Product Characteristics
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS
NAME OF THE MEDICINAL PRODUCT
Zutectra 500 IU solution for injection in pre-filled syringe
QUALITATIVE AND QUANTITATIVE COMPOSITION
One pre-filled syringe of 1 ml contains Human hepatitis B immunoglobulin 500 IU.
Human protein 150 mg/ml of which at least 96 % is IgG, with a content of antibodies to hepatitis B
virus surface antigen (HBs) of 500 IU/ml.
Distribution of IgG subclasses:
IgG1:
IgA content max. 6 mg/ml.
For a full list of excipients, see section 6.1.
Solution for injection in pre-filled syringe.
The solution is clear and pale yellow or light brown.
4.1 Therapeutic indications
Prevention of hepatitis B virus (HBV) re-infection in HBV-DNA negative patients ≥ 6 months after
liver transplantation for hepatitis B induced liver failure.
Zutectra is indicated in adults only.
The concomitant use of adequate virostatic agents should be considered, if appropriate, as standard of
hepatitis B re-infection prophylaxis.
4.2
Posology and method of administration
In HBV-DNA negative adults ≥ 6 months after liver transplantation:
-
patients with bodyweight < 75 kg: 500 IU (1 ml)/week
patients with bodyweight ≥ 75 kg: 1,000 IU (2 times 1 ml)/week
Prior to the initiation of subcutaneous treatment with Zutectra, anti-HBs serum levels should be
stabilised with an adequate intravenous hepatitis B immunoglobulin to levels at or above 300-500 IU/l.
The first Zutectra dose should be administered approximately 14-21 days after the last intravenous
dose at stabilised anti-HBs serum levels in order to ensure adequate anti-HBs coverage during the
transition from intravenous to subcutaneous dosing. The dose of Zutectra can be adapted up to
1,000 IU/week to maintain an anti-HBs serum level of > 100 IU/l in HBsAg-negative and HBV-DNA
negative patients.
Patients must be monitored for serum anti-HBs antibody levels regularly.
There is no relevant indication for use of Zutectra in children.
Zutectra should be administered via the subcutaneous route.
Zutectra is accompanied by a package leaflet with detailed instruction for use to be followed. Injection
of the medicinal product by the patient or by caregiver in a home treatment requires training by a
physician experienced in the guidance of patients for home treatment. The patient or caregiver will be
instructed in injection techniques, the keeping of a treatment diary and measures to be taken in case of
severe adverse events. A sufficient surveillance period with stable anti-HBs trough serum levels of
> 100 IU/l as well as a fixed dosage regimen is required. In addition patient or caregiver must comply
with the injection technique as well as with the dosing regimen to ensure anti-HBs trough serum levels
> 100 IU/l after extended periods between level controls.
Hypersensitivity to any of the components.
Hypersensitivity to human immunoglobulins.
Zutectra must not be administered intravascularly.
4.4 Special warnings and precautions for use
Ensure that Zutectra is not administered into a blood vessel, because of the risk of shock.
If the recipient is a carrier of HBsAg, there is no benefit in administering this medicinal product.
There is no data about efficacy in post-exposure prophylaxis.
True hypersensitivity reactions are rare.
Zutectra contains a small quantity of IgA. Individuals who are deficient in IgA have the potential for
developing IgA antibodies and may have anaphylactic reactions after administration of blood
components containing IgA. The physician must therefore weigh the benefit of treatment with Zutectra
against the potential risk of hypersensitivity reactions.
Rarely, human hepatitis B immunoglobulin can induce a fall in blood pressure with anaphylactic
reaction, even in patients who have tolerated previous treatment with human immunoglobulin.
Potential complications can often be avoided by ensuring that:
-
patients are not sensitive to human normal immunoglobulin, by first injecting the medicinal
product slowly;
patients are carefully monitored for any symptoms throughout the injection. The patients should
be observed for at least 20 minutes after administration.
Suspicion of allergic or anaphylactic type reactions requires immediate discontinuation of the
injection. In case of shock, standard medical treatment for shock should be implemented.
Standard measures to prevent infections resulting from the use of medicinal products prepared from
human blood or plasma include selection of donors, screening of individual donations and plasma
pools for specific markers of infection and the inclusion of effective manufacturing steps for the
inactivation/removal of viruses. Despite this, when medicinal products prepared from human blood or
plasma are administered, the possibility of transmitting infective agents cannot be totally excluded.
This also applies to unknown or emerging viruses and other pathogens.
The measures taken are considered effective for enveloped viruses such as HIV, HBV and HCV, and
for the non-enveloped virus HAV. The measures taken may be of limited value against non-enveloped
viruses such as parvovirus B19.
There is reassuring clinical experience regarding the lack of hepatitis A or parvovirus B19
transmission with immunoglobulins and it is also assumed that the antibody content makes an
important contribution to the viral safety.
It is strongly recommended that every time that Zutectra is administered to a patient, the name and
batch number of the medicinal product are recorded in order to maintain a link between the patient and
the batch of the medicinal product. This recommendation applies also for documentation in the
treatment diary during self-administration of the medicinal product in a home treatment.
4.5 Interaction with other medicinal products and other forms of interaction
Live attenuated virus vaccines
Immunoglobulin administration may interfere with the development of an immune response to live
attenuated virus vaccines such as rubella, mumps, measles and varicella for a period of 3 months.
After administration of this medicinal product, an interval of at least 3 months should elapse before
vaccination with live attenuated virus vaccines.
Human hepatitis B immunoglobulin should be administrated three to four weeks after vaccination with
such a live attenuated vaccine; in case administration of human hepatitis B immunoglobulin is
essential within three to four weeks after vaccination, then revaccination should be performed three
months after the administration of human hepatitis B immunoglobulin.
Interference with serological testing
After injection of immunoglobulin the transitory rise of the various passively transferred antibodies in
the patient’s blood may result in misleading positive results in serological testing.
Passive transmission of antibodies to erythrocyte antigens, e.g. A, B, D may interfere with some
serological tests for red cell antibodies, for example the antiglobulin test (Coombs’ test).
4.6 Pregnancy and lactation
The safety of this medicinal product for use in human pregnancy has not been established in controlled
clinical trials and therefore should only be given with caution to pregnant women and breast-feeding
mothers. Clinical experience with immunoglobulins suggests that no harmful effects on the course of
pregnancy, or on the foetus and the neonate are to be expected.
4.7 Effects on ability to drive and use machines
No effects on ability to drive and use machines have been observed.
The following undesirable effects have been reported in the context of 697 subcutaneous applications
of Zutectra during two completed clinical trials.
Most adverse drug reactions (ADRs) observed in the two clinical trials were mild to moderate in
nature.
The ADRs reported in the two trials are summarised and categorised according to the MedDRA
system organ class and frequency below. Frequency per injection has been evaluated using the
following criteria: very common (≥ 1/10), common (≥ 1/100 to < 1/10), uncommon (≥ 1/1,000 to
< 1/100), rare (≥ 1/10,000 to < 1/1,000), very rare (< 1/10,000), not known (cannot be estimated from
the available data).
The effects were grouped by system organ classes under relevant medical headings.
Unspecific hypersensitivity reactions:
MedDRA System
Organ Class
Gastrointestinal disorders
Injection site reactions:
MedDRA System
Organ Class
General disorders and
administration site conditions
Pain, urticaria, haematoma
Other:
Additionally the following single case reports have been received from completed clinical trials:
General disorders and administration site conditions: fatigue, tiredness.
With normal immunoglobulins adverse reactions such as chills, headache, fever, vomiting, allergic
reactions, nausea, arthralgia, low blood pressure and moderate low back pain may occur occasionally.
Rarely human normal immunoglobulins may cause a sudden fall in blood pressure and, in isolated
cases, anaphylactic shock, even when the patient has shown no hypersensitivity to previous
administration.
Local reactions at injection sites: swelling, soreness, redness, induration, local heat, itching, bruising
and rash.
For safety with respect to transmissible agents, see section 4.4.
Consequences of an overdose are not known.
PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Specific immunoglobulins, ATC code: J06BB04
Zutectra contains mainly immunoglobulin G (IgG) with a specifically high content of antibodies
against hepatitis B virus surface antigen (HBs).
The open, prospective, single-arm clinical trial (Biotest 958) enrolled 23 liver transplant recipients,
who had been receiving intravenous hepatitis B immunoglobulin prophylaxis and subsequently
switched to subcutaneous Zutectra. The weekly subcutaneous dose was 500 IU for patients with
bodyweight < 75 kg (a dose increase to 1000 IU was allowed, if medically required to maintain a
safety level of > 100 IU) and 1000 IU for patients with bodyweight ≥ 75 kg . 2 patients received a
higher and 2 patients received a lower dose than recommended by the weight based dosing regimen.
Serum anti-HBs trough levels of 100 IU/l and higher (primary efficacy endpoint) were maintained for
all patients during the 18 to 24 week trial period. The > 100 IU/l safety margin is the generally










accepted level of effective prevention against HBV re-infection in liver transplant patients at risk. No
patient experienced HBV re-infection. Self-administration was feasible for most patients.
The mean anti-HBs serum level before switching was 393±139 IU/l. All patients used antiviral
medication.
Using the Clopper Pearson method, the failure rate after 18 weeks was 0 % for patients of the ITT set
(95 % CI: [0, 14.8 %]). A failure rate of 0 % was also found for the facultative extension phase
(week 24) (95 % CI: [0, 20.6 %])
5.2 Pharmacokinetic properties
Zutectra is slowly absorbed into the recipient’s circulation and reaches a maximum after a delay of
2-7 days.
Zutectra has a half-life of about 3-4 weeks. This half-life may vary from patient to patient.
IgG and IgG-complexes are broken down in the reticuloendothelial system.
5.3 Preclinical safety data
Immunoglobulins are normal constituents of the human body, therefore toxicity testing in
heterologous species is of no relevance.
In a local tolerance trial in rabbits, there was no evidence of irritation attributable to Zutectra.
No other non-clinical trials have been carried out.
PHARMACEUTICAL PARTICULARS
Glycine
Water for injections
This medicinal product must not be mixed with other medicinal products.
No other preparations may be added to the Zutectra solution as any change in the electrolyte
concentration or the pH may result in precipitation or denaturisation of the proteins.
The solution should be administered immediately after opening the syringe.
6.4 Special precautions for storage
Store and transport refrigerated (2°C-8°C).
Keep the container in the outer carton in order to protect from light.
6.5
Nature and contents of container
One ml solution in a pre-filled syringe (Type I glass) with a stopper (bromobutyl) and a tip cap
(bromobutyl rubber).
Pack size of five syringes in a blistered pack.
6.6 Special precautions for use and handling and disposal
This medicinal product should be brought to room temperature (approx. 23°C-27°C) before use.
The solution can vary from colourless to pale yellow up to light brown.
Solutions that are cloudy or have deposits should not be used.
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
MARKETING AUTHORISATION HOLDER
Biotest Pharma GmbH
Landsteinerstrasse 5
D-63303 Dreieich
Germany
Tel.: +49 6103 801-0
Telefax: +49 6103 801-150 / +49 6103 801-727
MARKETING AUTHORISATION NUMBER(S)
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
Detailed information on this medicine is available on the European Medicines Agency (EMEA) web
site:
http://www.emea.europa.eu/
.
MANUFACTURER OF THE BIOLOGICAL ACTIVE
SUBSTANCE AND MANUFACTURING AUTHORISATION
HOLDER RESPONSIBLE FOR BATCH RELEASE
CONDITIONS OF THE MARKETING AUTHORISATION
A. MANUFACTURER OF THE BIOLOGICAL ACTIVE SUBSTANCE AND
MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
RELEASE
Name and address of the manufacturers of the biological active substance
Biotest Pharma GmbH
Landsteinerstr. 5
D-63303 Dreieich
Germany
Biotest AG
Landsteinerstr. 5
D-63303 Dreieich
Germany
Name and address of the manufacturer responsible for batch release
Biotest Pharma GmbH
Landsteinerstrasse 5
D-63303 Dreieich
Germany
B. CONDITIONS OF THE MARKETING AUTHORISATION
•
CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE IMPOSED ON
THE MARKETING AUTHORISATION HOLDER
Medicinal product subject to medical prescription.
•
CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
Pharmacovigilance system
The MAH must ensure that the system of pharmacovigilance, as described in version BE-T:0001-
00/02 presented in Module 1.8.1. of the Marketing Authorisation Application, is in place and
functioning before and whilst the product is on the market.
Risk Management Plan
The MAH commits to performing the studies and additional pharmacovigilance activities detailed in
the Pharmacovigilance Plan, as agreed in version 3.0 of the Risk Management Plan (RMP) presented
in Module 1.8.2. of the Marketing Authorisation Application and any subsequent updates of the RMP
agreed by the CHMP.
As per the CHMP Guideline on Risk Management Systems for medicinal products for human use, the
updated RMP should be submitted at the same time as the next Periodic Safety Update Report
(PSUR).
In addition, an updated RMP should be submitted
•
When new information is received that may impact on the current Safety Specification,
Pharmacovigilance Plan or risk minimisation activities
•
Within 60 days of an important (pharmacovigilance or risk minimisation) milestone being
reached
•
At the request of the EMEA
Official batch release: in accordance with Article 114 Directive 2001/83/EC as amended, the
official batch release will be undertaken by a state laboratory or a laboratory designated for that
purpose.
ANNEX III
LABELLING AND PACKAGE LEAFLET
PARTICULARS TO APPEAR ON THE OUTER PACKAGING
NAME OF THE MEDICINAL PRODUCT
Zutectra 500 IU solution for injection in pre-filled syringe
Human hepatitis B immunoglobulin
STATEMENT OF ACTIVE SUBSTANCE(S)
1 ml contains:
Human protein 150 mg of which at least 96 % is IgG, with a content of antibodies to hepatitis B virus
surface antigen (HBs) of 500 IU.
IgG subclass distribution:
59 % IgG1, 35 % IgG2, 3 % IgG3, 3 % IgG4
IgA content
≤
6 mg/ml
Excipients: Glycine, water for injections.
PHARMACEUTICAL FORM AND CONTENTS
Solution for injection in pre-filled syringe (500 IU/ml) - pack size of 5.
METHOD AND ROUTE(S) OF ADMINISTRATION
For subcutaneous use only. Do not inject intravenously.
Read the package leaflet before use.
SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE REACH AND SIGHT OF CHILDREN
Keep out of the reach and sight of children.
OTHER SPECIAL WARNING(S), IF NECESSARY
EXP
The solution should be administered immediately after opening the syringe.






















SPECIAL STORAGE CONDITIONS
Store and transport refrigerated (2°C-8°C).
Do not freeze.
Keep the container in the outer carton in order to protect from light.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
Biotest Pharma GmbH
Landsteinerstr. 5
D-63303 Dreieich
Germany
12. MARKETING AUTHORISATION NUMBER(S)
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription.
16. INFORMATION IN BRAILLE



















PACKAGE LEAFLET: INFORMATION FOR THE USER
Zutectra 500 IU solution for injection in pre-filled syringe
Human hepatitis B immunoglobulin
Read all of this leaflet carefully before you start using this medicine.
-
Keep this leaflet. You may want to read it again.
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet,
please tell your doctor or pharmacist.
What Zutectra is and what it is used for
How to inject Zutectra by yourself or by caregiver
WHAT ZUTECTRA
IS AND WHAT IT IS USED FOR
Zutectra contains antibodies against the hepatitis B virus which are the body's own defensive
substances to protect you from hepatitis B. Hepatitis B is an inflammation of the liver caused by the
hepatitis B virus.
What Zutectra is used for
Zutectra is used to prevent re-infection of hepatitis B in adults who have had a liver transplant at least
6 months ago because they had liver failure caused by hepatitis B.
Please read this section carefully. The information given should be taken into consideration by you
and your doctor before you receive Zutectra.
Zutectra
should not be used
-
if you are
allergic (hypersensitive) to human immunoglobulin or any of the other ingredients
of Zutectra
(see list of ingredients in Section 6).
An allergic reaction may include sudden wheeziness, difficulty in breathing, fast pulse, swelling of the
eyelids, face, lips, throat or tongue, rash or itching.
Please tell your doctor or healthcare professional prior to treatment
about any medicine or food
which, previously, you have not tolerated well.
Take special care with Zutectra
Zutectra is for subcutaneous injection under the skin only. Injection into a vein or a blood vessel may
result in allergic shock.
if you have been told that you have antibodies against immunoglobulins of the type IgA in your
blood. This is very rare and may result in allergic reactions.
You may be allergic (hypersensitive) to immunoglobulins
(antibodies) without knowing it, even if
you have tolerated previous treatments with human immunoglobulins. Particularly if you do not have
enough immunoglobulins of the type IgA in your blood, allergic reactions such as a sudden fall in
blood pressure or shock may occur.
You will be carefully observed during and shortly after the 1
st
injection with Zutectra
to make
sure that you do not suffer from a reaction. If you have an allergic reaction to Zutectra, the injection
will be stopped immediately. Please tell your doctor or healthcare professional immediately if you
notice such reactions during your injection with Zutectra.
If you are HBs antigen positive
you will not receive Zutectra since there is no benefit in
administering this medicine to you. Your doctor will be able to explain this to you.
For your own safety
you will be monitored for antibody levels regularly.
Information on the starting material of Zutectra
and the possibility of transmission of infectious
agents:
The starting material or what Zutectra is made from is human blood plasma (this is the liquid part of
the blood).
When medicines are made from human blood or plasma, certain measures are put in place to prevent
infections being passed on to patients. These include
-
careful selection of blood and plasma donors to make sure those at risk of carrying infections
are excluded,
and
-
the testing of each donation and pools of plasma for signs of virus/infections.
Manufacturers of these medicines also include steps in the processing of the blood or plasma that can
inactivate or remove viruses. Despite these measures, when medicines prepared from human blood or
plasma are administered, the possibility of passing on infection cannot be totally excluded. This also
applies to any unknown or emerging viruses or other types of infections.
The measures taken are considered effective for enveloped viruses such as human immunodeficiency
virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV), and for the non-enveloped hepatitis
A virus. The measures taken may be of limited value against non-enveloped viruses such as parvovirus
B19 virus.
Immunoglobulins like Zutectra have not been associated with hepatitis A or parvovirus B19 infections
possibly because the antibodies against these infections, which are contained in the product, are
protective.
It is strongly recommended that every time Zutectra is used (both at hospital or in a home treatment),
the name and batch number of the medicine
are recorded in order to maintain a record of the
batches used.
Taking other medicines
Please tell your doctor or healthcare professional if you are taking or have recently taken any other
medicines including medicines obtained without a prescription.
Zutectra can reduce the effectiveness of some vaccines (measles, rubella, mumps, chicken pox) for a
Please tell your doctor or healthcare professional prior to treatment
-
period of up to 3 months.
You may have to wait at least 3 months after the last injection of Zutectra before you can have some
vaccines.
Please tell your doctor about your treatment with Zutectra prior to any vaccination.
Blood tests
Zutectra might affect the results of certain blood tests (serological tests). Please tell your doctor about
your treatment with Zutectra prior to any blood test.
Pregnancy and breast-feeding
Please tell your doctor or healthcare professional if you are pregnant or breast-feeding. Your doctor
will decide whether you can receive Zutectra during pregnancy and breast-feeding.
Driving and using machines
Zutectra has no known effects on your ability to drive or use machines.
Zutectra is intended for subcutaneous (under the skin) injection. The contents of one syringe are
intended for use once only.
This medicine must not be injected into a blood vessel.
In most cases you will be given the injection by your doctor or nurse. However, if your antibody levels
are sufficient and you have a fixed dosage regimen, you or your caregiver may be trained to carry out
the injection at home (see below).
For the documentation of your injections of Zutectra it is strongly recommended to use the treatment
diary. Your doctor will explain you how to use it.
The dose will depend on your condition and your body weight. Your doctor will tell you how much
and how often you need to use Zutectra.
patients with bodyweight less than 75 kg: 500 IU (1 ml) per week
patients with bodyweight equal or more than 75 kg: 1000 IU (2 times 1 ml) per week
Up to 1000 IU per week can be given to get sufficient antibody levels.
Injecting by yourself or by caregiver
You can inject Zutectra yourself without the help of your doctor, if they have trained you to do this.
If
you are administering Zutectra
yourself, please read instructions in the section “HOW TO
INJECT ZUTECTRA BY YOURSELF OR BY CAREGIVER” carefully.
If you use more Zutectra
than you should
There are no known consequences of an overdose. However, if you have used more than the
prescribed dose of Zutectra, contact your doctor, healthcare professional or pharmacist straight away
for advice.
If you miss your usual dose
Do not take a double dose to make up for a forgotten injection. Talk to your doctor about managing
the dose. Your doctor will tell you how much and how often you need to use Zutectra.
Make sure you use Zutectra as prescribed and as instructed by your doctor to avoid the risk of a
hepatitis B re-infection.
Like all medicines, Zutectra can have side effects, although not everybody gets them.
The frequency of possible side effects listed below is defined using the following convention:
very common (affects more than 1 user in 10)
common (affects 1 to 10 users in 100)
uncommon (affects 1 to 10 users in 1,000)
rare (affects 1 to 10 users in 10,000)
very rare (affects less than 1 user in 10,000)
not known (frequency cannot be estimated from the available data).
If you notice any of the following effects, tell your doctor immediately:
-
swelling of the eyelids, face, lips, throat or tongue,
low blood pressure, fast pulse
This can be an allergic reaction (hypersensitivity reaction) or a serious allergic reaction
(anaphylactic shock).
The following side effects have been reported:
Common
(likely to occur in more than 1 in 100 but less than 1 in 10 patients):
-
injection site reactions: pain, hives (urticaria), haematoma (a collection of blood in tissue under the
skin)
Uncommon
(likely to occur in more than 1 in 1 000 but less than 1 in 100 patients):
-
headache
-
upper abdominal pain (from your chest to the belly button)
Furthermore the following single case reports have been reported:
-
With other human immunoglobulin preparations the following additional symptoms have been
reported:
-
mild hypersensitivity reactions (allergic reactions),
The following local reactions at the injection site may occur: swelling, soreness, redness, hardening of
the skin, local heat, itching, bruising and rash.
You must closely monitor and carefully observe yourself for any symptoms throughout the
injection. Any injection-related adverse events should be treated by stopping the injection and
you must speak to your doctor immediately.
Please tell your doctor, healthcare professional or pharmacist:
if any of the side effects gets serious,
or
if you notice a side effect not listed in this leaflet.
Keep out of the reach and sight of children.
Do not use Zutectra after the expiry date which is stated on the outer carton and the syringe
label after EXP. The expiry date refers to the last day of that month.
Store and transport refrigerated (2°C-8°C).
Keep the container in the outer carton in order to protect from light.
Zutectra must be brought to room temperature (approx. 23°C-27°C) before use. The solution
should be administered immediately after opening the syringe.
Do not use Zutectra if you notice that the solution is cloudy or has particles.
Any unused medicine or waste material should be disposed of in accordance with local
requirements. Once the injection has been completed, dispose of all needles, syringes and empty
glass containers without delay in a container intended for sharp objects you were provided with.
The
active substance
of Zutectra is human hepatitis B immunoglobulin 500 IU/ml.
Zutectra contains 150 mg/ml of human plasma protein of which at least 96 % is
immunoglobulin G (IgG). The maximum immunoglobulin A (IgA) content is 6 mg/ml.
The
other ingredients
are glycine and water for injections.
What Zutectra looks like and the contents of the pack
Zutectra is presented as a solution for injection provided in pre-filled syringes (500 IU/ml - pack size
of 5 in a blister). The colour of the solution can vary from clear to pale yellow or light brown.
One pre-filled syringe of 1 ml Zutectra contains 500 IU. Zutectra is supplied in a pack size containing
5 pre-filled syringes each in a blister pack.
Marketing Authorisation Holder and Manufacturer
Biotest Pharma GmbH
Landsteinerstrasse 5
D-63303 Dreieich
Germany
Tel.: + 49 6103 801-0
Fax: + 49 6103 801-150 / -727
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder:
België/Belgique/Belgien
Infarama bvba
Costermansstede 1
Luxembourg/Luxemburg
Biotest AG
Landsteinerstr. 5
B-1850 Grimbergen
Tél/Tel: + 32 22709522
D-63303 Dreieich
Tél/Tel: + 49 6103 801-0
България
АНТИСЕЛ Братя Селидис България ООД
Ул. „Свети Иван Рилски” 33-35
София 1606
Teл.: + 359 2 953 1224
Magyarország
Biotest Hungaria Kft.
Torbágy u. 15/A
H-2045 Törökbálint
Tel.: + 36 23 511 311
Česká republika
Reg-Pharm spol.s.r.o.
Fialková 45
CZ-10600 Praha 10
Tel: + 420 2 7265 4004
Malta
Rodel Ltd
55, Ravina
Triq ir-Russett
MT-Kappara SGN 4432
Tel: + 356 27 386221
Danmark
Biotest AG
Landsteinerstrasse 5
D-63303 Dreieich
Tel: + 49 6103 801-0
Nederland
Infarama bvba
Costermansstede 1
B-1850 Grimbergen
Tel: + 32 22709522
Deutschland
Biotest AG
Landsteinerstrasse 5
D-63303 Dreieich
Tel: + 49 6103 801-0
Norge
Biotest AG
Landsteinerstrasse 5
D-63303 Dreieich
Tel: + 49 6103 801-0
Eesti
Biotest AG
Landsteinerstrasse 5
D-63303 Dreieich
Tel: + 49 6103 801-0
Österreich
Biotest Austria GmbH
Einsiedlergasse 58
A-1053 Wien
Tel: + 43 1 545 15 61-0
Ελλάδα
BIOTEST ΕΛΛΑΣ Μ.Ε.Π.Ε
25, Κυπρίων Αγωνιστών
GR-151 26 Μαρούσι
Τηλ: + 30 210 8043-437
Polska
Nobipharm Sp. Z.o.o.
ul Rydygiera 8
PL–01-793 Warszawa
Tel.: + 48 22 8322638
España
Biotest Medical, S.L.U.
C/Beethoven, 15, 4a Planta
ES-08021 Barcelona
Tel: + 34 931 838 734
Portugal
SPCare Especialidades Farmacêuticas, Lda
Rua Luciano Cordeiro, nº 123, 1º dto.
PT-1600 139 Lisboa
Tel: + 351 21 193 14 20
France
Biotest S.a.r.l.
375 rue Morane Saulnier, BP 65
F-78534 Buc Cedex
Tél: + 33 1 3920 2080
România
HK & ZY Best Pharma Distribution SRL.
Str General Ştefan Burileanu, nr 8
Bucharest 013762 - RO
Tel: + 40 21 4109494
Ireland
Intrapharma Ltd. Pharmaceutical Suppliers
Unitech House Magna Business Park,
Slovenija
Blood Transfusion Centre of Slovenia (BTC)
Slajmerjeva 6
Magna Drive, Citywest Road
IRL-Dublin 24
Tel: + 353 1 404 8344
SI-1000 Ljubljana
Tel: + 386 1 54 38 100
Ísland
Biotest AG
Landsteinerstrasse 5
D-63303 Dreieich
Tel: + 49 6103 801-0
Slovenská republika
Reg-Pharm spol.s.r.o.
Fialková 45
CZ-10600 Praha 10
Tel: + 420 2 7265 4004
Italia
Biotest Italia S.r.l.
Via Leonardo da Vinci 43
I-20090 Trezzano sul Naviglio
Tel: + 39 02 4844 2951
Suomi/Finland
Biotest AG
Landsteinerstrasse 5
D-63303 Dreieich
Tel: + 49 6103 801-0
Κύπρος
ΑΚΗΣ ΠΑΝΑΓΙΩΤΟΥ & ΥΙΟΣ ΛΤΔ
Γ. ΚΡΑΝΙΔΙΩΤΗ
Τ. Θ. 22578 1522 ΛΕΥΚΩΣΙΑ
Κ Υ Π Ρ Ο Σ
Τηλ: + 357 22 611 038
Sverige
Biotest AG
Landsteinerstrasse 5
D-63303 Dreieich
Tel: + 49 6103 801-0
Latvija
Biotest AG
Landsteinerstrasse 5
D-63303 Dreieich
Tel: + 49 6103 801-0
United Kingdom
Biotest UK Ltd.
28 Monkspath Business Park, Highlands Road,
Shirley, Solihull
West Midlands B90 4NZ–UK
Tel: + 44 121 733 3393
Lietuva
Biotest AG
Landsteinerstrasse 5
D-63303 Dreieich
Tel: + 49 6103 801-0
This leaflet was last approved in
Detailed information on this medicine is available on the European Medicines Agency (EMEA) web
site:
http://www.emea.europa.eu/
.
HOW TO INJECT ZUTECTRA BY YOURSELF OR BY CAREGIVER
The following instructions are intended to explain how to inject Zutectra. Please read the instructions
carefully and follow them step by step. The doctor or his/her assistant will teach you the process of
administration.
Do not attempt to inject Zutectra until you are sure that you understand how to prepare the injection
solution and give the injection.
Keep the syringes and syringe disposal unit out of the reach of children; lock the supplies if
possible.
Try to take the injection at the same time of day. This makes it easier to remember it.
Always double-check the dosage.
The solution must be brought to room temperature before use.
Open each syringe only when you are ready for an injection. You should administer the
injection immediately after opening the syringe.
The colour of the solution can vary from clear to pale yellow up to light brown. Do not use
solutions that are cloudy or have particles.
This medicine must not be mixed with other medicines.
1.
Wash your hands. It is important to have your hands and the items you use as clean as possible.
2.
Lay out everything you need in advance. Find a clean place where you can spread out all the items
you are going to use:
one needle suitable for subcutaneous injection.
Please note that alcohol swabs and needles are not contained in the pack and you need to supply them
yourself.
3.
Before preparing the injection, decide where you are going to inject. You should inject Zutectra into
the fatty layer between the skin and muscle (about 8 to 12 mm under the skin). The best places for
injections are where the skin is loose and soft for example in the abdomen, arm, thigh or buttocks, and
away from joints, nerves, bones.
Important:
Do not use on any area where you can feel lumps, bumps, firm knots, pain or on an area
that is discoloured, indented, scabbed, or where the skin is broken. Talk to the doctor or healthcare
professional about these or any other unusual conditions you may find. You should rotate the injection
site at every injection. If some areas are too difficult for you to reach, you may need a caregiver to
help you with these injections.
4.
Prepare the Zutectra syringe:
-
Take the syringe out of the pack.
-
Examine the solution carefully. It should be clear
and contain no particles. If the solution is
discoloured, cloudy or contains particles, discard
it and start again with a new syringe.
-
Remove the protective cap from the syringe.
-
Take the needle out of its sterile pack and fit the
needle onto the syringe.
5.
Get rid of any air bubbles there may be in the syringe.
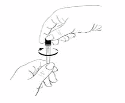

-
Hold the syringe with the needle pointing
upwards, and tap the syringe gently with your
fingers until the air has collected at the tip.
Carefully push the plunger in until the air bubbles
have disappeared.
1.
Choose the area where you will make the injection and make a note of it in the diary.
Abdomen
(stomach): Do not use the area within
one inch around the navel. Avoid using the belt
line area, as rubbing may irritate the injection site.
Avoid surgical scars. This is likely to be the easiest
place to inject if you are doing it yourself.
Thighs:
Use middle and outer areas where you can
pinch up tissue. You are likely to have more fatty
tissue the closer you are to the hip and the further
you are from the knee.
Arms:
The back of the upper arm should be used. It
is hard to pinch up the tissue and inject Zutectra
yourself using this site. If you do choose to inject
your arm yourself, try to pinch up the tissue by
placing your upper arm over the back of a chair or
brace it against a wall. It is much easier for
someone else to use this site if you do need help.
Buttocks:
Use any area where you can pinch up
tissue. It’s harder to give yourself an injection
here. Try standing in front of a mirror to locate the
site or you may want to ask your caregiver to give
you the injection.
It's important to
change (rotate) the injection sites
. This will help the skin stay supple and help the
medicine be absorbed evenly. Rotating sites means starting at one site and using all other sites before
going back to the first site you used. Then start the rotation again. It may be helpful to keep a record of
where you had the last injection to avoid problems.
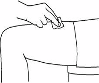
The administration in thighs is shown as an example in the following pictures:
2.
Wipe the intended area with an alcohol swab. Let
the skin air-dry.
3.
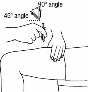
Gently pinch the skin together around the
disinfected injection site (to raise it up a little) and
push the needle into the skin with a rapid, confident
movement at an angle of 45 to 90 degrees. Inject
beneath the skin as you have been shown by the
doctor or nurse.




4.
Inject the liquid by pressing gently on the plunger.
Allow yourself enough time to inject the whole of the
solution until the syringe is empty.
5.
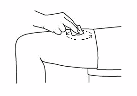
Then pull the needle out immediately and let go of
the pinched skin.
6.
Clean the injection site by wiping it in a circular
motion with the alcohol swab.
Dispose of all used items
Once the injection has been completed, dispose of all needles and empty glass containers without
delay in a container intended for sharp objects.

Source: European Medicines Agency
 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).
- If you wish to link to this page, you can do so by referring to the URL address below this line.
https://theodora.com/drugs/eu/zutectra.html
Copyright © 1995-2021 ITA all rights reserved.
|





























































































 - Please bookmark this page (add it to your favorites).
- Please bookmark this page (add it to your favorites).










































