Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Humalog 100 U/ml, solution for injection in vial
QUALITATIVE AND QUANTITATIVE COMPOSITION
One ml contains 100U (equivalent to 3.5mg) insulin lispro (recombinant DNA origin produced in
E.coli). Each container includes 10ml equivalent to 1000U insulin lispro.
For a full list of excipients, see section 6.1.
Humalog is a sterile, clear, colourless, aqueous solution.
4.1 Therapeutic indications
For the treatment of adults and children with diabetes mellitus who require insulin for the maintenance
of normal glucose homeostasis. Humalog is also indicated for the initial stabilisation of diabetes
mellitus.
4.2 Posology and method of administration
The dosage should be determined by the physician, according to the requirement of the patient.
Humalog may be given shortly before meals. When necessary Humalog can be given soon after meals.
Humalog preparations should be given by subcutaneous injection or by continuous subcutaneous
infusion pump (see section 4.2) and may, although not recommended, also be given by intramuscular
injection. If necessary, Humalog may also be administered intravenously, for example; for the control
of blood glucose levels during ketoacidosis, acute illnesses or during intra and post operative periods.
Subcutaneous administration should be in the upper arms, thighs, buttocks, or abdomen. Use of
injection sites should be rotated so that the same site is not used more than approximately once a
month.
When administered subcutaneously care should be taken when injecting Humalog to ensure that a
blood vessel has not been entered. After injection, the site of injection should not be massaged.
Patients must be educated to use the proper injection techniques.
Humalog takes effect rapidly and has a shorter duration of activity (2 to 5 hours) given subcutaneously
as compared with regular insulin. This rapid onset of activity allows a Humalog injection (or, in the
case of administration by continuous subcutaneous infusion, a Humalog bolus) to be given very close
to mealtime. The time course of action of any insulin may vary considerably in different individuals or
at different times in the same individual. The faster onset of action compared to soluble human insulin
is maintained regardless of injection site. As with all insulin preparations, the duration of action of
Humalog is dependent on dose, site of injection, blood supply, temperature, and physical activity.
Humalog can be used in conjunction with a longer-acting human insulin or oral sulphonylurea agents,
on the advice of a physician.
Use of Humalog in an insulin infusion pump
:
Only certain CE-marked insulin infusion pumps may be used to infuse insulin lispro. Before infusing
insulin lispro, the manufacturers instructions should be studied to ascertain the suitability or otherwise
for the particular pump. Read and follow the instructions that accompany the infusion pump. Use the
correct reservoir and catheter for the pump. Change the infusion set every 48 hours. Use aseptic
technique when inserting the infusion set. In the event of a hypoglycaemic episode, the infusion
should be stopped until the episode is resolved. If repeated or severe low blood glucose levels occur,
notify your health care professional and consider the need to reduce or stop your insulin infusion. A
pump malfunction or obstruction of the infusion set can result in a rapid rise in glucose levels. If an
interruption to insulin flow is suspected, follow the instructions in the product literature and if
appropriate, notify your health care professional. When used with an insulin infusion pump, Humalog
should not be mixed with any other insulin.
Intravenous administration of insulin
:
Intravenous injection of insulin lispro should be carried out following normal clinical practise for
intravenous injections, for example by an intravenous bolus or by an infusion system. Frequent
monitoring of the blood glucose levels is required.
Infusion systems at concentrations from 0.1U/ml to 1.0U/ml insulin lispro in 0.9% sodium chloride or
5% dextrose are stable at room temperature for 48 hours. It is recommended that the system is primed
before starting the infusion to the patient.
Hypersensitivity to insulin lispro or to any of the excipients.
4.4 Special warnings and precautions for use
Transferring a patient to another type or brand of insulin should be done under strict medical
supervision. Changes in strength, brand (manufacturer), type (regular, NPH, lente, etc.), species
(animal, human, human insulin analogue), and/or method of manufacture (recombinant DNA versus
animal-source insulin) may result in the need for a change in dosage. For fast-acting insulins, any
patient also on basal insulin must optimise dosage of both insulins to obtain glucose control across the
whole day, particularly nocturnal/fasting glucose control.
The shorter-acting Humalog should be drawn into the syringe first, to prevent contamination of the
vial by the longer-acting insulin. Mixing of the insulins ahead of time or just before the injection
should be on advice of the physician. However, a consistent routine must be followed.
Conditions which may make the early warning symptoms of hypoglycaemia different or less
pronounced include long duration of diabetes, intensified insulin therapy, diabetic nerve disease or
medications such as beta-blockers.
A few patients who have experienced hypoglycaemic reactions after transfer from animal-source
insulin to human insulin have reported that the early warning symptoms of hypoglycaemia were less
pronounced or different from those experienced with their previous insulin. Uncorrected
hypoglycaemic or hyperglycaemic reactions can cause loss of consciousness, coma, or death.
The use of dosages which are inadequate or discontinuation of treatment, especially in insulin-
dependent diabetics, may lead to hyperglycaemia and diabetic ketoacidosis; conditions which are
potentially lethal.
Insulin requirements may be reduced in the presence of renal impairment. Insulin requirements may be
reduced in patients with hepatic impairment due to reduced capacity for gluconeogenesis and reduced
insulin breakdown; however, in patients with chronic hepatic impairment, an increase in insulin
resistance may lead to increased insulin requirements.
Insulin requirements may be increased during illness or emotional disturbances.
Adjustment of dosage may also be necessary if patients undertake increased physical activity or
change their usual diet. Exercise taken immediately after a meal may increase the risk of
hypoglycaemia. A consequence of the pharmacodynamics of rapid-acting insulin analogues is that if
hypoglycaemia occurs, it may occur earlier after an injection when compared with soluble human
insulin.
Humalog should only be used in children in preference to soluble insulin when a fast action of insulin
might be beneficial. For example, in the timing of the injections in relation to meals.
4.5 Interaction with other medicinal products and other forms of interaction
Insulin requirements may be increased by medicinal products with hyperglycaemic activity, such as
oral contraceptives, corticosteroids, or thyroid replacement therapy, danazol, beta
2
stimulants (such as
ritodrine, salbutamol, terbutaline).
Insulin requirements may be reduced in the presence of medicinal products with hypoglycaemic
activity, such as oral hypoglycaemics, salicylates (for example, acetylsalicylic acid), sulpha
antibiotics, certain antidepressants (monoamine oxidase inhibitors, selective serotonin reuptake
inhibitors), certain angiotensin converting enzyme inhibitors (captopril, enalapril), angiotensin II
receptor blockers, beta-blockers, octreotide or alcohol.
The physician should be consulted when using other medications in addition to Humalog.
4.6 Pregnancy and lactation
Data on a large number of exposed pregnancies do not indicate any adverse effect of insulin lispro on
pregnancy or on the health of the foetus/newborn.
It is essential to maintain good control of the insulin-treated (insulin-dependent or gestational
diabetes) patient throughout pregnancy. Insulin requirements usually fall during the first trimester and
increase during the second and third trimesters. Patients with diabetes should be advised to inform
their doctor if they are pregnant or are contemplating pregnancy. Careful monitoring of glucose
control, as well as general health, is essential in pregnant patients with diabetes.
Patients with diabetes who are breast-feeding may require adjustments in insulin dose, diet or both.
4.7 Effects on ability to drive and use machines
The patient’s ability to concentrate and react may be impaired as a result of hypoglycaemia. This may
constitute a risk in situations where these abilities are of special importance (e.g. driving a car or
operating machinery).
Patients should be advised to take precautions to avoid hypoglycaemia whilst driving, this is
particularly important in those who have reduced or absent awareness of the warning signs of
hypoglycaemia or have frequent episodes of hypoglycaemia. The advisability of driving should be
considered in these circumstances.
Hypoglycaemia is the most frequent undesirable effect of insulin therapy that a patient with diabetes
may suffer. Severe hypoglycaemia may lead to loss of consciousness, and in extreme cases, death. No
specific frequency for hypoglycaemia is presented, since hypoglycaemia is a result of both the insulin
dose and other factors e.g. a patient`s level of diet and exercise.
Local allergy in patients is common (1/100 to <1/10). Redness, swelling, and itching can occur at the
site of insulin injection. This condition usually resolves in a few days to a few weeks. In some
instances, this condition may be related to factors other than insulin, such as irritants in the skin
cleansing agent or poor injection technique. Systemic allergy, which is rare (1/10,000 to <1/1,000)
but potentially more serious, is a generalised allergy to insulin. It may cause a rash over the whole
body, shortness of breath, wheezing, reduction in blood pressure, fast pulse, or sweating. Severe cases
of generalised allergy may be life-threatening.
Lipodystrophy at the injection site is uncommon (1/1,000 to <1/100).
Insulins have no specific overdose definitions because serum glucose concentrations are a result of
complex interactions between insulin levels, glucose availability and other metabolic processes.
Hypoglycaemia may occur as a result of an excess of insulin activity relative to food intake and
energy expenditure.
Hypoglycaemia may be associated with listlessness, confusion, palpitations, headache, sweating and
vomiting.
Mild hypoglycaemic episodes will respond to oral administration of glucose or other sugar or
saccharated products.
Correction of moderately severe hypoglycaemia can be accomplished by intramuscular or
subcutaneous administration of glucagon, followed by oral carbohydrate when the patient recovers
sufficiently. Patients who fail to respond to glucagon must be given glucose solution intravenously.
If the patient is comatose, glucagon should be administered intramuscularly or subcutaneously.
However, glucose solution must be given intravenously if glucagon is not available or if the patient
fails to respond to glucagon. The patient should be given a meal as soon as consciousness is
recovered.
Sustained carbohydrate intake and observation may be necessary because hypoglycaemia may recur
after apparent clinical recovery.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group : Fast-acting human insulin analogue. ATC code: A10A B04
The primary activity of insulin lispro is the regulation of glucose metabolism.
In addition, insulins have several anabolic and anti-catabolic actions on a variety of different tissues.
Within muscle tissue this includes increasing glycogen, fatty acid, glycerol and protein synthesis and
amino acid uptake, while decreasing glycogenolysis, gluconeogenesis, ketogenesis, lipolysis, protein
catabolism and amino acid output.
Insulin lispro has a rapid onset of action (approximately 15 minutes), thus allowing it to be given
closer to a meal (within zero to 15 minutes of the meal) when compared to regular insulin (30 to
45 minutes before). Insulin lispro takes effect rapidly and has a shorter duration of activity (2 to
5 hours) when compared to regular insulin.
Clinical trials in patients with type 1 and type 2 diabetes have demonstrated reduced postprandial
hyperglycaemia with insulin lispro compared to soluble human insulin.
As with all insulin preparations, the time course of insulin lispro action may vary in different
individuals or at different times in the same individual and is dependent on dose, site of injection,
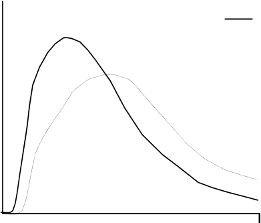
blood supply, temperature and physical activity. The typical activity profile following subcutaneous
injection is illustrated below.
The above representation reflects the relative amount of glucose over time required to maintain the
subject's whole blood glucose concentrations near fasting levels and is an indicator of the effect of
these insulins on glucose metabolism over time.
Clinical trials have been performed in children (61 patients aged 2 to 11) and children and adolescents
(481 patients aged 9 to 19 years), comparing insulin lispro to human soluble insulin. The
pharmacodynamic profile of insulin lispro in children is similar to that seen in adults.
When used in subcutaneous infusion pumps, treatment with insulin lispro has been shown to result in
lower glycosylated haemoglobin levels compared to soluble insulin. In a double-blind, crossover
study, the reduction in glycosylated haemoglobin levels after 12 weeks dosing was 0.37 percentage
points with insulin lispro, compared to 0.03 percentage points for soluble insulin (p = 0.004).
In patients with type 2 diabetes on maximum doses of sulphonyl urea agents, studies have shown that
the addition of insulin lispro significantly reduces HbA
1c
compared to sulphonyl urea alone. The
reduction of HbA
1c
would also be expected with other insulin products e.g. soluble or isophane
insulins.
Clinical trials in patients with type 1 and type 2 diabetes have demonstrated a reduced number of
episodes of nocturnal hypoglycaemia with insulin lispro compared to soluble human insulin. In some
studies, reduction of nocturnal hypoglycaemia was associated with increased episodes of daytime
hypoglycaemia.
The glucodynamic response to insulin lispro is not affected by renal or hepatic function impairment.
Glucodynamic differences between insulin lispro and soluble human insulin, as measured during a
glucose clamp procedure, were maintained over a wide range of renal function.
Insulin lispro has been shown to be equipotent to human insulin on a molar basis but its effect is more
rapid and of a shorter duration.
5.2 Pharmacokinetic properties
The pharmacokinetics of insulin lispro reflect a compound that is rapidly absorbed, and achieves peak
blood levels 30 to 70 minutes following subcutaneous injection. When considering the clinical











relevance of these kinetics, it is more appropriate to examine the glucose utilisation curves (as
discussed in 5.1).
Insulin lispro maintains more rapid absorption when compared to soluble human insulin in patients
with renal impairment. In patients with type 2 diabetes over a wide range of renal function the
pharmacokinetic differences between insulin lispro and soluble human insulin were generally
maintained and shown to be independent of renal function. Insulin lispro maintains more rapid
absorption and elimination when compared to soluble human insulin in patients with hepatic
impairment.
5.3 Preclinical safety data
In
in vitro
tests, including binding to insulin receptor sites and effects on growing cells, insulin lispro
behaved in a manner that closely resembled human insulin. Studies also demonstrate that the
dissociation of binding to the insulin receptor of insulin lispro is equivalent to human insulin. Acute,
one month and twelve month toxicology studies produced no significant toxicity findings.
Insulin lispro did not induce fertility impairment, embryotoxicity or teratogenicity in animal studies.
PHARMACEUTICAL PARTICULARS
m-
Cresol [3.15 mg/ml]
Glycerol
Dibasic sodium phosphate. 7H
2
O
Zinc oxide
Water for injections
Hydrochloric acid and sodium hydroxide maybe used to adjust pH to 7.0 – 7.8.
Humalog preparations should not be mixed with insulins produced by other manufacturers or with
animal insulin preparations. This medicinal product must not be mixed with other medicinal products
except those mentioned in section 6.6.
6.4 Special precautions for storage
Do not freeze. Do not expose to excessive heat or direct sunlight.
Unopened vials
Store in a refrigerator (2°C - 8°C).
After first use
Store in a refrigerator (2°C - 8°C) or below 30°C.
6.5 Nature and contents of container
The solution is contained in type I flint glass vials, sealed with butyl or halobutyl stoppers and secured
with aluminium seals. Dimeticone or silicone emulsion may be used to treat the vial stoppers.
Not all packs may be marketed.
1 x 10 ml Humalog vial.
2 x 10 ml Humalog vials.
5 x (1 x 10 ml) Humalog vials.
6.6 Special precautions for disposal and other handling
Any unused product or waste material should be disposed of in accordance with local requirements.
Instructions for use and handling
The vial is to be used in conjunction with an appropriate syringe (100 U markings).
Inspect the Humalog solution
.
It should be clear and colourless. Do not use Humalog if it appears
cloudy, thickened, or slightly coloured or if solid particles are visible.
If using a new vial, flip off the plastic protective cap, but
do not
remove the stopper.
If the therapeutic regimen requires the injection of basal insulin and Humalog at the same time,
the two can be mixed in the syringe. If mixing insulins, refer to the instructions for mixing that
follow in Section (ii) and 6.2.
Draw air into the syringe equal to the prescribed Humalog dose. Wipe the top of the vial with an
alcohol swab. Put the needle through the rubber top of the Humalog vial and inject the air into
the vial.
Turn the vial and syringe upside down. Hold the vial and syringe firmly in one hand.
Making sure the tip of the needle is in the Humalog, withdraw the correct dose into the syringe.
Before removing the needle from the vial, check the syringe for air bubbles that reduce the
amount of Humalog in it. If bubbles are present, hold the syringe straight up and tap its side
until the bubbles float to the top. Push them out with the plunger and withdraw the correct dose.
Remove the needle from the vial and lay the syringe down so that the needle does not touch
anything.
ii)
Mixing Humalog with longer-acting Human Insulins (see section 6.2)
Humalog should be mixed with longer-acting human insulins only on the advice of a doctor.
Draw air into the syringe equal to the amount of longer-acting insulin being taken. Insert the
needle into the longer-acting insulin vial and inject the air. Withdraw the needle.
Now inject air into the Humalog vial in the same manner, but
do not
withdraw the needle.
Turn the vial and syringe upside down.
Making sure the tip of the needle is in the Humalog, withdraw the correct dose of Humalog into
the syringe.
Before removing the needle from the vial, check the syringe for air bubbles that reduce the
amount of Humalog in it. If bubbles are present, hold the syringe straight up and tap its side
until the bubbles float to the top. Push them out with the plunger and withdraw the correct dose.
Remove the needle from the vial of Humalog and insert it into the vial of the longer-acting
insulin. Turn the vial and syringe upside down. Hold the vial and syringe firmly in one hand
and shake gently. Making sure the tip of the needle is in the insulin, withdraw the dose of
longer-acting insulin.
Withdraw the needle and lay the syringe down so that the needle does not touch anything.
Choose a site for injection.
Clean the skin as instructed.
Stabilise the skin by spreading it or pinching up a large area. Insert the needle and inject as
instructed.
Pull the needle out and apply gentle pressure over the injection site for several seconds. Do not
rub the area.
Dispose of the syringe and needle safely.
Use of the injection sites should be rotated so that the same is not used more than approximately
once a month.
Do not mix insulin in vials with insulin in cartridges. See section 6.2.
MARKETING AUTHORISATION HOLDER
Eli Lilly Nederland B.V., Grootslag 1-5, 3991 RA Houten, The Netherlands.
MARKETING AUTHORISATION NUMBERS
5 x (1 x 10 ml) Humalog vials
DATE OF FIRST AUTHORISATION/RENEWAL OF AUTHORISATION
Date of first authorisation: 30
th
April 1996
Date of last renewal: 30
th
April 2006
10.
DATE OF REVISION OF THE TEXT
NAME OF THE MEDICINAL PRODUCT
Humalog 100 U/ml, solution for injection in cartridge
QUALITATIVE AND QUANTITATIVE COMPOSITION
One ml contains 100U (equivalent to 3.5mg) insulin lispro (recombinant DNA origin produced in
E.coli). Each container includes 3ml equivalent to 300U insulin lispro.
For a full list of excipients, see section 6.1.
Humalog is a sterile, clear, colourless, aqueous solution.
4.1 Therapeutic indications
For the treatment of adults and children with diabetes mellitus who require insulin for the maintenance
of normal glucose homeostasis. Humalog is also indicated for the initial stabilisation of diabetes
mellitus.
4.2 Posology and method of administration
The dosage should be determined by the physician, according to the requirement of the patient.
Humalog may be given shortly before meals. When necessary Humalog can be given soon after meals.
Humalog preparations should be given by subcutaneous injection or by continuous subcutaneous
infusion pump (see section 4.2) and may, although not recommended, also be given by intramuscular
injection. If necessary, Humalog may also be administered intravenously, for example; for the control
of blood glucose levels during ketoacidosis, acute illnesses or during intra and post operative periods.
Subcutaneous administration should be in the upper arms, thighs, buttocks, or abdomen. Use of
injection sites should be rotated so that the same site is not used more than approximately once a
month.
When administered subcutaneously care should be taken when injecting Humalog to ensure that a
blood vessel has not been entered. After injection, the site of injection should not be massaged.
Patients must be educated to use the proper injection techniques.
Humalog takes effect rapidly and has a shorter duration of activity (2 to 5 hours) given subcutaneously
as compared with regular insulin. This rapid onset of activity allows a Humalog injection (or, in the
case of administration by continuous subcutaneous infusion, a Humalog bolus) to be given very close
to mealtime. The time course of action of any insulin may vary considerably in different individuals or
at different times in the same individual. The faster onset of action compared to soluble human insulin
is maintained regardless of injection site As with all insulin preparations, the duration of action of
Humalog is dependent on dose, site of injection, blood supply, temperature, and physical activity.
Humalog can be used in conjunction with a longer-acting human insulin or oral sulphonylurea agents,
on the advice of a physician.
Use of Humalog in an insulin infusion pump
:
Only certain CE-marked insulin infusion pumps may be used to infuse insulin lispro. Before infusing
insulin lispro, the manufacturers instructions should be studied to ascertain the suitability or otherwise
for the particular pump. Read and follow the instructions that accompany the infusion pump. Use the
correct reservoir and catheter for the pump. Change the infusion set every 48 hours. Use aseptic
technique when inserting the infusion set. In the event of a hypoglycaemic episode, the infusion
should be stopped until the episode is resolved. If repeated or severe low blood glucose levels occur,
notify your health care professional and consider the need to reduce or stop your insulin infusion. A
pump malfunction or obstruction of the infusion set can result in a rapid rise in glucose levels. If an
interruption to insulin flow is suspected, follow the instructions in the product literature and if
appropriate, notify your health care professional. When used with an insulin infusion pump, Humalog
should not be mixed with any other insulin.
Intravenous administration of insulin
:
Intravenous injection of insulin lispro should be carried out following normal clinical practise for
intravenous injections, for example by an intravenous bolus or by an infusion system. Frequent
monitoring of the blood glucose levels is required.
Infusion systems at concentrations from 0.1U/ml to 1.0U/ml insulin lispro in 0.9% sodium chloride or
5% dextrose are stable at room temperature for 48 hours. It is recommended that the system is primed
before starting the infusion to the patient.
Hypersensitivity to insulin lispro or to any of the excipients.
4.4 Special warnings and precautions for use
Transferring a patient to another type or brand of insulin should be done under strict medical
supervision. Changes in strength, brand (manufacturer), type (regular, NPH, lente, etc.), species
(animal, human, human insulin analogue), and/or method of manufacture (recombinant DNA versus
animal-source insulin) may result in the need for a change in dosage. For fast-acting insulins, any
patient also on basal insulin must optimise dosage of both insulins to obtain glucose control across the
whole day, particularly nocturnal/fasting glucose control.
Conditions which may make the early warning symptoms of hypoglycaemia different or less
pronounced include long duration of diabetes, intensified insulin therapy, diabetic nerve disease or
medications such as beta-blockers.
A few patients who have experienced hypoglycaemic reactions after transfer from animal-source
insulin to human insulin have reported that the early warning symptoms of hypoglycaemia were less
pronounced or different from those experienced with their previous insulin. Uncorrected
hypoglycaemic or hyperglycaemic reactions can cause loss of consciousness, coma, or death.
The use of dosages which are inadequate or discontinuation of treatment, especially in insulin-
dependent diabetics, may lead to hyperglycaemia and diabetic ketoacidosis; conditions which are
potentially lethal.
Insulin requirements may be reduced in the presence of renal impairment.
Insulin requirements may be reduced in patients with hepatic impairment due to reduced capacity for
gluconeogenesis and reduced insulin breakdown; however, in patients with chronic hepatic
impairment, an increase in insulin resistance may lead to increased insulin requirements.
Insulin requirements may be increased during illness or emotional disturbances.
Adjustment of dosage may also be necessary if patients undertake increased physical activity or
change their usual diet. Exercise taken immediately after a meal may increase the risk of
hypoglycaemia. A consequence of the pharmacodynamics of rapid-acting insulin analogues is that if
hypoglycaemia occurs, it may occur earlier after an injection when compared with soluble human
insulin.
If the 40 U/ml vial is the product normally prescribed, do not take insulin from a 100 U/ml cartridge
using a 40 U/ml syringe.
Humalog should only be used in children in preference to soluble insulin when a fast action of insulin
might be beneficial. For example, in the timing of the injections in relation to meals.
4.5 Interaction with other medicinal products and other forms of interaction
Insulin requirements may be increased by medicinal products with hyperglycaemic activity, such as
oral contraceptives, corticosteroids, or thyroid replacement therapy, danazol, beta
2
stimulants (such as
ritodrine, salbutamol, terbutaline).
Insulin requirements may be reduced in the presence of medicinal products with hypoglycaemic
activity, such as oral hypoglycaemics, salicylates (for example, acetylsalicylic acid), sulpha
antibiotics, certain antidepressants (monoamine oxidase inhibitors, selective serotonin reuptake
inhibitors), certain angiotensin converting enzyme inhibitors (captopril, enalapril), angiotensin II
receptor blockers, beta-blockers, octreotide or alcohol.
The physician should be consulted when using other medications in addition to Humalog .
4.6 Pregnancy and lactation
Data on a large number of exposed pregnancies do not indicate any adverse effect of insulin lispro on
pregnancy or on the health of the foetus/newborn.
It is essential to maintain good control of the insulin-treated (insulin-dependent or gestational
diabetes) patient throughout pregnancy. Insulin requirements usually fall during the first trimester and
increase during the second and third trimesters. Patients with diabetes should be advised to inform
their doctor if they are pregnant or are contemplating pregnancy. Careful monitoring of glucose
control, as well as general health, is essential in pregnant patients with diabetes.
Patients with diabetes who are breast-feeding may require adjustments in insulin dose, diet or both.
4.7 Effects on ability to drive and use machines
The patient’s ability to concentrate and react may be impaired as a result of hypoglycaemia. This may
constitute a risk in situations where these abilities are of special importance (e.g. driving a car or
operating machinery).
Patients should be advised to take precautions to avoid hypoglycaemia whilst driving, this is
particularly important in those who have reduced or absent awareness of the warning signs of
hypoglycaemia or have frequent episodes of hypoglycaemia. The advisability of driving should be
considered in these circumstances.
Hypoglycaemia is the most frequent undesirable effect of insulin therapy that a patient with diabetes
may suffer. Severe hypoglycaemia may lead to loss of consciousness, and in extreme cases, death. No
specific frequency for hypoglycaemia is presented, since hypoglycaemia is a result of both the insulin
dose and other factors e.g. a patient`s level of diet and exercise.
Local allergy in patients is common (1/100 to <1/10). Redness, swelling, and itching can occur at the
site of insulin injection. This condition usually resolves in a few days to a few weeks. In some
instances, this condition may be related to factors other than insulin, such as irritants in the skin
cleansing agent or poor injection technique. Systemic allergy, which is rare (1/10,000 to <1/1,000)
but potentially more serious, is a generalised allergy to insulin. It may cause a rash over the whole
body, shortness of breath, wheezing, reduction in blood pressure, fast pulse, or sweating. Severe cases
of generalised allergy may be life-threatening.
Lipodystrophy at the injection site is uncommon (1/1,000 to <1/100).
Insulins have no specific overdose definitions because serum glucose concentrations are a result of
complex interactions between insulin levels, glucose availability and other metabolic processes.
Hypoglycaemia may occur as a result of an excess of insulin activity relative to food intake and
energy expenditure.
Hypoglycaemia may be associated with listlessness, confusion, palpitations, headache, sweating and
vomiting.
Mild hypoglycaemic episodes will respond to oral administration of glucose or other sugar or
saccharated products.
Correction of moderately severe hypoglycaemia can be accomplished by intramuscular or
subcutaneous administration of glucagon, followed by oral carbohydrate when the patient recovers
sufficiently. Patients who fail to respond to glucagon must be given glucose solution intravenously.
If the patient is comatose, glucagon should be administered intramuscularly or subcutaneously.
However, glucose solution must be given intravenously if glucagon is not available or if the patient
fails to respond to glucagon. The patient should be given a meal as soon as consciousness is
recovered.
Sustained carbohydrate intake and observation may be necessary because hypoglycaemia may recur
after apparent clinical recovery.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group :Fast-acting human insulin analogue. ATC code: A10A B04
The primary activity of insulin lispro is the regulation of glucose metabolism.
In addition, insulins have several anabolic and anti-catabolic actions on a variety of different tissues.
Within muscle tissue this includes increasing glycogen, fatty acid, glycerol and protein synthesis and
amino acid uptake, while decreasing glycogenolysis, gluconeogenesis, ketogenesis, lipolysis, protein
catabolism and amino acid output.
Insulin lispro has a rapid onset of action (approximately 15 minutes), thus allowing it to be given
closer to a meal (within zero to 15 minutes of the meal) when compared to regular insulin (30 to
45 minutes before). Insulin lispro takes effect rapidly and has a shorter duration of activity (2 to
5 hours) when compared to regular insulin.
Clinical trials in patients with type 1 and type 2 diabetes have demonstrated reduced postprandial
hyperglycaemia with insulin lispro compared to soluble human insulin.
As with all insulin preparations, the time course of insulin lispro action may vary in different
individuals or at different times in the same individual and is dependent on dose, site of injection,
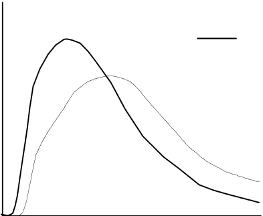
blood supply, temperature and physical activity. The typical activity profile following subcutaneous
injection is illustrated below.
The above representation reflects the relative amount of glucose over time required to maintain the
subject's whole blood glucose concentrations near fasting levels and is an indicator of the effect of
these insulins on glucose metabolism over time.
Clinical trials have been performed in children (61 patients aged 2 to 11) and children and adolescents
(481 patients aged 9 to 19 years), comparing insulin lispro to human soluble insulin. The
pharmacodynamic profile of insulin lispro in children is similar to that seen in adults.
When used in subcutaneous infusion pumps, treatment with insulin lispro has been shown to result in
lower glycosylated haemoglobin levels compared to soluble insulin. In a double-blind, crossover
study, the reduction in glycosylated haemoglobin levels after 12 weeks dosing was 0.37 percentage
points with insulin lispro, compared to 0.03 percentage points for soluble insulin (p = 0.004).
In patients with type 2 diabetes on maximum doses of sulphonyl urea agents, studies have shown that
the addition of insulin lispro significantly reduces HbA
1c
compared to sulphonyl urea alone. The
reduction of HbA
1c
would also be expected with other insulin products e.g. soluble or isophane
insulins.
Clinical trials in patients with type 1 and type 2 diabetes have demonstrated a reduced number of
episodes of nocturnal hypoglycaemia with insulin lispro compared to soluble human insulin. In some
studies, reduction of nocturnal hypoglycaemia was associated with increased episodes of daytime
hypoglycaemia.
The glucodynamic response to insulin lispro is not affected by renal or hepatic function impairment.
Glucodynamic differences between insulin lispro and soluble human insulin, as measured during a
glucose clamp procedure, were maintained over a wide range of renal function.
Insulin lispro has been shown to be equipotent to human insulin on a molar basis but its effect is more
rapid and of a shorter duration.
5.2 Pharmacokinetic properties












The pharmacokinetics of insulin lispro reflect a compound that is rapidly absorbed, and achieves peak
blood levels 30 to 70 minutes following subcutaneous injection. When considering the clinical
relevance of these kinetics, it is more appropriate to examine the glucose utilisation curves (as
discussed in 5.1).
Insulin lispro maintains more rapid absorption when compared to soluble human insulin in patients
with renal impairment. In patients with type 2 diabetes over a wide range of renal function the
pharmacokinetic differences between insulin lispro and soluble human insulin were generally
maintained and shown to be independent of renal function. Insulin lispro maintains more rapid
absorption and elimination when compared to soluble human insulin in patients with hepatic
impairment.
5.3 Preclinical safety data
In
in vitro
tests, including binding to insulin receptor sites and effects on growing cells, insulin lispro
behaved in a manner that closely resembled human insulin. Studies also demonstrate that the
dissociation of binding to the insulin receptor of insulin lispro is equivalent to human insulin. Acute,
one month and twelve month toxicology studies produced no significant toxicity findings.
Insulin lispro did not induce fertility impairment, embryotoxicity or teratogenicity in animal studies.
PHARMACEUTICAL PARTICULARS
m-
Cresol [3.15 mg/ml]
Glycerol
Dibasic sodium phosphate. 7H
2
O
Zinc oxide
Water for injections
Hydrochloric acid and sodium hydroxide maybe used to adjust pH to 7.0 – 7.8.
Humalog preparations should not be mixed with insulins produced by other manufacturers or with
animal insulin preparations.
Unused cartridge
3 years.
After cartridge insertion
28 days.
6.4 Special precautions for storage
Unused cartridge
Store in a refrigerator (2°C - 8°C). Do not freeze. Do not expose to excessive heat or direct sunlight.
After cartridge insertion
Store below 30°C. Do not refrigerate. The pen with the inserted cartridge should not be stored with the
needle attached.
6.5 Nature and contents of container
The solution is contained in type I flint glass cartridges, sealed with butyl or halobutyl disc seals and
plunger heads, and are secured with aluminium seals. Dimeticone or silicone emulsion may be used to
treat the cartridge plungers, and/or the glass cartridges.
Not all packs may be marketed.
5 x 3 ml Humalog cartridges for a 3 ml pen
2 x (5 x 3 ml) Humalog cartridges for a 3 ml pen
6.6 Special precautions for disposal and other handling
Any unused product or waste material should be disposed of in accordance with local requirements.
Instructions for use and handling
Humalog cartridges are to be used with a CE marked pen as recommended in the information provided
by the device manufacturer.
Inspect the Humalog solution. It should be clear and colourless. Do not use Humalog if it appears
cloudy, thickened, or slightly coloured or if solid particles are visible.
The following is a general description. The manufacturer’s instructions with each individual pen must
be followed for loading the cartridge, attaching the needle and administering the insulin injection.
Choose a site for injection.
Clean the skin as instructed.
Stabilise the skin by spreading it or pinching up a large area. Insert the needle as instructed.
Pull the needle out and apply gentle pressure over the injection site for several seconds. Do not
rub the area.
Using the outer needle cap, unscrew the needle and dispose of it safely.
Use of injection sites should be rotated so that the same site is not used more than
approximately once a month.
Do not mix insulin in vials with insulin in cartridges. See section 6.2.
MARKETING AUTHORISATION HOLDER
Eli Lilly Nederland B V., Grootslag 1-5, 3991 RA Houten, The Netherlands.
MARKETING AUTHORISATION NUMBERS
5 x 3 ml Humalog cartridges for a 3 ml pen
2 x (5 x 3 ml) Humalog cartridges for a 3 ml pen
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 30
th
April 1996
Date of last renewal: 30
th
April 2006
10. DATE OF REVISION OF THE TEXT
NAME OF THE MEDICINAL PRODUCT
Humalog Mix25 100 U/ml suspension for injection in vial
QUALITATIVE AND QUANTITATIVE COMPOSITION
One ml contains 100U (equivalent to 3.5mg) insulin lispro (recombinant DNA origin produced in
E.coli). Each container includes 10ml equivalent to 1000U insulin lispro.
Humalog Mix25 consists of 25% insulin lispro solution and 75% insulin lispro protamine suspension.
For a full list of excipients, see section 6.1.
Suspension for injection.
Humalog Mix25 is a white, sterile suspension.
4.1 Therapeutic indications
Humalog Mix25 is indicated for the treatment of patients with diabetes mellitus who require insulin
for the maintenance of normal glucose homeostasis.
4.2 Posology and method of administration
The dosage should be determined by the physician, according to the requirement of the patient.
Humalog Mix25 may be given shortly before meals. When necessary, Humalog Mix25 can be given
soon after meals. Humalog Mix25 should only be given by subcutaneous injection. Under no
circumstances should Humalog Mix25 be given intravenously.
Subcutaneous administration should be in the upper arms, thighs, buttocks, or abdomen. Use of
injection sites should be rotated so that the same site is not used more than approximately once a
month.
When administered subcutaneously care should be taken when injecting Humalog Mix25 to ensure
that a blood vessel has not been entered. After injection, the site of injection should not be massaged.
Patients must be educated to use the proper injection techniques.
The rapid onset and early peak of activity of Humalog itself is observed following the subcutaneous
administration of Humalog Mix25. This allows Humalog Mix25 to be given very close to mealtime.
The duration of action of the insulin lispro protamine suspension (BASAL) component of Humalog
Mix25 is similar to that of a basal insulin (NPH).
The time course of action of any insulin may vary considerably in different individuals or at different
times in the same individual. As with all insulin preparations, the duration of action of Humalog
Mix25 is dependent on dose, site of injection, blood supply, temperature, and physical activity.
Hypersensitivity to insulin lispro or to any of the excipients.
4.4 Special warnings and precautions for use
Under no circumstances should Humalog Mix25 be given intravenously.
Transferring a patient to another type or brand of insulin should be done under strict medical
supervision. Changes in strength, brand (manufacturer), type (regular, NPH, lente, etc.), species
(animal, human, human insulin analogue), and/or method of manufacture (recombinant DNA versus
animal-source insulin) may result in the need for a change in dosage.
Conditions which may make the early warning symptoms of hypoglycaemia different or less
pronounced include long duration of diabetes, intensified insulin therapy, diabetic nerve disease or
medications such as beta-blockers.
A few patients who have experienced hypoglycaemic reactions after transfer from animal-source
insulin to human insulin have reported that the early warning symptoms of hypoglycaemia were less
pronounced or different from those experienced with their previous insulin. Uncorrected
hypoglycaemic or hyperglycaemic reactions can cause loss of consciousness, coma, or death.
The use of dosages which are inadequate or discontinuation of treatment, especially in insulin-
dependent diabetics, may lead to hyperglycaemia and diabetic ketoacidosis; conditions which are
potentially lethal.
Insulin requirements may be reduced in the presence of renal impairment.
Insulin requirements may be reduced in patients with hepatic impairment due to reduced capacity for
gluconeogenesis and reduced insulin breakdown; however, in patients with chronic hepatic
impairment, an increase in insulin resistance may lead to increased insulin requirements.
Insulin requirements may be increased during illness or emotional disturbances.
Adjustment of dosage may also be necessary if patients undertake increased physical activity or
change their usual diet. Exercise taken immediately after a meal may increase the risk of
hypoglycaemia.
Administration of insulin lispro to children below 12 years of age should be considered only in case of
an expected benefit when compared to regular insulin.
4.5 Interaction with other medicinal products and other forms of interaction
Insulin requirements may be increased by substances with hyperglycaemic activity, such as oral
contraceptives, corticosteroids, or thyroid replacement therapy, danazol, beta
2
stimulants (such as
ritodrine, salbutamol, terbutaline).
Insulin requirements may be reduced in the presence of substances with hypoglycaemic activity, such
as oral hypoglycaemics, salicylates (for example, acetylsalicylic acid), sulphate antibiotics, certain
antidepressants (monoamine oxidase inhibitors, selective serotonin reuptake inhibitors), certain
angiotensin converting enzyme inhibitors (captopril, enalapril), angiotensin II receptor blockers, beta-
blockers, octreotide or alcohol.
Mixing Humalog Mix25 with other insulins has not been studied.
The physician should be consulted when using other medications in addition to Humalog Mix25.
4.6 Pregnancy and lactation
Data on a large number of exposed pregnancies do not indicate any adverse effect of insulin lispro on
pregnancy or on the health of the foetus/newborn.
It is essential to maintain good control of the insulin-treated (insulin-dependent or gestational
diabetes) patient throughout pregnancy. Insulin requirements usually fall during the first trimester and
increase during the second and third trimesters. Patients with diabetes should be advised to inform
their doctor if they are pregnant or are contemplating pregnancy. Careful monitoring of glucose
control, as well as general health, is essential in pregnant patients with diabetes.
Patients with diabetes who are breast-feeding may require adjustments in insulin dose, diet or both.
4.7 Effects on ability to drive and use machines
The patient’s ability to concentrate and react may be impaired as a result of hypoglycaemia. This may
constitute a risk in situations where these abilities are of special importance (e.g. driving a car or
operating machinery).
Patients should be advised to take precautions to avoid hypoglycaemia whilst driving, this is
particularly important in those who have reduced or absent awareness of the warning signs of
hypoglycaemia or have frequent episodes of hypoglycaemia. The advisability of driving should be
considered in these circumstances.
Hypoglycaemia is the most frequent undesirable effect of insulin therapy that a patient with diabetes
may suffer. Severe hypoglycaemia may lead to loss of consciousness, and in extreme cases, death. No
specific frequency for hypoglycaemia is presented, since hypoglycaemia is a result of both the insulin
dose and other factors e.g. a patient`s level of diet and exercise.
Local allergy in patients is common (1/100 to <1/10). Redness, swelling, and itching can occur at the
site of insulin injection. This condition usually resolves in a few days to a few weeks. In some
instances, this condition may be related to factors other than insulin, such as irritants in the skin
cleansing agent or poor injection technique. Systemic allergy, which is rare (1/10,000 to <1/1,000)
but potentially more serious, is a generalised allergy to insulin. It may cause a rash over the whole
body, shortness of breath, wheezing, reduction in blood pressure, fast pulse, or sweating. Severe cases
of generalised allergy may be life-threatening.
Lipodystrophy at the injection site is uncommon (1/1,000 to <1/100).
Insulins have no specific overdose definitions because serum glucose concentrations are a result of
complex interactions between insulin levels, glucose availability and other metabolic processes.
Hypoglycaemia may occur as a result of an excess of insulin activity relative to food intake and energy
expenditure.
Hypoglycaemia may be associated with listlessness, confusion, palpitations, headache, sweating and
vomiting.
Mild hypoglycaemic episodes will respond to oral administration of glucose or other sugar or
saccharated products.
Correction of moderately severe hypoglycaemia can be accomplished by intramuscular or
subcutaneous administration of glucagon, followed by oral carbohydrate when the patient recovers
sufficiently. Patients who fail to respond to glucagon must be given glucose solution intravenously.
If the patient is comatose, glucagon should be administered intramuscularly or subcutaneously.
However, glucose solution must be given intravenously if glucagon is not available or if the patient
fails to respond to glucagon. The patient should be given a meal as soon as consciousness is recovered.
Sustained carbohydrate intake and observation may be necessary because hypoglycaemia may recur
after apparent clinical recovery.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmaco-therapeutic group: ATC Code: A10A D04.
Humalog Mix25 is a premixed suspension consisting of insulin lispro (fast-acting human insulin
analogue) and insulin lispro protamine suspension (intermediate acting human insulin analogue).
The primary activity of insulin lispro is the regulation of glucose metabolism.
In addition, insulins have several anabolic and anti-catabolic actions on a variety of different tissues.
Within muscle tissue this includes increasing glycogen, fatty acid, glycerol and protein synthesis and
amino acid uptake, while decreasing glycogenolysis, gluconeogenesis, ketogenesis, lipolysis, protein
catabolism and amino acid output.
Insulin lispro has a rapid onset of action (approximately 15 minutes), thus allowing it to be given
closer to a meal (within zero to 15 minutes of the meal) when compared to regular insulin (30 to 45
minutes before). The rapid onset and early peak of activity of insulin lispro is observed following the
subcutaneous administration of Humalog Mix25. Humalog BASAL has an activity profile that is very
similar to that of a basal insulin (NPH) over a period of approximately 15 hours.
Clinical trials in patients with type 1 and type 2 diabetes have demonstrated reduced postprandial
hyperglycaemia with Humalog Mix25 compared to human insulin mixture 30/70. In one clinical study
there was a small (0.38 mmol/l) increase in blood glucose levels at night (3a.m.).
In the figure below the pharmacodynamics of Humalog Mix25 and BASAL are illustrated.
Humalog Mix25
Humalog Basal
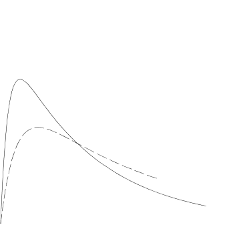




































The above representation reflects the relative amount of glucose over time required to maintain the
subject's whole blood glucose concentrations near fasting levels and is an indicator of the effect of
these insulins on glucose metabolism over time.
The glucodynamic response to insulin lispro is not affected by renal or hepatic function impairment.
Glucodynamic differences between insulin lispro and soluble human insulin, as measured during a
glucose clamp procedure, were maintained over a wide range of renal function.
Insulin lispro has been shown to be equipotent to human insulin on a molar basis but its effect is more
rapid and of a shorter duration.
In two 8-month open label crossover studies, type 2 diabetes patients who were either new to insulin
therapy or already using one or two injections of insulin, received 4 months of treatment with
Humalog Mix25 (used twice daily with metformin) and insulin glargine (used once daily with
metformin) in a randomised sequence. Detailed information can be found in the following table.
Insulin-Naive Patients
n = 78
Not Insulin-Naive Patients
n = 97
Mean total daily insulin dose at endpoint
Haemoglobin A1c –Reduction
1
1.30%
(mean at baseline = 8.7%)
1.00 %
(mean at baseline = 8.5%)
Reduction of the mean of combined
morning / evening two-hour postprandial
blood glucose
1
Reduction of the mean fasting blood
glucose
1
Incidence of hypoglycaemia at endpoint
1
from baseline to end of Humalog Mix25 treatment
2
in patients randomised to Humalog Mix25 during the first crossover period
5.2 Pharmacokinetic properties
The pharmacokinetics of insulin lispro reflect a compound that is rapidly absorbed, and achieves peak
blood levels 30 to 70 minutes following subcutaneous injection. The pharmacokinetics of insulin
lispro protamine suspension are consistent with those of an intermediate acting insulin such as NPH.
The pharmacokinetics of Humalog Mix25 are representative of the individual pharmacokinetic
properties of the two components. When considering the clinical relevance of these kinetics, it is more
appropriate to examine the glucose utilisation curves (as discussed in 5.1).
Insulin lispro maintains more rapid absorption when compared to soluble human insulin in patients
with renal impairment. In patients with type 2 diabetes over a wide range of renal function the
pharmacokinetic differences between insulin lispro and soluble human insulin were generally
maintained and shown to be independent of renal function. Insulin lispro maintains more rapid
absorption and elimination when compared to soluble human insulin in patients with hepatic
impairment.
5.3 Preclinical safety data
In
in vitro
tests, including binding to insulin receptor sites and effects on growing cells, insulin lispro
behaved in a manner that closely resembled human insulin. Studies also demonstrate that the
dissociation of binding to the insulin receptor of insulin lispro is equivalent to human insulin. Acute,
one month and twelve month toxicology studies produced no significant toxicity findings.
Insulin lispro did not induce fertility impairment, embryotoxicity or teratogenicity in animal studies.











PHARMACEUTICAL PARTICULARS
Protamine sulphate
m-
cresol [1.76 mg/ml]
Phenol [0.80 mg/ml]
Glycerol
Dibasic sodium phosphate.7H
2
O
Zinc oxide
Water for injections
Hydrochloric acid and sodium hydroxide may be used to adjust pH to 7.0 – 7.8.
Mixing Humalog Mix25 with other insulins has not been studied. In the absence of compatibility
studies, this medicinal product must not be mixed with other medicinal products.
6.4 Special precautions for storage
Do not freeze. Do not expose to excessive heat or direct sunlight.
Unopened vials
Store in a refrigerator (2°C - 8°C).
After first use
Store in a refrigerator (2°C - 8°C) or below 30°C.
6.5 Nature and contents of container
The suspension is contained in type I flint glass vials, sealed with butyl or halobutyl stoppers and
secured with aluminium seals. Dimeticone or silicone emulsion may have been used to treat the vial
stoppers.
1 x 10 ml Humalog Mix25 vial.
6.6 Special precautions for disposal and other handling
Any unused product or waste material should be disposed of in accordance with local requirements.
Instructions for use and handling
The vial is to be used in conjunction with an appropriate syringe (100 U markings).
Vials containing Humalog Mix25 should be rotated in the palms of the hands before use to resuspend
the insulin until it appears uniformly cloudy or milky. Do not shake vigorously as this may cause
frothing which may interfere with the correct measurement of the dose. The vials should be examined
frequently and should not be used if clumps of material are present or if solid white particles stick to
the bottom or wall of the vial, giving it a frosted appearance.
If using a new vial, flip off the plastic protective cap, but
do not
remove the stopper.
Draw air into the syringe equal to the prescribed Humalog Mix25 dose. Wipe the top of the vial
with an alcohol swab. Put the needle through rubber top of the Humalog Mix25 vial and inject
the air into the vial.
Turn the vial and syringe upside down. Hold the vial and syringe firmly in one hand.
Making sure the tip of the needle is in the Humalog Mix25, withdraw the correct dose into the
syringe.
Before removing the needle from the vial, check the syringe for air bubbles that reduce the
amount of Humalog Mix25 in it. If bubbles are present, hold the syringe straight up and tap its
side until the bubbles float to the top. Push them out with the plunger and withdraw the correct
dose.
Remove the needle from the vial and lay the syringe down so that the needle does not touch
anything.
Choose a site for injection.
Clean the skin as instructed.
Stabilise the skin by spreading it or pinching up a large area. Insert the needle and inject as
instructed.
Pull the needle out and apply gentle pressure over the injection site for several seconds. Do not
rub the area.
Dispose of the syringe and needle safely.
Use of the injection sites should be rotated so that the same is not used more than approximately
once a month.
MARKETING AUTHORISATION HOLDER
Eli Lilly Nederland B.V., Grootslag 1-5, 3991 RA Houten, The Netherlands.
MARKETING AUTHORISATION NUMBER
DATE OF FIRST AUTHORISATION/RENEWAL OF AUTHORISATION
Date of first authorisation: 30
th
April 1996
Date of last renewal: 30
th
April 2006
10. DATE OF REVISION OF THE TEXT
What Humalog BASAL 100 U/ml KwikPen, suspension for injection contains
-
The active substance is insulin lispro. Insulin lispro is made in the laboratory by a ‘recombinant
DNA technology’ process. It is a changed form of human insulin and so is different from other
human and animal insulins. Insulin lispro is closely related to human insulin which is a natural
hormone made by the pancreas.
The other ingredients are protamine sulphate, m-cresol, phenol, glycerol, dibasic sodium
phosphate 7H
2
O, zinc oxide and water for injection. Sodium hydroxide or hydrochloric acid
may have been used to adjust the acidity.
What Humalog BASAL 100 U/ml KwikPen, suspension for injection looks like and contents of
the pack
fever, infection or emotional stress.
Humalog BASAL 100 U/ml KwikPen, suspension for injection is a white, sterile suspension and
contains 100 units of insulin lispro in each millilitre (100 U/ml) suspension for injection. The insulin
lispro in Humalog BASAL is available in a suspension together with protamine sulphate. Each
Humalog BASAL KwikPen contains 300 units (3 millilitres). The Humalog BASAL KwikPen comes
in a pack of 5 pre-filled pens or a multipack of 2 x 5 pre-filled pens. Not all pack sizes may be
marketed. The Humalog BASAL in your KwikPen is the same as the Humalog BASAL, which
comes in separate Humalog BASAL cartridges. The KwikPen simply has a built in cartridge. When
the KwikPen is empty you cannot use it again.
Marketing Authorisation Holder and Manufacturer
Humalog BASAL 100 U/ml KwikPen, suspension for injection is made by:
•
Lilly France S.A.S., Rue du Colonel Lilly, 67640 Fegersheim, France
Lilly Pharma Fertigung und Distribution GmbH & Co. KG, Teichweg 3, 35396 Giessen,
Germany.
The product licence is held by: Eli Lilly Nederland B.V., Grootslag 1-5, 3991 RA Houten, The
Netherlands.
For any information about this medicinal product, please contact the local representative of the
Marketing Authorisation Holder:
Belgique/België/Belgien
Eli Lilly Benelux S.A.
Tél/Tel: + 32-(0)2 548 84 84
Luxembourg/Luxemburg
Eli Lilly Benelux S.A.
Tél/Tel: + 32-(0)2 548 84 84
България
ТП "Ели Лили Недерланд" Б.В. - България
тел. + 359 2 491 41 40
Magyarország
Lilly Hungária Kft.
Tel: + 36 1 328 5100
Česká republika
ELI LILLY ČR, s.r.o.
Tel: + 420 234 664 111
Malta
Charles de Giorgio Ltd.
Tel: + 356 25600 500
Danmark
Eli Lilly Danmark A/S
Tlf: +45 45 26 6100
Nederland
Eli Lilly Nederland B.V.
Tel: + 31-(0) 30 60 25 800
Deutschland
Lilly Deutschland GmbH
Tel. + 49-(0) 6172 273 2222
Norge
Eli Lilly Norge A.S.
Tlf: + 47 22 88 18 00
Eesti
Eli Lilly Holdings Limited Eesti filiaal
Tel:
+
3726441100
Österreich
Eli Lilly Ges. m.b.H.
Tel: + 43-(0) 1 711 780
Ελλάδα
ΦΑΡΜΑΣΕΡΒ-ΛΙΛΛΥ Α.Ε.Β.Ε.
Τηλ: +30 210 629 4600
Polska
Eli Lilly Polska Sp. z o.o.
Tel: +48 (0) 22 440 33 00
España
Lilly S.A.
Tel: + 34-91 663 50 00
Portugal
Lilly Portugal - Produtos Farmacêuticos, Lda
Tel: + 351-21-4126600
France
Lilly France S.A.S.
Tél: +33-(0) 1 55 49 34 34
România
Eli Lilly România S.R.L.
Tel: + 40 21 4023000
Ireland
Eli Lilly and Company (Ireland) Limited
Tel: + 353-(0) 1 661 4377
Slovenija
Eli Lilly farmacevtska družba, d.o.o.
Tel: +386 (0) 1 580 00 10
Ísland
Icepharma hf.
Sími + 354 540 8000
Slovenská republika
Eli Lilly Slovakia, s.r.o.
Tel: + 421 220 663 111
Italia
Eli Lilly Italia S.p.A.
Tel: + 39- 055 42571
Suomi/Finland
Oy Eli Lilly Finland Ab
Puh/Tel: + 358-(0) 9 85 45 250
Κύπρος
Phadisco Ltd
Τηλ: +357 22 715000
Sverige
Eli Lilly Sweden AB
Tel: + 46-(0) 8 7378800
Latvija
Eli Lilly Holdings Limited pārstāvniecība Latvijā
Tel:
+
371 67364000
United Kingdom
Eli Lilly and Company Limited
Tel: + 44-(0) 1256 315999
Lietuva
Eli Lilly Holdings Limited atstovybė
Tel. +370 (5) 2649600
This leaflet was last approved in
{MM/YYYY}.
Please see manual text later.
Detailed information on this medicine is available on the European Medicines Agency (EMEA) web
site: http://www.emea.europa.eu/.
KwikPen™
Insulin delivery device
The
KwikPen™
is designed for ease of use. It is a disposable pen containing 3 mL (300 units) of U-
100 insulin. You can inject from 1 to 60 units of insulin in one injection. You can dial your dose one
unit at a time. If you dial too many units, you can correct the dose without wasting any insulin.
Before using KwikPen, read the entire manual completely and follow the directions carefully. If you
do not follow these directions completely, you may get too much or too little insulin.
Your KwikPen must be used only for your injections. Do not share your pen or your needles. Use a
new needle for each injection.
DO NOT USE your pen if any part appears broken or damaged. Always carry an extra pen in case
yours is lost or damaged.
This pen is not recommended for use by the blind or visually impaired persons without the assistance
of a person trained in the proper use of the product.
•
Read and follow the directions provided in the insulin package leaflet .
•
Check the label on your pen before each injection for the expiration date and to make sure you are
using the correct type of insulin. Do not remove the pen label.
Note: The colour of your KwikPen Dose Knob matches the insulin-specific colour band shown
on the Pen Label. In this user manual, the Dose Knob is shown in grey. The Pen Body is
blue to indicate that it contains a Humalog family of products.
•
Your healthcare professional has prescribed the most appropriate type of insulin for you.
Any
changes in insulin therapy should be made only under medical supervision.
•
KwikPen
is recommended
for use with Becton, Dickinson and Company (BD) pen needles.
•
Be sure the needle is completely attached to the pen before use.
Dose Knob Colour-code key:













•
Keep these directions for future reference.
Frequently Asked Questions about Preparing KwikPen
•
What should my insulin look like?
Some insulins are cloudy while others are clear, be sure to
refer to your insulin package leaflet for the appearance of your specific insulin.
•
What do I do if my dose is higher than 60 units?
If your dose is higher than 60 units of insulin,
multiple injections will be required or you may contact your healthcare professional.
•
Why should I use a new needle for each injection?
If needles are reused, you may get the
wrong amount of insulin, a clogged needle, a jammed pen, or an infection, because sterility is not
ensured.
•
What should I do if I am not sure how much insulin remains in my cartridge?
Hold the pen
with the needle end pointing down. The scale on the clear Cartridge Holder shows an estimate of
the number of units remaining.
These numbers should NOT be used for measuring an insulin
dose.
•
What should I do, if I can’t remove the Pen Cap?
Pull the cap straight off. If you are having
difficulty removing the cap, gently twist the cap back and forth to realign, and then pull the cap
straight off.
•
Prime every time
. The pen must be primed to a stream of insulin before each injection to make
sure the pen is ready to dose.
•
If you do not prime
to a stream, you may get too much or too little insulin.
Frequently Asked Questions about Priming
•
Why should I prime my pen before each dose?
1.
Ensures that the pen is ready to dose.
2.
Confirms that a stream of insulin comes out of the tip of the needle when you push the
Dose Knob in.
3.
Removes air that may collect in the needle or insulin cartridge during normal use.
•
What should I do if I cannot completely push in the Dose Knob when priming KwikPen?
1.
Attach a new needle.
2.
Prime the pen.
•
What should I do if I see an air bubble in the cartridge?
You need to prime the pen.
Remember, do not store the pen with the needle attached as this may cause air bubbles to collect in
the insulin cartridge. A small air bubble will not affect your dose and you can continue to take
your dose as usual.
Important Notes
•
Follow the instructions for sanitary injection technique recommended by your healthcare
professional.
•
Make sure you receive your complete dose by pushing and holding the dose knob in and
count to
5 slowly
before removing the needle. If insulin is leaking from the pen you may not have held it in
your skin long enough.
•
The pen will not allow you to dial more than the number of units left in the pen.
•
If your dose is greater than the number of units left in the pen, you may either inject the amount
remaining in your current pen and then use a new pen to complete your dose, OR inject the full
dose with a new pen.
•
Do not attempt to inject your insulin by
turning
the Dose Knob. You will NOT receive your
insulin by turning the Dose Knob.
You must PUSH the Dose Knob straight in for the dose to
be delivered.
•
Do not attempt to change the dose while injecting.
•
The directions regarding needle handling are not intended to replace local, healthcare professional
and/or institutional policies.
•
Remove the needle after completing each injection.
Frequently Asked Questions about Injecting Your Dose
•
Why is it difficult to push the Dose Knob when I try to inject?
1.
Your needle may be clogged. Try attaching a new needle.
When you do this you may see
insulin come out of the needle. Then prime the pen.
2.
Pressing the Dose Knob quickly may make the Dose Knob harder to push. Pressing the
Dose Knob more slowly may make it easier.
3.
Using a larger diameter needle will make it easier to push the Dose Knob during your
injection. See your healthcare professional to determine which needle size is best for you.
4.
If the Dose Knob continues to be difficult to push after following the steps above, you
may need a new pen.
•
What should I do if my KwikPen is jammed?
Your pen is jammed if it is difficult to inject a
dose or dial a dose. To clear the jam:
1.
Attach a new needle. When you do this you may see insulin come out of the needle.
2.
Prime the pen.
3.
Dial your dose and inject.
Do not attempt to lubricate your pen as this may damage the mechanism.
The Dose Knob may become harder to push, if foreign material (dirt, dust, food, insulin, or other
liquids) get inside the pen. Avoid getting foreign material inside the pen.
•
Why is insulin leaking from the needle after I finished my dose?
You may have removed the
needle from your skin too quickly.
1.
Make sure you see a 0 in the Dose Window.
2.
For the next dose,
push and hold
the Dose Knob in and
count to 5 slowly
before
removing the needle.
•
What should I do if I dial a wrong dose (too high or too low)?
Turn the Dose Knob backward
or forward to correct the dose.
•
What should I do if I see insulin leaking from the pen needle while dialling the dose or
correcting the dose?
Do not inject the dose because you may not get your complete dose. Dial
•
What should I do if my dose is dialled and the Dose Knob is accidentally pushed in without a
needle attached?
1.
Dial back to zero.
2.
Attach a new needle.
3.
Prime the pen.
4.
Dial your dose and inject.
the pen down to zero and prime the pen again (see
Routine Use
section
“Priming KwikPen” steps
2B thru 2D). Dial your dose and inject.
•
What should I do if my full dose cannot be dialled?
The pen will not allow you to dial a dose
greater than the number of insulin units remaining in the cartridge. For example, if you need 31
units and only 25 units remain in the cartridge you will not be able to dial past 25. Do not attempt
to dial past this point. If a partial dose remains in the pen you may either:
1.
Give the partial dose and then give the remaining dose using a new pen.
or
2.
Give the full dose with a new pen.
•
Why can I not dial the dose to use the small amount of insulin that remains in my cartridge?
The pen is designed to deliver at least 300 units of insulin. The pen design prevents the cartridge
from being completely emptied because the small amount of insulin that remains cannot be
delivered accurately.
•
Before the first use store your pen in a refrigerator (2°C – 8°C). Do not use a pen if it has been
frozen.
•
Keep your pen in use at room temperature and away from heat and light.
•
Do not store the pen with the needle attached. If the needle is left on, insulin may leak from the
pen, insulin may dry inside the needle causing the needle to clog, or air bubbles may form in the
cartridge.
•
Refer to the package leaflet for complete insulin storage instructions.
•
After first use the pen should not be used beyond the time specified in the insulin package leaflet.
•
Dispose of used needles in a puncture-resistant container or as directed by your healthcare
professional.
•
Dispose of used pens as instructed by your healthcare professional and without the needle
attached.
•
Keep the pen out of the reach of children.
If you have any questions or problems with your KwikPen
,
contact your healthcare professional
for assistance.
Routine Use Follow these instructions for each injection
1. Preparing KwikPen
Pull Pen Cap straight
off to remove. Do not
twist the cap.
Do not
remove the Pen
Label.
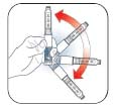
Gently
roll the
pen ten
times
and
invert
the pen
ten
times.
The
insulin
should
look
evenly
mixed.
Remove paper tab
from Outer Needle
Shield.
Push capped needle
straight
onto the
pen.
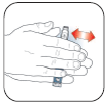
Use an alcohol swab to
wipe the Rubber Seal
on the end of the
Cartridge Holder.
Screw needle on
until secure.
Be sure to check your
insulin for:
•
Type
•
Expiration date
•
Appearance
Caution: Always
read the Pen Label
to ensure you are
using the correct
insulin type.

2. Priming KwikPen
Caution: If you do not prime to a stream before each injection, you may get too much or too little
insulin.
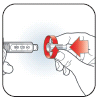
A.
•
With
needle
pointed up,
push Dose
Knob in
until it
stops and 0
is seen in
the Dose
Window.
•
Hold Dose
Knob in
and
count
to 5
slowly.
•
Priming is
complete
when a
stream of
insulin
appears
from the
needle tip.
•
If a stream
of insulin
does not
appear,
repeat
priming
steps 2B
thru 2D up
to four
times.
Pull off Outer
Needle Shield.
Do not
throw
away.
Dial 2 Units by
turning the Dose
Knob.
Tap Cartridge
Holder to collect
air at top.
Pull off Inner
Needle Shield
and throw away.
Note: If you do
not see a
stream of
insulin from
the needle tip
and dialling the
pen is more
difficult,
change the
needle and
prime the pen.






3. Injecting Your Dose
A.
Turn Dose Knob
to the number of
units you need to
inject. If you dial
too many units,
you can correct
the dose by
dialling
backwards.
Insert needle into
skin using
injection
technique
recommended by
your healthcare
professional.
To deliver the full dose,
hold Dose Knob in and
count to 5 slowly
.
Remove needle from
skin.
Carefully replace
the Outer Needle
Shield.
Unscrew the capped
needle and throw away
as directed by your
healthcare
professional.
Place your thumb
on the Dose Knob
and push firmly
until the Dose
Knob stops
moving.
Note: Check to make
sure you see 0 in the
Dose Window to
confirm you received
the complete dose.
Note: Remove the
needle after each
injection to keep
air out of the
cartridge.
Do not store the
pen with the
needle attached.
Replace Pen Cap by
aligning the Cap Clip
with the Dose Window
and pushing straight
on.

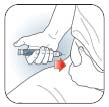

Note: The pen will not allow you to dial
more than the number of units left in the pen.
The
even
numbers are
printed on the
dial. The
odd
numbers, after the
number one, are
shown as full
lines.

Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/humalog.html
Copyright © 1995-2021 ITA all rights reserved.
|



























































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































