Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
InductOs 12 mg kit for implant
QUALITATIVE AND QUANTITATIVE COMPOSITION
One vial contains 12 mg dibotermin alfa*. After reconstitution, InductOs contains 1.5 mg/ml
dibotermin alfa.
*dibotermin alfa (recombinant human Bone Morphogenetic Protein-2; rhBMP-2) is a human protein
derived from a recombinant Chinese Hamster Ovary (CHO) cell line.
For a full list of excipients, see section 6.1.
The kit consists of a white powder for solution, a clear colourless solvent and a white matrix.
4.1 Therapeutic indications
InductOs is indicated for single-level (L4
– S1) anterior lumbar spine fusion as a substitute for
autogenous bone graft in adults with degenerative disc disease who have had at least 6 months of
non-operative treatment for this condition.
InductOs is indicated for the treatment of acute tibia fractures in adults, as an adjunct to standard care
using open fracture reduction and intramedullary unreamed nail fixation.
4.2 Posology and method of administration
InductOs should be used by an appropriately qualified surgeon.
The directions for preparation for each kit should be followed exactly, using the appropriate amount of
InductOs for the intended indication.
InductOs is prepared immediately prior to use from a kit containing all necessary components. Once
prepared, InductOs contains dibotermin alfa at a concentration of 1.5 mg/ml (12 mg per vial ).
InductOs should not be used in concentrations higher than 1.5 mg/ml (see section 4.9).
There is very limited experience of the efficacy and safety of the medicinal product in the elderly
(>65 years of age).
Paediatric use is not recommended until further data become available.
1. Using sterile technique, place one syringe, one needle and the matrix inner package in the sterile
field.
2. Disinfect the stoppers of the dibotermin alfa and solvent vials.
3. Using the remaining syringe and needle from the kit, reconstitute the dibotermin alfa vial with
8.4 ml of solvent. Slowly inject the solvent into the vial containing the lyophilised dibotermin alfa.
Swirl the vial gently to aid reconstitution. Do not shake. Discard syringe and needle after use.
4. Disinfect the stopper of the reconstituted dibotermin alfa vial.
5. Peel open the interior package of the matrix and leave the matrix in its tray.
6. Using aseptic transfer technique and the syringe and needle from step 1, withdraw 8 ml of the
reconstituted dibotermin alfa solution from the vial in the non-sterile field, holding up the inverted
vial to facilitate withdrawal.

7. Leaving the matrix in its tray, UNIFORMLY distribute the dibotermin alfa solution on the matrix,
following the pattern in the figure below.
8. Wait a MINIMUM of 15 minutes before using the prepared InductOs product. The product must
be used within 2 hours after preparation.
To prevent overloading the matrix, it is important to reconstitute the dibotermin alfa and to wet the
entire sponge as described above.
9. Follow instructions relevant to the planned surgery – anterior lumbar spine fusion or acute tibia
fracture repair.
Instructions for use in anterior lumbar spine fusion surgery
InductOs should not be used alone for this indication, but must be used with the LT-CAGE Lumbar
Tapered Fusion Device.
Failure to follow the product preparation instructions for InductOs may compromise its safety and
effectiveness. Care and caution should be used to prevent overfilling of the construct and/or
intervertebral space (see section 4.4).
Cut the wetted matrix of InductOs into 6 equal (approximately 2.5 x 5 cm) pieces. During cutting and
handling, avoid excessive fluid loss from InductOs. Do not squeeze.
The number of pieces of InductOs required is determined by the size of the LT-CAGE Lumbar
Tapered Fusion Device being used. Using the table below, identify the number of 2.5 x 5 cm pieces of
InductOs required for the size of LT-CAGE Lumbar Tapered Fusion Device.

LT-CAGE Lumbar Tapered Fusion Device
Size
(lead diameter x length)
Number of 2.5 x 5 cm pieces of InductOs per
LT-CAGE Lumbar Tapered Fusion Device
Using forceps to avoid excessive squeezing, carefully roll the required number of InductOs pieces for
each LT-CAGE device and insert each roll into the matching LT-CAGE Lumbar Tapered Fusion
Device, as shown in the figure below.
For instructions of implantation of the LT-CAGE Lumbar Tapered Fusion Device, please refer to the
package leaflet for the LT-CAGE device.
Once InductOs and the LT-CAGE device are implanted, do not irrigate the wound region.
If a surgical drain is required, place the drain remotely from the implantation site or, preferably, one
layer superficially to the implantation site.
Instructions for use in acute tibia fractures
•
Achieve definitive fracture reduction, fixation, and hemostasis prior to InductOs implantation.
•
InductOs does not provide mechanical stability and should not be used to fill spaces in the presence
of compressive forces.
•
Fold or cut InductOs as needed prior to implantation. During handling, avoid excessive
fluid loss
from InductOs. Do not squeeze. If the surgical setting requires that only a portion of the product is
needed, first prepare the entire InductOs product (following steps 1-8 above), cut the product to the
desired size, and discard the unused portion.
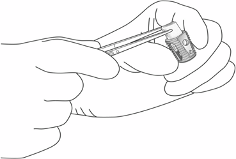











InductOs is implanted after the completion of standard fracture and wound management, i.e., at the
time of soft-tissue closure. The number of InductOs kits to use and the volume of InductOs to be
implanted are determined by the fracture anatomy and the ability to close the wound without overly
packing or compressing the product. Generally, each fracture site is treated with the contents of one
kit. The maximum dosage of InductOs is limited to 2 kits. To the extent possible, the accessible surface
area of the fracture (fracture lines and defects) should be covered with InductOs. Place InductOs so
that it bridges the fracture region and makes good contact with the major proximal and distal
fragments. It is not necessary to overlay the contents of multiple kits to achieve the desired effect.
During implantation, use forceps to handle InductOs to avoid excessive loss of fluid.
InductOs may be placed into a void (loosely packed), folded, rolled, or wrapped, as the geometry of
the fracture requires. Do not squeeze.
Once InductOs is implanted, do not irrigate the wound.
If a surgical drain is required, place the drain remotely from the implantation site or, preferably, one
layer superficially to the implantation site.
In order to achieve maximum potential efficacy, it is important to achieve complete soft-tissue
coverage of InductOs following its implantation.
InductOs is contraindicated for patients with:
•
Hypersensitivity to the active substance or to any of the excipients
•
Skeletal immaturity
•
Any active malignancy or patient undergoing treatment for a malignancy
•
An active infection at the operative site
•
Persistent compartment syndrome or neurovascular residua of compartment syndrome
•
Pathological fractures such as those observed in (but not limited to) Paget’s disease or in metastatic
bone
4.4 Special warnings and precautions for use
Failure to follow the product preparation instructions for InductOs may compromise its safety and
effectiveness. Care and caution should be used to prevent overfilling of the construct and/or
intervertebral space.
Localised oedema associated with the use of InductOs has been reported in patients undergoing
cervical spine surgery. The oedema was delayed in onset and, in some cases, severe enough to result in
airway compromise. The safety and efficacy of InductOs in cervical spine surgery have not been
established, and InductOs should not be used in this condition.
Formation of fluid collections (pseudocysts, localised oedema, implant site effusion), sometimes
encapsulated, in some cases resulting in nerve compression and pain, has been reported in patients
undergoing spine surgery associated with the use of InductOs. Many of these reports have occurred
when InductOs was used in unapproved approaches/devices or in a manner inconsistent with the
instructions for use. Clinical intervention (aspiration and/or surgical removal) may be required if
symptoms persist (see section 4.8).
There are no data on the efficacy and safety of the product in concomitant use with bone graft.
In the absence of any experience, the repeated use of the medicinal product is not recommended.
Nerve compression associated with ectopic bone formation and InductOs use has been reported.
Additional surgical intervention may be required.
InductOs can cause initial resorption of surrounding trabecular bone. Therefore, in the absence of
clinical data, the product should not be used for direct applications to trabecular bone when transient
bone resorption may create a risk of bone fragility. When InductOs was used with the LT-CAGE
device (section 4.2) in clinical trials for anterior lumbar spine fusion, the frequency and severity of
resorption of bone as evidenced by radiolucencies and/or device migration was similar to that observed
for patients treated with autogenous bone graft.
Device migration can occur after the use of InductOs in spinal fusion surgery, which may necessitate
surgical revision (see section 4.8).
Use of InductOs may cause heterotopic ossification in the surrounding tissues, which can result in
complications. Exuberant bone formation at the site of implantation and ectopic bone formation have
been observed.
InductOs should not be used in patients with history or clinical suspicion of malignancy at the site of
application (see section 4.3).
The safety and efficacy of the use of InductOs in patients with known autoimmune disease have not
been established. These autoimmune diseases include rheumatoid arthritis, systemic lupus
erythematosus, scleroderma, Sjögren's syndrome and dermatomyositis/polymyositis.
The safety and efficacy of InductOs have not been demonstrated in patients with metabolic bone
diseases.
No studies have been performed in patients with hepatic or renal impairment.
Both dibotermin alfa and bovine Type I collagen have been found to elicit immune responses in
patients.
Anti-dibotermin alfa antibodies:
In anterior lumbar spine fusion studies, 0.7% of patients receiving
InductOs developed antibodies versus 0.8% of patients receiving autogenous bone graft. In acute tibia
fracture studies, 4.4% of patients receiving InductOs developed antibodies versus 0.6% in the control
group.
Anti-bovine Type I collagen antibodies:
In anterior lumbar spine fusion studies, 19% of patients
receiving InductOs developed antibodies to bovine Type I collagen versus 13% of patients receiving
autogenous bone graft. In acute tibia fracture studies,15.7% of patients receiving InductOs developed
antibodies to bovine Type I collagen versus 11.8% of control patients. In either of the two indications,
no patients who tested positive for anti-bovine Type I collagen antibodies developed antibodies to
human Type I collagen.
Although no clear association with clinical outcome or undesirable effects could be observed in
clinical studies, the possibility of developing neutralising antibodies or hypersensitivity-type reactions
cannot be excluded. Special consideration of risks and benefits should be given for patients who have
previously received injectable collagen (see section 4.3). The possibility of an immune response to the
product should be evaluated in cases where an undesirable effect with immunological background is
suspected.
Special warnings and precautions for use specific to anterior lumbar spine fusion
The safety and efficacy of InductOs have not been established in the following conditions:
•
used with spinal implants other than the LT-CAGE device
•
implanted at locations other than L4 – S1
in the lower lumbar spine
•
used in surgical techniques other than anterior open or anterior laparoscopic approaches
When degenerative disc disease was treated by a posterior lumbar interbody fusion procedure with
cylindrical threaded cages and dibotermin alfa, posterior bone formation was observed in some
instances.
Special warnings and precautions for use specific to acute tibia fractures
InductOs is intended for use in patients with the following:
•
adequate fracture reduction and stabilization to ensure mechanical stability
•
adequate neurovascular status (e.g., absence of compartment syndrome, low risk of amputation)
•
adequate hemostasis (providing a relatively dry implantation site)
•
absence of large segmental defect repair of long bones, in which significant soft tissue
compression can occur
The implant may only be administered to the fracture site under adequate vision and with utmost care
(see section 4.2).
Efficacy information in tibia fracture is available only from controlled clinical trials in which open
tibial fractures were treated using intramedullary nail fixation (see section 5.1). In a clinical study in
which the intramedullary canal was reamed to cortical chatter, an increased rate of infection was
observed in the InductOs-treated group versus the standard of care control group (see section 4.8). The
use of InductOs with reamed nails in open tibial fracture repair is not recommended.
InductOs does not provide mechanical stability and should not be used to fill space in the presence of
compressive forces. Long-bone fracture and soft-tissue management procedures should be based on
standard practice, including control of infection.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed. As dibotermin alfa is a protein and has not been identified
in the general circulation, it is an unlikely candidate for pharmacokinetic drug-drug interactions.
Information from clinical studies in acute tibia fractures indicated that the use of InductOs in patients
receiving glucocorticoids was not associated with any apparent adverse effect. In preclinical studies,
concurrent administration of glucocorticoids depressed bone repair (measured as a % change from
control), but the effects of InductOs were not altered.
In acute tibia fracture clinical trials, more InductOs patients receiving concomitant NSAIDs for 14
consecutive days experienced mild or moderate adverse events related to wound healing (e.g., wound
drainage) than InductOs patients not taking NSAIDs. Although patient outcome was not affected, an
interaction between NSAIDs and InductOs cannot be excluded.
4.6 Pregnancy and lactation
There are no adequate data from the use of dibotermin alfa in pregnant women.
Studies in animals have shown reproductive toxicity (see section 5.3). The potential risk for humans is
unknown.
Animal studies have been conducted that cannot rule out effects of anti-dibotermin alfa antibodies on
embryo-foetal development (see section 5.3).
Due to the unknown risks to the foetus associated with
the potential development of neutralising antibodies to dibotermin alfa, InductOs should not be used
during pregnancy unless clearly necessary (see section 4.4). Women of childbearing potential should
be advised to use effective contraception up to at least 12 months after treatment.
It is unknown whether dibotermin alfa is excreted in human breast milk. The excretion of dibotermin
alfa has not been studied in animals. Lactation is not recommended during treatment with InductOs.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed, but since
InductOs has no systemic effect, it is not likely to interfere with the ability to drive or use machinery
.
Over 1,490 patients have been evaluated in clinical studies, of which more than 955 received InductOs
treatment. In the long-bone fracture studies, over 418 patients received InductOs. In the anterior
lumber spine fusion studies, over 288 patients received InductOs.
There have been post-marketing reports of localised oedema in patients undergoing cervical spine
surgery associated with the use of InductOs. The oedema was delayed in onset and, in some cases,
severe enough to result in airway compromise (see section 4.4).
There have been post-marketing reports of formation of fluid collections (pseudocysts, localised
oedema, implant site effusion), sometimes encapsulated, in some cases resulting in nerve compression
and pain in patients undergoing spine surgery with InductOs (see section 4.4).
Nerve compression associated with ectopic bone formation has been reported in patients undergoing
spine surgery with InductOs (see section 4.4).
Radiculitis after spinal fusion surgery can occur in patients who have received InductOs.
Device migration can occur after the use of InductOs in spinal fusion surgery, which may necessitate
surgical revision (see section 4.4). In some cases device migration has been reported in association
with bone resorption and formation of fluid collections (pseudocysts, localised oedema, implant site
effusion).
Placement of InductOs can cause initial resorption of trabecular bone (see sections 4.4 and 5.1).
Undesirable effects specific to use in anterior lumbar spine fusion
The undesirable effects observed in anterior lumbar spine fusion patients were generally representative
of the morbidity associated with spine fusion using autogenous bone graft taken from the iliac crest.
Very common (≥1/10) undesirable effects: accidental injury, neuralgia, back pain and bone disorder,
were similar in both control and InductOs treatment groups.
Undesirable effects specific to use in acute tibia fractures
The undesirable effects observed in long-bone fracture patients were generally representative of the
morbidity associated with either orthopaedic trauma or the surgical procedure.
Localised infection specific to the fractured limb occurred in >1/10 patients in a clinical study in which
the intramedullary canal was reamed to cortical chatter. An increased rate of infection was observed in
the InductOs-treated group versus the standard of care control group (19% versus 9%, respectively; see
section 4.4). For use with unreamed nails, estimated rates of infection were similar between treatment
groups in a study (21% versus 23%, respectively).
Common (≥1/100 to <1/10 ) undesirable effects were observed with equal incidence in control and
InductOs treatment groups, with the following four exceptions, which were observed significantly
more frequently in the InductOs treatment group than in the control group:
•
blood amylase increased (without overt signs of pancreatitis in InductOs-treated patients)
•
tachycardia
•
hypomagnesemia
•
headache
Use of InductOs in patients undergoing cervical spine surgery in concentrations or amounts greater
than those recommended in section 4.2 for the approved indications has been associated with reports of
localised oedema (see section 4.4).
In the case of patients receiving concentrations or amounts greater than those recommended, treatment
should be supportive.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Bone Morphogenetic Proteins; ATC code: M05BC01
Dibotermin alfa is an osteoinductive protein that results in the induction of new bone tissue at the site
of implantation. Dibotermin alfa binds to receptors on the surface of mesenchymal cells and causes
cells to differentiate into cartilage- and bone-forming cells. The differentiated cells form trabecular
bone as the matrix is degraded, with vascular invasion evident at the same time. The bone formation
process develops from the outside of the implant towards the center, until the entire InductOs implant
is replaced by trabecular bone.
Remodeling of the surrounding trabecular bone occurs in a manner that is consistent with the
biomechanical forces placed on it. Placement of InductOs into trabecular bone resulted in transient
resorption of the bone surrounding the implant, followed by replacement with new, more dense bone.
The ability of InductOs to support bone remodeling may be responsible for the biological and
biomechanical integration of the new bone induced by InductOs with that of the surrounding bone.
Radiographic, biomechanical, and histologic evaluation of the induced bone indicates that it functions
biologically and biomechanically as native bone. Furthermore, preclinical studies have indicated that
the bone induced by InductOs, if fractured, can repair itself in a manner indistinguishable from native
bone.
Preclinical studies have suggested that bone formation initiated by InductOs is a self-limiting process,
forming a well-defined volume of bone. This self-limitation is likely due to the loss of dibotermin alfa
from the implant site, as well as the presence of BMP inhibitors in the surrounding tissues. In addition,
several preclinical studies indicate that there is a negative feedback mechanism at the molecular level
that limits bone induction by BMPs.
Clinical pharmacology studies demonstrate that the matrix alone is not osteoinductive and is no longer
present in biopsies taken as early as 16 weeks post-implantation.
Pharmacodynamic information specific to anterior lumbar spine fusion studies
The efficacy and safety of InductOs were demonstrated in a randomised, controlled, multicenter,
non-inferiority study of 279 patients aged 19–78 years undergoing an open anterior lumbar interbody
fusion procedure. Patients had received at least six months of non-operative treatment prior to
treatment with InductOs for anterior lumbar spine fusion. Patients were randomised to receive the
LT-CAGE Lumbar Tapered Fusion Device filled with either InductOs or autogenous bone graft taken
from the iliac crest.
At 24 months post-operation, InductOs was demonstrated to be statistically non-inferior to autogenous
bone graft. The success rate for radiologically determined fusion was 94.4% for InductOs versus
88.9% (95% two-sided CI of the difference: -1.53, 12.46) for autogenous bone graft. For pain and
disability (Oswestry score), the success rate was 72.9% versus 72.5% (95% two-sided CI of the
difference: -11.2, 12.0). A single, multi-component endpoint, known as overall success was the
primary variable of the study. Overall success consists of the following primary efficacy and safety
considerations:
1.
Radiographically demonstrated fusion
2.
Oswestry pain/disability improvement
3.
Maintenance or improvement in neurological status
4.
No Grade 3 or 4 adverse event classified as implant-associated or implant/surgical
procedure-associated
5.
No additional surgical procedure performed that was classified as a “failure”
At 24 months post-operation, the overall success rate was 57.5% for InductOs versus 55.8% (95%
two-sided CI of the difference: -10.72, 14.01) for autogenous bone graft.
An additional, non-comparative study of 134 patients who received anterior lumbar interbody fusion
procedures via a laparoscopic surgical technique yielded similar success rates of 92.9% for fusion,
85.6% for pain and disability, and 90.3% for neurological status. The study confirmed the applicability
of anterior lumbar spine fusion using InductOs via laparoscopic surgical implantation techniques.
Pharmacodynamic information specific to acute tibia fracture studies
The efficacy of InductOs was demonstrated in a multinational, randomized, controlled, single-blind
study of 450 patients (age range 18 to 87 years; 81% male) with open tibial shaft fractures requiring
surgical management. Patients received (in a 1:1:1 ratio) standard care (control group) consisting of
intramedullary (IM) nail fixation and routine soft-tissue management, standard care plus InductOs
0.75 mg/ml, or standard care plus InductOs 1.5 mg/ml. Patients were followed for 12 months after
soft-tissue closure.
In the acute tibia fracture pivotal trial, InductOs increased the probability of fracture healing; patients
treated with InductOs 1.5 mg/ml had a 44% reduced risk for treatment failure (secondary intervention
to promote fracture healing) compared with patients in the standard-care group (RR = 0.56; 95%
CI = 0.40 to 0.78). These results were independently corroborated by a radiology panel blinded to
treatment. The number of secondary and subsequent interventions was significantly reduced for the
InductOs patients, particularly with regard to more invasive interventions, such as bone graft and
exchange nailing (P=0.0326).
The proportion of patients healed after treatment with InductOs 1.5 mg/ml was significantly higher at
all visits from 10 weeks to 12 months post-operative, suggesting accelerated fracture healing.
InductOs 1.5 mg/ml was significantly effective (compared to standard care) in patients both with or
without a history of smoking.
Severity of fractures:
Treatment with InductOs 1.5 mg/ml was significantly effective in all fracture
classes, including severe Gustilo IIIB fractures (52% reduced risk of secondary interventions as
compared to standard-care patients).
The proportion of patients with healed soft-tissue wounds was significantly higher at the 6-week
post-treatment visit in the InductOs 1.5 mg/ml group compared with the standard-care group (83%
versus 65%; P=0.0010). The proportion of patients with hardware failure (locking screws bent or
broken) was significantly lower in the InductOs 1.5 mg/ml group as compared to standard-care group
(11% versus 22%; P=0.0174).
5.2 Pharmacokinetic properties
InductOs is active at the site of implantation. In two exploratory studies, pre- and post-surgery serum
samples were collected from a few long-bone fracture patients. Dibotermin alfa was not detectable in
serum.
In animal studies (rats) using InductOs containing radiolabelled dibotermin alfa, the mean residence
time at the site of implantation was 4-8 days. Peak levels of circulating dibotermin alfa (0.1% of the
implanted dose) were observed within 6 hours following implantation. When injected intravenously,
the terminal half-life of dibotermin alfa was 16 minutes in rats and 6.7 minutes in cynomolgus
monkeys. It is concluded, therefore, that at the site of implantation, dibotermin alfa is slowly released
from the matrix and rapidly cleared when taken up into the systemic circulation.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans on conventional studies of pharmacology, acute
and repeat exposure toxicity.
In reproductive toxicity studies in rats, where dibotermin alfa was administered intravenously to
maximize systemic exposure, increased fetal weight and increased fetal ossification was observed and
a treatment-related effect could not be ruled out. The clinical relevance of these effects is unknown.
Anti-dibotermin antibodies have been investigated in pregnant rabbits following hyper
-
immunisation
with dibotermin alfa to experimentally induce anti-BMP-2 antibodies. In some foetuses with decreased
body weights, there were decreases in ossification of frontal and parietal bones (4 out of 151 foetuses),
which is generally considered to be reversible, and antibody related effects could not be ruled out.
There were no other alterations in foetal external, visceral, or skeletal morphology. Other animal
studies do not indicate direct or indirect harmful effects with respect to pregnancy, maternal toxicity,
embryolethality, or fetotoxicity.
InductOs has not been tested for
in vivo
carcinogenicity. Dibotermin alfa has demonstrated variable
effects on human tumour cell lines
in vitro
. Although the available
in vitro
data suggest a low potential
for promotion of tumour growth, the use of InductOs is contraindicated in patients with an active
malignancy or in patients undergoing treatment for a malignancy (see also section 4.3).
InductOs has been studied in a canine spinal implantation model. InductOs was implanted directly onto
the exposed dura following a laminectomy. Although narrowing of the neuroforamen and stenosis was
observed, no mineralization of the dura, no spinal cord stenosis, and no neurological deficits
subsequent to the application of InductOs were observed. The significance of these data for humans is
not known.
PHARMACEUTICAL PARTICULARS
Powder:
Sucrose
Glycine
Glutamic acid
Sodium chloride
Polysorbate 80
Sodium hydroxide
Solvent:
Water for injections
Matrix:
Bovine Type I collagen
This medicinal product must not be mixed with other medicinal products, except those mentioned in
section 6.6.
6.4 Special precautions for storage
Do not store above 30°C. Do not freeze
.
Store in the original package.
6.5 Nature and contents of container
Each kit of InductOs is provided with:
•
12 mg of sterile dibotermin alfa powder in a 20 ml vial (Type I glass) stoppered with a
bromobutyl rubber closure sealed with an aluminum flip-off seal and plastic cap.
•
Solvent for reconstitution in a 10 ml vial (Type I glass) stoppered with a bromobutyl rubber
closure sealed with an aluminum flip-off seal and plastic cap.
•
One sterile matrix in a polyvinyl chloride (PVC) blister package sealed with a Tyvek lid.
•
Two sterile 10 ml disposable polypropylene syringes.
•
Two sterile needles (stainless steel).
6.6 Special precautions for disposal and other handling
Any unused product or waste material should be disposed of in accordance with local requirements.
Dibotermin alfa must be used only with the accompanying solvent and matrix provided in the InductOs
kit. See section 4.2.
MARKETING AUTHORISATION HOLDER
Wyeth Europa Ltd.
Huntercombe Lane South
Taplow, Maidenhead
Berkshire, SL6 0PH
United Kingdom
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation
:
9 September 2002
Date of latest renewal: 9 September 2007
10 DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency
(EMA) http://www.ema.europa.eu
MANUFACTURER(S) OF THE BIOLOGICAL ACTIVE SUBSTANCE AND
MANUFACTURING AUTHORISATION HOLDER(S) RESPONSIBLE FOR
BATCH RELEASE
CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURER(S) OF THE BIOLOGICAL ACTIVE SUBSTANCE(S) AND
MANUFACTURING AUTHORISATION HOLDER(S) RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer(s) of the biological active substance(s)
Wyeth BioPharma
One Burtt Road
Andover
Massachusetts 01810
USA
Name and address of the manufacturer(s) responsible for batch release
Wyeth Pharmaceuticals
New Lane
Havant
Hants PO9 2NG
United Kingdom
B. CONDITIONS OF THE MARKETING AUTHORISATION
•
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to restricted medical prescription (See Annex I: Summary of Product
Characteristics, section 4.2)
•
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
The MAH must ensure that the system of pharmacovigilance, as described in version 3.0 presented in
Module 1.8.1. of the Marketing Authorisation, is in place and functioning before and whilst the
product is on the market.
The Marketing Authorisation Holder will continue to submit yearly PSURs.
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
InductOs 12 mg kit for implant
dibotermin alfa
STATEMENT OF ACTIVE SUBSTANCE(S)
Each kit contains 12 mg dibotermin alfa
.
When reconstituted, InductOs contains 1.5 mg/ml dibotermin
alfa.
Powder: sucrose, glycine, glutamic acid, sodium chloride, sodium hydroxide and polysorbate 80
Solvent: water for injections
Matrix: Type I bovine collagen
PHARMACEUTICAL FORM AND CONTENTS
Kit for implant contains:
1 vial of dibotermin alfa 12 mg powder for solution
1 vial of solvent for dibotermin alfa (10 ml water for injections)
1 sterile 7.5 x 10 cm matrix
2 sterile 10 ml syringes
2 sterile 20 gauge needles.
METHOD AND ROUTE(S) OF ADMINISTRATION
Kit for implant and single use only. For instructions for use, see section 4.2 of the enclosed Summary
of Product Characteristics.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY





















SPECIAL STORAGE CONDITIONS
Do not store above 30°C. Do not freeze. Store in the original package.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Wyeth Europa Ltd.
Huntercombe Lane South
Taplow, Maidenhead
Berkshire, SL6 0PH
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE
Justification for not including Braille accepted





















PARTICULARS TO APPEAR ON THE IMMEDIATE PACKAGING
NAME OF THE MEDICINAL PRODUCT
InductOs 12 mg kit for implant
dibotermin alfa
STATEMENT OF ACTIVE SUBSTANCE(S)
Each kit contains 12 mg dibotermin alfa. When reconstituted, contains 1.5 mg/ml dibotermin alfa.
Powder: sucrose, glycine, glutamic acid, sodium chloride, sodium hydroxide and polysorbate 80
Solvent: water for injections
Matrix: Type I bovine collagen
PHARMACEUTICAL FORM AND CONTENTS
Kit for implant contains:
1 vial of dibotermin alfa 12 mg powder for solution
1 vial of solvent for dibotermin alfa (10 ml water for injections)
1 sterile 7.5 x 10 cm matrix
2 sterile 10 ml syringes
2 sterile 20-gauge needles.
METHOD AND ROUTE(S) OF ADMINISTRATION
Kit for implant and single use only. For instructions for use, see section 4.2 of the enclosed Summary
of Product Characteristics.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY























SPECIAL STORAGE CONDITIONS
Do not store above 30°C. Do not freeze.
Store in original package.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Wyeth Europa Ltd.
Huntercombe Lane South
Taplow, Maidenhead
Berkshire, SL6 0PH
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE
Justification for not including Braille accepted



















MINIMUM PARTICULARS TO APPEAR ON SMALL IMMEDIATE PACKAGING UNITS
NAME OF THE MEDICINAL PRODUCT AND ROUTE(S) OF ADMINISTRATION
Powder for InductOs 12 mg kit for implant
dibotermin alfa
For instructions for use, see section 4.2 of the enclosed Summary of Product Characteristics
CONTENTS BY WEIGHT, BY VOLUME OR BY UNIT
12 mg dibotermin alfa; when reconstituted, contains 1.5 mg/ml.















MINIMUM PARTICULARS TO APPEAR ON SMALL IMMEDIATE PACKAGING UNITS
NAME OF THE MEDICINAL PRODUCT AND ROUTE(S) OF ADMINISTRATION
Solvent for InductOs 12 mg kit for implant
Water for injections
For instructions for use, see section 4.2 of the enclosed Summary of Product Characteristics
CONTENTS BY WEIGHT, BY VOLUME OR BY UNIT
10 ml water for injections















PACKAGE LEAFLET: INFORMATION FOR THE USER
InductOs 12 mg kit for implant
dibotermin alfa
Read all of this leaflet carefully before you are given this medicine.
−
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor.
If any of the side effects get serious or if you notice any side effects not listed in this leaflet,
please tell your doctor.
What InductOs is and what it is used for
Before you receive InductOs
WHAT INDUCTOS IS AND WHAT IT IS USED FOR
InductOs contains the active substance, dibotermin alfa. This is a protein that helps bone to grow and is
very similar to a protein that is already found in your body.
InductOs may be used either in lower back spine fusion surgery or to repair fractures of the shin bone.
Lower back spine fusion surgery
If you have a lot of pain from a damaged disc in your lower back, and other treatments have not proven
effective, you may be considered for lower back spine fusion surgery. InductOs is used instead of
taking a bone graft from your hip; this avoids the problems and pain that can be caused by an operation
to collect the bone graft.
When used in lower back fusion surgery, InductOs is used in combination with a metal cage, which
corrects the position of your spine. If you have any question about the cage, please ask your doctor.
Fractures of the shin bone
If you have broken your shin bone, InductOs is used to increase the chance that your broken bone will
heal, to help your fracture heal faster, and to reduce the need for additional surgeries to help your
fracture heal. InductOs is used in addition to standard treatment and care of shin bone fractures.
BEFORE YOU RECEIVE INDUCTOS
You should not receive InductOs
•
if you are allergic (hypersensitive) to dibotermin alfa or bovine collagen or any of the other
ingredients of InductOs.
•
if you are still growing (skeletally immature).
•
if you have an active infection at the surgery site.
•
if the doctor treating you decides that you have inadequate blood supply at the fracture site.
•
for treating a fracture that is disease-related (e.g., fractures due to Paget’s disease or cancer).
•
if you have been diagnosed with or are being treated for cancer.
The following are precautions for use of InductOs to be discussed with your doctor
•
You should inform your doctor if you have an autoimmune disease, such as rheumatoid arthritis,
sytemic lupus erythematosus, scleroderma, Sjögren's syndrome or dermatomyositis/polymyositis.
•
You should inform your doctor if you have any bone disease.
•
You should inform your doctor of any history of cancer.
•
The product should not be placed in direct contact with certain types of bones. Your surgeon will
know which bones to avoid.
•
Use of InductOs may cause bone formation (heterotopic ossification) in the surrounding tissues,
which can result in complications.
•
Some patients may develop antibodies (made by your body to fight a foreign protein) to InductOs.
While no harmful effects have been noted, the long-term effects are unknown.
•
You should inform your doctor if you have kidney or liver disease.
•
Some patients may develop nerve pain due to localised fluid collection, which would require
drainage or a surgical procedure to remove the fluid.
•
Localised swelling, in some cases resulting in breathing difficulties, has been reported in patients
when InductOs has been used in surgery of the upper (neck) region of the spine. The safety and
effectiveness of InductOs in cervical spine surgery have not been established, and InductOs should
not be used in this situation.
Using InductOs with other medicines
Some shin bone clinical trials have shown that if you are treated with InductOs and take pain
medication, such as aspirin or non-steroidal anti-inflammatory drugs (NSAIDs), like ibuprofen, for a
longer period of time (e.g., for more than 14 days) more fluid may discharge from your wound. This
additional fluid discharge has not been associated with problems in fracture or wound healing.
Please inform your doctor if you are taking or have recently taken any other medicines, including
medicines obtained without prescription.
Pregnancy and breast feeding
The effects of InductOs on pregnancy are not known. The use of the product in pregnant women is not
advised. Ask your doctor for advice if you become pregnant or intend to become pregnant. Your doctor
should advise you about using contraception for one year after having been treated with InductOs.
It is not known if InductOs passes into breast milk. Treatment with InductOs is not recommended in
mothers who are breastfeeding an infant. Ask your doctor for advice before breast feeding your baby.
Driving and using machines
InductOs will not affect your ability to drive or operate machinery.
Important information about some of the ingredients of InductOs
Some patients may develop antibodies (made by your body to fight a foreign protein) against
dibotermin alfa or against the collagen in the sponge.
In clinical studies, the presence of antibodies was not associated with side effects, e.g., allergies, nor
was it shown to decrease the effectiveness of InductOs.
The doctor treating you will administer InductOs during surgery. The medical staff will prepare
InductOs in the operating room.
If you are receiving InductOs for lower back spine fusion, your surgeon will remove the damaged disc
that is causing the pain and replace it with two metal cages filled with InductOs. The metal cages
correct the position of your spine, and InductOs encourages the bone to grow between the two
vertebrae to join them permanently in the correct position.
If you are receiving InductOs for a broken shin bone, your doctor will place InductOs around your
broken bone when your fracture is treated. The doctor will determine how many kits of InductOs you
will receive, depending on the size and number of fractures. Generally, one kit is used; however, a
maximum of two kits may be used.
Side effects specific to shin bone fracture surgery
Like all medicines, InductOs can cause side effects, although not everybody gets them.
The most frequent (likely to occur in more than 1 in 10 patients) events reported in clinical studies
were similar to those due to the traumatic injuries sustained by the patients or to the surgery itself.
These effects were pain, swelling, wound infection, and fever.
Common side effects were (likely to occur in fewer than 1 out of 10 patients), headache, signs
indicating malfunction of the pancreas (amylasaemia), decreased levels of magnesium in the blood,
and a transient increase in heart rate. These were observed slightly more frequently in patients treated
with InductOs for shin bone studies than in patients who did not receive the product.
Side effects specific to
spine surgery
The most common (more that 1 in 10 patients) side effects observed in spinal fusion studies were:
accidental injury, nerve pain, back pain and disorders of the bone, such as delayed healing. There have
been reports of nerve pain due to localised fluid collection, which would require drainage or a surgical
procedure to remove the fluid.
Spinal nerve pain resulting in pain in arm, back or leg has been reported
Localised swelling, in some cases resulting in breathing difficulties, has been reported in patients when
InductOs has been used in surgery of the upper (neck) region of the spine. After spinal fusion surgery,
undesirable movement of the metal cage has been reported.
If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell
your doctor.
Keep out of the reach and sight of children.
You will not be required to store this product.
What InductOs contains
The active substance in InductOs is dibotermin alfa (recombinant human Bone Morphogenetic
Protein-2), 12 mg. The other ingredients are sucrose, glycine, glutamic acid, sodium chloride, sodium
hydroxide and polysorbate 80, water for injections, and bovine Type I collagen.
What InductOs looks like and contents of the pack
InductOs is supplied to your doctor as a kit for implanting during surgery. The kit contains 12 mg of
dibotermin alfa powder, a solvent (water) and a sponge, which is made from a protein called collagen
obtained from cattle (otherwise known as bovine Type I collagen). Dibotermin alfa is supplied in the
kit as a white powder.
For use during surgery, it must be dissolved in the water provided to form a solution, which is used to
soak the sponge. The soaked sponge may then be placed where bone growth is desired. The sponge
and dibotermin alfa solution will gradually disappear as bone is formed.
Marketing Authorisation Holder
Wyeth Europa Ltd.
Huntercombe Lane South
Taplow, Maidenhead
Berkshire, SL6 0PH
United Kingdom
Manufacturer
Wyeth Pharmaceuticals
New Lane
Havant
Hants, PO9 2NG
United Kingdom
For any information about this medicinal product, please contact the local representative of the
Marketing Authorisation Holder.
België/Belgique/Belgien
Luxembourg/Luxemburg
Pfizer S.A. / N.V.
Tél/Tel: +32 (0)2 554 62 11
Magyarország
Pfizer Kft.
Tel: +36 1 488 3700
Malta
Vivian Corporation Ltd.
Tel: +35621 344610
Pfizer s.r.o.
Tel: +420-283-004-111
Danmark
Pfizer ApS
Tlf: +45 44 201 100
Nederland
Wyeth Pharmaceuticals B.V.
Tel: +31 23 567 2567
Deutschland
Pfizer Pharma GmbH
Tel: +49 (0)30 550055-51000
Norge
Pfizer AS
Tlf: +47 67 526 100
Österreich
Pfizer Corporation Austria Ges.m.b.H.
Tel: +43 (0)1 521 15-0
България/Eesti/Latvija/Lietuva/ Slovenija
Wyeth Whitehall Export GmbH
Teл/Tel/Tãlr:+43 1 89 1140
Polska
Pfizer Polska Sp. z o.o.,
Tel.: +48 22 335 61 00
Ελλάδα
Pfizer Hellas A.E.
Τηλ.: +30 210 6785 800
España
Pfizer, S.A.
Télf:+34914909900
Portugal
Laboratórios Pfizer, Lda.
Tel: (+351) 21 423 55 00
France
Pfizer
Tél +33 1 58 07 30 00
România
Pfizer Romania S.R.L
Tel: +40 (0) 21 207 28 00
Ireland
Wyeth Pharmaceuticals
Tel: +353 1 449 3500
Slovenská Republika
Pfizer Luxembourg SARL, organizačná
zložka
Tel: + 421 2 3355 5500
Ísland
Icepharma hf
Tel: +354 540 8000
Suomi/Finland
Pfizer Oy
Puh/Tel: +358 (0)9 430 040
Italia
Wyeth Lederle S.p.A.
Tel: +39 06 927151
Sverige
Pfizer AB
Tel: +46 (0)8 550 520 00
Kύπρος
Wyeth Hellas (Cyprus Branch) AEBE
Tηλ: +357 22 817690
United Kingdom
Wyeth Pharmaceuticals
Tel: +44 1628 415330
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency (EMA) web
site: http://www.ema.europa.eu
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/inductos.html
Copyright © 1995-2021 ITA all rights reserved.
|



























































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































