Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
XEPLION 25 mg prolonged release suspension for injection
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each pre-filled syringe contains 39 mg paliperidone palmitate equivalent to 25 mg paliperidone.
For a full list of excipients, see section 6.1.
Prolonged release suspension for injection.
The suspension is white to off-white. The suspension is pH neutral (approximately 7.0).
4.1 Therapeutic indications
XEPLION is indicated for maintenance treatment of schizophrenia in adult patients stabilised with
paliperidone or risperidone.
In selected adult patients with schizophrenia and previous responsiveness to oral paliperidone or
risperidone, XEPLION may be used without prior stabilisation with oral treatment if psychotic symptoms
are mild to moderate and a long-acting injectable treatment is needed.
4.2 Posology and method of administration
Recommended initiation of XEPLION is with a dose of 150 mg on treatment day 1 and 100 mg one week
later (day 8), both administered in the deltoid muscle in order to attain therapeutic concentrations rapidly
(see section 5.2). The recommended monthly maintenance dose is 75 mg; some patients may benefit from
lower or higher doses within the recommended range of 25 to 150 mg based on individual patient
tolerability and/or efficacy. Patients who are overweight or obese may require doses in the upper range
(see section 5.2). Following the second dose, monthly maintenance doses can be administered in either the
deltoid or gluteal muscle.
Adjustment of the maintenance dose may be made monthly. When making dose adjustments, the
prolonged release characteristics of XEPLION should be considered (see section 5.2), as the full effect of
maintenance doses may not be evident for several months.
Switching from oral paliperidone or oral risperidone
Previous oral paliperidone or oral risperidone can be discontinued at the time of initiation of treatment
with XEPLION. XEPLION should be initiated as described at the beginning of section 4.2 above.
Switching from Risperidone long acting injection.
When switching patients from risperidone long acting injection, initiate XEPLION therapy in place of the
next scheduled injection. XEPLION should then be continued at monthly intervals. The one-week
initiation dosing regimen including the intramuscular injections (day 1 and 8, respectively) as described in
section 4.2 above is not required. Patients previously stabilised on different doses of risperidone long
acting injection can attain similar paliperidone steady-state exposure during maintenance treatment with
XEPLION monthly doses according to the following:
Doses of Risperidone long acting injection and XEPLION needed to attain similar
paliperidone exposure at steady-state
Previous Risperidone long acting injection dose
Discontinuation of antipsychotic medicinal products should be made in accordance with appropriate
prescribing information. If XEPLION is discontinued, its prolonged release characteristics must be
considered. As recommended with other antipsychotic medicinal products, the need for continuing
existing extrapyramidal symptoms (EPS) medicine should be re-evaluated periodically.
Avoiding missed doses
It is recommended that the second initiation dose of XEPLION be given one week after the first dose. To
avoid a missed dose, patients may be given the second dose 2 days before or after the one-week (day 8)
time point. Similarly, the third and subsequent injections after the initiation regimen are recommended to
be given monthly. To avoid a missed monthly dose, patients may be given the injection up to 7 days
before or after the monthly time point.
If the target date for the second XEPLION injection (day 8 ± 2 days) is missed, the recommended
reinitiation depends on the length of time which has elapsed since the patient's first injection.
Missed second initiation dose (< 4 weeks from first injection)
If less than 4 weeks have elapsed since the first injection, then the patient should be administered the
second injection of 100 mg in the deltoid muscle as soon as possible. A third XEPLION injection of
75 mg in either the deltoid or gluteal muscles should be administered 5 weeks after the first injection
(regardless of the timing of the second injection). The normal monthly cycle of injections in either the
deltoid or gluteal muscle of 25 mg to 150 mg based on individual patient tolerability and/or efficacy
should be followed thereafter.
Missed second initiation dose (4-7 weeks from first injection)
If 4 to 7 weeks have elapsed since the first injection of XEPLION, resume dosing with two injections of
100 mg in the following manner:
1.
a deltoid injection as soon as possible,
another deltoid injection one week later,







resumption of the normal monthly cycle of injections in either the deltoid or gluteal muscle of
25 mg to 150 mg based on individual patient tolerability and/or efficacy.
Missed second initiation dose (> 7 weeks from first injection)
If more than 7 weeks have elapsed since the first injection of XEPLION, initiate dosing as described for
the initial recommended initiation of XEPLION above.
Missed monthly maintenance dose (1 month to 6 weeks)
After initiation, the recommended injection cycle of XEPLION is monthly. If less than 6 weeks have
elapsed since the last injection, then the previously stabilised dose should be administered as soon as
possible, followed by injections at monthly intervals.
Missed monthly maintenance dose (> 6 weeks to 6 months)
If more than 6 weeks have elapsed since the last injection of XEPLION, the recommendation is as
follows:
For patients stabilised with doses of 25 to 100
mg:
1. a deltoid injection as soon as possible at the same dose the patient was previously stabilised on
2. another deltoid injection (same dose) one week later (day 8)
3. resumption of the normal monthly cycle of injections in either the deltoid or gluteal muscle of
25 mg to 150 mg based on individual patient tolerability and/or efficacy
For patients stabilised with 150
mg:
1. a deltoid injection as soon as possible at the 100 mg dose
2. another deltoid injection one week later (day 8) at the 100 mg dose
3. resumption of the normal monthly cycle of injections in either the deltoid or gluteal muscle of
25 mg to 150 mg based on individual patient tolerability and/or efficacy
Missed monthly maintenance dose (> 6 months).
If more than 6 months have elapsed since the last
injection of XEPLION, initiate dosing as described for the initial recommended initiation of XEPLION
above.
Elderly population
Efficacy and safety in elderly > 65 years have not been established.
In general, recommended dosing of XEPLION for elderly patients with normal renal function is the same
as for younger adult patients with normal renal function. However, because elderly patients may have
diminished renal function, dose adjustment may be necessary (see
Renal impairment
below for dosing
recommendations in patients with renal impairment).
Renal impairment
XEPLION has not been systematically studied in patients with renal impairment (see section 5.2). For
patients with mild renal impairment (creatinine clearance ≥ 50 to < 80 ml/min), recommended initiation of
XEPLION is with a dose of 100 mg on treatment day 1 and 75 mg one week later, both administered in
the deltoid muscle. The recommended monthly maintenance dose is 50 mg with a range of 25 to 100 mg
based on patient tolerability and/or efficacy.
XEPLION is not recommended in patients with moderate or severe renal impairment (creatinine clearance
< 50 ml/min) (see section 4.4).
Hepatic impairment
Based on experience with oral paliperidone, no dose adjustment is required in patients with mild or
moderate hepatic impairment. As paliperidone has not been studied in patients with severe hepatic
impairment, caution is recommended in such patients.
Other special populations
No dose adjustment for XEPLION is recommended based on gender, race, or smoking status.
Paediatric population
The safety and efficacy of XEPLION in children < 18 years of age have not been established. No data are
available.
XEPLION is intended for intramuscular use only. It should be injected slowly, deep into the muscle. Each
injection should be administered by a health care professional. Administration should be in a single
injection. The dose should not be given in divided injections. The dose should not be administered
intravascularly or subcutaneously.
The day 1 and day 8 initiation doses must each be administered in the deltoid muscle in order to attain
therapeutic concentrations rapidly (see section 5.2). Following the second dose, monthly maintenance
doses can be administered in either the deltoid or gluteal muscle. A switch from gluteal to deltoid (and
vice versa) should be considered in the event of injection site pain if the injection site discomfort is not
well tolerated (see section 4.8). It is also recommended to alternate between left and right sides (see
below).
For instructions for use and handling of XEPLION, see package leaflet (information intended for medical
or healthcare professionals).
Deltoid muscle administration
The recommended needle size for initial and maintenance administration of XEPLION into the deltoid
muscle is determined by the patient’s weight. For those ≥ 90 kg, the 1½ inch, 22 gauge needle (38.1 mm x
0.72 mm) is recommended. For those < 90 kg, the 1-inch, 23 gauge needle (25.4 mm x 0.64 mm) is
recommended. Deltoid injections should be alternated between the two deltoid muscles.
Gluteal muscle administration
The recommended needle size for maintenance administration of XEPLION into the gluteal muscle is the
1½-inch, 22 gauge needle (38.1 mm x 0.72 mm). Administration should be made into the upper-outer
quadrant of the gluteal area. Gluteal injections should be alternated between the two gluteal muscles.
Hypersensitivity to the active substance, to risperidone or to any of the excipients.
4.4 Special warnings and precautions for use
Use in patients who are in an acutely agitated or severely psychotic state
XEPLION should not be used to manage acutely agitated or severely psychotic states when immediate
symptom control is warranted.
Caution should be exercised when paliperidone is prescribed in patients with known cardiovascular
disease or family history of QT prolongation, and in concomitant use with other medicinal products
thought to prolong the QT interval.
Neuroleptic malignant syndrome
Neuroleptic Malignant Syndrome (NMS), characterised by hyperthermia, muscle rigidity, autonomic
instability, altered consciousness, and elevated serum creatine phosphokinase levels has been reported to
occur with paliperidone. Additional clinical signs may include myoglobinuria (rhabdomyolysis) and acute
renal failure. If a patient develops signs or symptoms indicative of NMS, all antipsychotics, including
paliperidone, should be discontinued.
Medicinal products with dopamine receptor antagonistic properties have been associated with the
induction of tardive dyskinesia characterised by rhythmical, involuntary movements, predominantly of the
tongue and/or face. If signs and symptoms of tardive dyskinesia appear, the discontinuation of all
antipsychotics, including paliperidone, should be considered.
Rare cases of glucose related adverse reactions, e.g. increase in blood glucose, have been reported in
clinical trials with paliperidone. Appropriate clinical monitoring is advisable in diabetic patients and in
patients with risk factors for the development of diabetes mellitus.
Tissue culture studies suggest that cell growth in human breast tumours may be stimulated by prolactin.
Although no clear association with the administration of antipsychotics has so far been demonstrated in
clinical and epidemiological studies, caution is recommended in patients with relevant medical history.
Paliperidone should be used with caution in patients with possible prolactin-dependent tumours.
Paliperidone may induce orthostatic hypotension in some patients based on its alpha-blocking activity.
Based on pooled data from the three placebo-controlled, 6-week, fixed-dose trials with oral paliperidone
prolonged release tablets (3, 6, 9, and 12 mg), orthostatic hypotension was reported by 2.5% of subjects
treated with oral paliperidone compared with 0.8% of subjects treated with placebo. XEPLION should be
used with caution in patients with known cardiovascular disease (e.g., heart failure, myocardial infarction
or ischaemia, conduction abnormalities), cerebrovascular disease, or conditions that predispose the patient
to hypotension (e.g. dehydration and hypovolemia).
XEPLION should be used cautiously in patients with a history of seizures or other conditions that
potentially lower the seizure threshold.
The plasma concentrations of paliperidone are increased in patients with renal impairment and therefore,
dose adjustment is recommended in patients with mild renal impairment. XEPLION is not recommended
in patients with moderate or severe renal impairment (creatinine clearance < 50 ml/min) (see sections 4.2
and 5.2).
No data are available in patients with severe hepatic impairment (Child-Pugh class C). Caution is
recommended if paliperidone is used in such patients.
Elderly patients with dementia
XEPLION has not been studied in elderly patients with dementia. XEPLION should be used with caution
in elderly patients with dementia with risk factors for stroke.
The experience from risperidone cited below is considered valid also for paliperidone.
Overall mortality
In a meta-analysis of 17 controlled clinical trials, elderly patients with dementia treated with other atypical
antipsychotics, including risperidone, aripiprazole, olanzapine, and quetiapine had an increased risk of
mortality compared to placebo. Among those treated with risperidone, the mortality was 4% compared
with 3.1% for placebo.
Cerebrovascular adverse reactions
An approximately 3-fold increased risk of cerebrovascular adverse reactions has been seen in randomised
placebo-controlled clinical trials in the dementia population with some atypical antipsychotics, including
risperidone, aripiprazole, and olanzapine. The mechanism for this increased risk is not known.
Parkinson’s disease and dementia with Lewy bodies
Physicians should weigh the risks versus the benefits when prescribing XEPLION to patients with
Parkinson’s Disease or Dementia with Lewy Bodies (DLB) since both groups may be at increased risk of
Neuroleptic Malignant Syndrome as well as having an increased sensitivity to antipsychotics.
Manifestation of this increased sensitivity can include confusion, obtundation, postural instability with
frequent falls, in addition to extrapyramidal symptoms.
Antipsychotic medicinal products (including risperidone) with alpha-adrenergic blocking effects have
been reported to induce priapism. During postmarketing surveillance, priapism has also been reported with
oral paliperidone, which is the active metabolite of risperidone. Patients should be informed to seek urgent
medical care in case that priapism has not been resolved within 3-4 hours.
Body temperature regulation
Disruption of the body’s ability to reduce core body temperature has been attributed to antipsychotic
medicinal products. Appropriate care is advised when prescribing XEPLION to patients who will be
experiencing conditions which may contribute to an elevation in core body temperature, e.g. exercising
strenuously, exposure to extreme heat, receiving concomitant medicinal products with anticholinergic
activity or being subject to dehydration.
Cases of venous thromboembolism (VTE) have been reported with antipsychotic medicinal products.
Since patients treated with antipsychotics often present with acquired risk factors for VTE, all possible
risk factors for VTE should be identified before and during treatment with XEPLION and preventative
measures undertaken.
An antiemetic effect was observed in preclinical studies with paliperidone. This effect, if it occurs in
humans, may mask the signs and symptoms of overdosage with certain medicinal products or of
conditions such as intestinal obstruction, Reye’s syndrome and brain tumour.
Patients should be advised of the potential for weight gain. Weight should be measured regularly.
Care must be taken to avoid inadvertent injection of XEPLION into a blood vessel.
4.5 Interaction with other medicinal products and other forms of interaction
Caution is advised when prescribing XEPLION with medicinal products known to prolong the QT
interval, e.g. class IA antiarrhythmics (e.g., quinidine, disopyramide) and class III antiarrhythmics (e.g.
amiodarone, sotalol), some antihistaminics, some other antipsychotics and some antimalarials (e.g.
mefloquine). This list is indicative and not exhaustive.
Potential for XEPLION to affect other medicinal products
Paliperidone is not expected to cause clinically important pharmacokinetic interactions with medicinal
products that are metabolised by cytochrome P-450 isozymes.
Given the primary central nervous system (CNS) effects of paliperidone (see section 4.8), XEPLION
should be used with caution in combination with other centrally acting medicinal products, e.g.,
anxiolytics, most antipsychotics, hypnotics, opiates, etc. or alcohol.
Paliperidone may antagonise the effect of levodopa and other dopamine agonists. If this combination is
deemed necessary, particularly in end-stage Parkinson’s disease, the lowest effective dose of each
treatment should be prescribed.
Because of its potential for inducing orthostatic hypotension (see section 4.4), an additive effect may be
observed when XEPLION is administered with other therapeutic agents that have this potential, e.g., other
antipsychotics, tricyclics.
Caution is advised if paliperidone is combined with other medicinal products known to lower the seizure
threshold (i.e., phenothiazines or butyrophenones, tricyclics or SSRIs, tramadol, mefloquine, etc.).
Co-administration of oral paliperidone prolonged release tablets at steady-state (12 mg once daily) with
divalproex sodium prolonged release tablets (500 mg to 2000 mg once daily) did not affect the steady-
state pharmacokinetics of valproate.
No interaction study between XEPLION and lithium has been performed, however, a pharmacokinetic
interaction is not likely to occur.
Potential for other medicinal products to affect XEPLION
In vitro
studies indicate that CYP2D6 and CYP3A4 may be minimally involved in paliperidone
metabolism, but there are no indications
in vitro
nor
in vivo
that these isozymes play a significant role in
the metabolism of paliperidone. Concomitant administration of oral paliperidone with paroxetine, a potent
CYP2D6 inhibitor, showed no clinically significant effect on the pharmacokinetics of paliperidone.
Co-administration of oral paliperidone prolonged release once daily with carbamazepine 200 mg twice
daily caused a decrease of approximately 37% in the mean steady-state C
max
and AUC of paliperidone.
This decrease is caused, to a substantial degree, by a 35% increase in renal clearance of paliperidone likely
as a result of induction of renal P-gp by carbamazepine. A minor decrease in the amount of active
substance excreted unchanged in the urine suggests that there was little effect on the CYP metabolism or
bioavailability of paliperidone during carbamazepine co-administration. Larger decreases in plasma
concentrations of paliperidone could occur with higher doses of carbamazepine. On initiation of
carbamazepine, the dose of XEPLION should be re-evaluated and increased if necessary. Conversely, on
discontinuation of carbamazepine, the dose of XEPLION should be re-evaluated and decreased if
necessary.
Co-administration of a single dose of an oral paliperidone prolonged release tablet 12 mg with divalproex
sodium prolonged release tablets (two 500 mg tablets once daily) resulted in an increase of approximately
50% in the C
max
and AUC of paliperidone, likely as a result of increased oral absorption. Since no effect
on the systemic clearance was observed, a clinically significant interaction would not be expected between
divalproex sodium prolonged release tablets and XEPLION intramuscular injection. This interaction has
not been studied with XEPLION.
Concomitant use of XEPLION with risperidone
Risperidone administered orally or intramuscularly will be metabolised to a variable degree to
paliperidone. Consideration should be given if risperidone or oral paliperidone is co-administered with
XEPLION.
4.6 Fertility, pregnancy and lactation
There are no adequate data from the use of paliperidone during pregnancy. Intramuscularly injected
paliperidone palmitate and orally administered paliperidone were not teratogenic in animal studies, but
other types of reproductive toxicity were seen (see section 5.3). The use of antipsychotics during the last
trimester of pregnancy has resulted in long term but reversible neurological disturbances of
extrapyramidal nature in the infant. XEPLION should not be used during pregnancy unless clearly
necessary.
Paliperidone is excreted in the breast milk to such an extent that effects on the breastfed infant are likely if
therapeutic doses are administered to breastfeeding women. XEPLION should not be used while breast
feeding.
There were no relevant effects observed in the non-clinical studies.
4.7 Effects on ability to drive and use machines
Paliperidone can have minor or moderate influence on the ability to drive and use machines due to
potential nervous system and visual effects, such as sedation, somnolence, syncope, vision blurred (see
section 4.8). Therefore, patients should be advised not to drive or operate machines until their individual
susceptibility to XEPLION is known.
The most frequently reported adverse drug reactions (ADRs) reported in clinical trials were insomnia,
headache, weight increased, injection site reactions, agitation, somnolence, akathisia, nausea, constipation,
dizziness, tremor, vomiting, upper respiratory tract infection, diarrhoea, and tachycardia. Of these,
akathisia appeared to be dose-related.
The following are all ADRs that were reported in XEPLION-treated subjects in clinical trials. The
following terms and frequencies are applied:
very common
(≥ 1/10),
common
(≥ 1/100 to < 1/10),
uncommon
(≥ 1/1000 to < 1/100),
rare
(≥ 1/10,000 to < 1/1000),
very rare
(< 1/10,000), and
not known
(cannot be estimated from the available data).
Adverse Drug Reaction
Frequency
Infections and
infestations
upper respiratory tract
infection
Metabolism and
nutrition disorders
weight increased,
blood glucose
increased, blood
triglycerides
increased
hyperglycaemia,
hyperinsulinaemia,
increased appetite,
decreased appetite,
blood cholesterol
increased
headache dystonia,
parkinsonism,
akathisia, dyskinesia,
extrapyramidal
disorder, tremor,
dizziness,
somnolence
syncope, convulsion,
tardive dyskinesia,
dysarthria,
psychomotor
hyperactivity,
dizziness postural,
lethargy
neuroleptic
malignant
syndrome,
cerebrovascular
accident
eye rolling, eye
movement
disorder
Ear and labyrinth
disorders

















Adverse Drug Reaction
Frequency
sinus tachycardia,
conduction disorder,
atrioventricular block
first degree,
bradycardia, postural
orthostatic
tachycardia
syndrome,
palpitations,
electrocardiogram
QT prolonged,
electrocardiogram
abnormal
Gastrointestinal
disorders
vomiting, abdominal
discomfort/abdominal
pain upper, diarrhoea,
nausea, constipation,
toothache
Skin and
subcutaneous tissue
disorders
urticaria, pruritus
generalised, pruritus
Musculoskeletal and
connective tissue
disorders
back pain, pain in
extremity
Reproductive system
and breast disorders
gynaecomastia,
erectile dysfunction,
sexual dysfunction,
galactorrhoea,
amenorrhoea,
menstruation
irregular, menstrual
disorder,
menstruation delayed
General disorders and
administration site
conditions
asthenia, injection site
induration, fatigue,
injection site pain
injection site pruritus administration
site pain,
administration
site reaction,
injection site
nodule
The following is a list of additional ADRs that have been reported with oral paliperidone in the treatment
of schizophrenia:
















Infections and infestations
Common:
nasopharyngitis
Uncommon:
urinary tract infection, rhinitis
Rare:
anaphylactic reaction
Rare:
transient ischaemic attack, grand mal convulsion
Uncommon:
sinus arrhythmia
Rare:
bundle branch block left
Uncommon:
hypotension
Rare:
ischaemia
Respiratory, thoracic and
mediastinal disorders
Common:
cough,
pharyngolaryngeal pain, nasal congestion
Not known:
pneumonia aspiration
Gastrointestinal disorders
Common:
dyspepsia
Uncommon:
flatulence
Rare:
small intestinal obstruction
Not known:
swollen tongue
Skin and subcutaneous tissue
disorders
Rare:
angioedema, rash papular
Common:
arthralgia
Uncommon:
musculoskeletal pain
Renal and urinary disorders
Uncommon:
urinary retention
Rare:
urinary incontinence
Reproductive system and
breast disorders
Rare:
breast engorgement, breast pain, breast tenderness,
retrograde ejaculation
Not known:
priapism
General disorders and
administration site conditions
Uncommon:
oedema peripheral
Rare:
oedema
Description of selected adverse reactions
Injection site reactions
The most commonly reported injection site related adverse reaction was pain. The majority of these
reactions were reported to be of mild to moderate severity. Subject evaluations of injection site pain based
on a visual analogue scale tended to lessen in frequency and intensity over time in all Phase 2 and 3
studies. Injections into the deltoid were perceived as slightly more painful than corresponding gluteal
injections. Other injection site reactions were mostly mild in intensity and included induration (common),
pruritus (uncommon) and nodules (rare).
Musculoskeletal and
connective tissue disorders













Weight gain
In the 13-week study involving the 150 mg initiation dosing, the proportion of subjects with an abnormal
weight increase ≥ 7% showed a dose-related trend, with a 5% incidence rate in the placebo group
compared with rates of 6%, 8% and 13% in the XEPLION 25 mg, 100 mg, and 150 mg groups,
respectively.
During the 33-week open-label transition/maintenance period of the long-term recurrence prevention trial,
12% of XEPLION-treated subjects met this criterion (weight gain of ≥ 7% from double-blind phase to
endpoint); the mean (SD) weight change from open-label baseline was +0.7 (4.79) kg.
Serum prolactin
In clinical trials, median increases in serum prolactin were observed in subjects of both genders who
received XEPLION. Adverse reactions that may suggest increase in prolactin levels (e.g. amenorrhoea,
galactorrhoea and gynaecomastia) were reported overall in <1% of subjects.
QT prolongation, ventricular arrhythmias (ventricular fibrillation, ventricular tachycardia), sudden
unexplained death, cardiac arrest, and Torsade de pointes may occur with antipsychotics. Cases of venous
thromboembolism, including cases of pulmonary embolism and cases of deep vein thrombosis, have been
reported with antipsychotic medicinal products (frequency unknown).
In general, expected signs and symptoms are those resulting from an exaggeration of paliperidone’s
known pharmacological effects, i.e., drowsiness and sedation, tachycardia and hypotension, QT
prolongation, and extrapyramidal symptoms. Torsade de pointes and ventricular fibrillation have been
reported in a patient in the setting of overdose with oral paliperidone. In the case of acute overdose, the
possibility of multiple drug involvement should be considered.
Consideration should be given to the prolonged release nature of the medicinal product and the long
elimination half-life of paliperidone when assessing treatment needs and recovery. There is no specific
antidote to paliperidone. General supportive measures should be employed. Establish and maintain a clear
airway and ensure adequate oxygenation and ventilation.
Cardiovascular monitoring should commence immediately and should include continuous
electrocardiographic monitoring for possible arrhythmias. Hypotension and circulatory collapse should be
treated with appropriate measures such as intravenous fluid and/or sympathomimetic agents. In case of
severe extrapyramidal symptoms, anticholinergic agents should be administered. Close supervision and
monitoring should continue until the patient recovers.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Psycholeptics, other antipsychotics. ATC code: N05AX13
XEPLION contains a racemic mixture of (+)- and (-)-paliperidone.
Paliperidone is a selective blocking agent of monoamine effects, whose pharmacological properties are
different from that of traditional neuroleptics. Paliperidone binds strongly to serotonergic 5-HT2- and
dopaminergic D2-receptors. Paliperidone also blocks alpha 1-adrenergic receptors and slightly less,
H1-histaminergic and alpha 2-adrenergic receptors. The pharmacological activity of the (+)- and (-)-
paliperidone enantiomers are qualitatively and quantitatively similar.
Paliperidone is not bound to cholinergic receptors. Even though paliperidone is a strong D2-antagonist,
which is believed to relieve the positive symptoms of schizophrenia, it causes less catalepsy and decreases
motor functions less than traditional neuroleptics. Dominating central serotonin antagonism may reduce
the tendency of paliperidone to cause extrapyramidal side effects.
Acute treatment of schizophrenia
The efficacy of XEPLION in the acute treatment of schizophrenia was established in four short-term (one
9-week and three 13-week) double-blind, randomised, placebo-controlled, fixed-dose studies of acutely
relapsed adult inpatients who met DSM-IV criteria for schizophrenia. The fixed doses of XEPLION in
these studies were given on days 1, 8, and 36 in the 9-week study, and additionally on day 64 of the
13-week studies. No additional oral antipsychotic supplementation was needed during the acute treatment
of schizophrenia with XEPLION. The primary efficacy endpoint was defined as a decrease in Positive and
Negative Syndrome Scale (PANSS) total scores as shown in the table below. The PANSS is a validated
multi-item inventory composed of five factors to evaluate positive symptoms, negative symptoms,
disorganised thoughts, uncontrolled hostility/excitement and anxiety/depression. Functioning was
evaluated using the Personal and Social Performance (PSP) scale. The PSP is a validated clinician rated
scale that measures personal and social functioning in four domains: socially useful activities (work and
study), personal and social relationships, self-care and disturbing and aggressive behaviours.
In a 13-week study (n=636) comparing three fixed doses of XEPLION (initial deltoid injection of 150 mg
followed by 3 gluteal or deltoid doses of either 25 mg/4 weeks, 100 mg/4 weeks or 150 mg/4 weeks) to
placebo, all three doses of XEPLION were superior to placebo in improving the PANSS total score.
In this
study, both the 100 mg/4 weeks and 150 mg /4 weeks, but not the 25 mg/4 weeks, treatment groups
demonstrated statistical superiority to placebo for the PSP score. These results support efficacy across the
entire duration of treatment and improvement in PANSS and was observed as early as day 4 with
significant separation from placebo in the 25 mg and 150 mg XEPLION groups by day 8.
The results of the other studies yielded statistically significant results in favour of XEPLION, except for
the 50 mg dose in one study (see table below).
Positive and Negative Syndrome Scale for Schizophrenia (PANSS) Total Score - Change From Baseline to
End Point- LOCF for Studies R092670-SCH-201, R092670-PSY-3003, R092670-PSY-3004 and
R092670-PSY-3007: Primary Efficacy Analysis Set
R092670-PSY-3007
*
Mean baseline (SD)
Mean change (SD)
P-value (vs. Placebo)
n = 160
86.8 (10.31)
-2.9 (19.26)
--
n = 155
86.9 (11.99)
-8.0 (19.90)
0.034
n = 161
86.2 (10.77)
-11.6 (17.63)
<0.001
n = 160
88.4 (11.70)
-13.2 (18.48)
<0.001












R092670-PSY-3003
Mean baseline (SD)
Mean change (SD)
P-value (vs. Placebo)
n = 132
92.4 (12.55)
-4.1 (21.01)
--
n = 93
89.9 (10.78)
-7.9 (18.71)
0.193
n = 94
90.1 (11.66)
-11.0 (19.06)
0.019
n = 30
92.2 (11.72)
-5.5 (19.78)
--
R092670-PSY-3004
Mean baseline (SD)
Mean change (SD)
P-value (vs. Placebo)
n = 125
90.7 (12.22)
-7.0 (20.07)
--
n = 129
90.7 (12.25)
-13.6 (21.45)
0.015
n = 128
91.2 (12.02)
-13.2 (20.14)
0.017
n = 131
90.8 (11.70)
-16.1 (20.36)
<0.001
R092670-SCH-201
Mean baseline (SD)
Mean change (SD)
P-value (vs. Placebo)
n=66
87.8 (13.90)
6.2 (18.25)
--
n=63
88.0 (12.39)
-5.2 (21.52)
0.001
n=68
85.2 (11.09)
-7.8 (19.40)
<0.0001
*
For Study R092670-PSY-3007 an initiation dose of 150 mg was given to all subjects in the XEPLION treatment groups on
Day 1 followed by the assigned dose afterwards.
Note: Negative change in score indicates improvement.
Maintaining symptom control and delaying relapse of schizophrenia
The efficacy of XEPLION in maintaining symptomatic control and delaying relapse of schizophrenia was
established in a longer-term double-blind, placebo-controlled, flexible-dose study involving 849 non-
elderly adult subjects who met DSM-IV criteria for schizophrenia. This study included a 33-week open-
label acute treatment and stabilisation phase, a randomised, double-blind placebo-controlled phase to
observe for relapse, and a 52-week open-label extension period. In this study, doses of XEPLION
included 25, 50, 75, and 100 mg administered monthly; the 75 mg dose was allowed only in the 52-week
open-label extension. Subjects initially received flexible doses (25-100 mg) of XEPLION during a 9-week
transition period, followed by a 24-week maintenance period, where subjects were required to have a
PANSS score of ≤ 75. Dosing adjustments were only allowed in the first 12 weeks of the maintenance
period. A total of 410 stabilised patients were randomised to either XEPLION (median duration 171 days
[range 1 day to 407 days]) or to placebo (median duration 105 days [range 8 days to 441 days]) until they
experienced a relapse of schizophrenia symptoms in the variable length double-blind phase. The trial was
stopped early for efficacy reasons as a significantly longer time to relapse (p < 0.0001, Figure 1) was seen
in patients treated with XEPLION compared to placebo (hazard ratio = 4.32; 95% CI: 2.4-7.7).













The absolute bioavailability of paliperidone palmitate following XEPLION administration is 100%.
Following administration of paliperidone palmitate the (+) and (-) enantiomers of paliperidone
interconvert, reaching an AUC (+) to (-) ratio of approximately 1.6-1.8.
The plasma protein binding of racemic paliperidone is 74%.
Biotransformation and elimination
One week following administration of a single oral dose of 1 mg immediate-release
14
C-paliperidone, 59%
of the dose was excreted unchanged into urine, indicating that paliperidone is not extensively metabolised
in the liver. Approximately 80% of the administered radioactivity was recovered in urine and 11% in the
faeces. Four metabolic pathways have been identified
in vivo
, none of which accounted for more than
6.5% of the dose: dealkylation, hydroxylation, dehydrogenation, and benzisoxazole scission. Although
in vitro
studies suggested a role for CYP2D6 and CYP3A4 in the metabolism of paliperidone, there is no
evidence
in vivo
that these isozymes play a significant role in the metabolism of paliperidone. Population
pharmacokinetics analyses indicated no discernable difference on the apparent clearance of paliperidone
after administration of oral paliperidone between extensive metabolisers and poor metabolisers of
CYP2D6 substrates.
In vitro
studies in human liver microsomes showed that paliperidone does not
substantially inhibit the metabolism of medicinal products metabolised by cytochrome P450 isozymes,
including CYP1A2, CYP2A6, CYP2C8/9/10, CYP2D6, CYP2E1, CYP3A4, and CYP3A5.
In vitro
studies have shown that paliperidone is a P-gp substrate and a weak inhibitor of P-gp at high
concentrations. No
in vivo
data are available and the clinical relevance is unknown.
Long acting paliperidone palmitate injection versus oral prolonged release paliperidone
XEPLION
is designed to deliver paliperidone over a monthly period while prolonged release oral
paliperidone is administered on a daily basis. The initiation regimen for XEPLION (150 mg/100 mg in the
deltoid muscle on Day 1/Day 8) was designed to rapidly attain steady-state paliperidone concentrations
when initiating therapy without the use of oral supplementation.
In general, overall initiation plasma levels with XEPLION were within the exposure range observed with
6-12 mg prolonged release oral paliperidone. The use of the XEPLION initiation regimen allowed patients
to stay in this exposure window of 6-12 mg prolonged release oral paliperidone even on trough pre-dose
days (Day 8 and Day 36). Because of the difference in median pharmacokinetic profiles between the two
medicinal products, caution should be exercised when making a direct comparison of their
pharmacokinetic properties.
Paliperidone is not extensively metabolised in the liver. Although XEPLION was not studied on patients
with hepatic impairment, no dose adjustment is required in patients with mild or moderate hepatic
impairment. In a study with oral paliperidone in subjects with moderate hepatic impairment (Child-Pugh
class B), the plasma concentrations of free paliperidone were similar to those of healthy subjects.
Paliperidone has not been studied in patients with severe hepatic impairment.
The disposition of a single oral dose paliperidone 3 mg prolonged release tablet was studied in subjects
with varying degrees of renal function. Elimination of paliperidone decreased with decreasing estimated
creatinine clearance. Total clearance of paliperidone was reduced in subjects with impaired renal function
by 32% on average in mild (CrCl = 50 to < 80 ml/min), 64% in moderate (CrCl = 30 to < 50 ml/min), and
71% in severe (CrCl = 10 to < 30 ml/min) renal impairment, corresponding to an average increase in
exposure (AUC
inf
) of 1.5, 2.6, and 4.8 fold, respectively, compared to healthy subjects. Based on a limited
number of observations with XEPLION in subjects with mild renal impairment and pharmacokinetic
simulations, a reduced dose is recommended (see section 4.2).
No dose adjustment is recommended based on age alone. However, dose adjustment may be required
because of age-related decreases in creatinine clearance (see Renal impairment above and section 4.2).
Pharmacokinetic studies with paliperidone palmitate have shown somewhat lower (10-20%) plasma
concentrations of paliperidone in patients who are overweight or obese in comparison with normal weight
patients (see section 4.2).
Population pharmacokinetics analysis of data from studies with oral paliperidone revealed no evidence of
race-related differences in the pharmacokinetics of paliperidone following XEPLION administration.
No clinically significant differences were observed between men and women.
Based on
in vitro
studies utilising human liver enzymes, paliperidone is not a substrate for CYP1A2;
smoking should, therefore, not have an effect on the pharmacokinetics of paliperidone. A population
pharmacokinetic analysis based on data with oral paliperidone prolonged release tablets showed a slightly
lower exposure to paliperidone in smokers compared with non-smokers. The difference is unlikely to be
of clinical relevance, though. Smoking was not assessed for XEPLION.
5.3 Preclinical safety data
Repeat-dose toxicity studies of intramuscularly injected paliperidone palmitate and orally administered
paliperidone in rat and dog showed mainly pharmacological effects, such as sedation and prolactin-
mediated effects on mammary glands and genitals. In animals treated with paliperidone palmitate an
inflammatory reaction was seen at the intramuscular injection site. Occasionally abscess formation
occurred.
In rat reproduction studies with oral risperidone, which is extensively converted to paliperidone in rats and
humans, adverse effects were seen on the birth weight and survival of the offspring. No embryotoxicity or
malformations were observed following intramuscular administration of paliperidone palmitate to
pregnant rats up to the highest dose (160 mg/kg/day) corresponding to 4.1 times the exposure level in
humans at the maximum recommended dose of 150 mg. Other dopamine antagonists, when administered
to pregnant animals, have caused negative effects on learning and motor development in the offspring.
Paliperidone palmitate and paliperidone were not genotoxic. In oral carcinogenicity studies of risperidone
in rats and mice, increases in pituitary gland adenomas (mouse), endocrine pancreas adenomas (rat), and
mammary gland adenomas (both species) were seen. The carcinogenic potential of intramuscularly
injected paliperidone palmitate was assessed in rats. There was a statistically significant increase in
mammary gland adenocarcinomas in female rats at 10, 30 and 60 mg/kg/month. Male rats showed a
statistically significant increase in mammary gland adenomas and carcinomas at 30 and 60 mg/kg/month
which is 1.2 and 2.2 times the exposure level at the maximum recommended human 150 mg dose. These
tumours can be related to prolonged dopamine D2 antagonism and hyperprolactinemia. The relevance of
these tumour findings in rodents in terms of human risk is unknown.
PHARMACEUTICAL PARTICULARS
Polysorbate 20
Polyethylene glycol 4000
Citric acid monohydrate
Disodium hydrogen phosphate anhydrous
Sodium dihydrogen phosphate monohydrate
Sodium hydroxide (for pH adjustment)
Water for injections
This medicinal product must not be mixed with other medicinal products.
6.4 Special precautions for storage
6.5 Nature and contents of container
Pre-filled syringe (cyclic-olefin-copolymer) with a plunger stopper and tip cap (bromobutyl rubber) with a
22G 1½-inch safety needle (0.72 mm x 38.1 mm) and a 23G 1-inch safety needle (0.64 mm x 25.4 mm).
Pack sizes:
Pack contains 1 pre-filled syringe and 2 needles
6.6 Special precautions for disposal
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Janssen-Cilag International NV,
Turnhoutseweg 30,
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency:
http://www.ema.europa.eu/
NAME OF THE MEDICINAL PRODUCT
XEPLION 50 mg prolonged release suspension for injection
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each pre-filled syringe contains 78 mg paliperidone palmitate equivalent to 50 mg paliperidone.
For a full list of excipients, see section 6.1.
Prolonged release suspension for injection.
The suspension is white to off-white. The suspension is pH neutral (approximately 7.0).
4.1 Therapeutic indications
XEPLION is indicated for maintenance treatment of schizophrenia in adult patients stabilised with
paliperidone or risperidone.
In selected adult patients with schizophrenia and previous responsiveness to oral paliperidone or
risperidone, XEPLION may be used without prior stabilisation with oral treatment if psychotic symptoms
are mild to moderate and a long-acting injectable treatment is needed.
4.2 Posology and method of administration
Recommended initiation of XEPLION is with a dose of 150 mg on treatment day 1 and 100 mg one week
later (day 8), both administered in the deltoid muscle in order to attain therapeutic concentrations rapidly
(see section 5.2). The recommended monthly maintenance dose is 75 mg; some patients may benefit from
lower or higher doses within the recommended range of 25 to 150 mg based on individual patient
tolerability and/or efficacy. Patients who are overweight or obese may require doses in the upper range
(see section 5.2). Following the second dose, monthly maintenance doses can be administered in either the
deltoid or gluteal muscle.
Adjustment of the maintenance dose may be made monthly. When making dose adjustments, the
prolonged release characteristics of XEPLION should be considered (see section 5.2), as the full effect of
maintenance doses may not be evident for several months.
Switching from oral paliperidone or oral risperidone
Previous oral paliperidone or oral risperidone can be discontinued at the time of initiation of treatment
with XEPLION. XEPLION should be initiated as described at the beginning of section 4.2 above.
Switching from Risperidone long acting injection.
When switching patients from risperidone long acting injection, initiate XEPLION therapy in place of the
next scheduled injection. XEPLION should then be continued at monthly intervals. The one-week
initiation dosing regimen including the intramuscular injections (day 1 and 8, respectively) as described in
section 4.2 above is not required. Patients previously stabilised on different doses of risperidone long
acting injection can attain similar paliperidone steady-state exposure during maintenance treatment with
XEPLION monthly doses according to the following:
Doses of Risperidone long acting injection and XEPLION needed to attain similar
paliperidone exposure at steady-state
Previous Risperidone long acting injection dose
Discontinuation of antipsychotic medicinal products should be made in accordance with appropriate
prescribing information. If XEPLION is discontinued, its prolonged release characteristics must be
considered. As recommended with other antipsychotic medicinal products, the need for continuing
existing extrapyramidal symptoms (EPS) medicine should be re-evaluated periodically.
Avoiding missed doses
It is recommended that the second initiation dose of XEPLION be given one week after the first dose. To
avoid a missed dose, patients may be given the second dose 2 days before or after the one-week (day 8)
time point. Similarly, the third and subsequent injections after the initiation regimen are recommended to
be given monthly. To avoid a missed monthly dose, patients may be given the injection up to 7 days
before or after the monthly time point.
If the target date for the second XEPLION injection (day 8 ± 2 days) is missed, the recommended
reinitiation depends on the length of time which has elapsed since the patient's first injection.
Missed second initiation dose (< 4 weeks from first injection)
If less than 4 weeks have elapsed since the first injection, then the patient should be administered the
second injection of 100 mg in the deltoid muscle as soon as possible. A third XEPLION injection of
75 mg in either the deltoid or gluteal muscles should be administered 5 weeks after the first injection
(regardless of the timing of the second injection). The normal monthly cycle of injections in either the
deltoid or gluteal muscle of 25 mg to 150 mg based on individual patient tolerability and/or efficacy
should be followed thereafter.
Missed second initiation dose (4-7 weeks from first injection)
If 4 to 7 weeks have elapsed since the first injection of XEPLION, resume dosing with two injections of
100 mg in the following manner:
1.
a deltoid injection as soon as possible,
another deltoid injection one week later,







resumption of the normal monthly cycle of injections in either the deltoid or gluteal muscle of
25 mg to 150 mg based on individual patient tolerability and/or efficacy.
Missed second initiation dose (> 7 weeks from first injection)
If more than 7 weeks have elapsed since the first injection of XEPLION, initiate dosing as described for
the initial recommended initiation of XEPLION above.
Missed monthly maintenance dose (1 month to 6 weeks)
After initiation, the recommended injection cycle of XEPLION is monthly. If less than 6 weeks have
elapsed since the last injection, then the previously stabilised dose should be administered as soon as
possible, followed by injections at monthly intervals.
Missed monthly maintenance dose (> 6 weeks to 6 months)
If more than 6 weeks have elapsed since the last injection of XEPLION, the recommendation is as
follows:
For patients stabilised with doses of 25 to 100
mg:
1. a deltoid injection as soon as possible at the same dose the patient was previously stabilised on
2. another deltoid injection (same dose) one week later (day 8)
3. resumption of the normal monthly cycle of injections in either the deltoid or gluteal muscle of
25 mg to 150 mg based on individual patient tolerability and/or efficacy
For patients stabilised with 150
mg:
1. a deltoid injection as soon as possible at the 100 mg dose
2. another deltoid injection one week later (day 8) at the 100 mg dose
3. resumption of the normal monthly cycle of injections in either the deltoid or gluteal muscle of
25 mg to 150 mg based on individual patient tolerability and/or efficacy
Missed monthly maintenance dose (> 6 months).
If more than 6 months have elapsed since the last
injection of XEPLION, initiate dosing as described for the initial recommended initiation of XEPLION
above.
Elderly population
Efficacy and safety in elderly > 65 years have not been established.
In general, recommended dosing of XEPLION for elderly patients with normal renal function is the same
as for younger adult patients with normal renal function. However, because elderly patients may have
diminished renal function, dose adjustment may be necessary (see
Renal impairment
below for dosing
recommendations in patients with renal impairment).
Renal impairment
XEPLION has not been systematically studied in patients with renal impairment (see section 5.2). For
patients with mild renal impairment (creatinine clearance ≥ 50 to < 80 ml/min), recommended initiation of
XEPLION is with a dose of 100 mg on treatment day 1 and 75 mg one week later, both administered in
the deltoid muscle. The recommended monthly maintenance dose is 50 mg with a range of 25 to 100 mg
based on patient tolerability and/or efficacy.
XEPLION is not recommended in patients with moderate or severe renal impairment (creatinine clearance
< 50 ml/min) (see section 4.4).
Hepatic impairment
Based on experience with oral paliperidone, no dose adjustment is required in patients with mild or
moderate hepatic impairment. As paliperidone has not been studied in patients with severe hepatic
impairment, caution is recommended in such patients.
Other special populations
No dose adjustment for XEPLION is recommended based on gender, race, or smoking status.
Paediatric population
The safety and efficacy of XEPLION in children < 18 years of age have not been established. No data are
available.
XEPLION is intended for intramuscular use only. It should be injected slowly, deep into the muscle. Each
injection should be administered by a health care professional. Administration should be in a single
injection. The dose should not be given in divided injections. The dose should not be administered
intravascularly or subcutaneously.
The day 1 and day 8 initiation doses must each be administered in the deltoid muscle in order to attain
therapeutic concentrations rapidly (see section 5.2). Following the second dose, monthly maintenance
doses can be administered in either the deltoid or gluteal muscle. A switch from gluteal to deltoid (and
vice versa) should be considered in the event of injection site pain if the injection site discomfort is not
well tolerated (see section 4.8). It is also recommended to alternate between left and right sides (see
below).
For instructions for use and handling of XEPLION, see package leaflet (information intended for medical
or healthcare professionals).
Deltoid muscle administration
The recommended needle size for initial and maintenance administration of XEPLION into the deltoid
muscle is determined by the patient’s weight. For those ≥ 90 kg, the 1½ inch, 22 gauge needle (38.1 mm x
0.72 mm) is recommended. For those < 90 kg, the 1-inch, 23 gauge needle (25.4 mm x 0.64 mm) is
recommended. Deltoid injections should be alternated between the two deltoid muscles.
Gluteal muscle administration
The recommended needle size for maintenance administration of XEPLION into the gluteal muscle is the
1½-inch, 22 gauge needle (38.1 mm x 0.72 mm). Administration should be made into the upper-outer
quadrant of the gluteal area. Gluteal injections should be alternated between the two gluteal muscles.
Hypersensitivity to the active substance, to risperidone or to any of the excipients.
4.4 Special warnings and precautions for use
Use in patients who are in an acutely agitated or severely psychotic state
XEPLION should not be used to manage acutely agitated or severely psychotic states when immediate
symptom control is warranted.
Caution should be exercised when paliperidone is prescribed in patients with known cardiovascular
disease or family history of QT prolongation, and in concomitant use with other medicinal products
thought to prolong the QT interval.
Neuroleptic malignant syndrome
Neuroleptic Malignant Syndrome (NMS), characterised by hyperthermia, muscle rigidity, autonomic
instability, altered consciousness, and elevated serum creatine phosphokinase levels has been reported to
occur with paliperidone. Additional clinical signs may include myoglobinuria (rhabdomyolysis) and acute
renal failure. If a patient develops signs or symptoms indicative of NMS, all antipsychotics, including
paliperidone, should be discontinued.
Medicinal products with dopamine receptor antagonistic properties have been associated with the
induction of tardive dyskinesia characterised by rhythmical, involuntary movements, predominantly of the
tongue and/or face. If signs and symptoms of tardive dyskinesia appear, the discontinuation of all
antipsychotics, including paliperidone, should be considered.
Rare cases of glucose related adverse reactions, e.g. increase in blood glucose, have been reported in
clinical trials with paliperidone. Appropriate clinical monitoring is advisable in diabetic patients and in
patients with risk factors for the development of diabetes mellitus.
Tissue culture studies suggest that cell growth in human breast tumours may be stimulated by prolactin.
Although no clear association with the administration of antipsychotics has so far been demonstrated in
clinical and epidemiological studies, caution is recommended in patients with relevant medical history.
Paliperidone should be used with caution in patients with possible prolactin-dependent tumours.
Paliperidone may induce orthostatic hypotension in some patients based on its alpha-blocking activity.
Based on pooled data from the three placebo-controlled, 6-week, fixed-dose trials with oral paliperidone
prolonged release tablets (3, 6, 9, and 12 mg), orthostatic hypotension was reported by 2.5% of subjects
treated with oral paliperidone compared with 0.8% of subjects treated with placebo. XEPLION should be
used with caution in patients with known cardiovascular disease (e.g., heart failure, myocardial infarction
or ischaemia, conduction abnormalities), cerebrovascular disease, or conditions that predispose the patient
to hypotension (e.g. dehydration and hypovolemia).
XEPLION should be used cautiously in patients with a history of seizures or other conditions that
potentially lower the seizure threshold.
The plasma concentrations of paliperidone are increased in patients with renal impairment and therefore,
dose adjustment is recommended in patients with mild renal impairment. XEPLION is not recommended
in patients with moderate or severe renal impairment (creatinine clearance < 50 ml/min) (see sections 4.2
and 5.2).
No data are available in patients with severe hepatic impairment (Child-Pugh class C). Caution is
recommended if paliperidone is used in such patients.
Elderly patients with dementia
XEPLION has not been studied in elderly patients with dementia. XEPLION should be used with caution
in elderly patients with dementia with risk factors for stroke.
The experience from risperidone cited below is considered valid also for paliperidone.
Overall mortality
In a meta-analysis of 17 controlled clinical trials, elderly patients with dementia treated with other atypical
antipsychotics, including risperidone, aripiprazole, olanzapine, and quetiapine had an increased risk of
mortality compared to placebo. Among those treated with risperidone, the mortality was 4% compared
with 3.1% for placebo.
Cerebrovascular adverse reactions
An approximately 3-fold increased risk of cerebrovascular adverse reactions has been seen in randomised
placebo-controlled clinical trials in the dementia population with some atypical antipsychotics, including
risperidone, aripiprazole, and olanzapine. The mechanism for this increased risk is not known.
Parkinson’s disease and dementia with Lewy bodies
Physicians should weigh the risks versus the benefits when prescribing XEPLION to patients with
Parkinson’s Disease or Dementia with Lewy Bodies (DLB) since both groups may be at increased risk of
Neuroleptic Malignant Syndrome as well as having an increased sensitivity to antipsychotics.
Manifestation of this increased sensitivity can include confusion, obtundation, postural instability with
frequent falls, in addition to extrapyramidal symptoms.
Antipsychotic medicinal products (including risperidone) with alpha-adrenergic blocking effects have
been reported to induce priapism. During postmarketing surveillance, priapism has also been reported with
oral paliperidone, which is the active metabolite of risperidone. Patients should be informed to seek urgent
medical care in case that priapism has not been resolved within 3-4 hours.
Body temperature regulation
Disruption of the body’s ability to reduce core body temperature has been attributed to antipsychotic
medicinal products. Appropriate care is advised when prescribing XEPLION to patients who will be
experiencing conditions which may contribute to an elevation in core body temperature, e.g. exercising
strenuously, exposure to extreme heat, receiving concomitant medicinal products with anticholinergic
activity or being subject to dehydration.
Cases of venous thromboembolism (VTE) have been reported with antipsychotic medicinal products.
Since patients treated with antipsychotics often present with acquired risk factors for VTE, all possible
risk factors for VTE should be identified before and during treatment with XEPLION and preventative
measures undertaken.
An antiemetic effect was observed in preclinical studies with paliperidone. This effect, if it occurs in
humans, may mask the signs and symptoms of overdosage with certain medicinal products or of
conditions such as intestinal obstruction, Reye’s syndrome and brain tumour.
Patients should be advised of the potential for weight gain. Weight should be measured regularly.
Care must be taken to avoid inadvertent injection of XEPLION into a blood vessel.
4.5 Interaction with other medicinal products and other forms of interaction
Caution is advised when prescribing XEPLION with medicinal products known to prolong the QT
interval, e.g. class IA antiarrhythmics (e.g., quinidine, disopyramide) and class III antiarrhythmics (e.g.
amiodarone, sotalol), some antihistaminics, some other antipsychotics and some antimalarials (e.g.
mefloquine). This list is indicative and not exhaustive.
Potential for XEPLION to affect other medicinal products
Paliperidone is not expected to cause clinically important pharmacokinetic interactions with medicinal
products that are metabolised by cytochrome P-450 isozymes.
Given the primary central nervous system (CNS) effects of paliperidone (see section 4.8), XEPLION
should be used with caution in combination with other centrally acting medicinal products, e.g.,
anxiolytics, most antipsychotics, hypnotics, opiates, etc. or alcohol.
Paliperidone may antagonise the effect of levodopa and other dopamine agonists. If this combination is
deemed necessary, particularly in end-stage Parkinson’s disease, the lowest effective dose of each
treatment should be prescribed.
Because of its potential for inducing orthostatic hypotension (see section 4.4), an additive effect may be
observed when XEPLION is administered with other therapeutic agents that have this potential, e.g., other
antipsychotics, tricyclics.
Caution is advised if paliperidone is combined with other medicinal products known to lower the seizure
threshold (i.e., phenothiazines or butyrophenones, tricyclics or SSRIs, tramadol, mefloquine, etc.).
Co-administration of oral paliperidone prolonged release tablets at steady-state (12 mg once daily) with
divalproex sodium prolonged release tablets (500 mg to 2000 mg once daily) did not affect the steady-
state pharmacokinetics of valproate.
No interaction study between XEPLION and lithium has been performed, however, a pharmacokinetic
interaction is not likely to occur.
Potential for other medicinal products to affect XEPLION
In vitro
studies indicate that CYP2D6 and CYP3A4 may be minimally involved in paliperidone
metabolism, but there are no indications
in vitro
nor
in vivo
that these isozymes play a significant role in
the metabolism of paliperidone. Concomitant administration of oral paliperidone with paroxetine, a potent
CYP2D6 inhibitor, showed no clinically significant effect on the pharmacokinetics of paliperidone.
Co-administration of oral paliperidone prolonged release once daily with carbamazepine 200 mg twice
daily caused a decrease of approximately 37% in the mean steady-state C
max
and AUC of paliperidone.
This decrease is caused, to a substantial degree, by a 35% increase in renal clearance of paliperidone likely
as a result of induction of renal P-gp by carbamazepine. A minor decrease in the amount of active
substance excreted unchanged in the urine suggests that there was little effect on the CYP metabolism or
bioavailability of paliperidone during carbamazepine co-administration. Larger decreases in plasma
concentrations of paliperidone could occur with higher doses of carbamazepine. On initiation of
carbamazepine, the dose of XEPLION should be re-evaluated and increased if necessary. Conversely, on
discontinuation of carbamazepine, the dose of XEPLION should be re-evaluated and decreased if
necessary.
Co-administration of a single dose of an oral paliperidone prolonged release tablet 12 mg with divalproex
sodium prolonged release tablets (two 500 mg tablets once daily) resulted in an increase of approximately
50% in the C
max
and AUC of paliperidone, likely as a result of increased oral absorption. Since no effect
on the systemic clearance was observed, a clinically significant interaction would not be expected between
divalproex sodium prolonged release tablets and XEPLION intramuscular injection. This interaction has
not been studied with XEPLION.
Concomitant use of XEPLION with risperidone
Risperidone administered orally or intramuscularly will be metabolised to a variable degree to
paliperidone. Consideration should be given if risperidone or oral paliperidone is co-administered with
XEPLION.
4.6 Fertility, pregnancy and lactation
There are no adequate data from the use of paliperidone during pregnancy. Intramuscularly injected
paliperidone palmitate and orally administered paliperidone were not teratogenic in animal studies, but
other types of reproductive toxicity were seen (see section 5.3). The use of antipsychotics during the last
trimester of pregnancy has resulted in long term but reversible neurological disturbances of
extrapyramidal nature in the infant. XEPLION should not be used during pregnancy unless clearly
necessary.
Paliperidone is excreted in the breast milk to such an extent that effects on the breastfed infant are likely if
therapeutic doses are administered to breastfeeding women. XEPLION should not be used while breast
feeding.
There were no relevant effects observed in the non-clinical studies.
4.7 Effects on ability to drive and use machines
Paliperidone can have minor or moderate influence on the ability to drive and use machines due to
potential nervous system and visual effects, such as sedation, somnolence, syncope, vision blurred (see
section 4.8). Therefore, patients should be advised not to drive or operate machines until their individual
susceptibility to XEPLION is known.
The most frequently reported adverse drug reactions (ADRs) reported in clinical trials were insomnia,
headache, weight increased, injection site reactions, agitation, somnolence, akathisia, nausea, constipation,
dizziness, tremor, vomiting, upper respiratory tract infection, diarrhoea, and tachycardia. Of these,
akathisia appeared to be dose-related.
The following are all ADRs that were reported in XEPLION-treated subjects in clinical trials. The
following terms and frequencies are applied:
very common
(≥ 1/10),
common
(≥ 1/100 to < 1/10),
uncommon
(≥ 1/1000 to < 1/100),
rare
(≥ 1/10,000 to < 1/1000),
very rare
(< 1/10,000), and
not known
(cannot be estimated from the available data).
Adverse Drug Reaction
Frequency
Infections and
infestations
upper respiratory tract
infection
Metabolism and
nutrition disorders
weight increased,
blood glucose
increased, blood
triglycerides
increased
hyperglycaemia,
hyperinsulinaemia,
increased appetite,
decreased appetite,
blood cholesterol
increased
headache dystonia,
parkinsonism,
akathisia, dyskinesia,
extrapyramidal
disorder, tremor,
dizziness,
somnolence
syncope, convulsion,
tardive dyskinesia,
dysarthria,
psychomotor
hyperactivity,
dizziness postural,
lethargy
neuroleptic
malignant
syndrome,
cerebrovascular
accident
eye rolling, eye
movement
disorder
Ear and labyrinth
disorders

















Adverse Drug Reaction
Frequency
sinus tachycardia,
conduction disorder,
atrioventricular block
first degree,
bradycardia, postural
orthostatic
tachycardia
syndrome,
palpitations,
electrocardiogram
QT prolonged,
electrocardiogram
abnormal
Gastrointestinal
disorders
vomiting, abdominal
discomfort/abdominal
pain upper, diarrhoea,
nausea, constipation,
toothache
Skin and
subcutaneous tissue
disorders
urticaria, pruritus
generalised, pruritus
Musculoskeletal and
connective tissue
disorders
back pain, pain in
extremity
Reproductive system
and breast disorders
gynaecomastia,
erectile dysfunction,
sexual dysfunction,
galactorrhoea,
amenorrhoea,
menstruation
irregular, menstrual
disorder,
menstruation delayed
General disorders and
administration site
conditions
asthenia, injection site
induration, fatigue,
injection site pain
injection site pruritus administration
site pain,
administration
site reaction,
injection site
nodule
The following is a list of additional ADRs that have been reported with oral paliperidone in the treatment
of schizophrenia:
















Infections and infestations
Common:
nasopharyngitis
Uncommon:
urinary tract infection, rhinitis
Rare:
anaphylactic reaction
Rare:
transient ischaemic attack, grand mal convulsion
Uncommon:
sinus arrhythmia
Rare:
bundle branch block left
Uncommon:
hypotension
Rare:
ischaemia
Respiratory, thoracic and
mediastinal disorders
Common:
cough,
pharyngolaryngeal pain, nasal congestion
Not known:
pneumonia aspiration
Gastrointestinal disorders
Common:
dyspepsia
Uncommon:
flatulence
Rare:
small intestinal obstruction
Not known:
swollen tongue
Skin and subcutaneous tissue
disorders
Rare:
angioedema, rash papular
Common:
arthralgia
Uncommon:
musculoskeletal pain
Renal and urinary disorders
Uncommon:
urinary retention
Rare:
urinary incontinence
Reproductive system and
breast disorders
Rare:
breast engorgement, breast pain, breast tenderness,
retrograde ejaculation
Not known:
priapism
General disorders and
administration site conditions
Uncommon:
oedema peripheral
Rare:
oedema
Description of selected adverse reactions
Injection site reactions
The most commonly reported injection site related adverse reaction was pain. The majority of these
reactions were reported to be of mild to moderate severity. Subject evaluations of injection site pain based
on a visual analogue scale tended to lessen in frequency and intensity over time in all Phase 2 and 3
studies. Injections into the deltoid were perceived as slightly more painful than corresponding gluteal
injections. Other injection site reactions were mostly mild in intensity and included induration (common),
pruritus (uncommon) and nodules (rare).
Musculoskeletal and
connective tissue disorders













Weight gain
In the 13-week study involving the 150 mg initiation dosing, the proportion of subjects with an abnormal
weight increase ≥ 7% showed a dose-related trend, with a 5% incidence rate in the placebo group
compared with rates of 6%, 8% and 13% in the XEPLION 25 mg, 100 mg, and 150 mg groups,
respectively.
During the 33-week open-label transition/maintenance period of the long-term recurrence prevention trial,
12% of XEPLION-treated subjects met this criterion (weight gain of ≥ 7% from double-blind phase to
endpoint); the mean (SD) weight change from open-label baseline was +0.7 (4.79) kg.
Serum prolactin
In clinical trials, median increases in serum prolactin were observed in subjects of both genders who
received XEPLION. Adverse reactions that may suggest increase in prolactin levels (e.g. amenorrhoea,
galactorrhoea and gynaecomastia) were reported overall in <1% of subjects.
QT prolongation, ventricular arrhythmias (ventricular fibrillation, ventricular tachycardia), sudden
unexplained death, cardiac arrest, and Torsade de pointes may occur with antipsychotics. Cases of venous
thromboembolism, including cases of pulmonary embolism and cases of deep vein thrombosis, have been
reported with antipsychotic medicinal products (frequency unknown).
In general, expected signs and symptoms are those resulting from an exaggeration of paliperidone’s
known pharmacological effects, i.e., drowsiness and sedation, tachycardia and hypotension, QT
prolongation, and extrapyramidal symptoms. Torsade de pointes and ventricular fibrillation have been
reported in a patient in the setting of overdose with oral paliperidone. In the case of acute overdose, the
possibility of multiple drug involvement should be considered.
Consideration should be given to the prolonged release nature of the medicinal product and the long
elimination half-life of paliperidone when assessing treatment needs and recovery. There is no specific
antidote to paliperidone. General supportive measures should be employed. Establish and maintain a clear
airway and ensure adequate oxygenation and ventilation.
Cardiovascular monitoring should commence immediately and should include continuous
electrocardiographic monitoring for possible arrhythmias. Hypotension and circulatory collapse should be
treated with appropriate measures such as intravenous fluid and/or sympathomimetic agents. In case of
severe extrapyramidal symptoms, anticholinergic agents should be administered. Close supervision and
monitoring should continue until the patient recovers.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Psycholeptics, other antipsychotics. ATC code: N05AX13
XEPLION contains a racemic mixture of (+)- and (-)-paliperidone.
Paliperidone is a selective blocking agent of monoamine effects, whose pharmacological properties are
different from that of traditional neuroleptics. Paliperidone binds strongly to serotonergic 5-HT2- and
dopaminergic D2-receptors. Paliperidone also blocks alpha 1-adrenergic receptors and slightly less,
H1-histaminergic and alpha 2-adrenergic receptors. The pharmacological activity of the (+)- and (-)-
paliperidone enantiomers are qualitatively and quantitatively similar.
Paliperidone is not bound to cholinergic receptors. Even though paliperidone is a strong D2-antagonist,
which is believed to relieve the positive symptoms of schizophrenia, it causes less catalepsy and decreases
motor functions less than traditional neuroleptics. Dominating central serotonin antagonism may reduce
the tendency of paliperidone to cause extrapyramidal side effects.
Acute treatment of schizophrenia
The efficacy of XEPLION in the acute treatment of schizophrenia was established in four short-term (one
9-week and three 13-week) double-blind, randomised, placebo-controlled, fixed-dose studies of acutely
relapsed adult inpatients who met DSM-IV criteria for schizophrenia. The fixed doses of XEPLION in
these studies were given on days 1, 8, and 36 in the 9-week study, and additionally on day 64 of the
13-week studies. No additional oral antipsychotic supplementation was needed during the acute treatment
of schizophrenia with XEPLION. The primary efficacy endpoint was defined as a decrease in Positive and
Negative Syndrome Scale (PANSS) total scores as shown in the table below. The PANSS is a validated
multi-item inventory composed of five factors to evaluate positive symptoms, negative symptoms,
disorganised thoughts, uncontrolled hostility/excitement and anxiety/depression. Functioning was
evaluated using the Personal and Social Performance (PSP) scale. The PSP is a validated clinician rated
scale that measures personal and social functioning in four domains: socially useful activities (work and
study), personal and social relationships, self-care and disturbing and aggressive behaviours.
In a 13-week study (n=636) comparing three fixed doses of XEPLION (initial deltoid injection of 150 mg
followed by 3 gluteal or deltoid doses of either 25 mg/4 weeks, 100 mg/4 weeks or 150 mg/4 weeks) to
placebo, all three doses of XEPLION were superior to placebo in improving the PANSS total score.
In this
study, both the 100 mg/4 weeks and 150 mg /4 weeks, but not the 25 mg/4 weeks, treatment groups
demonstrated statistical superiority to placebo for the PSP score. These results support efficacy across the
entire duration of treatment and improvement in PANSS and was observed as early as day 4 with
significant separation from placebo in the 25 mg and 150 mg XEPLION groups by day 8.
The results of the other studies yielded statistically significant results in favour of XEPLION, except for
the 50 mg dose in one study (see table below).
Positive and Negative Syndrome Scale for Schizophrenia (PANSS) Total Score - Change From Baseline to
End Point- LOCF for Studies R092670-SCH-201, R092670-PSY-3003, R092670-PSY-3004 and
R092670-PSY-3007: Primary Efficacy Analysis Set
R092670-PSY-3007
*
Mean baseline (SD)
Mean change (SD)
P-value (vs. Placebo)
n = 160
86.8 (10.31)
-2.9 (19.26)
--
n = 155
86.9 (11.99)
-8.0 (19.90)
0.034
n = 161
86.2 (10.77)
-11.6 (17.63)
<0.001
n = 160
88.4 (11.70)
-13.2 (18.48)
<0.001












R092670-PSY-3003
Mean baseline (SD)
Mean change (SD)
P-value (vs. Placebo)
n = 132
92.4 (12.55)
-4.1 (21.01)
--
n = 93
89.9 (10.78)
-7.9 (18.71)
0.193
n = 94
90.1 (11.66)
-11.0 (19.06)
0.019
n = 30
92.2 (11.72)
-5.5 (19.78)
--
R092670-PSY-3004
Mean baseline (SD)
Mean change (SD)
P-value (vs. Placebo)
n = 125
90.7 (12.22)
-7.0 (20.07)
--
n = 129
90.7 (12.25)
-13.6 (21.45)
0.015
n = 128
91.2 (12.02)
-13.2 (20.14)
0.017
n = 131
90.8 (11.70)
-16.1 (20.36)
<0.001
R092670-SCH-201
Mean baseline (SD)
Mean change (SD)
P-value (vs. Placebo)
n=66
87.8 (13.90)
6.2 (18.25)
--
n=63
88.0 (12.39)
-5.2 (21.52)
0.001
n=68
85.2 (11.09)
-7.8 (19.40)
<0.0001
*
For Study R092670-PSY-3007 an initiation dose of 150 mg was given to all subjects in the XEPLION treatment groups on
Day 1 followed by the assigned dose afterwards.
Note: Negative change in score indicates improvement.
Maintaining symptom control and delaying relapse of schizophrenia
The efficacy of XEPLION in maintaining symptomatic control and delaying relapse of schizophrenia was
established in a longer-term double-blind, placebo-controlled, flexible-dose study involving 849 non-
elderly adult subjects who met DSM-IV criteria for schizophrenia. This study included a 33-week open-
label acute treatment and stabilisation phase, a randomised, double-blind placebo-controlled phase to
observe for relapse, and a 52-week open-label extension period. In this study, doses of XEPLION
included 25, 50, 75, and 100 mg administered monthly; the 75 mg dose was allowed only in the 52-week
open-label extension. Subjects initially received flexible doses (25-100 mg) of XEPLION during a 9-week
transition period, followed by a 24-week maintenance period, where subjects were required to have a
PANSS score of ≤ 75. Dosing adjustments were only allowed in the first 12 weeks of the maintenance
period. A total of 410 stabilised patients were randomised to either XEPLION (median duration 171 days
[range 1 day to 407 days]) or to placebo (median duration 105 days [range 8 days to 441 days]) until they
experienced a relapse of schizophrenia symptoms in the variable length double-blind phase. The trial was
stopped early for efficacy reasons as a significantly longer time to relapse (p < 0.0001, Figure 1) was seen
in patients treated with XEPLION compared to placebo (hazard ratio = 4.32; 95% CI: 2.4-7.7).













The absolute bioavailability of paliperidone palmitate following XEPLION administration is 100%.
Following administration of paliperidone palmitate the (+) and (-) enantiomers of paliperidone
interconvert, reaching an AUC (+) to (-) ratio of approximately 1.6-1.8.
The plasma protein binding of racemic paliperidone is 74%.
Biotransformation and elimination
One week following administration of a single oral dose of 1 mg immediate-release
14
C-paliperidone, 59%
of the dose was excreted unchanged into urine, indicating that paliperidone is not extensively metabolised
in the liver. Approximately 80% of the administered radioactivity was recovered in urine and 11% in the
faeces. Four metabolic pathways have been identified
in vivo
, none of which accounted for more than
6.5% of the dose: dealkylation, hydroxylation, dehydrogenation, and benzisoxazole scission. Although
in vitro
studies suggested a role for CYP2D6 and CYP3A4 in the metabolism of paliperidone, there is no
evidence
in vivo
that these isozymes play a significant role in the metabolism of paliperidone. Population
pharmacokinetics analyses indicated no discernable difference on the apparent clearance of paliperidone
after administration of oral paliperidone between extensive metabolisers and poor metabolisers of
CYP2D6 substrates.
In vitro
studies in human liver microsomes showed that paliperidone does not
substantially inhibit the metabolism of medicinal products metabolised by cytochrome P450 isozymes,
including CYP1A2, CYP2A6, CYP2C8/9/10, CYP2D6, CYP2E1, CYP3A4, and CYP3A5.
In vitro
studies have shown that paliperidone is a P-gp substrate and a weak inhibitor of P-gp at high
concentrations. No
in vivo
data are available and the clinical relevance is unknown.
Long acting paliperidone palmitate injection versus oral prolonged release paliperidone
XEPLION
is designed to deliver paliperidone over a monthly period while prolonged release oral
paliperidone is administered on a daily basis. The initiation regimen for XEPLION (150 mg/100 mg in the
deltoid muscle on Day 1/Day 8) was designed to rapidly attain steady-state paliperidone concentrations
when initiating therapy without the use of oral supplementation.
In general, overall initiation plasma levels with XEPLION were within the exposure range observed with
6-12 mg prolonged release oral paliperidone. The use of the XEPLION initiation regimen allowed patients
to stay in this exposure window of 6-12 mg prolonged release oral paliperidone even on trough pre-dose
days (Day 8 and Day 36). Because of the difference in median pharmacokinetic profiles between the two
medicinal products, caution should be exercised when making a direct comparison of their
pharmacokinetic properties.
Paliperidone is not extensively metabolised in the liver. Although XEPLION was not studied on patients
with hepatic impairment, no dose adjustment is required in patients with mild or moderate hepatic
impairment. In a study with oral paliperidone in subjects with moderate hepatic impairment (Child-Pugh
class B), the plasma concentrations of free paliperidone were similar to those of healthy subjects.
Paliperidone has not been studied in patients with severe hepatic impairment.
The disposition of a single oral dose paliperidone 3 mg prolonged release tablet was studied in subjects
with varying degrees of renal function. Elimination of paliperidone decreased with decreasing estimated
creatinine clearance. Total clearance of paliperidone was reduced in subjects with impaired renal function
by 32% on average in mild (CrCl = 50 to < 80 ml/min), 64% in moderate (CrCl = 30 to < 50 ml/min), and
71% in severe (CrCl = 10 to < 30 ml/min) renal impairment, corresponding to an average increase in
exposure (AUC
inf
) of 1.5, 2.6, and 4.8 fold, respectively, compared to healthy subjects. Based on a limited
number of observations with XEPLION in subjects with mild renal impairment and pharmacokinetic
simulations, a reduced dose is recommended (see section 4.2).
No dose adjustment is recommended based on age alone. However, dose adjustment may be required
because of age-related decreases in creatinine clearance (see Renal impairment above and section 4.2).
Pharmacokinetic studies with paliperidone palmitate have shown somewhat lower (10-20%) plasma
concentrations of paliperidone in patients who are overweight or obese in comparison with normal weight
patients (see section 4.2).
Population pharmacokinetics analysis of data from studies with oral paliperidone revealed no evidence of
race-related differences in the pharmacokinetics of paliperidone following XEPLION administration.
No clinically significant differences were observed between men and women.
Based on
in vitro
studies utilising human liver enzymes, paliperidone is not a substrate for CYP1A2;
smoking should, therefore, not have an effect on the pharmacokinetics of paliperidone. A population
pharmacokinetic analysis based on data with oral paliperidone prolonged release tablets showed a slightly
lower exposure to paliperidone in smokers compared with non-smokers. The difference is unlikely to be
of clinical relevance, though. Smoking was not assessed for XEPLION.
5.3 Preclinical safety data
Repeat-dose toxicity studies of intramuscularly injected paliperidone palmitate and orally administered
paliperidone in rat and dog showed mainly pharmacological effects, such as sedation and prolactin-
mediated effects on mammary glands and genitals. In animals treated with paliperidone palmitate an
inflammatory reaction was seen at the intramuscular injection site. Occasionally abscess formation
occurred.
In rat reproduction studies with oral risperidone, which is extensively converted to paliperidone in rats and
humans, adverse effects were seen on the birth weight and survival of the offspring. No embryotoxicity or
malformations were observed following intramuscular administration of paliperidone palmitate to
pregnant rats up to the highest dose (160 mg/kg/day) corresponding to 4.1 times the exposure level in
humans at the maximum recommended dose of 150 mg. Other dopamine antagonists, when administered
to pregnant animals, have caused negative effects on learning and motor development in the offspring.
Paliperidone palmitate and paliperidone were not genotoxic. In oral carcinogenicity studies of risperidone
in rats and mice, increases in pituitary gland adenomas (mouse), endocrine pancreas adenomas (rat), and
mammary gland adenomas (both species) were seen. The carcinogenic potential of intramuscularly
injected paliperidone palmitate was assessed in rats. There was a statistically significant increase in
mammary gland adenocarcinomas in female rats at 10, 30 and 60 mg/kg/month. Male rats showed a
statistically significant increase in mammary gland adenomas and carcinomas at 30 and 60 mg/kg/month
which is 1.2 and 2.2 times the exposure level at the maximum recommended human 150 mg dose. These
tumours can be related to prolonged dopamine D2 antagonism and hyperprolactinemia. The relevance of
these tumour findings in rodents in terms of human risk is unknown.
PHARMACEUTICAL PARTICULARS
Polysorbate 20
Polyethylene glycol 4000
Citric acid monohydrate
Disodium hydrogen phosphate anhydrous
Sodium dihydrogen phosphate monohydrate
Sodium hydroxide (for pH adjustment)
Water for injections
This medicinal product must not be mixed with other medicinal products.
6.4 Special precautions for storage
6.5 Nature and contents of container
Pre-filled syringe (cyclic-olefin-copolymer) with a plunger stopper and tip cap (bromobutyl rubber) with a
22G 1½-inch safety needle (0.72 mm x 38.1 mm) and a 23G 1-inch safety needle (0.64 mm x 25.4 mm).
Pack sizes:
Pack contains 1 pre-filled syringe and 2 needles
6.6 Special precautions for disposal
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Janssen-Cilag International NV,
Turnhoutseweg 30,
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency:
http://www.ema.europa.eu/
NAME OF THE MEDICINAL PRODUCT
XEPLION 75 mg prolonged release suspension for injection
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each pre-filled syringe contains 117 mg paliperidone palmitate equivalent to 75 mg paliperidone.
For a full list of excipients, see section 6.1.
Prolonged release suspension for injection.
The suspension is white to off-white. The suspension is pH neutral (approximately 7.0).
4.1 Therapeutic indications
XEPLION is indicated for maintenance treatment of schizophrenia in adult patients stabilised with
paliperidone or risperidone.
In selected adult patients with schizophrenia and previous responsiveness to oral paliperidone or
risperidone, XEPLION may be used without prior stabilisation with oral treatment if psychotic symptoms
are mild to moderate and a long-acting injectable treatment is needed.
4.2 Posology and method of administration
Recommended initiation of XEPLION is with a dose of 150 mg on treatment day 1 and 100 mg one week
later (day 8), both administered in the deltoid muscle in order to attain therapeutic concentrations rapidly
(see section 5.2). The recommended monthly maintenance dose is 75 mg; some patients may benefit from
lower or higher doses within the recommended range of 25 to 150 mg based on individual patient
tolerability and/or efficacy. Patients who are overweight or obese may require doses in the upper range
(see section 5.2). Following the second dose, monthly maintenance doses can be administered in either the
deltoid or gluteal muscle.
Adjustment of the maintenance dose may be made monthly. When making dose adjustments, the
prolonged release characteristics of XEPLION should be considered (see section 5.2), as the full effect of
maintenance doses may not be evident for several months.
Switching from oral paliperidone or oral risperidone
Previous oral paliperidone or oral risperidone can be discontinued at the time of initiation of treatment
with XEPLION. XEPLION should be initiated as described at the beginning of section 4.2 above.
Switching from Risperidone long acting injection.
When switching patients from risperidone long acting injection, initiate XEPLION therapy in place of the
next scheduled injection. XEPLION should then be continued at monthly intervals. The one-week
initiation dosing regimen including the intramuscular injections (day 1 and 8, respectively) as described in
section 4.2 above is not required. Patients previously stabilised on different doses of risperidone long
acting injection can attain similar paliperidone steady-state exposure during maintenance treatment with
XEPLION monthly doses according to the following:
Doses of Risperidone long acting injection and XEPLION needed to attain similar
paliperidone exposure at steady-state
Previous Risperidone long acting injection dose
Discontinuation of antipsychotic medicinal products should be made in accordance with appropriate
prescribing information. If XEPLION is discontinued, its prolonged release characteristics must be
considered. As recommended with other antipsychotic medicinal products, the need for continuing
existing extrapyramidal symptoms (EPS) medicine should be re-evaluated periodically.
Avoiding missed doses
It is recommended that the second initiation dose of XEPLION be given one week after the first dose. To
avoid a missed dose, patients may be given the second dose 2 days before or after the one-week (day 8)
time point. Similarly, the third and subsequent injections after the initiation regimen are recommended to
be given monthly. To avoid a missed monthly dose, patients may be given the injection up to 7 days
before or after the monthly time point.
If the target date for the second XEPLION injection (day 8 ± 2 days) is missed, the recommended
reinitiation depends on the length of time which has elapsed since the patient's first injection.
Missed second initiation dose (< 4 weeks from first injection)
If less than 4 weeks have elapsed since the first injection, then the patient should be administered the
second injection of 100 mg in the deltoid muscle as soon as possible. A third XEPLION injection of
75 mg in either the deltoid or gluteal muscles should be administered 5 weeks after the first injection
(regardless of the timing of the second injection). The normal monthly cycle of injections in either the
deltoid or gluteal muscle of 25 mg to 150 mg based on individual patient tolerability and/or efficacy
should be followed thereafter.
Missed second initiation dose (4-7 weeks from first injection)
If 4 to 7 weeks have elapsed since the first injection of XEPLION, resume dosing with two injections of
100 mg in the following manner:
1.
a deltoid injection as soon as possible,
another deltoid injection one week later,







resumption of the normal monthly cycle of injections in either the deltoid or gluteal muscle of
25 mg to 150 mg based on individual patient tolerability and/or efficacy.
Missed second initiation dose (> 7 weeks from first injection)
If more than 7 weeks have elapsed since the first injection of XEPLION, initiate dosing as described for
the initial recommended initiation of XEPLION above.
Missed monthly maintenance dose (1 month to 6 weeks)
After initiation, the recommended injection cycle of XEPLION is monthly. If less than 6 weeks have
elapsed since the last injection, then the previously stabilised dose should be administered as soon as
possible, followed by injections at monthly intervals.
Missed monthly maintenance dose (> 6 weeks to 6 months)
If more than 6 weeks have elapsed since the last injection of XEPLION, the recommendation is as
follows:
For patients stabilised with doses of 25 to 100
mg:
1. a deltoid injection as soon as possible at the same dose the patient was previously stabilised on
2. another deltoid injection (same dose) one week later (day 8)
3. resumption of the normal monthly cycle of injections in either the deltoid or gluteal muscle of
25 mg to 150 mg based on individual patient tolerability and/or efficacy
For patients stabilised with 150
mg:
1. a deltoid injection as soon as possible at the 100 mg dose
2. another deltoid injection one week later (day 8) at the 100 mg dose
3. resumption of the normal monthly cycle of injections in either the deltoid or gluteal muscle of
25 mg to 150 mg based on individual patient tolerability and/or efficacy
Missed monthly maintenance dose (> 6 months).
If more than 6 months have elapsed since the last
injection of XEPLION, initiate dosing as described for the initial recommended initiation of XEPLION
above.
Elderly population
Efficacy and safety in elderly > 65 years have not been established.
In general, recommended dosing of XEPLION for elderly patients with normal renal function is the same
as for younger adult patients with normal renal function. However, because elderly patients may have
diminished renal function, dose adjustment may be necessary (see
Renal impairment
below for dosing
recommendations in patients with renal impairment).
Renal impairment
XEPLION has not been systematically studied in patients with renal impairment (see section 5.2). For
patients with mild renal impairment (creatinine clearance ≥ 50 to < 80 ml/min), recommended initiation of
XEPLION is with a dose of 100 mg on treatment day 1 and 75 mg one week later, both administered in
the deltoid muscle. The recommended monthly maintenance dose is 50 mg with a range of 25 to 100 mg
based on patient tolerability and/or efficacy.
XEPLION is not recommended in patients with moderate or severe renal impairment (creatinine clearance
< 50 ml/min) (see section 4.4).
Hepatic impairment
Based on experience with oral paliperidone, no dose adjustment is required in patients with mild or
moderate hepatic impairment. As paliperidone has not been studied in patients with severe hepatic
impairment, caution is recommended in such patients.
Other special populations
No dose adjustment for XEPLION is recommended based on gender, race, or smoking status.
Paediatric population
The safety and efficacy of XEPLION in children < 18 years of age have not been established. No data are
available.
XEPLION is intended for intramuscular use only. It should be injected slowly, deep into the muscle. Each
injection should be administered by a health care professional. Administration should be in a single
injection. The dose should not be given in divided injections. The dose should not be administered
intravascularly or subcutaneously.
The day 1 and day 8 initiation doses must each be administered in the deltoid muscle in order to attain
therapeutic concentrations rapidly (see section 5.2). Following the second dose, monthly maintenance
doses can be administered in either the deltoid or gluteal muscle. A switch from gluteal to deltoid (and
vice versa) should be considered in the event of injection site pain if the injection site discomfort is not
well tolerated (see section 4.8). It is also recommended to alternate between left and right sides (see
below).
For instructions for use and handling of XEPLION, see package leaflet (information intended for medical
or healthcare professionals).
Deltoid muscle administration
The recommended needle size for initial and maintenance administration of XEPLION into the deltoid
muscle is determined by the patient’s weight. For those ≥ 90 kg, the 1½ inch, 22 gauge needle (38.1 mm x
0.72 mm) is recommended. For those < 90 kg, the 1-inch, 23 gauge needle (25.4 mm x 0.64 mm) is
recommended. Deltoid injections should be alternated between the two deltoid muscles.
Gluteal muscle administration
The recommended needle size for maintenance administration of XEPLION into the gluteal muscle is the
1½-inch, 22 gauge needle (38.1 mm x 0.72 mm). Administration should be made into the upper-outer
quadrant of the gluteal area. Gluteal injections should be alternated between the two gluteal muscles.
Hypersensitivity to the active substance, to risperidone or to any of the excipients.
4.4 Special warnings and precautions for use
Use in patients who are in an acutely agitated or severely psychotic state
XEPLION should not be used to manage acutely agitated or severely psychotic states when immediate
symptom control is warranted.
Caution should be exercised when paliperidone is prescribed in patients with known cardiovascular
disease or family history of QT prolongation, and in concomitant use with other medicinal products
thought to prolong the QT interval.
Neuroleptic malignant syndrome
Neuroleptic Malignant Syndrome (NMS), characterised by hyperthermia, muscle rigidity, autonomic
instability, altered consciousness, and elevated serum creatine phosphokinase levels has been reported to
occur with paliperidone. Additional clinical signs may include myoglobinuria (rhabdomyolysis) and acute
renal failure. If a patient develops signs or symptoms indicative of NMS, all antipsychotics, including
paliperidone, should be discontinued.
Medicinal products with dopamine receptor antagonistic properties have been associated with the
induction of tardive dyskinesia characterised by rhythmical, involuntary movements, predominantly of the
tongue and/or face. If signs and symptoms of tardive dyskinesia appear, the discontinuation of all
antipsychotics, including paliperidone, should be considered.
Rare cases of glucose related adverse reactions, e.g. increase in blood glucose, have been reported in
clinical trials with paliperidone. Appropriate clinical monitoring is advisable in diabetic patients and in
patients with risk factors for the development of diabetes mellitus.
Tissue culture studies suggest that cell growth in human breast tumours may be stimulated by prolactin.
Although no clear association with the administration of antipsychotics has so far been demonstrated in
clinical and epidemiological studies, caution is recommended in patients with relevant medical history.
Paliperidone should be used with caution in patients with possible prolactin-dependent tumours.
Paliperidone may induce orthostatic hypotension in some patients based on its alpha-blocking activity.
Based on pooled data from the three placebo-controlled, 6-week, fixed-dose trials with oral paliperidone
prolonged release tablets (3, 6, 9, and 12 mg), orthostatic hypotension was reported by 2.5% of subjects
treated with oral paliperidone compared with 0.8% of subjects treated with placebo. XEPLION should be
used with caution in patients with known cardiovascular disease (e.g., heart failure, myocardial infarction
or ischaemia, conduction abnormalities), cerebrovascular disease, or conditions that predispose the patient
to hypotension (e.g. dehydration and hypovolemia).
XEPLION should be used cautiously in patients with a history of seizures or other conditions that
potentially lower the seizure threshold.
The plasma concentrations of paliperidone are increased in patients with renal impairment and therefore,
dose adjustment is recommended in patients with mild renal impairment. XEPLION is not recommended
in patients with moderate or severe renal impairment (creatinine clearance < 50 ml/min) (see sections 4.2
and 5.2).
No data are available in patients with severe hepatic impairment (Child-Pugh class C). Caution is
recommended if paliperidone is used in such patients.
Elderly patients with dementia
XEPLION has not been studied in elderly patients with dementia. XEPLION should be used with caution
in elderly patients with dementia with risk factors for stroke.
The experience from risperidone cited below is considered valid also for paliperidone.
Overall mortality
In a meta-analysis of 17 controlled clinical trials, elderly patients with dementia treated with other atypical
antipsychotics, including risperidone, aripiprazole, olanzapine, and quetiapine had an increased risk of
mortality compared to placebo. Among those treated with risperidone, the mortality was 4% compared
with 3.1% for placebo.
Cerebrovascular adverse reactions
An approximately 3-fold increased risk of cerebrovascular adverse reactions has been seen in randomised
placebo-controlled clinical trials in the dementia population with some atypical antipsychotics, including
risperidone, aripiprazole, and olanzapine. The mechanism for this increased risk is not known.
Parkinson’s disease and dementia with Lewy bodies
Physicians should weigh the risks versus the benefits when prescribing XEPLION to patients with
Parkinson’s Disease or Dementia with Lewy Bodies (DLB) since both groups may be at increased risk of
Neuroleptic Malignant Syndrome as well as having an increased sensitivity to antipsychotics.
Manifestation of this increased sensitivity can include confusion, obtundation, postural instability with
frequent falls, in addition to extrapyramidal symptoms.
Antipsychotic medicinal products (including risperidone) with alpha-adrenergic blocking effects have
been reported to induce priapism. During postmarketing surveillance, priapism has also been reported with
oral paliperidone, which is the active metabolite of risperidone. Patients should be informed to seek urgent
medical care in case that priapism has not been resolved within 3-4 hours.
Body temperature regulation
Disruption of the body’s ability to reduce core body temperature has been attributed to antipsychotic
medicinal products. Appropriate care is advised when prescribing XEPLION to patients who will be
experiencing conditions which may contribute to an elevation in core body temperature, e.g. exercising
strenuously, exposure to extreme heat, receiving concomitant medicinal products with anticholinergic
activity or being subject to dehydration.
Cases of venous thromboembolism (VTE) have been reported with antipsychotic medicinal products.
Since patients treated with antipsychotics often present with acquired risk factors for VTE, all possible
risk factors for VTE should be identified before and during treatment with XEPLION and preventative
measures undertaken.
An antiemetic effect was observed in preclinical studies with paliperidone. This effect, if it occurs in
humans, may mask the signs and symptoms of overdosage with certain medicinal products or of
conditions such as intestinal obstruction, Reye’s syndrome and brain tumour.
Patients should be advised of the potential for weight gain. Weight should be measured regularly.
Care must be taken to avoid inadvertent injection of XEPLION into a blood vessel.
4.5 Interaction with other medicinal products and other forms of interaction
Caution is advised when prescribing XEPLION with medicinal products known to prolong the QT
interval, e.g. class IA antiarrhythmics (e.g., quinidine, disopyramide) and class III antiarrhythmics (e.g.
amiodarone, sotalol), some antihistaminics, some other antipsychotics and some antimalarials (e.g.
mefloquine). This list is indicative and not exhaustive.
Potential for XEPLION to affect other medicinal products
Paliperidone is not expected to cause clinically important pharmacokinetic interactions with medicinal
products that are metabolised by cytochrome P-450 isozymes.
Given the primary central nervous system (CNS) effects of paliperidone (see section 4.8), XEPLION
should be used with caution in combination with other centrally acting medicinal products, e.g.,
anxiolytics, most antipsychotics, hypnotics, opiates, etc. or alcohol.
Paliperidone may antagonise the effect of levodopa and other dopamine agonists. If this combination is
deemed necessary, particularly in end-stage Parkinson’s disease, the lowest effective dose of each
treatment should be prescribed.
Because of its potential for inducing orthostatic hypotension (see section 4.4), an additive effect may be
observed when XEPLION is administered with other therapeutic agents that have this potential, e.g., other
antipsychotics, tricyclics.
Caution is advised if paliperidone is combined with other medicinal products known to lower the seizure
threshold (i.e., phenothiazines or butyrophenones, tricyclics or SSRIs, tramadol, mefloquine, etc.).
Co-administration of oral paliperidone prolonged release tablets at steady-state (12 mg once daily) with
divalproex sodium prolonged release tablets (500 mg to 2000 mg once daily) did not affect the steady-
state pharmacokinetics of valproate.
No interaction study between XEPLION and lithium has been performed, however, a pharmacokinetic
interaction is not likely to occur.
Potential for other medicinal products to affect XEPLION
In vitro
studies indicate that CYP2D6 and CYP3A4 may be minimally involved in paliperidone
metabolism, but there are no indications
in vitro
nor
in vivo
that these isozymes play a significant role in
the metabolism of paliperidone. Concomitant administration of oral paliperidone with paroxetine, a potent
CYP2D6 inhibitor, showed no clinically significant effect on the pharmacokinetics of paliperidone.
Co-administration of oral paliperidone prolonged release once daily with carbamazepine 200 mg twice
daily caused a decrease of approximately 37% in the mean steady-state C
max
and AUC of paliperidone.
This decrease is caused, to a substantial degree, by a 35% increase in renal clearance of paliperidone likely
as a result of induction of renal P-gp by carbamazepine. A minor decrease in the amount of active
substance excreted unchanged in the urine suggests that there was little effect on the CYP metabolism or
bioavailability of paliperidone during carbamazepine co-administration. Larger decreases in plasma
concentrations of paliperidone could occur with higher doses of carbamazepine. On initiation of
carbamazepine, the dose of XEPLION should be re-evaluated and increased if necessary. Conversely, on
discontinuation of carbamazepine, the dose of XEPLION should be re-evaluated and decreased if
necessary.
Co-administration of a single dose of an oral paliperidone prolonged release tablet 12 mg with divalproex
sodium prolonged release tablets (two 500 mg tablets once daily) resulted in an increase of approximately
50% in the C
max
and AUC of paliperidone, likely as a result of increased oral absorption. Since no effect
on the systemic clearance was observed, a clinically significant interaction would not be expected between
divalproex sodium prolonged release tablets and XEPLION intramuscular injection. This interaction has
not been studied with XEPLION.
Concomitant use of XEPLION with risperidone
Risperidone administered orally or intramuscularly will be metabolised to a variable degree to
paliperidone. Consideration should be given if risperidone or oral paliperidone is co-administered with
XEPLION.
4.6 Fertility, pregnancy and lactation
There are no adequate data from the use of paliperidone during pregnancy. Intramuscularly injected
paliperidone palmitate and orally administered paliperidone were not teratogenic in animal studies, but
other types of reproductive toxicity were seen (see section 5.3). The use of antipsychotics during the last
trimester of pregnancy has resulted in long term but reversible neurological disturbances of
extrapyramidal nature in the infant. XEPLION should not be used during pregnancy unless clearly
necessary.
Paliperidone is excreted in the breast milk to such an extent that effects on the breastfed infant are likely if
therapeutic doses are administered to breastfeeding women. XEPLION should not be used while breast
feeding.
There were no relevant effects observed in the non-clinical studies.
4.7 Effects on ability to drive and use machines
Paliperidone can have minor or moderate influence on the ability to drive and use machines due to
potential nervous system and visual effects, such as sedation, somnolence, syncope, vision blurred (see
section 4.8). Therefore, patients should be advised not to drive or operate machines until their individual
susceptibility to XEPLION is known.
The most frequently reported adverse drug reactions (ADRs) reported in clinical trials were insomnia,
headache, weight increased, injection site reactions, agitation, somnolence, akathisia, nausea, constipation,
dizziness, tremor, vomiting, upper respiratory tract infection, diarrhoea, and tachycardia. Of these,
akathisia appeared to be dose-related.
The following are all ADRs that were reported in XEPLION-treated subjects in clinical trials. The
following terms and frequencies are applied:
very common
(≥ 1/10),
common
(≥ 1/100 to < 1/10),
uncommon
(≥ 1/1000 to < 1/100),
rare
(≥ 1/10,000 to < 1/1000),
very rare
(< 1/10,000), and
not known
(cannot be estimated from the available data).
Adverse Drug Reaction
Frequency
Infections and
infestations
upper respiratory tract
infection
Metabolism and
nutrition disorders
weight increased,
blood glucose
increased, blood
triglycerides
increased
hyperglycaemia,
hyperinsulinaemia,
increased appetite,
decreased appetite,
blood cholesterol
increased
headache dystonia,
parkinsonism,
akathisia, dyskinesia,
extrapyramidal
disorder, tremor,
dizziness,
somnolence
syncope, convulsion,
tardive dyskinesia,
dysarthria,
psychomotor
hyperactivity,
dizziness postural,
lethargy
neuroleptic
malignant
syndrome,
cerebrovascular
accident
eye rolling, eye
movement
disorder
Ear and labyrinth
disorders

















Adverse Drug Reaction
Frequency
sinus tachycardia,
conduction disorder,
atrioventricular block
first degree,
bradycardia, postural
orthostatic
tachycardia
syndrome,
palpitations,
electrocardiogram
QT prolonged,
electrocardiogram
abnormal
Gastrointestinal
disorders
vomiting, abdominal
discomfort/abdominal
pain upper, diarrhoea,
nausea, constipation,
toothache
Skin and
subcutaneous tissue
disorders
urticaria, pruritus
generalised, pruritus
Musculoskeletal and
connective tissue
disorders
back pain, pain in
extremity
Reproductive system
and breast disorders
gynaecomastia,
erectile dysfunction,
sexual dysfunction,
galactorrhoea,
amenorrhoea,
menstruation
irregular, menstrual
disorder,
menstruation delayed
General disorders and
administration site
conditions
asthenia, injection site
induration, fatigue,
injection site pain
injection site pruritus administration
site pain,
administration
site reaction,
injection site
nodule
The following is a list of additional ADRs that have been reported with oral paliperidone in the treatment
of schizophrenia:
















Infections and infestations
Common:
nasopharyngitis
Uncommon:
urinary tract infection, rhinitis
Rare:
anaphylactic reaction
Rare:
transient ischaemic attack, grand mal convulsion
Uncommon:
sinus arrhythmia
Rare:
bundle branch block left
Uncommon:
hypotension
Rare:
ischaemia
Respiratory, thoracic and
mediastinal disorders
Common:
cough,
pharyngolaryngeal pain, nasal congestion
Not known:
pneumonia aspiration
Gastrointestinal disorders
Common:
dyspepsia
Uncommon:
flatulence
Rare:
small intestinal obstruction
Not known:
swollen tongue
Skin and subcutaneous tissue
disorders
Rare:
angioedema, rash papular
Common:
arthralgia
Uncommon:
musculoskeletal pain
Renal and urinary disorders
Uncommon:
urinary retention
Rare:
urinary incontinence
Reproductive system and
breast disorders
Rare:
breast engorgement, breast pain, breast tenderness,
retrograde ejaculation
Not known:
priapism
General disorders and
administration site conditions
Uncommon:
oedema peripheral
Rare:
oedema
Description of selected adverse reactions
Injection site reactions
The most commonly reported injection site related adverse reaction was pain. The majority of these
reactions were reported to be of mild to moderate severity. Subject evaluations of injection site pain based
on a visual analogue scale tended to lessen in frequency and intensity over time in all Phase 2 and 3
studies. Injections into the deltoid were perceived as slightly more painful than corresponding gluteal
injections. Other injection site reactions were mostly mild in intensity and included induration (common),
pruritus (uncommon) and nodules (rare).
Musculoskeletal and
connective tissue disorders













Weight gain
In the 13-week study involving the 150 mg initiation dosing, the proportion of subjects with an abnormal
weight increase ≥ 7% showed a dose-related trend, with a 5% incidence rate in the placebo group
compared with rates of 6%, 8% and 13% in the XEPLION 25 mg, 100 mg, and 150 mg groups,
respectively.
During the 33-week open-label transition/maintenance period of the long-term recurrence prevention trial,
12% of XEPLION-treated subjects met this criterion (weight gain of ≥ 7% from double-blind phase to
endpoint); the mean (SD) weight change from open-label baseline was +0.7 (4.79) kg.
Serum prolactin
In clinical trials, median increases in serum prolactin were observed in subjects of both genders who
received XEPLION. Adverse reactions that may suggest increase in prolactin levels (e.g. amenorrhoea,
galactorrhoea and gynaecomastia) were reported overall in <1% of subjects.
QT prolongation, ventricular arrhythmias (ventricular fibrillation, ventricular tachycardia), sudden
unexplained death, cardiac arrest, and Torsade de pointes may occur with antipsychotics. Cases of venous
thromboembolism, including cases of pulmonary embolism and cases of deep vein thrombosis, have been
reported with antipsychotic medicinal products (frequency unknown).
In general, expected signs and symptoms are those resulting from an exaggeration of paliperidone’s
known pharmacological effects, i.e., drowsiness and sedation, tachycardia and hypotension, QT
prolongation, and extrapyramidal symptoms. Torsade de pointes and ventricular fibrillation have been
reported in a patient in the setting of overdose with oral paliperidone. In the case of acute overdose, the
possibility of multiple drug involvement should be considered.
Consideration should be given to the prolonged release nature of the medicinal product and the long
elimination half-life of paliperidone when assessing treatment needs and recovery. There is no specific
antidote to paliperidone. General supportive measures should be employed. Establish and maintain a clear
airway and ensure adequate oxygenation and ventilation.
Cardiovascular monitoring should commence immediately and should include continuous
electrocardiographic monitoring for possible arrhythmias. Hypotension and circulatory collapse should be
treated with appropriate measures such as intravenous fluid and/or sympathomimetic agents. In case of
severe extrapyramidal symptoms, anticholinergic agents should be administered. Close supervision and
monitoring should continue until the patient recovers.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Psycholeptics, other antipsychotics. ATC code: N05AX13
XEPLION contains a racemic mixture of (+)- and (-)-paliperidone.
Paliperidone is a selective blocking agent of monoamine effects, whose pharmacological properties are
different from that of traditional neuroleptics. Paliperidone binds strongly to serotonergic 5-HT2- and
dopaminergic D2-receptors. Paliperidone also blocks alpha 1-adrenergic receptors and slightly less,
H1-histaminergic and alpha 2-adrenergic receptors. The pharmacological activity of the (+)- and (-)-
paliperidone enantiomers are qualitatively and quantitatively similar.
Paliperidone is not bound to cholinergic receptors. Even though paliperidone is a strong D2-antagonist,
which is believed to relieve the positive symptoms of schizophrenia, it causes less catalepsy and decreases
motor functions less than traditional neuroleptics. Dominating central serotonin antagonism may reduce
the tendency of paliperidone to cause extrapyramidal side effects.
Acute treatment of schizophrenia
The efficacy of XEPLION in the acute treatment of schizophrenia was established in four short-term (one
9-week and three 13-week) double-blind, randomised, placebo-controlled, fixed-dose studies of acutely
relapsed adult inpatients who met DSM-IV criteria for schizophrenia. The fixed doses of XEPLION in
these studies were given on days 1, 8, and 36 in the 9-week study, and additionally on day 64 of the
13-week studies. No additional oral antipsychotic supplementation was needed during the acute treatment
of schizophrenia with XEPLION. The primary efficacy endpoint was defined as a decrease in Positive and
Negative Syndrome Scale (PANSS) total scores as shown in the table below. The PANSS is a validated
multi-item inventory composed of five factors to evaluate positive symptoms, negative symptoms,
disorganised thoughts, uncontrolled hostility/excitement and anxiety/depression. Functioning was
evaluated using the Personal and Social Performance (PSP) scale. The PSP is a validated clinician rated
scale that measures personal and social functioning in four domains: socially useful activities (work and
study), personal and social relationships, self-care and disturbing and aggressive behaviours.
In a 13-week study (n=636) comparing three fixed doses of XEPLION (initial deltoid injection of 150 mg
followed by 3 gluteal or deltoid doses of either 25 mg/4 weeks, 100 mg/4 weeks or 150 mg/4 weeks) to
placebo, all three doses of XEPLION were superior to placebo in improving the PANSS total score.
In this
study, both the 100 mg/4 weeks and 150 mg /4 weeks, but not the 25 mg/4 weeks, treatment groups
demonstrated statistical superiority to placebo for the PSP score. These results support efficacy across the
entire duration of treatment and improvement in PANSS and was observed as early as day 4 with
significant separation from placebo in the 25 mg and 150 mg XEPLION groups by day 8.
The results of the other studies yielded statistically significant results in favour of XEPLION, except for
the 50 mg dose in one study (see table below).
Positive and Negative Syndrome Scale for Schizophrenia (PANSS) Total Score - Change From Baseline to
End Point- LOCF for Studies R092670-SCH-201, R092670-PSY-3003, R092670-PSY-3004 and
R092670-PSY-3007: Primary Efficacy Analysis Set
R092670-PSY-3007
*
Mean baseline (SD)
Mean change (SD)
P-value (vs. Placebo)
n = 160
86.8 (10.31)
-2.9 (19.26)
--
n = 155
86.9 (11.99)
-8.0 (19.90)
0.034
n = 161
86.2 (10.77)
-11.6 (17.63)
<0.001
n = 160
88.4 (11.70)
-13.2 (18.48)
<0.001












R092670-PSY-3003
Mean baseline (SD)
Mean change (SD)
P-value (vs. Placebo)
n = 132
92.4 (12.55)
-4.1 (21.01)
--
n = 93
89.9 (10.78)
-7.9 (18.71)
0.193
n = 94
90.1 (11.66)
-11.0 (19.06)
0.019
n = 30
92.2 (11.72)
-5.5 (19.78)
--
R092670-PSY-3004
Mean baseline (SD)
Mean change (SD)
P-value (vs. Placebo)
n = 125
90.7 (12.22)
-7.0 (20.07)
--
n = 129
90.7 (12.25)
-13.6 (21.45)
0.015
n = 128
91.2 (12.02)
-13.2 (20.14)
0.017
n = 131
90.8 (11.70)
-16.1 (20.36)
<0.001
R092670-SCH-201
Mean baseline (SD)
Mean change (SD)
P-value (vs. Placebo)
n=66
87.8 (13.90)
6.2 (18.25)
--
n=63
88.0 (12.39)
-5.2 (21.52)
0.001
n=68
85.2 (11.09)
-7.8 (19.40)
<0.0001
*
For Study R092670-PSY-3007 an initiation dose of 150 mg was given to all subjects in the XEPLION treatment groups on
Day 1 followed by the assigned dose afterwards.
Note: Negative change in score indicates improvement.
Maintaining symptom control and delaying relapse of schizophrenia
The efficacy of XEPLION in maintaining symptomatic control and delaying relapse of schizophrenia was
established in a longer-term double-blind, placebo-controlled, flexible-dose study involving 849 non-
elderly adult subjects who met DSM-IV criteria for schizophrenia. This study included a 33-week open-
label acute treatment and stabilisation phase, a randomised, double-blind placebo-controlled phase to
observe for relapse, and a 52-week open-label extension period. In this study, doses of XEPLION
included 25, 50, 75, and 100 mg administered monthly; the 75 mg dose was allowed only in the 52-week
open-label extension. Subjects initially received flexible doses (25-100 mg) of XEPLION during a 9-week
transition period, followed by a 24-week maintenance period, where subjects were required to have a
PANSS score of ≤ 75. Dosing adjustments were only allowed in the first 12 weeks of the maintenance
period. A total of 410 stabilised patients were randomised to either XEPLION (median duration 171 days
[range 1 day to 407 days]) or to placebo (median duration 105 days [range 8 days to 441 days]) until they
experienced a relapse of schizophrenia symptoms in the variable length double-blind phase. The trial was
stopped early for efficacy reasons as a significantly longer time to relapse (p < 0.0001, Figure 1) was seen
in patients treated with XEPLION compared to placebo (hazard ratio = 4.32; 95% CI: 2.4-7.7).












The absolute bioavailability of paliperidone palmitate following XEPLION administration is 100%.
Following administration of paliperidone palmitate the (+) and (-) enantiomers of paliperidone
interconvert, reaching an AUC (+) to (-) ratio of approximately 1.6-1.8.
The plasma protein binding of racemic paliperidone is 74%.
Biotransformation and elimination
One week following administration of a single oral dose of 1 mg immediate-release
14
C-paliperidone, 59%
of the dose was excreted unchanged into urine, indicating that paliperidone is not extensively metabolised
in the liver. Approximately 80% of the administered radioactivity was recovered in urine and 11% in the
faeces. Four metabolic pathways have been identified
in vivo
, none of which accounted for more than
6.5% of the dose: dealkylation, hydroxylation, dehydrogenation, and benzisoxazole scission. Although
in vitro
studies suggested a role for CYP2D6 and CYP3A4 in the metabolism of paliperidone, there is no
evidence
in vivo
that these isozymes play a significant role in the metabolism of paliperidone. Population
pharmacokinetics analyses indicated no discernable difference on the apparent clearance of paliperidone
after administration of oral paliperidone between extensive metabolisers and poor metabolisers of
CYP2D6 substrates.
In vitro
studies in human liver microsomes showed that paliperidone does not
substantially inhibit the metabolism of medicinal products metabolised by cytochrome P450 isozymes,
including CYP1A2, CYP2A6, CYP2C8/9/10, CYP2D6, CYP2E1, CYP3A4, and CYP3A5.
In vitro
studies have shown that paliperidone is a P-gp substrate and a weak inhibitor of P-gp at high
concentrations. No
in vivo
data are available and the clinical relevance is unknown.
Long acting paliperidone palmitate injection versus oral prolonged release paliperidone
XEPLION
is designed to deliver paliperidone over a monthly period while prolonged release oral
paliperidone is administered on a daily basis. The initiation regimen for XEPLION (150 mg/100 mg in the
deltoid muscle on Day 1/Day 8) was designed to rapidly attain steady-state paliperidone concentrations
when initiating therapy without the use of oral supplementation.
In general, overall initiation plasma levels with XEPLION were within the exposure range observed with
6-12 mg prolonged release oral paliperidone. The use of the XEPLION initiation regimen allowed patients
to stay in this exposure window of 6-12 mg prolonged release oral paliperidone even on trough pre-dose
days (Day 8 and Day 36). Because of the difference in median pharmacokinetic profiles between the two
medicinal products, caution should be exercised when making a direct comparison of their
pharmacokinetic properties.
Paliperidone is not extensively metabolised in the liver. Although XEPLION was not studied on patients
with hepatic impairment, no dose adjustment is required in patients with mild or moderate hepatic
impairment. In a study with oral paliperidone in subjects with moderate hepatic impairment (Child-Pugh
class B), the plasma concentrations of free paliperidone were similar to those of healthy subjects.
Paliperidone has not been studied in patients with severe hepatic impairment.
The disposition of a single oral dose paliperidone 3 mg prolonged release tablet was studied in subjects
with varying degrees of renal function. Elimination of paliperidone decreased with decreasing estimated
creatinine clearance. Total clearance of paliperidone was reduced in subjects with impaired renal function
by 32% on average in mild (CrCl = 50 to < 80 ml/min), 64% in moderate (CrCl = 30 to < 50 ml/min), and
71% in severe (CrCl = 10 to < 30 ml/min) renal impairment, corresponding to an average increase in
exposure (AUC
inf
) of 1.5, 2.6, and 4.8 fold, respectively, compared to healthy subjects. Based on a limited
number of observations with XEPLION in subjects with mild renal impairment and pharmacokinetic
simulations, a reduced dose is recommended (see section 4.2).
No dose adjustment is recommended based on age alone. However, dose adjustment may be required
because of age-related decreases in creatinine clearance (see Renal impairment above and section 4.2).
Pharmacokinetic studies with paliperidone palmitate have shown somewhat lower (10-20%) plasma
concentrations of paliperidone in patients who are overweight or obese in comparison with normal weight
patients (see section 4.2).
Population pharmacokinetics analysis of data from studies with oral paliperidone revealed no evidence of
race-related differences in the pharmacokinetics of paliperidone following XEPLION administration.
No clinically significant differences were observed between men and women.
Based on
in vitro
studies utilising human liver enzymes, paliperidone is not a substrate for CYP1A2;
smoking should, therefore, not have an effect on the pharmacokinetics of paliperidone. A population
pharmacokinetic analysis based on data with oral paliperidone prolonged release tablets showed a slightly
lower exposure to paliperidone in smokers compared with non-smokers. The difference is unlikely to be
of clinical relevance, though. Smoking was not assessed for XEPLION.
5.3 Preclinical safety data
Repeat-dose toxicity studies of intramuscularly injected paliperidone palmitate and orally administered
paliperidone in rat and dog showed mainly pharmacological effects, such as sedation and prolactin-
mediated effects on mammary glands and genitals. In animals treated with paliperidone palmitate an
inflammatory reaction was seen at the intramuscular injection site. Occasionally abscess formation
occurred.
In rat reproduction studies with oral risperidone, which is extensively converted to paliperidone in rats and
humans, adverse effects were seen on the birth weight and survival of the offspring. No embryotoxicity or
malformations were observed following intramuscular administration of paliperidone palmitate to
pregnant rats up to the highest dose (160 mg/kg/day) corresponding to 4.1 times the exposure level in
humans at the maximum recommended dose of 150 mg. Other dopamine antagonists, when administered
to pregnant animals, have caused negative effects on learning and motor development in the offspring.
Paliperidone palmitate and paliperidone were not genotoxic. In oral carcinogenicity studies of risperidone
in rats and mice, increases in pituitary gland adenomas (mouse), endocrine pancreas adenomas (rat), and
mammary gland adenomas (both species) were seen. The carcinogenic potential of intramuscularly
injected paliperidone palmitate was assessed in rats. There was a statistically significant increase in
mammary gland adenocarcinomas in female rats at 10, 30 and 60 mg/kg/month. Male rats showed a
statistically significant increase in mammary gland adenomas and carcinomas at 30 and 60 mg/kg/month
which is 1.2 and 2.2 times the exposure level at the maximum recommended human 150 mg dose. These
tumours can be related to prolonged dopamine D2 antagonism and hyperprolactinemia. The relevance of
these tumour findings in rodents in terms of human risk is unknown.
PHARMACEUTICAL PARTICULARS
Polysorbate 20
Polyethylene glycol 4000
Citric acid monohydrate
Disodium hydrogen phosphate anhydrous
Sodium dihydrogen phosphate monohydrate
Sodium hydroxide (for pH adjustment)
Water for injections
This medicinal product must not be mixed with other medicinal products.
6.4 Special precautions for storage
6.5 Nature and contents of container
Pre-filled syringe (cyclic-olefin-copolymer) with a plunger stopper and tip cap (bromobutyl rubber) with a
22G 1½-inch safety needle (0.72 mm x 38.1 mm) and a 23G 1-inch safety needle (0.64 mm x 25.4 mm).
Pack sizes:
Pack contains 1 pre-filled syringe and 2 needles
6.6 Special precautions for disposal
Any unused product or waste material should be disposed of in accordance with local requirements.
MARKETING AUTHORISATION HOLDER
Janssen-Cilag International NV,
Turnhoutseweg 30,
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this product is available on the website of the European Medicines Agency:
http://www.ema.europa.eu/
What XEPLION contains
The active substance is paliperidone.
Each pre-filled syringe contains 156 mg paliperidone palmitate equivalent to 100 mg paliperidone.
Each pre-filled syringe contains 234 mg paliperidone palmitate equivalent to 150 mg paliperidone.
The other ingredients are:
Polysorbate 20
Polyethylene glycol 4000
Citric acid monohydrate
Disodium hydrogen phosphate anhydrous
Sodium dihydrogen phosphate monohydrate
Sodium hydroxide (for pH adjustment)
Water for injection
What XEPLION looks like and contents of the pack
XEPLION is a white to off-white prolonged release suspension for injection in a pre-filled syringe that is
provided by your doctor’s office or clinic.
Each pack contains 1 pre-filled syringe and 2 needles.
The Treatment initiation pack contains two initial doses: a pack containing first initiation dose of 150 mg
and a pack containing second initiation dose of 100 mg.
Marketing Authorisation Holder and Manufacturer
Janssen-Cilag International NV,
Turnhoutseweg 30,
B-2340 Beerse,
Belgium
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder.
België/Belgique/Belgien
JANSSEN-CILAG N.V./S.A.
Tel/Tél: +32 14 64 94 11
Luxembourg/Luxemburg
JANSSEN-CILAG N.V./S.A.
Tel/Tél: +32 14 64 94 11
България
Представителство на Johnson & Johnson,
Тел.:+359 2 489 9400
Magyarország
JANSSEN-CILAG Kft.
Tel. :+36 23 513 800
Česká republika
JANSSEN-CILAG s.r.o.
Tel:+420 227 012 222
Malta
AM MANGION LTD
Tel: +356 2397 6000
Danmark
JANSSEN-CILAG A/S
Tlf: +45 45 94 82 82
Nederland
JANSSEN-CILAG B.V.
Tel: +31 13 583 73 73
Deutschland
JANSSEN-CILAG GmbH
Tel: +49 2137-955-955
Norge
JANSSEN-CILAG AS
Tlf: + 47 24 12 65 00
Eesti
JANSSEN-CILAG
Tel.: + 372 617 7410
Österreich
JANSSEN-CILAG PHARMA GmbH
Tel: +43 1 610 300
Ελλάδα
JANSSEN-CILAG
Tηλ: +30 210 80 90 000
Polska
JANSSEN–CILAG Polska Sp. z o.o.
Tel.: + 48 22 –237 6000
España
JANSSEN-CILAG, S.A.
Tel: +34 91 722 81 00
Portugal
JANSSEN-CILAG FARMACEUTICA,
LDA
Tel: +351 21-4368835
France
JANSSEN-CILAG
Tel: 0800 25 50 75 / + 33 1 55 00 44 44
România
Johnson & Johnson d.o.o.
Tel: +40 21 207 1800
Ireland
JANSSEN-CILAG Ltd.
Tel: +44 1 494 567 567
Slovenija
Johnson & Johnson d.o.o.
Tel: + 386 1401 18 30
Ísland
JANSSEN-CILAG
C/o Vistor hf
Sími: +354 535 7000
Slovenská republika
JOHNSON & JOHNSON S.R.O,
Tel: +421 233 552 600
Italia
JANSSEN-CILAG SpA
Tel: +39 02/2510.1
Suomi/Finland
JANSSEN-CILAG OY
Puh/Tel: +358 207 531 300
Κύπρος
Βαρνάβας Χατζηπαναγής Λτδ
Tηλ: +357 22 755 214
Sverige
JANSSEN-CILAG AB
Tel: +46 8 626 50 00
Latvija
JANSSEN-CILAG Polska Sp. z.o.o. filiāle
Latvijā
Talr. +371 6789 3561
United Kingdom
JANSSEN-CILAG Ltd.
Tel: +44 1494 567 567
Lietuva
Johnson & Johnson UAB
Tel.: +370 5 278 68 88
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency web site:
http://www.ema.europa.eu/
The following information is intended for medical or healthcare professionals only:
The suspension for injection is for single use only. It should be inspected visually for foreign matter
before administration. Do not use if the syringe is not visually free of foreign matter.
The pack contains a pre-filled syringe and 2 safety needles (a 1½-inch 22 gauge needle [38.1 mm x
0.72 mm] and a 1-inch 23 gauge needle [25.4 mm x 0.64 mm]) for intramuscular injection. XEPLION is
also available in a Treatment initiation pack which contains a second pre-filled syringe and 2 additional
safety needles.
Shake the syringe vigorously for a minimum of 10 seconds to ensure a homogeneous suspension.
Select the appropriate needle.
For DELTOID injection, if the patient weighs < 90 kg, use the 1-inch,
23
gauge needle (25.4 mm x
0.64 mm) (needle with
blue
colored hub); if the patient weighs ≥ 90 kg, use the 1½-inch,
22
gauge
needle (38.1 mm x 0.72 mm) (needle with
grey
coloured hub).
For GLUTEAL injection, use the 1½-inch,
22
gauge needle (38.1 mm x 0.72 mm) (needle with
grey
coloured hub).


While holding the syringe upright, remove the rubber tip cap with a twisting motion.
Peel the safety needle blister pouch half way open. Grasp the needle sheath using the plastic peel
pouch. Attach the safety needle to the luer connection of the syringe with an easy clockwise
twisting motion.
Pull the needle sheath away from the needle with a straight pull. Do not twist the sheath as the
needle may be loosened from the syringe.

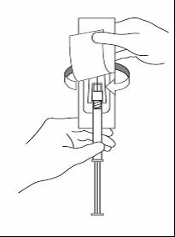
Bring the syringe with the attached needle in upright position to de-aerate. De-aerate the syringe by
moving the plunger rod carefully forward.
Inject the entire contents intramuscularly into the selected deltoid or gluteal muscle of the patient.
Do not administer intravascularly or subcutaneously.
After the injection is complete, use either thumb or finger of one hand (8a, 8b) or a flat surface (8c)
to activate the needle protection system. The system is fully activated when a ‘click’ is heard.
Discard the syringe with needle appropriately.


Any unused product or waste material should be disposed of in accordance with local requirements.
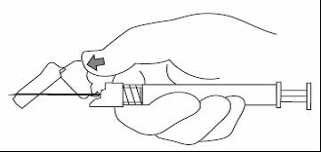


Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/xeplion.html
Copyright © 1995-2021 ITA all rights reserved.
|





























































































































































































































































































































































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































