Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Firazyr 30 mg solution for injection in pre-filled syringe
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each pre-filled syringe of 3 ml contains icatibant acetate equivalent to 30 mg icatibant.
Each ml of the solution contains 10 mg of icatibant.
For a full list of excipients, see section 6.1.
Solution for injection.
The solution is a clear and colourless liquid.
4.1 Therapeutic indications
Firazyr is indicated for symptomatic treatment of acute attacks of hereditary angioedema (HAE) in
adults (with C1-esterase-inhibitor deficiency).
4.2 Posology and method of administration
Firazyr is intended for subcutaneous administration preferably in the abdominal area.
Firazyr is intended for use under the guidance of a healthcare professional.
Firazyr may be self-administered or administered by a caregiver only after training in subcutaneous
injection technique by a healthcare professional.
The decision on initiating self-administration of Firazyr should only be taken by a physician
experienced in the diagnosis and treatment of hereditary angioedema (see section 4.4).
Patients with laryngeal attacks should be managed in an appropriate medical institution after injection
until the physician considers discharge to be safe.
Each Firazyr syringe is intended for single use only.
The recommended dose is a single subcutaneous injection of Firazyr 30 mg.
Firazyr solution for injection should be injected slowly due to the volume to be administered (3 ml).
In the majority of cases a single injection of Firazyr is sufficient to treat an attack. In case of
insufficient relief or recurrence of symptoms, a second injection of Firazyr can be administered after
6 hours. If the second injection produces insufficient relief or a recurrence of symptoms is observed, a
third injection of Firazyr can be administered after a further 6 hours. No more than 3 injections of
Firazyr should be administered in a 24 hour period.
In the clinical trials, not more than 8 injections of Firazyr per month have been administered.
Elderly patients
Limited information is available on patients older than 65 years of age.
Elderly patients have been shown to have increased systemic exposure to icatibant. The relevance of
this to the safety of Firazyr is unknown (see section 5.2).
Hepatic impairment
No dosage adjustment is required in patients with hepatic impairment.
Renal impairment
No dosage adjustment is required in patients with renal impairment.
Paediatric population
The safety and efficacy of Firazyr in children aged 0-18 years has not been established.
No pediatric data are available.
Hypersensitivity to the active substance or to any of the excipients.
Special warnings and precautions for use
For patients who never received Firazyr previously, the first treatment should be given in a medical
institution or under the guidance of a physician.
In case of insufficient relief or recurrence of symptoms after self-treatment, it is recommended that the
patient should seek medical advice and that subsequent doses are given in a medical institution (see
section 4.2).
Patients experiencing a laryngeal attack should always seek medical advice and be observed in a
medical institution also after having taken the injection at home.
Ischemic heart disease
Under ischemic conditions, a deterioration of cardiac function and a decrease in coronary blood flow
could theoretically arise from antagonism of bradykinin receptor type 2. Caution should therefore be
observed in the administration of Firazyr to patients with acute ischemic heart disease or unstable
angina pectoris (see section 5.3).
Stroke
Although there is evidence to support a beneficial effect of B2 receptor blockade immediately
following a stroke, there is a theoretical possibility that icatibant may attenuate the positive late phase
neuroprotective effects of bradykinin. Accordingly, caution should be observed in the administration
of icatibant to patients in the weeks following a stroke.
Interaction with other medicinal products and other forms of interaction
Pharmacokinetic drug interactions involving CYP450 are not expected (see section 5.2)
Co-administration of Firazyr with ACE inhibitors has not been studied. ACE inhibitors are
contraindicated in HAE patients due to possible enhancement of bradykinin levels.
4.6
Fertility, pregnancy and lactation
For icatibant, no clinical data on exposed pregnancies are available. Animal studies showed effects on
uterine implantation and parturition (see section 5.3), but the potential risk for humans is unknown.
Firazyr should be used during pregnancy only, if the potential benefit justifies the potential risk for the
foetus, (e.g for treatment of potentially life threatening laryngeal attacks).
Icatibant is excreted in the milk of lactating rats at concentrations similar to those in maternal blood.
No effects were detected in the post-natal development of rat pups.
It is unknown whether icatibant is excreted in human breast milk but it is recommended that
breastfeeding women who wish to take Firazyr should not breastfeed for 12 hours after treatment.
In immature animals repeated use of icatibant reversibly delayed sexual maturation (see section 5.3).
4.7 Effects on ability to drive and use machines
Firazyr has minor or moderate influence on the ability to drive and use machines. Fatigue, lethargy,
tiredeness, somnolence, and dizziness have been reported uncommonly following the use of Firazyr.
These symptoms may occur as a result of an attack of HAE. However, a causal relationship to the use
of Firazyr cannot be excluded. Patients should be advised not to drive and use machines if they feel
tired or dizzy.
The safety of icatibant has been established in 1304 subjects treated with various doses, regimens and
routes of administration during Phase I-III studies in various indications.
Sixty three (HAE) patients received icatibant in two Phase III trials for treatment of an attack in the
controlled phase and 126 patients were treated in the open label phase.
Almost all subjects who were treated with subcutaneous icatibant in clinical trials developed reactions
at the site of injection (characterised by skin irritation, swelling, pain, itchiness, erythema, burning
sensation). These reactions were generally mild in severity, transient, and resolved without further
intervention.
The frequency of adverse reactions listed in Table 1 is defined using the following convention:
Very common (≥1/10); common (≥1/100, <1/10); uncommon (≥1/1,000, <1/100); rare (≥1/10,000,
<1/1,000); very rare (<1/10,000).
Note: Due to the low number of patients, each of the uncommon events has only been reported in a
single patient.
Table 1: Adverse reactions reported with icatibant in the phase III clinical trials.
Congenital, familial
and genetic disorders
Gastrointestinal
disorders
General disorders and
administration site
conditions
Injections site reactions
(characterised by skin
irritation, swelling,
Asthenia, fatigue,
pyrexia










pain, itchiness,
erythema, burning
sensation)
Infections and
infestations
Herpes zoster
,
pharyngitis
Injury, poisoning and
procedural
complications
Blood creatinine
phosphokinase
increased, prothrombin
time prolonged
Weight increased,
blood glucose
increased, liver
function test abnormal
Metabolism and
nutrition disorders
Hyperuricaemia,
hyperglycaemia
Musculoskeletal and
connective tissue
disorders
Renal and urinary
disorders
Respiratory, thoracic
and mediastinal
disorders
Asthma, cough, nasal
congestion
Skin and subcutaneous
tissue disorders
* HAE attacks were reported as adverse reactions, however based on time of occurrence, the majority
were recurrent attacks and not related to treatment with Firazyr.
In an open-label study, the safety profile of the patients who self-administered Firazyr was similar to
that administered by healthcare professionals.
No clinical information on overdose is available.
A dose of 3.2 mg/kg intravenously (approximately 8 times the therapeutic dose) caused transient
erythema, itching or hypotension in healthy subjects. No therapeutic intervention was necessary.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: cardiac therapy, other cardiac preparations, ATC code: C01EB19.
















HAE (an autosomal dominant disease) is caused by an absence or dysfunction of C1-esterase-
inhibitor. HAE attacks are accompanied by an increased release of bradykinin, which is the key
mediator in the development of the clinical symptoms.
HAE manifests as intermittent attacks of subcutaneous and/or sub mucosal oedema involving the
upper respiratory tract, the skin and the gastrointestinal tract. An attack usually lasts between 2 to 5
days.
Icatibant is a selective competitive antagonist at the bradykinin type 2 (B2) receptor. It is a synthetic
decapeptide with a structure similar to bradykinin, but with 5 non-proteinogenic amino acids. In HAE
increased bradykinin concentrations are the key mediator in the development of the clinical symptoms.
In healthy young subjects, icatibant administered in doses of 0.8 mg/kg over 4 hours; 1.5 mg/kg/day or
0.15 mg/kg/day for 3 days, development of bradykinin-induced hypotension, vasodilatation and reflex
tachycardia was prevented. Icatibant was shown to be a competitive antagonist when the bradykinin
challenge dose was increased 4-fold.
Efficacy data were obtained from an initial open-label Phase II study and from two randomised,
double blind controlled multi centre Phase III studies (one with oral tranexamic acid as the comparator
and one placebo controlled). The pivotal Phase III studies were otherwise identical in design. A total
of 130 patients were randomized to receive either a 30 mg dose of icatibant (63 patients) or
comparator (either tranexamic acid, - 38 or placebo - 29 patients). Subsequent episodes of HAE were
treated in an open label extension. Patients with symptoms of laryngeal angioedema received open
label treatment with icatibant.
In the Phase III trials, the primary efficacy endpoint was time to onset of symptom relief using a visual
analogue scale (VAS). In both studies, patients on icatibant had a faster median time to onset of
symptom relief (2.0 and 2.5 hours, respectively) compared to tranexamic acid (12.0 hours) and
placebo (4.6 hours). The treatment effect of icatibant was confirmed by secondary efficacy endpoints.
The following table shows the results for the two pivotal trials
Controlled Clinical Study of FIRAZYR vs Tranexamic acid or Placebo: Efficacy Results
STUDY 1
Number of subjects
in ITT Population
Number of subjects
in ITT Population
Change from
baseline to 4 hours
Change from
baseline to 4 hours
Difference
between treatments
(95% CI, p-value)
-27.8 (-39.4, -16.2) p < 0.001
Difference
between treatments
(95% CI, p-value)
-23.3 (-37.1, -9.4) p = 0.002
Change from
baseline to
1
2 hours
Change from
baseline to
1
2 hours
Difference
between treatments
(95% CI, p-value)
-24.1 (-33.6, -14.6) p < 0.001
Difference
between treatments
(95% CI, p-value)
-15.2 (-28.6, -1.7) p = 0.028
Median time to
onset of symptom
relief (hours)
Median time to
onset of symptom
relief (hours)


















































Controlled Clinical Study of FIRAZYR vs Tranexamic acid or Placebo: Efficacy Results
STUDY 1
Response rate
(%, CI) at 4 hours
after start of
t
reatment
Response rate
(%, CI) at 4 hours
after start of
t
reatment
Median time to
onset of symptom
relief: all
symptoms (hours):
Abdominal pain
Skin swelling
Skinpain
Median time to
onset of symptom
relief: all
symptoms (hours):
Abdominalpain
Skin swelling
Skin pain
Median time to
almost complete
symptom relief
(hours)
Median time to
almost complete
symptom relief
(hours)
Median time to
regression of
symptoms, by
p
atient (hours)
Median time to
regression of
symptoms, by
p
atient (hours)
Median time to
overall patient
improvement, by
p
hysician (hours)
Median time to
overall patient
improvement, by
p
hysician (hours)
126 patients were treated in the open label extension (OLE) phase for a total of 714 separate attacks.
The efficacy results were similar to those seen in the controlled phase of the studies. The majority of
attacks (88.2% in Study 2 and 89.8% in Study 1) in both studies required only a single dose of
icatibant.
A total of 38 patients were treated for a total of 78 attacks of HAE affecting the larynx. The results
were similar to patients with non-laryngeal attacks of HAE with a median time to start of regression of
symptoms of 0.6 - 1.0 hours (controlled phase).
5.2 Pharmacokinetic properties
The pharmacokinetics of icatibant has been extensively characterized by studies using both
intravenous and subcutaneous administration to healthy volunteers and patients. The pharmacokinetic
profile of icatibant in patients with HAE is similar to that in healthy volunteers.







































































Absorption
Following subcutaneous administration, the absolute bioavailability of icatibant is 97%. The time to
maximum concentration is approximately 30 minutes.
Distribution
Icatibant volume of distribution (Vss) is about 20-25 L. Plasma protein binding is 44%.
Elimination
Icatibant is mainly eliminated by metabolism with less than 10% of the dose eliminated in the urine as
unchanged drug. Clearance is about 15-20 l/h and independent of dose. The terminal plasma half-life
is about 1-2 hours.
Metabolism
Icatibant is extensively metabolized by proteolytic enzymes to inactive metabolites that are primarily
excreted in the urine.
In vitro
studies have confirmed that icatibant is not degraded by oxidative metabolic pathways and is
not an inhibitor of major cytochrome P450 (CYP) isoenzymes (CYP 1A2, 2A6, 2B6, 2C8, 2C9, 2C19,
2D6, 2E1, and 3A4) and is not an inducer of CYP 1A2 and 3A4.
Special populations
Data suggest an age-related decline in clearance resulting in about 50-60% higher exposure in the
elderly (75-80 years) compared to patients aged 40 years. Data suggests that gender and weight do not
have a significant influence on icatibant pharmacokinetics.
Limited data suggest that icatibant exposure is not influenced by hepatic or renal impairment. The
influence of race on icatibant pharmacokinetics has not been evaluated. There are no pharmacokinetic
data in children.
5.3 Preclinical safety data
Repeated-dose studies of up to 3-months duration have been conducted in rat and dog. Maximum daily
exposures (AUC) at the No Observed Adverse Effect Levels in the 3-month study in rat were 3.6 times
and in the 4 week study in dog were 9.4 times the AUC in humans after a subcutaneous dose of 30 mg.
Long-term studies to determine the carcinogenic potential of icatibant have not been conducted to
date.
In a standard battery
of
in vitro
and
in vivo
tests icatibant was not genotoxic.
Icatibant was not teratogenic when administered by s.c. injection during early embryonic and fetal
development in rat (top dose 25 mg/kg/day) and rabbit (top dose 10 mg/kg/day). Icatibant is a potent
antagonist of bradykinin and therefore, at high dose levels, treatment can have effects on the uterine
implantation process and subsequent uterine stability in early pregnancy. These uterine effects also
manifest in late stage pregnancy where icatibant exhibits a tocolytic effect resulting in delayed
parturition in the rat, with increased fetal distress and perinatal death at high doses (10 mg/kg/day).
In immature rats and dogs, repeated use of icatibant reversibly delayed sexual maturation. The effects
appeared to be secondary to icatibant-induced changes in gonadotrophin levels, and were reversible.
Similar effects of icatibant on gonadotrophins also occurred in sexually mature dogs.
Icatibant had no effect on the fertility of male mice and rats.
Icatibant did not elicit any cardiac conduction change
in vitro
(hERG channel) or
in vivo
in normal
dogs or in various dog models (ventricular pacing, physical exertion and coronary ligation) where no
associated hemodynamic changes were observed. Icatibant has been shown to aggravate induced
cardiac ischemia in several non-clinical models, although a detrimental effect has not consistently
been shown in acute ischemia.
PHARMACEUTICAL PARTICULARS
Sodium chloride
Acetic acid, glacial (for pH adjustment)
Sodium hydroxide (for pH adjustment)
Water for injections
Special precautions for storage
Do not store above 25
○
C.
Do not freeze.
6.5 Nature and contents of container
3 ml of solution in a 3 ml pre-filled syringe (type I glass) with plunger stopper (bromobutyl coated
with fluorocarbon polymer). A hypodermic needle (25 G; 16 mm) is included in the pack.
Pack size of one pre-filled syringe with one needle or a multipack containing three pre-filled syringes
with three needles.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
The solution should be clear and colourless and free from visible particles. For single use only.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Jerini AG
Invalidenstr. 130
D-10115 Berlin
Germany
MARKETING AUTHORISATION NUMBER(S)
EU/1/08/461/001
EU/1/08/461/002
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10.
DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency
http://www.ema.europa.eu
MANUFACTURING AUTHORISATION HOLDER
RESPONSIBLE FOR BATCH RELEASE
CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer responsible for batch release
RENTSCHLER Biotechnologie GmbH
Erwin-Rentschler-Strasse 21
D-88471 Laupheim
Germany
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to medical prescription.
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
The MAH must ensure that the system of pharmacovigilance, as described in version 5.0 dated
11 April 2008 presented in Module 1.8.1. of the Marketing Authorisation Application, is in place and
functioning before and whilst the product is on the market.
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version 1.2 dated 15 April 2008 of the Risk Management
Plan (RMP) presented in Module 1.8.2. of the Marketing Authorisation Application and any
subsequent updates of the RMP agreed by the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, the
updated RMP should be submitted at the same time as the next Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted
•
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
•
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
•
At the request of the European Medicines Agency
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
Carton of unit pack
NAME OF THE MEDICINAL PRODUCT
Firazyr 30 mg solution for injection pre-filled syringe
Icatibant
STATEMENT OF ACTIVE SUBSTANCE(S)
Each 3 ml pre-filled syringe contains icatibant acetate equivalent to 30 mg icatibant.
Each ml of the solution contains 10mg of icatibant.
Contains: acetic acid glacial, sodium hydroxide, sodium chloride, water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection
One pre-filled syringe.
One 25G needle
METHOD AND ROUTE(S) OF ADMINISTRATION
Subcutaneous use
Read the package leaflet before use
For single use only
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
Do not store above 25ºC. Do not freeze.
























10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Marketing Authorisation Holder
Jerini AG
Invalidenstrasse. 130
D-10115 Berlin
Germany
MARKETING AUTHORISATION NUMBER(S)
GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.


















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
Outer Carton of Multi-Pack (Including Blue Box)
NAME OF THE MEDICINAL PRODUCT
Firazyr 30 mg solution for injection pre-filled syringe
Icatibant
STATEMENT OF ACTIVE SUBSTANCE(S)
Each 3 ml pre-filled syringe contains icatibant acetate equivalent to 30 mg icatibant.
Each ml of the solution contains 10mg of icatibant.
Contains: acetic acid glacial, sodium hydroxide, sodium chloride, water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection
Multipack containing three pre-filled syringes and three 25G needles
METHOD AND ROUTE(S) OF ADMINISTRATION
Subcutaneous use
Read the package leaflet before use
For single use only
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
Do not store above 25ºC. Do not freeze.





















10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Marketing Authorisation Holder
Jerini AG
Invalidenstrasse. 130
D-10115 Berlin
Germany
MARKETING AUTHORISATION NUMBER(S)
GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.


















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
Intermediate Carton of Multi-Pack (Without Blue Box)
NAME OF THE MEDICINAL PRODUCT
Firazyr 30 mg solution for injection pre-filled syringe
Icatibant
STATEMENT OF ACTIVE SUBSTANCE(S)
Each 3 ml pre-filled syringe contains icatibant acetate equivalent to 30 mg icatibant.
Each ml of the solution contains 10mg of icatibant.
Contains: acetic acid glacial, sodium hydroxide, sodium chloride, water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection
Component of a multipack containing one pre-filled syringe and one 25G needle
Not for individual sale
METHOD AND ROUTE(S) OF ADMINISTRATION
Subcutaneous use
Read the package leaflet before use
For single use only
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
Do not store above 25ºC. Do not freeze.























10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Marketing Authorisation Holder
Jerini AG
Invalidenstrasse. 130
D-10115 Berlin
Germany
MARKETING AUTHORISATION NUMBER(S)
GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.


















PACKAGE LEAFLET: INFORMATION FOR THE USER
Firazyr 30 mg solution for injection pre-filled syringe
Icatibant
Read all of this leaflet carefully before you start using this medicine.
-
Keep this leaflet. You may need to read it again.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
What Firazyr is and what it is used for
1.
WHAT FIRAZYR IS AND WHAT IT IS USED FOR
Firazyr is a solution for injection that contains the active substance icatibant.
Firazyr is used for treating the symptoms of hereditary angioedema (HAE) in adult patients.
In HAE levels of a substance in your bloodstream called bradykinin are increased and this leads to
symptoms like swelling, pain, nausea, and diarrhoea.
Firazyr blocks the activity of bradykinin and therefore ends the further progression of the symptoms of
an HAE attack.
2. BEFORE YOU USE FIRAZYR
If you are allergic (hypersensitive) to icatibant, or any of the other ingredients of Firazyr.
Take special care with Firazyr
-
Some of the side effects connected with Firazyr are similar to the symptoms of your disease.
Tell your doctor immediately if you notice that your symptoms of the attack get worse after
you received Firazyr.
-
If you are suffering from angina (reduced blood flow to the heart muscle), please consult your
doctor before using Firazyr.
-
If you have recently suffered a stroke, please consult your doctor before using Firazyr.
-
You must be trained on subcutaneous (under the skin) injection technique before you self-
inject Firazyr.
If you have any further questions, ask your doctor or pharmacist.
-
If you self-inject Firazyr or if your caregiver injects you with Firazyr while experiencing a
laryngeal attack (obstruction of the upper airway), you must seek medical care immediately.
-
If your symptoms are not resolved following one self-administered injection of Firazyr, you
should seek medical advice for further treatment.
Use in Children and adolescents
Firazyr is not recommended for use in children and adolescent under 18 years of age.
Firazyr is not known to interact with other medicines. If you are taking a medicine known as an
Angiotensin Converting Enzyme (ACE) inhibitor (for example: captopril, enalapril, ramipril,
quinapril, lisinopril) which is used to lower your blood pressure or for any other reason, you should
inform your doctor before receiving Firazyr.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
Taking Firazyr with food and drink
Food and drink have no effect on the action of Firazyr.
Pregnancy and breast feeding
If you are pregnant or plan becoming pregnant, discuss this with your doctor before starting to use
Firazyr.
If you are breast-feeding you should not breast-feed for 12 hours after you have received Firazyr.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Do not drive or use machines if you feel tired or dizzy as a result of your HAE attack or after using
Firazyr.
Important information about some of the ingredients of Firazyr
The injection solution contains less than 1 mmol (23 milligrams) of sodium, so it is essentially
‘sodium-free’.
If you have never received Firazyr previously, your first dose of Firazyr will always be injected by
your doctor or nurse. Your doctor will tell you when it is safe for you to go home.
After discussion with your doctor or nurse and after training in subcutaneous (under the skin) injection
technique, you may be able to inject yourself with Firazyr or your caregiver may inject Firazyr for you
when you have an HAE attack. It is important that Firazyr is injected subcutaneously (under the skin)
as soon as you notice the attack of hereditary angioedema. Your healthcare provider will teach you
and your caregiver how to safely inject Firazyr by following the instructions in the Package Leaflet.
When and how often should you use Firazyr?
Your doctor has determined the exact dose of Firazyr and will tell you how often it should be used.
The recommended dose of Firazyr is one injection (3 ml, 30 mg) applied subcutaneously (under the
skin) as soon as you notice the attack of hereditary angioedema (for example increased skin swelling,
particularly affecting the face and neck, or increasing tummy pain). If you experience no relief of
symptoms after 6 hours, an additional injection of Firazyr (3 ml) can be given. If after a further
6 hours you still experience no relief you might have a third injection Firazyr (3 ml).
You should not have more than 3 injections in a 24 hour period and no more than 8
injections of
Firazyr in total per month.
How should Firazyr be administered?
Firazyr is intended for subcutaneous injection (under the skin). Each syringe should only be used
once.
Firazyr is injected with a short needle into the fatty tissue under the skin in the abdomen (tummy).
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
The following step-by-step instruction is intended for self-administration only
The instructions include the following main steps:
1) General Important Information
2) Preparing the syringe and needle for injection
3) Preparing the injection site
4) Injecting the solution
5) Disposal of the injection material
Step-by-Step Instructions for Injection
1) General Important Information
•
Wash your hands with soap and water before beginning the process
•
Open the blister by peeling back the seal
•
Remove the pre-filled syringe from the blister tray
•
Remove the cap from the end of the pre-filled syringe by unscrewing the cap
•
Put down the pre-filled syringe after unscrewing the cap
2) Preparing the syringe and needle for injection
•
Remove the needle cap from the blister
•
Remove the seal from the needle cap (the needle should be still in the needle cap)
•
Grip the syringe firmly. Carefully attach the needle to the pre-filled syringe containing the
colourless solution
•
Screw the pre-filled syringe on the needle still fixed in the needle cap
•
Remove the needle from the needle cap by pulling the syringe. Do not pull up on the plunger
•
The syringe is now ready for injection
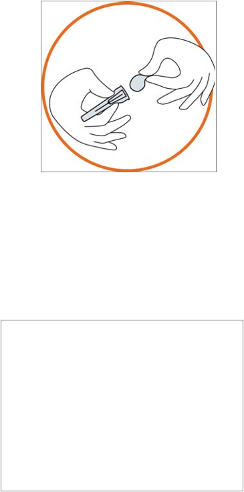

3) Preparing the injection site
•
Choose the injection site. The injection site should be a skin fold on your abdomen
approximately 5-10 cm (2-4 inches) below your navel on either side. This area should be at
least 5 cm (2 inches) away from any scars. Do not choose an area that is bruised, swollen, or
painful
•
Clean the injection site with a rubbing alcohol pad and allow it to dry
4) Injecting the Solution
•
Hold the syringe in one hand between two fingers with your thumb at the bottom of the
plunger
•
Make sure that there is no air bubble in the syringe by pressing the plunger until the first drop
appears on the tip of the needle


4) Injecting the Solution (cont’d)
•
Hold syringe between 45-90 degrees angle to skin with needle facing the skin
•
Keeping the syringe in one hand, use your other hand to gently hold a fold of skin between
your thumb and fingers at the previously disinfected injection site
•
Hold the fold of skin, bring the syringe to the skin and quickly insert the needle into the skin
fold
•
Slowly push the plunger of the syringe with a steady hand until all the fluid is injected into
the skin and no liquid remain in the syringe
•
Press slowly so that this takes approximately 30 seconds
•
Release the skin fold and gently pull the needle out
5) Disposal of the injection material
•
Discard the syringe, needle and needle cap into the sharp container for throwing away waste
that might hurt others if not handled properly.
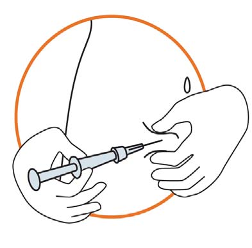

Like all medicines, Firazyr can cause side effects, although not everybody gets them. Almost all
patients receiving Firazyr will experience a reaction at the site of the injection (such as skin irritation,
swelling, pain, itchiness, erythema and burning sensation). These effects are usually mild and clear up
without the need for any additional treatment.
The frequency of possible side effects listed below is defined using the following convention: very
common (affects more than 1 user in 10), common (affects 1 to 10 users in 100), uncommon (affects 1
to 10 users in 1,000), rare (affects 1 to 10 users in 10,000), very rare (affects less than 1 user in
10,000), not known (frequency cannot be estimated from the available data).
Very common:
Injection site reactions (skin irritation, swelling, pain, itchiness, erythema and burning sensation).
Common:
Abnormal results from some blood test
Headache
Dizziness
Itching
Rash
Skin redness
Uncommon:
Abnormal liver function test
Asthma
Blocked nose
Bruising
Cough
Fever
Hot flushes
Increased amount of uric acid in the blood (which may indicate gout)
Increased amount of blood glucose
Muscle spasm
Nausea or vomiting
Hives
Shingles
Sore throat
Positive test for protein in your urine
Tiredness
Weakness
Weight gain
Tell your doctor immediately if you notice that the symptoms of your attack get worse after you
received Firazyr.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Firazyr after the expiry date stated on the label after ‘EXP’. The expiry date refers to the
last day of that month.
Do not store above 25
○
C. Do not freeze.
Firazyr should not be used if the syringe or needle packaging is damaged or if there are any visible
signs of deterioration, for example if the solution is cloudy, if it has floating particles, or if the colour
of the solution has changed.
Medicines should not be disposed of via waste water or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active substance is icatibant 30 milligrams (as acetate) in 3 ml solution for injection
in each
pre-filled syringe.
The other ingredients are sodium chloride, acetic acid glacial, sodium hydroxide and water for
injection. The solution does not contain any preservative.
What Firazyr looks like and contents of the pack
Firazyr is presented as a clear, colourless solution for injection in a pre-filled glass syringe of 3 ml.
Hypodermic needle (25 G; 16 mm) is included in the pack.
Firazyr is available as a single pack containing one pre-filled syringe with one needle or as a
multipack containing three pre-filled syringes with three needles.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Jerini AG
Invalidenstr. 130
10115 Berlin
Germany
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency website:
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/firazyr.html
Copyright © 1995-2021 ITA all rights reserved.
|



















































































































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































