Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Victoza 6 mg/ml
solution for injection in pre-filled pen
QUALITATIVE AND QUANTITATIVE COMPOSITION
One ml of solution contains 6 mg of liraglutide*. One pre-filled pen contains 18 mg liraglutide in 3 ml.
* human glucagon-like peptide-1 (GLP-1) analogue produced by recombinant DNA technology in
Saccharomyces cerevisiae
.
For a full list of excipients, see section 6.1.
Solution for injection
in pre-filled pen (injection).
Clear, colourless, isotonic solution; pH=8.15.
4.1 Therapeutic indications
Victoza is indicated for treatment of adults with type 2 diabetes mellitus to achieve glycaemic control:
In combination with:
– Metformin or a sulphonylurea, in patients with insufficient glycaemic control despite maximal
tolerated dose of monotherapy with metformin or sulphonylurea.
In combination with:
–
Metformin and a sulphonylurea or metformin and a thiazolidinedione in patients with
insufficient glycaemic control despite dual therapy.
4.2 Posology and method of administration
Posology
To improve gastro-intestinal tolerability, the starting dose is 0.6 mg liraglutide daily. After at least one
week, the dose should be increased to 1.2 mg. Some patients are expected to benefit from an increase
in dose from 1.2 mg to 1.8 mg and based on clinical response, after at least one week the dose can be
increased to 1.8 mg to further improve glycaemic control. Daily doses higher than 1.8 mg are not
recommended.
Victoza can be added to existing metformin or to a combination of metformin and thiazolidinedione
therapy. The current dose of metformin and thiazolidinedione can be continued unchanged.
Victoza can be added to existing sulphonylurea or to a combination of metformin and sulphonylurea
therapy. When Victoza is added to sulphonylurea therapy, a reduction in the dose of sulphonylurea
should be considered to reduce the risk of hypoglycaemia (see section 4.4).
Self-monitoring of blood glucose is not needed in order to adjust the dose of Victoza. However, when
initiating treatment with Victoza in combination with a sulphonylurea, blood glucose self-monitoring
may become necessary to adjust the dose of the sulphonylurea.
Special populations
Elderly
(>65 years old):
No dose adjustment is required based on age. Therapeutic experience in
patients ≥75 years of age is limited (see section 5.2).
Renal impairment:
No dose adjustment is required for patients with mild renal impairment (creatinine
clearance 60-90 ml/min). There is very limited therapeutic experience in patients with moderate renal
impairment (creatinine clearance of 30-59 ml/min) and no therapeutic experience in patients with
severe renal impairment (creatinine clearance below 30 ml/min). Victoza can currently not be
recommended for use in patients with moderate and severe renal impairment including patients with
end-stage renal disease (see section 5.2).
Hepatic impairment:
The therapeutic experience in patients with all degrees of hepatic impairment is
currently too limited to recommend the use in patients with mild, moderate or severe hepatic
impairment (see section 5.2).
Paediatric population:
Victoza is not recommended for use in children below 18 years of age due to
lack of data on its safety and efficacy.
Method of administration
Victoza should
not
be administered intravenously or intramuscularly.
Victoza is administered once daily at any time, independent of meals, and can be injected
subcutaneously in the abdomen, in the thigh or in the upper arm. The injection site and timing can be
changed without dose adjustment. However, it is preferable that Victoza is injected around the same
time of the day, when the most convenient time of the day has been chosen. For further instructions on
administration, see section 6.6.
Hypersensitivity to the active substance or to any of the excipients.
4.4 Special warnings and precautions for use
Victoza should not be used in patients with type 1 diabetes mellitus or for the treatment of diabetic
ketoacidosis.
There is limited experience in patients with congestive heart failure New York Heart Association
(NYHA) class I-II. There is no experience in patients with congestive heart failure NYHA class III-IV.
There is limited experience in patients with inflammatory bowel disease and diabetic gastroparesis and
Victoza is therefore not recommended in these patients. The use of Victoza is associated with transient
gastrointestinal adverse reactions, including nausea, vomiting and diarrhoea.
Use of GLP-1 analogues has been associated with the risk of pancreatitis. There have been few
reported events of acute pancreatitis. Patients should be informed of the characteristic symptom of
acute pancreatitis: persistent, severe abdominal pain. If pancreatitis is suspected, Victoza and other
potentially suspect medicinal products should be discontinued.
Thyroid adverse events, including increased blood calcitonin, goitre and thyroid neoplasm have been
reported in clinical trials in particular in patients with pre-existing thyroid disease (see section 4.8).
Patients receiving Victoza in combination with a sulphonylurea may have an increased risk of
hypoglycaemia (see section 4.8). The risk of hypoglycaemia can be lowered by a reduction in the dose
of sulphonylurea.
Sign and symptoms of dehydration, including altered renal function have been reported in patients
treated with Victoza. Patients treated with Victoza should be advised of the potential risk of
dehydration in relation to gastrointestinal side effects and take precautions to avoid fluid depletion
4.5 Interaction with other medicinal products and other forms of interaction
In vitro,
liraglutide has shown very low potential to be involved in pharmacokinetic interactions with
other active substances related to cytochrome P450 and plasma protein binding.
The small delay of gastric emptying with liraglutide may influence absorption of concomitantly
administered oral medicinal products. Interaction studies did not show any clinically relevant delay of
absorption. Few patients treated with liraglutide reported at least one episode of severe diarrhoea.
Diarrhoea may affect the absorption of concomitant oral medicinal products.
Paracetamol
Liraglutide did not change the overall exposure of paracetamol following a single dose of 1000 mg.
Paracetamol C
max
was decreased by 31% and median t
max
was delayed up to 15 min. No dose
adjustment for concomitant use of paracetamol is required.
Atorvastatin
Liraglutide did not change the overall exposure of atorvastatin to a clinical relevant degree following
single dose administration of atorvastatin 40 mg. Therefore, no dose adjustment of atorvastatin is
required when given with liraglutide. Atorvastatin C
max
was decreased by 38% and median t
max
was
delayed from 1 h to 3 h with liraglutide.
Griseofulvin
Liraglutide did not change the overall exposure of griseofulvin following administration of a single
dose of griseofulvin 500 mg. Griseofulvin C
max
increased by 37% while median t
max
did not change.
Dose adjustments of griseofulvin and other compounds with low solubility and high permeability are
not required.
Digoxin
A Single dose administration of digoxin 1 mg with liraglutide resulted in a reduction of digoxin AUC
by 16%; C
max
decreased by 31%. Digoxin median t
max
was delayed from 1 h to 1.5 h. No adjustment of
digoxin dose is required based on these results.
Lisinopril
A single dose administration of lisinopril 20 mg with liraglutide resulted in a reduction of lisinopril
AUC by 15% ; C
max
decreased by 27%. Lisinopril median t
max
was delayed from 6 h to 8 h with
liraglutide. No dose adjustment of lisinopril is required based on these results.
Oral contraceptives
Liraglutide lowered ethinyloestradiol and levonorgestrel C
max
by 12 and 13%, respectively, following
administration of a single dose of an oral contraceptive product. T
max
was delayed by 1.5 h with
liraglutide for both compounds. There was no clinically relevant effect on the overall exposure of
either ethinyloestradiol or levonorgestrel. The contraceptive effect is therefore anticipated to be
unaffected when co-administered with liraglutide.
Warfarin and other coumarin derivatives
No interaction study has been performed. A clinically relevant interaction with active substances with
poor solubility or with narrow therapeutic index such as warfarin cannot be excluded. Upon initiation
of liraglutide treatment in patients on warfarin or other coumarin derivatives more frequent monitoring
of INR (International Normalised Ratio) is recommended.
Insulin
Combination of liraglutide with insulin has not been evaluated and is therefore not recommended.
4.6 Pregnancy and lactation
Pregnancy
There are no adequate data from the use of Victoza in pregnant women. Studies in animals have
shown reproductive toxicity (see section 5.3). The potential risk for humans is unknown.
Victoza should not be used during pregnancy, and the use of insulin is recommended instead. If a
patient wishes to become pregnant, or pregnancy occurs, treatment with Victoza should be
discontinued.
Lactation
It is not known whether liraglutide is excreted in human milk. Animal studies have shown that the
transfer of liraglutide and metabolites of close structural relationship into milk is low. Non-clinical
studies have shown a treatment-related reduction of neonatal growth in suckling rat pups (see section
5.3). Because of lack of experience, Victoza should not be used during breast-feeding.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. Patients
should be advised to take precautions to avoid hypoglycaemia while driving and using machines, in
particular when Victoza is used in combination with a sulphonylurea.
In five large long-term clinical trials over 2500 patients have received treatment with Victoza alone or
in combination with metformin, a sulphonylurea (with or without metformin) or metformin plus
rosiglitazone.
Frequencies are defined as: Very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000
to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000); not known (cannot be estimated from
the available data). Within each frequency grouping, undesirable effects are presented in order of
decreasing seriousness.
The most frequently reported adverse reactions during clinical trials were gastrointestinal disorders:
nausea and diarrhoea were very common, whereas vomiting, constipation, abdominal pain, and
dyspepsia were common. At the beginning of Victoza therapy, these gastrointestinal adverse reactions
may occur more frequently. These reactions usually diminish within a few days or weeks on continued
treatment. Headache and nasopharyngitis were also common. Furthermore, hypoglycaemia was
common, and very common when Victoza is used in combination with a sulphonylurea. Major
hypoglycaemia has primarily been observed when combined with a sulphonylurea.
Table 1 lists related adverse reactions identified from Phase III combination-studies with Victoza. The
table presents adverse reactions that occurred with a frequency >5% if the frequency was higher
among Victoza-treated patients than patients treated with comparator. The table also includes adverse
reactions with a frequency ≥2% if the frequency was >2 times the frequency for comparator-treated
subjects.
Table 1 Adverse reactions identified from long-term controlled phase III studies
Adverse reaction
Frequency of adverse reaction by treatment group
Liraglutide
with
metformin
Liraglutide
with
glimepiride
Liraglutide with
metformin and
glimepiride
Liraglutide with
metformin and
rosiglitazone
Infections and
infestations
Metabolism and
nutrition
disorders
Appetite decreased
Common















Frequency of adverse reaction by treatment group
Liraglutide
with
metformin
Liraglutide
with
glimepiride
Liraglutide with
metformin and
glimepiride
Liraglutide with
metformin and
rosiglitazone
Gastrointestinal
disorders
Gastroesophageal
reflux disease
General disorders
and
administration
site conditions
In a clinical trial with Victoza as monotherapy rates of hypoglycaemia reported with Victoza were
lower than rates reported for patients treated with active comparator (glimepiride). The most
frequently reported adverse events were gastrointestinal and infections and infestations.
Hypoglycaemia
Most episodes of confirmed hypoglycaemia in clinical studies were minor. No episodes of major
hypoglycaemia were observed in the study with Victoza used as monotherapy. Major hypoglycaemia
may occur uncommonly and has primarily been observed when Victoza is combined with a
sulphonylurea (0.02 events/subject year). Very few episodes (0.001 events/subject year) were
observed with administration of Victoza in combination with oral antidiabetics other than
sulphonylureas.
Gastrointestinal adverse reactions
When combining Victoza with metformin, 20.7% of patients reported at least one episode of nausea,
and 12.6% of patients reported at least one episode of diarrhoea. When combining Victoza with a
sulphonylurea, 9.1% of patients reported at least one episode of nausea and 7.9% of patients reported
at least one episode of diarrhoea. Most episodes were mild to moderate and occurred in a dose-
dependent fashion. With continued therapy, the frequency and severity decreased in most patients who




























initially experienced nausea.
Patients >70 years may experience more gastrointestinal effects when treated with liraglutide.
Patients with mild renal impairment (creatinine clearance 60-90 ml/min) may experience more
gastrointestinal effects when treated with liraglutide.
Withdrawal
The incidence of withdrawal due to adverse reactions was 7.8% for Victoza-treated patients and 3.4%
for comparator-treated patients in the long-term controlled trials (26 weeks or longer). The most
frequent adverse reactions leading to withdrawal for Victoza-treated patients were nausea (2.8% of
patients) and vomiting (1.5%).
Immunogenicity
Consistent with the potentially immunogenic properties of medicinal products containing proteins or
peptides, patients may develop anti-liraglutide antibodies following treatment with Victoza. On
average, 8.6% of patients developed antibodies. Antibody formation has not been associated with
reduced efficacy of Victoza.
Few cases (0.05%) of angioedema have been reported during all long-term clinical trials with Victoza.
Injection site reactions
Injection site reaction has been reported in approximately 2% of subjects receiving Victoza in long-
term (26 weeks or longer) controlled trials. These reactions have usually been mild and did not lead to
discontinuation of Victoza.
Pancreatitis
Few cases (<0.2%) of acute pancreatitis have been reported during long-term clinical trials with
Victoza.
Thyroid events
The overall rates of thyroid adverse events in all intermediate and long-term trials are 33.5, 30.0 and
21.7 events per 1000 subject years of exposure for total liraglutide, placebo and total comparators; 5.4,
2.1 and 1.2 events, respectively concern serious thyroid adverse events.
Thyroid neoplasms, increased blood calcitonin and goiters were the most frequently thyroid adverse
events. The rates per 1000 subject years of exposure were 6.8, 10.9 and 5.4 of liraglutide treated
patients in comparison with 6.4, 10.7 and 2.1 of placebo treated and 2.4, 6.0 and 1.8 of total
comparator treated patients respectively.
In a clinical study of Victoza, one patient with type 2 diabetes experienced a single overdose of
17.4 mg subcutaneous (10 times the maximal recommended maintenance dose of 1.8 mg). Effects of
the overdose included severe nausea and vomiting, but not hypoglycaemia. The patient recovered
without complications.
In the event of overdose, appropriate supportive treatment should be initiated according to the
patient’s clinical signs and symptoms.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Other blood glucose lowering drugs, excl. insulins. ATC code: A10BX07
Mechanism of action
Liraglutide is a GLP-1 analogue with 97% sequence homology to human GLP-1 that binds to and
activates the GLP-1 receptor. The GLP-1 receptor is the target for native GLP-1, an endogenous
incretin hormone that potentiates glucose-dependent insulin secretion from the pancreatic beta cells.
Unlike native GLP-1, liraglutide has a pharmacokinetic and pharmacodynamic profile in humans
suitable for once daily administration. Following subcutaneous administration, the protracted action
profile is based on three mechanisms: self-association, which results in slow absorption; binding to
albumin; and higher enzymatic stability towards the dipeptidyl peptidase IV (DPP-IV) and neutral
endopeptidase (NEP) enzymes, resulting in a long plasma half-life.
Liraglutide action is mediated via a specific interaction with GLP-1 receptors, leading to an increase in
cyclic adenosine monophosphate (cAMP). Liraglutide stimulates insulin secretion in a glucose-
dependent manner. Simultaneously, liraglutide lowers inappropriately high glucagon secretion, also in
a glucose-dependent manner. Thus, when blood glucose is high, insulin secretion is stimulated and
glucagon secretion is inhibited. Conversely, during hypoglycaemia liraglutide diminishes insulin
secretion and does not impair glucagon secretion. The mechanism of blood glucose lowering also
involves a minor delay in gastric emptying. Liraglutide reduces body weight and body fat mass
through mechanisms involving reduced hunger and lowered energy intake.
Pharmacodynamic effects
Liraglutide has 24-hour duration of action and improves glycaemic control by lowering fasting and
postprandial blood glucose in patients with type 2 diabetes mellitus.
Clinical efficacy
Five double-blind, randomised, controlled clinical trials were conducted to evaluate the effects of
Victoza on glycaemic control. Treatment with Victoza produced clinically and statistically significant
improvements in glycosylated haemoglobin A
1c
(HbA
1c
), fasting plasma glucose and post-prandial
glucose compared with placebo.
These studies included 3,978 exposed patients with type 2 diabetes (2,501 subjects treated with
Victoza), 53.7% men and 46.3% women, 797 subjects (508 treated with Victoza) were ≥65 years of
age and 113 subjects (66 treated with Victoza) were ≥75 years of age.
There was an additional open-label randomised controlled study comparing liraglutide with exenatide.
Glycaemic control
Victoza in combination therapy, for 26 weeks, with metformin, glimepiride or metformin and
rosiglitazone resulted in statistically significant (p<0.0001) and sustained reductions in HbA
1c
compared with patients receiving placebo (Tables 2 and 3).
Results of two 26 week trials. Victoza in combination with metformin and Victoza in
combination with glimepiride.
1.8 mg liraglutide
+ metformin
3
1.2 mg liraglutide
+ metformin
3
Glimepiride
2
+ metformin
3
Mean HbA
1c
(%)
Baseline
Change from baseline
Patients (%) achieving
HbA
1c
<7%
Allpatients
PreviousOAD
monotherapy
Mean body weight (kg)
Baseline
Change from baseline
1.8 mg liraglutide 1.2 mg liraglutide Placebo



+ glimepiride
2
+ glimepiride
2
Mean HbA
1c
(%)
Baseline
Change from baseline
Patients (%) achieving
HbA
1c
<7%
All patients
Previous OAD
monotherapy
Mean body weight (kg)
Baseline
Change from baseline
1
Rosiglitazone 4 mg/day;
2
glimepiride 4 mg/day;
3
metformin 2000 mg/day
Table 3 Results of two 26 week trials. Victoza in combination with metformin + rosiglitazone
and Victoza in combination with glimepiride + metformin
.
Metformin + rosiglitazone
add-on therapy
1.8 mg liraglutide
+ metformin
2
+ rosiglitazone
3
1.2mg liraglutide
+ metformin
2
+ rosiglitazone
3
placebo
+ metformin
2
+ rosiglitazone
3
Mean HbA
1c
(%)
Baseline
Change from baseline
Patients (%) achieving
HbA
1c
<7%
All patients
Mean body weight (kg)
Baseline
Change from baseline
Metformin + glimepiride
add-on therapy
1.8 mg liraglutide
+ metformin
2
+ glimepiride
4
Placebo
+ metformin
2
+ glimepiride
4
insulin glargine
1
+ metformin
2
+ glimepiride
4
Mean HbA
1c
(%)
Baseline
Change from baseline
Patients (%) achieving
HbA
1c
<7%
All patients
85.2
1.62
1
The dosing of insulin glargine was open-labelled and was applied according to the following titration
guideline. Titration of the insulin glargine dose was managed by the patient after instruction by the
investigator.
Guideline for titration of insulin glargine
Self-measured FPG Increase in insulin glargine dose (IU)
≤5.5 mmol/l (≤100 mg/dl) Target No adjustment
>5.5 and <6.7 mmol/l (>100 and <120 mg/dl) 0 – 2 IU
a
≥6.7 mmol/l (≥120 mg/dl) 2 IU
a
According to the individualised recommendation by the investigator at the previous visit for example
depending on whether subject has experienced hypoglycaemia.
Mean body weight (kg)
Baseline
Change from baseline

















2
Metformin 2000 mg/day;
3
rosiglitazone 4 mg twice daily;
4
glimepiride 4 mg/day.
Proportion of patients achieving reductions in HbA
1c
Victoza in combination with metformin, glimepiride, or metformin and rosiglitazone resulted in a
statistically significant (p≤0.0001) greater proportion of patients achieving an HbA
1c
≤6.5% at
26 weeks compared with patients receiving these agents alone.
Fasting plasma glucose
Treatment with Victoza alone or in combination with one or two oral antidiabetic drugs resulted in a
reduction in fasting plasma glucose of 13-43.5 mg/dl (0.72-2.42 mmol/l). This reduction was observed
within the first two weeks of treatment.
Post-prandial glucose
Victoza reduces post-prandial glucose across all three daily meals by 31-49 mg/dl (1.68-2.71 mmol/l).
Beta-cell function
Clinical studies with Victoza indicate improved beta-cell function based on measures such as the
homeostasis model assessment for beta-cell function (HOMA-B) and the proinsulin to insulin ratio.
Improved first and second phase insulin secretion after 52 weeks treatment with Victoza was
demonstrated in a subset of patients with type 2 diabetes (N=29).
Body weight
Victoza in combination with metformin, metformin and glimepiride or metformin and rosiglitazone
was associated with sustained weight reduction over the duration of studies in a range from 1.0 kg to
2.8 kg.
Larger weight reduction was observed with increasing body mass index (BMI) at baseline.
Blood pressure
Over the duration of the studies Victoza decreased the systolic blood pressure on average of 2.3 to
6.7 mmHg from baseline and compared to active comparator the decrease was 1.9 to 4.5 mmHg
5.2 Pharmacokinetic properties
Absorption
The absorption of liraglutide following subcutaneous administration is slow, reaching maximum
concentration 8-12 hours post dosing. Estimated maximum liraglutide concentration was 9.4 nmol/l
for a subcutaneous single dose of liraglutide 0.6 mg. At 1.8 mg liraglutide, the average steady state
concentration of liraglutide (AUC
τ/24
) reached approximately 34 nmol/l. Liraglutide exposure
increased proportionally with dose. The intra-subject coefficient of variation for liraglutide AUC was
11% following single dose administration.
Absolute bioavailability of liraglutide following subcutaneous administration is approximately 55%.
Distribution
The apparent volume of distribution after subcutaneous administration is 11-17 l. The mean volume of
distribution after intravenous administration of liraglutide is 0.07 l/kg. Liraglutide is extensively bound
to plasma proteins (>98%).
Metabolism
During 24 hours following administration of a single radiolabelled [
3
H]-liraglutide dose to healthy
subjects, the major component in plasma was intact liraglutide. Two minor plasma metabolites were
detected (≤9% and ≤5% of total plasma radioactivity exposure). Liraglutide is metabolised in a similar
manner to large proteins without a specific organ having been identified as major route of elimination.
Elimination
Following a [
3
H]-liraglutide dose, intact liraglutide was not detected in urine or faeces. Only a minor
part of the administered radioactivity was excreted as liraglutide-related metabolites in urine or faeces
(6% and 5%, respectively). The urine and faeces radioactivity was mainly excreted during the first
6-8 days, and corresponded to three minor metabolites, respectively.
The mean clearance following subcutaneous administration of a single dose liraglutide is
approximately 1.2 l/h with an elimination half-life of approximately 13 hours.
Elderly
: Age had no clinically relevant effect on the pharmacokinetics of liraglutide based on the
results from a pharmacokinetic study in healthy subjects and population pharmacokinetic data analysis
of patients (18 to 80 years).
Gender
: Gender had no clinically meaningful effect on the pharmacokinetics of liraglutide based on
the results of population pharmacokinetic data analysis of male and female patients and a
pharmacokinetic study in healthy subjects.
Ethnic origin:
Ethnic origin had no clinically relevant effect on the pharmacokinetics of liraglutide
based on the results of population pharmacokinetic analysis which included subjects of White, Black,
Asian and Hispanic groups.
Obesity
: Population pharmacokinetic analysis suggests that body mass index (BMI) has no significant
effect on the pharmacokinetics of liraglutide.
Hepatic impairment:
The pharmacokinetics of liraglutide was evaluated in subjects with varying
degree of hepatic impairment in a single-dose trial. Liraglutide exposure was decreased by 13-23% in
subjects with mild to moderate hepatic impairment compared to healthy subjects.
Exposure was significantly lower (44%) in subjects with severe hepatic impairment (Child Pugh
score >9).
Renal impairment:
Liraglutide exposure was reduced in subjects with renal impairment compared to
individuals with normal renal function. Liraglutide exposure was lowered by 33%, 14%, 27% and
28%, respectively, in subjects with mild (creatinine clearance, CrCL 50-80 ml/min), moderate (CrCL
30-50 ml/min), and severe (CrCL <30 ml/min) renal impairment and in end-stage renal disease
requiring dialysis.
5.3 Preclinical safety data
Non-clinical data reveal no special hazards for humans based on conventional studies of safety
pharmacology, repeat-dose toxicity, or genotoxicity.
Non-lethal thyroid C-cell tumours were seen in 2-year carcinogenicity studies in rats and mice. In rats,
a no observed adverse effect level (NOAEL) was not observed. These tumours were not seen in
monkeys treated for 20 months. These findings in rodents are caused by a non-genotoxic, specific
GLP-1 receptor-mediated mechanism to which rodents are particularly sensitive. The relevance for
humans is likely to be low but cannot be completely excluded. No other treatment-related tumours
have been found.
Animal studies did not indicate direct harmful effects with respect to fertility but slightly increased
early embryonic deaths at the highest dose. Dosing with Victoza during mid-gestation caused a
reduction in maternal weight and foetal growth with equivocal effects on ribs in rats and skeletal
variation in the rabbit. Neonatal growth was reduced in rats while exposed to Victoza, and persisted in
the post-weaning period in the high dose group. It is unknown whether the reduced pup growth is
caused by reduced pup milk intake due to a direct GLP-1 effect or reduced maternal milk production
due to decreased caloric intake.
PHARMACEUTICAL PARTICULARS
Disodium phosphate dihydrate
Propylene glycol
Phenol
Water for injections
Substances added to Victoza may cause degradation of liraglutide. In the absence of compatibility
studies, this medicinal product must not be mixed with other medicinal products.
6.4 Special precautions for storage
Store in a refrigerator (2°C - 8°C).
Do not freeze.
Store away from the freezer compartment.
After first use
: Store below 30°C or store in a refrigerator (2°C - 8°C).
Do not freeze.
Keep the cap on the pen in order to protect from light.
6.5 Nature and contents of container
Cartridge (type 1 glass) with a plunger (bromobutyl) and a stopper (bromobutyl/polyisoprene)
contained in a pre-filled multidose disposable pen made of polyolefin and polyacetal.
Each pen contains 3 ml solution, delivering 30 doses of 0.6 mg, 15 doses of 1.2 mg or 10 doses of
1.8 mg.
Pack sizes of 1, 2, 3, 5 or 10 pre-filled pens.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
Victoza should not be used if it does not appear clear and colourless.
Victoza should not be used if it has been frozen.
Victoza can be administered with needles up to a length of 8 mm and as thin as 32G. The pen is
designed to be used with NovoFine or NovoTwist disposable needles.
Injection needles are not included.
The patient should be advised to discard the injection needle in accordance with local requirements
after each injection and store the Victoza pen without an injection needle attached. This prevents
contamination, infection, and leakage. It also ensures that the dosing is accurate.
MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
ANNEX II
A. MANUFACTURING AUTHORISATION HOLDER
RESPONSIBLE FOR BATCH RELEASE
B. CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer(s) responsible for batch release
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsvaerd
Denmark
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to medical prescription.
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
Pharmacovigilance system
The MAH must ensure that the system of pharmacovigilance, as described in version 7.0 presented in
Module 1.8.1. of the Marketing Authorisation Application, is in place and functioning before and
whilst the product is on the market.
Risk Management Plan
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version 5.0 of the Risk Management Plan (RMP) presented
in Module 1.8.2. of the Marketing Authorisation Application and any subsequent updates of the RMP
agreed by the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, the
updated RMP should be submitted at the same time as the next Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted
•
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
At the request of the EMEA
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
Victoza 6 mg/ml solution for injection
in pre-filled pen
Liraglutide
STATEMENT OF ACTIVE SUBSTANCE
One ml contains 6 mg of liraglutide. One pre-filled pen contains 18 mg liraglutide
Disodium phosphate dihydrate, propylene glycol, phenol, water for injections
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection
in pre-filled pen.
1 pen
2 pens
3 pens
5 pens
10 pens
Each pen contains 3 ml solution, delivering 30 doses of 0.6 mg, 15 doses of 1.2 mg or 10 doses of
1.8 mg.
METHOD AND ROUTE OF ADMINISTRATION
Read the package leaflet before use
Subcutaneous use
Victoza pen is designed to be used with NovoFine and NovoTwist disposable needles.
Needles are not included
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNINGS, IF NECESSARY
Do not store the pen with a needle attached.






















Discard pen 1 month after first use.
SPECIAL STORAGE CONDITIONS
Store in a refrigerator. Do not freeze.
After first use of the pen, store below 30°C or in a refrigerator. Do not freeze.
Keep the pen cap on in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
12. MARKETING AUTHORISATION NUMBERS
EU/1/09/529/001 1 x 3 ml
EU/1/09/529/002 2 x 3 ml
EU/1/09/529/003 3 x 3 ml
EU/1/09/529/004 5 x 3 ml
EU/1/09/529/005 10 x 3 ml
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
16. INFORMATION IN BRAILLE


























PACKAGE LEAFLET: INFORMATION FOR THE USER
Victoza 6 mg/ml solution for injection in pre-filled pen
Liraglutide
Read all of this leaflet carefully before you start using this medicine.
–
If you have any further questions, ask your doctor, nurse or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects get serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor, nurse or pharmacist.
What Victoza is and what it is used for
WHAT VICTOZA IS AND WHAT IT IS USED FOR
Victoza contains the active substance liraglutide. It helps your body reduce your blood sugar level
only when blood sugar is too high. It also slows food passage through your stomach.
Victoza is used to treat type 2 diabetes mellitus when:
–
metformin or a sulphonylurea alone (such as glimepiride or glibenclamide) despite the maximal
tolerated dose are not enough to control your blood sugar levels.
metformin in combination with a sulphonylurea (such as glimepiride or glibenclamide) or
metformin in combination with a glitazone (such as rosiglitazone or pioglitazone) are not
enough to control your blood sugar levels.
if you are allergic (hypersensitive) to liraglutide or any of the other ingredients of Victoza
(listed in section 6, ‘What Victoza contains’).
Take special care with Victoza
–
if you are also taking a sulphonylurea (such as glimepiride or glibenclamide), your doctor may
tell you to test your blood sugar levels. This will help your doctor to decide if the dose of the
sulphonylurea needs to be changed.
Victoza should not be used if you have type 1 diabetes or diabetic ketoacidosis. Victoza should not be
used in children and adolescents under 18 years.
The use of Victoza is not recommended in patients with inflammatory bowel disease and/or diabetic
gastroparesis.
If you have symptoms of acute pancreatitis, like persistent, severe abdominal pain, you should consult
your doctor.
When initiating treatment with Victoza, you may in some cases experience loss of fluids/ dehydration,
e.g. in case of vomiting, nausea and diarrhea. It is important to avoid dehydration by drinking plenty
Keep this leaflet. You may need to read it again.
of fluids. Contact your doctor if you have any questions or concerns.
Using other medicines
Please tell your doctor, nurse or pharmacist if you are taking or have recently taken any other
medicines, including medicines obtained without a prescription.
In particular, tell your doctor, nurse or pharmacist if you are using medicines for diabetes containing
any of the following active substances:
•
insulin. Victoza is not recommended if you are using insulin.
a sulphonylurea (such as glimepiride or glibenclamide). You may get hypoglycaemia (low
blood sugar) when using Victoza together with a sulphonylurea as sulphonylureas increase the
risk of hypoglycaemia. When you first start using these medicines together, your doctor may tell
you to lower the dose of the sulphonylurea medicine. Please see section 4 for the warnings signs
of low blood sugar.
Using Victoza with food and drink
You can use Victoza regardless of meals.
Pregnancy and breast-feeding
Tell your doctor if you are, you think you might be, or are planning to become pregnant. Victoza
should not be used during pregnancy. It is not known if Victoza may harm your unborn child.
It is not known if Victoza passes into breast milk. Do not use Victoza if you are breast-feeding.
Ask your doctor or pharmacist for advice before taking any medicine if you are pregnant or breast-
feeding.
Driving and using machines
While you are driving or using tools or machines, you should avoid getting low blood sugar
(hypoglycaemia), because this may reduce your ability to concentrate. Your doctor will tell you how
to do this.
Always use Victoza exactly as your doctor has told you. You should check with your doctor, nurse or
pharmacist if you are not sure.
• The starting dose is
0.6 mg
once a day, for at least one week.
• Your doctor will tell you when to increase it to
1.2 mg
once a day.
• Your doctor may tell you to further increase the dose to
1.8 mg
once a day, if your blood
glucose is not adequately controlled with a dose of 1.2 mg.
Do not change your dose unless your doctor has told you to.
Victoza is given as an injection under the skin (subcutaneous). Do not inject it into a vein or muscle.
The best places to give yourself the injection are the front of your thighs, the front of your waist
(abdomen), or your upper arm.
You can give yourself the injection at any time of the day, regardless of meals. When you have found
the most convenient time of the day it is preferred that you inject Victoza around the same time of the
day.
Before you use the pen for the first time, your doctor or nurse will show you how to use it.
Detailed instructions for use are provided on the other side of this leaflet.
If you use more Victoza than you should
If you use more Victoza than you should, talk to your doctor straight away. You may need medical
treatment. If you use too much Victoza, you may experience nausea or vomiting.
If you forget to use Victoza
If you forget a dose, use Victoza as soon as you remember.
However, if it is more than 12 hours since you should have used Victoza, skip the missed dose. Then
take your next dose as usual the following day.
Do not take an extra dose or increase the dose on the following day to make up for the missed dose.
If you stop using Victoza
Do not stop using Victoza without talking to your doctor. If you stop using it, your blood sugar levels
may increase.
If you have any further questions on the use of this medicine, ask your doctor, nurse or pharmacist.
Like all medicines, Victoza can cause side effects although not everybody gets them.
Side effects may occur with certain frequencies, which are defined as follows:
•
very common: affects more than 1 user in 10
uncommon: affects 1 to 10 users in 1,000
rare: affects 1 to 10 users in 10,000
very rare: affects less than 1 user in 10,000
not known: frequency cannot be estimated from the available data
Very common side effects
•
Nausea (feeling sick). This usually goes away over time.
Diarrhoea. This usually goes away over time.
Hypoglycaemia (low blood sugar). The warning signs of low blood sugar may come on
suddenly and can include: cold sweat, cool pale skin, headache, fast heart beat, feeling sick,
feeling very hungry, changes in vision, feeling sleepy, feeling weak, nervous, anxious,
confused, difficulty concentrating, shaking (tremor). Your doctor will tell you how to treat low
blood sugar and what to do if you notice these warning signs. If you are already taking a
sulphonylurea medicine when you start using Victoza, your doctor may tell you to reduce the
dose of the sulphonylurea.
Inflamed stomach (gastritis). The signs include stomach pain, nausea and vomiting.
Gastro-oesophageal reflux disease (GORD). The signs include heartburn.
Painful or swollen tummy (abdomen)
Viral infection in stomach
Injection site reactions (such as bruising, pain, irritation, itching and rash)
common: affects 1 to 10 users in 100
Thyroid events - like nodules, increased blood calcitonin and goiters
If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please
tell your doctor, nurse or pharmacist.
Keep out of the reach and sight of children.
Do not use Victoza after the expiry date which is stated on the pen label and carton after EXP. The
expiry date refers to the last day of that month.
Before opening
:
Store in a refrigerator (2˚C - 8˚C). Do not freeze. Keep away from the freezer compartment.
During use:
You can keep the pen for 1 month when stored at a temperature below 30˚C or in a refrigerator (2˚C -
8˚C), away from the freezer compartment. Do not freeze.
When you are not using the pen, keep the pen cap on in order to protect from light.
Do not use Victoza if the solution is not clear and colourless.
The active substance is liraglutide.
One ml solution for injection contains 6 mg liraglutide. One
pre-filled pen contains 18 mg liraglutide.
The other ingredients are disodium phosphate dihydrate, propylene glycol, phenol and water for
injections.
What Victoza looks like and contents of the pack
Victoza is supplied as a clear, colourless solution for injection in pre-filled pen. Each pen contains
3 ml of solution, delivering 30 doses of 0.6 mg, 15 doses of 1.2 mg or 10 doses of 1.8 mg.
Victoza is available in packs containing 1, 2, 3, 5 or 10 pens. Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency (EMEA)
Instructions for using the Victoza pen
Please read these instructions carefully before using your Victoza pen.
Your Victoza pen comes with 18 mg of liraglutide. You can select doses of 0.6 mg, 1.2 mg and
1.8 mg.
Victoza pen is designed to be used with NovoFine or NovoTwist disposable injection needles up to a
length of 8 mm and as thin as 32G.
Preparing your Victoza pen
B.
Pull off the paper tab from a new disposable needle. Screw the needle straight and tightly onto
your pen.
C.
Pull off the outer needle cap and keep it for later.
D.
Pull off the inner needle cap and throw it away.
Always use a new needle for each injection to prevent contamination.
Be careful not to bend or damage the needle.
Never put the inner needle cap back on when you have removed it from the needle. This reduces
the risk of hurting yourself with the needle.
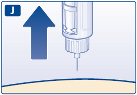
Always check the flow as follows before you inject with a new pen.






Flow check
symbol
s ele ct ed
E.
Turn the dose selector until the flow check symbol lines up with the pointer.
F.
Hold the pen with the needle pointing up. Tap the cartridge gently with your finger a few times.
This will make any air bubbles collect at the top of the cartridge.
G.
Keep the needle pointing up and press the dose button until 0 mg lines up with the pointer.
Repeat steps
E
to
G
until a drop of liraglutide appears at the needle tip. If no drop appears after
six times, change the needle and repeat steps
E
to
G
up to six more times. If you still see no
drop of liraglutide, the pen is broken and you must use a new one.
If you have dropped your pen against a hard surface or suspect that something is wrong with it,
always put on a new disposable needle and check the flow before you inject.
Always check that the pointer lines up with 0 mg.
H.
Turn the dose selector until your needed dose lines up with the pointer (0.6 mg, 1.2 mg or
1.8 mg).
If you selected a wrong dose by mistake, simply change it by turning the dose selector
backwards or forwards until the right dose lines up with the pointer. Be careful not to press the
dose button when turning the dose selector backwards, as liraglutide may come out.
If the dose selector stops before your needed dose lines up with the pointer, there is not enough
liraglutide left for a full dose. Then you can either:
Divide your dose into two injections:
Turn the dose selector in either direction until 0.6 mg or 1.2 mg lines up with the pointer. Inject
the dose. Prepare a new pen for injection and inject the remaining number of mg to complete





your dose.
Inject the full dose with a new pen:
If the dose selector stops before 0.6 mg lines up with the pointer, prepare a new pen and inject
the full dose with the new pen.
The dose selector clicks when you turn it. You must not use these clicks to select the amount of
liraglutide to inject.
Do not try to select other doses than 0.6 mg, 1.2 mg or 1.8 mg. The numbers in the display must
line up precisely with the pointer to ensure that you get a correct dose.
Insert the needle into your skin using the injection technique shown by your doctor or nurse.
Then follow the instructions below:
Press the dose button to inject until 0 mg lines up with the pointer. Be careful not to touch the
display with your other fingers or press the dose selector sideways when you inject. This is
because it may block the injection. Keep the dose button pressed down and leave the needle
under the skin for at least six seconds. This is to make sure that you get your full dose.
Pull out the needle.
After that, you may see a drop of liraglutide at the needle tip.
This is normal and has no effect on the dose you have just had.
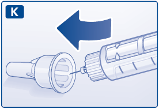
K.
Guide the needle tip into the outer needle cap without touching the outer needle cap.
L.
When the needle is covered, carefully push the outer needle cap completely on. Then unscrew
the needle. Carefully throw the needle away and put the pen cap back on. When the pen is
empty, carefully throw it away without a needle attached. Please throw the pen and needle away
in accordance with local requirements.
Always remove the needle after each injection and store your Victoza pen without a needle
attached.
This prevents contamination or infection or leakage of liraglutide. It also ensures that the dosing
is accurate.
Caring for your Victoza pen
Your Victoza pen is accurate and safe to use. But you must take care of it:
•
Keep your pen away from dust, dirt and all kinds of liquids.
Clean the pen with a cloth moistened with a mild detergent. Do not try to wash it, soak it or
lubricate it – this can harm the pen.
Do not use the cartridge scale to measure how much liraglutide to inject – it is not accurate
enough.
Caregivers should be very careful when handling used needles to avoid hurting themselves with
the needles.
Do not try to repair your pen or pull it apart.









Do not share your Victoza pen with anyone else.
Keep your Victoza pen out of reach of others, especially children.

Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/victoza.html
Copyright © 1995-2021 ITA all rights reserved.
|
























































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































