Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Zometa 4 mg powder and solvent for solution for infusion
QUALITATIVE AND QUANTITATIVE COMPOSITION
One vial contains 4 mg zoledronic acid (anhydrous), corresponding to 4.264 mg zoledronic acid
monohydrate.
For a full list of excipients, see section 6.1.
Powder and solvent for solution for infusion
4.1 Therapeutic indications
Prevention of skeletal related events (pathological fractures, spinal compression, radiation or
surgery to bone, or tumour-induced hypercalcaemia) in patients with advanced malignancies
involving bone.
Treatment of tumour-induced hypercalcaemia (TIH).
4.2 Posology and method of administration
Zometa must only be used by clinicians experienced in the administration of intravenous
bisphosphonates.
Zometa reconstituted solution must not be mixed with calcium or other divalent cation-containing
infusion solutions such as lactated Ringer’s solution, and should be administered as a single
intravenous solution in a separate infusion line.
Prevention of skeletal related events in patients with advanced malignancies involving bone
Adults and elderly
The recommended dose in the prevention of skeletal related events in patients with advanced
malignancies involving bone is 4 mg reconstituted and further diluted Zometa solution for infusion
(diluted with 100 ml 0.9% w/v sodium chloride or 5% w/v glucose solution), given in no less than a
15-minute intravenous infusion every 3 to 4 weeks.
Patients should also be administered an oral calcium supplement of 500 mg and 400 IU vitamin D
daily.
Treatment of TIH
Adults and elderly
The recommended dose in hypercalcaemia (albumin-corrected serum calcium ≥ 12.0 mg/dl or
3.0 mmol/l) is 4 mg reconstituted and further diluted Zometa solution for infusion (diluted with 100 ml
sterile 0.9% w/v sodium chloride or 5% w/v glucose solution), given as a single intravenous infusion
in no less than 15 minutes. Patients must be maintained well hydrated prior to and following
administration of Zometa.
Renal impairment
TIH:
Zometa treatment in TIH patients who also have severe renal impairment should be considered only
after evaluating the risks and benefits of treatment. In the clinical studies, patients with serum
creatinine > 400 µmol/l or > 4.5 mg/dl were excluded. No dose adjustment is necessary in TIH
p
atients with serum creatinine < 400 µmol/l or < 4.5 mg/dl (see section 4.4).
Prevention of skeletal related events in patients with advanced malignancies involving bone:
When initiating treatment with Zometa in patients with multiple myeloma or metastatic bone lesions
from solid tumours, serum creatinine and creatinine clearance (CLcr) should be determined. CLcr is
calculated from serum creatinine using the Cockcroft-Gault formula. Zometa is not recommended for
patients presenting with severe renal impairment prior to initiation of therapy, which is defined for this
population as CLcr < 30 ml/min. In clinical trials with Zometa, patients with serum creatinine
> 265 µmol/l or > 3.0 mg/dl were excluded.
In patients with bone metastases presenting with mild to moderate renal impairment prior to initiation
of therapy, which is defined for this population as CLcr 30–60 ml/min, the following Zometa dose is
recommended (see also section 4.4):
Baseline Creatinine Clearance (ml/min)
Zometa Recommended Dose*
> 60 4.0 mg
50–60 3.5 mg*
40–49 3.3 mg*
30–39 3.0 mg*
*
Doses have been calculated assuming target AUC of 0.66 (mg•hr/l) (CLcr=75 ml/min). The reduced
doses for patients with renal impairment are expected to achieve the same AUC as that seen in patients
with creatinine clearance of 75 ml/min.
Following initiation of therapy, serum creatinine should be measured prior to each dose of Zometa and
treatment should be withheld if renal function has deteriorated. In the clinical trials, renal deterioration
was defined as follows:
-
For patients with normal baseline serum creatinine (< 1.4 mg/dl or < 124 µmol/l), an increase of
0.5 mg/dl or 44 µmol/l;
For patients with an abnormal baseline creatinine (> 1.4 mg/dl or > 124 µmol/l), an increase of
1.0 mg/dl or 88 µmol/l.
In the clinical studies, Zometa treatment was resumed only when the creatinine level returned to
within 10% of the baseline value (see section 4.4). Zometa treatment should be resumed at the same
dose as that prior to treatment interruption.
Instructions for preparing reduced doses of Zometa
Withdraw an appropriate volume of the reconstituted solution (4 mg/5 ml) as needed:
-
For information on the reconstitution and dilution of Zometa, see section 6.6. The withdrawn amount
of reconstituted solution must be diluted in 100 ml of sterile 0.9% w/v sodium chloride solution or 5%
w/v glucose solution. The dose must be given as a single intravenous infusion over no less than
15 minutes
.
The use of Zometa in paediatric patients has been studied in 2 clinical trials in the treatment of severe
osteogenesis imperfecta (see section 5.1). Zometa should not be used in the paediatric population
because safety and efficacy in children have not been established (see sections 4.4 and 5.1).

Hypersensitivity to the active substance, to other bisphosphonates or to any of the excipients in
the formulation of Zometa
Breast-feeding (see section 4.6)
4.4 Special warnings and precautions for use
General
Patients must be assessed prior to administration of Zometa to ensure that they are adequately
hydrated.
Overhydration should be avoided in patients at risk of cardiac failure.
Standard hypercalcaemia-related metabolic parameters, such as serum levels of calcium, phosphate
and magnesium, should be carefully monitored after initiating Zometa therapy. If hypocalcaemia,
hypophosphataemia, or hypomagnesaemia occurs, short-term supplemental therapy may be necessary.
Untreated hypercalcaemia patients generally have some degree of renal function impairment, therefore
careful renal function monitoring should be considered.
Zometa contains the same active substance as found in Aclasta (zoledronic acid). Patients being
treated with Zometa should not be treated with Aclasta concomitantly.
The safety and efficacy of Zometa in paediatric patients have not been established (see section 5.1).
Renal insufficiency
Patients with TIH with evidence of deterioration in renal function should be appropriately evaluated
with consideration given as to whether the potential benefit of treatment with Zometa outweighs the
possible risk.
The decision to treat patients with bone metastases for the prevention of skeletal related events should
consider that the onset of treatment effect is 2–3 months.
As with other bisphosphonates, Zometa has been associated with reports of renal dysfunction. Factors
that may increase the potential for deterioration in renal function include dehydration, pre-existing
renal impairment, multiple cycles of Zometa and other bisphosphonates as well as use of other
nephrotoxic drugs. While the risk is reduced with a dose of Zometa 4 mg administered over
15 minutes, deterioration in renal function may still occur. Renal deterioration, progression to renal
failure and dialysis have been reported in patients after the initial dose or a single dose of Zometa.
Increases in serum creatinine also occur in some patients with chronic administration of Zometa at
recommended doses for prevention of skeletal related events, although less frequently.
Patients should have their serum creatinine levels assessed prior to each dose of Zometa. Upon
initiation of treatment in patients with bone metastases with mild to moderate renal impairment, lower
doses of Zometa are recommended. In patients who show evidence of renal deterioration during
treatment, Zometa should be withheld. Zometa should only be resumed when serum creatinine returns
to within 10% of baseline (see section 4.2).
In view of the potential impact of bisphosphonates, including Zometa, on renal function, the lack of
clinical safety data in patients with severe renal impairment (in clinical trials defined as serum
creatinine ≥ 400 µmol/l or ≥ 4.5 mg/dl for patients with TIH and ≥ 265 µmol/l or ≥ 3.0 mg/dl for
patients with cancer and bone metastases, respectively) at baseline and only limited pharmacokinetic
data in patients with severe renal impairment at baseline (creatinine clearance < 30 ml/min), the use of
Zometa is not recommended in patients with severe renal impairment.
Hepatic insufficiency
As only limited clinical data are available in patients with severe hepatic insufficiency, no specific
recommendations can be given for this patient population.
Osteonecrosis of the jaw
Osteonecrosis of the jaw has been reported in patients, predominantly those with cancer, receiving
treatment with bisphosphonates, including Zometa. Many of these patients were also receiving
chemotherapy and corticosteroids. The majority of reported cases have been associated with dental
procedures such as tooth extraction. Many had signs of local infection including osteomyelitis.
A dental examination with appropriate preventive dentistry should be considered prior to treatment
with bisphosphonates in patients with concomitant risk factors (e.g. cancer, chemotherapy,
corticosteroids, poor oral hygiene).
While on treatment, these patients should avoid invasive dental procedures if possible. For patients
who develop osteonecrosis of the jaw while on bisphosphonate therapy, dental surgery may exacerbate
the condition. For patients requiring dental procedures, there are no data available to suggest whether
discontinuation of bisphosphonate treatment reduces the risk of osteonecrosis of the jaw. Clinical
judgement of the treating physician should guide the management plan of each patient based on
individual benefit/risk assessment.
Musculoskeletal pain
In post-marketing experience, severe and occasionally incapacitating bone, joint, and/or muscle pain
have been reported in patients taking bisphosphonates. However, such reports have been infrequent.
This category of drugs includes Zometa (zoledronic acid). The time to onset of symptoms varied from
one day to several months after starting treatment. Most patients had relief of symptoms after stopping
treatment. A subset had recurrence of symptoms when rechallenged with the same drug or another
bisphosphonate.
4.5 Interaction with other medicinal products and other forms of interaction
In clinical studies, Zometa has been administered concomitantly with commonly used anticancer
agents, diuretics, antibiotics and analgesics without clinically apparent interactions occurring.
Zoledronic acid shows no appreciable binding to plasma proteins and does not inhibit human P450
enzymes
in vitro
(see section 5.2), but no formal clinical interaction studies have been performed.
Caution is advised when bisphosphonates are administered with aminoglycosides, since both agents
may have an additive effect, resulting in a lower serum calcium level for longer periods than required.
Caution is indicated when Zometa is used with other potentially nephrotoxic drugs. Attention should
also be paid to the possibility of hypomagnesaemia developing during treatment.
In multiple myeloma patients, the risk of renal dysfunction may be increased when intravenous
bisphosphonates are used in combination with thalidomide.
4.6 Pregnancy and lactation
Pregnancy
There are no adequate data on the use of zoledronic acid in pregnant women. Animal reproduction
studies with zoledronic acid have shown reproductive toxicity (see section 5.3). The potential risk for
humans is unknown. Zometa should not be used during pregnancy.
Lactation
It is not known whether zoledronic acid is excreted into human milk. Zometa is contraindicated in
breast-feeding women (see section 4.3).
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed.
Frequencies of adverse reactions for Zometa 4 mg are mainly based on data collection from chronic
treatment. Adverse reactions to Zometa are similar to those reported for other bisphosphonates and can
be expected to occur in approximately one third of patients. Intravenous administration has been most
commonly associated with a flu-like syndrome in about 9% of patients, including bone pain (9.1%),
fever (7.2%), fatigue (4.1%) and rigors (2.9%). Occasionally cases of arthralgia and myalgia in
approximately 3% have been reported. No information is available on the reversibility of these adverse
effects.
Frequently, the reduction in renal calcium excretion is accompanied by a fall in serum phosphate
levels (in approximately 20% of patients), which is asymptomatic not requiring treatment. The serum
calcium may fall to asymptomatic hypocalcaemic levels in approximately 3% of patients.
Gastrointestinal reactions, such as nausea (5.8%) and vomiting (2.6%) have been reported following
intravenous infusion of Zometa. Occasionally local reactions at the infusion site such as redness or
swelling and/or pain were also observed in less than 1% of the patients.
Anorexia was reported in 1.5% of patients treated with Zometa 4 mg.
Few cases of rash or pruritus have been observed (below 1%).
As with other bisphosphonates, cases of conjunctivitis in approximately 1% have been reported.
There have been some reports of impaired renal function (2.3%), although the aetiology appears to be
multifactorial in many cases.
Based on pooled analysis of placebo-controlled studies, severe anaemia (Hb < 8.0 g/dl) was reported
in 5.2% of patients receiving Zometa 4 mg versus 4.2% on placebo.
The following adverse reactions, listed in Table 1, have been accumulated from clinical studies
following predominantly chronic treatment with zoledronic acid:
Adverse reactions are ranked under headings of frequency, the most frequent first, using the following
convention: Very common (≥1/10), common (≥1/100, <1/10), uncommon (≥1/1,000, <1/100), rare
(≥1/10,000, <1/1,000), very rare (<1/10,000), not known (cannot be estimated from the available data).
Blood and lymphatic system disorders
Thrombocytopenia, leukopenia
Dizziness, paraesthesia, taste disturbance,
hypoaesthesia, hyperaesthesia, tremor
Anxiety, sleep disturbance
Gastrointestinal disorders
Diarrhoea, constipation, abdominal pain,
dyspepsia, stomatitis, dry mouth
Respiratory, thoracic and mediastinal disorders
Skin and subcutaneous tissue disorders
Pruritus, rash (including erythematous and
macular rash), increased sweating
Musculoskeletal and connective tissue disorders
Bone pain, myalgia, arthralgia, generalised
pain
Hypertension, hypotension
Renal and urinary disorders
Acute renal failure, haematuria, proteinuria
Hypersensitivity reaction
General disorders and administration site conditions
Fever, flu-like syndrome (including fatigue,
rigors, malaise and flushing)
Asthenia, peripheral oedema, injection site
reactions (including pain, irritation, swelling,
induration), chest pain, weight increase
Nausea, vomiting, anorexia














Blood creatinine and blood urea increased,
hypocalcaemia
Hypomagnesaemia, hypokalaemia
In one 3-year, randomised, double-blind controlled trial that evaluated the efficacy and safety of
zoledronic acid 5 mg once yearly vs. placebo in the treatment of postmenopausal osteoporosis (PMO),
the overall incidence of atrial fibrillation was 2.5% (96 out of 3,862) and 1.9% (75 out of 3,852) in
patients receiving zoledronic acid 5 mg and placebo, respectively. The rate of atrial fibrillation serious
adverse events was 1.3% (51 out of 3,862) and 0.6% (22 out of 3,852) in patients receiving zoledronic
acid 5 mg and placebo, respectively. The imbalance observed in this trial has not been observed in
other trials with zoledronic acid, including those with Zometa (zoledronic acid) 4 mg every 3-4 weeks
in oncology patients. The mechanism behind the increased incidence of atrial fibrillation in this single
clinical trial is unknown.
Post-marketing experience
The following adverse reactions have been reported during post-approval use of Zometa.
Cases of osteonecrosis (primarily of the jaws) have been reported, predominantly in cancer patients
treated with bisphosphonates, including Zometa. Many of these patients had signs of local infection
including osteomyelitis, and the majority of the reports refer to cancer patients following tooth
extractions or other dental surgeries. Osteonecrosis of the jaws has multiple documented risk factors
including a diagnosis of cancer, concomitant therapies (e.g. chemotherapy, radiotherapy,
corticosteroids) and co-morbid conditions (e.g. anaemia, coagulopathies, infection, pre-existing oral
disease). Although causality has not been determined, it is prudent to avoid dental surgery as recovery
may be prolonged (see section 4.4).
In very rare cases, the following events have been reported: hypotension leading to syncope or
circulatory collapse, primarily in patients with underlying risk factors, atrial fibrillation, somnolence,
bronchoconstriction, anaphylactic reaction/shock, urticaria, scleritis and orbital inflammation. Because
these reports are from a population of uncertain size and are subject to confounding factors, it is
difficult to assess causality and to estimate event incidence rates.
Paediatric population
Safety findings in the paediatric population are summarised in section 5.1.
Clinical experience with acute overdose of Zometa is limited. Patients who have received doses higher
than those recommended should be carefully monitored, since renal function impairment (including
renal failure) and serum electrolyte (including calcium, phosphorus and magnesium) abnormalities
have been observed. In the event of hypocalcaemia, calcium gluconate infusions should be
administered as clinically indicated.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Bisphosphonate, ATC code: M05 BA 08
Zoledronic acid belongs to the class of bisphosphonates and acts primarily on bone. It is an inhibitor of
osteoclastic bone resorption.
Hyperkalaemia, hypernatraemia



The selective action of bisphosphonates on bone is based on their high affinity for mineralised bone,
but the precise molecular mechanism leading to the inhibition of osteoclastic activity is still unclear. In
long-term animal studies, zoledronic acid inhibits bone resorption without adversely affecting the
formation, mineralisation or mechanical properties of bone.
In addition to being a potent inhibitor of bone resorption, zoledronic acid also possesses several anti-
tumour properties that could contribute to its overall efficacy in the treatment of metastatic bone
disease. The following properties have been demonstrated in preclinical studies:
-
In vivo:
Inhibition of osteoclastic bone resorption, which alters the bone marrow
microenvironment, making it less conducive to tumour cell growth, anti-angiogenic activity and
anti-pain activity.
In vitro:
Inhibition of osteoblast proliferation, direct cytostatic and pro-apoptotic activity on
tumour cells, synergistic cytostatic effect with other anti-cancer drugs, anti-adhesion/invasion
activity.
Clinical trial results in the prevention of skeletal related events in patients with advanced malignancies
involving bone
The first randomised, double-blind, placebo-controlled study compared Zometa to placebo for the
prevention of skeletal related events (SREs) in prostate cancer patients. Zometa 4 mg significantly
reduced the proportion of patients experiencing at least one skeletal related event (SRE), delayed the
median time to first SRE by > 5 months, and reduced the annual incidence of events per patient -
skeletal morbidity rate. Multiple event analysis showed a 36% risk reduction in developing SREs in
the Zometa group compared with placebo. Patients receiving Zometa reported less increase in pain
than those receiving placebo, and the difference reached significance at months 3, 9, 21 and 24. Fewer
Zometa patients suffered pathological fractures. The treatment effects were less pronounced in patients
with blastic lesions. Efficacy results are provided in Table 2.
In a second study including solid tumours other than breast or prostate cancer, Zometa 4 mg
significantly reduced the proportion of patients with an SRE, delayed the median time to first SRE by
> 2 months, and reduced the skeletal morbidity rate. Multiple event analysis showed 30.7% risk
reduction in developing SREs in the Zometa group compared with placebo. Efficacy results are
provided in Table 3.
Table 2:
Efficacy results (prostate cancer patients receiving hormonal therapy)
Radiation therapy
to bone
Proportion of patients
with SREs (%)
Median time to SRE
(days)
Risk reduction of
suffering from
multiple events** (%)
* Includes vertebral and non-vertebral fractures
** Accounts for all skeletal events, the total number as well as time to each event during the trial
NR Not Reached
NA Not Applicable



















Table 3:
Efficacy results (solid tumours other than breast or prostate cancer)
Radiation therapy
to bone
Proportion of patients
with SREs (%)
Median time to SRE
(days)
Risk reduction of
suffering from
multiple events** (%)
* Includes vertebral and non-vertebral fractures
** Accounts for all skeletal events, the total number as well as time to each event during the trial
NR Not Reached
NA Not Applicable
In a third phase III randomised, double-blind trial, 4 mg Zometa or 90 mg pamidronate every 3 to
4 weeks were compared in patients with multiple myeloma or breast cancer with at least one bone
lesion. The results demonstrated that Zometa 4 mg showed comparable efficacy to 90 mg pamidronate
in the prevention of SREs. The multiple event analysis revealed a significant risk reduction of 16% in
patients treated with Zometa 4 mg in comparison with patients receiving pamidronate. Efficacy results
are provided in Table 4.























Table 4:
Efficacy results (breast cancer and multiple myeloma patients)
Radiation therapy
to bone
Proportion of patients
with SREs (%)
Median time to SRE
(days)
Risk reduction of
suffering from
multiple events** (%)
* Includes vertebral and non-vertebral fractures
** Accounts for all skeletal events, the total number as well as time to each event during the trial
NR Not Reached
NA Not Applicable
Zometa was also studied in a double-blind, randomised, placebo-controlled trial in 228 patients with
documented bone metastases from breast cancer to evaluate the effect of Zometa on the skeletal
related event (SRE) rate ratio, calculated as the total number of SRE events (excluding hypercalcaemia
and adjusted for prior fracture), divided by the total risk period. Patients received either 4 mg Zometa
or placebo every four weeks for one year. Patients were evenly distributed between Zometa-treated
and placebo groups.
The SRE rate (events/person year) was 0.628 for Zometa and 1.096 for placebo. The proportion of
patients with at least one SRE (excluding hypercalcaemia) was 29.8% in the Zometa-treated group
versus 49.6% in the placebo group (p=0.003). Median time to onset of the first SRE was not reached
in the Zometa-treated arm at the end of the study and was significantly prolonged compared to placebo
(p=0.007). Zometa reduced the risk of SREs by 41% in a multiple event analysis (risk ratio=0.59,
p=0.019) compared with placebo.
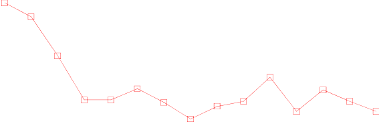
In the Zometa-treated group, statistically significant improvement in pain scores (using the Brief Pain
Inventory, BPI) was seen at 4 weeks and at every subsequent time point during the study, when
compared to placebo (Figure 1). The pain score for Zometa was consistently below baseline and pain
reduction was accompanied by a trend in reduced analgesics score.



















Figure 1. Mean changes from baseline in BPI scores. Statistically significant differences are marked
(*p<0.05) for between treatment comparisons (Zometa vs. Placebo)
Clinical trial results in the treatment of TIH
Clinical studies in tumour-induced hypercalcaemia (TIH) demonstrated that the effect of zoledronic
acid is characterised by decreases in serum calcium and urinary calcium excretion. In Phase I dose
finding studies in patients with mild to moderate tumour-induced hypercalcaemia (TIH), effective
doses tested were in the range of approximately 1.2–2.5 mg.
To assess the effects of Zometa versus pamidronate 90 mg, the results of two pivotal multicentre
studies in patients with TIH were combined in a pre-planned analysis. There was faster normalisation
of corrected serum calcium at day 4 for Zometa 8 mg and at day 7 for Zometa 4 mg and 8 mg. The
following response rates were observed:
Table 5:
Proportion of complete responders by day in the combined TIH studies
*p-values compared to pamidronate.
Median time to normocalcaemia was 4 days. Median time to relapse (re-increase of albumin-corrected
serum calcium ≥ 2.9 mmol/l) was 30 to 40 days for patients treated with Zometa versus 17 days for
those treated with pamidronate 90 mg (p-values: 0.001 for 4 mg and 0.007 for 8 mg). There were no
statistically significant differences between the two Zometa doses.
In clinical trials 69 patients who relapsed or were refractory to initial treatment (Zometa 4 mg, 8 mg or
pamidronate 90 mg) were retreated with Zometa 8 mg. The response rate in these patients was about
52%. Since those patients were retreated with the 8 mg dose only, there are no data available allowing
comparison with the 4 mg dose.
























In clinical trials performed in patients with tumour-induced hypercalcaemia (TIH), the overall safety
profile amongst all three treatment groups (zoledronic acid 4 and 8 mg and pamidronate 90 mg) was
similar in types and severity.
Clinical trial results in the treatment of severe osteogenesis imperfecta in paediatric patients aged 1 to
17 years
The effects of intravenous zoledronic acid in the treatment of paediatric patients (age 1 to 17 years)
with severe osteogenesis imperfecta (types I, III and IV) were compared to intravenous pamidronate in
one international, multicentre, randomised, open-label study with 74 and 76 patients in each treatment
group, respectively. The study treatment period was 12 months preceded by a 4- to 9-week screening
period during which vitamin D and elemental calcium supplements were taken for at least 2 weeks. In
the clinical programme patients aged 1 to < 3 years received 0.025 mg/kg zoledronic acid (up to a
maximum single dose of 0.35 mg) every 3 months and patients aged 3 to 17 years received 0.05 mg/kg
zoledronic acid (up to a maximum single dose of 0.83 mg) every 3 months. An extension study was
conducted in order to examine the long-term general and renal safety of once yearly or twice yearly
zoledronic acid over the 12-month extension treatment period in children who had completed one year
of treatment with either zoledronic acid or pamidronate in the core study.
The primary endpoint of the study was the percent change from baseline in lumbar spine bone mineral
density (BMD) after 12 months of treatment. Estimated treatment effects on BMD were similar, but
the trial design was not sufficiently robust to establish non-inferior efficacy for Zometa. In particular
there was no clear evidence of efficacy on incidence of fracture or on pain. Fracture adverse events of
long bones in the lower extremities were reported in approximately 24% (femur) and 14% (tibia) of
zoledronic acid-treated patients vs 12% and 5% of pamidronate-treated patients with severe
osteogenesis imperfecta, regardless of disease type and causality but overall incidence of fractures was
comparable for the zoledronic acid and pamidronate-treated patients: 43% (32/74) vs 41% (31/76).
Interpretation of the risk of fracture is confounded by the fact that fractures are common events in
patients with severe osteogenesis imperfecta as part of the disease process.
The type of adverse reactions observed in this population were similar to those previously seen in
adults with advanced malignancies involving the bone (see section 4.8). The adverse reactions ranked
under headings of frequency, are presented in Table 6. The following conventional classification is
used: very common (≥1/10), common (≥1/100, <1/10), uncommon (≥1/1,000, <1/100), rare
(≥1/10,000, <1/1,000), very rare (<1/10,000), not known (cannot be estimated from the available data).
Table 6:
Adverse reactions observed in paediatric patients with severe osteogenesis imperfecta
1
Respiratory, thoracic and mediastinal disorders
Gastrointestinal disorders
Musculoskeletal and connective tissue disorders
Pain in extremities, arthralgia, musculoskeletal
pain
General disorders and administration site conditions
Acute phase reaction, pain
Investigations
Very common: Hypocalcaemia
Common: Hypophosphataemia
1
Adverse events occurring with frequencies < 5% were medically assessed and it was shown that
these cases are consistent with the well established safety profile of Zometa (see section 4.8)
In paediatric patients with severe osteogenesis imperfecta, zoledronic acid seems to be associated with
more pronounced risks for acute phase reaction, hypocalcaemia and unexplained tachycardia, in
comparison to pamidronate, but this difference declined after subsequent infusions.
The European Medicines Agency has waived the obligation to submit the results of studies with
Zometa in all subsets of the paediatric population in the treatment of tumour-induced hypercalcaemia
and prevention of skeletal-related events in patients with advanced malignancies involving bone (see
section 4.2 for information on paediatric use).
5.2 Pharmacokinetic properties
Single and multiple 5- and 15-minute infusions of 2, 4, 8 and 16 mg zoledronic acid in 64 patients
with bone metastases yielded the following pharmacokinetic data, which were found to be dose
independent.
After initiating the infusion of zoledronic acid, the plasma concentrations of drug rapidly increased,
achieving their peak at the end of the infusion period, followed by a rapid decline to < 10% of peak
after 4 hours and < 1% of peak after 24 hours, with a subsequent prolonged period of very low
concentrations not exceeding 0.1% of peak prior to the second infusion of drug on day 28.
Intravenously administered zoledronic acid is eliminated by a triphasic process: rapid biphasic
disappearance from the systemic circulation, with half-lives of t
½α
0.24 and t
½β
1.87 hours, followed by
a long elimination phase with a terminal elimination half-life of t
½γ
146 hours. There was no
accumulation of drug in plasma after multiple doses of the drug given every 28 days. Zoledronic acid
is not metabolised and is excreted unchanged via the kidney. Over the first 24 hours, 39 ± 16% of the
administered dose is recovered in the urine, while the remainder is principally bound to bone tissue.
From the bone tissue it is released very slowly back into the systemic circulation and eliminated via
the kidney. The total body clearance is 5.04 ± 2.5 l/h, independent of dose, and unaffected by gender,
age, race, and body weight. Increasing the infusion time from 5 to 15 minutes caused a 30% decrease
in zoledronic acid concentration at the end of the infusion, but had no effect on the area under the
plasma concentration versus time curve.









The interpatient variability in pharmacokinetic parameters for zoledronic acid was high, as seen with
other bisphosphonates.
No pharmacokinetic data for zoledronic acid are available in patients with hypercalcaemia or in
patients with hepatic insufficiency. Zoledronic acid does not inhibit human P450 enzymes
in vitro
,
shows no biotransformation and in animal studies < 3% of the administered dose was recovered in the
faeces, suggesting no relevant role of liver function in the pharmacokinetics of zoledronic acid.
The renal clearance of zoledronic acid was correlated with creatinine clearance, renal clearance
representing 75 ± 33% of the creatinine clearance, which showed a mean of 84 ± 29 ml/min (range 22
to 143 ml/min) in the 64 cancer patients studied. Population analysis showed that for a patient with
creatinine clearance of 20 ml/min (severe renal impairment), or 50 ml/min (moderate impairment), the
corresponding predicted clearance of zoledronic acid would be 37% or 72%, respectively, of that of a
patient showing creatinine clearance of 84 ml/min. Only limited pharmacokinetic data are available in
patients with severe renal insufficiency (creatinine clearance < 30 ml/min).
Zoledronic acid shows no affinity for the cellular components of blood and plasma protein binding is
low (approximately 56%) and independent of the concentration of zoledronic acid.
Special populations
Paediatric patients
Limited pharmacokinetic data in children with severe osteogenesis imperfecta suggest that zoledronic
acid pharmacokinetics in children aged 3 to 17 years are similar to those in adults at a similar mg/kg
dose level. Age, body weight, gender and creatinine clearance appear to have no effect on zoledronic
acid systemic exposure.
5.3 Preclinical safety data
Acute toxicity
The highest non-lethal single intravenous dose was 10 mg/kg bodyweight in mice and 0.6 mg/kg in
rats.
Subchronic and chronic toxicity
Zoledronic acid was well tolerated when administered subcutaneously to rats and intravenously to
dogs at doses up to 0.02 mg/kg daily for 4 weeks. Administration of 0.001 mg/kg/day subcutaneously
in rats and 0.005 mg/kg intravenously once every 2–3 days in dogs for up to 52 weeks was also well
tolerated.
The most frequent finding in repeat-dose studies consisted of increased primary spongiosa in the
metaphyses of long bones in growing animals at nearly all doses, a finding that reflected the
compound’s pharmacological antiresorptive activity.
The safety margins relative to renal effects were narrow in the long-term repeat-dose parenteral animal
studies but the cumulative no adverse event levels (NOAELs) in the single dose (1.6 mg/kg) and
multiple dose studies of up to one month (0.06–0.6 mg/kg/day) did not indicate renal effects at doses
equivalent to or exceeding the highest intended human therapeutic dose. Longer-term repeat
administration at doses bracketing the highest intended human therapeutic dose of zoledronic acid
produced toxicological effects in other organs, including the gastrointestinal tract, liver, spleen and
lungs, and at intravenous injection sites.
Reproduction toxicity
Zoledronic acid was teratogenic in the rat at subcutaneous doses ≥ 0.2 mg/kg. Although no
teratogenicity or foetotoxicity was observed in the rabbit, maternal toxicity was found. Dystocia was
observed at the lowest dose (0.01 mg/kg bodyweight) tested in the rat.
Mutagenicity and carcinogenic potential
Zoledronic acid was not mutagenic in the mutagenicity tests performed and carcinogenicity testing did
not provide any evidence of carcinogenic potential.
PHARMACEUTICAL PARTICULARS
Powder vial: Mannitol
Sodium citrate
Solvent ampoule: Water for injections
To avoid potential incompatibilities, Zometa reconstituted solution is to be diluted with 0.9% w/v
sodium chloride solution or 5% w/v glucose solution.
Zometa reconstituted solution must not be mixed with calcium or other divalent cation-containing
infusion solutions such as lactated Ringer’s solution, and should be administered as a single
intravenous solution in a separate infusion line.
Studies with glass bottles, as well as several types of infusion bags and infusion lines made from
polyvinylchloride, polyethylene and polypropylene (prefilled with 0.9% w/v sodium chloride solution
or 5% w/v glucose solution), showed no incompatibility with Zometa.
The reconstituted solution is chemically and physically stable for 24 hours at 2°C – 8°C.
6.4 Special precautions for storage
No special precautions for storage.
After aseptic reconstitution and dilution, it is preferable to use the reconstituted and diluted product
immediately. If not used immediately, the duration and conditions of storage prior to use are the user’s
responsibility. The total time between reconstitution, dilution, storage in a refrigerator at 2°C – 8°C
and end of administration must not exceed 24 hours.
6.5 Nature and contents of container
Zometa 4 mg powder for solution for infusion is supplied as packs containing 1, 4 or 10 vials and 1, 4
or 10 ampoules of water for injections, respectively. Not all pack sizes may be marketed.
Powder vial: 6-ml colourless glass vial, hydrolytic glass type I (Ph. Eur.).
Solvent ampoule: 5-ml colourless glass ampoule.
6.6 Special precautions for disposal and other handling
The powder must first be reconstituted in the vial using 5 ml water for injections from the ampoule
supplied. Dissolution must be complete before the solution is withdrawn. The amount of reconstituted
solution as required is then further diluted with 100 ml of calcium-free infusion solution (0.9% w/v
sodium chloride solution or 5% w/v glucose solution). If refrigerated, the solution must be allowed to
reach room temperature before administration.
MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
MARKETING AUTHORISATION NUMBERS
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 20.03.2001
Date of first renewal: 20.03.2006
10. DATE OF REVISION OF THE TEXT
NAME OF THE MEDICINAL PRODUCT
Zometa 4 mg/5 ml concentrate for solution for infusion
QUALITATIVE AND QUANTITATIVE COMPOSITION
One vial with 5 ml concentrate contains 4 mg zoledronic acid (anhydrous).
One ml concentrate contains zoledronic acid (as monohydrate) corresponding to 0.8 mg zoledronic
acid (anhydrous).
For a full list of excipients, see section 6.1.
Concentrate for solution for infusion
4.1 Therapeutic indications
Prevention of skeletal related events (pathological fractures, spinal compression, radiation or
surgery to bone, or tumour-induced hypercalcaemia) in patients with advanced malignancies
involving bone.
Treatment of tumour-induced hypercalcaemia (TIH).
4.2 Posology and method of administration
Zometa must only be used by clinicians experienced in the administration of intravenous
bisphosphonates.
Zometa concentrate must not be mixed with calcium or other divalent cation-containing infusion
solutions such as lactated Ringer’s solution, and should be administered as a single intravenous
solution in a separate infusion line.
Prevention of skeletal related events in patients with advanced malignancies involving bone
Adults and elderly
The recommended dose in the prevention of skeletal related events in patients with advanced
malignancies involving bone is 4 mg zoledronic acid. The concentrate must be further diluted with
100 ml sterile 0.9% w/v sodium chloride or 5% w/v glucose solution and given in no less than a 15-
minute intravenous infusion every 3 to 4 weeks.
Patients should also be administered an oral calcium supplement of 500 mg and 400 IU vitamin D
daily.
Treatment of TIH
Adults and elderly
The recommended dose in hypercalcaemia (albumin-corrected serum calcium ≥ 12.0 mg/dl or
3.0 mmol/l) is 4 mg zoledronic acid. The concentrate must be further diluted with 100 ml sterile 0.9%
w/v sodium chloride or 5% w/v glucose solution and given as a single intravenous infusion in no less
than 15 minutes. Patients must be maintained well hydrated prior to and following administration of
Zometa.
Renal impairment
TIH:
Zometa treatment in TIH patients who also have severe renal impairment should be considered only
after evaluating the risks and benefits of treatment. In the clinical studies, patients with serum
creatinine > 400 µmol/l or > 4.5 mg/dl were excluded. No dose adjustment is necessary in TIH
p
atients with serum creatinine < 400 µmol/l or < 4.5 mg/dl (see section 4.4).
Prevention of skeletal related events in patients with advanced malignancies involving bone:
When initiating treatment with Zometa in patients with multiple myeloma or metastatic bone lesions
from solid tumours, serum creatinine and creatinine clearance (CLcr) should be determined. CLcr is
calculated from serum creatinine using the Cockcroft-Gault formula. Zometa is not recommended for
patients presenting with severe renal impairment prior to initiation of therapy, which is defined for this
population as CLcr < 30 ml/min. In clinical trials with Zometa, patients with serum creatinine
> 265 µmol/l or > 3.0 mg/dl were excluded.
In patients with bone metastases presenting with mild to moderate renal impairment prior to initiation
of therapy, which is defined for this population as CLcr 30–60 ml/min, the following Zometa dose is
recommended (see also section 4.4):
Baseline Creatinine Clearance (ml/min)
Zometa Recommended Dose*
> 60 4.0 mg
50–60 3.5 mg*
40–49 3.3 mg*
30–39 3.0 mg*
*
Doses have been calculated assuming target AUC of 0.66 (mg•hr/l) (CLcr=75 ml/min). The reduced
doses for patients with renal impairment are expected to achieve the same AUC as that seen in patients
with creatinine clearance of 75 ml/min.
Following initiation of therapy, serum creatinine should be measured prior to each dose of Zometa and
treatment should be withheld if renal function has deteriorated. In the clinical trials, renal deterioration
was defined as follows:
-
For patients with normal baseline serum creatinine (< 1.4 mg/dl or < 124 µmol/l), an increase of
0.5 mg/dl or 44 µmol/l;
For patients with an abnormal baseline creatinine (> 1.4 mg/dl or > 124 µmol/l), an increase of
1.0 mg/dl or 88 µmol/l.
In the clinical studies, Zometa treatment was resumed only when the creatinine level returned to
within 10% of the baseline value (see section 4.4). Zometa treatment should be resumed at the same
dose as that prior to treatment interruption.
Instructions for preparing reduced doses of Zometa
Withdraw an appropriate volume of the concentrate needed, as follows:
-
The withdrawn amount of concentrate must be further diluted in 100 ml of sterile 0.9% w/v sodium
chloride solution or 5% w/v glucose solution. The dose must be given as a single intravenous infusion
over no less than 15 minutes
.
The use of Zometa in paediatric patients has been studied in 2 clinical trials in the treatment of severe
osteogenesis imperfecta (see section 5.1). Zometa should not be used in the paediatric population
because safety and efficacy in children have not been established (see sections 4.4 and 5.1).

Hypersensitivity to the active substance, to other bisphosphonates or to any of the excipients in
the formulation of Zometa
Breast-feeding (see section 4.6)
4.4 Special warnings and precautions for use
General
Patients must be assessed prior to administration of Zometa to ensure that they are adequately
hydrated.
Overhydration should be avoided in patients at risk of cardiac failure.
Standard hypercalcaemia-related metabolic parameters, such as serum levels of calcium, phosphate
and magnesium, should be carefully monitored after initiating Zometa therapy. If hypocalcaemia,
hypophosphataemia, or hypomagnesaemia occurs, short-term supplemental therapy may be necessary.
Untreated hypercalcaemia patients generally have some degree of renal function impairment, therefore
careful renal function monitoring should be considered.
Zometa contains the same active substance as found in Aclasta (zoledronic acid). Patients being
treated with Zometa should not be treated with Aclasta concomitantly.
The safety and efficacy of Zometa in paediatric patients have not been established (see section 5.1).
Renal insufficiency
Patients with TIH with evidence of deterioration in renal function should be appropriately evaluated
with consideration given as to whether the potential benefit of treatment with Zometa outweighs the
possible risk.
The decision to treat patients with bone metastases for the prevention of skeletal related events should
consider that the onset of treatment effect is 2–3 months.
As with other bisphosphonates, Zometa has been associated with reports of renal dysfunction. Factors
that may increase the potential for deterioration in renal function include dehydration, pre-existing
renal impairment, multiple cycles of Zometa and other bisphosphonates as well as use of other
nephrotoxic drugs. While the risk is reduced with a dose of Zometa 4 mg administered over
15 minutes, deterioration in renal function may still occur. Renal deterioration, progression to renal
failure and dialysis have been reported in patients after the initial dose or a single dose of Zometa.
Increases in serum creatinine also occur in some patients with chronic administration of Zometa at
recommended doses for prevention of skeletal related events, although less frequently.
Patients should have their serum creatinine levels assessed prior to each dose of Zometa. Upon
initiation of treatment in patients with bone metastases with mild to moderate renal impairment, lower
doses of Zometa are recommended. In patients who show evidence of renal deterioration during
treatment, Zometa should be withheld. Zometa should only be resumed when serum creatinine returns
to within 10% of baseline (see section 4.2).
In view of the potential impact of bisphosphonates, including Zometa, on renal function, the lack of
clinical safety data in patients with severe renal impairment (in clinical trials defined as serum
creatinine ≥ 400 µmol/l or ≥ 4.5 mg/dl for patients with TIH and ≥ 265 µmol/l or ≥ 3.0 mg/dl for
patients with cancer and bone metastases, respectively) at baseline and only limited pharmacokinetic
data in patients with severe renal impairment at baseline (creatinine clearance < 30 ml/min), the use of
Zometa is not recommended in patients with severe renal impairment.
Hepatic insufficiency
As only limited clinical data are available in patients with severe hepatic insufficiency, no specific
recommendations can be given for this patient population.
Osteonecrosis of the jaw
Osteonecrosis of the jaw has been reported in patients, predominantly those with cancer, receiving
treatment with bisphosphonates, including Zometa. Many of these patients were also receiving
chemotherapy and corticosteroids. The majority of reported cases have been associated with dental
procedures such as tooth extraction. Many had signs of local infection including osteomyelitis.
A dental examination with appropriate preventive dentistry should be considered prior to treatment
with bisphosphonates in patients with concomitant risk factors (e.g. cancer, chemotherapy,
corticosteroids, poor oral hygiene).
While on treatment, these patients should avoid invasive dental procedures if possible. For patients
who develop osteonecrosis of the jaw while on bisphosphonate therapy, dental surgery may exacerbate
the condition. For patients requiring dental procedures, there are no data available to suggest whether
discontinuation of bisphosphonate treatment reduces the risk of osteonecrosis of the jaw. Clinical
judgement of the treating physician should guide the management plan of each patient based on
individual benefit/risk assessment.
Musculoskeletal pain
In post-marketing experience, severe and occasionally incapacitating bone, joint, and/or muscle pain
have been reported in patients taking bisphosphonates. However, such reports have been infrequent.
This category of drugs includes Zometa (zoledronic acid). The time to onset of symptoms varied from
one day to several months after starting treatment. Most patients had relief of symptoms after stopping
treatment. A subset had recurrence of symptoms when rechallenged with the same drug or another
bisphosphonate.
4.5 Interaction with other medicinal products and other forms of interaction
In clinical studies, Zometa has been administered concomitantly with commonly used anticancer
agents, diuretics, antibiotics and analgesics without clinically apparent interactions occurring.
Zoledronic acid shows no appreciable binding to plasma proteins and does not inhibit human P450
enzymes
in vitro
(see section 5.2), but no formal clinical interaction studies have been performed.
Caution is advised when bisphosphonates are administered with aminoglycosides, since both agents
may have an additive effect, resulting in a lower serum calcium level for longer periods than required.
Caution is indicated when Zometa is used with other potentially nephrotoxic drugs. Attention should
also be paid to the possibility of hypomagnesaemia developing during treatment.
In multiple myeloma patients, the risk of renal dysfunction may be increased when intravenous
bisphosphonates are used in combination with thalidomide.
4.6 Pregnancy and lactation
Pregnancy
There are no adequate data on the use of zoledronic acid in pregnant women. Animal reproduction
studies with zoledronic acid have shown reproductive toxicity (see section 5.3). The potential risk for
humans is unknown. Zometa should not be used during pregnancy.
Lactation
It is not known whether zoledronic acid is excreted into human milk. Zometa is contraindicated in
breast-feeding women (see section 4.3).
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed.
Frequencies of adverse reactions for Zometa 4 mg are mainly based on data collection from chronic
treatment. Adverse reactions to Zometa are similar to those reported for other bisphosphonates and can
be expected to occur in approximately one third of patients. Intravenous administration has been most
commonly associated with a flu-like syndrome in about 9% of patients, including bone pain (9.1%),
fever (7.2%), fatigue (4.1%) and rigors (2.9%). Occasionally cases of arthralgia and myalgia in
approximately 3% have been reported. No information is available on the reversibility of these adverse
effects.
Frequently, the reduction in renal calcium excretion is accompanied by a fall in serum phosphate
levels (in approximately 20% of patients), which is asymptomatic not requiring treatment. The serum
calcium may fall to asymptomatic hypocalcaemic levels in approximately 3% of patients.
Gastrointestinal reactions, such as nausea (5.8%) and vomiting (2.6%) have been reported following
intravenous infusion of Zometa. Occasionally local reactions at the infusion site such as redness or
swelling and/or pain were also observed in less than 1% of the patients.
Anorexia was reported in 1.5% of patients treated with Zometa 4 mg.
Few cases of rash or pruritus have been observed (below 1%).
As with other bisphosphonates, cases of conjunctivitis in approximately 1% have been reported.
There have been some reports of impaired renal function (2.3%), although the aetiology appears to be
multifactorial in many cases.
Based on pooled analysis of placebo-controlled studies, severe anaemia (Hb < 8.0 g/dl) was reported
in 5.2% of patients receiving Zometa 4 mg versus 4.2% on placebo.
The following adverse reactions, listed in Table 1, have been accumulated from clinical studies
following predominantly chronic treatment with zoledronic acid:
Adverse reactions are ranked under headings of frequency, the most frequent first, using the following
convention: Very common (≥1/10), common (≥1/100, <1/10), uncommon (≥1/1,000, <1/100), rare
(≥1/10,000, <1/1,000), very rare (<1/10,000), not known (cannot be estimated from the available data).
Blood and lymphatic system disorders
Thrombocytopenia, leukopenia
Dizziness, paraesthesia, taste disturbance,
hypoaesthesia, hyperaesthesia, tremor
Anxiety, sleep disturbance
Gastrointestinal disorders
Diarrhoea, constipation, abdominal pain,
dyspepsia, stomatitis, dry mouth
Respiratory, thoracic and mediastinal disorders
Skin and subcutaneous tissue disorders
Pruritus, rash (including erythematous and
macular rash), increased sweating
Musculoskeletal and connective tissue disorders
Bone pain, myalgia, arthralgia, generalised
pain
Hypertension, hypotension
Renal and urinary disorders
Acute renal failure, haematuria, proteinuria
Hypersensitivity reaction
General disorders and administration site conditions
Fever, flu-like syndrome (including fatigue,
rigors, malaise and flushing)
Asthenia, peripheral oedema, injection site
reactions (including pain, irritation, swelling,
induration), chest pain, weight increase
Nausea, vomiting, anorexia














Blood creatinine and blood urea increased,
hypocalcaemia
Hypomagnesaemia, hypokalaemia
In one 3-year, randomised, double-blind controlled trial that evaluated the efficacy and safety of
zoledronic acid 5 mg once yearly vs. placebo in the treatment of postmenopausal osteoporosis (PMO),
the overall incidence of atrial fibrillation was 2.5% (96 out of 3,862) and 1.9% (75 out of 3,852) in
patients receiving zoledronic acid 5 mg and placebo, respectively. The rate of atrial fibrillation serious
adverse events was 1.3% (51 out of 3,862) and 0.6% (22 out of 3,852) in patients receiving zoledronic
acid 5 mg and placebo, respectively. The imbalance observed in this trial has not been observed in
other trials with zoledronic acid, including those with Zometa (zoledronic acid) 4 mg every 3-4 weeks
in oncology patients. The mechanism behind the increased incidence of atrial fibrillation in this single
clinical trial is unknown.
Post-marketing experience
The following adverse reactions have been reported during post-approval use of Zometa.
Cases of osteonecrosis (primarily of the jaws) have been reported, predominantly in cancer patients
treated with bisphosphonates, including Zometa. Many of these patients had signs of local infection
including osteomyelitis, and the majority of the reports refer to cancer patients following tooth
extractions or other dental surgeries. Osteonecrosis of the jaws has multiple documented risk factors
including a diagnosis of cancer, concomitant therapies (e.g. chemotherapy, radiotherapy,
corticosteroids) and co-morbid conditions (e.g. anaemia, coagulopathies, infection, pre-existing oral
disease). Although causality has not been determined, it is prudent to avoid dental surgery as recovery
may be prolonged (see section 4.4).
In very rare cases, the following events have been reported: hypotension leading to syncope or
circulatory collapse, primarily in patients with underlying risk factors, atrial fibrillation, somnolence,
bronchoconstriction, anaphylactic reaction/shock, urticaria, scleritis and orbital inflammation. Because
these reports are from a population of uncertain size and are subject to confounding factors, it is
difficult to assess causality and to estimate event incidence rates.
Paediatric population
Safety findings in the paediatric population are summarised in section 5.1.
Clinical experience with acute overdose of Zometa is limited. Patients who have received doses higher
than those recommended should be carefully monitored, since renal function impairment (including
renal failure) and serum electrolyte (including calcium, phosphorus and magnesium) abnormalities
have been observed. In the event of hypocalcaemia, calcium gluconate infusions should be
administered as clinically indicated.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Bisphosphonate, ATC code: M05 BA 08
Zoledronic acid belongs to the class of bisphosphonates and acts primarily on bone. It is an inhibitor of
osteoclastic bone resorption.
Hyperkalaemia, hypernatraemia



The selective action of bisphosphonates on bone is based on their high affinity for mineralised bone,
but the precise molecular mechanism leading to the inhibition of osteoclastic activity is still unclear. In
long-term animal studies, zoledronic acid inhibits bone resorption without adversely affecting the
formation, mineralisation or mechanical properties of bone.
In addition to being a potent inhibitor of bone resorption, zoledronic acid also possesses several anti-
tumour properties that could contribute to its overall efficacy in the treatment of metastatic bone
disease. The following properties have been demonstrated in preclinical studies:
-
In vivo:
Inhibition of osteoclastic bone resorption, which alters the bone marrow
microenvironment, making it less conducive to tumour cell growth, anti-angiogenic activity and
anti-pain activity.
In vitro:
Inhibition of osteoblast proliferation, direct cytostatic and pro-apoptotic activity on
tumour cells, synergistic cytostatic effect with other anti-cancer drugs, anti-adhesion/invasion
activity.
Clinical trial results in the prevention of skeletal related events in patients with advanced malignancies
involving bone
The first randomised, double-blind, placebo-controlled study compared Zometa to placebo for the
prevention of skeletal related events (SREs) in prostate cancer patients. Zometa 4 mg significantly
reduced the proportion of patients experiencing at least one skeletal related event (SRE), delayed the
median time to first SRE by > 5 months, and reduced the annual incidence of events per patient -
skeletal morbidity rate. Multiple event analysis showed a 36% risk reduction in developing SREs in
the Zometa group compared with placebo. Patients receiving Zometa reported less increase in pain
than those receiving placebo, and the difference reached significance at months 3, 9, 21 and 24. Fewer
Zometa patients suffered pathological fractures. The treatment effects were less pronounced in patients
with blastic lesions. Efficacy results are provided in Table 2.
In a second study including solid tumours other than breast or prostate cancer, Zometa 4 mg
significantly reduced the proportion of patients with an SRE, delayed the median time to first SRE by
> 2 months, and reduced the skeletal morbidity rate. Multiple event analysis showed 30.7% risk
reduction in developing SREs in the Zometa group compared with placebo. Efficacy results are
provided in Table 3.
Table 2:
Efficacy results (prostate cancer patients receiving hormonal therapy)
Radiation therapy
to bone
Proportion of patients
with SREs (%)
Median time to SRE
(days)
Risk reduction of
suffering from
multiple events** (%)
* Includes vertebral and non-vertebral fractures
** Accounts for all skeletal events, the total number as well as time to each event during the trial
NR Not Reached
NA Not Applicable



















Table 3:
Efficacy results (solid tumours other than breast or prostate cancer)
Radiation therapy
to bone
Proportion of patients
with SREs (%)
Median time to SRE
(days)
Risk reduction of
suffering from
multiple events** (%)
* Includes vertebral and non-vertebral fractures
** Accounts for all skeletal events, the total number as well as time to each event during the trial
NR Not Reached
NA Not Applicable
In a third phase III randomised, double-blind trial, 4 mg Zometa or 90 mg pamidronate every 3 to
4 weeks were compared in patients with multiple myeloma or breast cancer with at least one bone
lesion. The results demonstrated that Zometa 4 mg showed comparable efficacy to 90 mg pamidronate
in the prevention of SREs. The multiple event analysis revealed a significant risk reduction of 16% in
patients treated with Zometa 4 mg in comparison with patients receiving pamidronate. Efficacy results
are provided in Table 4.























Table 4:
Efficacy results (breast cancer and multiple myeloma patients)
Radiation therapy
to bone
Proportion of patients
with SREs (%)
Median time to SRE
(days)
Risk reduction of
suffering from
multiple events** (%)
* Includes vertebral and non-vertebral fractures
** Accounts for all skeletal events, the total number as well as time to each event during the trial
NR Not Reached
NA Not Applicable
Zometa was also studied in a double-blind, randomised, placebo-controlled trial in 228 patients with
documented bone metastases from breast cancer to evaluate the effect of Zometa on the skeletal
related event (SRE) rate ratio, calculated as the total number of SRE events (excluding hypercalcaemia
and adjusted for prior fracture), divided by the total risk period. Patients received either 4 mg Zometa
or placebo every four weeks for one year. Patients were evenly distributed between Zometa-treated
and placebo groups.
The SRE rate (events/person year) was 0.628 for Zometa and 1.096 for placebo. The proportion of
patients with at least one SRE (excluding hypercalcaemia) was 29.8% in the Zometa-treated group
versus 49.6% in the placebo group (p=0.003). Median time to onset of the first SRE was not reached
in the Zometa-treated arm at the end of the study and was significantly prolonged compared to placebo
(p=0.007). Zometa reduced the risk of SREs by 41% in a multiple event analysis (risk ratio=0.59,
p=0.019) compared with placebo.
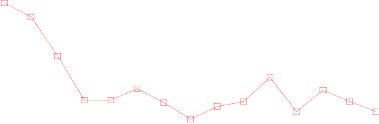
In the Zometa-treated group, statistically significant improvement in pain scores (using the Brief Pain
Inventory, BPI) was seen at 4 weeks and at every subsequent time point during the study, when
compared to placebo (Figure 1). The pain score for Zometa was consistently below baseline and pain
reduction was accompanied by a trend in reduced analgesics score.



















Figure 1. Mean changes from baseline in BPI scores. Statistically significant differences are marked
(*p<0.05) for between treatment comparisons (Zometa vs. Placebo)
Clinical trial results in the treatment of TIH
Clinical studies in tumour-induced hypercalcaemia (TIH) demonstrated that the effect of zoledronic
acid is characterised by decreases in serum calcium and urinary calcium excretion. In Phase I dose
finding studies in patients with mild to moderate tumour-induced hypercalcaemia (TIH), effective
doses tested were in the range of approximately 1.2–2.5 mg.
To assess the effects of Zometa versus pamidronate 90 mg, the results of two pivotal multicentre
studies in patients with TIH were combined in a pre-planned analysis. There was faster normalisation
of corrected serum calcium at day 4 for Zometa 8 mg and at day 7 for Zometa 4 mg and 8 mg. The
following response rates were observed:
Table 5:
Proportion of complete responders by day in the combined TIH studies
*p-values compared to pamidronate.
Median time to normocalcaemia was 4 days. Median time to relapse (re-increase of albumin-corrected
serum calcium ≥ 2.9 mmol/l) was 30 to 40 days for patients treated with Zometa versus 17 days for
those treated with pamidronate 90 mg (p-values: 0.001 for 4 mg and 0.007 for 8 mg). There were no
statistically significant differences between the two Zometa doses.
In clinical trials 69 patients who relapsed or were refractory to initial treatment (Zometa 4 mg, 8 mg or
pamidronate 90 mg) were retreated with Zometa 8 mg. The response rate in these patients was about
52%. Since those patients were retreated with the 8 mg dose only, there are no data available allowing
comparison with the 4 mg dose.





















In clinical trials performed in patients with tumour-induced hypercalcaemia (TIH), the overall safety
profile amongst all three treatment groups (zoledronic acid 4 and 8 mg and pamidronate 90 mg) was
similar in types and severity.
Clinical trial results in the treatment of severe osteogenesis imperfecta in paediatric patients aged 1 to
17 years
The effects of intravenous zoledronic acid in the treatment of paediatric patients (age 1 to 17 years)
with severe osteogenesis imperfecta (types I, III and IV) were compared to intravenous pamidronate in
one international, multicentre, randomised, open-label study with 74 and 76 patients in each treatment
group, respectively. The study treatment period was 12 months preceded by a 4- to 9-week screening
period during which vitamin D and elemental calcium supplements were taken for at least 2 weeks. In
the clinical programme patients aged 1 to < 3 years received 0.025 mg/kg zoledronic acid (up to a
maximum single dose of 0.35 mg) every 3 months and patients aged 3 to 17 years received 0.05 mg/kg
zoledronic acid (up to a maximum single dose of 0.83 mg) every 3 months. An extension study was
conducted in order to examine the long-term general and renal safety of once yearly or twice yearly
zoledronic acid over the 12-month extension treatment period in children who had completed one year
of treatment with either zoledronic acid or pamidronate in the core study.
The primary endpoint of the study was the percent change from baseline in lumbar spine bone mineral
density (BMD) after 12 months of treatment. Estimated treatment effects on BMD were similar, but
the trial design was not sufficiently robust to establish non-inferior efficacy for Zometa. In particular
there was no clear evidence of efficacy on incidence of fracture or on pain. Fracture adverse events of
long bones in the lower extremities were reported in approximately 24% (femur) and 14% (tibia) of
zoledronic acid-treated patients vs 12% and 5% of pamidronate-treated patients with severe
osteogenesis imperfecta, regardless of disease type and causality but overall incidence of fractures was
comparable for the zoledronic acid and pamidronate-treated patients: 43% (32/74) vs 41% (31/76).
Interpretation of the risk of fracture is confounded by the fact that fractures are common events in
patients with severe osteogenesis imperfecta as part of the disease process.
The type of adverse reactions observed in this population were similar to those previously seen in
adults with advanced malignancies involving the bone (see section 4.8). The adverse reactions ranked
under headings of frequency, are presented in Table 6. The following conventional classification is
used: very common (≥1/10), common (≥1/100, <1/10), uncommon (≥1/1,000, <1/100), rare
(≥1/10,000, <1/1,000), very rare (<1/10,000), not known (cannot be estimated from the available data).
Table 6:
Adverse reactions observed in paediatric patients with severe osteogenesis imperfecta
1
Respiratory, thoracic and mediastinal disorders
Gastrointestinal disorders
Musculoskeletal and connective tissue disorders
Pain in extremities, arthralgia, musculoskeletal
pain
General disorders and administration site conditions
Acute phase reaction, pain
Investigations
Very common: Hypocalcaemia
Common: Hypophosphataemia
1
Adverse events occurring with frequencies < 5% were medically assessed and it was shown that
these cases are consistent with the well established safety profile of Zometa (see section 4.8)
In paediatric patients with severe osteogenesis imperfecta, zoledronic acid seems to be associated with
more pronounced risks for acute phase reaction, hypocalcaemia and unexplained tachycardia, in
comparison to pamidronate, but this difference declined after subsequent infusions.
The European Medicines Agency has waived the obligation to submit the results of studies with
Zometa in all subsets of the paediatric population in the treatment of tumour-induced hypercalcaemia
and prevention of skeletal-related events in patients with advanced malignancies involving bone (see
section 4.2 for information on paediatric use).
5.2 Pharmacokinetic properties
Single and multiple 5- and 15-minute infusions of 2, 4, 8 and 16 mg zoledronic acid in 64 patients
with bone metastases yielded the following pharmacokinetic data, which were found to be dose
independent.
After initiating the infusion of zoledronic acid, the plasma concentrations of drug rapidly increased,
achieving their peak at the end of the infusion period, followed by a rapid decline to < 10% of peak
after 4 hours and < 1% of peak after 24 hours, with a subsequent prolonged period of very low
concentrations not exceeding 0.1% of peak prior to the second infusion of drug on day 28.
Intravenously administered zoledronic acid is eliminated by a triphasic process: rapid biphasic
disappearance from the systemic circulation, with half-lives of t
½α
0.24 and t
½β
1.87 hours, followed by
a long elimination phase with a terminal elimination half-life of t
½γ
146 hours. There was no
accumulation of drug in plasma after multiple doses of the drug given every 28 days. Zoledronic acid
is not metabolised and is excreted unchanged via the kidney. Over the first 24 hours, 39 ± 16% of the
administered dose is recovered in the urine, while the remainder is principally bound to bone tissue.
From the bone tissue it is released very slowly back into the systemic circulation and eliminated via
the kidney. The total body clearance is 5.04 ± 2.5 l/h, independent of dose, and unaffected by gender,
age, race, and body weight. Increasing the infusion time from 5 to 15 minutes caused a 30% decrease
in zoledronic acid concentration at the end of the infusion, but had no effect on the area under the
plasma concentration versus time curve.









The interpatient variability in pharmacokinetic parameters for zoledronic acid was high, as seen with
other bisphosphonates.
No pharmacokinetic data for zoledronic acid are available in patients with hypercalcaemia or in
patients with hepatic insufficiency. Zoledronic acid does not inhibit human P450 enzymes
in vitro
,
shows no biotransformation and in animal studies < 3% of the administered dose was recovered in the
faeces, suggesting no relevant role of liver function in the pharmacokinetics of zoledronic acid.
The renal clearance of zoledronic acid was correlated with creatinine clearance, renal clearance
representing 75 ± 33% of the creatinine clearance, which showed a mean of 84 ± 29 ml/min (range 22
to 143 ml/min) in the 64 cancer patients studied. Population analysis showed that for a patient with
creatinine clearance of 20 ml/min (severe renal impairment), or 50 ml/min (moderate impairment), the
corresponding predicted clearance of zoledronic acid would be 37% or 72%, respectively, of that of a
patient showing creatinine clearance of 84 ml/min. Only limited pharmacokinetic data are available in
patients with severe renal insufficiency (creatinine clearance < 30 ml/min).
Zoledronic acid shows no affinity for the cellular components of blood and plasma protein binding is
low (approximately 56%) and independent of the concentration of zoledronic acid.
Special populations
Paediatric patients
Limited pharmacokinetic data in children with severe osteogenesis imperfecta suggest that zoledronic
acid pharmacokinetics in children aged 3 to 17 years are similar to those in adults at a similar mg/kg
dose level. Age, body weight, gender and creatinine clearance appear to have no effect on zoledronic
acid systemic exposure.
5.3 Preclinical safety data
Acute toxicity
The highest non-lethal single intravenous dose was 10 mg/kg bodyweight in mice and 0.6 mg/kg in
rats.
Subchronic and chronic toxicity
Zoledronic acid was well tolerated when administered subcutaneously to rats and intravenously to
dogs at doses up to 0.02 mg/kg daily for 4 weeks. Administration of 0.001 mg/kg/day subcutaneously
in rats and 0.005 mg/kg intravenously once every 2–3 days in dogs for up to 52 weeks was also well
tolerated.
The most frequent finding in repeat-dose studies consisted of increased primary spongiosa in the
metaphyses of long bones in growing animals at nearly all doses, a finding that reflected the
compound’s pharmacological antiresorptive activity.
The safety margins relative to renal effects were narrow in the long-term repeat-dose parenteral animal
studies but the cumulative no adverse event levels (NOAELs) in the single dose (1.6 mg/kg) and
multiple dose studies of up to one month (0.06–0.6 mg/kg/day) did not indicate renal effects at doses
equivalent to or exceeding the highest intended human therapeutic dose. Longer-term repeat
administration at doses bracketing the highest intended human therapeutic dose of zoledronic acid
produced toxicological effects in other organs, including the gastrointestinal tract, liver, spleen and
lungs, and at intravenous injection sites.
Reproduction toxicity
Zoledronic acid was teratogenic in the rat at subcutaneous doses ≥ 0.2 mg/kg. Although no
teratogenicity or foetotoxicity was observed in the rabbit, maternal toxicity was found. Dystocia was
observed at the lowest dose (0.01 mg/kg bodyweight) tested in the rat.
Mutagenicity and carcinogenic potential
Zoledronic acid was not mutagenic in the mutagenicity tests performed and carcinogenicity testing did
not provide any evidence of carcinogenic potential.
PHARMACEUTICAL PARTICULARS
Mannitol
Sodium citrate
Water for injections
To avoid potential incompatibilities, Zometa concentrate is to be diluted with 0.9% w/v sodium
chloride solution or 5% w/v glucose solution.
Zometa concentrate must not be mixed with calcium or other divalent cation-containing infusion
solutions such as lactated Ringer’s solution, and should be administered as a single intravenous
solution in a separate infusion line.
Studies with glass bottles, as well as several types of infusion bags and infusion lines made from
polyvinylchloride, polyethylene and polypropylene (prefilled with 0.9% w/v sodium chloride solution
or 5% w/v glucose solution), showed no incompatibility with Zometa.
The Zometa solution is stable for 24 hours at 2°C – 8°C after further dilution in 100 ml physiological
saline or 5% w/v glucose solution.
6.4 Special precautions for storage
No special precautions for storage.
After aseptic dilution, it is preferable to use the diluted product immediately. If not used immediately,
the duration and conditions of storage prior to use are the user’s responsibility. The total time between
dilution, storage in a refrigerator at 2°C – 8°C and end of administration must not exceed 24 hours.
6.5 Nature and contents of container
Zometa 4 mg/5 ml concentrate for solution for infusion is supplied as packs containing 1, 4 or 10 vials.
Not all pack sizes may be marketed.
Vial: 5-ml plastic vial made of clear, colourless cycloolefine copolymer with fluoropolymer-coated
bromobutyl rubber stopper and aluminium cap with plastic flip-off component.
6.6 Special precautions for disposal and other handling
Prior to administration, 5.0 ml concentrate from one vial or the volume of the concentrate withdrawn
as required must be further diluted with 100 ml of calcium-free infusion solution (0.9% w/v sodium
chloride solution or 5% w/v glucose solution). If refrigerated, the solution must be allowed to reach
room temperature before administration.
MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
MARKETING AUTHORISATION NUMBERS
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 24.03.2003
Date of first renewal: 20.03.2006
10. DATE OF REVISION OF THE TEXT
A.
MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR
BATCH RELEASE
B.
CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturer responsible for batch release
Novartis Pharma GmbH
Roonstrasse 25
D-90429 Nuremberg
Germany
Manufacturing authorisation issued on 1 August 1997 by Regierungspräsidium Freiburg, Germany.
B. CONDITIONS OF THE MARKETING AUTHORISATION
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to restricted medical prescription (See Annex I: Summary of Product
Characteristics, 4.2).
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
Risk Management Plan
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version 2.0 of the Risk Management Plan (RMP) presented
in Module 1.8.2. of the Marketing Authorisation and any subsequent updates of the RMP agreed by
the CHMP.
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
FOLDING BOX FOR 1 VIAL AND 1 AMPOULE AS UNIT PACK (INCLUDING BLUE BOX)
FOLDING BOX FOR 4 VIALS AND 4 AMPOULES AS UNIT PACK (INCLUDING BLUE
BOX
NAME OF THE MEDICINAL PRODUCT
Zometa 4 mg
Powder and solvent for solution for infusion
Zoledronic acid
STATEMENT OF ACTIVE SUBSTANCE(S)
One vial contains 4 mg of zoledronic acid (anhydrous).
It also contains mannitol and sodium citrate.
The solvent ampoule contains water for injections.
PHARMACEUTICAL FORM AND CONTENTS
One vial 4 mg
One ampoule of solvent 5 ml
Four vials 4 mg
Four ampoules of solvent 5 ml
METHOD AND ROUTE(S) OF ADMINISTRATION
For intravenous use only.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
Use only as directed by a doctor.

























SPECIAL STORAGE CONDITIONS
No special precautions for storage.
The reconstituted solution is stable for 24 hours at 2°C – 8°C.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE




















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
FOLDING BOX FOR 1 VIAL AND 1 AMPOULE AS INTERMEDIATE PACK (WITHOUT
BLUE BOX)
NAME OF THE MEDICINAL PRODUCT
Zometa 4 mg
Powder and solvent for solution for infusion
Zoledronic acid
STATEMENT OF ACTIVE SUBSTANCE(S)
One vial contains 4 mg of zoledronic acid (anhydrous).
It also contains mannitol and sodium citrate.
The solvent ampoule contains water for injections.
PHARMACEUTICAL FORM AND CONTENTS
One vial 4 mg
One ampoule of solvent 5 ml
Component of a multipack comprising ten packs, each containing one vial and one ampoule
METHOD AND ROUTE(S) OF ADMINISTRATION
For intravenous use only.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
Use only as directed by a doctor.























SPECIAL STORAGE CONDITIONS
No special precautions for storage.
The reconstituted solution is stable for 24 hours at 2°C – 8°C.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE



















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
WRAPPER LABEL ON MULTIPACKS WRAPPED IN NON-TRANSPARENT FOIL
(INCLUDING BLUE BOX)
NAME OF THE MEDICINAL PRODUCT
Zometa 4 mg
Powder and solvent for solution for infusion
Zoledronic acid
STATEMENT OF ACTIVE SUBSTANCE(S)
One vial contains 4 mg of zoledronic acid (anhydrous).
It also contains mannitol and sodium citrate.
The solvent ampoule contains water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Multipack comprising ten packs, each containing one vial and one ampoule of solvent 5 ml.
METHOD AND ROUTE(S) OF ADMINISTRATION
For intravenous use only.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
Use only as directed by a doctor.





















SPECIAL STORAGE CONDITIONS
No special precautions for storage.
The reconstituted solution is stable for 24 hours at 2°C – 8°C.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE



















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
FOLDING BOX FOR 1 VIAL AS UNIT PACK (INCLUDING BLUE BOX)
FOLDING BOX FOR 4 VIALS AS UNIT PACK (INCLUDING BLUE BOX)
NAME OF THE MEDICINAL PRODUCT
Zometa 4 mg/5 ml concentrate for solution for infusion
Zoledronic acid
STATEMENT OF ACTIVE SUBSTANCE(S)
One vial contains 4 mg of zoledronic acid (anhydrous).
It also contains mannitol, sodium citrate and water for injections.
PHARMACEUTICAL FORM AND CONTENTS
One vial with 5 ml concentrate for solution for infusion
Four vials with 5 ml concentrate for solution for infusion
METHOD AND ROUTE(S) OF ADMINISTRATION
For intravenous use only.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
Use only as directed by a doctor.
SPECIAL STORAGE CONDITIONS
No special precautions for storage.
Stable for 24 hours at 2°C – 8°C after further dilution in 100 ml physiological saline or 5% w/v
glucose solution.



























10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE





















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
FOLDING BOX FOR 1 VIAL AS INTERMEDIATE PACK (WITHOUT BLUE BOX)
NAME OF THE MEDICINAL PRODUCT
Zometa 4 mg/5 ml concentrate for solution for infusion
Zoledronic acid
STATEMENT OF ACTIVE SUBSTANCE(S)
One vial contains 4 mg of zoledronic acid (anhydrous).
It also contains mannitol, sodium citrate and water for injections.
PHARMACEUTICAL FORM AND CONTENTS
One vial with 5 ml concentrate for solution for infusion
Component of a multipack comprising ten packs, each containing one vial
METHOD AND ROUTE(S) OF ADMINISTRATION
For intravenous use only.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
Use only as directed by a doctor.
SPECIAL STORAGE CONDITIONS
No special precautions for storage.
Stable for 24 hours at 2°C – 8°C after further dilution in 100 ml physiological saline or 5% w/v
glucose solution.

























10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE


















PARTICULARS TO APPEAR ON THE OUTER PACKAGING
WRAPPER LABEL ON MULTIPACKS WRAPPED IN NON-TRANSPARENT FOIL
(INCLUDING BLUE BOX)
NAME OF THE MEDICINAL PRODUCT
Zometa 4 mg/5 ml concentrate for solution for infusion
Zoledronic acid
STATEMENT OF ACTIVE SUBSTANCE(S)
One vial contains 4 mg of zoledronic acid (anhydrous).
It also contains mannitol, sodium citrate and water for injections.
PHARMACEUTICAL FORM AND CONTENTS
One vial with 5 ml concentrate for solution for infusion.
Multipack comprising ten packs, each containing one vial.
METHOD AND ROUTE(S) OF ADMINISTRATION
For intravenous use only.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
Use only as directed by a doctor.
SPECIAL STORAGE CONDITIONS
No special precautions for storage.
Stable for 24 hours at 2°C – 8°C after further dilution in 100 ml physiological saline or 5% w/v
glucose solution.

























10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE


















PACKAGE LEAFLET: INFORMATION FOR THE USER
Zometa 4 mg powder and solvent for solution for infusion
Zoledronic acid
Read all of this leaflet carefully before you are given Zometa.
−
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor, nurse or pharmacist.
If any of the side effects gets serious, or if you notice side effects not listed in this leaflet, please
tell your doctor, nurse or pharmacist.
What Zometa is and what it is used for
Before you are given Zometa
WHAT ZOMETA IS AND WHAT IT IS USED FOR
The active substance in Zometa is zoledronic acid, which belongs to a group of substances called
bisphosphonates. Zoledronic acid works by attaching itself to the bone and slowing down the rate of
bone change. It is used:
•
To treat bone metastases
(spread of cancer from the primary cancer site to the bone) and to
prevent associated/related bone complications, e.g. fractures.
To reduce the amount of calcium
in the blood in cases where it is too high due to the presence
of a tumour. Tumours can accelerate normal bone change in such a way that the release of
calcium from bone is increased. This condition is known as tumour-induced hypercalcaemia
(TIH).
BEFORE YOU ARE GIVEN ZOMETA
Follow carefully all instructions given to you by your doctor.
Your doctor will carry out blood tests before you start treatment with Zometa and will check your
response to treatment at regular intervals.
You should not be given Zometa:
−
if you are breast-feeding.
if you are allergic (hypersensitive) to zoledronic acid, another bisphosphonate (the group of
substances to which Zometa belongs), or any of the other ingredients of Zometa.
Before you are given Zometa, tell your doctor:
−
if you have or have had a
liver problem.
if you have or have had a
kidney problem
.
if you have or have had a
heart problem.
if you have or have had
pain, swelling or numbness
of the jaw, a feeling of heaviness in the
jaw or loosening of a tooth.
if you are having
dental treatment
or are due to undergo dental surgery, tell your dentist that
you are being treated with Zometa.
Using other medicines
Please tell your doctor if you are taking or have recently taken any other medicines, including
medicines obtained without a prescription. It is especially important that you tell your doctor if you are
also taking:
–
Aminoglycosides (medicines used to treat severe infections), since the combination of these
with bisphosphonates may cause the calcium level in the blood to become too low.
Thalidomide or any other medicines which may harm your kidneys.
Patients aged 65 years and over
Zometa can be given to people aged 65 years and over. There is no evidence to suggest that any extra
precautions are needed.
Use in children
There have been 2 studies on the use of Zometa in children with severe osteogenesis imperfecta (a
genetic disorder, also called “brittle bone disease”). However, it has not been established whether
these children will benefit from Zometa treatment.
Pregnancy and breast-feeding
You should not be given Zometa if you are pregnant. Tell your doctor if you are or think that you may
be pregnant.
You must not be given Zometa if you are breast-feeding.
Ask your doctor for advice before taking any medicine while you are pregnant or breast-feeding.
Driving and using machines
The effects of Zometa on driving, using machines and performing other tasks that need your full
attention have not been studied. However, there have been very rare cases of drowsiness with the use
of Zometa. You should therefore be careful when driving, using machinery or performing other tasks
that need full attention
.
Your doctor will recommend that you drink enough water before each treatment to help prevent
dehydration.
Carefully follow all the other instructions given to you by your doctor, nurse or pharmacist.
How much Zometa is given
–
If you have a kidney problem, your doctor will give you a lower dose depending on the severity
of your kidney problem.
Zometa is given as a drip (infusion) into a vein which should take at least 15 minutes and should
be administered as a single intravenous solution in a separate infusion line.
Patients whose blood calcium levels are not too high will also be prescribed calcium and vitamin D
supplements to be taken each day.
How often you will be given Zometa
–
If you are being treated for bone metastases, you will be given one infusion of Zometa every
three to four weeks.
If you are being treated to reduce the amount of calcium in your blood, you will normally only
be given one infusion of Zometa.
The usual single dose given is 4 mg.
If you are given more Zometa than you should be
If you have received doses higher than those recommended, you must be carefully monitored by your
doctor. This is because you may develop serum electrolyte abnormalities (e.g. abnormal levels of
calcium, phosphorus and magnesium) and/or changes in kidney function, including severe kidney
impairment. If your level of calcium falls too low, you may have to be given supplemental calcium by
infusion.
Like all medicines, Zometa can cause side effects, although not everybody gets them. The most
common ones are usually mild and will probably disappear after a short time.
The frequency of possible side effects listed below is defined using the following convention:
−
very common: affects more than 1 user in 10
common: affects 1 to 10 users in 100
uncommon: affects 1 to 10 users in 1,000
rare: affects 1 to 10 users in 10,000
very rare: affects less than 1 user in 10,000
not known: frequency cannot be estimated from the available data.
Tell your doctor about any of the following side effects as soon as possible:
Low level of phosphate in the blood.
Headache and a flu-like syndrome consisting of fever, fatigue, weakness, drowsiness, chills and
bone, joint and/or muscle ache. In most cases no specific treatment is required and the
symptoms disappear after a short time (couple of hours or days).
Gastrointestinal reactions such as nausea and vomiting as well as loss of appetite.
Conjunctivitis, as reported with other bisphosphonates (the group of substances to which
Zometa belongs).
Blood tests indicating changes in kidney function (higher levels of creatinine), including severe
kidney impairment. Such changes are also known to occur with other medicinal products of this
kind. In addition, some cases of kidney disease have been reported.
Low level of red blood cells (anaemia).
Low level of calcium in the blood.
Pain in the mouth, teeth and/or jaw, swelling or sores inside the mouth, numbness or a feeling of
heaviness in the jaw, or loosening of a tooth. These could be signs of bone damage in the jaw
(osteonecrosis). Tell your doctor and dentist immediately if you experience such symptoms.
Hypersensitivity reactions.
Skin reactions (redness and swelling) at the infusion site, rash, itching.
High blood pressure, shortness of breath, dizziness, sleep disturbances, tingling or numbness of
the hands or feet, diarrhoea.
Low counts of white blood cells and blood platelets.
Low level of magnesium and potassium in the blood. Your doctor will monitor this and take any
necessary measures.
Fainting due to low blood pressure.
Severe bone, joint and/or muscle pain, occasionally incapacitating.
Painful redness and/or swelling of the eye.
Irregular heart rhythm (atrial fibrillation) has been seen in patients receiving zoledronic acid for
postmenopausal osteoporosis. It is currently unclear whether zoledronic acid causes this irregular heart
rhythm but you should report it to your doctor if you experience such symptoms after you have
received zoledronic acid.
If any of the side effects gets serious,
or if you notice any side effects not listed in this leaflet, please
tell your doctor, nurse or pharmacist.
Your doctor, nurse or pharmacist knows how to store Zometa properly (see section 6).
The active substance of Zometa is zoledronic acid.
The other ingredients are: mannitol, sodium citrate.
What Zometa looks like and contents of the pack
Zometa is supplied as a powder in a vial. One vial contains 4 mg of zoledronic acid.
Each pack contains the vial with powder, together with an ampoule of 5 ml water for injections, which
is used to dissolve the powder. Zometa is supplied as packs containing 1, 4 or 10 vials and 1, 4 or
10 ampoules, respectively. Not all pack sizes may be marketed.
Marketing Authorisation Holder
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
Manufacturer
Novartis Pharma GmbH
Roonstrasse 25
D-90429 Nuremberg
Germany
For any further information about this medicine, please contact the local representative of the
Marketing Authorisation Holder:
België/Belgique/Belgien
Novartis Pharma N.V.
Tél/Tel: +32 2 246 16 11
Luxembourg/Luxemburg
Novartis Pharma GmbH
Tél/Tel: +49 911 273 0
България
Novartis Pharma Services Inc.
Тел.: +359 2 489 98 28
Magyarország
Novartis Hungária Kft. Pharma
Tel.: +36 1 457 65 00
Česká republika
Novartis s.r.o.
Tel: +420 225 775 111
Malta
Novartis Pharma Services Inc.
Tel: +356 2298 3217
Danmark
Novartis Healthcare A/S
Tlf: +45 39 16 84 00
Nederland
Novartis Pharma B.V.
Tel: +31 26 37 82 111
Deutschland
Novartis Pharma GmbH
Tel: +49 911 273 0
Norge
Novartis Norge AS
Tlf: +47 23 05 20 00
Eesti
Novartis Pharma Services Inc.
Tel: +372 60 62 400
Österreich
Novartis Pharma GmbH
Tel: +43 1 86 6570
Ελλάδα
Novartis (Hellas) A.E.B.E.
Τηλ: +30 210 281 17 12
Polska
Novartis Poland Sp. z o.o.
Tel.: +48 22 550 8888
España
Novartis Farmacéutica, S.A.
Tel: +34 93 306 42 00
Portugal
Novartis Farma - Produtos Farmacêuticos, S.A.
Tel: +351 21 000 8600
France
Novartis Pharma S.A.S.
Tél: +33 1 55 47 66 00
România
Novartis Pharma Services Inc.
Tel: +40 21 31299 01
Ireland
Novartis Ireland Limited
Tel: +353 1 260 12 55
Slovenija
Novartis Pharma Services Inc.
Tel: +386 1 300 75 77
Ísland
Vistor hf.
Sími: +354 535 7000
Slovenská republika
Novartis Slovakia s.r.o.
Tel: +421 2 5542 5439
Italia
Novartis Farma S.p.A.
Tel: +39 02 96 54 1
Suomi/Finland
Novartis Finland Oy
Puh/Tel: +358 9 61 33 22 11
Κύπρος
Δημητριάδης και Παπαέλληνας Λτδ
Τηλ: +357 22 690 690
Sverige
Novartis Sverige AB
Tel: +46 8 732 32 00
Latvija
Novartis Pharma Services Inc.
Tel: +371 7 887 070
United Kingdom
Novartis Pharmaceuticals UK Ltd.
Tel: +44 1276 698370
Lietuva
Novartis Pharma Services Inc.
Tel: +370 5 269 16 50
This leaflet was last approved in
INFORMATION FOR THE HEALTHCARE PROFESSIONAL
How to prepare and administer Zometa
To prepare an infusion solution containing 4 mg Zometa, add 5 ml of water for injections from
the ampoule supplied in the pack to the vial containing the Zometa powder under aseptic
conditions. Shake the vial gently to dissolve the powder.
Further dilute the Zometa reconstituted solution (5 ml) with 100 ml of calcium-free or other
divalent cation-free infusion solution. If a lower dose of Zometa is required, first withdraw the
appropriate volume of the reconstituted solution (4 mg/5 ml) as indicated below and then dilute
it further with 100 ml of infusion solution. To avoid potential incompatibilities, the infusion
solution used for dilution must be either 0.9% w/v sodium chloride or 5% w/v glucose solution.
Do not mix Zometa reconstituted solution with calcium-containing or other divalent
cation-containing solutions such as lactated Ringer’s solution.
Instructions for preparing reduced doses of Zometa:
Withdraw the appropriate volume of the reconstituted solution (4 mg/5 ml), as follows:
-
The Zometa infusion solution should preferably be used immediately. If the solution is not used
immediately, storage prior to use is the responsibility of the care provider and should be in a
refrigerator at 2°C – 8°C. Allow the refrigerated solution to reach room temperature before
administration.
The total time between reconstitution, dilution, storage in the refrigerator and end of
administration must not exceed 24 hours.
The solution containing Zometa is given as a single 15-minute intravenous infusion. The
hydration status of patients must be assessed prior to and following administration of Zometa to
assure that they are adequately hydrated.
Studies with glass bottles, several types of infusion bags and infusion lines made from
polyvinylchloride, polyethylene and polypropylene (prefilled with 0.9% w/v sodium chloride
solution or 5% w/v glucose solution) showed no incompatibility with Zometa.
Since no data are available on the compatibility of Zometa with other intravenously
administered substances, Zometa must not be mixed with other medications/substances and
should always be given through a separate infusion line.
Keep Zometa out of the reach and sight of children.
Do not use Zometa after the expiry date stated on the pack.
The Zometa infusion solution should preferably be used immediately. If the solution is not used
immediately, storage prior to use is the responsibility of the user and should be in a refrigerator
at 2°C – 8°C.
The total time between reconstitution, dilution, storage in the refrigerator and end of
administration must not exceed 24 hours.
PACKAGE LEAFLET: INFORMATION FOR THE USER
Zometa 4 mg/5 ml concentrate for solution for infusion
Zoledronic acid
Read all of this leaflet carefully before you are given Zometa.
−
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor, nurse or pharmacist.
If any of the side effects gets serious, or if you notice side effects not listed in this leaflet, please
tell your doctor, nurse or pharmacist.
What Zometa is and what it is used for
WHAT ZOMETA IS AND WHAT IT IS USED FOR
The active substance in Zometa is zoledronic acid, which belongs to a group of substances called
bisphosphonates. Zoledronic acid works by attaching itself to the bone and slowing down the rate of
bone change. It is used:
•
To treat bone metastases
(spread of cancer from the primary cancer site to the bone) and to
prevent associated/related bone complications, e.g. fractures.
To reduce the amount of calcium
in the blood in cases where it is too high due to the presence
of a tumour. Tumours can accelerate normal bone change in such a way that the release of
calcium from bone is increased. This condition is known as tumour-induced hypercalcaemia
(TIH).
BEFORE YOU ARE GIVEN ZOMETA
Follow carefully all instructions given to you by your doctor.
Your doctor will carry out blood tests before you start treatment with Zometa and will check your
response to treatment at regular intervals.
You should not be given Zometa:
−
if you are breast-feeding.
if you are allergic (hypersensitive) to zoledronic acid, another bisphosphonate (the group of
substances to which Zometa belongs), or any of the other ingredients of Zometa.
Before you are given Zometa, tell your doctor:
−
if you have or have had a
liver problem.
if you have or have had a
kidney problem.
if you have or have had a
heart problem.
if you have or have had
pain, swelling or numbness
of the jaw, a feeling of heaviness in the
jaw or loosening of a tooth.
if you are having
dental treatment
or are due to undergo dental surgery, tell your dentist that
you are being treated with Zometa.
Before you are given Zometa
Using other medicines
Please tell your doctor if you are taking or have recently taken any other medicines, including
medicines obtained without a prescription. It is especially important that you tell your doctor if you are
also taking:
–
Aminoglycosides (medicines used to treat severe infections), since the combination of these
with bisphosphonates may cause the calcium level in the blood to become too low.
Thalidomide or any other medicines which may harm your kidneys.
Patients aged 65 years and over
Zometa can be given to people aged 65 years and over. There is no evidence to suggest that any extra
precautions are needed.
Use in children
There have been 2 studies on the use of Zometa in children with severe osteogenesis imperfecta (a
genetic disorder, also called “brittle bone disease”). However, it has not been established whether
these children will benefit from Zometa treatment.
Pregnancy and breast-feeding
You should not be given Zometa if you are pregnant. Tell your doctor if you are or think that you may
be pregnant.
You must not be given Zometa if you are breast-feeding.
Ask your doctor for advice before taking any medicine while you are pregnant or breast-feeding.
Driving and using machines
The effects of Zometa on driving, using machines and performing other tasks that need your full
attention have not been studied. However, there have been very rare cases of drowsiness with the use
of Zometa. You should therefore be careful when driving, using machinery or performing other tasks
that need full attention
.
Your doctor will recommend that you drink enough water before each treatment to help prevent
dehydration.
Carefully follow all the other instructions given to you by your doctor, nurse or pharmacist.
How much Zometa is given
–
If you have a kidney problem, your doctor will give you a lower dose depending on the severity
of your kidney problem.
Zometa is given as a drip (infusion) into a vein which should take at least 15 minutes and should
be administered as a single intravenous solution in a separate infusion line.
Patients whose blood calcium levels are not too high will also be prescribed calcium and vitamin D
supplements to be taken each day.
How often you will be given Zometa
–
If you are being treated for bone metastases, you will be given one infusion of Zometa every
three to four weeks.
If you are being treated to reduce the amount of calcium in your blood, you will normally only
be given one infusion of Zometa.
The usual single dose given is 4 mg.
If you are given more Zometa than you should be
If you have received doses higher than those recommended, you must be carefully monitored by your
doctor. This is because you may develop serum electrolyte abnormalities (e.g. abnormal levels of
calcium, phosphorus and magnesium) and/or changes in kidney function, including severe kidney
impairment. If your level of calcium falls too low, you may have to be given supplemental calcium by
infusion.
Like all medicines, Zometa can cause side effects, although not everybody gets them. The most
common ones are usually mild and will probably disappear after a short time.
The frequency of possible side effects listed below is defined using the following convention:
−
very common: affects more than 1 user in 10
common: affects 1 to 10 users in 100
uncommon: affects 1 to 10 users in 1,000
rare: affects 1 to 10 users in 10,000
very rare: affects less than 1 user in 10,000
not known: frequency cannot be estimated from the available data.
Tell your doctor about any of the following side effects as soon as possible:
Low level of phosphate in the blood.
Headache and a flu-like syndrome consisting of fever, fatigue, weakness, drowsiness, chills and
bone, joint and/or muscle ache. In most cases no specific treatment is required and the
symptoms disappear after a short time (couple of hours or days).
Gastrointestinal reactions such as nausea and vomiting as well as loss of appetite.
Conjunctivitis, as reported with other bisphosphonates (the group of substances to which
Zometa belongs).
Blood tests indicating changes in kidney function (higher levels of creatinine), including severe
kidney impairment. Such changes are also known to occur with other medicinal products of this
kind. In addition, some cases of kidney disease have been reported.
Low level of red blood cells (anaemia).
Low level of calcium in the blood.
Pain in the mouth, teeth and/or jaw, swelling or sores inside the mouth, numbness or a feeling of
heaviness in the jaw, or loosening of a tooth. These could be signs of bone damage in the jaw
(osteonecrosis). Tell your doctor and dentist immediately if you experience such symptoms.
Hypersensitivity reactions.
Skin reactions (redness and swelling) at the infusion site, rash, itching.
High blood pressure, shortness of breath, dizziness, sleep disturbances, tingling or numbness of
the hands or feet, diarrhoea.
Low counts of white blood cells and blood platelets.
Low level of magnesium and potassium in the blood. Your doctor will monitor this and take any
necessary measures.
Fainting due to low blood pressure.
Severe bone, joint and/or muscle pain, occasionally incapacitating.
Painful redness and/or swelling of the eye.
Irregular heart rhythm (atrial fibrillation) has been seen in patients receiving zoledronic acid for
postmenopausal osteoporosis. It is currently unclear whether zoledronic acid causes this irregular heart
rhythm but you should report it to your doctor if you experience such symptoms after you have
received zoledronic acid.
If any of the side effects gets serious,
or if you notice any side effects not listed in this leaflet, please
tell your doctor, nurse or pharmacist.
Your doctor, nurse or pharmacist knows how to store Zometa properly (see section 6).
The active substance of Zometa is zoledronic acid.
The other ingredients are: mannitol, sodium citrate, water for injections.
What Zometa looks like and contents of the pack
Zometa is supplied as a liquid concentrate in a vial. One vial contains 4 mg of zoledronic acid.
Each pack contains the vial with concentrate. Zometa is supplied as packs containing 1, 4 or 10 vials.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Novartis Europharm Limited
Wimblehurst Road
Horsham
West Sussex, RH12 5AB
United Kingdom
Manufacturer
Novartis Pharma GmbH
Roonstrasse 25
D-90429 Nuremberg
Germany
For any further information about this medicine, please contact the local representative of the
Marketing Authorisation Holder:
België/Belgique/Belgien
Novartis Pharma N.V.
Tél/Tel: +32 2 246 16 11
Luxembourg/Luxemburg
Novartis Pharma GmbH
Tél/Tel: +49 911 273 0
България
Novartis Pharma Services Inc.
Тел.: +359 2 489 98 28
Magyarország
Novartis Hungária Kft. Pharma
Tel.: +36 1 457 65 00
Česká republika
Novartis s.r.o.
Tel: +420 225 775 111
Malta
Novartis Pharma Services Inc.
Tel: +356 2298 3217
Danmark
Novartis Healthcare A/S
Tlf: +45 39 16 84 00
Nederland
Novartis Pharma B.V.
Tel: +31 26 37 82 111
Deutschland
Novartis Pharma GmbH
Tel: +49 911 273 0
Norge
Novartis Norge AS
Tlf: +47 23 05 20 00
Eesti
Novartis Pharma Services Inc.
Tel: +372 60 62 400
Österreich
Novartis Pharma GmbH
Tel: +43 1 86 6570
Ελλάδα
Novartis (Hellas) A.E.B.E.
Τηλ: +30 210 281 17 12
Polska
Novartis Poland Sp. z o.o.
Tel.: +48 22 550 8888
España
Novartis Farmacéutica, S.A.
Tel: +34 93 306 42 00
Portugal
Novartis Farma - Produtos Farmacêuticos, S.A.
Tel: +351 21 000 8600
France
Novartis Pharma S.A.S.
Tél: +33 1 55 47 66 00
România
Novartis Pharma Services Inc.
Tel: +40 21 31299 01
Ireland
Novartis Ireland Limited
Tel: +353 1 260 12 55
Slovenija
Novartis Pharma Services Inc.
Tel: +386 1 300 75 77
Ísland
Vistor hf.
Sími: +354 535 7000
Slovenská republika
Novartis Slovakia s.r.o.
Tel: +421 2 5542 5439
Italia
Novartis Farma S.p.A.
Tel: +39 02 96 54 1
Suomi/Finland
Novartis Finland Oy
Puh/Tel: +358 9 61 33 22 11
Κύπρος
Δημητριάδης και Παπαέλληνας Λτδ
Τηλ: +357 22 690 690
Sverige
Novartis Sverige AB
Tel: +46 8 732 32 00
Latvija
Novartis Pharma Services Inc.
Tel: +371 7 887 070
United Kingdom
Novartis Pharmaceuticals UK Ltd.
Tel: +44 1276 698370
Lietuva
Novartis Pharma Services Inc.
Tel: +370 5 269 16 50
This leaflet was last approved in
INFORMATION FOR THE HEALTHCARE PROFESSIONAL
How to prepare and administer Zometa
To prepare an infusion solution containing 4 mg Zometa, further dilute the Zometa concentrate
(5.0 ml) with 100 ml of calcium-free or other divalent cation-free infusion solution. If a lower
dose of Zometa is required, first withdraw the appropriate volume as indicated below and then
dilute it further with 100 ml of infusion solution. To avoid potential incompatibilities, the
infusion solution used for dilution must be either 0.9% w/v sodium chloride or 5% w/v glucose
solution.
Do not mix Zometa concentrate with calcium-containing or other divalent cation-
containing solutions such as lactated Ringer’s solution.
Instructions for preparing reduced doses of Zometa:
Withdraw the appropriate volume of the liquid concentrate, as follows:
-
After preparation, Zometa infusion solution should preferably be used immediately. If the
solution is not used immediately, storage prior to use is the responsibility of the care provider
and should be in a refrigerator at 2°C – 8°C. Allow the refrigerated solution to reach room
temperature before administration.
The total time between dilution, storage in the refrigerator and end of administration must not
exceed 24 hours.
The solution containing Zometa is given as a single 15-minute intravenous infusion. The
hydration status of patients must be assessed prior to and following administration of Zometa to
ensure that they are adequately hydrated.
Studies with glass bottles, several types of infusion bags and infusion lines made from
polyvinylchloride, polyethylene and polypropylene (prefilled with 0.9% w/v sodium chloride
solution or 5% w/v glucose solution) showed no incompatibility with Zometa.
Since no data are available on the compatibility of Zometa with other intravenously
administered substances, Zometa must not be mixed with other medications/substances and
should always be given through a separate infusion line.
Keep Zometa out of the reach and sight of children.
Do not use Zometa after the expiry date stated on the pack.
The readily prepared Zometa infusion solution should preferably be used immediately. If the
solution is not used immediately, storage prior to use is the responsibility of the user and should
be in a refrigerator at 2°C – 8°C.
The total time between dilution, storage in the refrigerator and end of administration must not
exceed 24 hours.
Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/zometa.html
Copyright © 1995-2021 ITA all rights reserved.
|






















































































































































































































































































































































































































































































































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































