Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Oslif Breezhaler 150 microgram inhalation powder, hard capsules
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each capsule contains indacaterol maleate equivalent to 150 microgram indacaterol.
The delivered dose leaving the mouthpiece of the Oslif Breezhaler inhaler is indacaterol maleate
equivalent to 120 microgram indacaterol.
Excipients:
Each capsule contains 24.8 mg lactose.
For a full list of excipients, see section 6.1.
Inhalation powder, hard capsule
Clear colourless capsules containing a white powder, with “IDL 150” printed in black above and
company logo ( ) printed in black below a black bar.
4.1 Therapeutic indications
Oslif Breezhaler is indicated for maintenance bronchodilator treatment of airflow obstruction in adult
patients with chronic obstructive pulmonary disease (COPD).
4.2 Posology and method of administration
Posology
The recommended dose is the inhalation of the content of one 150 microgram capsule once a day,
using the Oslif Breezhaler inhaler. The dose should only be increased on medical advice.
The inhalation of the content of one 300 microgram capsule once a day, using the Oslif Breezhaler
inhaler has been shown to provide additional clinical benefit with regard to breathlessness, particularly
for patients with severe COPD. The maximum dose is 300 microgram once daily.
Oslif Breezhaler should be administered at the same time of the day each day.
If a dose is missed the next dose should be taken at the usual time the next day.
Elderly population
Maximum plasma concentration and overall systemic exposure increase with age but no dose
adjustment is required in elderly patients.
Paediatric population
There is no relevant use of Oslif Breezhaler in the paediatric population (under 18 years).
Hepatic impairment
No dose adjustment is required for patients with mild and moderate hepatic impairment. There are no
data available for use of Oslif Breezhaler in patients with severe hepatic impairment.
Renal impairment
No dose adjustment is required for patients with renal impairment.
Method of administration
For inhalation use only.
Oslif Breezhaler capsules must be administered only using the Oslif Breezhaler inhaler (see section
6.6).
Oslif Breezhaler capsules must not be swallowed.
Hypersensitivity to the active substance, to lactose or to any of the other excipients.
4.4 Special warnings and precautions for use
Asthma
Oslif Breezhaler should not be used in asthma due to the absence of long-term outcome data in asthma
with Oslif Breezhaler.
Paradoxical bronchospasm
As with other inhalation therapy, administration of Oslif Breezhaler may result in paradoxical
bronchospasm that may be life-threatening. If paradoxical bronchospasm occurs Oslif Breezhaler
should be discontinued immediately and alternative therapy substituted.
Deterioration of disease
Oslif Breezhaler is not indicated for the treatment of acute episodes of bronchospasm, i.e. as rescue
therapy. In the event of deterioration of COPD during treatment with Oslif Breezhaler, a re-evaluation
of the patient and of the COPD treatment regimen should be undertaken. An increase in the daily dose
of Oslif Breezhaler beyond the maximum dose of 300 microgram is not appropriate.
Systemic effects
Although no clinically relevant effect on the cardiovascular system is usually seen after the
administration of Oslif Breezhaler at the recommended doses, as with other beta
2
-adrenergic agonists,
indacaterol should be used with caution in patients with cardiovascular disorders (coronary artery
disease, acute myocardial infarction, cardiac arrhythmias, hypertension), in patients with convulsive
disorders or thyrotoxicosis, and in patients who are unusually responsive to beta
2
-adrenergic agonists.
Cardiovascular effects
Like other beta
2
-adrenergic agonists, indacaterol may produce a clinically significant cardiovascular
effect in some patients as measured by increases in pulse rate, blood pressure, and/or symptoms. In
case such effects occur, treatment may need to be discontinued. In addition, beta-adrenergic agonists
have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave and
ST segment depression, although the clinical significance of these observations is unknown.
Clinically relevant effects on prolongation of the QT
c
-interval have not been observed in clinical
studies of Oslif Breezhaler at recommended therapeutic doses (see section 5.1).
Hypokalaemia
Beta
2
-adrenergic agonists may produce significant hypokalaemia in some patients, which has the
potential to produce adverse cardiovascular effects. The decrease in serum potassium is usually
transient, not requiring supplementation. In patients with severe COPD, hypokalaemia may be
potentiated by hypoxia and concomitant treatment (see section 4.5), which may increase the
susceptibility to cardiac arrhythmias.
Hyperglycaemia
Inhalation of high doses of beta
2
-adrenergic agonists may produce increases in plasma glucose. Upon
initiation of treatment with Oslif Breezhaler plasma glucose should be monitored more closely in
diabetic patients.
During clinical studies, clinically notable changes in blood glucose were generally more frequent by
1-2% on Oslif Breezhaler at the recommended doses than on placebo. Oslif Breezhaler has not been
investigated in patients with not well controlled diabetes mellitus.
4.5 Interaction with other medicinal products and other forms of interaction
Sympathomimetic agents
Concomitant administration of other sympathomimetic agents (alone or as part of combination
therapy) may potentiate the undesirable effects of Oslif Breezhaler.
Oslif Breezhaler should not be used in conjunction with other long-acting beta
2
-adrenergic agonists or
medicinal products containing long-acting beta
2
-adrenergic agonists.
Hypokalaemic treatment
Concomitant hypokalaemic treatment with methylxanthine derivatives, steroids, or non-potassium-
sparing diuretics may potentiate the possible hypokalaemic effect of beta
2
-adrenergic agonists,
therefore use with caution (see section 4.4).
Beta-adrenergic blockers
Beta-adrenergic blockers may weaken or antagonise the effect of beta
2
-adrenergic agonists. Therefore
indacaterol should not be given together with beta-adrenergic blockers (including eye drops) unless
there are compelling reasons for their use. Where required, cardioselective beta-adrenergic blockers
should be preferred, although they should be administered with caution.
Metabolic and transporter based interactions
Inhibition of the key contributors of indacaterol clearance, CYP3A4 and P-glycoprotein (P-gp) raises
the systemic exposure of indacaterol by up to two-fold. The magnitude of exposure increases due to
interactions does not raise any safety concerns given the safety experience of treatment with Oslif
Breezhaler in clinical studies of up to one year at doses up to twice the maximum recommended
therapeutic dose.
Indacaterol has not been shown to cause interactions with co-medications.
In vitro
investigations have
indicated that indacaterol has negligible potential to cause metabolic interactions with medicinal
products at the systemic exposure levels achieved in clinical practice.
4.6 Pregnancy and lactation
Pregnancy
There are no data from the use of indacaterol in pregnant women available. Animal studies do not
indicate direct or indirect harmful effects with respect to reproductive toxicity at clinically relevant
exposures (see section 5.3). Like other beta
2
-adrenergic agonists, indacaterol may inhibit labour due to
a relaxant effect on uterine smooth muscle. Oslif Breezhaler should only be used during pregnancy if
the expected benefits outweigh the potential risks.
Lactation
It is not known whether indacaterol/metabolites are excreted in human milk. Available
pharmacokinetic/toxicological data in animals have shown excretion of indacaterol/metabolites in milk
(see section 5.3). A risk to the breast-fed child cannot be excluded. A decision must be made whether
to discontinue breast-feeding or to discontinue/abstain from Oslif Breezhaler therapy, taking into
account the benefit of breast-feeding for the child and the benefit of therapy for the woman.
Fertility
A decreased pregnancy rate has been observed in rats. Nevertheless, it is considered unlikely that
indacaterol will affect reproductive or fertility performance in humans following inhalation of the
maximum recommended dose (see section 5.3).
4.7 Effects on ability to drive and use machines
Oslif Breezhaler has no or negligible influence on the ability to drive and use machines
.
Summary of the safety profile
The most common adverse reactions at the recommended doses were nasopharyngitis (9.1%), cough
(6.8%), upper respiratory tract infection (6.2%) and headache (4.8%). These were in the vast majority
mild or moderate and became less frequent if treatment was continued.
At the recommended doses, the adverse reaction profile of Oslif Breezhaler in patients with COPD
shows clinically insignificant systemic effects of beta
2
-adrenergic stimulation. Mean heart rate changes
were less than one beat per minute, and tachycardia was infrequent and reported at a similar rate as
under placebo treatment. Relevant prolongations of QT
c
F were not detectable in comparison to
placebo. The frequency of notable QT
c
F intervals [i.e. >450 ms (males) and >470 ms (females)] and
reports of hypokalaemia were similar to placebo. The mean of the maximum changes in blood glucose
were similar between Oslif Breezhaler and placebo.
Tabulated summary of adverse reactions
The Oslif Breezhaler Phase III clinical development programme involved patients with a clinical
diagnosis of moderate to severe COPD. 2,154 patients were exposed to indacaterol up to one year at
doses up to twice the maximum recommended dose
.
Of these patients, 627 were on treatment with
150 microgram once daily and 853 on treatment with 300 microgram once daily. Approximately 40%
of patients had severe COPD. The mean age of patients was 63 years, with 47% of patients aged
65 years or older, and the majority (89%) was Caucasian.
Adverse reactions in Table 1
are listed according to MedDRA system organ class in the COPD safety
database. Within each system organ class, adverse reactions are ranked by frequency in descending
order according to the following convention (CIOMS III): Very common (≥1/10); common (≥1/100 to
<1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000), not
known (cannot be estimated from the available data).
Infections and infestations
Nasopharyngitis
Upper respiratory tract infection
Metabolism and nutrition disorders
Diabetes mellitus and hyperglycaemia
Nervous system disorders
Headache
Cardiac disorders
Ischaemic heart disease
Respiratory, thoracic and mediastinal disorders
Cough
Respiratory tract congestion
Musculoskeletal and connective tissue disorders
Muscle spasm
General disorders and administration site conditions
Peripheral oedema
At twice the maximum recommended dose, the safety profile of Oslif Breezhaler was overall similar to
that of recommended doses. Additional adverse reactions were tremor (common) and anaemia
(uncommon).
Description of selected adverse reactions
In Phase III clinical studies, healthcare providers observed during clinic visits that on average 17-20%
of patients experienced a sporadic cough that occurred usually within 15 seconds following inhalation
and typically lasted for 5 seconds (about 10 seconds in current smokers). It was observed with a higher
frequency in female than in male patients and in current smokers than in ex-smokers. This cough
experienced post inhalation was generally well tolerated and did not lead to any patient discontinuing
from the studies at the recommended doses (cough is a symptom in COPD and only 6.8% of patients
overall reported cough as an adverse event). There is no evidence that cough experienced post
inhalation is associated with bronchospasm, exacerbations, deteriorations of disease or loss of efficacy.
In COPD patients, single doses of 10 times the maximum recommended therapeutic dose were
associated with a moderate increase in pulse rate, systolic blood pressure and QT
c
interval.
An overdose of indacaterol is likely to lead to exaggerated effects typical of beta
2
-adrenergic
stimulants, i.e. tachycardia, tremor, palpitations, headache, nausea, vomiting, drowsiness, ventricular
arrhythmias, metabolic acidosis, hypokalaemia and hyperglycaemia.


Supportive and symptomatic treatment is indicated. In serious cases, patients should be hospitalised.
Use of cardioselective beta blockers may be considered, but only under the supervision of a physician
and with extreme caution since the use of beta-adrenergic blockers may provoke bronchospasm.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Long-acting beta
2
-adrenergic agonist, ATC code: R03AC18
Mechanism of action
The pharmacological effects of beta
2
-adrenoceptor agonists are at least in part attributable to
stimulation of intracellular adenyl cyclase, the enzyme that catalyses the conversion of adenosine
triphosphate (ATP) to cyclic-3’, 5’-adenosine monophosphate (cyclic monophosphate). Increased
cyclic AMP levels cause relaxation of bronchial smooth muscle.
In vitro
studies have shown that
indacaterol, a long-acting beta
2
-adrenergic agonist, has more than 24-fold greater agonist activity at
beta
2
-receptors compared to beta
1
-receptors and 20-fold greater agonist activity compared to beta
3
-
receptors.
When inhaled, indacaterol acts locally in the lung as a bronchodilator. Indacaterol is a partial agonist
at the human beta
2
-adrenergic receptor with nanomolar potency. In isolated human bronchus,
indacaterol has a rapid onset of action and a long duration of action.
Although beta
2
-receptors are the predominant adrenergic receptors in bronchial smooth muscle and
beta
1
-receptors are the predominant receptors in the human heart, there are also beta
2
-adrenergic
receptors in the human heart comprising 10-50% of the total adrenergic receptors. The precise function
of beta
2
-adrenergic receptors in the heart is not known, but their presence raises the possibility that
even highly selective beta
2
-adrenergic agonists may have cardiac effects.
Pharmacodynamic effects
Oslif Breezhaler, administered once a day at doses of 150 and 300 microgram consistently provided
clinically significant improvements in lung function (as measured by the forced expiratory volume in
one second, FEV
1
) over 24 hours across a number of clinical pharmacodynamic and efficacy studies.
There was a rapid onset of action within 5 minutes after inhalation, with an increase in FEV
1
relative
to baseline of 110-160 ml, comparable to the effect of the fast-acting beta
2
-agonist salbutamol
200 microgram and statistically significantly faster compared to salmeterol/fluticasone
50/500 microgram. Mean peak improvements in FEV
1
relative to baseline were 250-330 ml at steady
state.
The bronchodilator effect did not depend on the time of dosing, morning or evening.
Oslif Breezhaler was shown to reduce lung hyperinflation, resulting in increased inspiratory capacity
during exercise and at rest, compared to placebo.
Effects on cardiac electrophysiology
A double-blind, placebo- and active (moxifloxacin)-controlled study for 2 weeks in 404 healthy
volunteers demonstrated maximum mean (90% confidence intervals) prolongations of the QT
c
F
interval (in milliseconds) of 2.66 (0.55, 4.77) 2.98 (1.02, 4.93) and 3.34 (0.86, 5.82) following
multiple doses of 150 microgram, 300 microgram and 600 microgram, respectively. Therefore, this
shows no concern for a pro-arrhythmic potential related to QT-interval prolongations at recommended
therapeutic doses or at twice the maximum recommended dose. There was no evidence of a
concentration-delta QT
c
relationship in the range of doses evaluated.
As demonstrated in 605 patients with COPD in a 26-week, double-blind, placebo-controlled Phase III
study, there was no clinically relevant difference in the development of arrhythmic events monitored
over 24 hours, at baseline and up to 3 times during the 26-week treatment period, between patients
receiving recommended doses of Oslif Breezhaler treatment and those patients who received placebo
or treatment with tiotropium.
Clinical efficacy and safety
The clinical development programme included one 12-week, two six-month (one of which was
extended to one year to evaluate safety and tolerability) and one one-year randomised controlled
studies in patients with a clinical diagnosis of COPD. These studies included measures of lung
function and of health outcomes such as dyspnoea, exacerbations and health-related quality of life.
Lung function
Oslif Breezhaler, administered once a day at doses of 150 microgram and 300 microgram, showed
clinically meaningful improvements in lung function. At the 12-week primary endpoint (24-hour
trough FEV
1
), the 150 microgram dose resulted in a 130-180 ml increase compared to placebo
(p<0.001) and a 60 ml increase compared to salmeterol 50 microgram twice a day (p<0.001). The
300 microgram dose resulted in a 170-180 ml increase compared to placebo (p<0.001) and a 100 ml
increase compared to formoterol 12 microgram twice a day (p<0.001). Both doses resulted in an
increase of 40-50 ml over open-label tiotropium 18 microgram once a day (150 microgram, p=0.004;
300 microgram, p=0.01). The 24-hour bronchodilator effect of Oslif Breezhaler was maintained from
the first dose throughout a one-year treatment period with no evidence of loss in efficacy
(tachyphylaxis).
Symptomatic benefits
Both doses demonstrated statistically significant improvements in symptom relief over placebo for
dyspnoea and health status (as evaluated by Transitional Dyspnoea Index [TDI] and St. George’s
Respiratory Questionnaire [SGRQ], respectively). The magnitude of response was generally greater
than seen with active comparators (Table 2). In addition, patients treated with Oslif Breezhaler
required significantly less rescue medication, had more days when no rescue medication was needed
compared to placebo and had a significantly improved percentage of days with no daytime symptoms.
Pooled efficacy analysis over 6 months’ treatment demonstrated that the rate of COPD exacerbations
was statistically significantly lower than the placebo rate. Treatment comparison compared to placebo
showed a ratio of rates of 0.68 (95% CI [ 0.47, 0.98]; p-value 0.036) and 0.74 (95% CI [0.56, 0.96]; p-
value 0.026) for 150 microgram and 300 microgram, respectively.
Limited treatment experience is available in individuals of African descent.
Symptom relief at 6 months treatment duration
Treatment
Dose
(microgram)
Indacaterol
150
once a day
Indacaterol
300
once a day
Salmeterol
50
twice a day
Formoterol
12
twice a day
Percentage of
patients who
achieved MCID
TDI
†
Percentage of
patients who
achieved MCID
SGRQ
†
Reduction in
puffs/day of
rescue
medication use
vs. baseline
Percentage of
days with no
rescue
medication use
Study design with
a
: indacaterol 150 microgram, salmeterol and placebo;
b
: indacaterol 150 and
300 microgram, tiotropium and placebo;
c
: indacaterol 300 microgram, formoterol and placebo
†
MCID = minimal clinically important difference (≥1 point change in TDI, ≥4 point change in SGRQ)
n/e= not evaluated at six months
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Oslif
Breezhaler in all subsets of the paediatric population in chronic obstructive pulmonary disease
(COPD) (see section 4.2 for information on paediatric use).
5.2 Pharmacokinetic properties
Indacaterol is a chiral molecule with R-configuration.
Pharmacokinetic data were obtained from a number of clinical studies, from healthy volunteers and
COPD patients.
Absorption
The median time to reach peak serum concentrations of indacaterol was approximately 15 min after
single or repeated inhaled doses. Systemic exposure to indacaterol increased with increasing dose
(150 microgram to 600 microgram) in a dose proportional manner. Absolute bioavailability of
indacaterol after an inhaled dose was on average 43% to 45%. Systemic exposure results from a
composite of pulmonary and gastrointestinal absorption; about 75% of systemic exposure was from
pulmonary absorption and about 25% from gastrointestinal absorption.
Indacaterol serum concentrations increased with repeated once-daily administration. Steady state was
achieved within 12 to 14 days. The mean accumulation ratio of indacaterol, i.e. AUC over the 24-h
dosing interval on Day 14 compared to Day 1, was in the range of 2.9 to 3.5 for once-daily inhaled
doses between 150 microgram and 600 microgram.














Distribution
After intravenous infusion the volume of distribution of indacaterol during the terminal elimination
phase was 2557 litres indicating an extensive distribution. The
in vitro
human serum and plasma
protein binding was 94.1-95.3% and 95.1-96.2%, respectively.
Biotransformation
After oral administration of radiolabelled indacaterol in a human ADME (absorption, distribution,
metabolism, excretion) study, unchanged indacaterol was the main component in serum, accounting
for about one third of total drug-related AUC over 24 hours. A hydroxylated derivative was the most
prominent metabolite in serum. Phenolic O-glucuronides of indacaterol and hydroxylated indacaterol
were further prominent metabolites. A diastereomer of the hydroxylated derivative, a N-glucuronide of
indacaterol, and C- and N-dealkylated products were further metabolites identified.
In vitro
investigations indicated that UGT1A1 is the only UGT isoform that metabolised indacaterol to
the phenolic O-glucuronide. The oxidative metabolites were found in incubations with recombinant
CYP1A1, CYP2D6, and CYP3A4. CYP3A4 is concluded to be the predominant isoenzyme
responsible for hydroxylation of indacaterol.
In vitro
investigations further indicated that indacaterol is
a low affinity substrate for the efflux pump P-gp.
Elimination
In clinical studies which included urine collection, the amount of indacaterol excreted unchanged via
urine was generally lower than 2% of the dose. Renal clearance of indacaterol was, on average,
between 0.46 and 1.20 litres/hour. When compared with the serum clearance of indacaterol of
23.3 litres/hour, it is evident that renal clearance plays a minor role (about 2 to 5% of systemic
clearance) in the elimination of systemically available indacaterol.
In a human ADME study where indacaterol was given orally, the faecal route of excretion was
dominant over the urinary route. Indacaterol was excreted into human faeces primarily as unchanged
parent substance (54% of the dose) and, to a lesser extent, hydroxylated indacaterol metabolites (23%
of the dose). Mass balance was complete with ≥90% of the dose recovered in the excreta.
Indacaterol serum concentrations declined in a multi-phasic manner with an average terminal half-life
ranging from 45.5 to 126 hours. The effective half-life, calculated from the accumulation of
indacaterol after repeated dosing ranged from 40 to 52 hours which is consistent with the observed
time-to-steady state of approximately 12-14 days.
Special populations
A population pharmacokinetic analysis showed that there is no clinically relevant effect of age (adults
up to 88 years), sex, weight (32-168 kg) or race on the pharmacokinetics of indacaterol. It did not
suggest any difference between ethnic subgroups in this population.
Patients with mild and moderate hepatic impairment showed no relevant changes in C
max
or AUC of
indacaterol, nor did protein binding differ between mild and moderate hepatic impaired subjects and
their healthy controls. Studies in subjects with severe hepatic impairment were not performed.
Due to the very low contribution of the urinary pathway to total body elimination, a study in renally
impaired subjects was not performed.
5.3 Preclinical safety data
Effects on the cardiovascular system attributable to the beta
2
-agonistic properties of indacaterol
included tachycardia, arrhythmias and myocardial lesions in dogs. Mild irritancy of the nasal cavity
and larynx were seen in rodents. All these findings occurred at exposures sufficiently in excess of
those anticipated in humans.
Although indacaterol did not affect general reproductive performance in a rat fertility study, a decrease
in the number of pregnant F
1
offspring was observed in the peri- and post-developmental rat study at
an exposure 14-fold higher than in humans treated with Oslif Breezhaler. Indacaterol was not
embryotoxic or teratogenic in rats or rabbits.
Genotoxicity studies did not reveal any mutagenic or clastogenic potential. Carcinogenicity was
assessed in a two-year rat study and a six-month transgenic mouse study. Increased incidences of
benign ovarian leiomyoma and focal hyperplasia of ovarian smooth muscle in rats were consistent
with similar findings reported for other beta
2
-adrenergic agonists. No evidence of carcinogenicity was
seen in mice. Systemic exposures (AUC) in rats and mice at the no-observed adverse effect levels in
these studies were at least 7- and 49-fold higher, respectively, than in humans treated with Oslif
Breezhaler once a day at a dose of 300 microgram.
PHARMACEUTICAL PARTICULARS
Capsule content
Lactose monohydrate
6.4 Special precautions for storage
Oslif Breezhaler capsules must always be stored in the blister to protect from moisture and only
removed immediately before use.
6.5 Nature and contents of container
Oslif Breezhaler is a single-dose inhalation device. Inhaler body and cap are made from acrylonitrile
butadiene styrene, push buttons are made from methyl methacrylate acrylonitrile butadiene styrene.
Needles and springs are made from stainless steel.
PA/Alu/PVC - Alu blister packs, containing 10 hard capsules, with an inhaler made from plastic
materials provided in each pack.
Carton containing 10 capsules (1x10 capsule blister strips) and one Oslif Breezhaler inhaler.
Carton containing 30 capsules (3x10 capsule blister strips) and one Oslif Breezhaler inhaler.
Multipack comprising 2 packs (each containing 30 capsules and 1 inhaler).
Multipack comprising 3 packs (each containing 30 capsules and 1 inhaler).
Multipack comprising 30 packs (each containing 10 capsules and 1 inhaler).
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
The Oslif Breezhaler inhaler provided with each new prescription should be used. Dispose of each
inhaler after 30 days of use.
Instructions for handling and use
Open inhaler:
Hold the base of the inhaler firmly and tilt the
mouthpiece. This opens the inhaler.
Prepare capsule:
Immediately before use,
with dry hands, remove
one capsule from the blister.
Insert capsule:
Place the capsule into the capsule chamber.
Never place a capsule directly into the
mouthpiece.











Close the inhaler:
Close the inhaler until you hear a “click”.
Pierce the capsule:
Hold the inhaler upright with the
mouthpiece pointing up.
Pierce the capsule by firmly pressing
together both side buttons at the same time.
Do this only once.
You should hear a “click” as the capsule is
being pierced.
Release the side buttons fully.
Breathe out:
Before placing the mouthpiece in your mouth,
breathe out fully.
Do not blow into the mouthpiece.
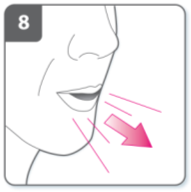
Inhale the medicine
To breathe the medicine deeply into your
airways:
Hold the inhaler as shown in the picture.
The side buttons should be facing left and
right. Do not press the side buttons.
Place the mouthpiece in your mouth and
close your lips firmly around it.
Breathe in rapidly but steadily and as
deeply as you can.



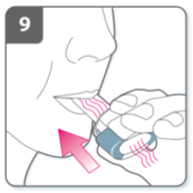








Note:
As you breathe in through the inhaler, the capsule
spins around in the chamber and you should hear
a whirring noise. You will experience a sweet
flavour as the medicine goes into your lungs.
Additional information
Occasionally, very small pieces of the capsule can
get past the screen and enter your mouth. If this
happens, you may be able to feel these pieces on
your tongue. It is not harmful if these pieces are
swallowed or inhaled. The chances of the capsule
shattering will be increased if the capsule is
accidentally pierced more than once (step 6).
If you do not hear a whirring noise:
The capsule may be stuck in the capsule chamber.
If this happens:
Open the inhaler and carefully loosen the
capsule by tapping the base of the inhaler.
Do not press the side buttons.
Inhale the medicine again by repeating
steps 8 and 9.
Hold breath:
After you have inhaled the medicine:
Hold your breath for at least 5-10 seconds
or as long as you comfortably can while
taking the inhaler out of your mouth.
Open the inhaler to see if any powder is left
in the capsule.
If there is powder left in the capsule:
Repeat steps 8, 9, 10 and 11.
Most people are able to empty the capsule with
one or two inhalations.
Additional information
Some people may occasionally cough briefly
soon after inhaling the medicine. If you do, don’t
worry. As long as the capsule is empty, you have
received enough of your medicine.
After you have finished taking
your medicine:
Open the mouthpiece again, and remove
the empty capsule by tipping it out of the
capsule chamber. Put the empty capsule in
your household waste.
Close the inhaler and replace the cap.
Do not store the capsules in the Oslif
Breezhaler inhaler.








Mark daily dose tracker:
On the inside of the pack there is a daily dose
tracker. Put a mark in today’s box if it helps to
remind you of when your next dose is due.
MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT





NAME OF THE MEDICINAL PRODUCT
Oslif Breezhaler 300 microgram inhalation powder, hard capsules
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each capsule contains indacaterol maleate equivalent to 300 microgram indacaterol.
The delivered dose leaving the mouthpiece of the Oslif Breezhaler inhaler is indacaterol maleate
equivalent to 240 microgram indacaterol.
Excipients:
Each capsule contains 24.6 mg lactose.
For a full list of excipients, see section 6.1.
Inhalation powder, hard capsule
Clear colourless capsules containing a white powder, with “IDL 300” printed in blue above and
company logo ( ) printed in blue below a blue bar.
4.1 Therapeutic indications
Oslif Breezhaler is indicated for maintenance bronchodilator treatment of airflow obstruction in adult
patients with chronic obstructive pulmonary disease (COPD).
4.2 Posology and method of administration
Posology
The recommended dose is the inhalation of the content of one 150 microgram capsule once a day,
using the Oslif Breezhaler inhaler. The dose should only be increased on medical advice.
The inhalation of the content of one 300 microgram capsule once a day, using the Oslif Breezhaler
inhaler has been shown to provide additional clinical benefit with regard to breathlessness, particularly
for patients with severe COPD. The maximum dose is 300 microgram once daily.
Oslif Breezhaler should be administered at the same time of the day each day.
If a dose is missed the next dose should be taken at the usual time the next day.
Elderly population
Maximum plasma concentration and overall systemic exposure increase with age but no dose
adjustment is required in elderly patients.
Paediatric population
There is no relevant use of Oslif Breezhaler in the paediatric population (under 18 years).
Hepatic impairment
No dose adjustment is required for patients with mild and moderate hepatic impairment. There are no
data available for use of Oslif Breezhaler in patients with severe hepatic impairment.

Renal impairment
No dose adjustment is required for patients with renal impairment.
Method of administration
For inhalation use only.
Oslif Breezhaler capsules must be administered only using the Oslif Breezhaler inhaler (see section
6.6).
Oslif Breezhaler capsules must not be swallowed.
Hypersensitivity to the active substance, to lactose or to any of the other excipients.
4.4 Special warnings and precautions for use
Asthma
Oslif Breezhaler should not be used in asthma due to the absence of long-term outcome data in asthma
with Oslif Breezhaler.
Paradoxical bronchospasm
As with other inhalation therapy, administration of Oslif Breezhaler may result in paradoxical
bronchospasm that may be life-threatening. If paradoxical bronchospasm occurs Oslif Breezhaler
should be discontinued immediately and alternative therapy substituted.
Deterioration of disease
Oslif Breezhaler is not indicated for the treatment of acute episodes of bronchospasm, i.e. as rescue
therapy. In the event of deterioration of COPD during treatment with Oslif Breezhaler, a re-evaluation
of the patient and of the COPD treatment regimen should be undertaken. An increase in the daily dose
of Oslif Breezhaler beyond the maximum dose of 300 microgram is not appropriate.
Systemic effects
Although no clinically relevant effect on the cardiovascular system is usually seen after the
administration of Oslif Breezhaler at the recommended doses, as with other beta
2
-adrenergic agonists,
indacaterol should be used with caution in patients with cardiovascular disorders (coronary artery
disease, acute myocardial infarction, cardiac arrhythmias, hypertension), in patients with convulsive
disorders or thyrotoxicosis, and in patients who are unusually responsive to beta
2
-adrenergic agonists.
Cardiovascular effects
Like other beta
2
-adrenergic agonists, indacaterol may produce a clinically significant cardiovascular
effect in some patients as measured by increases in pulse rate, blood pressure, and/or symptoms. In
case such effects occur, treatment may need to be discontinued. In addition, beta-adrenergic agonists
have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave and
ST segment depression, although the clinical significance of these observations is unknown.
Clinically relevant effects on prolongation of the QT
c
-interval have not been observed in clinical
studies of Oslif Breezhaler at recommended therapeutic doses (see section 5.1).
Hypokalaemia
Beta
2
-adrenergic agonists may produce significant hypokalaemia in some patients, which has the
potential to produce adverse cardiovascular effects. The decrease in serum potassium is usually
transient, not requiring supplementation. In patients with severe COPD, hypokalaemia may be
potentiated by hypoxia and concomitant treatment (see section 4.5), which may increase the
susceptibility to cardiac arrhythmias.
Hyperglycaemia
Inhalation of high doses of beta
2
-adrenergic agonists may produce increases in plasma glucose. Upon
initiation of treatment with Oslif Breezhaler plasma glucose should be monitored more closely in
diabetic patients.
During clinical studies, clinically notable changes in blood glucose were generally more frequent by
1-2% on Oslif Breezhaler at the recommended doses than on placebo. Oslif Breezhaler has not been
investigated in patients with not well controlled diabetes mellitus.
4.5 Interaction with other medicinal products and other forms of interaction
Sympathomimetic agents
Concomitant administration of other sympathomimetic agents (alone or as part of combination
therapy) may potentiate the undesirable effects of Oslif Breezhaler.
Oslif Breezhaler should not be used in conjunction with other long-acting beta
2
-adrenergic agonists or
medicinal products containing long-acting beta
2
-adrenergic agonists.
Hypokalaemic treatment
Concomitant hypokalaemic treatment with methylxanthine derivatives, steroids, or non-potassium-
sparing diuretics may potentiate the possible hypokalaemic effect of beta
2
-adrenergic agonists,
therefore use with caution (see section 4.4).
Beta-adrenergic blockers
Beta-adrenergic blockers may weaken or antagonise the effect of beta
2
-adrenergic agonists. Therefore
indacaterol should not be given together with beta-adrenergic blockers (including eye drops) unless
there are compelling reasons for their use. Where required, cardioselective beta-adrenergic blockers
should be preferred, although they should be administered with caution.
Metabolic and transporter based interactions
Inhibition of the key contributors of indacaterol clearance, CYP3A4 and P-glycoprotein (P-gp) raises
the systemic exposure of indacaterol by up to two-fold. The magnitude of exposure increases due to
interactions does not raise any safety concerns given the safety experience of treatment with Oslif
Breezhaler in clinical studies of up to one year at doses up to twice the maximum recommended
therapeutic dose.
Indacaterol has not been shown to cause interactions with co-medications.
In vitro
investigations have
indicated that indacaterol has negligible potential to cause metabolic interactions with medicinal
products at the systemic exposure levels achieved in clinical practice.
4.6 Pregnancy and lactation
Pregnancy
There are no data from the use of indacaterol in pregnant women available. Animal studies do not
indicate direct or indirect harmful effects with respect to reproductive toxicity at clinically relevant
exposures (see section 5.3). Like other beta
2
-adrenergic agonists, indacaterol may inhibit labour due to
a relaxant effect on uterine smooth muscle. Oslif Breezhaler should only be used during pregnancy if
the expected benefits outweigh the potential risks.
Lactation
It is not known whether indacaterol/metabolites are excreted in human milk. Available
pharmacokinetic/toxicological data in animals have shown excretion of indacaterol/metabolites in milk
(see section 5.3). A risk to the breast-fed child cannot be excluded. A decision must be made whether
to discontinue breast-feeding or to discontinue/abstain from Oslif Breezhaler therapy, taking into
account the benefit of breast-feeding for the child and the benefit of therapy for the woman.
Fertility
A decreased pregnancy rate has been observed in rats. Nevertheless, it is considered unlikely that
indacaterol will affect reproductive or fertility performance in humans following inhalation of the
maximum recommended dose (see section 5.3).
4.7 Effects on ability to drive and use machines
Oslif Breezhaler has no or negligible influence on the ability to drive and use machines
.
Summary of the safety profile
The most common adverse reactions at the recommended doses were nasopharyngitis (9.1%), cough
(6.8%), upper respiratory tract infection (6.2%) and headache (4.8%). These were in the vast majority
mild or moderate and became less frequent if treatment was continued.
At the recommended doses, the adverse reaction profile of Oslif Breezhaler in patients with COPD
shows clinically insignificant systemic effects of beta
2
-adrenergic stimulation. Mean heart rate changes
were less than one beat per minute, and tachycardia was infrequent and reported at a similar rate as
under placebo treatment. Relevant prolongations of QT
c
F were not detectable in comparison to
placebo. The frequency of notable QT
c
F intervals [i.e. >450 ms (males) and >470 ms (females)] and
reports of hypokalaemia were similar to placebo. The mean of the maximum changes in blood glucose
were similar between Oslif Breezhaler and placebo.
Tabulated summary of adverse reactions
The Oslif Breezhaler Phase III clinical development programme involved patients with a clinical
diagnosis of moderate to severe COPD. 2,154 patients were exposed to indacaterol up to one year at
doses up to twice the maximum recommended dose
.
Of these patients, 627 were on treatment with
150 microgram once daily and 853 on treatment with 300 microgram once daily. Approximately 40%
of patients had severe COPD. The mean age of patients was 63 years, with 47% of patients aged
65 years or older, and the majority (89%) was Caucasian.
Adverse reactions in Table 1
are listed according to MedDRA system organ class in the COPD safety
database. Within each system organ class, adverse reactions are ranked by frequency in descending
order according to the following convention (CIOMS III): Very common (≥1/10); common (≥1/100 to
<1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000), not
known (cannot be estimated from the available data).
Infections and infestations
Nasopharyngitis
Upper respiratory tract infection
Metabolism and nutrition disorders
Diabetes mellitus and hyperglycaemia
Nervous system disorders
Headache
Cardiac disorders
Ischaemic heart disease
Respiratory, thoracic and mediastinal disorders
Cough
Respiratory tract congestion
Musculoskeletal and connective tissue disorders
Muscle spasm
General disorders and administration site conditions
Peripheral oedema
At twice the maximum recommended dose, the safety profile of Oslif Breezhaler was overall similar to
that of recommended doses. Additional adverse reactions were tremor (common) and anaemia
(uncommon).
Description of selected adverse reactions
In Phase III clinical studies, healthcare providers observed during clinic visits that on average 17-20%
of patients experienced a sporadic cough that occurred usually within 15 seconds following inhalation
and typically lasted for 5 seconds (about 10 seconds in current smokers). It was observed with a higher
frequency in female than in male patients and in current smokers than in ex-smokers. This cough
experienced post inhalation was generally well tolerated and did not lead to any patient discontinuing
from the studies at the recommended doses (cough is a symptom in COPD and only 6.8% of patients
overall reported cough as an adverse event). There is no evidence that cough experienced post
inhalation is associated with bronchospasm, exacerbations, deteriorations of disease or loss of efficacy.
In COPD patients, single doses of 10 times the maximum recommended therapeutic dose were
associated with a moderate increase in pulse rate, systolic blood pressure and QT
c
interval.
An overdose of indacaterol is likely to lead to exaggerated effects typical of beta
2
-adrenergic
stimulants, i.e. tachycardia, tremor, palpitations, headache, nausea, vomiting, drowsiness, ventricular
arrhythmias, metabolic acidosis, hypokalaemia and hyperglycaemia.


Supportive and symptomatic treatment is indicated. In serious cases, patients should be hospitalised.
Use of cardioselective beta blockers may be considered, but only under the supervision of a physician
and with extreme caution since the use of beta-adrenergic blockers may provoke bronchospasm.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Long-acting beta
2
-adrenergic agonist, ATC code: R03AC18
Mechanism of action
The pharmacological effects of beta
2
-adrenoceptor agonists are at least in part attributable to
stimulation of intracellular adenyl cyclase, the enzyme that catalyses the conversion of adenosine
triphosphate (ATP) to cyclic-3’, 5’-adenosine monophosphate (cyclic monophosphate). Increased
cyclic AMP levels cause relaxation of bronchial smooth muscle.
In vitro
studies have shown that
indacaterol, a long-acting beta
2
-adrenergic agonist, has more than 24-fold greater agonist activity at
beta
2
-receptors compared to beta
1
-receptors and 20-fold greater agonist activity compared to beta
3
-
receptors.
When inhaled, indacaterol acts locally in the lung as a bronchodilator. Indacaterol is a partial agonist
at the human beta
2
-adrenergic receptor with nanomolar potency. In isolated human bronchus,
indacaterol has a rapid onset of action and a long duration of action.
Although beta
2
-receptors are the predominant adrenergic receptors in bronchial smooth muscle and
beta
1
-receptors are the predominant receptors in the human heart, there are also beta
2
-adrenergic
receptors in the human heart comprising 10-50% of the total adrenergic receptors. The precise function
of beta
2
-adrenergic receptors in the heart is not known, but their presence raises the possibility that
even highly selective beta
2
-adrenergic agonists may have cardiac effects.
Pharmacodynamic effects
Oslif Breezhaler, administered once a day at doses of 150 and 300 microgram consistently provided
clinically significant improvements in lung function (as measured by the forced expiratory volume in
one second, FEV
1
) over 24 hours across a number of clinical pharmacodynamic and efficacy studies.
There was a rapid onset of action within 5 minutes after inhalation, with an increase in FEV
1
relative
to baseline of 110-160 ml, comparable to the effect of the fast-acting beta
2
-agonist salbutamol
200 microgram and statistically significantly faster compared to salmeterol/fluticasone
50/500 microgram. Mean peak improvements in FEV
1
relative to baseline were 250-330 ml at steady
state.
The bronchodilator effect did not depend on the time of dosing, morning or evening.
Oslif Breezhaler was shown to reduce lung hyperinflation, resulting in increased inspiratory capacity
during exercise and at rest, compared to placebo.
Effects on cardiac electrophysiology
A double-blind, placebo- and active (moxifloxacin)-controlled study for 2 weeks in 404 healthy
volunteers demonstrated maximum mean (90% confidence intervals) prolongations of the QT
c
F
interval (in milliseconds) of 2.66 (0.55, 4.77) 2.98 (1.02, 4.93) and 3.34 (0.86, 5.82) following
multiple doses of 150 microgram, 300 microgram and 600 microgram, respectively. Therefore, this
shows no concern for a pro-arrhythmic potential related to QT-interval prolongations at recommended
therapeutic doses or at twice the maximum recommended dose. There was no evidence of a
concentration-delta QT
c
relationship in the range of doses evaluated.
As demonstrated in 605 patients with COPD in a 26-week, double-blind, placebo-controlled Phase III
study, there was no clinically relevant difference in the development of arrhythmic events monitored
over 24 hours, at baseline and up to 3 times during the 26-week treatment period, between patients
receiving recommended doses of Oslif Breezhaler treatment and those patients who received placebo
or treatment with tiotropium.
Clinical efficacy and safety
The clinical development programme included one 12-week, two six-month (one of which was
extended to one year to evaluate safety and tolerability) and one one-year randomised controlled
studies in patients with a clinical diagnosis of COPD. These studies included measures of lung
function and of health outcomes such as dyspnoea, exacerbations and health-related quality of life.
Lung function
Oslif Breezhaler, administered once a day at doses of 150 microgram and 300 microgram, showed
clinically meaningful improvements in lung function. At the 12-week primary endpoint (24-hour
trough FEV
1
), the 150 microgram dose resulted in a 130-180 ml increase compared to placebo
(p<0.001) and a 60 ml increase compared to salmeterol 50 microgram twice a day (p<0.001). The
300 microgram dose resulted in a 170-180 ml increase compared to placebo (p<0.001) and a 100 ml
increase compared to formoterol 12 microgram twice a day (p<0.001). Both doses resulted in an
increase of 40-50 ml over open-label tiotropium 18 microgram once a day (150 microgram, p=0.004;
300 microgram, p=0.01). The 24-hour bronchodilator effect of Oslif Breezhaler was maintained from
the first dose throughout a one-year treatment period with no evidence of loss in efficacy
(tachyphylaxis).
Symptomatic benefits
Both doses demonstrated statistically significant improvements in symptom relief over placebo for
dyspnoea and health status (as evaluated by Transitional Dyspnoea Index [TDI] and St. George’s
Respiratory Questionnaire [SGRQ], respectively). The magnitude of response was generally greater
than seen with active comparators (Table 2). In addition, patients treated with Oslif Breezhaler
required significantly less rescue medication, had more days when no rescue medication was needed
compared to placebo and had a significantly improved percentage of days with no daytime symptoms.
Pooled efficacy analysis over 6 months’ treatment demonstrated that the rate of COPD exacerbations
was statistically significantly lower than the placebo rate. Treatment comparison compared to placebo
showed a ratio of rates of 0.68 (95% CI [ 0.47, 0.98]; p-value 0.036) and 0.74 (95% CI [0.56, 0.96]; p-
value 0.026) for 150 microgram and 300 microgram, respectively.
Limited treatment experience is available in individuals of African descent.
Symptom relief at 6 months treatment duration
Treatment
Dose
(microgram)
Indacaterol
150
once a day
Indacaterol
300
once a day
Salmeterol
50
twice a day
Formoterol
12
twice a day
Percentage of
patients who
achieved MCID
TDI
†
Percentage of
patients who
achieved MCID
SGRQ
†
Reduction in
puffs/day of
rescue
medication use
vs. baseline
Percentage of
days with no
rescue
medication use
Study design with
a
: indacaterol 150 microgram, salmeterol and placebo;
b
: indacaterol 150 and
300 microgram, tiotropium and placebo;
c
: indacaterol 300 microgram, formoterol and placebo
†
MCID = minimal clinically important difference (≥1 point change in TDI, ≥4 point change in SGRQ)
n/e= not evaluated at six months
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Oslif
Breezhaler in all subsets of the paediatric population in chronic obstructive pulmonary disease
(COPD) (see section 4.2 for information on paediatric use).
5.2 Pharmacokinetic properties
Indacaterol is a chiral molecule with R-configuration.
Pharmacokinetic data were obtained from a number of clinical studies, from healthy volunteers and
COPD patients.
Absorption
The median time to reach peak serum concentrations of indacaterol was approximately 15 min after
single or repeated inhaled doses. Systemic exposure to indacaterol increased with increasing dose
(150 microgram to 600 microgram) in a dose proportional manner. Absolute bioavailability of
indacaterol after an inhaled dose was on average 43% to 45%. Systemic exposure results from a
composite of pulmonary and gastrointestinal absorption; about 75% of systemic exposure was from
pulmonary absorption and about 25% from gastrointestinal absorption.
Indacaterol serum concentrations increased with repeated once-daily administration. Steady state was
achieved within 12 to 14 days. The mean accumulation ratio of indacaterol, i.e. AUC over the 24-h
dosing interval on Day 14 compared to Day 1, was in the range of 2.9 to 3.5 for once-daily inhaled
doses between 150 microgram and 600 microgram.














Distribution
After intravenous infusion the volume of distribution of indacaterol during the terminal elimination
phase was 2557 litres indicating an extensive distribution. The
in vitro
human serum and plasma
protein binding was 94.1-95.3% and 95.1-96.2%, respectively.
Biotransformation
After oral administration of radiolabelled indacaterol in a human ADME (absorption, distribution,
metabolism, excretion) study, unchanged indacaterol was the main component in serum, accounting
for about one third of total drug-related AUC over 24 hours. A hydroxylated derivative was the most
prominent metabolite in serum. Phenolic O-glucuronides of indacaterol and hydroxylated indacaterol
were further prominent metabolites. A diastereomer of the hydroxylated derivative, a N-glucuronide of
indacaterol, and C- and N-dealkylated products were further metabolites identified.
In vitro
investigations indicated that UGT1A1 is the only UGT isoform that metabolised indacaterol to
the phenolic O-glucuronide. The oxidative metabolites were found in incubations with recombinant
CYP1A1, CYP2D6, and CYP3A4. CYP3A4 is concluded to be the predominant isoenzyme
responsible for hydroxylation of indacaterol.
In vitro
investigations further indicated that indacaterol is
a low affinity substrate for the efflux pump P-gp.
Elimination
In clinical studies which included urine collection, the amount of indacaterol excreted unchanged via
urine was generally lower than 2% of the dose. Renal clearance of indacaterol was, on average,
between 0.46 and 1.20 litres/hour. When compared with the serum clearance of indacaterol of
23.3 litres/hour, it is evident that renal clearance plays a minor role (about 2 to 5% of systemic
clearance) in the elimination of systemically available indacaterol.
In a human ADME study where indacaterol was given orally, the faecal route of excretion was
dominant over the urinary route. Indacaterol was excreted into human faeces primarily as unchanged
parent substance (54% of the dose) and, to a lesser extent, hydroxylated indacaterol metabolites (23%
of the dose). Mass balance was complete with ≥90% of the dose recovered in the excreta.
Indacaterol serum concentrations declined in a multi-phasic manner with an average terminal half-life
ranging from 45.5 to 126 hours. The effective half-life, calculated from the accumulation of
indacaterol after repeated dosing ranged from 40 to 52 hours which is consistent with the observed
time-to-steady state of approximately 12-14 days.
Special populations
A population pharmacokinetic analysis showed that there is no clinically relevant effect of age (adults
up to 88 years), sex, weight (32-168 kg) or race on the pharmacokinetics of indacaterol. It did not
suggest any difference between ethnic subgroups in this population.
Patients with mild and moderate hepatic impairment showed no relevant changes in C
max
or AUC of
indacaterol, nor did protein binding differ between mild and moderate hepatic impaired subjects and
their healthy controls. Studies in subjects with severe hepatic impairment were not performed.
Due to the very low contribution of the urinary pathway to total body elimination, a study in renally
impaired subjects was not performed.
5.3 Preclinical safety data
Effects on the cardiovascular system attributable to the beta
2
-agonistic properties of indacaterol
included tachycardia, arrhythmias and myocardial lesions in dogs. Mild irritancy of the nasal cavity
and larynx were seen in rodents. All these findings occurred at exposures sufficiently in excess of
those anticipated in humans.
Although indacaterol did not affect general reproductive performance in a rat fertility study, a decrease
in the number of pregnant F
1
offspring was observed in the peri- and post-developmental rat study at
an exposure 14-fold higher than in humans treated with Oslif Breezhaler. Indacaterol was not
embryotoxic or teratogenic in rats or rabbits.
Genotoxicity studies did not reveal any mutagenic or clastogenic potential. Carcinogenicity was
assessed in a two-year rat study and a six-month transgenic mouse study. Increased incidences of
benign ovarian leiomyoma and focal hyperplasia of ovarian smooth muscle in rats were consistent
with similar findings reported for other beta
2
-adrenergic agonists. No evidence of carcinogenicity was
seen in mice. Systemic exposures (AUC) in rats and mice at the no-observed adverse effect levels in
these studies were at least 7- and 49-fold higher, respectively, than in humans treated with Oslif
Breezhaler once a day at a dose of 300 microgram.
PHARMACEUTICAL PARTICULARS
Capsule content
Lactose monohydrate
6.4 Special precautions for storage
Oslif Breezhaler capsules must always be stored in the blister to protect from moisture and only
removed immediately before use.
6.5 Nature and contents of container
Oslif Breezhaler is a single-dose inhalation device. Inhaler body and cap are made from acrylonitrile
butadiene styrene, push buttons are made from methyl methacrylate acrylonitrile butadiene styrene.
Needles and springs are made from stainless steel.
PA/Alu/PVC - Alu blister packs, containing 10 hard capsules, with an inhaler made from plastic
materials provided in each pack.
Carton containing 10 capsules (1x10 capsule blister strips) and one Oslif Breezhaler inhaler.
Carton containing 30 capsules (3x10 capsule blister strips) and one Oslif Breezhaler inhaler.
Multipack comprising 2 packs (each containing 30 capsules and 1 inhaler).
Multipack comprising 3 packs (each containing 30 capsules and 1 inhaler).
Multipack comprising 30 packs (each containing 10 capsules and 1 inhaler).
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
The Oslif Breezhaler inhaler provided with each new prescription should be used. Dispose of each
inhaler after 30 days of use.
Instructions for handling and use
Open inhaler:
Hold the base of the inhaler firmly and tilt the
mouthpiece. This opens the inhaler.
Prepare capsule:
Immediately before use,
with dry hands, remove
one capsule from the blister.
Insert capsule:
Place the capsule into the capsule chamber.
Never place a capsule directly into the
mouthpiece.



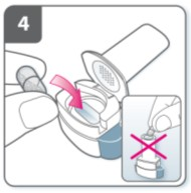







Close the inhaler:
Close the inhaler until you hear a “click”.
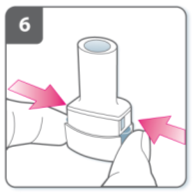
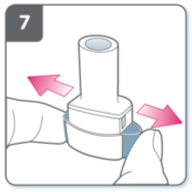
Pierce the capsule:
Hold the inhaler upright with the
mouthpiece pointing up.
Pierce the capsule by firmly pressing
together both side buttons at the same time.
Do this only once.
You should hear a “click” as the capsule is
being pierced.
Release the side buttons fully.
Breathe out:
Before placing the mouthpiece in your mouth,
breathe out fully.
Do not blow into the mouthpiece.
Inhale the medicine
To breathe the medicine deeply into your
airways:
Hold the inhaler as shown in the picture.
The side buttons should be facing left and
right. Do not press the side buttons.
Place the mouthpiece in your mouth and
close your lips firmly around it.
Breathe in rapidly but steadily and as
deeply as you can.











Note:
As you breathe in through the inhaler, the capsule
spins around in the chamber and you should hear
a whirring noise. You will experience a sweet
flavour as the medicine goes into your lungs.
Additional information
Occasionally, very small pieces of the capsule can
get past the screen and enter your mouth. If this
happens, you may be able to feel these pieces on
your tongue. It is not harmful if these pieces are
swallowed or inhaled. The chances of the capsule
shattering will be increased if the capsule is
accidentally pierced more than once (step 6).
If you do not hear a whirring noise:
The capsule may be stuck in the capsule chamber.
If this happens:
Open the inhaler and carefully loosen the
capsule by tapping the base of the inhaler.
Do not press the side buttons.
Inhale the medicine again by repeating
steps 8 and 9.
Hold breath:
After you have inhaled the medicine:
Hold your breath for at least 5-10 seconds
or as long as you comfortably can while
taking the inhaler out of your mouth.
Open the inhaler to see if any powder is left
in the capsule.
If there is powder left in the capsule:
Repeat steps 8, 9, 10 and 11.
Most people are able to empty the capsule with
one or two inhalations.
Additional information
Some people may occasionally cough briefly
soon after inhaling the medicine. If you do, don’t
worry. As long as the capsule is empty, you have
received enough of your medicine.
After you have finished taking
your medicine:
Open the mouthpiece again, and remove
the empty capsule by tipping it out of the
capsule chamber. Put the empty capsule in
your household waste.
Close the inhaler and replace the cap.
Do not store the capsules in the Oslif
Breezhaler inhaler.









Mark daily dose tracker:
On the inside of the pack there is a daily dose
tracker. Put a mark in today’s box if it helps to
remind you of when your next dose is due.
MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT





A. MANUFACTURING AUTHORISATION HOLDER
RESPONSIBLE FOR BATCH RELEASE
B. CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURING AUTHORISATION HOLDER(S) RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer responsible for batch release
Novartis Pharma GmbH
Roonstrasse 25
D-90429 Nürnberg
Germany
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to medical prescription.
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
The Marketing Authorisation Holder (MAH) shall ensure, at launch, that physicians who are expected
to prescribe/use Oslif Breezhaler and pharmacists are provided with an information card containing the
following elements:
Indication is for maintenance bronchodilator treatment of airflow obstruction in adult patients
with COPD.
Oslif Breezhaler should not be used in asthma due to the absence of long-term outcome data in
asthma with Oslif Breezhaler.
Recommended dose is the inhalation of the content of one 150 microgram capsule once a day,
using the Oslif Breezhaler inhaler. The dose should only be increased on medical advice.
All materials will refer to summary of product characteristics for full prescribing information.
Pharmacovigilance system
The MAH must ensure that the system of pharmacovigilance, presented in Module 1.8.1. of the
Marketing Authorisation, is in place and functioning before and whilst the product is on the market.
Risk Management Plan
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version version 4 (23 September 2009) of the Risk
Management Plan (RMP) presented in Module 1.8.2. of the Marketing Authorisation Application and
any subsequent updates of the RMP agreed by the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, the
updated RMP should be submitted at the same time as the next Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
At the request of the EMEA
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
OUTER CARTON OF UNIT PACK
NAME OF THE MEDICINAL PRODUCT
Oslif Breezhaler 150 microgram inhalation powder, hard capsules
Indacaterol
STATEMENT OF ACTIVE SUBSTANCE(S)
Each capsule contains indacaterol maleate equivalent to 150 microgram indacaterol.
Contains lactose monohydrate (see package leaflet for further information) and gelatin.
PHARMACEUTICAL FORM AND CONTENTS
10 capsules + 1 inhaler
30 capsules + 1 inhaler
METHOD AND ROUTE(S) OF ADMINISTRATION
Inhalation use
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
For use only with the inhaler provided in the pack.
Do not swallow capsules.
Lift here to open.























SPECIAL STORAGE CONDITIONS
Do not store above 30°C.
Store in the original package to protect from moisture and do not remove until immediately before use.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE





















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
OUTER CARTON OF MULTIPACK (INCLUDING BLUE BOX)
NAME OF THE MEDICINAL PRODUCT
Oslif Breezhaler 150 microgram inhalation powder, hard capsules
Indacaterol
STATEMENT OF ACTIVE SUBSTANCE(S)
Each capsule contains indacaterol maleate equivalent to 150 microgram indacaterol.
Contains lactose monohydrate (see package leaflet for further information) and gelatin.
PHARMACEUTICAL FORM AND CONTENTS
60 capsules + 2 inhalers
90 capsules + 3 inhalers
300 capsules + 30 inhalers
Multipack comprising 2 packs (each containing 30 capsules and 1 inhaler).
Multipack comprising 3 packs (each containing 30 capsules and 1 inhaler).
Multipack comprising 30 packs (each containing 10 capsules and 1 inhaler).
METHOD AND ROUTE(S) OF ADMINISTRATION
Inhalation use
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
For use only with the inhaler provided in the pack.
Do not swallow capsules.






















SPECIAL STORAGE CONDITIONS
Do not store above 30°C.
Store in the original package to protect from moisture and do not remove until immediately before use.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
300 capsules + 30 inhalers
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE
























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
INTERMEDIATE CARTON OF MULTIPACK (WITHOUT BLUE BOX)
NAME OF THE MEDICINAL PRODUCT
Oslif Breezhaler 150 microgram inhalation powder, hard capsules
Indacaterol
STATEMENT OF ACTIVE SUBSTANCE(S)
Each capsule contains indacaterol maleate equivalent to 150 microgram indacaterol.
Contains lactose monohydrate (see package leaflet for further information) and gelatin.
PHARMACEUTICAL FORM AND CONTENTS
Component of a multipack comprising 2 packs (each containing 30 capsules and 1 inhaler).
Component of a multipack comprising 3 packs (each containing 30 capsules and 1 inhaler).
Component of a multipack comprising 30 packs (each containing 10 capsules and 1 inhaler).
METHOD AND ROUTE(S) OF ADMINISTRATION
Inhalation use
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
For use only with the inhaler provided in the pack.
Do not swallow capsules.
Lift here to open.

























SPECIAL STORAGE CONDITIONS
Do not store above 30°C.
Store in the original package to protect from moisture and do not remove until immediately before use.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
300 capsules + 30 inhalers
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE
























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
INNER LID OF OUTER CARTON OF UNIT PACK AND OF INTERMEDIATE CARTON OF
MULTIPACK
See package leaflet for pictures and information on using Oslif Breezhaler.





PARTICULARS TO APPEAR ON THE OUTER PACKAGING
OUTER CARTON OF UNIT PACK
NAME OF THE MEDICINAL PRODUCT
Oslif Breezhaler 300 microgram inhalation powder, hard capsules
Indacaterol
STATEMENT OF ACTIVE SUBSTANCE(S)
Each capsule contains indacaterol maleate equivalent to 300 microgram indacaterol.
Contains lactose monohydrate (see package leaflet for further information) and gelatin.
PHARMACEUTICAL FORM AND CONTENTS
10 capsules + 1 inhaler
30 capsules + 1 inhaler
METHOD AND ROUTE(S) OF ADMINISTRATION
Inhalation use
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
For use only with the inhaler provided in the pack.
Do not swallow capsules.
Lift here to open.























SPECIAL STORAGE CONDITIONS
Do not store above 30°C.
Store in the original package to protect from moisture and do not remove until immediately before use.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE





















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
OUTER CARTON OF MULTIPACK (INCLUDING BLUE BOX)
NAME OF THE MEDICINAL PRODUCT
Oslif Breezhaler 300 microgram inhalation powder, hard capsules
Indacaterol
STATEMENT OF ACTIVE SUBSTANCE(S)
Each capsule contains indacaterol maleate equivalent to 300 microgram indacaterol.
Contains lactose monohydrate (see package leaflet for further information) and gelatin.
PHARMACEUTICAL FORM AND CONTENTS
60 capsules + 2 inhalers
90 capsules + 3 inhalers
300 capsules + 30 inhalers
Multipack comprising 2 packs (each containing 30 capsules and 1 inhaler).
Multipack comprising 3 packs (each containing 30 capsules and 1 inhaler).
Multipack comprising 30 packs (each containing 10 capsules and 1 inhaler).
METHOD AND ROUTE(S) OF ADMINISTRATION
Inhalation use
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
For use only with the inhaler provided in the pack.
Do not swallow capsules.






















SPECIAL STORAGE CONDITIONS
Do not store above 30°C.
Store in the original package to protect from moisture and do not remove until immediately before use.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
300 capsules + 30 inhalers
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE
























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
INTERMEDIATE CARTON OF MULTIPACK (WITHOUT BLUE BOX)
NAME OF THE MEDICINAL PRODUCT
Oslif Breezhaler 300 microgram inhalation powder, hard capsules
Indacaterol
STATEMENT OF ACTIVE SUBSTANCE(S)
Each capsule contains indacaterol maleate equivalent to 300 microgram indacaterol.
Contains lactose monohydrate (see package leaflet for further information) and gelatin.
PHARMACEUTICAL FORM AND CONTENTS
Component of a multipack comprising 2 packs (each containing 30 capsules and 1 inhaler).
Component of a multipack comprising 3 packs (each containing 30 capsules and 1 inhaler).
Component of a multipack comprising 30 packs (each containing 10 capsules and 1 inhaler).
METHOD AND ROUTE(S) OF ADMINISTRATION
Inhalation use
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
For use only with the inhaler provided in the pack.
Do not swallow capsules.
Lift here to open.

























SPECIAL STORAGE CONDITIONS
Do not store above 30°C.
Store in the original package to protect from moisture and do not remove until immediately before use.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
300 capsules + 30 inhalers
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE
























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
INNER LID OF OUTER CARTON OF UNIT PACK AND OF INTERMEDIATE CARTON OF
MULTIPACK
See package leaflet for pictures and information on using Oslif Breezhaler.





PACKAGE LEAFLET: INFORMATION FOR THE USER
Oslif Breezhaler 150 microgram inhalation powder, hard capsules
Oslif Breezhaler 300 microgram inhalation powder, hard capsules
Indacaterol maleate
Read all of this leaflet carefully before you start using this medicine.
-
If you have further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
What Oslif Breezhaler is and what it is used for
How to use Oslif Breezhaler
How to store Oslif Breezhaler
WHAT OSLIF BREEZHALER IS AND WHAT IT IS USED FOR
What Oslif Breezhaler is
Oslif Breezhaler contains the active substance indacaterol which belongs to a group of medicines
called bronchodilators. When you inhale it, it relaxes the muscles in the walls of the small air passages
in the lungs. This helps open up the airways, making it easier to get air in and out.
What Oslif Breezhaler is used for
Oslif Breezhaler is used in adults who have breathing difficulties due to a lung disease called chronic
obstructive pulmonary disease (COPD). It helps you breathe more easily and minimise the effects of
COPD.
BEFORE YOU USE OSLIF BREEZHALER
Follow all the doctor’s instructions carefully. They may differ from the general information contained
in this leaflet.
Do not use Oslif Breezhaler
-
if you are allergic (hypersensitive) to indacaterol, to lactose or to gelatin.
If this applies to you,
tell your doctor without taking Oslif Breezhaler.
If you think you may be
allergic, ask your doctor for advice.
Take special care with Oslif Breezhaler
if you have asthma (in this case you should not use Oslif Breezhaler).
if you have heart problems.
if you have epilepsy.
if you have thyroid gland problems (thyrotoxicosis).
if you have diabetes.
If any of the above applies to you (or you are not sure),
tell your doctor before using Oslif
Breezhaler
.
Keep this leaflet. You may need to read it again.
Before you use Oslif Breezhaler
Oslif Breezhaler
should not
be given to
children or adolescents below the age of 18 years
.
During treatment with Oslif Breezhaler,
Stop using the medicine and tell your doctor immediately
if you get tightness of the chest,
coughing, wheezing or breathlessness immediately after using the medicine. These may be signs
of a condition called bronchospasm.
Tell your doctor immediately
if your COPD symptoms (breathlessness, wheezing, cough) do
not improve or get worse.
Taking other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
In particular, please tell your doctor if you are using:
medicines for breathing problems that are similar to Oslif Breezhaler. You may be more likely
to get side effects.
medicines called beta blockers that are used for high blood pressure or other heart problems
(such as propranolol), or for the eye problem called glaucoma (such as timolol).
medicines that lower the amount of potassium in your blood. These include:
o
steroids (e.g. prednisolone),
diuretics (water tablets) used for high blood pressure such as hydrochlorothiazide,
medicines for breathing problems such as theophylline.
Using Oslif Breezhaler with food and drink
You can inhale Oslif Breezhaler anytime before or after food or drink.
Pregnancy and breast-feeding
If you are pregnant or think that you may be pregnant, or if you are breast-feeding, tell your doctor
before using Oslif Breezhaler. You should not use Oslif Breezhaler unless your doctor tells you so.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
It is unlikely that Oslif Breezhaler will affect your ability to drive and use machines.
HOW TO USE OSLIF BREEZHALER
Always use Oslif Breezhaler exactly as your doctor has told you. You should check with your doctor
or pharmacist if you are not sure.
How much Oslif Breezhaler to use
The usual dose is to inhale the content of one capsule each day. Your doctor may tell you to use
the 150 microgram capsule or the 300 microgram capsule depending on your condition and on
how you respond to the treatment. Do not use more than your doctor tells you to use.
Use your inhaler at the same time each day, the effects last for 24 hours. This ensures that there
is always enough medicine in your body to help you breathe more easily throughout the day and
night. It will also help you to remember to use it.
How to use Oslif Breezhaler
In this pack, you will find an inhaler and capsules (in blister strips) that contain the medicine as
inhalation powder. The Oslif Breezhaler inhaler enables you to inhale the medicine contained in
a capsule.
Only use the capsules with the inhaler provided in this pack (Oslif Breezhaler inhaler). The
capsules should remain in the blister strip until you need to use them.
When you start a new pack, use the new Oslif Breezhaler inhaler that is supplied in the pack.
Dispose of each inhaler after 30 days of use.
Do not swallow the capsules.
Please read the instructions at the end of this leaflet for more information about how to
use the inhaler.
If you use more Oslif Breezhaler than you should
If you have inhaled too much Oslif Breezhaler or if someone else uses your capsules, tell your doctor
immediately or go to the nearest emergency unit. Show the pack of Oslif Breezhaler. Medical attention
may be needed.
If you forget to use Oslif Breezhaler
If you forget to inhale a dose, inhale just one dose at the usual time the next day. Do not inhale a
double dose to make up for a forgotten dose.
How long to continue your treatment with Oslif Breezhaler
Keep using your treatment with Oslif Breezhaler for as long as your doctor tells you.
COPD is a long-term disease and you should use Oslif Breezhaler
every day
and not only when
you have breathing problems or other symptoms of COPD.
If you have questions about how long to continue your treatment with Oslif Breezhaler, talk to your
doctor or pharmacist.
Like all medicines, Oslif Breezhaler
can cause side effects, although not everybody gets them.
These side effects may occur with certain frequencies, which are defined as follows:
very common: affects more than 1 patient in 10
common: affects 1 to 10 patients in 100
uncommon: affects 1 to 10 patients in 1,000
rare: affects 1 to 10 patients in 10,000
very rare: affects less than 1 patient in 10,000
not known: frequency cannot be estimated from the available data.
Some side effects may be serious. Tell your doctor immediately
if you get crushing chest pain, or irregular heart beat.
if you get high levels of sugar in your blood (diabetes). You will feel tired, very thirsty and
hungry (without gaining weight) and will pass more urine than usual.
Other side effects may include:
feeling of pressure or pain in the cheeks and forehead (inflammation of the sinuses)
cold-like symptoms. You may get all or most of the following: sore throat, runny nose, blocked
nose, sneezing, coughing and a headache.
difficulty to breathe as with bronchitis
swollen hands, ankles and feet (oedema)
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Some people occasionally cough soon after inhaling the medicine. Cough is a common symptom in
COPD. If you experience coughing briefly after inhaling the medicine, do not worry. Check your
inhaler to see if the capsule is empty and that you have received the full dose. If the capsule is empty,
there is no need for concern. If the capsule is not empty then inhale again as directed.
HOW TO STORE OSLIF BREEZHALER
Keep out of the reach and sight of children.
Do not use after the expiry date shown on the box and blister strip. The expiry date refers to the last
day of that month.
Do not store above 30°C.
Store in the original package in order to protect from moisture and do not remove until immediately
before use.
Do not use Oslif Breezhaler if you notice that the pack is damaged or show signs of tampering.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
What Oslif Breezhaler contains
-
Each Oslif Breezhaler 150 microgram capsule contains 150 microgram indacaterol as
indacaterol maleate. The other ingredients are lactose and gelatin.
Each Oslif Breezhaler 300 microgram capsule contains 300 microgram indacaterol as
indacaterol maleate. The other ingredients are lactose and gelatin.
What Oslif Breezhaler looks like and content of the pack
In this pack, you will find an inhaler, together with capsules in blister strips. The capsules are clear
and colourless and contain a white powder.
Oslif Breezhaler 150 microgram capsules are clear and colourless. They have a
black
product
code “
IDL 150
” printed above and a
black
company logo ( ) printed below a
black
bar.
Oslif Breezhaler 300 microgram capsules are clear and colourless. They have a
blue
product
code “
IDL 300
” printed above and a
blue
company logo ( ) printed below a
blue
bar.
The following pack sizes are available:
Carton containing 10 capsules (1x10 capsule blister strips) and 1 inhaler.
Carton containing 30 capsules (3x10 capsule blister strips) and 1 inhaler.
Multipack comprising 2 packs (each containing 30 capsules and 1 inhaler).
Multipack comprising 3 packs (each containing 30 capsules and 1 inhaler).
Multipack comprising 30 packs (each containing 10 capsules and 1 inhaler).
Not all pack sizes or strengths may be available in your country.

Marketing Authorisation Holder
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
Manufacturer
Novartis Pharma GmbH
Roonstraße 25
D-90429 Nuremberg
Germany
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
Novartis Pharma N.V.
Tél/Tel: +32 2 246 16 11
Luxembourg/Luxemburg
Novartis Pharma N.V.
Tél/Tel: +32 2 246 16 11
България
Novartis Pharma Services Inc.
Тел.: +359 2 489 98 28
Magyarország
Novartis Hungária Kft. Pharma
Tel.: +36 1 457 65 00
Česká republika
Novartis s.r.o.
Tel: +420 225 775 111
Malta
Novartis Pharma Services Inc.
Tel: +356 2298 3217
Danmark
Novartis Healthcare A/S
Tlf: +45 39 16 84 00
Nederland
Novartis Pharma B.V.
Tel: +31 26 37 82 111
Deutschland
Novartis Pharma GmbH
Tel: +49 911 273 0
Norge
Novartis Norge AS
Tlf: +47 23 05 20 00
Eesti
Novartis Pharma Services Inc.
Tel: +372 66 30 810
Österreich
Novartis Pharma GmbH
Tel: +43 1 86 6570
Ελλά
Novartis (Hellas) A.E.B.E.
Τηλ: +30 210 281 17 12
Polska
Novartis Poland Sp. z o.o.
Tel.: +48 22 550 8888
España
Ferrer Internacional, S.A.
Tel: +34 93 600 37 00
Portugal
Laboratório Medinfar - Produtos Farmacêuticos,
S.A.
Tel: +351 21 499 7400
France
Pierre Fabre Médicament
Tél: +33 1 49 10 96 18
România
Novartis Pharma Services Inc.
Tel: +40 21 31299 01
Ireland
Novartis Ireland Limited
Tel: +353 1 260 12 55
Slovenija
Novartis Pharma Services Inc.
Tel: +386 1 300 75 50
Ísland
Vistor hf.
Sími: +354 535 7000
Slovenská republika
Novartis Slovakia s.r.o.
Tel: +421 2 5542 5439
Italia
Novartis Farma S.p.A.
Tel: +39 02 96 54 1
Suomi/Finland
Novartis Finland Oy
Puh/Tel: +358 (0)10 6133 200
Κύπρς
Δημητριάης κι Ππέλληνς Λτ
Τηλ: +357 22 690 690
Sverige
Novartis Sverige AB
Tel: +46 8 732 32 00
Latvija
Novartis Pharma Services Inc.
Tel: +371 67 887 070
United Kingdom
Novartis Pharmaceuticals UK Ltd.
Tel: +44 1276 698370
Lietuva
Novartis Pharma Services Inc.
Tel: +370 5 269 16 50
This leaflet was last approved in
INSTRUCTIONS FOR USE OF OSLIF BREEZHALER INHALER
Please read the following instructions carefully to learn how to use and care for your Oslif Breezhaler
inhaler.
Only use the Oslif Breezhaler inhaler contained in this pack.
Do not use Oslif Breezhaler
capsules with any other inhaler, and do not use Oslif Breezhaler inhaler to take any other
capsule medicine.
When you start a new pack, only use the new Oslif Breezhaler inhaler that is supplied in the
pack.
Dispose of each inhaler after 30 days of use.
Ask your pharmacist how to dispose of medicines and inhalers no longer required.
Do not swallow the capsules.
The powder in the capsules is for you to inhale.
Your Oslif Breezhaler pack:
Each Oslif Breezhaler pack contains:
one Oslif Breezhaler inhaler
one or more blister strips containing Oslif Breezhaler capsules to be used in the inhaler.
The Oslif Breezhaler inhaler enables you to inhale the medicine contained in an Oslif Breezhaler
capsule.

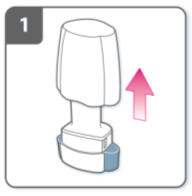




Open inhaler:
Hold the base of the inhaler firmly and tilt the mouthpiece.
This opens the inhaler.
Prepare capsule.
Immediately before use,
with dry hands, remove one
capsule from the blister.
Insert capsule:
Place the capsule into the capsule chamber.
Never place a capsule directly into the mouthpiece.
Close the inhaler:
Close the inhaler until you hear a “click”.



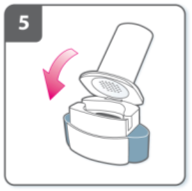
Hold the inhaler upright with the mouthpiece pointing
up.
Pierce the capsule by firmly pressing together both
side buttons at the same time. Do this only once.
You should hear a “click” as the capsule is being
pierced.
Release the side buttons fully.
Breathe out:
Before placing the mouthpiece in your mouth, breathe out
fully.
Do not blow into the mouthpiece.
Inhale the medicine:
To breathe the medicine deeply into your airways:
Hold the inhaler as shown in the picture. The side
buttons should be facing left and right. Do not press
the side buttons.
Place the mouthpiece in your mouth and close your
lips firmly around the mouthpiece.
Breathe in rapidly but steadily and as deeply as you
can.



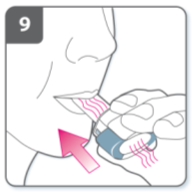
Note:
As you breathe in through the inhaler, the capsule spins
around in the chamber and you should hear a whirring noise.
You will experience a sweet flavour as the medicine goes
into your lungs.
Additional information
Occasionally, very small pieces of the capsule can get past
the screen and enter your mouth. If this happens, you may be
able to feel these pieces on your tongue. It is not harmful if
these pieces are swallowed or inhaled. The chances of the
capsule shattering will be increased if the capsule is
accidentally pierced more than once (step 6).
If you do not hear a whirring noise
:
The capsule may be stuck in the capsule chamber. If this
happens:
Open the inhaler and carefully loosen the capsule by
tapping the base of the inhaler. Do not press the side
buttons.
Inhale the medicine again by repeating steps 8 and 9.
Hold breath:
After you have inhaled the medicine:
Hold your breath for at least 5-10 seconds or as long
as you comfortably can while taking the inhaler out of
your mouth.
Open the inhaler to see if any powder is left in the
capsule.
If there is powder left in the capsule:
Repeat steps 8, 9, 10 and 11.
Most people are able to empty the capsule with one or two
inhalations.
Additional information
Some people may occasionally cough briefly soon after
inhaling the medicine. If you do, don’t worry. As long as the
capsule is empty, you have received enough of your
medicine.
After you have finished taking
your medicine:
Open the mouthpiece again, remove the empty capsule
by tipping it out of the capsule chamber. Put the
empty capsule in your household waste.
Close the inhaler and replace the cap.
Do not store the capsules in the Oslif Breezhaler inhaler.


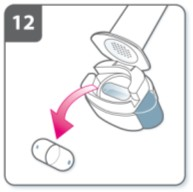
Mark daily dose tracker:
On the inside of the pack there is a daily dose tracker. Put a
mark in today’s box if it helps to remind you of when your
next dose is due.
Do not swallow Oslif Breezhaler capsules.
Only use the Oslif Breezhaler
inhaler contained in this pack.
Oslif Breezhaler capsules must always be stored in the blister, and only removed immediately
before use.
Never place a Oslif Breezhaler capsule directly into the mouthpiece of the Oslif Breezhaler
inhaler.
Do not press the side buttons more than once.
Never blow into the mouthpiece of the Oslif Breezhaler inhaler.
Always release the push buttons before inhalation.
Never wash the Oslif Breezhaler inhaler with water. Keep it dry. See “How to clean your
inhaler”.
Never take the Oslif Breezhaler inhaler apart.
Always use the new Oslif Breezhaler inhaler that comes with your new Oslif Breezhaler
medication pack. Dispose of each inhaler after 30 days of use.
Do not store the capsules in the Oslif Breezhaler inhaler.
Always keep the Oslif Breezhaler inhaler and Oslif Breezhaler capsules in a dry place.
How to clean your inhaler
Clean your inhaler once a week.
Wipe the mouthpiece inside and outside to remove any powder with a clean, dry lint-free cloth.
Do not wash your inhaler with water. Keep it dry.
Do not take the inhaler apart.

Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/oslif_breezhaler.html
Copyright © 1995-2021 ITA all rights reserved.
|































































































































































































































































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































