Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Rotavirus vaccine (live, oral)
QUALITATIVE AND QUANTITATIVE COMPOSITION
One 2-ml dose contains:
rotavirus serotype* G1
not less than 2.2 x 10
6
IU
1, 2
not less than 2.8 x 10
6
IU
1, 2
not less than 2.2 x 10
6
IU
1, 2
not less than 2.0 x 10
6
IU
1, 2
rotavirus serotype* P1[8]
not less than 2.3 x 10
6
IU
1, 2
* human-bovine rotavirus reassortants (live), produced in Vero cells.
1
Infectious Units
2
As lower confidence limit (p = 0.95)
Excipient:
This product contains sucrose 1080 mg (see section 4.4).
For a full list of excipients, see section 6.1.
Pale yellow clear liquid that may have a pink tint
4.1 Therapeutic indications
RotaTeq is indicated for the active immunisation of infants from the age of 6 weeks for prevention of
gastroenteritis due to rotavirus infection (see section 4.2).
In clinical trials, efficacy was demonstrated against gastroenteritis due to rotavirus of serotypes
G1P1[8], G2P[4], G3P1[8], G4P1[8], and G9P1[8]. See sections 4.4 and 5.1.
The use of RotaTeq should be in accordance with official recommendations.
4.2 Posology and method of administration
Posology
Three doses of RotaTeq should be administered.
The first dose may be administered from the age of six weeks and no later than the age of 12 weeks.
RotaTeq may be given to infants who were born prematurely provided that the period of gestation was
at least 25 weeks.
These infants should receive the first dose of RotaTeq at least six weeks after birth.
See sections 4.4 and 5.1.
There should be intervals of at least 4 weeks between doses.
It is preferable that all three doses should be administered before the age of 20-22 weeks.
All three doses should be given by the age of 26 weeks.
As no data exist regarding the interchangeability of RotaTeq with another rotavirus vaccine, it is
recommended that infants who receive RotaTeq for the first immunisation against rotavirus should
receive this same vaccine for the subsequent doses.
If it is observed or strongly suspected that an incomplete dose has been swallowed (e.g., infant spits or
regurgitates the vaccine), a single replacement dose may be given at the same vaccination visit
,
however, this has not been studied in clinical trials. If the problem recurs, additional replacement
doses should not be given.
No further doses are recommended after completion of the 3-dose series (see sections 4.4 and 5.1
regarding available information on persistence of protection).
Method of administration
For oral administration only.
RotaTeq SHOULD UNDER NO CIRCUMSTANCES BE INJECTED.
RotaTeq may be given without regard to food, liquid, or breast milk.
See section 6.6 for administration instructions.
Hypersensitivity to the active substance or to any of the excipients.
Hypersensitivity after previous administration of rotavirus vaccines.
Previous history of intussusception.
Subjects with congenital malformation of the gastrointestinal tract that could predispose to
intussusception.
Infants who have known or suspected immunodeficiency. Asymptomatic HIV infection is not
expected to affect the safety or efficacy of RotaTeq. However, in the absence of sufficient data,
administration of RotaTeq to asymptomatic HIV subjects is not recommended.
Administration of RotaTeq should be postponed in infants suffering from acute severe febrile illness.
The presence of a minor infection is not a contraindication for immunisation.
The administration of RotaTeq should be postponed in subjects suffering from acute diarrhoea or
vomiting.
4.4 Special warnings and precautions for use
No safety or efficacy data are available from clinical trials regarding administration of RotaTeq to
immunocompromised infants, infants infected with HIV or infants who have received a blood
transfusion or immunoglobulins within 42 days of dosing.
Cases of gastroenteritis associated with vaccine virus have been reported post marketing in infants
with severe combined immunodeficiency (SCID).
In trials, RotaTeq was shed in the stools of 8.9 % of vaccine recipients almost exclusively in the week
after dose 1 and in only one vaccine recipient (0.3 %) after dose 3. Peak excretion occured within
7 days of dosing. It is theoretically possible that transmission of vaccine virus may occur to
seronegative contacts. RotaTeq should be administered with caution to individuals with close contacts
who are immunodeficient (e.g., individuals with malignancies or who are otherwise
immunocompromised or individuals receiving immunosuppressive therapy). Also, those caring for
recent vaccinees should observe careful hygiene especially when handling excreta.
In a clinical study, RotaTeq was administered to approximately 1,000 infants who were born at a
gestational age of 25 to 36 weeks. The first dose was administered from 6 weeks after birth. The safety
and efficacy of RotaTeq were comparable between this subset of infants and infants born at term.
However, 19 of the approximately 1,000 infants were born at a gestational age of 25 to 28 weeks, 55
were born at a gestational age of 29 to 31 weeks and the remainder was born at a gestational age of
between 32 and 36 weeks. See sections 4.2 and 5.1.
Safety or efficacy data are not available for infants with active gastrointestinal illnesses (including
chronic diarrhoea) or growth retardation. Administration of RotaTeq may be considered with caution
in such infants when, in the opinion of the physician, withholding the vaccine entails a greater risk.
The level of protection provided by RotaTeq is based on the completion of all 3 doses. As with any
vaccine, vaccination with RotaTeq may not result in complete protection in all recipients. RotaTeq
does not protect against gastroenteritis due to other pathogens than rotavirus.
Clinical trials of efficacy against rotavirus gastroenteritis were performed in Europe, the United States,
Latin America, and Asia. During these trials, the most common circulating rotavirus serotype was
G1P1[8], while rotavirus serotypes G2P[4], G3P1[8], G4P1[8], and G9P1[8] were identified less
often. The extent of protection that RotaTeq
might provide against other rotavirus serotypes and in
other populations is unknown.
No clinical data are available on the use of RotaTeq for post-exposure prophylaxis.
RotaTeq contains sucrose. Patients with rare hereditary problems of fructose intolerance, glucose-
galactose malabsorption or sucrase-isomaltase insufficiency should not take this vaccine.
The potential risk of apnoea and the need for respiratory monitoring for 48-72h should be considered
when administering the primary immunisation series to very premature infants (born ≤ 28 weeks of
gestation) and particularly for those with a previous history of respiratory immaturity. As the benefit
of vaccination is high in this group of infants, vaccination should not be withheld or delayed.
4.5 Interaction with other medicinal products and other forms of interaction
Co-administration of RotaTeq with vaccines containing one or more of the following antigens at
approximately 2, 4 and 6 months of age demonstrated that the immune responses and the safety
profiles of the administered vaccines were unaffected:
-
Diphtheria-tetanus-acellular pertussis vaccine (DTaP)
-
Haemophilus influenzae
type b vaccine (Hib)
-
Inactivated poliomyelitis vaccine (IPV)
-
Hepatitis B vaccine (HBV)
Pneumococcal conjugate vaccine (PCV)
Co-administration of RotaTeq with DTaP-IPV-HBV-Hib vaccine (Infanrix hexa) at approximately 2,
3, and 4 months of age demonstrated that the immune responses and the safety profiles of the
co-administered vaccines were unaffected compared to separate administrations.
Co-administration of RotaTeq with a group C meningococcal conjugate vaccine (MenCC, the vaccine
studied was a tetanus toxoid conjugate) at 3 and 5 months of age (and mostly at the same time as
DTaP-IPV-Hib vaccine), followed by a third dose of RotaTeq at approximately 6 months of age,
demonstrated that the immune responses to RotaTeq and MenCC were unaffected. Co-administration
resulted in an acceptable safety profile.
Concomitant administration of RotaTeq and oral poliomyelitis vaccine (OPV) did not affect the
immune response to the poliovirus antigens. Although concomitant administration of OPV slightly
reduced the immune response to rotavirus vaccine, there is currently no evidence that clinical
protection against severe rotavirus gastroenteritis would be affected. The immune response to RotaTeq
was unaffected when OPV was administered two weeks after RotaTeq.
Therefore, RotaTeq can be given concomitantly with monovalent or combination infant vaccines
containing one or more of the following antigens: DTaP, Hib, IPV or OPV, HBV, PCV and MenCC.
4.6 Pregnancy and lactation
RotaTeq is intended for use in infants only. Thus human data on use during pregnancy or lactation are
not available and animal reproduction studies have not been performed.
4.7 Effects on ability to drive and use machines
In a subset of infants from 3 placebo-controlled clinical trials (n=6,130 recipients of RotaTeq and
5,560 placebo recipients), RotaTeq was evaluated for all adverse events within 42 days of vaccination
with or without concomitant use of other paediatric vaccines. Overall, 47 % of infants given RotaTeq
experienced an adverse reaction compared with 45.8 % of infants given placebo. The most commonly
reported adverse reactions that occurred more frequently with vaccine than with placebo were pyrexia
(20.9 %), diarrhoea (17.6 %) and vomiting (10.1 %).
Adverse reactions more common in the vaccine group are listed below per system organ class and
frequency. Based on pooled data from 3 clinical trials in which 6,130 infants received RotaTeq and
5,560 received placebo, the adverse reactions listed occurred with excess incidences in RotaTeq
recipients compared to placebo recipients of between 0.2 % and 2.5 %.
Frequencies are reported as:
Very Common (≥1/10); Common (≥1/100, <1/10); Uncommon (≥1/1,000, <1/100); Rare (≥1/10,000,
<1/1,000)
Infections and infestations
Common: Upper respiratory tract infection
Uncommon: Nasopharyngitis
Gastrointestinal disorders
Very common: Diarrhoea, Vomiting
Uncommon: Abdominal pain upper
Skin and subcutaneous tissue disorders
Uncommon: Rash
General disorders and administration site conditions
Very common: Pyrexia
Serious adverse reactions were assessed in all participants (36,150 recipients of RotaTeq and 35,536
placebo recipients) of 3 clinical trials for up to 42 days after each dose. The overall frequency of these
serious adverse reactions was 0.1 % among recipients of RotaTeq and 0.2 % among placebo
recipients.
Kawasaki disease was reported in 5 of 36,150 vaccine recipients (<0.1 %) and 1 of 35,536 placebo
recipients (<0.1 %) with a relative risk (RR) of 4.9 [95% CI, 0.6 – 239.1] (not statistically significant).
No increased risk of Kawasaki disease was observed among infants receiving RotaTeq in a large post-
marketing observational safety surveillance study (see section 5.1).
Otitis media and bronchospasm were reported in significantly more vaccine than placebo recipients
overall; however, among cases that were considered to be vaccine-related in the opinion of the study
investigator, the incidence of otitis media (0.3 %) and bronchospasm (<0.1 %) was the same for
vaccine and placebo recipients.
Haematochezia was reported as an adverse experience in 0.6 % (39/6,130) of vaccine recipients and
0.6% (34/5,560) of placebo recipients within 42 days of any dose (the difference was not statistically
significant).
Intussusception
The risk of intussusception has been evaluated in a placebo-controlled study in infants. During the
combined 42-day periods following each dose, there were 6 cases of intussusception in 34,837
recipients of RotaTeq compared with 5 cases in 34,788 placebo recipients. The 95% CI for the relative
risk were 0.4, 6.4. There was no clustering of cases among recipients of RotaTeq at any time period
after any dose.
No increased risk of intussusception was observed among infants receiving RotaTeq in a large post-
marketing observational safety surveillance study (see section 5.1).
Post-marketing reports
The following adverse experiences have been spontaneously reported with RotaTeq.
Gastrointestinal disorders:
Haematochezia
Skin and subcutaneous tissue disorders:
Urticaria
Additional information on special populations:
Apnoea in very premature infants (≤ 28 weeks of gestation) (see section 4.4)
There are no data with regard to overdose.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group:
viral vaccines
ATC code:
J07BH02
.
Efficacy
The protective efficacy of RotaTeq was evaluated in two ways in the placebo-controlled Rotavirus
Efficacy and Safety Trial (REST):
In 5,673 vaccinated infants (2,834 in the vaccine group) protective efficacy was measured as a
reduction in the incidence of rotavirus (RV) gastroenteritis caused by vaccine G serotypes (G1-
G4) that occurred at least 14 days after the third dose of vaccine through the first full rotavirus
season after vaccination.
In 68,038 vaccinated infants (34,035 in the vaccine group) protective efficacy was measured as
a reduction in the rate of hospitalisations and emergency department visits for RV
gastroenteritis from 14 days after the third dose.
The results of these analyses are presented in the following tables.
Reduction in incidence of RV gastroenteritis through one full season post-vaccination
(RotaTeq n=2,834) (% [95 % CI])
* Severe defined as a score >16/24 using a validated clinical scoring system based on the intensity and
duration of symptoms (fever, vomiting, diarrhoea and behavioural changes)
†
Statistically significant
Reduction in hospitalisations and emergency department visits for RV gastroenteritis for up to 2 years
post-vaccination
(RotaTeq n=34,035) (% [95 % CI])
†
Statistically significant
The reduction in incidence of RV gastroenteritis caused by G1-G4 during the second rotavirus season
after vaccination was 88.0 % [95 % CI 49.4, 98.7] for severe disease and 62.6 % [95 % CI 44.3, 75.4]
for disease of any severity.
Efficacy against G2P[4], G3P1[8], G4P1[8] and G9P1[8] rotavirus was based on fewer cases than for
G1. The efficacy observed against G2P[4] most likely resulted from the G2 component of the vaccine.
There was an extension of REST conducted in Finland only. This Finnish Extension Study (FES)
included a subset of 20,736 subjects that had been enrolled previously in REST. The infants were
followed for up to 3 years post-vaccination in the FES.
In REST there were 403 healthcare encounters (20 in the vaccine group and 383 in the placebo group)
associated with G1-G4 and G9 RV gastroenteritis in the per protocol population. The additional data
from the FES increased the number by 136 encounters in total, including 9 in the vaccine group and
127 in the placebo group. Overall, 31% and 25% of the encounters in the respective groups occurred
during the FES.
Based upon combined data from REST and the FES, the reduction up to 3 years post-vaccination in
the rate of hospitalisations and emergency department visits for RV gastroenteritis was 94.4% (95%
CI: 91.6, 96.2) for serotypes G1-G4
,
95.5% (95% CI: 92.8, 97.2) for serotype G1, 81.9% (95% CI:
16.1, 98.0) for serotype G2, 89.0% (95% CI: 53.3, 98.7) for serotype G3, 83.4% (95% CI: 51.2, 95.8)
for serotype G4, and 94.2% (95% CI: 62.2, 99.9) for serotype G9. During year 3, there were no health
care contacts for RV gastroenteritis in the vaccine group (n=3,112) and one (non-typeable) in the
placebo group (n=3,126).
A complete 3-dose vaccination series of RotaTeq should be administered (see section 4.2) to provide
the level and duration of protection against rotavirus gastroenteritis that was observed in the clinical
studies. However, post hoc analyses indicated that RotaTeq achieved some reduction in the numbers
of cases of rotavirus gastroenteritis of sufficient severity to require hospitalisation or an emergency
department visit before completion of all three doses (i.e. from approximately 14 days after
administration of the first dose onwards).

















Efficacy in premature infants
In REST, RotaTeq was administered to approximately 1,000 infants who were born at a gestational
age of 25 to 36 weeks. The efficacy of RotaTeq was comparable between this subset of infants and
infants born at term.
Post-marketing observational safety surveillance study
In a large prospective post-marketing observational study the risks of intussusception and Kawasaki
disease were analysed among 85,150 infants receiving one or more doses of RotaTeq (17,433 person-
years of follow-up)
During the 0-30 day follow-up period after vaccination, there were no statistically significant
differences in the rates of intussusception or Kawasaki disease compared with the expected
background rates. In addition, there was no statistically significant increased risk of these adverse
events during the 0-30 day follow-up period compared with a concurrent control group of infants who
received DTaP, but not RotaTeq (n=62,617, 12,339 person-years of follow-up). For intussusception, 6
confirmed cases were recorded among infants vaccinated with RotaTeq compared with 5 among the
concurrent controls vaccinated with DTaP (relative risk = 0.8, 95% CI: 0.22-3.52). For Kawasaki
disease, one chart-confirmed case was recorded among infants vaccinated with RotaTeq compared
with one chart-confirmed case among concurrent DTaP controls (relative risk = 0.7, 95% CI: 0.01-
55.56). In the general safety analyses, no specific safety concerns were identified.
Immunogenicity
The immunological mechanism by which RotaTeq protects against rotavirus gastroenteritis is not
completely understood. No immunological correlate of protection has currently been identified for
rotavirus vaccines. In phase III studies between 92.5 % and 100 % of recipients of RotaTeq achieved a
significant rise in serum anti-rotavirus IgA after a three-dose regimen. The vaccine induces an immune
response (i.e., appearance of serum neutralising antibody) to the five human-rotavirus proteins
expressed on the reassortants (G1, G2, G3, G4 and P1[8]).
5.2 Pharmacokinetic properties
Evaluation of pharmacokinetic properties is not required for vaccines.
5.3 Preclinical safety data
A single and repeated dose oral toxicity study in mice suggests no special hazard to humans. The dose
administered to mice was approximately 2.79 x 10
8
infectious units per kg (about 14-fold the projected
infant dose).
RotaTeq is unlikely to pose any environmental risk.
PHARMACEUTICAL PARTICULARS
Sucrose
Sodium citrate
Sodium dihydrogen phosphate monohydrate
Sodium hydroxide
Polysorbate 80
Culture media (containing inorganic salts, amino acids and vitamins)
Purified water
The vaccine must not be mixed with other medicinal products.
RotaTeq should be administered promptly after removal from refrigeration.
6.4 Special precautions for storage
Store in a refrigerator (2 °C – 8 °C).
Keep the dosing tube in the outer carton in order to protect from light.
6.5 Nature and contents of container
2 ml solution in a pre-filled squeezable tube (LDPE), with a twist-off cap (HDPE) in a protective bag,
pack size of 1 or in a pack of 10.
Not all pack sizes may be marketed.
Special precautions for disposal
The vaccine is to be administered orally without mixing with any other vaccines or solutions. Do not
dilute.
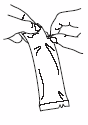
To administer the vaccine:
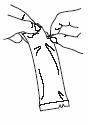
Tear open the protective bag and remove the dosing tube.
Clear the fluid from the dispensing tip by holding tube vertically and tapping the
twist-off cap.
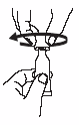
Open the dosing tube in 2 easy motions:
1.
Puncture the dispensing tip by screwing cap
clockwise
until it becomes
tight.
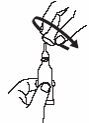
2.
Remove cap by turning it
counterclockwise
.









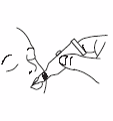
Administer dose by gently squeezing liquid into infant's mouth toward the inner
cheek until dosing tube is empty. (A residual drop may remain in the tip of the
tube.)
Discard the empty tube and cap in approved biological waste containers
according to local regulations.
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Sanofi Pasteur MSD, SNC
8, rue Jonas Salk
F-69007 LYON
France
MARKETING AUTHORISATION NUMBERS
EU/1/06/348/001
EU/1/06/348/002
DATE OF FIRST AUTHORISATION/RENEWAL OF AUTHORISATION
10. DATE OF REVISION OF THE TEXT





A. MANUFACTURER OF THE BIOLOGICAL ACTIVE
SUBSTANCE AND MANUFACTURING AUTHORISATION
HOLDER RESPONSIBLE FOR BATCH RELEASE
B. CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURER OF THE BIOLOGICAL ACTIVE SUBSTANCE AND
MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer of the biological active substance
Name of Company: Merck & Co., Inc
Address:
Sumneytown Pike – PO Box 4 – West Point – Pennsylvania 19486
Name and address of the manufacturer responsible for batch release
Name of Company: Merck Sharp and Dohme BV
Address:
Waarderweg 39, 2031 BN Haarlem, P.O. Box 581, 2003 PC Haarlem
The printed package leaflet of the medicinal product must state the name and address of the
manufacturer responsible for the release of the concerned batch.
B. CONDITIONS OF THE MARKETING AUTHORISATION
•
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to medical prescription
•
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
Pharmacovigilance system
The MAH must ensure that the system of pharmacovigilance, as described in version 2.0 presented in
Module 1.8.1. of the Marketing Authorisation Application, is in place and functioning before and
whilst the product is on the market.
Official batch release: in accordance with Article 114 Directive 2001/83/EC as amended, the official
batch release will be undertaken by a state laboratory or a laboratory designated for that purpose.
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
RotaTeq – Pack size of 1 single-dose(2ml) Tube
RotaTeq – Pack size of 10 single-dose(2ml) Tubes
NAME OF THE MEDICINAL PRODUCT
RotaTeq, oral solution
Rotavirus vaccine (live, oral)
STATEMENT OF ACTIVE SUBSTANCE(S)
One 2 ml dose contains rotavirus serotype*:
G1
* human-bovine rotavirus reassortants (live), produced in Vero cell.
PHARMACEUTICAL FORM AND CONTENTS
2 ml oral solution in a tube
pack size of 1 tube
pack size of 10 tubes
METHOD AND ROUTE(S) OF ADMINISTRATION
FOR ORAL USE ONLY
Read the package leaflet before use
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
























SPECIAL STORAGE CONDITIONS
Store in a refrigerator
Keep the dosing tube in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Please read the package leaflet for disposal of medicines no longer required
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Sanofi Pasteur MSD, SNC
8, rue Jonas Salk
F-69007 Lyon
France
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/06/348/001 pack of 1 tube
EU/1/06/348/002 pack of 10 tubes
13. MANUFACTURER’S BATCH NUMBER
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
16. INFORMATION IN BRAILLE

























PACKAGE LEAFLET: INFORMATION FOR THE USER
RotaTeq, oral solution
Rotavirus vaccine (live oral)
Read all of this leaflet before your child is vaccinated.
-
If you have any further questions, ask your doctor/health care professional.
This vaccine has been prescribed for your child. Do not pass it on to others.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor/health care professional.
What RotaTeq is and what it is used for
Before your child receives RotaTeq
WHAT RotaTeq IS AND WHAT IT IS USED FOR
Type of Medicine: vaccine against a virus
RotaTeq is an oral vaccine that helps protect infants and young children against gastroenteritis
(diarrhoea and vomiting) caused by rotavirus infection. The vaccine contains five types of live
rotavirus strains. When
an infant is given the vaccine, the immune system (the body’s natural
defences) will make antibodies against the most commonly occurring types of rotavirus. These
antibodies help protect against gastroenteritis caused by these types of rotavirus.
BEFORE YOUR CHILD RECEIVES RotaTeq
your child is allergic to any of the components of the vaccine (see section 6).
your child developed an allergic reaction after receiving a dose of RotaTeq or other rotavirus
vaccine.
your child has previously had intussusception (a bowel obstruction in which one segment of
bowel becomes enfolded within another segment).
your child was born with a malformation of the gastrointestinal system that might predispose for
intussusception.
your child has a severe infection with a high temperature. It might be necessary to postpone the
vaccination until recovery. A minor infection such as a cold should not be a problem, but talk to
your doctor first.
your child has diarrhoea or is vomiting. It might be necessary to postpone the vaccination until
recovery.
Take special care with RotaTeq:
Inform your doctor/health care professional if your child:
-
has received a blood transfusion or immunoglobulins within the last 6 weeks.
Keep this leaflet. You may need to read it again.
your child has any disease which reduces his/her resistance to infection.
has a close contact such as a household member who has a weakened immune system, e.g., a
person with cancer or who is taking medicines that may weaken the immune system.
has any disorder of the gastrointestinal system.
has not been gaining weight and growing as expected.
As always, please take care to wash your hands thoroughly after changing soiled nappies.
Also see
Important information about some of the ingredients of RotaTeq
below.
As with other vaccines, RotaTeq may not completely protect all children who are vaccinated even
after all three doses have been given.
If your child has already been infected with rotavirus but is not yet ill when vaccinated, RotaTeq may
not be able to prevent the illness.
RotaTeq does not protect against diarrhoea and vomiting due to causes other than rotavirus.
Using other medicines and other vaccines:
RotaTeq may be given at the same time as your child receives other normally recommended
vaccinations, such as diphtheria, tetanus, pertussis (whooping cough),
Haemophilu
s
influenzae
type b,
inactivated or oral poliomyelitis, hepatitis B, pneumococcal conjugate and meningococcus group C
conjugate vaccines.
Please tell your doctor/health care professional if your child is taking or has recently taken any other
medicine, including medicines obtained without a prescription.
Taking RotaTeq with food and drink:
There are no restrictions on taking
food or liquid, including breast milk, either before or after
vaccination with RotaTeq.
Important information about some of the ingredients of RotaTeq:
RotaTeq contains sucrose. If you have been told that your child has an intolerance to some sugars,
inform your doctor/health care professional before the vaccine is administered.
RotaTeq IS FOR ORAL USE ONLY.
A doctor or nurse will administer the recommended doses of RotaTeq to your child. The vaccine (2 ml
of liquid per dose) will be given by gently squeezing the tube and delivering the vaccine into your
child’s mouth. The vaccine can be given without regard to food, liquid, or breast milk. In case your
child spits out or regurgitates most of the vaccine dose, a single replacement dose may be given at the
same vaccination visit.
Under no circumstance should this vaccine be administered by injection.
The first dose of RotaTeq may be given from the age of 6 weeks and should be given before 12 weeks
of age (about 3 months). RotaTeq may be given to infants who were born early provided that the
pregnancy had lasted at least 25 weeks. These infants should receive the first dose of vaccine between
6 and 12 weeks after birth.
Your child will receive 3 doses of RotaTeq given at least four weeks apart. It is important that your
child receives all 3 doses of the vaccine for protection against rotavirus. It is preferred that all three
doses should be given by the age of 20-22 weeks and at latest all three doses should be given by the
age of 26 weeks.
When RotaTeq is given to your child for the first dose, it is recommended that your child also receives
RotaTeq (and not another rotavirus vaccine) to complete the vaccination course.
If you forget an appointment for RotaTeq:
It is important that you follow the instructions of your doctor/health care professional regarding your
child’s return visits for the follow-up doses. If you forget or are not able to go back to your
doctor/health care professional at the scheduled time, ask him or her for advice.
Like all medicines, RotaTeq can cause side effects, although not everybody gets them.
The following side effects were reported in clinical studies with the use of RotaTeq:
Very common (occurs in more than 1 in 10 infants): fever, diarrhoea, vomiting.
Common (occurs in more than 1 in 100 infants): infections of the upper respiratory system.
Uncommon (occurs in less than 1 in 100 infants): stomach pains, runny nose and sore throat, ear
infection, rash.
Rare (occurs in less than 1 in 1000 infants): bronchospasm (wheezing or coughing).
Side effects that have been reported during marketed use include:
blood in stool, hives.
In babies born very prematurely (at or before 28 weeks of gestation) longer gaps than normal between
breaths may occur for 2-3 days after vaccination.
Ask your doctor/health care professional if you want more information about side effects for RotaTeq.
If any of the side effects gets serious, or if you noticed any side effects not listed in this leaflet, please
tell your doctor/health care professional. If the condition persists or worsens, seek medical attention.
Keep out of the reach and sight of children.
Store in a refrigerator (2 °C to 8 °C). Keep the dosing tube in the outer carton in order to protect from
light.
Do not use any of the dosing tubes of RotaTeq after the expiry date which is stated on the label after
EXP. The expiry date refers to the last day of that month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active substances in RotaTeq are 5 human-bovine reassortant rotavirus strains:
G1
2.2 x 10
6
Infectious Units
2.8 x 10
6
Infectious Units
G3 2.2 x 10
6
Infectious Units
G4 2.0 x 10
6
Infectious Units
P1[8] 2.3 x 10
6
Infectious Units
The other ingredients in RotaTeq are: sucrose, sodium citrate, sodium dihydrogen phosphate
monohydrate, sodium hydroxide, polysorbate 80, culture media (containing inorganic salts, amino
acids and vitamins), and purified water.
What RotaTeq looks like and contents of the pack
This vaccine is contained in a single-dose tube and is a pale yellow clear liquid that may have a pink
tint.
RotaTeq is available in pack size of 1, 10. Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder: Sanofi Pasteur MSD SNC, 8, rue Jonas Salk, F-69007 Lyon, France
Manufacturer Responsible for Batch Release: Merck Sharp and Dohme, B.V., Waarderweg, 39, 2031
BN, Haarlem, The Netherlands
For any information about this medicinal product, please contact the local representative of the
Marketing Authorisation Holder.
België/Belgique/Belgien:
Sanofi Pasteur MSD, Tél/Tel: +32.2.726.95.84
България:
Мерк Шарп и Доум България ЕООД, тел. + 359 2 8193740
Česká republika:
Merck Sharp & Dohme IDEA, Inc., org. sl., Tel: +420.233.010.111
Danmark:
Sanofi Pasteur MSD, Tlf: +45.23.32.6929
Deutschland:
Sanofi Pasteur MSD GmbH, Tel: +49.6224.5940
Eesti:
Merck Sharp & Dohme OÜ, Tel: +372.613.9750
Ελλάδα:
ΒΙΑΝΕΞ Α.Ε., Τηλ: +30.210.8009111
España:
Sanofi Pasteur MSD S.A., Tel: +34.91.371.78.00
France:
Sanofi Pasteur MSD SNC, Tél: +33.4.37.28.40.00
Ireland:
Sanofi Pasteur MSD Ltd, Tel: +3531.468.5600
Ísland:
Sanofi Pasteur MSD, Sími: +32.2.726.95.84
Italia:
Sanofi Pasteur MSD Spa, Tel: +39.06.664.092.11
Kύπρος:
Merck Sharp & Dohme (Middle East) Limited., Τηλ: +357 22866700
Latvija:
SIA Merck Sharp & Dohme Latvija, Tel: +371.67364.224
Lietuva:
UAB Merck Sharp & Dohme, Tel. +370 5 2780 247
Luxembourg/Luxemburg:
Sanofi Pasteur MSD, Tél: +32.2.726.95.84
Magyarország:
MSD Magyarország Kft, Tel: + 36.1.888.5300
Malta:
Merck Sharp & Dohme (Middle East) Limited., Tel: +357 22866700
Nederland:
Sanofi Pasteur MSD, Tel: +31.23.567.96.00
Norge:
Sanofi Pasteur MSD, Tlf: +47.67.50.50.20
Österreich:
Sanofi Pasteur MSD GmbH, Tel: +43.1.866.70.22.202
Polska:
MSD Polska Sp. z o.o., Tel.: +48.22.549.51.00
Portugal
: Sanofi Pasteur MSD, SA, Tel: +351 21 470 45 50
România
: Merck Sharp & Dohme Romania S.R.L., Tel: + 4021 529 29 00
Slovenija:
Merck Sharp & Dohme, inovativna zdravila d.o.o., Tel: +386.1.520.4201
Slovenská republika:
Merck Sharp & Dohme IDEA, Inc., Tel: +421.2.58282010
Suomi/Finland:
Sanofi Pasteur MSD, Puh/Tel: +358.9.565.88.30
Sverige:
Sanofi Pasteur MSD, Tel: +46.8.564.888.60
United Kingdom:
Sanofi Pasteur MSD Ltd, Tel: +44.1.628.785.291
This leaflet was last approved in
:
The following information is intended for medical or health care professionals only:
To administer the vaccine:
Tear open the protective bag and remove the dosing tube.
Clear the fluid from the dispensing tip by holding tube vertically and
tapping the twist-off cap.
Open the dosing tube in 2 easy motions:
1.
Puncture the dispensing tip by screwing cap
clockwise
until it
becomes tight.
2.
Remove cap by turning it
counterclockwise
.
Administer dose by gently squeezing liquid into infant's mouth toward
the inner cheek until dosing tube is empty. (A residual drop may
remain in the tip of the tube.)
Discard the empty tube and cap in approved biological waste containers
according to local regulations.
Any unused product or waste material should be disposed of in accordance with local requirements.
See also section 3. HOW RotaTeq IS GIVEN.














Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/rotateq.html
Copyright © 1995-2021 ITA all rights reserved.
|



































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































