Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
PecFent 100 micrograms/spray nasal spray solution
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml of solution contains 1,000 micrograms fentanyl (as citrate)
1 spray (100 microliters) contains 100 micrograms fentanyl (as citrate)
Each bottle contains 1.55 ml (1.55 mg fentanyl) ensuring delivery of 8 sprays of 100 micrograms
Excipients
Each spray contains 0.02 mg propylhydroxybenzoate (E216).
For a full list of excipients, see section 6.1.
Nasal spray, solution (nasal spray)
A clear to practically clear colourless aqueous solution.
4.1 Therapeutic indications
PecFent is indicated for the management of breakthrough pain (BTP) in adults who are already
receiving maintenance opioid therapy for chronic cancer pain. Breakthrough pain is a transitory
exacerbation of pain that occurs on a background of otherwise controlled persistent pain.
Patients receiving maintenance opioid therapy are those who are taking at least 60 mg of oral
morphine daily, at least 25 micrograms of transdermal fentanyl per hour, at least 30 mg of oxycodone
daily, at least 8 mg of oral hydromorphone daily or an equianalgesic dose of another opioid for a week
or longer.
4.2
Posology and method of administration
Treatment should be initiated by and remain under the supervision of a physician experienced in the
management of opioid therapy in cancer patients. Physicians should keep in mind the potential for
abuse of fentanyl.
PecFent should be titrated to an “effective” dose that provides adequate analgesia and minimises
adverse reactions without causing undue (or intolerable) adverse reactions, for two consecutively
treated episodes of BTP. The efficacy of a given dose should be assessed over the ensuing 30 minute
period.
Patients should be carefully monitored until an effective dose is reached.
One dose of PecFent may include administration of 1 spray (100 microgram or 400 microgram doses)
or 2 sprays (200 microgram or 800 microgram doses) of the
same
dose strength (either 100 microgram
or 400 microgram strength).
Patients should not take more than 4 doses per day. Patients should wait at least 4 hours after a dose
before treating another BTP episode with PecFent.
The initial dose of PecFent to treat episodes of BTP is always 100 micrograms (one spray), even
in patients switching from other fentanyl containing products for their BTP.
Patients must wait at least 4 hours before treating another episode of BTP with PecFent.
Patients should be prescribed an initial titration supply of one bottle (8 sprays) of PecFent
100 micrograms/spray.
Patients whose initial dose is 100 micrograms and who need to titrate to a higher dose due to a
lack of effect can be instructed to use two 100 microgram sprays (one in each nostril) for their
next BTP episode. If this dose is not successful, the patient may be prescribed a bottle of
PecFent 400 micrograms/spray and instructed to change to one 400 microgram spray for their
next episode of pain. If this dose is not successful, the patient may be instructed to increase to
two 400 microgram sprays (one in each nostril).
From treatment initiation, patients should be closely followed and the dose titrated until an
effective dose is reached and confirmed for two consecutively treated episodes of BTP.
Titration in patients switching between immediate-release fentanyl containing products
Substantial differences may exist in the pharmacokinetic profile of immediate-release fentanyl
products, which result in clinically important differences in the rate and extent of absorption of
fentanyl. Therefore, when switching between fentanyl containing products indicated for treatment of
breakthrough pain, including intranasal formulations, it is essential that patients are again titrated with
the new product, and not switched on a dose-for-dose (microgram-for-microgram) basis.
Once an effective dose has been established during titration, patients should continue to take this dose
up to a maximum of 4 doses per day.
Generally, the maintenance dose of PecFent should be increased only where the current dose fails to
adequately treat the BTP for several consecutive episodes.
A review of the dose of the background opioid therapy may be required if patients consistently present
with more than four BTP episodes per 24 hours.
If adverse reactions are intolerable or persistent, the dose should be reduced or treatment with PecFent
replaced by another analgesic.
Discontinuation of therapy
PecFent should be discontinued immediately if the patient no longer experiences breakthrough pain
episodes. The treatment for persistent backgound pain should be kept as prescribed.
If discontinuation of all opioid therapy is required, the patient must be closely followed by the doctor
as gradual downward opioid titration therapy is necessary in order to avoid the possibility of abrupt
withdrawal effects.
Paediatric population
The safety and efficacy of PecFent in children aged below 18 years have not yet been established.
No data are available.
Use in the elderly (older than 65 years)
In the PecFent clinical trial programme, 104 (26.1%) of patients were over 60 years of age, 67 (16.8%)
over 65 years and 15 (3.8%) over 75 years. There was no indication that older patients tended to
titrate to lower doses or experience more adverse reactions. Nevertheless, in view of the importance of
renal and hepatic function in the metabolism and clearance of fentanyl, additional care should be
exercised in the use of PecFent in the elderly. No data on the pharmacokinetics of PecFent in elderly
patients are available.
Hepatic or renal impairment
PecFent should be administered with caution to patients with moderate or severe hepatic or renal
impairment (see section 4.4)
PecFent is for administration via the nasal route only.
PecFent can deliver 100, 200, 400 and 800 microgram doses as follows:
Dose required
micrograms)
Product strength
(micrograms)
One spray administered into one
nostril
One spray administered into each
nostril
One spray administered into one
nostril
One spray administered into each
nostril
The bottle should be removed from the child resistant container immediately prior to use and the
protective cap removed. The bottle must be primed before first use by holding upright and simply
pressing and releasing the finger grips either side of the nozzle until a green bar appears in the
counting window (should occur after four sprays).
If the product has not been used for more than 5 days or if it is more than 14 days since the product
was first used, the PecFent bottle should be discarded.
To administer PecFent the nozzle is placed a short distance (about 1 cm) into the nostril and pointed
slightly towards the bridge of the nose. A spray is then administered by pressing and releasing the
finger grips either side of the nozzle. An audible click will be heard and the number displayed on the
counter will advance by one.
Patients must be advised that they may not feel the spray being administered, and that they should
therefore rely on the audible click and the number on the counter advancing to confirm that a spray has
been delivered. The nasal spray pump permanently locks after the eighth spray has been administered.
The PecFent spray droplets form a gel in the nose. Patients should be advised not to blow their nose
immediately after PecFent administration.
The protective cap should be replaced after each use and the bottle returned to the child resistant
container for safe storage.









Hypersensitivity to the active substance or to any of the excipients.
Use in opioid naïve patients.
Severe respiratory depression or severe obstructive lung conditions.
4.4 Special warnings and precautions for use
Patients and their carers must be instructed that PecFent contains an active substance in an amount that
can be fatal to a child, and therefore to keep PecFent out of the reach and sight of children.
In order to minimise the risks of opioid-related adverse reactions and to identify the effective dose, it
is imperative that patients be monitored closely by health professionals during the titration process.
It is important that the long acting opioid treatment used to treat the patient’s persistent pain has been
stabilised before PecFent therapy begins.
Respiratory depression
There is a risk of clinically significant respiratory depression associated with the use of fentanyl.
Patients with pain who receive chronic opioid therapy develop tolerance to respiratory depression and
hence the risk of respiratory depression in these patients is reduced. The use of concomitant central
nervous system depressants may increase the risk of respiratory depression (see section 4.5).
Chronic pulmonary disease
In patients with chronic obstructive pulmonary diseases, fentanyl may cause more serious adverse
reactions. In these patients, opioids may decrease respiratory drive and increase airway resistance.
Increased intracranial pressure
PecFent should only be administered with extreme caution in patients who may be particularly
susceptible to the intracranial effects of CO
2
retention, such as those with evidence of increased
intracranial pressure or impaired consciousness. Opioids may obscure the clinical course of patients
with a head injury and should be used only if clinically warranted.
Cardiac disease
Intravenous fentanyl may produce bradycardia. PecFent should therefore be used with caution in
patients with pre-existing bradyarrhythmias.
Impaired hepatic or renal function
In addition, PecFent should be administered with caution to patients with hepatic or renal impairment.
The influence of hepatic and renal impairment on the pharmacokinetics of the medicinal product has
not been evaluated; however, when administered intravenously the clearance of fentanyl has been
shown to be altered in hepatic and renal impairment due to alterations in metabolic clearance and
plasma proteins. Therefore, special care should be taken during the titration process in patients with
moderate or severe hepatic or renal impairment.
Careful consideration should be given to patients with hypovolaemia and hypotension.
Abuse potential and tolerance
Tolerance and physical and/or psychological dependence may develop upon repeated administration
of opioids such as fentanyl. However, iatrogenic addiction following therapeutic use of opioids is rare.
Athletes should be informed that treatment with fentanyl could lead to positive doping tests.
Route of administration
PecFent is only intended for intranasal administration, and must not be administered by any other
route. Due to physico-chemical properties of excipients included in the formulation, intravenous or
intra-arterial injection must be avoided in particular.
Nasal conditions
If the patient experiences recurrent episodes of epistaxis or nasal discomfort while taking PecFent, an
alternative method of administration for treatment of breakthrough pain should be considered.
PecFent excipients
PecFent contains propylhydroxybenzoate (E216). In some patients this may cause allergic reactions
(possibly delayed) and, exceptionally, bronchospasm (if the product is not correctly administered).
4.5 Interaction with other medicinal products and other forms of interaction
Fentanyl is metabolised mainly via the human cytochrome P450 3A4 isoenzyme system (CYP3A4),
therefore potential interactions may occur when PecFent is given concurrently with agents that affect
CYP3A4 activity. Coadministration with agents that induce 3A4 activity may reduce the efficacy of
PecFent. The concomitant use of PecFent with strong CYP3A4 inhibitors (e.g. ritonavir, ketoconazole,
itraconazole, troleandomycin, clarithromycin, and nelfinavir) or moderate CYP3A4 inhibitors (e.g.
amprenavir, aprepitant, diltiazem, erythromycin, fluconazole, fosamprenavir, grapefruit juice, and
verapamil) may result in increased fentanyl plasma concentrations, potentially causing serious adverse
drug reactions including fatal respiratory depression. Patients receiving PecFent concomitantly with
moderate or strong CYP3A4 inhibitors should be carefully monitored for an extended period of time.
Dose increase should be undertaken with caution.
The concomitant use of other central nervous system depressants, including other opioids, sedatives or
hypnotics, general anaesthetics, phenothiazines, tranquillisers, skeletal muscle relaxants, sedating
antihistamines and alcohol may produce additive depressant effects.
PecFent is not recommended for use in patients who have received monoamine oxidase (MAO)
inhibitors within the previous 14 days because severe and unpredictable potentiation by MAO
inhibitors has been reported with opioid analgesics.
The concomitant use of partial opioid agonists/antagonists (e.g. buprenorphine, nalbuphine,
pentazocine) is not recommended. They have high affinity to opioid receptors with relatively low
intrinsic activity and therefore partially antagonise the analgesic effect of fentanyl and may induce
withdrawal symptoms in opioid dependant patients.
Concomitant use of nasally administered oxymetazoline has been shown to decrease the absorption of
PecFent (see section 5.2). The concomitant use of nasally administered vasoconstrictive decongestants
during titration is therefore not recommended as this may lead to patients titrating to a dose that is
higher than required. PecFent maintenance treatment may also be less effective in patients with rhinitis
when administered concomitantly with a nasal vasoconstrictive decongestant. If this occurs, patients
should be advised to discontinue their decongestant.
Concomitant use of PecFent and other medicinal products (other than oxymetazoline) administered via
the nose has not been evaluated in the clinical trials. Other nasally administered treatments should be
avoided within 15 minutes of dosing with PecFent.
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no adequate data from the use of fentanyl in pregnant women. Studies in animals have
shown reproductive toxicity (see section 5.3). The potential risk for humans is unknown. PecFent
should not be used during pregnancy unless clearly necessary.
Following long-term treatment, fentanyl may cause withdrawal in the new-born infant. It is advised
not to use fentanyl during labour and delivery (including caesarean section) because fentanyl passes
through the placenta and may cause respiratory depression in the foetus. If PecFent is administered, an
antidote for the child should be readily available.
Breastfeeding
Fentanyl passes into breast milk and may cause sedation and respiratory depression in the breast-fed
child. Fentanyl should not be used by breastfeeding women and breast-feeding should not be restarted
until at least 48 hours after the last administration of fentanyl.
Fertility
There are no clinical data on the effects of fentanyl on fertility.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed.
However, opioid analgesics may impair the mental and/or physical ability required for driving or
operating machinery.
Patients should be advised not to drive or operate machinery if they experience somnolence, dizziness,
or visual disturbance or other adverse reactions which can impair their ability to drive or operate
machinery.
Typical opioid adverse reactions are to be expected with PecFent. Frequently, these will cease or
decrease in intensity with continued use of the medicinal product, as the patient is titrated to the most
appropriate dose. However, the most serious adverse reactions are respiratory depression (potentially
leading to apnoea or respiratory arrest), circulatory depression, hypotension and shock and all patients
should be monitored for these.
The clinical studies of PecFent were designed to evaluate safety and efficacy in treating BTP and all
patients were also on background opioid therapies, such as sustained-release morphine or transdermal
fentanyl, for their persistent pain. Therefore it is not possible to definitively separate the effects of
PecFent alone.
The adverse reactions considered to be at least possibly-related to treatment, from Phase II and III
clinical studies were as follows (frequencies defined as very common (≥1/10); common (≥1/100 to
<1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000) within
each frequency grouping, adverse reactions are presented in order of decreasing seriousness).
Infections and infestations
Pneumonia
Nasopharyngitis
Pharyngitis
Rhinitis
Blood and lymphatic system
disorders
Metabolism and nutrition
disorders
Dehydration
Hyperglycaemia
Decreased appetite
Increased appetite










Drug abuse
Delirium
Hallucination
Confusional state
Depression
Attention deficit/hyperactivity
disorder
Anxiety
Euphoric mood
Nervousness
Dysgeusia
Dizziness
Somnolence
Headache
Loss of consciousness
Depressed level of consciousness
Convulsion
Ageusia
Anosmia
Memory impairment
Parosmia
Speech disorder
Sedation
Lethargy
Tremor
Ear and labyrinth disorders
Cardiovascular insufficiency
Lymphoedema
Hypotension
Hot flush
Respiratory, thoracic and
mediastinal disorders
Epistaxis
Rhinorrhoea
Nasal discomfort
Upper airway obstruction
Pharyngolaryngeal pain
Rhinalgia
Nasal mucosal disorder
Cough
Dyspnoea
Sneezing
Upper respiratory tract congestion
Nasal congestion
Intranasal hypoaesthesia
Throat irritiation
Postnasal drip
Nasal dryness
Gastrointestinal disorders
Vomiting
Nausea
Constipation
Intestinal perforation
Peritonitis
Oral hypoaesthesia
Oral paraesthesia
Diarrhoea
Retching
Abdominal pain
Tongue disorder
Mouth ulceration
Dyspepsia
Dry mouth
Skin and subcutaneous tissue
disorders














Musculoskeletal and connective
tissue disorders
Arthralgia
Muscle twitching
Renal and urinary disorders
Anuria
Dysuria
Proteinuria
Urinary hesitation
Reproductive system and breast
disorders
General disorders and
administration site conditions
Non-cardiac chest pain
Asthenia
Chills
Face oedema
Peripheral oedema
Gait disturbance
Pyrexia
Fatigue
Malaise
Thirst
Platelet count decreased
Weight increased
Injury, poisoning and procedural
complications
Fall
Intentional drug misuse
Medication error
The symptoms of fentanyl overdose via the nasal route are expected to be similar in nature to those of
intravenous fentanyl and other opioids, and are an extension of its pharmacological actions, with the
most serious significant effect being respiratory depression.
Immediate management of opioid overdose includes ensuring a patent airway, physical and verbal
stimulation of the patient, assessment of the level of consciousness, ventilatory and circulatory status,
and assisted ventilation (ventilatory support) if necessary.
For treatment of overdose (accidental ingestion) in the opioid-naïve person, intravenous access should
be obtained and naloxone or other opioid antagonists should be employed as clinically indicated. The
duration of respiratory depression following overdose may be longer than the effects of the opioid
antagonist’s action (e.g. the half life of naloxone ranges from 30 to 81 minutes) and repeated
administration may be necessary. Consult the Summary of Product Characteristics of the individual
opioid antagonist for details about such use.
For treatment of overdose in opioid-maintained patients, intravenous access should be obtained. The
judicious use of naloxone or another opioid antagonist may be warranted in some instances, but it is
associated with the risk of precipitating an acute withdrawal syndrome.
It should be noted that although statistically significant increases in C
max
levels were seen following a
second dose of PecFent given either one or two hours after the initial dose, this increase is not
considered to be large enough to suggest that clinically concerning accumulation or over-exposure
would occur, providing a wide safety margin for the recommended dose interval of four hours.
Although muscle rigidity interfering with respiration has not been seen following the use of PecFent,
this is possible with fentanyl and other opioids. If it occurs, it should be managed by the use of
assisted ventilation, by an opioid antagonist, and as a final alternative, by a neuromuscular blocking
agent.












PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Analgesics; phenylpiperidine derivatives; ATC code: N02A-B03.
Mechanism of action
Fentanyl is an opioid analgesic, interacting predominantly with the opioid µ-receptor. Its primary
therapeutic actions are analgesia and sedation. Secondary pharmacological effects are respiratory
depression, bradycardia, hypothermia, constipation, miosis, physical dependence and euphoria.
Pharmacodynamic effects
A double-blind, randomised, placebo-controlled crossover study has been conducted in which 114
patients who experienced on average 1 to 4 episodes of break through pain (BTP) per day while taking
maintenance opioid therapy were entered into an initial open-label titration phase in order to identify
an effective dose of PecFent (Study CP043). The patients entering the double-blind phase treated up to
10 episodes of BTP with either PecFent (7 episodes) or placebo (3 episodes) in a random order.
Of the patients entering the titration phase, only 7 (6.1 %) were unable to be titrated to an effective
dose due to lack of efficacy and 6 (5.3 %) withdrew due to adverse events.
The primary endpoint was the comparison between the summed pain intensity difference at 30 minutes
after dosing (SPID
30
), which was 6.57 in the PecFent-treated episodes compared to 4.45 for placebo
(p<0.0001 ). The SPID for PecFent-treated episodes was also significantly different to placebo at 10
15, 45 and 60 minutes after administration.
The mean pain intensity scores (73 patients) for all PecFent-treated episodes (459 episodes) compared
to those treated with placebo (200 episodes) were significantly lower at 5, 10, 15, 30, 45 and
60 minutes following administration (see Figure 1).
Figure 1: Mean (± SE) Pain Intensity Scores at Each Time Point (mITT Population)
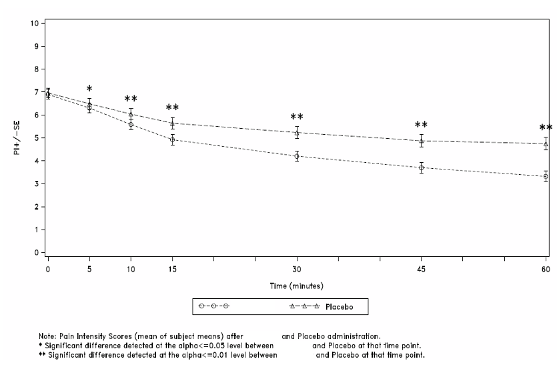
The superior efficacy of PecFent over placebo was supported by data from secondary endpoints
including the number of BTP episodes with clinically meaningful pain relief, defined as a reduction in
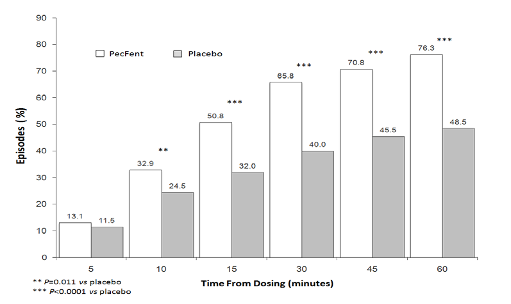
pain intensity score of at least 2 (Figure 2).
Figure 2: Clinically Meaningful Pain Relief – PecFent
vs
placebo: % Patients’ Episodes With ≥2 Point
Reduction in Pain Intensity
In a double-blind, randomized comparator-controlled study (Study 044) of similar design to Study 043
conducted in opioid-tolerant patients with breakthrough cancer pain on stable doses of regularly
scheduled opioids, PecFent was shown to be superior to immediate-release morphine sulfate (IRMS).
Superiority was demonstrated by the primary endpoint, Pain Intensity Difference within 15 minutes,
which was 3.02 in patients treated with PecFent compared to 2.69 in patients treated with IRMS
(p=0.0396).
In a long-term, open-label, safety study (Study 045), 355 patients entered the 16-week treatment
phase, during which 42,227 episodes of breakthrough cancer pain (BTP) were treated with PecFent.
One hundred of these patients continued treatment for up to 26 months in an extension phase. Of the
355 patients treated in the open-label treatment phase, 90 % required no increase in dose.
In the randomised, placebo-controlled study (CP043) 9.4% of 459 PecFent-treated BTP episodes in 73
patients required use of any further (rescue) medicinal products within 60 minutes of dosing. During
the longer-term, open-label study (CP045) this was 6.0 % of 42,227 episodes in 355 patients treated
with PecFent during up to 159 days of treatment.
5.2 Pharmacokinetic properties
Fentanyl is highly lipophilic and can be absorbed very rapidly through the nasal mucosa and more
slowly by the gastrointestinal route. It is subject to first pass hepatic and intestinal metabolism and the
metabolites do not contribute to fentanyl’s therapeutic effects.
PecFent utilises the PecSys nasal drug delivery system to modulate the delivery and absorption of
fentanyl. The PecSys system allows the product to be sprayed into the front area of the nasal cavity as
a fine mist of droplets, which gel on contact with the calcium ions present in the nasal mucosa.
Fentanyl diffuses from the gel and is absorbed through the nasal mucosa; this gel-modulated
A pharmacokinetic study was conducted to evaluate the absorption and tolerability of a single dose of
PecFent in patients with pollen-induced seasonal allergic rhinitis, comparing the un-challenged,
acutely challenged (rhinitic) and acutely challenged and then treated with oxymetazoline, states.
There was no clinically significant effect of acute rhinitis on C
max
, T
max
or overall exposure to fentanyl,
comparing the unchallenged with the acutely challenged states. Following treatment of the acute
rhinitic state with oxymetazoline, there were reductions in C
max
and exposure, and increases in T
max
that were statistically, and possibly clinically, significant.
Fentanyl is highly lipophilic and is well distributed beyond the vascular system, with a large apparent
volume of distribution. Animal data have shown that, following absorption, fentanyl is rapidly
distributed to the brain, heart, lungs, kidneys and spleen followed by a slower redistribution to muscles
and fat.
The plasma protein binding of fentanyl is 80 – 85 %. The main binding protein is alpha-1-acid
glycoprotein, but both albumin and lipoproteins contribute to some extent. The free fraction of
fentanyl increases with acidosis.
The metabolic pathways following nasal administration of PecFent have not been characterised in
clinical studies. Fentanyl is metabolised in the liver to norfentanyl by cytochrome CYP3A4 isoform.
Norfentanyl is not pharmacologically active in animal studies. It is more than 90 % eliminated by
biotransformation to N-dealkylated and hydroxylated inactive metabolites.
Disposition of fentanyl following intranasal administration of PecFent has not been characterised in a
mass balance study. Less than 7 % of an administered dose of fentanyl is excreted unchanged in the
urine and only about 1 % is excreted unchanged in the faeces. The metabolites are mainly excreted in
the urine, while faecal excretion is less important.
The total plasma clearance of fentanyl following intravenous administration is approximately 42 L/h.
Linearity/non-linearity
Dose-proportionality was demonstrated for C
max
and AUC in the dose range 100 micrograms to
800 micrograms.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, genotoxicity and carcinogenicity.
Embryo-foetal developmental toxicity studies conducted in rats and rabbits revealed no compound-
induced malformations or developmental variations when administered during the period of
organogenesis.
In a fertility and early embryonic development study in rats, a male-mediated effect was observed at
high doses (300 mcg/kg/day, s.c.) and is consistent with the sedative effects of fentanyl in animal
studies.
In studies on pre and postnatal development in rats the survival rate of offspring was significantly
reduced at doses causing severe maternal toxicity. Further findings at maternally toxic doses in F1
pups were delayed physical development, sensory functions, reflexes and behaviour. These effects
could either be indirect effects due to altered maternal care and/or decreased lactation rate or a direct
effect of fentanyl on the pups.
Carcinogenicity studies (26-week dermal alternative bioassay in Tg.AC transgenic mice; two-year
subcutaneous carcinogenicity study in rats) did not induce any findings indicative of oncogenic
potential.
PHARMACEUTICAL PARTICULARS
Pectin (E440)
Mannitol (E421)
Phenylethyl alcohol
Propyl hydroxybenzoate (E216)
Sucrose
Hydrochloric acid (0.36%) or sodium hydroxide (for pH adjustment)
Purified water
3 years
After first use: 14 days
After last actuation of the pump: 5 days
The patient should be advised to write the date of first use in the space provided on the label of the
child resistant container
6.4 Special precautions for storage
Do not store above 25
°
C.
Do not freeze.
Keep the bottle in the child resistant container in order to protect from light.
Store the bottle in the child resistant container at all times, even when finished.
6.5
Nature and contents of container
Bottle (clear Type I glass) with an attached metering pump incorporating an audible dose counter and
lock, and a protective cap. Packed in a clam-shell-like child resistant container.
Each bottle contains 1.55 ml ensuring delivery of 8 sprays.
Bottles in their child resistant containers are supplied in cartons containing 1, 4 or 12 bottles.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
As soon as PecFent is no longer needed, patients and members of their household must be advised to
systematically dispose of any bottles remaining from a prescription as soon as possible by returning
them to their child-resistant container and discarding them, according to local requirements or by
returning them to the pharmacy.
MARKETING AUTHORISATION HOLDER
Archimedes Development Ltd
Nottingham
NG7 2TN
United Kingdom
MARKETING AUTHORISATION NUMBER(S)
EU/1/10/644/001
EU/1/10/644/002
EU/1/10/644/005
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 31 August 2010
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
NAME OF THE MEDICINAL PRODUCT
PecFent 400 micrograms/spray nasal spray solution
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml of solution contains 4,000 micrograms fentanyl (as citrate)
1 spray (100 microliters) contains 400 micrograms fentanyl (as citrate)
Each bottle contains 1.55 ml (6.20 mg fentanyl) ensuring delivery of 8 sprays of 400 micrograms
Excipients
Each spray contains 0.02 mg propylhydroxybenzoate (E216).
For a full list of excipients, see section 6.1.
Nasal spray, solution (nasal spray)
A clear to practically clear colourless aqueous solution.
4.1 Therapeutic indications
PecFent is indicated for the management of breakthrough pain (BTP) in adults who are already
receiving maintenance opioid therapy for chronic cancer pain. Breakthrough pain is a transitory
exacerbation of pain that occurs on a background of otherwise controlled persistent pain.
Patients receiving maintenance opioid therapy are those who are taking at least 60 mg of oral
morphine daily, at least 25 micrograms of transdermal fentanyl per hour, at least 30 mg of oxycodone
daily, at least 8 mg of oral hydromorphone daily or an equianalgesic dose of another opioid for a week
or longer.
4.2
Posology and method of administration
Treatment should be initiated by and remain under the supervision of a physician experienced in the
management of opioid therapy in cancer patients. Physicians should keep in mind the potential for
abuse of fentanyl.
PecFent should be titrated to an “effective” dose that provides adequate analgesia and minimises
adverse reactions without causing undue (or intolerable) adverse reactions, for two consecutively
treated episodes of BTP. The efficacy of a given dose should be assessed over the ensuing 30 minute
period.
Patients should be carefully monitored until an effective dose is reached.
One dose of PecFent may include administration of 1 spray (100 microgram or 400 microgram doses)
or 2 sprays (200 microgram or 800 microgram doses) of the
same
dose strength (either 100 microgram
or 400 microgram strength).
Patients should not take more than 4 doses per day. Patients should wait at least 4 hours after a dose
before treating another BTP episode with PecFent.
The initial dose of PecFent to treat episodes of BTP is always 100 micrograms (one spray), even
in patients switching from other fentanyl containing products for their BTP.
Patients must wait at least 4 hours before treating another episode of BTP with PecFent.
Patients should be prescribed an initial titration supply of one bottle (8 sprays) of PecFent
100 micrograms/spray.
Patients whose initial dose is 100 micrograms and who need to titrate to a higher dose due to a
lack of effect can be instructed to use two 100 microgram sprays (one in each nostril) for their
next BTP episode. If this dose is not successful, the patient may be prescribed a bottle of
PecFent 400 micrograms/spray and instructed to change to one 400 microgram spray for their
next episode of pain. If this dose is not successful, the patient may be instructed to increase to
two 400 microgram sprays (one in each nostril).
From treatment initiation, patients should be closely followed and the dose titrated until an
effective dose is reached and confirmed for two consecutively treated episodes of BTP.
Titration in patients switching between immediate-release fentanyl containing products
Substantial differences may exist in the pharmacokinetic profile of immediate-release fentanyl
products, which result in clinically important differences in the rate and extent of absorption of
fentanyl. Therefore, when switching between fentanyl containing products indicated for treatment of
breakthrough pain, including intranasal formulations, it is essential that patients are again titrated with
the new product, and not switched on a dose-for-dose (microgram-for-microgram) basis.
Once an effective dose has been established during titration, patients should continue to take this dose
up to a maximum of 4 doses per day.
Generally, the maintenance dose of PecFent should be increased only where the current dose fails to
adequately treat the BTP for several consecutive episodes.
A review of the dose of the background opioid therapy may be required if patients consistently present
with more than four BTP episodes per 24 hours.
If adverse reactions are intolerable or persistent, the dose should be reduced or treatment with PecFent
replaced by another analgesic.
Discontinuation of therapy
PecFent should be discontinued immediately if the patient no longer experiences breakthrough pain
episodes. The treatment for persistent backgound pain should be kept as prescribed.
If discontinuation of all opioid therapy is required, the patient must be closely followed by the doctor
as gradual downward opioid titration therapy is necessary in order to avoid the possibility of abrupt
withdrawal effects.
Paediatric population
The safety and efficacy of PecFent in children aged below 18 years have not yet been established.
No data are available.
Use in the elderly (older than 65 years)
In the PecFent clinical trial programme, 104 (26.1%) of patients were over 60 years of age, 67 (16.8%)
over 65 years and 15 (3.8%) over 75 years. There was no indication that older patients tended to
titrate to lower doses or experience more adverse reactions. Nevertheless, in view of the importance of
renal and hepatic function in the metabolism and clearance of fentanyl, additional care should be
exercised in the use of PecFent in the elderly. No data on the pharmacokinetics of PecFent in elderly
patients are available.
Hepatic or renal impairment
PecFent should be administered with caution to patients with moderate or severe hepatic or renal
impairment (see section 4.4)
PecFent is for administration via the nasal route only.
PecFent can deliver 100, 200, 400 and 800 microgram doses as follows:
Dose required
micrograms)
Product strength
(micrograms)
One spray administered into one
nostril
One spray administered into each
nostril
One spray administered into one
nostril
One spray administered into each
nostril
The bottle should be removed from the child resistant container immediately prior to use and the
protective cap removed. The bottle must be primed before first use by holding upright and simply
pressing and releasing the finger grips either side of the nozzle until a green bar appears in the
counting window (should occur after four sprays).
If the product has not been used for more than 5 days or if it is more than 14 days since the product
was first used, the PecFent bottle should be discarded.
To administer PecFent the nozzle is placed a short distance (about 1 cm) into the nostril and pointed
slightly towards the bridge of the nose. A spray is then administered by pressing and releasing the
finger grips either side of the nozzle. An audible click will be heard and the number displayed on the
counter will advance by one.
Patients must be advised that they may not feel the spray being administered, and that they should
therefore rely on the audible click and the number on the counter advancing to confirm that a spray has
been delivered. The nasal spray pump permanently locks after the eighth spray has been administered.
The PecFent spray droplets form a gel in the nose. Patients should be advised not to blow their nose
immediately after PecFent administration.
The protective cap should be replaced after each use and the bottle returned to the child resistant
container for safe storage.









Hypersensitivity to the active substance or to any of the excipients.
Use in opioid naïve patients.
Severe respiratory depression or severe obstructive lung conditions.
4.4 Special warnings and precautions for use
Patients and their carers must be instructed that PecFent contains an active substance in an amount that
can be fatal to a child, and therefore to keep PecFent out of the reach and sight of children.
In order to minimise the risks of opioid-related adverse reactions and to identify the effective dose, it
is imperative that patients be monitored closely by health professionals during the titration process.
It is important that the long acting opioid treatment used to treat the patient’s persistent pain has been
stabilised before PecFent therapy begins.
Respiratory depression
There is a risk of clinically significant respiratory depression associated with the use of fentanyl.
Patients with pain who receive chronic opioid therapy develop tolerance to respiratory depression and
hence the risk of respiratory depression in these patients is reduced. The use of concomitant central
nervous system depressants may increase the risk of respiratory depression (see section 4.5).
Chronic pulmonary disease
In patients with chronic obstructive pulmonary diseases, fentanyl may cause more serious adverse
reactions. In these patients, opioids may decrease respiratory drive and increase airway resistance.
Increased intracranial pressure
PecFent should only be administered with extreme caution in patients who may be particularly
susceptible to the intracranial effects of CO
2
retention, such as those with evidence of increased
intracranial pressure or impaired consciousness. Opioids may obscure the clinical course of patients
with a head injury and should be used only if clinically warranted.
Cardiac disease
Intravenous fentanyl may produce bradycardia. PecFent should therefore be used with caution in
patients with pre-existing bradyarrhythmias.
Impaired hepatic or renal function
In addition, PecFent should be administered with caution to patients with hepatic or renal impairment.
The influence of hepatic and renal impairment on the pharmacokinetics of the medicinal product has
not been evaluated; however, when administered intravenously the clearance of fentanyl has been
shown to be altered in hepatic and renal impairment due to alterations in metabolic clearance and
plasma proteins. Therefore, special care should be taken during the titration process in patients with
moderate or severe hepatic or renal impairment.
Careful consideration should be given to patients with hypovolaemia and hypotension.
Abuse potential and tolerance
Tolerance and physical and/or psychological dependence may develop upon repeated administration
of opioids such as fentanyl. However, iatrogenic addiction following therapeutic use of opioids is rare.
Athletes should be informed that treatment with fentanyl could lead to positive doping tests.
Route of administration
PecFent is only intended for intranasal administration, and must not be administered by any other
route. Due to physico-chemical properties of excipients included in the formulation, intravenous or
intra-arterial injection must be avoided in particular.
Nasal conditions
If the patient experiences recurrent episodes of epistaxis or nasal discomfort while taking PecFent, an
alternative method of administration for treatment of breakthrough pain should be considered.
PecFent excipients
PecFent contains propylhydroxybenzoate (E216). In some patients this may cause allergic reactions
(possibly delayed) and, exceptionally, bronchospasm (if the product is not correctly administered).
4.5 Interaction with other medicinal products and other forms of interaction
Fentanyl is metabolised mainly via the human cytochrome P450 3A4 isoenzyme system (CYP3A4),
therefore potential interactions may occur when PecFent is given concurrently with agents that affect
CYP3A4 activity. Coadministration with agents that induce 3A4 activity may reduce the efficacy of
PecFent. The concomitant use of PecFent with strong CYP3A4 inhibitors (e.g. ritonavir, ketoconazole,
itraconazole, troleandomycin, clarithromycin, and nelfinavir) or moderate CYP3A4 inhibitors (e.g.
amprenavir, aprepitant, diltiazem, erythromycin, fluconazole, fosamprenavir, grapefruit juice, and
verapamil) may result in increased fentanyl plasma concentrations, potentially causing serious adverse
drug reactions including fatal respiratory depression. Patients receiving PecFent concomitantly with
moderate or strong CYP3A4 inhibitors should be carefully monitored for an extended period of time.
Dose increase should be undertaken with caution.
The concomitant use of other central nervous system depressants, including other opioids, sedatives or
hypnotics, general anaesthetics, phenothiazines, tranquillisers, skeletal muscle relaxants, sedating
antihistamines and alcohol may produce additive depressant effects.
PecFent is not recommended for use in patients who have received monoamine oxidase (MAO)
inhibitors within the previous 14 days because severe and unpredictable potentiation by MAO
inhibitors has been reported with opioid analgesics.
The concomitant use of partial opioid agonists/antagonists (e.g. buprenorphine, nalbuphine,
pentazocine) is not recommended. They have high affinity to opioid receptors with relatively low
intrinsic activity and therefore partially antagonise the analgesic effect of fentanyl and may induce
withdrawal symptoms in opioid dependant patients.
Concomitant use of nasally administered oxymetazoline has been shown to decrease the absorption of
PecFent (see section 5.2). The concomitant use of nasally administered vasoconstrictive decongestants
during titration is therefore not recommended as this may lead to patients titrating to a dose that is
higher than required. PecFent maintenance treatment may also be less effective in patients with rhinitis
when administered concomitantly with a nasal vasoconstrictive decongestant. If this occurs, patients
should be advised to discontinue their decongestant.
Concomitant use of PecFent and other medicinal products (other than oxymetazoline) administered via
the nose has not been evaluated in the clinical trials. Other nasally administered treatments should be
avoided within 15 minutes of dosing with PecFent.
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no adequate data from the use of fentanyl in pregnant women. Studies in animals have
shown reproductive toxicity (see section 5.3). The potential risk for humans is unknown. PecFent
should not be used during pregnancy unless clearly necessary.
Following long-term treatment, fentanyl may cause withdrawal in the new-born infant. It is advised
not to use fentanyl during labour and delivery (including caesarean section) because fentanyl passes
through the placenta and may cause respiratory depression in the foetus. If PecFent is administered, an
antidote for the child should be readily available.
Breastfeeding
Fentanyl passes into breast milk and may cause sedation and respiratory depression in the breast-fed
child. Fentanyl should not be used by breastfeeding women and breast-feeding should not be restarted
until at least 48 hours after the last administration of fentanyl.
Fertility
There are no clinical data on the effects of fentanyl on fertility.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed.
However, opioid analgesics may impair the mental and/or physical ability required for driving or
operating machinery.
Patients should be advised not to drive or operate machinery if they experience somnolence, dizziness,
or visual disturbance or other adverse reactions which can impair their ability to drive or operate
machinery.
Typical opioid adverse reactions are to be expected with PecFent. Frequently, these will cease or
decrease in intensity with continued use of the medicinal product, as the patient is titrated to the most
appropriate dose. However, the most serious adverse reactions are respiratory depression (potentially
leading to apnoea or respiratory arrest), circulatory depression, hypotension and shock and all patients
should be monitored for these.
The clinical studies of PecFent were designed to evaluate safety and efficacy in treating BTP and all
patients were also on background opioid therapies, such as sustained-release morphine or transdermal
fentanyl, for their persistent pain. Therefore it is not possible to definitively separate the effects of
PecFent alone.
The adverse reactions considered to be at least possibly-related to treatment, from Phase II and III
clinical studies were as follows (frequencies defined as very common (≥1/10); common (≥1/100 to
<1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000) within
each frequency grouping, adverse reactions are presented in order of decreasing seriousness).
Infections and infestations
Pneumonia
Nasopharyngitis
Pharyngitis
Rhinitis
Blood and lymphatic system
disorders
Metabolism and nutrition
disorders
Dehydration
Hyperglycaemia
Decreased appetite
Increased appetite










Drug abuse
Delirium
Hallucination
Confusional state
Depression
Attention deficit/hyperactivity
disorder
Anxiety
Euphoric mood
Nervousness
Dysgeusia
Dizziness
Somnolence
Headache
Loss of consciousness
Depressed level of consciousness
Convulsion
Ageusia
Anosmia
Memory impairment
Parosmia
Speech disorder
Sedation
Lethargy
Tremor
Ear and labyrinth disorders
Cardiovascular insufficiency
Lymphoedema
Hypotension
Hot flush
Respiratory, thoracic and
mediastinal disorders
Epistaxis
Rhinorrhoea
Nasal discomfort
Upper airway obstruction
Pharyngolaryngeal pain
Rhinalgia
Nasal mucosal disorder
Cough
Dyspnoea
Sneezing
Upper respiratory tract congestion
Nasal congestion
Intranasal hypoaesthesia
Throat irritiation
Postnasal drip
Nasal dryness
Gastrointestinal disorders
Vomiting
Nausea
Constipation
Intestinal perforation
Peritonitis
Oral hypoaesthesia
Oral paraesthesia
Diarrhoea
Retching
Abdominal pain
Tongue disorder
Mouth ulceration
Dyspepsia
Dry mouth
Skin and subcutaneous tissue
disorders














Musculoskeletal and connective
tissue disorders
Arthralgia
Muscle twitching
Renal and urinary disorders
Anuria
Dysuria
Proteinuria
Urinary hesitation
Reproductive system and breast
disorders
General disorders and
administration site conditions
Non-cardiac chest pain
Asthenia
Chills
Face oedema
Peripheral oedema
Gait disturbance
Pyrexia
Fatigue
Malaise
Thirst
Platelet count decreased
Weight increased
Injury, poisoning and procedural
complications
Fall
Intentional drug misuse
Medication error
The symptoms of fentanyl overdose via the nasal route are expected to be similar in nature to those of
intravenous fentanyl and other opioids, and are an extension of its pharmacological actions, with the
most serious significant effect being respiratory depression.
Immediate management of opioid overdose includes ensuring a patent airway, physical and verbal
stimulation of the patient, assessment of the level of consciousness, ventilatory and circulatory status,
and assisted ventilation (ventilatory support) if necessary.
For treatment of overdose (accidental ingestion) in the opioid-naïve person, intravenous access should
be obtained and naloxone or other opioid antagonists should be employed as clinically indicated. The
duration of respiratory depression following overdose may be longer than the effects of the opioid
antagonist’s action (e.g. the half life of naloxone ranges from 30 to 81 minutes) and repeated
administration may be necessary. Consult the Summary of Product Characteristics of the individual
opioid antagonist for details about such use.
For treatment of overdose in opioid-maintained patients, intravenous access should be obtained. The
judicious use of naloxone or another opioid antagonist may be warranted in some instances, but it is
associated with the risk of precipitating an acute withdrawal syndrome.
It should be noted that although statistically significant increases in C
max
levels were seen following a
second dose of PecFent given either one or two hours after the initial dose, this increase is not
considered to be large enough to suggest that clinically concerning accumulation or over-exposure
would occur, providing a wide safety margin for the recommended dose interval of four hours.
Although muscle rigidity interfering with respiration has not been seen following the use of PecFent,
this is possible with fentanyl and other opioids. If it occurs, it should be managed by the use of
assisted ventilation, by an opioid antagonist, and as a final alternative, by a neuromuscular blocking
agent.












PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Analgesics; phenylpiperidine derivatives; ATC code: N02A-B03.
Mechanism of action
Fentanyl is an opioid analgesic, interacting predominantly with the opioid µ-receptor. Its primary
therapeutic actions are analgesia and sedation. Secondary pharmacological effects are respiratory
depression, bradycardia, hypothermia, constipation, miosis, physical dependence and euphoria.
Pharmacodynamic effects
A double-blind, randomised, placebo-controlled crossover study has been conducted in which 114
patients who experienced on average 1 to 4 episodes of break through pain (BTP) per day while taking
maintenance opioid therapy were entered into an initial open-label titration phase in order to identify
an effective dose of PecFent (Study CP043). The patients entering the double-blind phase treated up to
10 episodes of BTP with either PecFent (7 episodes) or placebo (3 episodes) in a random order.
Of the patients entering the titration phase, only 7 (6.1 %) were unable to be titrated to an effective
dose due to lack of efficacy and 6 (5.3 %) withdrew due to adverse events.
The primary endpoint was the comparison between the summed pain intensity difference at 30 minutes
after dosing (SPID
30
), which was 6.57 in the PecFent-treated episodes compared to 4.45 for placebo
(p<0.0001 ). The SPID for PecFent-treated episodes was also significantly different to placebo at 10
15, 45 and 60 minutes after administration.
The mean pain intensity scores (73 patients) for all PecFent-treated episodes (459 episodes) compared
to those treated with placebo (200 episodes) were significantly lower at 5, 10, 15, 30, 45 and
60 minutes following administration (see Figure 1).
Figure 1: Mean (± SE) Pain Intensity Scores at Each Time Point (mITT Population)
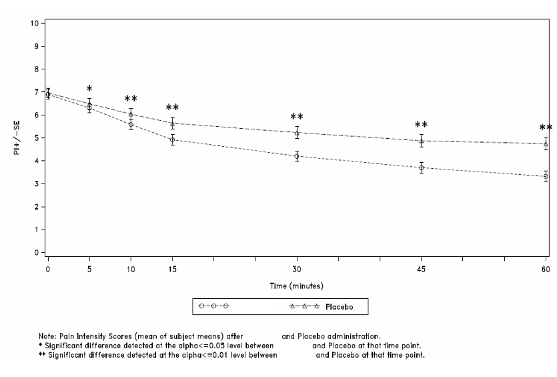
The superior efficacy of PecFent over placebo was supported by data from secondary endpoints
including the number of BTP episodes with clinically meaningful pain relief, defined as a reduction in
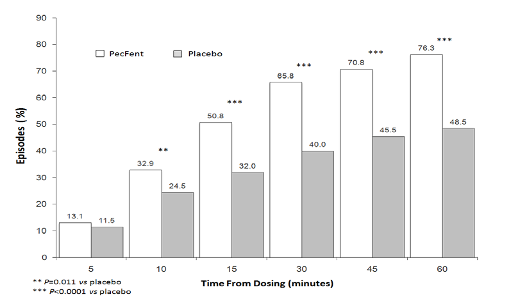
pain intensity score of at least 2 (Figure 2).
Figure 2: Clinically Meaningful Pain Relief – PecFent
vs
placebo: % Patients’ Episodes With ≥2 Point
Reduction in Pain Intensity
In a double-blind, randomized comparator-controlled study (Study 044) of similar design to Study 043
conducted in opioid-tolerant patients with breakthrough cancer pain on stable doses of regularly
scheduled opioids, PecFent was shown to be superior to immediate-release morphine sulfate (IRMS).
Superiority was demonstrated by the primary endpoint, Pain Intensity Difference within 15 minutes,
which was 3.02 in patients treated with PecFent compared to 2.69 in patients treated with IRMS
(p=0.0396).
In a long-term, open-label, safety study (Study 045), 355 patients entered the 16-week treatment
phase, during which 42,227 episodes of breakthrough cancer pain (BTP) were treated with PecFent.
One hundred of these patients continued treatment for up to 26 months in an extension phase. Of the
355 patients treated in the open-label treatment phase, 90 % required no increase in dose.
In the randomised, placebo-controlled study (CP043) 9.4% of 459 PecFent-treated BTP episodes in 73
patients required use of any further (rescue) medicinal products within 60 minutes of dosing. During
the longer-term, open-label study (CP045) this was 6.0 % of 42,227 episodes in 355 patients treated
with PecFent during up to 159 days of treatment.
5.2 Pharmacokinetic properties
Fentanyl is highly lipophilic and can be absorbed very rapidly through the nasal mucosa and more
slowly by the gastrointestinal route. It is subject to first pass hepatic and intestinal metabolism and the
metabolites do not contribute to fentanyl’s therapeutic effects.
PecFent utilises the PecSys nasal drug delivery system to modulate the delivery and absorption of
fentanyl. The PecSys system allows the product to be sprayed into the front area of the nasal cavity as
a fine mist of droplets, which gel on contact with the calcium ions present in the nasal mucosa.
Fentanyl diffuses from the gel and is absorbed through the nasal mucosa; this gel-modulated
A pharmacokinetic study was conducted to evaluate the absorption and tolerability of a single dose of
PecFent in patients with pollen-induced seasonal allergic rhinitis, comparing the un-challenged,
acutely challenged (rhinitic) and acutely challenged and then treated with oxymetazoline, states.
There was no clinically significant effect of acute rhinitis on C
max
, T
max
or overall exposure to fentanyl,
comparing the unchallenged with the acutely challenged states. Following treatment of the acute
rhinitic state with oxymetazoline, there were reductions in C
max
and exposure, and increases in T
max
that were statistically, and possibly clinically, significant.
Fentanyl is highly lipophilic and is well distributed beyond the vascular system, with a large apparent
volume of distribution. Animal data have shown that, following absorption, fentanyl is rapidly
distributed to the brain, heart, lungs, kidneys and spleen followed by a slower redistribution to muscles
and fat.
The plasma protein binding of fentanyl is 80 – 85 %. The main binding protein is alpha-1-acid
glycoprotein, but both albumin and lipoproteins contribute to some extent. The free fraction of
fentanyl increases with acidosis.
The metabolic pathways following nasal administration of PecFent have not been characterised in
clinical studies. Fentanyl is metabolised in the liver to norfentanyl by cytochrome CYP3A4 isoform.
Norfentanyl is not pharmacologically active in animal studies. It is more than 90 % eliminated by
biotransformation to N-dealkylated and hydroxylated inactive metabolites.
Disposition of fentanyl following intranasal administration of PecFent has not been characterised in a
mass balance study. Less than 7 % of an administered dose of fentanyl is excreted unchanged in the
urine and only about 1 % is excreted unchanged in the faeces. The metabolites are mainly excreted in
the urine, while faecal excretion is less important.
The total plasma clearance of fentanyl following intravenous administration is approximately 42 L/h.
Linearity/non-linearity
Dose-proportionality was demonstrated for C
max
and AUC in the dose range 100 micrograms to
800 micrograms.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, genotoxicity and carcinogenicity.
Embryo-foetal developmental toxicity studies conducted in rats and rabbits revealed no compound-
induced malformations or developmental variations when administered during the period of
organogenesis.
In a fertility and early embryonic development study in rats, a male-mediated effect was observed at
high doses (300 mcg/kg/day, s.c.) and is consistent with the sedative effects of fentanyl in animal
studies.
In studies on pre and postnatal development in rats the survival rate of offspring was significantly
reduced at doses causing severe maternal toxicity. Further findings at maternally toxic doses in F1
pups were delayed physical development, sensory functions, reflexes and behaviour. These effects
could either be indirect effects due to altered maternal care and/or decreased lactation rate or a direct
effect of fentanyl on the pups.
Carcinogenicity studies (26-week dermal alternative bioassay in Tg.AC transgenic mice; two-year
subcutaneous carcinogenicity study in rats) did not induce any findings indicative of oncogenic
potential.
PHARMACEUTICAL PARTICULARS
Pectin (E440)
Mannitol (E421)
Phenylethyl alcohol
Propyl hydroxybenzoate (E216)
Sucrose
Hydrochloric acid (0.36%) or sodium hydroxide (for pH adjustment)
Purified water
3 years
After first use: 14 days
After last actuation of the pump: 5 days
The patient should be advised to write the date of first use in the space provided on the label of the
child resistant container
6.4 Special precautions for storage
Do not store above 25
°
C.
Do not freeze.
Keep the bottle in the child resistant container in order to protect from light.
Store the bottle in the child resistant container at all times, even when finished.
6.5
Nature and contents of container
Bottle (clear Type I glass) with an attached metering pump incorporating an audible dose counter and
lock, and a protective cap. Packed in a clam-shell-like child resistant container.
Each bottle contains 1.55 ml ensuring delivery of 8 sprays.
Bottles in their child resistant containers are supplied in cartons containing 1, 4 or 12 bottles.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
As soon as PecFent is no longer needed, patients and members of their household must be advised to
systematically dispose of any bottles remaining from a prescription as soon as possible by returning
them to their child-resistant container and discarding them, according to local requirements or by
returning them to the pharmacy.
MARKETING AUTHORISATION HOLDER
Archimedes Development Ltd
Nottingham
NG7 2TN
United Kingdom
MARKETING AUTHORISATION NUMBER(S)
EU/1/10/644/003
EU/1/10/644/004
EU/1/10/644/006
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 31 August 2010
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
ANNEX II
A.
MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
B. CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer responsible for batch release
L. Molteni & C Dei. F. LLi Alitti Societá di Esercizio S.p.A
Strada Statale 67
Tosco Romagnola
Fraz. Granatieri
IT-50018 Scandicci (FI)
Italy
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to special and restricted medical prescription (See Annex I: Summary of
Product Characteristics, section 4.2).
•
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
Prior to launch in each member State the MAH shall agree the final educational material with the
National Competent Authority.
The MAH shall ensure that, at launch, all physicians, pharmacists and patients expected to
prescribe/use PecFent are provided with educational material informing about the correct and safe use
of the product.
Educational material for patients should highlight the following:
•
Instructions for use of the nasal spray device
•
Instructions for opening and closing of the child-resistant box
•
Information on the correct indication
•
Only use PecFent nasal spray if you are using other opioid pain medicine on a daily basis
•
Only use PecFent nasal spray if you have been experiencing breakthrough cancer pain
episodes
•
Do not use PecFent nasal spray to treat any other short-term pain or pain status
•
Do not use PecFent nasal spray for treatment of more than four breakthrough cancer pain
episodes a day
•
Only use PecFent nasal spray if you have received the proper information regarding the use of
the device and the safety precautions from the prescriber and/or the pharmacist
•
All unused devices or empty containers should be returned systematically according to the
local regulation
Educational material for physicians should highlight the following:
•
PecFent nasal spray should be prescribed only by physicians experienced in the management
of opioid therapy in cancer patients.
•
The prescribers of PecFent nasal spray must critically select the patients and closely follow
o
Instructions for use of the nasal spray device
o
Instructions for opening and closing of the child-resistant box
o
Information on the correct indication
•
PecFent nasal spray should not be used to treat any other short-term pain or pain status.
•
All unused devices or empty containers should be returned systematically according to the
local regulation.
•
The prescriber must make use of the checklist for prescribers
Educational material for pharmacists should highlight the following:
•
PecFent nasal spray is only indicated for the management of breakthrough pain in adults
already receiving maintenance opioid therapy for chronic cancer pain
•
PecFent nasal spray should not be used to treat any other short-term pain or pain status
•
The pharmacist must be familiar with the educational material of PecFent nasal spray before
using it in his/her organization
•
The PecFent nasal spray dose strengths can not be compared with other PecFent products
•
Instructions for use of the nasal spray device
•
Instructions for opening and closing of the child-resistant box
•
The pharmacist must inform the patients that in order to prevent theft and misuse of PecFent
nasal spray they have to keep it in a safe place to avoid misuse and diversion
•
All unused devices or empty containers should be returned systematically according to the
local regulation
•
The pharmacist must make use of the checklist for pharmacist
The MAH must ensure that the system of pharmacovigilance, as described in version 3.0 presented in
Module 1.8.1 of the Marketing Authorisation Application, is in place and functioning before and
whilst the product is on the market.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, the
updated RMP should be submitted at the same time as the next Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted:
•
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
•
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being reached
•
at the request of the EMA
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version 3.0 of the Risk Management Plan (RMP) presented
in Module 1.8.2 of the Marketing Authorisation application and any subsequent updates of the RMP
agreed by the CHMP.
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
PecFent 100 micrograms/spray nasal spray solution
Fentanyl
STATEMENT OF ACTIVE SUBSTANCE(S)
Each spray contains 100 micrograms of fentanyl (as citrate)
Each ml of solution contains 1,000 micrograms fentanyl (as citrate)
Also contains: pectin (E440), mannitol (E421), phenylethyl alcohol, propyl hydroxybenzoate (E216),
sucrose, purified water and hydrochloric acid (0.36%) or sodium hydroxide for pH adjustment. See
package leaflet for further information.
PHARMACEUTICAL FORM AND CONTENTS
Nasal spray, solution
1 bottle
4 bottles
12 bottles
METHOD AND ROUTE(S) OF ADMINISTRATION
Nasal use
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
This product should only be used by patients taking other opioids.
EXP
After first-use, use within 14 days
After last actuation, use within 5 days
























SPECIAL STORAGE CONDITIONS
Do not store above 25
°
C.
Keep the bottle in the child resistant container in order to protect from light.
Do not freeze.
Store the PecFent bottle in the child resistant container at all times, even when finished.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Archimedes Development Ltd
Nottingham
NG7 2TN
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/10/644/001
EU/1/10/644/002
EU/1/10/644/005
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE






















PARTICULARS TO APPEAR ON THE CHILD RESISTANT CONTAINER (CRC)
NAME OF THE MEDICINAL PRODUCT
PecFent 100 micrograms/spray nasal spray
Fentanyl
STATEMENT OF ACTIVE SUBSTANCE(S)
Each spray contains 100 micrograms of fentanyl (as citrate)
Also contains: pectin (E440), mannitol (E421), phenylethyl alcohol, propyl hydroxybenzoate (E216),
sucrose, purified water and hydrochloric acid (0.36%) or sodium hydroxide for pH adjustment. See
package leaflet for further information.
PHARMACEUTICAL FORM AND CONTENTS
METHOD AND ROUTE(S) OF ADMINISTRATION
Nasal use
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
This product should only be used by patients taking other opioids.
After first-use, use within 14 days
Date of first use: ……….
SPECIAL STORAGE CONDITIONS
Do not store above 25
°
C.
Keep the bottle in the child resistant container in order to protect from light.
Do not freeze.
Store the PecFent bottle in the child resistant container at all times, even when finished.
Keep in child resistant container.























10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Archimedes Development Ltd
Nottingham
NG7 2TN
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
16. INFORMATION IN BRAILLE

















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
PecFent 400 micrograms/spray nasal spray solution
Fentanyl
STATEMENT OF ACTIVE SUBSTANCE(S)
Each spray contains 400 micrograms of fentanyl (as citrate)
Each ml of solution contains 4,000 micrograms fentanyl (as citrate)
Also contains: pectin (E440), mannitol (E421), phenylethyl alcohol, propyl hydroxybenzoate (E216),
sucrose, purified water and hydrochloric acid (0.36%) or sodium hydroxide for pH adjustment. See
package leaflet for further information.
PHARMACEUTICAL FORM AND CONTENTS
Nasal spray, solution
1 bottle
4 bottles
12 bottles
METHOD AND ROUTE(S) OF ADMINISTRATION
Nasal use
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
This product should only be used by patients taking other opioids.
EXP
After first-use, use within 14 days.
After last actuation, use within 5 days.
























SPECIAL STORAGE CONDITIONS
Do not store above 25
°
C.
Keep the bottle in the child resistant container in order to protect from light.
Do not freeze.
Store the PecFent bottle in the child resistant container at all times, even when finished.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Archimedes Development Ltd
Nottingham
NG7 2TN
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/10/644/003
EU/1/10/644/004
EU/1/10/644/006
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE






















PARTICULARS TO APPEAR ON THE CHILD RESISTANT CONTAINER (CRC)
NAME OF THE MEDICINAL PRODUCT
PecFent 400 micrograms/spray nasal spray
Fentanyl
STATEMENT OF ACTIVE SUBSTANCE(S)
Each spray contains 400 micrograms of fentanyl (as citrate)
Also contains: pectin (E440), mannitol (E421), phenylethyl alcohol, propyl hydroxybenzoate (E216),
sucrose, purified water and hydrochloric acid (0.36%) or sodium hydroxide for pH adjustment. See
package leaflet for further information.
PHARMACEUTICAL FORM AND CONTENTS
METHOD AND ROUTE(S) OF ADMINISTRATION
Nasal use
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
This product should only be used by patients taking other opioids.
After first-use, use within 14 days
Date of first use: ……….
SPECIAL STORAGE CONDITIONS
Do not store above 25
°
C.
Keep the bottle in the child resistant container in order to protect from light.
Do not freeze.
Store the PecFent bottle in the child resistant container at all times, even when finished.
Keep in child resistant container.























10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Archimedes Development Ltd
Nottingham
NG7 2TN
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
16. INFORMATION IN BRAILLE

















PACKAGE LEAFLET: INFORMATION FOR THE USER
PecFent 100 micrograms/spray nasal spray, solution
PecFent 400 micrograms/spray nasal spray, solution
Fentanyl
Read all of this leaflet carefully before you start using this medicine.
•
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects get serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
In this leaflet
:
1. What PecFent is and what it is used for
2.
Before you use PecFent
3. How to use PecFent
4. Possible side effects
5.
WHAT PECFENT IS AND WHAT IT IS USED FOR
What PecFent is
PecFent is a strong pain-relieving medicine known as an opioid pain killer.
What PecFent is used for
PecFent is used in adults with cancer for a type of pain called ‘breakthrough’ pain.
•
Breakthrough pain comes on suddenly.
•
It comes on even though you have taken your usual opioid pain killer (such as morphine,
fentanyl, oxycodone or hydromorphone) to control your constant background pain.
PecFent is only to be used by adults who are already taking other opioid medicines daily for their
constant cancer pain.
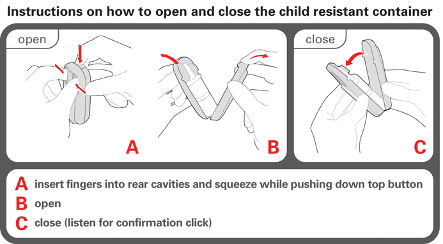
How PecFent works
PecFent is a nasal spray.
•
When you spray PecFent into your nose the very small spray droplets form a thin gel.
Fentanyl is absorbed quickly through the lining of your nose and into the blood stream.
This means the medicine gets into your system quickly to relieve your breakthrough pain.
Do not use PecFent if:
•
you are allergic (hypersensitive) to fentanyl or any of the other ingredients of PecFent (listed in
Section 6).
•
you are
not
regularly using a prescribed opioid medicine (e.g. codeine, fentanyl,
hydromorphone, morphine, oxycodone, pethidine) for your constant pain.
•
you have a serious breathing or lung problem.
Do not use PecFent if any of the above applies to you. If you are not sure, talk to your doctor or
pharmacist before using PecFent.
Take special care with PecFent
Keeping PecFent safe from children
•
You must keep PecFent in the child resistant storage container when you are not using it, even if
you have used all 8 sprays. This is because PecFent could be life-threatening if taken by a child
by accident.
•
PecFent is not approved for use in children under 18 years of age.
Check with your doctor or pharmacist before using PecFent if:
•
you have not been taking the same dose of your daily opioid medicine for your constant pain for
some time
•
you have breathing problems such as asthma, wheezing or shortness of breath
•
you suffer a severe blow to the head
•
you have heart problems such as a very slow heart rate
•
you have low blood pressure or a low amount of fluid in your circulation
•
you have liver or kidney problems. This is because it may affect the way in which your body
breaks down the medicine.
If any of the above apply to you (or you are not sure), talk to your doctor or pharmacist before using
PecFent.
If you are an athlete, taking PecFent may result in positive doping-tests.
Please also tell your doctor if after using PecFent:
•
you suffer from recurrent nose bleeding - he may advise an alternative treatment
you feel that PecFent is becoming less effective in treating your episodes of breakthrough pain
you think you are becoming dependent on PecFent
Using other medicines
Please tell your doctor or pharmacist if you are using or have recently used any other medicines. This
includes medicines you get without a prescription, such as herbal medicines.
In particular tell your doctor or pharmacist before using PecFent if you are taking or have recently
taken any of the following medicines:
•
medicines that might make you sleepy such as sleeping tablets, tranquilisers, muscle relaxants,
medicines for anxiety or medicines for allergies (anti-histamines)
medicines for depression called ‘monoamine-oxidase inhibitors’ (MAOI). Tell your doctor or
pharmacist if you have taken an MAOI medicine in the past 2 weeks before using PecFent
nasal sprays to treat a stuffy nose (containing a decongestant such as oxymetazoline)
medicines that might have an effect on the way your body breaks down PecFent. These include:
o
medicines for HIV infection (such as ritonavir, nelfinavir, amprenavir or fosamprenavir)
medicines for fungal infections (such as ketoconazole, itraconazole or fluconazole)
o
medicines for bacterial infections (such as troleandomycin, clarithromycin or
erythromycin)
o
‘aprepitant’ - used to stop you feeling sick
o
‘diltiazem’ and ‘verapamil’ - used for high blood pressure or heart problems.
o
Other pain killers like buprenorphine, nalbuphine, pentazocine
If any of the above applies to you (or you are not sure), talk to your doctor or pharmacist before using
PecFent.
Don’t use any other kind of nasal spray for at least 15 minutes after using PecFent.
Using PecFent with food and drink
•
Do not drink alcohol while using PecFent. It can increase the risk of getting serious side effects.
Do not drink grapefruit juice while using PecFent. It may affect the way your body breaks down
PecFent.
Pregnancy and breast-feeding
•
Do not use PecFent if you are pregnant or might get pregnant, unless your doctor has told you
to.
•
Do not use PecFent during child birth. This is because it may cause breathing problems in your
baby.
•
Do not use PecFent if you are breast-feeding, unless your doctor has told you to. This is because
the medicine can get into your breast milk and may cause side effects in the breast-fed child.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
•
Talk to your doctor about whether it will be safe for you to drive or use tools or machines after
using PecFent.
You may feel sleepy, dizzy or have problems with your eyesight after using PecFent. If this
happens, do not drive or use any tools or machines.
Do not drive or use tools or machines until you know how this medicine makes you feel.
Important information about some of the ingredients of PecFent
PecFent contains propylhydroxybenzoate (E216). May cause allergic reactions (possibly delayed), and
exceptionally, bronchospasm (if you do not use the nasal spray correctly).
Always use PecFent exactly as your doctor has told you. You should check with your doctor or
pharmacist if you are not sure.
PecFent comes in two different strengths:
a 100 microgram per spray bottle and a 400 microgram
per spray bottle. Make sure that you use the strength that your doctor has prescribed for you.
Do not use PecFent more than 4 times daily
Wait at least 4 hours to take the next dose of PecFent.
A dose to treat a breakthrough pain episode might be either 1 spray or 2 sprays (one in each
nostril). Your doctor will tell you how many sprays (1 or 2) you should use to treat your
breakthrough pain episode.
Do not use more than the dose your doctor prescribes for any single breakthrough pain
episode.
The starting dose is 100 micrograms.
This is a single spray into one nostril from the 100 microgram per spray bottle.
See ‘Using the PecFent bottle’ for instructions on how to take a dose.
Your doctor will then help you find the right dose to relieve your breakthrough pain. It is very
important to follow your doctor’s instructions.
Tell your doctor about your pain and how PecFent is working. Your doctor will decide if your
PecFent dose needs to be changed.
Do not change the dose yourself.
Once you have found the right dose
•
Tell your doctor if your dose of PecFent does not relieve your breakthrough pain. Your doctor
will decide if your dose needs to be changed.
Do not change the dose of PecFent or your
other pain medicines yourself.
•
Tell your doctor straight away if you have more than 4 episodes of breakthrough pain a day.
Your doctor may change the medicine for your constant pain. Once your constant pain is
controlled, your doctor may then change your dose of PecFent.
If you are not sure about the right dose or how much PecFent to use, ask your doctor.
PecFent is only to be used by spraying into your nostril.
Preparing the PecFent bottle for use
Before you use a new bottle of PecFent
you need to prepare it for use. This is called ‘priming’.
To prime the bottle, please follow the instructions below:
1.
A new bottle of PecFent will show two red lines in the counting window in the white plastic top
on the bottle (Figure 1 and Figure 3a).
2.
Take off the clear plastic protective cap from the nozzle (Figure 1).
3.
Aim the nasal spray away from you (and any other people or animals).
4.
Hold the PecFent nasal spray upright with your thumb on the bottom of the bottle, and your first
and middle fingers on the finger grips each side of the nozzle (Figure 2).
5.
Firmly press down on the finger grips until a ‘click’ is heard and then let go of the grips (Figure
2). You will hear a second ‘click’ and there should now be a single large red bar in the counting
window (Figure 3b).
6.
Repeat step 5 three times. As you repeat step 5, the red bar will become smaller and smaller
until you see a green bar in the counting window (Figure 3b-e). The green bar means the
PecFent nasal spray is ready to use.
7.
Wipe the nozzle with a tissue and flush the tissue down the toilet.
8.
If you are not going to take your medicine straight away, put the protective cap back on. Then
put the PecFent bottle in the child-resistant storage container. Once you have primed the
PecFent bottle you must start using it within 5 days.
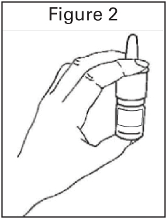
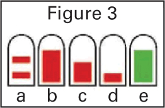
Dispose of the PecFent bottle and start a new one if:
•
it has been 14 days or more since you primed or used your bottle for the first time.
•
it has been 5 days or more since you last used this bottle of PecFent.
Dispose of the PecFent bottle and start a new one if either of the above apply to you.
Using PecFent
1.
Check that there is green bar or a number showing in the counting window (Figure 4): this
confirms that the PecFent
bottle has been primed (see ‘Preparing the PecFent bottle for use’
above).
2.
Blow your nose if you feel you need to.
3.
Sit down with your head upright.
4.
Take off the protective cap from the nozzle.
5.
Hold the PecFent bottle with your thumb on the bottom of the bottle and your first and middle
fingers on the finger grips (Figure 4).
6.
Put the nozzle a short distance (about 1 cm) into your nostril. Point it inwards towards the wall
of your nose. This will tilt the bottle slightly (Figure 5).
7.
Close the other nostril with a finger from your other hand (Figure 5).
8.
Firmly press down on the finger grips so that PecFent sprays into your nostril. When you hear a
click let go of the grips. Note: You may not feel anything happen in your nose at all – do not
trust this to mean the spray did not operate – rely on the click and number counter.
9.
Breathe in gently through your nose and out through your mouth.
10.
The number counter will move forward after each use and show how many sprays have been
used.
11.
If your doctor has prescribed a second spray, repeat steps 5 to 9, using the other nostril.
Do not use more than the dose that your doctor prescribes to treat any single pain episode.
12.
Put the bottle back in the child-resistant container after each use. Keep out of the reach and sight
of children (Figure 6)
13.
Stay sitting for at least 1 minute after using the nasal spray.
Number of sprays in a PecFent bottle
There are 8 sprays in each PecFent bottle.
•
After the first spray, number 1 will appear in the counting window. This will go up each time
the spray is used.
When you see a red 8 in the counting window, there are no more sprays left and the pump will
lock. Put the bottle back in the child resistant container.
If the PecFent nasal spray is blocked or does not spray properly
•
If the spray is blocked, aim the spray away from you (and any other people or animals) and push
firmly down on the pump. This should clear any blockage.
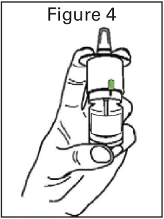
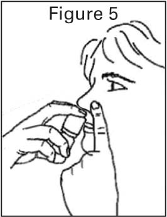
If your nasal spray is still not working properly, dispose of the faulty bottle and start a new one.
Tell your doctor what happened.
Never try to fix the nasal spray yourself or take it apart.
This is because it may then give you the wrong dose.
If you use more PecFent than you should
•
If you use more PecFent than you should, you may feel sleepy, sick, dizzy or have slow or
shallow breathing. If you feel very dizzy, very sleepy or have slow or shallow breathing, call an
ambulance or ask someone else to call one straight away.
If you stop using PecFent
If you no longer have breakthrough pain, talk to your doctor before stopping PecFent and follow
his/her advice. However, you should keep taking your other opioid medicine to treat your constant
pain. Your doctor may need to check the dose.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Like all medicines, PecFent can have side effects, although not everybody gets them.
Call an ambulance or ask someone else to call one straight away if you:
•
feel very dizzy or faint
•
feel very sleepy
•
get slow or shallow breathing
•
get cold clammy skin, look pale, have a weak pulse or other signs of shock.
If you or your carer notice any of the side effects above, call an ambulance straight away.
The frequency of possible side effects listed below is defined using the following convention:
Very common (affects more than 1 in every 10 users)
Common (affects 1 to 10 users in every 100 users)
Uncommon (affects 1 to 10 users in every 1,000 users)
Rare (affects 1 to 10 users in every 10,000 users)
Very rare (affects less than 1 user in every 10,000 users)
Not known (frequency cannot be estimated from the available data).
not knowing where you are (disorientated)
nose bleed, discomfort in the nose, runny nose
painful, sore or inflamed throat or nose
cough, sneezing, catarrh or cold, changes in the fluid produced by your nose
loss of or increase in appetite, weight increase
dehydration, feeling thirsty
abusing or misusing the medicine
seeing or hearing things that are not really there (hallucinations/delirium), feeling confused
depressed, worried, slow or nervous
a lack of concentration or increased activity
being less aware or responsive, losing consciousness
muscle convulsions or trembling
loss of taste, loss or change in sense of smell
heat and circulation not working properly, hot flush or fever, chills, excessive sweating
swelling of the soft tissue
blockage in the wind-pipe
tear in the intestine or inflammation of the stomach lining
numbness or tingling in the mouth or tongue, or other tongue problems, mouth ulcers, dry
mouth
retching, stomach pains, indigestion
difficulty in or inability to pass water
feeling tired or weak, problems moving
changes in blood cells (detected by laboratory tests)
If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep PecFent out of the reach and sight of children. PecFent could be life-threatening if taken
by a child by accident.
•
Do not use PecFent after the expiry date which is stated on the carton and bottle after EXP. The
expiry date refers to the last day of that month.
Do not use for more than 14 days after first use (either priming or using to treat a breakthrough
pain episode).
Write the date of first use in the space provided on the label of the child resistant container.
If it is more than 5 days since you last used the PecFent nasal spray, dispose of the bottle,
following the instructions below, and use a new bottle.
Do not store PecFent above 25
°
C. Do not freeze.
Keep the bottle in its child resistant container in order to protect from light
.
Store the PecFent bottle in the child resistant container at all times, even when finished.
PecFent that has passed the expiry date or is no longer required may still contain enough
medicine to be harmful to other people, especially children. PecFent should not be disposed of
via wastewater or household waste. Any used or unused bottles should be returned to their child
resistant container and discarded as soon as possible by taking them back to the pharmacy or
according to local requirements.
What PecFent contains
PecFent is available in two strengths: PecFent 100 micrograms/spray nasal spray solution and PecFent
400 micrograms/spray nasal spray solution.
The active substance is fentanyl.
PecFent 100 or 400 micrograms/spray nasal spray, solution
Each ml of solution contains 1,000 micrograms (100 microgram strength) or 4,000 micrograms
(400 microgram strength) fentanyl (as citrate).
1 spray (100 microliters) contains either 100 micrograms (100 microgram strength) or 400
micrograms (400 microgram strength) fentanyl.
The other ingredients are pectin (E440), mannitol (E421), phenylethyl alcohol, propyl
hydroxybenzoate (E216), sucrose, purified water and hydrochloric acid or sodium hydroxide for
pH adjustment.
What PecFent looks like and contents of the pack
The medicine is a clear to almost clear, colourless nasal spray, solution. It is contained in a clear glass
bottle, fitted with a metering pump. The pump has a spray counter that clicks, so you can hear as well
as see that the spray has been given, an end-of-use lock and a protective cap. After the PecFent bottle
has been primed (prepared for use) it delivers 8 sprays. The nasal spray pump locks after the eighth
spray has been used. Each PecFent bottle is supplied in a child resistant container.
PecFent bottles in their child resistant containers are supplied in cartons containing 1, 4 or 12 bottles.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Archimedes Development Ltd
Nottingham
NG7 2TN
United Kingdom
Manufacturer
L. Molteni & C. dei. F.lli Alitti Società di Esercizio S.p.A
Strada Statale 67 Tosco Romagnola,
Fraz. Granatieri – 50018 Scandicci (FI)
Italy
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
Luxembourg/Luxemburg
Royaume-Uni
Tél/Tel: + 44 (0)118 931 5050
Lietuva
Archimedes Pharma Ltd
Didžioji Britanija
(Jungtinė Karalystė
)
Tel: + 44 (0)118 931 5050
България
Великобритания (Обединеното кралство)
Teл.: + 44 (0)118 931 5050
Magyarország
Archimedes Pharma Ltd
Egyesült Királyság
(Nagy-Britannia)
Tel: + 44 (0)118 931 5050
Česká republika
Archimedes Pharma Ltd
Spojené království
(Velká Británie)
Tel: + 44 (0)118 931 5050
Malta
Archimedes Pharma Ltd
Ir-Renju Unit
Tel: + 44 (0)118 931 5050
Danmark
Archimedes Pharma Ltd
Det Forenede Kongerige
(
Storbritannien)
Tlf: + 44 (0)118 931 5050
Nederland
Archimedes Pharma Ltd
Verenigd Koninkrijk
Tel: + 44 (0)118 931 5050
Deutschland
Archimedes Pharma Germany GmbH
Tel: +49 (0) 621124704-20
Norge
Archimedes Pharma Ltd
Det forente kongerike
(Storbritannia)
Tlf: + 44 (0)118 931 5050
Eesti
Archimedes Pharma Ltd
Ühendkuningriik
Tel: + 44 (0)118 931 5050
Österreich
Archimedes Pharma Ltd
Vereinigtes Königreich
Tel: + 44 (0)118 931 5050
Ελλάδα
Archimedes Pharma Ltd
Ηνωμένο Βασίλειο
Τηλ: + 44 (0)118 931 5050
Polska
Molteni Farmaceutici Polska Sp. z o.o.
Tel: + 48 (012) 653 15 71
España
Archimedes Pharma Ibérica SL
Tel: + 34 (0) 91 3510373
Portugal
Archimedes Pharma Ltd
Reino UnidoTel: + 44 (0)118 931 5050
France
Archimedes Pharma France SARL
Tél : +33 (0) 155702320
România
Archimedes Pharma Ltd
Marea Britanie
Tel: + 44 (0)118 931 5050
Ireland
Archimedes Pharma Ltd
UK
Tel: + 44 (0)118 931 5050
Slovenija
Archimedes Pharma Ltd
Združeno kraljestvo
(Velika Britanija)
Tel: + 44 (0)118 931 5050
Ísland
Archimedes Pharma Ltd
Sameinaða konungsdæmið
(Bretland)
Sími: + 44 (0)118 931 5050
Slovenská republika
Archimedes Pharma Ltd
Spojené kráľovstvo (
Veľká Británia
)
Tel: + 44 (0)118 931 5050
Italia
L. Molteni & C. dei F.lli Alitti
Società di Esercizio S.p.A.
Tel: + 39.055.73611
Suomi/Finland
Archimedes Pharma Ltd
Yhdistynyt kuningaskunta
(Iso-Britannia)
Puh/Tel: + 44 (0)118 931 5050
Κύπρος
Archimedes Pharma Ltd
250 South Oak Way, Green Park
Ηνωμένο Βασίλειο
Τηλ: + 44 (0)118 931 5050
Sverige
Archimedes Pharma Ltd
Förenade Kungariket
(Storbritannien)
Tel: + 44 (0)118 931 5050
Latvija
Archimedes Pharma Ltd
Lielbritānija
Tel: + 44 (0)118 931 5050
United Kingdom
Archimedes Pharma Ltd
Tel: + 44 (0)118 931 5050
This leaflet was last approved in
{MM/YYYY}.
Detailed information on this medicine is available on the European Medicines Agency (EMA) web
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/pecfent.html
Copyright © 1995-2021 ITA all rights reserved.
|



















































































































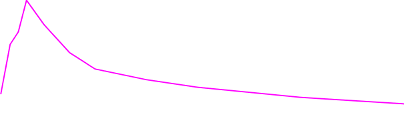





































































































































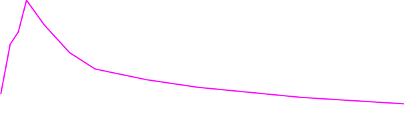









































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































