Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Nivestim 12 MU/ 0.2 ml solution for injection/infusion
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml of solution for injection or infusion contains 60 million units [MU] (600 micrograms) of filgrastim*.
Each pre-filled syringe contains 12 million units (MU) (120 micrograms) of filgrastim in 0.2 ml (0.6 mg/ml).
*recombinant methionyl granulocyte-colony stimulating factor [G-CSF] produced in
Escherichia Coli
(BL21)
by recombinant DNA technology.
Excipient(s)
: Each ml of solution contains 50 mg of sorbitol.
For a full list of excipients, see section 6.1.
Solution for injection/infusion (injection/ infusion).
Clear, colourlesss solution.
4.1 Therapeutic indications
Filgrastim is indicated for the reduction in the duration of neutropenia and the incidence of febrile
neutropenia in patients treated with established cytotoxic chemotherapy for malignancy (with the exception
of chronic myeloid leukaemia and myelodysplastic syndromes) and for the reduction in the duration of
neutropenia in patients undergoing myeloablative therapy followed by bone marrow transplantation
considered to be at increased risk of prolonged severe neutropenia.
The safety and efficacy of filgrastim are similar in adults and children receiving cytotoxic chemotherapy.
Filgrastim is indicated for the mobilisation of peripheral blood progenitor cells (PBPC).
In patients, children or adults, with severe congenital, cyclic, or idiopathic neutropenia with an absolute
neutrophil count (ANC) of ≤ 0.5 x 10
9
/l and a history of severe or recurrent infections, long term
administration of filgrastim is indicated to increase neutrophil counts and to reduce the incidence and
duration of infection-related events.
Filgrastim is indicated for the treatment of persistent neutropenia (ANC less than or equal to 1.0 x 10
9
/l) in
patients with advanced HIV infection, in order to reduce the risk of bacterial infections when other options to
manage neutropenia are inappropriate.
4.2
Posology and method of administration
Filgrastim therapy should only be given in collaboration with an oncology centre which has experience in G-
CSF treatment and haematology and has the necessary diagnostic facilities. The mobilisation and apheresis
procedures should be performed in collaboration with an oncology-haematology centre with acceptable
experience in this field and where the monitoring of haematopoietic progenitor cells can be correctly
performed.
Established cytotoxic chemotherapy
The recommended dose of filgrastim is 0.5 MU (5 micrograms)/kg/day. The first dose of filgrastim should
not be administered less than 24 hours following cytotoxic chemotherapy. Filgrastim may be given as a daily
subcutaneous injection or as a daily intravenous infusion diluted in glucose 50 mg/ml (5%) solution for
infusion given over 30 minutes (see section 6.6 for instructions on dilutions)
.
The subcutaneous route is preferred in most cases. There is some evidence from a study of single dose
administration that intravenous dosing may shorten the duration of effect. The clinical relevance of this
finding to multiple dose administration is not clear. The choice of route should depend on the individual
clinical circumstance. In randomised clinical trials, a subcutaneous dose of 230 micrograms/m
2
/day (4.0 to
8.4 micrograms/kg/day) was used.
Daily dosing with filgrastim should continue until the expected neutrophil nadir is passed and the neutrophil
count has recovered to the normal range. Following established chemotherapy for solid tumours,
lymphomas, and lymphoid leukaemias, it is expected that the duration of treatment required to fulfil these
criteria will be up to 14 days. Following induction and consolidation treatment for acute myeloid leukaemia
the duration of treatment may be substantially longer (up to 38 days) depending on the type, dose and
schedule of cytotoxic chemotherapy used.
In patients receiving cytotoxic chemotherapy, a transient increase in neutrophil counts is typically seen 1 to 2
days after initiation of filgrastim therapy. However, for a sustained therapeutic response, filgrastim therapy
should not be discontinued before the expected nadir has passed and the neutrophil count has recovered to
the normal range. Premature discontinuation of filgrastim therapy, prior to the time of the expected
neutrophil nadir, is not recommended.
In patients treated with myeloablative therapy followed by bone marrow transplantation
The recommended starting dose of filgrastim is 1.0 MU (10 micrograms)/kg/day given as a 30 minute or 24
hour intravenous infusion or 1.0 MU (10 micrograms)/kg/day given by continuous 24 hour subcutaneous
infusion. Filgrastim should be diluted in 20 ml of 50 mg/ml (5%) glucose solution for infusion (see section
6.6).
The first dose of filgrastim should not be administered less than 24 hours following cytotoxic chemotherapy
and within 24 hours of bone marrow infusion.
Once the neutrophil nadir has been passed, the daily dose of filgrastim should be titrated against the
neutrophil response as follows:
Filgrastim dose adjustment
> 1.0 x 10
9
/l for 3 consecutive days
Then, if ANC remains > 1.0 x 10
9
/l for 3 more
consecutive days
If the ANC decreases to < 1.0 x 10
9
/l during the treatment period the dose of filgrastim should be re-
escalated according to the above steps
For the mobilisation of peripheral blood progenitor cells (PBPC) in patients undergoing myelosuppressive or
myeloablative therapy followed by autologous peripheral blood progenitor cell transplantation
The recommended dose of filgrastim for PBPC mobilisation when used alone is 1.0 MU
(10 micrograms)/kg/day as a 24 hour subcutaneous continuous infusion or a single daily subcutaneous
injection for 5 to 7 consecutive days. For infusions filgrastim should be diluted in 20 ml of 50 mg/ml (5%)
glucose solution for infusion (see section 6.6). Timing of leukapheresis: one or two leukaphereses on days 5








and 6 are often sufficient. In other circumstances, additional leukaphereses may be necessary. Filgrastim
dosing should be maintained until the last leukapheresis.
The recommended dose of filgrastim for PBPC mobilisation after myelosuppressive chemotherapy is 0.5 MU
(5 micrograms)/kg/day given daily by subcutaneous injection from the first day after completion of
chemotherapy until the expected neutrophil nadir is passed and the neutrophil count has recovered to the
normal range. Leukapheresis should be performed during the period when the ANC rises from < 0.5 x 10
9
/l
to > 5.0 x 10
9
/l. For patients who have not had extensive chemotherapy, one leukapheresis is often sufficient.
In other circumstances, additional leukaphereses are recommended.
For the mobilisation of peripheral blood progenitor cells (PBPCs) in normal donors prior to allogeneic
peripheral blood progenitor cell transplantation
For PBPC mobilisation in normal donors, filgrastim should be administered at 10 micrograms/kg/day
subcutaneously for 4 to 5 consecutive days. Leukapheresis should be started at day 5 and continued until day
6 if needed in order to collect 4 x 10
6
CD34
+
cells/kg recipient bodyweight.
In patients with severe chronic neutropenia
Congenital neutropenia
: the recommended starting dose is 1.2 MU (12 micrograms)/kg/day subcutaneously
as a single dose or in divided doses.
Idiopathic or cyclic neutropenia
: the recommended starting dose is 0.5 MU (5 micrograms)/kg/day
subcutaneously as a single dose or in divided doses.
Dose adjustment
: Filgrastim should be administered daily by subcutaneous injection until the neutrophil
count has reached and can be maintained at more than 1.5 x 10
9
/l. When the response has been obtained the
minimal effective dose to maintain this level should be established. Long-term daily administration is
required to maintain an adequate neutrophil count. After one to two weeks of therapy, the initial dose may be
doubled or halved depending upon the patient's response. Subsequently the dose may be individually
adjusted every 1 to 2 weeks to maintain the average neutrophil count between 1.5 x 10
9
/l and 10 x 10
9
/l. A
faster schedule of dose escalation may be considered in patients presenting with severe infections. In clinical
trials, 97% of patients who responded had a complete response at doses ≤24 micrograms/kg/day. The long-
term safety of filgrastim administration above 24 micrograms/kg/day in patients with severe chronic
neutropenia has not been established.
In patients with HIV infection
For reversal of neutropenia
The recommended starting dose of filgrastim is 0.1 MU (1 micrograms)/kg/day given daily by subcutaneous
injection with titration up to a maximum of 0.4 MU (4 μg)/kg/day until a normal neutrophil count is reached
and can be maintained (ANC > 2.0 x10
9
/l). In clinical studies, > 90% of patients responded at these doses,
achieving reversal of neutropenia in a median of 2 days.
In a small number of patients (< 10%), doses up to 1.0 MU (10 micrograms)/kg/day were required to achieve
reversal of neutropenia.
For maintaining normal neutrophil counts
When reversal of neutropenia has been achieved, the minimal effective dose to maintain a normal neutrophil
count should be established. Initial dose adjustment to alternate day dosing with 30 MU
(300 micrograms)/day by subcutaneous injection is recommended. Further dose adjustment may be
necessary, as determined by the patient's ANC, to maintain the neutrophil count at > 2.0 x 10
9
/l. In clinical
studies, dosing with 30 MU (300 micrograms)/day on 1 to 7 days per week was required to maintain the
ANC > 2.0 x 10
9
/l, with the median dose frequency being 3 days per week. Long-term administration may be
required to maintain the ANC > 2.0 x 10
9
/l.
Clinical trials with filgrastim have included a small number of elderly patients but special studies have not
been performed in this group and therefore specific posology recommendations cannot be made.
Patients with renal or hepatic impairment
Studies of filgrastim in patients with severe impairment of renal or hepatic function demonstrate that it
exhibits a similar pharmacokinetic and pharmacodynamic profile to that seen in normal individuals. Dose
adjustment is not required in these circumstances.
Paediatric use in the severe chronic neutropenia (SCN) and cancer settings
Sixty-five percent of the patients studied in the SCN trial program were under 18 years of age. The efficacy
of treatment was clear for this age group, which included most patients with congenital neutropenia. There
were no differences in the safety profiles for paediatric patients treated for severe chronic neutropenia.
Data from clinical studies in paediatric patients indicate that the safety and efficacy of filgrastim are similar
in both adults and children receiving cytotoxic chemotherapy.
The posology recommendations in paediatric patients are the same as those in adults receiving
myelosuppressive cytotoxic chemotherapy.
Hypersensitivity to the active substance(s) or to any of the excipients.
4.4 Special warnings and precautions for use
Filgrastim should not be used to increase the dose of cytotoxic chemotherapy beyond established
posology regimens.
Filgrastim should not be administered to patients with severe congenital neutropenia (Kostmann's
syndrome) with abnormal cytogenetics.
Malignant cell growth
GCSF can promote growth of myeloid cells
in vitro
and similar effects may be seen on some non-myeloid
cells
in vitro
.
The safety and efficacy of filgrastim administration in patients with myelodysplastic syndrome, or chronic
myelogenous leukaemia have not been established.
Filgrastim is not indicated for use in these conditions. Particular care should be taken to distinguish the
diagnosis of blast transformation of chronic myeloid leukaemia from acute myeloid leukaemia.
In view of limited safety and efficacy data in patients with secondary AML, filgrastim should be
administered with caution.
The safety and efficacy of filgrastim administration in
de novo
AML patients aged < 55 years with good
cytogenetics (t(8;21), t(15;17), and inv(16)) have not been established.
Other special precautions
Monitoring of bone density may be indicated in patients with underlying osteoporotic bone diseases who
undergo continuous therapy with filgrastim for more than 6 months.
Rare (> 0.01% and <0.1%) pulmonary adverse events in particular interstitial pneumonia, have been reported
after G-CSF administration. Patients with a recent history of pulmonary infiltrates or pneumonia may be at
higher risk. The onset of pulmonary signs, such as cough, fever and dyspnoea in association with
radiological signs of pulmonary infiltrates and deterioration in pulmonary function may be preliminary signs
of Adult Respiratory Distress Syndrome (ARDS). Filgrastim should be discontinued and appropriate
treatment given.
Special precautions in cancer patients
Leukocytosis
White blood cell (WBC) counts of 100 x 10
9
/l or greater have been observed in less than 5% of patients
receiving filgrastim at doses above 0.3 MU/kg/day (3 μg/kg/day). No undesirable effects directly attributable
to this degree of leukocytosis have been reported. However, in view of the potential risks associated with
severe leukocytosis, a white blood cell count should be performed at regular intervals during filgrastim
therapy. If leukocyte counts exceed 50 x 10
9
/l after the expected nadir, filgrastim should be discontinued
immediately. However, during the period of administration of filgrastim for PBPC mobilisation, filgrastim
should be discontinued or its dosage should be reduced if the leukocyte counts rise to > 70 x 10
9
/l.
Risks associated with increased doses of chemotherapy
Special caution should be used when treating patients with high dose chemotherapy, because improved
tumour outcome has not been demonstrated and intensified doses of chemotherapeutic agents may lead to
increased toxicities including cardiac, pulmonary, neurologic, and dermatologic effects (please refer to the
prescribing information of the specific chemotherapy agents used).
Treatment with filgrastim alone does not preclude thrombocytopenia and anaemia due to myelosuppressive
chemotherapy. Because of the potential of receiving higher doses of chemotherapy (e.g. full doses on the
prescribed schedule) the patient may be at greater risk of thrombocytopenia and anaemia. Regular
monitoring of platelet count and haematocrit is recommended. Special care should be taken when
administering single or combination chemotherapeutic agents which are known to cause severe
thrombocytopenia.
The use of filgrastim-mobilised PBPCs has been shown to reduce the depth and duration of
thrombocytopenia following myelosuppressive or myeloablative chemotherapy.
Other special precautions
The effects of filgrastim in patients with substantially reduced myeloid progenitors have not been studied.
Filgrastim acts primarily on neutrophil precursors to exert its effect in elevating neutrophil counts. Therefore,
in patients with reduced precursors neutrophil response may be diminished (such as those treated with
extensive radiotherapy or chemotherapy, or those with bone marrow infiltration by tumour).
There have been reports of graft versus host disease GvHD and fatalities in patients receiving G-CSF after
allogeneic bone marrow transplantation (see section 5.1)
Known cases of Hereditary Fructose Intolerance (HFI).
Filgrastim contains sorbitol as an excipient at a concentration of 50 mg/ml. It is unlikely that as a
consequence of treatment with filgrastim alone that sufficient sorbitol will be infused to result in clinically
relevant toxicity in affected individuals. However, in cases of HFI caution is advised
The effect of filgrastim on GvHD has not been defined.
Increased haematopoietic activity of the bone marrow in response to growth factor therapy has been
associated with transient positive bone imaging findings. This should be considered when interpreting bone-
imaging results.
Special precautions in patients undergoing peripheral blood progenitor cell mobilisation
Mobilisation
There are no prospectively randomised comparisons of the two recommended mobilisation methods
(filgrastim alone, or in combination with myelosuppressive chemotherapy) within the same patient
population. The degree of variation between individual patients and between laboratory assays of CD34
+
cells mean that direct comparison between different studies is difficult. It is therefore difficult to recommend
an optimum method. The choice of mobilisation method should be considered in relation to the overall
objectives of treatment for an individual patient.
Prior exposure to cytotoxic agents
Patients who have undergone very extensive prior myelosuppressive therapy may not show sufficient
mobilisation of PBPC to achieve the recommended minimum yield (2.0 x 10
6
CD34
+
cells/kg) or acceleration
of platelet recovery, to the same degree.
Some cytotoxic agents exhibit particular toxicities to the haematopoietic progenitor pool, and may adversely
affect progenitor mobilisation. Agents such as melphalan, carmustine (BCNU), and carboplatin, when
administered over prolonged periods prior to attempts at progenitor mobilisation may reduce progenitor
yield. However, the administration of melphalan, carboplatin or BCNU together with filgrastim, has been
shown to be effective for progenitor mobilisation. When a peripheral blood progenitor cell transplantation is
envisaged it is advisable to plan the stem cell mobilisation procedure early in the treatment course of the
patient. Particular attention should be paid to the number of progenitors mobilised in such patients before the
administration of high-dose chemotherapy. If yields are inadequate, as measured by the criteria above,
alternative forms of treatment, not requiring progenitor support should be considered.
Assessment of progenitor cell yields
In assessing the number of progenitor cells harvested in patients treated with filgrastim, particular attention
should be paid to the method of quantitation. The results of flow cytometric analysis of CD34
+
cell numbers
vary depending on the precise methodology used and recommendations of numbers based on studies in other
laboratories need to be interpreted with caution.
Statistical analysis of the relationship between the number of CD34
+
cells re-infused and the rate of platelet
recovery after high-dose chemotherapy indicates a complex but continuous relationship.
The recommendation of a minimum yield of 2.0 x 10
6
CD34
+
cells/kg is based on published experience
resulting in adequate haematologic reconstitution. Yields in excess of this appear to correlate with more rapid
recovery, those below with slower recovery.
Special precautions in normal donors undergoing peripheral blood progenitor cell mobilisation
Mobilisation of PBPC does not provide a direct clinical benefit to normal donors and should only be
considered for the purposes of allogeneic stem cell transplantation.
PBPC mobilisation should be considered only in donors who meet normal clinical and laboratory eligibility
criteria for stem cell donation with special attention to haematological values and infectious disease.
The safety and efficacy of filgrastim have not been assessed in normal donors < 16 years or > 60 years.
Transient thrombocytopenia (platelets < 100 x 10
9
/l) following filgrastim administration and leukapheresis
was observed in 35% of subjects studied. Among these, two cases of platelets < 50 x 10
9
/l were reported and
attributed to the leukapheresis procedure.
If more than one leukapheresis is required, particular attention should be paid to donors with platelets < 100
x 10
9
/l prior to leukapheresis; in general apheresis should not be performed if platelets < 75 x 10
9
/l.
Leukapheresis should not be performed in donors who are anticoagulated or who have known defects in
haemostasis.
Filgrastim administration should be discontinued or its posology should be reduced if the leukocyte counts
rise to > 70 x10
9
/l.
Donors who receive G-CSFs for PBPC mobilisation should be monitored until haematological indices return
to normal.
Transient cytogenetic modifications have been observed in normal donors following G-CSF use. The
significance of these changes is unknown.
Long-term safety follow-up of donors is ongoing. Nevertheless, a risk of promotion of a malignant myeloid
clone can not be excluded. It is recommended that the apheresis centre perform a systematic record and
tracking of the stem cell donors to ensure monitoring of long-term safety.
Common but generally asymptomatic cases of splenomegaly and very rare cases of splenic rupture have been
reported in healthy donors (and patients) following administration of G-CSFs. Some cases of splenic rupture
were fatal. Therefore, spleen size should be carefully monitored (e.g. clinical examination, ultrasound). A
diagnosis of splenic rupture should be considered in donors and/or patients reporting left upper abdominal
pain or shoulder tip pain.
In normal donors, pulmonary adverse events (haemoptysis, pulmonary haemorrhage, pulmonary infiltrates,
dyspnoea and hypoxia) have been reported very rarely in post marketing experience with other filgrastim-
containing medicinal products. In case of suspected or confirmed pulmonary adverse events, discontinuation
of treatment with filgrastim should be considered and appropriate medical care given.
Special precautions in recipients of allogeneic peripheral blood progenitor cells mobilised with filgrastim
Current data indicate that immunological interactions between the allogeneic PBPC graft and the recipient
may be associated with an increased risk of acute and chronic Graft versus Host Disease (GvHD) when
compared with bone marrow transplantation.
Special precautions in severe chronic neutropenia (SCN) patients
Blood cell counts
Platelet counts should be monitored closely, especially during the first few weeks of filgrastim therapy.
Consideration should be given to intermittent cessation or decreasing the dose of filgrastim in patients who
develop thrombocytopenia, i.e. platelets consistently < 100 x 10
9
/l
.
Other blood cell changes occur, including anaemia and transient increases in myeloid progenitors, which
require close monitoring of cell counts.
Transformation to leukaemia or myelodysplastic syndrome
Special care should be taken in the diagnosis of severe chronic neutropenias to distinguish them from other
haematopoietic disorders such as aplastic anaemia, myelodysplasia, and myeloid leukaemia. Complete blood
cell counts with differential and platelet counts, and an evaluation of bone marrow morphology and
karyotype should be performed prior to treatment.
There was a low frequency (approximately 3%) of myelodysplastic syndromes (MDS) or leukaemia in
clinical trial patients with severe chronic neutropenia treated with filgrastim. This observation has only been
made in patients with congenital neutropenia. MDS and leukaemias are natural complications of the disease
and are of uncertain relation to filgrastim therapy. A subset of approximately 12% of patients who had
normal cytogenetic evaluations at baseline were subsequently found to have abnormalities, including
monosomy 7, on routine repeat evaluation. If patients with severe chronic neutropenia develop abnormal
cytogenetics, the risks and benefits of continuing filgrastim should be carefully weighed; filgrastim should be
discontinued if MDS or leukaemia occurs. It is currently unclear whether long-term treatment of patients
with severe chronic neutropenia will predispose patients to cytogenetic abnormalities, MDS or leukaemic
transformation. It is recommended to perform morphologic and cytogenetic bone marrow examinations in
patients at regular intervals (approximately every 12 months).
Other special precautions
Causes of transient neutropenia, such as viral infections should be excluded.
Splenic enlargement is a direct effect of treatment with filgrastim. Thirty-one percent (31%) of patients in
studies were documented as having palpable splenomegaly. Increases in volume, measured radiographically,
occurred early during filgrastim therapy and tended to plateau. Dose reductions were noted to slow or stop
the progression of splenic enlargement, and in 3% of patients a splenectomy was required. Spleen size
should be evaluated regularly. Abdominal palpation should be sufficient to detect abnormal increases in
splenic volume.
Haematuria/proteinuria occurred in a small number of patients. Regular urinanalysis should be performed to
monitor this event.
The safety and efficacy in neonates and patients with autoimmune neutropenia have not been established.
Special precautions in patients with HIV infection
Blood cell counts
Absolute neutrophil count (ANC) should be monitored closely, especially during the first few weeks of
filgrastim therapy. Some patients may respond very rapidly and with a considerable increase in neutrophil
count to the initial dose of filgrastim. It is recommended that the ANC is measured daily for the first 2 to
3 days of filgrastim administration. Thereafter, it is recommended that the ANC is measured at least twice
per week for the first two weeks and subsequently once per week or once every other week during
maintenance therapy. During intermittent dosing with 30 MU (300 μg)/day of filgrastim, there can be wide
fluctuations in the patient's ANC over time. In order to determine a patient's trough or nadir ANC, it is
recommended that blood samples are taken for ANC measurement immediately prior to any scheduled
dosing with filgrastim.
Risk associated with increased doses of myelosuppressive medicinal products
Treatment with filgrastim alone does not preclude thrombocytopenia and anaemia due to myelosuppressive
medicine. As a result of the potential to receive higher doses or a greater number of these medicinal products
with filgrastim therapy, the patient may be at higher risk of developing thrombocytopenia and anaemia.
Regular monitoring of blood counts is recommended (see above).
Infections and malignancies causing myelosuppression
Neutropenia may be due to bone marrow infiltrating opportunistic infections such as
Mycobacterium avium
complex or malignancies such as lymphoma. In patients with known bone marrow infiltrating infections or
malignancy, consider appropriate therapy for treatment of the underlying condition, in addition to
administration of filgrastim for treatment of neutropenia. The effects of filgrastim on neutropenia due to bone
marrow infiltrating infection or malignancy have not been well established.
Special precautions in sickle cell disease
Sickle cells crises, in some cases fatal, have been reported with the use of filgrastim in subjects with sickle
cell disease. Physicians should exercise caution when considering the use of filgrastim in patients with sickle
cell disease, and only after careful evaluation of the potential risks and benefits.
Excipients
Nivestim contains sorbitol. Patients with rare hereditary problems of fructose intolerance should not use this
medicinal product. It also contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially ‘sodium-free’.
4.5 Interaction with other medicinal products and other forms of interaction
The safety and efficacy of filgrastim given on the same day as myelosuppressive cytotoxic chemotherapy
have not been definitively established. In view of the sensitivity of rapidly dividing myeloid cells to
myelosuppressive cytotoxic chemotherapy, the use of filgrastim is not recommended in the period from
24 hours before to 24 hours after chemotherapy. Preliminary evidence from a small number of patients
treated concomitantly with filgrastim and 5-Fluorouracil indicates that the severity of neutropenia may be
exacerbated.
Possible interactions with other haematopoietic growth factors and cytokines have not yet been investigated
in clinical trials.
Since lithium promotes the release of neutrophils, lithium is likely to potentiate the effect of filgrastim.
Although this interaction has not been formally investigated, there is no evidence that such an interaction is
harmful
.
4.6 Pregnancy and lactation
The safety of filgrastim has not been established in pregnant women. There are reports in the literature where
the transplacental passage of filgrastim in pregnant women has been demonstrated. There is no evidence
from studies in rats and rabbits that filgrastim is teratogenic. An increased incidence of embryo-loss has been
observed in rabbits, but no malformation has been seen. In pregnancy, the possible risk of filgrastim use to
the foetus must be weighed against the expected therapeutic benefit.
It is not known whether filgrastim is excreted in human milk, therefore filgrastim is not recommended for
use in breast-feeding women.
4.7 Effects on ability to drive and use machines
Filgrastim has negligible influence on the ability to drive and use machines. If the patient is experiencing
fatigue, caution is advised when driving a car or operating machinery.
During clinical studies 183 cancer patients and 96 healthy volunteers were exposed to Nivestim.
The safety profile of filgrastim observed in these clinical studies was consistent with that reported with the
reference product used in these studies.
The following undesirable effects and their frequencies have been observed under treatment with filgrastim
based on published information.
The assessment of undesirable effects is based on the following frequency data:
Very common: ≥1/10
Common: ≥1/100 to <1/10
Uncommon: ≥1/1,000 to <1/100
Rare: ≥1/10,000 to <1/1,000
Very rare: <1/10,000
Not known: cannot be estimated from the available data
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
In cancer patients
In clinical trials, the most frequent undesirable effects attributable to filgrastim at the recommended dose
were mild or moderate musculoskeletal pain, occurring in 10%, and severe musculoskeletal pain in 3% of
patients. Musculoskeletal pain is usually controlled with standard analgesics. Less frequent undesirable
effects include urinary abnormalities predominantly mild or moderate dysuria.
In randomised, placebo-controlled clinical trials, filgrastim did not increase the incidence of undesirable
effects associated with cytotoxic chemotherapy. Undesirable effects reported with equal frequency in
patients treated with filgrastim chemotherapy and placebo/chemotherapy included nausea and vomiting,
alopecia, diarrhoea, fatigue, anorexia, mucositis, headache, cough, skin rash, chest pain, generalised
weakness, sore throat, constipation and unspecified pain.
Reversible, dose-dependent and usually mild or moderate elevations of lactate dehydrogenase, alkaline
phosphatase, serum uric acid, and gamma-glutamyl transpeptidase occurred with filgrastim in approximately
50%, 35%, 25%, and 10% of patients, respectively at recommended doses.
Transient decreases in blood pressure, not requiring clinical treatment, have been reported occasionally.
There have been reports of GvHD and fatalities in patients receiving G-CSF after allogeneic bone marrow
transplantation (see section 5.1).
Vascular disorders, including veno-occlusive disease and fluid volume disturbances, have been reported
occasionally in patients undergoing high dose chemotherapy followed by autologous bone marrow
transplantation. The causal association with filgrastim has not been established.
Very rare events of cutaneous vasculitis have been reported in patients treated with filgrastim. The
mechanism of vasculitis in patients receiving filgrastim is unknown.
The occurrence of Sweet's syndrome (acute febrile dermatosis) has been reported occasionally. However,
since a significant percentage of these patients were suffering from leukaemia, a condition known to be
associated with Sweet's syndrome, a causal relationship with filgrastim has not been established.
Exacerbation of rheumatoid arthritis has been observed in individual cases.
Rare pulmonary adverse events including interstitial pneumonia, pulmonary oedema, and pulmonary
infiltrates have been reported in some cases with an outcome of respiratory failure or adult respiratory
distress syndrome (ARDS), which may be fatal (see section 4.4).
Allergic Reactions: Allergic-type reactions, including anaphylaxis, skin rash, urticaria, angioedema,
dyspnoea and hypotension, occurring on initial or subsequent treatment have been reported in patients
receiving filgrastim. Overall, reports were more common after intravenous administration. In some cases,
symptoms have recurred with rechallenge, suggesting a causal relationship. filgrastim should be permanently
discontinued in patients who experience a serious allergic reaction.
Isolated cases of sickle cells crises have been reported in patients with sickle cell disease (see section 4.4).
Metabolism and nutrition
disorders
Elevated alkaline phosphatase, elevated LDH,
elevated uric acid
Respiratory, thoracic and
mediastinal disorders
Gastrointestinal disorders
Constipation, anorexia, diarrhoea, mucositis
Skin and subcutaneous tissue
disorders
Sweet’s syndrome, cutaneous vasculitis
Musculoskeletal and
connective tissue disorders
Chest pain, musculoskeletal pain
Rheumatoid arthritis exacerbation
Renal and urinary disorders
General disorders and
administration site conditions
Fatigue, generalised weakness
In peripheral blood progenitor cell mobilisation in normal donors























The most commonly reported undesirable effect was mild to moderate transient musculo-skeletal pain.
Leukocytosis (White Blood Cell (WBC) > 50 x 10
9
/l) was observed in 41% of donors and transient
thrombocytopenia (platelets < 100 x 10
9
/l) following filgrastim and leukapheresis was observed in 35% of
donors.
Transient, minor increases in alkaline phosphatase, LDH, SGOT and uric acid have been reported in normal
donors receiving filgrastim; these were without clinical sequelae.
Exacerbation of arthritic symptoms has been observed very rarely.
Symptoms suggestive of severe allergic reactions have been reported very rarely.
Headaches, believed to be caused by filgrastim, have been reported in PBPC donor studies.
Common but generally asymptomatic cases of splenomegaly and very rare cases of splenic rupture have been
reported in healthy donors and patients following administration of G-CSFs (see section 4.4).
In normal donors, pulmonary adverse events (haemoptysis, pulmonary haemorrhage, pulmonary infiltrates,
dyspnoea and hypoxia) have been reported very rarely in post marketing experience with other filgrastim-
containing medicinal products (see section 4.4).
Blood and lymphatic system
disorders
Leukocytosis, thrombocytopenia
Metabolism and nutrition
disorders
Elevated alkaline phosphatase,
elevated LDH
SGCT increased, hyperuricaemia
Musculoskeletal and connective
tissue disorders
Rheumatoid arthritis exacerbation
General disorders and
administration site conditions
In severe chronic neutropenia (SCN) patients
Undesirable effects related to filgrastim therapy in SCN patients have been reported and for some their
frequency tend to decrease with time.
The most frequent undesirable effects attributable to filgrastim were bone pain, and general musculoskeletal
pain.
Other undesirable effects seen include splenic enlargement, which may be progressive in a minority of cases
and thrombocytopenia. Headache and diarrhoea have been reported shortly after starting filgrastim therapy,
typically in less than 10% of patients. Anaemia and epistaxis have also been reported.
Transient increases with no clinical symptoms were observed in serum uric acid, lactic dehydrogenase, and
alkaline phosphatase. Transient, moderate decreases in non-fasting blood glucose have also been seen.
Undesirable effects possibly related to filgrastim therapy and typically occurring in < 2% of SCN patients
were injection site reaction, headache, hepatomegaly, arthralgia, alopecia, osteoporosis, and rash.
















During long term use cutaneous vasculitis has been reported in 2% of SCN patients. There have been very
few instances of proteinuria/haematuria.
Blood and lymphatic system
disorders
Metabolism and nutrition
disorders
Decreased glucose, Elevated alkaline
phosphatase, elevated LDH,
hyperuricaemia
Respiratory, thoracic and
mediastinal disorders
Gastrointestinal disorders
Skin and subcutaneous tissue
disorders
Alopecia, cutaneous vasculitis,
injection site pain, rash
Musculoskeletal and connective
tissue disorders
Renal and urinary disorders
In patients with HIV
In clinical studies, the only undesirable effects that were consistently considered to be related to filgrastim
administration were musculoskeletal pain, predominantly mild to moderate bone pain and myalgia. The
incidence of these events was similar to that reported in cancer patients.
Splenic enlargement was reported to be related to filgrastim therapy in < 3% of patients. In all cases this was
mild or moderate on physical examination and the clinical course was benign; no patients had a diagnosis of
hypersplenism and no patients underwent splenectomy. As splenic enlargement is a common finding in
patients with HIV infection and is present to varying degrees in most patients with AIDS, the relationship to
filgrastim treatment is unclear.
Blood and lymphatic system
disorders
Musculoskeletal and connective
tissue disorders
The effects of filgrastim overdose have not been established.
Discontinuation of filgrastim therapy usually results in a 50% decrease in circulating neutrophils within 1 to
2 days, with a return to normal levels in 1 to 7 days.






























PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: colony stimulating factors, ATC code: L03AA02.
Nivestim is a biosimilar medicinal product. Detailed information is available on the website of the
European Medicines Agency (EMA)
http://www.ema.europa.eu
Human G-CSF is a glycoprotein which regulates the production and release of functional neutrophils from
the bone marrow. Nivestim containing r-metHuG-CSF (filgrastim) causes marked increases in peripheral
blood neutrophil counts within twenty-four hours, with minor increases in monocytes. In some severe
chronic neutropenia patients filgrastim can also induce a minor increase in the number of circulating
eosinophils and basophils relative to baseline; some of these patients may present with eosinophilia or
basophilia already prior to treatment. Elevations of neutrophil counts are dose-dependent at recommended
doses. Neutrophils produced in response to filgrastim show normal or enhanced function as demonstrated by
tests of chemotactic and phagocytic function. Following termination of filgrastim therapy, circulating
neutrophil counts decrease by 50% within 1 to 2 days, and to normal levels within 1 to 7 days.
Use of filgrastim in patients undergoing cytotoxic chemotherapy leads to significant reductions in the
incidence, severity and duration of neutropenia and febrile neutropenia. Treatment with filgrastim
significantly reduces the duration of febrile neutropenia, antibiotic use and hospitalisation after induction
chemotherapy for acute myelogenous leukaemia or myeloablative therapy followed by bone marrow
transplantation. The incidence of fever and documented infections were not reduced in either setting. The
duration of fever was not reduced in patients undergoing myeloablative therapy followed by bone marrow
transplantation.
Use of filgrastim, either alone, or after chemotherapy, mobilises haematopoietic progenitor cells into
peripheral blood. These autologous peripheral blood progenitor cells (PBPCs) may be harvested and infused
after high-dose cytotoxic therapy, either in place of, or in addition to bone marrow transplantation. Infusion
of PBPCs accelerates haematopoietic recovery reducing the duration of risk for haemorrhagic complications
and the need for platelet transfusions.
Recipients of allogeneic peripheral blood progenitor cells mobilised with filgrastim experienced significantly
more rapid haematological recovery, leading to a significant decrease in time to unsupported platelet
recovery when compared with allogeneic bone marrow transplantation.
One retrospective European study evaluating the use of G-CSF after allogeneic bone marrow transplantation
in patients with acute leukaemias suggested an increase in the risk of GvHD, treatment related mortality
(TRM) and mortality when G-CSF was administered. In a separate retrospective International study in
patients with acute and chronic myelogenous leukaemias, no effect on the risk of GvHD, TRM and mortality
was seen. A meta-analysis of allogeneic transplant studies, including the results of nine prospective
randomized trials, 8 retrospective studies and 1 case-controlled study, did not detect an effect on the risks of
acute GvHD, chronic GvHD or early treatment-related mortality.
Relative Risk (95% CI) of GvHD and TRM
Following treatment with G-CSF after bone marrow transplantation
Publication
European Retrospective
Study (2004)











International Retrospective
Study (2006)
a
Analysis includes studies involving BM transplant during this period; some studies used GM-CSF
b
Analysis includes patients receiving BM transplant during this period
Prior to allogeneic PBPC transplantation, use of filgrastim for the mobilisation of PBPC in normal donors
allows a collection of 4 x 106 CD34+ cells/kg recipient body weight in the majority of the donors after two
leukaphereses. Normal donors are given a dose of a 10 μg/kg/day, administered subcutaneously for 4 to 5
consecutive days.
Use of filgrastim in patients, children or adults, with severe chronic neutropenia (severe congenital, cyclic,
and idiopathic neutropenia) induces a sustained increase in absolute neutrophil counts in peripheral blood
and a reduction of infection and related events.
Use of filgrastim in patients with HIV infection maintains normal neutrophil counts to allow scheduled
dosing of antiviral and/or other myelosuppressive medicine. There is no evidence that patients with HIV
infection treated with filgrastim show an increase in HIV replication.
As with other haematopoietic growth factors, G-CSF has shown
in vitro
stimulating properties on human
endothelial cells.
The efficacy and safety of Nivestim has been assessed in randomised, controlled phase III study in breast
cancer. There were no relevant differences between Nivestim and the reference product with regard to
duration of severe neutropenia and incidence of febrile neutropenia.
5.2 Pharmacokinetic properties
A randomised, open-label, single-dose, comparator-controlled, two-way crossover study in 46 healthy
volunteers showed that the pharmacokinetic profile of Nivestim was comparable to that of the reference
product after subcutaneous and intravenous administration. Another randomised, double-blind, multiple-
dose, comparator-controlled, two-way crossover study in 50 healthy volunteers showed that the
pharmacokinetic profile of Nivestim was comparable to that of the reference product after subcutaneous
administration.
Clearance of filgrastim has been shown to follow first-order pharmacokinetics after both subcutaneous and
intravenous administration. The serum elimination half-life of filgrastim is approximately 3.5 hours, with a
clearance rate of approximately 0.6 ml/min/kg. Continuous infusion with filgrastim over a period of up to
28 days, in patients recovering from autologous bone-marrow transplantation, resulted in no evidence of
drug accumulation and comparable elimination half-lives. There is a positive linear correlation between the
dose and the serum concentration of filgrastim, whether administered intravenously or subcutaneously.
Following subcutaneous administration of recommended doses, serum concentrations were maintained above
10 ng/ml for 8 to 16 hours. The volume of distribution in blood is approximately 150 ml/kg.
5.3 Preclinical safety data
There are no preclinical data of relevance to the prescriber which are additional to that already included in
other sections of the Summary of Product Characteristics.
PHARMACEUTICAL PARTICULARS










Acetic acid, glacial
Sodium hydroxide
Sorbitol (E420)
Polysorbate 80
Water for injections
Nivestim must not be diluted with sodium chloride solutions.
Diluted filgrastim may be adsorbed to glass and plastic materials unless it is diluted in 50mg/ml (5%)
glucose solution for infusion (see section 6.6).
This medicinal product must not be mixed with other medicinal products except those mentioned in section
6.6.
After dilution: Chemical and physical in-use stability of the diluted solution for infusion has been
demonstrated for 24 hours at 2°C to 8°C. From a microbiological point of view, the product should be used
immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility
of the user and would normally not be longer than 24 hours at 2°C to 8°C, unless dilution has taken place in
controlled and validated aseptic conditions.
6.4 Special precautions for storage
Store and transport refrigerated (2°C - 8°C).
Do not freeze. Keep the pre-filled syringe in the outer carton in order to protect from light.
Accidental exposure to freezing temperatures for up to 24 hours does not affect the stability of Nivestim. The
frozen pre-filled syringes can be thawed and then refrigerate for future use. If exposure has been greater then
24 hours or frozen more than once then Nivestim should NOT be used.
Within its shelf-life and for the purpose of ambulatory use, the patient may remove the product from the
refrigerator and store it at room temperature (not above 25°C) for one single period of up to 7 days. At the
end of this period, the product should not be put back in the refrigerator and should be disposed of.
For storage conditions of the diluted medicinal product, see section 6.3.
6.5
Nature and contents of container
Pre-filled syringe (type I glass), with injection needle (stainless steel) with a needle guard, containing 0.2 ml
solution for injection/ infusion.
Pack sizes of 1, 5 or 10 pre-filled syringes.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
If required, Nivestim may be diluted in 50 mg/ml (5%) glucose solution for infusion.
Dilution to a final concentration less than 0.2 MU (2 micrograms) per ml is not recommended at any time.
The solution should be visually inspected prior to use. Only clear solutions without particles should be used.
For patients treated with filgrastim diluted to concentrations below 1.5 MU (15 micrograms) per ml, human
serum albumin (HSA) should be added to a final concentration of 2 mg/ml.
Example: In a final injection volume of 20 ml, total doses of filgrastim less than 30 MU (300 micrograms)
should be given with 0.2 ml of 20% human albumin solution added.
When diluted in 50 mg/ml (5%) glucose solution for infusion, filgrastim is compatible with glass and a
variety of plastics including polyvinyl chloride (PVC), polyolefin (a co-polymer of polypropylene and
polyethylene) and polypropylene.
Nivestim contains no preservative. In view of the possible risk of microbial contamination, Nivestim
syringes are for single use only. Any unused product or waste material should be disposed of in accordance
with local requirements.
MARKETING AUTHORISATION HOLDER
Hospira UK Limited
Queensway
Royal Leamington Spa
Warwickshire CV31 3RW
United Kingdom
Tel: +44 (0)1926 820 820
Fax: +44 (0) 1926 821 049
MARKETING AUTHORISATION NUMBER(S)
EU/1/10/631/001
EU/1/10/631/002
EU/1/10/631/003
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
NAME OF THE MEDICINAL PRODUCT
Nivestim 30 MU/ 0.5 ml solution for injection/infusion.
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml of solution for injection or infusion contains 60 million units [MU] (600 micrograms) of filgrastim*.
Each pre-filled syringe contains 30 million units(MU) (300 micrograms) of filgrastim in 0.5 ml (0.6 mg/ml).
*recombinant methionyl granulocyte-colony stimulating factor [G-CSF] produced in
Escherichia Coli
(BL21)
by recombinant DNA technology.
Excipient(s): Each ml of solution contains 50 mg of sorbitol.
For a full list of excipients, see section 6.1.
Solution for injection/infusion (injection/ infusion).
Clear, colourlesss solution.
4.1 Therapeutic indications
Filgrastim is indicated for the reduction in the duration of neutropenia and the incidence of febrile
neutropenia in patients treated with established cytotoxic chemotherapy for malignancy (with the exception
of chronic myeloid leukaemia and myelodysplastic syndromes) and for the reduction in the duration of
neutropenia in patients undergoing myeloablative therapy followed by bone marrow transplantation
considered to be at increased risk of prolonged severe neutropenia.
The safety and efficacy of filgrastim are similar in adults and children receiving cytotoxic chemotherapy.
Filgrastim is indicated for the mobilisation of peripheral blood progenitor cells (PBPC).
In patients, children or adults, with severe congenital, cyclic, or idiopathic neutropenia with an absolute
neutrophil count (ANC) of ≤ 0.5 x 10
9
/l and a history of severe or recurrent infections, long term
administration of filgrastim is indicated to increase neutrophil counts and to reduce the incidence and
duration of infection-related events.
Filgrastim is indicated for the treatment of persistent neutropenia (ANC less than or equal to 1.0 x 10
9
/l) in
patients with advanced HIV infection, in order to reduce the risk of bacterial infections when other options to
manage neutropenia are inappropriate.
4.2
Posology and method of administration
Filgrastim therapy should only be given in collaboration with an oncology centre which has experience in G-
CSF treatment and haematology and has the necessary diagnostic facilities. The mobilisation and apheresis
procedures should be performed in collaboration with an oncology-haematology centre with acceptable
experience in this field and where the monitoring of haematopoietic progenitor cells can be correctly
performed.
Established cytotoxic chemotherapy
The recommended dose of filgrastim is 0.5 MU (5 micrograms)/kg/day. The first dose of filgrastim should
not be administered less than 24 hours following cytotoxic chemotherapy. Filgrastim may be given as a daily
subcutaneous injection or as a daily intravenous infusion diluted in glucose 50 mg/ml (5%) solution for
infusion given over 30 minutes (see section 6.6 for instructions on dilutions)
.
The subcutaneous route is preferred in most cases. There is some evidence from a study of single dose
administration that intravenous dosing may shorten the duration of effect. The clinical relevance of this
finding to multiple dose administration is not clear. The choice of route should depend on the individual
clinical circumstance. In randomised clinical trials, a subcutaneous dose of 230 micrograms/m
2
/day (4.0 to
8.4 micrograms/kg/day) was used.
Daily dosing with filgrastim should continue until the expected neutrophil nadir is passed and the neutrophil
count has recovered to the normal range. Following established chemotherapy for solid tumours,
lymphomas, and lymphoid leukaemias, it is expected that the duration of treatment required to fulfil these
criteria will be up to 14 days. Following induction and consolidation treatment for acute myeloid leukaemia
the duration of treatment may be substantially longer (up to 38 days) depending on the type, dose and
schedule of cytotoxic chemotherapy used.
In patients receiving cytotoxic chemotherapy, a transient increase in neutrophil counts is typically seen 1 to 2
days after initiation of filgrastim therapy. However, for a sustained therapeutic response, filgrastim therapy
should not be discontinued before the expected nadir has passed and the neutrophil count has recovered to
the normal range. Premature discontinuation of filgrastim therapy, prior to the time of the expected
neutrophil nadir, is not recommended.
In patients treated with myeloablative therapy followed by bone marrow transplantation
The recommended starting dose of filgrastim is 1.0 MU (10 micrograms)/kg/day given as a 30 minute or 24
hour intravenous infusion or 1.0 MU (10 micrograms)/kg/day given by continuous 24 hour subcutaneous
infusion. Filgrastim should be diluted in 20 ml of 50 mg/ml (5%) glucose solution for infusion (see section
6.6).
The first dose of filgrastim should not be administered less than 24 hours following cytotoxic chemotherapy
and within 24 hours of bone marrow infusion.
Once the neutrophil nadir has been passed, the daily dose of filgrastim should be titrated against the
neutrophil response as follows:
Filgrastim dose adjustment
> 1.0 x 10
9
/l for 3 consecutive days
Then, if ANC remains > 1.0 x 10
9
/l for 3 more
consecutive days
If the ANC decreases to < 1.0 x 10
9
/l during the treatment period the dose of filgrastim should be re-
escalated according to the above steps
For the mobilisation of peripheral blood progenitor cells (PBPC) in patients undergoing myelosuppressive or
myeloablative therapy followed by autologous peripheral blood progenitor cell transplantation
The recommended dose of filgrastim for PBPC mobilisation when used alone is 1.0 MU
(10 micrograms)/kg/day as a 24 hour subcutaneous continuous infusion or a single daily subcutaneous
injection for 5 to 7 consecutive days. For infusions filgrastim should be diluted in 20 ml of 50 mg/ml (5%)
glucose solution for infusion (see section 6.6). Timing of leukapheresis: one or two leukaphereses on days 5







and 6 are often sufficient. In other circumstances, additional leukaphereses may be necessary. Filgrastim
dosing should be maintained until the last leukapheresis.
The recommended dose of filgrastim for PBPC mobilisation after myelosuppressive chemotherapy is 0.5 MU
(5 micrograms)/kg/day given daily by subcutaneous injection from the first day after completion of
chemotherapy until the expected neutrophil nadir is passed and the neutrophil count has recovered to the
normal range. Leukapheresis should be performed during the period when the ANC rises from < 0.5 x 10
9
/l
to > 5.0 x 10
9
/l. For patients who have not had extensive chemotherapy, one leukapheresis is often sufficient.
In other circumstances, additional leukaphereses are recommended.
For the mobilisation of peripheral blood progenitor cells (PBPCs) in normal donors prior to allogeneic
peripheral blood progenitor cell transplantation
For PBPC mobilisation in normal donors, filgrastim should be administered at 10 micrograms/kg/day
subcutaneously for 4 to 5 consecutive days. Leukapheresis should be started at day 5 and continued until day
6 if needed in order to collect 4 x 10
6
CD34
+
cells/kg recipient bodyweight.
In patients with severe chronic neutropenia
Congenital neutropenia
: the recommended starting dose is 1.2 MU (12 micrograms)/kg/day subcutaneously
as a single dose or in divided doses.
Idiopathic or cyclic neutropenia
: the recommended starting dose is 0.5 MU (5 micrograms)/kg/day
subcutaneously as a single dose or in divided doses.
Dose adjustment
: Filgrastim should be administered daily by subcutaneous injection until the neutrophil
count has reached and can be maintained at more than 1.5 x 10
9
/l. When the response has been obtained the
minimal effective dose to maintain this level should be established. Long-term daily administration is
required to maintain an adequate neutrophil count. After one to two weeks of therapy, the initial dose may be
doubled or halved depending upon the patient's response. Subsequently the dose may be individually
adjusted every 1 to 2 weeks to maintain the average neutrophil count between 1.5 x 10
9
/l and 10 x 10
9
/l. A
faster schedule of dose escalation may be considered in patients presenting with severe infections. In clinical
trials, 97% of patients who responded had a complete response at doses ≤24 micrograms/kg/day. The long-
term safety of filgrastim administration above 24 micrograms/kg/day in patients with severe chronic
neutropenia has not been established.
In patients with HIV infection
For reversal of neutropenia
The recommended starting dose of filgrastim is 0.1 MU (1 micrograms)/kg/day given daily by subcutaneous
injection with titration up to a maximum of 0.4 MU (4 μg)/kg/day until a normal neutrophil count is reached
and can be maintained (ANC > 2.0 x10
9
/l). In clinical studies, > 90% of patients responded at these doses,
achieving reversal of neutropenia in a median of 2 days.
In a small number of patients (< 10%), doses up to 1.0 MU (10 micrograms)/kg/day were required to achieve
reversal of neutropenia.
For maintaining normal neutrophil counts
When reversal of neutropenia has been achieved, the minimal effective dose to maintain a normal neutrophil
count should be established. Initial dose adjustment to alternate day dosing with 30 MU
(300 micrograms)/day by subcutaneous injection is recommended. Further dose adjustment may be
necessary, as determined by the patient's ANC, to maintain the neutrophil count at > 2.0 x 10
9
/l. In clinical
studies, dosing with 30 MU (300 micrograms)/day on 1 to 7 days per week was required to maintain the
ANC > 2.0 x 10
9
/l, with the median dose frequency being 3 days per week. Long-term administration may be
required to maintain the ANC > 2.0 x 10
9
/l.
Clinical trials with filgrastim have included a small number of elderly patients but special studies have not
been performed in this group and therefore specific posology recommendations cannot be made.
Patients with renal or hepatic impairment
Studies of filgrastim in patients with severe impairment of renal or hepatic function demonstrate that it
exhibits a similar pharmacokinetic and pharmacodynamic profile to that seen in normal individuals. Dose
adjustment is not required in these circumstances.
Paediatric use in the severe chronic neutropenia (SCN) and cancer settings
Sixty-five percent of the patients studied in the SCN trial program were under 18 years of age. The efficacy
of treatment was clear for this age group, which included most patients with congenital neutropenia. There
were no differences in the safety profiles for paediatric patients treated for severe chronic neutropenia.
Data from clinical studies in paediatric patients indicate that the safety and efficacy of filgrastim are similar
in both adults and children receiving cytotoxic chemotherapy.
The posology recommendations in paediatric patients are the same as those in adults receiving
myelosuppressive cytotoxic chemotherapy.
Hypersensitivity to the active substance(s) or to any of the excipients.
4.4 Special warnings and precautions for use
Filgrastim should not be used to increase the dose of cytotoxic chemotherapy beyond established
posology regimens.
Filgrastim should not be administered to patients with severe congenital neutropenia (Kostmann's
syndrome) with abnormal cytogenetics.
Malignant cell growth
GCSF can promote growth of myeloid cells
in vitro
and similar effects may be seen on some non-myeloid
cells
in vitro
.
The safety and efficacy of filgrastim administration in patients with myelodysplastic syndrome, or chronic
myelogenous leukaemia have not been established.
Filgrastim is not indicated for use in these conditions. Particular care should be taken to distinguish the
diagnosis of blast transformation of chronic myeloid leukaemia from acute myeloid leukaemia.
In view of limited safety and efficacy data in patients with secondary AML, filgrastim should be
administered with caution.
The safety and efficacy of filgrastim administration in
de novo
AML patients aged < 55 years with good
cytogenetics (t(8;21), t(15;17), and inv(16)) have not been established.
Other special precautions
Monitoring of bone density may be indicated in patients with underlying osteoporotic bone diseases who
undergo continuous therapy with filgrastim for more than 6 months.
Rare (> 0.01% and <0.1%) pulmonary adverse events in particular interstitial pneumonia, have been reported
after G-CSF administration. Patients with a recent history of pulmonary infiltrates or pneumonia may be at
higher risk. The onset of pulmonary signs, such as cough, fever and dyspnoea in association with
radiological signs of pulmonary infiltrates and deterioration in pulmonary function may be preliminary signs
of Adult Respiratory Distress Syndrome (ARDS). Filgrastim should be discontinued and appropriate
treatment given.
Special precautions in cancer patients
Leukocytosis
White blood cell (WBC) counts of 100 x 10
9
/l or greater have been observed in less than 5% of patients
receiving filgrastim at doses above 0.3 MU/kg/day (3 μg/kg/day). No undesirable effects directly attributable
to this degree of leukocytosis have been reported. However, in view of the potential risks associated with
severe leukocytosis, a white blood cell count should be performed at regular intervals during filgrastim
therapy. If leukocyte counts exceed 50 x 10
9
/l after the expected nadir, filgrastim should be discontinued
immediately. However, during the period of administration of filgrastim for PBPC mobilisation, filgrastim
should be discontinued or its dosage should be reduced if the leukocyte counts rise to > 70 x 10
9
/l.
Risks associated with increased doses of chemotherapy
Special caution should be used when treating patients with high dose chemotherapy, because improved
tumour outcome has not been demonstrated and intensified doses of chemotherapeutic agents may lead to
increased toxicities including cardiac, pulmonary, neurologic, and dermatologic effects (please refer to the
prescribing information of the specific chemotherapy agents used).
Treatment with filgrastim alone does not preclude thrombocytopenia and anaemia due to myelosuppressive
chemotherapy. Because of the potential of receiving higher doses of chemotherapy (e.g. full doses on the
prescribed schedule) the patient may be at greater risk of thrombocytopenia and anaemia. Regular
monitoring of platelet count and haematocrit is recommended. Special care should be taken when
administering single or combination chemotherapeutic agents which are known to cause severe
thrombocytopenia.
The use of filgrastim-mobilised PBPCs has been shown to reduce the depth and duration of
thrombocytopenia following myelosuppressive or myeloablative chemotherapy.
Other special precautions
The effects of filgrastim in patients with substantially reduced myeloid progenitors have not been studied.
Filgrastim acts primarily on neutrophil precursors to exert its effect in elevating neutrophil counts. Therefore,
in patients with reduced precursors neutrophil response may be diminished (such as those treated with
extensive radiotherapy or chemotherapy, or those with bone marrow infiltration by tumour).
There have been reports of graft versus host disease GvHD and fatalities in patients receiving G-CSF after
allogeneic bone marrow transplantation (see section 5.1)
Known cases of Hereditary Fructose Intolerance (HFI).
Filgrastim contains sorbitol as an excipient at a concentration of 50 mg/ml. It is unlikely that as a
consequence of treatment with filgrastim alone that sufficient sorbitol will be infused to result in clinically
relevant toxicity in affected individuals. However, in cases of HFI caution is advised
The effect of filgrastim on GvHD has not been defined.
Increased haematopoietic activity of the bone marrow in response to growth factor therapy has been
associated with transient positive bone imaging findings. This should be considered when interpreting bone-
imaging results.
Special precautions in patients undergoing peripheral blood progenitor cell mobilisation
Mobilisation
There are no prospectively randomised comparisons of the two recommended mobilisation methods
(filgrastim alone, or in combination with myelosuppressive chemotherapy) within the same patient
population. The degree of variation between individual patients and between laboratory assays of CD34
+
cells mean that direct comparison between different studies is difficult. It is therefore difficult to recommend
an optimum method. The choice of mobilisation method should be considered in relation to the overall
objectives of treatment for an individual patient.
Prior exposure to cytotoxic agents
Patients who have undergone very extensive prior myelosuppressive therapy may not show sufficient
mobilisation of PBPC to achieve the recommended minimum yield (2.0 x 10
6
CD34
+
cells/kg) or acceleration
of platelet recovery, to the same degree.
Some cytotoxic agents exhibit particular toxicities to the haematopoietic progenitor pool, and may adversely
affect progenitor mobilisation. Agents such as melphalan, carmustine (BCNU), and carboplatin, when
administered over prolonged periods prior to attempts at progenitor mobilisation may reduce progenitor
yield. However, the administration of melphalan, carboplatin or BCNU together with filgrastim, has been
shown to be effective for progenitor mobilisation. When a peripheral blood progenitor cell transplantation is
envisaged it is advisable to plan the stem cell mobilisation procedure early in the treatment course of the
patient. Particular attention should be paid to the number of progenitors mobilised in such patients before the
administration of high-dose chemotherapy. If yields are inadequate, as measured by the criteria above,
alternative forms of treatment, not requiring progenitor support should be considered.
Assessment of progenitor cell yields
In assessing the number of progenitor cells harvested in patients treated with filgrastim, particular attention
should be paid to the method of quantitation. The results of flow cytometric analysis of CD34
+
cell numbers
vary depending on the precise methodology used and recommendations of numbers based on studies in other
laboratories need to be interpreted with caution.
Statistical analysis of the relationship between the number of CD34
+
cells re-infused and the rate of platelet
recovery after high-dose chemotherapy indicates a complex but continuous relationship.
The recommendation of a minimum yield of 2.0 x 10
6
CD34
+
cells/kg is based on published experience
resulting in adequate haematologic reconstitution. Yields in excess of this appear to correlate with more rapid
recovery, those below with slower recovery.
Special precautions in normal donors undergoing peripheral blood progenitor cell mobilisation
Mobilisation of PBPC does not provide a direct clinical benefit to normal donors and should only be
considered for the purposes of allogeneic stem cell transplantation.
PBPC mobilisation should be considered only in donors who meet normal clinical and laboratory eligibility
criteria for stem cell donation with special attention to haematological values and infectious disease.
The safety and efficacy of filgrastim have not been assessed in normal donors < 16 years or > 60 years.
Transient thrombocytopenia (platelets < 100 x 10
9
/l) following filgrastim administration and leukapheresis
was observed in 35% of subjects studied. Among these, two cases of platelets < 50 x 10
9
/l were reported and
attributed to the leukapheresis procedure.
If more than one leukapheresis is required, particular attention should be paid to donors with platelets < 100
x 10
9
/l prior to leukapheresis; in general apheresis should not be performed if platelets < 75 x 10
9
/l.
Leukapheresis should not be performed in donors who are anticoagulated or who have known defects in
haemostasis.
Filgrastim administration should be discontinued or its posology should be reduced if the leukocyte counts
rise to > 70 x10
9
/l.
Donors who receive G-CSFs for PBPC mobilisation should be monitored until haematological indices return
to normal.
Transient cytogenetic modifications have been observed in normal donors following G-CSF use. The
significance of these changes is unknown.
Long-term safety follow-up of donors is ongoing. Nevertheless, a risk of promotion of a malignant myeloid
clone can not be excluded. It is recommended that the apheresis centre perform a systematic record and
tracking of the stem cell donors to ensure monitoring of long-term safety.
Common but generally asymptomatic cases of splenomegaly and very rare cases of splenic rupture have been
reported in healthy donors (and patients) following administration of G-CSFs. Some cases of splenic rupture
were fatal. Therefore, spleen size should be carefully monitored (e.g. clinical examination, ultrasound). A
diagnosis of splenic rupture should be considered in donors and/or patients reporting left upper abdominal
pain or shoulder tip pain.
In normal donors, pulmonary adverse events (haemoptysis, pulmonary haemorrhage, pulmonary infiltrates,
dyspnoea and hypoxia) have been reported very rarely in post marketing experience with other filgrastim-
containing medicinal products. In case of suspected or confirmed pulmonary adverse events, discontinuation
of treatment with filgrastim should be considered and appropriate medical care given.
Special precautions in recipients of allogeneic peripheral blood progenitor cells mobilised with filgrastim
Current data indicate that immunological interactions between the allogeneic PBPC graft and the recipient
may be associated with an increased risk of acute and chronic Graft versus Host Disease (GvHD) when
compared with bone marrow transplantation.
Special precautions in severe chronic neutropenia (SCN) patients
Blood cell counts
Platelet counts should be monitored closely, especially during the first few weeks of filgrastim therapy.
Consideration should be given to intermittent cessation or decreasing the dose of filgrastim in patients who
develop thrombocytopenia, i.e. platelets consistently < 100 x 10
9
/l
.
Other blood cell changes occur, including anaemia and transient increases in myeloid progenitors, which
require close monitoring of cell counts.
Transformation to leukaemia or myelodysplastic syndrome
Special care should be taken in the diagnosis of severe chronic neutropenias to distinguish them from other
haematopoietic disorders such as aplastic anaemia, myelodysplasia, and myeloid leukaemia. Complete blood
cell counts with differential and platelet counts, and an evaluation of bone marrow morphology and
karyotype should be performed prior to treatment.
There was a low frequency (approximately 3%) of myelodysplastic syndromes (MDS) or leukaemia in
clinical trial patients with severe chronic neutropenia treated with filgrastim. This observation has only been
made in patients with congenital neutropenia. MDS and leukaemias are natural complications of the disease
and are of uncertain relation to filgrastim therapy. A subset of approximately 12% of patients who had
normal cytogenetic evaluations at baseline were subsequently found to have abnormalities, including
monosomy 7, on routine repeat evaluation. If patients with severe chronic neutropenia develop abnormal
cytogenetics, the risks and benefits of continuing filgrastim should be carefully weighed; filgrastim should be
discontinued if MDS or leukaemia occurs. It is currently unclear whether long-term treatment of patients
with severe chronic neutropenia will predispose patients to cytogenetic abnormalities, MDS or leukaemic
transformation. It is recommended to perform morphologic and cytogenetic bone marrow examinations in
patients at regular intervals (approximately every 12 months).
Other special precautions
Causes of transient neutropenia, such as viral infections should be excluded.
Splenic enlargement is a direct effect of treatment with filgrastim. Thirty-one percent (31%) of patients in
studies were documented as having palpable splenomegaly. Increases in volume, measured radiographically,
occurred early during filgrastim therapy and tended to plateau. Dose reductions were noted to slow or stop
the progression of splenic enlargement, and in 3% of patients a splenectomy was required. Spleen size
should be evaluated regularly. Abdominal palpation should be sufficient to detect abnormal increases in
splenic volume.
Haematuria/proteinuria occurred in a small number of patients. Regular urinanalysis should be performed to
monitor this event.
The safety and efficacy in neonates and patients with autoimmune neutropenia have not been established.
Special precautions in patients with HIV infection
Blood cell counts
Absolute neutrophil count (ANC) should be monitored closely, especially during the first few weeks of
filgrastim therapy. Some patients may respond very rapidly and with a considerable increase in neutrophil
count to the initial dose of filgrastim. It is recommended that the ANC is measured daily for the first 2 to
3 days of filgrastim administration. Thereafter, it is recommended that the ANC is measured at least twice
per week for the first two weeks and subsequently once per week or once every other week during
maintenance therapy. During intermittent dosing with 30 MU (300 μg)/day of filgrastim, there can be wide
fluctuations in the patient's ANC over time. In order to determine a patient's trough or nadir ANC, it is
recommended that blood samples are taken for ANC measurement immediately prior to any scheduled
dosing with filgrastim.
Risk associated with increased doses of myelosuppressive medicinal products
Treatment with filgrastim alone does not preclude thrombocytopenia and anaemia due to myelosuppressive
medicine. As a result of the potential to receive higher doses or a greater number of these medicinal products
with filgrastim therapy, the patient may be at higher risk of developing thrombocytopenia and anaemia.
Regular monitoring of blood counts is recommended (see above).
Infections and malignancies causing myelosuppression
Neutropenia may be due to bone marrow infiltrating opportunistic infections such as
Mycobacterium avium
complex or malignancies such as lymphoma. In patients with known bone marrow infiltrating infections or
malignancy, consider appropriate therapy for treatment of the underlying condition, in addition to
administration of filgrastim for treatment of neutropenia. The effects of filgrastim on neutropenia due to bone
marrow infiltrating infection or malignancy have not been well established.
Special precautions in sickle cell disease
Sickle cells crises, in some cases fatal, have been reported with the use of filgrastim in subjects with sickle
cell disease. Physicians should exercise caution when considering the use of filgrastim in patients with sickle
cell disease, and only after careful evaluation of the potential risks and benefits.
Excipients
Nivestim contains sorbitol. Patients with rare hereditary problems of fructose intolerance should not use this
medicinal product. It also contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially ‘sodium-free’.
4.5 Interaction with other medicinal products and other forms of interaction
The safety and efficacy of filgrastim given on the same day as myelosuppressive cytotoxic chemotherapy
have not been definitively established. In view of the sensitivity of rapidly dividing myeloid cells to
myelosuppressive cytotoxic chemotherapy, the use of filgrastim is not recommended in the period from
24 hours before to 24 hours after chemotherapy. Preliminary evidence from a small number of patients
treated concomitantly with filgrastim and 5-Fluorouracil indicates that the severity of neutropenia may be
exacerbated.
Possible interactions with other haematopoietic growth factors and cytokines have not yet been investigated
in clinical trials.
Since lithium promotes the release of neutrophils, lithium is likely to potentiate the effect of filgrastim.
Although this interaction has not been formally investigated, there is no evidence that such an interaction is
harmful
.
4.6 Pregnancy and lactation
The safety of filgrastim has not been established in pregnant women. There are reports in the literature where
the transplacental passage of filgrastim in pregnant women has been demonstrated. There is no evidence
from studies in rats and rabbits that filgrastim is teratogenic. An increased incidence of embryo-loss has been
observed in rabbits, but no malformation has been seen. In pregnancy, the possible risk of filgrastim use to
the foetus must be weighed against the expected therapeutic benefit.
It is not known whether filgrastim is excreted in human milk, therefore filgrastim is not recommended for
use in breast-feeding women.
4.7 Effects on ability to drive and use machines
Filgrastim has negligible influence on the ability to drive and use machines. If the patient is experiencing
fatigue, caution is advised when driving a car or operating machinery.
During clinical studies 183 cancer patients and 96 healthy volunteers were exposed to Nivestim.
The safety profile of filgrastim observed in these clinical studies was consistent with that reported with the
reference product used in these studies.
The following undesirable effects and their frequencies have been observed under treatment with filgrastim
based on published information.
The assessment of undesirable effects is based on the following frequency data:
Very common: ≥1/10
Common: ≥1/100 to <1/10
Uncommon: ≥1/1,000 to <1/100
Rare: ≥1/10,000 to <1/1,000
Very rare: <1/10,000
Not known: cannot be estimated from the available data
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
In cancer patients
In clinical trials, the most frequent undesirable effects attributable to filgrastim at the recommended dose
were mild or moderate musculoskeletal pain, occurring in 10%, and severe musculoskeletal pain in 3% of
patients. Musculoskeletal pain is usually controlled with standard analgesics. Less frequent undesirable
effects include urinary abnormalities predominantly mild or moderate dysuria.
In randomised, placebo-controlled clinical trials, filgrastim did not increase the incidence of undesirable
effects associated with cytotoxic chemotherapy. Undesirable effects reported with equal frequency in
patients treated with filgrastim chemotherapy and placebo/chemotherapy included nausea and vomiting,
alopecia, diarrhoea, fatigue, anorexia, mucositis, headache, cough, skin rash, chest pain, generalised
weakness, sore throat, constipation and unspecified pain.
Reversible, dose-dependent and usually mild or moderate elevations of lactate dehydrogenase, alkaline
phosphatase, serum uric acid, and gamma-glutamyl transpeptidase occurred with filgrastim in approximately
50%, 35%, 25%, and 10% of patients, respectively at recommended doses.
Transient decreases in blood pressure, not requiring clinical treatment, have been reported occasionally.
There have been reports of GvHD and fatalities in patients receiving G-CSF after allogeneic bone marrow
transplantation (see section 5.1).
Vascular disorders, including veno-occlusive disease and fluid volume disturbances, have been reported
occasionally in patients undergoing high dose chemotherapy followed by autologous bone marrow
transplantation. The causal association with filgrastim has not been established.
Very rare events of cutaneous vasculitis have been reported in patients treated with filgrastim. The
mechanism of vasculitis in patients receiving filgrastim is unknown.
The occurrence of Sweet's syndrome (acute febrile dermatosis) has been reported occasionally. However,
since a significant percentage of these patients were suffering from leukaemia, a condition known to be
associated with Sweet's syndrome, a causal relationship with filgrastim has not been established.
Exacerbation of rheumatoid arthritis has been observed in individual cases.
Rare pulmonary adverse events including interstitial pneumonia, pulmonary oedema, and pulmonary
infiltrates have been reported in some cases with an outcome of respiratory failure or adult respiratory
distress syndrome (ARDS), which may be fatal (see section 4.4).
Allergic Reactions: Allergic-type reactions, including anaphylaxis, skin rash, urticaria, angioedema,
dyspnoea and hypotension, occurring on initial or subsequent treatment have been reported in patients
receiving filgrastim. Overall, reports were more common after intravenous administration. In some cases,
symptoms have recurred with rechallenge, suggesting a causal relationship. filgrastim should be permanently
discontinued in patients who experience a serious allergic reaction.
Isolated cases of sickle cells crises have been reported in patients with sickle cell disease (see section 4.4).
Metabolism and nutrition
disorders
Elevated alkaline phosphatase, elevated LDH,
elevated uric acid
Respiratory, thoracic and
mediastinal disorders
Gastrointestinal disorders
Constipation, anorexia, diarrhoea, mucositis
Skin and subcutaneous tissue
disorders
Sweet’s syndrome, cutaneous vasculitis
Musculoskeletal and
connective tissue disorders
Chest pain, musculoskeletal pain
Rheumatoid arthritis exacerbation
Renal and urinary disorders
General disorders and
administration site conditions
Fatigue, generalised weakness
In peripheral blood progenitor cell mobilisation in normal donors























The most commonly reported undesirable effect was mild to moderate transient musculo-skeletal pain.
Leukocytosis (White Blood Cell (WBC) > 50 x 10
9
/l) was observed in 41% of donors and transient
thrombocytopenia (platelets < 100 x 10
9
/l) following filgrastim and leukapheresis was observed in 35% of
donors.
Transient, minor increases in alkaline phosphatase, LDH, SGOT and uric acid have been reported in normal
donors receiving filgrastim; these were without clinical sequelae.
Exacerbation of arthritic symptoms has been observed very rarely.
Symptoms suggestive of severe allergic reactions have been reported very rarely.
Headaches, believed to be caused by filgrastim, have been reported in PBPC donor studies.
Common but generally asymptomatic cases of splenomegaly and very rare cases of splenic rupture have been
reported in healthy donors and patients following administration of G-CSFs (see section 4.4).
In normal donors, pulmonary adverse events (haemoptysis, pulmonary haemorrhage, pulmonary infiltrates,
dyspnoea and hypoxia) have been reported very rarely in post marketing experience with other filgrastim-
containing medicinal products (see section 4.4).
Blood and lymphatic system
disorders
Leukocytosis, thrombocytopenia
Metabolism and nutrition
disorders
Elevated alkaline phosphatase,
elevated LDH
SGCT increased, hyperuricaemia
Musculoskeletal and connective
tissue disorders
Rheumatoid arthritis exacerbation
General disorders and
administration site conditions
In severe chronic neutropenia (SCN) patients
Undesirable effects related to filgrastim therapy in SCN patients have been reported and for some their
frequency tend to decrease with time.
The most frequent undesirable effects attributable to filgrastim were bone pain, and general musculoskeletal
pain.
Other undesirable effects seen include splenic enlargement, which may be progressive in a minority of cases
and thrombocytopenia. Headache and diarrhoea have been reported shortly after starting filgrastim therapy,
typically in less than 10% of patients. Anaemia and epistaxis have also been reported.
Transient increases with no clinical symptoms were observed in serum uric acid, lactic dehydrogenase, and
alkaline phosphatase. Transient, moderate decreases in non-fasting blood glucose have also been seen.
Undesirable effects possibly related to filgrastim therapy and typically occurring in < 2% of SCN patients
were injection site reaction, headache, hepatomegaly, arthralgia, alopecia, osteoporosis, and rash.
















During long term use cutaneous vasculitis has been reported in 2% of SCN patients. There have been very
few instances of proteinuria/haematuria.
Blood and lymphatic system
disorders
Metabolism and nutrition
disorders
Decreased glucose, Elevated alkaline
phosphatase, elevated LDH,
hyperuricaemia
Respiratory, thoracic and
mediastinal disorders
Gastrointestinal disorders
Skin and subcutaneous tissue
disorders
Alopecia, cutaneous vasculitis,
injection site pain, rash
Musculoskeletal and connective
tissue disorders
Renal and urinary disorders
In patients with HIV
In clinical studies, the only undesirable effects that were consistently considered to be related to filgrastim
administration were musculoskeletal pain, predominantly mild to moderate bone pain and myalgia. The
incidence of these events was similar to that reported in cancer patients.
Splenic enlargement was reported to be related to filgrastim therapy in < 3% of patients. In all cases this was
mild or moderate on physical examination and the clinical course was benign; no patients had a diagnosis of
hypersplenism and no patients underwent splenectomy. As splenic enlargement is a common finding in
patients with HIV infection and is present to varying degrees in most patients with AIDS, the relationship to
filgrastim treatment is unclear.
Blood and lymphatic system
disorders
Musculoskeletal and connective
tissue disorders
The effects of filgrastim overdose have not been established.
Discontinuation of filgrastim therapy usually results in a 50% decrease in circulating neutrophils within 1 to
2 days, with a return to normal levels in 1 to 7 days.






























PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: colony stimulating factors, ATC code: L03AA02.
Nivestim is a biosimilar medicinal product. Detailed information is available on the website of the
European Medicines Agency (EMA)
http://www.ema.europa.eu
Human G-CSF is a glycoprotein which regulates the production and release of functional neutrophils from
the bone marrow. Nivestim containing r-metHuG-CSF (filgrastim) causes marked increases in peripheral
blood neutrophil counts within twenty-four hours, with minor increases in monocytes. In some severe
chronic neutropenia patients filgrastim can also induce a minor increase in the number of circulating
eosinophils and basophils relative to baseline; some of these patients may present with eosinophilia or
basophilia already prior to treatment. Elevations of neutrophil counts are dose-dependent at recommended
doses. Neutrophils produced in response to filgrastim show normal or enhanced function as demonstrated by
tests of chemotactic and phagocytic function. Following termination of filgrastim therapy, circulating
neutrophil counts decrease by 50% within 1 to 2 days, and to normal levels within 1 to 7 days.
Use of filgrastim in patients undergoing cytotoxic chemotherapy leads to significant reductions in the
incidence, severity and duration of neutropenia and febrile neutropenia. Treatment with filgrastim
significantly reduces the duration of febrile neutropenia, antibiotic use and hospitalisation after induction
chemotherapy for acute myelogenous leukaemia or myeloablative therapy followed by bone marrow
transplantation. The incidence of fever and documented infections were not reduced in either setting. The
duration of fever was not reduced in patients undergoing myeloablative therapy followed by bone marrow
transplantation.
Use of filgrastim, either alone, or after chemotherapy, mobilises haematopoietic progenitor cells into
peripheral blood. These autologous peripheral blood progenitor cells (PBPCs) may be harvested and infused
after high-dose cytotoxic therapy, either in place of, or in addition to bone marrow transplantation. Infusion
of PBPCs accelerates haematopoietic recovery reducing the duration of risk for haemorrhagic complications
and the need for platelet transfusions.
Recipients of allogeneic peripheral blood progenitor cells mobilised with filgrastim experienced significantly
more rapid haematological recovery, leading to a significant decrease in time to unsupported platelet
recovery when compared with allogeneic bone marrow transplantation.
One retrospective European study evaluating the use of G-CSF after allogeneic bone marrow transplantation
in patients with acute leukaemias suggested an increase in the risk of GvHD, treatment related mortality
(TRM) and mortality when G-CSF was administered. In a separate retrospective International study in
patients with acute and chronic myelogenous leukaemias, no effect on the risk of GvHD, TRM and mortality
was seen. A meta-analysis of allogeneic transplant studies, including the results of nine prospective
randomized trials, 8 retrospective studies and 1 case-controlled study, did not detect an effect on the risks of
acute GvHD, chronic GvHD or early treatment-related mortality.
Relative Risk (95% CI) of GvHD and TRM
Following treatment with G-CSF after bone marrow transplantation
Publication
European Retrospective
Study (2004)











International Retrospective
Study (2006)
a
Analysis includes studies involving BM transplant during this period; some studies used GM-CSF
b
Analysis includes patients receiving BM transplant during this period
Prior to allogeneic PBPC transplantation, use of filgrastim for the mobilisation of PBPC in normal donors
allows a collection of 4 x 106 CD34+ cells/kg recipient body weight in the majority of the donors after two
leukaphereses. Normal donors are given a dose of a 10 μg/kg/day, administered subcutaneously for 4 to 5
consecutive days.
Use of filgrastim in patients, children or adults, with severe chronic neutropenia (severe congenital, cyclic,
and idiopathic neutropenia) induces a sustained increase in absolute neutrophil counts in peripheral blood
and a reduction of infection and related events.
Use of filgrastim in patients with HIV infection maintains normal neutrophil counts to allow scheduled
dosing of antiviral and/or other myelosuppressive medicine. There is no evidence that patients with HIV
infection treated with filgrastim show an increase in HIV replication.
As with other haematopoietic growth factors, G-CSF has shown
in vitro
stimulating properties on human
endothelial cells.
The efficacy and safety of Nivestim has been assessed in randomised, controlled phase III study in breast cancer.
There were no relevant differences between Nivestim and the reference product with regard to duration of severe
neutropenia and incidence of febrile neutropenia.
5.2 Pharmacokinetic properties
A randomised, open-label, single-dose, comparator-controlled, two-way crossover study in 46 healthy
volunteers showed that the pharmacokinetic profile of Nivestim was comparable to that of the reference
product after subcutaneous and intravenous administration. Another randomised, double-blind, multiple-
dose, comparator-controlled, two-way crossover study in 50 healthy volunteers showed that the
pharmacokinetic profile of Nivestim was comparable to that of the reference product after subcutaneous
administration.
Clearance of filgrastim has been shown to follow first-order pharmacokinetics after both subcutaneous and
intravenous administration. The serum elimination half-life of filgrastim is approximately 3.5 hours, with a
clearance rate of approximately 0.6 ml/min/kg. Continuous infusion with filgrastim over a period of up to
28 days, in patients recovering from autologous bone-marrow transplantation, resulted in no evidence of
drug accumulation and comparable elimination half-lives. There is a positive linear correlation between the
dose and the serum concentration of filgrastim, whether administered intravenously or subcutaneously.
Following subcutaneous administration of recommended doses, serum concentrations were maintained above
10 ng/ml for 8 to 16 hours. The volume of distribution in blood is approximately 150 ml/kg.
5.3 Preclinical safety data
There are no preclinical data of relevance to the prescriber which are additional to that already included in
other sections of the Summary of Product Characteristics.
PHARMACEUTICAL PARTICULARS










Acetic acid, glacial
Sodium hydroxide
Sorbitol (E420)
Polysorbate 80
Water for injections
Nivestim must not be diluted with sodium chloride solutions.
Diluted filgrastim may be adsorbed to glass and plastic materials unless it is diluted in 50mg/ml (5%)
glucose solution for infusion (see section 6.6).
This medicinal product must not be mixed with other medicinal products except those mentioned in section
6.6.
After dilution: Chemical and physical in-use stability of the diluted solution for infusion has been
demonstrated for 24 hours at 2°C to 8°C. From a microbiological point of view, the product should be used
immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility
of the user and would normally not be longer than 24 hours at 2°C to 8°C, unless dilution has taken place in
controlled and validated aseptic conditions.
6.4 Special precautions for storage
Store and transport refrigerated (2°C - 8°C).
Do not freeze. Keep the pre-filled syringe in the outer carton in order to protect from light.
Accidental exposure to freezing temperatures for up to 24 hours does not affect the stability of Nivestim. The
frozen pre-filled syringes can be thawed and then refrigerate for future use. If exposure has been greater then
24 hours or frozen more than once then Nivestim should NOT be used.
Within its shelf-life and for the purpose of ambulatory use, the patient may remove the product from the
refrigerator and store it at room temperature (not above 25°C) for one single period of up to 7 days. At the
end of this period, the product should not be put back in the refrigerator and should be disposed of.
For storage conditions of the diluted medicinal product, see section 6.3.
6.5 Nature and contents of container
Pre-filled syringe (type I glass), with injection needle (stainless steel) with a needle guard, containing 0.5 ml
solution for injection/ infusion
Pack sizes of 1, 5 or 10 pre-filled syringes.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
If required, Nivestim may be diluted in 50 mg/ml (5%) glucose solution for infusion.
Dilution to a final concentration less than 0.2 MU (2 micrograms) per ml is not recommended at any time.
The solution should be visually inspected prior to use. Only clear solutions without particles should be used.
For patients treated with filgrastim diluted to concentrations below 1.5 MU (15 micrograms) per ml, human
serum albumin (HSA) should be added to a final concentration of 2 mg/ml.
Example: In a final injection volume of 20 ml, total doses of filgrastim less than 30 MU (300 micrograms)
should be given with 0.2 ml of 20% human albumin solution added.
When diluted in 50 mg/ml (5%) glucose solution for infusion, filgrastim is compatible with glass and a
variety of plastics including polyvinyl chloride (PVC), polyolefin (a co-polymer of polypropylene and
polyethylene) and polypropylene.
Nivestim contains no preservative. In view of the possible risk of microbial contamination, Nivestim
syringes are for single use only. Any unused product or waste material should be disposed of in accordance
with local requirements.
MARKETING AUTHORISATION HOLDER
Hospira UK Limited
Queensway
Royal Leamington Spa
Warwickshire CV31 3RW
United Kingdom
Tel: +44 (0)1926 820 820
Fax: +44 (0) 1926 821 049
MARKETING AUTHORISATION NUMBER(S)
EU/1/10/631/004
EU/1/10/631/005
EU/1/10/631/006
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
NAME OF THE MEDICINAL PRODUCT
Nivestim 48 MU/ 0.5 ml solution for injection/infusion.
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml of solution for injection or infusion contains 96 million units [MU] (960 micrograms) of filgrastim*.
Each pre-filled syringe contains 48 million units (MU) (480 micrograms) of filgrastim in 0.5 ml
(0.96 mg/ml).
*recombinant methionyl granulocyte-colony stimulating factor [G-CSF] produced in
Escherichia Coli
(BL21)
by recombinant DNA technology.
Excipient(s): Each ml of solution contains 50 mg of sorbitol.
For a full list of excipients, see section 6.1.
Solution for injection/infusion (injection/ infusion).
Clear, colourlesss solution.
4.1 Therapeutic indications
Filgrastim is indicated for the reduction in the duration of neutropenia and the incidence of febrile
neutropenia in patients treated with established cytotoxic chemotherapy for malignancy (with the exception
of chronic myeloid leukaemia and myelodysplastic syndromes) and for the reduction in the duration of
neutropenia in patients undergoing myeloablative therapy followed by bone marrow transplantation
considered to be at increased risk of prolonged severe neutropenia.
The safety and efficacy of filgrastim are similar in adults and children receiving cytotoxic chemotherapy.
Filgrastim is indicated for the mobilisation of peripheral blood progenitor cells (PBPC).
In patients, children or adults, with severe congenital, cyclic, or idiopathic neutropenia with an absolute
neutrophil count (ANC) of ≤ 0.5 x 10
9
/l and a history of severe or recurrent infections, long term
administration of filgrastim is indicated to increase neutrophil counts and to reduce the incidence and
duration of infection-related events.
Filgrastim is indicated for the treatment of persistent neutropenia (ANC less than or equal to 1.0 x 10
9
/l) in
patients with advanced HIV infection, in order to reduce the risk of bacterial infections when other options to
manage neutropenia are inappropriate.
4.2
Posology and method of administration
Filgrastim therapy should only be given in collaboration with an oncology centre which has experience in G-
CSF treatment and haematology and has the necessary diagnostic facilities. The mobilisation and apheresis
procedures should be performed in collaboration with an oncology-haematology centre with acceptable
experience in this field and where the monitoring of haematopoietic progenitor cells can be correctly
performed.
Established cytotoxic chemotherapy
The recommended dose of filgrastim is 0.5 MU (5 micrograms)/kg/day. The first dose of filgrastim should
not be administered less than 24 hours following cytotoxic chemotherapy. Filgrastim may be given as a daily
subcutaneous injection or as a daily intravenous infusion diluted in glucose 50 mg/ml (5%) solution for
infusion given over 30 minutes (see section 6.6 for instructions on dilutions)
.
The subcutaneous route is preferred in most cases. There is some evidence from a study of single dose
administration that intravenous dosing may shorten the duration of effect. The clinical relevance of this
finding to multiple dose administration is not clear. The choice of route should depend on the individual
clinical circumstance. In randomised clinical trials, a subcutaneous dose of 230 micrograms/m
2
/day (4.0 to
8.4 micrograms/kg/day) was used.
Daily dosing with filgrastim should continue until the expected neutrophil nadir is passed and the neutrophil
count has recovered to the normal range. Following established chemotherapy for solid tumours,
lymphomas, and lymphoid leukaemias, it is expected that the duration of treatment required to fulfil these
criteria will be up to 14 days. Following induction and consolidation treatment for acute myeloid leukaemia
the duration of treatment may be substantially longer (up to 38 days) depending on the type, dose and
schedule of cytotoxic chemotherapy used.
In patients receiving cytotoxic chemotherapy, a transient increase in neutrophil counts is typically seen 1 to 2
days after initiation of filgrastim therapy. However, for a sustained therapeutic response, filgrastim therapy
should not be discontinued before the expected nadir has passed and the neutrophil count has recovered to
the normal range. Premature discontinuation of filgrastim therapy, prior to the time of the expected
neutrophil nadir, is not recommended.
In patients treated with myeloablative therapy followed by bone marrow transplantation
The recommended starting dose of filgrastim is 1.0 MU (10 micrograms)/kg/day given as a 30 minute or 24
hour intravenous infusion or 1.0 MU (10 micrograms)/kg/day given by continuous 24 hour subcutaneous
infusion. Filgrastim should be diluted in 20 ml of 50 mg/ml (5%) glucose solution for infusion (see section
6.6).
The first dose of filgrastim should not be administered less than 24 hours following cytotoxic chemotherapy
and within 24 hours of bone marrow infusion.
Once the neutrophil nadir has been passed, the daily dose of filgrastim should be titrated against the
neutrophil response as follows:
Filgrastim dose adjustment
> 1.0 x 10
9
/l for 3 consecutive days
Then, if ANC remains > 1.0 x 10
9
/l for 3 more
consecutive days
If the ANC decreases to < 1.0 x 10
9
/l during the treatment period the dose of filgrastim should be re-
escalated according to the above steps
For the mobilisation of peripheral blood progenitor cells (PBPC) in patients undergoing myelosuppressive or
myeloablative therapy followed by autologous peripheral blood progenitor cell transplantation
The recommended dose of filgrastim for PBPC mobilisation when used alone is 1.0 MU
(10 micrograms)/kg/day as a 24 hour subcutaneous continuous infusion or a single daily subcutaneous
injection for 5 to 7 consecutive days. For infusions filgrastim should be diluted in 20 ml of 50 mg/ml (5%)








glucose solution for infusion (see section 6.6). Timing of leukapheresis: one or two leukaphereses on days 5
and 6 are often sufficient. In other circumstances, additional leukaphereses may be necessary. Filgrastim
dosing should be maintained until the last leukapheresis.
The recommended dose of filgrastim for PBPC mobilisation after myelosuppressive chemotherapy is 0.5 MU
(5 micrograms)/kg/day given daily by subcutaneous injection from the first day after completion of
chemotherapy until the expected neutrophil nadir is passed and the neutrophil count has recovered to the
normal range. Leukapheresis should be performed during the period when the ANC rises from < 0.5 x 10
9
/l
to > 5.0 x 10
9
/l. For patients who have not had extensive chemotherapy, one leukapheresis is often sufficient.
In other circumstances, additional leukaphereses are recommended.
For the mobilisation of peripheral blood progenitor cells (PBPCs) in normal donors prior to allogeneic
peripheral blood progenitor cell transplantation
For PBPC mobilisation in normal donors, filgrastim should be administered at 10 micrograms/kg/day
subcutaneously for 4 to 5 consecutive days. Leukapheresis should be started at day 5 and continued until day
6 if needed in order to collect 4 x 10
6
CD34
+
cells/kg recipient bodyweight.
In patients with severe chronic neutropenia
Congenital neutropenia
: the recommended starting dose is 1.2 MU (12 micrograms)/kg/day subcutaneously
as a single dose or in divided doses.
Idiopathic or cyclic neutropenia
: the recommended starting dose is 0.5 MU (5 micrograms)/kg/day
subcutaneously as a single dose or in divided doses.
Dose adjustment
: Filgrastim should be administered daily by subcutaneous injection until the neutrophil
count has reached and can be maintained at more than 1.5 x 10
9
/l. When the response has been obtained the
minimal effective dose to maintain this level should be established. Long-term daily administration is
required to maintain an adequate neutrophil count. After one to two weeks of therapy, the initial dose may be
doubled or halved depending upon the patient's response. Subsequently the dose may be individually
adjusted every 1 to 2 weeks to maintain the average neutrophil count between 1.5 x 10
9
/l and 10 x 10
9
/l. A
faster schedule of dose escalation may be considered in patients presenting with severe infections. In clinical
trials, 97% of patients who responded had a complete response at doses ≤24 micrograms/kg/day. The long-
term safety of filgrastim administration above 24 micrograms/kg/day in patients with severe chronic
neutropenia has not been established.
In patients with HIV infection
For reversal of neutropenia
The recommended starting dose of filgrastim is 0.1 MU (1 micrograms)/kg/day given daily by subcutaneous
injection with titration up to a maximum of 0.4 MU (4 μg)/kg/day until a normal neutrophil count is reached
and can be maintained (ANC > 2.0 x10
9
/l). In clinical studies, > 90% of patients responded at these doses,
achieving reversal of neutropenia in a median of 2 days.
In a small number of patients (< 10%), doses up to 1.0 MU (10 micrograms)/kg/day were required to achieve
reversal of neutropenia.
For maintaining normal neutrophil counts
When reversal of neutropenia has been achieved, the minimal effective dose to maintain a normal neutrophil
count should be established. Initial dose adjustment to alternate day dosing with 30 MU
(300 micrograms)/day by subcutaneous injection is recommended. Further dose adjustment may be
necessary, as determined by the patient's ANC, to maintain the neutrophil count at > 2.0 x 10
9
/l. In clinical
studies, dosing with 30 MU (300 micrograms)/day on 1 to 7 days per week was required to maintain the
ANC > 2.0 x 10
9
/l, with the median dose frequency being 3 days per week. Long-term administration may be
required to maintain the ANC > 2.0 x 10
9
/l.
Elderly patients
Clinical trials with filgrastim have included a small number of elderly patients but special studies have not
been performed in this group and therefore specific posology recommendations cannot be made.
Patients with renal or hepatic impairment
Studies of filgrastim in patients with severe impairment of renal or hepatic function demonstrate that it
exhibits a similar pharmacokinetic and pharmacodynamic profile to that seen in normal individuals. Dose
adjustment is not required in these circumstances.
Paediatric use in the severe chronic neutropenia (SCN) and cancer settings
Sixty-five percent of the patients studied in the SCN trial program were under 18 years of age. The efficacy
of treatment was clear for this age group, which included most patients with congenital neutropenia. There
were no differences in the safety profiles for paediatric patients treated for severe chronic neutropenia.
Data from clinical studies in paediatric patients indicate that the safety and efficacy of filgrastim are similar
in both adults and children receiving cytotoxic chemotherapy.
The posology recommendations in paediatric patients are the same as those in adults receiving
myelosuppressive cytotoxic chemotherapy.
Hypersensitivity to the active substance(s) or to any of the excipients.
4.4 Special warnings and precautions for use
Filgrastim should not be used to increase the dose of cytotoxic chemotherapy beyond established
posology regimens.
Filgrastim should not be administered to patients with severe congenital neutropenia (Kostmann's
syndrome) with abnormal cytogenetics.
Malignant cell growth
GCSF can promote growth of myeloid cells
in vitro
and similar effects may be seen on some non-myeloid
cells
in vitro
.
The safety and efficacy of filgrastim administration in patients with myelodysplastic syndrome, or chronic
myelogenous leukaemia have not been established.
Filgrastim is not indicated for use in these conditions. Particular care should be taken to distinguish the
diagnosis of blast transformation of chronic myeloid leukaemia from acute myeloid leukaemia.
In view of limited safety and efficacy data in patients with secondary AML, filgrastim should be
administered with caution.
The safety and efficacy of filgrastim administration in
de novo
AML patients aged < 55 years with good
cytogenetics (t(8;21), t(15;17), and inv(16)) have not been established.
Other special precautions
Monitoring of bone density may be indicated in patients with underlying osteoporotic bone diseases who
undergo continuous therapy with filgrastim for more than 6 months.
Rare (> 0.01% and <0.1%) pulmonary adverse events in particular interstitial pneumonia, have been reported
after G-CSF administration. Patients with a recent history of pulmonary infiltrates or pneumonia may be at
higher risk. The onset of pulmonary signs, such as cough, fever and dyspnoea in association with
radiological signs of pulmonary infiltrates and deterioration in pulmonary function may be preliminary signs
of Adult Respiratory Distress Syndrome (ARDS). Filgrastim should be discontinued and appropriate
treatment given.
Special precautions in cancer patients
Leukocytosis
White blood cell (WBC) counts of 100 x 10
9
/l or greater have been observed in less than 5% of patients
receiving filgrastim at doses above 0.3 MU/kg/day (3 μg/kg/day). No undesirable effects directly attributable
to this degree of leukocytosis have been reported. However, in view of the potential risks associated with
severe leukocytosis, a white blood cell count should be performed at regular intervals during filgrastim
therapy. If leukocyte counts exceed 50 x 10
9
/l after the expected nadir, filgrastim should be discontinued
immediately. However, during the period of administration of filgrastim for PBPC mobilisation, filgrastim
should be discontinued or its dosage should be reduced if the leukocyte counts rise to > 70 x 10
9
/l.
Risks associated with increased doses of chemotherapy
Special caution should be used when treating patients with high dose chemotherapy, because improved
tumour outcome has not been demonstrated and intensified doses of chemotherapeutic agents may lead to
increased toxicities including cardiac, pulmonary, neurologic, and dermatologic effects (please refer to the
prescribing information of the specific chemotherapy agents used).
Treatment with filgrastim alone does not preclude thrombocytopenia and anaemia due to myelosuppressive
chemotherapy. Because of the potential of receiving higher doses of chemotherapy (e.g. full doses on the
prescribed schedule) the patient may be at greater risk of thrombocytopenia and anaemia. Regular
monitoring of platelet count and haematocrit is recommended. Special care should be taken when
administering single or combination chemotherapeutic agents which are known to cause severe
thrombocytopenia.
The use of filgrastim-mobilised PBPCs has been shown to reduce the depth and duration of
thrombocytopenia following myelosuppressive or myeloablative chemotherapy.
Other special precautions
The effects of filgrastim in patients with substantially reduced myeloid progenitors have not been studied.
Filgrastim acts primarily on neutrophil precursors to exert its effect in elevating neutrophil counts. Therefore,
in patients with reduced precursors neutrophil response may be diminished (such as those treated with
extensive radiotherapy or chemotherapy, or those with bone marrow infiltration by tumour).
There have been reports of graft versus host disease GvHD and fatalities in patients receiving G-CSF after
allogeneic bone marrow transplantation (see section 5.1)
Known cases of Hereditary Fructose Intolerance (HFI).
Filgrastim contains sorbitol as an excipient at a concentration of 50 mg/ml. It is unlikely that as a
consequence of treatment with filgrastim alone that sufficient sorbitol will be infused to result in clinically
relevant toxicity in affected individuals. However, in cases of HFI caution is advised
The effect of filgrastim on GvHD has not been defined.
Increased haematopoietic activity of the bone marrow in response to growth factor therapy has been
associated with transient positive bone imaging findings. This should be considered when interpreting bone-
imaging results.
Special precautions in patients undergoing peripheral blood progenitor cell mobilisation
Mobilisation
There are no prospectively randomised comparisons of the two recommended mobilisation methods
(filgrastim alone, or in combination with myelosuppressive chemotherapy) within the same patient
population. The degree of variation between individual patients and between laboratory assays of CD34
+
cells mean that direct comparison between different studies is difficult. It is therefore difficult to recommend
an optimum method. The choice of mobilisation method should be considered in relation to the overall
objectives of treatment for an individual patient.
Prior exposure to cytotoxic agents
Patients who have undergone very extensive prior myelosuppressive therapy may not show sufficient
mobilisation of PBPC to achieve the recommended minimum yield (2.0 x 10
6
CD34
+
cells/kg) or acceleration
of platelet recovery, to the same degree.
Some cytotoxic agents exhibit particular toxicities to the haematopoietic progenitor pool, and may adversely
affect progenitor mobilisation. Agents such as melphalan, carmustine (BCNU), and carboplatin, when
administered over prolonged periods prior to attempts at progenitor mobilisation may reduce progenitor
yield. However, the administration of melphalan, carboplatin or BCNU together with filgrastim, has been
shown to be effective for progenitor mobilisation. When a peripheral blood progenitor cell transplantation is
envisaged it is advisable to plan the stem cell mobilisation procedure early in the treatment course of the
patient. Particular attention should be paid to the number of progenitors mobilised in such patients before the
administration of high-dose chemotherapy. If yields are inadequate, as measured by the criteria above,
alternative forms of treatment, not requiring progenitor support should be considered.
Assessment of progenitor cell yields
In assessing the number of progenitor cells harvested in patients treated with filgrastim, particular attention
should be paid to the method of quantitation. The results of flow cytometric analysis of CD34
+
cell numbers
vary depending on the precise methodology used and recommendations of numbers based on studies in other
laboratories need to be interpreted with caution.
Statistical analysis of the relationship between the number of CD34
+
cells re-infused and the rate of platelet
recovery after high-dose chemotherapy indicates a complex but continuous relationship.
The recommendation of a minimum yield of 2.0 x 10
6
CD34
+
cells/kg is based on published experience
resulting in adequate haematologic reconstitution. Yields in excess of this appear to correlate with more rapid
recovery, those below with slower recovery.
Special precautions in normal donors undergoing peripheral blood progenitor cell mobilisation
Mobilisation of PBPC does not provide a direct clinical benefit to normal donors and should only be
considered for the purposes of allogeneic stem cell transplantation.
PBPC mobilisation should be considered only in donors who meet normal clinical and laboratory eligibility
criteria for stem cell donation with special attention to haematological values and infectious disease.
The safety and efficacy of filgrastim have not been assessed in normal donors < 16 years or > 60 years.
Transient thrombocytopenia (platelets < 100 x 10
9
/l) following filgrastim administration and leukapheresis
was observed in 35% of subjects studied. Among these, two cases of platelets < 50 x 10
9
/l were reported and
attributed to the leukapheresis procedure.
If more than one leukapheresis is required, particular attention should be paid to donors with platelets < 100
x 10
9
/l prior to leukapheresis; in general apheresis should not be performed if platelets < 75 x 10
9
/l.
Leukapheresis should not be performed in donors who are anticoagulated or who have known defects in
haemostasis.
Filgrastim administration should be discontinued or its posology should be reduced if the leukocyte counts
rise to > 70 x10
9
/l.
Donors who receive G-CSFs for PBPC mobilisation should be monitored until haematological indices return
to normal.
Transient cytogenetic modifications have been observed in normal donors following G-CSF use. The
significance of these changes is unknown.
Long-term safety follow-up of donors is ongoing. Nevertheless, a risk of promotion of a malignant myeloid
clone can not be excluded. It is recommended that the apheresis centre perform a systematic record and
tracking of the stem cell donors to ensure monitoring of long-term safety.
Common but generally asymptomatic cases of splenomegaly and very rare cases of splenic rupture have been
reported in healthy donors (and patients) following administration of G-CSFs. Some cases of splenic rupture
were fatal. Therefore, spleen size should be carefully monitored (e.g. clinical examination, ultrasound). A
diagnosis of splenic rupture should be considered in donors and/or patients reporting left upper abdominal
pain or shoulder tip pain.
In normal donors, pulmonary adverse events (haemoptysis, pulmonary haemorrhage, pulmonary infiltrates,
dyspnoea and hypoxia) have been reported very rarely in post marketing experience with other filgrastim-
containing medicinal products. In case of suspected or confirmed pulmonary adverse events, discontinuation
of treatment with filgrastim should be considered and appropriate medical care given.
Special precautions in recipients of allogeneic peripheral blood progenitor cells mobilised with filgrastim
Current data indicate that immunological interactions between the allogeneic PBPC graft and the recipient
may be associated with an increased risk of acute and chronic Graft versus Host Disease (GvHD) when
compared with bone marrow transplantation.
Special precautions in severe chronic neutropenia (SCN) patients
Blood cell counts
Platelet counts should be monitored closely, especially during the first few weeks of filgrastim therapy.
Consideration should be given to intermittent cessation or decreasing the dose of filgrastim in patients who
develop thrombocytopenia, i.e. platelets consistently < 100 x 10
9
/l
.
Other blood cell changes occur, including anaemia and transient increases in myeloid progenitors, which
require close monitoring of cell counts.
Transformation to leukaemia or myelodysplastic syndrome
Special care should be taken in the diagnosis of severe chronic neutropenias to distinguish them from other
haematopoietic disorders such as aplastic anaemia, myelodysplasia, and myeloid leukaemia. Complete blood
cell counts with differential and platelet counts, and an evaluation of bone marrow morphology and
karyotype should be performed prior to treatment.
There was a low frequency (approximately 3%) of myelodysplastic syndromes (MDS) or leukaemia in
clinical trial patients with severe chronic neutropenia treated with filgrastim. This observation has only been
made in patients with congenital neutropenia. MDS and leukaemias are natural complications of the disease
and are of uncertain relation to filgrastim therapy. A subset of approximately 12% of patients who had
normal cytogenetic evaluations at baseline were subsequently found to have abnormalities, including
monosomy 7, on routine repeat evaluation. If patients with severe chronic neutropenia develop abnormal
cytogenetics, the risks and benefits of continuing filgrastim should be carefully weighed; filgrastim should be
discontinued if MDS or leukaemia occurs. It is currently unclear whether long-term treatment of patients
with severe chronic neutropenia will predispose patients to cytogenetic abnormalities, MDS or leukaemic
transformation. It is recommended to perform morphologic and cytogenetic bone marrow examinations in
patients at regular intervals (approximately every 12 months).
Other special precautions
Causes of transient neutropenia, such as viral infections should be excluded.
Splenic enlargement is a direct effect of treatment with filgrastim. Thirty-one percent (31%) of patients in
studies were documented as having palpable splenomegaly. Increases in volume, measured radiographically,
occurred early during filgrastim therapy and tended to plateau. Dose reductions were noted to slow or stop
the progression of splenic enlargement, and in 3% of patients a splenectomy was required. Spleen size
should be evaluated regularly. Abdominal palpation should be sufficient to detect abnormal increases in
splenic volume.
Haematuria/proteinuria occurred in a small number of patients. Regular urinanalysis should be performed to
monitor this event.
The safety and efficacy in neonates and patients with autoimmune neutropenia have not been established.
Special precautions in patients with HIV infection
Blood cell counts
Absolute neutrophil count (ANC) should be monitored closely, especially during the first few weeks of
filgrastim therapy. Some patients may respond very rapidly and with a considerable increase in neutrophil
count to the initial dose of filgrastim. It is recommended that the ANC is measured daily for the first 2 to
3 days of filgrastim administration. Thereafter, it is recommended that the ANC is measured at least twice
per week for the first two weeks and subsequently once per week or once every other week during
maintenance therapy. During intermittent dosing with 30 MU (300 μg)/day of filgrastim, there can be wide
fluctuations in the patient's ANC over time. In order to determine a patient's trough or nadir ANC, it is
recommended that blood samples are taken for ANC measurement immediately prior to any scheduled
dosing with filgrastim.
Risk associated with increased doses of myelosuppressive medicinal products
Treatment with filgrastim alone does not preclude thrombocytopenia and anaemia due to myelosuppressive
medicine. As a result of the potential to receive higher doses or a greater number of these medicinal products
with filgrastim therapy, the patient may be at higher risk of developing thrombocytopenia and anaemia.
Regular monitoring of blood counts is recommended (see above).
Infections and malignancies causing myelosuppression
Neutropenia may be due to bone marrow infiltrating opportunistic infections such as
Mycobacterium avium
complex or malignancies such as lymphoma. In patients with known bone marrow infiltrating infections or
malignancy, consider appropriate therapy for treatment of the underlying condition, in addition to
administration of filgrastim for treatment of neutropenia. The effects of filgrastim on neutropenia due to bone
marrow infiltrating infection or malignancy have not been well established.
Special precautions in sickle cell disease
Sickle cells crises, in some cases fatal, have been reported with the use of filgrastim in subjects with sickle
cell disease. Physicians should exercise caution when considering the use of filgrastim in patients with sickle
cell disease, and only after careful evaluation of the potential risks and benefits.
Excipients
Nivestim contains sorbitol. Patients with rare hereditary problems of fructose intolerance should not use this
medicinal product. It also contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially ‘sodium-free’.
4.5 Interaction with other medicinal products and other forms of interaction
The safety and efficacy of filgrastim given on the same day as myelosuppressive cytotoxic chemotherapy
have not been definitively established. In view of the sensitivity of rapidly dividing myeloid cells to
myelosuppressive cytotoxic chemotherapy, the use of filgrastim is not recommended in the period from
24 hours before to 24 hours after chemotherapy. Preliminary evidence from a small number of patients
treated concomitantly with filgrastim and 5-Fluorouracil indicates that the severity of neutropenia may be
exacerbated.
Possible interactions with other haematopoietic growth factors and cytokines have not yet been investigated
in clinical trials.
Since lithium promotes the release of neutrophils, lithium is likely to potentiate the effect of filgrastim.
Although this interaction has not been formally investigated, there is no evidence that such an interaction is
harmful
.
4.6 Pregnancy and lactation
The safety of filgrastim has not been established in pregnant women. There are reports in the literature where
the transplacental passage of filgrastim in pregnant women has been demonstrated. There is no evidence
from studies in rats and rabbits that filgrastim is teratogenic. An increased incidence of embryo-loss has been
observed in rabbits, but no malformation has been seen. In pregnancy, the possible risk of filgrastim use to
the foetus must be weighed against the expected therapeutic benefit.
It is not known whether filgrastim is excreted in human milk, therefore filgrastim is not recommended for
use in breast-feeding women.
4.7 Effects on ability to drive and use machines
Filgrastim has negligible influence on the ability to drive and use machines. If the patient is experiencing
fatigue, caution is advised when driving a car or operating machinery.
During clinical studies 183 cancer patients and 96 healthy volunteers were exposed to Nivestim.
The safety profile of filgrastim observed in these clinical studies was consistent with that reported with the
reference product used in these studies.
The following undesirable effects and their frequencies have been observed under treatment with filgrastim
based on published information.
The assessment of undesirable effects is based on the following frequency data:
Very common: ≥1/10
Common: ≥1/100 to <1/10
Uncommon: ≥1/1,000 to <1/100
Rare: ≥1/10,000 to <1/1,000
Very rare: <1/10,000
Not known: cannot be estimated from the available data
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
In cancer patients
In clinical trials, the most frequent undesirable effects attributable to filgrastim at the recommended dose
were mild or moderate musculoskeletal pain, occurring in 10%, and severe musculoskeletal pain in 3% of
patients. Musculoskeletal pain is usually controlled with standard analgesics. Less frequent undesirable
effects include urinary abnormalities predominantly mild or moderate dysuria.
In randomised, placebo-controlled clinical trials, filgrastim did not increase the incidence of undesirable
effects associated with cytotoxic chemotherapy. Undesirable effects reported with equal frequency in
patients treated with filgrastim chemotherapy and placebo/chemotherapy included nausea and vomiting,
alopecia, diarrhoea, fatigue, anorexia, mucositis, headache, cough, skin rash, chest pain, generalised
weakness, sore throat, constipation and unspecified pain.
Reversible, dose-dependent and usually mild or moderate elevations of lactate dehydrogenase, alkaline
phosphatase, serum uric acid, and gamma-glutamyl transpeptidase occurred with filgrastim in approximately
50%, 35%, 25%, and 10% of patients, respectively at recommended doses.
Transient decreases in blood pressure, not requiring clinical treatment, have been reported occasionally.
There have been reports of GvHD and fatalities in patients receiving G-CSF after allogeneic bone marrow
transplantation (see section 5.1).
Vascular disorders, including veno-occlusive disease and fluid volume disturbances, have been reported
occasionally in patients undergoing high dose chemotherapy followed by autologous bone marrow
transplantation. The causal association with filgrastim has not been established.
Very rare events of cutaneous vasculitis have been reported in patients treated with filgrastim. The
mechanism of vasculitis in patients receiving filgrastim is unknown.
The occurrence of Sweet's syndrome (acute febrile dermatosis) has been reported occasionally. However,
since a significant percentage of these patients were suffering from leukaemia, a condition known to be
associated with Sweet's syndrome, a causal relationship with filgrastim has not been established.
Exacerbation of rheumatoid arthritis has been observed in individual cases.
Rare pulmonary adverse events including interstitial pneumonia, pulmonary oedema, and pulmonary
infiltrates have been reported in some cases with an outcome of respiratory failure or adult respiratory
distress syndrome (ARDS), which may be fatal (see section 4.4).
Allergic Reactions: Allergic-type reactions, including anaphylaxis, skin rash, urticaria, angioedema,
dyspnoea and hypotension, occurring on initial or subsequent treatment have been reported in patients
receiving filgrastim. Overall, reports were more common after intravenous administration. In some cases,
symptoms have recurred with rechallenge, suggesting a causal relationship. filgrastim should be permanently
discontinued in patients who experience a serious allergic reaction.
Isolated cases of sickle cells crises have been reported in patients with sickle cell disease (see section 4.4).
Metabolism and nutrition
disorders
Elevated alkaline phosphatase, elevated LDH,
elevated uric acid
Respiratory, thoracic and
mediastinal disorders
Gastrointestinal disorders
Constipation, anorexia, diarrhoea, mucositis
Skin and subcutaneous tissue
disorders
Sweet’s syndrome, cutaneous vasculitis
Musculoskeletal and
connective tissue disorders
Chest pain, musculoskeletal pain
Rheumatoid arthritis exacerbation
Renal and urinary disorders
General disorders and
administration site conditions
Fatigue, generalised weakness























In peripheral blood progenitor cell mobilisation in normal donors
The most commonly reported undesirable effect was mild to moderate transient musculo-skeletal pain.
Leukocytosis (White Blood Cell (WBC) > 50 x 10
9
/l) was observed in 41% of donors and transient
thrombocytopenia (platelets < 100 x 10
9
/l) following filgrastim and leukapheresis was observed in 35% of
donors.
Transient, minor increases in alkaline phosphatase, LDH, SGOT and uric acid have been reported in normal
donors receiving filgrastim; these were without clinical sequelae.
Exacerbation of arthritic symptoms has been observed very rarely.
Symptoms suggestive of severe allergic reactions have been reported very rarely.
Headaches, believed to be caused by filgrastim, have been reported in PBPC donor studies.
Common but generally asymptomatic cases of splenomegaly and very rare cases of splenic rupture have been
reported in healthy donors and patients following administration of G-CSFs (see section 4.4).
In normal donors, pulmonary adverse events (haemoptysis, pulmonary haemorrhage, pulmonary infiltrates,
dyspnoea and hypoxia) have been reported very rarely in post marketing experience with other filgrastim-
containing medicinal products (see section 4.4).
Blood and lymphatic system
disorders
Leukocytosis, thrombocytopenia
Metabolism and nutrition
disorders
Elevated alkaline phosphatase,
elevated LDH
SGCT increased, hyperuricaemia
Musculoskeletal and connective
tissue disorders
Rheumatoid arthritis exacerbation
General disorders and
administration site conditions
In severe chronic neutropenia (SCN) patients
Undesirable effects related to filgrastim therapy in SCN patients have been reported and for some their
frequency tend to decrease with time.
The most frequent undesirable effects attributable to filgrastim were bone pain, and general musculoskeletal
pain.
Other undesirable effects seen include splenic enlargement, which may be progressive in a minority of cases
and thrombocytopenia. Headache and diarrhoea have been reported shortly after starting filgrastim therapy,
typically in less than 10% of patients. Anaemia and epistaxis have also been reported.
Transient increases with no clinical symptoms were observed in serum uric acid, lactic dehydrogenase, and
alkaline phosphatase. Transient, moderate decreases in non-fasting blood glucose have also been seen.















Undesirable effects possibly related to filgrastim therapy and typically occurring in < 2% of SCN patients
were injection site reaction, headache, hepatomegaly, arthralgia, alopecia, osteoporosis, and rash.
During long term use cutaneous vasculitis has been reported in 2% of SCN patients. There have been very
few instances of proteinuria/haematuria.
Blood and lymphatic system
disorders
Metabolism and nutrition
disorders
Decreased glucose, Elevated alkaline
phosphatase, elevated LDH,
hyperuricaemia
Respiratory, thoracic and
mediastinal disorders
Gastrointestinal disorders
Skin and subcutaneous tissue
disorders
Alopecia, cutaneous vasculitis,
injection site pain, rash
Musculoskeletal and connective
tissue disorders
Renal and urinary disorders
In patients with HIV
In clinical studies, the only undesirable effects that were consistently considered to be related to filgrastim
administration were musculoskeletal pain, predominantly mild to moderate bone pain and myalgia. The
incidence of these events was similar to that reported in cancer patients.
Splenic enlargement was reported to be related to filgrastim therapy in < 3% of patients. In all cases this was
mild or moderate on physical examination and the clinical course was benign; no patients had a diagnosis of
hypersplenism and no patients underwent splenectomy. As splenic enlargement is a common finding in
patients with HIV infection and is present to varying degrees in most patients with AIDS, the relationship to
filgrastim treatment is unclear.
Blood and lymphatic system
disorders
Musculoskeletal and connective
tissue disorders
The effects of filgrastim overdose have not been established.





























Discontinuation of filgrastim therapy usually results in a 50% decrease in circulating neutrophils within 1 to
2 days, with a return to normal levels in 1 to 7 days.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: colony stimulating factors, ATC code: L03AA02.
Nivestim is a biosimilar medicinal product. Detailed information is available on the website of the
European Medicines Agency (EMA)
http://www.ema.europa.eu
Human G-CSF is a glycoprotein which regulates the production and release of functional neutrophils from
the bone marrow. Nivestim containing r-metHuG-CSF (filgrastim) causes marked increases in peripheral
blood neutrophil counts within twenty-four hours, with minor increases in monocytes. In some severe
chronic neutropenia patients filgrastim can also induce a minor increase in the number of circulating
eosinophils and basophils relative to baseline; some of these patients may present with eosinophilia or
basophilia already prior to treatment. Elevations of neutrophil counts are dose-dependent at recommended
doses. Neutrophils produced in response to filgrastim show normal or enhanced function as demonstrated by
tests of chemotactic and phagocytic function. Following termination of filgrastim therapy, circulating
neutrophil counts decrease by 50% within 1 to 2 days, and to normal levels within 1 to 7 days.
Use of filgrastim in patients undergoing cytotoxic chemotherapy leads to significant reductions in the
incidence, severity and duration of neutropenia and febrile neutropenia. Treatment with filgrastim
significantly reduces the duration of febrile neutropenia, antibiotic use and hospitalisation after induction
chemotherapy for acute myelogenous leukaemia or myeloablative therapy followed by bone marrow
transplantation. The incidence of fever and documented infections were not reduced in either setting. The
duration of fever was not reduced in patients undergoing myeloablative therapy followed by bone marrow
transplantation.
Use of filgrastim, either alone, or after chemotherapy, mobilises haematopoietic progenitor cells into
peripheral blood. These autologous peripheral blood progenitor cells (PBPCs) may be harvested and infused
after high-dose cytotoxic therapy, either in place of, or in addition to bone marrow transplantation. Infusion
of PBPCs accelerates haematopoietic recovery reducing the duration of risk for haemorrhagic complications
and the need for platelet transfusions.
Recipients of allogeneic peripheral blood progenitor cells mobilised with filgrastim experienced significantly
more rapid haematological recovery, leading to a significant decrease in time to unsupported platelet
recovery when compared with allogeneic bone marrow transplantation.
One retrospective European study evaluating the use of G-CSF after allogeneic bone marrow transplantation
in patients with acute leukaemias suggested an increase in the risk of GvHD, treatment related mortality
(TRM) and mortality when G-CSF was administered. In a separate retrospective International study in
patients with acute and chronic myelogenous leukaemias, no effect on the risk of GvHD, TRM and mortality
was seen. A meta-analysis of allogeneic transplant studies, including the results of nine prospective
randomized trials, 8 retrospective studies and 1 case-controlled study, did not detect an effect on the risks of
acute GvHD, chronic GvHD or early treatment-related mortality.
Relative Risk (95% CI) of GvHD and TRM
Following treatment with G-CSF after bone marrow transplantation
Publication










European Retrospective
Study (2004)
International Retrospective
Study (2006)
a
Analysis includes studies involving BM transplant during this period; some studies used GM-CSF
b
Analysis includes patients receiving BM transplant during this period
Prior to allogeneic PBPC transplantation, use of filgrastim for the mobilisation of PBPC in normal donors
allows a collection of 4 x 106 CD34+ cells/kg recipient body weight in the majority of the donors after two
leukaphereses. Normal donors are given a dose of a 10 μg/kg/day, administered subcutaneously for 4 to 5
consecutive days.
Use of filgrastim in patients, children or adults, with severe chronic neutropenia (severe congenital, cyclic,
and idiopathic neutropenia) induces a sustained increase in absolute neutrophil counts in peripheral blood
and a reduction of infection and related events.
Use of filgrastim in patients with HIV infection maintains normal neutrophil counts to allow scheduled
dosing of antiviral and/or other myelosuppressive medicine. There is no evidence that patients with HIV
infection treated with filgrastim show an increase in HIV replication.
As with other haematopoietic growth factors, G-CSF has shown
in vitro
stimulating properties on human
endothelial cells.
The efficacy and safety of Nivestim has been assessed in randomised, controlled phase III study in breast
cancer. There were no relevant differences between Nivestim and the reference product with regard to
duration of severe neutropenia and incidence of febrile neutropenia.
5.2 Pharmacokinetic properties
A randomised, open-label, single-dose, comparator-controlled, two-way crossover study in 46 healthy
volunteers showed that the pharmacokinetic profile of Nivestim was comparable to that of the reference
product after subcutaneous and intravenous administration. Another randomised, double-blind, multiple-
dose, comparator-controlled, two-way crossover study in 50 healthy volunteers showed that the
pharmacokinetic profile of Nivestim was comparable to that of the reference product after subcutaneous
administration.
Clearance of filgrastim has been shown to follow first-order pharmacokinetics after both subcutaneous and
intravenous administration. The serum elimination half-life of filgrastim is approximately 3.5 hours, with a
clearance rate of approximately 0.6 ml/min/kg. Continuous infusion with filgrastim over a period of up to
28 days, in patients recovering from autologous bone-marrow transplantation, resulted in no evidence of
drug accumulation and comparable elimination half-lives. There is a positive linear correlation between the
dose and the serum concentration of filgrastim, whether administered intravenously or subcutaneously.
Following subcutaneous administration of recommended doses, serum concentrations were maintained above
10 ng/ml for 8 to 16 hours. The volume of distribution in blood is approximately 150 ml/kg.
5.3 Preclinical safety data
There are no preclinical data of relevance to the prescriber which are additional to that already included in
other sections of the Summary of Product Characteristics.













PHARMACEUTICAL PARTICULARS
Acetic acid, glacial
Sodium hydroxide
Sorbitol (E420)
Polysorbate 80
Water for injections
Nivestim must not be diluted with sodium chloride solutions.
Diluted filgrastim may be adsorbed to glass and plastic materials unless it is diluted in 50mg/ml (5%)
glucose solution for infusion (see section 6.6).
This medicinal product must not be mixed with other medicinal products except those mentioned in section
6.6.
After dilution: Chemical and physical in-use stability of the diluted solution for infusion has been
demonstrated for 24 hours at 2°C to 8°C. From a microbiological point of view, the product should be used
immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility
of the user and would normally not be longer than 24 hours at 2°C to 8°C, unless dilution has taken place in
controlled and validated aseptic conditions.
6.4 Special precautions for storage
Store and transport refrigerated (2°C - 8°C).
Do not freeze. Keep the pre-filled syringe in the outer carton in order to protect from light.
Accidental exposure to freezing temperatures for up to 24 hours does not affect the stability of Nivestim. The
frozen pre-filled syringes can be thawed and then refrigerate for future use. If exposure has been greater then
24 hours or frozen more than once then Nivestim should NOT be used.
Within its shelf-life and for the purpose of ambulatory use, the patient may remove the product from the
refrigerator and store it at room temperature (not above 25°C) for one single period of up to 7 days. At the
end of this period, the product should not be put back in the refrigerator and should be disposed of.
For storage conditions of the diluted medicinal product, see section 6.3.
6.5 Nature and contents of container
Pre-filled syringe (type I glass), with injection needle (stainless steel) with a needle guard, containing 0.5 ml
solution for injection/ infusion
Pack sizes of 1, 5 or 10 pre-filled syringes.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
If required, Nivestim may be diluted in 50 mg/ml (5%) glucose solution for infusion.
Dilution to a final concentration less than 0.2 MU (2 micrograms) per ml is not recommended at any time.
The solution should be visually inspected prior to use. Only clear solutions without particles should be used.
For patients treated with filgrastim diluted to concentrations below 1.5 MU (15 micrograms) per ml, human
serum albumin (HSA) should be added to a final concentration of 2 mg/ml.
Example: In a final injection volume of 20 ml, total doses of filgrastim less than 30 MU (300 micrograms)
should be given with 0.2 ml of 20% human albumin solution added.
When diluted in 50 mg/ml (5%) glucose solution for infusion, filgrastim is compatible with glass and a
variety of plastics including polyvinyl chloride (PVC), polyolefin (a co-polymer of polypropylene and
polyethylene) and polypropylene.
Nivestim contains no preservative. In view of the possible risk of microbial contamination, Nivestim
syringes are for single use only. Any unused product or waste material should be disposed of in accordance
with local requirements.
MARKETING AUTHORISATION HOLDER
Hospira UK Limited
Queensway
Royal Leamington Spa
Warwickshire CV31 3RW
United Kingdom
Tel: +44 (0)1926 820 820
Fax: +44 (0) 1926 821 049
MARKETING AUTHORISATION NUMBER(S)
EU/1/10/631/007
EU/1/10/631/008
EU/1/10/631/009
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European
MANUFACTURER OF THE BIOLOGICAL ACTIVE SUBSTANCE AND
MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
CONDITIONS OF THE MARKETING AUTHORISATION
MANUFACTURER OF THE BIOLOGICAL ACTIVE SUBSTANCE AND
MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer of the biological active substance
Hospira Zagreb d.o.o
Prilaz Baruna Filipovica 27/D
10000 Zagreb
Croatia
Name and address of the manufacturer responsible for batch release
PLIVA Kraków, S.A.
ul. Mogilska 80
31-546 Kraków
Poland
B. CONDITIONS OF THE MARKETING AUTHORISATION
•
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON THE
MARKETING AUTHORISATION HOLDER
Medicinal product subject to restricted medical prescription (See Annex I: Summary of Product
Characteristics, section 4.2)
•
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND EFFECTIVE USE
OF THE MEDICINAL PRODUCT
The MAH must ensure that the system of pharmacovigilance, as described in version 5.4 presented in
Module 1.8.1. of the Marketing Authorisation Application, is in place and functioning before and whilst the
product is on the market.
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in the
Pharmacovigilance Plan, as agreed in version 4.0 of the Risk Management Plan (RMP) presented in Module
1.8.2. of the Marketing Authorisation Application and any subsequent updates of the RMP agreed by the
CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, the
updated RMP should be submitted at the same time as the next Periodic Safety Update Report (PSUR).
In addition, an updated RMP should be submitted
When new information is received that may impact on the current Safety Specification, Pharmacovigilance
Plan or risk minimisation activities
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being reached
At the request of the Agency.
ANNEX III
LABELLING AND PACKAGE LEAFLET

PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
Nivestim 12 MU/ 0.2 ml solution for injection/ infusion
Filgrastim
STATEMENT OF ACTIVE SUBSTANCE(S)
Each pre-filled syringe contains 12 million units (MU) (120 micrograms) of filgrastim in 0.2 ml (0.6 mg/ml)
Acetic acid glacial, sodium hydroxide, polysorbate 80, sorbitol (E420) and water for injections. See leaflet
for further information.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection/ infusion.
1 pre-filled syringe with 0.2 ml.
5 pre-filled syringes with 0.2 ml.
10 pre-filled syringes with 0.2 ml.
METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use.
For single use only.
For intravenous or subcutaneous use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT OF
THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
Needle guard is attached to the pre-filled syringe in order to protect from needle stick injury. See package
leaflet for direction for use of the needle safe device.
EXP:
After dilution use within 24 hours.
SPECIAL STORAGE CONDITIONS
Store and transport refrigerated (2°C - 8°C). Do not freeze.
Keep the pre-filled syringe in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS OR
WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF APPROPRIATE

























11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Hospira UK Limited
Queensway
Royal Leamington Spa
Warwickshire CV31 3RW
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/10/631/001
EU/1/10/631/002
EU/1/10/631/003
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE
















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
Nivestim 30 MU/ 0.5 ml solution for injection/ infusion
Filgrastim
STATEMENT OF ACTIVE SUBSTANCE(S)
Each pre-filled syringe contains 30 million units (300 micrograms) of filgrastim in 0.5 ml (0.6 mg/ml)
Acetic acid glacial, sodium hydroxide, polysorbate 80, sorbitol (E420) and water for injections. See leaflet
for further information.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection/ infusion.
1 pre-filled syringe with 0.5 ml
5 pre-filled syringes with 0.5 ml
10 pre-filled syringes with 0.5 ml
METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use.
For single use only.
For intravenous or subcutaneous use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT OF
THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
Needle guard is attached to the pre-filled syringe in order to protect from needle stick injury. See pacakge
leaflet for direction for use of the needle safe device.
EXP:
After dilution use within 24 hours.
SPECIAL STORAGE CONDITIONS
Store and transport refrigerated (2°C - 8°C). Do not freeze.
Keep the pre-filled syringe in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS OR
WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF APPROPRIATE

























11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Hospira UK Limited
Queensway
Royal Leamington Spa
Warwickshire CV31 3RW
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/10/631/004
EU/1/10/631/005
EU/1/10/631/006
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE
















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
Nivestim 48 MU/ 0.5 ml solution for injection/ infusion
Filgrastim
STATEMENT OF ACTIVE SUBSTANCE(S)
Each pre-filled syringe contains 48 million units (480 micrograms) of filgrastim in 0.5 ml (0.96 mg/ml)
Acetic acid glacial, sodium hydroxide, polysorbate 80, sorbitol (E420) and water for injections. See leaflet
for further information.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection/ infusion.
1 pre-filled syringe with 0.5 ml
5 pre-filled syringes with 0.5 ml
10 pre-filled syringes with 0.5 ml
METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use.
For single use only.
For intravenous or subcutaneous use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT OF
THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
Needle guard is attached to the pre-filled syringe in order to protect from needle stick injury. See pacakge
leaflet for direction for use of the needle safe device.
EXP:
After dilution use within 24 hours.
SPECIAL STORAGE CONDITIONS
Store and transport refrigerated (2°C - 8°C). Do not freeze.
Keep the pre-filled syringe in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS OR
WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF APPROPRIATE

























11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Hospira UK Limited
Queensway
Royal Leamington Spa
Warwickshire CV31 3RW
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/10/631/007
EU/1/10/631/008
EU/1/10/631/009
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE
















PACKAGE LEAFLET: INFORMATION FOR THE USER
Nivestim 12 MU/ 0.2 ml solution for injection/ infusion
Nivestim 30 MU/ 0.5 ml solution for injection/ infusion
Nivestim 48 MU/ 0.5 ml solution for injection/ infusion
Filgrastim
Read all of this leaflet carefully before you start using this medicine.
-
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their
symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
1. What Nivestim is and what it is used for
2. Before you use Nivestim
3. How to use
Nivestim
4. Possible side effects
5.
How to store Nivestim
6.
1.
WHAT NIVESTIM IS AND WHAT IT IS USED FOR
What Nivestim is
Nivestim contains the active substance filgrastim. It belongs to a group of proteins called cytokines and is
very similar to a natural protein (granulocyte-colony stimulating factor [G-CSF]) produced by your own
body. Filgrastim stimulates the bone marrow (the tissue where new blood cells are made) to produce more
blood cells, especially certain types of white cells. White cells are important as they help your body fight
infection.
What Nivestim is used for
Your doctor has prescribed Nivestim for you to help your body make more white blood cells. Your doctor
will tell you why you are being treated with Nivestim.
Nivestim is useful in several different conditions which are:
-
bone marrow transplantation,
severe chronic neutropenia (neutropenia is a condition of an abnormally low number of a particular
type of white blood cell called a neutrophil),
neutropenia in patients with HIV infection,
peripheral blood stem cell mobilisation.
2.
BEFORE YOU USE NIVESTIM
if you are allergic (hypersensitive) to filgrastim or any of the other ingredients of Nivestim
.
Take special care with Nivestim
-
if you are suffering from any other illness (especially if you think you may have an infection),
if you experience cough, fever and difficulty breathing. It could be due to a lung disorder (see section
4 “POSSIBLE SIDE EFFECTS”),
if you have sickle cell disease (an inherited blood disorder that affects red blood cells),
if you get left upper abdominal pain or pain at the tip of your shoulder. It could be a consequence
of a spleen disorder (see section 4 “POSSIBLE SIDE EFFECTS”).
You may need to have regular blood tests whilst being treated with Nivestim to count the number of
neutrophils and other white blood cells in your blood. This will tell your doctor how the treatment is working
and will also indicate if treatment needs to be continued.
Using other medicines
You should not receive Nivestim in the 24 hours before and the 24 hours after receiving chemotherapy.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including
medicines obtained without a prescription.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking any medicine.
Filgrastim has not been tested in pregnant women. It is important to tell your doctor if you are pregnant,
think you may be pregnant or plan to get pregnant, as your doctor may decide that you should not use this
medicine. Filgrastim could affect your ability to become pregnant or stay pregnant.
It is unknown whether filgrastim passes over to the breast milk. Therefore, your doctor may decide that
you should not use this medicine if you are breast-feeding.
Driving and using machines
Filgrastim has negligible influence on the ability to drive and use machines. If the patient is experiencing
fatigue, caution is advised when driving a car or operating machinery.
Important information about some of the ingredients of Nivestim
This medicine contains sorbitol (E420). If you have been told by your doctor that you have an intolerance to
some sugars (fructose), contact your doctor before taking this medicine. This medicine also contains sodium
less than 1mmol sodium (23 mg) per dose, i.e. essentially ‘sodium-free’.
Always use Nivestim exactly as your doctor tells you to.
This medicine is given by injection, either through an intravenous infusion (drip) or by a subcutaneous
injection into the tissue just under the skin.
If you are receiving this medicine by subcutaneous injection, your doctor may suggest that you learn how to
give yourself the injections. Your doctor or nurse will give you instructions on how to do this (see end of
leaflet for self administration information). Do not attempt to self-administer without this training. Some of
the information you require is given at the end of this leaflet, but proper treatment of your disease requires
close and constant co-operation with your doctor. The amount of Nivestim you need, will depend on the
condition you are taking Nivestim for and on your bodyweight.
Nivestim and neutropenia associated with chemotherapy
The usual dose for adults and children is 0.5 million units (5 micrograms) per kilogram of bodyweight each
day. For example, if you weigh 60 kg your daily dose will be 30 million units (300 micrograms). Your
treatment will usually last for about 14 days. In some disease types however, longer treatment lasting up to
about one month may be required.
Nivestim and bone marrow transplantation
The normal starting dose is 1 million units (10 micrograms) per kilogram of bodyweight each day given as
an infusion. For example, if you weigh 60 kg your daily dose will be 60 million units (600 micrograms). You
will normally receive your first dose of Nivestim at least 24 hours after your chemotherapy but within 24
hours of receiving your bone marrow transplantation. Your doctor may then test your blood to tell how well
your treatment is working and how long it should last.
Nivestim and severe chronic neutropenia
The normal starting dose is between 0.5 million (5 micrograms) and 1.2 million (12 micrograms) units per
kilogram bodyweight each day in a single or divided dose. Your doctor may then test your blood to see how
well your treatment is working and to find the dose that is best for you. Long-term treatment with Nivestim
is required for neutropenia.
Nivestim and neutropenia in patients with HIV infection
The normal starting dose is between 0.1 (1 micrograms) and 0.4 million units (4 micrograms) per kilogram
bodyweight each day. Your doctor may test your blood at regular intervals to see how well the treatment is
working and to decide on the dose necessary. Once the number of white cells in your blood have returned to
normal it may be possible to reduce the dose frequency to less than once per day. Long term treatment with
Nivestim may be required to maintain a normal number of white cells in your blood.
Nivestim and peripheral blood stem cell transplantation
If you are donating stem cells for yourself, the usual dose is 0.5 million (5 micrograms) to 1 million units
(10 micrograms) per kilogram bodyweight each day. Nivestim treatment will last for up to 2 weeks. Your
doctor will monitor your blood to determine the best time to collect the stem cells.
If you are acting as a stem cell donor for another person, the usual dose is 1 million units per kilogram
bodyweight each day. Nivestim treatment will last for 4 to 5 days.
If you use more Nivestim than you should
If you use more Nivestim than you should, contact your doctor or pharmacist as soon as possible.
If you forget to use Nivestim
If you have forgotten to inject a dose, speak to your doctor or pharmacist to find out when you should inject
the next dose. Do not use a double dose to make up for a forgotten injection.
If you stop using Nivestim
Your doctor will tell you when to stop using Nivestim. It is quite normal to have a number of courses of
Nivestim treatment.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Like all medicines, Nivestim can cause side effects, although not everyone gets them.
Allergic-type reactions to filgrastim, including skin rash, raised areas of the skin that itch and anaphylaxis
(weakness, drop in blood pressure, difficulty breathing and swelling of the face) have been reported. If you
think you are having this type of reaction, stop your Nivestim injection and get medical help immediately.
Increased spleen size and very rare cases of spleen ruptures have been reported. Some cases of rupture of the
spleen were fatal.
It is important that you contact your
doctor immediately
if you experience
pain in the upper left side of
the abdomen or left shoulder pain
since this may relate to a problem with your spleen.
It is also very important that you call your doctor if you think you may have an infection.
There are
many ways an infection may show itself. You should watch for a temperature of 37.8 °C or above, chills or
other signs of infection, such as a rash, sore throat, diarrhoea, ear-ache, difficult or painful breathing or
problems such as cough or wheezing. These symptoms could be signs of severe lung side effects, like
pneumonia and respiratory distress syndrome in adults, which may be fatal. If you have a fever or any of
these symptoms, contact your doctor immediately and go straight to your hospital.
If you have Sickle Cell Disease, make sure that you tell your doctor before you start taking Nivestim. Sickle
cell crisis has happened in some patients with Sickle Cell Disease who have been given filgrastim.
The frequency of possible side effects listed below is defined using the following convention:
Very common (affects more than 1 user in 10)
Common (affects 1 to 10 users in 100)
Uncommon (affects 1 to 10 users in 1,000)
Rare (affects 1 to 10 users in 10,000)
Very rare (affects less than 1 user in 10,000)
Very common side effects
•
Feeling or being sick
•
Bone and muscle pain
•
Nose bleeds
•
Decreased blood glucose levels
•
Raised level of some liver enzymes or altered blood chemicals. Your doctor will take blood tests for
these
•
Raised uric acid level which may present as gout
Common side effects
•
Fatigue
•
Generalised weakness
•
Headache
•
Constipation or diarrhoea
•
Loss of appetite
•
Inflammation and ulceration of the mouth and lining of the gut
•
Chest pain
•
Cough
•
Sore throat
•
Hair loss
•
Skin rash
•
Enlarged liver
•
Thinning of the bones
•
Injection site pain
•
Inflammation of the blood vessels
•
Reduction in platelets (cells involved in clotting) – which increases the risk of bleeding or bruising
Uncommon side effects
•
Unspecified pain
•
Blood or protein in your urine
Rare side effects
•
Problems with your blood vessels
The side effects that may be experienced by you if you are acting as a stem cell donor for another
person are:
Very common side effects
•
Headache
•
Bone or muscle pain
•
Changes in your white cells or blood platelets (your doctor will monitor for this by blood tests)
Common side effects
•
Raised levels of some liver enzymes (your doctor will monitor for this)
Uncommon side effects
•
Severe allergic reaction
•
Problems with your spleen
•
Raised uric acid levels which may present as gout
•
Worsening of existing rheumatoid arthritis
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your
doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Nivestim after the expiry date which is stated on the outer carton and on the pre-filled syringe
after EXP. The expiry date refers to the last day of that month.
Store and transport refrigerated (2°C – 8°C). Do not freeze. Keep the pre-filled syringe in the outer carton in
order to protect from light.
The syringe can be removed from the refrigerator and left at room temperature for a single period of
maximum 7 days (but not above 25°C).
Do not use Nivestim if you notice it is cloudy or there are particles in it.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
•
The active substance is filgrastim. Each ml contains 60 million units [MU] (600 micrograms) or
96 million units [MU] (960 micrograms) of filgrastim.
•
Nivestim 12 MU/ 0.2 ml solution for injection/ infusion: each pre-filled syringe contains 12 million
units (MU), 120 micrograms of filgrastim in 0.2 ml (corresponding to 0.6 mg/ml).
•
Nivestim 30 MU/ 0.5 ml solution for injection/ infusion: each pre-filled syringe contains 30 million
units (MU), 300 micrograms of filgrastim in 0.5 ml (corresponding to 0.6 mg/ml).
•
Nivestim 48 MU/ 0.5 ml solution for injection/ infusion: each pre-filled syringe contains 48 million
units (MU), 480 micrograms of filgrastim in 0.5 ml (corresponding to 0.96 mg/ml).
•
The other ingredients are acetic acid (glacial), sodium hydroxide, sorbitol E420, polysorbate 80, and
water for injections.
What Nivestim looks like and contents of the pack
Nivestim is a clear colourless solution for injection/ infusion in a glass pre-filled syringe with an injection
needle (stainless steel) with a needle guard. There are 1, 5 or 10 syringes in each pack.
Marketing Authorisation Holder
Hospira UK Limited
Queensway
Royal Leamington Spa
Warwickshire
CV31 3RW
United Kingdom
Tel: +44 (0)1926 820 820
Fax: +44 (0)1926 821 041
PLIVA Kraków, S.A.
ul. Mogilska 80
31-546 Kraków
Poland
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
Hospira Benelux BVBA
Tél/Tel: + 32 2 332 03 15
Luxembourg/Luxemburg
Hospira Benelux BVBA
Tél/Tel: + 32 2 332 03 15
България
Hospira UK Limited
Teл.: + 44 (0) 1926 820820
Magyarország
Pharmacenter Hungary Kft.
Tel.: + 36-1-209-5927
Česká republika
Movianto Česká republika s.r.o
Tel: + 420 548 134 400
Malta
Hospira UK Limited
Tel: + 44 (0) 1926 820820
Danmark
Hospira Nordic AB
Tlf: + 46 (0)8 672 85 00
Nederland
Hospira Benelux BVBA
Tel: + 32 2 332 03 15
Deutschland
Hospira Deutschland GmbH
Tel: + 49 (0) 89 43 77 77 0
Norge
Hospira Nordic AB
Tlf: + 46 (0)8 672 85 00
Eesti
Berren Medical, c/o Axellus OÜ
Österreich
Astro-Pharma Vertrieb und Handel von
pharmazeutischen Produkten GmbH
Tel: + 43 (0)1 961 93 13
Ελλάδα
Aenorasis S.A.
Τηλ: + 30 210 6136332
Polska
Zaklady Farmaceutyczne S.A.
Tel.: + 481 26178048
España
Hospira
Productos Farmacéuticos y Hospitalarios S.L.
Tel: + 34 914847100
Portugal
Hospira Portugal Lda
France
Hospira France
Tél: + 33 (0) 826 30 03 02
România
Hospira UK Limited
Tel: + 44 (0) 1926 820820
Ireland
Hospira Ireland Limited
Tel: + 353 (0) 1 2962102
Slovenija
Valentis Pharmaceuticals d.o.0
Tel: + 386 1 2000603
Ísland
Hospira Nordic AB
Sími: + 46 (0)8 672 85 00
Slovenská republika
Hospira UK Limited
Tel: + 44 (0) 1926 820820
Italia
Hospira Italia Srl
Tel: + 39 0812405912
Suomi/Finland
Hospira Nordic AB
Puh/Tel: + 46 (0)8 672 85 00
Κύπρος
Hospira UK Limited
Τηλ: + 44 (0) 1926 820820
Sverige
Hospira Nordic AB
Tel: + 46 (0)8 672 85 00
Latvija
Berren Medical, c/o Axellus SIA
Tel: + 371 721 1629
United Kingdom
Hospira UK Limited
Tel: + 44 (0) 1926 820820
Lietuva
Berren Medical, c/o Axellus UAB
Tel: + 370 5 231 0654
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency (EMA) web site:
http://www.ema.europa.eu
<-----------------------------------------------------------------------------------------------------------------------------
Information on self administration by the patient
This section contains information on how to give yourself an injection of Nivestim. It is important that you
do not try to give yourself the injection unless you have received special training from your doctor or nurse.
It is also important that you dispose of the syringe in a puncture-proof container. If you are not sure about
giving yourself the injection or you have any questions, please ask your doctor or nurse for help.
How do I administer m
y
Nivestim?
Nivestim is usually given once a day by injection, usually into the tissue just under the skin. This is known
as a subcutaneous injection.
Learning to give your own injections will mean that you will not have to wait at home for a nurse to call, nor
will you have to go to the hospital or clinic every day to receive your injections.
You will need to have your injections at about the same time every day. The most suitable places for
injection are:
the abdomen, except for the area around the navel.
It is better to change the injection site every day to avoid the risk of soreness at any one site.
Equipment required for administration
To give yourself a subcutaneous injection you will need the following items:
•
A new pre-filled syringe of Nivestim.
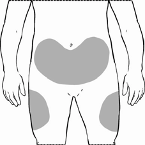
•
A sharps container (puncture proof container) for disposing of used syringes safely.
•
Antiseptic wipes (if recommended by your doctor or nurse).
How do I give my subcutaneous Nivestim injection?
1.
Try to self-inject at approximately the same time every day.
2.
Remove the Nivestim from the fridge and allow it to reach room temperature (approximately
25 °C). This will take 15–30 minutes. Check the date on the pack to make sure that the medicine
has not passed the expiry date. Make sure you have your sharps bin nearby.
3.
Find a comfortable well lit working place to give your injection and check the dose that you
have been prescribed.
4.
Wash your hands thoroughly with soap and water.
5.
Remove the syringe from the blister pack and check that the solution is clear, colourless and
practically free from visible particles. Do not use the Nivestim if the liquid has particles floating
in it or any of the liquid has leaked out of the syringe.
6.
Hold the syringe with the needle pointing upwards. Remove the protective cap from the injection
needle and expel any air from the syringe and needle by gently pressing the plunger upwards.
The syringe is now ready for use.
7.
Decide where to inject Nivestim - find a place on the front of your abdomen or the front of your
thigh. Choose a different injection site each time. Do not choose an area which is tender, red,
bruised or scarred. If your nurse or doctor recommends it, clean the area of skin with an
antiseptic wipe.
8.
Pinch a large area of skin, taking care not to touch the area you have cleaned.
9.
With your other hand, insert the needle at an approximate 45˚ angle.
10.
Pull the plunger back slightly to check if any blood appears in the syringe. If you do see blood
inside the syringe, remove the needle and re-insert it in a different site. Slowly push down the
plunger until all the contents of the syringe have been emptied.
11.
After injecting the solution remove the needle from the skin.
12.
Ensure needle guard covers the needle according to instructions for active needle guard or
passive needle guard below.
13.
Place the syringe into the sharps container. Do not try to replace the protective cap.
Remember
Most people can learn to give themselves a subcutaneous injection, but if you are experiencing a lot of
difficulty, please do not be afraid to ask for help and advice from your doctor or nurse.
Use of Active Ultrasafe Needle Guard
for
Nivestim 12 MU/ 0.2 ml solution for injection/ infusion
The pre-filled syringe has an UltraSafe Needle Guard attached in order to protect from needle stick injury.
When handling the pre-filled syringe, keep hands behind the needle.
1.
Perform the injection using the technique described above.
2.
When you have completed the injection, slide the needle guard forward until the needle is
completely covered (device ‘clicks’ into place).

Use of Ultrasafe Passive Needle Guard for
Nivestim 30 MU/ 0.5 ml solution for injection/ infusion and
Nivestim 48 MU/ 0.5 ml solution for injection/ infusion
The pre-filled syringe has an UltraSafe Needle Guard attached in order to protect from needle stick injury.
When handling the pre-filled syringe, keep hands behind the needle.
1.
Perform the injection using the technique described above.
2.
Depress the plunger while grasping the finger flange until the entire dose has been given. The
passive needle guard will NOT activate unless the ENTIRE dose has been given.
3.
Remove needle from your skin, then let go of the plunger and allow syringe to move up until the
entire needle is guarded and locks into place.
•
Keep used syringes out of the reach and sight of children
•
NEVER put used syringes into your normal household waste bin.
-----------------------------------------------------------------------------------------------------------------------------------
THE FOLLOWING INFORMATION IS INTENDED FOR MEDICAL OR HEALTHCARE
PROFESSIONALS ONLY:
Nivestim does not contain any preservative. In view of the possible risk of microbial contamination,
Nivestim syringes are for single use only.
Accidental exposure to freezing temperatures for up to 24 hours does not affect the stability of Nivestim. The
frozen pre-filled syringes can be thawed and then refrigerate for future use. If exposure has been greater then
24 hours or frozen more than once then Nivestim should NOT be used.














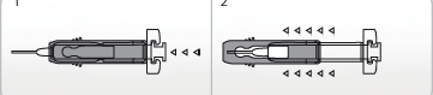











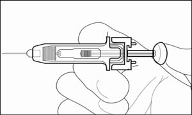

Nivestim should not be diluted with sodium chloride solution. This medicinal product must not be mixed
with other medicinal products except those mentioned below. Diluted filgrastim may be adsorbed to glass
and plastic materials except diluted, as mentioned below.
If required, Nivestim may be diluted in glucose 50 mg/ml (5%) solution for infusion. Dilution to a final
concentration less than 0.2 MU (2 micrograms) per ml is not recommended at any time. The solution should
be visually inspected prior to use. Only clear solutions without particles should be used. For patients treated
with filgrastim diluted to concentrations below 1.5 MU (15 micrograms) per ml, human serum albumin
(HSA) should be added to a final concentration of 2 mg/ml.
Example: In a final injection volume of 20 ml, total doses of filgrastim less than 30 MU (300 micrograms)
should be given with 0.2 ml of 200 mg/ml (20%) human albumin solution added. When diluted in glucose
50 mg/ml (5%) solution for infusion, Nivestim is compatible with glass and a variety of plastics including
PVC, polyolefin (a co-polymer of polypropylene and polyethylene) and polypropylene.
After dilution: Chemical and physical in-use stability of the diluted solution for infusion has been
demonstrated for 24 hours at 2 °C to 8 °C. From a microbiological point of view, the product should be used
immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility
of the user and would normally not be longer than 24 hours at 2 °C to 8 °C, unless dilution has taken place in
controlled and validated aseptic conditions.
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/nivestim.html
Copyright © 1995-2021 ITA all rights reserved.
|












































































































































































































































































































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































