Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Xyrem 500 mg/ml oral solution
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml of solution
contains 500 mg of sodium oxybate.
For a full list of excipients, see section 6.1.
The oral solution is clear to slightly opalescent.
4.1 Therapeutic indications
Treatment of narcolepsy with cataplexy in adult patients.
4.2 Posology and method of administration
Treatment should be initiated by and remain under the guidance of a physician experienced in the
treatment of sleep disorders. Due to the well known potential of abuse of sodium oxybate, physicians
should evaluate patients for a history of drug abuse (see section 4.4).
Posology
The recommended starting dose is 4.5 g/day sodium oxybate divided into two equal doses of
2.25 g/dose. The dose should be titrated to effect based on efficacy and tolerability (see section 4.4) up
to a maximum of 9 g/day divided into two equal doses of 4.5
g/dose by adjusting up or down in dose
increments of 1.5 g/day (i.e. 0.75 g/dose). A minimum of one to two weeks is recommended between
dose increments. The dose of 9
g/day should not be exceeded due to the possible occurrence of severe
symptoms at doses of 18
g/day or above (see section 4.4).
Single doses of 4.5 g should not be given unless the patient has been titrated previously to that dose
level.
The discontinuation effects of sodium oxybate have not been systematically evaluated in controlled
clinical trials (see section 4.4).
If the patient stops the medicinal product medication for more than 14 consecutive days, titration
should be restarted from the lowest dose
Special populations
Patients with hepatic impairment
The starting dose should be halved in all patients with hepatic impairment, and response to
dose
increments monitored closely (see section 4.4).
Patients with renal impairment
All patients with impaired renal function should consider a dietary recommendation to reduce sodium
intake (see section 4.4).
Elderly patients
Elderly patients should be monitored closely for impaired motor and/or cognitive function when
taking sodium oxybate (see section 4.4).
Paediatric population
The safety and efficacy of sodium oxybate in children and adolescents aged 0-18 years has not been
established. No data is available. Therefore the use of sodium oxybate in children and adolescents in
not recommended.
Method of administration
Xyrem should be taken orally upon getting into bed and again between 2.5 to 4 hours later. It is
recommended that both doses of Xyrem should be made up at the same time upon retiring to bed.
Xyrem is provided for use with a graduated measuring syringe and two 90 ml dosing cups with child
resistant caps. Each measured dose of Xyrem must be dispensed into the dosing cup and diluted with
60 ml of water prior to ingestion.
Because food significantly reduces the bioavailability of sodium oxybate, patients should eat at least
several (2-3) hours before taking the first dose of Xyrem at bedtime. Patients should always observe
the same timing of dosing in relation to meals
Hypersensitivity to the active substance or to any of the excipients.
Patients with succinic semialdehyde dehydrogenase deficiency.
Patients being treated with opioids or barbiturates.
4.4 Special warnings and precautions for use
Xyrem has the potential to induce respiratory depression
Respiratory depression
Sodium oxybate also has the potential to induce respiratory depression. Apnoea and respiratory
depression have been observed in a fasting healthy subject after a single intake of 4.5
g (twice the
recommended starting dose). Patients should be questioned regarding signs of Central Nervous System
(CNS) or respiratory depression. Special caution should be observed in patients with an underlying
respiratory disorder.
Approximately 80% of patients who received sodium oxybate during clinical trials maintained CNS
stimulant use. Whether this affected respiration during the night is unknown. Before increasing the
sodium oxybate dose (see section 4.2), prescribers should be aware that sleep apnoea occurs in up to
50% of patients with narcolepsy.
Abuse potential and dependence
Sodium oxybate, which is as the sodium salt of GHB, is a CNS depressant active substance with well
known abuse potential. Physicians should evaluate patients for a history of drug abuse and follow such
patients closely.
There have been case reports of dependence after illicit use of GHB at frequent repeated doses (18 to
250 g/day) in excess of the therapeutic dose range. Whilst there is no clear evidence of emergence of
dependence in patients taking sodium oxybate at therapeutic doses, this possibility cannot be excluded.



The combined use of alcohol or any CNS depressant medicinal product with sodium oxybate may
result in potentiation of the CNS-depressant effects of sodium oxybate. Therefore, patients should be
warned against the use of alcohol in conjunction with sodium oxybate.
Patients with porphyria
Sodium oxybate is considered to be unsafe in patients with porphyria because it has been shown to be
porphyrogenic in animals or
in vitro
systems.
Benzodiazepines
Given the possibility of increasing the risk of respiratory depression, the concomitant use of
benzodiazepines and sodium oxybate should be avoided.
Neuropsychiatric events
Patients may become confused while being treated with sodium oxybate. If this occurs, they should be
evaluated fully, and appropriate intervention considered on an individual basis. Other neuropsychiatric
events include psychosis, paranoia, hallucinations, and agitation. The emergence of thought disorders
and/or behavioural abnormalities when patients are treated with sodium oxybate requires careful and
immediate evaluation.
The emergence of depression when patients are treated with sodium oxybate requires careful and
immediate evaluation. Patients with a previous history of a depressive illness and/or suicide attempt
should be monitored especially carefully for the emergence of depressive symptoms while taking
sodium oxybate.
If a patient experiences urinary or faecal incontinence during sodium oxybate therapy, the prescriber
should consider pursuing investigations to rule out underlying aetiologies.
Sleepwalking has been reported in patients treated in clinical trials with sodium oxybate. It is unclear
if some or all of these episodes correspond to true somnambulism (a parasomnia occurring during non-
REM sleep) or to any other specific medical disorder. The risk of injury or self-harm should be borne
in mind in any patient with sleepwalking. Therefore, episodes of sleepwalking should be fully
evaluated and appropriate interventions considered.
Sodium intake
Patients taking sodium oxybate will have an additional daily intake of sodium that ranges from 0.82
g
(for a 4.5
g/day Xyrem dose) to 1.6
g (for a 9
g/day Xyrem dose). A dietary recommendation to reduce
sodium intake should be carefully considered in the management of patients with heart failure,
hypertension or compromised renal function. (see section 4.2).
Patients with compromised liver function
Patients with compromised liver function will have an increased elimination half-life and systemic
exposure to sodium oxybate (see Section 5.2). The starting dose should therefore
be halved in such
patients, and response to
dose increments monitored closely (see section 4.2).
Elderly
There is very limited experience with sodium oxybate in the elderly. Therefore, elderly patients
should be monitored closely for impaired motor and/or cognitive function when taking sodium
oxybate.
Childhood and adolescence
Safety and effectiveness in children and adolescents has not been established, therefore use in patients
under 18 years of age is not recommended.
Epileptic patients
Seizures have been observed in patients treated with sodium oxybate. In patients with epilepsy, the
safety and efficacy of sodium oxybate has not been established, therefore use is not recommended.
Rebound effects and withdrawal syndrome
The discontinuation effects of sodium oxybate have not been systematically evaluated in controlled
clinical trials. In some patients, cataplexy may return at a higher frequency on cessation of sodium
oxybate therapy, however this may be due to the normal variability of the disease. Although the
clinical trial experience with sodium oxybate in narcolepsy/cataplexy patients at therapeutic doses
does not show clear evidence of a withdrawal syndrome, in rare cases, events such as insomnia,
headache, anxiety, dizziness, sleep disorder, somnolence, hallucination, and psychotic disorders were
observed after GHB discontinuation.
4.5 Interaction with other medicinal products and other forms of interaction
The combined use of alcohol with sodium oxybate may result in potentiation of the central nervous
system-depressant effects of sodium oxybate. Patients should be warned against the use of any
alcoholic beverages in conjunction with sodium oxybate.
Sodium oxybate should not be used in combination with sedative hypnotics or other CNS depressants.
Drug interaction studies in healthy adults demonstrated no pharmacokinetic interactions between
sodium oxybate and protriptyline hydrochloride (an antidepressant), zolpidem tartrate (a hypnotic),
and modafinil (a stimulant). However, pharmacodynamic interactions with these medicinal products
have not been assessed.
The co-administration of omeprazole (a medicinal product that alters gastric pH) has no clinically
significant effect on the pharmacokinetics of sodium oxybate. The dose of sodium oxybate therefore
does not require adjustment when given concomitantly with proton pump inhibitors.
Studies
in vitro
with pooled human liver microsomes indicate that sodium oxybate does not
significantly inhibit the activities of the human isoenzymes (see section 5.2).
Since sodium oxybate is metabolised by GHB dehydrogenase there is a potential risk of an interaction
with medicinal products that stimulate or inhibit this enzyme (e.g. valproate, phenytoin or
ethosuximide). No interaction studies have been conducted in human subjects
Sodium oxybate has been administered concomitantly with CNS stimulant medicinal products in
approximately 80% of patients in clinical studies. Whether this affected respiration during the night is
unknown.
Antidepressants have been used in the treatment of cataplexy. A possible additive effect of
antidepressants and sodium oxybate cannot be excluded. The rate of adverse reactions has increased
when sodium oxybate is co-administered with tricyclic antidepressants.
4.6 Fertility, pregnancy and lactation
Pregnancy
Animal studies have shown no evidence of teratogenicity but embryolethality was seen in both rat and
rabbit studies (see section 5.3).
There are no adequate data on the use of sodium oxybate during the first trimester of pregnancy.
Limited data from pregnant patients during second and third trimester indicate no malformative nor
foeto/neonatal toxicity of sodium oxybate.
Sodium oxybate is not recommended during pregnancy.
Breastfeeding
It is not known whether sodium oxybate is excreted into breast milk. Breastfeeding is not
recommended when treating with sodium oxybate.
Fertility
There is no clinical data available on the effect of sodium oxybate on fertility. No effect on fertility
parameters in the rat were observed (see section 5.3)
4.7 Effects on ability to drive and use machines
Sodium oxybate has a major influence on the ability to drive and use machines.
For at least 6 hours after taking sodium oxybate, patients must not undertake activities requiring
complete mental alertness or motor co-ordination, such as operating machinery or driving.
When patients first start taking sodium oxybate, until they know whether this medicinal product will
still have some carryover effect on them the next day, they should use extreme care while driving a
car, operating heavy machines, or performing any other task that could be dangerous or require full
mental alertness.
The most commonly reported adverse reactions are dizziness, nausea, and headache, all occurring in
10% to 20% of patients.
Frequency estimate: very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1000 to <
1/100); rare (≥ 1/10,000 to < 1/1000); very rare (< 1/10,000); not known (cannot be estimated from the
available data).
Within each frequency grouping, adverse events are presented in order of decreasing seriousness
Immune system disorders
:
Not known (cannot be estimated from the available data):
hypersensitivity
Metabolism and nutrition disorders:
Common:
anorexia
Psychiatric disorders:
Common:
depression, cataplexy, anxiety, abnormal dreams, confusion, disorientation, nightmares,
sleepwalking, sleep disorder, insomnia, middle insomnia, nervousness
Uncommon:
suicide attempt, psychosis, paranoia, hallucination, abnormal thinking, agitation, initial
insomnia
Not known (cannot be estimated from the available data):
suicidal ideation
Nervous system disorders:
Very common:
dizziness, headache
Common:
sleep paralysis, somnolence, tremor, balance disorder, disturbance in attention,
hypoaesthesia, paraesthesia, sedation
Uncommon:
myoclonus, amnesia, restless legs syndrome
Not known (cannot be estimated from the available data):
convulsion
Eye disorders:
Common:
blurred vision
Vascular disorders:
Common:
hypertension
Respiratory, thoracic and mediastinal disorders:
Common:
dyspnoea, snoring
Not known (cannot be estimated from the available data)
: respiratory depression
Gastrointestinal disorders:
Very common:
nausea (the frequency of nausea is higher in women than men)
Common:
vomiting, diarrhoea, upper abdominal pain,
Uncommon
: faecal incontinence
Skin and subcutaneous tissue disorders:
Common:
sweating
Uncommon:
rash
Not known (cannot be estimated from the available data):
urticaria
Musculoskeletal, connective tissue and bone disorders:
Common:
arthralgia, muscle cramps
Renal and urinary disorders:
Common:
enuresis nocturna, urinary incontinence
General disorders and administration site conditions:
Common:
asthenia, fatigue, feeling drunk, oedema peripheral
Investigations:
Uncommon:
blood pressure increased, weight decreased
Injury, poisoning and procedural complications
Common:
fall
Description of selected adverse reactions
In some patients, cataplexy may return at a higher frequency on cessation of sodium oxybate therapy,
however this may be due to the normal variability of the disease. Although the clinical trial
experience with sodium oxybate in narcolepsy/cataplexy patients at therapeutic doses does not show
clear evidence of a withdrawal syndrome, in rare cases, adverse reactions such as insomnia, headache,
anxiety, dizziness, sleep disorder, somnolence, hallucination, and psychotic disorders were observed
after GHB discontinuation.
Information about signs and symptoms associated with overdose with sodium oxybate is limited.
Most data derives from the illicit use of GHB. Sodium oxybate is the sodium salt of GHB. Events
associated with withdrawal syndrome have been observed outside the therapeutic range.
Patients have exhibited varying degrees of depressed consciousness that may fluctuate rapidly between
a confusional, agitated combative state with ataxia and coma. Emesis (even with impaired
consciousness), diaphoresis, headache, and impaired psychomotor skills may be observed. Blurred
vision has been reported. An increasing depth of coma has been observed at higher doses. Myoclonus
and tonic-clonic seizures have been reported. There are reports of compromise in the rate and depth of
respiration and of life-threatening respiratory depression, necessitating intubation and ventilation.
Cheyne-Stokes respiration and apnoea have been observed. Bradycardia and hypothermia may
accompany unconsciousness, as well as
muscular hypotonia, but
tendon reflexes remain intact.
Bradycardia has been responsive to atropine intravenous administration.
Gastric lavage may be considered if co-ingestants are suspected. Because emesis may occur in the
presence of impaired consciousness, appropriate posture (left lateral recumbent position) and
protection of the airway by intubation may be warranted. Although gag reflex may be absent in
deeply comatose patients, even unconscious patients may become combative to intubation, and rapid
sequence induction (without the use of sedative) should be considered.
No reversal of the central depressant effects of sodium oxybate can be expected from flumazenil
administration. There is insufficient evidence to recommend the use of naloxone in the treatment of
overdose with GHB. The use of haemodialysis and other forms of extracorporeal medicinal product
removal have not been studied in sodium oxybate overdose. However, due to the rapid metabolism of
sodium oxybate, these measures are not warranted.
PHARMACOLOGICAL PROPERTIES
5.1
Pharmacodynamic properties
Pharmacotherapeutic group: Other nervous system medicinal products, ATC code: N07XX04
Sodium oxybate is a central nervous system depressant which reduces excessive daytime sleepiness
and cataplexy in patients with narcolepsy and modifies sleep architecture reducing fragmented
nighttime sleep. The precise mechanism by which sodium oxybate produces an effect is unknown,
however sodium oxybate is thought to act by promoting slow (delta) wave sleep and consolidating
night-time sleep. Sodium oxybate administered before nocturnal sleep increases Stages 3 and 4 sleep
and increases sleep latency, whilst reducing the frequency of sleep onset REM periods (SOREMPs).
Other mechanisms, which have yet to be elucidated, may also be involved.
In the clinical trial database, greater than 80 % of patients maintained concomitant stimulant use.
The effectiveness of sodium oxybate
for the treatment of narcolepsy symptoms was established in four
multicentre, randomised, double-blind, placebo-controlled, parallel-group trials (Trial 1, 2, 3 and 4) in
patients with narcolepsy with cataplexy except for trial 2 where cataplexy was not required for
enrolment Concomitant stimulant use was permitted in all trials (except for the active-treatment phase
of Trial 2); antidepressants were withdrawn prior to active treatment in all trials with the exception of
Trial 2.
In each trial, the daily dose was divided into two equal doses. The first dose each night was
taken at bedtime and the second dose was taken 2.5 to 4 hours later.
Summary of clinical trials performed using sodium oxybate for the treatment of
Active
treatment
and Dose
(g/d)
MWT/Sleep Architecture/
Cataplexy/Naps/FOSQ
8 weeks Xyrem 6 – 9
Modafinil
200-600 mg
Trial 3 Cataplexy 136 EDS (ESS)/CGIc/Naps 4 weeks Xyrem 3 - 9
Trial 4 Cataplexy 55 None 4 weeks Xyrem 3 - 9
EDS – Excessive daytime sleepiness; ESS – Epworth Sleepiness Scale; MWT – Maintenance of
Wakefulness Test; Naps – Number of inadvertent daytime naps; CGIc – Clinical Global Impression of
Change; FOSQ – Functional Outcomes of Sleep Questionnaire
Sleep Architecture/
ESS/CGIc/Naps
Trial 1 enrolled 246 patients with narcolepsy and incorporated a 1 week up-titration period. The
primary measures of efficacy were changes in excessive daytime sleepiness as measured by the
Epworth Sleepiness Scale (ESS), and the change in the overall severity of the patient’s narcolepsy
symptoms as assessed by the investigator using the Clinical Global Impressions of Change (CGI-c)
measure.










Summary of ESS in Trial 1
Epworth Sleepiness Scale (ESS; range 0-24)
Median Change
from Baseline
Change from Baseline
Compared to Placebo
(p-value)
Summary of CGI-c in Trial 1
Clinical Global Impressions of Change (CGI-c)
Dose Group [g/d (n)]
Change from Baseline
Compared to Placebo
(p-value)
* The CGI-c data were analysed by defining responders as those patients who were very much
improved or much improved.
Trial 2 compared the effects of orally administered sodium oxybate, modafinil and sodium oxybate +
modafinil, with placebo in the treatment of daytime sleepiness in narcolepsy. During the 8 week
double-blind period, patients took modafinil at their established dose or placebo equivalent. The
sodium oxybate or placebo equivalent dose was 6 g/day for the first 4 weeks and was increased to
9 g/day for the remaining 4 weeks. The primary measure of efficacy was excessive daytime sleepiness
as measured by objective response in MWT.
Summary of MWT in Trial 2
Mean Change from
Baseline
Endpoint
Compared to
Placebo
Sodium Oxybate +
Modafinil (57)
Trial 3 enrolled 136 narcoleptic patients with moderate to severe cataplexy (median of 21 cataplexy
attacks per week) at baseline.
The primary efficacy measure in this trial was the frequency of
cataplexy attacks.


































Summary of outcomes in Trial 3
Median Change
from Baseline
Trial 4 enrolled 55 narcoleptic patients who had been taking open-label sodium oxybate for 7 to 44
months. Patients were randomised to continued treatment with sodium oxybate at their stable dose or
to placebo. Trial 4 was designed specifically to evaluate the continued efficacy of sodium oxybate
after long-term use. The primary efficacy measure in this trial was the frequency of cataplexy attacks.
Summary of outcome in Trial 4
Median Change
from Baseline
In Trial 4, the response was numerically similar for patients treated with doses of 6 to 9 g/day, but
there was no effect seen in patients treated with doses less than 6 g/day.
5.2 Pharmacokinetic properties
Sodium oxybate is rapidly but incompletely absorbed after oral administration; absorption is delayed
and decreased by a high fat meal. It is eliminated mainly by metabolism with a half-life of 0.5 to 1
hour. Pharmacokinetics are nonlinear with the area under the plasma concentration curve (AUC)
versus time curve increasing 3.8-fold as dose is doubled from 4.5
g to 9
g. The pharmacokinetics are
not altered with repeat dosing.
Absorption
:
Sodium oxybate is absorbed rapidly following oral administration with an absolute
bioavailability of about 25 %. The average peak plasma concentrations (1
st
and 2
nd
peak) following
administration of a 9 g daily dose divided into two equivalent doses given four hours apart were 78
and 142
μg/ml, respectively. The average time to peak plasma concentration (T
max
) ranged from 0.5 to
2
hours in eight pharmacokinetic studies. Following oral administration, the plasma levels of sodium
oxybate increase more than proportionally with increasing dose. Single doses greater than 4.5 g have
not been studied. Administration of sodium oxybate immediately after a high fat meal resulted in
delayed absorption (average T
max
increased from 0.75 hr to 2.0 hr) and a reduction in peak plasma
level (C
max
) by a mean of 58% and of systemic exposure (AUC) by 37%.



















Distribution:
Sodium oxybate is a hydrophilic compound with an apparent volume of distribution
averaging 190-384 ml/kg. At sodium oxybate concentrations ranging from 3 to 300 μg/ml, less than
1% is bound to plasma proteins.
Biotransformation:
Animal studies indicate that metabolism is the major elimination pathway for
sodium oxybate, producing carbon dioxide and water via the tricarboxylic acid (Krebs) cycle and
secondarily by β-oxidation. The primary pathway involves a cytosolic NADP
+
-linked enzyme, GHB
dehydrogenase, that catalyses the conversion of sodium oxybate to succinic semialdehyde, which is
then biotransformed to succinic acid by the enzyme succinic semialdehyde dehydrogenase. Succinic
acid enters the Krebs cycle where it is metabolised to carbon dioxide and water. A second
mitochondrial oxidoreductase enzyme, a transhydrogenase, also catalyses the conversion to succinic
semialdehyde in the presence of α-ketoglutarate. An alternate pathway of biotransformation involves
β-oxidation via 3,4-dihydroxybutyrate to Acetyl CoA, which also enters the citric acid cycle to result
in the formation of carbon dioxide and water. No active metabolites have been identified.
Studies
in vitro
with pooled human liver microsomes indicate that sodium oxybate does not
significantly inhibit the activities of the human isoenzymes: CYP1A2, CYP2C9, CYP2C19, CYP2D6,
CYP2E1, or CYP3A up to the concentration of 3 mM (378 μg/ml). These levels are considerably
higher than levels achieved with therapeutic doses.
Elimination:
The clearance of sodium oxybate is almost entirely by biotransformation to carbon
dioxide, which is then eliminated by expiration. On average, less than 5% of unchanged medicinal
product appears in human urine within 6 to 8 hours after dosing. Faecal excretion is negligible.
Elderly patients
: The pharmacokinetics of sodium oxybate in patients greater than the age of 65
years
have not been studied.
Paediatric patients
: The pharmacokinetics of sodium oxybate in paediatric patients under the age of
18
years have not been studied.
Renal impairment
: Because the kidney does not have a significant role in the excretion of sodium
oxybate, no pharmacokinetic study in patients with renal dysfunction has been conducted; no effect of
renal function on sodium oxybate pharmacokinetics would be expected.
Hepatic disease
: Sodium oxybate undergoes significant presystemic (hepatic first-pass) metabolism.
After a single oral dose of 25
mg/kg, AUC values were double in cirrhotic patients, with apparent oral
clearance reduced from 9.1 in healthy adults to 4.5 and 4.1
ml/min/kg in Class A (without ascites) and
Class C (with ascites) patients, respectively. Elimination half-life was significantly longer in Class C
and Class A patients than in control subjects (mean t
1/2
of 59 and 32 versus 22 minutes). It is prudent
to reduce the starting dose of sodium oxybate by one-half in patients with liver dysfunction (see
Section 4.2).
Race
The effect of race on metabolism of sodium oxybate has not been evaluated.
5.3 Preclinical safety data
Repeat administration of sodium oxybate to rats (90 days and 26 weeks) and dogs (52 weeks) did not
result in any significant findings in clinical chemistry and micro- and macro pathology. Treatment-
related clinical signs were mainly related to sedation, reduced food consumption and secondary
changes in body weight, body weight gain and organ weights. The rat and dog exposures at the NOEL
were lower (~50%) than that in humans. Sodium oxybate was non-mutagenic and non-clastogenic in
in vitro
and
in vivo
assays.
Gamma Butyrolactone (GBL), a pro-drug of GHB tested at exposures similar to the expected in man
(1.21-1.64 times) has been classified by NTP as non-carcinogenic in rats and equivocal carcinogen in
mice, due to slight increase of pheochromocytomas which was difficult to interpret due to high
mortality in the high dose group. In a rat carcinogenicity study with oxybate no compound-related
tumours were identified.
GHB had no effect on mating, general fertility or sperm parameters and did not produce embryo-foetal
toxicity in rats exposed to up 1000 mg/kg/day GHB (1.64 times the human exposure calculated in
nonpregnant animals). Perinatal mortality was increased and mean pup weight was decreased during
the lactation period in high-dose F
1
animals. The association of these developmental effects with
maternal toxicity could not be established. In rabbits, slight foetotoxicity was observed.
Drug discrimination studies show that GHB produces a unique discriminative stimulus that in some
respects is similar to that of alcohol, morphine and certain GABA-mimetic medicinal products. Self-
administration studies in rats, mice and monkeys have produced conflicting results, whereas tolerance
to GHB as well as cross-tolerance to alcohol have been clearly demonstrated in rodents.
PHARMACEUTICAL PARTICULARS
Purified water
Malic acid for pH adjustment
Sodium hydroxide for pH adjustment
This medicinal product must not be mixed with other medicinal products.
After first opening
:
40 days
After dilution in the dosing cups (see section 4.2), the preparation should be used within 24 hours.
6.4 Special precautions for storage
This medicinal product does not require any special storage conditions.
For storage conditions of the diluted medicinal product see section 6.3.
6.5 Nature and contents of container
Amber oval PET bottle which is delivered with a plastic/foil seal and closed with a child resistant
closure composed of HDPE/polypropylene with a pulpboard inner liner.
Each carton contains one bottle of 180 ml solution, a press-in bottle adaptor consisting of an LDPE
bottle-well housing, a Silastic Biomedical ETR Elastomer valve, an acrylonitrile butadiene styrene
terpolymer valve retainer and LDPE tubing, a graduated measuring device (polypropylene syringe),
two polypropylene dosing cups and two HDPE child resistant screw closures.
6.6 Special precautions for disposal
MARKETING AUTHORISATION HOLDER
UCB Pharma Ltd
208 Bath Road
Slough
Berkshire
SL1 3WE
UK
MARKETING AUTHORISATION NUMBER
DATE OF FIRST AUTHORISATION/RENEWAL OF AUTHORISATION
Date for first Authorisation: 13/10/2005
Date of latest renewal: 18/10/2010
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicinal product is available on the website of the European Medicines
A.
MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR
BATCH RELEASE
B.
CONDITIONS OF THE MARKETING AUTHORISATION
MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer responsible for batch release
Recipharm Limited
Vale of Bardsley, Ashton under Lyne
Lancashire OL7 9RR
United Kingdom
UCB Pharma Ltd
208 Bath Road
Slough
Berkshire SL1 3WE
United Kingdom
The printed package leaflet of the medicinal product must state the name and address of the
manufacturer responsible for the release of the concerned batch.
CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to special and restricted medical prescription (See Annex I: Summary of
Product Characteristics, section 4.2
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
Pharmacovigilance system
The MAH must ensure that the system of pharmacovigilance, as described in version 1.0 of February
28
th
, 2010 presented in module 1.8.1 of the Marketing Authorisation, is in place and functioning
before and whilst the medicinal product is on the market.
Risk Management Plan (RMP)
The MAH commits to performing the additional pharmacovigilance activities detailed in the
Pharmacovigilance Plan as agreed in version 3 of March 8
th
, 2010 of the RMP presented in module
1.8.2 of the Marketing Authorisation and any subsequent updates agreed by the Committee for
Medicinal Products for Human Use (CHMP).
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, any
updated RMP should be submitted at the same time as the following Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted:
•
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
•
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
At the request of the European Medicines Agency
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
Xyrem 500 mg/ml oral solution
sodium oxybate
STATEMENT OF ACTIVE SUBSTANCE
Each ml of solution contains 500 mg sodium oxybate
PHARMACEUTICAL FORM AND CONTENTS
METHOD AND ROUTE OF ADMINISTRATION
Oral use. Read the package leaflet before use
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
Keep the container tightly closed.
SPECIAL STORAGE CONDITIONS
The medicinal product should be used within 40 days after the first opening.
After dilution in the dosing cups the preparation should be used within 24 hours
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
























OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
UCB Pharma Ltd
208 Bath Road
Slough
Berkshire
SL1 3WE.
UK.
12. MARKETING AUTHORISATION NUMBER
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE
Xyrem 500 mg/ml (applies to carton only)



















PACKAGE LEAFLET: INFORMATION FOR THE USER
Xyrem 500
mg/ml oral solution
Sodium oxybate
Read all of this leaflet carefully before you start using this medicine.
-
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or your pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even
if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
What Xyrem is and what it is used for
WHAT XYREM IS AND WHAT IT IS USED FOR
Xyrem works by consolidating night-time sleep, though its exact mechanism of action is unknown.
Xyrem is used to treat narcolepsy with cataplexy in adult patients.
Narcolepsy is a sleep disorder that may include attacks of sleep during normal waking hours, as well
as cataplexy, sleep paralysis, hallucinations and poor sleep. Cataplexy is the onset of sudden muscle
weakness or paralysis without losing consciousness, in response to a sudden emotional reaction such
as anger, fear, joy, laughter or surprise.
if you are allergic (hypersensitive) to sodium oxybate or any of the other ingredients of Xyrem
if you have succinic semialdehyde dehydrogenase deficiency (a rare metabolic disorder)
Take special care with Xyrem
-
if you have breathing or lung problems, because Xyrem has the potential to cause respiratory
depression
if you have or have previously had depressive illness
if you have heart failure, hypertension (high blood pressure), liver or kidney problems as your
dose may need to be adjusted
if you are taking other central nervous system depressants or alcohol
if you have previously had experience with drug abuse
if you suffer from epilepsy the use of Xyrem is not recommended
if you have porphyria (an uncommon metabolic disorder)
If any of these apply to you, tell your doctor before you take Xyrem.
While you are taking Xyrem, if you experience bed wetting and incontinence (both urine and faeces),
confusion, hallucinations, episodes of sleepwalking or abnormal thinking you should tell your doctor
if you are being treated with opioids or barbiturate medicines
straight away. Whilst these effects are uncommon, if they do occur they are usually mild-to-moderate
in nature.
If you are elderly, your doctor will monitor your condition carefully to check whether Xyrem is
having the desired effects.
Xyrem should not be taken by children and adolescents.
When you discontinue taking Xyrem you need to follow your doctor’s instructions as it may result in
side effects e.g. headache, lack of sleep, mood changes and hallucinations.
Xyrem has a well known abuse potential. No cases of abuse are known among individuals treated for
narcolepsy. However cases of dependency have occurred after the illicit use of sodium oxybate.
Taking other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines,
including medicines obtained without a prescription.
In particular Xyrem should not be taken together with sleep inducing medicines and medicines that
reduce central nervous system activity (the central nervous system is the part of the body related to the
brain and spinal cord).
Also care should be taken to tell your doctor and pharmacist if your are taking any of the following
types of medicines:
•
medicines that increase central nervous system activity and antidepressants
•
medicines that may be processed in a similar way by the body (eg valporate, phenytoin or
ethosuximide)
Taking Xyrem with food and drink
You must not drink alcohol while taking Xyrem, as its effects can be increased.
Xyrem is to be taken at a set time well after a meal (two - three hours) as food decreases the amount of
Xyrem that is absorbed by your body
You need to monitor the amount of salt you take as Xyrem contains sodium (which is found in table
salt) which may affect you if you have had high blood pressure, heart or kidney problems in the past.
If you take two 2.25 g doses of sodium oxybate each night you will take 0.82 g of sodium, or if you
take two 4.5 g doses of sodium oxybate each night you will take in 1.6 g sodium. You may need to
moderate your intake of salt.
Pregnancy and breast-feeding
The effects of Xyrem in pregnant women are not known, and so the use of Xyrem in pregnant women
is not recommended. Tell your doctor if you are pregnant or are planning to become pregnant.
It is not known whether Xyrem passes into breast milk. Patients taking Xyrem should stop breast
feeding.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Xyrem will affect you if you drive or operate tools or machines. Do not drive a car, operate heavy
machinery, or perform any activity that is dangerous or that requires mental alertness for at least 6
hours after taking Xyrem. When you first start taking Xyrem, until you know whether it makes you
sleepy the next day, use extreme care while driving a car, operating heavy machinery or doing
anything else that could be dangerous or needs you to be fully mentally alert.
Always take Xyrem exactly as your doctor has instructed you. You should check with your doctor or
pharmacist if you are unsure.
The usual starting dose is 4.5 g/day, given as two equally divided doses of 2.25 g/dose. Your doctor
may gradually increase your dose up to a maximum of 9 g/day given as two equally divided doses of
4.5 g/dose.
Take Xyrem orally two times each night. Take the first dose upon getting into bed and the second
dose 2.5 to 4 hours later. You may need to set an alarm clock to make sure you wake up to take the
second dose. Food decreases the amount of Xyrem that is absorbed by your body. Therefore, it is best
to take Xyrem at set times well after a meal (two-three hours). Prepare both doses before bedtime.
If you stop taking Xyrem for more than 14 consecutive days you should consult your doctor as you
should restart taking Xyrem at a reduced dose.
Instructions on how to dilute Xyrem
The following instructions explain how to prepare Xyrem. Please read the instructions carefully and
follow them step by step.
To help you, the Xyrem carton contains 1 bottle of medicine, a measuring syringe and two dosing cups
with child-resistant caps.
Remove the bottle cap by pushing down while turning the cap anticlockwise (to the left). After
removing the cap, set the bottle upright on a table-top. There is a plastic covered foil seal on the
top of the bottle, which must be removed before using the bottle for the first time. While
holding the bottle in its upright position, insert the press-in-bottle-adaptor into the neck of the
bottle. This needs only to be done the first time that the bottle is opened. The adaptor can then
be left in the bottle for all subsequent uses
Next, insert the tip of the measuring syringe into the centre opening of the bottle and press down
firmly (See Figure 1).
While holding the bottle and syringe with one hand, draw up the prescribed dose with the other
hand by pulling on the plunger. NOTE: Medicine will not flow into the syringe unless you keep
the bottle in its upright position (See Figure 2).


Remove the syringe from the centre opening of the bottle. Empty the medicine from the syringe
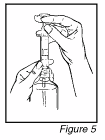
into one of the dosing cups provided by pushing on the plunger (See Figure 3). Repeat this step
for the second dosing cup. Then add about 60 ml of water to each dosing cup (60 ml is about 4
tablespoons)
Place the caps provided on the dosing cups and turn each cap clockwise (to the right) until it
clicks and locks into its child-resistant position (See Figure 4). Rinse out the syringe with water.
Just before going to sleep, place your second dose near your bed. You may need to set an alarm
so you wake up to take your second dose no earlier than 2.5 hours and no later than 4 hours
after your first dose. Remove the cap from the first dosing cup by pressing down on the child-
resistant locking tab and turning the cap anticlockwise (to the left). Drink all of the first dose
while sitting in bed, recap the cup, and then lie down right away
When you wake up 2.5 to 4 hours later, remove the cap from the second dosing cup. While
sitting in bed, drink all of the second dose right before lying down to continue sleeping. Recap
the second cup.
If you have the impression that the effect of Xyrem is too strong or too weak, talk to your doctor or
pharmacist.
If you take more Xyrem than you should
Symptoms of Xyrem overdose may include agitation, confusion, impaired movement, impaired
breathing, blurred vision, profuse sweating, headache, vomiting, decreased consciousness leading to
coma and seizures. If you take more Xyrem than you were told to take, or take it by accident, get
emergency medical help right away. You should take the labelled medicine bottle with you, even if it
is empty.
If you forget to take Xyrem
If you forget to take the first dose, take it as soon as you remember and then continue as before. If you
miss the second dose, skip that dose and do not take Xyrem again until the next night. Do not take a
double dose to make up for forgotten individual doses.
If you stop taking Xyrem
You should continue to take Xyrem for as long as instructed by your doctor. You may find that your
cataplexy attacks return if your medicine is stopped and you may experience insomnia, headache,
anxiety, dizziness, sleeping problems, sleepiness, hallucination and abnormal thinking.
If you have any further questions on the use of this product, ask your doctor or pharmacist.



Like all medicines, Xyrem can have side effects. These are usually mild to moderate. If you
experience any of these, tell your doctor straight away.
The frequency of possible side effects listed below is defined using the following convention:
very common (affects more than 1 user in 10)
common (affects 1 to 10 users in 100)
uncommon (affects 1 to 10 users in 1,000)
rare (affects 1 to 10 users in 10,000)
very rare (affects less than 1 user in 10,000)
not known (frequency cannot be estimated from the available data)
Very common side effects (affects more than 1 user in 10) include:
Nausea, dizziness, headache
Common side effects (affects 1 to 10 users in 100) include:
Sleeping problems including insomnia, blurred vision, vomiting, stomach pains, diarrhoea, anorexia,
weakness, abnormal dreams, tiredness, feeling drunk, sleep paralysis, sleepiness, trembling, confusion/
disorientation, nightmares, sleep walking, bed wetting, sweating, depression, muscle cramps,
swelling, fall, joint pain, cataplexy, balance disorder, disturbance in attention, disturbed sensitivity
particularly to touch, abnormal touch sensation, sedation, anxiety, difficulty in falling asleep in the
middle of the night, nervousness, urinary incontinence, shortness of breath, snoring
Uncommon side effects (affects 1 to 10 users in 1,000)
include:
Psychosis (a mental disorder that may involve hallucinations, incoherent speech, or disorganized and
agitated behaviour), paranoia, abnormal thinking, hallucination, agitation, suicide attempt, difficulty in
falling asleep, restless legs, weight loss, forgetfulness, myoclonus (involuntary contractions of
muscles), involuntary passage of faeces, rash, increased blood pressure
Side effects with unknown frequency (cannot be estimated from the available data) include:
Convulsion, decreased breathing depth or rate, hives, hypersensitivity, suicidal thoughts
If any of these affect you severely, tell your doctor.
If you are concerned about any side effect, or if you notice any side effects not mentioned in this
leaflet, please tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use Xyrem after the expiry date stated on the bottle. The expiry date refers to the last day of
that month.
Store in the original container.
Do not store Xyrem solutions diluted with water for more than 24 hours.
Once you open a bottle of Xyrem, any contents that you have not used with 40 days of opening should
be disposed of.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
The active substance is sodium oxybate. Each ml of solution contains 500 mg of sodium
oxybate.
The other ingredients are purified water, malic acid and sodium hydroxyde.
What Xyrem looks like and contents of the pack
Xyrem is supplied as an oral solution in a 180 ml amber plastic bottle, which is closed with a child-
resistant cap. When the bottle is delivered, there is a plastic covered foil seal which is on the top of the
bottle, underneath the cap. Each pack contains one bottle, a press-in-bottle-adaptor (PIBA), a plastic
measuring syringe and two dosing cups with child-resistant caps
Xyrem is a clear to slightly opalescent solution.
The amber plastic bottle contains 180 ml of oral solution.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder: UCB Pharma Ltd, 208 Bath Road, Slough, Berkshire, SL1 3WE,
United Kingdom.
Manufacturer:
Recipharm Limited, Vale of Bardsley, Ashton-under-Lyne, Lancashire, OL7 9RR, United Kingdom.
UCB Pharma Ltd, 208 Bath Road, Slough, Berkshire SL1 3WE, United Kingdom
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder.
You should have received a Xyrem Information Pack from your physician, which includes a booklet
all about Xyrem and a video showing you how to take the medicine. If you have not received this,
please contact the local representative of the Marketing Authorisation Holder, below.
België/Belgique/Belgien
UCB Pharma SA/NV
Tel/Tél: +32 / (0)2 559 92 00
Luxembourg/Luxemburg
UCB Pharma SA/NV
Tél/Tel: +32 / (0)2 559 92 00
България
Ю СИ БИ България ЕООД
Teл.: + 359 (0) 2 962 30 49
Magyarország
UCB Magyarország Kft.
Tel.: + 36-(1) 391 0060
Česká republika
UCB s.r.o.
Tel: + 420 221 773 411
Malta
Pharmasud Ltd.
Tel: +356 / 21 37 64 36
Danmark
UCB Nordic A/S
Tlf: + 45 / 32 46 24 00
Nederland
UCB Pharma B.V.
Tel.: +31 / (0)76-573 11 40
Deutschland
UCB Pharma GmbH
Tel: + 49 /(0) 2173 48 4848
Norge
UCB Nordic A/S
Tel: +45 / 32 46 24 00
Eesti
UCB Pharma Oy Finland
Tel: + 358 10 234 6800 (Soome)
Österreich
UCB Pharma GmbH
Tel: + 43 (1) 291 80 00
Ελλάδα
UCB Α.Ε.
Τηλ: +30 / 2109974000
Polska
UCB Pharma Sp. z o.o.
Tel.: + 48 (0) 22 696 99 20
España
UCB Pharma, S.A.
Tel: + 34 / 91 570 34 44
Portugal
UCB Pharma (Produtos Farmacêuticos), Lda
Tel: + 351 / 21 302 5300
France
UCB Pharma S.A.
Tél: + 33 / (0)1 47 29 44 66
România
UCB Pharma Romania S.R.L.
Tel: +40 21 300 29 04
Ireland
UCB (Pharma) Ireland Ltd.
Tel: + 353 / (0)1-46 37 395
Slovenija
Medis, d.o.o.
Tel: + 386 1 589 69 00
Ísland
Vistor hf.
Tel: +354 535 7000
Slovenská republika
UCB s.r.o.
, organizačná zložka
Tel: + 421 (0) 2 5920 2020
Italia
UCB Pharma S.p.A.
Tel: + 39 / 02 300 791
Suomi/Finland
UCB Pharma Oy Finland
Puh/ Tel: + 358 10 234 6800
Κύπρος
Lifepharma (Z.A.M.) Ltd
Τηλ: + 357 22 34 74 40
Sverige
UCB Nordic A/S
Tel: + 46 / (0) 40 29 49 00
Latvija
UCB Pharma Oy Finland
Tel: + 358 10 234 6800 (Somija)
United Kingdom
UCB Pharma Ltd.
Tel : +44 / (0)1753 534 655
Lietuva
UCB Pharma Oy Finland
Tel: + 358 10 234 6800 (Suomija)
<------------------------------------------------------------------------------------------------------------------------------
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency website:
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/xyrem.html
Copyright © 1995-2021 ITA all rights reserved.
|



















































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































