Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
NutropinAq 10 mg/2 ml (30 IU) solution for injection
QUALITATIVE AND QUANTITATIVE COMPOSITION
One cartridge contains 10 mg (30 IU) of somatropin*
* human growth hormone produced in
Escherichia coli
cells by recombinant DNA technology.
For a full list of excipients, see section 6.1.
Solution for injection.
NutropinAq is a solution for subcutaneous use. The clear, colourless, sterile solution for multidose use
is contained in a glass cartridge, closed with a rubber stopper and a rubber seal.
4.1 Therapeutic indications
Long-term treatment of children with growth failure due to inadequate endogenous growth
hormone secretion.
Treatment of prepubertal children with growth failure associated with chronic renal
insufficiency up to the time of renal transplantation.
Replacement of endogenous growth hormone in adults with growth hormone deficiency of
either childhood or adult-onset etiology. Growth hormone deficiency should be confirmed
appropriately prior to treatment (see section 4.4).
4.2 Posology and method of administration
Diagnosis and therapy with somatropin should be initiated and monitored by physicians who are
appropriately qualified and experienced in the diagnosis and management of patients with the
therapeutic indication of use.
The NutropinAq dosage and administration schedule should be individualised for each patient.
Growth failure in children due to inadequate growth hormone secretion
:
0.025 - 0.035 mg/kg bodyweight given as a daily subcutaneous injection.
Somatropin therapy should be continued in children and adolescents until their epiphysis are closed.
Growth failure associated with Turner syndrome:
Up to 0.05 mg/kg bodyweight given as a daily subcutaneous injection.
Somatropin therapy should be continued in children and adolescents until their epiphysis are closed.
Growth failure associated with chronic renal insufficiency:
Up to 0.05 mg/kg bodyweight given as a daily subcutaneous injection.
Somatropin therapy should be continued in children and adolescents until their epiphysis are closed, or
up to the time of renal transplantation.
Growth hormone deficiency in adults:
Long-term treatment of growth failure associated with Turner syndrome.
At the start of somatropin therapy, low initial doses of 0.15 - 0.3 mg are recommended, given as a
daily subcutaneous injection. The dose should be adjusted stepwise, controlled by serum Insulin-like
Growth Factor-1 (IGF-1) values. The recommended final dose seldom exceeds 1.0 mg/day. In general,
the lowest efficacious dose should be administered. In older or overweight patients, lower doses may
be necessary.
The solution for injection should be administered subcutaneously each day. The site of injection
should be changed.
For instructions for use and handling, see section 6.6.
Hypersensitivity to the active substance or to any of the excipients.
Somatropin should not be used for growth promotion in patients with closed epiphyses.
Growth hormone should not be used in patients with active neoplasm. NutropinAq therapy should be
discontinued if evidence of tumour growth develops.
Growth hormone should not be initiated to treat patients with acute critical illness due to complications
following open-heart or abdominal surgery, multiple accidental traumas or to treat patients having
acute respiratory failure.
4.4 Special warnings and precautions for use
In adults with growth hormone deficiency the diagnosis should be established depending on the
etiology:
Adult-onset:
The patient must have growth hormone deficiency as a result of hypothalamic or pituitary
disease, and at least one other hormone deficiency diagnosed (except for prolactin). Test for growth
hormone deficiency should not be performed until adequate replacement therapy for other hormone
deficiencies have been instituted.
Childhood-onset:
Patients who have had growth hormone deficiency as a child should be retested to
confirm growth hormone deficiency in adulthood before replacement therapy with NutropinAq is
started.
In patients with previous malignant disease, special attention should be given to signs and symptoms
of relapse.
Patients with a history of an intracranial lesion should be examined frequently for progression or
recurrence of the lesion.
NutropinAq is not indicated for the long-term treatment of paediatric patients who have growth failure
due to genetically confirmed Prader-Willi syndrome, unless they also have a diagnosis of growth
hormone deficiency. There have been reports of sleep apnoea and sudden death after initiating therapy
with growth hormone in paediatric patients with Prader-Willi syndrome who had one or more of the
following risk factors: severe obesity, history of upper airway obstruction or sleep apnoea, or
unidentified respiratory infection.
The effects of growth hormone on recovery were studied in two placebo-controlled clinical trials
involving 522 adult patients who were critically ill due to complications following open-heart or
abdominal surgery, multiple accidental traumas, or who were having acute respiratory failure.
Mortality was higher (41.9 % vs. 19.3 %) among growth hormone treated patients (doses 5.3 - 8
mg/day) compared to those receiving placebo.
The safety of continuing somatropin treatment in patients with acute critical illness in intensive care
units due to complications following open-heart or abdominal surgery, multiple accidental trauma or
acute respiratory failure receiving replacement doses for approved indications has not been
established. Therefore, the benefit-risk assessment for continuing treatment should be performed
carefully.
Patients with growth hormone failure secondary to CRI should be examined periodically for evidence
of progression of renal osteodystrophy. Slipped capital femoral epiphyses and aseptic necrosis of the
femoral head may be seen in children with advanced renal osteodystrophy and in growth hormone
deficiency, and it is uncertain whether these problems are affected by GH therapy. Physicians and
parents should be alert to the development of a limp or complaints of hip or knee pain in patients
treated with NutropinAq.
Scoliosis may progress in any child during rapid growth. Signs of scoliosis should be monitored
during treatment. However, growth hormone treatment has not been shown to increase the incidence
or severity of scoliosis.
Because somatropin may reduce insulin sensitivity, patients should be monitored for evidence of
glucose intolerance. For patients with diabetes mellitus, the insulin dose may require adjustment after
NutropinAq therapy is instituted. Patients with diabetes or glucose intolerance should be monitored
closely during somatropin therapy.
Intracranial hypertension with papilloedema, visual changes, headache, nausea and/or vomiting has
been reported in a small number of patients treated with somatropin. Symptoms usually occur within
the first eight weeks of the initiation of NutropinAq therapy. In all reported cases, intracranial
hypertension-associated signs and symptoms resolved after reduction of the somatropin dose or
termination of the therapy. Funduscopic examination is recommended at the initiation and periodically
during the course of treatment.
Hypothyroidism may develop during treatment with somatropin, and untreated hypothyroidism may
prevent optimal response to NutropinAq. Therefore, patients should have periodic thyroid function
tests and should be treated with thyroid hormone when indicated. Patients with severe hypothyroidism
should be treated accordingly prior to the start of NutropinAq therapy.
Since somatropin therapy following renal transplantation has not been adequately tested, NutropinAq
treatment should be terminated after that surgery
.
Concomitant treatment with glucocorticoids inhibits the growth-promoting effects of NutropinAq.
Patients with ACTH deficiency should have their glucocorticoid replacement therapy carefully
adjusted to avoid any inhibitory effect on growth. The use of NutropinAq in patients with chronic
renal insufficiency receiving glucocorticoid therapy has not been evaluated.
4.5 Interaction with other medicinal products and other forms of interaction
Limited published data indicate that growth hormone treatment increases cytochrome P450 mediated
antipyrine clearance in man. Monitoring is advisable when NutropinAq is administered in combination
with medicinal products known to be metabolised by CYP450 liver enzymes, such as corticosteroids,
sex steroids, anticonvulsants, and cyclosporin.
4.6 Pregnancy and lactation
For NutropinAq, no clinical data on exposed pregnancies are available. Thus, the risk for humans is
unknown. Although animal studies do not point to a potential risk during pregnancy, NutropinAq
should be discontinued if pregnancy occurs. During pregnancy, maternal somatropin will largely be
replaced by placental growth hormone.
It is not known whether somatropin is excreted in human milk, however, absorption of intact protein
from the gastrointestinal tract of the infant is unlikely.
4.7 Effects on ability to drive and use machines
No studies on the effects of NutropinAq on the ability to drive and use machines have been performed.
Somatropin has no known effect on the ability to drive or to use machines.
Safety data from 9829 patients treated with Nutropin or NutropinAq - derived from a post-marketing
surveillance survey in the United States - demonstrate that approximately 2% of patients have been
shown to experience drug-related adverse reactions. Most of these adverse drug reactions were
reported in the “general disorders and administration site conditions” system organ class.
The adverse reactions are listed in the table below, based on experience from clinical trials and a post-
marketing surveillance survey. Within the system organ classes, adverse reactions are listed under
headings of frequency using the following categories: very common (≥ 1/10),
common (≥ 1/100, < 1/10); uncommon (≥ 1/1000, < 1/100); rare (≥ 1/10,000, < 1/1,000).
Neoplasms benign, malignant and
unspecified (including cysts and polyps)
Uncommon
: neoplasm malignant, neoplasm
Blood and lymphatic system disorders
Common
: antibody building
Metabolism and nutrition disorders
Common
: glucose tolerance impaired
Uncommon
: hypoglycaemia, hyperphosphatemia
Rare
: diabetes mellitus
Uncommon
: personality disorder
Common
: headache, hypertonia
Uncommon
: somnolence, nystagmus
Rare
: neuropathy, intracranial pressure increased
Uncommon
: papilloedema, diplopia
Ear and labyrinth disorders
Uncommon
: tachycardia, hypertension
Gastrointestinal disorders
Uncommon
: vomiting, abdominal pain, flatulence,
nausea
Rare
: diarrhoea
Skin and subcutaneous tissue disorders
Uncommon
: lipodystrophy, skin atrophy, dermatitis
exfoliative, urticaria, hirsutism, skin hypertrophy
Musculoskeletal and connective tissue
disorders
Very common in adults, common in children:
arthralgia, myalgia
Uncommon
: muscle atrophy, bone pain, carpal tunnel
syndrome
Renal and urinary disorders
Uncommon
: urinary incontinence, haematuria,
polyuria,
Reproductive system and breast disorders
Uncommon
: genital discharge
General disorders and administration site
conditions
Very common in adults, common in children
: oedema,
peripheral oedema
Common
: injection site reactions, asthenia
Uncommon: injection site atrophy, injection site



















haemorrhage, injection site mass, hypertrophy
Rare
: renal function test abnormal
As with all medicinal products, a small percentage of patients may develop antibodies to the protein
somatropin. The binding capacity of growth hormone antibodies was lower than 2 mg/l in NutropinAq
subjects tested, which has not been associated with adversely affected growth rate.
Leukaemia has been reported in a small number of growth hormone deficient patients treated with
growth hormone. A causal relationship to somatropin therapy is unlikely.
Patients with endocrinological disorders are more prone to develop an epiphysiolysis.
Indication-specific adverse drug reactions from clinical trials
Paediatric patients:
Patients with growth failure due to inadequate growth hormone secretion
Common
: central nervous system neoplasm.
Patients with growth failure associated with Turner syndrome
Common
: menorrhagia.
Patients with growth failure associated with chronic renal insufficiency
Common
: renal failure, peritonitis, osteonecrosis, blood creatinine increase.
Children with chronic renal insufficiency receiving NutropinAq are more likely to develop intracranial
hypertension. The greatest risk is at the beginning of treatment.
Adults with growth hormone deficiency
Very common
: paraesthesia.
Common
: hyperglycaemia, hyperlipidaemia, insomnia, synovial disorder, arthrosis, muscular
weakness, back pain, breast pain, gynaecomastia.
Acute overdose could lead to hyperglycaemia. Long-term overdose could result in signs and
symptoms of gigantism and/or acromegaly consistent with the known effects of excess growth
hormone.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Somatropin and analogues, ATC Code: H01 AC 01
Somatropin stimulates growth rate and increases adult height in children who lack endogenous growth
hormone. Treatment of growth hormone deficient adults with somatropin results in reduced fat mass,
increased lean body mass and increased spine bone mineral density. Metabolic alterations in these
patients include normalisation of IGF-1 serum levels.
In vitro
and
in vivo
preclinical and clinical tests have demonstrated that somatropin is therapeutically
equivalent to human growth hormone of pituitary origin.





Actions that have been demonstrated for human growth hormone include:
Tissue Growth
1. Skeletal growth: growth hormone and its mediator IGF-1 stimulate skeletal growth in growth
hormone deficient children by an effect on the epiphyseal plates of long bones. This results in a
measurable increase in body length until these growth plates fuse at the end of puberty.
2. Cell growth: Treatment with somatropin results in an increase in both the number and size of
skeletal muscle cells.
3. Organ growth: Growth hormone increases the size of internal organs, including kidneys, and
increases red blood cell mass.
Protein metabolism
Linear growth is facilitated in part by growth hormone-stimulated protein synthesis. This is reflected
by nitrogen retention as demonstrated by a decline in urinary nitrogen excretion and blood urea
nitrogen during growth hormone therapy.
Carbohydrate metabolism
Patients with inadequate growth hormone secretion sometimes experience fasting hypoglycaemia that
is improved by treatment with somatropin. Growth hormone therapy may decrease insulin sensitivity
and impair glucose tolerance.
Mineral metabolism
Somatropin induces retention of sodium, potassium and phosphorus. Serum concentration of inorganic
phosphorus are increased in patients with growth hormone deficiency after NutropinAq therapy due to
metabolic activity associated with bone growth and increased tubular reabsorption in the kidney.
Serum calcium is not significantly altered by somatropin. Adults with growth hormone deficiency
show low bone mineral density and in the childhood-onset patient, NutropinAq has been shown to
increase spine bone mineral density in a dose-dependent manner.
Connective tissue metabolism
Somatropin stimulates the synthesis of chondroitin sulphate and collagen as well as the urinary
excretion of hydroxyproline.
Body composition
Adult growth hormone deficient patients treated with somatropin at a mean dosage of 0.014 mg/kg
bodyweight daily demonstrate a decrease in fat mass and increase in lean body mass. When these
alterations are coupled with the increase in total body water and bone mass, the overall effect of
somatropin therapy is to modify body composition, an effect that is maintained with continued
treatment.
5.2 Pharmacokinetic properties
The pharmacokinetic properties of NutropinAq have only been investigated in healthy adult males.
Absorption
:
The absolute bioavailability of recombinant human growth hormone after subcutaneous
administration is about 80%.
Distribution
:
Animal studies with somatropin showed that growth hormone localises to highly
perfused organs, particularly the liver and kidney. The volume of distribution at steady state for
somatropin in healthy adult males is about 50 ml/kg bodyweight, approximating the serum volume.
Metabolism
:
Both the liver and the kidney have been shown to be important protein catabolising
organs for growth hormone. Animal studies suggest that the kidney is the dominant organ of
clearance. Growth hormone is filtered at the glomerulus and reabsorbed in the proximal tubules. It is
then cleaved within renal cells into its constituent amino acids, which return to the systemic
circulation.
Elimination
:
After subcutaneous bolus administration, the mean terminal half-life t
½
of somatropin is
about 2.3 hours. After intravenous bolus administration of somatropin, the mean terminal half-life t
½
β
or t
½
γ is about 20 minutes and the mean clearance is reported to be in the range of 116 - 174 ml/h/kg.
Available literature data suggest that somatropin clearance is similar in adults and children.
Characteristics in patients
Clearance and mean terminal half-life t
½
of somatropin in adult and paediatric growth hormone
deficient patients are similar to those observed in healthy subjects.
Children and adults with chronic renal failure and end-stage renal disease tend to have decreased
clearance compared to normal subjects. Endogenous growth hormone production may also increase in
some individuals with end-stage renal disease. However, no somatropin accumulation has been
reported in children with chronic renal failure or end-stage renal disease dosed with current regimens.
Limited published data for exogenously-administered somatropin suggest absorption and elimination
half-lives and time of maximum concentration t
max
in Turner patients are similar to those observed in
both normal and growth hormone deficient populations.
In patients with severe liver dysfunction a reduction in somatropin clearance has been noted. The
clinical significance of this decrease is unknown.
5.3 Preclinical safety data
The toxicity of NutropinAq has been tested in rats and monkeys and no findings of toxicological
relevance were revealed.
Due to its hormonal activity, somatropin may exert a promotional effect on tumour growth in tumour-
bearing subjects. To date, this has not been confirmed in patients.
Local tolerance studies with NutropinAq showed no substantial adverse local reactions.
Studies in transgenic mice suggest a low antibody provoking potential of (aged) liquid Nutropin.
No common reproduction studies were performed. However, long-term treatment of monkeys during
pregnancy and lactation and of newborn animals until adolescence, sexual maturity and reproduction
did not indicate substantial disturbances of fertility, pregnancy, delivery, nursing or development of
progeny.
PHARMACEUTICAL PARTICULARS
Sodium chloride
Phenol
Polysorbate 20
Sodium citrate and citric acid anhydrous
Water for injections
In the absence of compatibility studies, this medicinal product
must not be mixed with other medicinal
products.
Chemical and physical in-use stability has been demonstrated for 28 days at 2°C - 8°C.
From a microbiological point of view, once opened, the product may be stored for a maximum of 28
days at 2°C - 8°C. NutropinAq is designed to withstand a nominal (one hour maximum) period of time
outside of the refrigerator on a daily basis.
6.4 Special precautions for storage
Store in a refrigerator (2°C - 8°C).
Do not freeze.
Keep the blister in the outer carton
For in-use storage conditions of the medicinal product, see section 6.3.
6.5 Nature and contents of container
2 ml of solution in a cartridge (Type I glass) closed with a stopper (butyl rubber) and a seal (rubber).
Pack sizes of 1, 3 and 6 cartridges.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
Instructions for use and handling
NutropinAq is supplied as a sterile solution with preservative for multiple use. The solution should be
clear immediately after removal from the refrigerator. If the solution is cloudy, the content must not be
injected. Gently swirl. Do not shake vigorously in order not to denature the protein.
NutropinAq is intended for use only with the NutropinAq Pen. Wipe the rubber seal of the
NutropinAq with rubbing alcohol or an antiseptic solution to prevent contamination of the contents by
microorganisms that may be introduced by repeated needle insertions. It is recommended that
NutropinAq be administered using sterile, disposable needles.
The NutropinAq Pen allows for administration of a minimum dose of 0.1 mg to a maximum dose of
4.0 mg, in 0.1 mg increments.
A cartridge that is in the pen should not be removed during injections.
MARKETING AUTHORISATION HOLDER
Ipsen Pharma
65 quai Georges Gorse
92100 Boulogne-Billancourt
France
MARKETING AUTHORISATION NUMBERS
EU/1/00/164/003
EU/1/00/164/004
EU/1/00/164/005
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation:16 February 2001
Date of last renewal: 16 February 2006
10.
DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
Agency (EMEA) http://www.emea.eu.int/
A.
MANUFACTURER(S) OF THE BIOLOGICAL ACTIVE
SUBSTANCE AND MANUFACTURING AUTHORISATION
HOLDER(S) RESPONSIBLE FOR BATCH RELEASE
B.
CONDITIONS OF THE MARKETING AUTHORISATION
MANUFACTURER(S) OF THE BIOLOGICAL ACTIVE SUBSTANCE AND
MANUFACTURING AUTHORISATION HOLDER(S) RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer of the biological active substance
Genentech Inc.
1 DNA Way
South San Francisco
CA 94080-4990
USA
Name and address of the manufacturer responsible for batch release
Ipsen Pharma Biotech SAS
Parc d’Activités du Plateau de Signes
Chemin Départemental no 402
83870 Signes
France
CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED
ON THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to restricted medical prescription (See Annex I: Summary of Product
Characteristics, section 4.2).
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING AND THE IMMEDIATE
PACKAGING
{CARTON - 1 CARTRIDGE}
NAME OF THE MEDICINAL PRODUCT
NutropinAq
10 mg/2 ml (30 IU), solution for injection
Somatropin
STATEMENT OF ACTIVE SUBSTANCE
2 ml of solution contains:
10 mg (30 IU) of somatropin
Other ingredients: sodium chloride, phenol, polysorbate 20, sodium citrate, citric acid anhydrous and
water for injections.
PHARMACEUTICAL FORM AND CONTENTS
1 cartridge containing 2 ml solution for injection
METHOD AND ROUTE(S) OF ADMINISTRATION
Subcutaneous use
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF
THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY



















SPECIAL STORAGE CONDITIONS
Store in a refrigerator.
Do not freeze.
Keep the blister in the outer carton.
Chemical and physical in-use stability has been demonstrated for 28 days at 2°C - 8°C.
From a microbiological point of view, once opened, the product may be stored for a maximum of 28
days at 2°C - 8°C.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Ipsen Pharma, 65 quai Georges Gorse, 92100 Boulogne-Billancourt, France
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE



















PARTICULARS TO APPEAR ON THE OUTER PACKAGING AND THE IMMEDIATE
PACKAGING
{CARTON - 3 CARTRIDGES}
NAME OF THE MEDICINAL PRODUCT
NutropinAq
10 mg/2 ml (30 IU), solution for injection
Somatropin
STATEMENT OF ACTIVE SUBSTANCE
2 ml of solution contains:
10 mg (30 IU) of somatropin
Other ingredients: sodium chloride, phenol, polysorbate 20, sodium citrate, citric acid anhydrous and
water for injections.
PHARMACEUTICAL FORM AND CONTENTS
3 cartridges containing 2 ml solution for injection
METHOD AND ROUTE(S) OF ADMINISTRATION
Subcutaneous use.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF
THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY



















SPECIAL STORAGE CONDITIONS
Store in a refrigerator.
Do not freeze.
Keep the blister in the outer carton.
Chemical and physical in-use stability has been demonstrated for 28 days at 2°C - 8°C.
From a microbiological point of view, once opened, the product may be stored for a maximum of 28
days at 2°C - 8°C.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Ipsen Pharma, 65 quai Georges Gorse, 92100 Boulogne-Billancourt, France
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE



















PARTICULARS TO APPEAR ON THE OUTER PACKAGING AND THE IMMEDIATE
PACKAGING
{CARTON - 6 CARTRIDGES}
NAME OF THE MEDICINAL PRODUCT
NutropinAq
10 mg/2 ml (30IU), solution for injection
Somatropin
STATEMENT OF ACTIVE SUBSTANCE
2 ml of solution contains:
10 mg (30 IU) of somatropin
Other ingredients: sodium chloride, phenol, polysorbate 20, sodium citrate, citric acid anhydrous and
water for injections.
PHARMACEUTICAL FORM AND CONTENTS
6 cartridges containing 2 ml solution for injection
METHOD AND ROUTE(S) OF ADMINISTRATION
Subcutaneous use.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF
THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY



















SPECIAL STORAGE CONDITIONS
Store in a refrigerator.
Do not freeze.
Keep the blister in the outer carton.
Chemical and physical in-use stability has been demonstrated for 28 days at 2°C - 8°C.
From a microbiological point of view, once opened, the product may be stored for a maximum of 28
days at 2°C - 8°C.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Ipsen Pharma, 65 quai Georges Gorse, 92100 Boulogne-Billancourt, France
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE



















MINIMUM PARTICULARS TO APPEAR ON BLISTERS OR STRIPS
{BLISTER}
NAME OF THE MEDICINAL PRODUCT
NutropinAq 10 mg/2 ml (30IU) solution for injection
Somatropin
NAME OF THE MARKETING AUTHORISATION HOLDER
Subcutaneous use.
Read the package leaflet before use.
Store in a refrigerator.













PACKAGE LEAFLET : INFORMATION FOR THE USER
NutropinAq 10 mg/2 ml (30 IU) solution for injection
Somatropin
Read all of this leaflet carefully before you start using this medicine.
-
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
In this leaflet
:
1. What NutropinAq is and what it is used for
2. Before you use NutropinAq
3. How to use NutropinAq
4. Possible side effects
5. How to store NutropinAq
6. Further Information
WHAT NutropinAq IS AND WHAT IT IS USED FOR
Somatropin has effects that are equivalent to human growth hormone of pituitary origin. Growth
hormone exerts significant effects directly on the production of other hormones, e.g. IGF-1, and on
metabolic actions. The anabolic and growth-promoting effects of somatropin are to some part indirect
effects mediated by IGF-1.
NutropinAq is indicated for:
Long-term treatment of children with growth failure due to inadequate endogenous growth
hormone secretion.
Long-term treatment of growth failure associated with Turner syndrome.
Treatment of prepubertal children with growth failure associated with chronic renal
insufficiency up to the time of renal transplantation.
Replacement of growth hormone in adults with growth hormone deficiency originating in either
childhood or adulthood.
2.
BEFORE YOU USE NutropinAq
If you are allergic (hypersensitive) to somatropin or any of the other ingredients of NutropinAq.
For growth promotion if growing is already finished.
If an active tumour arises. Somatropin therapy should be discontinued if evidence of tumour
growth develops.
During acute critical illness due to complications following open-heart or abdominal surgery,
multiple accidental trauma, or in case of acute respiratory failure.
Keep this leaflet. You may need to read it again.
Take special care with NutropinAq
In patients with previous malignant disease, the doctor should give special attention to signs and
symptoms of relapse.
In patients with a history of brain lesions, the patient should be examined frequently for a
progression or recurrence of the lesion.
Children with Prader-Willi syndrome should not be treated with NutropinAq unless they are
also suffering from growth hormone failure.
In patients with acute critical illness in intensive care units, the doctor should carefully evaluate
the safety of continuing somatropin treatment.
If during growing a limp or hip or knee pain develops, please ask the doctor for advice.
Scoliosis may progress in any child during rapid growth. Signs of scoliosis should be monitored
during treatment.
In patients receiving NutropinAq, evidence of glucose intolerance should be monitored. If the
patient also has diabetes mellitus, please consult the doctor regularly during NutropinAq
therapy. The insulin dose may require adjustment after somatropin therapy is started.
This medicinal product contains 8,2 mg of sodium per cartridge. To be taken in consideration by
patients on a controlled sodium diet.
If symptoms like visual changes, headache, nausea and/or vomiting occur, especially within the
first eight weeks after starting somatropin therapy, please ask the doctor for advice.
In case of an untreated decrease of thyroid gland function (hypothyroidism), the optimal
response to somatropin may be reduced. Severe hypothyroidism has to be treated prior to start
of NutropinAq therapy.
Since somatropin therapy following renal transplantation has not been adequately tested, growth
hormone treatment should be terminated after that surgery.
Adrenocorticotrophic Hormone (ACTH) -deficient patients should consult the doctor regularly
during growth hormone therapy. The glucocorticoid replacement therapy may require
adjustment after NutropinAq therapy has been started.
Concomitant treatment with glucocorticoids may reduce the growth-promoting effect of
somatropin.
Because somatropin may reduce insulin sensitivity, patients with diabetes mellitus may require
adjustment of their antidiabetic therapy.
During concomitant administration of somatropin with corticosteroids, sex steroids,
anticonvulsants or cyclosporin, please ask the doctor for advice.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription
Pregnancy and breast-feeding
NutropinAq therapy should be discontinued if pregnancy occurs.
It is not known whether somatropin is excreted in human milk. However, absorption of intact protein
from the gastrointestinal tract of the infant is unlikely.
Please ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
No studies on the effects of NutropinAq on the ability to drive and use machines have been performed.
Somatropin has no known effect on the ability to drive or to use machines.
The diagnosis and management of somatropin therapy should be initiated and monitored by
adequately experienced physicians.
The doctor will advise you about the individualised dose of NutropinAq. Please do not change the
dosage without consulting the doctor or pharmacist. In general, the dosage will be calculated
according to the following rules:
Growth failure in children due to inadequate growth hormone secretion
:
0.025 - 0.035 mg/kg bodyweight given as a daily subcutaneous injection.
Growth failure associated with Turner syndrome:
Up to 0.05 mg/kg bodyweight given as a daily subcutaneous injection.
Growth failure associated with chronic renal insufficiency:
Up to 0.05 mg/kg bodyweight given as a daily subcutaneous injection.
Somatropin therapy may be continued up to the time of renal transplantation.
Growth hormone deficiency in adults:
Low initial doses of 0.15 - 0.3 mg given as a daily subcutaneous injection. The dose may be increased
stepwise by the doctor according to the patient’s individual requirements. The final dose seldom
exceeds 1.0 mg/day. In general, the lowest efficacious dose should be received. For older or
overweight patients lower doses may be necessary.
NutropinAq is designed for use only with the NutropinAq Pen. Please administer the prescribed dose
of NutropinAq solution for injection subcutaneously each day and change the site of injection each
time. At the start of therapy it is recommended that a doctor or a nurse give the injection and train you
with the appropriate NutropinAq Pen to use with your NutropinAq cartridge.
After training, the injection can be given by the patient or his/her carer. The medicine is supplied in a
cartridge as a sterile solution with preservative for multiple use. For each single injection please use a
new, sterile injection needle. Do not use the solution unless it is clear and not cloudy. Please see also
the instructions for use (on reverse).
Treatment with somatropin is a long-term therapy. For further information please ask the doctor.
If you use more NutropinAq than you should
If more NutropinAq than recommended was injected, please consult the doctor.
Acute overdose could lead initially to a glucose decrease (hypoglycaemia) and subsequently to a
glucose increase (hyperglycaemia). Long-term overdose may result in an enhanced growth of ears,
nose, lips, tongue and cheekbone (gigantism and/or acromegaly). These signs are consistent with the
known effects of excess in human growth hormone.
If you forget to take NutropinAq
Do not take a double dose to make up for a forgotten dose. The prescribed dosage regimen should be
continued.
If you stop using NutropinAq
A disruption or early ending of the treatment with somatropin may impair the success of the growth
hormone therapy. Please ask the doctor for advice before stopping the treatment.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Like all medicines, NutropinAq can cause side effects, although not everybody gets them.
The
common side effects
reported were injection site reactions, headache, excessive tension of the
muscles (hypertonia), subnormal activity of the thyroid gland (hypothyroidism), impaired glucose
tolerance, loss of strength (asthenia) and development of antibodies to the protein somatropin.
An accumulation of fluids in the body with a mild transient swelling of the hands and feet (oedema),
muscular pain (myalgia) and arthralgia (neuralgic pain in one or more joints) were seen very
commonly in adult patients and commonly in children.
In
patients with Turner syndrome
, abnormally heavy bleeding at menstruation was commonly
reported.
In
patients with chronic renal insufficiency
, renal failure, peritonitis, bone necrosis, increase of
creatinine blood levels were commonly reported.
Abnormal sensations (paraesthesia) were seen very commonly in
adults with growth hormone
deficiency
. These patients also reported the following common side effects: abnormally high levels of
blood glucose, an excess of lipids in the blood, sleeplessness, joint disorders, arthrosis, muscular
debility, back pain, breast pain and breast enlargement (gynaecomastia).
Other
less common side effects
have been reported:
Those occurring at the injection site
: injection site atrophy, injection site haemorrhage, injection site
mass and hypertrophy.
Metabolic disorders
: decrease in blood glucose, increase in phosphate levels in the blood.
Nervous disorders
: somnolence, rapid involuntary movements of the eyes (nystagmus), personality
disorder, vertigo.
Those related to the heart:
abnormally rapid heart rate (tachycardia), high blood pressure.
Those related to the stomach
: vomiting, abdominal pain, flatulence, feeling of sickness, and rarely
diarrhoea.
Those affecting the skin
: lipodystrophy (disturbed fat metabolism), skin atrophy, exfoliative
inflammation of the skin, urticaria (allergic disorder marked by raised oedematous patches of skin or
mucus membrane), excessive growth of hair on the face and body (hirsutism), skin hypertrophy.
Those related to the musculoskeletal system
: decrease in muscle size (muscle atrophy), bone pain and
carpal tunnel syndrome.
Those affecting the urogenital system
: urinary incontinence, discharge of mucus from the vagina
(leukorrhoea), excessive secretion of urine, urine frequency, urine abnormality, urine haemorrhage.
Other possible side effects
: neoplasm, anaemia, papilloedema, diplopia.
Increased growth of birthmarks or moles (pre-existing naevi) may occur. The doctor should be
consulted as soon as possible if any changed appearance of the skin is noticed.
As with all protein medicines, in a few patients antibodies may develop to the protein somatropin.
Growth hormone antibodies with very low binding capacity have not been associated with adversely
affected growth rate.
Leukaemia has been reported in a small number of growth hormone deficient patients treated with
growth hormone. A causal relationship to somatropin therapy is unlikely.
Symptoms of benign increased brain pressure (benign intracranial hypertension), which are
papilloedema, visual changes, headache, nausea and vomiting, may occur more often in children with
chronic renal insufficiency receiving NutropinAq.
Patients with endocrinological disorders are more prone to develop an epiphysiolysis.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Store in a refrigerator (2°C - 8°C).
Do not freeze.
Keep the blister in the outer carton.
After first use, the cartridge may be stored for up to 28 days at 2°C - 8°C. Do not remove the cartridge
that is being used from the NutropinAq Pen between injections.
Do not use NutropinAq after the expiry date which is stated on the label of the cartridge and the carton
after EXP. The expiry date refers to the last day of that month.
Do not use NutropinAq if you notice that the solution is cloudy.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
What NutropinAq contains
−
The active substance of NutropinAq is somatropin*.
* human growth hormone produced in
Escherichia coli
cells by recombinant DNA technology.
The other ingredients are sodium chloride, phenol, polysorbate 20, sodium citrate, citric acid
anhydrous and water for injections.
What NutropinAq looks like and contents of the pack
NutropinAq is a solution for injection (in a cartridge (10 mg/2ml) - pack size of 1, 3 and 6). The
solution for multidose use is clear, colourless and sterile.
Not all pack sizes may be marketed.
Marketing Authorisation Holder:
Ipsen Pharma, 65 quai Georges Gorse, 92100 Boulogne-Billancourt, France
Manufacturer:
IPSEN PHARMA BIOTECH S.A.S., Parc d’Activités du Plateau de Signes, CD no 402, 83870
Signes, France
For any information about this medicinal product, please contact the local representative of the
Marketing Authorisation Holder.
België/Belgique/Belgien,
Luxembourg/Luxemburg
Ipsen NV
Guldensporenpark 87
B-9820 Merelbeke
België /Belgique/Belgien
Tél/Tel: + 32 - 9 - 243 96 00
Latvija
Beaufour Ipsen Pharma
Bauskas 58
Riga LV 1004
Tel: +371 67622233
Česká republika
Beaufour Ipsen Pharma
Evropská 136/810
CZ-160 00 Praha 6
Česká republika
Tel: + 420 242 481 821
Lietuva
Beaufour Ipsen Pharma Lietuvos filialas
Betygalos g. 2
LT-47183 Kaunas
Tel. + 370 37 337854
Danmark, Norge, Suomi/Finland, Sverige,
Ísland
Institut Produits Synthèse (IPSEN) AB
Kista Science Tower
Färögatan 33
SE- 164 51 Kista
Sverige/Ruotsi/Svíþjóð
Tlf/Puh/Tel/Sími: +46 8 588 370 70
Magyarország
Beaufour Ipsen Pharma SAS Magyarországi
Kereskedelmi Képviselet .
Kövér Lajos utca 45-47.
H- 1149 Budapest
Tel.: + 36 - 1 – 555-59-30
Deutschland, Österreich
Ipsen Pharma GmbH
Einsteinstr. 30
D-76275 Ettlingen
Deutschland
Tel: + 49 7243 184-80
Nederland
Ipsen Farmaceutica B.V.
Taurusavenue 33b
NL-2132 LS Hoofddorp
Tel: + 31 (0) 23 55 41 600
Eesti
ESTOBIIN OÜ
Udeselja 4-4
EE-11913 Tallinn
Tel: +372 51 55 810
Polska
Ipsen Poland Sp z o.o.
Al. jana Pawla II 29
PL-00- 867 Warszawa
Tel.: + 48 (0) 22 653 68 00
Ελλάδα, Κύπρος, Malta
Ipsen EΠΕ
Αγ. ∆ηµητρίου 63
Άλιµος
GR-17456 Αθήνα
Ελλάδα
Τηλ: + 30 - 210 - 984 3324
Portugal
Ipsen Portugal - Produtos Farmacêuticos S.A.
Alameda Fernão Lopes, n
o
16-11
o
, Miraflores
P-1495 - 190 Algés
Tel: + 351 - 21 - 412 3550
España
Ipsen Pharma S.A.
Ctra Laureà Miró 395
Sant Feliu de Llobregat
E-08980 Barcelona
Tel: + 34 - 936 - 858 100
România, България
Beaufour Ipsen Pharma
Aleea Alexandru, nr.10, Ap.1-3,
Sct.1 Bucuresti, 011823 - RO
Tel: + 40 21(0) 230 43 80
France
Ipsen Pharma
65 quai Georges Gorse
F-92100 Boulogne-Billancourt
Tél: + 33 - 1 - 58 33 50 00
Slovenija
PharmaSwiss d.o.o.
Dolenjska cesta 242c
SI-1000 Ljubljana
Tel: + 386 1 236 47 00
Ireland
Ipsen Pharmaceuticals Ltd.
7 Upper Leeson Street
IRL-Dublin 4
Tel: + 353 - 1 - 668 1377
Slovenská republika
Liek s.r.o.
19 Hviezdoslavova
SK-90301 Seneec
Tel: + 421 253 412 018
Italia
Ipsen SpA
Via A. Figino, 16
I-20156 Milano
Tel: + 39 - 02 - 39 22 41
United Kingdom
Ipsen Ltd.
190 Bath Road
Slough, Berkshire
SL1 3XE
Tel: + 44 - (0)1753 - 62 77 00
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency (EMEA) web
site:
http://www.emea.europa.eu/
.
NutropinAq Pen
Instructions for use with NutropinAq
DO NOT INJECT THE MEDICINE UNTIL YOUR DOCTOR OR NURSE HAS THOROUGHLY
TRAINED YOU IN THE PROPER TECHNIQUES.
Caution:
Before using your NutropinAq Pen, please read the following instructions carefully. We also suggest
you consult your doctor or nurse for a demonstration.
The NutropinAq Pen is designed for use only with cartridges of NutropinAq (for subcutaneous use
only).
As shown by the illustrations below, NutropinAq pen and cartridges are available in two designs (with
or without additional yellow colour).The working of the pen and the content of the cartridges are the
same for both designs. Either of the NutropinAq cartridge designs can be used with either of the
NutropinAq Pen designs.
Only use the pen needles recommended by your doctor or nurse.
The dosage scale located beside the window of the cartridge holder should not be used as a dose
measurement. It should only be used to estimate the dosage remaining in the cartridge. Always refer to
the LCD (Liquid Crystal Display), not audible clicks, for setting an injection of NutropinAq. The
clicks are only audible confirmation that the black dose knob has been moved.
Always store the pen and cartridges in a clean, safe place in the refrigerator at a temperature between
2-8°C and out of children’s reach and sight. Protect from intense light. Use a cooler to store your
NutropinAq Pen when travelling. The NutropinAq is designed to withstand a nominal (one hour
maximum) period of time outside of the refrigerator on a daily basis. Avoid areas of extreme
temperature. Check the expiry date of the cartridge before use.
To guard against the spread of infection, follow these safety measures:
Wash your hands thoroughly with soap and water before using your pen.
Clean the cartridge rubber seal with an alcohol swab or cotton ball saturated with alcohol.
Avoid touching the cartridge rubber seal at all times.
If you accidentally touch the cartridge rubber seal, clean it with an alcohol swab.
Do not use the same needle for more than one person.
NutropinAq Pen Components:


Shown below are the items necessary for giving an injection. Gather all of these components prior to
use.
Your NutropinAq cartridge and Pen will be supplied separately.
Part I: Preparing and Injecting
Follow the instructions in this section if you are using the pen for the first time or are replacing an
empty cartridge.
Inspect all new cartridges prior to use. Occasionally, after refrigeration, you may notice that small
colourless particles are present in the NutropinAq solution. This is not unusual for solutions containing
proteins like NutropinAq and does not affect the strength of the product. Allow the cartridge to come
to room temperature and gently swirl. Do not shake. If the solution is cloudy or hazy or contains any
solid matter, the cartridge should not be used. Return the cartridge to your pharmacist or prescribing
doctor.
1.
Remove the green pen cap and unscrew the cartridge holder from the pen. If
necessary, remove the empty cartridge and discard it properly.
2.
Press the white reset button.
3.
Turn the black dose knob counter-clockwise back to its starting position until it no
longer turns.
(See illustration.)
Then turn the dose knob clockwise until the first click
position is reached (approximately
1
/
4
turn). This ensures that the plunger push rod is
reset to the starting position. If this is not done when the dosage knob is first pushed
in, NutropinAq will be wasted or the cartridge may crack.
4.
Insert cartridge into the cartridge holder, then screw the cartridge holder back onto
the pen.
(Be careful not to touch the rubber seal.)
5.
Remove the paper seal from a new needle assembly and screw it onto the cartridge holder.
6.
Carefully remove both protective caps from the needle by pulling gently. Do not throw the larger
cap away as it will be used later for proper needle removal and disposal.
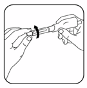


7.
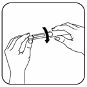
Holding the pen with the needle pointing upward, gently tap the cartridge holder to
move any air bubbles to the top. While still holding the pen in the upright position,
push in the black dose knob until it clicks into position. You should see a drop of
solution appear.
Be patient. If medicine doesn’t appear within a few seconds, you may need to
push the reset button again.
8.
If no drop of medicine appears, push the white reset button again. Now turn the
black dose knob clockwise
(See illustration)
by one click (0.1mg). If you accidentally
turn it too far, go back one click (0.1 mg).
9.
While still holding the pen in the upright position, push in the black dose knob again and watch the
needle tip for a drop of medicine to appear. Repeat steps 8 and 9 until it appears.
10.
Press the white reset button.
11.
Set the required dose by turning the black dose knob. If you cannot dial the full
dose, either start a new cartridge (as described in Part I), or inject the partial dose.
Then, start a new cartridge (as described in Part I) to administer the remaining portion
of your medication. Your doctor or nurse will advise you on the procedure for
administering the last dose in the cartridge.
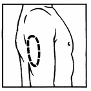
Prepare the injection site by wiping with an antiseptic impregnated swab. Injection sites include
the upper arms, abdomen, and upper thighs. Change the injection sites to avoid discomfort.
Even if you develop a preference for one site, you still should rotate the injection site.
12.
If you are using the passive shield (or no shield) proceed to step 13. If you are
using the active shield, slide the shield onto the pen and push the 2 black lock knobs
on the needle shield toward the tip.
13.
Set the tip of the pen on the prepared injection site and press the needle into the
skin by pushing the pen downward until the shield is totally depressed. Your doctor or nurse will show





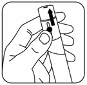
you how to do this. Now you are ready to administer the dose. Press down on the black dose knob.
Wait 5 seconds after the button is pushed, then withdraw the pen from the skin. A drop of blood may
appear. Put a plaster on the injection site if you wish.
14.
Pull the needle shield off the pen (if you have used one) and place the larger needle cap on a flat
surface. Slide the needle in to pick it up and push the cap completely down over the needle. Twist off
the needle and discard it properly. Your doctor or nurse will tell you how to dispose of the items you
have used for the injection. Always store your disposal container out of the reach of children.
15.
Attach the pen cap and return it to its case with the black dose knob pressed in. You should always
store the pen in a refrigerator. Do not remove cartridge between injections.
DO NOT FREEZE.
For subsequent injections with the NutropinAq Pen, attach a new needle, push the white reset
button and dial your dose.
Part II: Storage and Maintenance
Follow these tips to ensure proper care of your NutropinAq Pen:
Always keep your NutropinAq Pen and cartridge refrigerated and protected from light when not
in use.
You may remove the pen and cartridge from the refrigerator up to 45 minutes prior to use.
Do not let your NutropinAq Pen and/or cartridge freeze. Contact your doctor or nurse for a
replacement if either the pen or cartridge does not work.
Avoid excessive temperatures. The solution in the cartridge is stable for up to 28 days after first
use when stored at 2 - 8°C.
If your pen requires cleaning, do not place under water. Use a damp cloth to wipe away dirt. Do
not use alcohol.
When priming a new cartridge, you may need to repeat Part I, steps 8 and 9 up to a total of 6
times (0.6 mg) to remove air bubbles. Small bubbles may remain and will not affect the dose.
The pen should contain the NutropinAq that is being used. Do not remove cartridge between
injections.
The NutropinAq cartridge may be used for up to 28 days.
Do not store the NutropinAq Pen with needle attached.
Part III: Needles for the NutropinAq Pen
Your doctor or nurse will recommend a needle that is appropriate for you. Always use the needles
recommended.
Needles from other countries may not fit on your NutropinAq Pen. If you travel outside the European
Union, make sure you take enough needles for the duration of your stay.
Part IV: Commonly Asked Questions
Q: Do I need to change the needle every time I use my NutropinAq Pen?
A: Yes. A new needle should be used for every injection. The needle is only sterile on the first use.
Q: Where should I store my NutropinAq Pen?
A: Your NutropinAq Pen should be stored in the case, inside a refrigerator when a cartridge is
inserted. When travelling, place your pen case in a cooler.
DO NOT FREEZE.
Q: Why do I keep my medication in the refrigerator?
A: To maintain its strength.
Q: Can I store my NutropinAq Pen in the freezer?
A: No. Freezing will damage the pen and medicine.
Q: How long can I keep my NutropinAq Pen and cartridge outside the refrigerator?
A: We recommend no longer than one hour. Your doctor or nurse will advise you regarding pen
storage.
Q: What is the maximum dose the NutropinAq Pen can deliver in one injection?
A: The NutropinAq Pen can give a minimum dose of 0.1 mg up to a maximum dose of 4.0 mg (40
clicks). If you attempt to dose more than 4 mg at a time, the medicine will either be forced out of the
needle and wasted or excess pressure will be placed upon the cartridge and it may crack.
Q: Is it possible to turn the black dose knob back if I click too many times?
A: Yes. You can turn the black dose knob backwards until the correct number appears in the LCD.
Q: What should I do if there is not enough solution left in the cartridge for my next dose?
A: Your doctor or nurse will advise you what to do for the last dose in the cartridge.
Q: Why do I have to rewind the black dose knob on my NutropinAq Pen every time I replace the
cartridge?
A: This ensures that the plunger push rod completely resets itself back to the starting position. If this is
not done, liquid will come out of the needle when a new cartridge is placed into the pen.
Q: Can I use my NutropinAq Pen without the shields?
A: Yes. Your NutropinAq Pen works without shields. The shields are optional to help you administer
your injection.
Q: What should I do if I drop my NutropinAq Pen?
A: If you drop the NutropinAq Pen, check to see if the cartridge is damaged. You should also check
the pen to see that the black dose knob is moving up and down properly and that the LCD counter is
working. If your cartridge or pen is damaged, ask your doctor or nurse for a replacement.
Q: How long can I use my NutropinAq Pen?
A: The NutropinAq Pen is designed to last 24 months from the time you first use your pen.
Q: What does a blinking ‘bt’ mean in the LCD?
A: The battery in your NutropinAq Pen is losing its charge. Please contact your doctor or nurse for a
replacement pen. Batteries typically last 24 months and have a 4-week life from the time the ‘bt’ first
starts blinking.
Q: How do I replace my NutropinAq Pen?
A: Contact your doctor or nurse if you need a replacement part or if you need to replace your entire
pen.
For more information, please contact the local representative. Your local representative and the
manufacturer for the NutropinAq Pen device are the same as for the medicinal product detailed
overleaf. Please see section 6 overleaf for contact details.
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/nutropinaq.html
Copyright © 1995-2021 ITA all rights reserved.
|



















































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































