Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
NovoSeven 1.2 mg (60 KIU) - powder and solvent for solution for injection
QUALITATIVE AND QUANTITATIVE COMPOSITION
NovoSeven is presented as powder and solvent for solution for injection containing 1.2 mg eptacog
alfa (activated) per vial (corresponds to 60 KIU/vial).
1 KIU equals 1000 IU (International Units).
eptacog alfa (activated) is recombinant coagulation factor VIIa with a molecular mass of
approximately 50,000 Dalton produced by genetic engineering from baby hamster kidney cells (BHK
Cells).
After reconstitution the product contains 0.6 mg/ml eptacog alfa (activated) when reconstituted with
solvent.
For a full list of excipients, see Section 6.1.
Powder and solvent for solution for injection.
4.1 Therapeutic indications
NovoSeven is indicated for the treatment of bleeding episodes and for the prevention of bleeding in
those undergoing surgery or invasive procedures in the following patient groups:
•
in patients with congenital haemophilia with inhibitors to coagulation factors VIII or IX > 5
Bethesda Units (BU)
in patients with congenital haemophilia who are expected to have a high anamnestic response to
factor VIII or factor IX administration
in patients with congenital FVII deficiency
in patients with Glanzmann’s thrombasthenia with antibodies to GP IIb - IIIa and/or HLA, and
with past or present refractoriness to platelet transfusions.
4.2 Posology and method of administration
Treatment should be initiated under the supervision of a physician experienced in the treatment of
haemophilia and/or bleeding disorders.
Haemophilia A or B with inhibitors or expected to have a high anamnestic response
Dose
NovoSeven should be given as early as possible after the start of a bleeding episode. The
recommended initial dose, administered by intravenous bolus injection, is 90 g per kg body weight.
Following the initial dose of NovoSeven further injections may be repeated. The duration of treatment
and the interval between injections will vary with the severity of the haemorrhage, the invasive
procedures or surgery being performed.
in patients with acquired haemophilia
Dosing in children
Current clinical experience does not warrant a general differentiation in dosing between children and
adults, although children have faster clearance than adults. Therefore, higher doses of rFVIIa may be
needed in paediatric patients to achieve similar plasma concentrations as in adult patients (see Section
5.2).
Dose interval
Initially 2 - 3 hours to obtain haemostasis.
If continued therapy is needed, the dose interval can be increased successively once effective
haemostasis is achieved to every 4, 6, 8 or 12 hours for as long as treatment is judged as being
indicated.
Mild to moderate bleeding episodes (including home therapy)
Early intervention has been shown to be efficacious in the treatment of mild to moderate joint, muscle
and mucocutaneous bleeds. Two dosing regimens can be recommended:
1) Two to three injections of 90 µg per kg body weight administered at three-hour intervals
If further treatment is required, one additional dose of 90 µg per kg body weight can be
administered
2) One single injection of 270 µg per kg body weight
The duration of the home therapy should not exceed 24 hours.
There is no clinical experience with administration of a single dose of 270 µg per kg body weight in
elderly patients.
Serious bleeding episodes
An initial dose of 90 µg per kg body weight is recommended and could be administered on the way to
the hospital where the patient is usually treated. The following dose varies according to the type and
severity of the haemorrhage. Dosing frequency should initially be every second hour until clinical
improvement is observed. If continued therapy is indicated, the dose interval can then be increased to
3 hours for 1 - 2 days. Thereafter, the dose interval can be increased successively to every 4, 6, 8 or
12 hours for as long as treatment is judged as being indicated. A major bleeding episode may be
treated for 2 - 3 weeks but can be extended beyond this if clinically warranted.
Invasive procedure/surgery
An initial dose of 90 µg per kg body weight should be given immediately before the intervention. The
dose should be repeated after 2 hours and then at 2 - 3 hour intervals for the first 24 - 48 hours
depending on the intervention performed and the clinical status of the patient. In major surgery, the
dose should be continued at 2 - 4 hour intervals for 6 - 7 days. The dose interval may then be increased
to 6 - 8 hours for another 2 weeks of treatment. Patients undergoing major surgery may be treated for
up to 2 - 3 weeks until healing has occurred.
Dose and dose interval
NovoSeven should be given as early as possible after the start of a bleeding episode. The
recommended initial dose, administered by intravenous bolus injection, is 90 µg per kg body weight.
Following the initial dose of NovoSeven further injections may be given if required. The duration of
treatment and the interval between injections will vary with the severity of the haemorrhage, the
invasive procedures or the surgery being performed.
The initial dose interval should be 2 - 3 hours. Once haemostasis has been achieved, the dose interval
can be increased successively to every 4, 6, 8 or 12 hours for as long as treatment is judged to be
indicated.
Dose, dose range and dose interval
The recommended dose range for treatment of bleeding episodes and for the prevention of bleeding in
patients undergoing surgery or invasive procedures is 15 - 30 μg per kg body weight every 4 - 6 hours
until haemostasis is achieved. Dose and frequency of injections should be adapted to each individual.
Glanzmann’s thrombasthenia
Dose, dose range and dose interval
The recommended dose for treatment of bleeding episodes and for the prevention of bleeding in
patients undergoing surgery or invasive procedures is 90 µg (range 80 - 120 µg) per kg body weight at
intervals of two hours (1.5 - 2.5 hours). At least three doses should be administered to secure effective
haemostasis. The recommended route of administration is bolus injection as lack of efficacy may
appear in connection with continuous infusion.
For those patients who are not refractory, platelets are the first line treatment for Glanzmann’s
thrombasthenia.
Reconstitute the solution as described under section 6.6 and administer as an intravenous bolus
injection over 2 - 5 minutes.
Monitoring of treatment – laboratory tests
There is no requirement for monitoring of NovoSeven therapy. Severity of bleeding condition and
clinical response to NovoSeven administration must guide dosing requirements.
After administration of NovoSeven, prothrombin time (PT) and activated partial thromboplastin time
(aPTT) have been shown to shorten, however no correlation has been demonstrated between PT and
aPTT and clinical efficacy of NovoSeven.
Hypersensitivity to the active substance, or to any of the excipients, or to mouse, hamster or bovine
protein.
4.4 Special warnings and precautions for use
In pathological conditions in which tissue factor may be expressed more extensively than considered
normal, there may be a potential risk of development of thrombotic events or induction of
Disseminated Intravascular Coagulation (DIC) in association with NovoSeven treatment.
Such situations may include patients with advanced atherosclerotic disease, crush injury, septicaemia
or DIC. Because of the risk of thromboembolic complications, caution should be excercised when
administering NovoSeven to patients with a history of coronary heart disease, to patients with liver
disease, to patients post-operatively, to neonates, or to patients at risk of thromboembolic phenomena
or disseminated intravascular coagulation. In each of these situations, the potential benefit of treatment
with NovoSeven should be weighed against the risk of these complications.
As recombinant coagulation factor VIIa NovoSeven may contain trace amounts of mouse IgG, bovine
IgG and other residual culture proteins (hamster and bovine serum proteins), the remote possibility
exists that patients treated with the product may develop hypersensitivity to these proteins. In such
cases treatment with antihistamines i.v. should be considered.
If allergic or anaphylactic-type reactions occur, the administration should be discontinued
immediately. In case of shock, standard medical treatment for shock should be implemented. Patients
should be informed of the early signs of hypersensitivity reactions. If such symptoms occur, the
patient should be advised to discontinue use of the product immediately and contact their physician.
In case of severe bleeds the product should be administered in hospitals preferably specialized in
treatment of haemophilia patients with coagulation factor VIII or IX inhibitors, or if not possible in
close collaboration with a physician specialized in haemophilia treatment.
If bleeding is not kept under control hospital care is mandatory. Patients/carers should inform the
physician/supervising hospital at the earliest possible opportunity about all usages of NovoSeven.
Factor VII deficient patients should be monitored for prothrombin time and factor VII coagulant
activity before and after administration of NovoSeven. In case the factor VIIa activity fails to reach the
expected level or bleeding is not controlled after treatment with the recommended doses, antibody
formation may be suspected and analysis for antibodies should be performed. The risk of thrombosis
in factor VII deficient patients treated with NovoSeven is unknown.
4.5 Interaction with other medicinal products and other forms of interaction
The risk of a potential interaction between NovoSeven and coagulation factor concentrates is
unknown. Simultaneous use of prothrombin complex concentrates, activated or not, should be
avoided.
Anti-fibrinolytics have been reported to reduce blood loss in association with surgery in haemophilia
patients, especially in orthopaedic surgery and surgery in regions rich in fibrinolytic activity, such as
the oral cavity. Experience with concomitant administration of anti-fibrinolytics and NovoSeven
treatment is however limited.
4.6 Pregnancy and lactation
As a precautionary measure, it is preferable to avoid use of NovoSeven during pregnancy. Data on a
limited number of exposed pregnancies within approved indications indicate no adverse effects of
rFVIIa on pregnancy or on the health of the foetus/new-born child. To date, no other relevant
epidemiological data are available. Animal studies do not indicate direct or indirect harmful effects
with respect to pregnancy, embryonal/foetal development, parturition or postnatal development (see
Section 5.3).
It is unknown whether rFVIIa is excreted in human breast milk. The excretion of rFVIIa in milk has
not been studied in animals. A decision on whether to continue/discontinue breast-feeding or to
continue/discontinue therapy with NovoSeven should be made taking into account the benefit of
breast-feeding to the child and the benefit of NovoSeven therapy to the woman.
4.7 Effects on ability to drive and use machines
No studies on the effect on the ability to drive and use machines have been performed.
The frequencies of both serious and non-serious adverse drug reactions are listed by system organ
classes in the table below.
Blood and the lymphatic syste
m disorders
Rare (> 1/10,000, < 1/1,000)
Disseminated intravascular coagulation and related laboratory
findings including elevated levels of D-dimer and decreased levels of
AT (see Section 4.4)
Rare (> 1/10,000, < 1/1,000)
Hypersensitivity, (see Sections 4.3 and 4.4)
Rare (> 1/10,000, < 1/1,000)
Rare (> 1/10,000, < 1/1,000)
Arterial thromboembolic events (myocardial infarction, cerebral
infarction, cerebral ischaemia, cerebral artery occlusion,
cerebrovascular accident, renal artery thrombosis, peripheral
ischaemia, peripheral arterial thrombosis and intestinal ischaemia)
Uncommon (> 1/1,000,
< 1/100)
Venous thromboembolic events (deep vein thrombosis, thrombosis at
i.v. site, pulmonary embolism, thromboembolic events of the liver
including portal vein thrombosis, renal vein thrombosis,
thrombophlebitis, superficial thrombophlebitis and intestinal
ischaemia)
Rare (> 1/10,000, < 1/1,000)
Gastrointestinal disorders
Rare (> 1/10,000, < 1/1,000)
Skin and subcutaneous disord
ers
Uncommon (> 1/1,000,
< 1/100)
Rash (including allergic dermatitis and rash erythematous)
General disorders and admini
stration site conditions
Uncommon (> 1/1,000,
< 1/100)
Therapeutic response decreased*
Rare (> 1/10,000, < 1/1,000)
Injection site reaction including injection site pain.








































Rare (> 1/10,000, < 1/1,000)
Increased fibrin degradation products
Increase in alanine aminotransferase, alkaline phosphatase, lactate
dehydrogenase and prothrombin.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Adverse drug reaction reported post-marketing only (i.e. not in clinical trials) are presented with a frequency
of not known.
* Lack of efficacy (therapeutic response decreased) has been reported. It is important that the dosage
regimen of NovoSeven is compliant with the recommended dosage as stated in Section 4.2.
Patients with acquired haemophilia
Clinical trials conducted in 61 patients with acquired haemophilia with a total of 100 treatment
episodes, showed that certain adverse drug reactions were reported more frequent (1% based on
treatment episodes): Arterial thromboembolic events (cerebral artery occlusion, cerebrovascular
accident), venous thromboembolic events (pulmonary embolism and deep vein thrombosis), angina
pectoris, nausea, pyrexia, erythematous rash and investigation of increased levels of fibrin degradation
products.
Inhibitory antibody formation
In post-marketing experience, there have been no reports of antibodies against NovoSeven or FVII in
patients with haemophilia A or B.
In clinical trials of patients with factor VII deficiency, formation of antibodies against NovoSeven and
FVII is the only adverse drug reaction reported (frequency: common (≥ 1/100 to < 1/10)). In some
cases, the antibodies showed inhibitory effect
in vitro
. Risk factors that may have contributed to
antibody development including previous treatment with human plasma and/or plasma-derived factor
VII, severe mutation of FVII gene, and overdose of NovoSeven, were present. Patients with factor VII
deficiency treated with NovoSeven should be monitored for factor VII antibodies, (see Section 4.4).
When NovoSeven is administered to patients outside approved indications, arterial thromboembolic
events are common (≥ 1/100 to < 1/10). A higher risk of arterial thromboembolic adverse events (5.6%
in patients treated with NovoSeven versus 3.0% in placebo-treated patients) has been shown in a meta-
analysis of pooled data from placebo-controlled trials conducted outside current approved indications
in various clinical settings, each of these having distinct patient characteristics and hence different
underlying risk profiles.
Safety and efficacy of NovoSeven have not been established outside the approved indications and
therefore NovoSeven should not be used.
Thromboembolic events may lead to cardiac arrest.
Dose limiting toxicities of NovoSeven have not been investigated in clinical trials.
Three cases of overdose have been reported in patients with haemophilia in 13 years. The only
complication reported in connection with an overdose was a slight transient increase in blood pressure















in a 16 year-old patient receiving 24 mg rFVIIa instead of 5.5 mg.
No cases of overdose have been reported in patients with acquired haemophilia or Glanzmann’s
thrombasthenia.
In patients with factor VII deficiency, where the recommended dose is 15 – 30 µg/kg rFVIIa, one
episode of overdose has been associated with a thrombotic event (occipital stroke) in an elderly
(> 80 year) male patient treated with 10 – 20 times the recommended dose. In addition, the
development of antibodies against NovoSeven and FVII has been associated with overdose in one
patient with factor VII deficiency.
The dose schedule should not be intentionally increased above the recommended doses due to the
absence of information on the additional risk that may be incurred.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Blood coagulation factors, ATC code: B02BD08
NovoSeven contains activated recombinant coagulation factor VII. The mechanism of action includes
the binding of factor VIIa to exposed tissue factor. This complex activates factor IX into factor IXa
and factor X into factor Xa, leading to the initial conversion of small amounts of prothrombin into
thrombin. Thrombin leads to the activation of platelets and factors V and VIII at the site of injury and
to the formation of the haemostatic plug by converting fibrinogen into fibrin. Pharmacological doses
of NovoSeven activate factor X directly on the surface of activated platelets, localized to the site of
injury, independently of tissue factor. This results in the conversion of prothrombin into large amounts
of thrombin independently of tissue factor. Accordingly, the pharmacodynamic effect of factor VIIa
gives rise to an increased local formation of factor Xa, thrombin and fibrin.
A theoretical risk for the development of systemic activation of the coagulation system in patients
suffering from underlying diseases predisposing them to DIC cannot be totally excluded.
5.2 Pharmacokinetic properties
Using the FVII clotting assay, the pharmacokinetics of NovoSeven were investigated in 35 healthy
Caucasian and Japanese subjects in a dose-escalation study. Subjects were stratified according to sex
and ethnic group and dosed with 40, 80 and 160 µg NovoSeven per kg body weight and/or placebo
(3 doses each). The pharmacokinetic profiles indicated dose proportionality. The pharmacokinetics
were similar across sex and ethnic groups. The mean steady state volume of distribution ranged from
130 to 165 ml/kg, the mean values of clearance ranged from 33.3 to 37.2 ml/h×kg, and the mean
terminal half-life ranged from 3.9 to 6.0 hours.
Haemophilia A and B with inhibitors
Using the FVIIa assay, the pharmacokinetic properties of NovoSeven were studied in 12 paediatric
(2 - 12 years) and 5 adult patients in non bleeding state. Dose proportionality was established in
children for the investigated doses of 90 and 180 µg per kg body weight, which is in accordance with
previous findings at lower doses (17.5 - 70 µg/kg rFVIIa). Mean clearance was approximately 50%
higher in paediatric patients relative to adults (78 versus 53 ml/h×kg), whereas the mean terminal half
life was determined to 2.3 hours in both groups. Mean volume of distribution at steady state was
196 ml/kg in paediatric patients versus 159 ml/kg in adults. Clearance appears related with age,
therefore in younger patients clearance may be increased by more than 50%.
Single dose pharmacokinetics of NovoSeven, 15 and 30 μg per kg body weight, showed no significant
difference between the two doses used with regard to dose-independent parameters: total body
clearance (70.8 - 79.1 ml/h×kg), volume of distribution at steady state (280 - 290 ml/kg), mean
residence time (3.75 - 3.80 h), and half-life (2.82 - 3.11 h). The mean
in vivo
plasma recovery was
approximately 20%.
Glanzmann’s thrombasthenia
Pharmacokinetics of NovoSeven in patients with Glanzmann’s thrombasthenia have not been
investigated, but are expected to be similar to the pharmacokinetics in haemophilia A and B patients.
5.3 Preclinical safety data
All findings in the preclinical safety programme were related to the pharmacological effect of
NovoSeven.
PHARMACEUTICAL PARTICULARS
Powder
Sodium chloride
Calcium chloride dihydrate
Glycylglycine
Polysorbate 80
Mannitol
Hydrochloric acid (for pH-adjustment)
Sodium hydroxide (for pH-adjustment)
Solvent
Water for injections
NovoSeven must not be mixed with infusion solutions or be given in a drip.
The shelf life is 3 years for the product packed for sale.
After reconstitution, chemical and physical stability has been demonstrated for 24 hours at 25C.
From a microbiological point of view, the product should be used immediately. If not used
immediately, storage time and storage conditions prior to use are the responsibility of the user, and
would normally not be longer than 24 hours at 2C - 8C, unless reconstitution has taken place in
controlled and validated aseptic conditions.
6.4 Special precautions for storage
Store in original package in order to protect from light
Do not freeze to prevent damage to the solvent vial.
Store NovoSeven in a refrigerator (2°C - 8°C)
6.5 Nature and contents of container
Vials for NovoSeven:
Glass closed with a bromobutyl rubber stopper covered with an aluminium cap.
The closed vials are equipped with a tamper-evident snap-off cap which is made of polypropylene.
Vials for solvent:
Glass closed with a bromobutyl rubber disc with teflon, covered with an aluminium cap.
The closed vials are equipped with a tamper-evident snap-off cap which is made of polypropylene.
Syringe for reconstitution and administration:
The sterile disposable syringe is made of polypropylene.
The NovoSeven package contains:
–
1 vial (2 ml) with white powder (NovoSeven) for solution for injection
1 vial (2 ml) with solvent (Water for injections) for reconstitution
1 sterile disposable syringe for reconstitution and administration
1 sterile infusion set for administration
2 alcohol swabs for cleansing the rubber stoppers on the vials
Package leaflet with instructions for use.
6.6 Special precautions for disposal and other handling
Always use an aseptic technique
Bring the NovoSeven powder and water vials to room temperature (but not above 37°C). You
can do this by holding them in your hands. Remove the plastic caps from the two vials. If the
caps are loose or missing, do not use the vials. Clean the rubber stoppers on the vials with the
alcohol swabs and allow them to dry before use.
Remove the protective paper from the vial adapter without taking the vial adapter out of the
protective cap. Attach the vial adapter to the solvent vial. Take care not to touch the spike on the
vial adapter.
Once attached, remove the protective cap from the vial adapter.
Pull the plunger to draw in a volume of air that is equal to the amount of solvent in the solvent
vial (ml equals cc on the syringe).
Screw the syringe tightly onto the vial adapter on the solvent vial. Inject air into the vial by
pushing the plunger until you feel a clear resistance.
Hold the syringe with the water vial upside down and pull the plunger to draw the water into the
syringe.
Remove the empty water vial by tipping the syringe with the vial adapter.
Click the vial adapter, still attached to the syringe, onto the powder vial. Hold the syringe
slightly tilted with the vial facing downwards. Push the plunger slowly to inject the water into
the powder vial. Make sure not to aim the stream of water directly at the NovoSeven powder as
this will cause foaming.
1 sterile vial adapter for reconstitution
Gently swirl the vial until all the powder is dissolved. Do not shake the vial as this will cause
foaming.
NovoSeven reconstituted solution is colourless and should be inspected visually for particulate matter
and discolouration prior to administration.
The enclosed disposable syringe is compatible with the reconstituted preparation, but
do not
store
reconstituted NovoSeven in plastic syringes.
It is recommended to use NovoSeven immediately after reconstitution.
Ensure that the plunger is pushed all the way in before turning the syringe upside down (it may
have been pushed out by the pressure in the syringe). Hold the syringe with the vial upside
down and pull the plunger to draw all the solution into the syringe.
Unscrew the vial adapter with the empty vial.
NovoSeven is now ready for injection. Locate a suitable site, and slowly inject NovoSeven into
a vein over a period of 2 - 5 minutes without removing the needle from the injection site.
Safely dispose of the syringe, vial adapter, vials, infusion set and any unused product. Any unused
product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 23 February 1996
Date of latest renewal: 23 February 2006
10.
DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency
(EMA)
http://www.ema.europa.eu/
NAME OF THE MEDICINAL PRODUCT
NovoSeven 2.4 mg (120 KIU) - powder and solvent for solution for injection
QUALITATIVE AND QUANTITATIVE COMPOSITION
NovoSeven is presented as powder and solvent for solution for injection containing 2.4 mg eptacog
alfa (activated) per vial (corresponds to 120 KIU/vial).
1 KIU equals 1000 IU (International Units).
eptacog alfa (activated) is recombinant coagulation factor VIIa with a molecular mass of
approximately 50,000 Dalton produced by genetic engineering from baby hamster kidney cells (BHK
Cells).
After reconstitution the product contains 0.6 mg/ml eptacog alfa (activated) when reconstituted with
solvent.
For a full list of excipients, see Section 6.1.
Powder and solvent for solution for injection.
4.1 Therapeutic indications
NovoSeven is indicated for the treatment of bleeding episodes and for the prevention of bleeding in
those undergoing surgery or invasive procedures in the following patient groups:
•
in patients with congenital haemophilia with inhibitors to coagulation factors VIII or IX > 5
Bethesda Units (BU)
in patients with congenital haemophilia who are expected to have a high anamnestic response to
factor VIII or factor IX administration
in patients with acquired haemophilia
in patients with congenital FVII deficiency
in patients with Glanzmann’s thrombasthenia with antibodies to GP IIb - IIIa and/or HLA, and
with past or present refractoriness to platelet transfusions.
4.2 Posology and method of administration
Treatment should be initiated under the supervision of a physician experienced in the treatment of
haemophilia and/or bleeding disorders.
Haemophilia A or B with inhibitors or expected to have a high anamnestic response
Dose
NovoSeven should be given as early as possible after the start of a bleeding episode. The
recommended initial dose, administered by intravenous bolus injection, is 90 µg per kg body weight.
Following the initial dose of NovoSeven further injections may be repeated. The duration of treatment
and the interval between injections will vary with the severity of the haemorrhage, the invasive
procedures or surgery being performed.
Dosing in children
Current clinical experience does not warrant a general differentiation in dosing between children and
adults, although children have faster clearance than adults. Therefore, higher doses of rFVIIa may be
needed in paediatric patients to achieve similar plasma concentrations as in adult patients (see Section
5.2).
Dose interval
Initially 2 - 3 hours to obtain haemostasis.
If continued therapy is needed, the dose interval can be increased successively once effective
haemostasis is achieved to every 4, 6, 8 or 12 hours for as long as treatment is judged as being
indicated.
Mild to moderate bleeding episodes (including home therapy)
Early intervention has been shown to be efficacious in the treatment of mild to moderate joint, muscle
and mucocutaneous bleeds. Two dosing regimens can be recommended:
1) Two to three injections of 90 µg per kg body weight administered at three-hour intervals
If further treatment is required, one additional dose of 90 µg per kg body weight can be
administered
2) One single injection of 270 µg per kg body weight
The duration of the home therapy should not exceed 24 hours.
There is no clinical experience with administration of a single dose of 270 µg per kg body weight in
elderly patients.
Serious bleeding episodes
An initial dose of 90 µg per kg body weight is recommended and could be administered on the way to
the hospital where the patient is usually treated. The following dose varies according to the type and
severity of the haemorrhage. Dosing frequency should initially be every second hour until clinical
improvement is observed. If continued therapy is indicated, the dose interval can then be increased to
3 hours for 1 - 2 days. Thereafter, the dose interval can be increased successively to every 4, 6, 8 or
12 hours for as long as treatment is judged as being indicated. A major bleeding episode may be
treated for 2 - 3 weeks but can be extended beyond this if clinically warranted.
Invasive procedure/surgery
An initial dose of 90 µg per kg body weight should be given immediately before the intervention. The
dose should be repeated after 2 hours and then at 2 - 3 hour intervals for the first 24 - 48 hours
depending on the intervention performed and the clinical status of the patient. In major surgery, the
dose should be continued at 2 - 4 hour intervals for 6 - 7 days. The dose interval may then be increased
to 6 - 8 hours for another 2 weeks of treatment. Patients undergoing major surgery may be treated for
up to 2 - 3 weeks until healing has occurred.
Dose and dose interval
NovoSeven should be given as early as possible after the start of a bleeding episode. The
recommended initial dose, administered by intravenous bolus injection, is 90 µg per kg body weight.
Following the initial dose of NovoSeven further injections may be given if required. The duration of
treatment and the interval between injections will vary with the severity of the haemorrhage, the
invasive procedures or the surgery being performed.
The initial dose interval should be 2 - 3 hours. Once haemostasis has been achieved, the dose interval
can be increased successively to every 4, 6, 8 or 12 hours for as long as treatment is judged to be
indicated.
Dose, dose range and dose interval
The recommended dose range for treatment of bleeding episodes and for the prevention of bleeding in
patients undergoing surgery or invasive procedures is 15 - 30 μg per kg body weight every 4 - 6 hours
until haemostasis is achieved. Dose and frequency of injections should be adapted to each individual.
Glanzmann’s thrombasthenia
Dose, dose range and dose interval
The recommended dose for treatment of bleeding episodes and for the prevention of bleeding in
patients undergoing surgery or invasive procedures is 90 µg (range 80 - 120 µg) per kg body weight at
intervals of two hours (1.5 - 2.5 hours). At least three doses should be administered to secure effective
haemostasis. The recommended route of administration is bolus injection as lack of efficacy may
appear in connection with continuous infusion.
For those patients who are not refractory, platelets are the first line treatment for Glanzmann’s
thrombasthenia.
Reconstitute the solution as described under section 6.6 and administer as an intravenous bolus
injection over 2 - 5 minutes.
Monitoring of treatment – laboratory tests
There is no requirement for monitoring of NovoSeven therapy. Severity of bleeding condition and
clinical response to NovoSeven administration must guide dosing requirements.
After administration of NovoSeven, prothrombin time (PT) and activated partial thromboplastin time
(aPTT) have been shown to shorten, however no correlation has been demonstrated between PT and
aPTT and clinical efficacy of NovoSeven.
Hypersensitivity to the active substance, or to any of the excipients, or to mouse, hamster or bovine
protein.
4.4 Special warnings and precautions for use
In pathological conditions in which tissue factor may be expressed more extensively than considered
normal, there may be a potential risk of development of thrombotic events or induction of
Disseminated Intravascular Coagulation (DIC) in association with NovoSeven treatment.
Such situations may include patients with advanced atherosclerotic disease, crush injury, septicaemia
or DIC. Because of the risk of thromboembolic complications, caution should be excercised when
administering NovoSeven to patients with a history of coronary heart disease, to patients with liver
disease, to patients post-operatively, to neonates, or to patients at risk of thromboembolic phenomena
or disseminated intravascular coagulation. In each of these situations, the potential benefit of treatment
with NovoSeven should be weighed against the risk of these complications.
As recombinant coagulation factor VIIa NovoSeven may contain trace amounts of mouse IgG, bovine
IgG and other residual culture proteins (hamster and bovine serum proteins), the remote possibility
exists that patients treated with the product may develop hypersensitivity to these proteins. In such
cases treatment with antihistamines i.v. should be considered.
If allergic or anaphylactic-type reactions occur, the administration should be discontinued
immediately. In case of shock, standard medical treatment for shock should be implemented. Patients
should be informed of the early signs of hypersensitivity reactions. If such symptoms occur, the
patient should be advised to discontinue use of the product immediately and contact their physician.
In case of severe bleeds the product should be administered in hospitals preferably specialized in
treatment of haemophilia patients with coagulation factor VIII or IX inhibitors, or if not possible in
close collaboration with a physician specialized in haemophilia treatment.
If bleeding is not kept under control hospital care is mandatory. Patients/carers should inform the
physician/supervising hospital at the earliest possible opportunity about all usages of NovoSeven.
Factor VII deficient patients should be monitored for prothrombin time and factor VII coagulant
activity before and after administration of NovoSeven. In case the factor VIIa activity fails to reach the
expected level or bleeding is not controlled after treatment with the recommended doses, antibody
formation may be suspected and analysis for antibodies should be performed. The risk of thrombosis
in factor VII deficient patients treated with NovoSeven is unknown.
4.5 Interaction with other medicinal products and other forms of interaction
The risk of a potential interaction between NovoSeven and coagulation factor concentrates is
unknown. Simultaneous use of prothrombin complex concentrates, activated or not, should be
avoided.
Anti-fibrinolytics have been reported to reduce blood loss in association with surgery in haemophilia
patients, especially in orthopaedic surgery and surgery in regions rich in fibrinolytic activity, such as
the oral cavity. Experience with concomitant administration of anti-fibrinolytics and NovoSeven
treatment is however limited.
4.6 Pregnancy and lactation
As a precautionary measure, it is preferable to avoid use of NovoSeven during pregnancy. Data on a
limited number of exposed pregnancies within approved indications indicate no adverse effects of
rFVIIa on pregnancy or on the health of the foetus/new-born child. To date, no other relevant
epidemiological data are available. Animal studies do not indicate direct or indirect harmful effects
with respect to pregnancy, embryonal/foetal development, parturition or postnatal development (see
Section 5.3).
It is unknown whether rFVIIa is excreted in human breast milk. The excretion of rFVIIa in milk has
not been studied in animals. A decision on whether to continue/discontinue breast-feeding or to
continue/discontinue therapy with NovoSeven should be made taking into account the benefit of
breast-feeding to the child and the benefit of NovoSeven therapy to the woman.
4.7 Effects on ability to drive and use machines
No studies on the effect on the ability to drive and use machines have been performed.
The frequencies of both serious and non-serious adverse drug reactions are listed by system organ
classes in the table below.
Blood and the lymphatic system disord
ers
Rare (> 1/10,000, < 1/1,000)
Disseminated intravascular coagulation and related laboratory
findings including elevated levels of D-dimer and decreased
levels of AT (see Section 4.4)
Rare (> 1/10,000, < 1/1,000)
Hypersensitivity, (see Sections 4.3 and 4.4)
Rare (> 1/10,000, < 1/1,000)
Rare (> 1/10,000, < 1/1,000)
Arterial thromboembolic events (myocardial infarction, cerebral
infarction, cerebral ischaemia, cerebral artery occlusion,
cerebrovascular accident, renal artery thrombosis, peripheral
ischaemia, peripheral arterial thrombosis and intestinal
ischaemia)
Uncommon (> 1/1,000, < 1/100)
Venous thromboembolic events (deep vein thrombosis,
thrombosis at i.v. site, pulmonary embolism, thromboembolic
events of the liver including portal vein thrombosis, renal vein
thrombosis, thrombophlebitis, superficial thrombophlebitis and
intestinal ischaemia)
Rare (> 1/10,000, < 1/1,000)
Gastrointestinal disorders
Rare (> 1/10,000, < 1/1,000)
Skin and subcutaneous disorders
Uncommon (> 1/1,000, < 1/100)
Rash (including allergic dermatitis and rash erythematous)
General disorders and administration
site conditions
Uncommon (> 1/1,000, < 1/100)
Therapeutic response decreased*




























Rare (> 1/10,000, < 1/1,000)
Injection site reaction including injection site pain.
Rare (> 1/10,000, < 1/1,000)
Increased fibrin degradation products
Increase in alanine aminotransferase, alkaline phosphatase, lactate
dehydrogenase and prothrombin.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Adverse drug reaction reported post-marketing only (i.e. not in clinical trials) are presented with a frequency of
not known.
* Lack of efficacy (therapeutic response decreased) has been reported. It is important that the dosage
regimen of NovoSeven is compliant with the recommended dosage as stated in Section 4.2.
Patients with acquired haemophilia
Clinical trials conducted in 61 patients with acquired haemophilia with a total of 100 treatment
episodes, showed that certain adverse drug reactions were reported more frequent (1% based on
treatment episodes): Arterial thromboembolic events (cerebral artery occlusion, cerebrovascular
accident), venous thromboembolic events (pulmonary embolism and deep vein thrombosis), angina
pectoris, nausea, pyrexia, erythematous rash and investigation of increased levels of fibrin degradation
products.
Inhibitory antibody formation
In post-marketing experience, there have been no reports of antibodies against NovoSeven or FVII in
patients with haemophilia A or B.
In clinical trials of patients with factor VII deficiency, formation of antibodies against NovoSeven and
FVII is the only adverse drug reaction reported (frequency: common (≥ 1/100 to < 1/10)). In some
cases, the antibodies showed inhibitory effect
in vitro
. Risk factors that may have contributed to
antibody development including previous treatment with human plasma and/or plasma-derived factor
VII, severe mutation of FVII gene, and overdose of NovoSeven, were present. Patients with factor VII
deficiency treated with NovoSeven should be monitored for factor VII antibodies, (see Section 4.4).
When NovoSeven is administered to patients outside approved indications, arterial thromboembolic
events are common (≥ 1/100 to < 1/10). A higher risk of arterial thromboembolic adverse events (5.6%
in patients treated with NovoSeven versus 3.0% in placebo-treated patients) has been shown in a meta-
analysis of pooled data from placebo-controlled trials conducted outside current approved indications
in various clinical settings, each of these having distinct patient characteristics and hence different
underlying risk profiles.
Safety and efficacy of NovoSeven have not been established outside the approved indications and
therefore NovoSeven should not be used.
Thromboembolic events may lead to cardiac arrest.
Dose limiting toxicities of NovoSeven have not been investigated in clinical trials.
Three cases of overdose have been reported in patients with haemophilia in 13 years. The only















complication reported in connection with an overdose was a slight transient increase in blood pressure
in a 16 year-old patient receiving 24 mg of rFVIIa instead of 5.5 mg.
No cases of overdose have been reported in patients with acquired haemophilia or Glanzmann’s
thrombasthenia.
In patients with factor VII deficiency, where the recommended dose is 15 – 30 µg/kg rFVIIa, one
episode of overdose has been associated with a thrombotic event (occipital stroke) in an elderly
(> 80 year) male patient treated with 10 – 20 times the recommended dose. In addition, the
development of antibodies against NovoSeven and FVII has been associated with overdose in one
patient with factor VII deficiency.
The dose schedule should not be intentionally increased above the recommended doses due to the
absence of information on the additional risk that may be incurred.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Blood coagulation factors, ATC code: B02BD08
NovoSeven contains activated recombinant coagulation factor VII. The mechanism of action includes
the binding of factor VIIa to exposed tissue factor. This complex activates factor IX into factor IXa
and factor X into factor Xa, leading to the initial conversion of small amounts of prothrombin into
thrombin. Thrombin leads to the activation of platelets and factors V and VIII at the site of injury and
to the formation of the haemostatic plug by converting fibrinogen into fibrin. Pharmacological doses
of NovoSeven activate factor X directly on the surface of activated platelets, localized to the site of
injury, independently of tissue factor. This results in the conversion of prothrombin into large amounts
of thrombin independently of tissue factor. Accordingly, the pharmacodynamic effect of factor VIIa
gives rise to an increased local formation of factor Xa, thrombin and fibrin.
A theoretical risk for the development of systemic activation of the coagulation system in patients
suffering from underlying diseases predisposing them to DIC cannot be totally excluded.
5.2 Pharmacokinetic properties
Using the FVII clotting assay, the pharmacokinetics of NovoSeven were investigated in 35 healthy
Caucasian and Japanese subjects in a dose-escalation study. Subjects were stratified according to sex
and ethnic group and dosed with 40, 80 and 160 µg NovoSeven per kg body weight and/or placebo
(3 doses each). The pharmacokinetic profiles indicated dose proportionality. The pharmacokinetics
were similar across sex and ethnic groups. The mean steady state volume of distribution ranged from
130 to 165 ml/kg, the mean values of clearance ranged from 33.3 to 37.2 ml/h×kg, and the mean
terminal half-life ranged from 3.9 to 6.0 hours.
Haemophilia A and B with inhibitors
Using the FVIIa assay, the pharmacokinetic properties of NovoSeven were studied in 12 paediatric
(2 - 12 years) and 5 adult patients in non bleeding state. Dose proportionality was established in
children for the investigated doses of 90 and 180 µg per kg body weight, which is in accordance with
previous findings at lower doses (17.5 - 70 µg/kg rFVIIa). Mean clearance was approximately 50%
higher in paediatric patients relative to adults (78 versus 53 ml/h×kg), whereas the mean terminal half
life was determined to 2.3 hours in both groups. Mean volume of distribution at steady state was
196 ml/kg in paediatric patients versus 159 ml/kg in adults. Clearance appears related with age,
therefore in younger patients clearance may be increased by more than 50%.
Single dose pharmacokinetics of NovoSeven, 15 and 30 μg per kg body weight, showed no significant
difference between the two doses used with regard to dose-independent parameters: total body
clearance (70.8 - 79.1 ml/h×kg), volume of distribution at steady state (280 - 290 ml/kg), mean
residence time (3.75 - 3.80 h), and half-life (2.82 - 3.11 h). The mean
in vivo
plasma recovery was
approximately 20%.
Glanzmann’s thrombasthenia
Pharmacokinetics of NovoSeven in patients with Glanzmann’s thrombasthenia have not been
investigated, but are expected to be similar to the pharmacokinetics in haemophilia A and B patients.
5.3 Preclinical safety data
All findings in the preclinical safety programme were related to the pharmacological effect of
NovoSeven.
PHARMACEUTICAL PARTICULARS
Powder
Sodium chloride
Calcium chloride dihydrate
Glycylglycine
Polysorbate 80
Mannitol
Hydrochloric acid (for pH-adjustment)
Sodium hydroxide (for pH-adjustment)
Solvent
Water for injections
NovoSeven must not be mixed with infusion solutions or be given in a drip.
The shelf life is 3 years for the product packed for sale.
After reconstitution, chemical and physical stability has been demonstrated for 24 hours at 25°C.
From a microbiological point of view, the product should be used immediately. If not used
immediately, storage time and storage conditions prior to use are the responsibility of the user, and
would normally not be longer than 24 hours at 2°C - 8°C, unless reconstitution has taken place in
controlled and validated aseptic conditions.
6.4 Special precautions for storage
Store NovoSeven in a refrigerator (2°C - 8°C)
Store in original package in order to protect from light
Do not freeze to prevent damage to the solvent vial.
6.5 Nature and contents of container
Vials for NovoSeven:
Glass closed with a bromobutyl rubber stopper covered with an aluminium cap.
The closed vials are equipped with a tamper-evident snap-off cap which is made of polypropylene.
Vials for solvent:
Glass closed with a bromobutyl rubber disc with teflon, covered with an aluminium cap.
The closed vials are equipped with a tamper-evident snap-off cap which is made of polypropylene.
Syringe for reconstitution and administration:
The sterile disposable syringe is made of polypropylene.
The NovoSeven package contains:
–
1 vial (5 ml) with white powder (NovoSeven) for solution for injection
1 vial (5 ml) with solvent (Water for injections) for reconstitution
1 sterile disposable syringe for reconstitution and administration
1 sterile infusion set for administration
2 alcohol swabs for cleansing the rubber stoppers on the vials
Package leaflet with instructions for use.
6.6 Special precautions for disposal and other handling
Always use an aseptic technique
Bring the NovoSeven powder and water vials to room temperature (but not above 37°C). You
can do this by holding them in your hands. Remove the plastic caps from the two vials. If the
caps are loose or missing, do not use the vials. Clean the rubber stoppers on the vials with the
alcohol swabs and allow them to dry before use.
Remove the protective paper from the vial adapter without taking the vial adapter out of the
protective cap. Attach the vial adapter to the solvent vial. Take care not to touch the spike on the
vial adapter.
Once attached, remove the protective cap from the vial adapter.
Pull the plunger to draw in a volume of air that is equal to the amount of solvent in the solvent
vial (ml equals cc on the syringe).
Screw the syringe tightly onto the vial adapter on the solvent vial. Inject air into the vial by
pushing the plunger until you feel a clear resistance.
Hold the syringe with the water vial upside down and pull the plunger to draw the water into the
syringe.
Remove the empty water vial by tipping the syringe with the vial adapter.
Click the vial adapter, still attached to the syringe, onto the powder vial. Hold the syringe
slightly tilted with the vial facing downwards. Push the plunger slowly to inject the water into
the powder vial. Make sure not to aim the stream of water directly at the NovoSeven powder as
this will cause foaming.
1 sterile vial adapter for reconstitution
Gently swirl the vial until all the powder is dissolved. Do not shake the vial as this will cause
foaming.
NovoSeven reconstituted solution is colourless and should be inspected visually for particulate matter
and discolouration prior to administration.
The enclosed disposable syringe is compatible with the reconstituted preparation, but
do not
store
reconstituted NovoSeven in plastic syringes.
It is recommended to use NovoSeven immediately after reconstitution.
Ensure that the plunger is pushed all the way in before turning the syringe upside down (it may
have been pushed out by the pressure in the syringe). Hold the syringe with the vial upside
down and pull the plunger to draw all the solution into the syringe.
Unscrew the vial adapter with the empty vial.
NovoSeven is now ready for injection. Locate a suitable site, and slowly inject NovoSeven into
a vein over a period of 2 - 5 minutes without removing the needle from the injection site.
Safely dispose of the syringe, vial adapter, vials, infusion set and any unused product. Any unused
product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 23 February 1996
Date of latest renewal: 23 February 2006
10. DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency
(EMA)
http://www.ema.europa.eu/
NAME OF THE MEDICINAL PRODUCT
NovoSeven 4.8 mg (240 KIU) - powder and solvent for solution for injection
QUALITATIVE AND QUANTITATIVE COMPOSITION
NovoSeven is presented as powder and solvent for solution for injection containing 4.8 mg eptacog
alfa (activated) per vial (corresponds to 240 KIU/vial).
1 KIU equals 1000 IU (International Units).
eptacog alfa (activated) is recombinant coagulation factor VIIa with a molecular mass of
approximately 50,000 Dalton produced by genetic engineering from baby hamster kidney cells (BHK
Cells).
After reconstitution the product contains 0.6 mg/ml eptacog alfa (activated) when reconstituted with
solvent.
For a full list of excipients, see Section 6.1.
Powder and solvent for solution for injection.
4.1 Therapeutic indications
NovoSeven is indicated for the treatment of bleeding episodes and for the prevention of bleeding in
those undergoing surgery or invasive procedures in the following patient groups:
•
in patients with congenital haemophilia with inhibitors to coagulation factors VIII or IX > 5
Bethesda Units (BU)
in patients with congenital haemophilia who are expected to have a high anamnestic response to
factor VIII or factor IX administration
in patients with acquired haemophilia
in patients with congenital FVII deficiency
in patients with Glanzmann’s thrombasthenia with antibodies to GP IIb - IIIa and/or HLA, and
with past or present refractoriness to platelet transfusions.
4.2 Posology and method of administration
Treatment should be initiated under the supervision of a physician experienced in the treatment of
haemophilia and/or bleeding disorders.
Haemophilia A or B with inhibitors or expected to have a high anamnestic response
Dose
NovoSeven should be given as early as possible after the start of a bleeding episode. The
recommended initial dose, administered by intravenous bolus injection, is 90 µg per kg body weight.
Following the initial dose of NovoSeven further injections may be repeated. The duration of treatment
and the interval between injections will vary with the severity of the haemorrhage, the invasive
procedures or surgery being performed.
Dosing in children
Current clinical experience does not warrant a general differentiation in dosing between children and
adults, although children have faster clearance than adults. Therefore, higher doses of rFVIIa may be
needed in paediatric patients to achieve similar plasma concentrations as in adult patients (see Section
5.2).
Dose interval
Initially 2 - 3 hours to obtain haemostasis.
If continued therapy is needed, the dose interval can be increased successively once effective
haemostasis is achieved to every 4, 6, 8 or 12 hours for as long as treatment is judged as being
indicated.
Mild to moderate bleeding episodes (including home therapy)
Early intervention has been shown to be efficacious in the treatment of mild to moderate joint, muscle
and mucocutaneous bleeds. Two dosing regimens can be recommended:
1) Two to three injections of 90 µg per kg body weight administered at three-hour intervals
If further treatment is required, one additional dose of 90 µg per kg body weight can be
administered
2) One single injection of 270 µg per kg body weight
The duration of the home therapy should not exceed 24 hours.
There is no clinical experience with administration of a single dose of 270 µg per kg body weight in
elderly patients.
Serious bleeding episodes
An initial dose of 90 µg per kg body weight is recommended and could be administered on the way to
the hospital where the patient is usually treated. The following dose varies according to the type and
severity of the haemorrhage. Dosing frequency should initially be every second hour until clinical
improvement is observed. If continued therapy is indicated, the dose interval can then be increased to
3 hours for 1 - 2 days. Thereafter, the dose interval can be increased successively to every 4, 6, 8 or
12 hours for as long as treatment is judged as being indicated. A major bleeding episode may be
treated for 2 - 3 weeks but can be extended beyond this if clinically warranted.
Invasive procedure/surgery
An initial dose of 90 µg per kg body weight should be given immediately before the intervention. The
dose should be repeated after 2 hours and then at 2 - 3 hour intervals for the first 24 - 48 hours
depending on the intervention performed and the clinical status of the patient. In major surgery, the
dose should be continued at 2 - 4 hour intervals for 6 - 7 days. The dose interval may then be increased
to 6 - 8 hours for another 2 weeks of treatment. Patients undergoing major surgery may be treated for
up to 2 - 3 weeks until healing has occurred.
Dose and dose interval
NovoSeven should be given as early as possible after the start of a bleeding episode. The
recommended initial dose, administered by intravenous bolus injection, is 90 µg per kg body weight.
Following the initial dose of NovoSeven further injections may be given if required. The duration of
treatment and the interval between injections will vary with the severity of the haemorrhage, the
invasive procedures or the surgery being performed.
The initial dose interval should be 2 - 3 hours. Once haemostasis has been achieved, the dose interval
can be increased successively to every 4, 6, 8 or 12 hours for as long as treatment is judged to be
indicated.
Dose, dose range and dose interval
The recommended dose range for treatment of bleeding episodes and for the prevention of bleeding in
patients undergoing surgery or invasive procedures is 15 - 30 μg per kg body weight every 4 - 6 hours
until haemostasis is achieved. Dose and frequency of injections should be adapted to each individual.
Glanzmann’s thrombasthenia
Dose, dose range and dose interval
The recommended dose for treatment of bleeding episodes and for the prevention of bleeding in
patients undergoing surgery or invasive procedures is 90 µg (range 80 - 120 µg) per kg body weight at
intervals of two hours (1.5 - 2.5 hours). At least three doses should be administered to secure effective
haemostasis. The recommended route of administration is bolus injection as lack of efficacy may
appear in connection with continuous infusion.
For those patients who are not refractory, platelets are the first line treatment for Glanzmann’s
thrombasthenia.
Reconstitute the solution as described under section 6.6 and administer as an intravenous bolus
injection over 2 - 5 minutes.
Monitoring of treatment – laboratory tests
There is no requirement for monitoring of NovoSeven therapy. Severity of bleeding condition and
clinical response to NovoSeven administration must guide dosing requirements.
After administration of NovoSeven, prothrombin time (PT) and activated partial thromboplastin time
(aPTT) have been shown to shorten, however no correlation has been demonstrated between PT and
aPTT and clinical efficacy of NovoSeven.
Hypersensitivity to the active substance, or to any of the excipients, or to mouse, hamster or bovine
protein.
4.4 Special warnings and precautions for use
In pathological conditions in which tissue factor may be expressed more extensively than considered
normal, there may be a potential risk of development of thrombotic events or induction of
Disseminated Intravascular Coagulation (DIC) in association with NovoSeven treatment.
Such situations may include patients with advanced atherosclerotic disease, crush injury, septicaemia
or DIC. Because of the risk of thromboembolic complications, caution should be excercised when
administering NovoSeven to patients with a history of coronary heart disease, to patients with liver
disease, to patients post-operatively, to neonates, or to patients at risk of thromboembolic phenomena
or disseminated intravascular coagulation. In each of these situations, the potential benefit of treatment
with NovoSeven should be weighed against the risk of these complications.
As recombinant coagulation factor VIIa NovoSeven may contain trace amounts of mouse IgG, bovine
IgG and other residual culture proteins (hamster and bovine serum proteins), the remote possibility
exists that patients treated with the product may develop hypersensitivity to these proteins. In such
cases treatment with antihistamines i.v. should be considered.
If allergic or anaphylactic-type reactions occur, the administration should be discontinued
immediately. In case of shock, standard medical treatment for shock should be implemented. Patients
should be informed of the early signs of hypersensitivity reactions. If such symptoms occur, the
patient should be advised to discontinue use of the product immediately and contact their physician.
In case of severe bleeds the product should be administered in hospitals preferably specialized in
treatment of haemophilia patients with coagulation factor VIII or IX inhibitors, or if not possible in
close collaboration with a physician specialized in haemophilia treatment.
If bleeding is not kept under control hospital care is mandatory. Patients/carers should inform the
physician/supervising hospital at the earliest possible opportunity about all usages of NovoSeven.
Factor VII deficient patients should be monitored for prothrombin time and factor VII coagulant
activity before and after administration of NovoSeven. In case the factor VIIa activity fails to reach the
expected level or bleeding is not controlled after treatment with the recommended doses, antibody
formation may be suspected and analysis for antibodies should be performed. The risk of thrombosis
in factor VII deficient patients treated with NovoSeven is unknown.
4.5 Interaction with other medicinal products and other forms of interaction
The risk of a potential interaction between NovoSeven and coagulation factor concentrates is
unknown. Simultaneous use of prothrombin complex concentrates, activated or not, should be
avoided.
Anti-fibrinolytics have been reported to reduce blood loss in association with surgery in haemophilia
patients, especially in orthopaedic surgery and surgery in regions rich in fibrinolytic activity, such as
the oral cavity. Experience with concomitant administration of anti-fibrinolytics and NovoSeven
treatment is however limited.
4.6 Pregnancy and lactation
As a precautionary measure, it is preferable to avoid use of NovoSeven during pregnancy. Data on a
limited number of exposed pregnancies within approved indications indicate no adverse effects of
rFVIIa on pregnancy or on the health of the foetus/new-born child. To date, no other relevant
epidemiological data are available. Animal studies do not indicate direct or indirect harmful effects
with respect to pregnancy, embryonal/foetal development, parturition or postnatal development (see
Section 5.3).
It is unknown whether rFVIIa is excreted in human breast milk. The excretion of rFVIIa in milk has
not been studied in animals. A decision on whether to continue/discontinue breast-feeding or to
continue/discontinue therapy with NovoSeven should be made taking into account the benefit of
breast-feeding to the child and the benefit of NovoSeven therapy to the woman.
4.7 Effects on ability to drive and use machines
No studies on the effect on the ability to drive and use machines have been performed.
The frequencies of both serious and non-serious adverse drug reactions are listed by system organ
classes in the table below.
Blood and the lymphatic system di
sorders
Rare (> 1/10,000, < 1/1,000)
Disseminated intravascular coagulation and related laboratory
findings including elevated levels of D-dimer and decreased
levels of AT (see Section 4.4)
Rare (> 1/10,000, < 1/1,000)
Hypersensitivity, (see Sections 4.3 and 4.4)
Rare (> 1/10,000, < 1/1,000)
Rare (> 1/10,000, < 1/1,000)
Arterial thromboembolic events (myocardial infarction, cerebral
infarction, cerebral ischaemia, cerebral artery occlusion,
cerebrovascular accident, renal artery thrombosis, peripheral
ischaemia, peripheral arterial thrombosis and intestinal
ischaemia)
Uncommon (> 1/1,000, < 1/100)
Venous thromboembolic events (deep vein thrombosis,
thrombosis at i.v. site, pulmonary embolism, thromboembolic
events of the liver including portal vein thrombosis, renal vein
thrombosis, thrombophlebitis, superficial thrombophlebitis and
intestinal ischaemia)
Rare (> 1/10,000, < 1/1,000)
Gastrointestinal disorders
Rare (> 1/10,000, < 1/1,000)
Skin and subcutaneous disorders
Uncommon (> 1/1,000, < 1/100)
Rash (including allergic dermatitis and rash erythematous)
General disorders and administrat
ion site conditions
Uncommon (> 1/1,000, < 1/100)
Therapeutic response decreased*




























Rare (> 1/10,000, < 1/1,000)
Injection site reaction including injection site pain.
Rare (> 1/10,000, < 1/1,000)
Increased fibrin degradation products
Increase in alanine aminotransferase, alkaline phosphatase,
lactate dehydrogenase and prothrombin.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Adverse drug reaction reported post-marketing only (i.e. not in clinical trials) are presented with a frequency
of not known.
* Lack of efficacy (therapeutic response decreased) has been reported. It is important that the dosage
regimen of NovoSeven is compliant with the recommended dosage as stated in Section 4.2.
Patients with acquired haemophilia
Clinical trials conducted in 61 patients with acquired haemophilia with a total of 100 treatment
episodes, showed that certain adverse drug reactions were reported more frequent (1% based on
treatment episodes): Arterial thromboembolic events (cerebral artery occlusion, cerebrovascular
accident), venous thromboembolic events (pulmonary embolism and deep vein thrombosis), angina
pectoris, nausea, pyrexia, erythematous rash and investigation of increased levels of fibrin degradation
products.
Inhibitory antibody formation
In post-marketing experience, there have been no reports of antibodies against NovoSeven or FVII in
patients with haemophilia A or B.
In clinical trials of patients with factor VII deficiency, formation of antibodies against NovoSeven and
FVII is the only adverse drug reaction reported (frequency: common (≥ 1/100 to < 1/10)). In some
cases, the antibodies showed inhibitory effect
in vitro
. Risk factors that may have contributed to
antibody development including previous treatment with human plasma and/or plasma-derived factor
VII, severe mutation of FVII gene, and overdose of NovoSeven, were present. Patients with factor VII
deficiency treated with NovoSeven should be monitored for factor VII antibodies, (see Section 4.4).
When NovoSeven is administered to patients outside approved indications, arterial thromboembolic
events are common (≥ 1/100 to < 1/10). A higher risk of arterial thromboembolic adverse events (5.6%
in patients treated with NovoSeven versus 3.0% in placebo-treated patients) has been shown in a meta-
analysis of pooled data from placebo-controlled trials conducted outside current approved indications
in various clinical settings, each of these having distinct patient characteristics and hence different
underlying risk profiles.
Safety and efficacy of NovoSeven have not been established outside the approved indications and
therefore NovoSeven should not be used.
Thromboembolic events may lead to cardiac arrest.
Dose limiting toxicities of NovoSeven have not been investigated in clinical trials.
Three cases of overdose have been reported in patients with haemophilia in 13 years. The only












complication reported in connection with an overdose was a slight transient increase in blood pressure
in a 16 year-old patient receiving 24 mg rFVIIa instead of 5.5 mg.
No cases of overdose have been reported in patients with acquired haemophilia or Glanzmann’s
thrombasthenia.
In patients with factor VII deficiency, where the recommended dose is 15 – 30 µg/kg rFVIIa, one
episode of overdose has been associated with a thrombotic event (occipital stroke) in an elderly
(> 80 year) male patient treated with 10 – 20 times the recommended dose. In addition, the
development of antibodies against NovoSeven and FVII has been associated with overdose in one
patient with factor VII deficiency.
The dose schedule should not be intentionally increased above the recommended doses due to the
absence of information on the additional risk that may be incurred.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Blood coagulation factors, ATC code: B02BD08
NovoSeven contains activated recombinant coagulation factor VII. The mechanism of action includes
the binding of factor VIIa to exposed tissue factor. This complex activates factor IX into factor IXa
and factor X into factor Xa, leading to the initial conversion of small amounts of prothrombin into
thrombin. Thrombin leads to the activation of platelets and factors V and VIII at the site of injury and
to the formation of the haemostatic plug by converting fibrinogen into fibrin. Pharmacological doses
of NovoSeven activate factor X directly on the surface of activated platelets, localized to the site of
injury, independently of tissue factor. This results in the conversion of prothrombin into large amounts
of thrombin independently of tissue factor. Accordingly, the pharmacodynamic effect of factor VIIa
gives rise to an increased local formation of factor Xa, thrombin and fibrin.
A theoretical risk for the development of systemic activation of the coagulation system in patients
suffering from underlying diseases predisposing them to DIC cannot be totally excluded.
5.2 Pharmacokinetic properties
Using the FVII clotting assay, the pharmacokinetics of NovoSeven were investigated in 35 healthy
Caucasian and Japanese subjects in a dose-escalation study. Subjects were stratified according to sex
and ethnic group and dosed with 40, 80 and 160 µg NovoSeven per kg body weight and/or placebo
(3 doses each). The pharmacokinetic profiles indicated dose proportionality. The pharmacokinetics
were similar across sex and ethnic groups. The mean steady state volume of distribution ranged from
130 to 165 ml/kg, the mean values of clearance ranged from 33.3 to 37.2 ml/h×kg, and the mean
terminal half-life ranged from 3.9 to 6.0 hours.
Haemophilia A and B with inhibitors
Using the FVIIa assay, the pharmacokinetic properties of NovoSeven were studied in 12 paediatric
(2 - 12 years) and 5 adult patients in non bleeding state. Dose proportionality was established in
children for the investigated doses of 90 and 180 µg per kg body weight, which is in accordance with
previous findings at lower doses (17.5 - 70 µg/kg rFVIIa). Mean clearance was approximately 50%
higher in paediatric patients relative to adults (78 versus 53 ml/h×kg), whereas the mean terminal half
life was determined to 2.3 hours in both groups. Mean volume of distribution at steady state was
196 ml/kg in paediatric patients versus 159 ml/kg in adults. Clearance appears related with age,
therefore in younger patients clearance may be increased by more than 50%.
Single dose pharmacokinetics of NovoSeven, 15 and 30 μg per kg body weight, showed no significant
difference between the two doses used with regard to dose-independent parameters: total body
clearance (70.8 - 79.1 ml/h×kg), volume of distribution at steady state (280 - 290 ml/kg), mean
residence time (3.75 - 3.80 h), and half-life (2.82 - 3.11 h). The mean
in vivo
plasma recovery was
approximately 20%.
Glanzmann’s thrombasthenia
Pharmacokinetics of NovoSeven in patients with Glanzmann’s thrombasthenia have not been
investigated, but are expected to be similar to the pharmacokinetics in haemophilia A and B patients.
5.3 Preclinical safety data
All findings in the preclinical safety programme were related to the pharmacological effect of
NovoSeven.
PHARMACEUTICAL PARTICULARS
Powder
Sodium chloride
Calcium chloride dihydrate
Glycylglycine
Polysorbate 80
Mannitol
Hydrochloric acid (for pH-adjustment)
Sodium hydroxide (for pH-adjustment)
Solvent
Water for injections
NovoSeven must not be mixed with infusion solutions or be given in a drip.
The shelf life is 3 years for the product packed for sale.
After reconstitution, chemical and physical stability has been demonstrated for 24 hours at 25°C.
From a microbiological point of view, the product should be used immediately. If not used
immediately, storage time and storage conditions prior to use are the responsibility of the user, and
would normally not be longer than 24 hours at 2°C - 8°C, unless reconstitution has taken place in
controlled and validated aseptic conditions.
6.4 Special precautions for storage
Store NovoSeven in a refrigerator (2°C - 8°C)
Store in original package in order to protect from light
Do not freeze to prevent damage to the solvent vial.
6.5 Nature and contents of container
Vials for NovoSeven:
Glass closed with a bromobutyl rubber stopper covered with an aluminium cap.
The closed vials are equipped with a tamper-evident snap-off cap which is made of polypropylene.
Vials for solvent:
Glass closed with a bromobutyl rubber disc with teflon, covered with an aluminium cap.
The closed vials are equipped with a tamper-evident snap-off cap which is made of polypropylene.
Syringe for reconstitution and administration:
The sterile disposable syringe is made of polypropylene.
The NovoSeven package contains:
–
1 vial (12 ml) with white powder (NovoSeven) for solution for injection
1 vial (12 ml) with solvent (Water for injections) for reconstitution
1 sterile disposable syringe for reconstitution and administration
1 sterile infusion set for administration
2 alcohol swabs for cleansing the rubber stoppers on the vials
Package leaflet with instructions for use.
6.6 Special precautions for disposal and other handling
Always use an aseptic technique
Bring the NovoSeven powder and water vials to room temperature (but not above 37°C). You
can do this by holding them in your hands. Remove the plastic caps from the two vials. If the
caps are loose or missing, do not use the vials. Clean the rubber stoppers on the vials with the
alcohol swabs and allow them to dry before use.
Remove the protective paper from the vial adapter without taking the vial adapter out of the
protective cap. Attach the vial adapter to the solvent vial. Take care not to touch the spike on the
vial adapter.
Once attached, remove the protective cap from the vial adapter.
Pull the plunger to draw in a volume of air that is equal to the amount of solvent in the solvent
vial (ml equals cc on the syringe).
Screw the syringe tightly onto the vial adapter on the solvent vial. Inject air into the vial by
pushing the plunger until you feel a clear resistance.
Hold the syringe with the water vial upside down and pull the plunger to draw the water into the
syringe.
Remove the empty water vial by tipping the syringe with the vial adapter.
Click the vial adapter, still attached to the syringe, onto the powder vial. Hold the syringe
slightly tilted with the vial facing downwards. Push the plunger slowly to inject the water into
the powder vial. Make sure not to aim the stream of water directly at the NovoSeven powder as
this will cause foaming.
1 sterile vial adapter for reconstitution
Gently swirl the vial until all the powder is dissolved. Do not shake the vial as this will cause
foaming.
NovoSeven reconstituted solution is colourless and should be inspected visually for particulate matter
and discolouration prior to administration.
The enclosed disposable syringe is compatible with the reconstituted preparation, but
do not
store
reconstituted NovoSeven in plastic syringes.
It is recommended to use NovoSeven immediately after reconstitution.
Ensure that the plunger is pushed all the way in before turning the syringe upside down (it may
have been pushed out by the pressure in the syringe). Hold the syringe with the vial upside
down and pull the plunger to draw all the solution into the syringe.
Unscrew the vial adapter with the empty vial.
NovoSeven is now ready for injection. Locate a suitable site, and slowly inject NovoSeven into
a vein over a period of 2 - 5 minutes without removing the needle from the injection site.
Safely dispose of the syringe, vial adapter, vials, infusion set and any unused product. Any unused
product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 23 February 1996
Date of latest renewal: 23 February 2006
10. DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency
(EMA)
http://www.ema.europa.eu/
What NovoSeven contains
•
The active substance is recombinant coagulation factor VIIa (activated eptacog alfa).
Blood clots in the veins in lungs, legs or at site of injection
Keep out of the reach and sight of children.
Store powder and solvent below 25°C.
The other ingredients in the powder are sodium chloride, calcium chloride dihydrate,
glycylglycine, polysorbate 80, mannitol, sucrose, methionine, hydrochloric acid, sodium
hydroxide. The ingredients in the solvent are histidine, hydrochloric acid, sodium hydroxide,
water for injections.
The powder for solution for injection contains: 1 mg/vial (corresponding to 50 KIU/vial), 2 mg/vial
(corresponding to 100 KIU/vial), 5 mg/vial (corresponding to 250 KIU/vial) or 8 mg/vial
(corresponding to 400 KIU/vial).
After reconstitution 1 ml of the solution contains 1 mg eptacog alfa (activated).
1 KIU equals 1000 IU (International Units).
What NovoSeven looks like and contents of the pack
The powder vial contains white powder and the solvent vial contains a clear colourless solution. The
reconstituted solution for injection is colourless. Do not use the reconstituted solution if particle
formation or discolouration is noticed.
Each NovoSeven pack contains:
•
1 vial with white powder for solution for injection
1 vial with solvent for reconstitution
Pack sizes: 1 mg (50 KIU), 2 mg (100 KIU), 5 mg (250 KIU) and 8 mg (400 KIU).
Please refer to outer packaging for present pack size.
Marketing Authorisation Holder and Manufacturer
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd, Denmark
This leaflet was last approved in
NOVOSEVEN USER INSTRUCTIONS
Wash your hands. NovoSeven powder and solvent vials should be at room temperature at
reconstitution. Remove the plastic caps from the two vials. If the caps are loose or missing, do not use
the vials. Clean the rubber stoppers on the vials with alcohol swabs and allow them to dry before use.
Use a disposable syringe of an appropriate size and a vial adapter, transfer needle (20 - 26G) or other
suitable device.
A
Remove the protective paper from the vial adapter without taking it out of the protective cap. Attach
the vial adapter to the solvent vial. Once attached, remove the protective cap.
Take care not to touch
the spike on the vial adapter. If using a transfer needle, remove transfer needle from the packaging
without taking it out of the protective cap. Screw the transfer needle tightly onto the syringe.
B
Pull the plunger to draw in a volume of air that is equal to the amount of solvent in the solvent vial (ml
equals cc on the syringe).
C
Screw the syringe tightly onto the vial adapter on the solvent vial. If using a transfer needle, remove
the protective cap and insert the transfer needle into the rubber stopper of the solvent vial. Take care
not to touch the end of the transfer needle. Inject air into the vial by pushing the plunger until you feel
a clear resistance.
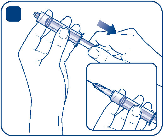



D
Hold the syringe with the solvent vial upside down. If you are using a transfer needle, make sure that
the transfer needle tip is in the solvent. Pull the plunger to draw the solvent into the syringe.
E
Remove the empty solvent vial. If you use a vial adapter, tip the syringe to remove it from the vial.
F
Attach the syringe with vial adapter or transfer needle to the powder vial. If you use a transfer needle,
make sure to penetrate the centre of the rubber stopper. Hold the syringe slightly tilted with the vial
facing downwards. Push the plunger slowly to inject the solvent into the powder vial. Make sure not to
aim the stream of solvent directly at the NovoSeven powder as this will cause foaming.
G
Gently swirl the vial until all the powder is dissolved. Do not shake the vial as this will cause foaming.
Check the solution for visible particles and discolouration. If you notice either, do not use it.
NovoSeven reconstituted product is a clear, colourless solution. Keep the vial adapter or transfer
needle attached to the vial.


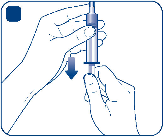

Although NovoSeven will be stable for 24 hours after it has been mixed, you should use it at once to
avoid infection. If you do not use it immediately after mixing, you should store the vial with the
syringe still attached in a refrigerator at 2°C to 8°C for no longer than 24 hours. Do not store the
solution without your doctor’s advice.
H
Ensure that the plunger is pushed all the way in before turning the syringe upside down (it may have
been pushed out by the pressure in the syringe). If you use a transfer needle, make sure that the
transfer needle tip is in the solution. Hold the syringe with the vial upside down and pull the plunger to
draw all the solution into the syringe.
I
If you use a vial adapter, unscrew the vial adapter with the empty vial. If you use a transfer needle,
remove the transfer needle from the vial, replace the transfer needle cap, and twist the transfer needle
off the syringe.
NovoSeven is now ready for injection. Follow the injection procedure as instructed by your health care
professional.

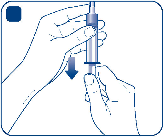

J
Safely dispose of the syringe, vials, any unused product and other waste materials as instructed by
your health care professional.

Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/novoseven.html
Copyright © 1995-2021 ITA all rights reserved.
|






















































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































