Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Valtropin 5 mg/1.5 ml powder and solvent for solution for injection
QUALITATIVE AND QUANTITATIVE COMPOSITION
One vial of powder contains 5 mg somatropin (corresponding to 15 IU).
After reconstitution with 1.5 ml solvent, 1 ml contains:
somatropin* 3.33 mg (corresponding to 10 IU)
* produced in Saccharomyces cerevisiae cells by recombinant DNA technology.
Excipients: 1.5 ml of solvent contains 4.5 mg metacresol.
For a full list of excipients, see section 6.1.
Powder and solvent for solution for injection.
White or almost white powder. The solvent is a clear solution.
After reconstitution with the solvent provided Valtropin has a pH of approximately 7.5 and an
osmolality of approximately 320 mOsm/kg.
4.1 Therapeutic indications
Long-term treatment of children with growth failure due to an inadequate secretion of normal
endogenous growth hormone.
Treatment of short stature in children with Turner syndrome, confirmed by chromosome
analysis.
Treatment of growth retardation in pre-pubertal children with chronic renal insufficiency.
Replacement therapy in adults with pronounced growth hormone deficiency of either childhood-
or adult-onset aetiology.
Patients with severe growth hormone deficiency in adulthood are defined as patients with known
hypothalamic-pituitary pathology and at least one additional known deficiency of a pituitary hormone
not being prolactin. These patients should undergo a single dynamic test in order to diagnose or
exclude a growth hormone deficiency. In patients with childhood-onset isolated growth hormone
deficiency (no evidence of hypothalamic-pituitary disease or cranial irradiation), two dynamic tests
should be recommended, except for those having low insulin-like growth factor-1 (IGF-1)
concentrations (< 2 standard deviation score (SDS)), who may be considered for one test. The cut-off
point of the dynamic test should be strict.
4.2 Posology and method of administration
Therapy with Valtropin should be initiated and monitored by physicians adequately experienced in the
diagnosis and management of patients with growth hormone deficiency.
The dosage and administration schedule should be individualised for each patient.
Dosage in paediatric patients
Growth hormone deficiency in children
The recommended dosage is 0.025 - 0.035 mg/kg body weight per day, given as a subcutaneous
injection.
Children with Turner syndrome
The recommended dosage is 0.045 - 0.050 mg/kg body weight per day, given as a subcutaneous
injection.
Pre-pubertal children with chronic renal insufficiency
The recommended dosage is 0.045 - 0.050 mg/kg body weight per day, given as a subcutaneous
injection.
Growth hormone deficiency in adults
The recommended starting dose is 0.15 - 0.30 mg/day, given as a subcutaneous injection. A lower
starting dose may be necessary in older and obese patients.
This dose should be gradually increased according to individual patient requirements based on the
clinical response and serum IGF-1 concentrations. Total daily dose usually does not exceed 1 mg.
IGF-1 concentrations should be maintained below the upper limit of the age-specific normal range.
The minimum effective dose should be used, and dose requirements may decline with increasing age.
The dosage of somatropin should be decreased in cases of persistent oedema or severe paraesthesia, in
order to avoid the development of carpal tunnel syndrome.
Valtropin is administered by subcutaneous injection.
The injection sites should be varied in order to avoid lipo-atrophy.
For further information on reconstitution and administration see section 6.6.
Hypersensitivity to the active substance or to any of the excipients (e.g. metacresol) (see
section 4.4).
Any evidence of activity of a tumour. Intracranial lesions must be inactive and anti-tumour
therapy complete prior to the institution of growth hormone therapy. Valtropin should be
discontinued if there is evidence of tumour growth.
Valtropin should not be used for growth promotion in children with closed epiphyses.
Patients with acute critical illness due to complications following open heart or abdominal
surgery, multiple accidental trauma, or patients having acute respiratory failure.
4.4 Special warnings and precautions for use
There is no evidence to suspect that growth hormone replacement influences the recurrence rate or
regrowth of intracranial neoplasms, but standard clinical practice requires regular pituitary imaging in
patients with a history of pituitary pathology. A baseline scan is recommended in these patients before
instituting growth hormone replacement therapy.
In cases of severe or recurrent headache, visual problems, nausea, and/or vomiting, a fundoscopy for
papilloedema is recommended. If papilloedema is confirmed, a diagnosis of benign intracranial
hypertension should be considered and, if appropriate, the growth hormone treatment should be
discontinued. At present, there is insufficient evidence to guide clinical decision making in patients
with resolved intracranial hypertension. If growth hormone treatment is restarted, careful monitoring
for symptoms of intracranial hypertension is necessary.
Because human growth hormone may induce a state of insulin resistance, patients treated with
somatropin should be monitored for evidence of glucose intolerance.
Growth hormone increases the extrathyroidal conversion of T4 to T3 and may, as such, unmask
incipient hypothyroidism. Monitoring of thyroid function should therefore be conducted in all patients.
In patients with hypopituitarism, standard replacement therapy must be closely monitored when
somatropin therapy is administered.
Patients with endocrine disorders, including growth hormone deficiency, may develop slipped capital
epiphyses more frequently. Any child with the onset of a limp during growth hormone therapy should
be evaluated.
Subjects who had been treated with growth hormone during childhood, until final height was attained,
should be re-evaluated for growth hormone deficiency after epiphyseal closure before replacement
therapy is commenced at the doses recommended for adults.
For children, the treatment should be continued until the end of the growth has been reached. It is
advisable not to exceed the recommended dosage in view of the potential risks of acromegaly,
hyperglycaemia, and glucosuria.
Valtropin is not indicated for the treatment of patients with growth failure due to Prader-Willi
syndrome unless they also have a diagnosis of growth hormone deficiency. There have been reports of
sleep apnoea and sudden death after initiating growth hormone therapy in patients with Prader-Willi
syndrome, who had one or more of the following risk factors: severe obesity, history of upper airway
obstruction or sleep apnoea, or unidentified respiratory infection.
Before instituting treatment with somatropin for growth retardation secondary to chronic renal
insufficiency, children should have been followed for one year to verify growth disturbance.
Conservative treatment for renal insufficiency (which includes control of acidosis,
hyperparathyroidism, and nutritional status for one year prior to the treatment) should have been
established and should be maintained during treatment. Treatment with somatropin should be
discontinued at the time of renal transplantation.
In order to reach the defined treatment goal, men may need lower growth hormone doses than women.
Oral oestrogen administration increases the dose requirements in women. An increasing sensitivity to
growth hormone (expressed as change in IGF-1 per growth hormone dose) over time may be observed,
particularly in men. The accuracy of the growth hormone dose should therefore be controlled every
6 months.
Valtropin should not be reconstituted with the supplied solvent for patients with a known sensitivity to
metacresol. If sensitivity to the accompanying solvent occurs, the vials should be reconstituted with
water for injections and used as a single use vial (see section 6.3).
Patients with Turner syndrome should be evaluated carefully for otitis media and other ear disorders
since these patients have an increased risk of ear or hearing disorders.
After accidental intramuscular injection, hypoglycaemia may appear.
Experience of somatropin treatment in patients above 60 years of age is limited.
Experience of prolonged treatment (over 5 years) with somatropin in adults is limited.
4.5 Interaction with other medicinal products and other forms of interaction
Excessive glucocorticoid therapy will inhibit the growth-promoting effect of human growth hormone.
Patients with co-existing adrenocorticotropic hormone (ACTH) deficiency should have their
glucocorticoid replacement dose carefully adjusted to avoid an inhibitory effect on growth.
In women taking oral oestrogens, a higher dose of somatropin may be required to achieve the
treatment goal.
Patients taking insulin for diabetes mellitus should be carefully monitored during treatment with
somatropin. An adjustment of the insulin dose may be required.
Data from an interaction study performed in growth hormone deficient adults, suggests that
somatropin administration may increase the clearance of compounds known to be metabolised by
cytochrome P450 isoenzymes. The clearance of compounds metabolised by cytochrome P 450 3A4
(e.g. sex steroids, corticosteroids, anticonvulsants and cyclosporine) may be especially increased
resulting in lower plasma levels of these compounds. The clinical significance of this is unknown.
4.6 Pregnancy and lactation
For Valtropin no clinical data on exposed pregnancies are available. Animal studies are insufficient
with respect to effects on pregnancy, embryofoetal development, parturition or postnatal development
(see section 5.3). Therefore Valtropin should not be used during pregnancy unless clearly necessary.
There have been no clinical studies conducted with Valtropin in breast-feeding women. It is not
known whether somatropin is excreted in human milk. Therefore caution should be exercised when
Valtropin is administered to breast-feeding women.
4.7 Effects on ability to drive and use machines
Valtropin has no or negligible influence on the ability to drive and use machines.
During clinical studies 128 children (98 children with growth hormone deficiency and 30 with Turner
syndrome) were exposed to Valtropin. The safety profile of Valtropin observed in these clinical
studies was consistent with that reported with the reference product used in these studies and other
somatropin containing products.
The following adverse reactions and their frequencies have been observed under treatment with
somatropin based on published information:
Very common (> 1/10), common (> 1/100, < 1/10), uncommon (> 1/1,000, < 1/100), rare (> 1/10,000,
< 1/1,000), very rare (< 1/10,000) including isolated reports
Endocrine disorders
Hypothyroidism: common
Metabolism and nutrition disorders
Mild hyperglycaemia: common (1% in children; 1% - 10% in adults)
Nervous system disorders
Benign intracranial hypertension: rare
Headache: very common in adults
Insomnia: very rare in children; common in adults
Paraesthesia: rare in children; very common in adults
Vascular disorders
Hypertension: rare in children; common in adults
Musculoskeletal and connective tissue disorders
Localised muscle pain (myalgia): common in adults
Joint pain and disorder (arthralgia): very common in adults
Reproductive system and breast disorders
Gynaecomastia: very rare in children; uncommon in adults
General disorders and administration site conditions
Weakness: uncommon
Injection site pain (reaction): common
Oedema (local or generalised): common (1% - 10% in children; 10% in adults)
As with all somatropin containing medicinal products a small percentage of patients may develop
antibodies to somatropin. In a clinical study with Valtropin, 3% of children with growth hormone
deficiency developed such antibodies. The binding capacity of these antibodies was low and there was
no effect on growth rate. Testing for antibodies to somatropin should be carried out in any patient who
fails to respond to therapy.
Anti-host cell protein (anti-
S. cerevisiae
) antibodies were uncommon in patients treated with
Valtropin. The generation of such antibodies with low binding capacity is unlikely to be clinically
relevant. In contrast to bacteria (
E. coli
), yeast has not been described to elicit adjuvant effects
modifying the immunological response.
There was one case (< 1%) of acute hypersensitivity involving urticaria and pruritus in a patient
administered Valtropin.
Paediatric patients
Mild and transient oedema was observed early during the course of treatment with somatropin.
Leukaemia has been reported in a small number of children who have been treated with somatropin.
However, there is no evidence that leukaemia incidence is increased in somatropin recipients without
predisposing factors.
Adult patients
In general, in patients with adult-onset growth hormone deficiency, oedema, muscle pain, joint pain
and disorders were reported early in therapy and tended to be transient. Adult patients treated with
somatropin, following diagnosis of growth hormone deficiency in childhood, reported undesirable
effects less frequently than those with adult-onset growth hormone deficiency.
Acute overdose could lead initially to hypoglycaemia and subsequently to hyperglycaemia. Long-term
overdose could result in signs and symptoms of acromegaly consistent with the known effects of
excess human growth hormone.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Somatropin and analogues; ATC code: H01A C01
Somatropin is a polypeptide hormone of recombinant DNA origin. It has 191 amino acid residues and
a molecular weight of 22,125 daltons. The amino acid sequence of the product is identical to that of
human growth hormone of pituitary origin. Valtropin is synthesised in yeast cells (
Saccharomyces
cerevisiae
).
The biological effects of somatropin are equivalent to those of human growth hormone of pituitary
origin.
The most prominent effect of somatropin is that it stimulates the growth plates of long bones.
Additionally, it promotes cellular protein synthesis and nitrogen retention.
Somatropin stimulates lipid metabolism; it increases plasma fatty acids and high-density lipoprotein
(HDL)-cholesterols, and decreases total plasma cholesterol.
Somatropin therapy has a beneficial effect on body composition in growth hormone-deficient patients,
in that body fat stores are reduced and lean body mass is increased. Long-term therapy in growth
hormone-deficient patients increases bone mineral density.
Somatropin may induce insulin resistance. Large doses of somatropin may impair glucose tolerance.
The efficacy and safety of Valtropin has been assessed in a randomised, double-blind, parallel,
controlled Phase III study in children with growth hormone deficiency. There were no relevant
differences between Valtropin and the reference product with regard to height velocity and height
velocity SDS.
An open single-arm Phase III study investigating the efficacy and safety of treatment with Valtropin in
girls with short stature associated with Turner syndrome showed a significant effect of study treatment
on height velocity.
5.2 Pharmacokinetic properties
A double-blind, randomised, single dose, crossover study in 24 healthy volunteers showed that the
pharmacokinetic profile of Valtropin was comparable to that of the reference product. Subcutaneous
administration of 0.073 mg/kg body weight of Valtropin resulted in a C
max
of 43.97 ng/ml and an
AUC
0-24 h
of 369.90 ng·h/ml. C
max
was reached at 4 h and t
½
was 3 h.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies with Valtropin of
repeated dose toxicity, genotoxicity and reproductive toxicity studies.
Animal studies with Valtropin are not sufficient to assess the reproductive toxicity potential. From
reproductive toxicity studies performed with other somatropin products there is no evidence of an
increased risk of adverse reactions for the embryo or foetus.
Long-term studies for carcinogenicity have not been performed. There are no specific local tolerance
studies in animals after subcutaneous injection of Valtropin. However, in single and repeat-dose
general toxicity studies no adverse reactions at the injection sites were reported.
PHARMACEUTICAL PARTICULARS
Powder:
Glycine
Mannitol
Sodium phosphate monobasic
Sodium phosphate dibasic
Sodium hydroxide (for pH adjustment)
Hydrochloric acid (for pH adjustment).
Solvent:
Metacresol
Water for injections.
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal
products.
After first opening or following reconstitution with the solvent provided:
After reconstitution with the solvent provided, chemical and physical in-use stability has been
demonstrated for 21 days at 2°C - 8°C (refrigerator).
From a microbiological point of view, once opened, the product may be stored for a maximum of
21 days at 2°C - 8°C (refrigerator).
Following reconstitution with water for injections:
After reconstitution with water for injections, the product must be used immediately and must be used
as a single use vial. If not used immediately, in-use storage times and conditions prior to use would
normally not be longer than 24 hours at 2°C - 8°C (refrigerator), unless reconstitution has taken place
in controlled and validated aseptic conditions.
6.4 Special precautions for storage
Store in a refrigerator (2°C - 8°C). Do not freeze.
For the purpose of transport and/or ambulatory use the non-reconstituted product can be kept at room
temperature (not above 25°C) for one single period of up to 4 weeks.
Patients may keep the non-reconstituted product at room temperature for one single period of up to 4
weeks before use.
For storage conditions of the reconstituted medicinal product, see section 6.3.
6.5 Nature and contents of container
5 mg of powder in a vial (Type I glass) closed with a stopper (butyl rubber) and a flip-off cap
(aluminium plastic).
1.5 ml of solvent in a pre-filled syringe (Type I glass) closed with a tip cap (FluroTec coated butyl
rubber)
Pack size of 1 vial and 1 pre-filled syringe.
6.6 Special precautions for disposal
For use and handling
Valtropin should not be reconstituted with the supplied solvent for patients with a known sensitivity to
metacresol (see section 4.3). If sensitivity to the accompanying solvent occurs, the vials should be
reconstituted with water for injections and used as a single use vial.
Reconstitution with the solvent provided
Each vial of Valtropin should be reconstituted using the accompanying solvent. The solvent should not
be used if it is discoloured or cloudy. The solvent should be injected into the vial by aiming the stream
of liquid against the glass wall. Following reconstitution, the vial should be swirled with a GENTLE
rotary motion until the contents are completely dissolved. DO NOT SHAKE. The resulting solution
should be clear, without particulate matter. If the solution is discoloured, cloudy or contains particulate
matter, the contents MUST NOT be injected. Before and after every injection, the septum of the vial
should be wiped with alcohol to prevent contamination of the contents by repeated needle insertions.
If reconstituted with the solvent, then the solution is for multidose use (see section 6.3).
Reconstitution with water for injections
After reconstitution with water for injections the product must be used immediately (see section 6.3)
and the solution is for single use only.
Administration
Sterile disposable syringes and needles should be used for administration of Valtropin. The volume of
the syringe should be small enough so that the prescribed dose can be withdrawn from the vial with
reasonable accuracy.
Disposal
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Germany
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
MANUFACTURER OF THE BIOLOGICAL ACTIVE
SUBSTANCE AND MANUFACTURING AUTHORISATION
HOLDER RESPONSIBLE FOR BATCH RELEASE
CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURER OF THE BIOLOGICAL ACTIVE SUBSTANCE AND
MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer of the biological active substance
LG Life Sciences Ltd., Iksan Plant, 601 Yongje-dong, Iksan-si, Jeonbuk-do 570-350, South Korea
Name and address of the manufacturer responsible for batch release
BioPartners GmbH, Kaiserpassage 11, D-72764 Reutlingen, Germany
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to restricted medical prescription (See Annex I: Summary of Product
Characteristics, section 4.2).
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
Pharmacovigilance system
The MAH must ensure that the system of pharmacovigilance is in place and functioning before the
product is placed on the market and for as long as the marketed product remains in use.
Risk Management plan
The MAH commits to performing additional pharmacovigilance activities as detailed in the
Pharmacovigilance Plan.
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
Valtropin 5 mg/1.5 ml powder and solvent for solution for injection
Somatropin
STATEMENT OF ACTIVE SUBSTANCE(S)
1 vial of powder contains 5 mg (15 IU) somatropin (3.33 mg/ml somatropin after reconstitution with
1.5 ml solvent).
Powder: glycine, mannitol, sodium phosphate monobasic, sodium phosphate dibasic.
pH adjustment: sodium hydroxide and hydrochloric acid.
Solvent: metacresol (see leaflet for further information) and water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Powder and solvent for solution for injection
1 vial (powder) and 1 pre-filled syringe (1.5 ml solvent).
METHOD AND ROUTE(S) OF ADMINISTRATION
Subcutaneous use
For information on reconstitution and use read the package leaflet.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
After reconstitution with the solvent provided: can be stored for 21 days in a refrigerator.
After reconstitution with water for injections: must be used immediately.






















SPECIAL STORAGE CONDITIONS
Store in a refrigerator. Do not freeze.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
BioPartners GmbH, Kaiserpassage 11, D-72764 Reutlingen, Germany
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE

















MINIMUM PARTICULARS TO APPEAR ON SMALL IMMEDIATE PACKAGING UNITS
PRE-FILLED SYRINGE OF SOLVENT
NAME OF THE MEDICINAL PRODUCT AND ROUTE(S) OF ADMINISTRATION
Valtropin 5 mg/1.5 ml
Solvent for solution for injection
Read the package leaflet before use.
CONTENTS BY WEIGHT, BY VOLUME OR BY UNIT
1.5 ml (water for injections with metacresol)
Store in a refrigerator. Do not freeze.
Contains 4.5 mg metacresol.

















PACKAGE LEAFLET: INFORMATION FOR THE USER
Valtropin 5 mg/1.5 ml powder and solvent for solution for injection
Somatropin
Read all of this leaflet carefully before you start using this medicine.
-
Keep this leaflet. You may need to read it again.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
What Valtropin is and what it is used for
WHAT Valtropin IS AND WHAT IT IS USED FOR
Your medicine is called Valtropin. It is a brand of human growth hormone, also called somatropin.
Valtropin is made in the laboratory by a special process known as a ‘recombinant DNA technology’
process in yeast cells (
Saccharomyces cerevisiae
). It has the same structure as the growth hormone that
the body produces in the pituitary glands. Growth hormone regulates the growth and development of
cells. When it stimulates growth of cells in the long bones of the legs and spine, it causes an increase
in height.
to treat
children
who do not develop to their normal height because of poor bone growth caused
by growth hormone deficiency (relative lack of growth hormone), Turner syndrome, or ‘chronic
renal insufficiency’ (a condition in which the kidneys gradually lose their ability to perform
their normal functions, such as the removal of wastes and extra fluid from the body).
to treat
adults
with severe growth hormone deficiency who already had growth hormone
deficiency when they were children or who do not have enough growth hormone during their
adult years for some other reason.
In this leaflet the patient is addressed as ‘you’. Caregivers administering Valtropin to their children
should consider that ‘you’ refers to the child.
Do not use Valtropin:
-
if you are
allergic (hypersensitive) to somatropin or any of the other ingredients of the
powder or solvent of Valtropin
, e.g. metacresol (see section 2, ‘Take special care with
Valtropin – Occurrence of certain side effects’)
to
promote growth if you have already
stopped growing
if you have had a
serious heart or abdominal operation
if you are
being treated for more than one injury following a serious accident
if you have
sudden serious breathing problems
If you have any further questions, ask your doctor or pharmacist.
if you have an
active brain tumour or any other tumour (cancer)
, since growth hormone will
make it worse. Tumours must be inactive and anti-tumour therapy complete before growth
hormone can be prescribed. If there is evidence of tumour growth, treatment with Valtropin
should be discontinued.
/!\
Take special care with Valtropin
Examinations before starting treatment:
-
A specialist doctor trained in hormone disorders must examine you to decide if it is safe to use
Valtropin.
If adults have been treated with growth hormone during childhood, they should be re-evaluated
for growth hormone deficiency before starting any further treatment with growth hormones.
Patients with Prader-Willi syndrome should not be treated with Valtropin unless they are also
suffering from growth hormone failure.
During or after serious illness:
-
If you have had a brain tumour, you should be re-examined frequently to make sure that the
tumour has not come back.
If children have had a kidney transplant, growth hormone treatment will be stopped.
If the child has Turner syndrome, the child’s doctor should carefully check for ear infections
such as otitis media, because Turner syndrome patients have an increased risk of ear or other
hearing disorders.
Occurrence of certain side effects:
-
If symptoms like headache (severe and recurrent), visual changes, nausea and/or vomiting
occur, please ask your doctor for advice.
If you have injected Valtropin by mistake into the muscle instead of under the skin, your blood
sugar may become too low (hypoglycaemia).
Too much growth hormone can cause greater than normal growth of ears, nose, lips, tongue and
cheekbone (acromegaly), high blood sugar (hyperglycaemia) and presence of sugar in the urine
(glucosuria). Always use Valtropin as recommended by your doctor.
If sensitivity to solvent occurs, the vials should be reconstituted with water for injections
without preservative and used as a single use vial (see section 5 ‘How to store Valtropin’).
Do
not use the supplied solvent if you have a known sensitivity to metacresol preservative
.
Monitoring during treatment by your doctor:
-
Valtropin may affect the way your body handles sugar from food and drink. Your doctor may
check the amount of sugar in your urine or blood.
Valtropin can affect the amount of thyroid hormone in the blood, so you must have thyroid
function tests from time to time. If the thyroid is not working properly, Valtropin may not work
as well as it should.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any of the following
medicines:
-
adrenal steroid hormone such as cortisone or prednisolone
-
insulin
-
oral oestrogen
-
other prescription medicines (e.g. sex steroids, corticosteroids, anticonvulsants or cyclosporine),
or non-prescription medicines.
Your doctor may need to adjust the dose of Valtropin or of the other medicine.
If the child begins to limp under treatment with Valtropin, please ask your doctor for advice.
Pregnancy
Valtropin should not be used during pregnancy unless clearly necessary. If you become pregnant, tell
your doctor immediately.
Breast-feeding
If you are breast-feeding or intend to breast-feed, please ask your doctor for advice before using
Valtropin.
Driving and using machines
Valtropin has no or negligible effect on the ability to drive and use machines.
Important information about some of the ingredients of Valtropin
The accompanying solvent of Valtropin contains metacresol. Do not use this solvent if you are allergic
(hypersensitive) to metacresol (see section 2,
‘
Do not use Valtropin’). If sensitivity to the solvent
occurs, the vials should be reconstituted with water for injections and used as a single use vial (see
section 5 ‘How to store Valtropin’).
Always use Valtropin exactly as your doctor has told you. You should check with your doctor or
pharmacist if you are not sure. Do not inject Valtropin yourself if you are not sure about the dose.
Dosage
Your doctor will tell you how much to use. This will vary according to your disease. Please do not
change the dosage without consulting your doctor.
The accuracy of the Valtropin dose should be checked every 6 months by your doctor.
In general the dosage will be calculated as described below. However, individual doses may vary, and
the doctor may change your dose based on your specific need.
Growth hormone deficiency in children
Inject 0.025 - 0.035 milligrams (mg) for each kilogramme of body weight once daily under the skin
(subcutaneously).
Children with Turner syndrome
Inject 0.045 - 0.050 milligrams (mg) for each kilogramme of body weight once daily under the skin
(subcutaneously).
Pre-pubertal children with chronic renal insufficiency
Inject 0.045 - 0.050 milligrams (mg) for each kilogramme of body weight once daily under the skin
(subcutaneously).
Growth hormone deficiency in adults
Inject 0.15 - 0.30 milligrams (mg) once daily under the skin (subcutaneously). A lower starting dose
may be necessary if you are older or overweight.
If necessary, your doctor will gradually increase this dose according to your individual requirements
based on the clinical outcome and measurement of your blood levels of a so called “growth factor”
(known as IGF-1). The total daily dose usually does not exceed 1 mg. IGF-1 concentrations need to be
regularly measured and should be maintained below the upper limit of the normal range for your age
and sex.
Your doctor will always prescribe the minimum effective dose to be used.
Dosage adjustment
In elderly patients a dose reduction may be necessary.
The dosage of somatropin should be reduced in cases of long lasting swelling (oedema) or severe
abnormal sensation (paraesthesia), in order to avoid the development of a rare side effect called carpal
tunnel syndrome (hand numbness and pain).
Following use of the medicine for some time, it may be necessary to reduce the dose, particularly in
men.
When using other medicines the dose of Valtropin or of the other medicine may need to be adjusted
(see section 2, ‘Using other medicines’).
Administration
Valtropin is intended for subcutaneous use after reconstitution. This means that after reconstitution of
the powder with the solvent provided the solution is injected with a short needle into the fatty tissue
just under the skin.
If you are injecting this medicine yourself you will be instructed how to prepare and give the injection.
Do not inject Valtropin yourself unless you have received training.
Detailed instructions for subcutaneous administration are provided with this leaflet (see section
‘Information on how to self-inject Valtropin’ at the end of this leaflet).
If you use more Valtropin than you should
In case more Valtropin was used than recommended, please consult your doctor.
If you have used too much Valtropin, initially your blood sugar may decrease and become too low
(hypoglycaemia) and subsequently increase and become too high (hyperglycaemia). If you have used
too much Valtropin over a longer period, this may result in a greater than normal growth of ears, nose,
lips, tongue and cheekbone (acromegaly).
If you forget to use Valtropin
Do not take a double dose to make up for forgotten doses. Continue with the prescribed dosage
regimen. If you have any doubts, please contact your doctor.
If you stop using Valtropin
Please ask your doctor for advice before stopping treatment. Interruption or early stopping of
treatment with Valtropin may impair the success of the growth hormone therapy.
Like all medicines, Valtropin can cause side effects, although not everybody gets them.
The side effects of medicines are classified as follows:
very common happening in more than 1 in 10 patients
common
happening in 1 in 100 to 1 in 10 patients





happening in 1 in 1,000 to 1 in 100 patients
happening in 1 in 10,000 to 1 in 1,000 patients
happening in less than 1 in 10,000 patients, including isolated reports
You may experience any of the following side effects after administration of Valtropin:
General and local effects
Pain or reaction at the injection site: common
Swelling (local or generalised oedema): common (more common in adults than in children)
Subnormal activity of the thyroid gland (hypothyroidism): common
Breast enlargement (gynaecomastia): very rare (children); uncommon (adults)
Mild increase in blood sugar (mild hyperglycaemia): common
Insulin resistance. Insulin resistance means that the normal effect of insulin on muscle, fat, and
liver cells is reduced.
Symptoms of benign increased brain pressure (benign intracranial hypertension): rare
Headache: very common (adults)
Insomnia: very rare (children); common (adults)
Abnormal sensation (paraesthesia): rare (children); very common (adults)
High blood pressure (hypertension): rare (children); common (adults)
Joint and muscle effects
Joint pain and disorder (arthralgia): very common (adults)
Localised muscle pain (myalgia): common (adults)
As with all medicines containing growth hormone a small percentage of patients may develop
blocking chemicals called antibodies to growth hormone. In a clinical study with Valtropin 3% of
children with growth hormone deficiency developed such antibodies. However, these antibodies did
not have an effect on the growth response to Valtropin. If you are failing to respond to therapy with
Valtropin, your doctor will test for antibodies.
There was one patient (< 1%) who experienced acute hypersensitivity reactions involving hives
(urticaria) and itching (pruritus) after administration of Valtropin.
Children
Leukaemia has been reported in a small number of children treated with somatropin. However, there is
no evidence that somatropin causes leukaemia when there are no other risk factors.
Adults
Swelling, muscle pain, joint pain and disorders have been reported early in therapy with somatropin
but these effects tended to be transient (short-lived).
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor or pharmacist.






Keep out of the reach and sight of children.
Do not use Valtropin after the expiry date which is stated on the labels and the carton. The
expiry date refers to the last day of that month.
Storage conditions of the unopened product:
Store in a refrigerator (2°C - 8°C). Do not freeze.
The non-reconstituted product can be kept at room temperature (not above 25°C) for one single
period of up to 4 weeks before use.
Shelf-life after reconstitution with solvent:
After reconstitution with the solvent provided the product may be stored in the refrigerator
(2°C - 8°C) for a maximum of 21 days.
Shelf-life after reconstitution with water for injections (NOT tap water):
After reconstitution with water for injections the product must be used immediately and as a
single use vial.
Do not use Valtropin if you notice that the solvent or the reconstituted solution is cloudy or
discoloured.
Medicines should not be disposed of via waste water or household waste. Ask your pharmacist how to
dispose of medicines that are no longer required. These measures will help to protect the environment.
Powder:
-
The active substance is somatropin. One vial of powder contains 5 mg somatropin
(corresponding to 15 IU). After reconstitution with 1.5 ml solvent, 1 ml contains 3.33 mg
somatropin (corresponding to 10 IU).
-
The other ingredients are glycine, mannitol, sodium phosphate monobasic, sodium phosphate
dibasic and for pH adjustment sodium hydroxide and hydrochloric acid.
Solvent:
-
The pre-filled syringe contains water for injections and metacresol (see section 2, ‘Important
information about some of the ingredients of Valtropin’).
What Valtropin looks like and contents of the pack
Valtropin is presented as a powder and solvent for solution for injection.
A single pack contains:
-
1 vial with white to almost white powder
-
1 pre-filled syringe with 1.5 ml of solvent for reconstitution as a clear solution.
Marketing Authorisation Holder and Manufacturer
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Germany
Tel: +49 (0) 7121 948 7756
Fax: +49 (0) 7121 346 255
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
Belgique/België/Belgien
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Allemagne/Deutschland
Tél/Tel : +49 (0) 7121 948 7756
Luxembourg/Luxemburg
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Allemagne/Deutschland
Tél/Tel : +49 (0) 7121 948 7756
България
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Германия
Teл.: + 49(0) 7121 948 7756
Magyarország
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Németország
Tel: +49 (0) 7121 948 7756
Česká republika
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Německo
Tel: +49 (0) 7121 948 7756
Malta
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Ġermanja
Tel: +49 (0) 7121 948 7756
Danmark
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Tyskland
Tlf: +49 (0) 7121 948 7756
Nederland
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Duitsland
Tel: +49 (0) 7121 948 7756
Deutschland
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Tel: + 49(0) 7121 948 7756
Norge
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Tyskland
Tlf: +49 (0) 7121 948 7756
Eesti
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Saksamaa
Tel: + 49 (0) 7121 948 7756
Österreich
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Deutschland
Tel: +49 (0) 7121 948 7756
Ελλάδα
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Γερμανία
Τηλ: +49 (0) 7121 948 7756
Polska
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Niemcy
Tel: +49 (0) 7121 948 7756
España
BioPartners GmbH
Kaiserpassage 11
Portugal
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Alemania
Tel: +49 (0) 7121 948 7756
D-72764 Reutlingen
Alemanha
Tel: +49 (0) 7121 948 7756
France
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Allemagne
Tél: + 49 (0) 7121 948 7756
România
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Germania
Tel. + 49 (0) 7121 948 7756
Ireland
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Germany
Tel: + 49 (0) 7121 948 7756
Slovenija
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Nemčija
Tel: + 49 (0) 7121 948 7756
Ísland
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Þýskaland
Tel: +49 (0) 7121 948 7756
Slovenská republika
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Nemecko
Tel: + 49 (0) 7121 948 7756
Italia
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Germania
Tel: + 49 (0) 7121 948 7756
Suomi/Finland
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Saksa
Puh/Tel: +49 (0) 7121 948 7756
Κύπρος
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Γερμανία
Τηλ: + 49 (0) 7121 948 7756
Sverige
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Tyskland
Tel: +49 (0) 7121 948 7756
Latvija
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Vācija
Tel: +49 (0) 7121 948 7756
United Kingdom
Cambridge Laboratories
Deltic House Kingfisher Way
Silverlink Business Park
Wallsend Tyne & Wear NE28 9NX - UK
Tel: +44 (0) 191 296 9369
Lietuva
BioPartners GmbH
Kaiserpassage 11
D-72764 Reutlingen
Vokietija
Tel: +49 (0) 7121 948 7756
This leaflet was last approved in
{MM/YYYY}
Detailed information on this medicine is available on the European Medicines Agency (EMEA) web
---------------------------------------------------------------------------------------------------------------------------
--
INFORMATION ON HOW TO SELF-INJECT Valtropin
Please read the following instructions carefully before using Valtropin.
Introduction
The following instructions explain how to inject Valtropin
yourself. Please read the instructions carefully
and follow them step by step. Your doctor or his/her assistant will instruct you how to self-inject Valtropin.
Do not attempt to inject yourself unless you are sure you understand the procedure and requirements of
self-injection.
General notes
For patients with a known sensitivity to metacresol, Valtropin should not be reconstituted with the
supplied solvent (see section 2, ‘Do not use Valtropin’). If sensitivity to the accompanying solvent
occurs, the vials should be reconstituted with water for injections (see section 5 ‘How to store
Valtropin’). Do not use tap water.
Collect the necessary items before you begin. These are:
Supplied in the pack
the Valtropin
vial with powder for solution for injection
the pre-filled syringe with 1.5 ml solvent for solution for injection
NOT supplied in the pack
sterile injection syringe and needles
disposal box for used syringes and needles.
Wash your hands thoroughly with soap and water before preparing the medicine.
Take the Valtropin carton out of the
refrigerator
and take the
powder vial
and
pre-filled syringe
with solvent out of the box. Check that the medicine is within the expiry date.
Remove the protective plastic cap from the powder vial.
Clean the rubber stopper on the top of the powder vial with an alcohol swab. After cleaning do not
touch the top of the vial.

Vial containing the powder of your medicine
Take the
pre-filled syringe
with solvent
supplied in the pack to prepare your medicine. Remove the
rubber tip cap and firmly attach a needle to the syringe. Your doctor or his/her assistant will tell you
what size of needle to use.
Remove the needle guard without touching the needle.
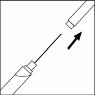
Slowly insert the needle straight through the centre of the rubber stopper of the vial.
Slowly inject all of the solvent (1.5 ml) into the powder vial aiming the stream of liquid against the
side of the vial.
DO NOT
aim it at the white powder at the bottom of the vial.
Before taking the syringe out of the vial, draw in the same amount of air (1.5 ml) as the solvent you
injected to reduce the pressure in the vial. Withdraw the syringe and replace the needle guard.
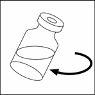
Swirl the vial
GENTLY
to completely dissolve the contents.
DO NOT SHAKE.
Dissolving up your medicine




10. The resulting solution should be clear, without particles.
11. Label the vial with the date on which you prepared the solution.
12. Clean the rubber stopper on the top of the vial with an alcohol swab again. After cleaning do not
touch the top of the vial.
Vial containing the solution of your medicine
13. Take the
injection syringe
and the needle supplied by your pharmacy or hospital, to withdraw the
solution of medicine. Remove the injection syringe from its sterile packaging and attach the needle to
the syringe.
14. Fill the syringe with air by pulling the plunger back to the level that represents your dose as
prescribed by your doctor.
15. Remove the needle guard without touching the needle.
16. Slowly insert the needle straight through the centre of the rubber stopper of the vial.
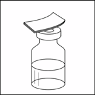




17. Gently push the plunger to discharge the air in the syringe into the vial.
18. Turn the vial upside down with the needle still in it and hold the vial in one hand. Hold the syringe
with the needle in the vial pointing up. Ensure that the tip of the needle is in the solution. Using your
other hand slowly pull back the plunger in a continuous motion to draw the correct dose into the
syringe ensuring that the needle tip remains in the solution.
Withdrawing the right volume of your medicine with the help of the syringe markings
19. Remove the syringe from the needle leaving the needle in the vial without touching the tip of the
syringe. Withdraw the needle, replace the needle guard and dispose in a closed container. For
handling the vial see ‘Injecting the solution’, step 32.
20. Take a new needle (one that is suitable for subcutaneous injection) and place it firmly onto the tip of
the syringe.
Syringe containing your medicine being attached to a new needle
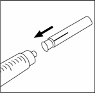
21. Remove the needle guard from the syringe needle and check for air bubbles in the syringe.
22. If you see any bubbles, pull the plunger slightly back; tap the syringe gently, with the needle pointing
up, until the bubbles disappear. Push the plunger slowly back up to the correct dose.
23. Replace the needle guard and place the syringe with the needle on a flat surface.



24. Ensure the solution is at room temperature. If the solution is cold, warm the syringe between your
palms.
25. Inspect the solution prior to administration: if the solution is discoloured or if you can see any solid
particles in the liquid the solution
MUST NOT
be injected.
26. Select the injection site according to the recommendation of your doctor. It is very important that you
vary the injection site
each time you give the medicine.
27. Cleanse the injection site with an alcohol swab and wait for the area to dry.
28. Check that the correct dose of Valtropin solution is in the syringe. Hold the syringe in your hand as
you would hold a pencil.
29. Squeeze a big skin fold between your thumb and index finger. Insert the needle into the pinched skin
at a 45° to 90° angle with a quick, firm motion. This hurts less than pushing the needle in slowly.
30. Slowly (over a few seconds) inject the solution by gently depressing the plunger until the syringe is
empty.
31. Withdraw the needle quickly and apply pressure over the injection site with a dry gauze or cotton pad
for several seconds. If there is bleeding, cover the injection site with an adhesive plaster.
32. Dispose the used syringe in a closed container. Be sure to
return the vial to the refrigerator
. When
empty, discard the vial as well. For shelf-life after reconstitution see section 5 ‘How to store
Valtropin’.
If the powder is reconstituted with water for injections, then the vial is for single use only. Any
unused solution should be discarded.
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/valtropin.html
Copyright © 1995-2021 ITA all rights reserved.
|





































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































