Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
1. NAME OF THE MEDICINAL PRODUCT
STELARA 45 mg solution for injection
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each single-use vial contains 45 mg ustekinumab in 0.5 ml.
Ustekinumab is a fully human IgG1κ monoclonal antibody to interleukin (IL)-12/23 produced in a
murine myeloma cell line using recombinant DNA technology.
For a full list of excipients, see section 6.1.
The solution is clear to slightly opalescent, colourless to light yellow.
4.1 Therapeutic indications
STELARA is indicated for the treatment of moderate to severe plaque psoriasis in adults who failed to
respond to, or who have a contraindication to, or are intolerant to other systemic therapies including
ciclosporin, methotrexate and PUVA (see section 5.1).
4.2 Posology and method of administration
STELARA is intended for use under the guidance and supervision of a physician experienced in the
diagnosis and treatment of psoriasis.
Posology
The recommended posology of STELARA is an initial dose of 45 mg administered subcutaneously,
followed by a 45 mg dose 4 weeks later, and then every 12 weeks thereafter.
Consideration should be given to discontinuing treatment in patients who have shown no response up
to 28 weeks of treatment.
Patients with body weight > 100 kg
For patients with a body weight > 100 kg the initial dose is 90 mg administered subcutaneously,
followed by a 90 mg dose 4 weeks later, and then every 12 weeks thereafter. In these patients, 45 mg
was also shown to be efficacious. However, 90 mg resulted in greater efficacy. (see section 5.1,
Table 2)
Elderly patients (≥ 65 years)
No dose adjustment is needed for elderly patients (see section 4.4).
Children and adolescents (< 18 years)
STELARA is not recommended for use in children and adolescents below age 18 due to a lack of data
on safety and efficacy.
Renal and hepatic impairment
STELARA has not been studied in these patient populations. No dose recommendations can be made.
Method of administration
STELARA is for subcutaneous injection. If possible, areas of the skin that show psoriasis should be
avoided as injection sites.
After proper training in subcutaneous injection technique, patients may self-inject STELARA if a
physician determines that it is appropriate. However, the physician should ensure appropriate
follow-up of patients. Patients should be instructed to inject the full amount of STELARA according
to the directions provided in the package leaflet. Comprehensive instructions for administration are
given in the package leaflet.
For further instructions on preparation and special precautions for handling, see section 6.6.
Hypersensitivity to the active substance or to any of the excipients (see section 6.1).
Clinically important, active infection (e.g. active tuberculosis).
4.4 Special warnings and precautions for use
Infections
Ustekinumab may have the potential to increase the risk of infections and reactivate latent infections.
In clinical studies, serious bacterial, fungal, and viral infections have been observed in patients
receiving STELARA (see section 4.8).
Caution should be exercised when considering the use of STELARA in patients with a chronic
infection or a history of recurrent infection (see section 4.3 for clinically important, active infection).
Prior to initiating treatment with STELARA, patients should be evaluated for tuberculosis infection.
STELARA must not be given to patients with active tuberculosis (see section 4.3). Treatment of latent
tuberculosis infection should be initiated prior to administering STELARA. Anti-tuberculosis therapy
should also be considered prior to initiation of STELARA in patients with a history of latent or active
tuberculosis in whom an adequate course of treatment cannot be confirmed. Patients receiving
STELARA should be monitored closely for signs and symptoms of active tuberculosis during and
after treatment.
Patients should be instructed to seek medical advice if signs or symptoms suggestive of an infection
occur. If a patient develops a serious infection, the patient should be closely monitored and STELARA
should not be administered until the infection resolves.
Malignancies
Immunosuppressants like ustekinumab have the potential to increase the risk of malignancy. Some
patients who received STELARA in clinical studies developed cutaneous and non-cutaneous
malignancies (see section 4.8).
No studies have been conducted that include patients with a history of malignancy or that continue
treatment in patients who develop malignancy while receiving STELARA. Thus, caution should be
exercised when considering the use of STELARA in these patients.
Hypersensitivity reactions
Serious allergic reactions have been reported in the postmarketing setting, in some cases several days
after treatment. Anaphylaxis and angioedema have occurred. If an anaphylactic or other serious
allergic reaction occurs, administration of STELARA should be discontinued immediately and
appropriate therapy instituted (see section 4.8).




It is recommended that live viral or live bacterial vaccines (such as Bacillus of Calmette and Guérin
(BCG)) should not be given concurrently with STELARA. Specific studies have not been conducted
in patients who had recently received live viral or live bacterial vaccines. No data are available on the
secondary transmission of infection by live vaccines in patients receiving STELARA. Before live viral
or live bacterial vaccination, treatment with STELARA should be withheld for at least 15 weeks after
the last dose and can be resumed at least 2 weeks after vaccination. Prescribers should consult the
Summary of Product Characteristics for the specific vaccine for additional information and guidance
on concomitant use of immunosuppressive agents post-vaccination.
Patients receiving STELARA may receive concurrent inactivated or non-live vaccinations.
Concomitant immunosuppressive therapy
The safety and efficacy of STELARA in combination with other immunosuppressants, including
biologics, or phototherapy have not been evaluated. Caution should be exercised when considering
concomitant use of other immunosuppressants and STELARA or when transitioning from other
immunosuppressive biologics (see section 4.5).
Immunotherapy
STELARA has not been evaluated in patients who have undergone allergy immunotherapy. It is not
known whether STELARA may affect allergy immunotherapy.
Special populations
Elderly patients (
65 years)
No overall differences in efficacy or safety in patients age 65 and older who received STELARA were
observed compared to younger patients. Because there is a higher incidence of infections in the elderly
population in general, caution should be used in treating the elderly.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed. In the population pharmacokinetic analysis of the phase
III studies, the effect of the most frequently used concomitant medicinal products in patients with
psoriasis (including paracetamol, ibuprofen, acetylsalicylic acid, metformin, atorvastatin,
levothyroxine) on pharmacokinetics of ustekinumab was explored. There were no indications of an
interaction with these concomitantly administered medicinal products. The basis for this analysis was
that at least 100 patients (> 5% of the studied population) were treated concomitantly with these
medicinal products for at least 90% of the study period.
Live vaccines should not be given concurrently with STELARA (see section 4.4).
The safety and efficacy of STELARA in combination with other immunosuppressants, including
biologics, or phototherapy have not been evaluated (see section 4.4).
4.6 Pregnancy and lactation
Pregnancy
There are no adequate data from the use of ustekinumab in pregnant women. Animal studies do not
indicate direct or indirect harmful effects with respect to pregnancy, embryonic/foetal development,
parturition or postnatal development (see section 5.3). As a precautionary measure, it is preferable to
avoid the use of STELARA in pregnancy. Women of childbearing potential should use effective
methods of contraception during treatment and up to 15 weeks after treatment.
Breastfeeding
It is unknown whether ustekinumab is excreted in human breast milk. Animal studies have shown
excretion of ustekinumab at low levels in breast milk. It is not known if ustekinumab is absorbed
systemically after ingestion. Because of the potential for adverse reactions in nursing infants from
ustekinumab, a decision on whether to discontinue breastfeeding during treatment and up to 15 weeks
after treatment or to discontinue therapy with STELARA must be made taking into account the benefit




of breastfeeding to the child and the benefit of STELARA therapy to the woman.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use of machines have been performed.
The safety data described below reflect exposure to ustekinumab in 3 studies of 2,266 patients,
including 1,970 exposed for at least 6 months, 1,285 exposed for at least 1 year, and 373 exposed for
at least 18 months.
The following serious adverse reactions were reported:
Serious infections
Malignancies
The most common adverse reactions (> 10%) in controlled and uncontrolled portions of the psoriasis
clinical studies with ustekinumab were nasopharyngitis and upper respiratory tract infection. Most
were considered to be mild and did not necessitate discontinuation of study treatment.
Table 1 provides a summary of adverse reactions from psoriasis clinical studies as well as adverse
reactions reported from post-marketing experience. The adverse reactions are classified by System
Organ Class and frequency, using the following convention: Very common ( 1/10), Common
( 1/100 to < 1/10), Uncommon ( 1/1,000 to < 1/100), Rare ( 1/10,000 to < 1/1,000), Very rare
(< 1/10,000), not known (cannot be estimated from the available data). Within each frequency
grouping, adverse reactions are presented in order of decreasing seriousness.
Table 1 Summary of adverse reactions in psoriasis clinical studies and from post-marketing
experience
System Organ Class
Frequency: Adverse reaction
Infections and infestations
Very common: Upper respiratory tract infection, nasopharyngitis
Common: Cellulitis, viral upper respiratory tract infection
Common: Hypersensitivity reactions (including rash, urticaria)
Rare: Serious allergic reactions (including anaphylaxis,
angioedema)
Common: Dizziness, headache
Respiratory, thoracic and
mediastinal disorders
Common: Pharyngolaryngeal pain, nasal congestion
Gastrointestinal disorders
Skin and subcutaneous tissue
disorders
Musculoskeletal and connective
tissue disorders
Common: Back pain, myalgia
General disorders and
administration site conditions
Common: Fatigue, injection site erythema
Uncommon: Injection site reactions (including pain, swelling,
pruritus, induration, haemorrhage, bruising and irritation)














In controlled studies of psoriasis patients, the rates of infection or serious infection were similar
between ustekinumab-treated patients and those treated with placebo. In the placebo-controlled period
of clinical studies of psoriasis patients, the rate of infection was 1.39 per patient-year of follow-up in
ustekinumab-treated patients, and 1.21 in placebo-treated patients. Serious infections occurred in 0.01
per patient-year of follow-up in ustekinumab-treated patients (5 serious infections in 407 patient-years
of follow-up) and 0.02 in placebo-treated patients (3 serious infections in 177 patient-years of
follow-up) (see section 4.4).
In the controlled and non-controlled portions of psoriasis clinical studies, the rate of infection was 1.24
per patient-year of follow-up in ustekinumab-treated patients, and the incidence of serious infections
was 0.01 per patient-year of follow-up in ustekinumab-treated patients (24 serious infections in
2,251 patient-years of follow-up) and serious infections reported included cellulitis, diverticulitis,
osteomyelitis, viral infections, gastroenteritis, pneumonia, and urinary tract infections.
In clinical studies, patients with latent tuberculosis who were concurrently treated with isoniazid did
not develop tuberculosis.
Malignancies
In the placebo-controlled period of the psoriasis clinical studies, the incidence of malignancies
excluding non-melanoma skin cancer was 0.25 per 100 patient-years of follow-up for
ustekinumab-treated patients (1 patient in 406 patient-years of follow-up) compared with 0.57 for
placebo-treated patients (1 patient in 177 patient-years of follow-up). The incidence of non-melanoma
skin cancer was 0.74 per 100 patient-years of follow-up for ustekinumab-treated patients (3 patients in
406 patient-years of follow-up) compared to 1.13 for placebo-treated patients (2 patients in
176 patient-years of follow-up).
In the controlled and non-controlled portions of psoriasis clinical studies, the incidence of
malignancies excluding non-melanoma skin cancers was 0.36 per 100 patient-years of follow-up for
ustekinumab-treated patients (8 patients in 2,249 patient-years of follow-up) and malignancies
reported included breast, colon, head and neck, kidney, prostate, and thyroid cancers. The rate of
malignancies reported in ustekinumab-treated patients was comparable to the rate expected in the
general population (standardised incidence ratio = 0.68 [95% confidence interval: 0.29, 1.34]). The
incidence of non-melanoma skin cancer was 0.80 per 100 patient-years of follow-up for
ustekinumab-treated patients (18 patients in 2,245 patient-years of follow-up) (see section 4.4).
Hypersensitivity reactions
In clinical studies of ustekinumab, rash and urticaria have each been observed in < 2% of patients.
Immunogenicity
Approximately 5% of ustekinumab-treated patients developed antibodies to ustekinumab, which were
generally low-titer. No apparent correlation of antibody development to injection site reactions was
seen. Efficacy tended to be lower in patients positive for antibodies to ustekinumab; however,
antibody positivity does not preclude a clinical response.
No cases of overdose have been reported.
Single doses up to 4.5 mg/kg have been administered intravenously in clinical studies without
dose-limiting toxicity. In case of overdose, it is recommended that the patient be monitored for any
signs or symptoms of adverse reactions and appropriate symptomatic treatment be instituted
immediately.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties


Pharmacotherapeutic group: Immunosuppressants, interleukin inhibitors, ATC code: L04AC05.
Mechanism of action
Ustekinumab is a fully human IgG1κ monoclonal antibody that binds with high affinity and specificity
to the p40 protein subunit of the human cytokines IL-12 and IL-23. Ustekinumab inhibits the activity
of human IL-12 and IL-23 by preventing these cytokines from binding to their IL-12R1 receptor
protein expressed on the surface of immune cells. Ustekinumab cannot bind to IL-12 or IL-23 that is
pre-bound to IL-12R1 cell surface receptors. Thus, ustekinumab is not likely to contribute to
complement- or antibody-mediated cytotoxicity of the receptor-bearing cell. IL-12 and IL-23 are
heterodimeric cytokines secreted by activated antigen presenting cells, such as macrophages and
dendritic cells. IL-12 and IL-23 participate in immune function by contributing to natural killer (NK)
cell activation and CD4+ T-cell differentiation and activation. However, abnormal regulation of IL-12
and IL-23 has been associated with immune-mediated diseases, such as psoriasis. Ustekinumab
prevents IL-12 and IL-23 contributions to immune cell activation, such as intracellular signaling and
cytokine secretion. Thus, ustekinumab is believed to interrupt signaling and cytokine cascades that are
relevant to psoriasis pathology.
Clinical efficacy
The safety and efficacy of ustekinumab was assessed in 1,996 patients in two randomised,
double-blind, placebo-controlled studies in patients with moderate to severe plaque psoriasis and who
were candidates for phototherapy or systemic therapy. In addition, a randomised, blinded assessor,
active-controlled study compared ustekinumab and etanercept in patients with moderate to severe
plaque psoriasis who had had an inadequate response to, intolerance to, or contraindication to
ciclosporin, methotrexate, or PUVA.
Psoriasis Study 1 (PHOENIX 1) evaluated 766 patients. 53% of these patients were either
non-responsive, intolerant, or had a contraindication to other systemic therapy. Patients randomised to
ustekinumab received 45 mg or 90 mg doses at Weeks 0 and 4 and followed by the same dose every
12 weeks. Patients randomised to receive placebo at Weeks 0 and 4 crossed over to receive
ustekinumab (either 45 mg or 90 mg) at Weeks 12 and 16 followed by dosing every 12 weeks. Patients
originally randomised to ustekinumab who achieved Psoriasis Area and Severity Index 75 response
(PASI improvement of at least 75% relative to baseline) at both Weeks 28 and 40 were re-randomised
to receive ustekinumab every 12 weeks or to placebo (i.e., withdrawal of therapy). Patients who were
re-randomised to placebo at Week 40 reinitiated ustekinumab at their original dosing regimen when
they experienced at least a 50% loss of their PASI improvement obtained at Week 40. All patients
were followed for up to 76 weeks following first administration of study treatment.
Psoriasis Study 2 (PHOENIX 2) evaluated 1,230 patients. 61% of these patients were either
non-responsive, intolerant, or had a contraindication to other systemic therapy. Patients randomised to
ustekinumab received 45 mg or 90 mg doses at Weeks 0 and 4 followed by an additional dose at
16 weeks. Patients randomised to receive placebo at Weeks 0 and 4 crossed over to receive
ustekinumab (either 45 mg or 90 mg) at Weeks 12 and 16. All patients were followed for up to
52 weeks following first administration of study treatment.
Psoriasis Study 3 (ACCEPT) evaluated 903 patients with moderate to severe psoriasis who
inadequately responded to, were intolerant to, or had a contraindication to other systemic therapy and
compared the efficacy of ustekinumab to etanercept and evaluated the safety of ustekinumab and
etanercept. During the 12-week active-controlled portion of the study, patients were randomised to
receive etanercept (50 mg twice a week), ustekinumab 45 mg at Weeks 0 and 4, or ustekinumab 90 mg
at Weeks 0 and 4.
Baseline disease characteristics were generally consistent across all treatment groups in Psoriasis
Studies 1 and 2 with a median baseline PASI score from 17 to 18, median baseline Body Surface Area
(BSA) 20, and median Dermatology Life Quality Index (DLQI) range from 10 to 12. Approximately
one third (Psoriasis Study 1) and one quarter (Psoriasis Study 2) of subjects had Psoriatic Arthritis
(PsA). Similar disease severity was also seen in Psoriasis Study 3.

The primary endpoint in these studies was the proportion of patients who achieved PASI 75 response
from baseline at Week 12 (see Tables 2 and 3).
Table 2 Summary of clinical response in Psoriasis Study 1 (PHOENIX 1) and Psoriasis Study 2
(PHOENIX 2)
Week 12
2 doses (Week 0 and Week 4)
Week 28
3 doses (Week 0, Week
4 and W
eek 16)
Psoriasis Study 1
Number of patients randomised
255
PGA
b
of cleared or minimal N
(%)
Number of patients ≤ 100 kg
PASI 75 response N (%) 6 (4%) 124 (74%) 107 (65%) 130 (79%) 124 (81%)
Number of patients > 100 kg 89 87 92 86 90
PASI 75 response N (%) 2 (2%) 47 (54%) 63 (68%) 48 (56%) 67 (74%)
Psoriasis Study 2
Number of patients randomised
410
PGA
b
of cleared or minimal N
(%)
Number of patients ≤ 100 kg 290 297 289 287 280
PASI 75 response N (%) 12 (4%) 218 (73%) 225 (78%) 217 (76%) 226 (81%)
Number of patients > 100 kg 120 112 121 110 119
PASI 75 response N (%) 3 (3%) 55 (49%) 86 (71%) 59 (54%) 88 (74%)
p < 0.001 for ustekinumab 45 mg or 90 mg in comparison with placebo (PBO).
PGA = Physician Global Assessment
Table 3 Summary of clinical response at Week 12 in Psoriasis Study 3 (ACCEPT)
Psoriasis Study 3
Etanercept
24 doses
(50 mg twice a
week)
Ustekinumab
2 doses (Week 0 and Week 4)
45 mg

































Number of patients randomised 347
PGA of cleared or minimal N (%) 170 (49%)
Number of patients ≤ 100 kg
PASI 75 response N (%) 154 (61%)
Number of patients > 100 kg
PASI 75 response N (%) 43 (45%)
p < 0.001 for ustekinumab 45 mg or 90 mg in comparison with etanercept.
p = 0.012 for ustekinumab 45 mg in comparison with etanercept.
In Psoriasis Study 1 maintenance of PASI 75 was significantly superior with continuous treatment
compared with treatment withdrawal (p < 0.001). Similar results were seen with each dose of
ustekinumab. At Week 52, 89% of patients re-randomised to maintenance treatment were PASI 75
responders compared with 63% of patients re-randomised to placebo (treatment withdrawal)
(p < 0.001). At week 76, 84% of patients re-randomised to maintenance treatment were PASI 75
responders compared with 19% of patients re-randomised to placebo (treatment withdrawal).
In patients re-randomised to placebo, and who reinitiated their original ustekinumab treatment regimen
after loss of ≥ 50% of PASI improvement 85% regained PASI 75 response within 12 weeks after
re-initiating therapy.
In Psoriasis Study 1, at Week 2 and Week 12, significantly greater improvements from baseline were
demonstrated in the DLQI in each ustekinumab treatment group compared with placebo. The
improvement was sustained through Week 28. Similarly, significant improvements were seen in
Psoriasis Study 2 at Week 4 and 12, which were sustained through Week 24. In Psoriasis Study 1,
improvements in nail psoriasis (Nail Psoriasis Severity Index), in the physical and mental component
summary scores of the SF-36 and in the Itch Visual Analogue Scale (VAS) were also significant in
each ustekinumab treatment group compared with placebo. In Psoriasis Study 2, the Hospital Anxiety
and Depression Scale (HADS) and Work Limitations Questionnaire (WLQ) were also significantly
improved in each ustekinumab treatment group compared with placebo.
5.2 Pharmacokinetic properties
Absorption
The median time to reach the maximum serum concentration (t
max
) was 8.5 days after a single 90 mg
subcutaneous administration in healthy subjects. The median t
max
values of ustekinumab following a
single subcutaneous administration of either 45 mg or 90 mg in patients with psoriasis were
comparable to those observed in healthy subjects.
The absolute bioavailability of ustekinumab following a single subcutaneous administration was
estimated to be 57.2% in patients with psoriasis.
Distribution
Median volume of distribution during the terminal phase (Vz) following a single intravenous
administration to patients with psoriasis ranged from 57 to 83 ml/kg.
Metabolism
The exact metabolic pathway for ustekinumab is unknown.


















Median systemic clearance (CL) following a single intravenous administration to patients with
psoriasis ranged from 1.99 to 2.34 ml/day/kg. Median half-life (t
1/2
) of ustekinumab was
approximately 3 weeks in patients with psoriasis, ranging from 15 to 32 days across all psoriasis
studies. In a population pharmacokinetic analysis, the apparent clearance (CL/F) and apparent volume
of distribution (V/F) were 0.465 l/day and 15.7 l, respectively, in patients with psoriasis. The CL/F of
ustekinumab was not impacted by gender. Population pharmacokinetic analysis showed that there was
a trend towards a higher clearance of ustekinumab in patients who tested positive for antibodies to
ustekinumab.
Dose linearity
The systemic exposure of ustekinumab (C
max
and AUC) increased in an approximately
dose-proportional manner after a single intravenous administration at doses ranging from 0.09 mg/kg
to 4.5 mg/kg or following a single subcutaneous administration at doses ranging from approximately
24 mg to 240 mg in patients with psoriasis.
Single dose vs. multiple doses
Serum concentration-time profiles of ustekinumab were generally predictable after single or multiple
subcutaneous dose administrations. Steady-state serum concentrations of ustekinumab were achieved
by Week 28 after initial subcutaneous doses at Weeks 0 and 4 followed by doses every 12 weeks. The
median steady-state trough concentration ranged from 0.21 μg/ml to 0.26 μg/ml (45 mg) and from
0.47 μg/ml to 0.49 μg/ml (90 mg). There was no apparent accumulation in serum ustekinumab
concentration over time when given subcutaneously every 12 weeks.
Impact of weight on pharmacokinetics
In a population pharmacokinetic analysis, body weight was found to be the most significant covariate
affecting the clearance of ustekinumab. The median CL/F in patients with weight > 100 kg was
approximately 55% higher compared to patients with weight 100 kg. The median V/F in patients
with weight > 100 kg was approximately 37% higher as compared to patients with weight 100 kg.
The median trough serum concentrations of ustekinumab in patients with higher weight (> 100 kg) in
the 90 mg group were comparable to those in patients with lower weight (≤ 100 kg) in the 45 mg
group.
Special populations
No pharmacokinetic data are available in patients with impaired renal or hepatic function.
No specific studies have been conducted in elderly patients.
In the population pharmacokinetic analysis, there were no indications of an effect of tobacco or
alcohol on the pharmacokinetics of ustekinumab.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard (e.g. organ toxicity) for humans based on studies of
repeated-dose toxicity and developmental and reproductive toxicity, including safety pharmacology
evaluations. In developmental and reproductive toxicity studies in cynomolgus monkeys, neither
adverse effects on male fertility indices nor birth defects or developmental toxicity were observed. No
adverse effects on female fertility indices were observed using an analogous antibody to IL-12/23 in
mice.
Dose levels in animal studies were up to approximately 45-fold higher than the highest equivalent
dose intended to be administered to psoriasis patients and resulted in peak serum concentrations in
monkeys that were more than 100-fold higher than observed in humans.
Carcinogenicity studies were not performed with ustekinumab due to the lack of appropriate models
for an antibody with no cross-reactivity to rodent IL-12/23 p40.
6. PHARMACEUTICAL PARTICULARS



Sucrose
L-histidine
L-histidine monohydrochloride monohydrate
Polysorbate 80
Water for injections
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
6.4 Special precautions for storage
Store in a refrigerator (2ºC - 8ºC). Do not freeze.
Keep the vial in the outer carton in order to protect from light.
6.5 Nature and contents of container
STELARA is supplied as a sterile solution in a single-use type I glass 2 ml vial closed with a coated
butyl rubber stopper. STELARA is available in a 1 vial pack.
6.6 Special precautions for disposal and other handling
The solution in the STELARA vial should not be shaken. The solution should be visually inspected for
particulate matter or discoloration prior to subcutaneous administration. The solution is clear to
slightly opalescent, colourless to light yellow and may contain a few small translucent or white
particles of protein. This appearance is not unusual for proteinaceous solutions. The product should
not be used if the solution is discoloured or cloudy, or if foreign particulate matter is present. Before
administration, STELARA should be allowed to reach room temperature (approximately half an hour).
Detailed instructions for use are provided in the package leaflet.
STELARA does not contain preservatives; therefore any unused product remaining in the vial and the
syringe should not be used. Any unused product or waste material should be disposed of in accordance
with local requirements.
7. MARKETING AUTHORISATION HOLDER
Janssen-Cilag International NV
Turnhoutseweg 30
2340 Beerse
Belgium
8. MARKETING AUTHORISATION NUMBER(S)
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 16 January 2009
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
Agency
http://www.ema.europa.eu/
1. NAME OF THE MEDICINAL PRODUCT
STELARA 90 mg solution for injection
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each single-use vial contains 90 mg ustekinumab in 1 ml.
Ustekinumab is a fully human IgG1κ monoclonal antibody to interleukin (IL)-12/23 produced in a
murine myeloma cell line using recombinant DNA technology.
For a full list of excipients, see section 6.1.
The solution is clear to slightly opalescent, colourless to light yellow.
4.1 Therapeutic indications
STELARA is indicated for the treatment of moderate to severe plaque psoriasis in adults who failed to
respond to, or who have a contraindication to, or are intolerant to other systemic therapies including
ciclosporin, methotrexate and PUVA (see section 5.1).
4.2 Posology and method of administration
STELARA is intended for use under the guidance and supervision of a physician experienced in the
diagnosis and treatment of psoriasis.
Posology
The recommended posology of STELARA is an initial dose of 45 mg administered subcutaneously,
followed by a 45 mg dose 4 weeks later, and then every 12 weeks thereafter.
Consideration should be given to discontinuing treatment in patients who have shown no response up
to 28 weeks of treatment.
Patients with body weight > 100 kg
For patients with a body weight > 100 kg the initial dose is 90 mg administered subcutaneously,
followed by a 90 mg dose 4 weeks later, and then every 12 weeks thereafter. In these patients, 45 mg
was also shown to be efficacious. However, 90 mg resulted in greater efficacy. (see section 5.1,
Table 2)
Elderly patients (≥ 65 years)
No dose adjustment is needed for elderly patients (see section 4.4).
Children and adolescents (< 18 years)
STELARA is not recommended for use in children and adolescents below age 18 due to a lack of data
on safety and efficacy.
Renal and hepatic impairment
STELARA has not been studied in these patient populations. No dose recommendations can be made.
Method of administration
STELARA is for subcutaneous injection. If possible, areas of the skin that show psoriasis should be
avoided as injection sites.
After proper training in subcutaneous injection technique, patients may self-inject STELARA if a
physician determines that it is appropriate. However, the physician should ensure appropriate
follow-up of patients. Patients should be instructed to inject the full amount of STELARA according
to the directions provided in the package leaflet. Comprehensive instructions for administration are
given in the package leaflet.
For further instructions on preparation and special precautions for handling, see section 6.6.
Hypersensitivity to the active substance or to any of the excipients (see section 6.1).
Clinically important, active infection (e.g. active tuberculosis).
4.4 Special warnings and precautions for use
Infections
Ustekinumab may have the potential to increase the risk of infections and reactivate latent infections.
In clinical studies, serious bacterial, fungal, and viral infections have been observed in patients
receiving STELARA (see section 4.8).
Caution should be exercised when considering the use of STELARA in patients with a chronic
infection or a history of recurrent infection (see section 4.3 for clinically important, active infection).
Prior to initiating treatment with STELARA, patients should be evaluated for tuberculosis infection.
STELARA must not be given to patients with active tuberculosis (see section 4.3). Treatment of latent
tuberculosis infection should be initiated prior to administering STELARA. Anti-tuberculosis therapy
should also be considered prior to initiation of STELARA in patients with a history of latent or active
tuberculosis in whom an adequate course of treatment cannot be confirmed. Patients receiving
STELARA should be monitored closely for signs and symptoms of active tuberculosis during and
after treatment.
Patients should be instructed to seek medical advice if signs or symptoms suggestive of an infection
occur. If a patient develops a serious infection, the patient should be closely monitored and STELARA
should not be administered until the infection resolves.
Malignancies
Immunosuppressants like ustekinumab have the potential to increase the risk of malignancy. Some
patients who received STELARA in clinical studies developed cutaneous and non-cutaneous
malignancies (see section 4.8).
No studies have been conducted that include patients with a history of malignancy or that continue
treatment in patients who develop malignancy while receiving STELARA. Thus, caution should be
exercised when considering the use of STELARA in these patients.
Hypersensitivity reactions
Serious allergic reactions have been reported in the postmarketing setting, in some cases several days
after treatment. Anaphylaxis and angioedema have occurred. If an anaphylactic or other serious
allergic reaction occurs, administration of STELARA should be discontinued immediately and
appropriate therapy instituted (see section 4.8).




It is recommended that live viral or live bacterial vaccines (such as Bacillus of Calmette and Guérin
(BCG)) should not be given concurrently with STELARA. Specific studies have not been conducted
in patients who had recently received live viral or live bacterial vaccines. No data are available on the
secondary transmission of infection by live vaccines in patients receiving STELARA. Before live viral
or live bacterial vaccination, treatment with STELARA should be withheld for at least 15 weeks after
the last dose and can be resumed at least 2 weeks after vaccination. Prescribers should consult the
Summary of Product Characteristics for the specific vaccine for additional information and guidance
on concomitant use of immunosuppressive agents post-vaccination.
Patients receiving STELARA may receive concurrent inactivated or non-live vaccinations.
Concomitant immunosuppressive therapy
The safety and efficacy of STELARA in combination with other immunosuppressants, including
biologics, or phototherapy have not been evaluated. Caution should be exercised when considering
concomitant use of other immunosuppressants and STELARA or when transitioning from other
immunosuppressive biologics (see section 4.5).
Immunotherapy
STELARA has not been evaluated in patients who have undergone allergy immunotherapy. It is not
known whether STELARA may affect allergy immunotherapy.
Special populations
Elderly patients (
65 years)
No overall differences in efficacy or safety in patients age 65 and older who received STELARA were
observed compared to younger patients. Because there is a higher incidence of infections in the elderly
population in general, caution should be used in treating the elderly.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed. In the population pharmacokinetic analysis of the phase
III studies, the effect of the most frequently used concomitant medicinal products in patients with
psoriasis (including paracetamol, ibuprofen, acetylsalicylic acid, metformin, atorvastatin,
levothyroxine) on pharmacokinetics of ustekinumab was explored. There were no indications of an
interaction with these concomitantly administered medicinal products. The basis for this analysis was
that at least 100 patients (> 5% of the studied population) were treated concomitantly with these
medicinal products for at least 90% of the study period.
Live vaccines should not be given concurrently with STELARA (see section 4.4).
The safety and efficacy of STELARA in combination with other immunosuppressants, including
biologics, or phototherapy have not been evaluated (see section 4.4).
4.6 Pregnancy and lactation
Pregnancy
There are no adequate data from the use of ustekinumab in pregnant women. Animal studies do not
indicate direct or indirect harmful effects with respect to pregnancy, embryonic/foetal development,
parturition or postnatal development (see section 5.3). As a precautionary measure, it is preferable to
avoid the use of STELARA in pregnancy. Women of childbearing potential should use effective
methods of contraception during treatment and up to 15 weeks after treatment.
Breastfeeding
It is unknown whether ustekinumab is excreted in human breast milk. Animal studies have shown
excretion of ustekinumab at low levels in breast milk. It is not known if ustekinumab is absorbed
systemically after ingestion. Because of the potential for adverse reactions in nursing infants from
ustekinumab, a decision on whether to discontinue breastfeeding during treatment and up to 15 weeks
after treatment or to discontinue therapy with STELARA must be made taking into account the benefit




of breastfeeding to the child and the benefit of STELARA therapy to the woman.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use of machines have been performed.
The safety data described below reflect exposure to ustekinumab in 3 studies of 2,266 patients,
including 1,970 exposed for at least 6 months, 1,285 exposed for at least 1 year, and 373 exposed for
at least 18 months.
The following serious adverse reactions were reported:
Serious infections
Malignancies
The most common adverse reactions (> 10%) in controlled and uncontrolled portions of the psoriasis
clinical studies with ustekinumab were nasopharyngitis and upper respiratory tract infection. Most
were considered to be mild and did not necessitate discontinuation of study treatment.
Table 1 provides a summary of adverse reactions from psoriasis clinical studies as well as adverse
reactions reported from post-marketing experience. The adverse reactions are classified by System
Organ Class and frequency, using the following convention: Very common ( 1/10), Common
( 1/100 to < 1/10), Uncommon ( 1/1,000 to < 1/100), Rare ( 1/10,000 to < 1/1,000), Very rare
(< 1/10,000), not known (cannot be estimated from the available data). Within each frequency
grouping, adverse reactions are presented in order of decreasing seriousness.
Table 1 Summary of adverse reactions in psoriasis clinical studies and from post-marketing
experience
System Organ Class
Frequency: Adverse reaction
Infections and infestations
Very common: Upper respiratory tract infection, nasopharyngitis
Common: Cellulitis, viral upper respiratory tract infection
Common: Hypersensitivity reactions (including rash, urticaria)
Rare: Serious allergic reactions (including anaphylaxis,
angioedema)
Common: Dizziness, headache
Respiratory, thoracic and
mediastinal disorders
Common: Pharyngolaryngeal pain, nasal congestion
Gastrointestinal disorders
Skin and subcutaneous tissue
disorders
Musculoskeletal and connective
tissue disorders
Common: Back pain, myalgia
General disorders and
administration site conditions
Common: Fatigue, injection site erythema
Uncommon: Injection site reactions (including pain, swelling,
pruritus, induration, haemorrhage, bruising and irritation)













In controlled studies of psoriasis patients, the rates of infection or serious infection were similar
between ustekinumab-treated patients and those treated with placebo. In the placebo-controlled period
of clinical studies of psoriasis patients, the rate of infection was 1.39 per patient-year of follow-up in
ustekinumab-treated patients, and 1.21 in placebo-treated patients. Serious infections occurred in 0.01
per patient-year of follow-up in ustekinumab-treated patients (5 serious infections in 407 patient-years
of follow-up) and 0.02 in placebo-treated patients (3 serious infections in 177 patient-years of
follow-up) (see section 4.4).
In the controlled and non-controlled portions of psoriasis clinical studies, the rate of infection was 1.24
per patient-year of follow-up in ustekinumab-treated patients, and the incidence of serious infections
was 0.01 per patient-year of follow-up in ustekinumab-treated patients (24 serious infections in
2,251 patient-years of follow-up) and serious infections reported included cellulitis, diverticulitis,
osteomyelitis, viral infections, gastroenteritis, pneumonia, and urinary tract infections.
In clinical studies, patients with latent tuberculosis who were concurrently treated with isoniazid did
not develop tuberculosis.
Malignancies
In the placebo-controlled period of the psoriasis clinical studies, the incidence of malignancies
excluding non-melanoma skin cancer was 0.25 per 100 patient-years of follow-up for
ustekinumab-treated patients (1 patient in 406 patient-years of follow-up) compared with 0.57 for
placebo-treated patients (1 patient in 177 patient-years of follow-up). The incidence of non-melanoma
skin cancer was 0.74 per 100 patient-years of follow-up for ustekinumab-treated patients (3 patients in
406 patient-years of follow-up) compared to 1.13 for placebo-treated patients (2 patients in
176 patient-years of follow-up).
In the controlled and non-controlled portions of psoriasis clinical studies, the incidence of
malignancies excluding non-melanoma skin cancers was 0.36 per 100 patient-years of follow-up for
ustekinumab-treated patients (8 patients in 2,249 patient-years of follow-up) and malignancies
reported included breast, colon, head and neck, kidney, prostate, and thyroid cancers. The rate of
malignancies reported in ustekinumab-treated patients was comparable to the rate expected in the
general population (standardised incidence ratio = 0.68 [95% confidence interval: 0.29, 1.34]). The
incidence of non-melanoma skin cancer was 0.80 per 100 patient-years of follow-up for
ustekinumab-treated patients (18 patients in 2,245 patient-years of follow-up) (see section 4.4).
Hypersensitivity reactions
In clinical studies of ustekinumab, rash and urticaria have each been observed in < 2% of patients.
Immunogenicity
Approximately 5% of ustekinumab-treated patients developed antibodies to ustekinumab, which were
generally low-titer. No apparent correlation of antibody development to injection site reactions was
seen. Efficacy tended to be lower in patients positive for antibodies to ustekinumab; however,
antibody positivity does not preclude a clinical response.
No cases of overdose have been reported.
Single doses up to 4.5 mg/kg have been administered intravenously in clinical studies without
dose-limiting toxicity. In case of overdose, it is recommended that the patient be monitored for any
signs or symptoms of adverse reactions and appropriate symptomatic treatment be instituted
immediately.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties


Pharmacotherapeutic group: Immunosuppressants, interleukin inhibitors, ATC code: L04AC05.
Mechanism of action
Ustekinumab is a fully human IgG1κ monoclonal antibody that binds with high affinity and specificity
to the p40 protein subunit of the human cytokines IL-12 and IL-23. Ustekinumab inhibits the activity
of human IL-12 and IL-23 by preventing these cytokines from binding to their IL-12R1 receptor
protein expressed on the surface of immune cells. Ustekinumab cannot bind to IL-12 or IL-23 that is
pre-bound to IL-12R1 cell surface receptors. Thus, ustekinumab is not likely to contribute to
complement- or antibody-mediated cytotoxicity of the receptor-bearing cell. IL-12 and IL-23 are
heterodimeric cytokines secreted by activated antigen presenting cells, such as macrophages and
dendritic cells. IL-12 and IL-23 participate in immune function by contributing to natural killer (NK)
cell activation and CD4+ T-cell differentiation and activation. However, abnormal regulation of IL-12
and IL-23 has been associated with immune-mediated diseases, such as psoriasis. Ustekinumab
prevents IL-12 and IL-23 contributions to immune cell activation, such as intracellular signaling and
cytokine secretion. Thus, ustekinumab is believed to interrupt signaling and cytokine cascades that are
relevant to psoriasis pathology.
Clinical efficacy
The safety and efficacy of ustekinumab was assessed in 1,996 patients in two randomised,
double-blind, placebo-controlled studies in patients with moderate to severe plaque psoriasis and who
were candidates for phototherapy or systemic therapy. In addition, a randomised, blinded assessor,
active-controlled study compared ustekinumab and etanercept in patients with moderate to severe
plaque psoriasis who had had an inadequate response to, intolerance to, or contraindication to
ciclosporin, methotrexate, or PUVA.
Psoriasis Study 1 (PHOENIX 1) evaluated 766 patients. 53% of these patients were either
non-responsive, intolerant, or had a contraindication to other systemic therapy. Patients randomised to
ustekinumab received 45 mg or 90 mg doses at Weeks 0 and 4 and followed by the same dose every
12 weeks. Patients randomised to receive placebo at Weeks 0 and 4 crossed over to receive
ustekinumab (either 45 mg or 90 mg) at Weeks 12 and 16 followed by dosing every 12 weeks. Patients
originally randomised to ustekinumab who achieved Psoriasis Area and Severity Index 75 response
(PASI improvement of at least 75% relative to baseline) at both Weeks 28 and 40 were re-randomised
to receive ustekinumab every 12 weeks or to placebo (i.e., withdrawal of therapy). Patients who were
re-randomised to placebo at Week 40 reinitiated ustekinumab at their original dosing regimen when
they experienced at least a 50% loss of their PASI improvement obtained at Week 40. All patients
were followed for up to 76 weeks following first administration of study treatment.
Psoriasis Study 2 (PHOENIX 2) evaluated 1,230 patients. 61% of these patients were either
non-responsive, intolerant, or had a contraindication to other systemic therapy. Patients randomised to
ustekinumab received 45 mg or 90 mg doses at Weeks 0 and 4 followed by an additional dose at
16 weeks. Patients randomised to receive placebo at Weeks 0 and 4 crossed over to receive
ustekinumab (either 45 mg or 90 mg) at Weeks 12 and 16. All patients were followed for up to
52 weeks following first administration of study treatment.
Psoriasis Study 3 (ACCEPT) evaluated 903 patients with moderate to severe psoriasis who
inadequately responded to, were intolerant to, or had a contraindication to other systemic therapy and
compared the efficacy of ustekinumab to etanercept and evaluated the safety of ustekinumab and
etanercept. During the 12-week active-controlled portion of the study, patients were randomised to
receive etanercept (50 mg twice a week), ustekinumab 45 mg at Weeks 0 and 4, or ustekinumab 90 mg
at Weeks 0 and 4.
Baseline disease characteristics were generally consistent across all treatment groups in Psoriasis
Studies 1 and 2 with a median baseline PASI score from 17 to 18, median baseline Body Surface Area
(BSA) 20, and median Dermatology Life Quality Index (DLQI) range from 10 to 12. Approximately
one third (Psoriasis Study 1) and one quarter (Psoriasis Study 2) of subjects had Psoriatic Arthritis
(PsA). Similar disease severity was also seen in Psoriasis Study 3.

The primary endpoint in these studies was the proportion of patients who achieved PASI 75 response
from baseline at Week 12 (see Tables 2 and 3).
Table 2 Summary of clinical response in Psoriasis Study 1 (PHOENIX 1) and Psoriasis Study 2
(PHOENIX 2)
Week 12
2 doses (Week 0 and Week 4)
Week 28
3 doses (Week 0, Week 4
and W
eek 16)
Psoriasis Study 1
Number of patients randomised
255
PGA
b
of cleared or minimal N
(%)
Number of patients ≤ 100 kg
PASI 75 response N (%) 6 (4%) 124 (74%) 107 (65%) 130 (79%) 124 (81%)
Number of patients > 100 kg 89 87 92 86 90
PASI 75 response N (%) 2 (2%) 47 (54%) 63 (68%) 48 (56%) 67 (74%)
Psoriasis Study 2
Number of patients randomised
410
PGA
b
of cleared or minimal N
(%)
Number of patients ≤ 100 kg 290 297 289 287 280
PASI 75 response N (%) 12 (4%) 218 (73%) 225 (78%) 217 (76%) 226 (81%)
Number of patients > 100 kg 120 112 121 110 119
PASI 75 response N (%) 3 (3%) 55 (49%) 86 (71%) 59 (54%) 88 (74%)
p < 0.001 for ustekinumab 45 mg or 90 mg in comparison with placebo (PBO).
PGA = Physician Global Assessment
Table 3 Summary of clinical response at Week 12 in Psoriasis Study 3 (ACCEPT)
Psoriasis Study 3
Etanercept
24 doses
(50 mg twice a
week)
Ustekinumab
2 doses (Week 0 and Week 4)


































Number of patients randomised 347
PGA of cleared or minimal N (%) 170 (49%)
Number of patients ≤ 100 kg
PASI 75 response N (%) 154 (61%)
Number of patients > 100 kg
PASI 75 response N (%) 43 (45%)
p < 0.001 for ustekinumab 45 mg or 90 mg in comparison with etanercept.
p = 0.012 for ustekinumab 45 mg in comparison with etanercept.
In Psoriasis Study 1 maintenance of PASI 75 was significantly superior with continuous treatment
compared with treatment withdrawal (p < 0.001). Similar results were seen with each dose of
ustekinumab. At Week 52, 89% of patients re-randomised to maintenance treatment were PASI 75
responders compared with 63% of patients re-randomised to placebo (treatment withdrawal)
(p < 0.001). At week 76, 84% of patients re-randomised to maintenance treatment were PASI 75
responders compared with 19% of patients re-randomised to placebo (treatment withdrawal).
In patients re-randomised to placebo, and who reinitiated their original ustekinumab treatment regimen
after loss of ≥ 50% of PASI improvement 85% regained PASI 75 response within 12 weeks after
re-initiating therapy.
In Psoriasis Study 1, at Week 2 and Week 12, significantly greater improvements from baseline were
demonstrated in the DLQI in each ustekinumab treatment group compared with placebo. The
improvement was sustained through Week 28. Similarly, significant improvements were seen in
Psoriasis Study 2 at Week 4 and 12, which were sustained through Week 24. In Psoriasis Study 1,
improvements in nail psoriasis (Nail Psoriasis Severity Index), in the physical and mental component
summary scores of the SF-36 and in the Itch Visual Analogue Scale (VAS) were also significant in
each ustekinumab treatment group compared with placebo. In Psoriasis Study 2, the Hospital Anxiety
and Depression Scale (HADS) and Work Limitations Questionnaire (WLQ) were also significantly
improved in each ustekinumab treatment group compared with placebo.
5.2 Pharmacokinetic properties
Absorption
The median time to reach the maximum serum concentration (t
max
) was 8.5 days after a single 90 mg
subcutaneous administration in healthy subjects. The median t
max
values of ustekinumab following a
single subcutaneous administration of either 45 mg or 90 mg in patients with psoriasis were
comparable to those observed in healthy subjects.
The absolute bioavailability of ustekinumab following a single subcutaneous administration was
estimated to be 57.2% in patients with psoriasis.
Distribution
Median volume of distribution during the terminal phase (Vz) following a single intravenous
administration to patients with psoriasis ranged from 57 to 83 ml/kg.
Metabolism
The exact metabolic pathway for ustekinumab is unknown.


















Median systemic clearance (CL) following a single intravenous administration to patients with
psoriasis ranged from 1.99 to 2.34 ml/day/kg. Median half-life (t
1/2
) of ustekinumab was
approximately 3 weeks in patients with psoriasis, ranging from 15 to 32 days across all psoriasis
studies. In a population pharmacokinetic analysis, the apparent clearance (CL/F) and apparent volume
of distribution (V/F) were 0.465 l/day and 15.7 l, respectively, in patients with psoriasis. The CL/F of
ustekinumab was not impacted by gender. Population pharmacokinetic analysis showed that there was
a trend towards a higher clearance of ustekinumab in patients who tested positive for antibodies to
ustekinumab.
Dose linearity
The systemic exposure of ustekinumab (C
max
and AUC) increased in an approximately
dose-proportional manner after a single intravenous administration at doses ranging from 0.09 mg/kg
to 4.5 mg/kg or following a single subcutaneous administration at doses ranging from approximately
24 mg to 240 mg in patients with psoriasis.
Single dose vs. multiple doses
Serum concentration-time profiles of ustekinumab were generally predictable after single or multiple
subcutaneous dose administrations. Steady-state serum concentrations of ustekinumab were achieved
by Week 28 after initial subcutaneous doses at Weeks 0 and 4 followed by doses every 12 weeks. The
median steady-state trough concentration ranged from 0.21 μg/ml to 0.26 μg/ml (45 mg) and from
0.47 μg/ml to 0.49 μg/ml (90 mg). There was no apparent accumulation in serum ustekinumab
concentration over time when given subcutaneously every 12 weeks.
Impact of weight on pharmacokinetics
In a population pharmacokinetic analysis, body weight was found to be the most significant covariate
affecting the clearance of ustekinumab. The median CL/F in patients with weight > 100 kg was
approximately 55% higher compared to patients with weight 100 kg. The median V/F in patients
with weight > 100 kg was approximately 37% higher as compared to patients with weight 100 kg.
The median trough serum concentrations of ustekinumab in patients with higher weight (> 100 kg) in
the 90 mg group were comparable to those in patients with lower weight (≤ 100 kg) in the 45 mg
group.
Special populations
No pharmacokinetic data are available in patients with impaired renal or hepatic function.
No specific studies have been conducted in elderly patients.
In the population pharmacokinetic analysis, there were no indications of an effect of tobacco or
alcohol on the pharmacokinetics of ustekinumab.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard (e.g. organ toxicity) for humans based on studies of
repeated-dose toxicity and developmental and reproductive toxicity, including safety pharmacology
evaluations. In developmental and reproductive toxicity studies in cynomolgus monkeys, neither
adverse effects on male fertility indices nor birth defects or developmental toxicity were observed. No
adverse effects on female fertility indices were observed using an analogous antibody to IL-12/23 in
mice.
Dose levels in animal studies were up to approximately 45-fold higher than the highest equivalent
dose intended to be administered to psoriasis patients and resulted in peak serum concentrations in
monkeys that were more than 100-fold higher than observed in humans.
Carcinogenicity studies were not performed with ustekinumab due to the lack of appropriate models
for an antibody with no cross-reactivity to rodent IL-12/23 p40.
6. PHARMACEUTICAL PARTICULARS



Sucrose
L-histidine
L-histidine monohydrochloride monohydrate
Polysorbate 80
Water for injections
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
6.4 Special precautions for storage
Store in a refrigerator (2ºC - 8ºC). Do not freeze.
Keep the vial in the outer carton in order to protect from light.
6.5 Nature and contents of container
STELARA is supplied as a sterile solution in a single-use type I glass 2 ml vial closed with a coated
butyl rubber stopper. STELARA is available in a 1 vial pack.
6.6 Special precautions for disposal and other handling
The solution in the STELARA vial should not be shaken. The solution should be visually inspected for
particulate matter or discoloration prior to subcutaneous administration. The solution is clear to
slightly opalescent, colourless to light yellow and may contain a few small translucent or white
particles of protein. This appearance is not unusual for proteinaceous solutions. The product should
not be used if the solution is discoloured or cloudy, or if foreign particulate matter is present. Before
administration, STELARA should be allowed to reach room temperature (approximately half an hour).
Detailed instructions for use are provided in the package leaflet.
STELARA does not contain preservatives; therefore any unused product remaining in the vial and the
syringe should not be used. Any unused product or waste material should be disposed of in accordance
with local requirements.
7. MARKETING AUTHORISATION HOLDER
Janssen-Cilag International NV
Turnhoutseweg 30
2340 Beerse
Belgium
8. MARKETING AUTHORISATION NUMBER(S)
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 16 January 2009
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
Agency
http://www.ema.europa.eu/
1. NAME OF THE MEDICINAL PRODUCT
STELARA 45 mg solution for injection in pre-filled syringe.
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each single-use pre-filled syringe contains 45 mg ustekinumab in 0.5 ml.
Ustekinumab is a fully human IgG1κ monoclonal antibody to interleukin (IL)-12/23 produced in a
murine myeloma cell line using recombinant DNA technology.
For a full list of excipients, see section 6.1.
Solution for injection in pre-filled syringe (injection).
The solution is clear to slightly opalescent, colourless to light yellow.
4.1 Therapeutic indications
STELARA is indicated for the treatment of moderate to severe plaque psoriasis in adults who failed to
respond to, or who have a contraindication to, or are intolerant to other systemic therapies including
ciclosporin, methotrexate and PUVA (see section 5.1).
4.2 Posology and method of administration
STELARA is intended for use under the guidance and supervision of a physician experienced in the
diagnosis and treatment of psoriasis.
Posology
The recommended posology of STELARA is an initial dose of 45 mg administered subcutaneously,
followed by a 45 mg dose 4 weeks later, and then every 12 weeks thereafter.
Consideration should be given to discontinuing treatment in patients who have shown no response up
to 28 weeks of treatment.
Patients with body weight > 100 kg
For patients with a body weight > 100 kg the initial dose is 90 mg administered subcutaneously,
followed by a 90 mg dose 4 weeks later, and then every 12 weeks thereafter. In these patients, 45 mg
was also shown to be efficacious. However, 90 mg resulted in greater efficacy. (see section 5.1,
Table 2)
Elderly patients (≥ 65 years)
No dose adjustment is needed for elderly patients (see section 4.4).
Children and adolescents (< 18 years)
STELARA is not recommended for use in children and adolescents below age 18 due to a lack of data
on safety and efficacy.
Renal and hepatic impairment
STELARA has not been studied in these patient populations. No dose recommendations can be made.
Method of administration
STELARA is for subcutaneous injection. If possible, areas of the skin that show psoriasis should be
avoided as injection sites.
After proper training in subcutaneous injection technique, patients may self-inject STELARA if a
physician determines that it is appropriate. However, the physician should ensure appropriate
follow-up of patients. Patients should be instructed to inject the full amount of STELARA according
to the directions provided in the package leaflet. Comprehensive instructions for administration are
given in the package leaflet.
For further instructions on preparation and special precautions for handling, see section 6.6.
Hypersensitivity to the active substance or to any of the excipients (see section 6.1).
Clinically important, active infection (e.g. active tuberculosis).
4.4 Special warnings and precautions for use
Infections
Ustekinumab may have the potential to increase the risk of infections and reactivate latent infections.
In clinical studies, serious bacterial, fungal, and viral infections have been observed in patients
receiving STELARA (see section 4.8).
Caution should be exercised when considering the use of STELARA in patients with a chronic
infection or a history of recurrent infection (see section 4.3 for clinically important, active infection).
Prior to initiating treatment with STELARA, patients should be evaluated for tuberculosis infection.
STELARA must not be given to patients with active tuberculosis (see section 4.3). Treatment of latent
tuberculosis infection should be initiated prior to administering STELARA. Anti-tuberculosis therapy
should also be considered prior to initiation of STELARA in patients with a history of latent or active
tuberculosis in whom an adequate course of treatment cannot be confirmed. Patients receiving
STELARA should be monitored closely for signs and symptoms of active tuberculosis during and
after treatment.
Patients should be instructed to seek medical advice if signs or symptoms suggestive of an infection
occur. If a patient develops a serious infection, the patient should be closely monitored and STELARA
should not be administered until the infection resolves.
Malignancies
Immunosuppressants like ustekinumab have the potential to increase the risk of malignancy. Some
patients who received STELARA in clinical studies developed cutaneous and non-cutaneous
malignancies (see section 4.8).
No studies have been conducted that include patients with a history of malignancy or that continue
treatment in patients who develop malignancy while receiving STELARA. Thus, caution should be
exercised when considering the use of STELARA in these patients.
Hypersensitivity reactions
Serious allergic reactions have been reported in the postmarketing setting, in some cases several days
after treatment. Anaphylaxis and angioedema have occurred. If an anaphylactic or other serious
allergic reaction occurs, administration of STELARA should be discontinued immediately and
appropriate therapy instituted (see section 4.8).



The needle cover on the syringe in the pre-filled syringe is manufactured from dry natural rubber (a
derivative of latex), which may cause allergic reactions in individuals sensitive to latex.
Vaccinations
It is recommended that live viral or live bacterial vaccines (such as Bacillus of Calmette and Guérin
(BCG)) should not be given concurrently with STELARA. Specific studies have not been conducted
in patients who had recently received live viral or live bacterial vaccines. No data are available on the
secondary transmission of infection by live vaccines in patients receiving STELARA. Before live viral
or live bacterial vaccination, treatment with STELARA should be withheld for at least 15 weeks after
the last dose and can be resumed at least 2 weeks after vaccination. Prescribers should consult the
Summary of Product Characteristics for the specific vaccine for additional information and guidance
on concomitant use of immunosuppressive agents post-vaccination.
Patients receiving STELARA may receive concurrent inactivated or non-live vaccinations.
Concomitant immunosuppressive therapy
The safety and efficacy of STELARA in combination with other immunosuppressants, including
biologics, or phototherapy have not been evaluated. Caution should be exercised when considering
concomitant use of other immunosuppressants and STELARA or when transitioning from other
immunosuppressive biologics (see section 4.5).
Immunotherapy
STELARA has not been evaluated in patients who have undergone allergy immunotherapy. It is not
known whether STELARA may affect allergy immunotherapy.
Special populations
Elderly patients (
65 years)
No overall differences in efficacy or safety in patients age 65 and older who received STELARA were
observed compared to younger patients. Because there is a higher incidence of infections in the elderly
population in general, caution should be used in treating the elderly.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed. In the population pharmacokinetic analysis of the phase
III studies, the effect of the most frequently used concomitant medicinal products in patients with
psoriasis (including paracetamol, ibuprofen, acetylsalicylic acid, metformin, atorvastatin,
levothyroxine) on pharmacokinetics of ustekinumab was explored. There were no indications of an
interaction with these concomitantly administered medicinal products. The basis for this analysis was
that at least 100 patients (> 5% of the studied population) were treated concomitantly with these
medicinal products for at least 90% of the study period.
Live vaccines should not be given concurrently with STELARA (see section 4.4).
The safety and efficacy of STELARA in combination with other immunosuppressants, including
biologics, or phototherapy have not been evaluated (see section 4.4).
4.6 Pregnancy and lactation
Pregnancy
There are no adequate data from the use of ustekinumab in pregnant women. Animal studies do not
indicate direct or indirect harmful effects with respect to pregnancy, embryonic/foetal development,
parturition or postnatal development (see section 5.3). As a precautionary measure, it is preferable to
avoid the use of STELARA in pregnancy. Women of childbearing potential should use effective
methods of contraception during treatment and up to 15 weeks after treatment.
Breastfeeding
It is unknown whether ustekinumab is excreted in human breast milk. Animal studies have shown





excretion of ustekinumab at low levels in breast milk. It is not known if ustekinumab is absorbed
systemically after ingestion. Because of the potential for adverse reactions in nursing infants from
ustekinumab, a decision on whether to discontinue breastfeeding during treatment and up to 15 weeks
after treatment or to discontinue therapy with STELARA must be made taking into account the benefit
of breastfeeding to the child and the benefit of STELARA therapy to the woman.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use of machines have been performed.
The safety data described below reflect exposure to ustekinumab in 3 studies of 2,266 patients,
including 1,970 exposed for at least 6 months, 1,285 exposed for at least 1 year, and 373 exposed for
at least 18 months.
The following serious adverse reactions were reported:
Serious infections
Malignancies
The most common adverse reactions (> 10%) in controlled and uncontrolled portions of the psoriasis
clinical studies with ustekinumab were nasopharyngitis and upper respiratory tract infection. Most
were considered to be mild and did not necessitate discontinuation of study treatment.
Table 1 provides a summary of adverse reactions from psoriasis clinical studies as well as adverse
reactions reported from post-marketing experience. The adverse reactions are classified by System
Organ Class and frequency, using the following convention: Very common ( 1/10), Common
( 1/100 to < 1/10), Uncommon ( 1/1,000 to < 1/100), Rare ( 1/10,000 to < 1/1,000), Very rare
(< 1/10,000), not known (cannot be estimated from the available data). Within each frequency
grouping, adverse reactions are presented in order of decreasing seriousness.
Table 1 Summary of adverse reactions in psoriasis clinical studies and from post-marketing
experience
System Organ Class
Frequency: Adverse reaction
Infections and infestations
Very common: Upper respiratory tract infection, nasopharyngitis
Common: Cellulitis, viral upper respiratory tract infection
Common: Hypersensitivity reactions (including rash, urticaria)
Rare: Serious allergic reactions (including anaphylaxis,
angioedema)
Common: Dizziness, headache
Respiratory, thoracic and
mediastinal disorders
Common: Pharyngolaryngeal pain, nasal congestion
Gastrointestinal disorders
Skin and subcutaneous tissue
disorders
Musculoskeletal and connective
tissue disorders
Common: Back pain, myalgia











General disorders and
administration site conditions
Common: Fatigue, injection site erythema
Uncommon: Injection site reactions (including pain, swelling,
pruritus, induration, haemorrhage, bruising and irritation)
Infections
In controlled studies of psoriasis patients, the rates of infection or serious infection were similar
between ustekinumab-treated patients and those treated with placebo. In the placebo-controlled period
of clinical studies of psoriasis patients, the rate of infection was 1.39 per patient-year of follow-up in
ustekinumab-treated patients, and 1.21 in placebo-treated patients. Serious infections occurred in 0.01
per patient-year of follow-up in ustekinumab-treated patients (5 serious infections in 407 patient-years
of follow-up) and 0.02 in placebo-treated patients (3 serious infections in 177 patient-years of
follow-up) (see section 4.4).
In the controlled and non-controlled portions of psoriasis clinical studies, the rate of infection was 1.24
per patient-year of follow-up in ustekinumab-treated patients, and the incidence of serious infections
was 0.01 per patient-year of follow-up in ustekinumab-treated patients (24 serious infections in
2,251 patient-years of follow-up) and serious infections reported included cellulitis, diverticulitis,
osteomyelitis, viral infections, gastroenteritis, pneumonia, and urinary tract infections.
In clinical studies, patients with latent tuberculosis who were concurrently treated with isoniazid did
not develop tuberculosis.
Malignancies
In the placebo-controlled period of the psoriasis clinical studies, the incidence of malignancies
excluding non-melanoma skin cancer was 0.25 per 100 patient-years of follow-up for
ustekinumab-treated patients (1 patient in 406 patient-years of follow-up) compared with 0.57 for
placebo-treated patients (1 patient in 177 patient-years of follow-up). The incidence of non-melanoma
skin cancer was 0.74 per 100 patient-years of follow-up for ustekinumab-treated patients (3 patients in
406 patient-years of follow-up) compared to 1.13 for placebo-treated patients (2 patients in
176 patient-years of follow-up).
In the controlled and non-controlled portions of psoriasis clinical studies, the incidence of
malignancies excluding non-melanoma skin cancers was 0.36 per 100 patient-years of follow-up for
ustekinumab-treated patients (8 patients in 2,249 patient-years of follow-up) and malignancies
reported included breast, colon, head and neck, kidney, prostate, and thyroid cancers. The rate of
malignancies reported in ustekinumab-treated patients was comparable to the rate expected in the
general population (standardised incidence ratio = 0.68 [95% confidence interval: 0.29, 1.34]). The
incidence of non-melanoma skin cancer was 0.80 per 100 patient-years of follow-up for
ustekinumab-treated patients (18 patients in 2,245 patient-years of follow-up) (see section 4.4).
Hypersensitivity reactions
In clinical studies of ustekinumab, rash and urticaria have each been observed in < 2% of patients.
Immunogenicity
Approximately 5% of ustekinumab-treated patients developed antibodies to ustekinumab, which were
generally low-titer. No apparent correlation of antibody development to injection site reactions was
seen. Efficacy tended to be lower in patients positive for antibodies to ustekinumab; however,
antibody positivity does not preclude a clinical response.
No cases of overdose have been reported.
Single doses up to 4.5 mg/kg have been administered intravenously in clinical studies without
dose-limiting toxicity. In case of overdose, it is recommended that the patient be monitored for any
signs or symptoms of adverse reactions and appropriate symptomatic treatment be instituted







5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Immunosuppressants, interleukin inhibitors, ATC code: L04AC05.
Mechanism of action
Ustekinumab is a fully human IgG1κ monoclonal antibody that binds with high affinity and specificity
to the p40 protein subunit of the human cytokines IL-12 and IL-23. Ustekinumab inhibits the activity
of human IL-12 and IL-23 by preventing these cytokines from binding to their IL-12R1 receptor
protein expressed on the surface of immune cells. Ustekinumab cannot bind to IL-12 or IL-23 that is
pre-bound to IL-12R1 cell surface receptors. Thus, ustekinumab is not likely to contribute to
complement- or antibody-mediated cytotoxicity of the receptor-bearing cell. IL-12 and IL-23 are
heterodimeric cytokines secreted by activated antigen presenting cells, such as macrophages and
dendritic cells. IL-12 and IL-23 participate in immune function by contributing to natural killer (NK)
cell activation and CD4+ T-cell differentiation and activation. However, abnormal regulation of IL-12
and IL-23 has been associated with immune-mediated diseases, such as psoriasis. Ustekinumab
prevents IL-12 and IL-23 contributions to immune cell activation, such as intracellular signaling and
cytokine secretion. Thus, ustekinumab is believed to interrupt signaling and cytokine cascades that are
relevant to psoriasis pathology.
Clinical efficacy
The safety and efficacy of ustekinumab was assessed in 1,996 patients in two randomised,
double-blind, placebo-controlled studies in patients with moderate to severe plaque psoriasis and who
were candidates for phototherapy or systemic therapy. In addition, a randomised, blinded assessor,
active-controlled study compared ustekinumab and etanercept in patients with moderate to severe
plaque psoriasis who had had an inadequate response to, intolerance to, or contraindication to
ciclosporin, methotrexate, or PUVA.
Psoriasis Study 1 (PHOENIX 1) evaluated 766 patients. 53% of these patients were either
non-responsive, intolerant, or had a contraindication to other systemic therapy. Patients randomised to
ustekinumab received 45 mg or 90 mg doses at Weeks 0 and 4 and followed by the same dose every
12 weeks. Patients randomised to receive placebo at Weeks 0 and 4 crossed over to receive
ustekinumab (either 45 mg or 90 mg) at Weeks 12 and 16 followed by dosing every 12 weeks. Patients
originally randomised to ustekinumab who achieved Psoriasis Area and Severity Index 75 response
(PASI improvement of at least 75% relative to baseline) at both Weeks 28 and 40 were re-randomised
to receive ustekinumab every 12 weeks or to placebo (i.e., withdrawal of therapy). Patients who were
re-randomised to placebo at Week 40 reinitiated ustekinumab at their original dosing regimen when
they experienced at least a 50% loss of their PASI improvement obtained at Week 40. All patients
were followed for up to 76 weeks following first administration of study treatment.
Psoriasis Study 2 (PHOENIX 2) evaluated 1,230 patients. 61% of these patients were either
non-responsive, intolerant, or had a contraindication to other systemic therapy. Patients randomised to
ustekinumab received 45 mg or 90 mg doses at Weeks 0 and 4 followed by an additional dose at
16 weeks. Patients randomised to receive placebo at Weeks 0 and 4 crossed over to receive
ustekinumab (either 45 mg or 90 mg) at Weeks 12 and 16. All patients were followed for up to
52 weeks following first administration of study treatment.
Psoriasis Study 3 (ACCEPT) evaluated 903 patients with moderate to severe psoriasis who
inadequately responded to, were intolerant to, or had a contraindication to other systemic therapy and
compared the efficacy of ustekinumab to etanercept and evaluated the safety of ustekinumab and
etanercept. During the 12-week active-controlled portion of the study, patients were randomised to
receive etanercept (50 mg twice a week), ustekinumab 45 mg at Weeks 0 and 4, or ustekinumab 90 mg
at Weeks 0 and 4.

Baseline disease characteristics were generally consistent across all treatment groups in Psoriasis
Studies 1 and 2 with a median baseline PASI score from 17 to 18, median baseline Body Surface Area
(BSA) 20, and median Dermatology Life Quality Index (DLQI) range from 10 to 12. Approximately
one third (Psoriasis Study 1) and one quarter (Psoriasis Study 2) of subjects had Psoriatic Arthritis
(PsA). Similar disease severity was also seen in Psoriasis Study 3.
The primary endpoint in these studies was the proportion of patients who achieved PASI 75 response
from baseline at Week 12 (see Tables 2 and 3).
Table 2 Summary of clinical response in Psoriasis Study 1 (PHOENIX 1) and Psoriasis Study 2
(PHOENIX 2)
Week 12
2 doses (Week 0 and Week 4)
Week 28
3 doses (Week 0, Week
4 and W
eek 16)
Psoriasis Study 1
Number of patients randomised
255
PGA
b
of cleared or minimal N
(%)
Number of patients ≤ 100 kg 166 168 164 164 153
PASI 75 response N (%) 6 (4%) 124 (74%) 107 (65%) 130 (79%) 124 (81%)
Number of patients > 100 kg 89 87 92 86 90
PASI 75 response N (%) 2 (2%) 47 (54%) 63 (68%) 48 (56%) 67 (74%)
Psoriasis Study 2
Number of patients randomised
410
PGA
b
of cleared or minimal N
(%)
Number of patients ≤ 100 kg
PASI 75 response N (%) 12 (4%) 218 (73%) 225 (78%) 217 (76%) 226 (81%)
Number of patients > 100 kg 120 112 121 110 119
PASI 75 response N (%) 3 (3%) 55 (49%) 86 (71%) 59 (54%) 88 (74%)
p < 0.001 for ustekinumab 45 mg or 90 mg in comparison with placebo (PBO).
PGA = Physician Global Assessment
Table 3 Summary of clinical response at Week 12 in Psoriasis Study 3 (ACCEPT)
























Etanercept
24 doses
(50 mg twice a
week)
Ustekinumab
2 doses (Week 0 and Week 4)
45 mg
Number of patients randomised 347
PGA of cleared or minimal N (%) 170 (49%)
Number of patients ≤ 100 kg
PASI 75 response N (%) 154 (61%)
Number of patients > 100 kg
PASI 75 response N (%) 43 (45%)
p < 0.001 for ustekinumab 45 mg or 90 mg in comparison with etanercept.
p = 0.012 for ustekinumab 45 mg in comparison with etanercept.
In Psoriasis Study 1 maintenance of PASI 75 was significantly superior with continuous treatment
compared with treatment withdrawal (p < 0.001). Similar results were seen with each dose of
ustekinumab. At Week 52, 89% of patients re-randomised to maintenance treatment were PASI 75
responders compared with 63% of patients re-randomised to placebo (treatment withdrawal)
(p < 0.001). At week 76, 84% of patients re-randomised to maintenance treatment were PASI 75
responders compared with 19% of patients re-randomised to placebo (treatment withdrawal).
In patients re-randomised to placebo, and who reinitiated their original ustekinumab treatment regimen
after loss of ≥ 50% of PASI improvement 85% regained PASI 75 response within 12 weeks after
re-initiating therapy.
In Psoriasis Study 1, at Week 2 and Week 12, significantly greater improvements from baseline were
demonstrated in the DLQI in each ustekinumab treatment group compared with placebo. The
improvement was sustained through Week 28. Similarly, significant improvements were seen in
Psoriasis Study 2 at Week 4 and 12, which were sustained through Week 24. In Psoriasis Study 1,
improvements in nail psoriasis (Nail Psoriasis Severity Index), in the physical and mental component
summary scores of the SF-36 and in the Itch Visual Analogue Scale (VAS) were also significant in
each ustekinumab treatment group compared with placebo. In Psoriasis Study 2, the Hospital Anxiety
and Depression Scale (HADS) and Work Limitations Questionnaire (WLQ) were also significantly
improved in each ustekinumab treatment group compared with placebo.
5.2 Pharmacokinetic properties
Absorption
The median time to reach the maximum serum concentration (t
max
) was 8.5 days after a single 90 mg
subcutaneous administration in healthy subjects. The median t
max
values of ustekinumab following a
single subcutaneous administration of either 45 mg or 90 mg in patients with psoriasis were
comparable to those observed in healthy subjects.
The absolute bioavailability of ustekinumab following a single subcutaneous administration was
estimated to be 57.2% in patients with psoriasis.
Distribution
Median volume of distribution during the terminal phase (Vz) following a single intravenous





















administration to patients with psoriasis ranged from 57 to 83 ml/kg.
Metabolism
The exact metabolic pathway for ustekinumab is unknown.
Elimination
Median systemic clearance (CL) following a single intravenous administration to patients with
psoriasis ranged from 1.99 to 2.34 ml/day/kg. Median half-life (t
1/2
) of ustekinumab was
approximately 3 weeks in patients with psoriasis, ranging from 15 to 32 days across all psoriasis
studies. In a population pharmacokinetic analysis, the apparent clearance (CL/F) and apparent volume
of distribution (V/F) were 0.465 l/day and 15.7 l, respectively, in patients with psoriasis. The CL/F of
ustekinumab was not impacted by gender. Population pharmacokinetic analysis showed that there was
a trend towards a higher clearance of ustekinumab in patients who tested positive for antibodies to
ustekinumab.
Dose linearity
The systemic exposure of ustekinumab (C
max
and AUC) increased in an approximately
dose-proportional manner after a single intravenous administration at doses ranging from 0.09 mg/kg
to 4.5 mg/kg or following a single subcutaneous administration at doses ranging from approximately
24 mg to 240 mg in patients with psoriasis.
Single dose vs. multiple doses
Serum concentration-time profiles of ustekinumab were generally predictable after single or multiple
subcutaneous dose administrations. Steady-state serum concentrations of ustekinumab were achieved
by Week 28 after initial subcutaneous doses at Weeks 0 and 4 followed by doses every 12 weeks. The
median steady-state trough concentration ranged from 0.21 μg/ml to 0.26 μg/ml (45 mg) and from
0.47 μg/ml to 0.49 μg/ml (90 mg). There was no apparent accumulation in serum ustekinumab
concentration over time when given subcutaneously every 12 weeks.
Impact of weight on pharmacokinetics
In a population pharmacokinetic analysis, body weight was found to be the most significant covariate
affecting the clearance of ustekinumab. The median CL/F in patients with weight > 100 kg was
approximately 55% higher compared to patients with weight 100 kg. The median V/F in patients
with weight > 100 kg was approximately 37% higher as compared to patients with weight 100 kg.
The median trough serum concentrations of ustekinumab in patients with higher weight (> 100 kg) in
the 90 mg group were comparable to those in patients with lower weight (≤ 100 kg) in the 45 mg
group.
Special populations
No pharmacokinetic data are available in patients with impaired renal or hepatic function.
No specific studies have been conducted in elderly patients.
In the population pharmacokinetic analysis, there were no indications of an effect of tobacco or
alcohol on the pharmacokinetics of ustekinumab.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard (e.g. organ toxicity) for humans based on studies of
repeated-dose toxicity and developmental and reproductive toxicity, including safety pharmacology
evaluations. In developmental and reproductive toxicity studies in cynomolgus monkeys, neither
adverse effects on male fertility indices nor birth defects or developmental toxicity were observed. No
adverse effects on female fertility indices were observed using an analogous antibody to IL-12/23 in
mice.
Dose levels in animal studies were up to approximately 45-fold higher than the highest equivalent
dose intended to be administered to psoriasis patients and resulted in peak serum concentrations in
monkeys that were more than 100-fold higher than observed in humans.





Carcinogenicity studies were not performed with ustekinumab due to the lack of appropriate models
for an antibody with no cross-reactivity to rodent IL-12/23 p40.
6. PHARMACEUTICAL PARTICULARS
Sucrose
L-histidine
L-histidine monohydrochloride monohydrate
Polysorbate 80
Water for injections
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
6.4 Special precautions for storage
Store in a refrigerator (2ºC - 8ºC). Do not freeze.
Keep the pre-filled syringe in the outer carton in order to protect from light.
6.5 Nature and contents of container
STELARA is supplied as a sterile solution in a single-use type I glass 1 ml syringe with a fixed
stainless steel needle and a needle cover containing dry natural rubber (a derivative of latex). The
syringe is fitted with a passive safety guard. STELARA is available in a pack of 1 pre-filled syringe.
6.6 Special precautions for disposal and other handling
The solution in the STELARA pre-filled syringe should not be shaken. The solution should be visually
inspected for particulate matter or discoloration prior to subcutaneous administration. The solution is
clear to slightly opalescent, colourless to light yellow and may contain a few small translucent or
white particles of protein. This appearance is not unusual for proteinaceous solutions. The product
should not be used if the solution is discoloured or cloudy, or if foreign particulate matter is present.
Before administration, STELARA should be allowed to reach room temperature (approximately half
an hour). Detailed instructions for use are provided in the package leaflet.
STELARA does not contain preservatives; therefore any unused product remaining in the syringe
should not be used. Any unused product or waste material should be disposed of in accordance with
local requirements.
7. MARKETING AUTHORISATION HOLDER
Janssen-Cilag International NV
Turnhoutseweg 30
2340 Beerse
Belgium
8. MARKETING AUTHORISATION NUMBER(S)
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 16 January 2009
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
Agency
http://www.ema.europa.eu/
1. NAME OF THE MEDICINAL PRODUCT
STELARA 90 mg solution for injection in pre-filled syringe.
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each single-use pre-filled syringe contains 90 mg ustekinumab in 1 ml.
Ustekinumab is a fully human IgG1κ monoclonal antibody to interleukin (IL)-12/23 produced in a
murine myeloma cell line using recombinant DNA technology.
For a full list of excipients, see section 6.1.
Solution for injection in pre-filled syringe (injection).
The solution is clear to slightly opalescent, colourless to light yellow.
4.1 Therapeutic indications
STELARA is indicated for the treatment of moderate to severe plaque psoriasis in adults who failed to
respond to, or who have a contraindication to, or are intolerant to other systemic therapies including
ciclosporin, methotrexate and PUVA (see section 5.1).
4.2 Posology and method of administration
STELARA is intended for use under the guidance and supervision of a physician experienced in the
diagnosis and treatment of psoriasis.
Posology
The recommended posology of STELARA is an initial dose of 45 mg administered subcutaneously,
followed by a 45 mg dose 4 weeks later, and then every 12 weeks thereafter.
Consideration should be given to discontinuing treatment in patients who have shown no response up
to 28 weeks of treatment.
Patients with body weight > 100 kg
For patients with a body weight > 100 kg the initial dose is 90 mg administered subcutaneously,
followed by a 90 mg dose 4 weeks later, and then every 12 weeks thereafter. In these patients, 45 mg
was also shown to be efficacious. However, 90 mg resulted in greater efficacy. (see section 5.1,
Table 2)
Elderly patients (≥ 65 years)
No dose adjustment is needed for elderly patients (see section 4.4).
Children and adolescents (< 18 years)
STELARA is not recommended for use in children and adolescents below age 18 due to a lack of data
on safety and efficacy.
Renal and hepatic impairment
STELARA has not been studied in these patient populations. No dose recommendations can be made.
Method of administration
STELARA is for subcutaneous injection. If possible, areas of the skin that show psoriasis should be
avoided as injection sites.
After proper training in subcutaneous injection technique, patients may self-inject STELARA if a
physician determines that it is appropriate. However, the physician should ensure appropriate
follow-up of patients. Patients should be instructed to inject the full amount of STELARA according
to the directions provided in the package leaflet. Comprehensive instructions for administration are
given in the package leaflet.
For further instructions on preparation and special precautions for handling, see section 6.6.
Hypersensitivity to the active substance or to any of the excipients (see section 6.1).
Clinically important, active infection (e.g. active tuberculosis).
4.4 Special warnings and precautions for use
Infections
Ustekinumab may have the potential to increase the risk of infections and reactivate latent infections.
In clinical studies, serious bacterial, fungal, and viral infections have been observed in patients
receiving STELARA (see section 4.8).
Caution should be exercised when considering the use of STELARA in patients with a chronic
infection or a history of recurrent infection (see section 4.3 for clinically important, active infection).
Prior to initiating treatment with STELARA, patients should be evaluated for tuberculosis infection.
STELARA must not be given to patients with active tuberculosis (see section 4.3). Treatment of latent
tuberculosis infection should be initiated prior to administering STELARA. Anti-tuberculosis therapy
should also be considered prior to initiation of STELARA in patients with a history of latent or active
tuberculosis in whom an adequate course of treatment cannot be confirmed. Patients receiving
STELARA should be monitored closely for signs and symptoms of active tuberculosis during and
after treatment.
Patients should be instructed to seek medical advice if signs or symptoms suggestive of an infection
occur. If a patient develops a serious infection, the patient should be closely monitored and STELARA
should not be administered until the infection resolves.
Malignancies
Immunosuppressants like ustekinumab have the potential to increase the risk of malignancy. Some
patients who received STELARA in clinical studies developed cutaneous and non-cutaneous
malignancies (see section 4.8).
No studies have been conducted that include patients with a history of malignancy or that continue
treatment in patients who develop malignancy while receiving STELARA. Thus, caution should be
exercised when considering the use of STELARA in these patients.
Hypersensitivity reactions
Serious allergic reactions have been reported in the postmarketing setting, in some cases several days
after treatment. Anaphylaxis and angioedema have occurred. If an anaphylactic or other serious
allergic reaction occurs, administration of STELARA should be discontinued immediately and
appropriate therapy instituted (see section 4.8).



The needle cover on the syringe in the pre-filled syringe is manufactured from dry natural rubber (a
derivative of latex), which may cause allergic reactions in individuals sensitive to latex.
Vaccinations
It is recommended that live viral or live bacterial vaccines (such as Bacillus of Calmette and Guérin
(BCG)) should not be given concurrently with STELARA. Specific studies have not been conducted
in patients who had recently received live viral or live bacterial vaccines. No data are available on the
secondary transmission of infection by live vaccines in patients receiving STELARA. Before live viral
or live bacterial vaccination, treatment with STELARA should be withheld for at least 15 weeks after
the last dose and can be resumed at least 2 weeks after vaccination. Prescribers should consult the
Summary of Product Characteristics for the specific vaccine for additional information and guidance
on concomitant use of immunosuppressive agents post-vaccination.
Patients receiving STELARA may receive concurrent inactivated or non-live vaccinations.
Concomitant immunosuppressive therapy
The safety and efficacy of STELARA in combination with other immunosuppressants, including
biologics, or phototherapy have not been evaluated. Caution should be exercised when considering
concomitant use of other immunosuppressants and STELARA or when transitioning from other
immunosuppressive biologics (see section 4.5).
Immunotherapy
STELARA has not been evaluated in patients who have undergone allergy immunotherapy. It is not
known whether STELARA may affect allergy immunotherapy.
Special populations
Elderly patients (
65 years)
No overall differences in efficacy or safety in patients age 65 and older who received STELARA were
observed compared to younger patients. Because there is a higher incidence of infections in the elderly
population in general, caution should be used in treating the elderly.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed. In the population pharmacokinetic analysis of the phase
III studies, the effect of the most frequently used concomitant medicinal products in patients with
psoriasis (including paracetamol, ibuprofen, acetylsalicylic acid, metformin, atorvastatin,
levothyroxine) on pharmacokinetics of ustekinumab was explored. There were no indications of an
interaction with these concomitantly administered medicinal products. The basis for this analysis was
that at least 100 patients (> 5% of the studied population) were treated concomitantly with these
medicinal products for at least 90% of the study period.
Live vaccines should not be given concurrently with STELARA (see section 4.4).
The safety and efficacy of STELARA in combination with other immunosuppressants, including
biologics, or phototherapy have not been evaluated (see section 4.4).
4.6 Pregnancy and lactation
Pregnancy
There are no adequate data from the use of ustekinumab in pregnant women. Animal studies do not
indicate direct or indirect harmful effects with respect to pregnancy, embryonic/foetal development,
parturition or postnatal development (see section 5.3). As a precautionary measure, it is preferable to
avoid the use of STELARA in pregnancy. Women of childbearing potential should use effective
methods of contraception during treatment and up to 15 weeks after treatment.
Breastfeeding
It is unknown whether ustekinumab is excreted in human breast milk. Animal studies have shown





excretion of ustekinumab at low levels in breast milk. It is not known if ustekinumab is absorbed
systemically after ingestion. Because of the potential for adverse reactions in nursing infants from
ustekinumab, a decision on whether to discontinue breastfeeding during treatment and up to 15 weeks
after treatment or to discontinue therapy with STELARA must be made taking into account the benefit
of breastfeeding to the child and the benefit of STELARA therapy to the woman.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use of machines have been performed.
The safety data described below reflect exposure to ustekinumab in 3 studies of 2,266 patients,
including 1,970 exposed for at least 6 months, 1,285 exposed for at least 1 year, and 373 exposed for
at least 18 months.
The following serious adverse reactions were reported:
Serious infections
Malignancies
The most common adverse reactions (> 10%) in controlled and uncontrolled portions of the psoriasis
clinical studies with ustekinumab were nasopharyngitis and upper respiratory tract infection. Most
were considered to be mild and did not necessitate discontinuation of study treatment.
Table 1 provides a summary of adverse reactions from psoriasis clinical studies as well as adverse
reactions reported from post-marketing experience. The adverse reactions are classified by System
Organ Class and frequency, using the following convention: Very common ( 1/10), Common
( 1/100 to < 1/10), Uncommon ( 1/1,000 to < 1/100), Rare ( 1/10,000 to < 1/1,000), Very rare
(< 1/10,000), not known (cannot be estimated from the available data). Within each frequency
grouping, adverse reactions are presented in order of decreasing seriousness.
Table 1 Summary of adverse reactions in psoriasis clinical studies and from post-marketing
experience
System Organ Class
Frequency: Adverse reaction
Infections and infestations
Very common: Upper respiratory tract infection, nasopharyngitis
Common: Cellulitis, viral upper respiratory tract infection
Common: Hypersensitivity reactions (including rash, urticaria)
Rare: Serious allergic reactions (including anaphylaxis,
angioedema)
Common: Dizziness, headache
Respiratory, thoracic and
mediastinal disorders
Common: Pharyngolaryngeal pain, nasal congestion
Gastrointestinal disorders
Skin and subcutaneous tissue
disorders
Musculoskeletal and connective
tissue disorders
Common: Back pain, myalgia











General disorders and
administration site conditions
Common: Fatigue, injection site erythema
Uncommon: Injection site reactions (including pain, swelling,
pruritus, induration, haemorrhage, bruising and irritation)
Infections
In controlled studies of psoriasis patients, the rates of infection or serious infection were similar
between ustekinumab-treated patients and those treated with placebo. In the placebo-controlled period
of clinical studies of psoriasis patients, the rate of infection was 1.39 per patient-year of follow-up in
ustekinumab-treated patients, and 1.21 in placebo-treated patients. Serious infections occurred in 0.01
per patient-year of follow-up in ustekinumab-treated patients (5 serious infections in 407 patient-years
of follow-up) and 0.02 in placebo-treated patients (3 serious infections in 177 patient-years of
follow-up) (see section 4.4).
In the controlled and non-controlled portions of psoriasis clinical studies, the rate of infection was 1.24
per patient-year of follow-up in ustekinumab-treated patients, and the incidence of serious infections
was 0.01 per patient-year of follow-up in ustekinumab-treated patients (24 serious infections in
2,251 patient-years of follow-up) and serious infections reported included cellulitis, diverticulitis,
osteomyelitis, viral infections, gastroenteritis, pneumonia, and urinary tract infections.
In clinical studies, patients with latent tuberculosis who were concurrently treated with isoniazid did
not develop tuberculosis.
Malignancies
In the placebo-controlled period of the psoriasis clinical studies, the incidence of malignancies
excluding non-melanoma skin cancer was 0.25 per 100 patient-years of follow-up for
ustekinumab-treated patients (1 patient in 406 patient-years of follow-up) compared with 0.57 for
placebo-treated patients (1 patient in 177 patient-years of follow-up). The incidence of non-melanoma
skin cancer was 0.74 per 100 patient-years of follow-up for ustekinumab-treated patients (3 patients in
406 patient-years of follow-up) compared to 1.13 for placebo-treated patients (2 patients in
176 patient-years of follow-up).
In the controlled and non-controlled portions of psoriasis clinical studies, the incidence of
malignancies excluding non-melanoma skin cancers was 0.36 per 100 patient-years of follow-up for
ustekinumab-treated patients (8 patients in 2,249 patient-years of follow-up) and malignancies
reported included breast, colon, head and neck, kidney, prostate, and thyroid cancers. The rate of
malignancies reported in ustekinumab-treated patients was comparable to the rate expected in the
general population (standardised incidence ratio = 0.68 [95% confidence interval: 0.29, 1.34]). The
incidence of non-melanoma skin cancer was 0.80 per 100 patient-years of follow-up for
ustekinumab-treated patients (18 patients in 2,245 patient-years of follow-up) (see section 4.4).
Hypersensitivity reactions
In clinical studies of ustekinumab, rash and urticaria have each been observed in < 2% of patients.
Immunogenicity
Approximately 5% of ustekinumab-treated patients developed antibodies to ustekinumab, which were
generally low-titer. No apparent correlation of antibody development to injection site reactions was
seen. Efficacy tended to be lower in patients positive for antibodies to ustekinumab; however,
antibody positivity does not preclude a clinical response.
No cases of overdose have been reported.
Single doses up to 4.5 mg/kg have been administered intravenously in clinical studies without
dose-limiting toxicity. In case of overdose, it is recommended that the patient be monitored for any
signs or symptoms of adverse reactions and appropriate symptomatic treatment be instituted







5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Immunosuppressants, interleukin inhibitors, ATC code: L04AC05.
Mechanism of action
Ustekinumab is a fully human IgG1κ monoclonal antibody that binds with high affinity and specificity
to the p40 protein subunit of the human cytokines IL-12 and IL-23. Ustekinumab inhibits the activity
of human IL-12 and IL-23 by preventing these cytokines from binding to their IL-12R1 receptor
protein expressed on the surface of immune cells. Ustekinumab cannot bind to IL-12 or IL-23 that is
pre-bound to IL-12R1 cell surface receptors. Thus, ustekinumab is not likely to contribute to
complement- or antibody-mediated cytotoxicity of the receptor-bearing cell. IL-12 and IL-23 are
heterodimeric cytokines secreted by activated antigen presenting cells, such as macrophages and
dendritic cells. IL-12 and IL-23 participate in immune function by contributing to natural killer (NK)
cell activation and CD4+ T-cell differentiation and activation. However, abnormal regulation of IL-12
and IL-23 has been associated with immune-mediated diseases, such as psoriasis. Ustekinumab
prevents IL-12 and IL-23 contributions to immune cell activation, such as intracellular signaling and
cytokine secretion. Thus, ustekinumab is believed to interrupt signaling and cytokine cascades that are
relevant to psoriasis pathology.
Clinical efficacy
The safety and efficacy of ustekinumab was assessed in 1,996 patients in two randomised,
double-blind, placebo-controlled studies in patients with moderate to severe plaque psoriasis and who
were candidates for phototherapy or systemic therapy. In addition, a randomised, blinded assessor,
active-controlled study compared ustekinumab and etanercept in patients with moderate to severe
plaque psoriasis who had had an inadequate response to, intolerance to, or contraindication to
ciclosporin, methotrexate, or PUVA.
Psoriasis Study 1 (PHOENIX 1) evaluated 766 patients. 53% of these patients were either
non-responsive, intolerant, or had a contraindication to other systemic therapy. Patients randomised to
ustekinumab received 45 mg or 90 mg doses at Weeks 0 and 4 and followed by the same dose every
12 weeks. Patients randomised to receive placebo at Weeks 0 and 4 crossed over to receive
ustekinumab (either 45 mg or 90 mg) at Weeks 12 and 16 followed by dosing every 12 weeks. Patients
originally randomised to ustekinumab who achieved Psoriasis Area and Severity Index 75 response
(PASI improvement of at least 75% relative to baseline) at both Weeks 28 and 40 were re-randomised
to receive ustekinumab every 12 weeks or to placebo (i.e., withdrawal of therapy). Patients who were
re-randomised to placebo at Week 40 reinitiated ustekinumab at their original dosing regimen when
they experienced at least a 50% loss of their PASI improvement obtained at Week 40. All patients
were followed for up to 76 weeks following first administration of study treatment.
Psoriasis Study 2 (PHOENIX 2) evaluated 1,230 patients. 61% of these patients were either
non-responsive, intolerant, or had a contraindication to other systemic therapy. Patients randomised to
ustekinumab received 45 mg or 90 mg doses at Weeks 0 and 4 followed by an additional dose at
16 weeks. Patients randomised to receive placebo at Weeks 0 and 4 crossed over to receive
ustekinumab (either 45 mg or 90 mg) at Weeks 12 and 16. All patients were followed for up to
52 weeks following first administration of study treatment.
Psoriasis Study 3 (ACCEPT) evaluated 903 patients with moderate to severe psoriasis who
inadequately responded to, were intolerant to, or had a contraindication to other systemic therapy and
compared the efficacy of ustekinumab to etanercept and evaluated the safety of ustekinumab and
etanercept. During the 12-week active-controlled portion of the study, patients were randomised to
receive etanercept (50 mg twice a week), ustekinumab 45 mg at Weeks 0 and 4, or ustekinumab 90 mg
at Weeks 0 and 4.

Baseline disease characteristics were generally consistent across all treatment groups in Psoriasis
Studies 1 and 2 with a median baseline PASI score from 17 to 18, median baseline Body Surface Area
(BSA) 20, and median Dermatology Life Quality Index (DLQI) range from 10 to 12. Approximately
one third (Psoriasis Study 1) and one quarter (Psoriasis Study 2) of subjects had Psoriatic Arthritis
(PsA). Similar disease severity was also seen in Psoriasis Study 3.
The primary endpoint in these studies was the proportion of patients who achieved PASI 75 response
from baseline at Week 12 (see Tables 2 and 3).
Table 2 Summary of clinical response in Psoriasis Study 1 (PHOENIX 1) and Psoriasis Study 2
(PHOENIX 2)
Week 12
2 doses (Week 0 and Week 4)
Week 28
3 doses (Week 0, Week 4
and Week 16)
Psoriasis Study 1
Number of patients randomised 255 255 256 250 243
PASI 50 response N (%) 26 (10%) 213 (84%)
a
220 (86%)
a
228 (91%) 234 (96%)
PASI 75 response N (%) 8 (3%) 171 (67%)
a
170 (66%)
a
178 (71%) 191 (79%)
PASI 90 response N (%) 5 (2%) 106 (42%)
a
94 (37%)
a
123 (49%) 135 (56%)
PGA
b
of cleared or minimal N (%) 10 (4%) 151 (59%)
a
156 (61%)
a
146 (58%) 160 (66%)
Number of patients ≤ 100 kg 166 168 164 164 153
PASI 75 response N (%) 6 (4%) 124 (74%) 107 (65%) 130 (79%) 124 (81%)
Number of patients > 100 kg 89 87 92 86 90
PASI 75 response N (%) 2 (2%) 47 (54%) 63 (68%) 48 (56%) 67 (74%)
Psoriasis Study 2
Number of patients randomised 410 409 411 397 400
PASI 50 response N (%) 41 (10%) 342 (84%)
a
367 (89%)
a
369 (93%) 380 (95%)
PASI 75 response N (%) 15 (4%) 273 (67%)
a
311 (76%)
a
276 (70%) 314 (79%)
PASI 90 response N (%) 3 (1%) 173 (42%)
a
209 (51%)
a
178 (45%) 217 (54%)
PGA
b
of cleared or minimal N (%) 18 (4%) 277 (68%)
a
300 (73%)
a
241 (61%) 279 (70%)
Number of patients ≤ 100 kg 290 297 289 287 280
PASI 75 response N (%) 12 (4%) 218 (73%) 225 (78%) 217 (76%) 226 (81%)
Number of patients > 100 kg 120 112 121 110 119
PASI 75 response N (%) 3 (3%) 55 (49%) 86 (71%) 59 (54%) 88 (74%)
p < 0.001 for ustekinumab 45 mg or 90 mg in comparison with placebo (PBO).
PGA = Physician Global Assessment
Table 3 Summary of clinical response at Week 12 in Psoriasis Study 3 (ACCEPT)






















Etanercept
24 doses
(50 mg twice a
week)
Ustekinumab
2 doses (Week 0 and Week 4)
Number of patients randomised 347
PGA of cleared or minimal N (%) 170 (49%)
Number of patients ≤ 100 kg
PASI 75 response N (%) 154 (61%)
Number of patients > 100 kg
PASI 75 response N (%) 43 (45%)
p < 0.001 for ustekinumab 45 mg or 90 mg in comparison with etanercept.
p = 0.012 for ustekinumab 45 mg in comparison with etanercept.
In Psoriasis Study 1 maintenance of PASI 75 was significantly superior with continuous treatment
compared with treatment withdrawal (p < 0.001). Similar results were seen with each dose of
ustekinumab. At Week 52, 89% of patients re-randomised to maintenance treatment were PASI 75
responders compared with 63% of patients re-randomised to placebo (treatment withdrawal)
(p < 0.001). At week 76, 84% of patients re-randomised to maintenance treatment were PASI 75
responders compared with 19% of patients re-randomised to placebo (treatment withdrawal).
In patients re-randomised to placebo, and who reinitiated their original ustekinumab treatment regimen
after loss of ≥ 50% of PASI improvement 85% regained PASI 75 response within 12 weeks after
re-initiating therapy.
In Psoriasis Study 1, at Week 2 and Week 12, significantly greater improvements from baseline were
demonstrated in the DLQI in each ustekinumab treatment group compared with placebo. The
improvement was sustained through Week 28. Similarly, significant improvements were seen in
Psoriasis Study 2 at Week 4 and 12, which were sustained through Week 24. In Psoriasis Study 1,
improvements in nail psoriasis (Nail Psoriasis Severity Index), in the physical and mental component
summary scores of the SF-36 and in the Itch Visual Analogue Scale (VAS) were also significant in
each ustekinumab treatment group compared with placebo. In Psoriasis Study 2, the Hospital Anxiety
and Depression Scale (HADS) and Work Limitations Questionnaire (WLQ) were also significantly
improved in each ustekinumab treatment group compared with placebo.
5.2 Pharmacokinetic properties
Absorption
The median time to reach the maximum serum concentration (t
max
) was 8.5 days after a single 90 mg
subcutaneous administration in healthy subjects. The median t
max
values of ustekinumab following a
single subcutaneous administration of either 45 mg or 90 mg in patients with psoriasis were
comparable to those observed in healthy subjects.
The absolute bioavailability of ustekinumab following a single subcutaneous administration was
estimated to be 57.2% in patients with psoriasis.
Distribution
Median volume of distribution during the terminal phase (Vz) following a single intravenous




















administration to patients with psoriasis ranged from 57 to 83 ml/kg.
Metabolism
The exact metabolic pathway for ustekinumab is unknown.
Elimination
Median systemic clearance (CL) following a single intravenous administration to patients with
psoriasis ranged from 1.99 to 2.34 ml/day/kg. Median half-life (t
1/2
) of ustekinumab was
approximately 3 weeks in patients with psoriasis, ranging from 15 to 32 days across all psoriasis
studies. In a population pharmacokinetic analysis, the apparent clearance (CL/F) and apparent volume
of distribution (V/F) were 0.465 l/day and 15.7 l, respectively, in patients with psoriasis. The CL/F of
ustekinumab was not impacted by gender. Population pharmacokinetic analysis showed that there was
a trend towards a higher clearance of ustekinumab in patients who tested positive for antibodies to
ustekinumab.
Dose linearity
The systemic exposure of ustekinumab (C
max
and AUC) increased in an approximately
dose-proportional manner after a single intravenous administration at doses ranging from 0.09 mg/kg
to 4.5 mg/kg or following a single subcutaneous administration at doses ranging from approximately
24 mg to 240 mg in patients with psoriasis.
Single dose vs. multiple doses
Serum concentration-time profiles of ustekinumab were generally predictable after single or multiple
subcutaneous dose administrations. Steady-state serum concentrations of ustekinumab were achieved
by Week 28 after initial subcutaneous doses at Weeks 0 and 4 followed by doses every 12 weeks. The
median steady-state trough concentration ranged from 0.21 μg/ml to 0.26 μg/ml (45 mg) and from
0.47 μg/ml to 0.49 μg/ml (90 mg). There was no apparent accumulation in serum ustekinumab
concentration over time when given subcutaneously every 12 weeks.
Impact of weight on pharmacokinetics
In a population pharmacokinetic analysis, body weight was found to be the most significant covariate
affecting the clearance of ustekinumab. The median CL/F in patients with weight > 100 kg was
approximately 55% higher compared to patients with weight 100 kg. The median V/F in patients
with weight > 100 kg was approximately 37% higher as compared to patients with weight 100 kg.
The median trough serum concentrations of ustekinumab in patients with higher weight (> 100 kg) in
the 90 mg group were comparable to those in patients with lower weight (≤ 100 kg) in the 45 mg
group.
Special populations
No pharmacokinetic data are available in patients with impaired renal or hepatic function.
No specific studies have been conducted in elderly patients.
In the population pharmacokinetic analysis, there were no indications of an effect of tobacco or
alcohol on the pharmacokinetics of ustekinumab.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard (e.g. organ toxicity) for humans based on studies of
repeated-dose toxicity and developmental and reproductive toxicity, including safety pharmacology
evaluations. In developmental and reproductive toxicity studies in cynomolgus monkeys, neither
adverse effects on male fertility indices nor birth defects or developmental toxicity were observed. No
adverse effects on female fertility indices were observed using an analogous antibody to IL-12/23 in
mice.
Dose levels in animal studies were up to approximately 45-fold higher than the highest equivalent
dose intended to be administered to psoriasis patients and resulted in peak serum concentrations in
monkeys that were more than 100-fold higher than observed in humans.





Carcinogenicity studies were not performed with ustekinumab due to the lack of appropriate models
for an antibody with no cross-reactivity to rodent IL-12/23 p40.
6. PHARMACEUTICAL PARTICULARS
Sucrose
L-histidine
L-histidine monohydrochloride monohydrate
Polysorbate 80
Water for injections
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
6.4 Special precautions for storage
Store in a refrigerator (2ºC - 8ºC). Do not freeze.
Keep the pre-filled syringe in the outer carton in order to protect from light.
6.5 Nature and contents of container
STELARA is supplied as a sterile solution in a single-use type I glass 1 ml syringe with a fixed
stainless steel needle and a needle cover containing dry natural rubber (a derivative of latex). The
syringe is fitted with a passive safety guard. STELARA is available in a pack of 1 pre-filled syringe.
6.6 Special precautions for disposal and other handling
The solution in the STELARA pre-filled syringe should not be shaken. The solution should be visually
inspected for particulate matter or discoloration prior to subcutaneous administration. The solution is
clear to slightly opalescent, colourless to light yellow and may contain a few small translucent or
white particles of protein. This appearance is not unusual for proteinaceous solutions. The product
should not be used if the solution is discoloured or cloudy, or if foreign particulate matter is present.
Before administration, STELARA should be allowed to reach room temperature (approximately half
an hour). Detailed instructions for use are provided in the package leaflet.
STELARA does not contain preservatives; therefore any unused product remaining in the syringe
should not be used. Any unused product or waste material should be disposed of in accordance with
local requirements.
7. MARKETING AUTHORISATION HOLDER
Janssen-Cilag International NV
Turnhoutseweg 30
2340 Beerse
Belgium
8. MARKETING AUTHORISATION NUMBER(S)
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 16 January 2009
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
Agency
http://www.ema.europa.eu/
A. MANUFACTURER OF THE BIOLOGICAL ACTIVE
SUBSTANCE AND MANUFACTURING AUTHORISATION
HOLDER RESPONSIBLE FOR BATCH RELEASE
B. CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURERS OF THE BIOLOGICAL ACTIVE SUBSTANCE AND
MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH RELEASE
Name and address of the manufacturers of the biological active substance
Centocor Biologics, LLC
4777 LeBourget Drive
St. Louis, MO 63134
USA
Janssen Biologics B.V.
Einsteinweg 101
NL-2333 CB Leiden
The Netherlands
Janssen Biologics (Ireland)
Barnahely
Ringaskiddy
Co. Cork
Ireland
Name and address of the manufacturer responsible for batch release
Janssen Biologics B.V.
Einsteinweg 101
NL-2333 CB Leiden
The Netherlands
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to restricted medical prescription (See Annex I: Summary of Product
Characteristics).
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
The Marketing authorisation Holder (MAH) shall ensure that prior to launch of Stelara, all healthcare
professionals who are expected to prescribe/use Stelara are provided with educational materials
containing the following:
Healthcare Professional educational pack
Patient information pack
The key messages and components included in the Healthcare Professional educational pack are
defined as follows:
Summary of Product Characteristics
Local Guidance for tuberculosis screening;
Risk of serious infections, including salmonella, tuberculosis, and other mycobacterial
infections;
Risk of hypersensitivity reactions, including latex allergy;
Risk of malignancies.

The key messages in the patient information pack are defined as follows:
Patient Information Leaflet
Risk of reactivation of latent tuberculosis and information about the screening for tuberculosis
according to the local guidance;
Risk of serious infections, including salmonella, tuberculosis, and other mycobacterial
infections;
Risk of hypersensitivity reactions, including latex allergy;
Potential risk of malignancies;
Appropriate techniques for self administration of Stelara, including use of the prefilled
syringes.
Pharmacovigilance system
The MAH must ensure that the system of pharmacovigilance, presented in Module 1.8.1. of the
Marketing Authorisation, is in place and functioning before and whilst the product is on the market.
Risk Management Plan
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version 3.0 (dated 14/08/2009) of the Risk Management Plan
(RMP) presented in Module 1.8.2. of the Marketing Authorisation Application and any subsequent
updates of the RMP agreed by the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, the
updated RMP should be submitted at the same time as the Periodic Safety Update Reports (PSUR).
Updated RMP should be submitted
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
At the request of the European Medicines Agency
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
1. NAME OF THE MEDICINAL PRODUCT
STELARA 45 mg solution for injection
ustekinumab
2. STATEMENT OF ACTIVE SUBSTANCE(S)
Each vial contains 45 mg of ustekinumab in 0.5 ml.
Excipients: Sucrose, L-histidine, L-histidine monohydrochloride monohydrate, polysorbate 80, water
for injections.
4. PHARMACEUTICAL FORM AND CONTENTS
Solution for injection
45 mg/0.5 ml
1 vial
5. METHOD AND ROUTE(S) OF ADMINISTRATION
Do not shake.
Subcutaneous use
Read the package leaflet before use.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
7. OTHER SPECIAL WARNING(S), IF NECESSARY
9. SPECIAL STORAGE CONDITIONS
























Do not freeze.
Keep the vial in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Janssen-Cilag International NV
Turnhoutseweg 30
2340 Beerse
Belgium
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE

















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
1. NAME OF THE MEDICINAL PRODUCT
STELARA 90 mg solution for injection
ustekinumab
2. STATEMENT OF ACTIVE SUBSTANCE(S)
Each vial contains 90 mg of ustekinumab in 1 ml.
Excipients: Sucrose, L-histidine, L-histidine monohydrochloride monohydrate, polysorbate 80, water
for injections.
4. PHARMACEUTICAL FORM AND CONTENTS
Solution for injection
90 mg/1 ml
1 vial
5. METHOD AND ROUTE(S) OF ADMINISTRATION
Do not shake.
Subcutaneous use
Read the package leaflet before use.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
7. OTHER SPECIAL WARNING(S), IF NECESSARY
9. SPECIAL STORAGE CONDITIONS
























Do not freeze.
Keep the vial in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Janssen-Cilag International NV
Turnhoutseweg 30
2340 Beerse
Belgium
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE

















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
PRE-FILLED SYRINGE CARTON TEXT (45 mg)
1. NAME OF THE MEDICINAL PRODUCT
STELARA 45 mg solution for injection in pre-filled syringe
ustekinumab
2. STATEMENT OF ACTIVE SUBSTANCE(S)
Each pre-filled syringe contains 45 mg of ustekinumab in 0.5 ml.
Excipients: Sucrose, L-histidine, L-histidine monohydrochloride monohydrate, polysorbate 80, water
for injections. The container of this medicinal product contains latex rubber. See the package leaflet
for further information.
4. PHARMACEUTICAL FORM AND CONTENTS
Solution for injection in pre-filled syringe
45 mg/0.5 ml
1 pre-filled syringe
5. METHOD AND ROUTE(S) OF ADMINISTRATION
Do not shake.
Subcutaneous use
Read the package leaflet before use.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
7. OTHER SPECIAL WARNING(S), IF NECESSARY
9. SPECIAL STORAGE CONDITIONS

























Store in a refrigerator.
Do not freeze.
Keep the pre-filled syringe in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Janssen-Cilag International NV
Turnhoutseweg 30
2340 Beerse
Belgium
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE

















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
PRE-FILLED SYRINGE CARTON TEXT (90 mg)
1. NAME OF THE MEDICINAL PRODUCT
STELARA 90 mg solution for injection in pre-filled syringe
ustekinumab
2. STATEMENT OF ACTIVE SUBSTANCE(S)
Each pre-filled syringe contains 90 mg of ustekinumab in 1 ml.
Excipients: Sucrose, L-histidine, L-histidine monohydrochloride monohydrate, polysorbate 80, water
for injections. The container of this medicinal product contains latex rubber. See the package leaflet
for further information.
4. PHARMACEUTICAL FORM AND CONTENTS
Solution for injection in pre-filled syringe
90 mg/1 ml
1 pre-filled syringe
5. METHOD AND ROUTE(S) OF ADMINISTRATION
Do not shake.
Subcutaneous use
Read the package leaflet before use.
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
7. OTHER SPECIAL WARNING(S), IF NECESSARY
9. SPECIAL STORAGE CONDITIONS


























Store in a refrigerator.
Do not freeze.
Keep the pre-filled syringe in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Janssen-Cilag International NV
Turnhoutseweg 30
2340 Beerse
Belgium
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE

















PACKAGE LEAFLET: INFORMATION FOR THE USER
STELARA 45 mg solution for injection
Ustekinumab
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
In this leaflet
:
1. What STELARA is and what it is used for
2. Before you use STELARA
3. How to use STELARA
4. Possible side effects
5. How to store STELARA
6. Further information
1. WHAT STELARA IS AND WHAT IT IS USED FOR
STELARA belongs to a group of medicines called immunosuppressants (medicines that inhibit your
immune system). STELARA contains the active substance ustekinumab, a monoclonal antibody.
Monoclonal antibodies are proteins that recognise and bind to other specific proteins in the body.
STELARA is used to treat moderate to severe plaque psoriasis in adult patients who cannot use or did
not respond to other medicines and phototherapy. This disease causes inflammation of skin and nails.
STELARA will reduce the inflammation and other signs of the disease.
2. BEFORE YOU USE STELARA
Do not use STELARA
If you are allergic (hypersensitive) to ustekinumab or to any of the other ingredients of
STELARA (listed in section 6 ‘What STELARA contains’).
If you have an active infection which your doctor considers important (see also below ‘Take
special care with STELARA’).
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before using
STELARA.
Take special care with STELARA
Your doctor will assess your health before each treatment. Make sure you tell your doctor about any
illness you have before each treatment. Check with your doctor before using STELARA if you have:
Infections
o
You must tell your doctor if you have any kind of infection
STELARA may make you less able to fight infections. Some infections could also
become serious.
o
Tell your doctor if you have any signs of infection, even if it is very minor. Signs may
include fever, feeling tired, cough, flu-like symptoms, diarrhoea, dental problems and
burning when urinating. If you are not sure, talk to your doctor straight away
o
It is particularly important to tell your doctor if you have an infection that will not go
away or keeps coming back
o
Tell your doctor if you have any open cuts or sores – they might get infected.
o
Tuberculosis (TB)
Tell your doctor if you have had tuberculosis. Also tell him or her if you have
recently been near anyone who might have tuberculosis
Your doctor will examine you for tuberculosis and perform a test to see if you have
tuberculosis, before you are given STELARA
If your doctor thinks that you are at risk of tuberculosis, you may be given
medicines for tuberculosis. This will be before you begin treatment with
STELARA, and during treatment with STELARA
Cancer.
Immunosuppressants like STELARA decrease the activity of the immune system. This
may increase the risk of cancer. Tell your doctor if you have ever had any type of cancer.
Vaccinations.
Tell your doctor if you have recently had or are going to have a vaccine.
Other therapies for psoriasis.
Tell your doctor if you are receiving any other
immunosuppressant or phototherapy (when your body is treated with specific ultraviolet (UV)
light) while using STELARA, which may also decrease the activity of your immune system.
The combination of these therapies has not been investigated and it may increase the risk of
diseases related to a weakened immune system.
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before using
STELARA.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
You should not be given certain types of vaccines while on treatment with STELARA.
Pregnancy and breastfeeding
Talk to your doctor before using STELARA:
If you are pregnant or are planning to become pregnant while using STELARA. The effects of
this medicine in pregnant women are not known. If you are a woman of childbearing potential,
you are advised to avoid becoming pregnant and must use adequate contraception while using
STELARA and for at least 15 weeks after the last STELARA treatment.
If you are breastfeeding or if you plan to breastfeed while using STELARA. Your doctor will
decide whether you should use this medicine.
Driving and using machines
It is not known if STELARA can affect the ability to drive or use machines.
Always use STELARA exactly as your doctor has told you. You should check with your doctor if you
are not sure. Make sure you discuss with your doctor when you will have your injections and your
follow-up appointments.
How much STELARA is given
Your doctor will decide how much STELARA you need and for how long
This may depend on your weight
The usual starting dose is 45 mg ustekinumab. After the starting dose, you will receive the next
dose 4 weeks later, and then every 12 weeks
Patients who weigh more than 100 kg may be given 90 mg instead of 45 mg.
Children and adolescents (under 18 years)
STELARA is not recommended for children and adolescents (under 18 years old) because it has
not been studied in this age group.
How STELARA is given
STELARA is given by injection under your skin (subcutaneously)
At the start, medical or nursing staff may inject STELARA. However, you and your doctor may
decide that you may inject STELARA yourself. In this case you will get training on how to
inject STELARA yourself.
Talk to your doctor if you have any questions about giving yourself an injection. See below in section
‘Instructions for administration’ for further information about how to inject STELARA.
If you use more STELARA than you should
If you have used or been given too much STELARA, talk to a doctor or pharmacist straight away.
Always have the outer carton of the medicine with you, even if it is empty.
If you forget to use STELARA
If you forget a dose, contact your doctor or pharmacist. Do not take a double dose to make up for a
forgotten dose.
If you stop using STELARA
It is not dangerous to stop using STELARA. However, the symptoms for which STELARA was
prescribed may come back.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Like all medicines, STELARA can cause side effects, although not everybody gets them.
Most side effects are mild to moderate. However, some patients may experience serious side effects
and may require treatment.
Tell your doctor straight away if you notice any of the following serious side effects – you may
need urgent medical treatment:
Signs of an allergic reaction such as swelling of the face, lips, mouth or throat which may make
it difficult to swallow or breathe; skin rash; hives; swelling of the hands, feet or ankles
Signs of infection (including tuberculosis) such as fever, feeling tired or short of breath, cough
which will not go away, flu-like symptoms, night sweats, diarrhoea, wounds, dental problems
and burning when urinating.
Side effects may occur with certain frequencies, which are defined as follows:
very common: affects more than 1 user in 10
common: affects 1 to 10 users in 100
uncommon: affects 1 to 10 users in 1,000
rare: affects 1 to 10 users in 10,000
very rare: affects less than 1 user in 10,000
not known: frequency cannot be estimated from the available data.
The following side effects have been observed with STELARA:
Very common:
Infection of the throat or airways.
Common:
Depression
Feeling dizzy
Headache
Sore throat
Blocked or stuffy nose
Diarrhoea
Itching
Rash
Itchy bumps
Back or muscle pain
Feeling tired
Redness of the injection site
Inflammation of tissue under the skin. The signs include warmth, swelling, redness and pain.
Uncommon:
Pain, swelling, itching, hardness, bleeding, bruising and irritation where the injection is given.
Rare:
Serious allergic reactions including anaphylaxis, angioedema. Symptoms of serious allergic
reaction may include wheezing, dizziness and swelling of the face, lips, mouth or throat which
may make it difficult to swallow or breathe.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Store in a refrigerator (2°C–8°C). Do not freeze.
Keep the vial in the outer carton in order to protect from light.
Do not shake STELARA vials. Prolonged vigorous shaking may damage the medicine.
Do not use STELARA
After the expiry date which is stated on the label and the carton after “EXP”. The expiry date
refers to the last day of that month
If the liquid is discoloured, cloudy or you can see other foreign particles floating in it (see
further section 6 ‘What STELARA looks like and contents of the pack’)
If you know, or think that it may have been exposed to extreme temperatures (such as
accidentally frozen or heated)
If the product has been shaken vigorously
If the seal is broken.
STELARA is for single use only. Any unused product remaining in the vial and the syringe should be
disposed of.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active substance is ustekinumab. Each vial contains 45 mg ustekinumab in 0.5 ml.
The other ingredients are sucrose, L-histidine, L-histidine monohydrochloride monohydrate,
polysorbate 80 and water for injections.
What STELARA looks like and contents of the pack
STELARA is a clear to slightly opalescent, colourless to light yellow solution for injection. The
solution may contain a few small translucent or white particles of protein. It is supplied as a carton
pack containing 1 single-dose, glass 2 ml vial. Each vial contains 45 mg ustekinumab in 0.5 ml of
solution for injection.
Marketing Authorisation Holder
Janssen-Cilag International NV
Turnhoutseweg 30
2340 Beerse
Belgium
Manufacturer
Janssen Biologics B.V.
Einsteinweg 101
2333 CB Leiden
The Netherlands
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
JANSSEN-CILAG NV
Antwerpseweg 15-17
B-2340 Beerse
Tél/Tel: +32 14 649 411
Luxembourg/Luxemburg
JANSSEN-CILAG NV
Antwerpseweg 15-17
B-2340 Beerse
Belgique/Belgien
Tél: +32 14 649 411
България
Johnson & Johnson d.o.o.
Бизнес Парк София,
Младост 4, сграда 4, етаж 3
София 1766
Тел.: +359 2 489 94 00
Magyarország
JANSSEN-CILAG Kft.
H-2045 Törökbálint, Tó Park
Tel: +36 23-510-919
Česká republika
JANSSEN-CILAG s.r.o.
Karla Engliše 3201/6
15000 Praha 5
Česká republika
Tel: +420 227 012 222
Malta
A.M. Mangion Ltd.
Mangion Building
Triq ġdida fi triq Valletta
Luqa LQA 6000
Malta
TEL: 00356 2397 6000/6412
Danmark
JANSSEN-CILAG A/S
Hammerbakken 19
DK-3460 Birkerød
Tlf: +45 45 94 82 82
Nederland
JANSSEN-CILAG B.V.
Dr. Paul Janssenweg 150
5026 RH Tilburg
Tel: +31 13 583 73 73
Deutschland
JANSSEN-CILAG GmbH
Johnson & Johnson Platz 1
D-41470 Neuss
Tel: +49 2137-955-955
Norge
JANSSEN-CILAG A.S.
Drammensveien 288
NO-0283 Oslo
Tlf: + 47 24 12 65 00
Eesti
Janssen-Cilag Polska Sp. z o.o.
Eesti filiaal
Lõõtsa 2
EE-11415 Tallinn
Tel: +372 617 7410
Österreich
JANSSEN-CILAG Pharma GmbH
Vorgartenstraße 206B
A-1020 Wien
Tel: +43 1 610 300
Ελλάδα
JANSSEN-CILAG Φαρμακευτική Α.Ε.Β.Ε.
Λεωφόρος Ειρήνης 56
GR-151 21 Πεύκη
Αθήνα
Tηλ: +30 210 80 90 000
Polska
JANSSEN–CILAG
POLSKA Sp. z o.o.
ul.Iłżecka 24
02–135 Warszawa
Tel.: + 48 22 237 60 00
España
JANSSEN-CILAG, S.A.
Paseo de las Doce Estrellas, 5-7
Campo de las Naciones
E-28042 Madrid
Tel: +34 91 722 81 00
Portugal
JANSSEN-CILAG FARMACÊUTICA, LDA.
Estrada Consiglieri Pedroso, 69 A
Queluz de Baixo
P-2734-503 Barcarena
Tel: +351 21 43 68 835
France
JANSSEN-CILAG
1, rue Camille Desmoulins
TSA 91003
92787 Issy Les Moulineaux
Cedex 9
Tél: 0800 25 50 75 / +33 1 55 00 44 44
România
Johnson & Johnson d.o.o.
Str. Tipografilor nr. 11-15,
Clădirea S-Park,
Corp A2, Etaj 5
013714 Bucureşti
Tel : +40 21 207 18 00
Ireland
JANSSEN-CILAG Ltd.
50 -100 Holmers Farm Way,
High Wycombe,
Buckinghamshire, HP12 4EG
Tel: +44 1 494 567 567
Slovenija
Johnson & Johnson d.o.o.
Šmartinska 53
SI-1000, Ljubljana
Tel. +386 1 401 18 30
Ísland
JANSSEN-CILAG
c/o Vistor hf.
Hörgatún 2
IS-210 Garðabær
Sími: +354 535 7000
Slovenská republika
Johnson & Johnson, s.r.o.
Plynárenská 7/B
824 78 Bratislava
Tel: +421 233 552 600
Italia
JANSSEN-CILAG SpA
Via M.Buonarroti, 23
I-20093 Cologno Monzese MI
Tel: +39 02/2510.1
Suomi/Finland
JANSSEN-CILAG OY
Vaisalantie/Vaisalavägen 2
FI-02130 Espoo/Esbo
Puh/Tel: +358 207 531 300
Κύπρος
Βαρνάβας Χατζηπαναγής Λτδ
7 Ανδροκλέους
CY-1060 Λευκωσία
Tηλ: +357 22 755 214
Sverige
JANSSEN-CILAG AB
Box 7073
192 07 Sollentuna
Tel +46 8 626 50 00
Latvija
Janssen-Cilag Polska Sp. z o.o.
filāle Latvijā
Bauskas iela 58A-3
LV-1004, Rīga
Tel: +371 678 93561
United Kingdom
JANSSEN-CILAG Ltd.
50 -100 Holmers Farm Way,
High Wycombe,
Buckinghamshire, HP12 4EG
Tel: +44 1 494 567 567
Lietuva
UAB “Johnson & Johnson”
Geležinio Vilko g. 18A
LT-08104 Vilnius
Tel: +370 5 278 68 88
This leaflet was last approved in
{MM/YYYY}.
Detailed information on this medicine is available on the European Medicines Agency web site:
http://www.ema.europa.eu/
.
INSTRUCTIONS FOR ADMINISTRATION
At the start of treatment, your healthcare provider assists you with your first injection. However, you
and your doctor may decide that you may inject STELARA yourself. If this happens, you will get
training on how to inject STELARA. Talk to your doctor if you have any questions about giving
yourself an injection.
Do not mix STELARA with other liquids for injection
Do not shake STELARA vials. This is because strong shaking may damage the medicine. Do
not use the medicine if it has been shaken strongly.
1. Check the number of vials and prepare the materials:
Take the vial(s) out of the refrigerator. Let the vial stand for about half an hour. This will let the liquid
come to a comfortable temperature for injection (room temperature).
Check the vial(s) to make sure
the number of vials and strength is correct
o
If your dose is 45 mg you will get one 45 mg vial of STELARA
o
If your dose is 90 mg you will get two 45 mg vials of STELARA and you will need to
give yourself two injections. Choose two different sites for these injections (e.g. one
injection in the right thigh and the other injection in the left thigh), and give the injections
one right after the other. Use a new needle and syringe for each injection.
it is the right medicine
it has not passed its expiry date
the vial is not damaged and the seal is not broken
the solution in the vial is clear to slightly opalescent (having a pearl-like shine) and colourless to
light yellow
the solution is not discoloured or cloudy and does not contain any foreign particles
the solution is not frozen.
Get everything together that you need and lay out on a clean surface. This includes a syringe,
needle, antiseptic wipes, a cotton ball or gauze, and a sharps container (see Figure 1).
2. Choose and prepare the injection site:
Choose an injection site (see Figure 2)
STELARA is given by injection under your skin (subcutaneously)
Good places for the injection are the upper thigh or around the belly (abdomen) at least 5 cm
away from the navel (belly button)
If possible, do not use areas of skin that show signs of psoriasis
If someone will assist in giving you the injection, then he or she may also choose the upper arms
as an injection site.

*Areas in gray are recommended injection sites.
Prepare the injection site
Wash your hands very well with soap and warm water
Wipe the injection site on the skin with an antiseptic wipe
Do not touch this area again before giving the injection.
3. Prepare the dose:
Take the cap off the top of the vial (see Figure 3)
Do not remove the stopper
Clean the stopper with an antiseptic swab
Put the vial on a flat surface.
Remove the needle cover
Do not touch the needle or let the needle touch anything
Push the needle through the rubber stopper
Turn the vial and the syringe upside down
Pull on the syringe plunger to fill the syringe with the required amount of liquid, as prescribed
by your doctor (0.5 ml)
It is important that the needle is always in the liquid. This stops air bubbles forming in the
syringe (see Figure 4)





Remove the needle from the vial
Hold the syringe with the needle pointing up to see if it has any air bubbles inside
If there are air bubbles, tap the side gently until the air bubbles go to the top of the syringe (see
Figure 5)
Then press the plunger until all of the air (but none of the liquid) has been removed
Do not lay the syringe down or allow the needle to touch anything.
4. Inject the dose:
Gently pinch the cleaned skin between your thumb and index finger. Do not squeeze it tightly
Push the needle into the pinched skin
Push the plunger with your thumb as far as it will go to inject all of the liquid. Push it slowly
and evenly, keeping the skin gently pinched
When the plunger is pushed as far as it will go, take out the needle and let go of the skin
5. After the injection:
Press an antiseptic wipe over the injection site for a few seconds after the injection
There may be a small amount of blood or liquid at the injection site. This is normal
You can press a cotton ball or gauze over the injection site and hold for 10 seconds
Do not rub your skin. You may cover the injection site with a small adhesive bandage, if
necessary.
6. Disposal:
Used syringes and needles should be placed in a puncture-resistant container, like a sharps
container. Do not ever re-use needles and syringes, for your safety and health and for the safety
of others. Dispose of your sharps container according to your local regulations
Empty vials, antiseptic wipes, and other supplies can be disposed of in your garbage.



PACKAGE LEAFLET: INFORMATION FOR THE USER
STELARA 90 mg solution for injection
Ustekinumab
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
In this leaflet
:
1. What STELARA is and what it is used for
2. Before you use STELARA
3. How to use STELARA
4. Possible side effects
5. How to store STELARA
6. Further information
1. WHAT STELARA IS AND WHAT IT IS USED FOR
STELARA belongs to a group of medicines called immunosuppressants (medicines that inhibit your
immune system). STELARA contains the active substance ustekinumab, a monoclonal antibody.
Monoclonal antibodies are proteins that recognise and bind to other specific proteins in the body.
STELARA is used to treat moderate to severe plaque psoriasis in adult patients who cannot use or did
not respond to other medicines and phototherapy. This disease causes inflammation of skin and nails.
STELARA will reduce the inflammation and other signs of the disease.
2. BEFORE YOU USE STELARA
Do not use STELARA
If you are allergic (hypersensitive) to ustekinumab or to any of the other ingredients of
STELARA (listed in section 6 ‘What STELARA contains’).
If you have an active infection which your doctor considers important (see also below ‘Take
special care with STELARA’).
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before using
STELARA.
Take special care with STELARA
Your doctor will assess your health before each treatment. Make sure you tell your doctor about any
illness you have before each treatment. Check with your doctor before using STELARA if you have:
Infections
o
You must tell your doctor if you have any kind of infection
STELARA may make you less able to fight infections. Some infections could also
become serious.
o
Tell your doctor if you have any signs of infection, even if it is very minor. Signs may
include fever, feeling tired, cough, flu-like symptoms, diarrhoea, dental problems and
burning when urinating. If you are not sure, talk to your doctor straight away
o
It is particularly important to tell your doctor if you have an infection that will not go
away or keeps coming back
o
Tell your doctor if you have any open cuts or sores – they might get infected.
o
Tuberculosis (TB)
Tell your doctor if you have had tuberculosis. Also tell him or her if you have
recently been near anyone who might have tuberculosis
Your doctor will examine you for tuberculosis and perform a test to see if you have
tuberculosis, before you are given STELARA
If your doctor thinks that you are at risk of tuberculosis, you may be given
medicines for tuberculosis. This will be before you begin treatment with
STELARA, and during treatment with STELARA
Cancer.
Immunosuppressants like STELARA decrease the activity of the immune system. This
may increase the risk of cancer. Tell your doctor if you have ever had any type of cancer.
Vaccinations.
Tell your doctor if you have recently had or are going to have a vaccine.
Other therapies for psoriasis.
Tell your doctor if you are receiving any other
immunosuppressant or phototherapy (when your body is treated with specific ultraviolet (UV)
light) while using STELARA, which may also decrease the activity of your immune system.
The combination of these therapies has not been investigated and it may increase the risk of
diseases related to a weakened immune system.
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before using
STELARA.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
You should not be given certain types of vaccines while on treatment with STELARA.
Pregnancy and breastfeeding
Talk to your doctor before using STELARA:
If you are pregnant or are planning to become pregnant while using STELARA. The effects of
this medicine in pregnant women are not known. If you are a woman of childbearing potential,
you are advised to avoid becoming pregnant and must use adequate contraception while using
STELARA and for at least 15 weeks after the last STELARA treatment.
If you are breastfeeding or if you plan to breastfeed while using STELARA. Your doctor will
decide whether you should use this medicine.
Driving and using machines
It is not known if STELARA can affect the ability to drive or use machines.
Always use STELARA exactly as your doctor has told you. You should check with your doctor if you
are not sure. Make sure you discuss with your doctor when you will have your injections and your
follow-up appointments.
How much STELARA is given
Your doctor will decide how much STELARA you need and for how long
This may depend on your weight
The usual starting dose is 45 mg ustekinumab. After the starting dose, you will receive the next
dose 4 weeks later, and then every 12 weeks
Patients who weigh more than 100 kg may be given 90 mg instead of 45 mg.
Children and adolescents (under 18 years)
STELARA is not recommended for children and adolescents (under 18 years old) because it has
not been studied in this age group.
How STELARA is given
STELARA is given by injection under your skin (subcutaneously)
At the start, medical or nursing staff may inject STELARA. However, you and your doctor may
decide that you may inject STELARA yourself. In this case you will get training on how to
inject STELARA yourself.
Talk to your doctor if you have any questions about giving yourself an injection. See below in section
‘Instructions for administration’ for further information about how to inject STELARA.
If you use more STELARA than you should
If you have used or been given too much STELARA, talk to a doctor or pharmacist straight away.
Always have the outer carton of the medicine with you, even if it is empty.
If you forget to use STELARA
If you forget a dose, contact your doctor or pharmacist. Do not take a double dose to make up for a
forgotten dose.
If you stop using STELARA
It is not dangerous to stop using STELARA. However, the symptoms for which STELARA was
prescribed may come back.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Like all medicines, STELARA can cause side effects, although not everybody gets them.
Most side effects are mild to moderate. However, some patients may experience serious side effects
and may require treatment.
Tell your doctor straight away if you notice any of the following serious side effects – you may
need urgent medical treatment:
Signs of an allergic reaction such as swelling of the face, lips, mouth or throat which may make
it difficult to swallow or breathe; skin rash; hives; swelling of the hands, feet or ankles
Signs of infection (including tuberculosis) such as fever, feeling tired or short of breath, cough
which will not go away, flu-like symptoms, night sweats, diarrhoea, wounds, dental problems
and burning when urinating.
Side effects may occur with certain frequencies, which are defined as follows:
very common: affects more than 1 user in 10
common: affects 1 to 10 users in 100
uncommon: affects 1 to 10 users in 1,000
rare: affects 1 to 10 users in 10,000
very rare: affects less than 1 user in 10,000
not known: frequency cannot be estimated from the available data.
The following side effects have been observed with STELARA:
Very common:
Infection of the throat or airways.
Depression
Feeling dizzy
Headache
Sore throat
Blocked or stuffy nose
Diarrhoea
Itching
Rash
Itchy bumps
Back or muscle pain
Feeling tired
Redness of the injection site
Inflammation of tissue under the skin. The signs include warmth, swelling, redness and pain.
Uncommon:
Pain, swelling, itching, hardness, bleeding, bruising and irritation where the injection is given.
Rare:
Serious allergic reactions including anaphylaxis, angioedema. Symptoms of serious allergic
reaction may include wheezing, dizziness and swelling of the face, lips, mouth or throat which
may make it difficult to swallow or breathe.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Store in a refrigerator (2°C–8°C). Do not freeze.
Keep the vial in the outer carton in order to protect from light.
Do not shake STELARA vials. Prolonged vigorous shaking may damage the medicine.
Do not use STELARA
After the expiry date which is stated on the label and the carton after “EXP”. The expiry date
refers to the last day of that month
If the liquid is discoloured, cloudy or you can see other foreign particles floating in it (see
further section 6 ‘What STELARA looks like and contents of the pack’)
If you know, or think that it may have been exposed to extreme temperatures (such as
accidentally frozen or heated)
If the product has been shaken vigorously
If the seal is broken.
STELARA is for single use only. Any unused product remaining in the vial and the syringe should be
disposed of.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
What STELARA contains
The active substance is ustekinumab. Each vial contains 90 mg ustekinumab in 1 ml.
The other ingredients are sucrose, L-histidine, L-histidine monohydrochloride monohydrate,
polysorbate 80 and water for injections.
What STELARA looks like and contents of the pack
STELARA is a clear to slightly opalescent, colourless to light yellow solution for injection. The
solution may contain a few small translucent or white particles of protein. It is supplied as a carton
pack containing 1 single-dose, glass 2 ml vial. Each vial contains 90 mg ustekinumab in 1 ml of
solution for injection.
Marketing Authorisation Holder
Janssen-Cilag International NV
Turnhoutseweg 30
2340 Beerse
Belgium
Manufacturer
Janssen Biologics B.V.
Einsteinweg 101
2333 CB Leiden
The Netherlands
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
JANSSEN-CILAG NV
Antwerpseweg 15-17
B-2340 Beerse
Tél/Tel: + 32 14 649 411
Luxembourg/Luxemburg
JANSSEN-CILAG NV
Antwerpseweg 15-17
B-2340 Beerse
Belgique/Belgien
Tél: +32 14 649 411
България
Johnson & Johnson d.o.o.
Бизнес Парк София,
Младост 4, сграда 4, етаж 3
София 1766
Тел.: +359 2 489 94 00
Magyarország
JANSSEN-CILAG Kft.
H-2045 Törökbálint, Tó Park
Tel: +36 23-510-919
Česká republika
JANSSEN-CILAG s.r.o.
Karla Engliše 3201/6
15000 Praha 5
Česká republika
Tel: +420 227 012 222
Malta
A.M. Mangion Ltd.
Mangion Building
Triq ġdida fi triq Valletta
Luqa LQA 6000
Malta
TEL: 00356 2397 6000/6412
Danmark
JANSSEN-CILAG A/S
Hammerbakken 19
DK-3460 Birkerød
Tlf: +45 45 94 82 82
Nederland
JANSSEN-CILAG B.V.
Dr. Paul Janssenweg 150
5026 RH Tilburg
Tel: +31 13 583 73 73
Deutschland
JANSSEN-CILAG GmbH
Johnson & Johnson Platz 1
D-41470 Neuss
Tel: +49 2137-955-955
Norge
JANSSEN-CILAG A.S.
Drammensveien 288
NO-0283 Oslo
Tlf: + 47 24 12 65 00
Eesti
Janssen-Cilag Polska Sp. z o.o.
Eesti filiaal
Lõõtsa 2
EE-11415 Tallinn
Tel: +372 617 7410
Österreich
JANSSEN-CILAG Pharma GmbH
Vorgartenstraße 206B
A-1020 Wien
Tel: +43 1 610 300
Ελλάδα
JANSSEN-CILAG Φαρμακευτική Α.Ε.Β.Ε.
Λεωφόρος Ειρήνης 56
GR-151 21 Πεύκη
Αθήνα
Tηλ: +30 210 80 90 000
Polska
JANSSEN–CILAG
POLSKA Sp. z o.o.
ul.Iłżecka 24
02–135 Warszawa
Tel.: + 48 22 237 60 00
España
JANSSEN-CILAG, S.A.
Paseo de las Doce Estrellas, 5-7
Campo de las Naciones
E-28042 Madrid
Tel: +34 91 722 81 00
Portugal
JANSSEN-CILAG FARMACÊUTICA, LDA.
Estrada Consiglieri Pedroso, 69 A
Queluz de Baixo
P-2734-503 Barcarena
Tel: +351 21 43 68 835
France
JANSSEN-CILAG
1, rue Camille Desmoulins
TSA 91003
92787 Issy Les Moulineaux
Cedex 9
Tél: 0800 25 50 75 / +33 1 55 00 44 44
România
Johnson & Johnson d.o.o.
Str. Tipografilor nr. 11-15,
Clădirea S-Park,
Corp A2, Etaj 5
013714 Bucureşti
Tel : +40 21 207 18 00
Ireland
JANSSEN-CILAG Ltd.
50 -100 Holmers Farm Way,
High Wycombe,
Buckinghamshire, HP12 4EG
Tel: +44 1 494 567 567
Slovenija
Johnson & Johnson d.o.o.
Šmartinska 53
SI-1000, Ljubljana
Tel. +386 1 401 18 30
Ísland
JANSSEN-CILAG
c/o Vistor hf.
Hörgatún 2
IS-210 Garðabær
Sími: +354 535 7000
Slovenská republika
Johnson & Johnson, s.r.o.
Plynárenská 7/B
824 78 Bratislava
Tel: +421 233 552 600
Italia
JANSSEN-CILAG SpA
Via M.Buonarroti, 23
I-20093 Cologno Monzese MI
Tel: +39 02/2510.1
Suomi/Finland
JANSSEN-CILAG OY
Vaisalantie/Vaisalavägen 2
FI-02130 Espoo/Esbo
Puh/Tel: +358 207 531 300
Κύπρος
Βαρνάβας Χατζηπαναγής Λτδ
7 Ανδροκλέους
CY-1060 Λευκωσία
Tηλ: +357 22 755 214
Sverige
JANSSEN-CILAG AB
Box 7073
192 07 Sollentuna
Tel +46 8 626 50 00
Latvija
Janssen-Cilag Polska Sp. z o.o.
filāle Latvijā
Bauskas iela 58A-3
LV-1004, Rīga
Tel: +371 678 93561
United Kingdom
JANSSEN-CILAG Ltd.
50 -100 Holmers Farm Way,
High Wycombe,
Buckinghamshire, HP12 4EG
Tel: +44 1 494 567 567
Lietuva
UAB “Johnson & Johnson”
Geležinio Vilko g. 18A
LT-08104 Vilnius
Tel: +370 5 278 68 88
This leaflet was last approved in
{MM/YYYY}.
Detailed information on this medicine is available on the European Medicines Agency web site:
http://www.ema.europa.eu/
.
INSTRUCTIONS FOR ADMINISTRATION
At the start of treatment, your healthcare provider assists you with your first injection. However, you
and your doctor may decide that you may inject STELARA yourself. If this happens, you will get
training on how to inject STELARA. Talk to your doctor if you have any questions about giving
yourself an injection.
Do not mix STELARA with other liquids for injection
Do not shake STELARA vials. This is because strong shaking may damage the medicine. Do
not use the medicine if it has been shaken strongly.
1. Check the number of vials and prepare the materials:
Take the vial(s) out of the refrigerator. Let the vial stand for about half an hour. This will let the liquid
come to a comfortable temperature for injection (room temperature).
Check the vial(s) to make sure
the number of vials and strength is correct
o
If your dose is 90 mg you will get one 90 mg vial of STELARA.
it is the right medicine
it has not passed its expiry date
the vial is not damaged and the seal is not broken
the solution in the vial is clear to slightly opalescent (having a pearl-like shine) and colourless to
light yellow
the solution is not discoloured or cloudy and does not contain any foreign particles
the solution is not frozen.
Get everything together that you need and lay out on a clean surface. This includes a syringe,
needle, antiseptic wipes, a cotton ball or gauze, and a sharps container (see Figure 1).
2. Choose and prepare the injection site:
Choose an injection site (see Figure 2)
STELARA is given by injection under your skin (subcutaneously)
Good places for the injection are the upper thigh or around the belly (abdomen) at least 5 cm
away from the navel (belly button)
If possible, do not use areas of skin that show signs of psoriasis
If someone will assist in giving you the injection, then he or she may also choose the upper arms
as an injection site.
*Areas in gray are recommended injection sites.
Prepare the injection site
Wash your hands very well with soap and warm water
Wipe the injection site on the skin with an antiseptic wipe
Do not touch this area again before giving the injection.
3. Prepare the dose:
Take the cap off the top of the vial (see Figure 3)
Do not remove the stopper
Clean the stopper with an antiseptic swab
Put the vial on a flat surface.
Remove the needle cover
Do not touch the needle or let the needle touch anything
Push the needle through the rubber stopper
Turn the vial and the syringe upside down
Pull on the syringe plunger to fill the syringe with the required amount of liquid, as prescribed
by your doctor (1 ml)
It is important that the needle is always in the liquid. This stops air bubbles forming in the
syringe (see Figure 4)





Remove the needle from the vial
Hold the syringe with the needle pointing up to see if it has any air bubbles inside
If there are air bubbles, tap the side gently until the air bubbles go to the top of the syringe (see
Figure 5)
Then press the plunger until all of the air (but none of the liquid) has been removed
Do not lay the syringe down or allow the needle to touch anything.
4. Inject the dose:
Gently pinch the cleaned skin between your thumb and index finger. Do not squeeze it tightly
Push the needle into the pinched skin
Push the plunger with your thumb as far as it will go to inject all of the liquid. Push it slowly
and evenly, keeping the skin gently pinched
When the plunger is pushed as far as it will go, take out the needle and let go of the skin
5. After the injection:
Press an antiseptic wipe over the injection site for a few seconds after the injection
There may be a small amount of blood or liquid at the injection site. This is normal
You can press a cotton ball or gauze over the injection site and hold for 10 seconds
Do not rub your skin. You may cover the injection site with a small adhesive bandage, if
necessary.
6. Disposal:
Used syringes and needles should be placed in a puncture-resistant container, like a sharps
container. Do not ever re-use needles and syringes, for your safety and health and for the safety
of others. Dispose of your sharps container according to your local regulations
Empty vials, antiseptic wipes, and other supplies can be disposed of in your garbage.




PACKAGE LEAFLET: INFORMATION FOR THE USER
STELARA 45 mg solution for injection in pre-filled syringe
Ustekinumab
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
In this leaflet
:
1. What STELARA is and what it is used for
2. Before you use STELARA
3. How to use STELARA
4. Possible side effects
5. How to store STELARA
6. Further information
1. WHAT STELARA IS AND WHAT IT IS USED FOR
STELARA belongs to a group of medicines called immunosuppressants (medicines that inhibit your
immune system). STELARA contains the active substance ustekinumab, a monoclonal antibody.
Monoclonal antibodies are proteins that recognise and bind to other specific proteins in the body.
STELARA is used to treat moderate to severe plaque psoriasis in adult patients who cannot use or did
not respond to other medicines and phototherapy. This disease causes inflammation of skin and nails.
STELARA will reduce the inflammation and other signs of the disease.
2. BEFORE YOU USE STELARA
Do not use STELARA
If you are allergic (hypersensitive) to ustekinumab or to any of the other ingredients of
STELARA (listed in section 6 ‘What STELARA contains’).
If you have an active infection which your doctor considers important (see also below ‘Take
special care with STELARA’).
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before using
STELARA.
Take special care with STELARA
Your doctor will assess your health before each treatment. Make sure you tell your doctor about any
illness you have before each treatment. Check with your doctor before using STELARA if you have:
Infections
o
You must tell your doctor if you have any kind of infection
STELARA may make you less able to fight infections. Some infections could also
become serious.
o
Tell your doctor if you have any signs of infection, even if it is very minor. Signs may
include fever, feeling tired, cough, flu-like symptoms, diarrhoea, dental problems and
burning when urinating. If you are not sure, talk to your doctor straight away
o
It is particularly important to tell your doctor if you have an infection that will not go
away or keeps coming back
o
Tell your doctor if you have any open cuts or sores – they might get infected.
o
Tuberculosis (TB)
Tell your doctor if you have had tuberculosis. Also tell him or her if you have
recently been near anyone who might have tuberculosis
Your doctor will examine you for tuberculosis and perform a test to see if you have
tuberculosis, before you are given STELARA
If your doctor thinks that you are at risk of tuberculosis, you may be given
medicines for tuberculosis. This will be before you begin treatment with
STELARA, and during treatment with STELARA
Latex sensitivity:
The container of this medicinal product contains latex rubber. This may
cause severe allergic reactions in people who are sensitive to latex. Tell your doctor if you have
ever had an allergic reaction to latex or developed any allergic reaction to STELARA injection.
Cancer.
Immunosuppressants like STELARA decrease the activity of the immune system. This
may increase the risk of cancer. Tell your doctor if you have ever had any type of cancer.
Vaccinations.
Tell your doctor if you have recently had or are going to have a vaccine.
Other therapies for psoriasis.
Tell your doctor if you are receiving any other
immunosuppressant or phototherapy (when your body is treated with specific ultraviolet (UV)
light) while using STELARA, which may also decrease the activity of your immune system.
The combination of these therapies has not been investigated and it may increase the risk of
diseases related to a weakened immune system.
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before using
STELARA.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
You should not be given certain types of vaccines while on treatment with STELARA.
Pregnancy and breastfeeding
Talk to your doctor before using STELARA:
If you are pregnant or are planning to become pregnant while using STELARA. The effects of
this medicine in pregnant women are not known. If you are a woman of childbearing potential,
you are advised to avoid becoming pregnant and must use adequate contraception while using
STELARA and for at least 15 weeks after the last STELARA treatment.
If you are breastfeeding or if you plan to breastfeed while using STELARA. Your doctor will
decide whether you should use this medicine.
Driving and using machines
It is not known if STELARA can affect the ability to drive or use machines.
Always use STELARA exactly as your doctor has told you. You should check with your doctor if you
are not sure. Make sure you discuss with your doctor when you will have your injections and your
follow-up appointments.
How much STELARA is given
Your doctor will decide how much STELARA you need and for how long
This may depend on your weight
The usual starting dose is 45 mg ustekinumab. After the starting dose, you will receive the next
dose 4 weeks later, and then every 12 weeks
Patients who weigh more than 100 kg may be given 90 mg instead of 45 mg.
Children and adolescents (under 18 years)
STELARA is not recommended for children and adolescents (under 18 years old) because it has
not been studied in this age group.
How STELARA is given
STELARA is given by injection under your skin (subcutaneously)
At the start, medical or nursing staff may inject STELARA. However, you and your doctor may
decide that you may inject STELARA yourself. In this case you will get training on how to
inject STELARA yourself.
Talk to your doctor if you have any questions about giving yourself an injection. See below in section
‘Instructions for administration’ for further information about how to inject STELARA.
If you use more STELARA than you should
If you have used or been given too much STELARA, talk to a doctor or pharmacist straight away.
Always have the outer carton of the medicine with you, even if it is empty.
If you forget to use STELARA
If you forget a dose, contact your doctor or pharmacist. Do not take a double dose to make up for a
forgotten dose.
If you stop using STELARA
It is not dangerous to stop using STELARA. However, the symptoms for which STELARA was
prescribed may come back.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Like all medicines, STELARA can cause side effects, although not everybody gets them.
Most side effects are mild to moderate. However, some patients may experience serious side effects
and may require treatment.
Tell your doctor straight away if you notice any of the following serious side effects – you may
need urgent medical treatment:
Signs of an allergic reaction such as swelling of the face, lips, mouth or throat which may make
it difficult to swallow or breathe; skin rash; hives; swelling of the hands, feet or ankles
Signs of infection (including tuberculosis) such as fever, feeling tired or short of breath, cough
which will not go away, flu-like symptoms, night sweats, diarrhoea, wounds, dental problems
and burning when urinating.
Side effects may occur with certain frequencies, which are defined as follows:
very common: affects more than 1 user in 10
common: affects 1 to 10 users in 100
uncommon: affects 1 to 10 users in 1,000
rare: affects 1 to 10 users in 10,000
very rare: affects less than 1 user in 10,000
not known: frequency cannot be estimated from the available data.
The following side effects have been observed with STELARA:
Very common:
Infection of the throat or airways.
Common:
Depression
Feeling dizzy
Headache
Sore throat
Blocked or stuffy nose
Diarrhoea
Itching
Rash
Itchy bumps
Back or muscle pain
Feeling tired
Redness of the injection site
Inflammation of tissue under the skin. The signs include warmth, swelling, redness and pain.
Uncommon:
Pain, swelling, itching, hardness, bleeding, bruising and irritation where the injection is given.
Rare:
Serious allergic reactions including anaphylaxis, angioedema. Symptoms of serious allergic
reaction may include wheezing, dizziness and swelling of the face, lips, mouth or throat which
may make it difficult to swallow or breathe.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Store in a refrigerator (2°C–8°C). Do not freeze.
Keep the pre-filled syringe in the outer carton in order to protect from light.
Do not shake STELARA pre-filled syringes. Prolonged vigorous shaking may damage the medicine.
Do not use STELARA
After the expiry date which is stated on the label and the carton after “EXP”. The expiry date
refers to the last day of that month
If the liquid is discoloured, cloudy or you can see other foreign particles floating in it (see
further section 6 ‘What STELARA looks like and contents of the pack’)
If you know, or think that it may have been exposed to extreme temperatures (such as
accidentally frozen or heated)
If the product has been shaken vigorously
STELARA is for single use only. Any unused product remaining in the syringe should be disposed of.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
What STELARA contains
The active substance is ustekinumab. Each pre-filled syringe contains 45 mg ustekinumab in
0.5 ml.
The other ingredients are sucrose, L-histidine, L-histidine monohydrochloride monohydrate,
polysorbate 80 and water for injections.
What STELARA looks like and contents of the pack
STELARA is a clear to slightly opalescent, colourless to light yellow solution for injection. The
solution may contain a few small translucent or white particles of protein. It is supplied as a carton
pack containing 1 single-dose, glass 1 ml pre-filled syringe. Each pre-filled syringe contains 45 mg
ustekinumab in 0.5 ml of solution for injection.
Marketing Authorisation Holder
Janssen-Cilag International NV
Turnhoutseweg 30
2340 Beerse
Belgium
Manufacturer
Janssen Biologics B.V.
Einsteinweg 101
2333 CB Leiden
The Netherlands
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
JANSSEN-CILAG NV
Antwerpseweg 15-17
B-2340 Beerse
Tél/Tel: + 32 14 649 411
Luxembourg/Luxemburg
JANSSEN-CILAG NV
Antwerpseweg 15-17
B-2340 Beerse
Belgique/Belgien
Tél: +32 14 649 411
България
Johnson & Johnson d.o.o.
Бизнес Парк София,
Младост 4, сграда 4, етаж 3
София 1766
Тел.: +359 2 489 94 00
Magyarország
JANSSEN-CILAG Kft.
H-2045 Törökbálint, Tó Park
Tel: +36 23-510-919
Česká republika
JANSSEN-CILAG s.r.o.
Karla Engliše 3201/6
15000 Praha 5
Česká republika
Tel: +420 227 012 222
Malta
A.M. Mangion Ltd.
Mangion Building
Triq ġdida fi triq Valletta
Luqa LQA 6000
Malta
TEL: 00356 2397 6000/6412
Danmark
JANSSEN-CILAG A/S
Hammerbakken 19
DK-3460 Birkerød
Tlf: +45 45 94 82 82
Nederland
JANSSEN-CILAG B.V.
Dr. Paul Janssenweg 150
5026 RH Tilburg
Tel: +31 13 583 73 73
Deutschland
JANSSEN-CILAG GmbH
Johnson & Johnson Platz 1
D-41470 Neuss
Tel: +49 2137-955-955
Norge
JANSSEN-CILAG A.S.
Drammensveien 288
NO-0283 Oslo
Tlf: + 47 24 12 65 00
Eesti
Janssen-Cilag Polska Sp. z o.o.
Eesti filiaal
Lõõtsa 2
EE-11415 Tallinn
Tel: +372 617 7410
Österreich
JANSSEN-CILAG Pharma GmbH
Vorgartenstraße 206B
A-1020 Wien
Tel: +43 1 610 300
Ελλάδα
JANSSEN-CILAG Φαρμακευτική Α.Ε.Β.Ε.
Λεωφόρος Ειρήνης 56
GR-151 21 Πεύκη
Αθήνα
Tηλ: +30 210 80 90 000
Polska
JANSSEN–CILAG
POLSKA Sp. z o.o.
ul.Iłżecka 24
02–135 Warszawa
Tel.: + 48 22 237 60 00
España
JANSSEN-CILAG, S.A.
Paseo de las Doce Estrellas, 5-7
Campo de las Naciones
E-28042 Madrid
Tel: +34 91 722 81 00
Portugal
JANSSEN-CILAG FARMACÊUTICA, LDA.
Estrada Consiglieri Pedroso, 69 A
Queluz de Baixo
P-2734-503 Barcarena
Tel: +351 21 43 68 835
France
JANSSEN-CILAG
1, rue Camille Desmoulins
TSA 91003
92787 Issy Les Moulineaux
Cedex 9
Tél: 0800 25 50 75 / +33 1 55 00 44 44
România
Johnson & Johnson d.o.o.
Str. Tipografilor nr. 11-15,
Clădirea S-Park,
Corp A2, Etaj 5
013714 Bucureşti
Tel : +40 21 207 18 00
Ireland
JANSSEN-CILAG Ltd.
50 -100 Holmers Farm Way,
High Wycombe,
Buckinghamshire, HP12 4EG
Tel: +44 1 494 567 567
Slovenija
Johnson & Johnson d.o.o.
Šmartinska 53
SI-1000, Ljubljana
Tel. +386 1 401 18 30
Ísland
JANSSEN-CILAG
c/o Vistor hf.
Hörgatún 2
IS-210 Garðabær
Sími: +354 535 7000
Slovenská republika
Johnson & Johnson, s.r.o.
Plynárenská 7/B
824 78 Bratislava
Tel: +421 233 552 600
Italia
JANSSEN-CILAG SpA
Via M.Buonarroti, 23
I-20093 Cologno Monzese MI
Tel: +39 02/2510.1
Suomi/Finland
JANSSEN-CILAG OY
Vaisalantie/Vaisalavägen 2
FI-02130 Espoo/Esbo
Puh/Tel: +358 207 531 300
Κύπρος
Βαρνάβας Χατζηπαναγής Λτδ
7 Ανδροκλέους
CY-1060 Λευκωσία
Tηλ: +357 22 755 214
Sverige
JANSSEN-CILAG AB
Box 7073
192 07 Sollentuna
Tel +46 8 626 50 00
Latvija
Janssen-Cilag Polska Sp. z o.o.
filāle Latvijā
Bauskas iela 58A-3
LV-1004, Rīga
Tel: +371 678 93561
United Kingdom
JANSSEN-CILAG Ltd.
50 -100 Holmers Farm Way,
High Wycombe,
Buckinghamshire, HP12 4EG
Tel: +44 1 494 567 567
Lietuva
UAB “Johnson & Johnson”
Geležinio Vilko g. 18A
LT-08104 Vilnius
Tel: +370 5 278 68 88
This leaflet was last approved in
{MM/YYYY}.
Detailed information on this medicine is available on the European Medicines Agency web site:
http://www.ema.europa.eu/
.
INSTRUCTIONS FOR ADMINISTRATION
At the start of treatment, your healthcare provider assists you with your first injection. However, you
and your doctor may decide that you may inject STELARA yourself. If this happens, you will get
training on how to inject STELARA. Talk to your doctor if you have any questions about giving
yourself an injection.
Do not mix STELARA with other liquids for injection
Do not shake STELARA pre-filled syringes. This is because strong shaking may damage the
medicine. Do not use the medicine if it has been shaken strongly.
Figure 1 shows what the pre-filled syringe looks like.
1. Check the number of pre-filled syringes and prepare the materials:
Preparing for use of the pre-filled syringe
Take the pre-filled syringe(s) out of the refrigerator. Let the pre-filled syringe stand outside the
box for about half an hour. This will let the liquid come to a comfortable temperature for
injection (room temperature). Do not remove the syringe’s needle cover while allowing it to
reach room temperature
Hold the pre-filled syringe by the body of the syringe with the covered needle pointing upward
Do not hold by the plunger head, plunger, needle guard wings, or needle cover
Do not pull back on the plunger at any time
Do not remove the needle cover from the pre-filled syringe until instructed to do so
Do not touch the needle guard activation clips (as indicated by asterisks * in Figure 1) to
prevent prematurely covering the needle with the needle guard.
Check the pre-filled syringe(s) to make sure
the number of pre-filled syringes and strength is correct
o If your dose is 45 mg you will get one 45 mg pre-filled syringe of STELARA
o If your dose is 90 mg you will get two 45 mg pre-filled syringes of STELARA and you
will need to give yourself two injections. Choose two different sites for these injections
(e.g. one injection in the right thigh and the other injection in the left thigh), and give the
injections one right after the other.
it is the right medicine
it has not passed its expiry date
the pre-filled syringe is not damaged
the solution in the pre-filled syringe is clear to slightly opalescent (having a pearl-like shine)
and colourless to light yellow
the solution in the pre-filled syringe is not discoloured or cloudy and does not contain any
foreign particles
the solution in the pre-filled syringe is not frozen.
Get everything together that you need and lay out on a clean surface. This includes antiseptic

wipes, a cotton ball or gauze, and a sharps container.
2. Choose and prepare the injection site:
Choose an injection site (see Figure 2)
STELARA is given by injection under your skin (subcutaneously)
Good places for the injection are the upper thigh or around the belly (abdomen) at least 5 cm
away from the navel (belly button)
If possible, do not use areas of skin that show signs of psoriasis
If someone will assist in giving you the injection, then he or she may also choose the upper arms
as an injection site.
*Areas in gray are recommended injection sites.
Prepare the injection site
Wash your hands very well with soap and warm water
Wipe the injection site on the skin with an antiseptic wipe
Do not
touch this area again before giving the injection.
3. Remove the needle cover (see Figure 3):
The needle cover should
not
be removed until you are ready to inject the dose
Pick up the pre-filled syringe, hold the body of the syringe with one hand
Pull the needle cover straight off and throw it away. Do not touch the plunger while you do this
You may notice an air bubble in the pre-filled syringe or a drop of liquid at the end of the
needle. These are both normal and do not need to be removed
Do not touch the needle or allow it to touch any surface
Do not use the pre-filled syringe if it is dropped without the needle cover in place. If this
happens, please contact your doctor or pharmacist
Inject the dose promptly after removing the needle cover.
4. Inject the dose:
Hold the pre-filled syringe with one hand between the middle and index fingers and place the
thumb on top of the plunger head and use the other hand to gently pinch the cleaned skin
between your thumb and index finger. Do not squeeze it tightly


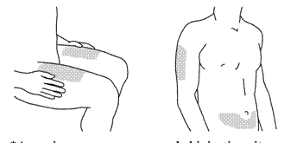
Do not pull back on the plunger at any time
In a single and swift motion, insert the needle through the skin as far as it will go (see Figure 4)
Inject all of the medication by pushing in the plunger until the plunger head is completely
between the needle guard wings (see Figure 5)
When the plunger is pushed as far as it will go, continue to keep the pressure on the plunger
head, take out the needle and let go of the skin (see Figure 6)
Slowly take your thumb off the plunger head to allow the empty syringe to move up until the
entire needle is covered by the needle guard, as shown by Figure 7:




5. After the injection:
Press an antiseptic wipe over the injection site for a few seconds after the injection.
There may be a small amount of blood or liquid at the injection site. This is normal.
You can press a cotton ball or gauze over the injection site and hold for 10 seconds.
Do not rub your skin. You may cover the injection site with a small adhesive bandage, if
necessary.
6. Disposal:
Used syringes should be placed in a puncture-resistant container, like a sharps container (see
Figure 8). Do not ever re-use a syringe, for your safety and health and for the safety of others.
Dispose of your sharps container according to your local regulations
Antiseptic wipes and other supplies can be disposed of in your garbage.
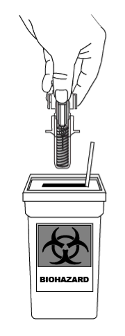
PACKAGE LEAFLET: INFORMATION FOR THE USER
STELARA 90 mg solution for injection in pre-filled syringe
Ustekinumab
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
In this leaflet
:
1. What STELARA is and what it is used for
2. Before you use STELARA
3. How to use STELARA
4. Possible side effects
5. How to store STELARA
6. Further information
1. WHAT STELARA IS AND WHAT IT IS USED FOR
STELARA belongs to a group of medicines called immunosuppressants (medicines that inhibit your
immune system). STELARA contains the active substance ustekinumab, a monoclonal antibody.
Monoclonal antibodies are proteins that recognise and bind to other specific proteins in the body.
STELARA is used to treat moderate to severe plaque psoriasis in adult patients who cannot use or did
not respond to other medicines and phototherapy. This disease causes inflammation of skin and nails.
STELARA will reduce the inflammation and other signs of the disease.
2. BEFORE YOU USE STELARA
Do not use STELARA
If you are allergic (hypersensitive) to ustekinumab or to any of the other ingredients of
STELARA (listed in section 6 ‘What STELARA contains’).
If you have an active infection which your doctor considers important (see also below ‘Take
special care with STELARA’).
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before using
STELARA.
Take special care with STELARA
Your doctor will assess your health before each treatment. Make sure you tell your doctor about any
illness you have before each treatment. Check with your doctor before using STELARA if you have:
Infections
o
You must tell your doctor if you have any kind of infection
STELARA may make you less able to fight infections. Some infections could also
become serious.
o
Tell your doctor if you have any signs of infection, even if it is very minor. Signs may
include fever, feeling tired, cough, flu-like symptoms, diarrhoea, dental problems and
burning when urinating. If you are not sure, talk to your doctor straight away
o
It is particularly important to tell your doctor if you have an infection that will not go
away or keeps coming back
o
Tell your doctor if you have any open cuts or sores – they might get infected.
o
Tuberculosis (TB)
Tell your doctor if you have had tuberculosis. Also tell him or her if you have
recently been near anyone who might have tuberculosis
Your doctor will examine you for tuberculosis and perform a test to see if you have
tuberculosis, before you are given STELARA
If your doctor thinks that you are at risk of tuberculosis, you may be given
medicines for tuberculosis. This will be before you begin treatment with
STELARA, and during treatment with STELARA
Latex sensitivity:
The container of this medicinal product contains latex rubber. This may
cause severe allergic reactions in people who are sensitive to latex. Tell your doctor if you have
ever had an allergic reaction to latex or developed any allergic reaction to STELARA injection.
Cancer.
Immunosuppressants like STELARA decrease the activity of the immune system. This
may increase the risk of cancer. Tell your doctor if you have ever had any type of cancer.
Vaccinations.
Tell your doctor if you have recently had or are going to have a vaccine.
Other therapies for psoriasis.
Tell your doctor if you are receiving any other
immunosuppressant or phototherapy (when your body is treated with specific ultraviolet (UV)
light) while using STELARA, which may also decrease the activity of your immune system.
The combination of these therapies has not been investigated and it may increase the risk of
diseases related to a weakened immune system.
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before using
STELARA.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
You should not be given certain types of vaccines while on treatment with STELARA.
Pregnancy and breastfeeding
Talk to your doctor before using STELARA:
If you are pregnant or are planning to become pregnant while using STELARA. The effects of
this medicine in pregnant women are not known. If you are a woman of childbearing potential,
you are advised to avoid becoming pregnant and must use adequate contraception while using
STELARA and for at least 15 weeks after the last STELARA treatment.
If you are breastfeeding or if you plan to breastfeed while using STELARA. Your doctor will
decide whether you should use this medicine.
Driving and using machines
It is not known if STELARA can affect the ability to drive or use machines.
Always use STELARA exactly as your doctor has told you. You should check with your doctor if you
are not sure. Make sure you discuss with your doctor when you will have your injections and your
follow-up appointments.
How much STELARA is given
Your doctor will decide how much STELARA you need and for how long
This may depend on your weight
The usual starting dose is 45 mg ustekinumab. After the starting dose, you will receive the next
dose 4 weeks later, and then every 12 weeks
Patients who weigh more than 100 kg may be given 90 mg instead of 45 mg.
Children and adolescents (under 18 years)
STELARA is not recommended for children and adolescents (under 18 years old) because it has
not been studied in this age group.
How STELARA is given
STELARA is given by injection under your skin (subcutaneously)
At the start, medical or nursing staff may inject STELARA. However, you and your doctor may
decide that you may inject STELARA yourself. In this case you will get training on how to
inject STELARA yourself.
Talk to your doctor if you have any questions about giving yourself an injection. See below in section
‘Instructions for administration’ for further information about how to inject STELARA.
If you use more STELARA than you should
If you have used or been given too much STELARA, talk to a doctor or pharmacist straight away.
Always have the outer carton of the medicine with you, even if it is empty.
If you forget to use STELARA
If you forget a dose, contact your doctor or pharmacist. Do not take a double dose to make up for a
forgotten dose.
If you stop using STELARA
It is not dangerous to stop using STELARA. However, the symptoms for which STELARA was
prescribed may come back.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Like all medicines, STELARA can cause side effects, although not everybody gets them.
Most side effects are mild to moderate. However, some patients may experience serious side effects
and may require treatment.
Tell your doctor straight away if you notice any of the following serious side effects – you may
need urgent medical treatment:
Signs of an allergic reaction such as swelling of the face, lips, mouth or throat which may make
it difficult to swallow or breathe; skin rash; hives; swelling of the hands, feet or ankles
Signs of infection (including tuberculosis) such as fever, feeling tired or short of breath, cough
which will not go away, flu-like symptoms, night sweats, diarrhoea, wounds, dental problems
and burning when urinating.
Side effects may occur with certain frequencies, which are defined as follows:
very common: affects more than 1 user in 10
common: affects 1 to 10 users in 100
uncommon: affects 1 to 10 users in 1,000
rare: affects 1 to 10 users in 10,000
very rare: affects less than 1 user in 10,000
not known: frequency cannot be estimated from the available data.
The following side effects have been observed with STELARA:
Very common:
Infection of the throat or airways.
Common:
Depression
Feeling dizzy
Headache
Sore throat
Blocked or stuffy nose
Diarrhoea
Itching
Rash
Itchy bumps
Back or muscle pain
Feeling tired
Redness of the injection site
Inflammation of tissue under the skin. The signs include warmth, swelling, redness and pain.
Uncommon:
Pain, swelling, itching, hardness, bleeding, bruising and irritation where the injection is given.
Rare:
Serious allergic reactions including anaphylaxis, angioedema. Symptoms of serious allergic
reaction may include wheezing, dizziness and swelling of the face, lips, mouth or throat which
may make it difficult to swallow or breathe.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Store in a refrigerator (2°C–8°C). Do not freeze.
Keep the pre-filled syringe in the outer carton in order to protect from light.
Do not shake STELARA pre-filled syringes. Prolonged vigorous shaking may damage the medicine.
Do not use STELARA
After the expiry date which is stated on the label and the carton after “EXP”. The expiry date
refers to the last day of that month
If the liquid is discoloured, cloudy or you can see other foreign particles floating in it (see
further section 6 ‘What STELARA looks like and contents of the pack’)
If you know, or think that it may have been exposed to extreme temperatures (such as
accidentally frozen or heated)
If the product has been shaken vigorously
STELARA is for single use only. Any unused product remaining in the syringe should be disposed of.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
What STELARA contains
The active substance is ustekinumab. Each pre-filled syringe contains 90 mg ustekinumab in
1 ml.
The other ingredients are sucrose, L-histidine, L-histidine monohydrochloride monohydrate,
polysorbate 80 and water for injections.
What STELARA looks like and contents of the pack
STELARA is a clear to slightly opalescent, colourless to light yellow solution for injection. The
solution may contain a few small translucent or white particles of protein. It is supplied as a carton
pack containing 1 single-dose, glass 1 ml pre-filled syringe. Each pre-filled syringe contains 90 mg
ustekinumab in 1 ml of solution for injection.
Marketing Authorisation Holder
Janssen-Cilag International NV
Turnhoutseweg 30
2340 Beerse
Belgium
Manufacturer
Janssen Biologics B.V.
Einsteinweg 101
2333 CB Leiden
The Netherlands
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
JANSSEN-CILAG NV
Antwerpseweg 15-17
B-2340 Beerse
Tél/Tel: + 32 14 649 411
Luxembourg/Luxemburg
JANSSEN-CILAG NV
Antwerpseweg 15-17
B-2340 Beerse
Belgique/Belgien
Tél: +32 14 649 411
България
Johnson & Johnson d.o.o.
Бизнес Парк София,
Младост 4, сграда 4, етаж 3
София 1766
Тел.: +359 2 489 94 00
Magyarország
JANSSEN-CILAG Kft.
H-2045 Törökbálint, Tó Park
Tel: +36 23-510-919
Česká republika
JANSSEN-CILAG s.r.o.
Karla Engliše 3201/6
15000 Praha 5
Česká republika
Tel: +420 227 012 222
Malta
A.M. Mangion Ltd.
Mangion Building
Triq ġdida fi triq Valletta
Luqa LQA 6000
Malta
TEL: 00356 2397 6000/6412
Danmark
JANSSEN-CILAG A/S
Hammerbakken 19
DK-3460 Birkerød
Tlf: +45 45 94 82 82
Nederland
JANSSEN-CILAG B.V.
Dr. Paul Janssenweg 150
5026 RH Tilburg
Tel: +31 13 583 73 73
Deutschland
JANSSEN-CILAG GmbH
Johnson & Johnson Platz 1
D-41470 Neuss
Tel: +49 2137-955-955
Norge
JANSSEN-CILAG A.S.
Drammensveien 288
NO-0283 Oslo
Tlf: + 47 24 12 65 00
Eesti
Janssen-Cilag Polska Sp. z o.o.
Eesti filiaal
Lõõtsa 2
EE-11415 Tallinn
Tel: +372 617 7410
Österreich
JANSSEN-CILAG Pharma GmbH
Vorgartenstraße 206B
A-1020 Wien
Tel: +43 1 610 300
Ελλάδα
JANSSEN-CILAG Φαρμακευτική Α.Ε.Β.Ε.
Λεωφόρος Ειρήνης 56
GR-151 21 Πεύκη
Αθήνα
Tηλ: +30 210 80 90 000
Polska
JANSSEN–CILAG
POLSKA Sp. z o.o.
ul.Iłżecka 24
02–135 Warszawa
Tel.: + 48 22 237 60 00
España
JANSSEN-CILAG, S.A.
Paseo de las Doce Estrellas, 5-7
Campo de las Naciones
E-28042 Madrid
Tel: +34 91 722 81 00
Portugal
JANSSEN-CILAG FARMACÊUTICA, LDA.
Estrada Consiglieri Pedroso, 69 A
Queluz de Baixo
P-2734-503 Barcarena
Tel: +351 21 43 68 835
France
JANSSEN-CILAG
1, rue Camille Desmoulins
TSA 91003
92787 Issy Les Moulineaux
Cedex 9
Tél: 0800 25 50 75 / +33 1 55 00 44 44
România
Johnson & Johnson d.o.o.
Str. Tipografilor nr. 11-15,
Clădirea S-Park,
Corp A2, Etaj 5
013714 Bucureşti
Tel : +40 21 207 18 00
Ireland
JANSSEN-CILAG Ltd.
50 -100 Holmers Farm Way,
High Wycombe,
Buckinghamshire, HP12 4EG
Tel: +44 1 494 567 567
Slovenija
Johnson & Johnson d.o.o.
Šmartinska 53
SI-1000, Ljubljana
Tel. +386 1 401 18 30
Ísland
JANSSEN-CILAG
c/o Vistor hf.
Hörgatún 2
IS-210 Garðabær
Sími: +354 535 7000
Slovenská republika
Johnson & Johnson, s.r.o.
Plynárenská 7/B
824 78 Bratislava
Tel: +421 233 552 600
Italia
JANSSEN-CILAG SpA
Via M.Buonarroti, 23
I-20093 Cologno Monzese MI
Tel: +39 02/2510.1
Suomi/Finland
JANSSEN-CILAG OY
Vaisalantie/Vaisalavägen 2
FI-02130 Espoo/Esbo
Puh/Tel: +358 207 531 300
Κύπρος
Βαρνάβας Χατζηπαναγής Λτδ
7 Ανδροκλέους
CY-1060 Λευκωσία
Tηλ: +357 22 755 214
Sverige
JANSSEN-CILAG AB
Box 7073
192 07 Sollentuna
Tel +46 8 626 50 00
Latvija
Janssen-Cilag Polska Sp. z o.o.
filāle Latvijā
Bauskas iela 58A-3
LV-1004, Rīga
Tel: +371 678 93561
United Kingdom
JANSSEN-CILAG Ltd.
50 -100 Holmers Farm Way,
High Wycombe,
Buckinghamshire, HP12 4EG
Tel: +44 1 494 567 567
Lietuva
UAB “Johnson & Johnson”
Geležinio Vilko g. 18A
LT-08104 Vilnius
Tel: +370 5 278 68 88
This leaflet was last approved in
{MM/YYYY}.
Detailed information on this medicine is available on the European Medicines Agency web site:
http://www.ema.europa.eu/
.
INSTRUCTIONS FOR ADMINISTRATION
At the start of treatment, your healthcare provider assists you with your first injection. However, you
and your doctor may decide that you may inject STELARA yourself. If this happens, you will get
training on how to inject STELARA. Talk to your doctor if you have any questions about giving
yourself an injection.
Do not mix STELARA with other liquids for injection
Do not shake STELARA pre-filled syringes. This is because strong shaking may damage the
medicine. Do not use the medicine if it has been shaken strongly.
Figure 1 shows what the pre-filled syringe looks like.
1. Check the number of pre-filled syringes and prepare the materials:
Preparing for use of the pre-filled syringe
Take the pre-filled syringe(s) out of the refrigerator. Let the pre-filled syringe stand outside the
box for about half an hour. This will let the liquid come to a comfortable temperature for
injection (room temperature). Do not remove the syringe’s needle cover while allowing it to
reach room temperature
Hold the pre-filled syringe by the body of the syringe with the covered needle pointing upward
Do not hold by the plunger head, plunger, needle guard wings, or needle cover
Do not pull back on the plunger at any time
Do not remove the needle cover from the pre-filled syringe until instructed to do so
Do not touch the needle guard activation clips (as indicated by asterisks * in Figure 1) to
prevent prematurely covering the needle with the needle guard.
Check the pre-filled syringe(s) to make sure
the number of pre-filled syringes and strength is correct
o If your dose is 90 mg you will get one 90 mg pre-filled syringe of STELARA.
it is the right medicine
it has not passed its expiry date
the pre-filled syringe is not damaged
the solution in the pre-filled syringe is clear to slightly opalescent (having a pearl-like shine)
and colourless to light yellow
the solution in the pre-filled syringe is not discoloured or cloudy and does not contain any
foreign particles
the solution in the pre-filled syringe is not frozen.
Get everything together that you need and lay out on a clean surface. This includes antiseptic
wipes, a cotton ball or gauze, and a sharps container.
2. Choose and prepare the injection site:
Choose an injection site (see Figure 2)

STELARA is given by injection under your skin (subcutaneously)
Good places for the injection are the upper thigh or around the belly (abdomen) at least 5 cm
away from the navel (belly button)
If possible, do not use areas of skin that show signs of psoriasis
If someone will assist in giving you the injection, then he or she may also choose the upper arms
as an injection site.
*Areas in gray are recommended injection sites.
Prepare the injection site
Wash your hands very well with soap and warm water
Wipe the injection site on the skin with an antiseptic wipe
Do not
touch this area again before giving the injection.
3. Remove the needle cover (see Figure 3):
The needle cover should
not
be removed until you are ready to inject the dose
Pick up the pre-filled syringe, hold the body of the syringe with one hand
Pull the needle cover straight off and throw it away. Do not touch the plunger while you do this
You may notice an air bubble in the pre-filled syringe or a drop of liquid at the end of the
needle. These are both normal and do not need to be removed
Do not touch the needle or allow it to touch any surface
Do not use the pre-filled syringe if it is dropped without the needle cover in place. If this
happens, please contact your doctor or pharmacist
Inject the dose promptly after removing the needle cover.
4. Inject the dose:
Hold the pre-filled syringe with one hand between the middle and index fingers and place the
thumb on top of the plunger head and use the other hand to gently pinch the cleaned skin
between your thumb and index finger. Do not squeeze it tightly


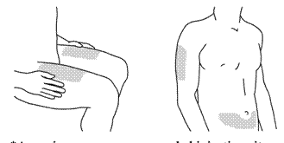
Do not pull back on the plunger at any time
In a single and swift motion, insert the needle through the skin as far as it will go (see Figure 4)
Inject all of the medication by pushing in the plunger until the plunger head is completely
between the needle guard wings (see Figure 5)
When the plunger is pushed as far as it will go, continue to keep the pressure on the plunger
head, take out the needle and let go of the skin (see Figure 6)
Slowly take your thumb off the plunger head to allow the empty syringe to move up until the
entire needle is covered by the needle guard, as shown by Figure 7:
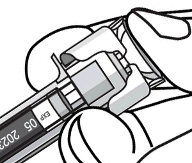
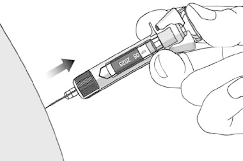



5. After the injection:
Press an antiseptic wipe over the injection site for a few seconds after the injection.
There may be a small amount of blood or liquid at the injection site. This is normal.
You can press a cotton ball or gauze over the injection site and hold for 10 seconds.
Do not rub your skin. You may cover the injection site with a small adhesive bandage, if
necessary.
6. Disposal:
Used syringes should be placed in a puncture-resistant container, like a sharps container (see
Figure 8). Do not ever re-use a syringe, for your safety and health and for the safety of others.
Dispose of your sharps container according to your local regulations
Antiseptic wipes and other supplies can be disposed of in your garbage.
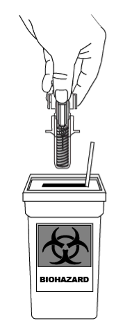
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/stelara.html
Copyright © 1995-2021 ITA all rights reserved.
|











































































































































































































































































































































































































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































