4.3, 4.4 and 5.2).
Paediatric population
The safety and efficacy of Faslodex in children from birth to 18 years of age have not been
established. No data are available.
Method of administration
Faslodex should be administered as two consecutive 5 ml injections by slow intramuscular injection
(1-2 minutes/injection), one in each buttock..
For detailed instructions for administration, see section 6.6.
Hypersensitivity to the active substance or to any of the excipients.
Pregnancy and lactation (see section 4.6).
Severe hepatic impairment (see sections 4.4 and 5.2).
4.4 Special warnings and precautions for use
Faslodex should be used with caution in patients with mild to moderate hepatic impairment (see
sections 4.2, 4.3 and 5.2).
Faslodex should be used with caution in patients with severe renal impairment (creatinine clearance
less than 30 ml/min).
Due to the intramuscular route of administration, Faslodex should be used with caution if treating
patients with bleeding diatheses, thrombocytopenia or those taking anticoagulant treatment.
Thromboembolic events are commonly observed in women with advanced breast cancer and have
been observed in clinical trials with Faslodex (see section 4.8). This should be taken into consideration
when prescribing Faslodex to patients at risk.
There are no long-term data on the effect of fulvestrant on bone. Due to the mechanism of action of
fulvestrant, there is a potential risk of osteoporosis.
4.5 Interaction with other medicinal products and other forms of interaction
A clinical interaction study with midazolam (substrate of CYP3A4) demonstrated that fulvestrant does
not inhibit CYP3A4. Clinical interaction studies with rifampicin (inducer of CYP3A4) and
ketoconazole (inhibitor of CYP3A4) showed no clinically relevant change in fulvestrant clearance.
Dose
adjustment is therefore not necessary in patients who are receiving fulvestrant and CYP3A4
inhibitors or inducers concomitantly.
4.6 Fertility, pregnancy and lactation
Women of childbearing potential
Patients of child-bearing potential should be advised to use effective contraception while on treatment.
Pregnancy
Faslodex is contraindicated in pregnancy (see section 4.3). Fulvestrant has been shown to cross the
placenta after single intramuscular doses in rat and rabbit. Studies in animals have shown reproductive
toxicity including an increased incidence of foetal abnormalities and deaths (see section 5.3). If
pregnancy occurs while taking Faslodex, the patient must be informed of the potential hazard to the
foetus and potential risk for loss of pregnancy.
Breastfeeding
Breastfeeding must be discontinued during treatment with Faslodex. Fulvestrant is excreted in milk in
lactating rats. It is not known whether fulvestrant is excreted in human milk. Considering the potential
for serious adverse reactions due to fulvestrant in breast-fed infants, use during lactation is
contraindicated (see section 4.3).
Fertility
The effects of Faslodex on fertility in humans has not been studied.
4.7 Effects on ability to drive and use machines
Faslodex has no or negligible influence on the ability to drive or use machines. However, since
asthenia has been reported very commonly with Faslodex, caution should be observed by those
patients who experience this adverse reaction when driving or operating machinery.
This section provides information based on all adverse reactions from clinical trials, post-marketing
studies or spontaneous reports. The most frequently reported adverse reactions are injection site
reactions, asthenia, nausea, and increased hepatic enzymes (ALT, AST, ALP).
The following frequency categories for adverse drug reactions (ADRs) were calculated based on the
Faslodex 500 mg treatment group in pooled safety analyses of the CONFIRM (Study D6997C00002),
FINDER 1 (Study D6997C00004), FINDER 2 (Study D6997C00006), and NEWEST (Study
D6997C00003) studies that compared Faslodex 500 mg with Faslodex 250 mg. The frequencies in the
following table were based on all reported adverse drug reactions, regardless of the investigator
assessment of causality.
Adverse reactions listed below are classified according to frequency and System Organ Class (SOC).
Frequency groupings are defined according to the following convention: Very common (≥1/10),
Common (≥1/100 to <1/10), Uncommon (≥1/1,000 to <1/100). Within each frequency grouping
adverse reactions are reported in order of decreasing seriousness.
Adverse reactions by system organ class and frequency
Infections and infestations
Hypersensitivity reactions
Metabolism and nutrition disorders
Venous thromboembolism
a
, hot
flushes
Gastrointestinal disorders
Increased hepatic enzymes (ALT,
AST, ALP)
a
Skin and subcutaneous tissue disorders
Musculoskeletal and connective tissue
disorders
Reproductive system and breast disorders Uncommon
Vaginal moniliasis, leukorrhea,
vaginal haemorrhage
General disorders and administration site
conditions
Asthenia
a
, injection site reactions
b
Injection site haemorrhage, injection
site haematoma
Includes adverse drug reactions for which the exact contribution of Faslodex cannot be assessed due to the
underlying disease.
The term injection site reactions does not include the terms injection site haemorrhage and injection site
haematoma.


















There is no human experience of overdose. Animal studies suggest that no effects other than those
related directly or indirectly to anti-estrogenic activity were evident with higher doses of fulvestrant
(see section 5.3). If overdose occurs, symptomatic supportive treatment is recommended.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Endocrine therapy, Anti-estrogens, ATC code: L02BA03
Mechanism of action and pharmacodynamic effects
Fulvestrant is a competitive estrogen receptor (ER) antagonist with an affinity comparable to estradiol.
Fulvestrant blocks the trophic actions of estrogens without any partial agonist (estrogen-like) activity.
The mechanism of action is associated with down-regulation of estrogen receptor protein levels.
Clinical trials in postmenopausal women with primary breast cancer have shown that fulvestrant
significantly down-regulates ER protein in ER positive tumours compared with placebo. There was
also a significant decrease in progesterone receptor expression consistent with a lack of intrinsic
estrogen agonist effects. It has also been shown that fulvestrant 500 mg downregulates ER and the
proliferation marker Ki67, to a greater degree than fulvestrant 250 mg in breast tumours in
postmenopausal neoadjuvant setting.
Clinical safety and efficacy in advanced breast cancer
A phase III clinical trial was completed in 736 postmenopausal women with advanced breast cancer
who had disease recurrence on or after adjuvant endocrine therapy or progression following endocrine
therapy for advanced disease. The study included 423 patients whose disease had recurred or
progressed during anti-estrogen therapy (AE subgroup) and 313 patients whose disease had recurred
or progressed during aromatase inhibitor therapy (AI subgroup). This trial compared the efficacy and
safety of Faslodex 500 mg (n=362) with Faslodex 250 mg (n=374). Progression-free survival (PFS)
was the primary endpoint; key secondary efficacy endpoints included objective response rate (ORR),
clinical benefit rate (CBR) and overall survival (OS). Efficacy results for the CONFIRM study are
summarized in Table 2.
Summary of results of the primary efficacy endpoint (PFS) and key
secondary efficacy endpoints in the CONFIRM study
Type of
estimate;
treatment
comparison
Comparison between groups
(Faslodex 500 mg/Faslodex 250 mg)
K-M median
in months;
hazard ratio
K-M median in
months;
hazard ratio
Type of
estimate;
treatment
comparison
Comparison between groups
(Faslodex 500 mg/Faslodex 250 mg)
% of patients
with OR;
absolute
difference in %
% of patients
with CB;
absolute
difference in %
a
Faslodex is indicated in patients whose disease had recurred or progressed on an anti-estrogen therapy. The results in
the AI subgroup are inconclusive.
b
ORR was assessed in patients who were evaluable for response at baseline (ie, those with measurable disease at
baseline: 240 patients in the Faslodex 500 mg group and 261 patients in the Faslodex 250 mg group).
c
Patients with a best objective response of complete response, partial response or stable disease ≥24 weeks.
PFS:Progression-free survival; ORR:Objective response rate; OR:Objective response; CBR:Clinical benefit rate; CB:Clinical
benefit; OS:Overall survival; K-M:Kaplan-Meier; CI:Confidence interval; AI:Aromatase inhibitor; AE:Anti-estrogen.
Two phase III clinical trials were completed in a total of 851 postmenopausal women with advanced
breast cancer who had disease recurrence on or after adjuvant endocrine therapy or progression
following endocrine therapy for advanced disease. 77% of the study population had estrogen receptor
positive breast cancer. These trials compared the safety and efficacy of monthly administration of
Faslodex 250 mg versus the daily administration of 1 mg anastrozole (aromatase inhibitor). Overall,
Faslodex at the 250 mg monthly dose was at least as effective as anastrozole in terms of progression-
free survival, objective response, and time to death. There were no statistically significant differences
in any of these endpoints between the two treatment groups. Progression-free survival was the primary








endpoint. Combined analysis of both trials showed that 83% of patients who received Faslodex
progressed, compared with 85% of patients who received anastrozole. Combined analysis of both
trials showed the hazard ratio of Faslodex 250 mg to anastrozole for progression-free survival was
0.95 (95% CI 0.82 to 1.10). The objective response rate for Faslodex 250 mg was 19.2% compared
with 16.5% for anastrozole. The median time to death was 27.4 months for patients treated with
Faslodex and 27.6 months for patients treated with anastrozole. The hazard ratio of Faslodex 250 mg
to anastrozole for time to death was 1.01 (95% CI 0.86 to 1.19).
Effects on the postmenopausal endometrium
Preclinical data do not suggest a stimulatory effect of fulvestrant on the postmenopausal endometrium
(see section 5.3). A 2-week study in healthy postmenopausal volunteers treated with 20 μg per day
ethinylestradiol showed that pre-treatment with Faslodex 250 mg resulted in significantly reduced
stimulation of the postmenopausal endometrium, compared to pre-treatment with placebo, as judged
by ultrasound measurement of endometrium thickness.
Neoadjuvant treatment for up to 16 weeks in breast cancer patients treated with either Faslodex
500 mg or Faslodex 250 mg did not result in clinically significant changes in endometrial thickness,
indicating a lack of agonist effect. There is no evidence of adverse endometrial effects in the breast
cancer patients studied. No data are available regarding endometrial morphology.
In two short-term studies (1 and 12 weeks) in premenopausal patients with benign gynaecologic
disease, no significant differences in endometrial thickness were observed by ultrasound measurement
between fulvestrant and placebo groups.
Effects on bone
There are no long-term data on the effect of fulvestrant on bone. Neoadjuvant treatment for up to
16 weeks in breast cancer patients with either Faslodex 500 mg or Faslodex 250 mg did not result in
clinically significant changes in serum bone-turnover markers.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with
Faslodex in all subsets of the paediatric population in breast cancer (see section 4.2 for information on
paediatric use).
5.2 Pharmacokinetic properties
Absorption
After administration of Faslodex long-acting intramuscular injection, fulvestrant is slowly absorbed
and maximum plasma concentrations (C
max
) are reached after about 5 days. Administration of
Faslodex 500 mg regimen achieves exposure levels at, or close to, steady state within the first month
of dosing (mean [CV]: AUC 475 [33.4%] ng.days/ml, C
max
25.1 [35.1%] ng/ml, C
min
16.3 [25.9%] ng/ml, respectively). At steady state, fulvestrant plasma concentrations are maintained
within a relatively narrow range with up to an approximately 3-fold difference between maximum and
trough concentrations. After intramuscular administration, the exposure is approximately
dose-proportional in the dose range 50 to 500 mg.
Distribution
Fulvestrant is subject to extensive and rapid distribution. The large apparent volume of distribution at
steady state (Vd
ss
) of approximately 3 to 5 l/kg suggests that distribution is largely extravascular.
Fulvestrant is highly (99%) bound to plasma proteins. Very low density lipoprotein (VLDL), low
density lipoprotein (LDL), and high density lipoprotein (HDL) fractions are the major binding
components. No interaction studies were conducted on competitive protein binding. The role of sex
hormone-binding globulin (SHBG) has not been determined.
The metabolism of fulvestrant has not been fully evaluated, but involves combinations of a number of
possible biotransformation pathways analogous to those of endogenous steroids. Identified metabolites
(includes 17-ketone, sulphone, 3-sulphate, 3- and 17-glucuronide metabolites) are either less active or
exhibit similar activity to fulvestrant in anti-estrogen models. Studies using human liver preparations
and recombinant human enzymes indicate that CYP3A4 is the only P450 isoenzyme involved in the
oxidation of fulvestrant; however, non-P450 routes appear to be more predominant
in vivo
.
In vitro
data suggest that fulvestrant does not inhibit CYP450 isoenzymes.
Elimination
Fulvestrant is eliminated mainly in metabolised form. The major route of excretion is via the faeces,
with less than 1% being excreted in the urine. Fulvestrant has a high clearance, 11±1.7 ml/min/kg,
suggesting a high hepatic extraction ratio. The terminal half-life (t
1/2
) after intramuscular
administration is governed by the absorption rate and was estimated to be 50 days.
Special populations
In a population pharmacokinetic analysis of data from phase III studies, no difference in fulvestrant’s
pharmacokinetic profile was detected with regard to age (range 33 to 89 years), weight (40-127 kg) or
race.
Renal impairment
Mild to moderate impairment of renal function did not influence the pharmacokinetics of fulvestrant to
any clinically relevant extent.
Hepatic impairment
The pharmacokinetics of fulvestrant has been evaluated in a single-dose clinical trial conducted in
subjects with mild to moderate hepatic impairment (Child-Pugh class A and B). A high dose of a
shorter duration intramuscular injection formulation was used. There was up to about 2.5-fold increase
in AUC in subjects with hepatic impairment compared to healthy subjects. In patients administered
Faslodex, an increase in exposure of this magnitude is expected to be well tolerated.
Subjects with
severe hepatic impairment (Child-Pugh class C) were not evaluated.
5.3 Preclinical safety data
The acute toxicity of fulvestrant is low.
Faslodex and other formulations of fulvestrant were well tolerated in animal species used in multiple
dose studies. Local reactions, including myositis and granulomata at the injection site were attributed
to the vehicle but the severity of myositis in rabbits increased with fulvestrant, compared to the saline
control. In toxicity studies with multiple intramuscular doses of fulvestrant in rats and dogs, the anti-
estrogenic activity of fulvestrant was responsible for most of the effects seen, particularly in the
female reproductive system, but also in other organs sensitive to hormones in both sexes. Arteritis
involving a range of different tissues was seen in some dogs after chronic (12 months) dosing.
In dog studies following oral and intravenous administration, effects on the cardiovascular system
(slight elevations of the S-T segment of the ECG [oral], and sinus arrest in one dog [intravenous])
were seen. These occurred at exposure levels higher than in patients (C
max
>15 times) and are likely to
be of limited significance for human safety at the clinical dose.
Fulvestrant showed no genotoxic potential.
Fulvestrant showed effects upon reproduction and embryo/foetal development consistent with its
anti-estrogenic activity, at doses similar to the clinical dose. In rats, a reversible reduction in female
fertility and embryonic survival, dystocia and an increased incidence of foetal abnormalities including
tarsal flexure were observed. Rabbits given fulvestrant failed to maintain pregnancy. Increases in
placental weight and post-implantation loss of foetuses were seen. There was an increased incidence
of foetal variations in rabbits (backwards displacement of the pelvic girdle and 27 pre-sacral
vertebrae).
A two-year oncogenicity study in rats (intramuscular administration of Faslodex) showed increased
incidence of ovarian benign granulosa cell tumours in female rats at the high dose, 10 mg/rat/15 days
and an increased incidence of testicular Leydig cell tumours in males. Induction of such tumours is
consistent with pharmacology-related endocrine feedback alterations. These findings are not of clinical
relevance for the use of fulvestrant in postmenopausal women with advanced breast cancer.
PHARMACEUTICAL PARTICULARS
Ethanol (96 per cent)
Benzyl alcohol
Benzyl benzoate
Castor oil
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
6.4 Special precautions for storage
Store in a refrigerator (2°C - 8°C).
Store the pre-filled syringe in the original package in order to protect from light.
6.5 Nature and contents of container
BD SafetyGlide is a trademark of Becton Dickinson and Company and is CE-marked: CE 0050.
The pre-filled syringe presentation consists of:
One clear type 1 glass pre-filled syringe with polystyrene plunger rod, fitted with a tamper-evident
closure, containing 5 ml Faslodex solution for injection.
A safety needle (BD SafetyGlide™) for connection to the barrel is also provided.
Or
Two clear type 1 glass pre-filled syringes with polystyrene plunger rod, fitted with a tamper-evident
closure, each containing 5 ml Faslodex solution for injection. Safety needles (BD SafetyGlide™) for
connection to each barrel are also provided.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
Instructions for administration
Warning - Do not autoclave safety needle (BD SafetyGlide Shielding Hypodermic Needle) before use.
Hands must remain behind the needle at all times during use and disposal.
For each of the two syringes:
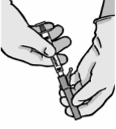
• Remove glass syringe barrel from tray and check that it is
not damaged.
• Break the seal of the white plastic cover on the syringe
Luer connector Luer-Lok to remove the cover with the
attached rubber tip cap (see Figure 1).
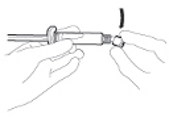
• Peel open the safety needle (BD SafetyGlide) outer
packaging. Attach the safety needle to the Luer-Lok (see
Figure 2)
• Twist until firmly seated.
• Twist to lock the needle to the Luer connector.
• Pull shield straight off needle to avoid damaging needle
point.
• Transport filled syringe to point of administration.
• Remove needle sheath.
• Parenteral solutions must be inspected visually for
particulate matter and discolouration prior to
administration.
• Expel excess gas from the syringe.
• Administer intramuscularly slowly (1-2 minutes/injection)
into the buttock. For user convenience, the needle bevel- up
position is oriented to the lever arm (see Figure 3).
• After injection, immediately apply a single-finger stroke to
the activation assisted lever arm to activate the shielding
mechanism (see Figure 4).
NOTE: Activate away from self and others. Listen for click
and visually confirm needle tip is fully covered.
Disposal
Pre-filled syringes are for single use
only
.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
AstraZeneca UK Limited
Alderley Park
Macclesfield
Cheshire
SK10 4TG
United Kingdom



MARKETING AUTHORISATION NUMBER(S)
EU/1/03/269/001
EU/1/03/269/002
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 10 March 2004
Date of last renewal: 10 March 2009
10. DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency
http://www.ema.europa.eu
MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE
FOR BATCH RELEASE
CONDITIONS OF THE MARKETING AUTHORISATION
A MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer responsible for batch release
AstraZeneca UK Limited
Silk Road Business Park,
Macclesfield, SK10 2NA
United Kingdom
CONDITIONS OF THE MARKETING AUTHORISATION
•
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to medical prescription.
Pharmacovigilance system
The MAH must ensure that the system of pharmacovigilance, as described in version 10.0 presented in
Module 1.8.1. of the Marketing Authorisation Application, is in place and functioning before and
whilst the product is on the market.
Risk Management Plan
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version 4.0 of the Risk Management Plan (RMP) presented
in Module 1.8.2. of the Marketing Authorisation Application and any subsequent updates of the RMP
agreed by the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, the
updated RMP should be submitted at the same time as the next Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted
• When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
• Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
• At the request of the European Medicines Agency.
ANNEX III
LABELLING AND PACKAGE LEAFLET

PARTICULARS TO APPEAR ON THE OUTER PACKAGING AND THE IMMEDIATE
PACKAGING
NAME OF THE MEDICINAL PRODUCT
Faslodex 250 mg solution for injection.
fulvestrant
STATEMENT OF ACTIVE SUBSTANCE(S)
One pre-filled syringe contains 250 mg fulvestrant in 5 ml solution
Ethanol (96 per cent), benzyl alcohol, benzyl benzoate and castor oil. See the package leaflet for
further information.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection in a pre-filled syringe.
1 pre-filled syringe (5 ml)
1 safety needle
2 pre-filled syringes (5 ml each)
2 safety needles
METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use.
Intramuscular use.
For single use only.
For full instructions on the administration of Faslodex and the use of the safety needle see enclosed,
Instructions for administration.
Two syringes must be administered to receive the 500 mg recommended monthly dose.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY

























SPECIAL STORAGE CONDITIONS
Store in a refrigerator.
Store the pre-filled syringe in the original package in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
AstraZeneca UK Limited
Alderley Park
Macclesfield
Cheshire
SK10 4TG
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/03/269/001
EU/1/03/269/002
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE
Justification for not including Braille accepted





















PACKAGE LEAFLET: INFORMATION FOR THE USER
Faslodex 250 mg solution for injection
Fulvestrant
Read all of this leaflet carefully before you start using this medicine.
-
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor, nurse or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor, nurse or pharmacist.
What Faslodex is and what it is used for
WHAT FASLODEX IS AND WHAT IT IS USED FOR
Faslodex contains the active substance fulvestrant, which belongs to the group of estrogen blockers.
Estrogens, a type of female sex hormones, can in some cases be involved in the growth of breast
cancer.
Faslodex is used to treat advanced or metastatic breast cancer in postmenopausal women.
if you are allergic (hypersensitive) to fulvestrant or to any of the other ingredients of Faslodex
(listed in section 6 ‘What Faslodex contains’)
if you are pregnant or breast-feeding
if you have severe liver problems
Take special care with Faslodex
Tell your doctor if any of these apply to you:
-
low numbers of platelets (which help blood clotting) or bleeding disorders
osteoporosis (loss of bone density)
Children
Faslodex is not indicated in children and adolescents under 18 years.
Using other medicines
Please tell your doctor, nurse or pharmacist if you are taking or have recently taken any other
medicines, including medicines obtained without a prescription.
In particular, you should tell your doctor if you are using anticoagulants (medicines to prevent blood
clots).
previous problems with blood clots
Pregnancy and breast-feeding
You must not use Faslodex if you are pregnant. If you can become pregnant, you should use effective
contraception while being treated with Faslodex.
You must not breast-feed while on treatment with Faslodex.
Driving and using machines
Faslodex is not expected to affect your ability to drive or use machines. However, if you feel tired
after treatment do not drive or use machines
.
Important information about some of the ingredients of Faslodex
This medicinal product contains 10% w/v ethanol (alcohol), i.e. up to 1000 mg per dose, equivalent to
20 ml beer or 8 ml wine per dose.
Harmful for those suffering from alcoholism.
To be taken into account in pregnant or breast-feeding women, children and high-risk groups such as
patients with liver disease, or epilepsy.
The usual dose is 500 mg fulvestrant (two 250 mg/5 ml injections) given once a month with an
additional 500 mg dose given 2 weeks after the initial dose.
Your doctor or nurse will give you Faslodex as a slow intramuscular injection, one into each of your
buttocks.
Like all medicines, Faslodex can cause side effects, although not everybody gets them.
These side effects may occur with certain frequencies, which are defined as follows:
• very common: affects more than 1 user in 10
• common: affects 1 to 10 users in 100
• uncommon: affects 1 to 10 users in 1,000
• rare: affects 1 to 10 users in 10,000
• very rare: affects less than 1 user in 10,000
• not known: frequency cannot be estimated from the available data.
Very common side effects
• Injection site reactions, such as pain and/or inflammation
• Abnormal levels of liver enzymes (in blood tests)*
• Nausea (feeling sick)
• Weakness, tiredness*
Common side effects
• Headache
• Hot flushes
• Vomiting, diarrhoea, or loss of appetite*
• Rash
• Urinary tract infections
• Back pain*
• Thromboembolism (Increased risk of blood clots)*
• Allergic (hypersensitivity) reactions, including swelling of the face, lips, tongue and/or throat
Uncommon side effects
• Vaginal bleeding, thick, whitish discharge and candidiasis (infection)
• Bruising and bleeding at the site of injection
* Includes side effects for which the exact role of Faslodex cannot be assessed due to the underlying
disease.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor, nurse or pharmacist.
Store in a refrigerator (2°C - 8°C)
Keep the pre-filled syringe in the original package, in order to protect from light.
Keep out of the reach and sight of children.
Do not use Faslodex after the expiry date which is stated on the carton or syringe labels after the
abbreviation EXP. The expiry date refers to the last day of that month.
Your health care professional will be responsible for the correct storage, use and disposal of Faslodex.
The active substance is fulvestrant. Each pre-filled syringe (5 ml) contains 250 mg fulvestrant.
What Faslodex looks like and contents of the pack
Faslodex is a clear, colourless to yellow, viscous solution in a pre-filled syringe fitted with a
tamper-evident closure, containing 5 ml solution for injection. Two syringes must be administered to
receive the 500 mg recommended monthly dose.
Faslodex has 2 pack presentations, either a pack containing 1 glass pre-filled syringe or a pack
containing 2 glass pre-filled syringes. Safety needles (BD SafetyGlide™) for connection to each barrel
are also provided.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
AstraZeneca UK Limited
Alderley Park
Macclesfield
Cheshire
SK10 4TG
United Kingdom
Manufacturer
AstraZeneca UK Limited
Silk Road Business Park
Macclesfield
Cheshire
SK10 2NA
The other ingredients are ethanol (96 per cent), benzyl alcohol, benzyl benzoate and castor oil.
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder (see contacts list):
België/Belgique/Belgien
NV AstraZeneca SA
Tel: +32 2 370 48 11
Luxembourg/Luxemburg
NV AstraZeneca SA
Tél/Tel: +32 2 370 48 11
България
ТП AstraZeneca UK Limited
Тел.: +359 2 971 25 33
Magyarország
AstraZeneca Kft
Tel: +36 1 883 6500
Česká republika
AstraZeneca Czech Republic s.r.o.
Tel: +420 222 807 111
Malta
Associated Drug Co. Ltd
Tel: +356 2277 8000
Danmark
AstraZeneca A/S
Tlf: +45 43
66 64 62
Nederland
AstraZeneca BV
Tel: +31 79 363 2222
Deutschland
AstraZeneca GmbH
Tel: +49 41 03 7080
Norge
AstraZeneca AS
Tlf: +47 21 00 64 00
Eesti
AstraZeneca
Tel: +372 6549 600
Österreich
AstraZeneca Österreich GmbH
Tel: +43 1 711 31 0
Ελλάδα
AstraZeneca A.E.
Τηλ: + 30 2 106871500
Polska
AstraZeneca Pharma Poland Sp. z o.o.
Tel.: +48 22 874 35 00
España
AstraZeneca Farmacéutica Spain, S.A.
Tel: +34 91 301 91 00
Portugal
AstraZeneca Produtos Farmacêuticos, Lda.
Tel: +351 21 434 61 00
France
AstraZeneca
Tél: +33 1 41 29 40 00
România
AstraZeneca Pharma SRL
Tel: +40 21 317 60 41
Ireland
AstraZeneca Pharmaceuticals (Ireland) Ltd
Tel: +353 1609 7100
Slovenija
AstraZeneca UK Limited
Tel: +386 1 51 35 600
Ísland
Vistor hf.
Sími: +354 535 7000
Slovenská republika
AstraZeneca AB o.z.
Tel.: +421 2 5737 7777
Italia
AstraZeneca S.p.A.
Tel: +39 02 9801 1
Suomi/Finland
AstraZeneca Oy
Puh/Tel: +358 10 23 010
Κύπρος
Αλέκτωρ Φαρµακευτική Λτδ
Τηλ: +357 22490305
Sverige
AstraZeneca AB
Tel: +46 8 553 26 000
Latvija
AstraZeneca AB pārstāvniecība Latvijā
Tel: +371 67377100
United Kingdom
AstraZeneca UK Ltd
Tel: +44 1582 836 836
Lietuva
UAB AstraZeneca Lietuva
Tel: +370 5 2660550
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency web site:
http://www.ema.europa.eu
The following information is intended for healthcare professionals only:
Faslodex 500 mg (2 x 250 mg/5 ml solution for injection) should be administered using two pre-filled
syringes, see section 3.
BD SafetyGlide is a trademark of Becton Dickinson and Company and is CE-marked: CE 0050.
Instructions for administration
Warning - Do not autoclave safety needle (BD SafetyGlide™ Shielding Hypodermic Needle) before
use. Hands must remain behind the needle at all times during use and disposal.
For each of the two syringes:
• Remove glass syringe barrel from tray and check that it is
not damaged.
• Break the seal of the white plastic cover on the syringe
Luer connector Luer-Lok to remove the cover with the
attached rubber tip cap (see Figure 1).
• Peel open the safety needle (BD SafetyGlide) outer
packaging. Attach the safety needle to the Luer-Lok (see
Figure 2)
• Twist until firmly seated.
• Twist to lock the needle to the Luer connector.
• Pull shield straight off needle to avoid damaging needle
point.
• Transport filled syringe to point of administration.
• Remove needle sheath.
• Parenteral solutions must be inspected visually for
particulate matter and discolouration prior to
administration.
• Expel excess gas from the syringe.
• Administer intramuscularly slowly (1-2 minutes/injection)
into the buttock. For user convenience, the needle bevel- up
position is oriented to the lever arm (see Figure 3).
• After injection, immediately apply a single-finger stroke to
the activation assisted lever arm to activate the shielding
mechanism (see Figure 4).
NOTE: Activate away from self and others. Listen for click
and visually confirm needle tip is fully covered.
Disposal
Pre-filled syringes are for single use
only
.
Any unused product or waste material should be disposed of in accordance with local requirements.



Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/faslodex.html
Copyright © 1995-2021 ITA all rights reserved.
|


































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































