Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
MEPACT 4 mg powder for suspension for infusion.
QUALITATIVE AND QUANTITATIVE COMPOSITION
One vial contains 4 mg mifamurtide*.
After reconstitution, each ml of suspension in the vial contains 0.08 mg mifamurtide.
*fully synthetic analogue of a component of
Mycobacterium sp.
cell wall.
For a full list of excipients, see section 6.1.
Powder for suspension for infusion.
White to off-white homogeneous lyophilised powder.
MEPACT is indicated in children, adolescents and young adults for the treatment of high-grade
resectable non-metastatic osteosarcoma after macroscopically complete surgical resection. It is
used in combination with post-operative multi-agent chemotherapy. Safety and efficacy have been
assessed in studies of patients 2 to 30 years of age at initial diagnosis (see section 5.1).
4.2
Posology and method of administration
MEPACT treatment should be initiated and supervised by specialist physicians experienced in the
diagnosis and treatment of osteosarcoma.
Posology
The recommended dose of mifamurtide for all patients is 2 mg/m
2
body surface area. It should be
administered as adjuvant therapy following resection: twice weekly at least 3 days apart for
12 weeks, followed by once-weekly treatments for an additional 24 weeks for a total of
48 infusions in 36 weeks
.
Paediatric patients
The safety and efficacy of MEPACT have been established in children from the age of 2 years. It
is not recommended for use in children below the age of 2 due to a lack of data on efficacy and
safety in this age group.
Elderly patients
None of the patients treated in the osteosarcoma studies were 65 or older and in the phase III
randomised study, only patients up to age 30 years were included. Therefore, there are not
sufficient data to recommend the use of MEPACT in patients >30 years of age.
Patients with impaired renal or hepatic function
The pharmacokinetics of mifamurtide in patients with renal or hepatic impairment have not been
formally studied. Caution should be used in these patients because dose adjustment information is
not available.
Continued monitoring of the kidney and liver function is recommended if MEPACT is used
beyond completion of chemotherapy until all therapy is completed.
Method of administration
MEPACT must be reconstituted, filtered using the filter provided and further diluted prior to
administration. The reconstituted, filtered and diluted suspension for infusion is a homogenous, white
to off-white, opaque liposomal suspension, free of visible particles and free of foam and lipid lumps.
After reconstitution, filtering using the filter provided and further dilution, MEPACT is administered
by intravenous infusion over a period of 1 hour.
MEPACT
must not
be administered as a bolus injection.
For further instructions on reconstitution, filtering using the filter provided and dilution prior to
administration, see section 6.6.
Hypersensitivity to the active substance or to any of the excipients.
Concurrent use with ciclosporin or other calcineurin inhibitors (see section 4.5).
Concurrent use with high-dose non-steroidal anti-inflammatory drugs (NSAIDs, cyclooxygenase
inhibitors) (see section 4.5).
Special warnings and precautions for use
Respiratory distress
In patients with a history of asthma or other chronic obstructive pulmonary disease, consideration
should be given to administration of bronchodilators on a prophylactic basis. Two patients with
pre-existing asthma developed mild to moderate respiratory distress associated with the treatment.
If a severe respiratory reaction occurs, administration of MEPACT should be discontinued and
appropriate treatment initiated.
Neutropenia
Administration of MEPACT was commonly associated with transient neutropenia, usually when
used in conjunction with chemotherapy. Episodes of neutropenic fever should be monitored and
managed appropriately. MEPACT may be given during periods of neutropenia, but subsequent
fever attributed to the treatment should be monitored closely. Fever or chills persisting for more
than 8 hours after administration of MEPACT should be evaluated for possible sepsis.
Inflammatory response
Association of MEPACT with signs of pronounced inflammatory response, including pericarditis
and pleuritis, was uncommon. It should be used with caution in patients with a history of
autoimmune, inflammatory or other collagen diseases. During MEPACT administration, patients
should be monitored for unusual signs or symptoms, such as arthritis or synovitis, suggestive of
uncontrolled inflammatory reactions.
Cardiovascular disorders
Patients with a history of venous thrombosis, vasculitis or unstable cardiovascular disorders
should be closely monitored during MEPACT administration. If symptoms are persistent and
worsening, administration should be delayed or discontinued. Haemorrhage was observed in
animals at very high doses. These are not expected at the recommended dose, however monitoring
of clotting parameters after the first dose and once again after several doses is recommended.
Allergic reactions
Occasional allergic reactions have been associated with MEPACT treatment, including rash,
shortness of breath and Grade 4 hypertension. It may be difficult to distinguish allergic reactions
from exaggerated inflammatory responses, but patients should be monitored for signs of allergic
reactions.
Gastrointestinal toxicity
Nausea, vomiting and loss of appetite are very common adverse reactions to MEPACT.
Gastrointestinal toxicity may be exacerbated when MEPACT is used in combination with high
dose, multi-agent chemotherapy and was associated with an increased use of parenteral nutrition.
Interaction with other medicinal products and other forms of interaction
Limited studies of the interaction of MEPACT with chemotherapy have been conducted. Although
these studies are not conclusive, there is no evidence of interference of MEPACT with the
anti-tumour effects of chemotherapy and vice versa.
It is recommended to separate the administration times of MEPACT and doxorubicin or other
lipophilic medicinal products if used in the same chemotherapy regimen.
The use of MEPACT concurrently with ciclosporin or other calcineurin inhibitors is
contraindicated due to their hypothesised effect on splenic macrophages and mononuclear
phagocytic function (see section 4.3).
Also, it has been demonstrated
in vitro
that high-dose NSAIDs (cyclooxygenase inhibitors) can
block the macrophage activating effect of liposomal mifamurtide. Therefore the use of high-dose
NSAIDs is contraindicated (see section 4.3).
Because mifamurtide acts through stimulation of the immune system, the chronic or routine use of
corticosteroids should be avoided during treatment with MEPACT.
In vitro
interaction studies showed that liposomal and non-liposomal mifamurtide do not inhibit
the metabolic activity of cytochrome P450 in pooled human liver microsomes. Liposomal and
non-liposomal mifamurtide do not induce the metabolic activity or the transcription of cytochrome
P450 in primary cultures of freshly isolated human hepatocytes. Mifamurtide is therefore not
expected to interact with the metabolism of substances that are hepatic cytochrome P450
substrates.
In a large controlled randomised study, MEPACT used at the recommended dose and schedule
with other medicinal products that have known renal (cisplatin, ifosfamide) or hepatic (high-dose
methotrexate, ifosfamide) toxicities did not exacerbate those toxicities and there was no need to
adjust mifamurtide dose.
4.6
Pregnancy and lactation
Pregnancy
There are no data from the use of mifamurtide in pregnant patients. Animal studies are insufficient
with respect to reproductive toxicity (see section 5.3). MEPACT should not be used during
pregnancy and in women not using effective contraception.
Lactation
It is unknown whether mifamurtide is excreted in human milk. The excretion of mifamurtide in
milk has not been studied in animals. A decision on whether to continue/discontinue
breast-feeding or to continue/discontinue therapy should be made taking into account the benefit
of breast-feeding to the child and the benefit of MEPACT therapy to the woman.
Effects on ability to drive and use machines
No studies of the effects on the ability to drive and use machines have been performed. Some very
common or common undesirable effects of MEPACT treatment (such as dizziness, vertigo, fatigue
and blurred vision) may have an effect on the ability to drive and use machines.
Each of the 248 patients treated with MEPACT during the early phase single arm studies in
patients with mostly advanced malignancies experienced at least one undesirable effect. Many of
the most frequently reported undesirable effects as shown in the following summary table are
thought to be related to the mechanism of action of mifamurtide. The majority of these events
were reported as either mild or moderate. This profile is consistent whether summarising all early
studies (n=248) or only those studies in osteosarcoma (n=51). It is likely that undesirable effects
also occurred in the large randomised study, but they were not recorded because only serious and
life-threatening adverse reactions were collected in that study.
Adverse reactions are classified according to system organ class and frequency. Frequency
groupings are defined according to the following convention: Very common (≥1/10), common
(≥1/100 to <1/10). Within each frequency grouping, undesirable effects are presented in order of
decreasing seriousness.
Table 1. Adverse reactions associated with MEPACT in ≥ 1/100 patients
Infections and infestations
Common: Sepsis, cellulitis, nasopharyngitis, catheter site infection, upper
respiratory tract infection, urinary tract infection, pharyngitis,
Herpes
simplex
infection
Neoplasms benign, malignant and unspecified (incl cysts and polyps)
Common: Cancer pain
Blood and lymphatic system disorders
Very common: Anaemia
Common: Leukopenia, thrombocytopenia, granulocytopenia
Metabolism and nutrition disorders
Very common:
Dehydration, hypokalaemia, decreased appetite
Psychiatric disorders
Common:
Confusional state, depression, insomnia, anxiety
Nervous system disorders
Very common: Headache, dizziness
Common: Paraesthesia, hypoaesthesia, tremor, somnolence, lethargy
Eye disorders
Common: Blurred vision
Ear and labyrinth disorders
Common: Vertigo, tinnitus, hearing loss
Cardiac disorders
Very common: Tachycardia
Common: Cyanosis, palpitations
Vascular disorders
Very common: Hypertension, hypotension
Common: Phlebitis, flushing, pallor
Respiratory, thoracic and mediastinal disorders
Very common:
Pleural effusion, exacerbated dyspnoea, productive cough, haemoptysis,
wheezing, epistaxis, exertional dyspnoea, sinus congestion, nasal
congestion, pharyngolaryngeal pain
Dyspnoea, tachypnoea, cough
Gastrointestinal disorders
Very common:
Vomiting, diarrhoea, constipation, abdominal pain, nausea
Upper abdominal pain, dyspepsia, abdominal distension, lower
abdominal pain
Hepatobiliary disorders
Common: Hepatic pain
Skin and subcutaneous tissue disorders
Very common: Hyperhidrosis
Common: Rash, pruritis, erythema, alopecia, dry skin
Musculoskeletal and connective tissue disorders
Very common: Myalgia, arthralgia, back pain, pain in extremity
Common: Muscle spasms, neck pain, groin pain, bone pain, shoulder pain, chest
wall pain, musculoskeletal stiffness
Renal and urinary disorders
Common: Haematuria, dysuria, pollakiuria
Reproductive system and breast disorders
Common: Dysmenorrhoea
General disorders and administration site conditions
Very common: Fever, chills, fatigue, hypothermia, pain, malaise, asthenia, chest pain
Common: Peripheral oedema, oedema, mucosal inflammation, infusion site
erythema, infusion site reaction, catheter site pain, chest discomfort,
feeling cold
Investigations
Common: Weight decreased
Surgical and medical procedures
Common:
Blood and lymphatic system disorders
Anaemia has most commonly been reported when MEPACT is used in conjunction with
chemotherapeutic agents. In a randomised controlled trial, the incidence of myeloid malignancy
(acute myeloid leukaemia/myelodysplastic syndrome) was the same in patients receiving
MEPACT plus chemotherapy as in patients receiving only chemotherapy (approximately 2.5%).
Metabolism and nutritional disorders
Anorexia (21%) was very commonly reported in trials of MEPACT in late stage cancer patients.
Nervous system disorders
Consistent with other generalised symptoms, the most common nervous system disorders were
headache (50%) and dizziness (17%).
Ear and labyrinth disorders
Although hearing loss may be attributable to ototoxic chemotherapy, like cisplatin, it is unclear
whether MEPACT in conjunction with multi-agent chemotherapy may increase hearing loss.
A higher percentage of objective and subjective hearing loss was observed overall in patients who
received MEPACT and chemotherapy (12 % and 7%, respectively) in the phase III study (see
Section 5.1 for a description of the trial) compared to those patients that received only
chemotherapy (7% and 1%). All patients received a total dose of cisplatin of 480 mg/m
2
as part of
their induction (neoadjuvant) and/or maintenance (adjuvant) chemotherapy regimen.
Cardiac and vascular disorders
Mild-moderate tachycardia (50%), hypertension (26%) and hypotension (29%) were commonly
reported in uncontrolled trials of MEPACT. One serious incident of subacute thrombosis was
reported in early studies, but no serious cardiac events were associated with MEPACT in a large
randomised controlled trial.
Respiratory disorders
Respiratory disorders, including dyspnoea (21%), cough (18%) and tachypnoea (13%) were very
commonly reported, and two patients with pre-existing asthma developed mild to moderate
respiratory distress associated with MEPACT treatment in a phase II study.
Gastrointestinal disorders
Gastrointestinal disorders were frequently associated with MEPACT administration, including
nausea (57%) and vomiting (44%) in about half of patients, constipation (17%), diarrhoea (13%)
and abdominal pain.
Skin and subcutaneous disorders
Hyperhidrosis (11%) was very common in patients receiving MEPACT in uncontrolled studies.
Musculoskeletal and connective tissue disorders
Low grade pain was common in patients receiving MEPACT, including myalgia (31%), back pain
(15%), extremity pain (12%) and arthralgia (10%).
General disorders and administration site conditions
The majority of patients experience chills (89%), fever (85%) and fatigue (53%). These are
typically mild to moderate, transient in nature and generally respond to palliative treatment (e.g.,
paracetamol for fever). Other generalised symptoms that were typically mild to moderate and very
common included hypothermia (23%), malaise (13%), pain (15%), asthenia (13%) and chest pain
(11%). Oedema, chest discomfort, local infusion or catheter site reactions and ‘feeling cold’ were
less frequently reported in these patients, mostly with late stage malignant disease.
Investigations
Increase in blood urea and blood creatinine was associated with MEPACT use in one patient with
osteosarcoma.
No case of overdose has been reported. The maximum tolerated dose in phase I studies was 4-
6 mg/m
2
with a high variability of adverse reactions. Signs and symptoms that were associated
with higher doses and/or were dose limiting were not life-threatening, and included fever, chills,
fatigue, nausea, vomiting, headache and hypo- or hypertension.
In the event of an overdose, it is recommended that appropriate supportive treatment be initiated.
Supportive measures should be based on institutional guidelines and the clinical symptoms
observed. Examples include paracetamol for fever, chills and headache and anti-emetics (other
than steroids) for nausea and vomiting.
PHARMACOLOGICAL PROPERTIES
Pharmacodynamic properties
Pharmacotherapeutic group: Other cytokines and immunomodulators, ATC code: L03AX15
Mechanism of action
Mifamurtide (muramyl tripeptide phosphatidyl ethanolamine, MTP-PE) is a fully synthetic
derivative of muramyl dipeptide (MDP), the smallest naturally-occurring immune stimulatory
component of cell walls from
Mycobacterium sp
. It has similar immunostimulatory effects as
natural MDP with the additional advantage of a longer half-life in plasma. MEPACT is a
liposomal formulation specifically designed for
in vivo
targeting to macrophages by intravenous
infusion.
MTP-PE is a specific ligand of NOD2, a receptor found primarily on monocytes, dendritic cells
and macrophages. MTP-PE is a potent activator of monocytes and macrophages. Activation of
human macrophages by MEPACT is associated with production of cytokines, including tumour
necrosis factor (TNF-α), interleukin-1 (IL-1β), IL-6, IL-8, and IL-12 and adhesion molecules,
including lymphocyte function-associated antigen-1 (LFA-1) and intercellular adhesion molecule-
1 (ICAM-1).
In vitro
-treated human monocytes killed allogeneic and autologous tumor cells
(including melanoma, ovarian, colon, and renal carcinoma), but had no toxicity towards normal
cells.
In vivo
administration of MEPACT resulted in the inhibition of tumour growth in mouse and rat
models of lung metastasis, skin and liver cancer, and fibrosarcoma. Significant enhancement of
disease-free survival was also demonstrated in the treatment of dog osteosarcoma and
hemangiosarcoma with MEPACT as adjuvant therapy. The exact mechanism by which MEPACT
activation of monocytes and macrophages leads to antitumour activity in animals and humans is
not yet known.
Clinical safety and efficacy
The safety of liposomal mifamurtide has been assessed in more than 700 patients with various
kinds and stages of cancer and in 21 healthy adult subjects (see section 4.8).
MEPACT significantly increased the overall survival of patients with newly-diagnosed resectable
high-grade osteosarcoma when used in conjunction with combination chemotherapy when
compared to chemotherapy alone. In a randomised phase III study of 678 patients (age range from
1.4 to 30.6 years) with newly-diagnosed resectable high-grade osetosarcoma, the addition of
adjuvant MEPACT to chemotherapy either doxorubicin cisplatin and methotrexate with or without
ifosfamide
resulted in a relative reduction in the risk of death of 28% (p = 0.0313, hazard ratio (HR) = 0.72
[95% confidence interval (CI): 0.53, 0.97]).
Pharmacokinetic properties
After intravenous administration in 21 healthy adult subjects mifamurtide was cleared rapidly
from plasma (minutes), resulting in a very low plasma concentration of total (liposomal and free)
mifamurtide. The mean AUC was 17.0 +/- 4.71 h x nM and Cmax was 15.7 +/- 3.72 nM. In
separate study in 14 patients, mean serum concentration-time curves of total and free mifamurtide
that were assessed after the first infusion of MEPACT and after a last infusion 11 or 12 weeks
later, were almost superimposable and the mean AUC values of the free mifamurtide after the first
and last infusion were similar. These data indicate that neither total nor free mifamurtide
accumulated during the treatment period.
At 6 hours after injection of radiolabelled liposomes containing 6 mg mifamurtide, radioactivity
was found in liver, spleen, nasopharynx, thyroid, and, to a lesser extent, in lung. The liposomes
were phagocytosed by cells of the reticuloendothelial system. In 2 of 4 patients with lung
metastases, radioactivity was associated with lung metastases. Mean half-life of radiolabelled
material was biphasic with an α phase of about 15 minutes and a terminal half-life of
approximately 18 hours.
In sensitive species (rabbit and dog) the highest daily dose of liposomal mifamurtide that did not
cause adverse effects was 0.1 mg/kg, corresponding to 1.2 and 2 mg/m
2
, respectively. The
no-adverse-effect level for MEPACT in animals corresponds roughly to the 2 mg/m
2
recommend
dose for humans.
Data from a six month dog study of daily intravenous injections of up to 0.5 mg/kg (10 mg/m
2
)
MEPACT provide an 8- to 19-fold cumulative exposure safety margin for overt toxicity for the
intended clinical dose in humans. Major toxic effects associated with these high daily and
cumulative doses of MEPACT were mainly exaggerated pharmacological effects: pyrexia, signs of
pronounced inflammatory response manifested as synovitis, bronchopneumonia, pericarditis and
inflammatory necrosis of the liver and bone marrow. The following events were also observed:
haemorrhage and prolongation of coagulation times, infarcts, morphological changes in the wall of
small arteries, oedema and congestion of the central nervous system, minor cardiac effects, and
slight hyponatraemia. MEPACT was not mutagenic and did not cause teratogenic effects in rats
and rabbits. Embryotoxic effects were observed only at maternal toxic levels.
There were no results from general toxicity studies that suggested harmful effects on male or
female reproductive organs. Specific studies addressing reproductive function, perinatal toxicity
and carcinogenic potential have not been performed.
PHARMACEUTICAL PARTICULARS
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC)
1,2-Dioleoyl-sn-glycero-3-phospho-L-serine monosodium salt (OOPS)
This medicinal product must not be mixed with other medicinal products except those mentioned
in section 6.6.
Unopened vial of powder
:
30 months
Reconstituted suspension
:
Chemical and physical stability has been demonstrated for 6 hours up to 25ºC.
From a microbiological point of view, immediate use is recommended. If not used immediately,
the reconstituted, filtered and diluted solution in-use storage times and conditions prior to use of
the reconstituted product are the responsibility of the user and must not be longer than 6 hours at
25ºC. Do not store in a refrigerator and do not freeze the solution.
Special precautions for storage
Store in a refrigerator (2°C – 8°C). Do not freeze.
Keep the vial in the outer carton in order to protect from light.
For storage conditions of the reconstituted medicinal product, see section 6.3.
6.5
Nature and contents of container
50 ml type I glass vial with a grey butyl rubber stopper, aluminium seal and plastic flip-off cap,
containing 4 mg of mifamurtide.
Each carton contains one vial and one single-use, non-pyrogenic, latex-free sterile Filter for
MEPACT supplied in a PVC-grade blister.
Special precautions for disposal and other handling
MEPACT must be reconstituted, filtered using the filter provided and further diluted using aseptic
technique.
Each vial should be reconstituted with 50 ml of sodium chloride 9 mg/ml (0.9 %) solution for
injection. After reconstitution, each ml suspension in the vial contains 0.08 mg mifamurtide. The
volume of reconstituted suspension corresponding to the calculated dose is extracted through the
filter provided and further diluted with additional 50 ml sodium chloride 9 mg/ml (0.9 %) solution
for injection according to the detailed instructions shown below.
Instructions for preparation of MEPACT for intravenous infusion
Materials provided in each package -
•
MEPACT powder for suspension for infusion (vial)
•
Filter for MEPACT
Materials required but not provided
-
•
Sodium chloride 9 mg/ml (0.9%) solution for injection, EP/USP 100 ml bag
•
One single use 60 or 100 ml sterile syringe with luer lock
•
Two medium (18) gauge sterile injection needles
It is recommended that the reconstitution of the liposomal suspension should be performed in a
laminar flow cabinet utilising sterile gloves using aseptic technique.
The lyophilised powder should be allowed to reach a temperature between approximately 20°C –
25°C prior to reconstitution, filtering using the filter provided and dilution. This should take
approximately 30 minutes.
1.
The cap of the vial should be removed and the stopper cleaned using an alcohol pad.
2.
The filter should be removed from the blister pack, and the cap removed from the filter spike.
The spike should then be inserted into the vial septum firmly until seated. The filter luer
connector cap should not be removed at this time.
3.
The 100 ml sodium chloride 9 mg/ml (0.9%) solution for injection bag, needle and syringe
should be unpacked (not provided in the pack).
4.
The site of the sodium chloride 9 mg/ml (0.9%) solution for injection bag where the needle is
going to be inserted should be swabbed with an alcohol pad.
5.
Using the needle and syringe, 50 ml of sodium chloride 9 mg/ml (0.9%) solution for injection
should be withdrawn from the bag.
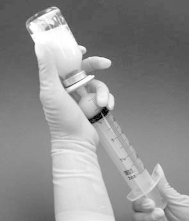
6. After removing the needle from the syringe, the syringe should be attached to the filter by
opening the filter luer connector cap (Figure 1).

7. The sodium chloride 9 mg/ml (0.9%) solution for injection is added to the vial by slow, firm
depression of the syringe plunger.
The filter and syringe must not be removed from the
vial
.
8. The vial should be allowed to stand undisturbed for one minute to ensure thorough hydration
of the dry substance.
9.
The vial should then be shaken vigorously for one minute while keeping the
filter and
syringe attached
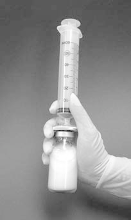
. During this time the liposomes are formed spontaneously (Figure 2).
10.
The desired dose may be withdrawn from the vial by inverting the vial and slowly pulling
back on the syringe plunger (Figure 3). Each ml reconstituted suspension contains 0.08 mg
mifamurtide. The volume of suspension to be withdrawn for dose quantities is calculated as
follows:
Volume to withdraw = [12.5 x calculated dose (mg)] ml
For convenience, the following table of concordance is provided:









11.
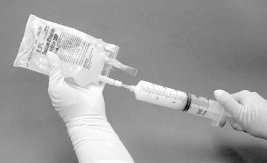
The syringe should then be removed from the filter and a new needle placed on the
suspension-filled syringe. The bag injection site should be wiped with an alcohol pad and the
suspension in the syringe should be injected into the original bag containing the remaining
50 ml of sodium chloride 9 mg/ml (0.9%) solution for injection (Figure 4).
12.
The bag should be gently swirled to mix the solution.
13.
Patient identification, time and date should be added to the label on the bag containing the
reconstituted, filtered and diluted liposomal suspension.
14.
Chemical and physical in-use stability has been demonstrated for 6 hours at room temperature
(between approximately 20°C – 25°C).
15.
From a microbiological point of view, the product should be used immediately. If not used
immediately, in-use storage times and conditions prior to use are the responsibility of the user
and would normally not be longer than 6 hours at room temperature.
16.
The liposomal suspension is infused intravenously over about one hour.
Disposal
No special requirements.
MARKETING AUTHORISATION HOLDER
IDM PHARMA SAS
11-15 Quai De Dion Bouton
92816 Puteaux Cedex
France
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines
Agency (EMEA)
http://www.emea.europa.eu/
MANUFACTURING AUTHORISATION HOLDER
RESPONSIBLE FOR BATCH RELEASE
CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer responsible for batch release
Takeda Ireland Ltd
Bray Business Park
Kilruddery
Co. Wicklow
Ireland
Takeda Italia Farmaceutici S.p.A
Via Crosa, 86
28065 Cerano (NO)
Italy
The printed package leaflet of the medicinal product must state the name and address of the
manufacturer responsible for the release of the concerned batch.
B. CONDITIONS OF THE MARKETING AUTHORISATION
•
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to restricted medical prescription (See Annex I: Summary of Product
Characteristics, section 4.2).
•
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
Pharmacovigilance system
The MAH must ensure that the system of pharmacovigilance, presented in Module 1.8.1. of the
Marketing Authorisation, is in place and functioning before and whilst the product is on the market.
Risk Management Plan
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version POL/500 v1 of the Risk Management Plan (RMP)
presented in Module 1.8.2. of the Marketing Authorisation Application and any subsequent updates of
the RMP agreed by the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, the
updated RMP should be submitted at the same time as the next Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted
•
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
•
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
•
At the request of the EMEA
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
MEPACT 4 mg powder for suspension for infusion
Mifamurtide
STATEMENT OF ACTIVE SUBSTANCE(S)
Each vial contains 4 mg of mifamurtide. After reconstitution, each ml of reconstituted suspension in
the vial contains 0.08 mg of mifamurtide.
Excipients: Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC),
1,2-Dioleoyl-sn-glycero-3-phospho-L-serine monosodium salt (OOPS)
PHARMACEUTICAL FORM AND CONTENTS
Powder for suspension for infusion
Pack of 1 vial of powder
containing 4 mg mifamurtide, 1 sterile Filter for MEPACT
METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use
For intravenous infusion after reconstitution, filtering using the filter provided and further dilution.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED
OUT OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
Store in a refrigerator. Do not freeze.
Keep the vial in the outer carton in order to protect from light.






















10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL
PRODUCTS OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL
PRODUCTS, IF APPROPRIATE
NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
IDM PHARMA SAS
11-15 Quai De Dion Bouton
92816 Puteaux Cedex
France
MARKETING AUTHORISATION NUMBER(S)
GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
Justification for not including Braille accepted


















PARTICULARS TO APPEAR ON THE IMMEDIATE PACKAGING
NAME OF THE MEDICINAL PRODUCT
MEPACT 4 mg powder for suspension for infusion
Mifamurtide
STATEMENT OF ACTIVE SUBSTANCE(S)
Each vial contains 4 mg of mifamurtide. After reconstitution, each ml of reconstituted suspension in
the vial contains 0.08 mg of mifamurtide.
Excipients: Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC),
1,2-Dioleoyl-sn-glycero-3-phospho-L-serine monosodium salt (OOPS)
PHARMACEUTICAL FORM AND CONTENTS
Powder for suspension for infusion
4 mg mifamurtide
METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use
For intravenous infusion after reconstitution, filtering using the filter provided and further dilution.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED
OUT OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
Store in a refrigerator. Do not freeze.
Keep the vial in the outer carton in order to protect from light.





















10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL
PRODUCTS OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL
PRODUCTS, IF APPROPRIATE
NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
IDM PHARMA SAS
11-15 Quai De Dion Bouton
92816 Puteaux Cedex
France
MARKETING AUTHORISATION NUMBER(S)
GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
Justification for not including Braille accepted


















MINIMUM PARTICULARS TO APPEAR ON BLISTERS OR STRIPS
BLISTER – FILTER FOR MEPACT
NAME OF THE MEDICINAL PRODUCT
NAME OF THE MARKETING AUTHORISATION HOLDER
Store at 2°C – 40°C
Read the package leaflet before use
Single-use/non-pyrogenic/latex-free
Manufactured by ARIES s.r.l.













PACKAGE LEAFLET: INFORMATION FOR THE USER
MEPACT 4 mg powder for suspension for infusion
Mifamurtide
Read all of this leaflet carefully before you start using this medicine.
-
Keep this leaflet. You may need to read it again.
-
If you have any further questions, ask your doctor.
-
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor.
In this leaflet
:
1. What MEPACT is and what it is used for
2. Before you use MEPACT
3. How to use MEPACT
4. Possible side effects
5.
How to store MEPACT
6.
1.
WHAT MEPACT IS AND WHAT IT IS USED FOR
MEPACT contains the active substance mifamurtide, similar to a component of the cell wall of
certain bacteria. It stimulates your immune system to help your body kill tumour cells.
MEPACT is used to treat osteosarcoma (bone cancer) in children, adolescents and young adults. It
is used after you have had surgery to remove the tumour and together with chemotherapy to kill
remaining cancer cells to reduce the risk of cancer coming back.
Do not use MEPACT
-
if you are allergic (hypersensitive) to mifamurtide or any of the other ingredients of MEPACT.
- if you are taking medicines containing ciclosporin or tacrolimus or high doses of NSAIDs (see
“Using other medicines” below).
Take special care with MEPACT
You should tell your doctor before using MEPACT if any of the following applies to you:
-
if you have or have had problems with your heart or blood vessels, like blood clots
(thrombosis), bleeding (haemorrhage) or inflammation of the veins (vasculitis). You should be
more closely monitored while receiving MEPACT treatment. If you have long-lasting or
worsening symptoms, you should contact your doctor, as MEPACT administration may need to be
delayed or discontinued.
-
if you have a history of asthma or other breathing disorders. Before using MEPACT, you
should discuss with your doctor whether you should take medicine for your asthma when using
MEPACT.
-
if you have a history of inflammatory or autoimmune disease or have been treated with
corticosteroids or other medicines that may affect your immune system.
Using other medicines
Please tell your doctor if you are taking or have recently taken any other medicines, including
medicines that may be obtained without a prescription. It is especially important to tell your doctor
if you are taking medicines containing any of the following active substances:
-
ciclosporin, tacrolimus, used after a transplant to prevent rejection of transplanted organs, or
other immunosuppressants used e.g. to treat psoriasis (a skin disease).
-
non-steroidal-anti-inflammatory drugs (NSAIDs), such as acetylsalicylic acid, ibuprofen, or
diclofenac, used for treatment of headaches, fever or pain. You must not use MEPACT with high
doses of NSAIDs.
-
corticosteroids, used to treat inflammations, allergies or asthma. You must not use MEPACT
with regular use of corticosteroids.
It is recommended to separate the times of administration of MEPACT and doxorubicin or other
medicines if used in the same chemotherapy treatment regimen.
Pregnancy and breast-feeding
MEPACT has not been tested in pregnant women. Therefore, MEPACT should not be used during
pregnancy and in women not using effective contraception. You should use effective
contraception if you are being treated with MEPACT. It is important to tell your doctor if you are
pregnant, think you may be pregnant, or are planning to get pregnant.
It is not known whether MEPACT passes to human milk. If you are breast-feeding, you should
discuss with your doctor.
Driving and using machines
Some very common and common side effects of MEPACT treatment (such as dizziness, vertigo,
fatigue and blurred vision) may affect your ability to drive and use machines.
Dose and schedule
The safety and efficacy of MEPACT have been established in patients aged 2 to 30 years. The
dose of MEPACT is 2 mg mifamurtide/m
2
body surface area. It will be given to you twice a week
(at least three days apart) for the first 12 weeks, then once a week for 24 more weeks.
The schedule of your MEPACT treatments can be adjusted to fit with your chemotherapy
schedule. It is not necessary to interrupt your schedule of MEPACT if your chemotherapy is
delayed; you should complete 36 weeks (9 months) of treatment with MEPACT without an
interruption.
How MEPACT is given
The freeze-dried powder has to be reconstituted into a liquid suspension, filtered using the filter
provided and further diluted before use. MEPACT is then infused directly into your vein
(intravenous) over about one hour. This is done by your doctor or a nurse, who will also monitor
you during that time. You do not need to be hospitalised to receive MEPACT. It can also be
administered as an outpatient.
If you use more MEPACT than you should
You may experience more severe side effects, including fever, chills, fatigue, nausea, vomiting,
headache and hypo- or hypertension. In the event of such an overdose, contact your doctor or
nearest hospital.
If you have any other questions on the use of this medicine, ask your doctor.
Like all medicines, MEPACT can cause side effects, although not everybody gets them.
The majority of patients experience chills, fever and fatigue. These are typically mild to moderate
and transient and can usually be treated by your doctor, e.g., with paracetamol for fever.
Contact your doctor
immediately
:
-
if you have continuing fever or chills more than 8 hours after your dose of MEPACT, because
this may be a sign of an infection or
-
if you experience rash or have any problems breathing (wheezing).
Side effects may occur with certain frequencies, which are defined as follows:
very common: affects more than 1 user in 10
common: affects 1 to 10 users in 100
uncommon: affects 1 to 10 users in 1,000
rare: affects 1 to 10 users in 10,000
very rare: affects less than 1 user in 10,000
not known: frequency cannot be estimated from the available data.
Very common side effects:
-
fever, shaking/shivering, weakness, tiredness or general discomfort
-
nausea and/or vomiting, diarrhoea or constipation
-
headache or dizziness
-
rapid beating of the heart
-
high blood pressure or low blood pressure
-
no appetite for food
-
sweating
-
pain, including general pain, pain in your muscles and/or joints and pain in back, chest,
abdomen, arm or leg
-
cough, trouble breathing or rapid breathing
-
low body temperature
-
low number of red blood cells
Common side effects:
-
blue colour of tissues such as the skin or gums caused by too little oxygen
-
perceptible increase in frequency or force of heartbeat
-
swelling in arms or legs or other swelling
-
chest discomfort
-
upset stomach, decreased appetite or weight loss
-
injection site or catheter site redness, swelling, infection or other local reaction
-
rash or redness, inflammation of the skin, itching, dry skin, pale or transient red appearance
-
inflammation of skin, tendons, muscles or similar tissues that support body structure
-
inflammation of a vein
-
upper abdominal or chest wall pain; abdominal bloating or pain
-
other pain, including neck, shoulder or throat pain
-
muscle spasms or stiffness
-
feeling cold
-
tired feeling, drowsiness or sleepiness
-
burning, pricking/tingling sensation or diminished sensitivity to sensation
-
involuntary shaking movement
-
dehydration
-
mucosal inflammation
-
nose, throat, or sinus congestion or inflammation
-
infections of the upper respiratory tract (such as a cold) or the urinary tract (such as a bladder
infection)
-
generalised infection
-
Herpes simplex
(virus) infection
-
productive cough, wheezing or exertional or exacerbated shortness of breath
-
spitting of blood or nosebleed
-
fluid in the lung cavity
-
blood in urine, difficulty or pain in urination or frequent urination
-
difficulty sleeping, depression, anxiety or confusion
-
dizziness
-
ears ringing
-
blurred vision
-
hair loss
-
difficult, painful menstruation
-
hearing loss
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor.
Keep out of the reach and sight of children.
Do not use MEPACT after the expiry date which is stated on the vial label and the carton.
Unopened vial
Store in a refrigerator (2°C – 8°C). Do not freeze.
Keep the vial in outer carton in order to protect from light.
Reconstituted suspension
Once reconstituted in sodium chloride 9 mg/ml (0.9%) solution, store at room temperature
(approximately 20ºC - 25ºC) and use within 6 hours.
What MEPACT contains
-
The active substance is mifamurtide. Each vial contains 4 mg of mifurtamide. After
reconstitution with 50 ml sodium chloride 9 mg/ml (0.9%) solution for injection, each ml of
suspension contains 0.08 mg of mifamurtide.
-
The other ingredients are 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and
1,2-Dioleoyl-sn-glycero-3-phospho-L-serine monosodium salt (OOPS).
What MEPACT looks like and contents of the pack
MEPACT is a white to off-white homogeneous
freeze-dried powder for suspension for infusion.
MEPACT is supplied in a carton that contains
•
One 50 ml vial with a grey butyl stopper, aluminium seal and plastic flip-off cap.
•
One sterile Filter for MEPACT supplied in a blister.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
IDM PHARMA SAS
11-15 Quai De Dion Bouton
92816 Puteaux Cedex
France
Manufacturer:
Takeda Ireland Ltd
Bray Business Park
Kilruddery
Co. Wicklow
Ireland
Takeda Italia Farmaceutici S.p.A
Via Crosa, 86
28065 Cerano (NO)
Detailed information on this medicine is available on the European Medicines Agency (EMEA)
website:
http://www.emea.europa.eu/
This leaflet was last approved in
----------------------------------------------------------------------------------------------------------------------
The following information is intended for medical or healthcare professionals only:
Instructions for preparation of MEPACT for intravenous infusion
Materials provided in each package -
•
1 vial of MEPACT (mifamurtide)
•
1 Filter for MEPACT
Materials required but not provided -
•
Sodium chloride 9 mg/ml (0.9%) solution for injection, EP/USP 100 ml bag
•
One single use 60 or 100 ml sterile syringe with luer lock
•
Two medium (18) gauge sterile injection needles
It is recommended that the reconstitution of the liposomal suspension should be performed in a
laminar flow cabinet utilising sterile gloves using aseptic technique.
The lyophilised powder should be allowed to reach a temperature between approximately 20°C –
25°C prior to reconstitution, filtering using the filter provided and dilution. This should take
approximately 30 minutes.
1.
The cap of the vial should be removed and the stopper cleaned using an alcohol pad.
2.
The filter should be removed from the blister pack, and the cap removed from the filter spike.
The spike should then be inserted into the vial septum firmly until seated. The filter luer
connector cap should not be removed at this time.
3.
The 100 ml sodium chloride 9 mg/ml (0.9%) solution for injection bag, needle and syringe
should be unpacked (not provided in the pack).
4.
The site of the sodium chloride 9 mg/ml (0.9%) solution for injection bag where the needle is
going to be inserted should be swabbed with an alcohol pad.
5.
Using the needle and syringe, 50 ml of sodium chloride 9 mg/ml (0.9%) solution for injection
should be withdrawn from the bag.
6. After removing the needle from the syringe, the syringe should be attached to the filter by
opening the filter luer connector cap (Figure 1).

7. The sodium chloride 9 mg/ml (0.9%) solution for injection is added to the vial by slow, firm
depression of the syringe plunger.
The filter and syringe must not be removed from the
vial.
8. The vial should be allowed to stand undisturbed for one minute to ensure thorough hydration
of the dry substance.
9.
The vial should then be shaken vigorously for one minute while keeping the
filter and
syringe attached.
During this time the liposomes are formed spontaneously (Figure 2).
10.
The desired dose may be withdrawn from the vial by inverting the vial and slowly pulling
back on the syringe plunger (Figure 3). Each ml reconstituted suspension contains 0.08 mg
mifamurtide. The volume of suspension to be withdrawn for dose quantities is calculated as
follows:
Volume to withdraw = [12.5 x calculated dose (mg)] ml
For convenience, the following table of concordance is provided:









11.
The syringe should then be removed from the filter and a new needle placed on the
suspension-filled syringe. The bag injection site should be wiped with an alcohol pad and the
suspension in the syringe should be injected into the original bag containing the remaining
50 ml of sodium chloride 9 mg/ml (0.9%) solution for injection (Figure 4).
12.
The bag should be gently swirled to mix the solution.
13.
Patient identification, time and date should be added to the label on the bag containing the
reconstituted, filtered and diluted liposomal suspension.
14.
Chemical and physical in-use stability has been demonstrated for 6 hours at room temperature
(between approximately 20°C – 25°C).
15.
From a microbiological point of view, the product should be used immediately. If not used
immediately, in-use storage times and conditions prior to use are the responsibility of the user
and would normally not be longer than 6 hours at room temperature.
Disposal
No special requirements.

Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/mepact.html
Copyright © 1995-2021 ITA all rights reserved.
|




















































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































