Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
SonoVue 8 microlitres / ml powder and solvent for dispersion for injection
QUALITATIVE AND QUANTITATIVE COMPOSITION
One ml contains 8 µl of sulphur hexafluoride microbubbles
On reconstitution as directed, 1 ml of the resulting dispersion contains 8 µl sulphur hexafluoride in the
microbubbles, equivalent to 45 microgrammes.
For a full list of excipients, see section 6.1
Powder and solvent for dispersion for injection.
SonoVue is a kit including
1 vial containing 25 mg of lyophilised powder
1 pre-filled syringe containing 5 ml sodium chloride
1 Mini-Spike transfer system
Information on the appearance of the reconstituted solution is given in section 6.6.
4.1 Therapeutic indications
This medicinal product is for diagnostic use only.
SonoVue is for use with ultrasound imaging to enhance the echogenicity of the blood, which results in
an improved signal to noise ratio.
SonoVue should only be used in patients where study without contrast enhancement is inconclusive.
SonoVue is a transpulmonary echocardiographic contrast agent for use in patients with suspected or
established cardiovascular disease to provide opacification of cardiac chambers and enhance left
ventricular endocardial border delineation.
Doppler of macrovasculature
SonoVue increases the accuracy in detection or exclusion of abnormalities in cerebral arteries and
extracranial carotid or peripheral arteries
by improving the Doppler signal to noise ratio.
SonoVue increases the quality of the Doppler flow image and the duration of clinically
-
useful signal
enhancement in portal vein assessment.
Doppler of microvasculature
SonoVue improves display of the vascularity of liver and breast lesions during Doppler sonography,
leading to more specific lesion characterisation.
4.2 Posology and method of administration
This product should only be used by physicians experienced in diagnostic ultrasound imaging.
The recommended doses of SonoVue are:
B-mode imaging of cardiac chambers, at rest or with stress: 2 ml.
Vascular Doppler imaging: 2.4 ml.
During a single examination, a second injection of the recommended dose can be made when deemed
necessary by the physician.
Elderly Patients
The dosage recommendations also apply to elderly patients.
Paediatric Patients
The safety and effectiveness of SonoVue in patients under 18 years old has not been established and
the product should not be used in these patients.
The microbubble dispersion is prepared before use by injecting through the septum 5 ml of sodium
chloride 9 mg/ml (0.9%) solution for injection to the contents of the vial. The vial is then shaken
vigorously for a few seconds until the lyophilisate is completely dissolved. The desired volume of the
dispersion can be drawn into a syringe any time up to six hours after reconstitution. Just before
drawing into the syringe, the vial should be agitated to re-suspend the microbubbles. SonoVue should
be administered immediately after drawing into the syringe by injection into a peripheral vein. Every
injection should be followed by a flush with 5 ml of sodium chloride 9 mg/ml (0.9%) solution for
injection.
For instructions for preparation see section 6.6.
SonoVue should not be administered to patients with known hypersensitivity to sulphur hexafluoride
or to any of the components of SonoVue.
SonoVue is contraindicated for use in patients
with recent acute coronary syndrome or clinically
unstable ischaemic cardiac disease, including: evolving or ongoing myocardial infarction, typical
angina at rest within last 7
days, significant worsening of cardiac symptoms within last 7
days, recent
coronary artery intervention or other factors suggesting clinical instability (for example, recent
deterioration of ECG, laboratory or clinical findings), acute cardiac failure, Class III/IV cardiac
failure, or severe rhythm disorders.
SonoVue is contraindicated in patients known to have right
-
to
-
left shunts, severe pulmonary
hypertension (pulmonary artery pressure >90
mmHg), uncontrolled systemic hypertension, and in
patients with adult respiratory distress syndrome.
The safety and efficacy of SonoVue have not been established in pregnant and lactating women
therefore, SonoVue should not be administered during pregnancy and lactation (see Section 4.6).
4.4 Special warnings and precautions for use
ECG monitoring should be performed in high
-
risk patients as clinically indicated.
It should be emphasised that stress echocardiography, which can mimic an ischaemic episode, could
potentially increase the risk of SonoVue utilisation. Therefore, if SonoVue is to be used in conjunction
with stress echocardiography patients must have a stable condition verified by absence of chest pain or
ECG modification during the two preceding days.
Moreover, ECG and blood pressure monitoring should be performed during SonoVue
-
enhanced
echocardiography with a pharmacological stress (e.g. with dobutamine).
Care should be taken in patients with ischaemic cardiac disease because in these patients allergy
-
like
and/or vasodilatory reactions may lead to life
-
threatening conditions.
Emergency equipment and personnel trained in its use must be readily available. Caution is advised
when SonoVue is administered to patients with clinically significant pulmonary disease, including
severe chronic obstructive pulmonary disease.
It is recommended to keep the patient under close medical supervision during and for at least
30
minutes following the administration of SonoVue.
Numbers of patients with the following conditions who were exposed to SonoVue in the clinical trials
were limited, and therefore, caution is advisable when administering the product to patients with
:
acute endocarditis, prosthetic valves,
acute systemic inflammation and/or sepsis, hyperactive
coagulation states and/or recent
thromboembolism, and end-stage renal or hepatic disease.
SonoVue is not suitable for use in ventilated patients, and those with unstable neurological diseases.
In animal studies, the application of echo
-
contrast agents revealed biological side effects (e.g.
endothelial cell injury, capillary rupture) by interaction with the ultrasound beam. Although these
biological side effects have not been reported in humans, the use of a low mechanical index is
recommended.
4.5 Interaction with other medicinal products and other forms of interaction
No specific interaction studies have been performed. There was no apparent relationship with respect
to occurrence of adverse events in the clinical studies for patients receiving various categories of the
most common concomitant medications.
4.6 Pregnancy and lactation
No clinical data on exposed pregnancies are available. Animal studies do not indicate harmful effects
with respect to pregnancy, embryonal/foetal development, parturition or postnatal development (see
section 5.3 Preclinical safety data). Caution should be exercised when prescribing to pregnant women.
It is not known if sulphur hexafluoride is excreted in human milk
.
Therefore, caution should be
exercised when SonoVue is administered to breast-feeding women.
4.7 Effects on ability to drive and use machines
On the basis of the pharmacokinetic and pharmacodynamic profiles, no or negligible influence is
expected with the use of SonoVue on the ability to drive or use machines.
The safety of Sonovue was evaluated in 4653 adult patients who participated in 58 clinical trials..
The adverse reactions are classified by System Organ Class and frequency, using the following
convention: Very common (≥ 1/10), Common (≥ 1/100 to < 1/10), Uncommon (≥ 1/1,000 to < 1/100),
Rare (≥ 1/10,000 to < 1/1,000), Very rare (< 1/10,000), not known (cannot be estimated from the
available data)
Adverse Drug Reactions
Frequency Category
Uncommon
(≥1/1,000to <1/100)
Rare
(≥1/10,000to<1/1000)
Not known
Cannot be estimated
from available data
Hypersensitivity,
anaphylactic reaction,
anaphylactoid reaction
Headache, paraesthesia,
dizziness, dysgeusia
Respiratory, thoracic and
mediastinal disorders
Gastrointestinal
disorders
Skin and subcutaneous
tissue disorders
Musculoskeletal,
connective tissue and
bone disorders
General disorders and
administration site
conditions
Chest pain, chest
discomfort, pain, fatigue,
injection site reaction,
feeling hot
In some of the cases of hypersensitivity, in patients with underlying coronary artery disease,
myocardial ischemia and/or myocardial infarctions were also reported.
In very rare cases, fatal outcomes have been reported in temporal association with the use of SonoVue.
In all these patients there was a high underlying risk for major cardiac complications, which could
have led to the fatal outcome
.
Since there have been no cases of overdose reported to date, neither signs nor symptoms of overdose
have been identified. In a Phase I study doses up to 56 ml of SonoVue were administered to normal
volunteers without serious adverse events being reported. In the event of overdose occurring, the
patient should be observed and treated symptomatically.





















PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Ultrasound contrast media
ATC code: VO8DA.
The addition of sodium chloride 9 mg/ml (0.9%) solution for injection to the lyophilised powder
followed by vigorous shaking results in the production of the microbubbles of sulphur hexafluoride.
The microbubbles have a mean diameter of about 2.5 µm, with 90% having a diameter less than 6 µm
and 99% having a diameter less than 11 µm. Each millilitre of SonoVue contains 8 µl of the
microbubbles
.
The interface between the sulphur hexafluoride bubble and the aqueous medium acts as
a reflector of the ultrasound beam thus enhancing blood echogenicity and increasing contrast between
the blood and the surrounding tissues.
The intensity of the reflected signal is dependent on concentration of the microbubbles and frequency
of the ultrasound beam. At the proposed clinical doses, SonoVue has been shown to provide marked
increase in signal intensity of more than 2 minutes for B-mode imaging in echocardiography and of
3 to 8 minutes for Doppler imaging of the macrovasculature and microvasculature.
Sulphur hexafluoride is an inert, innocuous gas, poorly soluble in aqueous solutions. There are
literature reports of the use of the gas in the study of respiratory physiology and in pneumatic
retinopexy.
5.2 Pharmacokinetic properties
The total amount of sulphur hexafluoride administered in a clinical dose is extremely small, (in a 2
ml
dose the microbubbles contain 16
µl of gas). The sulphur hexafluoride dissolves in the blood and is
subsequently exhaled.
After a single intravenous injection of 0.03 or 0.3 ml of SonoVue/kg (approximately 1 and 10 times
the maximum clinical dose) to human volunteers, the sulphur hexafluoride was cleared rapidly. The
mean terminal half-life was 12 minutes (range 2 to 33 minutes). More than 80% of the administered
sulphur hexafluoride was recovered in exhaled air within 2 minutes after injection and almost 100%
after 15 minutes.
In patients with diffuse interstitial pulmonary fibrosis, the percent of dose recovered in expired air
averaged 100% and the terminal half-life was similar to that measured in healthy volunteers.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, genotoxicity and toxicity to reproduction. Caecal lesions observed in some repeat-dose
studies with rats, but not in monkeys, are not relevant for humans under normal conditions of
administration.
PHARMACEUTICAL PARTICULARS
Powder:
Macrogol 4000
Distearoylphosphatidylcholine
Dipalmitoylphosphatidylglycerol Sodium
Palmitic acid
Solvent:
Sodium chloride 9 mg/ml (0.9%) solution for injection
In the absence of compatibility studies, SonoVue should not be admixed with any other medicinal
product except the solvent provided.
Once reconstituted, chemical and physical stability has been demonstrated for 6 hours. From a
microbiological point of view, the product should be used immediately. If not used immediately, in
use storage times and conditions prior to use are the responsibility of the user.
6.4 Special precautions for storage
The medicinal product does not require any special storage conditions.
For storage conditions of the reconstituted medicinal product, see section 6.3.
6.5 Nature and contents of container
Presentation 02 (with separate MiniSpike transfer system):
25 mg of dry, lyophilised powder in an atmosphere of sulphur hexafluoride in a colourless
Type I glass vial, with elastomeric closure.
Separate transfer system.
Type I glass pre-filled syringe containing 5 ml sodium chloride 9 mg/ml (0.9%) solution for injection.
6.6 Special precautions for disposal
Before use examine the product to ensure that the container and closure have not been damaged.
SonoVue must be prepared before use by injecting through the septum 5 ml of sodium chloride
9 mg/ml (0.9%) solution for injection to the contents of the vial. The vial is then shaken vigorously for
twenty seconds after which the desired volume of the dispersion can be drawn into a syringe as
follows, depending on the presentation:
Presentation 02 (with separate MiniSpike transfer system)
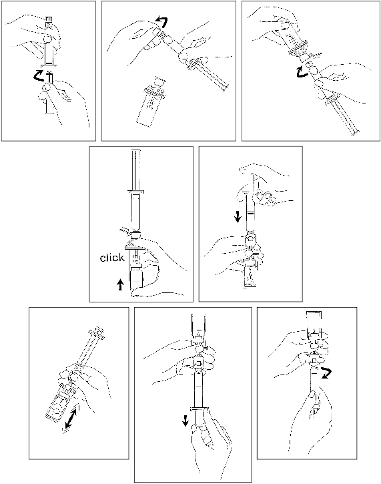
Open the MiniSpike transfer system blister and remove syringe tip cap.
Open the transfer system cap and connect the syringe to the transfer system by screwing it
in clockwise.
Remove Flipcap glass protective disk from the vial. Slide the vial into the transparent
sleeve of the transfer system and press firmly to lock the vial in place.
Shake vigorously for 20 seconds to mix all the contents in the vial (white milky liquid).
Invert the system and carefully withdraw SonoVue into the syringe.
Unscrew the syringe from the transfer system.
SonoVue should be administered immediately by injection into a peripheral vein.
After reconstitution, a homogeneous white milky liquid is obtained. If solid parts of the lyophilisate
are seen or the suspension is not homogeneous, the product should be discarded. If SonoVue is not
used immediately after reconstitution the microbubble dispersion should be shaken again before being
drawn up into a syringe. Chemical and physical stability of the microbubble dispersion has been
demonstrated for 6
hours.
The vial is for a single examination only. Any unused dispersion remaining at the end of an
examination or waste material must be discarded in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Bracco International B.V.
Strawinskylaan 3051
Connect the plunger rod by screwing it clockwise into the syringe.
Empty the contents of the syringe into the vial by pushing on the plunger rod.
NL - 1077 ZX Amsterdam
The Netherlands
MARKETING AUTHORISATION NUMBERS
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
26 March 2001 / 24 April 2006
10. DATE OF REVISION OF THE TEXT
A.
MANUFACTURING AUTHORISATION HOLDER
RESPONSIBLE FOR BATCH RELEASE
B.
CONDITIONS OF THE MARKETING AUTHORISATION
MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer responsible for batch release
Bracco Imaging S.p.A.
Via Ribes 5, Biondustry Park
Colleretto Giacosa - 10010 (TO)
Italy
CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to restricted medical prescription (See Annex I: Summary of Product
Characteristics, section 4.2)
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
•
OTHER CONDITIONS
Pharmacovigilance system
The MAH must ensure that the system of pharmacovigilance in Module 1.8.1. of the Marketing
Authorisation is in place and functioning before and whilst the product is on the market.
Risk Management Plan
The MAH commits to performing the studies and additional pharmacovigilance activities
detailed in the Pharmacovigilance Plan, as agreed in version 1 of the Risk Management Plan
(RMP) presented in Module 1.8.2. of the Marketing Authorisation and any subsequent updates
of the RMP agreed by the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, any
updated RMP should be submitted at the same time as the following Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted:
-
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
At the request of the European Medicines Agency
PSURs: The MAH will provide a PSUR covering the period 01/10/2005 to 30/09/2006 and continue
with yearly PSURs unless otherwise agreed with CHMP.
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING AND THE IMMEDIATE
PACKAGING
Outer label, presentation 02
NAME OF THE MEDICINAL PRODUCT
SonoVue sulphur hexafluoride microbubbles 8 microlitres/ml
Powder and solvent for dispersion for injection
STATEMENT OF ACTIVE SUBSTANCE(S)
One ml contains 8 µl of sulphur hexafluoride microbubbles
Macrogol 4000, distearoylphosphatidylcholine, dipalmitoylphosphatidylglycerol sodium, palmitic
acid.
Solvent: sodium chloride 9 mg/ml (0.9%) solution for injection.
PHARMACEUTICAL FORM AND CONTENTS
1 vial containing 25 mg lyophilized powder to be reconstituted with 5 ml sodium chloride 9 mg/ml
(0.9%) solution for injection
1 pre-filled syringe containing sodium chloride 9 mg/ml (0.9%) solution for injection
1 Mini-Spike Plus 6/8 (CE 0123) transfer system.
1 ml of the reconstituted dispersion contains 8 microlitres sulphur hexafluoride microbubbles.
METHOD AND ROUTE(S) OF ADMINISTRATION
Intravenous use
Read carefully the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED
OUT OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children
OTHER SPECIAL WARNING(S), IF NECESSARY

























SPECIAL STORAGE CONDITIONS
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
For single use only, discard any unused portion
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Bracco International B.V.,
Strawinskylaan 3051,
NL - 1077 ZX Amsterdam,
The Netherlands
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
16. INFORMATION IN BRAILLE



















PACKAGE LEAFLET: INFORMATION FOR THE USER
SonoVue 8 microlitres/ml powder and solvent for dispersion for injection
sulphur hexafluoride
Read all of this leaflet carefully before you start using this medicine.
Keep this leaflet. You may need to read it again.
If you have further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if
their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
What SonoVue is and what it is used for
WHAT SONOVUE IS AND WHAT IT IS USED FOR
SonoVue is a contrast agent for ultrasound scans of blood vessels and tissues of the body.
SonoVue is a dispersion containing millions of tiny bubbles (microbubbles). Each of these bubbles is
smaller than a red blood cell. The bubbles act as a reflector of the ultrasound beam and provide a
better echo than the tissues of the body.
SonoVue is a contrast agent that reflects ultrasound waves differently from the tissues of the body and
improves the picture from the scan. This helps your doctor to identify the part of your body or blood
vessel and to see any abnormalities. SonoVue can be used for scans of the chambers of the heart, scans
of large blood vessels and scans to assess lesions in the breast and the liver.
This medicine is for diagnostic use only.
2.
BEFORE YOU USE SONOVUE
Do not use SonoVue:
If you are allergic (hypersensitive) to sulphur hexafluoride or any of the other ingredients of SonoVue
or if you have:
-
had a myocardial infarction and you still suffer from frequent and/or repeated angina or chest
pain,
had a recent coronary artery intervention,
recent changes in your electrocardiogram,
frequent and/or repeated angina or chest pain in the past 7 days,
severe heart rhythm disorders,
right-to-left shunts of the heart,
severe increase in pulmonary artery blood pressure,
uncontrolled hypertension,
adult respiratory distress syndrome.



If you have had an allergic reaction in the past to SonoVue or any other ultrasound contrast agent tell
your doctor.
If you have to undergo echocardiography during stress, tell your doctor if in the past 2 days you have:
-
had frequent and/or repeated angina or chest pain, especially if you have history of heart
disease,
recent electrocardiography changes.
Take special care with SonoVue:
-
during SonoVue-enhanced echocardiography examination with a pharmacological stress, when
ECG and blood pressure should be carefully monitored,
in case you have severe lung disease and shortage of breath,
in case you have a neurological illness which is unstable, respiratory ventilation, acute
endocarditis, artificial heart valves, acute systemic inflammation and/or sepsis, hyperactive
coagulation states and/or recent thromboembolism, advanced kidney or liver diseases.
Taking other medicines:
There are no reports of reactions between SonoVue and other medicines. However, please tell your
doctor if you are taking or have recently taken any other medicines, including medicines obtained
without a prescription.
Pregnancy and breast
-
feeding
SonoVue has not been studied in pregnant women. Danger to a developing baby is not expected.
If you are pregnant, or think that you might be pregnant tell your doctor.
If you are breast feeding tell your doctor. It is not known if SonoVue passes into breast milk. Your
doctor will advise you if you should stop feeding for a short time after your ultrasound examination.
Ask your doctor for advice before taking any medicine.
After reconstitution, SonoVue is a homogeneous white milky dispersion. If solid parts are seen or the
dispersion is not homogeneous, the product will be discarded.
If SonoVue is not used immediately after reconstitution the dispersion will be shaken again before
being drawn up into a syringe.
SonoVue is injected into a vein, usually in your arm. The amount that you will be given depends on
which part of your body is being scanned. The usual dose is 2 or 2.4 ml of the dispersion. This dose
might be repeated. The medical staff supervising your scan will administer the injection of SonoVue to
you. The dose is the same in adult and elderly patients, however SonoVue should not be given to
patients under 18 years old.
You will be monitored for 30 minutes after your examination.
The product is for a single examination only. Any unused liquid remaining at the end of an
examination must be discarded.
If you have any further questions on the use of this product, ask your doctor.
Like all medicines, SonoVue can cause side effects, although not everybody gets them.
Most of the side effects are mild to moderate. However , some patients may experience serious side
effects and may require treatment .
Tell your doctor straight away if you notice any of the following serious side effects – you may need
urgent medical treatment:
- Signs of a severe allergic reaction such as swelling of the face, lips, mouth or throat which may make
it difficult to swallow or breathe; skin rash; hives; swelling of the hands, feet or ankles.
Side effects may occur with certain frequencies, which are defined as follows:
-
very common: affects more than 1 user in 10
uncommon: affects 1 to 10 users in 1,000
rare: affects 1 to 10 users in 10,000
rare: affects less than 1 user in 10,000
known: frequency cannot be estimated from the available data.
The following side effects have been observed with SonoVue:
Uncommon:
-
Strange taste in the mouth
Local reactions where the injection was given such as: pain or an unusual sensation at the
injection site
Increase in blood sugar levels
Pain or pressure in the forehead, cheeks, nose and between the eyes
Severe and less severe allergic reaction
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
inform your doctor or pharmacist.
Keep out of the reach and sight of children
Do not use after the expiry date stated on the label.
This medicinal product does not require any special storage conditions.
SonoVue dispersion should be administered to you within six hours of its preparation.
common: affects 1 to 10 users in 100
The active substance is sulphur hexafluoride in the form of microbubbles.
The other ingredients are: macrogol 4000, distearoylphosphatidylcholine,
dipalmitoylphosphatidylglycerol sodium, palmitic acid.
The glass syringe contains sodium chloride 9 mg/ml (0.9%) solution for injection.
What SonoVue looks like and content of the pack
SonoVue is a kit which includes a glass vial containing white powder, a glass syringe containing the
solvent and a transfer system.
Marketing Authorisation Holder:
Bracco International B.V.
Strawinskylaan 3051
NL - 1077 ZX Amsterdam
The Netherlands
Manufacturer:
Bracco Imaging S.p.A.
Via Ribes 5, Biondustry Park
Colleretto Giacosa - 10010 (TO)
Italy
This leaflet was last approved on
The following information is intended for medical or healthcare professionals only:
Presentation 02 (with separate MiniSpike transfer system (CE 0123))
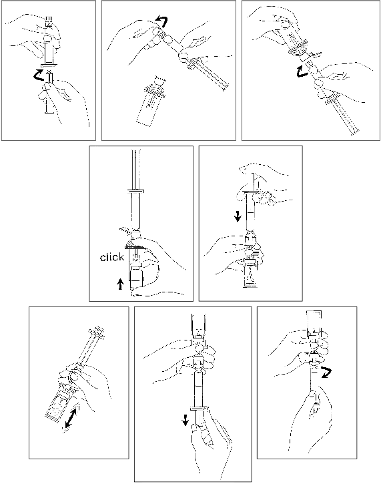
Connect the plunger rod by screwing it clockwise into the syringe.
Open the MiniSpike transfer system blister and remove syringe tip cap
.
Open the transfer system cap and connect the syringe to the transfer system by screwing it
in clockwise.
Remove Flipcap glass protective disk from the vial. Slide the vial into the transparent
sleeve of the transfer system and press firmly to lock the vial in place.
Empty the contents of the syringe into the vial by pushing on the plunger rod.
Shake vigorously for 20 seconds to mix all the contents in the vial (white milky liquid).
Invert the system and carefully withdraw SonoVue into the syringe.
Unscrew the syringe from the system.
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/sonovue.html
Copyright © 1995-2021 ITA all rights reserved.
|




































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































