Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender spot-on solution for small cats
Profender spot-on solution for medium cats
Profender spot-on solution for large cats
QUALITATIVE AND QUANTITATIVE COMPOSITION
Profender contains 21.4 mg/ml emodepside and 85.8 mg/ml praziquantel.
Each unit dose (pipette) of Profender delivers:
Profender for Small Cats
(
Profender for Medium Cats
(> 2.5 – 5 kg)
Profender for Large Cats
(> 5 – 8 kg)
5.4 mg/ml butylhydroxyanisole (E320; as antioxidant)
For a full list of excipients, see section 6.1.
Spot-on solution.
Clear yellow to brown solution.
4.2 Indications for use, specifying the target species
For cats suffering from, or at risk from, mixed parasitic infections caused by roundworms and
tapeworms of the following species:
Roundworms (Nematodes)
Toxocara cati
(mature adult, immature adult, L4 and L3)
Toxascaris leonina
(mature adult, immature adult and L4)
Ancylostoma tubaeforme
(mature adult, immature adult and L4)










Tapeworms (Cestodes)
Dipylidium caninum
(adult)
Taenia taeniaeformis
(adult)
Echinococcus multilocularis
(adult)
Do not use in kittens under 8 weeks of age or weighing less than 0.5 kg.
Shampooing or immersion of the animal in water directly after treatment may reduce the efficacy of
the product. Treated animals therefore should not be bathed until the solution has dried.
Parasite resistance to any particular class of anthelmintic may develop following frequent, repeated
use of an anthelmintic of that class.
4.5 Special precautions for use
Special precautions for use in animals
Apply only to the skin surface and on intact skin. Do not administer orally or parenterally.
Avoid the treated cat or other cats in the household licking the site of application while it is wet.
There is limited experience on the use of the product in sick and debilitated animals, thus the product
should only be used based on a benefit-risk assessment for these animals.
Special precautions to be taken by the person administering the veterinary medicinal product to
animals
Read the package leaflet before use.
Do not smoke, eat or drink during application.
Avoid direct contact with application area while it is wet. Keep children away from treated animals
during that time.
Wash hands after use.
In case of accidental spillage onto skin, wash off immediately with soap and water.
If the product accidentally gets into eyes, they should be thoroughly flushed with plenty of water.
If skin or eye symptoms persist, or in case of accidental ingestion, seek medical advice and show the
package leaflet or the label to the physician.
Care should be taken not to allow children to have prolonged intensive contact (for example, by
sleeping) with treated cats during the first 24 hours after application of the product.
Frequent users of the product (for example, veterinarians, professional cat breeders) should wear
disposable gloves when administering the product.
Although the product was well tolerated by pregnant cats, studies performed in rats and rabbits
suggest that emodepside may interfere with embryo-foetal development. Therefore, women of child-
bearing potential should avoid contact with, or wear disposable gloves when administering, the
product.
The solvent in this product may stain certain materials including leather, fabrics, plastics and finished
surfaces. Allow the application site to dry before permitting contact with such materials.
Echinococcosis represents a hazard for humans. As Echinococcosis is a notifiable disease to the OIE,
specific guidelines on the treatment and follow-up, and on the safeguard of persons, need to be
obtained from the relevant competent authority.
4.6 Adverse reactions (frequency and seriousness)
Salivation and vomiting may occur in very rare cases. This is thought to occur as a result of the cat
licking the application site immediately after treatment. In very rare cases following administration of
Profender transient alopecia, pruritus and/or inflammation were observed at the application site.
4.7 Use during pregnancy, lactation or lay
Can be used during pregnancy and lactation.
4.8 Interaction with other medicinal products and other forms of interaction
Emodepside is a substrate for P-glycoprotein. Co-treatment with other drugs that are P-glycoprotein
substrates/inhibitors (for example, ivermectin and other antiparasitic macrocyclic lactones,
erythromycin, prednisolone and cyclosporine) could give rise to pharmacokinetic drug interactions.
The potential clinical consequences of such interactions have not been investigated.
4.9 Amounts to be administered and administration route
Dosage and Treatment Schedule
The recommended minimum doses are 3 mg emodepside / kg body weight and 12 mg praziquantel /
kg body weight, equivalent to 0.14 ml Profender / kg body weight.
0.5 - 2.5 Profender for Small Cats
Profender for Medium Cats
Use an appropriate combination of pipettes
A single administration per treatment is effective.
Remove one pipette from package. Hold pipette in upright position, twist and pull off cap and use the
opposite end of the cap to break the seal.
Part the fur on the cat’s neck at the base of the skull until the skin is visible. Place the tip of the
pipette on the skin and squeeze firmly several times to empty the contents directly onto the skin.
Application on the base of the skull will minimise the ability of the cat to lick the product off.
4.10 Overdose (symptoms, emergency procedures, antidotes), if necessary
Salivation, vomiting and neurological signs (tremor) were observed occasionally when the product
was administered at up to 10 times the recommended dose in adult cats and up to 5 times the
recommended dose in kittens. These symptoms were thought to occur as a result of the cat licking the
application site. The symptoms were completely reversible.
There is no known specific antidote.











PHARMACOLOGICAL PROPERTIES
Pharmacotherapeutic group: therapeutic antiparasitic agent; ATCvet code: QP52AA51.
5.1 Pharmacodynamic properties
Emodepside
is a semi-synthetic compound belonging to the new chemical group of depsipeptides. It is
active against roundworms (ascarids and hookworms). In this product, emodepside is responsible for
the efficacy against
Toxocara cati
,
Toxascaris leonina
, and
Ancylostoma tubaeforme
.
It acts at the neuromuscular junction by stimulating presynaptic receptors belonging to the secretin
receptor family which results in paralysis and death of the parasites.
Praziquantel
is a pyrazinoisoquinoline derivative effective against tapeworms such as
Dipylidium
caninum
,
Echinococcus
multilocularis
, and
Taenia taeniaeformis
.
Praziquantel is rapidly adsorbed via the surface of the parasites and acts primarily by changing the
Ca
++
permeability of the parasite membranes. This results in severe damage to the parasite
integument, contraction and paralysis, disruption of metabolism and finally leads to the death of the
parasite.
5.2 Pharmacokinetic particulars
After topical application of this product to cats at the minimum therapeutic dose of
0.14 ml/kg bodyweight, mean maximum serum concentrations of 32.2 ± 23.9 µg emodepside/l and
61.3 ± 44.1 µg praziquantel/l were observed. Maximum concentrations were reached for emodepside
3.2 ± 2.7 days after application and 18.7 ± 47 hours for praziquantel. Both active substances are then
slowly eliminated from the serum with a half-life of 9.2 ± 3.9 days for emodepside and 4.1 ± 1.5 days
for praziquantel.
After oral application in the rat, emodepside is distributed to all organs. Highest concentration levels
are found in the fat. Faecal excretion predominates with unchanged emodepside and hydroxylated
derivatives as the major excretion products.
Studies in many different species show that praziquantel is rapidly metabolised in the liver. The main
metabolites are monohydroxycyclohexyl derivatives of praziquantel. Renal elimination predominates.
5.3 Environmental properties
PHARMACEUTICAL PARTICULARS
Butylhydroxyanisole
Isopropylidene glycerol
Lactic acid
Shelf life of the veterinary medicinal product as packaged for sale: 3 years
6.4 Special precautions for storage
Store in the original package in order to protect from moisture.
6.5 Nature and composition of immediate packaging
0.35 ml, 0.70 ml and 1.12 ml per pipette
Blister packs containing 2, 4, 12, 20, or 40 unit dose
pipettes; 0.70 ml pipette only: additional blister pack
containing 80 pipettes
White polypropylene pipettes with caps in aluminium
blisters
Not all pack sizes may be marketed.
6.6
Special precautions for the disposal of unused veterinary medicinal product or waste
materials derived from the use of such products
Profender should not be allowed to enter surface water as emodepside has shown harmful effects on
aquatic organisms.
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal
products should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Bayer Animal Health GmbH
D-51368 Leverkusen
Germany
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
27 July 2005/1
st
July 2010
10. DATE OF REVISION OF THE TEXT
Detailed information on this veterinary medicinal product is available on the website of the European
Medicines Agency
http://www.ema.europa.eu/
.
PROHIBITION OF SALE, SUPPLY AND/OR USE
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender spot-on solution for cats
QUALITATIVE AND QUANTITATIVE COMPOSITION
Profender contains 21.4 mg/ml emodepside and 85.8 mg/ml praziquantel.
5.4 mg/ml butylhydroxyanisole (E320; as antioxidant)
For a full list of excipients, see section 6.1.
Spot-on solution.
Clear yellow to brown solution.
4.2 Indications for use, specifying the target species
For cats suffering from, or at risk from, mixed parasitic infections caused by roundworms and
tapeworms of the following species:
Roundworms (Nematodes)
Toxocara cati
(mature adult, immature adult, L4 and L3)
Toxascaris leonina
(mature adult, immature adult and L4)
Ancylostoma tubaeforme
(mature adult, immature adult and L4)
Tapeworms (Cestodes)
Dipylidium caninum
(adult)
Taenia taeniaeformis
(adult)
Echinococcus multilocularis
(adult)
Do not use in kittens under 8 weeks of age or weighing less than 0.5 kg.
Shampooing or immersion of the animal in water directly after treatment may reduce the efficacy of
the product. Treated animals therefore should not be bathed until the solution has dried.
Parasite resistance to any particular class of anthelmintic may develop following frequent, repeated
use of an anthelmintic of that class.
4.5 Special precautions for use
Special precautions for use in animals
Apply only to the skin surface and on intact skin. Do not administer orally or parenterally.
Avoid the treated cat or other cats in the household licking the site of application while it is wet.
There is limited experience on the use of the product in sick and debilitated animals, thus the product
should only be used based on a benefit-risk assessment for these animals.
Special precautions to be taken by the person administering the veterinary medicinal product to
animals
Read the package leaflet before use.
Do not smoke, eat or drink during application.
Avoid direct contact with application area while it is wet. Keep children away from treated animals
during that time.
Wash hands after use.
In case of accidental spillage onto skin, wash off immediately with soap and water.
If the product accidentally gets into eyes, they should be thoroughly flushed with plenty of water.
If skin or eye symptoms persist, or in case of accidental ingestion, seek medical advice and show the
package leaflet or the label to the physician.
Care should be taken not to allow children to have prolonged intensive contact (for example, by
sleeping) with treated cats during the first 24 hours after application of the product.
Frequent users of the product (for example, veterinarians, professional cat breeders) should wear
disposable gloves when administering the product.
Although the product was well tolerated by pregnant cats, studies performed in rats and rabbits
suggest that emodepside may interfere with embryo-foetal development. Therefore, women of child-
bearing potential should avoid contact with, or wear disposable gloves when administering, the
product.
The solvent in this product may stain certain materials including leather, fabrics, plastics and finished
surfaces. Allow the application site to dry before permitting contact with such materials.
Echinococcosis represents a hazard for humans. As Echinococcosis is a notifiable disease to the OIE,
specific guidelines on the treatment and follow-up, and on the safeguard of persons, need to be
obtained from the relevant competent authority.
4.6 Adverse reactions (frequency and seriousness)
Salivation and vomiting may occur in very rare cases. This is thought to occur as a result of the cat
licking the application site immediately after treatment. In very rare cases following administration of
Profender transient alopecia, pruritus and/or inflammation were observed at the application site.
4.7 Use during pregnancy, lactation or lay
Can be used during pregnancy and lactation.
4.8 Interaction with other medicinal products and other forms of interaction
Emodepside is a substrate for P-glycoprotein. Co-treatment with other drugs that are P-glycoprotein
substrates/inhibitors (for example, ivermectin and other antiparasitic macrocyclic lactones,
erythromycin, prednisolone and cyclosporine) could give rise to pharmacokinetic drug interactions.
The potential clinical consequences of such interactions have not been investigated.
4.9 Amounts to be administered and administration route
Dosage and Treatment Schedule
The recommended minimum doses are 3 mg emodepside / kg body weight and 12 mg praziquantel /
kg body weight, equivalent to 0.14 ml Profender / kg body weight.
Either calculate the exact dose based on the individual body weight, or use the following dose
volumes recommended for the different weight ranges:
Appropriate combination of volumes
A single administration per treatment is effective.
Take the adapter, remove protective cover from the spike and insert spike into the central area of the
stopper. Remove screw cap. Take a standard disposable 1 ml syringe with luer nozzle and connect it
to the adapter. Then turn bottle up-side down, and withdraw the necessary volume. Replace screw cap
after use.
Part the fur on the cat’s neck at the base of the skull until the skin is visible. Place the tip of the
syringe on the skin and empty the contents directly onto the skin.
Application on the base of the skull will minimise the ability of the cat to lick the product off.
4.10 Overdose (symptoms, emergency procedures, antidotes), if necessary
Salivation, vomiting and neurological signs (tremor) were observed occasionally when the product
was administered at up to 10 times the recommended dose in adult cats and up to 5 times the
recommended dose in kittens. These symptoms were thought to occur as a result of the cat licking the
application site. The symptoms were completely reversible.
There is no known specific antidote.












PHARMACOLOGICAL PROPERTIES
Pharmacotherapeutic group: therapeutic antiparasitic agent; ATCvet code: QP52AA51.
5.1 Pharmacodynamic properties
Emodepside
is a semi-synthetic compound belonging to the new chemical group of depsipeptides. It is
active against roundworms (ascarids and hookworms). In this product, emodepside is responsible for
the efficacy against
Toxocara cati
,
Toxascaris leonina
, and
Ancylostoma tubaeforme
.
It acts at the neuromuscular junction by stimulating presynaptic receptors belonging to the secretin
receptor family which results in paralysis and death of the parasites.
Praziquantel
is a pyrazinoisoquinoline derivative effective against tapeworms such as
Dipylidium
caninum
,
Echinococcus
multilocularis
, and
Taenia taeniaeformis
.
Praziquantel is rapidly adsorbed via the surface of the parasites and acts primarily by changing the
Ca
++
permeability of the parasite membranes. This results in severe damage to the parasite
integument, contraction and paralysis, disruption of metabolism and finally leads to the death of the
parasite.
5.2 Pharmacokinetic particulars
After topical application of this product to cats at the minimum therapeutic dose of
0.14 ml/kg bodyweight, mean maximum serum concentrations of 32.2 ± 23.9 µg emodepside/l and
61.3 ± 44.1 µg praziquantel/l were observed. Maximum concentrations were reached for emodepside
3.2 ± 2.7 days after application and 18.7 ± 47 hours for praziquantel. Both active substances are then
slowly eliminated from the serum with a half-life of 9.2 ± 3.9 days for emodepside and 4.1 ± 1.5 days
for praziquantel.
After oral application in the rat, emodepside is distributed to all organs. Highest concentration levels
are found in the fat. Faecal excretion predominates with unchanged emodepside and hydroxylated
derivatives as the major excretion products.
Studies in many different species show that praziquantel is rapidly metabolised in the liver. The main
metabolites are monohydroxycyclohexyl derivatives of praziquantel. Renal elimination predominates.
5.3 Environmental properties
PHARMACEUTICAL PARTICULARS
Butylhydroxyanisole
Isopropylidene glycerol
Lactic acid
Shelf life of the veterinary medicinal product as packaged for sale: 3 years
Shelf life after first opening the immediate container: 3 months
6.4 Special precautions for storage
This veterinary medicinal product does not require any special storage conditions.
6.5 Nature and composition of immediate packaging
Pack size: 14 ml
Container: Amber coloured glass bottle with teflon-coated stopper and micro-spike adapter with
luer-port
6.6
Special precautions for the disposal of unused veterinary medicinal product or waste
materials derived from the use of such products
Profender should not be allowed to enter surface water as emodepside has shown harmful effects on
aquatic organisms.
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal
products should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Bayer Animal Health GmbH
D-51368 Leverkusen
Germany
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
27 July 2005/1
st
July 2010
10. DATE OF REVISION OF THE TEXT
Detailed information on this veterinary medicinal product is available on the website of the European
Medicines Agency
http://www.ema.europa.eu/
.
PROHIBITION OF SALE, SUPPLY AND/OR USE
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender 15 mg/3 mg modified-release Tablets for Small Dogs
Profender 50 mg/10 mg modified-release Tablets for Medium Dogs
Profender 150 mg/30 mg modified-release Tablets for Large Dogs
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each tablet of Profender contains:
Profender Tablets for Small Dogs
Profender Tablets for Medium Dogs
Profender Tablets for Large Dogs
For a full list of excipients, see section 6.1.
Modified-release tablets.
Brown, bone-shaped tablets with a score mark on each side.
The tablets can be divided into equal halves.
4.2 Indications for use, specifying the target species
For dogs suffering from, or at risk from, mixed parasitic infections caused by roundworms and
tapeworms of the following species:
Roundworms (Nematodes):
Toxocara canis
(mature adult, immature adult, L4 and L3)
Toxascaris leonina
(mature adult, immature adult and L4)
Ancylostoma caninum
(mature adult and immature adult)
Uncinaria stenocephala
(mature adult and immature adult)
Trichuris vulpis
(mature adult, immature adult)
Tapeworms (Cestodes):
Dipylidium caninum
Taenia
spp.
Echinococcus multilocularis
(mature adult and immature)
Echinococcus granulosus
(mature adult and immature)








Do not use in puppies under 12 weeks of age or weighing less than 1 kg.
Do not use in case of hypersensitivity to the active substances or to any of the excipients.
Parasite resistance to any particular class of anthelmintic may develop following frequent, repeated
use of an anthelmintic of that class.
4.5 Special precautions for use
Special precautions for use in animals
Administer only to fasted dogs. For example: Overnight fasting if the dog is to be treated in the
morning. No food should be given until 4 hours after treatment.
When
D. caninum
infection is present, concomitant treatment against intermediate hosts such as fleas
and lice should be considered to prevent reinfection.
No studies have been performed with severely debilitated dogs or individuals with seriously
compromised kidney or liver function. Therefore, the veterinary medicinal product should only be
used in such animals according to a benefit/risk assessment by the responsible veterinarian.
Special precautions to be taken by the person administering the veterinary medicinal product to
animals
In the interests of good hygiene, wash your hands after administering the tablets to the dog.
In case of accidental ingestion, especially in the case of children, seek medical advice and show the
package leaflet or the label to the physician.
Echinococcosis represents a hazard for humans. As Echinococcosis is a notifiable disease to the
World Organisation for Animal Health (OIE), specific guidelines on the treatment and follow-up, and
on the safeguard of persons, need to be obtained from the relevant competent authority.
4.6 Adverse reactions (frequency and seriousness)
Transient mild digestive tract disorders (e.g. hypersalivation, vomiting) were observed in very rare
cases.
Transient mild neurological disorders (e.g. tremors, incoordination) were observed in very rare cases.
Non compliance with fasting requirements tended to be a feature of those cases. In addition, signs of
neurological disorders may be more severe (e.g. convulsion) in mdr1 mutant (-/-) Collies, Shelties and
Australian Shepherds.
Specific antidotes are not known.
4.7 Use during pregnancy or lactation
The safety of the veterinary medicinal product has not been investigated in pregnant and lactating
dogs. Use in these dogs is therefore not recommended.
4.8 Interaction with other medicinal products and other forms of interaction
Emodepside is a substrate for P-glycoprotein. Co-treatment with other drugs that are P-glycoprotein
substrates/inhibitors (for example, ivermectin and other antiparasitic macrocyclic lactones,
erythromycin, prednisolone and cyclosporine) could give rise to pharmacokinetic drug interactions.
The potential clinical consequences of such interactions have not been investigated.
4.9 Amounts to be administered and administration route
Dosage and Treatment Schedule
Profender is to be administered at a minimum dose of 1 mg/kg body weight emodepside and 5 mg/kg
body weight praziquantel, according to the following dosage table.
A single administration per treatment is effective.
Number of Profender tablets for
For oral use in dogs from 12 weeks of age and weighing at least 1 kg. Profender tablets are meat
flavoured and usually dogs will accept them without any food.
Administer only to fasted dogs. For example: Overnight fasting if the dog is to be treated in the
morning. No food should be given until 4 hours after treatment.
4.10 Overdose (symptoms, emergency procedures, antidotes), if necessary
Transient muscular tremors, incoordination and depression were occasionally observed when the
veterinary product was administered at overdoses of up to 5 times the recommended dose. In mdr1
mutant (-/-) Collies the margin of safety appears lower compared to the normal dog population, with
mild transient tremor and/or ataxia occasionally observed after twice the recommended dose, in dogs
fasted as recommended.
The symptoms were completely self-resolving without any treatment. Feeding can increase the
incidence and intensity of such overdose symptoms and occasionally vomiting may occur.
Specific antidotes are not known.
PHARMACOLOGICAL PROPERTIES
Pharmacotherapeutic group: therapeutic antiparasitic agent; ATCvet code: QP52AA51.






















5.1 Pharmacodynamic properties
Emodepside
is a semi-synthetic compound belonging to the new chemical group of depsipeptides. It is
active against roundworms (ascarids, hookworms and whipworms). In this product, emodepside is
responsible for the efficacy against
Toxocara canis, Toxascaris leonina, Ancylostoma caninum
,
Uncinaria stenocephala
and
Trichuris vulpis
.
It acts at the neuromuscular junction by stimulating presynaptic receptors belonging to the secretin
receptor family which results in paralysis and death of the parasites.
Praziquantel
is a pyrazinoisoquinoline derivative effective against tapeworms such as
Dipylidium
caninum, Taenia
spp.,
Echinococcus multilocularis
and
Echinococcus granulosus
.
Praziquantel is rapidly adsorbed via the surface of the parasites and acts primarily by changing the
calcium (Ca
++
) permeability of the parasite membranes. This results in severe damage to the parasite
integument, contraction and paralysis, disruption of metabolism and finally leads to the death of the
parasite.
5.2 Pharmacokinetic particulars
After treatment with a dose of 1.5 mg emodepside and 7.5 mg praziquantel per kg bodyweight,
geometric mean maximum plasma concentrations of 47 µg emodepside/l and 593 µg praziquantel/l
were observed. Maximum concentrations were reached 2 hours after treatment for both active
substances. Both active substances were then eliminated from the plasma with a half-life of 1.4 to
1.7 hours.
After oral application in the rat, emodepside is distributed to all organs. Highest concentration levels
are found in the fat. Unchanged emodepside and hydroxylated derivatives are the major excretion
products. The excretion of emodepside has not been investigated in dogs.
Studies in many different species show that praziquantel is rapidly metabolised in the liver. The main
metabolites are monohydroxycyclohexyl derivatives of praziquantel. Renal excretion of metabolites
predominates.
PHARMACEUTICAL PARTICULARS
Calcium hydrogen phosphate anhydrous
Cellulose, microcrystalline
Silica, colloidal anhydrous
Croscarmellose sodium
Magnesium stearate
Povidone
Artificial beef flavour
Shelf life of the veterinary medicinal product as packaged for sale:
36 months
6.4 Special precautions for storage
Store in the original package in order to protect from moisture.
6.5 Nature and composition of immediate packaging
Cardboard boxes containing aluminium foil blister strips. The following pack sizes are available:
Profender 15 mg/3 mg tablets for small dogs
(3 blister strips with 8 tablets each)
(5 blister strips with 10 tablets each)
Profender 50 mg/10 mg tablets for medium dogs
-
2 tablets (1 blister strip)
-
4 tablets (1 blister strip)
-
6 tablets (1 blister strip)
-
24 tablets (4 blister strips with 6 tablets each)
-
102 tablets (17 blister strips with 6 tablets each)
Profender 150 mg/30 mg tablets for large dogs
(6 blister strips with 4 tablets each)
(13 blister strips with 4 tablets each)
Not all pack sizes may be marketed.
6.6
Special precautions for the disposal of unused veterinary medicinal product or waste
materials derived from the use of such products
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal
products should be disposed of in accordance with local requirements.
Unused half tablets must not be stored for future use and should be disposed of in accordance with local
requirements.
MARKETING AUTHORISATION HOLDER
Bayer Animal Health GmbH
D-51368 Leverkusen
Germany
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
25 August 2008/1
st
July 2010
10. DATE OF REVISION OF THE TEXT
Detailed information on this veterinary medicinal product is available on the website of the European
Medicines Agency
http://www.ema.europa.eu/
.
PROHIBITION OF SALE, SUPPLY AND/OR USE
MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR
BATCH RELEASE
CONDITIONS OR RESTRICTIONS OF THE MARKETING
AUTHORISATION REGARDING SUPPLY OR USE
CONDITIONS OR RESTRICTIONS OF THE MARKETING
AUTHORISATION WITH REGARD TO SAFE AND EFFECTIVE USE
A. MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer responsible for batch release
KVP Pharma + Veterinär Produkte GmbH
Projensdorfer Str. 324
D-24106 Kiel
Germany
B. CONDITIONS OR RESTRICTIONS OF THE MARKETING AUTHORISATION
REGARDING SUPPLY OR USE
To be supplied only on veterinary prescription.
C. CONDITIONS OR RESTRICTIONS OF THE MARKETING AUTHORISATION WITH
REGARD TO THE SAFE AND EFFECTIVE USE OF THE PRODUCT
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGE
Profender spot-on solution for small cats
Outer carton, pack size of 2 (or 4) pipettes
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender spot-on solution for small cats
STATEMENT OF ACTIVE AND OTHER SUBSTANCES
Each 0.35 ml pipette contains:
Active substances: 7.5 mg emodepside, 30 mg praziquantel
5.4 mg/ml butylhydroxyanisole (E320; as antioxidant)
Roundworms:
Toxocara cati, Toxascaris leonina, Ancylostoma tubaeforme
Tapeworms:
Dipylidium caninum, Taenia taeniaeformis, Echinococcus multilocularis
For the complete indication, including the larval stages, read the package leaflet.
METHOD AND ROUTE(S) OF ADMINISTRATION
For external use only.
Read the package leaflet before use.























SPECIAL WARNING(S), IF NECESSARY
11. SPECIAL STORAGE CONDITIONS
Store in the original package in order to protect from moisture.
12. SPECIAL PRECAUTIONS FOR THE DISPOSAL OF UNUSED PRODUCTS OR
WASTE MATERIALS, IF ANY
13. THE WORDS “FOR ANIMAL TREATMENT ONLY” AND CONDITIONS OR
RESTRICTIONS REGARDING SUPPLY AND USE, if applicable
For animal treatment only - to be supplied only on veterinary prescription.
14. THE WORDS “KEEP OUT OF THE REACH AND SIGHT OF CHILDREN”
Keep out of the reach and sight of children.
15. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Bayer Animal Health GmbH, D-51368 Leverkusen, Germany
16. MARKETING AUTHORISATION NUMBER(S)
EU/2/05/054/001 2 pipettes
EU/2/05/054/002 4 pipettes
17. MANUFACTURER’S BATCH NUMBER






















PARTICULARS TO APPEAR ON THE OUTER PACKAGE
Profender spot-on solution for small cats
Outer carton, pack size of 12 (20 or 40) pipettes
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender spot-on solution for small cats
STATEMENT OF ACTIVE AND OTHER SUBSTANCES
Each 0.35 ml pipette contains:
Active substances: 7.5 mg emodepside, 30 mg praziquantel
5.4 mg/ml butylhydroxyanisole (E320; as antioxidant)
12 pipettes
20 pipettes
40 pipettes
Roundworms:
Toxocara cati, Toxascaris leonina, Ancylostoma tubaeforme
Tapeworms:
Dipylidium caninum, Taenia taeniaeformis, Echinococcus multilocularis
For the complete indication, including the larval stages, read the package leaflet.
METHOD AND ROUTE(S) OF ADMINISTRATION
For external use only.
Read the package leaflet before use.

























SPECIAL WARNING(S), IF NECESSARY
Do not use in kittens under 8 weeks of age or weighing less than 0.5 kg.
For user safety warnings – read the package leaflet before use.
11. SPECIAL STORAGE CONDITIONS
Store in the original package in order to protect from moisture.
12. SPECIAL PRECAUTIONS FOR THE DISPOSAL OF UNUSED PRODUCTS OR
WASTE MATERIALS, IF ANY
Profender should not be allowed to enter surface water as emodepside has shown harmful effects on
aquatic organisms.
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal
products should be disposed of in accordance with local requirements.
13. THE WORDS “FOR ANIMAL TREATMENT ONLY” AND CONDITIONS OR
RESTRICTIONS REGARDING SUPPLY AND USE, if applicable
For animal treatment only - to be supplied only on veterinary prescription.
14. THE WORDS “KEEP OUT OF THE REACH AND SIGHT OF CHILDREN”
Keep out of the reach and sight of children.
15. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Bayer Animal Health GmbH, D-51368 Leverkusen, Germany
16. MARKETING AUTHORISATION NUMBER(S)
EU/2/05/054/003 12 pipettes
EU/2/05/054/004 20 pipettes
EU/2/05/054/005 40 pipettes




















17. MANUFACTURER’S BATCH NUMBER



PARTICULARS TO APPEAR ON THE OUTER PACKAGE
Profender spot-on solution for medium cats
Outer carton, pack size of 2 (or 4) pipettes
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender spot-on solution for medium cats
STATEMENT OF ACTIVE AND OTHER SUBSTANCES
Each 0.70 ml pipette contains:
Active substances: 15 mg emodepside, 60 mg praziquantel
5.4 mg/ml butylhydroxyanisole (E320; as antioxidant)
For medium cats > 2.5 kg – 5 kg
Roundworms:
Toxocara cati, Toxascaris leonina, Ancylostoma tubaeforme
Tapeworms:
Dipylidium caninum, Taenia taeniaeformis, Echinococcus multilocularis
For the complete indication, including the larval stages, read the package leaflet.
METHOD AND ROUTE(S) OF ADMINISTRATION
For external use only.
Read the package leaflet before use.























SPECIAL WARNING(S), IF NECESSARY
11. SPECIAL STORAGE CONDITIONS
Store in the original package in order to protect from moisture.
12. SPECIAL PRECAUTIONS FOR THE DISPOSAL OF UNUSED PRODUCTS OR
WASTE MATERIALS, IF ANY
13. THE WORDS “FOR ANIMAL TREATMENT ONLY” AND CONDITIONS OR
RESTRICTIONS REGARDING SUPPLY AND USE, if applicable
For animal treatment only - to be supplied only on veterinary prescription.
14. THE WORDS “KEEP OUT OF THE REACH AND SIGHT OF CHILDREN”
Keep out of the reach and sight of children.
15. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Bayer Animal Health GmbH, D-51368 Leverkusen, Germany
16. MARKETING AUTHORISATION NUMBER(S)
EU/2/05/054/006 2 pipettes
EU/2/05/054/007 4 pipettes
17. MANUFACTURER’S BATCH NUMBER






















PARTICULARS TO APPEAR ON THE OUTER PACKAGE
Profender spot-on solution for medium cats
Outer carton, pack size of 12 (20, 40 or 80) pipettes
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender spot-on solution for medium cats
STATEMENT OF ACTIVE AND OTHER SUBSTANCES
Each 0.70 ml pipette contains:
Active substances: 15 mg emodepside, 60 mg praziquantel
5.4 mg/ml butylhydroxyanisole (E320; as antioxidant)
12 pipettes
20 pipettes
40 pipettes
80 pipettes
For medium cats > 2.5 kg – 5 kg
Roundworms:
Toxocara cati, Toxascaris leonina, Ancylostoma tubaeforme
Tapeworms:
Dipylidium caninum, Taenia taeniaeformis, Echinococcus multilocularis
For the complete indication, including the larval stages, read the package leaflet.
METHOD AND ROUTE(S) OF ADMINISTRATION
For external use only.
Read the package leaflet before use.

























SPECIAL WARNING(S), IF NECESSARY
Do not use in kittens under 8 weeks of age or weighing less than 0.5 kg.
For user safety warnings – read the package leaflet before use.
11. SPECIAL STORAGE CONDITIONS
Store in the original package in order to protect from moisture.
12. SPECIAL PRECAUTIONS FOR THE DISPOSAL OF UNUSED PRODUCTS OR
WASTE MATERIALS, IF ANY
Profender should not be allowed to enter surface water as emodepside has shown harmful effects on
aquatic organisms.
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal
products should be disposed of in accordance with local requirements.
13. THE WORDS “FOR ANIMAL TREATMENT ONLY” AND CONDITIONS OR
RESTRICTIONS REGARDING SUPPLY AND USE, if applicable
For animal treatment only - to be supplied only on veterinary prescription.
14. THE WORDS “KEEP OUT OF THE REACH AND SIGHT OF CHILDREN”
Keep out of the reach and sight of children.
15. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Bayer Animal Health GmbH, D-51368 Leverkusen, Germany
16. MARKETING AUTHORISATION NUMBER(S)
EU/2/05/054/008 12 pipettes
EU/2/05/054/009 20 pipettes
EU/2/05/054/010 40 pipettes
EU/2/05/054/011 80 pipettes




















17. MANUFACTURER’S BATCH NUMBER



PARTICULARS TO APPEAR ON THE OUTER PACKAGE
Profender spot-on solution for large cats
Outer carton, pack size of 2 (or 4) pipettes
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender spot-on solution for large cats
STATEMENT OF ACTIVE AND OTHER SUBSTANCES
Each 1.12 ml pipette contains:
Active substances: 24 mg emodepside, 96 mg praziquantel
5.4 mg/ml butylhydroxyanisole (E320; as antioxidant)
For large cats > 5 kg – 8 kg
Roundworms:
Toxocara cati, Toxascaris leonina, Ancylostoma tubaeforme
Tapeworms:
Dipylidium caninum, Taenia taeniaeformis, Echinococcus multilocularis
For the complete indication, including the larval stages, read the package leaflet.
METHOD AND ROUTE(S) OF ADMINISTRATION
For external use only.
Read the package leaflet before use.























SPECIAL WARNING(S), IF NECESSARY
11. SPECIAL STORAGE CONDITIONS
Store in the original package in order to protect from moisture.
12. SPECIAL PRECAUTIONS FOR THE DISPOSAL OF UNUSED PRODUCTS OR
WASTE MATERIALS, IF ANY
13. THE WORDS “FOR ANIMAL TREATMENT ONLY” AND CONDITIONS OR
RESTRICTIONS REGARDING SUPPLY AND USE, if applicable
For animal treatment only - to be supplied only on veterinary prescription.
14. THE WORDS “KEEP OUT OF THE REACH AND SIGHT OF CHILDREN”
Keep out of the reach and sight of children.
15. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Bayer Animal Health GmbH, D-51368 Leverkusen, Germany
16. MARKETING AUTHORISATION NUMBER(S)
EU/2/05/054/012 2 pipettes
EU/2/05/054/013 4 pipettes
17. MANUFACTURER’S BATCH NUMBER






















PARTICULARS TO APPEAR ON THE OUTER PACKAGE
Profender spot-on solution for large cats
Outer carton, pack size of 12 (20 or 40) pipettes
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender spot-on solution for large cats
STATEMENT OF ACTIVE AND OTHER SUBSTANCES
Each 1.12 ml pipette contains:
Active substances: 24 mg emodepside, 96 mg praziquantel
5.4 mg/ml butylhydroxyanisole (E320; as antioxidant)
12 pipettes
20 pipettes
40 pipettes
For large cats > 5 kg – 8 kg
Roundworms:
Toxocara cati, Toxascaris leonina, Ancylostoma tubaeforme
Tapeworms:
Dipylidium caninum, Taenia taeniaeformis, Echinococcus multilocularis
For the complete indication, including the larval stages, read the package leaflet.
METHOD AND ROUTE(S) OF ADMINISTRATION
For external use only.
Read the package leaflet before use.

























SPECIAL WARNING(S), IF NECESSARY
Do not use in kittens under 8 weeks of age or weighing less than 0.5 kg.
For user safety warnings – read the package leaflet before use.
11. SPECIAL STORAGE CONDITIONS
Store in the original package in order to protect from moisture.
12. SPECIAL PRECAUTIONS FOR THE DISPOSAL OF UNUSED PRODUCTS OR
WASTE MATERIALS, IF ANY
Profender should not be allowed to enter surface water as emodepside has shown harmful effects on
aquatic organisms.
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal
products should be disposed of in accordance with local requirements.
13. THE WORDS “FOR ANIMAL TREATMENT ONLY” AND CONDITIONS OR
RESTRICTIONS REGARDING SUPPLY AND USE, if applicable
For animal treatment only - to be supplied only on veterinary prescription.
14. THE WORDS “KEEP OUT OF THE REACH AND SIGHT OF CHILDREN”
Keep out of the reach and sight of children.
15. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Bayer Animal Health GmbH, D-51368 Leverkusen, Germany
16. MARKETING AUTHORISATION NUMBER(S)
EU/2/05/054/014 12 pipettes
EU/2/05/054/015 20 pipettes
EU/2/05/054/016 40 pipettes




















17. MANUFACTURER’S BATCH NUMBER



PARTICULARS TO APPEAR ON THE OUTER PACKAGE
Profender spot-on solution for cats
Outer carton, Multi-dose bottle
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender spot-on solution for cats
STATEMENT OF ACTIVE AND OTHER SUBSTANCES
Active substances: 21.4 mg/ml emodepside, 85.8 mg/ml praziquantel
5.4 mg/ml butylhydroxyanisole (E320; as antioxidant)
Roundworms:
Toxocara cati, Toxascaris leonina, Ancylostoma tubaeforme
Tapeworms:
Dipylidium caninum, Taenia taeniaeformis, Echinococcus multilocularis
For the complete indication, including the larval stages, read the package leaflet.
METHOD AND ROUTE(S) OF ADMINISTRATION
For external use only.
Read the package leaflet before use.





















SPECIAL WARNING(S), IF NECESSARY
For user safety warnings – read the package leaflet before use.
EXP {month/year}
Shelf life after first opening the immediate container: 3 months
11. SPECIAL STORAGE CONDITIONS
12. SPECIAL PRECAUTIONS FOR THE DISPOSAL OF UNUSED PRODUCTS OR
WASTE MATERIALS, IF ANY
13. THE WORDS “FOR ANIMAL TREATMENT ONLY” AND CONDITIONS OR
RESTRICTIONS REGARDING SUPPLY AND USE, if applicable
For animal treatment only - to be supplied only on veterinary prescription.
14. THE WORDS “KEEP OUT OF THE REACH AND SIGHT OF CHILDREN”
Keep out of the reach and sight of children.
15. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Bayer Animal Health GmbH, D-51368 Leverkusen, Germany
16. MARKETING AUTHORISATION NUMBER(S)
17. MANUFACTURER’S BATCH NUMBER



















MINIMUM PARTICULARS TO APPEAR ON SMALL IMMEDIATE PACKAGING UNITS
Profender spot-on solution for cats
Bottle label
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender spot-on solution for cats
QUANTITY OF THE ACTIVE SUBSTANCE(S)
21.4 mg/ml emodepside, 85.8 mg/ml praziquantel
CONTENTS BY WEIGHT, BY VOLUME OR BY NUMBER OF DOSES
ROUTE(S) OF ADMINISTRATION
Spot-on use.
For external use only.
Once opened, use by…………….{leave space for the date to be inserted}.
THE WORDS “FOR ANIMAL TREATMENT ONLY”
For animal treatment only.





















PARTICULARS TO APPEAR ON THE OUTER PACKAGE
Profender 15 mg / 3 mg Tablets for Small Dogs
Outer carton, pack size of 2 (or 4) tablets
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender 15 mg / 3 mg modified-release Tablets for Small Dogs
STATEMENT OF ACTIVE AND OTHER SUBSTANCES
3 mg emodepside, 15 mg praziquantel.
Dewormer against roundworms and tapeworms.
For the complete indication, including species and larval stages, read the package leaflet.
METHOD AND ROUTE(S) OF ADMINISTRATION
For oral use.
Read the package leaflet before use.
SPECIAL WARNING(S), IF NECESSARY























11. SPECIAL STORAGE CONDITIONS
Store in the original package in order to protect from moisture.
12. SPECIAL PRECAUTIONS FOR THE DISPOSAL OF UNUSED PRODUCTS OR
WASTE MATERIALS, IF ANY
Disposal: Read package leaflet.
13. THE WORDS “FOR ANIMAL TREATMENT ONLY” AND CONDITIONS OR
RESTRICTIONS REGARDING SUPPLY AND USE, if applicable
For animal treatment only - to be supplied only on veterinary prescription.
14. THE WORDS “KEEP OUT OF THE REACH AND SIGHT OF CHILDREN”
Keep out of the reach and sight of children.
15. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Bayer Animal Health GmbH, D-51368 Leverkusen, Germany
16. MARKETING AUTHORISATION NUMBER(S)
EU/2/05/054/018 2 tablets
EU/2/05/054/019 4 tablets
17. MANUFACTURER’S BATCH NUMBER


















PARTICULARS TO APPEAR ON THE OUTER PACKAGE
Profender 15 mg / 3 mg Tablets for Small Dogs
Outer carton, pack size of 10 (24 or 50) tablets
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender 15 mg / 3 mg modified-release Tablets for Small Dogs
STATEMENT OF ACTIVE AND OTHER SUBSTANCES
3 mg emodepside, 15 mg praziquantel.
10 tablets
24 tablets
50 tablets
Dewormer against roundworms and tapeworms.
For the complete indication, including species and larval stages, read the package leaflet.
METHOD AND ROUTE(S) OF ADMINISTRATION
For oral use.
Read the package leaflet before use.
SPECIAL WARNING(S), IF NECESSARY
Do not use in puppies under 12 weeks of age or weighing less than 1 kg.

























11. SPECIAL STORAGE CONDITIONS
Store in the original package in order to protect from moisture.
12. SPECIAL PRECAUTIONS FOR THE DISPOSAL OF UNUSED PRODUCTS OR
WASTE MATERIALS, IF ANY
Disposal: Read package leaflet.
13. THE WORDS “FOR ANIMAL TREATMENT ONLY” AND CONDITIONS OR
RESTRICTIONS REGARDING SUPPLY AND USE, if applicable
For animal treatment only - to be supplied only on veterinary prescription.
14. THE WORDS “KEEP OUT OF THE REACH AND SIGHT OF CHILDREN”
Keep out of the reach and sight of children.
15. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Bayer Animal Health GmbH, D-51368 Leverkusen, Germany
16. MARKETING AUTHORISATION NUMBER(S)
EU/2/05/054/020 10 tablets
EU/2/05/054/021 24 tablets
EU/2/05/054/022 50 tablets
17. MANUFACTURER’S BATCH NUMBER




















PARTICULARS TO APPEAR ON THE OUTER PACKAGE
Profender 50 mg / 10 mg Tablets for Medium Dogs
Outer carton, pack size of 2 (or 4) tablets
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender 50 mg / 10 mg modified-release Tablets for Medium Dogs
STATEMENT OF ACTIVE AND OTHER SUBSTANCES
10 mg emodepside, 50 mg praziquantel.
Dewormer against roundworms and tapeworms.
For the complete indication, including species and larval stages, read the package leaflet.
METHOD AND ROUTE(S) OF ADMINISTRATION
For oral use.
Read the package leaflet before use.
SPECIAL WARNING(S), IF NECESSARY























11. SPECIAL STORAGE CONDITIONS
Store in the original package in order to protect from moisture.
12. SPECIAL PRECAUTIONS FOR THE DISPOSAL OF UNUSED PRODUCTS OR
WASTE MATERIALS, IF ANY
Disposal: Read package leaflet.
13. THE WORDS “FOR ANIMAL TREATMENT ONLY” AND CONDITIONS OR
RESTRICTIONS REGARDING SUPPLY AND USE, if applicable
For animal treatment only - to be supplied only on veterinary prescription.
14. THE WORDS “KEEP OUT OF THE REACH AND SIGHT OF CHILDREN”
Keep out of the reach and sight of children.
15. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Bayer Animal Health GmbH, D-51368 Leverkusen, Germany
16. MARKETING AUTHORISATION NUMBER(S)
EU/2/05/054/023 2 tablets
EU/2/05/054/024 4 tablets
17. MANUFACTURER’S BATCH NUMBER


















PARTICULARS TO APPEAR ON THE OUTER PACKAGE
Profender 50 mg / 10 mg Tablets for Medium Dogs
Outer carton, pack size of 6 (24 or 102) tablets
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender 50 mg / 10 mg modified-release Tablets for Medium Dogs
STATEMENT OF ACTIVE AND OTHER SUBSTANCES
10 mg emodepside, 50 mg praziquantel.
6 tablets
24 tablets
102 tablets
Dewormer against roundworms and tapeworms.
For the complete indication, including species and larval stages, read the package leaflet.
METHOD AND ROUTE(S) OF ADMINISTRATION
For oral use.
Read the package leaflet before use.
SPECIAL WARNING(S), IF NECESSARY
Do not use in puppies under 12 weeks of age or weighing less than 1 kg.

























11. SPECIAL STORAGE CONDITIONS
Store in the original package in order to protect from moisture.
12. SPECIAL PRECAUTIONS FOR THE DISPOSAL OF UNUSED PRODUCTS OR
WASTE MATERIALS, IF ANY
Disposal: Read package leaflet.
13. THE WORDS “FOR ANIMAL TREATMENT ONLY” AND CONDITIONS OR
RESTRICTIONS REGARDING SUPPLY AND USE, if applicable
For animal treatment only - to be supplied only on veterinary prescription.
14. THE WORDS “KEEP OUT OF THE REACH AND SIGHT OF CHILDREN”
Keep out of the reach and sight of children.
15. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Bayer Animal Health GmbH, D-51368 Leverkusen, Germany
16. MARKETING AUTHORISATION NUMBER(S)
EU/2/05/054/025 6 tablets
EU/2/05/054/026 24 tablets
EU/2/05/054/027 102 tablets
17. MANUFACTURER’S BATCH NUMBER




















PARTICULARS TO APPEAR ON THE OUTER PACKAGE
Profender 150 mg / 30 mg Tablets for Large Dogs
Outer carton, pack size of 2 tablets
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender 150 mg / 30 mg modified-release Tablets for Large Dogs
STATEMENT OF ACTIVE AND OTHER SUBSTANCES
30 mg emodepside, 150 mg praziquantel.
Dewormer against roundworms and tapeworms.
For the complete indication, including species and larval stages, read the package leaflet.
METHOD AND ROUTE(S) OF ADMINISTRATION
For oral use.
Read the package leaflet before use.
SPECIAL WARNING(S), IF NECESSARY























11. SPECIAL STORAGE CONDITIONS
Store in the original package in order to protect from moisture.
12. SPECIAL PRECAUTIONS FOR THE DISPOSAL OF UNUSED PRODUCTS OR
WASTE MATERIALS, IF ANY
Disposal: Read package leaflet.
13. THE WORDS “FOR ANIMAL TREATMENT ONLY” AND CONDITIONS OR
RESTRICTIONS REGARDING SUPPLY AND USE, if applicable
For animal treatment only - to be supplied only on veterinary prescription.
14. THE WORDS “KEEP OUT OF THE REACH AND SIGHT OF CHILDREN”
Keep out of the reach and sight of children.
15. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Bayer Animal Health GmbH, D-51368 Leverkusen, Germany
16. MARKETING AUTHORISATION NUMBER(S)
EU/2/05/054/028 2 tablets
17. MANUFACTURER’S BATCH NUMBER















PARTICULARS TO APPEAR ON THE OUTER PACKAGE
Profender 150 mg / 30 mg Tablets for Large Dogs
Outer carton, pack size of 4 (24 or 52) tablets
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender 150 mg / 30 mg modified-release Tablets for Large Dogs
STATEMENT OF ACTIVE AND OTHER SUBSTANCES
30 mg emodepside, 150 mg Praziquantel.
4 tablets
24 tablets
52 tablets
Dewormer against roundworms and tapeworms.
For the complete indication, including species and larval stages, read the package leaflet.
METHOD AND ROUTE(S) OF ADMINISTRATION
For oral use.
Read the package leaflet before use.
SPECIAL WARNING(S), IF NECESSARY
Do not use in puppies under 12 weeks of age or weighing less than 1 kg.

























11. SPECIAL STORAGE CONDITIONS
Store in the original package in order to protect from moisture.
12. SPECIAL PRECAUTIONS FOR THE DISPOSAL OF UNUSED PRODUCTS OR
WASTE MATERIALS, IF ANY
Disposal: Read package leaflet.
13. THE WORDS “FOR ANIMAL TREATMENT ONLY” AND CONDITIONS OR
RESTRICTIONS REGARDING SUPPLY AND USE, if applicable
For animal treatment only - to be supplied only on veterinary prescription.
14. THE WORDS “KEEP OUT OF THE REACH AND SIGHT OF CHILDREN”
Keep out of the reach and sight of children.
15. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Bayer Animal Health GmbH, D-51368 Leverkusen, Germany
16. MARKETING AUTHORISATION NUMBER(S)
EU/2/05/054/029 4 tablets
EU/2/05/054/030 24 tablets
EU/2/05/054/031 52 tablets
17. MANUFACTURER’S BATCH NUMBER




















MINIMUM PARTICULARS TO APPEAR ON BLISTERS OR STRIPS
Profender 15 mg / 3 mg Tablets for Small Dogs
Blister
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender Tablets for Small Dogs
NAME OF THE MARKETING AUTHORISATION HOLDER
THE WORDS “FOR ANIMAL TREATMENT ONLY”
For animal treatment only.













MINIMUM PARTICULARS TO APPEAR ON BLISTERS OR STRIPS
Profender 50 mg / 10 mg Tablets for Medium Dogs
Blister
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender Tablets for Medium Dogs
NAME OF THE MARKETING AUTHORISATION HOLDER
THE WORDS “FOR ANIMAL TREATMENT ONLY”
For animal treatment only.













MINIMUM PARTICULARS TO APPEAR ON BLISTERS OR STRIPS
Profender 150 mg / 30 mg Tablets for Large Dogs
Blister
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender Tablets for Large Dogs
NAME OF THE MARKETING AUTHORISATION HOLDER
THE WORDS “FOR ANIMAL TREATMENT ONLY”
For animal treatment only.













PACKAGE LEAFLET
Profender spot-on solution for small cats
Profender spot-on solution for medium cats
Profender spot-on solution for large cats
NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER AND OF
THE MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE, IF DIFFERENT
Marketing authorisation holder
:
Bayer Animal Health GmbH
D-51368 Leverkusen
Germany
Manufacturer for the batch release
:
KVP Pharma + Veterinär Produkte GmbH
Projensdorfer Str. 324
D-24106 Kiel
Germany
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender spot-on solution for small cats
Profender spot-on solution for medium cats
Profender spot-on solution for large cats
STATEMENT OF THE ACTIVE SUBSTANCES AND OTHER INGREDIENTS
Profender contains 21.4 mg/ml emodepside and 85.8 mg/ml praziquantel.
Each unit dose (pipette) of Profender delivers:
Profender for Small Cats
(
Profender for Medium Cats
(> 2.5 – 5 kg)
Profender for Large Cats
(> 5 – 8 kg)
5.4 mg/ml butylhydroxyanisole (E320; as antioxidant)










For cats suffering from, or at risk from, mixed parasitic infections caused by roundworms and
tapeworms of the following species:
Roundworms (Nematodes)
Toxocara cati
(mature adult, immature adult, L4 and L3)
Toxascaris leonina
(mature adult, immature adult and L4)
Ancylostoma tubaeforme
(mature adult, immature adult and L4)
Tapeworms (Cestodes)
Dipylidium caninum
(adult)
Taenia taeniaeformis
(adult)
Echinococcus multilocularis
(adult)
Do not use in kittens under 8 weeks of age or weighing less than 0.5 kg.
Salivation and vomiting may occur in very rare cases. This is thought to occur as a result of the cat
licking the application site immediately after treatment. In very rare cases following administration of
Profender transient alopecia, pruritus and/or inflammation were observed at the application site.
If you notice any serious effects or other effects not mentioned in this leaflet, please inform your
veterinary surgeon.
DOSAGE, ROUTE AND METHOD OF ADMINISTRATION
Dosage and Treatment Schedule
The recommended minimum doses are 3 mg emodepside / kg body weight and 12 mg praziquantel /
kg body weight, equivalent to 0.14 ml Profender / kg body weight.
0.5 - 2.5 Profender for Small Cats
Profender for Medium Cats
Use an appropriate combination of pipettes
A single administration per treatment is effective.














ADVICE ON CORRECT ADMINISTRATION
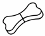
Remove one pipette from package. Hold pipette in upright position, twist and pull off cap and use the
opposite end of the cap to break the seal.
Part the fur on the cat’s neck at the base of the skull until the skin is visible. Place the tip of the
pipette on the skin and squeeze firmly several times to empty the contents directly onto the skin.
Application on the base of the skull will minimise the ability of the cat to lick the product off. Apply
only to the skin surface and on intact skin.
10. SPECIAL STORAGE PRECAUTIONS
Keep out of the reach and sight of children.
Store in the original package in order to protect from moisture.
Do not use after the expiry date stated on the label and carton.
Do not administer orally or parenterally.
Avoid the treated cat or other cats in the household licking the site of application while it is wet.
There is limited experience on the use of the product in sick and debilitated animals, thus the product
should only be used based on a benefit-risk assessment for these animals.
Shampooing or immersion of the animal in water directly after treatment may reduce the efficacy of
the product. Treated animals therefore should not be bathed until the solution has dried.
To the user:
Do not smoke, eat or drink during application.
Avoid direct contact with application area while it is wet. Keep children away from treated animals
during that time.
Wash hands after use.
In case of accidental spillage onto skin, wash off immediately with soap and water.
If the product accidentally gets into eyes, they should be thoroughly flushed with plenty of water.
If skin or eye symptoms persist, or in case of accidental ingestion, seek medical advice and show the
package leaflet or the label to the physician.
Care should be taken not to allow children to have prolonged intensive contact (for example, by
sleeping) with treated cats during the first 24 hours after application of the product.
Although the product was well tolerated by pregnant cats, studies performed in rats and rabbits
suggest that emodepside may interfere with embryo-foetal development. Therefore, women of child-
bearing potential should avoid contact with, or wear disposable gloves when administering, the
product.
Frequent users of the product (for example, veterinarians, professional cat breeders) should wear
disposable gloves when administering the product.
The solvent in this product may stain certain materials including leather, fabrics, plastics and finished
surfaces. Allow the application site to dry before permitting contact with such materials.
12. SPECIAL PRECAUTIONS FOR THE DISPOSAL OF UNUSED PRODUCT OR
WASTE MATERIALS
Profender should not be allowed to enter surface water as emodepside has shown harmful effects on
aquatic organisms.
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal
products should be disposed of in accordance with local requirements.
13. DATE ON WHICH THE PACKAGE LEAFLET WAS LAST APPROVED
Profender can be used during pregnancy and lactation.
Parasite resistance to any particular class of anthelmintic may develop following frequent, repeated
use of an anthelmintic of that class.
Echinococcosis represents a hazard for humans. As Echinococcosis is a notifiable disease to the OIE,
specific guidelines on the treatment and follow-up, and on the safeguard of persons, need to be
obtained from the relevant competent authority.
Not all pack sizes may be marketed.
For any information about this veterinary medicinal product, please contact the local representative of
the marketing authorisation holder.
België/Belgique/Belgien
Bayer SA-NV
J.E. Mommaertslaan 14
B–1831 Diegem (Machelen)
Tel/Tél: +32 2 535 66 54
Luxembourg/Luxemburg
Bayer SA-NV
J.E. Mommaertslaan 14
B–1831 Diegem (Machelen)
Belgique/Belgien
Tél/Tel: +32 2 535 66 54
Република България
Алапис България ЕООД
ул. “Атанас Дуков” № 29
BG София 1407
Teл: + 359 2 862 46 98
Magyarország
Bayer Hungária Kft.
H-1123 Budapest
Alkotás u. 50
Tel: +36 1 487 4100
Česká republika
Bayer s.r.o.,
Animal Health
Litvínovská 609/3
CZ-190 21 Praha 9
Tel: +420 2 66 10 14 71
Malta
Bayer Animal Health GmbH
D-51368 Leverkusen
Germany
Tel: +49 2173 38 4012
Danmark
Bayer A/S, Bayer HealthCare
Animal Health Division
Nørgaardsvej 32
DK-2800 Kgs. Lyngby
Tlf: +45 4523 5000
Nederland
Bayer B.V., Animal Health Division
Energieweg 1
NL-3641 RT Mijdrecht
Tel: +31 297 280 467
Deutschland
Bayer Vital GmbH
Geschäftsbereich Tiergesundheit
D-51368 Leverkusen
Tel: +49 214 301
Norge
Bayer AS
Bayer HealthCare
Animal Health Division
Drammensveien 147 B
N-0277 Oslo
Tlf: +47 24 11 18 00
Eesti
Magnum Veterinaaria AS
Vae 16
EE-76401 Laagri
Tel: +372 650 1920
Österreich
Bayer Austria GmbH
Geschäftsbereich Tiergesundheit
Herbststraße 6 – 10
A-1160 Wien
Tel: +43 1 71146 2850
Ελλάδα
ALAPIS ABEE
GR–19300, Ασπρόπυργος
Αττικής, Τ.Θ. 26
Τηλ: +30 210 5575770
Polska
Bayer Sp. z o.o. Animal Health
Al. Jerozolimskie 158
PL-02-326 Warszawa
Tel: +48 22 572 35 00
España
Química Farmacéutica Bayer, S.L.
División Sanidad Animal
Av. Baix Llobregat, 3-5
E-08970 Sant Joan Despí (Barcelona)
Tel: +34 93 4956500
Portugal
Bayer Portugal S.A.
Divisão de Saúde Animal
Rua da Quinta do Pinheiro, 5
P-2794-003 Carnaxide
Tel: +351 21 4172121
France
Bayer Santé
Division Santé Animale
13, rue Jean Jaurès
F–92807 Puteaux cédex
Tél: +33 1 49 06 58 19
România
S.C. Alapis România S.R.L.
Str. Leordeni 13
Bragadiru, Ilfov 077025 - RO
Tel: +40 21 314 59 31
Ireland
Bayer Limited, Animal Health Division
The Atrium,
Blackthorn Road
IRL - Dublin 18
Tel: +353 1 2999313
Slovenija
Bayer d.o.o.
Bravničarjeva 13
SI-1000 Ljubljana
Tel: +386 1 5814 451
Ísland
Icepharma hf.
Lynghálsi 13
IS-110 Reykjavík
Sími: +354 540 8000
Slovenská republika
Bayer s.r.o.,
Animal Health
Litvínovská 609/3
CZ-190 21 Praha 9
Česká republika
Tel: +420 2 66 10 14 71
Italia
Bayer S.p.A.
Viale Certosa, 130
I-20156 Milano
Tel: +39 02 3978 1
Suomi/Finland
Orion Oyj
ORION PHARMA ELÄINLÄÄKKEET
Tengströminkatu 8, PL/PB 425
FIN-20101 Turku/Åbo
Puh/Tel: +358 10 4261
Κύπρος
PHARMACARE Ltd.
Τ.Θ.28351
CY-2093, ΛΕΥΚΩΣΙΑ
Τηλ: +357-22-323060
Sverige
Bayer A/S, Bayer HealthCare
Animal Health Division
Nørgaardsvej 32
DK-2800 Kgs. Lyngby
Danmark
Tel: +46 (0)8-580 223 00
Latvija
Magnum Veterinaaria AS
Vae 16
EE-76401 Laagri
Tel: +372 650 1920
United Kingdom
Bayer plc, Animal Health Division,
Bayer House,
Strawberry Hill,
Newbury,
Berkshire RG14 1JA-UK
Tel: +44 1635 563000
Lietuva
Magnum Veterinaaria AS
Vae 16
EE-76401 Laagri
Tel: +372 650 1920
PACKAGE LEAFLET
Profender spot-on solution for cats
NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER AND OF
THE MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE, IF DIFFERENT
Marketing authorisation holder
:
Bayer Animal Health GmbH
D-51368 Leverkusen
Germany
Manufacturer for the batch release
:
KVP Pharma + Veterinär Produkte GmbH
Projensdorfer Str. 324
D-24106 Kiel
Germany
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender spot-on solution for cats
STATEMENT OF THE ACTIVE SUBSTANCES AND OTHER INGREDIENTS
Profender contains 21.4 mg/ml emodepside and 85.8 mg/ml praziquantel.
5.4 mg/ml butylhydroxyanisole (E320; as antioxidant)
For cats suffering from, or at risk from, mixed parasitic infections caused by roundworms and
tapeworms of the following species:
Roundworms (Nematodes)
Toxocara cati
(mature adult, immature adult, L4 and L3)
Toxascaris leonina
(mature adult, immature adult and L4)
Ancylostoma tubaeforme
(mature adult, immature adult and L4)
Tapeworms (Cestodes)
Dipylidium caninum
(adult)
Taenia taeniaeformis
(adult)
Echinococcus multilocularis
(adult)
Do not use in kittens under 8 weeks of age or weighing less than 0.5 kg.
Salivation and vomiting may occur in very rare cases. This is thought to occur as a result of the cat
licking the application site immediately after treatment. In very rare cases following administration of
Profender transient alopecia, pruritus and/or inflammation were observed at the application site.
If you notice any serious effects or other effects not mentioned in this leaflet, please inform your
veterinary surgeon.
DOSAGE, ROUTE AND METHOD OF ADMINISTRATION
Dosage and Treatment Schedule
The recommended minimum doses are 3 mg emodepside / kg body weight and 12 mg praziquantel /
kg body weight, equivalent to 0.14 ml Profender / kg body weight.
Either calculate the exact dose based on the individual body weight, or use the following dose
volumes recommended for the different weight ranges:
Appropriate combination of volumes
A single administration per treatment is effective.
ADVICE ON CORRECT ADMINISTRATION
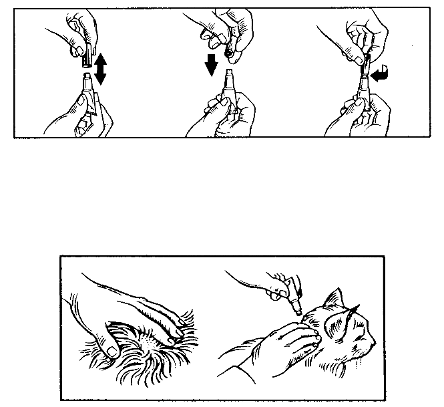
Take the adapter, remove protective cover from the spike and insert spike into the central area of the
stopper (1). Remove screw cap (2). Take a standard disposable 1 ml syringe with luer nozzle and
connect it to the adapter (3). Then turn bottle up-side down, and withdraw the necessary volume (4).
Replace screw cap after use. Part the fur on the cat’s neck at the base of the skull until the skin is
visible. Place the tip of the syringe on the skin and empty the contents directly onto the skin (5).















Application on the base of the skull will minimise the ability of the cat to lick the product off. Apply
only to the skin surface and on intact skin.
10. SPECIAL STORAGE PRECAUTIONS
Keep out of the reach and sight of children.
Do not use after the expiry date stated on the label and carton.
Shelf life after first opening the immediate container: 3 months
Do not administer orally or parenterally.
Avoid the treated cat or other cats in the household licking the site of application while it is wet.
There is limited experience on the use of the product in sick and debilitated animals, thus the product
should only be used based on a benefit-risk assessment for these animals.
Shampooing or immersion of the animal in water directly after treatment may reduce the efficacy of
the product. Treated animals therefore should not be bathed until the solution has dried.
To the user:
Do not smoke, eat or drink during application.
Avoid direct contact with application area while it is wet. Keep children away from treated animals
during that time.
Wash hands after use.
In case of accidental spillage onto skin, wash off immediately with soap and water.
If the product accidentally gets into eyes, they should be thoroughly flushed with plenty of water.
If skin or eye symptoms persist, or in case of accidental ingestion, seek medical advice and show the
package leaflet or the label to the physician.
Care should be taken not to allow children to have prolonged intensive contact (for example, by
sleeping) with treated cats during the first 24 hours after application of the product.
Although the product was well tolerated by pregnant cats, studies performed in rats and rabbits
suggest that emodepside may interfere with embryo-foetal development. Therefore, women of child-
bearing potential should avoid contact with, or wear disposable gloves when administering, the
product.
Frequent users of the product (for example, veterinarians, professional cat breeders) should wear
disposable gloves when administering the product.
The solvent in this product may stain certain materials including leather, fabrics, plastics and finished
surfaces. Allow the application site to dry before permitting contact with such materials.
12. SPECIAL PRECAUTIONS FOR THE DISPOSAL OF UNUSED PRODUCT OR
WASTE MATERIALS
Profender should not be allowed to enter surface water as emodepside has shown harmful effects on
aquatic organisms.
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal
products should be disposed of in accordance with local requirements.
13. DATE ON WHICH THE PACKAGE LEAFLET WAS LAST APPROVED
Profender can be used during pregnancy and lactation.
Parasite resistance to any particular class of anthelmintic may develop following frequent, repeated
use of an anthelmintic of that class.
Echinococcosis represents a hazard for humans. As Echinococcosis is a notifiable disease to the OIE,
specific guidelines on the treatment and follow-up, and on the safeguard of persons, need to be
obtained from the relevant competent authority.
Not all pack sizes may be marketed.
For any information about this veterinary medicinal product, please contact the local representative of
the marketing authorisation holder.
België/Belgique/Belgien
Bayer SA-NV
J.E. Mommaertslaan 14
B–1831 Diegem (Machelen)
Tel/Tél: +32 2 535 66 54
Luxembourg/Luxemburg
Bayer SA-NV
J.E. Mommaertslaan 14
B–1831 Diegem (Machelen)
Belgique/Belgien
Tél/Tel: +32 2 535 66 54
Република България
Алапис България ЕООД
ул. “Атанас Дуков” № 29
BG София 1407
Teл: + 359 2 862 46 98
Magyarország
Bayer Hungária Kft.
H-1123 Budapest
Alkotás u. 50
Tel: +36 1 487 4100
Česká republika
Bayer s.r.o.,
Animal Health
Litvínovská 609/3
CZ-190 21 Praha 9
Tel: +420 2 66 10 14 71
Malta
Bayer Animal Health GmbH
D-51368 Leverkusen
Germany
Tel: +49 2173 38 4012
Danmark
Bayer A/S, Bayer HealthCare
Animal Health Division
Nørgaardsvej 32
DK-2800 Kgs. Lyngby
Tlf: +45 4523 5000
Nederland
Bayer B.V., Animal Health Division
Energieweg 1
NL-3641 RT Mijdrecht
Tel: +31 297 280 467
Deutschland
Bayer Vital GmbH
Geschäftsbereich Tiergesundheit
D-51368 Leverkusen
Tel: +49 214 301
Norge
Bayer AS
Bayer HealthCare
Animal Health Division
Drammensveien 147 B
N-0277 Oslo
Tlf: +47 24 11 18 00
Eesti
Magnum Veterinaaria AS
Vae 16
EE-76401 Laagri
Tel: +372 650 1920
Österreich
Bayer Austria GmbH
Geschäftsbereich Tiergesundheit
Herbststraße 6 – 10
A-1160 Wien
Tel: +43 1 71146 2850
Ελλάδα
ALAPIS ABEE
GR–19300, Ασπρόπυργος
Αττικής, Τ.Θ. 26
Τηλ: +30 210 5575770
Polska
Bayer Sp. z o.o. Animal Health
Al. Jerozolimskie 158
PL-02-326 Warszawa
Tel: +48 22 572 35 00
España
Química Farmacéutica Bayer, S.L.
División Sanidad Animal
Av. Baix Llobregat, 3-5
E-08970 Sant Joan Despí (Barcelona)
Tel: +34 93 4956500
Portugal
Bayer Portugal S.A.
Divisão de Saúde Animal
Rua da Quinta do Pinheiro, 5
P-2794-003 Carnaxide
Tel: +351 21 4172121
France
Bayer Santé
Division Santé Animale
13, rue Jean Jaurès
F–92807 Puteaux cédex
Tél: +33 1 49 06 58 19
România
S.C. Alapis România S.R.L.
Str. Leordeni 13
Bragadiru, Ilfov 077025 - RO
Tel: +40 21 314 59 31
Ireland
Bayer Limited, Animal Health Division
The Atrium,
Blackthorn Road
IRL - Dublin 18
Tel: +353 1 2999313
Slovenija
Bayer d.o.o.
Bravničarjeva 13
SI-1000 Ljubljana
Tel: +386 1 5814 451
Ísland
Icepharma hf.
Lynghálsi 13
IS-110 Reykjavík
Sími: +354 540 8000
Slovenská republika
Bayer s.r.o.,
Animal Health
Litvínovská 609/3
CZ-190 21 Praha 9
Česká republika
Tel: +420 2 66 10 14 71
Italia
Bayer S.p.A.
Viale Certosa, 130
I-20156 Milano
Tel: +39 02 3978 1
Suomi/Finland
Orion Oyj
ORION PHARMA ELÄINLÄÄKKEET
Tengströminkatu 8, PL/PB 425
FIN-20101 Turku/Åbo
Puh/Tel: +358 10 4261
Κύπρος
PHARMACARE Ltd.
Τ.Θ.28351
CY-2093, ΛΕΥΚΩΣΙΑ
Τηλ: +357-22-323060
Sverige
Bayer A/S, Bayer HealthCare
Animal Health Division
Nørgaardsvej 32
DK-2800 Kgs. Lyngby
Danmark
Tel: +46 (0)8-580 223 00
Latvija
Magnum Veterinaaria AS
Vae 16
EE-76401 Laagri
Tel: +372 650 1920
United Kingdom
Bayer plc, Animal Health Division,
Bayer House,
Strawberry Hill,
Newbury,
Berkshire RG14 1JA-UK
Tel: +44 1635 563000
Lietuva
Magnum Veterinaaria AS
Vae 16
EE-76401 Laagri
Tel: +372 650 1920
PACKAGE LEAFLET
Profender Modified-Release Tablets for Dogs
NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER AND OF
THE MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Marketing authorisation holder
:
Bayer Animal Health GmbH
D-51368 Leverkusen
Germany
Manufacturer for the batch release
:
KVP Pharma + Veterinär Produkte GmbH
Projensdorfer Str. 324
D-24106 Kiel
Germany
NAME OF VETERINARY THE MEDICINAL PRODUCT
Profender 15 mg/3 mg modified-release Tablets for Small Dogs
Profender 50 mg/10 mg modified-release Tablets for Medium Dogs
Profender 150 mg/30 mg modified-release Tablets for Large Dogs
Praziquantel / Emodepside
STATEMENT OF THE ACTIVE SUBSTANCES
Each tablet of Profender contains:
Profender Tablets for Small Dogs
Profender Tablets for Medium Dogs
Profender Tablets for Large Dogs
For dogs suffering from, or at risk from, mixed parasitic infections caused by roundworms and
tapeworms of the following species:
Roundworms (Nematodes):
Toxocara canis
(mature adult, immature adult, L4 and L3)
Toxascaris leonina
(mature adult, immature adult and L4)
Ancylostoma caninum
(mature adult and immature adult)
Uncinaria stenocephala
(mature adult and immature adult)
Trichuris vulpis
(mature adult, immature adult)








Tapeworms (Cestodes):
Dipylidium caninum
Taenia
spp.
Echinococcus multilocularis
(mature adult and immature)
Echinococcus granulosus
(mature adult and immature)
Do not use in puppies under 12 weeks of age or weighing less than 1 kg.
Do not use in case of hypersensitivity to the active substances or to any of the excipients.
Transient mild digestive tract disorders (e.g. hypersalivation, vomiting) were observed in very rare
cases.
Transient mild neurological disorders (e.g. tremors, incoordination) were observed in very rare cases.
Non compliance with fasting requirements tended to be a feature of those cases. In addition, signs of
neurological disorders may be more severe (e.g. convulsion) in mdr1 mutant (-/-) Collies, Shelties and
Australian Shepherds.
Specific antidotes are not known.
If you notice any serious effects or other effects not mentioned in this leaflet, please inform your
veterinary surgeon.
DOSAGE, ROUTE AND METHOD OF ADMINISTRATION
For oral use in dogs from 12 weeks of age and weighing at least 1 kg.
Profender is to be administered at a minimum dose of 1 mg/kg body weight emodepside and 5 mg/kg
body weight praziquantel, according to the following dosage table.
A single administration per treatment is effective.
Number of Profender tablets for
ADVICE ON CORRECT ADMINISTRATION
Profender tablets are meat flavoured and usually dogs will accept them without any food.
Administer only to fasted dogs. For example: Overnight fasting if the dog is to be treated in the
morning. No food should be given until 4 hours after treatment.
10. SPECIAL STORAGE PRECAUTIONS
Keep out of the reach and sight of children.
Store in the original package in order to protect from moisture.
Do not use after the expiry date stated on the carton or blister.
Administer only to fasted dogs. For example: Overnight fasting if the dog is to be treated in the
morning. No food should be given until 4 hours after treatment.
The safety of this veterinary medicinal product has not been investigated in pregnant and lactating
dogs. Use in these dogs is therefore not recommended.
No studies have been performed with severely debilitated dogs or individuals with seriously
compromised kidney or liver function. Therefore, the veterinary medicinal product should only be
used in such animals according to a benefit/risk assessment by the responsible veterinarian.
Temporary trembling, incoordination and depression were occasionally observed when the veterinary
product was administered at overdoses of up to 5 times the recommended dose. In mdr1 mutant (-/-)
Collies the margin of safety appears lower compared to the normal dog population, with mild transient
tremor and/or ataxia occasionally observed after twice the recommended dose, in dogs fasted as
recommended. The symptoms were completely self-resolving without any treatment. Feeding can
increase the frequency and intensity of such overdose symptoms and occasionally vomiting may
occur. Specific antidotes are not known.
Parasite resistance to any particular class of anthelmintic may develop following frequent, repeated
use of an anthelmintic of that class.






















When
D. caninum
infection is present, concomitant treatment against intermediate hosts such as fleas
and lice should be considered to prevent reinfection.
Emodepside is a substrate for P-glycoprotein. Co-treatment with other drugs that are P-glycoprotein
substrates/inhibitors (for example, ivermectin and other antiparasitic macrocyclic lactones,
erythromycin, prednisolone and cyclosporine) could give rise to pharmacokinetic drug interactions.
The potential clinical consequences of such interactions have not been investigated.
In the interests of good hygiene, wash your hands after administering the tablets to the dog. In case of
accidental ingestion, especially in the case of children, seek medical advice and show the package
leaflet or the label to the physician.
Echinococcosis represents a hazard for humans. As Echinococcosis is a notifiable disease to the
World Organisation for Animal Health (OIE), specific guidelines on the treatment and follow-up, and
on the safeguard of persons, need to be obtained from the relevant competent authority.
12. SPECIAL PRECAUTIONS FOR THE DISPOSAL OF UNUSED PRODUCT OR
WASTE MATERIALS
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal
products should be disposed of in accordance with local requirements.
Unused half tablets must not be stored for future use and should be disposed of in accordance with local
requirements.
13. DATE ON WHICH THE PACKAGE LEAFLET WAS LAST APPROVED
Profender 15 mg / 3 mg modified-release Tablets for Small Dogs
(3 blister strips with 8 tablets each)
(5 blister strips with 10 tablets each)
Profender 50 mg / 10 mg modified-release Tablets for Medium Dogs
-
2 tablets (1 blister strip)
-
4 tablets (1 blister strip)
-
6 tablets (1 blister strip)
-
24 tablets (4 blister strips with 6 tablets each)
-
102 tablets (17 blister strips with 6 tablets each)
Profender 150 mg / 30 mg modified-release Tablets for Large Dogs
(6 blister strips with 4 tablets each)
(13 blister strips with 4 tablets each)
Not all pack sizes may be marketed.
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/profender_veterinary.html
Copyright © 1995-2021 ITA all rights reserved.
|




































































































































































































































































































































































































































































































































































































































































































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































