Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
1. NAME OF THE MEDICINAL PRODUCT
Temozolomide Teva 5 mg hard capsules
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each hard capsule contains 5 mg temozolomide.
Excipient: Each hard capsule contains 87 mg of anhydrous lactose.
For a full list of excipients, see section 6.1.
The hard capsules have a white opaque body and cap with two stripes in green ink on the cap and with
“T 5 mg” in green ink on the body.
4.1 Therapeutic indications
Temozolomide Teva hard capsules is indicated for the treatment of:
-
adult patients with newly-diagnosed glioblastoma multiforme concomitantly with radiotherapy
(RT) and subsequently as monotherapy treatment.
children from the age of three years, adolescents and adult patients with malignant glioma, such
as glioblastoma multiforme or anaplastic astrocytoma, showing recurrence or progression after
standard therapy.
4.2 Posology and method of administration
Temozolomide Teva hard capsules should only be prescribed by physicians experienced in the
oncological treatment of brain tumours.
Anti-emetic therapy may be administered (see section 4.4).
Adult patients with newly-diagnosed glioblastoma multiforme
Temozolomide Teva hard capsules is administered in combination with focal radiotherapy
(concomitant phase) followed by up to 6 cycles of temozolomide (TMZ) monotherapy (monotherapy
phase).
Concomitant phase
TMZ is administered orally at a dose of 75 mg/m² daily for 42 days concomitant with focal
radiotherapy (60 Gy administered in 30 fractions). No dose reductionsare recommended, but delay or
discontinuation of TMZ administration should be decided weekly according to haematological and
non-haematological toxicity criteria. TMZ administration can be continued throughout the 42 day
concomitant period (up to 49 days) if all of the following conditions are met:
-
absolute neutrophil count (ANC) ≥ 1.5 x 10
9
/l
thrombocyte count ≥ 100 x 10
9
/l
- common toxicity criteria (CTC) non-haematological toxicity ≤ Grade 1 (except for alopecia,
nausea and vomiting).
During treatment a complete blood count should be obtained weekly. TMZ administration should be
temporarily interrupted or permanently discontinued during the concomitant phase according to the
haematological and non-haematological toxicity criteria as noted in Table 1.
Table 1. TMZ dosing interruption or discontinuation during
concomitant radiotherapy and TMZ
Absolute Neutrophil Count
≥ 0.5 and < 1.5 x 10
9
/l
Thrombocyte Count
≥ 10 and < 100 x 10
9
/l
CTC Non-haematological
toxicity (except for alopecia,
nausea, vomiting)
Treatment with concomitant TMZ can be continued when all of the following conditions are met:
absolute neutrophil count ≥ 1.5 x 10
9
/l; thrombocyte count ≥ 100 x 10
9
/l; CTC non-haematological
toxicity ≤ Grade 1 (except for alopecia, nausea, vomiting).
Four weeks after completing the TMZ + RT concomitant phase, TMZ is administered for up to 6
cycles of monotherapy treatment. Dose in Cycle 1 (monotherapy) is 150 mg/m² once daily for 5 days
followed by 23 days without treatment. At the start of Cycle 2, the dose is escalated to 200 mg/m² if
the CTC nonhaematological toxicity for Cycle 1 is Grade ≤ 2 (except for alopecia, nausea and
vomiting), absolute neutrophil count (ANC) is ≥ 1.5 x 10
9
/l, and the thrombocyte count is
≥ 100 x 10
9
/l. If the dose was not escalated at Cycle 2, escalation should not be done in subsequent
cycles. Once escalated, the dose remains at 200 mg/m² per day for the first 5 days of each subsequent
cycle except if toxicity occurs. Dose reductions and discontinuations during the monotherapy phase
should be applied according to
Tables 2 and 3
.
During treatment a complete blood count should be obtained on Day 22 (21 days after the first dose of
TMZ). The dose should be reduced or administration discontinued according to
Table 3
.
Table 2. TMZ dose levels for monotherapy treatment
Reduction for prior toxicity
Dose during Cycles 2-6 in absence of toxicity
Table 3. TMZ dose reduction or discontinuation during monothera
py treatment
Toxicity
Reduce TMZ by 1 dose level
a
Absolute Neutrophil Count
CTC Non-haematological Toxicity
(except for alopecia, nausea, vomiting)
a: TMZ dose levels are listed in Table 2.
b: TMZ is to be discontinued if:
•
dose level -1 (100 mg/m²) still results in unacceptable toxicity
the same Grade 3 non-haematological toxicity (except for alopecia, nausea, vomiting) recurs
after dose reduction.
Adult and paediatric patients 3 years of age or older with recurrent or progressive malignant glioma
A treatment cycle comprises 28 days. In patients previously untreated with chemotherapy, TMZ is
administered orally at a dose of 200 mg/m² once daily for the first 5 days followed by a 23 day

































treatment interruption (total of 28 days). In patients previously treated with chemotherapy, the initial
dose is 150 mg/m² once daily, to be increased in the second cycle to 200 mg/m² once daily, for 5 days
if there is no haematological toxicity (see section 4.4)
In patients 3 years of age or older, TMZ is only to be used in recurrent or progressive malignant
glioma. There is no clinical experience with use of TMZ in children under the age of 3 years.
Experience in older children is very limited (see sections 4.4 and 5.1).
Patients with hepatic or renal impairment
The pharmacokinetics of TMZ were comparable in patients with normal hepatic function and in those
with mild or moderate hepatic impairment. No data are available on the administration of TMZ in
patients with severe hepatic impairment (Child’s Class C) or with renal impairment. Based on the
pharmacokinetic properties of TMZ, it is unlikely that dose reductions are required in patients with
severe hepatic impairment or any degree of renal impairment. However, caution should be exercised
when TMZ is administered in these patients.
Based on a population pharmacokinetic analysis in patients 19-78 years of age, clearance of TMZ is
not affected by age. However, elderly patients (> 70 years of age) appear to be at increased risk of
neutropenia and thrombocytopenia (see section 4.4).
Temozolomide Teva hard capsules should be administered in the fasting state.
The capsules must be swallowed whole with a glass of water and must not be opened or chewed.
If vomiting occurs after the dose is administered, a second dose should not be administered that day.
Hypersensitivity to the active substance or to any of the excipients.
Hypersensitivity to dacarbazine (DTIC).
Severe myelosuppression (see section 4.4).
4.4 Special warnings and precautions for use
Pneumocystis carinii pneumonia
Patients who received concomitant TMZ and RT in a pilot trial for the prolonged 42-day schedule
were shown to be at particular risk for developing
Pneumocystis carinii
pneumonia (PCP). Thus,
prophylaxis against PCP is required for all patients receiving concomitant TMZ and RT for the 42-day
regimen (with a maximum of 49 days) regardless of lymphocyte count. If lymphopenia occurs, they
are to continue the prophylaxis until recovery of lymphopenia to grade ≤ 1.
There may be a higher occurrence of PCP when TMZ is administered during a longer dosing regimen.
However, all patients receiving TMZ, particularly patients receiving steroids, should be observed
closely for the development of PCP, regardless of the regimen.
Cases of myelodysplastic syndrome and secondary malignancies, including myeloid leukaemia, have
also been reported very rarely (see section 4.8).
Nausea and vomiting are very commonly associated with TMZ.
Anti-emetic therapy may be administered prior to or following administration of TMZ.
Adult patients with newly-diagnosed glioblastoma multiforme
Anti-emetic prophylaxis is recommended prior to the initial dose of concomitant phase and it is
strongly recommended during the monotherapy phase.
Patients with recurrent or progressive malignant glioma
Patients who have experienced severe (Grade 3 or 4) vomiting in previous treatment cycles may
require anti-emetic therapy.
Prior to dosing, the following laboratory parameters must be met: ANC ≥ 1.5 x 10
9
/l and platelet count
≥ 100 x 10
9
/l. A complete blood count should be obtained on Day 22 (21 days after the first dose) or
within 48 hours of that day, and weekly until ANC > 1.5 x 10
9
/l and platelet count > 100 x10
9
/l. If
ANC falls to < 1.0 x 10
9
/l or the platelet count is < 50 x 10
9
/l during any cycle, the next cycle should
be reduced one dose level (see section 4.2). Dose levels include 100 mg/m², 150 mg/m², and
200 mg/m². The lowest recommended dose is 100 mg/m².
There is no clinical experience with use of TMZ in children under the age of 3 years. Experience in
older children and adolescents is very limited (see sections 4.2 and 5.1).
Elderly patients (> 70 years of age)
Elderly patients appear to be at increased risk of neutropenia and thrombocytopenia, compared with
younger patients. Therefore, special care should be taken when TMZ is administered in elderly
patients.
Men being treated with TMZ should be advised not to father a child up to 6 months after receiving the
last dose and to seek advice on cryoconservation of sperm prior to treatment (see section 4.6).
This medicinal product contains lactose. Patients with rare hereditary problems of galactose
intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this
medicine.
4.5 Interaction with other medicinal products and other forms of interaction
Interaction studies have only been performed in adults.
In a separate phase I study, administration of TMZ with ranitidine did not result in alterations in the
extent of absorption of temozolomide or the exposure to its active metabolite monomethyl
triazenoimidazole carboxamide (MTIC).
Administration of TMZ with food resulted in a 33% decrease in C
max
and a 9% decrease in area under
the curve (AUC).
As it cannot be excluded that the change in C
max
is clinically significant, Temozolomide Teva should
be administered without food.
Based on an analysis of population pharmacokinetics in phase II trials, co-administration of
dexamethasone, prochlorperazine, phenytoin, carbamazepine, ondansetron, H
2
receptor antagonists, or
phenobarbital did not alter the clearance of TMZ. Co-administration with valproic acid was associated
with a small but statistically significant decrease in clearance of TMZ.
No studies have been conducted to determine the effect of TMZ on the metabolism or elimination of
other medicinal products. However, since TMZ does not undergo hepatic metabolism and exhibits low
protein binding, it is unlikely that it would affect the pharmacokinetics of other medicinal products
(see section 5.2).
Use of TMZ in combination with other myelosuppressive agents may increase the likelihood of
myelosuppression.
4.6 Pregnancy and lactation
There are no data in pregnant women. In preclinical studies in rats and rabbits receiving 150 mg/m²
TMZ, teratogenicity and/or foetal toxicity were demonstrated (see section 5.3). Temozolomide Teva
hard capsules should not be administered to pregnant women. If use during pregnancy must be
considered, the patient should be apprised of the potential risk to the foetus. Women of childbearing
potential should be advised to use effective contraception to avoid pregnancy while they are receiving
TMZ.
It is not known whether TMZ is excreted in human milk; thus, breast-feeding should be discontinued
while receiving treatment with TMZ.
TMZ can have genotoxic effects. Therefore, men being treated with it should be advised not to father a
child up to 6 months after receiving the last dose and to seek advice on cryoconservation of sperm
prior to treatment, because of the possibility of irreversible infertility due to therapy with TMZ.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. The ability to
drive and use machines may be impaired in patients treated with TMZ due to fatigue and somnolence.
Clinical trial experience
In patients treated with TMZ, whether used in combination with RT or as monotherapy following RT
for newly-diagnosed glioblastoma multiforme, or as monotherapy in patients with recurrent or
progressive glioma, the reported very common adverse reactions were similar: nausea, vomiting,
constipation, anorexia, headache and fatigue. Convulsions were reported very commonly in the newly-
diagnosed glioblastoma multiforme patients receiving monotherapy, and rash was reported very
commonly in newly-diagnosed glioblastoma multiforme patients receiving TMZ concurrent with RT
and also as monotherapy, and commonly in recurrent glioma. Most haematologic adverse reactions
were reported commonly or very commonly in both indications (Tables 4 and 5); the frequency of
grade 3-4 laboratory findings is presented after each table.
In the tables undesirable effects are classified according to System Organ Class and frequency.
Frequency groupings are defined according to the following convention: Very common (≥ 1/10);
Common (≥ 1/100 to < 1/10); Uncommon (≥ 1/1,000 to < 1/100). Within each frequency grouping,
undesirable effects are presented in order of decreasing seriousness.
Newly-diagnosed glioblastoma multiforme
Table 4 provides treatment-emergent adverse events in patients with newly-diagnosed glioblastoma
multiforme during the concomitant and monotherapy phases of treatment.
Table 4: Treatment-emergent events during concomitant and monotherapy treatment
phases in patients with newly-diagnosed glioblastoma multiforme
TMZ + concomitant RT
n=288*
Infections and infestations
Common:
Infection
, Herpes simplex
, wound
infection, pharyngitis, candidiasis
oral
Infection, candidiasis oral
Herpes simplex
, Herpes zoster,
influenza–like symptoms
Blood and lymphatic system disorders
Common:
Neutropenia, thrombocytopenia,
lymphopenia, leukopenia
Febril neutropenia,
thrombocytopenia, anaemia,
leukopenia
Febrile neutropenia, anaemia
Endocrine disorders
Uncommon:
Metabolism and nutrition disorders
Very Common:
Hyperglycaemia, weight decreased
Hypokalemia, alkaline phosphatase
increased, weight increased
Hyperglycaemia, weight
increased
Psychiatric disorders
Common:
Anxiety, emotional lability, insomnia
Anxiety, depression, emotional
lability, insomnia
Agitation, apathy, behaviour
disorder, depression, hallucination
Nervous system disorders
Very Common:
Convulsions, consciousness
decreased, somnolence, aphasia,
balance impaired, dizziness,
confusion, memory impairment,
Hemiparesis, aphasia, balance
impaired, somnolence,
confusion, dizziness, memory
impairment, concentration
























concentration impaired, neuropathy,
paresthesia, speech disorder,
tremor
impaired, dysphasia,
neurological disorder (NOS),
neuropathy, peripheral
neuropathy, paresthesia, speech
disorder, tremor
Status epilepticus, extrapyramidal
disorder, hemiparesis, ataxia,
cognition impaired, dysphasia, gait
abnormal, hyperesthesia,
hypoesthesia, neurological disorder
(NOS), peripheral neuropathy
Hemiplegia, ataxia, coordination
abnormal, gait abnormal,
hyperesthesia, sensory
disturbance
Visual field defect, vision
blurred, diplopia
Hemianopia, visual acuity reduced,
vision disorder, visual field defect,
eye pain
Visual acuity reduced, eye pain,
eyes dry
Ear and labyrinth disorders
Common:
Hearing impairment, tinnitus
Otitis media, tinnitus, hyperacusis,
earache
Deafness, vertigo, earache
Cardiac disorders
Uncommon:
Vascular disorders
Common:
Haemorrhage, oedema, oedema leg
Haemorrhage, deep venous
thrombosis, oedema leg
Cerebral haemorrhage, hypertension
Embolism pulmonary, oedema,
oedema peripheral
Respiratory, thoracic and mediastinal disorders
Common:
Pneumonia, upper respiratory
infection, nasal congestion
Pneumonia, sinusitis, upper
respiratory infection, bronchitis
Gastrointestinal disorders
Very Common:
Constipation, nausea, vomiting
Constipation, nausea, vomiting
Stomatitis, diarrhoea, abdominal
pain, dyspepsia, dysphagia
Stomatitis, diarrhoea, dyspepsia,
dysphagia, mouth dry
Abdominal distension, fecal
incontinence, gastrointestinal
disorder (NOS), gastroenteritis,
haemorrhoids
Skin and subcutaneous tissue disorders
Very Common:
Dermatitis, dry skin, erythema,
pruritus
Skin exfoliation, photosensitivity
reaction, pigmentation abnormal
Erythema, pigmentation
abnormal, sweating increased
Musculoskeletal and connective tissue disorders





























Muscle weakness, arthralgia
Muscle weakness, arthralgia,
musculoskeletal pain, myalgia
Myopathy, back pain,
musculoskeletal pain, myalgia
Renal and urinary disorders
Common:
Micturition frequency, urinary
incontinence
Reproductive system and breast disorders
Uncommon:
Vaginal haemorrhage,
menorrhagia, amenorrhea,
vaginitis, breast pain
General disorders and administration site conditions
Very Common:
Allergic reaction, fever, radiation
injury, face oedema, pain, taste
perversion
Allergic reaction, fever,
radiation injury, pain, taste
perversion
Asthenia, flushing, hot flushes,
condition aggravated, rigors, tongue
discolouration, parosmia, thirst
Asthenia, face oedema, pain,
condition aggravated, rigors,
tooth disorder, taste perversion
Hepatic enzymes increased, Gamma
GT increased, AST increased
*A patient who was randomised to the RT arm only, received TMZ + RT.
Myelosuppression (neutropenia and thrombocytopenia), which is known dose-limiting toxicity for
most cytotoxic agents, including TMZ, was observed. When laboratory abnormalities and adverse
events were combined across concomitant and monotherapy treatment phases, Grade 3 or Grade 4
neutrophil abnormalities including neutropenic events were observed in 8% of the patients. Grade 3 or
Grade 4 thrombocyte abnormalities, including thrombocytopenic events were observed in 14% of the
patients who received TMZ.
Recurrent or progressive malignant glioma
In clinical trials, the most frequently occurring treatment-related undesirable effects were
gastrointestinal disorders, specifically nausea (43%) and vomiting (36%). These reactions were
usually Grade 1 or 2 (0 – 5 episodes of vomiting in 24 hours) and were either self-limiting or readily
controlled with standard anti-emetic therapy. The incidence of severe nausea and vomiting was 4%.
Table 5 includes adverse reactions reported during clinical trials for recurrent or progressive malignant
glioma and following the marketing of TMZ.
Table 5. Adverse reactions in patients with recurrent or progressive malignant glioma
Infections and infestations
Rare:
Opportunistic infections, including PCP
Blood and lymphatic system disorders
Very common:
Neutropenia or lymphopenia (grade 3-4),
thrombocytopenia (grade 3-4)



























Pancytopenia, anaemia (grade 3-4), leukopenia
Metabolism and nutrition disorders
Very common:
Nervous system disorders
Very common:
Somnolence, dizziness, paresthesia
Respiratory, thoracic and mediastinal disorders
Common:
Gastrointestinal disorders
Very common:
Vomiting, nausea, constipation
Diarrhoea, abdominal pain, dyspepsia
Skin and subcutaneous tissue disorders
Common:
Erythema multiforme, erythroderma, urticaria,
exanthema
General disorders and administration site conditions
Very common:
Fever, asthenia, rigors, malaise, pain, taste
perversion
Allergic reactions, including anaphylaxis,
angioedema
Grade 3 or 4 thrombocytopenia and neutropenia occurred in 19% and 17% respectively, of patients
treated for malignant glioma. This led to hospitalisation and/or discontinuation of TMZ in 8% and 4%,
respectively. Myelosuppression was predictable (usually within the first few cycles, with the nadir
between Day 21 and Day 28), and recovery was rapid, usually within 1-2 weeks. No evidence of
cumulative myelosuppression was observed. The presence of thrombocytopenia may increase the risk
of bleeding, and the presence of neutropenia or leukopenia may increase the risk of infection.
In a population pharmacokinetics analysis of clinical trial experience there were 101 female and 169
male subjects for whom nadir neutrophil counts were available and 110 female and 174 male subjects
for whom nadir platelet counts were available. There were higher rates of Grade 4 neutropenia (ANC
< 0.5 x 10
9
/l), 12%
vs
5%, and thrombocytopenia (< 20 x 10
9
/l), 9%
vs
3%, in women
vs
men in the
first cycle of therapy. In a 400 subject recurrent glioma data set, Grade 4 neutropenia occurred in 8%
of female
vs
4% of male subjects and Grade 4 thrombocytopenia in 8% of female
vs
3% of male
subjects in the first cycle of therapy. In a study of 288 subjects with newly-diagnosed glioblastoma
multiforme, Grade 4 neutropenia occurred in 3% of female
vs
0% of male subjects and Grade 4
thrombocytopenia in 1% of female
vs
0% of male subjects in the first cycle of therapy.
Post-marketing experience
Antineoplastic agents, and notably alkylating agents, have been associated with a potential risk of
myelodysplastic syndrome (MDS) and secondary malignancies, including leukaemia. Very rare cases
of MDS and secondary malignancies, including myeloid leukaemia have been reported in patients
treated with regimens that included TMZ. Prolonged pancytopenia, which may result in aplastic
anaemia has been reported very rarely
.
Cases of toxic epidermal necrolysis and Stevens-Johnson syndrome have been reported very rarely.
Cases of interstitial pneumonitis/pneumonitis have been reported very rarely.






















Doses of 500, 750, 1,000, and 1,250 mg/m² (total dose per cycle over 5 days) have been evaluated
clinically in patients. Dose-limiting toxicity was haematological and was reported with any dose but is
expected to be more severe at higher doses. An overdose of 10,000 mg (total dose in a single cycle,
over 5 days) was taken by one patient and the adverse reactions reported were pancytopenia, pyrexia,
multiorgan failure and death. There are reports of patients who have taken the recommended dose for
more than 5 days of treatment (up to 64 days) with adverse events reported including bone marrow
suppression, with or without infection, in some cases severe and prolonged and resulting in death. In
the event of an overdose, haematological evaluation is needed. Supportive measures should be
provided as necessary.
5. PHARMACOLOGICAL PROPERTIES
5.1
Pharmacodynamic properties
Pharmacotherapeutic group: Other alkylating agents, ATC code: L01A X03
Temozolomide is a triazene, which undergoes rapid chemical conversion at physiologic pH to the
active monomethyl triazenoimidazole carboxamide (MTIC). The cytotoxicity of MTIC is thought to
be due primarily to alkylation at the O6 position of guanine with additional alkylation also occurring at
the N7 position. Cytotoxic lesions that develop subsequently are thought to involve aberrant repair of
the methyl adduct.
Newly-diagnosed glioblastoma multiforme
A total of 573 patients were randomised to receive either TMZ + RT (n=287) or RT alone (n=286).
Patients in the TMZ + RT arm received concomitant TMZ (75 mg/m²) once daily, starting the first day
of RT until the last day of RT, for 42 days (with a maximum of 49 days). This was followed by
monotherapy TMZ (150 - 200 mg/m²) on Days 1 - 5 of every 28-day cycle for up to 6 cycles, starting
4 weeks after the end of RT. Patients in the control arm received RT only.
Pneumocystis carinii
pneumonia (PCP) prophylaxis was required during RT and combined TMZ therapy.
TMZ was administered as salvage therapy in the follow-up phase in 161 patients of the 282 (57%) in
the RT alone arm, and 62 patients of the 277 (22%) in the TMZ + RT arm.
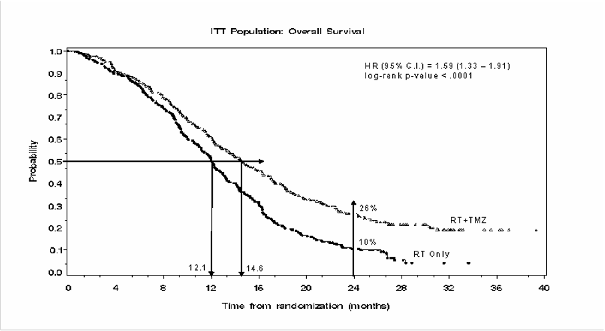
The hazard ratio (HR) for overall survival was 1.59 (95% CI for HR=1.33 -1.91) with a log-rank
p < 0.0001 in favour of the TMZ arm. The estimated probability of surviving 2 years or more (26%
vs
10%) is higher for the RT + TMZ arm. The addition of concomitant TMZ to RT, followed by TMZ
monotherapy in the treatment of patients with newly-diagnosed glioblastoma multiforme demonstrated
a statistically significant improvement in overall survival (OS) compared with RT alone
(Figure 1).

Figure 1 Kaplan-Meier curves for overall survival (intent-to-treat population)
The results from the trial were not consistent in the subgroup of patients with a poor performance
status (WHO PS=2, n=70), where overall survival and time to progression were similar in both arms.
However, no unacceptable risks appear to be present in this patient group.
Recurrent or progressive malignant glioma
Data on clinical efficacy in patients with glioblastoma multiforme (Karnofsky performance status
[KPS] ≥ 70), progressive or recurrent after surgery and RT, were based on two clinical trials with oral
TMZ. One was a non-comparative trial in 138 patients (29% received prior chemotherapy), and the
other was a randomised active-controlled trial of TMZ
vs
procarbazine in a total of 225 patients (67%
received prior treatment with nitrosourea based chemotherapy). In both trials, the primary endpoint
was progression-free survival (PFS) defined by MRI scans or neurological worsening. In the
noncomparative trial, the PFS at 6 months was 19%, the median progression-free survival was 2.1
months, and the median overall survival 5.4 months. The objective response rate (ORR) based on MRI
scans was 8%.
In the randomised active-controlled trial, the PFS at 6 months was significantly greater for TMZ than
for procarbazine (21%
vs
8%, respectively – chi-square p = 0.008) with median PFS of 2.89 and 1.88
months respectively (log rank p = 0.0063). The median survival was 7.34 and 5.66 months for TMZ
and procarbazine, respectively (log rank p = 0.33). At 6 months, the fraction of surviving patients was
significantly higher in the TMZ arm (60 %) compared with the procarbazine arm (44%) (chi-square
p = 0.019). In patients with prior chemotherapy a benefit was indicated in those with a KPS ≥ 80.
Data on time to worsening of neurological status favoured TMZ over procarbazine as did data on time
to worsening of performance status (decrease to a KPS of < 70 or a decrease by at least 30 points).
Themedian times to progression in these endpoints ranged from 0.7 to 2.1 months longer for TMZ
than for procarbazine (log rank p = < 0.01 to 0.03).
Recurrent anaplastic astrocytoma
In a multicentre, prospective phase II trial evaluating the safety and efficacy of oral TMZ in the
treatment of patients with anaplastic astrocytoma at first relapse, the 6 month PFS was 46%. The
median PFS was 5.4 months. Median overall survival was 14.6 months. Response rate, based on the
central reviewer assessment, was 35% (13 CR and 43 PR) for the intent-to-treat population (ITT)
n=162. In 43 patients stable disease was reported. The 6-month event-free survival for the ITT
population was 44% with a median event-free survival of 4.6 months, which was similar to the results
for the progression-free survival. For the eligible histology population, the efficacy results were
similar. Achieving a radiological objective response or maintaining progression-free status was
strongly associated with maintained or improved quality of life.
Oral TMZ has been studied in paediatric patients (age 3-18 years) with recurrent brainstem glioma or
recurrent high grade astrocytoma, in a regimen administered daily for 5 days every 28 days. Tolerance
to TMZ is similar to adults.
5.2
Pharmacokinetic properties
TMZ is spontaneously hydrolyzed at physiologic pH primarily to the active species, 3-methyl-(triazen-
1-yl)imidazole-4-carboxamide (MTIC). MTIC is spontaneously hydrolyzed to 5-amino-imidazole-4-
carboxamide (AIC), a known intermediate in purine and nucleic acid biosynthesis, and to
methylhydrazine, which is believed to be the active alkylating species. The cytotoxicity of MTIC is
thought to be primarily due to alkylation of DNA mainly at the O
6
and N
7
positions of guanine.
Relative to the AUC of TMZ, the exposure to MTIC and AIC is ~ 2.4% and 23%, respectively.
In
vivo
, the t
1/2
of MTIC was similar to that of TMZ, 1.8 hr.
After oral administration to adult patients, TMZ is absorbed rapidely, with peak concentrations
reached as early as 20 minutes post-administration (mean time between 0.5 and 1.5 hours). After oral
administration of
14
C-labelled TMZ, mean faecal excretion of
14
C over 7 days post-dose was 0.8%
indicationg complete absorption.
TMZ demonstrates low protein binding (10% to 20%), and thus it is not expected to interact with
highly protein-bound substances.
PET studies in humans and preclinical data suggest that TMZ crosses the blood-brain barrier rapidly
and is present in the CSF. CSF penetration was confirmed in one patient; CSF exposure based on AUC
of TMZ was approximately 30% of that in plasma, which is consistent with animal data.
The half-life (t
1/2
) in plasma is approximately 1.8 hours. The major route of
14
C elimination is renal.
Following oral administration, approximately 5% to 10% of the dose is recovered unchanged in the
urine over 24 hours, and the remainder excreted as temozolomide acid, 5-aminoimidazole-4-
carboxamide (AIC) or unidentified polar metabolites.
Plasma concentrations increase in a dose-related manner. Plasma clearance, volume of distribution and
half-life are independent of dose.
Analysis of population-based pharmacokinetics of TMZ revealed that plasma TMZ clearance was
independent of age, renal function or tobacco use. In a separate pharmacokinetic study, plasma
pharmacokinetic profiles in patients with mild to moderate hepatic impairment were similar to those
observed in patients with normal hepatic function.
Paediatric patients had a higher AUC than adult patients; however, the maximum tolerated dose
(MTD) was 1,000 mg/m² per cycle both in children and in adults.
5.3
Preclinical safety data
Single-cycle (5-day dosing, 23 days non-treatment), 3- and 6-cycle toxicity studies were conducted in
rats and dogs. The primary targets of toxicity included the bone marrow, lymphoreticular system,
testes, the gastrointestinal tract and, at higher doses, which were lethal to 60% to 100% of rats and
dogs tested, degeneration of the retina occurred. Most of the toxicity showed evidence of reversibility,
except for adverse events on the male reproductive system and retinal degeneration. However, because
the doses implicated in retinal degeneration were in the lethal dose range, and no comparable effect
has been observed in clinical studies, this finding was not considered to have clinical relevance.
TMZ is an embryotoxic, teratogenic and genotoxic alkylating agent. TMZ is more toxic to the rat and
dog than to humans, and the clinical dose approximates the minimum lethal dose in rats and dogs.
Doserelated reductions in leukocytes and platelets appear to be sensitive indicators of toxicity. A
variety of neoplasms, including mammary carcinomas, keratocanthoma of the skin and basal cell
adenoma were observed in the 6-cycle rat study while no tumours or pre-neoplastic changes were
evident in dog studies. Rats appear to be particularly sensitive to oncogenic effects of TMZ, with the
occurrence of first tumours within 3 months of initiating dosing. This latency period is very short even
for an alkylating agent.
Results of the Ames/salmonella and Human Peripheral Blood Lymphocyte (HPBL) chromosome
aberration tests showed a positive mutagenicity response.
PHARMACEUTICAL PARTICULARS
Capsule contents
Anhydrous lactose
Sodium starch glycolate Type A
Colloidal anhydrous silica
Tartaric acid
Stearic acid
Capsule shell
Gelatin
Titanium dioxide (E171)
Printing ink
Shellac
Propylene Glycol
Titanium dioxide (E171)
Yellow iron oxide (E172)
Indigo carmine (E132) aluminium lake
6.4 Special precautions for storage
Store in the original package.
Keep the bottle tightly closed in order to protect from moisture.
6.5
Nature and contents of container
Amber glass bottle with white polypropylene child-resistant screw cap equipped with an induction seal
of polyethylene
containing 5 or 20 capsules.
Not all pack sizes may be marketed.
6.6
Special precautions for disposal and other handling
Capsules should not be opened. If a capsule becomes damaged, contact of the powder contents with
skin or mucous membrane must be avoided. If Temozolomide Teva comes into contact with skin or
mucosa, it should be washed immediately and thoroughly with soap and water.
Patients should be advised to keep capsules out of the reach and sight of children, preferably in a
locked cupboard. Accidental ingestion can be lethal for children.
Any unused product or waste material should be disposed of in accordance with local requirements.
7. MARKETING AUTHORISATION HOLDER
Teva Pharma B.V.
Computerweg 10
3542 DR Utrecht
The Netherlands
MARKETING AUTHORISATION NUMBER(S)
EU/1/09/606/001
EU/1/09/606/002
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
Agency http://www.ema.europa.eu/
1. NAME OF THE MEDICINAL PRODUCT
Temozolomide Teva 20 mg hard capsules
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each hard capsule contains 20 mg temozolomide.
Excipient: Each hard capsule contains 72 mg of anhydrous lactose and sunset yellow FCF (E110).
For a full list of excipients, see section 6.1.
The hard capsules have a white opaque body and cap with two stripes in orange ink on the cap and
with “T 20 mg” in orange ink on the body.
4.1 Therapeutic indications
Temozolomide Teva hard capsules is indicated for the treatment of:
-
adult patients with newly-diagnosed glioblastoma multiforme concomitantly with radiotherapy
(RT) and subsequently as monotherapy treatment.
children from the age of three years, adolescents and adult patients with malignant glioma, such
as glioblastoma multiforme or anaplastic astrocytoma, showing recurrence or progression after
standard therapy.
4.2 Posology and method of administration
Temozolomide Teva hard capsules should only be prescribed by physicians experienced in the
oncological treatment of brain tumours.
Anti-emetic therapy may be administered (see section 4.4).
Adult patients with newly-diagnosed glioblastoma multiforme
Temozolomide Teva hard capsules is administered in combination with focal radiotherapy
(concomitant phase) followed by up to 6 cycles of temozolomide (TMZ) monotherapy (monotherapy
phase).
Concomitant phase
TMZ is administered orally at a dose of 75 mg/m² daily for 42 days concomitant with focal
radiotherapy (60 Gy administered in 30 fractions). No dose reductionsare recommended, but delay or
discontinuation of TMZ administration should be decided weekly according to haematological and
non-haematological toxicity criteria. TMZ administration can be continued throughout the 42 day
concomitant period (up to 49 days) if all of the following conditions are met:
-
absolute neutrophil count (ANC) ≥ 1.5 x 10
9
/l
thrombocyte count ≥ 100 x 10
9
/l
- common toxicity criteria (CTC) non-haematological toxicity ≤ Grade 1 (except for alopecia,
nausea and vomiting).
During treatment a complete blood count should be obtained weekly. TMZ administration should be
temporarily interrupted or permanently discontinued during the concomitant phase according to the
haematological and non-haematological toxicity criteria as noted in Table 1.
Table 1. TMZ dosing interruption or discontinuation during
concomitant radiotherapy and TMZ
Absolute Neutrophil Count
≥ 0.5 and < 1.5 x 10
9
/l
Thrombocyte Count
≥ 10 and < 100 x 10
9
/l
CTC Non-haematological
toxicity (except for alopecia,
nausea, vomiting)
absolute neutrophil count ≥ 1.5 x 10
9
/l; thrombocyte count ≥ 100 x 10
9
/l; CTC non-haematological
toxicity ≤ Grade 1 (except for alopecia, nausea, vomiting).
Four weeks after completing the TMZ + RT concomitant phase, TMZ is administered for up to 6
cycles of monotherapy treatment. Dose in Cycle 1 (monotherapy) is 150 mg/m² once daily for 5 days
followed by 23 days without treatment. At the start of Cycle 2, the dose is escalated to 200 mg/m² if
the CTC nonhaematological toxicity for Cycle 1 is Grade ≤ 2 (except for alopecia, nausea and
vomiting), absolute neutrophil count (ANC) is ≥ 1.5 x 10
9
/l, and the thrombocyte count is
≥ 100 x 10
9
/l. If the dose was not escalated at Cycle 2, escalation should not be done in subsequent
cycles. Once escalated, the dose remains at 200 mg/m² per day for the first 5 days of each subsequent
cycle except if toxicity occurs. Dose reductions and discontinuations during the monotherapy phase
should be applied according to Tables 2 and 3.
During treatment a complete blood count should be obtained on Day 22 (21 days after the first dose of
TMZ). The dose should be reduced or administration discontinued according to Table 3.
Table 2. TMZ dose levels for monotherapy treatment
Reduction for prior toxicity
Dose during Cycles 2-6 in absence of toxicity
Table 3. TMZ dose reduction or discontinuation during monothera
py treatment
Toxicity
Reduce TMZ by 1 dose level
a
Absolute Neutrophil Count
CTC Non-haematological Toxicity
(except for alopecia, nausea, vomiting)
a: TMZ dose levels are listed in Table 2.
b: TMZ is to be discontinued if:
•
the same Grade 3 non-haematological toxicity (except for alopecia, nausea, vomiting) recurs
after dose reduction.
Adult and paediatric patients 3 years of age or older with recurrent or progressive malignant glioma
Treatment with concomitant TMZ can be continued when all of the following conditions are met:
dose level -1 (100 mg/m²) still results in unacceptable toxicity

































A treatment cycle comprises 28 days. In patients previously untreated with chemotherapy, TMZ is
administered orally at a dose of 200 mg/m² once daily for the first 5 days followed by a 23 day
treatment interruption (total of 28 days). In patients previously treated with chemotherapy, the initial
dose is 150 mg/m² once daily, to be increased in the second cycle to 200 mg/m² once daily, for 5 days
if there is no haematological toxicity (see section 4.4)
In patients 3 years of age or older, TMZ is only to be used in recurrent or progressive malignant
glioma. There is no clinical experience with use of TMZ in children under the age of 3 years.
Experience in older children is very limited (see sections 4.4 and 5.1).
Patients with hepatic or renal impairment
The pharmacokinetics of TMZ were comparable in patients with normal hepatic function and in those
with mild or moderate hepatic impairment. No data are available on the administration of TMZ in
patients with severe hepatic impairment (Child’s Class C) or with renal impairment. Based on the
pharmacokinetic properties of TMZ, it is unlikely that dose reductions are required in patients with
severe hepatic impairment or any degree of renal impairment. However, caution should be exercised
when TMZ is administered in these patients.
Based on a population pharmacokinetic analysis in patients 19-78 years of age, clearance of TMZ is
not affected by age. However, elderly patients (> 70 years of age) appear to be at increased risk of
neutropenia and thrombocytopenia (see section 4.4).
Temozolomide Teva hard capsules should be administered in the fasting state.
The capsules must be swallowed whole with a glass of water and must not be opened or chewed.
If vomiting occurs after the dose is administered, a second dose should not be administered that day.
Hypersensitivity to the active substance or to any of the excipients.
Hypersensitivity to dacarbazine (DTIC).
Severe myelosuppression (see section 4.4).
4.4 Special warnings and precautions for use
Pneumocystis carinii pneumonia
Patients who received concomitant TMZ and RT in a pilot trial for the prolonged 42-day schedule
were shown to be at particular risk for developing
Pneumocystis carinii
pneumonia (PCP). Thus,
prophylaxis against PCP is required for all patients receiving concomitant TMZ and RT for the 42-day
regimen (with a maximum of 49 days) regardless of lymphocyte count. If lymphopenia occurs, they
are to continue the prophylaxis until recovery of lymphopenia to grade ≤ 1.
There may be a higher occurrence of PCP when TMZ is administered during a longer dosing regimen.
However, all patients receiving TMZ, particularly patients receiving steroids, should be observed
closely for the development of PCP, regardless of the regimen.
Cases of myelodysplastic syndrome and secondary malignancies, including myeloid leukaemia, have
also been reported very rarely (see section 4.8).
Nausea and vomiting are very commonly associated with TMZ.
Anti-emetic therapy may be administered prior to or following administration of TMZ.
Adult patients with newly-diagnosed glioblastoma multiforme
Anti-emetic prophylaxis is recommended prior to the initial dose of concomitant phase and it is
strongly recommended during the monotherapy phase.
Patients with recurrent or progressive malignant glioma
Patients who have experienced severe (Grade 3 or 4) vomiting in previous treatment cycles may
require anti-emetic therapy.
Prior to dosing, the following laboratory parameters must be met: ANC ≥ 1.5 x 10
9
/l and platelet count
≥ 100 x 10
9
/l. A complete blood count should be obtained on Day 22 (21 days after the first dose) or
within 48 hours of that day, and weekly until ANC > 1.5 x 10
9
/l and platelet count > 100 x 10
9
/l. If
ANC falls to < 1.0 x 10
9
/l or the platelet count is < 50 x 10
9
/l during any cycle, the next cycle should
be reduced one dose level (see section 4.2). Dose levels include 100 mg/m², 150 mg/m², and
200 mg/m². The lowest recommended dose is 100 mg/m².
There is no clinical experience with use of TMZ in children under the age of 3 years. Experience in
older children and adolescents is very limited (see sections 4.2 and 5.1).
Elderly patients (> 70 years of age)
Elderly patients appear to be at increased risk of neutropenia and thrombocytopenia, compared with
younger patients. Therefore, special care should be taken when TMZ is administered in elderly
patients.
Men being treated with TMZ should be advised not to father a child up to 6 months after receiving the
last dose and to seek advice on cryoconservation of sperm prior to treatment (see section 4.6).
This medicinal product contains lactose. Patients with rare hereditary problems of galactose
intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this
medicine.
The excipient sunset yellow FCF (E110) included in the capsules shell may cause allergic reactions.
4.5 Interaction with other medicinal products and other forms of interaction
Interaction studies have only been performed in adults.
In a separate phase I study, administration of TMZ with ranitidine did not result in alterations in the
extent of absorption of temozolomide or the exposure to its active metabolite monomethyl
triazenoimidazole carboxamide (MTIC).
Administration of TMZ with food resulted in a 33% decrease in C
max
and a 9% decrease in area under
the curve (AUC).
As it cannot be excluded that the change in C
max
is clinically significant, Temozolomide Teva should
be administered without food.
Based on an analysis of population pharmacokinetics in phase II trials, co-administration of
dexamethasone, prochlorperazine, phenytoin, carbamazepine, ondansetron, H2 receptor antagonists, or
phenobarbital did not alter the clearance of TMZ. Co-administration with valproic acid was associated
with a small but statistically significant decrease in clearance of TMZ.
No studies have been conducted to determine the effect of TMZ on the metabolism or elimination of
other medicinal products. However, since TMZ does not undergo hepatic metabolism and exhibits low
protein binding, it is unlikely that it would affect the pharmacokinetics of other medicinal products
(see section 5.2).
Use of TMZ in combination with other myelosuppressive agents may increase the likelihood of
myelosuppression.
4.6 Pregnancy and lactation
There are no data in pregnant women. In preclinical studies in rats and rabbits receiving 150 mg/m²
TMZ, teratogenicity and/or foetal toxicity were demonstrated (see section 5.3). Temozolomide Teva
hard capsules should not be administered to pregnant women. If use during pregnancy must be
considered, the patient should be apprised of the potential risk to the foetus. Women of childbearing
potential should be advised to use effective contraception to avoid pregnancy while they are receiving
TMZ.
It is not known whether TMZ is excreted in human milk; thus, breast-feeding should be discontinued
while receiving treatment with TMZ.
TMZ can have genotoxic effects. Therefore, men being treated with it should be advised not to father a
child up to 6 months after receiving the last dose and to seek advice on cryoconservation of sperm
prior to treatment, because of the possibility of irreversible infertility due to therapy with TMZ.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. The ability to
drive and use machines may be impaired in patients treated with TMZ due to fatigue and somnolence.
Clinical trial experience
In patients treated with TMZ, whether used in combination with RT or as monotherapy following RT
for newly-diagnosed glioblastoma multiforme, or as monotherapy in patients with recurrent or
progressive glioma, the reported very common adverse reactions were similar: nausea, vomiting,
constipation, anorexia, headache and fatigue. Convulsions were reported very commonly in the newly-
diagnosed glioblastoma multiforme patients receiving monotherapy, and rash was reported very
commonly in newly-diagnosed glioblastoma multiforme patients receiving TMZ concurrent with RT
and also as monotherapy, and commonly in recurrent glioma. Most haematologic adverse reactions
were reported commonly or very commonly in both indications (Tables 4 and 5); the frequency of
grade 3-4 laboratory findings is presented after each table.
In the tables undesirable effects are classified according to System Organ Class and frequency.
Frequency groupings are defined according to the following convention: Very common (≥ 1/10);
Common (≥ 1/100 to < 1/10); Uncommon (≥ 1/1,000 to < 1/100). Within each frequency grouping,
undesirable effects are presented in order of decreasing seriousness.
Newly-diagnosed glioblastoma multiforme
Table 4 provides treatment-emergent adverse events in patients with newly-diagnosed glioblastoma
multiforme during the concomitant and monotherapy phases of treatment.
Table 4: Treatment-emergent events during concomitant and monotherapy treatment
phases in patients with newly-diagnosed glioblastoma multiforme
TMZ + concomitant RT
n=288*
Infections and infestations
Common:
Infection
, Herpes simplex
, wound
infection, pharyngitis, candidiasis
oral
Infection, candidiasis oral
Herpes simplex
, Herpes zoster,
influenza–like symptoms
Blood and lymphatic system disorders
Common:
Neutropenia, thrombocytopenia,
lymphopenia, leukopenia
Febril neutropenia,
thrombocytopenia, anaemia,
leukopenia
Febrile neutropenia, anaemia
Endocrine disorders
Uncommon:
Metabolism and nutrition disorders
Very Common:
Hyperglycaemia, weight decreased
Hypokalemia, alkaline phosphatase
increased, weight increased
Hyperglycaemia, weight
increased
Psychiatric disorders
Common:
Anxiety, emotional lability, insomnia
Anxiety, depression, emotional
lability, insomnia
Agitation, apathy, behaviour
disorder, depression, hallucination
Nervous system disorders
Very Common:
Convulsions, consciousness
Hemiparesis, aphasia, balance
























decreased, somnolence, aphasia,
balance impaired, dizziness,
confusion, memory impairment,
concentration impaired, neuropathy,
paresthesia, speech disorder,
tremor
impaired, somnolence,
confusion, dizziness, memory
impairment, concentration
impaired, dysphasia,
neurological disorder (NOS),
neuropathy, peripheral
neuropathy, paresthesia, speech
disorder, tremor
Status epilepticus, extrapyramidal
disorder, hemiparesis, ataxia,
cognition impaired, dysphasia, gait
abnormal, hyperesthesia,
hypoesthesia, neurological disorder
(NOS), peripheral neuropathy
Hemiplegia, ataxia, coordination
abnormal, gait abnormal,
hyperesthesia, sensory
disturbance
Visual field defect, vision
blurred, diplopia
Hemianopia, visual acuity reduced,
vision disorder, visual field defect,
eye pain
Visual acuity reduced, eye pain,
eyes dry
Ear and labyrinth disorders
Common:
Hearing impairment, tinnitus
Otitis media, tinnitus, hyperacusis,
earache
Deafness, vertigo, earache
Cardiac disorders
Uncommon:
Vascular disorders
Common:
Haemorrhage, oedema, oedema leg
Haemorrhage, deep venous
thrombosis, oedema leg
Cerebral haemorrhage, hypertension
Embolism pulmonary, oedema,
oedema peripheral
Respiratory, thoracic and mediastinal disorders
Common:
Pneumonia, upper respiratory
infection, nasal congestion
Pneumonia, sinusitis, upper
respiratory infection, bronchitis
Gastrointestinal disorders
Very Common:
Constipation, nausea, vomiting
Constipation, nausea, vomiting
Stomatitis, diarrhoea, abdominal
pain, dyspepsia, dysphagia
Stomatitis, diarrhoea, dyspepsia,
dysphagia, mouth dry
Abdominal distension, fecal
incontinence, gastrointestinal
disorder (NOS), gastroenteritis,
haemorrhoids
Skin and subcutaneous tissue disorders
Very Common:
Dermatitis, dry skin, erythema,
pruritus



























Skin exfoliation, photosensitivity
reaction, pigmentation abnormal
Erythema, pigmentation
abnormal, sweating increased
Musculoskeletal and connective tissue disorders
Common:
Muscle weakness, arthralgia
Muscle weakness, arthralgia,
musculoskeletal pain, myalgia
Myopathy, back pain,
musculoskeletal pain, myalgia
Renal and urinary disorders
Common:
Micturition frequency, urinary
incontinence
Reproductive system and breast disorders
Uncommon:
Vaginal haemorrhage,
menorrhagia, amenorrhea,
vaginitis, breast pain
General disorders and administration site conditions
Very Common:
Allergic reaction, fever, radiation
injury, face oedema, pain, taste
perversion
Allergic reaction, fever,
radiation injury, pain, taste
perversion
Asthenia, flushing, hot flushes,
condition aggravated, rigors, tongue
discolouration, parosmia, thirst
Asthenia, face oedema, pain,
condition aggravated, rigors,
tooth disorder, taste perversion
Hepatic enzymes increased, Gamma
GT increased, AST increased
*A patient who was randomised to the RT arm only, received TMZ + RT.
Myelosuppression (neutropenia and thrombocytopenia), which is known dose-limiting toxicity for
most cytotoxic agents, including TMZ, was observed. When laboratory abnormalities and adverse
events were combined across concomitant and monotherapy treatment phases, Grade 3 or Grade 4
neutrophil abnormalities including neutropenic events were observed in 8 % of the patients. Grade 3 or
Grade 4 thrombocyte abnormalities, including thrombocytopenic events were observed in 14% of the
patients who received TMZ.
Recurrent or progressive malignant glioma
In clinical trials, the most frequently occurring treatment-related undesirable effects were
gastrointestinal disorders, specifically nausea (43%) and vomiting (36%). These reactions were
usually Grade 1 or 2 (0 – 5 episodes of vomiting in 24 hours) and were either self-limiting or readily
controlled with standard anti-emetic therapy. The incidence of severe nausea and vomiting was 4%.
Table 5 includes adverse reactions reported during clinical trials for recurrent or progressive malignant
glioma and following the marketing of TMZ.




















Table 5. Adverse reactions in patients with recurrent or progressive malignant glioma
Infections and infestations
Rare:
Opportunistic infections, including PCP
Blood and lymphatic system disorders
Very common:
Neutropenia or lymphopenia (grade 3-4),
thrombocytopenia (grade 3-4)
Pancytopenia, anaemia (grade 3-4), leukopenia
Metabolism and nutrition disorders
Very common:
Nervous system disorders
Very common:
Somnolence, dizziness, paresthesia
Respiratory, thoracic and mediastinal disorders
Common:
Gastrointestinal disorders
Very common:
Vomiting, nausea, constipation
Diarrhoea, abdominal pain, dyspepsia
Skin and subcutaneous tissue disorders
Common:
Erythema multiforme, erythroderma, urticaria,
exanthema
General disorders and administration site conditions
Very common:
Fever, asthenia, rigors, malaise, pain, taste
perversion
Allergic reactions, including anaphylaxis,
angioedema
Grade 3 or 4 thrombocytopenia and neutropenia occurred in 19% and 17% respectively, of patients
treated for malignant glioma. This led to hospitalisation and/or discontinuation of TMZ in 8% and 4%,
respectively. Myelosuppression was predictable (usually within the first few cycles, with the nadir
between Day 21 and Day 28), and recovery was rapid, usually within 1-2 weeks. No evidence of
cumulative myelosuppression was observed. The presence of thrombocytopenia may increase the risk
of bleeding, and the presence of neutropenia or leukopenia may increase the risk of infection.
In a population pharmacokinetics analysis of clinical trial experience there were 101 female and 169
male subjects for whom nadir neutrophil counts were available and 110 female and 174 male subjects
for whom nadir platelet counts were available. There were higher rates of Grade 4 neutropenia (ANC
< 0.5 x 10
9
/l), 12%
vs
5 %, and thrombocytopenia (< 20 x 10
9
/l), 9%
vs
3%, in women
vs
men in the
first cycle of therapy. In a 400 subject recurrent glioma data set, Grade 4 neutropenia occurred in 8%
of female
vs
4% of male subjects and Grade 4 thrombocytopenia in 8% of female
vs
3% of male
subjects in the first cycle of therapy. In a study of 288 subjects with newly-diagnosed glioblastoma
multiforme, Grade 4 neutropenia occurred in 3% of female
vs
0% of male subjects and Grade 4
thrombocytopenia in 1% of female
vs
0% of male subjects in the first cycle of therapy.
Post-marketing experience
Antineoplastic agents, and notably alkylating agents, have been associated with a potential risk of
myelodysplastic syndrome (MDS) and secondary malignancies, including leukaemia. Very rare cases
of MDS and secondary malignancies, including myeloid leukaemia have been reported in patients



























treated with regimens that included TMZ. Prolonged pancytopenia, which may result in aplastic
anaemia has been reported very rarely
.
Cases of toxic epidermal necrolysis and Stevens-Johnson syndrome have been reported very rarely.
Cases of interstitial pneumonitis/pneumonitis have been reported very rarely.
Doses of 500, 750, 1,000, and 1,250 mg/m² (total dose per cycle over 5 days) have been evaluated
clinically in patients. Dose-limiting toxicity was haematological and was reported with any dose but is
expected to be more severe at higher doses. An overdose of 10,000 mg (total dose in a single cycle,
over 5 days) was taken by one patient and the adverse reactions reported were pancytopenia, pyrexia,
multiorgan failure and death. There are reports of patients who have taken the recommended dose for
more than 5 days of treatment (up to 64 days) with adverse events reported including bone marrow
suppression, with or without infection, in some cases severe and prolonged and resulting in death. In
the event of an overdose, haematological evaluation is needed. Supportive measures should be
provided as necessary.
5. PHARMACOLOGICAL PROPERTIES
5.1
Pharmacodynamic properties
Pharmacotherapeutic group: Other alkylating agents, ATC code: L01A X03
Temozolomide is a triazene, which undergoes rapid chemical conversion at physiologic pH to the
active monomethyl triazenoimidazole carboxamide (MTIC). The cytotoxicity of MTIC is thought to
be dueprimarily to alkylation at the O6 position of guanine with additional alkylation also occurring at
the N7 position. Cytotoxic lesions that develop subsequently are thought to involve aberrant repair of
the methyl adduct.
Newly-diagnosed glioblastoma multiforme
A total of 573 patients were randomised to receive either TMZ + RT (n=287) or RT alone (n=286).
Patients in the TMZ + RT arm received concomitant TMZ (75 mg/m²) once daily, starting the first day
of RT until the last day of RT, for 42 days (with a maximum of 49 days). This was followed by
monotherapy TMZ (150 - 200 mg/m²) on Days 1 - 5 of every 28-day cycle for up to 6 cycles, starting
4 weeks after the end of RT. Patients in the control arm received RT only.
Pneumocystis carinii
pneumonia (PCP) prophylaxis was required during RT and combined TMZ therapy.
TMZ was administered as salvage therapy in the follow-up phase in 161 patients of the 282 (57%) in
the RT alone arm, and 62 patients of the 277 (22%) in the TMZ + RT arm.
The hazard ratio (HR) for overall survival was 1.59 (95% CI for HR=1.33 -1.91) with a log-rank
p < 0.0001 in favour of the TMZ arm. The estimated probability of surviving 2 years or more (26%
vs
10 %) is higher for the RT + TMZ arm. The addition of concomitant TMZ to RT, followed by TMZ
monotherapy in the treatment of patients with newly-diagnosed glioblastoma multiforme demonstrated
a statistically significant improvement in overall survival (OS) compared with RT alone (Figure 1).
Figure 1 Kaplan-Meier curves for overall survival (intent-to-treat population)
The results from the trial were not consistent in the subgroup of patients with a poor performance
status (WHO PS=2, n=70), where overall survival and time to progression were similar in both arms.
However, no unacceptable risks appear to be present in this patient group.
Recurrent or progressive malignant glioma
Data on clinical efficacy in patients with glioblastoma multiforme (Karnofsky performance status
[KPS] ≥ 70), progressive or recurrent after surgery and RT, were based on two clinical trials with oral
TMZ. One was a non-comparative trial in 138 patients (29% received prior chemotherapy), and the
other was a randomised active-controlled trial of TMZ
vs
procarbazine in a total of 225 patients (67%
received prior treatment with nitrosourea based chemotherapy). In both trials, the primary endpoint
was progression-free survival (PFS) defined by MRI scans or neurological worsening. In the
noncomparative trial, the PFS at 6 months was 19%, the median progression-free survival was 2.1
months, and the median overall survival 5.4 months. The objective response rate (ORR) based on MRI
scans was 8%.
In the randomised active-controlled trial, the PFS at 6 months was significantly greater for TMZ than
for procarbazine (21%
vs
8%, respectively – chi-square p = 0.008) with median PFS of 2.89 and 1.88
months respectively (log rank p = 0.0063). The median survival was 7.34 and 5.66 months for TMZ
and procarbazine, respectively (log rank p = 0.33). At 6 months, the fraction of surviving patients was
significantly higher in the TMZ arm (60%) compared with the procarbazine arm (44%) (chi-square
p = 0.019). In patients with prior chemotherapy a benefit was indicated in those with a KPS ≥ 80.
Data on time to worsening of neurological status favoured TMZ over procarbazine as did data on time
to worsening of performance status (decrease to a KPS of < 70 or a decrease by at least 30 points). The
HR median times to progression in these endpoints ranged from 0.7 to 2.1 months longer for TMZ
than for procarbazine (log rank p = < 0.01 to 0.03).
Recurrent anaplastic astrocytoma
In a multicentre, prospective phase II trial evaluating the safety and efficacy of oral TMZ in the
treatment of patients with anaplastic astrocytoma at first relapse, the 6 month PFS was 46 %. The
median PFS was 5.4 months. Median overall survival was 14.6 months. Response rate, based on the
central reviewer assessment, was 35% (13 CR and 43 PR) for the intent-to-treat population (ITT)
n=162. In 43 patients stable disease was reported. The 6-month event-free survival for the ITT
population was 44% with a median event-free survival of 4.6 months, which was similar to the results
for the progression-free survival. For the eligible histology population, the efficacy results were
similar. Achieving a radiological objective response or maintaining progression-free status was
strongly associated with maintained or improved quality of life.
Oral TMZ has been studied in paediatric patients (age 3-18 years) with recurrent brainstem glioma or
recurrent high grade astrocytoma, in a regimen administered daily for 5 days every 28 days. Tolerance
to TMZ is similar to adults.
5.2 Pharmacokinetic properties
TMZ is spontaneously hydrolyzed at physiologic pH primarily to the active species, 3-methyl-(triazen-
1-yl)imidazole-4-carboxamide (MTIC). MTIC is spontaneously hydrolyzed to 5-amino-imidazole-4-
carboxamide (AIC), a known intermediate in purine and nucleic acid biosynthesis, and to
methylhydrazine, which is believed to be the active alkylating species. The cytotoxicity of MTIC is
thought to be primarily due to alkylation of DNA mainly at the O
6
and N
7
positions of guanine.
Relative to the AUC of TMZ, the exposure to MTIC and AIC is ~ 2.4% and 23%, respectively.
In
vivo
, the t
1/2
of MTIC was similar to that of TMZ, 1.8 hr.
After oral administration to adult patients, TMZ is absorbed rapidely, with peak concentrations
reached as early as 20 minutes post-administration (mean time between 0.5 and 1.5 hours). After oral
administration of
14
C-labelled TMZ, mean faecal excretion of
14
C over 7 days post-dose was 0.8 %
indicationg complete absorption.
TMZ demonstrates low protein binding (10% to 20%), and thus it is not expected to interact with
highly protein-bound substances.
PET studies in humans and preclinical data suggest that TMZ crosses the blood-brain barrier rapidly
and is present in the CSF. CSF penetration was confirmed in one patient; CSF exposure based on AUC
of TMZ was approximately 30% of that in plasma, which is consistent with animal data.
The half-life (t
1/2
) in plasma is approximately 1.8 hours. The major route of
14
C elimination is renal.
Following oral administration, approximately 5% to 10% of the dose is recovered unchanged in the
urine over 24 hours, and the remainder excreted as temozolomide acid, 5-aminoimidazole-4-
carboxamide (AIC) or unidentified polar metabolites.
Plasma concentrations increase in a dose-related manner. Plasma clearance, volume of distribution and
half-life are independent of dose.
Analysis of population-based pharmacokinetics of TMZ revealed that plasma TMZ clearance was
independent of age, renal function or tobacco use. In a separate pharmacokinetic study, plasma
pharmacokinetic profiles in patients with mild to moderate hepatic impairment were similar to those
observed in patients with normal hepatic function.
Paediatric patients had a higher AUC than adult patients; however, the maximum tolerated dose
(MTD) was 1,000 mg/m² per cycle both in children and in adults.
5.3 Preclinical safety data
Single-cycle (5-day dosing, 23 days non-treatment), 3- and 6-cycle toxicity studies were conducted in
rats and dogs. The primary targets of toxicity included the bone marrow, lymphoreticular system,
testes, the gastrointestinal tract and, at higher doses, which were lethal to 60% to 100% of rats and
dogs tested, degeneration of the retina occurred. Most of the toxicity showed evidence of reversibility,
except for adverse events on the male reproductive system and retinal degeneration. However, because
the doses implicated in retinal degeneration were in the lethal dose range, and no comparable effect
has been observed in clinical studies, this finding was not considered to have clinical relevance.
TMZ is an embryotoxic, teratogenic and genotoxic alkylating agent. TMZ is more toxic to the rat and
dog than to humans, and the clinical dose approximates the minimum lethal dose in rats and dogs.
Doserelated reductions in leukocytes and platelets appear to be sensitive indicators of toxicity. A
variety of neoplasms, including mammary carcinomas, keratocanthoma of the skin and basal cell
adenoma were observed in the 6-cycle rat study while no tumours or pre-neoplastic changes were
evident in dog studies. Rats appear to be particularly sensitive to oncogenic effects of TMZ, with the
occurrence of first tumours within 3 months of initiating dosing. This latency period is very short even
for an alkylating agent.
Results of the Ames/salmonella and Human Peripheral Blood Lymphocyte (HPBL) chromosome
aberration tests showed a positive mutagenicity response.
6. PHARMACEUTICAL PARTICULARS
Anhydrous lactose
Sodium starch glycolate Type A
Colloidal anhydrous silica
Tartaric acid
Stearic acid
Capsule shells
Gelatin
Titanium dioxide (E171)
Printing ink
Shellac
Propylene glycol
Titanium dioxide (E171)
Sunset yellow FCF Aluminium Lake (E110)
6.4 Special precautions for storage
Store in the original package.
Keep the bottle tightly closed in order to protect from moisture.
6.5 Nature and contents of container
Amber glass bottle with white polypropylene child-resistant screw cap equipped with an induction seal
of polyethylene
containing 5 or 20 capsules.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
Capsules should not be opened. If a capsule becomes damaged, contact of the powder contents with
skin or mucous membrane must be avoided. If Temozolomide Teva comes into contact with skin or
mucosa, it should be washed immediately and thoroughly with soap and water.
Patients should be advised to keep capsules out of the reach and sight of children, preferably in a
locked cupboard. Accidental ingestion can be lethal for children.
Any unused product or waste material should be disposed of in accordance with local requirements.
7. MARKETING AUTHORISATION HOLDER
Teva Pharma B.V.
Computerweg 10
3542 DR Utrecht
The Netherlands
8. MARKETING AUTHORISATION NUMBER(S)
EU/1/09/606/003
EU/1/09/606/004
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
Agency http://www.ema.europa.eu/
1. NAME OF THE MEDICINAL PRODUCT
Temozolomide Teva 100 mg hard capsules
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each hard capsule contains 100 mg temozolomide.
Excipient: Each hard capsule contains 84 mg of anhydrous lactose.
For a full list of excipients, see section 6.1.
The hard capsules have a white opaque body and cap with two stripes in pink ink on the cap and with
“T 100 mg” in pink ink on the body.
4.1 Therapeutic indications
Temozolomide Teva hard capsules is indicated for the treatment of:
-
adult patients with newly-diagnosed glioblastoma multiforme concomitantly with radiotherapy
(RT) and subsequently as monotherapy treatment.
children from the age of three years, adolescents and adult patients with malignant glioma, such
as glioblastoma multiforme or anaplastic astrocytoma, showing recurrence or progression after
standard therapy.
4.2 Posology and method of administration
Temozolomide Teva hard capsules should only be prescribed by physicians experienced in the
oncological treatment of brain tumours.
Anti-emetic therapy may be administered (see section 4.4).
Adult patients with newly-diagnosed glioblastoma multiforme
Temozolomide Teva hard capsules is administered in combination with focal radiotherapy
(concomitant phase) followed by up to 6 cycles of temozolomide (TMZ) monotherapy (monotherapy
phase).
Concomitant phase
TMZ is administered orally at a dose of 75 mg/m² daily for 42 days concomitant with focal
radiotherapy (60 Gy administered in 30 fractions). No dose reductionsare recommended, but delay or
discontinuation of TMZ administration should be decided weekly according to haematological and
non-haematological toxicity criteria. TMZ administration can be continued throughout the 42 day
concomitant period (up to 49 days) if all of the following conditions are met:
-
absolute neutrophil count (ANC) ≥ 1.5 x 10
9
/l
thrombocyte count ≥ 100 x 10
9
/l
- common toxicity criteria (CTC) non-haematological toxicity ≤ Grade 1 (except for alopecia,
nausea and vomiting).
During treatment a complete blood count should be obtained weekly. TMZ administration should be
temporarily interrupted or permanently discontinued during the concomitant phase according to the
haematological and non-haematological toxicity criteria as noted in Table 1.
Table 1. TMZ dosing interruption or discontinuation during concomitant radiotherapy and TMZ
Toxicity
Absolute Neutrophil Count
≥ 0.5 and < 1.5 x 10
9
/l
Thrombocyte Count
≥ 10 and < 100 x 10
9
/l
CTC Non-haematological
toxicity (except for alopecia,
nausea, vomiting)
Treatment with concomitant TMZ can be continued when all of the following conditions are met:
absolute neutrophil count ≥ 1.5 x 10
9
/l; thrombocyte count ≥ 100 x 10
9
/l; CTC non-haematological
toxicity ≤ Grade 1 (except for alopecia, nausea, vomiting).
Four weeks after completing the TMZ + RT concomitant phase, TMZ is administered for up to 6
cycles of monotherapy treatment. Dose in Cycle 1 (monotherapy) is 150 mg/m² once daily for 5 days
followed by 23 days without treatment. At the start of Cycle 2, the dose is escalated to 200 mg/m² if
the CTC nonhaematological toxicity for Cycle 1 is Grade ≤ 2 (except for alopecia, nausea and
vomiting), absolute neutrophil count (ANC) is ≥ 1.5 x 10
9
/l, and the thrombocyte count is
≥ 100 x 10
9
/l. If the dose was not escalated at Cycle 2, escalation should not be done in subsequent
cycles. Once escalated, the dose remains at 200 mg/m² per day for the first 5 days of each subsequent
cycle except if toxicity occurs. Dose reductions and discontinuations during the monotherapy phase
should be applied according to Tables 2 and 3.
During treatment a complete blood count should be obtained on Day 22 (21 days after the first dose of
TMZ). The dose should be reduced or administration discontinued according to Table 3.
Table 2. TMZ dose levels for monotherapy treatment
Reduction for prior toxicity
Dose during Cycles 2-6 in absence of toxicity
Table 3. TMZ dose reduction or discontinuation during monothera
py treatment
Toxicity
Reduce TMZ by 1 dose level
a
Absolute Neutrophil Count
CTC Non-haematological Toxicity
(except for alopecia, nausea, vomiting)
a: TMZ dose levels are listed in Table 2.
b: TMZ is to be discontinued if:
•
the same Grade 3 non-haematological toxicity (except for alopecia, nausea, vomiting) recurs
after dose reduction.
Adult and paediatric patients 3 years of age or older with recurrent or progressive malignant glioma
A treatment cycle comprises 28 days. In patients previously untreated with chemotherapy, TMZ is
administered orally at a dose of 200 mg/m² once daily for the first 5 days followed by a 23 day
treatment interruption (total of 28 days). In patients previously treated with chemotherapy, the initial
dose level -1 (100 mg/m²) still results in unacceptable toxicity

































dose is 150 mg/m² once daily, to be increased in the second cycle to 200 mg/m² once daily, for 5 days
if there is no haematological toxicity (see section 4.4)
In patients 3 years of age or older, TMZ is only to be used in recurrent or progressive malignant
glioma. There is no clinical experience with use of TMZ in children under the age of 3 years.
Experience in older children is very limited (see sections 4.4 and 5.1).
Patients with hepatic or renal impairment
The pharmacokinetics of TMZ were comparable in patients with normal hepatic function and in those
with mild or moderate hepatic impairment. No data are available on the administration of TMZ in
patients with severe hepatic impairment (Child’s Class C) or with renal impairment. Based on the
pharmacokinetic properties of TMZ, it is unlikely that dose reductions are required in patients with
severe hepatic impairment or any degree of renal impairment. However, caution should be exercised
when TMZ is administered in these patients.
Based on a population pharmacokinetic analysis in patients 19-78 years of age, clearance of TMZ is
not affected by age. However, elderly patients (> 70 years of age) appear to be at increased risk of
neutropenia and thrombocytopenia (see section 4.4).
Temozolomide Teva hard capsules should be administered in the fasting state.
The capsules must be swallowed whole with a glass of water and must not be opened or chewed.
If vomiting occurs after the dose is administered, a second dose should not be administered that day.
Hypersensitivity to the active substance or to any of the excipients.
Hypersensitivity to dacarbazine (DTIC).
Severe myelosuppression (see section 4.4).
4.4 Special warnings and precautions for use
Pneumocystis carinii pneumonia
Patients who received concomitant TMZ and RT in a pilot trial for the prolonged 42-day schedule
were shown to be at particular risk for developing
Pneumocystis carinii
pneumonia (PCP). Thus,
prophylaxis against PCP is required for all patients receiving concomitant TMZ and RT for the 42-day
regimen (with a maximum of 49 days) regardless of lymphocyte count. If lymphopenia occurs, they
are to continue the prophylaxis until recovery of lymphopenia to grade ≤ 1.
There may be a higher occurrence of PCP when TMZ is administered during a longer dosing regimen.
However, all patients receiving TMZ, particularly patients receiving steroids, should be observed
closely for the development of PCP, regardless of the regimen.
Cases of myelodysplastic syndrome and secondary malignancies, including myeloid leukaemia, have
also been reported very rarely (see section 4.8).
Nausea and vomiting are very commonly associated with TMZ.
Anti-emetic therapy may be administered prior to or following administration of TMZ.
Adult patients with newly-diagnosed glioblastoma multiforme
Anti-emetic prophylaxis is recommended prior to the initial dose of concomitant phase and it is
strongly recommended during the monotherapy phase.
Patients with recurrent or progressive malignant glioma
Patients who have experienced severe (Grade 3 or 4) vomiting in previous treatment cycles may
require anti-emetic therapy.
Prior to dosing, the following laboratory parameters must be met: ANC ≥ 1.5 x 10
9
/l and platelet count
≥ 100 x 10
9
/l. A complete blood count should be obtained on Day 22 (21 days after the first dose) or
within 48 hours of that day, and weekly until ANC > 1.5 x10
9
/l and platelet count > 100 x 10
9
/l. If
ANC falls to < 1.0 x 10
9
/l or the platelet count is < 50 x 10
9
/l during any cycle, the next cycle should
be reduced one dose level (see section 4.2). Dose levels include 100 mg/m², 150 mg/m², and
200 mg/m². The lowest recommended dose is 100 mg/m².
There is no clinical experience with use of TMZ in children under the age of 3 years. Experience in
older children and adolescents is very limited (see sections 4.2 and 5.1).
Elderly patients (> 70 years of age)
Elderly patients appear to be at increased risk of neutropenia and thrombocytopenia, compared with
younger patients. Therefore, special care should be taken when TMZ is administered in elderly
patients.
Men being treated with TMZ should be advised not to father a child up to 6 months after receiving the
last dose and to seek advice on cryoconservation of sperm prior to treatment (see section 4.6).
This medicinal product contains lactose. Patients with rare hereditary problems of galactose
intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this
medicine.
4.5 Interaction with other medicinal products and other forms of interaction
Interaction studies have only been performed in adults.
In a separate phase I study, administration of TMZ with ranitidine did not result in alterations in the
extent of absorption of temozolomide or the exposure to its active metabolite monomethyl
triazenoimidazole carboxamide (MTIC).
Administration of TMZ with food resulted in a 33% decrease in C
max
and a 9% decrease in area under
the curve (AUC).
As it cannot be excluded that the change in C
max
is clinically significant, Temozolomide Teva should
be administered without food.
Based on an analysis of population pharmacokinetics in phase II trials, co-administration of
dexamethasone, prochlorperazine, phenytoin, carbamazepine, ondansetron, H2 receptor antagonists, or
phenobarbital did not alter the clearance of TMZ. Co-administration with valproic acid was associated
with a small but statistically significant decrease in clearance of TMZ.
No studies have been conducted to determine the effect of TMZ on the metabolism or elimination of
other medicinal products. However, since TMZ does not undergo hepatic metabolism and exhibits low
protein binding, it is unlikely that it would affect the pharmacokinetics of other medicinal products
(see section 5.2).
Use of TMZ in combination with other myelosuppressive agents may increase the likelihood of
myelosuppression.
4.6 Pregnancy and lactation
There are no data in pregnant women. In preclinical studies in rats and rabbits receiving 150 mg/m²
TMZ, teratogenicity and/or foetal toxicity were demonstrated (see section 5.3). Temozolomide Teva
hard capsules should not be administered to pregnant women. If use during pregnancy must be
considered, the patientshould be apprised of the potential risk to the foetus. Women of childbearing
potential should be advised to use effective contraception to avoid pregnancy while they are receiving
TMZ. .
It is not known whether TMZ is excreted in human milk; thus, breast-feeding should be discontinued
while receiving treatment with TMZ.
TMZ can have genotoxic effects. Therefore, men being treated with it should be advised not to father a
child up to 6 months after receiving the last dose and to seek advice on cryoconservation of sperm
prior to treatment, because of the possibility of irreversible infertility due to therapy with TMZ.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. The ability to
drive and use machines may be impaired in patients treated with TMZ due to fatigue and somnolence.
Clinical trial experience
In patients treated with TMZ, whether used in combination with RT or as monotherapy following RT
for newly-diagnosed glioblastoma multiforme, or as monotherapy in patients with recurrent or
progressive glioma, the reported very common adverse reactions were similar: nausea, vomiting,
constipation, anorexia, headache and fatigue. Convulsions were reported very commonly in the newly-
diagnosed glioblastoma multiforme patients receiving monotherapy, and rash was reported very
commonly in newly-diagnosed glioblastoma multiforme patients receiving TMZ concurrent with RT
and also as monotherapy, and commonly in recurrent glioma. Most haematologic adverse reactions
were reported commonly or very commonly in both indications (Tables 4 and 5);the frequency of
grade 3-4 laboratory findings is presented after each table.
In the tables undesirable effects are classified according to System Organ Class and frequency.
Frequency groupings are defined according to the following convention: Very common (≥ 1/10);
Common (≥ 1/100 to < 1/10); Uncommon (≥ 1/1,000 to < 1/100). Within each frequency grouping,
undesirable effects are presented in order of decreasing seriousness.
Newly-diagnosed glioblastoma multiforme
Table 4 provides treatment-emergent adverse events in patients with newly-diagnosed glioblastoma
multiforme during the concomitant and monotherapy phases of treatment.
Table 4: Treatment-emergent events during concomitant and monotherapy treatment
phases in patients with newly-diagnosed glioblastoma multiforme
TMZ + concomitant RT
n=288*
Infections and infestations
Common:
Infection
, Herpes simplex
, wound
infection, pharyngitis, candidiasis
oral
Infection, candidiasis oral
Herpes simplex
, Herpes zoster,
influenza–like symptoms
Blood and lymphatic system disorders
Common:
Neutropenia, thrombocytopenia,
lymphopenia, leukopenia
Febril neutropenia,
thrombocytopenia, anaemia,
leukopenia
Febrile neutropenia, anaemia
Endocrine disorders
Uncommon:
Metabolism and nutrition disorders
Very Common:
Hyperglycaemia, weight decreased
Hypokalemia, alkaline phosphatase
increased, weight increased
Hyperglycaemia, weight
increased
Psychiatric disorders
Common:
Anxiety, emotional lability, insomnia
Anxiety, depression, emotional
lability, insomnia
Agitation, apathy, behaviour
disorder, depression, hallucination
Nervous system disorders
Very Common:
Convulsions, consciousness
decreased, somnolence, aphasia,
balance impaired, dizziness,
confusion, memory impairment,
concentration impaired, neuropathy,
paresthesia, speech disorder,
tremor
Hemiparesis, aphasia, balance
impaired, somnolence,
confusion, dizziness, memory
impairment, concentration
impaired, dysphasia,
neurological disorder (NOS),
neuropathy, peripheral
























neuropathy, paresthesia, speech
disorder, tremor
Status epilepticus, extrapyramidal
disorder, hemiparesis, ataxia,
cognition impaired, dysphasia, gait
abnormal, hyperesthesia,
hypoesthesia, neurological disorder
(NOS), peripheral neuropathy
Hemiplegia, ataxia, coordination
abnormal, gait abnormal,
hyperesthesia, sensory
disturbance
Visual field defect, vision
blurred, diplopia
Hemianopia, visual acuity reduced,
vision disorder, visual field defect,
eye pain
Visual acuity reduced, eye pain,
eyes dry
Ear and labyrinth disorders
Common:
Hearing impairment, tinnitus
Otitis media, tinnitus, hyperacusis,
earache
Deafness, vertigo, earache
Cardiac disorders
Uncommon:
Vascular disorders
Common:
Haemorrhage, oedema, oedema leg
Haemorrhage, deep venous
thrombosis, oedema leg
Cerebral haemorrhage, hypertension
Embolism pulmonary, oedema,
oedema peripheral
Respiratory, thoracic and mediastinal disorders
Common:
Pneumonia, upper respiratory
infection, nasal congestion
Pneumonia, sinusitis, upper
respiratory infection, bronchitis
Gastrointestinal disorders
Very Common:
Constipation, nausea, vomiting
Constipation, nausea, vomiting
Stomatitis, diarrhoea, abdominal
pain, dyspepsia, dysphagia
Stomatitis, diarrhoea, dyspepsia,
dysphagia, mouth dry
Abdominal distension, fecal
incontinence, gastrointestinal
disorder (NOS), gastroenteritis,
haemorrhoids
Skin and subcutaneous tissue disorders
Very Common:
Dermatitis, dry skin, erythema,
pruritus
Skin exfoliation, photosensitivity
reaction, pigmentation abnormal
Erythema, pigmentation
abnormal, sweating increased
Musculoskeletal and connective tissue disorders
Common:
Muscle weakness, arthralgia
Muscle weakness, arthralgia,
musculoskeletal pain, myalgia






























Myopathy, back pain,
musculoskeletal pain, myalgia
Renal and urinary disorders
Common:
Micturition frequency, urinary
incontinence
Reproductive system and breast disorders
Uncommon:
Vaginal haemorrhage,
menorrhagia, amenorrhea,
vaginitis, breast pain
General disorders and administration site conditions
Very Common:
Allergic reaction, fever, radiation
injury, face oedema, pain, taste
perversion
Allergic reaction, fever,
radiation injury, pain, taste
perversion
Asthenia, flushing, hot flushes,
condition aggravated, rigors, tongue
discolouration, parosmia, thirst
Asthenia, face oedema, pain,
condition aggravated, rigors,
tooth disorder, taste perversion
Hepatic enzymes increased, Gamma
GT increased, AST increased
*A patient who was randomised to the RT arm only, received TMZ + RT.
Myelosuppression (neutropenia and thrombocytopenia), which is known dose-limiting toxicity for
most cytotoxic agents, including TMZ, was observed. When laboratory abnormalities and adverse
events were combined across concomitant and monotherapy treatment phases, Grade 3 or Grade 4
neutrophil abnormalities including neutropenic events were observed in 8% of the patients. Grade 3 or
Grade 4 thrombocyte abnormalities, including thrombocytopenic events were observed in 14% of the
patients who received TMZ.
Recurrent or progressive malignant glioma
In clinical trials, the most frequently occurring treatment-related undesirable effects were
gastrointestinal disorders, specifically nausea (43%) and vomiting (36%). These reactions were
usually Grade 1 or 2 (0 – 5 episodes of vomiting in 24 hours) and were either self-limiting or readily
controlled with standard anti-emetic therapy. The incidence of severe nausea and vomiting was 4%.
Table 5 includes adverse reactions reported during clinical trials for recurrent or progressive malignant
glioma and following the marketing of TMZ.
Table 5. Adverse reactions in patients with recurrent or progressive malignant glioma
Infections and infestations
Rare:
Opportunistic infections, including PCP
Blood and lymphatic system disorders
Very common:
Neutropenia or lymphopenia (grade 3-4),
thrombocytopenia (grade 3-4)
Pancytopenia, anaemia (grade 3-4), leukopenia
Metabolism and nutrition disorders
Very common:





























Nervous system disorders
Very common:
Somnolence, dizziness, paresthesia
Respiratory, thoracic and mediastinal disorders
Common:
Gastrointestinal disorders
Very common:
Vomiting, nausea, constipation
Diarrhoea, abdominal pain, dyspepsia
Skin and subcutaneous tissue disorders
Common:
Erythema multiforme, erythroderma, urticaria,
exanthema
General disorders and administration site conditions
Very common:
Fever, asthenia, rigors, malaise, pain, taste
perversion
Allergic reactions, including anaphylaxis,
angioedema
Grade 3 or 4 thrombocytopenia and neutropenia occurred in 19% and 17% respectively, of patients
treated for malignant glioma. This led to hospitalisation and/or discontinuation of TMZ in 8% and 4%,
respectively. Myelosuppression was predictable (usually within the first few cycles, with the nadir
between Day 21 and Day 28), and recovery was rapid, usually within 1-2 weeks. No evidence of
cumulative myelosuppression was observed. The presence of thrombocytopenia may increase the risk
of bleeding, and the presence of neutropenia or leukopenia may increase the risk of infection.
In a population pharmacokinetics analysis of clinical trial experience there were 101 female and 169
male subjects for whom nadir neutrophil counts were available and 110 female and 174 male subjects
for whom nadir platelet counts were available. There were higher rates of Grade 4 neutropenia(ANC
< 0.5 x 10
9
/l), 12%
vs
5%, and thrombocytopenia (< 20 x 10
9
/l), 9 %
vs
3 %, in women
vs
men in the
first cycle of therapy. In a 400 subject recurrent glioma data set, Grade 4 neutropenia occurred in 8%
of female
vs
4% of male subjects and Grade 4 thrombocytopenia in 8% of female
vs
3% of male
subjects in the first cycle of therapy. In a study of 288 subjects with newly-diagnosed glioblastoma
multiforme, Grade 4 neutropenia occurred in 3% of female
vs
0% of male subjects and Grade 4
thrombocytopenia in 1% of female
vs
0% of male subjects in the first cycle of therapy.
Post-marketing experience
Antineoplastic agents, and notably alkylating agents, have been associated with a potential risk of
myelodysplastic syndrome (MDS) and secondary malignancies, including leukaemia. Very rare cases
of MDS and secondary malignancies, including myeloid leukaemia have been reported in patients
treated with regimens that included TMZ. Prolonged pancytopenia, which may result in aplastic
anaemia has been reported very rarely
.
Cases of toxic epidermal necrolysis and Stevens-Johnson syndrome have been reported very rarely.
Cases of interstitial pneumonitis/pneumonitis have been reported very rarely.
Doses of 500, 750, 1,000, and 1,250 mg/m² (total dose per cycle over 5 days) have been evaluated
clinically in patients. Dose-limiting toxicity was haematological and was reported with any dose but is



















expected to be more severe at higher doses. An overdose of 10,000 mg (total dose in a single cycle,
over5 days) was taken by one patient and the adverse reactions reported were pancytopenia, pyrexia,
multiorgan failure and death. There are reports of patients who have taken the recommended dose for
more than 5 days of treatment (up to 64 days) with adverse events reported including bone marrow
suppression, with or without infection, in some cases severe and prolonged and resulting in death. In
the event of an overdose, haematological evaluation is needed. Supportive measures should be
provided as necessary.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Other alkylating agents, ATC code: L01A X03
Temozolomide is a triazene, which undergoes rapid chemical conversion at physiologic pH to the
active monomethyl triazenoimidazole carboxamide (MTIC). The cytotoxicity of MTIC is thought to
be due primarily to alkylation at the O6 position of guanine with additional alkylation also occurring at
the N7 position. Cytotoxic lesions that develop subsequently are thought to involve aberrant repair of
the methyl adduct.
Newly-diagnosed glioblastoma multiforme
A total of 573 patients were randomised to receive either TMZ + RT (n=287) or RT alone (n=286).
Patients in the TMZ + RT arm received concomitant TMZ (75 mg/m²) once daily, starting the first day
of RT until the last day of RT, for 42 days (with a maximum of 49 days). This was followed
bymonotherapy TMZ (150 - 200 mg/m²) on Days 1 - 5 of every 28-day cycle for up to 6 cycles,
starting 4 weeks after the end of RT. Patients in the control arm received RT only.
Pneumocystis
carinii
pneumonia (PCP) prophylaxis was required during RT and combined TMZ therapy.
TMZ was administered as salvage therapy in the follow-up phase in 161 patients of the 282 (57%) in
the RT alone arm, and 62 patients of the 277 (22%) in the TMZ + RT arm.
The hazard ratio (HR) for overall survival was 1.59 (95% CI for HR=1.33 -1.91) with a log-rank
p < 0.0001 in favour of the TMZ arm. The estimated probability of surviving 2 years or more (26%
vs
10%) is higher for the RT + TMZ arm. The addition of concomitant TMZ to RT, followed by
TMZmonotherapy in the treatment of patients with newly-diagnosed glioblastoma multiforme
demonstrated a statistically significant improvement in overall survival (OS) compared with RT alone
(Figure 1).

Figure 1 Kaplan-Meier curves for overall survival (intent-to-treat population)
The results from the trial were not consistent in the subgroup of patients with a poor performance
status (WHO PS=2, n=70), where overall survival and time to progression were similar in both arms.
However, no unacceptable risks appear to be present in this patient group.
Recurrent or progressive malignant glioma
Data on clinical efficacy in patients with glioblastoma multiforme (Karnofsky performance status
[KPS] ≥ 70), progressive or recurrent after surgery and RT, were based on two clinical trials with oral
TMZ. One was a non-comparative trial in 138 patients (29% received prior chemotherapy), and the
other was a randomised active-controlled trial of TMZ
vs
procarbazine in a total of 225 patients (67%
received prior treatment with nitrosourea based chemotherapy). In both trials, the primary endpoint
was progression-free survival (PFS) defined by MRI scans or neurological worsening. In the
noncomparative trial, the PFS at 6 months was 19%, the median progression-free survival was 2.1
months, and the median overall survival 5.4 months. The objective response rate (ORR) based on MRI
scans was 8%.
In the randomised active-controlled trial, the PFS at 6 months was significantly greater for TMZ than
forprocarbazine (21%
vs
8%, respectively – chi-square p = 0.008) with median PFS of 2.89 and 1.88
months respectively (log rank p = 0.0063). The median survival was 7.34 and 5.66 months for TMZ
and procarbazine, respectively (log rank p = 0.33). At 6 months, the fraction of surviving patients was
significantly higher in the TMZ arm (60%) compared with the procarbazine arm (44%) (chi-square
p = 0.019). In patients with prior chemotherapy a benefit was indicated in those with a KPS ≥ 80.
Data on time to worsening of neurological status favoured TMZ over procarbazine as did data on time
to worsening of performance status (decrease to a KPS of < 70 or a decrease by at least 30 points). The
median times to progression in these endpoints ranged from 0.7 to 2.1 months longer for TMZ than for
procarbazine (log rank p = < 0.01 to 0.03).
Recurrent anaplastic astrocytoma
In a multicentre, prospective phase II trial evaluating the safety and efficacy of oral TMZ in the
treatment of patients with anaplastic astrocytoma at first relapse, the 6 month PFS was 46%. The
median PFS was 5.4 months. Median overall survival was 14.6 months. Response rate, based on the
central reviewer assessment, was 35% (13 CR and 43 PR) for the intent-to-treat population (ITT)
n=162. In 43 patients stable disease was reported. The 6-month event-free survival for the ITT
population was 44% with a median event-free survival of 4.6 months, which was similar to the results
for the progression-free survival. For the eligible histology population, the efficacy results were
similar. Achieving a radiological objective response or maintaining progression-free status was
strongly associated with maintained or improved quality of life.
Oral TMZ has been studied in paediatric patients (age 3-18 years) with recurrent brainstem glioma or
recurrent high grade astrocytoma, in a regimen administered daily for 5 days every 28 days. Tolerance
to TMZ is similar to adults.
5.2
Pharmacokineticproperties
TMZ is spontaneously hydrolyzed at physiologic pH primarily to the active species, 3-methyl-(triazen-
1-yl)imidazole-4-carboxamide (MTIC). MTIC is spontaneously hydrolyzed to 5-amino-imidazole-4-
carboxamide (AIC), a known intermediate in purine and nucleic acid biosynthesis, and to
methylhydrazine, which is believed to be the active alkylating species. The cytotoxicity of MTIC is
thought to be primarily due to alkylation of DNA mainly at the O
6
and N
7
positions of guanine.
Relative to the AUC of TMZ, the exposure to MTIC and AIC is ~ 2.4% and 23%, respectively.
In
vivo
, the t
½
of MTIC was similar to that of TMZ, 1.8 hr.
After oral administration to adult patients, TMZ is absorbed rapidely, with peak concentrations
reached as early as 20 minutes post-administration (mean time between 0.5 and 1.5 hours). After oral
administration of
14
C-labelled TMZ, mean faecal excretion of
14
C over 7 days post-dose was 0.8%
indicationg complete absorption.
TMZ demonstrates low protein binding (10% to 20%), and thus it is not expected to interact with
highly protein-bound substances.
PET studies in humans and preclinical data suggest that TMZ crosses the blood-brain barrier rapidly
and is present in the CSF. CSF penetration was confirmed in one patient; CSF exposure based on AUC
of TMZ was approximately 30% of that in plasma, which is consistent with animal data.
The half-life (t1/2) in plasma is approximately 1.8 hours. The major route of
14
C elimination is renal.
Following oral administration, approximately 5% to 10% of the dose is recovered unchanged in the
urine over 24 hours, and the remainder excreted as temozolomide acid, 5-aminoimidazole-4-
carboxamide (AIC) or unidentified polar metabolites.
Plasma concentrations increase in a dose-related manner. Plasma clearance, volume of distribution and
half-life are independent of dose.
Analysis of population-based pharmacokinetics of TMZ revealed that plasma TMZ clearance was
independent of age, renal function or tobacco use. In a separate pharmacokinetic study, plasma
pharmacokinetic profiles in patients with mild to moderate hepatic impairment were similar to those
observed in patients with normal hepatic function.
Paediatric patients had a higher AUC than adult patients; however, the maximum tolerated dose
(MTD) was 1,000 mg/m² per cycle both in children and in adults.
5.3 Preclinical safety data
Single-cycle (5-day dosing, 23 days non-treatment), 3- and 6-cycle toxicity studies were conducted in
rats and dogs. The primary targets of toxicity included the bone marrow, lymphoreticular system,
testes, the gastrointestinal tract and, at higher doses, which were lethal to 60% to 100% of rats and
dogs tested, degeneration of the retina occurred. Most of the toxicity showed evidence of reversibility,
except for adverse events on the male reproductive system and retinal degeneration. However, because
the doses implicated in retinal degeneration were in the lethal dose range, and no comparable effect
has been observed in clinical studies, this finding was not considered to have clinical relevance.
TMZ is an embryotoxic, teratogenic and genotoxic alkylating agent. TMZ is more toxic to the rat and
dog than to humans, and the clinical dose approximates the minimum lethal dose in rats and dogs.
Doserelated reductions in leukocytes and platelets appear to be sensitive indicators of toxicity. A
variety of neoplasms, including mammary carcinomas, keratocanthoma of the skin and basal cell
adenoma were observed in the 6-cycle rat study while no tumours or pre-neoplastic changes were
evident in dog studies. Rats appear to be particularly sensitive to oncogenic effects of TMZ, with the
occurrence of first tumours within 3 months of initiating dosing. This latency period is very short even
for an alkylating agent.
Results of the Ames/salmonella and Human Peripheral Blood Lymphocyte (HPBL) chromosome
aberration tests showed a positive mutagenicity response.
6. PHARMACEUTICAL PARTICULARS
Anhydrous lactose
Sodium starch glycolate Type A
Colloidal anhydrous silica
Tartaric acid
Stearic acid
Capsule shell
Gelatin
Titanium dioxide (E171)
Printing ink
Shellac
Propylene Glycol
Red iron oxide (E172)
Yellow iron oxide (E172)
Titanium dioxide (E171)
6.4 Special precautions for storage
Store in the original package.
Keep the bottle tightly closed in order to protect from moisture.
6.5 Nature and contents of container
Amber glass bottle with white polypropylene child-resistant screw cap equipped with an induction seal
of polyethylene
containing 5 or 20 capsules.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
Capsules should not be opened. If a capsule becomes damaged, contact of the powder contents with
skin or mucous membrane must be avoided. If Temozolomide Teva comes into contact with skin or
mucosa, it should be washed immediately and thoroughly with soap and water.
Patients should be advised to keep capsules out of the reach and sight of children, preferably in a
locked cupboard. Accidental ingestion can be lethal for children.
Any unused product or waste material should be disposed of in accordance with local requirements.
7. MARKETING AUTHORISATION HOLDER
Teva Pharma B.V.
Computerweg 10
3542 DR Utrecht
The Netherlands
8. MARKETING AUTHORISATION NUMBER(S)
EU/1/09/606/005
EU/1/09/606/006
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
Agency http://www.ema.europa.eu/
What Temozolomide Teva contains
-
The other ingredients are:
o
capsule content:
anhydrous lactose, colloidal anhydrous silica, sodium starch glycolate
type A, tartaric acid, stearic acid.
o
capsule shell (including printing ink):
gelatin, titanium dioxide (E 171), shellac,
propylene glycol, black iron oxide (E 172).
What Temozolomide Teva looks like and contents of the pack
Temozolomide Teva
250 mg hard capsules have a white opaque body and cap with two stripes in
black ink on the cap and with “T 250 mg” in black ink on the body.
The hard capsules for oral use are dispensed in amber glass bottles containing 5 or 20 capsules.
The active substance is temozolomide. Each capsule contains 250 mg temozolomide.
Not all pack sizes may be marketed.
Marketing Authorization Holder
Teva Pharma B.V.
Computerweg 10
3542 DR Utrecht
The Netherlands
Manufacturer
NerPharMa S.r.l.
Viale Pasteur, 10
20014 Nerviano (MI)
Italy
Pharmachemie BV.
Swensweg 5
2031 GA Haarlem
The Netherlands
TEVA Santé SA
Rue Bellocier
89107 Sens
France
Haupt Pharma Amareg GmbH
Donaustaufer Straβe 378
93055 Regensburg
Germany
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
Teva Pharma Belgium N.V./S.A.
Tel/Tél: +32 3 820 73 73
Luxembourg/Luxemburg
Teva Pharma Belgium S.A.
Tél: +32 3 820 73 73
България
Тева Фармасютикълс България ЕООД
Teл: +359 2 489 95 82
Magyarország
Teva Magyarország Zrt
Tel.: +36 1 288 64 00
Česká republika
Teva Pharmaceuticals CR, s.r.o.
Tel: +420 251 007 111
Malta
Teva Ελλάς Α.Ε.
Τel: +30 210 72 79 099
Danmark
Teva Denmark A/S
Tlf: +45 44 98 55 11
Nederland
Teva Nederland B.V.
Tel: +31 (0) 800 0228400
Deutschland
Teva Generics GmbH
Tel: (49) 351 834 0
Norge
Teva Sweden AB
Tlf: (46) 42 12 11 00
Eesti
Teva Eesti esindus UAB Sicor Biotech
Eesti filiaal
Tel: +372 611 2409
Österreich
Teva Generics GmbH
Tel: (49) 351 834 0
Ελλάδα
Teva Ελλάς Α.Ε.
Τηλ: +30 210 72 79 099
Polska
Teva Pharmaceuticals Polska Sp. z o.o.
Tel.: +(48) 22 345 93 00
España
Teva Genéricos Española, S.L.U.
Tél: +(34) 91 387 32 80
Portugal
Teva Pharma - Produtos Farmacêuticos Lda
Tel: (351) 214 235 910
France
Teva Santé
Tél: +(33) 1 55 91 7800
România
Teva Pharmaceuticals S.R.L
Tel: +4021 212 08 90
Ireland
Teva Pharmaceuticals Ireland
Tel: +353 (0)42 9395 892
Slovenija
Pliva Ljubljana d.o.o.
Tel: +386 1 58 90 390
Ísland
Teva UK Limited
Sími: +(44) 1323 501 111.
Slovenská republika
Teva Pharmaceuticals Slovakia s.r.o.
Tel: +(421) 2 5726 7911
Italia
Teva Italia S.r.l.
Tel: +(39) 0289179805
Suomi/Finland
Teva Sweden AB
Puh/Tel: +(46) 42 12 11 00
Κύπρος
Teva Ελλάς Α.Ε.
Τηλ: +30 210 72 79 099
Sverige
Teva Sweden AB
Tel: +(46) 42 12 11 00
Latvija
UAB Sicor Biotech filiāle Latvijā
Tel: +371 67 784 980
United Kingdom
Teva UK Limited
Tel: +(44) 1323 501 111
Lietuva
UAB “Sicor Biotech”
Tel: +370 5 266 02 03
This leaflet was last approved in
<Detailed information on this medicine is available on the European Medicines Agency web site:
http://www.ema.europa.eu/.
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/temozolomide_teva.html
Copyright © 1995-2021 ITA all rights reserved.
|




















































































































































































































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































