Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
1. NAME OF THE MEDICINAL PRODUCT
Relistor 8 mg solution for injection in pre-filled syringe
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each pre-filled syringe of 0.4 ml contains 8 mg methylnaltrexone bromide.
One ml of solution contains 20 mg methylnaltrexone bromide.
For a full list of excipients, see section 6.1.
Solution for injection in pre-filled syringe (injection).
Clear solution, colourless to pale-yellow, essentially free from visible particulates.
4.1 Therapeutic indications
Treatment of opioid-induced constipation in advanced illness patients who are receiving palliative care
when response to usual laxative therapy has not been sufficient.
4.2 Posology and method of administration
Relistor should be added to induce prompt bowel movements when response to usual laxative therapy
has not been sufficient.
The recommended dose of methylnaltrexone bromide is 8 mg (0.4 ml Relistor) (for patients weighing
38-61 kg) or 12 mg (0.6 ml Relistor) (for patients weighing 62-114 kg).
The usual administration schedule is one single dose every other day. Doses may also be given with
longer intervals, as per clinical need.
Patients may receive two consecutive doses 24 hours apart, only when there has been no response
(bowel movement) to the dose on the preceding day.
Patients weighing less than 38 kg or greater than 114 kg should use Relistor vials because the
recommended mg/kg dose cannot be accurately delivered with the pre-filled syringe.
In patients with severe renal impairment (creatinine clearance less than 30 ml/min), the dose of
methylnaltrexone bromide should be reduced from 12 mg to 8 mg (0.4 ml Relistor) for those weighing
62 to 114 kg. Patients with severe renal impairment whose weight falls outside the 62 to 114 kg range
(see section 5.2) need to reduce their mg/kg dose by 50 %. These patients should use Relistor vials and
not the pre-filled syringe. There are no data available from patients with end-stage renal impairment on
dialysis, and Relistor is not recommended in these patients (see section 4.4).
Hepatic impairment
No dose adjustment is necessary in patients with mild to moderate hepatic impairment (see section
5.2).
There are no data available from patients with severe hepatic impairment (Child-Pugh Class C), and
Relistor is not recommended in these patients (see section 4.4).
Paediatric population
No data are available. There is no experience in children under the age of 18 (see section 5.2).
Therefore, methylnaltrexone bromide should not be used in the paediatric age group until further data
become available.
No dose adjustment is recommended based on age (see section 5.2).
Relistor is given as a subcutaneous injection.
It is recommended to rotate injection sites. It is not recommended to inject into areas where the skin is
tender, bruised, red, or hard. Areas with scars or stretch marks should be avoided.
The three areas of the body recommended for injection of Relistor are upper legs, abdomen, and upper
arms.
Relistor can be injected without regard to food.
Hypersensitivity to the active substance or to any of the excipients.
Use of methylnaltrexone bromide in patients with known or suspected mechanical gastrointestinal
obstruction or acute surgical abdomen is contraindicated.
4.4 Special warnings and precautions for use
Cases of gastrointestinal (GI) perforation have been reported in the postauthorisation period in patients
using Relistor. Although patients had medical conditions that may be associated with localised or
diffuse reduction of structural integrity in the wall of the GI tract (e.g., cancer, peptic ulcer, pseudo-
obstruction), the use of Relistor may have contributed to these events.
Use Relistor with caution in patients with known or suspected lesions of the GI tract.
Advise patients to promptly report severe, persistent, and/or worsening symptoms.
The activity of methylnaltrexone bromide has been studied in patients with constipation induced by
opioids. Therefore, Relistor should not be used for treatment of patients with constipation not related
to opioid use.
If severe or persistent diarrhoea occurs during treatment, patients should be advised not to continue
therapy with Relistor and consult their physician.
Data from clinical trials suggest treatment with methylnaltrexone bromide can result in the rapid onset
(within 30 to 60 minutes on average) of a bowel movement.
Methylnaltrexone bromide treatment has not been studied in clinical trials for longer than 4 months,
and should therefore only be used for a limited period (see section 5.2).
Relistor should only be used in patients who are receiving palliative care. It is added to usual laxative
treatment.
Relistor is not recommended in patients with severe hepatic impairment or with end-stage renal
impairment requiring dialysis (see section 4.2).
Use of methylnaltrexone bromide in patients with colostomy, peritoneal catheter, active diverticular
disease or fecal impaction has not been studied. Therefore, Relistor should only be administered with
caution in these patients.
This medicinal product contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially sodium-
free.
4.5 Interaction with other medicinal products and other forms of interaction
Methylnaltrexone bromide does not affect the pharmacokinetics of medicinal products metabolised
by cytochrome P450 (CYP) isozymes. Methylnaltrexone bromide is minimally metabolised by CYP
isozymes.
In vitro
metabolism studies suggest that methylnaltrexone bromide does not inhibit the
activity of CYP1A2, CYP2E1, CYP2B6, CYP2A6, CYP2C9, CYP2C19 or CYP3A4, while it is a
weak inhibitor of the metabolism of a model CYP2D6 substrate. In a clinical drug interaction study in
healthy adult male subjects, a subcutaneous dose of 0.3 mg/kg of methylnaltrexone bromide did not
significantly affect the metabolism of dextromethorphan, a CYP2D6 substrate.
The organic cation transporter (OCT)-related drug-drug interaction potential between
methylnaltrexone bromide and an OCT inhibitor was studied in 18 healthy subjects by comparing
the single-dose pharmacokinetic profiles of methylnaltrexone bromide before and after multiple
400 mg doses of cimetidine. The renal clearance of methylnaltrexone bromide was reduced following
multiple-dose administration of cimetidine (from 31 l/h to 18 l/h). However, this resulted in a small
reduction in total clearance (from 107 l/h to 95 l/h). Consequently, no meaningful change in AUC
of methylnaltrexone bromide, in addition to C
max
, was observed before and after multiple-dose
administration of cimetidine.
4.6 Pregnancy and lactation
There are no adequate data with the use of methylnaltrexone bromide in pregnant women. Studies
in animals have shown reproductive toxicity at high doses (see section 5.3). The potential risk for
humans is unknown. Relistor should not be used during pregnancy unless clearly necessary.
It is unknown whether methylnaltrexone bromide is excreted in human breast milk. Animal studies
have shown excretion of methylnaltrexone bromide in breast milk. A decision on whether to continue/
discontinue breast-feeding or to continue/discontinue therapy with Relistor should be made, taking
into account the benefit of breast-feeding to the child and the benefit of Relistor therapy to the woman.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. However, as a
pure peripherally restricted opioid antagonist, the likelihood that Relistor will affect such activities is
low.
Dizziness may occur, and this may have an effect on driving and use of machines (see section 4.8).
The most common drug-related adverse reactions in all patients exposed to methylnaltrexone bromide
during all phases of placebo-controlled studies were abdominal pain, nausea, diarrhoea and flatulence.
Generally, these reactions were mild or moderate.
The adverse reactions are classified as: Very common (≥1/10); Common (≥1/100 to <1/10);
Uncommon (≥1/1,000 to <1/100); Rare (≥1/10,000 to <1/1,000); Very rare (<1/10,000); Not known
(cannot be estimated from the available data). Within each frequency grouping, adverse reactions are
presented in order of decreasing seriousness:
Gastrointestinal disorders
Very Common: Abdominal pain, nausea, diarrhoea, flatulence
Skin and subcutaneous tissue disorders
Common: Injection site reactions (e.g. stinging, burning, pain , redness, oedema), hyperhidrosis
Post Marketing Experience
Cases of gastrointestinal perforation have been reported in patients using Relistor (see section 4.4):
frequency unknown.
A study of healthy volunteers noted orthostatic hypotension associated with a dose of 0.64 mg/kg
administered as an intravenous bolus.
In the event of an overdose, signs and symptoms of orthostatic hypotension should be monitored and
reported to a physician. Treatment should be initiated as appropriate.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Peripheral opioid receptor antagonists, ATC code: A06AH01
Methylnaltrexone bromide is a selective antagonist of opioid binding at the mu-receptor.
In vitro
studies have shown methylnaltrexone bromide to be a mu-opioid receptor antagonist (inhibition
constant [K
i
] = 28 nM), with 8-fold less potency for kappa opioid receptors (K
i
= 230 nM) and much
reduced affinity for delta opioid receptors.
As a quaternary amine, the ability of methylnaltrexone bromide to cross the blood-brain barrier is
restricted. This allows methylnaltrexone bromide to function as a peripherally acting mu-opioid
antagonist in tissues such as the gastrointestinal tract, without impacting opioid-mediated analgesic
effects on the central nervous system.
Clinical efficacy and safety
The efficacy and safety of methylnaltrexone bromide in the treatment of opioid-induced constipation
in patients receiving palliative care was demonstrated in two randomised, double-blind, placebo-
controlled studies. In these studies, the median age was 68 years (range 21-100); 51 % were females.
In both studies, patients had advanced terminal illness and limited life expectancy, with the majority
having a primary diagnosis of incurable cancer; other primary diagnoses included end-stage COPD/
emphysema, cardiovascular disease/heart failure, Alzheimer’s disease/dementia, HIV/AIDS, or other
advanced illnesses. Prior to screening, patients had opioid-induced constipation defined as either <3
bowel movements in the preceding week or no bowel movement for >2 days.
Study 301 compared methylnaltrexone bromide given as a single, double-blind, subcutaneous dose
of 0.15 mg/kg, or 0.3 mg/kg versus placebo. The double-blind dose was followed by an open-
label, 4-week dosing period, where methylnaltrexone bromide could be used as needed, no more
frequently than 1 dose in a 24-hour period. Throughout both study periods, patients maintained
their usual laxative regimen. A total of 154 patients (methylnaltrexone bromide 0.15 mg/kg, n = 47;
methylnaltrexone bromide 0.3 mg/kg, n = 55, placebo, n = 52) were treated in the double-blind period.
The primary endpoint was the proportion of patients with a rescue-free laxation within 4 hours of
the double-blind dose of study medicinal product. Methylnaltrexone bromide-treated patients had a
significantly higher rate of laxation within 4 hours of the double-blind dose (62 % for 0.15 mg/kg and
58 % for 0.3 mg/kg) than placebo-treated patients (14 %); p<0.0001 for each dose versus placebo.
Study 302 compared double-blind, subcutaneous doses of methylnaltrexone bromide given every
other day for 2 weeks versus placebo. During the first week (days 1, 3, 5, 7), patients received either
methylnaltrexone bromide 0.15 mg/kg or placebo. In the second week, a patient’s assigned dose
could be increased to 0.30 mg/kg if the patient had 2 or fewer rescue-free laxations up to day 8.
At any time, the patient’s assigned dose could be reduced based on tolerability. Data from 133 (62
methylnaltrexone bromide, 71 placebo) patients were analysed. There were 2 primary endpoints:
proportion of patients with a rescue-free laxation within 4 hours of the first dose of study medicinal
product and proportion of patients with a rescue-free laxation within 4 hours after at least 2 of the
first 4 doses of medicinal product. Methylnaltrexone bromide-treated patients had a higher rate of
laxation within 4 hours of the first dose (48 %) than placebo-treated patients (16 %); p<0.0001.
Methylnaltrexone bromide-treated patients also had significantly higher rates of laxation within 4
hours after at least 2 of the first 4 doses (52 %) than did placebo-treated patients (9 %); p<0.0001.
Stool consistency was not meaningfully improved in patients who had soft stool at baseline.
In both studies, there was no evidence to suggest differential effects of age or gender on safety or
efficacy. The effect on race could not be analysed because the study population was predominantly
Caucasian (88 %).
Durability of response was demonstrated in Study 302, in which the laxation response rate was
consistent from dose 1 through dose 7 over the course of the 2-week, double-blind period.
The efficacy and safety of methylnaltrexone bromide were also demonstrated in open-label treatment
administered from Day 2 through Week 4 in Study 301, and in two open-label extension studies
(301EXT and 302EXT) in which methylnaltrexone bromide was given as needed for up to 4 months
(only 8 patients up to this point). A total of 136, 21, and 82 patients received at least one open-label
dose in studies 301, 301EXT, and 302EXT, respectively. Relistor was administered every 3.2 days
(median dosing interval, with a range of 1-39 days).
The rate of laxation response was maintained throughout the extension studies for those patients who
continued treatment.
There was no significant relationship between baseline opioid dose and laxation response in
methylnaltrexone bromide-treated patients in these studies. In addition, median daily opioid dose did
not vary meaningfully from baseline in either methylnaltrexone bromide-treated patients or in placebo-
treated patients. There were no clinically relevant changes in pain scores from baseline in either the
methylnaltrexone bromide or placebo-treated patients.
Effect on cardiac repolarisation
In a double-blind, randomised, parallel-group ECG study of single, subcutaneous doses of
methylnaltrexone bromide (0.15, 0.30 and 0.50 mg/kg), in 207 healthy volunteers, no signal of
QT/QTc prolongation or any evidence of an effect on secondary ECG parameters or waveform
morphology was detected as compared to placebo and a positive control (orally administered 400 mg
moxifloxacin).
5.2 Pharmacokinetic properties
Methylnaltrexone bromide is absorbed rapidly, with peak concentrations (C
max
) achieved at
approximately 0.5 hours following subcutaneous administration. The C
max
and area under the plasma
concentration-time curve (AUC) increase with dose increase from 0.15 mg/kg to 0.5 mg/kg in a dose-
proportional manner. Absolute bioavailability of a 0.30 mg/kg subcutaneous dose versus a 0.30 mg/kg
intravenous dose is 82 %.
Methylnaltrexone bromide undergoes moderate tissue distribution. The steady-state volume of
distribution (Vss) is approximately 1.1 l/kg. Methylnaltrexone bromide is minimally bound to human
plasma proteins (11.0 % to 15.3 %) as determined by equilibrium dialysis.
Methylnaltrexone bromide is metabolised to a modest extent in humans based on the amount of
methylnaltrexone bromide metabolites recovered from excreta. Conversion to methyl-6-naltrexol
isomers and methylnaltrexone sulphate appears to be the primary pathway to metabolism. Each of the
methyl-6-naltrexol isomers has somewhat less antagonist activity than parent compound, and a low
exposure in plasma of approximately 8 % of the drug-related materials. Methylnaltrexone sulphate
is an inactive metabolite and present in plasma at a level of approximately 25 % of drug related
materials. N-demethylation of methylnaltrexone bromide to produce naltrexone is not significant,
accounting for 0.06 % of the administered dose.
Methylnaltrexone bromide is eliminated primarily as the unchanged active substance. Approximately
half of the dose is excreted in the urine and somewhat less in faeces. The terminal disposition half-life
(t
1/2
) is approximately 8 hours.
The effect of mild and moderate hepatic impairment on the systemic exposure to methylnaltrexone
bromide has been studied in 8 subjects each, with Child-Pugh Class A and B, compared to healthy
subjects. Results showed no meaningful effect of hepatic impairment on the AUC or C
max
of
methylnaltrexone bromide. The effect of severe hepatic impairment on the pharmacokinetics of
methylnaltrexone bromide has not been studied.
Renal impairment
In a study of volunteers with varying degrees of renal impairment receiving a single dose of 0.30 mg/
kg methylnaltrexone bromide, renal impairment had a marked effect on the renal excretion of
methylnaltrexone bromide. The renal clearance of methylnaltrexone bromide decreased with
increasing severity of renal impairment. Severe renal impairment decreased the renal clearance of
methylnaltrexone bromide by 8- to 9-fold; however, this resulted in only a 2-fold increase in total
methylnaltrexone bromide exposure (AUC). C
max
was not significantly changed. No studies were
performed in patients with end-stage renal impairment requiring dialysis.
No studies have been performed in the paediatric population (see section 4.2).
In a study comparing single and multiple-dose pharmacokinetic profiles of intravenous
methylnaltrexone bromide at a dose of 24 mg between healthy, young (18 to 45 years of age
n = 10) and elderly (65 years of age and over n = 10) subjects, the effect of age on exposure to
methylnaltrexone bromide was found to be minor. The mean steady-state C
max
and AUC for the elderly
were 545 ng/ml and 412 ng•h/ml, approximately 8.1 % and 20 %, respectively, greater than those for
young subjects. Therefore, no dose adjustment is recommended based on age.
No meaningful gender differences have been observed.
An integrated analysis of pharmacokinetic data from healthy subjects indicated that methylnaltrexone
bromide mg/kg dose-adjusted exposure increased as body weight increased. The mean
methylnaltrexone bromide exposure at 0.15 mg/kg over a weight range of 38 to 114 kg was
179 (range = 139-240) ng•h/ml. This exposure for the 0.15 mg/kg dose can be achieved with a weight-
band-based dose adjustment using an 8 mg dose for body weight 38 to less than 62 kg and a 12 mg
dose for body weight 62 to 114 kg, yielding a mean exposure of 187 (range = 148-220) ng•h/ml.
In addition, the analysis showed that 8 mg dose for body weight 38 to less than 62 kg and a 12 mg
dose for body weight 62 to 114 kg correspond to mean doses of 0.16 (range = 0.21-0.13) mg/kg
and 0.16 (range = 0.19-0.11) mg/kg, respectively, based on the body weight distribution of patients
participating in studies 301 and 302.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, and genotoxicity. Cardiac effects were observed in some non-
clinical studies in canines (prolongation of action potentials in Purkinje fibers or prolongation of the
QTc interval). The mechanism of this effect is unknown; however, the human cardiac potassium ion
channel (hERG) appears not to be involved.
Subcutaneous injections of Relistor at 150 mg/kg/day decreased fertility in rats. Doses up to 25 mg/
kg/day (18 times the exposure [AUC] in humans at a subcutaneous dose of 0.3 mg/kg) did not affect
fertility or general reproductive performance.
There was no evidence of teratogenicity in rats or rabbits. Subcutaneous injections of Relistor at
150/100 mg/kg/day to rats resulted in decreased offspring weights; doses up to 25 mg/kg/day (18 times
the exposure [AUC] in humans at a subcutaneous dose of 0.3 mg/kg) had no effect on labour, delivery,
or offspring survival and growth.
Methylnaltrexone bromide is excreted via the milk of lactating rats.
Studies have been conducted in juvenile rats and dogs. Following intravenous injection of
methylnaltrexone bromide, juvenile rats were found to be more sensitive than adult rats to
methylnaltrexone-related toxicity. In juvenile rats administered intravenous methylnaltrexone bromide
for 13 weeks, adverse clinical signs (incidences of convulsions and labored breathing) occurred
at dosages (≥ 3 mg/kg/day) and exposures (5.4 times the exposure {AUC} in adult humans at a
subcutaneous dose of 0.15 mg/kg) that were lower than those that caused similar toxicity in adult rats
(20 mg/kg/day). No adverse effects occurred in juvenile rats at 1 mg/kg/day or in adult rats at 5 mg/
kg/day (1.6 times and 7.8 times, respectively, the exposure {AUC} in adult humans at a subcutaneous
dose of 0.15 mg/kg).
Following intravenous injection of methylnaltrexone bromide for 13 weeks, similar methylnaltrexone
related toxicity was observed in both juvenile and adult dogs. In adult and juvenile dogs given
methylnaltrexone bromide at 20 mg/kg/day, clinical signs indicative of CNS toxicity and prolongation
of QTc interval were observed. No adverse effects occurred in either juvenile or adult dogs at a dose of
5 mg/kg/day (44 times the exposure {AUC} in adult humans at a subcutaneous dose of 0.15 mg/kg).
Carcinogenicity studies have not been conducted with Relistor.
6. PHARMACEUTICAL PARTICULARS
Hydrochloric acid (to adjust pH)
Sodium hydroxide (to adjust pH)
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
6.4 Special precautions for storage
Keep the pre-filled syringe in the outer carton in order to protect from light.
6.5 Nature and contents of container
Each pre-filled syringe contains 0.4 ml of solution for injection.
Pre-filled syringe of clear type I glass with stainless-steel needle, plastic plunger, and polypropylene
rigid needle cover.
Pack sizes of 4, 7, 8 and 10 pre-filled syringes.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
7. MARKETING AUTHORISATION HOLDER
8. MARKETING AUTHORISATION NUMBERS
EU/1/08/463/007
EU/1/08/463/006
EU/1/08/463/005
EU/1/08/463/004
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 02 July 2008
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
1. NAME OF THE MEDICINAL PRODUCT
Relistor 12 mg/0.6 ml solution for injection
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each vial of 0.6 ml contains 12 mg methylnaltrexone bromide.
One ml of solution contains 20 mg methylnaltrexone bromide.
For a full list of excipients, see section 6.1.
Clear solution, colourless to pale-yellow, essentially free from visible particulates.
4.1 Therapeutic indications
Treatment of opioid-induced constipation in advanced illness patients who are receiving palliative care
when response to usual laxative therapy has not been sufficient.
4.2 Posology and method of administration
Relistor should be added to induce prompt bowel movements when response to usual laxative therapy
has not been sufficient.
The recommended dose of methylnaltrexone bromide is 8 mg (0.4 ml Relistor) (for patients weighing
38-61 kg) or 12 mg (0.6 ml Relistor) (for patients weighing 62-114 kg).
The usual administration schedule is one single dose every other day. Doses may also be given with
longer intervals, as per clinical need.
Patients may receive two consecutive doses 24 hours apart, only when there has been no response
(bowel movement) to the dose on the preceding day.
Patients whose weight falls outside of the ranges should be dosed at 0.15 mg/kg. The injection volume
for these patients should be calculated:
Dose (ml) = patient weight (kg) x 0.0075
In patients with severe renal impairment (creatinine clearance less than 30 ml/min), the dose of
methylnaltrexone bromide should be reduced from 12 mg to 8 mg (0.4 ml Relistor) for those weighing
62 to 114 kg, or from 0.15 mg/kg to 0.075 mg/kg for those whose weight falls outside the 62 to 114 kg
range (see section 5.2). There are no data available from patients with end-stage renal impairment on
dialysis, and Relistor is not recommended in these patients (see section 4.4).
Hepatic impairment
No dose adjustment is necessary in patients with mild to moderate hepatic impairment (see section
5.2).
There are no data available from patients with severe hepatic impairment (Child-Pugh Class C), and
Relistor is not recommended in these patients (see section 4.4).
Paediatric population
No data are available. There is no experience in children under the age of 18 (see section 5.2).
Therefore, methylnaltrexone bromide should not be used in the paediatric age group until further data
become available.
No dose adjustment is recommended based on age (see section 5.2).
Relistor is given as a subcutaneous injection.
It is recommended to rotate injection sites. It is not recommended to inject into areas where the skin is
tender, bruised, red, or hard. Areas with scars or stretch marks should be avoided.
The three areas of the body recommended for injection of Relistor are upper legs, abdomen, and upper
arms.
Relistor can be injected without regard to food.
Hypersensitivity to the active substance or to any of the excipients.
Use of methylnaltrexone bromide in patients with known or suspected mechanical gastrointestinal
obstruction or acute surgical abdomen is contraindicated.
4.4 Special warnings and precautions for use
Cases of gastrointestinal (GI) perforation have been reported in the postauthorisation period in patients
using Relistor. Although patients had medical conditions that may be associated with localised or
diffuse reduction of structural integrity in the wall of the GI tract (e.g., cancer, peptic ulcer, pseudo-
obstruction), the use of Relistor may have contributed to these events.
Use Relistor with caution in patients with known or suspected lesions of the GI tract.
Advise patients to promptly report severe, persistent, and/or worsening symptoms.
The activity of methylnaltrexone bromide has been studied in patients with constipation induced by
opioids. Therefore, Relistor should not be used for treatment of patients with constipation not related
to opioid use.
If severe or persistent diarrhoea occurs during treatment, patients should be advised not to continue
therapy with Relistor and consult their physician.
Data from clinical trials suggest treatment with methylnaltrexone bromide can result in the rapid onset
(within 30 to 60 minutes on average) of a bowel movement.
Methylnaltrexone bromide treatment has not been studied in clinical trials for longer than 4 months,
and should therefore only be used for a limited period (see section 5.2).
Relistor should only be used in patients who are receiving palliative care. It is added to usual laxative
treatment.
Relistor is not recommended in patients with severe hepatic impairment or with end-stage renal
impairment requiring dialysis (see section 4.2).
Use of methylnaltrexone bromide in patients with colostomy, peritoneal catheter, active diverticular
disease or fecal impaction has not been studied. Therefore, Relistor should only be administered with
caution in these patients.
This medicinal product contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially sodium-
free.
4.5 Interaction with other medicinal products and other forms of interaction
Methylnaltrexone bromide does not affect the pharmacokinetics of medicinal products metabolised
by cytochrome P450 (CYP) isozymes. Methylnaltrexone bromide is minimally metabolised by CYP
isozymes.
In vitro
metabolism studies suggest that methylnaltrexone bromide does not inhibit the
activity of CYP1A2, CYP2E1, CYP2B6, CYP2A6, CYP2C9, CYP2C19 or CYP3A4, while it is a
weak inhibitor of the metabolism of a model CYP2D6 substrate. In a clinical drug interaction study in
healthy adult male subjects, a subcutaneous dose of 0.3 mg/kg of methylnaltrexone bromide did not
significantly affect the metabolism of dextromethorphan, a CYP2D6 substrate.
The organic cation transporter (OCT)-related drug-drug interaction potential between
methylnaltrexone bromide and an OCT inhibitor was studied in 18 healthy subjects by comparing
the single-dose pharmacokinetic profiles of methylnaltrexone bromide before and after multiple
400 mg doses of cimetidine. The renal clearance of methylnaltrexone bromide was reduced following
multiple-dose administration of cimetidine (from 31 l/h to 18 l/h). However, this resulted in a small
reduction in total clearance (from 107 l/h to 95 l/h). Consequently, no meaningful change in AUC
of methylnaltrexone bromide, in addition to C
max
, was observed before and after multiple-dose
administration of cimetidine.
4.6 Pregnancy and lactation
There are no adequate data with the use of methylnaltrexone bromide in pregnant women. Studies
in animals have shown reproductive toxicity at high doses (see section 5.3). The potential risk for
humans is unknown. Relistor should not be used during pregnancy unless clearly necessary.
It is unknown whether methylnaltrexone bromide is excreted in human breast milk. Animal studies
have shown excretion of methylnaltrexone bromide in breast milk. A decision on whether to continue/
discontinue breast-feeding or to continue/discontinue therapy with Relistor should be made, taking
into account the benefit of breast-feeding to the child and the benefit of Relistor therapy to the woman.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. However, as a
pure peripherally restricted opioid antagonist, the likelihood that Relistor will affect such activities is
low.
Dizziness may occur, and this may have an effect on driving and use of machines (see section 4.8).
The most common drug-related adverse reactions in all patients exposed to methylnaltrexone bromide
during all phases of placebo-controlled studies were abdominal pain, nausea, diarrhoea and flatulence.
Generally, these reactions were mild or moderate.
The adverse reactions are classified as: Very common (≥1/10); Common (≥1/100 to <1/10);
Uncommon (≥1/1,000 to <1/100); Rare (≥1/10,000 to <1/1,000); Very rare (<1/10,000); Not known
(cannot be estimated from the available data). Within each frequency grouping, adverse reactions are
presented in order of decreasing seriousness:
Gastrointestinal disorders
Very Common: Abdominal pain, nausea, diarrhoea, flatulence
Skin and subcutaneous tissue disorders
Common: Injection site reactions (e.g. stinging, burning, pain , redness, oedema), hyperhidrosis
Post Marketing Experience
Cases of gastrointestinal perforation have been reported in patients using Relistor (see section 4.4):
frequency unknown.
A study of healthy volunteers noted orthostatic hypotension associated with a dose of 0.64 mg/kg
administered as an intravenous bolus.
In the event of an overdose, signs and symptoms of orthostatic hypotension should be monitored and
reported to a physician. Treatment should be initiated as appropriate.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Peripheral opioid receptor antagonists, ATC code: A06AH01
Methylnaltrexone bromide is a selective antagonist of opioid binding at the mu-receptor.
In vitro
studies have shown methylnaltrexone bromide to be a mu-opioid receptor antagonist (inhibition
constant [K
i
] = 28 nM), with 8-fold less potency for kappa opioid receptors (K
i
= 230 nM) and much
reduced affinity for delta opioid receptors.
As a quaternary amine, the ability of methylnaltrexone bromide to cross the blood-brain barrier is
restricted. This allows methylnaltrexone bromide to function as a peripherally acting mu-opioid
antagonist in tissues such as the gastrointestinal tract, without impacting opioid-mediated analgesic
effects on the central nervous system.
Clinical efficacy and safety
The efficacy and safety of methylnaltrexone bromide in the treatment of opioid-induced constipation
in patients receiving palliative care was demonstrated in two randomised, double-blind, placebo-
controlled studies. In these studies, the median age was 68 years (range 21-100); 51 % were females.
In both studies, patients had advanced terminal illness and limited life expectancy, with the majority
having a primary diagnosis of incurable cancer; other primary diagnoses included end-stage COPD/
emphysema, cardiovascular disease/heart failure, Alzheimer’s disease/dementia, HIV/AIDS, or other
advanced illnesses. Prior to screening, patients had opioid-induced constipation defined as either <3
bowel movements in the preceding week or no bowel movement for >2 days.
Study 301 compared methylnaltrexone bromide given as a single, double-blind, subcutaneous dose
of 0.15 mg/kg, or 0.3 mg/kg versus placebo. The double-blind dose was followed by an open-
label, 4-week dosing period, where methylnaltrexone bromide could be used as needed, no more
frequently than 1 dose in a 24-hour period. Throughout both study periods, patients maintained
their usual laxative regimen. A total of 154 patients (methylnaltrexone bromide 0.15 mg/kg, n = 47;
methylnaltrexone bromide 0.3 mg/kg, n = 55, placebo, n = 52) were treated in the double-blind period.
The primary endpoint was the proportion of patients with a rescue-free laxation within 4 hours of
the double-blind dose of study medicinal product. Methylnaltrexone bromide-treated patients had a
significantly higher rate of laxation within 4 hours of the double-blind dose (62 % for 0.15 mg/kg and
58 % for 0.3 mg/kg) than placebo-treated patients (14 %); p<0.0001 for each dose versus placebo.
Study 302 compared double-blind, subcutaneous doses of methylnaltrexone bromide given every
other day for 2 weeks versus placebo. During the first week (days 1, 3, 5, 7), patients received either
methylnaltrexone bromide 0.15 mg/kg or placebo. In the second week, a patient’s assigned dose
could be increased to 0.30 mg/kg if the patient had 2 or fewer rescue-free laxations up to day 8.
At any time, the patient’s assigned dose could be reduced based on tolerability. Data from 133 (62
methylnaltrexone bromide, 71 placebo) patients were analysed. There were 2 primary endpoints:
proportion of patients with a rescue-free laxation within 4 hours of the first dose of study medicinal
product and proportion of patients with a rescue-free laxation within 4 hours after at least 2 of the
first 4 doses of medicinal product. Methylnaltrexone bromide-treated patients had a higher rate of
laxation within 4 hours of the first dose (48 %) than placebo-treated patients (16 %); p<0.0001.
Methylnaltrexone bromide-treated patients also had significantly higher rates of laxation within 4
hours after at least 2 of the first 4 doses (52 %) than did placebo-treated patients (9 %); p<0.0001.
Stool consistency was not meaningfully improved in patients who had soft stool at baseline.
In both studies, there was no evidence to suggest differential effects of age or gender on safety or
efficacy. The effect on race could not be analysed because the study population was predominantly
Caucasian (88 %).
Durability of response was demonstrated in Study 302, in which the laxation response rate was
consistent from dose 1 through dose 7 over the course of the 2-week, double-blind period.
The efficacy and safety of methylnaltrexone bromide were also demonstrated in open-label treatment
administered from Day 2 through Week 4 in Study 301, and in two open-label extension studies
(301EXT and 302EXT) in which methylnaltrexone bromide was given as needed for up to 4 months
(only 8 patients up to this point). A total of 136, 21, and 82 patients received at least one open-label
dose in studies 301, 301EXT, and 302EXT, respectively. Relistor was administered every 3.2 days
(median dosing interval, with a range of 1-39 days).
The rate of laxation response was maintained throughout the extension studies for those patients who
continued treatment.
There was no significant relationship between baseline opioid dose and laxation response in
methylnaltrexone bromide-treated patients in these studies. In addition, median daily opioid dose did
not vary meaningfully from baseline in either methylnaltrexone bromide-treated patients or in placebo-
treated patients. There were no clinically relevant changes in pain scores from baseline in either the
methylnaltrexone bromide or placebo-treated patients.
Effect on cardiac repolarisation
In a double-blind, randomised, parallel-group ECG study of single, subcutaneous doses of
methylnaltrexone bromide (0.15, 0.30 and 0.50 mg/kg), in 207 healthy volunteers, no signal of
QT/QTc prolongation or any evidence of an effect on secondary ECG parameters or waveform
morphology was detected as compared to placebo and a positive control (orally administered 400 mg
moxifloxacin).
5.2 Pharmacokinetic properties
Methylnaltrexone bromide is absorbed rapidly, with peak concentrations (C
max
) achieved at
approximately 0.5 hours following subcutaneous administration. The C
max
and area under the plasma
concentration-time curve (AUC) increase with dose increase from 0.15 mg/kg to 0.5 mg/kg in a dose-
proportional manner. Absolute bioavailability of a 0.30 mg/kg subcutaneous dose versus a 0.30 mg/kg
intravenous dose is 82 %.
Methylnaltrexone bromide undergoes moderate tissue distribution. The steady-state volume of
distribution (Vss) is approximately 1.1 l/kg. Methylnaltrexone bromide is minimally bound to human
plasma proteins (11.0 % to 15.3 %) as determined by equilibrium dialysis.
Methylnaltrexone bromide is metabolised to a modest extent in humans based on the amount of
methylnaltrexone bromide metabolites recovered from excreta. Conversion to methyl-6-naltrexol
isomers and methylnaltrexone sulphate appears to be the primary pathway to metabolism. Each of the
methyl-6-naltrexol isomers has somewhat less antagonist activity than parent compound, and a low
exposure in plasma of approximately 8 % of the drug-related materials. Methylnaltrexone sulphate
is an inactive metabolite and present in plasma at a level of approximately 25 % of drug related
materials. N-demethylation of methylnaltrexone bromide to produce naltrexone is not significant,
accounting for 0.06 % of the administered dose.
Methylnaltrexone bromide is eliminated primarily as the unchanged active substance. Approximately
half of the dose is excreted in the urine and somewhat less in faeces. The terminal disposition half-life
(t
1/2
) is approximately 8 hours.
The effect of mild and moderate hepatic impairment on the systemic exposure to methylnaltrexone
bromide has been studied in 8 subjects each, with Child-Pugh Class A and B, compared to healthy
subjects. Results showed no meaningful effect of hepatic impairment on the AUC or C
max
of
methylnaltrexone bromide. The effect of severe hepatic impairment on the pharmacokinetics of
methylnaltrexone bromide has not been studied.
Renal impairment
In a study of volunteers with varying degrees of renal impairment receiving a single dose of 0.30 mg/
kg methylnaltrexone bromide, renal impairment had a marked effect on the renal excretion of
methylnaltrexone bromide. The renal clearance of methylnaltrexone bromide decreased with
increasing severity of renal impairment. Severe renal impairment decreased the renal clearance of
methylnaltrexone bromide by 8- to 9-fold; however, this resulted in only a 2-fold increase in total
methylnaltrexone bromide exposure (AUC). C
max
was not significantly changed. No studies were
performed in patients with end-stage renal impairment requiring dialysis.
No studies have been performed in the paediatric population (see section 4.2).
In a study comparing single and multiple-dose pharmacokinetic profiles of intravenous
methylnaltrexone bromide at a dose of 24 mg between healthy, young (18 to 45 years of age
n = 10) and elderly (65 years of age and over n = 10) subjects, the effect of age on exposure to
methylnaltrexone bromide was found to be minor. The mean steady-state C
max
and AUC for the elderly
were 545 ng/ml and 412 ng•h/ml, approximately 8.1 % and 20 %, respectively, greater than those for
young subjects. Therefore, no dose adjustment is recommended based on age.
No meaningful gender differences have been observed.
An integrated analysis of pharmacokinetic data from healthy subjects indicated that methylnaltrexone
bromide mg/kg dose-adjusted exposure increased as body weight increased. The mean
methylnaltrexone bromide exposure at 0.15 mg/kg over a weight range of 38 to 114 kg was
179 (range = 139-240) ng•h/ml. This exposure for the 0.15 mg/kg dose can be achieved with a weight-
band-based dose adjustment using an 8 mg dose for body weight 38 to less than 62 kg and a 12 mg
dose for body weight 62 to 114 kg, yielding a mean exposure of 187 (range = 148-220) ng•h/ml.
In addition, the analysis showed that 8 mg dose for body weight 38 to less than 62 kg and a 12 mg
dose for body weight 62 to 114 kg correspond to mean doses of 0.16 (range = 0.21-0.13) mg/kg
and 0.16 (range = 0.19-0.11) mg/kg, respectively, based on the body weight distribution of patients
participating in studies 301 and 302.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, and genotoxicity. Cardiac effects were observed in some non-
clinical studies in canines (prolongation of action potentials in Purkinje fibers or prolongation of the
QTc interval). The mechanism of this effect is unknown; however, the human cardiac potassium ion
channel (hERG) appears not to be involved.
Subcutaneous injections of Relistor at 150 mg/kg/day decreased fertility in rats. Doses up to 25 mg/
kg/day (18 times the exposure [AUC] in humans at a subcutaneous dose of 0.3 mg/kg) did not affect
fertility or general reproductive performance.
There was no evidence of teratogenicity in rats or rabbits. Subcutaneous injections of Relistor at
150/100 mg/kg/day to rats resulted in decreased offspring weights; doses up to 25 mg/kg/day (18 times
the exposure [AUC] in humans at a subcutaneous dose of 0.3 mg/kg) had no effect on labour, delivery,
or offspring survival and growth.
Methylnaltrexone bromide is excreted via the milk of lactating rats.
Studies have been conducted in juvenile rats and dogs. Following intravenous injection of
methylnaltrexone bromide, juvenile rats were found to be more sensitive than adult rats to
methylnaltrexone-related toxicity. In juvenile rats administered intravenous methylnaltrexone bromide
for 13 weeks, adverse clinical signs (incidences of convulsions and labored breathing) occurred
at dosages (≥ 3 mg/kg/day) and exposures (5.4 times the exposure {AUC} in adult humans at a
subcutaneous dose of 0.15 mg/kg) that were lower than those that caused similar toxicity in adult rats
(20 mg/kg/day). No adverse effects occurred in juvenile rats at 1 mg/kg/day or in adult rats at 5 mg/
kg/day (1.6 times and 7.8 times, respectively, the exposure {AUC} in adult humans at a subcutaneous
dose of 0.15 mg/kg).
Following intravenous injection of methylnaltrexone bromide for 13 weeks, similar methylnaltrexone
related toxicity was observed in both juvenile and adult dogs. In adult and juvenile dogs given
methylnaltrexone bromide at 20 mg/kg/day, clinical signs indicative of CNS toxicity and prolongation
of QTc interval were observed. No adverse effects occurred in either juvenile or adult dogs at a dose of
5 mg/kg/day (44 times the exposure {AUC} in adult humans at a subcutaneous dose of 0.15 mg/kg).
Carcinogenicity studies have not been conducted with Relistor.
6. PHARMACEUTICAL PARTICULARS
Hydrochloric acid (to adjust pH)
Sodium hydroxide (to adjust pH)
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
After withdrawal in the injection syringe:
Due to light sensitivity, the solution for injection should be used within 24 hours.
6.4 Special precautions for storage
This medicinal product does not require any special temperature storage conditions.
Keep the vial in the outer carton in order to protect from light.
For storage of the medicinal product in the syringe, see section 6.3.
6.5 Nature and contents of container
Clear, Type I, flint glass, single-use vial, grey butyl rubber stopper, and aluminium overseal with flip-
off-cap.
Each vial contains 0.6 ml of solution for injection.
The presentations of Relistor are:
1 vial of solution for injection
2 vials of solution for injection
2 sterile 1 ml injection syringes with retractable injection needle
7 vials of solution for injection
7 sterile 1 ml injection syringes with retractable injection needle
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
7. MARKETING AUTHORISATION HOLDER
8. MARKETING AUTHORISATION NUMBERS
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 02 July 2008
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
1. NAME OF THE MEDICINAL PRODUCT
Relistor 12 mg solution for injection in pre-filled syringe
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each pre-filled syringe of 0.6 ml contains 12 mg methylnaltrexone bromide
One ml of solution contains 20 mg methylnaltrexone bromide.
For a full list of excipients, see section 6.1.
Solution for injection in pre-filled syringe (injection).
Clear solution, colourless to pale-yellow, essentially free from visible particulates.
4.1 Therapeutic indications
Treatment of opioid-induced constipation in advanced illness patients who are receiving palliative care
when response to usual laxative therapy has not been sufficient.
4.2 Posology and method of administration
Relistor should be added to induce prompt bowel movements when response to usual laxative therapy
has not been sufficient.
The recommended dose of methylnaltrexone bromide is 8 mg (0.4 ml Relistor) (for patients weighing
38-61 kg) or 12 mg (0.6 ml Relistor) (for patients weighing 62-114 kg).
The usual administration schedule is one single dose every other day. Doses may also be given with
longer intervals, as per clinical need.
Patients may receive two consecutive doses 24 hours apart, only when there has been no response
(bowel movement) to the dose on the preceding day.
Patients weighing less than 38 kg or greater than 114 kg should use Relistor vials because the
recommended mg/kg dose cannot be accurately delivered with the pre-filled syringe.
In patients with severe renal impairment (creatinine clearance less than 30 ml/min), the dose of
methylnaltrexone bromide should be reduced from 12 mg to 8 mg (0.4 ml Relistor) for those weighing
62 to 114 kg. Patients with severe renal impairment whose weight falls outside the 62 to 114 kg range
(see section 5.2) need to reduce their mg/kg dose by 50 %. These patients should use Relistor vials and
not the pre-filled syringe. There are no data available from patients with end-stage renal impairment on
dialysis, and Relistor is not recommended in these patients (see section 4.4).
Hepatic impairment
No dose adjustment is necessary in patients with mild to moderate hepatic impairment (see section
5.2).
There are no data available from patients with severe hepatic impairment (Child-Pugh Class C), and
Relistor is not recommended in these patients (see section 4.4).
Paediatric population
No data are available. There is no experience in children under the age of 18 (see section 5.2).
Therefore, methylnaltrexone bromide should not be used in the paediatric age group until further data
become available.
No dose adjustment is recommended based on age (see section 5.2).
Relistor is given as a subcutaneous injection.
It is recommended to rotate injection sites. It is not recommended to inject into areas where the skin is
tender, bruised, red, or hard. Areas with scars or stretch marks should be avoided.
The three areas of the body recommended for injection of Relistor are upper legs, abdomen, and upper
arms.
Relistor can be injected without regard to food.
Hypersensitivity to the active substance or to any of the excipients.
Use of methylnaltrexone bromide in patients with known or suspected mechanical gastrointestinal
obstruction or acute surgical abdomen is contraindicated.
4.4 Special warnings and precautions for use
Cases of gastrointestinal (GI) perforation have been reported in the postauthorisation period in patients
using Relistor. Although patients had medical conditions that may be associated with localised or
diffuse reduction of structural integrity in the wall of the GI tract (e.g., cancer, peptic ulcer, pseudo-
obstruction), the use of Relistor may have contributed to these events.
Use Relistor with caution in patients with known or suspected lesions of the GI tract.
Advise patients to promptly report severe, persistent, and/or worsening symptoms.
The activity of methylnaltrexone bromide has been studied in patients with constipation induced by
opioids. Therefore, Relistor should not be used for treatment of patients with constipation not related
to opioid use.
If severe or persistent diarrhoea occurs during treatment, patients should be advised not to continue
therapy with Relistor and consult their physician.
Data from clinical trials suggest treatment with methylnaltrexone bromide can result in the rapid onset
(within 30 to 60 minutes on average) of a bowel movement.
Methylnaltrexone bromide treatment has not been studied in clinical trials for longer than 4 months,
and should therefore only be used for a limited period (see section 5.2).
Relistor should only be used in patients who are receiving palliative care. It is added to usual laxative
treatment.
Relistor is not recommended in patients with severe hepatic impairment or with end-stage renal
impairment requiring dialysis (see section 4.2).
Use of methylnaltrexone bromide in patients with colostomy, peritoneal catheter, active diverticular
disease or fecal impaction has not been studied. Therefore, Relistor should only be administered with
caution in these patients.
This medicinal product contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially sodium-
free.
4.5 Interaction with other medicinal products and other forms of interaction
Methylnaltrexone bromide does not affect the pharmacokinetics of medicinal products metabolised
by cytochrome P450 (CYP) isozymes. Methylnaltrexone bromide is minimally metabolised by CYP
isozymes.
In vitro
metabolism studies suggest that methylnaltrexone bromide does not inhibit the
activity of CYP1A2, CYP2E1, CYP2B6, CYP2A6, CYP2C9, CYP2C19 or CYP3A4, while it is a
weak inhibitor of the metabolism of a model CYP2D6 substrate. In a clinical drug interaction study in
healthy adult male subjects, a subcutaneous dose of 0.3 mg/kg of methylnaltrexone bromide did not
significantly affect the metabolism of dextromethorphan, a CYP2D6 substrate.
The organic cation transporter (OCT)-related drug-drug interaction potential between
methylnaltrexone bromide and an OCT inhibitor was studied in 18 healthy subjects by comparing
the single-dose pharmacokinetic profiles of methylnaltrexone bromide before and after multiple
400 mg doses of cimetidine. The renal clearance of methylnaltrexone bromide was reduced following
multiple-dose administration of cimetidine (from 31 l/h to 18 l/h). However, this resulted in a small
reduction in total clearance (from 107 l/h to 95 l/h). Consequently, no meaningful change in AUC
of methylnaltrexone bromide, in addition to C
max
, was observed before and after multiple-dose
administration of cimetidine.
4.6 Pregnancy and lactation
There are no adequate data with the use of methylnaltrexone bromide in pregnant women. Studies
in animals have shown reproductive toxicity at high doses (see section 5.3). The potential risk for
humans is unknown. Relistor should not be used during pregnancy unless clearly necessary.
It is unknown whether methylnaltrexone bromide is excreted in human breast milk. Animal studies
have shown excretion of methylnaltrexone bromide in breast milk. A decision on whether to continue/
discontinue breast-feeding or to continue/discontinue therapy with Relistor should be made, taking
into account the benefit of breast-feeding to the child and the benefit of Relistor therapy to the woman.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. However, as a
pure peripherally restricted opioid antagonist, the likelihood that Relistor will affect such activities is
low.
Dizziness may occur, and this may have an effect on driving and use of machines (see section 4.8).
The most common drug-related adverse reactions in all patients exposed to methylnaltrexone bromide
during all phases of placebo-controlled studies were abdominal pain, nausea, diarrhoea and flatulence.
Generally, these reactions were mild or moderate.
The adverse reactions are classified as: Very common (≥1/10); Common (≥1/100 to <1/10);
Uncommon (≥1/1,000 to <1/100); Rare (≥1/10,000 to <1/1,000); Very rare (<1/10,000); Not known
(cannot be estimated from the available data). Within each frequency grouping, adverse reactions are
presented in order of decreasing seriousness:
Gastrointestinal disorders
Very Common: Abdominal pain, nausea, diarrhoea, flatulence
Skin and subcutaneous tissue disorders
Common: Injection site reactions (e.g. stinging, burning, pain , redness, oedema), hyperhidrosis
Post Marketing Experience
Cases of gastrointestinal perforation have been reported in patients using Relistor (see section 4.4):
frequency unknown.
A study of healthy volunteers noted orthostatic hypotension associated with a dose of 0.64 mg/kg
administered as an intravenous bolus.
In the event of an overdose, signs and symptoms of orthostatic hypotension should be monitored and
reported to a physician. Treatment should be initiated as appropriate.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Peripheral opioid receptor antagonists, ATC code: A06AH01
Methylnaltrexone bromide is a selective antagonist of opioid binding at the mu-receptor.
In vitro
studies have shown methylnaltrexone bromide to be a mu-opioid receptor antagonist (inhibition
constant [K
i
] = 28 nM), with 8-fold less potency for kappa opioid receptors (K
i
= 230 nM) and much
reduced affinity for delta opioid receptors.
As a quaternary amine, the ability of methylnaltrexone bromide to cross the blood-brain barrier is
restricted. This allows methylnaltrexone bromide to function as a peripherally acting mu-opioid
antagonist in tissues such as the gastrointestinal tract, without impacting opioid-mediated analgesic
effects on the central nervous system.
Clinical efficacy and safety
The efficacy and safety of methylnaltrexone bromide in the treatment of opioid-induced constipation
in patients receiving palliative care was demonstrated in two randomised, double-blind, placebo-
controlled studies. In these studies, the median age was 68 years (range 21-100); 51 % were females.
In both studies, patients had advanced terminal illness and limited life expectancy, with the majority
having a primary diagnosis of incurable cancer; other primary diagnoses included end-stage COPD/
emphysema, cardiovascular disease/heart failure, Alzheimer’s disease/dementia, HIV/AIDS, or other
advanced illnesses. Prior to screening, patients had opioid-induced constipation defined as either <3
bowel movements in the preceding week or no bowel movement for >2 days.
Study 301 compared methylnaltrexone bromide given as a single, double-blind, subcutaneous dose
of 0.15 mg/kg, or 0.3 mg/kg versus placebo. The double-blind dose was followed by an open-
label, 4-week dosing period, where methylnaltrexone bromide could be used as needed, no more
frequently than 1 dose in a 24-hour period. Throughout both study periods, patients maintained
their usual laxative regimen. A total of 154 patients (methylnaltrexone bromide 0.15 mg/kg, n = 47;
methylnaltrexone bromide 0.3 mg/kg, n = 55, placebo, n = 52) were treated in the double-blind period.
The primary endpoint was the proportion of patients with a rescue-free laxation within 4 hours of
the double-blind dose of study medicinal product. Methylnaltrexone bromide-treated patients had a
significantly higher rate of laxation within 4 hours of the double-blind dose (62 % for 0.15 mg/kg and
58 % for 0.3 mg/kg) than placebo-treated patients (14 %); p<0.0001 for each dose versus placebo.
Study 302 compared double-blind, subcutaneous doses of methylnaltrexone bromide given every
other day for 2 weeks versus placebo. During the first week (days 1, 3, 5, 7), patients received either
methylnaltrexone bromide 0.15 mg/kg or placebo. In the second week, a patient’s assigned dose
could be increased to 0.30 mg/kg if the patient had 2 or fewer rescue-free laxations up to day 8.
At any time, the patient’s assigned dose could be reduced based on tolerability. Data from 133 (62
methylnaltrexone bromide, 71 placebo) patients were analysed. There were 2 primary endpoints:
proportion of patients with a rescue-free laxation within 4 hours of the first dose of study medicinal
product and proportion of patients with a rescue-free laxation within 4 hours after at least 2 of the
first 4 doses of medicinal product. Methylnaltrexone bromide-treated patients had a higher rate of
laxation within 4 hours of the first dose (48 %) than placebo-treated patients (16 %); p<0.0001.
Methylnaltrexone bromide-treated patients also had significantly higher rates of laxation within 4
hours after at least 2 of the first 4 doses (52 %) than did placebo-treated patients (9 %); p<0.0001.
Stool consistency was not meaningfully improved in patients who had soft stool at baseline.
In both studies, there was no evidence to suggest differential effects of age or gender on safety or
efficacy. The effect on race could not be analysed because the study population was predominantly
Caucasian (88 %).
Durability of response was demonstrated in Study 302, in which the laxation response rate was
consistent from dose 1 through dose 7 over the course of the 2-week, double-blind period.
The efficacy and safety of methylnaltrexone bromide were also demonstrated in open-label treatment
administered from Day 2 through Week 4 in Study 301, and in two open-label extension studies
(301EXT and 302EXT) in which methylnaltrexone bromide was given as needed for up to 4 months
(only 8 patients up to this point). A total of 136, 21, and 82 patients received at least one open-label
dose in studies 301, 301EXT, and 302EXT, respectively. Relistor was administered every 3.2 days
(median dosing interval, with a range of 1-39 days).
The rate of laxation response was maintained throughout the extension studies for those patients who
continued treatment.
There was no significant relationship between baseline opioid dose and laxation response in
methylnaltrexone bromide-treated patients in these studies. In addition, median daily opioid dose did
not vary meaningfully from baseline in either methylnaltrexone bromide-treated patients or in placebo-
treated patients. There were no clinically relevant changes in pain scores from baseline in either the
methylnaltrexone bromide or placebo-treated patients.
Effect on cardiac repolarisation
In a double-blind, randomised, parallel-group ECG study of single, subcutaneous doses of
methylnaltrexone bromide (0.15, 0.30 and 0.50 mg/kg), in 207 healthy volunteers, no signal of
QT/QTc prolongation or any evidence of an effect on secondary ECG parameters or waveform
morphology was detected as compared to placebo and a positive control (orally administered 400 mg
moxifloxacin).
5.2 Pharmacokinetic properties
Methylnaltrexone bromide is absorbed rapidly, with peak concentrations (C
max
) achieved at
approximately 0.5 hours following subcutaneous administration. The C
max
and area under the plasma
concentration-time curve (AUC) increase with dose increase from 0.15 mg/kg to 0.5 mg/kg in a dose-
proportional manner. Absolute bioavailability of a 0.30 mg/kg subcutaneous dose versus a 0.30 mg/kg
intravenous dose is 82 %.
Methylnaltrexone bromide undergoes moderate tissue distribution. The steady-state volume of
distribution (Vss) is approximately 1.1 l/kg. Methylnaltrexone bromide is minimally bound to human
plasma proteins (11.0 % to 15.3 %) as determined by equilibrium dialysis.
Methylnaltrexone bromide is metabolised to a modest extent in humans based on the amount of
methylnaltrexone bromide metabolites recovered from excreta. Conversion to methyl-6-naltrexol
isomers and methylnaltrexone sulphate appears to be the primary pathway to metabolism. Each of the
methyl-6-naltrexol isomers has somewhat less antagonist activity than parent compound, and a low
exposure in plasma of approximately 8 % of the drug-related materials. Methylnaltrexone sulphate
is an inactive metabolite and present in plasma at a level of approximately 25 % of drug related
materials. N-demethylation of methylnaltrexone bromide to produce naltrexone is not significant,
accounting for 0.06 % of the administered dose.
Methylnaltrexone bromide is eliminated primarily as the unchanged active substance. Approximately
half of the dose is excreted in the urine and somewhat less in faeces. The terminal disposition half-life
(t
1/2
) is approximately 8 hours.
The effect of mild and moderate hepatic impairment on the systemic exposure to methylnaltrexone
bromide has been studied in 8 subjects each, with Child-Pugh Class A and B, compared to healthy
subjects. Results showed no meaningful effect of hepatic impairment on the AUC or C
max
of
methylnaltrexone bromide. The effect of severe hepatic impairment on the pharmacokinetics of
methylnaltrexone bromide has not been studied.
Renal impairment
In a study of volunteers with varying degrees of renal impairment receiving a single dose of 0.30 mg/
kg methylnaltrexone bromide, renal impairment had a marked effect on the renal excretion of
methylnaltrexone bromide. The renal clearance of methylnaltrexone bromide decreased with
increasing severity of renal impairment. Severe renal impairment decreased the renal clearance of
methylnaltrexone bromide by 8- to 9-fold; however, this resulted in only a 2-fold increase in total
methylnaltrexone bromide exposure (AUC). C
max
was not significantly changed. No studies were
performed in patients with end-stage renal impairment requiring dialysis.
No studies have been performed in the paediatric population (see section 4.2).
In a study comparing single and multiple-dose pharmacokinetic profiles of intravenous
methylnaltrexone bromide at a dose of 24 mg between healthy, young (18 to 45 years of age
n = 10) and elderly (65 years of age and over n = 10) subjects, the effect of age on exposure to
methylnaltrexone bromide was found to be minor. The mean steady-state C
max
and AUC for the elderly
were 545 ng/ml and 412 ng•h/ml, approximately 8.1 % and 20 %, respectively, greater than those for
young subjects. Therefore, no dose adjustment is recommended based on age.
No meaningful gender differences have been observed.
An integrated analysis of pharmacokinetic data from healthy subjects indicated that methylnaltrexone
bromide mg/kg dose-adjusted exposure increased as body weight increased. The mean
methylnaltrexone bromide exposure at 0.15 mg/kg over a weight range of 38 to 114 kg was
179 (range = 139-240) ng•h/ml. This exposure for the 0.15 mg/kg dose can be achieved with a weight-
band-based dose adjustment using an 8 mg dose for body weight 38 to less than 62 kg and a 12 mg
dose for body weight 62 to 114 kg, yielding a mean exposure of 187 (range = 148-220) ng•h/ml.
In addition, the analysis showed that 8 mg dose for body weight 38 to less than 62 kg and a 12 mg
dose for body weight 62 to 114 kg correspond to mean doses of 0.16 (range = 0.21-0.13) mg/kg
and 0.16 (range = 0.19-0.11) mg/kg, respectively, based on the body weight distribution of patients
participating in studies 301 and 302.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, and genotoxicity. Cardiac effects were observed in some non-
clinical studies in canines (prolongation of action potentials in Purkinje fibers or prolongation of the
QTc interval). The mechanism of this effect is unknown; however, the human cardiac potassium ion
channel (hERG) appears not to be involved.
Subcutaneous injections of Relistor at 150 mg/kg/day decreased fertility in rats. Doses up to 25 mg/
kg/day (18 times the exposure [AUC] in humans at a subcutaneous dose of 0.3 mg/kg) did not affect
fertility or general reproductive performance.
There was no evidence of teratogenicity in rats or rabbits. Subcutaneous injections of Relistor at
150/100 mg/kg/day to rats resulted in decreased offspring weights; doses up to 25 mg/kg/day (18 times
the exposure [AUC] in humans at a subcutaneous dose of 0.3 mg/kg) had no effect on labour, delivery,
or offspring survival and growth.
Methylnaltrexone bromide is excreted via the milk of lactating rats.
Studies have been conducted in juvenile rats and dogs. Following intravenous injection of
methylnaltrexone bromide, juvenile rats were found to be more sensitive than adult rats to
methylnaltrexone-related toxicity. In juvenile rats administered intravenous methylnaltrexone bromide
for 13 weeks, adverse clinical signs (incidences of convulsions and labored breathing) occurred
at dosages (≥ 3 mg/kg/day) and exposures (5.4 times the exposure {AUC} in adult humans at a
subcutaneous dose of 0.15 mg/kg) that were lower than those that caused similar toxicity in adult rats
(20 mg/kg/day). No adverse effects occurred in juvenile rats at 1 mg/kg/day or in adult rats at 5 mg/
kg/day (1.6 times and 7.8 times, respectively, the exposure {AUC} in adult humans at a subcutaneous
dose of 0.15 mg/kg).
Following intravenous injection of methylnaltrexone bromide for 13 weeks, similar methylnaltrexone
related toxicity was observed in both juvenile and adult dogs. In adult and juvenile dogs given
methylnaltrexone bromide at 20 mg/kg/day, clinical signs indicative of CNS toxicity and prolongation
of QTc interval were observed. No adverse effects occurred in either juvenile or adult dogs at a dose of
5 mg/kg/day (44 times the exposure {AUC} in adult humans at a subcutaneous dose of 0.15 mg/kg).
Carcinogenicity studies have not been conducted with Relistor.
6. PHARMACEUTICAL PARTICULARS
Hydrochloric acid (to adjust pH)
Sodium hydroxide (to adjust pH)
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
6.4 Special precautions for storage
Keep the pre-filled syringe in the outer carton in order to protect from light.
6.5 Nature and contents of container
Each pre-filled syringe contains 0.6 ml of solution for injection.
Pre-filled syringe of clear type I glass with stainless-steel needle, plastic plunger, and polypropylene
rigid needle cover.
Pack sizes of 4, 7, 8 and 10 pre-filled syringes.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
7. MARKETING AUTHORISATION HOLDER
8. MARKETING AUTHORISATION NUMBERS
EU/1/08/463/011
EU/1/08/463/010
EU/1/08/463/009
EU/1/08/463/008
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 02 July 2008
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
ANNEX II
A. MANUFACTURING AUTHORISATION HOLDERS RESPONSIBLE FOR BATCH
RELEASE
B. CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURING AUTHORISATION HOLDERS RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturers responsible for batch release
The printed package leaflet of the medicinal product must state the name and address of the
manufacturer responsible for the release of the concerned batch.
B. CONDITIONS OF THE MARKETING AUTHORISATION
•
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to medical prescription.
•
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
The MAH must ensure that the system of pharmacovigilance, as described in version 3.0 presented
in Module 1.8.1. of the Marketing Authorisation Application, is in place and functioning before and
whilst the product is on the market.
The MAH must ensure that the system of pharmacovigilance, as described in version 3.0 presented
in Module 1.8.1. of the Marketing Authorisation Application, is in place and functioning before and
whilst the product is on the market.
Risk Management Plan
Risk Management Plan
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version 1.3 of the Risk Management Plan (RMP) presented
in Module 1.8.2. of the Marketing Authorisation Application and any subsequent updates of the RMP
agreed by the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use,
the updated RMP should be submitted at the same time as the next Periodic Safety Update Report
(PSUR).
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use,
the updated RMP should be submitted at the same time as the next Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted
In addition, an updated RMP should be submitted
• When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
• Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
• At the request of the European Medicines Agency.
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
CARTON TEXT (VIAL PRESENTATION)
1. NAME OF THE MEDICINAL PRODUCT
Relistor 12 mg/0.6 ml solution for injection
2. STATEMENT OF ACTIVE SUBSTANCE(S)
Each vial of 0.6 ml contains 12 mg of methylnaltrexone bromide.
One ml of solution contains 20 mg of methylnaltrexone bromide.
Sodium chloride, sodium calcium edetate, glycine hydrochloride, water for injections, hydrochloric
acid (to adjust pH), sodium hydroxide (to adjust pH).
4. PHARMACEUTICAL FORM AND CONTENTS
2 sterile 1 ml injection syringes with retractable injection needle
5. METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.



























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
CARTON TEXT (VIAL PRESENTATION)
1. NAME OF THE MEDICINAL PRODUCT
Relistor 12 mg/0.6 ml solution for injection
2. STATEMENT OF ACTIVE SUBSTANCE(S)
Each vial of 0.6 ml contains 12 mg of methylnaltrexone bromide.
One ml of solution contains 20 mg of methylnaltrexone bromide.
Sodium chloride, sodium calcium edetate, glycine hydrochloride, water for injections, hydrochloric
acid (to adjust pH), sodium hydroxide (to adjust pH).
4. PHARMACEUTICAL FORM AND CONTENTS
5. METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
7. OTHER SPECIAL WARNING(S), IF NECESSARY



































9. SPECIAL STORAGE CONDITIONS
Keep the vial in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
12. MARKETING AUTHORISATION NUMBER
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
CARTON TEXT (VIAL PRESENTATION)
1. NAME OF THE MEDICINAL PRODUCT
Relistor 12 mg/0.6 ml solution for injection
2. STATEMENT OF ACTIVE SUBSTANCE(S)
Each vial of 0.6 ml contains 12 mg of methylnaltrexone bromide.
One ml of solution contains 20 mg of methylnaltrexone bromide.
Sodium chloride, sodium calcium edetate, glycine hydrochloride, water for injections, hydrochloric
acid (to adjust pH), sodium hydroxide (to adjust pH).
4. PHARMACEUTICAL FORM AND CONTENTS
7 sterile 1 ml injection syringes with retractable injection needle
5. METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.



























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
CARTON TEXT (PRE-FILLED SYRINGE PRESENTATION)
1. NAME OF THE MEDICINAL PRODUCT
Relistor 8 mg solution for injection in pre-filled syringe
2. STATEMENT OF ACTIVE SUBSTANCE(S)
Each pre-filled syringe of 0.4 ml contains 8 mg of methylnaltrexone bromide.
Sodium chloride, sodium calcium edetate, glycine hydrochloride, water for injections, hydrochloric
acid (to adjust pH), sodium hydroxide (to adjust pH).
4. PHARMACEUTICAL FORM AND CONTENTS
Solution for injection in a pre-filled syringe
5. METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
7. OTHER SPECIAL WARNING(S), IF NECESSARY



































9. SPECIAL STORAGE CONDITIONS
Keep the pre-filled syringe in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
12. MARKETING AUTHORISATION NUMBER
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
CARTON TEXT (PRE-FILLED SYRINGE PRESENTATION)
1. NAME OF THE MEDICINAL PRODUCT
Relistor 8 mg solution for injection in pre-filled syringe
2. STATEMENT OF ACTIVE SUBSTANCE(S)
Each pre-filled syringe of 0.4 ml contains 8 mg of methylnaltrexone bromide.
Sodium chloride, sodium calcium edetate, glycine hydrochloride, water for injections, hydrochloric
acid (to adjust pH), sodium hydroxide (to adjust pH).
4. PHARMACEUTICAL FORM AND CONTENTS
Solution for injection in a pre-filled syringe
5. METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
7. OTHER SPECIAL WARNING(S), IF NECESSARY



































9. SPECIAL STORAGE CONDITIONS
Keep the pre-filled syringe in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
12. MARKETING AUTHORISATION NUMBER
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
CARTON TEXT (PRE-FILLED SYRINGE PRESENTATION)
1. NAME OF THE MEDICINAL PRODUCT
Relistor 8 mg solution for injection in pre-filled syringe
2. STATEMENT OF ACTIVE SUBSTANCE(S)
Each pre-filled syringe of 0.4 ml contains 8 mg of methylnaltrexone bromide.
Sodium chloride, sodium calcium edetate, glycine hydrochloride, water for injections, hydrochloric
acid (to adjust pH), sodium hydroxide (to adjust pH).
4. PHARMACEUTICAL FORM AND CONTENTS
Solution for injection in a pre-filled syringe
5. METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
7. OTHER SPECIAL WARNING(S), IF NECESSARY



































9. SPECIAL STORAGE CONDITIONS
Keep the pre-filled syringe in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
12. MARKETING AUTHORISATION NUMBER
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
CARTON TEXT (PRE-FILLED SYRINGE PRESENTATION)
1. NAME OF THE MEDICINAL PRODUCT
Relistor 8 mg solution for injection in pre-filled syringe
2. STATEMENT OF ACTIVE SUBSTANCE(S)
Each pre-filled syringe of 0.4 ml contains 8 mg of methylnaltrexone bromide.
Sodium chloride, sodium calcium edetate, glycine hydrochloride, water for injections, hydrochloric
acid (to adjust pH), sodium hydroxide (to adjust pH).
4. PHARMACEUTICAL FORM AND CONTENTS
Solution for injection in a pre-filled syringe
5. METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
7. OTHER SPECIAL WARNING(S), IF NECESSARY



































9. SPECIAL STORAGE CONDITIONS
Keep the pre-filled syringe in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
12. MARKETING AUTHORISATION NUMBER
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
CARTON TEXT (PRE-FILLED SYRINGE PRESENTATION)
1. NAME OF THE MEDICINAL PRODUCT
Relistor 12 mg solution for injection in pre-filled syringe
2. STATEMENT OF ACTIVE SUBSTANCE(S)
Each pre-filled syringe of 0.6 ml contains 12 mg of methylnaltrexone bromide.
Sodium chloride, sodium calcium edetate, glycine hydrochloride, water for injections, hydrochloric
acid (to adjust pH), sodium hydroxide (to adjust pH).
4. PHARMACEUTICAL FORM AND CONTENTS
Solution for injection in a pre-filled syringe
5. METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
7. OTHER SPECIAL WARNING(S), IF NECESSARY



































9. SPECIAL STORAGE CONDITIONS
Keep the pre-filled syringe in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
12. MARKETING AUTHORISATION NUMBER
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
CARTON TEXT (PRE-FILLED SYRINGE PRESENTATION)
1. NAME OF THE MEDICINAL PRODUCT
Relistor 12 mg solution for injection in pre-filled syringe
2. STATEMENT OF ACTIVE SUBSTANCE(S)
Each pre-filled syringe of 0.6 ml contains 12 mg of methylnaltrexone bromide.
Sodium chloride, sodium calcium edetate, glycine hydrochloride, water for injections, hydrochloric
acid (to adjust pH), sodium hydroxide (to adjust pH).
4. PHARMACEUTICAL FORM AND CONTENTS
Solution for injection in a pre-filled syringe
5. METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
7. OTHER SPECIAL WARNING(S), IF NECESSARY



































9. SPECIAL STORAGE CONDITIONS
Keep the pre-filled syringe in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
12. MARKETING AUTHORISATION NUMBER
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
CARTON TEXT (PRE-FILLED SYRINGE PRESENTATION)
1. NAME OF THE MEDICINAL PRODUCT
Relistor 12 mg solution for injection in pre-filled syringe
2. STATEMENT OF ACTIVE SUBSTANCE(S)
Each pre-filled syringe of 0.6 ml contains 12 mg of methylnaltrexone bromide.
Sodium chloride, sodium calcium edetate, glycine hydrochloride, water for injections, hydrochloric
acid (to adjust pH), sodium hydroxide (to adjust pH).
4. PHARMACEUTICAL FORM AND CONTENTS
Solution for injection in a pre-filled syringe
5. METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
7. OTHER SPECIAL WARNING(S), IF NECESSARY



































9. SPECIAL STORAGE CONDITIONS
Keep the pre-filled syringe in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
12. MARKETING AUTHORISATION NUMBER
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
CARTON TEXT (PRE-FILLED SYRINGE PRESENTATION)
1. NAME OF THE MEDICINAL PRODUCT
Relistor 12 mg solution for injection in pre-filled syringe
2. STATEMENT OF ACTIVE SUBSTANCE(S)
Each pre-filled syringe of 0.6 ml contains 12 mg of methylnaltrexone bromide.
Sodium chloride, sodium calcium edetate, glycine hydrochloride, water for injections, hydrochloric
acid (to adjust pH), sodium hydroxide (to adjust pH).
4. PHARMACEUTICAL FORM AND CONTENTS
Solution for injection in a pre-filled syringe
5. METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
7. OTHER SPECIAL WARNING(S), IF NECESSARY



































9. SPECIAL STORAGE CONDITIONS
Keep the pre-filled syringe in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
12. MARKETING AUTHORISATION NUMBER
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.























PACKAGE LEAFLET: INFORMATION FOR THE USER
Relistor 12 mg/0.6 ml solution for injection
Methylnaltrexone bromide
Read all of this leaflet carefully before you start using this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
In this leaflet:
1. What Relistor is and what it is used for
2. Before you use Relistor
3. How to use Relistor
4. Possible side effects
5. How to store Relistor
6. Further information
1. WHAT RELISTOR IS AND WHAT IT IS USED FOR
Relistor (methylnaltrexone bromide) acts by blocking the gastrointestinal effects of opioid pain
medicines.
Relistor treats constipation that is caused by medicines for moderate to severe pain called opioids
(for example morphine or codeine), in patients receiving supportive care for their advanced illness,
when other medicines for constipation, called laxatives, have not worked well enough. Opioids are
prescribed by your doctor. Relistor is given on top of your usual laxatives.
Relistor is for use in adults (aged 18 and over).
2. BEFORE YOU USE RELISTOR
Do not use Relistor
• If you are allergic (hypersensitive) to methylnaltrexone bromide or any of the other ingredients.
• If you or your doctor know that your bowels are obstructed or your bowels are in a state
where there is an immediate need for surgical intervention (which has to be diagnosed by your
doctor).
Take special care with Relistor
• If you have severe, persistent, and/or worsening abdominal symptoms contact your doctor
immediately because these could be symptoms of intestinal perforation.
• If you have severe liver or kidney disease.
• If you develop severe or persistent diarrhoea (passing of frequent watery stools), discontinue
therapy and contact your doctor immediately.
• It is important to be near a toilet with assistance available if necessary, since bowel movement
may happen within 30 minutes after injection of the medicine.
• Please talk to your doctor if you experience persistent stomach pain, nausea, (feeling sick in the
stomach) or vomiting that is new or worsened.
• Please also talk to your doctor if you have a colostomy, a tube in your abdomen (peritoneal
catheter), or already known disease called diverticular disease or faecal impaction.
• Relistor should only be used in patients who are receiving palliative care. It is added to usual
laxative therapy.
• Relistor should be used only for a limited period of time (it has not been studied for more than
4 months).
• Relistor should not be used for treatment of patients with constipation not related to opioid use.
If you have suffered from constipation before you had to take opioids (for pain), please talk to
your doctor.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
Your doctor may allow you to take other medicines, including those used for constipation.
Using Relistor with food and drink
Relistor can be taken with or without food.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking any medicine.
The effects of methylnaltrexone bromide in pregnant women are not known, and so the use of Relistor
during pregnancy is not recommended.
Women using Relistor should not breast-feed, since it is not known if methylnaltrexone bromide
passes into human breast milk.
Driving and using machines
Relistor may cause dizziness, and this may have an effect on driving and use of machines.
Important information about some of the ingredients of Relistor
This medicine contains less than 1 mmol sodium (23 mg) per dose i.e., essentially “sodium free.”
Always use Relistor exactly as your doctor has told you. You should check with your doctor or
pharmacist if you are not sure.
The usual dose is 8 mg methylnaltrexone bromide (0.4 ml Relistor) for patients weighing 38-61 kg or
12 mg (0.6 ml Relistor) for patients weighing 62-114 kg. The dose is given every 48 hours (every two
days) as an injection under the skin. Your doctor will determine your dose.
Relistor is given by an injection under the skin (by subcutaneous injection) in either (1) your upper
legs (thighs), (2) your abdomen (stomach), and (3) your upper arm (if not self-injecting). (See
INSTRUCTIONS FOR PREPARING AND GIVING AN INJECTION OF RELISTOR at the end of
this leaflet.)
You may have a bowel movement within a few minutes to a few hours of the injection; therefore, it is
recommended to have a toilet facility or bedpan near you.
If you use more Relistor than you should
If you have used more Relistor than you should (either by injecting too much on a single occasion or
by using more than one injection in 24 hours), talk to a doctor or pharmacist immediately. Always
have the outer carton of the medicine with you, even if it is empty.
If you forget to use Relistor
If you forget a dose, talk to your doctor or pharmacist as soon as possible.
If you stop using Relistor
If you stop using Relistor, talk to a doctor or pharmacist.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Like all medicines, Relistor can cause side effects, although not everybody gets them.
The most common side effects likely to occur in more than 1 in 10 patients:
• Abdominal pain (belly ache)
• Nausea (feeling sick in the stomach)
• Diarrhoea (passing of frequent watery stools)
• Flatulence (passing wind)
Common side effects reported in more than one in 100 patients, but in less than one in ten patients
receiving Relistor are:
• Dizziness (light-headed)
• Reaction at the site of injection (e.g., stinging, burning, pain, redness, oedema)
• Sweating
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Relistor after the expiry date which is stated on the carton and vial.
This medicinal product does not require any special temperature storage conditions.
Keep the vial in the outer carton in order to protect from light.
Only use Relistor if the solution is clear, colourless to pale yellow, and does not contain flakes or
particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
What Relistor contains
The active substance is methylnaltrexone bromide.
Each vial of 0.6 ml contains 12 mg methylnaltrexone bromide.
One ml of solution contains 20 mg methylnaltrexone bromide.
The other ingredients are sodium chloride, sodium calcium edetate, glycine hydrochloride, water for
injections, hydrochloric acid (to adjust pH) and sodium hydroxide (to adjust pH).
What Relistor looks like and contents of the pack
Relistor is a solution for injection. It is clear, colourless to pale yellow, and does not contain flakes or
particles.
Each vial contains 0.6 ml of solution.
Packs of more than one vial contain trays consisting of: one vial, one 1 ml injection syringe with
retractable injection needle, and two alcohol swabs.
The following packs are available:
Pack containing 2 vials, 2 injection syringes with retractable injection needle, and 4 alcohol swabs (i.e.
2 trays).
Pack containing 7 vials, 7 injection syringes with retractable injection needle, and 14 alcohol swabs
(i.e. 7 trays).
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Wyeth Europa Ltd.
Manufacturer
Wyeth Lederle S.p.A.
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
Luxembourg/Luxemburg
Pfizer S.A. / N.V.
Tél/Tel: +32 (0)2 554 62 11
Magyarország
Pfizer Kft.
Tel: +36 1 488 3700
България/Eesti/Latvija/Lietuva/
Slovenija
Wyeth Whitehall Export GmbH
Teл./Tel/Tãlr: +43 1 89 1140
Malta
Vivian Corporation Ltd.
Tel: +356 213 44616
Česká republika
Pfizer s.r.o.
Tel: +420-283-004-111
Nederland
Wyeth Pharmaceuticals B.V.
Tel: +31 23 567 2567
Danmark
Pfizer ApS
Tlf: +45 44 201 100
Norge
Pfizer AS
Tlf: +47 67 526 100
Deutschland
Pfizer Pharma GmbH
Tel: +49 (0)30 550055-51000
Österreich
Pfizer Corporation Austria Ges.m.b.H.
Tel: +43 (0)1 521 15-0
Ελλάδα
Pfizer Hellas A.E.
Τηλ.: +30 210 6785 800
Polska
Pfizer Polska Sp. z o.o.,
Tel.: +48 22 335 61 00
España
Pfizer, S.A.
Télf: +34 91 490 99 00
Portugal
Laboratórios Pfizer, Lda.
Tel: (+351) 21 423 55 00
France
Pfizer
Tél +33 1 58 07 30 00
România
Pfizer Romania S.R.L
Tel: +40 (0) 21 207 28 00
Ireland
Wyeth Pharmaceuticals
Tel:+353 1 449 3500
Slovenská republika
Pfizer Luxembourg SARL, organizačná
zložka
Tel: + 421 2 3355 5500
Ísland
Icepharma hf.
Tel:+354 540 8000
Suomi/Finland
Pfizer Oy
Puh/Tel: +358 (0)9 430 040
Italia
Wyeth Lederle S.p.A.
Tel:+39 06 927151
Sverige
Pfizer AB
Tel: +46 (0)8 550 520 00
Kύπρος
Wyeth Hellas (Cyprus Branch) AEBE
Τηλ:+357 22 817690
United Kingdom
Wyeth Pharmaceuticals
Tel: +44 845 3670098
This leaflet was last approved in {MM/YYYY}.
Detailed information on this medicine is available on the European Medicines Agency (EMEA) web






This section contains important questions that you will need to answer before you take Relistor, and
during treatment with Relistor.
If you answer No to any of the following questions during the course of your medication please
contact your doctor or health care professional.
1. Are you receiving opioid therapy for your illness?
2. Has it been 48 hours or longer since your last bowel movement?
3. Are you familiar with the technique of self injection or have you discussed this with your
doctor (or health care professional)?
4. Are you mobile enough to reach the toilet, or do you have a caregiver looking after you who
can help?
5. Do you have a contact number for your community nurse or the health centre?
INSTRUCTIONS FOR PREPARING AND GIVING AN INJECTION OF RELISTOR
This section is divided into the following subsections:
Step 1: Setting up for an injection
Step 2: Preparing the injection syringe
Step 3: Choosing and preparing an injection site
Step 4a: Injecting Relistor using a pack containing injection syringe with retractable injection needle
Step 4b: Injecting Relistor using a standard injection syringe and injection needle
Step 5 Disposing of supplies
The following instructions explain how to inject Relistor. Please read the instructions carefully and
follow them step by step. You will be instructed by your healthcare professional on the techniques of
self-administration. Do not attempt to administer an injection until you are sure that you understand
how to give the injection. This injection should not be mixed in the same syringe with any other
medicine.
You may receive either a pack containing a tray with everything needed for the injection, or a single
vial only. If you receive only the vial, you will need to obtain alcohol swabs and an injection syringe.
Step 1: Setting up for an injection
1. Select a flat, clean, well-lit working surface where you can lay out the contents of your Relistor
carton. Make sure you have set aside a proper amount of time to complete the injection.
2. Wash your hands thoroughly with soap and warm water.
3. Assemble the supplies you will need for your injection. These include the Relistor vial, a 1
ml injection syringe (with or without retractable needle), 2 alcohol swabs, and a cotton ball or
gauze.
4. Make sure the solution in the vial is clear and colourless to pale yellow, and does not contain
flakes or particles. If it is not, do not use the solution. Contact your pharmacist, nurse or doctor
for assistance.
Step 2: Preparing the injection syringe
1. Remove the protective plastic cap from the vial.
2. Wipe the vial’s rubber stopper with an alcohol swab and place it on your flat work surface.
Make sure not to touch the rubber stopper again.
3. Pick up the syringe from your work surface. Hold the barrel of the syringe with one hand and
pull the needle cover straight off. Place the needle cover back on the work surface. DO NOT
touch the needle or allow it to come into contact with any other surface.

Carefully pull back the plunger on the syringe to either the 0.4 ml mark for 8 mg of Relistor or
the 0.6 ml mark for 12 mg Relistor. Your healthcare professional will have advised you which
dose they have prescribed for you and how often you need to take it. The usual doses are given
in the table below. The dose is normally given every 48 hours (every two days) as an injection
under the skin.
Patient weight in kg
Fill syringe to ml level (dose)
Less than 38 kg 0.15 mg/kg
38-61 kg 0.4 ml (8mg)
62-114 kg 0.6 ml (12 mg)
More than 114 kg 0.15 mg/kg
4. Insert the needle straight down into the centre of the vial stopper. Do not insert it at an angle as
the needle may bend or break. Hold the vial on the work surface with the other hand so that it
can not slip off. You will feel a slight resistance as the needle passes through the stopper. Look
for the needle tip inside the vial.
5. In order to get the air out of the syringe, gently push the plunger down to inject the air into the
vial.



6. If you are using the supplied injection syringe with retractable injection needle, DO NOT
PUSH THE PLUNGER DOWN COMPLETELY. Make sure you stop pushing the plunger
when you feel resistance. If you push the plunger completely, you will hear a ‘click’ sound.
This will mean that the safety mechanism has been activated, and the needle will disappear into
the syringe. If this happens, discard the product and start again using another vial and syringe.
With the needle still in the vial, turn the vial upside-down. Hold the syringe at eye level so
that you can see the dosing marks and make sure the tip of the needle is in the fluid all of the
time. Slowly pull the plunger down to the 0.4 ml or 0.6 ml mark on the syringe or as advised,
depending on the dose prescribed by your healthcare professional. You may see some fluid or
bubbles inside the vial when the syringe is properly filled. This is normal.
7. With the needle still inserted in the upside down vial, check for air bubbles in the syringe.
Gently tap the syringe to make any air bubbles rise to the top of the syringe; be sure that
you still hold onto the vial and syringe. Slowly push the plunger up until all air bubbles are
removed. If you push solution back into the vial, slowly pull back the plunger to draw the
correct amount of solution back into the syringe. Due to the safety design of the syringe, a
small air bubble may be resistant to removal. There is no need to worry about this as it will not
affect the accuracy of the dose or pose any risk to your health.

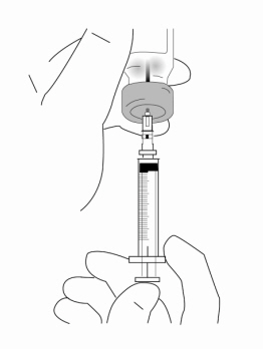
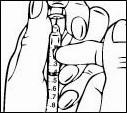
8. Always make sure you have the correct dose in the syringe. If unsure, please contact your
healthcare professional.
9. Remove the syringe and needle from the vial. Keep the needle attached to the syringe. Do not
touch the needle or allow the needle to touch any surface.
Step 3: Choosing and preparing an injection site


1. The three areas of the body recommended for injection of Relistor are: (1) your upper legs
(thighs), (2) your abdomen (stomach), and (3) your upper arm (only if injecting another
person).
2. It is recommended to move to a different site each time an injection is given. Avoid repeated
injections at the exact same spot previously used. Do not inject into areas where the skin is
tender, bruised, red, or hard. Avoid areas with scars or stretch marks.
3. To prepare the area of skin where Relistor is to be injected, wipe the injection site with an
alcohol swab. DO NOT TOUCH THIS AREA AGAIN BEFORE GIVING THE INJECTION.
Allow the injection site to air-dry before injecting.
Step 4a: Injecting Relistor using a pack containing injection syringe with retractable injection
needle


1. Holding the filled syringe with the needle pointing up, recheck the syringe for air bubbles. If
there are bubbles, gently tap the syringe with your finger until the air bubbles rise to the top of
the syringe. Slowly push the plunger up to force the air bubbles out of the syringe.
2. Hold the syringe in one hand like a pencil. Use the other hand to gently pinch the cleaned area
of skin and hold it firmly.
3. Push the full length of the needle into the skin at a slight angle (45 degrees) with a quick, short
motion.
4. After the needle is inserted, let go of the skin and slowly push the plunger all the way down
until the syringe is empty and you hear a click to inject Relistor.
5. When you hear a click sound that means the entire contents were injected. The needle will
automatically retract from the skin and be capped. There may be a little bleeding at the
injection site. You can press a cotton ball or gauze over the injection site. Do not rub the
injection site. If needed, you may cover the injection site with a plaster.
Step 4b: Injecting Relistor using a standard injection syringe and injection needle


1. Holding the filled syringe with the needle pointing up, recheck the syringe for air bubbles. If
there are bubbles, gently tap the syringe with your finger until the air bubbles rise to the top of
the syringe. Slowly push the plunger up to force the air bubbles out of the syringe.
2. Hold the syringe in one hand like a pencil. Use the other hand to gently pinch the cleaned area
of skin and hold it firmly.
3. Push the full length of the needle into the skin at a slight angle (45 degrees) with a quick, short
motion.
4. After the needle is inserted, let go of the skin and slowly push the plunger all the way down to
inject Relistor.
5. When the syringe is empty, quickly pull the needle out of the skin, being careful to keep it at
the same angle as inserted. There may be a little bleeding at the injection site. You can press
a cotton ball or gauze over the injection site. Do not rub the injection site. If needed, you may
cover the injection site with a plaster.
Step 5: Disposing of supplies
The capped syringe or syringe and needle should NEVER be reused. NEVER recap the needle.
Dispose of the capped syringe or needle and syringe in a closable puncture-resistant container as
instructed by your doctor, nurse or pharmacist.
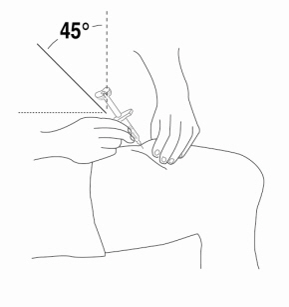
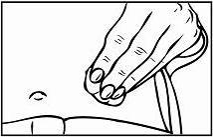
Frequently asked questions
Why have I been prescribed Relistor ?
Relistor treats constipation that is caused by medicines used to manage pain and other symptoms
called opioids (for example: morphine, codeine). Opioids are prescribed by your healthcare
professional. Relistor is used together with laxatives.
Relistor is for use in adults (aged 18 and over).
Relistor blocks the constipating effects of opioids in a different way to laxatives. It will not stop the
effect of your painkillers.
How should I use Relistor?
Relistor is administered by an injection just under the skin. Your healthcare professional will have
chosen the right dose for you.
Always use Relistor exactly as your healthcare professional has told you.
You should ask your healthcare professional if you are unsure about anything.
How quickly does it work?
Relistor may work within a few minutes to a few hours of the injection. Therefore, it is important to be
near toilet facilities soon after receiving your dose with assistance available if necessary.
What are the possible side-effects?
As with all medicines, some patients may experience side effects, although not everybody will get
them. The most common side effects (more than 1 in 10 patients) which have been reported are
stomach pain, feeling sick, wind, and diarrhoea.
Common side effects (more than 1 in 100 patients - but less than 1 in 10 patients) are dizziness,
sweating, and reactions at the site of injection.
Relistor may cause dizziness, and this may have an effect on driving and use of machines.
If any of these side effects gets serious or if you notice any side effect not listed here, please tell your
healthcare professional. If severe diarrhoea occurs during treatment, you should stop taking Relistor
and contact your healthcare professional immediately.
Will my painkillers stop working?
No, Relistor has been shown to have no effect on the painkilling effect of opioids.
Do I need to stop taking laxatives?
Do not stop taking laxatives unless your healthcare professional tells you to.
What if I am taking other medicines?
Please tell your healthcare professional if you are taking or have recently taken other medicines,
including medicines obtained without a prescription. You healthcare professional may allow you to
take other medicines, including those used for constipation.
How often do I need to take it?
You healthcare professional will have decided on how much you should take and how often you
should take it. Always use Relistor exactly as your healthcare professional has told you. You should
check with your healthcare professional if you are not sure.
This medicine should be stored in its carton to protect it from light. It should not be used after the
expiry date shown on the carton and vial. As with all medicines, it must be kept safely out of the
reach and sight of children. Only use it if the solution is clear, colourless to pale yellow, and does not
contain flakes or particles.
What do I need to do if my injection does not work?
Patients respond differently and some patients may not respond to every dose. It is important to
continue to keep your healthcare professional informed of your response.
There are some instances where Relistor should only be used with special care. For instance, if you
have severe liver or kidney disease, or if you have a colostomy, peritoneal catheter, diverticular
disease, or faecal impaction.
Ask your healthcare professional for advice before taking Relistor, or any medicine, if you are
pregnant, think you might be pregnant or you are breast feeding.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their
symptoms are the same as yours. If you have any further questions, ask your healthcare professional.
When should I not use Relistor?
If you are allergic (hypersensitive) to methylnaltrexone bromide or any of the other ingredients, or
if you or your healthcare professional know that your bowels are blocked by something other than
constipation.
What should I do if I use more than I should?
If you have administered more Relistor than you should have done, (either by injecting too much on
a single occasion or by using more than one injection in 24 hours), you must contact your healthcare
professional immediately and always have the outer carton with you even if is empty.
What should I do if I forget to take my Relistor?
If you forget a dose, do not worry. Talk to your healthcare professional.
How should I dispose of my injection supplies?
The vial and needle/syringe should not be re-used. Do not re-cap the needle. Once you have finished
with the needle and syringe, they should both be placed in a sealable, puncture-resistant container,
such as a dedicated ‘sharps bin’ (e.g. yellow biohazard container), hard plastic container (e.g.
detergent bottle) or, metal container (e.g. an empty drink can). Ask your healthcare professional for
instructions on how to properly dispose of the container after use if you are at all unsure.
What does my medicine look like?
It is a solution for injection. It is clear, colourless to pale yellow, and does not contain any flakes or
particles.
Each vial contains 0.6 ml of solution.
Cartons of more than one vial contain trays which have a 1 ml injection syringe with retractable needle
and two alcohol swabs.
What are the ingredients?
The active substance is methylnaltrexone bromide. Each vial of 0.6 ml contains 12 mg
methylnaltrexone bromide.
The other ingredients are sodium chloride, sodium calcium edetate, glycine hydrochloride, water for
injections, hydrochloric acid (to adjust pH) and sodium hydroxide (to adjust pH).
PACKAGE LEAFLET: INFORMATION FOR THE USER
Relistor 8 mg solution for injection in pre-filled syringe
Relistor 12 mg solution for injection in pre-filled syringe
Methylnaltrexone bromide
Read all of this leaflet carefully before you start using this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
In this leaflet:
1. What Relistor is and what it is used for
2. Before you use Relistor
3. How to use Relistor
4. Possible side effects
5. How to store Relistor
6. Further information
1. WHAT RELISTOR IS AND WHAT IT IS USED FOR
Relistor (methylnaltrexone bromide) acts by blocking the gastrointestinal effects of opioid pain
medicines.
Relistor treats constipation that is caused by medicines for moderate to severe pain called opioids
(for example morphine or codeine), in patients receiving supportive care for their advanced illness,
when other medicines for constipation, called laxatives, have not worked well enough. Opioids are
prescribed by your doctor. Relistor is given on top of your usual laxatives.
Relistor is for use in adults (aged 18 and over).
2. BEFORE YOU USE RELISTOR
Do not use Relistor
• If you are allergic (hypersensitive) to methylnaltrexone bromide or any of the other ingredients.
• If you or your doctor know that your bowels are obstructed or your bowels are in a state
where there is an immediate need for surgical intervention (which has to be diagnosed by your
doctor).
Take special care with Relistor
• If you have severe, persistent, and/or worsening abdominal symptoms contact your doctor
immediately because these could be symptoms of intestinal perforation.
• If you have severe liver or kidney disease.
• If you develop severe or persistent diarrhoea (passing of frequent watery stools), discontinue
therapy and contact your doctor immediately.
• It is important to be near a toilet with assistance available if necessary, since bowel movement
may happen within 30 minutes after injection of the medicine.
• Please talk to your doctor if you experience persistent stomach pain, nausea, (feeling sick in the
stomach) or vomiting that is new or worsened.
• Please also talk to your doctor if you have a colostomy, a tube in your abdomen (peritoneal
catheter), or already known disease called diverticular disease or faecal impaction.
• Relistor should only be used in patients who are receiving palliative care. It is added to usual
laxative therapy.
• Relistor should be used only for a limited period of time (it has not been studied for more than
4 months).
• Relistor should not be used for treatment of patients with constipation not related to opioid use.
If you have suffered from constipation before you had to take opioids (for pain), please talk to
your doctor.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
Your doctor may allow you to take other medicines, including those used for constipation.
Using Relistor with food and drink
Relistor can be taken with or without food.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking any medicine.
The effects of methylnaltrexone bromide in pregnant women are not known, and so the use of Relistor
during pregnancy is not recommended.
Women using Relistor should not breast-feed, since it is not known if methylnaltrexone bromide
passes into human breast milk.
Driving and using machines
Relistor may cause dizziness, and this may have an effect on driving and use of machines.
Important information about some of the ingredients of Relistor
This medicine contains less than 1 mmol sodium (23 mg) per dose i.e., essentially “sodium free.”
Always use Relistor exactly as your doctor has told you. You should check with your doctor or
pharmacist if you are not sure.
The usual dose is 8 mg methylnaltrexone bromide (0.4 ml Relistor) for patients weighing 38-61 kg or
12 mg (0.6 ml Relistor) for patients weighing 62-114 kg. The dose is given every 48 hours (every two
days) as an injection under the skin. Your doctor will determine your dose.
If you weigh less than 38 kg or more than 114 kg you should use Relistor vials because the correct
dose cannot be accurately delivered with these pre-filled syringes.
Relistor is given by an injection under the skin (by subcutaneous injection) in either (1) your upper
legs (thighs), (2) your abdomen (stomach), and (3) your upper arm (if not self-injecting). (See
INSTRUCTIONS FOR PREPARING AND GIVING AN INJECTION OF RELISTOR at the end of
this leaflet.)
You may have a bowel movement within a few minutes to a few hours of the injection; therefore, it is
recommended to have a toilet facility or bedpan near you.
If you use more Relistor than you should
If you have used more Relistor than you should (either by injecting too much on a single occasion or
by using more than one injection in 24 hours), talk to a doctor or pharmacist immediately. Always
have the outer carton of the medicine with you, even if it is empty.
If you forget to use Relistor
If you forget a dose, talk to your doctor or pharmacist as soon as possible.
If you stop using Relistor
If you stop using Relistor, talk to a doctor or pharmacist.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Like all medicines, Relistor can cause side effects, although not everybody gets them.
The most common side effects likely to occur in more than 1 in 10 patients:
• Abdominal pain (belly ache)
• Nausea (feeling sick in the stomach)
• Diarrhoea (passing of frequent watery stools)
• Flatulence (passing wind)
Common side effects reported in more than one in 100 patients, but in less than one in ten patients
receiving Relistor are:
• Dizziness (light-headed)
• Reaction at the site of injection (e.g., stinging, burning, pain, redness, oedema)
• Sweating
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Relistor after the expiry date which is stated on the carton, tray lid and syringe label.
Keep the pre-filled syringe in the outer carton in order to protect from light.
Only use Relistor if the solution is clear, colourless to pale yellow, and does not contain flakes or
particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
What Relistor contains
The active substance is methylnaltrexone bromide.
Each syringe of 0.4 ml contains 8 mg methylnaltrexone bromide.
Each syringe of 0.6 ml contains 12 mg methylnaltrexone bromide.
One ml of solution contains 20 mg methylnaltrexone bromide.
The other ingredients are sodium chloride, sodium calcium edetate, glycine hydrochloride, water for
injections, hydrochloric acid (to adjust pH) and sodium hydroxide (to adjust pH).
What Relistor looks like and contents of the pack
Relistor is a solution for injection. It is clear, colourless to pale yellow, and does not contain flakes or
particles.
The following packs are available:
Pack containing 4, 7, 8 or 10 pre-filled syringes with a needle shield.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Wyeth Europa Ltd.
Manufacturer
Wyeth Lederle S.p.A.
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
Luxembourg/Luxemburg
Pfizer S.A. / N.V.
Tél/Tel: +32 (0)2 554 62 11
Magyarország
Pfizer Kft.
Tel: +36 1 488 3700
България/Eesti/Latvija/Lietuva/
Slovenija
Wyeth Whitehall Export GmbH
Teл./Tel/Tãlr: +43 1 89 1140
Malta
Vivian Corporation Ltd.
Tel: +356 213 44616
Česká republika
Pfizer s.r.o.
Tel: +420-283-004-111
Nederland
Wyeth Pharmaceuticals B.V.
Tel: +31 23 567 2567
Danmark
Pfizer ApS
Tlf: +45 44 201 100
Norge
Pfizer AS
Tlf: +47 67 526 100
Deutschland
Pfizer Pharma GmbH
Tel: +49 (0)30 550055-51000
Österreich
Pfizer Corporation Austria Ges.m.b.H.
Tel: +43 (0)1 521 15-0
Ελλάδα
Pfizer Hellas A.E.
Τηλ.: +30 210 6785 800
Polska
Pfizer Polska Sp. z o.o.,
Tel.: +48 22 335 61 00
España
Pfizer, S.A.
Télf: +34 91 490 99 00
Portugal
Laboratórios Pfizer, Lda.
Tel: (+351) 21 423 55 00
France
Pfizer
Tél +33 1 58 07 30 00
România
Pfizer Romania S.R.L
Tel: +40 (0) 21 207 28 00
Ireland
Wyeth Pharmaceuticals
Tel:+353 1 449 3500
Slovenská republika
Pfizer Luxembourg SARL, organizačná
zložka
Tel: + 421 2 3355 5500
Ísland
Icepharma hf.
Tel:+354 540 8000
Suomi/Finland
Pfizer Oy
Puh/Tel: +358 (0)9 430 040
Italia
Wyeth Lederle S.p.A.
Tel:+39 06 927151
Sverige
Pfizer AB
Tel: +46 (0)8 550 520 00
Kύπρος
Wyeth Hellas (Cyprus Branch) AEBE
Τηλ:+357 22 817690
United Kingdom
Wyeth Pharmaceuticals
Tel: +44 845 3670098
This leaflet was last approved in {MM/YYYY}.
Detailed information on this medicine is available on the European Medicines Agency (EMEA) web






This section contains important questions that you will need to answer before you take Relistor, and
during treatment with Relistor.
If you answer No to any of the following questions during the course of your medication please
contact your doctor or health care professional.
1. Are you receiving opioid therapy for your illness?
2. Has it been 48 hours or longer since your last bowel movement?
3. Are you familiar with the technique of self injection or have you discussed this with your
doctor (or health care professional)?
4. Are you mobile enough to reach the toilet, or do you have a caregiver looking after you who
can help?
5. Do you have a contact number for your community nurse or the health centre?
INSTRUCTIONS FOR PREPARING AND GIVING AN INJECTION OF RELISTOR
This section is divided into the following subsections:
Step 1: Preparing for an injection
Step 2: Choosing and preparing an injection site
Step 3: Injecting Relistor pre-filled syringe
Step 4: Disposing of supplies
The following instructions explain how to prepare and give an injection of Relistor when using a pre-
filled syringe. Please read and follow them step by step. You will be instructed by your doctor, nurse
or pharmacist on the techniques of self-injection. Do not attempt to administer an injection until you
are sure that you understand how to prepare and give an injection.
•
Do not use a Relistor pre-filled syringe more than one time, even if there is medicine in
the syringe.
•
Safely throw away the Relistor pre-filled syringe after use (Step 4).
•
To avoid needle-stick injuries, do not recap used needles.
Gather the supplies you will need for your injection:
1. Relistor pre-filled syringe
2. Alcohol swab
3. Cotton ball or gauze
4. Adhesive plaster
Step 1: Preparing for an injection
1. Select a flat, clean, well-lit working surface where you can lay out the contents of your Relistor
carton. Make sure you have set aside a proper amount of time to complete the injection.
2. Wash your hands thoroughly with soap and warm water.
3. Look at the pre-filled syringe. Make sure that the dose prescribed by your doctor matches the
dose on the pre-filled syringe label.
4. Make sure the liquid in the pre-filled syringe is clear and colourless to pale yellow, and does
not have any particles in it. If not, do not use the pre-filled syringe and call your nurse, doctor
or pharmacist.
5. Firmly hold the barrel of the pre-filled syringe and pull the needle cap straight off.
Do not touch the needle or allow it to touch any surface.
Step 2: Choosing and preparing an injection site

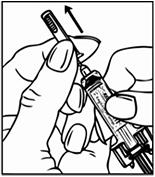
1. The three areas of the body recommended for injection of Relistor are: (1) your upper legs
(thighs), (2) your abdomen (stomach), and (3) your upper arm (only if injecting another
person).
2. It is recommended to move to a different site each time an injection is given. Avoid repeated
injections at the exact same spot previously used. Do not inject into areas where the skin is
tender, bruised, red, or hard. Avoid areas with scars or stretch marks.
3. Clean the injection site with an alcohol swab and let it dry. Do not touch this area again before
giving the injection.
Step 3: Injecting Relistor pre-filled syringe


1. Hold the syringe in one hand like a pencil. Use the other hand to gently pinch the cleaned area
of skin and hold it firmly.
2. Push the full length of the needle into the skin at a slight angle (45 degrees) with a quick, short
motion.
3. After the needle is inserted, let go of the skin and slowly push the plunger all the way down
until the pre-filled syringe is empty.
4. Quickly pull the needle out of the skin, being careful to keep it at the same angle as it was
inserted. Release your thumb from the plunger to allow the protective sleeve to cover the
needle. There may be a little bleeding at the injection site.
5. You can press a cotton ball or gauze over the injection site. Do not rub the injection site. If
needed, you may cover the injection site with a plaster.



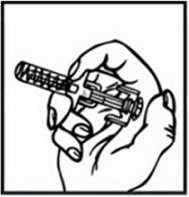
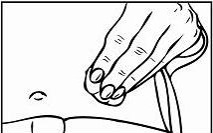
Step 4: Disposing of supplies
The pre-filled syringe should
NEVER
be reused.
NEVER
recap the needle. Dispose of the pre-filled
syringe as instructed by your doctor, nurse or pharmacist.
Place used pre-filled syringe in a closable, puncture-resistant container. You may use a sharps
container (such as a yellow biohazard container). Ask your doctor, nurse or pharmacist for instructions
on the right way to throw away (dispose of) the container. There may be local laws about how you
should throw away used needles and syringes.
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/relistor.html
Copyright © 1995-2021 ITA all rights reserved.
|





































































































































































































































































































































































































































































































































































































































































































































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































