Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Thalidomide Celgene 50 mg hard capsules
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each capsule contains 50 mg of thalidomide.
Excipient:
Each capsule contains 257.2 mg of anhydrous lactose.
For a full list of excipients, see section 6.1.
Hard capsule.
White opaque capsules marked “Thalidomide 50 mg Celgene”.
4.1 Therapeutic indications
Thalidomide Celgene in combination with melphalan and prednisone as first line treatment of patients
with untreated multiple myeloma, aged ≥ 65 years or ineligible for high dose chemotherapy.
Thalidomide Celgene is prescribed and dispensed according to the Thalidomide Celgene Pregnancy
Prevention Programme (see section 4.4).
4.2
Posology and method of administration
Thalidomide treatment must be initiated and monitored under the supervision of physicians with
expertise in managing immunomodulatory or chemotherapeutic agents and a full understanding of the
risks of thalidomide therapy and monitoring requirements (see section 4.4).
Recommended posology in adults:
The recommended oral dose is 200 mg per day.
A maximum number of 12 cycles of 6 weeks should be used.
Thalidomide Celgene should be taken as a single dose at bedtime, to reduce the impact of somnolence.
Thalidomide Celgene can be taken with or without food.
Patients should be monitored for: thromboembolic events; peripheral neuropathy; rash/skin reactions;
bradycardia, syncope and somnolence (see sections 4.4 and 4.8). Dose delay, reduction or
discontinuation, dependent upon the NCI CTC grade, may be necessary.
Thromboembolic events:
Thromboprophylaxis should be administered for at least the first 5 months of treatment especially in
patients with additional thrombotic risk factors. Prophylactic antithrombotic medicinal products, such
as low molecular weight heparins or warfarin, should be recommended. The decision to take
antithrombotic prophylactic measures should be made after careful assessment of an individual
patient’s underlying risk factors (see sections 4.4, 4.5 and 4.8).
If the patient experiences any thromboembolic events, treatment must be discontinued and standard
anticoagulation therapy started. Once the patient has been stabilised on the anticoagulation treatment
and any complications of the thromboembolic event have been managed, the thalidomide treatment
may be restarted at the original dose dependent upon a benefit risk assessment. The patient should
continue anticoagulation therapy during the course of thalidomide treatment.
Peripheral neuropathy:
Dose modifications due to peripheral neuropathy are described in Table 1.
Table 1: Recommended dose modifications for Thalidomide Celgene related neuropathy in first line
treatment of multiple myeloma.
Modification of dose and regimen
Grade 1 (paraesthesia, weakness and/or loss of
reflexes) with no loss of function
Continue to monitor the patient with clinical
examination. Consider reducing dose if
symptoms worsen. However, dose reduction is
not necessarily followed by improvement of
symptoms.
Grade 2 (interfering with function but not with
activities of daily living)
Reduce dose or interrupt treatment and
continue to monitor the patient with clinical
and neurological examination. If no
improvement or continued worsening of the
neuropathy, discontinue treatment. If the
neuropathy resolves to Grade 1 or better, the
treatment may be restarted, if the benefit/risk is
favourable.
Grade 3 (interfering with activities of daily
living)
Grade 4 (neuropathy which is disabling)
Elderly:
No specific dose adjustments are recommended for the elderly.
Patients with renal or hepatic impairment:
Thalidomide Celgene has not formally been studied in patients with impaired renal or hepatic function.
No specific dose recommendations for these patient populations are available. Patients with severe
organ impairment should be carefully monitored for adverse reactions.
Paediatric patients:
Thalidomide Celgene is not recommended for use in children below 18 years of age as safety and
efficacy have not been established.
−
Hypersensitivity to thalidomide or to any of the excipients.
−
Pregnant women (see section 4.6).
−
Women of childbearing potential unless all the conditions of the Thalidomide Celgene Pregnancy
Prevention Programme are met (see sections 4.4 and 4.6).
−
Patients unable to follow or comply with the required contraceptive measures (see section 4.4).








4.4 Special warnings and precautions for use
Teratogenic effects:
Thalidomide is a powerful human teratogen, inducing a high frequency of severe and life-
threatening birth defects. Thalidomide must never be used by women who are pregnant or by
women who could become pregnant unless all the conditions of the Thalidomide Celgene
Pregnancy Prevention Programme are met.
The conditions of the Thalidomide Celgene Pregnancy
Prevention Programme must be fulfilled for all male and female patients.
Criteria for women of non-childbearing potential:
A female patient or a female partner of a male patient is considered to have childbearing potential
unless she meets at least one of the following criteria:
•
Age ≥ 50 years and naturally amenorrhoeic for ≥ 1 year*.
•
Premature ovarian failure confirmed by a specialist gynaecologist.
•
Previous bilateral salpingo-oophorectomy, or hysterectomy.
•
XY genotype, Turner’s syndrome, uterine agenesis.
*Amenorrhoea following cancer therapy does not rule out childbearing potential.
Counselling:
For women of childbearing potential, thalidomide is contraindicated unless all of the following are
met:
•
She understands the teratogenic risk to the unborn child
•
She understands the need for effective contraception, without interruption, 4 weeks before
starting treatment, throughout the entire duration of treatment, and 4 weeks after the end of
treatment
•
Even if a woman of childbearing potential has amenorrhea she must follow all the advice on
effective contraception
•
She should be capable of complying with effective contraceptive measures
•
She is informed and understands the potential consequences of pregnancy and the need to
rapidly consult if there is a risk of pregnancy
•
She understands the need to commence the treatment as soon as thalidomide is dispensed
following a negative pregnancy test
•
She understands the need and accepts to undergo pregnancy testing every 4 weeks
•
She acknowledges that she understands the hazards and necessary precautions associated with
the use of thalidomide.
As thalidomide is found in semen, male patients taking thalidomide must meet the following
conditions:
•
Understand the teratogenic risk if engaged in sexual activity with a pregnant woman.
•
Understand the need for the use of a condom if engaged in sexual activity with a pregnant
woman or a woman of childbearing potential not using effective contraception.
The prescriber must ensure that:
•
The patient complies with the conditions of the Thalidomide Celgene Pregnancy Prevention
Programme
•
The patient confirms that he (she) understand the aforementioned conditions.
Contraception:
Women of childbearing potential must use one effective method of contraception for 4 weeks before
therapy, during therapy, and during 4 weeks after thalidomide therapy and even in case of dose
interruption unless the patient commits to absolute and continuous abstinence confirmed on a monthly
basis. If not established on effective contraception, the patient must be referred preferably to an
appropriately trained healthcare professional for contraceptive advice in order that contraception can
be initiated.



The following can be considered to be examples of effective methods of contraception:
•
Subcutaneous hormonal implant
•
Levonorgestrel-releasing intrauterine system (IUS)
•
Medroxyprogesterone acetate depot
•
Tubal sterilisation
•
Sexual intercourse with a vasectomised male partner only; vasectomy must be confirmed by two
negative semen analyses
•
Ovulation inhibitory progesterone-only pills (i.e., desogestrel)
Because of the increased risk of venous thromboembolism in patients with multiple myeloma,
combined oral contraceptive pills are not recommended (see section 4.5). If a patient is currently using
combined oral contraception the patient should switch to one of the effective method listed above. The
risk of venous thromboembolism continues for 4−6 weeks after discontinuing combined oral
contraception.
Pregnancy testing:
Medically supervised pregnancy tests with a minimum sensitivity of 25 mIU/ml must be performed for
women of childbearing potential as outlined below. This requirement includes women of childbearing
potential who practice absolute and continuous abstinence.
Prior to starting treatment
A medically supervised pregnancy test should be performed during the consultation, when thalidomide
is prescribed or in the 3 days prior to the visit to the prescriber once the patient had been using
effective contraception for at least 4 weeks. The test should ensure the patient is not pregnant when she
starts treatment with thalidomide.
Follow-up and end of treatment
A medically supervised pregnancy test should be repeated every 4 weeks, including 4 weeks after the
end of treatment. These pregnancy tests should be performed on the day of the prescribing visit or in
the 3 days prior to the visit to the prescriber.
Men:
As thalidomide is found in semen, male patients must use condoms during treatment and for 1 week
after dose interruption and/or cessation of treatment if their partner is pregnant or is of childbearing
potential not using effective contraception.
Prescribing and dispensing restrictions:
For women of childbearing potential, prescriptions of Thalidomide Celgene should be limited to 4
weeks of treatment and continuation of treatment requires a new prescription. Ideally, pregnancy
testing, issuing a prescription and dispensing should occur on the same day. Dispensing of thalidomide
should occur within a maximum of 7 days of the prescription.
For all other patients, prescriptions of Thalidomide Celgene should be limited to 12 weeks and
continuation of treatment requires a new prescription.
Additional precautions:
Patients should be instructed never to give this medicinal product to another person and to return any
unused capsules to their pharmacist at the end of treatment.
Patients should not donate blood or semen during therapy or for 1 week following discontinuation of
thalidomide.
Educational materials:
In order to assist patients in avoiding foetal exposure to thalidomide and to provide additional
important safety information, the Marketing Authorisation holder will provide educational material to
healthcare professionals. The Thalidomide Celgene Pregnancy Prevention Programme reinforces the
warnings about the teratogenicity of thalidomide, provides advice on contraception before therapy is
started and provides guidance on the need for pregnancy testing. Full patient information about the
teratogenic risk and the pregnancy prevention measures as specified in the Thalidomide Celgene
Pregnancy Prevention Programme should be given by the physician to women of childbearing
potential and, as appropriate, to male patients.
Deep venous thrombosis and pulmonary embolism:
An increased risk of deep venous thrombosis (DVT) and pulmonary embolus (PE) has been reported in
patients treated with thalidomide (see section 4.8). The risk appears to be greatest during the first
5 months of therapy. Thromboprophylaxis and dosing/anticoagulation therapy recommendations are
provided in section 4.2.
Previous history of thromboembolic events or concomitant administration of erythropoietic agents or
other agents such as hormone replacement therapy, may also increase thrombotic risk in these patients.
Therefore, these agents should be used with caution in multiple myeloma patients receiving
thalidomide with prednisone and melphalan. Particularly, a haemoglobin concentration above 12g/dl
should lead to discontinuation of erythropoietic agents.
Patients and physicians are advised to be observant for the signs and symptoms of thromboembolism.
Patients should be instructed to seek medical care if they develop symptoms such as shortness of
breath, chest pain, arm or leg swelling.
Peripheral neuropathy:
Peripheral neuropathy is a very common, potentially severe, adverse reaction to treatment with
thalidomide that may result in irreversible damage (see section 4.8). In a phase 3 study, the median
time to first neuropathy event was 42.3 weeks.
If the patient experiences peripheral neuropathy, follow the dose and schedule modification instruction
provided in
section 4.2.
Careful monitoring of patients for symptoms of neuropathy is recommended. Symptoms include
paraesthesia, dysaesthesia, discomfort, abnormal co-ordination or weakness.
It is recommended that clinical and neurological examinations are performed in patients prior to
starting thalidomide therapy, and that routine monitoring is carried out regularly during treatment.
Medicinal products known to be associated with neuropathy should be used with caution in patients
receiving thalidomide (see section 4.5).
Thalidomide may also potentially aggravate existing neuropathy and should therefore not be used in
patients with clinical signs or symptoms of peripheral neuropathy unless the clinical benefits outweigh
the risks.
Syncope and bradycardia:
Patients should be monitored for syncope and bradycardia and dose reduction or discontinuation may
be required.
Skin reactions:
If at anytime the patient experiences a toxic skin reaction e.g. Stevens-Johnson Syndrome, the
treatment should be discontinued permanently.
Somnolence:
Thalidomide frequently causes somnolence. Patients should be instructed to avoid situations where
somnolence may be a problem and to seek medical advice before taking other medicinal products
known to cause somnolence. Patients should be monitored and dose reduction may be required.
Patients should be advised as to the possible impairment of mental and/or physical abilities required
for the performance of hazardous tasks (see
section 4.7).
Tumour lysis syndrome:
The patients at risk of tumour lysis syndrome are those with high tumour burden prior to treatment.
These patients should be monitored closely and appropriate precautions taken.
Patients with renal or hepatic impairment:
Studies conducted in healthy subjects and patients with multiple myeloma suggest that Thalidomide is
not influenced to any significant extent by renal or hepatic function (see section 5.2). However, this
has not formally been studied in patients with impaired renal or hepatic function; therefore patients
with severe renal or hepatic impairment should be carefully monitored for any adverse effects.
Lactose intolerance:
The capsules contain lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp
lactase deficiency or glucose-galactose malabsorption should not take this medicinal product.
4.5
Interaction with other medicinal products and other forms of interaction
Thalidomide is a poor substrate for cytochrome P450 isoenzymes and therefore clinically important
interactions with medicinal products metabolized by this enzyme system are unlikely. Non-enzymatic
hydrolysis suggests that the potential for drug-drug interactions with thalidomide is low.
Increase of sedative effects of other medicinal products:
Thalidomide has sedative properties thus may enhance the sedation induced by anxiolytics, hypnotics,
antipsychotics, H1 anti-histamines, opiate derivatives, barbiturates and alcohol. Caution should be
used when thalidomide is given in combination with medicinal products that cause drowsiness.
Bradycardic effect:
Due to thalidomide’s potential to induce bradycardia, caution should be exercised with medicinal
products having the same pharmacodynamic effect such as active substances known to induce torsade
de pointes, beta blockers or anticholinesterase agents.
Medicinal products known to cause peripheral neuropathy:
Medicinal products known to be associated with peripheral neuropathy (e.g. vincristine and
bortezomib) should be used with caution in patients receiving thalidomide.
Hormonal contraceptives:
Thalidomide does not interact with hormonal contraceptives. In 10 healthy women, the
pharmacokinetic profiles of norethindrone and ethinyl estradiol following administration of a single
dose containing 1.0 mg of norethindrone acetate and 0.75 mg of ethinyl estradiol were studied. The
results were similar with and without co-administration of thalidomide 200 mg/day to steady-state
levels. However, combined hormonal contraceptives are not recommended due to the increased risk of
venous thrombo-embolic disease.
Warfarin:
Multiple dose administration of 200 mg thalidomide q.d. for 4 days had no effect on the international
normalized ratio (INR) in healthy volunteers. However, due to the increased risk of thrombosis in
cancer patients, and a potentially accelerated metabolism of warfarin with corticosteroids, close
monitoring of INR values is advised during thalidomide-prednisone combination treatment as well as
during the first weeks after ending these treatments.
Digoxin:
Thalidomide does not interact with digoxin. In 18 healthy male volunteers, multiple dose
administration of 200 mg thalidomide had no apparent effect on the single dose pharmacokinetics of
digoxin. In addition, single dose administration of 0.5 mg digoxin had no apparent effect on
thalidomide pharmacokinetics. It is not known whether the effect will be different in multiple
myeloma patients.
4.6 Fertility, pregnancy and lactation
Thalidomide Celgene is contraindicated during pregnancy and in women of childbearing potential
unless all the conditions of the Thalidomide Celgene Pregnancy Prevention Programme are met (see
section 4.3)
Thalidomide is a powerful human teratogen, inducing a high frequency (about 30%) of severe and
live-threatening birth defects such as: ectromelia (amelia, phocomelia, hemimelia) of the upper and/or
lower extremities, microtia with abnormality of the external acoustic meatus (blind or absent), middle
and internal ear lesions (less frequent), ocular lesions (anophthalmia, microphthalmia), congenital
heart disease, renal abnormalities. Other less frequent abnormalities have also been described.
Women of childbearing potential:
Women of childbearing potential must use one effective method of contraception for 4 weeks before
therapy, during therapy, and during 4 weeks after thalidomide therapy (see section 4.4).
If pregnancy occurs in a woman treated with thalidomide, treatment must be stopped
immediately
and
the patient should be referred to a physician specialised or experienced in teratology for evaluation and
advice.
Male patients with female partners of childbearing potential:
As thalidomide is found in semen, male patients must use condoms during treatment and for 1 week
after dose interruption and/or cessation of treatment when having sexual intercourse with a pregnant
woman or with a woman with childbearing potential who is not using effective contraception.
If pregnancy occurs in a partner of male patient taking thalidomide, the female partner should be
referred to a physician specialised or experienced in teratology for evaluation and advice.
Lactation:
It is unknown whether thalidomide is excreted in human breast milk. Animal studies have shown
excretion of thalidomide in breast milk. Therefore breast-feeding should be discontinued during
therapy with thalidomide.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed.
However thalidomide may cause somnolence and blurred vision (see section 4.8). If affected, patients
should be instructed not to drive cars, use machines or perform hazardous tasks while being treated
with thalidomide.
Most patients taking thalidomide can be expected to experience adverse reactions. The most
commonly observed adverse reactions associated with the use of thalidomide in combination with
melphalan and prednisone are: neutropenia, leukopenia, constipation, somnolence, paraesthesia,
peripheral neuropathy, anaemia, lymphopenia, thrombocytopenia, dizziness, dysaesthesia, tremor and
peripheral oedema.
The clinically important adverse reactions associated with the use of thalidomide in combination with
melphalan and prednisone or dexamethasone include: deep vein thrombosis and pulmonary embolism,
peripheral neuropathy, severe skin reactions including Stevens Johnson Syndrome and toxic epidermal
necrolysis, syncope, bradycardia, and dizziness (see sections 4.2, 4.4 and 4.5).
Table 2 contains only the adverse reactions for which a causal relationship with medicinal product
treatment could reasonably be established. Frequencies given are based on the observations during a
pivotal comparative clinical study investigating the effect of thalidomide in combination with
melphalan and prednisone in previously untreated multiple myeloma patients. In addition to the
adverse reactions noted in the pivotal study, adverse reactions related to thalidomide in combination
with dexamethasone and also those based on post-marketing experience with the medicinal product are
provided after Table 2.
Frequencies are defined as: very common (≥1/10), common (≥1/100 to <1/10); uncommon (≥1/1000 to
<1/100); rare (≥1/10,000 to <1/1000); very rare (<1/10,000, not known (cannot be estimated from the
available data)). Within each frequency grouping, adverse reactions are presented in order of
decreasing seriousness.
Table 2: Frequency of adverse reactions with thalidomide in combination with melphalan and
prednisone
Cardiac failure
Bradycardia
Blood and lymphatic system disorders
Neutropenia
Leukopenia
Anaemia
Lymphopenia
Thrombocytopenia
Peripheral neuropathy*
Tremor
Dizziness
Paraesthesia
Dysaesthesia
Somnolence
Respiratory, thoracic and mediastinal
disorders
Pulmonary embolism*
Interstitial lung disease
Bronchopneumopathy
Dyspnea
Gastrointestinal disorders
Skin and subcutaneous tissue disorders
Toxic skin eruption
Rash
Dry skin
Infections and infestations
General disorders and administration site
conditions
Confusional state
Depression
* - See detailed section below
In addition to the adverse reactions outlined above thalidomide in combination with dexamethasone in
other clinical studies led to the very common adverse reaction of fatigue; common adverse reactions of
transient ischaemic event, syncope, vertigo, hypotension, mood altered, anxiety, blurred vision, nausea
and dyspepsia; and uncommon adverse reactions of cerebrovascular accident, diverticular perforation,
peritonitis, orthostatic hypotension and bronchitis.
Additional adverse reactions related to post-marketing experience with thalidomide and not seen in the
pivotal study include: toxic epidermal necrolysis, intestinal obstruction, hypothyroidism, sexual
dysfunction, tumour lysis syndrome, gastro-intestinal perforations, hypersensitivity, hearing impaired
or deafness and renal failure.
Blood and lymphatic system disorders:
Adverse reactions for haematological disorders are provided compared to the comparator arm, as the
comparator has a significant effect on these disorders (Table 3)
















Table 3: Comparison of haematological disorders for the melphalan, prednisone (MP) and melphalan,
prednisone, thalidomide (MPT) combinations in study IFM 99-06 (see section 5.1)
Thrombocytopenia
19 (9.8)
Additional adverse reactions from post-marketing experience with thalidomide and not seen in the
pivotal study include febrile neutropenia and pancytopenia.
Teratogenicity:
The risk of intra-uterine death or severe birth defects, primarily phocomelia, is extremely high.
Thalidomide must not be used at any time during pregnancy (see sections 4.4 and 4.6).
Thromboembolic events:
An increased risk of DVT and PE has been reported in patients treated with thalidomide (see section
4.4).
Peripheral neuropathy:
Peripheral neuropathy is a very common, potentially severe, adverse reaction of treatment with
thalidomide that may result in irreversible damage (see section 4.4). Peripheral neuropathy generally
occurs following chronic use over a period of months. However, reports following relatively short-
term use also exist. Incidence of neuropathy events leading to discontinuation, dose reduction or
interruption increases with cumulative dose and duration of therapy. Symptoms may occur some time
after thalidomide treatment has been stopped and may resolve slowly or not at all.
Eighteen cases of overdose have been reported in the literature concerning doses up to 14.4 g. No
fatalities have been reported and all overdose patients recovered without sequelae. There is no specific
antidote for a thalidomide overdose. In the event of an overdose, the patient’s vital signs should be
monitored and appropriate supportive care given to maintain blood pressure and respiratory status.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: immunosuppressive agent, ATC code: L04AX 02.
Thalidomide has a chiral centre and is used clinically as a racemate of (+)-(R)- and (-)-(S)-
thalidomide. The spectrum of activity of thalidomide is not fully characterised.
Thalidomide shows immunomodulatory anti-inflammatory and potential anti-neoplastic activities.
Data from
in vitro
studies and clinical trials suggest that the immunomodulatory, anti-inflammatory
and anti-neoplastic effects of thalidomide may be related to suppression of excessive tumour necrosis
factor-alpha (TNF-α) production, down-modulation of selected cell surface adhesion molecules
involved in leukocyte migration and anti-angiogenic activity. Thalidomide is also a non-barbiturate
centrally active hypnotic sedative. It has no anti-bacterial effects.












Clinical efficacy:
Results from IFM 99-06, a Phase 3, randomised, open label, parallel group, multicentre study have
demonstrated a survival advantage when thalidomide is used in combination with melphalan and
prednisone for 12 cycles of 6 weeks in the treatment of newly diagnosed multiple myeloma patients. In
this study the age range of patients was 65-75 years, with 41% (183/447) of patients 70 years old or
older. The median dose of thalidomide was 217 mg and >40% of patients received 9 cycles. Melphalan
and prednisone were dosed at 0.25 mg/kg/day and 2 mg/kg/day respectively on days 1 to 4 of each
6 weeks cycle.
Further to the per protocol analysis, an update was conducted for the IFM 99-06 study providing an
additional 15 months follow-up data. The median overall survival (OS) was 51.6 ± 4.5 and 33.2 ±
3.2 months in the MPT and MP groups, respectively (97.5% CI 0.42 to 0.84). This 18 month
difference was statistically significant with a hazard ratio of reduction of risk of death in the MPT arm
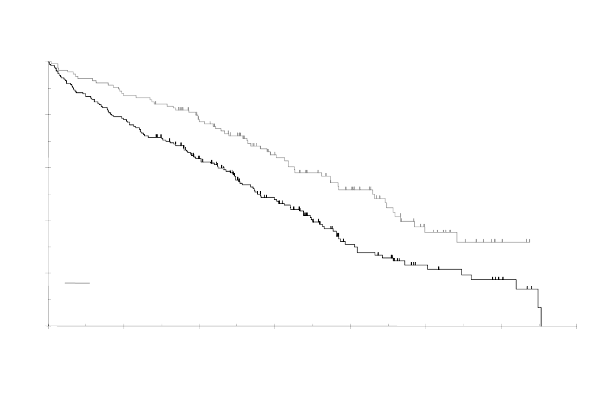
of 0.59, 97.5% confidence interval of 0.42-0.84 and p-value of <0.001 (see Figure 1).
Figure 1: Overall survival according to treatment
Survival time
median ± se (month)
Time from randomization (month )
5.2 Pharmacokinetic properties
Absorption:
Absorption of thalidomide is slow after oral administration. The maximum plasma concentrations are
reached 1-5 hours after administration. Co-administration of food delayed absorption but did not alter
the overall extent of absorption.
Distribution:
The plasma protein binding of the (+)-(R) and (-)-(S) enantiomers was found to be 55% and 65%
respectively. Thalidomide is present in the semen of male patients at levels similar to plasma
concentrations. Therefore, because of the known severe teratogenic effects of the product, during
treatment with thalidomide and for 1 week after stopping the treatment, male patients must use
condoms if their partner is pregnant or is of childbearing potential not using effective contraception
(see section 4.4).
Metabolism:
Thalidomide is metabolised almost exclusively by non-enzymatic hydrolysis. In plasma, unchanged
thalidomide represents 80% of the circulatory components. Unchanged thalidomide was a minor
component (<3% of the dose) in urine. In addition to thalidomide, hydrolytic products N-(o-
carboxybenzoyl) glutarimide and phthaloyl isoglutamine formed via non-enzymatic processes are also
present in plasma and in majority in urine. Oxidative metabolism does not contribute significantly to
the overall metabolism of thalidomide. There is minimal cytochrome P450 catalysed hepatic
metabolism of thalidomide. There are in vitro data indicating that prednisone may give rise to enzyme
induction which could reduce the systemic exposure of concomitantly used medicinal products. The in
vivo relevance of these findings is unknown.
Elimination:
The mean elimination half-life of thalidomide in plasma following single oral doses between 50 mg
and 400 mg was 5.5 to 7.3 hours. Total systemic exposure (AUC) is proportional to dose at single-dose
conditions. No time dependency of the pharmacokinetics has been observed. Following a single oral
dose of 400mg of radio-labelled thalidomide, the total mean recovery was 93.6% of the administered
dose by Day 8. The majority of the radioactive dose was excreted within 48 hour following dose
administration. The major route of excretion was via the urine (>90%) while faecal excretion was
minor.
There is a linear relationship between body weight and estimated thalidomide clearance; in MM
patients with body weight from 47-133kg, thalidomide clearance ranged from approximately 6-12 L/h,
representing an increase in thalidomide clearance of 0.621 L/h per 10kg body weight increase.
However, the distribution of thalidomide is not influenced by age, gender, renal function and blood
chemistry variables, to any significant level.
Hepatic and renal insufficiency:
The extent of thalidomide metabolism by the liver cytochrome P450 system is minimal and intact
thalidomide is not excreted by the kidney. Measures of renal function (CLcr) and liver function (blood
chemistry) indicate minimal effect of kidney and liver function on the pharmacokinetics of
thalidomide. As such the metabolism of thalidomide is not expected to be affected by hepatic or renal
dysfunction. Data from patients with end-stage renal disease suggest no impact of kidney function on
thalidomide pharmacokinetics. However, considering that pharmacologically active metabolites are
eliminated via urine, it is advised that patients with severe renal impairment should be carefully
monitored for any adverse reactions.
5.3
Preclinical safety data
In the male dog, after one year of dosing, reversible bile plugs in canaliculi were observed at exposures
greater than 1.9 fold the human exposure.
Decreased platelet counts were noted in the mouse and rat studies. The latter appears to be related to
thalidomide and occurred at exposures greater than 2.4 fold the human exposure. This decrease did not
result in clinical signs.
In a one-year dog study, enlarged and/or blue discoloration of mammary glands and prolonged estrus
were observed in females at exposures equal to 1.8 or greater than 3.6-fold the human exposure,
respectively. The relevance to humans is unknown.
The effect of thalidomide on thyroid function was assessed in both rats and dogs. No effects were
observed in dogs; however in rats, there was an apparent dose-dependent decrease in total and free T4
that was more consistent in the female.
No mutagenic or genotoxic effect has been revealed when thalidomide was assayed in a standard
battery of genotoxicity tests. No evidence of carcinogenicity was observed at exposures approximately
15, 13 and 39 times the estimated clinical AUC at the recommended starting dose in mice, male rats
and female rats respectively.
Animal studies have demonstrated differences in species susceptibility to the teratogenic effects of
thalidomide. In humans, thalidomide is a proven teratogen.
A study in rabbits demonstrated no effect on fertility indices in males or females although testicular
degeneration was observed in males.
A peri and postnatal toxicity study performed in rabbits with thalidomide administered at doses up to
500 mg/kg/day resulted in abortions, increased stillbirths and decreased pup viability during lactation.
Pups from mothers treated with thalidomide had increased abortions, reduced body weight gain,
alterations in learning and memory, decreased fertility, and reduced pregnancy index.
PHARMACEUTICAL PARTICULARS
Capsule contents:
Anhydrous lactose
Microcrystalline cellulose
Crospovidone (Type A)
Povidone (K90)
Stearic acid
Colloidal anhydrous silica
Capsule shell:
Gelatin
Titanium dioxide (E171)
Printing ink:
Shellac
Black iron oxide (E172)
Propylene glycol
6.4
Special precautions for storage
This medicinal product does not require any special storage conditions.
6.5
Nature and contents of container
PVC/PE/Aclar/aluminium blister containing 14 capsules
Pack sizes: 28 capsules (two blisters) in a wallet card.
6.6 Special precautions for disposal
All unused capsules should be returned to the pharmacist at the end of treatment.
MARKETING AUTHORISATION HOLDER
Celgene Europe Ltd
Riverside House
Riverside Walk
Windsor
SL4 1NA
United Kingdom
Tel: +44 (0)1753 240600
Fax: +44 (0)1753 240899
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
HOLDER RESPONSIBLE FOR BATCH RELEASE
CONDITIONS OF THE MARKETING AUTHORISATION
MANUFACTURING AUTHORISATION
A. MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer responsible for batch release
Penn Pharmaceutical Services Limited
Tafarnaubach Industrial Estate
Tredegar
Gwent
NP22 3AA
United Kingdom
Celgene Europe Limited
Riverside House
Riverside Walk
Windsor
SL4 1NA
United Kingdom.
The printed package leaflet of the medicinal product must state the name and address of the
manufacturer responsible for the release of the concerned batch.
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to restricted medical prescription.(see Annex 1: Summary of Product
Characteristics, section 4.2.).
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
1.
The MAH shall agree the details of a controlled distribution system with the National
Competent Authorities and must implement such programme nationally to ensure that:
o
Prior to launch, all doctors and pharmacists who intend to prescribe or dispense
Thalidomide Celgene receive a Dear Healthcare Professional letter as described below.
o
Prior to prescribing all healthcare professionals who intend to prescribe (and in agreement
with the National Competent Authority, dispense) Thalidomide Celgene are provided
with an Educational Healthcare Professional’s Kit containing the following:
o
Healthcare professional booklet
o
Patient booklets
o
Patient cards
o
Summary of Product Characteristics, Package Leaflet and Labelling
2.
The MAH shall implement a Pregnancy Prevention Programme (PPP) in each Member
State. Details of the PPP should be agreed wth the National Competent Authorities in each
Member State and put in place prior to the marketing of the medicinal product.
3.
The MAH should agree the final text of the Dear Healthcare Professional letter and the
contents of the Educational Healthcare Professional’s Kit with the National Competent
Authority in each Member State prior to marketing of the product and ensure that the
materials contain the key elements as described below.
4.
The MAH should agree on the implementation of the patient card system in each Member
State.
5.
The MAH should ensure that the educational materials are provided to and reviewed by the
national patients’ organisations or if such an organisation does not exist or can not be
involved, by a relevant patients group. Patients involved should be preferably naïve to the
history of thalidomide. Results of the user testing will have to be provided to the national
competent authority and final materials validated at a national level.
6.
The MAH should also agree with each Member State prior to the launch of the product:
o
The most appropriate strategies to monitor the off label use within national territories
o
The collection of detailed data with at least patient demographics and indication in order
to monitor closely the off-label use within national territory
o
The set-up of national measures to assess the effectiveness of and compliance with the
PPP.
7. The MAH shall notify the EMA and the appropriate national patients and victims
representatives of the proposed launch date before launch in each Member State.
Key elements to be included
Dear Healthcare Professional letter
The Dear Healthcare Professional letter will consist of two parts:
•
Core text as agreed by the CHMP
•
National specific requirements agreed with the National Competent Authority regarding:
o
Distribution of the product
o
Procedures to ensure that all appropriate measures have been performed prior to
thalidomide being dispensed
Educational healthcare professional’s kit
The educational healthcare professional’s kit shall contain the following elements:
•
Healthcare professional booklet
o
History of thalidomide, background on Thalidomide Celgene and its licensed
indication
o
Posology
o
Maximum duration of prescription
4 weeks for women with childbearing potential
12 weeks for men and women without childbearing potential
o
Teratogenicity and the need to avoid foetal exposure
o
Obligations of healthcare professionals who intend to prescribe or dispense
Thalidomide Celgene including
The need to provide comprehensive advice and counselling to patients
That patients should be capable of complying with the requirements for the
safe use of thalidomide
Need to provide patients with the appropriate patient educational material
Report any pregnancy, neuropathy or other adverse events to Celgene and the
local health authority (if applicable to a Member State) using the forms
provided in the “Educational Healthcare Professional’s Kit”
o
Safety advice relevant to all patients
Description and management of thromboembolic and cardiovascular events,
and peripheral neuropathy
Disposal of unwanted medicine
Not to donate blood during treatment and for one week after treatment ends
o
Algorithm for Pregnancy Prevention Plan implementation
This shall assist with patient categorisation, and determination of required
pregnancy prevention and testing measures.
o
Pregnancy Prevention Programme information
Definition of women of childbearing potential (WCBP) and actions the
prescriber should take if childbearing status is unclear
Information on what is effective contraception
Safety advice for WCBP
•
Need to avoid foetal exposure
•
Pregnancy prevention requirement, definition and need for adequate
contraceptive methods
•
That if she needs to change or stop using her method of contraception
she should inform:
- the physician prescribing her contraception that she is on
thalidomide
- the physician prescribing thalidomide that she has stopped or
changed her method of contraception
•
Pregnancy testing requirements
o
Advice on suitable tests
o
Frequency (before commencing, monthly during treatment
and after finishing treatment)
•
Need to stop Thalidomide Celgene immediately upon suspicion of
pregnancy
•
Need to tell treating doctor immediately upon suspicion of pregnancy
Safety advice for men
•
The need to avoid foetal exposure
•
That thalidomide is found in semen and the need to use condoms if
sexual partner is pregnant or is a women with childbearing potential
not using effective contraception
•
That if his partner becomes pregnant he should inform his treating
doctor immediately and always use a condom during intercourse
•
That he should not donate semen during therapy and for one week
after discontinuation of thalidomide
o
Pregnancy reporting requirements
Stop Thalidomide Celgene immediately upon suspicion of pregnancy
Refer patient to physician specialised or experienced in dealing with
teratology for advice and evaluation
Complete pregnancy reporting form as provided in the “Educational
Healthcare Professional’s Kit”
Local contact details for reporting of any suspected pregnancy
•
Pregnancy initial and outcome reporting forms
•
Post-marketing and compliance assessment (as applicable to a Member State)
•
Neuropathy and adverse reaction reporting forms
o
All Treatment Initiation Forms should contain the following elements:
Teratogenicity warning
Date of counselling
Affirmation of patient understanding regarding the risk of thalidomide and the
PPP measures
Patient details, signature and date
Prescriber name, signature and date
•
Treatment initiation forms
o
There should be 3 types of treatment initiation forms:
Female patient of childbearing potential
Female patient of non-childbearing potential
Male patient
Aim of this document i.e. as stated in the PPP: “The aim of the treatment
initiation form is to protect patients and any possible foetuses by ensuring that
patients are fully informed of and understand the risk of teratogenicity and
other adverse reactions associated with the use of thalidomide. It is not a
contract and does not absolve anybody from his/her responsibilities with
regard to the safe use of the product and prevention of foetal exposure.”
o
Treatment initiation forms for female patients with childbearing potential should also
include:
Confirmation that the physician has discussed the following:
•
The need to avoid foetal exposure
•
That if she is pregnant or plans to be, she must not take Thalidomide
Celgene
•
The need for effective contraception, without interruption, 4 weeks
before starting treatment, throughout the entire duration of treatment,
and 4 weeks after the end of treatment
•
That if she needs to change or stop using her method of contraception
she should inform:
- the physician prescribing her contraception that she is on
thalidomide
- the physician prescribing thalidomide that she has stopped or
changed her method of contraception
•
The need for pregnancy tests i.e. before treatment, every 4 weeks
during treatment and after treatment
•
The need to stop Thalidomide Celgene immediately upon suspicion of
pregnancy
•
The need to contact their doctor immediately upon suspicion of
pregnancy
•
That she should not share the treatment with any other person
•
That she should not donate blood during therapy and for one week
following discontinuation of thalidomide
•
That she should return the capsules to the pharmacist at the end of
treatment
o
Treatment initiation forms for male patients should also include:
Confirmation that the physician has discussed the following:
•
The need to avoid foetal exposure
•
That thalidomide is found in semen and the need to use condoms if
sexual partner is pregnant or is a women with childbearing potential
not on effective contraception
•
That if his partner becomes pregnant he should inform his treating
doctor immediately and always use a condom
•
That he should not donate blood or semen during therapy and for one
week following discontinuation of thalidomide
•
That he should not share the treatment with any other person
•
That he should return the capsules to the pharmacist at the end of
treatment
o
Treatment initiation forms for female patients with no childbearing potential should
also include:
Confirmation that the physician has discussed the following:
•
That she should not share the treatment with any other person
•
That she should not donate blood during therapy and for one week
following discontinuation of thalidomide
•
That she should return the capsules to the pharmacist at the end of
treatment
•
Patient cards and/or equivalent tools:
o
verification that appropriate counselling has taken place
o
documentation of childbearing status potential
o
check box (or similar) which physician ticks to confirm that patient is using effective
contraception (if female with childbearing potential)
o
verification of initial negative pregnancy test prior to start of treatment (if female with
childbearing potential)
o
pregnancy test dates and results
•
Educational patient booklets:
o
The booklets can be of 3 types or a single patient booklet that combines the
information for each patient category:
Booklet for women of childbearing potential and their partners
Booklet for women patients who are not of childbearing potential
Booklet for male patients
o
All booklets should contain the following information
That Thalidomide Celgene is teratogenic
That Thalidomide Celgene may cause thromboembolism, cardiovascular
events and neuropathy
Description of the patient card and its use in the individual Member State
National or other applicable specific arrangements for a prescription for
thalidomide to be dispensed
That Thalidomide Celgene must not be given to any other person
That the patient should not donate blood
That the patient should tell their doctor about any adverse events
That any unused capsules should be returned to the pharmacist at the end of
the treatment
o
The following information should also be provided in the appropriate booklets:
Female patient of childbearing potential
•
The need to avoid foetal exposure
•
The need for effective contraception
•
That if she needs to change or stop using her method of contraception
she should inform:
- the physician prescribing her contraception that she is on
thalidomide
- the physician prescribing thalidomide that she has stopped or
changed her method of contraception
•
The need for pregnancy tests i.e. before treatment, every 4 weeks
during treatment and after treatment
•
The need to stop Thalidomide Celgene immediately upon suspicion of
pregnancy
•
The need to contact their doctor immediately upon suspicion of
pregnancy
Male patients
•
The need to avoid foetal exposure
•
That thalidomide is found in semen and the need to use condoms if
sexual partner is pregnant or is a women with childbearing potential
not on effective contraception
•
That if his partner becomes pregnant he should inform his treating
doctor immediately and always use a condom
•
That he should not donate semen during therapy and for one week
following discontinuation of thalidomide
Pharmacovigilance system
The MAH must ensure that the system of pharmacovigilance, presented in Module 1.8.1. of the
Marketing Authorisation, is in place and functioning before and whilst the product is on the market.
Risk Management Plan
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version 10.0 of the Risk Management Plan (RMP) presented
in Module 1.8.2. of the Marketing Authorisation and any subsequent updates of the RMP agreed by
the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, the
updated RMP should be submitted at the same time as the next Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted
•
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
•
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
•
At the request of the European Medicines Agency
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
Thalidomide Celgene 50 mg hard capsules
Thalidomide
STATEMENT OF ACTIVE SUBSTANCE(S)
Each capsule contains 50 mg of thalidomide.
Also contains: Lactose. See the package leaflet for further information.
PHARMACEUTICAL FORM AND CONTENTS
METHOD AND ROUTE(S) OF ADMINISTRATION
For oral use.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
WARNING: Thalidomide causes birth defects and foetal death.
Patients must follow the Thalidomide Celgene Pregnancy Prevention Programme.
May cause drowsiness, if affected do not drive or operate machines.






















SPECIAL STORAGE CONDITIONS
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
Unused medicinal product should be returned to your pharmacist.
NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Celgene Europe Ltd
Riverside House
Riverside Walk
Windsor
SL4 1NA
United Kingdom
MARKETING AUTHORISATION NUMBER(S)
GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.



















PACKAGE LEAFLET: INFORMATION FOR THE USER
Thalidomide Celgene 50 mg hard capsules
Thalidomide
WARNING
Thalidomide causes birth defects and foetal death. Do not take thalidomide if you are pregnant
or could become pregnant. You must follow the contraception advice given to you by your
doctor.
Read all of this leaflet carefully before you start taking this medicine.
•
Keep this leaflet. You may need to read it again
•
If you have further questions, please ask your doctor or pharmacist
•
This medicine has been prescribed for you. Never pass it on to others. It may harm them, even if
their symptoms are the same as yours
•
If any of your side effects get serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist
In this leaflet:
1. What Thalidomide Celgene is and what it is used for
2. Before you take Thalidomide Celgene
3.
How to take Thalidomide Celgene
4.
Possible side effects
5. How to store Thalidomide Celgene
6. Further information
WHAT THALIDOMIDE CELGENE IS AND WHAT IT IS USED FOR
What Thalidomide Celgene is:
Thalidomide Celgene contains an active substance called thalidomide. This belongs to a group of
medicines known as ‘immunosuppressive’ medicines. These work by acting on the cells involved in
your immune system. The immune system is part of the body’s defence which helps to fight illness
and infection.
What Thalidomide Celgene is used for:
Thalidomide Celgene is used to treat multiple myeloma, a cancer of the bone marrow. Multiple
myeloma is a type of blood cancer that affects a subgroup of white blood cells that produce
antibodies. It is used in combination with two other medicines, melphalan and prednisone, for the first
line treatment of multiple myeloma in patients ≥ 65 years or those who cannot receive high dose
chemotherapy. In the principal clinical trial, this combination was associated with a prolongation of
the survival time by an average of 18 months.
To find out more about these medicines, please ask your doctor or read the Package Leaflet that comes
with these medicines.
2. BEFORE YOU TAKE THALIDOMIDE CELGENE
You will have been given specific instructions by your doctor, particularly on the effects of
thalidomide on unborn babies (outlined in the Thalidomide Celgene Pregnancy Prevention
Programme).
You will have been given a patient card or any other relevant document by your doctor. Read it
carefully and follow the related instructions.



If you do not fully understand these instructions, please ask your doctor to explain them again before
you take thalidomide. See also further information in this section under “Take special care with
Thalidomide Celgene” and “Pregnancy”.
Do not take Thalidomide Celgene:
•
If you are pregnant or think you may be pregnant or are planning to become pregnant,
as
Thalidomide Celgene causes birth defects and foetal death.
•
If you are able to become pregnant but are unable to follow the necessary pregnancy prevention
measures (outlined in the Thalidomide Celgene Pregnancy Prevention Programme).
•
If you are allergic (hypersensitive) to thalidomide or any of the other ingredients of Thalidomide
Celgene (listed in section 6. "What Thalidomide Celgene contains:”).
Do not take Thalidomide Celgene if any of the above applies to you. If you are not sure, talk to your
doctor or pharmacist before taking Thalidomide Celgene.
Take special care with Thalidomide Celgene:
Talk to your doctor before taking this medicine in the following situations:
For all patients
taking Thalidomide Celgene
Check with your doctor before taking this medicine if:
•
You are at high risk of a blood clot developing in your veins (deep vein thrombosis) or in your
lungs (pulmonary embolism).
•
You do not understand the contraception advice given to you by your doctor or if you do not feel
able to follow this advice.
•
You have nerve damage, such as numbness, tingling or pain in your hands or feet.
For women
taking Thalidomide Celgene
Before starting the treatment, you should ask your doctor if you are able to become pregnant, even if
you think this is unlikely.
If you are able to become pregnant:
•
Your doctor will make sure that you have pregnancy tests
o
before treatment
o
every 4 weeks during treatment
o
4 weeks after stopping treatment
•
You must use one effective method of contraception:
o
for 4 weeks before starting treatment
o
during treatment
o
until 4 weeks after stopping treatment
Your doctor will tell you what method of contraception to use.
If you are able to become pregnant, your doctor will record with each prescription that the necessary
measures, as outlined above, have been taken and will give you a record of this on your patient card or
any other relevant documents.
For men
taking Thalidomide Celgene
Thalidomide passes into semen. Therefore do not have unprotected intercourse.
•
Pregnancy and any exposure during pregnancy must be avoided. Always use a condom:
o
during treatment
o
for 1 week after stopping treatment
•
You must not donate semen:
o
during treatment
o
for 1 week after stopping treatment
Donating Blood:
You must not donate blood during Thalidomide Celgene treatment and for 1 week after stopping
treatment.
If you are not sure if any of the above apply to you, talk to your doctor before taking Thalidomide
Celgene.
Taking other medicines:
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines since
other medicines could interfere with the effect of thalidomide. This includes medicines obtained
without a prescription, including herbal medicines.
Make sure you tell your doctor if you are taking any medicines which cause sleepiness as thalidomide
may increase their effects.
Taking Thalidomide Celgene with food and drink:
Thalidomide Celgene can be taken with or without food (see section 3, “How to take Thalidomide
Celgene”).
Do not drink alcohol while you are taking Thalidomide Celgene. This is because alcohol can make you
sleepy and Thalidomide Celgene can make you even sleepier.
Pregnancy:
Thalidomide causes severe birth defects or death to an unborn baby.
•
As little as one capsule taken by a pregnant woman can cause a baby to have serious birth defects.
•
These defects can include shortened arms or legs, malformed hands or feet, eye or ear defects, and
problems with internal organs.
If you are pregnant, you must not take Thalidomide Celgene. In addition, you must not become
pregnant while taking Thalidomide Celgene.
You must use one effective method of contraception if you are a woman who is able to become
pregnant (see section 2, “Take special care with Thalidomide Celgene”).
You must stop treatment and inform your doctor straight away if:
•
You miss or think you have missed a period, or you have unusual menstrual bleeding, or
suspect you are pregnant.
•
You have heterosexual intercourse without using an effective method of contraception.
If you do become pregnant during the treatment with thalidomide, you must stop the treatment and
inform your doctor immediately.
For men taking Thalidomide Celgene who have a female partner who is able to become pregnant,
please see section 2 “Take special care with Thalidomide Celgene”. If your partner becomes pregnant
whilst you are taking thalidomide, you should inform your doctor immediately.
Breast-feeding:
Do not breastfeed when taking Thalidomide Celgene as it is not known if thalidomide is passed into
human breast milk.
Driving and using machines:
Do not drive or use any tools or machines if you experience side effects, such as dizziness, tiredness,
sleepiness or blurred vision.
Important information about some of the ingredients of Thalidomide Celgene:
Thalidomide Celgene contains lactose (a type of sugar). If you have been told by your doctor that you
have an intolerance to some sugars, contact your doctor before taking this medicine.
Always take Thalidomide Celgene exactly as your doctor has told you to. You should check with your
doctor if you are not sure.
HOW TO TAKE THALIDOMIDE CELGENE
Thalidomide Celgene is not suitable under the age of 18.
How much to take:
The usual dose is 200 mg (4 capsules) a day. However your doctor will choose the dose for you,
monitor your progress and may adjust your dose. Your doctor will tell you how to take Thalidomide
Celgene and for how long you will need to take it.
Thalidomide Celgene is taken daily in treatment cycles, each cycle lasting 6 weeks, in combination
with melphalan and prednisone which are taken on days 1 to 4 of each 6 week cycle.
Taking this medicine:
•
Take this medicine by mouth
•
Swallow the capsules whole with a full glass of water
•
Do not crush or chew
•
Take the capsules as a single dose before going to bed. This will make you less likely to feel
sleepy at other times.
If you take more Thalidomide Celgene than you should:
If you take more Thalidomide Celgene than you should, talk to a doctor or go to a hospital
straightaway. If possible, take the medicine pack and this leaflet with you.
If you forget to take Thalidomide Celgene:
If you forget to take
Thalidomide Celgene at your regular time and
•
less than 12 hours have passed: take your capsules immediately.
•
more than 12 hours have passed: do not take your capsules. Take your next capsules at the
usual time the next day.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Like all medicines, Thalidomide Celgene can cause side effects although not everybody gets them.
The frequency of side effects is classified into the following categories:
Affects more than 1 user in 10
Affects 1 to 10 users in 100
Affects 1 to 10 users in 1,000
Affects 1 to 10 users in 10,000
Affects less than 1 user in 10,000
The following side effects may happen with this medicine:
Stop taking Thalidomide Celgene and see a doctor straight away if you notice the following
serious side effects – you may need urgent medical treatment:
•
Severe skin reactions including rashes, which is a common side effect and blistering of the skin
and mucosa (Stevens Johnson Syndrome and toxic epidermal necrolysis, which are rare side
effects). You may have a high temperature (fever) at the same time.
Tell your doctor straight away if you notice any of the following serious side effects:
•
Numbness, tingling, abnormal co-ordination or pain in your hands and feet.








This may be due to nerve damage (called ‘peripheral neuropathy’), which is a very common side
effect. It may become very severe, painful and disabling. If you experience such symptoms, speak
to your doctor straight away, who may reduce the dose or discontinue the treatment. This side
effect usually happens after you have been taking this medicine for several months but can happen
sooner than this. It can also happen some time after treatment has stopped. It may not go away, or
may go away slowly.
•
Sudden pain in your chest or difficulty in breathing.
This may be due to blood clots in the arteries leading to your lungs (called ‘pulmonary
embolism’), which is a common side effect. These can happen during treatment, or after treatment
has stopped.
•
Pain or swelling in your legs, especially in your lower leg or calves.
This may be due to blood clots in the veins of your leg (deep vein thrombosis), which is a common
side effect. These can happen during treatment, or after treatment has stopped.
Other side effects include:
Very common
•
Constipation.
•
Feeling dizzy.
•
Sleepiness, feeling tired.
•
Shaking (tremor).
•
Swelling of hands and feet.
•
Numbness and tingling
•
Low blood cell counts. This may mean that you are more likely to develop infections. Your
doctor may monitor your blood cell counts during treatment with Thalidomide Celgene.
Common
•
Indigestion, feeling sick (nausea), being sick (vomiting), dry mouth.
•
Rash, dryness of the skin.
•
Feeling weak, faint or unsteady, lack of energy or strength, low blood pressure.
•
Fever, feeling generally unwell.
•
A spinning feeling in your head, making it difficult to stand up and move normally.
•
Having difficulty in seeing or speaking, which is temporary. This may be due to a clot in an
artery in the brain.
•
Blurred vision.
•
Shortness of breath, difficulty breathing.
•
Chest infection (pneumonia), lung disease.
•
A slow heart rate, heart failure.
•
Depression, confusion, mood changes, anxiety.
Uncommon
•
Having difficulty seeing or speaking due to bleeding from an artery in the brain.
•
Feeling dizzy when you stand up.
•
Inflammation and swelling of the tubes in your lungs (bronchitis).
•
Inflammation of the cells lining your stomach wall.
•
A hole in part of your large bowel (colon) which can cause infection.
Additional side effects have been reported after this medicine was marketed. These include
•
very serious skin reaction (toxic epidermal necrolysis)
•
underactive thyroid (hypothyroidism)
•
bowel obstruction
•
sexual dysfunction, for example impotence
•
a fall in the number of white blood cells (neutropenia) accompanied by fever and infection
•
a fall in the number of red and white blood cells and platelets at the same time (pancytopenia)
•
Tumour Lysis Syndrome - metabolic complications that can occur during the treatment of
cancer and sometimes even without treatment. These complications are caused by the break-
down products of dying cancer cells and may include the following: changes to blood
chemistry; high potassium, phosphorus, uric acid, and low calcium consequently leading to
changes in kidney function, heart beat, seizures, and sometimes death.
•
allergic reactions such as a localised or generalised pruritic rash
•
hearing decreased or deafness
•
kidney disease (renal failure)
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.
HOW TO STORE THALIDOMIDE CELGENE
Keep out of the reach and sight of children and any other person not directly involved in the treatment.
Do not use after the expiry date stated on the wallet card and the blister after EXP. The expiry date
refers to the last day of that month.
This medicinal product does not require any special storage conditions.
At the end of your treatment you should return all unused capsules to the pharmacist or doctor. These
measures will prevent misuse.
What Thalidomide Celgene contains:
•
The active substance is thalidomide. Each capsule contains 50mg of thalidomide.
•
The other ingredients are anhydrous lactose, microcrystalline cellulose, povidone (K90), stearic
acid, colloidal anhydrous silica, and crospovidone (Type A). The capsule shell contains gelatin
and titanium dioxide (E171). The printing ink is composed of shellac, black iron oxide (E172) and
propylene glycol.
What Thalidomide Celgene looks like and contents of the pack:
Thalidomide Celgene are white hard capsules marked “Thalidomide 50 mg Celgene”. The capsules
are supplied in a wallet card containing 28 capsules (2 blisters of 14 capsules each).
Marketing Authorisation Holder:
Celgene Europe Ltd, Riverside House, Riverside Walk, Windsor, SL4 1NA, United Kingdom.
Manufacturer:
Penn Pharmaceutical Services Limited, Tafarnaubach Industrial Estate, Tredegar, Gwent, NP22 3AA,
United Kingdom.
Celgene Europe Limited, Riverside House, Riverside Walk, Windsor, Berkshire, SL4 1NA, United
Kingdom.
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency web site:
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/thalidomide_celgene.html
Copyright © 1995-2021 ITA all rights reserved.
|









































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































