Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Mixtard 30 40 IU/ml suspension for injection in a vial.
QUALITATIVE AND QUANTITATIVE COMPOSITION
Insulin human, rDNA (produced by recombinant DNA technology in
Saccharomyces cerevisiae
).
1 ml contains 40 IU of insulin human.
1 vial contains 10 ml equivalent to 400 IU.
One IU (International Unit) corresponds to 0.035 mg of anhydrous human insulin.
Mixtard is a mixture of dissolved insulin and isophane (NPH) insulin.
Mixtard 30 consists of 30% dissolved insulin and 70% isophane insulin.
For a full list of excipients, see section 6.1.
Suspension for injection in a vial.
Cloudy, white, aqueous suspension.
4.1 Therapeutic indications
Treatment of diabetes mellitus.
4.2 Posology and method of administration
Mixtard is a dual-acting insulin. It is biphasic formulation containing both fast-acting and long-acting
insulin.
Premixed insulin products are usually given once or twice daily when a rapid initial effect together
with a more prolonged effect is desired.
Dosage
Dosage is individual and determined in accordance with the needs of the patient. The individual
insulin requirement is usually between 0.3 and 1.0 IU/kg/day. The daily insulin requirement may be
higher in patients with insulin resistance (e.g. during puberty or due to obesity) and lower in patients
with residual, endogenous insulin production.
In patients with diabetes mellitus optimised glycaemic control delays the onset of late diabetic
complications. Close blood glucose monitoring is therefore recommended.
An injection should be followed within 30 minutes by a meal or snack containing carbohydrates.
Dosage adjustment
Concomitant illness, especially infections and feverish conditions, usually increases the patient's
insulin requirement.
Renal or hepatic impairment may reduce insulin requirement.
Adjustment of dosage may also be necessary if patients change physical activity or their usual diet.
Dosage adjustment may be necessary when transferring patients from one insulin preparation to
another (see section 4.4 ).
Administration
For subcutaneous use. Insulin suspensions are never to be administered intravenously.
Mixtard is administered subcutaneously in the thigh or abdominal wall. If convenient, the gluteal
region or the deltoid region may also be used.
Subcutaneous injection into the abdominal wall ensures a faster absorption than from other injection
sites.
Injection into a lifted skin fold minimises the risk of unintended intramuscular injection.
The needle should be kept under the skin for at least 6 seconds to make sure the entire dose is injected.
Injection sites should be rotated within an anatomic region in order to avoid lipodystrophy.
The vials are for use with insulin syringes with a corresponding unit scale.
Mixtard is accompanied by a package leaflet with detailed instruction for use to be followed.
Hypersensitivity to the active substance or to any of the excipients (see section 6.1).
Hypoglycaemia.
4.4 Special warnings and precautions for use
Inadequate dosage or discontinuation of treatment, especially in type 1 diabetes, may lead to
hyperglycaemia
.
Usually, the first symptoms of hyperglycaemia set in gradually, over a period of hours or days. They
include thirst, increased frequency of urination, nausea, vomiting, drowsiness, flushed dry skin, dry
mouth, loss of appetite as well as acetone odour of breath.
In type 1 diabetes, untreated hyperglycaemic events eventually lead to diabetic ketoacidosis, which is
potentially lethal.
Hypoglycaemia
may occur if the insulin dose is too high in relation to the insulin requirement (see
sections 4.8 and 4.9).
Omission of a meal or unplanned, strenuous physical exercise may lead to hypoglycaemia.
Patients whose blood glucose control is greatly improved e.g. by intensified insulin therapy, may
experience a change in their usual warning symptoms of hypoglycaemia and should be advised
accordingly.
Usual warning symptoms may disappear in patients with longstanding diabetes.
Transferring a patient to another type or brand of insulin should be done under strict medical
supervision. Changes in strength, brand (manufacturer), type (fast-, dual-, long-acting insulin etc.),
origin (animal, human or analogue insulin) and/or method of manufacture (recombinant DNA versus
animal source insulin) may result in a need for a change in dosage. If an adjustment is needed when
switching the patients to Mixtard, it may occur with the first dose or during the first several weeks or
months.
As with any insulin therapy, injection site reactions may occur and include pain, itching, hives,
swelling and inflammation. Continuous rotation of the injection site within a given area may help to
reduce or prevent these reactions. Reactions usually resolve in a few days to a few weeks. On rare
occasions, injection site reactions may require discontinuation of Mixtard.
A few patients who have experienced hypoglycaemic reactions after transfer from animal source
insulin have reported that early warning symptoms of hypoglycaemia were less pronounced or
different from those experienced with their previous insulin.
Before travelling between different time zones, the patient should be advised to consult the physician,
since this may mean that the patient has to take insulin and meals at different times.
Insulin suspensions are not to be used in insulin infusion pumps.
Mixtard contains metacresol, which may cause allergic reactions.
4.5 Interaction with other medicinal products and other forms of interaction
A number of medicinal products are known to interact with glucose metabolism. The physician must
therefore take possible interactions into account and should always ask his patients about any
medicinal products they take.
The following substances may reduce insulin requirement:
Oral hypoglycaemic agents (OHA),
monoamine oxidase inhibitors (MAOI), non-selective beta-
blocking agents, angiotensin converting enzyme (ACE) inhibitors, salicylates, alcohol, anabolic
steroids and sulphonamides.
The following substances may increase insulin requirement:
Oral contraeptives, thiazides, glucocorticoids, thyroid hormones and beta-sympathomimetics, growth
hormone and danazol.
Beta-blocking agents may mask the symptoms of hypoglycaemia and delay recovery from
hypoglycaemia.
Octreotide/lanreotide may both decrease and increase insulin requirement.
Alcohol may intensify and prolong the hypoglycaemic effect of insulin.
4.6 Pregnancy and lactation
There are no restrictions on treatment of diabetes with insulin during pregnancy, as insulin does not
pass the placental barrier.
Both hypoglycaemia and hyperglycaemia, which can occur in inadequately controlled diabetes
therapy, increase the risk of malformations and death
in utero
. Intensified control in the treatment of
pregnant women with diabetes is therefore recommended throughout pregnancy and when
contemplating pregnancy.
Insulin requirements usually fall in the first trimester and subsequently increase during the second and
third trimesters.
After delivery, insulin requirements return rapidly to pre-pregnancy values.
Insulin treatment of the nursing mother presents no risk to the baby. However, the Mixtard dosage
may need to be adjusted.
4.7 Effects on ability to drive and use machines
The patient’s ability to concentrate and react may be impaired as a result of hypoglycaemia. This may
constitute a risk in situations where these abilities are of special importance (e.g. driving a car or
operating machinery).
Patients should be advised to take precautions to avoid hypoglycaemia whilst driving. This is
particularly important in those who have reduced or absent awareness of the warning signs of
hypoglycaemia or have frequent episodes of hypoglycaemia. The advisability of driving should be
considered in these circumstances.
As for other insulin products, in general, hypoglycaemia is the most frequently occurring undesirable
effect. It may occur if the insulin dose is too high in relation to the insulin requirement. In clinical
trials and during marketed use, the frequency varies with patient population and dose regimens.
Therefore, no specific frequency can be presented. Severe hypoglycaemia may lead to
unconsciousness and/or convulsions and may result in temporary or permanent impairment of brain
function or even death.
Frequencies of adverse drug reactions from clinical trials that are considered related to Mixtard are
listed below. The frequencies are defined as: uncommon (≥1/1,000 to <1/100). Isolated spontaneous
cases are presented as very rare defined as <1/10,000 including isolated reports.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Nervous system disorders
Uncommon - Peripheral neuropathy
Fast improvement in blood glucose control may be associated with a condition termed “acute painful
neuropathy”, which is usually reversible.
Eye disorders
Very rare - Refraction disorders
Refraction anomalies may occur upon initiation of insulin therapy. These symptoms are usually of
transitory nature.
Uncommon - Diabetic retinopathy
Long-term improved glycaemic control decreases the risk of progression of diabetic retinopathy.
However, intensification of insulin therapy with abrupt improvement in glycaemic control may be
associated with temporary worsening of diabetic retinopathy.
Skin and subcutaneous tissue disorders
Uncommon - Lipodystrophy
Lipodystrophy may occur at the injection site as a consequence of failure to rotate injection sites
within an area.
General disorders and administration site conditions
Uncommon - Injection site reactions
Injection site reactions (redness, swelling, itching, pain and haematoma at the injection site) may occur
during treatment with insulin. Most reactions are transitory and disappear during continued treatment.
Uncommon - Oedema
Oedema may occur upon initiation of insulin therapy. These symptoms are usually of transitory nature.
Immune system disorders
Uncommon - Urticaria, rash
Very rare - Anaphylactic reactions
Symptoms of generalised hypersensitivity may include generalised skin rash, itching, sweating,
gastrointestinal upset, angioneurotic oedema, difficulties in breathing, palpitation, reduction in blood
pressure and fainting/loss of consciousness. Generalised hypersensitivity reactions are potentially life-
threatening.
A specific overdose of insulin cannot be defined. However, hypoglycaemia may develop over
sequential stages:
Mild hypoglycaemic episodes can be treated by oral administration of glucose or sugary
products. It is therefore recommended that the diabetic patients carry some sugar lumps, sweets,
biscuits or sugary fruit juice.
Severe hypoglycaemic episodes, where the patient has become unconscious, can be treated by
glucagon (0.5 to 1 mg) given intramuscularly or subcutaneously by a person who has received
appropriate instruction, or by glucose given intravenously by a medical professional. Glucose
must also be given intravenously, if the patient does not respond to glucagon within 10 to
15 minutes.
Upon regaining consciousness, administration of oral carbohydrate is recommended for the
patient in order to prevent relapse.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Insulins and analogues for injection, intermediate-acting combined with
fast-acting, insulin (human). ATC code: A10A D01.
The blood glucose lowering effect of insulin is due to the facilitated uptake of glucose following
binding of insulin to receptors on muscle and fat cells and to the simultaneous inhibition of glucose
output from the liver.
Mixtard is a dual-acting insulin.
Onset of action is within ½ hour, reaches a maximum effect within 2-8 hours and the entire duration of
action is up to 24 hours.
5.2 Pharmacokinetic properties
Insulin in the blood stream has a half-life of a few minutes. Consequently, the time-action profile of an
insulin preparation is determined solely by its absorption characteristics.
This process is influenced by several factors (e.g. insulin dosage, injection route and site, thickness of
subcutaneous fat, type of diabetes). The pharmacokinetics of insulin products are therefore affected by
significant intra- and inter-individual variation.
Absorption
The absorption profile is due to the product being a mixture of insulin products with fast and
protracted absorption respectively. The maximum plasma concentration of the fast-acting insulin is
reached within 1.5-2.5 hours after subcutaneous administration.
Distribution
No profound binding to plasma proteins, except circulating insulin antibodies (if present) has been
observed.
Metabolism
Human insulin is reported to be degraded by insulin protease or insulin-degrading enzymes and
possibly protein disulfide isomerase. A number of cleavage (hydrolysis) sites on the human insulin
molecule have been proposed; none of the metabolites formed following the cleavage are active.
Elimination
The terminal half-life is determined by the rate of absorption from the subcutaneous tissue. The
terminal half-life (t
½
) is therefore a measure of the absorption rather than of the elimination per se of
insulin from plasma (insulin in the blood stream has a t
½
of a few minutes). Trials have indicated a t
½
of about 5-10 hours.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction.
PHARMACEUTICAL PARTICULARS
Zinc chloride
Glycerol
Metacresol
Phenol
Disodium phosphate dihydrate
Sodium hydroxide (for pH adjustment)
Hydrochloric acid (for pH adjustment)
Protamine sulphate
Water for injections
Insulin products should only be added to compounds with which it is known to be compatible.
Insulin suspensions should not be added to infusion fluids.
30 months when stored between 2°C - 8°C.
6 weeks when used or stored at room temperature (below 25°C).
6.4 Special precautions for storage
Before use: store in a refrigerator (2°C - 8°C).
Do not store them in or too near the freezer section or cooling element.
Do not freeze.
During use: do not refrigerate. Do not store above 25°C.
Keep the vial in the outer carton in order to protect from light.
Protect from excessive heat and sunlight.
6.5 Nature and contents of container
10 ml glass vial (type 1) closed with a bromobutyl/polyisoprene rubber stopper and a protective
tamper-proof cap.
Pack sizes: 1 and 5 vials x 10 ml and a multipack with 5 x (1 x 10 ml) vials.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
Insulin preparations which have been frozen must not be used.
After removing Mixtard vial from the refrigerator it is recommended to allow the vial to reach room
temperature (not above 25°C) before resuspending the insulin as instructed for first time use.
Insulin suspensions should not be used if they do not appear uniformly white and cloudy after
resuspension.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 07 October 2002
Date of latest renewal: 18 September 2007
10. DATE OF REVISION OF THE TEXT
NAME OF THE MEDICINAL PRODUCT
Mixtard 30 100 IU/ml suspension for injection in a vial.
QUALITATIVE AND QUANTITATIVE COMPOSITION
Insulin human, rDNA (produced by recombinant DNA technology in
Saccharomyces cerevisiae
).
1 ml contains 100 IU of insulin human.
1 vial contains 10 ml equivalent to 1000 IU.
One IU (International Unit) corresponds to 0.035 mg of anhydrous human insulin.
Mixtard is a mixture of dissolved insulin and isophane (NPH) insulin.
Mixtard 30 consists of 30% dissolved insulin and 70% isophane insulin.
For a full list of excipients, see section 6.1.
Suspension for injection in a vial.
Cloudy, white, aqueous suspension.
4.1 Therapeutic indications
Treatment of diabetes mellitus.
4.2 Posology and method of administration
Mixtard is a dual-acting insulin. It is biphasic formulation containing both fast-acting and long-acting
insulin.
Premixed insulin products are usually given once or twice daily when a rapid initial effect together
with a more prolonged effect is desired.
Dosage
Dosage is individual and determined in accordance with the needs of the patient. The individual
insulin requirement is usually between 0.3 and 1.0 IU/kg/day. The daily insulin requirement may be
higher in patients with insulin resistance (e.g. during puberty or due to obesity) and lower in patients
with residual, endogenous insulin production.
In patients with diabetes mellitus optimised glycaemic control delays the onset of late diabetic
complications. Close blood glucose monitoring is therefore recommended.
An injection should be followed within 30 minutes by a meal or snack containing carbohydrates.
Dosage adjustment
Concomitant illness, especially infections and feverish conditions, usually increases the patient's
insulin requirement.
Renal or hepatic impairment may reduce insulin requirement.
Adjustment of dosage may also be necessary if patients change physical activity or their usual diet.
Dosage adjustment may be necessary when transferring patients from one insulin preparation to
another (see section 4.4 ).
Administration
For subcutaneous use. Insulin suspensions are never to be administered intravenously.
Mixtard is administered subcutaneously in the thigh or abdominal wall. If convenient, the gluteal
region or the deltoid region may also be used.
Subcutaneous injection into the abdominal wall ensures a faster absorption than from other injection
sites.
Injection into a lifted skin fold minimises the risk of unintended intramuscular injection.
The needle should be kept under the skin for at least 6 seconds to make sure the entire dose is injected.
Injection sites should be rotated within an anatomic region in order to avoid lipodystrophy.
The vials are for use with insulin syringes with a corresponding unit scale.
Mixtard is accompanied by a package leaflet with detailed instruction for use to be followed.
Hypersensitivity to the active substance or to any of the excipients (see section 6.1).
Hypoglycaemia.
4.4 Special warnings and precautions for use
Inadequate dosage or discontinuation of treatment, especially in type 1 diabetes, may lead to
hyperglycaemia
.
Usually, the first symptoms of hyperglycaemia set in gradually, over a period of hours or days. They
include thirst, increased frequency of urination, nausea, vomiting, drowsiness, flushed dry skin, dry
mouth, loss of appetite as well as acetone odour of breath.
In type 1 diabetes, untreated hyperglycaemic events eventually lead to diabetic ketoacidosis, which is
potentially lethal.
Hypoglycaemia
may occur if the insulin dose is too high in relation to the insulin requirement (see
sections 4.8 and 4.9).
Omission of a meal or unplanned, strenuous physical exercise may lead to hypoglycaemia.
Patients whose blood glucose control is greatly improved e.g. by intensified insulin therapy, may
experience a change in their usual warning symptoms of hypoglycaemia and should be advised
accordingly.
Usual warning symptoms may disappear in patients with longstanding diabetes.
Transferring a patient to another type or brand of insulin should be done under strict medical
supervision. Changes in strength, brand (manufacturer), type (fast-, dual-, long-acting insulin etc.),
origin (animal, human or analogue insulin) and/or method of manufacture (recombinant DNA versus
animal source insulin) may result in a need for a change in dosage. If an adjustment is needed when
switching the patients to Mixtard, it may occur with the first dose or during the first several weeks or
months.
As with any insulin therapy, injection site reactions may occur and include pain, itching, hives,
swelling and inflammation. Continuous rotation of the injection site within a given area may help to
reduce or prevent these reactions. Reactions usually resolve in a few days to a few weeks. On rare
occasions, injection site reactions may require discontinuation of Mixtard.
A few patients who have experienced hypoglycaemic reactions after transfer from animal source
insulin have reported that early warning symptoms of hypoglycaemia were less pronounced or
different from those experienced with their previous insulin.
Before travelling between different time zones, the patient should be advised to consult the physician,
since this may mean that the patient has to take insulin and meals at different times.
Insulin suspensions are not to be used in insulin infusion pumps.
Mixtard contains metacresol, which may cause allergic reactions.
4.5 Interaction with other medicinal products and other forms of interaction
A number of medicinal products are known to interact with glucose metabolism. The physician must
therefore take possible interactions into account and should always ask hispatients about any
medicinal products they take.
The following substances may reduce insulin requirement:
Oral hypoglycaemic agents (OHA),
monoamine oxidase inhibitors (MAOI), non-selective beta-
blocking agents, angiotensin converting enzyme (ACE) inhibitors, salicylates, alcohol, anabolic
steroids and sulphonamides.
The following substances may increase insulin requirement:
Oral contraeptives, thiazides, glucocorticoids, thyroid hormones and beta-sympathomimetics, growth
hormone and danazol.
Beta-blocking agents may mask the symptoms of hypoglycaemia and delay recovery from
hypoglycaemia.
Octreotide/lanreotide may both decrease and increase insulin requirement.
Alcohol may intensify and prolong the hypoglycaemic effect of insulin.
4.6 Pregnancy and lactation
There are no restrictions on treatment of diabetes with insulin during pregnancy, as insulin does not
pass the placental barrier.
Both hypoglycaemia and hyperglycaemia, which can occur in inadequately controlled diabetes
therapy, increase the risk of malformations and death
in utero
. Intensified control in the treatment of
pregnant women with diabetes is therefore recommended throughout pregnancy and when
contemplating pregnancy.
Insulin requirements usually fall in the first trimester and subsequently increase during the second and
third trimesters.
After delivery, insulin requirements return rapidly to pre-pregnancy values.
Insulin treatment of the nursing mother presents no risk to the baby. However, the Mixtard dosage
may need to be adjusted.
4.7 Effects on ability to drive and use machines
The patient’s ability to concentrate and react may be impaired as a result of hypoglycaemia. This may
constitute a risk in situations where these abilities are of special importance (e.g. driving a car or
operating machinery).
Patients should be advised to take precautions to avoid hypoglycaemia whilst driving. This is
particularly important in those who have reduced or absent awareness of the warning signs of
hypoglycaemia or have frequent episodes of hypoglycaemia. The advisability of driving should be
considered in these circumstances.
As for other insulin products, in general, hypoglycaemia is the most frequently occurring undesirable
effect. It may occur if the insulin dose is too high in relation to the insulin requirement. In clinical
trials and during marketed use, the frequency varies with patient population and dose regimens.
Therefore, no specific frequency can be presented. Severe hypoglycaemia may lead to
unconsciousness and/or convulsions and may result in temporary or permanent impairment of brain
function or even death.
Frequencies of adverse drug reactions from clinical trials that are considered related to Mixtard are
listed below. The frequencies are defined as: uncommon (≥1/1,000 to <1/100). Isolated spontaneous
cases are presented as very rare defined as < 1/10,000 including isolated reports.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Nervous system disorders
Uncommon - Peripheral neuropathy
Fast improvement in blood glucose control may be associated with a condition termed “acute painful
neuropathy”, which is usually reversible.
Eye disorders
Very rare - Refraction disorders
Refraction anomalies may occur upon initiation of insulin therapy. These symptoms are usually of
transitory nature.
Uncommon - Diabetic retinopathy
Long-term improved glycaemic control decreases the risk of progression of diabetic retinopathy.
However, intensification of insulin therapy with abrupt improvement in glycaemic control may be
associated with temporary worsening of diabetic retinopathy.
Skin and subcutaneous tissue disorders
Uncommon - Lipodystrophy
Lipodystrophy may occur at the injection site as a consequence of failure to rotate injection sites
within an area.
General disorders and administration site conditions
Uncommon - Injection site reactions
Injection site reactions (redness, swelling, itching, pain and haematoma at the injection site) may occur
during treatment with insulin. Most reactions are transitory and disappear during continued treatment.
Uncommon - Oedema
Oedema may occur upon initiation of insulin therapy. These symptoms are usually of transitory nature.
Immune system disorders
Uncommon - Urticaria, rash
Very rare - Anaphylactic reactions
Symptoms of generalised hypersensitivity may include generalised skin rash, itching, sweating,
gastrointestinal upset, angioneurotic oedema, difficulties in breathing, palpitation, reduction in blood
pressure and fainting/loss of consciousness. Generalised hypersensitivity reactions are potentially life-
threatening.
A specific overdose of insulin cannot be defined. However, hypoglycaemia may develop over
sequential stages:
Mild hypoglycaemic episodes can be treated by oral administration of glucose or sugary
products. It is therefore recommended that the diabetic patients carry some sugar lumps, sweets,
biscuits or sugary fruit juice.
Severe hypoglycaemic episodes, where the patient has become unconscious, can be treated by
glucagon (0.5 to 1 mg) given intramuscularly or subcutaneously by a person who has received
appropriate instruction, or by glucose given intravenously by a medical professional. Glucose
must also be given intravenously, if the patient does not respond to glucagon within 10 to
15 minutes.
Upon regaining consciousness, administration of oral carbohydrate is recommended for the
patient in order to prevent relapse.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Insulins and analogues for injection, intermediate-acting combined with
fast-acting, insulin (human). ATC code: A10A D01.
The blood glucose lowering effect of insulin is due to the facilitated uptake of glucose following
binding of insulin to receptors on muscle and fat cells and to the simultaneous inhibition of glucose
output from the liver.
Mixtard is a dual-acting insulin.
Onset of action is within ½ hour, reaches a maximum effect within 2-8 hours and the entire duration of
action is up to 24 hours.
5.2 Pharmacokinetic properties
Insulin in the blood stream has a half-life of a few minutes. Consequently, the time-action profile of an
insulin preparation is determined solely by its absorption characteristics.
This process is influenced by several factors (e.g. insulin dosage, injection route and site, thickness of
subcutaneous fat, type of diabetes). The pharmacokinetics of insulin products are therefore affected by
significant intra- and inter-individual variation.
Absorption
The absorption profile is due to the product being a mixture of insulin products with fast and
protracted absorption respectively. The maximum plasma concentration of the fast-acting insulin is
reached within 1.5-2.5 hours after subcutaneous administration.
Distribution
No profound binding to plasma proteins, except circulating insulin antibodies (if present) has been
observed.
Metabolism
Human insulin is reported to be degraded by insulin protease or insulin-degrading enzymes and
possibly protein disulfide isomerase. A number of cleavage (hydrolysis) sites on the human insulin
molecule have been proposed; none of the metabolites formed following the cleavage are active.
Elimination
The terminal half-life is determined by the rate of absorption from the subcutaneous tissue. The
terminal half-life (t
½
) is therefore a measure of the absorption rather than of the elimination per se of
insulin from plasma (insulin in the blood stream has a t
½
of a few minutes). Trials have indicated a t
½
of about 5-10 hours.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction.
PHARMACEUTICAL PARTICULARS
Zinc chloride
Glycerol
Metacresol
Phenol
Disodium phosphate dihydrate
Sodium hydroxide (for pH adjustment)
Hydrochloric acid (for pH adjustment)
Protamine sulphate
Water for injections
Insulin products should only be added to compounds with which it is known to be compatible.
Insulin suspensions should not be added to infusion fluids.
30 months when stored between 2ºC - 8ºC.
6 weeks when used or stored at room temperature (below 25ºC).
6.4 Special precautions for storage
Before use: store in a refrigerator (2°C - 8°C).
Do not store them in or too near the freezer section or cooling element.
Do not freeze.
During use: do not refrigerate. Do not store above 25°C.
Keep the vial in the outer carton in order to protect from light.
Protect from excessive heat and sunlight.
6.5 Nature and contents of container
10 ml glass vial (type 1) closed with a bromobutyl/polyisoprene rubber stopper and a protective
tamper-proof cap.
Pack sizes: 1 and 5 vials x 10 ml and a multipack with 5 x (1 x 10 ml) vials.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
Insulin preparations which have been frozen must not be used.
After removing Mixtard vial from the refrigerator it is recommended to allow the vial to reach room
temperature (not above 25°C) before resuspending the insulin as instructed for first time use.
Insulin suspensions should not be used if they do not appear uniformly white and cloudy after
resuspension.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 07 October 2002
Date of latest renewal: 18 September 2007
10. DATE OF REVISION OF THE TEXT
NAME OF THE MEDICINAL PRODUCT
Mixtard 30 Penfill 100 IU/ml suspension for injection in a cartridge.
QUALITATIVE AND QUANTITATIVE COMPOSITION
Insulin human, rDNA (produced by recombinant DNA technology in
Saccharomyces cerevisiae
).
1 ml contains 100 IU of insulin human.
1 cartridge contains 3 ml equivalent to 300 IU.
One IU (International Unit) corresponds to 0.035 mg of anhydrous human insulin.
Mixtard is a mixture of dissolved insulin and isophane (NPH) insulin.
Mixtard 30 consists of 30% dissolved insulin and 70% isophane insulin.
For a full list of excipients, see section 6.1.
Suspension for injection in a cartridge.
Cloudy, white, aqueous suspension.
4.1 Therapeutic indications
Treatment of diabetes mellitus.
4.2 Posology and method of administration
Mixtard is a dual-acting insulin. It is biphasic formulation containing both fast-acting and long-acting
insulin.
Premixed insulin products are usually given once or twice daily when a rapid initial effect together
with a more prolonged effect is desired.
Dosage
Dosage is individual and determined in accordance with the needs of the patient. The individual
insulin requirement is usually between 0.3 and 1.0 IU/kg/day. The daily insulin requirement may be
higher in patients with insulin resistance (e.g. during puberty or due to obesity) and lower in patients
with residual, endogenous insulin production.
In patients with diabetes mellitus optimised glycaemic control delays the onset of late diabetic
complications. Close blood glucose monitoring is therefore recommended.
An injection should be followed within 30 minutes by a meal or snack containing carbohydrates.
Dosage adjustment
Concomitant illness, especially infections and feverish conditions, usually increases the patient's
insulin requirement.
Renal or hepatic impairment may reduce insulin requirement.
Adjustment of dosage may also be necessary if patients change physical activity or their usual diet.
Dosage adjustment may be necessary when transferring patients from one insulin preparation to
another (see section 4.4 ).
Administration
For subcutaneous use. Insulin suspensions are never to be administered intravenously.
Mixtard is administered subcutaneously in the thigh or abdominal wall. If convenient, the gluteal
region or the deltoid region may also be used.
Subcutaneous injection into the abdominal wall ensures a faster absorption than from other injection
sites.
Injection into a lifted skin fold minimises the risk of unintended intramuscular injection.
The needle should be kept under the skin for at least 6 seconds to make sure the entire dose is injected.
Injection sites should be rotated within an anatomic region in order to avoid lipodystrophy.
The cartridges are designed to be used with Novo Nordisk delivery systems (durable devices for
repeated use) and NovoFine or NovoTwist needles. Detailed instruction accompanying the delivery
system must be followed.
Mixtard is accompanied by a package leaflet with detailed instruction for use to be followed.
Hypersensitivity to the active substance or to any of the excipients (see section 6.1).
Hypoglycaemia.
4.4 Special warnings and precautions for use
Inadequate dosage or discontinuation of treatment, especially in type 1 diabetes, may lead to
hyperglycaemia
.
Usually, the first symptoms of hyperglycaemia set in gradually, over a period of hours or days. They
include thirst, increased frequency of urination, nausea, vomiting, drowsiness, flushed dry skin, dry
mouth, loss of appetite as well as acetone odour of breath.
In type 1 diabetes, untreated hyperglycaemic events eventually lead to diabetic ketoacidosis, which is
potentially lethal.
Hypoglycaemia
may occur if the insulin dose is too high in relation to the insulin requirement (see
sections 4.8 and 4.9).
Omission of a meal or unplanned, strenuous physical exercise may lead to hypoglycaemia.
Patients whose blood glucose control is greatly improved e.g. by intensified insulin therapy, may
experience a change in their usual warning symptoms of hypoglycaemia and should be advised
accordingly.
Usual warning symptoms may disappear in patients with longstanding diabetes.
Transferring a patient to another type or brand of insulin should be done under strict medical
supervision. Changes in strength, brand (manufacturer), type (fast-, dual-, long-acting insulin etc.),
origin (animal, human or analogue insulin) and/or method of manufacture (recombinant DNA versus
animal source insulin) may result in a need for a change in dosage. If an adjustment is needed when
switching the patients to Mixtard, it may occur with the first dose or during the first several weeks or
months.
As with any insulin therapy, injection site reactions may occur and include pain, itching, hives,
swelling and inflammation. Continuous rotation of the injection site within a given area may help to
reduce or prevent these reactions. Reactions usually resolve in a few days to a few weeks. On rare
occasions, injection site reactions may require discontinuation of Mixtard.
A few patients who have experienced hypoglycaemic reactions after transfer from animal source
insulin have reported that early warning symptoms of hypoglycaemia were less pronounced or
different from those experienced with their previous insulin.
Before travelling between different time zones, the patient should be advised to consult the physician,
since this may mean that the patient has to take insulin and meals at different times.
Insulin suspensions are not to be used in insulin infusion pumps.
Mixtard contains metacresol, which may cause allergic reactions.
4.5 Interaction with other medicinal products and other forms of interaction
A number of medicinal products are known to interact with glucose metabolism. The physician must
therefore take possible interactions into account and should always ask his patients about any
medicinal products they take.
The following substances may reduce insulin requirement:
Oral hypoglycaemic agents (OHA),
monoamine oxidase inhibitors (MAOI), non-selective beta-
blocking agents, angiotensin converting enzyme (ACE) inhibitors, salicylates, alcohol, anabolic
steroids and sulphonamides.
The following substances may increase insulin requirement:
Oral contraeptives, thiazides, glucocorticoids, thyroid hormones and beta-sympathomimetics, growth
hormone and danazol.
Beta-blocking agents may mask the symptoms of hypoglycaemia and delay recovery from
hypoglycaemia.
Octreotide/lanreotide may both decrease and increase insulin requirement.
Alcohol may intensify and prolong the hypoglycaemic effect of insulin.
4.6 Pregnancy and lactation
There are no restrictions on treatment of diabetes with insulin during pregnancy, as insulin does not
pass the placental barrier.
Both hypoglycaemia and hyperglycaemia, which can occur in inadequately controlled diabetes
therapy, increase the risk of malformations and death
in utero
. Intensified control in the treatment of
pregnant women with diabetes is therefore recommended throughout pregnancy and when
contemplating pregnancy.
Insulin requirements usually fall in the first trimester and subsequently increase during the second and
third trimesters.
After delivery, insulin requirements return rapidly to pre-pregnancy values.
Insulin treatment of the nursing mother presents no risk to the baby. However, the Mixtard dosage
may need to be adjusted.
4.7 Effects on ability to drive and use machines
The patient’s ability to concentrate and react may be impaired as a result of hypoglycaemia. This may
constitute a risk in situations where these abilities are of special importance (e.g. driving a car or
operating machinery).
Patients should be advised to take precautions to avoid hypoglycaemia whilst driving. This is
particularly important in those who have reduced or absent awareness of the warning signs of
hypoglycaemia or have frequent episodes of hypoglycaemia. The advisability of driving should be
considered in these circumstances.
As for other insulin products, in general, hypoglycaemia is the most frequently occurring undesirable
effect. It may occur if the insulin dose is too high in relation to the insulin requirement. In clinical
trials and during marketed use, the frequency varies with patient population and dose regimens.
Therefore, no specific frequency can be presented. Severe hypoglycaemia may lead to
unconsciousness and/or convulsions and may result in temporary or permanent impairment of brain
function or even death.
Frequencies of adverse drug reactions from clinical trials that are considered related to Mixtard are
listed below. The frequencies are defined as: uncommon (≥1/1,000 to <1/100). Isolated spontaneous
cases are presented as very rare defined as <1/10,000 including isolated reports.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Nervous system disorders
Uncommon - Peripheral neuropathy
Fast improvement in blood glucose control may be associated with a condition termed “acute painful
neuropathy”, which is usually reversible.
Eye disorders
Very rare - Refraction disorders
Refraction anomalies may occur upon initiation of insulin therapy. These symptoms are usually of
transitory nature.
Uncommon - Diabetic retinopathy
Long-term improved glycaemic control decreases the risk of progression of diabetic retinopathy.
However, intensification of insulin therapy with abrupt improvement in glycaemic control may be
associated with temporary worsening of diabetic retinopathy.
Skin and subcutaneous tissue disorders
Uncommon - Lipodystrophy
Lipodystrophy may occur at the injection site as a consequence of failure to rotate injection sites
within an area.
General disorders and administration site conditions
Uncommon - Injection site reactions
Injection site reactions (redness, swelling, itching, pain and haematoma at the injection site) may occur
during treatment with insulin. Most reactions are transitory and disappear during continued treatment.
Uncommon - Oedema
Oedema may occur upon initiation of insulin therapy. These symptoms are usually of transitory nature.
Immune system disorders
Uncommon - Urticaria, rash
Very rare - Anaphylactic reactions
Symptoms of generalised hypersensitivity may include generalised skin rash, itching, sweating,
gastrointestinal upset, angioneurotic oedema, difficulties in breathing, palpitation, reduction in blood
pressure and fainting/loss of consciousness. Generalised hypersensitivity reactions are potentially life-
threatening.
A specific overdose of insulin cannot be defined. However, hypoglycaemia may develop over
sequential stages:
•
Mild hypoglycaemic episodes can be treated by oral administration of glucose or sugary
products. It is therefore recommended that the diabetic patients carry some sugar lumps, sweets,
biscuits or sugary fruit juice.
Severe hypoglycaemic episodes, where the patient has become unconscious, can be treated by
glucagon (0.5 to 1 mg) given intramuscularly or subcutaneously by a person who has received
appropriate instruction, or by glucose given intravenously by a medical professional. Glucose
must also be given intravenously, if the patient does not respond to glucagon within 10 to
15 minutes.
Upon regaining consciousness, administration of oral carbohydrate is recommended for the
patient in order to prevent relapse.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Insulins and analogues for injection, intermediate-acting combined with
fast-acting, insulin (human). ATC code: A10A D01.
The blood glucose lowering effect of insulin is due to the facilitated uptake of glucose following
binding of insulin to receptors on muscle and fat cells and to the simultaneous inhibition of glucose
output from the liver.
Mixtard is a dual-acting insulin.
Onset of action is within ½ hour, reaches a maximum effect within 2-8 hours and the entire duration of
action is up to 24 hours.
5.2 Pharmacokinetic properties
Insulin in the blood stream has a half-life of a few minutes. Consequently, the time-action profile of an
insulin preparation is determined solely by its absorption characteristics.
This process is influenced by several factors (e.g. insulin dosage, injection route and site, thickness of
subcutaneous fat, type of diabetes). The pharmacokinetics of insulin products are therefore affected by
significant intra- and inter-individual variation.
Absorption
The absorption profile is due to the product being a mixture of insulin products with fast and
protracted absorption respectively. The maximum plasma concentration of the fast-acting insulin is
reached within 1.5-2.5 hours after subcutaneous administration.
Distribution
No profound binding to plasma proteins, except circulating insulin antibodies (if present) has been
observed.
Metabolism
Human insulin is reported to be degraded by insulin protease or insulin-degrading enzymes and
possibly protein disulfide isomerase. A number of cleavage (hydrolysis) sites on the human insulin
molecule have been proposed; none of the metabolites formed following the cleavage are active.
Elimination
The terminal half-life is determined by the rate of absorption from the subcutaneous tissue. The
terminal half-life (t
½
) is therefore a measure of the absorption rather than of the elimination per se of
insulin from plasma (insulin in the blood stream has a t
½
of a few minutes). Trials have indicated a t
½
of about 5-10 hours.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction.
PHARMACEUTICAL PARTICULARS
Zinc chloride
Glycerol
Metacresol
Phenol
Disodium phosphate dihydrate
Sodium hydroxide (for pH adjustment)
Hydrochloric acid (for pH adjustment)
Protamine sulphate
Water for injections
Insulin products should only be added to compounds with which it is known to be compatible.
Insulin suspensions should not be added to infusion fluids.
30 months when stored between 2°C - 8°C.
6 weeks when used or carried as a spare (below 30°C).
6.4 Special precautions for storage
Before use: Store in a refrigerator (2°C - 8°C).
Do not store them in or too near the freezer section or cooling element.
Do not freeze.
During use: do not refrigerate. Do not store above 30°C.
Keep the cartridge in the outer carton in order to protect from light.
Protect from excessive heat and sunlight.
6.5 Nature and contents of container
3 ml glass cartridge (type 1) with a bromobutyl rubber plunger and a bromobutyl/polyisoprene rubber
stopper. The cartridge contains a glass ball to facilitate the re-suspension.
Pack sizes: 1, 5 and 10 cartridges x 3 ml.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
Cartridges should only be used in combination with products that are compatible with them and allow
the cartridge to function safely and effectively.
Mixtard Penfill is for single person use only. The container must not be refilled.
Insulin preparations which have been frozen must not be used.
After removing Mixtard Penfill from the refrigerator it is recommended to allow the Penfill to reach
room temperature (not above 25°C) before resuspending the insulin as instructed for first time use.
Insulin suspensions should not be used if they do not appear uniformly white and cloudy after
resuspension.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 07 October 2002
Date of latest renewal: 18 September 2007
10. DATE OF REVISION OF THE TEXT
The active substance
is insulin human made by recombinant biotechnology (30% as soluble
insulin and 70% as isophane insulin). 1 ml contains 100 IU of insulin human. 1 pre-filled pen
contains 3 ml equivalent to 300 IU
The other ingredients are
zinc chloride, glycerol, metacresol, phenol, disodium phosphate
dihydrate, sodium hydroxide, hydrochloric acid, protamine sulphate and water for injections.
What Mixtard looks like and contents of the pack
The suspension for injection comes as a cloudy, white, aqueous suspension in packs.
It is supplied of 1, 5 or 10 pre-filled pens of 3 ml. Not all packs may be marketed.
Marketing Authorisation Holder
Novo Nordisk A/S
Novo Allé, DK-2880 Bagsværd, Denmark
Manufacturer
The manufacturer can be identified by the batch number printed on the slip of the carton and on the
label:
If the second and third characters are W5, S6, P5, K7, or ZF Novo Nordisk A/S, Novo Allé,
DK-2880 Bagsværd, Denmark is the manufacturer
If the second and third characters are H7 or T6 Novo Nordisk Production SAS, 45 Avenue
d’Orléans, F-28002 Chartres, France is the manufacturer.
Now turn over for
information on how to use your
FlexPen.
This leaflet was last approved in
Please read the following instructions carefully before using your Mixtard 30 FlexPen.
Your FlexPen is a unique dial-a-dose insulin pen. You can select doses from 1 to 60 units in
increments of 1 unit. FlexPen is designed and tested to be used with NovoFine or NovoTwist
disposable needles up to a length of 8 mm. As a precautionary measure, always carry a spare insulin
delivery device in case your FlexPen is lost or damaged.
The colour of the pen in the illustrations differs from your FlexPen.
Your FlexPen is designed to work accurately and safely. It must be handled with care. If it is dropped
or crushed, there is a risk of damage and leakage of insulin.
You can clean the exterior of your FlexPen by wiping it with a medicinal swab. Do not soak it, wash
or lubricate it as it may damage the pen.
Do not refill your FlexPen.
Preparing your Mixtard FlexPen
Check the label to make sure that your FlexPen contains the correct type of insulin. Before your
first injection with a new FlexPen you must resuspend the insulin:
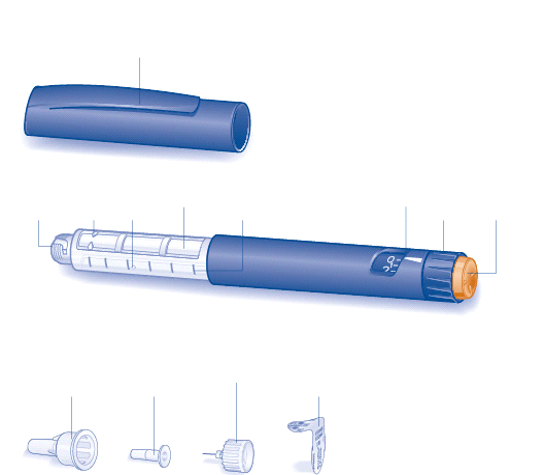
Let the insulin reach room temperature before you use it.
This makes it easier to resuspend.
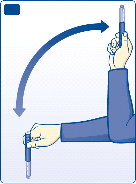
B
Move the pen up and down twenty times between the two positions as shown, so the glass ball moves
from one end of the cartridge to the other. Repeat until the liquid appears uniformly white and cloudy.
For every following injection
move the pen up and down between the two positions at least ten times
until the liquid appears uniformly white and cloudy.
After you have resuspended the insulin, complete all the following steps of injection without delay.
Always check there are at least 12 units of insulin left in the cartridge to allow resuspension. If
there are less than 12 units left, use a new FlexPen.
Disinfect the rubber membrane with a medicinal swab.
C
Remove the protective tab from a new disposable needle.
Screw the needle straight and tightly onto your FlexPen.
D
Pull off the big outer needle cap and keep it for later.


E
Pull off the inner needle cap and dispose of it.
Always use a new needle for each injection to prevent contamination.
Be careful not to bend or damage the needle before use.
To reduce the risk of unexpected needle sticks, never put the inner needle cap back on when you
have removed it from the needle.
Checking the insulin flow
Prior to each injection small amounts of air may collect in the cartridge during normal use. To
avoid injection of air and ensure proper dosing:
F
Turn the dose selector to select 2 units.
G
Hold your FlexPen with the needle pointing upwards and tap the cartridge gently with your finger a
few times to make any air bubbles collect at the top of the cartridge.



H
Keeping the needle upwards, press the push-button all the way in. The dose selector returns to 0.
A drop of insulin should appear at the needle tip. If not, change the needle and repeat the procedure no
more than six times.
If a drop of insulin still does not appear, the pen is defective, and you must use a new one.
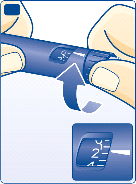
Check that the dose selector is set at 0.
I
Turn the dose selector to select the number of units you need to inject.
The dose can be corrected either up or down by turning the dose selector in either direction until the
correct dose lines up with the pointer. When turning the dose selector be careful not to push the push-
button as insulin will come out.
You cannot select a dose larger than the number of units left in the cartridge.
Do not use the residual scale to measure your dose of insulin.
Insert the needle into your skin. Use the injection technique shown by your doctor or nurse.


Inject the dose by pressing the push-button all the way in until 0 lines up with the pointer. Be careful
only to push the push-button when injecting.
Turning the dose selector will not inject insulin.
K
Keep the push-button fully depressed after the injection until the needle has been withdrawn from the
skin.
The needle must remain under the skin for at least six seconds. This will ensure that the full dose has
been injected.
L
Lead the needle into the big outer needle cap without touching the big outer needle cap. When the
needle is covered, carefully push the big outer needle cap completely on and then unscrew the needle.
Dispose of it carefully and put the pen cap back on.
Always remove the needle after each injection and store your FlexPen without the needle
attached. Otherwise, the liquid may leak out which can cause inaccurate dosing.
Caregivers should be most careful when handling used needles to avoid needle sticks.
Dispose of the used FlexPen carefully without the needle attached.
Do not share your FlexPen with anyone else.



Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/mixtard.html
Copyright © 1995-2021 ITA all rights reserved.
|













 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































