Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
1. NAME OF THE MEDICINAL PRODUCT
Rotarix powder and solvent for
oral
suspension
Rotavirus vaccine, live
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
After reconstitution, 1 dose (1 ml) contains:
Human rotavirus RIX4414 strain (live, attenuated)*
not less than 10
6.0
CCID
50
Excipients:
This product contains sucrose 9 mg and sorbitol 13.5 mg (see section 4.4)
For a full list of excipients, see section 6.1.
Powder and solvent for
oral
suspension.
The powder is white.
The solvent is a turbid liquid with a slow settling white deposit and a colourless supernatant.
4.1 Therapeutic indications
Rotarix is indicated for the active immunisation of infants aged 6 to 24 weeks for prevention of gastro-
enteritis due to rotavirus infection (see section 4.2).
In clinical trials, efficacy was demonstrated against gastro-enteritis due to rotavirus of types G1P[8],
G2P[4], G3P[8], G4P[8] and G9P[8] (see sections 4.4 and 5.1).
The use of Rotarix should be based on official recommendations.
4.2 Posology and method of administration
The vaccination course consists of two doses. The first dose may be administered from the age of 6
weeks. There should be an interval of at least 4 weeks between doses. The vaccination course should
preferably be given before 16 weeks of age, but must be completed by the age of 24 weeks.
Rotarix may be given with the same posology to preterm infants born after at least 27 weeks of
gestational age (see sections 4.8 and 5.1).
In clinical trials, spitting or regurgitation of the vaccine has rarely been observed and, under such
circumstances, a replacement dose was not given. However, in the unlikely event that an infant spits
out or regurgitates most of the vaccine dose, a single replacement dose may be given at the same
vaccination visit.
It is recommended that infants who receive a first dose of Rotarix complete the 2-dose regimen with
Rotarix. There are no data on safety, immunogenicity or efficacy when Rotarix is administered for the
first dose and another rotavirus vaccine is administered for the second dose or vice versa.
Rotarix should not be used in children over 24 weeks of age.
Rotarix is for
oral
use only.
Rotarix should under no circumstances be injected.
Hypersensitivity to the active substance or to any of the excipients.
Hypersensitivity after previous administration of rotavirus vaccines.
Previous history of intussusception.
Subjects with uncorrected congenital malformation of the gastrointestinal tract that would predispose
for intussusception.
Subjects with Severe Combined Immunodeficiency (SCID) disorder (see section 4.8).
Administration of Rotarix should be postponed in subjects suffering from acute severe febrile
illness. The presence of a minor infection is not a contra-indication for immunisation.
The administration of Rotarix should be postponed in subjects suffering from diarrhoea or vomiting.
4.4 Special warnings and precautions for use
It is good clinical practice that vaccination should be preceded by a review of the medical history
especially with regard to the contraindications and by a clinical examination.
There are no data on the safety and efficacy of Rotarix in infants with gastrointestinal illnesses or
growth retardation. Administration of Rotarix may be considered with caution in such infants when, in
the opinion of the physician, withholding the vaccine entails a greater risk.
Although no causal relationship has been established between vaccination with Rotarix and
intussusception (see section 4.8), as a precaution, healthcare professionals should follow-up on any
symptoms indicative of intussusception (severe abdominal pain, persistent vomiting, bloody stools,
abdominal bloating and/or high fever). Parents/guardians should be advised to promptly report such
symptoms.
Asymptomatic and mildly symptomatic HIV infections are not exp ected to affect the safety or efficacy
of Rotarix. A clinical study in a limited number of asymptomatic or mildly symptomatic HIV positive
infants showed no apparent safety problems (see section 4.8).
Administration of Rotarix to infants who have known or suspected immunodeficiency should be based
on careful consideration of potential benefits and risks.
Excretion of the vaccine virus in the stools is known to occur after vaccination with peak excretion
around the 7th day. Viral antigen particles detected by ELISA were found in 50% of stools after the
first dose and 4% of stools after the second dose. When these stools were tested for the presence of
live vaccine strain, only 17% were positive.
Cases of transmission of this excreted vaccine virus to seronegative contacts of vaccinees have been
observed without causing any clinical symptom.
Rotarix should be administered with caution to individuals with immunodeficient close contacts,
such as individuals with malignancies, or who are otherwise immunocompromised or individuals
receiving immunosuppressive therapy.
Contacts of recent vaccinees should observe personal hygi ene (e.g. wash their hands after changing
child’s nappies).
The potential risk of apnoea and the need for respiratory monitoring for 48-72h should be considered
when administering the primary immunisation series to very premature infants (born≤ 28 weeks of
gestation) and particularly for those with a previous history of respiratory immaturity.
As the benefit of the vaccination is high in this group of infants, vaccination should not be withheld or
delayed.
A protective immune response may not be elicited in all vaccinees (see section 5.1).
In clinical trials, efficacy was demonstrated against gastro-enteritis due to rotavirus of types G1P[8],
G2P[4], G3P[8], G4P[8] and G9P[8]. The ext ent of protection that Rotarix might provide against other
serotypes is unknown. Clinical studies from which efficacy data were derived were conducted in
Europe and Central and South America (see section 5.1).
Rotarix does not protect against gastro-enteritis due to other pathogens than rotavirus.
No data are available on the use of Rotarix for post-exposure prophylaxis.
Rotarix should under no circumstances be injected.
The vaccine contains sucrose and sorbitol as excipients. Patients with rare hereditary problems of
fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency should not
take this vaccine.
4.5 Interaction with other medicinal products and other forms of interaction
Rotarix can be given concomitantly with any of the following monovalent or combination vaccines
[including hexavalent vaccines (DTPa-HBV-IPV/Hib)]: diphtheria-tetanus-whole cell pertussis
vaccine (DTPw), diphtheria-tetanus-acellular pertussis vaccine (DTPa),
Haemophilus influenzae
type b vaccine (Hib), inactivated polio vaccine (IPV), hepatitis B vaccine (HBV), pneumococcal
conjugate vaccine and meningococcal serogroup C conjugate vaccine. Clinical studies demonstrated
that the immune responses and the safety profiles of the administered vaccines were unaffected.
Concomitant administration of Rotarix and oral polio vaccine (OPV) does not affect the immune
response to the polio antigens. Although concomitant administration of OPV may slightly reduce the
immune response to rotavirus vaccine, clinical protection against severe rotavirus gastro-enteritis was
shown to be maintained in a clinical trial involving more than 4200 subjects who received Rotarix
concomitantly with OPV.
There are no restrictions on the infant’s consumption of food or liquid, either before or after
vaccination.
4.6 Fertility, pregnancy and lactation
Rotarix is not intended for use in adults. There are no data on the use of Rotarix during pregnancy and
lactation.
Based on evidence generated in clinical trials, breast-feeding does not reduce the protection against
rotavirus gastro-enteritis afforded by Rotarix. Therefore, breast-feeding may be continued during the
vaccination schedule.
4.7 Effects on ability to drive and use machines
The safety profile presented below is based on data from clinical trials conducted with either the
lyophilised or the liquid formulation of Rotarix.
In a total of four clinical trials, approximately 3800 doses of Rotarix liquid formulation were
administered to approximately 1900 infants. Those trials have shown that the safety profile of the
liquid formulation is comparable to the lyophilised formulation.
In a total of twenty-three clinical trials, approximately 106000 doses of Rotarix (lyophilised or liquid
formulation)
were administered to approximately 51000 infants.
In three placebo-controlled clinical trials (Finland, India and Bangladesh), in which Rotarix was
administered alone (administration of routine paediatric vaccines was staggered), the incidence and
severity of the solicited events (collected 8 days post-vaccination), diarrhoea, vomiting, loss of
appetite, fever, irritability and cough/runny nose were not significantly different in the group receiving
Rotarix when compared to the group receiving placebo. No increase in the incidence or severity of
these events was seen with the second dose.
In a pooled analysis from seventeen placebo-controlled clinical trials (Europe, North America, Latin
America, Asia, Africa) including trials in which Rotarix was co-administered with routine paediatric
vaccines (see section 4.5), the following adverse reactions (collected 31 days post-vaccination) were
considered as possibly related to vaccination.
Adverse reactions are listed below per system organ class and frequency.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Frequencies are defined as:
Very common (≥1/10)
Common (≥1/100, <1/10)
Uncommon (≥1/1,000, <1/100)
Rare (≥1/10,000, <1/1,000)
Gastrointestinal disorders
Uncommon
: abdominal pain, flatulence
Skin and subcutaneous tissue disorders
General disorders and administration site conditions
The risk of intussusception has been evaluated in a large safety trial conducted in Latin America and
Finland where 63225 infants were enrolled. This trial gave evidence of no increased risk of
intussusception in the Rotarix group when compared with the placebo group as shown in the table
below.
Intussusception within 31 days
after administration of:
Safety in preterm infants
In a clinical study, 670 pre-term infants from 27 to 36 weeks of gestational age were administered
Rotarix and 339 received placebo. The first dose was administered from 6 weeks after birth. Serious
adverse events were observed in 5.1% of recipients of Rotarix as compared with 6.8% of placebo
recipients. Similar rates of other adverse events were observed in Rotarix and placebo recipients. No
cases of intussuscept ion were reported.
Safety in infants with human immunodeficiency (HIV) infection
In a clinical study, 100 infants with HIV infection were administered Rotarix or placebo. The safety
profile was similar between Rotarix and placebo recipients.
Post marketing surveillance:
Becaus e these events were reported spontaneous ly, it is not possible to reliably estimate their
frequency.
Respiratory, thoracic and mediastinal disorders:
Apnoea in very premature infants (≤ 28 weeks of gestation) (see section 4.4)
Gastrointestinal disorders:
Gastroenteritis with vaccine viral shedding in infants with Severe Combined Immunodeficiency
(SCID) disorder
In post-marketing experience, cases of intussusception have been reported in temporal association
with Rotarix. Most cases were reported within seven days following the first dose (see section 4.4).
No case of overdose has been reported.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmaco-therapeutic group: rotavirus diarrhoea vaccines, ATC code: J07BH01
Clinical studies have been conducted in Europe and Latin America to evaluate the protective efficacy
of Rotarix against any and severe rotavirus gastro-enteritis.
A clinical study performed in Europe evaluated Rotarix given according to different European
schedules (2, 3 months; 2, 4 months; 3, 4 months; 3, 5 months) in 4000 subjects. Severity of gastro-
enteritis was defined according to the Vesikari 20-point scale which evaluates the full clinical picture








of rotavirus gastro-enteritis by taking into account the severity and duration of diarrhoea and vomiting,
the severity of fever and dehydration as well as the need for treatment.
After two doses of Rotarix, the protective vaccine efficacy observed during the first and second year
of life is presented in the following table:
2
nd
year of life
Rotarix N=2554;
Placebo N=1294 (§)
Vaccine efficacy (%) against any and severe rotavirus gastro-enteritis
[95% CI]
1
st
year of life
Rotarix N=2572;
Placebo N=1302 (§)
Strains with P[8]
genotype
Circulating
rotavirus strains
85.6*
[75.8;91.9]
Vaccine efficacy (%) against rotavirus gastro-enteritis requiring medical
attention
[95% CI]
76.2*
[63.0;85.0]
Vaccine efficacy (%) against hospitalisation due to rotavirus gastro-enteritis
[95% CI]
92.2*
[65.6;99.1]
†
Severe gastro-enteritis defined as a score ≥11 on the Vesikari scale
(§) ATP cohort for efficacy
* Statistically significant (p < 0.05)
Vaccine efficacy during the first year of life progressively increased with increasing disease severity,
reaching 100% (95% CI: 84.7;100) for Vesikari scores ≥17.
A clinical study performed in Latin America evaluated Rotarix in more than 20000 subjects. Severity
of gastro-enteritis was defined according to WHO criteria. The protective vaccine efficacy against
severe rotavirus gastro-enteritis requiring hospitalisation and/or rehydration therapy in a medical
facility and the type specific vaccine efficacy after two doses of Rotarix are presented in the table
below:
Severe rotavirus gastro-
enteritis (1
st
year of life)
Rotarix N=9009;
Placebo N=8858
(§)
Severe rotavirus gastro-
enteritis (2
nd
year of life)
Rotar ix N=7175;
Placebo N=7062 (§)
Circulating
rotavirus strains
Circulating
rotavirus strains



























Strains with P[8]
genotype
(§)ATP cohort for efficacy
* Statistically significant (p < 0.05)
# The numbers of cases, on which the estimates of efficacy against G4P[8] were based, were very
small (1 case in the Rotarix group and 2 cases in the placebo group).
A pool ed analysis of five efficacy studies*, showed a 71.4% (95% CI:20.1;91.1) efficacy against
severe rotavirus gastro-enteritis (Vesikari score ≥11) caused by rotavirus G2P[4] type during the first
year of life.
* In these studi es, the poi nt estimates and confidence intervals were respectively: 100% (95% CI: -
1858.0;100), 100% (95% CI: 21.1;100), 45.4% (95% CI: -81.5;86.6), 74.7 (95% CI :-386.2;99.6). No
point estimate was available for the remaining study.
The immunologic mechanism by which Rotarix protects against rotavirus gastro-enteritis is not
completely understood. A relationship between antibody responses to rotavirus vaccination and
protection against rotavirus gastro-enteritis has not been established.
The following table shows the percentage of subjects with serum anti-rotavirus IgA antibody titers ≥
20U/ml (by ELISA) one to two months after the second dose of vaccine or placebo as observed in
different studies.
2, 3 months
France,
Germany
3, 5 months
Finland, Italy 180
3, 4 months
Czech
Republic
Latin
America; 11
countries
Immune response in preterm infants
In a clinical study conducted in preterm infants, born after at least 27 weeks of gestational age, the
immunogenicity of Rotarix was assessed in a subset of 147 subjects and showed that Rotarix is
immunogenic in this population; 85.7% (95% CI: 79.0;90.9) of subjects achieved serum anti-rotavirus
IgA antibody titers ≥ 20U/ml (by ELISA) one month after the second dose of vaccine.

























5.2 Pharmacokinetic properties
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of repeated dose
toxicity.
6. PHARMACEUTICAL PARTICULARS
Dextran
Sorbitol
Amino acids
Dulbecco’s Modified Eagle Medium (DMEM)
Xanthan gum
Sterile water
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
After reconstitution
After the reconstitution, the vaccine should be administered immediately. If not used immediately,
in-use storage should not be longer than 24 hours and at a temperature between 2°C-25°C.
6.4 Special precautions for storage
Store in a refrigerator (2°C – 8°C).
Do not freeze.
Store in the original package, in order to protect from light.
For storage conditions of the reconstituted product, see section 6.3.
6.5 Nature and contents of container
1 dose of powder in a glass container (type I glass) with a stopper (rubber butyl)
1 ml of solvent in an
oral
applicator (type I glass) with a plunger stopper and a protective tip cap
(rubber butyl).
Transfer adapter for reconstitution (1/dose)
in the following pack sizes:
-
pack size of 1 glass container of powder plus 1
oral
applicator of solvent
-
pack size of 5 glass containers of powder plus 5
oral
applicators of solvent
-
pack size of 10 glass containers of powder plus 10
oral
applicators of solvent
-
pack size of 25 glass containers of powder plus 25
oral
applicators of solvent
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
Before reconstitution:
A white deposit and clear supernatant is observed upon storage of the
oral
applicator containing the
solvent.
The solvent should be inspected visually for any foreign particulate matter and/or abnormal physical
appearance prior to reconstitution.
After reconstitution:
The reconstituted vaccine is slightly more turbid than the solvent and is milky white in appearance.
The reconstituted vaccine should also be inspected visually for any foreign particulate matter and/or
abnormal physical appearance prior to administration. In the event of either being observed, discard
the vaccine.
Any unused vaccine or waste material should be disposed of in accordance with local requirements.
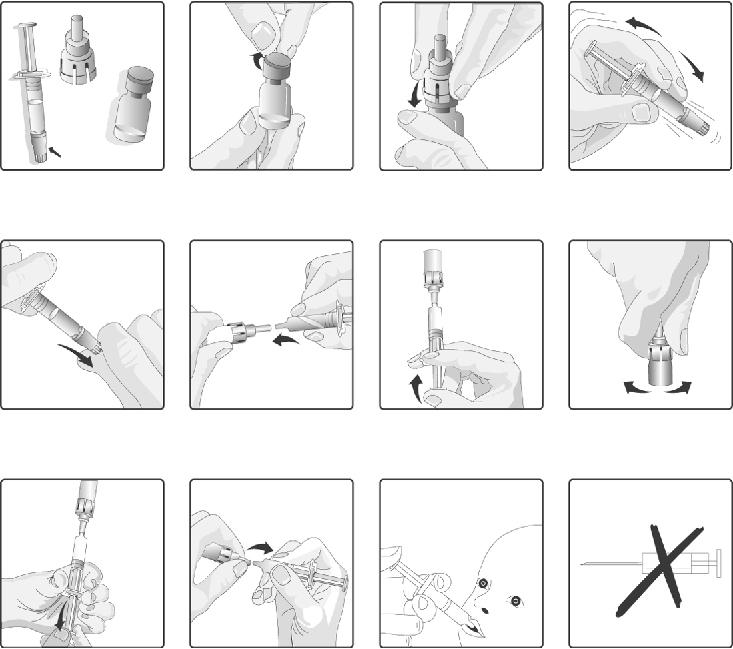
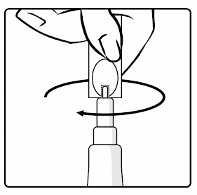
Instructions for reconstitution and administration of the vaccine
1.
Remove the plastic cover from the glass container containing the powder.
2.
Connect the transfer adapter onto the glass container by pushing it downwards until the transfer
adapter is properly and securely placed.
3.
Shake the
oral
applicator containing the solvent vigorously. The shaken suspension will appear as
a turbid liquid with a slow settling white deposit.
4.
Remove the protective tip cap from the
oral
applicator.
5.
Connect the
oral
applicator into the transfer adapter by pushing it firmly on this device.
6.
Transfer the entire content of the
oral
applicator into the glass container containing the powder.
7.
With the
oral
applicator still attached, shake the glass container and examine it for complete
suspension of the powder. The reconstituted vaccine will appear more turbid than the solvent
alone. This appearance is normal.
8.
Withdraw the entire mixture back into the
oral
applicator.
9.
Remove the
oral
applicator from the transfer adapter.
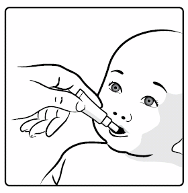
10.
This vaccine is for
oral administration only
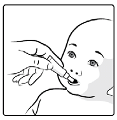
. The child should be seated in a reclining position.
Administer the entire content of the
oral
applicator
orally
(by administering the entire content of
the
oral
applicator on the inside of the cheek).
11.
Do not inject
.
If the reconstituted vaccine is to be stored temporarily before administration, replace the protective tip
cap on the
oral
applicator. The
oral
applicator containing the reconstituted vaccine should be shaken
gently again before
oral
administration.
1. Remove the plastic cover from the
glass container containing the powder
2. Connect the transfer adapter onto
the glass container by pushing it
downwards until the transfer adapter
is properly and securely placed
3. Shake the
oral
applicator
containing the solvent vigorously. The
shaken suspension will appear as a
turbid liquid with a slow settling white
deposit
4. Remove the protective tip cap from
the
oral
applicator
5. Connect the
oral
applicator into the
transfer adapter by pushing it firmly
on this device
6. Transfer the entire content of the
oral
applicator into the glass
container containing the powder
7. With the
oral
applicator still attached,
shake the glass container and examine it
for complete suspension of the powder.
The reconstituted vaccine will appear more
turbid than the solvent alone. This
appearance is normal
8. Withdraw the entire mixture back
into the
oral
applicator
9. Remove the
oral
applicator from
the transfer adapter
10. This vaccine is for
oral
administration only
. The child
should be seated in a reclining
position. Administer the entire content
of the
oral
applicator
orally
(by
administering the entire content of the
oral
applicator on the inside of the
cheek)





MARKETING AUTHORISATION HOLDER
GlaxoSmithKline Biologicals s.a.
Rue de l'Institut 89
B-1330 Rixensart, Belgium
8. MARKETING AUTHORISATION NUMBER(S)
EU/1/05/330/001
EU/1/05/330/002
EU/1/05/330/003
EU/1/05/330/004
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 21 February 2006
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
1. NAME OF THE MEDICINAL PRODUCT
Rotarix
oral
suspension in pre-filled
oral
applicator
Rotavirus vaccine, live
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
1 dose (1.5 ml) contains:
Human rotavirus RIX4414 strain (live, attenuated)*
not less than 10
6.0
CCID
50
Excipient:
This product contains sucrose 1073 mg (see section 4.4).
For a full list of excipients, see section 6.1.
Oral
suspension.
Rotarix is a clear and colourless liquid.
4.1 Therapeutic indications
Rotarix is indicated for the active immunisation of infants aged 6 to 24 weeks for prevention of gastro-
enteritis due to rotavirus infection (see section 4.2).
In clinical trials, efficacy was demonstrated against gastro-enteritis due to rotavirus of types G1P[8],
G2P[4], G3P[8], G4P[8] and G9P[8] (see sections 4.4 and 5.1).
The use of Rotarix should be based on official recommendations.
4.2 Posology and method of administration
The vaccination course consists of two doses. The first dose may be administered from the age of 6
weeks. There should be an interval of at least 4 weeks between doses. The vaccination course should
preferably be given before 16 weeks of age, but must be completed by the age of 24 weeks.
Rotarix may be given with the same posology to preterm infants born after at least 27 weeks of
gestational age (see sections 4.8 and 5.1).
In clinical trials, spitting or regurgitation of the vaccine has rarely been observed and, under such
circumstances, a replacement dose was not given. However, in the unlikely event that an infant spits
out or regurgitates most of the vaccine dose, a single replacement dose may be given at the same
vaccination visit.
It is recommended that infants who receive a first dose of Rotarix complete the 2-dose regimen with
Rotarix. There are no data on safety, immunogenicity or efficacy when Rotarix is administered for the
first dose and another rotavirus vaccine is administered for the second dose or vice versa.
Rotarix should not be used in children over 24 weeks of age.
Rotarix is for
oral
use only.
Rotarix should under no circumstances be injected.
Hypersensitivity to the active substance or to any of the excipients.
Hypersensitivity after previous administration of rotavirus vaccines.
Previous history of intussusception.
Subjects with uncorrected congenital malformation of the gastrointestinal tract that would predispose
for intussusception.
Subjects with Severe Combined Immunodeficiency (SCID) disorder (see section 4.8).
Administration of Rotarix should be postponed in subjects suffering from acute severe febrile
illness. The presence of a minor infection is not a contra-indication for immunisation.
The administration of Rotarix should be postponed in subjects suffering from diarrhoea or vomiting.
4.4 Special warnings and precautions for use
It is good clinical practice that vaccination should be preceded by a review of the medical history
especially with regard to the contraindications and by a clinical examination.
There are no data on the safety and efficacy of Rotarix in infants with gastrointestinal illnesses or
growth retardation. Administration of Rotarix may be considered with caution in such infants when, in
the opinion of the physician, withholding the vaccine entails a greater risk.
Although no causal relationship has been established between vaccination with Rotarix and
intussusception (see section 4.8), as a precaution, healthcare professionals should follow-up on any
symptoms indicative of intussusception (severe abdominal pain, persistent vomiting, bloody stools,
abdominal bloating and/or high fever). Parents/guardians should be advised to promptly report such
symptoms.
Asymptomatic and mildly symptomatic HIV infections are not expected to affect the safety or efficacy
of Rotarix. A clinical study in a limited number of asymptomatic or mildly symptomatic HIV positive
infants showed no apparent safety problems (see section 4.8).
Administration of Rotarix to infants who have known or suspected immunodeficiency should be based
on careful consideration of potential benefits and risks.
Excretion of the vaccine virus in the stools is known to occur after vaccination with peak excretion
around the 7th day. Viral antigen particles detected by ELISA were found in 50% of stools after the
first dose of Rotarix lyophilised formulation and 4% of stools after the second dose. When these stools
were tested for the presence of live vaccine strain, only 17% were positive. In two comparative
controlled trials, vaccine shedding after vaccination with Rotarix liquid formulation was comparable
to that observed after vaccination with Rotarix lyophilised formulation.
Cases of transmission of this excreted vaccine virus to seronegative contacts of vaccinees have been
observed without causing any clinical symptom.
Rotarix should be administered with caution to individuals with immunodeficient close contacts,
such as individuals with malignancies, or who are otherwise immunocompromised or individuals
receiving immunosuppressive therapy.
Contacts of recent vaccinees should observe personal hygi ene (e.g. wash their hands after changing
child’s nappies).
The potential risk of apnoea and the need for respiratory monitoring for 48-72h should be considered
when administering the primary immunisation series to very premature infants (born ≤ 28 weeks of
gestation) and particularly for those with a previous history of respiratory immaturity.
As the benefit of the vaccination is high in this group of infants, vaccination should not be withheld or
delayed.
A protective immune response may not be elicited in all vaccinees (see section 5.1).
In clinical trials, efficacy was demonstrated against gastro-enteritis due to rotavirus of types G1P[8],
G2P[4], G3P[8], G4P[8] and G9P[8]. The extent of protection that Rotarix might provide against other
serotypes is unknown. Clinical studies from which efficacy data were derived were conducted in
Europe and Central and South America (see section 5.1).
Rotarix does not protect against gastro-enteritis due to other pathogens than rotavirus.
No data are available on the use of Rotarix for post-exposure prophylaxis.
Rotarix should under no circumstances be injected.
The vaccine contains sucrose as an excipient. Patients with rare hereditary problems of fructose
intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency should not take this
vaccine.
4.5 Interaction with other medicinal products and other forms of interaction
Rotarix can be given concomitantly with any of the following monovalent or combination vaccines
[including hexavalent vaccines (DTPa-HBV-IPV/Hib)]: diphtheria-tetanus-whole cell pertussis
vaccine (DTPw), diphtheria-tetanus-acellular pertussis vaccine (DTPa),
Haemophilus influenzae
type b vaccine (Hib), inactivated polio vaccine (IPV), hepatitis B vaccine (HBV), pneumococcal
conjugate vaccine and meningococcal serogroup C conjugate vaccine. Clinical studies demonstrated
that the immune responses and the safety profiles of the administered vaccines were unaffected.
Concomitant administration of Rotarix and oral polio vaccine (OPV) does not affect the immune
response to the polio antigens. Although concomitant administration of OPV may slightly reduce the
immune response to rotavirus vaccine, clinical protection against severe rotavirus gastro-enteritis was
shown to be maintained in a clinical trial involving more than 4200 subjects who received Rotarix
concomitantly with OPV.
There are no restrictions on the infant’s consumption of food or liquid, either before or after
vaccination.
4.6 Fertility, pregnancy and lactation
Rotarix is not intended for use in adults. There are no data on the us e of Rotarix during pregnancy and
lactation.
Based on evidence generated in clinical trials, breast-feeding does not reduce the protection against
rotavirus gastro-enteritis afforded by Rotarix. Therefore, breast-feeding may be continued during the
vaccination schedule.
4.7 Effects on ability to drive and use machines
The safety profile presented below is based on data from clinical trials conducted with either the
lyophilised or the liquid formulation of Rotarix.
In a total of four clinical trials, approximately 3800 doses of Rotarix liquid formulation were
administered to approximately 1900 infants. Those trials have shown that the safety profile of the
liquid formulation is comparable to the lyophilised formulation.
In a total of twenty-three clinical trials, approximately 106000 doses of Rotarix (lyophilised or liquid
formulation)
were administered to approximately 51000 infants.
In three placebo-controlled clinical trials (Finland, India and Bangladesh), in which Rotarix was
administered alone (administration of routine paediatric vaccines was staggered), the incidence and
severity of the solicited events (collected 8 days post-vaccination), diarrhoea, vomiting, loss of
appetite, fever, irritability and cough/runny nose were not significantly different in the group receiving
Rotarix when compared to the group receiving placebo. No increase in the incidence or severity of
these events was seen with the second dose.
In a pooled analysis from seventeen placebo-controlled clinical trials (Europe, North America, Latin
America, Asia, Africa) including trials in which Rotarix was co-administered with routine paediatric
vaccines (see section 4.5), the following adverse reactions (collected 31 days post-vaccination) were
considered as possibly related to vaccination.
Adverse reactions are listed below per system organ class and frequency.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Frequencies are defined as:
Very common (≥1/10)
Common (≥1/100, <1/10)
Uncommon (≥1/1,000, <1/100)
Rare (≥1/10,000, <1/1,000)
Gastrointestinal disorders
Uncommon
: abdominal pain, flatulence
Skin and subcutaneous tissue disorders
General disorders and administration site conditions
The risk of intussusception has been evaluated in a large safety trial conducted in Latin America and
Finland where 63225 infants were enrolled. This trial gave evidence of no increased risk of
intussusception in the Rotarix group when compared with the placebo group as shown in the table
below.
Intussusception within 31 days
after administration of:
Safety in preterm infants
In a clinical study, 670 pre-term infants from 27 to 36 weeks of gestational age were administered
Rotarix lyophilised formulation and 339 received placebo. The first dose was administered from 6
weeks after birth. Serious adverse events were observed in 5.1% of recipients of Rotarix as compared
with 6.8% of placebo recipients. Similar rates of other adverse events were observed in Rotarix and
placebo recipients. No cases of intussusception were reported.
Safety in infants with human immunodeficiency (HIV) infection
In a clinical study, 100 infants with HIV infection were administered Rotarix lyophilised formulation
or placebo. The safety profile was similar between Rotarix and placebo recipients.
Post marketing surveillance:
Because these events were reported spontaneously, it is not possible to reliably estimate their
frequency.
Respiratory, thoracic and mediastinal disorders:
Apnoea in very premature infants (≤ 28 weeks of gestation) (see section 4.4)
Gastrointestinal disorders:
Gastroenteritis with vaccine viral shedding in infants with Severe Combined Immunodeficiency
(SCID) disorder
In post-marketing experience, cases of intussusception have been reported in temporal association
with Rotarix. Most cases were reported within seven days following the first dose (see section 4.4).
No case of overdose has been reported.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmaco-therapeutic group: rotavirus diarrhoea vaccines, ATC code: J07BH01
Protective efficacy of the lyophilised formulation
Clinical studies have been conducted in Europe and Latin America to evaluate the protective efficacy
of Rotarix against any and severe rotavirus gastro-enteritis.
A clinical study performed in Europe evaluated Rotarix given according to different European
schedules (2, 3 months; 2, 4 months; 3, 4 months; 3, 5 months) in 4000 subjects. Severity of gastro-
enteritis was defined according to the Vesikari 20-point scale which evaluates the full clinical picture
of rotavirus gastro-enteritis by taking into account the severity and duration of diarrhoea and vomiting,
the severity of fever and dehydration as well as the need for treatment.








After two doses of Rotarix, the protective vaccine efficacy observed during the first and second year
of life is presented in the following table:
2
nd
year of life
Rotarix N=2554;
Placebo N=1294 (§)
Vaccine efficacy (%) against any and severe rotavirus gastro-enteritis
[95% CI]
1
st
year of life
Rotarix N=2572;
Placebo N=1302 (§)
Strains with P[8]
genotype
Circulating
rotavirus strains
85.6*
[75.8;91.9]
Vaccine efficacy (%) against rotavirus gastro-enteritis requiring medical
attention
[95% CI]
76.2*
[63.0;85.0]
Vaccine efficacy (%) against hospitalisation due to rotavirus gastro-enteritis
[95% CI]
92.2*
[65.6;99.1]
†
Severe gastro-enteritis defined as a score ≥11 on the Vesikari scale
(§) ATP cohort for efficacy
* Statistically significant (p < 0.05)
Vaccine efficacy during the first year of life progressively increased with increasing disease severity,
reaching 100% (95% CI: 84.7;100) for Vesikari scores ≥17.
A clinical study performed in Latin America evaluated Rotarix in more than 20000 subjects. Severity
of gastro-enteritis was defined according to WHO criteria. The protective vaccine efficacy against
severe rotavirus gastro-enteritis requiring hospitalisation and/or rehydration therapy in a medical
facility and the type specific vaccine efficacy after two doses of Rotarix are presented in the table
below:
Severe rotavirus gastro-
enteritis (1
st
year of life)
Rotarix N=9009;
Placebo N=8858
(§)
Severe rotavirus gastro-
enteritis (2
nd
year of life)
Rotar ix N=7175;
Placebo N=7062 (§)
Circulating
rotavirus strains
Circulating
rotavirus strains




























Strains with P[8]
genotype
(§)ATP cohort for efficacy
* Statistically significant (p < 0.05)
# The numbers of cases, on which the estimates of efficacy against G4P[8] were based, were very
small (1 case in the Rotarix group and 2 cases in the placebo group).
A pool ed analysis of five efficacy studies*, showed a 71.4% (95% CI:20.1;91.1) efficacy against
severe rotavirus gastro-enteritis (Vesikari score ≥11) caused by rotavirus G2P[4] type during the first
year of life.
* In these studies, the point estimates and confidence intervals were respectively: 100% (95% CI: -
1858.0;100), 100% (95% CI: 21.1;100), 45.4% (95% CI: -81.5;86.6), 74.7 (95% CI :-386.2;99.6). No
point estimate was available for the remaining study.
Protective efficacy of the liquid formulation:
Since the immune response observed after 2 doses of Rotarix liquid formulation was comparable to
the immune response observed after 2 doses of Rotarix lyophilised formulation, the levels of vaccine
efficacy observed with the lyophilised formulation can be extrapolated to the liquid formulation.
The immunologic mechanism by which Rotarix protects against rotavirus gastro-enteritis is not
completely understood. A relationship between antibody responses to rotavirus vaccination and
protection against rotavirus gastro-enteritis has not been established.
The following table shows the percentage of subjects with serum anti-rotavirus IgA antibody titers
≥20U/ml (by ELISA) one to two months after the second dose of vaccine or placebo as observed in
different studies with Rotarix lyophilised formulation.
2, 3 months
France,
Germany
3, 5 months
Finland, Italy 180
3, 4 months
Czech
Republic
Latin
America; 11
countries
In three comparative controlled trials, the immune response elicited by Rotarix liquid formulation was
comparable to the one elicited by Rotarix lyophilised formulation.

























Immune response in preterm infants
In a clinical study conducted in preterm infants, born after at least 27 weeks of gestational age, the
immunogenicity of Rotarix was assessed in a subset of 147 subjects and showed that Rotarix is
immunogenic in this population; 85.7% (95% CI: 79.0;90.9) of subjects achieved serum anti-rotavirus
IgA antibody titers ≥ 20U/ml (by ELISA) one month after the second dose of vaccine.
5.2 Pharmacokinetic properties
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of repeated dose
toxicity.
6. PHARMACEUTICAL PARTICULARS
Sucrose
Di-sodium Adipate
Dulbecco’s Modified Eagle Medium (DMEM)
Sterile water
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
3 years.
The vaccine should be used immediately after opening.
6.4 Special precautions for storage
Store in a refrigerator (2°C – 8°C).
Do not freeze.
Store in the original package, in order to protect from light.
6.5 Nature and contents of container
1.5 ml of
oral
suspension in a pre-filled
oral
applicator (type I glass) with a plunger stopper (rubber
butyl) and a protective tip cap (rubber butyl) in pack sizes of 1, 5, 10 or 25.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
The vaccine is presented as a clear, colourless liquid, free of visible particles, for
oral
administration.
The vaccine is ready to use (no reconstitution or dilution is required).
The vaccine is to be administered
orally
without mixing with any other vaccines or solutions.
The vaccine should be inspected visually for any foreign particulate matter and/or abnormal physical
appearance. In the event of either being observed, discard the vaccine.
Any unused vaccine or waste material should be disposed of in accordance with local requirements.
Instructions for administration of the vaccine
1. Remove the protective tip cap from the
oral
applicator.
2. This vaccine is for
oral administration only
. The child should be seated in a reclining position.
Administer
orally
(i.e. into the child’s mouth,
towards the inner cheek) the entire content of the
oral
applicator.
3.
Do not inject.
1. Remove the protective tip cap from
the
oral
applicator.
2. This vaccine is for
oral
administration only
. The child
should be seated in a reclining
position. Administer
orally
(i.e. into
the child’s mouth,
towards the inner
cheek) the entire content of the
oral
applicator.
Discard the empty
oral
applicator and tip cap in approved biological waste containers according to
local regulations.
MARKETING AUTHORISATION HOLDER
GlaxoSmithKline Biologicals s.a.
Rue de l'Institut 89
B-1330 Rixensart, Belgium
8. MARKETING AUTHORISATION NUMBER(S)
EU/1/05/330/005
EU/1/05/330/006
EU/1/05/330/007
EU/1/05/330/008
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 21 February 2006
10. DATE OF REVISION OF THE TEXT



Detailed information on this medicinal product is available on the website of the European Medicines
1. NAME OF THE MEDICINAL PRODUCT
Rotarix
oral
suspension
Rotavirus vaccine, live
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
1 dose (1.5 ml) contains:
Human rotavirus RIX4414 strain (live, attenuated)*
not less than 10
6.0
CCID
50
Excipient:
This product contains sucrose 1073 mg (see section 4.4).
For a full list of excipients, see section 6.1.
Oral
suspension.
Rotarix is a clear and colourless liquid.
4.1 Therapeutic indications
Rotarix is indicated for the active immunisation of infants aged 6 to 24 weeks for prevention of gastro-
enteritis due to rotavirus infection (see section 4.2).
In clinical trials, efficacy was demonstrated against gastro-enteritis due to rotavirus of types G1P[8],
G2P[4], G3P[8], G4P[8] and G9P[8] (see sections 4.4 and 5.1).
The use of Rotarix should be based on official recommendations.
4.2 Posology and method of administration
The vaccination course consists of two doses. The first dose may be administered from the age of 6
weeks. There should be an interval of at least 4 weeks between doses. The vaccination course should
preferably be given before 16 weeks of age, but must be completed by the age of 24 weeks.
Rotarix may be given with the same posology to preterm infants born after at least 27 weeks of
gestational age (see sections 4.8 and 5.1).
In clinical trials, spitting or regurgitation of the vaccine has rarely been observed and, under such
circumstances, a replacement dose was not given. However, in the unlikely event that an infant spits
out or regurgitates most of the vaccine dose, a single replacement dose may be given at the same
vaccination visit.
It is recommended that infants who receive a first dose of Rotarix complete the 2-dose regimen with
Rotarix. There are no data on safety, immunogenicity or efficacy when Rotarix is administered for the
first dose and another rotavirus vaccine is administered for the second dose or vice versa.
Rotarix should not be used in children over 24 weeks of age.
Rotarix is for
oral
use only.
Rotarix should under no circumstances be injected.
Hypersensitivity to the active substance or to any of the excipients.
Hypersensitivity after previous administration of rotavirus vaccines.
Previous history of intussusception.
Subjects with uncorrected congenital malformation of the gastrointestinal tract that would predispose
for intussusception.
Subjects with Severe Combined Immunodeficiency (SCID) disorder (see section 4.8).
Administration of Rotarix should be postponed in subjects suffering from acute severe febrile
illness. The presence of a minor infection is not a contra-indication for immunisation.
The administration of Rotarix should be postponed in subjects suffering from diarrhoea or vomiting.
4.4 Special warnings and precautions for use
It is good clinical practice that vaccination should be preceded by a review of the medical history
especially with regard to the contraindications and by a clinical examination.
There are no data on the safety and efficacy of Rotarix in infants with gastrointestinal illnesses or
growth retardation. Administration of Rotarix may be considered with caution in such infants when, in
the opinion of the physician, withholding the vaccine entails a greater risk.
Although no causal relationship has been established between vaccination with Rotarix and
intussusception (see section 4.8), as a precaution, healthcare professionals should follow-up on any
symptoms indicative of intussusception (severe abdominal pain, persistent vomiting, bloody stools,
abdominal bloating and/or high fever). Parents/guardians should be advised to promptly report such
symptoms.
Asymptomatic and mildly symptomatic HIV infections are not expected to affect the safety or efficacy
of Rotarix. A clinical study in a limited number of asymptomatic or mildly symptomatic HIV positive
infants showed no apparent safety problems (see section 4.8).
Administration of Rotarix to infants who have known or suspected immunodeficiency should be based
on careful consideration of potential benefits and risks.
Excretion of the vaccine virus in the stools is known to occur after vaccination with peak excretion
around the 7th day. Viral antigen particles detected by ELISA were found in 50% of stools after the
first dose of Rotarix lyophilised formulation and 4% of stools after the second dose. When these stools
were tested for the presence of live vaccine strain, only 17% were positive. In two comparative
controlled trials, vaccine shedding after vaccination with Rotarix liquid formulation was comparable
to that observed after vaccination with Rotarix lyophilised formulation.
Cases of transmission of this excreted vaccine virus to seronegative contacts of vaccinees have been
observed without causing any clinical symptom.
Rotarix should be administered with caution to individuals with immunodeficient close contacts,
such as individuals with malignancies, or who are otherwise immunocompromised or individuals
receiving immunosuppressive therapy.
Contacts of recent vaccinees should observe personal hygi ene (e.g. wash their hands after changing
child’s nappies).
The potential risk of apnoea and the need for respiratory monitoring for 48-72h should be considered
when administering the primary immunisation series to very premature infants (born ≤ 28 weeks of
gestation) and particularly for those with a previous history of respiratory immaturity.
As the benefit of the vaccination is high in this group of infants, vaccination should not be withheld or
delayed.
A protective immune response may not be elicited in all vaccinees (see section 5.1).
In clinical trials, efficacy was demonstrated against gastro-enteritis due to rotavirus of types G1P[8],
G2P[4], G3P[8], G4P[8] and G9P[8]. The ext ent of protection that Rotarix might provide against other
serotypes is unknown. Clinical studies from which efficacy data were derived were conducted in
Europe and Central and South America (see section 5.1).
Rotarix does not protect against gastro-enteritis due to other pathogens than rotavirus.
No data are available on the use of Rotarix for post-exposure prophylaxis.
Rotarix should under no circumstances be injected.
The vaccine contains sucrose as an excipient. Patients with rare hereditary problems of fructose
intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency should not take this
vaccine.
4.5 Interaction with other medicinal products and other forms of interaction
Rotarix can be given concomitantly with any of the following monovalent or combination vaccines
[including hexavalent vaccines (DTPa-HBV-IPV/Hib)]: diphtheria-tetanus-whole cell pertussis
vaccine (DTPw), diphtheria-tetanus-acellular pertussis vaccine (DTPa),
Haemophilus influenzae
type b vaccine (Hib), inactivated polio vaccine (IPV), hepatitis B vaccine (HBV), pneumococcal
conjugate vaccine and meningococcal serogroup C conjugate vaccine. Clinical studies demonstrated
that the immune responses and the safety profiles of the administered vaccines were unaffected.
Concomitant administration of Rotarix and oral polio vaccine (OPV) does not affect the immune
response to the polio antigens. Although concomitant administration of OPV may slightly reduce the
immune response to rotavirus vaccine, clinical protection against severe rotavirus gastro-enteritis was
shown to be maintained in a clinical trial involving more than 4200 subjects who received Rotarix
concomitantly with OPV.
There are no restrictions on the infant’s consumption of food or liquid, either before or after
vaccination.
4.6 Fertility, pregnancy and lactation
Rotarix is not intended for use in adults. There are no data on the us e of Rotarix during pregnancy and
lactation.
Based on evidence generated in clinical trials, breast-feeding does not reduce the protection against
rotavirus gastro-enteritis afforded by Rotarix. Therefore, breast-feeding may be continued during the
vaccination schedule.
4.7 Effects on ability to drive and use machines
The safety profile presented below is based on data from clinical trials conducted with either the
lyophilised or the liquid formulation of Rotarix.
In a total of four clinical trials, approximately 3800 doses of Rotarix liquid formulation were
administered to approximately 1900 infants. Those trials have shown that the safety profile of the
liquid formulation is comparable to the lyophilised formulation.
In a total of twenty-three clinical trials, approximately 106000 doses of Rotarix (lyophilised or liquid
formulation)
were administered to approximately 51000 infants.
In three placebo-controlled clinical trials (Finland, India and Bangladesh), in which Rotarix was
administered alone (administration of routine paediatric vaccines was staggered), the incidence and
severity of the solicited events (collected 8 days post-vaccination), diarrhoea, vomiting, loss of
appetite, fever, irritability and cough/runny nose were not significantly different in the group receiving
Rotarix when compared to the group receiving placebo. No increase in the incidence or severity of
these events was seen with the second dose.
In a pooled analysis from seventeen placebo-controlled clinical trials (Europe, North America, Latin
America, Asia, Africa) including trials in which Rotarix was co-administered with routine paediatric
vaccines (see section 4.5), the following adverse reactions (collected 31 days post-vaccination) were
considered as possibly related to vaccination.
Adverse reactions are listed below per system organ class and frequency.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Frequencies are defined as:
Very common (≥1/10)
Common (≥1/100, <1/10)
Uncommon (≥1/1,000, <1/100)
Rare (≥1/10,000, <1/1,000)
Gastrointestinal disorders
Uncommon
: abdominal pain, flatulence
Skin and subcutaneous tissue disorders
General disorders and administration site conditions
The risk of intussusception has been evaluated in a large safety trial conducted in Latin America and
Finland where 63225 infants were enrolled. This trial gave evidence of no increased risk of
intussusception in the Rotarix group when compared with the placebo group as shown in the table
below.
Intussusception within 31 days
after administration of:
Safety in preterm infants
In a clinical study, 670 pre-term infants from 27 to 36 weeks of gestational age were administered
Rotarix lyophilised formulation and 339 received placebo. The first dose was administered from 6
weeks after birth. Serious adverse events were observed in 5.1% of recipients of Rotarix as compared
with 6.8% of placebo recipients. Similar rates of other adverse events were observed in Rotarix and
placebo recipients. No cases of intussusception were reported.
Safety in infants with human immunodeficiency (HIV) infection
In a clinical study, 100 infants with HIV infection were administered Rotarix lyophilised formulation
or placebo. The safety profile was similar between Rotarix and placebo recipients.
Post marketing surveillance:
Becaus e these events were reported spontaneous ly, it is not possible to reliably estimate their
frequency.
Respiratory, thoracic and mediastinal disorders:
Apnoea in very premature infants (≤ 28 weeks of gestation) (see section 4.4)
Gastrointestinal disorders:
Gastroenteritis with vaccine viral shedding in infants with Severe Combined Immunodeficiency
(SCID) disorder
In post-marketing experience, cases of intussusception have been reported in temporal association
with Rotarix. Most cases were reported within seven days following the first dose (see section 4.4).
No case of overdose has been reported.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmaco-therapeutic group: rotavirus diarrhoea vaccines, ATC code: J07BH01
Protective efficacy of the lyophilised formulation
Clinical studies have been conducted in Europe and Latin America to evaluate the protective efficacy
of Rotarix against any and severe rotavirus gastro-enteritis.
A clinical study performed in Europe evaluated Rotarix given according to different European
schedules (2, 3 months; 2, 4 months; 3, 4 months; 3, 5 months) in 4000 subjects. Severity of gastro-
enteritis was defined according to the Vesikari 20-point scale which evaluates the full clinical picture
of rotavirus gastro-enteritis by taking into account the severity and duration of diarrhoea and vomiting,
the severity of fever and dehydration as well as the need for treatment.








After two doses of Rotarix, the protective vaccine efficacy observed during the first and second year
of life is presented in the following table:
2
nd
year of life
Rotarix N=2554;
Placebo N=1294 (§)
Vaccine efficacy (%) against any and severe rotavirus gastro-enteritis
[95% CI]
1
st
year of life
Rotarix N=2572;
Placebo N=1302 (§)
Strains with P[8]
genotype
Circulating
rotavirus strains
85.6*
[75.8;91.9]
Vaccine efficacy (%) against rotavirus gastro-enteritis requiring medical
attention
[95% CI]
76.2*
[63.0;85.0]
Vaccine efficacy (%) against hospitalisation due to rotavirus gastro-enteritis
[95% CI]
92.2*
[65.6;99.1]
†
Severe gastro-enteritis defined as a score ≥11 on the Vesikari scale
(§) ATP cohort for efficacy
* Statistically significant (p < 0.05)
Vaccine efficacy during the first year of life progressively increased with increasing disease severity,
reaching 100% (95% CI: 84.7;100) for Vesikari scores ≥17.
A clinical study performed in Latin America evaluated Rotarix in more than 20000 subjects. Severity
of gastro-enteritis was defined according to WHO criteria. The protective vaccine efficacy against
severe rotavirus gastro-enteritis requiring hospitalisation and/or rehydration therapy in a medical
facility and the type specific vaccine efficacy after two doses of Rotarix are presented in the table
below:
Severe rotavirus gastro-
enteritis (1
st
year of life)
Rotarix N=9009;
Placebo N=8858
(§)
Severe rotavirus gastro-
enteritis (2
nd
year of life)
Rotar ix N=7175;
Placebo N=7062 (§)
Circulating
rotavirus strains
Circulating
rotavirus strains




























Strains with P[8]
genotype
(§)ATP cohort for efficacy
* Statistically significant (p < 0.05)
# The numbers of cases, on which the estimates of efficacy against G4P[8] were based, were very
small (1 case in the Rotarix group and 2 cases in the placebo group).
A pool ed analysis of five efficacy studies*, showed a 71.4% (95% CI:20.1;91.1) efficacy against
severe rotavirus gastro-enteritis (Vesikari score ≥11) caused by rotavirus G2P[4] type during the first
year of life.
* In these studies, the point estimates and confidence intervals were respectively: 100% (95% CI: -
1858.0;100), 100% (95% CI: 21.1;100), 45.4% (95% CI: -81.5;86.6), 74.7 (95% CI :-386.2;99.6). No
point estimate was available for the remaining study.
Protective efficacy of the liquid formulation:
Since the immune response observed after 2 doses of Rotarix liquid formulation was comparable to
the immune response observed after 2 doses of Rotarix lyophilised formulation, the levels of vaccine
efficacy observed with the lyophilised formulation can be extrapolated to the liquid formulation.
The immunologic mechanism by which Rotarix protects against rotavirus gastro-enteritis is not
completely understood. A relationship between antibody responses to rotavirus vaccination and
protection against rotavirus gastro-enteritis has not been established.
The following table shows the percentage of subjects with serum anti-rotavirus IgA antibody titers
≥20U/ml (by ELISA) one to two months after the second dose of vaccine or placebo as observed in
different studies with Rotarix lyophilised formulation.
2, 3 months
France,
Germany
3, 5 months
Finland, Italy 180
3, 4 months
Czech
Republic
Latin
America; 11
countries
In three comparative controlled trials, the immune response elicited by Rotarix liquid formulation was
comparable to the one elicited by Rotarix lyophilised formulation.

























Immune response in preterm infants
In a clinical study conducted in preterm infants, born after at least 27 weeks of gestational age, the
immunogenicity of Rotarix was assessed in a subset of 147 subjects and showed that Rotarix is
immunogenic in this population; 85.7% (95% CI: 79.0;90.9) of subjects achieved serum anti-rotavirus
IgA antibody titers ≥ 20U/ml (by ELISA) one month after the second dose of vaccine.
5.2 Pharmacokinetic properties
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of repeated dose
toxicity.
6. PHARMACEUTICAL PARTICULARS
Sucrose
Di-sodium Adipate
Dulbecco’s Modified Eagle Medium (DMEM)
Sterile water
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
3 years.
The vaccine should be used immediately after opening.
6.4 Special precautions for storage
Store in a refrigerator (2°C – 8°C).
Do not freeze.
Store in the original package, in order to protect from light.
6.5 Nature and contents of container
1.5 ml of
oral
suspension in a squeezable tube (polyethylene) fitted with a tip seal and a tube cap
(polypropylene) in pack sizes of 1, 10 or 50.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
The vaccine is presented as a clear, colourless liquid, free of visible particles, for
oral
administration.
The vaccine is ready to use (no reconstitution or dilution is required).
The vaccine is to be administered
orally
without mixing with any other vaccines or solutions.
The vaccine should be inspected visually for any foreign particulate matter and/or abnormal physical
appearance. In the event of either being observed, discard the vaccine.
Any unused vaccine or waste material should be disposed of in accordance with local requirements.
Instructions for administration of the vaccine
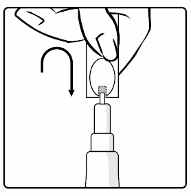
1. Pull off the cap from the top of the tube.
2. Turn the cap upside-down and replace the cap vertically over the tip seal as shown in the diagram.
3. Twist the cap to remove the tip seal (do not snap it off) leaving a hole through which the vaccine
can be expelled.
4.
Ensure that the tip seal has been properly removed. A hole should be clearly visible at the tip of
the tube and the tip seal should be inside the top of the tube cap.
In the event that the tip seal is accidentally pushed into the tube, discard the vaccine. This is just a
precaution, since it is unlikely that the tip seal could be expelled from the tube while
administering the vaccine.
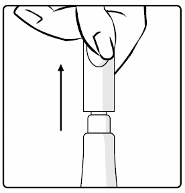
5. This vaccine is for
oral administration only
. The child should be seated in a reclining position.
Administer
orally
(i.e. into the child’s mouth,
towards the inner cheek) the entire content of the
tube by gently squeezing the tube several times. (A residual drop may remain in the tip of the
tube).
1. Pull off the cap from the top of the
tube.
2. Turn the cap upside-down and
replace the cap vertically over the
tip seal as shown in the diagram.
3. Twist the cap to remove the tip seal (do
not snap it off) leaving a hole through
which the vaccine can be expelled.
4. Ensure that the tip seal has been
properly removed. A hole should be clearly
visible at the tip of the tube and
the tip seal should be inside the top
of the tube cap.
In the event that the tip seal is accidentally
pushed into the tube, discard the vaccine.
This is just a precaution, since it is unlikely
that the tip seal could be expelled from the
tube while administering the vaccine.
5. This vaccine is for
oral administration
only
. The child should be seated in a
reclining position. Administer
orally
(i.e.
into the child’s mouth,
towards the inner
cheek) the entire content of the tube by
gently squeezing the tube several times.
(A residual drop may remain in the tip of
the tube).
Discard the empty tube and cap in approved biological waste containers according to local regulations.
MARKETING AUTHORISATION HOLDER
GlaxoSmithKline Biologicals s.a.
Rue de l'Institut 89
B-1330 Rixensart, Belgium
8. MARKETING AUTHORISATION NUMBER(S)
EU/1/05/330/009
EU/1/05/330/010
EU/1/05/330/011
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 21 February 2006
10. DATE OF REVISION OF THE TEXT










Detailed information on this medicinal product is available on the website of the European Medicines
MANUFACTURER(S) OF THE BIOLOGICAL ACTIVE
SUBSTANCE(S) AND MANUFACTURING
AUTHORISATION HOLDER(S) RESPONSIBLE FOR
BATCH RELEASE
B. CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURER(S) OF THE BIOLOGICAL ACTIVE SUBSTANCE(S) AND
MANUFACTURING AUTHORISATION HOLDER(S) RESPONSIBLE FOR
BATCH RELEASE
Name and address of the manufacturer(s) of the biological active substance(s)
GlaxoSmithKline Biologicals s.a.
Rue de l’Institut 89
1330 Rixensart
Belgium
Name and address of the manufacturer(s) responsible for batch release
GlaxoSmithKline Biologicals s.a.
Rue de l’Institut 89
1330 Rixensart
Belgium
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to medical prescription.
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
Official batch release: in accordance with Article 114 Directive 2001/83/EC as amended, the official
batch release will be undertaken by a state laboratory or a laboratory designated for that purpose.
The MAH must ensure that the system of pharmacovigilance presented in Module 1.8.1. of the
Marketing Authorisation, is in place and functioning before and whilst the product is on the market.
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version 4 of the Risk Management Plan (RMP) presented in
Module 1.8.2. of the Marketing Authorisation Application and any subsequent updates of the RMP
agreed by the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, the
updated RMP should be submitted at the same time as the next Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted
-
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
-
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
-
At the request of the European Medicines Agency
In order to address the presence of porcine circovirus type 1(PCV-1) in Rotarix, the MAH
commits to :
- Develop a PCV-free vaccine according to an implementation plan to be agreed with CHMP.
The plan should be submitted no later than 31 December 2010
- Provide updates on the progress being made on a 6-month basis
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
GLASS CONTAINER WITH ORAL APPLICATOR AND TRANSFER ADAPTER, PACK
SIZE OF 1, 5, 10 OR 25
NAME OF THE MEDICINAL PRODUCT
Rotarix powder and solvent for
oral
suspension
Rotavirus vaccine, live
STATEMENT OF ACTIVE SUBSTANCE(S)
After reconstitution, 1 dose (1 ml) contains:
Human rotavirus RIX4414 strain (live, attenuated)*
not less than 10
6.0
CCID
50
See the package leaflet for further information
PHARMACEUTICAL FORM AND CONTENTS
Powder and solvent for
oral
suspension
1 glass container: powder
1
oral
applicator: solvent
1 transfer adapter
1 dose (1 ml)
5 glass containers: powder
5
oral
applicators: solvent
5 transfer adapters
5 x 1 dose (1 ml)
10 glass containers: powder
10
oral
applicators: solvent
10 transfer adapters
10 x 1 dose (1 ml)
25 glass containers: powder
25
oral
applicators: solvent
25 transfer adapters
25 x 1 dose (1 ml)
METHOD AND ROUTE(S) OF ADMINISTRATION




















Do not inject!
Shake before us e
Read the package leaflet before use
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
Store in a refrigerator
Do not freeze
Store in the original package in order to protect from light
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Dispose of in accordance with local regulations
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
GlaxoSmithKline Biologicals s.a.
Rue de l’Institut 89
B-1330 Rixensart, Belgium
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/05/330/001 – pack of 1 (glass container +
oral
applicator + transfer adapter)
EU/1/05/330/002 – pack of 5 (glass container +
oral
applicator + transfer adapter)
EU/1/05/330/003 – pack of 10 (glass container +
oral
applicator + transfer adapter)
EU/1/05/330/004 – pack of 25 (glass container +
oral
applicator + transfer adapter)
14. GENERAL CLASSIFICATION FOR SUPPLY


























Medicinal product subject to medical prescription
16. INFORMATION IN BRAILLE
Justification for not including Braille accepted






PARTICULARS TO APPEAR ON THE OUTER PACKAGING
PRE-FILLED ORAL APPLICATOR, PACK SIZE OF 1, 5, 10 OR 25
NAME OF THE MEDICINAL PRODUCT
Rotarix
oral
suspension in pre-filled
oral
applicator
Rotavirus vaccine, live
STATEMENT OF ACTIVE SUBSTANCE(S)
1 dose (1.5 ml) contains:
Human rotavirus RIX4414 strain (live, attenuated)
not less than 10
6.0
CCID
50
See the package leaflet for further information
PHARMACEUTICAL FORM AND CONTENTS
Oral
suspension in pre-filled
oral
applicator
1 pre-filled
oral
applicator
1 dose (1.5 ml)
5 pre-filled
oral
applicators
5 x 1 dose (1.5 ml)
10 pre-filled
oral
applicators
10 x 1 dose (1.5 ml)
25 pre-filled
oral
applicators
25 x 1 dose (1.5 ml)
METHOD AND ROUTE(S) OF ADMINISTRATION
Oral
use
Do not inject!
Read the package leaflet before use
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children
OTHER SPECIAL WARNING(S), IF NECESSARY

























Ready to use
No reconstitution required
SPECIAL STORAGE CONDITIONS
Store in a refrigerator
Do not freeze
Store in the original package in order to protect from light
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Dispose of in accordance with local regulations
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
GlaxoSmithKline Biologicals s.a.
Rue de l’Institut 89
B-1330 Rixensart, Belgium
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/05/330/005 – pack of 1 pre-filled
oral
applicator
EU/1/05/330/006 – pack of 5 pre-filled
oral
applicators
EU/1/05/330/007 – pack of 10 pre-filled
oral
applicators
EU/1/05/330/008 – pack of 25 pre-filled
oral
applicators
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
16. INFORMATION IN BRAILLE
Justification for not including Braille accepted

























PARTICULARS TO APPEAR ON THE OUTER PACKAGING
TUBE, PACK SIZES OF 1, 10 OR 50
NAME OF THE MEDICINAL PRODUCT
Rotarix
oral
suspension
Rotavirus vaccine, live
STATEMENT OF ACTIVE SUBSTANCE(S)
1 dose (1.5 ml) contains:
Human rotavirus RIX4414 strain (live, attenuated)
not less than 10
6.0
CCID
50
See the package leaflet for further information
PHARMACEUTICAL FORM AND CONTENTS
Oral
suspension
1 tube
1 dose (1.5 ml)
10 tubes
10 x 1 dose (1.5 ml)
50 tubes
50 x 1 dose (1.5 ml)
METHOD AND ROUTE(S) OF ADMINISTRATION
Oral
use
Do not inject!
Read the package leaflet before use
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children
OTHER SPECIAL WARNING(S), IF NECESSARY

























SPECIAL STORAGE CONDITIONS
Store in a refrigerator
Do not freeze
Store in the original package in order to protect from light
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Dispose of in accordance with local regulations
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
GlaxoSmithKline Biologicals s.a.
Rue de l’Institut 89
B-1330 Rixensart, Belgium
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/05/330/009 – pack of 1 tube
EU/1/05/330/010 – pack of 10 tubes
EU/1/05/330/011 – pack of 50 tubes
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
























16. INFORMATION IN BRAILLE
Justification for not including Braille accepted




PACKAGE LEAFLET: INFORMATION FOR THE USER
Rotarix powder and solvent for oral suspension
Rotavirus vaccine, live
Read all of this leaflet carefully before your child receives this vaccine.
-
Keep this leaflet. You may need to read it again.
This vaccine has been prescribed for your child. Do not pass it on to others.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
In this leaflet:
1.
What Rotarix is and what it is used for
2.
Before your child receives Rotarix
3.
How Rotarix is given
4.
Possible side effects
5.
How to store Rotarix
6.
Further information
WHAT ROTARIX IS AND WHAT IT IS USED FOR
Rotarix is a viral vaccine, containing live, attenuated human rotavirus, that helps to protect your child,
from the age of 6 weeks, against gastro-enteritis (diarrhoea and vomiting) caused by rotavirus
infection.
Rotavirus infection is the most common cause of severe diarrhoea in infants and young children.
Rotavirus is easily spread from hand-to-mouth due to contact with stools from an infected person.
Most children with rotavirus diarrhoea recover on their own. However, some children become very ill
with severe vomiting, diarrhoea and life-threatening loss of fluids that requires hospitalisation.
When a person is given the vaccine, the immune system (the body’s natural defences) will make
antibodies against the most commonly occurring types of rotavirus. These antibodies protect against
disease caus ed by these types of rotavirus.
As with all vaccines, Rotarix may not completely protect all people who are vaccinated against the
rotavirus infections it is intended to prevent.
BEFORE YOUR CHILD RECEIVES ROTARIX
Rotarix should not be given:
•
if your child has previously had any allergic reaction to rotavirus vaccines or any component
contained in Rotarix. The active substances and other ingredients in Rotarix are listed at the end
of the leaflet. Signs of an allergic reaction may include itchy skin rash, shortness of breath and
swelling of the face or tongue.
if your child has previously had intussusception (a bowel obstruction in which one segment of
bowel becomes enfolded within another segment).
if your child was born with a malformation of the gut that could lead to intussusception.
if your child has a rare inherited illness which affects their immune system called Severe
Combined Immunodeficiency (SCID).
if your child has a severe infection with a high temperature. It might be necessary to postpone
the vaccination until recovery. A minor infection such as a cold should not be a problem, but
talk to your doctor first.
If you have any further questions, ask your doctor or pharmacist.
if your child has diarrhoea or is vomiting. It might be necessary to postpone the vaccination
until recovery.
Take special care with Rotarix
Before your child receives Rotarix, inform your doctor/health care professional if he/she:
•
has a close contact such as a household member who has a weakened immune system, e.g., a
person with cancer or who is taking medicines that may weaken the immune system.
has any disorder of the gastrointestinal system.
has not been gaining weight and growing as expected.
has any disease or is taking any medicine which reduces his/her resistance to infection.
After your child has received Rotarix, contact your doctor/health care professional right away if your
child exp eriences severe stomach pain, persistent vomiting, blood in stools, a swollen belly and/or
high fever.
As always, please take care to wash your hands thoroughly after changing soiled nappies.
Using other vaccines
Please tell your doctor if your child is taking or has recently taken any other medicines, including
medicines obtained without a prescription or has recently received any other vaccine.
Rotarix may be given at the same time your child receives other normally recommended vaccines,
such as diphtheria, tetanus, pertussis (whooping cough),
Haemophilus influenzae
type b, oral or
inactivated polio, hepatitis B vaccines as well as pneumococcal and meningococcal serogroup C
conjugate vaccines.
Using Rotarix with food and drink
There are no restrictions on your child’s consumption of food or liquids, either before or after
vaccination.
Breast-feeding
Based on evidence generated in clinical trials, breast-feeding does not reduce the protection against
rotavirus gastro-enteritis afforded by Rotarix. Therefore, breast-feeding may be continued during the
vaccination schedule.
Important information about some of the ingredients of Rotarix
If you have been told by your doctor that the child being vaccinated has an intolerance to some sugars,
contact your doctor before receiving this vaccine.
The doctor or nurse will administer the recommended dose of Rotarix to your child. The vaccine (1 ml
liquid) will be given
orally
. Under no circumstance should this vaccine be administered by injection.
Your child will receive two doses of the vaccine. Each dose will be given on a separate occasion with
an interval of at least 4 weeks between the two doses. The first dose may be given from the age of 6
weeks. The two doses of the vaccine must have been given by the age of 24 weeks, although they
should preferably have been given before 16 weeks of age.
Rotarix may be given according to the same vaccination course to infants who were born prematurely,
provided that the pregnancy had lasted at least 27 weeks.
In case your child spits out or regurgitates most of the vaccine dose, a single replacement dose may be
given at the same vaccination visit
.
When Rotarix is given to your child for the first dose, it is recommended that your child also receives
Rotarix (and not another rotavirus vaccine) for the second dose.
It is important that you follow the instructions of your doctor or nurse regarding return visits. If you
forget to go back to your doctor at the scheduled time, ask your doctor for advice.
Like all medicines, Rotarix can cause side effects, although not everybody gets them.
Side effects that occurred during clinical trials with Rotarix were as follows:
♦
Common (These may occur with up to 1 in 10 doses of the vaccine):
•
diarrhoea
•
irritability
♦
Uncommon (These may occur with up to 1 in 100 doses of the vaccine):
•
abdominal pain, flatulence
•
inflammation of the skin
Side effects that have been reported during marketed use of Rotarix include:
•
blood in stools
•
in babies born very prematurely (at or before 28 weeks of gestation) longer gaps than normal
between breaths may occur for 2-3 days after vaccination.
•
children with a rare inherited illness called Severe Combined Immunodeficiency (SCID)
may have an inflamed stomach or gut (gastroenteritis) and pass the vaccine virus in their
stools. The signs of gastroenteritis may include feeling sick, being sick, stomach cramps or
diarrhoea.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Rotarix after the expiry date which is stated on the carton. The exp iry date refers to the last
day of that month.
Store in a refrigerator (2°C – 8°C).
Do not freeze.
Store in the original package in order to protect from light.
After reconstitution, the vaccine contained in the
oral
applicator should be administered promptly. If
the reconstituted vaccine is not used within 24 hours, it should be discarded.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active subs tances are:
Human rotavirus RIX4414 strain (live, attenuated)*
not less than 10
6.0
CCID
50
The other ingredients in Rotarix are:
Powder
: sucrose, dextran, sorbitol, amino acids, Dulbecco’s Modified Eagle Medium (DMEM)
Solvent
: calcium carbonate, xanthan gum, sterile water
What Rotarix looks like and contents of the pack
Powder and solvent for
oral
suspension
Rotarix is supplied as a whitish powder in a single dose glass container and a separate
oral
applicator
of solvent which contains a slow settling white deposit and a colourless supernatant. There is also a
transfer adapter which allows easy transfer of the solvent into the glass container containing the
powder for mixing the different components of the vaccine.
Both components must be mixed together before your child receives the vaccine. The mixed vaccine
will appear more turbid than the solvent alone.
Rotarix is available in a pack of 1, 5, 10 or 25.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
GlaxoSmithKline Biologicals s.a.
Rue de l’Institut 89
B-1330 Rixensart
Belgium
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
GlaxoSmithKline s.a./n.v.
Tél/Tel: + 32 2 656 21 11
Luxembourg/Luxemburg
GlaxoSmithKline s.a./n.v.
Tél/Tel: + 32 2 656 21 11
България
ГлаксоСмитКлайн ЕООД
ул. Димитър Манов бл.10
София 1408
Тел. + 359 2 953 10 34
Magyarország
GlaxoSmithKline Kft.
Tel.: + 36-1-2255300
Česká republika
GlaxoSmithKline s.r.o.
Tel: + 420 2 22 00 11 11
czmail@gsk.com
Malta
GlaxoSmithKline (Malta) Ltd
Tel: + 356 21 238131
Danmark
GlaxoSmithKline Pharma A/S
Tlf: + 45 36 35 91 00
dk-info@gsk.com
Nederland
GlaxoSmithKline BV
Tel: + 31 (0)30 69 38 100
nlinfo@gsk.com
Deutschland
GlaxoSmithKline GmbH & Co. KG
Tel: + 49 (0)89 360448701
produkt.info@gsk.com
Tlf: + 47 22 70 20 00
firmapost@gsk.no
Eesti
GlaxoSmithKline Eesti OÜ
Tel: +372 667 6900
estonia@gsk.com
Österreich
GlaxoSmithKline Pharma GmbH.
Tel: + 43 1 970 75-0
at.info@gsk.com
Ελλάδα
GlaxoSmithKline A.E.B.E
Tηλ: + 30 210 68 82 100
Polska
GSK Commercial Sp. z o.o.
Tel.: + 48 (22) 576 9000
España
GlaxoSmithKline, S.A.
Tel: + 34 902 202 700
es-ci@gsk.com
Portugal
Smith Kline & French Portuguesa, Produtos
Farmacêuticos, Lda.
Tel: + 351 21 412 95 00
FI.PT@gsk.com
France
Laboratoire GlaxoSmithKline
Tél: + 33 (0) 1 39 17 84 44
diam@gsk.com
România
GlaxoSmithKline (GSK) SRL
Tel: +40 (0)21 3028 208
Ireland
GlaxoSmithKline (Ireland) Ltd
Tel: + 353 (0)1 4955000
Slovenija
GlaxoSmithKline d.o.o.
Tel: + 386 (0) 1 280 25 00
medical.x.si@gsk.com
Ísland
GlaxoSmithKline ehf.
Sími: +354-530 3700
Slovenská republika
GlaxoSmithKline Slovakia s.r.o.
Tel: + 421 (0)2 48 26 11 11
recepcia.sk@gsk.com
Italia
GlaxoSmithKline S.p.A.
Tel:+ 39 04 59 21 81 11
Suomi/Finland
GlaxoSmithKline Oy
Puh/Tel: + 358 10 30 30 30
Finland.tuoteinfo@gsk.com
Κύπρος
GlaxoSmithKline (Cyprus) Ltd
Τηλ: + 357 22 39 70 00
Sverige
GlaxoSmithKline AB
Tel: + 46 (0)8 638 93 00
info.produkt@gsk.com
Latvija
GlaxoSmithKline Latvia SIA
Tel: + 371 67312687
lv-epasts@gsk.com
United Kingdom
GlaxoSmithKline UK
Tel: + 44 (0)808 100 9997
customercontactuk@gsk.com
Lietuva
GlaxoSmithKline Lietuva UAB
Tel: +370 5 264 90 00
info.lt@gsk.com
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency web site:
-------------------------------------------------------------------------------------------------------------------------
The following information is intended for medical or healthcare professionals only:
Before reconstitution:
A white deposit and clear supernatant is observed upon storage of the
oral
applicator containing the
solvent.
The solvent should be inspected visually for any foreign particulate matter and/or abnormal physical
appearance prior to reconstitution.
After reconstitution:
The reconstituted vaccine is slightly more turbid than the solvent and is milky white in appearance.
The reconstituted vaccine should also be inspected visually for any foreign particulate matter and/or
abnormal physical appearance prior to administration. In the event of either being observed, discard
the vaccine.
Any unused vaccine or waste material should be disposed of in accordance with local requirements.
Instructions for reconstitution and administration of the vaccine
1.
Remove the plastic cover from the glass container containing the powder.
2.
Connect the transfer adapter onto the glass container by pushing it downwards until the transfer
adapter is properly and securely placed.
3.
Shake the
oral
applicator containing the solvent vigorously. The shaken suspension will appear as
a turbid liquid with a slow settling white deposit.
4.
Remove the protective tip cap from the
oral
applicator.
5.
Connect the
oral
applicator into the transfer adapter by pushing it firmly on this device.
6.
Transfer the entire content of the
oral
applicator into the glass container containing the powder.
7.
With the
oral
applicator still attached, shake the glass container and examine it for complete
suspension of the powder. The reconstituted vaccine will appear more turbid than the solvent
alone. This appearance is normal.
8.
Withdraw the entire mixture back into the
oral
applicator.
9.
Remove the
oral
applicator from the transfer adapter.
10.
This vaccine is for
oral administration only
. The child should be seated in a reclining position.
Administer the entire content of the
oral
applicator
orally
(by administering the entire content of
the
oral
applicator on the inside of the cheek).
11.
Do not inject.
If the reconstituted vaccine is to be stored temporarily before administration, replace the protective tip
cap on the
oral
applicator. The
oral
applicator containing the reconstituted vaccine should be shaken
gently again before
oral
administration.
1. Remove the plastic cover from the
glass container containing the powder
2. Connect the transfer adapter onto
the glass container by pushing it
downwards until the transfer adapter
is properly and securely placed
3. Shake the
oral
applicator
containing the solvent vigorously. The
shaken suspension will appear as a
turbid liquid with a slow settling white
deposit
4. Remove the protective tip cap from
the
oral
applicator
5. Connect the
oral
applicator into the
transfer adapter by pushing it firmly
on this device
6. Transfer the entire content of the
oral
applicator into the glass
container containing the powder
7. With the
oral
applicator still attached,
shake the glass container and examine it
for complete suspension of the powder.
The reconstituted vaccine will appear more
turbid than the solvent alone. This
appearance is normal
8. Withdraw the entire mixture back
into the
oral
applicator
9. Remove the
oral
applicator from
the transfer adapter
10. This vaccine is for
oral
administration only
. The child
should be seated in a reclining
position. Administer the entire content
of the
oral
applicator
orally
(by
administering the entire content of the
oral
applicator on the inside of the
cheek)
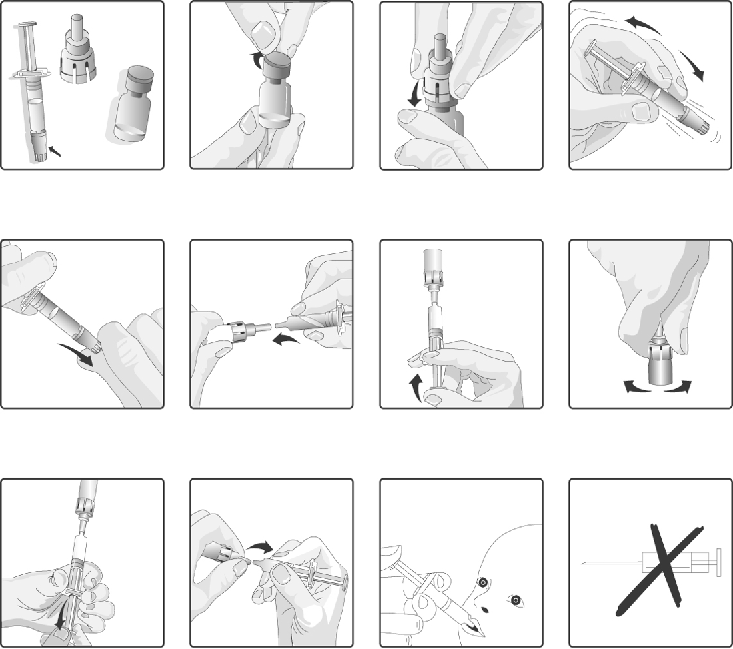






PACKAGE LEAFLET: INFORMATION FOR THE USER
Rotarix oral suspension in pre-filled oral applicator
Rotavirus vaccine, live
Read all of this leaflet carefully before your child receives this vaccine.
-
Keep this leaflet. You may need to read it again.
This vaccine has been prescribed for your child. Do not pass it on to others.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
In this leaflet:
1.
What Rotarix is and what it is used for
2.
Before your child receives Rotarix
3.
How Rotarix is given
4.
Possible side effects
5.
How to store Rotarix
6.
Further information
WHAT ROTARIX IS AND WHAT IT IS USED FOR
Rotarix is a viral vaccine, containing live, attenuated human rotavirus, that helps to protect your child,
from the age of 6 weeks, against gastro-enteritis (diarrhoea and vomiting) caused by rotavirus
infection.
Rotavirus infection is the most common cause of severe diarrhoea in infants and young children.
Rotavirus is easily spread from hand-to-mouth due to contact with stools from an infected person.
Most children with rotavirus diarrhoea recover on their own. However, some children become very ill
with severe vomiting, diarrhoea and life-threatening loss of fluids that requires hospitalisation.
When a person is given the vaccine, the immune system (the body’s natural defences) will make
antibodies against the most commonly occurring types of rotavirus. These antibodies protect against
disease caus ed by these types of rotavirus.
As with all vaccines, Rotarix may not completely protect all people who are vaccinated against the
rotavirus infections it is intended to prevent.
BEFORE YOUR CHILD RECEIVES ROTARIX
Rotarix should not be given:
•
if your child has previously had any allergic reaction to rotavirus vaccines or any component
contained in Rotarix. The active substances and other ingredients in Rotarix are listed at the end
of the leaflet. Signs of an allergic reaction may include itchy skin rash, shortness of breath and
swelling of the face or tongue.
if your child has previously had intussusception (a bowel obstruction in which one segment of
bowel becomes enfolded within another segment).
if your child was born with a malformation of the gut that could lead to intussusception.
if your child has a rare inherited illness which affects their immune system called Severe
Combined Immunodeficiency (SCID).
if your child has a severe infection with a high temperature. It might be necessary to postpone
the vaccination until recovery. A minor infection such as a cold should not be a problem, but
talk to your doctor first.
If you have any further questions, ask your doctor or pharmacist.
if your child has diarrhoea or is vomiting. It might be necessary to postpone the vaccination
until recovery.
Take special care with Rotarix
Before your child receives Rotarix, inform your doctor/health care professional if he/she:
•
has a close contact such as a household member who has a weakened immune system, e.g., a
person with cancer or who is taking medicines that may weaken the immune system.
has any disorder of the gastrointestinal system.
has not been gaining weight and growing as expected.
has any disease or is taking any medicine which reduces his/her resistance to infection.
After your child has received Rotarix, contact your doctor/health care professional right away if your
child exp eriences severe stomach pain, persistent vomiting, blood in stools, a swollen belly and/or
high fever.
As always, please take care to wash your hands thoroughly after changing soiled nappies.
Using other vaccines
Please tell your doctor if your child is taking or has recently taken any other medicines, including
medicines obtained without a prescription or has recently received any other vaccine.
Rotarix may be given at the same time your child receives other normally recommended vaccines,
such as diphtheria, tetanus, pertussis (whooping cough),
Haemophilus influenzae
type b, oral or
inactivated polio, hepatitis B vaccines as well as pneumococcal and meningococcal serogroup C
conjugate vaccines.
Using Rotarix with food and drink
There are no restrictions on your child’s consumption of food or liquids, either before or after
vaccination.
Breast-feeding
Based on evidence generated in clinical trials, breast-feeding does not reduce the protection against
rotavirus gastro-enteritis afforded by Rotarix. Therefore, breast-feeding may be continued during the
vaccination schedule.
Important information about some of the ingredients of Rotarix
If you have been told by your doctor that the child being vaccinated has an intolerance to some sugars,
contact your doctor before receiving this vaccine.
The doctor or nurse will administer the recommended dose of Rotarix to your child. The vaccine (1.5
ml liquid) will be given
orally
. Under no circumstance should this vaccine be administered by
injection.
Your child will receive two doses of the vaccine. Each dose will be given on a separate occasion with
an interval of at least 4 weeks between the two doses. The first dose may be given from the age of 6
weeks. The two doses of the vaccine must have been given by the age of 24 weeks, although they
should preferably have been given before 16 weeks of age.
Rotarix may be given according to the same vaccination course to infants who were born prematurely,
provided that the pregnancy had lasted at least 27 weeks.
In case your child spits out or regurgitates most of the vaccine dose, a single replacement dose may be
given at the same vaccination visit
.
When Rotarix is given to your child for the first dose, it is recommended that your child also receives
Rotarix (and not another rotavirus vaccine) for the second dose.
It is important that you follow the instructions of your doctor or nurse regarding return visits. If you
forget to go back to your doctor at the scheduled time, ask your doctor for advice.
Like all medicines, Rotarix can cause side effects, although not everybody gets them.
Side effects that occurred during clinical trials with Rotarix were as follows:
♦
Common (These may occur with up to 1 in 10 doses of the vaccine):
•
diarrhoea
•
irritability
♦
Uncommon (These may occur with up to 1 in 100 doses of the vaccine):
•
abdominal pain, flatulence
•
inflammation of the skin
Side effects that have been reported during marketed use of Rotarix include:
•
blood in stools
•
in babies born very prematurely (at or before 28 weeks of gestation) longer gaps than normal
between breaths may occur for 2-3 days after vaccination.
•
children with a rare inherited illness called Severe Combined Immunodeficiency (SCID)
may have an inflamed stomach or gut (gastroenteritis) and pass the vaccine virus in their
stools. The signs of gastroenteritis may include feeling sick, being sick, stomach cramps or
diarrhoea.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Rotarix after the expiry date which is stated on the carton. The expiry date refers to the last
day of that month.
Store in a refrigerator (2°C – 8°C).
Do not freeze.
Store in the original package in order to protect from light.
The vaccine should be used immediately after opening.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active subs tances are:
Human rotavirus RIX4414 strain (live, attenuated)*
not less than 10
6.0
CCID
50
The other ingredients in Rotarix are: sucrose, Di-sodium Adipate, Dulbecco’s Modified Eagle
Medium (DMEM), sterile water
What Rotarix looks like and contents of the pack
Oral
suspension in pre-filled
oral
applicator.
Rotarix is supplied as clear and colourless liquid in a single dose pre-filled
oral
applicator (1.5 ml).
Rotarix is available in a pack of 1, 5, 10 or 25.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
GlaxoSmithKline Biologicals s.a.
Rue de l’Institut 89
B-1330 Rixensart
Belgium
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
GlaxoSmithKline s.a./n.v.
Tél/Tel: + 32 2 656 21 11
Luxembourg/Luxemburg
GlaxoSmithKline s.a./n.v.
Tél/Tel: + 32 2 656 21 11
България
ГлаксоСмитКлайн ЕООД
ул. Димитър Манов бл.10
София 1408
Тел. + 359 2 953 10 34
Magyarország
GlaxoSmithKline Kft.
Tel.: + 36-1-2255300
Česká republika
GlaxoSmithKline s.r.o.
Tel: + 420 2 22 00 11 11
czmail@gsk.com
Malta
GlaxoSmithKline (Malta) Ltd
Tel: + 356 21 238131
Danmark
GlaxoSmithKline Pharma A/S
Tlf: + 45 36 35 91 00
dk-info@gsk.com
Nederland
GlaxoSmithKline BV
Tel: + 31 (0)30 69 38 100
nlinfo@gsk.com
Deutschland
GlaxoSmithKline GmbH & Co. KG
Tel: + 49 (0)89 360448701
produkt.info@gsk.com
Norge
GlaxoSmithKline AS
Tlf: + 47 22 70 20 00
firmapost@gsk.no
Eesti
GlaxoSmithKline Eesti OÜ
Tel: +372 667 6900
estonia@gsk.com
Österreich
GlaxoSmithKline Pharma GmbH.
Tel: + 43 1 970 75-0
at.info@gsk.com
Ελλάδα
GlaxoSmithKline A.E.B.E
Tηλ: + 30 210 68 82 100
Polska
GSK Commercial Sp. z o.o.
Tel.: + 48 (22) 576 9000
España
GlaxoSmithKline, S.A.
Tel: + 34 902 202 700
es-ci@gsk.com
Portugal
Smith Kline & French Portuguesa, Produtos
Farmacêuticos, Lda.
Tel: + 351 21 412 95 00
FI.PT@gsk.com
France
Laboratoire GlaxoSmithKline
Tél: + 33 (0) 1 39 17 84 44
diam@gsk.com
România
GlaxoSmithKline (GSK) SRL
Tel: +40 (0)21 3028 208
Ireland
GlaxoSmithKline (Ireland) Ltd
Tel: + 353 (0)1 4955000
Slovenija
GlaxoSmithKline d.o.o.
Tel: + 386 (0) 1 280 25 00
medical.x.si@gsk.com
Ísland
GlaxoSmithKline ehf.
Sími: +354-530 3700
Slovenská republika
GlaxoSmithKline Slovakia s.r.o.
Tel: + 421 (0)2 48 26 11 11
recepcia.sk@gsk.com
Italia
GlaxoSmithKline S.p.A.
Tel:+ 39 04 59 21 81 11
Suomi/Finland
GlaxoSmithKline Oy
Puh/Tel: + 358 10 30 30 30
Finland.tuoteinfo@gsk.com
Κύπρος
GlaxoSmithKline Cyprus Ltd
Τηλ: + 357 22 39 70 00
Sverige
GlaxoSmithKline AB
Tel: + 46 (0)8 638 93 00
info.produkt@gsk.com
Latvija
GlaxoSmithKline Latvia SIA
Tel: + 371 67312687
lv-epasts@gsk.com
United Kingdom
GlaxoSmithKline UK
Tel: + 44 (0)808 100 9997
customercontactuk@gsk.com
Lietuva
GlaxoSmithKline Lietuva UAB
Tel: +370 5 264 90 00
info.lt@gsk.com
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency web site:
-------------------------------------------------------------------------------------------------------------------------
The following information is intended for medical or healthcare professionals only:
The vaccine is presented as a clear, colourless liquid, free of visible particles, for
oral
administration.
The vaccine is ready to use (no reconstitution or dilution is required).
The vaccine is to be administered
orally
without mixing with any other vaccines or solutions.
The vaccine should be inspected visually for any foreign particulate matter and/or abnormal physical
appearance. In the event of either being observed, discard the vaccine.
Any unused vaccine or waste material should be disposed of in accordance with local requirements.
Instructions for administration of the vaccine
1. Remove the protective tip cap from the
oral
applicator.
2. This vaccine is for
oral administration only
. The child should be seated in a reclining position.
Administer
orally
(i.e. into the child’s mouth,
towards the inner cheek) the entire content of the
oral
applicator.
3.
Do not inject.
1. Remove the protective tip cap from
the
oral
applicator.
2. This vaccine is for
oral
administration only
. The child
should be seated in a reclining
position. Administer
orally
(i.e. into
the child’s mouth,
towards the inner
cheek) the entire content of the
oral
applicator.
Discard the empty
oral
applicator and tip cap in approved biological waste containers according to
local regulations.
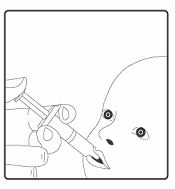



PACKAGE LEAFLET: INFORMATION FOR THE USER
Rotarix oral suspension
Rotavirus vaccine, live
Read all of this leaflet carefully before your child receives this vaccine.
-
Keep this leaflet. You may need to read it again.
This vaccine has been prescribed for your child. Do not pass it on to others.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
In this leaflet:
1.
What Rotarix is and what it is used for
2.
Before your child receives Rotarix
3.
How Rotarix is given
4.
Possible side effects
5.
How to store Rotarix
6. Further information
WHAT ROTARIX IS AND WHAT IT IS USED FOR
Rotarix is a viral vaccine, containing live, attenuated human rotavirus, that helps to protect your child,
from the age of 6 weeks, against gastro-enteritis (diarrhoea and vomiting) caused by rotavirus
infection.
Rotavirus infection is the most common cause of severe diarrhoea in infants and young children.
Rotavirus is easily spread from hand-to-mouth due to contact with stools from an infected person.
Most children with rotavirus diarrhoea recover on their own. However, some children become very ill
with severe vomiting, diarrhoea and life-threatening loss of fluids that requires hospitalisation.
When a person is given the vaccine, the immune system (the body’s natural defences) will make
antibodies against the most commonly occurring types of rotavirus. These antibodies protect against
disease caus ed by these types of rotavirus.
As with all vaccines, Rotarix may not completely protect all people who are vaccinated against the
rotavirus infections it is intended to prevent.
BEFORE YOUR CHILD RECEIVES ROTARIX
Rotarix should not be given:
•
if your child has previously had any allergic reaction to rotavirus vaccines or any component
contained in Rotarix. The active substances and other ingredients in Rotarix are listed at the end
of the leaflet. Signs of an allergic reaction may include itchy skin rash, shortness of breath and
swelling of the face or tongue.
if your child has previously had intussusception (a bowel obstruction in which one segment of
bowel becomes enfolded within another segment).
if your child was born with a malformation of the gut that could lead to intussusception.
if your child has a rare inherited illness which affects their immune system called Severe
Combined Immunodeficiency (SCID).
if your child has a severe infection with a high temperature. It might be necessary to postpone
the vaccination until recovery. A minor infection such as a cold should not be a problem, but
talk to your doctor first.
If you have any further questions, ask your doctor or pharmacist.
if your child has diarrhoea or is vomiting. It might be necessary to postpone the vaccination
until recovery.
Take special care with Rotarix
Before your child receives Rotarix, inform your doctor/health care professional if he/she:
•
has a close contact such as a household member who has a weakened immune system, e.g., a
person with cancer or who is taking medicines that may weaken the immune system.
has any disorder of the gastrointestinal system.
has not been gaining weight and growing as expected.
has any disease or is taking any medicine which reduces his/her resistance to infection.
After your child has received Rotarix, contact your doctor/health care professional right away if your
child exp eriences severe stomach pain, persistent vomiting, blood in stools, a swollen belly and/or
high fever.
As always, please take care to wash your hands thoroughly after changing soiled nappies.
Using other vaccines
Please tell your doctor if your child is taking or has recently taken any other medicines, including
medicines obtained without a prescription or has recently received any other vaccine.
Rotarix may be given at the same time your child receives other normally recommended vaccines,
such as diphtheria, tetanus, pertussis (whooping cough),
Haemophilus influenzae
type b, oral or
inactivated polio, hepatitis B vaccines as well as pneumococcal and meningococcal serogroup C
conjugate vaccines.
Using Rotarix with food and drink
There are no restrictions on your child’s consumption of food or liquids, either before or after
vaccination.
Breast-feeding
Based on evidence generated in clinical trials, breast-feeding does not reduce the protection against
rotavirus gastro-enteritis afforded by Rotarix. Therefore, breast-feeding may be continued during the
vaccination schedule.
Important information about some of the ingredients of Rotarix
If you have been told by your doctor that the child being vaccinated has an intolerance to some sugars,
contact your doctor before receiving this vaccine.
The doctor or nurse will administer the recommended dose of Rotarix to your child. The vaccine (1.5
ml liquid) will be given
orally
. Under no circumstance should this vaccine be administered by
injection.
Your child will receive two doses of the vaccine. Each dose will be given on a separate occasion with
an interval of at least 4 weeks between the two doses. The first dose may be given from the age of 6
weeks. The two doses of the vaccine must have been given by the age of 24 weeks, although they
should preferably have been given before 16 weeks of age.
Rotarix may be given according to the same vaccination course to infants who were born prematurely,
provided that the pregnancy had lasted at least 27 weeks.
In case your child spits out or regurgitates most of the vaccine dose, a single replacement dose may be
given at the same vaccination visit
.
When Rotarix is given to your child for the first dose, it is recommended that your child also receives
Rotarix (and not another rotavirus vaccine) for the second dose.
It is important that you follow the instructions of your doctor or nurse regarding return visits. If you
forget to go back to your doctor at the scheduled time, ask your doctor for advice.
Like all medicines, Rotarix can cause side effects, although not everybody gets them.
Side effects that occurred during clinical trials with Rotarix were as follows:
♦
Common (These may occur with up to 1 in 10 doses of the vaccine):
•
diarrhoea
•
irritability
♦
Uncommon (These may occur with up to 1 in 100 doses of the vaccine):
•
abdominal pain, flatulence
•
inflammation of the skin
Side effects that have been reported during marketed use of Rotarix include:
•
blood in stools
•
in babies born very prematurely (at or before 28 weeks of gestation) longer gaps than normal
between breaths may occur for 2-3 days after vaccination.
•
children with a rare inherited illness called Severe Combined Immunodeficiency (SCID)
may have an inflamed stomach or gut (gastroenteritis) and pass the vaccine virus in their
stools. The signs of gastroenteritis may include feeling sick, being sick, stomach cramps or
diarrhoea.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Rotarix after the expiry date which is stated on the carton. The expiry date refers to the last
day of that month.
Store in a refrigerator (2°C – 8°C).
Do not freeze.
Store in the original package in order to protect from light.
The vaccine should be used immediately after opening.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active subs tances are:
Human rotavirus RIX4414 strain (live, attenuated)*
not less than 10
6.0
CCID
50
The other ingredients in Rotarix are: sucrose, Di-sodium Adipate, Dulbecco’s Modified Eagle
Medium (DMEM), sterile water
What Rotarix looks like and contents of the pack
Rotarix is supplied as clear and colourless liquid in a single dose squeezable tube (1.5 ml).
Rotarix is available in a pack of 1, 10 or 50.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
GlaxoSmithKline Biologicals s.a.
Rue de l’Institut 89
B-1330 Rixensart
Belgium
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
GlaxoSmithKline s.a./n.v.
Tél/Tel: + 32 2 656 21 11
Luxembourg/Luxemburg
GlaxoSmithKline s.a./n.v.
Tél/Tel: + 32 2 656 21 11
България
ГлаксоСмитКлайн ЕООД
ул. Димитър Манов бл.10
София 1408
Тел. + 359 2 953 10 34
Magyarország
GlaxoSmithKline Kft.
Tel.: + 36-1-2255300
Česká republika
GlaxoSmithKline s.r.o.
Tel: + 420 2 22 00 11 11
czmail@gsk.com
Malta
GlaxoSmithKline (Malta) Ltd
Tel: + 356 21 238131
Danmark
GlaxoSmithKline Pharma A/S
Tlf: + 45 36 35 91 00
dk-info@gsk.com
Nederland
GlaxoSmithKline BV
Tel: + 31 (0)30 69 38 100
nlinfo@gsk.com
Deutschland
GlaxoSmithKline GmbH & Co. KG
Tel: + 49 (0)89 360448701
produkt.info@gsk.com
Norge
GlaxoSmithKline AS
Tlf: + 47 22 70 20 00
firmapost@gsk.no
Eesti
GlaxoSmithKline Eesti OÜ
Tel: +372 667 6900
estonia@gsk.com
Österreich
GlaxoSmithKline Pharma GmbH.
Tel: + 43 1 970 75-0
at.info@gsk.com
Ελλάδα
GlaxoSmithKline A.E.B.E
Tηλ: + 30 210 68 82 100
Polska
GSK Commercial Sp. z o.o.
Tel.: + 48 (22) 576 9000
España
GlaxoSmithKline, S.A.
Tel: + 34 902 202 700
es-ci@gsk.com
Portugal
Smith Kline & French Portuguesa, Produtos
Farmacêuticos, Lda.
Tel: + 351 21 412 95 00
FI.PT@gsk.com
France
Laboratoire GlaxoSmithKline
Tél: + 33 (0) 1 39 17 84 44
diam@gsk.com
România
GlaxoSmithKline (GSK) SRL
Tel: +40 (0)21 3028 208
Ireland
GlaxoSmithKline (Ireland) Ltd
Tel: + 353 (0)1 4955000
Slovenija
GlaxoSmithKline d.o.o.
Tel: + 386 (0) 1 280 25 00
medical.x.si@gsk.com
Ísland
GlaxoSmithKline ehf.
Sími: +354-530 3700
Slovenská republika
GlaxoSmithKline Slovakia s.r.o.
Tel: + 421 (0)2 48 26 11 11
recepcia.sk@gsk.com
Italia
GlaxoSmithKline S.p.A.
Tel:+ 39 04 59 21 81 11
Suomi/Finland
GlaxoSmithKline Oy
Puh/Tel: + 358 10 30 30 30
Finland.tuoteinfo@gsk.com
Κύπρος
GlaxoSmithKline Cyprus Ltd
Τηλ: + 357 22 39 70 00
Sverige
GlaxoSmithKline AB
Tel: + 46 (0)8 638 93 00
info.produkt@gsk.com
Latvija
GlaxoSmithKline Latvia SIA
Tel: + 371 67312687
lv-epasts@gsk.com
United Kingdom
GlaxoSmithKline UK
Tel: + 44 (0)808 100 9997
customercontactuk@gsk.com
Lietuva
GlaxoSmithKline Lietuva UAB
Tel: +370 5 264 90 00
info.lt@gsk.com
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency web site:
-------------------------------------------------------------------------------------------------------------------------
The following information is intended for medical or healthcare professionals only:
The vaccine is presented as a clear, colourless liquid, free of visible particles, for
oral
administration.
The vaccine is ready to use (no reconstitution or dilution is required).
The vaccine is to be administered
orally
without mixing with any other vaccines or solutions.
The vaccine should be inspected visually for any foreign particulate matter and/or abnormal physical
appearance. In the event of either being observed, discard the vaccine.
Any unused vaccine or waste material should be disposed of in accordance with local requirements.
Instructions for administration of the vaccine
1. Pull off the cap from the top of the tube.
2. Turn the cap upside-down and replace the cap vertically over the tip seal as shown in the diagram.
3. Twist the cap to remove the tip seal (do not snap it off) leaving a hole through which the vaccine
can be expelled.
4.
Ensure that the tip seal has been properly removed. A hole should be clearly visible at the tip of
the tube and the tip seal should be inside the top of the tube cap.
In the event that the tip seal is accidentally pushed into the tube, discard the vaccine. This is just a
precaution, since it is unlikely that the tip seal could be expelled from the tube while
administering the vaccine.
5. This vaccine is for
oral administration only
. The child should be seated in a reclining position.
Administer
orally
(i.e. into the child’s mouth,
towards the inner cheek) the entire content of the
tube by gently squeezing the tube several times. (A residual drop may remain in the tip of the
tube).
1. Pull off the cap from the top of the
tube.
2. Turn the cap upside-down and
replace the cap vertically over the
tip seal as shown in the diagram.
3. Twist the cap to remove the tip seal (do
not snap it off) leaving a hole through
which the vaccine can be expelled.
4. Ensure that the tip seal has been
properly removed. A hole should be clearly
visible at the tip of the tube and
the tip seal should be inside the top
of the tube cap.
In the event that the tip seal is accidentally
pushed into the tube, discard the vaccine.
This is just a precaution, since it is unlikely
that the tip seal could be expelled from the
tube while administering the vaccine.
5. This vaccine is for
oral administration
only
. The child should be seated in a
reclining position. Administer
orally
(i.e.
into the child’s mouth,
towards the inner
cheek) the entire content of the tube by
gently squeezing the tube several times.
(A residual drop may remain in the tip of
the tube).
Discard the empty tube and cap in approved biological waste containers according to local regulations.
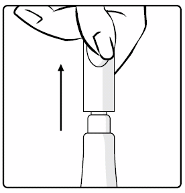
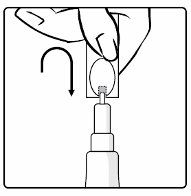
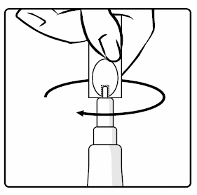
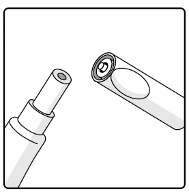










ANNEX IV
GROUNDS FOR ONE ADDITIONAL RENEWAL
GROUNDS FOR ONE ADDITIONAL RENEWAL
Based upon the data that have become available since the granting of the Marketing Authorisation, the
CHMP considers that the benefit-risk balance of Rotarix remains positive, but considers that its safety
profile is to be closely monitored for the following reasons :
Final data from post-authorisation safety studies are still awaited and the CHMP considers that the
Rotarix safety profile remains to be closely monitored. Given the remaining questions regarding the
risk of intussusception (IS) following administration of Rotarix, the regulatory authorities should be
kept updated of the results of the ongoing post-authorisation safety studies to further study IS, as well
as analyses of spontaneously reported IS cases. The CHMP considers that more safety experience
needs to be gained about IS and recommended that the MAH should continue to submit yearly PSURs.
Therefore, based on the safety profile of Rotarix, which requires the submission of yearly PSURs, the
CHMP concluded that the MAH should submit one additional renewal application in 5 years time.
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/rotarix.html
Copyright © 1995-2021 ITA all rights reserved.
|






































































































































































































































































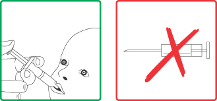





















































































































































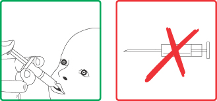










































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































