Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Tasigna 150 mg hard capsules
QUALITATIVE AND QUANTITATIVE COMPOSITION
One hard capsule contains 150 mg nilotinib (as hydrochloride monohydrate).
Excipient
Lactose monohydrate: 117.08 mg per capsule.
For a full list of excipients, see section 6.1.
White to yellowish powder in red opaque hard gelatin capsules, size 1 with black axial imprint
“NVR/BCR”.
4.1 Therapeutic indications
Tasigna is indicated for the treatment of adult patients with newly diagnosed Philadelphia
chromosome positive chronic myelogenous leukaemia (CML) in the chronic phase.
4.2 Posology and method of administration
Therapy should be initiated by a physician experienced in the diagnosis and the treatment of patients
with CML.
Posology
The recommended dose of Tasigna is 300 mg twice daily. Treatment should be continued as long as
the patient continues to benefit.
For a dose of 400 mg once daily (see dose adjustments below), 200 mg capsules are available.
Dose adjustments or modifications
Tasigna may need to be temporarily withheld and/or dose reduced for haematological toxicities
(neutropenia, thrombocytopenia) that are not related to the underlying leukaemia (see Table 1).
Dose adjustments for neutropenia and thrombocytopenia
Newly diagnosed
chronic phase CML
at 300 mg twice
daily
ANC* <1.0 x 10
9
/l and/or platelet
counts <50 x 10
9
/l
1.
Treatment with Tasigna must be
interrupted and blood count
monitored.
2.
Treatment must be resumed within
2 weeks at prior dose if ANC
>1.0 x 10
9
/l and/or platelets
>50 x 10
9
/l.
3.
If blood counts remain low, a dose
reduction to 400 mg once daily may
be required.
*ANC = absolute neutrophil count





If clinically significant moderate or severe non-haematological toxicity develops, dosing should be
interrupted, and may be resumed at 400 mg once daily once the toxicity has resolved. If clinically
appropriate, re-escalation of the dose to 300 mg twice daily should be considered.
Elevated serum lipase: For Grade 3-4 serum lipase elevations, doses should be reduced to 400 mg once
daily or interrupted. Serum lipase levels should be tested monthly or as clinically indicated (see
section 4.4).
Elevated bilirubin and hepatic transaminases: For Grade 3-4 bilirubin and hepatic transaminase
elevations, doses should be reduced to 400 mg once daily or interrupted. Bilirubin and hepatic
transaminases levels should be tested monthly or as clinically indicated.
Paediatric population
The safety and efficacy of Tasigna in paediatric patients from birth to less then 18 years have not yet
been established (see section 5.1). Therefore its use in paediatric patients is not recommended due to a
lack of data on safety and efficacy.
Elderly patients
Approximately 12% of subjects in the clinical study were 65 years of age or over. No major
differences were observed for safety and efficacy in patients ≥65 years of age as compared to adults
aged 18 to 65 years.
Patients with renal impairment
Clinical studies have not been performed in patients with impaired renal function.
Since nilotinib and its metabolites are not renally excreted, a decrease in total body clearance is not
anticipated in patients with renal impairment.
Patients with hepatic impairment
Hepatic impairment has a modest effect on the pharmacokinetics of nilotinib. Dose adjustment is not
considered necessary in patients with hepatic impairment. However, patients with hepatic impairment
should be treated with caution (see section 4.4).
Cardiac disorders
In clinical studies, patients with uncontrolled or significant cardiac disease (e.g. recent myocardial
infarction, congestive heart failure, unstable angina or clinically significant bradycardia) were
excluded. Caution should be exercised in patients with relevant cardiac disorders (see section 4.4).
Method of administration
Tasigna should be taken twice daily approximately 12 hours apart and must not be taken with food.
The capsules should be swallowed whole with water. No food should be consumed for 2 hours before
the dose is taken and for at least one hour after.
If a dose is missed the patient should not take an additional dose, but take the usual prescribed next
dose.
For patients who are unable to swallow capsules, the content of each capsule may be dispersed in one
teaspoon of apple sauce (puréed apple) and should be taken immediately. Not more than one teaspoon
of apple sauce and no food other than apple sauce must be used (see sections 4.4 and 5.2).
Tasigna may be given in combination with haematopoietic growth factors such as erythropoietin or
granulocyte colony-stimulating factor (G-CSF) if clinically indicated. It may be given with
hydroxyurea or anagrelide if clinically indicated.
Hypersensitivity to the active substance or to any of the excipients.
4.4 Special warnings and precautions for use
Myelosuppression
Treatment with Tasigna is associated with (National Cancer Institute Common Toxicity Criteria
grade 3-4) thrombocytopenia, neutropenia and anaemia. Complete blood counts should be performed
every two weeks for the first 2 months and then monthly thereafter, or as clinically indicated.
Myelosuppression was generally reversible and usually managed by withholding Tasigna temporarily
or dose reduction (see section 4.2).
QT prolongation
Tasigna has been shown to prolong cardiac ventricular repolarisation as measured by the QT interval
on the surface ECG in a concentration-dependent manner.
In the Phase III study in patients with newly diagnosed CML in chronic phase receiving 300 mg
nilotinib twice daily, the change from baseline in mean time-averaged QTcF interval at steady state
was 6 msec. No patient had a QTcF >480 msec. No episodes of torsade de pointes were observed.
In a healthy volunteer study with exposures that were comparable to the exposures observed in
patients, the time-averaged mean placebo-subtracted QTcF change from baseline was 7 msec
(CI ± 4 msec). No subject had a QTcF >450 msec. Additionally, no clinically relevant arrhythmias
were observed during the conduct of the trial. In particular, no episodes of torsade de pointes (transient
or sustained) were observed.
Significant prolongation of the QT interval may occur when nilotinib is inappropriately taken with
strong CYP3A4 inhibitors and/or medicinal products with a known potential to prolong QT, and/or
food (see section 4.5). The presence of hypokalaemia and hypomagnesaemia may further enhance this
effect. Prolongation of the QT interval may expose patients to the risk of fatal outcome.
Tasigna should be used with caution in patients who have or who are at significant risk of developing
prolongation of QTc, such as those:
-
with congenital long QT prolongation
-
with uncontrolled or significant cardiac disease including recent myocardial infarction,
congestive heart failure, unstable angina or clinically significant bradycardia.
-
taking anti-arrhythmic medicinal products or other substances that lead to QT prolongation.
Close monitoring for an effect on the QTc interval is advisable and a baseline ECG is recommended
prior to initiating therapy with Tasigna and as clinically indicated. Hypokalaemia or
hypomagnesaemia must be corrected prior to Tasigna administration and should be monitored
periodically during therapy.
Sudden death
Uncommon cases (0.1 to 1%) of sudden deaths have been reported in patients with imatinib-resistant
or intolerant CML in chronic phase or accelerated phase with a past medical history of cardiac disease
or significant cardiac risk factors. Co-morbidities in addition to the underlying malignancy were also
frequently present as were concomitant medicinal products. Ventricular repolarisation abnormalities
may have been contributory factors. No cases of sudden death were reported in the Phase III study in
newly diagnosed patients with CML in chronic phase.
Interactions with other medicinal products
The administration of Tasigna with agents that are strong CYP3A4 inhibitors (including, but not
limited to, ketoconazole, itraconazole, voriconazole, clarithromycin, telithromycin, ritonavir) should
be avoided. Should treatment with any of these agents be required, it is recommended that therapy
with Tasigna be interrupted if possible (see section 4.5). If transient interruption of treatment is not
possible, close monitoring of the individual for prolongation of the QT interval is indicated (see
sections 4.2, 4.5 and 5.2).
Concomitant use of Tasigna with medicinal products that are potent inducers of CYP3A4 (e.g.
phenytoin, rifampicin, carbamazepine, phenobarbital and St. John’s Wort) is likely to reduce exposure
to nilotinib to a clinically relevant extent. Therefore, in patients receiving Tasigna, co-administration
of alternative therapeutic agents with less potential for CYP3A4 induction should be selected (see
section 4.5).
Food effect
The bioavailability of nilotinib is increased by food. Tasigna should not be taken in conjunction with
food (see sections 4.2 and 4.5) and should be taken 2 hours after a meal. No food should be consumed
for at least one hour after the dose is taken. Grapefruit juice and other foods that are known to inhibit
CYP3A4 should be avoided. For patients who are unable to swallow capsules, the content of each
capsule may be dispersed in one teaspoon of apple sauce and should be taken immediately. Not more
than one teaspoon of apple sauce and no food other than apple sauce must be used (see section 5.2).
Hepatic impairment
Hepatic impairment has a modest effect on the pharmacokinetics of nilotinib. Single dose
administration of 200 mg of nilotinib resulted in increases in AUC of 35%, 35% and 19% in subjects
with mild, moderate and severe hepatic impairment, respectively, compared to a control group of
subjects with normal hepatic function. The predicted steady-state C
max
of nilotinib showed an increase
of 29%, 18% and 22%, respectively. Clinical studies have excluded patients with alanine transaminase
(ALT) and/or aspartate transaminase (AST) >2.5 (or >5, if related to disease) times the upper limit of
the normal range and/or total bilirubin >1.5 times the upper limit of the normal range. Metabolism of
nilotinib is mainly hepatic. Patients with hepatic impairment might therefore have increased exposure
to nilotinib and should be treated with caution (see section 4.2).
Serum lipase
Elevation in serum lipase has been observed. Caution is recommended in patients with previous
history of pancreatitis. In case lipase elevations are accompanied by abdominal symptoms, Tasigna
should be interrupted and appropriate diagnostic measures considered to exclude pancreatitis.
Total gastrectomy
The bioavailability of nilotinib might be reduced in patients with total gastrectomy (see section 5.2).
More frequent follow-up of these patients should be considered.
Lactose
Tasigna capsules contain lactose. Patients with rare hereditary problems of galactose intolerance, the
Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicinal product.
4.5 Interaction with other medicinal products and other forms of interaction
Substances that may increase nilotinib serum concentrations
Nilotinib is mainly metabolised in the liver and is also a substrate for the multi-drug efflux pump, P-
glycoprotein (P-gp). Therefore, absorption and subsequent elimination of systemically absorbed
nilotinib may be influenced by substances that affect CYP3A4 and/or P-gp. Concomitant
administration of nilotinib with imatinib (a substrate and moderator of P-gp and CYP3A4), had a
slight inhibitory effect on CYP3A4 and/or P-gp. The AUC of imatinib was increased by 18% to 39%,
and the AUC of nilotinib was increased by 18% to 40%. These changes are unlikely to be clinically
important.
The exposure to nilotinib in healthy subjects was increased 3-fold when co-administered with the
strong CYP3A4 inhibitor ketoconazole. Concomitant treatment with strong CYP3A4 inhibitors,
including ketoconazole, itraconazole, voriconazole, ritonavir, clarithromycin, and telithromycin,
should therefore be avoided (see sections 4.2 and 4.4). Increased exposure to nilotinib might also be
expected with moderate CYP3A4 inhibitors. Alternative concomitant medicinal products with no or
minimal CYP3A4 inhibition should be considered.
Substances that may decrease nilotinib serum concentrations
Rifampicin, a potent CYP3A4 inducer, decreases nilotinib C
max
by 64% and reduces nilotinib AUC by
80%. Rifampicin and nilotinib should not be used concomitantly.
The concomitant administration of other medicinal products that induce CYP3A4 (e.g. phenytoin,
carbamazepine, phenobarbital and St. John’s Wort) is likewise likely to reduce exposure to nilotinib to
a clinically relevant extent. In patients for whom CYP3A4 inducers are indicated, alternative agents
with less enzyme induction potential should be selected.
Nilotinib has pH dependent solubility, with lower solubility at higher pH. In healthy subjects receiving
esomeprazole at 40 mg once daily for 5 days, gastric pH was markedly increased, but nilotinib
absorption was only decreased modestly (27% decrease in C
max
and 34% decrease in AUC0-∞).
Nilotinib may be used concurrently with esomeprazole or other proton pump inhibitors as needed.
Substances that may have their systemic concentration altered by nilotinib
Nilotinib is a relatively strong inhibitor of CYP3A4, CYP2C8, CYP2C9, CYP2D6 and UGT1A1
in
vitro
, with Ki value being lowest for CYP2C9 (Ki=0.13 microM).
A single-dose drug-drug interaction study in healthy volunteers with 25 mg warfarin, a sensitive
CYP2C9 substrate, and 800 mg nilotinib did not result in any changes in warfarin pharmacokinetic
parameters or warfarin pharmacodynamics measured as prothrombin time (PT) and international
normalised ratio (INR). There are no steady-state data. This study suggests that a clinically meaningful
drug-drug interaction between nilotinib and warfarin is less likely up to a dose of 25 mg of warfarin.
Due to lack of steady-state data, control of warfarin pharmacodynamic markers (INR or PT) following
initiation of nilotinib therapy (at least during the first 2 weeks) is recommended.
In addition, single-dose administration of Tasigna with orally administered midazolam to healthy
subjects increased midazolam exposure by 30%. It cannot be excluded that the effect of nilotinib is
greater at steady state. Caution should be exercised when co-administering Tasigna with substrates of
these enzymes that have a narrow therapeutic index [e.g. astemizole, terfenadine, cisapride, pimozide,
quinidine, bepridil or ergot alkaloids (ergotamine, dihydroergotamine)].
Anti-arrhythmic medicinal products and other substances that may prolong QT
Nilotinib should be used with caution in patients who have or may develop prolongation of QT,
including those patients taking anti-arrhythmic medicinal products such as amiodarone, disopyramide,
procainamide, quinidine and sotalol or other medicinal products that may lead to QT prolongation
such as chloroquine, halofantrine, clarithromycin, haloperidol, methadone and moxifloxacin (see
section 4.4).
Other interactions that may affect serum concentrations
The absorption of Tasigna is increased if it is taken with food, resulting in a higher serum
concentration (see sections 4.2, 4.4 and 5.2). Grapefruit juice and other foods that are known to inhibit
CYP3A4 should be avoided.
4.6 Fertility, pregnancy and lactation
Women of childbearing potential
Women of childbearing potential have to use effective contraception during treatment with Tasigna.
Pregnancy
There are no adequate data from the use of nilotinib in pregnant women. Studies in animals have
shown reproductive toxicity (see section 5.3). Tasigna should not be used during pregnancy unless the
clinical condition of the woman requires treatment with nilotinib. If it is used during pregnancy, the
patient must be informed of the potential risk to the foetus.
Breast-feeding
It is unknown whether nilotinib is excreted in human milk. Available toxicological data in animals
have shown excretion of nilotinib in milk (see section 5.3). A risk to the newborns/infants cannot be
excluded. Tasigna should not be used during breast-feeding.
Fertility
Animal studies did not show an effect on fertility in male and female rats (see section 5.3).
4.7 Effects on ability to drive and use machines
No studies on the effects of nilotinib on the ability to drive and use machines have been performed.
Patients experiencing dizziness, fatigue, visual impairment or other undesirable effects with a potential
impact on the ability to drive or use machines safely should refrain from these activities as long as the
undesirable effects persist (see section 4.8).
The data described below reflect exposure to Tasigna in 279 patients from a randomised Phase III
study in newly diagnosed patients with CML in chronic phase treated with 300 mg of nilotinib twice
daily. At data cut-off, 64% of patients had a duration of exposure of more than 12 months and 16.5%
of patients had a duration of exposure of more than 18 months. The median duration of exposure was
14 months.
The most frequent non-haematological adverse reactions were rash, pruritus, headache, nausea, fatigue
and myalgia. Most of these adverse reactions were mild to moderate in severity. Upper abdominal
pain, alopecia, constipation, diarrhoea, asthenia, dry skin, muscle spasms, arthralgia, vomiting,
abdominal pain and peripheral oedema were observed less commonly and have been of mild to
moderate severity. Discontinuation for adverse events regardless of causality was observed in 6.8% of
patients.
Treatment-emergent haematological toxicities include myelosuppression: thrombocytopenia (17%),
neutropenia (14%) and anaemia (6%). Pleural and pericardial effusions occurred in <1% of patients
receiving Tasigna. Gastrointestinal haemorrhage was reported in <1% of patients.
The change from baseline in mean time-averaged QTcF interval at steady state was 6 msec. No patient
had a QTcF >480 msec. QTcF increase from baseline exceeding 60 msec was observed <1% of
patients. No sudden deaths or episodes of torsade de pointes (transient or sustained) have been
observed. No decrease from baseline in mean left ventricular ejection fraction (LVEF) was observed at
any time during treatment. No patient had a LVEF of <45% during treatment nor an absolute reduction
in LVEF of more than 15%.
Non-haematological adverse reactions (excluding laboratory abnormalities) that are reported in at least
5% of the patients treated with 300 mg of nilotinib twice daily in the randomised Phase III study are
shown in Table 2. These are ranked under heading of frequency using the following convention: very
common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to
<1/1,000), very rare (<1/10,000); not known (cannot be estimated from the available data). Within
each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Non-haematological adverse reactions (≥5% of all patients)
Gastrointestinal disorders
Skin and subcutaneous tissue
disorders
Musculoskeletal and
connective tissue disorders
General disorders and
administration site conditions
The following adverse reactions were reported in the Tasigna Phase III study at a frequency of less
than 5%. For laboratory abnormalities, very common events (1/10) not included in Table 2 are also
reported. These adverse reactions are included based on clinical relevance and ranked in order of
decreasing seriousness within each category.
Infections and infestations:
Common: folliculitis
Uncommon: upper respiratory tract infection.
Not known: respiratory tract infection, herpes virus infection, oral candidiasis, subcutaneous abscess,
anal abscess, furuncle, nasopharyngitis, rhinitis, tinea pedis.
Neoplasms benign, malignant and unspecified (including cysts and polyps):
Common: skin papilloma.
Not known: papilloma.
Blood and lymphatic system disorders:
Common: lymphopenia.
Uncommon: pancytopenia.
Not known: febrile neutropenia.
Immune system disorders:
Not known: hypersensitivity.
Endocrine disorders
:
Not known: hyperparathyroidism secondary.

























Metabolism and nutrition disorders:
Common: hypokalaemia, diabetes mellitus, hypercholesterolaemia, hyperlipidaemia, hyperglycaemia,
hypophosphataemia, anorexia.
Uncommon: hyperkalaemia, hypocalcaemia, decreased appetite.
Not known: hyperuricaemia, gout, hypoglycaemia, dyslipidaemia, appetite disorder.
Psychiatric disorders:
Common: insomnia.
Uncommon: anxiety.
Not known: depressed mood, amnesia, dysphoria.
Nervous system disorders:
Common: dizziness, hypoaesthesia.
Uncommon: paraesthesia.
Not known: syncope, migraine, tremor, peripheral neuropathy, lethargy, dysaesthesia.
Eye disorders:
Common: eye pruritus, conjunctivitis, dry eye.
Uncommon: eyelid oedema, photopsia.
Not known: periorbital oedema, eye irritation, blepharitis, eye pain, chorioretinopathy, conjunctival
haemorrhage, conjunctivitis allergic, conjunctival hyperaemia, ocular hyperaemia, ocular surface
disease, scleral hyperaemia.
Ear and labyrinth disorders:
Common: vertigo.
Cardiac disorders:
Common: electrocardiogram QT prolonged, palpitations.
Uncommon: cyanosis.
Not known: arrhythmia, ejection fraction decrease, pericardial effusion, sinus bradycardia.
Vascular disorders:
Common: hypertension, flushing.
Not known: haematoma.
Respiratory, thoracic and mediastinal disorders:
Common: dyspnoea, cough.
Not known: pleural effusion, dyspnoea exertional, pleurisy, epistaxis.
Gastrointestinal disorders:
Common: abdominal distension, abdominal discomfort, dyspepsia, flatulence.
Uncommon: pancreatitis, oesophageal pain, dysgeusia.
Not known: oesophageal ulcer, gastric ulcer, stomatitis, dry mouth, gastritis, haemorrhoids, hiatus
hernia, rectal haemorrhage, sensitivity of teeth, gingivitis.
Hepatobiliary disorders:
Common: hepatic function abnormal.
Uncommon: hepatitis, jaundice.
Not known: hepatotoxicity.
Skin and subcutaneous tissue disorders:
Common: erythema, hyperhidrosis, contusion, acne, dermatitis, night sweats.
Uncommon: drug eruption, skin pain.
Not known: eczema, urticaria, blister, dermal cyst, sebaceous hyperplasia, swelling face, skin atrophy,
skin hypertrophy, skin exfoliation, skin hyperpigmentation, skin discolouration.
Musculoskeletal and connective tissue disorders:
Common: bone pain, pain in extremity, back pain.
Uncommon: muscular pain, pain.
Not known: muscular weakness, flank pain.
Renal and urinary disorders:
Not known: dysuria, pollakiuria, chromaturia.
Reproductive system and breast disorders:
Not known: gynaecomastia, breast induration, menorrhagia, nipple swelling.
General disorders and administration site conditions:
Common: pyrexia, chest pain, chest discomfort.
Uncommon: chills.
Not known: face oedema, malaise, feeling hot, localised oedema.
Investigations:
Common: platelet count decreased, blood amylase increased, blood alkaline phosphatase increased,
weight increased.
Uncommon: haemoglobin decreased, neutrophil count decreased, blood phosphorus decreased,
gamma-glutamyltransferase increased.
Not known: blood insulin increased, very low density lipoprotein increased, blood parathyroid
hormone increased, blood potassium increased, blood pressure increased, white blood cell count
decreased, weight decreased.
Clinically relevant or severe abnormalities of routine haematological or biochemistry laboratory
values are presented in Table 3.
Grade 3-4 laboratory abnormalities
Haematological parameters
- Elevated bilirubin (total)
Isolated reports of intentional overdose with nilotinib were reported, where an unspecified number of
Tasigna capsules were ingested in combination with alcohol and other medicinal products. Events
included neutropenia, vomiting and drowsiness. No ECG changes or hepatotoxicity were reported.
Outcomes were reported as recovered.
In the event of overdose, the patient should be observed and appropriate supportive treatment given.
















PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Protein kinase inhibitors, ATC code: L01XE08
Nilotinib is a potent inhibitor of the Abl tyrosine kinase activity of the Bcr-Abl oncoprotein both in
cell lines and in primary Philadelphia-chromosome positive leukaemia cells. The substance binds with
high affinity to the ATP-binding site in such a manner that it is a potent inhibitor of wild-type Bcr-Abl
and maintains activity against 32/33 imatinib-resistant mutant forms of Bcr-Abl. As a consequence of
this biochemical activity, nilotinib selectively inhibits the proliferation and induces apoptosis in cell
lines and in primary Philadelphia-chromosome positive leukaemia cells from CML patients. In murine
models of CML, as a single agent nilotinib reduces tumour burden and prolongs survival following
oral administration.
Nilotinib has little or no effect against the majority of other protein kinases examined, including Src,
except for the PDGF, Kit and Ephrin receptor kinases, which it inhibits at concentrations within the
range achieved following oral administration at therapeutic doses recommended for the treatment of
CML (see Table 4).
Kinase profile of nilotinib (phosphorylation IC
50
nM)
Clinical studies in newly diagnosed CML in chronic phase
An open-label, multicentre, randomised Phase III study was conducted to determine the efficacy of
Nilotinib versus imatinib in 846 adult patients with cytogenetically confirmed newly diagnosed
Philadelphia chromosome positive CML in the chronic phase. Patients were within six months of
diagnosis and were previously untreated, with the exception of hydroxyurea and/or anagrelide.
Patients were randomised 1:1:1 to receive either nilotinib 300 mg twice daily (n=282), nilotinib
400 mg twice daily (n=281) or imatinib 400 mg once daily (n=283). Randomisation was stratified by
Sokal risk score at the time of diagnosis.
Baseline characteristics were well balanced between the three treatment arms. Median age was
47 years in both nilotinib arms and 46 years in the imatinib arm, with 12.8%, 10.0% and 12.4% of
patients were ≥65 years of age in the nilotinib 300 mg twice daily, nilotinib 400 mg twice daily and
imatinib 400 mg once daily treatment arms, respectively. There were slightly more male than female
patients (56.0%, 62.3% and 55.8%, in the nilotinib 300 mg twice daily, 400 mg twice daily and
imatinib 400 mg once daily arm, respectively). More than 60% of all patients were Caucasian and 25%
of all patients were Asian.
The time point for the primary data analysis was when all 846 patients completed 12 months of
treatment (or discontinued earlier). The median time on treatment was 14 months. More than 60% of
all patients had received treatment for longer than 12 months. The median actual dose intensity was
592 mg/day for nilotinib 300 mg twice daily, 779 mg/day for nilotinib 400 mg twice daily and
400 mg/day for imatinib 400 mg once daily.
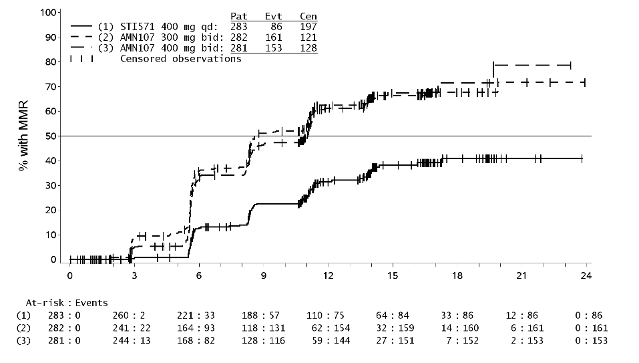
The primary efficacy endpoint was major molecular response (MMR) at 12 months. MMR was
defined as ≤0.1% Bcr-Abl/Abl % by international scale measured by RQ-PCR, which corresponds to a
≥3 log reduction of Bcr-Abl transcript from standardised baseline. The MMR rate at 12 months was
statistically significantly higher for nilotinib 300 mg twice daily compared to imatinib 400 mg once
daily (44.3% versus 22.3%, p<0.0001). The rate of MMR at 12 months, was also statistically
significantly higher for nilotinib 400 mg twice daily compared to imatinib 400 mg once daily (42.7%
versus 22.3%, p<0.0001).







The rates of MMR at 3, 6, 9 and 12 months were 8.9%, 33.0%, 43.3% and 44.3% for nilotinib 300 mg
twice daily, 5.0%, 29.5%, 38.1% and 42.7% for nilotinib 400 mg twice daily and 0.7%, 12.0%, 18.0%
and 22.3% for imatinib 400 mg once daily.
The Kaplan-Meier analysis of time to first MMR is shown in Figure 1. The probability of achieving
MMR at different time points was higher for both nilotinib at 300 mg and 400 mg twice daily
compared to imatinib 400 mg once daily (HR=2.6 and stratified log-rank p<0.0001 between nilotinib
300 mg twice daily and imatinib 400 mg once daily, HR=1.6 and stratified log-rank p<0.0001 between
nilotinib 400 mg twice daily and imatinib 400 mg once daily). The proportion of patients who
achieved a Bcr-Abl ratio of ≤0.01% (4 log reduction) and ≤0.0032% (4.5 log reduction) at 12 months
were statistically significantly higher for both nilotinib 300 mg twice daily (11.7% and 4.3%,
respectively) and nilotinib 400 mg twice daily (8.5% and 4.6%, respectively) compared to 400 mg
imatinib once daily (3.9% and 0.4% respectively). For all Sokal risk groups, the response rates were
higher for both nilotinib at 300 mg and 400 mg twice daily than for imatinib 400 mg once daily.
Major molecular response (MMR) rate at 12 months
Tasigna (nilotinib)
AMN107
300 mg twice daily
n=282
(%)
Tasigna (nilotinib)
AMN107
400 mg twice daily
n=281
(%)
Glivec (imatinib)
STI571
400 mg once daily
n=283
(%)
CMH* test p-value for response
rate (vs. imatinib 400 mg once
daily)
*CMH = Cochran-Mantel-Haenszel
Figure 1 Kaplan-Meier estimate of time to first major molecular response (MMR)
Time since randomisation (months)
Complete cytogenetic response (CCyR) was defined as 0% Ph+ metaphases in the bone marrow based
on a minimum of 20 metaphases evaluated. Best CCyR rate by 12 months (including patients who













achieved CCyR at or before the 12 month time point as responders) was statistically higher for both
nilotinib 300 mg and 400 mg twice daily compared to imatinib 400 mg once daily.
Best complete cytogenetic response (CCyR) rate by 12 months
Tasigna (nilotinib)
300 mg twice daily
n=282
(%)
Tasigna (nilotinib)
400 mg twice daily
n=281
(%)
Glivec (imatinib)
400 mg once daily
n=283
(%)
CMH test p-value for response
rate (vs. imatinib 400 mg once
daily)
Progression to accelerated phase or blast crisis on treatment was observed in a total of 14 patients:
2 patients on nilotinib 300 mg twice daily, 1 patient on nilotinib 400 mg twice daily and 11 patients on
imatinib 400 mg once daily. None of the patients who progressed had achieved MMR, while 3 of these
patients receiving imatinib 400 mg once daily achieved CCyR. There was a statistically significant
difference in progression to accelerated phase or blast crisis between nilotinib 300 mg twice daily and
imatinib 400 mg once daily (p=0.0095) and between nilotinib 400 mg twice daily and imatinib 400 mg
once daily (p=0.0037) in favour of nilotinib.
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with
Tasigna in paediatric patients from birth to less then 18 years in the treatment of Philadelphia
chromosome positive chronic myeloid leukaemia (see 4.2 for information on paediatric use).
5.2 Pharmacokinetic properties
Absorption
Peak concentrations of nilotinib are reached 3 hours after oral administration. Nilotinib absorption
following oral administration was approximately 30%. In healthy volunteers, C
max
and area under the
serum concentration-time curve (AUC) of nilotinib are increased by 112% and 82%, respectively,
compared to fasting conditions when Tasigna is given with food. Administration of Tasigna
30 minutes or 2 hours after food increased bioavailability of nilotinib by 29% or 15%, respectively
(see sections 4.2, 4.4 and 4.5).
Single-dose administration of 400 mg nilotinib, using 2 capsules of 200 mg whereby the content of
each capsule was dispersed in one teaspoon of apple sauce, was shown to be bioequivalent with a
single-dose administration of 2 intact capsules of 200 mg.
Nilotinib absorption (relative bioavailability) might be reduced by approximately 48% and 22% in
patients with total gastrectomy and partial gastrectomy, respectively.
Distribution
The blood-to-plasma ratio of nilotinib is 0.71. Plasma protein binding is approximately 98% on the
basis of
in vitro
experiments.
Biotransformation
Main metabolic pathways identified in healthy subjects are oxidation and hydroxylation. Nilotinib is
the main circulating component in the serum. None of the metabolites contribute significantly to the
pharmacological activity of nilotinib. Nilotinib is primarily metabolised by CYP3A4, with possible
minor contribution from CYP2C8.









Elimination
After a single dose of radiolabelled nilotinib in healthy subjects, more than 90% of the dose was
eliminated within 7 days, mainly in faeces (94% of the dose). Parent drug accounted for 69% of the
dose.
Linearity / non-linearity
Steady-state nilotinib exposure was dose-dependent, with less than dose-proportional increases in
systemic exposure at dose levels higher than 400 mg given as once-daily dosing. Daily serum
exposure to nilotinib with 400 mg twice-daily dosing at steady state was 35% higher than with 800 mg
once-daily dosing. Systemic exposure (AUC) of nilotinib at steady state at a dose level of 400 mg
twice daily was approximately 13.4% higher than at a dose level of 300 mg twice daily. The average
nilotinib trough and peak concentrations over 12 months were approximately 15.7% and 14.8% higher
following 400 mg twice daily dosing compared to 300 mg twice daily. There was no relevant increase
in exposure to nilotinib when the dose was increased from 400 mg twice daily to 600 mg twice daily.
Characteristics in patients
Steady-state conditions were essentially achieved by day 8. An increase in serum exposure to nilotinib
between the first dose and steady state was approximately 2-fold for daily dosing and 3.8-fold for
twice-daily dosing. The apparent elimination half-life estimated from the multiple-dose
pharmacokinetics with daily dosing was approximately 17 hours. Inter-patient variability in nilotinib
pharmacokinetics was moderate to high.
5.3 Preclinical safety data
Nilotinib has been evaluated in safety pharmacology, repeated dose toxicity, genotoxicity,
reproductive toxicity and phototoxicity studies.
Nilotinib did not have effects on CNS or respiratory functions.
In vitro
cardiac safety studies
demonstrated a preclinical signal for QT prolongation, based upon block of hERG currents and
prolongation of the action potential duration in isolated rabbit hearts by nilotinib. No effects were seen
in ECG measurements in dogs or monkeys treated for up to 39 weeks or in a special telemetry study in
dogs.
Repeated-dose toxicity studies in dogs of up to 4 weeks’ duration and in cynomolgus monkeys of up
to 9 months’ duration revealed the liver as the primary target organ of toxicity of nilotinib. Alterations
included increased alanine aminotransferase and alkaline phosphatase activity and histopathology
findings (mainly sinusoidal cell or Kupffer cell hyperplasia/hypertrophy, bile duct hyperplasia and
periportal fibrosis). In general the changes in clinical chemistry were fully reversible after a four-week
recovery period and the histological alterations showed partial reversibility. Exposures at the lowest
dose levels at which the liver effects were seen were lower than the exposure in humans at a dose of
800 mg/day. Only minor liver alterations were seen in mice or rats treated for up to 26 weeks. Mainly
reversible increases in cholesterol levels were seen in rats, dogs and monkeys.
Genotoxicity studies in bacterial
in vitro
systems and in mammalian
in vitro
and
in vivo
systems with
and without metabolic activation did not reveal any evidence for a mutagenic potential of nilotinib.
Nilotinib did not induce teratogenicity, but did show embryo- and foetotoxicity at doses that also
showed maternal toxicity. Increased post-implantation loss was observed in both the fertility study,
which involved treatment of both males and females, and the embryotoxicity study, which involved
treatment of females. Embryo-lethality and foetal effects (mainly decreased foetal weights, premature
fusion of the facial bones (fused maxilla/zygomatic) visceral and skeletal variations) in rats and
increased resorption of foetuses and skeletal variations in rabbits were present in the embryotoxicity
studies. In a pre- and postnatal development study in rats, maternal exposure to nilotinib caused
reduced pup body weight with associated changes in physical development parameters as well as
reduced mating and fertility indices in the offspring. Exposure to nilotinib in females at No-Observed-
Adverse-Effect-Levels was generally less or equal to that in humans at 800 mg/day.
In a juvenile development study, nilotinib was administered via oral gavage to juvenile rats from the
first week post partum through young adult (day 70 post partum) at doses of 2, 6 and 20 mg/kg/day.
Besides standard study parameters, evaluations of developmental landmarks, CNS effects, mating and
fertility were performed. Based on a reduction in body weight in both genders and a delayed preputial
separation in males (which may be associated with the reduction in weight), the No-Observed-Effect-
Level in juvenile rats was considered to be 6 mg/kg/day. The juvenile animals did not exert increased
sensitivity to nilotinib relative to adults. In addition, the toxicity profile in juvenile rats was
comparable to that observed in adult rats.
No effects on sperm count/motility or on fertility were noted in male and female rats up to the highest
tested dose, approximately 5 times the recommended dosage for humans.
Nilotinib was shown to absorb light in the UV-B and UV-A range, is distributed into the skin and
showed a phototoxic potential
in vitro
, but no effects have been observed
in vivo
. Therefore the risk
that nilotinib causes photosensitisation in patients is considered very low.
Carcinogenicity studies with nilotinib have not been performed.
PHARMACEUTICAL PARTICULARS
Capsule content
Lactose monohydrate
Crospovidone
Poloxamer 188
Silica, colloidal anhydrous
Magnesium stearate
Capsule shell
Gelatin
Titanium dioxide (E171)
Red iron oxide (E172)
Yellow iron oxide (E172)
Printing ink
Shellac
Black iron oxide (E172)
6.4 Special precautions for storage
Store in the original package in order to protect from moisture.
6.5 Nature and contents of container
Tasigna is available in weekly and monthly packs:
The weekly pack contains 28 capsules (7 daily blisters, each containing 4 capsules).
The monthly pack contains 112 capsules (4 individual weekly packs, each containing
28 capsules).
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency
http://www.ema.europa.eu
NAME OF THE MEDICINAL PRODUCT
Tasigna 200 mg hard capsules
QUALITATIVE AND QUANTITATIVE COMPOSITION
One hard capsule contains 200 mg nilotinib (as hydrochloride monohydrate).
Excipient
Lactose monohydrate: 156.11 mg per capsule.
For a full list of excipients, see section 6.1.
White to yellowish powder in light yellow opaque hard gelatin capsules, size 0 with red axial imprint
“NVR/TKI”.
4.1 Therapeutic indications
Tasigna is indicated for the treatment of adult patientswith:
-
newly diagnosed Philadelphia chromosome positive chronic myelogenous leukaemia (CML) in
the chronic phase,
chronic phase and accelerated phase Philadelphia chromosome positive CML with resistance or
intolerance to prior therapy including imatinib. Efficacy data in patients with CML in blast
crisis are not available.
4.2 Posology and method of administration
Therapy should be initiated by a physician experienced in the diagnosis and the treatment of patients
with CML.
Posology
The recommended dose of Tasigna is:
-
300 mg twice daily in newly diagnosed patients with CML in the chronic phase,
-
400 mg twice daily in patients with chronic or accelerated phase CML with resistance or
intolerance to prior therapy.
Treatment should be continued as long as the patient continues to benefit.
For a dose of 300 mg twice daily, 150 mg capsules are available.
Dose adjustments or modifications
Tasigna may need to be temporarily withheld and/or dose reduced for haematological toxicities
(neutropenia, thrombocytopenia) that are not related to the underlying leukaemia (see Table 1).
Dose adjustments for neutropenia and thrombocytopenia
Newly diagnosed
chronic phase CML
at 300 mg twice
daily
and
imatinib-resistant or
intolerant CML in
chronic phase at
400 mg twice daily
ANC* <1.0 x 10
9
/l and/or platelet
counts <50 x 10
9
/l
1.
Treatment with Tasigna must be interrupted
and blood count monitored.
2.
Treatment must be resumed within 2 weeks
at prior dose if ANC >1.0 x 10
9
/l and/or
platelets >50 x 10
9
/l.
3.
If blood counts remain low, a dose reduction
to 400 mg once daily may be required.
Imatinib-resistant or
intolerant CML in
accelerated phase at
400 mg twice daily
ANC* <0.5 x 10
9
/l and/or platelet
counts <10 x 10
9
/l
1.
Treatment with Tasigna must be interrupted
and blood count monitored.
2.
Treatment must be resumed within 2 weeks at
prior dose if ANC >1.0 x 10
9
/l and/or
platelets >20 x 10
9
/l.
3.
If blood counts remain low, a dose reduction
to 400 mg once daily may be required.
*ANC = absolute neutrophil count
If clinically significant moderate or severe non-haematological toxicity develops, dosing should be
interrupted, and may be resumed at 400 mg once daily once the toxicity has resolved. If clinically
appropriate, re-escalation of the dose to the starting dose of 300 mg twice daily in newly diagnosed
patients with CML in the chronic phase or to 400 mg twice daily in patients with imatinib-resistant or
intolerant CML in chronic phase and accelerated phase should be considered.
Elevated serum lipase: For Grade 3-4 serum lipase elevations, doses should be reduced to 400 mg once
daily or interrupted. Serum lipase levels should be tested monthly or as clinically indicated (see
section 4.4).
Elevated bilirubin and hepatic transaminases: For Grade 3-4 bilirubin and hepatic transaminase
elevations, doses should be reduced to 400 mg once daily or interrupted. Bilirubin and hepatic
transaminases levels should be tested monthly or as clinically indicated.
Paediatric population
The safety and efficacy of Tasigna in paediatric patients from birth to less than 18 years have not yet
been established (see section 5.1). Therefore its use in paediatric patients is not recommended due to a
lack of data on safety and efficacy.
Elderly patients
Approximately 12% of subjects in the Phase III study in patients with newly diagnosed CML in
chronic phase and approximately 30% of subjects in the Phase II study in patients with imatinib-
resistant or intolerant CML in chronic phase and accelerated phase were 65 years of age or over. No
major differences were observed for safety and efficacy in patients ≥65 years of age as compared to
adults aged 18 to 65 years.
Patients with renal impairment
Clinical studies have not been performed in patients with impaired renal function.
Since nilotinib and its metabolites are not renally excreted, a decrease in total body clearance is not
anticipated in patients with renal impairment.






Patients with hepatic impairment
Hepatic impairment has a modest effect on the pharmacokinetics of nilotinib. Dose adjustment is not
considered necessary in patients with hepatic impairment. However, patients with hepatic impairment
should be treated with caution (see section 4.4).
Cardiac disorders
In clinical studies, patients with uncontrolled or significant cardiac disease (e.g. recent myocardial
infarction, congestive heart failure, unstable angina or clinically significant bradycardia) were
excluded. Caution should be exercised in patients with relevant cardiac disorders (see section 4.4).
Method of administration
Tasigna should be taken twice daily approximately 12 hours apart and must not be taken with food.
The capsules should be swallowed whole with water. No food should be consumed for 2 hours before
the dose is taken and no food should be consumed for at least one hour after the dose is taken.
If a dose is missed the patient should not take an additional dose, but take the usual prescribed next
dose.
For patients who are unable to swallow capsules, the content of each capsule may be dispersed in one
teaspoon of apple sauce (puréed apple) and should be taken immediately. Not more than one teaspoon
of apple sauce and no food other than apple sauce must be used (see sections 4.4 and 5.2).
Tasigna may be given in combination with haematopoietic growth factors such as erythropoietin or
granulocyte colony-stimulating factor (G-CSF) if clinically indicated. It may be given with
hydroxyurea or anagrelide if clinically indicated.
Hypersensitivity to the active substance or to any of the excipients.
4.4 Special warnings and precautions for use
Myelosuppression
Treatment with Tasigna is associated with (National Cancer Institute Common Toxicity Criteria
grade 3-4) thrombocytopenia, neutropenia and anaemia.
Occurrence is more frequent in patients with
imatinib-resistant or intolerant CML, in particular in patients with accelerated-phase CML. Complete
blood counts should be performed every two weeks for the first 2 months and then monthly thereafter,
or as clinically indicated. Myelosuppression was generally reversible and usually managed by
withholding Tasigna temporarily or dose reduction (see section 4.2).
QT prolongation
Tasigna has been shown to prolong cardiac ventricular repolarisation as measured by the QT interval
on the surface ECG in a concentration-dependent manner.
In the Phase III study in patients with newly diagnosed CML in chronic phase receiving 300 mg
nilotinib twice daily, the change from baseline in mean time-averaged QTcF interval at steady state
was 6 msec. No patient had a QTcF >480 msec. No episodes of torsade de pointes were observed.
In the Phase II study in imatinib-resistant and intolerant CML patients in chronic and accelerated
phase receiving 400 mg nilotinib twice daily, the change from baseline in mean time-averaged QTcF
interval at steady state was 5 and 8 msec, respectively. QTcF of >500 msec was observed in <1% of
these patients. No episodes of torsade de pointes were observed in clinical studies.
In a healthy volunteer study with exposures that were comparable to the exposures observed in
patients, the time-averaged mean placebo-subtracted QTcF change from baseline was 7 msec
(CI ± 4 msec). No subject had a QTcF >450 msec. Additionally, no clinically relevant arrhythmias
were observed during the conduct of the trial. In particular, no episodes of torsade de pointes (transient
or sustained) were observed.
Significant prolongation of the QT interval may occur when nilotinib is inappropriately taken with
strong CYP3A4 inhibitors and/or medicinal products with a known potential to prolong QT, and/or
food (see section 4.5). The presence of hypokalaemia and hypomagnesaemia may further enhance this
effect. Prolongation of the QT interval may expose patients to the risk of fatal outcome.
Tasigna should be used with caution in patients who have or who are at significant risk of developing
prolongation of QTc, such as those:
-
with congenital long QT prolongation
-
with uncontrolled or significant cardiac disease including recent myocardial infarction,
congestive heart failure, unstable angina or clinically significant bradycardia.
-
taking anti-arrhythmic medicinal products or other substances that lead to QT prolongation.
Close monitoring for an effect on the QTc interval is advisable and a baseline ECG is recommended
prior to initiating therapy with Tasigna and as clinically indicated. Hypokalaemia or
hypomagnesaemia must be corrected prior to Tasigna administration and should be monitored
periodically during therapy.
Sudden death
Uncommon cases (0.1 to 1%) of sudden deaths have been reported in patients with imatinib-resistant
or intolerant CML in chronic phase or accelerated phase with a past medical history of cardiac disease
or significant cardiac risk factors. Co-morbidities in addition to the underlying malignancy were also
frequently present as were concomitant medicinal products. Ventricular repolarisation abnormalities
may have been contributory factors. No cases of sudden death were reported in the Phase III study in
newly diagnosed patients with CML in chronic phase.
Interactions with other medicinal products
The administration of Tasigna with agents that are strong CYP3A4 inhibitors (including, but not
limited to, ketoconazole, itraconazole, voriconazole, clarithromycin, telithromycin, ritonavir) should
be avoided. Should treatment with any of these agents be required, it is recommended that therapy
with Tasigna be interrupted if possible (see section 4.5). If transient interruption of treatment is not
possible, close monitoring of the individual for prolongation of the QT interval is indicated (see
sections 4.2, 4.5 and 5.2).
Concomitant use of Tasigna with medicinal products that are potent inducers of CYP3A4 (e.g.
phenytoin, rifampicin, carbamazepine, phenobarbital and St. John’s Wort) is likely to reduce exposure
to nilotinib to a clinically relevant extent. Therefore, in patients receiving Tasigna, co-administration
of alternative therapeutic agents with less potential for CYP3A4 induction should be selected (see
section 4.5).
Food effect
The bioavailability of nilotinib is increased by food. Tasigna should not be taken in conjunction with
food (see sections 4.2 and 4.5) and should be taken 2 hours after a meal. No food should be consumed
for at least one hour after the dose is taken. Grapefruit juice and other foods that are known to inhibit
CYP3A4 should be avoided. For patients who are unable to swallow capsules, the content of each
capsule may be dispersed in one teaspoon of apple sauce and should be taken immediately. Not more
than one teaspoon of apple sauce and no food other than apple sauce must be used (see section 5.2).
Hepatic impairment
Hepatic impairment has a modest effect on the pharmacokinetics of nilotinib. Single dose
administration of 200 mg of nilotinib resulted in increases in AUC of 35%, 35% and 19% in subjects
with mild, moderate and severe hepatic impairment, respectively, compared to a control group of
subjects with normal hepatic function. The predicted steady-state C
max
of nilotinib showed an increase
of 29%, 18% and 22%, respectively. Clinical studies have excluded patients with alanine transaminase
(ALT) and/or aspartate transaminase (AST) >2.5 (or >5, if related to disease) times the upper limit of
the normal range and/or total bilirubin >1.5 times the upper limit of the normal range. Metabolism of
nilotinib is mainly hepatic. Patients with hepatic impairment might therefore have increased exposure
to nilotinib and should be treated with caution (see section 4.2).
Serum lipase
Elevation in serum lipase has been observed. Caution is recommended in patients with previous
history of pancreatitis. In case lipase elevations are accompanied by abdominal symptoms, Tasigna
should be interrupted and appropriate diagnostic measures considered to exclude pancreatitis.
Total gastrectomy
The bioavailability of nilotinib might be reduced in patients with total gastrectomy (see section 5.2).
More frequent follow-up of these patients should be considered.
Lactose
Tasigna capsules contain lactose. Patients with rare hereditary problems of galactose intolerance, the
Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicinal product.
4.5 Interaction with other medicinal products and other forms of interaction
Substances that may increase nilotinib serum concentrations
Nilotinib is mainly metabolised in the liver and is also a substrate for the multi-drug efflux pump, P-
glycoprotein (P-gp). Therefore, absorption and subsequent elimination of systemically absorbed
nilotinib may be influenced by substances that affect CYP3A4 and/or P-gp. Concomitant
administration of nilotinib with imatinib (a substrate and moderator of P-gp and CYP3A4), had a
slight inhibitory effect on CYP3A4 and/or P-gp. The AUC of imatinib was increased by 18% to 39%,
and the AUC of nilotinib was increased by 18% to 40%. These changes are unlikely to be clinically
important.
The exposure to nilotinib in healthy subjects was increased 3-fold when co-administered with the
strong CYP3A4 inhibitor ketoconazole. Concomitant treatment with strong CYP3A4 inhibitors,
including ketoconazole, itraconazole, voriconazole, ritonavir, clarithromycin, and telithromycin,
should therefore be avoided (see sections 4.2 and 4.4). Increased exposure to nilotinib might also be
expected with moderate CYP3A4 inhibitors. Alternative concomitant medicinal products with no or
minimal CYP3A4 inhibition should be considered.
Substances that may decrease nilotinib serum concentrations
Rifampicin, a potent CYP3A4 inducer, decreases nilotinib C
max
by 64% and reduces nilotinib AUC by
80%. Rifampicin and nilotinib should not be used concomitantly.
The concomitant administration of other medicinal products that induce CYP3A4 (e.g. phenytoin,
carbamazepine, phenobarbital and St. John’s Wort) is likewise likely to reduce exposure to nilotinib to
a clinically relevant extent. In patients for whom CYP3A4 inducers are indicated, alternative agents
with less enzyme induction potential should be selected.
Nilotinib has pH dependent solubility, with lower solubility at higher pH. In healthy subjects receiving
esomeprazole at 40 mg once daily for 5 days, gastric pH was markedly increased, but nilotinib
absorption was only decreased modestly (27% decrease in C
max
and 34% decrease in AUC0-∞).
Nilotinib may be used concurrently with esomeprazole or other proton pump inhibitors as needed.
Substances that may have their systemic concentration altered by nilotinib
Nilotinib is a relatively strong inhibitor of CYP3A4, CYP2C8, CYP2C9, CYP2D6 and UGT1A1
in
vitro
, with Ki value being lowest for CYP2C9 (Ki=0.13 microM).
A single-dose drug-drug interaction study in healthy volunteers with 25 mg warfarin, a sensitive
CYP2C9 substrate, and 800 mg nilotinib did not result in any changes in warfarin pharmacokinetic
parameters or warfarin pharmacodynamics measured as prothrombin time (PT) and international
normalised ratio (INR). There are no steady-state data. This study suggests that a clinically meaningful
drug-drug interaction between nilotinib and warfarin is less likely up to a dose of 25 mg of warfarin.
Due to lack of steady-state data, control of warfarin pharmacodynamic markers (INR or PT) following
initiation of nilotinib therapy (at least during the first 2 weeks) is recommended.
In addition, single-dose administration of Tasigna with orally administered midazolam to healthy
subjects increased midazolam exposure by 30%. It cannot be excluded that the effect of nilotinib is
greater at steady state. Caution should be exercised when co-administering Tasigna with substrates of
these enzymes that have a narrow therapeutic index [e.g. astemizole, terfenadine, cisapride, pimozide,
quinidine, bepridil or ergot alkaloids (ergotamine, dihydroergotamine)].
Anti-arrhythmic medicinal products and other substances that may prolong QT
Nilotinib should be used with caution in patients who have or may develop prolongation of QT,
including those patients taking anti-arrhythmic medicinal products such as amiodarone, disopyramide,
procainamide, quinidine and sotalol or other medicinal products that may lead to QT prolongation
such as chloroquine, halofantrine, clarithromycin, haloperidol, methadone and moxifloxacin (see
section 4.4).
Other interactions that may affect serum concentrations
The absorption of Tasigna is increased if it is taken with food, resulting in higher serum concentration
(see sections 4.2, 4.4 and 5.2). Grapefruit juice and other foods that are known to inhibit CYP3A4
should be avoided.
4.6 Fertility, pregnancy and lactation
Women of childbearing potential
Women of childbearing potential have to use effective contraception during treatment with Tasigna.
Pregnancy
There are no adequate data from the use of nilotinib in pregnant women. Studies in animals have
shown reproductive toxicity (see section 5.3). Tasigna should not be used during pregnancy unless the
clinical condition of the woman requires treatment with nilotinib. If it is used during pregnancy, the
patient must be informed of the potential risk to the foetus.
Breast-feeding
It is unknown whether nilotinib is excreted in human milk. Available toxicological data in animals
have shown excretion of nilotinib in milk (see section 5.3). A risk to the newborns/infants cannot be
excluded. Tasigna should not be used during breast-feeding.
Fertility
Animal studies did not show an effect on fertility in male and female rats (see section 5.3).
4.7 Effects on ability to drive and use machines
No studies on the effects of nilotinib on the ability to drive and use machines have been performed.
Patients experiencing dizziness, fatigue, visual impairment or other undesirable effects with a potential
impact on the ability to drive or use machines safely should refrain from these activities as long as the
undesirable effects persist (see section 4.8).
The data described below reflect exposure to Tasigna in a total of 717 patients from a randomised
Phase III study in newly diagnosed patients with CML in chronic phase treated at the recommended
dose of 300 mg twice daily (n=279) and from an open-label multicentre Phase II study in patients with
imatinib-resistant or intolerant CML in chronic phase (n=318) and accelerated phase (n=120) treated
at the recommended dose of 400 mg twice daily.
Newly diagnosed CML in chronic phase
At data cut-off in the Phase III study, 64% of the patients had a duration of exposure of more than
12 months and 16.5% of patients had a duration of exposure of more than 18 months; the median
duration of exposure was 14 months.
The most frequent non-haematological adverse reactions were rash, pruritus, headache, nausea, fatigue
and myalgia. Most of these adverse reactions were mild to moderate in severity. Upper abdominal
pain, alopecia, constipation, diarrhoea, asthenia, dry skin, muscle spasms, arthralgia, vomiting,
abdominal pain and peripheral oedema were observed less commonly and have been of mild to
moderate severity. Discontinuation for adverse events regardless of causality was observed in 6.8% of
patients.
Treatment-emergent haematological toxicities include myelosuppression: thrombocytopenia (17%),
neutropenia (14%) and anaemia (6%). Pleural and pericardial effusions occurred in <1% of patients
receiving Tasigna. Gastrointestinal haemorrhage was reported in <1% of patients.
The change from baseline in mean time-averaged QTcF interval at steady state was 6 msec. No patient
had a QTcF >480 msec. QTcF increase from baseline exceeding 60 msec was observed <1% of
patients. No sudden deaths or episodes of torsade de pointes (transient or sustained) have been
observed. No decrease from baseline in mean left ventricular ejection fraction (LVEF) was observed at
any time during treatment. No patient had a LVEF of <45% during treatment nor an absolute reduction
in LVEF of more than 15%.
Imatinib-resistant or intolerant CML in chronic phase and accelerated phase
The data described below reflect exposure to Tasigna in 458 patients in an open-label multicentre
Phase II study in patients with imatinib-resistant or intolerant CML in chronic phase (n=321) and
accelerated phase (n=137) treated at the recommended dose of 400 mg twice daily. The median
duration of exposure in days was 561 (1-1,096) for the CML-CP patients and 264 (2-1,160) for the
CML-AP patients.
The most frequent non-haematological drug-related adverse events were rash, pruritus, nausea, fatigue,
headache, abdominal pain, constipation and diarrhoea. Most of these adverse events were mild to
moderate in severity. Vomiting, myalgia, alopecia, muscle spasms, anorexia, arthralgia, bone pain,
peripheral oedema and asthenia were observed commonly and have been of mild to moderate severity
(Grade 1 or 2). Discontinuation for drug-related adverse reactions was observed in 16% of CP and
10% of AP patients.
Treatment-emergent haematological toxicities include myelosuppression: thrombocytopenia (31%),
neutropenia (17%) and anaemia (14%). Pleural and pericardial effusions as well as complications of
fluid retention occurred in <1% of patients receiving Tasigna. Cardiac failure was observed in <1% of
patients. Gastrointestinal and CNS haemorrhage were reported in 1% and <1% of patients,
respectively.
QTcF exceeding 500 msec was observed in <1% of patients. No episodes of torsade de pointes
(transient or sustained) were observed.
Most frequently reported adverse reactions in Tasigna clinical studies
Non-haematological adverse reactions (excluding laboratory abnormalities) that are reported in at least
5% of the patients in Tasigna clinical studies are shown in Table 2. These are ranked under heading of
frequency using the following convention: very common (≥1/10), common (≥1/100 to <1/10),
uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to <1/1,000), very rare (<1/10,000); not known
[cannot be estimated from the available data]. Within each frequency grouping, undesirable effects are
presented in order of decreasing seriousness.
The following adverse reactions were reported in patients in the Tasigna clinical studies at a frequency
of less than 5%. For laboratory abnormalities, very common events (1/10) not included in Table 2 are
also reported. These adverse reactions are included based on clinical relevance and ranked in order of
decreasing seriousness within each category.
Infections and infestations:
Common: folliculitis.
Uncommon: pneumonia, urinary tract infection, gastroenteritis, upper respiratory tract infection,
bronchitis, herpes virus infection, candidiasis.
Not known: sepsis, respiratory tract infection, subcutaneous abscess, anal abscess, furuncle,
nasopharyngitis, rhinitis, tinea pedis.
Neoplasms benign, malignant and unspecified (including cysts and polyps):
Common: skin papilloma.
Not known: papilloma.
Blood and lymphatic system disorders:
Common: febrile neutropenia, pancytopenia, lymphopenia.
Uncommon: thrombocythaemia, leukocytosis.
Immune system disorders:
Not known: hypersensitivity.
Endocrine disorders
:
Uncommon: hyperthyroidism, hypothyroidism.
Not known: hyperparathyroidism secondary, thyroiditis.
Metabolism and nutrition disorders:
Common: electrolyte imbalance (including hypomagnesaemia, hyperkalaemia, hypokalaemia,
hyponatraemia, hypocalcaemia, hypophosphataemia, hypercalcaemia, hyperphosphataemia), diabetes
mellitus, hyperglycaemia, hypercholesterolaemia, hyperlipidaemia.
Uncommon: dehydration, decreased appetite, increased appetite.
Not known: hyperuricaemia, gout, hypoglycaemia, dyslipidaemia.
Psychiatric disorders:
Common: depression, insomnia.
Uncommon: anxiety.
Not known: disorientation, confusional state, amnesia, dysphoria.
Nervous system disorders:
Common: dizziness, hypoaesthesia, paraesthesia.
Uncommon: intracranial haemorrhage, migraine, loss of consciousness (including syncope), tremor,
disturbance in attention, hyperaesthesia.
Not known: brain oedema, optic neuritis, peripheral neuropathy, lethargy, dysaesthesia.
Eye disorders:
Common: eye haemorrhage, periorbital oedema, eye pruritus, conjunctivitis, dry eye.
Uncommon: visual impairment, vision blurred, visual acuity reduced, eyelid oedema, photopsia, eye
irritation.
Not known: papilloedema, chorioretinopathy, diplopia, photophobia, eye swelling, blepharitis, eye
pain, conjunctival haemorrhage, conjunctivitis allergic, conjunctival hyperaemia, ocular hyperaemia,
ocular surface disease, scleral hyperaemia.
Ear and labyrinth disorders:
Common: vertigo.
Not known: hearing impaired, ear pain, tinnitus.
Cardiac disorders:
Common: angina pectoris, arrhythmia (including atroventricular block, cardiac flutter, extrasystoles,
tachycardia, atrial fibrillation, bradycardia), palpitations, electrocardiogram QT prolonged.
Uncommon: cardiac failure, pericardial effusion, coronary artery disease, cardiac murmur, cyanosis.
Not known: myocardial infarction, ventricular dysfunction, pericarditis, ejection fraction decreased.
Vascular disorders:
Common: hypertension, flushing.
Uncommon: hypertensive crisis, haematoma.
Not known: shock haemorrhagic, hypotension, thrombosis.
Respiratory, thoracic and mediastinal disorders:
Common: dyspnoea, dyspnoea exertional, epistaxis, cough, dysphonia.
Uncommon: pulmonary oedema, pleural effusion, interstitial lung disease, pleuritic pain, pleurisy,
pharyngolaryngeal pain, throat irritation.
Not known: pulmonary hypertension, wheezing.
Gastrointestinal disorders:
Common: pancreatitis, abdominal discomfort, abdominal distension, dyspepsia, flatulence.
Uncommon: gastrointestinal haemorrhage, melaena, mouth ulceration, gastroesophageal reflux,
stomatitis, oesophageal pain, dysgeusia, dry mouth.
Not known: gastrointestinal ulcer perforation, retroperitoneal haemorrhage, haematemesis, gastric
ulcer, oesophagitis ulcerative, subileus, gastritis, haemorrhoids, hiatus hernia, rectal haemorrhage,
sensitivity of teeth, gingivitis.
Hepatobiliary disorders:
Common: hepatic function abnormal.
Uncommon: hepatitis, jaundice.
Not known: cholestasis, hepatotoxicity, hepatomegaly.
Skin and subcutaneous tissue disorders:
Common: night sweats, eczema, urticaria, erythema, hyperhidrosis, contusion, acne, dermatitis, dry
skin.
Uncommon: exfoliative rash, drug eruption, skin pain, ecchymosis, swelling face.
Not known: erythema nodosum, skin ulcer, palmar-plantar erythrodysaesthesia syndrome, petechiae,
photosensitivity, blister, dermal cysts, sebaceous hyperplasia, skin atrophy, skin discolouration, skin
exfoliation, skin hyperpigmentation, skin hypertrophy.
Musculoskeletal and connective tissue disorders:
Common: musculoskeletal chest pain, musculoskeletal pain, pain in extremity, back pain.
Uncommon: flank pain, musculoskeletal stiffness, pain, muscular weakness, joint swelling.
Not known: arthritis.
Renal and urinary disorders:
Common: pollakiuria.
Uncommon: dysuria, micturition urgency, nocturia.
Not known: renal failure, haematuria, urinary incontinence, chromaturia.
Reproductive system and breast disorders:
Uncommon: breast pain, gynaecomastia, erectile dysfunction.
Not known: breast induration, menorrhagia, nipple swelling.
General disorders and administration site conditions:
Common: chest pain, pain (including neck pain and back pain), pyrexia, chest discomfort, malaise.
Uncommon: face oedema, gravitational oedema, influenza-like illness, chills.
Not known: feeling hot, localised oedema.
Investigations:
Common: platelet count decreased, blood amylase increased, blood alkaline phosphatase increased,
gamma-glutamyltransferase increased, blood creatinine phosphokinase increased, weight decreased,
weight increased.
Uncommon: haemoglobin decreased, neutrophil count decreased, blood lactate dehydrogenase
increased, blood glucose decreased, blood urea increased, blood phosphorus decreased.
Not known: blood insulin increased, very low density lipoprotein increased, blood parathyroid
hormone increased, blood potassium increased, blood pressure increased, white blood cell count
decreased.
Clinically relevant or severe abnormalities of routine haematological or biochemistry laboratory
values are presented in Table 3.
Grade 3-4 laboratory abnormalities
Newly diagnosed
CML-CP
300 mg twice
daily
Imatinib-resistant or intolerant
CML-CP and CML-AP
400 mg twice daily
Haematological parameters
- Elevated bilirubin (total)
Sudden death
Uncommon cases (0.1 to 1%) of sudden deaths have been reported in Tasigna clinical trials and/or
compassionate use programs in patients with imatinib-resistant or intolerant CML in chronic phase or
accelerated phase with a past medical history of cardiac disease or significant cardiac risk factors (see
section 4.4).
Isolated reports of intentional overdose with nilotinib were reported, where an unspecified number of
Tasigna capsules were ingested in combination with alcohol and other medicinal products. Events
included neutropenia, vomiting and drowsiness. No ECG changes or hepatotoxicity were reported.
Outcomes were reported as recovered.
In the event of overdose, the patient should be observed and appropriate supportive treatment given.



















PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Protein kinase inhibitors, ATC code: L01XE08
Nilotinib is a potent inhibitor of the Abl tyrosine kinase activity of the Bcr-Abl oncoprotein both in
cell lines and in primary Philadelphia-chromosome positive leukaemia cells. The substance binds with
high affinity to the ATP-binding site in such a manner that it is a potent inhibitor of wild-type Bcr-Abl
and maintains activity against 32/33 imatinib-resistant mutant forms of Bcr-Abl. As a consequence of
this biochemical activity, nilotinib selectively inhibits the proliferation and induces apoptosis in cell
lines and in primary Philadelphia-chromosome positive leukaemia cells from CML patients. In murine
models of CML, as a single agent nilotinib reduces tumour burden and prolongs survival following
oral administration.
Nilotinib has little or no effect against the majority of other protein kinases examined, including Src,
except for the PDGF, Kit and Ephrin receptor kinases, which it inhibits at concentrations within the
range achieved following oral administration at therapeutic doses recommended for the treatment of
CML (see Table 4).
Kinase profile of nilotinib (phosphorylation IC
50
nM)
Clinical studies in newly diagnosed CML in chronic phase
An open-label, multicentre, randomised Phase III study was conducted to determine the efficacy of
Nilotinib versus imatinib in 846 adult patients with cytogenetically confirmed newly diagnosed
Philadelphia chromosome positive CML in the chronic phase. Patients were within six months of
diagnosis and were previously untreated, with the exception of hydroxyurea and/or anagrelide.
Patients were randomised 1:1:1 to receive either nilotinib 300 mg twice daily (n=282), nilotinib
400 mg twice daily (n=281) or imatinib 400 mg once daily (n=283). Randomisation was stratified by
Sokal risk score at the time of diagnosis.
Baseline characteristics were well balanced between the three treatment arms. Median age was
47 years in both nilotinib arms and 46 years in the imatinib arm, with 12.8%, 10.0% and 12.4% of
patients were ≥65 years of age in the nilotinib 300 mg twice daily, nilotinib 400 mg twice daily and
imatinib 400 mg once daily treatment arms, respectively. There were slightly more male than female
patients (56.0%, 62.3% and 55.8%, in the nilotinib 300 mg twice daily, 400 mg twice daily and
imatinib 400 mg once daily arm, respectively). More than 60% of all patients were Caucasian and 25%
of all patients were Asian.
The time point for the primary data analysis was when all 846 patients completed 12 months of
treatment (or discontinued earlier). The median time on treatment was 14 months. More than 60% of
all patients had received treatment for longer than 12 months. The median actual dose intensity was
592 mg/day for nilotinib 300 mg twice daily, 779 mg/day for nilotinib 400 mg twice daily and
400 mg/day for imatinib 400 mg once daily.
The primary efficacy endpoint was major molecular response (MMR) at 12 months. MMR was
defined as ≤0.1% Bcr-Abl/Abl % by international scale measured by RQ-PCR, which corresponds to a
≥3 log reduction of Bcr-Abl transcript from standardised baseline. The MMR rate at 12 months was
statistically significantly higher for nilotinib 300 mg twice daily compared to imatinib 400 mg once
daily (44.3% versus 22.3%, p<0.0001). The rate of MMR at 12 months, was also statistically
significantly higher for nilotinib 400 mg twice daily compared to imatinib 400 mg once daily (42.7%
versus 22.3%, p<0.0001).






The rates of MMR at 3, 6, 9 and 12 months were 8.9%, 33.0%, 43.3% and 44.3% for nilotinib 300 mg
twice daily, 5.0%, 29.5%, 38.1% and 42.7% for nilotinib 400 mg twice daily and 0.7%, 12.0%, 18.0%
and 22.3% for imatinib 400 mg once daily.
The Kaplan-Meier analysis of time to first MMR is shown in Figure 1. The probability of achieving
MMR at different time points was higher for both nilotinib at 300 mg and 400 mg twice daily
compared to imatinib 400 mg once daily (HR=2.6 and stratified log-rank p<0.0001 between nilotinib
300 mg twice daily and imatinib 400 mg once daily, HR=1.6 and stratified log-rank p<0.0001 between
nilotinib 400 mg twice daily and imatinib400 mg once daily). The proportion of patients who achieved
a Bcr-Abl ratio of ≤0.01% (4 log reduction) and ≤0.0032% (4.5 log reduction) at 12 months were
statistically significantly higher for both nilotinib 300 mg twice daily (11.7% and 4.3%, respectively)
and nilotinib 400 mg twice daily (8.5% and 4.6%, respectively) compared to 400 mg imatinib once
daily (3.9% and 0.4% respectively). For all Sokal risk groups, the response rates were higher for both
nilotinib at 300 mg and 400 mg twice daily than for imatinib 400 mg once daily.
Major molecular response (MMR) rate at 12 months
Tasigna (nilotinib)
AMN107
300 mg twice
daily
n=282
(%)
Tasigna (nilotinib)
AMN107
400 mg twice
daily
n=281
(%)
Glivec (imatinib)
STI571
400 mg once daily
n=283
(%)
CMH* test p-value for response rate
(vs. imatinib 400 mg once daily)
*CMH = Cochran-Mantel-Haenszel
Figure 1 Kaplan-Meier estimate of time to first major molecular response (MMR)
Time since randomisation (months)














Complete cytogenetic response (CCyR) was defined as 0% Ph+ metaphases in the bone marrow based
on a minimum of 20 metaphases evaluated. Best CCyR rate by 12 months (including patients who
achieved CCyR at or before the 12 month time point as responders) was statistically higher for both
nilotinib 300 mg and 400 mg twice daily compared to imatinib 400 mg once daily.
Best complete cytogenetic response (CCyR) rate by 12 months
Tasigna (nilotinib)
300 mg twice daily
n=282
(%)
Tasigna (nilotinib)
400 mg twice daily
n=281
(%)
Glivec (imatinib)
400 mg once daily
n=283
(%)
CMH test p-value for response rate
(vs. imatinib 400 mg once daily)
Progression to accelerated phase or blast crisis on treatment was observed in a total of 14 patients:
2 patients on nilotinib 300 mg twice daily, 1 patient on nilotinib 400 mg twice daily and 11 patients on
imatinib 400 mg once daily. None of the patients who progressed had achieved MMR, while 3 of these
patients receiving imatinib 400 mg once daily achieved CCyR. There was a statistically significant
difference in progression to accelerated phase or blast crisis between nilotinib 300 mg twice daily and
imatinib 400 mg once daily (p=0.0095) and between nilotinib 400 mg twice daily and imatinib 400 mg
once daily (p=0.0037) in favour of nilotinib.
Clinical studies in imatinib-resistant or intolerant CML in chronic phase and accelerated phase
An open-label, uncontrolled, multicentre Phase II study was conducted to determine the efficacy of
Tasigna in patients with imatinib resistant or intolerant CML with separate treatment arms for chronic
and accelerated phase disease. The study is ongoing. Efficacy was based on 321 CP patients and
137 AP patients enrolled. Median duration of treatment was 561 days for CP patients and 264 days for
AP patients (see Table 7). Tasigna was administered on a continuous basis (twice daily 2 hours after a
meal and with no food for at least one hour after administration) unless there was evidence of
inadequate response or disease progression. The dose was 400 mg twice daily and dose escalation to
600 mg twice daily was allowed.
Duration of exposure with Tasigna
Median duration of therapy in days
(25th-75th percentiles)
Resistance to imatinib included failure to achieve a complete haematological response (by 3 months),
cytogenetic response (by 6 months) or major cytogenetic response (by 12 months) or progression of
disease after a previous cytogenetic or haematological response. Imatinib intolerance included patients
who discontinued imatinib because of toxicity and were not in major cytogenetic response at time of
study entry.
















Overall, 73% of patients were imatinib-resistant, while 27% were imatinib-intolerant. The majority of
patients had a long history of CML that included extensive prior treatment with other antineoplastic
agents, including imatinib, hydroxyurea, interferon, and some had even failed organ transplant
(Table 8). The median highest prior imatinib dose had been 600 mg/day. The highest prior imatinib
dose was 600 mg/day in 74% of all patients, with 40% of patients receiving imatinib doses
800 mg/day.
CML disease history characteristics
Accelerated phase
(n=137)*
Median time since diagnosis in months
(range)
Imatinib
Resistant
Intolerant without MCyR
Median time of imatinib treatment in
days
(25th-75
th
percentiles)
Prior bone marrow transplant
* Missing information on imatinib-resistant/intolerant status for one patient.
The primary endpoint in the CP patients was major cytogenetic response (MCyR), defined as
elimination (CCyR, complete cytogenetic response) or significant reduction to <35% Ph+ metaphases
(partial cytogenetic response) of Ph+ haematopoietic cells. Complete haematological response (CHR)
in CP patients was evaluated as a secondary endpoint. The primary endpoint in the AP patients was
overall confirmed haematological response (HR), defined as either a complete haematological
response, no evidence of leukaemia or return to chronic phase.
Chronic Phase
The MCyR rate in 321 CP patients was 51%. Most responders achieved their MCyR rapidly within
3 months (median 2.8 months) of starting Tasigna treatment and these were sustained. The median
time to achieve CCyR was just past 3 months (median 3.4 months). Of the patients who achieved
MCyR, 77% (95% CI: 70% - 84%) were maintaining response at 24 months. Median duration of
MCyR has not been reached. Of the patients who achieved CCyR, 85% (95% CI: 78% - 93%) were
maintaining response at 24 months. Median duration of CCyR has not been reached. Patients with a
CHR at baseline achieved a MCyR faster (1.9 vs. 2.8 months). Of CP patients without a baseline CHR,
70% achieved a CHR, median time to CHR was 1 month and median duration of CHR was
32.8 months. The estimated 24-month overall survival rate in CML-CP patients was 87%.
Accelerated Phase
The overall confirmed HR rate in 137 AP patients was 50%. Most responders achieved a HR early
with Tasigna treatment (median 1.0 months) and these have been durable (median duration of
confirmed HR was 24.2 months). Of the patients who achieved HR, 53% (95% CI: 39% - 67%) were
maintaining response at 24 months. MCyR rate was 30% with a median time to response of
2.8 months. Of the patients who achieved MCyR, 63% (95% CI: 45% - 80%) were maintaining
response at 24 months. Median duration of MCyR was 32.7 months. The estimated 24-month overall
survival rate in CML-AP patients was 70%.











The rates of response for the two treatment arms are reported in Table 9.
Haematological
Response (%)
Overall (95%CI)
Complete
NEL
Return to CP
Cytogenetic
Response (%)
Major (95%CI)
Complete
Partial
NEL = no evidence of leukaemia/marrow response
1
114 CP patients had a CHR at baseline and were therefore not assessable for complete
haematological response
* Missing information on imatinib-resistant/intolerant status for one patient.
Efficacy data in patients with CML-BC are not yet available. Separate treatment arms were also
included in the Phase II study to investigate Tasigna in a group of CP and AP patients who had been
extensively pre-treated with multiple therapies including a tyrosine kinase inhibitor agent in addition
to imatinib. The study is ongoing. Of these patients 30/36 (83%) were treatment resistant not
intolerant. In 22 CP patients evaluated for efficacy Tasigna induced a 32% MCyR rate and a 50%
CHR rate. In 11 AP patients, evaluated for efficacy, treatment induced a 36% overall HR rate.
After imatinib failure, 24 different Bcr-Abl mutations were noted in 42% of chronic phase and 54% of
accelerated phase CML patients who were evaluated for mutations. Tasigna demonstrated efficacy in
patients harboring a variety of Bcr-Abl mutations associated with imatinib resistance, except T315I.
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with
Tasigna in paediatric patients from birth to less then 18 years in the treatment of Philadelphia
chromosome positive chronic myeloid leukaemia (see 4.2 for information on paediatric use).
5.2 Pharmacokinetic properties
Absorption
Peak concentrations of nilotinib are reached 3 hours after oral administration. Nilotinib absorption
following oral administration was approximately 30%. In healthy volunteers, C
max
and area under the
serum concentration-time curve (AUC) of nilotinib are increased by 112% and 82%, respectively,
compared to fasting conditions when Tasigna is given with food. Administration of Tasigna
30 minutes or 2 hours after food increased bioavailability of nilotinib by 29% or 15%, respectively
(see sections 4.2, 4.4 and 4.5).
Single-dose administration of 400 mg nilotinib, using 2 capsules of 200 mg whereby the content of
each capsule was dispersed in one teaspoon of apple sauce, was shown to be bioequivalent with a
single-dose administration of 2 intact capsules of 200 mg.















Nilotinib absorption (relative bioavailability) might be reduced by approximately 48% and 22% in
patients with total gastrectomy and partial gastrectomy, respectively.
Distribution
The blood-to-plasma ratio of nilotinib is 0.71. Plasma protein binding is approximately 98% on the
basis of
in vitro
experiments.
Biotransformation
Main metabolic pathways identified in healthy subjects are oxidation and hydroxylation. Nilotinib is
the main circulating component in the serum. None of the metabolites contribute significantly to the
pharmacological activity of nilotinib. Nilotinib is primarily metabolised by CYP3A4, with possible
minor contribution from CYP2C8.
Elimination
After a single dose of radiolabelled nilotinib in healthy subjects, more than 90% of the dose was
eliminated within 7 days, mainly in faeces (94% of the dose). Parent drug accounted for 69% of the
dose.
Linearity / non-linearity
Steady-state nilotinib exposure was dose-dependent, with less than dose-proportional increases in
systemic exposure at dose levels higher than 400 mg given as once-daily dosing. Daily serum
exposure to nilotinib with 400 mg twice-daily dosing at steady state was 35% higher than with 800 mg
once-daily dosing. Systemic exposure (AUC) of nilotinib at steady state at a dose level of 400 mg
twice daily was approximately 13.4% higher than at a dose level of 300 mg twice daily. The average
nilotinib trough and peak concentrations over 12 months were approximately 15.7% and 14.8% higher
following 400 mg twice-daily dosing compared to 300 mg twice daily. There was no relevant increase
in exposure to nilotinib when the dose was increased from 400 mg twice daily to 600 mg twice daily.
Characteristics in patients
Steady-state conditions were essentially achieved by day 8. An increase in serum exposure to nilotinib
between the first dose and steady state was approximately 2-fold for daily dosing and 3.8-fold for
twice-daily dosing. The apparent elimination half-life estimated from the multiple-dose
pharmacokinetics with daily dosing was approximately 17 hours. Inter-patient variability in nilotinib
pharmacokinetics was moderate to high.
5.3 Preclinical safety data
Nilotinib has been evaluated in safety pharmacology, repeated dose toxicity, genotoxicity,
reproductive toxicity and phototoxicity studies.
Nilotinib did not have effects on CNS or respiratory functions.
In vitro
cardiac safety studies
demonstrated a preclinical signal for QT prolongation, based upon block of hERG currents and
prolongation of the action potential duration in isolated rabbit hearts by nilotinib. No effects were seen
in ECG measurements in dogs or monkeys treated for up to 39 weeks or in a special telemetry study in
dogs.
Repeated-dose toxicity studies in dogs of up to 4 weeks’ duration and in cynomolgus monkeys of up
to 9 months’ duration revealed the liver as the primary target organ of toxicity of nilotinib. Alterations
included increased alanine aminotransferase and alkaline phosphatase activity and histopathology
findings (mainly sinusoidal cell or Kupffer cell hyperplasia/hypertrophy, bile duct hyperplasia and
periportal fibrosis). In general the changes in clinical chemistry were fully reversible after a four-week
recovery period and the histological alterations showed partial reversibility. Exposures at the lowest
dose levels at which the liver effects were seen were lower than the exposure in humans at a dose of
800 mg/day. Only minor liver alterations were seen in mice or rats treated for up to 26 weeks. Mainly
reversible increases in cholesterol levels were seen in rats, dogs and monkeys.
Genotoxicity studies in bacterial
in vitro
systems and in mammalian
in vitro
and
in vivo
systems with
and without metabolic activation did not reveal any evidence for a mutagenic potential of nilotinib.
Nilotinib did not induce teratogenicity, but did show embryo- and foetotoxicity at doses that also
showed maternal toxicity. Increased post-implantation loss was observed in both the fertility study,
which involved treatment of both males and females, and the embryotoxicity study, which involved
treatment of females. Embryo-lethality and foetal effects (mainly decreased foetal weights, premature
fusion of the facial bones (fused maxilla/zygomatic) visceral and skeletal variations) in rats and
increased resorption of foetuses and skeletal variations in rabbits were present in the embryotoxicity
studies. In a pre- and postnatal development study in rats, maternal exposure to nilotinib caused
reduced pup body weight with associated changes in physical development parameters as well as
reduced mating and fertility indices in the offspring. Exposure to nilotinib in females at No-Observed-
Adverse-Effect-Levels was generally less or equal to that in humans at 800 mg/day.
In a juvenile development study, nilotinib was administered via oral gavage to juvenile rats from the
first week post partum through young adult (day 70 post partum) at doses of 2, 6 and 20 mg/kg/day.
Besides standard study parameters, evaluations of developmental landmarks, CNS effects, mating and
fertility were performed. Based on a reduction in body weight in both genders and a delayed preputial
separation in males (which may be associated with the reduction in weight), the No-Observed-Effect-
Level in juvenile rats was considered to be 6 mg/kg/day. The juvenile animals did not exert increased
sensitivity to nilotinib relative to adults. In addition, the toxicity profile in juvenile rats was
comparable to that observed in adult rats.
No effects on sperm count/motility or on fertility were noted in male and female rats up to the highest
tested dose, approximately 5 times the recommended dosage for humans.
Nilotinib was shown to absorb light in the UV-B and UV-A range, is distributed into the skin and
showed a phototoxic potential
in vitro
, but no effects have been observed
in vivo
. Therefore the risk
that nilotinib causes photosensitisation in patients is considered very low.
Carcinogenicity studies with nilotinib have not been performed.
PHARMACEUTICAL PARTICULARS
Capsule content
Lactose monohydrate
Crospovidone
Poloxamer 188
Silica, colloidal anhydrous
Magnesium stearate
Capsule shell
Gelatin
Titanium dioxide (E171)
Yellow iron oxide (E172)
Printing ink
Shellac
Red iron oxide (E172)
Soya lecithin (E322)
6.4 Special precautions for storage
Store in the original package in order to protect from moisture.
6.5 Nature and contents of container
PVC/PVDC/Al and PA/Al/PVC/Al blisters.
Tasigna is available in weekly and monthly packs:
The weekly pack contains 28 capsules.
The monthly pack contains 112 capsules (4 individual weekly wallets, each containing
28 capsules).
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency
http://www.ema.europa.eu
MANUFACTURING AUTHORISATION HOLDER
RESPONSIBLE FOR BATCH RELEASE
CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer responsible for batch release
Novartis Pharma GmbH
Roonstraße 25
D-90429 Nuremberg
Germany
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to restricted medical prescription (See Annex I: Summary of Product
Characteristics, section 4.2).
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
The MAH shall ensure that prior to launch, all doctors who intend to prescribe Tasigna, and all
pharmacists who may dispense Tasigna, are provided with a healthcare professional information
pack containing the following:
Educational brochure
Summary of Product Characteristics (SPC) and Package Leaflet and Labelling
Key elements to be included in the educational brochure
Brief background on Tasigna, its authorised indication and posology
Information on the cardiac risks associated with the use of Tasigna
o
That Tasigna can cause prolongation of the QT interval and that patients at risk of
arrythmia, especially torsade de pointes, should not be prescribed Tasigna.
o
The need to avoid co-prescription with any other medicines that might prolong the
QT interval
o
Caution in prescribing to patients with a history of or risk factors for coronary heart
disease
o
That Tasigna may cause fluid retention, cardiac failure and pulmonary oedema
That Tasigna is metabolised by CYP3A4 and that strong inhibitors or inducers of this
enzyme may significantly affect exposure to Tasigna.
o
That inhibitors may increase the potential for adverse drug reactions in particular
QT interval prolongation.
o
To warn patients about OTC medicines in particular St John’s Wort
The need to inform patients about the effects of food on Tasigna
o
Not to eat within two hours before and one hour after taking Tasigna
o
The need to avoid foods such as grapefruit juice which inhibit CYP3A4 enzymes
Pharmacovigilance system
The MAH must ensure that the system of pharmacovigilance, presented in Module 1.8.1. of the
Marketing Authorisation, is in place and functioning before and whilst the product is on the market.
Risk Management Plan
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version 8.1 of the Risk Management Plan (RMP) presented
in Module 1.8.2. of the Marketing Authorisation and any subsequent updates of the RMP agreed by
the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, any
updated RMP should be submitted at the same time as the following Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted:
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
At the request of the European Medicines Agency
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
Tasigna 150 mg hard capsules
Nilotinib
STATEMENT OF ACTIVE SUBSTANCE(S)
One hard capsule contains 150 mg nilotinib (as hydrochloride monohydrate).
Contains lactose – see the package leaflet for further information.
PHARMACEUTICAL FORM AND CONTENTS
METHOD AND ROUTE(S) OF ADMINISTRATION
Oral use.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
Do not store above 30°C.
Store in the original package in order to protect from moisture.






















10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE

















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
CARTON OF MONTHLY PACK (INCLUDING BLUE BOX)
NAME OF THE MEDICINAL PRODUCT
Tasigna 150 mg hard capsules
Nilotinib
STATEMENT OF ACTIVE SUBSTANCE(S)
One hard capsule contains 150 mg nilotinib (as hydrochloride monohydrate).
Contains lactose – see the package leaflet for further information.
PHARMACEUTICAL FORM AND CONTENTS
112 hard capsules
Monthly pack containing 4 weekly packs.
METHOD AND ROUTE(S) OF ADMINISTRATION
Oral use.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
Do not store above 30°C.
Store in the original package in order to protect from moisture.






















10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE

















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
INTERMEDIATE CARTON OF MONTHLY PACK (WITHOUT BLUE BOX)
NAME OF THE MEDICINAL PRODUCT
Tasigna 150 mg hard capsules
Nilotinib
STATEMENT OF ACTIVE SUBSTANCE(S)
One hard capsule contains 150 mg nilotinib (as hydrochloride monohydrate).
Contains lactose – see the package leaflet for further information.
PHARMACEUTICAL FORM AND CONTENTS
28 hard capsules
Component of a monthly pack containing 4 weekly packs.
METHOD AND ROUTE(S) OF ADMINISTRATION
Oral use.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
Do not store above 30°C.
Store in the original package in order to protect from moisture.





















10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE

















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
Tasigna 200 mg hard capsules
Nilotinib
STATEMENT OF ACTIVE SUBSTANCE(S)
One hard capsule contains 200 mg nilotinib (as hydrochloride monohydrate).
Contains lactose – see the package leaflet for further information.
PHARMACEUTICAL FORM AND CONTENTS
METHOD AND ROUTE(S) OF ADMINISTRATION
Oral use.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
Do not store above 30°C.
Store in the original package in order to protect from moisture.





















10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE


















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
Tasigna 200 mg hard capsules
Nilotinib
STATEMENT OF ACTIVE SUBSTANCE(S)
One hard capsule contains 200 mg nilotinib (as hydrochloride monohydrate).
Contains lactose – see the package leaflet for further information.
PHARMACEUTICAL FORM AND CONTENTS
112 hard capsules
Monthly pack containing 4 wallets.
METHOD AND ROUTE(S) OF ADMINISTRATION
Oral use.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
Do not store above 30°C.
Store in the original package in order to protect from moisture.






















10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE


















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
INTERMEDIATE WALLET OF MONTHLY PACK
NAME OF THE MEDICINAL PRODUCT
Tasigna 200 mg hard capsules
Nilotinib
STATEMENT OF ACTIVE SUBSTANCE(S)
One hard capsule contains 200 mg nilotinib (as hydrochloride monohydrate).
Contains lactose – see the package leaflet for further information.
PHARMACEUTICAL FORM AND CONTENTS
28 hard capsules
Component of a monthly pack containing 4 wallets.
METHOD AND ROUTE(S) OF ADMINISTRATION
Oral use.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
SPECIAL STORAGE CONDITIONS
Do not store above 30°C.
Store in the original package in order to protect from moisture.





















10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE


















PACKAGE LEAFLET: INFORMATION FOR THE USER
Tasigna 150 mg hard capsules
Nilotinib
Read all of this leaflet carefully before you start taking this medicine.
-
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
What Tasigna is and what it is used for
WHAT TASIGNA IS AND WHAT IT IS USED FOR
What Tasigna is
Tasigna is a medicine containing an active substance called nilotinib.
What Tasigna is used for
Tasigna is used to treat a type of leukaemia called Philadelphia chromosome positive chronic myeloid
leukaemia (Ph-positive CML). CML is a cancer of the blood which makes the body produce too many
abnormal white blood cells.
Tasigna is used in patients with newly diagnosed CML.
How Tasigna works
In patients with CML, a change in DNA (genetic material) triggers a signal that tells the body to
produce abnormal white blood cells. Tasigna blocks this signal, and thus stops the production of these
cells.
Monitoring your Tasigna treatment
You will have regular tests, including blood tests, during treatment. These will monitor the amount of
blood cells (white blood cells, red blood cells and platelets) in your body to see how Tasigna is
tolerated.
If you have any questions about how Tasigna works or why it has been prescribed for you, ask your
doctor.
Follow all the doctor’s instructions carefully. They may differ from the general information contained
in this leaflet.
Do not take Tasigna
-
if you are
allergic
(hypersensitive) to nilotinib or any of the other ingredients of Tasigna listed
at the end of this leaflet.
If you think you may be allergic, tell your doctor
before taking Tasigna
.
Take special care with Tasigna
-
if you have a
heart disorder
, such as an abnormal electrical signal called “prolongation of the
QT interval”.
-
if you are being
treated with medicines
that affect the heart beat (anti-arrhythmics) or the liver
(see
Taking other medicines
).
-
if you suffer from lack of potassium or magnesium.
-
if you have been treated with a medicine of the type called anthracyclines (frequently used in
leukaemia therapy).
-
if you have a liver or pancreas disorder.
If any of these apply to you, tell your doctor.
During treatment with Tasigna
-
if you faint (loss of consciousness) or have an irregular heart beat while taking Tasigna,
tell
your doctor immediately
as this may be a sign of a serious heart condition. Prolongation of the
QT interval or an irregular heart beat may lead to sudden death. Uncommon cases of sudden
death have been reported in patients taking Tasigna.
Taking other medicines
Tasigna may interfere with some other medicines.
Tell your doctor
or pharmacist
before taking Tasigna
if you are taking or have recently taken any
other medicines, including medicines obtained without a prescription. This includes in particular:
-
antiarrhythmics – used to treat irregular heart beat;
-
chloroquine, halofantrine, clarithromycin, haloperidol, methadone, moxifloxacin - medicines
that may have an unwanted effect on the function of the heart;
-
ketoconazole, itraconazole, voriconazole, clarithromycin, telithromycin – used to treat
infections;
-
ritonavir – a medicine from the class “ antiproteases” used to treat HIV;
-
carbamazepine, phenobarbital, phenytoin – used to treat epilepsy;
-
rifampicin – used to treat tuberculosis;
-
St. John’s Wort – a herbal product used to treat depression and other conditions (also known as
Hypericum perforatum
);
midazolam – used to relieve anxiety before surgery;
warfarin – used to treat blood coagulation disorders (such as blood clots or thromboses);
astemizole, terfenadine, cisapride, pimozide, quinidine, bepridil or ergot alkaloids (ergotamine,
dihydroergotamine).
These medicines should be avoided during your treatment with Tasigna. If you are taking any of these,
your doctor might prescribe other alternative medicines.
You should also tell your doctor
if you are already taking Tasigna
and you are prescribed a new
medicine that you have not taken previously during Tasigna treatment.
Taking Tasigna with food and drink
-
Do not take Tasigna with food.
Take the capsules at least 2 hours after any food and then wait
at least 1 hour before eating again. For more information, see under “When to take Tasigna” in
section 3.
-
Do not drink grapefruit juice or eat grapefruit. It may increase the amount of Tasigna in the
blood, possibly to a harmful level.
If you are unable to swallow capsules, you may sprinkle the content of each capsule in one teaspoon of
apple sauce (puréed apple) and take it immediately.
Do not use more than one teaspoon of apple
sauce for each capsule and do not use any food other than apple sauce.
Older people (age 65 years and over)
Tasigna can be used by people aged 65 years and over at the same dose as for other adults.
Pregnancy and breast-feeding
-
Tasigna is not recommended during pregnancy
unless clearly necessary. If you are pregnant
or think that you may be, tell your doctor who will discuss with you whether you can take
Tasigna during your pregnancy.
-
Women who might get pregnant
are advised to use effective contraception during treatment.
-
Breast-feeding is not recommended
during treatment with Tasigna. Tell your doctor if you are
breast-feeding.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
If you experience side effects (such as dizziness or visual disorders) with a potential impact on the
ability to safely drive or use any tools or machines after taking Tasigna, you should refrain from these
activities until the effect has disappeared.
Important information about some of the ingredients of Tasigna
This medicine contains lactose (also known as milk sugar). If you have been told by your doctor that
you have an intolerance to some sugars, contact your doctor before taking this medicine.
Always take Tasigna exactly as your doctor has told you. You should check with your doctor or
pharmacist if you are not sure.
How much Tasigna to take
-
The starting dose is 600 mg per day. This dose is achieved by taking two capsules of 150 mg
twice a day.
Your doctor may prescribe a lower dose depending on how you respond to treatment.
When to take Tasigna
Take the capsules:
-
twice a day (approximately every 12 hours);
-
at least 2 hours after any food;
-
then wait 1 hour before eating again.
If you have questions about when to take Tasigna, talk to your doctor or pharmacist. Taking Tasigna at
the same time each day will help you remember when to take your capsules.
Do not take any food together with the capsules.
Do not open the capsules unless you are unable to swallow them. If so, you may sprinkle the
content of each capsule in
one
teaspoon of apple sauce and take it immediately. Do not use
more than one teaspoon of apple sauce for each capsule and do not use any food other than
apple sauce.
Swallow the capsules whole with water.
How long to take Tasigna
Continue taking Tasigna every day for as long as your doctor tells you. This is a long-term treatment.
Your doctor will regularly monitor your condition to check that the treatment is having the desired
effect.
If you have questions about how long to take Tasigna, talk to your doctor.
If you take more Tasigna than you should
If you have taken more Tasigna than you should have, or if someone else accidentally takes your
capsules, contact a doctor or hospital for advice straight away. Show them the pack of capsules and
this package leaflet. Medical treatment may be necessary.
If you forget to take Tasigna
If you miss a dose, take your next dose as scheduled. Do not take a double dose to make up for the
forgotten capsules.
If you stop taking Tasigna
Do not stop taking Tasigna unless your doctor tells you to.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Like all medicines, Tasigna can cause side effects, although not everybody gets them. Most of the side
effects are mild to moderate and will generally disappear after a few days to a few weeks of treatment.
Some side effects could be serious.
These side effects are common, uncommon or have been reported in very few patients.
-
rapid weight gain,
swelling of hands, ankles, feet or face (signs of water retention)
-
chest pain, high blood pressure, irregular heart rhythm, blue discolouration of the lips, tongue or
skin (signs of heart disorders)
-
difficulty breathing, cough, wheezing, swelling of the feet or legs (signs of lung disorders)
-
fever, easy bruising, frequent infections (signs of blood disorders)
-
blurred vision, loss of vision, blood in eye (signs of eye disorders)
-
swelling and pain in one part of the body (signs of clotting within a vein)
-
abdominal pain, nausea, constipation, swollen abdomen (signs of gastrointestinal disorders)
-
severe upper abdominal pain (sign of pancreatitis)
-
yellow skin and eyes, nausea, loss of appetite, light-coloured urine (signs of liver disorders)
-
rash, painful red lumps, pain in joints and muscles (signs of skin disorders)
-
excessive thirst, high urine output, increased appetite with weight loss, tiredness (signs of high
level of sugar in the blood)
If you get any of these,
tell your doctor straight away
.
Some side effects are very common
(may affect more than 10 in every 100 patients)
-
headache
-
tiredness
-
muscle pain
-
itching, rash, hives
If any of these affects you severely, tell your doctor.
Some side effects are common
(may affect between 1 and 10 in every 100 patients)
-
diarrhoea, vomiting, abdominal discomfort, stomach discomfort after meals, flatulence, swelling
or bloating of the abdomen
-
bone pain, pain in joints, muscle spasms, pain in extremity, back pain
-
eye irritation, swelling, discharge, itching or redness, dry eye (signs of eye disorders)
-
skin reddening, dry skin, acne, wart, decreased skin sensitivity
-
loss of appetite, weight increase
-
hair loss
-
insomnia
-
night sweats, excessive sweating, hot flushes
-
dizziness, spinning sensation
-
palpitations (sensation of rapid heart beat)
If any of these affects you severely, tell your doctor.
Some side effects are uncommon
(may affect less than 1 in every 100 patients)
-
skin pain
-
swelling of eyelids
-
disturbed sense of taste
-
nose bleed
-
anxiety
-
flu-like symptoms
-
tingling or numbness
-
visual disturbances
If any of these affects you severely, tell your doctor.
The following other side effects have been reported in very few patients treated with Tasigna:
-
memory loss, disturbed or depressed mood, lack of energy, generally feeling unwell
-
oral thrush, bacterial infection of the skin
-
blister, skin cyst, oily skin, thinning of the skin, dark patches of skin, skin discolouration
-
increased skin sensitivity
-
sensitivity of teeth, bleeding, tender or enlarged gums
-
runny or stuffy nose, sneezing
-
dry mouth, sore throat, mouth sores
-
trembling
-
eye pain or redness, pain, itching of the eyelids
-
painful and swollen joints (gout), muscle weakness, pain in the side of your body
-
unconsciousness
-
difficulty and pain when passing urine, exaggerated sense of needing to urinate
-
frequent urine output, abnormal urine colour
-
haemorrhoids
-
feeling of hardening in the breasts, heavy periods, nipple swelling
-
feeling hot
-
appetite disorder, weight decreased
-
severe headache often accompanied by nausea, vomiting and sensitivity to light
-
heartburn
-
breast enlargement in men
If any of these affects you severely, tell your doctor.
During Tasigna treatment, you may also have some abnormal blood test results such as low level of
blood cells (white cells, red cells, platelets), high blood level of lipase or amylase (pancreas function),
high blood level of bilirubin (liver function) or high blood level of creatinine (kidney function).
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Tasigna after the expiry date which is stated on the carton and blister. The expiry
date refers to the last day of that month.
Store in the original package in order to protect from moisture.
Do not use any pack that is damaged or shows signs of tampering.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist
how to dispose of medicines no longer required. These measures will help to protect the
environment.
The active substance is nilotinib. Each capsule contains 150 mg nilotinib (as hydrochloride
monohydrate).
The other ingredients are lactose monohydrate, crospovidone, poloxamer 188, silica colloidal
anhydrous, magnesium stearate. The capsule shell is composed of gelatin, titanium dioxide
(E171), red and yellow iron oxide (E172) and, shellac and black iron oxide (E172) for stamping
of the imprint.
What Tasigna looks like and contents of the pack
Tasigna is supplied as hard capsules. The capsules are red. A black imprint is stamped on each capsule
(“NVR/BCR”).
Tasigna is available in weekly and monthly packs:
-
The monthly pack contains 112 capsules (4 individual weekly packs, each containing
28 capsules).
Not all packs may be marketed in your country.
Marketing Authorisation Holder
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
Manufacturer
Novartis Pharma GmbH
Roonstraße 25
D-90429 Nuremberg
Germany
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
Novartis Pharma N.V.
Tél/Tel: +32 2 246 16 11
Luxembourg/Luxemburg
Novartis Pharma GmbH
Tél/Tel: +49 911 273 0
The weekly pack contains 28 capsules (7 daily blisters, each containing 4 capsules).
България
Novartis Pharma Services Inc.
Тел.: +359 2 489 98 28
Magyarország
Novartis Hungária Kft. Pharma
Tel.: +36 1 457 65 00
Česká republika
Novartis s.r.o.
Tel: +420 225 775 111
Malta
Novartis Pharma Services Inc.
Tel: +356 2298 3217
Danmark
Novartis Healthcare A/S
Tlf: +45 39 16 84 00
Nederland
Novartis Pharma B.V.
Tel: +31 26 37 82 111
Deutschland
Novartis Pharma GmbH
Tel: +49 911 273 0
Norge
Novartis Norge AS
Tlf: +47 23 05 20 00
Eesti
Novartis Pharma Services Inc.
Tel: +372 66 30 810
Österreich
Novartis Pharma GmbH
Tel: +43 1 86 6570
Ελλά
Novartis (Hellas) A.E.B.E.
Τηλ: +30 210 281 17 12
Polska
Novartis Poland Sp. z o.o.
Tel.: +48 22 550 8888
España
Novartis Farmacéutica, S.A.
Tel: +34 93 306 42 00
Portugal
Novartis Farma - Produtos Farmacêuticos, S.A.
Tel: +351 21 000 8600
France
Novartis Pharma S.A.S.
Tél: +33 1 55 47 66 00
România
Novartis Pharma Services Inc.
Tel: +40 21 31299 01
Ireland
Novartis Ireland Limited
Tel: +353 1 260 12 55
Slovenija
Novartis Pharma Services Inc.
Tel: +386 1 300 75 50
Ísland
Vistor hf.
Sími: +354 535 7000
Slovenská republika
Novartis Slovakia s.r.o.
Tel: +421 2 5542 5439
Italia
Novartis Farma S.p.A.
Tel: +39 02 96 54 1
Suomi/Finland
Novartis Finland Oy
Puh/Tel: +358 (0)10 6133 200
Κύπρς
Novartis Pharma Services Inc.
Τηλ: +357 22 690 690
Sverige
Novartis Sverige AB
Tel: +46 8 732 32 00
Latvija
Novartis Pharma Services Inc.
Tel: +371 67 887 070
United Kingdom
Novartis Pharmaceuticals UK Ltd.
Tel: +44 1276 698370
Lietuva
Novartis Pharma Services Inc.
Tel: +370 5 269 16 50
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency web site:
http://www.ema.europa.eu/. There are also links to other websites about rare diseases and treatments.
PACKAGE LEAFLET: INFORMATION FOR THE USER
Tasigna 200 mg hard capsules
Nilotinib
Read all of this leaflet carefully before you start taking this medicine.
-
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
What Tasigna is and what it is used for
WHAT TASIGNA IS AND WHAT IT IS USED FOR
What Tasigna is
Tasigna is a medicine containing an active substance called nilotinib.
What Tasigna is used for
Tasigna is used to treat a type of leukaemia called Philadelphia chromosome positive chronic myeloid
leukaemia (Ph-positive CML). CML is a cancer of the blood which makes the body produce too many
abnormal white blood cells.
Tasigna is used in patients with newly diagnosed CML or in patients with CML who are no longer
benefiting from previous treatment including imatinib. It is also used in patients who experienced
serious side effects with previous treatment and are not able to continue taking it.
How Tasigna works
In patients with CML, a change in DNA (genetic material) triggers a signal that tells the body to
produce abnormal white blood cells. Tasigna blocks this signal, and thus stops the production of these
cells.
Monitoring your Tasigna treatment
You will have regular tests, including blood tests, during treatment. These will monitor the amount of
blood cells (white blood cells, red blood cells and platelets) in your body to see how Tasigna is
tolerated.
If you have any questions about how Tasigna works or why it has been prescribed for you, ask your
doctor.
Keep this leaflet. You may need to read it again.
Follow all the doctor’s instructions carefully. They may differ from the general information contained
in this leaflet.
Do not take Tasigna
-
if you are
allergic
(hypersensitive) to nilotinib or any of the other ingredients of Tasigna listed
at the end of this leaflet.
If you think you may be allergic, tell your doctor
before taking Tasigna
.
Take special care with Tasigna
-
if you have a
heart disorder
, such as an abnormal electrical signal called “prolongation of the
QT interval”.
-
if you are being
treated with medicines
that affect the heart beat (anti-arrhythmics) or the liver
(see
Taking other medicines
).
-
if you suffer from lack of potassium or magnesium.
-
if you have been treated with a medicine of the type called anthracyclines (frequently used in
leukaemia therapy).
-
if you have a liver or pancreas disorder.
If any of these apply to you, tell your doctor.
During treatment with Tasigna
-
if you faint (loss of consciousness) or have an irregular heart beat while taking Tasigna,
tell
your doctor immediately
as this may be a sign of a serious heart condition. Prolongation of the
QT interval or an irregular heart beat may lead to sudden death. Uncommon cases of sudden
death have been reported in patients taking Tasigna.
Taking other medicines
Tasigna may interfere with some other medicines.
Tell your doctor
or pharmacist
before taking Tasigna
if you are taking or have recently taken any
other medicines, including medicines obtained without a prescription. This includes in particular:
-
antiarrhythmics – used to treat irregular heart beat;
-
chloroquine, halofantrine, clarithromycin, haloperidol, methadone, moxifloxacin - medicines
that may have an unwanted effect on the function of the heart;
-
ketoconazole, itraconazole, voriconazole, clarithromycin, telithromycin – used to treat
infections;
-
ritonavir – a medicine from the class “ antiproteases” used to treat HIV;
-
carbamazepine, phenobarbital, phenytoin – used to treat epilepsy;
-
rifampicin – used to treat tuberculosis;
-
St. John’s Wort – a herbal product used to treat depression and other conditions (also known as
Hypericum perforatum
);
midazolam – used to relieve anxiety before surgery;
warfarin – used to treat blood coagulation disorders (such as blood clots or thromboses);
astemizole, terfenadine, cisapride, pimozide, quinidine, bepridil or ergot alkaloids (ergotamine,
dihydroergotamine).
These medicines should be avoided during your treatment with Tasigna. If you are taking any of these,
your doctor might prescribe other alternative medicines.
You should also tell your doctor
if you are already taking Tasigna
and you are prescribed a new
medicine that you have not taken previously during Tasigna treatment.
Taking Tasigna with food and drink
-
Do not take Tasigna with food.
Take the capsules at least 2 hours after any food and then wait
at least 1 hour before eating again. For more information, see under “When to take Tasigna” in
section 3.
-
Do not drink grapefruit juice or eat grapefruit. It may increase the amount of Tasigna in the
blood, possibly to a harmful level.
If you are unable to swallow capsules, you may sprinkle the content of each capsule in
one
teaspoon
of apple sauce (puréed apple) and take it immediately.
Do not use more than one teaspoon of apple
sauce for each capsule and do not use any food other than apple sauce.
Older people (age 65 years and over)
Tasigna can be used by people aged 65 years and over at the same dose as for other adults.
Pregnancy and breast-feeding
-
Tasigna is not recommended during pregnancy
unless clearly necessary. If you are pregnant
or think that you may be, tell your doctor who will discuss with you whether you can take
Tasigna during your pregnancy.
-
Women who might get pregnant
are advised to use effective contraception during treatment.
-
Breast-feeding is not recommended
during treatment with Tasigna. Tell your doctor if you are
breast-feeding.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
If you experience side effects (such as dizziness or visual disorders) with a potential impact on the
ability to safely drive or use any tools or machines after taking Tasigna, you should refrain from these
activities until the effect has disappeared.
Important information about some of the ingredients of Tasigna
This medicine contains lactose (also known as milk sugar). If you have been told by your doctor that
you have an intolerance to some sugars, contact your doctor before taking this medicine.
Always take Tasigna exactly as your doctor has told you. You should check with your doctor or
pharmacist if you are not sure.
How much Tasigna to take
-
The starting dose is 800 mg per day. This dose is achieved by taking two capsules of 200 mg
twice a day.
Your doctor may prescribe a lower dose depending on how you respond to treatment.
When to take Tasigna
Take the capsules:
-
twice a day (approximately every 12 hours);
-
at least 2 hours after any food;
-
then wait 1 hour before eating again.
If you have questions about when to take Tasigna, talk to your doctor or pharmacist. Taking Tasigna at
the same time each day will help you remember when to take your capsules.
Swallow the capsules whole with water.
Do not open the capsules unless you are unable to swallow them. If so, you may sprinkle the
content of each capsule in
one
teaspoon of apple sauce and take it immediately. Do not use
more than one teaspoon of apple sauce for each capsule and do not use any food other than
apple sauce.
Do not take any food together with the capsules.
How long to take Tasigna
Continue taking Tasigna every day for as long as your doctor tells you. This is a long-term treatment.
Your doctor will regularly monitor your condition to check that the treatment is having the desired
effect.
If you have questions about how long to take Tasigna, talk to your doctor.
If you take more Tasigna than you should
If you have taken more Tasigna than you should have, or if someone else accidentally takes your
capsules, contact a doctor or hospital for advice straight away. Show them the pack of capsules and
this package leaflet. Medical treatment may be necessary.
If you forget to take Tasigna
If you miss a dose, take your next dose as scheduled. Do not take a double dose to make up for the
forgotten capsules.
If you stop taking Tasigna
Do not stop taking Tasigna unless your doctor tells you to.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Like all medicines, Tasigna can cause side effects, although not everybody gets them. Most of the side
effects are mild to moderate and will generally disappear after a few days to a few weeks of treatment.
Some side effects could be serious.
These side effects are common, uncommon or have been reported in very few patients.
-
rapid weight gain,
swelling of hands, ankles, feet or face (signs of water retention)
-
chest pain, high blood pressure, irregular heart rhythm, blue discolouration of the lips, tongue or
skin (signs of heart disorders)
-
difficulty breathing, cough, wheezing, swelling of the feet or legs (signs of lung disorders)
-
fever, easy bruising, frequent infections (signs of blood disorders)
-
weakness or paralysis of the limbs or face, difficulty speaking, severe headache, seeing, feeling
or hearing things that are not there (signs of nervous system disorders)
-
thirst, dry skin, irritability, dark urine, decreased urine output (signs of kidney disorders)
-
blurred vision, loss of vision, blood in eye (signs of eye disorders)
-
swelling and pain in one part of the body (signs of clotting within a vein)
-
abdominal pain, nausea, vomiting of blood, black stools, constipation, swollen abdomen (signs
of gastrointestinal disorders)
-
severe upper abdominal pain (sign of pancreatitis)
-
yellow skin and eyes, nausea, loss of appetite, light-coloured urine (signs of liver disorders)
-
rash, painful red lumps, pain in joints and muscles (signs of skin disorders)
-
excessive thirst, high urine output, increased appetite with weight loss, tiredness (signs of high
level of sugar in the blood)
-
fast heartbeat, bulging eyes, weight loss, swelling at the front of the neck (signs of overactive
thyroid gland)
If you get any of these,
tell your doctor straight away
.
Some side effects are very common
(may affect more than 10 in every 100 patients)
-
diarrhoea
-
headache
-
tiredness
-
muscle pain
-
itching, rash, hives
If any of these affects you severely, tell your doctor.
Some side effects are common
(may affect between 1 and 10 in every 100 patients)
-
vomiting, abdominal discomfort, stomach discomfort after meals, flatulence, swelling or
bloating of the abdomen
-
bone pain, pain in joints, muscle spasms
-
pain including back pain, neck pain and pain in extremity
-
eye irritation, swelling, discharge, itching or redness, dry eye (signs of eye disorders)
-
skin reddening, dry skin, acne, wart, decreased skin sensitivity
-
loss of appetite, weight decrease or increase
-
hair loss
-
insomnia, depression
-
night sweats, excessive sweating, hot flushes
-
dizziness, generally feeling unwell, spinning sensation
-
tingling or numbness
-
voice disorder
-
nose bleed
-
frequent urine output
-
palpitations (sensation of rapid heart beat)
If any of these affects you severely, tell your doctor.
Some side effects are uncommon
(may affect less than 1 in every 100 patients)
-
increased skin sensitivity, skin pain
-
swelling of the eyelids
-
dry mouth, sore throat, mouth sores, disturbed sense of taste
-
heartburn
-
breast pain
-
increased appetite
-
anxiety, attention disorder
-
difficulty and pain when urinating, exaggerated sense of needing to urinate
-
inability to achieve or maintain an erection
-
breast enlargement in men
-
flu-like symptoms, muscle weakness
-
trembling
-
decreased sharpness of vision
-
severe headache often accompanied by nausea, vomiting and sensitivity to light
-
visual disturbances
-
oral or vaginal thrush
-
pain or discomfort in the side of the body
-
muscle and joint stiffness
-
unconsciousness
-
weight gain, feeling cold
If any of these affects you severely, tell your doctor.
The following other side effects have been reported in very few patients treated with Tasigna:
-
confusion, disorientation, memory loss, disturbed mood, lack of energy
bacterial infection of the skin
blister, skin cyst, oily skin, thinning of the skin, dark patches of skin, skin discolouration
sensitivity of teeth, bleeding, tender or enlarged gums
runny or stuffy nose, sneezing
reddening and/or swelling and possibly peeling on the palms and soles (so called hand-foot
syndrome)
increased sensitivity of the eyes or the skin to light
eye pain or redness, pain, itching of the eyelids
difficulty hearing, ear pain, noises (ringing) in the ears
painful and swollen joints (gout)
blood in urine, abnormal urine colour, urinary incontinence
-
feeling of hardening in the breasts, heavy periods, nipple swelling
-
feeling hot
If any of these affects you severely, tell your doctor.
During Tasigna treatment, you may also have some abnormal blood test results such as low level of
blood cells (white cells, red cells, platelets), high blood level of lipase or amylase (pancreas function),
high blood level of bilirubin (liver function) or high blood level of creatinine (kidney function).
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Tasigna after the expiry date which is stated on the carton and blister. The expiry
date refers to the last day of that month.
Store in the original package in order to protect from moisture.
Do not use any pack that is damaged or shows signs of tampering.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist
how to dispose of medicines no longer required. These measures will help to protect the
environment.
The active substance is nilotinib. Each capsule contains 200 mg nilotinib (as hydrochloride
monohydrate).
The other ingredients are lactose monohydrate, crospovidone, poloxamer 188, silica colloidal
anhydrous, magnesium stearate. The capsule shell is composed of gelatin, titanium dioxide
(E171), yellow iron oxide (E172) and, shellac, red iron oxide (E172) and soya lecithin (E322)
for stamping of the imprint.
What Tasigna looks like and contents of the pack
Tasigna is supplied as hard capsules. The capsules are light yellow. A red imprint is stamped on each
capsule (“NVR/TKI”).
Tasigna is available in weekly and monthly packs:
-
The monthly pack contains 112 capsules (4 individual weekly wallets, each containing
28 capsules).
Not all packs may be marketed in your country.
Marketing Authorisation Holder
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
The weekly pack contains 28 capsules.
Manufacturer
Novartis Pharma GmbH
Roonstraße 25
D-90429 Nuremberg
Germany
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
Novartis Pharma N.V.
Tél/Tel: +32 2 246 16 11
Luxembourg/Luxemburg
Novartis Pharma GmbH
Tél/Tel: +49 911 273 0
България
Novartis Pharma Services Inc.
Тел.: +359 2 489 98 28
Magyarország
Novartis Hungária Kft. Pharma
Tel.: +36 1 457 65 00
Česká republika
Novartis s.r.o.
Tel: +420 225 775 111
Malta
Novartis Pharma Services Inc.
Tel: +356 2298 3217
Danmark
Novartis Healthcare A/S
Tlf: +45 39 16 84 00
Nederland
Novartis Pharma B.V.
Tel: +31 26 37 82 111
Deutschland
Novartis Pharma GmbH
Tel: +49 911 273 0
Norge
Novartis Norge AS
Tlf: +47 23 05 20 00
Eesti
Novartis Pharma Services Inc.
Tel: +372 66 30 810
Österreich
Novartis Pharma GmbH
Tel: +43 1 86 6570
Ελλά
Novartis (Hellas) A.E.B.E.
Τηλ: +30 210 281 17 12
Polska
Novartis Poland Sp. z o.o.
Tel.: +48 22 550 8888
España
Novartis Farmacéutica, S.A.
Tel: +34 93 306 42 00
Portugal
Novartis Farma - Produtos Farmacêuticos, S.A.
Tel: +351 21 000 8600
France
Novartis Pharma S.A.S.
Tél: +33 1 55 47 66 00
România
Novartis Pharma Services Inc.
Tel: +40 21 31299 01
Ireland
Novartis Ireland Limited
Tel: +353 1 260 12 55
Slovenija
Novartis Pharma Services Inc.
Tel: +386 1 300 75 50
Ísland
Vistor hf.
Sími: +354 535 7000
Slovenská republika
Novartis Slovakia s.r.o.
Tel: +421 2 5542 5439
Italia
Novartis Farma S.p.A.
Tel: +39 02 96 54 1
Suomi/Finland
Novartis Finland Oy
Puh/Tel: +358 (0)10 6133 200
Κύπρς
Novartis Pharma Services Inc.
Τηλ: +357 22 690 690
Sverige
Novartis Sverige AB
Tel: +46 8 732 32 00
Latvija
Novartis Pharma Services Inc.
Tel: +371 67 887 070
United Kingdom
Novartis Pharmaceuticals UK Ltd.
Tel: +44 1276 698370
Lietuva
Novartis Pharma Services Inc.
Tel: +370 5 269 16 50
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency website:
http://www.ema.europa.eu/. There are also links to other websites about rare diseases and treatments.
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/tasigna.html
Copyright © 1995-2021 ITA all rights reserved.
|




















































































































































































































































































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































