Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
INTANZA 9 microgram/strain suspension for injection
Influenza vaccine (split virion, inactivated)
QUALITATIVE AND QUANTITATIVE COMPOSITION
Influenza virus (inactivated, split) of the following strains*:
A/California/7/2009 (H1N1) – derived strain used NYMC X-179A ……………9 micrograms HA**
A/Perth/16/2009 (H3N2) – like strain used NYMC X-187 derived from A/Victoria/210/2009
…………………………………………………………………………………….9 micrograms HA**
B/Brisbane/60/2008 ………………………………………………………………9 micrograms HA**
* propagated in fertilised hens’ eggs from healthy chicken flocks
** haemagglutinin
This vaccine complies with the WHO recommendations (Northern Hemisphere) and EU decision for
the 2010/2011 season.
For a full list of excipients, see section 6.1.
INTANZA contains residues of eggs such as ovalbumin.
Suspension for injection.
Colourless and opalescent suspension.
4.1 Therapeutic indications
Prophylaxis of influenza in adults up to 59 years of age, especially in those who run an increased risk
of associated complications.
The use of INTANZA should be based on official recommendations.
4.2 Posology and method of administration
Posology
Adults up to 59 years of age: 0.1 ml.
Paediatric population:
INTANZA is not recommended for use in children and adolescents below 18 years due to insufficient
data on safety and efficacy.
Method of administration
Immunisation should be carried out by intradermal route.


The recommended site of administration is the region of the deltoid.
Precaution to be taken before manipulating or administering the product
For instructions for preparation of the medicinal product before administration, see section 6.6.
Hypersensitivity to the active substances, to any of the excipients, to residues of eggs, such as
ovalbumin, and to chicken proteins. INTANZA does not contain more than 0.05 microgram
ovalbumin per dose
The vaccine may also contain residues of the following substances: neomycin, formaldehyde and
octoxinol 9.
Immunisation shall be postponed in subjects with febrile illness or acute infection.
4.4 Special warnings and precautions for use
As with all injectable vaccines, appropriate medical treatment and supervision should always be
readily available in case of an anaphylactic event following the administration of the vaccine.
INTANZA should under no circumstances be administered intravascularly.
Antibody response in patients with endogenous or iatrogenic immunosuppression may be insufficient.
In case of presence of liquid at the injection site after vaccine administration, re-vaccination is not
required.
Interference with serological testing: See section 4.5.
4.5 Interaction with other medicinal products and other forms of interaction
INTANZA may be given at the same time as other vaccines. Immunisation should be carried out on
separate limbs. It should be noted that the adverse reactions may be intensified.
The immunological response may be diminished if the patient is undergoing immunosuppressant
treatment.
Following influenza vaccination, false positive results in serology tests using the ELISA method to
detect antibodies against HIV1, Hepatitis C and especially HTLV1 have been observed. The Western
Blot technique disproves the false-positive ELISA test results. The transient false positive reactions
could be due to the IgM response by the vaccine.
4.6 Fertility, pregnancy and lactation
Pregnancy
For INTANZA no clinical data on exposed pregnancies are available. In general data from
intramuscular influenza vaccinations in pregnant women do not indicate adverse fetal and maternal
outcomes attributable to the vaccine. One animal study with INTANZA did not indicate direct or
indirect harmful effects with respect to pregnancy, embryonic/fetal development, parturition or
postnatal development
.
The use of INTANZA may be considered from the second trimester of pregnancy. For pregnant
women with medical conditions that increase their risk of complications from influenza,
administration of the vaccine is recommended, irrespective of their stage of pregnancy
.
Breast-feeding
The vaccine INTANZA may be used during breast-feeding.
Fertility
No fertility data are available in Humans.
One animal study with INTANZA did not indicate harmful
effects on female fertility.
4.7 Effects on ability to drive and use machines
INTANZA has no or negligible influence on the ability to drive and use machines.
a. Summary of the safety profile
The safety of INTANZA has been assessed in 2 open-label randomised clinical trials in which 2,384
vaccinees received an injection of INTANZA.
Safety evaluation was performed for all subjects during the first 3 weeks following vaccination and
serious adverse reactions were collected during six months of follow-up.
The most common reactions occurring after vaccine administration were local reactions at injection
site.
Apparent local reactions after intradermal administration were more frequent than after the
comparator vaccine administered intramuscularly.
Most reactions resolved spontaneously within 1 to 3 days after onset.
Systemic safety profile of INTANZA is similar to the comparator vaccine administered
intramuscularly.
After repetitive yearly injections the safety profile of INTANZA is similar to the previous injections.
b. Tabulated summary of adverse reactions
The data below summarizes the frequencies of the adverse reactions that were recorded following
vaccination from clinical trials, using the following convention: very common (≥1/10); common
(≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare
(<1/10,000), not known (cannot be estimated from available data).

Blood and
lymphatic system
disorders
Skin and
subcutaneous
tissue disorders
Musculoskeletal
and connective
tissue disorders
General disorders
and
administration
site conditions
Local
reactions:
redness*,
swelling,
induration
pain,
pruritus
Local reactions:
ecchymosis
* In some cases, local redness lasted up to 7 days
c. Potentiels adverse events
Based on the experience with trivalent inactivated influenza vaccines administered by intramuscular
or deep subcutaneous injection, the following events may be reported:
Blood and lymphatic system disorders
Transient thrombocytopenia
Immune system disorders
Allergic reactions, in rare cases leading to shock, angioedema
Nervous system disorders
Neuralgia, febrile convulsions, neurological disorders, such as encephalomyelitis, neuritis and
Guillain Barré syndrome
Vascular disorders
Vasculitis associated in very rare cases with transient renal involvement
Skin and subcutaneous tissue disorders
Generalised skin reactions including urticaria
Overdose is unlikely to have any untoward effect.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Influenza vaccines, ATC code: J07BB02













Seroprotection is generally obtained within 2 to 3 weeks. The duration of postvaccinal immunity to
homologous strains or to strains closely related to the vaccine strains varies but is usually 6-
12 months.
In a randomised comparative phase III trial, 1,796 subjects from 18 to 59 years of age received 0.1 ml
of INTANZA by intradermal route and 453 subjects from 18 to 59 years of age received 0.5 ml of
trivalent inactivated influenza vaccine administered by intramuscular route.
In this comparative trial the seroprotection rate*, seroconversion or significant increase rate** and the
geometric mean titre ratio (GMTR) for anti-HA antibody (measured by HI) were assessed according
to predefined criteria.
Data were as follows (values in brackets show the 95% confidence intervals):
Strain specific anti-HA antibody
A/H1N1
A/New Caledonia
/ 20/99
N=1,296
A/H3N2
A/Wisconsin
/ 67/2005
N=1,297
B
B/Malaysia/
2506/2004
N=1,294
Seroconversion/ Significant
increase rate
*Seroprotection = HI titre ≥ 40
** Seroconversion = negative pre-vaccination HI titre and post vaccination HI titre ≥ 40, Significant
increase = positive pre-vaccination HI titre and at least a 4-fold increase in post-vaccination HI titre
GMTR: Geometric mean titre ratio of individual (post-/pre-vaccination titre).
INTANZA is as immunogenic as the comparator trivalent inactivated influenza vaccine administered
by intramuscular route for each of the 3 influenza strains in subjects from 18 to 59 years of age.
Across all three influenza strains, for the comparator intramuscular vaccine seroprotection rates
ranged between 74.8% and 95.4%, seroconversion or significant increase rates ranged between 56.4%
and 69.3% and GMTRs ranged between 6.63 and 11.2-fold over baseline HI titres.
5.2 Pharmacokinetic properties
5.3 Preclinical safety data
Non-clinical data revealed no special hazard for humans based on animal studies. The vaccine was
immunogenic in mice and rabbits. In repeated-dose toxicity studies in rabbits there was no significant
evidence of systemic toxicity. Nevertheless, single and repeated administration led to transient local
erythema and oedema. Genotoxicity and carcinogenic potential were not assessed because these
studies are not appropriate for a vaccine. Fertility and toxicity studies to reproduction in females have
not identified any specific potential hazard for humans.
PHARMACEUTICAL PARTICULARS










Sodium chloride
Potassium chloride
Disodium phosphate dihydrate
Potassium dihydrogen phosphate
Water for injections
In the absence of compatibility studies, this medicinal product must not be mixed with other
medicinal products.
6.4 Special precautions for storage
Store in a refrigerator (2°C-8°C). Do not freeze.
Keep the syringe in the outer carton in order to protect from light.
6.5 Nature and contents of container
0.1 ml of suspension in a pre-filled syringe (glass) with a Micro-Injection System, with attached
micro-needle, equipped with an elastomer plunger stopper (chlorobutyl), a tip cap (thermoplastic
elastomer and polypropylene) and a needle shielding system. Pack size of 1 or 10 or 20.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
Any unused vaccine or waste material should be disposed of in accordance with local requirements.
The vaccine should be allowed to reach room temperature before use.
The vaccine should not be used if foreign particles are present in the suspension.
It is not necessary to shake the vaccine before use.
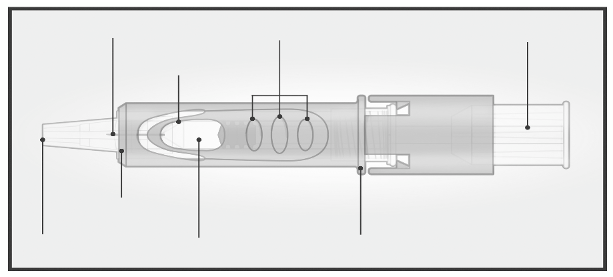
The Micro-Injection System for intradermal injection consists of a pre-filled syringe with a micro-
needle (1.5 mm) and a needle shielding system.
The needle shielding system is designed to cover the microneedle after use.
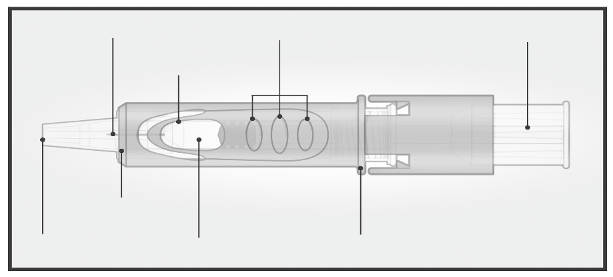
Please read the instruction before use
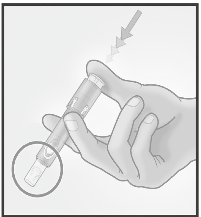
2/ HOLD MICRO-INJECTION SYSTEM
BETWEEN THUMB & MIDDLE FINGER
Remove the needle
cap from the
Micro-Injection
System.
Hold the system by
placing the thumb and
middle finger only on
the finger pads; the
index finger remains
free.
Do not purge air
through the
needle.
Do not place fingers
on the windows.
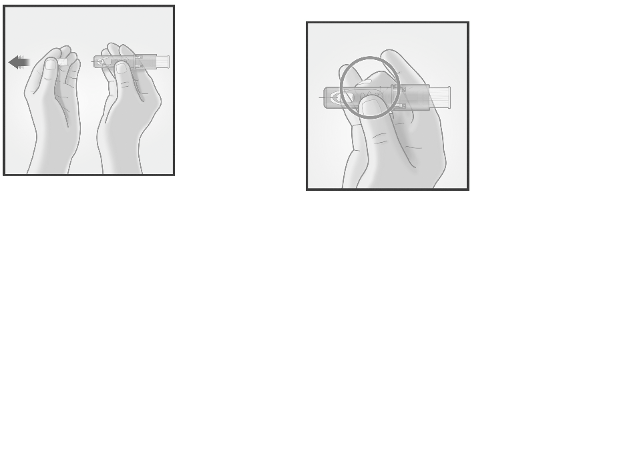
3/ INSERT NEEDLE RAPIDLY
PERPENDICULAR TO THE SKIN
4/ INJECT USING THE INDEX FINGER
Once the micro-needle
has been inserted,
maintain a light
pressure on the surface
of the skin and inject
using the index finger
to push on the plunger.
The vein test is
unnecessary.
Insert the needle
perpendicular to
the skin, in the
region of the
deltoid, in a short,
quick movement.
5/ ACTIVATE NEEDLE SHIELD BY PUSHING FIRMLY ON PLUNGER
Remove the needle from the skin.
Orient the needle away from you and others.
With the same hand, push very firmly with the thumb on the
plunger to activate the needle shield.
You hear a click and a shield comes out to cover the needle.
Immediately dispose of the system in the nearest sharps collector.
Injection is considered successful whether or not the presence of a
wheal is observed.
In case of presence of liquid at the injection site after vaccine
administration, re-vaccination is not required.





MARKETING AUTHORISATION HOLDER
Sanofi Pasteur MSD SNC, 8 rue Jonas Salk, F-69007 Lyon, France.
MARKETING AUTHORISATION NUMBER(S)
EU/1/08/505/001
EU/1/08/505/002
EU/1/08/505/003
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
Agency http://www.ema.europa.eu
NAME OF THE MEDICINAL PRODUCT
INTANZA 15 microgram/strain suspension for injection
Influenza vaccine (split virion, inactivated)
QUALITATIVE AND QUANTITATIVE COMPOSITION
Influenza virus (inactivated, split) of the following strains*:
A/California/7/2009 (H1N1) – derived strain used NYMC X-179A …………….15 micrograms HA**
A/Perth/16/2009 (H3N2) – like strain used NYMC X-187 derived from A/Victoria/210/2009
…………………………………………………………………………………….15 micrograms HA**
B/Brisbane/60/2008 ………………………………………………………………15 micrograms HA**
* propagated in fertilised hens’ eggs from healthy chicken flocks
** haemagglutinin
This vaccine complies with the WHO recommendations (Northern Hemisphere) and EU decision for
the 2010/20011 season.
For a full list of excipients, see section 6.1.
INTANZA contains residues of eggs such as ovalbumin.
Suspension for injection.
Colourless and opalescent suspension.
4.1 Therapeutic indications
Prophylaxis of influenza in individuals 60 years of age and over, especially in those who run an
increased risk of associated complications.
The use of INTANZA should be based on official recommendations.
4.2 Posology and method of administration
Posology
Individuals 60 years of age and over: 0.1 ml.
Paediatric population
INTANZA is not recommended for use in children and adolescents below 18 years due to insufficient
data on safety and efficacy.
Method of administration
Immunisation should be carried out by intradermal route.
The recommended site of administration is the region of the deltoid.


Precaution to be taken before manipulating or administering the product
For instructions for preparation of the medicinal product before administration,, see section 6.6.
Hypersensitivity to the active substances, to any of the excipients, to residues of eggs, such as
ovalbumin, and to chicken proteins. INTANZA does not contain more than 0.05 microgram
ovalbumin per dose.
The vaccine may also contain residues of the following substances: neomycin, formaldehyde and
octoxinol 9.
Immunisation shall be postponed in subjects with febrile illness or acute infection.
4.4 Special warnings and precautions for use
As with all injectable vaccines, appropriate medical treatment and supervision should always be
readily available in case of an anaphylactic event following the administration of the vaccine.
INTANZA should under no circumstances be administered intravascularly.
Antibody response in patients with endogenous or iatrogenic immunosuppression may be insufficient.
In case of presence of liquid at the injection site after vaccine administration, re-vaccination is not
required.
Interference with serological testing: See section 4.5.
4.5 Interaction with other medicinal products and other forms of interaction
INTANZA may be given at the same time as other vaccines. Immunisation should be carried out on
separate limbs. It should be noted that the adverse reactions may be intensified.
The immunological response may be diminished if the patient is undergoing immunosuppressant
treatment.
Following influenza vaccination, false positive results in serology tests using the ELISA method to
detect antibodies against HIV1, Hepatitis C and especially HTLV1 have been observed. The Western
Blot technique disproves the false-positive ELISA test results. The transient false positive reactions
could be due to the IgM response by the vaccine.
4.6 Fertility, pregnancy and lactation
This vaccine is intended for individuals 60 years of age and over. Therefore, this information is not
applicable.
4.7 Effects on ability to drive and use machines
INTANZA has no or negligible influence on the ability to drive and use machines.
a. Summary of the safety profile
The safety of INTANZA has been assessed in 3 open-label randomised clinical trials, 3,372 vaccinees
received an injection of INTANZA.
Safety evaluation was performed for all subjects during the first 3 weeks following vaccination and
serious adverse reactions were collected during six months of follow-up for 2,974 subjects
(population of two out of the three clinical trials).
The most common reactions occurring after vaccine administration were local reactions at injection
site.
Apparent local reactions after intradermal administration were more frequent than after intramuscular
administration of an adjuvanted or non-adjuvanted comparator vaccine.
Most reactions resolved spontaneously within 1 to 3 days after onset.
Systemic safety profile of INTANZA is similar to the comparator vaccine, adjuvanted or non-
adjuvanted, administered intramuscularly.
After repetitive yearly injections the safety profile of INTANZA is similar to the previous injections.
b. Tabulated summary of adverse reactions
The data below summarizes the frequencies of the adverse reactions that were recorded following
vaccination from clinical trials, using the following convention: very common (≥1/10); common
(≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1000); very rare
(<1/10,000), not known (cannot be estimated from available data).
Skin and
subcutaneous
tissue disorders
Musculoskeletal
and connective
tissue disorders
General disorders
and
administration
site conditions
Local
reactions:
redness*,
induration
swelling,
pruritus,
pain
Malaise,
shivering,
fever,
Local
reactions:
ecchymosis
*In some cases, local redness lasted up to 7 days.
c. Potential adverse events
Based on the experience with trivalent inactivated influenza vaccines administered by intramuscular
or deep subcutaneous injection, the following events may be reported:
Blood and lymphatic system disorders
Transient thrombocytopenia, transient lymphadenopathy
Immune system disorders
Allergic reactions, in rare cases leading to shock, angioedema












Nervous system disorders
Neuralgia, febrile convulsions, neurological disorders, such as encephalomyelitis and Guillain Barré
syndrome
Vascular disorders
Vasculitis associated in very rare cases with transient renal involvement
Skin and subcutaneous tissue disorders
Generalised skin reactions including urticaria
Overdose is unlikely to have any untoward effect.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Influenza vaccines, ATC code: J07BB02
Seroprotection is generally obtained within 2 to 3 weeks. The duration of postvaccinal immunity to
homologous strains or to strains closely related to the vaccine strains varies but is usually 6-
12 months.
In a pivotal randomised comparative phase III trial, 2,606 subjects over 60 years of age received
0.1 ml of INTANZA by intradermal route and 1,089 subjects over 60 years of age received 0.5 ml of a
trivalent inactivated influenza vaccine administered by intramuscular route.
In this comparative trial the geometric mean titres (GMTs), seroprotection rate*, seroconversion or
significant increase rate** and the geometric mean titre ratio (GMTR) for anti-HA antibody
(measured by HI) were assessed according to predefined criteria.
Data were as follows (values in brackets show the 95% confidence intervals):
Geometric mean of titre (1/dil)
Seroprotection rate (%) *
Seroconversion or significant
increase rate (%) **
Geometric mean of titre ratio
(GMTR)
*Seroprotection = HI titre ≥ 40














** Seroconversion = negative pre-vaccination HI titre and post vaccination HI titre ≥ 40, Significant
increase = positive pre-vaccination HI titre and at least a 4-fold increase in post-vaccination HI titre
GMTR: Geometric mean titre ratio of individual (post-/pre-vaccination titre).
INTANZA is at least as immunogenic as the comparator trivalent inactivated influenza vaccine
administered by intramuscular route for each of the 3 influenza strains in subjects from 60 years of
age and over.
Across all three influenza strains, for the comparator intramuscular vaccine GMTs ranged between
34.8 (1/dil) and 181.0 (1/dil), seroprotection rates ranged between 48.9% and 87.9%, seroconversion
or significant increase rates ranged between 30.0% and 46.9% and GMTRs ranged between 3.04 and
5.35-fold over baseline HI titres.
In a randomised comparative phase III trial, 398 subjects over 65 years of age received, 0.1 ml of
INTANZA by intradermal route and 397 subjects over 65 years of age received 0.5 ml of a trivalent
inactivated adjuvanted (MF-59 containing) influenza vaccine at the same dosage administered by
intramuscular route.
INTANZA is as immunogenic as the comparator trivalent adjuvanted (MF-59 containing) vaccine in
terms of GMT for each of the 3 influenza strains with the SRH method and for 2 strains with the HI
method.
5.2 Pharmacokinetic properties
5.3 Preclinical safety data
Non-clinical data revealed no special hazard for humans based on animal studies. The vaccine was
immunogenic in mice and rabbits. In repeated-dose toxicity studies in rabbits there was no significant
evidence of systemic toxicity. Nevertheless, single and repeated administrations led to transient local
erythema and oedema. Genotoxicity and carcinogenic potential were not assessed because these
studies are not appropriate for a vaccine. Fertility and toxicity studies to reproduction in females have
not identified any specific potential hazard for humans.
PHARMACEUTICAL PARTICULARS
Sodium chloride
Potassium chloride
Disodium phosphate dihydrate
Potassium dihydrogen phosphate
Water for injections
In the absence of compatibility studies, this medicinal product must not be mixed with other
medicinal products.
6.4 Special precautions for storage
Store in a refrigerator (2°C-8°C). Do not freeze.
Keep the syringe in the outer carton in order to protect from light.
6.5 Nature and contents of container
0.1 ml of suspension in a pre-filled syringe (glass) with a Micro-Injection System, with attached
micro-needle, equipped with an elastomer plunger stopper (chlorobutyl), a tip cap (thermoplastic
elastomer and polypropylene) and a needle shielding system. Pack size of 1 or 10 or 20.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
Any unused vaccine or waste material should be disposed of in accordance with local requirements.
The vaccine should be allowed to reach room temperature before use.
The vaccine should not be used if foreign particles are present in the suspension.
It is not necessary to shake the vaccine before use.
The Micro-Injection System for intradermal injection consists of a pre-filled syringe with a micro-
needle (1.5 mm) and a needle shielding system.
The needle shielding system is designed to cover the microneedle after use.
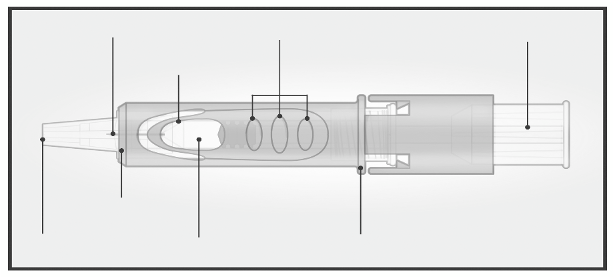
Please read the instruction before use
2/ HOLD MICRO-INJECTION SYSTEM
BETWEEN THUMB & MIDDLE FINGER
Remove the needle
cap from the
Micro-Injection
System.
Hold the system by
placing the thumb and
middle finger only on
the finger pads; the
index finger remains
free.
Do not purge air
through the
needle.
Do not place fingers
on the windows.
3/ INSERT NEEDLE RAPIDLY
PERPENDICULAR TO THE SKIN
4/ INJECT USING THE INDEX FINGER
Once the micro-needle
has been inserted,
maintain a light
pressure on the surface
of the skin and inject
using the index finger
to push on the plunger.
The vein test is
unnecessary.
Insert the needle
perpendicular to
the skin, in the
region of the
deltoid, in a short,
quick movement.
5/ ACTIVATE NEEDLE SHIELD BY PUSHING FIRMLY ON PLUNGER
Remove the needle from the skin.
Orient the needle away from you and others.
With the same hand, push very firmly with the thumb on the
plunger to activate the needle shield.
You hear a click and a shield comes out to cover the needle.
Immediately dispose of the system in the nearest sharps collector.
Injection is considered successful whether or not the presence of a
wheal is observed.
In case of presence of liquid at the injection site after vaccine
administration, re-vaccination is not required.




MARKETING AUTHORISATION HOLDER
Sanofi Pasteur MSD SNC, 8 rue Jonas Salk, F-69007 Lyon, France.
MARKETING AUTHORISATION NUMBER(S)
EU/1/08/505/004
EU/1/08/505/005
EU/1/08/505/006
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
Agency http://www.ema.europa.eu
MANUFACTURER(S) OF THE BIOLOGICAL ACTIVE
SUBSTANCE(S) AND MANUFACTURING
AUTHORISATION HOLDER(S) RESPONSIBLE FOR
BATCH RELEASE
CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURER OF THE BIOLOGICAL ACTIVE SUBSTANCE AND
MANUFACTURING AUTHORISATION HOLDERS RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer of the biological active substance
Sanofi Pasteur
Parc Industriel d'Incarville
27100 Val-de-Reuil
France
Name and address of the manufacturers responsible for batch release
Sanofi Pasteur
Parc Industriel d’Incarville
27100 Val-de-Reuil
France
Sanofi Pasteur
Campus Mérieux
1541 avenue Marcel Mérieux
69280 Marcy L’Etoile
France
The printed package leaflet of the medicinal product must state the name and address of the
manufacturer responsible for the release of the concerned batch.
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to medical prescription.
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
Pharmacovigilance system
The MAH must ensure that the system of pharmacovigilance, as described in version 2.0 presented in
Module 1.8.1. of the Marketing Authorisation Application, is in place and functioning before and
whilst the product is on the market.
Risk Management Plan
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version 3.0 of the Risk Management Plan (RMP) presented
in Module 1.8.2. of the Marketing Authorisation Application and any subsequent updates of the RMP
agreed by the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, the
updated RMP should be submitted at the same time as the next Periodic Safety Update Report
(PSUR).

In addition, an updated RMP should be submitted
·
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
At the request of the European Medicines Agency
Official batch release: in accordance with Article 114 Directive 2001/83/EC as amended, the official
batch release will be undertaken by a state laboratory or a laboratory designated for that purpose.
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
Pack of 1 or 10 or 20 pre-filled syringe(s) with a Micro-Injection System
NAME OF THE MEDICINAL PRODUCT
INTANZA 9 microgram/strain, suspension for injection
Influenza vaccine (split virion, inactivated).
Strains 2010/2011
STATEMENT OF ACTIVE SUBSTANCE(S)
Influenza virus (inactivated, split) of the following strains*:
A/California/7/2009 (H1N1) – derived strain…………………………………….. 9 micrograms HA**
A/Perth/16/2009 (H3N2) – like strain…………………………………… 9 micrograms HA**
B/Brisbane/60/2008…………………………………………………… 9 micrograms HA**
* propagated in fertilised hens’ eggs from healthy chicken flocks
** haemagglutinin
Sodium chloride, potassium chloride, disodium phosphate dihydrate, potassium dihydrogen phosphate
and water for injections
PHARMACEUTICAL FORM AND CONTENTS
Suspension for injection
Pre-filled syringe with a Micro-Injection System (0.1 ml) - pack of 1
Pre-filled syringe with a Micro-Injection System (0.1 ml) - pack of 10
Pre-filled syringe with a Micro-Injection System (0.1 ml) - pack of 20
METHOD AND ROUTE(S) OF ADMINISTRATION
Intradermal use.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.


























OTHER SPECIAL WARNING(S), IF NECESSARY
INTANZA does not contain more than 0.05 microgram ovalbumin per dose.
SPECIAL STORAGE CONDITIONS
Store in a refrigerator. Do not freeze.
Keep the syringe in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Sanofi Pasteur MSD SNC
8, rue Jonas Salk
F-69007 Lyon
France
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/08/505/001 - pack of 1 pre-filled syringe with a Micro-Injection System
EU/1/08/505/002 - pack of 10 pre-filled syringes with a Micro-Injection System
EU/1/08/505/003 - pack of 20 pre-filled syringes with a Micro-Injection System
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE
Justification for not including Braille is accepted.



























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
Pack of 1 or 10 or 20 pre-filled syringe(s) with a Micro-Injection System
NAME OF THE MEDICINAL PRODUCT
INTANZA 15 microgram/strain, suspension for injection
Influenza vaccine (split virion, inactivated).
Strains 2010/2011
STATEMENT OF ACTIVE SUBSTANCES
Influenza virus (inactivated, split) of the following strains*:
A/California/7/2009 (H1N1) – derived strain…………………………………… 15 micrograms HA**
A/Perth/16/2009 (H3N2) – like strain ……………………………………………15 micrograms HA**
B/Brisbane/60/2008 ………………………………………………………………15 micrograms HA**
* propagated in fertilised hens’ eggs from healthy chicken flocks
** haemagglutinin
Sodium chloride, potassium chloride, disodium phosphate dihydrate, potassium dihydrogen phosphate
and water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Suspension for injection
Pre-filled syringe with a Micro-Injection System (0.1 ml) - pack of 1
Pre-filled syringe with a Micro-Injection System (0.1 ml) - pack of 10
Pre-filled syringe with a Micro-Injection System (0.1 ml) - pack of 20
METHOD AND ROUTE OF ADMINISTRATION
Intradermal use.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.


























OTHER SPECIAL WARNING(S), IF NECESSARY
INTANZA does not contain more than 0.05 microgram ovalbumin per dose.
SPECIAL STORAGE CONDITIONS
Store in refrigerator. Do not freeze.
Keep the syringe in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Sanofi Pasteur MSD SNC
8, rue Jonas Salk
F-69007 Lyon
France
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/08/505/004 - pack of 1 pre-filled syringe with a Micro-Injection System
EU/1/08/505/005 - pack of 10 pre-filled syringes with a Micro-Injection System
EU/1/08/505/006 - pack of 20 pre-filled syringes with a Micro-Injection System
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE
Justification for not including Braille is accepted.



























PACKAGE LEAFLET: INFORMATION FOR THE USER
INTANZA 9 microgram/strain suspension for injection
Influenza vaccine (split virion, inactivated)
Read all of this leaflet carefully before you receive this vaccine.
-
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
This vaccine has been prescribed for you. Do not pass it on to others.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
1. What INTANZA is and what it is used for
2. Before you use INTANZA
3. How to use INTANZA
4. Possible side effects
5.
How to store INTANZA
6.
1.
WHAT INTANZA IS AND WHAT IT IS USED FOR
INTANZA
is a vaccine. This vaccine is recommended to help to protect you against flu.
The vaccine may be administered to adults up to 59 years of age, especially in those who run an
increased risk of associated complications.
When an injection of INTANZA is given, the immune system (body's natural defences) will develop
protection against flu infection.
INTANZA will help to protect you against the three strains of virus contained in the vaccine, or other
strains closely related to them. Full effect of the vaccine is generally achieved 2-3 weeks after the
vaccination.
2.
BEFORE YOU USE INTANZA
If you are allergic (hypersensitive) to:
-
Any of the other ingredients of INTANZA listed in section 6 of this leaflet in the section
"FURTHER INFORMATION",
Residues of eggs such as ovalbumin, to chicken proteins, to neomycin, formaldehyde and
octoxinol 9.
If you have an illness with fever or acute infection, the vaccination shall be postponed until
after you have recovered.
Take special care with INTANZA
You should tell your doctor before vaccination if you have a poor immune response
(immunosuppression) due to disease or medicines, because the vaccine may not work very well
in this case.
INTANZA should under no circumstances be administered into a vein (intravascularly).
If, for any reason, you have a blood test within a few days following an influenza vaccination,
please tell your doctor. Tests for HIV-1, hepatitis C virus and HTLV-1 may be affected.
Other vaccines: INTANZA can be given at the same time as other vaccines by using separate
limbs. It should be noted that the side effects may be intensified.
Tell your doctor if you have been treated with medicines that may reduce your immune
response such as corticosteroids (for example cortisone), medicines against cancer
(chemotherapy), radiotherapy or other medicines affecting the immune system. In this case, the
vaccine may not work very well.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
Pregnancy and breast-feeding
Pregnancy
Tell your doctor or pharmacist if you are pregnant or think you may be pregnant.
Your doctor or pharmacist will be able to decide if you should receive INTANZA.
Breast - feeding
The vaccine INTANZA may be used during breast-feeding.
Ask your doctor or pharmacist for advice before taking any medicines.
Driving and using machines
INTANZA has no or negligible influence on the ability to drive and use machines.
INTANZA is administered to you by your doctor or nurse.
Adults from 18 to 59 of age receive one 0.1 ml dose.
Use in children and adolescents
INTANZA is not recommended for use in children and adolescents below 18 years.
INTANZA is given as an injection into the upper layer of the skin (preferably the muscle of the upper
arm).
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Like all medicines, INTANZA can cause side effects, although not everybody gets them.
During clinical trials, the following side effects were reported with the use of INTANZA:
The frequency of possible side effects listed below is defined using the following convention:
Very common (affects more than 1 user in 10)
Common (affects 1 to 10 users in 100)
Uncommon (affects 1 to 10 users in 1,000)
Rare (affects 1 to 10 users in 10,000)
Very rare (affects less than 1 user in 10,000)
Not known (frequency cannot be estimated from the available data).
At the injection site: redness, swelling, hardness, itching and pain.
Feeling generally unwell, headache and muscular pain.
Bruising at the injection site
Shivering and fever (38.0°C or higher).
Tiredness, swelling of the glands in the neck, armpit or groin, tingling or numbness, joint pain,
itching and rash.
Most of side effects listed above disappeared without treatment within 1 to 3 days after onset. In
some cases, redness at the injection site lasted up to 7 days.
The following side effects have been reported with other vaccines given to prevent flu. These side
effects may occur with INTANZA:
·
Temporary reduction in the number of blood particles called platelets which can result in
bruising or bleeding
Allergic reactions which can lead in rare cases:
- to a failure of the circulatory system (shock) leading to medical emergency
- to swollen face, tongue or pharynx, difficulty to swallow, hives and difficulties to breathe
(angioedema)
Pain located on the nerve route, convulsions associated with fever, nervous system disorders
including inflammation of the brain or spinal cord, inflammation of nerves, or Guillain-Barré
syndrome which causes extreme weakness and paralysis
Vessel inflammation which may result in very rare cases in temporary kidney problems
Skin reactions that may spread throughout the body including hives.
You should see your doctor immediately if you experience symptoms of angioedema, such as:
·
Swollen face, tongue or pharynx
Hives and difficulties to breathe.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use INTANZA after the expiry date which is stated on the carton after EXP. The expiry date
refers to the last day of that month.
Store in a refrigerator (2°C - 8°C). Do not freeze. Keep the syringe in the outer carton in order to
protect from light.



Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active substances are Influenza virus (inactivated, split) of the following strains*:
A/California/7/2009 (H1N1) – derived strain used NYMC X-179A ……………9 micrograms HA**
A/Perth/16/2009 (H3N2) – like strain used NYMC X-187 derived from A/Victoria/210/2009…
………………………………………………………………………………..…..9 micrograms HA**
B/Brisbane/60/2008……………………………………………….…………….. 9 micrograms HA**
* propagated in fertilised hens’ eggs from healthy chicken flocks
** haemagglutinin
This vaccine complies with the WHO recommendations (Northern Hemisphere) and EU decision for
the 2010/2011 season.
The other ingredients are: sodium chloride, potassium chloride, disodium phosphate dihydrate,
potassium dihydrogen phosphate and water for injections.
What INTANZA looks like and contents of the pack
The vaccine is a colourless and opalescent suspension.
INTANZA is a suspension for injection in a pre-filled syringe of 0.1 ml with a Micro-Injection
System in packs of 1, 10 or 20.
Not all pack sizes may be marketed
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder: Sanofi Pasteur MSD SNC, 8 rue Jonas Salk, F-69007 Lyon, France.
Manufacturer:
sanofi pasteur - Parc Industriel d’Incarville- 27100 Val-de-Reuil- France
sanofi pasteur, Campus Mérieux – 1541, avenue Marcel Mérieux – 69280 Marcy l’Etoile - France
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
Sanofi Pasteur MSD
Tél/Tel: +32 2 726.9584
Luxembourg/Luxemburg
Sanofi Pasteur MSD
Tél: +32 2 726.9584
България
Sanofi Pasteur representative Office
Teл.: +359 2 980 08 33
Magyarország
sanofi-aventis zrt
Tel.: +36 1 505 2723








Česká republika
Sanofi Pasteur
Odd. vakcín Sanofi-aventis, s.r.o.
Tel: +420 233 086 111
Malta
Cherubino Ltd
Tel.: +356 21 343270
Danmark
Sanofi Pasteur MSD
Tlf: +45 23 32 69 29
Nederland
Sanofi Pasteur MSD
Tel: +31.23.567.96.00
Deutschland
Sanofi Pasteur MSD GmbH
Tel: +49 6224.594.0
Norge
Sanofi Pasteur MSD
Tlf: +47.67.50.50.20
Eesti
Sanofi-Aventis Estonia OÜ
Tel.: +372 627 3488
Österreich
Sanofi Pasteur MSD GmbH
Tel: +43.1.866.70.22.202
Ελλάδα
ΒΙΑΝΕΞ Α.Ε.
Τηλ: +30.210.8009111
Polska
Sanofi Pasteur Sp. z o.o.
Tel.: +48 22 280 05 00
España
Sanofi Pasteur MSD S.A.
Tel: +34.91.371.78.00
Portugal
Sanofi Pasteur MSD, SA
Tel: +351 21 470 4550
France
Sanofi Pasteur MSD SNC
Tél: +33.4.37.28.40.00
România
Sanofi - Aventis Romania SRL
Tel.: +40(21) 317 31 36
Ireland
Sanofi Pasteur MSD Ltd
Tel: +353 1 468 5600
Slovenija
ALPE s.p.
Tel.: +386 (0)1 432 62 38
Ísland
Sanofi Pasteur MSD
Sími: +32.2.726.95.84
Slovenská
republika
sanofi-aventis Pharma Slovakia s.r.o.
divízia vakcín Sanofi Pasteur
Tel.: +421 2 33 100 100
Italia
Sanofi Pasteur MSD Spa
Tel: +39 06.664.09.211
Suom
i/
Finland
Sanofi Pasteur MSD
Puh/Tel: +358.9.565.88.30
Κύπρος
Γ. Α. Σταάτης & Σια Λτδ.
Τηλ.: +357 - 22 76 62 76
Sverige
Sanofi Pasteur MSD
Tel: +46.8.564.888.60
Latvija
Sanofi Aventis Latvia SIA Vakcīnu nodaĜa
Tel.: +371 67114978
United Kingdom
Sanofi Pasteur MSD Ltd
Tel: +44.1.628.785.291
Lietuva
Sanofi – Aventis Lietuva, UAB
Tel.: +370 5 2730967
















This leaflet was last approved in
{MM/YYYY}.
Detailed information on this medicine is available on the European Medicines Agency web site:
http://www.ema.europa.eu
---------------------------------------------------------------------------------------------------------------------------
The following information is intended for medical or healthcare professionals only:
As with all injectable vaccines, appropriate medical treatment and supervision should always be
readily available in case of anaphylactic event following the administration of the vaccine.
The vaccine should be allowed to reach room temperature before use.
The vaccine should not be used if foreign particles are present in the suspension.
It is not necessary to shake the vaccine before use.
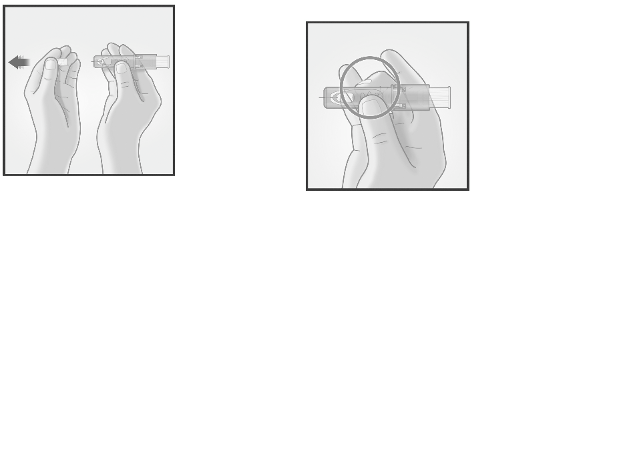
The Micro-Injection System for intradermal injection consists of a pre-filled syringe with a
micro-needle (1.5 mm) and a needle shielding system.
The needle shielding system is designed to cover the microneedle after use.
Please read the instruction before use
2/ HOLD MICRO-INJECTION SYSTEM
BETWEEN THUMB & MIDDLE FINGER
Remove the needle
cap from the
Micro-Injection
System.
Hold the system by
placing the thumb and
middle finger only on
the finger pads; the
index finger remains
free.
Do not purge air
through the
needle.
Do not place fingers
on the windows.
3/ INSERT NEEDLE RAPIDLY
PERPENDICULAR TO THE SKIN
4/ INJECT USING THE INDEX FINGER
Once the micro-needle
has been inserted,
maintain a light
pressure on the surface
of the skin and inject
using the index finger
to push on the plunger.
The vein test is
unnecessary.
Insert the needle
perpendicular to
the skin, in the
region of the
deltoid, in a short,
quick movement.
5/ ACTIVATE NEEDLE SHIELD BY PUSHING FIRMLY ON PLUNGER
Remove the needle from the skin.
Orient the needle away from you and others.
With the same hand, push very firmly with the thumb on the
plunger to activate the needle shield.
You hear a click and a shield comes out to cover the needle.
Immediately dispose of the system in the nearest sharps collector.
Injection is considered successful whether or not the presence of a
wheal is observed.
In case of presence of liquid at the injection site after vaccine
administration, re-vaccination is not required.
See also section 3. HOW TO USE INTANZA




PACKAGE LEAFLET: INFORMATION FOR THE USER
INTANZA 15 microgram/strain suspension for injection
Influenza vaccine (split virion, inactivated)
Read all of this leaflet carefully before you receive this vaccine.
-
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
This vaccine has been prescribed for you. Do not pass it on to others.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
1.
What INTANZA is and what it is used for
2.
Before you use INTANZA
3.
How to use INTANZA
4.
Possible side effects
5.
How to store INTANZA
6.
Further information
WHAT INTANZA IS AND WHAT IT IS USED FOR
INTANZA
is a vaccine.
This vaccine is recommended to help to protect you against flu.
The vaccine may be administered to individuals of 60 years of age and over, especially in those who
run an increased risk of associated complications.
When an injection of INTANZA
is given, the immune system (body's natural defences) will develop
protection against flu infection.
INTANZA will help you to protect you against the three strains of virus contained in the vaccine, or
other strains closely related to them. Full effect of the vaccine is generally achieved 2-3 weeks after
the vaccination.
If you are allergic (hypersensitive) to:
-
Any of the other ingredients of INTANZA listed in section 6 of this leaflet in the section
"FURTHER INFORMATION",
Residues of eggs such as ovalbumin, to chicken proteins, to neomycin, formaldehyde and
octoxinol 9.
If you have an illness with fever or acute infection, the vaccination shall be postponed until
after you have recovered.
Take special care with INTANZA
You should tell your doctor before vaccination if you have a poor immune response
(immunosuppression) due to disease or medicines, because the vaccine may not work very well
in this case.
INTANZA should under no circumstances be administered into a vein (intravascularly).
If, for any reason, you have a blood test within a few days following an influenza vaccination,
please tell your doctor. Tests for HIV-1, hepatitis C virus and HTLV-1 may be affected.
Other vaccines: INTANZA can be given at the same time as other vaccines by using separate
limbs. It should be noted that the side effects may be intensified.
Tell your doctor if you have been treated with medicines that may reduce your immune
response such as corticosteroids (for example cortisone), medicines against cancer
(chemotherapy), radiotherapy or other medicines affecting the immune system. In this case, the
vaccine may not work very well.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
Pregnancy and breast-feeding
This vaccine is intended for individuals 60 years of age and over. Therefore, this information is not
applicable.
Driving and using machines
INTANZA has no or negligible influence on the ability to drive and use machines.
INTANZA is administered to you by your doctor or nurse.
Individuals 60 years of age and over receive one 0.1 ml dose.
INTANZA is given as an injection
into the upper layer of the skin (preferably the muscle of the
upper arm).
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Like all medicines, INTANZA can cause side effects, although not everybody gets them.
During clinical trials, the following side effects were reported with the use of INTANZA:
The frequency of possible side effects listed below is defined using the following convention:
Very common (affects more than 1 user in 10)
Common (affects 1 to 10 users in 100)
Uncommon (affects 1 to 10 users in 1,000)
Rare (affects 1 to 10 users in 10,000)
Very rare (affects less than 1 user in 10,000)
Not known (frequency cannot be estimated from the available data).
At the injection site: redness, hardness, swelling, itching and pain.
Headache and muscular pain.
Bruising at the injection site

Feeling generally unwell, fever (38.0°C or higher) and shivering.
Tiredness, joint pain and increased sweating.
Tingling or numbness, inflammation of nerves, itching and rash.
Most of side effects listed above disappeared without treatment within 1 to 3 days after onset. In
some cases, redness at the injection site lasted up to 7 days.
The following side effects have been reported with other vaccines given to prevent flu. These side
effects may occur with INTANZA:
·
Temporary reduction in the number of blood particles called platelets which can result in
bruising or bleeding, temporary swelling of the glands in the neck, armpit or groin
Allergic reactions which can lead in rare cases:
- to a failure of the circulatory system (shock) leading to medical emergency
- to swollen face, tongue or pharynx, difficulty to swallow, hives and difficulties to breathe
(angioedema)
Pain located on the nerve route, convulsions associated with fever, nervous system disorders
including inflammation of the brain or spinal cord or Guillain-Barré syndrome which causes
extreme weakness and paralysis
Vessel inflammation which may result in very rare cases in temporary kidney problems
Skin reactions that may spread throughout the body including hives.
You should see your doctor immediately if you experience symptoms of angioedema, such as:
·
Swollen face, tongue or pharynx
Hives and difficulties to breathe.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use INTANZA after the expiry date which is stated on the carton after EXP. The expiry date
refers to the last day of that month.
Store in a refrigerator (2°C - 8°C). Do not freeze. Keep the syringe in the outer carton in order to
protect from light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active substances are Influenza virus (inactivated, split) of the following strains*:
A/California/7/2009 (H1N1) – derived strain used NYMC X-179A …………….15 micrograms HA**


A/Perth/16/2009 (H3N2) – like strain used NYMC X-187 derived from A/Victoria/210/2009
…………………………………………………………………………………….15 micrograms HA**
B/Brisbane/60/2008 ………………………………………………………………15 micrograms HA**
Per 0.1 ml dose
* propagated in fertilised hens’ eggs from healthy chicken flocks
** haemagglutinin
This vaccine complies with the WHO recommendations (Northern Hemisphere) and EU decision for
the 2010/2011 season.
The other ingredients are: sodium chloride, potassium chloride, disodium phosphate dihydrate,
potassium dihydrogen phosphate and water for injections.
What INTANZA looks like and contents of the pack
The vaccine is a colourless and opalescent suspension.
INTANZA is a suspension for injection in a pre-filled syringe of 0.1 ml with a Micro-Injection
System in packs of 1, 10 or 20.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder: Sanofi Pasteur MSD SNC, 8 rue Jonas Salk, F-69007 Lyon, France.
Manufacturer:
sanofi pasteur - Parc Industriel d’Incarville - 27100 Val-de-Reuil- France
sanofi pasteur, Campus Mérieux – 1541, avenue Marcel Mérieux – 69280 Marcy l’Etoile - France
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
Sanofi Pasteur MSD
Tél/Tel: +32 2 726.9584
Luxembourg/Luxemburg
Sanofi Pasteur MSD
Tél: +32 2 726.9584
България
Sanofi Pasteur representative Office
Teл.: +359 2 980 08 33
Magyarország
sanofi-aventis zrt
Tel.: +36 1 505 2723
Česká republika
Sanofi Pasteur
Odd. vakcín Sanofi-aventis, s.r.o.
Tel: +420 233 086 111
Malta
Cherubino Ltd
Tel.: +356 21 343270
Danmark
Sanofi Pasteur MSD
Tlf: +45 23 32 69 29
Nederland
Sanofi Pasteur MSD
Tel: +31.23.567.96.00










Deutschland
Sanofi Pasteur MSD GmbH
Tel: +49 6224.594.0
Norge
Sanofi Pasteur MSD
Tlf: +47.67.50.50.20
Eesti
Sanofi-Aventis Estonia OÜ
Tel.: +372 627 3488
Österreich
Sanofi Pasteur MSD GmbH
Tel: +43.1.866.70.22.202
Ελλάδα
ΒΙΑΝΕΞ Α.Ε.
Τηλ: +30.210.8009111
Polska
Sanofi Pasteur Sp. z o.o.
Tel.: +48 22 280 05 00
España
Sanofi Pasteur MSD S.A.
Tel: +34.91.371.78.00
Portugal
Sanofi Pasteur MSD, SA
Tel: +351 21 470 4550
France
Sanofi Pasteur MSD SNC
Tél: +33.4.37.28.40.00
România
Sanofi -Aventis Romania SRL
Tel.: +40(21) 317 31 36
Ireland
Sanofi Pasteur MSD Ltd
Tel: +353 1 468 5600
Slovenija
ALPE s.p.
Tel.: +386 (0)1 432 62 38
Ísland
Sanofi Pasteur MSD
Sími: +32.2.726.95.84
Slovenská
republika
sanofi-aventis Pharma Slovakia s.r.o.
divízia vakcín Sanofi Pasteur
Tel.: +421 2 33 100 100
Italia
Sanofi Pasteur MSD Spa
Tel: +39 06.664.09.211
Suom
i/
Finland
Sanofi Pasteur MSD
Puh/Tel: +358.9.565.88.30
Κύπρος
Γ. Α. Σταάτης & Σια Λτδ.
Τηλ.: +357 - 22 76 62 76
Sverige
Sanofi Pasteur MSD
Tel: +46.8.564.888.60
Latvija
Sanofi Aventis Latvia SIA Vakcīnu nodaĜa
Tel.: +371 67114978
United Kingdom
Sanofi Pasteur MSD Ltd
Tel: +44.1.628.785.291
Lietuva
Sanofi – Aventis Lietuva, UAB
Tel.: +370 5 2730967
This leaflet was last approved in
{MM/YYYY}.
Detailed information on this medicine is available on the European Medicines Agency web site:
http://www.ema.europa.eu
---------------------------------------------------------------------------------------------------------------------------
The following information is intended for medical or healthcare professionals only:
As with all injectable vaccines, appropriate medical treatment and supervision should always be
readily available in case of anaphylactic event following the administration of the vaccine.














The vaccine should be allowed to reach room temperature before use.
The vaccine should not be used if foreign particles are present in the suspension.
It is not necessary to shake the vaccine before use.
The Micro-Injection System for intradermal injection consists of a pre-filled syringe with a
micro-needle (1.5 mm) and a needle shielding system.
The needle shielding system is designed to cover the microneedle after use.

Please read the instruction before use
2/ HOLD MICRO-INJECTION SYSTEM
BETWEEN THUMB & MIDDLE FINGER
Remove the needle
cap from the
Micro-Injection
System.
Hold the system by
placing the thumb and
middle finger only on
the finger pads; the
index finger remains
free.
Do not purge air
through the
needle.
Do not place fingers
on the windows.
3/ INSERT NEEDLE RAPIDLY
PERPENDICULAR TO THE SKIN
4/ INJECT USING THE INDEX FINGER
Once the micro-needle
has been inserted,
maintain a light
pressure on the surface
of the skin and inject
using the index finger
to push on the plunger.
The vein test is
unnecessary.
Insert the needle
perpendicular to
the skin, in the
region of the
deltoid, in a short,
quick movement.
5/ ACTIVATE NEEDLE SHIELD BY PUSHING FIRMLY ON PLUNGER
Remove the needle from the skin.
Orient the needle away from you and others.
With the same hand, push very firmly with the thumb on the
plunger to activate the needle shield.
You hear a click and a shield comes out to cover the needle.
Immediately dispose of the system in the nearest sharps collector.
Injection is considered successful whether or not the presence of a
wheal is observed.
In case of presence of liquid at the injection site after vaccine
administration, re-vaccination is not required.
See also section 3. HOW TO USE INTANZA




Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/intanza.html
Copyright © 1995-2021 ITA all rights reserved.
|

















































































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































