Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Luveris 75 IU powder and solvent for solution for injection.
QUALITATIVE AND QUANTITATIVE COMPOSITION
One vial contains 75 IU of lutropin alfa (recombinant human Luteinising Hormone, r-hLH). Lutropin
alfa is produced in genetically engineered Chinese Hamster Ovary (CHO) cells.
For a full list of excipients, see section 6.1.
Powder and solvent for solution for injection.
Appearance of the powder: white lyophilised pellet
Appearance of the solvent: clear colourless solution
The pH of the reconstituted solution is 7.5 - 8.5.
4.1 Therapeutic indications
Luveris in association with a Follicle Stimulating Hormone (FSH) preparation is recommended for the
stimulation of follicular development in adult women with severe Luteinising Hormone (LH) and FSH
deficiency. In clinical trials these patients were defined by an endogenous serum LH level <1.2 IU/l.
4.2 Posology and method of administration
Treatment with Luveris should be initiated under the supervision of a physician experienced in the
treatment of fertility problems.
In LH and FSH deficient women, the objective of Luveris therapy in association with FSH is to
develop a single mature Graafian follicle from which the oocyte will be liberated after the
administration of human chorionic gonadotropin (hCG). Luveris should be given as a course of daily
injections simultaneously with FSH. Since these patients are amenorrhoeic and have low endogenous
oestrogen secretion, treatment can commence at any time.
Luveris should be adminstered concomitantly with follitropin alfa.
Treatment should be tailored to the individual patient’s response as assessed by measuring follicle size
by ultrasound and oestrogen response. A recommended regimen commences at 75 IU of lutropin alfa
(ie. one vial of Luveris) daily with 75-150 IU FSH.
In clinical trials, Luveris has been shown to increase the ovarian sensitivity to follitropin alfa. If an
FSH dose increase is deemed appropriate, dose adaptation should preferably be after 7-14 day
intervals and preferably by 37.5 IU-75 IU increments. It may be acceptable to extend the duration of
stimulation in any one cycle to up to 5 weeks.
When an optimal response is obtained, a single injection of 250 micrograms of r-hCG or 5,000 IU to
10,000 IU hCG should be administered 24-48 hours after the last Luveris and FSH injections. The
patient is recommended to have coitus on the day of, and on the day following, hCG administration.
Alternatively, intrauterine insemination (IUI) may be performed.
Luteal phase support may be considered since lack of substances with luteotrophic activity (LH/hCG)
after ovulation may lead to premature failure of the corpus luteum.
If an excessive response is obtained, treatment should be stopped and hCG withheld. Treatment should
recommence in the next cycle at a dose of FSH lower than that of the previous cycle.
Elderly
There is no relevant indication for the use of Luveris in the elderly population. Safety and
effectiveness of Luveris in elderly patients have not been established.
Renal or hepatic impaired patients
Safety, efficacy, and pharmacokinetics of Luveris in patients with renal or hepatic impairment have
not been established.
Paediatric patients
There is no relevant indication for the use of Luveris in the paediatric population.
Luveris is intended for subcutaneous use. The powder should be reconstituted, immediately prior to
use, with the solvent provided.
Self-administration of this medicinal product should only be performed by patients who are well-
motivated, adequately trained and with access to expert advice.
Luveris is contraindicated in patients with:
hypersensitivity to gonadotropins or to any of the excipients
ovarian, uterine, or mammary carcinoma
tumours of the hypothalamus and pituitary gland
ovarian enlargement or ovarian cyst unrelated to polycystic ovarian disease and of unknown
origin
gynaecological haemorrhages of unknown origin
Luveris must not be used when a condition exists which would make a normal pregnancy impossible,
such as:
malformations of sexual organs incompatible with pregnancy
fibroid tumours of the uterus incompatible with pregnancy
4.4 Special warnings and precautions for use
Before starting treatment, the couple's infertility should be assessed as appropriate and putative
contraindications for pregnancy evaluated. In addition, patients should be evaluated for
hypothyroidism, adrenocortical deficiency and hyperprolactinemia and appropriate specific treatment
given.
In patients with porphyria or a family history of porphyria Luveris may increase the risk of an acute
attack. Deterioration or a first appearance of this condition may require cessation of treatment.
Ovarian Hyperstimulation Syndrome (OHSS)
A certain degree of ovarian enlargement is an expected effect of controlled ovarian stimulation. It is
more commonly seen in women with polycystic ovarian syndrome and usually regresses without
treatment.
In distinction to uncomplicated ovarian enlargement, OHSS is a condition that can manifest itself with
increasing degrees of severity. It comprises marked ovarian enlargement, high serum sex steroids, and
an increase in vascular permeability which can result in an accumulation of fluid in the peritoneal,
pleural and, rarely, in the pericardial cavities.
Mild manifestations of OHSS may include abdominal pain, abdominal discomfort and distension, or
enlarged ovaries. Moderate OHSS may additionally present with nausea, vomiting, ultrasound
evidence of ascites or marked ovarian enlargement.
Severe OHSS further includes symptoms such as severe ovarian enlargement, weight gain, dyspnoea
or oliguria. Clinical evaluation may reveal signs such as hypovolaemia, haemoconcentration,
electrolyte imbalances, ascites, pleural effusions, or acute pulmonary distress. Very rarely, severe
OHSS may be complicated by ovarian torsion or thromboembolic events, such as pulmonary
embolism, ischaemic stroke or myocardial infarction.
Independent risk factors for developing OHSS include young age, lean body mass, polycystic ovarian
syndrome, higher doses of exogenous gonadotropins, high absolute or rapidly rising serum estradiol
levels and previous episodes of OHSS, large number of developing ovarian follicles and large number
of oocytes retrieved in ART cycles.
Adherence to recommended Luveris and FSH dosage and regimen of administration can minimise the
risk of ovarian hyperstimulation. Monitoring of stimulation cycles by ultrasound scans as well as
estradiol measurements are recommended to early identify risk factors.
There is evidence to suggest that hCG plays a key role in triggering OHSS and that the syndrome may
be more severe and more protracted if pregnancy occurs. Therefore, if signs of ovarian
hyperstimulation occur, it is recommended that hCG be withheld
and the patient be advised to refrain
from coitus or use barrier contraceptive methods for at least 4 days. As OHSS may progress rapidly
(within 24 hours) or over several days to become a serious medical event, patients should be followed
for at least two weeks after hCG administration.
Mild or moderate OHSS usually resolves spontaneously. If severe OHSS occurs, it is recommended
that gonadotropin treatment be stopped if still ongoing and that the patient be hospitalised and
appropriate therapy be started.
Ovarian torsion
Ovarian torsion has been reported after treatment with other gonadotropins. This may be associated
with other risk factors such as OHSS, pregnancy, previous abdominal surgery, past history of ovarian
torsion, previous or current ovarian cyst and polycystic ovarian syndrome. Damage to the ovary due to
reduced blood supply can be limited by early diagnosis and immediate detorsion.
Multiple pregnancy
In patients undergoing induction of ovulation, the incidence of multiple pregnancy is increased
compared with natural conception. The majority of multiple conceptions are twins. Multiple
pregnancy, especially high order, carry an increased risk of adverse maternal and perinatal outcomes
To minimise the risk of higher order multiple pregnancy, careful monitoring of ovarian response is
recommended.
In patients undergoing Assisted Reproductive Technology (ART) procedures the risk of multiple
pregnancy is related mainly to the number of embryos replaced, their quality and the patient age.
The incidence of pregnancy loss by miscarriage or abortion is higher in patients undergoing
stimulation of follicular growth for ovulation induction than following natural conception.
Ectopic pregnancy
Women with a history of tubal disease are at risk of ectopic pregnancy, whether the pregnancy is
obtained by spontaneous conception or with fertility treatments. The prevalence of ectopic pregnancy
after ART was reported to be higher than in the general population.
Congenital anomalies
The prevalence of congenital malformations after ART may be slightly higher than after spontaneous
conceptions. This could be due to parental factors (e.g. maternal age, genetics), ART procedures and
multiple pregnancies.
Thromboembolic events
In women with recent or ongoing thromboembolic disease or women with generally recognised risk
factors for thromboembolic events, such as personal or family history, thrombophilia or severe obesity
(body mass index >30 kg/m
2
), treatment with gonadotropins may further increase the risk for
aggravation or occurrence of such events. In these women, the benefits of gonadotropin administration
need to be weighed against the risks. It should be noted however, that pregnancy itself, as well as
OHSS, also carries an increased risk of thromboembolic events.
Reproductive system neoplasms
There have been reports of ovarian and other reproductive system neoplasms, both benign and
malignant, in women who have undergone multiple drug regimens for infertility treatment. It is not yet
established whether or not treatment with gonadotropins increases the risk of these tumours in infertile
women.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed with Luveris.
Luveris should not be administered as a mixture with other medicinal products, in the same injection,
except follitropin alfa for which studies have shown that co-administration does not significantly alter
the activity, stability, pharmacokinetic nor pharmacodynamic properties of the active substances.
4.6 Fertility,pregnancy and lactation
Pregnancy
There is no indication for the use of Luveris during pregnancy.
Data on a limited number of exposed pregnancies indicate no adverse reactions of gonadotropins on
pregnancy, embryonal or foetal development, parturition or postnatal development following
controlled ovarian stimulation. No teratogenic effect of Luveris has been observed in animal studies.
In case of exposure during pregnancy, clinical data are not sufficient to exclude a teratogenic effect of
Luveris.
Lactation
Luveris is not indicated during breastfeeding.
Fertility
Luveris is indicated for the stimulation of follicular development, in association with FSH (see
section 4.1).
4.7 Effects on ability to drive and use machines
Luveris has no or negligible influence on the ability to drive and use machines.
Luveris is used for the stimulation of follicular development in association with follitropin alfa. In this
context, it is difficult to attribute adverse reactions to any one of the substances used.
In a clinical trial, mild and moderate injection site reactions (bruising, pain, redness, itching or
swelling) were reported in 7.4% and 0.9% of the injections, respectively. No severe injection site
reactions were reported. Ovarian Hyper-Stimulation Syndrome (OHSS) was observed in less than 6%
of patients treated with Luveris. No severe ovarian hyperstimulation syndrome was reported
(section 4.4).
In rare instances, adnexal torsion (a complication of ovarian enlargement), and haemoperitoneum have
been associated with human menopausal gonadotropin therapy. Although these adverse reactions were
not observed, there is the possibility that they may also occur with Luveris.
Ectopic pregnancy may also occur, especially in women with a history of prior tubal disease.
The following definitions apply to the frequency terminology used hereafter:
Very common (≥1/10)
Common (≥1/100 to <1/10)
Uncommon (≥1/1,000 to <1/100)
Rare (≥1/10,000 to <1/1,000)
Very rare (<1/10,000)
Frequency not known (cannot be estimated from the available data)
The following adverse reactions may be observed after administration of Luveris.
Immune system disorders
Very rare: Mild to severe hypersensitivity reactions including anaphylactic reactions and shock.
Nervous system disorders
Common: Headache.
Vascular disorders
Very rare: Thromboembolism, usually associated with severe OHSS.
Gastrointestinal disorders
Common: Abdominal pain
,
abdominal discomfort
,
nausea
,
vomiting, diarrhoea.
Reproductive system and breast disorders
Common: Mild or moderate OHSS (including associated symptomatology), ovarian cyst, breast pain,
pelvic pain.
General disorders and administration site conditions:
Common: Injection site reaction (e.g. pain, erythema, haematoma, swelling and/or irritation at the site
of injection).
The effects of an overdose of Luveris are unknown. Nevertheless there is a possibility that OHSS may
occur, which is further described in section 4.4.
Single doses of up to 40,000 IU of lutropin alfa have been administered to healthy female volunteers
without serious adverse reactions and were well tolerated.
Management
Treatment is directed to symptoms.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Sex hormones and modulators of the genital system, gonadotropins. ATC
code: G03GA07
Lutropin alfa is a recombinant human luteinising hormone, a glycoprotein composed of non-
covalently bound α- and β-subunits. Luteinising hormone binds on the ovarian theca (and granulosa)
cells and testicular Leydig cells, to a receptor shared with human chorionic gonadotropin hormone
(hCG). This LH/CG transmembrane receptor is a member of the super-family of G protein-coupled
receptors; specifically, it has a large extra-cellular domain.
In vitro
the affinity binding of recombinant
hLH to the LH/CG receptor on Leydig tumour cells (MA-10) is between that for hCG and that of
pituitary hLH, but within the same order of magnitude.
In the ovaries, during the follicular phase, LH stimulates theca cells to secrete androgens, which will
be used as the substrate by granulosa cell aromatase enzyme to produce estradiol, supporting
FSH-induced follicular development. At mid-cycle, high levels of LH trigger corpus luteum formation
and ovulation. After ovulation, LH stimulates progesterone production in the corpus luteum by
increasing the conversion of cholesterol to pregnenolone.
In the stimulation of follicular development in anovulatory women deficient in LH and FSH, the
primary effect resulting from administration of lutropin alfa is an increase in estradiol secretion by the
follicles, the growth of which is stimulated by FSH.
In clinical trials, patients were defined by an endogenous serum LH level <1.2 IU/l as measured in a
central laboratory. However, it should be taken into account that there are variations between LH
measurements performed in different laboratories.
In these trials the ovulation rate per cycle was 70-75%.
5.2 Pharmacokinetic properties
The pharmacokinetics of lutropin alfa have been studied in pituitary desensitised female volunteers
from 75 IU up to 40,000 IU.
The pharmacokinetic profile of lutropin alfa is similar to that of urinary-derived hLH.
Following intravenous administration, lutropin alfa is rapidly distributed with an initial half-life of
approximately one hour and eliminated from the body with a terminal half-life of about 10-12 hours.
The steady state volume of distribution is around 10-14 l. Lutropin alfa shows linear
pharmacokinetics, as assessed by AUC which is directly proportional to the dose administered. Total
clearance is around 2 l/h, and less than 5% of the dose is excreted in the urine. The mean residence
time is approximately 5 hours.
Following subcutaneous administration, the absolute bioavailability is approximately 60%; the
terminal half-life is slightly prolonged. The lutropin alfa pharmacokinetics following single and
repeated administration of Luveris are comparable and the accumulation ratio of lutropin alfa is
minimal. There is no pharmacokinetic interaction with follitropin alfa when administered
simultaneously.
5.3 Preclinical safety data
Nonclinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential. As expected from the
heterologous protein nature of the hormone, lutropin alfa raised an antibody response in experimental
animals after a period that reduced the measurable serum LH levels but did not fully prevent its
biological action. No signs of toxicity due to the development of antibodies to lutropin alfa were
observed.
At doses of 10 IU/kg/day and greater, repeated administration of lutropin alfa to pregnant rats and
rabbits caused impairment of reproductive function including resorption of foetuses and reduced body
weight gain of the dams. However, drug-related teratogenesis was not observed in either animal
model.
Other studies have shown that lutropin alfa is not mutagenic.
PHARMACEUTICAL PARTICULARS
Sucrose
Disodium phosphate dihydrate
Sodium dihydrogen phosphate monohydrate
Polysorbate 20
Phosphoric acid, concentrated (for pH adjustment)
Sodium hydroxide (for pH adjustment)
Methionine
Nitrogen
Solvent: Water for injection
This medicinal product must not be administered as a mixture in the same injection with other
medicinal products except follitropin alfa.
6.4 Special precautions for storage
Do not store above 25°C. Store in the original package in order to protect from light.
6.5 Nature and contents of container
The powder is packaged in 3 ml neutral colourless glass (type I) vials. The vials are sealed with
bromobutyl stoppers protected by aluminium seal rings and flip-off caps. The solvent is packaged
either in 2 or 3 ml neutral colourless glass (type I) vials with a Teflon-coated rubber stopper or in 2 ml
neutral colourless glass (type I) ampoules.
The medicinal product is supplied in packs of 1, 3 or 10 vials with the corresponding number of
solvent vials or ampoules. Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
For immediate and single use following first opening and reconstitution.
The powder must be reconstituted with the solvent before use by gentle swirling.
The reconstituted solution should not be administered if it contains particles or is not clear.
Luveris may be mixed with follitropin alfa and co-administered as a single injection.
In this case Luveris should be reconstituted first and then used to reconstitute the follitropin alfa
powder.
In order to avoid the injection of large volumes, one vial of Luveris can be reconstituted together with
one or two ampoule(s)/vial(s) of follitropin alfa, 37.5 IU, 75 IU or 150 IU, in 1 ml of solvent.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Merck Serono Europe Limited,
56 Marsh Wall,
London E14 9TP
United Kingdom
MARKETING AUTHORISATION NUMBER(S)
EU/1/00/155/001
EU/1/00/155/002
EU/1/00/155/003
EU/1/00/155/004
EU/1/00/155/005
EU/1/00/155/006
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 29
th
November 2000.
Date of last renewal: 30
th
November 2005.
10.
DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency
http://www.ema.europa.eu/.
NAME OF THE MEDICINAL PRODUCT
Luveris 450 IU solution for injection.
QUALITATIVE AND QUANTITATIVE COMPOSITION
One cartridge contains 450 IU/0.72 ml of lutropin alfa (recombinant human Luteinising Hormone,
r-hLH) and allows delivery of 6 doses of 75 IU of lutropin alfa.
Lutropin alfa is produced in genetically engineered Chinese Hamster Ovary (CHO) cells.
For a full list of excipients, see section 6.1.
Solution for injection.
The solution is clear and colourless.
The pH of the solution is 7.5 - 8.5. Osmolality is 250 – 450 mOsm/kg.
4.1 Therapeutic indications
Luveris in association with a Follicle Stimulating Hormone (FSH) preparation is recommended for the
stimulation of follicular development in adult women with severe Luteinising Hormone (LH) and FSH
deficiency. In clinical trials these patients were defined by an endogenous serum LH level <1.2 IU/l.
4.2 Posology and method of administration
Treatment with Luveris should be initiated under the supervision of a physician experienced in the
treatment of fertility problems.
In LH and FSH deficient women, the objective of Luveris therapy in association with FSH is to
develop a single mature Graafian follicle from which the oocyte will be liberated after the
administration of human chorionic gonadotropin (hCG). Luveris should be given as a course of daily
injections simultaneously with FSH. Since these patients are amenorrhoeic and have low endogenous
oestrogen secretion, treatment can commence at any time.
Luveris should be administered concomitantly with follitropin alfa.
Treatment should be tailored to the individual patient’s response as assessed by measuring follicle size
by ultrasound and oestrogen response. A recommended regimen commences at 75 IU of lutropin alfa
daily with 75-150 IU FSH.
In clinical trials, Luveris has been shown to increase the ovarian sensitivity to follitropin alfa. If an
FSH dose increase is deemed appropriate, dose adaptation should preferably be after 7-14 day
intervals and preferably by 37.5 IU-75 IU increments. It may be acceptable to extend the duration of
stimulation in any one cycle to up to 5 weeks.
Luveris solution for injection in a cartridge must be administered with the re-usable autoinjector
device provided separately. For administration refer to the instructions provided with the device.
When an optimal response is obtained, a single injection of 250 micrograms of r-hCG or 5,000 IU to
10,000 IU hCG should be administered 24-48 hours after the last Luveris and FSH injections. The
patient is recommended to have coitus on the day of, and on the day following, hCG administration.
Alternatively, intrauterine insemination (IUI) may be performed.
Luteal phase support may be considered since lack of substances with luteotrophic activity (LH/hCG)
after ovulation may lead to premature failure of the corpus luteum.
If an excessive response is obtained, treatment should be stopped and hCG withheld. Treatment should
recommence in the next cycle at a dose of FSH lower than that of the previous cycle.
Elderly
There is no relevant indication for the use of Luveris in the elderly population. Safety and
effectiveness of Luveris in elderly patients have not been established.
Renal or hepatic impaired patients
Safety, efficacy, and pharmacokinetics of Luveris in patients with renal or hepatic impairment have
not been established.
Paediatric patients
There is no relevant indication for the use of Luveris in the paediatric population.
Luveris is intended for subcutaneous use.
Self-administration of this medicinal product should only be performed by patients who are well-
motivated, adequately trained and with access to expert advice.
Luveris is contraindicated in patients with:
hypersensitivity to gonadotropins or to any of the excipients
ovarian, uterine, or mammary carcinoma
tumours of the hypothalamus and pituitary gland
ovarian enlargement or ovarian cyst unrelated to polycystic ovarian disease and of unknown
origin
gynaecological haemorrhages of unknown origin
Luveris must not be used when a condition exists which would make a normal pregnancy impossible,
such as:
malformations of sexual organs incompatible with pregnancy
fibroid tumours of the uterus incompatible with pregnancy
4.4 Special warnings and precautions for use
Before starting treatment, the couple's infertility should be assessed as appropriate and putative
contraindications for pregnancy evaluated. In addition, patients should be evaluated for
hypothyroidism, adrenocortical deficiency and hyperprolactinemia and appropriate specific treatment
given.
In patients with porphyria or a family history of porphyria Luveris may increase the risk of an acute
attack. Deterioration or a first appearance of this condition may require cessation of treatment.
Ovarian Hyperstimulation Syndrome (OHSS)
A certain degree of ovarian enlargement is an expected effect of controlled ovarian stimulation. It is
more commonly seen in women with polycystic ovarian syndrome and usually regresses without
treatment.
In distinction to uncomplicated ovarian enlargement, OHSS is a condition that can manifest itself with
increasing degrees of severity. It comprises marked ovarian enlargement, high serum sex steroids, and
an increase in vascular permeability which can result in an accumulation of fluid in the peritoneal,
pleural and, rarely, in the pericardial cavities.
Mild manifestations of OHSS may include abdominal pain, abdominal discomfort and distension, or
enlarged ovaries. Moderate OHSS may additionally present with nausea, vomiting, ultrasound
evidence of ascites or marked ovarian enlargement.
Severe OHSS further includes symptoms such as severe ovarian enlargement, weight gain, dyspnoea
or oliguria. Clinical evaluation may reveal signs such as hypovolaemia, haemoconcentration,
electrolyte imbalances, ascites, pleural effusions, or acute pulmonary distress. Very rarely, severe
OHSS may be complicated by ovarian torsion or thromboembolic events, such as pulmonary
embolism, ischaemic stroke or myocardial infarction.
Independent risk factors for developing OHSS include young age, lean body mass, polycystic ovarian
syndrome, higher doses of exogenous gonadotropins, high absolute or rapidly rising serum estradiol
levels and previous episodes of OHSS, large number of developing ovarian follicles and large number
of oocytes retrieved in ART cycles.
Adherence to recommended Luveris and FSH dosage and regimen of administration can minimise the
risk of ovarian hyperstimulation. Monitoring of stimulation cycles by ultrasound scans as well as
estradiol measurements are recommended to early identify risk factors.
There is evidence to suggest that hCG plays a key role in triggering OHSS and that the syndrome may
be more severe and more protracted if pregnancy occurs. Therefore, if signs of ovarian
hyperstimulation occur, it is recommended that hCG be withheld
and the patient be advised to refrain
from coitus or use barrier contraceptive methods for at least 4 days. As OHSS may progress rapidly
(within 24 hours) or over several days to become a serious medical event, patients should be followed
for at least two weeks after hCG administration.
Mild or moderate OHSS usually resolves spontaneously. If severe OHSS occurs, it is recommended
that gonadotropin treatment be stopped if still ongoing and that the patient be hospitalised and
appropriate therapy be started.
Ovarian torsion
Ovarian torsion has been reported after treatment with other gonadotropins. This may be associated
with other risk factors such as OHSS, pregnancy, previous abdominal surgery, past history of ovarian
torsion, previous or current ovarian cyst and polycystic ovarian syndrome. Damage to the ovary due to
reduced blood supply can be limited by early diagnosis and immediate detorsion.
Multiple pregnancy
In patients undergoing induction of ovulation, the incidence of multiple pregnancy is increased
compared with natural conception. The majority of multiple conceptions are twins. Multiple
pregnancy, especially high order, carry an increased risk of adverse maternal and perinatal outcomes
To minimise the risk of higher order multiple pregnancy, careful monitoring of ovarian response is
recommended.
In patients undergoing Assisted Reproductive Technology (ART) procedures the risk of multiple
pregnancy is related mainly to the number of embryos replaced, their quality and the patient age.
Pregnancy loss
The incidence of pregnancy loss by miscarriage or abortion is higher in patients undergoing
stimulation of follicular growth for ovulation induction than following natural conception.
Ectopic pregnancy
Women with a history of tubal disease are at risk of ectopic pregnancy, whether the pregnancy is
obtained by spontaneous conception or with fertility treatments. The prevalence of ectopic pregnancy
after ART was reported to be higher than in the general population.
Congenital anomalies
The prevalence of congenital malformations after ART may be slightly higher than after spontaneous
conceptions. This could be due to parental factors (e.g. maternal age, genetics), ART procedures and
multiple pregnancies.
Thromboembolic events
In women with recent or ongoing thromboembolic disease or women with generally recognised risk
factors for thromboembolic events, such as personal or family history, thrombophilia or severe obesity
(body mass index >30 kg/m
2
), treatment with gonadotropins may further increase the risk for
aggravation or occurrence of such events. In these women, the benefits of gonadotropin administration
need to be weighed against the risks. It should be noted however, that pregnancy itself, as well as
OHSS, also carries an increased risk of thromboembolic events.
Reproductive system neoplasms
There have been reports of ovarian and other reproductive system neoplasms, both benign and
malignant, in women who have undergone multiple drug regimens for infertility treatment. It is not yet
established whether or not treatment with gonadotropins increases the risk of these tumours in infertile
women.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed with Luveris.
Co-administration of lutropin alfa and follitropin alfa does not significantly alter neither the activity,
stability, pharmacokinetic, nor the pharmacodynamic properties of the active substances.
4.6 Fertility,pregnancy and lactation
Pregnancy
There is no indication for the use of Luveris during pregnancy.
Data on a limited number of exposed pregnancies indicate no adverse reactions of gonadotropins on
pregnancy, embryonal or foetal development, parturition or postnatal development following
controlled ovarian stimulation. No teratogenic effect of Luveris has been observed in animal studies.
In case of exposure during pregnancy, clinical data are not sufficient to exclude a teratogenic effect of
Luveris.
Lactation
Luveris is not indicated during breastfeeding.
Fertility
Luveris is indicated for the stimulation of follicular development, in association with FSH (see
section 4.1).
4.7 Effects on ability to drive and use machines
Luveris has no or negligible influence on the ability to drive and use machines.
Luveris is used for the stimulation of follicular development in association with follitropin alfa. In this
context, it is difficult to attribute adverse reactions to any one of the substances used.
In a clinical trial, mild and moderate injection site reactions (bruising, pain, redness, itching or
swelling) were reported in 7.4% and 0.9% of the injections, respectively. No severe injection site
reactions were reported. Ovarian Hyper-Stimulation Syndrome (OHSS) was observed in less than 6%
of patients treated with Luveris. No severe ovarian hyperstimulation syndrome was reported
(section 4.4).
In rare instances, adnexal torsion (a complication of ovarian enlargement), and haemoperitoneum have
been associated with human menopausal gonadotropin therapy. Although these adverse reactions were
not observed, there is the possibility that they may also occur with Luveris.
Ectopic pregnancy may also occur, especially in women with a history of prior tubal disease.
The following definitions apply to the frequency terminology used hereafter:
Very common (≥1/10)
Common (≥1/100 to <1/10)
Uncommon (≥1/1,000 to <1/100)
Rare (≥1/10,000 to <1/1,000)
Very rare (<1/10,000)
Frequency not known (cannot be estimated from the available data)
The following adverse reactions may be observed after administration of Luveris:
Immune system disorders
Very rare: Mild to severe hypersensitivity reactions including anaphylactic reactions and shock.
Nervous system disorders
Common: Headache.
Vascular disorders
Very rare: Thromboembolism, usually associated with severe OHSS.
Gastrointestinal disorders
Common: Abdominal pain
,
abdominal discomfort
,
nausea
,
vomiting, diarrhoea.
Reproductive system and breast disorders
Common: Mild or moderate OHSS (including associated symptomatology), ovarian cyst, breast pain,
pelvic pain.
General disorders and administration site conditions:
Common: Injection site reaction (e.g. pain, erythema, haematoma, swelling and/or irritation at the site
of injection).
The effects of an overdose of Luveris are unknown. Nevertheless there is a possibility that OHSS may
occur, which is further described in section 4.4.
Single doses of up to 40,000 IU of lutropin alfa have been administered to healthy female volunteers
without serious adverse reactions and were well tolerated.
Management
Treatment is directed to symptoms.
PHARMACOLOGICAL PROPERTIE
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Sex hormones and modulators of the genital system, gonadotropins. ATC
code: G03GA07
Lutropin alfa is a recombinant human luteinising hormone, a glycoprotein composed of non-
covalently bound α- and β-subunits. Luteinising hormone binds on the ovarian theca (and granulosa)
cells and testicular Leydig cells, to a receptor shared with human chorionic gonadotropin hormone
(hCG). This LH/CG transmembrane receptor is a member of the super-family of G protein-coupled
receptors; specifically, it has a large extra-cellular domain.
In vitro
the affinity binding of recombinant
hLH to the LH/CG receptor on Leydig tumour cells (MA-10) is between that for hCG and that of
pituitary hLH, but within the same order of magnitude.
In the ovaries, during the follicular phase, LH stimulates theca cells to secrete androgens, which will
be used as the substrate by granulosa cell aromatase enzyme to produce estradiol, supporting
FSH-induced follicular development. At mid-cycle, high levels of LH trigger corpus luteum formation
and ovulation. After ovulation, LH stimulates progesterone production in the corpus luteum by
increasing the conversion of cholesterol to pregnenolone.
In the stimulation of follicular development in anovulatory women deficient in LH and FSH, the
primary effect resulting from administration of lutropin alfa is an increase in estradiol secretion by the
follicles, the growth of which is stimulated by FSH.
In clinical trials, patients were defined by an endogenous serum LH level <1.2 IU/l as measured in a
central laboratory. However, it should be taken into account that there are variations between LH
measurements performed in different laboratories.
In these trials the ovulation rate per cycle was 70-75%.
5.2 Pharmacokinetic properties
The pharmacokinetics of lutropin alfa have been studied in pituitary desensitised female volunteers
from 75 IU up to 40,000 IU.
The pharmacokinetic profile of lutropin alfa is similar to that of urinary-derived hLH.
Following intravenous administration, lutropin alfa is rapidly distributed with an initial half-life of
approximately one hour and eliminated from the body with a terminal half-life of about 10-12 hours.
The steady state volume of distribution is around 10-14 l. Lutropin alfa shows linear
pharmacokinetics, as assessed by AUC which is directly proportional to the dose administered. Total
clearance is around 2 l/h, and less than 5% of the dose is excreted in the urine. The mean residence
time is approximately 5 hours.
Following subcutaneous administration, the absolute bioavailability is approximately 60%; the
terminal half-life is slightly prolonged. The lutropin alfa pharmacokinetics following single and
repeated administration of Luveris are comparable and the accumulation ratio of lutropin alfa is
minimal. There is no pharmacokinetic interaction with follitropin alfa when administered
simultaneously.
A comparative study between the registered powder and solvent and the solution for injection
formulations showed bioequivalence between the two formulations.
5.3 Preclinical safety data
Nonclinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential. As expected from the
heterologous protein nature of the hormone, lutropin alfa raised an antibody response in experimental
animals after a period that reduced the measurable serum LH levels but did not fully prevent its
biological action. No signs of toxicity due to the development of antibodies to lutropin alfa were
observed.
At doses of 10 IU/kg/day and greater, repeated administration of lutropin alfa to pregnant rats and
rabbits caused impairment of reproductive function including resorption of foetuses and reduced body
weight gain of the dams. However, drug-related teratogenesis was not observed in either animal
model.
Other studies have shown that lutropin alfa is not mutagenic.
PHARMACEUTICAL PARTICULARS
L-arginine HCl
Disodium phosphate dihydrate
Sodium dihydrogen phosphate monohydrate
Sodium hydroxide (for pH-adjustment)
Phosphoric acid (for pH-adjustment)
Polysorbate 20
L-methionine
Phenol
Water for injection
2 years.
Once opened: 28 days (within the shelf life).
6.4 Special precautions for storage
Store in a refrigerator (2ºC – 8ºC). Do not freeze.
Store in the original package in order to protect from light.
Once opened, the cartridge with the autoinjector must be stored for a maximum of 28 days in a
refrigerator (2ºC – 8ºC). (See section 6.3).
6.5 Nature and contents of container
Cartridge (type I glass), with a dark grey bromobutyl rubber plunger and a crimp cap made with
rubber stopper septum and aluminium, containing 0.72 ml of solution for injection.
6.6 Special precautions for disposal and other handling
After first use do not remove the cartridge from the autoinjector device.
The solution should not be administered if it contains particles or is not clear.
Any unused solution must be discarded not later than 28 days after first opening.
Discard used needles immediately after injection.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Merck Serono Europe Limited,
56 Marsh Wall
London E14 9TP
United Kingdom
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 29
th
November 2000.
Date of last renewal: 30
th
November 2005.
10.
DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency
http://www.ema.europa.eu/.
MANUFACTURER OF THE BIOLOGICAL ACTIVE
SUBSTANCE AND MANUFACTURING AUTHORISATION
HOLDER RESPONSIBLE FOR BATCH RELEASE
B. CONDITIONS OF THE MARKETING AUTHORISATION
A.
MANUFACTURER OF THE BIOLOGICAL ACTIVE SUBSTANCE AND
MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer of the biological active substance
Merck Serono SA
1170 Aubonne
Switzerland
Name and address of the manufacturer responsible for batch release
Merck Serono S.p.A.
Via delle Magnolie 15
I-70026 Modugno (Bari)
Italy
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to restricted medical prescription (See Annex I: Summary of Product
Characteristics, 4.2).
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
Pharmacovigilance system
The MAH must ensure that the system of pharmacovigilance, presented in Module 1.8.1. of the
Marketing Authorisation, is in place and functioning before and whilst the product is on the market.
Risk Management Plan
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version 2.1 of the Risk Management Plan (RMP) presented
in Module 1.8.2. of the Marketing Authorisation Application and any subsequent updates of the RMP
agreed by the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, the
updated RMP should be submitted at the same time as the next Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted:
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
At the request of the European Medicines Agency.
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
LUVERIS 75 IU, VIALS
NAME OF THE MEDICINAL PRODUCT
Luveris 75 IU powder and solvent for solution for injection.
Lutropin alfa
STATEMENT OF ACTIVE SUBSTANCE(S)
Composition: one vial of powder contains: lutropin alfa 75 IU.
Other ingredients: polysorbate 20, sucrose, sodium dihydrogen phosphate monohydrate, disodium
phosphate dihydrate, phosphoric acid, concentrated, sodium hydroxide, methionine and nitrogen.
One ampoule of solvent contains: 1 ml water for injection.
PHARMACEUTICAL FORM AND CONTENTS
1 vial of powder for solution for injection / 1 ampoule of solvent.
3 vials of powder for solution for injection / 3 ampoules of solvent.
10 vials of powder for solution for injection / 10 ampoules of solvent.
1 vial of powder for solution for injection / 1 vial of solvent.
3 vials of powder for solution for injection / 3 vials of solvent.
10 vials of powder for solution for injection / 10 vials of solvent.
METHOD AND ROUTE(S) OF ADMINISTRATION
Subcutaneous use.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
One vial of solvent contains: 1 ml water for injection.

























SPECIAL STORAGE CONDITIONS
Do not store above 25°C. Store in the original package in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Any unused product or waste material should be disposed of in accordance with local requirements.
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Merck Serono Europe Limited
56 Marsh Wall,
London E14 9TP
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/00/155/001 (1vial/ 1 ampoule)
EU/1/00/155/002 (3 vials/ 3 ampoules)
EU/1/00/155/003 (10 vials/ 10 ampoules)
EU/1/00/155/004 (1 vial/ 1 vial)
EU/1/00/155/005 (3 vial/ 3 vials)
EU/1/00/155/006 (10 vials/ 10 vials)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE






















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
CARTON BOX OF 1 CARTRIDGE
NAME OF THE MEDICINAL PRODUCT
Luveris 450 IU solution for injection.
Lutropin alfa
STATEMENT OF ACTIVE SUBSTANCE(S)
One multidose cartridge contains: lutropin alfa 450 IU/0.72 ml and allows delivery of 6 doses of 75 IU
of lutropin alfa.
Other ingredients: L-arginine HCl, disodium phosphate dihydrate, sodium dihydrogen phosphate
monohydrate, sodium hydroxide, phosphoric acid, polysorbate 20, L-methionine, phenol and water for
injections.
PHARMACEUTICAL FORM AND CONTENTS
1 cartridge of solution for injection.
METHOD AND ROUTE(S) OF ADMINISTRATION
Subcutaneous use.
For multiple injections only.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY





















SPECIAL STORAGE CONDITIONS
Store in a refrigerator. Do not freeze.
Store in the original package in order to protect from light.
Once opened, the cartridge within the autoinjector must be stored in a refrigerator for a maximum of
28 days.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Any unused solution or waste material should be disposed of in accordance with local requirements.
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Merck Serono Europe Limited
56 Marsh Wall
London E14 9TP
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE



















PACKAGE LEAFLET: INFORMATION FOR THE USER
Solvent in ampoules
Luveris 75 IU powder and solvent for solution for injection
Lutropin alfa
Read all of this leaflet carefully before you start using this medicine.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
In this leaflet:
1.
What Luveris is and what it is used for
2.
Before you use Luveris
3.
How to use Luveris
4.
Possible side effects
5.
How to store Luveris
6.
Further information
1.
WHAT LUVERIS IS AND WHAT IT IS USED FOR
What Luveris is
Luveris is a medicine containing lutropin alfa, a recombinant Luteinising Hormone (LH) which is
essentially similar to the hormone found naturally in humans, but is made by means of biotechnology.
It belongs to the family of hormones called gonadotropins, which are involved in the normal control of
reproduction.
What Luveris is used for
Luveris is recommended for the treatment of adult women who have been shown to produce very low
levels of some of the hormones involved in the natural reproductive cycle. The medicine is used
together with another hormone called Follicle Stimulating Hormone (FSH) to bring about the
development of follicles, which are in the ovary, the structures maturing the eggs (ova). It is followed
by treatment with a single dose of human chorionic gonadotropin (hCG), which leads to the release of
an egg from the follicle (ovulation).
2.
BEFORE YOU USE LUVERIS
If you are allergic (hypersensitive) to gonadotopins (such as luteinising hormone, follicle
stimulating hormone or human chorionic gonadotropin), or any of the other ingredients of
Luveris.
If you have cancer of ovaries, uterus or breast.
If you have had a brain tumour diagnosed.
If you have ovarian enlargement or sacs of fluid within the ovaries (ovarian cyst) of unknown
origin.
If you have unexplained vaginal bleeding.
Do not use Luveris if any of the above applies to you. If you are not sure, talk to your doctor or
pharmacist before using this medicine.
Take special care with Luveris
You and your partner's fertility should be evaluated before the treatment is started.
It is recommended not to use Luveris if you have any condition that usually makes a normal
pregnancy impossible, such as ovaries that do not work because of a condition called primary ovarian
failure, or malformations of sexual organs.
Porphyria
Tell your doctor before you start treatment, if you or any member of your family have porphyria (an
inability to break down porphyrins that may be passed on from parents to children).
Ovarian Hyper-Stimulation Syndrome (OHSS)
This medicine increases your risk of developing a condition called ovarian hyperstimulation syndrome
(OHSS). This is when your follicles develop too much and become large cysts. If you get lower
abdominal pain, gain any weight rapidly, feel sick or are vomiting or if you have difficulty in
breathing, talk to your doctor straight away who might ask you to stop using this medicine (see
section 4).
In case you are not ovulating, and if the recommended dose and schedule of administration are
adhered to, the occurrence of OHSS is less likely. Luveris treatment seldom causes severe OHSS
unless the medicine that is used for final follicular maturation (containing human Chorionic
Gonadotropin, hCG) is administered. If you are developing OHSS your doctor may not give you any
hCG in this treatment cycle and you may be told not to have sex or to use a barrier contraceptive
method for at least four days.
Your doctor will ensure, careful monitoring of ovarian response, based on ultrasound and blood
sampling before and during the course of treatment.
Multiple pregnancy
When using Luveris, you have a higher risk of being pregnant with more than one child at the same
time (“multiple pregnancy”, mostly twins), than if you conceived naturally. Multiple pregnancy may
lead to medical complications for you and your babies. You can reduce the risk of multiple pregnancy
by using the right dose of Luveris at the right times. When undergoing assisted reproductive
technology the risk of having a multiple pregnancy is related to your age, the quality and the number
of fertilised eggs or embryos placed inside you.
Miscarriage
When undergoing assisted reproductive technology or stimulation of your ovaries to produce eggs,
you are more likely to have a miscarriage than the average woman.
Ectopic pregnancy
Women with a history of tubal disease are at risk of ectopic pregnancy (pregnancy where the embryo
is implanted outside the womb), whether the pregnancy is obtained by spontaneous conception or with
fertility treatments.
Blood clotting problems (thromboembolic events)
If you had in the past or recently blood clots in the leg or in the lung, or a heart attack or stroke, or if
those happened in your family, then you might have a higher risk that these problems occur or become
worse with Luveris treatment.
Tumours of sexual organs
There have been reports of tumours of the ovary and other reproductive organs, both benign and
malignant, in women who have undergone multiple drug regimens for infertility treatment.
Birth defects
Birth defects after Assisted Reproductive Technology (ART) may be slightly higher than after
spontaneous conceptions. This could be due to differences in parental factors like maternal age,
genetics, as well as the ART procedures and multiple pregnancies.
Children
Luveris is not for use in children.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
Pregnancy and breast-feeding
Luveris is not indicated if you are pregnant or breast-feeding.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Luveris has no or negligible influence on the ability to drive and use machines.
Important information about some of the ingredients of Luveris
Luveris contains less than 1 mmol sodium (23 mg) per dose of 75 IU, i.e. it is essentially “sodium
free”.
Always use Luveris exactly as your doctor has told you. You should check with your doctor if you are
not sure.
Using this medicine
Your doctor will decide on the dose and schedule of administration, which are most appropriate for
you during this course of treatment.
How much to use
Luveris is usually used every day for up to three weeks simultaneously with injections of FSH.
The usual starting dose is 75 IU (1 vial) of Luveris together with 75 IU or 150 IU of FSH.
According to your response, your doctor may increase your dose of FSH by preferably 37.5-75 IU at 7
to 14-day intervals.
Your physician may decide to extend your treatment up to 5 weeks.
When the desired response has been obtained, a single injection of hCG is given 24 to 48 hours after
the last injections of Luveris and FSH. You are recommended to have sexual intercourse on the day of,
and the day following, administration of the hCG. Alternatively, Intrauterine Insemination (IUI) may
be performed.
If an excessive response is obtained, treatment should be stopped and hCG withheld (see section
headed ‘Possible Side Effects’). For the following cycle, your doctor will prescribe FSH at a lower
dose than that of the previous cycle.
Luveris
is intended for subcutaneous use which means it is given by injection under the skin. Each vial
is for single use only.
If you administer Luveris to yourself, please carefully read the following instructions:
Wash your hands. It is important that your hands and the items you use be as clean as possible.
Assemble everything you need. Find a clean area and lay out everything:
-
one reconstitution needle for dissolving the powder in the solvent,
a fine-bore needle for subcutaneous injection,
a sharps container for safe disposal of glass and needles.
Open the
ampoule of solvent
: On the head of the solvent ampoule, you will see a small
coloured dot. Directly below it is where the neck of the
ampoule has been treated to make it easier to break.
Gently flick the top section of the ampoule so that any
fluid in the neck of the ampoule drops into the bottom
chamber. Now press the ampoule firmly over the neck,
and break the ampoule away from the coloured dot.
Carefully place the open ampoule upright on the work-
surface.
Draw up the solvent: Attach the
reconstitution needle
to the syringe, with the syringe in one
hand, pick up the open ampoule, insert the needle and
draw up all of the solvent. Carefully set the syringe down
on the work-surface, taking care not to touch the needle.
Prepare the injection solution: Remove the protective cap from the
Luveris powder vial
, pick
up your syringe and slowly inject the solvent into the vial
of Luveris. Swirl gently without removing the syringe.
Do not shake.
After the powder has dissolved (which usually occurs immediately), check that the resulting
solution is clear and does not contain any particles. Turn
the vial upside down and gently draw the solution back
into the syringe.
You may also mix Luveris and follitropin alfa as an alternative to injecting each product separately.
After dissolving the Luveris powder, draw the solution back into the syringe and re-inject it into the
container with the follitropin alfa powder. Once the powder has dissolved, draw the solution back into
the syringe. Inspect for particles as before, and do not use if the solution is not clear.
Up to 3 containers of powder may be dissolved in 1 ml of solvent.




Change the needle for the
fine-bore needle
and remove any air bubbles: If you see air bubbles
in the syringe, hold the syringe with the needle pointing
upwards and gently flick the syringe until all the air
collects at the top. Gently push the plunger until the air
bubbles are gone.
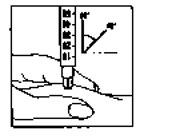
Immediately inject the solution: Your doctor or nurse will have already advised you where to
inject (e.g. tummy, front of thigh). Wipe the chosen area
with an alcohol swab. Firmly pinch the skin together and
insert the needle at a 45° to 90° angle using a dart-like
motion. Inject under the skin, as you were taught. Do not
inject directly into a vein. Inject the solution by pushing
gently on the plunger. Take as much time as you need to
inject all the solution. Immediately withdraw the needle
and clean the skin with an alcohol swab using a circular
motion.
Dispose of all used items: Once you have finished your injection, immediately discard all
needles and empty glass containers in the sharps container provided. Any unused solution must
be discarded.
If you use more Luveris than you should
The effects of an overdose of Luveris are unknown, nevertheless there is a possibility that ovarian
hyperstimulation syndrome may occur, which is further described in ‘Possible side effects'. However
this will only occur if hCG is administered (see section headed ‘Take special care with Luveris’).
If you forget to use Luveris
Do not use a double dose to make up for a forgotten dose. Please contact your doctor.
If you have any further question on the use of this medicine, ask your doctor or pharmacist.
Like all medicines, Luveris can cause side effects, although not everybody gets them.
The frequency of possible side effects listed below is defined using the following convention:
very common (affects more than 1 user in 10)
common (affects 1 to 10 users in 100)
uncommon (affects 1 to 10 users in 1,000)
rare (affects 1 to 10 users in 10,000)
very rare (affects less than 1 user in 10,000)
not known (frequency cannot be estimated from the available data)
Allergic reactions such as rash, red skin, hives, swelling of your face with difficulty breathing
can sometimes be serious. This side effect is very rare.
Lower abdominal pain together with nausea or vomiting may be the symptoms of Ovarian
Hyper-Stimulation Syndrome (OHSS). This may indicate that the ovaries over-reacted to the
treatment and that large ovarian cysts developed (see also section 2 “Take special care with
Luveris”). This side effect is common.
Serious blood clotting complications (thromboembolic events) usually with severe OHSS may

be found very rarely. This could cause chest pain, breathlessness, stroke or heart attack (see also
section 2 “Take special care with Luveris”).
Other common side effects
Nausea, vomiting, diarrhoea, abdominal discomfort or abdominal pain
Ovarian cysts, breast pain and pelvic pain.
Local reactions at the injection site, such as pain, redness or swelling
Torsion of the ovary and bleeding into the abdomen have not been reported with Luveris, however,
there have been rare cases reported following treatment with human menopausal gonadotropin (hMG),
a urine-derived medication also containing LH.
Ectopic pregnancy (embryo implanted outside the womb) may occur especially in women with a
history of prior tubal disease.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Luveris after the expiry date, which is stated on the vials after EXP. The expiry date refers
to the last day of that month.
Do not store above 25°C.
Store in the original package in order to protect from light.
Do not use Luveris if you notice any visible signs of deterioration, such as discolouration of the
powder or damage to the container.
The medicine should be administered immediately after dissolving the powder.
The solution should not be administered if it contains particles or is not clear.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
What Luveris contains
The active substance is lutropin alfa. One vial of powder for injection contains 75 IU (International
Units).
Lutropin alfa is recombinant human luteinising hormone, r-hLH, produced by recombinant DNA
technology.
The other ingredients are polysorbate 20, sucrose, sodium dihydrogen phosphate monohydrate,
disodium phosphate dihydrate, concentrated phosphoric acid, sodium hydroxide, methionine and
nitrogen.
The solvent is water for injection.
What Luveris looks like and contents of the pack
Luveris is provided as a powder and solvent for solution for injection.
It is supplied in packs containing 1, 3 or 10 vials of powder, together with the same number of solvent
ampoules.
Each vial of powder contains 75 IU of lutropin alfa and each ampoule of solvent contains 1 ml of
water for injection.
Marketing Authorisation Holder
Merck Serono Europe Limited
56 Marsh Wall
London E14 9TP
United Kingdom
Manufacturer
Merck Serono S.p.A.
Via delle Magnolie 15
I-70026 Modugno (Bari)
Italy
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder.
België/Belgique/Belgien
MERCK NV/SA
Brusselsesteenweg 288
B-3090 Overijse
Tél/Tel: +32-2-686 07 11
Luxembourg/Luxemburg
MERCK NV/SA
Brusselsesteenweg 288
B-3090 Overijse, Belgique/Belgien
Tél/Tel: +32-2-686 07 11
България
Мерк България ЕАД
Бул. „Проф. Цветан Лазаров“ 83
София 1582
България
Teл: +359 28075 111
Magyarország
Merck Kft.
Bocskai út 134-146.
H-1113 Budapest
Tel: +36-1-463-8100
Česká republika
Merck spol. s r.o.
Na Hrebenech II. 1718/10
CZ-140 00 Praha 4
Tel. +420 272084 211
Malta
Cherubino Ltd
Delf Building
Sliema Road
MT-GZR 06 Gzira Malta
Tel: +356-21-343270/1/2/3/4
Danmark
Merck A/S
Strandvejen 102 B, 4th
DK-2900 Hellerup
Tlf: +45 35253550
Nederland
Merck BV
Tupolevlaan 41-61
NL-1119 NW Schiphol-Rijk
Tel: +31-20-6582800
Deutschland
Merck Serono GmbH
Alsfelder Straße 17
D-64289 Darmstadt
Tel: +49-6151-6285-0
Norge
Merck Serono Norge
Luhrtoppen 2
N-1470 Lørenskog
Tlf: +47 67 90 35 90
Eesti
Merck Serono OÜ
Tornimäe 7 - 132
EE-10145, Tallinn
Tel: +372 682 5882
Österreich
Merck GesmbH.
Zimbagasse 5
A-1147 Wien
Tel: +43 1 57600-0
Ελλάδα
Merck A.E.
Κηφισίας 41-45, Κτίριο Β
GR-151 23 Μαρούσι
Αθήνα
T: +30-210-61 65 100
Polska
Merck Sp. z o.o.
Al. Jerozolimskie 178
PL-02-486 Warszawa
Tel.: +48 22 53 59 700
España
Merck S.L.
María de Molina, 40
E-28006 Madrid
Línea de Información: 900 200 400
Tel: +34-91-745 44 00
Portugal
Merck, s.a.
Rua Alfredo da Silva, 3-C
P-1300-040 Lisboa
Tel: +351-21-361 35 00
France
Merck Serono s.a.s.
37, rue Saint-Romain
F-69379 Lyon cedex 08
Tél.: +33-4-72 78 25 25
Numéro vert : 0 800 888 024
România
MERCK d.o.o.,
Dunajska cesta 119
SI-1000 Lubliana, Slovenia
Tel: +386 1 560 3 800
Ireland
Merck Serono Ltd
Bedfont Cross, Stanwell Road
Feltham, Middlesex TW14 8NX
United Kingdom
Tel: +44-20 8818 7200
Slovenija
MERCK d.o.o.
Dunajska cesta 119
SI-1000 Ljubljana
Tel: +386 1 560 3 800
Ísland
Icepharma hf
Lynghálsi 13
IS-110 Reykjavík
Tel: + 354 540 8000
Slovenská
republika
Merck spol. s r.o.
Tuhovská 3
SK-831 06 Bratislava
Tel: + 421 2 49 267 111
Italia
Merck Serono S.p.A.
Via Casilina 125
I-00176 Roma
Tel: +39-06-70 38 41
Suomi
/
Finland
Merck Oy
Pihatörmä 1 C
FIN-02240 Espoo
Puh/Tel: +358-9-8678 700
Κύπρος
Χρ. Γ. Παπαλοϊζου Λτδ
Λeωfόρος Κιλκίς 35,
CY-2234 Λatsιά, Λευκωσία
Τηλ: +357-22-490305
Sverige
Merck AB
Box 3033
169 03 Solna
Tel: +46-8-562 445 00
Latvija
Merck Serono SIA
Duntes iela 23A
LV-1005, Rīga
Tel: +371 67152500
United Kingdom
Merck Serono Ltd
Bedfont Cross, Stanwell Road
Feltham, Middlesex TW14 8NX – UK
Tel: +44-20 8818 7200
Lietuva
Merck Serono UAB
Savanoriu pr. 192,
LT-44151 Kaunas
Tel: +370 37320603
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency web site:
http://www.ema.europa.eu.
PACKAGE LEAFLET: INFORMATION FOR THE USER
Solvent in vials
Luveris 75 IU powder and solvent for solution for injection
Lutropin alfa
Read all of this leaflet carefully before you start using this medicine.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if
their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
What Luveris is and what it is used for
WHAT LUVERIS IS AND WHAT IT IS USED FOR
What Luveris is
Luveris is a medicine containing lutropin alfa, a recombinant Luteinising Hormone (LH) which is
essentially similar to the hormone found naturally in humans, but is made by means of biotechnology.
It belongs to the family of hormones called gonadotropins, which are involved in the normal control of
reproduction.
What Luveris is used for
Luveris is recommended for the treatment of adult women who have been shown to produce very low
levels of some of the hormones involved in the natural reproductive cycle. The medicine is used
together with another hormone called Follicle Stimulating Hormone (FSH) to bring about the
development of follicles which are in the ovary, the structures maturing the eggs (ova). It is followed
by treatment with a single dose of human chorionic gonadotropin (hCG), which leads to the release of
an egg from the follicle (ovulation).
If you are allergic (hypersensitive) to gonadotropins (such as luteinising hormone, follicle
stimulating hormone or human chorionic gonadotropin), or any of the other ingredients of
Luveris.
If you have cancer of ovaries, uterus or breast.
If you have had a brain tumour diagnosed.
If you have ovarian enlargement or sac of fluid within the ovaries (ovarian cyst) of unknown
origin.
If you have unexplained vaginal bleeding.
Do not use Luveris if any of the above applies to you. If you are not sure, talk to your doctor or
pharmacist before using this medicine.
Take special care with Luveris
You and your partner's fertility should be evaluated before the treatment is started.
It is recommended not to use Luveris if you have any condition that usually makes a normal
pregnancy impossible, such as ovaries that do not work because of a condition called primary ovarian
failure, or malformations of sexual organs.
Porphyria
Tell your doctor before you start treatment, if you or any member of your family have porphyria (an
inability to break down porphyrins that may be passed on from parents to children).
Ovarian Hyper-Stimulation Syndrome (OHSS)
This medicine increases your risk of developing a condition called ovarian hyperstimulation syndrome
(OHSS). This is when your follicles develop too much and become large cysts. If you get lower
abdominal pain, gain any weight rapidly, feel sick or are vomiting or if you have difficulty in
breathing, talk to your doctor straight away who might ask you to stop using this medicine (see
section 4).
In case you are not ovulating, and if the recommended dose and schedule of administration are
adhered to, the occurrence of OHSS is less likely. Luveris treatment seldom causes severe OHSS
unless the medicine that is used for final follicular maturation (containing human Chorionic
Gonadotropin, hCG) is administered. If you are developing OHSS your doctor may not give you any
hCG in this treatment cycle and you may be told not to have sex or to use a barrier contraceptive
method for at least four days.
Your doctor will ensure, careful monitoring of ovarian response, based on ultrasound and blood
sampling before and during the course of treatment.
Multiple pregnancy
When using Luveris, you have a higher risk of being pregnant with more than one child at the same
time (“multiple pregnancy”, mostly twins), than if you conceived naturally. Multiple pregnancy may
lead to medical complications for you and your babies. You can reduce the risk of multiple pregnancy
by using the right dose of Luveris at the right times. When undergoing assisted reproductive
technology the risk of having a multiple pregnancy is related to your age, the quality and the number
of fertilised eggs or embryos placed inside you.
Miscarriage
When undergoing assisted reproductive technology or stimulation of your ovaries to produce eggs,
you are more likely to have a miscarriage than the average woman.
Ectopic pregnancy
Women with a history of tubal disease are at risk of ectopic pregnancy (pregnancy where the embryo
is implanted outside the womb), whether the pregnancy is obtained by spontaneous conception or with
fertility treatments.
Blood clotting problems (thromboembolic events)
If you had in the past or recently blood clots in the leg or in the lung, or a heart attack or stroke, or if
those happened in your family, then you might have a higher risk that these problems occur or become
worse with Luveris treatment.
Tumours of sexual organs
There have been reports of tumours of the ovary and other reproductive organs, both benign and
malignant, in women who have undergone multiple drug regimens for infertility treatment.
Birth defects
Birth defects after Assisted Reproductive Technology (ART) may be slightly higher than after
spontaneous conceptions. This could be due to differences in parental factors like maternal age,
genetics, as well as the ART procedures and multiple pregnancies.
Children
Luveris is not for use in children.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
Pregnancy and breast-feeding
Luveris is not indicated if you are pregnant or breast-feeding.
Ask your doctor or pharmacist for advice before taking any medicine
Driving and using machines
Luveris has no or negligible influence on the ability to drive and use machines.
Important information about some of the ingredients of Luveris
Luveris contains less than 1 mmol sodium (23 mg) per dose of 75 IU, i.e. it is essentially “sodium
free”.
Always use Luveris exactly as your doctor has told you. You should check with your doctor if you are
not sure.
Using this medicine
Your doctor will decide on the dose and schedule of administration, which are most appropriate for
you during this course of treatment.
How much to use
Luveris is usually used every day for up to three weeks simultaneously with injections of FSH.
The usual starting dose is 75 IU (1 vial) of Luveris together with 75 IU or 150 IU of FSH.
According to your response, your doctor may increase your dose of FSH by preferably 37.5-75 IU at 7
to 14-day intervals.
Your physician may decide to extend your treatment up to 5 weeks.
When the desired response has been obtained, a single injection of hCG is given 24 to 48 hours after
the last injections of Luveris and FSH. You are recommended to have sexual intercourse on the day of,
and the day following, administration of the hCG. Alternatively, intrauterine insemination (IUI) may
be performed.
If an excessive response is obtained, treatment should be stopped and hCG withheld (see section
headed ‘Possible Side Effects’). For the following cycle, your doctor will prescribe FSH at a lower
dose than that of the previous cycle.
Luveris is intended for subcutaneous use which means it is given by injection under the skin. Each vial
is for single use only.
If you administer Luveris to yourself, please carefully read the following instructions:
Wash your hands. It is important that your hands and the items you use be as clean as possible.
Assemble everything you need. Find a clean area and lay out everything:
-
one reconstitution needle for dissolving the powder in the solvent,
a fine-bore needle for subcutaneous injection,
a sharps container for safe disposal of glass and needles.
Remove the protective cap from the
solvent vial.
Attach the
reconstitution
needle
to the
syringe and draw up some air into the syringe by pulling
the plunger to approximately the 1 ml mark. Then, insert
the needle into the vial, push the plunger to expel the air,
turn the vial upside down and gently draw up all the
solvent.
Set the syringe down carefully on the work-surface
taking care not to touch the needle.
Prepare the injection solution: Remove the protective cap from the
Luveris powder
vial
, pick
up your syringe and slowly inject the solvent into the vial
of Luveris. Swirl gently without removing the syringe.
Do not shake.
After the powder has dissolved (which usually occurs immediately), check that the resulting
solution is clear and does not contain any particles. Turn the vial upside down and gently draw
the solution back into the syringe.
You may also mix Luveris and follitropin alfa as an alternative to injecting each product separately.
After dissolving the Luveris powder, draw the solution back into the syringe and re-inject it into the
container with the follitropin alfa powder. Once the powder has dissolved, draw the solution back into
the syringe. Inspect for particles as before, and do not use if the solution is not clear.
Up to 3 containers of powder may be dissolved in 1 ml of solvent.
Change the needle for the
fine-bore needle
and remove any air bubbles: If you see air bubbles
in the syringe, hold the syringe with the needle pointing
upwards and gently flick the syringe until all the air
collects at the top. Gently push the plunger until the air
bubbles are gone.



Immediately inject the solution: Your doctor or nurse will have already advised you where to
inject (e.g. tummy, front of thigh). Wipe the chosen area
with an alcohol swab. Firmly pinch the skin together and
insert the needle at a 45° to 90° angle using a dart-like
motion. Inject under the skin, as you were taught. Do not
inject directly into a vein. Inject the solution by pushing
gently on the plunger. Take as much time as you need to
inject all the solution. Immediately withdraw the needle
and clean the skin with an alcohol swab using a circular
motion.
Dispose of all used items: Once you have finished your injection, immediately discard all
needles and empty glass containers in the sharps container provided. Any unused solution must
be discarded.
If you use more Luveris than you should
The effects of an overdose of Luveris are unknown, nevertheless there is a possibility that ovarian
hyperstimulation syndrome may occur, which is further described in ‘Possible side effects'. However
this will only occur if hCG is administered (see section headed ‘Take special care with Luveris’).
If you forget to use Luveris
Do not use a double dose to make up for a forgotten dose. Please contact your doctor.
If you have any further question on the use of this medicine, ask your doctor or pharmacist
Like all medicines, Luveris can cause side effects, although not everybody gets them.
The frequency of possible side effects listed below is defined using the following convention:
very common (affects more than 1 user in 10)
common (affects 1 to 10 users in 100)
uncommon (affects 1 to 10 users in 1,000)
rare (affects 1 to 10 users in 10,000)
very rare (affects less than 1 user in 10,000)
not known
(
frequency cannot be estimated from the available data)
Allergic reactions such as rash, red skin, hives, swelling of your face with difficulty breathing
can sometimes be serious. This side effect is very rare.
Lower abdominal pain together with nausea or vomiting may be the symptoms of Ovarian
Hyper-Stimulation Syndrome (OHSS). This may indicate that the ovaries over-reacted to the
treatment and that large ovarian cysts developed (see also section 2 “Take special care with
Luveris”). This side effect is common.
Serious blood clotting complications (thromboembolic events) usually with severe OHSS may
be found very rarely. This could cause chest pain, breathlessness, stroke or heart attack (see also
section 2 “Take special care with Luveris”).
Other common side effects
Nausea, vomiting, diarrhoea, abdominal discomfort or abdominal pain
Ovarian cysts, breast pain and pelvic pain.
Local reactions at the injection site, such as pain, redness or swelling
Torsion of the ovary and bleeding into the abdomen have not been reported with Luveris, however,
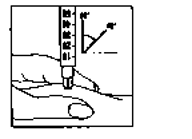
there have been rare cases reported following treatment with human menopausal gonadotropin (hMG),
a urine-derived medication also containing LH.
Ectopic pregnancy (embryo implanted outside the womb) may occur especially in women with a
history of prior tubal disease.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Luveris after the expiry date, which is stated on the vials after EXP. The expiry date refers
to the last day of that month.
Do not store above 25°C.
Store in the original package in order to protect from light.
Do not use Luveris if you notice any visible signs of deterioration, such as discolouration of the
powder or damage to the container.
The medicine should be administered immediately after dissolving the powder.
The solution should not be administered if it contains particles or is not clear.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
What Luveris contains
The active substance is lutropin alfa. One vial of powder for injection contains 75 IU (International
Units).
Lutropin alfa is recombinant human luteinising hormone, r-hLH, produced by recombinant DNA
technology.
The other ingredients are polysorbate 20, sucrose, sodium dihydrogen phosphate monohydrate,
disodium phosphate dihydrate, concentrated phosphoric acid, sodium hydroxide, methionine and
nitrogen.
The solvent is water for injection.
What Luveris looks like and contents of the pack
Luveris is provided as a powder and solvent for solution for injection.
It is supplied in packs containing 1, 3 or 10 vials of powder, together with the same number of solvent
vials.
Each vial of powder contains 75 IU of lutropin alfa and each vial of solvent contains 1 ml of water for
injection.
Marketing Authorisation Holder
Merck Serono Europe Limited
56 Marsh Wall
London E14 9TP
United Kingdom
Manufacturer
Merck Serono S.p.A.
Via delle Magnolie 15
I-70026 Modugno (Bari)
Italy
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder.
België/Belgique/Belgien
MERCK NV/SA
Brusselsesteenweg 288
B-3090 Overijse
Tél/Tel: +32-2-686 07 11
Luxembourg/Luxemburg
MERCK NV/SA
Brusselsesteenweg 288
B-3090 Overijse, Belgique/Belgien
Tél/Tel: +32-2-686 07 11
България
Мерк България ЕАД
Бул. „Проф. Цветан Лазаров“ 83
София 1582
България
Teл: +359 28075 111
Magyarország
Merck Kft.
Bocskai út 134-146.
H-1113 Budapest
Tel: +36-1-463-8100
Česká republika
Merck spol. s r.o.
Na Hrebenech II. 1718/10
CZ-140 00 Praha 4
Tel. +420 272084 211
Malta
Cherubino Ltd
Delf Building
Sliema Road
MT-GZR 06 Gzira Malta
Tel: +356-21-343270/1/2/3/4
Danmark
Merck A/S
Strandvejen 102 B, 4th
DK-2900 Hellerup
Tlf: +45 35253550
Nederland
Merck BV
Tupolevlaan 41-61
NL-1119 NW Schiphol-Rijk
Tel: +31-20-6582800
Deutschland
Merck Serono GmbH
Alsfelder Straße 17
D-64289 Darmstadt
Tel: +49-6151-6285-0
Norge
Merck Serono Norge
Luhrtoppen 2
N-1470 Lørenskog
Tlf: +47 67 90 35 90
Eesti
Merck Serono OÜ
Tornimäe 7 - 132
EE-10145, Tallinn
Tel: +372 682 5882
Österreich
Merck GesmbH.
Zimbagasse 5
A-1147 Wien
Tel: +43 1 57600-0
Ελλάδα
Merck A.E.
Κηφισίας 41-45, Κτίριο Β
GR-151 23 Μαρούσι
Αθήνα
T: +30-210-61 65 100
Polska
Merck Sp. z o.o.
Al. Jerozolimskie 178
PL-02-486 Warszawa
Tel.: +48 22 53 59 700
España
Merck S.L.
María de Molina, 40
E-28006 Madrid
Línea de Información: 900 200 400
Tel: +34-91-745 44 00
Portugal
Merck, s.a.
Rua Alfredo da Silva, 3-C
P-1300-040 Lisboa
Tel: +351-21-361 35 00
France
Merck Serono s.a.s.
37, rue Saint-Romain
F-69379 Lyon cedex 08
Tél.: +33-4-72 78 25 25
Numéro vert : 0 800 888 024
România
MERCK d.o.o.,
Dunajska cesta 119
SI-1000 Lubliana, Slovenia
Tel: +386 1 560 3 800
Ireland
Merck Serono Ltd
Bedfont Cross, Stanwell Road
Feltham, Middlesex TW14 8NX
United Kingdom
Tel: +44-20 8818 7200
Slovenija
MERCK d.o.o.
Dunajska cesta 119
SI-1000 Ljubljana
Tel: +386 1 560 3 800
Ísland
Icepharma hf
Lynghálsi 13
IS-110 Reykjavík
Tel: + 354 540 8000
Slovenská
republika
Merck spol. s r.o.
Tuhovská 3
SK-831 06 Bratislava
Tel: + 421 2 49 267 111
Italia
Merck Serono S.p.A.
Via Casilina 125
I-00176 Roma
Tel: +39-06-70 38 41
Suomi
/
Finland
Merck Oy
Pihatörmä 1 C
FIN-02240 Espoo
Puh/Tel: +358-9-8678 700
Κύπρος
Χρ. Γ. Παπαλοϊζου Λτδ
Λeωfόρος Κιλκίς 35,
CY-2234 Λatsιά, Λευκωσία
Τηλ: +357-22-490305
Sverige
Merck AB
Box 3033
169 03 Solna
Tel: +46-8-562 445 00
Latvija
Merck Serono SIA
Duntes iela 23A
LV-1005, Rīga
Tel: +371 67152500
United Kingdom
Merck Serono Ltd
Bedfont Cross, Stanwell Road
Feltham, Middlesex TW14 8NX – UK
Tel: +44-20 8818 7200
Lietuva
Merck Serono UAB
Savanoriu pr. 192,
LT-44151 Kaunas
Tel: +370 37320603
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency web site:
http://www.ema.europa.eu.
PACKAGE LEAFLET: INFORMATION FOR THE USER
Luveris 450 IU solution for injection
Lutropin alfa
Read all of this leaflet carefully before you start using this medicine.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if
their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
What Luveris is and what it is used for
WHAT LUVERIS IS AND WHAT IT IS USED FOR
What Luveris is
Luveris is a medicine containing lutropin alfa, a recombinant Luteinising Hormone (LH) which is
essentially similar to the hormone found naturally in humans, but is made by means of biotechnology.
It belongs to the family of hormones called gonadotropins, which are involved in the normal control of
reproduction.
What Luveris is used for
Luveris is recommended for the treatment of adult women who have been shown to produce very low
levels of some of the hormones involved in the natural reproductive cycle. The medicine is used
together with another hormone called Follicle Stimulating Hormone (FSH) to bring about the
development of follicles which are in the ovary, the structures maturing the eggs (ova). It is followed
by treatment with a single dose of human chorionic gonadotropin (hCG), which leads to the release of
an egg from the follicle (ovulation).
If you are allergic (hypersensitive) to gonadotropins (such as luteinising hormone, follicle
stimulating hormone or human chorionic gonadotropin), or any of the other ingredients of
Luveris.
If you have cancer of ovaries, uterus or breast.
If you have had a brain tumour diagnosed.
If you have ovarian enlargement or sacs of fluid within the ovaries (ovarian cyst) of unknown
origin.
If you have unexplained vaginal bleeding.
Do not use Luveris if any of the above applies to you. If you are not sure, talk to your doctor or
pharmacist before using this medicine.
Take special care with Luveris
You and your partner's fertility should be evaluated before the treatment is started.
It is recommended not to use Luveris if you have any condition that usually makes a normal
pregnancy impossible, such as ovaries that do not work because of a condition called primary ovarian
failure, or malformations of sexual organs.
Porphyria
Tell your doctor before you start treatment, if you or any member of your family have porphyria (an
inability to break down porphyrins that may be passed on from parents to children).
Ovarian Hyper-Stimulation Syndrome (OHSS)
This medicine increases your risk of developing a condition called ovarian hyperstimulation syndrome
(OHSS). This is when your follicles develop too much and become large cysts. If you get lower
abdominal pain, gain any weight rapidly, feel sick or are vomiting or if you have difficulty in
breathing, talk to your doctor straight away who might ask you to stop using this medicine (see
section 4).
In case you are not ovulating, and if the recommended dose and schedule of administration are
adhered to, the occurrence of OHSS is less likely. Luveris treatment seldom causes severe OHSS
unless the medicine that is used for final follicular maturation (containing human Chorionic
Gonadotropin, hCG) is administered. If you are developing OHSS your doctor may not give you any
hCG in this treatment cycle and you may be told not to have sex or to use a barrier contraceptive
method for at least four days.
Your doctor will ensure, careful monitoring of ovarian response, based on ultrasound and blood
sampling before and during the course of treatment.
Multiple pregnancy
When using Luveris, you have a higher risk of being pregnant with more than one child at the same
time (“multiple pregnancy”, mostly twins), than if you conceived naturally. Multiple pregnancy may
lead to medical complications for you and your babies. You can reduce the risk of multiple pregnancy
by using the right dose of Luveris at the right times. When undergoing assisted reproductive
technology the risk of having a multiple pregnancy is related to your age, the quality and the number
of fertilised eggs or embryos placed inside you.
Miscarriage
When undergoing assisted reproductive technology or stimulation of your ovaries to produce eggs,
you are more likely to have a miscarriage than the average woman.
Ectopic pregnancy
Women with a history of tubal disease are at risk of ectopic pregnancy (pregnancy where the embryo
is implanted outside the womb), whether the pregnancy is obtained by spontaneous conception or with
fertility treatments.
Blood clotting problems (thromboembolic events)
If you had in the past or recently blood clots in the leg or in the lung, or a heart attack or stroke, or if
those happened in your family, then you might have a higher risk that these problems occur or become
worse with Luveris treatment.
Tumours of sexual organs
There have been reports of tumours of the ovary and other reproductive organs, both benign and
malignant, in women who have undergone multiple drug regimens for infertility treatment.
Birth defects
Birth defects after Assisted Reproductive Technology (ART) may be slightly higher than after
spontaneous conceptions. This could be due to differences in parental factors like maternal age,
genetics, as well as the ART procedures and multiple pregnancies.
Children
Luveris is not for use in children.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
Pregnancy and breast-feeding
Luveris is not indicated if you are pregnant or breast-feeding.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Luveris has no or negligible influence on the ability to drive and use machines.
Important information about some of the ingredients of Luveris
Luveris contains less than 1 mmol sodium (23 mg) per dose of 75 IU, i.e. it is essentially “sodium
free”.
Always use Luveris exactly as your doctor has told you. You should check with your doctor if you are
not sure.
Using this medicine
Your doctor will decide on the dose and schedule of administration, which are most appropriate for
you during this course of treatment.
How much to use
Luveris is usually used every day for up to three weeks simultaneously with injections of FSH.
The usual starting dose
is 75 IU of Luveris together with 75 IU or 150 IU of FSH.
According to your response, your doctor may increase your dose of FSH by preferably 37.5-75 IU at 7
to 14-day intervals.
Your physician may decide to extend your treatment up to 5 weeks.
When the desired response has been obtained, a single injection of hCG is given 24 to 48 hours after
the last injections of Luveris and FSH. You are recommended to have sexual intercourse on the day of,
and the day following, administration of the hCG. Alternatively, Intrauterine Insemination (IUI) may
be performed.
If an excessive response is obtained, treatment should be stopped and hCG withheld (see section
headed ‘Possible Side Effects’). For the following cycle, your doctor will prescribe FSH at a lower
dose than that of the previous cycle.
Luveris is intended for subcutaneous use which means it is given by injection under the skin. The
cartridge is intended for multiple injections (6 doses).
Luveris cartridge is to be used with the autoinjector device provided separately.
If you administer Luveris to yourself:
Wash your hands. It is important that your hands and the items you use be as clean as possible.
Wipe the area chosen for the injection with an alcohol swab.
For injection, please read carefully the autoinjector device Instructions for Use provided with
the device.
Do not inject directly into a vein.
After the injection clean the skin with an alcohol swab using a circular motion.
Once you have finished your injection, immediately discard the needle.
If you use more Luveris than you should
The effects of an overdose of Luveris are unknown, nevertheless there is a possibility that ovarian
hyperstimulation syndrome may occur, which is further described in ‘Possible side effects'. However
this will only occur if hCG is administered (see section headed ‘Take special care with Luveris’).
If you forget to use Luveris
Do not use a double dose to make up for a forgotten dose. Please contact your doctor.
If you have any further question on the use of this medicine, ask your doctor or pharmacist.
Like all medicines, Luveris can cause side effects, although not everybody gets them.
The frequency of possible side effects listed below is defined using the following convention:
very common (affects more than 1 user in 10)
common (affects 1 to 10 users in 100)
uncommon (affects 1 to 10 users in 1,000)
rare (affects 1 to 10 users in 10,000)
very rare (affects less than 1 user in 10,000)
not known (frequency cannot be estimated from the available data)
Allergic reactions such as rash, red skin, hives, swelling of your face with difficulty breathing
can sometimes be serious. This side effect is very rare.
Lower abdominal pain together with nausea or vomiting may be the symptoms of Ovarian
Hyper-Stimulation Syndrome (OHSS). This may indicate that the ovaries over-reacted to the
treatment and that large ovarian cysts developed (see also section 2 “Take special care with
Luveris”). This side effect is common.
Serious blood clotting complications (thromboembolic events) usually with severe OHSS may
be found very rarely. This could cause chest pain, breathlessness, stroke or heart attack (see also
section 2 “Take special care with Luveris”).
Other common side effects
Nausea, vomiting, diarrhoea, abdominal discomfort or abdominal pain
Ovarian cysts, breast pain and pelvic pain.
Local reactions at the injection site, such as pain, redness or swelling
Torsion of the ovary and bleeding into the abdomen have not been reported with Luveris, however,
there have been rare cases reported following treatment with human menopausal gonadotropin (hMG),
a urine-derived medication also containing LH.
Ectopic pregnancy (embryo implanted outside the womb) may occur especially in women with a
history of prior tubal disease.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Luveris after the expiry date, which is stated on the cartridge after EXP. The expiry date
refers to the last day of that month.
Store in a refrigerator (2ºC – 8ºC). Do not freeze.
Store in the original package in order to protect from light.
After first use do not remove the cartridge from the autoinjector device.
Once opened, the cartridge with the autoinjector must be stored for a maximum of 28 days in a
refrigerator.
Do not use Luveris if you notice any visible signs of deterioration.
The solution should not be administered if it contains particles or is not clear.
Any unused solution must be discarded not later than 28 days after first opening.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
What Luveris contains
The active substance is lutropin alfa .
Lutropin alfa is recombinant human luteinising hormone, r-hLH, produced by recombinant DNA
technology. \
Each cartridge of Luveris contains 450 IU of lutropin alfa in 0.72 ml water for injections that allows
delivering six doses of 75 IU of lutropin alfa.
The other ingredients are L-arginine HCl, disodium phosphate dihydrate, sodium dihydrogen
phosphate monohydrate, sodium hydroxide, phosphoric acid, polysorbate 20, L-methionine, phenol
and water for injections.
What Luveris looks like and contents of the pack
Luveris 450 IU/0.72 ml is presented as a solution for injection in a cartridge.
It is supplied in packs of 1 cartridge containing 6 doses.
An appropriate autoinjector device is supplied separately.
The solution is clear and colourless.
Marketing Authorisation Holder
Merck Serono Europe Limited
56 Marsh Wall
London E14 9TP
United Kingdom
Manufacturer
Merck Serono S.p.A.
Via delle Magnolie 15
I-70026 Modugno (Bari)
Italy
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder.
België/Belgique/Belgien
MERCK NV/SA
Brusselsesteenweg 288
B-3090 Overijse
Tél/Tel: +32-2-686 07 11
Luxembourg/Luxemburg
MERCK NV/SA
Brusselsesteenweg 288
B-3090 Overijse, Belgique/Belgien
Tél/Tel: +32-2-686 07 11
България
Мерк България ЕАД
Бул. „Проф. Цветан Лазаров“ 83
София 1582
България
Teл: +359 28075 111
Magyarország
Merck Kft.
Bocskai út 134-146.
H-1113 Budapest
Tel: +36-1-463-8100
Česká republika
Merck spol. s r.o.
Na Hrebenech II. 1718/10
CZ-140 00 Praha 4
Tel. +420 272084 211
Malta
Cherubino Ltd
Delf Building
Sliema Road
MT-GZR 06 Gzira Malta
Tel: +356-21-343270/1/2/3/4
Danmark
Merck A/S
Strandvejen 102 B, 4th
DK-2900 Hellerup
Tlf: +45 35253550
Nederland
Merck BV
Tupolevlaan 41-61
NL-1119 NW Schiphol-Rijk
Tel: +31-20-6582800
Deutschland
Merck Serono GmbH
Alsfelder Straße 17
D-64289 Darmstadt
Tel: +49-6151-6285-0
Norge
Merck Serono Norge
Luhrtoppen 2
N-1470 Lørenskog
Tlf: +47 67 90 35 90
Eesti
Merck Serono OÜ
Tornimäe 7 - 132
EE-10145, Tallinn
Tel: +372 682 5882
Österreich
Merck GesmbH.
Zimbagasse 5
A-1147 Wien
Tel: +43 1 57600-0
Ελλάδα
Merck A.E.
Κηφισίας 41-45, Κτίριο Β
GR-151 23 Μαρούσι
Αθήνα
T: +30-210-61 65 100
Polska
Merck Sp. z o.o.
Al. Jerozolimskie 178
PL-02-486 Warszawa
Tel.: +48 22 53 59 700
España
Merck S.L.
María de Molina, 40
E-28006 Madrid
Línea de Información: 900 200 400
Tel: +34-91-745 44 00
Portugal
Merck, s.a.
Rua Alfredo da Silva, 3-C
P-1300-040 Lisboa
Tel: +351-21-361 35 00
France
Merck Serono s.a.s.
37, rue Saint-Romain
F-69379 Lyon cedex 08
Tél.: +33-4-72 78 25 25
Numéro vert : 0 800 888 024
România
MERCK d.o.o.,
Dunajska cesta 119
SI-1000 Lubliana, Slovenia
Tel: +386 1 560 3 800
Ireland
Merck Serono Ltd
Bedfont Cross, Stanwell Road
Feltham, Middlesex TW14 8NX
United Kingdom
Tel: +44-20 8818 7200
Slovenija
MERCK d.o.o.
Dunajska cesta 119
SI-1000 Ljubljana
Tel: +386 1 560 3 800
Ísland
Icepharma hf
Lynghálsi 13
IS-110 Reykjavík
Tel: + 354 540 8000
Slovenská
republika
Merck spol. s r.o.
Tuhovská 3
SK-831 06 Bratislava
Tel: + 421 2 49 267 111
Italia
Merck Serono S.p.A.
Via Casilina 125
I-00176 Roma
Tel: +39-06-70 38 41
Suomi
/
Finland
Merck Oy
Pihatörmä 1 C
FIN-02240 Espoo
Puh/Tel: +358-9-8678 700
Κύπρος
Χρ. Γ. Παπαλοϊζου Λτδ
Λeωfόρος Κιλκίς 35,
CY-2234 Λatsιά, Λευκωσία
Τηλ: +357-22-490305
Sverige
Merck AB
Box 3033
169 03 Solna
Tel: +46-8-562 445 00
Latvija
Merck Serono SIA
Duntes iela 23A
LV-1005, Rīga
Tel: +371 67152500
United Kingdom
Merck Serono Ltd
Bedfont Cross, Stanwell Road
Feltham, Middlesex TW14 8NX – UK
Tel: +44-20 8818 7200
Lietuva
Merck Serono UAB
Savanoriu pr. 192,
LT-44151 Kaunas
Tel: +370 37320603
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency web site:
http://www.ema.europa.eu
EU SPC LAB PL Lutropin 2011Feb11sub II-45 Rto2ndRSI
en.doc
Directory: \\Fxmadrid\Work\05_Dpt\TS\Andres\9190\60_To_FQA\00
Source\Updated source
Template: H04EN v4.0.dot
Title: Luveris, INN-lutropin alfa
Subject: EPAR
Author: CHMP
Keywords: Luveris, INN-lutropin alfa
Comments:
Creation Date: 3/14/2011 12:41:00 PM
Change Number: 4
Last Saved On: 3/14/2011 12:42:00 PM
Last Saved By: II-45 Day+5
Total Editing Time: 2 Minutes
Last Printed On: 3/14/2011 12:42:00 PM
As of Last Complete Printing
Number of Pages: 54
Number of Words: 14.943 (approx.)
Number of Characters: 85.177 (approx.)
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/luveris.html
Copyright © 1995-2021 ITA all rights reserved.
|





































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































