Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Xolair 75 mg powder and solvent for solution for injection
QUALITATIVE AND QUANTITATIVE COMPOSITION
One vial contains 75 mg of omalizumab*.
*Omalizumab is a humanised monoclonal antibody manufactured by recombinant DNA technology in
a Chinese hamster ovary (CHO) mammalian cell line.
After reconstitution one vial contains 125 mg/ml of omalizumab (75 mg in 0.6 ml).
For a full list of excipients, see section 6.1.
Powder and solvent for solution for injection.
Xolair is an off-white lyophilised powder.
4.1 Therapeutic indications
Xolair is indicated in adults, adolescents and children (6 to <12 years of age).
Xolair treatment should only be considered for patients with convincing IgE (immunoglobulin E)
mediated asthma (see section 4.2).
Adults and adolescents (12 years of age and older)
Xolair is indicated as add-on therapy to improve asthma control in patients with severe persistent
allergic asthma who have a positive skin test or
in vitro
reactivity to a perennial aeroallergen and who
have reduced lung function (FEV
1
<80%) as well as frequent daytime symptoms or night-time
awakenings and who have had multiple documented severe asthma exacerbations despite daily high-
dose inhaled corticosteroids, plus a long-acting inhaled beta2-agonist.
Children (6 to <12 years of age)
Xolair is indicated as add-on therapy to improve asthma control in patients with severe persistent
allergic asthma who have a positive skin test or
in vitro
reactivity to a perennial aeroallergen and
frequent daytime symptoms or night-time awakenings and who have had multiple documented severe
asthma exacerbations despite daily high-dose inhaled corticosteroids, plus a long-acting inhaled beta2-
agonist.
4.2 Posology and method of administration
Xolair treatment should be initiated by physicians experienced in the diagnosis and treatment of severe
persistent asthma.
Posology
The appropriate dose and frequency of Xolair is determined by baseline IgE (IU/ml), measured before
the start of treatment, and body weight (kg). Prior to administration of the initial dose, patients should
have their IgE level determined by any commercial serum total IgE assay for their dose assignment.
Based on these measurements, 75 to 600 mg of Xolair in 1 to 4 injections may be needed for each
administration.
Patients with IgE lower than 76 IU/ml were less likely to experience benefit (see section 5.1).
Prescribing physicians should ensure that adult and adolescent patients with IgE below 76 IU/ml and
children (6 to < 12 years of age) with IgE below 200 IU/ml have unequivocal
in vitro
reactivity
(RAST) to a perennial allergen before starting therapy.
See Table 1 for a conversion chart and Tables 2 and 3 for the dose determination charts in adults,
adolescents and children (6 to <12 years of age).
Patients whose baseline IgE levels or body weight in kilograms are outside the limits of the dose table
should not be given Xolair.
The maximum recommended dose is 600 mg omalizumab every two weeks.
Table 1: Conversion from dose to number of vials, number of injections and total injection
volume for each administration
Total injection volume (ml)
a
0.6 ml = maximum delivered volume per vial (Xolair 75 mg).
b
1.2 ml = maximum delivered volume per vial (Xolair 150 mg).
c
or use 0.6 ml from a 150 mg vial.
Table 2: ADMINISTRATION EVERY 4 WEEKS. Xolair doses (milligrams per dose)
administered by subcutaneous injection every 4 weeks
>20-25 >25-30 >30-40 >40-50 >50-60 >60-70 >70-80 >80-90
ADMINISTRATION EVERY 2 WEEKS
SEE TABLE 3































Special populations
Elderly (65 years of age and older)
There are limited data available on the use of Xolair in patients older than 65 years but there is no
evidence that elderly patients require a different dose from younger adult patients.
Renal or hepatic impairment
There have been no studies on the effect of impaired renal or hepatic function on the pharmacokinetics
of Xolair. Because omalizumab clearance at clinical doses is dominated by the reticular endothelial
system (RES) it is unlikely to be altered by renal or hepatic impairment. While no particular dose
adjustment is recommended for these patients, Xolair should be administered with caution (see section
4.4).
Paediatric population
The safety and efficacy of Xolair in children below age 6 have not been established. No data are
available.
Method of administration
For subcutaneous administration only. Do not administer by the intravenous or intramuscular route.
The injections are administered subcutaneously in the deltoid region of the arm. Alternatively, the
injections can be administered in the thigh if there is any reason precluding administration in the
deltoid region.
There is limited experience with self-administration of Xolair. Therefore treatment is intended to be
administered by a healthcare provider only.
For instructions on reconstitution of Xolair before administration, see section 6.6 and also information
for the healthcare professional section of the package leaflet.
Hypersensitivity to the active substance or to any of the excipients.
4.4 Special warnings and precautions for use
General
Xolair is not indicated for the treatment of acute asthma exacerbations, acute bronchospasm or status
asthmaticus.
Xolair has not been studied in patients with hyperimmunoglobulin E syndrome or allergic
bronchopulmonary aspergillosis or for the prevention of anaphylactic reactions, including those
provoked by food allergy
, atopic dermatitis, or allergic rhinitis
. Xolair is not indicated for the
treatment of these conditions.
Xolair therapy has not been studied in patients with autoimmune diseases, immune complex-mediated
conditions, or pre-existing renal or hepatic impairment (see section 4.2). Caution should be exercised
when administering Xolair in these patient populations.
Abrupt discontinuation of systemic or inhaled corticosteroids after initiation of Xolair therapy is not
recommended. Decreases in corticosteroids should be performed under the direct supervision of a
physician and may need to be performed gradually.
Immune system disorders
Allergic reactions type I
Type I local or systemic allergic reactions, including anaphylaxis and anaphylactic shock, may occur
when taking omalizumab, also with onset after a long duration of treatment. Most of these reactions
occurred within 2 hours after the first and subsequent injections of Xolair but some started beyond
2 hours and even beyond 24 hours after the injection. Therefore medicinal products for the treatment
of anaphylactic reactions should always be available for immediate use following administration of
Xolair. Patients should be informed that such reactions are possible and prompt medical attention
should be sought if allergic reactions occur.
Anaphylactic reactions were rare in clinical trials (see section 4.8).
Antibodies to omalizumab have been detected in a low number of patients in clinical trials. The
clinical relevance of anti-Xolair antibodies is not well understood.
Serum sickness
Serum sickness and serum sickness-like reactions, which are delayed allergic type III reactions, have
been seen in patients treated with humanised monoclonal antibodies including omalizumab. The
suggested pathophysiologic mechanism includes immune-complex formation and deposition due to
development of antibodies against omalizumab. The onset has typically been 1-5 days after
administration of the first or subsequent injections, also after long duration of treatment. Symptoms
suggestive of serum sickness include arthritis/arthralgias, rash (urticaria or other forms), fever and
lymphadenopathy. Antihistamines and corticosteroids may be useful for preventing or treating this
disorder, and patients should be advised to report any suspected symptoms.
Churg-Strauss syndrome and hypereosinophilic syndrome
Patients with severe asthma may rarely present systemic hypereosinophilic syndrome or allergic
eosinophilic granulomatous vasculitis (Churg-Strauss syndrome), both of which are usually treated
with systemic corticosteroids.
In rare cases, patients on therapy with anti-asthma medicinal products, including omalizumab, may
present or develop systemic eosinophilia and vasculitis. These events are commonly associated with
the reduction of oral corticosteroid therapy.
In these patients, physicians should be alert to the development of marked eosinophilia, vasculitic
rash, worsening pulmonary symptoms, paranasal sinus abnormalities, cardiac complications, and/or
neuropathy.
Discontinuation of omalizumab should be considered in all severe cases with the above mentioned
immune system disorders.
Malignancies
During clinical trials in adults and adolescents 12 years of age and older, there was a numerical
imbalance in cancers arising in the Xolair (0.5%; 25 cancers in 5,015 patients) treatment group
compared with the control (0.18%; 5 cancers in 2,854 patients) group. Malignancies were uncommon
(<1/100) in both the active and the control group. The diversity in the type of cancers observed, the
relatively short duration of exposure and the clinical features of the individual cases render a causal
relationship unlikely. The overall observed incidence rate of malignancy in the Xolair clinical trial
programme was comparable to that reported in the general population.
Parasitic (helminth) infections
IgE may be involved in the immunological response to some helminth infections. In patients at chronic
high risk of helminth infection, a placebo-controlled trial showed a slight increase in infection rate
with omalizumab, although the course, severity, and response to treatment of infection were unaltered.
The helminth infection rate in the overall clinical programme, which was not designed to detect such
infections, was less than 1 in 1,000 patients. However, caution may be warranted in patients at high
risk of helminth infection, in particular when travelling to areas where helminthic infections are
endemic. If patients do not respond to recommended anti-helminth treatment, discontinuation of
Xolair should be considered.
4.5 Interaction with other medicinal products and other forms of interaction
Cytochrome P450 enzymes, efflux pumps and protein-binding mechanisms are not involved in the
clearance of omalizumab; thus, there is little potential for drug-drug interactions. Medicinal product or
vaccine interaction studies have not been performed with Xolair. There is no pharmacological reason
to expect that commonly prescribed medicinal products used in the treatment of asthma will interact
with omalizumab.
In clinical studies Xolair was commonly used in conjunction with inhaled and oral corticosteroids,
inhaled short-acting and long-acting beta agonists, leukotriene modifiers, theophyllines and oral
antihistamines. There was no indication that the safety of Xolair was altered with these other
commonly used anti-asthma medicinal products. Limited data are available on the use of Xolair in
combination with specific immunotherapy (hypo-sensitisation therapy). In a clinical trial where Xolair
was co-administered with immunotherapy, the safety and efficacy of Xolair in combination with
specific immunotherapy were found to be no different to that of Xolair alone.
Xolair may indirectly reduce the efficacy of medicinal products for the treatment of helminthic or
other parasitic infections (see section 4.4).
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no adequate data from the use of omalizumab in pregnant women. Animal studies do not
indicate direct or indirect harmful effects with respect to pregnancy, embryonal/foetal development,
parturition or postnatal development (see section 5.3). Omalizumab crosses the placental barrier and
the potential for harm to the foetus is unknown. Omalizumab has been associated with age-dependent
decreases in blood platelets in non-human primates, with a greater relative sensitivity in juvenile
animals (see section 5.3). Xolair should not be used during pregnancy unless clearly necessary.
Breast-feeding
It is not known whether omalizumab is excreted in human breast milk. Omalizumab is excreted into
non-human primate breast milk and risk to the suckling child cannot be excluded. Women should not
breast-feed during Xolair therapy.
Fertility
There are no human fertility data for omalizumab. In non-clinical studies, no impairment of fertility
and no genotoxicity have been observed (see section 5.3).
4.7 Effects on ability to drive and use machines
Xolair has no or negligible influence on the ability to drive and use machines.
Over 4,400 allergic asthma patients were randomised in controlled efficacy trials with Xolair.
During clinical trials in adult and adolescent patients 12 years of age and older, the most commonly
reported adverse reactions were injection site reactions, including injection site pain, swelling,
erythema and pruritus, and headaches. In clinical trials in children 6 to <12 years of age, the most
commonly reported adverse reactions suspected of being related to the medicinal product were
headache, pyrexia and upper abdominal pain. Most of the reactions were mild or moderate in severity.
Table 4 lists the adverse reactions recorded in clinical studies in the total safety population treated with
Xolair by MedDRA system organ class and frequency. Within each frequency grouping, adverse
reactions are presented in order of decreasing seriousness. Frequency categories are defined as: very
common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to
<1/1,000) and very rare (<1/10,000). Reactions reported in the post-marketing setting are listed with
frequency not known (cannot be estimated from the available data).
Table 4: Adverse reactions
Infections and infestations
Uncommon Pharyngitis
Rare Parasitic infection
Blood and lymphatic system disorders
Not known
Idiopathic severe thrombocytopenia
Immune system disorders
Rare
Anaphylactic reaction, other serious allergic conditions
Serum sickness, may include fever and lymphadenopathy
Nervous system disorders
Common
Syncope, paraesthesia, somnolence, dizziness
Vascular disorders
Uncommon Postural hypotension, flushing
Respiratory, thoracic and mediastinal disorders
Uncommon
Allergic bronchospasm, coughing
Allergic granulomatous vasculitis (i.e. Churg-Strauss syndrome)
Gastrointestinal disorders
Common Abdominal pain upper**
Uncommon Dyspeptic signs and symptoms, diarrhoea, nausea
Skin and subcutaneous tissue disorders
Uncommon Photosensitivity, urticaria, rash, pruritus
Rare Angioedema
Not known Alopecia
Musculoskeletal and connective tissue disorders
Not known Arthralgia, myalgia, joint swelling
General disorders and administration site conditions
Very common Pyrexia**
Common Injection site reactions such as swelling, erythema, pain, pruritus
Uncommon Influenza-like illness, swelling arms, weight increase, fatigue
*: Very common in children 6 to <12 years of age
**: In children 6 to <12 years of age
Immune system disorders
For further information, see section 4.4.
Malignancies
The overall observed incidence rate of malignancy in adults and in adolescents 12 years of age and
older in the Xolair clinical trial programme was comparable to that reported in the general population
(see section 4.4).
There were no cases of malignancy with omalizumab in the clinical trials in children 6 to <12 years of
age; there was a single case of malignancy in the control group.






















Arterial thromboembolic events (ATE)
In controlled clinical trials and an ongoing observational study, a numerical imbalance of ATEs was
observed. ATE included stroke, transient ischaemic attack, myocardial infarction, unstable angina, and
cardiovascular death (including death from unknown cause). The rate of ATE in patients in the
controlled clinical trials was 6.29 for Xolair-treated patients (17/2703 patient years) and 3.42 for
control patients (6/1755 patient years). In Cox proportional hazards model, Xolair was not associated
with ATE risk (hazard ratio 1.86; 95% confidence interval 0.73-4.72). In the observational study, the
rate of ATE was 5.59 (79/14140 patients years) for Xolair-treated patients and 3.71 (31/8366 patient
years) for control patients.In a multivariate analysis controlling for baseline cardiovascular risk
factors, Xolair was not associated with ATE risk (hazard ratio 1.11; 95% confidence interval
0.70-1.76).
Platelets
In clinical trials few patients had platelet counts below the lower limit of the normal laboratory range.
None of these changes were associated with bleeding episodes or a decrease in haemoglobin. No
pattern of persistent decrease in platelet counts, as observed in non-human primates (see section 5.3),
has been reported in humans (patients above 6 years of age), even though isolated cases of idiopathic
thrombocytopenia have been reported in the post-marketing setting.
Parasitic infections
In patients at chronic high risk of helminth infection, a placebo-controlled trial showed a slight
numerical increase in infection rate with omalizumab that was not statistically significant. The course,
severity, and response to treatment of infections were unaltered (see section 4.4).
Maximum tolerated dose of Xolair has not been determined. Single intravenous doses up to 4,000 mg
have been administered to patients without evidence of dose-limiting toxicities. The highest
cumulative dose administered to patients was 44,000 mg over a 20-week period and this dose did not
result in any untoward acute effects.
If an overdose is suspected, the patient should be monitored for any abnormal signs or symptoms.
Medical treatment should be sought and instituted appropriately.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Drugs for obstructive airway diseases, other systemic drugs for
obstructive airway diseases, ATC code: R03DX05
Omalizumab is a recombinant DNA-derived humanised monoclonal antibody that selectively binds to
human immunoglobulin E (IgE). The antibody is an IgG1 kappa that contains human framework
regions with the complementary-determining regions of a murine parent antibody that binds to IgE.
Omalizumab binds to IgE and prevents binding of IgE to FCRI (high-affinity IgE receptor), thereby
reducing the amount of free IgE that is available to trigger the allergic cascade. Treatment of atopic
subjects with omalizumab resulted in a marked down-regulation of FCRI receptors on basophils.
Furthermore, the
in vitro
histamine release from basophils isolated from Xolair-treated subjects was
reduced by approximately 90% following stimulation with an allergen compared to pre-treatment
values.
In clinical studies, serum free IgE levels were reduced in a dose-dependent manner within one hour
following the first dose and maintained between doses. One year after discontinuation of Xolair
dosing, the IgE levels had returned to pre-treatment levels with no observed rebound in IgE levels
after washout of the medicinal product.
Clinical experience
Adults and adolescents ≥12 years of age
The efficacy and safety of Xolair were demonstrated in a 28-week double-blind placebo-controlled
study (study 1) involving 419 severe allergic asthmatics, ages 12-79 years, who had reduced lung
function (FEV
1
40-80% predicted) and poor asthma symptom control despite receiving high dose
inhaled corticosteroids and a long-acting beta2-agonist. Eligible patients had experienced multiple
asthma exacerbations requiring systemic corticosteroid treatment or had been hospitalised or attended
an emergency room due to a severe asthma exacerbation in the past year despite continuous treatment
with high-dose inhaled corticosteroids and a long-acting beta2-agonist. Subcutaneous Xolair or
placebo were administered as add-on therapy to >1,000 micrograms beclomethasone dipropionate (or
equivalent) plus a long-acting beta2-agonist. Oral corticosteroid, theophylline and leukotriene-
modifier maintenance therapies were allowed (22%, 27%, and 35% of patients, respectively).
The rate of asthma exacerbations requiring treatment with bursts of systemic corticosteroids was the
primary endpoint. Omalizumab reduced the rate of asthma exacerbations by 19% (p = 0.153). Further
evaluations which did show statistical significance (p<0.05) in favour of Xolair included reductions in
severe exacerbations (where patient‟s lung function was reduced to below 60% of personal best and
requiring systemic corticosteroids) and asthma-related emergency visits (comprised of
hospitalisations, emergency room, and unscheduled doctor visits), and improvements in Physician‟s
overall assessment of treatment effectiveness, Asthma-related Quality of Life (AQL), asthma
symptoms and lung function.
In a subgroup analysis, patients with pre-treatment total IgE ≥76 IU/ml were more likely to experience
clinically meaningful benefit to Xolair. In these patients in study 1 Xolair reduced the rate of asthma
exacerbations by 40% (p = 0.002). In addition more patients had clinically meaningful responses in the
total IgE ≥76 IU/ml population across the Xolair severe asthma programme. Table 5 includes results in
the study 1 population.
Table 5: Results of study 1
% reduction, p-value for rate ratio
Severe asthma exacerbations
% reduction, p-value for rate ratio
% reduction, p-value for rate ratio
Physician’s overall assessment
% of patients ≥0.5 improvement
* marked improvement or complete control
** p-value for overall distribution of assessment
Study 2 assessed the efficacy and safety of Xolair in a population of 312 severe allergic asthmatics
which matched the population in study 1. Treatment with Xolair in this open label study led to a 61%
reduction in clinically significant asthma exacerbation rate compared to current asthma therapy alone.
Four additional large placebo-controlled supportive studies of 28 to 52 weeks duration in 1,722 adults
and adolescents (studies 3, 4, 5, 6) assessed the efficacy and safety of Xolair in patients with severe
persistent asthma. Most patients were inadequately controlled but were receiving less concomitant
asthma therapy than patients in studies 1 or 2. Studies 3-5 used exacerbation as primary endpoint,
whereas study 6 primarily evaluated inhaled corticosteroid sparing.
In studies 3, 4 and 5 patients treated with Xolair had respective reductions in asthma exacerbation rates
of 37.5% (p = 0.027), 40.3% (p<0.001) and 57.6% (p<0.001) compared to placebo.
In study 6, significantly more severe allergic asthma patients on Xolair were able to reduce their
fluticasone dose to 500 micrograms/day without deterioration of asthma control (60.3%) compared to
the placebo group (45.8%, p<0.05).
Quality of life scores were measured using the Juniper Asthma-related Quality of Life Questionnaire.
For all six studies there was a statistically significant improvement from baseline in quality of life
scores for Xolair patients versus the placebo or control group.







Physician‟s overall assessment of treatment effectiveness:
Physician‟s overall assessment was performed in five of the above studies as a broad measure of
asthma control performed by the treating physician. The physician was able to take into account PEF
(peak expiratory flow), day and night time symptoms, rescue medication use, spirometry and
exacerbations. In all five studies a significantly greater proportion of Xolair treated patients were
judged to have achieved either a marked improvement or complete control of their asthma compared
to placebo patients.
Children 6 to <12 years of age
The primary support for safety and efficacy of Xolair in the group aged 6 to <12 years comes from one
randomised, double-blind, placebo-controlled, multi-centre trial (study 7).
Study 7 was a placebo-controlled trial which included a specific subgroup (n=235) of patients as
defined in the present indication, who were treated with high-dose inhaled corticosteroids
(≥500 µg/day fluticasone equivalent) plus long-acting beta agonist.
A clinically significant exacerbation was defined as a worsening of asthma symptoms as judged
clinically by the investigator, requiring doubling of the baseline inhaled corticosteroid dose for at least
3 days and/or treatment with rescue systemic (oral or intravenous) corticosteroids for at least 3 days.
In the specific subgroup of patients on high dose inhaled corticosteroids, the omalizumab group had a
statistically significantly lower rate of clinically significant asthma exacerbations than the placebo
group. At 24 weeks, the difference in rates between treatment groups represented a 34% (rate ratio
0.662, p = 0.047) decrease relative to placebo for omalizumab patients. In the second double-blind 28-
week treatment period the difference in rates between treatment groups represented a 63% (rate ratio
0.37, p<0.001) decrease relative to placebo for omalizumab patients.
During the 52-week double-blind treatment period (including the 24-week fixed-dose steroid phase
and the 28-week steroid adjustment phase) the difference in rates between treatment groups
represented a 50% (rate ratio 0.504, p<0.001) relative decrease in exacerbations for omalizumab
patients.
The omalizumab group showed greater decreases in beta-agonist rescue medication use than the
placebo group at the end of the 52-week treatment period, although the difference between treatment
groups was not statistically significant. For the global evaluation of treatment effectiveness at the end
of the 52-week double-blind treatment period in the subgroup of severe patients on high-dose inhaled
corticosteroids plus long-acting beta agonists, the proportion of patients rated as having „excellent‟
treatment effectiveness was higher, and the proportions having „moderate‟ or „poor‟ treatment
effectiveness lower in the omalizumab group compared to the placebo group; the difference between
groups was statistically significant (p<0.001), while there were no differences between the
omalizumab and placebo groups for patients‟ subjective Quality of Life ratings.
5.2 Pharmacokinetic properties
The pharmacokinetics of omalizumab have been studied in adult and adolescent patients with allergic
asthma.
Absorption
After subcutaneous administration, omalizumab is absorbed with an average absolute bioavailability
of 62%. Following a single subcutaneous dose in adult and adolescent patients with asthma,
omalizumab was absorbed slowly, reaching peak serum concentrations after an average of 7-8 days.
The pharmacokinetics of omalizumab are linear at doses greater than 0.5 mg/kg. Following multiple
doses of omalizumab, areas under the serum concentration-time curve from Day 0 to Day 14 at steady
state were up to 6-fold of those after the first dose.
Distribution
In vitro
, omalizumab forms complexes of limited size with IgE. Precipitating complexes and
complexes larger than one million Daltons in molecular weight are not observed
in vitro
or
in vivo
.
The apparent volume of distribution in patients following subcutaneous administration was
78 ± 32 ml/kg.
Elimination
Clearance of omalizumab involves IgG clearance processes as well as clearance via specific binding
and complex formation with its target ligand, IgE. Liver elimination of IgG includes degradation in the
reticuloendothelial system and endothelial cells. Intact IgG is also excreted in bile. In asthma patients
the omalizumab serum elimination half-life averaged 26 days, with apparent clearance averaging
2.4 1.1 ml/kg/day. In addition, doubling of body weight approximately doubled apparent clearance.
Characteristics in patient populations
Age, Race/Ethnicity, Gender, Body Mass Index
The population pharmacokinetics of Xolair were analysed to evaluate the effects of demographic
characteristics. Analyses of these limited data suggest that no dose adjustments are necessary for age
(6-76 years), race/ethnicity, gender or Body Mass Index (see section 4.2).
Renal and hepatic impairment
There are no pharmacokinetic or pharmacodynamic data in patients with renal or hepatic impairment
(see sections 4.2 and 4.4).
5.3 Preclinical safety data
The safety of omalizumab has been studied in the cynomolgus monkey, since omalizumab binds to
cynomolgus and human IgE with similar affinity. Antibodies to omalizumab were detected in some
monkeys following repeated subcutaneous or intravenous administration. However, no apparent
toxicity, such as immune complex-mediated disease or complement-dependent cytotoxicity, was seen.
There was no evidence of an anaphylactic response due to mast-cell degranulation in cynomolgus
monkeys.
Chronic administration of high dose levels (up to 250 mg/kg) of omalizumab was well tolerated in
non-human primates (both adult and juvenile animals), with the exception of a dose-related and age-
dependent decrease in blood platelets, with a greater sensitivity in juvenile animals. The serum
concentration required to attain a 50% drop in platelets from baseline in adult cynomolgus monkeys
was roughly 4- to 20-fold higher than anticipated maximum clinical serum concentrations. In addition,
acute haemorrhage and inflammation were observed at injection sites in cynomolgus monkeys.
Formal carcinogenicity studies have not been conducted with omalizumab.
In reproduction studies in cynomolgus monkeys, subcutaneous doses up to 75 mg/kg (about 12-fold
exposure ratio based on 28-day AUC values at 75 mg/kg versus the clinical maximum dose) did not
elicit maternal toxicity, embryotoxicity or teratogenicity when administered throughout organogenesis
and did not elicit adverse effects on foetal or neonatal growth when administered throughout late
gestation, delivery and nursing.
Omalizumab is excreted in milk in cynomolgus monkeys. Milk levels of omalizumab were 1.5% of the
maternal blood concentration.
PHARMACEUTICAL PARTICULARS
Powder
Sucrose
Histidine
Histidine hydrochloride monohydrate
Polysorbate 20
Solvent
Water for injections
This medicinal product must not be mixed with other medicinal products except those mentioned in
section 6.6.
After reconstitution: The chemical and physical stability of the reconstituted medicinal product have
been demonstrated for 8 hours at 2°C to 8°C and for 4 hours at 30°C.
From a microbiological point of view, the medicinal product should be used immediately after
reconstitution. If not used immediately, in-use storage times and conditions prior to use are the
responsibility of the user and would normally not be longer than 8 hours at 2°C to 8°C or 4 hours at
30°C.
6.4 Special precautions for storage
Store in a refrigerator (2C - 8C).
Do not freeze.
For storage conditions of the reconstituted medicinal product, see section 6.3.
6.5 Nature and contents of container
Powder vial: Clear, colourless type I glass vial with a butyl rubber stopper and grey flip-off seal.
Solvent ampoule: Clear, colourless type I glass ampoule containing 2 ml water for injections.
Pack containing one vial of powder for solution for injection and one ampoule of water for injections.
6.6 Special precautions for disposal and other handling
The lyophilised medicinal product takes 15-20 minutes to dissolve, although in some cases it may take
longer. The fully reconstituted medicinal product will appear clear or slightly opaque and may have a
few small bubbles or foam around the edge of the vial. Because of the viscosity of the reconstituted
medicinal product care must be taken to withdraw all of the medicinal product from the vial before
expelling any air or excess solution from the syringe in order to obtain the 0.6 ml.
To prepare Xolair 75 mg vials for subcutaneous administration, please adhere to the following
instructions:
Draw
0.9 ml of water for injections from the ampoule into a syringe equipped with a large-bore
18-gauge needle.
With the vial placed upright on a flat surface, insert the needle and transfer the water for
injections into the vial containing the lyophilised powder using standard aseptic techniques,
directing the water for injections directly on to the powder.
Keeping the vial in an upright position, vigorously swirl it (do not shake) for approximately
1 minute to evenly wet the powder.
To aid in dissolution after completing step 3, gently swirl the vial for 5-10 seconds
approximately every 5 minutes in order to dissolve any remaining solids.
Note that in some cases it may take longer than 20 minutes for the powder to dissolve
completely. If this is the case, repeat step 4 until there are no visible gel-like particles in the
solution.
When the medicinal product is fully dissolved, there should be no visible gel-like particles in
the solution. Small bubbles or foam around the edge of the vial are common. The reconstituted
medicinal product will appear clear or slightly opaque. Do not use if solid particles are present.
Invert the vial for at least 15 seconds in order to allow the solution to drain towards the stopper.
Using a new 3-ml syringe equipped with a large-bore, 18-gauge needle, insert the needle into
the inverted vial. Keeping the vial inverted position the needle tip at the very bottom of the
solution in the vial when drawing the solution into the syringe. Before removing the needle
from the vial, pull the plunger all the way back to the end of the syringe barrel in order to
remove all of the solution from the inverted vial.
Replace the 18-gauge needle with a 25-gauge needle for subcutaneous injection.
Expel air, large bubbles, and any excess solution in order to obtain the required 0.6 ml dose. A
thin layer of small bubbles may remain at the top of the solution in the syringe. Because the
solution is slightly viscous, it may take 5-10 seconds to administer the solution by subcutaneous
injection.
The vial delivers 0.6 ml (75 mg) of Xolair.
The injections are administered subcutaneously in the deltoid region of the arm or the thigh.
Xolair 75 mg powder for solution for injection is supplied in a single-use vial.
From a microbiological point of view, the medicinal product should be used immediately after
reconstitution (see section 6.3).
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 25/10/2005
Date of latest renewal: 25/10/2010
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
Agency http://www.ema.europa.eu
NAME OF THE MEDICINAL PRODUCT
Xolair 150 mg powder and solvent for solution for injection
QUALITATIVE AND QUANTITATIVE COMPOSITION
One vial contains 150 mg of omalizumab*.
*Omalizumab is a humanised monoclonal antibody manufactured by recombinant DNA technology in
a Chinese hamster ovary (CHO) mammalian cell line.
After reconstitution one vial contains 125 mg/ml of omalizumab (150 mg in 1.2 ml).
For a full list of excipients, see section 6.1.
Powder and solvent for solution for injection.
Xolair is an off-white lyophilised powder.
4.1 Therapeutic indications
Xolair is indicated in adults, adolescents and children (6 to <12 years of age).
Xolair treatment should only be considered for patients with convincing IgE (immunoglobulin E)
mediated asthma (see section 4.2).
Adults and adolescents (12 years of age and older)
Xolair is indicated as add-on therapy to improve asthma control in patients with severe persistent
allergic asthma who have a positive skin test or
in vitro
reactivity to a perennial aeroallergen and who
have reduced lung function (FEV
1
<80%) as well as frequent daytime symptoms or night-time
awakenings and who have had multiple documented severe asthma exacerbations despite daily high-
dose inhaled corticosteroids, plus a long-acting inhaled beta2-agonist.
Children (6 to <12 years of age)
Xolair is indicated as add-on therapy to improve asthma control in patients with severe persistent
allergic asthma who have a positive skin test or
in vitro
reactivity to a perennial aeroallergen and
frequent daytime symptoms or night-time awakenings and who have had multiple documented severe
asthma exacerbations despite daily high-dose inhaled corticosteroids, plus a long-acting inhaled beta2-
agonist.
4.2 Posology and method of administration
Xolair treatment should be initiated by physicians experienced in the diagnosis and treatment of severe
persistent asthma.
Posology
The appropriate dose and frequency of Xolair is determined by baseline IgE (IU/ml), measured before
the start of treatment, and body weight (kg). Prior to administration of the initial dose, patients should
have their IgE level determined by any commercial serum total IgE assay for their dose assignment.
Based on these measurements, 75 to 600 mg of Xolair in 1 to 4 injections may be needed for each
administration.
Patients with IgE lower than 76 IU/ml were less likely to experience benefit (see section 5.1).
Prescribing physicians should ensure that adult and adolescent patients with IgE below 76 IU/ml and
children (6 to < 12 years of age) with IgE below 200 IU/ml have unequivocal
in vitro
reactivity
(RAST) to a perennial allergen before starting therapy.
See Table 1 for a conversion chart and Tables 2 and 3 for the dose determination charts in adults,
adolescents and children (6 to <12 years of age).
Patients whose baseline IgE levels or body weight in kilograms are outside the limits of the dose table
should not be given Xolair.
The maximum recommended dose is 600 mg omalizumab every two weeks.
Table 1: Conversion from dose to number of vials, number of injections and total injection
volume for each administration
Total injection volume (ml)
a
0.6 ml = maximum delivered volume per vial (Xolair 75 mg).
b
1.2 ml = maximum delivered volume per vial (Xolair 150 mg).
c
or use 0.6 ml from a 150 mg vial.
Table 2: ADMINISTRATION EVERY 4 WEEKS. Xolair doses (milligrams per dose)
administered by subcutaneous injection every 4 weeks
>20-25 >25-30 >30-40 >40-50 >50-60 >60-70 >70-80 >80-90
ADMINISTRATION EVERY 2 WEEKS
SEE TABLE 3































Special populations
Elderly (65 years of age and older)
There are limited data available on the use of Xolair in patients older than 65 years but there is no
evidence that elderly patients require a different dose from younger adult patients.
Renal or hepatic impairment
There have been no studies on the effect of impaired renal or hepatic function on the pharmacokinetics
of Xolair. Because omalizumab clearance at clinical doses is dominated by the reticular endothelial
system (RES) it is unlikely to be altered by renal or hepatic impairment. While no particular dose
adjustment is recommended for these patients, Xolair should be administered with caution (see section
4.4).
Paediatric population
The safety and efficacy of Xolair in children below age 6 have not been established. No data are
available.
Method of administration
For subcutaneous administration only. Do not administer by the intravenous or intramuscular route.
The injections are administered subcutaneously in the deltoid region of the arm. Alternatively, the
injections can be administered in the thigh if there is any reason precluding administration in the
deltoid region.
There is limited experience with self-administration of Xolair. Therefore treatment is intended to be
administered by a healthcare provider only.
For instructions on reconstitution of Xolair before administration, see section 6.6 and also information
for the healthcare professional section of the package leaflet.
Hypersensitivity to the active substance or to any of the excipients.
4.4 Special warnings and precautions for use
General
Xolair is not indicated for the treatment of acute asthma exacerbations, acute bronchospasm or status
asthmaticus.
Xolair has not been studied in patients with hyperimmunoglobulin E syndrome or allergic
bronchopulmonary aspergillosis or for the prevention of anaphylactic reactions, including those
provoked by food allergy, atopic dermatitis, or allergic rhinitis. Xolair is not indicated for the
treatment of these conditions.
Xolair therapy has not been studied in patients with autoimmune diseases, immune complex-mediated
conditions, or pre-existing renal or hepatic impairment (see section 4.2). Caution should be exercised
when administering Xolair in these patient populations.
Abrupt discontinuation of systemic or inhaled corticosteroids after initiation of Xolair therapy is not
recommended. Decreases in corticosteroids should be performed under the direct supervision of a
physician and may need to be performed gradually.
Immune system disorders
Allergic reactions type I
Type I local or systemic allergic reactions, including anaphylaxis and anaphylactic shock, may occur
when taking omalizumab, also with onset after a long duration of treatment. Most of these reactions
occurred within 2 hours after the first and subsequent injections of Xolair but some started beyond
2 hours and even beyond 24 hours after the injection. Therefore medicinal products for the treatment
of anaphylactic reactions should always be available for immediate use following administration of
Xolair. Patients should be informed that such reactions are possible and prompt medical attention
should be sought if allergic reactions occur.
Anaphylactic reactions were rare in clinical trials (see section 4.8).
Antibodies to omalizumab have been detected in a low number of patients in clinical trials. The
clinical relevance of anti-Xolair antibodies is not well understood.
Serum sickness
Serum sickness and serum sickness-like reactions, which are delayed allergic type III reactions, have
been seen in patients treated with humanised monoclonal antibodies including omalizumab. The
suggested pathophysiologic mechanism includes immune-complex formation and deposition due to
development of antibodies against omalizumab. The onset has typically been 1-5 days after
administration of the first or subsequent injections, also after long duration of treatment. Symptoms
suggestive of serum sickness include arthritis/arthralgias, rash (urticaria or other forms), fever and
lymphadenopathy. Antihistamines and corticosteroids may be useful for preventing or treating this
disorder, and patients should be advised to report any suspected symptoms.
Churg-Strauss syndrome and hypereosinophilic syndrome
Patients with severe asthma may rarely present systemic hypereosinophilic syndrome or allergic
eosinophilic granulomatous vasculitis (Churg-Strauss syndrome), both of which are usually treated
with systemic corticosteroids.
In rare cases, patients on therapy with anti-asthma medicinal products, including omalizumab, may
present or develop systemic eosinophilia and vasculitis. These events are commonly associated with
the reduction of oral corticosteroid therapy.
In these patients, physicians should be alert to the development of marked eosinophilia, vasculitic
rash, worsening pulmonary symptoms, paranasal sinus abnormalities, cardiac complications, and/or
neuropathy.
Discontinuation of omalizumab should be considered in all severe cases with the above mentioned
immune system disorders.
Malignancies
During clinical trials in adults and adolescents 12 years of age and older, there was a numerical
imbalance in cancers arising in the Xolair (0.5%; 25 cancers in 5,015 patients) treatment group
compared with the control (0.18%; 5 cancers in 2,854 patients) group. Malignancies were uncommon
(<1/100) in both the active and the control group. The diversity in the type of cancers observed, the
relatively short duration of exposure and the clinical features of the individual cases render a causal
relationship unlikely. The overall observed incidence rate of malignancy in the Xolair clinical trial
programme was comparable to that reported in the general population.
Parasitic (helminth) infections
IgE may be involved in the immunological response to some helminth infections. In patients at chronic
high risk of helminth infection, a placebo-controlled trial showed a slight increase in infection rate
with omalizumab, although the course, severity, and response to treatment of infection were unaltered.
The helminth infection rate in the overall clinical programme, which was not designed to detect such
infections, was less than 1 in 1,000 patients. However, caution may be warranted in patients at high
risk of helminth infection, in particular when travelling to areas where helminthic infections are
endemic. If patients do not respond to recommended anti-helminth treatment, discontinuation of
Xolair should be considered.
4.5 Interaction with other medicinal products and other forms of interaction
Cytochrome P450 enzymes, efflux pumps and protein-binding mechanisms are not involved in the
clearance of omalizumab; thus, there is little potential for drug-drug interactions. Medicinal product or
vaccine interaction studies have not been performed with Xolair. There is no pharmacological reason
to expect that commonly prescribed medicinal products used in the treatment of asthma will interact
with omalizumab.
In clinical studies Xolair was commonly used in conjunction with inhaled and oral corticosteroids,
inhaled short-acting and long-acting beta agonists, leukotriene modifiers, theophyllines and oral
antihistamines. There was no indication that the safety of Xolair was altered with these other
commonly used anti-asthma medicinal products. Limited data are available on the use of Xolair in
combination with specific immunotherapy (hypo-sensitisation therapy). In a clinical trial where Xolair
was co-administered with immunotherapy, the safety and efficacy of Xolair in combination with
specific immunotherapy were found to be no different to that of Xolair alone.
Xolair may indirectly reduce the efficacy of medicinal products for the treatment of helminthic or
other parasitic infections (see section 4.4).
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no adequate data from the use of omalizumab in pregnant women. Animal studies do not
indicate direct or indirect harmful effects with respect to pregnancy, embryonal/foetal development,
parturition or postnatal development (see section 5.3). Omalizumab crosses the placental barrier and
the potential for harm to the foetus is unknown. Omalizumab has been associated with age-dependent
decreases in blood platelets in non-human primates, with a greater relative sensitivity in juvenile
animals (see section 5.3). Xolair should not be used during pregnancy unless clearly necessary.
Breast-feeding
It is not known whether omalizumab is excreted in human breast milk. Omalizumab is excreted into
non-human primate breast milk and risk to the suckling child cannot be excluded. Women should not
breast-feed during Xolair therapy.
Fertility
There are no human fertility data for omalizumab. In non-clinical studies, no impairment of fertility
and no genotoxicity have been observed (see section 5.3).
4.7 Effects on ability to drive and use machines
Xolair has no or negligible influence on the ability to drive and use machines.
Over 4,400 allergic asthma patients were randomised in controlled efficacy trials with Xolair.
During clinical trials in adult and adolescent patients 12 years of age and older, the most commonly
reported adverse reactions were injection site reactions, including injection site pain, swelling,
erythema and pruritus, and headaches. In clinical trials in children 6 to <12 years of age, the most
commonly reported adverse reactions suspected of being related to the medicinal product were
headache, pyrexia and upper abdominal pain. Most of the reactions were mild or moderate in severity.
Table 4 lists the adverse reactions recorded in clinical studies in the total safety population treated with
Xolair by MedDRA system organ class and frequency. Within each frequency grouping, adverse
reactions are presented in order of decreasing seriousness. Frequency categories are defined as: very
common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to
<1/1,000) and very rare (<1/10,000). Reactions reported in the post-marketing setting are listed with
frequency not known (cannot be estimated from the available data).
Table 4: Adverse reactions
Infections and infestations
Uncommon Pharyngitis
Rare Parasitic infection
Blood and lymphatic system disorders
Not known
Idiopathic severe thrombocytopenia
Immune system disorders
Rare
Serum sickness, may include fever and lymphadenopathy
Nervous system disorders
Common
Syncope, paraesthesia, somnolence, dizziness
Vascular disorders
Uncommon Postural hypotension, flushing
Respiratory, thoracic and mediastinal disorders
Uncommon
Allergic bronchospasm, coughing
Allergic granulomatous vasculitis (i.e. Churg-Strauss syndrome)
Gastrointestinal disorders
Common Abdominal pain upper**
Uncommon Dyspeptic signs and symptoms, diarrhoea, nausea
Skin and subcutaneous tissue disorders
Uncommon Photosensitivity, urticaria, rash, pruritus
Rare Angioedema
Not known Alopecia
Musculoskeletal and connective tissue disorders
Not known Arthralgia, myalgia, joint swelling
General disorders and administration site conditions
Very common Pyrexia**
Common Injection site reactions such as swelling, erythema, pain, pruritus
Uncommon Influenza-like illness, swelling arms, weight increase, fatigue
*: Very common in children 6 to <12 years of age
**: In children 6 to <12 years of age
Immune system disorders
For further information, see section 4.4.
Malignancies
The overall observed incidence rate of malignancy in adults and in adolescents 12 years of age and
older in the Xolair clinical trial programme was comparable to that reported in the general population
(see section 4.4).
There were no cases of malignancy with omalizumab in the clinical trials in children 6 to <12 years of
age; there was a single case of malignancy in the control group.
Anaphylactic reaction, other serious allergic conditions























Arterial thromboembolic events (ATE)
In controlled clinical trials and an ongoing observational study, a numerical imbalance of ATEs was
observed. ATE included stroke, transient ischaemic attack, myocardial infarction, unstable angina, and
cardiovascular death (including death from unknown cause). The rate of ATE in patients in the
controlled clinical trials was 6.29 for Xolair-treated patients (17/2703 patient years) and 3.42 for
control patients (6/1755 patient years). In Cox proportional hazards model, Xolair was not associated
with ATE risk (hazard ratio 1.86; 95% confidence interval 0.73-4.72). In the observational study, the
rate of ATE was 5.59 (79/14140 patients years) for Xolair-treated patients and 3.71 (31/8366 patient
years) for control patients.In a multivariate analysis controlling for baseline cardiovascular risk
factors, Xolair was not associated with ATE risk (hazard ratio 1.11; 95% confidence interval
0.70-1.76).
Platelets
In clinical trials few patients had platelet counts below the lower limit of the normal laboratory range.
None of these changes were associated with bleeding episodes or a decrease in haemoglobin. No
pattern of persistent decrease in platelet counts, as observed in non-human primates (see section 5.3),
has been reported in humans (patients above 6 years of age), even though isolated cases of idiopathic
thrombocytopenia have been reported in the post-marketing setting.
Parasitic infections
In patients at chronic high risk of helminth infection, a placebo-controlled trial showed a slight
numerical increase in infection rate with omalizumab that was not statistically significant. The course,
severity, and response to treatment of infections were unaltered (see section 4.4).
Maximum tolerated dose of Xolair has not been determined. Single intravenous doses up to 4,000 mg
have been administered to patients without evidence of dose-limiting toxicities. The highest
cumulative dose administered to patients was 44,000 mg over a 20-week period and this dose did not
result in any untoward acute effects.
If an overdose is suspected, the patient should be monitored for any abnormal signs or symptoms.
Medical treatment should be sought and instituted appropriately.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Drugs for obstructive airway diseases, other systemic drugs for
obstructive airway diseases, ATC code: R03DX05
Omalizumab is a recombinant DNA-derived humanised monoclonal antibody that selectively binds to
human immunoglobulin E (IgE). The antibody is an IgG1 kappa that contains human framework
regions with the complementary-determining regions of a murine parent antibody that binds to IgE.
Omalizumab binds to IgE and prevents binding of IgE to FCRI (high-affinity IgE receptor), thereby
reducing the amount of free IgE that is available to trigger the allergic cascade. Treatment of atopic
subjects with omalizumab resulted in a marked down-regulation of FCRI receptors on basophils.
Furthermore, the
in vitro
histamine release from basophils isolated from Xolair-treated subjects was
reduced by approximately 90% following stimulation with an allergen compared to pre-treatment
values.
In clinical studies, serum free IgE levels were reduced in a dose-dependent manner within one hour
following the first dose and maintained between doses. One year after discontinuation of Xolair
dosing, the IgE levels had returned to pre-treatment levels with no observed rebound in IgE levels
after washout of the medicinal product.
Clinical experience
Adults and adolescents ≥12 years of age
The efficacy and safety of Xolair were demonstrated in a 28-week double-blind placebo-controlled
study (study 1) involving 419 severe allergic asthmatics, ages 12-79 years, who had reduced lung
function (FEV
1
40-80% predicted) and poor asthma symptom control despite receiving high dose
inhaled corticosteroids and a long-acting beta2-agonist. Eligible patients had experienced multiple
asthma exacerbations requiring systemic corticosteroid treatment or had been hospitalised or attended
an emergency room due to a severe asthma exacerbation in the past year despite continuous treatment
with high-dose inhaled corticosteroids and a long-acting beta2-agonist. Subcutaneous Xolair or
placebo were administered as add-on therapy to >1,000 micrograms beclomethasone dipropionate (or
equivalent) plus a long-acting beta2-agonist. Oral corticosteroid, theophylline and leukotriene-
modifier maintenance therapies were allowed (22%, 27%, and 35% of patients, respectively).
The rate of asthma exacerbations requiring treatment with bursts of systemic corticosteroids was the
primary endpoint. Omalizumab reduced the rate of asthma exacerbations by 19% (p = 0.153). Further
evaluations which did show statistical significance (p<0.05) in favour of Xolair included reductions in
severe exacerbations (where patient‟s lung function was reduced to below 60% of personal best and
requiring systemic corticosteroids) and asthma-related emergency visits (comprised of
hospitalisations, emergency room, and unscheduled doctor visits), and improvements in Physician‟s
overall assessment of treatment effectiveness, Asthma-related Quality of Life (AQL), asthma
symptoms and lung function.
In a subgroup analysis, patients with pre-treatment total IgE ≥76 IU/ml were more likely to experience
clinically meaningful benefit to Xolair. In these patients in study 1 Xolair reduced the rate of asthma
exacerbations by 40% (p = 0.002). In addition more patients had clinically meaningful responses in the
total IgE ≥76 IU/ml population across the Xolair severe asthma programme. Table 5 includes results in
the study 1 population.
Table 5: Results of study 1
% reduction, p-value for rate ratio
Severe asthma exacerbations
% reduction, p-value for rate ratio
% reduction, p-value for rate ratio
Physician’s overall assessment
% of patients ≥0.5 improvement
* marked improvement or complete control
** p-value for overall distribution of assessment
Study 2 assessed the efficacy and safety of Xolair in a population of 312 severe allergic asthmatics
which matched the population in study 1. Treatment with Xolair in this open label study led to a 61%
reduction in clinically significant asthma exacerbation rate compared to current asthma therapy alone.
Four additional large placebo-controlled supportive studies of 28 to 52 weeks duration in 1,722 adults
and adolescents (studies 3, 4, 5, 6) assessed the efficacy and safety of Xolair in patients with severe
persistent asthma. Most patients were inadequately controlled but were receiving less concomitant
asthma therapy than patients in studies 1 or 2. Studies 3-5 used exacerbation as primary endpoint,
whereas study 6 primarily evaluated inhaled corticosteroid sparing.
In studies 3, 4 and 5 patients treated with Xolair had respective reductions in asthma exacerbation rates
of 37.5% (p = 0.027), 40.3% (p<0.001) and 57.6% (p<0.001) compared to placebo.
In study 6, significantly more severe allergic asthma patients on Xolair were able to reduce their
fluticasone dose to 500 micrograms/day without deterioration of asthma control (60.3%) compared to
the placebo group (45.8%, p<0.05).
Quality of life scores were measured using the Juniper Asthma-related Quality of Life Questionnaire.
For all six studies there was a statistically significant improvement from baseline in quality of life
scores for Xolair patients versus the placebo or control group.







Physician‟s overall assessment of treatment effectiveness:
Physician‟s overall assessment was performed in five of the above studies as a broad measure of
asthma control performed by the treating physician. The physician was able to take into account PEF
(peak expiratory flow), day and night time symptoms, rescue medication use, spirometry and
exacerbations. In all five studies a significantly greater proportion of Xolair treated patients were
judged to have achieved either a marked improvement or complete control of their asthma compared
to placebo patients.
Children 6 to <12 years of age
The primary support for safety and efficacy of Xolair in the group aged 6 to <12 years comes from one
randomised, double-blind, placebo-controlled, multi-centre trial (study 7).
Study 7 was a placebo-controlled trial which included a specific subgroup (n=235) of patients as
defined in the present indication, who were treated with high-dose inhaled corticosteroids
(≥500 µg/day fluticasone equivalent) plus long-acting beta agonist.
A clinically significant exacerbation was defined as a worsening of asthma symptoms as judged
clinically by the investigator, requiring doubling of the baseline inhaled corticosteroid dose for at least
3 days and/or treatment with rescue systemic (oral or intravenous) corticosteroids for at least 3 days.
In the specific subgroup of patients on high dose inhaled corticosteroids, the omalizumab group had a
statistically significantly lower rate of clinically significant asthma exacerbations than the placebo
group. At 24 weeks, the difference in rates between treatment groups represented a 34% (rate ratio
0.662, p = 0.047) decrease relative to placebo for omalizumab patients. In the second double-blind 28-
week treatment period the difference in rates between treatment groups represented a 63% (rate ratio
0.37, p<0.001) decrease relative to placebo for omalizumab patients.
During the 52-week double-blind treatment period (including the 24-week fixed-dose steroid phase
and the 28-week steroid adjustment phase) the difference in rates between treatment groups
represented a 50% (rate ratio 0.504, p<0.001) relative decrease in exacerbations for omalizumab
patients.
The omalizumab group showed greater decreases in beta-agonist rescue medication use than the
placebo group at the end of the 52-week treatment period, although the difference between treatment
groups was not statistically significant. For the global evaluation of treatment effectiveness at the end
of the 52-week double-blind treatment period in the subgroup of severe patients on high-dose inhaled
corticosteroids plus long-acting beta agonists, the proportion of patients rated as having „excellent‟
treatment effectiveness was higher, and the proportions having „moderate‟ or „poor‟ treatment
effectiveness lower in the omalizumab group compared to the placebo group; the difference between
groups was statistically significant (p<0.001), while there were no differences between the
omalizumab and placebo groups for patients‟ subjective Quality of Life ratings.
5.2 Pharmacokinetic properties
The pharmacokinetics of omalizumab have been studied in adult and adolescent patients with allergic
asthma.
Absorption
After subcutaneous administration, omalizumab is absorbed with an average absolute bioavailability
of 62%. Following a single subcutaneous dose in adult and adolescent patients with asthma,
omalizumab was absorbed slowly, reaching peak serum concentrations after an average of 7-8 days.
The pharmacokinetics of omalizumab are linear at doses greater than 0.5 mg/kg. Following multiple
doses of omalizumab, areas under the serum concentration-time curve from Day 0 to Day 14 at steady
state were up to 6-fold of those after the first dose.
Distribution
In vitro
, omalizumab forms complexes of limited size with IgE. Precipitating complexes and
complexes larger than one million Daltons in molecular weight are not observed
in vitro
or
in vivo
.
The apparent volume of distribution in patients following subcutaneous administration was
78 ± 32 ml/kg.
Elimination
Clearance of omalizumab involves IgG clearance processes as well as clearance via specific binding
and complex formation with its target ligand, IgE. Liver elimination of IgG includes degradation in the
reticuloendothelial system and endothelial cells. Intact IgG is also excreted in bile. In asthma patients
the omalizumab serum elimination half-life averaged 26 days, with apparent clearance averaging
2.4 1.1 ml/kg/day. In addition, doubling of body weight approximately doubled apparent clearance.
Characteristics in patient populations
Age, Race/Ethnicity, Gender, Body Mass Index
The population pharmacokinetics of Xolair were analysed to evaluate the effects of demographic
characteristics. Analyses of these limited data suggest that no dose adjustments are necessary for age
(6-76 years), race/ethnicity, gender or Body Mass Index (see section 4.2).
Renal and hepatic impairment
There are no pharmacokinetic or pharmacodynamic data in patients with renal or hepatic impairment
(see sections 4.2 and 4.4).
5.3 Preclinical safety data
The safety of omalizumab has been studied in the cynomolgus monkey, since omalizumab binds to
cynomolgus and human IgE with similar affinity. Antibodies to omalizumab were detected in some
monkeys following repeated subcutaneous or intravenous administration. However, no apparent
toxicity, such as immune complex-mediated disease or complement-dependent cytotoxicity, was seen.
There was no evidence of an anaphylactic response due to mast-cell degranulation in cynomolgus
monkeys.
Chronic administration of high dose levels (up to 250 mg/kg) of omalizumab was well tolerated in
non-human primates (both adult and juvenile animals), with the exception of a dose-related and age-
dependent decrease in blood platelets, with a greater sensitivity in juvenile animals. The serum
concentration required to attain a 50% drop in platelets from baseline in adult cynomolgus monkeys
was roughly 4- to 20-fold higher than anticipated maximum clinical serum concentrations. In addition,
acute haemorrhage and inflammation were observed at injection sites in cynomolgus monkeys.
Formal carcinogenicity studies have not been conducted with omalizumab.
In reproduction studies in cynomolgus monkeys, subcutaneous doses up to 75 mg/kg (about 12-fold
exposure ratio based on 28-day AUC values at 75 mg/kg versus the clinical maximum dose) did not
elicit maternal toxicity, embryotoxicity or teratogenicity when administered throughout organogenesis
and did not elicit adverse effects on foetal or neonatal growth when administered throughout late
gestation, delivery and nursing.
Omalizumab is excreted in milk in cynomolgus monkeys. Milk levels of omalizumab were 1.5% of the
maternal blood concentration.
PHARMACEUTICAL PARTICULARS
Powder
Sucrose
Histidine
Histidine hydrochloride monohydrate
Polysorbate 20
Solvent
Water for injections
This medicinal product must not be mixed with other medicinal products except those mentioned in
section 6.6.
After reconstitution: The chemical and physical stability of the reconstituted medicinal product have
been demonstrated for 8 hours at 2°C to 8°C and for 4 hours at 30°C.
From a microbiological point of view, the medicinal product should be used immediately after
reconstitution. If not used immediately, in-use storage times and conditions prior to use are the
responsibility of the user and would normally not be longer than 8 hours at 2°C to 8°C or 4 hours at
30°C.
6.4 Special precautions for storage
Store in a refrigerator (2C - 8C).
Do not freeze.
For storage conditions of the reconstituted medicinal product, see section 6.3.
6.5 Nature and contents of container
Powder vial: Clear, colourless type I glass vial with a butyl rubber stopper and blue flip-off seal.
Solvent ampoule: Clear, colourless type I glass ampoule containing 2 ml water for injections.
Xolair 150 mg powder and solvent for solution for injection is supplied as packs containing 1, 4 or
10 vials of powder and 1, 4 or 10 ampoules of water for injections, respectively.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
The lyophilised medicinal product takes 15-20 minutes to dissolve, although in some cases it may take
longer. The fully reconstituted medicinal product will appear clear or slightly opaque and may have a
few small bubbles or foam around the edge of the vial. Because of the viscosity of the reconstituted
medicinal product care must be taken to withdraw all of the medicinal product from the vial before
expelling any air or excess solution from the syringe in order to obtain the 1.2 ml.
To prepare Xolair 150 mg vials for subcutaneous administration, please adhere to the following
instructions:
Draw 1.4 ml of water for injections from the ampoule into a syringe equipped with a large-bore
18-gauge needle.
With the vial placed upright on a flat surface, insert the needle and transfer the water for
injections into the vial containing the lyophilised powder using standard aseptic techniques,
directing the water for injections directly on to the powder.
Keeping the vial in an upright position, vigorously swirl it (do not shake) for approximately
1 minute to evenly wet the powder.
To aid in dissolution after completing step 3, gently swirl the vial for 5-10 seconds
approximately every 5 minutes in order to dissolve any remaining solids.
Note that in some cases it may take longer than 20 minutes for the powder to dissolve
completely. If this is the case, repeat step 4 until there are no visible gel-like particles in the
solution.
When the medicinal product is fully dissolved, there should be no visible gel-like particles in
the solution. Small bubbles or foam around the edge of the vial are common. The reconstituted
medicinal product will appear clear or slightly opaque. Do not use if solid particles are present.
Invert the vial for at least 15 seconds in order to allow the solution to drain towards the stopper.
Using a new 3-ml syringe equipped with a large-bore, 18-gauge needle, insert the needle into
the inverted vial. Keeping the vial inverted position the needle tip at the very bottom of the
solution in the vial when drawing the solution into the syringe. Before removing the needle
from the vial, pull the plunger all the way back to the end of the syringe barrel in order to
remove all of the solution from the inverted vial.
Replace the 18-gauge needle with a 25-gauge needle for subcutaneous injection.
Expel air, large bubbles, and any excess solution in order to obtain the required 1.2 ml dose. A
thin layer of small bubbles may remain at the top of the solution in the syringe. Because the
solution is slightly viscous, it may take 5-10 seconds to administer the solution by subcutaneous
injection.
The vial delivers 1.2 ml (150 mg) of Xolair. For a 75 mg dose, draw up 0.6 ml into the syringe
and discard the remaining solution.
The injections are administered subcutaneously in the deltoid region of the arm or the thigh.
Xolair 150 mg powder for solution for injection is supplied in a single-use vial.
From a microbiological point of view, the medicinal product should be used immediately after
reconstitution (see section 6.3).
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
MARKETING AUTHORISATION NUMBER(S)
EU/1/05/319/002
EU/1/05/319/003
EU/1/05/319/004
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 25/10/2005
Date of latest renewal: 25/10/2010
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
Agency http://www.ema.europa.eu
NAME OF THE MEDICINAL PRODUCT
Xolair 75 mg solution for injection
Omalizumab
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each pre-filled syringe of 0.5 ml solution contains 75 mg of omalizumab*.
*Omalizumab is a humanised monoclonal antibody manufactured by recombinant DNA technology in
a Chinese hamster ovary (CHO) mammalian cell line.
For a full list of excipients, see section 6.1.
Clear to opalescent, slightly yellow to brown solution.
4.1 Therapeutic indications
Xolair is indicated in adults, adolescents and children (6 to <12 years of age).
Xolair treatment should only be considered for patients with convincing IgE (immunoglobulin E)
mediated asthma (see section 4.2).
Adults and adolescents (12 years of age and older)
Xolair is indicated as add-on therapy to improve asthma control in patients with severe persistent
allergic asthma who have a positive skin test or
in vitro
reactivity to a perennial aeroallergen and who
have reduced lung function (FEV
1
<80%) as well as frequent daytime symptoms or night-time
awakenings and who have had multiple documented severe asthma exacerbations despite daily high-
dose inhaled corticosteroids, plus a long-acting inhaled beta2-agonist.
Children (6 to <12 years of age)
Xolair is indicated as add-on therapy to improve asthma control in patients with severe persistent
allergic asthma who have a positive skin test or
in vitro
reactivity to a perennial aeroallergen and
frequent daytime symptoms or night-time awakenings and who have had multiple documented severe
asthma exacerbations despite daily high-dose inhaled corticosteroids, plus a long-acting inhaled beta2-
agonist.
4.2 Posology and method of administration
Xolair treatment should be initiated by physicians experienced in the diagnosis and treatment of severe
persistent asthma.
Posology
The appropriate dose and frequency of Xolair is determined by baseline IgE (IU/ml), measured before
the start of treatment, and body weight (kg). Prior to administration of the initial dose, patients should
have their IgE level determined by any commercial serum total IgE assay for their dose assignment.
Based on these measurements, 75 to 600 mg of Xolair in 1 to 4 injections may be needed for each
administration.
Patients with IgE lower than 76 IU/ml were less likely to experience benefit (see section 5.1).
Prescribing physicians should ensure that adult and adolescent patients with IgE below 76 IU/ml and
children (6 to < 12 years of age) with IgE below 200 IU/ml have unequivocal
in vitro
reactivity
(RAST) to a perennial allergen before starting therapy.
See Table 1 for a conversion chart and Tables 2 and 3 for the dose determination charts in adults,
adolescents and children (6 to <12 years of age).
Patients whose baseline IgE levels or body weight in kilograms are outside the limits of the dose table
should not be given Xolair.
The maximum recommended dose is 600 mg omalizumab every two weeks.
Table 1: Conversion from dose to number of syringes, number of injections and total injection
volume for each administration
Dose (mg) Number of syringes
Total injection volume (ml)
Table 2: ADMINISTRATION EVERY 4 WEEKS. Xolair doses (milligrams per dose)
administered by subcutaneous injection every 4 weeks
>20-25 >25-30 >30-40 >40-50 >50-60 >60-70 >70-80 >80-90
ADMINISTRATION EVERY 2 WEEKS
SEE TABLE 3































Special populations
Elderly (65 years of age and older)
There are limited data available on the use of Xolair in patients older than 65 years but there is no
evidence that elderly patients require a different dose from younger adult patients.
Renal or hepatic impairment
There have been no studies on the effect of impaired renal or hepatic function on the pharmacokinetics
of Xolair. Because omalizumab clearance at clinical doses is dominated by the reticular endothelial
system (RES) it is unlikely to be altered by renal or hepatic impairment. While no particular dose
adjustment is recommended for these patients, Xolair should be administered with caution (see section
4.4).
Paediatric population
The safety and efficacy of Xolair in children below age 6 have not been established. No data are
available.
Method of administration
For subcutaneous administration only. Do not administer by the intravenous or intramuscular route.
The injections are administered subcutaneously in the deltoid region of the arm. Alternatively, the
injections can be administered in the thigh if there is any reason precluding administration in the
deltoid region.
There is limited experience with self-administration of Xolair. Therefore treatment is intended to be
administered by a healthcare provider only (see section 6.6 and also information for the healthcare
professional section of the package leaflet).
Hypersensitivity to the active substance or to any of the excipients.
4.4 Special warnings and precautions for use
General
Xolair is not indicated for the treatment of acute asthma exacerbations, acute bronchospasm or status
asthmaticus.
Xolair has not been studied in patients with hyperimmunoglobulin E syndrome or allergic
bronchopulmonary aspergillosis or for the prevention of anaphylactic reactions, including those
provoked by food allergy, atopic dermatitis, or allergic rhinitis. Xolair is not indicated for the
treatment of these conditions.
Xolair therapy has not been studied in patients with autoimmune diseases, immune complex-mediated
conditions, or pre-existing renal or hepatic impairment (see section 4.2). Caution should be exercised
when administering Xolair in these patient populations.
Abrupt discontinuation of systemic or inhaled corticosteroids after initiation of Xolair therapy is not
recommended. Decreases in corticosteroids should be performed under the direct supervision of a
physician and may need to be performed gradually.
Immune system disorders
Allergic reactions type I
Type I local or systemic allergic reactions, including anaphylaxis and anaphylactic shock, may occur
when taking omalizumab, also with onset after a long duration of treatment. Most of these reactions
occurred within 2 hours after the first and subsequent injections of Xolair but some started beyond
2 hours and even beyond 24 hours after the injection. Therefore medicinal products for the treatment
of anaphylactic reactions should always be available for immediate use following administration of
Xolair. Patients should be informed that such reactions are possible and prompt medical attention
should be sought if allergic reactions occur.
Anaphylactic reactions were rare in clinical trials (see section 4.8).
Antibodies to omalizumab have been detected in a low number of patients in clinical trials. The
clinical relevance of anti-Xolair antibodies is not well understood.
Serum sickness
Serum sickness and serum sickness-like reactions, which are delayed allergic type III reactions, have
been seen in patients treated with humanised monoclonal antibodies including omalizumab. The
suggested pathophysiologic mechanism includes immune-complex formation and deposition due to
development of antibodies against omalizumab. The onset has typically been 1-5 days after
administration of the first or subsequent injections, also after long duration of treatment. Symptoms
suggestive of serum sickness include arthritis/arthralgias, rash (urticaria or other forms), fever and
lymphadenopathy. Antihistamines and corticosteroids may be useful for preventing or treating this
disorder, and patients should be advised to report any suspected symptoms.
Churg-Strauss syndrome and hypereosinophilic syndrome
Patients with severe asthma may rarely present systemic hypereosinophilic syndrome or allergic
eosinophilic granulomatous vasculitis (Churg-Strauss syndrome), both of which are usually treated
with systemic corticosteroids.
In rare cases, patients on therapy with anti-asthma medicinal products, including omalizumab, may
present or develop systemic eosinophilia and vasculitis. These events are commonly associated with
the reduction of oral corticosteroid therapy.
In these patients, physicians should be alert to the development of marked eosinophilia, vasculitic
rash, worsening pulmonary symptoms, paranasal sinus abnormalities, cardiac complications, and/or
neuropathy.
Discontinuation of omalizumab should be considered in all severe cases with the above mentioned
immune system disorders.
Malignancies
During clinical trials in adults and adolescents 12 years of age and older, there was a numerical
imbalance in cancers arising in the Xolair (0.5%; 25 cancers in 5,015 patients) treatment group
compared with the control (0.18%; 5 cancers in 2,854 patients) group. Malignancies were uncommon
(<1/100) in both the active and the control group. The diversity in the type of cancers observed, the
relatively short duration of exposure and the clinical features of the individual cases render a causal
relationship unlikely. The overall observed incidence rate of malignancy in the Xolair clinical trial
programme was comparable to that reported in the general population.
Parasitic (helminth) infections
IgE may be involved in the immunological response to some helminth infections. In patients at chronic
high risk of helminth infection, a placebo-controlled trial showed a slight increase in infection rate
with omalizumab, although the course, severity, and response to treatment of infection were unaltered.
The helminth infection rate in the overall clinical programme, which was not designed to detect such
infections, was less than 1 in 1,000 patients. However, caution may be warranted in patients at high
risk of helminth infection, in particular when travelling to areas where helminthic infections are
endemic. If patients do not respond to recommended anti-helminth treatment, discontinuation of
Xolair should be considered.
4.5 Interaction with other medicinal products and other forms of interaction
Cytochrome P450 enzymes, efflux pumps and protein-binding mechanisms are not involved in the
clearance of omalizumab; thus, there is little potential for drug-drug interactions. Medicinal product or
vaccine interaction studies have not been performed with Xolair. There is no pharmacological reason
to expect that commonly prescribed medicinal products used in the treatment of asthma will interact
with omalizumab.
In clinical studies Xolair was commonly used in conjunction with inhaled and oral corticosteroids,
inhaled short-acting and long-acting beta agonists, leukotriene modifiers, theophyllines and oral
antihistamines. There was no indication that the safety of Xolair was altered with these other
commonly used anti-asthma medicinal products. Limited data are available on the use of Xolair in
combination with specific immunotherapy (hypo-sensitisation therapy). In a clinical trial where Xolair
was co-administered with immunotherapy, the safety and efficacy of Xolair in combination with
specific immunotherapy were found to be no different to that of Xolair alone.
Xolair may indirectly reduce the efficacy of medicinal products for the treatment of helminthic or
other parasitic infections (see section 4.4).
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no adequate data from the use of omalizumab in pregnant women. Animal studies do not
indicate direct or indirect harmful effects with respect to pregnancy, embryonal/foetal development,
parturition or postnatal development (see section 5.3). Omalizumab crosses the placental barrier and
the potential for harm to the foetus is unknown. Omalizumab has been associated with age-dependent
decreases in blood platelets in non-human primates, with a greater relative sensitivity in juvenile
animals (see section 5.3). Xolair should not be used during pregnancy unless clearly necessary.
Breast-feeding
It is not known whether omalizumab is excreted in human breast milk. Omalizumab is excreted into
non-human primate breast milk and risk to the suckling child cannot be excluded. Women should not
breast-feed during Xolair therapy.
Fertility
There are no human fertility data for omalizumab. In non-clinical studies, no impairment of fertility
and no genotoxicity have been observed (see section 5.3).
4.7 Effects on ability to drive and use machines
Xolair has no or negligible influence on the ability to drive and use machines.
Over 4,400 allergic asthma patients were randomised in controlled efficacy trials with Xolair.
During clinical trials in adult and adolescent patients 12 years of age and older, the most commonly
reported adverse reactions were injection site reactions, including injection site pain, swelling,
erythema and pruritus, and headaches. In clinical trials in children 6 to <12 years of age, the most
commonly reported adverse reactions suspected of being related to the medicinal product were
headache, pyrexia and upper abdominal pain. Most of the reactions were mild or moderate in severity.
Table 4 lists the adverse reactions recorded in clinical studies in the total safety population treated with
Xolair by MedDRA system organ class and frequency. Within each frequency grouping, adverse
reactions are presented in order of decreasing seriousness. Frequency categories are defined as: very
common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to
<1/1,000) and very rare (<1/10,000). Reactions reported in the post-marketing setting are listed with
frequency not known (cannot be estimated from the available data).
Table 4: Adverse reactions
Infections and infestations
Uncommon Pharyngitis
Rare Parasitic infection
Blood and lymphatic system disorders
Not known
Idiopathic severe thrombocytopenia
Immune system disorders
Rare
Anaphylactic reaction, other serious allergic conditions
Serum sickness, may include fever and lymphadenopathy
Nervous system disorders
Common
Syncope, paraesthesia, somnolence, dizziness
Vascular disorders
Uncommon Postural hypotension, flushing
Respiratory, thoracic and mediastinal disorders
Uncommon
Allergic bronchospasm, coughing
Allergic granulomatous vasculitis (i.e. Churg-Strauss syndrome)
Gastrointestinal disorders
Common Abdominal pain upper**
Uncommon Dyspeptic signs and symptoms, diarrhoea, nausea
Skin and subcutaneous tissue disorders
Uncommon Photosensitivity, urticaria, rash, pruritus
Rare Angioedema
Not known Alopecia
Musculoskeletal and connective tissue disorders
Not known
Arthralgia, myalgia, joint swelling
General disorders and administration site conditions
Very common Pyrexia**
Common Injection site reactions such as swelling, erythema, pain, pruritus
Uncommon Influenza-like illness, swelling arms, weight increase, fatigue
*: Very common in children 6 to <12 years of age
**: In children 6 to <12 years of age
Immune system disorders
For further information, see section 4.4.
Malignancies
The overall observed incidence rate of malignancy in adults and in adolescents 12 years of age and
older in the Xolair clinical trial programme was comparable to that reported in the general population
(see section 4.4).
There were no cases of malignancy with omalizumab in the clinical trials in children 6 to <12 years of
age; there was a single case of malignancy in the control group.






















Arterial thromboembolic events (ATE)
In controlled clinical trials and an ongoing observational study, a numerical imbalance of ATEs was
observed. ATE included stroke, transient ischaemic attack, myocardial infarction, unstable angina, and
cardiovascular death (including death from unknown cause). The rate of ATE in patients in the
controlled clinical trials was 6.29 for Xolair-treated patients (17/2703 patient years) and 3.42 for
control patients (6/1755 patient years). In Cox proportional hazards model, Xolair was not associated
with ATE risk (hazard ratio 1.86; 95% confidence interval 0.73-4.72). In the observational study, the
rate of ATE was 5.59 (79/14140 patients years) for Xolair-treated patients and 3.71 (31/8366 patient
years) for control patients.In a multivariate analysis controlling for baseline cardiovascular risk
factors, Xolair was not associated with ATE risk (hazard ratio 1.11; 95% confidence interval
0.70-1.76).
Platelets
In clinical trials few patients had platelet counts below the lower limit of the normal laboratory range.
None of these changes were associated with bleeding episodes or a decrease in haemoglobin. No
pattern of persistent decrease in platelet counts, as observed in non-human primates (see section 5.3),
has been reported in humans (patients above 6 years of age), even though isolated cases of idiopathic
thrombocytopenia have been reported in the post-marketing setting.
Parasitic infections
In patients at chronic high risk of helminth infection, a placebo-controlled trial showed a slight
numerical increase in infection rate with omalizumab that was not statistically significant. The course,
severity, and response to treatment of infections were unaltered (see section 4.4).
Maximum tolerated dose of Xolair has not been determined. Single intravenous doses up to 4,000 mg
have been administered to patients without evidence of dose-limiting toxicities. The highest
cumulative dose administered to patients was 44,000 mg over a 20-week period and this dose did not
result in any untoward acute effects.
If an overdose is suspected, the patient should be monitored for any abnormal signs or symptoms.
Medical treatment should be sought and instituted appropriately.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Drugs for obstructive airway diseases, other systemic drugs for
obstructive airway diseases, ATC code: R03DX05
Omalizumab is a recombinant DNA-derived humanised monoclonal antibody that selectively binds to
human immunoglobulin E (IgE). The antibody is an IgG1 kappa that contains human framework
regions with the complementary-determining regions of a murine parent antibody that binds to IgE.
Omalizumab binds to IgE and prevents binding of IgE to FCRI (high-affinity IgE receptor), thereby
reducing the amount of free IgE that is available to trigger the allergic cascade. Treatment of atopic
subjects with omalizumab resulted in a marked down-regulation of FCRI receptors on basophils.
Furthermore, the
in vitro
histamine release from basophils isolated from Xolair-treated subjects was
reduced by approximately 90% following stimulation with an allergen compared to pre-treatment
values.
In clinical studies, serum free IgE levels were reduced in a dose-dependent manner within one hour
following the first dose and maintained between doses. One year after discontinuation of Xolair
dosing, the IgE levels had returned to pre-treatment levels with no observed rebound in IgE levels
after washout of the medicinal product.
Clinical experience
Adults and adolescents ≥12 years of age
The efficacy and safety of Xolair were demonstrated in a 28-week double-blind placebo-controlled
study (study 1) involving 419 severe allergic asthmatics, ages 12-79 years, who had reduced lung
function (FEV
1
40-80% predicted) and poor asthma symptom control despite receiving high dose
inhaled corticosteroids and a long-acting beta2-agonist. Eligible patients had experienced multiple
asthma exacerbations requiring systemic corticosteroid treatment or had been hospitalised or attended
an emergency room due to a severe asthma exacerbation in the past year despite continuous treatment
with high-dose inhaled corticosteroids and a long-acting beta2-agonist. Subcutaneous Xolair or
placebo were administered as add-on therapy to >1,000 micrograms beclomethasone dipropionate (or
equivalent) plus a long-acting beta2-agonist. Oral corticosteroid, theophylline and leukotriene-
modifier maintenance therapies were allowed (22%, 27%, and 35% of patients, respectively).
The rate of asthma exacerbations requiring treatment with bursts of systemic corticosteroids was the
primary endpoint. Omalizumab reduced the rate of asthma exacerbations by 19% (p = 0.153). Further
evaluations which did show statistical significance (p<0.05) in favour of Xolair included reductions in
severe exacerbations (where patient‟s lung function was reduced to below 60% of personal best and
requiring systemic corticosteroids) and asthma-related emergency visits (comprised of
hospitalisations, emergency room, and unscheduled doctor visits), and improvements in Physician‟s
overall assessment of treatment effectiveness, Asthma-related Quality of Life (AQL), asthma
symptoms and lung function.
In a subgroup analysis, patients with pre-treatment total IgE ≥76 IU/ml were more likely to experience
clinically meaningful benefit to Xolair. In these patients in study 1 Xolair reduced the rate of asthma
exacerbations by 40% (p = 0.002). In addition more patients had clinically meaningful responses in the
total IgE ≥76 IU/ml population across the Xolair severe asthma programme. Table 5 includes results in
the study 1 population.
Table 5: Results of study 1
% reduction, p-value for rate ratio
Severe asthma exacerbations
% reduction, p-value for rate ratio
% reduction, p-value for rate ratio
Physician’s overall assessment
% of patients ≥0.5 improvement
* marked improvement or complete control
** p-value for overall distribution of assessment







Study 2 assessed the efficacy and safety of Xolair in a population of 312 severe allergic asthmatics
which matched the population in study 1. Treatment with Xolair in this open label study led to a 61%
reduction in clinically significant asthma exacerbation rate compared to current asthma therapy alone.
Four additional large placebo-controlled supportive studies of 28 to 52 weeks duration in 1,722 adults
and adolescents (studies 3, 4, 5, 6) assessed the efficacy and safety of Xolair in patients with severe
persistent asthma. Most patients were inadequately controlled but were receiving less concomitant
asthma therapy than patients in studies 1 or 2. Studies 3-5 used exacerbation as primary endpoint,
whereas study 6 primarily evaluated inhaled corticosteroid sparing.
In studies 3, 4 and 5 patients treated with Xolair had respective reductions in asthma exacerbation rates
of 37.5% (p = 0.027), 40.3% (p<0.001) and 57.6% (p<0.001) compared to placebo.
In study 6, significantly more severe allergic asthma patients on Xolair were able to reduce their
fluticasone dose to 500 micrograms/day without deterioration of asthma control (60.3%) compared to
the placebo group (45.8%, p<0.05).
Quality of life scores were measured using the Juniper Asthma-related Quality of Life Questionnaire.
For all six studies there was a statistically significant improvement from baseline in quality of life
scores for Xolair patients versus the placebo or control group.
Physician‟s overall assessment of treatment effectiveness:
Physician‟s overall assessment was performed in five of the above studies as a broad measure of
asthma control performed by the treating physician. The physician was able to take into account PEF
(peak expiratory flow), day and night time symptoms, rescue medication use, spirometry and
exacerbations. In all five studies a significantly greater proportion of Xolair treated patients were
judged to have achieved either a marked improvement or complete control of their asthma compared
to placebo patients.
Children 6 to <12 years of age
The primary support for safety and efficacy of Xolair in the group aged 6 to <12 years comes from one
randomised, double-blind, placebo-controlled, multi-centre trial (study 7).
Study 7 was a placebo-controlled trial which included a specific subgroup (n=235) of patients as
defined in the present indication, who were treated with high-dose inhaled corticosteroids
(≥500 µg/day fluticasone equivalent) plus long-acting beta agonist.
A clinically significant exacerbation was defined as a worsening of asthma symptoms as judged
clinically by the investigator, requiring doubling of the baseline inhaled corticosteroid dose for at least
3 days and/or treatment with rescue systemic (oral or intravenous) corticosteroids for at least 3 days.
In the specific subgroup of patients on high dose inhaled corticosteroids, the omalizumab group had a
statistically significantly lower rate of clinically significant asthma exacerbations than the placebo
group. At 24 weeks, the difference in rates between treatment groups represented a 34% (rate ratio
0.662, p = 0.047) decrease relative to placebo for omalizumab patients. In the second double-blind 28-
week treatment period the difference in rates between treatment groups represented a 63% (rate ratio
0.37, p<0.001) decrease relative to placebo for omalizumab patients.
During the 52-week double-blind treatment period (including the 24-week fixed-dose steroid phase
and the 28-week steroid adjustment phase) the difference in rates between treatment groups
represented a 50% (rate ratio 0.504, p<0.001) relative decrease in exacerbations for omalizumab
patients.
The omalizumab group showed greater decreases in beta-agonist rescue medication use than the
placebo group at the end of the 52-week treatment period, although the difference between treatment
groups was not statistically significant. For the global evaluation of treatment effectiveness at the end
of the 52-week double-blind treatment period in the subgroup of severe patients on high-dose inhaled
corticosteroids plus long-acting beta agonists, the proportion of patients rated as having „excellent‟
treatment effectiveness was higher, and the proportions having „moderate‟ or „poor‟ treatment
effectiveness lower in the omalizumab group compared to the placebo group; the difference between
groups was statistically significant (p<0.001), while there were no differences between the
omalizumab and placebo groups for patients‟ subjective Quality of Life ratings.
5.2 Pharmacokinetic properties
The pharmacokinetics of omalizumab have been studied in adult and adolescent patients with allergic
asthma.
Absorption
After subcutaneous administration, omalizumab is absorbed with an average absolute bioavailability
of 62%. Following a single subcutaneous dose in adult and adolescent patients with asthma,
omalizumab was absorbed slowly, reaching peak serum concentrations after an average of 7-8 days.
The pharmacokinetics of omalizumab are linear at doses greater than 0.5 mg/kg. Following multiple
doses of omalizumab, areas under the serum concentration-time curve from Day 0 to Day 14 at steady
state were up to 6-fold of those after the first dose.
Distribution
In vitro
, omalizumab forms complexes of limited size with IgE. Precipitating complexes and
complexes larger than one million Daltons in molecular weight are not observed
in vitro
or
in vivo
.
The apparent volume of distribution in patients following subcutaneous administration was
78 ± 32 ml/kg.
Elimination
Clearance of omalizumab involves IgG clearance processes as well as clearance via specific binding
and complex formation with its target ligand, IgE. Liver elimination of IgG includes degradation in the
reticuloendothelial system and endothelial cells. Intact IgG is also excreted in bile. In asthma patients
the omalizumab serum elimination half-life averaged 26 days, with apparent clearance averaging
2.4 1.1 ml/kg/day. In addition, doubling of body weight approximately doubled apparent clearance.
Characteristics in patient populations
Age, Race/Ethnicity, Gender, Body Mass Index
The population pharmacokinetics of Xolair were analysed to evaluate the effects of demographic
characteristics. Analyses of these limited data suggest that no dose adjustments are necessary for age
(6-76 years), race/ethnicity, gender or Body Mass Index (see section 4.2).
Renal and hepatic impairment
There are no pharmacokinetic or pharmacodynamic data in patients with renal or hepatic impairment
(see sections 4.2 and 4.4).
5.3 Preclinical safety data
The safety of omalizumab has been studied in the cynomolgus monkey, since omalizumab binds to
cynomolgus and human IgE with similar affinity. Antibodies to omalizumab were detected in some
monkeys following repeated subcutaneous or intravenous administration. However, no apparent
toxicity, such as immune complex-mediated disease or complement-dependent cytotoxicity, was seen.
There was no evidence of an anaphylactic response due to mast-cell degranulation in cynomolgus
monkeys.
Chronic administration of high dose levels (up to 250 mg/kg) of omalizumab was well tolerated in
non-human primates (both adult and juvenile animals), with the exception of a dose-related and age-
dependent decrease in blood platelets, with a greater sensitivity in juvenile animals. The serum
concentration required to attain a 50% drop in platelets from baseline in adult cynomolgus monkeys
was roughly 4- to 20-fold higher than anticipated maximum clinical serum concentrations. In addition,
acute haemorrhage and inflammation were observed at injection sites in cynomolgus monkeys.
Formal carcinogenicity studies have not been conducted with omalizumab.
In reproduction studies in cynomolgus monkeys, subcutaneous doses up to 75 mg/kg (about 12-fold
exposure ratio based on 28-day AUC values at 75 mg/kg versus the clinical maximum dose) did not
elicit maternal toxicity, embryotoxicity or teratogenicity when administered throughout organogenesis
and did not elicit adverse effects on foetal or neonatal growth when administered throughout late
gestation, delivery and nursing.
Omalizumab is excreted in breast milk in cynomolgus monkeys. Milk levels of omalizumab were
1.5% of the maternal blood concentration.
PHARMACEUTICAL PARTICULARS
L-arginine hydrochloride
L-histidine hydrochloride
L-histidine
Polysorbate 20
Water for injections
This medicinal product must not be mixed with other medicinal products.
6.4 Special precautions for storage
Store in a refrigerator (2C – 8C).
Do not freeze.
Store in the original package in order to protect from light.
6.5 Nature and contents of container
0.5 ml solution in a pre-filled syringe barrel (type I glass) with staked needle (stainless steel), (type I)
plunger stopper (latex-free rubber) and needle shield (latex-free). Pack sizes of 1, 4 or 10.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
Prior to completion of the injection, avoid contact with the device activation clips to keep from
prematurely covering the needle with the needle guard.
Pull off the needle cap from the syringe and discard it. Do not touch the exposed needle.
Gently pinch the skin at the injection site and insert the needle.
Holding onto the finger flange, slowly depress the plunger as far as it will go. If any solution
leaks from the injection site, insert the needle further.
Keeping the plunger fully depressed, carefully lift the needle straight out from the injection site.
Slowly release the plunger and allow the needle guard to automatically cover the exposed
needle.
Disposal instructions
Dispose of the used syringe immediately in a sharps container.
MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
MARKETING AUTHORISATION NUMBER(S)
EU/1/05/319/005
EU/1/05/319/006
EU/1/05/319/007
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 25/10/2005
Date of latest renewal: 25/10/2010
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
Agency http://www.ema.europa.eu
The active substance is omalizumab. One syringe of 0.5 ml solution contains 75 mg
omalizumab. One syringe of 1 ml solution contains 150 mg omalizumab.
The other ingredients are L-arginine hydrochloride, L-histidine hydrochloride, L-histidine,
Polysorbate 20 and water for injections.
What Xolair looks like and contents of the pack
Xolair solution for injection is supplied as a clear to opalescent, slightly yellow to brown solution in a
pre-filled syringe, and is available as 75 mg and 150 mg solution for injection.
Xolair 75 mg solution for injection is available in packs containing 1 pre-filled syringe and in
multipacks comprising 4 or 10 intermediate packs, each containing 1 pre-filled syringe.
Xolair 150 mg solution for injection is available in packs containing 1 pre-filled syringe and in
multipacks comprising 4 or 10 intermediate packs, each containing 1 pre-filled syringe.
Not all pack sizes may be marketed in your country.
Marketing Authorisation Holder
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
Manufacturer
Novartis Pharma S.A.S.
Centre de Biotechnologie
8, rue de l‟Industrie
F-68330 Huningue
France
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder.
België/Belgique/Belgien
Novartis Pharma N.V.
Tél/Tel: +32 2 246 16 11
Luxembourg/Luxemburg
Novartis Pharma N.V.
Tél/Tel: +32 2 246 16 11
България
Novartis Pharma Services Inc.
Тел.: +359 2 489 98 28
Magyarország
Novartis Hungária Kft. Pharma
Tel.: +36 1 457 65 00
Česká republika
Novartis s.r.o.
Tel: +420 225 775 111
Malta
Novartis Pharma Services Inc.
Tel: +356 2298 3217
Danmark
Novartis Healthcare A/S
Tlf: +45 39 16 84 00
Nederland
Novartis Pharma B.V.
Tel: +31 26 37 82 111
Deutschland
Novartis Pharma GmbH
Tel: +49 911 273 0
Norge
Novartis Norge AS
Tlf: +47 23 05 20 00
Eesti
Novartis Pharma Services Inc.
Tel: +372 66 30 810
Österreich
Novartis Pharma GmbH
Tel: +43 1 86 6570
Ελλά
Novartis (Hellas) A.E.B.E.
Τηλ: +30 210 281 17 12
Polska
Novartis Poland Sp. z o.o.
Tel.: +48 22 550 8888
España
Novartis Farmacéutica, S.A.
Tel: +34 93 306 42 00
Portugal
Novartis Farma - Produtos Farmacêuticos, S.A.
Tel: +351 21 000 8600
France
Novartis Pharma S.A.S.
Tél: +33 1 55 47 66 00
România
Novartis Pharma Services Inc.
Tel: +40 21 31299 01
Ireland
Novartis Ireland Limited
Tel: +353 1 260 12 55
Slovenija
Novartis Pharma Services Inc.
Tel: +386 1 300 75 50
Ísland
Vistor hf.
Sími: +354 535 7000
Slovenská republika
Novartis Slovakia s.r.o.
Tel: +421 2 5542 5439
Italia
Novartis Farma S.p.A.
Tel: +39 02 96 54 1
Suomi/Finland
Novartis Finland Oy
Puh/Tel: +358 (0)10 6133 200
Κύπρς
Novartis Pharma Services Inc.
Τηλ: +357 22 690 690
Sverige
Novartis Sverige AB
Tel: +46 8 732 32 00
Latvija
Novartis Pharma Services Inc.
Tel: +371 67 887 070
United Kingdom
Novartis Pharmaceuticals UK Ltd.
Tel: +44 1276 698370
Lietuva
Novartis Pharma Services Inc.
Tel: +370 5 269 16 50
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency web site:
http://www.ema.europa.eu
INFORMATION FOR THE HEALTHCARE PROFESSIONAL
The following information is intended for medical or healthcare professionals only:
Before using the syringe, please read the following information carefully.
Each Xolair pack contains a pre-filled syringe individually sealed in a plastic wrapper.
Parts of the pre-filled syringe
Viewing window
Label and expiration date
Xolair syringes are intended to be used by a healthcare professional only.
Preparing the syringe for use
Prior to completion of the injection, avoid contact with the device activation clips (see first
illustration) to keep from prematurely covering the needle with the needle guard.
1.
Take the box containing the syringe out of the refrigerator and leave it
unopened
for about
20 minutes so that it reaches room temperature.
When you are ready to use the syringe, wash your hands thoroughly with soap and water.
Remove the plastic tray from the box, peel back the paper cover, and remove the syringe.
Inspect the syringe. DO NOT USE if it is broken or if the liquid looks cloudy or contains
particles. In all these cases, return the entire pack to the pharmacy.
Holding the syringe horizontally (as shown below), look into the viewing window to check the
dose (75 mg or 150 mg) of medicine and the expiry date printed on the label. Note: Rotate the
inner part of the syringe assembly as shown below so that the label can be read in the viewing
window.
DO NOT USE if the product has expired or if the
dose is incorrect. In both these cases, return the
entire pack to the pharmacy.
Hold the syringe vertically with the plunger uppermost and tap the side of the syringe against
your finger to allow the air bubble to rise. Slowly push the plunger up to force the air bubble out
of the syringe without inadvertently expelling solution.
Clean the injection site.
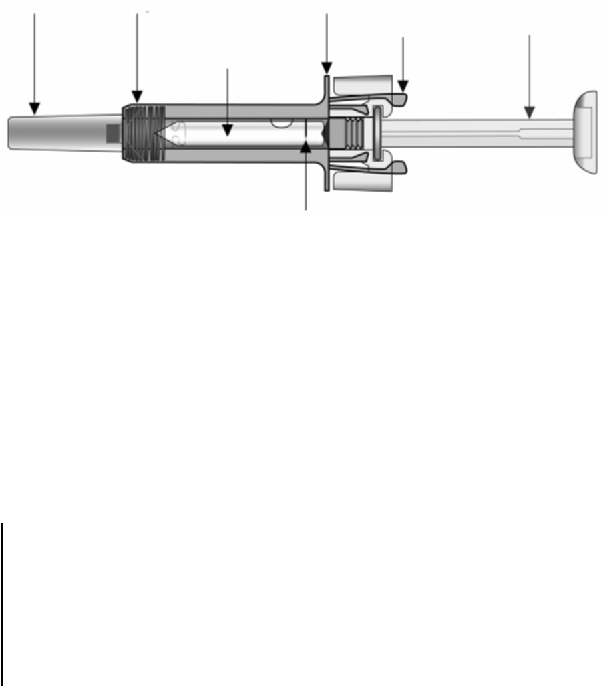
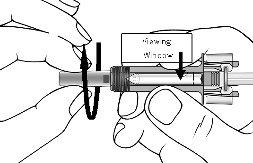


Check to see if the liquid level is at or above the minimum fill line. If the liquid is below the fill
line, return the entire pack to the pharmacy.
Carefully pull off the needle cap from the syringe
and discard it. Do not touch the exposed needle.
Gently pinch the skin at the injection site and
insert the needle.
Holding onto the finger flange, slowly depress the
plunger as far as it will go.
If any solution leaks from the injection site, insert
the needle further.
Keeping the plunger fully depressed, carefully lift
the needle straight out from the injection site.
















Slowly release the plunger and allow the needle
guard to automatically cover the exposed needle.
Hold gauze on the injection site for approximately
30 seconds.
Disposal instructions
Dispose of the used syringe immediately in a sharps container.







Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/xolair.html
Copyright © 1995-2021 ITA all rights reserved.
|












































































































































































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































